Abstract
Background
Parenteral fluids are commonly used in people with acute stroke with poor oral fluid intake. However, the balance between benefit and harm for different fluid regimens is unclear.
Objectives
To assess whether different parenteral fluid regimens lead to differences in death, or death or dependence, after stroke based on fluid type, fluid volume, duration of fluid administration, and mode of delivery.
Search methods
We searched the Cochrane Stroke Group Trials Register (May 2015), the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) (Cochrane Library 2015, Issue 5), MEDLINE (2008 to May 2015), EMBASE (2008 to May 2015), and CINAHL (1982 to May 2015). We also searched ongoing trials registers (May 2015) and reference lists, performed cited reference searches, and contacted authors.
Selection criteria
Randomised trials of parenteral fluid regimens in adults with ischaemic or haemorrhagic stroke within seven days of stroke onset that reported death or dependence.
Data collection and analysis
One review author screened titles and abstracts. We obtained the full‐text articles of relevant studies, and two review authors independently selected trials for inclusion and extracted data. We used Cochrane's tool for bias assessment.
Main results
We included 12 studies (2351 participants: range 27 to 841).
Characteristics: The 12 included studies compared hypertonic (colloids) with isotonic fluids (crystalloids); of these, five studies (1420 participants) also compared 0.9% saline with another fluid. No data were available to make other comparisons. Delay from stroke to recruitment varied from less than 24 hours to 72 hours. Duration of fluid delivery was between two hours and 10 days.
Bias assessment: Investigators and participants in eight of the 12 included studies were blind to treatment allocation, seven of the 12 included studies gave details of randomisation, and eight of the 12 included studies reported all outcomes measured.
Results: There were no relevant completed trials that addressed the effect of volume, duration, or mode of fluid delivery on death or dependence in people with stroke.
The odds of death or dependence were similar in participants allocated to colloids or crystalloid fluid regimens (odds ratio (OR) 0.97, 95% confidence interval (CI) 0.79 to 1.21, five studies, I² = 58%, low‐quality evidence), and between 0.9% saline or other fluid regimens (OR 1.04, 95% CI 0.82 to 1.32, three studies, I² = 71%, low‐quality evidence). There was substantial heterogeneity in these estimates.
The odds of death were similar between colloids and crystalloids (OR 1.02, 95% CI 0.82 to 1.27, 12 studies, I² = 24%, moderate‐quality evidence), and 0.9% saline and other fluids (OR 0.87, 95% CI 0.67 to 1.12, five studies, I² = 53%, low‐quality evidence). The odds of pulmonary oedema were higher in participants allocated to colloids (OR 2.34, 95% CI 1.28 to 4.29, I² = 0%). Although the studies observed a higher risk of cerebral oedema (OR 0.20, 95% CI 0.02 to 1.74) and pneumonia (OR 0.58, 95% CI 0.17 to 2.01) with crystalloids, we could not exclude clinically important benefits or harms.
Authors' conclusions
We found no evidence that colloids were associated with lower odds of death or dependence in the medium term after stroke compared with crystalloids, though colloids were associated with greater odds of pulmonary oedema. We found no evidence to guide the best volume, duration, or mode of parenteral fluid delivery for people with acute stroke.
Plain language summary
Fluids for people with stroke
Review question
What is the best fluid type, fluid volume, mode of fluid delivery, and duration of fluid treatment to reduce the risk of death or dependence in people with acute stroke?
Background
Fluids given into a vein (intravenous, or iv) or under the skin (subcutaneous) are commonly used in people with stroke, but there are no clear guidelines on the best fluid management in such cases. There are a number of possible different types of fluid that can be used: isotonic fluids, or crystalloids are solutions that contain similar amounts of dissolved salts as in normal cells and blood, whilst hypertonic fluids, or colloids usually contain more (or larger) dissolved particles than in normal cells and blood. Fluid can also be given in different volumes, or for different durations. Hence, we searched the available literature to find answers to these questions.
Study characteristics
The evidence is current to May 2015. We found 12 relevant studies (with 2351 participants) comparing colloids with crystalloids. Eleven of these studies included people with ischaemic stroke (stroke sustained due to a clot), whilst one study included people with haemorrhagic stroke (stroke due to a bleed). Five of these studies (1420 participants) also made a comparison between 0.9% saline, the most commonly prescribed iv fluid, and another fluid type. The largest study had 841 participants, whilst the smallest had 27 participants. The length of time that fluids were given varied between trials, from two hours to 10 days.
Ten studies revealed a source of funding. Of these, two studies were funded by fluid manufacturers.
Key results
We did not find any studies that examined the best fluid volume, mode of fluid delivery, or duration of fluid treatment.
We found that people with acute stroke given crystalloids (including 0.9% saline) had about the same risk of death or dependence as people given other fluid types. People given crystalloids also had a lower risk of pulmonary oedema, a complication that can lead to breathlessness due to excess collection of watery fluid in the lungs. From the evidence we obtained, it was difficult to make any concrete conclusions about which fluids were better for reducing brain swelling (cerebral oedema) or a serious lung infection (pneumonia).
We found no evidence to guide the best volume, duration, or mode of parenteral fluid delivery for people with acute stroke.
Quality of the evidence
The majority of studies had a low to moderate risk of bias based on study limitations and inconsistency. Most studies reported the outcomes they stated they would.
Summary of findings
Background
In 2010, there were 16.9 million strokes globally, of which 5.9 million were fatal (Feigin 2014). Stroke is the second‐leading cause of death and third‐leading cause of disability worldwide (Feigin 2005). Any small improvement in stroke management would therefore benefit many people.
Parenteral fluids are a commonly used intervention in people with stroke who cannot swallow, and in whom dehydration is common (Rowat 2012). Although swallow recovers in more than 80% of people within two to four weeks of stroke onset in the immediate poststroke period (Gordon 1987), those with a poor swallow are at higher‐than‐average risk of dehydration.
Parenteral fluids might be beneficial by reducing the ischaemic penumbra and improving cerebral perfusion (Jauch 2013), or influencing stroke progression (Britton 1980). They may reduce the risk of complications of dehydration such as infection, deep vein thrombosis, constipation, and the exacerbation of delirium (Finestone 2001; Kelly 2004; Kositzke 1990). Hence, parenteral fluids are widely used.
However, parenteral fluids may also cause harm. Too much fluid can lead to cerebral or pulmonary oedema, cardiac failure, or hyponatraemia. Infection can develop around the sites of intravenous or subcutaneous cannulas that may lead to cellulitis or systemic infection. Indirect evidence that increased fluids might cause harm comes from the Glucose Insulin in Stroke Trial (GIST) trial, in which people who received the greatest volumes of infusion had the worst outcomes (although this may well be confounded by baseline glucose levels) (Scott 1999). Evidence from other conditions suggests that lower replacement volumes may be beneficial, for example in children with acute infection and in adults in intensive care (Maitland 2011; SAFE 2004). Furthermore, a sodium load with intravenous saline may lead to renal stress or hypertension (Myburgh 2013), and subcutaneous fluid regimens may not adequately replace potassium.
There is little evidence to support different fluid regimens in people with acute stroke. Haemodilution regimens (which aim to reduce blood viscosity) have been studied in many trials, though they are not widely used in clinical practice. A Cochrane review concluded that there was "no clear evidence of benefit of haemodilution therapy for acute ischaemic stroke" (Chang 2014).
A systematic review that provided clear information on evidence related to the effects of different fluid regimens on important clinical outcomes in people with acute stroke would therefore be very useful for clinicians and researchers. Strong evidence would assist healthcare professionals in better managing parenteral fluid prescription in people with acute stroke.
Description of the condition
In 1978, the World Health Organization defined stroke as a "neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours." (WHO 1978). About 80% of strokes are ischaemic (caused by interruption of the blood supply to an area of the brain), and the remaining 20% are haemorrhagic (mainly due to an abnormal vascular structure or to rupture of a vessel) (Sims 2010). We will not consider subarachnoid haemorrhage, which has a different cause and prognosis from ischaemic and haemorrhagic strokes, in this review. A significant proportion of stroke survivors are left with an impairment of physical, psychological, and social domains (Lopez‐Espuela 2014; Reith 1997). Between 20% and 50% die within the first month, depending on the type and severity of the stroke, the age of the person, comorbidities, and effectiveness of treatment for complications (Truelsen 2000).
Description of the intervention
Parenteral intravenous or subcutaneous fluids replace or supplement oral fluid intake. The UK National Institute for Health and Care Excellence (NICE) guidance on fluid prescription in all medical inpatients suggests that the volume and type of fluid therapy should be modified depending on patient weight, acid base and electrolyte disturbances and insensible losses (NICE 2013). However, there is little randomised evidence to support this strategy in people with stroke.
We aimed to explore the effects of the following aspects of parenteral fluid delivery.
Type of fluid.
Volume of fluid.
Duration of fluid administration.
Mode of delivery.
How the intervention might work
Parenteral fluids in people with acute stroke may lead to different outcomes, depending on fluid type, volume, duration, and mode of delivery. Possible effects of each of these are as follows.
Type of fluid
-
Isotonic (or crystalloids)
Isotonic saline: a solution of 0.9% sodium chloride weight by volume ('normal saline'). When given parenterally, it distributes throughout the extracellular fluid compartment, with only 25% remaining in the intravascular compartment. Because it has a high sodium content, large volumes can lead to water and salt retention (NICE 2013).
Glucose (dextrose) 5%: this fluid is usually used to replace losses of free water; such losses may lead to worsening of cerebral oedema. However, it can increase the risk of hyponatraemia and lead to hyperglycaemia in people with diabetes.
Intravenous 0.18% saline/4% glucose solution: there is a risk of hyponatraemia if given rapidly or in excess.
Balanced crystalloid solutions (e.g. Ringer's lactate, Hartmann's, Plasma‐Lyte, Sterofundin): these fluids have similar properties to 0.9% saline in terms of volume of distribution. However, because of the lower concentration of sodium, they may be associated with less sodium retention. These might be useful in maintenance regimens, and are frequently used after surgery.
-
Hypertonic (largely colloids)
Colloid solutions: colloid solutions contain macromolecules such as albumin, dextran, mannitol, or hydroxyethyl starch. They may expand intravascular volume more rapidly than crystalloids; however, their cost, risk of anaphylactic reactions, and association with worsening renal function may outweigh any putative physiological advantages.
Volume of fluid
Fluid therapy can be targeted based on a person's renal function or hydration state. However, fluid targets may be too generous, or not give sufficient replacement, leading to problems with:
greater volumes of fluid: cerebral oedema, respiratory distress, cardiac failure, and hyponatraemia;
lesser volumes of fluid: acute kidney injury, deep vein thrombosis, delirium, and infection.
Duration of fluid administration
A longer period of fluid administration could lead to complications associated with overhydration and intravenous or subcutaneous cannulation.
A shorter period of fluid administration could lead to complications associated with dehydration.
Mode of delivery
Intravenous fluids might lead to more rapid fluid resuscitation than subcutaneous fluids, though their use may increase the risk of cannula‐associated infection.
Use of subcutaneous fluids might be easier and carries a lower infection risk (Challiner 1994). However, this practice limits the rate of fluid administration, can cause unsightly skin blebs, and may lead to hypokalaemia, as potassium replacement is not possible through the subcutaneous route.
Why it is important to do this review
The current NICE guideline for the use of intravenous fluids in medical patients highlights the lack of systematic reviews and clinical trials to guide fluid treatment of inpatients, including people with stroke (NICE 2013). The guideline found no trial evidence to support different types of maintenance fluid, different durations of fluid replacement, or optimal volume of fluid. We sought to determine whether there was evidence to support parenteral fluid prescription in people with stroke.
Objectives
To assess whether different parenteral fluid regimens lead to differences in death, or death or dependence, after stroke based on fluid type, fluid volume, duration of fluid administration, and mode of delivery.
Methods
Criteria for considering studies for this review
Types of studies
We considered all published and unpublished randomised controlled trials (RCTs) in which parenteral fluids had been used as an intervention to improve outcome after stroke. We also considered cluster‐randomised trials. We included trials with or without blinding of assessors or participants. We excluded quasi‐randomised, non‐randomised, and cross‐over trials.
Types of participants
We included trials of parenteral fluid replacement therapy within seven days of stroke onset in adults (aged 18 years or older) of any gender or ethnicity. We planned to examine subgroups of participants by their ability to swallow, if these data were available.
We included trials of participants with ischaemic or haemorrhagic stroke (but not subarachnoid haemorrhage). We included all types, severities, and stages of stroke, and confirmation of the diagnosis using imaging was not compulsory. The diagnosis should be made by a doctor on either clinical or radiological grounds.
This review focused on the immediate period (within one week) poststroke; therefore, trials in all settings (for example rehabilitation ward, community, nursing home) in that period were eligible.
