Abstract
Background
Inhaled short‐acting anticholinergics (SAAC) and short‐acting beta₂‐agonists (SABA) are effective therapies for adult patients with acute asthma who present to the emergency department (ED). It is unclear, however, whether the combination of SAAC and SABA treatment is more effective in reducing hospitalisations compared to treatment with SABA alone.
Objectives
To conduct an up‐to‐date systematic search and meta‐analysis on the effectiveness of combined inhaled therapy (SAAC + SABA agents) vs. SABA alone to reduce hospitalisations in adult patients presenting to the ED with an exacerbation of asthma.
Search methods
We searched MEDLINE, Embase, CINAHL, SCOPUS, LILACS, ProQuest Dissertations & Theses Global and evidence‐based medicine (EBM) databases using controlled vocabulary, natural language terms, and a variety of specific and general terms for inhaled SAAC and SABA drugs. The search spanned from 1946 to July 2015. The Cochrane Airways Group provided search results from the Cochrane Airways Group Register of Trials which was most recently conducted in July 2016. An extensive search of the grey literature was completed to identify any other potentially relevant studies.
Selection criteria
Included studies were randomised or controlled clinical trials comparing the effectiveness of combined inhaled therapy (SAAC and SABA) to SABA treatment alone to prevent hospitalisations in adults with acute asthma in the emergency department. Two independent review authors assessed studies for inclusion using pre‐determined criteria.
Data collection and analysis
For dichotomous outcomes, we calculated individual and pooled statistics as risk ratios (RR) or odds ratios (OR) with 95% confidence intervals (CI) using a random‐effects model and reporting heterogeneity (I²). For continuous outcomes, we reported individual trial results using mean differences (MD) and pooled results as weighted mean differences (WMD) or standardised mean differences (SMD) with 95% CIs using a random‐effects model.
Main results
We included 23 studies that involved a total of 2724 enrolled participants. Most studies were rated at unclear or high risk of bias.
Overall, participants receiving combination inhaled therapy were less likely to be hospitalised (RR 0.72, 95% CI 0.59 to 0.87; participants = 2120; studies = 16; I² = 12%; moderate quality of evidence). An estimated 65 fewer patients per 1000 would require hospitalisation after receiving combination therapy (95% 30 to 95), compared to 231 per 1000 patients receiving SABA alone. Although combination inhaled therapy was more effective than SABA treatment alone in reducing hospitalisation in participants with severe asthma exacerbations, this was not found for participants with mild or moderate exacerbations (test for difference between subgroups P = 0.02).
Participants receiving combination therapy were more likely to experience improved forced expiratory volume in one second (FEV₁) (MD 0.25 L, 95% CI 0.02 to 0.48; participants = 687; studies = 6; I² = 70%; low quality of evidence), peak expiratory flow (PEF) (MD 36.58 L/min, 95% CI 23.07 to 50.09; participants = 1056; studies = 12; I² = 25%; very low quality of evidence), increased percent change in PEF from baseline (MD 24.88, 95% CI 14.83 to 34.93; participants = 551; studies = 7; I² = 23%; moderate quality of evidence), and were less likely to return to the ED for additional care (RR 0.80, 95% CI 0.66 to 0.98; participants = 1180; studies = 5; I² = 0%; moderate quality of evidence) than participants receiving SABA alone.
Participants receiving combination inhaled therapy were more likely to experience adverse events than those treated with SABA agents alone (OR 2.03, 95% CI 1.28 to 3.20; participants = 1392; studies = 11; I² = 14%; moderate quality of evidence). Among patients receiving combination therapy, 103 per 1000 were likely to report adverse events (95% 31 to 195 more) compared to 131 per 1000 patients receiving SABA alone.
Authors' conclusions
Overall, combination inhaled therapy with SAAC and SABA reduced hospitalisation and improved pulmonary function in adults presenting to the ED with acute asthma. In particular, combination inhaled therapy was more effective in preventing hospitalisation in adults with severe asthma exacerbations who are at increased risk of hospitalisation, compared to those with mild‐moderate exacerbations, who were at a lower risk to be hospitalised. A single dose of combination therapy and multiple doses both showed reductions in the risk of hospitalisation among adults with acute asthma. However, adults receiving combination therapy were more likely to experience adverse events, such as tremor, agitation, and palpitations, compared to patients receiving SABA alone.
Plain language summary
Combined beta‐agonists and anticholinergics compared to beta‐agonists alone for adults with asthma treated in emergency departments
Review question
We looked at if combined treatment of short‐acting beta‐agonists and anticholinergics were more effective to improve outcomes in adults with asthma who were treated in emergency departments compared to treatment with beta‐agonists alone.
Background
Asthma attacks result from airway passages to the lungs becoming constricted due to inflammation, resulting in wheezing, coughing, and difficulty breathing. People experiencing asthma attacks often go to emergency departments, and are usually treated using short‐acting inhaled beta‐agonists, although some patients may be treated with short‐acting inhaled anticholinergics.
Some research looks at whether treating people with asthma in emergency departments with a combination of beta‐agonists and anticholinergics is more effective than beta‐agonists alone.
Search date
The search was current to July 2016.
Study characteristics
We included 23 studies that compared the effectiveness of combined treatment with beta‐agonists and anticholinergics versus treatment with beta‐agonists alone. A total of 2724 adult participants were enrolled in the studies. Salbutamol (also called albuterol) was the most common beta‐agonist investigated and ipratropium bromide was the most common anticholinergic assessed.
Study fundin g sources
We found that most studies did not report sources of funding (14 studies); one study was supported by a hospital; another received support from a pharmaceutical company, but indicated that there was no involvement from the company in conducting or reporting research. Two studies were part‐funded and four were funded by pharmaceutical companies.
Key results
Patients with severe asthma who received combined treatment of beta‐agonists and anticholinergics were less likely to be admitted to hospital. An estimated 65 fewer patients per 1000 would require hospital admission after receiving combined inhaled therapy in the emergency department. Among patients with mild ‐to‐moderate asthma, combined inhaled therapy was less effective in preventing admission to hospital compared with people with severe asthma. Patients receiving combined treatment were less likely to return to the emergency department with worsening asthma symptoms and had better outcomes in most lung function tests. On the other hand, 103 more participants per 1000 who receive combined inhaled therapy would experience side effects compared to people who receive beta‐agonists alone.
Quality of the evidence
Quality of the evidence that combination inhaled therapy can improve health outcomes compared to treatment with beta‐agonists alone ranged from very low to moderate. Our confidence about the effects of combination inhaled therapy on hospital admissions, peak expiratory flow, percent change in peak expiratory flow from baseline, and relapse was moderate because of the overall risk of bias among included studies. Factors associated with inconsistency and imprecision were additional aspects that reduced the quality of the evidence for forced expiratory volume in one second, and percent predicted peak expiratory flow.
Summary of findings
Summary of findings for the main comparison. Combination inhaled therapy compared with SABA alone for acute asthma.
| Combination inhaled therapy compared with SABA alone for acute asthma | |||||
|
Patient or population: Adults with acute asthma Intervention: Combined inhaled therapy (SAAC + SABA) Comparison: SABA alone Settings: Emergency Department | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with SABA alone | Risk difference with combination therapy | ||||
| Hospitalisation | 231 per 1000 | 65 fewer per 1000 (from 30 fewer to 95 fewer) | RR 0.72 (0.59 to 0.86) | 2120 (16 studies) | ⊕⊕⊕⊝ moderate1 |
| Total adverse events | 131 per 1000 | 103 more per 1000 (from 31 more to 195 more) | OR 2.03 (1.28 to 3.20) | 1392 (11 studies) | ⊕⊕⊕⊝ moderate2 |
| FEV₁ | Control group range 1.36 to 2.4 Litres | MD 0.25 higher (0.02 to 0.48 higher) |
687 (6 studies) |
⊕⊕⊝⊝ low1,3 | |
| Percent change FEV₁(%) | Control group range 32 to 106% | MD 21.28 higher (5.62 lower to 48.18 higher) |
578 (5 studies) |
⊕⊝⊝⊝ very low1,3,4 | |
| Peak expiratory flow (PEF) | Control group range 190 to 313 litres/min | MD 36.58 higher (23.07 to 50.09 higher) |
1056 (12 studies) |
⊕⊕⊕⊝ moderate1 | |
| Percent change from baseline PEF (%) | Control group range 32 to 82% | MD 24.88 higher (14.83 to 34.93 higher) |
551 (7 studies) |
⊕⊕⊕⊝ moderate1 | |
| Relapse rates | 250 per 1000 | 50 fewer per 1000 (from 5 fewer to 85 fewer) | RR 0.8 (0.66 to 0.98) | 1180 (5 studies) | ⊕⊕⊕⊝ moderate1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; OR: Odds Ratio; MD: Mean Difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Most studies had an overall unclear of high risk of bias. Methods of randomisation or blinding were frequently unclear.
2 Potential selective reporting bias. Several studies did not report adverse events that enabled inclusion in the meta‐analysis.
3 Inconsistency. Large differences in effects between studies.
4 Imprecision around the pooled effect including both benefit, harm, and no effect.
Background
Description of the condition
Acute asthma is a common cause for visits to the emergency department (ED). Although most people with acute asthma are safely discharged home, some require admission to hospital for continued care. Of those presenting to an ED for acute asthma, approximately 11% (Hasegawa 2013) to 13% (Rowe 2010) were hospitalised in Japan and Canada respectively. The percentage of people who reported being hospitalised for asthma in the previous year ranged from 7% in Europe (Rabe 2000), 9% in the United States (Adams 2002), 15% in the Asia‐Pacific region (Lai 2003) and 22% in Latin America (Neffen 2005). The direct costs (including prescriptions, hospitalisations, clinic and ED visits) for asthma in the United States are approximately USD 5.1 billion (Smith 1997), while in Canada, direct costs were approximately CAD 306 million (Krahn 1996). In Europe the estimated total costs of asthma are approximately EUR 17.7 billion (Braman 2006).
Description of the intervention
Generally, adults presenting to the ED with acute asthma are treated with inhaled bronchodilators. There are two inhaled bronchodilators which have been proven to be particularly effective in reducing airway bronchospasm: short‐acting anticholinergics (SAAC; Aaron 2001) and short‐acting beta₂ ‐agonists (SABA; Price 1989). While SABA agents have become the first‐line treatment for people with acute asthma, some researchers have examined if there could be a synergistic effect in combining SAAC with SABA to improve important outcomes, such as improvements in pulmonary function, reduced hospitalisations, and improved quality of life.
How the intervention might work
The combination of inhaled SAAC and SABA agents potentially improves pulmonary function because each has a different mechanism of action designed to reduce airway bronchospasm. While SABA agents are known for their strong bronchodilating effect through their effect on airway smooth muscle and quick onset of action, SAAC agents act through different receptors, reduce airway secretions, and are weaker bronchodilators. Although SAAC agents have a slower onset of action, they are longer‐acting (Lanes 1998; Rebuck 1987). The combination of inhaled SAAC and SABA agents may provide prolonged and enhanced bronchodilation, and reduce need for hospitalisation compared to traditional treatment with SABA agents alone. Indeed, Rebuck 1987, found that one‐second forced expiratory volume (FEV₁) was significantly improved among patients receiving combined inhaled therapy of SAAC and SABA agents than those receiving either SAAC or SABA agents alone. Additional studies suggest that combination inhaled therapy may provide greater improvements in pulmonary function than treatment with SABA agents alone (Garrett 1997; Lin 1998; Nakano 2000; Rodrigo 2000).
Why it is important to do this review
Although some evidence supports the use of combination inhaled therapy, some studies found no significant difference between combination inhaled therapy or SABA alone in changes to pulmonary function or hospitalisation (Cydulka 2010; FitzGerald 1997; Salo 2006; Weber 1999). Accordingly, some reviews have attempted to pool and summarise the available evidence. A Cochrane review that considered children with acute asthma found combination inhaled therapy reduced the risk of hospitalisation, improved pulmonary function, and reduced the risk of adverse events (Griffiths 2013). With regard to adults with acute asthma, a pooled analysis of three studies reported a small benefit from combination inhaled therapy to improve pulmonary function and reduce risk of hospitalisation (Lanes 1998). Similarily, a systematic review of 16 studies found that combination inhaled therapy reduced hospitalisation and improved pulmonary function in adults with asthma (Rodrigo 2005). However, it is important to note that Rodrigo 2005 included studies that assessed patients either in the ED or hospital, as well as studies that provided patients with either long‐acting anticholinergics (LAAC) or SAAC agents as part of the combination inhaled therapy.
Since 2005, there have been several studies (Cydulka 2010; Hossain 2013; Salo 2006) which may impact the results of earlier systematic reviews. We found sufficient new evidence on the use of combination inhaled therapy (SAAC + SABA agents) vs. SABA alone for the treatment of acute asthma to indicate that a Cochrane review was necessary. The aim of this Cochrane review was to provide patients and healthcare professionals with current evidence to inform updating asthma guidelines on the use of combination inhaled therapy for adults in the ED.
Objectives
To conduct an up‐to‐date systematic search and meta‐analysis on the effectiveness of combined inhaled therapy (SAAC + SABA agents) vs. SABA alone to reduce hospitalisations in adult patients presenting to the ED with an exacerbation of asthma.
Methods
Criteria for considering studies for this review
Types of studies
Only prospective randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing the effectiveness of combined inhaled therapy of short‐acting anticholinergics (SAAC) and short‐acting beta₂‐agonists (SABA) vs. treatment with SABA alone in the emergency department (ED) were eligible for inclusion.
Types of participants
Studies including adult (aged ≥ 16 years) participants presenting to an ED or other equivalent acute care setting with an uncomplicated exacerbation of asthma were considered for inclusion in this review. The asthma diagnosis needed to have been made using international or national clinical criteria or spirometric assessment results or both. Studies involving children or patients already admitted to hospital were excluded. Studies that enrolled participants with either chronic obstructive pulmonary disease (COPD) or asthma were included only if COPD participants made up fewer than 20% of the total participant population, or if outcome data from the asthma only participants could be extracted for analysis. Outcomes that included more than 20% of COPD participants were not extracted for this review.
Types of interventions
Participants received either single or repeated doses of inhaled or nebulised SAAC agents either alongside or combined with SABA agents. Control group participants received SABA agents with or without placebo. Studies examining long‐acting anticholinergic (LAAC) agents, such as tiotropium, glycopyrrolate, or aclidinium bromide, were excluded. There were no limitations on co‐interventions participants with acute asthma could receive while being managed in the ED or at discharge, including additional treatments such as beta₂‐agonists, corticosteroids, theophylline compounds, and antihistamines. There were no limitations on inclusion based on types of interventions patients could have received before presenting to the ED. Co‐interventions provided are reported in Characteristics of included studies tables..
Types of outcome measures
Primary outcomes
The primary dichotomous outcome included:
the proportion of participants requiring hospitalisation.
Hospitalisation was defined as a decision by the treating physician to continue to provide continuing asthma care in an inpatient setting. Asthma severity, receiving corticosteroids as co‐interventions, and single or multiple doses of combination inhaled therapy were considered for subgroup analysis. We performed as reported and worst‐case scenario intention‐to‐treat (ITT) analyses. For worst‐case scenario ITT, withdrawals from the study by the participant or attending physician due to a lack of improvement after receiving treatment were considered to have been hospitalised.
Secondary outcomes
We assessed the following secondary outcomes for this review:
ED length of stay;
adverse events;
continuous data from pulmonary function testing (including: percent change of forced expiratory volume in one second (FEV₁), and percent predicted FEV₁, peak expiratory flow (PEF), percent change from baseline PEF, percent predicted PEF);
symptom scores;
quality of life;
number of additional bronchodilator treatments required; and
relapse proportions.
Search methods for identification of studies
Electronic searches
We conducted a systematic search of bibliographic databases: MEDLINE (Appendix 1), Embase (Appendix 2), CINAHL (Appendix 3), SCOPUS (Appendix 4), LILACS (Appendix 5), ProQuest Dissertations and Theses Global (Appendix 6) and evidence‐based medicine (EBM) reviews sources (Appendix 7). These included: Cochrane Database of Systematic Reviews (2005 to July 2015), ACP Journal Club (1991 to July 2015), Database of Abstracts of Reviews of Effects (DARE) (second quarter 2015), Cochrane Central Register of Controlled Trials (CENTRAL) (June 2015), Cochrane Methodology Register (third quarter 2012), Health Technology Assessment (second quarter 2015), and NHS Economic Evaluation Database (second quarter 2015). This search spanned from 1946 to July 2015. We also searched the Cochrane Airways Group register of trials which was most recently conducted on July 2016 (Appendix 8).
Search terms were adapted for each database using controlled vocabulary (e.g. MESH, EMTREE, etc.) and natural language terms and a variety of specific and general terms for beta₂‐agonists and short‐acting anticholinergic drugs. Searches in MEDLINE and Embase were restricted to adult populations. No other limits were applied including year of publication or language. Articles published in languages other than English and unpublished articles were included. We sought translation of studies by fluent bilingual speakers, but if this could not occur, articles were translated using Google Translate.
Searching other resources
The search of the grey literature for additional studies included:
clinical trial registries (Cochrane Central Register of Controlled Trials, controlled‐trials.com and ClinicalTrials.gov);
Google Scholar;
reference lists of included studies and reviews;
SCOPUS forward search of a sentinel paper (Rebuck 1987); and
Hand‐searches of the most recent emergency medicine conference abstracts associated with Canadian (Canadian Association of Emergency Physicians; Canadian Journal of Emergency Medicine, 2008 to 2016), US (American College of Emergency Physicians; Annals of Emergency Medicine, 2008 to 2015) and international (Society for Academic Emergency Medicine; Academic Emergency Medicine, 2008 to 2016) emergency medicine research meetings.
Data collection and analysis
Selection of studies
At least two independent review authors (CV, AD, BV, RC, TN, SWK) identified potentially relevant studies of citations by assessing titles, abstracts and MESH terms. Once identified, the full text of potentially relevant studies were assessed using pre‐defined inclusion and exclusion criteria by at least two independent review authors (TN, AD, RC, BV, SWK). Disagreements were resolved and discussed using third party adjudication (BHR) to achieve consensus. (Figure 1).
1.
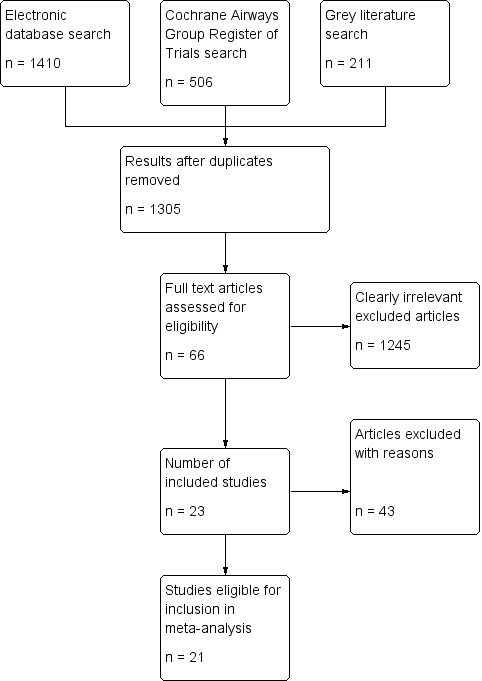
Study flow diagram
Data extraction and management
Data were extracted independently by at least two review authors (TN, AD, RC, BV, SWK) into a standardised form to collate information about participants, methods, interventions, outcomes, and adverse events provided in the articles. Data were verified (SWK) to ensure accuracy of the extraction process. Discrepancies were resolved by discussion and confirmation of the results from the text of the articles. Attempts were made to contact all primary authors for clarification of any missing or unclear data and to inquire if they could provide original study data.
Assessment of risk of bias in included studies
Quality assessment of included studies was completed using Cochrane's risk of bias (RoB) assessment tool (Higgins 2011). Two independent review authors (SWK, CV, BV) assessed seven different categories of bias including:
Sequence generation;
Allocation concealment;
Blinding of participants and personnel;
Blinding of outcome assessors;
Incorporation of outcome data (attrition and exclusions);
Selective reporting; and
Other potential sources of bias.
Disagreements were resolved and discussed by third party adjudication (BHR) to achieve consensus.
Measures of treatment effect
For dichotomous variables, individual and pooled statistics were calculated as risk ratios (RR) with 95% confidence intervals (CI) using a random‐effects model. For clinically rare dichotomous events, such as adverse events, odds ratios (OR) were calculated with 95% CI using a random‐effects model. A random‐effects model was chosen over fixed‐effect because it was assumed that the intervention effect would vary among included studies due to factors other than the intervention due to heterogeneity in study methodology, participant characteristics, co‐interventions and interventions received. For continuous outcomes, individual trial results were reported using mean differences (MD) and pooled results as weighted mean differences (WMD) or standardised mean differences (SMD) with 95% CIs using a random‐effects model. The weights given to each study in the pooled analysis were based on the inverse variance method.
Unit of analysis issues
The unit of analysis was the participants in the included studies.
