Abstract
Background
Hospital charges for lumbar spinal stenosis have increased significantly worldwide in recent times, with great variation in the costs and rates of different surgical procedures. There have also been significant increases in the rate of complex fusion and the use of spinal spacer implants compared to that of traditional decompression surgery, even though the former is known to incur costs up to three times higher. Moreover, the superiority of these new surgical procedures over traditional decompression surgery is still unclear.
Objectives
To determine the efficacy of surgery in the management of patients with symptomatic lumbar spinal stenosis and the comparative effectiveness between commonly performed surgical techniques to treat this condition on patient‐related outcomes. We also aimed to investigate the safety of these surgical interventions by including perioperative surgical data and reoperation rates.
Search methods
Review authors performed electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, AMED, Web of Science, LILACS and three trials registries from their inception to 16 June 2016. Authors also conducted citation tracking on the reference lists of included trials and relevant systematic reviews.
Selection criteria
This review included only randomised controlled trials that investigated the efficacy and safety of surgery compared with no treatment, placebo or sham surgery, or with another surgical technique in patients with lumbar spinal stenosis.
Data collection and analysis
Two reviewers independently assessed the studies for inclusion and performed the 'Risk of bias' assessment, using the Cochrane Back and Neck Review Group criteria. Reviewers also extracted demographics, surgery details, and types of outcomes to describe the characteristics of included studies. Primary outcomes were pain intensity, physical function or disability status, quality of life, and recovery. The secondary outcomes included measurements related to surgery, such as perioperative blood loss, operation time, length of hospital stay, reoperation rates, and costs. We grouped trials according to the types of surgical interventions being compared and categorised follow‐up times as short‐term when less than 12 months and long‐term when 12 months or more. Pain and disability scores were converted to a common 0 to 100 scale. We calculated mean differences for continuous outcomes and relative risks for dichotomous outcomes. We pooled data using the random‐effects model in Review Manager 5.3, and used the GRADE approach to assess the quality of the evidence.
Main results
We included a total of 24 randomised controlled trials (reported in 39 published research articles or abstracts) in this review. The trials included 2352 participants with lumbar spinal stenosis with symptoms of neurogenic claudication. None of the included trials compared surgery with no treatment, placebo or sham surgery. Therefore, all included studies compared two or more surgical techniques. We judged all trials to be at high risk of bias for the blinding of care provider domain, and most of the trials failed to adequately conceal the randomisation process, blind the participants or use intention‐to‐treat analysis. Five trials compared the effects of fusion in addition to decompression surgery. Our results showed no significant differences in pain relief at long‐term (mean difference (MD) ‐0.29, 95% confidence interval (CI) ‐7.32 to 6.74). Similarly, we found no between‐group differences in disability reduction in the long‐term (MD 3.26, 95% CI ‐6.12 to 12.63). Participants who received decompression alone had significantly less perioperative blood loss (MD ‐0.52 L, 95% CI ‐0.70 L to ‐0.34 L) and required shorter operations (MD ‐107.94 minutes, 95% CI ‐161.65 minutes to ‐54.23 minutes) compared with those treated with decompression plus fusion, though we found no difference in the number of reoperations (risk ratio (RR) 1.25, 95% CI 0.81 to 1.92). Another three trials investigated the effects of interspinous process spacer devices compared with conventional bony decompression. These spacer devices resulted in similar reductions in pain (MD ‐0.55, 95% CI ‐8.08 to 6.99) and disability (MD 1.25, 95% CI ‐4.48 to 6.98). The spacer devices required longer operation time (MD 39.11 minutes, 95% CI 19.43 minutes to 58.78 minutes) and were associated with higher risk of reoperation (RR 3.95, 95% CI 2.12 to 7.37), but we found no difference in perioperative blood loss (MD 144.00 mL, 95% CI ‐209.74 mL to 497.74 mL). Two trials compared interspinous spacer devices with decompression plus fusion. Although we found no difference in pain relief (MD 5.35, 95% CI ‐1.18 to 11.88), the spacer devices revealed a small but significant effect in disability reduction (MD 5.72, 95% CI 1.28 to 10.15). They were also superior to decompression plus fusion in terms of operation time (MD 78.91 minutes, 95% CI 30.16 minutes to 127.65 minutes) and perioperative blood loss (MD 238.90 mL, 95% CI 182.66 mL to 295.14 mL), however, there was no difference in rate of reoperation (RR 0.70, 95% CI 0.32 to 1.51). Overall there were no differences for the primary or secondary outcomes when different types of surgical decompression techniques were compared among each other. The quality of evidence varied from 'very low quality' to 'high quality'.
Authors' conclusions
The results of this Cochrane review show a paucity of evidence on the efficacy of surgery for lumbar spinal stenosis, as to date no trials have compared surgery with no treatment, placebo or sham surgery. Placebo‐controlled trials in surgery are feasible and needed in the field of lumbar spinal stenosis. Our results demonstrate that at present, decompression plus fusion and interspinous process spacers have not been shown to be superior to conventional decompression alone. More methodologically rigorous studies are needed in this field to confirm our results.
Plain language summary
Effectiveness of surgery for people with leg or back pain due to symptomatic spinal stenosis
Review question
How well do different types of surgery work for lumbar spinal stenosis?
Background
Spinal stenosis is the narrowing of the spinal canal in the lower back region caused by thickening of the soft tissues and bones. It is a common condition for which surgery is usually performed after non‐surgical treatments (such as physiotherapy) have failed to bring sufficient relief to patients. Spinal stenosis is a common cause of low back pain that radiates to the legs, and it is more common in older adults. Surgery for lumbar spinal stenosis normally involves taking pressure off the spinal cord or spinal nerves (known as decompression) by removing bone and soft tissues from around the spinal canal. Another common surgical approach is to fuse two or more vertebrae together after decompression in the patient whose spine seems to be unstable. The usefulness of some types of surgery for lumbar spinal stenosis, however, has been questioned, and previous studies have reported that patients who receive fusion are more likely to have major complications and higher costs when compared with patients who undergo decompression only. More recently, spinal implants were created to help indirectly reduce pressure in the spinal canal and at the same time stabilise the bones. However, these implants have also been linked to worse outcomes (e.g., higher reoperation rates) when compared to conventional decompression.
Search date
This review includes all trials published up to June 2016.
Study characteristics
We included all trials that compared any surgical technique with no surgery or placebo surgery, and also trials comparing different surgical techniques with each other, including fusion and spinal implants. All the patients included in these studies were diagnosed with lumbar spinal stenosis and had symptoms in the leg or thigh that worsened by walking or standing and were generally relieved by a change in position, such as bending forward or sitting. The main measure we used to compare how well the different types of surgery worked was how much less pain people felt as they went about their daily lives. We also looked at whether their leg pain improved, how much blood they lost during surgery, how long the surgery took, how long they had to stay in hospital, how many patients had to have another operation for the problem and how much the treatment cost.
Key results and quality of the evidence
Twenty‐four randomised controlled trials were included with a total of 2352 people. We did not find trials that compared surgery with no treatment or placebo surgery, so all included trials compared different surgical techniques. The quality of the evidence from these studies varied from very low quality to high quality. This large variation was mainly due to different study protocols, surgical techniques and quality of reporting according to the 'Risk of bias' assessment. We found that patients who had decompression plus fusion fared no better than those who underwent decompression surgery alone. In fact, decompression plus fusion resulted in more blood loss during surgery than decompression alone. Although the spinal spacers were slightly better than decompression plus fusion in terms of improvements on daily activities, there were no differences when they were compared with decompression alone. Finally, we found no differences between different forms of decompression.
Summary of findings
Summary of findings for the main comparison. SUMMARY OF FINDINGS FOR DECOMPRESSION VERSUS FUSION.
| Decompression alone compared with decompression plus fusion for lumbar spinal stenosis | ||||||
|
Patient or population: patients with lumbar spinal stenosis Settings: inpatient care Intervention: decompression alone Comparison: decompression plus fusion | ||||||
| Outcomes | Comparisons | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Decompression | Decompression with fusion | |||||
|
Pain Long‐term (≥ 12 months) Pain scores converted to 0 to 100 scale to allow for comparison of different disability scales (VAS, NRS) |
The mean pain score ranged across decompression groups from 9.50 to 48.10 points | The mean pain in the decompression with fusion groups was 0.29 higher (6.74 lower to 7.32 higher) | Mean difference ‐0.29 (‐7.32, 6.74) | 380 (4) | ⊕⊝⊝⊝ Very low | The difference is not statistically or clinically significant |
|
Disability Long‐term (≥ 12 months) Disability scores converted to 0 to 100 scale to allow for comparison of different disability scales (RMDQ, ODI, JOA) |
The mean pain score ranged across decompression groups from 17.90 to 56.29 points | The mean disability score in the decompression with fusion group was 3.26 lower (6.12 lower to 12.63 higher) | Mean difference 3.26 (‐6.12, 12.63) | 335 (3) | ⊕⊝⊝⊝ Very low | The difference is not statistically or clinically significant |
|
Operation time Duration of operation reported in minutes |
The mean operation time ranged across decompression groups from 88.46 minutes to 124.40 minutes | The mean operation time in the decompression with fusion groups was 107.94 higher (54.23 to 161.65 higher) | Mean difference ‐107.94 (‐161.65, ‐54.23) | 381 (4) | ⊕⊝⊝⊝ Very low | The difference is clinically significant |
|
Blood loss Amount of perioperative blood loss reported in L |
The mean perioperative blood loss ranged across decompression groups from 0.08 to 0.34 L | The mean perioperative blood loss in the decompression with fusion groups was 0.52 L higher (0.34 L to 0.70 L higher) | Mean difference ‐0.52 (‐0.70, ‐0.34) | 383 (4) | ⊕⊝⊝⊝ Very low | The difference is clinically significant |
|
Reoperations Number of patients requiring a revision surgery |
36 of 185 (19 per 100) participants had reoperation | 38 of 258 (15 per 100) participants had reoperation | Risk ratio 1.25 (0.81, 1.92) | 443 (5) | ⊕⊕⊕⊝ Moderate | The difference is not statistically or clinically significant |
| CI: confidence interval; VAS: visual analogue scale; NRS: numerical rating scale; RMDQ: Roland‐Morris Disability Questionnaire; ODI: Oswestry Disability Index; JOA: Japanese Orthopedic Association | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. SUMMARY OF FINDINGS FOR DECOMPRESSION VERSUS INTERSPINOUS SPACERS.
| Decompression compared with interspinous spacers for lumbar spinal stenosis | ||||||
|
Patient or population: patients with lumbar spinal stenosis Settings: inpatient care Intervention: decompression Comparison: interspinous process spacer devices | ||||||
| Outcomes | Comparisons | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Decompression | Interspinous spacers | |||||
|
Pain Long‐term (≥ 12 months) Pain scores converted to 0 to 100 scale to allow for comparison of different disability scales (VAS, NRS) |
The mean pain score ranged across decompression groups from 21.65 to 32.00 points | The mean pain score in the interspinous spacers groups was 0.55 higher (6.99 lower to 8.08 higher) | Mean difference ‐0.55 (‐8.08, 6.99) | 328 (3) | ⊕⊕⊕⊝ Moderate | The difference is not statistically or clinically significant |
|
Disability Long‐term (≥ 12 months) Disability scores converted to 0 to 100 scale to allow for comparison of different disability scales (RMDQ, ODI, JOA) |
The mean disability score ranged across decompression groups from 18.30 to 45.00 points | The mean disability score in the interspinous spacers groups was 1.25 lower (6.98 lower to 4.48 higher) | Mean difference 1.25 (‐4.48, 6.98) | 327 (3) | ⊕⊕⊝⊝ Low | The difference is not statistically or clinically significant |
|
Operation time Duration of operation reported in minutes |
The mean operation time ranged across decompression groups from 43.00 to 112.90 minutes | The mean operation time in the interspinous spacers groups was 39.11 minutes lower (19.43 to 58.78 lower) | Mean difference 39.11 (19.43, 58.78) | 340 (3) | ⊕⊕⊝⊝ Low | The difference is clinically significant |
|
Blood loss Amount of perioperative blood loss reported in mL |
The mean perioperative blood loss in the decompression group was 184 mL | The mean perioperative blood loss in the interspinous spacers group was 144 mL lower (209.74 mL lower to 497.74 mL higher) | Mean difference 144.00 (‐209.74, 497.74) | 81 (1) | ⊕⊕⊝⊝ Low | The difference is not statistically or clinically significant |
|
Reoperations Number of patients requiring a revision surgery |
11 of 163 (7 per 100) participants had reoperation | 44 of 163 (27 per 100) participants had reoperation | Risk ratio 0.25 (0.14, 0.47) | 326 (3) | ⊕⊕⊕⊕ High | The difference is clinically significant |
| CI: confidence interval; VAS: visual analogue scale; NRS: numerical rating scale; RMDQ: Roland‐Morris Disability Questionnaire; ODI: Oswestry Disability Index; JOA: Japanese Orthopedic Association | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. SUMMARY OF FINDINGS FOR FUSION VERSUS INTERSPINOUS SPACERS.
| Decompression plus fusion compared with interspinous spacers for lumbar spinal stenosis | ||||||
|
Patient or population: patients with lumbar spinal stenosis Settings: inpatient care Intervention: decompression plus fusion Comparison: interspinous process spacer devices | ||||||
| Outcomes | Comparisons | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Decompression and fusion | Interspinous spacer devices | |||||
|
Pain Long‐term (≥ 12 months) Pain scores converted to 0 to 100 scale to allow for comparison of different disability scales (VAS, NRS) |
The mean pain score ranged across fusion groups from 24.10 to 35.50 points | The mean pain score in the interspinous spacers groups was 5.35 lower (11.88 lower to 1.18 higher) | Mean difference 5.35 (‐1.18, 11.88) | 308 (2) | ⊕⊕⊝⊝ Low | The difference is not statistically or clinically significant |
|
Disability Long‐term (≥ 12 months) Disability scores converted to 0 to 100 scale to allow for comparison of different disability scales (RMDQ, ODI, JOA) |
The mean disability score ranged across fusion groups from 26.70 to 34.50 points | The mean disability score in the interspinous spacers groups was 5.72 lower (1.28 to 10.15 lower) | Mean difference 5.72 (1.28, 10.15) | 308 (2) | ⊕⊕⊝⊝ Low | The difference is not clinically significant |
|
Operation time Duration of operation reported in minutes |
The mean operation time ranged across fusion groups from 150.00 to 153.20 minutes | The mean operation time in the interspinous spacers groups was 78.91 lower (30.16 to 127.65 lower) | Mean difference 78.91 (30.16, 127.65) | 381 (2) | ⊕⊝⊝⊝ Very low | The difference is clinically significant |
|
Blood loss Amount of perioperative blood loss reported in mL |
The mean perioperative blood loss in the fusion group was 348.60 mL | The mean perioperative blood loss in the interspinous spacers groups was 238.90 mL lower (182.66 to 295.14 mL lower) | Mean difference 238.90 (182.66, 295.14) | 320 (1) | ⊕⊕⊕⊝ Moderate | The difference is clinically significant |
|
Reoperations Number of patients requiring a revision surgery |
8 of 107 (7 per 100) participants had reoperation | 23 of 215 (11 per 100) participants had reoperation | Risk ratio 0.70 (0.32, 1.51) | 322 (1) | ⊕⊕⊕⊕ High | The difference is not statistically or clinically significant |
| CI: confidence interval; RR: risk ratio; VAS: visual analogue scale; NRS: numerical rating scale; RMDQ: Roland‐Morris Disability Questionnaire; ODI: Oswestry Disability Index; JOA: Japanese Orthopedic Association | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Lumbar spinal stenosis is a narrowing of the spinal canal or the intervertebral foramina by surrounding bone and soft tissues that compromises neural structures (Bailey 1911; Portal 1803). Although it can be an incidental finding (Boden 1990), lumbar spinal stenosis may cause leg or lower back symptoms and disability, particularly in the older population (Kalichman 2009; Katz 2008). Radiographic findings of spinal stenosis are highly prevalent among those older than 60 years of age and can be as high as 80% in specific populations (Ishimoto 2013). Only 30%, however, present severe lumbar stenosis and about 17% have long‐term symptoms of intermittent neurogenic claudication. Neurogenic claudication is the most important feature of lumbar spinal stenosis as it limits patients' walking ability and causes a major impact on their quality of life. Intermittent neurogenic claudication is defined as uni‐ or bilateral radicular pain during walking or standing that is relieved by sitting down or flexing the lumbar spine (Blau 1961).
The differential diagnosis from vascular intermittent claudication is sometimes challenging as poor circulation in the muscles of the legs might mimic neurogenic claudication. Pain sensation while standing and pain relief with lumbar flexion are important characteristics of neurogenic claudication that may help distinguish between these conditions. Lumbar spinal stenosis can be classified as primary (congenital) or secondary stenosis (degenerative, iatrogenic, spondylotic, post‐traumatic and miscellaneous; Arnoldi 1976;Katz 2008;Siebert 2009). It is also anatomically classified as central, lateral or foraminal and it can be a result of multiple factors, such as intervertebral disc protrusion, loss of intervertebral space height, hypertrophy of joint capsules and ligaments, and osteophytes (Siebert 2009).
Description of the intervention
Bony decompression by laminectomy was first described by Alban Smith (Smith 1829), and first reported in a patient with spinal stenosis in 1893 (Lane 1893). This surgical procedure is still considered the gold standard of surgery and the most common technique for lumbar spinal stenosis (Gibson 2005; Jansson 2003). After intubation and anaesthesia the patient is positioned prone on the operating table, and imaging techniques guide a midline or posterolateral muscle splitting incision. The paraspinal muscles are stripped to expose the lamina and retracted laterally. The surgeon performs partial removal of both osseous (vertebrae lamina, spinous process, facet joints) and soft tissue elements (posterior ligamentous complex), but at least 50% of each facet joint complex is preserved to avoid iatrogenic instability. In cases of instability, lumbar fusion may be necessary in addition to decompression (Taylor 1994), which usually involves the use of spinal implants to stabilise the fused segments, though recent trials have questioned this view (Forsth 2016; Ghogawala 2016). In the United States, the rate of fusion for lumbar spinal stenosis has increased significantly in recent times (Deyo 2010). However, this procedure is associated with higher reoperation rates, post‐surgical complications, and costs when compared with decompression alone (Deyo 2013). Furthermore, it is still debatable whether the addition of fusion is more effective than decompression alone. To overcome the complications associated with fusion, less invasive surgical techniques have been developed, such as the interspinous process spacer devices (Coflex, Paradigm Spine USA and X‐Stop, Medtronic Spine USA). These spacer devices were created to promote an indirect decompression and provide stabilisation while preserving the bony structures of the spinal column (Senegas 1991). However, the most recent evidence on this topic has shown that these spacer devices alone are not only more costly than conventional decompression, but are also associated with higher reoperation rates (Deyo 2013).
Alternatives to conventional decompression by laminectomy have been developed to minimise the damage on posterior structures of the lumbar spine. Minimally invasive decompressive techniques used to treat lumbar spinal stenosis include uni‐ or bilateral laminotomies and spinal process‐splitting laminectomy. These techniques are also frequently performed with the use of an endoscope or microscope. The bilateral laminotomy technique preserves the neural arch of the vertebrae and protects the dura. In multisegmental stenosis this technique allows the reattachment of the paravertebral muscles to the spinous processes. The surgeon partially removes the laminae and ligamentum flavum but preserves the facet joint complex and the muscles attached to it (Aryanpur 1988). Unilateral laminotomy refers to partial resection of the facets and the medial portion of the lamina, and complete removal of the ligamentum flavum (Spetzger 1997). This technique was developed to overcome the disadvantage of surgically induced instability (Spetzger 1997a). More recently, the spinous process‐splitting laminectomy was developed (Watanabe 2005). In this technique, the lamina is exposed by longitudinally splitting the spinous process into halves, allowing muscles and ligamentous attachments to be left intact. Recently, another Cochrane review showed that these posterior decompression techniques delivered no different results in terms of leg pain or disability reduction compared to conventional laminectomy (Overdevest 2015).
How the intervention might work
Increasing the cross‐sectional area of the spinal canal at the level of stenosis (decompression) may decrease pain that is generated from increased pressure on the nerves within the stenosed segment. The complete removal of the vertebrae lamina and spinal process in an extensive conventional laminectomy is, however, linked to postsurgical spinal instability (Abumi 1990; Hopp 1988; Lee 1983). Therefore, techniques that increase spinal stability after decompression, such as fusion, might have an advantage compared with decompression alone. In a conventional laminectomy procedure, the paraspinal muscles are detached extensively from the spinal processes, vertebrae lamina and facets. Such muscle damage is associated with significant atrophy of paraspinal muscles (Kawaguchi 1996; See 1975), and the spinal process‐splitting decompression technique has been proposed to preserve muscle integrity. In addition, other minimally invasive decompression techniques (e.g., uni‐ or bilateral laminotomies) preserve spinal integrity and are potentially capable of reducing postoperative complications such as muscle atrophy, weakness, postoperative pain, perioperative blood loss, operation time and length of hospital stay. Endoscopic assisted decompressive surgery has also been proposed to avoid scaring of the epidural space (Cooper 1991).
Why it is important to do this review
Surgery for lumbar spinal stenosis is believed to be more effective than conservative treatment when the latter has failed for up to six months (Kovacs 2011;May 2013). However, the most recent evidence does not confirm this belief. For instance, in the Spine Patient Outcomes Research Trial (SPORT) patients treated surgically did not report any difference in outcomes compared with those treated non‐surgically in the intention‐to‐treat analyses, although the as‐treated analyses showed statistically significant but small differences in terms of pain and function favouring surgery (Weinstein 2008). Further, a recent trial has also shown that surgical decompression yielded similar effects to a physiotherapy programme (Delitto 2015). In this review we did not include trials comparing surgery with non‐surgical interventions, because this is covered in another Cochrane review (Zaina 2016). Given most of the evidence supporting the use of surgery for lumbar spinal stenosis comes largely from trials comparing surgery with non‐surgical interventions, it is not possible to distinguish the specific effects of surgery from the effects of time, regression to the mean, or placebo effects (Flum 2006). Moreover, many surgical techniques are available for the management of lumbar spinal stenosis, and the lack of evidence to support the rapid evolution of surgical techniques has led clinicians to rely on their own opinions and experiences to choose the surgical technique for their patients (Katz 1997), which leads to practice variation. The conflicting results from current randomised trials (Cavusoglu 2007; Grob 1995; Stromqvist 2013), and the emerging evidence on this topic (Forsth 2016; Ghogawala 2016) demand a synthesis of the available evidence.
Objectives
To determine the efficacy of surgery (i.e., surgery versus no treatment, or placebo/sham surgery) in the management of patients with symptomatic lumbar spinal stenosis and the comparative effectiveness of commonly performed surgical techniques to treat this condition on patient‐related outcomes. We also aimed to investigate the safety of these surgical interventions by including perioperative surgical data and reoperation rates.
Methods
Criteria for considering studies for this review
Types of studies
We only included published randomised controlled trials.
Types of participants
The participants included in our review consisted of adults with symptomatic degenerative lumbar spinal stenosis, despite its anatomical classification (central, foraminal or lateral) or diagnostic criteria (physical examination or radiographic imaging). There were no restrictions regarding intensity or duration of symptoms. Studies of participants with trauma, tumour and previous spine surgery were excluded. As degenerative spondylolisthesis is a common finding in patients with lumbar spinal stenosis, only trials including participants with spondylolisthesis up to Meyerding grade I (translation of the cranial vertebra of up to 25%) were included (Meyerding 1932).
Types of interventions
We considered studies that compared the efficacy of surgery with no treatment, placebo or sham surgery. We also included trials that compared the effectiveness of different surgical techniques for lumbar spinal stenosis. However, trials comparing different fusion techniques or interspinous spacer devices, and surgery for cervical spinal stenosis, were excluded. We also excluded trials that compared surgery with non‐surgical interventions, as this is covered in another recent Cochrane review (Zaina 2016).
Types of outcome measures
We included patient‐centred outcomes of clinical relevance, as well as safety and perioperative surgical outcomes. We did not consider radiographic and biomechanical outcomes.
Primary outcomes
The primary outcomes of this review comprised:
pain intensity;
physical function or disability status;
quality of life; and
recovery.
Pain intensity outcomes were back pain, leg pain or overall pain reported in visual analogue scales or numeric rating scales. Disability outcomes measures included Roland‐Morris Disability Questionnaire (RMDQ), Owestry Disability Index (ODI) or any other disability instrument used in low back pain research, and walking ability. Physical function was included if measured using the Zurich Claudication Questionnaire (ZCQ). Quality of life outcomes were, for example, total scores of the 36‐item or 12‐item Short Form Health Survey (SF‐36, SF‐12), or the EuroQol questionnaire (EQ‐5D). Trials that reported individual item scores, rather than the total scores, of the quality of life scales were not included in the meta‐analysis. Recovery was measured using the differences between preoperative and postoperative Japanese Orthopaedic Association (JOA) scores as reported by the included trials.
Secondary outcomes
Secondary outcomes were:
perioperative blood loss;
operation time;
length of hospital stay;
reoperation rate; and
costs.
Search methods for identification of studies
Electronic searches
Review authors developed the search strategy based on the Back and Neck Review Group methods guidelines and a specialist was consulted to revise it. Electronic searches of the following databases were performed up to 16 June 2016:
Cochrane Back and Neck Review Group Trials Register (OvidSP, 1991 to May 2016).
Cochrane Central Register of Controlled Trials (CENTRAL; OvidSP, Issue 5, 2016).
MEDLINE (OvidSP, 1946 to June Week 2 2016).
Embase (Embase.com, 1947 to 16 June 2016).
CINAHL (EBSCO, 1981 to 16 June 2016).
AMED (OvidSP, 1985 to 16 June 2016).
Web of Science (Thomson Reuters, 1900 to 16 June 2016).
