Abstract
Background
Acute hypoxaemia de novo or on a background of chronic hypoxaemia is a common reason for admission to intensive care and for provision of mechanical ventilation. Various refinements of mechanical ventilation or adjuncts are employed to improve patient outcomes. Mortality from acute respiratory distress syndrome, one of the main contributors to the need for mechanical ventilation for hypoxaemia, remains approximately 30‐40%. Ventilation in the prone position may improve lung mechanics and gas exchange and could improve outcomes.
Objectives
The objectives of this review are to ascertain whether prone ventilation offers a mortality advantage when compared with traditional supine or semi recumbent ventilation in adult patients with severe acute respiratory failure requiring conventional invasive artificial ventilation.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, CINAHL and LILACS up to May 2020 for eligible randomized controlled trials using an updated version of the search strategy from the earlier version of the review. We added a search in the Cochrane COVID 19 Register. We also searched for studies by hand‐searching reference lists and citations of relevant articles, by contacting colleagues, by hand‐searching published proceedings of relevant journals. We searched trial registers for ongoing studies in November 2020. We applied no language or publication status constraints.
Selection criteria
We included randomized controlled trials (RCTs) that examined the effects of prone position versus supine/semi recumbent position during conventional mechanical ventilation in adult participants with acute hypoxaemia.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We analysed data using Review Manager software and pooled included studies to determine the risk ratio (RR) for mortality and the risk ratio or mean difference (MD) for secondary outcomes; we also performed subgroup analyses and sensitivity analyses.
Main results
We identified nine relevant open‐label (unblinded) RCTs (12 publications), which enrolled a total of 2165 participants. All recruited participants suffered from disorders of lung function causing moderate to severe hypoxaemia and requiring mechanical ventilation, so they were fairly comparable within what is the great diversity of specific disease diagnoses in intensive care. Blinding of participants, carers, clinical trialists and other decision‐makers to treatment allocation was not possible (face‐up vs face‐down). This predisposes to bias with regards to use of co‐interventions and also initiation of with‐holding or withdrawing life‐support, a common practice in intensive care.
Primary analyses of short‐ and longer‐term mortality pooled from six trials demonstrated an RR of 0.84 to 0.86 in favour of the prone position (PP), but findings were not statistically significant: In the short term, mortality for those ventilated prone was 33.4% (363/1086) and supine 38.3% (395/1031). This resulted in an RR of 0.84 (95% confidence interval (CI) 0.69 to 1.02). For longer‐term mortality, results showed 41.7% (462/1107) for prone and 47.1% (490/1041) for supine positions, with an RR of 0.86 (95% CI 0.72 to 1.03). The quality of the evidence for both outcomes was rated as low as a result of important potential bias and serious inconsistency.
Subgroup analyses for mortality identified three groups consistently favouring PP: those recruited within 48 hours of meeting entry criteria (five trials; 1024 participants; RR of 0.75 (95% CI 0.59 to 94)); those treated in the PP for 16 or more hours per day (five trials; 1005 participants; RR of 0.77 (95% CI 0.61 to 0.99)); and participants with more severe hypoxaemia at trial entry (six trials; 1108 participants; RR of 0.77 (95% CI 0.65 to 0.92)). The quality of the evidence for these outcomes was rated as moderate as a result of potentially important risk of bias.
Prone positioning appeared to influence adverse effects: pressure ulcers (four trials; 823 participants) with an RR of 1.25 (95% CI 1.06 to 1.48) and tracheal tube obstruction with an RR of 1.78 (95% CI 1.22 to 2.60) were increased with prone ventilation. Reports of arrhythmias were reduced with PP, with an RR of 0.64 (95% CI 0.47 to 0.87).
Authors' conclusions
We found no convincing evidence of benefit nor harm from universal application of PP in adults with hypoxaemia, mechanically ventilated in intensive care units (ICUs). This is despite the benefits observed in one of the open‐label trials restricted to participants with greater disease severity. Three subgroups (early implementation of PP, prolonged adoption of PP and severe hypoxaemia at study entry) suggested that prone positioning may confer a benefit for mortality, but these results should be interpreted with caution. Additional adequately powered studies would be required to definitively confirm or refute these observations of subgroup benefit. This is problematic, given the results of the most recent open‐label trial showing a benefit and recommendations derived from several published subgroup analyses. If replication and confirmation of such trial results, which would be desirable, are not realistic, formal meta‐analysis of individual patient data and post‐trial observational studies (as occur after phase III clinical drug trials) could be utilised to confirm apparent benefit in at‐risk populations. Complications such as tracheal tube obstruction and pressure ulcers are increased with the use of prone ventilation. Long‐term mortality data (12 months and beyond), as well as functional, neuro‐psychological and quality of life data, are required if future studies are to better inform the role of PP in the management of hypoxaemic respiratory failure in the ICU.
Plain language summary
Prone (face‐down) position for mechanical ventilation of adults with acute respiratory failure
Review question
This review sought to investigate whether face‐down ventilation could improve important outcomes by, for instance, reducing the death rate (mortality) among individuals requiring mechanical ventilation in intensive care. We also wanted to identify disadvantages and complications associated with prone positioning, as well as long‐term benefits.
Background
People who are admitted to an intensive care unit and need assistance with breathing provided by a ventilator (mechanical ventilation) because of lung damage caused by illness have a high risk of dying. Lungs that are affected by conditions such as pneumonia will consist of normal and abnormal or diseased areas. Recovery of diseased areas takes time, and a person may need support with ventilation while this occurs. Ventilation support is potentially lifesaving, as it maintains proper oxygen levels in the blood while removing carbon dioxide waste. However, the ventilator itself can cause inflammation and thus additional lung complications. The harder a ventilator has to work to achieve normal oxygenation and removal of carbon dioxide, the more likely it is that healthy, normal areas of the lung may be damaged, and the person's condition made worse. Ventilation with the person lying face‐down (prone) instead of face‐up (supine) might improve how well the ventilator works, thereby reducing these undesirable side effects.
Search date
The evidence is current to 01 May 2020.
Study characteristics
We identified and included in this review randomized controlled trials of adults that compared conventional mechanical ventilation in the face‐down versus the face‐up position.
Key results
Reports from nine trials of 2165 participants (12 publications) show that prone ventilation did not appear to be of benefit for all participants requiring ventilation. The evidence suggested some situations in which it may improve survival. One group of participants with the most severe lung damage appeared to have reduced mortality, as did participants who received treatment early and for prolonged periods. Complications were described. The most common of these were pressure sores (or ulcers) and tracheal tube blockage or obstruction. Low blood pressure and abnormal heart rhythms were also seen. The application of prone position to all participants in intensive care who have low oxygen levels was not supported by the evidence identified, but some particular groups of participants, for example, those with especially low oxygen levels, may benefit from prone positioning. Further clinical trials would assist in clarifying potential benefits for such patient groups but further trials may not take place because of the very large treatment benefit observed in the most recent clinical trial of participants with very low oxygen levels. In the absence of new trials, meta‐analysis of individual patient data may facilitate further assessment as well as further observational studies in at risk populations.
Quality of the evidence
The quality of the evidence for primary outcomes of this systematic review was low as a result of serious inconsistency and important potential bias.
Summary of findings
Summary of findings 1. Mortality: prone position compared with supine for acute respiratory failure in adults requiring mechanical ventilation in intensive care.
| Mortality: prone position compared with supine for acute respiratory failure in adults requiring mechanical ventilation in intensive care | ||||||
| Patient or population: adults with acute respiratory failure Settings: Intervention: mortality: prone position compared with supine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Mortality: prone position compared with supine | |||||
| Short‐term mortality (STM) Alive or dead Follow‐up: 10 to 30 days | Study population | RR 0.84 (0.69 to 1.02) | 2117 (8 studies) | ⊕⊕⊝⊝ Lowa,b | ||
| 383 per 1000 | 322 per 1000 (264 to 391) | |||||
| Moderate | ||||||
| 450 per 1000 | 378 per 1000 (310 to 459) | |||||
| Longer‐term mortality (LTM) Alive or dead Follow‐up: 31 to 180 daysc | Study population | RR 0.86 (0.72 to 1.03) | 2141 (8 studies) | ⊕⊕⊝⊝ Lowa,b | ||
| 470 per 1000 | 404 per 1000 (339 to 484) | |||||
| Moderate | ||||||
| 525 per 1000 | 452 per 1000 (378 to 541) | |||||
| Subgroup analysis of longer‐term mortality: severe hypoxaemia Alive or dead Follow‐up: 31 to 180 daysc | Study population | RR 0.77 (0.65 to 0.92) | 977 (7 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 547 per 1000 | 421 per 1000 (356 to 503) | |||||
| Moderate | ||||||
| 653 per 1000 | 503 per 1000 (424 to 601) | |||||
| Subgroup analysis of longer‐term mortality: lower tidal volume ventilation Alive or dead Follow‐up: 31 to 180 daysc | Study population | RR 0.73 (0.55 to 0.96) | 911 (5 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 451 per 1000 | 329 per 1000 (248 to 433) | |||||
| Moderate | ||||||
| 523 per 1000 | 382 per 1000 (288 to 502) | |||||
| Subgroup analysis of longer‐term mortality: ARDS only Alive or dead Follow‐up: 31 to 180 daysc | Study population | RR 0.85 (0.71 to 1.01) | 1758 (8 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 483 per 1000 | 411 per 1000 (343 to 488) | |||||
| Moderate | ||||||
| 522 per 1000 | 444 per 1000 (371 to 527) | |||||
| Subgroup analysis of longer‐term mortality: ≥ 16 hours/d prone Alive or dead Follow‐up: 31 to 180 daysc | Study population | RR 0.77 (0.61 to 0.99) | 1005 (5 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 470 per 1000 | 362 per 1000 (286 to 465) | |||||
| Moderate | ||||||
| 526 per 1000 | 405 per 1000 (321 to 521) | |||||
| Subgroup analysis of longer‐term mortality: enrolment ≤ 48 hours after entry criteria/ventilation Alive or dead Follow‐up: 31 to 180 daysc | Study population | RR 0.75 (0.59 to 0.94) | 1024 (5 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 469 per 1000 | 352 per 1000 (277 to 441) | |||||
| Moderate | ||||||
| 523 per 1000 | 392 per 1000 (309 to 492) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aBlinding of participants and carers was not possible. Researchers also may not have been adequately blinded. All analyses were downgraded because of this important potential bias, leading the quality of all subgroup analyses to be rated as moderate bFor the primary outcomes, inconsistency across studies reflected different patient populations, different management strategies generally and differences in adaptations to resulting effects of the intervention. This led to further downgrading of the quality of evidence for the primary outcomes to low cLonger‐term mortality = 31 to 180 days OR hospital mortality
Background
Description of the condition
Acute respiratory failure can arise from numerous diseases or disease processes, and is a common reason for admission to hospital. Patients with profound gas exchange abnormalities unresponsive to ward‐based strategies (such as oxygen therapy or continuous positive airways pressure (CPAP) for hypoxaemic respiratory failure, or non‐invasive ventilation (NIV) for hypercapnic ventilatory failure) may be referred to intensive care units (ICUs) for further management. Patients whose problems are predominantly related to oxygenation include those with pneumonia, pulmonary oedema, pulmonary aspiration pneumonitis and pulmonary thromboembolism. Acute lung injury and acute respiratory distress syndrome (Bellani 2016; Bernard 2005; MacCallum 2005; Matthay 2019; Phua 2009; Rubenfeld 2007) are reported in an important subset of patients treated for hypoxaemia within the ICU. Acute respiratory distress syndrome (ARDS) as the phrase suggests is only a syndrome and not a specific or single disease process, It may arise as a result of a wide variety of disparate pulmonary and extrapulmonary disease processes (Rezoagli 2017; Walkey 2012), as can its less physiologically severe counterpart previously called acute lung injury (ALI). Redefinition of ARDS, which has occurred since most studies were designed or published, ALI has been renamed as "mild ARDS" (ARDS definition workforce 2012). For the new criteria of mild, moderate and severe ARDS, mortality is quoted as 27%, 32% and 45% (ARDS definition workforce 2012). The commonly described histological finding of diffuse alveolar damage is found in only 50% of cases (de Hemptinne 2009), and doubt has been cast on the usefulness of the ARDS paradigm (Marini 2008; Soni 2010). Nevertheless, the concept is considered clinically useful by most intensivists (Bernard 2005). Recently different ARDS phenotypes are recognised as important (Reilly 2019; Matthay 2019; Wilson 2020). This has been especially so for ARDS associated with COVID‐19 pneumonia (Marini 2020; Robba 2020). ARDS, originally an abbreviation for Adult Respiratory Distress Syndrome remains a signature medical condition process that intensive care personnel strive to research and improve patient outcomes but is only a subset of conditions that cause acute respiratory failure in adult intensive care.
Mechanical ventilation is also used in younger patients (neonates, infants, children through to adolescents) for paediatric ARDS (PARDS). It is important to stress that the adult‐based definitions of ARDS may not be applicable to paediatrics for a variety of reasons (Cheifetz 2017). These include anatomic and physiologic differences which render infants and children more vulnerable to severe respiratory insult, greater metabolic demand and less cardiorespiratory reserve than adolescents and adults. Special considerations are often necessary to optimise management approaches across the heterogeneous paediatric spectrum ranging from neonates to adolescents. Thomas and colleagues also note children have considerable variability in the predisposing conditions and etiology; their response to therapy was different and often better; and pre‐existing conditions and underlying etiology appeared to influence outcome to a greater extent than the severity of the lung injury itself. They (Thomas 2013) highlight a medical axiom that children should not be considered as "little adults." Kneyber et al also note that with regards to ventilator induced lung injury (VILI) that given the physiological and biological differences in the respiratory systems of infants, children, and adults, it is difficult to directly extrapolate clinical practice from adults to children (Kneyber 2014). For the above discussed reasons children are not considered together with adults in this systematic review. Children and neonates have also been the subject of separate Cochrane Systematic Reviews (Gillies 2012; Rivas‐Fernandez 2016)
Patients with ventilatory failure and hypercapnia include those with chronic obstructive pulmonary disease (COPD) and those receiving central nervous system depressant drugs; patients with neuromuscular problems may also require mechanical ventilation. Thus a wide variety of patients may require mechanical ventilation within the adult ICU. Variability is great with regards to severity of illness and severity of structural lung damage. The reversibility of disease‐driving processes is also inconsistent.
Description of the intervention
Patients with profound hypoxaemia present a significant challenge for carers in dealing with both hypoxaemia and underlying process(es). Although hypoxaemia often is not perceived as the ultimate cause of death in these patients, it does have deleterious effects (Strachan 2001). Avoidance of profound hypoxaemia is one of the goals of supportive therapy in ICU, and a variety of manoeuvres are employed to ameliorate hypoxaemia. Including: positive end‐expiratory pressure (PEEP), inverse ratio ventilation (IRV), alveolar recruitment manoeuvres, restrictive fluid administration strategies, inhaled pulmonary vasodilators such as nitric oxide and prostacyclin, corticosteroids and neuromuscular blockers and mechanical ventilation in the prone position (Adhikari 2004; Adhikari 2007; ARDSnet 2006b; ARDSnet 2006a; Cranshaw 2002;Fielding‐Singh 2018; Klein 2004; Mentzelopoulos 2005; Papazian 2010). All of these therapies have been shown to improve oxygenation, but few have demonstrated a mortality benefit in randomized controlled trials (Diaz 2010; Papazian 2010; Petrucci 2013). Athough a systematic review of high‐frequency oscillation (HFO) suggested their possible utility in the management of ARDS (Fan 2010; Sud 2013), two RCTs reported in 2013 have not confirmed benefit (Ferguson 2013; Young 2013). Notably one of the trials was discontinued early as a result of increased mortality in the treatment group (Ferguson 2013). Extracorporeal membrane oxygenation (ECMO) has been shown to be of benefit (Noah 2011; Noble 2010; Aoyama 2019) but is available only in relatively few specialized centres. Traditional mechanical ventilation, which normally is utilized in supine and semi recumbent patients, while ensuring short‐term survival, may also contribute to lung injury and other deleterious effects (Soni 2008). Ventilator‐induced lung injury has been demonstrated in both experimental animal models and in human participants to perpetuate or even accentuate the original injury to the lung and can cause dysfunction of distant organs (Verbrugge 2007) in the form of barotrauma, volutrauma and biotrauma.
Under normal circumstances, patients ventilated in ICUs are cared for in the semi‐recumbent position, often described as the supine position. This supine or semi‐recumbent position allows better access for carers to provide interventions such as mouth care and airway and vascular access procedures. It is also more appropriate for critical manoeuvres such as cardiopulmonary resuscitation, should this be required. This position is more comfortable for patients and allows them better interaction with their environment when compared with the prone, face‐down position.
This systematic review explores the intervention of placing patients in the prone position (face‐down) while they are mechanically ventilated for severe hypoxaemic respiratory failure via a tracheal tube.
How the intervention might work
Chatte 1997 and others have showed that ventilation provided with the patient in the prone position could have beneficial effects on oxygenation (Gattinoni 2001; Mure 2001). Recent studies in humans and in experimental animal models, have confirmed that ventilation in the prone position is associated with improved oxygenation in most individuals. More than 70% of patients with lung injury show clinical improvement in oxygenation with prone mechanical ventilation. In a retrospective multi‐variate analysis, prone positioning was independently correlated with positive outcomes in patients with ARDS (Venet 2003). The mechanisms by which prone position improves gas exchange include alveolar recruitment, redistribution of ventilation towards dorsal areas that remain well perfused, homogenization of tidal volume distribution and possible improved postural drainage of secretions (Gattinoni 2006; Guerin 2006). Postural drainage has also been suggested to reduce ventilator‐associated pneumonia, although theoretically this could spread organisms and inflammatory mediators within lung tissue, leading to increased damage (Graf 2008; Marini 2010). Homogenization of tidal volume distribution may reduce tissue stress/strain and consequently may diminish the well‐described injurious effects of mechanical ventilation, thus providing additional benefit over and above that associated with improved oxygenation (Gattinoni 2012; Gattinoni 2013; Mentzelopoulos 2005; Slutsky 2013). Other effects include improvements in haemodynamics (Guerin 2014).
Thus, three phenomena might improve survival among patients (Charron 2011).
Reduced extent and duration of severe hypoxaemia.
Reduced propensity to ventilator‐induced lung injury.
Reduced occurrence of nosocomial or ventilator‐associated pneumonia.
Adverse effects associated with the prone position for ventilation most notably include (Faculty of Intensive Care 2019):
unplanned extubation and risk of an episode of potentially catastrophic hypoxaemia;
inadvertent bronchial intubation, which will also worsen hypoxaemia and increase risk of barotrauma (e.g. pneumothorax);
development of pressure sores / ulcers (most cited injury);
facial / periorbital edema;
ocular complications including severe corneal abrasions and possible ischaemic neuropathy with permanent sight loss;
cardiovascular instability;
intravenous line displacement;
kidney dialysis / filtration line flow problems interfering with renal support;
intracranial hypertension, which can compromise cerebral circulation;
brachial plexus injuries; and
staff injuries, especially if insufficient trained staff are available to perform required turns in sedated intubated patients
Improved oxygenation with the prone position could allow additional time for lung reparative processes, and, by reducing secondary lung infection or injury, has the potential to accelerate recovery and lessen mortality among adults with acute respiratory failure. Adverse effects and complications related to the prone position might reduce the overall impact of these potential benefits.
Why it is important to do this review
Patients admitted to the ICU with severe hypoxaemia are at high risk for mortality (ARDS definition workforce 2012; Walkey 2012). For example, Phua and colleagues reported overall mortality in the important subset of ICU patients with ARDS of 44% in observational studies and 36% in randomized controlled trials. This rate of mortality does not seem to have been significantly reduced since 1994, when the American‐European Consensus Conference redefined ARDS (Phua 2009). Any intervention that reduces mortality, especially one that can be easily implemented at little additional cost, requires adequate exploration. This interim amendment of the original review (Bloomfield 2015) seeks to update evidence from any new randomized clinical trials and evaluate all evidence in the context of current theory and practice. This amendment may be superseded by a complete review update in the future.
Objectives
The objectives of this review are (1) to ascertain whether prone ventilation offers a mortality advantage when compared with traditional supine or semi recumbent ventilation in participants with severe acute respiratory failure requiring conventional invasive artificial ventilation, and (2) to supplement previous systematic reviews on prone ventilation for hypoxaemic respiratory failure in an adult population.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) comparing conventional modes of mechanical ventilation in the supine or semi recumbent position versus mechanical ventilation in the prone position in adults with acute respiratory failure. We did not include observational studies due to the perceived higher risk of bias.
We included unpublished studies and abstracts when identified.
We imposed no language restrictions.
Types of participants
We included studies on adults with critical illness in an ICU setting requiring conventional mechanical ventilation for acute severe respiratory failure.
We excluded studies primarily investigating participants with chronic respiratory impairment such as COPD. This review focused on acute severe respiratory failure.
We excluded studies on neonates or paediatric participants (i.e. younger than 16 years), which are covered separately in updated Cochrane reviews (Gillies 2012; Rivas‐Fernandez 2016) and because of other differences in physiology, aetiology, definitions, comorbidities, cointerventions and rescue treatments for paediatric patients with acute respiratory failure (Cheifetz 2017; Kneyber 2014; Thomas 2013).
Types of interventions
We examined interventions comparing conventional methods of ventilation in the supine or semi recumbent position (which could encompass lateral positioning as part of routine pressure care) versus the prone position.
We excluded studies that used primary positions other than supine or semi recumbent. We excluded rotational therapies provided in the prone position. We excluded studies comparing conventional prone ventilation versus other experimental modes of ventilation such as high‐frequency jet ventilation (HFJV) or high‐frequency oscillation (HFO).
Types of outcome measures
We sought information on the following main outcomes.
Primary outcomes
Short‐term mortality (10 to 30 days, or ICU mortality).
Longer‐term mortality (> 30 days, or hospital mortality).
Secondary outcomes
We also sought information on the following.
Rate of ventilator‐associated pneumonia, as defined in the original studies.
Number of days on a ventilator.
Length of ICU stay.
Length of hospital stay.
Improvement in oxygenation.
Adverse events.
Quality of life.
Economic outcomes.
Search methods for identification of studies
Electronic searches
We searched for eligible randomized controlled trials as described in the Cochrane Handbook of Systematic reviews of Interventions Chapter 4 (Lefebvre 2019).
We applied no language or publication status constraints.
We searched the following databases:
CENTRAL, Cochrane Central Register of Controlled Trials (2020, Issue 4)
MEDLINE ALL (Ovid SP; 2014 to 01 2020 May)
EMBASE (Ovid SP; 2014 to 01 May 2020)
CINAHL, Cumulative Index to Nursing and Allied Health Literature (Ebsco; 2014 to 01 May 2020)
LILACS, Latin American Caribbean Health Sciences Literature (2014 to 01 May 2020)
Cochrane COVID 19 Register
We updated the search strategy of Bloomfield 2015 with extra search terms and added filters for randomized controlled trials. The searches were run from 1 January 2014 to 1 May 2020 using the search strategy provided in Appendix 1.
For the earlier version of this review (Bloomfield 2015), we searched: CENTRAL (2014, Issue 1), MEDLINE (Ovid SP; 1950 to 31 January 2014), EMBASE (Ovid SP; 1980 to 31 January 2014), CINAHL (1982 to 31 January 2014) and LILACS (1992 to 31 January 2014).
Searching other resources
We also searched for studies by:
hand‐searching reference lists and citations of previous trials and review articles to May 2020;
hand‐searching books related to critical care and mechanical ventilation;
communicating with colleagues, particularly published trialists; and
-
performing a subject‐specific search of the following journals to look for published proceedings abstracts of clinical trials.
American Journal of Respiratory and Critical Care Medicine, volumes 175 to 189, 2007 to January 2014.
Critical Care, volumes 11 to 18, 2007 to January 2014.
Critical Care Medicine, volumes 35 to 42, 2007 to January 2014.
Intensive Care Medicine, volumes 33 to 40, 2007 to January 2014.
We searched for relevant ongoing trials at the following websites (searched 20 November 2020):
ClinicalTrials.gov (https://www.clinicaltrials.gov/)
ISRCTN Registry (http://www.isrctn.com/)
World Health Organization ‐ International Clinical Trials Registry Platform (ICTRP) (www.who.int/clinical-trials-registry-platform)
Cochrane Covid‐19 study register (https://covid-19.cochrane.org/)
We checked the included studies for retractions in the Retraction Watch Database and in PubMed.
Data collection and analysis
Selection of studies
Two review authors (RB and DWN) independently screened and classified all citations as potential primary studies, review articles or others for inclusion. Two review authors (RB and DWN) examined all potential primary studies and decided whether they should be included in the review. We resolved all disagreements by discussion.
Data extraction and management
Two review authors (RB and DWN) independently extracted in duplicate from each study data on methods and outcomes (Appendix 2). A third review author (AS) checked data subsequently entered onto a Microsoft Excel spreadsheet.
Assessment of risk of bias in included studies
We judged study quality using the Cochrane Risk of bias tool on the basis of criteria and mechanisms described in Table 8.5.d by Higgins et al (Higgins 2011a), which were based on:
adequacy of randomization;
allocation concealment;
blinding of participants and investigators;
blinding of outcome assessment;
completeness of outcome data;
selective reporting; and
other relevant potential bias.
We addressed the impact of methodological quality on results and performed a sensitivity analysis that excluded studies at high risk of bias. We rated the quality of evidence by using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c), as described below.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs). We used odds ratios (ORs) when outcomes were rare. We used standardized mean differences (SMDs) or mean differences (MDs) as appropriate for continuous outcomes.
Unit of analysis issues
We identified no specific issues.
Dealing with missing data
We did not specify in the original protocol how missing data issues would be managed (Bloomfield 2009).
Assessment of heterogeneity
We measured heterogeneity by using the Higgins test, whereby an I2 statistic greater than 25% is considered to show significant heterogeneity. This test describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2003). We used a fixed‐effect model when the Higgins test showed good homogeneity (low heterogeneity) between studies; otherwise, we used a random‐effects model. Higgins has defined an I2 statistic of 25%, 50% or 75% as low, moderate or high (Higgins 2003). When present, we planned to explore heterogeneity by considering these options listed in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Avoiding formal meta‐analysis when fewer than two studies provided quantitative data for primary outcomes.
Ignoring heterogeneity.
Using random‐effects meta‐analysis to ascertain mean effect and confidence intervals of the mean effect size. (Deeks 2019)
Exploring heterogeneity by using pre‐specified subgroup analyses or meta‐regression when we identified sufficient (> 10) studies.
Excluding studies as part of a sensitivity analysis (Deeks 2011).
Assessment of reporting biases
We used a funnel plot to assess the risk of small study effects or reporting bias (Higgins 2011a).
Data synthesis
We reviewed data from included studies qualitatively, and then, when possible, we combined data quantitatively by population, intervention and outcome, using Cochrane's statistical software, RevMan 5.40. We based quantitative analyses of outcomes on "intention‐to‐treat" results (i.e. results based on the intention‐to‐treat principle). We followed the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions regarding data synthesis. In cases of very substantial (high) heterogeneity (Higgins 2003), we would not pool the results to perform statistical analysis. We used a fixed‐effect model when homogeneity between studies was good, and a random‐effects model when it was not.
Subgroup analysis and investigation of heterogeneity
We planned to undertake the following subgroup analyses (SGA).
Duration of daily ventilation in the prone position (< 16 hours/d vs ≥ 16 hours/d). As any benefit from prone ventilation may be a dose (time)‐related phenomenon, daily duration of time in that position would appear potentially important.
Duration of supine ventilation before randomization. As ventilatory‐induced lung injury is relatively rapid in onset, any randomized trials and outcomes reporting very limited exposure to supine ventilation before randomization should be identified.
Outcome according to severity (oxygenation index; PaO2/FIO2 ratio or quotient; severity of illness score, e.g. Simplified Acute Physiology Score II (SAPS II)). As patients with more severe lung injury benefit from prone ventilation, this may be an important subgroup to explore. SAPS II and similar scores may indirectly reflect the severity of inciting injury and may be relevant.
Tidal volume (size of the mechanical breath given to the participant) in relation to body weight has been shown to affect survival and outcomes between high tidal volume (> 10 mL/kg of ideal body weight), moderate tidal volume (8 to 10 mL/kg of ideal body weight) and low tidal volume (≤ 8 mL/kg of ideal or predicted body weight) and will be explored if the data permit. (Actual body weight exceeds ideal or predicted body weight by a mean factor of as much as 1.25 (Bloomfield 2006) and therefore underestimates the standard metric of tidal volume, which is based on ideal body weight.) We considered "ideal" and "predicted body weight" as interchangeable and based on height and sex of participants.
We analysed studies of acute lung injury (ALI) together with acute respiratory distress syndrome (ARDS) separately from those examining other causes of acute severe hypoxaemic respiratory failure.
We further sub‐classified acute lung injury and ARDS into pulmonary and extrapulmonary causes and as conditions that may behave differently with different ventilatory strategies (Walkey 2012; Ware 2000). We planned to explore differences in outcomes in these subcategories if collected data allowed.
We explored evidence of substantial heterogeneity in primary outcomes as indicated by the Higgins test by performing subgroup analyses, and, if we identified more than 10 primary studies, by performing meta‐regression (Deeks 2011). We did not employ statistical techniques or adjustments for multiple comparisons when conducting these prespecified analyses.
Sensitivity analysis
We undertook a sensitivity analysis of primary outcomes with regards to the quality of data. We excluded from the sensitivity analysis studies with two or more "red flags" due to the high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We used the principles of the GRADE system (Guyatt 2008; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c) to assess the quality of the body of evidence associated with specific outcomes. In the case of RCTs, the GRADE system allows downgrading of the overall rating of evidence from "high quality" by one or two grades on the basis of study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
We applied the GRADE system to these primary outcome measures.
Short‐term mortality.
Longer‐term mortality.
We also applied this system to four subgroup analyses related to longer‐term mortality.
Participants with severe hypoxaemia.
Mechanical ventilation with lower tidal volumes.
Outcomes for participants with ALI or ARDS.
Maintenance of the prone position for 16 or more hours per day.
Enrolment within 48 hours of meeting study criteria.
We chose these outcomes for GRADE analysis after the protocol was published, as this tool was not used by The Cochrane Collaboration at that time.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
Details of the original search are reported in Bloomfield 2015. The results of the updated search from 1st January 2013 to 1st May 2020 are presented in a study flow diagram (Figure 1).
1.
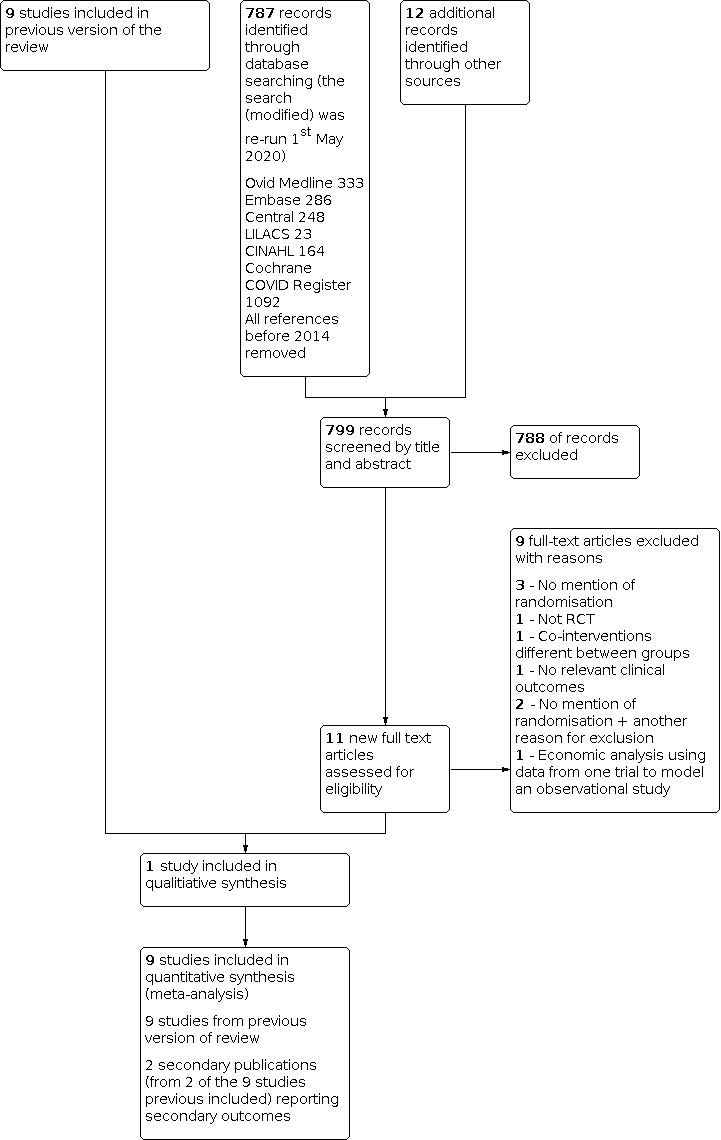
Flow diagram of results from updated search (January 2014 to 1st May 2020)
A search of proceedings supplements of the American Journal of Respiratory and Critical Care Medicine, Critical Care, Critical Care Medicine and Intensive Care Medicine yielded no additional relevant RCTs.
Included studies
We extracted data from nine primary studies reported in 13 publications. Eight were primary studies published in full form in peer‐reviewed journals (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005). One study was reported as an abstract, with supplementary information given in presentation slides provided by Jan Friederich (Leal 1997). We found additional data regarding some of the primary studies in seven journal publications (Ayzac 2016; Chiumello 2012; Gattinoni 2010; Girard 2014; Sud 2008b; Sud 2010; Sud 2014). These provided additional information on subgroups of participants with very severe hypoxaemia from four studies (Gattinoni 2001; Guerin 2004; Mancebo 2006; Taccone 2009) and on an ARDS subgroup from one study (Sud 2014). Chiumello et al (Chiumello 2012) reported one‐year mortality at five of the centres that contributed to the Taccone 2009 study. Sud et al provided additional data regarding severity of illness through SAPS II scores (Sud 2008b). Chiumello et al reported pulmonary function and quality of life data (Chiumello 2012). Most studies recruited participants with ALI or ARDS, although the largest single study (Guerin 2004) also recruited individuals who would have been excluded from the ALI/ARDS trials. Some information for this subgroup was later made available (Sud 2014).
Two secondary publications were identified in the latest search update and reported new data from the primary trial of Guerin (Guerin 2013) regarding pressure ulcers (Girard 2014) and ventilator associated pneumonia (Ayzac 2016). They are incorporated into this amendment.
Excluded studies
We excluded five studies that were included in other published meta‐analyses. Two studies (Demory 2007; Papazian 2005) investigated the short‐term effects of high‐frequency oscillatory ventilation (HFOV) in prone and supine participants. One was a prevention study (Beuret 2002) in patients with coma, and one (Watanabe 2002) applied an intervention of neuromuscular blockade to the prone group that has been associated with improved outcomes in some but not all trials (Ho 2020; Papazian 2010) and failed to report mortality data. Another study (Curley 2005) included predominantly very young children with a median age of two years and no adults. Two of these studies (Beuret 2002; Watanabe 2002) were conducted in the pre‐low tidal volume ventilation era.
The updated search for this amendment identified eight further studies for possible inclusion (Cao 2014; Cheng 2016; Li G 2015; Li J 2015; Peng 2018; Wang 2015; Yan 2015; Zhou 2014) two directly from the literature search and six from the systematic review of Du (Du 2018). All were ultimately excluded from analysis (Characteristics of excluded studies). Five (Cao 2014; Cheng 2016; Li J 2015; Wang 2015; Yan 2015 ) did not mention randomization. One (Li G 2015) was a retrospective study. The 60 patient 4‐limb randomized clinical study of Peng et al (Peng 2018) was primarily a short‐term physiological investigation with a single episode of prone intervention. It did not report mortality and had major baseline imbalances with regards to age and APACHE scores of participants. Such deficiencies in reporting of trials in the Chinese literature have been previously documented (Zhang 2008). The randomized trial of Zhou et al (Zhou 2014) compared supine position alone versus two interventions, prone position combined with recruitment manoeuvres and was also excluded.
One systematic review (Yue 2017) listed Charron 2011 as a randomized clinical trial in their meta‐analysis. Re‐examination of this study data confirmed results were derived from a clinical database and not a randomized clinical trial.
Studies awaiting classification
There are no additional studies awaiting classification.
Ongoing studies
Three ongoing studies were identified. This included one trial (NCT03891212) comparing prone versus supine positioning in mechanically ventilated patients with severe pneumonia, and two trials (NCT04139733; NCT04607551) comparing prone versus supine positioning in mechanically ventilated patients receiving ECMO (see Characteristics of ongoing studies).
Risk of bias in included studies
We have graphically presented in Figure 2 review authors' assessment of bias within individual studies, and in Figure 3, bias across studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation sequence generation was of a high standard overall, with most studies employing computer‐generated sequences and blinding sequence allocation by using a computer‐telephone allocation system of sealed opaque envelopes. Allocation bias seems likely for the study of Chan et al (Chan 2007).
Blinding
Complete blinding of participants and clinical staff to allocation would be impossible because participants were placed either with face and feet up or with face and feet down. Some blinding of processes and decision making could be incorporated into trial procedures together with some standardised or protocolised approaches to important co‐interventions to improve methodological rigour. Such measures have not been systematically applied across studies.
Lack of blinding could affect application of important related co‐interventions (Cummings 2013) such as decisions on futility, non‐escalation of treatment, withdrawal of treatment or continuation of active treatment (Forbes 2013; Morgan 2014; Stapleton 2005; Turnbull 2014). We downgraded the quality of evidence assessed by the GRADE system in all analyses in which it was utilized as important clinical decisions would be made with full knowledge of treatment allocation (Jadad 2007). A double downgrade was considered but not applied. It is not clear whether investigators were blind to participant allocation when performing data analyses.
Incomplete outcome data
Most studies reported participants lost to follow‐up and used the intention‐to‐treat principle. Missing data for primary and some major secondary outcomes were small (0 to 5% of participants), and this was unlikely to affect interpretation of findings. However, for some secondary outcomes, the disparity in reporting rates between studies suggests differences in outcome definition, priority for data collection or efforts to minimize these complications.
Selective reporting
Selective reporting within studies was unlikely for the primary outcomes and for subgroup analyses in which death was the outcome of interest. Most studies were conducted at multiple centres, and a pre‐study protocol would define criteria for most reported study endpoints. Post hoc analyses might be at risk of selective reporting e.g. choice of 88 mmHg (11.7 kPa) PaO2/FIO2 quotient as cutoff for post hoc mortality subgroup analysis (Gattinoni 2001).
Other potential sources of bias
For most studies, cross‐over of participants from one limb of the study to another was modest. However, cross‐over or non‐adherence to the protocol was considerable for the study of Guerin 2004. Eighty‐one (21%) participants crossed over from supine to prone, and 170/413 (41%) in the prone group were never actually put in prone position, or the prone position was discontinued before the study met prone weaning criteria (Guerin 2004; Sud 2008). This effect would assume even greater importance in subgroup analyses of sicker participants that included an even higher proportion of cross‐over participants. Although strictly not bias, this will reduce the efficiency (Lipsey 1990) of a trial and will "silently" increase the risk of type II statistical error. Supplementary per‐protocol analysis (Hernan 2017; Sheiner 1995) would be useful for assessing the impact of cross‐overs and of partial and full protocol violations. In addition, one study described differential use of co‐interventions (e.g. blood transfusion, neuromuscular blockers) that would be expected to influence outcomes (Voggenreiter 2005). Participant losses for main analyses were small (0 to 4.2%), and reasons were detailed. Supplementary analyses were not performed as a result of these small participant losses. Several of the trials (Chan 2007; Fernandez 2008; Gattinoni 2001; Mancebo 2006; Voggenreiter 2005 ) were discontinued early which can also be a source of bias (Bassler 2010).
Effects of interventions
See: Table 1
Before the last published trial (Guerin 2013) was included, statistical heterogeneity was low overall as assessed by the I2 method. Most analyses had an I2 statistic = 0. With inclusion of this last trial (Guerin 2013), heterogeneity was substantial, and heterogeneity for the primary analyses moved from 0% without the last study to 60% or greater with its inclusion (Analysis 1.1). Among subgroup analyses of mortality that included data from this trial (Guerin 2013), only one had an I2 statistic = 0, and the remaining nine analyses demonstrated I2 values ranging from 39% to 56%. With the last trial of Guerin excluded from the same analyses, all 10 trials had an I2 statistic = 0 (Analysis 1.3; Analysis 1.4; Analysis 1.6; Analysis 1.9; Analysis 1.11).
1.1. Analysis.