Dealing with missing data
We attempted to contact study authors to obtain missing or unclear data. If a study did not provide values for standard deviation, and attempts to retrieve the original data from study authors were unsuccessful, imputation was employed or standard deviation estimated from figures using GraphClick software (Version 3.0; Arizona Software, San Francisco, United States).
Assessment of heterogeneity
Heterogeneity was assessed visually, methodologically, and statistically (using Chi² and I² statistics). The I² values of 25%, 50% and 75% were assessed to represent low, moderate, and high degrees of heterogeneity, respectively (Higgins 2011). Heterogeneity was assessed using I² in RevMan (RevMan 2014).
Assessment of reporting biases
A funnel plot of the primary outcome was created to assess publication bias using RevMan 2014 (Figure 2).
2.
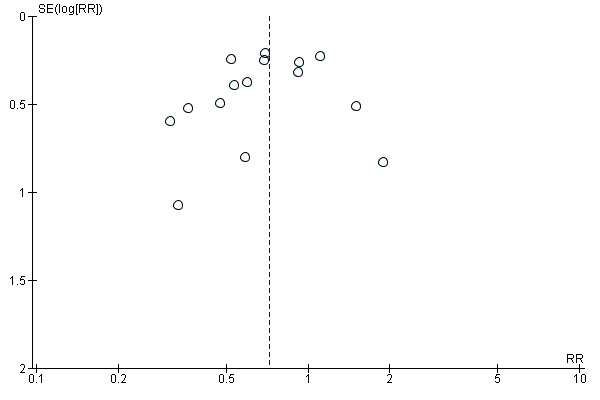
Funnel plot of comparison: 1 Hosptialisation rates, outcome: 1.1 Hospitalisation rates
Data synthesis
Data were extracted by review authors (TN, AD, RC, BV) and checked for reliability (SWK). Studies were pooled only if they represented similar populations, outcomes, and designs, and the review authors judged that clinical heterogeneity was sufficiently low. The PRISMA checklist was used to ensure that standard outcomes were reported (Moher 2009). Statistical analyses were performed using RevMan 2014. We compiled a summary of findings table for outcomes including hospitalisations, adverse events, PEF, percent change from baseline PEF (%), FEV₁, percent change FEV₁ (%), and relapse using GRADEpro (GRADEpro 2014) (Table 1). The quality of the primary outcome and important secondary outcomes were assessed using GRADE (Grades of Recommendation, Assessment, Development and Evaluation) based on the criteria developed by the GRADE Working Group (GRADE Working Group 2004). The quality of the evidence was either upgraded or downgraded based on the following criteria:
Limitations in study design or execution (risk of bias);
Inconsistency of results;
Indirectness of evidence;
Imprecision; and
Publication bias.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analysis was established a priori to examine the effects of single vs. multiple doses of combination inhaled therapy, exacerbation severity (mild, moderate, severe), co‐interventions with corticosteroids (received/did not receive corticosteroids in the ED), and type of SAAC used (ipratropium bromide vs. other SAAC) on heterogeneity.
Sensitivity analysis
Planned sensitivity testing included study quality (studies with an overall low vs. unclear vs. high risk of bias) and use of random‐effects vs. fixed‐effect models.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The literature search identified a total of 2127 records (see Figure 1). There were 1305 records following removal of duplicates. Following assessment based on titles and abstracts, we identified 66 potentially relevant studies that were obtained in full‐text. Following assessment we excluded 43 studies. We included 23 studies in this review. Two included studies were available only as abstracts, and did not include data that could be extracted for meta‐analysis (Canete 1991; Rahman 2006). Attempts to retrieve original data were unsuccessful, and as a result, the review included outcome data from 21 studies. Five included studies were exclusive to the search of the Cochrane Airways Group's Register of Trials (Canete 1991; Hossain 2013; Kohistani 2007; Rahman 2006; Rashid 2010). Solarte 2004 was identified from a search of the grey literature. Two articles were translated from Spanish (Canete 1991; Rodrigo 1995). A funnel plot based on the primary hospitalisation outcome did not show obvious publication bias (Figure 2).
Included studies
Design
Most included studies (n = 19) were published as journal articles; four were available only as abstracts (Canete 1991; Rahman 2006; Rashid 2010; Solarte 2004). All included studies reported prospective RCTs or CCTs.
Participants
The included studies enrolled a total of 2724 adult participants with acute asthma presenting to the ED. Five included studies were conducted in South Asia: India (Aggarwal 2002), Bangladesh (Hossain 2013; Rahman 2006; Rashid 2010), and Pakistan (Kohistani 2007). The remaining studies were conducted in Australia (Summers 1990), Canada (FitzGerald 1997; Rebuck 1987), Colombia (Solarte 2004), Japan (Kamei 1999; Nakano 2000), New Zealand (Garrett 1997), Spain (Canete 1991), United Kingdom (O'Driscoll 1989), United States (Cydulka 2010; Diaz 1997; Karpel 1996; Lin 1998; Owens 1991; Salo 2006; Weber 1999) and Uruguay (Rodrigo 1995; Rodrigo 2000). Two studies (O'Driscoll 1989; Rebuck 1987) included both asthma and COPD patients. Although people with the COPD made up more than 20% of the total patient population both O'Driscoll 1989 and Rebuck 1987 reported data on pulmonary function for only adult patients. The occurrence of other outcomes, such as hospitalisation and adverse events, was not provided. Attempts to contact the study authors to obtain additional data for the asthma population were unsuccessful, and as a result, only results for pulmonary function could be included in the meta‐analysis.
Only three studies classified the severity of asthma exacerbations among participants (Cydulka 2010; Nakano 2000; Rodrigo 1995). An attempt was made to estimate and categorise exacerbation severity among the included studies based on the pulmonary function eligibility criteria established by the study, in addition to the percentage of patients hospitalised in the SABA alone group, as developed and reported in a previous review (Rowe 2000a; Rowe 2000b). If studies reported forced expiatory volume in one second (FEV₁) or peak expiratory flow (PEF) of less than 50% predicted, the overall severity of acute asthma among participants was considered to be severe (Cydulka 2010; Nakano 2000; Rodrigo 2000). Studies reporting FEV₁ or PEF of less than 70%, or a peak expiratory flow rate (PEFR) of less than 200 L/minute, were estimated to have an overall exacerbation severity of mild, moderate, or severe based on the proportion of participants who were hospitalised. A percentage of hospitalisations of less than 10%, between 10% and 30%, and over 30% in the comparison groups were used to estimate the overall exacerbation severity of participants as mild (Kamei 1999), moderate (Diaz 1997; FitzGerald 1997; Garrett 1997; Karpel 1996; Owens 1991; Salo 2006; Solarte 2004), or severe (Kohistani 2007; Lin 1998; Rodrigo 1995; Weber 1999) (Table 2). If studies did not report an eligibility criterion based on pulmonary function, then the overall estimate of acute asthma severity was based on the proportions of participants hospitalised in the SABA alone group and classified as either mild (Aggarwal 2002) or moderate (Diaz 1997; Solarte 2004).
1. Exacerbation severity subgroups to examine the effectiveness of combination therapy to prevent hospitalisation.
| Studies | Pulmonary function: Eligibility criteria | Placebo group admission rate (%) |
| Mild subgroup | ||
| Aggarwal 2002 | Not defined | 0 |
| Kamei 1999 | FEV₁ < 70% predicted | 6 |
| Moderate subgroup | ||
| Diaz 1997 | Not defined | 26 |
| FitzGerald 1997 | FEV₁ < 70% predicted | 11 |
| Garrett 1997 | FEV₁ < 70% predicted | 22 |
| Karpel 1996 | FEV₁ < 60% predicted | 14 |
| Owens 1991 | FEV₁ < 2 L | 20 |
| Salo 2006 | PEFR < 70% predicted | 17 |
| Solarte 2004 | Not defined | 27 |
| Severe subgroup | ||
| Cydulka 2010* | FEV₁ < 50% predicted | 47 |
| Kohistani 2007 | PEFR < 200 L per minute | 37 |
| Lin 1998 | PEFR < 200 L per minute | 36 |
| Nakano 2000* | PEF < 50% normal predictive value | 28 |
| Rodrigo 1995* | FEV₁ and PEF < 50% predicted | 27 |
| Rodrigo 2000 | FEV₁1 < 50% predicted | 39 |
| Weber 1999 | PEFR < 70% predicted after treatment with bronchodilator treatment | 39 |
* Study reported to strictly enrolling patients presenting to the emergency department with severe exacerbations
Abbreviations:
FEV ‐ forced expiratory volume PEFR ‐
Interventions
All included studies compared combined inhaled therapy of SAAC with SABA vs. SABA treatment alone provided in the ED. Most included studies (n = 19) provided participants with ipratropium bromide as the SAAC agent (Aggarwal 2002; Canete 1991; Cydulka 2010; FitzGerald 1997; Garrett 1997; Hossain 2013; Karpel 1996; Kohistani 2007; Lin 1998; O'Driscoll 1989; Rahman 2006; Rashid 2010; Rebuck 1987; Rodrigo 1995; Rodrigo 2000; Salo 2006; Solarte 2004; Summers 1990; Weber 1999). Four studies used either atropine sulphate (Diaz 1997; Owens 1991) or oxitropium bromide (Kamei 1999; Nakano 2000).
Salbutamol (albuterol) was the most commonly‐used SABA agent (Aggarwal 2002; Canete 1991; Diaz 1997; FitzGerald 1997; Garrett 1997; Hossain 2013; Karpel 1996; Kohistani 2007; Lin 1998; Nakano 2000; O'Driscoll 1989; Rahman 2006; Rashid 2010; Rodrigo 1995; Rodrigo 2000; Salo 2006; Solarte 2004; Summers 1990; Weber 1999). Other SABA agents used were levabuterol (Cydulka 2010), fenoterol (Kamei 1999; Rebuck 1987), and metaproterenol (Owens 1991). Most studies administered the interventions via nebulisers; seven studies used a metered‐dose inhaler (MDI) and spacer devices (Canete 1991; Kamei 1999; Nakano 2000; Rahman 2006; Rashid 2010; Rodrigo 1995; Rodrigo 2000).
We included 12 studies that administered multiple doses of the drugs, including five puffs (Kamei 1999) four puffs (Nakano 2000; Rahman 2006; Rashid 2010; Rodrigo 1995; Rodrigo 2000), three puffs (Cydulka 2010; Hossain 2013; Solarte 2004), or two puffs (Diaz 1997; Karpel 1996). Canete 1991 did not specify the total number of puffs participants received.
There were 12 studies that administered a single dose of combined inhaled therapy (Aggarwal 2002; Diaz 1997; FitzGerald 1997; Garrett 1997; Kohistani 2007; Lin 1998; O'Driscoll 1989; Owens 1991; Rebuck 1987; Salo 2006; Summers 1990; Weber 1999). Diaz 1997 compared the effectiveness of single vs. multiple doses of combination inhaled therapy to SABA monotherapy. Two studies provided a single continuous dose of combined inhaled therapy or SABA agents alone for a two (Salo 2006) or three (Weber 1999) hour period.
Outcomes
Hospitalisation was assessed in 15 of the 23 included studies (Aggarwal 2002; Cydulka 2010; Diaz 1997; FitzGerald 1997; Garrett 1997; Kamei 1999; Karpel 1996; Kohistani 2007; Lin 1998; Nakano 2000; Owens 1991; Rodrigo 2000; Salo 2006; Solarte 2004; Weber 1999). Criteria for hospitalisation were defined in only five studies (Diaz 1997; Kohistani 2007; Lin 1998; Nakano 2000; Weber 1999) (Table 3).
2. Admission criteria of included studies.
| Study ID | Admission criteria |
| Diaz 1997 | Considered to by admitted patients if any of the following criteria were met:
|
| Kohistani 2007 | Admission criteria included the presence of any of the following after treatment:
|
| Lin 1998 | Admission criteria included the presence of any of the following after treatment:
|
| Nakano 2000 | Considered eligible for discharge if patients were:
Patients not meeting these criteria were given additional treatment with IV aminophylline and/or inhaled bronchodilators. If these patients still did not meet the discharge requirements, they were admitted to hospital |
| Weber 1999 | Decision to admit patients based on the 1991 guidelines in the National Asthma Education Program Expert Panel Report of the National Heart, Lung, and Blood Institute |
The total number of participants reporting adverse events was commonly reported, although several studies reported non‐significant differences in the occurrence of adverse events between groups and did not include any data which could be extracted. The frequency of particular adverse events including dry mouth, tremor, anxiety, palpitations, nausea, headache, blurred vision, agitation, and chest retractions, were inconsistently reported across studies, resulting in limited analysis of specific adverse events.
Meaningful analysis of proposed secondary outcomes including ED length of stay, symptom scores, and quality of life could not be completed as planned due to a lack of available data. Only one study reported on ED length of stay (Weber 1999). No studies reported symptoms scores or quality of life. Pulmonary function results were reported in most studies; however, the measures used to assess pulmonary function (PEF, FEV₁) differed. The final assessment of pulmonary function after the administration of study medications in all included studies was used in the meta‐analysis. Only studies that reported percent change in PEF from baseline to the last PEF value taken after treatment were extracted and included in the analysis.
Relapse and need for additional bronchodilator treatment in the ED were assessed in five (Cydulka 2010; FitzGerald 1997; Garrett 1997; Karpel 1996; Weber 1999) and four (Aggarwal 2002; Karpel 1996; Nakano 2000; Owens 1991) studies, respectively. Two studies reported relapse as a return to a healthcare provider within 24 hours after discharge (Karpel 1996; Weber 1999); two other studies assessed relapse within two weeks after discharge (Cydulka 2010; FitzGerald 1997).
No studies reported any participant deaths.
Supplemental information for Rashid 2010, was retrieved from another abstract that presented the same data (Rashid 2012). Supplemental data on relapse and hospitalisations for three studies (FitzGerald 1997; Garrett 1997; Karpel 1996) were retrieved from a previously published pooled analysis (Lanes 1998), and the proportion of patients who were hospitalised in Rodrigo 1995 was retrieved from a later systematic review (Rodrigo 2005). In several cases, standard deviation was estimated from standard error or confidence intervals (FitzGerald 1997; Garrett 1997; Kamei 1999; Owens 1991; Rodrigo 2000; Summers 1990; Weber 1999) or from a figure (O'Driscoll 1989). In two cases, pulmonary function data were not provided in the text, and were estimated from figures using GraphClick software (Kamei 1999; Nakano 2000).
Co‐interventions
Most studies provided participants with additional treatments during their stay in the ED (see Characteristics of included studies). Five studies did not state whether participants were provided with co‐interventions in the ED (Kohistani 2007; Owens 1991; Rahman 2006; Rashid 2010; Solarte 2004). Co‐interventions varied, but frequently included oxygen and intravenous (IV) aminophylline. No studies reported on whether patients received long‐acting anticholinergics or beta‐agonists as a co‐intervention in the ED or at discharge. Oral (Cydulka 2010; Lin 1998; Salo 2006; Weber 1999), intramuscular (Hossain 2013), and IV (Aggarwal 2002; FitzGerald 1997; Garrett 1997; Kamei 1999; Nakano 2000; Rebuck 1987; Rodrigo 1995; Summers 1990) corticosteroids were administered in 13 studies. The route of corticosteroid administration was unclear in two studies (Canete 1991; Diaz 1997). Several studies stated that all participants received corticosteroids as a co‐intervention along with combination inhaled therapy or SABA alone (Cydulka 2010; FitzGerald 1997; Garrett 1997; Nakano 2000; Rodrigo 1995; Salo 2006; Weber 1999) and some studies left the decision to provide corticosteroids to the discretion of attending physicians (Aggarwal 2002; Canete 1991; Diaz 1997; Kamei 1999; Lin 1998; O'Driscoll 1989; Rebuck 1987; Summers 1990). Diaz 1997 did not provide corticosteroids until after the participant's discharge disposition had been made, and Kamei 1999 only provided participants with IV corticosteroids if inhalation therapy was found to be ineffective.
Excluded studies
We excluded 43 studies following full‐text assessment. Reasons for exclusion were: studies did not report not a prospective RCTs or CCTs; did not include participants with acute asthma; settings were not EDs, or did not compare inhaled SAAC + SABA vs. SABA alone. See Characteristics of excluded studies.
Risk of bias in included studies
Most studies were assessed at high (Diaz 1997; FitzGerald 1997; Garrett 1997; Kamei 1999; Karpel 1996; Lin 1998; Nakano 2000; O'Driscoll 1989; Owens 1991; Rahman 2006; Rashid 2010; Solarte 2004; Summers 1990; Weber 1999) or unclear (Aggarwal 2002; Canete 1991; Hossain 2013; Kohistani 2007; Rebuck 1987; Rodrigo 1995; Rodrigo 2000; Salo 2006) risk of bias (Figure 3; Figure 4). Only one study was assessed at overall low risk of bias (Cydulka 2010).
3.
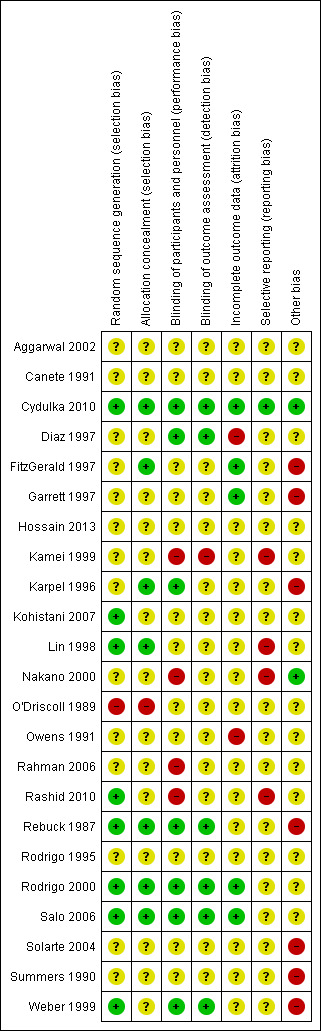
Risk of bias summary
4.
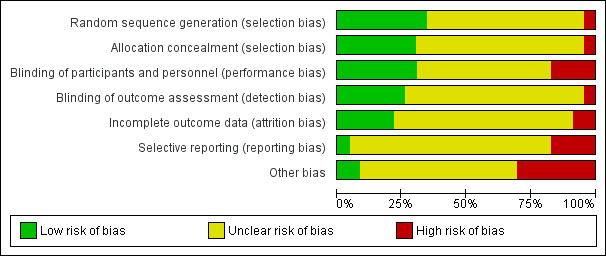
Risk of bias graph
Allocation
Although all studies reported being randomised, fewer than half provided adequate information on randomisation methods to enable assessment of selection bias (Cydulka 2010; Kohistani 2007; Lin 1998; O'Driscoll 1989; Rashid 2010; Rebuck 1987; Rodrigo 2000; Salo 2006; Weber 1999). Most studies provided insufficient information on methods of allocation concealment. Authors of three studies (FitzGerald 1997; Garrett 1997; Kamei 1999) provided additional clarification about methods of allocation concealment.
Blinding
Most studies were reported to be double‐blinded, although only seven adequately described methodology to enable assessment of low risk of bias (Cydulka 2010; Garrett 1997; Karpel 1996; Rebuck 1987; Rodrigo 2000; Salo 2006; Weber 1999). The four studies which were assessed at high risk of bias for this domain were self‐described as single‐blinded (Nakano 2000; Rahman 2006; Rashid 2010) and open‐labelled (Kamei 1999) studies. Only six studies were assessed at low risk of detection bias (Cydulka 2010; Garrett 1997; Rebuck 1987; Rodrigo 2000; Salo 2006; Weber 1999).
Incomplete outcome data
Most studies did not provide adequate information about numbers of participants screened for the study, including those who refused or were excluded from the study, to enable clear assessment of bias. Two studies (Diaz 1997; Owens 1991) were assessed at potentially high risk of bias because no information was provided on to which groups excluded participants belonged.
Selective reporting
Most studies were assessed at unclear risk of reporting bias due to a lack of an available protocol. Several studies reported side‐effects as an outcome; however, they did not provide data suitable for meta‐analysis, resulting in high risk of bias assessment (Kamei 1999; Lin 1998; Nakano 2000; Rashid 2010). Two studies (FitzGerald 1997; Karpel 1996) provided additional outcome data, but were assessed at unclear risk of bias. Cydulka 2010 was the only included study that published a protocol.
Other potential sources of bias
Most studies (n = 14) did not report sources of funding. Of two studies assessed at low risk of bias assessment, one reported receiving funding from a hospital (Nakano 2000), and the other received industry funding, but included a statement that the sponsor did not influence manuscript preparation or outcome reporting (Cydulka 2010). Seven studies reported receiving funding from pharmaceutical companies, but did not provide statements about funders' involvement in manuscript preparation or outcome reporting (FitzGerald 1997; Garrett 1997; Karpel 1996; Rebuck 1987; Solarte 2004; Summers 1990; Weber 1999).
Effects of interventions
See: Table 1
Hospitalisation
We included 15 studies, involving 2047 participants, that compared hospitalisation proportions in adults receiving combined inhaled therapy vs. SABA alone. Participants receiving combination inhaled therapy were less likely to be hospitalised than participants receiving SABA alone (RR 0.72, 95% CI 0.59 to 0.87; participants = 2120; studies = 16; I² = 12%; Analysis 1.1). Similiarily, worst‐case intention‐to‐treat (ITT) analysis found participants receiving combined inhaled therapy in the ED were less likely to be hospitalised (RR 0.76, 95% CI 0.63 to 0.91; participants = 2085; studies = 15; I² = 19%; Analysis 1.2) compared to participants receiving SABA only.