Latin American and Caribbean Health Sciences Literature (LILACS; 1967 to 16 June 2016).
There were no restrictions on language or publication date. The search strategy for each database can be found in Appendix 1.
Searching other resources
Authors also searched ClinicalTrials.gov, Australian New Zealand Clinical Trials Registry (ANZCTR), and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for registered, ongoing or completed trials and contacted the main investigators of the relevant trials to identify any publication of the study. The keywords used for these searches included spinal stenosis, surgery and decompression.
Data collection and analysis
Selection of studies
One reviewer (GM) performed the first screening for relevant records based on titles and abstracts. Two independent reviewers (GM and MP/MR/RY) performed the screening of full texts, used consensus to resolve any disagreement and consulted a third reviewer (MF) when consensus could not be reached.
Data extraction and management
Using a standardised data extraction form, two reviewers (GM and MP/RY) independently extracted data from each included study and used consensus to resolve any disagreement. From each study, the reviewers extracted participants’ characteristics (age, disease duration and diagnostic criteria), type of surgery, type of comparison and outcomes. Pain and disability outcome measures were converted to scales from 0 (no pain or disability) to 100 (worst possible pain or disability).
Assessment of risk of bias in included studies
Reviewers evaluated the risk of bias in the included trials using the 'Risk of bias' assessment tool as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Back and Neck Review Group (Furlan 2015). Two reviewers (GM and MP/RY) independently performed the 'Risk of bias' assessment of the included trials, used consensus if there was any disagreement and consulted a third reviewer (MF) when consensus could not be reached. We scored each study as having 'high', 'low' or 'unclear' risk of bias for each criterion (see Table 4 and Table 5).
1. Sources of Risk of Bias.
| Bias Domain | Source of Bias | PossibleAnswers |
| Selection | (1) Was the method of randomization adequate? | Yes/No/Unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/No/Unsure |
| Performance | (3) Was the patient blinded to the intervention? | Yes/No/Unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/No/Unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/No/Unsure |
| Attrition | (6) Was the drop‐out rate described and acceptable? | Yes/No/Unsure |
| Attrition | (7) Were all randomized participants analysed in the group to which they were allocated? | Yes/No/Unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/No/Unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/No/Unsure |
| Performance | (10) Were cointerventions avoided or similar? | Yes/No/Unsure |
| Performance | (11) Was the compliance acceptable in all groups? | Yes/No/Unsure |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/No/Unsure |
| Other | (13) Are other sources of potential bias unlikely? | Yes/No/Unsure |
2. Criteria for a Judgment of ‘‘Yes’’ for the Sources of Risk of Bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2
groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of
ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered
sealed envelopes, sequentially‐ordered vials, telephone call to a central office, and preordered list of
treatment assignments.Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| 3 | Index and control groups are indistinguishable for the patients or if the success of blinding was tested among the patients and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored
‘‘yes’’ if the success of blinding was tested among the outcome assessors and it was successful or: ‐for patient‐reported outcomes in which the patient is the outcome assessor (e.g., pain, disability): the blinding procedure is adequate for outcome assessors if participant blinding is scored ‘‘yes’’ ‐for outcome criteria assessed during scheduled visit and that supposes a contact between participants and outcome assessors (e.g., clinical examination): the blinding procedure is adequate if patients are blinded, and the treatment or adverse effects of the treatment cannot be noticed during clinical examination ‐for outcome criteria that do not suppose a contact with participants (e.g., radiography, magnetic resonance imaging): the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed when assessing the main outcome ‐for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g., cointerventions, hospitalisation length, treatment failure), in which the care provider is the outcome assessor: the blinding procedure is adequate for outcome assessors if item ‘‘4’’ (caregivers) is scored ‘‘yes’’ ‐for outcome criteria that are assessed from data of the medical forms: the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed on the extracted data |
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop‐outs does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias a ‘‘yes’’ is scored. (N.B. these percentages are arbitrary, not supported by literature). |
| 7 | All randomized patients are reported/analysed in the group they were allocated to by randomization for the most important moments of effect measurement (minus missing values) irrespective of noncompliance and cointerventions. |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgment. |
| 9 | Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s). |
| 10 | If there were no cointerventions or they were similar between the index and control groups. |
| 11 | The reviewer determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore it is necessary to assess how many sessions each patient attended. For single‐session interventions (e.g., surgery), this item is irrelevant. |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 13 | Other types of biases. For example: ‐When the outcome measures were not valid. There should be evidence from a previous or present scientific study that the primary outcome can be considered valid in the context of the present. ‐Industry‐sponsored trials. The conflict of interest (COI) statement should explicitly state that the researchers have had full possession of the trial process from planning to reporting without funders with potential COI having any possibility to interfere in the process. If, for example, the statistical analyses have been done by a funder with a potential COI, usually ‘‘unsure’’ is scored. |
Measures of treatment effect
Trials were grouped according to the types of surgical interventions being compared, outcomes and assessment time points. We extracted sample sizes, means (final values) and standard deviations (SD) for continuous outcomes and quantified the treatment effects as mean differences (MD), or standardised mean differences (SMD) when trials used different methods to assess the same outcome. For dichotomous outcomes, the number of cases and the total sample size were used to estimate risk ratios (RR). We, therefore, used MD, SMD or RR and 95% confidence intervals (CI) as measures of treatment effects.
Unit of analysis issues
We did not include cluster‐randomised trials or cross‐over trials. When multiple pain measures were reported we extracted the most severe measure at baseline. For disability, we chose the scale defined in the study as the primary outcome. For data synthesis, follow‐up times were categorised as short‐term (closest to three months) and long‐term (closest to 12 months). When studies reported results for more than two intervention groups, we combined similar groups according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
If trials reported incomplete data, we contacted authors to request further information. If authors were unavailable or when authors refused to provided data, we imputed data according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For example, we calculated missing SDs from reported standard errors or 95% CIs and sample size, or we imputed missing SDs from the average SD reported in similar studies. We also estimated SDs from graphs when these estimates were missing in tables or not reported in the text of included trials. When studies reported medians and interquartile ranges (IQR), we considered that the median was equivalent to the mean and the IQR was 1.35 times the SD (Higgins 2011).
Assessment of heterogeneity
We grouped similar trials (e.g., similar types of surgical comparison, outcomes, and assessment time points) into clusters and performed a separate analysis for each cluster. To assess heterogeneity for each pooled analysis we used the I² statistic to estimate the total variation across studies that was due to heterogeneity, and considered heterogeneity values greater than 50% to be high (Higgins 2002).
Assessment of reporting biases
We planned to assess reporting bias for each meta‐analysis with a minimum of 10 trials using visual inspection of funnel plots and Egger's test. However, the number of studies in each meta‐analysis was insufficient for assessing this type of bias.
Data synthesis
Treatment effects were calculated using random‐effects models with inverse variance weighting for all meta‐analyses. A summary of findings table was created in Review Manager 5.3 and we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE, see Appendix 2) to assess the quality of the evidence for each outcome measure (Guyatt 2008). The quality of evidence was downgraded by one level according to the following criteria: limitation of study design (> 25% of the studies with high risk of bias (at least one of the bias domain judged as high risk)), inconsistency of results (statistically significant heterogeneity (I² > 50%) or ≤ 75% of trials with findings in the same direction), and imprecision (wide confidence intervals or the total number of participants was fewer than 400 participants in the comparison for continuous data or fewer than 300 events for dichotomous data for each pooled analysis). The indirectness criterion was not considered in this review because we included a specific population with relevant outcomes and direct comparisons. Where only single trials were available, evidence from studies with less than 400 participants was downgraded for imprecision and rated as 'moderate quality' evidence. The quality of the evidence could be further downgraded to 'low quality' evidence if limitations of study design were found. The quality of evidence was defined as: 'high quality', 'moderate quality', 'low quality' or 'very low quality' (Guyatt 2008).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned according to type of surgical intervention (e.g., decompression alone versus decompression plus fusion) for all outcomes and duration of follow‐up (e.g., short‐term and long‐term). Although we planned analyses of sources of heterogeneity according to different factors (e.g., surgeon's experience) we did not have enough studies in each meta‐analysis to report accurate results.
Sensitivity analysis
We aimed to perform sensitivity analysis to investigate whether our judgment of risk of bias of individual studies and time point definition would affect our conclusions. However, this analysis was not possible due to the limited number of studies in each meta‐analysis.
Results
Description of studies
The description of included studies is summarised in Characteristics of included studies.
Results of the search
Our search identified a total of 7494 records. After excluding duplicates, we screened 5358 titles and abstracts, and assessed 145 full text records. Of these, 24 randomised controlled trials (reported in 39 published research articles or abstracts) remained eligible for inclusion in our review (Azzazi 2010; Bridwell 1993; Cavusoglu 2007; Celik 2010; Cho 2007; Davis 2013; Forsth 2016; Ghogawala 2016; Grob 1995; Gurelik 2012; Hallett 2007; Komp 2015; Liu 2013; Lonne 2015; Mobbs 2014; Moojen 2013; Postacchini 1993; Rajasekaran 2013; Ruetten 2009; Stromqvist 2013; Thome 2005; Usman 2013; Watanabe 2011; Yagi 2009). The flow chart of studies with the main reasons for exclusion are shown in Figure 1. All trials included in this review were published in English and therefore no translation was required.
1.
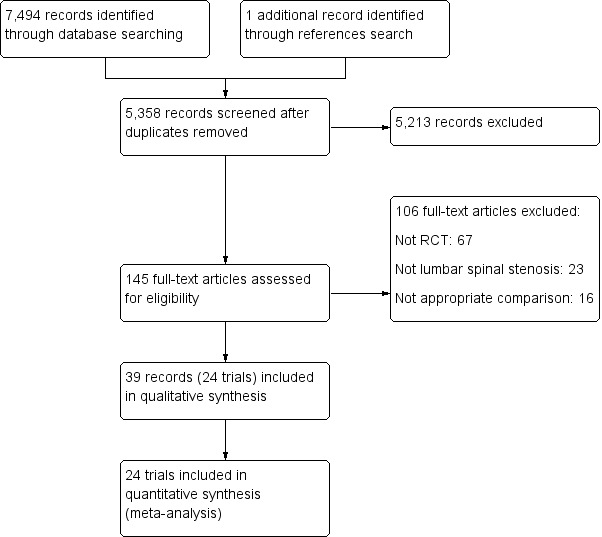
Study flow diagram.
Included studies
The 24 included trials investigated a total of 2352 participants and most studies defined lumbar spinal stenosis based on clinical assessment with a concordant imaging diagnosis (Azzazi 2010; Bridwell 1993; Cavusoglu 2007; Celik 2010; Cho 2007; Davis 2013; Grob 1995; Gurelik 2012; Forsth 2016; Ghogawala 2016; Hallett 2007; Lonne 2015; Mobbs 2014; Moojen 2013; Rajasekaran 2013; Ruetten 2009; Stromqvist 2013; Thome 2005; Usman 2013; Watanabe 2011; Yagi 2009). One study included participants based solely on imaging diagnosis (Postacchini 1993), and two studies used clinical assessment only (Komp 2015; Liu 2013). Nineteen out of 24 trials (80%) explicitly reported including only participants who had failed to improve with conservative treatment (Azzazi 2010; Bridwell 1993; Cavusoglu 2007; Celik 2010; Cho 2007; Davis 2013; Grob 1995; Gurelik 2012; Hallett 2007; Komp 2015; Lonne 2015; Moojen 2013; Rajasekaran 2013; Ruetten 2009; Stromqvist 2013; Thome 2005; Usman 2013; Watanabe 2011; Yagi 2009). The mean age of participants in included trials ranged from 56 to 73 years, and trials were conducted in a range of countries, including the United States, Australia, Turkey, Pakistan, Switzerland, Sweden, the United Kingdom, and Japan. See Characteristics of included studies for additional information.
Excluded studies
We excluded 106 reports from our review; see Characteristics of excluded studies. The reasons for exclusion were:
not a randomised controlled trial (67): Abdu 2009; Anderson 2011; Asazuma 2004; Bazan 2002; Blumenthal 2013; Bresnahan 2009; Cakir 2009; Cannone 2010; Carrasco 1986; Cassinelli 2007; Choi 2009; Dantas 2007; Delank 2002; Desai 2012; Epstein 2006; Escobar 2003; Fan 2009; Fast 1985; Fitzgerald 1976; Försth 2013; Fu 2008; Fujiya 1990; Ghahreman 2010; González 1992; Gotfryd 2012; Gotfryd 2012a; Gu 2009; Halm 2010; Herkowitz 1991; Hong 2010; Hong 2011; Ikuta 2005; Imagama 2009; Ito 2010; Katz 1997; Kawaguchi 2004; Kim 2007; Kim 2007a; Konno 2000; Kornblum 2004; Lee 2009; Liao 2011; Pappas 1994; Parker 2013; Radcliff 2012; Rapp 2009; Rapp 2011; Richter 2010; Rompe 1995; Rosa 2012; Rowland 2009; Satomi 1992; Schnake 2006; Sengupta 2006; Skidmore 2011; Smoljanovic 2010; Smorgick 2013; Steffee 1993; Tani 2002; Tenhula 2000; Tsutsumimoto 2009; Valesin 2009; Wang 1998; Willén 2008; Yamada 2012; Yang 2011; Yu 2008;
not lumbar spinal stenosis (23): Andersen 2008; Aoki 2012; Arriagada 2000; Benli 2006; Bjarke 2002; Carragee 1997; Carreon 2009; Chen 2010; Cheng 2009; Dahdaleh 2013; Delawi 2010; Dimar 2009; Feng 2011; Hwang 2010; Kim 2006; Korovessis 2004; Lian 2010; Ledonio 2012; Michielsen 2013; Videbaek 2010; Xiao 2007; Xiao 2007a; Zdeblick 1993; and
inappropriate comparison (16): Auerbach 2012; Altaf 2011; Auerbach 2011; Dirisio 2011; Dryer 2012; Haley 2012; Haley 2012a; Mahir 2012; McConnell 2011; Radcliff 2011; Repantis 2009; Sears 2012; Shapiro 2005; Weinstein 2007; Whang 2013; Zucherman 2004.
Risk of bias in included studies
As blinding of the therapist in surgical trials is not possible, we judged all studies to be at high risk of bias for this domain. We judged half of the included trials to be at low or unclear risk for all of the remaining domains of the 'Risk of bias' assessment. Only one trial (Moojen 2013) had all bias domains (except therapist blinding) judged as low risk. Most of the trials failed to adequately conceal the randomisation process, blind the participants or use an intention‐to‐treat analysis. The results from the risk of bias assessments for the included studies are summarised in Figure 2.
2.
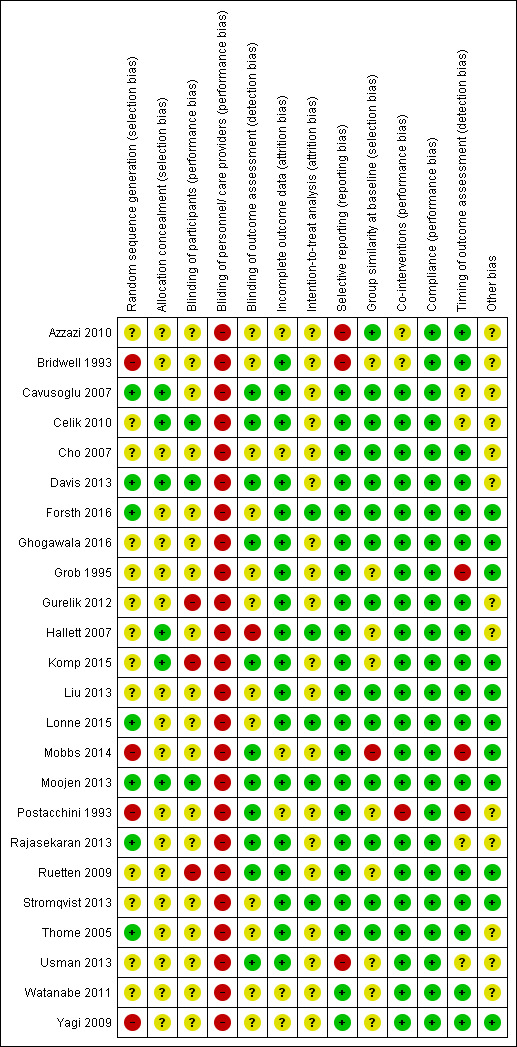
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only seven trials reported an appropriate method of randomisation, such as a computer‐generated randomisation list. Although 13 trials mentioned that study participants were randomised, they failed to describe the method used for randomisation and we therefore judged them to be at unclear risk of bias. Two trials reported that participants were randomly allocated according to the sequence of presentation to study site and we therefore considered them to be at high risk of bias (Mobbs 2014; Yagi 2009). In two trials, the authors reported that the randomisation protocol was broken and we also considered these trials at high risk of selection bias (Bridwell 1993; Postacchini 1993). Only six trials reported an appropriate method of allocation concealment, and 18 failed to report the method (Figure 2).
Blinding
In surgical clinical trials, it is not possible to blind care providers (i.e., surgeons), therefore we judged all included studies to be at high risk of bias for this domain. Only three studies blinded participants (Celik 2010; Davis 2013; Moojen 2013), while three trials reported not blinding participants leading us to judge them as being at high risk of bias (Gurelik 2012; Komp 2015; Ruetten 2009). The remaining 18 trials failed to provide information on blinding of participants, so we considered them to be at unclear risk for this bias domain. Eleven trials reported blinding of outcome assessors; 12 did not report this information and so we judged them as being at unclear risk of bias. Only one trial mentioned that outcome assessors were not blinded and we therefore considered it to be at high risk of bias (Hallett 2007).
Incomplete outcome data
We considered most of the trials (n = 17) to be at low risk of bias as they reported less than 15% drop‐out. One study reported that nearly 22% of participants were lost, but the number of drop‐outs and reasons were similar between the groups, therefore we judged this trial as being at low risk of bias for this outcome (Mobbs 2014). Six trials did not mention the number of participants withdrawn from the study and we thus judged them as being at unclear risk.
Selective reporting
We judged three trials as being at high risk of bias for selective reporting. Azzazi 2010 mentioned collecting short‐term follow‐up data in the methods section, but failed to report results. Also, although the authors mentioned measuring the amount of blood lost during surgery, these data were not reported in the published manuscript. Bridwell 1993 failed to report relevant patient‐related outcome measures (i.e., pain, disability), and Usman 2013 reported that recovery rate was one of the outcome measures of the trial, but it was not reported in the results section. We attempted to contact authors in order to have access to these data, but none replied.
Other potential sources of bias
Eleven trials reported not receiving funds for conducting the trial or disclosed any conflicts of interest; we therefore judged them as being at low risk of bias. The remaining trials did not provide a conflict of interest or funding statement so we considered them to be at unclear risk for other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
We did not identify trials comparing surgery with no treatment, placebo or sham surgery. Therefore, all trials included in this review compared different types of surgical interventions for lumbar spinal stenosis. We divided the included trials into six comparisons according to the surgical techniques being compared.
Decompression alone versus decompression plus fusion
The addition of fusion to bony decompression by either conventional laminectomy (Bridwell 1993; Forsth 2016; Ghogawala 2016; Grob 1995) or foraminotomy (Hallett 2007) was investigated in five randomised trials reporting data from 446 participants. Overall, the studies included in this review were fairly homogeneous, thus most of our meta‐analyses revealed no important heterogeneity (I² < 50%). A few pooled analyses resulted in considerable heterogeneity however (I² > 75%), especially the analysis on operation time, where a great variability of estimates were reported in included trials.
Primary outcomes
Our analyses showed no difference between groups on pain reduction in the short‐ (MD 4.50, 95% CI ‐0.70 to 9.70; Ghogawala 2016) and long‐term (MD ‐0.29, 95% CI ‐7.32 to 6.74; see Figure 3). Similarly, we found that decompression plus fusion was not superior to decompression alone on disability reduction at both short‐ (MD 5.20, 95% CI ‐3.50 to 13.90; Ghogawala 2016) and long‐term follow‐up (MD 3.26, 95% CI ‐6.12 to 12.63). We judged the quality of evidence in the short‐term for both outcomes as 'low quality' (downgraded for imprecision and inconsistency), and further downgraded it to 'very low quality' for limitation of study design in the long‐term. Three trials evaluated the effects of decompression plus fusion compared with decompression alone on walking ability (i.e., participants were considered improved when able to increase their walking distance by 50% at follow‐up). This analysis provided 'very low quality' evidence (downgraded for imprecision, inconsistency, and limitation of study design) of no difference between groups (RR 0.99, 95% CI 0.79 to 1.24; see Table 1).
3.
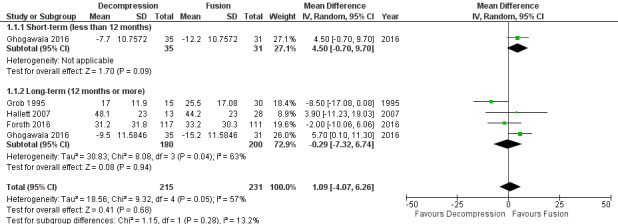
Forest plot of comparison: 1 Decompression alone versus decompression plus fusion, outcome: 1.1 Pain.
Secondary outcomes
Two trials reported the mean direct surgery cost per patient. Forsth 2016 showed lower costs for decompression alone (USD 10,392) compared with decompression plus fusion (USD 16,115). Similarly, Hallett 2007 revealed that decompression incurred half the cost of fusion surgery (USD 5,400 versus USD 12,200). However, no measures of variability or inferential statistics were reported for this outcome. We found 'very low quality' evidence (downgraded for imprecision, inconsistency, and limitation of study design) that decompression alone required shorter operation time (MD ‐107.94 minutes, 95% CI ‐161.65 minutes to ‐54.23 minutes; ) and was associated with less perioperative blood loss (MD ‐0.52 L, 95% CI ‐0.70 L to ‐0.34 L) compared with decompression plus fusion. 'Moderate quality' evidence (downgraded for limitation of study design) revealed no difference in the number of reoperations (RR 1.25, 95% CI 0.81 to 1.92), and 'low quality' evidence (downgraded for imprecision and inconsistency) showed shorter hospital stays after decompression alone (MD ‐1.69 days, 95% CI ‐2.12 days to ‐1.26 days) compared with decompression plus fusion operations.
Decompression versus interspinous spacer
Three trials reported data of 355 participants comparing bony decompression (laminectomy or laminotomy) with the X‐Stop or Coflex interspinous process spacer devices (Lonne 2015; Moojen 2013; Stromqvist 2013).
Primary outcomes
At short‐term, 'low quality' evidence (downgraded for imprecision and inconsistency) showed no difference on pain reduction (MD ‐0.93, 95% CI ‐9.86 to 8.00). Likewise, 'moderate quality' evidence (downgraded for imprecision) revealed no long‐term difference on pain between the groups (MD ‐0.55, 95% CI ‐8.08 to 6.99; see Figure 4). For disability, 'moderate quality evidence' (downgraded for imprecision) did not reveal any difference in the short‐term (MD 1.30, 95% CI ‐3.64 to 6.25), and 'low quality' evidence (downgraded for imprecision and inconsistency) also showed no superior benefits of interspinous spacers in the long‐term (MD 1.25, 95% CI ‐4.48 to 6.98). Pooling revealed 'moderate quality' evidence (downgraded for imprecision) that improvement of function (as measured by the ZCQ function sub scale) was similar in the two groups at short‐ (MD ‐0.06, 95% CI ‐0.27 to 0.14) and long‐term follow‐up (MD ‐0.00, 95% CI ‐0.30 to 0.29). One study (Lonne 2015) provided 'moderate quality' evidence (downgraded for imprecision) that there were no differences between decompression and interspinous spacers for quality of life improvement in the short‐ (MD ‐0.12, 95% CI ‐0.25 to 0.01) and long‐term (MD ‐0.05, 95% CI ‐0.18 to 0.07; see Table 2).
4.
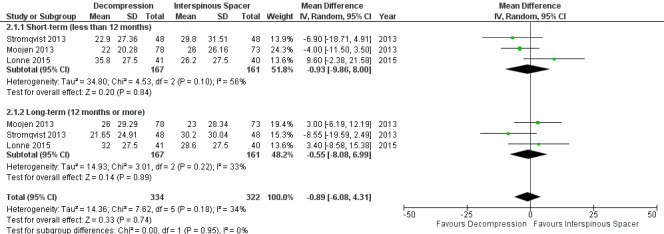
Forest plot of comparison: 2 Decompression versus interspinous spacer, outcome: 2.1 Pain.
Secondary outcomes
Results from 'low quality' evidence (downgraded for imprecision and inconsistency) showed that participants receiving interspinous spacers required longer operation time (MD 39.11 minutes, 95% CI 19.43 minutes to 58.78 minutes), but there were no differences in terms of length of hospital stay (MD 0.51 days, 95% CI ‐0.58 days to 1.60 days) and perioperative blood loss (MD 144.00 mL, 95% CI ‐209.74 mL to 497.74 mL). However, 'high quality' evidence demonstrated higher reoperation rates after interspinous spacers (RR 3.95, 95% CI 2.12 to 7.37) compared with conventional decompression. Two trials (Lonne 2015; Moojen 2013) providing 'moderate quality' evidence (downgraded for imprecision) reported the total health care cost associated with surgical procedures, and revealed a significantly higher cost associated with the interspinous spacers; the incremental cost for an implant was estimated at EUR 2,856.34 (95% CI EUR 1,970.40 to EUR 3,742.28) or USD 3,103.84 (95% CI USD 2,141.14 to USD 4,066.55).
Decompression plus fusion versus interspinous spacer
Two trials compared decompression plus fusion with the X‐Stop or Coflex interspinous spacer devices (Azzazi 2010; Davis 2013), including a total of 382 participants analysed at long‐term follow‐up only.