We considered the inclusion of studies in which only a subset of participants had suffered a stroke if a relevant outcome measure was reported for these participants.
Types of interventions
We sought studies that compared:
-
types of fluid:
use of colloids versus crystalloids;
use of 0.9% saline versus other fluid types (e.g. colloids, other crystalloids);
-
volumes of fluids:
greater versus lesser fluid volumes or no fluid;
administration of a fixed volume versus a targeted volume;
-
durations of fluid administration:
continuously versus intermittently;
longer duration versus shorter duration;
-
mode of delivery:
intravenous versus subcutaneous.
We anticipated that studies of these comparisons would report variations in dosage or intensity, mode of delivery (intravenous versus subcutaneous), personnel delivering the intervention, frequency of delivery, and duration and timing of delivery. If data were available on these factors, we aimed to collect them. We were unable to define thresholds for the comparison of more versus less or no fluid, or the duration of treatment, as the standard volume or duration of treatment is not defined in guidelines. We had planned to dichotomise our analysis at median term duration or fluid volume delivered. However, we found no completed studies that addressed these questions. We documented the regimen and delivery of the intervention and attempted to contact study authors when this information was unavailable or unclear.
Types of outcome measures
Our primary outcome was death or dependence less than six months after stroke. Our secondary outcomes were short‐term complications of fluid therapies.
A number of scales have been used in stroke research to assess functional outcome poststroke (De Haan 1993); the most common is the modified Rankin Scale (mRS) (Banks 2007), which measures activity limitation and participation restriction. The mRS dichotomises people who are dependent or dead (mRS 3 to 6), and people who are independent, and also people who are dead versus those who are alive.
The mRS measures functional outcome poststroke as:
0: no symptoms at all;
1: no significant disability despite symptoms; ability to carry out all usual duties and activities;
2: slight disability; inability to carry out all previous activities with ability to look after own affairs without assistance;
3: moderate disability requiring some help but with ability to walk without assistance;
4: moderately severe disability; inability to walk without assistance and inability to attend to own bodily needs;
5: severe disability; state of being bedridden and incontinent and requiring constant nursing care and attention;
6: death.
We anticipated that most studies would use the mRS to assess outcome poststroke. We considered sensible dichotomies of other outcome scales such as the Oxford Handicap Scale and the Barthel Index to define dependence (Bamford 1989; Granger 1979).
Primary outcomes
Death or dependence less than six months poststroke (measured as mRS grade 2 or more, or measured with other outcome scales).
Secondary outcomes
Death at 12 months or sooner after stroke
Complications potentially attributable to fluid therapy: pneumonia, pulmonary oedema, cerebral oedema, and renal failure.
If we had gathered sufficient data, we aimed to examine the following subgroups.
Stroke type (i.e. ischaemic versus haemorrhagic).
Ability to swallow.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and formats and arranged for translation of relevant papers published in languages other than English. We adhered to copyright legislation and the terms of database licensing agreements.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (May 2015) and the following electronic databases and trials registers.
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library 2015, Issue 5) (Appendix 1).
Cochrane Database of Systematic Reviews (CDSR) (Cochrane Library 2015, Issue 5) (Appendix 1).
Database of Abstracts of Reviews of Effects (DARE) (Cochrane Library 2015, Issue 5) (Appendix 1).
MEDLINE (Ovid) (January 2008 to May 2015) (Appendix 2).
EMBASE (Ovid) (January 2008 to May 2015) (Appendix 3).
CINAHL (EBSCO) (1982 to May 2015) (Appendix 4).
Stroke Trials Registry (www.strokecenter.org/trials/) (May 2015) (Appendix 5).
National Research Register Archive (https://portal.nihr.ac.uk/Pages/NRRArchiveSearch.aspx) (May 2015) (Appendix 5).
ClinicalTrials.gov (http://clinicaltrials.gov/) (May 2015) (Appendix 5).
ISRCTN Registry (http://www.isrctn.com/) (May 2015) (Appendix 5).
We developed the search strategies in collaboration with the Cochrane Stroke Group Trials Search Co‐ordinator. Using a comprehensive search strategy, the Cochrane Stroke Group Trials Search Co‐ordinator had already completed a retrospective search of MEDLINE and EMBASE for all stroke trials to January 2008 and added all relevant trials to the Stroke Group Trials Register. To avoid duplication of effort we have limited the search of MEDLINE and EMBASE from January 2008 onwards.
Searching other resources
We attempted to identify further published, unpublished, and ongoing trials by:
searching reference lists from included studies, published reviews, and relevant papers;
using Science Citation Index Cited Reference Search for forward tracking of relevant papers;
contacting study authors and researchers active in the field.
Data collection and analysis
Selection of studies
We managed all records identified from the electronic searches with reference management software and removed duplicate records. One review author (AV) screened titles and abstracts of references obtained as a result of our search and excluded irrelevant papers. Two review authors (WW and AV) retrieved full‐text articles of the remaining references and independently assessed each study for inclusion in the review on the basis of eligibility criteria. We identified and recorded the reasons for exclusion of ineligible studies. To avoid double counting the same participants, if a study published data more than once, we used the publication with the largest number of participants.
Both WW and AV independently collected data, including names of study authors, specific details of interventions (for example dose, frequency), numbers of participants, date and duration of the study, and results and then applied the eligibility criteria to all of the screened studies.
Together, the same two review authors (WW and AV) ran a pilot test on a sample of reports (approximately 10 to 12 papers) to ensure consistency between review authors. One review author (MD) was available to resolve disagreements.
We recorded the selection process and completed a PRISMA flow diagram (Figure 1).
1.

Study flow diagram.
Data extraction and management
We used a predesigned data extraction form to extract data from studies that met the inclusion criteria. Two review authors (WW and AV) independently documented the following information.
Source of data, including study ID, report ID, review author ID, and citation and contact details.
Methods: study design, total study duration, method of randomisation, allocation concealment, risk of bias, blinding, and follow‐up.
Participants: total number, setting, age, sex, type of stroke, method of stroke diagnosis, severity of disability recorded, and assessment of swallow.
Interventions: type of intervention poststroke, more versus less fluid, type of fluid given, and duration and timing of fluids.
Outcomes: primary and secondary outcomes and time to outcome measure.
Miscellaneous: any funding source, references to other relevant studies, and comments by review authors.
The review authors resolved data extraction discrepancies through discussion. We consulted the third review author (MD) to resolve uncertainty or continued disagreement. We managed extracted data by entering them directly into RevMan 5.3 (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (AV and WW) independently assessed each included study using Cochrane's tool for assessing risk of bias, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011). We extracted data into 'Risk of bias' tables, where each entry was graded as 'low risk', 'high risk', or 'unclear risk'. We created plots of 'Risk of bias' assessments using RevMan 5.3. As per the 'Risk of bias' tool, we considered the following domains.
Sequence generation (selection bias)
Allocation sequence concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other potential sources of bias
We tested assessments of the risk of bias within our review team on a pilot sample with varying range of bias to ensure that we applied the criteria consistently and that we could reach consensus. We discussed disagreements and when necessary consulted a third review author (MD) to achieve a consensus.
Measures of treatment effect
We used RevMan 5.3 to meta‐analyse study level measures of effect (RevMan 2014). We used odds ratios (ORs) with 95% confidence intervals (CIs) to report results for dichotomous (binary) data. We calculated the absolute differences in event rates using the GRADEpro software (GRADEpro GDT 2015) and used total event rate in the control arm of the pooled results (Table 1; Table 2). We used fixed‐effect meta‐analyses to summarise the effect of fluids on outcome after stroke and random‐effects models for sensitivity analyses.
Summary of findings for the main comparison. Colloids versus crystalloids for improving functional outcome in people with acute stroke.
| Colloids versus crystalloids for improving functional outcome in people with acute stroke | ||||||
| Patient or population: people with acute ischaemic or haemorrhagic stroke Settings: all trials were undertaken in a hospital setting in various parts of the world including USA, UK, Europe, Israel, Australia, and Hong Kong Intervention: colloids versus crystalloids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Colloids versus crystalloids | |||||
| Death or dependence Follow‐up: 3‐12 months | Study population | OR 0.97 (0.79 to 1.21) | 1420 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 589 per 1000 | 581 per 1000 (531 to 634) | |||||
| Moderate | ||||||
| 639 per 1000 | 632 per 1000 (583 to 682) | |||||
| Death Follow‐up: 3‐12 months | Study population | OR 1.02 (0.82 to 1.27) | 2351 (12 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 202 per 1000 | 206 per 1000 (172 to 244) | |||||
| Moderate | ||||||
| 219 per 1000 | 222 per 1000 (187 to 263) | |||||
| Pneumonia | Study population | OR 0.58 (0.17 to 2.01) | 416 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 33 per 1000 | 20 per 1000 (6 to 65) | |||||
| Moderate | ||||||
| 33 per 1000 | 19 per 1000 (6 to 64) | |||||
| Cerebral oedema Follow‐up: median 3 months | Study population | OR 0.20 (0.02 to 1.74) | 200 (1 study) | ⊕⊕⊝⊝ low5 | ||
| 49 per 1000 | 10 per 1000 (1 to 82) | |||||
| Moderate | ||||||
| 49 per 1000 | 10 per 1000 (1 to 82) | |||||
| Pulmonary oedema Follow‐up: median 90 days | Study population | OR 2.34 (1.28 to 4.29) | 730 (3 studies) | ⊕⊕⊝⊝ low6 | ||
| 45 per 1000 | 99 per 1000 (57 to 168) | |||||
| Moderate | ||||||
| 49 per 1000 | 108 per 1000 (62 to 181) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 Downgraded due to study limitations and inconsistency with substantial heterogeneity 2 Substantial heterogeneity 3 The confidence interval did not exclude important benefits or harms 4 Based on two studies, study limitations acknowledged 5 Data from one study only, no comparisons 6 Based on three studies, OR above 2.0
Summary of findings 2. 0.9% saline versus other fluid for improving functional outcome in people with acute stroke.
| 0.9% saline versus other fluid for improving functional outcome in people with acute stroke | ||||||
| Patient or population: people with acute ischaemic or haemorrhagic stroke Settings: all trials were undertaken in a hospital setting in various parts of the world including USA, UK, Europe, Israel, and Hong Kong Intervention: 0.9% saline versus other fluid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 0.9% saline versus other fluid | |||||
| Death or dependence Follow‐up: 3‐12 months | Study population | OR 1.04 (0.82 to 1.32) | 1120 (3 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 553 per 1000 | 563 per 1000 (503 to 620) | |||||
| Moderate | ||||||
| 540 per 1000 | 550 per 1000 (490 to 608) | |||||
| Death | Study population | OR 0.87 (0.67 to 1.12) | 1760 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 210 per 1000 | 188 per 1000 (151 to 229) | |||||
| Moderate | ||||||
| 208 per 1000 | 186 per 1000 (150 to 227) | |||||
| Pneumonia | Study population | OR 2.00 (0.36 to 11.16) | 216 (1 study) | ⊕⊝⊝⊝ very low3,4,5 | ||
| 19 per 1000 | 37 per 1000 (7 to 175) | |||||
| Moderate | ||||||
| 19 per 1000 | 37 per 1000 (7 to 178) | |||||
| Pulmonary oedema Follow‐up: mean 48 hours | Study population | OR 0.32 (0.14 to 0.69) | 424 (1 study) | ⊕⊕⊕⊝ moderate6 | ||
| 121 per 1000 | 41 per 1000 (19 to 87) | |||||
| Moderate | ||||||
| 121 per 1000 | 41 per 1000 (19 to 87) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 Study limitations acknowledged 2 Substantial heterogeneity 3 Unclear of blinding 4 Sample size lower than calculated 5 Incomplete reporting 6 Early termination of study
Unit of analysis issues
We took into account the level at which randomisation occurred in each study. We obtained only study level data. We examined outcomes at three to six months poststroke. We analysed the outcomes of dependence and death as dichotomous data. We used 2 × 2 tables, with effects of the intervention expressed as ORs.
Dealing with missing data
When necessary, we attempted to contact the original investigators to request missing data. We did not use replacement values in place of missing data.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). We considered values of 50% and above as representing significant heterogeneity.
Assessment of reporting biases
We carefully assessed each included study for risk of bias as outlined above. We used a comprehensive search strategy to minimise reporting bias by searching relevant electronic sources and other trials registers. We tried to identify unpublished studies and considered their inclusion. We considered the use of funnel plots to investigate publication bias (Egger 1997), but there were too few trials.
Data synthesis
We constructed forest plots to compare four intervention groups: type of fluid (0.9% saline versus others, colloids versus crystalloids), volume of fluid (more versus less (or none), fixed versus targeted), duration of fluid administration (intermittent versus continuous, longer versus shorter), and mode of delivery (intravenous versus subcutaneous), where data, if any, were available. We analysed dichotomous outcome data as dependence or death versus independence.
