Abstract
Mycoplasma is the most common contaminant and greatly affects host cells. The influence of mycoplasma on microglia cells remains unknown. Here, we investigated the influence of mycoplasma contamination on BV2 cells (a microglia cell line). We found that mycoplasma contamination increased the phosphorylation of NF-kB and MAPK signal pathway and induced the activation of BV2 cells. These mycoplasma-contaminated BV2 cells exhibited a transition of cell morphology and slower proliferation, as well as increased gene expression and protein secretion of inflammatory factors. Furthermore, mycoplasma-contaminated BV2 cells had decreased sensitivity to lipopolysaccharide stimulation. These findings suggested that mycoplasma contamination greatly influenced the characteristics and function of microglia cells. It is important to prevent and exclude mycoplasma contamination in our research.
Keywords: Mycoplasma contamination, Microglia cell, Immune cell, Cell culture
Introduction
Cell culture is a very important and common method in research. The cultures maybe primary cultures, which are taken directly from tissue or organs, or immortalized cell lines, which may be from normal or cancerous tissues. Unfortunately, cultures can have contaminants of various kinds, including bacteria (Chen et al. 2012), fungal (Mirjalili et al. 2005a), viruses (Uphoff et al. 2015) or other cells (Kniss and Summerfield 2014). Of the bacteria, mycoplasma is the most frequent contaminant and because most antibiotics fail to eradicate them, mycolplasma is a serious problem in animal cell cultures (Harlin and Gajewski 2008; Mirjalili et al. 2005; Nikfarjam and Farzaneh 2012; Drexler and Uphoff 2002).
Mycoplasmas are the smallest and simplest prokaryotic microorganism that lack cell wall and are highly polymorphic, they are parasitic microorganism and colonize animals or plants (Razin et al. 1998). Most of mycoplasmas are non-pathogenic except several types, such as mycoplasma pneumoniae (He et al. 2016), mycoplasma urealyticum (Shimada et al. 2014), mycoplasma hominis (Capoccia et al. 2013), mycoplasma gallisepticum (Xiao et al. 2016). It is difficult to prevent mycoplamsa infection partly because of lacking successful vaccination strategies (Lee et al. 2018; Leigh et al. 2018). Unfortunately, more and more cell lines were found to be contaminated by mycoplasma in research laboratories all over the world. It is reported that mycoplasma contamination changed the characteristics, protein expression and functions of host cells, which greatly influenced research work, especially for immune cells (Hoff et al. 2018; Jimbo et al. 2017; Nolan et al. 2016). Thus, mycoplasma contamination has become a troublesome problem (Callaway 2014).
BV2 cell is a murine microglia cell line that is commonly used in the research of brain inflammation. BV2 cell activated by lipopolysaccharide (LPS) stimulation is used as an inflammation research model in vitro (Ko et al. 2018; Latta et al. 2015). However, the influence of mycoplasma contamination on BV2 cells remains unknown. In our study which used BV2 cell as a cell model, we noticed that BV2 cells appeared greatly changes after accidental mycoplasma contamination. In order to understand the influence of mycoplasma contamination on BV2 cells, we compared the differences of normal BV2 cells, mycoplasma-contaminated BV2 cells and myco-3 (a kind of mycoplasma scavenger) treated mycoplasma-contaminated BV2 cells in morphology, proliferation and sensitivity to LPS stimulation.
Materials and methods
Cell culture
The immortalized murine microglial cell line BV2 and human neuroblastoma cell line SH-SY5Y were obtained from the Cell Resource Center, Peking Union Medical College (which is the headquarters of National Infrastructure of Cell Line Resource, NSTI). Both cell lines were cultured in dulbecco’s modified eagle’s medium (DMEM) (Gibco, Grand island, NY, USA) supplied with 10% fetal bovine serum (FBS) (Hyclone, South America) at 37 °C with 5% CO2 saturation. Passage was performed every 3 days at a ratio of 1:3–6 for BV2 and 1:3 for SH-SY5Y. Mycoplasma-contaminated BV2 cells were obtained occasionally from normal BV2 cells which were cultured in medium containing mycoplasma-contaminated fetal bovine serum. To stimulate the activation of microglia, BV2 cells were digested and seeded at 4 × 105/well in a six-well plate, 50 ng/ml LPS was added into the culture medium, 6 h later, the cell supernatant was filtered with 0.22 μm filter and collected as BV2 condition medium.
CCK8 assay
The cytotoxic effect of BV2 condition medium on SH-SY5Y was examined by CCK8 assay. Briefly, SH-SY5Y were seeded at 1.5 × 104/well in 96-well plate. Cell culture medium was changed into BV2 condition medium and incubated for 24 h. Then, 10 μl CCK8 reagent (KeyGEN BioTECH, Nanjing, China) was added into each well and incubated for 1–3 h. Optical density (OD) was measured at 450 nm using a microplate reader (Thermo scientific, USA). Cell viability was showed as OD and presented as mean ± SD. OD of cells cultured in DMEM containing 10% FBS was regarded as control, cell viability was calculated according to the following formula: cell viability = OD (experiment group-background)/OD (control group-background) × 100%.