Primary outcomes
There was 'low quality' evidence (downgraded for imprecision and limitation of study design) of no difference between groups on pain reduction (MD 5.35, 95% CI ‐1.18 to 11.88; see Figure 5), and 'moderate quality' evidence (downgraded for imprecision) also showed no superior benefit of interspinous spacers in terms of quality of life (MD ‐3.10, 95% CI ‐6.30 to 0.10). However, we found 'low quality' evidence (downgraded for imprecision and limitation of study design) that interspinous spacers were slightly more effective than fusion on disability reduction (MD 5.72, 95% CI 1.28 to 10.15; see Table 3).
5.
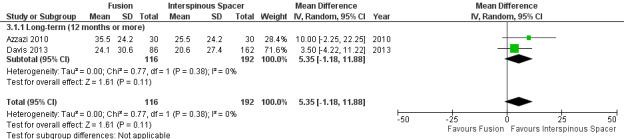
Forest plot of comparison: 3 Decompression plus fusion versus interspinous spacer, outcome: 3.1 Pain.
Secondary outcomes
We found 'moderate quality' evidence (downgraded for imprecision) that decompression plus fusion resulted in more perioperative blood loss (MD 238.90 mL, 95% CI 182.66 mL to 295.14 mL; Davis 2013) compared with interspinous spacers. 'Very low quality' evidence (downgraded for imprecision, inconsistency and limitation of study design) revealed longer operation time (MD 78.91 minutes, 95% CI 30.16 minutes to 127.65 minutes) and length of hospital stay (MD 1.58 days, 95% CI 0.90 days to 2.27 days) for decompression plus fusion. However, there was no difference in reoperation rates between the two groups (RR 0.70, 95% CI 0.32 to 1.51; Davis 2013) from 'high quality' evidence.
Laminectomy versus laminotomy
Six randomised controlled trials reporting data from 475 participants compared laminectomy to unilateral (Cavusoglu 2007; Gurelik 2012; Liu 2013; Thome 2005) or bilateral laminotomy (Celik 2010; Postacchini 1993; Thome 2005). Data from unilateral and bilateral laminotomy groups were combined according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Primary outcomes
We found 'moderate quality' evidence (downgraded for imprecision) that laminotomy is not superior to laminectomy in reducing pain in the short‐term (MD 0.32, 95% CI ‐2.39 to 3.04), and 'low quality' evidence (downgraded for inconsistency and limitation of study design) of no difference in the long‐term (MD ‐1.92, 95% CI ‐8.19 to 4.35; see Figure 6). Likewise, 'moderate quality' evidence (downgraded for imprecision) revealed no between‐group differences on disability reduction at short‐ (MD 1.56, 95% CI ‐1.02 to 4.13) and long‐term follow‐up (MD ‐0.43, 95% CI ‐4.37 to 3.52). For walking ability (i.e., walking distance in metres without radicular pain), we found 'low quality' evidence (downgraded for imprecision and limitation of study design) of no difference between these techniques in the short‐term (SMD ‐0.07, 95% CI ‐0.33 to 0.20). 'Moderate quality' evidence (downgraded for imprecision) also showed no difference in walking ability in the long‐term (SMD ‐0.02, 95% CI ‐0.33 to 0.28).
6.
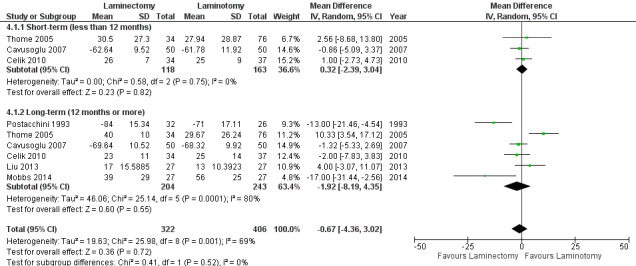
Forest plot of comparison: 4 Laminectomy versus laminotomy, outcome: 4.1 Pain.
Secondary outcomes
Our results revealed 'low quality' evidence (downgraded for imprecision and limitation of study design) of no difference between the two surgical procedures on the duration of operation (MD ‐6.25 minutes, 95% CI ‐13.76 minutes to 1.27 minutes). However, there was significantly more blood loss (MD 38.80 mL, 95% CI 17.81 mL to 59.80 mL) and longer hospital stay (MD 1.55, 95% CI 0.61 to 2.50) for laminectomy when compared with laminotomy. 'Moderate quality' evidence (downgraded for imprecision) demonstrated no difference in the number of participants having a revision surgery (RR 2.61, 95% CI 0.78 to 8.78).
Decompression versus split‐decompression
Four trials reported data of 218 participants comparing decompression (laminectomy) with spinous process split‐decompression (Cho 2007; Liu 2013; Rajasekaran 2013; Watanabe 2011). Only long‐term follow‐up data was available in included trials.
Primary outcomes
Pooling showed 'low quality' evidence (downgraded for inconsistency and imprecision) of no differences between treatments on pain reduction (MD 6.35, 95% CI ‐3.35 to 16.04). 'Moderate quality' evidence (downgraded for imprecision) also revealed no differences between the two groups on disability reduction (MD 1.87, 95% CI ‐2.82 to 6.57). 'Low quality' evidence (downgraded for inconsistency and imprecision) suggested no superior benefits of split‐decompression on long‐term recovery (MD ‐5.18, 95% CI ‐19.81 to 9.45), as assessed by the JOA recovery score (range 0 to 100), compared with conventional decompression.
Secondary outcomes
We found no differences between the two groups based on 'low quality' evidence (downgraded for inconsistency and imprecision) in terms of operation time (MD ‐10.57 minutes, 95% CI ‐34.39 minutes to 13.25 minutes), perioperative blood loss (MD ‐1.83 mL, 95% CI ‐27.65 mL to 23.98 mL), and length of hospital stay (MD 1.49 days, 95% CI ‐1.70 days to 4.67 days). 'Moderate quality' evidence (downgraded for imprecision) also demonstrated that the number of participants requiring reoperation was similar between the groups (RR 1.22, 95% CI 0.22 to 6.85).
Decompression versus endoscopic decompression
The efficacy of endoscopic–assisted decompression was investigated in three randomised trials including 393 participants (Komp 2015; Ruetten 2009; Yagi 2009).
Primary outcomes
Our meta‐analysis revealed 'low quality evidence' (downgraded for imprecision and limitation of study design) of a small but significant short‐term disability reduction of endoscopic approaches compared with conventional decompression (MD 4.12, 95% CI 0.91 to 7.33). However, 'very low quality evidence' (downgraded for inconsistency, imprecision and limitation of study design) showed no difference between these surgical interventions for disability in the long‐term (MD 1.44, 95% CI ‐2.66 to 5.54). Komp 2015 did not report estimates of between‐group differences or measures of variability for each treatment group, therefore we could not calculate a treatment effect for this trial.
Secondary outcomes
'Very low quality' evidence (downgraded for inconsistency, imprecision, and limitation of study design) showed no between‐group difference on operation time (MD 10.05 minutes, 95% CI ‐2.09 minutes to 22.18 minutes). However, Yagi 2009 provided 'low quality' evidence (downgraded for imprecision and limitation of study design) that conventional decompression was associated with more perioperative blood loss (MD 34.00 mL, 95% CI 30.40 mL to 37.60 mL) and longer hospital stay (MD 8.56 days, 95% CI 6.78 days to 10.34 days) compared with endoscopic decompression. 'Moderate quality' evidence (downgraded for limitation of study design) suggested that the number of participants having a revision surgery was similar between the surgical interventions (RR 0.81, 95% CI 0.22 to 2.97).
Discussion
Summary of main results
Our results revealed a paucity of evidence on the efficacy of surgery for lumbar spinal stenosis. We found no trials investigating the efficacy of surgery for lumbar spinal stenosis compared with no treatment, placebo or sham surgery. Therefore, the effects of time, regression to the mean, and patients' expectations (placebo effect) regarding surgery remain unknown. Previous research has shown that placebo‐controlled trials in surgery are feasible and a powerful tool to show the efficacy of surgical interventions (Wartolowska 2014). We identified 24 published randomised trials that compared the effects of different surgical techniques for this condition. In our main comparison, we found that fusion does not add benefits in terms of pain or disability reduction compared with decompression alone for the treatment of lumbar spinal stenosis. In addition, we found no differences on pain, disability and quality of life between interspinous process spacer devices and conventional bony decompression. However, the interspinous spacers resulted in significantly higher reoperation rates. We found no further differences in outcomes among the other surgical decompression techniques for lumbar spinal stenosis. In sum, at present, newer surgical techniques have not proven superior to conventional decompression for patients with lumbar spinal stenosis.
Overall completeness and applicability of evidence
Given the number of surgical techniques for the treatment of lumbar spinal stenosis, the need for placebo‐controlled trials has never been greater. Through our search, we could not find published placebo‐controlled surgical trials in patients with lumbar spinal stenosis. Previous studies have demonstrated the appropriate ethical considerations for placebo surgery (Horng 2003), and confirmed their feasibility (Wartolowska 2014). Such trials, investigating the efficacy of surgery compared with placebo for other spinal conditions, such as painful osteoporotic vertebral fractures, have been conducted and recently published. Buchbinder 2009 performed sham surgery by inserting a blunt stylet and gently tapping the vertebral body and compared this with conventional vertebroplasty. Likewise, Flum 2006 has suggested performing minimally invasive approaches simulating the decompressive technique to the spine for patients with lumbar spinal stenosis, but without actually removing any bone tissue.
The addition of fusion to decompression is commonly performed in this population, although a recent study has shown that fusion is not only more costly but highly associated with major complications and deaths when compared with decompression alone (Deyo 2010). Our review provides relevant information on this topic, showing that the addition of fusion was not associated with better outcomes (pain or disability) compared with decompression alone. In fact, fusion was significantly associated with longer operation time (nearly two hours difference) and more blood loss during operation (over 500 mL difference), confirming the higher risk for complications when performing this type of surgery. However, more studies are needed as we only included five trials providing 'very low quality' to 'moderate quality' evidence. For patients who present spinal instability and thus require stabilisation of spinal segments after decompression, the interspinous spacer devices might be an alternative as they were linked to less perioperative blood loss and shorter operation time and hospital length of stay. The interspinous spacer devices, however, should not replace conventional decompression surgery when only decompression of the spinal canal is warranted (i.e., no further fusion). These devices failed to be superior to conventional decompression on patient‐relevant outcomes, and resulted in significantly higher reoperation rates. Moreover, our results showed that these implants can cost on average 1.5 times more than conventional decompression. Considering the higher risks and costs, we would not recommend the spacer devices as an alternative to conventional decompression surgery for lumbar spinal stenosis.
One may argue that differences in the proportion of patients with mild spondylolisthesis included in the trials may affect the results. In trials that investigated fusion compared with interspinous spacers, both Davis 2013 and Azzazi 2010 included only participants with up to grade I stable degenerative spondylolisthesis. In Davis 2013, the proportion of participants with spondylolisthesis was 47%; however, Azzazi 2010 did not report the proportion of these participants. In the other included trials, the proportion of participants with up to grade I spondylolisthesis varied. For example, Ghogawala 2016 included only participants with lumbar spinal stenosis and grade I spondylolisthesis, whereas Forsth 2016 stratified the randomisation process to the presence or absence of degenerative spondylolisthesis, and Cavusoglu 2007 reported that 15% of included participants had mild spondylolisthesis. Although the differences between groups for some outcomes were not statistically significant, some might be considered clinically relevant. As most studies were very small, they were likely underpowered. Larger studies are needed to confirm these findings, for example the difference in revision rates between laminectomy and laminotomy.
This review provides valuable information for clinical decision making regarding the best surgical technique for patients with lumbar spinal stenosis, and should be used to inform clinical practice guidelines about the benefits and harms of different surgical options for this condition.
Quality of the evidence
Overall, the methodological quality of included studies was poor. Whereas blinding of the caregiver in surgical trials is typically not possible, eleven trials reported blinding of outcome assessors and only three studies reported that participants were blinded. The quality of the available evidence (GRADE) ranged from 'high quality' to 'very low quality'. In most cases where the evidence was downgraded, this was done because we found inconsistency of findings (I² > 50%) or imprecision (pooled sample size < 300 or 400), hence the evidence was judged as 'moderate quality'. In some pooled analyses, the evidence was downgraded for both inconsistency and imprecision, being judged as 'low quality'. In a few cases, evidence was further downgraded by one level because of limitation of study design, resulting in 'very low quality' evidence. More high quality trials comparing the effects between surgical techniques are needed to support our findings.
Potential biases in the review process
Although we tried to minimise various biases during the review process, the reporting of data was poor among some included studies, and in some circumstances we had to estimate data of treatment effects from graphs or use imputation of data from similar included trials. To overcome this issue, we recommend that future clinical trial authors adequately follow the instructions outlined in the CONSORT statement (Schulz 2010). It is also possible that we have underestimated the rates of reoperation, and our conclusions on harms of included interventions should be interpreted with caution. This is because safety reporting across included trials varied largely and not all trials have reported this outcome. Information on safety of surgical procedures is paramount for clinical decision making, therefore future trials should include complications and reoperations as outcomes and report them appropriately (Ioannidis 2004). We acknowledge the limited number of trials in each comparison, which also limited our ability to perform additional subgroup or sensitivity analyses. The search strategy was limited to humans in some of the databases (MEDLINE, EMBASE), so it is possible that we missed potentially relevant studies not indexed as humans. However, we searched a variety of sources as a way of trying to capture all relevant studies.
Agreements and disagreements with other studies or reviews
This review is an update of a recently published systematic review (Machado 2015), and included an additional seven randomised trials (10 records). A recent Cochrane review has also investigated the effects of decompression techniques for lumbar spinal stenosis, but limited the inclusion criteria to posterior decompression techniques that did not involve fusion or the use of interspinous process spacer devices (Overdevest 2015). Our results agree with those from this recent publication showing that different decompression techniques have similar effects on functional disability and leg pain.
Another systematic review has also investigated the effectiveness of interspinous process spacer devices for lumbar spinal stenosis, suggesting that spacer devices are superior to bony decompression (Chou 2011). However, this review could not find randomised trials that made a direct comparison between spacer devices and conventional decompression, therefore its conclusions were based on indirect comparisons through a network meta‐analysis. Similarly, a second systematic review failed to identify trials directly comparing these two techniques (Moojen 2011). As the first randomised trial comparing these techniques was published in 2013, these older systematic reviews did not include any randomised studies. More recently, a systematic review of direct comparisons was published (Wu 2014), but included both randomised and non‐randomised studies in their meta‐analysis. Results of this review also found higher reoperation rates and costs associated with spacer devices when compared with conventional decompression.
Authors' conclusions
Implications for practice.
There is relatively limited evidence to guide the use of surgery for the management of lumbar spinal stenosis, as there are no published placebo‐controlled trials investigating the effects of surgery for this condition. Most of the evidence supporting the use of surgery comes from randomised trials comparing surgery with non‐surgical interventions, with conflicting conclusions. The addition of fusion to decompression is not only more costly, but also leads to more intraoperative blood loss and longer operation time, and fails to result in superior clinical outcomes when compared with decompression alone. Operation using interspinous spacer devices is quicker, and results in less blood loss and shorter hospital length of stay than fusion. These devices, however, do not provide better outcomes than conventional decompression, and are associated with higher reoperation rates. This review provides valuable information for patients and clinicians to help decide the best surgical option for this condition.
Implications for research.
Future research should include high quality randomised placebo‐controlled trials, and trials comparing surgery with conservative care in order to investigate the specific effects of surgery for lumbar spinal stenosis. More methodologically rigorous studies are needed to compare the effects of the addition of fusion to decompression as we only identified five trials. Trials should incorporate a double‐blinded (patient and assessors) design and include an adequate randomisation process. The standardisation of outcomes is also crucial and trials should report patient‐related outcome measures, such as leg pain intensity using a visual analogue pain scale; function measured by the ZCQ or the ODI; walking ability using accelerometers; quality of life as reported using the SF‐36 or the EQ‐5D; as well as surgically relevant outcomes (i.e., perioperative blood loss, operation time, length of hospital stay), and reoperation rates. Also, future trials should include and report clinically important complications, such as infections, blood transfusions, and dural tears. Most included trials in this review reported one‐ or two‐year follow‐up, so future research should focus on longer follow‐up times (i.e., five years) to establish the long‐term effects of surgery in this population.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. GCM and MBP are supported by an international postgraduate research scholarship/postgraduate award from the Australian Department of Education and Training. CGM is supported by a principal research fellowship from the National Health and Medical Research Council. MLF is supported by a Sydney Medical Foundation Fellowship from the Sydney Medical School, The University of Sydney.
Appendices
Appendix 1. Search strategy
CENTRAL
Last searched 16 June 2016
1. spinal stenosis.mp. or Spinal Stenosis/
2. canal stenosis.mp.
3. lumbar stenosis.mp.
4. 1 or 2 or 3
5. neurosurgery/ or orthopedics/
6. decompression.mp. or Decompression, Surgical/
7. Spinal Fusion/
8. surgery.mp.
9. 5 or 6 or 7 or 8
10. 4 and 9
MEDLINE
Last searched 16 June 2016
1. Exp spinal stenosis/
2. "canal stenosis".mp.
3. (spin* adj3 stenosis).mp.
4. (lumbar adj3 stenosis).mp.
5. (lateral adj3 stenosis).mp.
6. (central adj3 stenosis).mp.
7. (foramin* adj3 stenosis).mp.
8. "neurogenic claudication".mp.
9. Exp radiculopathy/
10. Radiculopathy.mp.
11. "radicular pain".mp.
12. "lumbar radicular pain".mp.
13. Exp spondylolisthesis/
14. Spondylolisthesis.mp.
15. (lumb* adj5 spondyl*).mp.
16. Exp spondylosis/
17. Spondylosis.mp
18. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19. Exp general surgery/
20. Surgery.mp.
21. Exp decompression, surgical/
22. "decompres* surgery".mp.
23. Decompression.mp
24. (spin* adj3 decompress*).mp.
25. Exp laminectomy/
26. Laminectom*.mp.
27. Laminotom*.mp.
28. Laminoplasty.mp.
29. Exp spinal fusion/
30. (spin* adj3 fusion).mp.
31. (pedicle adj3 screw).mp.
32. "lumbar fusion".mp.
33. "vertebrae fusion".mp.
34. "vertebral fixation".mp.
35. "spinal fixation".mp.
36. Spondylodesis.mp
37. Spondylosyndesis.mp
38. Arthrodesis.mp. Or exp arthrodesis/
39. (posterolateral adj3 fusion).mp
40. (interbody adj3 fusion).mp
41. (anterior adj3 fusion).mp
42. (posterior adj3 fusion).mp
43. (transforaminal adj3 fusion).mp
44. (transpsoas adj3 fusion).mp
45. (facet adj3 fusion).mp
46. (bone adj3 graft).mp
47. (fixation adj3 spin*).mp
48. (pedicle adj3 fusion).mp
49. Graft.mp
50. (cage adj3 fusion).mp
51. (screw adj3 fusion).mp
52. Foraminotomy.mp. Or exp foraminotomy/
53. Foraminectomy.mp
54. Exp surgical procedures, minimally invasive/
55. "minim* invasive".mp.
56. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55
57. 18 and 56
58. Exp randomized controlled trial/
59. Randomized controlled trial.pt.
60. "randomized controlled trial".mp.
61. (random* adj3 trial).ab,ti.
62. Exp controlled clinical trial/
63. "controlled clinical trial".mp.
64. Randomized.ab,ti.
65. Placebo.ab,ti.
66. Randomly.ab,ti.
67. Random*.ab,ti.
68. Trial.ab,ti.
69. Exp clinical trial/
70. "clinical trial".pt.
71. "clinical trial".mp.
72. "clinical study".ab,ti.
73. 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72
74. 57 and 73
75. Limit 74 to humans
EMBASE
Last searched 16 June 2016
1. 'vertebral canal stenosis'/exp OR 'vertebral canal stenosis'
2. 'spine NEAR/3 stenosis'
3. 'lumbar NEAR/3 stenosis'
4. 'lateral NEAR/3 stenosis'
5. 'central stenosis'
6. 'foraminal stenosis'
7. 'neurogenic claudication'
8. 'radiculopathy'/exp OR radiculopathy
9. 'radicular pain'/exp OR 'radicular pain'
10. 'lumbar radicular pain'
11. 'spondylolisthesis'/exp OR spondylolisthesis
12. 'spondylosis'/exp OR spondylosis
13. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
14. 'surgery'/exp OR surgery
15. 'decompression surgery'/exp OR 'decompression surgery'
16. 'decompression spinal cord'/exp OR 'decompression spinal cord'
17. 'decompression'/exp OR decompression
18. 'laminectomy'/exp OR laminectomy
19. laminotomy
20. 'laminoplasty'/exp OR laminoplasty
21. 'spine fusion'/exp OR 'spine fusion'
22. 'spinal fusion'/exp OR 'spinal fusion'
23. 'lumbar NEAR/3 fusion'
24. 'vertebrae fusion'
25. 'vertebral fixation'
26. 'spondylodesis'/exp OR spondylodesis
27. 'spinal fixation'
28. 'spinal fixation device'/exp OR 'spinal fixation device'
29. 'spondylosyndesis'/exp OR spondylosyndesis
30. posterolateral NEAR/3 fusion
31. interbody NEAR/3 fusion
32. anterior NEAR/3 fusion
33. posterior NEAR/3 fusion
34. transforaminal NEAR/3 fusion
35. 'transpsoas fusion'
36. facet NEAR/3 fusion
37. 'arthrodesis'/exp OR arthrodesis
38. bone NEAR/5 graft
39. fixation NEAR/5 spin*
40. pedicle NEAR/5 fusion
41. cage NEAR/5 fusion
42. screw NEAR/5 fusion
43. pedicle NEAR/5 screw
44. 'foraminotomy'/exp OR foraminotomy
45. foraminectomy
46. 'minimally invasive procedures'/exp OR 'minimally invasive procedures'
47. 'minim$ invasive'
48. 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47
49. 13 AND 48
50. 'randomized controlled trial'/exp OR 'randomized controlled trial'
51. 'controlled clinical trial'/exp OR 'controlled clinical trial'
52. 'clinical trial'/exp OR 'clinical trial'
53. randomized:ab
54. placebo:ab
55. randomly:ab
56. trial:ab
57. 'clinical study':ab
58. 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34
59. 26 AND 35
60. 59 AND 'human'/de
CINAHL
Last searched 16 June 2016
57. 19 AND 56
56. 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55
55. (MH "Minimally Invasive Procedures") OR "Minimally Invasive"
54. "Foraminectomy"
53. "Foraminotomy"
52. "Screw fusion"
51. "Cage fusion"
50. "Pedicle fusion"
49. (MH "Grafts+") OR "Bone graft"
48. (MH "Arthrodesis+")
47. "Facet fusion"
46. "Transpsoas fusion"
45. "Transforaminal fusion"
44. "Posterior fusion"
43. "Anterior fusion"
42. "Anterior near/5 fusion"
41. "Interbody fusion"
40. "Posterolateral fusion"
39. "Spondylosyndesis"
38. "Spinal fixation" OR (MH "Orthopedic Fixation Devices+")
37. "Spondylodesis"
36. "vertebral fixation"
35. "vertebrae fusion"
34. "lumbar fusion"
33. (MH "Orthopedic Fixation Devices+") OR "pedicle screw"
32. "spin* fusion"
31. (MH "Arthrodesis+") OR "arthrodesis"
30. (MH "Spinal Fusion") OR "Spinal Fusion"
29. "Laminoplasty"
28. "Laminotom*"
27. "Laminectom*"
26. (MH "Laminectomy") OR "Laminectomy"
25. "lumbar decompress*"
24. "spin* decompress*"
23. "Decompres* surgery"
22. (MH "Decompression, Surgical+") OR "Decompression"
21. "surgery"
20. (MH "Surgery, Operative+")
19. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18
18. (MH "Spondylolysis+") OR "spondilolisys"
17. "Spondylosis"
16. (MH "Spondylosis+")
15. "lumb* spondyl*"
14. (MH "Spondylolisthesis") OR "Spondylolisthesis"
13. "lumbar radicular pain"
12. "radicular pain"
11. (MH "Radiculopathy") OR "Radiculopathy"
10. "neurogenic claudication"
9. (MH "Intermittent Claudication")
8. "foramin* stenosis"
7. "central stenosis"
6. "lateral stenosis"
5. "lumbar stenosis"
4. "Canal stenosis"
3. "spin* stenosis"
2. "spinal stenosis"
1. (MH "Spinal Stenosis")
AMED
Last searched 16 June 2016
1. Exp Spinal stenosis/
2. Canal stenosis.mp.
3. (spin* adj3 stenosis).mp.
4. (lumbar adj3 stenosis).mp.
5. (lateral adj3 stenosis).mp.
6. (central adj3 stenosis).mp.
7. (foramin* adj3 stenosis).mp.
8. "neurogenic claudication".mp.
9. Radiculopathy.mp.
10. "radicular pain".mp.
11. "lumbar radicular pain".mp.
12. Exp Spondylolisthesis/
13. Spondylolisthesis.mp.
14. (lumb* adj5 spondyl*).mp.
15. Spondylosis.mp.
16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. Exp surgery/
18. Surgery.mp.
19. Surgery operative.mp.
20. Decompression.mp.
21. "Decompres* surgery".mp.
22. (spin* adj3 decompress*).mp.
23. Exp Laminectomy/
24. Laminectom*.mp.
25. Laminotomy.mp.
26. Laminoplasty.mp.
27. Exp arthrodesis/
28. (spin* adj3 fusion).mp.
29. (pedicle adj3 screw).mp.
30. "lumbar fusion".mp.
31. "vertebrae fusion".mp.
32. "Vertebral fixation".mp.
33. "Spinal fixation".mp.
34. Spondylodesis.mp.
35. Spondylosyndesis.mp.
36. Exp Arthrodesis/ or Arthrodesis.mp.
37. (Posterolateral adj3 fusion).mp.
