Abstract
Background
The effectiveness of interventions to increase the uptake of influenza vaccination in people aged 60 and older is uncertain.
Objectives
To assess access, provider, system and societal interventions to increase the uptake of influenza vaccination in people aged 60 years and older in the community.
Search methods
We searched CENTRAL (2014, Issue 5), MEDLINE (January 1950 to May week 3 2014), EMBASE (1980 to June 2014), AgeLine (1978 to 4 June 2014), ERIC (1965 to June 2014) and CINAHL (1982 to June 2014).
Selection criteria
Randomised controlled trials (RCTs) of interventions to increase influenza vaccination uptake in people aged 60 and older.
Data collection and analysis
Two review authors independently assessed study quality and extracted influenza vaccine uptake data.
Main results
This update identified 13 new RCTs; the review now includes a total of 57 RCTs with 896,531 participants. The trials included community‐dwelling seniors in high‐income countries. Heterogeneity limited meta‐analysis. The percentage of trials with low risk of bias for each domain was as follows: randomisation (33%); allocation concealment (11%); blinding (44%); missing data (49%) and selective reporting (100%).
Increasing community demand (32 trials, 10 strategies)
The interventions with a statistically significant result were: three trials (n = 64,200) of letter plus leaflet/postcard compared to letter (odds ratio (OR) 1.11, 95% confidence interval (CI) 1.07 to 1.15); two trials (n = 614) of nurses/pharmacists educating plus vaccinating patients (OR 3.29, 95% CI 1.91 to 5.66); single trials of a phone call from a senior (n = 193) (OR 3.33, 95% CI 1.79 to 6.22), a telephone invitation versus clinic drop‐in (n = 243) (OR 2.72, 95% CI 1.55 to 4.76), a free groceries lottery (n = 291) (OR 1.04, 95% CI 0.62 to 1.76) and nurses educating and vaccinating patients (n = 485) (OR 152.95, 95% CI 9.39 to 2490.67).
We did not pool the following trials due to considerable heterogeneity: postcard/letter/pamphlets (16 trials, n = 592,165); tailored communications (16 trials, n = 388,164); customised letter/phone‐call (four trials, n = 82,465) and client‐based appraisals (three trials, n = 4016), although several trials showed the interventions were effective.
Enhancing vaccination access (10 trials, six strategies)
The interventions with a statistically significant result were: two trials (n = 2112) of home visits compared to clinic invitation (OR 1.30, 95% CI 1.05 to 1.61); two trials (n = 2251) of free vaccine (OR 2.36, 95% CI 1.98 to 2.82) and one trial (n = 321) of patient group visits (OR 24.85, 95% CI 1.45 to 425.32). One trial (n = 350) of a home visit plus vaccine encouragement compared to a home visit plus safety advice was non‐significant.
We did not pool the following trials due to considerable heterogeneity: nurse home visits (two trials, n = 2069) and free vaccine compared to no intervention (two trials, n = 2250).
Provider‐ or system‐based interventions (17 trials, 11 strategies)
The interventions with a statistically significant result were: two trials (n = 2815) of paying physicians (OR 2.22, 95% CI 1.77 to 2.77); one trial (n = 316) of reminding physicians about all their patients (OR 2.47, 95% CI 1.53 to 3.99); one trial (n = 8376) of posters plus postcards (OR 2.03, 95% CI 1.86 to 2.22); one trial (n = 1360) of chart review/feedback (OR 3.43, 95% CI 2.37 to 4.97) and one trial (n = 27,580) of educational outreach/feedback (OR 0.77, 95% CI 0.72 to 0.81).
Trials of posters plus postcards versus posters (n = 5753), academic detailing (n = 1400) and increasing staff vaccination rates (n = 26,432) were non‐significant.
We did not pool the following trials due to considerable heterogeneity: reminding physicians (four trials, n = 202,264) and practice facilitators (three trials, n = 2183), although several trials showed the interventions were effective.
Interventions at the societal level
We identified no RCTs of interventions at the societal level.
Authors' conclusions
There are interventions that are effective for increasing community demand for vaccination, enhancing access and improving provider/system response. Heterogeneity limited pooling of trials.
Keywords: Aged; Humans; Middle Aged; Reminder Systems; Attitude of Health Personnel; Community Participation; Health Services Needs and Demand; Immunization Programs; Immunization Programs/methods; Influenza Vaccines; Influenza Vaccines/administration & dosage; Influenza, Human; Influenza, Human/prevention & control; Randomized Controlled Trials as Topic; Vaccination; Vaccination/utilization
Interventions to increase influenza (flu) vaccination uptake for people aged 60 and older
Many health authorities recommend influenza vaccination of older people. However, vaccination uptake in people aged 60 and older varies across countries, socioeconomic and health‐risk groups. It is important to identify effective interventions to increase influenza vaccination uptake.
We included 57 randomised controlled trials (RCTs) with 896,531 participants (all were community‐dwelling seniors in high‐income countries). Thirty‐six trials compared the intervention to a no‐intervention control group. Of the 57 RCTs, 33% randomised participants using a method that produced a low risk of bias and 61% used a method with an unclear risk. For missing data, 49% of the RCTs had a low risk of bias and 39% had an unclear risk.
Included trials all focused on increasing influenza vaccination uptake and did not report adverse effects. Trials were varied and we needed to use caution when pooling results.
Increasing community demand for vaccination (32 trials, 10 strategies)
Effective interventions in this comparison were a letter plus leaflet/postcard compared to a letter, nurses/pharmacists educating plus vaccinating patients, a phone call from a senior, a telephone invitation rather than clinic drop‐in, free groceries lottery, and nurses educating and vaccinating patients. We were unable to pool trials of postcard/letter/pamphlets, communications tailored to patients, a customised letter/phone‐call or client‐based appraisals, but several trials of these interventions showed they were effective.
Enhancing vaccination access (eight trials, six strategies)
Effective interventions in this comparison were: home visits compared to an invitation to attend clinic, offers of free vaccine (in USA) and patient group‐visits to physicians. We were unable to pool trials of nurse home‐visits or free vaccine compared to no intervention (USA).
Improving provision by providers or the healthcare system (17 trials, 11 strategies)
Effective interventions in this comparison were: paying physicians, reminding physicians about all patients, posters plus postcards, chart review/feedback and educational outreach/feedback.
Trials of posters plus postcards versus posters, academic detailing and increasing staff vaccination rates showed that these interventions were not effective.
We did not pool the following trials due to considerable heterogeneity: reminding physicians (four trials, n = 202,264) and practice facilitators, although several of these trials showed the interventions were effective.
We found no low risk of bias RCTs or cohort studies that studied whether these interventions reduce morbidity or hospitalisation of seniors.
Evidence is current to 4 June 2014.
Societal level: No RCTs
Summary of findings
Summary of findings for the main comparison.
Summary of effects of interventions to increase influenza vaccination uptake
|
Population: all ≥ 60, any country Settings: living in the community (no RCTs were found for seniors living in institutions) Intervention: any intervention to increase influenza vaccinations | |||||
| Interventions | Number of participants in control (C) and intervention (I) Number of (RCTs) | Comparison | Outcomes: vaccination rates |
Quality of evidence (GRADE) |
Comments |
| I. Increasing community demand: reminders to participants | I = 30,377; C = 162,609 (10) | No intervention | 3 of 10 RCTs (and 3 of 4 largest) showed positive effect with entire 95% CI > 1 | ⊕⊕ 1 Low |
Data could not be pooled |
| I. Increasing community demand: tailored reminders to participants | I = 40,301; C = 166,927 (11) | No intervention | 6 of 11 RCTs (and all 5 of largest) showed positive effect with entire 95% CI > 1 | ⊕⊕ 2 Low |
Data could not be pooled due to heterogeneity |
| I. Increasing community demand: educating and vaccinating participants plus offer of vaccination | I = 293; C = 321 (2) | No intervention | Pooled OR 3.29 (95% CI 1.91 to 5.66); P value < 0.0001 | ⊕ 3 Very low |
|
| I. Increasing community demand: health risk appraisal plus offer of vaccination | I = 1228; C = 781 (1) | No intervention | OR 2.17 (95% CI 1.70 to 2.77); P value < 0.00001 | ⊕⊕4 Low |
|
| II. Increasing access: home visits | I = 710; C 1402 (2) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ I = 73; C = 69 (1) vaccination plus care plan developed with physician ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ I = 198; C = 152 (1) |
"usual care" ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ No intervention ‐‐‐‐‐‐‐‐‐‐‐‐‐ Safety intervention |
For 2 studies which could be pooled OR 1.30 (95% CI 1.05 to 1.61); P value = 0.01 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ OR 8.15 (95% CI 3.28 to 20.29); P value < 0.00001 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ OR 0.98 (95% CI 0.64 to 1.50); P value = 0.92 |
⊕⊕⊕ 5 Moderate |
2 studies were not pooled due to heterogeneity of the interventions |
| II. Increasing access: free vaccine | I = 1125; C = 1126 (2) | Patient paid | Pooled OR = 2.36 (95% CI = 1.98 to 2.82); P value < 0.0001 | ⊕ 6 Very low |
|
| III. Provider‐ or system‐based interventions: reminders to physicians | I = 979; C = 2437 (4) | No intervention | 1 of 4 RCTs showed positive effect with entire 95% CI > 1 | ⊕⊕⊕ 7 Moderate | Data could not be pooled due to heterogeneity |
| III. Provider‐ or system‐based interventions: Facilitators working with practices |
I = 95,987; C = 90.272 (4) | No intervention | 3 of 4 RCTs showed positive effect with entire 95% CI > 1 | ⊕⊕⊕ 8 Moderate |
Data could not be pooled due to heterogeneity |
| III. Provider‐ or system‐based interventions: education and feedback to physicians | I = 15,017; C = 15,323 (3) | Chart review and feedback | 1 RCT which compared chart review and feedback plus benchmarking to the vaccination rates achieved by the top 10% of physicians found OR 3.43 (95% CI 2.37 to 4.97); P value < 0.0001 1 RCT found no effect and 1 found educational outreach and feedback less effective than written feedback (OR 0.77, 95% CI 0.72 to 0.81); P value < 0.00001 |
⊕ 9 Very low |
Data could not be pooled due to heterogeneity |
| III. Provider‐ or system‐based interventions: financial incentives to physicians | I = 1559; C = 1256 (2) | Payment per vaccination | Pooled OR 2.22 (95% CI 1.77 to 2.77); P value < 0.0001 | ⊕⊕ 10 Low |
|
C: control CI: confidence interval I: intervention OR: odds ratio
GRADE quality of evidence (based on risk of bias, heterogeneity, indirectness, imprecision and reporting bias) ⊕⊕⊕⊕: High quality. Further research is very unlikely to change our confidence in the estimate of effect. ⊕⊕⊕ Moderate quality. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. ⊕⊕ Low quality. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. ⊕ Very low quality. We are very uncertain about the estimate.
1Only two RCTs reported adequate sequence generation, one concealment, two blinding, five addressed incomplete data and eight were free of selective reporting. 2Only two RCTs reported adequate sequence generation, none concealment, one blinding, seven addressed incomplete data and 10 were free of selective reporting.
3Neither RCT reported adequate sequence generation or concealment or blinding, one addressed incomplete data and both were free of selective reporting.
4This RCT did not report adequate sequence generation, concealment or blinding, but addressed incomplete data and was free of selective reporting.
5Two RCTs reported adequate sequence generation, one concealment, one blinding and all four addressed incomplete data and were free of selective reporting.
6Neither RCT reported adequate sequence generation, concealment, blinding or addressed incomplete data, but both were free of selective reporting.
7Two RCTs reported adequate sequence generation, one concealment, one blinding and all four addressed incomplete data and were free of selective reporting.
8Two RCTs reported adequate sequence generation, concealment and blinding, three addressed incomplete data and all four were free of selective reporting.
9None of the RCTs reported adequate sequence generation, concealment, blinding or addressed incomplete data, and two of the three were free of selective reporting.
10Neither RCT reported adequate sequence generation, concealment or blinding, but both addressed incomplete data and were free of selective reporting.
Background
Description of the condition
A review of the effectiveness of influenza vaccine in seniors included 75 studies and 100 data sets (Jefferson 2010). One randomised controlled trial (RCT) showed benefits against influenza symptoms but was underpowered to detect effects on complications (1348 participants). Other data sets were not randomised and were likely to contain biases. The review was unable to reach conclusions about the effects of the vaccines in persons 65 or older.
Nevertheless, since 1964 the Advisory Committee on Immunization Practices of the US Public Health Service has recommended influenza vaccination of high‐risk individuals, including older people (Ganguly 1990), and the US Task Force on Community Preventive Services has made detailed recommendations on how to achieve this goal (Willis 2005). Lu 2008, for the US National Health Interview Surveys, found that influenza vaccination rates for those aged 65 or older increased from 30.1% in 1989 to 70% in 2004. The influenza vaccination rate in the US in 2008 was 70% for Caucasians, 55% for Hispanics and 50% for African Americans (Michaelidis 2011). Telephone household surveys in the UK in 2006 found that 79% of the UK population aged 65 or older reported receiving an influenza vaccination (Holm 2007), and surveys in the UK, Germany, Italy, France and Spain, conducted from 2003 to 2005, found the vaccination rate for those aged 65 and older in 2005 computed a group rate for the five countries of 63.7% (Müller 2007). Household telephone surveys in 2007/8 found that the highest rates were among those aged 70 to 74 in the UK (87%) and Spain (72.8%) and those 75 or over in Germany (70.7%), France (72.7%) and Italy (72.4%) (Blank 2009). A survey in Sweden in 2005 found a lower rate of 46% for those aged 65 or older, attributed to vaccination being a responsibility of individual counties and multiple possible vaccinators and remuneration methods in each Swedish county (Kroneman 2007). Surveys of those over 65 in 2006 in several regions found low rates in China (4%), Turkey (5%), Romania (10%), Poland (12%) and South Africa (14%) and higher rates in Australia (over 60%) and South Korea (74%) (de Lataillade 2009).
Kamal 2003 assessed factors relating to influenza vaccination among those aged 65 or older in a retrospective, random national sample of the data from the 1999 Behavioral Risk Factor Surveillance System survey of the US Centers for Disease Control and Prevention. He found that average influenza vaccination rates were 66.7%, with differences between Caucasians (68.3%) and African Americans (52.9%), unemployed (61.8%), employed (57.4%) and retired (68.3%), those with annual household income less than USD 15,000 (58.4%) and those earning USD 50,000 or more (69.6%). Not surprisingly, the greatest difference was between those with health insurance (67.1%) and those without (46.4%).
It is important to use documented influenza vaccination as outcome data. Zimmerman 2003a telephoned 1642 individuals aged 66 or over and obtained data from 919 who agreed to have their reported vaccination status checked against their medical records: 80% reported receiving influenza vaccination but the medical records documented vaccination in only 51%. MacDonald 1999 surveyed 500 randomly selected outpatients in the Minneapolis Veterans Affairs clinics, obtained a response rate of 77% and found self report of vaccination status agreed 89% with chart documentation and 92% for a sample of those aged 65 or over in a Group Health organisation.
Description of the intervention
Studies have identified patient, administrative, healthcare worker and societal factors that affect influenza vaccination uptake in older people. The US Task Force on Community Preventive Services has classified interventions to increase vaccination uptake into three types: increasing community demand, enhancing access and provider‐ or system‐based (CDC 2014). To make this review of maximal use we have adopted their three‐fold classification and provide examples of each.
I. Interventions to increase community demand
Interventions include increasing the perception of seniors that they are susceptible to influenza, increasing beliefs that the vaccine is effective and appropriately decreasing concern over side effects. Methods of contacting seniors have included postcards, letters, tailored letters, pamphlets, patient education (Herman 1994) or telephone campaigns (Hull 2002). One study used financial incentives (Moran 1996) and one used seniors to advocate vaccination (Krieger 2000). Some studies have explored the cost‐effectiveness of different ways of encouraging patients to be vaccinated, such as reminder letters followed up by a phone call (Frank 1985). There is a need to overcome barriers to vaccination perceived by physicians and patients (De Wals 1996). Some studies have queried whether there is a ceiling effect where all those who will respond to such cues have responded (Ganguly 1995).
II. Interventions to enhance access
Interventions include providing more clinics, better clinic hours, including vaccination during existing home visits (Dalby 2000; Fabacher 1994), arranging home visits specifically to provide vaccination (Dixon‐Woods 2004), and decreasing administrative barriers such as paperwork. Decreasing economic barriers includes making vaccine available free or at a low cost. Decreasing administrative barriers for staff can include annual standing vaccine orders (Lawson 2000) and transferring responsibility to other staff (for example, from physicians to nurses). System‐wide administrative initiatives include quality improvement activities.
III. Provider‐ or system‐based interventions
Interventions with healthcare workers include information to change their personal beliefs and attitudes about the susceptibility of their patients and themselves to influenza, whether vaccination is effective and safe for their patients and themselves, and strategies to increase motivation and willingness to vaccinate patients (Ballada 1994). Changing professional healthcare workers behaviours includes increasing the frequency of taking a vaccination history, documenting vaccinations (Buffington 1991), identifying high‐risk patients (Wrenn 1994), organising reminders (Baker 1998; Chambers 1991; Chan 2002; Clayton 1999; Dexter 2001; Kelterman 2000), providing reminders during annual physical examinations (Cowan 1992), and organising and participating in educational campaigns or meetings for healthcare workers to promote vaccination for patients (Calkins 1995; Herman 1994; Karuza 1995). Some studies have identified that recommendations by healthcare workers are important in vaccine acceptance by older people (Ashby‐Hughes 1999; Nichol 1996; Nichol 2001; Shefer 1999). In the telephone household surveys of the UK, Germany, France, Italy and Spain from 2001 to 2006, attitudes to vaccination were not separately presented by age group, but the main reasons for vaccination in all the surveys were that the family physician or nurse advised it and because influenza is perceived as a serious illness (Holm 2007; Müller 2007). Other studies have investigated campaigns by healthcare workers such as pharmacists (Ginson 2000; Grabenstein 1992).
IV. Societal interventions
We added a fourth category to the three Centers for Disease Control and Prevention categories: interventions on a societal level, including administrative frameworks and campaigns that differ between societies and affect vaccination uptake (Bennett 1994; Hak 2000; Nichol 1990; Remmen 2002). These include government policies and mandated programmes, such as changes from risk‐based to age‐based targeting for vaccination programmes (De Wals 1996), remuneration to healthcare workers for increasing vaccination uptake (Ives 1994), or being paid for achieving specific vaccination targets, as in the UK. We did not expect to find randomised controlled trials at this level and planned to report evaluations on a societal level which are at low risk of bias. Currently, the US, in addition to recommending influenza immunisation for persons at high risk of complications from influenza or who live with persons at high risk of complications, explicitly recommends vaccination for persons aged 50 years or older (Fiore 2009). Germany, Austria, Hungary and the Spanish autonomous region of Catalonia recommend vaccination for those aged 60 years and older.
How the intervention might work
Each of the four types of interventions is designed to change predisposing or enabling factors at the level of patient, provider or system.
Why it is important to do this review
There are Cochrane Reviews assessing the effects of influenza vaccines in people affected by chronic obstructive pulmonary disease (Poole 2009), asthma (Cates 2013) and cystic fibrosis (Dharmaraj 2011). No Cochrane Review assessing interventions to increase influenza vaccination in older people in institutions and the community is available. The reviews by Gross 1995, Ndiaye 2005, Ompad 2006, Sarnoff 1998, Shea 1996, Stone 2002 and Szilagyi 2000 require updating. Vu 2002 shows several methodological weaknesses that are likely to undermine the authors' conclusions (for example, the exclusion of studies with denominators smaller than 30 and quantitative pooling of studies of different design). The Report of the Task Force on Community Preventive Services identified 12 studies reporting interventions to increase influenza vaccination uptake among those under 65. The systematic review by Kohlhammer 2007 of surveys to ascertain vaccination rates among those aged 65 and older mixed surveys of small areas with some national telephone surveys. The Shojania 2010 review was limited to point‐of‐care computer reminders to physicians and identified six studies on vaccination. Lau 2012 made an extensive search of the literature but limited the search to English language studies. They used the Downs‐Black measure of study quality, which has minimal literature on its validity and reliability (Downs 1998). They pooled together RCTs and other designs and pooled some studies with high I2 statistic measures of heterogeneity.
An accurate assessment of the effectiveness of interventions to increase influenza vaccination uptake in those aged 60 years and older the community, and the costs and benefits of these interventions, is essential to allow rational choice about whether there should be universal recommendations to vaccinate older people in the community. A separate review needs to be undertaken of those living in institutions or temporarily in institutions (such as emergency departments or hospitals).
Objectives
To assess access, provider, system and societal interventions to increase the uptake of influenza vaccination in people aged 60 years and older in the community.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of interventions to increase influenza vaccination uptake in those aged 60 years and older in the community, with recording of influenza vaccination status either through clinic records or billing data, or local or national vaccination registers. We included studies with either individual or group data.
We searched for RCTs (Appendix 1) and assessed and entered data on standard data abstraction forms (Appendix 2). We excluded studies without a case definition, retrospective designs based only on individual recall of disease, or studies comparing different types of vaccines or different schedules or doses without a control group.
Types of participants
Those aged 60 years or older living in the community. Healthcare workers affecting the provision of vaccination include physicians, nurses, pharmacists and administrators. To ensure comparability with other Cochrane Reviews on influenza vaccination we used the same age groupings (less than 60 and 60 years and older). We used data for those aged 65 or over if they were the only data presented in a study and we were unable to obtain data for those aged 60 or over from the authors.
Types of interventions
Any intervention to increase uptake of influenza vaccination in those aged 60 or over, in any dose, preparation or time schedule, compared to another intervention or no intervention. We assessed these types of interventions separately.
To increase community demand, for example, interventions to increase patients' perceptions of their susceptibility to influenza, the effectiveness of vaccination and decrease concerns about side effects, using postcards, letters, brochures, telephone calls, computer reminders, educational campaigns, media campaigns, vaccination campaigns, incentives for patients or client‐held records.
To enhance access, for example, more clinics, more available clinic hours, home visits, fewer administrative barriers, standing annual vaccine orders, free vaccine or vaccine at reduced out‐of‐pocket cost in the administrative area studied, or transfer of responsibility to other staff groups (for example, from physicians to nurses), home visits or increasing the effectiveness of vaccination activities through quality improvement activities.
Provider‐ or system‐based, for example, to increase healthcare workers beliefs that older people are susceptible to influenza and that vaccination is effective and safe for themselves and their patients; to increase healthcare worker professional behaviours such as the frequency of taking a vaccination history, documenting vaccination and identifying high‐risk patients; organising reminders, reminders during annual physical examinations and organising and participating in educational campaigns or meetings for healthcare workers.
Societal interventions, for example, administrative frameworks or decisions that differ between societies or regions of societies and affect vaccination uptake, such as increased remuneration to healthcare workers for increasing vaccination uptake.
Types of outcome measures
We looked for the effects of interventions on both immediate and long‐term changes in influenza vaccination uptake. The most important predictor of being vaccinated against influenza is being vaccinated the previous year, therefore we ascertained baseline rates in the year before the intervention. We excluded studies reporting only serological outcomes if they did not include and report an intervention to increase vaccination uptake as well as an outcome of actual vaccination uptake. We excluded studies that ascertained outcomes only by self report.
Primary outcomes
Uptake of vaccination against influenza in those aged 60 or over.
Secondary outcomes
None.
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 5) (accessed 2 June 2014), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (2010 to May week 3 2014), EMBASE (2010 to June 2014), ERIC (2010 to June 2014) and CINAHL (2010 to June 2014).
We searched MEDLINE and CENTRAL using the search strategy described in Appendix 3. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the MEDLINE search strategy to search the other databases. See Appendix 4 for previous search details and search strategies for the other databases. We applied no language or publication restrictions.
Searching other resources
We searched the trials registries WHO ICTRP (www.who.int/ictrp) and ClinicalTrials.gov (http://clinicaltrials.gov/) for completed and ongoing trials (latest search 2 June 2014). In addition, we scanned the bibliographies of included studies, followed up every reference in the reviews and systematic reviews, and contacted first or corresponding authors of relevant studies to identify further published or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (RET, DLL) independently assessed all abstracts for study design, reporting of influenza vaccination uptake for those aged 60 or over in the community and an intervention to increase vaccination uptake. Two review authors (RET, DLL) then independently assessed the full text of studies that appeared eligible for inclusion.
Data extraction and management
Two review authors (RET, DLL) independently entered the following data on data abstraction sheets.
Methods (purpose, design, duration of study, interval between intervention and when outcome was measured, power computation, statistics).
Participants (country, setting, eligible participants and health status, age, gender).
Interventions (intervention 1, intervention 2, control).
Outcomes (outcome measured, time points from the study that are considered in the review or measured or reported in the study, percentage vaccinated).
Funding.
Assessment of risk of bias in included studies
Two review authors (RET, DLL) independently assessed risk of bias for each study using RevMan 2014 and the detailed specifications in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Adequate sequence generation? Low, unclear or high risk of bias.
Allocation concealment? Low, unclear or high risk of bias.
Blinding of participants, personnel and outcome assessors? Low, unclear or high risk of bias.
Incomplete outcome data addressed? Low, unclear or high risk of bias.
Free of selective reporting? Low, unclear or high risk of bias.
Free of other bias? Low, unclear or high risk of bias.
We summarised the risk of bias for each of the above outcomes within RCTs and for each of the outcomes across RCTs.
Measures of treatment effect
There was only one outcome measure, the numbers of seniors who received influenza vaccination.
Unit of analysis issues
Of the 57 RCTs, 25 were cluster‐RCTs (C‐RCTs) and in 13 the cluster effect was corrected statistically by the authors.
1. Thirteen C‐RCTs with the effects of clustering controlled for in the analysis
Seven C‐RCTs were randomised by practice, four by physician and two by household.
In seven C‐RCTs randomisation was by clinic or practice. In Abramson 2011, randomisation by clinics was corrected with the Rao‐Scott procedure in computing odds ratios with an intra‐class correlation coefficient (ICC) of 0.015. In Lemelin 2001, randomisation by practice was corrected by general linear model repeated‐measures analysis of variance. Hull 2002 and Kerse 1999 corrected randomisation by household within practices by adjusting for clustering by generalised linear models. Kouides 1998 randomised physicians to the intervention (additional remuneration for influenza vaccination uptake of 70% or above, with each physician's individual vaccination uptake displayed on posters in clinics, or to usual remuneration). Baseline differences were controlled for by linear regression equations by practices with seven potential confounders. Satterthwaite 1997 corrected for clustering using the Rao‐Scott method. Siriwardena 2002 corrected randomisation of practices to educational outreach, audit and feedback compared to audit and feedback as follows: "Because the target of the intervention and therefore the unit of randomisation was the practice, cluster‐randomised methodology was used." They used Egret and SPSS programs for analysis and "Poisson regression was used to detect significant differences between intervention and control groups in vaccination uptake change, using population at risk as an offset and taking account of the stratification." The ICCs are not provided but the authors did state that they took account of the clustered design.
Four C‐RCTs were randomised by physician. Chan 2002 corrected randomisation by physiatrist by general linear mixed models. Dapp 2011 corrected randomisation by physician by generalised estimating equations. Kiefe 2001 corrected nesting of patients within physicians by controlling for baseline performance and by generalised linear models (but 27 of 97 physicians were lost to follow‐up). Kim 1999 corrected randomisation by physician (to receive either ongoing education, academic detailing and feedback or ongoing education) by mixed model ANOVA with patients nested within physicians. Although the authors do not explicitly say that the effects of clustering were assessed, the analysis probably accomplished this.
Two C‐RCTs were randomised by household. Berg 2008 corrected clustering effects of randomisation by household by using the 'proc genmod' command repeated option in SAS. Hogg 1998 randomised participants and then their entire family was included in the group the patient was assigned to; group baseline inequivalence in age, family size and number of procedures achieved by baseline were corrected for in the analysis and thus the groups were made equivalent (there were no data on the percentage of letters not delivered).
Interaction among patients or among health team members was an explicit part of the research design in these C‐RCTs: for example, in Lemelin 2001 and Hogg 2008 facilitators visited practices and worked with practice team members to encourage increased uptake and in Kerse 1999 the intervention was an educational programme for general practitioners.
2. Twelve C‐RCTs with the effects of clustering not controlled for in the analysis
The Cochrane Handbook for Systematic Reviews of Interventions identifies five particular biases to consider in C‐RCTs (Higgins 2011): (1) recruitment bias when individuals are recruited to the trial after the clusters have been randomised; (2) "chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance." (3) loss of clusters and missing outcomes for individuals within clusters; (4) "not taking the clustering into account. ... Such analyses create a 'unit of analysis error' and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis"; and (5) if there is "a herd effect in the cluster‐randomized trials ... such contamination would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and herd effects may be different for different types of cluster."
The solution is to correct each C‐RCT by its intra‐class correlation coefficient (ICC) but the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) comments that "In fact this is seldom available in published reports. A common approach is to use external estimates obtained from similar studies."
Four were randomised by practice, three by physician, two by household and three by place of residence.
(a) Randomisation by practice
In Buffington 1991, for a group of 13 private group practices the 45 physicians were randomised either to have a poster in their office displaying the number of influenza vaccinations they had given, or to display the poster plus their patients were sent a reminder postcard, or to a no intervention control group. There are no data on whether the physicians or the patients in their practices were similar. An e‐mail from Dr. Marc LaForce described the interest among the control group physicians and competition between physicians. Hogg 2008 randomised solo or group practices to either intervention (27 practices) or control (27 practices) and two nurses with a Master's degree were assigned (one to 13 and another to 14 of the intervention practices). The control group had 58.7% female physicians per practice (intervention 33.2%) and 59.2% had practice nurses (intervention 51.8%) but the practices were similar in numbers of physicians per practice, hours booked/week, date of graduation from medical school and scores on the pre‐intervention preventive performance index. Thus the clusters could differ by patients, physicians or intervention nurse. Outcomes were summarised at the practice level. Karuza 1995 randomised 13 group practices either to receive an intervention to use group discussion to adopt and implement a CDC influenza vaccination guideline, or to a non‐intervention control. The intervention physicians had more visits per patient during the influenza vaccination season (2.1 versus 1.6, P < 0.05) and more arthritis patients (21% versus 11%, P < 0.05), but were otherwise similar. There were no outcome differences between the 13 practice groups and so data were analyzed for the 51 physicians as a group. Eleven per cent of charts were not available for review at study end. Outcomes were analyzed at the physician level. There was opportunity for interaction between participants, physicians and team members. Morrissey 1995 randomised patients to received a nursing intervention within practices from nurses or physician assistants.
(b) Randomisation by physician
Chambers 1991 randomised internal medicine residents into three groups (all their patients received a reminder, or half their patients received a reminder, or none of their patients received a reminder). There were baseline group differences in patient age, risk level and number of visits and regression analyses were run to assess the effects of these differences but they were not corrected for in the overall results. Kumar 1999 from a list of all primary care physicians in Louisiana randomly selected 750 to be the intervention group and a listing of their Medicare patient pool immunisation rate and missed opportunities and "were encouraged to evaluate ways in which their practices might improve upon the baseline immunisation status and were offered assistance in designing quality improvement projects to effect such a change. The information provided to the physicians included computed uptake for all selected physicians which allowed them to compare their uptake with those of other physicians." Nexøe 1997 randomised 13 solo physicians either for their patients to receive a postcard inviting them to receive free influenza vaccination, or a postcard to receive vaccine at their own cost, or to no postcard. There are no data on whether the practices or physicians were similar.
(c) Randomisation by household
Clayton 1999 randomised households; the groups were equivalent at baseline on age, gender and state of residence; there was no information on the percentage of postcards not received and 8% of participants received a reminder call from their GP (not part of the design). Kellerman 2000 randomised households; there were no data on group baseline equivalence and only 66% of phone calls were successful.
(d) Randomisation by place of residence
McMahon 1995a and McMahon 1995b randomised regions (composed of zip code aggregates) in states (Montana and Wyoming; there were no data on baseline equivalence or the percentage of letters not received. McCaul 2002 stated: "First, we randomly assigned counties to either the reminder‐letter (n = 17), action‐letter (n = 12), or no letter (n = 20) conditions. Within the reminder‐letter counties we then randomly assigned individuals within each county to either the reminder‐only, reminder plus positive frame, or reminder plus negative frame conditions. Within the action letter counties, all individuals received the same letter from their county public health offices." The study design is thus clustered but random individual allocation within the reminder letter group. There were no data on group baseline equivalence but there was only 6% subject loss, mostly due to returned letters.
Conclusions about the C‐RCTs not corrected by the authors for clustering effects
For the C‐RCTs randomised by practice or physician to intervention or control, there may be discussions between some team members, some physician participants may differ in level of motivation, organisation and persuasiveness, and the patients may speak to each other in the waiting room before making a decision about vaccination. Those where the physician was designated as the focus of the intervention (and not just a way of administratively reaching patients) may be expected to have the strongest clustering effects. Hogg 2008 noted that the practices and the physicians were similar, Karuza 1995 that the physicians were similar. Kouides 1998 controlled for baseline differences by regression equations.
Clustering within households should have an effect only if the household members had different attitudes to vaccination or receiving interventions.
For the studies which randomised by place of residence (US states) there were no data on baseline equivalence but it is most unlikely there were conversations between potential participants and differences between groups could arise only from differences in socioeconomic status or culture that affect willingness to receive vaccination or interventions.
None of these C‐RCTs studies stated intra‐class correlation coefficients (ICCs) and there are no standard ICCs published for this kind of intervention, so we were not able to correct for clustering in those C‐RCTS where the authors had not corrected for clustering. The only ICC reported was in the study by Abramson 2011, who noted an ICC of 0.015, but the intervention was vaccinating physicians (with the hope that this would increase physicians' motivation to vaccinate patients) with no intervention to vaccinate patients.
The limited number of these C‐RCTs and the variability of the method of randomisation (by practice, physician, household or geographic area) meant that we did not have any ICCs from other studies with which to correct for clustering.
We did not find any C‐RCTs where individuals joined clusters after randomisation.
3. Thirty‐two RCTs in which individuals were randomised
The remaining 32 studies were RCTs of individual participants and did not involve clustering.
Some studies initially appeared to be C‐RCTs but were not. In McDowell 1986, although families were selected, only one patient was selected per family and then randomised. In Frank 2004, individual participants were randomised by the last digit of their family medical record number to intervention (and physicians then received automatic electronic reminders for 12 preventive care interventions) or control; groups were equivalent at baseline but physicians were not blinded to group of allocation. In Beck 1997, six internists and their nursing staff participated and participants were randomised within each physician's practice to either the intervention or control group. The intervention group received visits to their physician and nurse at the clinic in groups (average size eight) for (a) a 15‐minute warm‐up and socialisation with information on specific disease processes; (b) a 15‐minute break for socialisation and the nurse checked blood pressure, immunisation status, immediate needs and arranged a visit with their physician, (c) 15 minutes of questions and answers and planned next visit and (d) 30 minutes for the visit to their physician. It was part of the intervention that participants would socialise and exchange information but randomisation was by individual patient. Maglione 2002a, Maglione 2002b, Maglione 2002c and Maglione 2002d did not provide enough information for us to know whether individuals were randomised or randomisation was by region within states (unlike McMahon 1995a and McMahon 1995b, which provided information on randomisation by region within states).
Dealing with missing data
For missing data we contacted the trial authors. We did not replace missing data and we evaluated the effect of excluding outlier studies.
Assessment of heterogeneity
We inspected the data for heterogeneity within each category and used the Chi2 test to examine heterogeneity between studies and the I2 statistic to assess variability in estimates of effect due to heterogeneity. We performed a meta‐analysis if the I2 statistic was less than 50% for a group of studies. We looked at various strategies for meta‐regression (by quality and by sample size) and for each of the interventions that had more than three RCTs we carried out sensitivity analyses by removing serially the studies with the highest risk of bias, but this did not change the heterogeneity. We then serially removed the smallest RCTs and this also did not remove the heterogeneity.
Assessment of reporting biases
We constructed funnel plots (plots of the effect estimate from each study against the sample size or effect standard error) to assess the potential for bias related to the size of the trials, which could indicate possible publication bias. We only constructed them for interventions with five or more RCTs, as a funnel plot for smaller numbers of RCTs would be hard to interpret.
Data synthesis
All C‐RCTs and RCTs provided the numbers of vaccinated and unvaccinated individuals and we were thus able to synthesise the data with odds ratios (ORs) using the random‐effects model. We performed meta‐analysis on groups of RCTs where exposure, populations and outcomes were homogenous, where the I2 statistic was less than 50%.
Subgroup analysis and investigation of heterogeneity
We analyzed the C‐RCTs and RCTs according to the intervention used. The interventions differed markedly (increasing demand, increasing access, provider‐ or system‐interventions), therefore we did not aggregate these subgroups.
Sensitivity analysis
We conducted sensitivity analyses only where interventions were tested by five or more trials.
Results
Description of studies
Results of the search
For the first publication of this review (Thomas 2010), we identified 4495 titles from the electronic searches, independently read 359 full‐text articles that appeared to meet the inclusion criteria, placed 315 in the Excluded studies section and included 44 RCTs. For this 2014 update we identified 5119 titles. Two review authors (RET, DLL) independently assessed the titles and abstracts of the additional 624 and identified and independently read the full text of 371 studies that appeared to meet the inclusion criteria. However, we evaluated 207 as not relevant enough to be in the Excluded studies section (i.e. not meeting enough inclusion criteria but still of interest to other researchers of this topic), placed an additional 33 studies in the Characteristics of excluded studies table that other researchers might wish to read, and included 13 new RCTs for a total of 57 RCTs in this updated review. Two studies in Korean are awaiting translation before the full text can be reviewed (Lee 2003; Song 2000).
Included studies
We identified 57 RCTs, of which 34 were from the US, seven from Canada, four each from Australia and the UK, three from Spain and one each from Denmark, Germany, Israel, New Zealand and Puerto Rico.
The key predictor of influenza vaccination is whether the patient received it the previous year, therefore we initially separately analyzed the RCTs which reported baseline influenza vaccination uptake for both treatment and control groups for the year before the intervention and the RCTs with no baseline data.
Appendix 5 shows that for the 28 RCTs with previous year uptake, the difference in vaccination uptake in the treatment and control groups was 0% to 2% in 18 RCTs, 3% to 4% in seven RCTs and 5% or more in three RCTs. Randomisation had thus been relatively effective in producing intervention and control groups with similar uptake of influenza vaccination in the year before the intervention. We therefore decided that it would be appropriate to analyze together the studies with and without baseline influenza uptake (Appendix 6), in order to increase power and avoid the complexity of presenting outcomes for intervention groups 1, 2 and 3 for RCTs with baseline data and again separately for RCTs without baseline data for the year before the intervention.
We independently assessed all the non‐randomised studies and decided that with the data provided in the articles we could not evaluate the effect of known and unknown confounders (Appendix 7 and Characteristics of excluded studies table). We did not include data from these studies.
The population served and the healthcare system will affect the barriers to vaccination, motivations to implement vaccination, the resources made available and the effectiveness of interventions. It is thus difficult to compare studies carried out in different countries or areas. Differences due to the healthcare system will occur by socioeconomic area (for example, suburban populations where many people regularly see their own GP), by distance from any healthcare facility (for example, rural areas) or by transient work situations (for example, agricultural or mining communities).
Excluded studies
We excluded studies that by title or abstract appeared potentially includable but then the full text showed (a) they did not include individuals aged 60 or over or such individuals were not separable from the rest of the participants (and we were not able to obtain the data from the authors), or (b) there was no intervention to increase influenza vaccination uptake, or (c) vaccination status was measured only by unvalidated self report, or (d) there were serious problems in execution that would have led to very high risks of unknown bias in including them (for example Wadhwa 1997 failed to contact 57% of the people in the telephone arm of his RCT). We retrieved the full text whenever the abstract was not adequate to make these decisions and wrote to the authors when the full text was not adequate. For the first publication of this review we identified 4495 titles and abstracts and we excluded 4451 citations. For this review update we identified 5119 titles and abstracts and we excluded an additional 312 trials (with two in Korean awaiting translation).
We independently entered data for non‐RCTs on standard data abstraction forms and assessed risk of bias. Nearly all the exclusions were because there was no control group, regional vaccination data for the previous years were used as 'historical controls', or insufficient data were provided to assess known confounders (Appendix 7).
Risk of bias in included studies
Figure 1.
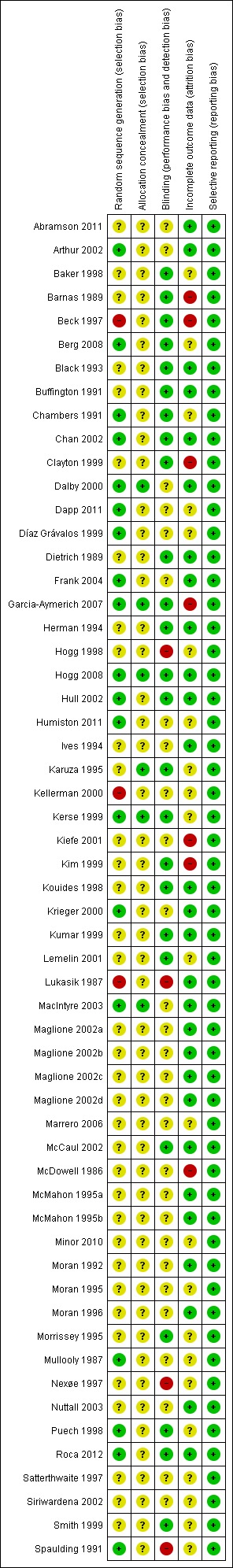
'Risk of bias' summary: review authors' judgments about each risk of bias item for each included study.
Figure 2.
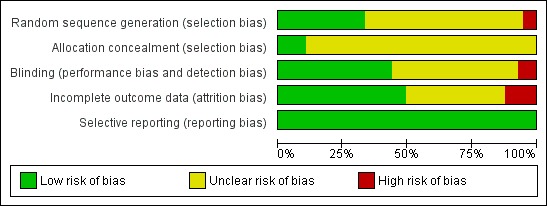
'Risk of bias' graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
For randomisation, 19 (33%) of the trials were at low risk of bias, 35 (61%) unclear and three (6%) at high risk. For concealment of sequence generation six (11%) were at low risk and 51 (89%) unclear. Concealment from the research director as to whether participants were allocated to the intervention or control arm could have been achieved by an independent statistician or researcher using a computer program.
Blinding
Twenty‐five (44%) of the trials were at low risk, 28 (49%) at unclear risk and four (7%) at high risk of bias. Studies which reported independent verification of vaccination status after the trial from databases were at lower risk of detection bias, especially if the databases were independently maintained by government agencies.
Incomplete outcome data
In 28 trials (49%) there was low risk of incomplete data, in 22 (39%) there was an unclear risk and in seven (12%) there was a high risk.
Influenza vaccination uptake was recorded in computers or ascertained from computerised records or review of clinic records in 53 RCTs; by two research assistants through phone calls or home visits in Black 1993; from records during the vaccination campaign in Díaz Grávalos 1999; from hospital records or letters to GPs in MacIntyre 2003; and from the records of the pharmacy where the RCT was conducted in Marrero 2006.
Selective reporting
All 57 trials (100%) were free of selective reporting.
Other potential sources of bias
We constructed funnel plots for interventions where there were five or more RCTs. There were only two such groups: reminders to participants and tailored reminders to participants. Their funnel plots do not show evidence of publication bias (Figure 3; Figure 4).
Figure 3.
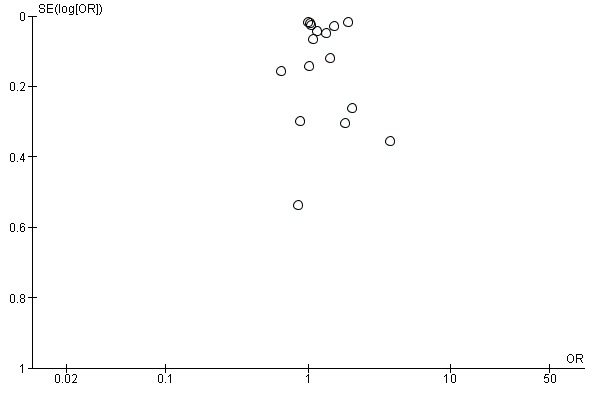
Funnel plot of comparison: 1 Increasing community demand, outcome: 1.1 Client reminder and recall (letter or postcard or pamphlet) compared to no intervention.
Figure 4.
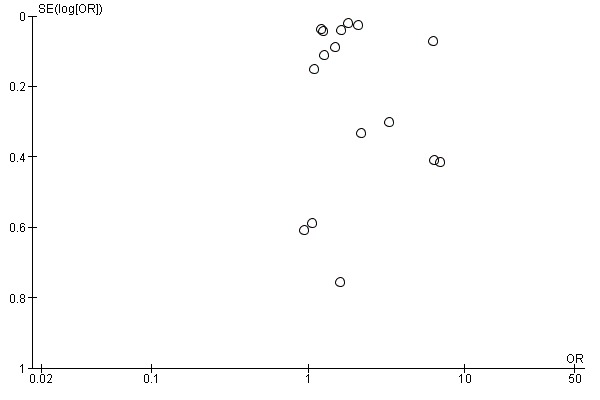
Funnel plot of comparison: 1 Increasing community demand, outcome: 1.2 Client reminder and recall (tailored letter or postcard or phone call) compared to no intervention.
Effects of interventions
See: Table 1
Primary outcome
For all interventions the outcome measure was any change in the percentage of patients who received influenza vaccination.
I. Increasing community demand
(a) Client reminders
(i) Client reminders: intervention compared to no intervention
The simplest kind of intervention was a patient reminder postcard compared to no intervention. There were 16 RCTs, with 124,600 participants in the intervention and 467,565 in the control group (Baker 1998; Barnas 1989; Berg 2008; Clayton 1999; Hogg 1998; Maglione 2002a; Maglione 2002b; Maglione 2002c; McCaul 2002; McMahon 1995a; McMahon 1995b; Minor 2010; Moran 1992; Moran 1995; Moran 1996; Puech 1998). However, there was marked heterogeneity (Chi2 = 880.09, P value < 0.00001; I2 statistic = 98%) and the data could not be pooled (Analysis 1.1; Figure 5). We assessed randomisation as at low risk of bias in two trials (and for these two trials the I2 statistic was 99%) and at unclear risk in the other 14. We assessed attrition as at low risk of bias in one trial, high risk in two and unclear risk in the other 13, so sensitivity analyses were not feasible.
Analysis 1.1.
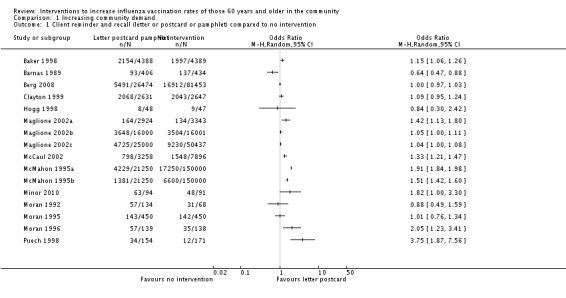
Comparison 1 Increasing community demand, Outcome 1 Client reminder and recall (letter or postcard or pamphlet) compared to no intervention.
Figure 5.
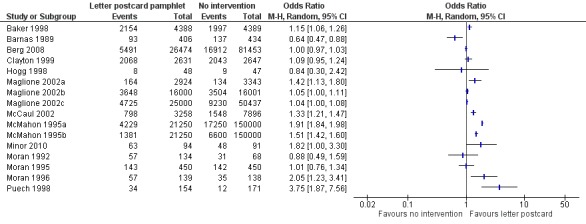
Forest plot of comparison: 1 Increasing community demand, outcome: 1.1 Client reminder and recall (letter or postcard or pamphlet) compared to no intervention.