Detection and elimination of mycoplasma contamination
Mycoplasma contamination of BV2 cells was detected by polymerase chain reaction (PCR) method with PCR mycoplasma detection kit (KeyGEN BioTECH, Nanjing, China) according to manufacturer’s instruction. Briefly, the cell supernatant of BV2 cells was transfered to a new tube and centrifuged at 13000 rpm for 5 min. Then the cell supernatant was incubated in 100 °C for 15 min and collected as template for the following PCR detection. For PCR reaction, 5 μl cell supernatant was mixed with 5 μl 10 × PCR buffer, 4 μl MgCl2, 2 μl dNTP mixture, 1 μl forward primer, 1 μl reverse primer, 1 μl taq polymerase and 31 μl water. The mixture was undergo PCR reaction under following conditions: first, pre-denatuation at 95 °C for 5 min; second, denatuation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extention at 72 °C for 1 min, for 40 cycles; third, extention at 72 °C for 10 min. The product was loaded on 1.5% agarose gel containing GeneGreen Nucleic Acid Stain (TianGen Biotech, Beijing, China) and observed with a G-box imaging analyzer (Gene company, Hongkong, China).
To remove mycoplasma contamination in cell culture, mycoplasma scavenger myco-3 (AppliChem, Darmstadt, Germany) was added into culture medium with the concentration of 1 μg/ml, medium was changed 2–3 days, cells were cultured in medium containing mycoplasma scavenger for 14 days. Finally, after another 14 days of culture in medium without myco-3, cells were collected for the detection of mycoplasma to ensure totally elimination of mycoplasma.
Western blot analysis
BV2 cells were seeded in 100 mm Poly-l-ornithine (Sigma-Aldrich, Shanghai, China) pre-coated dishes, all cells were washed with PBS and lysed with RIPA buffer (Beyotime, Beijing, China), the supernatant was collected and transfered to a new tube, after quantification with BCA detection kit (Beyotime, Beijing, China), samples were separated with 10% SDS-PAGE (Beyotime, Beijing, China), and proteins were blotted into a PVDF membrane (Millipore, Germany). Then the PVDF membrane was incubated with primary antibodies overnight at 4 °C, followed by incubation of corresponding HRP-conjugated secondary antibodies for 1 h at room temperature. The protein expression was colored with ECL substrate (Millipore, Germany) and detected with a BG-gdsAUTO710 Mini chemiluminescence imaging system (BAYGENE, Beijing, China). The following primary antibodies were used: rabbit anti-P44/42 MAPK (ERK1/2), anti-phospho-P44/42 MAPK (p-ERK1/2), anti-JNK, anti-phospho-SAPK/JNK (p-JNK), anti-NF-kB P65 (P65), anti-phospho-NF-kB P65 (p-P65), anti-P38 MAPK (P38), anti-phospho-P38 MAPK (p-P38), mouse anti-β-actin (Cell Signaling Technology, Shanghai, China). Secondary antibodies were peroxidase-conjugated goat anti-rabbit IgG(H + L), peroxidase-conjugated goat anti-mouse IgG(H + L) (Zhongshanjinqiao, Beijing, China). The gray value of all bands was measured by image J software and the relative expression of respective protein was normalized to β-actin.
RNA isolation and real-time PCR
Total RNA was extracted by Trizol reagent (Thermo Fisher Scientific, Carlsbad, CA, USA) according to manufacturer’s instruction. 1 μg total RNA was transcripted to cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Shanghai, China) according to manufacturer’s protocol. Quantitative real-time PCR was performed with SYBR premix Ex Taq (Roche) and detected by a Lightcycler 480 real-time PCR system (Roche, mannheim, Germany). GAPDH was used as internal control gene. Primer information was listed in Table 1. The PCR amplified under following conditions: denaturing at 95 °C for 10 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s, then extention at 70 °C for 5 min. Relative expression level was defined using the 2−ΔΔct method and normalized to GAPDH.
Table 1.
Primer information
| Gene | Forward | Reverse |
|---|---|---|
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| TNF-α | ACCCTCACACTCACAAACCAC | ACAAGGTACAACCCATCGGC |
| iNOS | TCCCTTCCGAAGTTTCTGGC | CTCTCTTGCGGACCATCTCC |
| COX-2 | TGGGGGAAGAAATGTGCCAA | AGAAGCGTTTGCGGTACTCA |
| GAPDH | GGAGAGTGTTTCCTCGTCCC | ACTGTGCCGTTGAATTTGCC |
Enzyme-linked immunosorbent assay (ELISA) analysis
To detect the secretion of inflammatory factors in BV2 cells, the culture medium was replaced 6 h before collection and centrifuged at 1500 rpm for 5 min at 4 °C; the secretion of Tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) were measured by enzyme-linked immunosorbent assay kits (R&D system, minneapolis, USA) according to the manufacturer’s instructions.
Statistical analysis
All experiments were conducted in triplicate. Data was presented as mean ± SD. Differences between groups were analyzed by one way ANOVA using SPSS 17.0. P < 0.05 was considered as statistical significance.