38. (Interbody adj3 fusion).mp.
39. (Anterior adj3 fusion).mp.
40. (Posterior adj3 fusion).mp.
41. (Transforaminal adj3 fusion).mp.
42. (Transpsoas adj3 fusion).mp.
43. (Facet adj3 fusion).mp.
44. (Bone adj3 graft).mp.
45. (Fixation adj3 spin*).mp.
46. (Pedicle adj3 fusion).mp.
47. Graft.mp.
48. (Cage adj3 fusion).mp.
49. (Screw adj3 fusion).mp.
50. Foraminotomy.mp.
51. "Minim* invasive".mp.
52. 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51
53. 16 and 52
Web of Science
Last searched 16 June 2016
54. 53 and 43
53. 52 or 51 or 50 or 49 or 48 or 47 or 46 or 45 or 44
52. "clinical study"
51. "clinical trial"
50. Trial)
49. Random*
48. Placebo
47. Randomized
46. "controlled clinical trial"
45. "randomized clinical trial"
44. "randomized controlled trial"
43. 42 and 14
42.41 or 40 or 39 or 38 or 37 or 36 or 35 or 34 or 33 or 32 or 31 or 30 or 29 or 28 or 27 or 26 or 25 or 24 or 23 or 22 or 21 or 20 or 19 or 18 or 17 or 16 or 15
41. "minimally invasive"
40. Foraminectomy
39. Foraminotomy
38. "pedicle screw"
37. "cage fusion”
36. Arthrodesis
35. "facet fusion"
34. "transpsoas fusion"
33. "transforaminal fusion"
32. "posterior fusion"
31. "anterior fusion"
30. "interbody fusion"
29. "posterolateral fusion"
28. Spondylosyndesis
27. "spinal fixation"
26. Spondylodesis
25. "vertebral fixation"
24. "vertebrae fusion"
23. Arthrodesis
22. "lumbar fusion"
21. "spin* fusion"
20. Laminoplasty
19. Laminotom*
18. Laminectomy
17. Decompressive
16. Decompression
15. Surgery
14. 13 or 12 or 11 or 10 or 9 or 8 or 7 or 6 or 5 or 4 or 3 or 2 or 1
13. Spondylolysis
12. Spondylosis
11. "spondylolisthesis"
10. "lumbar radicular pain"
9." radicular pain"
8. "radiculopathy"
7. "neurogenic claudication"
6. "foramin* stenosis"
5. "central stenosis"
4. "lateral stenosis"
3. "lumbar stenosis"
2. "canal stenosis"
1. "spin* stenosis"
LILACS
Last searched 16 June 2016
("spine stenosis" OR "spinal stenosis" OR "canal stenosis" OR "lumbar stenosis" OR "central stenosis" OR "lateral stenosis" OR "foraminal stenosis" OR "spondylolisthesis" OR spondylosis OR "neurogenic claudication" OR radiculopathy OR "radicular pain") AND (surgery OR decompression OR decompressive OR laminectomy OR laminotomy OR laminoplasty OR "spinal fusion" OR "spine fusion" OR arthrodesis OR "lumbar fusion" OR "vertebrae fusion" OR "vertebral fixation" OR spondylodesis OR "spinal fixation" OR spondylosyndesis OR "posterolateral fusion" OR "interbody fusion" OR "anterior fusion" OR "posterior fusion" OR "transforaminal fusion" OR "transpsoas fusion" OR "facet fusion" OR "bone graft" OR "pedicle fusion" OR "cage fusion" OR "screw fusion" OR "pedicle screw" OR screw OR rod OR foraminotomy OR foraminectomy OR "surgical procedure" OR "minimally invasive")
ClinicalTrials.gov, WHO ICTRP and ANZCTR
Last searched 16 June 2016
ClinicalTrials.gov: Search: (surgery OR decompression) AND Condition: spinal stenosis
WHO ICTRP: Title: (surgery OR decompression) AND Condition: spinal stenosis
ANZCTR: Search terms: (surgery OR decompression) AND Health condition(s) or problem(s) studied: spinal stenosis
Appendix 2. The GRADE approach to evidence synthesis
The quality of evidence will be categorised as follows:
High (⊕⊕⊕⊕): further research is very unlikely to change the confidence in the estimate of effect.
Moderate (⊕⊕⊕⊝): further research is likely to have an important impact in the confidence in the estimate of effect.
Low (⊕⊕⊝⊝): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very Low (⊕⊝⊝⊝): any estimate of effect is very uncertain.
The evidence available to answer each sub‐question will be graded on the domains in the following manner:
1. Risk of bias
Limitations in the study design and implementation may bias the estimates of the treatment effect. Our confidence in the estimate of the effect and in the following recommendation decreases if studies suffer from major limitations. We will examine all studies on five types of biases:
a) Selection (random sequence generation, allocation concealment, group similarities at baseline)
b) Performance (blinding of participants, blinding of healthcare providers)
c) Attrition (dropouts and intention‐to‐treat analysis)
d) Measurement (blinding of the outcome assessors and timing of outcome assessment)
e) Reporting bias (selective reporting)
The quality of evidence will be downgraded as follows:
by one level: when most of the evidence comes from individual studies either with a crucial limitation for one criterion, or with some limitations for multiple criteria
by two levels: when most of the evidence comes from individual studies with crucial limitations for multiple criteria
2. Inconsistency
Inconsistency refers to an unexplained heterogeneity of results. Widely differing estimates of the treatment effect (i.e. heterogeneity or variability in results) across studies suggest true differences in underlying treatment effect. Inconsistency may arise from differences in: populations (e.g. drugs may have larger relative effects in sicker populations), interventions (e.g. larger effects with higher drug doses), or outcomes (e.g. diminishing treatment effect with time).
The quality of evidence will be downgraded as follows:
by one level: when the heterogeneity or variability in results is large.
by two levels: when the heterogeneity or variability in results is large AND there was inconsistency arising from populations, interventions, or outcomes.
3. Indirectness
Indirect population, intervention, comparator, or outcome: the question being addressed in this systematic review is different from the available evidence regarding the population, intervention, comparator, or an outcome in the included randomised trial.
The quality of evidence will be downgraded as follows:
by one level: when there is indirectness in only one area
by two levels: when there is indirectness in two or more areas
4. Imprecision
Results are imprecise when studies include relatively few participants and few events and thus have wide confidence intervals around the estimate of the effect. In such a case we judge the quality of the evidence to be lower than it otherwise would be because of uncertainty in the results. Each outcome is considered separately.
For dichotomous outcomes
We will consider imprecision for either of the following two reasons:
1. There is only one study (unless the study provide data from more than 300 participants). When there is more than one study, the total number of events is less than 300 (a threshold rule‐of‐thumb value) (Guyatt 2011).
2. 95% confidence interval around the pooled or best estimate of effect includes both 1) no effect and 2) appreciable benefit or appreciable harm. The threshold for ’appreciable benefit’ or ’appreciable harm’ is a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%.
The quality of the evidence will be downgraded as follows:
by one level: when there is imprecision due to (1) or (2)
by two levels: when there is imprecision due to (1) and (2)
For continuous outcomes
We will consider imprecision for either of the following two reasons:
1. There is only one study (unless the study provide data from more than 400 participants). When there is more than one study, total population size is less than 400 (a threshold rule‐of‐thumb value; using the usual α and β , and an effect size of 0.2 standard deviations, representing a small effect).
2. 95% confidence interval includes no effect and the upper or lower confidence limit crosses an effect size (standardised mean difference) of 0.5 in either direction.
The quality of the evidence will be downgraded as follows:
by one level: when there is imprecision due to (1) or (2)
by two levels: when there is imprecision due to (1) and (2)
5. Publication bias
Publication bias is a systematic underestimate or an overestimate of the underlying beneficial or harmful effect due to the selective publication of studies.
The quality of evidence will be downgraded as follows:
by one level: when the funnel plot suggests publication bias
Data and analyses
Comparison 1. Decompression alone versus decompression plus fusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | 446 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐4.07, 6.26] |
| 1.1 Short‐term (less than 12 months) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 4.50 [‐0.70, 9.70] |
| 1.2 Long‐term (12 months or more) | 4 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐7.32, 6.74] |
| 2 Disability | 3 | 401 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐3.37, 10.11] |
| 2.1 Short‐term (less than 12 months) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 5.2 [‐3.50, 13.90] |
| 2.2 Long‐term (12 months or more) | 3 | 335 | Mean Difference (IV, Random, 95% CI) | 3.26 [‐6.12, 12.63] |
| 3 Walking ability | 3 | 316 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 3.1 Long‐term (12 months or more) | 3 | 316 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 4 Operation time | 4 | 381 | Mean Difference (IV, Random, 95% CI) | ‐107.94 [‐161.65, ‐54.23] |
| 5 Blood loss | 4 | 383 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.70, ‐0.34] |
| 6 Reoperations | 5 | 443 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.81, 1.92] |
| 7 Hospitalisation | 2 | 295 | Mean Difference (IV, Fixed, 95% CI) | ‐1.69 [‐2.12, ‐1.26] |
1.1. Analysis.
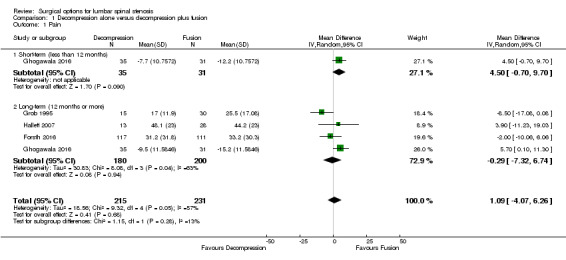
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 1 Pain.
1.2. Analysis.
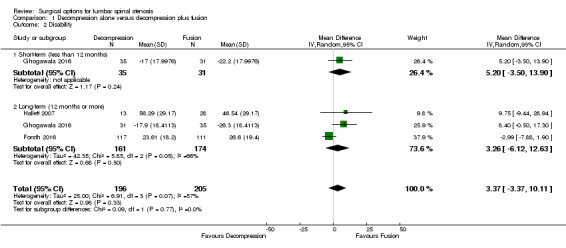
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 2 Disability.
1.3. Analysis.
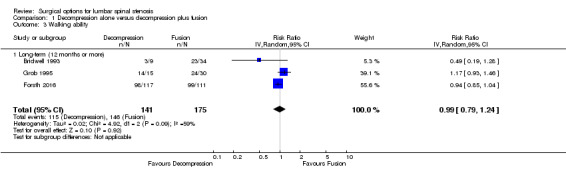
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 3 Walking ability.
1.4. Analysis.
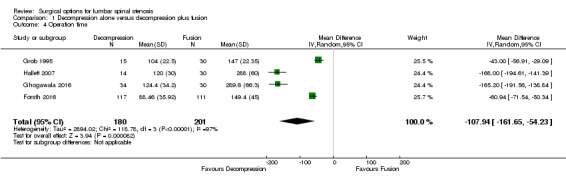
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 4 Operation time.
1.5. Analysis.
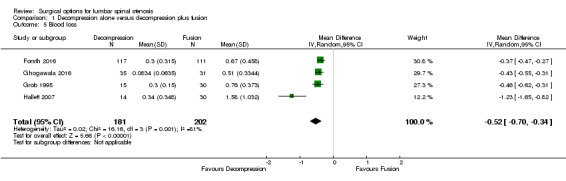
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 5 Blood loss.
1.6. Analysis.
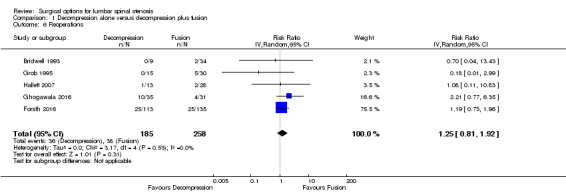
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 6 Reoperations.
1.7. Analysis.
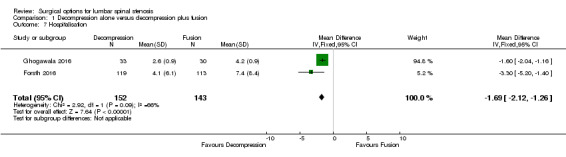
Comparison 1 Decompression alone versus decompression plus fusion, Outcome 7 Hospitalisation.
Comparison 2. Decompression versus interspinous spacer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | 656 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐6.08, 4.31] |
| 1.1 Short‐term (less than 12 months) | 3 | 328 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐9.86, 8.00] |
| 1.2 Long‐term (12 months or more) | 3 | 328 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐8.08, 6.99] |
| 2 Disability | 3 | 656 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐2.01, 4.69] |
| 2.1 Short‐term (less than 12 months) | 3 | 329 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐3.64, 6.25] |
| 2.2 Long‐term (12 months or more) | 3 | 327 | Mean Difference (IV, Random, 95% CI) | 1.25 [‐4.48, 6.98] |
| 3 Function | 2 | 360 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.19, 0.12] |
| 3.1 Short‐term (less than 12 months) | 2 | 185 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.27, 0.14] |
| 3.2 Long‐term (12 months or more) | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.29] |
| 4 Quality of life | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, 0.00] |
| 4.1 Short‐term (less than 12 months) | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 4.2 Long‐term (12 months or more) | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.18, 0.07] |
| 5 Costs | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 2856.34 [1970.40, 3742.28] |
| 6 Operation time | 3 | 340 | Mean Difference (IV, Random, 95% CI) | 39.11 [19.43, 58.78] |
| 7 Blood loss | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 144.0 [‐209.74, 497.74] |
| 8 Reoperations | 3 | 326 | Risk Ratio (IV, Random, 95% CI) | 3.95 [2.12, 7.37] |
| 9 Hospitalisation | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.58, 1.60] |
2.1. Analysis.

Comparison 2 Decompression versus interspinous spacer, Outcome 1 Pain.
2.2. Analysis.
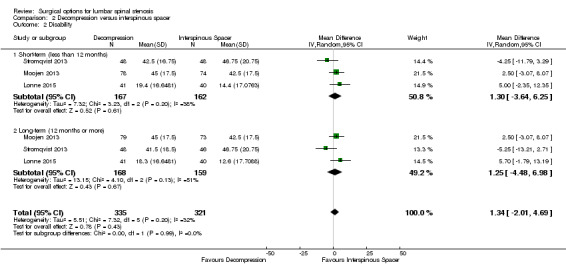
Comparison 2 Decompression versus interspinous spacer, Outcome 2 Disability.
2.3. Analysis.
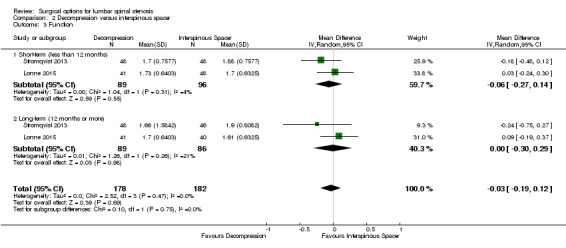
Comparison 2 Decompression versus interspinous spacer, Outcome 3 Function.
2.4. Analysis.
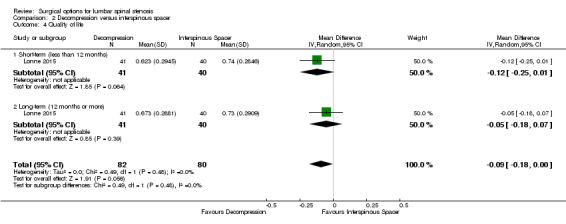
Comparison 2 Decompression versus interspinous spacer, Outcome 4 Quality of life.
2.5. Analysis.
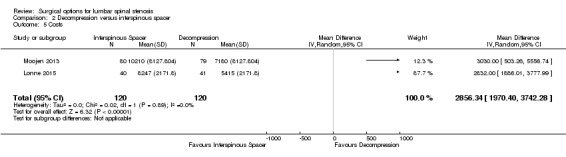
Comparison 2 Decompression versus interspinous spacer, Outcome 5 Costs.
2.6. Analysis.
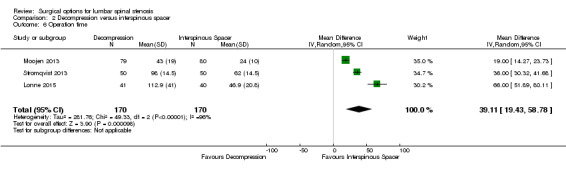
Comparison 2 Decompression versus interspinous spacer, Outcome 6 Operation time.
2.7. Analysis.
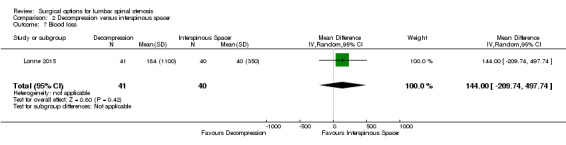
Comparison 2 Decompression versus interspinous spacer, Outcome 7 Blood loss.
2.8. Analysis.
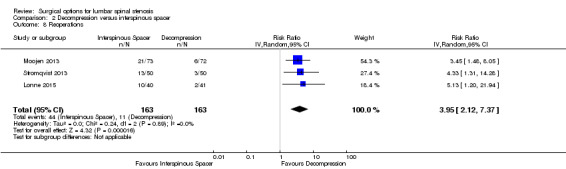
Comparison 2 Decompression versus interspinous spacer, Outcome 8 Reoperations.
2.9. Analysis.
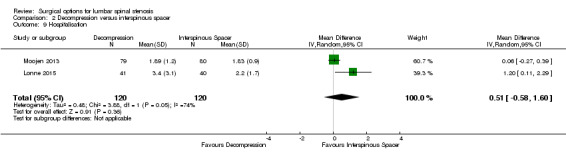
Comparison 2 Decompression versus interspinous spacer, Outcome 9 Hospitalisation.
Comparison 3. Decompression plus fusion versus interspinous spacer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 5.35 [‐1.18, 11.88] |
| 1.1 Long‐term (12 months or more) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 5.35 [‐1.18, 11.88] |
| 2 Disability | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 5.72 [1.28, 10.15] |
| 2.1 Long‐term (12 months or more) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 5.72 [1.28, 10.15] |
| 3 Quality of life | 1 | 226 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.30, 0.10] |
| 3.1 Long‐term (12 months or more) | 1 | 226 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.30, 0.10] |
| 4 Operation time | 2 | 381 | Mean Difference (IV, Random, 95% CI) | 78.91 [30.16, 127.65] |
| 5 Blood loss | 1 | 320 | Mean Difference (IV, Random, 95% CI) | 238.90 [182.66, 295.14] |
| 6 Reoperations | 1 | 322 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.32, 1.51] |
| 7 Hospitalisation | 2 | 382 | Mean Difference (IV, Random, 95% CI) | 1.58 [0.90, 2.27] |
3.1. Analysis.
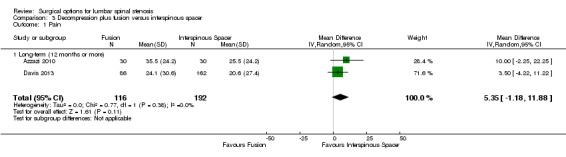
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 1 Pain.
3.2. Analysis.
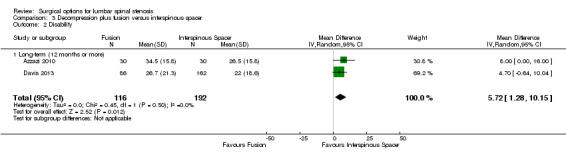
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 2 Disability.
3.3. Analysis.
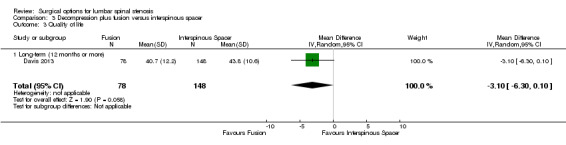
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 3 Quality of life.
3.4. Analysis.
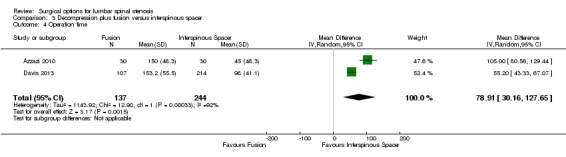
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 4 Operation time.
3.5. Analysis.
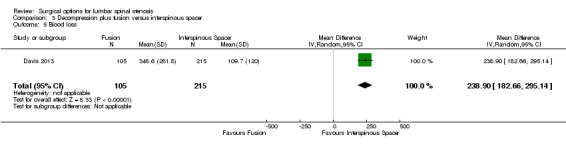
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 5 Blood loss.
3.6. Analysis.
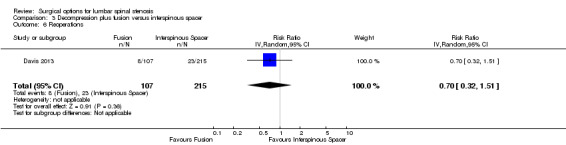
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 6 Reoperations.
3.7. Analysis.
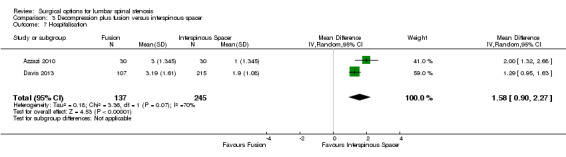
Comparison 3 Decompression plus fusion versus interspinous spacer, Outcome 7 Hospitalisation.
Comparison 4. Laminectomy versus laminotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 6 | 728 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐4.36, 3.02] |
| 1.1 Short‐term (less than 12 months) | 3 | 281 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐2.39, 3.04] |
| 1.2 Long‐term (12 months or more) | 6 | 447 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐8.19, 4.35] |
| 2 Disability | 6 | 722 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.81, 2.90] |
| 2.1 Short‐term (less than 12 months) | 4 | 333 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐1.02, 4.13] |
| 2.2 Long‐term (12 months or more) | 5 | 389 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐4.37, 3.52] |
| 3 Walking ability | 3 | 414 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.15] |
| 3.1 Short‐term (less than 12 months) | 3 | 233 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.33, 0.20] |
| 3.2 Long‐term (12 months or more) | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.33, 0.28] |
| 4 Operation time | 5 | 339 | Mean Difference (IV, Random, 95% CI) | ‐6.25 [‐13.76, 1.27] |
| 5 Blood loss | 5 | 381 | Mean Difference (IV, Random, 95% CI) | 38.80 [17.81, 59.80] |
| 6 Reoperations | 2 | 182 | Risk Ratio (IV, Random, 95% CI) | 2.61 [0.78, 8.78] |
| 7 Hospitalisation | 2 | 139 | Mean Difference (IV, Random, 95% CI) | 1.55 [0.61, 2.50] |
4.1. Analysis.
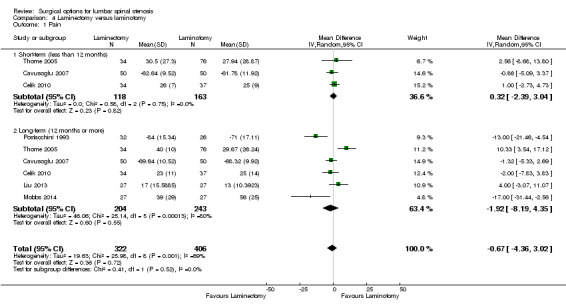
Comparison 4 Laminectomy versus laminotomy, Outcome 1 Pain.
4.2. Analysis.
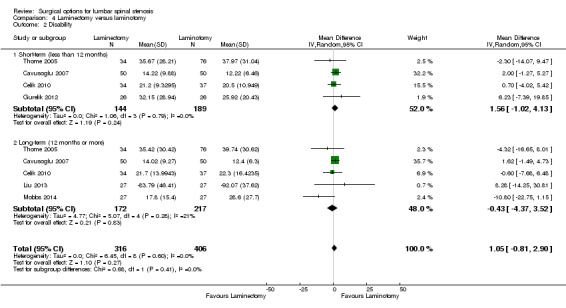
Comparison 4 Laminectomy versus laminotomy, Outcome 2 Disability.
4.3. Analysis.
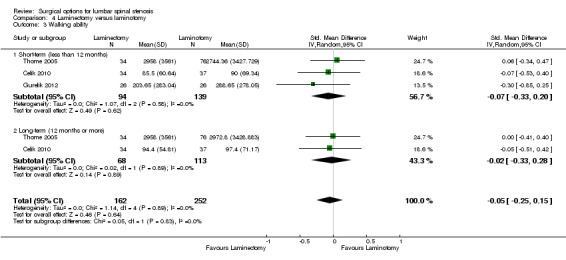
Comparison 4 Laminectomy versus laminotomy, Outcome 3 Walking ability.
4.4. Analysis.
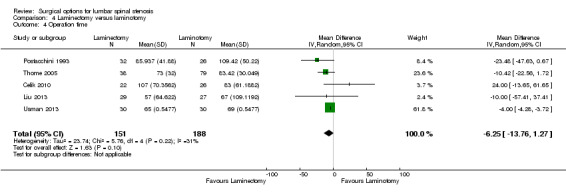
Comparison 4 Laminectomy versus laminotomy, Outcome 4 Operation time.
4.5. Analysis.
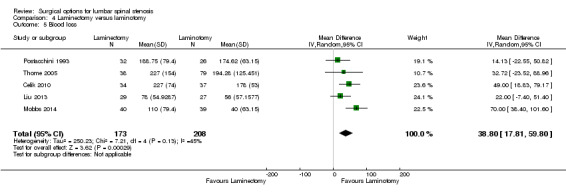
Comparison 4 Laminectomy versus laminotomy, Outcome 5 Blood loss.
4.6. Analysis.
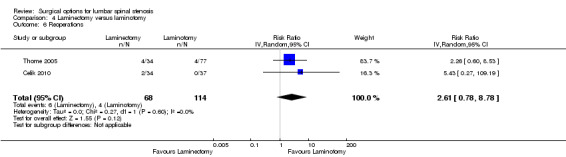
Comparison 4 Laminectomy versus laminotomy, Outcome 6 Reoperations.