We calculated the effects of the intervention with a fixed‐effect model in RevMan 5.3 and reported them as ORs with 95% CIs (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We had planned to carry out the following analyses (if sufficient data were available):
different stroke type: ischaemic versus haemorrhagic;
ability to swallow.
However, during our review process, we did not find data to perform these analyses.
Sensitivity analysis
We considered performing sensitivity analysis if results were reported by stroke subtype or the ability to swallow. However, none of our studies reported the latter. For the former, only one included study studied people with haemorrhagic stroke, hence this analysis was not possible.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Two trials had completed recruitment but not published results at the time of the review (Oghbaei 2012; Shalilahmadi 2013). We were unable to contact the authors to determine a publication date. We were also unable to extract enough data from one conference proceeding (Greenhall 1974).
From our initial protocol, we had planned four comparisons:
type of fluids (colloids versus crystalloids, 0.9% saline versus others);
volume of fluids (greater versus less (or none), fixed versus targeted);
duration of fluid delivery (continuous versus intermittent, longer versus shorter duration); and
mode of delivery (intravenous versus subcutaneous).
Data were only available for a comparison between fluid types (colloids versus crystalloids and 0.9% saline versus others).
Results of the search
We identified 14,199 publications from our database searches and searches from other sources. After the removal of 670 duplicates, 13,529 publications remained for screening. After initial screening of titles and abstracts, 183 publications remained for further evaluation. We obtained the full‐text articles for these records, and two review authors (AV and WW) independently screened them. After discussion, we excluded a further 130 records, leaving 53 records for further evaluation. Eight required translation (two German, one Dutch, one Italian, one Hebrew, and three Chinese) (see PRISMA flow diagram Figure 1).
After we screened the full texts and extracted data, 12 studies met our inclusion criteria, and we excluded 41 studies (see Characteristics of included studies; Characteristics of excluded studies). One study is still recruiting participants (due completion 2016) (NCT02003794; see Characteristics of ongoing studies).
Included studies
Of the 12 relevant studies, 11 studies recruited only people with ischaemic stroke, and one study, Yu 1992, recruited people with haemorrhagic stroke. The largest trial, ALIAS 2, randomised 841 participants to 25% albumin versus 0.9% sodium chloride (Ginsberg 2013). This trial was stopped early due to futility. The smallest trial recruited and randomised 27 participants into two arms: dextran 40 versus 5% dextrose (Larsson 1976).
The 12 included studies recruited a total of 2351 participants: 1185 allocated to colloids and 1166 allocated to crystalloids. All participants were in a hospital setting. Timing between the stroke to recruitment varied between trials: seven trials recruited participants within 24 hours of a stroke (Aichner 1998; Borenstein 1981; Ginsberg 2011; Ginsberg 2013; Larsson 1976; Rudolf 2002; Yu 1992), three trials recruited participants within 48 hours (Bayer 1987; Fawer 1978; Matthews 1976), and one trial within 72 hours (Gilroy 1969). In one study the timing after stroke for recruitment was unclear (Friedli 1975).
The trials studied fluids that were hypertonic (colloids): hydroethyl starch (Aichner 1998; Rudolf 2002), 10% glycerol (Bayer 1987; Fawer 1978; Friedli 1975; Larsson 1976; Yu 1992), dextran 40 (Borenstein 1981; Gilroy 1969; Matthews 1976), albumin (Ginsberg 2011; Ginsberg 2013); or isotonic (crystalloids): Ringer's lactate (Aichner 1998), Hartmann's (Borenstein 1981), dextrose (5% or 10%) (Friedli 1975; Larsson 1976; Matthews 1976), dextrose saline mixtures (Fawer 1978; Gilroy 1969), 0.9% saline (Bayer 1987; Ginsberg 2011; Ginsberg 2013; Rudolf 2002; Yu 1992), relative to plasma. All 12 trials compared colloids versus crystalloids. Of the 12 trials, five compared 0.9% saline with another fluid type that tended to be colloids (for example albumin, glycerol, and hydroethyl starch).
Ten studies reported their funding source: six studies were supported by national grants (from international research centres, institutes of health, or hospital research organisations) (Bayer 1987; Fawer 1978; Gilroy 1969; Ginsberg 2011; Ginsberg 2013; Matthews 1976); two studies were supported by the hospital where the study took place or university respectively (Larsson 1976; Yu 1992); and two studies were supported by makers of intravenous fluid products (Aichner 1998; Rudolf 2002).
Excluded studies
Of the 53 studies evaluated for inclusion in the review, we excluded 41 (see Characteristics of excluded studies).
The reasons for exclusion were:
results were not fully reported, and although we made contact with authors, we did not receive a reply (9/41);
no disability outcome was reported (11/41);
use of venesection (6/41);
unclear if the design was randomised (6/41);
outcomes not reported with absolute data (4/41); and
data not extractable from summary/abstract available (2/41)
Spudis 1973 did not report a control group (1/41);
Staedt 1987 compared two colloids, which did not fit our study comparison criteria (1/41); and
Frei 1987 id not report results according to the randomized groups; hence raising ambiguity about the effect of the intervention (1/41).
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Of the 12 included trials, seven gave details of randomisation. These trials used a table of random numbers (1/7), series of envelopes (2/7), a stratified randomised block design (2/7), or coin‐based minimisation algorithm (2/7). The other five trials did not fully explain the randomisation process (Bayer 1987; Borenstein 1981; Friedli 1975; Larsson 1976; Rudolf 2002).
Allocation sequence concealment
Five trials adequately concealed randomisation, and four did not (Bayer 1987; Fawer 1978; Friedli 1975; Matthews 1976). Three studies had an unclear risk, as although concealment was mentioned, information on how this was done was insufficient (Borenstein 1981; Larsson 1976; Rudolf 2002).
Blinding
Of the 12 included studies, eight studies blinded participants and clinicians who measured outcome, two trials blinded clinicians who measured outcome but not participants (Friedli 1975; Matthews 1976), and two trials offered no information on blinding (Gilroy 1969; Rudolf 2002). Although Yu 1992 was said to be a double‐blind study, this was not consistently justified throughout the study protocol.
Incomplete outcome data
Six of the 12 included studies reported outcomes on all participants randomised. A further five did not report all outcomes in all randomised participants (Aichner 1998; Ginsberg 2011; Ginsberg 2013; Larsson 1976; Yu 1992). It was unclear how many participants were lost to follow‐up in Rudolf 2002.
Selective reporting
Although planned, ALIAS 1 did not report a long‐term disability outcome (Ginsberg 2011). Larsson 1976 did not report all final specified outcomes at follow‐up. Ginsberg 2013 and Yu 1992 both had unclear risks for selective outcome reporting biases.
Other potential sources of bias
The ALIAS trials were halted prior to reaching their planned sample size (Ginsberg 2011; Ginsberg 2013). Truncated RCTs may systematically overestimate treatment effects for the outcome (Bassler 2010; Howard 2011). In Yu 1992, it was unclear if the same assessor followed up participants, which could account for observer variability.
Effects of interventions
The 12 studies recruited a total of 2351 people; most studies were small. The number of participants ranged from 27 to 841; the median was 103.
Effect of fluids
Colloids versus crystalloids
Twelve studies (2351 participants) compared colloids with crystalloids and reported death, death or dependence, or secondary outcomes of pneumonia, cerebral oedema, or pulmonary oedema.
We found no difference in the odds of death or dependence, or death, at three to 12 months between participants allocated to colloids compared with participants allocated to crystalloids, although the 95% CI did not exclude important harms or important benefits from either regimen (OR for death or dependence 0.97, 95% CI 0.79 to 1.21, I² = 58%, Analysis 1.1; OR for death 1.02, 95% CI 0.82 to 1.27, I² = 24%, Analysis 1.2). There was significant between‐trial heterogeneity in our summary estimates of the effect of colloids compared with crystalloids on death or dependence. For this reason, we graded the evidence as moderate quality for the outcome death, and as low quality for the outcome death or dependence due to study limitations and inconsistency for the outcome.
1.1. Analysis.

Comparison 1 Colloids versus crystalloids, Outcome 1 Death or dependence.
1.2. Analysis.

Comparison 1 Colloids versus crystalloids, Outcome 2 Death.
Based on the two studies reporting the outcome pneumonia between participants allocated to colloids versus crystalloids, the confidence interval of the odds of this outcome did not exclude harms or benefits from either regimen (OR 0.58, 95% CI 0.17 to 2.01, I² = 0%, two studies, Analysis 1.3). The two studies reporting this outcome compared 10% hydroethyl starch with Ringer's lactate and glycerol with 0.9% saline (Aichner 1998; Yu 1992).
1.3. Analysis.

Comparison 1 Colloids versus crystalloids, Outcome 3 Pneumonia.
Aichner 1998 reported cerebral oedema. Based on this one study, the confidence interval of odds of cerebral oedema between participants allocated to colloids versus crystalloids did not exclude significant harms, or significant benefits from either regimen (OR 0.20, 95% CI 0.02 to 1.74, Analysis 1.4).
1.4. Analysis.

Comparison 1 Colloids versus crystalloids, Outcome 4 Cerebral oedema.
The odds of pulmonary oedema were approximately doubled with colloids compared with crystalloids (OR 2.34, 95% CI 1.28 to 4.29, I² = 0%, three studies, Analysis 1.5) (Aichner 1998; Ginsberg 2011; Rudolf 2002). The crystalloid comparators were Ringer's lactate in Aichner 1998 and 0.9% saline in Ginsberg 2011 and Rudolf 2002.
1.5. Analysis.

Comparison 1 Colloids versus crystalloids, Outcome 5 Pulmonary oedema.
We downgraded the quality of evidence once for pneumonia (from high to moderate) and once for cerebral and pulmonary oedema (from moderate to low) due to study limitations.
Only Yu 1992 reported the outcomes of death and pneumonia in people with haemorrhagic stroke. The estimates for participants with haemorrhagic stroke were similar to participants with ischaemic stroke, and the CIs were consistent with the estimates for studies of participants with ischaemic stroke.
At the request of our editorial reviewers, we performed post‐hoc sensitivity analyses to compare the results of fixed‐effect meta‐analysis with random‐effects meta‐analyses. There were no important differences in the size or the statistical significance of our findings with this method of meta‐analysis.
0.9% saline versus other fluids
A total of five studies with 1760 participants compared 0.9% saline with other fluid regimens. Outcomes reported were death, death or dependence, and secondary outcomes of pneumonia and pulmonary oedema.
There was no significant difference in the odds of death or dependence for those randomised to 0.9% saline compared with those randomised to other fluids (OR 1.04, 95% CI 0.82 to 1.32, I² = 71%, three studies, Analysis 2.1) (Bayer 1987; Ginsberg 2011; Rudolf 2002). There was no difference in the odds of death between participants treated with 0.9% saline versus participants treated with other fluids, although the CI did not exclude a beneficial effect or a smaller harmful effect (OR 0.87, 95% CI 0.67 to 1.12, I² = 53%, five studies, Analysis 2.2). We downgraded the quality of evidence to low for both these outcomes due to study limitations and inconsistency, with substantial heterogeneity.
2.1. Analysis.

Comparison 2 0.9% saline versus other fluid, Outcome 1 Death or dependence.
2.2. Analysis.

Comparison 2 0.9% saline versus other fluid, Outcome 2 Death.
Yu 1992 was the only study that reported pneumonia for this comparison: four participants developed pneumonia in the 0.9% saline group versus two in the other‐fluid group (OR 2.00, 95% CI 0.36 to 11.16, Analysis 2.3). Ginsberg 2011 was the only study to report pulmonary oedema, with nine events in the saline group and 25 in the other‐fluid group (OR 0.32, 95% CI 0.14 to 0.69, Analysis 2.4), favouring 0.9% saline.
2.3. Analysis.
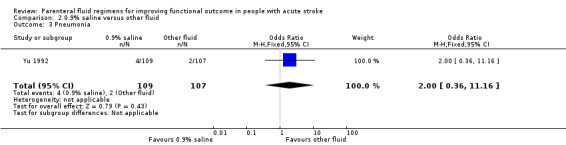
Comparison 2 0.9% saline versus other fluid, Outcome 3 Pneumonia.
2.4. Analysis.

Comparison 2 0.9% saline versus other fluid, Outcome 4 Pulmonary oedema.
Discussion
Summary of main results
We found no compelling evidence that early fluid replacement with colloids rather than crystalloids led to a difference in the odds of death, or death or dependence, three to 12 months after stroke, although colloids did lead to a doubling in the odds of pulmonary oedema. The summary estimate was consistent with both a modest increase and a modest reduction in the odds of death, or death or dependence, with either regimen.
We did not find any evidence to guide the best volume, duration, or route of administration of parenteral fluids in people with acute stroke.