Results
Mycoplasma contamination influenced cell morphology and proliferation of BV2 cells
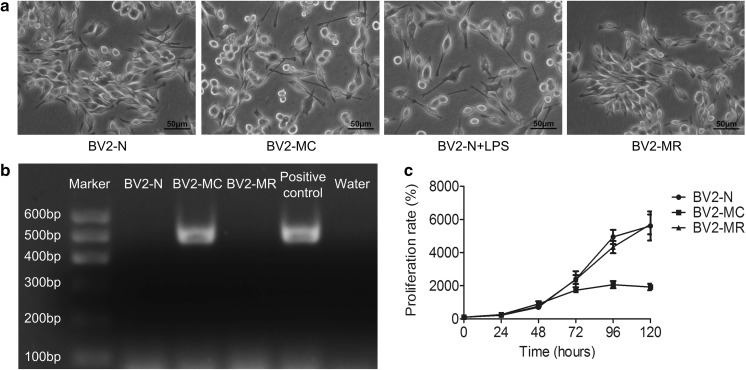
Normal BV2 cells displayed spindle or round morphology with small cell body, all cells had good refraction and smooth cell membrane, similar with the shape of “eyes”, which showed a healthy state. While, after mycoplasma contamination, “eyes” almost disappeared, some cells retracted and turned round with crumpled membrane, other cells stretched out slender branches, all cells showed activated morphology similar with BV2 cells which were stimulated with LPS. However, after treated with mycoplasma scavenger, all cells returned to a healthy state like normal BV2 cells (Fig. 1a).
Fig. 1.
Cell morphology and proliferative capability of mycoplasma-contaminated BV2 cells and PCR detection of mycoplasma. a Cell morphology of BV2-N, BV2-MC, LPS-treated BV2-N (BV2-N + LPS) and BV2-MR. b PCR detection of mycoplasma showed that BV2-MC had one specific band at the position of 502-520 bp, similar with positive control. c BV2-MC had a decreased proliferative capability compared to BV2-N. The proliferation of BV2-MC greatly decreased in BV2-MC but restored after mycoplasma elimination. (BV2-N: normal BV2 cell, BV2-MC: mycoplasma-contaminated BV2 cell, BV2-MR: mycoplasma-removed BV2-MC cell) (bar = 50 μm)
In order to determine whether cells were contaminated with mycoplasma in molecular level, cell samples were collected and detected by PCR kit, the primer set allowed detection of various mycoplasma species appeared in cell culture (including M. fermentans, M. hyorhinis, M. arginini, M. orale, M. salivarium, M. hominis, M. pulmonis, M. arthritidis, M. neurolyticum, M. Hyponeumoniae, M. Capricolum and so on). We found that BV2 cells with obvious morphology transition (mycoplasma-contaminated BV2 cells, BV2-MC) showed one specific band at the position of 502–520 bp similar with positive control, while normal BV2 cells (BV2-N) showed no band. Furthermore, after treated with mycoplasma scavenger, mycoplasma-contaminated BV2 cells (that is mycoplasma remove, BV2-MR) restored their normal morphology and there was also no any band observed after PCR detection, which was similar with BV2-N (Fig. 1b). This result showed that BV2 cell exhibited a transition of cell morphology after being contaminated by mycoplasma and this transition could be reversed by mycoplasma elimination.
To investigate the effect of mycoplasma contamination on cell proliferation, BV2-N, BV2-MC and BV2-MR were seeded at 200 cells per well in 96-well plate separately. Cell proliferation was detected by CCK8 reagent every day for 6 days. Cell proliferation rate was calculated according to the following formula: proliferation rate = OD (Day N-background)/OD (Day 0-background) × 100%, N represented different time. We found that, all cells grown with similar proliferation rate at the first 48 h, then the growth of BV2-MC began much slower than BV2-N and BV2-MR from 72 h, no difference was observed between BV2-N and BV2-MR group (Fig. 1c). This result indicated that mycoplasma contamination suppressed cell proliferation greatly, but cell restored proliferative capability after mycoplasma removement.