The next level of intervention was a letter, postcard or phone call personalised to the participant's health status compared to no intervention. There were 16 RCTs with 65,005 participants in the intervention and 323,159 in the control group (Baker 1998; Díaz Grávalos 1999; Dietrich 1989; Hogg 1998; Hull 2002; Humiston 2011; Kellerman 2000; McCaul 2002; McDowell 1986; McMahon 1995a; McMahon 1995b; Minor 2010; Mullooly 1987; Roca 2012; Smith 1999; Spaulding 1991). However, there was marked heterogeneity (Chi2 = 546.71, P value < 0.00001; I2 statistic = 97%) and the data could not be pooled (Analysis 1.2; Figure 6). We assessed randomisation as at low risk of bias in six trials (the I2 statistic was 99% so they could not be pooled), high risk of bias in one and unclear risk in the other nine. We assessed attrition as at low risk of bias in six trials (the I2 statistic was 90% so they could not be pooled), high risk in one and unclear risk in the other nine, so sensitivity analyses were not feasible.
Analysis 1.2.
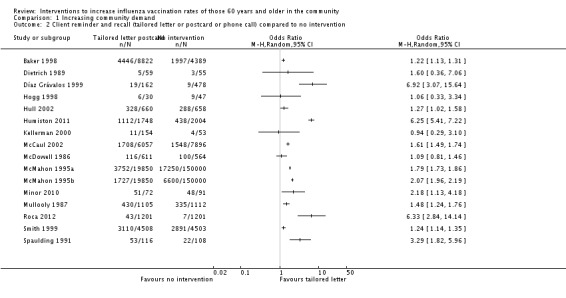
Comparison 1 Increasing community demand, Outcome 2 Client reminder and recall (tailored letter or postcard or phone call) compared to no intervention.
Figure 6.
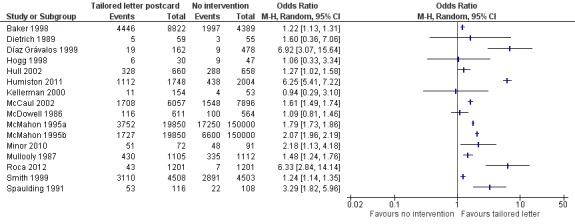
Forest plot of comparison: 1 Increasing community demand, outcome: 1.2 Client reminder and recall (tailored letter or postcard or phone call) compared to no intervention.
(ii) Client reminders: comparisons of two interventions
Three trials compared a reminder letter plus leaflet (or postcard) to a reminder letter, with 32,112 participants in the intervention and 32,088 in the control group (Maglione 2002b; Maglione 2002d; Nuttall 2003). The odds ratio (OR) was 1.11 (95% confidence interval (CI) 1.07 to 1.15, P value < 0.00001, I2 statistic = 0%) (Analysis 1.3).
Analysis 1.3.
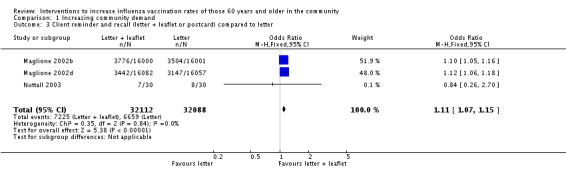
Comparison 1 Increasing community demand, Outcome 3 Client reminder and recall (letter + leaflet or postcard) compared to letter.
Four trials compared a customised letter or phone call to a form letter, with 39,798 in the intervention and 42,667 in the control group (Hogg 1998; McMahon 1995a; McMahon 1995b; Minor 2010) (Analysis 1.4). However, there was marked heterogeneity (Chi2 = 74.39, P value < 0.00001; I2 statistic = 96%) and the trials could not be pooled. For randomisation we assessed all four trials as at unclear risk of bias. For attrition we assessed two trials as at low risk (I2 = 99%) and two as at unclear risk and so we performed no sensitivity analysis.
Analysis 1.4.
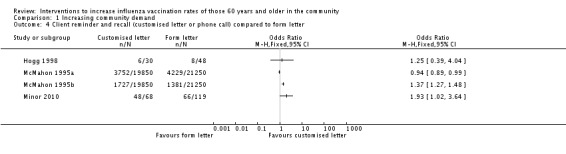
Comparison 1 Increasing community demand, Outcome 4 Client reminder and recall (customised letter or phone call) compared to form letter.
Krieger 2000, with 102 participants in the intervention and 91 in the control group, compared a telephone call from a trained senior plus an educational brochure to "usual publicity". The OR was 3.33 (95% CI 1.79 to 6.22, P value < 0.0002) (Analysis 1.5). However, for the participants who had been vaccinated the previous year, vaccination uptake in the intervention group declined from 100% to 98.5% and in the control group from 100% to 94.7%: a non‐significant difference.
Analysis 1.5.
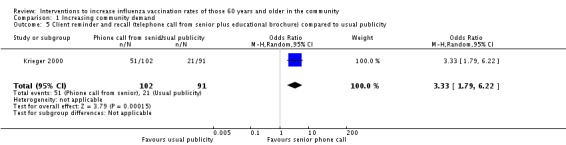
Comparison 1 Increasing community demand, Outcome 5 Client reminder and recall (telephone call from senior plus educational brochure) compared to usual publicity.
Lukasik 1987, with 120 participants in the intervention and 123 in the control group, compared a telephone invitation to be vaccinated to an invitation to be vaccinated when participants "dropped in" to the clinic. The OR was 2.72 (95% CI 1.55 to 4.76, P value = 0.0005) (Analysis 1.6).
Analysis 1.6.
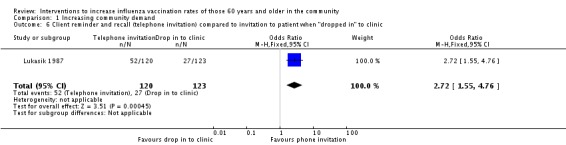
Comparison 1 Increasing community demand, Outcome 6 Client reminder and recall (telephone invitation) compared to invitation to patient when "dropped in" to clinic.
Moran 1996 compared a brochure plus a lottery for free groceries to no intervention, with 153 in the intervention and 138 in the control group. The OR was 1.04 (95% CI 0.62 to 1.76, P value = 0.88) (Analysis 1.7).
Analysis 1.7.
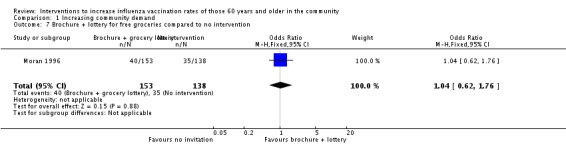
Comparison 1 Increasing community demand, Outcome 7 Brochure + lottery for free groceries compared to no intervention.
(b) Client‐based education and vaccination
Three trials, with 2226 participants in the intervention and 1790 in the control groups, compared a health risk appraisal plus an offer of influenza vaccination to no intervention (Garcia‐Aymerich 2007; Ives 1994; Morrissey 1995). Heterogeneity was high (Chi2 = 33.87; I2 statistic = 94%) and the data could not be pooled (Analysis 1.8).
Analysis 1.8.
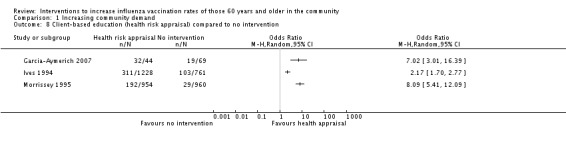
Comparison 1 Increasing community demand, Outcome 8 Client‐based education (health risk appraisal) compared to no intervention.
Two RCTs, with 293 participants in the intervention and 321 in the control group, compared nurses or pharmacists educating participants about influenza vaccination and nurses vaccinating participants to no intervention (Herman 1994; Marrero 2006). The OR was 3.29 (95% CI 1.91 to 5.66, P value < 0.0001). Heterogeneity was low (Chi2 = 1.12, P value = 0.27, I2 statistic = 18%) (Analysis 1.9). Herman 1994, also with 243 participants in the intervention and 242 in the control group, compared nurses or pharmacists educating participants and nurses vaccinating participants to only educating participants and found the vaccination uptake in the intervention group increased 23.8% and declined in the education only group by 2.1% (P value = 0.0001). The OR was 152.95 (95% CI 9.39 to 2490.67, P value = 0.0004) (Analysis 1.10).
Analysis 1.9.
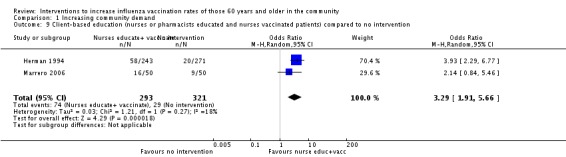
Comparison 1 Increasing community demand, Outcome 9 Client‐based education (nurses or pharmacists educated and nurses vaccinated patients) compared to no intervention.
Analysis 1.10.
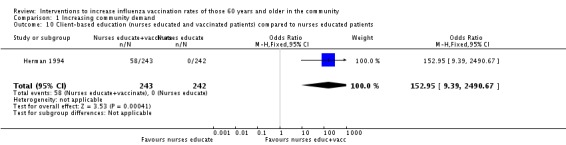
Comparison 1 Increasing community demand, Outcome 10 Client‐based education (nurses educated and vaccinated patients) compared to nurses educated patients.
2. Enhancing vaccination access
(a) Group visits by patients to physicians and nurses
Beck 1997, with 160 participants in the intervention and 161 in the control group, compared visits by groups of participants to a physician and nurse to "usual care" by a physician. The OR was 24.85 (95% CI 1.45 to 425.32, P value = 0.03). The uptake in the intervention group increased from 74% in the previous year to 81% and in the control group declined from 72% to 64%; this decline cannot be entered in the dichotomous data entry table and the result would be stronger if the decline could be recorded (Analysis 2.1).
Analysis 2.1.
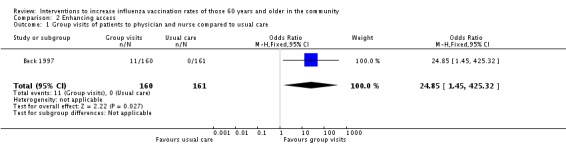
Comparison 2 Enhancing access, Outcome 1 Group visits of patients to physician and nurse compared to usual care.
(b) Home visits
Arthur 2002 compared a home visit with an offer of influenza vaccination to a letter inviting participants to attend a vaccination clinic. The OR was 1.28 (95% CI 1.03 to 1.58). Nuttall 2003, in a very small study, compared a home visit with an offer of influenza vaccination to "usual care". Their combined total was 710 participants in the intervention and 1402 in the control group. The pooled OR was 1.30 (95% CI 1.05 to 1.61, P value = 0.01), with low heterogeneity (Chi2 = 0.86, P value = 0.35; I2 statistic = 0%) (Analysis 2.2).
Analysis 2.2.
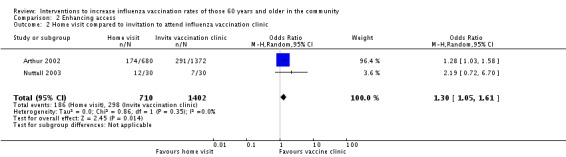
Comparison 2 Enhancing access, Outcome 2 Home visit compared to invitation to attend influenza vaccination clinic.
Black 1993, with 198 participants in the intervention and 152 in the control group, compared home visits, which included an encouragement to receive influenza vaccination, to home visits with a safety intervention. The OR was 0.98 (95% CI 0.64 to 1.50, P value = 0.92) (Analysis 2.3). Black noted: "Another 45 clients had been assigned to the influenza group but did not receive the promotion because the public health nurse found that they had already been administered influenza vaccine. These 45 participants and those who were missed (n = 9) were included in the analysis in their originally allocated group (an "intention to treat" analysis); thus a total sample of 359 was analysed." However, Black does not state the distribution of these 45 between the intervention and the control groups and an uneven distribution could positively or negatively affect the apparent effect of the intervention.
Analysis 2.3.
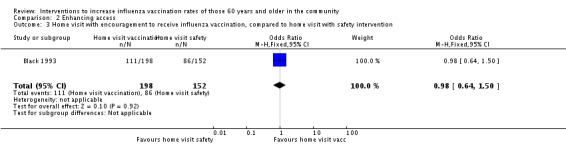
Comparison 2 Enhancing access, Outcome 3 Home visit with encouragement to receive influenza vaccination, compared to home visit with safety intervention.
Two trials assessed the effects of a home visit by a nurse with encouragement to receive influenza vaccination, with 647 in the intervention and 1422 in the control group (Dalby 2000; Dapp 2011). There was marked heterogeneity (Chi2 = 10.99, P value = 0.0009; I2 statistic = 91%) and they could not be pooled (Analysis 2.4). The Dapp 2011 study was much larger (574 intervention, 1353 control), with a complex intervention (health risk appraisal, individualised recommendations, health information, reinforcement by home visit or group sessions). The OR was 1.68 (95% CI 1.37 to 2.07, P value < 0.0001). Dalby 2000 was a small study with 73 participants in the intervention and 69 in the control group and also had a complex intervention (home visits with an encouragement to receive influenza vaccination plus a care plan developed with a physician). The OR was 8.15 (95% CI 3.28 to 20.29, P value < 0.00001) (Analysis 2.4). The group was unusual in being older (average age 78) and included women who had been widowed, hospitalised or experienced a degree of functional loss in the previous six months. Although the study scored a low risk of bias for randomisation, there was a marked gender imbalance, with 71% female in the experimental group and 62% in the control group.
Analysis 2.4.
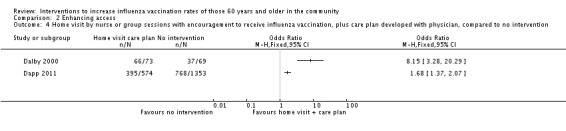
Comparison 2 Enhancing access, Outcome 4 Home visit by nurse or group sessions with encouragement to receive influenza vaccination, plus care plan developed with physician, compared to no intervention.
(c) Free influenza vaccination
Two RCTs, with a combined total of 1125 participants in the intervention and 1125 in the control group, compared an offer of free influenza vaccination to an invitation to be vaccinated but the participant paid (Nexøe 1997; Satterthwaite 1997). The OR was 2.36 (95% CI 1.98 to 2.82, P value < 0.00001). Heterogeneity was low (Chi2 = 0.42, P value = 0.52; I2 statistic = 0%) (Analysis 2.5).
Analysis 2.5.
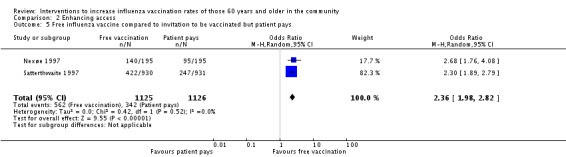
Comparison 2 Enhancing access, Outcome 5 Free influenza vaccine compared to invitation to be vaccinated but patient pays.
The same two RCTs compared an offer of free vaccination to no intervention. However, the trials could not be pooled due to high heterogeneity (Chi2 = 6.72, P value = 0.010; I2 statistic = 85%). Individually, Nexøe 1997 found an OR of 7.80 (95% CI 4.97 to 12.24, P value ≤ 0.00001) and Satterthwaite 1997 an OR of 4.03 (95% CI 3.25 to 4.99, P value ≤ 0.00001) (Analysis 2.6).
Analysis 2.6.
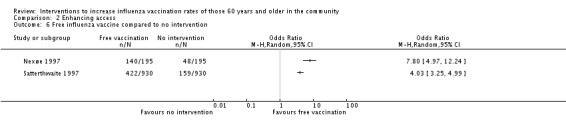
Comparison 2 Enhancing access, Outcome 6 Free influenza vaccine compared to no intervention.
3. Provider‐ or system‐based interventions
(a) Reminders to physicians
(i) Reminders to physicians
Four trials, with 71,845 in the intervention and 130,419 in the control group, compared a reminder to physicians to no intervention (Chambers 1991; Chan 2002; Frank 2004; Kumar 1999). There was marked heterogeneity (Chi2 = 30.66, P value < 0.00001; I2 statistic = 90%) and the trials could not be pooled (Analysis 3.1). Chambers 1991 included a separate comparison within his study, with 198 participants in the intervention (reminder to physicians about all their patients) and 118 in the control group (reminder to physicians about half of their patients). The OR was 2.47 (95% CI 1.53 to 3.99, P value = 0.0002) (Analysis 3.2). For both randomisation and attrition we assessed three trials as at low risk of bias and one as unclear and thus a sensitivity analysis was not feasible.
Analysis 3.1.
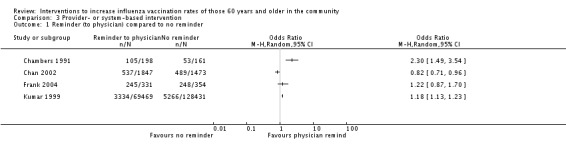
Comparison 3 Provider‐ or system‐based intervention, Outcome 1 Reminder (to physician) compared to no reminder.
Analysis 3.2.
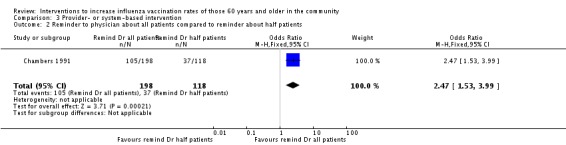
Comparison 3 Provider‐ or system‐based intervention, Outcome 2 Reminder to physician about all patients compared to reminder about half patients.
MacIntyre 2003, with 70 hospitalised participants in the intervention and 61 in the control group, compared a reminder to hospital staff to vaccinate the participants to a reminder letter to the participants' GP on the day of discharge. The OR was 1.70 (95% CI 0.51 to 5.70, P value = 0.39) (Analysis 3.3).
Analysis 3.3.
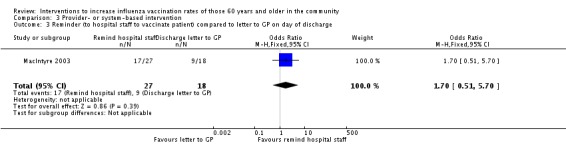
Comparison 3 Provider‐ or system‐based intervention, Outcome 3 Reminder (to hospital staff to vaccinate patient) compared to letter to GP on day of discharge.
(ii) Posters in clinics as a reminder to physicians, participants and staff
Buffington 1991, with 3604 participants in the intervention and 4772 in the control group, compared displaying posters in clinics with the influenza vaccination uptake by individual physicians, to encourage physicians to compete plus postcards to participants, to no intervention. The OR was 2.03 (95% CI 1.86 to 2.22, P value < 0.00001) (Analysis 3.4). The same RCT, with 3604 participants in the intervention and 2149 in the control group, compared posters in clinics displaying vaccination uptake and also sending postcards to participants, to posters in clinics displaying vaccination uptake. The OR was 1.06 (95% CI 0.95 to 1.19, P value = 0.32) (Analysis 3.5).
Analysis 3.4.
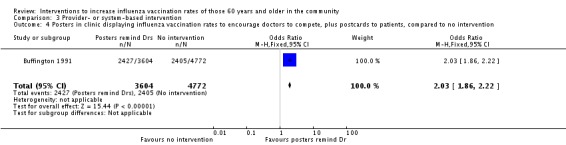
Comparison 3 Provider‐ or system‐based intervention, Outcome 4 Posters in clinic displaying influenza vaccination rates to encourage doctors to compete, plus postcards to patients, compared to no intervention.
Analysis 3.5.
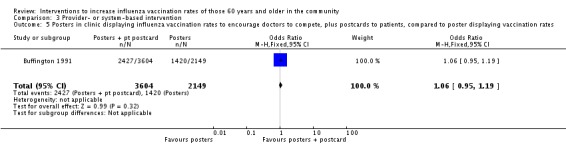
Comparison 3 Provider‐ or system‐based intervention, Outcome 5 Posters in clinic displaying influenza vaccination rates to encourage doctors to compete, plus postcards to patients, compared to poster displaying vaccination rates.
(b) Facilitator encouragement of prevention manoeuvres
Three RCTs, with a combined total of 1013 participants in the intervention and 1170 in the control group, compared facilitator encouragement to perform prevention manoeuvres, including influenza vaccination, to no intervention (Hogg 2008; Karuza 1995, Kerse 1999). Heterogeneity was high (Chi2 = 34.74, P value < 0.0001; I2 statistic = 94%) and the data could not be pooled (Analysis 3.6). Hogg 2008 found an OR of 2.11 (95% CI 1.27 to 3.49, P value = 0.0004) and Karuza 1995 an OR of 292.81 (95% CI 18.16 to 4721.62, P value ≤ 0.0001). Hogg 2008 did not obtain baseline influenza vaccination data from the previous year. Lemelin 2001 did not present numbers of participants aged 65 or older so could not be included in the meta‐analysis, but the increase in vaccination uptake in the intervention group was 18.7% and in the control 4.0% (P value < 0.01).
Analysis 3.6.
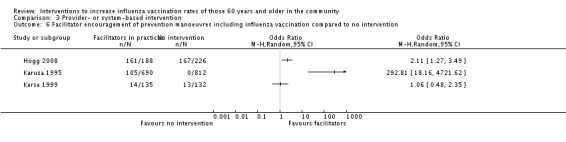
Comparison 3 Provider‐ or system‐based intervention, Outcome 6 Facilitator encouragement of prevention manoeuvres including influenza vaccination compared to no intervention.
The best predictor of vaccination is having been vaccinated the previous year, so if baseline vaccination data were presented for the previous year, we assessed the effect of the intervention by counting only new vaccinations. However, for Karuza 1995 the increase in the intervention group was from 47.56% to 62.78% and in the control group from 46.5% to 46.07%, which explains the very skewed OR and 95% CI.
(c) Physician education and feedback
Kim 1999, with 706 participants in the intervention and 694 in the control group, compared educational reminders, academic detailing and peer comparisons to other physicians, to mailed educational materials. The OR was 1.13 (95% CI 0.80 to 1.58, P value = 0.50) (Analysis 3.7).
Analysis 3.7.
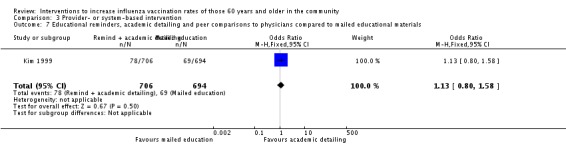
Comparison 3 Provider‐ or system‐based intervention, Outcome 7 Educational reminders, academic detailing and peer comparisons to physicians compared to mailed educational materials.
Kiefe 2001, with 678 participants in the intervention and 682 in the control group, compared chart review and feedback to physicians plus benchmarking to the vaccination uptake achieved by the top 10% of physicians, to chart review and feedback. The OR was 3.43 (95% CI 2.37 to 4.97, P value < 0.00001) (Analysis 3.8).
Analysis 3.8.
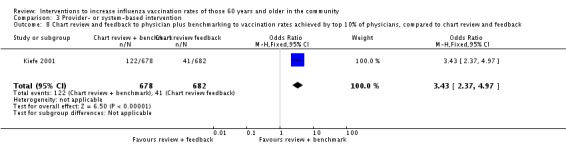
Comparison 3 Provider‐ or system‐based intervention, Outcome 8 Chart review and feedback to physician plus benchmarking to vaccination rates achieved by top 10% of physicians, compared to chart review and feedback.
Siriwardena 2002, with 13,633 participants in the intervention and 13,947 in the control group, found that educational outreach and feedback to practice teams was less effective than written feedback to practice teams. The OR was 0.77 (95% CI 0.72 to 0.81, P value < 0.00001) (Analysis 3.9).
Analysis 3.9.
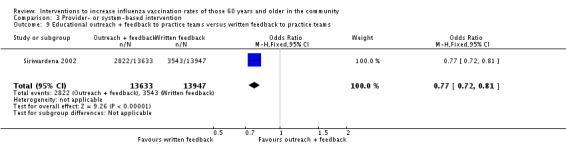
Comparison 3 Provider‐ or system‐based intervention, Outcome 9 Educational outreach + feedback to practice teams versus written feedback to practice teams.
(d) Payment to physicians for influenza vaccinations
Ives 1994 and Kouides 1998, with 1559 participants in the intervention and 1256 in the control group, compared capitated payments to payment per vaccination. The OR was 2.22 (95% CI 1.77 to 2.77, P value < 0.00001), with minimal heterogeneity (Chi2 = 0.23, P value = 0.63; I2 statistic = 0%) (Analysis 3.10).
Analysis 3.10.
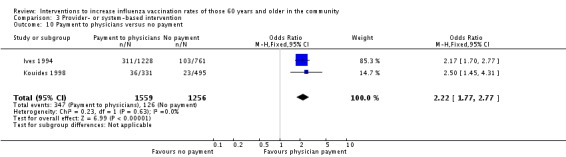
Comparison 3 Provider‐ or system‐based intervention, Outcome 10 Payment to physicians versus no payment.
(e) Interventions to increase staff influenza uptake
Abramson 2011 encouraged primary care physicians to receive influenza vaccination, hoping that would encourage them to vaccinate their patients. The physicians in the intervention group cared for 11,325 patients and those in the control group 15,097 patients. For vaccination of patients the OR was 1.04 (95% CI 0.97 to 1.12, P value = 0.24) (Analysis 3.11).
Analysis 3.11.
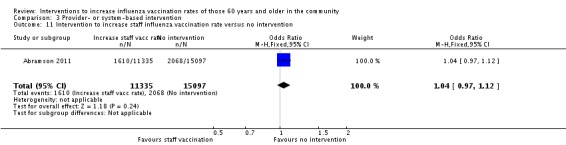
Comparison 3 Provider‐ or system‐based intervention, Outcome 11 Intervention to increase staff influenza vaccination rate versus no intervention.
4. Interventions at the societal level
There are no RCTs at the societal level.
Joseph 2005 assessed the effects of the change in influenza vaccination policy in the UK from a purely risk‐based policy to one which stated that age itself is a risk, because of the increasing risks from influenza with age and also because age is associated with risk factors that may be unknown to older people. In 1998 it was recommended that those aged 75 or older should be offered influenza vaccination and in 2000 to those aged 65 or older. For those aged 65 to 74 uptake rose from 34.6% in 1989 to 1990 to 55.8% in 1999 to 2000, and then to 65.8% in 2000 to 2001 and 72.1% in 2003 to 2004, showing a higher uptake after the introduction of the policy in 2000 to vaccinate those aged 65 or older.
The UK introduced the Quality and Outcomes Framework as an evidence‐based new General Medical Services Contract on 1 April 2004, which allowed GPs to earn 23% of their total income from targeted quality care. McGovern 2008 performed a serial cross‐sectional study of the recording of coronary heart disease (CHD) related health indicators and medications in 301 general practices in Scotland. Before the contract on 31 March 2004, 3.7% of participants over the age of 16 had a computer record of CHD and post‐contract on 31 March 2005, 4.9%. Of these, 57.4% had received influenza vaccination before and 85.5% after the contract, although the data do not separate those younger than 60 and 60 and older.
In the UK 'clinical governance' is a National Health Service quality assurance framework. Siriwardena 2003b reported on the impact of a clinical governance aim of immunising 60% of participants older than 65 years against influenza in 2000 in the West Lincolnshire Primary Care Trust. All 39 practices in this geographic area signed a clinical governance contract to participate and agreed to a practice audit (compulsory audit for CHD and voluntary audit for influenza vaccination). Practices that completed their agreement also received additional payments. The baseline audit was done in May 2000 and the audit was repeated in April 2001. Changes in vaccination uptake were calculated for the 24 practices which completed the audit cycle and uptakes were compared using paired t‐tests. There was a mean improvement of 24% (95% CI 19.7 to 28.4, P value < 0.001) in vaccination uptake in participants aged 65 years or older (mean at baseline 48.9%, at follow‐up 73.0%).
Jansen 2008 noted that in the Netherlands before the 1996 to 1997 respiratory season that influenza vaccination was only recommended for individuals with high‐risk medical conditions and after that was extended to all those aged 65 or older. Uptake for those aged 65 or older increased from 30% in 1991 to 45% in 1995 and 87% in 2002.
Remmen 2002 studied variations in influenza vaccination uptake in a group practice physically located in Belgium but near to the Netherlands border, which included participants from both Belgium and the Netherlands. Patients shared the same language and socioeconomic characteristics but were provided with services as related to their country of residence. Since 2000 in both countries vaccination has been recommended for persons aged 65 years or older, as well as for others with health conditions that place them at high risk of influenza complications. In Belgium, approximately 75% of the cost of obtaining a vaccine from a pharmacy and having it administered by a physician is covered by insurance, in contrast to the Netherlands where vaccination is obtained from physicians' offices with no direct cost to the patient. Among those aged 65 years or older, 64.3% of the Belgian compared to 77.5% of the Dutch participants were immunised in 2000 to 2001.
Two reports evaluated the effect of including influenza vaccination as a US Medicare B benefit from 1988 to 1992 for two million individuals aged 65 or older in intervention sites in 10 states. Hutton 1993 assessed the impact on influenza vaccination by telephone surveys. In 1988 to 1989 the vaccination uptake was 35% and 37% in comparison sites in the 10 states and in 1991 to 1992 it was 62% in the intervention and 50% in the comparison sites. However, claims rates were only 51% in 1991 to 1992, indicating that most individuals did not have Medicare pay for their influenza vaccination. Schmitz 1993a indicated that extensive publicity campaigns and mail‐out of an informative and persuasive letter had accompanied the implementation of this demonstration project. Over the period of the demonstration, vaccination uptake increased in both intervention and demonstration areas. For those aged 65 to 74 years the difference in coverage between intervention and comparison groups increased from +3% for 1988 to 1989 to +8% for 1989 to 1990 and to +12% for 1990 to 1991. For those aged 75 to 84 years, the differences were +1%, +4% and +12%, respectively. Among those aged 85 years or older, the respective differences were ‐5%, ‐5% and +12%.
Frick 2004 assessed the effect of including influenza vaccination as a Medicare benefit by using data from the Women's Health and Aging Study for 12 zip codes in Baltimore and interviewed 71% of the 1409 eligible females. However, uptake increased in the two years before the introduction of Medicare and the uptake afterwards decreased for Afro‐Americans and dipped then slightly increased for white females.
Jha 2003 assessed the effects of the US Veterans Affairs Department re‐engineering initiative from 1995, which implemented quality‐of‐care indicators and compared the vaccination uptake to those of the Medicare fee‐for‐service system. Influenza vaccination uptake for those aged 65 or over in the Veterans Affairs system increased from 28% in 1994 to 1995 before re‐engineering to 78% in 2000. They were 71% in 1997 to 1999 (compared to 66% for Medicare) and 78% in 2000 (compared to 71% for Medicare 2000 to 2001). There is no assessment of the differences in population characteristics or medical resources of the two systems.
The 2001 Japanese immunisation law subsidised routine influenza vaccinations for those aged 65 years or older or aged 60 years or older with specific health conditions. Co‐payments are determined by each local government every year and excess costs beyond co‐payments are subsidised by central and local governments directly to the medical institutions that provide vaccinations. Ohkusa 2005 compared the amount of the co‐payment provided by local government in 12 large cities to the influenza immunisation uptake for older people. Compared to the 2001 to 2002 season, the vaccination uptake increased in 2002 to 2003 and the magnitude of the association was negatively related to the amount of the co‐payment.
These interventions on the societal level are the hardest to evaluate because of unknown biases due to secular trends of increasing influenza vaccination rates in most societies, multiple and often unknown co‐interventions in the form of, for example, newspaper and magazine articles and alerts, and initiatives by organisations on many levels from individual practices to regional campaigns. Overall these societal interventions are correlated with increases in influenza vaccination rates.
Discussion
Of the 57 RCTs, 32 were published in 1999 or earlier and 25 in 2000 or later (Abramson 2011; Arthur 2002; Berg 2008; Chan 2002; Dalby 2000; Dapp 2011; Garcia‐Aymerich 2007; Hogg 2008; Hull 2002; Humiston 2011; Kellerman 2000; Kiefe 2001; Krieger 2000; Lemelin 2001; MacIntyre 2003; Maglione 2002a; Maglione 2002b; Maglione 2002c; Maglione 2002d; Marrero 2006; McCaul 2002; Minor 2010; Nuttall 2003; Roca 2012; Siriwardena 2002). However, in few cases was the research work undertaken during the avian influenza and H1N1 scares, which has changed the level of concern of both the public and the health professions, with many interventions at international, societal and regional levels and often with nightly news bulletins on the radio, TV and in the press during those episodes. There is thus a question as to whether all of the current body of research is relevant during pandemic scares and whether it remains relevant during routine influenza seasons.
Researchers have tested a wide range of interventions relevant to increasing community demand for influenza vaccination, increasing access and provider‐ and system‐based interventions. The percentage of the included trials that we assessed as being at low risk of bias for sequence generation was 33%, allocation concealment 11%, blinding 44%, attrition 49% and selective reporting 100%.
For the letter, postcard and phone call interventions, which included very large numbers of participants, there was marked heterogeneity and thus meta‐analysis was not possible for these interventions. The wide variety of interventions that could not logically be grouped together also reduces the power of this systematic review in drawing conclusions.
The recommendations of the version of Centers for Disease Control and Prevention (CDC) Community Guide Services available at that time are in the review by Briss 2000. The execution of each study was characterised as good, fair or limited based on the total number of categories with limitations. Good studies had zero or one limitation, fair studies had two to four and limited studies had five or more. Studies with limited execution did not qualify for the review. The overall approach of the CDC Community Guide Services to assessing study quality is presented in CDC 2014. The figure for each type of intervention in Briss 2000 included RCTs and other designs, interventions for different types of vaccine and age groups other than those aged 60 or older.
This Cochrane systematic review is based on a comprehensive search in all languages updated to 4 June 2014. It includes only RCTs (we did not include studies using other designs because of unknown confounders and non‐comparable hemi‐cohorts), includes only those aged 60 or older and assesses the risk of bias in each study using the Cochrane RevMan 2014 software and the 'Risk of bias' tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Summary of main results
I. Interventions to increase community demand
(a) Reminders to patients
Sixteen randomised controlled trials (RCTs), with a total of 592,165 participants, tested the effect of a reminder postcard or letter but the studies could not be pooled due to heterogeneity. The lower 95% confidence interval (CI) of six was entirely above unity.
Sixteen RCTs, with a total of 388,164 participants, tested the effect of a personalised postcard, letter or phone call but they could not be pooled due to heterogeneity. For seven trials the lower 95% CI was above unity.
Three RCTs, with 64,200 participants, found that client reminder and recall using a leaflet plus letter or postcard was more effective than a letter (odds ratio (OR) 1.11, 95% CI 1.07 to 1.15, P value < 0.00001).
Four RCTs, with 82,465 participants compare client reminder and recall using a customised letter or phone call to a form letter but the I2 statistic was 96% and the studies could not be pooled.
Krieger 2000, in a small study with 193 participants, found that a phone call from a senior (teachers well known in the community) was related to increased vaccination uptake (OR 3.33, 95% CI 1.79 to 6.22, P value < 0.0002). Lukasik 1987, in another small study with 243 participants, found that a phone call increased vaccination uptake compared to an invitation to be vaccinated when participants dropped into the clinic (OR 2.72, 95% CI 1.55 to 4.76, P value = 0.0005).
(b) Educating and vaccinating patients
Three RCTs, with 4016 participants, compared a health risk appraisal to no intervention but the I2 statistic was 94% and too high to permit pooling. For all three the lower 95% CI was above unity. Two RCTs, with 614 participants, compared nurses or pharmacists educating and then vaccinating patients to no intervention (OR 3.29, 95% CI 1.91 to 5.66, P value < 0.0001).
2. Interventions to increase access
(a) Group visits by patients to healthcare professionals
There was one RCT, with 321 participants, of group visits involving education about influenza vaccination (OR 24.85, 95% CI 1.45 to 425.32, P value = 0.03).
(b) Home visits with an encouragement to receive influenza vaccination
Two RCTs, with 2112 participants, compared a home visit to an invitation to attend an influenza vaccination clinic (OR 1.30, 95% CI 1.05 to 1.61, P value = 0.01). One RCT, with 1927 participants, compared home visits by a nurse or group sessions with encouragement to receive influenza vaccination plus a care plan developed with a physician to no intervention (OR 1.68, 95% CI 1.37 to 2.07, P value < 0.0001). When combined with a small (n = 142), similar study the I2 statistic was too high to permit pooling.
(c) Offer of free influenza vaccination
Two RCTs (n=2250) compared an offer of free influenza vaccination to no intervention (OR 2.36, 95% CI 1.98 to 2.82, P value < 0.00001).
3. Provider‐ or system‐based interventions
(a) Reminders to physicians
Four RCTs, with 202,264 participants, compared reminders to physicians to no reminder and we found a non‐significant pooled result. One small RCT, with 316 participants, found that a reminder to physicians about all their patients was more effective than reminding them about half their patients (OR 2.47, 95% CI 1.53 to 3.99, P value = 0.0002). One RCT, with 8376 participants, found that posters in clinics displaying influenza vaccination uptake to encourage physicians to compete, plus postcards, was more effective than no intervention (OR 2.03, 95% CI 1.86 to 2.22, P value < 0.00001), but not significant when compared to posters in clinics.
(b) Facilitators working with physicians and other healthcare workers in practices
Four RCTs, with 3583 participants, introduced facilitators into practices to achieve improvements in a group of health outcomes, including influenza vaccination uptake for those aged 60 and older. Hogg 2008 found an OR of 2.11 (95% CI 1.27 to 3.49, P value = 0.0004). Lemelin 2001 did not present the numbers vaccinated for those aged over 60 but the improvement in uptake was 18.7% in the intervention group and 4% in the control (P value < 0.01). Karuza 1995 had a very wide 95% CI and Kerse 1999 had a non‐significant result. Due to high heterogeneity (I2 statistic = 95%) the RCTs could not be pooled.
(c) Education and feedback to physicians
There were three RCTs of providing education and feedback to physicians. For Kiefe 2001, with 1360 participants, the OR was 3.43 (95% CI 2.37 to 4.97, P value < 0.00001). Siriwardena 2002, with 27,580 participants, obtained a negative result (OR 0.77, 95% CI 0.72 to 0.81, P value < 0.00001) and Kim 1999, with 1400 participants, obtained a non‐significant result.
(d) Financial incentives to physicians for increasing influenza vaccination uptake
Two RCTs (n = 2815) compared paying physicians to increase influenza vaccination uptake to no intervention (OR 2.2, 95% CI 1.77 to 2.77, P value < 0.00001) (Ives 1994; Kouides 1998).
(e) Increasing staff vaccination uptake
Abramson 2011, with 26,442 participants, compared an intervention to increase clinic health staff vaccination uptake to no intervention, hoping that this would increase staff behaviours to vaccinate clinic patients, but they found no significant increase in patient vaccination uptake.
4. Interventions on the societal level
There were no RCTs at the societal level and identifying the roles of policy changes about vaccination, educational interventions, media discussions and societal trends in affecting vaccination uptake is difficult. Interventions on the societal level are the hardest to evaluate because of unknown biases due to secular trends of increasing influenza vaccination rates in most societies, multiple and often unknown co‐interventions in the form of, for example, newspaper and magazine articles and alerts and initiatives by organisations on many levels from individual practices to regional campaigns. Overall these societal interventions are correlated with increases in influenza vaccination rates.
Overall completeness and applicability of evidence
We identified 57 RCTs, with 39 (68%) from the US, seven from Canada, four Australia, three Spain and one each from Denmark, Germany, Israel, New Zealand and Puerto Rico. The majority of studies thus reflect the US medical and financial structure. Interventions were tested comprehensively for effect in three parts of the healthcare system: participants, health care providers (physicians, nurses and pharmacists) and overall healthcare systems. However, a key problem is measuring how complete the assessment of influenza vaccination was, as in most of the US studies it was possible for participants to receive vaccination at walk‐in clinics and during campaigns instead of their regular clinics and some studies did not perform independent verification of the accuracy and completeness of their clinic records or financial billings (for the US participants aged 65 or older this is Medicare).
Quality of the evidence
Thirty of the RCTs were published before 2000, which may affect both the rigour of study design and data analysis.
For randomisation 19 (33%) of the trials were at low risk of bias, 35 (61%) unclear and three (6%) at high risk. The assessment of unclear risk of bias was usually because the description was limited to the words "were randomised". For concealment of allocation six (11%) were at low risk and 51 (89%) unclear because there was no statement in the text.
In 28 (49%) of trials there was low risk of incomplete data, in 22 (39%) an unclear risk and in seven (12%) a high risk. Influenza vaccination uptake was recorded in computers or ascertained from computerised records or review of clinic records in 53 RCTs; by two research assistants through phone calls or home visits in Black 1993; from records during the vaccination campaign in Díaz Grávalos 1999; from hospital records or phone calls and letters to GPs in MacIntyre 2003 and from the records of the pharmacy where the RCT was conducted in Marrero 2006.
All 57 (100%) of the trials were free of selective reporting.
Potential biases in the review process
All stages in the review process were accomplished independently, with data checking by the other review author. As this systematic review is unfunded, we were unable to afford translations and we included articles in languages that the review authors could read (English, French, German, Italian, Portuguese and Spanish) or for which the English language abstract provided sufficient information.
Agreements and disagreements with other studies or reviews
There are four previous systematic reviews specifically about increasing influenza vaccination uptake.
Briss 2000 identified 16 RCTs and four time series of interventions to increase adult influenza vaccination uptake, but did not state the period for the literature search and compared current year outcomes for intervention and control groups without deducting baseline uptake from the prior year, whereas we deducted prior year uptake from current year uptake for all RCTs where we had the data, so his results are not comparable to our review. Bordley 2000 searched MEDLINE from 1966 to 1997 for studies of the effect of audit and feedback on immunisation uptake and has been superseded by Ivers 2012. Sarnoff 1998, Gyorkos 1994 and Litt 1993 are also outdated.
This review adopted the three intervention categories of the US Task Force on Community Preventive Services as published in the Guide to Community Preventive Services (CDC 2014): 1. increasing community demand for vaccinations; 2. enhancing access to vaccination services; and 3. provider‐ or system‐based interventions. Their literature review (Chapter 6) included and added together the results from several types of study designs. They recommended these interventions for universally recommended vaccinations: 1. increasing community demand for vaccinations (client reminder and recall systems, multi‐component interventions that include education and vaccination requirements for child care, school and college attendance); 2. enhancing access to vaccination services (reducing out‐of‐pocket costs, expanding access in healthcare settings as part of a multi‐component intervention, vaccination programmes in women, infant and child (WIC) settings, vaccination programmes in schools and home visits) and 3. provider‐ or system‐based interventions (provider reminder and recall systems, assessment plus feedback for vaccination providers and standing orders for adults). The review synthesised results across age groups (children, adults and elders) and many different vaccines, but included studies of influenza vaccine among elders, rather than specifically focusing on interventions to increase influenza vaccination only among older people. They recommended combining interventions: one or more interventions to increase community demand plus at least one provider‐ or system‐based intervention plus at least one intervention to enhance access. The strategies for increasing community demand that were recommended included the use of client reminder/recall and multi‐component interventions that include education; those for enhancing access included both reducing out‐of‐pocket costs and home visiting. Recommended provider‐ or system‐based interventions included reminder/recall systems for providers, assessment and feedback of vaccination information to providers and the use of standing orders. Our review focuses on older persons and influenza vaccination and includes more recently published studies.
There are two Cochrane systematic reviews of interventions to change health professionals' behaviour, which include interventions to increase adult influenza vaccination uptake. Ivers 2012 reviewed the effects of audit and feedback and we included the four studies they found of interventions to increase adult influenza vaccination uptake (Buffington 1991; Kiefe 2001; Kim 1999; Siriwardena 2002); we excluded the others because either the intervention was not to increase influenza vaccination uptake, the outcome uptake for those aged 60 or older could not be separately identified, or seniors were not studied. Our conclusions are thus based on a very different set of studies. Jacobson 2009 reviewed patient reminder and recall systems for improving vaccination uptake and identified 16 RCTs of interventions to increase adult influenza vaccination uptake, of which we included three (Lukasik 1987; McDowell 1986; Puech 1998), but excluded the others as the results for those aged 60 or older could not be separately identified or influenza vaccination depended on self report. Krishna 2002 undertook a systematic review of telephone educational messages and identified one RCT of an intervention to increase influenza vaccination uptake.
Lau 2012 undertook a comprehensive search of the literature in English but excluded other languages. Study quality was assessed with the Downs and Black tool (Downs 1998), but this was tested with a very small number of studies and no further work has been undertaken on it since 1998. They analyzed randomised and non‐randomised studies together.
Authors' conclusions
For the non‐randomised designs we could not evaluate the effect of unknown confounders and unknown biases with the data provided in the articles (Appendix 7) and these are excluded from the analysis.
I. Interventions to increase community demand
Three randomised controlled trials (RCTs) (n = 64,200) found that client reminder with a letter plus leaflet or postcard was more effective than a letter (odds ratio (OR) 1.11, 95% confidence interval (CI) 1.07 to 1.15, P value < 0.00001) (Maglione 2002b; Maglione 2002d; Nuttall 2003). Two RCTs (n = 614) found that nurses or pharmacists educating then vaccinating patients was more effective than no intervention (OR 3.29, 95% CI 1.91 to 5.66 P value < 0.0001) (Herman 1994; Marrero 2006). A small RCT (n = 193) found that client reminder by a senior plus an educational brochure was more effective than usual publicity (OR 3.33, 95% CI 1.79 to 6.22, P value < 0.002) (Krieger 2000). A small RCT (n = 243) of client reminder found a telephone call more effective than an invitation to the patient when the patient dropped into the clinic (OR 2.72, 95% CI 1.55 to 4.76, P value = 0.0005) (Lukasik 1987). A small RCT (n = 291) of a lottery for free groceries to encourage vaccination found no effect (OR 1.04, 95% CI 0.62 to 1.76, P value = 0.88) (Moran 1992).
The groups of RCTs which could not be pooled due to high heterogeneity were: 16 RCTs (n = 592,165) of letter or postcard or telephone reminders to participants; 16 RCTs (n = 388,164) of letter, card or phone reminders personalised to the patient's health status; four RCTs (n = 82,465) comparing a customised letter or phone call to a form letter; and three RCTs (n = 4016) of a health risk appraisal compared to no intervention. Readers should consult the individual trial results.
There is thus evidence that some low‐ and higher‐intensity interventions to increase community demand are effective.
II. Interventions to enhance vaccination access
Two RCTs (n = 2112) found a home visit more effective than an invitation to attend an influenza vaccination clinic (OR 1.30, 95% CI 1.05 to 1.61, P value = 0.01) (Arthur 2002; Nuttall 2003). Two RCTs (n = 2251) found that offering free influenza vaccination was more effective than no intervention (OR 5.43, 95% CI 2.85 to 10.35, P value < 0.00001) (Nexøe 1997; Satterthwaite 1997). A small RCT (n = 321) found group visits of patients to the nurse and physician more effective than usual care (OR 24.85, 95% CI 1.45 to 425.32, P value = 0.03) (Beck 1997). One RCT (n = 350) compared a home visit with encouragement to be vaccinated with a home visit with a safety intervention and found no significant difference (Black 1993).
The groups of RCTs which could not be pooled due to high heterogeneity were: two RCTs (n = 2069) of home visits and two RCTs (n = 2250) of free influenza vaccination compared to no intervention. One of the RCTs of home visits (n = 1927) had a complex intervention with a home visit by a nurse or group sessions with encouragement to receive influenza vaccination plus a care plan developed with a physician; it was more effective than no intervention (OR 1.68, 95% CI 1.37 to 2.07, P value < 0.0001) (Dapp 2011).
There is thus evidence that home visits and offers of free vaccination are effective.
III. Provider‐ or system‐based interventions
Two RCTs (n = 2815) found payment to physicians for vaccinations more effective than no payment (OR 2.22, 95% CI 1.77 to 2.77, P value < 0.0001) (Ives 1994; Kouides 1998). A large RCT (n = 27,580) found educational outreach and feedback to practice teams less effective than written feedback to practice teams (OR 0.77, 95% CI 0.72 to 0.81, P value < 0.00001) (Siriwardena 2002). One small RCT (n = 316) found reminding physicians about all their patients more effective than reminding them about half (OR 2.47, 95% CI 1.53 to 3.99, P value = 0.0002) (Chambers 1991). Kiefe 2001 (n = 1360) found chart review and feedback to physicians plus benchmarking to the influenza vaccination uptake achieved by the top 10% of physicians more effective than chart review and feedback (OR 3.43, 95% CI 2.37 to 4.97, P value < 0.00001). One RCT (n = 8376) found that displaying influenza vaccination rates in clinics to encourage physicians was more effective than no intervention (OR 2.03, 95% CI 1.86 to 2.22, P value < 0.00001) (Buffington 1991).