1.1. Analysis.
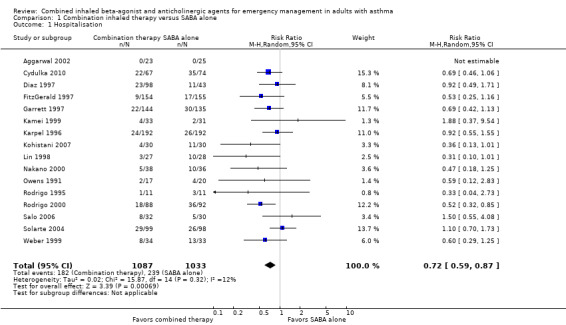
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 1 Hospitalisation.
1.2. Analysis.
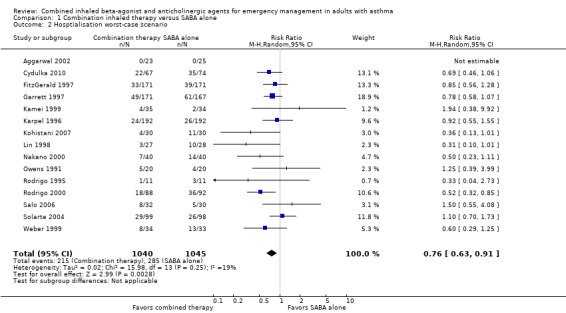
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 2 Hosptialisation worst‐case scenario.
Subgroup analyses
Subgroup analysis did not reveal whether single or multiple doses of combination inhaled therapy were more effective in mitigating the risk of hospitalisation (P = 0.29) (Analysis 2.1). Similarily, a subgroup analysis of participants who received or did not receive corticosteroids as a co‐intervention was unable to determine whether receiving additional corticosteroids modified the impact of combination therapy on reducing the risk for hospitalisation (P = 0.48) (Analysis 2.2).
2.1. Analysis.
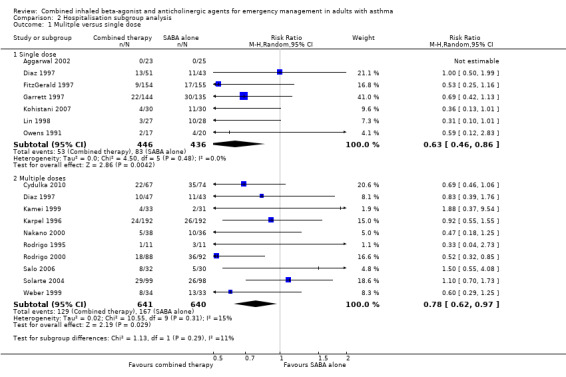
Comparison 2 Hospitalisation subgroup analysis, Outcome 1 Mulitple versus single dose.
2.2. Analysis.
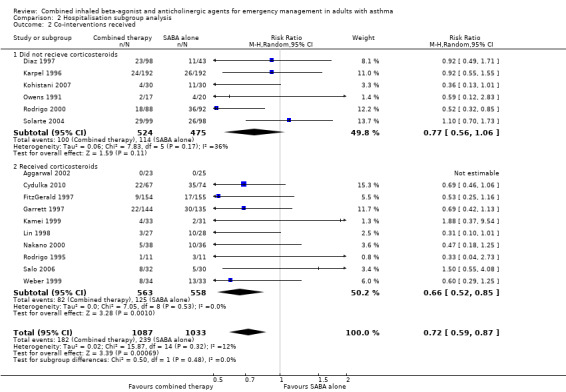
Comparison 2 Hospitalisation subgroup analysis, Outcome 2 Co‐interventions received.
Subgroup analysis of exacerbation severity did reveal a significant subgroup difference on the effects of combination inhaled therapy on mild, moderate and severe exacerbations (test for subgroup differences: P = 0.02). Although combination inhaled therapy was more effective than SABA alone in reducing hospitalisation in participants with severe exacerbations (RR 0.56, 95% CI 0.43 to 0.72; participants = 599; studies = 7; I² = 0%), no significant differences between combination inhaled therapy and SABA alone were found for participants with mild (RR 1.88, 95% CI 0.37 to 9.54; participants = 112; studies = 2; I² = 0%), or moderate (RR 0.88, 95% CI 0.69 to 1.11; participants = 1409; studies = 7; I² = 0%) exacerbations (Analysis 2.3). An analysis of exacerbation severity using risk difference revealed similar results, in which combination inhaled therapy was more effective in preventing hospitalisation among participants with severe acute asthma (RD ‐0.18, 95% CI ‐0.25 to ‐0.11; participants = 599; studies = 7; I² = 0%) compared to those with mild (RD 0.01, 95% CI ‐0.05 to 0.08; participants = 112; studies = 2; I² = 0%) or moderate (RD ‐0.03, 95% CI ‐0.07 to 0.01; participants = 1409; studies = 7; I² = 0%) acute asthma.
2.3. Analysis.
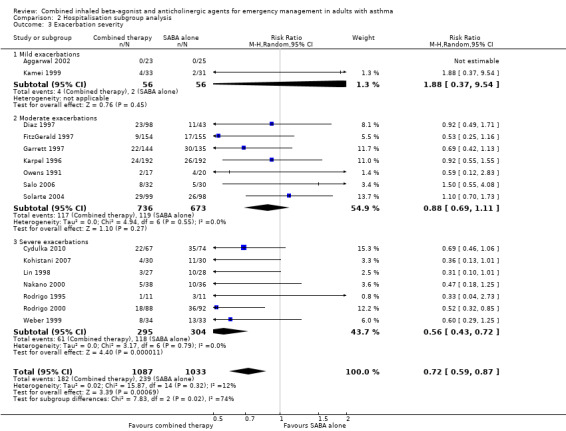
Comparison 2 Hospitalisation subgroup analysis, Outcome 3 Exacerbation severity.
No subgroup differences were found in regard to the type of SAAC therapy provided to participants (P = 0.62) (Analysis 2.4).
2.4. Analysis.
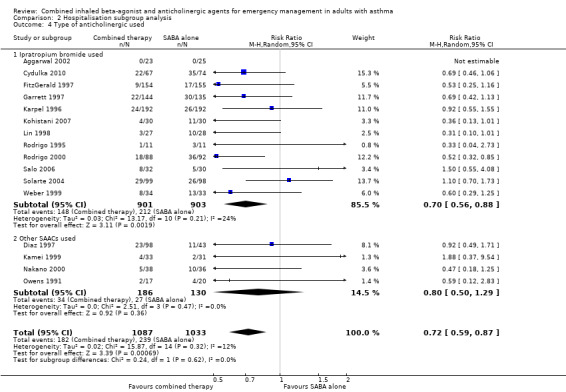
Comparison 2 Hospitalisation subgroup analysis, Outcome 4 Type of anticholinergic used.
Sensitivity analyses
Sensitivity analysis found that despite the removal of high risk of bias studies, participants receiving combination inhaled therapy were less likely to be hospitalised compared with participants receiving SABA alone (RR 0.63, 95% CI 0.44 to 0.90; participants = 513; studies = 6; I² = 22%; Analysis 3.1). Similar results were very similar using random‐effects (RR 0.72, 95% CI 0.59 to 0.87; participants = 2120; studies = 16; I² = 12%) and fixed‐effect models (RR 0.72, 95% CI 0.60 to 0.85; participants = 2120; studies = 16; I² = 12%; Analysis 3.2).
3.1. Analysis.
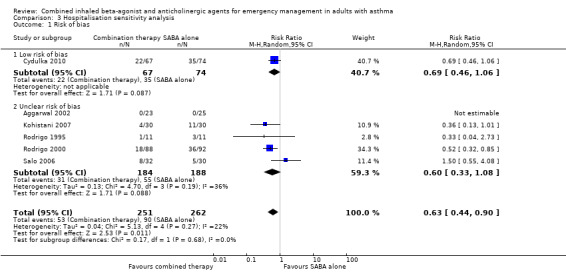
Comparison 3 Hospitalisation sensitivity analysis, Outcome 1 Risk of bias.
3.2. Analysis.
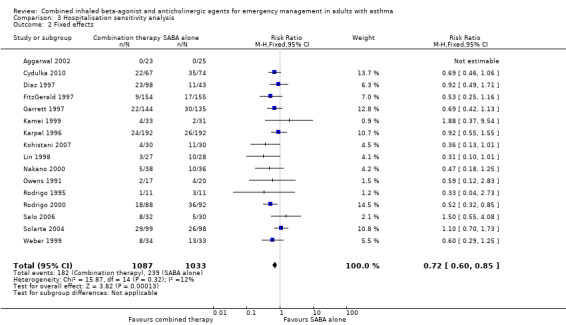
Comparison 3 Hospitalisation sensitivity analysis, Outcome 2 Fixed effects.
Adverse events
There were 11 studies involving 1392 participants that compared the frequency of adverse events after treatment with combination inhaled therapy vs. SABA alone. Participants who received combination inhaled therapy were more likely to experience adverse events than those who received SABA agents alone (OR 2.03, 95% CI 1.28 to 3.20; participants = 1392; studies = 11; I² = 14%; Analysis 1.3). Only a few studies reported the frequency of specific side effects related to inhaled SAAC or SABA use, such as tremor or dry mouth.
1.3. Analysis.
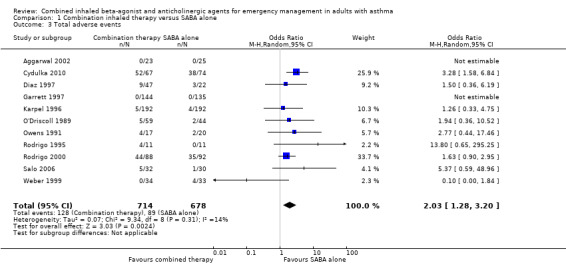
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 3 Total adverse events.
Additional analysis did not reveal differences in the frequency of specific adverse events including dry mouth (OR 2.08, 95% CI 0.84 to 5.12; participants = 447; studies = 5; I² = 54%; Analysis 1.4), tremor (OR 1.33, 95% CI 0.88 to 2.01; participants = 804; studies = 5; I² = 0%; Analysis 1.5), anxiety (OR 0.82, 95% CI 0.31 to 2.17; participants = 564; studies = 2; I² = 0%; Analysis 1.6), palpitations (OR 1.03, 95% CI 0.17 to 6.06; participants = 809; studies = 5; I² = 79%; Analysis 1.7), nausea (OR 0.65, 95% CI 0.19 to 2.17; participants = 245; studies = 3; I² = 0%; Analysis 1.8), headache (OR 1.46, 95% CI 0.31 to 6.78; participants = 247; studies = 2; I² = 13%; Analysis 1.9), blurred vision (OR 0.73, 95% CI 0.12 to 4.50; participants = 141; studies = 1; I² = 100%; Analysis 1.10), or agitation (OR 2.90, 95% CI 0.11 to 74.10; participants = 62; studies = 1; I² = 0%; Analysis 1.11) between participants receiving combined inhaled therapy vs. SABA treatment alone.
1.4. Analysis.
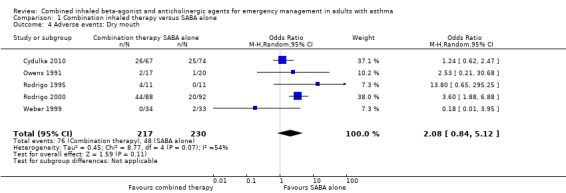
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 4 Adverse events: Dry mouth.
1.5. Analysis.
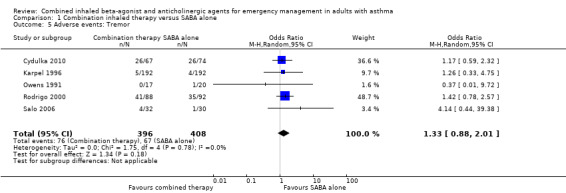
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 5 Adverse events: Tremor.
1.6. Analysis.
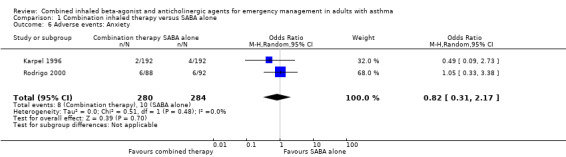
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 6 Adverse events: Anxiety.
1.7. Analysis.
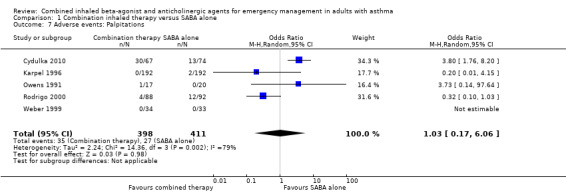
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 7 Adverse events: Palpitations.
1.8. Analysis.
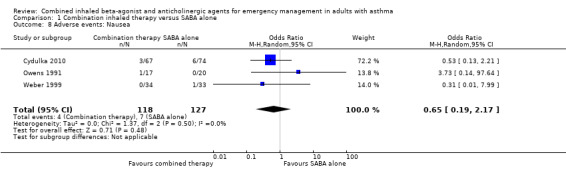
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 8 Adverse events: Nausea.
1.9. Analysis.
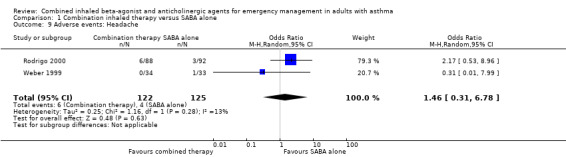
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 9 Adverse events: Headache.
1.10. Analysis.
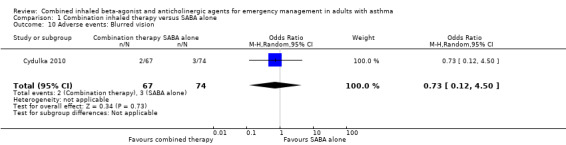
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 10 Adverse events: Blurred vision.
1.11. Analysis.
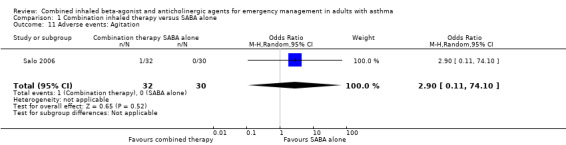
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 11 Adverse events: Agitation.
Pulmonary function
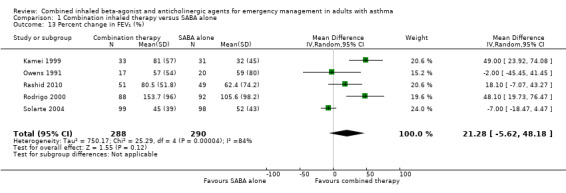
We assessed six studies that compared changes in FEV₁ between combination inhaled therapy and SABA alone. Participants who received combination inhaled therapy were more likely to exhibit improved FEV₁ by the end of the study period (MD 0.25 L, 95% CI 0.02 to 0.48; participants = 687; studies = 6); however, heterogeneity was high (I² = 70%; Analysis 1.12). In contrast, no significant differences were found in percent change in FEV₁ between participants who received combination inhaled therapy or SABA alone (MD 21.28% predicted, 95% CI ‐5.62 to 48.18; participants = 578; studies = 5), although heterogeneity was very high (I² = 84%; Analysis 1.13).
1.12. Analysis.
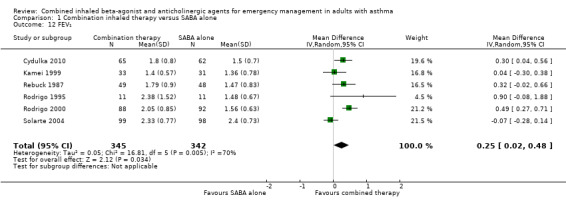
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 12 FEV₁.
1.13. Analysis.
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 13 Percent change in FEV₁ (%).
There were 12 studies that assessed lung functions using PEF. Participants who received combined inhaled therapy demonstrated improved PEF compared to those who received SABA only (MD 36.58 L/min, 95% CI 23.07 to 50.09; participants = 1056; studies = 12; I² = 25%; Analysis 1.14).
1.14. Analysis.
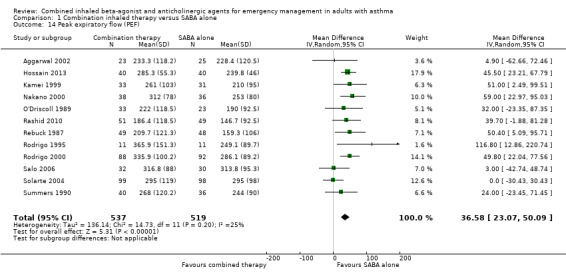
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 14 Peak expiratory flow (PEF).
Six studies compared the effects of combined inhaled treatment vs. SABA alone on percent change in PEF from baseline to the final PEF assessed after treatment. Participants who received combined inhaled therapy were more likely to have higher percent improvement in PEF compared to those who received SABA treatment alone (MD 24.88% improvement, 95% CI 14.83 to 34.93; participants = 551; studies = 7; I² = 23%; Analysis 1.15). Only two studies reported the percent predicted PEF, which was found to be higher among participants who received combination inhaled therapy compared to those who received SABA only (MD 13.67% predicted, 95% CI 3.88 to 23.46; participants = 102; studies = 2; I² = 50%; Analysis 1.16).
1.15. Analysis.
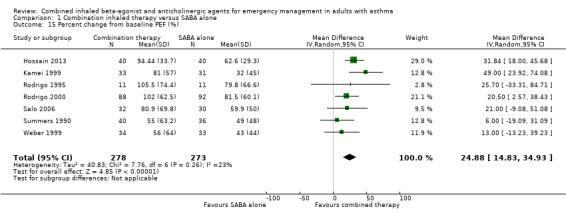
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 15 Percent change from baseline PEF (%).
1.16. Analysis.
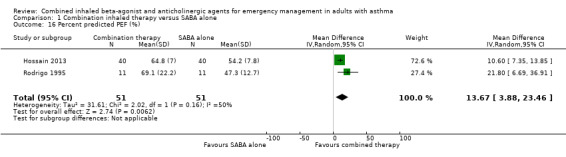
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 16 Percent predicted PEF (%).
Additional care
The need for additional treatments in the ED were examined in four studies. Only Nakano 2000 defined what was provided to participants as part of the additional ED treatments: these included IV aminophylline, inhaled bronchodilators, or both. Participants who received combined inhaled therapy did not show a difference in the need for additional treatment in the ED compared with participants who received SABA alone (RR 0.85, 95% CI 0.64 to 1.13; participants = 543; studies = 4); heterogeneity was moderate (I² = 27%; Analysis 1.17).
1.17. Analysis.
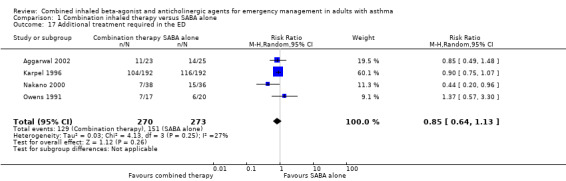
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 17 Additional treatment required in the ED.
Relapse
Five studies assessed whether participants needed to return to the ED after discharge due to a lack of improvement or worsening of symptoms. Participants who received combined inhaled therapy were less likely to return to the ED with worsening symptoms after discharge compared with those who received SABA treatment alone (RR 0.80, 95% CI 0.66 to 0.98; participants = 1180; studies = 5; I² = 0%; Analysis 1.18).
1.18. Analysis.
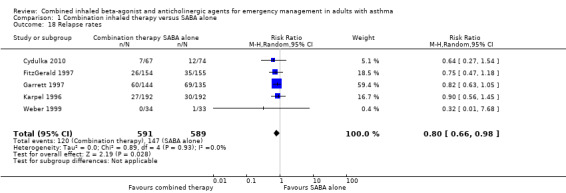
Comparison 1 Combination inhaled therapy versus SABA alone, Outcome 18 Relapse rates.
Discussion
Summary of main results
By using a comprehensive search strategy, and techniques to mitigate selection and publication bias, we identified 23 studies that included 2724 adult participants which compared combination inhaled treatment with inhaled short‐acting anticholinergics (SAAC) and short‐acting beta₂‐agonists (SABA) to treatment with inhaled SABA alone for the management of adults with acute asthma in the emergency department (ED). Only RCTs, CCTs and trials involving direct comparisons were eligible for inclusion. However, a lack of available data in two studies meant that 21 studies were included in the meta‐analysis for the primary outcome; even fewer studies could be meta‐analysed for the secondary outcomes.
The overall quality of the included studies was moderate to low; most were assessed at unclear risk of bias, and some at high risk of bias.
We identified several important findings regarding the effectiveness of combination therapy to mitigate hospitalisations.
First, combination inhaled therapy was shown to be more effective in reducing hospitalisations compared to treatment with inhaled SABA alone, particularly in participants with severe exacerbations who are at high risk for hospitalisation. Caution is warranted in the interpretation of this subgroup analysis due to the heterogeneity in assessing asthma severity employed across the studies.