Mycoplasma contamination activated BV2 cells and increased the production of inflammatory factors
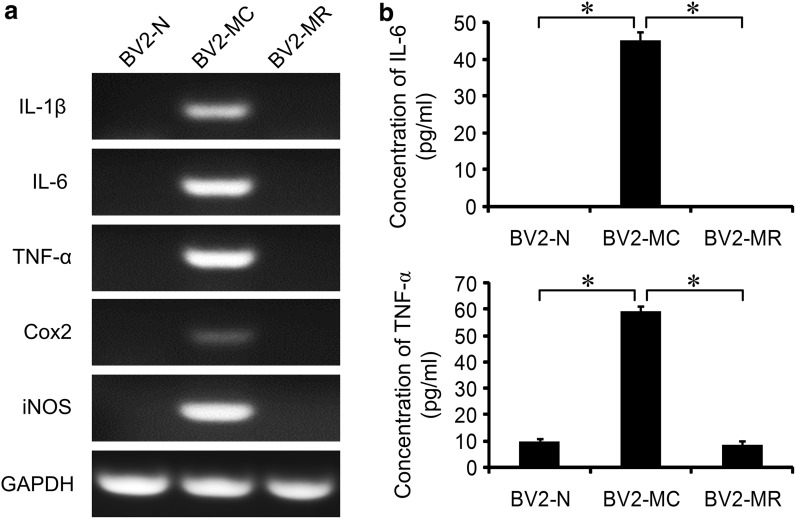
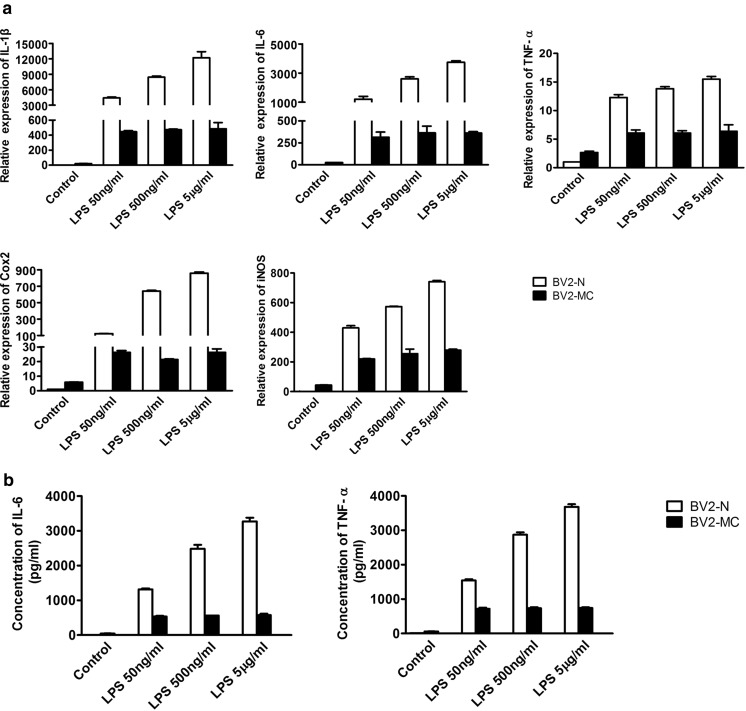
After mycoplasma contamination, BV2 cell obtained an activated morphology. To determine whether mycoplasma contamination activated BV2 cells, total RNA of BV2-N, BV2-MC and BV2-MR were extracted and cell supernatant were collected. Semi-quantitative PCR was used to detect the expression of inflammatory genes: Interleukin 1β (IL-1β), IL-6, TNF-α, Cox2 and iNOS, the secretion of IL-6 and TNF-α were measured by ELISA analysis. We didn’t detect mRNA expression of inflammatory factors in BV2-N and BV2-MR. In contrast, high expression of IL-1β, IL-6, TNF-α, Cox2 and iNOS were observed in BV2-MC cells (Fig. 2a). This result was consistent with the secretion of IL-6 and TNF-α, no production of IL-6 was detected in BV2-N and BV2-MR, while the concentration of IL-6 in the cell supernatant of BV2-MC was 45.17 ± 2.29 pg/ml. There were very low secretion of TNF-α in BV2-N and BV2-MR, that were 9.80 ± 1.11 pg/ml and 8.63 ± 1.41 pg/ml, after mycoplasma contamination, secretion of TNF-α greatly increased and reached to 59.30 ± 1.81 pg/ml (Fig. 2b). These results showed that mycoplasma contamination could activate BV2 cell and promote gene expression as well as protein secretion of inflammatory factors. However, this effect was reversible, BV2-MC cells could return back to non-activated state after mycoplasma elimination.
Fig. 2.
Mycoplasma contamination increased the gene expression and protein secretion of inflammatory factors. a There were strong expression of IL-1β, IL-6, TNF-α, Cox2 and iNOS in BV2-MC by semi-quantitative RT-PCR detection. b, c ELISA analysis showed that secretion of IL-6 and TNF-α increased significantly after mycoplasma contamination. (BV2-N: normal BV2 cell, BV2-MC: mycoplasma-contaminated BV2 cell, BV2-MR: mycoplasma-removed BV2-MC cell). (*P < 0.05)
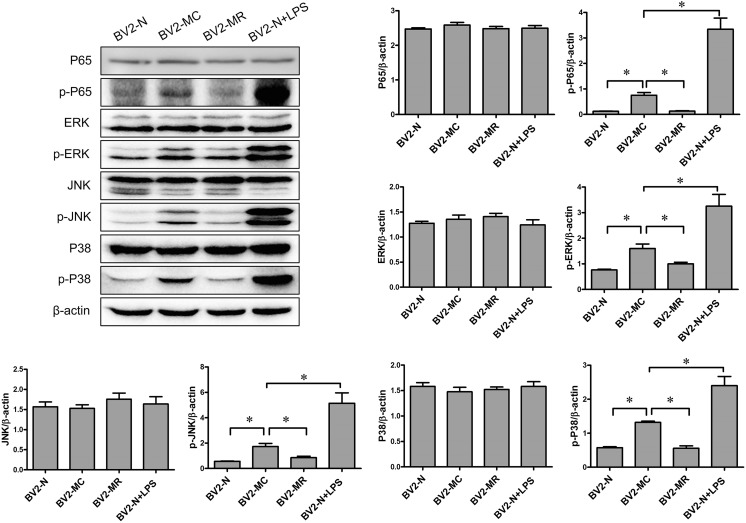
Mycoplasma contamination increased the activation of NF-kB and MAPK signal pathway in BV2 cells
Both of NF-kB and MAPK signal pathway play important role in inflammatory reaction (Oh et al. 2013; Zhao et al. 2018). It was reported that NF-kB and MAPK modulated the gene expression of pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 (Jaimes et al. 2017; Li et al. 2018; Wang et al. 2018). The influence of mycoplasma contamination on NF-kB and MAPK signal pathway is still unclear. Here, we detected the activation of NF-kB P65 (an important molecular in NF-kB signal pathway) and ERK1/2, JNK as well as P38 (three subgroups of MAPK signal pathway) in BV2-N, BV2-MC and BV2-MR. The result showed that there was slightly activation of NF-kB p-P65, p-ERK1/2, p-JNK as well as p-P38 in BV2-N and BV2-MR, while the activation increased significantly in BV2-MC, similar with activated BV2-N which was treated with LPS (Fig. 3). Obviously, mycoplasma contamination could increase the activation of NF-kB and MAPK signal pathway, and promoted the transition of BV2-N from resting state to activated state.