One RCT (n = 1400) did not find educational reminders, academic detailing and peer comparisons to other physicians more effective than mailed educational materials (OR 1.13, 95% CI 0.80 to 1.58, P value = 0.50) (Kim 1999). One RCT (n = 8376) did not find posters displaying influenza vaccination rates in clinics plus postcards more effective than posters (Buffington 1991). One RCT (n = 26,432) did not find that encouraging clinic staff to be vaccinated increased clinic patient vaccination uptake (Abramson 2011).
The groups of RCTs which could not be pooled due to high heterogeneity were: four RCTs (n = 202,264) of reminders to physicians and four RCTs of facilitators in practices. Three facilitator RCTs found positive results: Lemelin 2001 (P value < 0.01), Hogg 2008 (OR 2.11, 95% CI 1.27 to 3.49, P value = 0.0004) and Karuza 1995 (OR 292.81, 95% CI 18.16 to 4721.62, P value < 0.0001).
There is thus evidence that placing facilitators in clinics to encourage preventive interventions (including influenza vaccination) is effective. Paying physicians is also effective, but reminding physicians is not effective unless it is high‐profile (competitive posters of vaccination uptake in clinic). Chart review and more intensive audit and feedback was only effective in one study involving benchmarking the results to those of the top 10% of physicians (Kiefe 2001).
The Cochrane review by Jefferson 2010 found evidence from only one RCT to support influenza vaccination in persons aged 65 and over and the remainder of the 100 data sets were non‐RCTs subject to unknown biases. There were no RCTs or cohort studies at low risk of bias to answer the question of whether influenza vaccination leads to lower morbidity or hospitalisation of seniors. Jefferson 2010 recommends that an adequately powered, publicly funded (to avoid influences from drug companies), placebo‐controlled RCT needs to be conducted over several influenza seasons. Evidence from such a RCT is thus required to prove that the interventions which we identified as effective should be implemented. We have not yet established the secure evidence base required to prove that vaccination of those 65 and over is effective. The RCT recommended by Jefferson 2010, to measure the effectiveness of influenza vaccine in older persons, should maximise uptake of vaccine by those 65 or older, by implementing the strategies that we have found in this review to be effective.
IV. Societal interventions
No RCTs were found.
I. Interventions to increase community demand
For(a) reminders to participants and (b) educating and vaccinating participants there is need for further research of excellent quality, which brings interventions up to date with the current influenza challenge, particularly with SARS, H5N5, H1N1 and other new viral combinations.
II. Interventions to enhance vaccination access
(a) Group visits. It is likely that group visits of older people with chronic diseases and visits to multidisciplinary teams will become more frequent. For complex interactions with multiple health professionals and other participants it is important to conduct other RCTs to identify how to maximise vaccination uptake. (b) Home visits. Home visits are effective. There are increasing numbers of older people and an increasing desire to keep them in their own homes. It is therefore important to conduct further RCTs to compare home visits which encourage influenza vaccination to home visits or other outreach interventions to provide influenza vaccination, and to find out how best to combine assessment with senior‐oriented education on these visits to maximise vaccination uptake. If an influenza recommendation occurred in the context of a home visit for another purpose it would not add extra costs, but if planned solely for that purpose it would be very expensive compared to other methods. (c) Free vaccination. The two RCTs showed that free vaccination is effective and further studies at low risk of bias are needed.
III. Provider‐ or system‐based interventions
(a) Reminders to physicians and posters. We are now in an era when physicians are overwhelmed with guidelines and directives and more research on reminders to teams linked to guidelines ('Just in time CME') may be of value. We graded the one study of posters continuously updating vaccination levels and making them visible to encourage physicians, staff and participants to achieve higher levels of vaccination as at high risk of bias. Further high‐quality evidence and further research is needed. (b) Facilitators in practices. There are four RCTs that introduced facilitators into practices to achieve improvements in a group of health outcomes, including influenza vaccination uptake for those aged 60 and older. Further research to keep facilitator interventions up to date and more efficient to achieve higher vaccination uptake is needed because this is an expensive intervention. (c) Education and feedback to physicians. A huge amount of money and effort is spent endeavouring to educate physicians and other health professionals by paper, e‐mail, meetings at work and in conferences. Further RCTs are needed to assess the effectiveness of the educational interventions health professionals receive about influenza vaccination, to improve their incorporation of it into their practices. Research into whether the interventions correspond to the best researched and most effective models of learning would also be helpful. There is an overlap between learning and facilitation, as the facilitation RCTs often include education. (d) Financial incentives for physicians. There were two RCTs of incentives to increase influenza vaccination uptake but the risk of bias was high. Further research on the size of the incentives and optimum increments for higher levels of vaccination would be valuable.
IV. Societal interventions
An RCT at the national level of the interventions found in this review to be effective and integrating computerised reminder, recall and checking systems at the practice, community and national level and assigning designated individuals with the authority, staff and finances to identify unvaccinated individuals and get them vaccinated would be more valuable in ensuring completion than more ineffective reminders or 'education' directed to physicians without links to individual participants.
Baseline data. All future RCTs should obtain baseline data on influenza vaccination uptake for the years prior to the intervention.
Accuracy of the categorisation of types of interventions. Multi‐component interventions are most common, therefore researchers should carefully analyze and categorise their interventions according to the three Centers for Disease Control and Prevention (CDC 2014) criteria to ensure the comparability of future research.
Outcome data. Many studies could have improved the accuracy and completeness of vaccination recording. Future studies should validate vaccination histories, comparing and testing for completeness multiple hard data sources such as vaccination registries, clinic records and billing data. Some individuals may go 'off site' to walk‐in clinics or vaccination clinics in shopping malls, therefore researchers need to take careful vaccination histories, ask to see vaccination record cards and integrate this self report data. RCTs which rely only on self reported data should be discouraged.
Feedback
Interventions to increase influenza vaccination rates of those 60 years and older in the community, 27 October 2010
Summary
In the systematic review by Thomas et al. (Thomas 2010) titled Interventions to increase influenza vaccination rates of those 60 years and older in the community, the authors, in our opinion, fail to emphasize 2 key issues. While we do not dispute the findings that the methods proposed may increase compliance in influenza vaccine use, we question the relevance of reporting these results.
(1) The authors acknowledge the findings of a recently published systematic review Vaccines for preventing influenza in the elderly (Jefferson 2010), which concludes that ?available evidence is of poor quality and provides no guidance regarding the safety, efficacy or effectiveness of influenza vaccines for people aged 65 years or older.? Despite the recognition that current evidence is limited and is of poor quality, the authors proceed to defer to clinical practice guidelines in place since 1964 rather than stressing the importance that a large‐scale, publicly‐funded placebo‐controlled RCT is required to assess the value of vaccinating the community‐dwelling elderly population.
(2) In their review, Jefferson et al. found no difference in rates of adverse events between people who received vaccination and those who did not. However, adverse events occurring within one week of vaccine administration were assessed. Jefferson et al. also mention rare adverse events from vaccination but do not provide any detail, presumably because this data is from observational studies, as opposed to an RCT. Although the current literature on risk of serious adverse events is conflicting, this should not preclude patients and clinicians from being made aware of potential adverse effects of influenza vaccination. In addition, the prevalence of adverse events may substantially increase when a larger population is exposed to the vaccine.
(3) In our opinion, the conclusion of the review by Thomas et al. should include a definitive statement regarding the need for more robust evidence from properly designed studies on influenza vaccination, as well as an appeal to readers to consider the major gaps in the evidence. We think the conclusion should say that there is insufficient evidence that the vaccine improves clinical outcomes in the elderly. In addition, one cannot rule out the possibility that the vaccine increases the risk of serious harm. That being said, there is evidence that certain methods increase vaccination rates (e.g. postcards to patients) however this finding is of limited clinical importance based on the aforementioned concerns.
We look forward to hearing your comments.
Reference: Jefferson T, Di Pietrantonj C, Al‐Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2010, Issue 2. Art. No.: CD004876. DOI: 10.1002/14651858.CD004876.pub3.
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
The reply is keyed to the numbers in the feedback above.
(1) The opening sentence of the present review is: “A review (Jefferson 2010) of the effectiveness of influenza vaccine in seniors includes 75 studies and 100 data sets. One RCT showed benefits against influenza symptoms but was underpowered to detect effects on complications (1348 participants). Other data sets were not randomised and were which were likely to contain biases. The review was unable to reach conclusions about the effects of the vaccines in persons 65 or older.”
The ACIP statement for 2010 (www.cdc.gov downloaded on 27 May 2011) may not have been formulated when the results of the Jefferson (2010) Cochrane review were available and stated that the recommendations for influenza vaccination for 2010 are:
All persons aged 6 months and older should be vaccinated annually.
Protection of persons at higher risk for influenza‐related complications should continue to be a focus of vaccination efforts as providers and programs transition to routine vaccination of all persons aged 6 months and older.
-
When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to persons who:
are aged 6 months‐‐4 years (59 months);
are aged 50 years and older;
have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus);
are immunosuppressed (including immunosuppression caused by medications or by human immunodeficiency virus);
are or will be pregnant during the influenza season;
are aged 6 months‐‐18 years and receiving long‐term aspirin therapy and who therefore might be at risk for experiencing Reye syndrome after influenza virus infection;
are residents of nursing homes and other chronic‐care facilities;
are American Indians/Alaska Natives;
are morbidly obese (body‐mass index is 40 or greater);
are health‐care personnel;
are household contacts and caregivers of children aged younger than 5 years and adults aged 50 years and older, with particular emphasis on vaccinating contacts of children aged younger than 6 months; and
are household contacts and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza.
The present review and the Jefferson (2010) review were conducted in the same time frame and their conclusions became available at about the same time and neither group of reviewers could have anticipated the utility or conclusions of their review compared to the other review or the ACIP recommendations (which their systematic reviews were planned to test).
(2) The commentators are correct that minimal data about potential harms is available. The Jefferson (2010) review concluded:
"Seven studies included in our safety assessment are described below: Four RCTs (Govaert 1993; Keitel 1996; Margolis 1990a; Treanor 1994). Three surveillance studies with a non‐comparative design assessing rare events (Guillan Barré Syndrome (GBS)) (Kaplan 1982; Lasky 1998; Schonberger 1979) were commented on in the text but were not included in our meta‐analysis. One RCT assessed a vaccine which has not been in production for decades (Stuart 1969). Its harms data were not extracted."
One of the purposes of the larger publicly funded RCT advocated in the conclusions of both reviews would be to assess potential harms.
(3) The conclusions of the present review made precisely the recommendation that the commentators make above and recommended using the findings of the present study (how to increase uptake of vaccine) to improve execution of the larger publicly funded study of vaccine effectiveness both reviews recommend:
“The review by Jefferson 2010, which was updated at the same time as this review was being completed, found evidence only from one RCT to support influenza vaccination in persons 65 and over and the remainder of the 100 data sets were non‐RCTs subject to unknown biases. In the present review, out of 44 RCTs only five RCTs were found to be at low risk and six at moderate risk of bias. They included three of 13 personalized postcard interventions (all three with the 95% CI above unity), two of the four home visit interventions (both with 95% CI above unity but one a small study), three of the four reminder to physicians interventions (none with 95% CI above unity) and three of the four facilitator interventions (one with 95% CI above unity and one P < 0.01). The other 33 RCTs were at high risk of bias and no recommendations for practice can be drawn. Jefferson 2010 recommends that an adequately powered publicly‐funded (to avoid influences from drug companies) placebo‐controlled RCT needs to be conducted over several influenza seasons. Evidence from such an RCT is thus required to prove that the interventions which we identified as effective should be implemented. These two reviews have identified that we have not yet established the secure evidence base required to prove that vaccination of those 65 and over is effective. The RCT recommended by Jefferson 2010 to measure the effectiveness of influenza vaccine in older persons should maximize uptake of vaccine by implementing the strategies we found effective in increasing influenza vaccination rates."
Contributors
Michelle Co, BScPharm Hayley Coe, BScPharm Sarah West, BSc, BScPharm Aaron Tejani, BScPharm, PharmD
Acknowledgements
We thank Janine Morrison, Emily Medd and Wendy Spragins for retrieving all the full‐text articles for the first edition. For comments on the draft review we thank Vicky Debold, Amy Zelmer, Ann Mayo, Tony Arthur, Mark Jones and Matthew Thompson. We thank Dr. Margaret Russell for her excellent and invaluable organisational and critical work on the first edition of this review. For this update we thank Tony Arthur, Janet Wale, Conor Teljeur and Matthew Thompson.
Appendices
Appendix 1. Included studies design
A randomised controlled trial (RCT) is any study on humans in which the individuals (or other experimental units) followed in the study were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using random allocation.
Appendix 2. Data extraction form
| Methods |
Purpose: Design: Duration of study: Interval between intervention and when outcome was measured: Power computation: Statistics: |
| Participants | Country: Setting: Eligible participants: (health status) Age: Sex: |
| Interventions | Intervention 1: Intervention 2: Control: |
| Outcomes |
Outcome measured: Time points from the study that are considered in the review or measured or reported in the study: % vaccinated by |
| Notes | Funding: |
Appendix 3. MEDLINE (Ovid) search strategy
MEDLINE (OVID)
1 Influenza, Human/ 2 exp Influenza A virus/ 3 exp Influenzavirus B/ 4 Influenzavirus C/ 5 (influenza or flu or h1n1).tw. 6 or/1‐5 7 exp Immunization/ 8 exp Vaccines/ 9 (immuni* or vaccin*).tw. 10 or/7‐9 11 6 and 10 12 Influenza Vaccines/ 13 11 or 12 14 exp aged/ or middle aged/ 15 ((old* or age*) adj3 (people* or person* or adult* or women* or men* or citizen* or residen*)).tw. 16 (pension* or retire* or elderly or senior* or geriatric*).tw. 17 long‐term care/ or nursing care/ or palliative care/ 18 homes for the aged/ or nursing homes/ 19 nursing home*.tw. 20 Hospitals/ 21 residential facilities/ or assisted living facilities/ 22 Health Services for the Aged/ 23 (institution* adj3 elderly*).tw. 24 (aged care or hospice* or old people* home*).tw. 25 ("50 years or older" or "55 years or older" or "60 years or older" or "65 years or older" or "70 years or older" or "75 years or older" or "80 years or older").tw. 26 ("older than 50" or "older than 55" or "older than 60" or "older than 65" or "older than 70" or "older than 75" or "older than 80").tw. 27 or/14‐26 28 13 and 27
Appendix 4. Electronic database search strategies
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 5), which contains the Cochrane Acute Respiratory Infections Group's Specialized Register, to 4 June 2014, MEDLINE (January 2010 to 4 June 2014), PubMed (January 2010 to 4 June 2014), EMBASE (January 2010 to 4 June 2014), ERIC (January 2010 to 4 June 2014) and CINAHL (January 2010 to 4 June 2014).
MEDLINE (OVID)
1 Influenza, Human/ 2 exp Influenza A virus/ 3 exp Influenzavirus B/ 4 Influenzavirus C/ 5 (influenza or flu or h1n1).tw. 6 or/1‐5 7 exp Immunization/ 8 exp Vaccines/ 9 (immuni* or vaccin*).tw. 10 or/7‐9 11 6 and 10 12 Influenza Vaccines/ 13 11 or 12 14 exp aged/ or middle aged/ 15 ((old* or age*) adj3 (people* or person* or adult* or women* or men* or citizen* or residen*)).tw. 16 (pension* or retire* or elderly or senior* or geriatric*).tw. 17 long‐term care/ or nursing care/ or palliative care/ 18 homes for the aged/ or nursing homes/ 19 nursing home*.tw. 20 Hospitals/ 21 residential facilities/ or assisted living facilities/ 22 Health Services for the Aged/ 23 (institution* adj3 elderly*).tw. 24 (aged care or hospice* or old people* home*).tw. 25 ("50 years or older" or "55 years or older" or "60 years or older" or "65 years or older" or "70 years or older" or "75 years or older" or "80 years or older").tw. 26 ("older than 50" or "older than 55" or "older than 60" or "older than 65" or "older than 70" or "older than 75" or "older than 80").tw. 27 or/14‐26 28 13 and 27
The study designs filter used is based on the RCT highly sensitive search strategy defined by The Cochrane Collaboration and detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The RCT filter terms listed below are based on the most recent Cochrane recommendations.
MEDLINE (OVID)
(controlled clinical trial or meta analysis or randomised controlled trial).pt.
drug therapy.fs.
(groups or placebo* or random* or trial*).tw.
1 or 2 or 3
limit 4 to animals
limit 4 to (humans and animals)
5 not 6
4 not 7
No language or publication restrictions were applied.
Cochrane Central Register of Controlled Trials (CENTRAL)
influenza, human or exp influenzavirus a/ or exp influenzavirus b/ or influenzavirus c/
(influenza* or flu).tw.
1 or 2
vaccines/ or exp immunization/
(immuni* or vaccin*).tw.
4 or 5
3 and 6
influenza vaccines/
7 or 8
limit 9 to (“middle aged (45 plus years” or “all aged (65 and over)” or “aged (80 and over)”
exp middle aged/ or exp aged/ or homes for the aged/ or health services for the aged/
(elderly or senior*).tw.
11 or 12
9 and 13
10 or 14
PubMed
influenza, human[MeSH] or influenzavirus a[MeSH] or influenzavirus b[MeSH] or influenzavirus c[MeSH]
influenza[tiab] or flu[tiab]
1 or 2
Vaccines[MeSH:noexp] or immunization[MeSH]
(immuni*[tiab] or vaccin*[tiab]
4 or 5
3 and 6
influenza vaccines[MeSH]
7 or 8
limit 9 to (“middle aged (45 plus years” or “all aged (65 and over)” or “aged (80 and over)”
middle aged[MeSH] or aged[MeSH] or homes for the aged[MeSH] or health services for the aged[MeSH]
elderly[tiab] or senior*[tiab]
11 or 12
9 and 13
10 or 14
controlled clinical trial[pt] or randomized controlled trial[pt]
drug therapy[sh]
(groups[tiab] or placebo[tiab] or randomized[tiab] or randomly[tiab] or trial[tiab]
16 or 17 or 18
15 and 19
animals [mh] NOT humans [mh]
20 not 21
EMBASE (Ovid)
influenza/ or influenza A/ or exp influenza virus/
(influenza or flu).tw.
1 or 2
exp immunization/ or exp vaccine/
(immun* or vaccin*).tw.
4 or 5
3 and 6
influenza vaccine/ or influenza vaccination/
7 or 8
limit 9 to (adult <18 to 64 years> or aged (<65+ years>)
aged/ or exp elderly care/
(elderly or senior*).tw.
11 or 12
9 and 13
10 or 14
crossover procedure/ or double blind procedure/ o randomized controlled trial/ or single blind procedure/
((single or double or triple or treble) adj3 (blind* or mask*)).tw.
(allocat* or assign* or crossover* or cross over* or factorial or placebo* or random* or trial* or volunteer*).tw.
16 or 17 or 18
15 and 19
limit 20 to human
limit 20 to animal studies
22 not 21
20 not 23
ERIC (ProQuest)
((influenza* or flu or h1n1) AND (immuni* or vaccin*)) AND ((elderly OR senior* OR retire* OR pension* OR geriatric*) OR (old* NEAR/3 people* OR old* NEAR/3 person* OR old* NEAR/3 adult* OR old* NEAR/3 women* OR old* NEAR/3 men* OR old* NEAR/3 citizen* OR old* NEAR/3 residen*) OR (aged NEAR/3 people* OR aged NEAR/3 person* OR aged NEAR/3 adult* OR aged NEAR/3 women* OR aged NEAR/3 men* OR aged NEAR/3 citizen* OR aged NEAR/3 residen*) OR (nursing NEAR/2 home* OR home* NEAR/3 aged OR "aged care" OR retire* NEAR/2 home*) OR ("50 years or older" OR "55 years or older" OR "60 years or older" OR "65 years or older" OR "70 years or older" OR "75 years or older" OR "80 years or older") OR ("older than 50" OR "older than 55" OR "older than 60" OR "older than 65" OR "older than 70" OR "older than 75" OR "older than 80"))
CINAHL (EBSCOhost)
(MH “influenza vaccine”)
AB (influenza or flu) or TI (influenza or flu)
AB (vaccin* or immuni*) or TI (vaccin* or immuni*)
2 and 3
1 or 4
(MH “aged”) or (MH “aged, 80 and over”)
AB (aged or elderly or senior*) or TI (aged or elderly or senior*)
6 or 7
5 and 8
Limit 9 to Publication Type: Clinical Trial, Systematic Review
((MH "Clinical Trials") or (MH "Meta Analysis") or (MH "Systematic Review") or (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") or (MH "Placebos") or (MH "Evaluation Research")
TI ((single or double or triple or treble) and (blind* or mask*))
AB ((single or double or triple or treble) and (blind* or mask*))
TI ((systematic or synthesis) and (review* or overview*))
AB ((systematic or synthesis) and (review* or overview*))
TI (allocat* or assign* or control* or crossover* or cross over* or factorial or groups or metaanalys* or meta analys* or metanalys* or placebo* or rct* or random* or trial* or volunteer*)
AB (allocat* or assign* or control* or crossover* or cross over* or factorial or groups or metaanalys* or meta analys* or metanalys* or placebo* or rct* or random* or trial* or volunteer*)
11 or 12 or 13 or 14 or 15 or 16 or 17
9 and 18
10 or 19
Appendix 5. Differences in influenza vaccination percentages in the year before intervention for those RCTs which provided the information
| Author and date | Allocation concealment | Baseline influenza vaccination rate treatment group (%) | Baseline influenza vaccination rate control group (%) |
| Difference 2% | Or less | ||
| Abramson 2011 | Unclear | 43.4 | 44.4 |
| Arthur 2002 | Unclear | 48.7 | 46.7 |
| Barnas 1989 | Unclear | 5 | 5 |
| Beck 1997 | No | 74 | 72 |
| Clayton 1999 | Unclear | 0% for not vaccinated 100% for vaccinated |
0% for not vaccinated 100% for vaccinated |
| Frank 2004 | Yes | 65 | 66 |
| Ives 1994 | Unclear | 41.3 | 40.6 |
| Karuza 1995 | Unclear | 47.5 | 46.5 |
| Kiefe 2001 | Unclear | 40 | 40 |
| Kim 1999 | Unclear | 79 | 80 |
| Kouides 1998 | Unclear | 57.6 | 58 |
| Krieger 2000 | Yes | 0% for not vaccinated 100% for vaccinated |
0% for not vaccinated 100% for vaccinated |
| McCaul 2002 | Unclear | 0 | 0 |
| McDowell 1986 | Unclear | 0 | 0 |
| McMahon 1995b (McMahon Wyoming) | Unclear | Participants who received a personal letter 23.8 Participants who received a form letter 20.5 |
Participants who received no letter 21.6 |
| Moran 1995 | Unclear | 16.7 | 16.6 |
| Nuttall 2003 | Unclear | 0 | 0 |
| Roca 2012 | Unclear | 50.9 | 49.1 |
| Difference | 3% to 4% | ||
| Dietrich 1989 | Unclear | 36 | 39 |
| Herman 1994 | Unclear | 31.3 | 34.3 |
| Lemelin 2001 | Unclear | 46.1 | 49.4 |
| Lukasik 1987 | No | 7.3 | 4.5 |
| MacIntyre 2003 | Yes | 61 | 64 |
| McMahon 1995b (McMahon Montana 1994) | Unclear | Participants who received a personal letter 41.2 Participants who received a form letter 46 |
Participants who received no letter 42.3 |
| Siriwardena 2002 | Unclear | 48.6 | 44.7 |
| Difference | 5% or more | ||
| Chan 2002 | Unclear | 31.8 solo 42.5 group practice |
37.8 solo 30.1 group practice |
| Puech 1998 | Yes | 32 | 38 |
| Marrero 2006 | Unclear | 36 | 14 |
Appendix 6. RCTs without baseline influenza vaccination rates for the year before the intervention
Baker 1998; Berg 2004; Black 1993; Buffington 1991; Chambers 1991; Dalby 2000; Dapp 2011; Díaz Grávalos 1999; Garcia‐Aymerich 2007; Hogg 1998; Hogg 2008; Hull 2002; Humiston 2011; Kellerman 2000; Kerse 1999; Maglione 2002a; Maglione 2002b; Maglione 2002c; Maglione 2002d; Minor 2010; Moran 1992; Moran 1996; Morrissey 1995; Mullooly 1987; Nexøe 1997; Satterthwaite 1997; Smith 1999; Spaulding 1991. Incomplete prior year vaccination rates for Moran 1996
Appendix 7. Cohort, case‐control and time series studies and reasons for exclusion
| Author and date | Ref ID | Description of groups | Reason for exclusion |
| 'Historically controlled studies' | |||
| Barton 1990 | 1647 | 1983‐4 baseline rates 1984 postcard reminders 1985 postcard reminders + feedback to service chiefs 1986 postcard reminders + feedback to service chiefs + feedback to physicians |
Excluded as cannot assess secular trends for increase in rest of population |
| Chodroff 1990 | 1986 historical baseline 1986‐1990 residents given preventive checklists |
Excluded as cannot assess secular trends for increase in rest of population | |
| Davidson 1984 | 1772 | Intervention for nurse reminder: 50% of eligibles in 2 consecutive years Control: rest of eligible participants (called historical controls but are same years) |
Excluded as cannot assess secular trends for increase in rest of population |
| De Wals 1988 | 1677 | 1984 baseline 1985 information campaign by family physicians 1986 same + collective info campaign |
Excluded as cannot assess secular trends for increase in rest of population |
| Donato 2007 | 2016 | 2002 nurses screened participants' reminders 2003 standing orders 2004 education campaign |
Excluded as cannot assess secular trends for increase in rest of population |
| Gill 2000 | 1114,1251, 1311 |
1997 baseline rates 1998 reminder to nurse and physician during visit |
Excluded as cannot assess secular trends for increase in rest of population |
| Harris 1990 | 1633 | Retrospective analysis 1979‐80 baseline 1981 nurse prompt 1984 computer prompt |
Excluded as cannot assess secular trends for increase in rest of population |
| Humair 2002 | 2607 | 1995 baseline 1996 intervention |
Excluded as cannot assess secular trends for increase in rest of population |
| Hutchinson 1991 | 1982‐3 historical baseline 1987‐88 reminder placed on all charts |
Excluded as cannot assess secular trends for increase in rest of population | |
| Knoell 1991 | 1619 | 1987‐8 baseline 1989 intervention |
Excluded as cannot assess secular trends for increase in rest of population |
| Malmvall 2007 | 293 | 1999‐2001 baseline date (rates were increasing) 2002‐2005 same intervention in each of 4 years Appears initially to be a time series but is a series of same repeated interventions) |
Excluded as cannot assess secular trends for increase in rest of population |
| 2 GEOGRAPHICAL AREAS (“Non‐randomized controlled trials”) | |||
| Etkind 1996 | 1405 | 2 Massachusetts counties One reimbursement for vaccination + education campaigns One usual care |
Excluded, non‐comparable control |
| Harris 2006 | 34 | S Adelaide; intervention N and W Adelaide; control |
Excluded, non‐comparable control |
| Honkanen 1997 (same data bases as Honkanen 2006) | Admin Area A: risk of disease‐based influenza vaccination programme Admin Area B: age‐based vaccination programme offered Autumn 1993 and 1994 Admin Area C: age‐based vaccination programme offered 1992‐94 |
Non randomised; control areas may not be comparable | |
| Honkanen 2006 | 404 | 14 municipalities: risk of disease‐based intervention x 2 years 29 municipalities: age‐based intervention x 2 years 12 municipalities; cross‐over from disease‐based intervention in 1992 to age‐based intervention in 1993 |
Excluded, control areas may not be comparable |
| RETROSPECTIVE CHART REVIEWS | |||
| Goebel 2005 | 564 | Retrospective chart review of physicians who used standing orders and did not | Excluded, non‐comparable control |
| Jacobs 2001 | 1045 | Retrospective chart review of use of interpreters and non‐use | Excluded, non‐comparable control |
| COHORTS, NOT HISTORICAL | |||
| Bou‐Mias 2006 | 450 | 1 group assigned voice mail reminders 1 group no voice mail reminders |
Excluded, non‐comparable control |
| Charles 1994 | 120 | Allocated by physician team: Control Intervention |
Excluded, non‐comparable control |
| Crawford 2005 | 507 | 1 group assigned voice mail reminders 1 group no voice mail reminders |
Excluded, non‐comparable control |
| Leirer 1989 | 1661 | 2 groups assigned voice mail reminders 2 groups no voice mail reminders |
Excluded, non‐comparable control |
| Margolis 1992 | No ref ID as found by reading reference lists | 2 clinics assigned as intervention and 2 as control clinics | Excluded, non‐comparable control |
| CASE‐CONTROL | |||
| Earle 2003 | 846 | Comparison of participants in SEER (Survival, Epidemiology and End Results Tumour Registry) area with case‐matched controls |
Data and analyses
Comparison 1.
Increasing community demand
Comparison 2.
Enhancing access
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Group visits of patients to physician and nurse compared to usual care | 1 | 321 | Odds Ratio (M‐H, Fixed, 95% CI) | 24.85 [1.45, 425.32] |
| 2 Home visit compared to invitation to attend influenza vaccination clinic | 2 | 2112 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.05, 1.61] |
| 3 Home visit with encouragement to receive influenza vaccination, compared to home visit with safety intervention | 1 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.64, 1.50] |
| 4 Home visit by nurse or group sessions with encouragement to receive influenza vaccination, plus care plan developed with physician, compared to no intervention | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Free influenza vaccine compared to invitation to be vaccinated but patient pays | 2 | 2251 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [1.98, 2.82] |
| 6 Free influenza vaccine compared to no intervention | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3.
Provider‐ or system‐based intervention
What's new
Last assessed as up‐to‐date: 4 June 2014.
| Date | Event | Description |
|---|---|---|
| 4 June 2014 | New search has been performed | Searches updated. We included 13 new trials (Abramson 2011; Dapp 2011; Garcia‐Aymerich 2007; Humiston 2011; Kumar 1999; Maglione 2002a; Maglione 2002b; Maglione 2002c; Maglione 2002d; Minor 2010; Moran 1996; Morrissey 1995; Roca 2012) and identified two potentially relevant trials which are awaiting translation (Lee 2003; Song 2000). |
| 4 June 2014 | New citation required and conclusions have changed | In this update we concluded that letters and postcards, tailored letters/postcards or phone calls, educating patients, home visits, offering free vaccination, some reminders to physicians, paying physicians for improved vaccination rates and using facilitators in clinics were all effective in increasing influenza vaccination rates. However, using educational reminders and feedback to physicians were not effective. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 3 May 2011 | Feedback has been incorporated | Feedback comment added to review. |
| 30 January 2008 | Amended | Converted to new review format. |
| 23 November 2007 | New citation required and major changes | Substantive amendment. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abramson 2011
| Methods | Purpose: to compare influenza vaccination uptake of those ≥ 65 attending primary care clinics which received an intervention to increase staff influenza vaccination uptake, or control (no staff intervention). No influenza intervention for patients Design: C‐RCT (intervention provided to staff in 13 intervention clinics and not provided in 14 control clinics) Duration of study: data extracted from HMO computers for 2007 to 2008 (intervention year) and previous year (2006 to 2007) Interval between intervention and when outcome was measured: 2007 to 2008 (intervention year) (no further details) Power computation: based on 2006 2007 imputed ICC = 0.019, for the sample of patients in 2007 to 2008 ≥ 65, alpha = 0.05, power = 80% for increase in vaccination uptake from 50% to 58%, and power of 90% for increase in vaccination uptake to 60% for the healthcare workers, based on previous year staff vaccination uptake, predicted 156 healthcare workers required in each of intervention and control groups for power = 90% to detect relative increase in staff immunisation from 30% to 50%, with alpha = 0.05 Statistics: odds ratios and 95% CI corrected for clustering, logistic regression |
|
| Participants | Country: Israel Setting: 27 primary care community clinics Eligible participants: (health status); all healthcare workers in the 13 intervention clinics; all patients ≥ 65 in 13 intervention and 14 control clinics Age: ≥ 65; staff were all 344 physicians, nurses, pharmacists, administrative and ancillary staff with direct patient contact Gender of patients: 58% f |
|
| Interventions | Intervention 1: intervention to increase staff influenza vaccination uptake in the Jerusalem area Control: no staff intervention Co‐interventions: none |
|
| Outcomes | Outcome measured: % ≥ 65 influenza vaccination (intervention clinics 2006 to 2007 avg influenza vaccination uptake 58.1% (43.4% 2006 to 2007); control 56.7% (44.7%). Data are from Table 1, text offers different %s Time points reported in the study: 2007 to 2008 was intervention year (time points not stated) |
|
| Notes | Funding: none stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clinics randomly selected for staff intervention (method not stated) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline 11,755 in 13 intervention clinics; 420 (3.6%) excluded as died or left clinics or moved to sheltered accomodation before end of intervention period; 15,660 in 14 control clinics, 503 (3.2%) excluded |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Arthur 2002
| Methods | Purpose: to compare the effect of offering home health checks to appointments in a vaccination clinic on increasing influenza vaccination uptake Design: randomised 1/3 participants to receive 30‐minute health check and offer of influenza vaccine at home, and 2/3 to receive personal letter to attend vaccination clinic in surgery Duration of study: October to 4 December 2000 Interval between intervention and when outcome was measured: letters mailed October 2000; health checks undertaken 2 October to 4 December 2000 Power computation: 99% power at alpha = 0.05 for uptake of 64% in health check group compared to 50% in personal letter group Statistics: Chi2 to analyse difference in uptake between trial arms; ITT | |
| Participants | Country: UK Setting: 34 general practice physicians in Leicestershire Eligible participants: (health status) all 2052 participants >= 75 living in community Age: ≥ 75 years Gender: 60% female | |
| Interventions | Intervention 1: health check at home Intervention 2: invitation to attend vaccination clinic | |
| Outcomes | Outcome measured: % influenza vaccination; how receipt of vaccine was recorded not stated, but as is single practice, sole purpose of this intervention in influenza vaccination, and vaccination clinics and home visits are by practice nurses can be expected to be complete Time points from the study that are considered in the review or measured or reported in the study: 2 October to 4 December 2000 % vaccinated by 31 December 2000 | |
| Notes | Funding: Melton, Rutland and Harborough Primary Care Group, Leicestershire Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SAS data analysis program assigned codes |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 2408 participants, 356 in nursing home or sheltered accomodation; of 680 randomised to health check, 468 received health check and 680 followed up; of 1372 randomised to personal letter, 66 received flu vaccine at home and 1372 followed up |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Baker 1998
| Methods | Purpose: to compare generic postcard recommending immunisation, personalised postcard from physician, personalised letter from physician tailored to their health risk and no intervention Design: participants randomised to 3 interventions and 1 control group Duration of study: reminders posted 3rd week of September 1995; date of end of study not stated Interval between intervention and when outcome was measured: not stated Power computation: not performed Statistics: percentages, odds ratios and 95% CIs | |
| Participants | Country: US Setting: Henry Ford multispecialty clinics, south east Michigan Eligible participants: (health status): all participants ≥ 65 Age: ≥ 65 Gender: 57.7% f | |
| Interventions | Intervention 1: generic postcard recommending immunisation Intervention 2: personalised postcard from physician Intervention 3: personalised letter from physician tailored to their health risk Control: no intervention Co‐interventions: walk‐in influenza clinics October; printed materials based on Health Beliefs Model; toll‐free telephone line | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: computer‐generated reminders sent last week September 1995, date of end of study not stated % vaccinated by: not stated | |
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised into one of four groups" (no method stated) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, but computerised billing data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cohort = 24,743, ≥ 65 = 17,598; < 65 with chronic condition = 10,573; ≥ 65 with chronic condition = 3431, so there is overlap and those < 65 and ≥ 65 total 28,171, 3428 more than the cohort. We were unable to contact the authors after numerous e‐mail attempts including colleagues and organisations |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Barnas 1989
| Methods | Purpose: to compare pre‐appointment postcard with message encouraging influenza vaccination, to pre‐appointment card with no message Design: RCT, participants randomised Duration of study: "fall of 1986" Interval between intervention and when outcome was measured: not stated Power computation: not performed Statistics: Chi2, probabilities | |
| Participants | Country: USA Setting: Primary Care Clinic, Milwaukee County Medical Complex Eligible participants: (health status): 988 participants ≥ 65 were randomised and of the 840 (85%) who kept their appointments and were seen at the clinic 406 received the message and 434 did not Age: ≥ 65 Gender: not stated | |
| Interventions | Intervention 1: pre‐appointment postcard with message encouraging influenza vaccination Control: pre‐appointment card with no message | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: "Fall of 1986" % vaccinated by: not stated | |
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All 988 participants ... were randomised..." (no method stated) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement; computerised billing data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "988 participants ≥ 65 ... were randomised, ... of the 840 (85%) who kept their appointments and were seen at the clinic 406 received the message and 434 did not." Computerised billing data |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Beck 1997
| Methods | Purpose: to compare group visits of chronically ill older participants to a physician to usual care Design: RCT; individual participants randomised Duration of study: 1 year Interval between intervention and when outcome was measured: not stated Power computation: not performed Statistics: Chi2 for dichotomous data, ANOVA for continuous data; not ITT | |
| Participants | Country: USA Setting: 1 office of Colorado Permanente Medical Care Program, a group HMO in Denver Eligible participants: (health status) patients 65 or older with a chronic illness based on chart review (heart, lung or joint disease or diabetes) or high health utilisation in past 12 months (1 or more outpatient visits/month or 1 or more calls to nurse or physician per 2 months); 68% arthritis, 62 % hypertension, 30% heart disease, 31% liver disease, 15% cancer, 15% diabetes Age: average intervention 72, usual care 75 (P = 0.008) Gender: intervention 69%, control 64% female (ns). Baseline N: 419 contacted, of whom 300 returned questionnaires (of whom 77 said not interested, 3 termination from programme, 4 transfers to another clinic, 9 lack of transport, 3 died, 2 low utilisers, 1 home bound). Then 113 additional participants added. Randomised to (1) group visits (160, of whom 20 no shows, 19 drop‐outs, 2 no transport, 5 deaths, 1 skilled nursing facility, 1 transferred clinic), and (2) usual care (161, of whom 9 deaths, 7 belonged to Kaiser Permanente; 2 skilled nursing facility, 3 transferred clinic) | |
| Interventions | Intervention group 1: visits to physician and nurse at clinic in groups average size 8, for (a) 15‐minute warm‐up and socialisation with information on specific disease processes; (b) 15‐minute break for socialisation, and nurse checked blood pressure, immunisation status, immediate needs and arranged visit with physician, (c) 15 minutes of questions and answers, and planned next visit, (d) 30 minutes for visit to physician Control: usual visits to physician | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: not stated % vaccinated by: date not stated | |
| Notes | Funding: Garfield Memorial Fund, Research and Development Fund Kaiser Health Plan of Colorado data from administrative databases and chart review used to measure vaccination uptake No intended or unintended co‐interventions recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 113 participants added but did not receive the baseline Senior Health Questionnaire, and not stated if randomly assigned; groups were equivalent at baseline in important characteristics related to the outcome except age (P = 0.008) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | However, data from administrative databases and chart review used to measure vaccination uptake |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In intervention group participants attended average 6.62 group visits (55% of those scheduled) and no process analysis whether active involvement/participation by individual participants in group activities 48 drop‐outs from intervention group (30%) and 21 (9%) from control, not equivalent in composition: 20 no‐shows, 19 drop‐outs and 5 deaths in intervention and no‐shows or drop‐outs and 5 deaths in control |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Berg 2008
| Methods | Purpose: to test hypotheses that mailed advice to receive an influenza vaccine or to call a telephonic nurse service would reduce condition related inpatient bed days and emergency department visit Design: RCT Duration of study: 5 months Interval between intervention and when outcome was measured: not stated Power computation: no information provided Statistics: unit of study is household, not individual. Clustered analyses were done, including for differences in vaccination uptake using Chi2 statistics generated by the 'proc genmod' command using the 'repeated' option in SAS to account for the clustering effect on variance Data are presented such that the reader can do a comparison of the influenza vaccination uptake between groups as a secondary analysis but the trial was not explicitly designed to test if the interventions would make a difference to influenza vaccination uptake | |
| Participants | Country: USA Setting: subscribers (households) and their dependents over the age of 65 years enrolled in the Blue Cross & Blue Shield Government‐wide Service Benefit Plan in the states of Oklahoma, Rhode Island, Kentucky, California, Arizona, Utah and Colorado in October 2002. Subscribers were current or retired federal employees Eligible participants: (health status): no data provided on health status; however the 'participants' are actually 'households' Age: 65 years or older Gender: 60% female | |
| Interventions | Intervention 1: postal cue encouraging influenza vaccination (N = 26,474 people) Intervention 2: postal cue to call a nurse advice service if symptoms consistent with influenza‐like illness developed (26,846 people) Control: no postal cues sent (81453 people) | |
| Outcomes | Outcome measured: claims made to the insurance providers for inpatient bed days, emergency department visits, physician evaluation and management visits and other outpatient visits for selected respiratory or congestive heart failure ICD‐9‐CM code diagnoses claims. Physician evaluation and management visits were examined using clinical procedural terminology codes However, although not a primary outcome planned for this study, data were obtained for influenza vaccination uptake which are presented in Tables 2 and 3 in the form of rates calculated as (number of events/N in sample) x 10,000 |
|
| Notes | Funding: Blue Cross Blue Shield Association, McKesson Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Households in all states had an equal probability of assignment into the intervention group." "The simple randomisation code was developed by using a computer random number generator between the values of 0 and 1 so that the control group was 3 times as large as the intervention group." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement; outcome data based on billing claims |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition of participants not addressed. "Because the mailings were sent out in bulk, no information was available on undeliverable pieces." Incomplete data points for participants? Cannot assess. "Influenza vaccinations often are given in settings that do not generate claims, thus limiting the reliability of evidence of influenza vaccinations as seen via administrative claims." Analysis of whether differential attrition could affect outcomes? Not performed The study was not designed to evaluate uptake of influenza vaccination as a primary outcome, and because it is possible that participants might have received influenza vaccination from a source that did not result in a claim being made to the insurers from which the outcomes were ascertained, there is likely underestimation of the influenza vaccination uptake for all 3 study groups. However, one might argue that one would not necessarily a priori expect to see systematic difference in utilisation of uncaptured sources of influenza vaccination between these groups unless there was differential drop‐out between the groups over time. No information was presented on persons who might have dropped out because of death during the study or on persons who might have lost their insurance benefits during the study period. This is a threat to the validity of both the cardinal outcomes and the analysis of secondary outcomes we performed |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Black 1993
| Methods | Purpose: to compare effects on influenza vaccination uptake of a home visit including an intervention promoting influenza vaccination to a home visit with an intervention promoting safety Design: RCT Duration of study: not stated Interval between intervention and when outcome was measured: not stated Power computation: post hoc power computation showed 80% power α = 0.05 to detect 50% difference Statistics: percentages; multiple logistic regression | |
| Participants | Country: Canada Setting: Hamilton, Ontario Eligible participants: (health status): 1011 clients ≥ 65 referred to public health nurses in Hamilton Age: 78 Gender: 71% f in influenza intervention group, 62% f in safety intervention | |
| Interventions | Intervention 1: home visit including an intervention promoting influenza vaccination Intervention 2: home visit including an intervention promoting safety Control: no control group E‐mail from author: "our high rates post intervention in the intervention and control groups may have been due to attention bias, although we tried to minimize it in the 'safety' group by asking the PHNs to avoid discussing immunization history with safety group subjects. However, at that time the province and federal governments had become more active with media campaigns and that too could explain the high rates in both groups." | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: not stated % vaccinated by: not stated | |
| Notes | Funding: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly assigned" (no method stated) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement; "outcome data were obtained through telephone interview (or home visit) by two research assistants who were unaware of group membership." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 589 of 1011 eligibles excluded because of cognitive impairment or not active clients; and 57 declined; 157 received influenza and 148 safety promotion; 45 clients assigned to influenza group had already received influenza vaccine and were included in influenza group for ITT analysis Outcome data collected by 2 research assistants either through phone calls or home visits |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Buffington 1991
| Methods | Purpose: to compare displaying clinic and individual physician influenza vaccination uptake on posters plus postcard reminders to participants to displaying clinic and individual physician influenza vaccination uptake on posters to no intervention Design: RCT, clinics as unit of randomisation Duration of study: 23 September to 30 December 1989 Interval between intervention and when outcome was measured: from 23 September to 30 December 1989 Power computation: not performed Statistics: not stated; probabilities reported | |
| Participants | Country: USA Setting: 45 physicians in 3 offices associated with Genesee Hospital, Rochester, NY Eligible participants: (health status): ≥ 65 Age: ≥ 65 Gender: not stated | |
| Interventions | Intervention 1: display of clinic and individual physician influenza vaccination uptake on posters plus postcard reminders to participants Intervention 2: display of clinic and individual physician influenza vaccination uptake on posters Control: no intervention E‐mail from author: "What was interesting was the competition that evolved in those physicians that used the target model. Physicians using the target model did compare their progress with other physician's results. The whole effort generated a pretty positive attitude toward getting the elderly immunized against influenza." | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 23 September to 30 December 1989 % vaccinated by: 30 December | |
| Notes | Funding: Medicare Influenza Demonstration Project sponsored by US Health Care Finance Administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Practices were stratified according to size and randomised." (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, but influenza vaccination uptake from computerised billing codes, or line listing of vaccinees in practices not computerised |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2149 in Group 2 (poster), 3604 in group 3 (poster and postcard) and 4772 in Group 3 (control), but no statement how many letters returned undelivered; influenza vaccination uptake from computerised billing codes, or line listing of vaccinees in practices not computerised |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Chambers 1991
| Methods | Purpose: to compare reminders for all, half or none of their participants to internal medicine residents to give influenza vaccination Design: RCT, resident physicians randomised Duration of study: 2 months Interval between intervention and when outcome was measured: 1 October to 30 November 1987 Power computation: not performed Statistics: Chi2, multiple logistic regression | |
| Participants | Country: USA Setting: Family Practice Center of Thomas Jefferson University, Philadelphia Eligible participants: (health status); all participants ≥ 65 Age: ≥ 65 Gender 74% f | |
| Interventions | Intervention 1: reminders to internal medicine residents for all participants to give influenza vaccination Intervention 2: reminders to half of participants Control: no reminders | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 1 October to 30 November 1987 % vaccinated by: 30 November 1987 | |
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All physicians in the practice were stratified based on level of training and randomly assigned to one of three groups via a computerised randomization program" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, but influenza vaccinations recorded by computerised billing system |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2493 eligibles, of whom 864 visited clinic during 2‐month study period, of these 168 excluded (had already received influenza vaccine or saw several physicians), 24 made drop‐in visits, leaving 686 for randomisation, of whom 464 ≥ 65; average 10% had received influenza vaccination previous year |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Chan 2002
| Methods | Purpose: comparison of 4 reminders monthly to physiatrists to offer influenza vaccination compared to no reminders Design: RCT; intervention and control groups switched in 1998 Duration of study: intervention administered "during influenza season" Interval between intervention and when outcome was measured: all Medicare claims for influenza vaccination in 1997 and 1998 Power computation: not performed Statistics: t tests; random effects log‐binomial model and generalise programed linear mixed model to estimate RR of vaccination, controlling for patient age, gender and number of claims | |
| Participants | Country: USA Setting: physiatrists (rehabilitation physicians) in Washington State and their participants Eligible participants: (health status) 105 physiatrists in Washington State in 1996 with 4300 participants > 65 in 1997 and 4025 in 1998; exclusions: any patient seen by more than 1 physiatrist (n = 1065); 1 physiatrist who received intervention in both 1997 and 1998 and was excluded in 1998; 5 physiatrists who did not submit Medicare claims in 1997 Age: 1997 70.2; 1998 69.5 Gender: 60% f | |
| Interventions | Intervention 1: in 1997 the solo practitioners were randomised to receive either 4 reminders or none; group practices also randomised to receive 4 reminders or none; in 1998 within each practice group intervention and control groups were switched Control: no reminders in alternate years | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: all Medicare claims for influenza vaccination in 1997 and 1998 % vaccinated by 31 December 1998 | |