Second, the benefit combination inhaled therapy does not appear to be related to whether or not participants were administered systemic corticosteroids. It is important to note, however, that co‐interventions were inconsistently reported across the studies, so it is possible that more studies could have provided corticosteroids in the ED, but did not report it.
Third, combination inhaled therapy appears to be effective regardless of whether or not ipratropium bromide or other SAACs were provided.
Finally, there was inconclusive evidence regarding the effectiveness of single versus multiple doses of combined inhaled therapy to prevent hospitalisation. Additional studies assessing direct comparisons between single and multiple doses of combination inhaled therapy are needed to directly compare these approaches. Overall, the effectiveness of combined inhaled therapy to prevent hospitalisations were robust in the face of sensitivity analyses which included random‐effects vs. fixed‐effect results and study quality.
Participants who received combination inhaled therapy were more likely to experience improvements in pulmonary function testing representing higher forced expiratory volume in one second (FEV₁), peak expiratory flow (PEF), and higher percent improvement in PEF. Standard recommendations for the minimally clinically important difference in most guidelines are 12% (Global Initiative for Asthma 2016); however, data from asthma trials suggest minimally clinically important difference change from baseline percentages for FEV (10%) and PEF (6%) may be even lower (Santanello 1999). In addition, participants receiving the combination inhaled treatment experienced less relapses after discharge. No significant differences were noted between participants receiving combination inhaled therapy or SABA alone with regard to percent improved FEV₁ and the need for additional bronchodilators in the ED. Although it is unclear why no significant improvement in percent improved FEV₁ was found, despite an improvement in FEV₁, results showed considerable inconsistency and imprecision. Furthermore, although the effect was moderate, caution is warranted in the interpretation of these results due to the heterogeneity in assessing and reporting airway obstruction employed across the studies.
Participants who received combination inhaled therapy were more likely to report adverse events compared to those treated with SABA agents alone. Despite this finding representing a picture of the overall symptoms experienced by participants, studies frequently failed to report in sufficient detail on the frequency of individual adverse events, such as dry mouth, termor, palpitations, and headache. As such, although results from this review suggest that participants who received combination inhaled therapy were more likely to report adverse events, we were unable to report on which particular adverse event participants could experience.
These findings provide important outcomes that should assist clinicians in informing patients and balancing treatment benefit with risk. It is important to note that most adverse events would not be considered serious and many would be self‐limiting.
Overall completeness and applicability of evidence
Overall, we believe the completeness and applicability of the evidence to be high. This is a moderately‐sized review with 23 studies including 2724 participants. The studies were conducted in EDs in the Americas, Europe, Asia, and Pacific regions.
Most included studies enrolled adult participants with a minimum age of 18 years. There were two studies which set the minimum age for enrolment as 13 years and 15 years (Aggarwal 2002; Canete 1991), respectively. After consideration, it was decided that these studies would be included in the review because the stated median ages were frequently between 30 and 42, suggesting that most included participants were adults aged over 16 years.
We included only studies in which participants presented to the ED with acute asthma. Studies that included participants with either asthma or other airway diseases, such as chronic obstructive pulmonary disease (COP, were excluded unless data were available for only asthma participants, or if the sample of asthma participants made up at least 80% of the study population.
Most included studies assessed hospitalisation as a primary outcome. As a result, we believe the review results are applicable to adults presenting to the ED with acute asthma. Unfortunately, some proposed secondary outcomes, such as quality of life, symptoms scores, and ED length of stay, were not reported widely and could not be included in the meta‐analysis as planned. In addition, pulmonary function measures and adverse events were inconsistently reported, and in some cases, were reported incompletely in the text, and could not be extracted for meta‐ analysis. Our attempts to contact study authors to provide clarification of their data were successful in some cases (Cydulka 2010; Garrett 1997; Salo 2006), particularly with regard to frequency of adverse events.
Quality of the evidence
The quality of the included studies was generally considered to be low or unclear. We assessed 14 studies at high risk of bias due to lack of double blinding, incomplete reporting of adverse events, and receiving industry funding with no clarification of the role that company had on outcome reporting or manuscript preparation (Diaz 1997; FitzGerald 1997; Garrett 1997; Kamei 1999; Karpel 1996; Lin 1998; Nakano 2000; O'Driscoll 1989; Owens 1991; Rahman 2006; Rashid 2010; Solarte 2004; Summers 1990; Weber 1999). Only Cydulka 2010 was assessed as being a high quality study.
On GRADE assessment, the overall quality of outcomes reported ranged from very low to moderate. The primary outcome, hospitalisation, was reduced to moderate quality because most studies were assessed at unclear or high risk of bias, frequently due to inadequate (or no) reporting of randomisation, allocation concealment or blinding. The quality of the evidence for adverse events was considered moderate due to the high risk bias relating to selective reporting.
Despite that the quality of the evidence for PEF, percent change PEF from baseline, and relapse were considered moderate (due to overall unclear and high risk of biases found in the studies), the quality of the evidence regarding FEV₁ and percent change in FEV₁, was found to be low and very low respectively due to inconsistency and imprecision of the results.
A limitation of this review is that the included studies tended to be small, and despite the low‐moderate statistical heterogeneity identified across the outcomes of this review, clinical heterogeneity, including participant characteristics, treatment dosing, and settings (in regard to different healthcare systems) exists.
Differences in admission criteria may have influenced the results of this review, because studies may have applied more liberal or conservative admission criteria. Only five of the included studies provided defined admission criteria, and it is unclear what criteria the remaining studies used to decide whether or not participants should be hospitalised. Moreover, the influence of funding, such as payment models for admission, and hybrid models of care, such as short‐stay units and observation units, on these results could not be determined from the available data.
Potential biases in the review process
As with all reviews, there was a risk of potential screening and study selection bias, although strategies were applied to minimise this risk. Extensive searches of electronic databases, grey literature, and the Cochrane Airways Group register of trials were conducted with no limits on language, publication type or year of publication. Several articles published in languages other than English were identified, and were included or excluded based on the information translated from the text. Where information provided in studies did not inform a clear inclusion or exclusion decision, attempts were made to contact the authors to clarify information provided in the text. Screening and study selection was completed independently by several trained review authors in an effort to limit the possibility of bias. Despite this, it is recognised that some articles may have been missed. The funnel plot (Figure 2) was not indicative of potential publication bias for the primary outcome.
Agreements and disagreements with other studies or reviews
Our results align with previous systematic reviews which found combination inhaled therapy to be more effective in reducing hospitalisation and improving pulmonary function measures than treatment with SABA alone in adults (Rodrigo 1999; Rodrigo 2005; Stoodly 1999) and children (Griffiths 2013; Rodrigo 2005) with acute asthma. Lanes 1998, a pooled analysis of three studies (FitzGerald 1997;Garrett 1997;Karpel 1996) also reported a significant reduction in hospitalisation and improvement in FEV₁ among participants who received combination therapy.
There were several disagreements between findings of this review and previous reviews. Rodrigo 1999 and Rodrigo 2005 reported a similar rate of adverse events, such as tremor, between participants who received combination inhaled therapy and SABA alone, whereas we found more side effects with combination therapy. The reason for this difference is likely due to the increased number of studies included in this review, as well as additional information which was received from study authors regarding the occurrence of adverse events. In addition, we reported similar effectiveness of single and multi‐dose combination inhaled therapy to mitigate hospitalisation, which appears to differ from other reviews. For example, Rodrigo 2005 reported a "trend" toward reduced risk of hospitalisation in adults receiving multi‐dose combination therapy; however, unlike this review, the authors did not conduct a statistical subgroup comparison of the trials using multiple or single doses of combination inhaled therapy. Furthermore, we identified more studies for inclusion than Rodrigo 2005, and featured a more up‐to‐date and extensive search of the electronic and grey literature, which is likely to be the greatest contributor to reported differences in results.
Authors' conclusions
Implications for practice.
Overall, combination inhaled therapy appears to be effective in reducing the risk of hospitalisation among adult patients at high risk for hospitalisation presenting to the emergency department (ED) with acute asthma.
In particular, combination inhaled therapy is more effective at preventing hospitalisation in adult patients with severe exacerbations who are at increased risk for hospitalisation, compared to those patients with mild‐moderate exacerbations who are at a lower risk of hospitalisation.
It is unclear whether there is a difference between single or multiple doses of combination inhaled therapy in mitigating hospitalisation.
The beneficial effects of combination inhaled therapy appear to be independent of co‐treatment with corticosteroids in the ED.
While effective at mitigating the risk for hospitalisations, patients who received combination inhaled therapy were at increased risk for adverse events.
Implications for research.
Additional research comparing the effectiveness of combination inhaled therapy for mild, moderate, and severe exacerbations of asthma is needed to better understand how to optimise care. Researchers need to improve on reporting of the severity of acute asthma among study participants.
Additional research conducting direct comparisons between the effectiveness of multiple vs. single doses of combination inhaled therapy is needed.
Additional research needs to examine the effects of combination inhaled therapy on ED length of stay, quality of life, and symptom scores. Further standardisation of techniques to assess pulmonary function are required.
Additional research is needed to better understand the relationship of combination inhaled therapy and relapse proportions.
We included several studies which reported no differences in the frequency of adverse events; however, these studies provided no data for inclusion in the text of the study. This prohibited several studies from being included in the meta‐analysis. It is very important for studies examining interventions to provide results for important outcomes such as adverse events. Around half of the included studies did not report data on the overall occurrence of adverse events, and even fewer provided details on the specific adverse events experienced. Despite the lack of reporting, a significant difference in the frequency of adverse events was found. Consistent reporting of the frequency of adverse events in future research is needed.
Future researchers need to clearly report methods of randomisation, allocation concealment, blinding, participant attrition rates during study recruitment, and sources of funding. Several included studies were funded by the pharmaceutical industry with no statements indicating companies' influence on the study or the content of the manuscript.
Acknowledgements
The review authors would like to thank Drs Praveen Aggarwal, Ken Chapman, Rita Cydulka, Mark FitzGerald, Jeffrey Garrett, Jill Karpel, Robert Lin, Frederic Manresa, Anthony Rebuck, David Salo, and Ellen Weber for responding to our requests for additional information on their studies. We would like to thank Alan Davidson, Rajiv Chetram, and Dr Maria Ospina for their assistance with this review. We would like to thank Dr Cristina Villa‐Roel for her assistance with Spanish to English translation. Dr Rowe’s research is supported by Tier I Canada Research Chair in Evidence‐based Emergency Medicine from Canadian Institute of Health Research (CIHR) through the Government of Canada (Ottawa, Ontario).
Jimmy Chong was the Editor for this review and commented critically on the review. We would like to thank Jessica Thomas, Chris Cates, Emma Dennett, and Elizabeth Stovold for their editorial support. We would like to thank Elizabeth Stovold for conducting the search of the Cochrane Airways Register.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE, 1946 to July 17, 2015 Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. exp asthma/ 2. asthma*.mp. 3. 1 or 2 4. exp Emergency Service, Hospital/ or (acute or relaps* or exacerbat*).ti,ab. 5. (emergency adj3 (room* or ward or wards or department* or doctor* or nurse* or clincian* or practitioner*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 6. ("critical care" or "acute care").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 7. 4 or 5 or 6 8. 3 and 7 9. anticholinergic*.mp. 10. (ipratropium or atrovent or oxitropium or oxivent).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 11. exp Ipratropium/ 12. cholinergic.mp. or exp Cholinergic Agents/ 13. PARASYMPATHOMIMETICS.mp. or exp Parasympathomimetics/ 14. limit 13 to yr="1975 ‐ 1994" 15. 9 or 10 or 11 or 12 or 14 16. 8 and 15 17. salbutamol.mp. or exp Albuterol/ 18. ("levalbuterol hydrochloride" or sultanol or albuterol or "2‐t‐butylamino‐1‐(4‐hydroxy‐3‐hydroxy‐3‐hydroxymethyl)phenylethanol" or ventolin or "levosalbutamol hydrochloride" or proventil or "hydrochloride levalbuterol" or "xopenex levalbuterol").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 19. exp Adrenergic beta‐2 Receptor Agonists/ 20. 17 or 18 or 19 21. 16 and 20 (336) 22. (combivent or berodual).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 23. 16 and 22 24. 21 or 23 25. limit 24 to "all child (0 to 18 years)" 26. limit 25 to "all adult (19 plus years)" 27. 24 not 25 28. 26 or 27
Appendix 2. Embase search strategy
Database: Embase 1974 to 17 July 2015 Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. exp asthma/ 2. (asthma* or wheezing or bronchial constriction or bronchial restriction).mp. 3. 1 or 2 4. anticholinergic*.mp. 5. (atropine or ipratropium or atrovent or oxitropium or oxivent).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. cholinergic.mp. or exp Cholinergic Agents/ 7. 4 or 5 or 6 8. salbutamol.mp. or exp Albuterol/ 9. ("levalbuterol hydrochloride" or sultanol or albuterol or "2‐t‐butylamino‐1‐(4‐hydroxy‐3‐hydroxy‐3‐hydroxymethyl)phenylethanol" or ventolin or "levosalbutamol hydrochloride" or proventil or "hydrochloride levalbuterol" or "xopenex levalbuterol").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 10. exp Adrenergic beta‐2 Receptor Agonists/ 11. beta adrenergic receptor stimulating agent/ or fenoterol/ or exp levalbuterol/ or salbutamol/ or salbutamol sulfate/ 12. (salbutamol or levalbuterol or fenoterol or phenoterol or albuterol or metaproterenol).mp. 13. 8 or 9 or 10 or 11 or 12 14. exp ipratropium bromide/ 15. exp oxitropium bromide/ 16. 7 or 14 or 15 17. 13 and 16 18. combivent.mp. or exp ipratropium bromide plus salbutamol sulfate/ 19. berodual.mp. or exp fenoterol plus ipratropium bromide/ 20. 17 or 18 or 19 21. exp emergency treatment/ 22. emergency physician/ 23. emergency nursing/ 24. (emergency adj2 (care or service* or medic* or department* or unit or area or ward or physician* or doctor* or nurs*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 25. 21 or 22 or 23 or 24 26. 3 and 20 and 25 27. limit 26 to (infant <to one year> or child <unspecified age> or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>) 28. limit 27 to (adult <18 to 64 years> or aged <65+ years>) 29. 27 not 28 30. 26 not 27 31. 29 or 30
Appendix 3. CINAHL search strategy
1. (MH "Cholinergic Antagonists+")
2. anticholinergic* or atrovent or oxivent
3. "ipratropium bromide" OR (MH "Ipratropium")
4. oxtiropium bromide
5. (MH "Atropine") OR "atropine"
6. (MH "Albuterol") OR "salbuterol"
7. "levalbuterol"
8. "albuterol"
9. "fenoterol"
10. "phenoterol"
11. (MH "Ociprenaline") OR "metaproterenol"
12. "beta n3 agonist"
13. 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
14. (MH "Emergency Service+") OR (MH "Physicians, Emergency") OR (MH "Emergencies+") OR (MH "Emergency Patients") OR "emergency"
15. (MH Asthma+")
16 "wheezing"
17. "bronchial restriction" OR (MH Bronchial Spasm")
18. 15 OR 16 OR 17
19. "combivent"
20. berodual
21. 19 OR 20
22. 1 OR 2 OR 3 OR 4 OR 5
23. 13 AND 22
24. 21 OR 23
25. 14 AND 18 AND 24
Appendix 4. SCOPUS search strategy
1. (salbutamol OR levalbuterol OR fenoterol OR phenoterol OR albuterol OR metaproterenol OR beta w/2 agonist*)
2. (emergency w/2 (care or service* or medic* or department* or unit or area or ward or physician* or doctor* or nurs*))
3. (ematropine OR ipratropium OR atrovent OR oxitropium OR oxivent OR antichol*)
4. (asthma*) OR (bronchial w/1 constrict*) OR (bronchial w/1 restrict*) OR (wheezing*)
5. 1 AND 2 AND 3 AND 4 AND 5
Appendix 5. LILACS search strategy
1. antichol* OR ipratropium OR atrovent OR oxitropium OR oxivent
2. salbutamol OR albuterol OR ventolin
3. (emergen* OR acute OR relapse* OR exacerbat*) AND asthma
4. 1 AND 2 AND 3
Appendix 6. ProQuest Dissertations & Theses Global search strategy
1. (atropine OR ipratropium OR atrovent OR oxitropium OR oxivent OR antichol*)
2. (salbutamol OR levalbuterol OR fenoterol OR phenoterol OR albuterol OR metaproterenol OR beta w/2 agonist*)
3. 1 AND 2
Appendix 7. Evidence‐Based Medicine Reviews search strategy
Databases searched for EBM reviews: Cochrane Database of Systematic Reviews 2005 to June 2015 ACP Journal Club 1991 to July 2015 Database of Abstracts of Reviews of Effects (DARE)Second Quarter 2015 Cochrane Central Register of Controlled Trials (CENTRAL)June 2015 Cochrane Methodology Register third quarter 2012 Health Technology Assessment second quarter 2015 NHS Economic Evaluation Database second quarter 2015. Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. exp asthma/ 2. asthma*.mp. 3. 1 or 2 4. exp Emergency Service, Hospital/ or (acute or relaps* or exacerbat*).ti,ab. 5. (emergency adj3 (room* or ward or wards or department* or doctor* or nurse* or clincian* or practitioner*)).mp. [mp=ti, ab, tx, kw, ct, ot, sh, hw] 6. ("critical care" or "acute care").mp. [mp=ti, ab, tx, kw, ct, ot, sh, hw] 7. 4 or 5 or 6 8. 3 and 7 9. anticholinergic*.mp. 10. (ipratropium or atrovent or oxitropium or oxivent).mp. [mp=ti, ab, tx, kw, ct, ot, sh, hw] 11. exp Ipratropium/ 12. cholinergic.mp. or exp Cholinergic Agents/ 13. PARASYMPATHOMIMETICS.mp. or exp Parasympathomimetics/ 14. limit 13 to yr="1975 ‐ 1994" [Limit not valid in DARE; records were retained] 15. 9 or 10 or 11 or 12 or 14 16. 8 and 15 17. salbutamol.mp. or exp Albuterol/ 18. ("levalbuterol hydrochloride" or sultanol or albuterol or "2‐t‐butylamino‐1‐(4‐hydroxy‐3‐hydroxy‐3‐hydroxymethyl)phenylethanol" or ventolin or "levosalbutamol hydrochloride" or proventil or "hydrochloride levalbuterol" or "xopenex levalbuterol").mp. [mp=ti, ab, tx, kw, ct, ot, sh, hw] 19. exp Adrenergic beta‐2 Receptor Agonists/ 20. 17 or 18 or 19 21. 16 and 20 22. (combivent or berodual).mp. [mp=ti, ab, tx, kw, ct, ot, sh, hw] 23. 16 and 22 24. 21 or 23 25. limit 24 to "all child (0 to 18 years)" [Limit not valid in CDSR,ACP Journal Club,DARE,CCTR,CLCMR; records were retained] 26. limit 25 to "all adult (19 plus years)" [Limit not valid in CDSR,ACP Journal Club,DARE,CCTR,CLCMR; records were retained] 27. 24 not 25 28. 26 or 27 29. from 28 keep 1‐240
Appendix 8. Cochrane Airways Group register of trials search strategy
1. AST:MISC1
2. MeSH DESCRIPTOR Asthma Explode All
3. asthma*:ti,ab
4. 1 or 2 or 3
5. MeSH DESCRIPTOR Cholinergic Antagonists Explode All
6. anticholinergic* or anti‐cholinergic*
7. ipratropium*
8. MeSH DESCRIPTOR Ipratropium
9. Atrovent
10. MeSH DESCRIPTOR Atropine
11. atropine*
12. oxitropium*
13. Oxivent
14. muscarinic*
15. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16. acute* or status* or sever* or emerg* or exacerbat* or hospital* or crisis*
17. 4 and 15 and 16
Data and analyses
Comparison 1. Combination inhaled therapy versus SABA alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalisation | 16 | 2120 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.87] |
| 2 Hosptialisation worst‐case scenario | 15 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |
| 3 Total adverse events | 11 | 1392 | Odds Ratio (M‐H, Random, 95% CI) | 2.03 [1.28, 3.20] |
| 4 Adverse events: Dry mouth | 5 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.84, 5.12] |
| 5 Adverse events: Tremor | 5 | 804 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.88, 2.01] |
| 6 Adverse events: Anxiety | 2 | 564 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.31, 2.17] |
| 7 Adverse events: Palpitations | 5 | 809 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.17, 6.06] |
| 8 Adverse events: Nausea | 3 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.19, 2.17] |
| 9 Adverse events: Headache | 2 | 247 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.31, 6.78] |
| 10 Adverse events: Blurred vision | 1 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.12, 4.50] |
| 11 Adverse events: Agitation | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 2.90 [0.11, 74.10] |
| 12 FEV₁ | 6 | 687 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.02, 0.48] |
| 13 Percent change in FEV₁ (%) | 5 | 578 | Mean Difference (IV, Random, 95% CI) | 21.28 [‐5.62, 48.18] |
| 14 Peak expiratory flow (PEF) | 12 | 1056 | Mean Difference (IV, Random, 95% CI) | 36.58 [23.07, 50.09] |
| 15 Percent change from baseline PEF (%) | 7 | 551 | Mean Difference (IV, Random, 95% CI) | 24.88 [14.83, 34.93] |
| 16 Percent predicted PEF (%) | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 13.67 [3.88, 23.46] |
| 17 Additional treatment required in the ED | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 18 Relapse rates | 5 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.98] |
Comparison 2. Hospitalisation subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mulitple versus single dose | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Single dose | 7 | 882 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.86] |
| 1.2 Multiple doses | 10 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.97] |
| 2 Co‐interventions received | 16 | 2120 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.87] |
| 2.1 Did not recieve corticosteroids | 6 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.06] |
| 2.2 Received corticosteroids | 10 | 1121 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.52, 0.85] |
| 3 Exacerbation severity | 16 | 2120 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.87] |
| 3.1 Mild exacerbations | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.37, 9.54] |
| 3.2 Moderate exacerbations | 7 | 1409 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.11] |
| 3.3 Severe exacerbations | 7 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.43, 0.72] |
| 4 Type of anticholinergic used | 16 | 2120 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.87] |
| 4.1 Ipratropium bromide used | 12 | 1804 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.88] |
| 4.2 Other SAACs used | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.29] |
Comparison 3. Hospitalisation sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of bias | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.90] |
| 1.1 Low risk of bias | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.06] |
| 1.2 Unclear risk of bias | 5 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.08] |
| 2 Fixed effects | 16 | 2120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aggarwal 2002.