Fig. 3.
The phosphorylation of NF-kB and MAPK signal pathway in BV2-N, BV2-MC, BV2-MR, BV2-N + LPS (determined by western blot and normalized to β-actin). Mycoplasma contamination promoted the activation of NF-kB and MAPK signal pathway. The phosphorylation of NF-kB P65, ERK, JNK and P38 were greatly increased in BV2-MC, which was similar with LPS treated BV2-N (BV2-N + LPS). (BV2-N: normal BV2 cell, BV2-MC: mycoplasma-contaminated BV2 cell, BV2-MR: mycoplasma-removed BV2-MC cell). (*P < 0.05)
Mycoplasma contamination decreased the sensitivity of BV2 cells to LPS stimulation
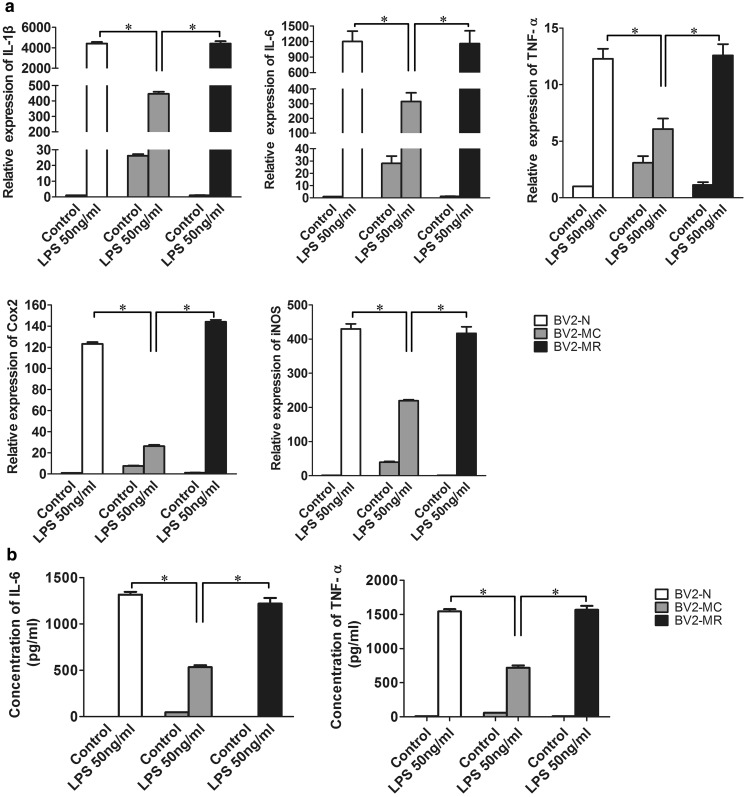
Mycoplasma contamination influences functions of many cells, whether it influences the function of BV2 cells remains unknown. Since activated BV2 cells were usually used as inflammatory cell model. We examined the response of BV2-MC to LPS. BV2-N, BV2-MC and BV2-MR were treated with 50 ng/ml LPS, 6 h later, total RNA and cell supernatant were collected. After quantitative real time PCR detection, we found that the expression of IL-1β, IL-6, TNF-α, Cox2 and iNOS increased after treated with LPS in BV2-N, BV2-MC and BV2-MR. However, compared to BV2-N, the fold change of gene expression in BV2-MC was much lower, there were 4413.19 ± 154.47 and 445.40 ± 14.39 for IL-1β, 1201.26 ± 199.89 and 314.87 ± 58.67 for IL-6, 12.29 ± 0.88 and 6.07 ± 0.93 for TNF-α, 123.12 ± 3.00 and 26.32 ± 2.09 for Cox2, 429.77 ± 25.34 and 219.46 ± 5.51 for iNOS in LPS-treated BV2-N and BV2-MC (Fig. 4a). The secretion of inflammatory factors in LPS-treated BV2-N and BV2-MC were 1316.51 ± 29.95 pg/ml and 533.51 ± 21.03 pg/ml for IL-6, 1543.61 ± 34.33 pg/ml and 719.86 ± 32.59 pg/ml for TNF-α, both were much lower in BV2-MC than BV2-N (Fig. 4b). Furthermore, consistent with previous results, there was no difference between BV2-N and BV2-MR. This meant that BV2-MC had decreased sensitivity to LPS stimulation and this was reversible after mycoplasma elimination.