Overall completeness and applicability of evidence
We have searched publicly available electronic databases up to and including the beginning of May 2015 comprehensively for trials of parenteral fluids in people with acute stroke. Due to limited resources we did not search the grey literature extensively, although we did search the Cochrane Stroke Group database of RCTs, which contains an up‐to‐date register of published and unpublished trials. Data from two trials that had completed recruitment but had not published results and one trial in progress were unavailable. It is therefore possible that we missed relevant studies.
There was substantial statistical heterogeneity in the odds ratios for death and death or dependence with colloids and crystalloids between the trials. Potential clinical reasons for this heterogeneity are the different types of colloids in different trials; the substantial time from the earliest to the latest trials (from 1969 to 2013), and hence differences in brain scanning, widespread use of nasogastric fluids, and easily titratable fluid delivery systems; the different hospital settings of different trials; or chance. Our summary estimate, whilst the best available, should therefore be interpreted with caution. More data to guide the volume, duration, and mode of delivery of parenteral fluids would be useful for clinical practice.
We did not find any evidence for renal impairment with different fluid types after stroke, and there was very little evidence for important clinical outcomes such as cerebral oedema.
Quality of the evidence
For assessments of the overall quality of evidence for each outcome that included pooled data for RCTs only, we graded evidence as moderate quality (for the primary outcome, death or dependence) and low quality for the rest of the outcome measures due to study limitations, indirectness of evidence, inconsistency, and imprecision. Inconsistency was the main reason for most of these downgradings, as evidenced by high heterogeneity values. Similarly, we graded evidence as low quality in our second comparison (0.9% saline versus other fluids) primarily due to risk of bias and inconsistency of results across the small number of included studies. As many trials were performed prior to the widespread adoption of Consolidated Standards of Reporting Trials (CONSORT) guidelines, randomisation and allocation concealment were inadequately described, particularly in the earlier studies. The modest heterogeneity across studies requires further research to determine the effects of fluids on death, or death or dependence.
Potential biases in the review process
Whilst we comprehensively searched the electronic databases literature, we may have missed some studies in the grey literature. We used the Cochrane Stroke Group's trials register, which does not include dissertations, and we did not handsearch non‐stroke related journals.
Due to lack of resources for the review, the initial screening and data entry were performed by one, rather than two review authors.
Prior to the review, we were unable to define thresholds of fluid volume, or the duration of fluid treatment that would divide studies into relevant comparison groups. Although we did not find any studies to answer questions about fluid volume or duration, it may arise in future that studies are available with different doses of an intervention (for example oxygen therapy, rehabilitation), and there is no generally agreed threshold.
Agreements and disagreements with other studies or reviews
Although there are numerous papers and trials on haemodilution after an acute ischaemic stroke, and discussions around hypervolaemic, isovolaemic and hypovolaemic haemodilution with the use of venesection or the combination of venesection and infusion of fluids (Heros 1989; IASS 1988; Koller 1990; Mast 1991; SSS 1987), there have actually been few trials that have compared the use of fluid regimens without venesection. A Cochrane review concluded that haemodilution with or without venesection had no place in the routine treatment of people with acute stroke (Chang 2014).
The most recent major review of parenteral fluids was guidance from the UK National Institute for Health and Care Excellence (NICE 2013). The review commented on special circumstances for maintaining intravenous fluid management in people with stroke, although it found no evidence to support fluid prescription in people with stroke. The review planned to determine the best fluid type in hospitalised patients, although it found no randomised evidence on the superiority of any crystalloids solution over any other solution. Four small studies of surgical inpatients were found that compared fluid volume after abdominal surgery, but none that were relevant to medical inpatients or people with stroke. This review was therefore consistent with our own, although we have identified more studies comparing fluid types in people with stroke.
Authors' conclusions
Implications for practice.
Parenteral 0.9% saline and other crystalloids are widely used after acute stroke. We found no evidence that colloids were associated with lower odds of death or dependence in the medium term after stroke than saline or other crystalloids, but colloids were associated with greater odds of pulmonary oedema. There is therefore no evidence to support a change from using crystalloids in clinical practice.
Implications for research.
Fluids are very commonly prescribed in acute stroke, although there is little evidence to support the type, volume, duration, or route of administration of this intervention. Further RCTs to address this uncertainty might lead to better fluid regimens in stroke, better advice for clinicians, and better outcomes for people with stroke.
The review authors recommend collaborative trials in adults with acute stroke (ischaemic or haemorrhagic) in a hospital setting studying type, volume, varying administration routes, and frequency of fluid therapy over a fixed duration. Addressing all four questions might require more than one trial. Such trials should measure important clinical outcomes such as early and late death, dependence, and secondary complications of fluid therapy (for example pulmonary oedema, cerebral oedema, pneumonia).
Acknowledgements
We would like to thank Mrs Brenda Thomas for her help in developing the MEDLINE search strategy and Professor John Starr and Dr Zhang Quili Zhang for their help with translating papers from Hebrew and Chinese, respectively.
Appendices
Appendix 1. Cochrane Library databases
Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE)
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "cerebrovascular trauma"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"vasospasm, intracranial"] or [mh ^"brain injuries"]
#2 (stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex*):ti,ab
#3 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracranial) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#5 brain next injur*:ti,ab
#6 #1 or #2 or #3 or #4 or #5
#7 [mh "fluid therapy"] or [mh "water‐electrolyte balance"/TH] or [mh ^"water‐electrolyte imbalance"/TH] or [mh ^dehydration/TH]
#8 [mh ^"rehydration solutions"] or [mh ^"hypertonic solutions"] or [mh ^"glucose solution, hypertonic"] or [mh ^"saline solution, hypertonic"] or [mh ^"hypotonic solutions"] or [mh ^"isotonic solutions"]
#9 [mh ^"hydroxyethyl starch derivatives"] or [mh ^albumins] or [mh "serum albumin"] or [mh colloids] or [mh dextrans] or [mh ^"plasma substitutes"] or [mh ^polygeline] or [mh ^povidone] or [mh ^glucose]
#10 [mh ^"sodium bicarbonate"] or [mh ^"potassium chloride"] or [mh ^"sodium chloride"]
#11 [mh ^plasma [mj]] or [mh ^serum [mj]] or [mh ^electrolytes/TU]
#12 ((fluid* or volume or electrolyte* or plasma) near/3 (therap* or intravenous or IV or infusion* or subcutaneous or drip or drips or administration or substitute* or restor* or resuscitat* or replac*)):ti,ab
#13 (rehydrate* or hydrat*):ti,ab
#14 ((blood or plasma) near/3 (substitut* or expan*)):ti,ab
#15 (colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl next starch* or dextran* or gelofus* or hemaccel* or haemaccel* or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartman* or "sodium chloride" or "potassium chloride" or saline or glucose or dextrose or bicarbonate):ti
#16 ((colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl next starch* dextran* or gelofus* or hemaccel* or haemaccel* or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartmann* or sodium or potassium or saline or glucose or dextrose or bicarbonate) near/3 (solution* or fluid* or therap* or intravenous or IV or infusion* or subcutaneous or drip or drips or administration or substitute* or restor* or resuscitat* or replac*)):ti,ab
#17 [mh ^"infusions, parenteral"] or [mh ^"infusions, intravenous"] or [mh "infusions, subcutaneous"]
#18 (colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl next starch* or dextran* or gelofus* or hemaccel* or haemaccel* or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartman* or sodium or potassium or saline or glucose or dextrose or bicarbonate):ti,ab
#19 #17 and #18
#20 (volume near/3 expan*):ti,ab
#21 ((balanced or physiologic* or parenteral) near/3 (fluid* or solution*)):ti,ab
#22 ((fresh or frozen) near/3 plasma):ti,ab
#23 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #19 or #20 or #21 or #22
#24 #6 and #23
#25 (neonat* or newborn* or "new born" or pediatric or paediatric or birth or infant or infants or perinatal or "peri‐natal" or baby or babies or child or children):ti
#26 #24 not #25
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or brain injuries/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. brain injur$.tw. 6. 1 or 2 or 3 or 4 or 5 7. exp fluid therapy/ or exp water‐electrolyte balance/th or water‐electrolyte imbalance/th or dehydration/th 8. rehydration solutions/ or hypertonic solutions/ or glucose solution, hypertonic/ or saline solution, hypertonic/ or hypotonic solutions/ or isotonic solutions/ 9. hydroxyethyl starch derivatives/ or albumins/ or exp serum albumin/ or exp colloids/ or exp dextrans/ or plasma substitutes/ or polygeline/ or povidone/ or glucose/ 10. sodium bicarbonate/ or potassium chloride/ or sodium chloride/ 11. exp *plasma/ or exp *serum/ or electrolytes/tu 12. ((fluid$ or volume or electrolyte$ or plasma) adj3 (therap$ or intravenous or IV or infusion$ or subcutaneous or drip or drips or administration or substitute$ or restor$ or resuscitat$ or replac$)).tw. 13. (rehydrate$ or hydrat$).tw. 14. ((blood or plasma) adj3 (substitut$ or expan$)).tw. 15. (colloid$ or hydrocolloid$ or crystalloid$ or albumin$ or albumen$ or hydroxyethylstarch$ or hydroxyethyl starch$ or dextran$ or gelofus$ or hemaccel$ or haemaccel$ or hetastarch or isotonic or hypertonic or hypotonic or ringer$ or gelatin$ or gentran$ or pentastarch$ or pentaspan$ or hartman$ or sodium chloride or potassium chloride or saline or glucose or dextrose or bicarbonate).ti. 16. ((colloid$ or hydrocolloid$ or crystalloid$ or albumin$ or albumen$ or hydroxyethylstarch$ or hydroxyethyl starch$ dextran$ or gelofus$ or hemaccel$ or haemaccel$ or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer$ or gelatin$ or gentran$ or pentastarch$ or pentaspan$ or hartmann$ or sodium or potassium or saline or glucose or dextrose or bicarbonate) adj3 (solution$ or fluid$ or therap$ or intravenous or IV or infusion$ or subcutaneous or drip or drips or administration or substitute$ or restor$ or resuscitat$ or replac$)).tw. 17. infusions, parenteral/ or infusions, intravenous/ or exp infusions, subcutaneous/ 18. (colloid$ or hydrocolloid$ or crystalloid$ or albumin$ or albumen$ or hydroxyethylstarch$ or hydroxyethyl starch$ or dextran$ or gelofus$ or hemaccel$ or haemaccel$ or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer$ or gelatin$ or gentran$ or pentastarch$ or pentaspan$ or hartman$ or sodium or potassium or saline or glucose or dextrose or bicarbonate).tw. 19. 17 and 18 20. (volume adj3 expan$).tw. 21. ((balanced or physiologic$ or parenteral) adj3 (fluid$ or solution$)).tw. 22. ((fresh or frozen) adj3 plasma).tw. 23. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 19 or 20 or 21 or 22 24. Randomized Controlled Trials as Topic/ 25. random allocation/ 26. Controlled Clinical Trials as Topic/ 27. control groups/ 28. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 29. double‐blind method/ 30. single‐blind method/ 31. Placebos/ 32. placebo effect/ 33. cross‐over studies/ 34. Therapies, Investigational/ 35. Research Design/ 36. randomized controlled trial.pt. 37. controlled clinical trial.pt. 38. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 39. (random$ or RCT or RCTs).tw. 40. (controlled adj5 (trial$ or stud$)).tw. 41. (clinical$ adj5 trial$).tw. 42. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 46. (cross‐over or cross over or crossover).tw. 47. (placebo$ or sham).tw. 48. trial.ti. 49. (assign$ or allocat$).tw. 50. controls.tw. 51. or/24‐50 52. 6 and 23 and 51 53. exp animals/ not humans.sh. 54. 52 not 53 55. (neonat$ or newborn$ or new born or pediatric or paediatric or birth or infant or infants or perinatal or peri‐natal or baby or babies or child or children).ti. 56. 54 not 55
Appendix 3. EMBASE search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or vertebrobasilar insufficiency/ or stroke/ or stroke patient/ or stroke unit/ or brain injury/ or acquired brain injury/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. brain injur$.tw.
6. 1 or 2 or 3 or 4 or 5
7. fluid therapy/ or fluid resuscitation/ or rehydration/ or hypodermoclysis/ or electrolyte disturbance/th or dehydration/th
8. hypertonic solution/ or hypotonic solution/ or isotonic solution/
9. hetastarch/ or hetastarch derivative/ or exp plasma substitute/ or dextran/ or fresh frozen plasma/ or polygeline/ or blood substitute/ or glucose/ or exp albuminoid/ or exp colloid/ or crystalloid/ or povidone/
10. bicarbonate/ or potassium chloride/ or sodium chloride/
11. exp *plasma/ or exp *serum/ or electrolyte/
12. ((fluid$ or volume or electrolyte$ or plasma) adj3 (therap$ or intravenous or IV or infusion$ or subcutaneous or drip or drips or administration or substitute$ or restor$ or resuscitat$ or replac$)).tw.