| Notes | Funding: Health Care Financing Administration We entered the vaccination uptake in the control groups in 1997 as the baseline prior year uptake for the intervention group in 1998; the 1998 trial was a cross‐over of the 1997 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We performed a randomised crossover trial..." E‐mail from author: "This project was done through Medicare's Division of Clinic Standards and Quality as a quality improvement project. I think that we went to a table of random numbers assigned each provider a random number. The even numbers got one arm, the odd number got the other arm" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | E‐mail from author: "Staff were blinded to the allocation." Outcome was influenza Medicare claims |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all 1997 and 1998 participants |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Clayton 1999
| Methods | Purpose: to compare educational material plus postcard to educational materials to encourage influenza vaccination Design: RCT, households randomised Duration of study: October to December 1997 Interval between intervention and when outcome was measured: October to December 1997 Power computation: 99% power to detect 5% difference Statistics: binomial test for differences in proportions; Chi2 for association between demographic variables and group assignment | |
| Participants | Country: USA Setting: Kaiser Permanente Northeast Eligible participants: (health status); 10,700 ≥ 65 Age: 73.5 Sex: 57% f | |
| Interventions | Patients with a record of influenza vaccination previous year (n = 5278) Intervention 1: mailed educational materials plus reminder postcard (N = 2631) Intervention 2: mailed educational materials (N = 2647) Patients with no record of influenza vaccination previous year (n = 5422) Intervention 1: mailed educational materials plus reminder postcard (N = 5422) No control group | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: October to December 1997 % vaccinated by: December 1997 | |
| Notes | Funding: Kaiser Permanente | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... half were randomly selected to receive the postcard reminder in addition to the standard member educational materials (intervention group), and the other half did not receive a postcard (control group)." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "... the vaccination rates were estimated through administrative data." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Because the sensitivity of administrative data is somewhat limited (estimated to be 62.4%, according to Kaiser Permanente Northeast Division studies), the vaccination rates presented are underestimates of the true rates." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Dalby 2000
| Methods | Purpose: to compare encouragement by visiting nurse to receive influenza vaccination to no intervention Design: RCT Duration of study: 14 months Interval between intervention and when outcome was measured: within 14 months of study Power computation: α = 0.05, β = 0.8, difference = 15%, requires n = 128 Statistics: Chi2, Fisher's exact; Student's t‐test, Mann‐Whitney U test | |
| Participants | Country: Canada Setting: practices of 2 physicians in Stoney Creek, Ontario Eligible participants: (health status): individuals ≥ 70 and functional impairment or admission to hospital or bereavement in past 6 months Age: ≥ 70, avg 78.5 Gender: 71% f in nurse group, 62% in control | |
| Interventions | Intervention 1: encouragement by visiting nurse during comprehensive assessments to receive influenza vaccination, care plan developed with physician Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 14 months, dates not stated % vaccinated by: not stated | |
| Notes | Funding: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned ... by a research assistant not affiliated with the HSO using a random number table. The randomization schedule was developed by another research assistant, who was not involved in the randomization process." |
| Allocation concealment (selection bias) | Low risk | "The randomizations schedule was kept within the Health Services Delivery Research Unit of the St. Joseph's Community Health centre throughout the trial." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "... a research nurse conducted a detailed audit of all participants' medical records" |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Dapp 2011
| Methods | Purpose: to assess the effects of health risk appraisal, personal reinforcement and quality circles for older people to improve preventative care and health behaviour Design: RCT (patients of solo GPs individually randomly assigned by computer to intervention or control). The 21 solo GPs were allocated to 3 clusters of GPs matched by age, gender and qualification Duration of study: recruitment over a 9‐month period. Follow‐up at 1 year (duration of intervention not stated) Interval between intervention and when outcome was measured: follow‐up at 1 year (duration from end of intervention not stated) Power computation: 763 in intervention and 1525 required in control to detect 30% difference in preventive care or health behaviour, alpha = 0.05, power = 80%, assuming 20% preventive behaviour in controls and 20% drop‐out Statistics: generalised estimating equations; for missing data multiple imputations |
|
| Participants | Country: Germany Setting: 21 solo GP practices in Hamburg Eligible participants: (health status): 500 GP practices in Hamburg, 21 agreed to participate; each practice provided completed list of ≥ 60, and "eligibles" from practices who returned brief questionnaire and consent form were randomised (total n eligibles not stated); 2580 randomised and 746 who were not randomised were placed in a "concurrent comparison" group Age: avg 72 Gender: 62% f |
|
| Interventions | Intervention 1: health risk appraisal, individualised recommendations, health information, reinforcement by home visit or group sessions Control: usual care (but GPs had received training to care for the intervention group patients) Comparison group: usual care, no training provided to GPs Co‐interventions: none |
|
| Outcomes | Outcome measured: % influenza vaccination (and 8 other preventive care outcomes and 6 health behaviours) Time points reported in the study: follow‐up 1 year, time from end of intervention to follow‐up not stated |
|
| Notes | Funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer based at independent centre (patients individually randomised within solo GP practices, GPs were allocated ‐ 7 to intervention, 7 to control and 7 to "concurrent comparison" group) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not possible as treating GPs received summary statements about patients as part of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total eligibles not stated; 2580 baseline in RCT (878 intervention, 1702 control), baseline characteristics similar, 746 in "concurrent comparison" group; at 1 year follow‐up 587 (70.6%) and 1376 (83.8%) in control group returned questionnaire; no differential attrition analysis |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Dietrich 1989
| Methods | Purpose: to compare effects of reminder letters and checklists to obtain influenza vaccination to no intervention Design: RCT, participants randomised Duration of study: enrolment during 3 months in "fall of 1984" Interval between intervention and when outcome was measured: 12 months before and after randomisation Power computation: not performed Statistics: t tests; Chi2 | |
| Participants | Country: USA Setting: community practice in New England with 5 family physicians and 1 internist Eligible participants: (health status) > 65 with office visits during 3‐month enrolment period in 1984; exclusions: no telephone, transient, blind, demented, terminally ill; 156 potential participants, 31 not eligible; 117 returned baseline questionnaire; 2 died and 1 moved during study Age: 74 Gender: 68% f | |
| Interventions | Intervention: mailed personal prevention checklists, letters encouraging use of checklists to keep track of preventive health care Control: no intervention | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: 12 months before and after randomisation % vaccinated by 12 months after randomisation | |
| Notes | Funding: American Academy of Family Physicians and US Public Health Service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were assigned randomly" (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, chart audit for vaccinations (not stated who performed chart audit, but was retrospective), and questionnaires for vaccination received elsewhere |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 114 recruited patients were followed to the end of the study; chart audit for vaccinations, and questionnaires for vaccination received elsewhere |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Díaz Grávalos 1999
| Methods | Purpose: to compare personalised postcard to encourage influenza vaccination to no intervention Design: RCT, participants randomised Duration of study: 1 October to 4 December 1998 Interval between intervention and when outcome was measured: 1 October to 4 December 1998 Power computation: p1 = 0.05; p2 = 0.15, α = 0.05, ß = 0.90, requires n = 152 Statistics: RRs, 95% CIs | |
| Participants | Country: Spain Setting: San Cristovo de Cea, Ourense Eligible participants: (health status): residents ≥ 65 (n = 640) who had not been vaccinated after 50 days (3/4 of influenza vaccination campaign) had elapsed, and 162 were randomly assigned to receive a reminder postcard Age: ≥ 65, avg 76.5 Gender: 58.6% f | |
| Interventions | Intervention 1: personalised postcard to encourage influenza vaccination Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 1 October to 4 December 1998 % vaccinated by: 4 December 1998 |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... aleatorio simple, mediante tabla de números aleatorios generada por EPIDAT" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on how many of the 162 were assessed at the end of the study although "... se siguieron controlando todas las vacunaciones" |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Frank 2004
| Methods | Purpose: comparison of opportunistic on‐screen reminders to physicians about preventive care compared to no reminders Design: RCT Duration of study: 9 March 1998 to 8 March 1999 Interval between intervention and when outcome was measured: between 9 March 1998 to 8 March 1999 Power computation: not performed Statistics: univariate binomial regression with GEE; ITT analysis (Very helpful e‐mail from Dr. Frank, 23 August 2008: "Our study looked at whether each opportunity to provide a preventive service in a consultation was taken. This is a different way of looking at the question from the more usual approach of asking what proportion of participants who had attended during the influenza immunization season had received the vaccine by the end of the season (in other words, efficacy), or from asking what proportion of participants of the practice had received the vaccine by the end of the season (effectiveness) We were interested in what happened in each consultation in which influenza vaccination was indicated and due for the patient. We were able to do this very data‐intensive exercise only because we set out to use a practice that kept all clinical and billing data electronically and because I custom wrote software to analyze the practice's electronic data automatically. To my knowledge, this study is unique in its intensive automated analysis of each consultation The GPs actually performed slightly worse when reminded to give influenza vaccine. We don't know why this occurred, but it may be because the rate of giving influenza vaccine to participants 65 years and over in Australia was already quite high, possibly making our reminders redundant In our approach, we were not interested in numbers of participants, but in the number of opportunities that arose in consultations for the participants who did attend. Our approach to examining the question of opportunistic performance of preventive services is almost unique, in that we looked closely at every opportunity that arose, and did not take a snapshot of the practice population at one point in time, which is what almost all other studies have done. In retrospect, it would have been useful to collect data about efficacy so that we could compare our results more easily with those other studies.") | |
| Participants | Country: Australia Setting: urban practice with 10 GPs Eligible participants: (health status): 10,507 for all reminder activities, of whom 1847 were ≥ 65 and eligible for the influenza intervention Age: ≥ 65 Gender: 57% f | |
| Interventions | Intervention: computer‐generated reminder Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 9 March 1998 to 30 June 1998 (these dates are from e‐mail from author) % vaccinated by 30 June 1998 | |
| Notes | Funding: not stated (PhD thesis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All quotes are from e‐mail from author 18 August 2008: "Randomization of participants was automated. Patients were randomised by the last digit of their family's five digit number within the practice. Family numbers had been allocated sequentially by the practice's computer system without regard to any characteristics of the patient or the family. We were satisfied that this method was not likely to cause any bias in the randomization." |
| Allocation concealment (selection bias) | Unclear risk | "Allocation was not concealed. However, I believe that in the daily rush of seeing participants, most of the GPs were unlikely to have had time or energy to look at the patient's family number in order to work out to which group the patient had been randomised." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Blinding, in the sense of blinding the investigators, was not necessary because the judgement of whether a preventive activity (including the administration of influenza vaccine) had been performed was made by searching the practice's electronic clinical record automatically". "Vaccinations were recorded by the doctors in their clinical record system's immunization module which used coded data entry to make the entries consistent and therefore machine‐searchable. If our search found a record of influenza vaccine being given between 9th March (the start of our trial) and the end of June (the end of the useful immunization season), this was counted as influenza immunisation having been performed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We analysed all data by intention to treat. All participants who were enrolled and randomised (both of which occurred automatically at their first visit during the trial) were included in the analyses." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Garcia‐Aymerich 2007
| Methods | Purpose: evaluate the effects of an integrated care intervention on outcomes of patients with COPD Design: RCT ‐ patients randomised Duration: 1 year Power computation: not performed Statistics: "Results are expressed as mean (SD), median (P25–P75), or as number (percentage) in the corresponding categories. To assess the possibility of selection bias, comparisons of baseline characteristics between UC and IC, both for the followed‐up and for the lost subjects were performed using independent t‐tests, Kruskal–Wallis test or the Chi‐square test" |
|
| Participants | Country: Spain Setting: Barcelona tertiary hospital Participants: 113 COPD patients discharged from hospital Age: avg 73 Gender: 84% male |
|
| Interventions | Intervention group received: 1. "a comprehensive assessment of the patient at discharge...by a specialized nurse"; 2. a 2‐hour education session focusing on disease education, treatment, self management,social support and call centre support; 3. tailored treatment plan, home visit by specialised nurse and primary care team within 72 hours after discharge and follow‐up phone calls at 3 and 9 months to reinforce self management strategies; and online access to a specialised nurse Control group received usual care Participants in intervention and control group were assessed via a questionnaire | |
| Outcomes | No significant difference in influenza vaccination uptake between intervention and control (90% versus 78%, P = 0.442) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned;" "blindly assigned (1:2 ratio) using computer generated random numbers either to integrated care (IC) or to usual care (UC)." |
| Allocation concealment (selection bias) | Low risk | "blindly assigned (1:2 ratio) using computer generated random numbers either to integrated care (IC) or to usual care (UC)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "blindly assigned (1:2 ratio) using computer generated random numbers either to integrated care (IC) or to usual care (UC)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21/44 of integrated care and 41/69 conventional care assessed at 12 months; "subjects who were lost for the present analysis had a higher number of COPD admissions in the previous year and in the follow‐up year, and they were using long‐term oxygen therapy in a higher proportion than those subjects who participated in the 12 months assessment." (no differential analysis by group) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Herman 1994
| Methods | Purpose: to compare patient education to patient education and vaccination by nurses before the participants were seen by the physician and to no intervention Design: RCT Duration of study: 1 October 1989 to 31 March 1990 Interval between intervention and when outcome was measured: 1 October 1989 to 31 January 1990 Power computation: not performed Statistics: Chi2; ANOVA; logistic regression controlling for prior baseline vaccination status, age, race, gender, high risk comorbidity and physicians' level of training | |
| Participants | Country: USA Setting: Metro‐Health Medical Center, teaching hospital of Case Western Reserve University Participants: (health status) 1202 participants > 65 seen during 1988/9 and 1989/90 influenza seasons, of whom 756 seen during both seasons Age: 74 Gender: 69% f | |
| Interventions | Intervention 1 "patient education group": educational materials (background papers, guidelines, lectures) plus nurses educated participants with National Institute on Aging "Shots for Safety" and material on influenza vaccination from Ohio Dept of Health Intervention 2 "prevention team group": same as 1 but nurses allowed to vaccinate participants before seen by doctor and maintained health maintenance flow sheet for each patient Control: no intervention for participants Co‐interventions: physicians and nurse practitioners in all 3 groups received educational materials and opportunities to attend lectures | |
| Outcomes | Outcome measured: % vaccinated, by billing data, researcher chart review, health maintenance flow sheets Time points from the study that are considered in the review or measured or reported in the study: 1 October 1989 to 31 January 1990 % vaccinated by: 31 January 1990 | |
| Notes | Funding: Case Western Reserve University Teaching Nursing Home Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The three ... practices were assigned randomly" (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | However, daily billing forms reviewed by trained research assistant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 1202 participants analysed |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Hogg 1998
| Methods | Purpose: to compare customised letters recommending preventive procedures to form letters and to no intervention Design: RCT, participants randomised, then entire family included in the intervention group to which the individual patient had been randomised Duration of study: letters sent September 1990 to March 1991; data collected months after letters sent Interval between intervention and when outcome was measured: 6 months Power computation: the smallest increase to be detected was for Pap smears, so sample powered with α = 0.05, β = 0.8 (% difference to be detected not stated), with allowance for participants who would leave the practice Statistics: Chi2, ANOVA, Kruskal‐Wallis one‐way ANOVA | |
| Participants | Country: Canada Setting: Wakefield Family Medicine Centre, western Québec Eligible participants: (health status); 8770 families, from whom 719 families randomly selected; "The random selection of the study sample was applied to individual patient registration numbers in the medical record software system." Age: ≥ 65 Gender: not stated separately for ≥ 65 | |
| Interventions | Intervention 1: customised letters recommending preventive procedures Intervention 2: form letters recommending preventive procedures Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: letters sent September 1990 to March 1991; data collected months after letters sent % vaccinated by: September 1991 | |
| Notes | Funding: National Health Research & Development Program, Health Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study used a randomised controlled trial design." "Once an individual was selected, his or her entire family was randomly assigned to one of the three arms of the study." (method not stated) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The study was not blinded in that physicians could be aware that a patient was a member of a family in the study if the patient mentioned that the family had received a letter." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 682 randomised to no letter, 676 to form letter and 613 to customised letter; final comparison among groups (Table 2) lists 249, 245, 192; initial randomisation resulted in unevenly sized groups with fewer in the control group |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Hogg 2008
| Methods | Purpose: to compare a comprehensive preventive intervention programme to no intervention Design: cluster‐RCT, match‐paired; "The unit of randomization and analysis was the practice; the unit of observation was the patient." Duration of study: 11.5 months Interval between intervention and when outcome was measured: "The intervention lasted 11.5 months." "Data were collected ... up to 2 months after the intervention." Power computation: 24 practices were needed to detect a mean difference of 0.07 in the primary outcome between intervention and control groups ("The delta selected (0.07) approximates the 10% change in care frequently associated with care improvement interventions"), SD = 0.083, α = 0.05, β = 0.83, and 27 practices were recruited to allow for 15% attrition Statistics: Chi2, paired t‐tests | |
| Participants | Country: Canada Setting: 2 letters and brochure to 351 primary care practices in eastern Ontario; 54 practices participated Eligible participants: (health status): ≥ 65 Age: ≥ 65 Gender: not stated | |
| Interventions | Intervention 1: comprehensive preventive intervention programme; facilitators were assigned 13 to 14 practices and visited them monthly, average duration of visit 46 minutes; facilitators encouraged 26 preventive manoeuvres; with baseline audit, feedback and consensus building, and periodic follow‐up and consensus building Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination for each practice Time points from the study that are considered in the review or measured or reported in the study: "The intervention lasted 11.5 months." "Data were collected ... up to 2 months after the intervention." % vaccinated by: "up to 2 months after the intervention" | |
| Notes | Funding: Canadian Institutes of Health Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were matched on solo versus group practice, presence of nursing staff and location (rural or urban) and each pair member was randomly assigned using the Statistical Analysis software package." |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was kept locked and unavailable to the administrative staff until the time of assignment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Physicians and facilitators were blinded to the actual manoeuvres that would be included in the preventive performance index." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 practices randomised, data from 54 analysed (27 intervention, 27 control practices) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Hull 2002
| Methods | Purpose: to compare phone call by receptionist to attend influenza vaccination clinic to no intervention Design: RCT Duration of study: 25 September to 6 October 2000 Interval between intervention and when outcome was measured: data on influenza vaccination status was submitted mid‐December 2000 Power computation: for α = 0.05, β = 0.8, would require 384 participants to show increase in vaccination uptake from 40% to 50% Statistics: Chi2, ITT, generalised linear models for clustered data | |
| Participants | Country: UK Setting: 3 general practices in East London and Essex Eligible participants: (health status); 1820 participants 65 to 74 not previously in an influenza vaccination recall system; exclusions: asthma, diabetes, COPD, IHD, renal disease Age: 69 Gender: 54% f | |
| Interventions | Intervention 1: phone call by receptionist to attend influenza vaccination clinic Control: no intervention Co‐interventions: East London and City Health Authority sent letter to every patient ≥ 65 asking them to contact GP for influenza vaccination; national campaign September promoting influenza vaccination | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 25 September to 6 October 2000 % vaccinated by: 6 October 2001 | |
| Notes | Funding: ELENoR infrastructure grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... households, which were randomised to either the control or intervention group by the study co‐ordinator using a computer program (STATA)" |
| Allocation concealment (selection bias) | Unclear risk | "... households, which were randomised to either the control or intervention group by the study co‐ordinator using a computer program (STATA)" (unclear if, once randomised, study co‐ordinator referred back to randomisation lists) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Nurses who undertook the vaccination clinics were unaware of the household allocation to control or intervention group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | E‐mail from author: "We did an intention to treat analysis, all households in the original randomisation were included in the analysis." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Humiston 2011
| Methods | Purpose: to compare tracking patient influenza vaccination uptake, providing reminders, patient recall and outreach to patients to standard care in each of 7 clinics Design: RCT, individual seniors were randomised within each clinic to intervention or control Duration of study: 29 September to 13 October 2004 (depending on arrival of influenza vaccine) to 22 January 2004 Interval between intervention and when outcome was measured: 15 weeks Power computation: 170 patients/group to demonstrate 15% difference in vaccination uptake (control rate = 50%) P < 0.05, power 0.80, 2‐tailed; as interest was also to collect data across multiple sites and ethic groups, more patients were enrolled than required by power computation Statistics: Chi2, Fisher's exact, logistic regression; intention‐to‐treat |
|
| Participants | Country: USA Setting: 7 clinics in Rochester, NY Eligible participants: (health status): 2004 (control), 1748 (intervention); 50% White, 33% African American, 10% Hispanic, 7% Other Age: avg 74.2 Gender: 62% f |
|
| Interventions | Intervention 1: outreach workers in each of 7 clinics tracked patient influenza vaccination uptake, provided reminders, recalled patients, recalled and phoned patients Control: standard routine for each clinic Co‐interventions: none |
|
| Outcomes | Outcome measured: % influenza vaccination Time points reported in the study: from 29 September to 13 October 2004 (depending on arrival of influenza vaccine) to 22 January 2004 |
|
| Notes | Funding; Centers for Disease Control National Immunization Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "individual seniors within PCCs to intervention or standard‐of‐care control groups" according to whether last digit of SSN odd or even |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not possible due to recalls and prompts |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3752 eligibles randomised (patients who died during the trial were analyzed as randomised). However: "Each outreach worker was responsible for tracking approximately 900 to 1,000 eligible patients" (which implies for 7 clinics total eligibles = 6300 to 7000) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Ives 1994
| Methods | Purpose: to compare offer of free influenza vaccination in capitated care groups to fee‐for‐service care groups and to no offer Design: RCT, participants randomised Duration of study: 1 May to 31 December 1989 Interval between intervention and when outcome was measured: April 1991 to March 1992 Power computation: not provided Statistics: Chi2; logistic regression controlling for age, gender, marital status, education, insurance and intervention group | |
| Participants | Country: USA Setting: community‐dwelling Medicare beneficiaries 65 to 79 in rural Pennsylvania Eligible participants: (health status) 3884 enrolled in demonstration project, of whom 3606 (92.8%) completed follow‐up telephone interview; then limited study population to those interviewed between April 1991 and March 1992 = 1989 community‐dwelling Medicare beneficiaries 65 to 79. Exclusions: institutionalised, non‐ambulatory, life‐threatening dx cancer in previous 5 years Age: 65 Gender: not stated | |
| Interventions | Intervention 1: patients participating in capitated payment group: after health risk appraisal interview randomly assigned to offer of no cost influenza immunisation Intervention 2: patients participating in fee‐for‐service group; after health risk appraisal interview randomly assigned to offer of no cost influenza immunisation; physicians only paid if they received and submitted payment voucher from participants Control: given their health risk appraisals but not offered immunisation This helpful e‐mail was received from Dr. Diane Ives: "Regarding the issues of bias, this was a community based demonstration project to see if Medicare beneficiaries would use prevention programs if offered at no cost. Everyone enrolled in Medicare Part B was potentially eligible and contacted to invite participation. Due to the nature of the programs, it was impossible to blind the providers or participants. However, subjects were randomly assigned to one of the 3 comparison groups (hospital based, physician based and control/no free services), with the exception that spouse pairs were assigned to the same group for feasibility of both using the services. The 2 references below detail the characteristics of people who came into the program based on various recruitment methods, and also describe those who did not participate. We found people who participated had more disease history and risk factors, people who were contacted but refused to participate were the healthiest and possibly refused because they felt they did not have the risk factors targeted by the interventions, and those unable to be reached had highest levels of disease based on Medicare claims data and may have been too ill to participate Ives DG, Kuller LH, Schulz R, Traven ND, Lave JR. Comparison of recruitment strategies and associated disease prevalence for health promotion in rural elderly. Preventive Medicine 1992;21:582‐591 Ives DG, Traven ND, Kuller LH, Schulz R. Selection bias and nonresponse to health promotion in older adults. Epidemiology 1994;5:456‐461." | |
| Outcomes | Outcome measured: % vaccinated, measured by self report and by completed flu vouchers for payment to physician by Medicare Time points from the study that are considered in the review or measured or reported in the study: April 1991 to March 1992 % vaccinated by March 1992 (2.5 years after study began, 1.5 years after offer of influenza vaccine) | |
| Notes | Funding: Health Care Financing Administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... participants were randomly assigned" (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Measured by self report, but also by completed flu vouchers for payment to physician by Medicare |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 1989 participants enrolled were analysed |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Karuza 1995
| Methods | Purpose: to compare focus groups of physicians discussing adoption of influenza guideline for participants ≥ 65 to focus groups of physicians about an unrelated topic Design: RCT, practices as the unit of randomisation Duration of study: 4 months Interval between intervention and when outcome was measured: 4 months Power computation: not performed Statistics: ANOVA for differences in uptake between study arms | |
| Participants | Country: USA Setting: Health Maintenance Organisation in Buffalo, NY Eligible participants: (health status) 13 practices in prepaid Health Maintenance Organisation in Buffalo, NY; all physicians volunteered to participate; 8 physicians dropped out due to sickness or reassignment, and 6 physicians were omitted as they did not have 5 eligible participants Age: participants were > 65, not institutionalised Gender: 63.5% f | |
| Interventions | Intervention 1: focus group of physicians with expert presenting guideline of Immunisation practices of the Advisory Committee of the Centers for Disease Control and Prevention, with discussion with facilitator, with a plan that intervention practices would develop their own methods such as reminder letters to participants or reminders on charts Intervention 2: focus group on non‐influenza topic (steroid use and GI bleeding) Control: none | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: pre‐intervention base uptake measured 1 October 1990 through 31 January 1991; intervention uptake measured during vaccination season 1 October 1991 to 31 January 1992 % vaccinated by 31 January 1992 | |
| Notes | Funding: US Bureau of Health Professions, US Health Resources and Services Administration, and Agency for Health Care Policy and Research, US Public Health Service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Thirteen group practices and their primary care physicians (mean size, 5) were assigned randomly to intervention or control arms." |
| Allocation concealment (selection bias) | Low risk | "The vaccination data were obtained through prechart and postchart reviews conducted at these sites by trained outside reviewers." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The vaccination data were obtained through prechart and postchart reviews conducted at these sites by trained outside reviewers." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Active participants who were not seen during the influenza vaccination season were counted as not receiving the vaccine." "...10% of the charts were reviewed again by a different reviewer. For the key measures the inter‐judge reliability of the chart review was better than 98% agreement." "Because of expected patient attrition (e.g. mortality, moving out of town, and changing physicians) and clerical error, an average of 11% of the charts was unavailable at the post chart review per physician." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Kellerman 2000
| Methods | Purpose: to compare a phone call reminder about influenza vaccination or no intervention Design: RCT, participants randomised Duration of study: 23 September to 23 October 1996 Interval between intervention and when outcome was measured: 1 month Power computation: not performed Statistics: percentages, probabilities | |
| Participants | Country: USA Setting: Smoky Hill Family Practice Center, Salina, Kansas Eligible participants: (health status): all 475 individuals ≥ 65 were sent a postcard reminder, eligibles are those who did not respond; exclusions = those resident in nursing homes Age: ≥ 65 Gender: not stated | |
| Interventions | All 475 individuals ≥ 65 were sent a postcard reminding them about influenza vaccination; non‐respondents were then randomised to either: Intervention 1: 1 to 2 phone calls Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 23 September to 23 October 1996 % vaccinated by: 23 October 1996 | |
| Notes | Funding: no funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate randomisation of alphabetised households |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Vaccination uptake for the whole practice for the 2 preceding years are provided, but not for the intervention and control groups. Not stated how immunisation data were recorded or whether the practice was computerised (however, participants were all ≥ 65 and thus Medicare beneficiaries so there was an incentive to record data to obtain payment) "For the purposes of this study, only immunizations administered at the Family Practice Center were considered in assessing the study's outcome. During the telephone intervention, Family Practice Center staff recorded any patient comments about prior immunization for that season or subsequent intentions for immunization." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Kerse 1999
| Methods | Purpose: to compare an educational programme for General Practitioners about social and physical activity, prescribing and vaccination practices for elderly participants with audit, to no intervention Design: RCT, general practices were unit of allocation Duration of study: November 1995 to April 1997 Interval between intervention and when outcome was measured: November 1995 to April 1997 Power computation: website stated 93 participants needed in each group to detect 20% change with α = 0.05, β = 0.8, allowing for clustering Statistics: ITT. "We adjusted for the effect of clustered design with a cross sectional time series iterative programed least squares regression." | |
| Participants | Country: Australia Setting: 42 GPs in Melbourne Eligible participants: (health status) a number was assigned to 398 GPs in metropolitan Melbourne then randomly selected 193 with no computerised recall system for influenza vaccination; exclusions from the 193 were: 6 were not contactable, 25 moved or had died, 28 had partners already enrolled in trial, 25 worked < 12 hours/week, 7 were retiring, 13 had no elderly participants or participants who did not speak English, and 7 had computerised recall systems. Then 42 of 82 eligibles were enrolled; then using random number table average 397 charts were reviewed per practitioner and 10 elderly participants identified per practitioner; 267 (64%) of invited participants participated Age: ≥ 65 Gender: 54% f | |
| Interventions | Intervention 1: educational programme in 5 stages for GPs about social and physical activity, prescribing and vaccination practices for elderly participants Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: November 1995 to February 1996 and at 1‐year follow‐up (December 1996 to April 1997) % vaccinated by: April 1997 E‐mail from Dr. Kerse indicated data on baseline influenza uptake for the year before the intervention would be supplied but further e‐mail not received | |
| Notes | Funding: Victoria Health Promotion Foundation; doctoral scholarship for Dr. Kerse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent research assistant at a distant site used computer randomisation to allocate general practitioners to intervention or control group and this was concealed until the interview began." |
| Allocation concealment (selection bias) | Low risk | "An independent research assistant at a distant site used computer randomization to allocate general practitioners to intervention or control group and this was concealed until the interview began." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Interviewers evaluating outcomes were blinded to the intervention group of participants and general practitioners at all times, and participants were unaware of the group allocation of their general practitioner." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In Table 1 135 participants are listed in the intervention group (but only 120 are listed as either "yes" or "no" for influenza vaccination) and 132 in the control (but only 112 listed "yes" or "no" for influenza vaccination status) "Influenza vaccination rates increased by almost 10% in both groups" (but no n's for these outcomes are cited) After 1 year 34 participants could not be followed up, and they were correctly counted in the groups to which they were randomised in an ITT analysis Immunisation data ascertained by chart review (all practices were deliberately selected as being not computerised) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Kiefe 2001
| Methods | Purpose: to compare a multimodal improvement intervention with chart review and feedback to physicians, to the same intervention plus feedback about the performance of the top 10% of physicians Design: RCT, physicians randomly assigned; 20 records for each physician randomly assessed at baseline and a different set of 20 records at follow‐up Duration of study: baseline was performance of physicians 1 January 1994 through 30 June 1995; intervention during 1996; follow‐up through 30 June 1998 Interval between intervention and when outcome was measured: 1 January 1997 to 30 June 1998 Power computation: (E‐mail from author Dr. C Kiefe; "We did perform an a priori power computation to have at least 80% power to detect an effect on at least one of the indicators. Because the study was positive, this became meaningless and we did not include this is the paper.") Statistics: t tests; generalised linear models with nesting of participants within physicians and controlling for baseline performance (no adjustments for patient characteristics as "each quality measure specified a group of participants who were ideal candidates for intervention") | |
| Participants | Country: USA Setting: 561 eligible physicians in Alabama Eligible participants: (health status) random sample of 97 Alabama fee‐for‐service physicians (of whom 70 completed the study; the 27 who did not complete the study practised in a different environment, or were retired or deceased) from a group of 561 Alabama family physicians, internists and endocrinologists. The 70 physicians had 2978 diabetic participants. Exclusions were: end‐stage renal disease, in a skilled nursing home, dead at baseline. (E‐mail from author Dr C Kiefe: "Community physicians who were participating in CMS (then [Alabama Health Quality Assurance Foundation] HCFA) Ambulatory Care Quality Improvement Project (ACQIP). The analyses were at the patient level, because the outcomes were measured at the patient level. Patients were Medicare beneficiaries with diabetes.") Age: average age 76 Gender: not stated; ("We have archived the original data and we could find the exact % female, but it would be fairly burdensome. I seem to remember that this older Medicare population had about 75% women") | |
| Interventions | Intervention 1: Ambulatory Care Quality Improvement Project; physicians given performance feedback on diabetes care, then quality improvement (n = 49 physicians, 14 lost to follow‐up) Intervention 2: same as 1 + achievable benchmark based on performance of top 10% of physicians being assessed (n = 48 physicians, 13 lost to follow‐up) No control group | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: baseline was performance of physicians 1 January 1994 through 30 June 1995; intervention during 1996; follow‐up 1 January 1997 to 30 June 1998 % vaccinated by: 20 June 1998 | |
| Notes | Funding: Agency for Health Care Research and Quality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... this group‐randomized trial"; (E‐mail from author Dr. C Kiefe: "We randomised the physicians and then reviewed the medical records of their participants to ascertain whether flu vaccine was documented.") |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement, but vaccination status assessed by chart review using protocol tested by pilot |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes for physicians who did not complete study not presented. E‐mail from author Dr. C Kiefe: ("It was not possible to review records for physicians who no longer wished to participate or were lost to follow‐up.") |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Kim 1999
| Methods | Purpose: to compare the effect of providing education, peer‐comparison feedback and academic detailing to physicians with providing education to physicians, on the number of preventive services and the % of participants to which they were offered Design: RCT, physicians randomised to the 2 interventions Duration of study: 2.5 years Interval between intervention and when outcome was measured: February 1992 to February 1994 Power computation: not performed Statistics: mixed model ANOVA, participants nested within physicians | |
| Participants | Country: USA Setting: Kaiser Permanente Woodland Hills HMO San Fernando Valley, California Eligible participants: (health status) 48 family physicians, internists and sub‐specialists providing primary care for at least 60 participants (of whom 7 dropped out leaving 41); 9233 participants were 65 to 75 and eligible; surveys mailed to a random sample of 3249, of whom 2237 completed baseline and follow‐up surveys, 299 then excluded as their physician left the group, sample = 1810 participants Age: avg 73 Gender: participants 50% f | |
| Interventions | Intervention 1: mailed educational materials about 7 preventive care services Intervention 2: same as 1 + anonymous 15 minutes academic detailing and peer‐comparison feedback from pharmacist at beginning of study and 6 and 12 months later Control: no control group | |
| Outcomes | Outcome measured: % vaccinated; measured by chart review and patient survey (23% to 26% over‐estimation by participants compared to chart review) Time points from the study that are considered in the review or measured or reported in the study: surveys of participants January to May 1992, and December 1995 to January 1996 Vaccinated by: January 1996 | |
| Notes | Funding: Sidney Garfield Memorial Fund, S Kaiser Permanente | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... physicians were randomly assigned" (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, but chart review by 4 trained personnel using standardised forms, interrater reliability = 100% |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2337 participants completed both baseline and follow‐up surveys, but outcomes for the 7 physicians who dropped out and their 128 participants, and a further 299 participants because their physician was not part of the group, are not presented; and final outcome data are presented only for 1810 participants |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Kouides 1998
| Methods | Purpose: to assess the effect of financial incentives to physicians for influenza vaccinations on achieving vaccination targets Design: RCT, physician practices randomised Duration of study: September 1991 to 1 January 1992 Interval between intervention and when outcome was measured: September 1991 to 1 January 1992 Power computation: not performed Statistics: t tests for normally distributed continuous variables; Wilcoxon Rank sum tests for nonparametric variables; Chi2, Fisher's exact test for discrete variables; multiple linear regression, controlling for number of elderly participants in the practice, type of practice, percent immunised in baseline year 1990, routine use of phone calls, postcards or flowcharts as reminders for preventive services, and total number of visits by study personnel to the practice | |
| Participants | Country: USA Setting: Medicare Influenza Demonstration Project, Monroe County, NY Eligible participants: (health status) 54 practices. Exclusions were physicians who provided care to < 50 participants, did not participate in Medicare Influenza Demonstration Project, or had participated in a previous study Age: > 65 Gender: not stated | |
| Interventions | Intervention: physicians received free influenza vaccine, were paid USD 8 per vaccination, were asked to enter cumulative weekly vaccinations on an office poster (target population = all active non‐nursing home participants with office visits 1991 or 1992); if they achieved 70% vaccination coverage they received an additional USD 0.80 per vaccination for vaccinations given in their office, and if they achieved 85% coverage they received an additional USD 1.60 per vaccination Control: no intervention Co‐interventions: extensive community media campaign, beneficiary letters to all Medicare recipients, extended schedule for public vaccination clinics (Koudies 1993 describes a non‐randomised study comparing patient vaccination uptake for physicians admitting to 2 hospitals) | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: September 1991 to 1 January 1992 % vaccinated by: 1 January 1992 | |
| Notes | Funding: Medicare Influenza Demonstration Project, Monroe County, NY | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All physicians...were randomised." (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, but vaccination status measured by Medicare billing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat with intervention group n = 21,196 and control group n = 17,608 |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Krieger 2000
| Methods | Purpose: to assess the effect of peer‐to‐peer telephone outreach by seniors to increase vaccination uptake Design: RCT, seniors randomised Duration of study: baseline survey September 1996; intervention 3rd week of October 1996 for 6 weeks; follow‐up survey March 1997 Interval between intervention and when outcome was measured: intervention 3rd week of October 1996 for 6 weeks; follow‐up survey March 1997 Power computation: "We estimated that 1000 participants divided into 2 groups of equal size would provide at least 80% power to detect a 25% difference in the proportions of subjects receiving a recommended immunization, given control‐group immunization uptake ranging from 40%–80% and a 5 0.05. Analyses included only the 1083 participants who completed both surveys." Statistics: "The chi‐square (with Yates correction), t test, analysis of variance, and Wilcoxon matched‐pairs signed‐rank and rank‐sum procedures were used to test for differences between groups, and McNemar test was used for assessing baseline to follow‐up differences within groups." | |
| Participants | Country: USA Setting: Seattle Partners for Healthy Communities Seattle Senior Immunization Project Eligible participants: (health status) recruited from senior centre and a marketing database of seniors in 5 contiguous zip codes; 5512 invited; of whom 1246 (23%) completed baseline survey; 163 (13%) dropped out Age: avg age 75 Gender: intervention 42.8% f; control 47.8% f | |
| Interventions | Intervention 1: mailed educational brochure, senior volunteers called 25 participants using script (4 hours training), follow‐up phone call, plus same interventions as control Control: usual senior centre and community immunisation newspaper articles, health fair, pamphlets, posters, media announcements, mailed letter from regional Medicare office to 10% of seniors, vaccine available at senior centre | |
| Outcomes | Outcome measured: % vaccinated, self report by survey (medical records were not audited because seniors obtained influenza vaccination from several locations) Time points from the study that are considered in the review or measured or reported in the study: baseline survey September 1996; intervention 3rd week of October 1996 for 6 weeks; follow‐up survey March 1997 % vaccinated by: March 1997 | |
| Notes | Funding: Centers for Disease Control and Prevention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... systematic allocation of alternate respondents to either control or intervention" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Volunteers ... made a follow‐up contact to ascertain whether immunization(s) were received." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 163 (13%) lost to follow‐up, similar proportions in intervention and control groups; "computerized registry to track the contact and immunization status of each subject." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Kumar 1999
| Methods | Purpose: to assess the effect of a physician‐targeted intervention to increase the influenza vaccination uptake among seniors Design: RCT, physicians randomised Duration of study: 1 September to 31 December 1997 Power computation: none provided Statistics: percentage of total Medicare beneficiaries immunised | |
| Participants | Country: USA Setting: Louisiana physician offices Participants: non‐HMO Medicare providers. 750 physicians assigned to intervention group; 1167 assigned to control group Age: patients >= 65 Gender: not reported |
|
| Interventions | Intervention group received a "... cover letter and their Medicare patient pool influenza immunization and missed opportunity indicator uptake in October 1997" and "... were encouraged to evaluate ways in which their practices might improve upon the baseline immunization status and were offered assistance in designing quality improvement projects to effect such a change. The information provided to the physicians included computed rates for all selected physicians which allowed them to compare their rates with rates of other physicians." The control group did not receive any educational or other materials | |
| Outcomes | % influenza vaccination Although the influenza vaccination uptake increased from 1996 to 1997 in both the intervention group (4.21% versus 5.23%) and the control (3.74% versus 4.5%) the intervention group uptake increased significantly more (P = 0.03) than that of the control |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly selected "intervention group" of physicians (n = 750)" and "... another group of physicians, with similar characteristics, was also randomly selected and designated as the "control group" (n= 1,167).)" (no statement about method of randomisation) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement, but outcomes ascertained from Medicare Part B claims |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Identified all Louisiana Medicare‐certified providers; analysed 1996 and 1997 Medicare Part B claims files for influenza vaccinations |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Lemelin 2001
| Methods | Purpose: to compare the effect of facilitators using 7 intervention strategies to encourage 8 recommended and discourage 5 not recommended preventive care manoeuvres, compared to no intervention Design: RCT, practices as unit of randomisation Duration of study: 18 months Interval between intervention and when outcome was measured: 18 months after last patient visit Power computation: 40 practices needed to detect mean difference of 0.09 in preventive performance index used in this study between intervention and control groups with α = 0.05, power = 80% Statistics: "Cross tabulations using Chi2 test and Fisher's exact test were used to examine categorical data and compare groups. We used Student's t‐test for independent groups for comparisons of continuous data. To test for significant differences in end points between the intervention and control groups, we analysed end points using GLE repeated‐measures ANOVA, where end points measured at baseline and follow‐up were treated as within‐subject factors ... and the intervention group was the between‐subjects factor ... Significant interaction effects were further analysed with a least‐significant‐difference post‐hoc test to evaluate mean differences. We used a GLE ANOVA to test for differences between the study groups in preventive performance index." | |
| Participants | Country: Canada Setting: Health Service Organisations in Ontario Eligible participants: (health status): 100 Health Service Organisations, of which 46 were recruited and 45 remained in study Age: Canadian Task Force on Preventive Care recommended ≥ 65 years Gender: 53.6% f | |
| Interventions | Intervention: facilitators used 7 strategies (audit and ongoing feedback, consensus building, opinion leaders and networking, academic detailing and education materials, reminder systems, patient‐mediated activities, and patient education materials) to increase uptake of 8 preventive care manoeuvres recommended by the Canadian Task Force on Preventive Care and discourage 5 not recommended Control: no intervention | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: intervention July 1997 to December 1998 % vaccinated by: 31 December 1998 E‐mail from Dr Bill Hogg: "Unfortunately the paper does not report the age break down of the participants in the intervention and control groups (only the average age) and so the information cannot be derived from the paper. I would have to go back to trial data to produce the numbers requested. I'm on sabbatical and away from home so can't manage this." | |