4.7. Analysis.
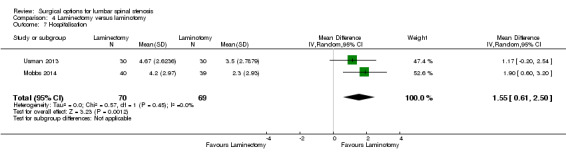
Comparison 4 Laminectomy versus laminotomy, Outcome 7 Hospitalisation.
Comparison 5. Decompression versus split‐decompression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | 175 | Mean Difference (IV, Random, 95% CI) | 6.35 [‐3.35, 16.04] |
| 1.1 Long‐term (12 months or more) | 3 | 175 | Mean Difference (IV, Random, 95% CI) | 6.35 [‐3.35, 16.04] |
| 2 Disability | 4 | 207 | Mean Difference (IV, Random, 95% CI) | 1.87 [‐2.82, 6.57] |
| 2.1 Long‐term (12 months or more) | 4 | 207 | Mean Difference (IV, Random, 95% CI) | 1.87 [‐2.82, 6.57] |
| 3 Recovery | 4 | 207 | Mean Difference (IV, Random, 95% CI) | ‐5.18 [‐19.81, 9.45] |
| 4 Operation time | 4 | 211 | Mean Difference (IV, Random, 95% CI) | ‐10.57 [‐34.39, 13.25] |
| 5 Blood loss | 4 | 211 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐27.65, 23.98] |
| 6 Reoperations | 3 | 153 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.22, 6.85] |
| 7 Hospitalisation | 2 | 121 | Mean Difference (IV, Random, 95% CI) | 1.49 [‐1.70, 4.67] |
5.1. Analysis.
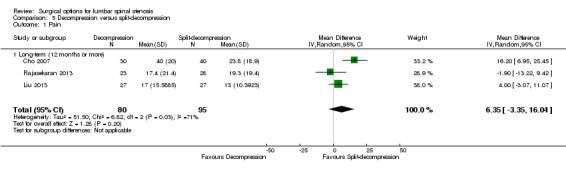
Comparison 5 Decompression versus split‐decompression, Outcome 1 Pain.
5.2. Analysis.
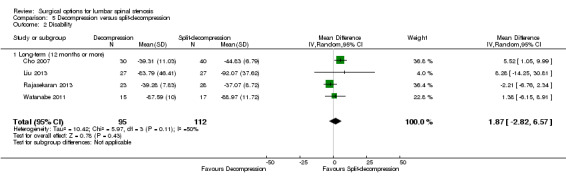
Comparison 5 Decompression versus split‐decompression, Outcome 2 Disability.
5.3. Analysis.

Comparison 5 Decompression versus split‐decompression, Outcome 3 Recovery.
5.4. Analysis.
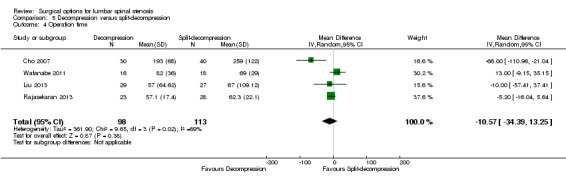
Comparison 5 Decompression versus split‐decompression, Outcome 4 Operation time.
5.5. Analysis.
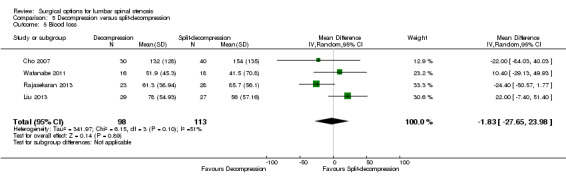
Comparison 5 Decompression versus split‐decompression, Outcome 5 Blood loss.
5.6. Analysis.
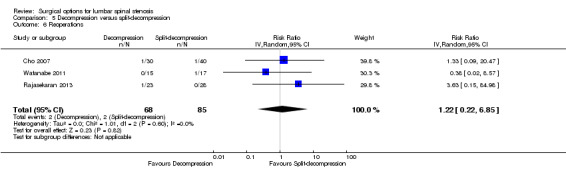
Comparison 5 Decompression versus split‐decompression, Outcome 6 Reoperations.
5.7. Analysis.

Comparison 5 Decompression versus split‐decompression, Outcome 7 Hospitalisation.
Comparison 6. Decompression versus endoscopic decompression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disability | 3 | 724 | Mean Difference (IV, Random, 95% CI) | 2.86 [0.26, 5.45] |
| 1.1 Short‐term (less than 12 months) | 3 | 362 | Mean Difference (IV, Random, 95% CI) | 4.12 [0.91, 7.33] |
| 1.2 Long‐term (12 months or more) | 3 | 362 | Mean Difference (IV, Random, 95% CI) | 1.44 [‐2.66, 5.54] |
| 2 Operation time | 3 | 393 | Mean Difference (IV, Random, 95% CI) | 10.05 [‐2.09, 22.18] |
| 3 Blood loss | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 34.0 [30.40, 37.60] |
| 4 Reoperations | 2 | 321 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.22, 2.97] |
| 5 Hospitalisation | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 8.56 [6.78, 10.34] |
6.1. Analysis.
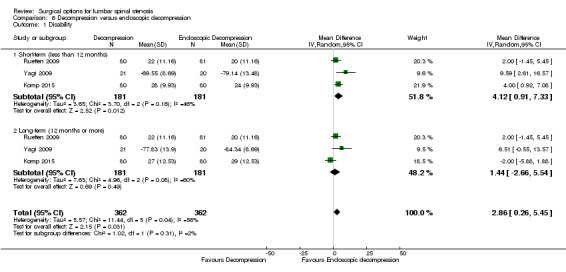
Comparison 6 Decompression versus endoscopic decompression, Outcome 1 Disability.
6.2. Analysis.
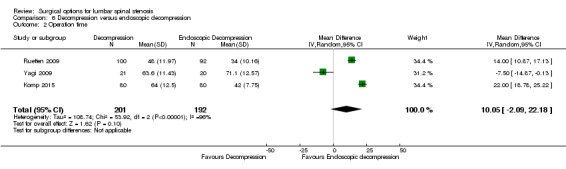
Comparison 6 Decompression versus endoscopic decompression, Outcome 2 Operation time.
6.3. Analysis.
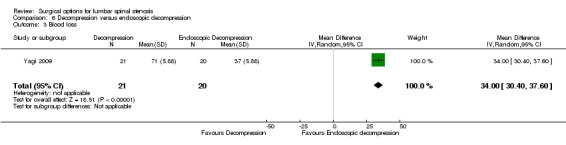
Comparison 6 Decompression versus endoscopic decompression, Outcome 3 Blood loss.
6.4. Analysis.
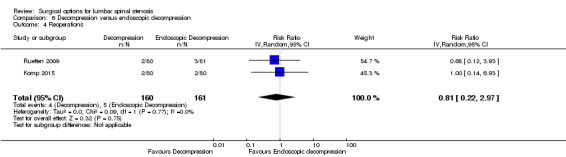
Comparison 6 Decompression versus endoscopic decompression, Outcome 4 Reoperations.
6.5. Analysis.
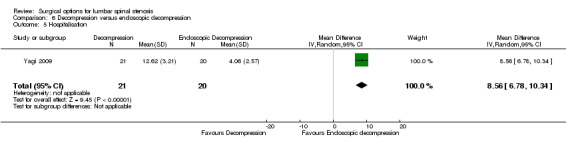
Comparison 6 Decompression versus endoscopic decompression, Outcome 5 Hospitalisation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azzazi 2010.
| Methods | Single‐centre RCT Setting: not reported Country: Egypt Period: March 2005 to May 2007 |
|
| Participants |
Number: 60 patients (30/30) Diagnosis: physical and neurological examinations and assessment of imaging studies (computerized tomography and magnetic resonance imaging) Included: degenerative spondylolisthesis up to grade I; lateral or central spinal stenosis; predominant component of leg pain (preoperative score of 40 mm on a 100 mm VAS) rather than back pain symptoms; moderate disability; unresponsive to conservative treatment for a minimum of three months Excluded: previous lumbar fusion, decompression or total facetectomy; trauma; diseases that preclude surgical management; patients younger than 20 years or older than 80 years of age; BMI greater than 40 Age (years): mean (range) 56.3 (27–79) BMI (kg/m²): mean 27/29 Lumbar stenosis duration (years): mean (range) 5.3 (0.2–36.9) |
|
| Interventions |
Group 1: decompression plus transpedicular screw fixation Group 2: interspinous process spacer device (X‐Stop) Follow‐up: 24 months |
|
| Outcomes |
Pain: 100 mm visual analogue scale leg pain Disability: ODI Operation time Complications Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "60 patients enrolled and randomized to be treated with either..." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about drop‐outs. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | The authors report in the methods that perioperative blood loss was recorded and patients returned for follow‐up evaluations 3 weeks, then 3, 6, 12 and 24 months after surgery. The results for blood loss were not reported and only 24‐month data were reported. Attempts to access these data from the authors was unsuccessful. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 1. |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Bridwell 1993.
| Methods | Single‐centre RCT Setting: Barnes‐Jewish Hospital, St. Louis Missouri Country: USA Period: February 1985 to March 1990 |
|
| Participants |
Number: 44 patients (9/11/24) Diagnosis: magnetic resonance and computed tomographic imaging. Spinal claudication caused by spinal stenosis at the spondylolisthesis level Included: no previous spine surgery Excluded: not reported Age (years): mean (range) 66.1 (46‐79) |
|
| Interventions |
Group 1: decompression alone. Surgical decompression comprised of laminectomy with preservation of bilateral facet joints without discectomy or extensive foraminotomy Group 2: decompression plus posterolateral (transverse processes) fusion without instrumentation or posterolateral (facets and transverse processes) fusion with instrumentation. All fusions were performed with autogenous iliac bone graft. Follow‐up: 37.2 months |
|
| Outcomes |
Disability: Walking ability: worse, same or significantly better after surgery Complications Reoperations |
|
| Notes |
Surgeon's experience: not reported Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "the patients were randomized so that". The authors report an error in the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43/44=97.7% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | Protocol not available, and relevant outcomes were not reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patient characteristics at baseline. |
| Co‐interventions (performance bias) | Unclear risk | Only the surgical technique differed between treatment groups. No concomitant discectomy, but foraminotomy was performed in some patients. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest not reported. Financial support was not reported in this study. |
Cavusoglu 2007.
| Methods | Single‐centre RCT Setting: Sisli Etfal State Hospital, Istanbul Country: Turkey Period: January 2000 to January 2002 |
|
| Participants |
Number: 100 patients (50/50) Diagnosis: physical examination, preoperative radiological investigations with plain roentgenogram, magnetic resonance and computed tomographic images Included: symptoms of neurogenic claudication or radiculopathy; radiological/neuroimaging evidence of lumbar stenosis; absence of associated pathology; no history of spinal surgery; non‐respondents to minimal 3 months of conservative care Excluded: not reported Age (years): mean (SD) 69.2 (12.2) Lumbar stenosis duration (years): range 0.7 to 5.0 |
|
| Interventions |
Group 1: hemi‐laminectomy with preservation of posterior midline structures Group 2: unilateral laminotomy for bilateral decompression. Decompression of the lateral recess was performed in the unilateral laminectomy group preserving the facet joints, and discectomy was performed if necessary. Follow‐up: 64.8 months |
|
| Outcomes |
Pain: 100‐point SF‐36 body pain Disability: ODI Complications |
|
| Notes |
Surgeon's experience: not reported Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a concealed computer‐generated randomization list was used to assign the patient to one of the two treatment groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "a concealed computer‐generated randomization list..." |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a single radiologist blinded to the clinical results of decompression reviewed all pre and postoperative studies" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97/100 = 97% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 1. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Unclear risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Celik 2010.
| Methods | Single‐centre RCT Setting: Department of Neurosurgery, Beyoglu State Hospital, Istanbul Country: Turkey Period: July 2001 to May 2003 |
|
| Participants |
Number: 80 patients (40/40) Diagnosis: dynamic x‐rays, thin‐sliced CT and MRI; severe back/leg pain and neurogenic claudication; anteroposterior diameter less than 10 mm of the lumbar spinal canal by CT scan and MRI Included: patients who had not responded to conservative medical therapy and physical therapy; more than 41% in ODI; more than 7 in VAS pain; walking distance less than 30 meters; severe lumbar spinal stenosis clinically Excluded: patients requiring discectomy or showing any kind of instability before the surgery Age (years): mean (SD) 61 (13)/59 (14) |
|
| Interventions |
Group 1: total laminectomy Group 2: bilateral micro decompressive laminotomy. Medial facetectomy and wide foraminotomies were performed at the level of stenosis, preserving the lateral aspect of the facet joints. No patient received discectomy. Follow‐up: 60 months |
|
| Outcomes |
Pain: 10 cm VAS leg pain Disability: ODI, walking distance Operation time Perioperative blood loss Complications Reoperations |
|
| Notes |
Surgeon's experience: "both groups of patients were operated by the same senior surgeon in the same time period" Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a chart system was used to process randomizaton". |
| Allocation concealment (selection bias) | Low risk | Quote: "a registered nurse informed surgeons about the type of surgery before the operation". |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "patients were not informed as which group they would be placed". |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the patients were preoperative examined and followed at regular intervals by the operating neurosurgeons and by a neurology specialist blinded to the study protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 71/80 = 89% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes. |
| Group similarity at baseline (selection bias) | Low risk | There were no preoperative differences between groups, based on Tables 1 to 3. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Unclear risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Cho 2007.
| Methods | Single‐centre RCT Setting: China Medical University and Hospital Country: China Period: May 2005 to January 2006 |
|
| Participants |
Number: 70 patients (30/40) Diagnosis: CT and MRI: antero‐posterior diameter of the spinal canal less than 11 mm, an interpediculate distance of less than 16 mm, and a lateral recess distance of less than 3 mm; clinical symptoms of lumbago and intermittent claudication Included: patients with lumbar stenosis with surgical indication for repair Excluded: patients > 80 years of age with high anaesthetic risks or severe co‐morbidity; patients requiring concomitant fusion Age (years): mean (SD) 61 (11)/59 (15) Lumbar stenosis duration (years): mean (SD) 4.0 (0.7)/5.3 (0.7) |
|
| Interventions |
Group 1: laminectomy Group 2: split‐spinous process laminotomy Follow‐up: 15 months |
|
| Outcomes |
Disability: JOA Operation time Perioperative blood loss Complications Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about drop‐outs. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on the Table 3. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. Similar percentage of concomitant discectomy. All participants received the same postoperative care. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Davis 2013.
| Methods | Multi‐centre RCT Setting: 21 sites in the United States Country: USA Period: 2006 to 2010 |
|
| Participants |
Number: 322 patients (215/107) Diagnosis: central, foraminal or lateral stenosis; more than 25% reduction of the anteroposterior dimension compared with the next adjacent normal level, with nerve root crowding compared with the normal level, as determined by the investigator on CT or MRI Included: patients with moderate radiographical diagnosis of spinal stenosis with low back pain; spondylolisthesis up to Meyerding grade I; minimum ODI of 20 (0‐50), and VAS back pain score of 50 or more (0‐100); minimum 6 months of conservative care Excluded: prior lumbar surgery; trauma or tumour; isthmic spondylolisthesis; spondylolysis; scoliosis > 25 degrees; disc herniation; serious disease Age (years): mean (SD) 64.1 (9.0)/62.1 (9.2) |
|
| Interventions |
Group 1: decompression plus transpedicular screw fixation Group 2: Coflex interspinous process spacer device (Paradigm spine, LLC, New York, NY) Follow‐up: 24 months |
|
| Outcomes |
Pain: 100 mm VAS leg pain Disability: ODI Operation time Perioperative blood loss Complications Reoperations Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: "Paradigm Spine, LLC (New York, NY) funds were received in support of this work. Relevant financial activities outside the submitted work: consultancy, royalties, payment for lecture, payment for manuscript preparation, patents, payment for development of educational presentations" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization codes" |
| Allocation concealment (selection bias) | Low risk | Quote: "centralized by the study sponsor" |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "study subjects were blinded until after surgery" |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "site study personnel were blinded to the treatment assignment up until 5 days prior to surgery" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Tables 4 to 9. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Quote: "Paradigm Spine, LLC (New York, NY) funds were received in support of this work. Relevant financial activities outside the submitted work: consultancy, royalties, payment for lecture, payment for manuscript preparation, patents, payment for development of educational presentations." |
Forsth 2016.
| Methods | Multi‐centre RCT Setting: 7 Swedish hospitals Country: Sweden Period: October 2006 to June 2012 |
|
| Participants |
Number: 247 patients (124/123) Diagnosis: pseudoclaudication and image findings as per inclusion criteria Included: pseudoclaudication in one or both legs and back pain (score on VAS > 30), 1 or 2 adjacent stenotic segments (cross‐section area of the dural sac ≤ 75 mm²) between L2 and the sacrum on MRI, duration of symptoms > 6 months Excluded: spondylolysis, degenerative lumbar scoliosis, history of lumbar spinal surgery for spinal stenosis or instability, stenosis not caused by degenerative changes, stenosis caused by a herniated disk, other specific spinal conditions, history of vertebral compression fractures in affected segments, psychological disorders Age (years): mean (SD) 66.0 (8.0)/66.0 (9.0) |
|
| Interventions |
Group 1: decompression alone Group 2: decompression plus fusion. The surgical technique was determined solely by the surgeon Follow‐up: 24 months |
|
| Outcomes |
Pain: 100 mm VAS leg pain Disability: ODI Operation time Perioperative blood loss Complications Reoperations Length of hospital stay Costs |
|
| Notes |
Surgeon's experience: all the trial surgeons were senior consultants and were highly experienced in performing the two trial interventions Funding: funded by an Uppsala institutional Avtal om Läkarutbildning och Forskning |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization was performed with the use of a Web‐based system that enabled computer‐generated random treatment assignment" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Authors used a modified intention‐to‐treat analysis that included 9 patients who did not initially receive the assigned treatment but did undergo subsequent surgery. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 2. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "No institution or company had a role in the data analysis, the preparation of the manuscript, or the decision to submit the manuscript for publication." |
Ghogawala 2016.
| Methods | Multi‐centre RCT Setting: 5 hospitals Country: USA Period: March 2002 to August 2009 |
|
| Participants |
Number: 66 patients (35/31) Diagnosis: standardized radiographic and magnetic resonance images Included: patients with grade I lumbar spondylolisthesis (degree of spondylolisthesis: 3 to 14 mm) with lumbar stenosis and neurogenic claudication with or without lumbar radiculopathy Excluded: radiography revealed lumbar instability (motion of > 3 mm at the level of listhesis, as measured on flexion–extension radiographs of the lumbar spine), previous lumbar spinal surgery, severe systemic disease Age (years): mean (SD) 66.5 (8.0)/66.7 (7.2) |
|
| Interventions |
Group 1: decompression alone by a complete laminectomy with partial removal of the medial facet joint Group 2: decompression plus fusion. Patients in the fusion group underwent a lumbar laminectomy as well as implantation of pedicle screws and titanium alloy rods across the level of listhesis, with a bone graft harvested from the iliac crest Follow‐up: 24 months |
|
| Outcomes |
Pain: SF‐36 bodily pain subscale Disability: ODI Operation time Perioperative blood loss Reoperations Length of hospital stay |
|
| Notes |
Surgeon's experience: all surgeons routinely performed both operations tested in the trial; each of the surgeons had performed at
least 100 laminectomies and 100 posterolateral fusions for lumbar spondylolisthesis before joining the trial. Funding: There was no industry funding or any other industry involvement in the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "independent study coordinator who was not aware of the study hypothesis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 1. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "There was no industry funding or any other industry involvement in the SLIP trial." |
Grob 1995.
| Methods | Single‐centre RCT Setting: Schutthess Hospital, Zurich Country: Switzerland Period: November 1989 to November 1990 |
|
| Participants |
Number: 45 patients (15/15/15) Diagnosis: history and clinical examination; CT and MRI (mid‐sagittal diameter of the spinal canal of less than 11 mm) Included: degenerative spinal stenosis Excluded: systemic disease; instability of the spine; previous operation Age (years): mean (range) 67 (48‐87) Lumbar stenosis duration (years): mean (range) 1.3 (0.5‐3.1) |
|
| Interventions |
Group 1: decompression alone. Decompression involved widening of the lateral recess, undercut of lamina, and discectomy or foraminotomy in some patients Group 2: decompression plus arthrodesis of the most stenotic segment Group 3: decompression plus arthrodesis of all of the decompressed vertebral segments Follow‐up: 28 months |
|
| Outcomes |
Pain: 10 cm VAS overall pain Disability: walking ability Operation time Perioperative blood loss: Complications Reoperations |
|
| Notes |
Surgeon's experience: All the operations were performed by the same surgeon Funding: "no funds were received in support to this study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomly assigned to the three treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the patients completed the follow‐up. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patients characteristics at baseline. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. Similar percentage of concomitant discectomy. All participants received the same postoperative care. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | High risk | Patients were assessed at different time points. The average duration of follow‐up was 38 months (range: 24 to 32). |
| Other bias | Low risk | Quote: "no funds were received in support to this study" |
Gurelik 2012.
| Methods | Single‐centre RCT Setting: Department of Neurosurgery, Van Training and Research Hospital, Van Country: Turkey Period: January 2006 to February 2009 |
|
| Participants |
Number: 52 patients (26/26) Diagnosis: MRI of degenerative lumbar spinal stenosis with symptoms of neurogenic claudication or radiculopathy Included: symptoms of neurogenic claudication or radiculopathy; radiological evidence of degenerative lumbar stenosis; absence of associated pathological entities such as instability and significant disc herniation; absence of previous surgery for lumbar spine disorder; non‐respondents to conservative care Excluded: not reported Age (years): mean (SD) 57.5 (8.5)/60.7 (10.0) |
|
| Interventions |
Group 1: laminectomy Group 2: unilateral laminotomy. Unilateral laminotomy was performed followed by ipsilateral medial facetectomy and foraminotomy, and the ligamentum flavum were resected partially. For both procedures, the medial aspects of the contralateral facet joints were resected partially. Follow‐up: 6 months |
|
| Outcomes | Disability: ODI, walking distance | |
| Notes |
Surgeon's experience: "all operations were performed by one author" Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to one of the following groups" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "patients were made aware of the method" and "told which operative procedure they were going to have" |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the patients completed the follow‐up. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 1. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Hallett 2007.
| Methods | Single‐centre RCT Setting: Spinal Unit, Royal Infirmary of Edinburgh, Edinburgh Country: Scotland, UK Period: January 1998 to August 2001 |
|
| Participants |
Number: 44 patients (14/15/15) Diagnosis: plain radiographs and magnetic resonance images Included: foraminal stenosis; single‐level degenerative disc disease; uni or bilateral leg pain, with or without positive root tension sign, muscle weakness and/or sensory loss; minimum 3 months of conservative care Excluded: spondylolisthesis Grade II or greater; vertebral translocation > 1 cm (instability); disc space narrowing of greater than 50%; serious disease Age (years): mean (range) 57 (34–75) |
|
| Interventions |
Group 1: decompression (single or bilateral foraminotomy) Group 2: decompression plus instrumented pedicular postero‐lateral fusion Group 3: decompression plus fusion with pedicular screw instrumentation with titanium interbody cages filled with autologous bone. Minimal microdiscectomy was performed if necessary Follow‐up: 60 months |
|
| Outcomes |
Pain: 10 cm VAS from the Low Back Outcome Score Disability: RMDQ Costs Operation time Perioperative blood loss Reoperations |
|
| Notes |
Surgeon's experience: All surgery was performed by the same surgeon in a laminar ventilated theatre. Funding: "supported by a grant from DePuy Ltd., U.K. Corporate/Industry funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "type of treatment was randomly allocated immediately before surgery." |
| Allocation concealment (selection bias) | Low risk | Quote: "shuffled, closed, opaque envelopes, that were numbered 1 to 150 and opened in sequence." |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the 3 observers were not blinded and any dispute was resolved by discussion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93.1% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Quote: "analysis of the results was by intention to treat." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patient characteristics at baseline. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. Similar percentage of concomitant discectomy. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Quote: "supported by a grant from DePuy Ltd., U.K. Corporate/ Industry funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript." |
Komp 2015.
| Methods | Single‐centre RCT Setting: not reported Country: Germany Period: not reported |
|
| Participants |
Number: 160 patients (80/ 80) Diagnosis: clinical assessment Included: predominant leg symptoms; neurogenic claudication with or without paresis; back pain maximum 30/100 on the VAS; conservative therapy exhausted or no longer indicated due to the symptoms; mono segmental central stenosis caused by facet hypertrophy; hypertrophy of the ligamentum flavum; and disc protrusions or a combination of those Excluded: predominant back pain, foraminal stenosis in the lower level, fresh soft disc herniations with bony stenosis; degenerative spondylolisthesis more than Meyerding Grade I; multidirectional rotation slide; scoliosis more than 20°; prior surgery in the same segment; and cauda equina syndrome Age (years): mean (SD) 62 (41‐84) Lumbar stenosis duration (months): mean 17 |
|
| Interventions |
Group 1: conventional microsurgical interlaminar decompression. The conventional decompression operation was performed using the bilateral laminotomy technique with partial facetectomy and flavum resection Group 2: full‐endoscopic interlaminar decompression. Follow‐up: 24 months |
|
| Outcomes |
Pain: 100 mm VAS leg pain Disability: ODI Operation time Perioperative blood loss Complications Reoperations |
|
| Notes |
Surgeon's experience: All operations were performed by 2 surgeons with many years of experience in both techniques Funding: "there was no external funding in the preparation of this manuscript". The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the randomization was carried out as a block randomization." |
| Allocation concealment (selection bias) | Low risk | Quote: "the secretary provided scheduling in a closed envelope." |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "randomization was not blinded, since the patients may identify the operation procedure." |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the follow‐up investigators were not informed of which surgical procedure had been carried out." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 153/160 = 96% of the patients completed the 3‐month follow‐up and 84% completed the 24‐month follow‐up. The number of drop‐outs was similar in each group and the reasons for drop‐out are also reported and are unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patient characteristics at baseline |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. Similar percentage of concomitant discectomy. All participants received the same postoperative care. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "there was no external funding in the preparation of this manuscript". The authors declared no conflicts of interest. |
Liu 2013.