Fig. 4.
Gene expression and protein secretion of inflammatory factors in BV2-N, BV2-MC and BV2-MR after 50 ng/ml LPS stimulation. a Gene expression of IL-1β, IL-6, TNF-α, Cox2 and iNOS was much lower in LPS-treated BV2-MC than BV2-N and BV2-MR. b the production of IL-6 and TNF-α in the supernatant of LPS-treated BV2-MC was much less than BV2-N and BV2-MR. (BV2-N: normal BV2 cell, BV2-MC: mycoplasma-contaminated BV2 cell, BV2-MR: mycoplasma-removed BV2-MC cell). (*P < 0.05)
To investigate the influence of LPS concentration on the decreased sensitivity of BV2-MC cells to LPS stimulation. BV2-N and BV2-MC cells were treated with LPS at different concentration of 0 ng/ml, 50 ng/ml, 500 ng/ml and 5 μg/ml for 6 h. Total RNA and cell supernatant were collected. Different with BV2-N cells, no difference was observed in gene expression of inflammatory factors in BV2-MC as the concentration of LPS increased (Fig. 5a). This result was consistent with protein secretion of IL-6 and TNF-α by ELISA detection, the amount of IL-6 and TNF-α in cell supernatant of BV2-N was improved as the concentration of LPS increased. However, the increased LPS concentration had few effect on the secretion of IL-6 and TNF-α (Fig. 5b). Both these results indicated that mycoplasma contamination made BV2 cells “blunt” and reduced the sensitivity of BV2 cells to LPS, thus changed the function of BV2 cells.
Fig. 5.
Gene expression and secretion of inflammatory factors in LPS-treated BV2-N and LPS-treated BV2-MC at different concentration (0 ng/ml, 50 ng/ml, 500 ng/ml and 5 μg/ml). Increased gene expression (a) and protein secretion (b) of inflammatory factors in BV2-N was observed as the concentration of LPS increased, but no difference was observed in BV2-MC (BV2-N: normal BV2 cell, BV2-MC: mycoplasma-contaminated BV2 cell)
Mycoplasma contamination decreased the cytotoxic effect of BV2 cells
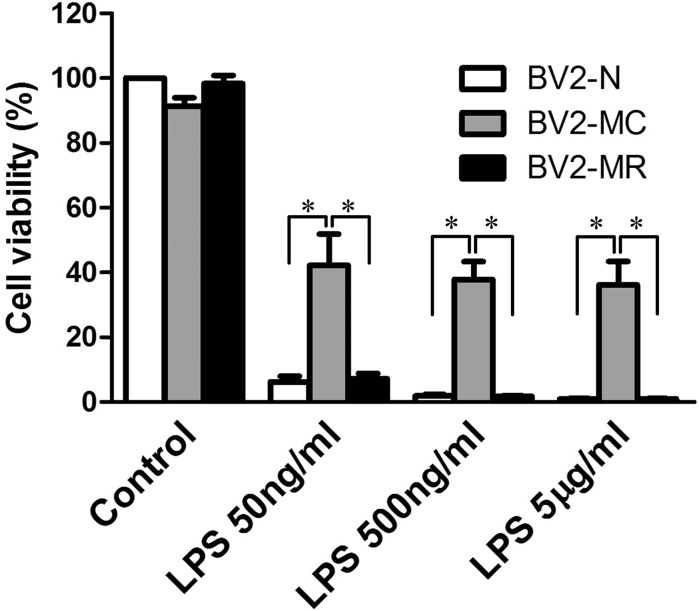
Microglia cell is a kind of immune cell which is important in central nervous system. However, over-activated microglia cells can secret large amount of inflammatory factors which are toxic to surrounding cells (Block and Hong 2005). To further determine the influence of mycoplasma contamination on the activation and protein secretion of BV2 cells after LPS exposure, we detected the cytotoxic effect of activated BV2-MC on the survival of SH-SY5Y cells. SH-SY5Y were cultured in the condition medium of 0 ng/ml, 50 ng/ml, 500 ng/ml and 5 μg/ml LPS-treated BV2-N, BV2-MC, BV2-MR. 24 h later, decreased cell viability was observed in all groups. Compared with BV2-N and BV2-MR group, cell viability in BV2-MC group was much higher, that meant less cytotoxicity. There was no difference between BV2-N and BV2-MR group. Moreover, the increase of LPS concentration had no influence on the cytotoxic effect of LPS-treated BV2-MC (Fig. 6), it was most probably that BV2-MC had decreased sensitivity to LPS and secreted less inflammatory factors. This was consistent with the changes of gene expression and protein secretion. (The effect of LPS on SH-SY5Y was excluded by culturing SH-SY5Y in medium with 0 ng/ml, 50 ng/ml, 500 ng/ml and 5 μg/ml LPS, no changes of cell viability was observed by CCK8 detection (this data was not show)).
Fig. 6.