| Notes | Funding: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The primary care practice (1 to 6 doctors) was the unit of randomization and the unit of analysis." (no statement of method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The chart auditors were blinded as to the status of the practices and assessment of outcomes." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For the performance of preventive manoeuvres: "The concordance between auditors was 85.4% (kappa = 0.71) at baseline and 84.4% (kappa = 0.69) at follow‐up." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Lukasik 1987
| Methods | Purpose: to compare phone invitations to receive influenza vaccination to a statement of vaccine availability when participants "dropped in" to the clinic Design: RCT Duration of study: mid September to December 1985 Interval between intervention and when outcome was measured: 0 to 3.5 months Power computation: not performed Statistics: not stated, appears to be comparison of percentages | |
| Participants | Country: Canada Setting: university family medicine clinic in London, Ontario, Canada Eligible participants: (health status): participants ≥ 65 Age: ≥ 65, average not stated Gender: not stated | |
| Interventions | Intervention 1: phone call to participants to inform them that influenza vaccine was available and they could receive it during a regular visit or a vaccine clinic Intervention 2: invitation to receive influenza vaccine during "drop‐in" visit to clinic Control: historical data from 1983 and 1984 (not used in this review as they are historical controls with no information about secular trends) | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: mid September to December 1985 (date in December not stated) % vaccinated by: December 1985 (date not stated) | |
| Notes | Funding: no funding stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "After a random start participants were alternately assigned to each group, though related participants and those living in a single household were kept in the same group." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | High risk | "A brightly coloured sticker was applied to the charts of the entire study population as a reminder to the health‐care team that the study was under way and that they were expected to promote the flu vaccine." "The patients would be told, whether by telephone or in the office, that the vaccine was available, and that they would be given a shot if they wished." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The analysis was done with participants in their originally assigned groups ... an "intention to treat analysis." Vaccination ascertained by chart review by research collaborators, outcomes for all 243 patients were tracked |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
MacIntyre 2003
| Methods | Purpose: for hospitalised participants ≥ 65 to compare an alert system for hospital staff to vaccinate them against influenza and a reminder letter sent to their GP on the day of their discharge Design: RCT, individuals randomised Duration of study: for participants admitted May to September 1998 Interval between intervention and when outcome was measured: day of discharge (arm A) or 1 month and 3 months after discharge (arm B) Power computation: 100 required for 10% difference in vaccination with 95% confidence and 80% power Statistics: odds ratios | |
| Participants | Country: Australia Setting: Royal Melbourne Hospital Eligible participants: (health status); 606 participants ≥ 65 admitted to a Melbourne hospital; of whom 238 already vaccinated, 35 vaccination history not verified, 88 unable to obtain consent, 113 refused, leaving 131 consented Age: 74 Gender: 56% f | |
| Interventions | Intervention 1: reminder in chart and face‐to‐face reminder to nursing and medical staff Intervention 2: reminder to GP on day of discharge Control: no control group | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: from admission (May to September 1998) up to day of discharge for hospital arm and up to 3 months after discharge for GP arm 1 % vaccinated by day of discharge for hospital arm and 3 months after discharge for GP arm | |
| Notes | Funding: Department of Human Services, Victoria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... research nurse picked a sealed envelope from a randomization box" |
| Allocation concealment (selection bias) | Low risk | "... research nurse picked a sealed envelope from a randomization box" (so likely researchers not aware of allocation) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 244 eligibles, 131 consented; all those who consented followed through to randomisation and receipt of vaccine. Vaccination for those vaccinated in hospital arm ascertained by discharge records and for those in GP arm by phone call then letter to GP |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Maglione 2002a
| Methods | Purpose: to compare a letter and brochure to no intervention in Minnesota. Other interventions were a postcard compared to no intervention (Utah‐Nevada Maglione 2002c), a letter plus postcard compared to no intervention (Washington State, Maglione 2002d), and a letter to a postcard compared to a letter and postcard and to no intervention (New Jersey, Maglione 2002b) Design: RCT; Peer Review Organizations in US states are required to conduct quality improvement projects and report results as part of the Health Care Quality Improvement Project (HCQIP). Maglione 2002a searched the HCQIP database for these reports, and identified published reports about Montana (McMahon 1995a) and Wyoming (McMahon 1995b) and unpublished reports about Minnesota, Utah‐Nevada, New Jersey and Washington State. Authors independently abstracted, compared and resolved discrepancies in data for study design, number and characteristics of patients, setting. Location and target of intervention, time from intervention to outcome measurement and results Duration of study: not stated (McMahon 1995a and McMahon 1995b were 3 months) Interval between intervention and when outcome was measured: brochure or letter mailed: not stated. All 4 unpublished RCTs were reported as being performed in 1996 Power computation: not performed Statistics: percentages | |
| Participants | Total number: Minnesota (letter plus brochure 2924; no intervention 3343); Utah‐Nevada (postcard 25,000, no intervention 50,437); Washington State (letter plus postcard 16,082, no intervention 16,057); New Jersey (letter 16,000, postcard 16,001, letter plus postcard 16,000, no intervention 16,001) Setting: Minnesota, Utah‐Nevada, Washington State, New Jersey, all Medicare Part B beneficiaries Diagnostic criteria: % receiving influenza vaccination, validated by HCFA billing claims Gender: not stated Age: ≥ 65 Country: USA [Co‐morbidity not stated] [Socio‐demographics not stated] [Ethnicity not stated] [Date of studies 1996] |
|
| Interventions | Intervention 1: letter Intervention 2: postcard Intervention 3: brochure Control: no intervention [Integrity of intervention: not stated] |
|
| Outcomes | Outcome measured: % vaccinated as measured by HCFA billing claims Time points from the study that are considered in the review or measured or reported in the study: 1996 % vaccinated during 1996 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "RCT" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96% of those ≥ 65 are covered by Medicare Part B, which processes all billing claims for influenza vaccination |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Maglione 2002b
| Methods | Data are reported for New Jersey. For details see Maglione 2002a | |
| Participants | See Maglione 2002a | |
| Interventions | See Maglione 2002a | |
| Outcomes | See Maglione 2002a | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "RCT" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96% of those ≥ 65 are covered by Medicare Part B, which processes all billing claims for influenza vaccination |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Maglione 2002c
| Methods | Data are reported for Utah‐Nevada. For details see Maglione 2002a | |
| Participants | See Maglione 2002a | |
| Interventions | See Maglione 2002a | |
| Outcomes | See Maglione 2002a | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "RCT" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96% of those ≥ 65 are covered by Medicare Part B, which processes all billing claims for influenza vaccination |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Maglione 2002d
| Methods | Data are reported for Washington State. For details see Maglione 2002a | |
| Participants | See Maglione 2002a | |
| Interventions | See Maglione 2002a | |
| Outcomes | See Maglione 2002a | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "RCT" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96% of those ≥ 65 are covered by Medicare Part B, which processes all billing claims for influenza vaccination |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Marrero 2006
| Methods | Purpose: to compare an educational session about influenza and vaccination clinic in a pharmacy to "usual care" (no intervention) Design: RCT Duration of study: 12 months Interval between intervention and when outcome was measured: 12 months Power computation: not performed Statistics: percentages, ANOVA | |
| Participants | Country: Puerto Rico Setting: pharmacy in San Lorenzo Eligible participants: (health status); pharmacy customers ≥ 65 who visited pharmacy June or July 2000 Age: ≥ 65 Gender: 62% f | |
| Interventions | Intervention 1: offer of educational session about influenza and to attend vaccination clinic Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: 12 months % vaccinated by; 12 months from intervention | |
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Los participantes se dividieron alcatoriamente (selección simple) en grupo control y grupo experimental." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 46/50 from intervention and 37/50 from control group received vaccination at 3 months; 42/50 from intervention and 31/50 from control group assessed clinical results after 12 months (no differential attrition analysis) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
McCaul 2002
| Methods | Purpose: to compare letter informing participants of importance of flu shot to reminder letter stating date and time of clinic Design: RCT, clustered by counties Duration of study: not reported Interval between intervention and when outcome was measured: not stated Power computation: not performed Statistics: t tests | |
| Participants | Country: USA Setting: 29 North Dakota counties Eligible participants: (health status): 6730 male and 9107 female Medicare recipients who had not submitted Medicare reimbursement requests for flu shots the previous year Age: ≥ 65 Gender: 57.5% f | |
| Interventions | Intervention 1: card reminding recipients of advantages of flu shots Intervention 2: letter reminding recipients of advantages of flu shots and stating time, date and place of flu shot clinics Control: no intervention | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: not stated % vaccinated by: not stated |
|
| Notes | Funding: Health Care Financing Administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we randomly assigned counties to either the reminder letter (n = 17), action‐letter (n = 12), or no‐letter (n = 20) conditions. Within the reminder‐letter counties, we then randomly assigned individuals within each county to either the reminder‐only, reminder plus positive frame, or reminder plus negative frame conditions. Within the action‐letter counties, all individuals received the same action letter" (no statement about method of randomisation) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No statement about blinding, but assessment based on Medicare reimbursement claims |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | E‐mail from author states "... subject loss was 6%, most of which was letters being returned." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
McDowell 1986
| Methods | Purpose: to compare reminders to receive influenza vaccination by telephone reminder by physician, telephone by nurse, or by letter Design: cluster‐RCT, participants randomised by family Duration of study: 23 October to 31 December 1984 Interval between intervention and when outcome was measured: 23 October to 31 December 1984 Power computation: sample sizes offered power to detect 10% to 15% difference in proportions (alpha not stated) Statistics: Chi2 | |
| Participants | Country: Canada Setting: Ottawa Civic Hospital Family Practice Clinics Eligible participants: (health status); 13,345 eligible patients, of whom 1420 > 65; 2 physicians refused to participate, leaving 939 participants; 113 had been vaccinated before the trial and were excluded; leaving 201 available for a personal reminder by physician, 208 for a phone call by nurse, 239 for a letter and 215 in a randomised control group Age: > 65 Gender: not stated Intervention group 1 (physician reminder): 1122 families, 1471 people Intervention group 2 (telephone reminder group): 1104 families, 1468 people Intervention group 3 (letter reminder group): 1168 families, 1541 people Control group: 1056 families, 1403 people eligible participants Exclusions: not clear | |
| Interventions | Intervention 1 (physician reminder): a computer‐generated reminder was included on the routinely printed encounter form before any visit to the office to remind the physician of outstanding preventive procedures Intervention 2 (telephone reminder): the practice nurse attempted to contact the family, making a maximum of 5 calls during working hours, and completed an action form for each listed patient. Once contact was made the nurse advised the patient about the indicated procedures and then attempted to arrange for them to be performed. The person answering the telephone was asked to relay the message to other family members Intervention 3 (letter reminder): computer‐generated letter, signed by their physician and nurse, describing the procedures that were overdue for each member of the family and the importance of having them performed. After 21 days a second reminder was sent out to non‐respondents Control: no action was taken to remind the physicians or the participants that a procedure was overdue. Non‐randomised control group: the participants of 2 doctors who refused were not randomised and were treated as a second control group to assess the effects of the increased preventive activity in the practices In the 1990 article in Family Medicine, McDowell provided baseline vaccination data for the 1984, year before the 2‐year intervention in 1985 and 1986, and grouped the letter, nurse and physician reminders into one treatment group compared to a control, and we have followed this reporting of the results in the final publication in their series | |
| Outcomes | Outcome measured: % vaccinated by 31 December 1984, recorded in clinic computer Time points from the study that are considered in the review or measured or reported in the study: intervention 23 October 1984 to 31 December 1984, vaccine receipt assessed until 31 December 1984 % vaccinated by: 31 December 1984 Intervention 1 (physician reminder): 766/1471 persons visited the practice in the study year; 22.9% of group were vaccinated but the denominator for this proportion is not stated (i.e. cannot tell if it was 766 persons versus 1471 persons versus 1122 families) Intervention 2 (telephone reminder): 1104 of the 1468 families assigned to telephone required a reminder for one or more interventions and 684 families were actually contacted. 37% of group were vaccinated but denominator for proportion not stated (i.e. cannot tell if it was 1104 families versus 684 families versus 1468 persons that comprised the 1104 families versus unknown number of persons in the 684 families actually reached) Intervention 3 (letter reminder): 164 of 1442 persons sent letters had letters returned as not deliverable. 35.2% were vaccinated but cannot tell which denominator was used (i.e. 1442 versus 978 persons) Control: 9.8% "of study group" were vaccinated. Not stated if the denominator is families or individual persons | |
| Notes | Funding: Dept National Health and Welfare, Ontario Ministry of Health, Career Health Scientist Award to Dr. McDowell; follow‐up in 1985 showed no difference between intervention and control groups (McDowell 1990) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... participants were randomly allocated by family" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement about blinding, but vaccinations recorded in clinic computer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 239 letters sent only 2 returned; nurses unable to contact 31 (15%) by phone Intervention 1: 766/1471 persons visited the practice in the study year; 22.9% of group vaccinated but the denominator for this proportion is not stated (cannot tell if it was 766 persons versus 1471 persons versus 1122 families) Intervention 2: 1104 of the 1468 families assigned to telephone required a reminder for 1 or more interventions and 684 families were actually contacted; 37% of group were vaccinated but denominator for proportion not stated (cannot tell if it was 1104 families versus 684 families versus 1468 persons that comprised the 1104 families versus unknown number of persons in the 684 families actually reached) Intervention 3: 164 of 1442 persons sent letters had letters returned as not deliverable; 35.2% were vaccinated but cannot tell which denominator was used (1442 versus 978 persons) Control: 9.8% "of study group" were vaccinated. Not stated if the denominator is families or individual persons "8 weeks after the study ended we called random samples of patients from each study group who had apparently not ben vaccinated to estimate the extent of underreporting." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
McMahon 1995a
| Methods | Purpose: to compare an individual letter plus an informational brochure about influenza vaccination to a form letter plus brochure to no intervention in Montana Design: RCT; Montana was divided into 24 geographic regions and Wyoming into 16 by zip codes and 4 regions randomly assigned from each to intervention Duration of study: 3 months Interval between intervention and when outcome was measured: brochure or letter mailed 23 to 30 September 1994; vaccination uptake assessed 1 to 31 December 1993 and 1994 Power computation: not performed Statistics: logistic regression to examine relationship of letter plus brochure and influenza vaccination; Egret® statistical software to adjust for confounding variables | |
| Participants | Total number: Montana: personalised letter 19,850, form letter 21,250, no letter 150,000; Wyoming same numbers Setting: all Medicare beneficiaries in Montana and Wyoming Diagnostic criteria: % receiving influenza vaccination recorded as influenza vaccination claims submitted to HCFA (Medicare pays for influenza vaccination for all those enrolled in Medicare Part B, and 96% of those ≥ 65 in the US are enrolled in Medicare Part B) Gender: not stated Age: ≥ 65 Country: USA [Co‐morbidity not stated] [Socio‐demographics not stated] [Ethnicity not stated ] [Date of study 1994] |
|
| Interventions | Intervention 1: individual letter plus an informational brochure about influenza vaccination Intervention 2: form letter plus brochure Control: no intervention [Integrity of Intervention not stated] |
|
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: intervention in September, influenza vaccination claims October 1 through 31 December 1993 and 1994 % vaccinated by: 31 December 1985 Note: n's in McMahon 1995a and McMahon 1995b differ from those in Maglione 2002a. We adopted the n's in Maglione 2002a because the authors reported extracting data independently in duplicate, comparing them and resolving discrepancies |
|
| Notes | Funding: Montana‐Wyoming Foundation for Medical Care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The two states were divided into 40 geographic regions defined by zip code aggregates (24 in Montana, 16 in Wyoming); in each state four regions were randomly selected as intervention sites." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Influenza vaccination data are collected by Medicare as billing claims |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
McMahon 1995b
| Methods | Data are for Wyoming. See McMahon 1995b | |
| Participants | See McMahon 1995b | |
| Interventions | See McMahon 1995b | |
| Outcomes | See McMahon 1995b | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The two states were divided into 40 geographic regions defined by zip code aggregates (24 in Montana, 16 in Wyoming); in each state four regions were randomly selected as intervention sites." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Influenza vaccination data are collected by Medicare as billing claims; 96% of those ≥ 65 are covered by Medicare Part B, which processes all billing claims for influenza vaccination |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Minor 2010
| Methods | Purpose: increase influenza vaccination uptake by phone versus mail reminders Design: RCT of attendees at hypertension clinic to phone, mail or control Duration of study: mid‐November to "the following spring" Interval between intervention and when outcome was measured: Intervention began after mid‐November, follow up "in the following Spring" Power computation: not performed Statistics: %s; ORs and 95% CIs |
|
| Participants | Country: USA Setting: University of Mississippi Hypertension Clinic Eligible participants: (health status): 257 > 65 Age: 257 > 65 Gender: 62% f for whole sample < 50 to > 65 |
|
| Interventions | Intervention 1: letter plus CDC Influenza Vaccine Information Statement Intervention 2: phone call with same information Control: standard clinic practice Co‐interventions: none |
|
| Outcomes | Outcome measured: % influenza vaccination Time points reported in the study: "Mid November"; "following Spring" |
|
| Notes | Funding: none stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1712 eligibles had clinic visit in preceding 15 months; 341 had received influenza vaccination; 487 not contactable after 5 attempts; sample = 884, of whom 257 > 65 |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Moran 1992
| Methods | Purpose: to compare 1 and 2 reminder letters offering free influenza vaccine to no intervention Design: RCT, participants randomised Duration of study: mid‐October Interval between intervention and when outcome was measured: not reported Power computation: "Sample size was sufficient to detect a 20% change in immunization (40% to 60%) with 80% power at ? = 0.05." Statistics: percentages | |
| Participants | Country: USA Setting: urban community health centre (location not stated but first author was located in Winston‐Salem, N. Carolina) Eligible participants: (health status): "High‐risk participants seen at an urban community health center." (eligible n not stated) Age: ≥ 65 Gender: 61% f | |
| Interventions | Intervention 1: 1 letter offering free influenza vaccine Intervention 2: 2 letters offering free influenza vaccine Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: first letter sent mid‐October 1990, second letter (to intervention group which received 2 letters) 1 month later Vaccinated by: not stated | |
| Notes | Funding: US National Research Service Award, National Institute on Aging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomised, single‐blind, controlled trial ..." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "single‐blind" but does not state if it was participants or researchers blinded; data entered on computer clinical tracking programme |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients randomised to intervention group 1 (n = 135) and intervention group 2 (n = 138) and 136 to control, of whom 66, 68 and 68 were ≥ 65, and vaccination status of all participants reported; immunisation reported in clinic computers |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Moran 1995
| Methods | Purpose: to compare the effect of a mailed educational brochure on influenza vaccination uptake compared to no intervention Design: RCT, participants as unit of randomisation Duration of study: 4 months Interval between intervention and when outcome was measured: "The educational brochures were mailed to the intervention group when the influenza vaccine became available at the beginning of October." (Year not stated) Power computation: 900 participants required to detect 20% difference if baseline rate 20%, 90% power, α = 0.05 Statistics: not stated (probabilities computed) | |
| Participants | Country: USA Setting: general internal medicine and gerontology service, Wake Forest University, N. Carolina Eligible participants: (health status): 1583, then excluded residents of long‐term care facilities, leaving 1251, of whom 900 were randomised to treatment and control groups Age: ≥ 65; avg = 76 Gender: 65.4% f | |
| Interventions | Intervention: mailed brochure encouraging influenza vaccination Control: no intervention | |
| Outcomes | Outcome measured: % vaccinated Time points from the study that are considered in the review or measured or reported in the study: October to following January (year not stated) % vaccinated by: January following intervention in October | |
| Notes | Funding: National Institute on Aging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... two random samples of 450 were selected for the intervention and control groups." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement, vaccination status entered on computer clinical tracking program |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clinic immunisation and financial logs showed 80 in intervention and 71 in control group received influenza vaccination; 666/900 responded to the postcard survey and a total of 218 in intervention group said had been vaccinated in clinic and elsewhere and 213 in control |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Moran 1996
| Methods | Purpose: "To determine whether an educational brochure or a lottery‐type incentive increases influenza immunization rates." Design: RCT ‐ patients randomised Duration of study: 3 months Power computation: not reported Statistics: Chi2, Wilcoxon, logistic regression, odds ratios with CI, percentage patients receiving influenza vaccination in 4 groups |
|
| Participants | Country: United States Setting: urban community health centre Participants: "All high‐risk ambulatory patients seen at the community health centre within the preceding 18 months" Age: > 18 to 99 years of age, mean age 66 (n = 797) Gender: male and female |
|
| Interventions | Patients were randomly assigned to 1 of 4 groups: control (n = 202), mailed educational brochure (n = 198), mailed lottery incentive wherein patients who obtained an influenza vaccination would be eligible to win 1 of 3 grocery gift certificates (n = 198), and a mailed combined educational brochure and lottery incentive (n = 199) | |
| Outcomes | Odds ratio of patients in the 4 groups obtaining an influenza vaccination. Odds ratio for patients in the brochure group obtaining influenza immunisation when compared with the control (odds ratio 2.29, 95% CI 1.45 to 3.61), odds ratio for incentive group compared with control: (OR = 1.68, 95% CI 1.05 to 2.68). "Immunization for the group mailed both interventions was not significantly different from control (OR = 1.41, 95% confidence interval CI 0.88‐2.27). For the subset of individuals for whom prior immunization status was known, the impact of the educational brochure was even more significant (OR = 3.95,95% CI 1.92 to S.lO), but the groups mailed incentive or both interventions were not significantly different". For those aged 65+, the study reports on the percentage in each group that received vaccination: 25% control, 41% brochure, 30% incentive, 24% brochure and incentive | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "High‐risk patients were randomly allocated to one of four groups." (no statement about method of randomisation) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "... all high‐risk patients (n = 797) seen in the preceding 18 months" were reported in the final outcome (Table II) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Morrissey 1995
| Methods | Purpose: to evaluate the effects of a free package of preventive healthcare services, including influenza vaccinations, on the health outcomes of seniors Design: RCT, patients randomised within practices Duration: 2 years Power computation: all eligible patients at the practices were evaluated for study inclusion Statistics: Chi2, analysis of covariance and regression analysis |
|
| Participants | Country: USA Setting: 10 primary care practices in 13 locations in central North Carolina Participants: 1914 patients (954 intervention, 960 control) Age: >= 65 years Gender: 61.1% women |
|
| Interventions | "The health promotion service package contained a set of procedures and nursing interventions that address important risk factors and premature mortality, institutionalization, and increased disability for older people. Health promotion sessions, in this demonstration were conducted in physician offices using an individual counseling strategy that involved the nurse/physician assistant and patient in mutual planning..." Practices were sent monthly reminders by research team to schedule intervention patients for preventive care and health promotion care services. Nurses were provided with training in administering the services. The control group received the usual preventive services offered by their practice at the usual costs | |
| Outcomes | Medical chart audits were performed on 3 heterogeneous practices (231 intervention patients and 224 controls) to determine whether or not there was an increase in the number of preventive care procedures performed in the intervention group. The percentage of patients who received the Fluvax vaccine during the 1st year of the study increased in the intervention group as compared to the control after randomisation (72% versus 52%, P < 0.001) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomised by strata into intervention or control" (no statement about method) |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Although contamination of the control group is sometimes a concern with such a design, it was not an issue here for two reasons: first, the financial intervention involved full Medicare reimbursement to physicians for preventive‐care and health promotion packages only for those patients randomised to the intervention group; and second, the office system intervention was in effect only for patients receiving the intervention group. The control group was not identified to the practice, there was no prompting, no form, and no special preventive visit for the control‐group patients" "Patients were informed of their random assignment only after they came into the practice for the interview" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 1914 patients recruited: "... it was not feasible to conduct chart reviews in every practice, so we chose three diverse groups: a three‐physician family practice.. a ten‐physician community health center, a six physician suburban internal medicine practice..." "Of 458 patients eligible for chart audit, charts were located and reviewed for 455 (231 intervention, 224 control)" |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Mullooly 1987
| Methods | Purpose: to compare personalised letter with no intervention Design: RCT, individuals randomised Duration of study: interval between intervention and when outcome was measured: "Kaiser Permanente ...operates seasonal influenza clinics." Power computation: not performed Statistics: percentages | |
| Participants | Country: USA Setting: Kaiser Permanente Northeast Region HMO in Portland, Oregon/Vancouver and Washington metropolitan area Eligible participants: (health status): ≥ 65, discharged alive from hospital October 1983 to September 1984 with diagnoses of cardiovascular, pulmonary, renal, metabolic/nutritional, neurologic or malignant diseases Age: ≥ 65 Gender: intervention 48.1% f; control 52.7% f | |
| Interventions | Intervention 1: personalised recommendation to obtain influenza vaccination, and information about where and when to obtain vaccination Control: no intervention | |
| Outcomes | Outcome measured: % influenza vaccination Time points from the study that are considered in the review or measured or reported in the study: not stated: "Kaiser Permanente ...operates seasonal influenza clinics." % vaccinated by; not stated | |
| Notes | Funding: not stated; we e‐mailed the author for influenza vaccination uptake in the year before the intervention but no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study group population was randomised into intervention and control groups based on a pseudo random digit of the individual membership ID number." |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No statement: "Medical records were retrospectively reviewed at the end of the study period to ascertain whether subjects had received influenza vaccine" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Medical records were retrospectively reviewed at the end of the study period to ascertain whether subjects had received influenza vaccine ..." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Nexøe 1997
| Methods | Purpose: to compare offer of free influenza vaccination to postal reminder with fee for vaccination paid by the participants Design: RCT Duration of study: 25 September to 15 December 1995 Interval between intervention and when outcome was measured: not clear Power computation: no information provided Statistics: Chi2 statistic for proportions, 2‐way analysis of variance at alpha = 0.05. No adjustments were made for within‐practice clustering or for prior year influenza vaccination status | |
| Participants | Country: Denmark Setting: 13 solo general practices in the counties of Funene and Vejle, 25 September to 15 December 1995. Eligible practices had not sent mailed reminders to participants in previous years and were required to have at least 45 elderly participants aged 65 years or older with a medical indication for influenza vaccination Eligible participants (health status): 585 persons. These included 45 participants from the practice of each GP who were aged over 65 years and with a medical indication for influenza vaccination (treated for chronic pulmonary or cardiovascular disorder; acquired or congenital immunodeficiency, other chronic disease such that the doctor perceived the person to be at increased risk for influenza related complications or nursing home resident) Age: all aged over 65 years, no age distribution provided Sex: no data presented | |
| Interventions | Intervention 1: free influenza vaccination (15 from each practice, i.e. 1/3 of participants from each practice) Intervention 2: invitation for influenza vaccination but requirement to pay the usual GP fee (USD 40 to 60) (15 from each practice, i.e. 1/3 of participants from each practice) Control: no invitation, vaccinated only at their own request (15 from each practice, i.e. 1/3 of participants from each practice) | |
| Outcomes | Outcome measured: % vaccinated within each group as "registered" Time points from the study that are considered in the review or measured or reported in the study: registration occurred from 25 September to 15 December 1995 % vaccinated by 15 December 1995 | |
| Notes | Patients were randomised within each practice Explicit definition of "registered" not provided by the context of the phrase suggests that this was by chart audit or records review In the control group 83% of the participants had been vaccinated in the previous year. Overall, 25% of all participating participants had been vaccinated prior year (only aggregated data across all practices provided). Authors do not provide practice specific denominators, only practice specific numerators for outcomes Funding: Danish Research foundation for General Practice Fees for vaccination and vaccine were paid for by the State Serum Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Randomisation was blinded for the GPs. However, GPs were paid the equivalent of USD 36 for each patient vaccinated without patient fee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition of participants: no explicit statement as to follow‐up Incomplete data points for participants No analysis if differential attrition could affect outcomes Given that data were obtained from the GP records, would appear to be complete although there is no explicit statement of records audit being done. Completeness of ascertainment would be best for the free vaccination group as it is stated that "the GP’s were paid for each patient vaccinated without patient fee." |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Nuttall 2003
| Methods | Purpose: test hypothesis that an invitation letter to attend GP for influenza immunisation plus home visit to discuss influenza vaccination is more likely to increase influenza vaccine uptake than an invitation letter to attend GP for immunisation alone, or invitation letter plus pamphlet promoting influenza immunisation Design: RCT: eligible participants were stratified by age (< 72 years; 72 years or older to ensure equal numbers each age group within each intervention group). Within each age group randomly allocated into 3 groups. A total of 30 persons were allocated to each intervention Interval between intervention and when outcome was measured: not explicitly stated: intervention was to be completed the start of the influenza immunisation programme at the GP surgery; health records audited "following completion of the influenza immunization program." Power computation: not done Statistics: simple comparison of proportions immunised across groups (ITT) | |
| Participants | Country: UK Setting: a single GP practice in East Lancashire Eligible participants (health status): 90 participants aged 65 to 90 years registered to the practice who had failed to attend for the influenza immunisation prior year (i.e. 2000 to 2001 campaign (N = 393) who agreed to participate, were not confused, did not have egg allergy (i.e. 90 participants) Age: 50% were aged 65 to 72 years, 50% were aged over 72 years Gender: no information provided | |
| Interventions | Intervention 1: invitation letter to attend GP for influenza immunisation plus leaflet promoting influenza vaccination Intervention 2: letter plus home visit Control: letter alone | |
| Outcomes | Outcome measured: % vaccinated based upon audit of health records Time points from the study that are considered in the review or measured or reported in the study: research project started following ethical approval (received 2 August 2001) and was completed by June 2002 % vaccinated by: not explicitly stated | |
| Notes | No source of funding mentioned Author comments that a smaller proportion of those immunised at outcome had received a prior vaccination, but a larger proportion of those immunised at outcome had a qualifying health condition at baseline 90 participants were eligible and consented of 393 who had failed to attend for the influenza immunisation prior year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The 90 respondents were divided in half by age (< 72 years, 72 years or older). The participants in each age group were allocated into the 3 intervention groups, using the stratified randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition of participants? Implied to be none, not explicitly stated Incomplete data points for participants? No Analysis if differential attrition could affect outcomes? No information provided Vaccination data assessed by chart review (RCT was of a single practice) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Puech 1998
| Methods | Purpose: to determine if a single postcard reminder for participants aged 65 years or older would improve influenza vaccination uptake in a 3‐partner general practice Design: RCT Duration of study: 1 April to 31 July 1996 Interval between intervention and when outcome was measured: postcard mailed on 1 April 1996. Outcomes ascertained "end of July 1996" ‐ 4 months later Power computation: study power to detect a difference of 20% in immunisation rates at 0.05 (2 sided): 61% for males, 81% for females Statistics: randomisation was done within sex strata, analysis controlled (logistic regression) for 1995 immunisation status and study factor but did not control for proximity to practice. Separate regressions done for males and females | |
| Participants | Country: Australia Site: Leichhardt general practice (a 3‐partner practice) in suburban Sydney, Australia Eligible participants: 325 participants aged 65 years or older identified from a computerised age‐sex‐disease registry maintained by the general practice who had made at least 3 visits to the practice, one of which had to have occurred in the 2 years prior to study Age: 65 to 69 years: 86/325 (26.5%) 70 to 74 years: 78/325 (24.0%) 75 to 79 years: 58/325 (17.8%) 80 to 84 years: 62/325 (19.1%) 85 years or older: 41/325 (12.6%) Gender: 38.5% male, 61.5% female Exclusions: 1) Nursing home residents were excluded as not on the computerised register; 2) flu vaccination received prior to 1 April 1996; 3) participants who had left practice, gone to a nursing home or died since most recent update of the practice register, 4) those known to be allergic to egg protein, 5) known by practice to object to flu vaccination, or having severe or terminal illness, dementia or unstable psychiatric conditions | |
| Interventions | Intervention 1: postcard mailed 1 April 1996 reminding them to attend the practice for an influenza vaccination before the end of the month and providing information on disease and vaccine, vaccine availability and vaccine cost Control: usual care: "ad hoc approach" co‐interventions: "influenced by news coverage of outbreaks, media campaigns by vaccine manufacturers, opportunistic reminders and secular events" | |
| Outcomes | Outcome measured: % vaccinated in 1996 (end of July) as validated by chart review Time points from the study that are considered in the review or measured or reported in the study: postcards mailed to intervention group on 1 April 1996. Practice records reviewed for documentation of receiving vaccination at the end of July 1996 | |
| Notes | Chart review of practice: assessor blind to patient group allocation; required documentation in chart that vaccination, not just prescription for vaccine actually provided. However, no information provided as to whether or not chart review would have captured any vaccinations obtained from outside of the practice Funding: no information provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants stratified by sex, then computer‐generated random numbers; however for married couples once identified as married, both randomly allocated to same intervention |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | General practitioners were blind to allocation but no information provided on methods of blinding. Person who assessed outcome was blind to the patient group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes were ascertained from patient chart and participants were considered immunised if either immunisation documented in patient record OR a prescription given for flu vaccine but no record of the actual vaccination in the notes. Authors provide no information on loss to follow‐up, thus it is possible that persons recorded as not vaccinated might in theory have received it from another practice |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Roca 2012
| Methods | Purpose: to assess the effects of a mail‐out education campaign on influenza vaccination uptake among seniors Design: RCT Duration: 1 week in September 2009 Power computation: "On the basis of the percentage of participants vaccinated in 2008 and results of previous studies, we calculated that a sample size of 1187 participants in each group was needed to find a vaccination rate difference of at least 5% between the EPG and the NPG (42.5% and 37.5% respectively) with a level of significance of P=.05 and a power of 80%" Statistics: t‐tests, Mann‐Whitney U, Wilcoxon, Kruskal Wallis, regression analysis |
|
| Participants | Country: Spain Setting: a health centre in Castellon, Spain Participants: 2402 patients in family practices of 13 physicians Age: >= 60 years old Gender: 55.7% f |
|
| Interventions | A personalised letter was sent to patients in the intervention group providing them with information about influenza and answers to common questions/concerns with respect to the influenza vaccine. The control group did not receive any letter | |
| Outcomes | Although there was an increase in vaccination uptake for both groups as compared with the previous year, there was a greater increase in the intervention group as compared with the control 9.4% versus 1.6% increase, P < 0.01) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a computer random number generator and a 1:1 ratio to randomly assign participants to 1 of 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study was open for participants but blinded for the healthcare workers responsible for caring for the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 2402 patients recruited were followed through the 2009 vaccination season |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Satterthwaite 1997
| Methods | Purpose: compare personalised invitation recommending a visit to doctor to be vaccinated where patient required to pay for vaccination to personalised invitation to be provided with free vaccination to no intervention on influenza immunisation uptake Design: RCT Duration of study: not stated Interval between intervention and when outcome was measured: not stated Power computation: not stated Statistics: Chi2 statistic of significance adjusted for design effect of within practice clustering. Design effect for contrast of intervention 1 versus control was 1.09. Design effect of contrast for intervention 2 versus control was 4.05 | |
| Participants | Country: New Zealand Setting: 31 active general practitioners in the Auckland region randomly selected from the cervical screening program were invited to participate. Eligible practitioners were able to generate a list of names and addresses of all participants over 65 years of age, normally provided influenza vaccine to participants, worked at least 8/10 full time equivalent and did not currently have in place a postal reminder system for influenza vaccination for participants over 65 years. 8 doctors were not eligible, 7 were eligible but did not wish to participate and 16 were eligible and participated. Within each practice, up to 210 participants were randomly allocated to interventions Eligible participants: (health status) 2791 persons aged over 65 years Age: within each practice, participants aged over 65 years. Age distribution of participants not stated Gender: sex distribution of participants not stated No information provided on exclusion of participants | |
| Interventions | Intervention 1 (N = 931): personalised invitation sent to people (mail) recommending that they visit their general practitioner to receive a flu vaccination. Those who accepted the invitation would have had to pay about NZD 20 for vaccination Intervention 2 (N = 930): personalised invitation sent to people recommending that they visit their general practitioner to receive a flu vaccination at no charge Control (N = 930): no intervention. These persons would have had to pay about NZD 20 for vaccine | |
| Outcomes | Outcome measured: % participants vaccinated after intervention as recorded by practice staff, validated by authors only for participants who received intervention 2 Time points from the study that are considered in the review or measured or reported in the study: no information provided | |
| Notes | No information provided on year study was done. Internal evidence in the article suggested prior February 1997. Authors note that in 1997 flu season, government policy will change to make influenza vaccination free for persons over 65 years of age No information provided on vaccination status prior year Data are not presented by practice Funding: vaccine provided at no cost by Rhone Poulenc and distributed to practitioners by Ebos Group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly allocated" (no method stated) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 930 in group 2 (invitation letter), 930 in group 3 (free vaccine letter) and 930 in group 1 (control); no data on attrition |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Siriwardena 2002
| Methods | Purpose: to compare the effect of an educational outreach visit to primary healthcare teams on influenza and pneumococcal vaccination uptake to written feedback Design: stratified cluster‐RCT Duration of study: 8 months Interval between intervention and when outcome was measured: 6 months Power computation: based on vaccination rate per practice as primary outcome. Sample size was based upon attainment of an increase in vacation uptake of 20%. To detect a difference between control rates and the desired targets of at least 1 standard deviation, the Student's t‐test with power 0.8 and size 0.05 would require 17 practices per group or 9 per group to detect an effect of 1.5 standard deviations with same power Statistics: Poisson regression using population at risk as an offset and taking account of the stratification. Rates were expressed as mean vaccination rates, odds ratios and confidence intervals | |
| Participants | Country: UK Setting: 20 primary care practices in the West Lincolnshire Primary Care Trust and the 10 Trent Focus Collaborative Research Network Eligible participants: (health status) 30 practices had participants aged 65 years or older or who had coronary heart disease, diabetes or splenectomy on their registers. A total of 27,580 participants aged 65 years or older were included in the 30 practices Age: no information provided on age distribution of participants in practices Gender: no information provided on sex distribution of participants in practices | |
| Interventions | Intervention 1: 1‐hour educational outreach visit (based on principles of academic detailing) to practice teams; delivered by one of the research team that included feedback of practice vaccination uptake in relation to other practices in the study and national targets Control: written feedback on their vaccination uptake compared with other participating practices | |
| Outcomes | Outcome measured: mean vaccination uptake (adjusted for initial level and stratification) based upon practice records, for
Time points from the study that are considered in the review or measured or reported in the study: baseline data collection began in August 2000. Interventions delivered at the start of the annual influenza vaccination campaign of October 2000. Outcomes ascertained 6 months after the educational outreach visit, i.e. 8 months after baseline data collection |
|
| Notes | Baseline data collection was in August 2000 and was done by practice staff The unit of cluster was the practice. However, because of ceiling effects (capacity to increase immunisation uptake depends on baseline, possibly easier to increase from low baseline), practices were stratified on baseline uptake of influenza vaccination for diabetics as this had been previously shown to be correlated with risk group. Within strata, practices were randomly allocated to intervention or control 20/39 practices in the West Lincolnshire Primary Trust participated as did 10/50 from the Trent Focus Collaborative Research Network Participating and non‐participating practices were similar in number of partners, list size, whether or not they were dispensing practices and rurally Funding: Trent Focus and West Lincolnshire Primary Care Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Fifteen practices were randomised to intervention and 15 to the control group after stratifying for baseline vaccination rate." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not possible with this design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13,633 in intervention group and 13,947 in control group, but no data on attrition; vaccination status assessed from clinic records |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Smith 1999
| Methods | Purpose: to determine the effectiveness of mailed reminders on influenza vaccination uptake Design: RCT Duration of study: 3 months Interval between intervention and when outcome was measured: first measurement was made on 9 February 1996 (minimum 8+ weeks after intervention) Power computation: not discussed Statistics: logistic regression analysis adjusting for age, gender, residency in medium or low compared to high population density counties. In sensitivity analysis, the logistic regression had data from both immunisation data and survey results with chronic disease variables | |
| Participants | Country: USA Setting: 10 counties in Indiana Eligible participants: 9011 persons (4508 intervention group, 4503 control group) registered in the Medicare eligibility file who were age 65 years or older, had no evidence of having died, had an allowable charge in the prior year, who were not residents of nursing homes and were not members of an HMO who lived in one of 10 eligible counties were randomly selected for the study in 1995 Intervention group: 4508 eligible participants Control group: 4503 eligible participants Age: 65 years or older; mean age of control group was 75.4 years, for intervention group 75.5 years Gender: 61.9% female (control group), 61.2% female (intervention group) Exclusions: those who were found to reside in an nursing home, who had an invalid address, who were dead or who refused to participate (intervention group: 497; control group: 492) | |
| Interventions | Intervention 1: a reminder letter adapted from the Health Belief Model that advised that costs were covered by Medicare, provided a state board of health phone number for those without access to physicians plus information about influenza vaccination. Letter was signed by the principal investigator, the state health commissioner and the medical director of Medicare for Indiana Control: no letters were sent | |
| Outcomes | Outcome measured: N, % vaccinated against influenza (self report by postal survey or by having a claim filed for immunisation between 1 October 1995 and 31 January 1996). Self reported immunisation was validated by survey (99.6% agreement between survey and Medicare claims for influenza vaccination) Time points from the study that are considered in the review or measured or reported in the study: letter was sent on 3 November 1995 and a reminder (same letter) sent again on 22 December 1995 | |
| Notes | The eligible counties were selected by multistage random sampling from the 56 Indiana counties that did not abut state borders: the county with highest population density of elders, 4 counties randomly selected with a medium density of elders (19.6/sq miles) and 5 with low population density of elders (random number generator). The reason for exclusion of border counties was that residents of those counties were perceived to be more likely to use out of state health services which would reduce ability to track outcomes Intensive follow‐up was done to ascertain outcomes: non‐responders to the 9 February 1996 postal survey were sent a second survey 16 April 1996 and 14 July 1996. A sample of those who did not respond after the 14 July mail‐out and who did not submit a claim for influenza immunisation or were not identified in mortality files were telephoned to determine immunisation status. Interviewers were blind to intervention assignment Funding: no information provided No data on vaccination prior to 1995 were collected or reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random selection was by a random number generator; ? "... and then randomised within county to control and intervention groups." No explicit statement that random allocation used a random number generator |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | In follow‐ups, telephone interviewers were blinded to intervention; no information provided as to blinding for postal surveys or Medicare claims. However, doubtful that contamination would have occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10,000 Medicare beneficiaries randomly selected; 5000 randomised to intervention and 5000 to control; 4503 eligibles in control, 4508 eligibles in intervention; 3487 in control responded to survey or filed claim and 3454 in intervention responded to survey or filed claim (no differential attrition analysis) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