| Methods | ‐ Prospective RCT. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Randomisation accomplished via random numbers tables. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Adult participants who presented to the ED in with acute bronchial asthma during working hours and had a previous diagnosis or treatment of bronchial asthma. ‐ Set in India. ‐ Ages: 15 to 30 years. ‐ Asthma exacerbation severity of presenting patients estimated as mild. |
|
| Interventions | ‐ Single dose of combination inhaled therapy provided. Study interventions provided via ultrasonic nebuliser. ‐ Group one received salbutamol (5 mg) over a period of five minutes at 0 and 60 minutes. ‐ Group two received ipratropium bromide (500 μg) over a period of five minutes at 0 and 60 minutes. ‐ Group three received a single dose of combined ipratropium bromide (500 μg) and salbutamol (5 mg) over a period of 5 minutes, followed by placebo nebulisation 60 minutes later. For the purposes of this review, group two was not included in the analysis. ‐ Additional co‐interventions provided in the ED included IV hydrocortisone, and supplemental oxygen. |
|
| Outcomes | ‐ Outcome measurements include hospitalisation, ED length of stay, vital signs, adverse events, and additional bronchodilator treatments. ‐ Only the outcomes of groups one and three were extracted. ‐ Outcomes measurements were performed at baseline, as well as 15, 60, 75, and 120 minutes after treatment. |
|
| Notes | ‐ Author was contacted to retrieve the original database for subgroup comparisons but the author stated that he no longer had access to the original data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random numbers drawn from random numbers table. Quote (p. 354): "For randomisation of the patients into three groups, random numbers were drawn from the random number table to decide allocation group of patients well in advance". |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided on whether participants or personnel were blinded were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on number of patients excluded during the screening process. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Canete 1991.
| Methods | ‐ Prospective RCT. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients presenting to the ED for exacerbation of asthma. ‐ Set in Spain. ‐ Ages: 13 to 85 years. ‐ Asthma exacerbation severity of patients presenting unclear. Insufficient information provided. |
|
| Interventions | ‐ Mutliple doses of combined inhaled therapy. Study interventions provided via nebuliser. Abstract states that patients received study interventions every two hours, but it is unclear how many doses patients received. ‐ Group one received ipratropium bromide (0.1 mg) and salbutamol (2.5 mg) every two hours. ‐ Group two received salbutamol (5.0 mg) alone every two hours. ‐ Additional co‐interventions provided in the ED included IV corticosteroids, IV aminophylline and supplemental oxygen according to need. |
|
| Outcomes | ‐ Outcome measurements included adverse events, and vital signs. ‐ Not enough information provided to extract data on pulmonary function or adverse events. ‐ Outcome measurements were performed at baseline and at two hours after the start of treatment. |
|
| Notes | ‐ Contacted primary author to clarify their methodology and results but they stated that they no longer had access to the original data. ‐ No full‐text, only an abstract available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on method of randomisation. Quote (p. 32): "Aleatoriamente se distribuyeron en dos grupos de tratamiento." (Translation: "They were randomised into two treatment groups.") |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information provided. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Cydulka 2010.
| Methods | ‐ Prospective, double‐blinded RCT. ‐ Comparison of ipratropium bromide and levabuterol vs. levabuterol alone. ‐ Randomisation was accomplished using computer‐generated block random numbers table (blocks of 15 by site). ‐ Allocation concealment was reported and discussed as pharmacy controlled. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of acute asthma. ‐ Set in United States. ‐ FEV on presentation to the ED was < 50% of predicted (In compliance with National Asthma Education and Prevention Program definition of severe asthma exacerbation). ‐ Age: 18 to 45 years. ‐ Asthma exacerbation severity of presenting patients was severe. Estimates of asthma severity based on control hospitalisation rates were estimated as moderate. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received three doses of levabuterol (1.25 mg) combined with ipratropium bromide (0.5 mg). ‐ Group two received three doses of levabuterol alone (1.25 mg). ‐ Additional co‐interventions provided in the ED include a single dose of oral prednisone (60 mg); discharged patients received an additional two day supply. ‐ Interventions provided only at discharge included SABA and inhaled corticosteroids if patient had a history of chronic persistent asthma. |
|
| Outcomes | ‐ Primary outcome was the change in FEV percent predicted over time. ‐ Additional outcomes included hospitalisation, ED discharge, adverse events and pulmonary testing. ‐ Outcome measurements were performed at baseline, 30 and 60 minutes after treatment. |
|
| Notes | ‐ Author was contacted and provided additional data on adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised via computer‐generated numbers table. Quote (p. 1095): "Patients were randomised to treatment group using a computer‐generated, block random numbers table (blocks of 15 by site)." |
| Allocation concealment (selection bias) | Low risk | Centrally allocated, pharmacy controlled. Quote (p. 1095): "The medication for both treatment groups was premixed in three vials by the pharmacy in a total of 3 mL normal saline solution. The pharmacist packed the treatments in brown numbered envelopes for the ED. The sequence assignment sheet was stored in a locked cabinet in the hospital pharmacy and concealed from the research nurses enrolling patients and assessing participants." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinding. Nurses, physicians, and patients were blind to the contents of the envelopes. Quote (p. 1095): "The research nurses were required to use the brown envelope containing medications in pre numbered sequence and record the sequence number on the data collection form. The brown envelopes contained all medications to use during the study. All vials contained in the envelope looked identical to one another. Physicians were asked to assess the patients before and between scheduled treatments. Blinding to group assignment was maintained throughout the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (research nurses) blinded. Quote (p. 1096): "At all times, the research nurses, patients, and treating physicians were blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 1095). |
| Selective reporting (reporting bias) | Low risk | Protocol available from ClinicalTrials.gov (NCT00583778). All proposed outcomes, including FEV, hospitalisations, relapse, and side effects were reported. The authors were contacted to provide additional data on adverse events to enable meta‐analysis. |
| Other bias | Low risk | Quote (p. 1099): "The study was supported by a grant from Sepracor (authors note: Sepracor is now known as Sunovion). The authors alone are responsible for the content and writing of the paper." Sunovion is a pharmaceutical manufacturer offering products to treat respiratory conditions. |
Diaz 1997.
| Methods | ‐ Prospective, randomised, double‐blinded, placebo‐controlled study. ‐ Comparison of atropine sulphate (multidose) and albuterol vs. atropine sulphate (single dose) and albuterol vs. albuterol alone. ‐ Method of randomisation unclear. ‐ Allocation concealment was reported and discussed as pharmacy controlled. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma and had a history of asthma. ‐ Set in United States. ‐ History of recurrent, episodic exacerbations of reversible bronchospasms were considered to have asthma. ‐ Age: 18 to 70 years. ‐ Asthma exacerbation severity of presenting patients estimated as moderate. |
|
| Interventions | ‐ Study assessed single and multiple doses of combination inhaled therapy. Study interventions provided via nebuliser. ‐ All groups received albuterol (2.5 mg) every 30 minutes for 3 doses (0, 30, 60 minutes). ‐ Group one received two additional doses of 2 mg of atropine sulphate at time 0, as well as an additional 2 mg at 60 minutes (multidose). ‐ Group two received one additional dose of 2 mg of atropine sulphate at time 0 only (single dose). ‐ Group three received only albuterol in the doses stated above. ‐ No additional co‐interventions in the ED provided. Systematic steroids, additional beta agonists, and IV therapy given only at discharge or upon admittance to hospital. |
|
| Outcomes | ‐ Outcomes included hospitalisations, pulmonary testing, ED length of stay, and presence of adverse events. ‐ Outcome measurements were performed at baseline, as well as 30, 60 and 90 minutes after treatment. |
|
| Notes | ‐ Unable to contact authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear, not enough information provided. Quote (p. 108): "A block‐design randomization scheme was devised prior to patient enrolment." |
| Allocation concealment (selection bias) | Unclear risk | No information provided on where the separate confidential location was located. Unclear if centrally allocated. Quote (p. 102): "The study agent (2 mg of atropine sulfate or an equal volume of normal saline) was prepared in advance and coded. All patients had a coded syringe added to the first and third nebulizers (time 0 and 60 minutes). The contents of the syringe were unknown to the treating physician, nurse, and patient. The code key was kept in a separate confidential location." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study. Study medications kept in coded identical syringes. Contents of syringe were unknown to the treating physician, nurse and patient. Quote (p. 102): "The contents of the syringe were unknown to the treating physician, nurse, and patient." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded study. Study medications kept in coded identical syringes. Contents of syringe were unknown to the treating physician, nurse and patient. Quote (p. 102): "The contents of the syringe were unknown to the treating physician, nurse, and patient." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported several patients were withdrawn or excluded after inclusion into the study. Did not specify from which groups patients were excluded or withdrew. Quote (p. 110): "A total of 153 patients satisfied enrolment criteria and 148 were randomised into 1 of the 3 treatment groups (5 patients were excluded due to previous entry into the study population). Another 4 patients were withdrawn from the study because they quickly decompensated during treatment and needed additional therapy. An additional 3 patients were excluded from analyses due to insufficient essential data (i.e. pulmonary function tests at > 1 time point). A total of 141 patients were analyzed with the intention to treat." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
FitzGerald 1997.
| Methods | ‐ Multicentre, double‐blind randomised, active‐controlled trial. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ Allocation concealment achieved via central allocation, telephone; drugs were kept in pharmacy. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma and had a diagnosis of asthma consistent with ATS guidelines. ‐ Set in Canada. ‐ Could perform reproducible spirometry. ‐ Initial FEV < 70% of predicted normal value. ‐ Age: 18 to 55 years. ‐ Asthma exacerbation severity of presenting participants estimated as moderate. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received ipratropium bromide (0.5 mg) and salbutamol (3.0 mg). ‐ Group two received salbutamol alone (3.0 mg). ‐ Additional co‐interventions provided in the ED included 125 mg of IV methylprednisolone within 15 minutes of nebulisation, as well as supplemental oxygen given continuously. |
|
| Outcomes | ‐ Outcomes measured included hospitalisations, ED discharge, pulmonary testing and relapse. ‐ Outcome measurements were performed at baseline, as well as 45, and 90 minutes. Relapse and hospitalisation assessed for two weeks after discharge from the ED. |
|
| Notes | ‐ Study authors contacted and provided clarification of some methodology and study results including relapse rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on method of randomisation. Quote (p. 312): "Following enrolment and measurement of baseline FEV₁ patients were randomised to receive in double‐blind fashion either a fixed‐dose combination of ipratropium bromide and salbutamol sulfate (0.5 mg and 3.0 mg, respectively) or salbutamol sulfate alone (3.0 mg)". |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. Information retrieved from personnel communication with authors. Personal communication: "Randomisation was centralised for NZ and Canadian Study and probably using similar software as this was by BI and Nebulisers were maintained in pharmacy" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reported as double‐blinded but no information provided on methods to ensure double‐blinding. Quote (p. 312): "Following enrolment and measurement of baseline FEV₁ patients were randomised to receive in double‐blind fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Extensive information provided on study attrition. Patient withdrawals evenly balanced between groups. Quote (p. 312): "Of 952 patients screened against inclusion or exclusion criteria for this study, 606 were found to be ineligible. Patients were excluded for the following reasons: smoking history greater than 10 pack‐years, 155; in extremis or with severe obstruction, 43; ATS definition of chronic obstructive lung disease, 10; previously recruited into the study, 19; receiving treatment for or suspected of having glaucoma, three; uncontrolled hypertension, three; known allergy or contraindications to study drugs or their excipients, 12; known or suspected to be pregnant or nursing, 17; suspected to have pneumonia, pneumothorax, or pneumomediastinum, 26; history of chest surgery, 13; other respiratory conditions, 13; required treatment of asthma attack other than study treatment regimen, 18; had been in other clinical trials within 3 months previously, 18; had an acute myocardial infarction, pulmonary edema, or other life‐threatening disease, six; or had obvious or previously diagnosed serious hepatic or renal impairment or bladder neck obstruction, six.Patients failing to meet the inclusion criteria were as follows: no diagnosis of asthma according to ATS criteria, outside the age range, nine; unable to perform spirometry, 60; FEVX > 70% of predicted normal, 259; and unwilling or unable to sign witnessed informed consent form, 156. The remaining 342 patients were randomised into the study with 171 patients in each treatment group. Of 342 patients randomised, two patients received no study drugs. Of the 342 patients randomised, 17 patients in the combination therapy group and 16 patients in the salbutamol alone group were either withdrawn by the study physician or requested to be withdrawn early." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Funding provided by Boehringer Ingelheim. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 311): "Supported in part by a research grant from Boehringer Ingelheim (Canada) Ltd." |
Garrett 1997.
| Methods | ‐ Prospective, randomised, double‐blind parallel‐group study. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ Allocation concealment achieved via central allocation by telephone. |
|
| Participants | ‐ Participants who presented to the ED with an exacerbation of asthma, capable of performing a forced respiratory maneuver. ‐ Set in New Zealand. ‐ Patients who had received a nebulised bronchodilator within 6 hours of presentation were not excluded. ‐ Asthma exacerbation was defined as a FEV < 70% predicted. ‐ Ages: 18 to 55 years. ‐ Asthma exacerbation severity of presenting patients reported as severe. Estimates of asthma severity based on control hospitalisation rates were estimated as moderate. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser mask. ‐ Group one received ipratropium bromide (0.5 mg) and salbutamol (2.5 mg). ‐ Group two received salbutamol alone (2.5 mg). ‐ Co‐interventions provided in the ED included IV hydrocortisone (200 mg). Isotonic IV fluid was only given if needed. |
|
| Outcomes | ‐ Primary outcomes included absolute change in FEV₁ at 90 minutes. ‐ Additional outcomes included pulmonary function, adverse events, vital signs, hospitalisation, ED discharge, and relapse rates. ‐ Outcome measurements were performed at baseline, as well as 45, and 90 minutes after treatment. Unclear from original text when relapse was assessed but supplemental information from Lanes 1998 suggests relapse was assessed at 48 hours after discharge. |
|
| Notes | ‐ Study authors contacted who provided clarification of some methodology, as well as frequency of adverse events and relapse rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 165): "Two New Zealand EDs participated in a double‐blind, randomised, active‐controlled, parallel‐group study comparing the bronchodilating effect of a fixed combination of nebulized ipratropium (0.5 rag) and salbutamol (2.5 rag) (Combivent) with nebulized salbutamoI (2.5 mg) alone in patients with acute severe asthma." |
| Allocation concealment (selection bias) | Unclear risk | Central allocation. Information retrieved from personnel communication with authors. Personal communication: "Randomisation was centralised for NZ and Canadian Study and probably using similar software as this was by BI and Nebulisers were maintained in pharmacy". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blinded. Study medications were kept in indistinguishable vials. Quote (p. 166): "Indistinguishable unit dose vials of 2.5 ml were developed for the Combivent and salbutamol solutions." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Extensive information provided on study attrition. Patient withdrawals evenly balanced between groups. Quote (p. 167): "Fifty‐nine of 338 patients recruited into the study were withdrawn before the primary outcome measurement of FEV₁ at 90 minutes (ΔFEV₁ 90) was obtained; 13 requested early withdrawal, (9 receiving Combivent and 4 receiving salbutamol), 45 were withdrawn early by the ED doctor because of a lack of satisfactory improvement (18 receiving Combivent and 27 receiving salbutamol), and one was withdrawn before treatment was administered because he was unable to provide blood samples." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Funding provided by Boehringer Ingelheim. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 165): "Supported by Boehringer Ingelheim Ltd." |
Hossain 2013.
| Methods | ‐ Prospective, randomised, single‐centre study. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma. ‐ Set in Bangladesh. ‐ Asthma exacerbation was defined as PEF < 50% predicted. ‐ Ages: 18 to 65 years. ‐ Asthma exacerbation severity of presenting patients reported as severe. Unable to assess estimates of asthma severity based on control hospitalisation rates due to lack of information. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received three doses of salbutamol alone (2.5 mg diluted in 2 mL of normal saline) every 20 minutes. ‐ Group two received three doses of ipratropium bromide (250 μg in 2 mL solution) and salbutamol (2.5 mg diluted in 2 mL of normal saline) every 20 minutes. ‐ Additional co‐interventions provided in the ED included supplemental oxygen and injection hydrocortisone. |
|
| Outcomes | ‐ The primary outcome was pulmonary function at 30 and 60 minutes after nebulisation. ‐ Outcome measurements were performed as baseline, as well as 30 and 60 minutes after treatment. |
|
| Notes | ‐ Study authors contacted and provided clarification of some methodology and study results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 347): A total of 80 patients were randomly assigned to two treatment groups,..." |
| Allocation concealment (selection bias) | Unclear risk | Study did not address allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study did not address blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study did not address blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement. Quote (p. 347): "A total 80 patients were randomly assigned to two treatment groups. Forty (40) received Salbutamol alone (Group A) and 40 received combination Ipratropium Bromide and Salbutomol (group B)..." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Kamei 1999.
| Methods | ‐ Prospective, multicenter, randomised open trial. ‐ Comparison of oxitropium bromide and fenoterol vs. fenoterol alone. ‐ Method of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients who present to the ED with an exacerbation of asthma and a previous diagnosis of asthma according to ATS guidelines. ‐ Patients capable of performing spirometry test. ‐ Asthma exacerbation defined as a PEF ≤ 70% of predicted value. ‐ Ages: 18 to 65 years. ‐ Asthma exacerbation severity in presenting participants estimated as moderate. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via MDI with a spacer device. ‐ Group one received fenoterol alone (200 μg/puff), taking one puff/minute for five minutes for a total of five puffs. ‐ Group two received oxitropium bromide (200 μg/puff) and fenoterol (200 μg/puff), taking one puff/minute each for five minutes for a total of five puffs. ‐ Additional co‐interventions provided in the ED included successive IV glucocorticoids and IV aminophylline if inhalation therapy was not effective. |
|
| Outcomes | ‐ Outcomes included pulmonary function, hospitalisations, ED discharge, and adverse events. ‐ Outcome measurements were performed at baseline, as well as 1, 15, 30, 60 minutes after treatment. |
|
| Notes | ‐ Unable to contact authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 68): "This study was a multicenter, randomised, open trial conducted at seven academic and nonacademic centers." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial study. Quotes: "This study was a multicenter, randomised, open trial conducted at seven academic and nonacademic centers." (p. 68) "Because we used an MDI with an InspirEase device, it was impossible to perform this study in a double‐blinded fashion." (p. 74) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial study. Quote (p. 68): "This study was a multicenter, randomised, open trial conducted at seven academic and nonacademic centers." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded patients balanced between groups. No information provided on number of patients screened. Quote (p. 69): "Thirty‐five patients were entered in the combination group and 34 patients were entered in the fenoterol‐only group. On the basis of chart review, 5 of the 69 patients were found to be ineligible. Before the study, four patients used inhalation therapy of oxitropium bromide on their own, and one patient received intravenous aminophylline, which was considered to be a physician’s protocol violation. Therefore, 33 patients were evaluated in the combination group and 31 patient were evaluated in the fenoterol‐only group." |
| Selective reporting (reporting bias) | High risk | No protocol available. The frequency of side effects were incompletely reported. Quote (p. 70): "There was no significant difference in the number of adverse reactions between the groups (data not shown)." |
| Other bias | Unclear risk | No source of funding provided. |
Karpel 1996.