| Methods | Single‐centre RCT Setting: Department of Orthopedic Surgery, Qilu Hospital of Shandong University, Jinan, Shandong Country: China Period: not reported |
|
| Participants |
Number: 56 patients (27/29) Diagnosis: lumbar spinal stenosis diagnosis by an experienced spine specialist Included: patients with lumbar spinal stenosis without degenerative spondylolisthesis or interbody instability Excluded: not reported Age (years): mean (SD) 59.4 (4.7)/61.1 (3.1) Lumbar stenosis duration (years): mean (range) 6.5/5.9 (0.6‐13) |
|
| Interventions |
Group 1: conventional laminectomy Group 2: spinous process‐splitting unilateral laminotomy. The spinous process and the interspinous ligaments were split longitudinally, preserving the paraspinal muscles. Then unilateral laminotomy was conducted for bilateral decompression with removal of the cranial and the caudal portion of the ipsilateral lamina, ligamentum flavum, and medial part of the facet Follow‐up: 24 months |
|
| Outcomes |
Pain: 10 cm VAS leg pain Disability: JOA Operation time Perioperative blood loss |
|
| Notes |
Surgeon's experience: all patients were diagnosed and assessed by experienced spine specialists Funding: "no funds were received in support of this work. No relevant financial activities outside the submitted work" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomly categorized into 2 groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54/57=94.7% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Tables 1 and 2. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "no funds were received in support of this work. No relevant financial activities outside the submitted work." |
Lonne 2015.
| Methods | Multi‐centre RCT Setting: 6 different Norwegian hospitals Country: Norway Period: June 2007 to September 2011 |
|
| Participants |
Number: 96 patients (49/47) Diagnosis: symptoms of neurogenic intermittent claudication and magnetic resonance images and radiographs Included: patients with 1 or 2 stenotic levels (from L2 to L5) and with minor spondylolisthesis (Meyerding, grade 1) Excluded: spinal stenosis at more than 2 levels; previous low back surgery; unilateral radiculopathy; severe paresis; cauda equina syndrome; degenerative spondylolisthesis > grade 1; isthmic spondylolisthesis; severe scoliosis, idiopathic or degenerative (Cobb angle > 10° or sagittally imbalanced); osteoporosis or suspected osteoporotic fractures in lumbar spine; symptomatic coxarthrosis; vascular intermittent claudication; polyneuropathy; malignant disease Age (years): mean (SD) 67 (8.7)/67 (8.8) BMI (kg/m²): mean (SD) 28 (3.8)/28 (4.7) Lumbar stenosis duration (years): more than 2 years for the majority of patients in both groups |
|
| Interventions |
Group 1: minimally invasive decompression (bilateral laminotomy). Decompression was performed by a partial excision of the lower part of the lamina and the medial aspects of the facet joint. Group 2: interspinous process spacer device (X‐Stop). The X‐Stop was inserted between the spinous processes through the interspinous ligament and was secured by the supraspinous ligament posteriorly and by the lamina anteriorly Follow‐up: 24 months |
|
| Outcomes |
Pain: 11‐point numerical rating scale leg pain Disability: ODI Quality of life: EQ‐5D Costs Operation time Perioperative blood loss Complications Reoperations Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: "the study was supported by non‐commercial organisations (South‐East Regional Health Authority, Norway and the National Advisory Unit on Spinal Surgery, St. Olavs Hospital, Norway)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized with randomly selected block sizes by a computer‐based web solution." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81/96 = 84% of the patients completed the follow‐up. The number of drop‐outs was similar in each group and the reasons for drop‐out are also reported and are unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Quote: "in the main evaluation, not only was an intention‐to‐treat analysis performed..." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Tables 2 and 4. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "the study was supported by non‐commercial organisations (South‐East Regional Health Authority, Norway and the National Advisory Unit on Spinal Surgery, St. Olavs Hospital, Norway)." |
Mobbs 2014.
| Methods | Single‐centre RCT Setting: Prince of Wales Hospital, Randwick, Sydney Country: Australia Period: 2007 to 2009 |
|
| Participants |
Number: 79 patients (40/39) Diagnosis: clinical assessment, MRI and CT myelogram Included: symptomatic lumbar spinal stenosis with radiculopathy, neurogenic claudication, urinary dysfunction; radiologically confirmed spinal stenosis caused by degenerative changes; canal stenosis at a maximum of 2 levels Excluded: concomitant fusion, instrumentation placement or lumbar laminectomy involving discectomy; previous lumbar surgeries at the same level; spondylolisthesis of any grade or degenerative scoliosis; evidence of instability on dynamic radiographs Age (years): mean (SD) 65.8 (14.3)/72.7 (10.4) |
|
| Interventions |
Group 1: conventional laminectomy. In the laminectomy group, the spinous process, lamina, ligamentum flavum and portion of the facet joints were removed Group 2: microscopic unilateral laminectomy for bilateral decompression. In the unilateral laminectomy group, a medial ipsilateral facetectomy was performed, and if necessary, a contralateral foraminotomy Follow‐up: 44.3 (15)/36.9 (4.3) months |
|
| Outcomes |
Pain: 10 cm VAS leg pain Disability: ODI Perioperative blood loss Complications Length of hospital stay |
|
| Notes |
Surgeon's experience: surgery performed by a single senior neurosurgeon with extensive experience in lumbar spine surgery and minimally invasive spine surgery Funding: The authors reported no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "assigned to either open decompressive laminectomy or microscopic ULBD in a 1:1 split according to their sequence of presentation." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the observer and statistician were blinded to treatment group by the use of reference numbers." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 54/79 = 68.4%. Similar and proportional number of drop‐outs in each group and similar reasons for withdraw. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | High risk | Patiant characteristics varied substantially for important variables, based on Table 3. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | High risk | Quote: "the mean duration of follow‐up was higher in the open‐surgery group than in the ULBD group." |
| Other bias | Low risk | The authors reported no conflict of interest. |
Moojen 2013.
| Methods | Multi‐centre double blinded RCT Setting: 5 neurosurgical centres in the Netherlands Country: Netherlands Period: October 2008 to September 2011 |
|
| Participants |
Number: 159 patients (79/80) Diagnosis: clinical diagnosis of neurogenic claudication by a neurologist with MRI findings of spinal canal stenosis Included: patients between 40 and 85 years; 3 months of neurogenic claudication; single or 2‐level degenerative lumbar canal stenosis; indication for surgery Excluded: cauda equina syndrome; herniated disc needing discectomy; history of surgery; significant scoliosis Age (years): mean 64/66 BMI (kg/m²): mean (range) 28 (20‐37)/27 (20‐48) Lumbar stenosis duration (years): mean (range) 1.9 (0.1‐17) |
|
| Interventions |
Group 1: decompression (laminotomy, flavectomy, facetectomy). In the decompression group, a partial resection of the adjacent laminas was executed, followed by a flavectomy with bilateral opening of the lateral recess and, if necessary, a medial facetectomy was done Group 2: interspinous process spacer device (Paradigm Spine, USA). In the experimental group, no bony decompression was done and the interspinous process device was implanted by a posterior midline approach. Follow‐up: 12 months |
|
| Outcomes |
Pain: 100 mm VAS leg pain Disability: RMDQ Function: ZCQ (physical function) Costs Operation time Perioperative blood loss Complications Reoperations Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: "Paradigm Spine funded this trial. Paradigm Spine had no role in data collection, design of the study, data analysis, interpretation of data, or writing the report and had no influence over whether to submit the manuscript. All the researchers were individually independent from funders" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized design with variable block sizes, with allocations stratified according to center." |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque, coded and sealed envelopes". "After induction of anaesthesia, the prepared envelope was opened and the patient allocated to one of the treatment arms." |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "patients, nurses on the hospital wards, and research nurses remained blind to the allocated treatment during the follow‐up period of one year." |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all caregivers blind to the allocated treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 151/159 = 95% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Quote: "we compared groups on the basis of an intention to treat analysis." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 1. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. All participants received the same postoperative care. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "Paradigm Spine funded this trial. Paradigm Spine had no role in data collection, design of the study, data analysis, interpretation of data, or writing the report and had no influence over whether to submit the manuscript. All the researchers were individually independent from funders." |
Postacchini 1993.
| Methods | RCT Setting: not reported Country: Italy Period: not reported |
|
| Participants |
Number: 67 patients (26/9/32) Diagnosis: all patients had plain and flexion‐extension radiographs of the lumbar spine with one or more of myelography, plain or contrast‐enhanced computed tomographic, and magnetic resonance imaging Included: patients with central lumbar stenosis who required surgery Excluded: not reported Age (years): mean (range) 57 (43‐79) |
|
| Interventions |
Group 1: multiple laminotomies Group 2: scheduled multiple laminotomies converted to total laminectomy Group 3: total laminectomy. Disc excision was performed at a single level in four patients. A unilateral or bilateral intertransverse fusion was performed in four patients with degenerative spondylolisthesis Follow‐up: 3.7 years (2.2‐5.3) |
|
| Outcomes |
Pain: 100 mm VAS leg pain (radicular symptoms) Operation time Perioperative blood loss Complications |
|
| Notes |
Surgeon's experience: all the patients were operated on by the senior author. Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "we aimed to randomise the choice of surgical procedure, but had to allow the protocol to be broken when multiple laminotomy appeared to be inadequate to obtain sufficient decompression." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "at the latest follow‐up, each patient was interviewed and examined by one of the authors, who was unaware of the type of decompression performed." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 67/70 = 95.7% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patient characteristics at baseline. |
| Co‐interventions (performance bias) | High risk | Concomitant discectomy and fusion were performed at different rates between the groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | High risk | Quote: "the mean follow‐up was 3.7 years (2.2 to 5.3)." |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Rajasekaran 2013.
| Methods | Singe‐centre RCT Setting: Department of Orthopaedics and Spine Surgery, Ganga Hospital, Coimbatore, Tamil Nadu Country: India Period: not reported |
|
| Participants |
Number: 51 patients (28/23) Diagnosis: MRI exam correlating with typical neurogenic claudication symptoms due to degenerative lumbar canal stenosis Included: degenerative lumbar canal stenosis affecting 3 or less levels; typical neurogenic claudication symptoms; MRI demonstrating good clinical correlation; and failure of conservative methods of treatment for a minimum period of 6 months Excluded: spondylolisthesis with slip Meyerding grade 2 or greater; instability at the level of stenosis (as defined by > 3 mm translation or > 10° angular change on flexion extension lateral radiographs); concomitant symptomatic cervical or thoracic stenosis; comorbidities such as cardiopulmonary insufficiency; peripheral neuropathy; peripheral vascular disease, prior lumbar spine surgery; severe hip or knee disease Age (years): mean (SD) 57.3 (11.2)/54.5 (8.2) |
|
| Interventions |
Group 1: lumbar spinous process splitting decompression. In the experimental group, the interspinous and supra spinous ligaments were cut longitudinally in line with the spinous processes, then decompression proceeded according to the conventional method Group 2: conventional midline decompression. In the conventional decompression group the overhanging portion of the proximal spinous process, the interspinous and the supraspinous ligaments were removed, and the ligamentum flavum and the distal half of the proximal lamina were excised. Facetal undercutting was performed as needed Follow‐up: 16 months |
|
| Outcomes |
Pain: 10 cm VAS leg pain (neurogenic claudication) Disability: JOA Recovery Operation time Perioperative blood loss Complications Reoperations Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: "AO Spine India research grant and the Ganga Orthopaedic Research and Education Foundation funds were received in support of this work" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the study was a prospective randomized controlled study" and "surgical treatment method for the patients was determined by an automated computer‐generated block randomization chart." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients outcomes were assessed by an independent observer who was blinded to the type of surgery that a particular patient has undergone." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the patients completed the follow‐up. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Tables 1, 2 and 4. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Unclear risk | Quote: "the mean duration of follow‐up was 14.2 ± 2.9 months (12–16 mo)." |
| Other bias | Unclear risk | Quote: "AO Spine India research grant and the Ganga Orthopaedic Research and Education Foundation funds were received in support of this work." |
Ruetten 2009.
| Methods | Single‐centre RCT Setting: Centre for Orthopaedics and Traumatology, St. Anna‐Hospital Herne, University of Witten/Herdecke, Herne Country: Germany Period: 2003 to 2005 |
|
| Participants |
Number: 192 patients (100/92) Diagnosis: MRI and CT Included: neurogenic claudication with unilateral leg pain with or without paresis; back pain with maximum score of 20/100 points on the VAS; and conservative therapy exhausted or no longer indicated due to the symptoms; monosegmental recess stenosis; no foraminal stenosis in the lower level; no disc herniation; degenerative spondylolisthesis with maximum Meyerding Grade I; no multidirectional rotation slide; scoliosis with maximum curvature of 20°; no prior surgery in the same segment Excluded: not reported Age (years): mean (range) 64 (38‐86) Lumbar stenosis duration (years): mean (range) 1.6 (0.17‐6.5) |
|
| Interventions |
Group 1: conventional microsurgical decompression. Decompression was accomplished by cranial and caudal laminotomy, partial facetectomy, and ligamentum flavum resection Group 2: full‐endoscopic transforaminal decompression. The operating instruments and optics were products supplied by Richard Wolf GmbH Follow‐up: 24 months |
|
| Outcomes |
Disability: ODI Operation time Complications Reoperations |
|
| Notes |
Surgeon's experience: all operations were performed by 2 surgeons who have many years of experience in both techniques. Funding: "the authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper". Financial support was not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized assignment was made by nonphysician study staff. This was accomplished using balanced block randomization." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "the patients are able to identify the surgical procedure." |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The later examiners were not informed about which operative procedure was applied." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 184/192 = 95.8% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patients characteristics at baseline. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "the authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper". Financial support was not reported in this study. |
Stromqvist 2013.
| Methods | Multi‐centre RCT Setting: 3 Swedish spine centres Country: Sweden Period: not reported |
|
| Participants |
Number: 100 patients (50/50) Diagnosis: MRI verified spinal stenosis on 1 or 2 levels in the lumbar spine Included: symptoms of neurogenic claudication for minimum 6 months elicited by walking and relieved by flexion of the spine or sitting down; age 40 years or more was required; spinal stenosis was allowed to be present at maximum 2 levels and minor spondylolisthesis (Meyerding, grade 1) was accepted Excluded: Previous spine surgery (except for successful disc surgery); infection or malignant disorder; osteoporosis diagnosed before referral for surgery and subjected to medical treatment; stenosis of the L5–S1‐level due to the small spinous process of S1 Age (years): mean (range) 69 (49‐89) |
|
| Interventions |
Group 1: decompression alone. The decompressive procedures were performed using laminectomy or laminotomies with facet‐joint sparing techniques Group 2: interspinous process spacer device (X‐Stop) All operations included open procedures Follow‐up: 24 months |
|
| Outcomes |
Pain: 100 mm VAS leg pain Disability: ZCQ (physical function) Operation time Complications Reoperations |
|
| Notes |
Surgeon's experience: not reported Funding: "no funds were received in support of this work" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was performed by using envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was performed by using envelopes." |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96/100 = 96% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Quote: "in the main evaluation, not only was intention‐to‐treat analysis used, but also as‐treated analysis was performed." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 1. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "no funds were received in support of this work." |
Thome 2005.
| Methods | Single‐centre RCT Setting: Departments of Neurosurgery, Neurology, and Neuroradiology, University Hospital Mannheim Country: Germany Period: not reported |
|
| Participants |
Number: 120 patients (40/40/40) Diagnosis: Radiological/neuroimaging evidence of lumbar stenosis Included: symptoms of neurogenic claudication or radiculopathy; radiological/neuroimaging evidence of degenerative lumbar stenosis; absence of associated pathological entities such as disc herniations or instability; no history of surgery for lumbar stenosis or lumbar fusion Excluded: patients who required discectomy Age (years): mean (range) 68 (44‐86) BMI (kg/m²): mean (SD) 28 (4)/29 (6)/29 (4) Lumbar stenosis duration (years): mean (SD) 1.7 (2.5) |
|
| Interventions |
Group 1: laminectomy Group 2: unilateral laminotomy Group 3: bilateral laminotomy An operating microscope and high‐speed burrs and Kerrison rongeurs were used in all procedures. Special care was taken in all three groups to minimize facet joint resection by using an undercutting technique Follow‐up: 12 months |
|
| Outcomes |
Pain: 10 cm VAS overall pain Disability: RMDQ Operation time Perioperative blood loss Complications Reoperations |
|
| Notes |
Surgeon's experience: not reported Funding: Conflict of interest and financial support were not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization list was used to assign the patient to one of the treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 110/120=91.6% of the patients completed the follow‐up. The number of drop‐outs is unlikely to affect the results. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on the Table 1. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Conflict of interest not reported. Financial support was not reported in this study. |
Usman 2013.
| Methods | Single‐centre RCT Setting: Neurosurgery department of PGMI, Lady Reading Hospital, Peshawar Country: Pakistan Period: January 2010 to December 2010 |
|
| Participants |
Number: 60 patients (30/30) Diagnosis: physical examination and radiological/neuroimaging evidence Included: patients with symptoms of radiculopathy or neurogenic claudication; radiological/neuroimaging evidence of lumbar spinal stenosis involving the central canal and/or foraminal stenosis; failure of conservative treatment with medication and physiotherapy for a minimum of three months Excluded: Patients with spondylolisthesis; associated co‐morbid conditions; recurrent lumbar spinal stenosis Age (years): 73.4% between 31‐50 years old |
|
| Interventions |
Group 1: conventional laminectomy Group 2: unilateral approach for bilateral decompression. Unilateral laminotomy was performed with partial resection of the inferior aspect of the cranial hemilamina and the superior aspect of the caudal hemilamina. Bilateral flavectomy was performed, and the lateral recess and neural foramina were decompressed contralaterally Follow‐up: minimum 3 months |
|
| Outcomes |
Operation time Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: Conflict of interest and financial support were not reported in this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 60 patients with lumbar stenosis were randomly assigned to undergo either a conventional laminectomy, or a unilateral approach." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a database was compiled using inpatients and outpatients medical records by an independent observer who was not part of the operative team and/or in patient care." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the patients completed the follow‐up. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | In the methods the authors reported that recovery rate was assessed as an outcomes measure. However, in the results the authors do not report data for this outcome. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patients characteristics at baseline. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Conflict of interest and financial support were not reported in this study. |
Watanabe 2011.
| Methods | Single‐centre RCT Setting: Department of Orthopaedic Surgery, National Hospital Organization, Murayama Medical Center, Tokyo Country: Japan Period: December 2004 to December 2005 |
|
| Participants |
Number: 41 (22/19) Diagnosis: radiography of the lumbar spine, myelography, CT and MRI Included: presence of neurogenic claudication; non‐respondents to minimum 6 months of conservative care; clinical symptoms corresponding to MRI or myelography results; 1‐2 level decompression necessary Excluded: spinal stenosis due to congenital, spondylolytic, traumatic and iatrogenic causes; previous surgery; presence of specific disorders; intermittent claudication due to arterial disease; severe osteoarthrosis or arthritis in the lower limbs; neurological disease causing impaired lower limb function; psychiatric disorders; 3 or more level requiring decompression Age (years): mean (SD) 69 (10)/71 (8) |
|
| Interventions |
Group 1: conventional laminectomy. In the conventional laminectomy group, the spinous processes were detached from the lamina Group 2: lumbar spinous process–splitting laminectomy. The cortex of the tip of the spinous process is removed at the midline using a high‐speed drill with a fine 2 mm diamond‐tipped bur, and then, using an osteotome, the spinous process is divided to the base and detached from the lamina. The supra‐ and interspinous ligaments were also split longitudinally with a scalpel Follow‐up: 12 months |
|
| Outcomes |
Disability: JOA Recovery Operation time Perioperative blood loss Reoperations |
|
| Notes |
Surgeon's experience: not reported Funding: "The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper". Financial support was not reported in this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, randomized, controlled study." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 34/41 = 82.9%. "The reasons for the withdrawal were the extension of decompression levels or the conversion of the procedure from decompression to fusion after randomization. However, we do not think that these withdrawals had a major impact on the results." |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patients characteristics at baseline. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Unclear risk | Quote: "The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper". Financial support was not reported in this study. |
Yagi 2009.
| Methods | Single‐centre RCT Setting: Department of Orthopedic Surgery, Kawasaki Municipal Hospital, Kawasaki city Country: Japan Period: not reported |
|
| Participants |
Number: 41 patients (21/20) Diagnosis: computed tomographic myelography and MRI Included: symptoms of neurogenic claudication referable to the lumbar spine; failure of conservative treatments; absence of associated pathological condition; 1‐level spondylosis Excluded: not reported Age (years): mean (range) 73.3 (63‐79)/70.8 (66‐73) |
|
| Interventions |
Group 1: conventional laminectomy Group 2: median approach microendoscopic laminectomy. The operating microscope was moved into the field and centralized on the laminar base. An osteotomy of the spinous process at the involved level was performed Follow‐up: 24 months |
|
| Outcomes |
Disability: JOA Operation time Perioperative blood loss Length of hospital stay |
|
| Notes |
Surgeon's experience: not reported Funding: "The authors received technical support from Medtronic Sofamor Danek. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patients were divided into 2 groups by turns when they came to our hospital." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) All outcomes | Unclear risk | No mention of any attempts to blind the participants. |
| Bliding of personnel/ care providers (performance bias) | High risk | The surgeon could not have been blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | No information about intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Group similarity at baseline (selection bias) | Unclear risk | No information about patient characteristics at baseline. |
| Co‐interventions (performance bias) | Low risk | Only the surgical technique differed between treatment groups. |
| Compliance (performance bias) | Low risk | Compliance in both treatment groups: 100% (surgery). |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
| Other bias | Low risk | Quote: "The authors received technical support from Medtronic Sofamor Danek. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper." |
RCT: randomized controlled trial
VAS: visual analogue scale
BMI: body mass index
ODI: Oswestry Disability Index
EQ‐5D: EuroQol
SD: standard deviation
SF‐36: Short Form (36‐item) Health Survey
CT: computerized tomography
MRI: magnetic resonance imaging
JOA: Japanese Orthopedic Association scale
RMDQ: Roland‐Morris Disability questionnaire
ZDQ: Zurich Claudication Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdu 2009 | Not a randomised controlled trial |
| Altaf 2011 | Not appropriate comparison |
| Andersen 2008 | Not lumbar spinal stenosis |
| Anderson 2011 | Not a randomised controlled trial |
| Aoki 2012 | Not lumbar spinal stenosis |
| Arriagada 2000 | Not lumbar spinal stenosis |
| Asazuma 2004 | Not a randomised controlled trial |
| Auerbach 2011 | Not appropriate comparison |
| Auerbach 2012 | Not appropriate comparison |
| Bazan 2002 | Not a randomised controlled trial |
| Benli 2006 | Not lumbar spinal stenosis |
| Bjarke 2002 | Not lumbar spinal stenosis |
| Blumenthal 2013 | Not a randomised controlled trial |
| Bresnahan 2009 | Not a randomised controlled trial |
| Cakir 2009 | Not a randomised controlled trial |
| Cannone 2010 | Not a randomised controlled trial |
| Carragee 1997 | Not lumbar spinal stenosis |
| Carrasco 1986 | Not a randomised controlled trial |
| Carreon 2009 | Not lumbar spinal stenosis |
| Cassinelli 2007 | Not a randomised controlled trial |
| Chen 2010 | Not lumbar spinal stenosis |
| Cheng 2009 | Not lumbar spinal stenosis |
| Choi 2009 | Not a randomised controlled trial |
| Dahdaleh 2013 | Not lumbar spinal stenosis |
| Dantas 2007 | Not a randomised controlled trial |
| Delank 2002 | Not a randomised controlled trial |
| Delawi 2010 | Not lumbar spinal stenosis |
| Desai 2012 | Not a randomised controlled trial |
| Dimar 2009 | Not lumbar spinal stenosis |
| Dirisio 2011 | Not appropriate comparison |
| Dryer 2012 | Not appropriate comparison |
| Epstein 2006 | Not a randomised controlled trial |
| Escobar 2003 | Not a randomised controlled trial |
| Fan 2009 | Not a randomised controlled trial |
| Fast 1985 | Not a randomised controlled trial |
| Feng 2011 | Not lumbar spinal stenosis |
| Fitzgerald 1976 | Not a randomised controlled trial |
| Fu 2008 | Not a randomised controlled trial |
| Fujiya 1990 | Not a randomised controlled trial |
| Försth 2013 | Not a randomised controlled trial |
| Ghahreman 2010 | Not a randomised controlled trial |
| González 1992 | Not a randomised controlled trial |
| Gotfryd 2012 | Not a randomised controlled trial |
| Gotfryd 2012a | Not a randomised controlled trial |
| Gu 2009 | Not a randomised controlled trial |
| Haley 2012 | Not appropriate comparison |
| Haley 2012a | Not appropriate comparison |
| Halm 2010 | Not a randomised controlled trial |
| Herkowitz 1991 | Not a randomised controlled trial |
| Hong 2010 | Not a randomised controlled trial |
| Hong 2011 | Not a randomised controlled trial |
| Hwang 2010 | Not lumbar spinal stenosis |
| Ikuta 2005 | Not a randomised controlled trial |
| Imagama 2009 | Not a randomised controlled trial |
| Ito 2010 | Not a randomised controlled trial |
| Katz 1997 | Not a randomised controlled trial |
| Kawaguchi 2004 | Not a randomised controlled trial |
| Kim 2006 | Not lumbar spinal stenosis |
| Kim 2007 | Not a randomised controlled trial |
| Kim 2007a | Not a randomised controlled trial |
| Konno 2000 | Not a randomised controlled trial |
| Kornblum 2004 | Not a randomised controlled trial |
| Korovessis 2004 | Not lumbar spinal stenosis |
| Ledonio 2012 | Not lumbar spinal stenosis |
| Lee 2009 | Not a randomised controlled trial |
| Lian 2010 | Not lumbar spinal stenosis |
| Liao 2011 | Not a randomised controlled trial |
| Mahir 2012 | Not appropriate comparison |
| McConnell 2011 | Not appropriate comparison |
| Michielsen 2013 | Not lumbar spinal stenosis |
| Pappas 1994 | Not a randomised controlled trial |
| Parker 2013 | Not a randomised controlled trial |
| Radcliff 2011 | Not appropriate comparison |
| Radcliff 2012 | Not a randomised controlled trial |
| Rapp 2009 | Not a randomised controlled trial |
| Rapp 2011 | Not a randomised controlled trial |
| Repantis 2009 | Not appropriate comparison |
| Richter 2010 | Not a randomised controlled trial |
| Rompe 1995 | Not a randomised controlled trial |
| Rosa 2012 | Not a randomised controlled trial |
| Rowland 2009 | Not a randomised controlled trial |
| Satomi 1992 | Not a randomised controlled trial |
| Schnake 2006 | Not a randomised controlled trial |
| Sears 2012 | Not appropriate comparison |
| Sengupta 2006 | Not a randomised controlled trial |
| Shapiro 2005 | Not appropriate comparison |
| Skidmore 2011 | Not a randomised controlled trial |
| Smoljanovic 2010 | Not a randomised controlled trial |
| Smorgick 2013 | Not a randomised controlled trial |
| Steffee 1993 | Not a randomised controlled trial |
| Tani 2002 | Not a randomised controlled trial |
| Tenhula 2000 | Not a randomised controlled trial |
| Tsutsumimoto 2009 | Not a randomised controlled trial |
| Valesin 2009 | Not a randomised controlled trial |
| Videbaek 2010 | Not lumbar spinal stenosis |
| Wang 1998 | Not a randomised controlled trial |
| Weinstein 2007 | Not surgical comparison |
| Whang 2013 | Not appropriate comparison |
| Willén 2008 | Not a randomised controlled trial |
| Xiao 2007 | Not lumbar spinal stenosis |
| Xiao 2007a | Not lumbar spinal stenosis |
| Yamada 2012 | Not a randomised controlled trial |
| Yang 2011 | Not a randomised controlled trial |
| Yu 2008 | Not a randomised controlled trial |
| Zdeblick 1993 | Not lumbar spinal stenosis |
| Zucherman 2004 | Not surgical comparison |
Differences between protocol and review
This is an update of a review published in PLoS One (Machado 2015). The study protocol was previously registered on PROSPERO (registration number CRD42013005901). We followed the new recommendations of the Cochrane Back and Neck Group in this review (Furlan 2015), which was not stated in the protocol or previous version of this review as it was not yet published. There were no substantial changes from the protocol or the previous version of this review.