Spaulding 1991
| Methods | Purpose: to compare a postcard reminder sent to high‐risk participants on influenza immunisation uptake to usual care (no postcard) Design: RCT Duration of study: 6 months Time: 1983/1984 influenza season Outcome measured: % vaccinated against influenza for the 1983 to 1984 season by sex, rank of military sponsor and age group (including those aged > 64 years) Interval between intervention and when outcome was measured: 6 months were allowed for people to be vaccinated and it is clear that the intervention ante‐dated the measurement of outcome Power computation: no information provided Statistics: Chi2 statistic to compare proportions vaccinated each group. Multivariate analysis using Mantel‐Haenszel (M‐H) Chi2 statistic and M‐H adjusted risk ratio. Within‐family clustering was not addressed | |
| Participants | Country: USA Setting: Department of Family practice at Madigan Army Medical Center, Ft Lewis Washington Eligible participants: 1068 military retirees or the family members of active or retired members of the military who had one or more high‐risk diagnoses for influenza complications according to the US Immunization Practices Advisory Committee criteria of 1983 Age: persons of all ages 0 to 20 years: 153 (71 intervention group 1; 82 control) 21 to 40 years: 130 (63 intervention group 1; 70 control) 41 to 64 years: 289 (269 intervention group 1; 289 control) 65 years or older: 224 (116 intervention group 1; 108 control) Sex: males 56.3%, females 43.7% Males: 573 (519 intervention group 1; 549 control) Females: 496 (257 intervention group 1; 238 control) Exclusions: persons who did not have high‐risk health conditions | |
| Interventions | Intervention 1: 519 participants in intervention group were mailed a reminder postcard advising them that their physician had determined that they were at high risk of complications should they catch the flu and strongly urging them to come to the Family Practice Clinic for intervention. Postcard sent 2 weeks before availability of the influenza vaccine used during the 1983/84 season Control: 549 participants who received routine care, were not sent a postcard | |
| Outcomes | Outcome measured: % receiving influenza vaccine based on office records of being vaccinated Time points from the study that are considered in the review or measured or reported in the study: from time postcard sent 2 weeks before vaccine availability to 6 months after vaccine became available Intervention: postcard sent 2 weeks before availability of the influenza vaccine used during the 1983/84 season % vaccinated by 6 months after the influenza vaccine used in the 1983/1984 season became available |
|
| Notes | Potential participants were assigned a code number that included 2 digits to identify if they were members of the same family. These data were not used in analysis (i.e. within‐family clustering was not addressed in the data analysis) There was no cost to patient for influenza immunisation No data are provided on influenza vaccination prior year Funding: no information provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individuals were assigned to intervention or control group by a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Physicians in the Department of Family Practice were aware that a study was in progress and that some of their participants might receive postcards about influenza immunisation. Vaccine was offered to all eligible participants on a walk‐in basis. Patients who presented for immunisation read and signed an informed consent document It is not stated if the physicians were those who performed the vaccinations. However, it is likely that participants might have told their vaccinator whether or not they had received a postcard |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on attrition or incomplete data points. No analysis whether differential attrition could affect results; vaccination status assessed from records at US Army Medical Centre |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
avg: average CDC: Centers for Disease Control and Prevention CI: confidence interval COPD: chronic obstructive pulmonary disease C‐RCT: cluster‐randomised controlled trial dx: diagnosis f: female GI: gastrointestinal GLE ANOVA: general linear model repeated‐measures analysis of variance GPs: general practitioners HMO: health maintenance organisation ICD‐9‐CM: International Classification of Diseases 9th Revision Clinical Modification IHD: ischaemic heart disease ITT: intention‐to‐treat n: number ns: non‐significant OR: odds ratio RCT: randomised controlled trial RR: risk ratio SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2004 | RCT; intervention to increase influenza vaccination rates; but cannot separate outcomes for 60 to 64 years from 18 to 64 years; E‐mail from Dr. Faruque Ahmed 3 April 2013: "We generated a random number for each employer using the RANUNI function in SAS. We randomised to the study arms based on the random number using defined cut‐offs. I am not sure whether we still have the data." |
| Alemi 1996 | Not RCT; children |
| Alexy 1998 | Not RCT; intervention to increase influenza vaccination rate and influenza vaccination rate outcomes; prospective cohort without control group (and those who participated through either the mobile health unit or a home visit received the same level of intervention and thus no comparison could be made for different levels of intervention) |
| Allsup 2004 | RCT. However, focus was invitation from practices to participate in a RCT. Once invitees agreed to participate they were randomised to receive either influenza vaccination or placebo, but there was no control group which did not receive an invitation to participate. The primary focus of analysis was occurrence of GP assessed pneumonia or ILI |
| Anderson 1979 | Not RCT; survey of sub‐sample asked about swine flu |
| Anon 2003 | Not appropriate study design (not RCT, cohort, case‐control or time series). Article is a note about policy change by Centers for Medicare and Medicaid to remove requirement for physician signature on orders for influenza vaccination |
| Armstrong 1999 | Not RCT; 8596 community‐dwelling residents who received care at University of Pennsylvania primary care site; reminder postcard to receive influenza vaccination mailed to random sample of 5000; brochure mailed to 390 of remaining 3596; no control; no baseline data; excluded as cannot assess secular trend in rest of population |
| Arthur 2001 | Not RCT; offer of health assessment, but no control group |
| Bakare 2007 | Not RCT; retrospective survey of physician‐ and nurse‐initiated influenza vaccination in acute care hospital |
| Balalagué 1993 | Not RCT; survey of vaccination rates |
| Baldo 1999 | RCT; vaccination outcomes, focus on side effects; no intervention to increase vaccination rates |
| Bardenheier 2005 | Not RCT; survey of interventions in 14 US states used to increase influenza vaccination rates |
| Bardenheier 2010 | Survey of vaccination policies and influenza vaccination rates |
| Bardenheier 2011 | Survey of vaccination policies and influenza vaccination rates |
| Barker 1999 | Not RCT; cohort comparing Monroe Country and Onondaga County NY; no data on comparability of cohorts; Bennett 1994 and Kouides 1993 also describe this non‐RCT |
| Barton 1990 | Not RCT; an intervention to increase influenza vaccination rates was used. For HMO in Boston 1983‐4 = baseline rates as historical control; 1984 postcard reminders for high‐risk individuals < 65; 1985 chart reminders for > 65 plus feedback to service chiefs; 1986 chart reminders plus feedback to service chiefs plus feedback to physicians plus lists of unimmunised patients; excluded as historical controls; excluded as cannot assess secular trend in rest of population |
| Beardsworth 2004 | Not RCT; coalition helped family physicians purchase influenza vaccine, educational pamphlets and provided a hotline; no control group |
| Becker 1989 | Not RCT; 40 to 60 years of age; preventive care reminders |
| Bekker 2003 | Not RCT; survey of attitudes of those ≥ 65 to influenza vaccination |
| Belcher 1990 | RCT; interventions to increase influenza rates: comparing education and feedback to physicians, patient education and a health promotion clinic; no baseline influenza vaccination rates; data for those ≥ 60 not separately available. We e‐mailed the author for data for ≥ 60, but received no response |
| Bennett 1994 | Not RCT; no control group; intervention to increase influenza vaccination rates: community‐wide demonstration project in Monroe County, New York, to enrol all Medicare B enrollees ≥ 65 to increase influenza vaccination rates |
| Berg 2004 | RCT; intervention to increase influenza vaccination rates: informational sheet; publication does not state baseline data or data for those < 60 and ≥ 60 separately. We e‐mailed the trial authors for data but received no reply |
| Berg 2005 | Matched patients randomly assigned from geographic regions; 78% of patients < 65 |
| Birchmeier 2002 | Not RCT; cohort; no control; residents offered influenza vaccination to patients in clinic |
| Bloom 1988 | Not RCT (no control group); patients ≥ 65; intervention to increase influenza vaccination rates |
| Bloom 1999 | Not RCT; for patients ≥ 65 a fax was sent to family physician requesting they administer influenza and pneumococcal vaccines |
| Bond 2011 | RCT; cannot identify outcomes for those ≥ 65 |
| Bou‐Mias 2006 | Not RCT; individuals 60 to 64 in urban health centre in Spain; non‐random allocation to receive phone call about influenza vaccination or no call; no baseline rates for year before intervention |
| Bovier 2001 | Not RCT; survey of attitudes of ≥ 65 to influenza vaccination |
| Brady 1988 | RCT; cannot separate results for < 60 and ≥ 60 |
| Breen 2003 | Not RCT; pneumococcal vaccination campaign |
| Brimberry 1988 | RCT; article states no baseline influenza vaccination rates available; vaccination rates not separately available for those > 60 |
| Browngoehl 1997 | Not RCT, retrospective cohort; children |
| Buchner 1987 | RCT; intervention to increase influenza vaccination; ≥ 65 years; but self report of influenza vaccination by questionnaire |
| Burns 2005 | Survey of attitudes to vaccination |
| Call 2005 | No intervention to increase influenza vaccination; article describes the clinical diagnosis of ILI |
| Cardozo 1998 | Not appropriate study design (not RCT, cohort, case‐control or time series). Article is a retrospective chart review |
| Carey 1991 | Not RCT; audit of 13 preventive manoeuvres including influenza vaccination |
| Carman 2000 | RCT; but no intervention to increase vaccination in elderly (one group of long‐term care hospitals had an "opt in" policy for influenza vaccination and another group an "opt out" policy; focus was on vaccinating healthcare workers |
| Carter 1986 | RCT; design of brochure to promote influenza vaccination; unable to contact author for more baseline and outcome numbers and %s for those ≥ 60; self report of influenza vaccination |
| Chami 2012 | RCT in nursing homes to use hygienic measures to reduce infections; no influenza vaccine intervention |
| Chan 1999 | No intervention to increase vaccination rates. Article is a survey of influenza vaccination rates of female Medicare beneficiaries |
| Charles 1994 | Not RCT; patients at Sunnybrook Health Science Centre Family Practice Unit, Toronto; 4 physician teams divided into 2 groups and "patients of two of the four teams were designated as subjects and patients of the remaining two were designated as controls," then "simple random selection of patients from the roster of each team physician to participate in the study." (Patients ≥ 65) |
| Chen 2007 | No intervention to increase vaccination rates. Article is a telephone survey of attitudes to influenza vaccination |
| Cheney 1987 | RCT; intervention to increase influenza vaccination rates: internal medicine residents were randomised to receive preventive care checklists; no baseline pre‐intervention influenza vaccination rates; no numbers for outcomes, only graphical presentation on small graphs so cannot assess numbers. We e‐mailed authors for numbers for outcomes but did not receive a reply |
| Chi 2006 | No intervention to increase vaccination rates. Article is a telephone survey of factors influencing influenza vaccination |
| Chodroff 1990 | Not RCT; 1986 historical controls; 1986 to 1990 residents given preventive care checklists |
| Christenson 2001 | Not RCT; no control group; intervention to increase influenza vaccination rates: all individuals in Stockholm county ≥ 65 (n = 259,627) invited to participate in influenza plus pneumococcal vaccination campaign; 100,242 received vaccine; focus on effectiveness of vaccination in reducing hospitalisation and pneumonia |
| Clancy 2003 | RCT; publication does not provide separate data for those < 60 and ≥ 60, or baseline influenza vaccination data for year prior to intervention; unable to locate author |
| Cohen 1982 | RCT; no baseline data for influenza vaccination rates; influenza rates for patients > 60 not separately available |
| Cohen 2004 | Not appropriate study design (not RCT, cohort, case‐control or time series). Article is an observational study of how physicians offer vaccination during consultations |
| Colombo 2005 | Not appropriate study design (not RCT, cohort, case‐control or time series). Article is an economic analysis of vaccination strategies |
| Correa‐de‐Araujo 2006 | Secondary analysis of differences in immunization rates by ethnic group in Medical Expenditure Panel Survey; no intervention to increase vaccination rates |
| Costa Tadeo 1994 | Not appropriate study design (not RCT, cohort, case‐control or time series). Article is a prospective cross‐over without control; results for ≥ 60 not available |
| Cowan 1992 | RCT; 16 residents in intervention, 13 in control group; no data that residents or patients groups similar; retrospective chart review of 107 charts (62 intervention, 45 control), also random sample of charts seen by first year residents (different residents from current sample) previous year |
| Cowan 2006 | No intervention to increase vaccination rates. Article is about attitudes to vaccination among healthcare workers |
| Crawford 2005 | Not RCT; patients in a Managed Care Organization (MCO) in "the eastern United States;" For breast cancer screening, cervical cancer screening or influenza vaccination (≥ 65 years) interactive voice reminders were sent; no control group; no data on secular trends; baseline data for year before intervention available |
| Crawford 2011 | No intervention; survey of patient characteristics of those ≥ 65 accepting influenza vaccination |
| Crouse 1994 | Not RCT; 6 community hospitals in N. Minnesota assessed 3 strategies to increase influenza vaccination rates: standing orders, physician chart reminders, physician education; excluded as cannot assess secular trend in rest of population |
| Curry 2006 | Survey of factors associated with influenza vaccination; no intervention to increase vaccination rates |
| Daniels 2007 | RCT; intervention to increase influenza vaccination rates: onsite adult vaccination in churches; abstract states patients ≥ 65, but Table 1 states mean age is 65 with SD = + or ‐ 14, so clearly includes patients younger than 60 |
| Dannetun 2003 | Survey of reasons for not being vaccinated by seniors in Linköping, Sweden; no intervention to increase vaccination rates |
| Davidse 1995 | Not RCT; GPs selected patients in Brabant for vaccination; cannot separate ≥ 60, no publication by this author since 1995 in MEDLINE to obtain e‐mail address |
| Davidson 1984 | Not RCT; intervention to increase influenza vaccination rates: university‐based internal medicine practice in N. Carolina; 50% sample selected 1 July 1979 to 30 June 1980 to receive nurse reminder for influenza vaccination, then another 50% sample selected 1 January to 31 December 1981; 50% not selected in each period served as controls; not stated what overlap occurred between intervention groups in the 2 periods, or controls in 2 periods; excluded as cannot assess secular trend in rest of population |
| Davis 2005 | Focus groups with physicians about barriers to influenza vaccination |
| De Wals 1989 | Not RCT; intervention to increase vaccination rates: patients of GPs in Braine‐le‐Château, Belgium; 1984 baseline; 1985 information campaign by GPs; 1986 information campaign by posters, newspaper editorials and lectures for retired individuals; excluded as cannot assess secular trend in rest of population |
| De Wals 1996 | Not RCT; survey of influenza vaccination rates in long‐term care facilities in Québec |
| Denis 1996 | Not RCT; intervention in Charleroi, Belgium, to increase influenza vaccination rates in those ≥ 65 |
| Desbiens 2005 | Not RCT; observational study of All‐Inclusive Care for the Elderly programme in Chattanooga, Tennessee. No control group |
| Dexter 2001 | RCT; intervention to increase influenza vaccination rates in hospitalised patients; cannot separate those ≥ 60 |
| Dickey 1990 | Not appropriate study design (not RCT, cohort, case‐control or time series). Survey of US family physicians about interest in using patient‐held health passport preventive care checklist |
| Dickey 1992 | Not RCT. Health Passport preventive care checklists used for preventive services in university family medicine clinic, but key table listing preventive services is omitted from article |
| Dickey 1993 | Literature review of paediatric and adult patient‐held preventive healthcare cards |
| Dini 1996 | No intervention to increase vaccination rates and not appropriate age group. (Audit of childhood vaccinations in Georgia, USA) |
| Donato 2007 | Not RCT; intervention to increase vaccination rates: 650‐bed community hospital in Pennsylvania; 2002 nurses screened patients for influenza vaccination, put reminder stickers on front of chart and orders in chart for physician to sign; 2003 nurses screened patients and standing order for influenza vaccination before discharge; 2004 same as 2003 plus Grand Rounds and nursing education sessions on each unit; excluded as cannot assess secular trend in rest of population |
| Douglas 1990 | Not RCT; no intervention to increase influenza vaccination rates. Retrospective audit in Kansas City family medicine residency programme clinics |
| Earle 2003 | Not RCT; survey of patients with colorectal cancer in SEER (US National Cancer Institute Survival, Epidemiology, and End Results) programme and factors associated with vaccination; average age 79; no baseline data for year before case‐control study; no control |
| Egido Polo 1989 | Data for those ≥ 60 not available; e‐mail for author not available |
| Etkind 1996 | Not RCT; in Essex county, Massachusetts, letters sent to all health care providers, press releases, newspaper articles, radio and TV announcements, lectures at senior centres, influenza vaccination clinic schedules sent to all community and elder organizations, Grand Rounds at each Essex County hospital; in Worcester county "usual care"; excluded as not RCT, geographical areas may not be comparable |
| Evans 2003 | No intervention to increase vaccination rates. Survey of reasons for not being vaccinated against influenza |
| Fairbrother 1999 | Not target age group (childhood vaccinations) |
| Fedson 1989 | No intervention to increase vaccination rates (guidelines for influenza vaccination in institutional settings) |
| Fedson 1994 | No intervention to increase vaccination rates (article presenting guidelines for prevention and control of influenza in hospitals and hospital staff) |
| Fedson 1996 | No intervention to increase vaccination rates (review of effectiveness of influenza vaccine) |
| Fernández Silvela 1994 | Not RCT; cohort; no control group; no baseline data |
| Ferrante 2010 | Cross‐sectional data from RCT on colon cancer screening; 23% received influenza vaccination, but no report of comparison to control group |
| Fiebach 1991 | Survey of reasons for accepting or refusing influenza vaccination |
| Fishbein 2006a | Observational study of missed opportunities for influenza vaccination |
| Fishbein 2006b | Cohort; average age 46‐8; cannot separate outcomes for those > 65; no reply to e‐mail to author |
| Fisher 2003 | Cross‐sectional analysis of spending patterns in Medicare regions and influenza vaccination rates; no intervention to increase vaccination rates in elderly |
| Fitzner 2001 | Theoretical model of cost‐effectiveness of influenza vaccination in Hong Kong |
| Fitzpatrick 2004 | Not RCT; retrospective case‐control; no intervention to increase vaccination rates in elderly |
| Flach 2004 | Secondary analysis of survey of relationship of patient‐centred care and vaccination rates in Veterans Administration Hospitals |
| Fontanesi 2004 | Analysis of workflow observations of care of patients ≥ 50 in convenience sample of 16 ambulatory care settings in San Diego (California) and Rochester (New York); development of model of 7 critical organisational, temporal and clinical activities that predicted 93% of influenza immunisations |
| Fowles 1998 | Not RCT; survey of influenza vaccination rates in seniors in HMO in Minneapolis‐St. Paul comparing staff, multispecialty or primary care practices |
| Frame 1994 | RCT; 10 preventive items; no influenza vaccination data |
| Francisco 2006 | Survey of reasons for not receiving influenza vaccination among those ≥ 60 in São Paulo, Brazil |
| Frank 1985 | Not RCT; cohort, no control; reminder letters and phone calls for influenza vaccination |
| Frick 2004 | Analysis of changes in influenza vaccination rates by race in US among disabled seniors |
| Furey 2001 | Not RCT; feedback to GPs on influenza vaccination rates in ≥ 75 in Merton Sutton and Wandsworth Health Authority, UK |
| Galasso 1977 | Review of clinical trials of influenza vaccination 1976 |
| Ganguly 1989 | Survey of reasons for acceptance/refusal of vaccination |
| Ganguly 1995 | Survey of vaccination status of veterans in a nursing home |
| Gannon 2012 | Not RCT, cohort study or time series; no control; team intervention to improve multiple vaccination rates; no data on secular trends |
| Garrett 2005 | Not RCT; pre‐post cohort; study of employed workers, i.e. < 65; ages not stated |
| Gauthey 1999 | Survey of influenza vaccination rates and motivations for receiving influenza vaccine among those > 65 in the State of Geneva |
| Gelfman 1986 | Before and after one group study; no control group; physicians were not prompted to offer influenza and pneumococcal vaccinations to high‐risk patients at the beginning of the influenza season, then were prompted later in the influenza season by reminders placed on charts at the Medical College of Virginia |
| Gerace 1988 | Not RCT; cohort, no control; comparison of letter in 1985 and phone call in 1986 |
| Giles 2003 | Summary of articles by Arthur 2002 and Hull 2002 |
| Gill 2000 | Not RCT; Christiana Care Foulk Road Family Medicine Center, Delaware; 1997 baseline rates; 1998 reminder to nurse and physician during visit; excluded as cannot assess secular trend in rest of population |
| Gill 2005 | Not RCT; retrospective cohort; impact of "Providing a Medical Home to the Uninsured" in Delaware, US; cannot separately identify those ≥ 60 |
| Goebel 2005 | Not RCT; retrospective chart review of physicians who used standing orders and those who did not |
| Grabenstein 1990 | Survey of vaccination status at Walter Reed Army Hospital |
| Grabenstein 1992 | Cost‐effectiveness model of pharmacists advocating and providing influenza vaccine |
| Grabenstein 2001 | No RCT; survey of influenza vaccination in Washington State (where pharmacists can give influenza vaccinations) and Oregon (where they cannot) |
| Granolllers 1993 | Not RCT; not ≥ 60; nursing staff preventive care interventions |
| Green 2003 | Survey of the relationship of functional status, depression and treatment for psychiatric problems, to rates of influenza vaccination in those ≥ 65 In the Kaiser Permanente Northeast HMO |
| Greene 2001 | Survey of uptake of preventive care |
| Groll 2006 | Not RCT; study of Universal Influenza Campaign in Ontario; data for those ≥ 60 not separately available |
| Gutiérrez 2005 | Economic evaluation of influenza vaccination for those ≥ 65 in Mexico |
| Gutschi 1998 | RCT; intervention to increase influenza rates; no vaccination rates for year before intervention; cannot separate rates for those ≥ 60 |
| Hahn 1990 | Not RCT; use of a health maintenance protocol in a family practice clinic; no influenza intervention or outcomes |
| Halliday 2003 | Survey of 19 residential care facilities in Australian Capital Territory on staff vaccination |
| Hanna 2001 | Not RCT; survey of pneumococcal and influenza vaccine rates in Indigenous population in New Zealand, and monitoring after local physicians encouraged to offer vaccination; no control group; no information on secular trends; cannot separate outcomes for those ≥ 60 |
| Hannah 2005 | Not RCT, CCT, cohort or time series; description of intervention programme in W. Virginia; no patient outcome data |
| Harari 2008 | RCT; influenza vaccination only recorded for year before study (Table 3) |
| Harbarth 1998 | Not RCT, cohort or time series (concurrent comparison group) |
| Harris 1990 | Retrospective chart review; N. Carolina Memorial Hospital Department of Medicine Polyclinic Practice; time series: 1979 to 1980 no prompts; 1981 nursing prompt; 1984 computer prompt; excluded as cannot assess secular trend in rest of population; cannot assess n's in target groups from Figure 2 |
| Harris 2006 | Not RCT; 249 patients with COPD recently discharged from hospital in Adelaide, Australia, for COPD intervention group (received Manual of Cochrane Collaboration systematic review summaries related to COPD) and control groups allocated to separate geographical areas; author sent PhD and we were able to verify it was not a RCT |
| Hedlund 2003 | Not RCT; study of influenza and pneumococcal vaccination campaign for individuals ≥ 65 in Stockholm County, Sweden, 1998; no control group; baseline data for year before intervention not available |
| Henk 1975 | Not RCT; cohort, no control; age lists used to identify patients for influenza vaccination |
| Hermiz 2002 | RCT; no intervention to increase influenza vaccination; no statement whether vaccinated patients had received vaccination before or after intervention |
| Hirdes 2006 | Survey of predictors of vaccination in Ontario nursing homes |
| Hoey 1982 | Not RCT; intervention to increase vaccination rates: nurses offered influenza vaccination to half patients seen in morning clinics, and patients were vaccinated by physicians in afternoon clinics; patients ≥ 60 cannot be identified |
| Honkanen 1996 | Survey of knowledge about influenza vaccination |
| Honkanen 1997 | Not RCT; for 3 administrative areas in Finland; Admin Area A: risk disease based influenza vaccination programme; admin area B: age‐based vaccination programme offered Autumn 1993 and 1994; admin area C: age‐based vaccination programme offered 1992 to 1994; areas not necessarily identical |
| Honkanen 2006 | Not RCT; northern Finland; 14 municipalities risk of disease‐based intervention x 2 years; 29 municipalities: age‐based intervention x 2 years. 12 municipalities cross‐over from disease‐based intervention in 1992 to age‐based intervention in 1993; excluded as not RCT; geographical areas may not be comparable |
| Humair 2002 | Not RCT; primary care clinic of Department of Community Medicine, Geneva University Hospital; 1995 baseline; 1996 leaflets and posters at reception desk and waiting areas, walk‐in immunisation clinic, 1.5‐hour training workshop on influenza for physicians, computer reports q 2 weeks to residents on vaccination performance compared to other residents; reminder stickers for records of high‐risk patients; excluded as cannot assess secular trend in rest of population |
| Hutchinson 1995 | Not RCT; survey of influenza vaccination in clinic patients |
| Hutchison 1991 | Not RCT; historical control 1982 to 1983; reminder letter 1987 to 1988 |
| Hutt 2010 | Quasi‐experimental mixed methods; cohort (8 nursing homes in Denver; no data on comparability of 8 non‐intervention nursing homes in Missouri and Kansas); survey of implementation of guidelines on nursing home‐acquired pneumonia and hospitalisation; data on influenza vaccination rates 2004 to 2007 |
| Jacobs 2001 | Not RCT; retrospective chart review of use and non‐use of interpreters for clinical and preventive services |
| Jain 1998 | Survey; no intervention to increase influenza vaccination |
| Jans 2000 | Cohort of 14 medical practices with 16 physicians implementing 8 guidelines for care of COPD and asthma, compared to 5 control practices with 5 physicians "located in the same region" (non‐comparable intervention and control groups: practices differed P value = 0.04 in "troublesome symptoms" and P value < 0.01 in type of disease (COPD versus asthma)) |
| Jefferson 1996 | Economic evaluation of influenza vaccination |
| Jiménez‐Garcia 2007 | Survey of influenza vaccination rates of COPD patients in Catalonia |
| Jin 2003 | Secondary analysis of Alberta administrative data for influenza vaccination rates for those ≥ 65 |
| Johnson 2005 | C‐RCT; no outcome data for influenza |
| Kassam 2001 | C‐RCT; cannot separate outcomes for influenza vaccination from pneumococcal vaccination |
| Kelly 1988 | Not RCT; children |
| Kemper 1993 | RCT; children |
| Kendal 1985 | Survey of vaccination rates in nursing homes in the USA |
| Kennedy 1994 | Not RCT; tracking system for paediatric vaccinations in a Medicaid managed care organisation |
| Kern 1990 | Not RCT; preventive care audit by faculty of charts of patients seen by internal medicine residents; influenza vaccine outcomes not separately available for those ≥ 65 |
| Klachko 1989 | Not RCT; survey of influenza vaccination rates in diabetic clinic; data not available separately for those > 60 |
| Knoell 1991 | Not RCT; General Internal Medicine Group Practices at University of California at San Francisco; 1987 to 1988 baseline; 1989 pharmacist presented 3 in‐services to nursing staff about influenza vaccination, patients > 65 received information sheet in clinic, campaign to provide vaccination with or without a visit; excluded as cannot assess secular trend in rest of population |
| Korn 1988 | Not RCT; preventive medicine checklist placed on charts, including influenza for those ≥ 65; faculty audit of charts of 15 internal medicine residents exposed to intervention and 13 who had not been; no assessment if residents similar; no data on secular trends in practice |
| Kosiak 2006 | Secondary analysis of influenza vaccination rates for those ≥ 65 in 2004 National Healthcare Quality Report and National Healthcare Disparities Report |
| Kunze 1998 | Editorial; no intervention to increase vaccination rates |
| Kwong 2006 | Secondary analysis of influenza vaccination rates in 1996 to 1997 National Population Health Survey of Canada and Population Health Survey of Canada 2000 to 2001 and 2003, including those ≥ 65 |
| Kyaw 2002 | Survey of influenza vaccination rates and vaccination policies in 53 general practices in Scotland 1993 to 1999 |
| Landis 1995 | Not RCT; vaccine manager to increase use of 4 vaccines; no data on influenza vaccination |
| Landon 2004 | Secondary analysis of Centers for Medicare & Medicaid Services data on influenza vaccination rates for ≥ 65 |
| Larson 1979 | Not RCT; reminder letter to those ≥ 65 and high‐risk patients University of Washington family medicine centre; cannot separate outcomes for those ≥ 65 from high‐risk patients |
| Larson 1982 | RCT; intervention to increase influenza vaccination rates: postcard reminders; correspondence from author was neither able to provide precise baseline influenza vaccination rates before intervention (Dr Larson estimated them from a survey with a 75% response rate at 50%), nor provide data separately for those ≥ 60; self report of vaccination |
| Lau 2006 | Telephone survey of influenza vaccination rates among residents of Hong Kong ≥ 65 |
| Lawson 2000 | Not RCT; standing orders for influenza vaccination; no control group (community rate used as control rate, no details on characteristics of community group) |
| Lazorik 2001 | Not RCT; no intervention to increase vaccination rates; article summarising preventive care options |
| LeBaron 1997 | Not RCT; annual measurement and feedback programme; children |
| Lees 2005 | Secondary analysis of 2000 US National Health Interview on influenza vaccination rates |
| Leirer 1989 | Not RCT; intervention to increase influenza vaccination rates: 321 older people who attended community supported lunch program at a senior citizen centre (location not stated, authors' professional address is Stanford, California); 64 individuals ≥ 65 "randomly selected" from those who attended ≥ 1 per week; and 257 "randomly selected" from those attending less frequently; (however 64 + 257 = 321, leaving no degrees of freedom so the second sample could not have been randomly selected); frequency of attendance does not control for potential confounders; no baseline data |
| Leirer 1991 | Not RCT; no influenza outcomes, n = only 16 |
| Levy 1996 | French economic evaluations of influenza vaccination |
| Lieberman 2003 | Not RCT; no intervention to increase vaccination rates. Discussion article about managing respiratory infections |
| Lindley 2006 | Telephone survey of Medicare beneficiaries about vaccination rates |
| Loeser 1983 | Not RCT; report of computerised vaccination register for children in Montréal; no influenza outcomes |
| Lu 2005 | Secondary analysis of 1989 to 2002 US National Health Interview Surveys for influenza vaccination rates in those ≥ 65, and factors predicting vaccination |
| Lynd 2005 | Article about antivirals for influenza |
| Macdonald 1985 | Not RCT; mass campaign; children |
| Maciosek 2006 | Literature review of cost‐effectiveness of influenza vaccination |
| Madlon‐Kay 1987 | Not RCT; audit of 8 preventive care items but influenza not audited as seasonal administration |
| Mair 1974 | RCT with outcomes of antigenicity and reactogenicity. No intervention to increase vaccination rates |
| Malmvall 2007 | Not RCT; intervention to increase influenza vaccination rates: inhabitants ≥ 65 in Jönköping county, Sweden; 1999 to 2001 baseline; 90% of GPs informed of vaccination campaign 2002; education meetings encouraging senior practice nurses to vaccinate seniors each year 2002 to 2005; excluded as cannot assess secular trend in rest of population |
| Mandel 1985 | Not RCT; audit of 9 preventive care items but influenza not included |
| Mangione 2006 | Not RCT; secondary analysis of influenza vaccination status of random sample of 8661 patients with diabetes in 7 US health plans 2000 to 2001, and description of physician reminders, performance feedback and structured care management |
| Mangtani 2006 | Survey of attitudes to influenza vaccination of 844 community dwelling individuals ≥ 75 in the UK 2004 Medical Research Council Trial of Assessment and Management of Older People in the Community |
| Margolis 1988 | Not RCT; Veterans Affairs clinic in Minneapolis with patients in 3 sub‐specialty clinics as historical controls |
| Margolis 1992 | Not RCT; informational mailing to patients; standing vaccination orders; vaccination reminders on daily patient lists; walk‐in vaccination visits; no n's from control clinic; comparator is 2 clinics "similar location" |
| Marra 2011 | Random allocation of 12 communities in British Columbia to an intervention for pharmacists to offer influenza vaccination and 13 control communities; no data on vaccination rates in control communities |
| Marsteller 2006 | Secondary analysis of the Canadian 1999 National Nursing Home Survey of the influenza vaccination status of a random sample of 73,350 individuals ≥ 65 in 1423 nursing facilities |
| Martinen 2004 | Not RCT; cohort; no control; managing congestive heart failure in long‐term care |
| Mayo 2004 | No intervention to increase vaccination rates. Study of perceived barriers for hospital patients to receiving influenza vaccination |
| McArthur 1999 | Survey of factors affecting vaccination rates in all 1520 Canadian long‐term care facilities in 1991 |
| McDonald 1984 | RCT; intervention to increase influenza vaccination rates: residents randomly allocated to receive computer analyses of patient charts with care reminders including CDC recommendations for influenza vaccination; influenza outcomes; no pre‐intervention baseline data |
| McDonald 1992 | RCT; intervention to increase influenza vaccination rates: computer‐generated influenza vaccination reminders; publication does not provide separate data for those < 60 and ≥ 60, or baseline influenza vaccination data for year prior to intervention; unable to locate author |
| McKinney 1989 | Not RCT; survey of factors related to physician ordering of influenza vaccination in the Primary Care Clinic at Milwaukee County Medical Complex |
| McLeod 2001 | Analysis of influenza outbreaks in seniors' lodges in Calgary 1997 to 2000 |
| Merkel 1994 | Not RCT; cohort; reminder data sheet; influenza vaccination baseline data available for only 75% of cohort; no control |
| Milman 2005 | Not RCT, no control group; effect of patient care team on influenza decisions |
| Mody 2005 | Not RCT; survey of infection control practices in nursing homes in south‐east Michigan |
| Morrow 1995 | Not RCT; audit of 3 preventive items; no influenza data |
| Mosesso 2003 | Not RCT; prospective observational cohort study of influenza vaccination by emergency services in Pittsburgh |
| Mukamel 2001 | Not RCT, no control group, no influenza outcome data |
| Mulet Pons 1995 | Telephone survey of influenza vaccination status of those ≥ 65 in a health centre in Alicante, Spain, and reasons for refusing vaccination |
| Murphy 1996 | Not RCT; intervention to increase childhood 0 to 5 vaccination rates in an inner city Dublin family practice using postcard reminders and an improved vaccination record system |
| Métrailler 2003 | Not RCT; no intervention to increase vaccination rates |
| Müller 2005 | Not RCT, no intervention to increase vaccination rates |
| Nakatani 2002 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Ndiaye 2005 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates. In this review, none of the results are presented for people aged 60 years or older ‐ summary just shows "high risk" and occasionally results for those less than 65 years |
| Nichol 1990 | Cohort design. However, self reported vaccination status without validation |
| Nichol 1992 | No intervention to increase vaccination rates |
| Nichol 1998 | Not appropriate study design (not RCT, cohort, case‐control or ITS). Too few data points to qualify as time series). Had multicomponent interventions (over time) to increase vaccination rates for influenza and pneumococcal vaccines in the patient population of the Minneapolis Department of Veterans Affairs (VA) Medical Center; self report of vaccination |
| Nichol 2006 | No intervention to increase vaccination rates |
| Nicoleau 2001 | Not appropriate study design (not RCT, cohort or time series); interviews with patients about vaccination intentions |
| Nowalk 2004a | No intervention to increase vaccination rates |
| Nowalk 2004b | No intervention to increase vaccination rates |
| Nowalk 2004c | Not appropriate study design (not RCT, cohort or time series); no control group; outcome is office and patient factors associated with vaccination |
| Nowalk 2008 | Not RCT; "Two of the intervention sites were faith based, one was a federally qualified health center (FQHC), and one was a FQHC look‐alike; two intervention sites were University of Pittsburgh family medicine residency practices"; data for those ≥ 60 not separately identifiable |
| O'Connor 1996 | RCT. Not target age group |
| O'Connor 1998 | Not appropriate study design (not RCT, cohort, case‐control or ITS). Also unable to extract vaccination data for target age group |
| O'Malley 2006 | No intervention to increase vaccination rates |
| O'Reilly 2002 | No intervention to increase vaccination rates |
| Ohmit 1995 | Not appropriate study design. 4 counties in south‐central and southwestern Michigan were randomised to the intervention and 3 contiguous counties "... assigned to be the comparison area." (does not state were randomised). Cases were those > 65 hospitalised with pneumonia. 2 controls per case "... similar in age, gender and zip code, were randomly selected from current study area Medicare beneficiary files." (but had not had pneumonia, so differ from cases on a key characteristic) |
| Ompad 2006 | Not appropriate study design (literature summary of vaccination in different settings) |
| Ornstein 1991 | Not influenza vaccination |
| Overhage 1996 | Not influenza vaccination |
| Padiyara 2011 | Cohort (1 group had 1 visit to the pharmacist, other group had 2 or more visits); groups were similar in gender, age, ethnicity diabetes and hypertension rates |
| Parchman 2004 | No intervention to increase vaccination rates |
| Parry 2004 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Pasquarella 2003 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Patel 2004 | Not target age group. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Patel 2006 | No intervention to increase vaccination rates |
| Patriarca 1985 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Payaprom 2011 | Not RCT; cannot identify outcomes for those > 65 |
| Pearson 2005 | Not appropriate study design (cohort, no control); patients presenting to an emergency department were invited to receive influenza and pneumococcal vaccinations |
| Piedra 1995 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Pleis 2002 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Ploeg 1994 | Not influenza vaccine. The study included interventions to address several health behaviours, however the focus of this article is on outcomes other than vaccination (i.e. safety changes to prevent injury) |
| Poole 2010 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Postma 2005 | Not target age group. Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Prati 2012 | RCT; individuals ≥ 65; no influenza vaccination outcomes (only risk perception, efficacy and self efficacy) |
| Puig‐Barbera 1999 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Quinley 2004 | Not influenza vaccination |
| Rantz 2001 | Not influenza vaccination, no intervention to increase vaccination rates |
| Reichert 2001 | Not target age group. No intervention to increase vaccination rates |
| Resnick 2001 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Ressel 2003 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Retchin 1991 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Rimple 2006 | Not appropriate study design (Not RCT, cohort or time series); offer of vaccination to patients in an emergency department; no control group |
| Robare 2011 | RCT; however, the Brief Education and Counselling Intervention and BECI plus physical activity group outcomes were pooled and no control group |
| Rodewald 1999 | Not target age group |
| Rodriguez 1993 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Rodriguez‐Rodriguez 2006 | No intervention to increase vaccination rates |
| Roffey 1998 | No intervention to increase vaccination rates |
| Russell 2000 | No intervention to increase vaccination rates |
| Rust 1999 | Not target age group. Not influenza vaccine |
| Ryan 1984 | Not target age group. No intervention to increase vaccination rates. Assesses impact of adverse events/side effects of prior vaccination on influenza vaccine acceptance in subsequent season among persons of all ages |
| Sambamoorthi 2005 | No intervention to increase vaccination rates |
| Sansom 2003 | Not influenza vaccination |
| Sarnoff 1998 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Schectman 1995 | No intervention to increase vaccination rates, not influenza vaccination |
| Schensul 2009 | RCT (2 buildings randomised); multi‐level intervention to increase influenza vaccination rates; average age of male participants = 57, female = 62; cannot identify results for those ≥ 60. E‐mail from Dr. Schensul 31 March 2013: "We have only baseline and endline data for our treatment and control groups and no data on vaccination rates prior to intervention baseline. With respect to randomization, our CDC funded study was a pilot that used a quasiexperimental design, with buildings matched by number of residential units and as best we could, by ethnicity. We could not apply randomization to our intervention assignment, as our pilot funding was not sufficient to allow us to randomize and work in multiple buildings, and the intervention was a "community" intervention designed to have an effect on the entire population of the intervention building." |
| Schluter 1999 | Not appropriate study design (cohort study without control); nursing homes in Colorado were surveyed for policies to provide influenza vaccination to staff, and influenza vaccination rates were measured 1995/6 and 1997/8 |
| Schmitz 1993a | Not appropriate study design (not RCT, cohort or time series; survey of vaccination rates in nursing homes) |
| Schmitz 1993b | Not appropriate study design (not RCT, cohort or time series; survey of vaccination rates in nursing homes) |
| Schneider 2001 | Not appropriate study design (not RCT, cohort or time series); 1996 Medicare Current Beneficiaries Survey interviewed individuals and compared vaccination status in managed care and fee‐for‐service practices |
| Schreiner 1988 | Not appropriate study design, not influenza vaccination |
| Schwartz 2006 | Not appropriate study design (not RCT or time series); cohort without control group; patients in 7 clinics offered vaccination by non‐physician staff members |
| Schwarz 2005 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Scott 1996 | No intervention to increase vaccination rates |
| Setia 1985 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Shah 2006 | Not RCT; emergency services screened adults for needed preventive interventions |
| Shahrabani 2006 | No intervention to increase vaccination rates |
| Shank 1989 | Not appropriate study design |
| Shenson 2005 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Shenson 2007 | No intervention to increase vaccination rates |
| Shenson 2011 | Not RCT; survey of screening received by those ≥ 65 |
| Shugarman 2006 | Retrospective cross‐sectional study; nursing homes; outcome = ILI |
| Siebers1985 | Not influenza vaccination |
| Simor 2002 | No intervention to increase vaccination rates |
| Siriwardena 2003a | Not appropriate study design (not RCT, time series); audit and anonymised feedback but no control group and no data on vaccination trends in Lincolnshire in non‐participating practices |
| Slobodkin 1998 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Soljak 1987 | Not target age group |
| Stancliff 2000 | Not appropriate study design; not appropriate age group; syringe exchange programme |
| Stehr‐Green 1993 | Not target age group |
| Stenqvist 2006 | Not appropriate study design |
| Steyer 2004 | Not RCT, cohort or time series; survey of vaccination rates in US states where pharmacists can and cannot give influenza vaccinations |
| Stott 1998 | No intervention to increase vaccination rates |
| Straits‐Troster 2006 | No intervention to increase vaccination rates |
| Stratis Health 1997 | Not RCT; intervention to increase influenza vaccination: postcard sent to 38,000 households with Medicare B beneficiary in Ramsey County, Minnesota; letter to sent to 2983 households with Medicare B beneficiary in selected zip codes; as comparator Hennepin county selected as urban county with similar demographics; individuals ≥ 65 |
| Stuart 1969 | No intervention to increase vaccination rates. Assessed vaccine efficacy related to outbreak investigation |
| Sylvan 2003 | Not appropriate study design |
| Szilagyi 1992 | Not target age group |
| Szilagyi 2005 | No intervention to increase vaccination rates |
| Szilagyi 2006 | Not target age group |
| Szucs 2006 | No intervention to increase vaccination rates |
| Tabbarah 2005 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates |
| Tacken 2002 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Tape 1993 | Not appropriate study design (i.e. not a RCT): this was an intervention study but allocation was not randomised. Results were presented but it was not possible to extract age‐specific results |
| Terrell‐Perica 2001 | RCT with intervention to increase vaccination rates. Excluded as not possible to extract results for persons over age 60 |
| Tierney 2005 | RCT; outcomes for those ≥ 60 cannot be separately identified |
| Tollestrup1991 | Not target age group, not influenza vaccination |
| Toscani 2003 | No intervention to increase vaccination rates |
| Traeger 2006 | Not appropriate study design (not RCT or time series); Whiteriver Services Unit in Arizona reported vaccination rates; no control group |
| Trick 2009 | Not RCT; electronic reminder intervention to increase influenza vaccination rates; average age of participants = 52; cannot identify individuals ≥ 60 |
| Tucker 1987 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Turner 1989 | Not influenza vaccination; not appropriate study design |
| Turner 1990 | RCT comparing computer prompts for physicians and computer prompts for physicians plus card prompts for their patients on performance of multiple preventive interventions including influenza vaccination. However, it is not possible to extract outcomes by age group |
| Turner 2003 | Not appropriate study design (not RCT, cohort, case‐control or ITS). No intervention to increase vaccination rates. Not influenza vaccination |
| Tymchuk 1991 | No intervention to increase vaccination rates |
| Usami 2009 | RCT; intervention to increase influenza vaccination rates (pharmacists explained risk of influenza and benefits of vaccine); participants ≥ 65; excluded as influenza vaccination rate by self report |
| Van Amburgh 2001 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Van den Hooven 2006 | No intervention to increase vaccination rates |
| van Essen 1997 | Results specific to the age group of interest to this review are not presented |
| Van Hoof 2001 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| van Lieshout 2012 | Not RCT; survey of cardiovascular care |
| Wadhwa 1997 | RCT; patients ≥ 65; but 57% of those in the phone arm were not contacted either by voice or machine, so excluded as unknown large risk of bias |
| Walker 1992 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Walsh 2012 | RCT; cannot separate outcome data for those 60 and older |
| Wang 2005 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Warren 1995 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Watkinson 2004 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Weatherill 2004 | Not appropriate study design; campaign to vaccinate high risk populations in disadvantaged area in Vancouver; no control; no data on secular trends; cannot separate outcomes for those ≥ 60 |
| Weaver 2001 | Not appropriate study design (not RCT, cohort, case‐control or ITS). The data for this study derive from a RCT; however, the focus of this article is a cost‐effectiveness analysis of a community‐based outreach initiative to promote pneumococcal and influenza vaccines for people aged 65 years or older. The full report of the RCT is presented in Krieger 2000 |
| Weaver 2003 | Not target age group. Although elderly persons were included, outcomes data could not be extracted by age group. The study design is best described as a cohort study |
| Wee 2001 | Not appropriate study design (not RCT, cohort or time series); chart review; no intervention |
| Wei 2007 | No intervention to increase vaccination rates |
| Whelan 2013 | Effect of request for proxy assent on recruitment to RCT of vaccination in care homes; no influenza vaccination outcome data |
| While 2005 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Wiese‐Posselt 2006 | No intervention to increase vaccination rates |
| Wilkinson 2002 | Not target age group. This was a pilot study and patients were randomly allocated to intervention; however, it is not possible to extract outcomes by age group |
| Williams 1987 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Wilson 1989 | Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Winston 2006a | Not appropriate study design (not RCT, cohort or time series); telephone survey in 5 US states; no control group; no intervention to increase vaccination rates |
| Winston 2006b | Not appropriate study design (not RCT, cohort or time series); chart review after introduction of vaccination policy in 4 Michigan hospitals; no control group |
| Wood 1998 | Not target age group |
| Wortley 2005 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Wray 2009 | RCT; intervention to increase influenza vaccination rates (vaccine safety message versus vaccine information statement); no influenza vaccination outcomes; cannot separate results for ≥ 60 |
| Wright 2011 | RCT; outcome data for those 60 and older cannot be identified; no reply from e‐mail to author |
| Wuorenma 1994 | Not target age group. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Yoo 2006 | No intervention to increase vaccination rates. Not appropriate study design (not RCT, cohort, case‐control or ITS) |
| Young 1980 | Not target age group |
| Zimmerman 2003a | No intervention to increase vaccination rates; survey of self report compared to medical record of influenza and pneumococcal vaccination |
| Zimmerman 2003b | No intervention to increase vaccination rates; survey of vaccination rates |
| Zimmerman 2003c | Not appropriate study design (not RCT or time series); cohort study compared vaccination rates in 2 health centres which could choose which interventions to implement; no control; Health Centre A chose clinic posters, mailed reminders, free vaccine, community posters, staff education, chart reminders, standing orders, designated vaccination times; Health Centre B chose clinic posters, free vaccine, community posters, staff education, reminder card in chart, standing orders, any time vaccination and off‐site vaccination clinics. It was thus not possible to disentangle the effects of interventions |
| Zimmerman 2004 | No intervention to increase vaccination rates; survey of factors associated with vaccination |
COPD: chronic obstructive pulmonary disease CDC: Centers for Disease Control CCT: controlled clinical trial C‐RCT: cluster‐randomised controlled trial GP: general practitioner HMO: Health Maintenance Organization ILI: influenza‐like illness ITS: interrupted time series RCT: randomised controlled trial SD: standard deviation
Characteristics of studies awaiting assessment [ordered by study ID]
Lee 2003
| Methods | Awaiting translation from Korean |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Song 2000
| Methods | Awaiting translation from Korean |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Contributions of authors
Roger E Thomas (RET) and Margaret Russell (MLR) for the first publication identified the question and planned the methodological approach using the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). RET and Diane Lorenzetti (DLL) planned and conducted the literature search for the first, second and third publications; RET for the first, second and third publications, MLR for the first publication and DLL for the second and third publications independently reviewed all citations for possible relevance. RET and MLR independently for the first publication and RET and DLL for the second and third publications assessed whether the studies were RCTs that contained data on increasing influenza vaccination uptake of seniors, extracted outcome data and entered data into data abstraction forms. RET undertook the analyses and wrote the text of the first, second and third publications of the review, MLR and DLL reviewed the text of the first and RET and DLL the second and third publications, and DLL wrote the search strategies for all publications.
Sources of support
Internal sources
None, Other.
External sources
No sources of support, Other.