13. (rehydrate$ or hydrat$).tw.
14. ((blood or plasma) adj3 (substitut$ or expan$)).tw.
15. (colloid$ or hydrocolloid$ or crystalloid$ or albumin$ or albumen$ or hydroxyethylstarch$ or hydroxyethyl starch$ or dextran$ or gelofus$ or hemaccel$ or haemaccel$ or hetastarch or isotonic or hypertonic or hypotonic or ringer$ or gelatin$ or gentran$ or pentastarch$ or pentaspan$ or hartman$ or sodium chloride or potassium chloride or saline or glucose or dextrose or bicarbonate).ti.
16. ((colloid$ or hydrocolloid$ or crystalloid$ or albumin$ or albumen$ or hydroxyethylstarch$ or hydroxyethyl starch$ dextran$ or gelofus$ or hemaccel$ or haemaccel$ or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer$ or gelatin$ or gentran$ or pentastarch$ or pentaspan$ or hartmann$ or sodium or potassium or saline or glucose or dextrose or bicarbonate) adj3 (solution$ or fluid$ or therap$ or intravenous or IV or infusion$ or subcutaneous or drip or drips or administration or substitute$ or restor$ or resuscitat$ or replac$)).tw.
17. infusions, parenteral/ or infusions, intravenous/ or exp infusions, subcutaneous/
18. (colloid$ or hydrocolloid$ or crystalloid$ or albumin$ or albumen$ or hydroxyethylstarch$ or hydroxyethyl starch$ or dextran$ or gelofus$ or hemaccel$ or haemaccel$ or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer$ or gelatin$ or gentran$ or pentastarch$ or pentaspan$ or hartman$ or sodium or potassium or saline or glucose or dextrose or bicarbonate).tw.
19. 17 and 18
20. (volume adj3 expan$).tw.
21. ((balanced or physiologic$ or parenteral) adj3 (fluid$ or solution$)).tw.
22. ((fresh or frozen) adj3 plasma).tw.
23. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 19 or 20 or 21 or 22
24. Randomized Controlled Trial/
25. Randomization/
26. Controlled Study/
27. control group/
28. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
29. Crossover Procedure/
30. Double Blind Procedure/
31. Single Blind Procedure/ or triple blind procedure/
32. placebo/
33. (random$ or RCT or RCTs).tw.
34. (controlled adj5 (trial$ or stud$)).tw.
35. (clinical$ adj5 trial$).tw.
36. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
37. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
38. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
39. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
40. (cross‐over or cross over or crossover).tw.
41. (placebo$ or sham).tw.
42. trial.ti.
43. (assign$ or allocat$).tw.
44. controls.tw.
45. or/24‐44
46. 6 and 23 and 45
47. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
48. 46 not 47
49. (neonat$ or newborn$ or new born or pediatric or paediatric or birth or infant or infants or perinatal or peri‐natal or baby or babies or child or children).ti.
50. 48 not 49
Appendix 4. CINAHL search strategy
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") or (MH "Hypoxia, Brain") OR (MH "Brain Damage, Chronic") OR (MH "Brain Injuries")
S2 .(MH "Stroke Patients") OR (MH "Stroke Units")
S3 .TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex)
S4 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S6 .S4 and S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial)
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 and S8
S10 .TI brain injur* OR AB brain injur*
S11 .S1 OR S2 OR S3 OR S6 OR S9 OR S10
S12 .(MH "Fluid Therapy") OR (MH "Fluid Resuscitation") OR (MH "Hypodermoclysis")
S13 .(MH "Fluid‐Electrolyte Balance+/TH") OR (MH "Fluid‐Electrolyte Imbalance+/TH") OR (MH "Dehydration/TH")
S14 .(MH "Rehydration Solutions") OR (MH "Crystalloid Solutions") OR (MH "Lactated Ringer's Solution") OR (MH "Hypertonic Solutions") OR (MH "Saline Solution, Hypertonic") OR (MH "Hypotonic Solutions") OR (MH "Isotonic Solutions") OR (MH "Normal Saline")
S15 .(MH "Hydroxyethyl Starch") OR (MH "Albumins") OR (MH "Serum Albumin") OR (MH "Colloids") OR (MH "Dextrans") OR (MH "Plasma Substitutes") OR (MH "Blood Substitutes") OR (MH "Glucose") OR (MH "Povidone")
S16 .(MH "Sodium Bicarbonate") OR (MH "Potassium Chloride") OR (MH "Sodium Chloride")
S17 .(MM "Plasma") OR (MM "Serum") OR (MH "Electrolytes/TU")
S18 .TI ( ((fluid* or volume or electrolyte* or plasma) N3 (therap* or intravenous or IV or infusion* or subcutaneous or drip or drips or administration or substitute* or restor* or resuscitat* or replac*)) ) OR AB ( ((fluid* or volume or electrolyte* or plasma) N3 (therap* or intravenous or IV or infusion* or subcutaneous or drip or drips or administration or substitute* or restor* or resuscitat* or replac*)) )
S19 .TI ( (rehydrat* or hydrat*) ) OR AB ( (rehydrat* or hydrat*) )
S20 .TI ( ((blood or plasma) N3 (substitut* or expan*)) ) OR AB ( ((blood or plasma) N3 (substitut* or expan*)) )
S21 .TI (colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl starch* or dextran* or gelofus* or hemaccel* or haemaccel* or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartman* or sodium chloride or potassium chloride or saline or glucose or dextrose or bicarbonate)
S22 .TI ((colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl starch* dextran* or gelofus* or hemaccel* or haemaccel* or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartmann* or sodium or potassium or saline or glucose or dextrose or bicarbonate) N3 (solution* or fluid* or therap* or intravenous or IV or infusion* or subcutaneous or drip or drips or administration or substitute* or restor* or resuscitat* or replac*))
S23 .AB ((colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl starch* dextran* or gelofus* or hemaccel* or haemaccel* or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartmann* or sodium or potassium or saline or glucose or dextrose or bicarbonate) N3 (solution* or fluid* or therap* or intravenous or IV or infusion* or subcutaneous or drip or drips or administration or substitute* or restor* or resuscitat* or replac*))
S24 .(MH "Infusions, Parenteral+") OR (MH "Infusions, Intravenous") OR (MH "Infusions, Subcutaneous+")
S25 .TI ( (colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl starch* or dextran* or gelofus* or hemaccel* or haemaccel* or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartman* or sodium or potassium or saline or glucose or dextrose or bicarbonate) ) OR AB ( (colloid* or hydrocolloid* or crystalloid* or albumin* or albumen* or hydroxyethylstarch* or hydroxyethyl starch* or dextran* or gelofus* or hemaccel* or haemaccel* or serum or hetastarch or isotonic or hypertonic or hypotonic or ringer* or gelatin* or gentran* or pentastarch* or pentaspan* or hartman* or sodium or potassium or saline or glucose or dextrose or bicarbonate) )
S26 .S24 AND S25
S27 .TI (volume N3 expan*) OR AB (volume N3 expan*)
S28 .TI ( ((balanced or physiologic* or parenteral) N3 (fluid* or solution*)) ) OR AB ( ((balanced or physiologic* or parenteral) N3 (fluid* or solution*)) )
S29 .TI ( ((fresh or frozen) N3 plasma) ) OR AB ( ((fresh or frozen) N3 plasma) )
S30 .S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S26 OR S27 OR S28 OR S29
S31 .S11 AND S30
S32 .PT randomized controlled trial or clinical trial
S33 .(MH "Random Assignment") or (MH "Random Sample+")
S34 .(MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies")
S35 .(MH "Control (Research)") or (MH "Control Group")
S36 .(MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials")
S37 .(MH "Placebo Effect") or (MH "Placebos")
S38 .(MH "Clinical Research") or (MH "Clinical Nursing Research")
S39 .(MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design")
S40 .TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S41 .TI ( controlled N5 (trial* or stud*) ) OR AB ( controlled N5 (trial* or stud*) )
S42 .TI (clinical N5 trial*) OR AB (clinical N5 trial*)
S43 .TI ( (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) ) OR AB ( (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) )
S44 .TI ( quasi‐random* or quasi random* or pseudo‐random* or pseudo random* ) OR AB ( quasi‐random* or quasi random* or pseudo‐random* or pseudo random* )
S45 .TI ( (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) ) OR AB ( (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) )
S46 .TI ( (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) )
S47 .TI ( cross‐over or cross over or crossover ) OR AB ( cross‐over or cross over or crossover )
S48 .TI trial
S49 .TI ( assign* or allocate* ) OR AB ( assign* or allocate* )
S50 .TI controls OR AB controls
S51 .S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50
S52 .S31 AND S51
S53 .TI (neonat* or newborn* or new born or pediatric or paediatric or birth or infant or infants or perinatal or peri‐natal or baby or babies or child or children)
S54 .S52 not S53
Appendix 5. Trials registry and ongoing trials search strategy
Advanced search combinations:
Stroke (as a condition) and fluid* (as an intervention)
Cerebrovascular event and fluid or parenteral fluid
Data and analyses
Comparison 1. Colloids versus crystalloids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependence | 5 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.21] |
| 2 Death | 12 | 2351 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.27] |
| 3 Pneumonia | 2 | 416 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 2.01] |
| 4 Cerebral oedema | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.74] |
| 5 Pulmonary oedema | 3 | 730 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.28, 4.29] |
Comparison 2. 0.9% saline versus other fluid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependence | 3 | 1120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.32] |
| 2 Death | 5 | 1760 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.12] |
| 3 Pneumonia | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.36, 11.16] |
| 4 Pulmonary oedema | 1 | 424 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aichner 1998.
| Methods | RCT, double blind Randomisation by a permutation of random numbers in blocks, identical bottles Disability measures: Graded Neurological Scale, Barthel Index, Mathew Scale |
|
| Participants | People with an ischaemic stroke in the MCA territory and < 70 in the Mathew Scale; within 6 hours | |
| Interventions | 10% HES 200/0.5 versus Ringer's lactate (control) for 5 days | |
| Outcomes | Death Disability: neurological and functional Complications secondary to fluids |
|
| Notes | Intended number of participants was 400. However, study was interrupted and interim analysis was performed at 200 participants due to slow recruitment and financial pressures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation scheme was generated by a permutation of random numbers in 150 blocks with a length of 4 participants each |
| Allocation concealment (selection bias) | Low risk | Medication boxes were identical; stained yellow to ensure consistency |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ''Double blinded''. Randomisation by random numbers, medication boxes identical, sealed envelopes contained randomisation number in case of an emergency |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome was measured by the same investigator at specified intervals. The investigator was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 200 participants, only 77 in the HES and 81 in the control group completed the study protocol |
| Selective reporting (reporting bias) | Low risk | Neurological outcome and disability (Graded Neurological Scale and Mathew Scale) were reported. Complications were also reported |
| Other bias | Low risk | — |
Bayer 1987.
| Methods | RCT, double blind Randomisation procedure not fully explained: ''Predetermined random order'' Disability measurement scaled via a ''defined system'' with maximum score = 228 |
|
| Participants | Participants with a hemiparesis from an ischaemic stroke within 48 hours | |
| Interventions | 10% glycerol versus saline (control) for 6 days | |
| Outcomes | Death Disabled |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were allocated by a predetermined random order; no explanation is provided as to this order |
| Allocation concealment (selection bias) | High risk | No explanation provided regarding the predetermined random order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ''Double blinded''. Infusion bottles coded, but of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ''Double blind''. Same investigator performed neurological and functional assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exact numbers reported; few dropouts. Outcomes (neurological, functional disability, complications, and mortality) all reported |
| Selective reporting (reporting bias) | Low risk | Grades of disability and functional decline reported as agreed in protocol |
| Other bias | Low risk | — |
Borenstein 1981.
| Methods | RCT, double blind Randomisation by coded numbers, although procedure not explained Disability outcome measured by clinical neurological score, Mathew Scale |
|
| Participants | Participants with an acute ischaemic stroke within 24 hours | |
| Interventions | Dextran in 5% dextrose versus Hartmann's (control) for 72 hours | |
| Outcomes | Death Change in Mathew score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not explained |
| Allocation concealment (selection bias) | Unclear risk | Rule for allocating participants to category and subsequent concealment of allocation not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | — |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data fully reported |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | — |
Fawer 1978.