Comparison 1: Mortality, Outcome 1: Mortality
1.3. Analysis.

Comparison 1: Mortality, Outcome 3: SGA of mortality prone ≥ 16 hours/d
1.4. Analysis.

Comparison 1: Mortality, Outcome 4: SGA of mortality: enrolled ≤ 48 hours after entry criteria met/ventilation
1.6. Analysis.

Comparison 1: Mortality, Outcome 6: SGA of severe hypoxaemia at entry
1.9. Analysis.

Comparison 1: Mortality, Outcome 9: SGA of low tidal volume (mean 6 to 8 mL/kg IBW)
1.11. Analysis.

Comparison 1: Mortality, Outcome 11: SGA of ARDS only
For reasons of inconsistency and heterogeneity, the quality of the evidence for primary outcomes based on the GRADE system was down‐rated further and was classified as low. The option of not exploring and analysing these results was avoided, and a random‐effects model was employed. The number of studies (<10) available for analysis were insufficient to justify meta‐regression techniques (Deeks 2019).
Fixed‐effect and random‐effects models were determined by I2 statistical value and are presented in the text as appropriate. Presentation of short‐ and longer‐term outcomes in the same forest plot has restricted use of one or the other model for these data pairs, and for some outcomes, minor discrepancies between text and figures may be noted for this reason.
Primary outcomes
Short‐term mortality
Eight clinical trials with 2117 participants reported on short‐term mortality (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009) and were included in this analysis (Analysis 1.1). Overall mortality of participants was 756/2117 (35.7%). Mortality for those ventilated prone was 33.4% (363/1086), and supine 38.3% (395/1031) resulting in a risk ratio of 0.84 (95% CI 0.69 to 1.02) in favour of the prone position.
Prior to the inclusion of the most recent study (Guerin 2013), which exclusively enrolled participants with more severe hypoxaemia than was seen in earlier studies and had a risk ratio for mortality of 0.49, the risk ratio from other seven studies was close to unity at 0.95. In the Higgins test, the I2 statistic moved from 0% to 60% with inclusion of the most recent study (Guerin 2013). Visual inspection of the funnel plot was not supportive of major small study effect or reporting bias (Figure 4). Removal of one study, which applied prone ventilation for only one day (Leal 1997), did not alter the risk ratio, which remained at 0.84.
4.

Funnel plot of comparison: 1 Mortality, outcome: 1.1 Mortality.
Longer‐term mortality
Eight clinical trials with 2140 participants (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005) reported longer‐term mortality (952/2140), which overall approximated 44.5% (Analysis 1.1). Mortality for prone (462/1099; 42.0%) and supine (490/1041; 47.1%) ventilated participants resulted in a risk ratio of 0.86 (95% CI 0.72 to 1.03) in favour of the prone position. Before inclusion of the last study (Guerin 2013), which had an individual risk ratio of 0.58, the risk ratio from the other studies was again close to unity at 0.97. In the Higggins test (Higgins 2003), the I2 statistic moved from undetected (0%) to "moderate" heterogeneity at 61%, with the addition of Guerin 2013. Visual inspection of the funnel plot was not supportive of major small study effect or reporting bias (Figure 4).
Use of the GRADE system to assess the quality of evidence for major risks of bias from lack of blinding (Guyatt 2011a) and from inconsistency of effect (Guyatt 2011c) reduced the overall rating of evidence quality to "low" for both of these primary outcomes.
One‐year mortality
Only one study (and therefore not part of the quantitative meta‐analysis) reported 12‐month mortality (113/187) in a secondary publication approximating 60% mortality overall (Chiumello 2012). This population of 187 participants accounted for approximately 55% of the original study population. The point estimate for the risk ratio in this secondary study was 1.13 (95% CI 0.89 to 1.42) in favour of supine positioning in this single‐study post hoc analysis. This is a reversal from the findings the parent study (Taccone 2009) which reported a 6 month mortality risk ratio of 0.90 (95% CI 0.73‐1.11) in favour of prone positioning.
Results for primary outcomes and for selected subgroup analyses are included in Table 1.
Secondary outcomes
Rate of ventilator‐associated pneumonia
Five studies (Ayzac 2016; Fernandez 2008; Guerin 2004; Mancebo 2006; Voggenreiter 2005) reported rates of ventilator‐associated pneumonia (VAP) with 1473 participants for analysis (Analysis 3.1). The overall rate of VAP for participants (226/1007) was 22.7%. The proportion of participants with VAP for prone positioning was 0.21 compared with 0.22 for the supine position. The risk ratio for VAP of 0.97 (95% CI 0.80 to 1.18) close to unity. The additional data from the PROSEVA trial (Ayzac 2016) for this amendment had the effect of shifting the point estimate closer to unity for this complication.
3.1. Analysis.

Comparison 3: Pneumonia, Outcome 1: Pneumonia
Number of days on a ventilator
Three studies (Fernandez 2008; Guerin 2004; Voggenreiter 2005) reported duration of mechanical ventilation in 871 participants (Analysis 4.1). Mean duration of ventilation was reduced by 0.47 days (95% CI ‐1.53 to 0.59) for participants in the prone position.
4.1. Analysis.

Comparison 4: Duration of mechanical ventilation, Outcome 1: Duration of mechanical ventilation
Length of ICU stay
Five studies (Fernandez 2008; Guerin 2004;Guerin 2013Mancebo 2006; Taccone 2009) with 1775 participants reported ICU length of stay (Analysis 5.1), which was increased for participants in the prone position by a mean of 1.06 (95% CI ‐1.13 to 3.26) days.
5.1. Analysis.

Comparison 5: Length of stay (LOS), Outcome 1: ICU LOS
Analysis of log (base 10) transformed data provided similar results, with an increase (geometric mean) of 1.07 days for participants treated in the prone position (95% CI ‐1.3 to 1.5 days).
Length of hospital stay
Only one study (and therefore not part of the quantitative meta‐analysis) of 40 participants reported hospital length of stay. This very small study favoured the supine position by a mean of 5.8 (range ‐7.9 to 19.5) days.
Improvement in oxygenation
Four studies (Gattinoni 2001; Guerin 2004; Mancebo 2006; Voggenreiter 2005) with 827 participants reported improvement in oxygenation (PaO2/FIO2 ratio or quotient) over seven to 10 days (Analysis 6.1). The mean difference in improvement in the PaO2/FIO2 quotient was 24.6 mmHg (95% CI 13.9 to 35.2 mmHg) compared with baseline measurements at study entry. This equates to 3.3 kPa (95% CI 1.8 to 4.7 kPa) (P value < 0.00001). Variance for two studies (Guerin 2004; Mancebo 2006) was estimated by using the higher of the "paired" variances for the study population. Inflating the entered standard deviations by approximately 50% to 120.0 for these two studies had no impact on inferences. The second study of Guerin (Guerin 2013) was not included in the analysis because of the formal reduction in PEEP mandated by improving oxygenation.
6.1. Analysis.

Comparison 6: Mean change in PaO2/FIO2 quotient (mmHg), Outcome 1: Mean increase in PaO2/FIO2 quotient (mmHg) at 7 or 10 days
One study (and therefore not part of the quantitative meta‐analysis) provided data for the PaO2/FIO2 quotient for a very small subset of participants (26) (Chiumello 2012) included in one of the primary studies (Taccone 2009; 342 participants) at 12‐month follow‐up. PaO2 data (Chiumello 2012) on air showed a change in the PaO2/FIO2 quotient of 43 mmHg (95% CI 15.8 to 70.2 mmHg) in favour of supine ventilation (P value = 0.002). This equates to a PaO2/FIO2 quotient of 5.7 kPa (95% CI 2.1 to 9.4 kPa).
Adverse events
Several adverse events were documented across studies and were reported in two different ways: Most reported events per participant group, but two studies reported some data as events per participant day. These were analysed separately.
New pressure sores or ulcers
Four studies (Chan 2007; Gattinoni 2001; Girard 2014; Voggenreiter 2005) with 823 participants reported pressure ulcer (or sore) events per participant per group (Analysis 7.1), and one additional study of 791 participants and 10,944 event days presented results on pressure sores as events per day. The four studies reported an event rate of 43% for participants ventilated prone and 34.2% for those ventilated supine, with a risk ratio of 1.25 (95% CI 1.06 to 1.48; P value = 0.01). The addition of data from the PROSEVA trial (Girard 2014) with a doubling of participants for analysis moved the point estimate closer to unity and increased the precision of that analysis. The single study (Guerin 2004) reporting events per day (and therefore not part of the quantitative meta‐analysis) reported pressure sores on 3.6% of event days in prone groups and 3.0% in supine groups, with an odds ratio of 1.20 (95% CI 0.97 to 1.48; P value = 0.09). Both analyses favoured the supine position to avoid this adverse event.
7.1. Analysis.

Comparison 7: Adverse events, Outcome 1: Adverse events
Tracheal tube displacement
Eight studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Taccone 2009; Voggenreiter 2005) of 2021 participants provided information on tracheal tube displacement or accidental extubation (Analysis 7.1). Participants ventilated prone experienced a 10.5% event rate compared with 9.2% among those ventilated supine. The risk ratio was 1.09 (95% CI 0.85 to 1.39).
Tracheal tube obstruction
Three studies (Guerin 2004; Guerin 2013; Taccone 2009) of 1599 participants reported the complication of tracheal obstruction. These three studies strongly favoured the supine position, although moderate heterogeneity was noted, with I2 statistic= 31% requiring use of a random‐effects model for analysis (Analysis 7.1). The overall incidence of tracheal obstruction for those ventilated prone was 15.9% compared with supine, which was 9.7%. The risk ratio was 1.78 (95% CI 1.22 to 2.60); P value = 0.003).
Pneumothorax
Four studies (Chan 2007; Fernandez 2008; Guerin 2013; Mancebo 2006) of 664 participants reported an event rate for pneumothoraces (Analysis 7.1), and one study (Guerin 2004) of 791 participants (and therefore not part of the quantitative meta‐analysis) reported pneumothoraces per participant day. The four studies reported an overall event rate of 6.6% for participants ventilated prone and 5.4% for those ventilated supine, with a risk ratio of 1.16 (95% CI 0.65 to 2.08). The largest single study reported risk for pneumothorax per participant day of 0.38% for prone ventilated participants and 0.54% for supine ventilated participants, with an odds ratio of 0.71 (95% CI 0.40 to 1.24).
Arrhythmias
Three studies (Guerin 2013; Mancebo 2006; Voggenreiter 2005) of 642 participants reported on the prevalence of arrhythmias including bradyarrhythmias and cardiac arrest, noting a rate of 15.3% for those ventilated prone and 24.7% for those ventilated supine (Analysis 7.1). This analysis was dominated by one study (Guerin 2013) which had a risk ratio of 0.64 (95% CI 0.47 to 0.87). One other study (Guerin 2004) of 791 participants and 10,942 event days (and therefore not part of the quantitative meta‐analysis) reported bradycardic episodes per participant day as well as cardiac arrests per participant day. For bradycardic episodes per participant day, the rate was 1.41% for prone ventilation and 1.39% for supine ventilation, with an odds ratio of 1.01 (95% CI 0.74 to 1.40). For cardiac arrest, the prevalence for prone ventilation was 1.51% per participant day and for supine 1.70% per participant day, with an odds ratio of 0.89 (95% CI 0.66 to 1.20).
Composite outcome of hypotension, arrhythmias and increased vasopressor use per participant day
This composite outcome was reported by one study (and therefore not part of the quantitative meta‐analysis) of 342 participants and 5524 participant days (Taccone 2009). The reported rate of such cardiovascular compromise was 18% for participants ventilated prone and 12.4% for those ventilated supine, with a risk ratio of 1.45 (1.28 to 1.65; P value < 0.00001) favouring the supine position.
Quality of life
Only one study (Chiumello 2012) (and therefore not part of the quantitative meta‐analysis) reported on quality of life for a small subset of 26 participants from five centres (187 participants) followed up from the Taccone 2009 study of 342 participants. The main quality of life metrics employed were the Short Form‐36 (SF‐36) questionnaire, which reported on eight items, and the Saint George's Respiratory Questionnaire (SGRQ), which reported on four items. For all domains in the SF‐36 questionnaire, results were similar for both groups. For all four SGRQ items, the prone group performed better, but none of these results were statistically significant.
With regards to pulmonary function assessment with standard pulmonary function tests and quantitative evaluation of CT scans, 15 items were evaluated. Two results were statistically significant, and one was of borderline significance. These were PaO2 (P value = 0.03) ‐ reported as the PaO2/FIO2 quotient in the oxygenation section above; and over‐aerated lung tissue on CT scan analysis of 12.5% of total lung weight for participants treated prone versus 5.3% for those treated supine (P value = 0.008). Mean results for well‐aerated lung tissue between groups showed 64.0% for participants treated prone versus 70.2% for those treated supine (P value = 0.052).
Economic outcomes
None of the identified primary papers provided data on economic outcomes. One economic analysis (Baston 2019) is available modelled on results from most recent trial of Guerin (Guerin 2013) and an observational study (Bellani 2016). They conclude based on short‐term mortality outcomes and after extensive modelling, interventions that increase utilization of prone positioning would be cost‐effective from both societal and hospital perspectives under many plausible cost and benefit assumptions. However this modelling is based on the results of a single unblinded trial that has been described as having, "a treatment effect virtually unprecedented in modern medicine." (Soo Hoo 2013). Baston et al did not take into account any requirement for increased staffing required to accomplish patient‐turning in sparsely staffed ICUs.
Planned subgroup analyses (SGA)
Analyses combining short‐ and longer‐term mortality allow for one model (fixed‐effect or random‐effects) only per analysis. In two cases (Analysis 1.6; Analysis 1.10), short‐ and longer‐term analyses required different models. Results presented in the text show actual result based on the correct model. All other analyses presented are correct.
1.10. Analysis.

Comparison 1: Mortality, Outcome 10: SGA of high tidal volume (> 8 mL/kg IBW)
Duration of daily ventilation in the prone position (< 16 hours/d vs ≥ 16 hours/d)
Mean daily application of prone ventilation for the nine included studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009; Voggenreiter 2005) was 16.3 hours (range 7 to 24 hours/d) given over a mean of 6.2 days (range 1 to 11.9 days). Mean total hours of prone ventilation for participants in each study ranged from 24 hours (Leal 1997) to 238 hours (Fernandez 2008), with a mean of 100 hours across included studies.
Two studies (Gattinoni 2001; Guerin 2004) of 1095 participants reported short‐term mortality for participants ventilated less than 16 hours/d in the prone position (Analysis 1.2). Mortality was 37.3% for participants ventilated prone and 36.2% for those ventilated supine, yielding a risk ratio for mortality of 1.04 (95% CI 0.89 to 1.21). Three studies (Gattinoni 2001; Guerin 2004; Voggenreiter 2005) of 1135 participants reported on longer‐term mortality for participants ventilated in the prone position for less than 16 hours per day. Mortality was 47.1% for participants ventilated prone and 45.9% for those ventilated supine, yielding a risk ratio for mortality of 1.03 (95% CI 0.92 to 1.17).
1.2. Analysis.

Comparison 1: Mortality, Outcome 2: Sub‐group analysis (SGA) of mortality < 16 hours/d prone
Six studies (Chan 2007; Fernandez 2008; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009) of 1022 participants reported on short‐term mortality for participants ventilated 16 or more hours per day, with moderate heterogeneity identified (I2 statistic = 41%) (Analysis 1.3). Short‐term mortality was 29.2% for participants ventilated prone and 40.5% for those ventilated supine, yielding a risk ratio of 0.73 (95% CI 0.58 to 0.93; P value = 0.01). Five studies (Chan 2007; Fernandez 2008; Guerin 2013; Mancebo 2006; Taccone 2009) of 1005 participants also reported longer‐term mortality of participants ventilated prone for 16 or more hours per day. Longer‐term mortality was 36.1% for those ventilated prone and 48.6% for those ventilated supine, with a risk ratio of 0.77 (95% CI 0.61 to 0.99; P value = 0.04).
The statistical test for subgroup differences for longer‐term outcomes regarding daily duration of prone ventilation was significant (P value = 0.03; I2 statistic = 78.0%), which provides stronger evidence for the benefit of longer duration prone position ventilation (Analysis 2.1). The quality of the evidence as rated by GRADE was moderate (Table 1), with downgrading based on the potential for risk of bias.
2.1. Analysis.