Contributions of authors
GCM drafted the manuscript. GCM, MBP, MR and RIY selected eligible studies from the systematic search and performed data extraction. GCM, MBP and RIY performed the 'Risk of bias' assessments. MLF, CGM, PHF and IAH are responsible for the concept and design of the review. BWK and MvT contributed with critical revision of the review for important intellectual content. All review authors participated in reading and approving the final manuscript.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
The review authors declare that they have no competing interests and received no external funding to perform this systematic review. GCM and MBP are supported by an international postgraduate research scholarship/postgraduate award from the Australian Department of Education and Training. CGM is supported by a principal research fellowship from the National Health and Medical Research Council. MLF is supported by a Sydney Medical Foundation Fellowship from the Sydney Medical School, The University of Sydney. BWK is co‐author of one of the included trials (Moojen 2013), but was not involved in the quality assessment or data extraction of this trial. The remaining authors have noting to declare.
New
References
References to studies included in this review
Azzazi 2010 {published data only}
- Azzazi A, Elhawary Y. Dynamic stabilization using X‐stop versus transpedicular screw fixation in the treatment of lumbar canal stenosis: comparative study of the clinical outcome. Neurosurgery Quartely 2010; Vol. 20:165‐9.
Bridwell 1993 {published data only}
- Bridwell KH, Sedgewick TA, O'Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. Journal of Spinal Disorders 1993;6:461‐72. [DOI] [PubMed] [Google Scholar]
Cavusoglu 2007 {published data only}
- Cavusoglu H, Turkmenoglu O, Kaya RA, Tuncer C, Colak I, Sahin Y, et al. Efficacy of unilateral laminectomy for bilateral decompression in lumbar spinal stenosis. Turkish Neurosurgery 2007;17:100‐8. [PubMed] [Google Scholar]
Celik 2010 {published and unpublished data}
- Celik SE, Celik S, Goksu K, Kara A, Ince I. Microdecompressive laminatomy with a 5‐year follow‐up period for severe lumbar spinal stenosis. Journal of Spinal Disorders & Techniques 2010;23:229‐35. [DOI] [PubMed] [Google Scholar]
Cho 2007 {published data only}
- Cho DY, Lin HL, Lee WY, Lee HC. Split‐spinous process laminotomy and discectomy for degenerative lumbar spinal stenosis: a preliminary report. Journal of Neurosurgery: Spine 2007;6:229‐39. [DOI] [PubMed] [Google Scholar]
Davis 2013 {published data only}
- Auerbach J, Davis R, Errico T, Bae H. Direct decompression and coflex interlaminar stabilization compared with laminectomy and posterior spinal fusion with pedicle screw instrumentation for spinal stenosis with back pain or degenerative spondylolisthesis: two‐year results from the prospective, randomized, multicenter FDA IDE trial. Spine Journal 2011;11:86S‐7S. [Google Scholar]
- Auerbach J, Zigler J, Davis R, Pettine K, Yeung A. Comparative cost‐effectiveness analysis of coflex interlaminar stabilization versus posterolateral fusion for lumbar stenosis and lowgrade spondylolisthesis. European Spine Journal 2012;21:S311‐2. [Google Scholar]
- Auerbach JD, Field JS. Two‐level Coflex interlaminar stabilization compared to two‐level lumbar spinal fusion for the treatment of spinal stenosis with low‐grade spondylolisthesis. Spine Journal 2013;13:155S‐6S. [Google Scholar]
- Auerbach JD, Schmier J, Ong KL, Lau E. Cost‐effectiveness of interlaminar stabilization compared with instrumented posterior spinal fusion for spinal stenosis and spondylolisthesis. Spine Journal 2012;12:13S. [Google Scholar]
- Cano WG, Block JE, Haley T, Kuznits S, Miller LE. Lumbar spinal stenosis treatment with an interspinous spacer: preliminary results of a multicenter, randomized, controlled FDA‐IDE trial. PM&R 2011;3:S213. [Google Scholar]
- Davis R, Auerbach JD, Bae H, Errico TJ. Can low‐grade spondylolisthesis be effectively treated by either coflex interlaminar stabilization or laminectomy and posterior spinal fusion: two‐year clinical and radiographic results from the randomized, prospective, multicenter US investigational device exemption trial. Journal of Neurosurgery: Spine 2013;19:174‐84. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Auerbach JD, Bae H, Errico TJ. Low‐grade spondylolisthesis can be effectively treated by either coflex interlaminar stabilization or laminectomy and posterior spinal fusion: 2‐year clinical and radiographic results from the US IDE trial. European Spine Journal 2012;21:S257‐8. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Errico TJ, Bae H, Auerbach JD. Decompression and coflex interlaminar stabilization compared with decompression and instrumented spinal fusion for spinal stenosis and low‐grade degenerative spondylolisthesis: two‐year results from the prospective, randomized, multicenter, food and drug administration investigational device exemption trial. Spine 2013;38:1529‐39. [DOI] [PubMed] [Google Scholar]
- Pettine KA. Lumbar decompression followed by coflex interlaminar implant vs. pedicle screw posterior lateral fusion for treatment of stenosis. The Spine Journal 2010; Vol. 10:S11.
- Schmier JK, Ong KL, Lau E, Zigler JD, Auerbach JD. Cost‐effectiveness of coflex interlaminar stabilization compared with instrumented posterior spinal fusion for spinal stenosis and spondylolisthesis. Value in Health 2012;15:A69. [Google Scholar]
Forsth 2016 {published data only}
- Forsth P, Olafsson G, Carlsson T, Frost A, Borgstrom F, Fritzell P, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. New England Journal of Medicine 2016;374:1413‐23. [DOI] [PubMed] [Google Scholar]
Ghogawala 2016 {published data only}
- Ghogawala Z, Benzel EC, Magge SN, Coumans JV, Harrington JF, Barker FG. Lumbar spinal fusion reduces risk of re‐operation after laminectomy for lumbar spinal stenosis associated with grade I degenerative spondylolisthesis: initial results from the SLIP trial. Neurosurgery 2010;67:542‐3. [Google Scholar]
- Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. New England Journal of Medicine 2016;374:1424‐34. [DOI] [PubMed] [Google Scholar]
Grob 1995 {published data only}
- Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. Journal of Bone & Joint Surgery ‐ American Volume 1995;77:1036‐41. [DOI] [PubMed] [Google Scholar]
- Grob D, Humke T, Dvorak J. [Significance of simultaneous fusion and surgical decompression in lumbar spinal stenosis]. Orthopade 1993;22:243‐9. [PubMed] [Google Scholar]
Gurelik 2012 {published data only}
- Gurelik M, Bozkina C, Kars Z, Karadag O, Unal O, Bayrakli F. Unilateral laminotomy for decompression of lumbar stenosis is effective and safe: a prospective randomized comparative study. Journal of Neurological Sciences 2012;29:744‐53. [Google Scholar]
Hallett 2007 {published data only}
- Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single‐level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine 2007;32:1375‐80. [DOI] [PubMed] [Google Scholar]
Komp 2015 {published data only}
- Komp M, Hahn P, Oezdemir S, Giannakopoulos A, Heikenfeld R, Kasch R, et al. Bilateral spinal decompression of lumbar central stenosis with the full‐endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician 2015;18:61‐70. [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu X, Yuan S, Tian Y. Modified unilateral laminotomy for bilateral decompression for lumbar spinal stenosis: technical note. Spine 2013;38:E732‐7. [DOI] [PubMed] [Google Scholar]
Lonne 2015 {published data only}
- Lonne G, Johnsen LG, Aas E, Lydersen S, Andresen H, Ronning R, et al. Comparing cost‐effectiveness of X‐stop to minimally invasive decompression in lumbar spinal stenosis: a randomized controlled trial. Spine (Phila Pa 1976) 2015;40:514‐20. [DOI] [PubMed] [Google Scholar]
- Lonne G, Johnsen LG, Rossvoll I, Andresen H, Storheim K, Zwart JA, et al. Minimally invasive decompression versus x‐stop in lumbar spinal stenosis: a randomized controlled multicenter study. Spine (Phila Pa 1976) 2015;40:77‐85. [DOI] [PubMed] [Google Scholar]
Mobbs 2014 {published data only}
- Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy. Journal of Neurosurgery: Spine 2014;21:179‐86. [DOI] [PubMed] [Google Scholar]
Moojen 2013 {published and unpublished data}
- Moojen W A, Arts M P, Jacobs W C, Zwet E W, Akker‐van Marle M E, Koes B W, et al. Interspinous process device versus standard conventional surgical decompression for lumbar spinal stenosis: randomized controlled trial. BMJ 2013;347:f6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moojen WA, Arts MP, Jacobs WC, Zwet EW, Akker‐van Marle ME, Koes BW, et al. IPD without bony decompression versus conventional surgical decompression for lumbar spinal stenosis: 2‐year results of a double‐blind randomized controlled trial. European Spine Journal 2015;24:2295‐305. [DOI] [PubMed] [Google Scholar]
- Akker‐van Marle ME, Moojen WA, Arts MP, Vleggeert‐Lankamp CL, Peul WC, Leiden‐The Hague Spine Intervention Prognostic Study Group. Interspinous process devices versus standard conventional surgical decompression for lumbar spinal stenosis: cost utility analysis. Spine Journal 2016;16(6):702‐10. [DOI] [PubMed] [Google Scholar]
Postacchini 1993 {published data only}
- Postacchini F, Cinotti G, Perugia D, Gumina S. The surgical treatment of central lumbar stenosis. Multiple laminotomy compared with total laminectomy. Journal of Bone & Joint Surgery ‐ British Volume 1993;75:386‐92. [DOI] [PubMed] [Google Scholar]
Rajasekaran 2013 {published data only}
- Rajasekaran S, Thomas A, Kanna R M, Shetty AP. Lumbar spinous process splitting decompression provides equivalent outcomes to conventional midline decompression in degenerative lumbar canal stenosis: a prospective, randomised controlled study of 51 patients. Spine 2013;38:1737‐43. [DOI] [PubMed] [Google Scholar]
Ruetten 2009 {published data only}
- Ruetten S, Komp M, Merk H, Godolias G. Surgical treatment for lumbar lateral recess stenosis with the full‐endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. Journal of Neurosurgery: Spine 2009;10:476‐85. [DOI] [PubMed] [Google Scholar]
Stromqvist 2013 {published and unpublished data}
- Stromqvist BH, Berg S, Gerdhem P, Johnsson R, Moller A, Sahlstrand T, et al. X‐stop versus decompressive surgery for lumbar neurogenic intermittent claudication: randomized controlled trial with 2‐year follow‐up. Spine 2013;38:1436‐42. [DOI] [PubMed] [Google Scholar]
Thome 2005 {published data only}
- Thome C, Schubert GA, Stier R, Hegewald AA, Schmiedek P. Long‐term outcome after less invasive surgery for decompression of lumbar stenosis ‐ a randomized comparison of unilateral laminotomy, bilateral laminotomy and laminectomy. Journal of Neurosurgery: Spine 2010;113:A432‐3. [DOI] [PubMed] [Google Scholar]
- Thome C, Zevgaridis D, Leheta O, Bazner H, Pockler‐Schoniger C, Wohrle J, et al. Outcome after less‐invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. Journal of Neurosurgery: Spine 2005;3:129‐41. [DOI] [PubMed] [Google Scholar]
Usman 2013 {published data only}
- Usman M, Ali M, Khanzada K, Ishaq M, Naeem ul Haq, Aman R, et al. Unilateral approach for bilateral decompression of lumbar spinal stenosis: a minimal invasive surgery. Journal of the College of Physicians & Surgeons Pakistan 2013;23:852‐6. [PubMed] [Google Scholar]
Watanabe 2011 {published data only}
- Watanabe K, Matsumoto M, Ikegami T, Nishiwaki Y, Tsuji T, Ishii K, et al. Reduced postoperative wound pain after lumbar spinous process‐splitting laminectomy for lumbar canal stenosis: a randomized controlled study. Journal of Neurosurgery: Spine 2011;14:51‐8. [DOI] [PubMed] [Google Scholar]
Yagi 2009 {published data only}
- Yagi M, Okada E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral‐approach microendoscopic midline decompression for degenerative spinal stenosis. Journal of Neurosurgery: Spine 2009;10:293‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdu 2009 {published data only}
- Abdu WA, Lurie JD, Spratt KF, Tosteson AN, Zhao W, Tosteson TD, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four‐year results of the spine patient outcomes research trial. Spine 2009;34:2351‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altaf 2011 {published data only}
- Altaf F, Jalgaonkar A, Raman SA. Prospective study of posterior lumbar interbody fusion and posterolateral fusion for degenerative spondylolisthesis. Spine Journal 2011;11:112S‐3S. [Google Scholar]
Andersen 2008 {published data only}
- Andersen T, Videbaek TS, Hansen ES, Bunger C, Christensen FB. The positive effect of posterolateral lumbar spinal fusion is preserved at long‐term follow‐up: a RCT with 11‐13 year follow‐up. European Spine Journal 2008;17:272‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2011 {published data only}
- Anderson DG, Patel A, Maltenfort M, Vaccaro AR, Ratliff J, Hilibrand A, et al. Lumbar decompression using a traditional midline Approach versus a tubular retractor system: comparison of patient‐based clinical outcomes. Spine 2011;36:E320‐5. [DOI] [PubMed] [Google Scholar]
Aoki 2012 {published data only}
- Aoki Y, Yamagata M, Ikeda Y, Nakajima F, Ohtori S, Nakagawa K, et al. A prospective randomized controlled study comparing transforaminal lumbar interbody fusion techniques for degenerative spondylolisthesis: unilateral pedicle screw and 1 cage versus bilateral pedicle screws and 2 cages. Journal of Neurosurgery: Spine 2012;17:153‐9. [DOI] [PubMed] [Google Scholar]
Arriagada 2000 {published data only}
- Arriagada LO. Manejo quirúrgico con instrumentación transpedicular de la espondilolistesis. Revista Chilena de Neurocirugía 2000;15:29‐36. [Google Scholar]
Asazuma 2004 {published data only}
- Asazuma T, Yamugishi M, Sato M, Ichimura S, Fujikawa K, Crock HV. Posterior spinal fusion for lumbar degenerative diseases using the Crock‐Yamagishi (C‐Y) spinal fixation system. Journal of Spinal Disorders and Techniques 2004;17:174‐7. [DOI] [PubMed] [Google Scholar]
Auerbach 2011 {published data only}
- Auerbach J, Davis R. Direct versus indirect decompression and stabilization: a comparison of clinical outcomes with coflex interlaminar stabilization, laminectomy and spinal fusion, and X‐STOP to treat spinal stenosis and low‐grade degenerative spondylolisthesis. Spine Journal 2011;11:104S‐5S. [Google Scholar]
Auerbach 2012 {published data only}
- Auerbach J, McGowan K, Halevi M, Maislin G. Mitigating the potential for adverse event reporting bias: utilization of an independent clinical events committee. European Spine Journal 2012;21:S284. [Google Scholar]
Bazan 2002 {published data only}
- Bazan O. Estenosis del conducto raquideo lumbar: artrodesis posterolateral. Revista de la Asociacion Argentina de Ortopedia y Traumatologia 2002;67:296. [Google Scholar]
Benli 2006 {published data only}
- Benli I T, Cicek H, Kaya A. Comparison of sagittal plane realignment and reduction with posterior instrumentation in developmental low or high dysplastic spondylolisthesis. Kobe Journal of Medical Sciences 2006;52:151‐69. [PubMed] [Google Scholar]
Bjarke 2002 {published data only}
- Bjarke Christensen F, Stender Hansen E, Laursen M, Thomsen K, Bunger CE. Long‐term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion: randomized clinical study with a 5‐year follow‐up. Spine 2002;27:1269‐77. [DOI] [PubMed] [Google Scholar]
Blumenthal 2013 {published data only}
- Blumenthal C, Curran J, Benzel EC, Potter R, Magge SN, Harrington Jr JF, et al. Radiographic predictors of delayed instability following decompression without fusion for degenerative Grade I lumbar spondylolisthesis: clinical article. Journal of Neurosurgery: Spine 2013;18:340‐6. [DOI] [PubMed] [Google Scholar]
Bresnahan 2009 {published data only}
- Bresnahan L, Ogden AT, Natarajan RN, Fessler RG. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine 2009;34:17‐23. [DOI] [PubMed] [Google Scholar]
Cakir 2009 {published data only}
- Cakir B, Carazzo C, Schmidt R, Mattes T, Reichel H, Käfer W. Adjacent segment mobility after rigid and semirigid instrumentation of the lumbar spine. Spine 2009;34:1287‐91. [DOI] [PubMed] [Google Scholar]
Cannone 2010 {published data only}
- Cannone J. Spinal Stenosis and Fusion Surgery. Positive Health 2010;175:1. [Google Scholar]
Carragee 1997 {published data only}
Carrasco 1986 {published data only}
- Carrasco M, Simonovich Z. Conducto raquídeo estrechado. Revista de la Asociación Argentina de Ortopedia y Traumatología 1986;51:349‐62. [Google Scholar]
Carreon 2009 {published data only}
- Carreon LY, Glassman SD, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, et al. RhBMP‐2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: a cost‐utility study. Spine 2009;34:238‐43. [DOI] [PubMed] [Google Scholar]
Cassinelli 2007 {published data only}
- Cassinelli EH, Eubanks J, Vogt M, Furey C, Yoo J, Bohlman HH. Risk factors for the development of perioperative complications in elderly patients undergoing lumbar decompression and arthrodesis for spinal stenosis: an analysis of 166 patients. Spine 2007;32:230‐5. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen KX, Yang QY, Liu XC, Li HJ. [Treatment of degenerative lumbar spondylolisthesis through posterolateral fusion and fixation with pedicle screws]. Zhongguo Gu Shang. 2010; Vol. 23:254‐6. [PubMed]
Cheng 2009 {published data only}
- Cheng L, Nie L, Zhang L. Posterior lumbar interbody fusion versus posterolateral fusion in spondylolisthesis: a prospective controlled study in the Han nationality. International Orthopaedics 2009; Vol. 33:1043‐7. [DOI] [PMC free article] [PubMed]
Choi 2009 {published data only}
- Choi Y, Kim K, So K. Adjacent segment instability after treatment with a Graf ligament at minimum 8 years' followup. Clinical Orthopaedics & Related Research 2009;467:1740‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahdaleh 2013 {published data only}
- Dahdaleh NS, Nixon AT, Lawton CD, Wong AP, Smith ZA, Fessler RG. Outcome following unilateral versus bilateral instrumentation in patients undergoing minimally invasive transforaminal lumbar interbody fusion: a single‐center randomized prospective study. Neurosurgical Focus 2013;35:E13. [DOI] [PubMed] [Google Scholar]
Dantas 2007 {published data only}
Delank 2002 {published data only}
- Delank KS, Eysel P, Zollner J, Drees P, Nafe B, Rompe JD. Undercutting decompression versus laminectomy. Clinical and radiological results of a prospective controlled trial. Orthopade 2002;31:1048‐56. [DOI] [PubMed] [Google Scholar]
Delawi 2010 {published data only}
- Delawi D, Dhert WJ, Rillardon L, Gay E, Prestamburgo D, Garcia‐Fernandez C, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein‐1 in instrumented posterolateral fusions: report on safety and feasibility. Spine 2010;35:1185‐91. [DOI] [PubMed] [Google Scholar]
Desai 2012 {published data only}
- Desai A, Bekelis K, Ball P, Lurie J, Mirza S, Tosteson TD, et al. Variability in outcomes after surgery for spinal stenosis and degenerative spondylolisthesis. Neurosurgery 2012;71:E545. [Google Scholar]
Dimar 2009 {published data only}
- Dimar Ii JR, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Clinical and radiographic analysis of an optimized rhBMP‐2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. Journal of Bone and Joint Surgery ‐ American Volume 2009;91:1377‐86. [DOI] [PubMed] [Google Scholar]
Dirisio 2011 {published data only}
- Dirisio DJ, Regan JJ, Hartjen CA, Dryer RF, Rodgers WB, Pettine K, et al. Treatment of 110 symptomatic lumbar stenosis patients at 16 centers in an FDA IDE study. Journal of Neurosurgery 2011;115:A408‐9. [Google Scholar]
Dryer 2012 {published data only}
- Dryer R, Youssef JA, Lorio MP, DiRisio DJ, Myer J, Baker K. Acadia facet replacement system ide clinical trial: preliminary two‐year outcomes. Spine Journal 2012;12:140S‐1S. [Google Scholar]
Epstein 2006 {published data only}
- Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. Journal of Spinal Disorders & Techniques 2006;19:424‐9. [DOI] [PubMed] [Google Scholar]
Escobar 2003 {published data only}
- Escobar E, Transfeldt E, Garvey T, Ogilvie J, Graber J, Schultz L. Video‐assisted versus open anterior lumbar spine fusion surgery: a comparison of four techniques and complications in 135 patients. Spine 2003;28:729‐32. [DOI] [PubMed] [Google Scholar]
Fan 2009 {published data only}
- Fan J, Yu GR, Liu F, Zhao J, Zhao WD. Biomechanical changes of modified Scott wiring fixation in treating 12 young patients with lumbar spondylolysis. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13:4245‐8. [Google Scholar]
Fast 1985 {published data only}
- Fast A, Robin GC, Floman Y. Surgical treatment of lumbar spinal stenosis in the elderly. Archives of Physical Medicine & Rehabilitation 1985;66:149‐51. [PubMed] [Google Scholar]
Feng 2011 {published data only}
- Feng ZZ, Cao YW, Jiang C, Jiang XX. Short‐term outcome of bilateral decompression via a unilateral paramedian approach for transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Orthopedics 2011;34:364. [DOI] [PubMed] [Google Scholar]
Fitzgerald 1976 {published data only}
- Fitzgerald JA, Newman PH. Degenerative spondylolisthesis. Journal of Bone & Joint Surgery ‐ British Volume 1976;58:184‐92. [DOI] [PubMed] [Google Scholar]
Försth 2013 {published data only}
- Försth P, Michaëlsson K, Sandén B. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis?: A two‐year follow‐up study involving 5390 patients. Bone & Joint Journal 2013;95‐B:960‐5. [DOI] [PubMed] [Google Scholar]
Fu 2008 {published data only}
- Fu YS, Zeng BF, Xu JG. Long‐term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine 2008;33:514‐8. [DOI] [PubMed] [Google Scholar]
Fujiya 1990 {published data only}
- Fujiya M, Saita M, Kaneda K, Abumi K. Clinical study on stability of combined distraction and compression rod instrumentation with posterolateral fusion for unstable degenerative spondylolisthesis. Spine 1990;15:1216‐22. [DOI] [PubMed] [Google Scholar]
Ghahreman 2010 {published data only}
González 1992 {published data only}
- González AG, Espinosa ES. Tratamiento quirúrgico del conducto lumbar estrecho degenerativo. Revista Mexicana de Ortopedia y Traumatología 1992;6:214‐6. [Google Scholar]
Gotfryd 2012 {published data only}
- Gotfryd AO, Henriques GG, Poletto PR. Influência da extensão da artrodese lombossacra nos resultados clínicos e funcionais. Coluna/Columna 2012;11:13‐6. [Google Scholar]
Gotfryd 2012a {published data only}
- Gotfryd AO, Spolidoro DR, Poletto PR. Descompressão neural isolada ou associada à fusão póstero‐lateral nas afecções degenerativas lombossacras: avaliação da qualidade de vida e incapacidade funcional pós‐operatória. Coluna/Columna 2012;11:17‐20. [Google Scholar]
Gu 2009 {published data only}
- Gu Y, Chen L, Yang HL, Chen XQ, Dong RB, Han GS, et al. Efficacy of surgery and type of fusion in patients with degenerative lumbar spinal stenosis. Journal of Clinical Neuroscience 2009;16:1291‐5. [DOI] [PubMed] [Google Scholar]
Haley 2012 {published data only}
- Haley TR, Dankmyer C, Miller LE, Block J. Novel interspinous spacer for treatment of moderate lumbar spinal stenosis: 1‐year results of a randomized controlled IDE trial. Pain Medicine 2012;13:305‐6. [Google Scholar]
Haley 2012a {published data only}
- Haley TR, Lichten D, Miller LE. Prospective randomized controlled trial of interspinous spacer treatment for moderate lumbar spinal stenosis. Pain Medicine 2012;13:1101. [Google Scholar]
Halm 2010 {published data only}
- Halm HFH, Richter A. Microsurgical decompression vs. microsurgical decompression plus interspinous stabilization in lumbar spinal stenosis. A prospective comparison of 60 patients. Spine Journal 2010; Vol. 10:S66.