| Methods | RCT, double blind Table of random numbers used for randomisation Own scoring system (total = 215) for measurement of disability |
|
| Participants | People with acute ischaemic stroke in the MCA territory within 48 hours | |
| Interventions | Glycerol versus glucose saline (control) for 6 days | |
| Outcomes | Death | |
| Notes | Number disabled difficult to interpret | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ''Patients who met the inclusion and exclusion criteria were divided into 2 groups by a table of random numbers'' |
| Allocation concealment (selection bias) | High risk | Table of random numbers was used to divide the participants into 2 groups, however there is no mention of concealed allocation after the generation of these random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ''Double blind''. Bottles were coded and identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind". Outcome assessment of functional and neurological recovery were performed by the same investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on mortality and functional recovery were reported. However, due to lack of cutoffs for the scale used, it was difficult to interpret absolute numbers of disabled |
| Selective reporting (reporting bias) | Low risk | Results reported as per scales mentioned |
| Other bias | Low risk | — |
Friedli 1975.
| Methods | RCT, single blinded (investigator) Randomisation procedure not explained Disability measured by a scoring system of mild/moderate or severe |
|
| Participants | People with an ischaemic stroke, timing not mentioned | |
| Interventions | 10% glycerol versus 5% dextrose (control) for 6 days | |
| Outcomes | Death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were ''randomly allocated''. No further clarification provided |
| Allocation concealment (selection bias) | High risk | It is unclear how "random allocation" occurred, as well as concealment of further participant allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded. The investigator was blinded, but participants were not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator was blinded to the treatment arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of neurological deficit and death reported as mentioned |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as mentioned |
| Other bias | Low risk | — |
Gilroy 1969.
| Methods | RCT Information on blinding not provided Randomisation by numerical series of envelopes Disability outcome measured by a ''scoring method'' (total = 50 points) |
|
| Participants | People with an ischaemic stroke within 72 hours | |
| Interventions | Dextran 40 versus saline dextrose (0.2 N/5%) as control for 3 days | |
| Outcomes | Death Change in neurological score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned in a ''random manner'' |
| Allocation concealment (selection bias) | Low risk | Randomisation by numerical series of envelopes, so allocation of participants remained concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes to be reported were recorded under the results section |
| Selective reporting (reporting bias) | Low risk | All results were reported, though absolute numbers for disability were difficult to interpret (change in neurological score) |
| Other bias | Low risk | — |
Ginsberg 2011.
| Methods | RCT, double blind Randomisation via centralised step‐forward web‐based 1:1 randomisation with biased‐coin minimisation algorithm Disability outcome measured by a number of scales: NIHSS, mRS, Barthel, stroke‐specific Quality of Life, Trail Making A & B |
|
| Participants | Participants with an ischaemic stroke over age 18 within 5 hours of stroke onset | |
| Interventions | 25% albumin versus 0.9% sodium chloride (placebo) over 120 minutes | |
| Outcomes | Death Complications of therapy, e.g. pulmonary oedema |
|
| Notes | 434 participants were enrolled. However, interim analysis was performed in 225 participants at 3 months, and the trial was suspended because of safety concerns | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised web‐based randomisation approach |
| Allocation concealment (selection bias) | Low risk | Drug kits were encased in blinding boxes; so further allocation of participants to treatment arms was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind approach |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of disability outcome (apart from death) at 3 months |
| Selective reporting (reporting bias) | High risk | No disability scale outcome reported |
| Other bias | High risk | Trial suspended early due to safety concerns |
Ginsberg 2013.
| Methods | RCT, double blind Randomisation via centralised 1:1 web randomisation biased‐coin minimisation approach Disability outcomes measured by NIHSS, mRS, Barthel, stroke‐specific Quality of Life, Trail Making A & B |
|
| Participants | People with an ischaemic stroke within 5 hours of onset; aged 18 to 83 years | |
| Interventions | 25% albumin versus 0.9% sodium chloride over 120 minutes | |
| Outcomes | Death Disability |
|
| Notes | Intention was to follow up participants for 1 year. However, enrolment was halted for futility with the third scheduled interim analysis. Hence 841 participants already in the trial were to be followed up for 90 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web randomisation, biased‐coin minimisation approach |
| Allocation concealment (selection bias) | Low risk | Sealed kit with bottles. Cardboard blinding boxes and opaque sheathing to conceal intravenous tubing |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study was terminated early |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported at 3 months, not the intended 1 year |
| Other bias | High risk | Early termination of study |
Larsson 1976.
| Methods | RCT, double blind Randomisation method unclear: ''Randomly allocated to treatment with prepared batches of intravenous solutions'' Disability measure by a ''neurological scoring system'' (max 54 points) |
|
| Participants | People with an ischaemic stroke within 6 hours | |
| Interventions | 10% glycerol in 5% dextrose versus 10% dextrose (placebo) for 6 days | |
| Outcomes | Death | |
| Notes | Disability outcome uninterpretable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is stated that participants were randomly allocated to treatment, but there is no mention of how this was done |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the randomisation and further allocation concealment took place |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ''Double blind'' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded, and 2 investigators performed the neurological scoring systems at specified intervals |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Death not reported at final follow‐up of 3 months |
| Selective reporting (reporting bias) | High risk | As above |
| Other bias | Low risk | — |
Matthews 1976.
| Methods | RCT, single blinded (evaluators only) Randomisation via series of coded envelopes Disability measure via ''6 point scale/Power of Limb Scale'' |
|
| Participants | People with an ischaemic stroke within 48 hours | |
| Interventions | Dextran 40 versus 5% dextrose (control) for 3 days | |
| Outcomes | Death Disabled: functional, neurological, need for institutional care |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated by opening the next one in a series of envelopes |
| Allocation concealment (selection bias) | High risk | Coded envelopes do not appear to be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Appears that assessors at follow‐up were blinded to the treatment received by each participant. However, evaluators do not seem to have been blinded at allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiners at follow‐up had not met the participants before, did not know the initial examination findings or the group the participant had been in |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Suggested outcomes were reported |
| Selective reporting (reporting bias) | Low risk | As above |
| Other bias | Low risk | — |
Rudolf 2002.
| Methods | RCT, no information on blinding Randomisation procedure not explained Disability outcome measured via NIHSS, Glasgow Outcome Scale, mRS, and Barthel Index |
|
| Participants | People with an ischaemic stroke within 6 hours | |
| Interventions | 10% HES 130/0.4 versus 0.9% saline (control) in a 2:1 ratio for 6.3 days | |
| Outcomes | Death Disabilty measured by functional outcome and neurological scores |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomised". No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Randomisation and allocation procedure not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of method of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of outcome evaluator |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear as to how many participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Disability outcome and severe adverse events reported |
| Other bias | Low risk | — |
Yu 1992.
| Methods | RCT, double blind Randomisation via group sequential approach; stratified randomised block design Disability outcome measures: Glasgow Coma Scale, Scandinavian Stroke Scale, Barthel Index |
|
| Participants | Participants with a haemorrhagic stroke within 24 hours | |
| Interventions | 10% glycerol versus 0.9% saline (control) for 6 days | |
| Outcomes | Death Change in neurological scores Cardiac complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A group sequential approach was adopted to randomly allocate treatment |
| Allocation concealment (selection bias) | Low risk | Treatment was allocated according to a stratified randomised block design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Though "double blind" is mentioned, this is not justified in the body of the protocol |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large proportion lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Secondary to large proportion lost to follow‐up |
| Other bias | Unclear risk | It is unclear if the same assessor was following up the participants; if not, observer variability could account for a source of bias |
HES: hydroethyl starch MCA; middle cerebral artery mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balcarce 1991 | Randomised trial; single‐blinded participants only. Involves venesection, which is not considered standard fluid intervention |
| Brownsell 2000 | People with stroke randomised to 3 different modes of hydration: slow subcutaneous fluids, bolus subcutaneous fluids, and intravenous fluids. Serum osmolality, weights, and infection rate as outcomes. No disability outcome measured |
| Challiner 1994 | Randomised trial of intravenous versus subcutaneous fluid therapies. No disability outcome measured |
| Diringer 2011 | Randomised trial of 2 osmotic agents; blinding unclear. NIHSS measured at baseline, but no disability outcome reported |
| Frei 1987 | RCT, double blind. Reports death as an outcome, but these results are not reported according to the randomised groups |
| Ginsberg 2006 | Multiple‐tier, open‐label, dose‐escalation design. Trial of people with ischaemic stroke; both groups received albumin, but the differing factor appears to be tissue plasminogen activator. Disability scales (Barthel Index, mRS, and NIHSS) measured at start of trial, but only death and other complications (pneumonia, cerebral oedema, pulmonary oedema, haemorrhage, pulmonary embolism) reported with no mention of measurement of disability at 3 months |
| Goslinga 1992 | RCT, blinding not mentioned. Involves venesection, which is not considered standard practise |
| Grauer 1999 | RCT, double blind. Clinical neurological score mentioned, but no disability outcome reported |
| Grauer 2001 | RCT, double blind. No disability outcome reported. Effects on haemostaseological parameters reported as outcome |
| Greenhall 1974 | Summary from conference proceeding. Full text unavailable. No data extractable from available summary |
| Haass 1986 | No disability outcome. Outcomes measured included plasma viscosity and erythrocyte aggregation (haemorheological factors) |
| Hamamoto 1992 | Randomised trial of hypervolaemic haemodilution. No information given on details of therapy. Graded Neurological Scale mentioned, but no effects of treatment reported |
| Han 2012 | Randomised double‐blind study comparing the effects of sodium chloride versus HES 130/0.4 on brain hypoperfusion. Study due completion April 2011 with disability outcome measured by NIHSS/Barthel and mRS scales. No results subsequently published. Authors contacted; no reply obtained |
| Haring 1994 | RCT, double blind. Randomisation method unknown. HES 200/0.5 versus HES 200/0.62 for 5 days. Disability outcome measured by Mathew Scale; change in neurological score provided but no absolute numbers of those dead or disabled, making interpretation of absolute data difficult |
| Hartmann 1987 | RCT of HES versus no specific treatment for 7 days. Randomisation procedure not mentioned; unclear blinding. Outcome measured is cerebral blood flow; no disability outcome reported |
| Hemmati 2012 | RCT, double blind. 140 people with stroke allocated to albumin versus saline. Functional outcome at 1 week and 3 months. Recruitment complete in 2012. Unclear current state of study; no results published. Author contacted, but no response received |
| HSSG 1989 | Randomised, unblinded study where the intervention group received HES for 72 to 96 hours with haemodynamic monitoring with Swan‐Ganz catheter and venesection in selected participants. Hence multiple interventions were used in the intervention group in comparison to the control, making direct comparisons of outcomes difficult to interpret. Also, venesection is not considered standard practise for the purposes of this review |
| Koltringer 1992 | Randomised trial of hypervolaemic haemodilution in 3 different groups of participants. Blinding unclear. Effect on haemorheological changes. No disability outcome reported |
| Kramer 1981 | Controlled trial, unclear if randomised |
| Kroemer 1987 | Unclear if randomised. No disability outcome. Outcomes measured include plasma viscosity and hematocrit |
| Lee 2012 | RCT, double blind. Albumin versus saline in people with acute ischaemic stroke. mRS and NIHSS as measures of disability outcome. Due completion of recruitment in September 2014; however, study terminated early, no interim results published. Author contacted, no response received |
| Lin 2013 | Study of hydration status and stroke in evolution. Unclear if randomised. Results of disability (as measured by the NIHSS scale) not fully reported with no absolute numbers revealed. Authors contacted, no reply obtained |
| Lye 2012 | A multicentre RCT comparing intravenous versus subcutaneous fluids in poststroke hydration. Trial reported as completed, but no outcome measures listed and no results published. Lead researcher contacted, no reply obtained |
| Morgenthaler 2010 | Randomised pilot study of 2 established HES solutions. No disability outcome measure. Interested outcome is on haemostatic effects |
| Oghbaei 2012 | RCT on the effect of albumin on morbidity and mortality of intracranial haemorrhage. Unclear of current status of trial; recruitment completed in September 2011. No results published. Author contacted, no reply obtained |
| Popa 1989 | RCT of venesection and dextran versus no specific treatment. Involves venesection, which is not considered standard practise |
| Qizilbash 1993 | Abstract of meta‐analysis of 5 trials on the use of dextran in acute stroke. We were unable to extract data from the abstract, exclusion of pre‐analysed data (meta‐analysis) |
| Schneider 1985 | Trial comparing HES and dextran 40. Unclear if randomised. No overall effect on death or neurological outcome noted |
| Shalilahmadi 2013 | Randomised trial of albumin versus saline on haematoma volume of people admitted with haemorrhagic stroke. Recruitment completed in 2013; unclear of current status of trial. No results published. Author contacted; no reply obtained |
| Shin 2007 | Trial of albumin versus saline in people with ischaemic stroke within 24 hours. Not randomised, as treatment received depended on the day of admission |
| Spudis 1973 | RCT; unclear of blinding. The use of dextran 40 in people with ischaemic stroke within 24 hours. Outcomes measured include death and change in neurological scores. No mention of the type of control used |
| Staedt 1987 | Randomised trial, no placebo control with comparison of 2 hypertonic solutions, which did not fit our inclusion criteria. No information regarding blinding. Outcomes of death and change in neurological score reported. Change in neurological score difficult to interpret with no absolute numbers reported |
| Tessitore 1990 | Unclear if the trial was randomised. Involves venesection in the intervention group, which is not standard practise. The control group seems to have obtained antioedema and antithrombosis treatment, but further clarification on these is not given |
| Torun 1995 | RCT of isovolaemic haemodilution involving venesection and HES. Functional outcome or survival data not reported |
| Treib 1999 | RCT, double blind comparing 2 different HES compounds with different molecular weights. Only abstract available. Results in available abstract not fully reported. ''32 showed an improved neurological score, 11 remain unchanged and none deteriorated''. No absolute figures or disability outcome in individual treatment arms mentioned. Author contacted; no reply obtained |
| Vorstrup 1989 | Unclear if this is a randomised trial. Involves haemodilution by venesection, which is not considered standard practise |
| Wagner 2011 | Not a randomised trial; the intervention group received hypertonic saline, and results were compared to historical controls |
| Woessner 2003 | RCT, double blind, comparing HES versus crystalloid solution in people with ischaemic stroke. Barthel Index measured at start of trial, day 1, 5, and 90. However, no absolute data given at 90 days: ''The increase in Barthel Index in HES group was 2x than in the electrolyte group at 90 days''. Author contacted to obtain unpublished data, no reply obtained |
| Wu 1993 | RCT of mannitol versus dextran in acute cerebral infarction. Outcome is curative rate; no absolute data provided |
| Yu 2003 | Comparison of combination therapy with mannitol; not solely fluid intervention. Outcome measures not reported with absolute data |
| Zhao 1987 | RCT of mannitol versus dextran in ischaemic strokes. Randomisation procedure not mentioned. Curative rate as outcome; percentages provided but not absolute numbers |
HES: hydroethyl starch mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02003794.