Declarations of interest
Roger E Thomas: none known. Diane L Lorenzetti: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Abramson ZH, Avni O, Levi O, Miskin IN. Is the influenza vaccination rate of elderly patients affected by raising the vaccination rate of the staff at their primary health care clinics?. Israel Medical Association Journal 2011;13:325‐8. [PubMed] [Google Scholar]; Abramson ZH, Avni O, Levi O, Miskin IN. Randomized trial of a program to increase staff influenza vaccination in primary care clinics. Annals of Family Medicine 2010;8:293‐8. [DOI: 10.1370/afm.1132] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur AJ, Matthews RJ, Jagger C, Clarke M, Hipkin A, Bennison DP. Improving uptake of influenza vaccination among older people: a randomised controlled trial. British Journal of General Practice 2002;52(482):717‐22. [PMC free article] [PubMed] [Google Scholar]
- Baker AM, McCarthy B, Gurley VF, Yood MU. Influenza immunization in a managed care organization. Journal of General Internal Medicine 1998;13(7):469‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnas GP, McKinney WP. Postcard reminders and influenza vaccination. Journal of the American Geriatrics Society 1989;37(2):195. [DOI] [PubMed] [Google Scholar]
- Beck A, Scott J, Williams P, Robertson B, Jackson D, Gade G, et al. A randomized controlled trial of group outpatient visits for chronically ill older HMO members: The Cooperative Health Care Clinic. Journal of the American Geriatrics Society 1997;45:543‐9. [DOI] [PubMed] [Google Scholar]
- Berg GD, Silverstein S, Thomas E, Korn AM. Cost and utilization avoidance with mail prompts: a randomized controlled trial. American Journal of Managed Care 2008;14(11):748‐54. [PubMed] [Google Scholar]
- Black ME, Ploeg J, Walter SD, Hutchinson BG, Scott EA, Chambers LW. The impact of a public health nurse intervention on influenza vaccine acceptance. American Journal of Public Health 1993;83(12):1751‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington J, Bell KM, LaForce FM. A target‐based model for increasing influenza immunizations in private practice. Genesee Hospital Medical Staff. Journal of General Internal Medicine 1991;6(3):204‐9. [DOI] [PubMed] [Google Scholar]
- Chambers CV, Balaban DJ, Carlson BL, Grasberger DM. The effect of microcomputer‐generated reminders on influenza vaccination rates in a university‐based family practice center. Journal of the American Board of Family Practice 1991;4(1):19‐26. [PubMed] [Google Scholar]
- Chan L, MacLehose RF, Houck PM. Impact of physician reminders on the use of influenza vaccinations: a randomized trial. Archives of Physical Medicine and Rehabilitation 2002;83(3):371‐5. [DOI] [PubMed] [Google Scholar]
- Clayton AE, McNutt LA, Homestead HL, Hartman TW, Senecal S. Public health in managed care: a randomized controlled trial of the effectiveness of postcard reminders. American Journal of Public Health 1999;89(8):1235‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby DM, Sellors JW, Fraser FD, Fraser C, Ineveld C, Howard M. Effect of preventive home visits by a nurse on the outcomes of frail elderly people in the community: a randomized controlled trial. Canadian Medical Association Journal 2000;162(4):497‐500. [PMC free article] [PubMed] [Google Scholar]
- Dapp U, Anders J, Renteln‐Kruse W, Meier‐Baumgartner HP. Active health promotion in old age: methodology of a preventive intervention programme provided by an interdisciplinary health advisory team for independent older people. Journal of Public Health 2005;13:122‐7. [Google Scholar]; Dapp U, Anders JAM, Renteln‐Kruse W, Minder CE, Meier‐Baumgartner HP, Swift CG, et al. A randomized trial of effects of health risk appraisal combined with group sessions or home visits on preventive behaviors in older adults. Journal of Gerontology A Biological Medical Science 2011;66A(5):591‐8. [DOI] [PubMed] [Google Scholar]
- Diaz Gravalos GJ, Palmeiro FG, Vazquez Fernandez LA, Casado Gorriz I, Fernandez Bernardez MA, Sobrado Palomares JR. Annual influenza vaccination. Causes of non‐compliance among patients aged over 65 years [Vacunación antigripal anual: causa de incumplimiento en mayores de 65 años]. Medifam ‐ Revisita de Medicina Familiar y Comunitaria 1999;9(4):222‐6. [Google Scholar]
- Dietrich AJ, Duhamel M. Improving geriatric preventive care through a patient‐held checklist. Family Medicine 1989;21(3):195‐8. [PubMed] [Google Scholar]
- Frank O, Litt J, Beilby J. Opportunistic electronic reminders. Improving performance of preventive care in general practice. Australian Family Physician 2004;33(1‐2):87‐90. [PubMed] [Google Scholar]
- Garcia‐Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez‐Roisin R, Anto JM. Roca J. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101(7):1462‐9. [DOI] [PubMed] [Google Scholar]
- Herman CJ, Speroff T, Cebul RD. Improving compliance with immunization in the older adult: results of a randomized cohort study. Journal of the American Geriatrics Society 1994;42(11):1154‐9. [DOI] [PubMed] [Google Scholar]
- Hogg WE, Bass M, Calonge N, Crouch H, Satenstein G. Randomized controlled study of customized preventive medicine reminder letters in a community practice. Canadian Family Physician 1998;44:81‐8. [PMC free article] [PubMed] [Google Scholar]
- Hogg W, Lemelin J, Graham ID, Grimshaw J, Martin C, Moore L, et al. Improving prevention in primary care: evaluating the effectiveness of outreach facilitation. Family Practice 2008;25(1):40‐8. [DOI] [PubMed] [Google Scholar]
- Hull S, Hagdrup N, Hart B, Griffiths C, Hennessy E. Boosting uptake of influenza immunisation: a randomised controlled trial of telephone appointing in general practice. British Journal of General Practice 2002;52(482):712‐6. [PMC free article] [PubMed] [Google Scholar]
- Humiston SG, Bennett NM, Long C, Eberly S, Arvelo L, Stankaitis J, et al. Increasing inner‐city adult influenza vaccination rates: a randomized controlled trial. Public Health Reports 2011;126(Suppl 2):39‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives DG, Lave JR, Traven ND, Kuller LH. Impact of Medicare reimbursement on influenza vaccination rates in the elderly. Preventive Medicine 1994;23(2):134‐41. [DOI] [PubMed] [Google Scholar]
- Karuza J, Calkins E, Feather J, Hershey CO, Katz L, Majeroni B. Enhancing physician adoption of practice guidelines. Dissemination of influenza vaccination guideline using a small‐group consensus process. Archives of Internal Medicine 1995;155(6):625‐32. [PubMed] [Google Scholar]
- Kellerman RD, Allred CT, Frisch LE. Enhancing influenza immunization. Postcard and telephone reminders and the challenge of immunization site shift. Archives of Family Medicine 2000;9(4):368‐72. [DOI] [PubMed] [Google Scholar]
- Kerse NM, Flicker L, Jolley D, Arroll B, Young D. Improving the health behaviours of elderly people: randomised controlled trial of a general practice education programme. BMJ 1999;319(7211):683‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA 2001;285(22):2871‐9. [DOI] [PubMed] [Google Scholar]
- Kim CS, Kristopaitis RJ, Stone E, Pelter M, Sandhu M, Weingarten SR. Physician education and report cards: do they make the grade? Results from a randomized controlled trial. American Journal of Medicine 1999;107(6):556‐60. [DOI] [PubMed] [Google Scholar]
- Kouides RW, Bennett NM, Lewis B, Cappuccio JD, Barker WH, LaForce FM. Performance‐based physician reimbursement and influenza immunization rates in the elderly. The Primary‐Care Physicians of Monroe County. American Journal of Preventive Medicine 1998;14(2):89‐95. [DOI] [PubMed] [Google Scholar]; Kouides RW, Lewis B, Bennett NM, Bell KM, Barker WH, Black ER, et al. A performance‐based incentive program for influenza immunization in the elderly. American Journal of Preventive Medicine 1993;9:250‐4. [PubMed] [Google Scholar]
- Krieger JW, Castorina JS, Walls ML, Weaver MR, Ciske S. Increasing influenza and pneumococcal immunization rates: a randomized controlled study of a senior center‐based intervention. American Journal of Preventive Medicine 2000;18(2):123‐31. [DOI] [PubMed] [Google Scholar]
- Kumar S, Deichman RE, Sarkar I. Effect of physician‐specific mailouts aimed at increasing influenza immunization rates. Journal of the Louisiana State Medical Society 1999;151:558‐65 . [PubMed] [Google Scholar]
- Lemelin J, Hogg W, Baskerville N. Evidence to action: a tailored multifaceted approach to changing family physician practice patterns and improving preventive care. Canadian Medical Association Journal 2001;164(6):757‐63. [PMC free article] [PubMed] [Google Scholar]
- Lukasik MH, Pratt G. The telephone: an overlooked technology for prevention in family medicine. Canadian Family Physician 1987;33:1997‐2001. [PMC free article] [PubMed] [Google Scholar]
- MacIntyre CR, Kainer MA, Brown GV. A randomised, clinical trial comparing the effectiveness of hospital and community‐based reminder systems for increasing uptake of influenza and pneumococcal vaccine in hospitalised patients aged 65 years and over. Gerontology 2003;49(1):33‐40. [DOI] [PubMed] [Google Scholar]
- Maglione MA, Stone EG, Shekelle PG. Mass mailings have little effect on utilization of influenza vaccine among medicare beneficiaries. American Journal of Preventive Medicine 2002;23(1):43‐6. [DOI] [PubMed] [Google Scholar]
- Maglione MA, Stone EG, Shekelle PG. Mass mailings have little effect on utilization of influenza vaccine among medicare beneficiaries. American Journal of Preventive Medicine 2002;23(1):43‐6. [DOI] [PubMed] [Google Scholar]
- Maglione MA, Stone EG, Shekelle PG. Mass mailings have little effect on utilization of influenza vaccine among medicare beneficiaries. American Journal of Preventive Medicine 2002;23(1):43‐6. [DOI] [PubMed] [Google Scholar]
- Maglione MA, Stone EG, Shekelle PG. Mass mailings have little effect on utilization of influenza vaccine among medicare beneficiaries. American Journal of Preventive Medicine 2002;23(1):43‐6. [DOI] [PubMed] [Google Scholar]
- Marrero W, Hernandez L, Garcia R, Gutierrez LM. Immunization program against influenza for adults 65 years or older at a community pharmacy in Puerto Rico. Puerto Rico Health Sciences Journal 2006;25(1):35‐42. [PubMed] [Google Scholar]
- McCaul KD, Johnson RJ, Rothman AJ. The effects of framing and action instructions on whether older adults obtain flu shots. Health Psychology 2002;21(6):624‐8. [DOI] [PubMed] [Google Scholar]
- McDowell I, Newell C, Rosser W. A follow‐up study of patients advised to obtain influenza immunizations. Family Medicine 1990;22:303‐6. [PubMed] [Google Scholar]; McDowell I, Newell C, Rosser W. Comparison of three methods of recalling patients for influenza vaccination. Canadian Medical Association Journal 1986;135(9):991‐7. [PMC free article] [PubMed] [Google Scholar]
- McMahon JW, Hillman JR, McInerney M, Kileen MJ, Christensen C. Increasing influenza vaccination rates for Medicare beneficiaries ‐ Montana and Wyoming, 1994. Morbidity & Mortality Weekly Report 1995;44(40):741‐4. [PubMed] [Google Scholar]
- McMahon JW, Hillman JR, McInerney M, Kileen MJ, Christensen C. Increasing influenza vaccination rates for Medicare beneficiaries ‐ Montana and Wyoming, 1994. Morbidity & Mortality Weekly Report 1995;44(40):741‐4. [PubMed] [Google Scholar]
- Minor DS, Eubanks JT, Butler KR Jr, Wofford MR, Penman D, Replogle WH. Improving influenza vaccination rates by targeting individuals not seeking early seasonal vaccination. American Journal of Medicine 2010;123:1031‐5. [DOI] [PubMed] [Google Scholar]
- Moran WP, Nelson K, Wofford JL, Velez R. Computer‐generated mailed reminders for influenza immunization: a clinical trial. Journal of General Internal Medicine 1992;7(5):535‐7. [DOI] [PubMed] [Google Scholar]
- Moran WP, Wofford JL, Velez R. Assessment of influenza immunization of community elderly: illustrating the need for community level health information. Carolina Health Services Review 1995;3:21‐9. [Google Scholar]
- Moran WP, Nelson K, Wofford JL, Velez R, Case LD. Increasing influenza immunization among high‐risk patients: education or financial incentive?. American Journal of Medicine 1996;101(6):612‐20. [DOI] [PubMed] [Google Scholar]
- Morrissey JP, Harris RP, Kincade‐Norburn J, McLaughlin C, Garrett JM, Jackman AM, et al. Medicare reimbursement for preventive care: changes in performance of services, quality of life, and health care costs. Journal of the American Geriatric Society 1995;33(4):315‐31. [PubMed] [Google Scholar]
- Mullooly JP. Increasing influenza vaccination among high‐risk elderly: a randomized controlled trial of a mail cue in an HMO setting. American Journal of Public Health 1987;77(5):626‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nexøe J, Kragstrup J, Ronne T. Impact of postal invitations and user fee on influenza vaccination rates among the elderly. A randomized controlled trial in general practice. Scandinavian Journal of Primary Health Care 1997;15:109‐12. [DOI] [PubMed] [Google Scholar]
- Nuttall D. The influence of health professionals on the uptake of the influenza immunization. British Journal of Community Nursing 2003;8(9):391‐6. [DOI] [PubMed] [Google Scholar]
- Puech M, Ward J, Lajoie V. Postcard reminders from GPs for influenza vaccine: are they more effective than an ad hoc approach?. Australian and New Zealand Journal of Public Health 1998;22(2):254‐6. [DOI] [PubMed] [Google Scholar]
- Roca B, Herrero E, Resino E, Torres V, Penades M, Andreu C. Impact of education program on influenza vaccination rates in Spain. American Journal of Managed Care 2012;18(12):e446‐52. [PubMed] [Google Scholar]
- Satterthwaite P. A randomised intervention study to examine the effect on immunisation coverage of making influenza vaccine available at no cost. New Zealand Medical Journal 1997;110(1038):58‐60. [PubMed] [Google Scholar]
- Siriwardena AN, Rashid A, Johnson MR, Dewey ME. Cluster randomised controlled trial of an educational outreach visit to improve influenza and pneumococcal immunisation rates in primary care. British Journal of General Practice 2002;52(482):735‐40. [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Zhou XH, Weinberger M, Smith F, McDonald RC. Mailed reminders for area‐wide influenza immunization: a randomized controlled trial. Journal of the American Geriatric Society 1999;47(1):1‐5. [DOI] [PubMed] [Google Scholar]
- Spaulding SA, Kugler JP. Influenza immunization: the impact of notifying patients of high‐risk status. Journal of Family Practice 1991;33(5):495‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
- Ahmed F, Friedman C, Franks A, Latts LM, Nugent EW, France EK, et al. Effect of the frequency of delivery of reminders and an influenza tool kit on increasing influenza vaccination rates among adults with high‐risk conditions. American Journal of Managed Care 2004;10:698‐702. [PubMed] [Google Scholar]
- Alemi F, Alemagno SA, Goldhagen J, Ash L, Finkelstein B, Lavin A, et al. Computer reminders improve on‐time immunization rates. Medical Care 1996;34(Suppl 10):45‐51. [DOI] [PubMed] [Google Scholar]
- Alexy BB, Elnitsky C. Rural mobile health unit: outcomes. Public Health Nursing 1998;15(1):3‐11. [DOI] [PubMed] [Google Scholar]
- Allsup S, Haycox A, Regan M, Gosney M. Is influenza vaccination cost effective for healthy people between ages 65 and 74 years? A randomised controlled trial. Vaccine 2004;23:639‐45. [DOI] [PubMed] [Google Scholar]
- Anderson C, Martin H. Effectiveness of patient recall system on immunization rates for influenza. Journal of Family Practice 1979;9(4):727‐30. [PubMed] [Google Scholar]
- Anonymous. Facilitating influenza and pneumococcal vaccination through standing order programs. Morbidity & Mortality Weekly Report 2003;52(4):68‐9. [PubMed] [Google Scholar]
- Armstrong K, Berlin M, Schwartz JS, Propert K, Ubel PA. Educational content and the effectiveness of influenza vaccination reminders. Journal of General Internal Medicine 1999;14(11):695‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur AJ. The effect of health assessments by practice nurses on uptake of influenza vaccination among older people in the UK. Journal of Clinical Nursing 2001;10(5):716‐7. [DOI] [PubMed] [Google Scholar]
- Bakare M, Shrivastava R, Jeevanantham V, Navaneethan SD. Impact of two different models on influenza and pneumococcal vaccination in hospitalized patients. Southern Medical Journal 2007;100(2):140‐4. [DOI] [PubMed] [Google Scholar]
- Balague GL, Ruiz Martinez MC, Mercade Mercade MA. The evaluation of an influenza vaccination campaign in a health sector. Atencion Primaria 1993;11(4):202. [PubMed] [Google Scholar]
- Baldo V, Menegon T, Buoro S, Scalici C, Vesco A, Peale S, et al. Vaccination against influenza in the elderly. Experience with adjuvant vaccines [Vaccinazione antinfluenzale in anziani. Esperienza con vaccini adiuvati]. Annali di Igiene 1999;11:369‐73. [PubMed] [Google Scholar]
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long‐term care facilities: the CMS‐CDC Standing Orders Program Project. Journal of the American Medical Directors Association 2005;6(5):291‐9. [DOI] [PubMed] [Google Scholar]
- Bardenheier BH, Shefer AM, Remsburg RE, Marsteller JA. Are standing order programs associated with influenza vaccination? ‐ NNHS, 2004. Journal of the American Medical Directors Association 2010;11:654‐61. [DOI] [PubMed] [Google Scholar]
- Bardenheier B, Shefer A, Ahmed F, Remsburg R, Rowland Hogue CJ, Gravenstein S. Do vaccination strategies implemented in nursing homes narrow the racial gap in receipt of influenza vaccinations in the United States?. Journal of the American Geriatric Society 2011;59:687‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker WH, Bennett NM, LaForce FM, Waltz EC, Weiner LB. "McFlu". The Monroe County, New York, Medicare vaccine demonstration. American Journal of Preventive Medicine 1999;16(Suppl 3):118‐27. [DOI] [PubMed] [Google Scholar]
- Barton MB, Schoenbaum SC. Improving influenza vaccination performance in an HMO setting: the use of computer‐generated reminders and peer comparison feedback. American Journal of Public Health 1990;80(5):534‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsworth A, Maxim R, Bertrand T. The power of coalition ‐ improving Rhode Island's adult immunization rate ‐ the Ocean State Adult Immunization Coalition. Medicine & Health, Rhode Island 2004;87(3):72‐4. [PubMed] [Google Scholar]
- Becker DM, Gomez EB, Kaiser DL, Yoshihasi A, Hodge RH Jr. Improving preventive care at a medical clinic: how can the patient help?. American Journal of Preventive Medicine 1989;5(6):353‐9. [PubMed] [Google Scholar]
- Bekker HL, Gough D, Williams M. Attendance choices about the influenza immunization programme: evidence for targeting patients' beliefs. Psychology Health & Medicine 2003;8(3):279‐88. [Google Scholar]
- Belcher DW. Implementing preventive services. Success and failure in an outpatient trial. Archives of Internal Medicine 1990;150(12):2533‐41. [DOI] [PubMed] [Google Scholar]
- Bennett NM, Lewis B, Doniger AS, Bell K, Kouides R, LaForce FM, et al. A coordinated, community‐wide program in Monroe County, New York, to increase influenza immunization rates in the elderly. Archives of Internal Medicine 1994;154:1741‐5. [PubMed] [Google Scholar]
- Berg GD, Thomas E, Silverstein S, Neel CL, Mireles M. Reducing medical service utilization by encouraging vaccines: randomized controlled trial. American Journal of Preventive Medicine 2004;27(4):284‐8. [DOI] [PubMed] [Google Scholar]
- Berg GD, Fleegler E, Vonno CJ, Thomas E. A matched‐cohort study of health services utilization for a heart failure disease management program. Disease Management 2005;8(1):35‐41. [DOI] [PubMed] [Google Scholar]
- Birchmeier M, Favrat B, Pecoud A, Abetel G, Karly M, Landry P, et al. Improving influenza vaccination rates in the elderly. Journal of Family Practice 2002;51(10):856. [PubMed] [Google Scholar]
- Bloom HG, Bloom JS, Krasnoff L, Frank AD. Increased utilization of influenza and pneumococcal vaccines in an elderly hospitalized population. Journal of the American Geriatrics Society 1988;36(10):897‐901. [DOI] [PubMed] [Google Scholar]
- Bloom HG, Wheeler DA, Linn J. A managed care organization's attempt to increase influenza and pneumococcal immunizations for older adults in an acute care setting. Journal of the American Geriatrics Society 1999;47(1):106‐10. [DOI] [PubMed] [Google Scholar]
- Bond TC, Patel PR, Krisher J, Sauls L, Deans J, Strott K, et al. A group‐randomized evaluation of a quality improvement intervention to improve influenza vaccination rates in dialysis centers. American Journal of Kidney Disease 2011;57(2):283‐90. [DOI] [PubMed] [Google Scholar]
- Bou‐Mias C, Zwart‐Salmeron M, Calvet‐Freixas E, Bunuel‐Alvarez JC. Telephone recruitment for flu vaccination. Atencion Primaria 2006;37(3):176‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovier PA, Chamot E, Gallacchi MB, Loutan L. Importance of patients' perceptions and general practitioners' recommendations in understanding missed opportunities for immunisations in Swiss adults. Vaccine 2001;19(32):4760‐7. [DOI] [PubMed] [Google Scholar]
- Brady WJ, Hissa DC, McConnell M, Wones RG. Should physicians perform their own quality assurance audits?. Journal of General Internal Medicine 1988;3(6):560‐5. [DOI] [PubMed] [Google Scholar]
- Breen D. Pneumococcal vaccination programme in over 65s and at‐risk groups: the Dumfries and Galloway experience. Communicable Disease & Public Health 2003;6(3):228‐30. [PubMed] [Google Scholar]
- Brimberry R. Vaccination of high‐risk patients for influenza. A comparison of telephone and mail reminder methods. Journal of Family Practice 1988;26(4):397‐400. [PubMed] [Google Scholar]
- Browngoehl K, Kennedy K, Krotki K, Mainzer H. Increasing immunization: a Medicaid managed care model. Pediatrics 1997;99:E4. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Larson EB, White RF. Influenza vaccination in community elderly. A controlled trial of postcard reminders. Journal of the American Geriatrics Society 1987;35(8):755‐60. [DOI] [PubMed] [Google Scholar]
- Burns VE, Ring C, Carroll D. Factors influencing influenza vaccination uptake in an elderly, community‐based sample. Vaccine 2005;23(27):3604‐8. [DOI] [PubMed] [Google Scholar]
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza?. JAMA 2005;293(8):987‐97. [DOI] [PubMed] [Google Scholar]
- Cardozo LJ, Steinberg J, Lepczyk MB, Binnus‐Emerick L, Cardozo YM, Aranha AN. Delivery of preventive healthcare to older African‐American patients: a performance comparison from two practice models. American Journal of Managed Care 1998;4(6):809‐16. [PubMed] [Google Scholar]
- Carey TS, Levis D, Pickard CG, Bernstein J. Development of a model quality‐of‐care assessment program for adult preventive care in rural medical practices. Quality Review Bulletin 1991;17(2):54‐9. [DOI] [PubMed] [Google Scholar]
- Carman WF, Elder AG, Wallace LA, McAulay K, Walker A, Murray GD, et al. Effects of influenza vaccination of health‐care workers on mortality of elderly people in long‐term care: a randomised controlled trial. Lancet 2000;355(9198):93‐7. [DOI] [PubMed] [Google Scholar]
- Carter WB, Beach LR, Inui TS. The flu shot study: using multiattribute utility theory to design a vaccination intervention. Organizational Behavior & Human Decision Processes 1986;38(3):378‐91. [DOI] [PubMed] [Google Scholar]
- Chami K, Gavazzi G, Bar‐Hen A, Carrat F, Wazière B, Lejeune B, et al. A short‐term multicomponent infection control program in nursing homes: a cluster randomized controlled trial. Journal of the American Association of Medical Directors 2012;13:569.e9‐e17. [DOI] [PubMed] [Google Scholar]
- Chan L, Doctor JN, MacLehose RF, Lawson H, Rosenblatt RA, Baldwin L, et al. Do Medicare patients with disabilities receive preventive services? A population‐based study. Archives of Physical Medicine & Rehabilitation 1999;80(6):642‐6. [DOI] [PubMed] [Google Scholar]
- Charles J, Lewis J. Requiring elderly patients to give signed consent for influenza vaccine. Does it affect acceptance?. Canadian Family Physician 1994;40:474‐7. [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Fox SA, Cantrell CH, Stockdale SE, Kagawa‐Singer M. Health disparities and prevention: racial/ethnic barriers to flu vaccinations. Journal of Community Health 2007;32(1):5‐20. [DOI] [PubMed] [Google Scholar]
- Cheney C, Ramsdell JW. Effect of medical records' checklists on implementation of periodic health measures. American Journal of Medicine 1987;83(1):129‐36. [DOI] [PubMed] [Google Scholar]
- Chi RC, Reiber GE, Neuzil KM. Influenza and pneumococcal vaccination in older veterans: results from the behavioral risk factor surveillance system. Journal of the American Geriatrics Society 2006;54(2):217‐23. [DOI] [PubMed] [Google Scholar]
- Chodroff CH. Cancer screening and immunization quality assurance using a personal computer. Quality Review Bulletin 1990;16(8):279‐87. [DOI] [PubMed] [Google Scholar]
- Christenson B, Lundbergh P, Hedlund J, Ortqvisit A. Effects of a large‐scale intervention with influenza and 23‐valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet 2001;357(9261):1008‐11. [DOI] [PubMed] [Google Scholar]
- Clancy DE, Cope DW, Magruder KM, Huang P, Wolfman TE. Evaluating concordance to American Diabetes Association standards of care for type 2 diabetes through group visits in an uninsured or inadequately insured patient population. Diabetes Care 2003;26(7):2032‐6. [DOI] [PubMed] [Google Scholar]
- Cohen DI, Littenberg B, Wetzel C, Neuhauser D. Improving physician compliance with preventive medicine guidelines. Medical Care 1982;20(10):1040‐5. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cicco‐Bloom B, Strickland PO, Headley A, Orzano J, Levine J, et al. Opportunistic approaches for delivering preventive care in illness visits. Preventive Medicine 2004;38(5):565‐73. [DOI] [PubMed] [Google Scholar]
- Colombo GL, Serra G, Morlotti L, Fara GM. The role of economic evaluation for the implementation of vaccination strategies [Ruolo della valutazione economica nell'implementazione di strategie vaccinali]. Annali di Igiene 2005;17(6):479‐90. [PubMed] [Google Scholar]
- Correa‐de‐Araujo R, McDermott K, Moy E. Gender differences across racial and ethnic groups in the quality of care for diabetes. Women's Health Issues 2006;16(2):56‐65. [DOI] [PubMed] [Google Scholar]
- Costa TX, Rodriguez AA, Perez PN, Begines CM, Cabello Ortega RC, Romero GA. Influenza vaccination in high‐risk groups. Role of the nursing staff. Atencion Primaria 1994;13(5):256‐8. [PubMed] [Google Scholar]
- Cowan JA, Heckerling PS, Parker JB. Effect of a fact sheet reminder on performance of the periodic health examination: a randomized controlled trial. American Journal of Preventive Medicine 1992;8:104‐9. [PubMed] [Google Scholar]
- Cowan AE, Winston CA, Davis MM, Wortley PM, Clark SJ. Influenza vaccination status and influenza‐related perspectives and practices among US physicians. American Journal of Infection Control 2006;34(4):164‐9. [DOI] [PubMed] [Google Scholar]
- Crawford AG, Sikirica V, Goldfarb N, Popiel RG, Patel M, Wang C, et al. Interactive voice response reminder effects on preventive service utilization. American Journal of Medical Quality 2005;20(6):329‐36. [DOI] [PubMed] [Google Scholar]
- Crawford VLS, O'Hanlon A, McGee H. The effect of patient characteristics upon uptake of the influenza vaccination: a study comparing community‐based older adults in two healthcare systems. Age and Aging 2011;40:35‐41. [DOI] [PubMed] [Google Scholar]
- Crouse BJ, Nichol K, Peterson DC, Grimm MB. Hospital‐based strategies for improving influenza vaccination rates. Journal of Family Practice 1994;38(3):258‐61. [PubMed] [Google Scholar]
- Curry E, Kerr N, Yang J, Briggs S. Influenza immunisation rate for 2005 and factors associated with receiving this vaccine in patients aged 65 years and over admitted to a general medical ward at Auckland City Hospital. New Zealand Medical Journal 2006;119(1243):U2254. [PubMed] [Google Scholar]
- Daniels NA, Juarbe T, Moreno‐John G, Perez‐Stable EJ. Effectiveness of adult vaccination programs in faith‐based organizations. Ethnicity and Disease 2007;17(1):S1. [PubMed] [Google Scholar]
- Dannetun E, Tegnell A, Normann B, Garpenholt O, Giesecke J. Influenza vaccine coverage and reasons for non‐vaccination in a sample of people above 65 years of age, in Sweden, 1998‐2000. Scandinavian Journal of Infectious Diseases 2003;35(6‐7):389‐93. [DOI] [PubMed] [Google Scholar]
- Davidse W, Perenboom RJ. Increase of degree of vaccination against influenza in at‐risk patients by directed primary care invitation. Nederlands Tijdschrift Voor Geneeskunde 1995;139(42):2149‐52. [PubMed] [Google Scholar]
- Davidson RA, Fletcher SW, Retchin S, Duh S. A nurse‐initiated reminder system for the periodic health examination. Implementation and evaluation. Archives of Internal Medicine 1984;144(11):2167‐70. [PubMed] [Google Scholar]
- Davis MM, Halasyamani LK, Sneller V‐P, Bishop KR, Clark SJ. Provider response to different formats of the adult immunization schedule. American Journal of Preventive Medicine 2005;29(1):34‐40. [DOI] [PubMed] [Google Scholar]
- Denis B, Lambrechts T, Lambeau JL, Soetens G. Immunization against influenza among elderly in general practice. Louvain Medical 1996;115(1):12‐9. [Google Scholar]
- Desbiens NA. A 5‐year experience with influenza prevention and containment in a program of all‐inclusive care for elderly adults. American Journal of Infection Control 2005;33(4):238‐42. [DOI] [PubMed] [Google Scholar]
- Wals P, Vienne A, Lemaire G, Tamigniau P, Demolin A, Hecquet P, et al. Acceptability of vaccination against influenza. Revue Medicale de Bruxelles 1989;10(1‐2):49‐52. [PubMed] [Google Scholar]
- Wals P, Carbonneau M, Payette H, Niyonsenga T. Influenza and pneumococcal vaccination in long term care facilities in two regions of Quebec. Canadian Journal of Infectious Diseases 1996;7(5):296‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. New England Journal of Medicine 2001;345(13):965‐70. [DOI] [PubMed] [Google Scholar]
- Dickey LL, Petitti D. Assessment of a patient‐held minirecord for adult health maintenance. Journal of Family Practice 1990;31(4):431‐8. [PubMed] [Google Scholar]
- Dickey LL, Petitti D. A patient‐held minirecord to promote adult preventive care. Journal of Family Practice 1992;34(4):457‐63. [PubMed] [Google Scholar]
- Dickey LL. Promoting preventive care with patient‐held minirecords: a review. Patient Education & Counseling 1993;20(1):37‐47. [DOI] [PubMed] [Google Scholar]
- Dini EF, Chaney M, Moolenaar RL, LeBaron CW. Information as intervention: how Georgia used vaccination coverage data to double public sector vaccination coverage in seven years. Journal of Public Health Management Practice 1996;2(1):45‐9. [DOI] [PubMed] [Google Scholar]
- Donato AA, Motz LM, Wilson G, Lloyd BJ. Efficacy of multiple influenza vaccine delivery systems in a single facility. Infection Control & Hospital Epidemiology 2007;28(2):219‐21. [DOI] [PubMed] [Google Scholar]
- Douglas KC, Rush DR, O'Dell M, Monroe A, Ausmus M. Adult immunization in a network of family practice residency programs. Journal of Family Practice 1990;31(5):513‐20. [PubMed] [Google Scholar]
- Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non‐breast cancer health maintenance among elderly breast cancer survivors. Journal of Clinical Oncology 2003;21(8):1447‐51. [DOI] [PubMed] [Google Scholar]
- Egido PA, Abat DX, Marimon Amenos MR, Andujar GA, Albiol PM. Influenza vaccination: evaluation of an integrated program in a basic urban health area. Atencion Primaria 1989;6(8):578‐82. [PubMed] [Google Scholar]
- Etkind P, Simon M, Shannon S, Bottum C, Goldstein R, Werner B, et al. The impact of the Medicare Influenza Demonstration Project on influenza vaccination in a county in Massachusetts, 1988‐1992. Journal of Community Health 1996;21(3):199‐209. [DOI] [PubMed] [Google Scholar]
- Evans MR, Watson PA. Why do older people not get immunised against influenza? A community survey. Vaccine 2003;21(19‐20):2421‐7. [DOI] [PubMed] [Google Scholar]
- Fairbrother G, Hanson KL, Friedman S, Butts GC. The impact of physician bonuses, enhanced fees, and feedback on childhood immunization coverage rates. American Journal of Public Health 1999;89(2):171‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedson DS. Prevention and control of influenza in institutional settings. Hospital Practice 1989;24(9A):87‐94. [PubMed] [Google Scholar]
- Fedson DS. Influenza and pneumococcal vaccination of the elderly: newer vaccines and prospects for clinical benefits at the margin. Preventive Medicine 1994;23(5):751‐5. [DOI] [PubMed] [Google Scholar]
- Fedson DS. Evaluating the impact of influenza vaccination a North American perspective. Pharmacoeconomics 1996;9(Suppl 3):54‐61. [DOI] [PubMed] [Google Scholar]
- Fernández SA, Lindoso LT, Valencia BS, Alvarez OS, varez Mazariegos JA. Influenza vaccination campaigns. A comparative evaluation [Campanas de vacunacion antigripal. Evaluacion comparativa]. Revisita de Enfermeria 1994;17(191‐2):13‐8. [PubMed] [Google Scholar]
- Ferrante JM, Balasubramanian BA, Hudson SV, Crabtree BF. Principles of the patient‐centered medical home and preventive services delivery. Annals of Family Medicine 2010;8:108‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach NH, Viscoli CM. Patient acceptance of influenza vaccination. American Journal of Medicine 1991;91(4):393‐400. [DOI] [PubMed] [Google Scholar]
- Fishbein DB, Fontanesi J, Kopald D, Stevenson J, Bennett NM, Stryker DW, et al. Why do not patients receive influenza vaccine in December and January?. Vaccine 2006;24(6):798‐802. [DOI] [PubMed] [Google Scholar]
- Fishbein DB, Willis BC, Cassidy WM, Marioneaux D, Winston CA. A comprehensive patient assessment and physician reminder tool for adult immunization: effect on vaccine administration. Vaccine 2006;25:3971‐83. [DOI] [PubMed] [Google Scholar]
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Annals of Internal Medicine 2003;138(4):273‐87. [DOI] [PubMed] [Google Scholar]
- Fitzner KA, Shortridge KF, McGhee SM, Hedley AJ. Cost‐effectiveness study on influenza prevention in Hong Kong. Health Policy 2001;56(3):215‐34. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F, Harrington P, Mahony D. The "silver‐haired" general medical services patient. Clinical activity of the non‐means tested over‐70's during their first six months. Irish Medical Journal 2004;97(4):111‐4. [PubMed] [Google Scholar]
- Flach SD, McCoy KD, Vaughn TE, Ward MM, Boots Miller BJ, Doebbeling BN. Does patient‐centered care improve provision of preventive services?. Journal of General Internal Medicine 2004;19(10):1019‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi J, Shefer AM, Fishbein DB, Bennett NM, Guire M, Kopald D, et al. Operational conditions affecting the vaccination of older adults. American Journal of Preventive Medicine 2004;26(4):265‐70. [DOI] [PubMed] [Google Scholar]
- Fowles JB, Beebe TJ. Failure to immunize the elderly: a systems problem or a statement of personal values?. Joint Commission Journal on Quality Improvement 1998;24(12):704‐10. [DOI] [PubMed] [Google Scholar]
- Frame PS, Zimmer JG, Werth PL, Hall WJ, Eberly SW. Computer‐based vs manual health maintenance tracking. A controlled trial. Archives of Family Medicine 1994;3(7):581‐8. [DOI] [PubMed] [Google Scholar]
- Francisco PM, Donalisio MR, Barros MB, Cesar CL, Carandina L, Goldbaum M. Factors associated with vaccination against influenza in the elderly. Pan American Journal of Public Health 2006;19(4):259‐64. [DOI] [PubMed] [Google Scholar]
- Frank JW, Henderson M, McMurray L. Influenza vaccination in the elderly: 1. Determinants of acceptance. Canadian Medical Association Journal 1985;132(4):371‐5. [PMC free article] [PubMed] [Google Scholar]
- Frick KD, Scanlon DP, Bandeen‐Roche K, Kasper JD, Simonsick EM, Sullivan EM. Influenza vaccination by race among disabled community dwelling older women. Journal of Health Care for the Poor & Underserved 2004;15(2):220‐36. [DOI] [PubMed] [Google Scholar]
- Furey A, Robinson E, Young Y. Improving influenza immunisation coverage in 2000‐2001: a baseline survey, review of the evidence and sharing of best practice. Communicable Disease & Public Health 2001;4(3):183‐7. [PubMed] [Google Scholar]
- Galasso GJ, Tyeryar FJ, M Jr. Overview of clinical trials of influenza vaccines, 1976. Journal of Infectious Diseases 1977;136(Suppl):425‐8. [DOI] [PubMed] [Google Scholar]
- Ganguly R, Cameron D. Factors affecting immunization rate in a cohort of elderly veterans: a retrospective pilot study of influenza vaccine compliance. Vaccine 1989;7(5):462‐4. [DOI] [PubMed] [Google Scholar]
- Ganguly R, Webster TB. Influenza vaccination in the elderly. Journal of Investigational Allergology & Clinical Immunology 1995;5(2):73‐7. [PubMed] [Google Scholar]
- Gannon M, Qaseem A, Snooks Q, Snow V. Improving adult immunization practices using a team approach in the primary care setting. American Journal of Public Health 102;7:e46‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DG, Bluml BM. Patient self‐management program for diabetes: first‐year clinical, humanistic, and economic outcomes. Journal of the American Pharmacists Association 2005;45(2):130‐7. [DOI] [PubMed] [Google Scholar]
- Gauthey L, Toscani L, Chamot E, Larequi T, Robert CF. Influenza vaccination coverage in the geriatric population of the State of Geneva, Switzerland. European Journal of Public Health 1999;9(1):36‐40. [Google Scholar]
- Gelfman DM, Witherspoon JM, Buchsbaum DG, Centor RM. Short‐term results of an immunization compliance program. Virginia Medical 1986;113(9):532‐4. [PubMed] [Google Scholar]
- Gerace TM, Sangster JF. Influenza vaccination: a comparison of two outreach strategies. Family Medicine 1988;20(1):43‐5. [PubMed] [Google Scholar]
- Giles SG. A home based health check combined with influenza vaccination improved uptake of influenza vaccination in people > than or = to 75 years of age. Evidence‐Based Nursing 2003;6:52‐3. [PubMed] [Google Scholar]
- Gill JM, Saldarriaga AM. The impact of a computerized physician reminder and a mailed patient reminder on influenza immunizations for older patients. Delaware Medical Journal 2000;72(10):425‐30. [PubMed] [Google Scholar]
- Gill JM, Fagan HB, Townsend B, Mainous AG III. Impact of providing a medical home to the uninsured: evaluation of a statewide program. Journal of Health Care for the Poor & Underserved 2005;16(3):515‐35. [DOI] [PubMed] [Google Scholar]
- Goebel LJ, Neitch SM, Mufson MA. Standing orders in an ambulatory setting increases influenza vaccine usage in older people. Journal of the American Geriatrics Society 2005;53(6):1008‐10. [DOI] [PubMed] [Google Scholar]
- Grabenstein JD, Smith LJ, Watson RR, Summers RJ. Immunization outreach using individual need assessments of adults at an army hospital. Public Health Reports 1990;105(3):311‐6. [PMC free article] [PubMed] [Google Scholar]
- Grabenstein JD, Hartzema AG, Guess HA, Johnston WP, Rittenhouse BE. Community pharmacists as immunization advocates. Cost‐effectiveness of a cue to influenza vaccination. Medical Care 1992;30(6):503‐13. [DOI] [PubMed] [Google Scholar]
- Grabenstein JD, Guess HA, Hartzema AG, Koch GG, Konrad TR. Effect of vaccination by community pharmacists among adult prescription recipients. Medical Care 2001;39(4):340‐8. [DOI] [PubMed] [Google Scholar]
- Granollers Mercarder S, Pont RA. Nurse care in primary health care: diagnosis and follow‐up of health problems [Cuidados de enfermeria en atencion primaria: diagnostico y seguimiento de problemas de salud]. Atencion Primaria 1993;11(2):64‐8. [PubMed] [Google Scholar]
- Green CA, Polen MR, Brody KK. Depression, functional status, treatment for psychiatric problems, and the health‐related practices of elderly HMO members. American Journal of Health Promotion 2003;17(4):269‐75. [DOI] [PubMed] [Google Scholar]
- Greene J, Blustein J, Laflamme KA. Use of preventive care services, beneficiary characteristics, and Medicare HMO performance. Health Care Financing Review 2001;22(4):141‐53. [PMC free article] [PubMed] [Google Scholar]
- Groll DL, Thomson DJ. Incidence of influenza in Ontario following the Universal Influenza Immunization Campaign. Vaccine 2006;24(24):5245‐50. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JP, Bertozzi SM. Influenza vaccination in the elderly population in Mexico: economic considerations [Vacunacion contra influenza para adultos mayores en Mexico: consideraciones economicas]. Salud Publica de Mexico 2005;47(3):234‐9. [DOI] [PubMed] [Google Scholar]
- Gutschi LM, Vaillancourt R. Effect of pharmacist interventions on pneumococcal and influenza vaccination rates: a seamless care approach. Canadian Pharmaceutical Journal 1998;131(8):32‐8. [Google Scholar]
- Hahn DL, Berger MG. Implementation of a systematic health maintenance protocol in a private practice. Journal of Family Practice 1990;31(5):492‐502. [PubMed] [Google Scholar]
- Halliday L, Thomson JA, Roberts L, Bowen S, Mead C. Influenza vaccination of staff in aged care facilities in the ACT: how can we improve the uptake of influenza vaccine?. Australian and New Zealand Journal of Public Health 2003;27(1):70. [DOI] [PubMed] [Google Scholar]
- Hanna JN, Young DM, Brookes DL, Dostie BG, Murphy DM. The initial coverage and impact of the pneumococcal and influenza vaccination program for at‐risk indigenous adults in Far North Queensland. Australian and New Zealand Journal of Public Health 2001;25(6):543‐6. [DOI] [PubMed] [Google Scholar]
- Hannah KL, Schade CP, Cochran R, Brehm JG. Promoting influenza and pneumococcal immunization in older adults. Joint Commission Journal on Quality & Patient Safety 2005;31(5):286‐93. [DOI] [PubMed] [Google Scholar]
- Harari D, Iliffe S, Kharicha K, Egger M, Gillman G, Renteln‐Kruse W, et al. Promotion of health in older people: a randomised controlled trial of health risk appraisal in British general practice. Age and Ageing 2008;37:565–71. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Siegrist C, Schira J, Wunderli W, Pittet D. Influenza immunization: improving compliance of healthcare workers. Infection Control and Hospital Epidemiology 1998;19:337–42. [DOI] [PubMed] [Google Scholar]
- Harris RP, O'Malley MS, Fletcher SW, Knight BP. Prompting physicians for preventive procedures: a five‐year study of manual and computer reminders. American Journal of Preventive Medicine 1990;6(3):145‐52. [PubMed] [Google Scholar]
- Harris LM, Chin NP, Fiscella K, Humiston S. Barrier to pneumococcal and influenza vaccinations in Black elderly communities: mistrust. Journal of the National Medical Association 2006;98(10):1678‐84. [PMC free article] [PubMed] [Google Scholar]
- Hedlund J, Christenson B, Lundbergh P, Ortqvisit A. Effects of a large‐scale intervention with influenza and 23‐valent pneumococcal vaccines in elderly people: a 1‐year follow‐up. Vaccine 2003;21(25‐6):3906‐11. [DOI] [PubMed] [Google Scholar]
- Henk M, Froom J. Outreach by primary‐care physicians. JAMA 1975;233(3):256‐9. [PubMed] [Google Scholar]
- Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002;26(325(7370)):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirdes JP, Dalby DM, Knight SR, Iain CG, Bernabei R, Morris JN, et al. Predictors of influenza immunization among home care clients in Ontario. Canadian Journal of Public Health 2006;97(4):335‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey JR, McCallum HP, Lepage EM. Expanding the nurse's role to improve preventive service in an outpatient clinic. Canadian Medical Association Journal 1982;127(1):27‐8. [PMC free article] [PubMed] [Google Scholar]
- Honkanen PO, Keistinen T, Kivela SL. Factors associated with influenza vaccination coverage among the elderly: role of health care personnel. Public Health 1996;110(3):163‐8. [DOI] [PubMed] [Google Scholar]
- Honkanen PO, Keistinen T, Kivela SL. The impact of vaccination strategy and methods of information on influenza and pneumococcal vaccination coverage in the elderly population. Vaccine 1997;15(3):317‐20. [DOI] [PubMed] [Google Scholar]
- Honkanen P, Laara E, Pyhala R, Kivela SL, Helena MP. Comparison of two vaccination programmes in preventing influenza‐related hospitalization among the elderly during two consecutive seasons. Scandinavian Journal of Infectious Diseases 2006;38(6‐7):506‐11. [DOI] [PubMed] [Google Scholar]
- Humair J‐P, Buchs CR, Stalder H. Promoting influenza vaccination of elderly patients in primary care. Family Practice 2002;19(4):383‐9. [DOI] [PubMed] [Google Scholar]
- Hutchinson HL, Norman LA. Compliance with influenza immunization: a survey of high‐risk patients at a family medicine clinic. Journal of the American Board of Family Practice 1995;8:448–51. [PubMed] [Google Scholar]
- Hutchison BG, Shannon HS. Effect of repeated annual reminder letters on influenza immunization among elderly patients. Journal of Family Practice 1991;33(2):187‐9. [PubMed] [Google Scholar]
- Hutt E, Radcliff TA, Oman KS, Fink R, Ruscin M, Linnebur S, et al. Impact of NHAP guideline implementation intervention on staff and resident vaccination rates. Journal of the American Medical Directors Association 2010;11:365‐70. [DOI] [PubMed] [Google Scholar]; Hutt E, Ruscin M, Linnebur SA, Fish DN, Oman KS, Fink RM, et al. A multifaceted intervention to implement guidelines did not affect hospitalization rates for nursing‐home acquired pneumonia. Journal of the American Medical Directors Association 2011;12:499‐507. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Lauderdale DS, Meltzer D, Shorey JM, Levinson W, Thisted RA. Impact of interpreter services on delivery of health care to limited‐English‐proficient patients. Journal of General Internal Medicine 2001;16(7):468‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Avins AL, Mendelson T. Preventive health services and access to care for male veterans compared with their spouses. Western Journal of Medicine 1998;168(6):499‐503. [PMC free article] [PubMed] [Google Scholar]
- Jans MP, Schellevis FG, Hensbergen W, Eijk JT. Improving general practice care of patients with asthma or chronic obstructive pulmonary disease: evaluation of a quality system. Effective Clinical Practice 2000;3(1):16‐24. [PubMed] [Google Scholar]
- Jefferson T, Demicheli V. Economic evaluation of influenza vaccination and economic modelling. Can results be pooled?. Pharmacoeconomics 1996;9(Suppl 3):67‐72. [DOI] [PubMed] [Google Scholar]
- Jiménez‐Garcia R, Rinez‐Fernandez MC, Hernandez‐Barrera V, Garcia‐Carballo MM, Miguel AG, Carrasco‐Garrido P. Compliance with influenza and pneumococcal vaccination among patients with chronic obstructive pulmonary disease consulting their medical practitioners in Catalonia, Spain. Journal of Infection 2007;54(1):65‐74. [DOI] [PubMed] [Google Scholar]
- Jin Y, Carriere KC, Predy G, Johnson DH, Marrie TJ. The association between influenza immunization coverage rates and hospitalization for community‐acquired pneumonia in Alberta. Canadian Journal of Public Health 2003;94(5):341‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EA, Webb WL, McDowall JM, Chasson LL, Oser CS, Grandpre JR, et al. A field‐based approach to support improved diabetes care in rural states. Preventing Chronic Disease 2005;2(4):1‐9. [PMC free article] [PubMed] [Google Scholar]
- Kassam R, Farris KB, Burback L, Volume CI, Cox CE, Cave A. Pharmaceutical care research and education project: pharmacists' interventions. Journal of the American Pharmaceutical Association 2001;41(3):401‐10. [DOI] [PubMed] [Google Scholar]
- Kelly SD. The impact of a microcomputer on a general practice immunisation clinic. Practitioner 1988;232(1443):197, 200‐1. [PubMed] [Google Scholar]
- Kemper KJ, Goldberg H. Do computer‐generated reminder letters improve the rate of influenza immunization in an urban pediatric clinic?. American Journal of Diseases of Children 1993;147(7):717‐8. [DOI] [PubMed] [Google Scholar]
- Kendal AP, Patriarca PA, Arden NH. Policies and outcomes for control of influenza among the elderly in the USA. Vaccine 1985;3(3):274‐6. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Browngoehl K. A "high‐tech," "soft‐touch" immunization program for members of a Medicaid managed care organization. HMO Practice 1994;8(3):115‐20, 21. [PubMed] [Google Scholar]
- Kern DE, Harris WL, Boekeloo BO, Barker LR, Hogeland P. Use of an outpatient medical record audit to achieve educational objectives: changes in residents' performances over six years. Journal of General Internal Medicine 1990;5(3):218‐24. [DOI] [PubMed] [Google Scholar]
- Klachko DM, Wright DL, Gardner DW. Effect of a microcomputer‐based registry on adult immunizations. Journal of Family Practice 1989;29(2):169‐72. [PubMed] [Google Scholar]
- Knoell KR, Leeds AL. Influenza vaccination program for elderly outpatients. American Journal of Hospital Pharmacy 1991;48(2):256‐9. [PubMed] [Google Scholar]
- Korn JE, Schlossberg LA, Rich EC. Improved preventive care following an intervention during an ambulatory care rotation: carryover to a second setting. Journal of General Internal Medicine 1988;3(2):156‐60. [DOI] [PubMed] [Google Scholar]
- Kosiak B, Sangl J, Correa‐de‐Araujo R. Quality of health care for older women: what do we know?. Women's Health Issues 2006;16(2):89‐99. [DOI] [PubMed] [Google Scholar]
- Kunze M. The contribution of social medicine to vaccination in Austria. Wiener Medizinische Wochenschrift 1998;148(8‐9):191‐7. [PubMed] [Google Scholar]
- Kwong JC, Sambell C, Johansen H, Stukel TA, Manuel DG. The effect of universal influenza immunization on vaccination rates in Ontario. Health Reports 2006;17(2):31‐40. [PubMed] [Google Scholar]
- Kyaw MH, Wayne B, Chalmers J, Jones IG, Campbell H. Influenza and pneumococcal vaccine distribution and use in primary care and hospital settings in Scotland: coverage, practice and policies. Epidemiology & Infection 2002;128(3):445‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis S, Scarbrough ML. Using a vaccine manager to enhance in‐hospital vaccine administration. Journal of Family Practice 1995;41(4):364‐9. [PubMed] [Google Scholar]
- Landon BE, Zaslavsky AM, Bernard SL, Cioffi MJ, Cleary PD. Comparison of performance of traditional Medicare vs Medicare managed care. JAMA 2004;291(14):1744‐52. [DOI] [PubMed] [Google Scholar]
- Larson EB, Olsen E, Cole W, Shortell S. The relationship of health beliefs and a postcard reminder to influenza vaccination. Journal of Family Practice 1979;8(6):1207‐11. [PubMed] [Google Scholar]
- Larson EB, Bergman J, Heidrich F, Alvin BL, Schneeweiss R. Do postcard reminders improve influenza compliance? A prospective trial of different postcard "cues". Medical Care 1982;20(6):639‐48. [DOI] [PubMed] [Google Scholar]
- Lau JTF, Yang X, Tsui HY, Kim JH. Prevalence of influenza vaccination and associated factors among community‐dwelling Hong Kong residents of age 65 or above. Vaccine 2006;24(26):5526‐34. [DOI] [PubMed] [Google Scholar]
- Lawson F, Baker V, Au D, McElhaney JE. Standing orders for influenza vaccination increased vaccination rates in inpatient settings compared with community rates. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 2000;55(9):522‐6. [DOI] [PubMed] [Google Scholar]
- Lazorik D. The role of emergency nurses in the prevention and control of influenza and pneumococcal disease. Journal of Emergency Nursing 2001;27(5):454‐60. [DOI] [PubMed] [Google Scholar]
- LeBaron CW, Chaney M, Baughman AL, Dini EF, Maes E, Dietz V, et al. Impact of measurement and feedback on vaccination coverage in public clinics, 1988‐1994. JAMA 1997;277(8):631‐5. [PubMed] [Google Scholar]
- Lees KA, Wortley PM, Coughlin SS. Comparison of racial/ethnic disparities in adult immunization and cancer screening. American Journal of Preventive Medicine 2005;29(5):404‐11. [DOI] [PubMed] [Google Scholar]