Comparison 2: Intervention comparisons and interactions, Outcome 1: Longer duration vs shorter duration of proning: longer‐term mortality
Duration of supine ventilation before randomization (< 48 hours vs ≥ 48 hours)
Five studies (Fernandez 2008; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009) of 1000 participants enrolled most participants within 48 hours of initiation of mechanical ventilation, allowing exploration of effects on short‐term mortality (Analysis 1.4). Moderate heterogeneity was noted (I2 statistic = 51%). Short‐term mortality among prone participants was 29.0% compared with 40.6% among those in the supine group, with a risk ratio of 0.72 (95% CI 0.56 to 0.93; P value = 0.01). Five studies (Fernandez 2008; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005) of 1024 participants enrolled most of their participants up to 48 hours after initiation of mechanical ventilation with regards to longer‐term mortality (Analysis 1.4). Moderate heterogeneity (I2 statistic = 50%) was noted. Longer‐term mortality among prone participants was 34.3% compared with 46.9% in participants assigned to supine ventilation, with a risk ratio of 0.75 (95% CI 0.59 to 0.94; P value = 0.01).
Three studies (Chan 2007; Gattinoni 2001; Guerin 2004) of 1117 participants enrolled most participants more than 48 hours after initiation of mechanical ventilation (Analysis 1.5). Short‐term mortality for prone participants was 37.7% compared with 36.2% in the supine group, with a risk ratio of 1.04 (95% CI 0.89 to 1.21). For longer‐term mortality three studies (Chan 2007; Gattinoni 2001; Guerin 2004) of 1116 participants enrolled participants after 48 hours or did not state enrolment time. For those ventilated prone, reported mortality was 48.6% compared with 47.2% for those ventilated supine. The overall risk ratio was 1.04 (95% CI 0.92 to 1.17).
1.5. Analysis.

Comparison 1: Mortality, Outcome 5: SGA of mortality: enrolled > 48 hours after entry criteria met/ventilation
The statistical test for subgroup differences for longer‐term outcomes regarding timing of enrolment for prone ventilation was significant (P value = 0.01; I2 statistic = 84.4%), providing stronger evidence for the benefit of longer duration of prone positioning (Analysis 2.2). The quality of evidence as rated by GRADE was moderate (Table 1), with downgrading based on the potential for risk of bias.
2.2. Analysis.

Comparison 2: Intervention comparisons and interactions, Outcome 2: Early enrolment vs later enrolment to intervention: longer‐term mortality
Outcome according to severity (PaO2/FIO2 ratio; severity of illness score, e.g. Simplified Acute Physiology Score II (SAPS II); oxygenation index)
With regards to short‐term outcomes, six studies (Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009) of 744 participants explored the effects of prone position in a subset of participants with severe hypoxaemia ‐ PaO2/FIO2 quotient < 150 mmHg (< 20.0 kPa) (Leal 1997); < 105 mmHg Guerin 2013); or < 100 mmHg (< 13.3 kPa) ‐ and in four others based on reanalysis of original data (Gattinoni 2010; Sud 2010). For short‐term mortality among participants with severe hypoxaemia (Analysis 1.6), adoption of prone ventilation was associated with 40.6% mortality in comparison with mortality of 50.1% for those ventilated supine, yielding a risk ratio of 0.80 (95% CI 0.68 to 0.93; P value = 0.003) when a fixed‐effect model was used with I2 statistic = 0. (The figure in the analysis presents results of the random‐effects model, as longer‐term outcomes required application of a random‐effects model.) Twenty‐eight‐day mortality data (as opposed to short‐term mortality) resulting from the combination of original studies and a review (Gattinoni 2010) yielded near identical results, with a risk ratio of 0.80. For longer‐term mortality, seven studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009) of 977 participants with severe hypoxaemia recorded mortality of 41.5% for participants ventilated prone and 54.7% for those ventilated supine, with a risk ratio of 0.77 (95% CI 0.65 to 0.92; P value = 0.003) when a random‐effects model was used (Analysis 1.6). Moderate heterogeneity was detected (I2 statistic = 39%). These results for short‐term and longer‐term mortality remained significant without inclusion of the most recent trial (Guerin 2013), which itself recorded highly significant results for short‐ and longer‐term mortality (P value < 0.001 for both time periods in favour of prone positioning). The quality of the evidence as rated by GRADE was moderate (Table 1), with downgrading based on the potential for risk of bias.
For participants with less severe hypoxaemia, no apparent benefit was observed (Analysis 1.7). Short‐term mortality from four trials (Gattinoni 2001; Guerin 2004; Mancebo 2006; Taccone 2009) of 1095 participants with milder hypoxaemia (PaO2/FIO2 ≥ 100 mmHg to 300 mmHg) failed to establish benefit of prone positioning, with a risk ratio of 1.03 (95% CI 0.87 to 1.21; I2 statistic = 0). Although data from one other study (Guerin 2013) were available, this study included only participants with PaO2/FIO2 ≥ 105 mmHg to 150 mmHg and did not reflect the full spectrum of milder disease, as was evident in the other studies. Therefore, this study was excluded from the analysis. Data were also available from six studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Mancebo 2006; Taccone 2009) of 1108 participants undertaken to explore longer‐term effects of the prone position applied in milder hypoxaemia (PaO2/FIO2 > 100 mmHg to 300 mmHg). The risk ratio for this group of participants was 1.06 (95% CI 0.93 to 1.21) and heterogeneity was negligible (I2 statistic = 1%). For long‐term outcomes with regards to severity of hypoxaemia, the statistical test for subgroup differences (Analysis 2.3) was significant (P value = 0.005; I2 statistic = 85.7%).
1.7. Analysis.

Comparison 1: Mortality, Outcome 7: SGA of less severe hypoxaemia at entry
2.3. Analysis.

Comparison 2: Intervention comparisons and interactions, Outcome 3: Severe vs less‐severe hypoxaemia: longer‐term mortality
Two studies (Gattinoni 2001; Mancebo 2006) provided sufficient data to allow exploration of the effects of physiological severity of illness on outcome, with data for one study derived from a journal comment (Sud 2008b). Heterogeneity between studies was considerable. For participants (327) with an SAPS II score of 49 or less, short‐term mortality was 25.8% among those ventilated prone in comparison with 28.5% in those ventilated supine, yielding a risk ratio of 0.85 (95% CI 0.45 to 1.60; I2 statistic = 69%) (Analysis 1.8). For participants (113) with greater severity of illness (SAPS II ≥ 50), short‐term mortality among those ventilated prone was 34.7% and 56.8% for those ventilated supine, with a risk ratio of 0.60 (95% CI 0.25 to 1.40; I2 statistic = 79%). Amalgamated data for two different time intervals (Gattinoni 2001 provided 10‐day mortality data and Mancebo 2006 provided ICU mortality data) were not available for analysis of longer‐term mortality.
1.8. Analysis.

Comparison 1: Mortality, Outcome 8: SGA of SAPS II ≤ 49/≥ 50: short‐term mortality
Data regarding oxygenation index, most commonly reported in paediatric studies, were not available for analysis. (Oxygenation index was calculated as mean airway pressure × FIO2 × 100/PaO2 in mmHg and was expressed as a unit‐less number.)
Tidal volume (6 to 8 mL/kg vs > 8.0 mL/kg of ideal body weight)
Three studies (Chan 2007; Guerin 2013; Taccone 2009) of 830 participants reported on the effects of ventilation with lower tidal volumes (mean of 6 to 8 mL/kg ideal body weight) on short‐term mortality (Analysis 1.9). Mortality for the prone subgroup was 25.5% compared with 36.7% among those ventilated supine. The risk ratio was 0.72 (95% CI 0.43 to 1.20; P value = 0.2). Substantial heterogeneity was noted (I2 statistic = 76%). Five studies (Chan 2007; Fernandez 2008; Guerin 2013; Taccone 2009; Voggenreiter 2005) of 910 participants reported on longer‐term mortality among participants ventilated with low tidal volumes (mean 6 to 8 mL/kg ideal body weight). Mortality for those ventilated prone was 32.5% and for those ventilated supine 45.1%, with a risk ratio of 0.73 (95% CI 0.53 to 0.96; P value = 0.02). Heterogeneity was moderate (I2 statistic = 43%).
Three studies (Gattinoni 2001; Guerin 2004; Mancebo 2006) of 1231 participants utilized high tidal volumes (mean > 8.0 mL/kg ideal body weight) and reported on short‐term mortality (Analysis 1.10). Mortality for participants ventilated prone was 38.1% and for participants ventilated supine was 38.5%, with a risk ratio of 0.99 (95% CI 0.86 to 1.14; I2 statistic = 38%; random‐effects model). Those studies (Gattinoni 2001; Guerin 2004; Mancebo 2006) with 1231 participants also reported on longer‐term mortality among participants ventilated with high tidal volumes. Mortality for those ventilated prone was 48.8% and for participants ventilated supine 48.5%, with a risk ratio of 1.01 (95% CI 0.9 to 1.13; I2 statistic = 19%; fixed‐effect model).
We categorized studies on the basis of mean tidal volumes (mL/kg) derived from ideal body weight (IBW) as provided by primary studies or imputed from actual body weight data. Two studies provided no measurements (Leal 1997; Voggenreiter 2005). The upper 95% confidence limit for tidal volumes was 7.3, 9.4, 9.8, 11.3, 14.6, 14.7 and 15.8 mL/kg IBW for the seven studies of Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006 and Taccone 2009. Notably, only one study (Guerin 2013) actually achieved the 6 to 8 mL/kg ideal body weight envelope in terms of the 95% CI approximating what is currently considered best clinical practice (Needham 2012).
A random‐effects model used to test for subgroup differences between low and high tidal volume ventilation strategies yielded significant findings (P value = 0.04; I2 statistic = 76.5%), strengthening evidence for the prone position in combination with lower tidal volumes (Analysis 2.4). The quality of evidence as rated by GRADE was moderate (Table 1), with downgrading based on the potential for risk of bias.
2.4. Analysis.