| Trial name or title | Randomised controlled study of the effectiveness of iv fluid infusion in people with acute ischaemic stroke (IVIS) |
| Methods | RCT, interventional, single blinded (outcomes assessor) |
| Participants | Adults (age 18 to 80) with acute ischaemic stroke, NIHSS > 1, within 72 hours of stroke onset |
| Interventions | 0.9% saline, 100 mL/hour for 3 days versus no iv fluids |
| Outcomes | Dependence (NIHSS score, mRS scores) |
| Starting date | May 2013 |
| Contact information | Supparat Charnwut: fern2_95@yahoo.com; Aurauma Chutinet: aurauma@yahoo.com |
| Notes | Sponsored by Chulalongkorn University and National Research Council of Thailand (NRCT) |
iv: intravenous mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial
Differences between protocol and review
We changed the term 'hypotonic' to 'crystalloids' throughout the review as we felt this to be more appropriate and easier to understand. We have also changed the word 'hypertonic' to 'colloids', as all included studies reported the use of colloids as the hypertonic comparator, and the review authors felt this would be easier to understand.
Due to the lack of trials studying outcomes in people with haemorrhagic stroke, we were unable to perform any subgroup analysis. No trials subcategorised participants in terms of their ability to swallow.
We updated the 'Objectives' section to clarify the different fluid regimens being compared.
We changed the wording of our primary outcome measure in the review from "independence at six months or less poststroke" to "death or dependence less than six months poststroke", as most studies reported the latter; this enabled easier data analysis and reporting.
We changed our first secondary outcome to death at 12 months or earlier after stroke (instead of three to six months) to ensure that we did not miss potentially relevant studies with a longer follow‐up time point.
Methods for future updates
For any future cluster‐randomised trials that may be relevant, we will extract information that is a direct estimate of the required effect measure.
We will consider sensitivity analyses based on stroke subtype and ability to swallow, if possible.
Contributions of authors
Akila Visvanathan: Searched for studies, extracted data from studies, performed meta‐analyses, produced the first draft of the manuscript. Martin Dennis: Commented critically on the final version of the manuscript. William Whiteley: Extracted data from studies, commented critically on the manuscript.
Sources of support
Internal sources
No internal sources of support, Other.
External sources
No external sources of support, Other.
Declarations of interest
Akila Visvanathan: None known. Martin Dennis: None known. William Whiteley: Funded by a Medical Research Council Clinician Scientist Fellowship.
New
References
References to studies included in this review
Aichner 1998 {published data only}
- Aichner FT, Fazekas F, Brainin M, Polz W, Mamoli B, Zeiler K. Hypervolemic hemodilution in acute ischemic stroke. The Multicenter Austrian Hemodilution Stroke Trial (MAHST). Stroke 1998;29:743‐9. [DOI] [PubMed] [Google Scholar]
Bayer 1987 {published data only}
- Bayer AJ, Pathy MSJ, Newcombe R. Double‐blind randomised trial of intravenous glycerol in acute stroke. The Lancet 1987;1:405‐8. [DOI] [PubMed] [Google Scholar]
Borenstein 1981 {published data only}
- Borenstein N, Regev I, Flechater S, Streifler M, Vardi J. Dextran‐40 in nonhemorrhagic cerebrovascular accidents. Harefuah 1981;100(10):455. [PubMed] [Google Scholar]
Fawer 1978 {published data only}
- Fawer R, Justafre JC, Berger JP, Schelling JL. Intravenous glycerol in cerebral infarction: a controlled 4 month trial. Stroke 1978;9:484‐6. [DOI] [PubMed] [Google Scholar]
Friedli 1975 {published data only}
- Friedli W, Imbach P, Ghisleni‐Steinegger S, Schwarz C, Maire P. Treatment with 10% glycerin in acute ischaemic cerebral infarct. Double blind study. Schweizerische Medizinische Wochenschrift 1979;109(20):737‐42. [PubMed] [Google Scholar]
Gilroy 1969 {published data only}
- Gilroy J, Barnhart M, Meyer JS. Treatment of acute stroke with Dextran 40. JAMA 1969;210(2):293‐8. [PubMed] [Google Scholar]
Ginsberg 2011 {published data only}
- Ginsberg MD, Palesch YY, Martin RH, Hill MD, Moy CS, Waldman BD, et al. The albumin in acute stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke 2011;42:119‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginsberg 2013 {published data only}
- Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, et al. High‐dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double‐blind, phase 3, placebo‐controlled trial. The Lancet Neurology 2013;12:1049‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsson 1976 {published data only}
- Larsson O, Marinovich N, Barber K. Double‐blind trial of glycerol therapy in early stroke. The Lancet 1976;1:832‐4. [DOI] [PubMed] [Google Scholar]
Matthews 1976 {published data only}
- Matthews WB, Oxbury JM, Grainger KM, Greenhall RC. A blind controlled trial of dextran 40 in the treatment of ischaemic stroke. Brain 1976;99:193‐206. [DOI] [PubMed] [Google Scholar]
Rudolf 2002 {published data only}
- Rudolf J. Hydroxyethyl starch for hypervolemic hemodilution in patients with acute ischemic stroke: a randomized, placebo‐controlled phase II safety study. Cerebrovascular Diseases 2002;14(1):33‐41. [DOI] [PubMed] [Google Scholar]
Yu 1992 {published data only}
- Yu Y, Kumana CR, Lauder IJ, Cheung YK, Chan FL, Kou M, et al. Treatment of acute cerebral hemorrhage with intravenous glycerol. A double‐blind, placebo‐controlled, randomized trial. Stroke 1992;23(7):967‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Balcarce 1991 {published data only}
- Balcarce PE, Vila JF, Fustinoni O. Isovolaemic haemodilution with plasma in acute ischaemic stroke. Revista Neurológica Argentina 1991;16:154‐60. [Google Scholar]
Brownsell 2000 {published data only}
- Brownsell MD, Mealey PJ, Watkins CL, Jack C, Leathley MJ. A feasibility study of differing methods of parenteral hydration post‐stroke. Proceedings of the Consensus Conference on Stroke Treatment and Service Delivery. 7‐8 November 2000; Edinburgh, UK. Royal College of Physicians of Edinburgh, 2000:41.
Challiner 1994 {published data only}
- Challiner YC, Jarrett D, Hayward MJ, al‐Jubouri MA, Julious SA. A comparison of intravenous and subcutaneous hydration in elderly acute stroke patients. Postgraduate Medical Journal 1994;70(821):196‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diringer 2011 {published data only}
- Diringer MN, Scalfani MT, Zazulia AR, Videen TO, Dhar R. Cerebral hemodynamic and metabolic effects of equi‐osmolar doses mannitol and 23.4% saline in patients with edema following large ischemic stroke. NeuroCritical Care 2011;14(1):11‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frei 1987 {published data only}
- Frei A, Cottier C, Wunderlich P. Glycerol and dextran combined in the therapy of acute stroke. A placebo‐controlled, double‐blind trial with a planned interim analysis. Stroke 1987;18(2):373‐9. [DOI] [PubMed] [Google Scholar]
Ginsberg 2006 {published data only}
- Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D. The ALIAS Pilot Trial: a dose‐escalation and safety study of albumin therapy for acute ischemic stroke ‐ I: physiological responses and safety results. Stroke 2006;37:2100‐16. [DOI] [PubMed] [Google Scholar]
Goslinga 1992 {published data only}
- Goslinga H, Eijzenbach V, Heuvelmans JH, Laan de Vries, Melis V, Bezemer PD. Custom‐tailored hemodilution with albumin and crystalloids in acute ischemic stroke. Stroke 1992;23(2):181‐8. [DOI] [PubMed] [Google Scholar]
Grauer 1999 {published data only}
- Grauer MT, Woessner R, Seltmann A, Windisch F, Schenk JF, Wenzel E, et al. Therapeutic safety of a high‐dose volume therapy with hydroxyethyl starch in patients with acute stroke under intensive care monitoring. Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 1999;34:S87‐8. [Google Scholar]
Grauer 2001 {published data only}
- Grauer MT, Baus D, Woessner R, Bepperling F, Kahles T, Georgi S, et al. Effects on general safety and coagulation after long‐term, high‐dose volume therapy with 6% hydroethyl starch 130/0.4 in patients with acute ischemic stroke. Results of a randomized, placebo‐controlled, double‐blind study. Critical Care 2001;5(1):S53‐4. [Google Scholar]
Greenhall 1974 {published data only}
- Greenhall RCD. Clinical and neuropathological observations in a controlled trial of low molecular weight dextran (LMD) in acute cerebral infarction. Neuropathology and Applied Neurobiology 1974;1(1):108. [Google Scholar]
Haass 1986 {published data only}
- Haass A, Kroemer H, Jager H, Muller K, Decker I, Wagner EM, et al. Dextran 40 or HES 200/0.5? Haemorheology on long‐time treatment of ischaemic stroke. Deutsche Medizinische Wochenschrift 1986;111:1681‐6. [DOI] [PubMed] [Google Scholar]
Hamamoto 1992 {published data only}
- Hamamoto M, Nagao T, Kanda A, Ichiseki H, Miyazaki T, Terashi A. Hypervolemic hemodilution therapy for the elderly patients with atherothrombotic and hemodynamic ischemic stroke. Journal of Stroke and Cerebrovascular Diseases 1992;2(1):S136. [Google Scholar]
Han 2012 {published and unpublished data}
- Han J, Yang F, Jiang W, Zhang G, Liu Z, Liu X, et al. Hydroxyethyl starch 130/0.4 and sodium chloride injection as adjunctive therapy in patients with cerebral hypoperfusion. BMC Neurology 2012;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haring 1994 {published data only}
- Haring HP, Pohl P, Schimetta W, Polz W, Aichner F. Hydroxyethyl starch ‐ influence of molar substitution on haemorheologic parameters: a clinical double blind study. Clinical Hemorheology 1994;14(1):11‐8. [Google Scholar]
Hartmann 1987 {published data only}
- Hartmann A, Tsuda Y, Lagreze H. Effect of hypervolaemic haemodilution of regional cerebral blood flow in patients with acute ischaemic stroke: a controlled study with hydroethyl starch. Journal of Neurology 1987;235:34‐8. [DOI] [PubMed] [Google Scholar]
Hemmati 2012 {published and unpublished data}
- Hemmati A. Assessment the effect of albumin compared with placebo on functional outcome after ischemic stroke in 18‐83 year old patients with ischemic stroke in MCA territory referred to Golestan Hospital of Ahvaz. Iranian Registry of Clinical Trials http://www.irct.ir/searchresult.php?keyword=randomly&field=&id=4606&number=1&prt=1084&total=10&m=1 2012.