- Leirer VO, Morrow DG, Pariante G, Doksum T. Increasing influenza vaccination adherence through voice mail. Journal of the American Geriatrics Society 1989;37(12):1147‐50. [DOI] [PubMed] [Google Scholar]
- Leirer VO, Morrow DG, Tanke ED, Pariante GM. Elders' nonadherence: its assessment and medication reminding by voice mail. Gerontologist 1991;31(4):514‐20. [DOI] [PubMed] [Google Scholar]
- Levy E. French economic evaluations of influenza and influenza vaccination. Pharmacoeconomics 1996;9(Suppl 3):62‐6. [DOI] [PubMed] [Google Scholar]
- Lieberman D, Lieberman D. Management of respiratory infections in the elderly. Expert Review of Antiinfective Therapy 2003;1(3):505‐16. [DOI] [PubMed] [Google Scholar]
- Lindley MC, Wortley PM, Winston CA, Bardenheier BH. The role of attitudes in understanding disparities in adult influenza vaccination. American Journal of Preventive Medicine 2006;31(4):281‐5. [DOI] [PubMed] [Google Scholar]
- Loeser H, Zvagulis I, Hercz L, Pless IB. The organization and evaluation of a computer‐assisted, centralized immunization registry. American Journal of Public Health 1983;73(11):1298‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P‐J, Singleton JA, Rangel MC, Wortley PM, Bridges CB. Influenza vaccination trends among adults 65 years or older in the United States, 1989‐2002. Archives of Internal Medicine 2005;165(16):1849‐56. [DOI] [PubMed] [Google Scholar]
- Lynd LD, Goeree R, O'Brien BJ. Antiviral agents for influenza: a comparison of cost‐effectiveness data. Pharmacoeconomics 2005;23(11):1083‐106. [DOI] [PubMed] [Google Scholar]
- Macdonald H, Roder D. The planning, implementation and evaluation of an immunization promotion campaign in South Australia. Hygiene 1985;4(2):13‐7. [PubMed] [Google Scholar]
- Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Influenza vaccination health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. American Journal of Preventive Medicine 2006;31(1):72‐9. [DOI] [PubMed] [Google Scholar]
- Madlon‐Kay DJ. Improving the periodic health examination: use of a screening flow chart for patients and physicians. Journal of Family Practice 1987;25(5):470‐3. [PubMed] [Google Scholar]
- Mair HJ, Sansome DA, Tillett HE. A controlled trial of inactivated monovalent influenza A vaccines in general practice. Journal of Hygiene 1974;73(2):317‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmvall BE, Franzen I, Abom PE, Hugosson MB. The rate of influenza immunization to people aged 65 years and older was increased from 45% to 70% by a primary health care‐based multiprofessional approach. Quality Management in Health Care 2007;16(1):51‐9. [PubMed] [Google Scholar]
- Mandel I, Franks P, Dickinson J. Improving physician compliance with preventive medicine guidelines. Journal of Family Practice 1985;21(3):223‐4. [PubMed] [Google Scholar]
- Mangione CM, Gerzoff RB, Williamson DF, Steers WN, Kerr EA, Brown AF, et al. The association between quality of care and the intensity of diabetes disease management programs. Annals of Internal Medicine 2006;145(2):107‐16. [DOI] [PubMed] [Google Scholar]
- Mangtani P, Breeze E, Stirling S, Hanciles S, Kovats S, Fletcher A. Cross‐sectional survey of older peoples' views related to influenza vaccine uptake. BMC Public Health 2006;6:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Lofgren RP, Korn JE. Organizational strategies to improve influenza vaccine delivery. A standing order in a general medicine clinic. Archives of Internal Medicine 1988;148(10):2205‐7. [PubMed] [Google Scholar]
- Margolis KL, Nichol KL, Wuorenma J, Sternberg TL. Exporting a successful influenza vaccination program from a teaching hospital to a community outpatient setting. Journal of the American Geriatrics Society 1992;40(10):1021‐3. [DOI] [PubMed] [Google Scholar]
- Marra F, Marra C, Kaczorowski J, Gastonguay L. Pharmacy‐based immunization in rural communities strategy (PhICS): interim results. Canadian Pharmacists Journal 2011;14(5):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsteller JA, Tiggle R, Remsburg R, Shefer A, Bardenheier B. Influenza immunization in nursing homes: who does not get immunized and whose status is unknown?. Infection Control & Hospital Epidemiology 2006;27(4):388‐96. [DOI] [PubMed] [Google Scholar]
- Martinen M, Freundl M. Managing congestive heart failure in long‐term care. Journal of Gerontological Nursing 2004;30(12):5‐12. [DOI] [PubMed] [Google Scholar]
- Mayo AM, Cobler S. Flu vaccines and patient decision making: what we need to know. Journal of the American Academy of Nurse Practitioners 2004;16(9):402‐10. [DOI] [PubMed] [Google Scholar]
- McArthur MA, Simor AE, Campbell B, McGeer A. Influenza vaccination in long‐term‐care facilities: structuring programs for success. Infection Control and Hospital Epidemiology 1999;20(7):499‐503. [DOI] [PubMed] [Google Scholar]
- McDonald CJ, Hui SL, Smith DM, Tierney WM, Cohen SJ, Weinberger M, et al. Reminders to physicians from an introspective computer medical record. A two‐year randomized trial. Annals of Internal Medicine 1984;100(1):130‐8. [DOI] [PubMed] [Google Scholar]
- McDonald CJ, Hui SL, Tierney WM. Effects of computer reminders for influenza vaccination on morbidity during influenza epidemics. MD Computing: Computers in Medical Practice 1992;9(5):304‐12. [PubMed] [Google Scholar]
- McKinney WP, Barnas GP. Influenza immunization in the elderly: knowledge and attitudes do not explain physician behavior. American Journal of Public Health 1989;79(10):1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod L, Lau WW. Decreasing influenza impact in lodges: 1997‐2000 Calgary Regional Health Authority. Canadian Journal of Public Health Revue Canadienne de Sante Publique 2001;92(4):291‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel PA, Caputo GC. Evaluation of a simple office‐based strategy for increasing influenza vaccine administration and the effect of differing reimbursement plans on the patient acceptance rate. Journal of General Internal Medicine 1994;9(12):679‐83. [DOI] [PubMed] [Google Scholar]
- Métrailler A, Emery G, Zuber A, Robyr M, Mabillard F, Dolt G, et al. Can we improve the general and nutritional management of elderly individuals living at a medical‐social facility? A team work [Peut‐on ameliorer la prise en charge generale et nutritionnelle des personnes agees vivant en etablissement medico‐social? Un travail d'equipe]. Revue Medicale de la Suisse Romande 2003;123(3):197‐200. [PubMed] [Google Scholar]
- Milman U, Ben‐Moshe S, Hermoni D. The role of the patient care team in elderly people decision on influenza vaccination. Patient Education & Counseling 2005;58(2):203‐8. [DOI] [PubMed] [Google Scholar]
- Mody L, Langa KM, Saint S, Bradley SF. Preventing infections in nursing homes: a survey of infection control practices in southeast Michigan. American Journal of Infection Control 2005;33(8):489‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow RW, Gooding AD, Clark C. Improving physicians' preventive health care behavior through peer review and financial incentives. Archives of Family Medicine 1995;4(2):165‐9. [DOI] [PubMed] [Google Scholar]
- Mosesso VN Jr, Packer CR, McMahon J, Auble TE, Paris PM. Influenza immunizations provided by EMS agencies: the MEDICVAX Project. Prehospital Emergency Care 2003;7(1):74‐8. [DOI] [PubMed] [Google Scholar]
- Mukamel DB, Gold HT, Bennett NM. Cost utility of public clinics to increase pneumococcal vaccines in the elderly. American Journal of Preventive Medicine 2001;21(1):29‐34. [DOI] [PubMed] [Google Scholar]
- Mulet Pons MJ, Sarrion Ferre MT, Barea MA, Marin RN, Blanquer Gregori JJ, Melchor Penella MA. Evaluation of completion of with anti‐flu vaccinations [Evaluacion del cumplimiento de la vacunacion antigripal]. Atencion Primaria 1995;16(7):423‐7. [PubMed] [Google Scholar]
- Muller D, Szucs TD. Coverage rates of influenza vaccine in Italy during the 2002/3 and 2003/04 seasons: a cross‐sectional study. Annali di Igiene 2005;17(4):351‐63. [PubMed] [Google Scholar]
- Murphy AW, Harrington M, Bury G, O'Doherty K, O'Kelly F, Smith M, et al. Impact of a collaborative immunisation programme in an inner city practice. Irish Medical Journal 1996;89(6):220‐1. [PubMed] [Google Scholar]
- Nakatani H, Sano T, Iuchi T. Development of vaccination policy in Japan: current issues and policy directions. Journal of Infectious Diseases 2002;55(4):101‐11. [PubMed] [Google Scholar]
- Ndiaye SM, Hopkins DP, Shefer AM, Hinman AR, Briss PA, Rodewald L, et al. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high‐risk adults: a systematic review. American Journal of Preventive Medicine 2005;28(Suppl 5):248‐79. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Korn JE, Margolis KL, Poland GA, Petzel RA, Lofgren RP. Achieving the national health objective for influenza immunization: success of an institution‐wide vaccination program. American Journal of Medicine 1990;89(2):156‐60. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Lofgren RP, Gapinski J. Influenza vaccination. Knowledge, attitudes, and behavior among high‐risk outpatients. Archives of Internal Medicine 1992;152(1):106‐10. [DOI] [PubMed] [Google Scholar]
- Nichol KL. Ten‐year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high‐risk adults. American Journal of Medicine 1998;105(5):385‐92. [DOI] [PubMed] [Google Scholar]
- Nichol K, Nordin J, Mullooly J. Influence of clinical outcome and outcome period definitions on estimates of absolute clinical and economic benefits of influenza vaccination in community dwelling elderly persons. Vaccine 2006;24(10):1562‐8. [DOI] [PubMed] [Google Scholar]
- Nicoleau A, Nicoleau CA, Balzora JD, Oboh A, Siddiqui N, Rosenberg C. Elderly African‐Americans and the influenza vaccine: the impact of the primary care physician. Journal of the American Medical Directors Association 2001;2(2):56‐9. [PubMed] [Google Scholar]
- Nowalk MP, Zimmerman RK, Feghali J. Missed opportunities for adult immunization in diverse primary care office settings. Vaccine 2004;22(25‐6):3457‐63. [DOI] [PubMed] [Google Scholar]
- Nowalk MP, Zimmerman RK, Shen S, Jewell IK, Raymund M. Barriers to pneumococcal and influenza vaccination in older community‐dwelling adults. Journal of the American Geriatrics Society 2004;52(1):25‐30. [DOI] [PubMed] [Google Scholar]
- Nowalk MP, Bardella IJ, Zimmerman RK, Shen S. The physician's office: can it influence adult immunization rates?. American Journal of Managed Care 2004;10(1):13‐9. [PubMed] [Google Scholar]
- Nowalk MP, Zimmerman RK, Lin CJ, Raymund M, Tabbarah M, Wilson SA, et al. Raising adult vaccination rates over 4 years among racially diverse patients at inner‐city health centers. Journal of the American Geriatrics Society 2008;56(7):1177‐82. [DOI] [PubMed] [Google Scholar]
- O'Connor AM, Pennie RA, Dales RE. Framing effects on expectations, decisions, and side effects experienced: the case of influenza immunization. Journal of Clinical Epidemiology 1996;49(11):1271‐6. [DOI] [PubMed] [Google Scholar]
- O'Connor PJ, Desai J, Rush WA, Cherney LM, Solberg LI, Bishop DB. Is having a regular provider of diabetes care related to intensity of care and glycemic control?. Journal of Family Practice 1998;47(4):290‐7. [PubMed] [Google Scholar]
- O'Malley AS, Forrest CB. Immunization disparities in older Americans: determinants and future research needs. American Journal of Preventive Medicine 2006;31(2):150‐8. [DOI] [PubMed] [Google Scholar]
- O'Reilly D, Gormley G, Gilliland A, Cuene‐Grandidier H, Rafferty C, Reilly P, et al. Influenza vaccinations in Northern Ireland: are older patients missing out?. Age and Ageing 2002;31(5):385‐90. [DOI] [PubMed] [Google Scholar]
- Ohmit SE, Furumoto‐Dawson A, Monto AS, Fasano N. Influenza vaccine use among an elderly population in a community intervention. American Journal of Preventive Medicine 1995;11(4):271‐6. [PubMed] [Google Scholar]
- Ompad DC, Galea S, Vlahov D. Distribution of influenza vaccine to high‐risk groups. Epidemiologic Reviews 2006;28:54‐70. [DOI] [PubMed] [Google Scholar]
- Ornstein SM, Garr DR, Jenkins RG, Rust PF, Arnon A. Computer‐generated physician and patient reminders. Tools to improve population adherence to selected preventive services. Journal of Family Practice 1991;32(1):82‐90. [PubMed] [Google Scholar]
- Overhage JM, Tierney WM, McDonald CJ. Computer reminders to implement preventive care guidelines for hospitalized patients. Archives of Internal Medicine 1996;156:1551‐6. [PubMed] [Google Scholar]
- Padiyara RS, D'Souza JJ, Rihani RS. Clinical pharmacist intervention and the proportion of diabetes patients attaining prevention objectives in a multispecialty medical group. Journal of Managed Care Pharmacy 2011;17(6):456‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchman ML, Burge SK. The patient‐physician relationship, primary care attributes, and preventive services. Family Medicine 2004;36(1):22‐7. [PubMed] [Google Scholar]
- Parry MF, Grant B, Iton A, Parry PD, Baranowsky D. Influenza vaccination: a collaborative effort to improve the health of the community. Infection Control and Hospital Epidemiology 2004;25(11):929‐32. [DOI] [PubMed] [Google Scholar]
- Pasquarella A, Perria C, D'Amato M, Billi P, Marceca M, Volpe E, et al. Management of vaccination practices in adults: the influenza vaccination campaign in Lazio region, Italy [Modelli organizzativi di profilassi vaccinale nell'adulto: l'esperienza della campagna di vaccinazione antinfluenzale nella regione Lazio]. Annali di Igiene 2003;15(6):871‐9. [PubMed] [Google Scholar]
- Patel PH, Welsh C, Foggs MB. Improved asthma outcomes using a coordinated care approach in a large medical group. Disease Management 2004;7(2):102‐11. [DOI] [PubMed] [Google Scholar]
- Patel MS, Davis MM. Could a federal program to promote influenza vaccination among elders be cost‐effective?. Preventive Medicine 2006;42(3):240‐6. [DOI] [PubMed] [Google Scholar]
- Patriarca PA, Weber JA, Meissner MK, Stricof RL, Dateno B, Braun JE, et al. Use of influenza vaccine in nursing homes. Journal of the American Geriatrics Society 1985;33(7):463‐6. [DOI] [PubMed] [Google Scholar]
- Payaprom Y, Alabaster E, Bennett P, Tantipong H. Using the health action process approach and implementation intentions to increase flu vaccine uptake in high risk Thai individuals: a controlled before‐after trial. Health Psychology 2011;30(4):492‐500. [DOI] [PubMed] [Google Scholar]
- Pearson E, Lang E, Colacone A, Farooki N, Afilalo M. Successful implementation of a combined pneumococcal and influenza vaccination program in a Canadian emergency department. Canadian Journal of Emergency Medical Care 2005;7(6):371‐7. [PubMed] [Google Scholar]
- Piedra PA. Influenza virus pneumonia: pathogenesis, treatment, and prevention. Seminars in Respiratory Infections 1995;10(4):216‐23. [PubMed] [Google Scholar]
- Pleis JR, Gentleman JF. Using the National Health Interview Survey: time trends in influenza vaccinations among targeted adults. Effective Clinical Practice: ECP 2002;5(Suppl 3):E3. [PubMed] [Google Scholar]
- Ploeg J, Black ME, Hutchison BG, Walter SD, Scott EA, Chambers LW. Personal, home and community safety promotion with community‐dwelling elderly persons: response to a public health nurse intervention. Canadian Journal of Public Health 1994;85(3):188‐91. [PubMed] [Google Scholar]
- Poole P, Chacko EE, Wood‐Baker R, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD002733.pub2] [DOI] [Google Scholar]
- Postma MJ, Jansema P, Scheijbeler HW, Genugten ML. Scenarios on costs and savings of influenza treatment and prevention for Dutch healthy working adults. Vaccine 2005;23(46‐7):5365‐71. [DOI] [PubMed] [Google Scholar]
- Prati G, Pietrantoni L, Zani B. Influenza vaccination: the persuasiveness of messages among people aged 65 years and older. Health Communication 2012;27:413‐20. [DOI] [PubMed] [Google Scholar]
- Puig‐Barbera J, Ors ZP, Vilchez PC, Lloria PF. Impact of various strategies on the rates of flu vaccination in the elderly [Impacto de distintas estrategias en las tasas de vacunacion antigripal en ancianos. Estudio del Impacto de Diversas Actividades en la Cobertura Vacunal (GEDAC)]. Atencion Primaria 1999;23(6):339‐45. [PubMed] [Google Scholar]
- Quinley JC, Shih A. Improving physician coverage of pneumococcal vaccine: a randomized trial of a telephone intervention. Journal of Community Health 2004;29(2):103‐15. [DOI] [PubMed] [Google Scholar]
- Rantz MJ, Popejoy L, Petroski GF, Madsden RW, Mehr DR, Zwygart‐Stauffacher M, et al. Randomized clinical trial of quality improvement interventions in nursing homes. Gerontologist 2001;41(4):525‐38. [DOI] [PubMed] [Google Scholar]
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. New England Journal of Medicine 2001;344(12):889‐96. [DOI] [PubMed] [Google Scholar]
- Resnick B. Promoting health in older adults: a four‐year analysis. Journal of the American Academic Nurse 2001;13(1):23‐33. [DOI] [PubMed] [Google Scholar]
- Ressel GW, Advisory Committee on Immunization Practices. ACIP releases 2003 guidelines on the prevention and control of influenza. American Family Physician 2003;68(7):1426‐30. [PubMed] [Google Scholar]
- Retchin SM, Preston J. Effects of cost containment on the care of elderly diabetics. Archives of Internal Medicine 1991;151(11):2244‐8. [PubMed] [Google Scholar]
- Rimple D, Weiss SJ, Brett M, Ernst AA. An emergency department‐based vaccination program: overcoming the barriers for adults at high risk for vaccine‐preventable diseases. Academic Emergency Medicine 2006;13(9):922‐30. [DOI] [PubMed] [Google Scholar]
- Robare JF, Bayles CM, Newman AB, Williams K, Milas C, Boudreau R, et al. The "10 Keys" to Healthy Aging: 24‐month follow‐up results from an innovative community‐based prevention program. Health Education & Behavior 2011;38(4):379‐88. [DOI] [PubMed] [Google Scholar]
- Rodewald L, Szilagyi P, Humiston S, Barth R, Kraus R, Raubertas R. A randomized study of tracking with outreach and provider prompting to improve immunization coverage and primary care. Pediatrics 1999;103(1):31‐8. [DOI] [PubMed] [Google Scholar]
- Rodriguez RM, Baraff LJ. Emergency department immunization of the elderly with pneumococcal and influenza vaccines. Annals of Emergency Medicine 1993;22(11):1729‐32. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Rodriguez MI, Gaton Del Amo M, Robles‐Marinas V, Rubio‐Dominguez J. Factors determining flu vaccination in the over‐65s [Factores determinantes de vacunacion antigripal en mayores de 65 anos]. Atencion Primaria 2006;37(7):381‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffey VE. Vaccination of health care workers working in geriatric long term care hospitals reduced patient mortality. Evidence Based Nursing 1998;1:18. [Google Scholar]
- Russell ML, Maxwell CJ. The prevalence and correlates of influenza vaccination among a home care population. Canadian Journal of Public Health 2000;91(6):441‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust CT, Sisk FA, Kuo AR, Smith J, Miller R, Sullivan KM. Impact of resident feedback on immunization outcomes. Archives of Pediatrics & Adolescent Medicine 1999;153(11):1165‐9. [DOI] [PubMed] [Google Scholar]
- Ryan MP, MacLeod AF. A comparison of adverse effects of two influenza vaccines, and the influence on subsequent uptake. Journal of the Royal College of General Practitioners 1984;34(265):442‐4. [PMC free article] [PubMed] [Google Scholar]
- Sambamoorthi U, Findley PA. Who are the elderly who never receive influenza immunization?. Preventive Medicine 2005;40(4):469‐78. [DOI] [PubMed] [Google Scholar]
- Sansom S, Rudy E, Strine T, Douglas W. Hepatitis A and B vaccination in a sexually transmitted disease clinic for men who have sex with men. Sexually Transmitted Diseases 2003;30(9):685‐8. [DOI] [PubMed] [Google Scholar]
- Sarnoff R, Rundall T. Meta‐analysis of effectiveness of interventions to increase influenza immunization rates among high‐risk population groups. Medical Care Research & Review 1998;55(4):432‐56. [DOI] [PubMed] [Google Scholar]
- Schectman JM, Kanwal NK, Schroth WS, Elinsky EG. The effect of an education and feedback intervention on group‐model and network‐model health maintenance organisation physician prescribing behavior. Medical Care 1995;33:139‐44. [PubMed] [Google Scholar]
- Schensul JJ, Radda K, Coman E, Vazquez E. Multi‐level intervention to prevent influenza infections in older low income and minority adults. American Journal of Community Psychology 2009;43(3‐4):313‐29. [DOI] [PubMed] [Google Scholar]
- Schluter WW, Ralston DL, Delaney RJ, Sauaia A, Dunn TR. Increasing influenza and pneumococcal vaccination and tuberculosis screening among residents of Colorado long‐term care facilities. Evaluation & the Health Professions 1999;22(4):466‐83. [DOI] [PubMed] [Google Scholar]
- Schmitz R, Schwartz A. The Medicare influenza vaccine cost‐effectiveness study nursing home survey. International Congress Series; options for the control of influenza International Congress Series: 1019 1993;1:123‐6. [Google Scholar]
- Schmitz RJ, Schwartz AM. Medicare coverage, vaccine promotion and rates of influenza vaccination among the Medicare population: 1998‐1991. International Congress Series; options for the control of influenza International Congress Series: 1019. Elsevier Science Publishers B.V.; Elsevier Science Publishing Co., Inc 1993;1:115‐22. [Google Scholar]
- Schneider EC, Cleary PD, Zaslavsky AM, Epstein AM. Racial disparity in influenza vaccination: does managed care narrow the gap between African Americans and whites?. JAMA 2001;286(12):1455‐60. [DOI] [PubMed] [Google Scholar]
- Schreiner DT, Petrus ER, Rettie CS, Kluge RM. Improving compliance with preventive medicine procedures in a house staff training program. Southern Medical Journal 1988;81(12):1553‐7. [DOI] [PubMed] [Google Scholar]
- Schwartz KL, Neale AV, Northrup J, Monsur J, Patel DA, Tobar R Jr, et al. Racial similarities in response to standardized offer of influenza vaccination: a MetroNet study. Journal of General Internal Medicine 2006;21(4):346‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Chavarri H, Ortuno Lopez JL, Lattur Vilchez A, Pedrera Carbonell V, Orozco Beltran D, Gil Guillen V. Flu vaccination in primary care: analysis of the process and proposals for increasing coverage [Vacunacion antigripal en atencion primaria: analisis del proceso y propuestas para aumentar las tasas de cobertura]. Atencion Primaria 2005;36(7):390‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WG, Scott HM. Economic evaluation of vaccination against influenza in New Zealand. Pharmacoeconomics 1996;9(1):51‐60. [DOI] [PubMed] [Google Scholar]
- Setia U, Serventi I, Lorenz P. Factors affecting the use of influenza vaccine in the institutionalized elderly. Journal of the American Geriatrics Society 1985;33(12):856‐8. [DOI] [PubMed] [Google Scholar]
- Shah MN, Clarkson L, Lerner EB, Fairbanks RJ, McCann R, Schneider SM. An emergency medical services program to promote the health of older adults. Journal of the American Geriatrics Society 2006;54:956‐62. [DOI] [PubMed] [Google Scholar]
- Shahrabani S, Benzion U. The effects of socioeconomic factors on the decision to be vaccinated: the case of flu shot vaccination. Israel Medical Association Journal 2006;8(9):630‐4. [PubMed] [Google Scholar]
- Shank JC, Powell T, Llewelyn J. A five‐year demonstration project associated with improvement in physician health maintenance behavior. Family Medicine 1989;21(4):273‐8. [PubMed] [Google Scholar]
- Shenson D, Bolen J, Adams M, Seeff L, Blackman D. Are older adults up‐to‐date with cancer screening and vaccinations?. Preventing Chronic Disease 2005;2(3):A04. [PMC free article] [PubMed] [Google Scholar]
- Shenson D, Bolen J, Adams M. Receipt of preventive services by elders based on composite measures, 1997‐2004. American Journal of Preventive Medicine 2007;32(1):11‐8. [DOI] [PubMed] [Google Scholar]
- Shenson D, Adams M, Bolen J, Anderson L. Routine checkups don't ensure that seniors get preventive services. Journal of Family Practice 2011;60(1):E1‐10. [PubMed] [Google Scholar]
- Shugarman LR, Hales C, Setodji CM, Bardenheier B, Lynn J. The influence of staff and resident immunization rates on influenza‐like illness outbreaks in nursing homes. Journal of the American Medical Directors Association 2006;7:562‐7. [DOI] [PubMed] [Google Scholar]
- Siebers M, Hunt V. Increasing the pneumococcal vaccination rate of elderly patients in a general internal medicine clinic. Journal of the American Geriatrics Society 1985;33(3):175‐8. [DOI] [PubMed] [Google Scholar]
- Simor AE. Influenza outbreaks in long‐term‐care facilities: how can we do better?. Infection Control and Hospital Epidemiology 2002;23(10):564‐7. [DOI] [PubMed] [Google Scholar]
- Siriwardena AN, Rashid A, Johnson M, Hazelwood L, Wilburn T. Improving influenza and pneumococcal vaccination uptake in high‐risk groups in Lincolnshire: a quality improvement report from a large rural county. Quality in Primary Care 2003;11(1):19‐28. [Google Scholar]
- Slobodkin D, Kitlas J, Zielske P. Opportunities not missed ‐ systematic influenza and pneumococcal immunization in a public inner‐city emergency department. Vaccine 1998;16(19):1795‐802. [DOI] [PubMed] [Google Scholar]
- Soljak M, Handford S. Early results from the Northland immunisation register. New Zealand Medical Journal 1987;100(822):244‐6. [PubMed] [Google Scholar]
- Stancliff S, Salomon N, Perlman DC, Russell PC. Provision of influenza and pneumococcal vaccines to injection drug users at a syringe exchange. Journal of Substance Abuse and Treatment 2000;18(3):263‐5. [DOI] [PubMed] [Google Scholar]
- Stehr‐Green P, Dini E, Lindegren M, Patriarca P. Evaluation of telephoned computer‐generated reminders to improve immunization coverage at inner‐city clinics. Public Health Reports 1993;108(4):426‐30. [PMC free article] [PubMed] [Google Scholar]
- Stenqvist K, Hellvin MA, Hellke P, Hoglund D, Sydow H. Influenza work on the regional level in Sweden: an integrated program for vaccination of risk groups, surveillance and pandemic planning which focuses on the role of the health care worker. Vaccine 2006;24(44‐46):6712‐6. [DOI] [PubMed] [Google Scholar]
- Steyer TE, Ragucci KR, Pearson WS, Mainous AG 3rd. The role of pharmacists in the delivery of influenza vaccinations. Vaccine 2004;22(8):1001‐6. [DOI] [PubMed] [Google Scholar]
- Stott DJ, Murray GD, Elder A, Carman WB. Influenza vaccination of health care workers in long‐term care protects elderly patients. Age and Ageing 1998;27(Suppl 2):45‐6. [Google Scholar]
- Straits‐Troster KA, Kahwati LC, Kinsinger LS, Orelien J, Burdick MB, Yevich SJ. Racial/ethnic differences in influenza vaccination in the Veterans Affairs Healthcare System. American Journal of Preventive Medicine 2006;31(5):375‐82. [DOI] [PubMed] [Google Scholar]
- Stratis Health. Minnesota. Influenza immunization, beneficiary based. Document MNS31, 1997. MNS31 ‐ 1 ‐ Stratis Health 19971997:1‐19.
- Stuart WH, Dull HB, Newton LH, McQueen JL, Schiff ER. Evaluation of monovalent influenza vaccine in a retirement community during the epidemic of 1965‐66. JAMA 1969;209(2):232‐8. [PubMed] [Google Scholar]
- Sylvan S, Eriksson G, Berglund K, Pauksen K, Bergqvisit S. Low vaccine coverage rate for influenza and pneumococcal vaccination in an elderly population in Uppsala County, Sweden. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy 2003;43:Abstract no. G‐884. [Google Scholar]
- Szilagyi P, Rodewald L, Savageau J, Yoos L, Doane C. Improving influenza immunization vaccination rates in children with asthma: a test of a computerized reminder system and an analysis of factors predicting vaccination. Pediatrics 1992;90(6):871‐5. [PubMed] [Google Scholar]
- Szilagyi PG, Shone LP, Barth R, Kouides RW, Long C, Humiston SG, et al. Physician practices and attitudes regarding adult immunizations. Preventive Medicine 2005;40(2):152‐61. [DOI] [PubMed] [Google Scholar]
- Szilagyi PG, Schaffer S, Barth R, Shone LP, Humiston SG, Ambrose S, et al. Effect of telephone reminder/recall on adolescent immunization and preventive visits: results from a randomized clinical trial. Archives of Pediatrics and Adolescent Medicine 2006;160:157‐63. [DOI] [PubMed] [Google Scholar]
- Szucs TD, Wahle K, Muller D. Influenza vaccination in Germany. A population‐based cross‐sectional analysis of three seasons between 2002 and 2005 [Grippeimpfung in Deutschland. Eine bevolkerungsbezongene Querschnittsanalyse der derie Influenzasasons von 2002 bis 2005]. Medizinische Klinik 2006;10(7):537‐45. [DOI] [PubMed] [Google Scholar]
- Tabbarah M, Zimmerman RK, Nowalk MP, Janosky JE, Troy JA, Raymund M, et al. What predicts influenza vaccination status in older Americans over several years?. Journal of the American Geriatrics Society 2005;53(8):1354‐9. [DOI] [PubMed] [Google Scholar]
- Tacken M, Braspenning J, Spreeuwenberg P, Hoogen H, Essen G, Bakker D, et al. Patient characteristics determine differences in the influenza vaccination rate more so than practice features. Preventive Medicine 2002;35(4):401‐6. [DOI] [PubMed] [Google Scholar]
- Tape TG, Campbell JR. Computerized medical records and preventive health care: success depends on many factors. American Journal of Medicine 1993;94(6):619‐25. [DOI] [PubMed] [Google Scholar]
- Terrell‐Perica SM, Effler PV, Houck PM, Lee L, Crosthwaite GH. The effect of a combined influenza/pneumococcal immunization reminder letter. American Journal of Preventive Medicine 2001;21(4):256‐60. [DOI] [PubMed] [Google Scholar]
- Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou X‐H, Eckert GJ, et al. Can computer‐generated evidence‐based care suggestions enhance evidence‐based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Services Research 2005;40(2):477‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollestrup K, Hubbard B. Evaluation of a follow‐up system in a county health department's immunization clinic. American Journal of Preventive Medicine 1991;7(1):24‐8. [PubMed] [Google Scholar]
- Toscani L, Gauthey L, Robert CF. The information network of senior citizens in Geneva, Switzerland, and progress in flu vaccination coverage between 1991 and 2000. Vaccine 2003;21(5‐6):393‐8. [DOI] [PubMed] [Google Scholar]
- Traeger M, Thompson A, Dickson E, Provencio A. Bridging disparity: a multidisciplinary approach for influenza vaccination in an American Indian community. American Journal of Public Health 2006;96(5):921‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick WE, Das K, Gerard MN, Charles‐Damte M, Murphy G, Benson I, et al. Clinical trial of standing‐orders strategies to increase the inpatient influenza vaccination rate. Infection Control and Hospital Epidemiology 2009;30(1):86‐8. [DOI] [PubMed] [Google Scholar]
- Tucker JB, DeSimone JP. Patient response to mail cues recommending influenza vaccine. Family Medicine 1987;19(3):209‐12. [PubMed] [Google Scholar]
- Turner BJ, Day SC, Borenstein B. A controlled trial to improve delivery of preventive care: physician or patient reminders. Journal of General Internal Medicine 1989;4:403‐9. [DOI] [PubMed] [Google Scholar]
- Turner RC, Waivers LE, O'Brien K. The effect of patient‐carried reminder cards on the performance of health maintenance measures. Archives of Internal Medicine 1990;150:645‐7. [PubMed] [Google Scholar]
- Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technology Assessment 2003;7(35):iii‐iv, xi‐xiii, 1‐170. [DOI] [PubMed] [Google Scholar]
- Tymchuk AJ, Ouslander JG. Informed consent: does position of information have an effect upon what elderly people in long‐term care remember?. Educational Gerontology 1991;17(1):11‐9. [DOI] [PubMed] [Google Scholar]
- Usami T, Hashiguchi M, Kouhara T, Ishii A, Nagata T, Mochizuki M. Impact of community pharmacists advocating immunization on influenza vaccination rates among the elderly. Yakugaku Zasshi 2009;129(9):1063‐8. [DOI] [PubMed] [Google Scholar]
- Amburgh JA, Waite NM, Hobson EH, Migden H. Improved influenza vaccination rates in a rural population as a result of a pharmacist‐managed immunization campaign. Pharmacotherapy 2001;21(9):1115‐22. [DOI] [PubMed] [Google Scholar]
- Hooven E, Hoes A, Nichol K, Hak E. Influence of design and analytical factors on confounding in non‐randomised influenza vaccine effect studies. European Journal of Epidemiology 2006;21(Suppl S):52‐3. [Google Scholar]
- Essen GA, Kuyvenhoven MM, Melker RA. Implementing the Dutch College of General Practitioner's guidelines for influenza vaccination: an intervention study. British Journal of General Practice 1997;47(414):25‐9. [PMC free article] [PubMed] [Google Scholar]
- Hoof TJ, Holmboe ES, Barr JK, Reisine S, Cohen KL, Wang Y, et al. Preventive service utilization in older adults: follow‐up results of a quality improvement project. Preventive Medicine in Managed Care 2001;2(3):115‐24. [Google Scholar]
- Lieshout J, Capell EF, Ludt S, Grol R, Wensing M. What components of chronic care organisation relate to better primary care for coronary heart diease patients? An observational study. BMJ Open 2012;0:e001344. [DOI: 10.1136/bmjopen-2012-001344] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa S, Lavizzo‐Mourey R, Taylor L, Pasupathy S, Shulkin D. Improving influenza vaccination among the elderly members of a large health care network: a randomized controlled trial of letter and voice reminders. Journal of General Internal Medicine 1997;12(Suppl 1):112. [Google Scholar]
- Walker CL, Patterson R, Wu A, Bennett E. Influenza vaccination: a successful outpatient program. Allergy Proceedings 1992;13(6):317‐9. [DOI] [PubMed] [Google Scholar]
- Walsh JME, Gildengorin G, Green LW, Jenkins J, Potter MB. The FLU‐FOBT Program in community clinics: durable benefits of a randomized controlled trial. Health Education Research 2012;27(5):886‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ST, Lee LT, Chen LS, Chen TH. Economic evaluation of vaccination against influenza in the elderly: an experience from a population‐based influenza vaccination program in Taiwan. Vaccine 2005;23(16):1973‐80. [DOI] [PubMed] [Google Scholar]
- Warren SS, Nguyen‐Van‐Tam JS, Pearson JC, Madeley RJ. Practices and policies for influenza immunization in old people's homes in Nottingham (UK) during the 1992‐1993 season: potential for improvement. Journal of Public Health Medicine 1995;17(4):392‐6. [PubMed] [Google Scholar]
- Watkinson M. Group visits improved concordance with American Diabetes Association practice guidelines in type 2 diabetes. Evidence Based Nursing 2004;7:57. [DOI] [PubMed] [Google Scholar]
- Weatherill SA, Buxton JA, Daly PC. Immunization programs in non‐traditional settings. Canadian Journal of Public Health 2004;95(2):133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Krieger J, Castorina J, Walls M, Ciske S. Cost‐effectiveness of combined outreach for the pneumococcal and influenza vaccines. Archives of Internal Medicine 2001;161(1):111‐30. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Goldstein B, Evans CT, Legro MW, LaVela S, Smith B, et al. Influenza vaccination among veterans with spinal cord injury: Part 2. Increasing vaccination rates. Journal of Spinal Cord Medicine 2003;26(3):210‐8. [DOI] [PubMed] [Google Scholar]
- Wee CC, Phillips RS, Burstin HR, Cook EF, Puopolo AL, Brennan TA, et al. Influence of financial productivity incentives on the use of preventive care. American Journal of Medicine 2001;110(3):181‐7. [DOI] [PubMed] [Google Scholar]
- Wei W, Findley PA, Sambamoorthi U. Disability and receipt of clinical preventive services among women. Women's Health Issues 2007;16(6):286‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan PJ, Walwyn R, Gaughran F, Macdonald A. Impact of the demand for 'proxy' assent on recruitment to a randomised controlled trial of vaccination testing in care homes. Journal of Medical Ethics 2013;39:36‐40. [DOI] [PubMed] [Google Scholar]
- While A, George C, Murgatroyd B. Promoting influenza vaccination in older people: rationale and reality. British Journal of Community Nursing 2005;10(9):427‐30. [DOI] [PubMed] [Google Scholar]
- Wiese‐Posselt M, Leitmeyer K, Hamouda O, Bocter N, Zollner I, Haas W, et al. Influenza vaccination coverage in adults belonging to defined target groups, Germany, 2003/2004. Vaccine 2006;24(14):2560‐6. [DOI] [PubMed] [Google Scholar]
- Wilkinson CR, Williams M. Strengthening patient‐provider relationships. Lippincott's Case Management 2002;7(3):86‐99. [DOI] [PubMed] [Google Scholar]
- Williams DM, Daugherty LM, Aycock DG, Lindley CM, Harris MJ. Effectiveness of improved targeting efforts for influenza immunization in an ambulatory care setting. Hospital Pharmacy 1987;22(5):462‐4. [PubMed] [Google Scholar]
- Wilson RW, Patterson MA, Alford DM. Services for maintaining independence. Journal of Gerontological Nursing 1989;15(6):31‐7. [DOI] [PubMed] [Google Scholar]
- Winston CA, Wortley PM, Lees KA. Factors associated with vaccination of medicare beneficiaries in five U.S. communities: results from the racial and ethnic adult disparities in immunization initiative survey, 2003. Journal of the American Geriatrics Society 2006;54(2):303‐10. [DOI] [PubMed] [Google Scholar]
- Winston CA, Lindley MC, Wortley PM. Lessons learned from inpatient vaccination in Michigan. American Journal of Medical Quality 2006;21(2):125‐33. [DOI] [PubMed] [Google Scholar]
- Wood D, Halfon N, Donald‐Sherbourne C, Mazel R, Schuster M, Hamlin J, et al. Increasing immunization rates among inner‐city, African American children. A randomized trial of case management. JAMA 1998;279(1):29‐34. [DOI] [PubMed] [Google Scholar]
- Wortley P. Who's getting shots and who's not: racial/ethnic disparities in immunization coverage. Ethnicity & Disease 2005;15(Suppl 3):S3‐4‐S3‐6. [PubMed] [Google Scholar]
- Wray RJ, Buskirk TD, Jupka K, Lapka C, Jacobsen H, Pakpahan R, et al. Influenza vaccination concerns among older blacks: a randomized controlled trial. American Journal of Preventive Medicine 2009;36(5):429‐34. [DOI] [PubMed] [Google Scholar]
- Wright A, Poon EG, Wald J, Feblowitz J, Pang JE, Schnipper JL, et al. Randomized controlled trial of health maintenance reminders provided directly to patients through an electronic PHR. Journal of General Internal Medicine 2011;27(1):85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuorenma J, Nichol K, Vonsternberg T. Implementing a mass influenza vaccination program. Nursing Management 1994;25(5):81. [PubMed] [Google Scholar]
- Yoo BK, Grosse S, Frick KD. Self‐selection and evaluation of influenza vaccination effectiveness among elderly. Vaccine 2006;24(40‐1):6374‐5. [DOI] [PubMed] [Google Scholar]
- Young S, Halpin T, Johnson D, Irvin J, Marks J. Effectiveness of a mailed reminder on the immunization levels of infants at high risk of failure to complete immunizations. American Journal of Public Health 1980;70(4):422‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RK, Raymund M, Janosky JE, Nowalk MP, Fine MJ. Sensitivity and specificity of patient self‐report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003;13‐14(1):1486‐91. [DOI] [PubMed] [Google Scholar]
- Zimmerman RK, Santibanez TA, Janosky JE, Fine MJ, Raymund M, Wilson SA, et al. What affects influenza vaccination rates among older patients? An analysis from inner‐city, suburban, rural, and Veterans Affairs practices. American Journal of Medicine 2003;114(1):31‐8. [DOI] [PubMed] [Google Scholar]
- Zimmerman RK, Nowalk MP, Raymund M, Tabbarah M, Hall DG, Wahrenberger JT, et al. Tailored interventions to increase influenza vaccination in neighborhood health centers serving the disadvantaged. American Journal of Public Health 2003;93(10):1699‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RK, Nowalk MP, Bardella IJ, Fine MJ, Janosky JE, Santibanez TA, et al. Physician and practice factors related to influenza vaccination among the elderly. American Journal of Preventive Medicine 2004;26(1):1‐10. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Lee SS. Is the use of physician reminder sticker on medical records effective for improving the rate of recommending influenza vaccination?. Korean Academy of Family Medicine 2003;24(8):715‐20. [Google Scholar]
- Song Y, Oh J, Han S, Choi C. Effectiveness of telephone and postcard reminders for the influenza vaccination: a study in the elderly who have visited a family practice center in a tertiary care hospital. Korean Journal of Preventive Medicine 2000;33(1):109‐16. [Google Scholar]
Additional references
- Ashby‐Hughes B, Nickerson N. Provider endorsement: the strongest cue in prompting high‐risk adults to receive influenza and pneumococcal immunizations. Clinical Excellence for Nurse Practitioners 1999;3(2):97‐104. [PubMed] [Google Scholar]
- Ballada D, Biasio LR, Cascio G, D'Alessandro D, Donatelli I, Fara GM, et al. Attitudes and behavior of health care personnel regarding influenza vaccination. European Journal of Epidemiology 1994;10:63‐8. [DOI] [PubMed] [Google Scholar]
- Blank PR, Schwenkglenks M, Szucs TD. Disparities in influenza vaccination coverage rates by target group in five European countries: trends over seven consecutive seasons. Infection 2009;37(5):390‐400. [DOI] [PubMed] [Google Scholar]
- Bordley WC, Chelminski A, Margolis PA, Kraus R, Szilagyi PG, Vann JJ. The effect of audit and feedback on immunization delivery: a systematic review. American Journal of Preventive Medicine 2000;18(4):343‐50. [DOI] [PubMed] [Google Scholar]
- Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernie RR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. American Journal of Preventive Medicine 2000;18(Suppl 1):97‐140. [DOI] [PubMed] [Google Scholar]
- Calkins E, Katz LA, Karuza J, Wagner A. The small group consensus process for changing physician practices: influenza vaccination. HMO Practice 1995;9(3):107‐10. [PubMed] [Google Scholar]
- Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD000364.pub4] [DOI] [Google Scholar]
- Centers for Disease Control and Prevention. The community guide. Centers for Disease Control (http:www.the communityguide.org/vaccines/index.html) 2014 (accessed 27 June 2014).
- Lataillade C, Auvergne S, Delannoy I. 2005 and 2006 seasonal influenza vaccination coverage rates in 10 countries in Africa, Asia Pacific, Europe, Latin America and the Middle East. Journal of Public Health Policy 2009;30(1):83‐101. [DOI] [PubMed] [Google Scholar]
- Dharmaraj P, Smyth RL. Vaccines for preventing influenza in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD001753.pub2] [DOI] [Google Scholar]
- Dixon‐Woods M, Brown H, Arthur A, Matthews R, Jagger C. Organising services for influenza vaccination for older people. Journal of Health Services Research & Policy 2004;9(2):85‐90. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health interventions. Journal of Epidemiology and Community Health 1998;52(6):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabacher D, Josephson K, Pietruszka F, Linderborn K, Morley JE, Rubenstein LZ. An in‐home preventive assessment program for independent older adults: a randomized controlled trial. Journal of the American Geriatrics Society 1994;42:630‐8. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. Morbidity & Mortality Weekly Report. Recommendations & Reports 2009;58(RR‐8):1‐52. [PubMed] [Google Scholar]
- Ganguly R, Webster TB, Chmel H, Yangco BV. Influence of physician's recommendation on influenza immunization: perception and acceptance among a group of institutionalized elderly. Serodiagnosis and Immunotherapy in Infectious Disease 1990;4:167‐71. [Google Scholar]
- Ginson SH, Malmberg CM, French DJ. Impact on vaccination rates of a pharmacist‐initiated influenza and pneumococcal vaccination program. Canadian Journal of Hospital Pharmacy 2000;53:270‐5. [Google Scholar]
- Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature. Annals of Internal Medicine 1995;123:518‐27. [DOI] [PubMed] [Google Scholar]
- Gyorkos TW, Tannenbaum TN, Abrahamowicz M, Bedard L, Carsley J, Franco ED, et al. Evaluation of the effectiveness of immunization delivery methods. Canadian Journal of Public Health 1994;85 Suppl 1:S14‐30. [PubMed] [Google Scholar]
- Hak E, Hermens RPMG, Hoes AW, Verheij TJM, Kuyvenhoven MM, Essen GA. Effectiveness of a co‐ordinated nation‐wide programme to improve influenza immunization rates in The Netherlands. Scandinavian Journal of Primary Health Care 2000;18:237‐41. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Holm MV, Blank PR, Szucs TD. Developments in influenza vaccination coverage in England, Scotland and Wales covering five consecutive seasons from 2001 to 2006. Vaccine 2007;25:7931‐8. [DOI] [PubMed] [Google Scholar]
- Hutton E, Meitl J, Johnston B, Kidder D, Schmitz R, Hassol A, et al. Medicare influenza vaccine demonstration: summary of accomplishments. International Congress Series; Options for the Control of Influenza II1993:99‐107.
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson Vann JC, Szilagyi P. Patient reminder and recall systems to improve immunization rates. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AGSC, Sanders EAM, Nichol KL, Loon AM, Hoes AW, Hak E. Decline in influenza‐associated mortality among Dutch elderly following the introduction of a nationwide vaccination program. Vaccine 2008;26:5567‐74. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Pietrantonj C, Al‐Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD004876.pub3] [DOI] [PubMed] [Google Scholar]
- Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the veterans affairs health care system on the quality of care. New England Journal of Medicine 2003;348(22):2218‐27. [DOI] [PubMed] [Google Scholar]
- Joseph C, Goddard N, Gelb D. Influenza vaccine uptake and distribution in England and Wales using data from the General Practice Research Database, 1989/90‐2003/04. Journal of Public Health 2005;27(4):371‐7. [DOI] [PubMed] [Google Scholar]
- Kamal KM, Madhavan SS, Amonkar MM. Determinants of adult influenza and pneumonia immunization rates. Journal of the American Pharmacists Association 2003;43(3):403‐11. [DOI] [PubMed] [Google Scholar]
- Kelterman R, Allred CT, Frisch LE. Enhancing influenza immunization. Postcard and telephone reminders and the challenge of immunization site shift. Archives of Family Medicine 2000;9:368‐72. [DOI] [PubMed] [Google Scholar]
- Kohlhammer Y, Schnoor M, Schwartz M, Raspe H, Schafer T. Determinants of influenza and pneumococcal vaccination in elderly people: a systematic review. Public Health 2007;121:742‐51. [DOI] [PubMed] [Google Scholar]
- Krishna S, Balas EA, Maglaveras N. Patient acceptance of educational voice messages: a review of controlled clinical studies. Methods of Information in Medicine 2002;41:360‐9. [PubMed] [Google Scholar]
- Kroneman MW, Essen GA. Variations in influenza vaccination coverage among the high‐risk population in Sweden in 2003/4 and 2004/5: a population survey. BMC Public Health 2007;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community‐dwelling adults: a systematic review and meta‐analysis. Annals of Family Medicine 2012;10(6):538‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Wiley‐Blackwell, 2011. [Google Scholar]
- Litt JCB, Lake PB. Improving influenza vaccine coverage in at‐risk groups. Medical Journal of Australia 1993;159(8):542‐7. [DOI] [PubMed] [Google Scholar]
- Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U. S., 1989‐2005. Vaccine 2008;26:1786‐93. [DOI] [PubMed] [Google Scholar]
- MacDonald R, Baken L, Nelson A, Nichol KL. Validation of self‐report of influenza and pneumococcal vaccination status in elderly outpatients. American Journal of Preventive Medicine 1999;16(3):173‐7. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Boroujerdi MA, Taylor MW, Williams DJ, Hannaford PC, Lefevre KE, et al. The effect of the UK incentive‐based contract on the management of patients with coronary heart disease in primary care. Family Practice 2008;25(1):33‐9. [DOI] [PubMed] [Google Scholar]
- Michaelidis CI, Zimmerman RK, Nowalk MP, Smith KJ. Estimating the cost‐effectiveness of a national program to eliminate disparities in influenza vaccination rates among elderly minority groups. Vaccine 2011;29(19):3525‐30. [DOI] [PubMed] [Google Scholar]
- Muller D, Szucs TD. Influenza vaccination coverage rates in 5 European countries: a population‐based cross‐sectional analysis of the seasons 02/03, 03/04 and 04/05. Infection 2007;35(5):308‐19. [DOI] [PubMed] [Google Scholar]
- Nichol KL, MacDonald R, Hauge M. Factors associated with influenza and pneumococcal vaccination behaviour among high‐risk adults. Journal of General Internal Medicine 1996;11:673‐7. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Zimmerman R. Generalist and subspecialist physicians' knowledge, attitudes, and practices regarding influenza and pneumococcal vaccinations for elderly and other high‐risk patients: a nationwide survey. Archives of Internal Medicine 2001;61:2702‐8. [DOI] [PubMed] [Google Scholar]
- Ohkusa Y. Policy evaluation for the subsidy for influenza vaccination in elderly. Vaccine 2005;23(17‐18):2256‐60. [DOI] [PubMed] [Google Scholar]
- Poole PJ, Chacko E, Wood‐Baker RWB, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002733.pub2] [DOI] [Google Scholar]
- Remmen R, Seuntjens R, Vriens V, Lesaffer C, Hermann I, Damme P, et al. Efficacy of influenza immunisation programmes: comparison of two European systems in one practice. European Journal of General Practice 2002;8(4):159‐62. [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Shea S, DuMouchel W, Bahamonde L. A meta‐analysis of 16 randomized controlled trials to evaluate computer‐based clinical reminder systems for preventive care in the ambulatory setting. Journal of the Medical Informatics Association 1996;3:399‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer A, Briss P, Rodewald L, Bernier R, Strikas R, Yusuf H, et al. Improving immunization coverage rates: an evidence‐based review of the literature. Epidemiology Review 1999;21(1):96‐142. [DOI] [PubMed] [Google Scholar]
- Shojania KG, Jennings A, Mayhew A, Ramsay C, Eccles M, Grimshaw J. Effect of point‐of care computer reminders on physician behaviour: a systematic review. Canadian Medical Association Journal 2010;182(5):E216‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwardena AN, Wilburn T, Hazelwood L. Increasing influenza and pneumococcal vaccination rates in high risk groups in one primary care trust as part of a clinical governance programme. Clinical Governance: an International Journal 2003;8(3):200‐7. [Google Scholar]
- Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, et al. Interventions that increase use of adult immunization and cancer screening services: a meta‐analysis. Annals of Internal Medicine 2002;136:641‐51. [DOI] [PubMed] [Google Scholar]
- Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA, et al. Effect of patient reminder/recall interventions on immunization rates. A review. JAMA 2000;284(14):1820‐7. [DOI] [PubMed] [Google Scholar]
- Vu T, Farish S, Jenkins M, Kelly H. A meta‐analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine 2002;20:1831‐6. [DOI] [PubMed] [Google Scholar]
- Willis BC, Ndiaye SM, Hopkins DP, Shefer A. Improving influenza, pneumococcal polysaccharide and hepatitis B vaccination coverage among adults aged < 65 years at high risk. A report on recommendations of the Task Force on Community Preventive Services. Morbidity and Mortality Weekly Report 2005;54(RR‐5):1‐11. [PubMed] [Google Scholar]
- Wrenn K, Zeldin M, Miller O. Influenza and pneumococcal vaccination in the emergency department. Journal of General Internal Medicine 1994;9:425‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Thomas RE, Russell M, Lorenzetti D. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD005188.pub2] [DOI] [PubMed] [Google Scholar]