Comparison 2: Intervention comparisons and interactions, Outcome 4: Lower tidal volume (TV) ventilation vs higher TV ventilation: longer‐term mortality
Analysis of studies of ALI and ARDS separate from other causes of acute severe hypoxaemic respiratory failure
Seven studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009) were included in this subgroup analysis of 1326 participants ventilated for ARDS with regards to short‐term mortality (Analysis 1.11). Data from Guerin's first study were not included, as inclusion criteria for participants were broader than the other clinical trials (Guerin 2004). Mortality for those ventilated prone in these subgroups of ARDS participants was 34.0% compared with 42.6% for those ventilated supine. The risk ratio was 0.79 (95% CI 0.63 to 1.00; P value = 0.05). Heterogeneity was moderate (I2 statistic = 57%).
Eight studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005) were included in this subgroup analysis of 1758 participants ventilated for ARDS with regards to longer‐term mortality. Ninety‐day mortality for ARDS participants (PaO2/FIO2 quotient < 300 mmHg) from the first study of Guerin (Guerin 2004) became available through a recent meta‐analysis (Sud 2014). Very minor adjustments were made to original data from another trial (Gattinoni 2001) on the basis of information obtained from this same meta‐analysis. Mortality for those ventilated prone in these subgroups of ARDS participants was 41.3% compared with 48.3% for those ventilated supine. The risk ratio was 0.85 (95% CI 0.71 to 1.01; P value = 0.07), and heterogeneity was moderate (I2 statistic = 56%). The quality of the evidence as rated by GRADE was moderate (Table 1), with downgrading based on the potential for risk of bias.
Pulmonary and extrapulmonary causes of ALI or ARDS
Data were insufficient to allow any analysis.
Use of meta‐regression to explore heterogeneity between subgroups
Identified studies were insufficient to meet criteria for use of meta‐regression techniques (Deeks 2011).
Sensitivity analysis based on potential risk of bias or confounding
Removing studies on the basis of potential risk of bias or confounding had little effects on most results. Excluding the studies of Guerin (Guerin 2004) on the basis of the high percentage of cross‐over participants; of Voggenreiter (Voggenreiter 2005) because of the hugely disparate blood transfusion requirements between groups and differential use of muscle relaxants; and of Chan (Chan 2007) because all risk of bias assessments were rated as "unclear" or "high" (Figure 2), produced a small shift in risk ratio for short‐term mortality from 0.84 to 0.79 (95% CI 0.61 to 1.01), and from 0.87 to 0.82 (95% CI 0.65 to 1.04) for longer‐term mortality.
Discussion
Summary of main results
The main findings of this review are presented here.
Primary outcomes
Among all‐comers entered into identified randomized but unblinded clinical trials, a statistically insignificant signal of benefit was seen for prone ventilation, with risk ratios of 0.84 and 0.86 for short‐term and longer‐term mortality. The last published trial (Guerin 2013) approximately halved mortality in its cohort of more severely hypoxaemic participants compared with other included studies, and changed heterogeneity as measured by the I2 statistic from 0% to 60% or more for both primary outcomes. The risk ratio before publication and inclusion of this study was close to unity. This suggests that unselected participants with moderate to severe hypoxaemia requiring mechanical ventilation and ventilated in the prone position may be too diffuse a target population for this intervention. When the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system was applied, evidence for benefit in unselected populations was rated low (Table 1), as the overall rating of quality of evidence was downgraded as a result of risk of bias and inconsistency (Guyatt 2008; Guyatt 2011a; Guyatt 2011c). Longer‐term mortality would seem a more important patient‐centred outcome when compared with short‐term mortality for primary outcomes and for prespecified subgroup analyses of mortality (Hough 2012; Wang 2014; Williams 2008), and formal application of the GRADE approach was limited to primary outcomes and to selected subgroup outcomes for longer‐term mortality only.
One trial later reported a subset of 187 participants, for whom additional 12‐month mortality data were reported (Chiumello 2012). This subset from five centres based in or near Milan, Italy, comprised more than 50% of the population of the original or primary trial (Taccone 2009). For this subset, there was no evidence of benefit for prone ventilation in unselected participants with regards to 12‐month mortality, as the risk ratio was slightly greater than unity (favouring supine positioning), although findings were statistically insignificant. We note this trial (Taccone 2009) was the second most recent published trial and employed low tidal volume ventilation.
With regards to heterogeneity, primary outcomes fell into the moderate heterogeneity category (Higgins 2003), and formal analysis was undertaken with a random‐effects model, wherein the I2 statistic exceeded 25%. In addition, recommended approaches for exploring heterogeneity through pre‐specified subgroup analyses and for excluding studies in sensitivity analyses were undertaken. With only eight studies available for each of the two primary outcomes (short‐term mortality ‐ Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009; and longer‐term mortality ‐ Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005), we did not utilize meta‐regression techniques, in accordance with best practice (Deeks 2011).
Secondary outcomes
Pneumonia
Ventilator associated pneumonia (VAP) is an important complication, which definitely increases resource utilization and prolongs intensive care unit (ICU) and hospital length of stay with uncertain attributable mortality. Analysis of data from nearly 1500 participants after the addition of data from the PROSEVA trial (Ayzac 2016) showed little signal of benefit from prone positioning with regards to VAP. The incidence of VAP varied across studies, and to what extent this was driven by different patient populations (patient diversity) and criteria used to diagnose pneumonia (methodological diversity) is uncertain. The diagnosis of ventilator‐associated pneumonia is problematic, given the various criteria in clinical use (Klompas 2008; O'Brien 2011; Stevens 2014; Sud 2008) over the 15‐year time span of these clinical trials.
Duration of mechanical ventilation
Duration of mechanical ventilation was reported in three studies of more than 800 participants (Fernandez 2008; Guerin 2004; Voggenreiter 2005). A small signal suggested benefit from prone positioning. However, the point estimate amounted to less than a half‐day and cannot be considered a major patient‐centred benefit. Such data are complicated to interpret due to differential patient mortality between groups, which will affect time data such as duration of mechanical ventilation and length of stay.
Length of stay (LOS)
Length of stay (LOS) data are difficult to interpret in that they are often skewed (Weissman 1997), and LOS will be influenced in diverse and opposite ways by the effectiveness of treatment. LOS may be reported as median and interquartile range (IQR), and sometimes as mean and standard deviation. The former data pose difficulties for meta‐analysis, although they can be used if it is assumed that the median approximates the mean together with a statistical multiplication factor used to impute the standard deviation (Higgins 2011b). For skewed data, reporting of means and standard deviations may be misleading. It is recommended that both should be reported (Weissman 1997). Furthermore, effective treatment may reduce the LOS of participants already destined to survive but will lengthen the stay of those who would have died but have survived as a result of receiving the intervention. The overall effect will be determined by the balance of these two effects.
In this systematic review, mean and standard deviation were imputed for one study on the basis of non‐parametric data (Taccone 2009). Point estimates for ICU LOS were similar with and without this study and favoured supine, with the mean difference in LOS between 1.34 and 1.37 days and not statistically significant. Only one study (Fernandez 2008) of 40 participants reported on hospital LOS; this again favoured supine ventilation, with the point estimate showing a difference of 5.8 days. Reduction in ICU LOS was not apparent with the use of prone ventilation in the four relevant studies (Fernandez 2008; Guerin 2004; Mancebo 2006; Taccone 2009). Data for hospital LOS were too sparse to allow meaningful comment.
Improved oxygenation
Improved oxygenation in participants ventilated prone was apparent at seven to 10 days in the outcome studies included in this systematic review. The mean difference in the PaO2/FIO2 quotient of 24.6 mmHg or 3.3 kPa is clinically meaningful and was statistically highly significant (P value < 0.00001). In a very small subset of participants (26) from the original Taccone trial (384 participants) examined at 12 months, residual benefit was not apparent, and a small but statistically significant benefit favoured supine‐ventilated participants (Chiumello 2012). Thus oxygenation was substantially improved while participants were critically ill (Analysis 6.1), but robust evidence that this benefit is carried on to or beyond 12 months was not available.
We did not include the second study of Guerin (Guerin 2013) in the analyses, as formally mandated reduction in PEEP would negatively impact oxygenation and would confound such results.
Adverse effects
Studies show that pressure sores and tracheal tube obstruction were increased by mechanical ventilation provided in the prone position. Cardiac arrhythmias were reduced with use of the prone position. This was described in different ways by different studies and required separate analyses for the same complication, sometimes leading to inconsistent results. An important factor for interpretation is how vigorously these events were classified and sought within individual studies (Bent 2006; Ioannidis 2006).
With regards to pressure ulcers, four studies (Chan 2007; Gattinoni 2001; Girard 2014; Voggenreiter 2005) demonstrated a statistically significant effect of pressure sores per participant among those ventilated prone (P value = 0.02). Another single study, reporting in such a way that it could not be included in the meta‐analysis, demonstrated the same trend but with borderline statistical significance (P value = 0.09). All five studies favoured the supine position with regards to development of pressure sores. This finding would be consistent with the area of tissue in contact with the bed during supine and prone ventilation and is biologically plausible.
Tracheal tube displacement, which might be considered a natural consequence of moving patients from supine to prone position and back, showed differing results across studies, with moderate statistical heterogeneity. For example, the short‐term (24‐hour) study of Leal 1997 reported 50% tracheal tube displacement, whereas Chan 2007, which utilized at least 72 hours of prone positioning per participant, reported a zero rate of displacement. Overall the risk ratio was 1.09 and was statistically insignificant. However, a clear increase in risk of tracheal tube obstruction was evident. Such obstruction might result from increased inspissated secretions or from kinking of the tracheal tube in the face‐down participant. The risk ratio for this complication was 1.78 and was statistically significant (P value = 0.003).
Pneumothoraces were counted as events per participant in four studies (Chan 2007; Fernandez 2008; Guerin 2013; Mancebo 2006), and as events per participant day in one study (Guerin 2004). Three studies favoured the supine position (Chan 2007; Guerin 2013; Mancebo 2006), and two favoured the prone position (Fernandez 2008; Guerin 2004): Evidence favouring one position or another is weak.
With regards to arrhythmias or bradycardias results from different studies are not concordant. The most recent study (Guerin 2013) heavily influences overall findings for the incidence of arrhthymias The point estimate was 0.64 (P value = 0.005) in favour of the prone position. A statistically insignificant trend from a single study (Guerin 2004) also favoured the prone position with regards to cardiac arrests. However, Taccone 2009, when using the composite endpoint of "hypotension, arrhythmias or increased vasopressor requirements", strongly favoured the supine position (P value = 0.001) (Glantz 2005).
Small clinical trials may not reliably quantitate the incidence of uncommon adverse effects: Ocular complications are described among patients undergoing undergoing general anaesthesia, for therapeutic purposes, with prone positioning an associated risk factor, and the incidence of perioperative visual loss ranges between 0.028% and 1.3% (Uribe 2012) in this setting. Permanent blindness can result from ischaemic optic neuropathy or from central artery occlusion, and the zero numerator from this systematic review of 1107 prone participants still indicates a 95% confidence interval of 0 to 0.27% for the incidence of such events (Hanley 1983). Clinicians should remain vigilant for ocular complications, given the potentially important long‐term consequences; a recent abstract has highlighted one such case (Ayoubieh 2014). Brachial Plexus injury is also a potential serious long‐term injury that may result from prone positioning (Goettler 2002)
It is difficult to gauge the relevance of some of the reported findings in terms of the methodological rigour of their detection (Ioannidis 2006) and their clinical importance. Guidelines have been produced to assist prevention of such complications (Faculty of Intensive Care 2019).
Quality of life (QOL)
With regards to quality of life (QOL), only one study of 26 participants has addressed this issue ‐ Chiumello 2012. No clear benefit was seen in this very small study, which encompassed a subgroup of participants originating in the Taccone 2009 trial. Firm conclusions with such a small number of participants would seem inappropriate. More evidence is required.
Intensive care patients comprise a diverse population of individuals admitted from all different hospital specialities, and the aetiology of acute respiratory distress syndrome (ARDS) is also varied (Walkey 2012). The more inclusive umbrella of hypoxaemic respiratory failure is even more disparate. This participant heterogeneity reduces the power of studies and increases the sample size required to avoid type II statistical error (Lipsey 1990). The power of studies to detect differences in outcome is hindered by participants who have crossed over to the opposite treatment, when results are analysed using intention to treat analyses only. Cross‐overs occurred in several studies to a greater or lesser extent (34% of participants in one study, Guerin 2004). This may be important for this systematic review, for which participant numbers available for exploratory analyses are only modest to moderate. Given the importance of optimum treatment of severely hypoxaemic patients in ICUs, it is disappointing that five studies had to be terminated prematurely because of poor participant accrual (Chan 2007; Fernandez 2008; Gattinoni 2001; Mancebo 2006; Voggenreiter 2005).
The almost complete absence of long‐term survival data (12 months and beyond), physiological function data, QOL life data and economic data is notable and is a major limitation with regards to overall evaluation of the benefits (or otherwise) of prone positioning as an adjunct to conventional mechanical ventilation. Such data are likely to be more relevant to patients, their families and society, when compared with short‐term mortality outcomes alone (Herridge 2011; Hough 2012; Wang 2014).
Subgroup analyses
Given clinical diversity in terms of diagnoses and severity of illness (hypoxaemia), as well as methodological diversity, which has contributed to substantial statistical heterogeneity, pre‐specified exploration of subgroups was warranted, and data will be of interest to stakeholders.
Important issues and limitations associated with subgroup analysis include reduced statistical power, increasing type II statistical error; multiple statistical testing, increasing type I error and ecological fallacy in study‐level meta‐analyses. The multiple subgroup analyses reported below should be viewed in this light (Counsell 1994; Lagakos 2006; Oxman 1992; Reade 2008; Sun 2014) as well as noting the relatively few studies (8 or less) which were available for analysis in each subgroup.
Subgroup analyses are presented for short‐term (10 to 30 days) and longer‐term mortality (31 to 180 days, or hospital mortality). As mentioned earlier, longer‐term mortality would seem more important than short‐term mortality from the patient perspective, given reduced longer‐term survival and the ongoing burden of disease following acute lung injury and intensive care stay (Herridge 2011; Hough 2012; Iwashyna 2010; Wang 2014; Williams 2008). Five subgroups are included in the Table 1, and all subgroup analyses were downgraded on the basis of potential for important risk of bias. Downgrading of evidence as a result of imprecision was considered on the basis of Figure 5 and Table 1 of Guyatt and colleagues (Guyatt 2011b), both of which base achievement of optimal information size (OIS) on sample size or number of events. Four of the subgroup analyses of longer‐term mortality (Analysis 1.3; Analysis 1.4; Analysis 1.6; Analysis 1.11) clearly met suggested requirements for OIS, with the number of events (deaths) in the subgroup analyses ranging from 411 to 736. For the remaining analysis (Analysis 1.9) exploring the subset of participants ventilated with lower tidal volumes (Table 1), events were fewer, at 353. Nevertheless, we also judged the OIS to have been met by taking into consideration the control event rate and the actual relative risk reduction in this subgroup. Although all subgroup analyses demonstrated substantial heterogeneity, as indexed by I2 statistical methods, further downgrading on the basis of inconsistency for these analyses was considered but not implemented, also in keeping with the approach recommended by Guyatt and colleagues (Guyatt 2011c).
A relatively small number of studies are available for these analyses and they must all be interpreted with appropriate caution. For random effects models adjustments or alternatives to the DerSimmonian‐Laird approach used in REVMAN such as the Hartung‐Knapp‐Sidik‐Jonkman adjustment are recommended in the current Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). These approaches would likely widen confidence intervals in random‐effects model analyses (Borenstein 2019; Graham 2020).
Duration of prone ventilation
First, the duration or "dose" of prone ventilation might have an impact on outcomes, and we examined the effects of short periods and longer periods of prone ventilation per day. Short periods of ventilation in the prone position appeared to confer no benefit for participants with a risk ratio very close to unity. Periods of prone ventilation for 16 or more hours per day yielded point estimates of 0.73 and 0.75, which were statistically significant for both short‐term (P value = 0.01) and longer‐term mortality (P value = 0.04). We rated the quality of evidence as moderate using the GRADE structure (Table 1). We calculated dose of prone ventilation (with a two‐hour change in cutoff) as per our published protocol (Bloomfield 2009). Other methods not employed in this review might also be considered. Total dosage of prone positioning might be equally valid and might produce different results. For example, the most recent trial (Guerin 2013), despite prone positioning participants for 17 hours per day, applied it for only four days (68 hours), and in terms of total hours prone for participants ranks as the fourth shortest across the nine included trials (Characteristics of included studies), after Leal 1997, Gattinoni 2001 and Guerin 2004. Two of the studies excluded from our review but not from others (Characteristics of excluded studies) applied prone ventilation for a single episode of 12 hours (Demory 2007; Papazian 2005), and two other excluded studies applied prone positioning over six days, for a total of 24 hours only (Beuret 2002; Watanabe 2002).
Timing of intervention
We investigated whether early intervention offered an advantage as a strategy with the potential to ameliorate lung injury, as adoption later in the illness might limit benefit. Short‐term mortality and longer‐term mortality analyses of available data showed a risk ratio close to unity for three studies (Chan 2007; Gattinoni 2001; Guerin 2004), which enrolled more than 1000 participants after 48 hours. Participants enrolled earlier in five other studies (short‐term mortality ‐ Fernandez 2008; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009; longer‐term mortality ‐ Fernandez 2008; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005) demonstrated a large effect favouring prone ventilation, with risk ratios of 0.72 and 0.75, which were statistically significant (P values = 0.01 and 0.01) for short‐term and longer‐term mortality for a participant sample of 1000 or more. The quality of the evidence was rated as moderate when the GRADE structure was applied (Table 1).
Severity of injury
It has been postulated that prone positioning might benefit participants with the most severe lung insult, and data are available from original trials and from further published analyses of the original trials (Gattinoni 2010; Sud 2010). With all trials included that provided data for participants with the most severe hypoxaemia, a large and statistically significant apparent benefit can be seen, with point estimates of 0.80 and 0.74 for short‐ and longer‐term mortality (P values = 0.003 and 0.003). The quality of the evidence was rated as moderate according to the GRADE structure (Table 1). Removing data for two additional studies (Gattinoni 2001; Guerin 2004) provided by Sud et al, in which the precise timing of mortality assessment was not specified (Sud 2010), did not change the outcome of this subgroup analysis. Prone ventilation of participants with milder hypoxaemia appeared to confer no benefit, with risk ratios of 1.03 and 1.06 for short‐ and longer‐term mortality. However, the second study of Guerin et al (Guerin 2013) was influential in the overall analysis: Overall short‐term mortality in control groups of the other studies that reported participants with severe hypoxaemia was 58.5%, with mortality of 49.6% among participants treated prone. These rates differ substantially from those of Guerin et al (Guerin 2013), who reported percentage rates of 33.9 and 20.7 with absolute risk reductions of 8.9% for all other studies and 13.2% for Guerin et al. Of particular note is the much lower control mortality observed in the study of Guerin et al compared with other studies which might be explained by the more rigid application of available evidence‐based practices not fully utilized by earlier investigators (ARDS Network 2000). However, this does not fully explain the apparent differences in absolute risk reduction resulting from application of prone positioning.
With regards to overall severity of acute illness (SAPS II scoring system), heterogeneity between studies was considerable. Total numbers of participants studied and actual events (deaths) were low, generating unacceptable imprecision (Guyatt 2011b) which further reduces the relevance and credibility of this analysis. Additionally, two different short‐term outcomes were reported and amalgamated (Gattinoni 2001;.Mancebo 2006; Alsaghir 2008; Sud 2008b): Gattinoni 2001 provided 10‐day mortality data for analysis (70 deaths), whereas Mancebo 2006 provided ICU mortality data. ICU mortality data from Gattinoni 2001 would have provided another 80 deaths for analysis, and the comment in this paper that “These differences in [10 day] mortality rate did not persist after discharge from the intensive care unit (data not shown)” suggests limited relevance of these published data. Use of a random‐effects model to combine data demonstrated lower mortality for participants ventilated in the prone position for both lower and higher severity of illness, but with wide confidence intervals. Both studies were conducted during the high tidal volume ventilation era, making their results even less relevant in the low tidal volume ventilation era. For multiple reasons, as listed above, available data do not support severity of acute illness (SAPS II) as a basis for selection of participants for application of prone ventilation.
Ventilation strategy
As mentioned in the previous section, before publication of the first ARDS Network study (ARDS Network 2000), high tidal volume ventilation was in widespread use, but since low tidal volume ventilation has become the standard of care in clinical trials of adult patients, it is increasingly used in clinical practice (ARDS Network 2000; Needham 2012; Petrucci 2013). Studies conducted before adoption of this standard may be carried out differently and may be less relevant to current practice (Verbrugge 2007). For studies using higher tidal volume ventilation, both short‐term and longer‐term risk ratios for mortality were close to unity. All four studies (Gattinoni 2001; Guerin 2004; Leal 1997; Mancebo 2006) totaling 1200 participants commenced recruitment of participants before the ARDS Network study was published (ARDS Network 2000). Slightly smaller numbers of participants (830 to 911) from identified trials were available for analysis of low tidal volume strategies. All of these trials except the most recent (Guerin 2013) did not achieve tidal volumes of 6 mL/kg of ideal body weight, and low tidal volumes were considered to be 6 to 8 mL/kg of ideal body weight for pragmatic reasons. These showed a risk ratio in favour of prone ventilation for both short‐term (0.72) and longer‐term mortality (0.73), which was statistically significant for longer‐term mortality only (P value = 0.02). For longer‐term mortality, this analysis also utilized the Voggenreiter 2005 study, which, although initiated before publication of the ARDS Network study (ARDS Network 2000), states it employed a low tidal volume lung‐protective strategy. The quality of the evidence was rated as moderate according to the GRADE structure (Table 1). Analysis of more than 1200 participants ventilated with higher tidal volumes demonstrated modest or little benefit, with a risk ratio of 0.97 for longer‐term mortality. As use of higher tidal volumes is no longer recommended because of the propensity of this approach to cause ventilator‐induced lung injury (Slutsky 2013), some may consider these older trials that pre‐date lung‐protective ventilation to have very limited relevance to current clinical practice. Notably, only the most recent study (Guerin 2013) truly employed an evidence‐based low tidal volume lung‐protective ventilation strategy (ARDS Network 2000), which is now considered a standard of care by many (Needham 2012). Guerin (Guerin 2013) reported a mean tidal volume of 6.1 mL/kg ideal body weight (95% CI 4.9 to 7.3). For all other studies, the 95% CI breached the 8 mL/kg ideal body weight upper limit for "lung‐protective ventilation" (Characteristics of included studies), and a substantial proportion of participants in studies identified as having received "lung‐protective ventilation" will not have achieved this if reported data were normally distributed.
Aetiology of hypoxaemia
Many aetiologies of hypoxaemia have been identified in ICU populations. Some involve specific treatments and may be effectively and rapidly reversible with non‐ventilatory treatments such as diuretics or haemofiltration for fluid overload and pulmonary oedema. A major group of conditions collectively labelled as acute respiratory distress syndrome (ARDS) (Walkey 2012; Matthay 2019) are of particular interest to intensivists and warrant separate exploration. Acute lung injury, recently re‐termed 'mild ARDS' (ARDS definition workforce 2012), is a less severe form of ARDS, is less threatening to life and is less of an immediate concern for intensive care physicians compared with ARDS (Walkey 2012; ARDS definition workforce 2012).
Only one study of 791 participants included a large proportion of participants with conditions other than ARDS or ALI (Guerin 2004). Outcomes of participants from this clinical trial (Guerin 2004) became available in a recent systematic review (Sud 2014), providing more than 1700 participants for analysis of longer‐term outcomes, but these results changed little overall. The subgroup of participants with predominantly ARDS or ALI (mild ARDS) demonstrated risk ratios of 0.79 and 0.82 (P values = 0.05 and 0.07). This compares with analyses of all‐comers with hypoxaemia who had risk ratios of 0.84 and 0.86 for short‐ and longer‐term mortality. The quality of the evidence was rated as moderate in accordance with the GRADE structure (Table 1).
The first study conducted by Guerin et al also reported a high rate (32%) of cross‐over (Guerin 2004; Sud 2008), and removal of this trial on the basis of inclusion of patients without ARDS serves as a sensitivity analysis for excessive cross‐overs ‐ that is, removal from the above analysis also results in exclusion of the only study with a very large cross‐over population.
Overall completeness and applicability of evidence
Primary outcomes analyses of all available studies provides only weak evidence of benefit for application of prone ventilation to all‐comers with hypoxaemic failure who met trial entry criteria in the included studies. Evidence suggests that targeted application to certain subgroups would be appropriate.
The most recently published study (Guerin 2013) has had a large impact on overall results and interpretation of the impact of prone ventilation on primary analyses and subgroup analyses for that intervention. This study investigated the effectiveness of prone ventilation in what would be a population subset of most of the other cited studies (more severe hypoxaemia as a fundamental trial entry criterion). In the study of Guerin et al (Guerin 2013), mortality was approximately halved, which is extraordinary for any medical intervention. The accompanying editorial for this study noted, "The 28‐day mortality with prone ventilation was halved (16.0% vs. 32.8% with supine ventilation, P<0.001), a treatment effect virtually unprecedented in modern medicine" ‐ (Soo Hoo 2013). Consequently, this single study has had a large impact on overall effect size and on assessment of heterogeneity in this systematic review.
Additionally, many studies (Gattinoni 2001; Guerin 2004; Leal 1997; Mancebo 2006) were conducted in an era before lung‐protective ventilation became a standard of practice, thus reducing external validity; results of those studies are likely to be very limited in relation to current practice. Restriction to studies that employed a more lung‐protective strategy with means of 6 to 8 mL/kg tidal volume did result in a risk ratio point estimate of 0.71 for longer‐term mortality, which was statistically significant (P value = 0.05). The study of Voggenreiter et al (Voggenreiter 2005) was also conducted (1999‐2001) before low tidal volume became a standard of care in clinical trials but the text indicates they used a low tidal volume strategy even before the low‐tidal volume era.
The intervention of prone positioning would involve very low cost with adequate staffing (or equipment) to safely turn patients from the supine to the prone position and back again, as required. If the intervention was effective with regards to patient‐centred outcomes, this would almost certainly be translated into an intervention that was also cost‐effective, provided that complications for patients and long‐term injuries to staff from additional lifting of patients did not occur. Cost‐effectiveness data and modelling or other economic data based on more than one study are currently not available.
One subgroup analysis that requires specific mention is the analysis of mortality of patients with severe hypoxaemia. For short‐term mortality in six studies of 744 participants (Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009), the risk ratio was 0.80 (95% CI 0.68 to 0.93; P value = 0.003) in favour of prone positioning with a fixed‐effect model. For longer‐term mortality, seven studies of 977 participants (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009) also demonstrated a risk ratio in favour of prone positioning of 0.77 (95% CI 0.70 to 0.95; P value = 0.003) using a random‐effects model. Although the quality of the evidence was rated as moderate according to the GRADE structure (Table 1), findings appear consistent in that the most recent trial (Guerin 2013), which focuses on participants with more severe hypoxaemia, on its own has shown a remarkable survival advantage for participants. This subgroup meta‐analysis with the study included is highly statistically significant, and the subgroup meta‐analysis remains significant when this recent study is removed from the analysis. The subgroup exploration fully supports the conclusions of the most recent randomized controlled trial (Guerin 2013) and adds support to use of prone position ventilation in this specific patient population.
This systematic review found no important signal of benefit among participants recruited after 48 hours upon meeting entry criteria and ventilated prone for less than 16 hours per day, nor among those ventilated with higher tidal volumes or among those with milder hypoxaemia. An earlier systematic review (Sud 2010) also suggested no benefit for patients with moderate hypoxaemia only.
Quality of the evidence
With regards to the primary outcome of mortality, eight studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Leal 1997; Mancebo 2006; Taccone 2009) were available for short‐term mortality, and eight studies (Chan 2007; Fernandez 2008; Gattinoni 2001; Guerin 2004; Guerin 2013; Mancebo 2006; Taccone 2009; Voggenreiter 2005) were available for longer‐term mortality. Each analysis included data from more than 2100 participants, and internal validity of results must be considered good in that regard. Clinical diversity and methodological diversity (for some outcomes at least) may have contributed to statistical heterogeneity as assessed by the Higgins (I2 statistical) method.
The trials were not fully blinded, and some bias would seem inevitable. With regards to bias, Cummings et al state (Cummings 2013), "In a randomized trial blinding is as important as randomization. Randomization minimises the effects of confounding variables at the time of randomization but has no impact on differences that develop between groups during follow‐up. Blinding minimizes post‐randomization sources of bias such as co‐interventions and biased outcome ascertainment and adjudication". Jadad and Enkin consider, "... that the control of bias is the reason d' êtrefor clinical trials and accept control of bias is the most important factor in diminishing inevitable error." (Jadad 2007).
We rated all studies to be at risk of bias in at least one domain (performance bias): all primary studies had at least one risk, as it would be difficult to blind participants, carers and carer‐researchers from their treatment allocation (face‐up or face‐down). Although actual assessment of alive or dead could not be affected (Guyatt 2011a), end‐of‐life practices are universal but varied and poorly delineated clinically within and between ICUs. End of life practices were not standardized in the included trials in a fashion to minimise such bias by carers as well as trialists. The decision to continue with active management for individual participants or to actively withdraw life‐sustaining treatments, or to move to non‐escalation of treatment (Morgan 2014; Turnbull 2014) is perhaps the most fundamental decision / intervention made in ICU. Differential employment of these or other co‐interventions for trial participants, could be influenced by personal views. It is our assessment that some level of bias with regards to patient management is highly probable at various levels in all included studies. Centres were not chosen at random, but rather by interest in this topic: An early study (Gattinoni 2001) was terminated "largely [because of] an increasing unwillingness among caregivers to forgo the use of prone positioning". Another study (Guerin 2013) stated, "Patients were recruited from 26 ICUs in France and 1 in Spain, all of which have used prone positioning in daily practice for more than 5 years". Unblinded trials are associated with exaggerated estimates of effect (Forbes 2013; Karanicolas 2010; Psaty 2010), and, although outcomes such as death are usually considered as relatively immune to bias, we believe this is not the case in intensive care, where mortality is high over a short time frame and death often is not directly related to hypoxaemia (Stapleton 2005; Turnbull 2014) but to active withholding or withdrawal of care. Of note Ferrand found that "In France, there are no guidelines available on withholding and withdrawal of life‐sustaining treatments, and information on the frequency of such decisions is scarce", (Ferrand 2001). More recent studies also document the similar issues (Ay 2020; Gristina 2018; Mark 2015). It is surprising that important potential bias has been overlooked, disregarded or dismissed by some contributors. For example, in the Scandinavian clinical practice guideline on mechanical ventilation in adults with the acute respiratory distress syndrome with regards to downgrading GRADE ratings they state, "In keeping with the GRADE methodology, the quality of evidence for an intervention (i.e. our confidence in the effect estimates) was rated down for identified risks of bias (e.g. due to lack of blinding...)... Importantly, however, when the outcome in question was death at any stage, we did not downgrade evidence due to lack of blinded outcome assessment."(Claesson 2015). Aoyama and colleagues (Aoyama 2019) adopted a similar approach, "For performance and detection bias domains, we judged that, because mortality is objective it was unlikely to be influenced by lack of blinding....". Others (Griffiths 2019) disagree with that view and identify serious risk of bias also stating, "All trials demonstrated performance bias, because of the impossibility of blinding patients and carers with respect to the intervention. All trials also demonstrated detection bias, where outcome assessors were not blinded to intervention allocation".