HSSG 1989 {published data only}
- Hemodilution in Stroke Study Group. Hypervolemic hemodilution treatment of acute stroke. Results of a randomized multicenter trial using pentastarch. Stroke 1989;20(3):317‐23. [DOI] [PubMed] [Google Scholar]
Koltringer 1992 {published data only}
- Koltringer P, Langsteger W, Reisecker F, Eber O. Hypervolemic haemodilution and completed stroke: how important is the application time for its effect?. Neurological Research 1992;14:171‐3. [DOI] [PubMed] [Google Scholar]
Kramer 1981 {published data only}
- Kramer W, Rompel C, Umlauf B, Ulm K. Controlled, comparative blind study on the effect of glycerin infusions in recent ischemic crises. Medizinische Welt 1981;32:813‐6. [PubMed] [Google Scholar]
Kroemer 1987 {published data only}
- Kroemer H, Haass A, Muller K, Jager H, Wagner EM, Heimburg P, et al. Haemodilution therapy in ischaemic stroke: plasma concentrations and plasma viscosity during long‐term infusion of dextran 40 or hydroethyl starch 200/0.5. European Journal of Clinical Pharmacology 1987;31:705‐10. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee KS. A placebo comparative, double blinded, randomized, multicenter study to evaluate the efficacy and safety of albumin therapy in acute ischemic stroke patients in Korea. Clinical Research Information Service (CRIS) Republic of Korea 2012.
Lin 2013 {published and unpublished data}
- Lin LC, Hsiao KY, Tsai YH, Lai SL, Lei CC, Hsiao CT. Hydration status and stroke‐in‐evolution after ischemic stroke: a preliminary study. International Journal of Stroke 2013;8(7):E52. [DOI] [PubMed] [Google Scholar]
Lye 2012 {published and unpublished data}
- Lye M. Comparison of intravenous and subcutaneous bolus infusion in post‐stroke hydration. www.controlled‐trials.com/mrct/trial/stroke/1046/18319.html 2012.
Morgenthaler 2010 {published data only}
- Morgenthaler M, Woessner R, Treib J. Is there new safety information to be gained out of the use of thrombelastography in stroke patients receiving hydroxyethyl starches for treatment?. Cerebrovascular Diseases 2010;29 Suppl 2:235. [Google Scholar]
Oghbaei 2012 {published and unpublished data}
- Oghbaei M. The effect of intravenous albumin in mortality and morbidity of intracranial hemorrhage; a randomized clinical trial. Iranian Registry of Clinical Trials http://irct.ir/searchresult.php?keyword=fibrinogen&id=8240&field=&number=1&prt=31&total=5&m=1 2012.
Popa 1989 {published data only}
- Popa G, Popa C, Stanescu A, Ionescu G, Lugoji G, Radula D, et al. Haemodilution therapy in acute ischaemic stroke. Revue Roumaine de Medecine Neurologie et Psychiatrie 1989;27:79‐90. [PubMed] [Google Scholar]
Qizilbash 1993 {published data only}
- Qizilbash N, Murphy M. Meta‐analysis of trials of dextran in acute stroke. Age and Ageing 1993;22:14. [Google Scholar]
Schneider 1985 {published data only}
- Schneider R, Hacke W, Kiesewetter H, Jung F. Hemodilution in acute ischemic insults. Comparative study on the clinical and hemorheologic effectiveness of 10% HES 200/0.5 and 10% dextran 40. Fortschritte der Medizin 1985;103(44):1031‐4. [PubMed] [Google Scholar]
Shalilahmadi 2013 {published and unpublished data}
- Shalilahmadi D. Assessment of the effect of albumin compared with placebo on the reduction of the hematoma volume in patients with hemorrhagic stroke referred to Ahvaz Golestan Hospital. Iranian Registry of Clinical Trials http://irct.ir/searchresult.php?keyword=fibrinogen&id=8574&field=&number=1&prt=73&total=5&m=1 2013.
Shin 2007 {published data only}
- Shin DH, Moon GJ, Bang OY. Albumin therapy in acute stroke patients. Journal of Neurology 2007;254(7):870‐8. [DOI] [PubMed] [Google Scholar]
Spudis 1973 {published data only}
- Spudis EV, Torre E, Pikula L. Management of completed strokes with dextran 40. A community hospital failure. Stroke 1973;4(6):895‐7. [DOI] [PubMed] [Google Scholar]
Staedt 1987 {published data only}
- Staedt U, Schlierf G, Oster P, Mechtersheimer U, Baessler G, Seufzer U. Hypervolemic hemodilution with 10% HES 200/0.5 and 10% Dextran 40 in patients with ischemic stroke. Cerebral Ischemia and Hemorheology. Springer, 1987:429‐35. [Google Scholar]
Tessitore 1990 {published data only}
- Tessitore A, Diaco S, Battaglia A. Hydroxyethyl starch in the treatment of ischemic stroke. Quaderni Di Medicina E Chirurgia 1990;6(3):350‐2. [Google Scholar]
Torun 1995 {published data only}
- Torun S, Ozoner Z, Gucuyener D, Uzuner N, Ozdemir G. The effects of isovolemic hemodilution in acute ischemic stroke (a randomized controlled clinical trial). European Journal of Neurology 1995;2 Suppl 1:78. [Google Scholar]
Treib 1999 {published and unpublished data}
- Treib J, Behnke S, Seltmann A, Fischer C, Windisch F, Grauer MT, et al. Effects of a high‐dose hypervolemic volume therapy using HES 200/0.5 or HES 70/0.5 on hemodynamics, hemorheology and coagulation in patients suffering from acute stroke. Results of a randomised double‐blind study. Cerebrovascular Diseases 1999;9 Suppl 1:101. [Google Scholar]
Vorstrup 1989 {published data only}
- Vorstrup S, Andersen A, Juhler M, Brun B, Boysen G. Hemodilution increases cerebral blood flow in acute ischemic stroke. Stroke 1989;20:84‐9. [DOI] [PubMed] [Google Scholar]
Wagner 2011 {published data only}
- Wagner I, Hauer E‐M, Staykov D, Volbers B, Dorfler A, Schwab S, et al. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke 2011;42(6):1540. [DOI] [PubMed] [Google Scholar]
Woessner 2003 {published and unpublished data}
- Woessner R, Grauer MT, Dieterich HJ, Bepperling F, Baus D, Kahles T, et al. Influence of a long‐term, high‐dose volume therapy with 6% hydroxyethyl starch 130/0.4 or crystalloid solution on hemodynamics, rheology and hemostasis in patients with acute ischemic stroke. Pathophysiology of Haemostasis and Thrombosis 2004;33(3):121‐6. [DOI] [PubMed] [Google Scholar]
Wu 1993 {published data only}
- Wu SH. Mannitol in the treatment of acute cerebral infarction (50 cases analyses). Journal of Stroke and Neurological Diseases 1993;10(3):177‐8. [Google Scholar]
Yu 2003 {published data only}
- Yu L, Zhao Y. Efficacy of aescine sodium, mannitol, and albumin on cerebral post‐infarction. China Pharmacist 2003;6(12):801‐2. [Google Scholar]
Zhao 1987 {published data only}
- Zhao PX. Clinical observation on the effect of 20% mannitol in treating cerebral thrombosis. Chinese Journal of Neurology & Psychiatry 1987;20(3):169‐72. [PubMed] [Google Scholar]
References to ongoing studies
NCT02003794 {published data only}
- Suwanwela NC. Randomised controlled study of the effectiveness of iv fluid infusion in patients with acute ischemic stroke (IVIS). clinicaltrials.gov/ct2/show/NCT02003794 (accessed May 2015).
Additional references
Bamford 1989
Banks 2007
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38(3):1091‐6. [PUBMED: 17272767] [DOI] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. JAMA 2010;303(12):1180‐7. [DOI] [PubMed] [Google Scholar]
Britton 1980
- Britton M, Faire U, Helmers C. Hazards of therapy for excessive hypertension in acute stroke. Acta Medica Scandinavica 1980;207(4):253‐7. [PUBMED: 7386220] [DOI] [PubMed] [Google Scholar]
Chang 2014
- Chang TS, Jensen MB. Haemodilution for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD000103.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Haan 1993
- Haan R, Horn J, Limburg M, Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke 1993;24(8):1178‐81. [PUBMED: 8342193] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigin 2005
- Feigin VL. Stroke epidemiology in the developing world. The Lancet 2005;365(9478):2160‐1. [PUBMED: 15978910] [DOI] [PubMed] [Google Scholar]
Feigin 2014
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. The Lancet 2014;18(383):245‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finestone 2001
- Finestone HM, Foley NC, Woodbury MG, Greene‐Finestone L. Quantifying fluid intake in dysphagic stroke patients: a preliminary comparison of oral and nonoral strategies. Archives of Physical Medicine and Rehabilitation 2001;82(12):1744‐6. [PUBMED: 11733894] [DOI] [PubMed] [Google Scholar]
Gordon 1987
- Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. British Medical Journal 1987;295(6595):411‐4. [PUBMED: 3115478] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Granger 1979
- Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: analysis of repeated Barthel Index measures. Archives of Physical Medicine and Rehabilitation 1979;60(1):14‐7. [PUBMED: 420565] [PubMed] [Google Scholar]
Heros 1989
- Heros R, Korosue K. Hemodilution for cerebral ischaemia. Stroke 1989;20:423‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Howard 2011
- Howard G, Hess DC, Howard VJ. Wisdom and determination in the ongoing pursuit of the ever‐elusive neuroprotective agent. Stroke 2011;42(6):1505‐6. [DOI] [PubMed] [Google Scholar]
IASS 1988
- Italian Acute Stroke Study Group. Haemodilution in acute stroke: results of the Italian haemodilution trial. The Lancet 1988;1:318‐21. [PubMed] [Google Scholar]
Jauch 2013
- Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870‐947. [PUBMED: 23370205] [DOI] [PubMed] [Google Scholar]
Kelly 2004
- Kelly J, Rudd A, Lewis RR, Coshall C, Parmar K, Moody A, et al. Screening for proximal deep vein thrombosis after acute ischemic stroke: a prospective study using clinical factors and plasma D‐dimers. Journal of Thrombosis and Haemostasis 2004;2:1321‐6. [DOI] [PubMed] [Google Scholar]
Koller 1990
- Koller M, Haenny P, Hess K, Weniger D, Zangger P. Adjusted hypervolemic hemodilution in acute ischemic stroke. Stroke 1990;21(10):1429‐34. [DOI] [PubMed] [Google Scholar]
Kositzke 1990
- Kositzke JA. A question of balance—dehydration in the elderly. Journal of Gerontological Nursing 1990;16(5):4‐11. [PUBMED: 2358648] [DOI] [PubMed] [Google Scholar]
Lopez‐Espuela 2014
- Lopez‐Espuela F, Zamorano JD, Ramírez‐Moreno JM, Jiménez‐Caballero PE, Portilla‐Cuenca JC, Lavado‐García JM, et al. Determinants of quality of life in stroke survivors after 6 months, from a comprehensive stroke unit: a longitudinal study. Biological Research for Nursing 2014;epub ahead of print:pii: 1099800414553658. [DOI] [PubMed] [Google Scholar]
Maitland 2011
- Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot‐Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. The New England Journal of Medicine 2011;364(26):2483‐95. [DOI] [PubMed] [Google Scholar]
Mast 1991
- Mast H, Marx P. Neurological deterioration under isovolemic hemodilution with hydroxyethyl starch in acute cerebral ischemia. Stroke 1991;22:955. [DOI] [PubMed] [Google Scholar]
Myburgh 2013
- Myburgh JA, Mythen MG. Resuscitation fluids. The New England Journal of Medicine 2013;369(13):1243‐51. [PUBMED: 24066745] [DOI] [PubMed] [Google Scholar]
NICE 2013
- NICE: National Institute for Health and Care Excellence. Intravenous fluid therapy in adults in hospital. http://www.nice.org.uk/guidance/index.jsp?action=byID&o=14330 2013.
Reith 1997
- Reith J, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study. Stroke 1997;28(8):1585‐9. [PUBMED: 9259753] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowat 2012
- Rowat A, Graham C, Dennis M. Dehydration in hospital‐admitted stroke patients. Stroke 2012;43:857‐9. [DOI] [PubMed] [Google Scholar]
SAFE 2004
- SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. The New England Journal of Medicine 2004;350:2247‐56. [DOI] [PubMed] [Google Scholar]
Scott 1999
- Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke Trial (GIST). Stroke 1999;30(4):793‐9. [PUBMED: 10187881] [DOI] [PubMed] [Google Scholar]
Sims 2010
- Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochimica et Biophysica Acta 2010;1802(1):80‐91. [PUBMED: 19751827] [DOI] [PubMed] [Google Scholar]
SSS 1987
- Scandinavian Stroke Study Group. Multicenter trial of hemodilution in acute ischemic stroke I. Results in the total patient population. Stroke 1987;18:691‐9. [DOI] [PubMed] [Google Scholar]
Truelsen 2000
- Truelsen T, Begg S, Mathers C. The global burden of cerebrovascular disease. World Health Organization. http://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf 2000.
WHO 1978
- World Health Organization. Cerebrovascular Disorders. Geneva: World Health Organization, 1978. [Google Scholar]