Herkowitz 1991 {published data only}
- Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. Journal of Bone and Joint Surgery. American Volume 1991; Vol. 73:802‐8. [PubMed]
Hong 2010 {published data only}
- Hong SW, Lee HY, Kim KH, Lee SH. Interspinous ligamentoplasty in the treatment of degenerative spondylolisthesis: Midterm clinical results. Journal of Neurosurgery: Spine 2010;13:27‐35. [DOI] [PubMed] [Google Scholar]
Hong 2011 {published data only}
- Hong SW, Choi KY, Ahn Y, Baek OK, Wang JC, Lee SH, et al. A comparison of unilateral and bilateral laminotomies for decompression of L4‐L5 spinal stenosis. Spine 2011;36:E172‐8. [DOI] [PubMed] [Google Scholar]
Hwang 2010 {published data only}
- Hwang CJ, Vaccaro AR, Hong J, Lawrence JP, Fischgrund JS, Alaoui‐Ismaili MH, et al. Immunogenicity of osteogenic protein 1: results from a prospective, randomized, controlled, multicenter pivotal study of uninstrumented lumbar posterolateral fusion. Journal of Neurosurgery: Spine 2010;13:484‐93. [DOI] [PubMed] [Google Scholar]
Ikuta 2005 {published data only}
- Ikuta K, Arima J, Tanaka T, Oga M, Nakano S, Sasaki K, et al. Short‐term results of microendoscopic posterior decompression for lumbar spinal stenosis. Technical note. Journal of Neurosurgery Spine 2005;2:624‐33. [DOI] [PubMed] [Google Scholar]
Imagama 2009 {published data only}
- Imagama S, Kawakami N, Matsubara Y, Kanemura T, Tsuji T, Ohara T. Preventive effect of artificial ligamentous stabilization on the upper adjacent segment impairment following posterior lumbar interbody fusion. Spine 2009;34:2775‐81. [DOI] [PubMed] [Google Scholar]
Ito 2010 {published data only}
- Ito Z, Matsuyama Y, Sakai Y, Imagama S, Wakao N, Ando K, et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine 2010;35:E1101‐5. [DOI] [PubMed] [Google Scholar]
Katz 1997 {published data only}
- Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine 1997;22:1123‐31. [DOI] [PubMed] [Google Scholar]
Kawaguchi 2004 {published data only}
- Kawaguchi Y, Kanamori M, Ishihara H, Kikkawa T, Matsui H, Tsuji H, et al. Clinical and radiographic results of expansive lumbar laminoplasty in patients with spinal stenosis. Journal of Bone & Joint Surgery ‐ American Volume 2004;86A:292‐9. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim KT, Lee SH, Lee YH, Bae SC, Suk KS. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine 2006;31:1351‐7. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim JW, Park HC, Yoon SH, Oh SH, Roh SW, Rim DC, et al. A multi‐center clinical study of posterior lumbar interbody fusion with the expandable stand‐alone cage (Tychea cage) for degenerative lumbar spinal disorders. Journal of Korean Neurosurgical Society 2007;42:251‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2007a {published data only}
- Kim SW, Ju CI, Kim CG, Lee SM, Shin H. Minimally invasive lumbar spinal decompression: A comparative study between bilateral laminotomy and unilateral laminotomy for bilateral decompression. Journal of Korean Neurosurgical Society 2007;42:195‐9. [Google Scholar]
Konno 2000 {published data only}
- Konno S, Kikuchi S. Prospective study of surgical treatment of degenerative spondylolisthesis: comparison between decompression alone and decompression with Graf system stabilization. Spine 2000;25:1533‐7. [DOI] [PubMed] [Google Scholar]
Kornblum 2004 {published data only}
- Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long‐term study comparing fusion and pseudarthrosis. Spine 2004;29:726‐33; discussion 733‐4. [DOI] [PubMed] [Google Scholar]
Korovessis 2004 {published data only}
Ledonio 2012 {published data only}
- Ledonio CGT, Polly DW, Ghogawala Z, Rampersaud RY, Santos ERG, Sembrano JN, et al. Societal cost impact of decompression alone versus decompression and fusion for degenerative spondylolisthesis. Spine Journal 2012;12:125S‐6S. [Google Scholar]
Lee 2009 {published data only}
- Lee SC, Chen JF, Wu CT, Lee ST. In situ local autograft for instrumented lower lumbar or lumbosacral posterolateral fusion. Journal of Clinical Neuroscience 2009;16:37‐43. [DOI] [PubMed] [Google Scholar]
Lian 2010 {published data only}
- Lian XF, Xu JG, Zeng BF, Zhou W, Kong WQ, Hou TS. Noncontiguous anterior decompression and fusion for multilevel cervical spondylotic myelopathy: a prospective randomized control clinical study. European Spine Journal 2010;19:713‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2011 {published data only}
- Liao JC, Chen WJ, Chen LH, Niu CC, Keorochana G. Surgical outcomes of degenerative spondylolisthesis with L5‐S1 disc degeneration: comparison between lumbar floating fusion and lumbosacral fusion at a minimum 5‐year follow‐up. Spine 2011;36:1600‐7. [DOI] [PubMed] [Google Scholar]
Mahir 2012 {published data only}
- Mahir S, Marsh G. The 3 year results of a prospective randomized controlled trial to assess the efficacy with the dynamic stabilisation of Wallis ligament as an interspinus implant in lumbar spine decompression. European Spine Journal 2012;21:S262. [DOI] [PubMed] [Google Scholar]
McConnell 2011 {published data only}
- McConnell J. A comparison of ß‐TCP+BMA versus RhBMP‐2 in anterior lumbar interbody fusion: a prospective, randomized trial with two‐year clinical and radiographic outcomes. Spine Journal 2011;11:64S‐5S. [Google Scholar]
Michielsen 2013 {published data only}
- Michielsen J, Sys J, Rigaux A, Bertrand C. The effect of recombinant human bone morphogenetic protein‐2 in single‐level posterior lumbar interbody arthrodesis. Journal of Bone & Joint Surgery ‐ American Volume 2013;95:873‐80. [DOI] [PubMed] [Google Scholar]
Pappas 1994 {published data only}
- Pappas CTE, Sonntag VKH. Lumbar stenosis in the elderly. Neurosurgery Quarterly 1994;4:102‐12. [Google Scholar]
Parker 2013 {published data only}
- Parker SL, Adogwa O, Davis BJ, Fulchiero E, Aaronson O, Cheng J, et al. Cost‐utility analysis of minimally invasive versus open multilevel hemilaminectomy for lumbar stenosis. Journal of Spinal Disorders & Techniques 2013;26:42‐7. [DOI] [PubMed] [Google Scholar]
Radcliff 2011 {published data only}
- Radcliff K, Hwang R, Hilibrand A, Smith HE, Gruskay J, Lurie JD, et al. Does iliac crest autograft affect the outcome of fusion in the setting of degenerative spondylolisthesis? A subgroup analysis of the SPORT study. Spine Journal 2011;11:60S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radcliff 2012 {published data only}
- Radcliff K, Hwang R, Hilibrand A, Smith HE, Gruskay J, Lurie JD, et al. The effect of iliac crest autograft on the outcome of fusion in the setting of degenerative spondylolisthesis: a subgroup analysis of the Spine Patient Outcomes Research Trial (SPORT). Journal of Bone and Joint Surgery ‐ Series A 2012;94:1685‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapp 2009 {published data only}
- Rapp SM. Decompression for stenosis less costly than fusion, especially when instrumented. Orthopedics Today 2009;29:38. [Google Scholar]
Rapp 2011 {published data only}
- Rapp SM. Greater spondylolisthesis pain relief found with bilateral vs. unilateral pedicle screws. Orthopedics Today 2011;31:61. [Google Scholar]
Repantis 2009 {published data only}
- Repantis T, Korovessis P, Papazisis Z. Effect of sagittal spinal alignment on low back pain following decompression and stabilisation with dynamic, semirigid and rigid instrumentation. A multifactorial analysis. Journal of Bone & Joint Surgery ‐ British Volume 2009; Vol. 91:149.
Richter 2010 {published data only}
- Richter A, Schutz C, Hauck M, Halm H. Does an interspinous device (coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One‐year follow up of a prospective case control study of 60 patients. European Spine Journal 2010;19:283‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rompe 1995 {published data only}
- Rompe JD, Eysel P, Hopf C, Heine J. Surgical management of central lumbar spinal stenosis ‐ results with decompressive laminectomy only and with concomitant instrumented fusion with the Cotrel‐Dubousset‐instrumentation. Neuro‐Orthopedics 1995; Vol. 19:17‐31.
Rosa 2012 {published data only}
- Rosa FWF, Foizer GA, Yoshino CV, Yonezaki AM, Ueno FH, Valesin Filho ES, et al. Avaliação dos pacientes submetidos à descompressão e artrodese póstero‐lateral devido à espondilolistese degenerativa com dois anos de acompanhamento. Coluna/Columna 2012;11:200‐3. [Google Scholar]
Rowland 2009 {published data only}
- Rowland KA. Spinal stenosis: new study purports to show surgery better than medical management. Evidence‐Based Practice 2009;12:1‐3. [Google Scholar]
Satomi 1992 {published data only}
- Satomi K, Hirabayashi K, Toyama Y, Fujimura Y. A clinical study of degenerative spondylolisthesis. Radiographic analysis and choice of treatment. Spine 1992;17:1329‐36. [DOI] [PubMed] [Google Scholar]
Schnake 2006 {published data only}
- Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine 2006;31:442‐9. [DOI] [PubMed] [Google Scholar]
Sears 2012 {published data only}
- Sears WR, Davis RJ, Auerbach JD. The Coflex versus fusion US FDA IDE trial: an in vivo biomechanical study of adjacent segment motion following fusion. Spine Journal 2012;12:29S. [Google Scholar]
Sengupta 2006 {published data only}
- Sengupta DK, Truumees E, Patel CK, Kazmierczak C, Hughes B, Elders G, et al. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine 2006;31:985‐91. [DOI] [PubMed] [Google Scholar]
Shapiro 2005 {published data only}
- Shapiro S, Rodgers RB, Sloan R, Altstadt T, Miller J. A randomized trial of instrumented posterior lumbar interbody fusion using machined cortical wedges/local bone with or without rhBMP2 in the treatment of degenerative lumbar spondylolisthesis with stenosis. Neurosurgery 2005;57:398. [Google Scholar]
Skidmore 2011 {published data only}
- Skidmore G, Ackerman SJ, Bergin C, Ross D, Butler J, Suthar M, et al. Cost‐effectiveness of the X‐STOP interspinous spacer for lumbar spinal stenosis: a comparison with conservative care and laminectomy. Spine 2011;36:E345‐56. [DOI] [PubMed] [Google Scholar]
Smoljanovic 2010 {published data only}
- Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six‐year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein‐2. Journal of Bone & Joint Surgery ‐ American Volume 2009;91:1181‐9. [DOI] [PubMed] [Google Scholar]
Smorgick 2013 {published data only}
- Smorgick Y, Park DK, Baker KC, Lurie JD, Tosteson TD, Zhao W, et al. Single‐versus multilevel fusion for single‐level degenerative spondylolisthesis and multilevel lumbar stenosis: four‐year results of the spine patient outcomes research trial. Spine 2013;38:797‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steffee 1993 {published data only}
- Steffee AD, Brantigan JW. The variable screw placement spinal fixation system. Report of a prospective study of 250 patients enrolled in Food and Drug Administration clinical trials. Spine 1993;18:1160‐72. [DOI] [PubMed] [Google Scholar]
Tani 2002 {published data only}
- Tani T, Ushida T, Ishida K, Iai H, Noguchi T, Yamamoto H. Relative safety of anterior microsurgical decompression versus laminoplasty for cervical myelopathy with a massive ossified posterior longitudinal ligament. Spine 2002;27:2491‐8. [DOI] [PubMed] [Google Scholar]
Tenhula 2000 {published data only}
- Tenhula J, Lenke LG, Bridwell KH, Gupta P, Riew D. Prospective functional evaluation of the surgical treatment of neurogenic claudication in patients with lumbar spinal stenosis. Journal of Spinal Disorders 2000;13:276‐82. [DOI] [PubMed] [Google Scholar]
Tsutsumimoto 2009 {published data only}
- Tsutsumimoto T, Shimogata M, Ohta H, Misawa H. Mini‐open versus conventional open posterior lumbar interbody fusion for the treatment of lumbar degenerative spondylolisthesis: comparison of paraspinal muscle damage and slip reduction. Spine 2009;34:1923‐8. [DOI] [PubMed] [Google Scholar]
Valesin 2009 {published data only}
- Valesin Filho ES, Ueno FH, Cabral LTB, Yonezaki AM, Nicolau RJ, Rodrigues LMR. Estudo prospectivo de avaliação de dor e incapacidade de pacientes operados de estenose de canal lombar com seguimento mínimo de dois anos. Coluna/Columna 2009;8:390‐4. [Google Scholar]
Videbaek 2010 {published data only}
- Videbaek TS, Egund N, Christensen FB, Grethe Jurik A, Bunger CE. Adjacent segment degeneration after lumbar spinal fusion: the impact of anterior column support: a randomized clinical trial with an eight‐ to thirteen‐year magnetic resonance imaging follow‐up. Spine 2010;35:1955‐64. [DOI] [PubMed] [Google Scholar]
Wang 1998 {published data only}
- Wang PJ, Chen WJ, Chen LH, Niu CC. Spinal fusion and pedicle screw instrumentation in the treatment of spondylolisthesis over the age of 60. Changgeng Yi Xue Za Zhi 1998;21:436‐41. [PubMed] [Google Scholar]
Weinstein 2007 {published data only}
- Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson ANA, Blood EA, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. New England Journal of Medicine 2007;356:2257‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whang 2013 {published data only}
- Whang PG, Patel VV, Bradley WD, Block JE. Midterm outcomes of a prospective multicenter randomized controlled trial comparing the clinical efficacy of interspinous spacers as a treatment for moderate lumbar spinal stenosis. Spine Journal 2013;13:136S‐7S. [Google Scholar]
Willén 2008 {published data only}
- Willén J, Wessberg PJ, Danielsson B. Surgical results in hidden lumbar spinal stenosis detected by axial loaded computed tomography and magnetic resonance imaging: an outcome study. Spine 2008;33:E109‐15. [DOI] [PubMed] [Google Scholar]
Xiao 2007 {published data only}
- Xiao R, Li Q, Tang Z. [Comparative study of lumbar spondylolisthesis treated by three different materials]. Chinese Journal of Reparative and Reconstructive Surgery 2007; Vol. 21:453‐6. [PubMed]
Xiao 2007a {published data only}
- Xiao RC, Xiao ZM, Li Q, Tang ZH, Hu JZ, Zou GY. Bone morphogenetic protein and interbody fusion cage change the height of intervertebral space in patients with lumbar spondylolisthesis. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11:1443‐6. [Google Scholar]
Yamada 2012 {published data only}
- Yamada T, Yoshii T, Sotome S, Yuasa M, Kato T, Arai Y, et al. Hybrid grafting using bone marrow aspirate combined with porous β‐tricalcium phosphate and trephine bone for lumbar posterolateral spinal fusion: a prospective, comparative study versus local bone grafting. Spine 2012;37:E174‐9. [DOI] [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang B, Chen R, Xie P, Liu B, Dong J, Rong L. [Microendoscopic decompression via unilateral approach for lumbar spinal stenosis]. Chung‐Kuo Hsiu Fu Chung Chien Wai Ko Tsa Chih/Chinese Journal of Reparative & Reconstructive Surgery 2011;25:1158‐63. [PubMed] [Google Scholar]
Yu 2008 {published data only}
- Yu CH, Wang CT, Chen PQ. Instrumented posterior lumbar interbody fusion in adult spondylolisthesis. Clinical Orthopaedics & Related Research 2008;466:3034‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zdeblick 1993 {published data only}
- Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine 1993;18:983‐91. [DOI] [PubMed] [Google Scholar]
Zucherman 2004 {published data only}
- Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, et al. A prospective randomized multi‐center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1‐year results. European Spine Journal 2004;13:22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abumi 1990
- Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142‐7. [DOI] [PubMed] [Google Scholar]
Arnoldi 1976
- Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clinical Orthopaedics and Related Research 1976;115:4‐5. [PubMed] [Google Scholar]
Aryanpur 1988
- Aryanpur J, Ducker T. Multilevel lumbar laminotomies for focal spinal stenosis: case report. Neurosurgery 1988;23:111‐5. [DOI] [PubMed] [Google Scholar]
Bailey 1911
- Bailey P, Casamajor L. Osteo‐arthritis of the spine as a cause of compression of the spinal cord and its roots: with report of five cases. The Journal of Nervous and Mental Disease 1911;38:588‐609. [Google Scholar]
Blau 1961
- Blau JN, Logue V. Intermittent claudication of the cauda equina: an unusual syndrome resulting from central protrusion of a lumbar intervertebral disc. Lancet 1961;277:1081‐6. [Google Scholar]
Boden 1990
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic‐resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. The Journal of Bone & Joint Surgery ‐ American Edition 1990;72:403‐8. [PubMed] [Google Scholar]
Buchbinder 2009
- Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. New England Journal of Medicine 2009;361:557‐68. [DOI] [PubMed] [Google Scholar]
Chou 2011
- Chou D, Lau D, Hermsmeyer J, Norvell D. Efficacy of interspinous device versus surgical decompression in the treatment of lumbar spinal stenosis: a modified network analysis. Evidence‐Based Spine‐Care Journal 2011;2:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper RG, Mitchell WS, Illingworth KJ, Forbes WS, Gillespie JE, Jayson MI. The role of epidural fibrosis and defective fibrinolysis in the persistence of postlaminectomy back pain. Spine (Phila Pa 1976) 1991;16:1044‐8. [DOI] [PubMed] [Google Scholar]
Delitto 2015
- Delitto A, Piva SR, Moore CG, Fritz JM, Wisniewski SR, Josbeno DA, et al. Surgery versus nonsurgical treatment of lumbar spinal stenosis: a randomized trial. Annals of Internernal Medicine 2015;162:465‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deyo 2010
- Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deyo 2013
- Deyo RA, Martin BI, Ching A, Tosteson AN, Jarvik JG, Kreuter W, et al. Interspinous spacers compared with decompression or fusion for lumbar stenosis: complications and repeat operations in the medicare population. Spine (Phila Pa 1976) 2013;38:865‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flum 2006
- Flum DR. Interpreting surgical trials with subjective outcomes: avoiding UnSPORTsmanlike conduct. JAMA 2006;296:2483‐5. [DOI] [PubMed] [Google Scholar]
Furlan 2015
Gibson 2005
- Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine 2005;30:2312‐20. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence‐‐imprecision. J Clin Epidemiol 2011;64:1283‐93. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Hopp 1988
- Hopp E, Tsou PM. Postdecompression lumbar instability. Clinical Orthopaedics and Related Research 1988;227:143‐51. [PubMed] [Google Scholar]
Horng 2003
- Horng S, Miller FG. Ethical framework for the use of sham procedures in clinical trials. Critical Care Medicine 2003;31:S126‐30. [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141:781‐8. [DOI] [PubMed] [Google Scholar]
Ishimoto 2013
- Ishimoto Y, Yoshimura N, Muraki S, Yamada H, Nagata K, Hashizume H, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthritis Cartilage 2013;21:783‐8. [DOI] [PubMed] [Google Scholar]
Jansson 2003
- Jansson KA, Blomqvist P, Granath F, Nemeth G. Spinal stenosis surgery in Sweden 1987‐1999. European Spine Journal 2003;12:535‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalichman 2009
- Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine Journal 2009;9:545‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2008
- Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. New England Journal of Medicine 2008;358:818‐25. [DOI] [PubMed] [Google Scholar]
Kawaguchi 1996
- Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine (Phila Pa 1976) 1996;21:941‐4. [DOI] [PubMed] [Google Scholar]
Kovacs 2011
- Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine 2011;36:E1335‐51. [DOI] [PubMed] [Google Scholar]
Lane 1893
- Lane A. Case of spondylolisthesis associated with progressive paraplegia; laminectomy. Lancet 1893;1:991‐2. [Google Scholar]
Lee 1983
- Lee CK. Lumbar spinal instability (olisthesis) after extensive posterior spinal decompression. Spine (Phila Pa 1976) 1983;8:429‐33. [DOI] [PubMed] [Google Scholar]
Machado 2015
- Machado GC, Ferreira PH, Harris IA, Pinheiro MB, Koes BW, Tulder M, et al. Effectiveness of surgery for lumbar spinal stenosis: a systematic review and meta‐analysis. PLoS One 2015;10:e0122800. [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2013
- May S, Comer C. Is surgery more effective than non‐surgical treatment for spinal stenosis, and which non‐surgical treatment is more effective? A systematic review. Physiotherapy 2013;99:12‐20. [DOI] [PubMed] [Google Scholar]
Meyerding 1932
- Meyerding HW. Spondylolisthesis. Surgery, Gynecology & Obstetrics 1932;54:37‐377. [Google Scholar]
Moojen 2011
- Moojen WA, Arts MP, Bartels RH, Jacobs WC, Peul WC. Effectiveness of interspinous implant surgery in patients with intermittent neurogenic claudication: a systematic review and meta‐analysis. European Spine Journal 2011;20:1596‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overdevest 2015
- Overdevest GM, Jacobs W, Vleggeert‐Lankamp C, Thome C, Gunzburg R, Peul W. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010036.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Portal 1803
- Portal A. Cours d'anatomie médicale, ou éléments de l'anatomie de l'homme. 1. Paris: Baudouin, 1803. [Google Scholar]
Review Manager [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
See 1975
- See DH, Kraft GH. Electromyography in paraspinal muscles following surgery for root compression. Archives of Physical Medicine and Rehabilitation 1975;56:80‐3. [PubMed] [Google Scholar]
Senegas 1991
- Senegas J. [Surgery of the intervertebral ligaments, alternative to arthrodesis in the treatment of degenerative instabilities]. Acta Orthopaedica Belgica 1991;57 Suppl 1:221‐6. [PubMed] [Google Scholar]
Siebert 2009
- Siebert E, Pruss H, Klingebiel R, Failli V, Einhaupl KM, Schwab JM. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nature Reviews Neurology 2009;5:392‐403. [DOI] [PubMed] [Google Scholar]
Smith 1829
- Smith AG. Account of a case in which portions of three dorsal vertebrae were removed for the relief of paralysis from fracture, with partial success. North American Medical and Surgical Journal 1829;8:94‐7. [Google Scholar]
Spetzger 1997
- Spetzger U, Bertalanffy H, Naujokat C, Keyserlingk DG, Gilsbach JM. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part I: Anatomical and surgical considerations. Acta Neurochirurgica 1997;139:392‐6. [DOI] [PubMed] [Google Scholar]
Spetzger 1997a
- Spetzger U, Bertalanffy H, Reinges MHT, Gilsbach JM. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part II: Clinical experiences. Acta Neurochirurgica 1997;139:397‐403. [DOI] [PubMed] [Google Scholar]
Taylor 1994
- Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976) 1994;19:1207‐12; discussion 13. [DOI] [PubMed] [Google Scholar]
Wartolowska 2014
- Wartolowska K, Judge A, Hopewell S, Collins GS, Dean BJ, Rombach I, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ 2014;348:g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 2005
- Watanabe K, Hosoya T, Shiraishi T, Matsumoto M, Chiba K, Toyama Y. Lumbar spinous process‐splitting laminectomy for lumbar canal stenosis. Technical note. Journal of Neurosurgery Spine 2005;3:405‐8. [DOI] [PubMed] [Google Scholar]
Weinstein 2008
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Blood E, Hanscom B, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. New England Journal of Medicine 2008;358:794‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014
- Wu AM, Zhou Y, Li QL, Wu XL, Jin YL, Luo P, et al. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta‐analysis. PLoS One 2014;9:e97142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaina 2016
- Zaina F, Tomkins‐Lane C, Carragee E, Negrini S. Surgical versus non‐surgical treatment for lumbar spinal stenosis. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD010264.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