Our GRADE assessment of quality of evidence for the primary outcomes of this systematic review was rated weak on the basis of bias (as discussed above) (Guyatt 2011a) and inconsistency (Guyatt 2011c). The Cochrane risk of bias assessment indicated that one trial had three different risks (Chan 2007) and two primary trials had two different risks (Guerin 2004; Voggenreiter 2005). These trials were subjected to a sensitivity analysis.
A small post hoc subgroup of 187 participants from one study (Chiumello 2012) allowed analysis of results at 12 months, which showed no signal of benefit for the prone position and so was inconsistent with the primary outcome measures. The quality of evidence for secondary outcomes and subgroup analyses varied in terms of numbers of participants available for analysis (113 to 1350) and quality of measurement, aspects of which have been mentioned in the secondary outcomes and subgroup analysis sections of the Discussion. Subgroups may not be independent, and this underscores the need to consider most as hypothesis‐generating only in this study‐level meta‐analysis e.g. Subgroups with the worst hypoxaemia will have the highest levels of PEEP, an analysis of one will be inextricably linked to the other and the analyses cannot be considered as independent.
Removal of the Voggenreiter study (Voggenreiter 2005) from our meta‐analysis as part of a sensitivity analysis, which did include a higher proportion of participants with acute lung injury (mild ARDS) and yielded a threefold higher rate of transfusion in the supine group as well as increased usage of neuromuscular blockers among participants treated prone, made little difference to this analysis. Also with regards to change in trial findings over time, the discrepancy between study initiation or completion and study publication was not constant. The time lag between study start (a surrogate of final trial protocol) and publication of results varied between five and eight years for published studies in this review. Time lag from study completion to publication varied from one to four years. Publication date may not be of greatest relevance for tagging studies with regards to the start of the lung‐protective ventilation era and may lead to incorrect data interpretation. The study of Voggenreiter 2005, although commenced in 1999 and completed in 2001, actually started using lung‐protective ventilation before the influential ARDS Network study was published (ARDS Network 2000).
Potential biases in the review process
We believe that the likelihood of bias in the review process of evaluated material is low. Processes used to identify relevant studies included in this review have been supplemented by several high‐quality systematic reviews, which also have involved nearly all of the key trialists. Therefore, it is likely that all relevant studies up to 1st May 2020 were identified. Adherence to most prespecified study inclusion and exclusion criteria in the protocol stage was maintained in the review stage. The cutoff in one subgroup analysis (long‐duration prone positioning) was changed by two hours from 18 hours per day to 16 hours per day post hoc in an effort to allocate and analyse the last published study (Guerin 2013) in the most biologically plausible manner consistent with the original protocol. Ecological fallacy or bias cannot be excluded in a study‐level systematic review of participant subgroups (Porta 2008; Reade 2008).
Agreements and disagreements with other studies or reviews
At least 21 other systematic reviews of prone ventilation have been published and been considered in this review (Abroug 2008; Abroug 2011; Alsaghir 2008; Aoyama 2019; Ball 1999; Beitler 2014; Chang 2014; Curley 1999; Du 2018; Gattinoni 2010; Hu 2014; Kopterides 2009; Lee 2014; Mora‐Arteaga 2015; Munshi 2017; Park 2015; Sud 2008; Sud 2010; Sud 2014Tiruvoipati 2008; Yue 2017). Eleven have been published as full papers since the last major and influential clinical trial of Guerin et al (Guerin 2013). In addition we identified recent abstracts which did not contain additional data (Sim 2014; Sud 2011a; Tabula 2015; Yankech 2017). We also identified one selective overview of systematic reviews which examined seven recent systematic reviews (Dalmedico 2017).
The primary studies the systematic reviews include are tabulated, Figure 5 . None of the 19 other meta‐analyses appear to have published protocols pre‐specifying their approach to data collection, management and analysis, which, since 2009, is a preferred option according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement (Moher 2009), with the possible exception of Sud 2014. Publication of protocols which to some extent protects from the consequences of data dredging, is an important safeguard and is an integral part of Cochrane Systematic Reviews.
5.
Primary RCTs incorporated in various published Systematic Reviews.
Green denotes primary study was incorporated; Pink denotes primary study was not utilised; Yellow denotes primary study was not available to reviewers. *Yue_17 also included non‐RCT.
Three systematic reviews (Gattinoni 2010; Sud 2010; Sud 2014) are extensively represented by authors of primary studies: In the systematic reviews of Sud et al (Sud 2010; Sud 2014), 13 of 17 listed review authors represent 10 of the 13 primary studies with mortality data. In the systematic review of Gattinoni (Gattinoni 2010), five of six review authors represent the four primary studies included in those analyses. In the recent systematic review of Munshi et al (Munshi 2017) Mancebo and Pesenti are co‐authors so several influential systematic reviews are authored by primary study investigators. Although benefits regarding data clarification and of cooperation are evident, independence and potential conflict of interest issues must also be considered in assessment of these systematic reviews.
The earliest systematic reviews we identified (Ball 1999; Curley 1999) did not contain meta‐analyses and pre‐dated the seminal outcome trial conducted by Gattinoni and colleagues (Gattinoni 2001).
Some subsequent systematic reviews (Abroug 2008; Alsaghir 2008; Curley 1999; Gattinoni 2010; Kopterides 2009; Sud 2008; Tiruvoipati 2008) did not include the two most recent trials (Guerin 2013; Taccone 2009) and despite some valuable insights are now less relevant in terms of both current clinical practice and volume of appropriate data for analysis. Inclusion criteria have differed between reviews, and those with larger numbers of studies have not included exactly the same studies for analyses, even analyses carried out by the same group (Figure 5). For example, Abroug et al included the study of Curley 2005 in one meta‐analysis (Abroug 2008) but not in their updated review (Abroug 2011). In this regard, two of the most recent reviews (Beitler 2014; Lee 2014) identified different numbers of studies for analysis: One (Beitler 2014) identified seven primary studies only and split one study into two to achieve eight data sets for their subgroup meta‐analysis; the other (Lee 2014) identified 11 studies for inclusion in their analyses. Two of the analyses of Sud et al included 10 of 13 primary studies with mortality data (Sud 2010; Sud 2014), but review authors excluded three studies from their original systematic review (Sud 2008). In their most recent review, the review authors state, "Reviewers were in total agreement about the included studies"; however, reviewers were authors of 10 of 11 of the included primary investigations (Sud 2014).
Our systematic review specified at the protocol stage which studies would be included and which excluded; resulting in fewer studies for analysis compared with some of the reviews mentioned above (Bloomfield 2009). In particular, our review consisted of adult participants receiving conventional mechanical ventilation as treatment for hypoxaemia. Thus trials that studied children, prevention and non‐conventional modes of ventilation were not considered for inclusion, and this largely accounts for the differences in numbers of participants studied in primary, secondary and subgroup analyses. Our review did include the small study of Leal (Leal 1997) which had only been included in one other systematic review (Sud 2008) as it met our pre‐specified inclusion criteria. Different studies have also provided different analyses. For example, in this systematic review, mortality was partitioned into short‐term mortality, longer‐term mortality and post hoc to 12‐month mortality. Other reviews have aggregated the latest available data for mortality and have not split data into short‐term and longer‐term categories (Sud 2010). This has resulted in the advantages of increasing numbers of studies and participants available for analysis but obscures where any advantages for participants lie: longer‐term mortality may be of greater relevance to participants (and patient‐centred) than short‐term‐mortality if "short‐term" still equates to being on a ventilator, or still requiring ICU care with the associated discomfort, invasive procedures and clinical burden that it entails. Nevertheless, the primary outcomes of this systematic review are in agreement with most other systematic reviews on this topic ‐ analysis of all studies fails to demonstrate unequivocal benefit for all participants with acute respiratory failure in terms of short‐ or longer‐term mortality. It also is consistent with all reviews in finding that oxygenation is improved and pressure ulcers/sores are increased (Sud 2010). This systematic review (Sud 2010) used data provided by the original investigators in many of the original trials and included many as co‐review authors: their report provided data (from last follow‐up) on participants with severe hypoxaemia and moderate (or less severe) hypoxaemia. These data indicate that participants with moderate hypoxaemia only, did not appear to benefit from prone ventilation, with a risk ratio 1.07 (95% CI 0.93 to 1.22; P value = 0.36) in favour of supine ventilation, which is consistent with the risk ratio of 1.06 obtained in our analysis.
One published systematic review (Abroug 2011) reached some conclusions that differed from the findings of our review. They concluded that no increase occurs in airway complications related to the prone position. However, we found an increase in airway obstruction, as did Sud et al (Sud 2010), and clinicians should be aware of this potentially serious problem. In this regard, we found a risk ratio of 1.78 in favour of the supine position (P value = 0.003) when using a random‐effects model for analysis. Analysing data from four studies only, Sud et al observed a statistically significant mortality benefit in a subgroup of studies enrolling ARDS participants only. They excluded the study of Gattinoni (Gattinoni 2001), although nearly 95% of participants in that study met ARDS criteria. Only with publication of the most recent study (Guerin 2013) could we identify any strong signal of support, and this study included only participants with more severe ARDS. Even with inclusion of this study, statistical significance was not achieved for longer‐term mortality in our analysis.
Early systematic reviews (Abroug 2008; Alsaghir 2008; Kopterides 2009; Sud 2008; Tiruvoipati 2008) generally considered the totality of evidence for prone positioning, but more recent aggregate‐level systematic reviews (Abroug 2011; Beitler 2014; Hu 2014; Munshi 2017; Park 2015; Sud 2010; Sud 2014) have focused on particular subgroups of patients and therefore are potentially subject to the problem of ecological fallacy as well as other biases eg data‐dredging bias (Jadad 2007) and limitations of subgroup analysis which means they should be considered as "entirely observational in their nature" (Borenstein 2019; Deeks 2019) and preclude causal inference.
We have evaluated three recent systematic reviews (Beitler 2014; Lee 2014; Sud 2014), which include the most recent clinical trial of Guerin and colleagues (Guerin 2013).
One review (Lee 2014) focusing on ARDS included studies that we specifically excluded (Beuret 2002; Demory 2007; Papazian 2005) for reasons listed in the Characteristics of excluded studies section. Inclusion of these studies reduced statistical heterogeneity but increased clinical diversity in the meta‐analysis (Bloomfield 2014). The former effect resulted in a statistically significant result for their primary analysis but we remain sceptical that inclusion of these three primary studies is justified on scientific grounds (Bloomfield 2014). Our published protocol specifically excluded such studies a priori. The immense influence of the PROSEVA study (Guerin 2013) was not highlighted. Review authors also rated the risk of bias from lack of blinding of participants and personnel as low, in contrast to our rating of such bias as discussed earlier. Further, they excluded Gattinoni et al (Gattinoni 2001) from their ARDS analysis, although nearly 95% of participants in that study met ARDS criteria (Bloomfield 2014). This meta‐analysis has drawn criticism from other workers similar to the criticisms described above (Iftikhar 2015).
The second systematic review (Beitler 2014), which focused on ARDS in the low tidal volume era, did not include the small study of Chan (Chan 2007), listing it as an observational study without a clearly defined control group, nor that of Leal (Leal 1997). This review explored reasons for heterogeneity but did not address the consistent and most important determinant of heterogeneity in this regard ‐ results from the PROSEVA study of Guerin et al (Guerin 2013). Review authors split the data from one trial (Taccone 2009) into two groups for their analysis, which we consider methodologically problematic. In this last regard, application of mechanical ventilation to more severely ill participants will almost inevitably lead to reduced mechanical ventilation tidal volumes (which was their justification for splitting one trial into two), as clinicians strive to reduce mean and peak ventilatory pressures, even though the two subgroups form one study sharing a single protocol over one study epoch. Their exploration of heterogeneity is puzzling in that almost unprecedented effect size of the PROSEVA trial (Guerin 2013) is not discussed at all (Soo Hoo 2013; Tekwani 2014; Tonelli 2014).
Sud and colleagues (Sud 2014) have updated their original meta‐analysis and have adopted a restrictive approach with regards to study selection for their primary analysis focused on ARDS. This review identified 11 studies, of which only six were used for the primary analysis. Thirteen of the 17 authors of this review contributed to 10 of the original studies, and they selected 11 for inclusion in their study‐level meta‐analysis. The primary analysis was restricted to a subset of studies describing participants with a diagnosis of ARDS and was further restricted to those receiving low tidal volume ventilation. Review authors included studies specifically excluded in this review (Beuret 2002; Curley 2005; Watanabe 2002) for reasons already described (Characteristics of excluded studies). Only one was used in the primary analysis (Curley 2005), which studied a paediatric population with a median age of two years. Review authors rated the quality of findings of their systematic review as high according to the GRADE structure in comparison with our and others (Griffiths 2019) assessment of low quality for our primary analyses and moderate quality for our main subgroup analyses (Table 1). A finding of low heterogeneity was emphasized, but no emphasis was placed on the substantial increase since the previous review (Sud 2010), most obviously resulting from inclusion of the most recent trial (Guerin 2013). The classification of heterogeneity is highly subjective, and a variety of ranges are suggested (Deeks 2011; Hatala 2005; Higgins 2003) for low, moderate and high heterogeneity using the I2 approach. We used a more conservative approach and would have rated most of these analyses as demonstrating moderate heterogeneity. Likewise, the GRADE approach to assessment of the quality of evidence is also subjective. We considered the risk of bias to be high, as others have (Griffiths 2019; Park 2015). The inconsistency and increased heterogeneity of results before and after inclusion of the most recent trial (Guerin 2013) could have led to further downgrading of the quality of evidence for this subgroup analysis of participants with an ARDS diagnosis. In this regard, analyses performed before publication of this trial (Guerin 2013) and those that did not include it (nine of 11 analyses) yielded an I2 statistic of 0% for short‐term mortality. For longer‐term mortality, nine of 11 studies also had an I2 statistic = 0, and 10 of 11 had an I2 statistic less than 25%. In contrast, with inclusion of the trial for short‐term mortality, only one of six analyses revealed an I2 statistic = 0 or less than 25%, and for longer‐term mortality, zero analyses of six had an I2 statistic = 0% or less than 25% (i.e. 19 of 22 analyses had zero or low heterogeneity without inclusion of data from Guerin 2013, in contrast to one of 12 analyses with inclusion of these data). With regards to statistical significance, despite increased heterogeneity and increased use of the random‐effects model, the pattern was opposite: A probability value of 0.05 or less was observed in two of the 22 analyses that did not include this influential study but in eight of 12 analyses that included it. The substantial impact of this trial is also apparent upon visual inspection of forest plots for mortality.
Our subgroup analysis results agree in part with the main findings of Sud (Sud 2014), in that longer‐term mortality appears reduced among participants receiving lower tidal volume ventilation (RR 0.73, 95% CI 0.55 to 0.96) and in severely hypoxaemic participants (RR 0.77, 95% CI 0.65 to 0.92), but results for participants with ARDS are only borderline in the analysis (RR 0.85, 95% CI 0.71 to 1.01). All three are only subgroup analyses of the greater body of evidence and in our view show moderate quality when assessed by the GRADE structure.
For this amendment, additional systematic reviews were identified.
Chang (Chang 2014) and colleagues presented a systematic review which incorporated nine randomized or pseudo‐randomized controlled trials and 10 non‐randomized ("comparable cohort / case‐control") studies. It did not identify the recent clinical trials of Taccone et al nor that of Guerin et al nor some older ones and so was not considered further.
Hu and co‐workers also published a systematic review of prone positioning (Hu 2014) and identified eight primary investigation of adult participants and one paediatric study (Figure 5) for their primary outcome of 28‐30 day mortality. An apparent benefit of prone positioning was demonstrated in a subgroup analysis. A proposed benefit of higher positive end‐expiratory pressure (PEEP) in subgroup analyses is doubtful in our view because of primary study misclassification and because of the association of higher PEEP levels with other factors explored in other subgroup analyses. The PROSEVA trial supplement indicates mean PEEP levels below 10cm water (low PEEP category) but appears in the Hu analysis as in the 10‐13cm (high PEEP category). The substantial impact of the PROSEVA trial, exploration of bias and limitations of subgroup analysis are inadequately explored, in our view.
Mora‐Arteaga and colleagues published their systematic review (Mora‐Arteaga 2015) with the primary objective of determining whether ventilation in the prone position reduces mortality in patients with ARDS compared with traditional ventilation in the supine position. With regards to bias assessment they stated, "it was not possible to blind the patients or the treating medical team‐‐‐though we consider that this had no effect upon the results". No basis for this judgement is provided. In the Cochrane Risk of Bias tool all seven primary studies were assessed as having low risk of performance bias (blinding of participants and personnel). They explored multiple sub‐group analyses with stratification and presented results broadly similar to other groups who have also explored the same data. The limitations of subgroup analyses are not highlighted.
Park et al (Park 2015) also published a systematic review with the aim "to evaluate the effects of prone positioning on mortality rates, particularly with respect to the duration and concurrent use of protective lung strategies". The primary outcome measure was the overall mortality at the longest available follow‐up. Notably, with regards to bias they stated, "Because the prone position with ventilator was always shown and patient progress was explained to the family and patients in the intensive care unit, blinding of participants or outcome measure was not possible. Therefore, there were high selection and detection biases in all included studies ". (Our emphasis). This seems reasonable and we are in agreement but their view contrasts the assessment of nearly all other systematic reviews on this topic. In particular, their Cochrane risk of bias assessment figure is substantially different to that of Mora‐Arteaga et al (Mora‐Arteaga 2015). As with several other systematic reviews on prone positioning they categorised statistical heterogeneity to be low for I2=25‐49%, moderate for I2= 50‐ 74%, and high for I2≥75% and all their analyses are presented as fixed effect models. Notably, the authors requested raw data for all included primary studies, to allow for analysis of subgroups of patients, "however most authors did not respond and one author refused our request". This is unfortunate scientifically, in our opinion, as many primary study authors have previously provided such data for other systematic reviews. Finally although Park et al describe limitations of available evidence they do not specifically discuss the limitations of analysis of multiple subgroup effects.
Munshi et al aimed to determine the effect on mortality (primary outcome) in adults with ARDS in the prone position versus ventilation exclusively in the supine position (Munshi 2017) to inform European and North‐American Societies of Intensive Care formulating mechanical ventilation guidelines (Fan 2017). They considered a study’s overall risk of bias to be high if any domain was judged to be at high risk of bias, with the exception of caregiver blinding, which effectively increased their quality of evidence assessments by GRADE which they rated as moderate to high for their subgroup analyses. In contrast using the same GRADE system we rated the quality of evidence as moderate (due to bias) or low (due to bias and inconsistency) Table 1. A recent guideline (Griffiths 2019) generally accords with our GRADE assessments. Many of the primary subgroup analyses of Munshi et al are in broad agreement with our own analyses presented in this Cochrane systematic review. For one subgroup analysis exploring moderate to severe ARDS they excluded the Gattinoni study (Gattinoni 2001) even though nearly 95% of participants in that trial met the the criteria for moderate to severe ARDS. In a secondary publication from that trial (Gattinoni 2010) data for severe ARDS was made available to researchers exploring the impact of disease severity. We do not understand why such data was excluded from analysis. In our own analyses of short and longer term mortality using their selected studies, exclusion and inclusion of Gattinoni's RCT (Gattinoni 2001) would change the conclusions that might be made from this particular subgroup analysis and so their conclusions cannot be considered robust. This systematic review (Munshi 2017) also only managed to identify and reference two other systematic reviews (Beitler 2014; Lee 2014) published after the PROSEVA trial, thus failing to identify contributions made by Hu, Sud, Park and Mora‐Arteaga groups (Hu 2014; Sud 2014; Mora‐Arteaga 2015; Park 2015) as well as the earlier version of this Cochrane Systematic Review. Additional insights generated by this review to the understanding of prone positioning seem limited (Møller 2017; Ioannidis 2016) given the systematic reviews listed above. We also consider their assessment of carer‐researcher bias substantially underestimates its potential impact in an ICU setting.The limitations of subgroup analysis (Guyatt 2008a) and the cautions required making inferences from a multiplicity of subgroup analyses (Borenstein 2019; Deeks 2019) are inadequately highlighted in our view.
Yue and co‐workers (Yue 2017) published a cumulative meta‐analysis in Chinese of nine primary English language studies categorised as randomized controlled trials (RCTs). Results of this cumulative meta‐analysis are invalidated by inclusion of an influential study which clearly is not a RCT (Charron 2011).
Du and colleagues (Du 2018) have provided a systematic review of prone positioning predominantly from the Chinese Language literature encompassing all age groups with pneumonia with the primary outcome of interest, oxygenation. They interrogated PubMed, EMBASE, Cochrane Library, Wanfang, VIP, CNKI databases and identified 12 randomized clinical trials in total. They did not identify or include any of the trials used in this or other systematic reviews but their systematic review provided an additional six studies of adult participants (Cao 2014; Li G 2015; Li J 2015; Wang 2015; Yan 2015; Cheng 2016) for potential inclusion in this amended Cochrane systematic review. Ultimately none were suitable for inclusion (Excluded studies). The other trials identified by Du et al were of paediatric populations and therefore not relevant to this review.
Aoyama et al conducted a network meta‐analysis (Aoyama 2019) with three studies only chosen from randomized trials of prone positioning (Fernandez 2008; Guerin 2013; Taccone 2009). They concluded with regard to prone positioning, that "Specifically our study supports the use of prone positioning (i.e. significant reduction of mortality and high ranking).....". With regards to their approach to potential bias they stated, "that for performance and detection of bias domains, we judged that because mortality is objective, that it was unlikely to be influenced by lack of blinding as long as a strict protocol for both groups was provided". As intensive care support is routinely and subjectively withheld or withdrawn by care providers as part of end of life care we remain surprised by such statements and consider this judgement of lack of bias over‐optimistic. We also note that if we had restricted our Cochrane systematic review to these three studies only, clear and unequivocal evidence of benefit would not be apparent despite inclusion of the PROSEVA study (Guerin 2013) which has been described as having "a treatment effect virtually unprecedented in modern medicine" (Soo Hoo 2013). Our post hoc analysis (generated by the study of Aoyama et al) using these three trials only found the mean effect size confidence interval for longer‐term mortality wide 0.73 (95%CI 0.51‐1.03) breaching unity, not statistically significant and so not in concordance with the results of this network meta‐analysis.
Generally in the more recent systematic reviews discussion of bias is largely limited to "publication bias" and tests thereof in contrast to very little consideration of more serious uncontrolled performance bias with lack of blinding of carers (nurses, attending physicians, other therapists, trialists etc). There were wide differences between studies in Risk of Bias Tool assessments and wide discrepancies regarding judged quality of evidence using the GRADE tool. Subgroup analyses were prominent in recent systematic reviews but their limitations (Oxman 2012; Sun 2014) received relatively little attention. Newer techniques that are now recommended by Cochrane (Deeks 2019) for random effects meta‐analyses to provide better effect size estimates (eg HKSJ adjustment IntHout 2014) have not been applied in this interim amendment nor in the systematic reviews listed in this section.
It is worth noting that despite recent contributions incorporating the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology (Mustafa 2013) that findings and recommendations based on largely the same data are substantially discordant (Claesson 2015; Fan 2017; Griffiths 2019; Hashimoto 2017) despite apparently transparent defined processes (Packer 2016).
Authors' conclusions
Implications for practice.
Results of this systematic review do not provide strong support for use in all patients with hypoxaemic respiratory failure who are intubated and mechanically ventilated in intensive care. Although the risk ratio for short‐term mortality and longer‐term mortality with inclusion of the last published study provided a signal for benefit, evidence was weak for all participants with acute respiratory failure (Table 1). Indeed, post hoc analysis of a small subset of 187 participants examined at 12 months (Chiumello 2012) actually favoured supine ventilation for these participants but with wide confidence intervals. It must also be borne in mind that all studies of prone position for acute respiratory failure included in this systematic review are open‐label and not blinded. Thus the potential for important bias due to deviations from intended interventions and measurement of outcomes must also be considered in any critical appraisal (Cummings 2013; Jadad 2007).
Oxygenation improvement persisted after adoption of the prone position seven to 10 days after enrolment, but many studies have shown that improvement in oxygenation (e.g. with use of nitric oxide) is not a reliable surrogate for improved patient‐centred outcomes (Adhikari 2007; Diaz 2010). This again appears to the case for the primary analyses of this systematic review of prone ventilation.
Complications were increased for pressure sores and for airway and some cardiovascular events, and, although these did not translate into excessive mortality, clinicians need to be aware of these issues. A widely varying rate was reported for some of these complications across studies, reflecting perhaps that "low priority data" were provided by these studies (Ioannidis 2006), or that better implementation of preventative measures in some studies led to reduced complication rates.
Exploratory subgroup analyses, which are widely utilized in published studies and in this systematic review, must be treated with caution. Risks of type I and type II statistical errors are increased through reduced statistical power derived from decreased participant numbers and increased numbers of hypotheses tested and by risks of ecological fallacy associated with aggregate‐level meta‐analyses (Lagakos 2006; Oxman 1992; Reade 2008; Sun 2014). Thus subgroup analyses must be interpreted with all these weaknesses in mind. Analyses of subgroups from this systematic review revealed subgroups in which prone ventilation appeared to have no benefit, with a risk ratio of close to unity; subgroups with point estimates suggestive of benefit but with wide confidence intervals; and subgroups for which the point estimate was both suggestive of benefit and statistically significant.
In particular, point estimates close to unity for prone ventilation of short daily duration, later application of prone ventilation after onset of hypoxaemia, milder hypoxaemia and ventilation with > 8 mL/kg ideal body weight indicated that prone positioning in these circumstances would be unlikely to provide benefit.
In contrast, ventilation prone for 16 or more hours per day, early intervention with prone positioning after entry criteria were met, and presentation of severe hypoxaemia point estimates suggest that prone positioning might be beneficial in these circumstances. Point estimates for risk ratio ranged from 0.72 to 0.80 in favour of prone ventilation. In the absence of alternative evidence, it is recommended that prone position should be actively considered for such patients. With regards to severe hypoxaemia, meta‐analysis of studies before publication of the most recent contribution of Guerin et al (Guerin 2013) strongly suggests benefit; the study of Guerin et al itself demonstrates clinically important and statistically significant benefit; and the combination of that study with other studies reaffirms that benefit. Thus although this is one of several subgroup analyses, evidence strongly favours prone positioning of mechanically ventilated patients exhibiting severe hypoxaemia.
Implications for research.
Clinical practices should not and will not be universally adopted unless data are robust (Noble 2004), but with the addition of the most recent study (Guerin 2013) to systematic reviews, residual uncertainty as to whether prone positioning can improve important patient‐centred outcomes in patients with severe hypoxaemia has seemingly been reduced by this single moderate‐sized trial, as well as by this and other meta‐analyses suggestive of survival benefit. There are important caveats. The issue that all of the included clinical trials were open label, must be considered and weighed. Treatment allocation would be known to caregivers so important bias may be present in all these studies particularly when death occurs after a decision to withdraw or with‐hold life‐sustaining supportive care. Also with regard to the PROSEVA trial (Guerin 2013) which dominates findings in this systematic review, a large number of baseline differences favouring outcomes for prone positioning were present (Festic 2016; Tekwani 2014) and may have exaggerated the substantial reduction mortality risk from application of prone positioning in this study. These factors would need to be considered against substantial apparent benefits before any future judgement as to the need to conduct further clinical trials.
Deficiencies in current knowledge arise from:
reliance on aggregate‐level subgroup analyses to identify more specific populations that might better benefit from this intervention in the short term or over the longer term;
limited data from the lung‐protective ventilation era, as only four reported trials (n ~ 830) were initiated after 2001 (Chan 2007; Fernandez 2008; Taccone 2009; Guerin 2013). Although one trial (n = 40) used a lung‐protective strategy before this date (Voggenreiter 2005), investigators did not provide recorded data. These five studies account for approximately 40% of participants in this review;
uncertain effect of bias as all data are from open label studies;
limited generalizability worldwide, as more than 95% of all participants were enrolled by three European Research Groups;
very sparse data for participants beyond six months, with the available data showing no signal of benefit for prone ventilation in terms of mortality at 12 months (Chiumello 2012);
lack of sufficient functional, physiological, neuropsychological or quality of life data (Bernard 2017; Chiumello 2016; Lamas 2014). Only one study of 26 patients (Chiumello 2012) has provided such data;
overall lack of healthcare economics data (Sud 2011); and
inconsistent reporting of data amongst studies, making analyses and aggregation of data for meta‐analysis more difficult.
Current evidence may be enhanced by the use and publication of formal individual patient data (IPD) meta‐analyses (Reade 2010; Riley 2010; Sud 2011a). Gattinoni et al (Gattinoni 2010) combined data from four primary studies in a review in what they describe as an "individual patient meta‐analysis". However, their paper does not provide any methodological description of the procedures undertaken to combine data from the four included studies (Stewart 2015; Tierney 2019).