| Methods | ‐ Prospective, randomised, double blind controlled study with a parallel group design. ‐ Comparison of ipratropium bromide and albuterol vs. albuterol alone. ‐ Randomisation was accomplished using computer‐generated random numbers via software however method of randomisation was unclear. ‐ Allocation concealment was done via central allocation, telephone. Medication was kept in pharmacy. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma. ‐ Set in the United States. ‐ Had to be capable of performing a forced expiratory maneuver. ‐ Asthma exacerbation was defined as FEV ≤ 60% of predicted value with a 12% adjustment for persons of African‐American heritage based on the equations of Morris 1988. ‐ Ages: 18 to 55 years. ‐ Asthma exacerbation severity of presenting patients estimated as moderate. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via updraft nebuliser. ‐ Group one received two doses of albuterol (0.5 mL of 0.5% solution) mixed with saline solution (2.5 mL). The second dose was provided 45 minutes after the first dose. ‐ Group two received two doses of ipratropium bromide (2.5 mL of 0.02% solution) and albuterol (0.5 mL of 0.5% solution). The second dose was provided 45 minutes after the first dose. ‐ Additional co‐interventions provided in the ED included supplemental oxygen delivered at 3 L/min at all times throughout the course of the study. |
|
| Outcomes | ‐ Outcomes included pulmonary function, hospitalisation, vital signs, ED discharge, ICU admission, adverse events and relapse. ‐ Outcome measurements were performed at baseline, as well as 45 and 90 minutes after the first dose. Relapse was assessed 24 hours after discharge from the ED. |
|
| Notes | ‐ Study authors contacted who provided clarification of some methodology and study results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation via site specific randomisation schedule using software, but no information available on method of randomisation. Quote (p. 612): "Patients were assigned to receive one of the two treatment regimens according to a center‐specific randomization schedule using software (ADLS‐11 software; Almedica Corp; Waldwick, NJ). |
| Allocation concealment (selection bias) | Low risk | Central allocation. Information retrieved from personnel communication with authors. Personal communication (May 7, 2014): "I think we called them & was assigned # over phone." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study. Study medications provided in identical, previously coded vials. Quote (p. 612): "The albuterol was obtained from unblinded multidose bottles. The blinded solution (either normal saline solution or Atrovent) was provided in identical, previously coded unit dose vials." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if staff were followed up with patients to assess relapse were blinded. Quote (p. 612): "Patients discharged from the ED were followed up for 24 h to assess the need for repeated ED visits." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded patients balanced between groups. No information provided on number of patients screened. Quote (p. 612): "Three hundred eighty‐four patients were randomised into the trial and 380 completed it. Two patients withdrew consent during the study: one withdrew due to worsening asthma, and one was withdrawn due an administrative problem." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Funding provided by Boehringer Ingelheim. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 611): "Supported by a grant from Boerhinger Ingelheim Pharmaceuticals, Inc." |
Kohistani 2007.
| Methods | ‐ Prospective, comparative study. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Randomisation was accomplished using a random numbers table. ‐ Allocation concealment unclear, treatment designation placed in envelopes, unclear if opaque or sealed. Held by uninvolved staff. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma and had a history or diagnosis of asthma. ‐ Set in Pakistan. ‐ Asthma was defined as being physician diagnosed, having a bronchodilator prescribed by a physician, or having prior episodes of wheezing that improved with beta agonist inhalers. ‐ Ages: 18 to 45 years. ‐ Asthma exacerbation severity of presenting patients estimated as severe. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via continuous nebuliser. ‐ Group one received a single dose ipratropium bromide (0.5 mg) and salbutamol (5.0 mg). Patients then received salbutamol alone at 30 and 60 minutes after the initial treatment with combination therapy. ‐ Group two received salbutamol (5.0 mg) alone. Patients received an additional dose of salbutamol alone at 30 and 60 minutes after the initial treatment with salbutamol alone. ‐ No additional co‐interventions in the ED stated. |
|
| Outcomes | ‐ Primary outcomes measured included pulmonary function. ‐ Secondary outcomes included hospitalisation and vital signs. ‐ Outcome measurements were performed at baseline, as well as 30, 60, and 90 minutes after start of the study protocol. |
|
| Notes | ‐ Unable to contact primary author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. Quote (p. 587): "Randomization was performed on the basis of a random assignment list generated using the random table." |
| Allocation concealment (selection bias) | Unclear risk | Treatment designation placed in envelopes and held by uninvolved ED staff, however unclear if envelopes were opaque or sealed. Quote (p. 587): "Each treatment designation was placed in a closed envelop the uninvolved E.D. staff used to administer treatment according to the treatment designation to which the patient would to do and the staff would not communicate the details of the treatment to the study physician who happened to be the resident physician on duty in the E.D." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study medications provided by uninvolved ED staff who did not inform staff the identity of the medications provided. No details provided on how patients were blinded. Quote (p. 587): "Each treatment designation was placed in a closed envelop the uninvolved E.D. staff used to administer treatment according to the treatment designation to which the patient would to do and the staff would not communicate the details of the treatment to the study physician who happened to be the resident physician on duty in the E.D." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided on patient attrition during screening process. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Lin 1998.
| Methods | ‐ Prospective, double‐blind, placebo‐controlled trial. ‐ Comparison of ipratropium bromide and albuterol vs. albuterol alone. ‐ Randomisation was accomplished via random assignment list generated by computer. ‐ Allocation concealment was reported, sealed envelopes stored in a locked cabinet were used. |
|
| Participants | ‐ Patients presenting to the ED with an exacerbation of asthma and had prior episodes of wheezing that improved with beta‐agonist inhalers. ‐ Set in the United States. ‐ Asthma exacerbation defined as PEF < 200 L/min. ‐ Patients had to be capable of performing PEF. ‐ Ages: 18 years or older. ‐ Asthma exacerbation severity in presenting patients estimated as severe. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via acorn nebuliser. ‐ Group one received a single dose of ipratropium bromide (3.5 mL) and albuterol (2.5 mg), followed by albuterol (2.5 mg) alone every 20 minutes for a total of two doses. ‐ Group two received a dose of albuterol (2.5 mg/3 doses) alone every 20 minutes for a total of three doses. ‐ Additional co‐interventions provided in the ED included supplemental oxygen and oral methylprednisolone if treatment given was believed to be inadequate. |
|
| Outcomes | ‐ Primary outcome assessed changes in pulmonary function. ‐ Additional outcomes included hospitalisation, vital signs, and adverse events. ‐ Outcome measurements were performed at baseline, as well as 20, 40 and 60 minutes after the start of treatment. |
|
| Notes | ‐ Contacted authors to clarify missing data but was informed they no longer had access to the original data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. Quote (p. 209): "Randomization was performed on the basis of a random assignment list generated by computer." |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes stored in a locked cabinet. Quote (p. 209): "Each treatment designation was placed in sealed, opaque envelopes stored in a locked cabinet." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded study. Study medications prepared by uninvolved ED staff without any communication of its contents to the ED staff. No details provided on how patients were blinded. Quote (p. 209): "The initial nebulized mixture was prepared and placed into a nebulizer by an uninvolved ED staff member without any communication of its contents to the study physician. A double‐blind study design was thus employed on a convenience sample of patients selected as described above." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions between groups were balanced. No details provided on patients attrition during the screening process. Quote (p. 210): "Among 60 patients recruited for the study, 4 did not receive the protocol, and a fifth patient was inadvertently studied twice, leaving 55 patients available for analysis." |
| Selective reporting (reporting bias) | High risk | No protocol available. The frequency of side effects were incompletely reported. Quote (p. 211): "The proportion of patients with tremor, agitation, and accessory muscle use did not significantly differ between the two groups at any time points (data not shown)." |
| Other bias | Unclear risk | No source of funding provided. |
Nakano 2000.
| Methods | ‐ Prospective, randomised, single‐blinded trial. ‐ Comparison of oxitropium bromide and salbutamol vs. salbutamol alone. ‐ Randomisation was accomplished but no details were given. ‐ Allocation concealment was reported as using sealed envelopes but no mention if they were opaque or sequentially numbered. |
|
| Participants | ‐ Patients who present to the ED with an exacerbation of asthma who met the criteria for asthma from ATS guidelines. ‐ Set in Japan. ‐ Had PEF ≤ 50% normal predicted value. ‐ Ages: 18 to 55 years. ‐ Asthma exacerbation severity of presenting patients was severe. Estimates of asthma severity based on control hospitalisation rates were estimated as moderate. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via MDI with a spacer device. ‐ Group one received a combination of oxitropium bromide (100 μg/puff) and salbutamol (100 μg/puff) at 4 puffs each at 0, 20, and 40 minutes. ‐ Group two received salbutamol alone (100 μg/puff) with placebo propellant gas at 4 puffs each at 0, 20, and 40 minutes. ‐ Additional co‐interventions provided in the ED included a single dose of IV betamethasone (8 mg) given to all patients in addition to supplemental oxygen. After 120 minutes, patients who showed no improvements received additional inhaled bronchodilators and IV aminophylline. |
|
| Outcomes | ‐ Primary outcome was pulmonary function. ‐ Additional outcomes included hospitalisation, adverse events, the need for additional ED treatment, and frequency of intubation. ‐ Outcome measurements were performed at baseline, as well as 20, 40, 60, and 120 minutes after treatment. |
|
| Notes | ‐ Unable to contact authors to clarify missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no information provided. Quote (p. 473): "Patients who agreed to participate in the study were randomly assigned to one of two treatments by means of sealed envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used but no mention if they were opaque or sequentially numbered. Quote (p. 473): "Patients who agreed to participate in the study were randomly assigned to one of two treatments by means of sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded study. Quote (p. 472): "Methods: A randomised, single‐blind, placebo‐controlled study was performed in 74 patients between 18 and 55 years old presenting to the emergency department (ED) for treatment of acute asthma with a peak expiratory flow (PEF) of 50% or less than the normal predicted value." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions between groups were balanced. No details provided on patients attrition during the screening process. Quote (p. 473): "Of the 80 patients who were enrolled and randomised, 6 patients (2 receiving combination therapy and 4 receiving salbutamol alone) requested early withdrawal and were excluded. The remaining 74 patients were analyzed." |
| Selective reporting (reporting bias) | High risk | No protocol available. The frequency of side effects were incompletely reported. Quote (p. 475): "There was no statistically significant difference in incidence of tremor, palpitations, cough, dry mouth, or bad taste between the groups." |
| Other bias | Low risk | Funding provided by grant from the Hammamatsu Rosai Hospital. Quote (p. 472): "Supported by a department grant of Hamamatsu Rosai Hospital, Hamamatsu, Japan." |
O'Driscoll 1989.
| Methods | ‐ Prospective, double‐blind trial. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Patients randomised into groups via year of birth (odd vs. even). |
|
| Participants | ‐ Patients who presented to the ED with an acute airflow obstruction. ‐ Set in the United Kingdom. ‐ Patients were classified has having either asthma or COPD according to the criteria of the ATS guidelines. ‐ Ages: 17 years and older. ‐ Asthma exacerbation severity of presenting patients was unclear. Not enough information provided to estimate asthma severity based on hospitalisations. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser with oxygen. ‐ Group one received a single dose of ipratropium bromide (0.5 mg) and salbutamol (10 mg). ‐ Group two received a single dose of salbutamol alone (10 mg) with additional 2 ml saline solution. ‐ Additional co‐interventions included supplemental oxygen. Intravaneous hydrocortisone and IV aminophylline was provided only if physicians determine further treatment was necessary. |
|
| Outcomes | ‐ Outcomes included pulmonary function, admission to the ICU, need for mechanical ventilation, and adverse events. ‐ Outcome measurements were performed at baseline and one hour after treatment. |
|
| Notes | ‐ Contacted authors to clarify results but no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation generated by odd/even days of birth. Quote (p. 1418): "The solutions were coded and treatment was determined by the patient’s year of birth (odd or even numbers)." |
| Allocation concealment (selection bias) | High risk | No allocation concealment, patients grouped based on date of birth. Quote (p. 1418): "The solutions were coded and treatment was determined by the patient’s year of birth (odd or even numbers)." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported staff were blinded to treatments but no details provided on whether participants were blinded. Quote (p. 1418): "The staff were blind to the treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded patients were equally balanced between groups. No information provided on attrition during the screening process prior to enrolment. Quote (p. 1419): "125 consecutive patients were entered in the study. 2 patients wished to go home within 60 min of starting nebulised treatment and a further 20 patients were transferred to a hospital ward within this period, either because accident and emergency beds were needed for other patients or because the patient was assigned to another hospital. No patient needed urgent admission to the intensive care unit or mechanical ventilation. The 22 patients who did not complete the trial were equally divided between the treatment groups." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Owens 1991.
| Methods | ‐ Prospective, randomised, double‐blind study. ‐ Comparison of atropine sulphate and metaproterenol vs. metaproterenol alone. ‐ Methods of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma and had a history of asthma as defined by the ATS guidelines. ‐ Set in the United States. ‐ Asthma exacerbation was defined as having an FEV < 2 L prior to beginning the study. ‐ Ages: 18 to 65 years. ‐ Asthma exacerbation severity of presenting patients estimated as moderate. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received a single dose of atropine sulphate (2.5 mg) and metaproterenol (0.3 mL, 5% solution). ‐ Group two received a single dose of metaproterenol alone (0.3 mL, 5% solution). ‐ No additional ED co‐interventions stated. |
|
| Outcomes | ‐ Outcomes included pulmonary function, hospitalisation, adverse events, and additional treatment in the ED. ‐ Outcome measurements were performed at baseline, as well as 30, 60, and 120 minutes after treatment. |
|
| Notes | ‐ Unable to contact authors to clarify original data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no information provided. Quote (p. 1084): "Patients who meet the admission criteria were randomised in a double‐blinded fashion to receive either one dose of nebulized metaproterenol (5 percent solution, 0.3 ml) alone or combined with atropine sulfate (2.5 mg) in 3 ml normal saline solution." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Drug medications prepared in packages in advance and coded. Selected at random by treating physician and neither physician or the patient knew which medications were administered. Unclear whether medication packaging were identical. Quote (p. 1084): "To ensure double‐blind treatment packages were prepared in advance and coded. These were then selected randomly by the treating physician, but neither this physician nor the patient knew which medications were administered." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patient attrition unbalanced between groups. The only three patients excluded from the intervention study for being too sick. No information on patient attrition during the screening process. Quote (p. 1085): "Forty patients satisfied all entry criteria and were randomised to one of the two treatment groups. Three of these patients were withdrawn from the study during the 2‐hour observation period because they were too ill and needed additional therapy." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Rahman 2006.
| Methods | ‐ Prospective, single blind, randomised study. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ No information provided on allocation concealment. |
|
| Participants | ‐ Adult patients with acute asthma presenting to the ED. ‐ Set in Bangladesh. ‐ Ages: Adults (exact ages of participants not provided). ‐ Asthma exacerbation of presenting patients unclear. Insufficient information provided, unable to estimate severity of asthma based on hospitalisation. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via MDI. ‐ Group one received four puffs of ipratropium bromide (20 μg/puff) and salbutamol (100 μg/puff) over 10 minutes. ‐ Group two received four puffs of salbutamol (100 μg/puff) alone over 10 minutes. ‐ No additional ED co‐interventions stated. |
|
| Outcomes | ‐ Outcomes included pulmonary function and vital signs. ‐ Outcome measurements were performed at baseline, as well as 30, 60, and 90 minutes after treatment. |
|
| Notes | ‐ The study author was contacted to obtain missing data. No response was received. ‐ No full‐text, only an abstract available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodology of randomisation not stated. Quote (p. ): "Single‐blind, randomised, prospective study..." |
| Allocation concealment (selection bias) | Unclear risk | No details provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded study. Quote (p. ): "Single‐blind, randomised, prospective study..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many patients were screened or how many patients refused or were excluded. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Rashid 2010.
| Methods | ‐ Prospective, randomised, single‐blind controlled study. ‐ A comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Randomisation was accomplished using a random number table. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma and had an FEV of 30% to 50% predicted. ‐ Set in Bangledesh. ‐ Ages: 18 years or older. ‐ Asthma exacerbation severity of presenting patients was unclear. Not enough information provided. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via volumetric spacer. ‐ Group one received four puffs of ipratropium bromide (20 μg/puff) and salbutamol (100 μg/puff) over 1.5 hours. ‐ Group two received four puffs of salbutamol (100 μg/puff) over 1.5 hours. ‐ No additional ED co‐interventions stated. |
|
| Outcomes | ‐ Outcomes included pulmonary function. ‐ Presence of side effects reported, but no details given. ‐ Outcome measurements were performed at baseline, as well as 30, 60, and 90 minutes after treatment. |
|
| Notes | ‐ The study author was contacted to retrieve missing data. No response was received. ‐ No full‐text, only an abstract available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. Quote (p. 56): "... and were divided into two groups randomly using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded study. Quote (p. 56): "This single‐blinded, randomised, controlled study..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on the number of patients screened, refused, or excluded. |
| Selective reporting (reporting bias) | High risk | No protocol available. The frequency of side effects were incompletely reported. Quote (p. 56): "Side effect profiles were minimal in both groups" |
| Other bias | Unclear risk | No source of funding provided. |
Rebuck 1987.
| Methods | ‐ Prospective, randomised, double‐blind study. ‐ Comparison of ipratropium bromide and fenoterol vs. fenoterol alone. ‐ Randomisation was accomplished using centre specific computer‐generated randomised schedule. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma or COPD, with an FEV ≤ 70% of the predicted value. ‐ Set in Canada. ‐ Participants who were able to perform a forced expiratory manoeuvre. ‐ Ages: 18 years or older. ‐ Asthma exacerbation severity of presenting patients was unclear. Not enough information provided. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser mask. ‐ Group one received a single dose of ipratropium bromide (0.5 mg) and fenoterol (1.25 mg). ‐ Group two received a single dose of fenoterol alone (1.25 mg). ‐ Additional co‐interventions provided in the ED included IV aminophylline or IV hydrocortisone at the discretion of the attending physician. All patients received supplemental oxygen. |
|
| Outcomes | ‐ Outcomes included pulmonary function and adverse events. ‐ Outcome measurements were performed at baseline, as well as 45 and 90 minutes after treatment. |
|
| Notes | ‐ Study authors contacted to clarify data, but they no longer had access to the original database. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centre specified computer‐generated randomised schedule. Quote (p. 60): "Center specific computer‐generated randomised schedule..." |
| Allocation concealment (selection bias) | Low risk | Study medications identical in appearance and coded. Quote (p. 60): "Unit‐dose vials containing these drugs were coded but identical in appearance." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study. Study medications identical in appearance and coded. Quote (p. 60): "Each studied 50 patients in double‐blind, randomised fashion." "Unit‐dose vials containing these drugs were coded but identical in appearance." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded. Outcome assessors blinded. Data accumulated centrally for analysis. Uncoded results were not revealed to investigates until all studies were completed. Quote (p. 60): "An identical protocol was adhered to by all investigators, and data were accumulated centrally for subsequent analysis. Although uncoded results were not revealed to Investigators until all studies were completed, an interim independent review of results was performed to ensure that no regimen was hazardous." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on the number of patients screened, refused, or excluded. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Funded by Boehringer Ingelheim. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 59): "This work was supported by a research grant from Boehringer Ingelheim (Canada) Ltd." |
Rodrigo 1995.
| Methods | ‐ Prospective, randomised, double‐blind study. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients presenting to the ED with an exacerbation of asthma with an FEV and PEF ≤ 50% of predicted value. ‐ Set in Uruguay. ‐ Ages: 18 to 50 years. ‐ Asthma exacerbation severity of presenting patients was unclear. Insufficient information presented. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via MDI spacer. ‐ Group one received four puffs of ipratropium bromide (20 μg/puff) and salbutamol (100 μg/puff) every 10 minutes for 3 hours. ‐ Group two received four puffs of salbutamol (100 μg/puff) along with placebo (propellant) every 10 minutes for 3 hours. ‐ Additional co‐interventions provided in the ED included IV hydrocortisone (500 mg) after upon completion of initial treatment for all patients. |
|
| Outcomes | ‐ Outcomes included pulmonary function and adverse events. ‐ Outcome measurements were performed at baseline, as well as 30, 60, 90, 120, 150, and 180 minutes after the start of treatment. |
|
| Notes | ‐ Contacted authors for additional information but no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no information provided. Quote (p. 177): "Los sujetos fueron asignados aleatoriamente a uno de dos grupos de tratamiento." (T ranslation: "Subjects were randomly assigned to one of two treatment groups"). |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blinded but no information was provided. Quote (p. 177): "Se utilizaron procedimiento de tipo doble ciego." (Translation: "We used double‐blind procedures.") |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many patients were screened, refused or excluded. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Rodrigo 2000.