Cytotoxic effect of the supernatant from LPS-treated BV2-N, BV2-MC and BV2-MR on SH-SY5Y. There was less cytotoxity in supernatant from LPS-treated BV2-MC than BV2-N and BV2-MR. As the concentration of LPS increased, the cytotoxic effect of the supernatant from LPS-treated BV2-N and BV2-MR increased, no changes was detected on the cytotoxicity of supernatant from LPS-treated BV2-MC. (BV2-N: normal BV2 cell, BV2-MC: mycoplasma-contaminated BV2 cell, BV2-MR: mycoplasma-removed BV2-MC cell). (*P < 0.05)
Discussion
Mycoplasma contamination is a common problem in cell culture and affects many aspects of cell physiology (Kagemann et al. 2005a; Yu et al. 2016). In this study, we found that mycoplasma contamination could increase the phosphorylation of NF-kB and MAPK signal pathway and induce the activation of BV2 cells. These activated BV2 cells exhibited a transition of cell morphology and slower proliferation, as well as increased gene expression and protein secretion of inflammatory factors. However, though mycoplasma contamination could activate BV2 cells, it greatly decreased the sensitivity of BV2 cells to LPS stimulation, which confirmed by lower gene expression and protein secretion of inflammatory factors, and lower cytotoxic effect in BV2-MC than BV2-N as well. All these results meant that mycoplasma contamination greatly affected the cell state and suppressed the immune function of BV2 microglia cells. Thus, prevention and detection of mycoplasma contamination are important in our research, because it greatly interferes experiment results and perverts our judgement.
It is reported that mycoplasma may influence many aspects of host cells, including metabolism, physiological function. Yu et al. found that mycoplasma contamination caused significant metabolic changes in PANC-1 cells (a pancreatic cancer cell line), 23 metabolites that involved in arginine, purine metabolism and energy supply were identified (Yu et al. 2016). Kagemann et al. observed that there was decreased arginase activity in hyorhinis infected murine keratinocytes, probably because that hyorhinis infection affected l-arginine metabolism and the synthesis of NO (Kagemann et al. 2005a). All these observations indicated that mycoplasma contamination had great effect on cell metabolism. Furthermore, mycoplasma contamination can influence the application of experiment methods. In 1999, Denecke found that mycoplasma-contaminated cells could reduce the tetrazolium when performed a tetrazolium based MTT assay in a cell cytotoxicity assay (Denecke et al. 1999). Moreover, it is well known that mycoplasma can produce ribonuclease, and siRNA (small interfering RNA) transfection is a common used method in gene knockdown. However, RNA oligonucleotides were degraded immediately after transfection in mycoplasma-contaminated cells (Hernandez et al. 2012). The effect of mycoplasma on experiment methods may cause great influence in our study, which may interfere the objectivity of the results, mislead our decision and analysis, and affect reproducibility of the result, all of these cause waste of time and money.
Microglia cell is a kind of neuroglia cell and mainly resides in brain. It is the most abundant and best studied macrophage cell in the central nervous system and plays important role in inflammatory reaction in brain (Perry and Teeling 2013; Sohrabji and Williams 2013). It is reported that, contamination of mycoplasma changes the psychological function of immune cells (Bai et al. 2013; Damte et al. 2015; Fang et al. 2016; Hwang et al. 2011; Kandasamy et al. 2011). Damte et al. found that the expression of inflammatory factors in mouse alveolar macrophages greatly increased after exposed to intact mycoplasma hyopneumoniae protein (Damte et al. 2015). Nolan et al. detected the response of mycoplasma-contaminated human monocytes and macrophages to LPS stimulation and found that, cells had lower response to LPS after mycoplasma contamination, followed by reduced secretion of inflammatory factors such as IL-1β, IL-6, TNF-α, Cox2 and iNOS, and impaired phagocytic function, which meant that contamination of mycoplasma inhibited the function of macrophages (Nolan et al. 2016). Zinöcker observed that mycoplasma contamination inhibited the immune-suppression function of mesenchymal stromal cells in vivo and decreased the proliferation of lymphocytes in mixed lymphocyte reaction (Zinocker et al. 2011). Sometimes, mycoplasma contamination may induce apoptosis of immune cells. It is reported that mycoplasma hyopneumoniae-derived lipid-associated membrane proteins could increase the activation of caspase-3 and secretion of NO, which induced the apoptosis of porcine alveolar macrophages (Bai et al. 2013). Whatever, these results showed that mycoplasma was a “pathogenic” factor for most cell, it could cause immune cell “sick” or die, and sequently reduced the response of immune cells to other stimulus and inhibited their functions. Consistent with previous reports, mycoplasma was a “bad factor” in our study, we also found that, BV2 cells were activated after mycoplasma contamination, but had lower sensitivity to LPS stimulation, as well as decreased cytotoxicity. However, different with LPS stimulation, activation of BV2 cells by mycoplasma contamination was reversible. It meant that, after mycoplasma elimination, microglia cell could restore its characteristics such as morphology, proliferation and immune function, it was further confirmed by the decreased p-P65, p-P38, p-JNK and p-ERK1/2 after removal of mycoplasma in BV2-MC, which also indicated that there may exist different mechanisms between activation by mycoplasma and LPS.