With the publication of the most recent trial and the benefits demonstrated (Guerin 2013), equipoise to allow future studies to be undertaken would seem problematic, unless long‐term outcomes (12 months or longer) indicate transient benefits only. Nevertheless, it must also be noted that many promising therapies based on randomized trials are not always confirmed in follow‐up trials (Goodwin 2012) and ideally replication of the PROSEVA trial would occur (KNAW 2018; The Academy of Medical Sciences 2015). Further insights on particular groups of patients and adequately described ARDS phenotypes (Marini 2020; Matthay 2019) will require formal individual patient data meta‐analyses (Stewart 2015) rather than additional study‐level meta‐analyses, as well as examination of outcomes beyond mortality data alone. Outcome data is very limited in scope. In this regard, it is hoped that investigators in some of the trials conducted to date might be able to provide stakeholders with information regarding long‐term outcomes and functional data (Wunsch 2010), as has already been provided for a limited number of patients by one group of investigators (Chiumello 2012; Taccone 2009). Funding bodies and critical care organisations should consider supporting long term outcome mortality analyses from previous trials conducted in the low tidal volume ventilation era and studies that will ascertain long term functional outcomes of survivors. Prospective observational data collection and analysis should have a role too (Frieden 2017) as it does in the pharmaceutical industry in the form of phase IV drug trials (Strom 2006). Future observational and epidemiological studies as well as interventional trials of ARDS even though not directly addressing prone positioning should report in detail on its use (Cheung 2020; Constantin 2019). This serves two purposes. Firstly such data can corroborate and perhaps calibrate the impact of prone position ventilation on mortality (Fletcher 2005) as suggested by selective subgroup analyses in this and other systematic reviews and by the results of the PROSEVA trial (Guerin 2013). Secondly, the internal and external validity of these new studies of ARDS would be strengthened by taking into account an intervention that may reduce mortality by more than 40% in some ARDS populations.
Searches of trial registries indicated that there are few ongoing studies addressing the use of prone versus supine positioning in patients receiving mechanical ventilation for acute respiratory failure (Characteristics of ongoing studies). The majority of ongoing trials investigating this intervention in the context of emergency and critical care are focused on determining the effectiveness of early use of this intervention in non‐mechanically ventilated (i.e., awake, non‐intubated) patients. In addition, some ongoing trials address questions regarding the specific prone positioning protocols used in mechanically ventilated patients. These trials include comparisons of early versus later initiation of prone positioning, longer versus shorter duration of prone positioning, and the use of prone positioning in patients receiving ECMO. These may be relevant questions to address in a future update of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 24 November 2020 | Amended | An updated search of databases was run in May 2020. No new trials were included in this rapid update. Review text was updated in the context of advances in knowledge since the original review and based on the availability of some secondary analyses from already included studies. There was no change to the conclusions. An updated search of trial registries was run in November 2020. Three ongoing studies were identified. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
The present interim amendment includes an updated search (May 2020, no new trials identified), and search of trial registries (November 2020, 3 ongoing studies identified). Review text was updated to reflect advances in knowledge since the original review and the availability of new data from secondary analyses; however, a complete update of this review is pending.
Acknowledgements
We would like to thank Dr Harald Herkner (Content Editor) and Dr Claude Guerin and Dr Spyros D. Mentzelopoulos (Peer Reviewers) for help and editorial advice provided during preparation of the protocol (Bloomfield 2009).
In addition, we would like to thank Dr Harald Herkner (Content Editor); Nathan Pace (Statistical Editor); Jean‐Louis Vincent, Spyros D Mentzelopoulos and Thordis Thomsen (Peer Reviewers); and Anne Lyddiatt (Consumer) for help and editorial advice provided during preparation of the original version of this systematic review (Bloomfield 2015).
We would like to thank Xuan Yu, Qiangqiang Guo for their assistance with study translation. We also thank Dr Zhou Quanhong MSc MD PhD for assessment and translation of some studies published in Chinese.
Finally we wish to acknowledge the invaluable assistance of Teo Aminah Wasteneys Quay and Janne Vendt for this update as well as the enormous amount encouragement and help of Jane Cracknell in preparation of the original version of this Cochrane Review. Insert full acknowledgements for this update.
Appendices
Appendix 1. Search strategy for updated review
The following strategies were used for this update.
Database: Ovid MEDLINE(R) ALL <1946 to May 01, 2020>
1 exp Respiratory Insufficiency/
2 (respiratory adj2 (insufficien* or failure* or depression)).mp.
3 Respiratory Distress Syndrome, Adult/
4 exp Severe Acute Respiratory Syndrome/
5 (ards or sars or respiratory distress syndrome* or acute respiratory syndrome*).mp.
6 ((acute or adult) adj2 respiratory distress).mp.
7 exp Lung Injury/
8 lung injur*.mp.
9 exp Pneumonia/
10 pneumon*.mp.
11 exp Pulmonary Embolism/
12 ((pulmonary or lung) adj2 embolism).mp.
13 Pulmonary Edema/
14 (Pulmonary adj2 (oedema* or edema*)).mp.
15 Shock, Cardiogenic/
16 left ventricular failure*.mp.
17 exp Heart Failure/
18 ((cardiac or heart) adj2 failure*).mp.
19 exp coronavirus/ or exp Coronavirus Infections/ or (coronavirus* or corona‐virus* or 2019‐nCoV or nCoV or COVID‐19 or Covid19 or SARS‐CoV* or SARSCov* or ncov* or Pandemi*2).mp.
20 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21 (prone* or proning or pronation).mp.
22 Prone Position/
23 positioning in.mp.
24 face down.mp.
25 ventral position*.mp.
26 21 or 22 or 23 or 24 or 25
27 20 and 26
28 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).dt,ez,yr,dp,ed.
29 27 and 28
30 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.)
31 29 and 30
Database: Embase <1974 to 2020 May 01>
1 exp respiratory failure/
2 (respiratory adj2 (insufficien* or failure* or depression)).mp.
3 adult respiratory distress syndrome/
4 severe acute respiratory syndrome/
5 (ards or sars or respiratory distress syndrome* or acute respiratory syndrome*).mp.
6 ((acute or adult) adj2 respiratory distress).mp.
7 exp lung injury/
8 lung injur*.mp.
9 exp pneumonia/
10 pneumon*.mp.
11 lung embolism/
12 ((pulmonary or lung) adj2 embolism).mp.
13 lung edema/
14 ((Pulmonary or lung) adj2 (oedema* or edema*)).mp.
15 cardiogenic shock/
16 left ventricular failure*.mp.
17 exp heart failure/
18 ((cardiac or heart) adj2 failure*).mp.
19 exp coronavirinae/
20 exp coronaviridae infection/
21 (coronavirus* or corona‐virus* or 2019‐nCoV or nCoV or COVID‐19 or Covid19 or SARS‐CoV* or SARSCov* or ncov* or Pandemi*2).mp.
22 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
23 (prone* or proning or pronation).mp.
24 prone position/
25 positioning in.mp.
26 face down.mp.
27 ventral position*.mp.
28 23 or 24 or 25 or 26 or 27
29 exp intensive care/
30 exp intensive care unit/
31 exp Respiration, Artificial/
32 critical illness/
33 (ICU or ICUs or ITU or CCU or ((intensive or critical) adj3 (care or unit*)) or (critical* adj3 ill*)).mp.
34 (artificial* adj3 respirat*).mp.
35 ventilat*.mp.
36 29 or 30 or 31 or 32 or 33 or 34 or 35
37 22 and 28 and 36
38 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).dc,dp,yr.
39 37 and 38
40 (randomized controlled trial/ or controlled clinical study/ or random$.ti,ab. or randomization/ or intermethod comparison/ or placebo.ti,ab. or (compare or compared or comparison).ti. or ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. or (open adj label).ti,ab. or ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. or double blind procedure/ or parallel group$1.ti,ab. or (crossover or cross over).ti,ab. or ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. or (assigned or allocated).ti,ab. or (controlled adj7 (study or design or trial)).ti,ab. or (volunteer or volunteers).ti,ab. or human experiment/ or trial.ti.) not (((random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)) or (cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)) or (((case adj control$) and random$) not randomi?ed controlled).ti,ab. or (Systematic review not (trial or study)).ti. or (nonrandom$ not random$).ti,ab. or Random field$.ti,ab. or (random cluster adj3 sampl$).ti,ab. or ((review.ab. and review.pt.) not trial.ti.) or (we searched.ab. and (review.ti. or review.pt.)) or update review.ab. or (databases adj4 searched).ab. or ((rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/) or (Animal experiment/ not (human experiment/ or human/)))
41 39 and 40
Central Cochrane Database of Systematic Reviews: Issue 5 of 12, May 2020
#1 MeSH descriptor: [Respiratory Insufficiency] explode all trees
#2 (respiratory near/2 (insufficien* or failure* or depression))
#3 MeSH descriptor: [Respiratory Distress Syndrome, Adult] explode all trees
#4 MeSH descriptor: [Severe Acute Respiratory Syndrome] explode all trees
#5 (ards or sars or (respiratory next distress next syndrome*) or (acute next respiratory next syndrome*))
#6 ((acute or adult) near/2 (respiratory next distress))
#7 MeSH descriptor: [Lung Injury] explode all trees
#8 lung next injur*
#9 MeSH descriptor: [Pneumonia] explode all trees
#10 pneumon*
#11 MeSH descriptor: [Pulmonary Embolism] explode all trees
#12 ((pulmonary or lung) near/2 embolism)
#13 MeSH descriptor: [Pulmonary Edema] explode all trees
#14 (Pulmonary near/2 (oedema* or edema*))
#15 MeSH descriptor: [Shock, Cardiogenic] explode all trees
#16 left next ventricular next failure*
#17 MeSH descriptor: [Heart Failure] explode all trees
#18 ((cardiac or heart) near/2 failure*)
#19 MeSH descriptor: [Coronavirus] explode all trees
#20 MeSH descriptor: [Coronavirus Infections] explode all trees
#21 (coronavirus* or corona‐virus* or corona next virus* or 2019 next nCoV or nCoV or COVID next 19 or Covid19 or SARS next CoV* or SARSCov* or ncov* or Pandemi next 2)
#22 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21
#23 MeSH descriptor: [Prone Position] explode all trees
#24 prone* or proning or pronation
#25 positioning next in
#26 face next down
#27 ventral next position*
#28 #23 or #24 or #25 or #26 or #27
#29 #22 and #28
#30 #29 with Cochrane Library publication date Between Jan 2014 and Dec 2020, in Trials
Cinahl
S1 (MH "Respiratory Failure+")
S2 TX (respiratory N2 (insufficien* or failure* or depression))
S3 (MH "Respiratory Distress Syndrome, Acute")
S4 (MH "Severe Acute Respiratory Syndrome")
S5 TX (ards or sars or (respiratory distress syndrome*) or (acute respiratory syndrome*))
S6 TX ((acute or adult) N2 respiratory distress)
S7 (MH "Lung Injury+")
S8 (MH "Acute Lung Injury+")
S9 TX (lung injur*)
S10 (MH "Pneumonia+")
S11 TX (pneumon*)
S12 (MH "Pulmonary Embolism")
S13 TX ((pulmonary or lung) N2 embolism)
S14 (MH "Pulmonary Edema+")
S15 TX (Pulmonary N2 (oedema* or edema*))
S16 (MH "Shock, Cardiogenic")
S17 TX (left ventricular failure*)
S18 (MH "Heart Failure+")
S19 TX ((cardiac or heart) N2 failure*)
S20 (MH "Coronavirus+") OR (MH "Coronavirus Infections+")
S21 (coronavirus* or corona‐virus* or corona virus or 2019‐nCoV or nCoV or COVID‐19 or Covid19 or SARS‐CoV* or SARSCov* or ncov* or Pandemi*2)
S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
S23 (MH "Prone Position")
S24 TX (prone* or proning or pronation)
S25 TX (positioning in)
S26 TX (face down)
S27 TX (ventral position*)
S28 S23 OR S24 OR S25 OR S26 OR S27
S29 S22 AND S28
S30 S22 AND S28 Published Date: 20140101‐20201231
S31 (MH (randomized controlled trials) OR MH (double‐blind studies) OR MH (single‐blind studies) OR MH (random assignment) OR MH (pretest‐posttest design) OR MH (cluster sample) OR TI
(randomised OR randomized) OR AB (random*) OR TI (trial) OR (MH (sample size) AND AB (assigned OR allocated OR control)) OR MH (placebos) OR PT (randomized controlled trial) OR
AB (control W5 group) OR MH (crossover design) OR MH (comparative studies) OR AB (cluster W3 RCT)) NOT ((MH (animals+) OR MH (animal studies) OR TI (animal model*)) NOT MH (human))
S32 S30 AND S31
LILACS
(ARDS or respiratory distress syndrome or Pneumonia or Pneumonitis or Respiratory failure or Respiratory insufficiency or Respiratory depression or Pulmonary edema or Pulmonary oedema or Pulmonary embolism or corona virus or coronavirus or covid19 or covid 19 or SARSCov or ncov) [Words] and (prone or proning or pronation) [Words] 2014‐2020
Cochrane Covid 19 register
Filtered by: prone* or proning or pronation:
Appendix 2. Original data extraction form
DATA EXTRACTION FORM – PRONE POSITION FOR ACUTE RESPIRATORY FAILURE IN ADULTS
(CARG 038)
| Reviewer name | |
| Study author & date | |
| Journal, volume, pages & MEDLINE ID | |
| Title | |
| Location of study | |
| Enrolment finished (month & year) | |
| Period of study |
Verification of study eligibility:
| Yes/No | Query or comments | |
| Is this a randomized controlled trial? | ||
| All patients are adults and required mechanical ventilation for acute respiratory failure? | ||
| Relevant clinical outcomes? |
Study population:
| Population description | |
| Inclusion criteria | |
| Exclusion criteria |
PARTICIPANTS:
| Number of eligible participants | Number enrolled in study | ||
| Number of males | Number of females |
STUDY POPULATION:
| Baseline characteristics | Intervention group | Control group | Overall |
| Number for which data are given | |||
| Sex | |||
| Age (range, mean, SD) | |||
| SAPS II score | |||
| Other severity score (specify) |
|||
| Number of organ dysfunctions | |||
| Organ dysfunction score (specify) | |||
| PaO2 | |||
| FIO2 | |||
| PaO2/FIO2 | |||
| PaCO2 | |||
| PEEP | |||
| PIP | |||
| Plat Press | |||
| Vt (mL/kg) Unspecified mode ‐ MV Volume controlled Pressure controlled |
|||
| ALI (%) | |||
| ARDS (%) | |||
| Pulmonary cause | |||
| Extrapulmonary cause | |||
| Pneumonia | |||
| Shock | |||
| Aspiration | |||
| Septic shock | |||
| Acute on chronic RF | |||
| Coma | |||
| Postoperative | |||
| Non‐pulmonary sepsis | |||
| Acute cardiogenic pulmonary oedema (%) | |||
| NIV before MV | |||
| Number of participants with vasopressors | |||
| Planned duration (dose) of prone ventilation (< 18 hours vs > 18 hours/d) | |||
| Duration (days) of interventions | |||
| Early randomization to prone vs undefined OR late | |||
| Severity of process (PaO2/FIO2, oxygenation index OR LIS) | |||
| CO‐INTERVENTIONS | |||
| Inhaled nitric oxide | |||
| Renal replacement therapy | |||
| Packed red cells/participant (units) | |||
| Pulmonary artery catheter |
Quality of concealment of random allocation:
| Allocation was not concealed (e.g. quasi‐randomization) D | |
| Allocation concealment was inadequate C | |
| Methods of concealment were unclear B | |
| Concealment was adequate (e.g. numbered, sealed opaque envelopes drawn NON‐consecutively) A |
|
| Inclusion and exclusion criteria were not clearly defined in the text | |
| Outcomes of participants who withdrew or were excluded after allocation were NEITHER detailed separately NOR included in an intention‐to‐treat analysis | |
| Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals | |
| Treatment and control groups were NOT adequately described at entry | |
| Treatment and control groups were adequately described at entry. A minimum of 4 admission details were described |
METHODS:
| Yes | No | Unclear | |
| Subject ‐ blinded (N/A – sedated) | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
| Physician ‐ blinded (N/A ‐ impossible to achieve, as body position cannot be concealed) | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
| Outcome assessor ‐ blinded |
Modified Jadad quality assessment tool (see Appendix for guidance*):
| Yes/No (Y = 1) | Points | |
| 1. Was the study described as randomized? | ||
| 2. Was the study described as blinded for assessments? | ||
| 3. Was there a description of withdrawals? | ||
| Additional point if study randomization appropriate* | ||
| Deducted point if study randomization inappropriate* |
Total points __________
*Give one additional point if: For question 1, the method used to generate the sequence was described, and it was appropriate (table of random numbers, computer‐generated, etc.)
Deduct one point if: For question 1, the method used to generate the sequence of randomization was described, and it was inappropriate (participants were allocated alternately or according to date of birth, hospital number, etc.)
RESULTS AND OUTCOMES:
| Intervention group | Control group | Overall | |
| 10‐Day mortality | |||
| ICU mortality | |||
| 28‐Day mortality | |||
| Hospital mortality | |||
| 90‐Day mortality | |||
| Duration of MV | |||
| Time to extubation | |||
| ICU length of stay | |||
| Hospital LOS | |||
| Days with ARDS | |||
| Days with ALI | |||
| Days with LIS > 2 | |||
| VAP rate | |||
| Percentage of participants with VAP/Prevalence of pneumonia | |||
| Days on vasopressors | |||
| FIO2 d4 | |||
| PaO2 d4 | |||
| PaO2/FIO2 d4 | |||
| PaCO2 d4 | |||
| Vt d4 | |||
| FIO2 d10 | |||
| PaO2 d10 | |||
| PaO2/FIO2 d10 | |||
| PaCO2 d10 | |||
| Vt d10 | |||
| Economic evaluation |
COMPLICATIONS:
| Intervention group | Control group | Overall | |
| New pressure sores | |||
| Arrhythmias | |||
| Pneumothorax | |||
| Unplanned extubation/“Displacement” of tracheal tube/Obstruction | |||
| Intracranial hypertension | |||
| Total number of complications documented for both groups |
SUB‐GROUP ANALYSES:
| Intervention group | Control group | Overall | |
| 10‐Day mortality/SAPS > 49 | |||
| ICU mortality SAPS > 49 | |||
| ICU mortality SAPS ≤ 49 | |||
| ICU or 10‐day mortality according to SAPS cutoffs | |||
| Vt ≥ 12 mL/kg | |||
| PaO2/FIO2 ≤ 88 |
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Short‐term mortality | 8 | 2117 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 1.1.2 Longer‐term mortality | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 1.2 Sub‐group analysis (SGA) of mortality < 16 hours/d prone | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Short‐term mortality | 2 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 1.2.2 Longer‐term mortality | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.17] |
| 1.3 SGA of mortality prone ≥ 16 hours/d | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Short‐term mortality | 6 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.93] |
| 1.3.2 Longer‐term mortality prone | 5 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 1.4 SGA of mortality: enrolled ≤ 48 hours after entry criteria met/ventilation | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Short‐term mortality | 5 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.93] |
| 1.4.2 Longer‐term mortality | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 1.5 SGA of mortality: enrolled > 48 hours after entry criteria met/ventilation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Short‐term mortality | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 1.5.2 Longer‐term mortality | 3 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
| 1.6 SGA of severe hypoxaemia at entry | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Short‐term mortality | 6 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 1.6.2 Longer‐term mortality | 7 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 1.7 SGA of less severe hypoxaemia at entry | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Short‐term mortality | 4 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.21] |
| 1.7.2 Longer‐term mortality | 6 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 1.8 SGA of SAPS II ≤ 49/≥ 50: short‐term mortality | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 SAPS II ≤ 49 | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.60] |
| 1.8.2 SAPS II ≥ 50 | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.40] |
| 1.9 SGA of low tidal volume (mean 6 to 8 mL/kg IBW) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.9.1 Short‐term mortality | 3 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.20] |
| 1.9.2 Longer‐term mortality | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 1.10 SGA of high tidal volume (> 8 mL/kg IBW) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Short‐term mortality | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 1.10.2 Longer‐term mortality | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 1.11 SGA of ARDS only | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.11.1 Short‐term mortality | 7 | 1326 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 1.00] |
| 1.11.2 Longer‐term mortality | 8 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
Comparison 2. Intervention comparisons and interactions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Longer duration vs shorter duration of proning: longer‐term mortality | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.73, 1.04] |
| 2.1.1 > 16 hours | 5 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 2.1.2 ≤ 16 hours prone | 3 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 2.2 Early enrolment vs later enrolment to intervention: longer‐term mortality | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 2.2.2 Late enrolment > 48 hours | 3 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 2.2.3 Early enrolment ≤ 48 hours | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 2.3 Severe vs less‐severe hypoxaemia: longer‐term mortality | 7 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 2.3.1 Severe hypoxaemia | 7 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 2.3.2 Less severe hypoxaemia | 6 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.26] |
| 2.4 Lower tidal volume (TV) ventilation vs higher TV ventilation: longer‐term mortality | 8 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 2.4.1 Lower TV ‐ mean 6 to 8 mL/kg IBW | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 2.4.2 High TV ‐ mean > 8 mL/kg IBW | 4 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.12] |
Comparison 3. Pneumonia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pneumonia | 5 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.18] |
Comparison 4. Duration of mechanical ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Duration of mechanical ventilation | 3 | 871 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.53, 0.59] |
Comparison 5. Length of stay (LOS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 ICU LOS | 5 | 1775 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐1.13, 3.26] |
Comparison 6. Mean change in PaO2/FIO2 quotient (mmHg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mean increase in PaO2/FIO2 quotient (mmHg) at 7 or 10 days | 4 | 827 | Mean Difference (IV, Fixed, 95% CI) | 24.03 [13.35, 34.71] |
| 6.1.1 Change data provided | 2 | 268 | Mean Difference (IV, Fixed, 95% CI) | 16.71 [0.11, 33.32] |
| 6.1.2 Calculated change data | 2 | 559 | Mean Difference (IV, Fixed, 95% CI) | 29.19 [15.24, 43.14] |
Comparison 7. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Adverse events | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 Pressure ulcers | 4 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.48] |
| 7.1.2 Tracheal tube displacement | 8 | 2021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.39] |
| 7.1.3 Tracheal tube obstruction | 3 | 1597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.35, 2.18] |
| 7.1.4 Pneumothorax | 4 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.65, 2.08] |
| 7.1.5 Arrhythmias | 3 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ayzac 2016.
| Study characteristics | ||
| Methods | Further analysis of PROSEVA randomized controlled trial (Guerin 2013) | |
| Participants | 466 Participants of PROSEVA trial. Analysed on an "Intention To Treat " basis ARDS ‐ American–European Consensus Conference criteria
|
|
| Interventions | Prone position for ≥ 16 hours/d vs semi recumbent position Tidal volume: 6.1 mL/kg IBW (~ 95% CI 4.9 to 7.3 mL/kg IBW) |
|
| Outcomes | Ventilator‐associated pneumonia | |
| Notes | Initial VAP diagnosis made by principal investigator for each site and not blinded to patient allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest post‐randomization bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Site investigator screened patients for VAP. Patients with labelled as having VAP then further adjudicated by blinded independent assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat listed as method of analysis. Eight participants from original cohort excluded (with explanation) |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | Low risk | Low cross‐over rate |
Chan 2007.
| Study characteristics | ||
| Methods | Participants were assigned to supine (n = 11) or prone (n = 11) position ventilation according to the discretion of the physician in charge. All participants had a Swan‐Ganz catheter, and an arterial line was inserted for haemodynamic monitoring and blood sampling. Oxygen saturations were measured with a pulse oximeter. Sedation was given to all participants via continuous infusion of midazolam and neuromuscular blockade with atracurium besylate. Antibiotics were given to participants according to American Thoracic Society guidelines for CAP and based on the clinical judgement of the in‐charge physician. All participants were intubated and underwent volume‐controlled mechanical ventilation | |
| Participants | 22 patients with community‐acquired pneumonia (fever plus cough with purulent sputum production and infiltrates on chest x‐ray within 72 hours of admission) during an SARS epidemic. All patients met the criteria for ARDS as defined by the American‐European Consensus Conference, with onset within 72 hours before enrolment | |
| Interventions | Prone position ventilation vs supine Participants in the intervention group were ventilated in the prone position and were maintained in this position for ≥ 72 hours. Participants were turned supine once they maintained an SpO2 > 90% with FIO2< 60 for more than 24 hours after 72 hours of prone positioning Tidal volume: 7.7 mL/kg IBW (95% CI ~ 5.6 to 9.8 mL/kg IBW) |
|
| Outcomes | Primary outcomes: plasma cytokine levels at baseline and at 24 hours and 72 hours after enrolment Secondary outcomes: PaO2/FIO2 and complications. 14‐day mortality is recorded |
|
| Notes | Randomization methods were unclear, with contradictory comments included in the manuscript and in subsequent correspondence. Described as "prospective observational study" in original paper, which also stated, "Patients were assigned to either continuous prone position ventilation (PRONE) or traditional supine ventilation (SUPINE) according to the in‐charge physician's decision." In subsequent correspondence, study authors stated, "after agreement of the in‐charge physician patients were enrolled and then assigned to either PRONE or SUPINE according to a computer run randomization table" (Chan 2008). Trial was discontinued for slow enrolment due to SARS outbreak. Trial commenced in 2002, was completed in 2003 and was published in 2007 Mean of 105.6 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The conflicting statements (above) make assessment unclear |
| Allocation concealment (selection bias) | High risk | Bias stated by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Bias stated by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinded assessment not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Few endpoints and short follow‐up, but comments regarding physician decisions and effects of the SARS outbreak make the risk of this sort of bias unclear |
| Selective reporting (reporting bias) | Unclear risk | No pre‐specified protocol reported |
| Other bias | Unclear risk | Study was ended prematurely, and such studies have been associated with inflated effect size (Bassler 2010) |
Chiumello 2012.
| Study characteristics | ||
| Methods | Follow‐up of subgroup of participants from Taccone 2009. Five Italian centres (of the 25 original centres ‐ 23 Italian and 2 Spanish) | |
| Participants | Quality of life and physiological data available for 26 participants (13 prone, 13 supine) from 67 eligible patients 12 months after enrolment. (The original study recruited 344 patients.) Mortality data from 187 patients also available | |
| Interventions | Randomly assigned to receive supine or prone ventilation for acute respiratory distress syndrome (see Taccone 2009) | |
| Outcomes | 12‐Month mortality; blood gas analysis; pulmonary function tests including CO diffusion; walking test; health‐related quality of life using Short Form‐36 (SF‐36) and St George's Respiratory Questionnaire (SGRQ); quantitative lung CT scan analysis Mortality at 12 month follow‐up, 60% overall. | |
| Notes | Small subgroup of participants with large attrition rate; participant samples may not be representative. Low power to detect clinically meaningful differences with regards to outcomes. Trial commenced in 2004, was completed in 2008 and was published in 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized telephone randomization system ‐ as for main study (Taccone 2009) |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants may remember and divulge allocation to "blinded" assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very high dropout rate. 13/29 assessed in the prone group. 13/38 assessed in the supine group. Not likely to be random |
| Selective reporting (reporting bias) | Unclear risk | Post hoc follow‐up tests |
| Other bias | Low risk | No other bias identified |
Fernandez 2008.
| Study characteristics | ||
| Methods | Multi‐centre, open, randomized controlled trial over 12 months. Participants were randomly assigned by computer‐generated random sequence to supine (n = 19) or early (within 48 hours) and continuous prone (n = 21) ventilation with further stratification of randomization according to severity using the SAPS II score and the type of ARDS | |
| Participants | 40 mechanically ventilated patients with early, refractory ARDS despite early protective supine ventilation 25 male, 15 female Inclusion: intubated adult patients within 48 hours of ARDS diagnosis (North American‐European Consensus Conference (NAECC) criteria) Exclusion: severe hypotension requiring vasopressors (cardiovascular SOFA score 3 to 4), traumatic brain injury (TBI), unstable pelvic or spinal column fracture, moribund condition or enrolment in another trial |
|
| Interventions | After a 1‐hour protocolized ventilation period, participants were placed in the assigned position (prone or supine), in which they were maintained for up to 20 hours per day. Prone participants were turned supine once PaO2/FIO2 quotient was < 250 mmHg (33.3 kPa) for longer than 12 hours. Mechanical ventilation appeared to be volume controlled and pressure limited Tidal volume: 7.25 mL/kg IBW (~ 95% CI 5.1 to 9.4 mL/kg IBW) |
|
| Outcomes | Primary: 60‐day survival. NB ICU mortality identical, as no participant died after discharge up to the 60 days studied Secondary: length of mechanical ventilation and ICU stay |
|
| Notes | Study was prematurely stopped because of low participant recruitment. Two participants were lost to follow‐up (4.8%) (1/group) and 2 supine participants were crossed over to prone. Both cross‐over participants died. Criteria for new pneumonia (ventilator‐associated pneumonia) not defined Trial commenced in 2003, was completed in 2004 and was published in 2008 Mean hours prone per participant not clear from text. Clarification sought but not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized control centre produced randomization codes |
| Allocation concealment (selection bias) | Low risk | The above would minimize selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes (e.g. pressure sores) have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants lost to follow‐up and 2 crossed over to prone ventilation during first week of care |
| Selective reporting (reporting bias) | Low risk | No mention of pre‐study publication protocol, but multi‐centre trial would require explicit protocol for each centre |
| Other bias | Unclear risk | Study was ended prematurely, and such studies have been associated with inflated effect size (Bassler 2010) |
Gattinoni 2001.
| Study characteristics | ||
| Methods | Multi‐centre, randomized trial over 34 consecutive months. Randomization to supine (n = 152) or prone (n = 152) position ventilation was done centrally by telephone based on a permuted block algorithm, allowing for stratification according to intensive care unit | |
| Participants | 304 mechanically ventilated patients with ALI or ARDS 214 males, 90 females Inclusion: PFR < 200 with PEEP > 5, or PFR < 300 with PEEP > 10, bilateral pulmonary infiltrates, pulmonary‐capillary wedge pressure ≤ 18 mmHg or absence of clinical evidence of left atrial hypertension Exclusion: < 16 years old, cardiogenic pulmonary oedema, cerebral oedema or intracranial hypertension, proning contraindications or severe haemodynamic instability |
|
| Interventions | Participants randomly assigned to the prone group were maintained in the prone position continuously for ≥ 6 hours per day for 10 days Tidal volume: 10.3 mL/kg IBW (~ 95% CI 4.8 to 15.8 mL/kg IBW) |
|
| Outcomes | Primary: 10‐day mortality (end of the prone period), mortality at discharge from ICU and 6 months post randomization Secondary: improvement in respiratory failure and organ dysfunction at 10 days |
|
| Notes | Twelve participants (7.9%) were crossed over from supine to prone position during the trial. 41 of 152 (27.0%) participants missed ≥ 1 scheduled proning sessions. Subgroup percentages were provided for more severely ill participants, etc, but not numbers of participants. Possible selection reporting bias for subgroup cutoffs (e.g. PaO2/FIO2 quotient of 88 mmHg (11.7 kPa). Compare these results vs the cutoff of 100 mmHg (13.3 kPa) in their recent systematic review (Gattinoni 2010). No apparent loss to follow‐up and apparent strict Intention‐to‐treat analysis with supplementary per‐protocol analyses. Trial was discontinued early by investigators and data and monitoring safety board because of slow recruitment ascribed to increasing unwillingness of investigators to forgo the use of prone positioning. Trial commenced in 1996, was completed in 1999 and was published in 2001 Mean of 32.9 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central telephone service |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest selection bias following randomization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias. Pressure sore assessment was well described in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat listed as method of analysis. No mention of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No mention of pre‐study publication protocol, but multi‐centre trial would require explicit protocol for each centre. Possible bias in reporting post hoc analyses (e.g. outcomes of participants with PaO2/FIO2 quotient of 88 mmHg (11.7 kPa) |
| Other bias | Unclear risk | Study was ended prematurely; such studies have been associated with inflated effect size (Bassler 2010), although little signal of effect was evident |
Girard 2014.
| Study characteristics | ||
| Methods | Further analysis of PROSEVA randomized controlled trial (Guerin 2013) | |
| Participants | ARDS ‐ American–European Consensus Conference criteria
|
|
| Interventions | Prone position for ≥ 16 hours/d vs semi recumbent position Tidal volume: 6.1 mL/kg IBW (~ 95% CI 4.9 to 7.3 mL/kg IBW) |
|
| Outcomes | Pressure ulcers (sores) using National Pressure Ulcer Advisory Panel's Updated Pressure Ulcer Staging System (NPAUP) | |
| Notes | Provides additional information on a secondary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest post‐randomization bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified. Some participants had treatment withdrawn. Prone 14/237 vs supine 30/229; bias regarding differential use of co‐interventions is also possible (providing a treatment or with‐holding a treatment). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators making assessments were not blinded but assessments were described as being standardized using the NPAUP scoring system.. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat listed as method for primary analysis. Eight participants from original cohort excluded (with explanation) from primary study. A further attrition of 10 patients described, 5 at randomization and five at day 7 (patients died or were discharged). |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | Low risk | Low cross‐over rate |