| Methods | ‐ Prospective, randomised, double‐blind trial. ‐ Comparison of ipratropium bromide and albuterol vs. albuterol alone. ‐ Randomisation was accomplished using a random number table. ‐ Allocation concealment reported and discussed, hospital pharmacy prepared the drugs and sealed drugs in opaque envelope. |
|
| Participants | ‐ Patients who presented to the ED with an exacerbation of asthma, who met the diagnosis criteria of asthma. ‐ Set in Uruguay. ‐ Exacerbation of asthma was defined as having an FEV < 50% predicted value. ‐ Ages: 18 to 50 years. ‐ Asthma exacerbation severity of presenting patients estimated as severe. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via MDI. ‐ Group one received four puffs of ipratropium bromide (21 μg/puff) and albuterol (120 μg/puff) at 10 minutes intervals over 3 hours. ‐ Group two received four puffs of albuterol (120 μg/puff) alone at 10 minutes intervals over 3 hours. ‐ Additional co‐interventions provided in the ED included supplemental oxygen if the patients oxygen saturation decreased to < 92%, however the study reveals this did not occur. |
|
| Outcomes | ‐ Primary outcomes included pulmonary function and hospitalisation. ‐ Additional outcomes included frequency of adverse events. ‐ Outcome measurements were performed at baseline, as well as 30, 60, 90, 120, 150, and 180 minutes after the start of treatment. |
|
| Notes | ‐ Contacted authors for additional information but no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. Quote (p. 1863): "The hospital pharmacy prepared the IB and control treatments in random sequence, using a random number table,..." |
| Allocation concealment (selection bias) | Low risk | Central allocation. Hospital pharmacy prepared the study medications. Stored in opaque envelopes. Quote (p. 1863): "The hospital pharmacy prepared the IB and control treatments in random sequence, using a random number table, in identical canisters, which were then numbered consecutively. For each study patient, the treatment nurse selected the next numbered canister from an opaque envelope,..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. Study medications kept in identical, consecutively numbered canisters. Study nurse selected the canisters from an opaque envelope. Quote (p. 1863): "The hospital pharmacy prepared the IB and control treatments in random sequence, using a random number table, in identical canisters, which were then numbered consecutively. For each study patient, the treatment nurse selected the next numbered canister from an opaque envelope,..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded. All outcome measures made by investigators unaware of the patients group assignment. Quote (p. 1863): "... and all measures were made by investigators unaware of the patients’ group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on patient attrition provided and balanced between groups. Quote (p. 1863): "One hundred ninety‐five patients were assessed in the ED. Of these, 15 (eight in the control group and seven in the IB group) did not fit the inclusion criteria for the study because they did not meet the age requirement (seven patients), or the FEV₁ requirement (five patients), or had cardiac disease (three patients). Of the remaining 180 patients, mean age ± SD, 34.4 ± 10.5 years), 88 were randomly assigned to the IB group and 92 to the control group. Analyses were by intention‐to‐treat, although no withdrawals occurred." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Salo 2006.
| Methods | ‐ Prospective, randomised, double‐blind, controlled clinical trial. ‐ Comparison of ipratropium bromide and albuterol vs. albuterol alone. ‐ Randomisation was using a computerised random numbers table. ‐ Allocation concealment was reported and discussed as using identical drug containers, with medication being kept in locked room. |
|
| Participants | ‐ Patients presenting to the ED with an exacerbation of asthma and had a history of prior episodes of asthma. ‐ Set in the United States. ‐ Exacerbation of asthma defined as having a PEF < 70% of the predicted value. ‐ Age: 18 years and older. ‐ Asthma exacerbation severity of presenting patients estimated as moderate. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received ipratropium bromide (2 mg) and albuterol (15 mg) taken continuously over a 2 hour period. ‐ Group two received albuterol (15 mg) alone taken continuously over a 2 hour period. ‐ Additional co‐interventions provided in the ED included 1 mg/kg of oral prednisone (maximum 60 mg) at time of enrolment. At discharge from the ED, patients received a prescription for oral corticosteroids for 5 days (1 mg/kg up to 60 mg per day). |
|
| Outcomes | ‐ Outcomes included pulmonary function and hospitalisation. Additional data on adverse events was retrieved from the study authors. ‐ Outcome measurements were performed at baseline, as well as 60 and 120 minutes after the start of treatment. |
|
| Notes | ‐ Contacted authors for additional information on the study. Study authors provided additional clarification on results for adverse events and pulmonary function. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers tables. Quote (p. 372): "Randomization into study groups was done using a computerized random numbers table." |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered medications which were identical in appearance. An ED nurse not involved in the direct care of administration of the study medications to the patients, prepared the medications in a separate locked medication room. Medications were prepared in separate locked medication room. Quotes (p. 372): "Patients who verbally consented during this brief assessment phase were then asked to review and provide full informed written consent while a previously inserviced ED nurse, not involved in direct care or administration of study medication to the patient (usually the charge nurse), prepared the study medication in a separate locked medication room. Before study startup, all study medications, a B&B Hope Nebulizer (B&B Medical Technologies Inc., Orangevale, CA), sterile saline, and instructions on how to mix medications were placed into sealed, sequentially marked bags, which were kept secured in the locked medication room." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study. ED staff not involved with the patient or administration of the study medications prepared the study medications for the research staff and instructed to not inform anyone involved in the study the contents of the mixtures. Medications were sequentially numbered and identical in appearance. Quote (pp. 372‐3): "The nurse preparing the medication selected the next numerically marked bag, recorded the patient’s name, medical record and bag number on a data enrolment form and was instructed not to divulge to anyone involved in the study the contents of the Hope Nebulizer. Both study mixtures were clear, colorless solutions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors in the ED were not informed of the study medications by the ED nurse preparing the study medications. Quote (pp. 372‐3): "The nurse preparing the medication selected the next numerically marked bag, recorded the patient’s name, medical record and bag number on a data enrolment form and was instructed not to divulge to anyone involved in the study the contents of the Hope Nebulizer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on patient attrition provided and balanced between groups with a flow diagram provided. See p. 373. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding stated. |
Solarte 2004.
| Methods | ‐ Prospective RCT. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Method of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Adults presenting to the ED with an exacerbation of asthma. ‐ Set in Columbia. ‐ Ages: 18 to 65 years. ‐ Asthma exacerbation severity of presenting patients was estimated as moderate. |
|
| Interventions | ‐ Multiple doses of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received one dose of ipratropium bromide (500 mg) and salbutamol (2.5 mg) every 20 minutes for one hour, for a total of three doses. ‐ Group two received one dose of salbutamol (2.5 mg) alone every 20 minutes for one hour, for a total of three doses. ‐ No co‐interventions stated. |
|
| Outcomes | ‐ The primary outcome was change in FEV₁. ‐ Secondary outcomes included peak flow, clinical signs and symptoms, adverse events, and hospitalisation. ‐ Outcome measurements were performed at baseline and 120 minutes after the start of treatment. |
|
| Notes | ‐ Contacted authors for missing data but no response received. ‐ No full‐text, only an abstract available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no information provided. Quote (p. 1): "Consecutive adult patients (18‐65) consulting to emergency room, with clinical and functional AAE were randomly assigned to receive..." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on patient attrition provided. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Funded by AstraZeneca. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 1): "Supported by an educational grant from AstraZeneca." |
Summers 1990.
| Methods | ‐ Prospective, randomised, double‐blind study. ‐ Comparison of ipratropium bromide and salbutamol vs. salbutamol alone. ‐ Methods of randomisation unclear. ‐ No information on allocation concealment provided. |
|
| Participants | ‐ Patients presenting to the ED with an exacerbation of acute asthma. ‐ Set in Australia. ‐ Patients must be able to perform PEF. ‐ Ages: 16 to 70 years. ‐ Asthma exacerbation severity of presenting patients was unclear. Insufficient information provided to estimate asthma severity based on hospitalisations. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser. ‐ Group one received a single dose of salbutamol (5 mg) alone, followed by a single dose of Ipratropium bromide (0.5 mg) one hour later. ‐ Group two received a single dose of ipratropium bromide (0.5 mg) alone, followed by a single dose of salbutamol (5 mg) one hour later. ‐ Group three received a single dose of ipratropium bromide (0.5 mg) and salbutamol (5 mg), followed by placebo one hour later. ‐ Additional co‐interventions provided in the ED included IV hydrocortisone and IV aminophylline if deemed necessary by the attending physician. |
|
| Outcomes | ‐ Outcomes included pulmonary function. ‐ Outcome measurements were performed at baseline, as well as 15 minutes, 60, 75, and 120 minutes after treatment. Only pulmonary function data measured 15 minutes after treatment exposure in groups one and three were extracted. Pulmonary data measured at 60, 75 and 120 minutes after treatment was not extracted because group one received Ipratropium bromide one hour after receiving salbutamol. |
|
| Notes | ‐ Unable to contact authors for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no information provided. Quote (p. 426): "The study was double‐blind and randomised, and there were three treatment groups as follow:..." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blinded but no information provided. Quote (p. 426): "The study was double‐blind..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many patients were screened, refused or excluded. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Funding provided by GlaxoSmithKline. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 429): "... and Glaxo Australia for financial support." |
Weber 1999.
| Methods | ‐ Prospective, randomised, double‐blind, placebo‐controlled study. ‐ Comparison of ipratropium bromide and albuterol vs. albuterol alone. ‐ Randomisation was accomplished using a random numbers table. ‐ Allocation concealment was reported and discussed as pharmacy controlled. |
|
| Participants | ‐ Patients presenting to the ED with an exacerbation of asthma, who had a PEF < 70% of the predicted value. ‐ Set in the United States. ‐ Ages: 18 years or older. ‐ Asthma exacerbation severity of presenting patients estimated as severe. |
|
| Interventions | ‐ Single dose of combination inhaled therapy. Study interventions provided via nebuliser. ‐ First group received ipratropium bromide (1.0 mg/hour) and albuterol (10 mg/hour) taken continuously over a three hour period. ‐ Second group received albuterol (10 mg/hour) alone taken continuously over a three hour period. ‐ Additional co‐interventions provided in the ED included oral prednisone and albuterol (2.5 mg) provided to all patients upon presentation to the ED. Supplemental oxygen was given if patients S0₂ was < 90%. |
|
| Outcomes | ‐ Primary outcomes included pulmonary function, hospitalisation and ED length of stay. ‐ Seconary outcomes included vital signs, symptom scores, and adverse events. ‐ Outcome measurements were performed at baseline, as well as one, two, and three hours after the start of treatment. |
|
| Notes | ‐ Contacted primary author who stated that they no longer had access to the original data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers tables. Quote (p. 938): "The combination and control treatments were prepared by the hospital pharmacy in random sequence using a random number table..." |
| Allocation concealment (selection bias) | Unclear risk | Central allocation, pharmacy‐controlled. Quote (p. 938): "The combination and control treatments were prepared by the hospital pharmacy..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. Study medications placed in consecutively numbered identical brown‐tinted bottles. Treating physicians, respiratory therapist, patients and investigators were blind to treatment. Quotes (pp. 938‐9): "The combination and control treatments were prepared by the hospital pharmacy in random sequence using a random number table and were placed in identical 4‐oz brown‐tinted bottles, which were then numbered consecutively." "The RT, treating physician, and patient were blinded to treatment, and the code for drug assignment was not known to the investigators until data for all patients had been entered into the study database." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians, and respiratory therapists were blinded to treatment. Code for drug assignment were unknown to the study investigators until all of the patients data have been entered into the study database. Quote (p. 939): "The RT, treating physician, and patient were blinded to treatment, and the code for drug assignment was not known to the investigators until data for all patients had been entered into the study database." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on why most screened patients were not enrolled into the study. Quote (p. 939): "There were 465 patients who presented to the ED with acute bronchospasm during the study period but were not enrolled in the study." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Ipratropium bromide and pharmacy costs provided by Boehringer Ingelheim. No statement provided on influence of funding on preparation of the manuscript. Quote (p. 937): "Ipratropium bromide and pharmacy costs were provided by Boehringer Ingelheim. |
Abbreviations: ATS ‐ American Thoracic Society COPD ‐ chronic obstructive pulmonary disease ED ‐ emergency department FEV ‐ forced expiratory volume ICU ‐ intensive care unit IV ‐ intravenous MDI ‐ metered‐dose inhaler PEF ‐ peak expiratory flow RCT ‐ randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1994 | Not a prospective RCT or CCT |
| Barrett 2014 | Not a prospective RCT or CCT |
| Beck 1985 | Included children |
| Bonsignore 1986 | Not treated for acute asthma, not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Bourcereau 1988 | Not treated for acute asthma, no comparison of SAAC + SABA vs. SABA alone |
| Brenner 1988 | Included children, not treated for acute asthma, not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Britton 1988 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Bryant 1985 | Not recruited in ED or acute care settings |
| Bryant 1990 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Chen 1989 | Not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Chhabra 2002 | Not treated for acute asthma, not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Cydulka 1994 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Garcia 2012 | Not a prospective RCT or CCT |
| Gaur 2008 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Gilman 1990 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Higgins 1988 | Not recruited in ED or acute care settings |
| Hunt 1983 | Not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs SABA alone |
| Janson 1988 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Kaik 1980 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Karpel 1986 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Kerstjens 2011 | Not treated for acute asthma, not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs SABA alone |
| Koumbourlis 2015 | Not a prospective RCT or CCT |
| Lanes 1998 | Not a prospective RCT or CCT |
| Leahy 1983 | Not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs SABA alone |
| Lin 1999 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Lin 2004 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Louw 1990 | Not recruited in ED or acute care settings |
| Maesen 1997 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Mazzei 1986 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Nana 1995 | Unable to confirm with study authors if patients were recruited in ED or acute care settings, or the age range of included participants |
| Patrick 1990 | Not treated for acute asthma, not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Rodrigo 1999 | Not a prospective RCT or CCT |
| Roeseler 1987 | Not treated for acute asthma |
| Salome 1988 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Schlueter 1978 | Not treated for acute asthma, not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Schneider 2012 | No comparison of inhaled SAAC + SABA vs. SABA alone |
| Stoodly 1999 | Not a prospective RCT or CCT |
| Tamura 2014 | Not a prospective RCT or CCT, no comparison of inhaled SAAC + SABA vs SABA alone |
| Toda 1992 | Unable to confirm study design, if participants were recruited in ED or acute care settings, or the age range of included participants |
| Vogt 1974 | Not treated for acute asthma, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Ward 1981 | Not recruited in ED or acute care settings, no comparison of inhaled SAAC + SABA vs. SABA alone |
| Youngchaiyud 1989 | Not treated for acute asthma, not recruited in ED or acute care settings |
| Zaritsky 1999 | Not a prospective RCT or CCT |
Abbreviations:
CCT ‐ clinical controlled trial ED ‐ emergency department RCT ‐ randomised controlled trial SAAC ‐ short‐acting anticholinergics SABA ‐ short‐acting beta₂‐agonists
Differences between protocol and review
There are several differences between the initial protocol and the final review.
We made changes to the inclusion and exclusion criteria. LAAC agents, such as tiotropium were explicitly excluded from the review as a result of a decision to focus primarily on SAAC agents before searching the electronic databases.
Studies including participants aged 16 years or older were eligible for inclusion in the review, rather than 18 years or older as stated in the protocol. This change was made to allow for any variations in defining adult populations globally. The included studies enrolled primarily
participants over the age of 18 years. No studies reported mean ages between 16 years and 17 years.
In addition to the search provided by the Cochrane Airways Group, the review presents results of searches of seven electronic databases using keywords and subject headings provided by a health librarian.
In the protocol, the grey literature search consisted solely of handsearching the top 20 respiratory care journals; however, for the review, this was expanded to include: a forward search on SCOPUS of the sentinel paper, Google Scholar, clinical trial registries, reference lists of reviews and included studies, and handsearching the top three evidence‐based emergency medicine journals.
We amended secondary outcomes measures proposed in the protocol, and excluded physiological measures, such as vital signs and SaO₂.
Because there were few adverse events reported, we calculated OR analyses.
Risk of bias assessment was completed using the Cochrane Risk of Bias tool, as recommended by Cochrane, rather than the Jadad.
Changes regarding data analysis included calculating random‐effects risk ratios for dichotomous variables for individual studies instead of odds ratios as mentioned in the protocol. Due to the rare occurrence of adverse events, OR analysis were calculated.
Heterogeneity was assessed using the more widely accepted I² statistic with I² values of 25, 50, and 75% representing low, moderate, and high degrees of heterogeneity respectively.
The reported subgroups based on single‐dose vs. multiple doses for all of the reported comparisons were not assessed in the final review; however, they were reported for the primary outcome. In addition, sensitivity analysis based on the Jadad score, Cochrane criteria, dosing agents and time of assessment was not assessed in the review.
The final review included a summary of findings table of the primary outcome and important secondary outcomes, including an assessment of the quality of evidence using GRADE, which was not included in the initial protocol.
The text of the final review varied considerably from the initial protocol due to a change in the authors involved in the study and its preparation of the final manuscript
The use of ipratropium bromide vs. other SAAC was added in the final review as a subgroup comparison.
Based on feedback provided by post peer review comments, the methods of estimating and categorising exacerbation severity was modified to include the pulmonary function eligibility criteria, in addition to the percentage of patients hospitalised in the SABA alone group.
Contributions of authors
SWK: assisted with protocol development, performed the literature search, selection of studies, quality assessment, data extraction, data analysis and manuscript preparation.
CV: assisted with the selection of studies, quality assessment, and manuscript preparation.
TN: assisted with study selection and manuscript preparation.
BV: assisted with study selection, quality assessment, and manuscript preparation.
SC: developed the search terms and performed the literature search of the systematic literature search and assisted with manuscript preparation.
BHR: initiated the review, assisted with protocol development, selection of studies, risk of bias assessment, data analysis, and manuscript preparation.
Sources of support
Internal sources
-
Department of Emergency Medicine, University of Alberta, AB, Canada, Canada.
Provided in‐kind resources
Emergency Strategic Clinical Network (SWK), AB, Canada.
External sources
-
Canadian Institutes of Health Research (CIHR), Ottawa, ON (BH Rowe), Canada, Canada.
This review was funded by a grant received from the CIHR. The funders were not involved in the conduct of this review, or the contents and preparation of the manuscript.
Declarations of interest
SWK: none known
CV: none known
BV: none known
TN: none known
SC: none known
BHR: Since 2013 Dr Rowe has received funding from GSK for speaking, study enrolment fees from MedImmune and funding for research from GSK.