Guerin 2004.
| Study characteristics | ||
| Methods | Prospective, unblinded, multi‐centre, randomized controlled trial over 48 consecutive months. Randomization was computer‐generated and was done separately for each ICU, with participants to supine (n = 378) or prone (n = 413) position ventilation | |
| Participants | 791 participants 593 males, 198 females Inclusion: mechanical ventilation (oral or nasal tracheal intubation or tracheostomy), PaO2/FIO2 ≤ 300, ≥ 18 years of age, expected duration of mechanical ventilation > 48 hours, written informed consent from next of kin Exclusion: prone position for ≥ 6 hours per day in the 4 days preceding enrolment, contraindications to proning (ICP > 30 mmHg, cerebral perfusion < 60 mmHg, massive haemoptysis, bronchopleural fistula, tracheal surgery or sternotomy in the past 15 days, MAP < 65 with or without vasopressors, DVT, pacemaker inserted for fewer than 2 days, unstable fracture), therapeutic limitation indicated in the first 24 hours of ICU admission, high risk of death in the next 48 hours, chronic respiratory failure requiring mechanical ventilation and inclusion in another protocol with mortality as a primary endpoint |
|
| Interventions | Participants were randomly assigned to the supine or the prone group, in which they were placed in a prone position for ≥ 8 hours per day Tidal volume: 10.1 mL/kg IBW* (~ 95% CI 5.5 to 14.7 mL/kg IBW). *Imputed from measured body weight data (Bloomfield 2006) |
|
| Outcomes | Primary: 28‐day mortality Secondary: 90‐day mortality, duration of mechanical ventilation, rate of ventilator‐associated pneumonia and oxygenation |
|
| Notes | VAP was well defined; 11 of 802 participants (1.4%) recruited, lost from final analysis Trial commenced in 1998, was completed in 2002 and was published in 2004 Mean of 36.9 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Detailed methods provided |
| Allocation concealment (selection bias) | Low risk | Detailed methods provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 of 802 participants (1.4%) recruited, lost from final analysis |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | High risk | Very high cross‐over rates reported: "At day 28, 83 (27.9%) of 297 patients in the supine group died, 36 (44.4%) of the 81 patients who had crossed over from the supine group died, 76 (31.3%) of 243 patients in the prone group died, and 58 (34.1%) of 170 patients who crossed over from the prone group died (P value = .85)". Overall, 32% of participants in the trial were crossed over to the opposite limb of the study. This level of cross‐over events makes reported effects difficult to interpret. This level of selective cross‐over of participants impairs the statistical power of the study and leads to bias against a positive result (Lipsey 1990; Porta 2008) |
Guerin 2013.
| Study characteristics | ||
| Methods | Multi‐centre, randomized, controlled, open‐label trial conducted in France and Spain | |
| Participants | ARDS ‐ American–European Consensus Conference criteria
|
|
| Interventions | Prone position for ≥ 16 hours/d vs semi recumbent position Tidal volume: 6.1 mL/kg IBW (~ 95% CI 4.9 to 7.3 mL/kg IBW) |
|
| Outcomes | Primary endpoint: 28‐day all‐cause mortality Secondary endpoints: mortality at day 90; rate of successful extubation; time to successful extubation; length of stay in the ICU; complications; use of non‐invasive ventilation; tracheotomy rate; number of days free from organ dysfunction; and ventilator settings of arterial blood gases and respiratory system mechanics measurements during the first week after randomization |
|
| Notes | 30 deaths in the supine group (n = 229) had an end‐of‐life decision; 14 deaths in the prone group (n = 237) had an end‐of‐life decision. Assist/control ventilation mode is not commonly utilized in Europe. PEEP table mandated high levels of PEEP. Improved oxygenation allowed reduction of these high levels ‐ so differential PEEP reduction of mandated PEEP may be a potential mechanism of benefit (Soni 2008) that accentuates benefit of prone positioning in this study. 8 of 474 participants recruited (1.7%) were lost from the final analysis Trial commenced in 2008, was completed in 2011 and was published in 2013 Mean of 68 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest post‐randomization bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified. Some participants had treatment withdrawn. Prone 14/237 vs supine 30/229; bias regarding differential use of co‐interventions is also possible (providing a treatment or with‐holding a treatment). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat listed as method of analysis. Eight participants from original cohort excluded (with explanation) |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | Low risk | Low cross‐over rate |
Leal 1997.
| Study characteristics | ||
| Methods | Single‐centre RCT, sequential sealed envelope allocation, no cross‐overs | |
| Participants | 16* patients with ARDS (8 participants per group). PaO2/FIO2 quotient < 150 mmHg and diagnosis to enrolment time < 24 hours (additional information from Sud 2008a) *2 additional participants were included in the actual meeting presentation (made available in Microsoft PowerPointTM slides by Dr Jan Friederich, Toronto, Canada) |
|
| Interventions | 24 hours prone ventilation (fixed duration and single application only) | |
| Outcomes | Mortality; complications; early effects on gas exchange Tidal volume not listed |
|
| Notes | Abstract and Microsoft PowerPoint presentation of original authors supplied by Dr Jan Friederich through Professor Brian Cuthbertson. Data limited. Outcomes assumed to be short‐term data in line with physiological nature of the study. Although single application lasted for 24 hours, the total application time during mechanical ventilation in the ICU was therefore limited Trial commencement and finish dates not available; abstract published in 1997. 50% of participants placed prone had airway complications despite proning only once per study participant Mortality in original abstract occurred in 5 of 7 participants in each group. With the addition of 1 participant to each group, mortality became 5 of 8 for participants randomly assigned to prone vs 6 of 8 randomly assigned to supine. The investigation was short‐term (72 hours), and mortality rates are assumed to be short‐term Mean of 24 hours total prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not stated |
| Allocation concealment (selection bias) | Low risk | Sequential sealed envelope allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data not described. Small single‐centre study; not able to assess risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | No mention of pre‐study publication protocol |
| Other bias | Low risk | No other bias identified |
Mancebo 2006.
| Study characteristics | ||
| Methods | Multi‐centre, randomized controlled trial over 45 months. Randomization was computer‐generated, assigning participants to the supine (n = 60) or the prone (n = 76) position group. Participants were enrolled within 48 hours of tracheal intubation for severe ARDS | |
| Participants | 136 participants 86 males, 50 females Inclusion: intubation, mechanical ventilation, > 18 years of age, ARDS (American‐European Consensus Conference definition), diffuse bilateral infiltrates on chest x‐ray Exclusion: > 48 hours since inclusion criteria were met, participation in other trials, pregnancy, systolic BP < 80 despite vasopressors, pelvic or spinal fracture, cranial trauma and/or clinical suspicion of raised ICP, considered moribund |
|
| Interventions | Participants were randomly assigned to the supine group or to the prone group, which received continuous prone position ventilation for 20 hours per day. Mechanical ventilation with volume assist‐control mode Tidal volume: 10.6 mL/kg IBW* (~ 95% CI 6.5 to 14.6 mL/kg IBW) *Correction for use of measured body weight rather than IBW (Bloomfield 2006) |
|
| Outcomes | Primary: ICU mortality Secondary: hospital mortality, associated complications and length of stay |
|
| Notes | Study was prematurely stopped because of low participant recruitment. 5 participants crossed over to prone ventilation from original assignment. (All died.) High tidal volumes were used. Up to 10 mg/kg actual body weight was allowed in the protocol and maximum plateau pressures up to 40 cm H2O. Some participants received tidal volumes in excess of 10 mL/kg and in excess of their 2 targets of 35 and 40 cm H2O. (See supplement.) This decreases relevance to currently accepted targets of tidal volumes of 6 mL/kg ideal body weight and plateau pressures < 30 cm H2O. 5 cross‐overs from the supine group to the prone group were reported. 6 of 142 participants (4.2%) enrolled were lost after randomization Trial commenced in 1998, was completed in 2002 and was published in 2006 Mean of 171.7 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants (4.2%) were not included in the final analysis: 3 because of lost forms, 2 because data were lacking and 1 as the result of transfer to a cardiac surgery centre for possible surgery |
| Selective reporting (reporting bias) | Low risk | No mention of pre‐study publication protocol, but multi‐centre trial would require an explicit protocol for each centre |
| Other bias | Unclear risk | Study was ended prematurely; such studies have been associated with inflated effect size (Bassler 2010) |
Taccone 2009.
| Study characteristics | ||
| Methods | Multi‐centre, unblinded, randomized controlled trial. Randomization to the supine (n = 174) or the prone (n = 168) position group was computer‐generated, and participants were stratified according to severity of hypoxaemia and participating centre. Prospective subgroup analysis defined the moderate subgroup as PaO2/FIO2 quotient of 100 to 200 mmHg, and severe as PaO2/FIO2 < 100 mmHg | |
| Participants | 342 participants 244 males, 98 females Inclusion: ARDS criteria (PFR ≤ 200 mmHg for PEEP 5 to 10 cm H2O) Exclusion: < 16 yo, > 72 hours since diagnosis of ARDS, history of solid organ or bone marrow transplantation, contraindication to proning (raised ICP, spine/pelvic fracture) |
|
| Interventions | Participants were randomly assigned to supine or prone position ventilation, which required maintaining prone position ≥ 20 hours per day until resolution of ARDS or the end of the 28‐day study period Tidal volume: 8.0 mL/kg IBW (~ 95% CI 4.7 to 11.3 mL/kg IBW) |
|
| Outcomes | Primary: 28‐day all‐cause mortality Secondary: 6‐month and ICU discharge mortality, organ dysfunction, complication rate related to prone positioning |
|
| Notes | It is noted that more participants randomly assigned to prone ventilation received increased sedation or muscle relaxants. This co‐intervention can improve survival (Papazian 2010) Trial commenced in 2004, was completed in 2008 and was published in 2009. Participants were enrolled a median of 0 days (IQR 0 to 1) after mechanical ventilation. 20 participants (11.5%) randomly assigned to the supine position were crossed over to the prone group as rescue therapy for hypoxaemia. 34 participants (20.2%) assigned to prone did not receive the intervention but were included in the ITT analysis. Ventilator‐associated pneumonia was not defined Mean of 149.4 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized telephone randomization system |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants in each group were lost to follow‐up. All 4 were assumed alive for the follow‐up period. 2 additional participants (1 per group) was ineligible; both were removed before protocol initiation |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | Unclear risk | 11.5% of participants randomly assigned to supine position were crossed over to the prone position as part of the pre‐defined rescue protocol. 34 participants (20.2%) assigned to prone did not receive intervention but were included in the ITT analysis |
Voggenreiter 2005.
| Study characteristics | ||
| Methods | 2 (trauma)‐centre prospective randomized trial. Randomization assigned participants to supine (n = 19) or prone (n = 21) position group and was conducted centrally by telephone, using a permuted‐block algorithm, allowing for stratification according to ICU, participant age, ISS, AIS‐chest, AIS‐head and interval between injury and randomization | |
| Participants | 40 participants 33 males, 7 females Inclusion: multiple trauma patients 18 to 80 years of age; ISS > 16; modified ALI/ARDS criteria (PaO2/FIO2 quotient for ALI or ARDS; "lung infiltrates"; and absence of evidence of left atrial hypertension) Exclusion: cardiogenic pulmonary oedema, cerebral oedema, ↑ ICP, other contraindications to prone (e.g. haemodynamic instability, unstable fracture) |
|
| Interventions | Participants were randomly assigned to the supine or the prone ventilation group, in which participants were continuously maintained in the prone position ≥ 8 hours and for a maximum of 23 hours per day. Mean of 11 hours (SD 5) of prone applied, and applied on a mean of 7 (SD 4) occasions | |
| Outcomes | Primary: duration of mechanical ventilation Secondary: days with ARDS (PaO2:FIO2 < 200), ALI (PaO2:FIO2 200 to 300); days with LIS > 2, course of PaO2:FIO2, Qs/Qt score, total static lung compliance, PIP, PEEP, LIS, TISS‐28, SOFA score, sepsis, prevalence of pneumonia, mortality within the 90‐day study period, complications/adverse events and ARDS following ALI |
|
| Notes | Participants in the supine limb received 3 times as many packed red cells (mean of 28.2 vs 9.5 packs of red cells per participant) (i.e. 19 more packs of red cells per participant, on average). Possible fluid overload and effects of RCC on infection and leucocytosis could confound pneumonia diagnosis. Neuromuscular blockers were used more in participants ventilated prone (7.8 days/patient vs 5.6 days/patient; P value = 0.06). Neuromuscular blockade is an intervention that could independently improve mortality (Papazian 2010) Trial commenced in 1999, was completed in 2001 and was published in 2005. A variety of modes of ventilation were used: BIPAP (n = 19), CPPV (n = 20) and SIMV (n = 1), but "lung protective strategy" was used. Actual data for tidal volumes are not available. Listed as enrolled < 48 hours from meeting criteria (Sud 2010). Pneumonia (VAP), new pneumonia within 90 days ‐ reasonably well‐defined criteria ‐ but results could be affected by differential RCC transfusion, as noted above. No apparent loss to follow‐up Mean of 77 hours prone per participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized telephone randomization system |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. 2‐centre study |
| Other bias | High risk | Markedly different red cell transfusion rates for 2 groups of participants (high risk). Sample size not predefined (unclear risk). Study was ended prematurely, and such studies have been associated with inflated effect size (Bassler 2010) (unclear risk) |
AIS = Abbreviated injury scale; ALI = Acute lung injury; ARDS = Acute respiratory distress syndrome; BIPAP = Bi‐phasic positive airways pressure; BP = Blood pressure; CAP = Community‐acquired pneumonia; CO = Cardiac output; CPPV = Controlled positive‐pressure ventilation; CT = Computed tomography; DVT = Deep venous thrombosis; IBW = Predicted ideal body weight; ICP = Intracranial pressure; ICU = Intensive care unit; ISS = Injury severity score; ITT = Intention‐to‐treat; IQR = Interquartile range; LIS = Lung injury score; MAP = Mean arterial pressure; n = number; NAECC = North American‐European Consensus Criteria; NB = Note well; PEEP = Positive end‐expiratory pressure; PFR = Pulmonary arterial‐fractional inspired oxygen ratio; PIP = Peak inspiratory pressure; RCC = Red cell concentrate; RCT = Randomized controlled trial; SAPS = Simplified acute physiology score; SARS = Severe acute respiratory syndrome; SD = Standard deviation; SF‐36 = Short Form‐36; SGRQ = St George's Respiratory Questionnaire; SIMV = Synchronized intermittent mechanical ventilation; SOFA = Sequential organ failure assessment; TBI = Traumatic brain injury; TISS = Therapeutic intervention scoring system; VAP = Ventilator‐associated pneumonia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beuret 2002 | The primary reason for use of mechanical ventilation in this cohort of patients was brain injury causing reduced level of consciousness (Glasgow Coma Scale of 9 or less). Such patients require intubation airway protection and mechanical ventilation to maintain target PaCO2. Brain injury in this study was as a result of trauma, intracranial haemorrhage, Ischaemic stroke, anoxic encephalopathy, intracranial infection and other miscellaneous causes of coma. Randomization to ventilation in the prone position was investigated as a means of prevention of hypoxaemic respiratory failure (as stated in the title of the study) and not as a treatment. Mean PaO2/FIO2 quotient exceeded 40 kPa (300 mmHg) in both groups which does not meet the criteria for even mild ARDS by The Berlin Criteria (ARDS definition workforce 2012). This study has been incorporated into three systematic reviews of prone positioning (Abroug 2008; Sud 2008;Sud 2010). Total hours prone for duration of study = 24 (4 hours for 6.0 days) |
| Cao 2014 | No mention of randomization in text. |
| Charron 2011 | Not a randomized controlled trial (retrospective analysis of database) but reconsidered because it was incorporated in to the cumulative meta‐analysis of Yue et al (Yue 2017). |
| Cheng 2016 | No mention of randomization in text. Primarily reports on physiological results from PiCCO monitor and some data derived from ventilator |
| Curley 2005 | This study has been incorporated into other meta‐analyses. However, the study population (n = 102) predominantly consisted of very young children, with 49% ≤ 2 years of age and 73% ≤ 8 years of age. Our protocol specifically excluded children (Bloomfield 2009) for several reasons listed in the text. |
| Demory 2007 | Non‐conventional ventilation employed: 12 hours of high‐frequency oscillatory ventilation (HFOV) following 12 hours of conventional ventilation in the prone or the supine position. Non‐conventional ventilation was specifically excluded from our protocol. Treatment interaction could not be excluded, as not a factorial design (Fleiss 1986; Friedman 1998). Total hours prone in study = 12 (12 hours prone for 1 day) |
| Li G 2015 | The study is a retrospective analysis. |
| Li J 2015 | Percussion/vibration was used as an intervention for prone position patients only. There is no indication of randomization, no mention how they performed prone position, how long, how often etc. |
| Papazian 2005 | Non‐conventional ventilation employed: comparison of non‐conventional mechanical ventilation vs high‐frequency oscillatory ventilation (HFOV) used in 12‐hour protocol only Non‐conventional ventilation was specifically excluded from our protocol. Treatment interaction could not be excluded, as not a factorial design (Fleiss 1986; Friedman 1998). Total hours prone = 12 (12 hours prone for 1 day only) |
| Peng 2018 | RCT with 2 interventions and 4 study limbs but short term physiological intervention with no mortality outcomes presented. We also note large baseline imbalances between groups (eg age and APACHE scores). |
| Wang 2015 | Randomization is not mentioned in the text of this large study of 73 obstetric patients at high altitude who suffered severe pneumonia and underwent Caesarean Section (CS). The study was excluded because randomization is not specified.Thirty four patients received prone positioning.Mechanical ventilation occurred after the CS. Blood gas analysis and respiratory mechanics are reported as are duration of mechanical ventilation (6.3 days for prone position group and 10.2 days in control group) and the ICU length of stay (10.6 days in prone position group and 14.8 days in control group). |
| Watanabe 2002 | Infusion of muscle relaxants given to prone participants only; this co‐intervention has been associated with improved survival in patients with lung injury in some studies (Papazian 2010). Mortality outcomes not published |
| Yan 2015 | Randomisation is not mentioned in text. There are no descriptions regarding prone positioning. Blood‐gas analyses were their primary outcome. |
| Zhou 2014 | RCT but supine positioning alone is compared with prone positioning together with an additional respiratory intervention (recruitment manoeuvres). Different sedation regime (bolus and infusions of midazolam) and neuromuscular blockers (vecuronium) employed in the prone position group during prone positioning. |
HFOV = High‐frequency oscillatory ventilation; n = number.
Characteristics of ongoing studies [ordered by study ID]
NCT03891212.
| Study name | The Effect of Prone Position Drainage on the Efficacy of Severe Pneumonia, a Multicenter Randomized Controlled Trial |
| Methods | Randomized controlled trial |
| Participants | Estimated number of participants = 500 Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: Placed in prone position for at least 16 consecutive hours a day; Control: Placed in supine position for at least 16 consecutive hours |
| Outcomes |
|
| Starting date | Not reported |
| Contact information | Pinhua Pan: pinhuapan668@126.com |
| Notes |
NCT04139733.
| Study name | Early Use of Prone Position in ECMO for Severe ARDS |
| Methods | Randomized single‐blind parallel trial |
| Participants | Estimated number of participants = 110
Age: 18 years to 75 years Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: Prone position within 6 hours after randomization. Prone position for at least conservative hours per days during a minimum number of days; Control: Conventional supine position ventilation, no prone position. |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | 3 September 2020 |
| Contact information | Rui Wang, Dr.; +8618601342030; xuanben1985@163.com |
| Notes |
NCT04607551.
| Study name | PRONing to Facilitate Weaning From ECMO in Patients With Refractory Acute Respiratory Distress Syndrome (PRONECMO) |
| Methods | Randomized open‐label parallel assignment trial |
| Participants | Estimated number of participants = 170
Age: 18 Years to 75 Years Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: Prone positioning ‐ 4 to 5 persons required for the procedure, one of them being dedicated to the management of the head of the patient, the endotracheal tube, the jugular ECMO cannula and the ventilator lines and another dedicated to the femoral ECMO cannula. The person at the head of the bed will coordinate the steps. The other persons will stand at each side of the bed. The direction of the rotation will be decided giving priority to the side of the central venous lines. The length of vascular and ventilator lines will be checked for appropriateness, the endotracheal tube and gastric tube will be secured, and the patient's knees, forehead, chest, and iliac crests will be protected using adhesive pads. The patient will be then moved along the horizontal plane to the opposite side of the bed selected for the direction of rotation. Patients will be proned at least four times during the first days on ECMO. Each prone session will stand for at least 16 hours; Control: Supine position‐ Patients assigned to supine will remain in a semi‐recumbent position. |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | November 2020 |
| Contact information | Matthieu SCHMIDT, MD; + 33 1 42 16 29 37; matthieu.schmidt@aphp.fr |
| Notes |
Differences between protocol and review
We made the following changes to the protocol (Bloomfield 2009).
We replaced Nigel Webster, who had insufficient time to assist with the review process, with Alexis Sudlow.
We added references and Background text.
We made the definition of tidal volume more specific for the purpose of identifying studies that employed "lung‐protective ventilation" (i.e. tidal volume should be expressed in mL per kg of ideal body weight.
Risk of bias methods for a Cochrane review have changed since protocol publication, and we reanalysed data for the new format.
The GRADE‐based summary of findings table has been introduced since protocol publication, and we have therefore retrospectively chosen the analyses displayed.
We did not employ the Q‐partitioning method (Deeks 2011) in exploring heterogeneity and used the I2 approach recommended instead.
We made minor modifications to search strategies for Ovid MEDLINE, EMBASE, CENTRAL, CINAHL and LILACS to better suit their updated search engines.
We changed the cutoff for prolonged duration of prone positioning from 18 or more hours per day to 16 or more hours per day. This placed the last published study (Guerin 2013) in the more biologically appropriate category for analysis. (The mean duration of proning in the short‐duration proning group was 8.3 hours per day, and for the long‐duration proning group 18.1 hours per day, and one study aiming for 20 hours per day actually achieved 17 hours per day (Mancebo 2006). The study of Guerin et al (Guerin 2013) also achieved proning for a mean of 17 hours per day.)
Contributions of authors
Conceiving of the review: Roxanna Bloomfield (RB), David W. Noble (DWN). Co‐ordinating the review: RB, DWN. Undertaking manual searches: RB, DWN. Screening search results: RB, DWN. Organizing retrieval of papers: RB, DWN. Screening retrieved papers against inclusion criteria: RB, DWN. Appraising quality of papers: RB, DWN, Alexis Sudlow (AS). Abstracting data from papers: RB, DWN, AS. Writing to authors of papers for additional information: DWN. Providing additional data about papers: Obtaining and screening data on unpublished studies: RB, DWN. Managing data for the review: RB, DWN. Entering data into Review Manager (RevMan 5.40): RB, DWN, AS. Analysing RevMan statistical data: DWN. Performing other statistical analyses not using RevMan: DWN Performing double entry of data: (data entered by person one: RB; data entered by person two: DWN). Interpreting data: RB, DWN, AS. Making statistical inferences: RB, DWN. Writing the review: RB, DWN, AS. Securing funding for the review: Performing previous work that served as the foundation of the present study: Serving as guarantor for the review (one review author): DWN. Taking responsibility for reading and checking the review before submission: AS.
Sources of support
Internal sources
NHS Grampian, UK
External sources
No sources of support supplied
Declarations of interest
Roxanna Bloomfield: none known.
David W Noble: none known.
Alexis Sudlow: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Ayzac 2016 {published data only}
- Ayzac L, Girard R, Baboi L, Beuret P, Rabilloud M, Richard J C, et al. Ventilator-associated pneumonia in ARDS patients: the impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Medicine 2016;42(5):871-8. [DOI: 10.1007/s00134-015-4167-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan MC, Hsu JY, Liu HH, Lee YL, Pong SC, Chang LY, et al. Effects of prone position on inflammatory parkers in patients with ARDS due to community-acquired pneumonia. Journal of the Formosan Medical Association 2007;106(9):708-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiumello 2012 {published data only}
- Chiumello D, Taccone P, Berto V, Marino A, Migliara G, Lazzerini M, et al. Long-term outcomes in survivors of acute respiratory distress syndrome ventilated in supine or prone position. Intensive Care Medicine 2012;38(2):221-9. [DOI: 10.1007/s00134-011-2445-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fernandez 2008 {published data only}
- Fernandez R, Trenchs X, Klamburg J, Castedo J, Serrano JM, Besso G, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Medicine 2008;34(8):1487-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gattinoni 2001 {published data only}
- Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. New England Journal of Medicine 2001;345(8):568-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Girard 2014 {published data only}
- Girard R, Baboi L, Ayzac L, Richard J-C, Guerin C. The impact of patient positioning on pressure ulcers in patients with severe ARDS: results from a multicentre randomised controlled trial on prone positioning. Intensive Care Medicine 2014;40(3):397-403. [DOI: 10.1007/s00134-013-3188-1 ] [PMID: 24352484] [DOI] [PubMed] [Google Scholar]
Guerin 2004 {published data only}
- Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 2004;292(19):2379-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guerin 2013 {published data only}
- Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. New England Journal of Medicine 2013;368(23):2059-68. [DOI: 10.1056/NEJMoa1214103] [ NCT00527813] [DOI] [PubMed] [Google Scholar]
Leal 1997 {published data only}
- Leal RP, Gonzales R, Gaona C, Garcia G, Maldonado A, Dominguez-Cherit G. Randomized trial compares prone v supine position in patients with ARDS. American Journal of Respiratory & Critical Care Medicine 1997;155:A745. [Google Scholar]
Mancebo 2006 {published data only}
- Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. American Journal of Respiratory & Critical Care Medicine 2006;173(11):1233-9. [DOI: 10.1164/rccm.200503-353OC ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taccone 2009 {published data only}
- Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009;302(18):1977-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Voggenreiter 2005 {published data only}
- Voggenreiter G, Aufmkolk M, Stiletto MJ, Baacke MG, Waydhas C, Ose C, et al. Prone positioning improves oxygenation in post-traumatic lung injury—A prospective randomized trial. Journal of Trauma 2005;59(2):333-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beuret 2002 {published data only}
- Beuret P, Carton M-J, Nourdine K, Kaaki M, Tramoni G, Ducreux J-C. Prone position as prevention of lung injury in comatose patients: a prospective randomized controlled study. Intensive Care Medicine 2002;28(5):564-9. [DOI: 10.1007/s00134-002-1266-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cao 2014 {published data only}
- Cao FT, Wan F, Fan XC, et al. Effects of ventilation under prone position in the elderly with severe pneumonia combined with acute respiratory failure [俯卧位通气对合并急性呼吸衰竭的老年重症肺炎的影响]. Jiangsu Medical Journal 2014;40(5):578-80. [Google Scholar]
Charron 2011 {published data only}
- Charron C, Bouferrache K, Caille V, Castro S, Aegerter P, Page B, et al. Routine prone positioning in patients with severe ARDS: feasibility and impact on prognosis. Intensive Care Medicine 2011;37(5):785–90. [DOI: DOI 10.1007/s00134-011-2180-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cheng 2016 {published data only}
- Cheng FQ, Zhao LH, Lan F, Jiang L,Zhao Xue Q,Wang H-Z. The application of prone position ventilation on elderly patients with severe pneumonia and respiratory failure [俯卧位通气在老年重症肺炎合并呼 吸衰竭中的应用]. Today Nurse 2016;10:20-1. [Google Scholar]
Curley 2005 {published data only}
- Curley MAQ, Hibberd PL, Fineman LD, Wypij D, Shih M-C, Thompson JE, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 2005;294(2):229-37. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demory 2007 {published data only}
- Demory D, Michelet P, Arnal J-M, Donati S, Forel J-M, Gainnier M, et al. High-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Critical Care Medicine 2007;35(1):106-11. [DOI: 10.1097/01.CCM.0000251128.60336.FE] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li G 2015 {published data only}
- Li GZ. Clinical research of prone position ventilation on severe pneumonia patients [俯卧位与仰卧位机械通气治疗重症肺炎临床疗 效的比较研究]. Medical Frontier 2015;5(23):166-7. [DOI: 10.3969/j.issn.2095-1752.2015.23.155] [DOI] [Google Scholar]
Li J 2015 {published data only}
- Li J, Xi JY. Comparative study for clinical effect on severe pneumonia between prone-position and supine-position mechanical ventilation [俯卧位机械通气应用于重症肺炎治疗的临床研究]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2015;23(10):85-7. [DOI: 10.3969/j.issn.1008-5971.2015.10.023.] [DOI] [Google Scholar]
Papazian 2005 {published data only}
- Papazian L, Gainnier M, Marin V, Donati S, Arnal J-M, Demory D, et al. Comparison of prone positioning and high-frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Critical Care Medicine 2005;33:2162-71. [DOI: 10.1097/01.CCM.0000181298.05474.2B] [PMID: ] [DOI] [PubMed] [Google Scholar]
Peng 2018 {published data only}
- Peng X, He L, Chen J. Comparison of the curative effect of supine position and prone position ventilation combined with vibration sputumin* patients with acute respiratory distress syndrome (*sputum)? [急性呼吸窘迫综合征病人仰卧位、俯卧位通气联合振动排痰的疗效比较]. Chinese Nursing Research 2018;32(9):1387-92. [Google Scholar]
Wang 2015 {published data only}
- Wang WX, Dong Y, Xu B, et al. Impact of prone position on patients with late pregnancy combined with severe pneumonia in high altitude area [王文欣,董颖,徐波,等.高海拔地区晚期妊娠并重症肺炎剖宫 产术后患者俯卧位通气的临床应用[J].高原医学杂志]. Journal of High Altitude Medicine 2015;3:38-42. [Google Scholar]
Watanabe 2002 {published data only}
- Watanabe I, Fujihara H, Sato K, Honda T, Ohashi S, Endoh H, et al. Beneficial effect of a prone position for patients with hypoxemia after transthoracic esophagectomy. Critical Care Medicine 2002;30(8):1799-02. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yan 2015 {published data only}
- Yan HS, Wang ZF. Treatment of prone and supine position ventilation on patients with severe pneumonia [俯卧位和仰卧位机械通气在重症肺炎治疗中 的应用效果研究]. Heilongjiang Med 2015;39(9):1021-2. [DOI: 10.3969/j.issn.1004-5775. 2015.09.012.] [Google Scholar]
Zhou 2014 {published data only}
- Zhou X, Liu D, Long Y, Zhang Q, Cui N, He H, et al. The effects of prone position ventilation combined with recruitment maneuvers on outcomes in patients with severe acute respiratory distress syndrome [Chinese]. Chung-Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 2014;53(6):437-41. [PMID: ] [PubMed] [Google Scholar]
References to ongoing studies
NCT03891212 {published data only}
- The Effect of Prone Position Drainage on the Efficacy of Severe Pneumonia, a Multicenter Randomized Controlled Trial. https://clinicaltrials.gov/ct2/show/NCT03891212 First Posted 26 March 2019. [CLINICALTRIALS.GOV IDENTIFIER: NCT03891212]
NCT04139733 {published data only}
- Early Use of Prone Position in ECMO for Severe ARDS. https://clinicaltrials.gov/ct2/show/NCT04139733 First Posted 25 October 2019. [CLINICALTRIALS.GOV IDENTIFIER: NCT04139733]
NCT04607551 {published data only}
- PRONing to Facilitate Weaning From ECMO in Patients With Refractory Acute Respiratory Distress Syndrome (PRONECMO). https://clinicaltrials.gov/ct2/show/NCT04607551 First Posted: 29 October 2020. [CLINICALTRIALS.GOV IDENTIFIER: NCT04607551]
Additional references
Abroug 2008
- Abroug F, Ouanes-Besbes L, Elatrous S, Brochard L. The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: a meta-analysis. Areas of uncertainty and recommendations for research. Intensive Care Medicine 2008;34(6):1002–11. [DOI: 10.1007/s00134-008-1062-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Abroug 2011
- Abroug F, Ouanes-Besbes L, Dachraoui F, Ouanes I, Brochard L. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Critical Care 2011;15(1):R6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adhikari 2004
- Adhikari N, Burns KEA, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD004477. [DOI: 10.1002/14651858.CD004477.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adhikari 2007
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007;334(7597):779-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsaghir 2008
- Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Critical Care Medicine 2008;36(2):603-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aoyama 2019
- Aoyama H, Uchida K, Aoyama K, Pechlivanoglou P, Englesakis M, Yamada Y, et al. Assessment of Therapeutic Interventions and Lung ProtectiveVentilation in Patients With Moderate to Severe Acute RespiratoryDistress Syndrome. A Systematic Review and Network Meta-analysis. JAMA Network Open 2019;2(7):e198116. [DOI: 10.1001/jamanetworkopen.2019.8116] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ARDS definition workforce 2012
- The ARDS Definition Workforce. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307(23):2526-33. [DOI: 10.1001/jama.2012.5669 ] [DOI] [PubMed] [Google Scholar]
ARDSnet 2006a
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. New England Journal of Medicine 2006;354(24):2564-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
ARDSnet 2006b
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. New England Journal of Medicine 2006;354(16):1671-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
ARDS Network 2000
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1301-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ay 2020
- Ay E, Weigand MA, Röhrig R, Gruss M. Dying in the Intensive Care Unit (ICU): A Retrospective Descriptive Analysis of Deaths in the ICU in a Communal Tertiary Hospital in Germany. Anesthesiology Research and Practice 2020;2020:Article ID 2356019. [DOI: 10.1155/2020/2356019] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayoubieh 2014
- Ayoubieh H, Alkhalili E, Kassis YE, Faza N, Khalaf S, Lystad L, et al. Ischemic optic neuropathy after prone ventilation for ARDS. Critical Care Medicine 2014;42(Suppl 1):A1641-A1642. [DOI: 10.1097/01.ccm.0000458670.13219.ba] [EMBASE: 71707880] [DOI] [Google Scholar]
Ball 1999
- Ball C. Use of prone position in management of acute respiratory failure. Clinical Effectiveness in Nursing 1999;3:36-46. [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA 2010;303(12):1180-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Baston 2019
- Baston CM, Coe NB, Guerin C, Mancebo J, Halpern S. The Cost-Effectiveness of Interventions to Increase Utilization of Prone Positioning for Severe Acute Respiratory Distress Syndrome. Critical Care Medicine 2019;47(3):e198-e205. [DOI: 10.1097/CCM.0000000000003617] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beitler 2014
- Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Medicine 2014;40(3):332-41. [DOI: 10.1007/s00134-013-3194-3 ] [PMID: 24435203] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellani 2016
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315(8):788-800. [DOI: 10.1001/jama.2016.0291] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bent 2006
- Bent S, Padula, Avins AL. Brief communication: better ways to question patients about adverse medical events: a randomized, controlled trial. Annals of Internal Medicine 2006;144(4):257-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernard 2005
- Bernard GR. Acute respiratory distress syndrome: a historical perspective. American Journal of Respiratory and Critical Care Medicine 2005;172(7):798-806. [DOI: 10.1164/rccm.200504-663OE ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernard 2017
- Bernard G. Acute Lung Failure — Our Evolving Understanding of ARDS. New England Journal of Medicine 2017;377(6):507-509. [DOI: 10.1056/NEJMp1706595] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bloomfield 2006
- Bloomfield R, Steel E, MacLennan G, Noble DW. Accuracy of weight and height estimation in an intensive care unit: implications for clinical practice and research. Critical Care Medicine 2006;34(8):2153-7. [PMID: 16763505 ] [DOI] [PubMed] [Google Scholar]
Bloomfield 2014
- Bloomfield R, Noble DW. Systematic review of prone positioning - study selection and analysis. Critical Care Medicine 2014;42(8):e598-9. [DOI: 10.1097/CCM.0000000000000380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Borenstein 2019
- Borenstein M. Common mistakes in meta-analysis and how to avoid to avoid them. 1st edition. New Jersey: Biostat Inc., 2019. [ISBN 978-1-7334367-1-7] [Google Scholar]
Chan 2008
- Chan M, Hsu J, Liu H, Lee Y, Pong S, Chan L, et al. Reply to Friederich et al. Journal of the Formosan Medical Association 2008;107(2):192. [Google Scholar]
Chang 2014
- Chang H-C, Chien H-T, Hwu J-Y. Effects of Prone Positioning on Oxygenation and Complications in Patients With Acute Respiratory Distress Syndrome (ARDS) in the Intensive Care Unit: A Systematic Review and Meta-Analysis [俯臥對加護病房急性呼吸窘迫症候群病人之氧合與合併症成效—系統性回顧暨統合分析]. Journal of Nursing & Healthcare Research 2014;10(3):178-189. [DOI: 10.6225/JNHR.10.3.178 ] [Google Scholar]
Chatte 1997
- Chatte G, Sab JM, Dubois JM, Sirodot M, Gaussorgues P, Robert D. Prone position in mechanically ventilated patients with severe acute respiratory failure. American Journal of Respiratory & Critical Care Medicine 1997;155(2):473-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cheifetz 2017
- Cheifetz IR. Pediatric ARDS. Respiratory Care 2017;62(6):718-31. [DOI: 10.4187/respcare.05591] [DOI] [PubMed] [Google Scholar]
Cheung 2020
- Cheung JCH, Lam PKN. Oxygen therapy in the ICU. New England Journal of Medicine 2020;382(26):2577. [DOI: 10.1056/NEJMc2009489] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiumello 2016
- Chiumello D, Coppola S, Froio S, Gotti M. What’s Next After ARDS: Long-Term Outcomes. Respiratory care 2016;61(5):689-99. [DOI: 10.4187/respcare.04644] [PMID: ] [DOI] [PubMed] [Google Scholar]
Claesson 2015
- Claesson J, Freundlich M, Gunnarsson I, Laake JH, Vandvik PO, Varpula T, et al. Scandinavian clinical practice guideline on mechanical ventilation in adults with the acute respiratory distress syndrome. Acta Anaesthesiologica Scandinavica 2015;59(3):286-97. [DOI: 10.1111/aas.12449] [PMID: ] [DOI] [PubMed] [Google Scholar]
Constantin 2019
- Constantin J-M, Jabaudon M, Lefrant J-Y, Jaber S, Quenot J-P, Langeron O, et al for the AZUREA network. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respiratory Medicine 2019;7(10):870-80. [DOI: 10.1016/S2213-2600(19)30138-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Counsell 1994
- Counsell CE, Clarke MJ, Slattery J, Sandercock PAG. The miracle of DICE therapy for acute stroke: fact or fictional product of subgroup analysis. BMJ 1994;309(6970):1677-81. [DOI: 10.1136/bmj.309.6970.1677] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cranshaw 2002
- Cranshaw J, Griffiths MJD, Evans TW. Non-ventilatory strategies in ARDS. Thorax 2002;57(9):823-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2013
- Cummings SR, Grady D, Hulley SB. Designing a randomized blinded trial. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB, editors(s). Designing Clinical Research. 4th edition. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins, 2013:145-149. [ISBN 978-1-60831-804-9] [Google Scholar]
Curley 1999
- Curley MAQ. Prone positioning of patients with acute respiratory distress syndrome: a systematic review. American Journal of Critical Care 1999;8(6):397-405. [PMID: ] [PubMed] [Google Scholar]
Dalmedico 2017
- Dalmedico MM, Salas D, Maciel de Oliveira A, Baran FDP, Meardi JT, Santos MC. Efficacy of prone position in acute respiratory distress syndrome: overview of systematic reviews. Revista da Escola de Enfermagem da USP (Journal of the School of Nursing, University of Sao Paulo) 2017;51:e03251. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org. New York: John Wiley & Sons, 2011. [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT Thomas J, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Second edition. Chichester: Wiley Blackwell, 2019:241-284. [ISBN 9781119536628] [Google Scholar]
de Hemptinne 2009
- Hemptinne Q, Remmelink M, Brimioulle S, Salmon I, Vincent J-L. ARDS: a clinicopathological confrontation. Chest 2009;135(4):944-9. [DOI: 10.1378/chest.08-1741] [PMID: ] [DOI] [PubMed] [Google Scholar]
Diaz 2010
- Diaz JV, Brower R, Calfee CS, Matthay MA. Therapeutic strategies for severe acute lung injury. Critical Care Medicine 2010;38(8):1644-50. [DOI: DOI: 10.1097/CCM.0b013e3181e795ee] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2018
- Du Y, Li Y, Sun R, Yuan B, Gao M, Wang Li. Meta analysis of observing prone position ventilation role in the oxygenation of severe pneumonia patients [俯卧位通气对重症肺炎患者氧合影响的Meta 分析]. Chinese Critical Care Medicine 2018;30(4):327-31. [DOI: 10.3760/cma.j.issn.2095-4352.2018.04.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faculty of Intensive Care 2019
- Bamford P, Denmore C, Newmarch C, Shirley P, Singer B, Webb S, et al. Guidance for: prone positioning in adult critical care. Faculty of Intensive Care Medicine (UK) 2019:1-40.
Fan 2010
- Fan E, Rubenfeld GD. High frequency oscillation in acute lung injury and ARDS. BMJ 2010;340:c2315. [DOI: 10.1136/bmj.c2315] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fan 2017
- Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ et al. An Official American Thoracic Society / European Society of Intensive Care Medicine / Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. American Journal of Respiratory & Crititical Care Medicine 2017;195(9):1253-63. [DOI: 10.1164/rccm.201703-0548ST] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2013
- Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. New England Journal of Medicine 2013;368(9):795-805. [DOI: DOI: 10.1056/NEJMoa1215554] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferrand 2001
- Ferrand E, Robert R, Ingrand P, Lemaire F, for the French LATAREA group. Withholding and withdrawal of life support in intensive-care units in France: a prospective survey. Lancet 2001;357(9249):9-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Festic 2016
- Festic E, Rawal B, Gajic O. How to improve assessment of balance in baseline characteristics of clinical trial participants - example from the PROSEVA trial data. Annals of Translational Medicine 2016;4(4):79. [DOI: 10.3978/j.issn.2305-5839.2016.01030] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fielding‐Singh 2018
- Fielding-Sing V, Mathay MA, Calfee CS. Beyond Low Tidal Volume Ventilation: Treatment Adjuncts for Severe Respiratory Failure in Acute Respiratory Distress Syndrome. Critical Care Medicine 2018;46(11):1820-31. [DOI: 10.1097/CCM.0000000000003406] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleiss 1986
- Fleiss JL. The Design and Analysis of Clinical Experiments. New York: John Wiley & Sons, 1986. [ISBN 0-471-82047-4] [Google Scholar]
Fletcher 2005
- Fletcher RW, Fletcher SW. Cause. In: Clinical Epidemiology. The Essentials. 4th edition. Philadelphia: Lippincott William & Wilkins, 2005:199-201. [ISBN 0-7817-5215-9] [Google Scholar]
Forbes 2013
- Forbes D. Blinding: an essential component in decreasing risk of bias in experimental designs. Evidence-Based Nursing 2013;16(3):70-1. [DOI: 10.1136/eb-2013-101382] [PMID: ] [DOI] [PubMed] [Google Scholar]
Frieden 2017
- Frieden HR. Evidence for Health Decision Making —Beyond Randomized, Controlled Trials. New England Journal of Medicine 2017;377(5):465-75. [DOI: 10.1056/NEJMra1614394] [PMID: ] [DOI] [PubMed] [Google Scholar]
Friedman 1998
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 3rd edition. New York: Springer Verlag, 1998. [ISBN 0-387-98586-7] [Google Scholar]
Gattinoni 2006
- Gattinoni L, Valenza F, Pelosi P, Mascheroni D. Prone positioning in acute respiratory failure. In: Tobin MJ, editors(s). Principles and Practice of Mechanical Ventilation. 2nd edition. New York: McGraw-Hill, 2006:1081-92. [Google Scholar]
Gattinoni 2010
- Gattinoni L, Carlesso E, Taccone P, Pollo F, Guerin C, Mancebo J. Prone positioning improves survival in ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiologica 2010;76(6):448-54. [PMID: ] [PubMed] [Google Scholar]
Gattinoni 2012
- Gattinoni L, Carlesso E, Caironi P. Stress and strain within the lung. Current Opinion in Critical Care 2012;18(1):42-7. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gattinoni 2013
- Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome: rationale, indications, and limits. American Journal of Respiratory Critical Care Medicine 2013;188(11):1286-93. [DOI: 10.1164/rccm.201308-1532CI ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gillies 2012
- Gillies D, Wells D, Bhandari AP. Positioning for acute respiratory distress in hospitalised infants and children. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD003645. [DOI: 10.1002/14651858.CD003645.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glantz 2005
- Glantz SA. Primer of biostatistics: The program version 6.0. 6th edition. New York: McGraw Hill, 2005. [ISBN 0-07-143822-X] [Google Scholar]
Goettler 2002
- Goettler CE, PryorJP, Reilly PM. Brachial plexopathy after prone positioning. Critical Care 2002;6(6):540-542.. [DOI: 10.1186/cc1823] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodwin 2012
- Goodwin AJ. Critical care clinical trials: getting off the roller coaster. Chest 2012;42(3):563-567. [DOI: 10.1378/chest.12-0519] [PMID: ] [DOI] [PubMed] [Google Scholar]
Graf 2008
- Graf J, Marini JJ. Do airway secretions play an under appreciated role in acute respiratory distress syndrome? Current Opinion in Critical Care 2008;14(1):44-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Graham 2020
- Graham PL, Moran JL. ECMO, ARDS and meta-analyses: Bayes to the rescue? Journal of Critical Care 2020;59:49-54. [DOI: 10.1016/j.jcrc.2020.05.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 2019
- Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respiratory Research 2019;6(1):e000420. [DOI: 10.1136/bmjresp-2019-000420] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gristina 2018
- Gristina GR, Baroncelli F, Vergano M. Forgoing life-sustaining treatments in the ICU. To withhold or to withdraw: is that the question? Minerva Anestesiologica 2018;84(6):756-65. [DOI: 10.23736/S0375-9393.18.12299-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guerin 2006
- Guerin C. Ventilation in the prone position in patients with acute lung injury/acute respiratory distress syndrome. Current Opinion in Critical Care 2006;12(1):50-4. [MEDLINE: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guerin 2014
- Guerin C, Baboi L, Richard JC. Mechanisms of prone positioning in acute respiratory distress syndrome. Intensive Care Medicine 2014;40(11):1634-42. [DOI: 10.1007/s00134-014-3500-8 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt, GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt G, Rennie D, Meade MO, Cook DJ. Users' Guides to the Medical Literature. Essentials of Evidence-Based Practice. 2nd Edition. Toronto: McGraw-Hill, 2008. [ISBN 978-0-07-159038-9] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Vistb G, Kunzc R, Brozeka J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [DOI: 10.1016/j.jclinepi.2010.07.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcocke J, Brozeka J, Helfand JM, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI: 10.1016/j.jclinepi.2011.03.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hanley 1983
- Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 1983;249(13):1743-5. [PMID: ] [PubMed] [Google Scholar]
Hashimoto 2017
- Hashimoto S, Sanui M, Egi M, Ohshimo S, Shiotsuka J, Seo R, et al. The clinical practice guideline for the management of ARDS in Japan. Journal of Intensive Care 2017;5:50. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatala 2005
- Hatala R, Keitz S, Wyer P, Guyatt G, Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2005;172(5):661-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernan 2017
- Hernan MA, Robins JM. Per-protocol analyses of pragmatic trials. New England Journal of Medicine 2017;377(14):1391-8. [DOI: 10.1056/NEJMsm1605385] [PMID: ] [DOI] [PubMed] [Google Scholar]
Herridge 2011
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine 2011;364(14):1293-304. [PMID: 21470008] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PMID: 12958120 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JC. Assessing risk of bias. In: Higgins JPT, Green S, editors(s). Cochrane Handbook of Systematic Reviews for Interventions. 5.1.0 edition. Chichester: Wiley, March 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks J. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. New York: Wiley-Blackwell, Version 5.1.0 [updated March 2011]. [Google Scholar]
Ho 2020
- Ho, ATN, Patolia S, Guervilly C. Neuromuscular blockade in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Journal of Intensive Care 2020;8:12. [DOI: 10.1186/s40560-020-0431-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hough 2012
- Hough CL, Herridge MS. Long-term outcome after acute lung injury. Current Opinion in Critical Care 2012;18(1):8-15. [DOI: 10.1097/MCC.0b013e32834f186d] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hu 2014
- Hu SL, He HL, Pan C, Liu AR, Liu SQ, Liu L, et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Critical Care 2014;18(3):R109. [DOI: 10.1186/cc13896] [PMID: 24887034 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iftikhar 2015
- Iftikhar IH, Donley MA, Owens WB. Prone positioning in acute respiratory distress syndrome. Critical Care Medicine 2015;43(2):e55-6. [DOI: 10.1097/CCM.0000000000000761] [PMID: ] [DOI] [PubMed] [Google Scholar]
IntHout 2014
- IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straight forward and considerably outperforms the standard DerSimonian-Laird method. BMC Medical Research Methodology 2014;14:25. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2006
- Ioannidis JP, Mulrow CD, Goodman SN. Adverse events: the more you search, the more you find. Annals of Internal Medicine 2006;114(4):298-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2016
- Ioannidis JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. The Milbank Quarterly 2016;94(3):485-514. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwashyna 2010
- Iwasyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Annals of Internal Medicine 2010;153(3):204-5. [DOI: ] [PMID: 20679565] [DOI] [PubMed] [Google Scholar]
Jadad 2007
- Jadad AR, Enkin MW. Bias in Randomized Controlled Trials. In: Randomized Controlled Trials - Questions, Answers and Musings. 2nd edition. Oxford: Blackwell, BMJ Books, 2007:29-47. [ISBN 978-1-4051-3266-4] [Google Scholar]
Karanicolas 2010
- Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how? Canadian Journal of Surgery 2010;53(5):345-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Klein 2004
- Klein Y, Blackbourne L, Barquist ES. Non-ventilatory-based strategies in the management of acute respiratory distress syndrome. Journal of Trauma, Injury, Infection and Critical Care 2004;57(4):915-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Klompas 2008
- Klompas M, Kulldorff M, Platt R. Risk of misleading ventilator-associated pneumonia rates with use of standard clinical and microbiological criteria. Clinical Infectious Diseases 2008;46(9):1443-6. [DOI: 10.1086/587103] [PMID: ] [DOI] [PubMed] [Google Scholar]
KNAW 2018
- KNAW (Royal Netherlands Academy of Arts and Sciences). Replication studies – Improving reproducibility in the empirical sciences. Amsterdam: KNAW, 2018. [ISBN 978-90-6984-720-7] [Google Scholar]
Kneyber 2014
- Kneyber MCJ Zhan H, Slutsky AS. Ventilator-induced lung injury. Similarity and differences between children and adults. American Journal of Respiratory and Critical Care Medicine 2014;190(3):258-65. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kopterides 2009
- Kopterides P, Siempos II, Armaganidis A. Prone positioning in hypoxemic respiratory failure: meta-analysis of randomized controlled trials. Journal of Critical Care 2009;24(1):89-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lagakos 2006
- Lagakos SW. The challenge of subgroup analyses - Reporting without distorting. New England Journal of Medicine 2006;354:1667-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lamas 2014
- Lamas D. Chronic critical illness. New England Journal of Medicine 2014;370(2):175-7. [DOI: 10.1056/NEJMms1310675] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee JM, Bae W, Lee YJ, Cho Y-J. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Critical Care Medicine 2014;42(5):1252-62. [DOI: 10.1097/CCM.0000000000000122] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. [AVAILABLE AT:: https://training.cochrane.org/handbook/current/chapter-04] [Google Scholar]
Lipsey 1990
- Lipsey MW. Design sensitivity: Statistical power for experimental research. London: Sage, 1990. [Google Scholar]
MacCallum 2005
- MacCallum NS, Evans TE. Epidemiology of acute lung injury. Current Opinion in Critical Care 2005;11(1):43-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marini 2008
- Marini JJ. Lung injury: settle for a sketch or design a blueprint? Critical Care Medicine 2008;36(10):2922-5. [DOI: 10.1097/CCM.0b013e318186a443] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marini 2010
- Marini JJ. Can we prevent the spread of focal lung inflammation? Critical Care Medicine 2010;38(10 Suppl):S574-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marini 2020
- Marini JJ Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA 2020;323(22):2329-30. [DOI: 10.1001/jama.2020.6825] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mark 2015
- Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Medicine 2015;41(9):1572-85. [DOI: 10.1007/s00134-015-3810-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Matthay 2019
- Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nature Review Disease Primers 2019;5(1):18. [DOI: 10.1038/s41572-019-0069-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mentzelopoulos 2005
- Mentzelopoulos SD, Roussos C, Zakynthinos SG. Prone position reduces lung stress in severe acute respiratory distress syndrome. European Respiratory Journal 2005;25(3):534-44. [MEDLINE: 15738300 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI: 10.1136/bmj.b2535] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mora‐Arteaga 2015
- Mora-Arteaga JA, Bernal-Ramírez OJ, Rodríguez SJ. The effects of prone position ventilation in patients with acute respiratory distress syndrome. A systematic review and metaanalysis. Medicina Intensiva 2015;39(6):359-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2014
- Morgan CK, Varas GM, Pedroza C, Almoosa KF. Defining the practice of “No Escalation of Care” in the ICU. Critical Care Medicine 2014;42(2):357-61. [DOI: 10.1097/CCM.0b013e3182a276c9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Munshi 2017
- Munshi L, Del Sorbo L, Adhikari NKJ, Hodgson CL, Wunsch H, Meade MO, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Annals of the American Thoracic Society 2017;14(4 Suppl):S280-8. [DOI: DOI: 10.1513/AnnalsATS.201704-343OT] [DOI] [PubMed] [Google Scholar]
Mure 2001
- Mure M, Lindahl SGE. Prone position improves gas exchange – but how? Acta Anaesthesiologica Scandinavica 2001;45(2):150-9. [PMID: ] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santessoa N, Brozeka J, Akla EA, Waltera SD, Normana G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42. [DOI: 10.1016/j.jclinepi.2013.02.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Møller 2017
- Møller MH, Ioannidis JPA, Darmon M. Are systematic reviews and meta‑analyses still useful research? We are not sure. Intensive Care Medicine 2018;44(4):518-20. [DOI: 10.1007/s00134-017-5039-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Needham 2012
- Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Himmelfarb CRD, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ 2012;344:e2124. [DOI: 10.1136/bmj.e2124 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noah 2011
- Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011;306(15):1659-68. [PMID: 21976615 ] [DOI] [PubMed] [Google Scholar]
Noble 2004
- Noble DW. Practice variations in acute respiratory distress syndrome: detrimental ignorance or healthy skepticism? Critical Care Medicine 2004;32(4):1079-80. [DOI: 10.1097/01.CCM.0000121429.21527.91] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noble 2010
- Noble DW, Peek GJ. Extracorporeal membrane oxygenation for respiratory failure: past, present and future. Anaesthesia 2010;65(10):971-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Brien 2011
- O'Brien JM, Prescott HC. More randomized controlled trials in acute lung injury? Not so fast, my friend. Critical Care Medicine 2011;39(12):2763-4. [DOI: 10.1097/CCM.0b013e31822a5a56] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Oxman 2012
- Oxman A. Subgroup analyses: The devil is in the interpretation. BMJ 2012;344:e2022. [DOI: 10.1136/bmj.e2022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Packer 2016
- Packer M. The room where it happens: a skeptic’s analysis of the New Heart Failure Guidelines. Journal of Cardiac Failure 2016;22(9):726-30. [DOI: 10.1016/j.cardfail.2016.07.433 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papazian 2010
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. New England Journal of Medicine 2010;363(12):1107-16. [PMID: 20843245 ] [DOI] [PubMed] [Google Scholar]
Park 2015
- Park SY, Kim HJ, Yoo KH, Park YB, Kim SW, Lee SJ, et al. The efficacy and safety of prone positioning in adults patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Journal of Thoracic Diseases 2015;7(3):356-67. [DOI: 10.3978/j.issn.2072-1439.2014.12.49] [PMID: 25922713] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrucci 2013
- Petrucci N, DeFeo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD003844. [DOI: 10.1002/14651858.CD003844.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phua 2009
- Phua J, Badia JR, Adhikari NKJ, Friedrich JO, Fowler RA, Singh JM, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. American Journal of Respiratory & Critical Care Medicine 2009;179(3):220-7. [DOI: 10.1164/rccm.200805-722OC ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Porta 2008
- Porta M (ed). A Dictionary of Epidemiology. 5th edition. Oxford: Oxford University Press, 2008. [Google Scholar]
Psaty 2010
- Psaty BM, Prentice RL. Minimizing bias in randomized trials: the importance of blinding. JAMA 2010;304(7):793-4. [MEDLINE: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reade 2008
- Reade MC, Delaney A, Bailey MJ, Angus DC. Bench-to-bedside review: avoiding pitfalls in critical care meta-analysis - funnel plots, risk estimates, types of heterogeneity, baseline risk and the ecologic fallacy. Critical Care 2008;12(4):220. [DOI: 10.1186/cc6941] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reade 2010
- Reade MC, Delaney A, Bailey MJ, Harrison DA, Yealy DM, Jones PG, et al. Prospective meta-analysis using individual patient data in intensive care medicine. Intensive Care Medicine 2010;36(1):11-21. [DOI: ] [PMID: 19760395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reilly 2019
- Reilly JP, Calfee CS, Christie JD. Acute Respiratory Distress Syndrome Phenotypes. Seminars in Respiratory & Critical Care Medicine 2019;40(1):19-30. [DOI: 10.1055/s-0039-1684049] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.40 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.40 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rezoagli 2017
- Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Annals of Translational Medicine 2017;5(14):282. [DOI: 10.21037/atm.2017.06.62] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2010
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct and reporting. BMJ 2010;340:c221. [DOI: 10.1136/bmj.c221] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rivas‐Fernandez 2016
- Rivas-Fernandez M, Roqué i Figuls M, Diez-Izquierdo A, Escribano J, Balaguer A. Infant position in neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD003668. [DOI: 10.1002/14651858.CD003668.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robba 2020
- Robba C, Battaglinia D, Balla L, Patronitia N, Locontea M, Brunetti L et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respiratory Physiology & Neurobiology 2020;279:103455. [DOI: 10.1016/j.resp.2020.103455] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubenfeld 2007
- Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest 2007;131(2):554-62. [DOI: 10.1378/chest.06-1976] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sheiner 1995
- Sheiner LB, Rubin DB. Intention-to-treat-analysis and the goals of clinical trials. Clinical Pharmacology and Therapeutics 1995;57(1):6-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sim 2014
- Sim YS, Jung H, Shin TR, Kim DG, Park SM. The efficacy and safety of prone positioning in adult patients with acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. Respirology (2014) 19 (Suppl. 3), 63–253 2014;19(Suppl 3):79. [DOI: doi: 10.1111/resp.12417] [Google Scholar]
Slutsky 2013
- Slutsky AS, Ranieri VM. Ventilator- induced lung injury. New England Journal of Medicine 2013;369(22):2126-36. [DOI: DOI: 10.1056/NEJMra1208707] [PMID: ] [DOI] [PubMed] [Google Scholar]
Soni 2008
- Soni N, Williams P. Positive pressure ventilation: what is the real cost? British Journal of Anaesthesia 2008;101(4):446-57. [DOI: 10.1093/bja/aen240] [PMID: ] [DOI] [PubMed] [Google Scholar]
Soni 2010
- Soni N. ARDS, acronyms and the Pinocchio effect. Anaesthesia 2010;65(10):976-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soo Hoo 2013
- Soo Hoo GW. In prone ventilation, one good turn deserves another. New England Journal of Medicine 2013;368(23):2227-8. [DOI: 10.1056/NEJMe1304349] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stapleton 2005
- Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 2005;128(2):525-32. [PMID: 16100134] [DOI] [PubMed] [Google Scholar]
Stevens 2014
- Stevens JP, Kachniarz B, Wright SB, Gillis J, Talmor D, Clardy P, et al. When policy gets it right: variability in U.S. hospitals’ diagnosis of ventilator-associated pneumonia. Critical Care Medicine 2014;42(3):497-503. [DOI: 10.1097/CCM.0b013e3182a66903] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2015
- Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al for the PRISMA-IPD Development Group. Preferred reporting items for a systematic review and meta-analysis of Individual participant data. The PRISMA-IPD Statement. JAMA 2015;313(16):1657-65. [DOI: 10.1001/jama.2015.3656] [PMID: ] [DOI] [PubMed] [Google Scholar]
Strachan 2001
- Strachan L, Noble DW. Hypoxia and surgical patients - prevention and treatment of an unnecessary cause of morbidity and mortality. Journal of The Royal College of Surgeons of Edinburgh 2001;46(5):297-302. [PMID: ] [PubMed] [Google Scholar]
Strom 2006
- Strom BL. How the US drug safety system should be changed. JAMA 2006;295(17):2072-5. [DOI: 10.1001/jama.295.17.2072] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sud 2008
- Sud S, Sud M, Friedrich JO, Adhikari NK. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. CMAJ Canadian Medical Association Journal 2008;178(9):1153-61. [PMID: 18427090 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sud 2008a
- Sud S, Sud M, Friedrich JO, Adhikari NKJ. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2008;178(9):1153-61. [DOI: 10.1503/cmaj.071802 ] [PMID: 18427090 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sud 2008b
- Sud S, Sud M, Friedrich J, Shing L-K, Adhikari NKJ. Effect of prone positioning in patients with acute respiratory distress syndrome and high Simplified Acute Physiology Score II. Critical Care Medicine 2008;36(9):2711-2. [DOI: 10.1097/CCM.0b013e3181846fc0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sud 2010
- Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NKJ, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Medicine 2010;36(4):585-99. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sud 2011
- Sud S, Cuthbertson BH. Understanding health economic analysis in critical care: insights from recent randomized controlled trials. Current Opinion in Critical Care 2011;17(5):504-9. [DOI: 10.1097/MCC.0b013e32834a4bc1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sud 2011a
- Sud S, Friederich J, Sud M, Adhikari N. Meta-Analysis, Meta-Regression, And Patient- And Trial- Level Data Subgroup Analysis: An Example of Ecological Bias In A Meta-Analysis Of Prone Ventilation In Patients With Severe Hypoxemic RespiratoryFailure Due To ALI/ARDS. American Journal of Respiratory and Critical Care Medicine 2011;183:A3738. [Google Scholar]
Sud 2013
- Sud S, Sud M, Friederich JO, Wunsch H, Meade MO, Ferguson ND, et al. High-frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD004085. [DOI: 10.1002/14651858.CD004085.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sud 2014
- Sud S, Friedrich JO, Adhikari NK, Taccone P, Mancebo J, Polli F, et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2014;186(10):E381-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2014
- Sun X, Ioannidis JPA, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users' guide to the medical literature. JAMA 2014;311(4):405-11. [DOI: 10.1001/jama.2013.285063] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tabula 2015
- Tabula J, Ordillo LS, Remalante PP, Wang A. The effect of prone versus supine positioning in mortality among adult patients with moderate to severe acute respiratory distress syndrome: a meta-analysis. Intensive Care Medicine Experimental 2015;3(Suppl 1):A93. [DOI: ] [Google Scholar]
Tekwani 2014
- Tekwani S, Murugan R. ‘To prone or not to prone’ in severe ARDS:questions answered, but others remain. Critical Care 2014;18(3):305. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
The Academy of Medical Sciences 2015
- Bishop D, Cantrell D, Johnson P, Kapur S, Macleod M, Savage C et al. Reproducibility and reliability of biomedical research: Improving research practice - Symposium Report. The Academy of Medical Sciences 2015:1-77.
Thomas 2013
- Thomas NJ, Jouvet P, Willson D. Acute lung injury in children - kids really aren't just "little adults". Pediatric Critical Care Medicine 2013;14(4):429-32. [DOI: 10.1097/PCC.0b013e31827456aa] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tierney 2019
- Tierney JF, Stewart LA, Clarke M. Ch 26: Individual Participant Data. In: Higgins JPT Thomas J, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester: Wiley Blackwell, 2019:643-658. [ISBN 9781119536628] [Google Scholar]
Tiruvoipati 2008
- Tiruvoipati R, Bangash M, Manktelow B, Peek GJ. Efficacy of prone ventilation in adult patients with acute respiratory failure: a meta-analysis. Journal of Critical Care 2008;23(1):101-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tonelli 2014
- Tonelli AR, Zein J, Adams J, Ioannidis JPA. Effects of Interventions on Survival in Acute Respiratory Distress Syndrome: an Umbrella Review of 159 Published Randomized Trials and 29 Meta-analyses. Intensive Care Medicine 2014;40(6):769-87. [DOI: 10.1007/s00134-014-3272-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnbull 2014
- Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Critical Care Medicine 2014;42(2):296-302. [DOI: 10.1097/CCM.0b013e3182a272db] [PMID: 23989178 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uribe 2012
- Uribe AA, Baig MA, Puente EG, Viloria A, Mendel E, Bergese SD. Current intraoperative devices to reduce visual loss after spine surgery. Neurosurgical Focus 2012;33(2):E14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Venet 2003
- Venet C, Guyomarc'h S, Pingat J, Michard C, Laporte S, Bertrand M, et al. Prognostic factors in acute respiratory distress syndrome: a retrospective multivariate analysis including prone positioning in management strategy. Intensive Care Medicine 2003;29(9):1435-41. [PMID: 12827238] [DOI] [PubMed] [Google Scholar]
Verbrugge 2007
- Verbrugge SJC, Lachmann B, Kesecioglu J. Lung protective ventilatory strategies in acute lung injury and acute respiratory distress syndrome: from experimental findings to clinical application. Clinical Physiology and Functional Imaging 2007;27(2):67-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walkey 2012
- Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clinical Epidemiology 2012;4:159-69. [DOI: 10.2147/CLEP.S28800] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2014
- Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Medicine 2014;40(3):388-96. [DOI: 10.1007/s00134-013-3186-3] [PMID: 24435201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 2000
- Ware LB, Matthay MA. Acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1334-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weissman 1997
- Weissman C. Analyzing intensive care unit length of stay data: problems and possible solutions. Critical Care Medicine 1997;25(9):1594-600. [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, et al. Determinants of long-term survival after intensive care. Critical Care Medicine 2008;36(5):1523-30. [DOI: 10.1097/CCM.0b013e318170a405] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilson 2020
- Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Critical Care 2020;24(1):102. [DOI: 10.1186/s13054-020-2778-x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wunsch 2010
- Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA 2010;303(9):849-56. [DOI: 10.1001/jama.2010.216] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yankech 2017
- Yankech L, Campbell C. Does the use of prone positioning improve survival in patients with ARDS. Canadian Journal of Respiratory Therapy 2017;53 (poster abstracts)(3):53. [Google Scholar]
Young 2013
- Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High-frequency oscillation for acute respiratory distress syndrome. New England Journal of Medicine 2013;368(9):806-13. [DOI: DOI: 10.1056/NEJMoa1215716] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yue 2017
- Yue W, Zhang Z, Zhang C, YangL, He J, Hou Y, et al. Effect of prone positioning ventilation for mortality in severe acute respiratory distress syndrome patients: a cumulative meta-analysis [俯卧位通气治疗急性呼吸窘迫综合征患者病死率的累积 Meta 分析]. Chinese Journal of Evidence-based Medicine 2017;17(7):792-7. [DOI: 10.7507/1672-2531.201702002] [DOI] [Google Scholar]
Zhang 2008
- Zhang D, Yin P, Freemantle N, Jordan R, Zhong, Cheng KK. An assessment of the quality of randomised controlled trials conducted in China. Trials 2008;9:22. [DOI: 10.1186/1745-6215-9-22] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bloomfield 2009
- Bloomfield R, Noble DW, Webster NR. Prone position for acute respiratory failure in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD008095. [DOI: 10.1002/14651858.CD008095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloomfield 2015
- Bloomfield R, Noble DW, Sudlow A. Prone position for acute respiratory failure in adults. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD008095. [ART. NO.: ] [DOI: 10.1002/14651858.CD008095.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