New
References
References to studies included in this review
Aggarwal 2002 {published data only (unpublished sought but not used)}
- Aggarwal P, Singh O, Wali JP, Handa R, Dwivedi SN, Biswas A, et al. Efficacy of nebulised ipratropium in acute bronchial asthma. Journal, Indian Academy of Clinical Medicine 2002;3(4):353‐9. [Google Scholar]
Canete 1991 {published data only (unpublished sought but not used)}
- Canete C, Esteban LL, Sanchon BR, Manresa F. Treatment with anticholinergics in the asthmatic crisis [Tratamiento con anticholinérgicos en la crisis asmática]. Archivos De Bronconeumologia 1991;27(Suppl 1):44. [Google Scholar]
Cydulka 2010 {published and unpublished data}
- Cydulka RK, Emerman CL, Muni A. Levelbuterol versus lebalbuterol plus ipratropium in the treatment of severe acute asthma. Journal of Asthma 2010;47(10):1094‐100. [DOI] [PubMed] [Google Scholar]
Diaz 1997 {published and unpublished data}
- Diaz JE, Dubin R, Gaeta TJ, Pelczar P, Bradley K. Efficacy of atropine sulfate in combination with albuterol in the treatment for acute asthma. Academic Emergency Medicine 1997;4(2):107‐13. [DOI] [PubMed] [Google Scholar]
FitzGerald 1997 {published and unpublished data}
- FitzGerald JM, Grunfeld A, Pare PD, Levy RD, Newhouse MT, Hodder R, et al. The clinical efficacy of combination nebulized anticholinergic and adrenergic bronchodilators vs nebulized adrenergic bronchodilator alone in acute asthma. Canadian Combivent Study Group. Chest 1997;111(2):311‐5. [DOI] [PubMed] [Google Scholar]
Garrett 1997 {published and unpublished data}
- Garrett JE, Town GI, Rodwell P, Kelly AM. Nebulized salbutamol with and without ipratropium bromide in the treatment of acute asthma. Journal of Allergy and Clinical Immunology 1997;100(2):165‐70. [DOI] [PubMed] [Google Scholar]
Hossain 2013 {published and unpublished data}
- Hossain AS, Barua UK, Roy GC, Sutradhar SR, Rahman I, Rahman G. Comparison of salbutamol and ipratropium bromide versus salbutamol alone in the treatment of acute severe asthma. Mymensingh Medical Journal 2013;22(2):345‐52. [PubMed] [Google Scholar]
Kamei 1999 {published data only (unpublished sought but not used)}
- Kamei T, Fujita J, Okada H, Nakamura H, Kishimoto T, Momoi A, et al. Comparison between fenoterol and fenoterol plus oxitropium bromide delivered by metered‐dose inhaler with inspirease to relieve acute asthma attack. Journal of Asthma 1999;36(1):67‐75. [DOI] [PubMed] [Google Scholar]
Karpel 1996 {published and unpublished data}
- Karpel JP, Schacter EN, Fanta C, Levey D, Spiro P, Aldrich T, et al. A comparison of ipratropium and albuterol vs. albuterol alone for the treatment of acute asthma. Chest 1996;110:611‐6. [DOI] [PubMed] [Google Scholar]
Kohistani 2007 {published data only (unpublished sought but not used)}
- Kohistani TA. Acute severe asthma; salbutamol plus ipratropium bromide nebulization versus salbutomol nebulization alone. Professional Medical Journal 2007;14(4):586‐90. [Google Scholar]
Lin 1998 {published data only (unpublished sought but not used)}
- Lin RY, Pesola GR, Bakalchuk L, Morgan JP, Heyl GT, Freyberg CW, et al. Superiority of ipratropium plus albuterol over albuterol alone in the emergency department management of adult asthma: a randomized clinical trial. Annals of Emergency Medicine 1998;31(2):208‐13. [DOI] [PubMed] [Google Scholar]
Nakano 2000 {published data only (unpublished sought but not used)}
- Nakano Y, Enomoto N, Kawamoto A, Hirai R, Chida K. Efficacy of adding multiple doses of oxitropium bromide to salbutomol delivered by means of a metered‐dose inhaler with a spacer device in adults with acute severe asthma. Journal of Allergy and Clinical Immunology 2000;106(3):472‐8. [DOI] [PubMed] [Google Scholar]
O'Driscoll 1989 {published data only (unpublished sought but not used)}
- O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulised salbutomol with and without ipratropium bromide in acute airflow obstruction. Lancet 1989;333(8652):1418‐20. [DOI] [PubMed] [Google Scholar]
Owens 1991 {published data only (unpublished sought but not used)}
- Owens MW, George RB. Nebulized atropine sulfate in the treatment of acute asthma. Chest 1991;99(5):1084‐7. [DOI] [PubMed] [Google Scholar]
Rahman 2006 {published data only (unpublished sought but not used)}
- Rahman SMS, Hossain AH, Hasan R, Mahmood AM. First‐line therapy for adult patient with acute asthma receiving a multiple‐dose regime of ipratropium bromide plus salbutamol in the emergency department. Indian Journal of Allergy Asthma and Immunology 2006;20(2):129. [Google Scholar]
Rashid 2010 {published data only (unpublished sought but not used)}
- Rashid M, Khair M, Hossain A, Hassan R. A newer treatment regime for adult acute asthmatics with inhaled ipratropium bromide and salbutamol in multiple intermittent doses using a volumetric spacer in emergency setting. Respirology 2010;15(Suppl 2):56. [Google Scholar]
Rebuck 1987 {published data only (unpublished sought but not used)}
- Rebuck AS, Chapman KR, Abboud R, Pare PD, Kreisman H, Wolkove N, et al. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. American Journal of Medicine 1987;82:59‐64. [DOI] [PubMed] [Google Scholar]
Rodrigo 1995 {published data only (unpublished sought but not used)}
- Rodrigo Gorina CP, Rodrigo Gorina GJ. Treatment or asthma with high doses of salbutamol and ipratropium bromide administered through metered dose inhaler and holding chamber [Tratamiento de la crisis asmática con altas dosis de salbutamol y brumuro de ipratropio administrados mediante inhalador de dosis medida e inhalocámara]. Paciente Critico 1995;8(3):175‐84. [Google Scholar]
Rodrigo 2000 {published data only (unpublished sought but not used)}
- Rodrigo GJ, Rodrigo C. First‐line therapy for adult patients with acute asthma receiving a multiple‐dose protocol of ipratropium bromide plus albuterol in the emergency department. American Journal of Respiratory and Critical Care Medicine 2000;161(6):1862‐8. [DOI] [PubMed] [Google Scholar]
Salo 2006 {published and unpublished data}
- Salo D, Tuel M, Lavery RF, Reischel U, Lebowitz J, Moore T. A randomized, clinical trial comparing the efficacy of continuous nebulized albuterol (15 mg) versus continuous nebulized albuterol (15 mg) plus ipratropium bromide (2 mg) for the treatment of acute asthma. Journal of Emergency Medicine 2006;31(4):371‐6. [DOI] [PubMed] [Google Scholar]
Solarte 2004 {published data only (unpublished sought but not used)}
- Solarte I, Sanchez LY, Lasso JI, Londono D. Ipratropium bromide plus salbutamol in the emergency management of acute asthma exacerbation (AAE). American Journal of Respiratory and Critical Care Medicine 2004;169(Suppl 7):A37. [Google Scholar]
Summers 1990 {published data only (unpublished sought but not used)}
- Summers QA, Tarala RA. Nebulized ipratropium in the treatment of acute asthma. Chest 1990;97(2):425‐9. [DOI] [PubMed] [Google Scholar]
Weber 1999 {published data only (unpublished sought but not used)}
- Weber EJ, Levitt MA, Covington JK, Gambrioli E. Effect of continuously nebulized ipratropium bromide plus albuterol on emergency department length of stay and hospital admission rates in patients with acute bronchospasm. A randomized, controlled trial. Chest 1999;115(4):937‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1994 {published data only}
- Anonymous. Terbutaline and ipratropium for nebulization. Prescire International 1994;3(11):71‐4. [Google Scholar]
Barrett 2014 {published data only}
- Barrett MJ. Combined anti‐cholinergic and short‐acting beta‐agonist therapy reduces hospital admissions for acute asthma. Journal of Paediatric Child Health 2014;50(7):577‐8. [DOI] [PubMed] [Google Scholar]
Beck 1985 {published data only}
- Beck R, Robertson C, Galdes‐Sebaldt M, Levison H. Combined salbutomol and ipratropium bromide by inhalation in the treatment of severe acute asthma. Journal of Pediatrics 1985;107(4):605‐8. [DOI] [PubMed] [Google Scholar]
Bonsignore 1986 {published data only}
- Bonsignore G, Bellia V, Peralta G, Alessi N, Migliara G. The combination of fenoterol and ipratropium bromide in bronchial asthma: Comparison of the acute effects of two different dosages. Respiration 1986;50(Suppl 2):148‐51. [DOI] [PubMed] [Google Scholar]
Bourcereau 1988 {published data only}
- Bourcereau J, Prosper M, Dy NR. Nebulized ipratropium bromide in the treatment of acute attacks of asthma and exacerbation of chronic bronchitis. European Respiratory Journal 1988;1:338s. [Google Scholar]
Brenner 1988 {published data only}
- Brenner M, Berkowitz R, Marshall N, Strunk RC. Need for theophylline in severe steroid‐requiring asthmatics. Clinical Allergy 1998;18(2):143‐50. [DOI] [PubMed] [Google Scholar]
Britton 1988 {published data only}
- Britton J, Hanley SP, Garrett HV, Hadfield JW, Tatterfield AE. Dose related effects of salbutamol and ipratropium bromide on airway calibre and reactivity in subjects with asthma. Thorax 1988;43:300‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryant 1985 {published data only}
- Bryant DH. Nebulized ipratropium bromide in the treatment of acute asthma. Chest 1985;88(1):24‐9. [DOI] [PubMed] [Google Scholar]
Bryant 1990 {published data only}
- Bryant DH, Rogers P. Oxitropium bromide: An acute dose response study of a new anticholinergic drug in combination with fenoterol in asthma and chronic bronchitis. Pulmonary Pharmacology 1990;3(2):55‐8. [DOI] [PubMed] [Google Scholar]
Chen 1989 {published data only}
- Chen L, Deng K, Ye YQ. Effects of ipratropium bromide (IPB) and its combination with salbutamol sulfate (SAS) in acute asthmatic attack. Zhonghua Nei Ke Za Zhi [Chinese Journal of Internal Medicine] 1989;28(11):657‐60. [PubMed] [Google Scholar]
Chhabra 2002 {published data only}
- Chhabra SK, Pandey KK. Comparison of acute bronchodilator effects of inhaled ipratropium bromide and salbutamol in bronchial asthma. Journal of Asthma 2002;39(5):375‐81. [DOI] [PubMed] [Google Scholar]
Cydulka 1994 {published data only}
- Cydulka RK, Emerman CL. Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of asthma. Annals of Emergency Medicine 1994;23(2):270‐4. [DOI] [PubMed] [Google Scholar]
Garcia 2012 {published data only}
- Garcia G, Bergna M, Nannini L, Neffen H, Rodrigo G, Mercurio S. Acute asthma in Buenos Aires. European Respiratory Journal 2012;40(56):1010. [Google Scholar]
Gaur 2008 {published data only}
- Gaur SN, Singh A, Kumar R. Evaluating role of inhaled magnesium sulphate as an adjunct to salbutamol and ipratropium in severe acute asthma. Chest 2008;134(4):91003. [Google Scholar]
Gilman 1990 {published data only}
- Gilman MJ, Meyer L, Carter J, Slovis C. Comparison of aerosolized glycopyrrolate and metaproterenol in acute asthma. Chest 1990;98(5):1095‐8. [DOI] [PubMed] [Google Scholar]
Higgins 1988 {published data only}
- Higgins RM, Stradling JR, Lane DJ. Should ipratropium bromide be added to beta‐agonists in treatment of acute severe asthma?. Chest 1988;94(4):718‐22. [DOI] [PubMed] [Google Scholar]
Hunt 1983 {published data only}
- Hunt D, MacDonald GF, Reilly P, Plumley D, Kazim F. Bronchodilator response to several doses of ipratropium bromide in asthmatics and bronchitis. Current Therapeutic Research, Clinical and Experimental 1983;33(4):651‐8. [Google Scholar]
Janson 1988 {published data only}
- Janson C, Herala M, Sjögren I. Nebulization versus injection in ambulatory treatment of acute asthma: A comparative study. British Journal of Diseases of the Chest 1988;82(4):347‐51. [DOI] [PubMed] [Google Scholar]
Kaik 1980 {published data only}
- Kaik VG, Kaik B, Laggner A. Clinical pharmacological trials of a new metered dose inhaler, IK‐6, a combination of the bronchodilators fenoterol and ipratropium bromide. Wiener Medizinische Wochenschrift 1980;130(11):385‐93. [PubMed] [Google Scholar]
Karpel 1986 {published data only}
- Karpel JP, Appel D, Breidbart D, Fusco MJ. A comparison of atropine sulfate and metaproterenol sulfate in the emergency treatment of asthma. American Review of Respiratory Diseases 1986;133(5):727‐9. [DOI] [PubMed] [Google Scholar]
Kerstjens 2011 {published data only}
- Kerstjens HAM, Disse B, Schröder‐Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: A randomized controlled trial. Journal of Allergy and Clinical Immunology 2011;128(2):308‐14. [DOI] [PubMed] [Google Scholar]
Koumbourlis 2015 {published data only}
- Koumbourlis A, Mastropietro C. Continuous inhalation of ipratropium bromide for acute asthma refractory to ß2‐agonist treatment. Journal of Pediatric Pharmacological and Therapeutics 2015;20(1):66‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanes 1998 {published data only}
- Lanes SF, Garrett JE, Wentworth CH, FitzGerald JM, Karpel JP. The effect of adding ipratropium bromide to salbutamol in the treatment of acute asthma: A pooled analysis of three trials. Chest 1998;114(2):365‐72. [DOI] [PubMed] [Google Scholar]
Leahy 1983 {published data only}
- Leahy BC, Gomm SA, Allen SC. Comparison of nebulized salbutamol with nebulized ipratropium bromide in acute asthma. British Journal of Diseases of the Chest 1983;77(2):159‐63. [DOI] [PubMed] [Google Scholar]
Lin 1999 {published data only}
- Lin RY, Pesola GR, Bakalchuk L, Heyl GT, Dow AM, Tenenbaum C, et al. Rapid improvement of peak flow in asthmatic patients treated with parenteral methylprednisolone in the emergency department: A randomized controlled study. Annals of Emergency Medicine 1999;33(5):487‐94. [DOI] [PubMed] [Google Scholar]
Lin 2004 {published data only}
- Lin RY, Pesola GR, Bakalchuk L, Curry A, Nelson M, Lee H, et al. The effect of early parenteral administration of corticosteroids in severe asthma: A study not employing concomitant ipratropium treatment. Internet Journal of Asthma, Allergy and Immunology 2004; Vol. 3, issue 1. [URL: http://ispub.com/IJAAI/3/1/4365]
Louw 1990 {published data only}
- Louw SJ, Goldin JG, Isaacs S. Relative efficacy of nebulized ipratropium bromide and fenoterol in acute severe asthma. South African Medical Journal 1990;77(1):24‐6. [PubMed] [Google Scholar]
Maesen 1997 {published data only}
- Maesen FP, Greefhorst LP, Smeets JJ, Wald FD, Cornelissen PJ. Therapeutic equivalence of a novel HFA134a‐containing metered‐dose inhaler and the conventional CFC inhaler (berodual) for the delivery of a fixed combination of fenoterol/ipratropium bromide. A randomized double‐blind placebo‐controlled crossover study in patients with asthma. Respiration 1997;64(4):273‐80. [DOI] [PubMed] [Google Scholar]
Mazzei 1986 {published data only}
- Mazzei JA, Torres J. Blind randomized cross‐over comparative study of salbutamol and the combination fenoterol‐ipratropium IK6 in patients with bronchial asthma. Respiration 1986;50(Suppl 2):313‐7. [DOI] [PubMed] [Google Scholar]
Nana 1995 {published data only}
- Nana A. Combination of nebulized ipratropium bromide and beta‐agonists in the treatment of acute severe asthma. European Respiratory Journal 1995;8(Suppl 19):455s. [Google Scholar]
Patrick 1990 {published data only}
- Patrick DM, Dales RE, Stark RM, Laliberte G, Dickinson G. Severe exacerbations of COPD and asthma. Incremental benefit of adding ipratropium to usual therapy. Chest 1990;96(2):295‐7. [DOI] [PubMed] [Google Scholar]
Rodrigo 1999 {published data only}
- Rodrigo G, Rodrigo C, Burschtin O. A meta‐analysis of the effects of ipratropium bromide in adults with acute asthma. American Journal of Medicine 1999;107(4):363‐70. [DOI] [PubMed] [Google Scholar]
Roeseler 1987 {published data only}
- Roeseler J, Reynaert MS. A comparison of fenoterol and fenoterol‐ipratropium nebulisation treatment in acute asthma. Acta Therapeutica 1987;13(6):571‐8. [Google Scholar]
Salome 1988 {published data only}
- Salome CM, Wright W, Sedgwick CJ, Woolcock AJ. Acute effects of fenoterol (Berotec) and ipratropium bromide (Atrovent) alone and in combination on bronchial hyper‐responsiveness in asthmatic subjects. Progress in Clinical and Biological Research 1988;263:405‐19. [PubMed] [Google Scholar]
Schlueter 1978 {published data only}
- Schlueter DP, Neumann JL. Double blind comparison of acute bronchial and ventilation‐perfusion changes to atrovent and isoproterenol. Chest 1978;73(6):982‐3. [DOI] [PubMed] [Google Scholar]
Schneider 2012 {published data only}
- Schneider J, Matsuda K, House SL, Ferguson I, Aubuchon K, Lewis L. Dyspnea scores may be a better predictor of hospital admissions than FEV1 for patients with acute asthma exacerbations. Academic Emergency Medicine. 2012; Vol. 19:S312.
Stoodly 1999 {published data only}
- Stoodley RG, Aaron SD, Dales RE. The role of ipratropium bromide in the emergency management of acute asthma exacerbation: A meta analysis of randomized clinical trials. Annals of Emergency Medicine 1999;34(1):8‐18. [DOI] [PubMed] [Google Scholar]
Tamura 2014 {published data only}
- Tamura T, Chung T, Kabaroff A, Liss K, Villa‐Roel C, Rowe BH. Pre‐hospital management of patients with history of asthma and COPD in one Canadian urban setting. Canadian Journal of Emergency Medicine 2014;15(Suppl 1):S75. [Google Scholar]
Toda 1992 {published data only}
- Toda M, Fukuda T, Motojima S, Makino S. Effect of the combination of fenoterol with oxitropium on asthmatic patients with acute air‐way obstructions. European Respiratory Journal. 1992; Vol. 5:209s.
Vogt 1974 {published data only}
- Vogt R, Rychel F. Acute test comparing ipratropium bromide (SCH 1000), fenoterol, and placebo, by measured dose aerosol, in obstructive lung disease [Akuter vergleich von Sch 1000‐, berotec‐ und placebo‐dosieraerosol bei obstruktiven lungenerkrankungen]. Therapiewoche 1974;24(39):4228‐34. [Google Scholar]
Ward 1981 {published data only}
- Ward MJ, Fentem PH, Roderick Smith WH, Davies D. Ipratropium bromide in acute asthma. British Medical Journal 1981;282(6264):598‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Youngchaiyud 1989 {published data only}
- Youngchaiyud P, Charoenratanakul S, Suthamsmai T, Sriwatanakul K. Comparison of fenoterol, ipratropium bromide and their combination in asthma. Siriraj Hospital Gazette 1989;41(4):197‐201. [Google Scholar]
Zaritsky 1999 {published data only}
- Zaritsky A, Qureshi F. Ipratropium does indeed reduce admissions to hospital with severe asthma. British Medical Journal 1999;318(7185):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aaron 2001
- Aaron SD. The use of ipratropium bromide for the management of acute asthma exacerbation in adults and children: A systematic review. Journal of Asthma 2001;38(7):521‐30. [DOI] [PubMed] [Google Scholar]
Adams 2002
- Adams RJ, Fuhlbriggie A, Guilbert T, Lozano P, Martinez F. Inadequate use of asthma medication in the United States: Results of the Asthma in America national population survey. Journal of Allergy and Clinical Immunology 2002;110(1):58‐64. [DOI] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130(1 Suppl):4S‐12S. [DOI] [PubMed] [Google Scholar]
Global Initiative for Asthma 2016
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2016. www.ginaasthma.org.
GRADE Working Group 2004
- Grade Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro [www.gradepro.org]. Version [3.6]. McMaster University, 2014.
Griffiths 2013
- Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short‐acting beta2‐agonists for initial treatment of acute asthma in children. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD000060.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasegawa 2013
- Hasegawa K, Chiba T, Hagiwara Y, Watase H, Tsugawa Y, Brown DFM, et al. Japanese Emergency Medicine Network Investigators. Quality of care for acute asthma in emergency departments in Japan: A multicenter observational study. Journal of Allergy and Clinical Immunology: In Practice 2013;1(5):509‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. The Cochrane Collaboration 2011:Available from www.cochrane‐handbook.org.
Krahn 1996
- Krahn MD, Berka C, Langlois P, Detsky AS. Direct and indirect costs of asthma in Canada. Canadian Medical Association Journal 1996;154(6):821‐31. [PMC free article] [PubMed] [Google Scholar]
Lai 2003
- Lai CKW, Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, et al. Asthma Insights and Reality in Asia‐Pacific Steering Committee. Asthma control in the Asia‐Pacific region: The Asthma Insights and reality in Asia‐Pacific study. Journal of Allergy and Clinical Immunology 2003;111(2):263‐8. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Open Medicine 2009;3:123‐9. [PMC free article] [PubMed] [Google Scholar]
Morris 1988
- Morris JF, Koski A, Temple WP, Claremont A, Thomas DR. Fifteen year interval spirometric evaluation of the Oregon pedictive equations. Chest 1988;92:123‐27. [DOI] [PubMed] [Google Scholar]
Neffen 2005
- Neffen H, Fritscher C, Schacht FC, Levy G, Chiarella P, Soriano JB, et al. Asthma control in Latin America: the Asthma Insights and Reality in Latin America (AIRLA) survey. Revista Panamericana de Salud Publica 2005;17(3):191‐7. [DOI] [PubMed] [Google Scholar]
Price 1989
- Price AH, Clissold SP. Salbutamol in the 1980s. A reappraisal of its clinical efficacy. Drugs 1989;38(1):77‐122. [DOI] [PubMed] [Google Scholar]
Rabe 2000
- Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. European Respiratory Journal 2000;16(5):802‐7. [DOI] [PubMed] [Google Scholar]
Rashid 2012
- Rashid MM, Khair MM, Hossain MA, Hassan MR. Newer effective treatment for adult patients with acute asthma with a multi‐dose regimen of ipratropium bromide plus salbutamol using a spacer device in emergency department. Respirology 2012;17(Suppl 2):2. [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Rodrigo 2005
- Rodrigo GJ, Castro‐Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta‐analysis. Thorax 2005;60(9):740‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2000a
- Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: A systematic review of the literature. Annals of Emergency Medicine 2000;36(3):181‐90. [DOI] [PubMed] [Google Scholar]
Rowe 2000b
- Rowe BH, Bretzlaff J, Bourdon C, Bota G, Blitz S, Camargo Jr CA. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No.: CD001490. [DOI: 10.1002/14651858.CD001490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2010
- Rowe BH, Villa‐Roel C, Abu‐Laban RB, Stenstrom R, Mackey D, Stiell IG, et al. Admissions to Canadian hospitals for acute asthma: A prospective, multicentre study. Canadian Respiratory Journal 2010;17(1):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santanello 1999
- Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are the minimal important changes for asthma measures in a clinical trial?. European Respiratory Journal 1999;14(1):23‐7. [DOI] [PubMed] [Google Scholar]
Smith 1997
- Smith DH, Malone DC, Lawson KA, Okamoto LJ, Battista C, Saunders WB. A national estimate of the economic costs of asthma. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):787‐93. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Spooner 1998
- Spooner C, Spooner GR, Jones AP, Rowe BH. Inhaled beta2‐agonist and anticholinergic agents for emergency management of asthma in adults. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001284] [DOI] [PMC free article] [PubMed] [Google Scholar]