Lots of laboratories are contaminated by mycoplasma with different levels. It is difficult to discover it because different cells have different responses to mycoplasma. Whether the experiment results are influenced remains unknown. All these remind us that it is important to detect and prevent mycoplasma contamination to ensure the accuracy and reliability of our experiment data.
Acknowledgements
This research was supported by the Beijing Natural science Foundation (No. 7164261) and Basics-Clinical Research Cooperation Fund of Capital Medical University (No. 16JL31).
Author contributions
Nianhua Feng conceived the study, designed and performed the experiment, wrote the manuscript; Xiaoxi Huang supervised the study and provide some of reagents; Yanjun Jia supervised the study.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bai F, Ni B, Liu M, Feng Z, Xiong Q, Xiao S, Shao G. Mycoplasma hyopneumoniae-derived lipid-associated membrane proteins induce apoptosis in porcine alveolar macrophage via increasing nitric oxide production, oxidative stress, and caspase-3 activation. Vet Immunol Immunopathol. 2013;155:155–161. doi: 10.1016/j.vetimm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Callaway E. Contamination hits cell work. Nature. 2014;511:518. doi: 10.1038/511518a. [DOI] [PubMed] [Google Scholar]
- Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. 2013;26:231–240. doi: 10.1097/QCO.0b013e328360db58. [DOI] [PubMed] [Google Scholar]
- Chen J, Bergevin J, Kiss R, Walker G, Battistoni T, Lufburrow P, Lam H, Vinther A. Case study: a novel bacterial contamination in cell culture production–leptospira licerasiae. PDA J Pharm Sci Technol. 2012;66:580–591. doi: 10.5731/pdajpst.2012.00892. [DOI] [PubMed] [Google Scholar]
- Damte D, Lee SJ, Birhanu BT, Suh JW, Park SC. Sonicated protein fractions of mycoplasma hyopneumoniae induce inflammatory responses and differential gene expression in a murine alveolar macrophage cell line. J Microbiol Biotechnol. 2015;25:2153–2159. doi: 10.4014/jmb.1506.06049. [DOI] [PubMed] [Google Scholar]
- Denecke J, Becker K, Jurgens H, Gross R, Wolff JE. Falsification of tetrazolium dye (MTT) based cytotoxicity assay results due to mycoplasma contamination of cell cultures. Anticancer Res. 1999;19:1245–1248. [PubMed] [Google Scholar]
- Drexler HG, Uphoff CC. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology. 2002;39:23–38. doi: 10.1023/A:1022913015916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Zhao W, Xu J, Tu F, Wang X, Li B, Fu Y, Ren S. CYP1A1 mediates the suppression of major inflammatory cytokines in pulmonary alveolar macrophage (PAM) cell lines caused by Mycoplasma hyponeumoniae. Dev Comp Immunol. 2016;65:132–138. doi: 10.1016/j.dci.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Harlin H, Gajewski TF. Diagnosis and treatment of mycoplasma-contaminated cell cultures. Curr Protoc Cytom Appendix. 2008;3:3C. doi: 10.1002/0471142956.cya03cs43. [DOI] [PubMed] [Google Scholar]
- He J, Liu M, Ye Z, Tan T, Liu X, You X, Zeng Y, Wu Y. Insights into the pathogenesis of Mycoplasma pneumoniae (Review) Mol Med Rep. 2016;14:4030–4036. doi: 10.3892/mmr.2016.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez FJ, Stockdale KR, Huang L, Horswill AR, Behlke MA, Mcnamara JN. Degradation of nuclease-stabilized RNA oligonucleotides in mycoplasma-contaminated cell culture media. Nucl Acid Ther. 2012;22:58–68. doi: 10.1089/nat.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff FW, Hu CW, Qutub AA, Qiu Y, Graver E, Hoang G, Chauhan M, de Bont E, Kornblau SM. Mycoplasma contamination of leukemic cell lines alters protein expression determined by reverse phase protein arrays. Cytotechnology. 2018;70:1529–1535. doi: 10.1007/s10616-018-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MH, Damte D, Lee JS, Gebru E, Chang ZQ, Cheng H, Jung BY, Rhee MH, Park SC. Mycoplasma hyopneumoniae induces pro-inflammatory cytokine and nitric oxide production through NFkappaB and MAPK pathways in RAW264.7 cells. Vet Res Commun. 2011;35:21–34. doi: 10.1007/s11259-010-9447-5. [DOI] [PubMed] [Google Scholar]
- Jaimes Y, Naaldijk Y, Wenk K, Leovsky C, Emmrich F. Mesenchymal stem cell-derived microvesicles modulate Lipopolysaccharides-induced inflammatory responses to Microglia cells. Stem Cells. 2017;35:812–823. doi: 10.1002/stem.2541. [DOI] [PubMed] [Google Scholar]
- Jimbo S, Suleman M, Maina T, Prysliak T, Mulongo M, Perez-Casal J. Effect of Mycoplasma bovis on bovine neutrophils. Vet Immunol Immunopathol. 2017;188:27–33. doi: 10.1016/j.vetimm.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Kagemann G, Henrich B, Kuhn M, Kleinert H, Schnorr O. Impact of Mycoplasma hyorhinis infection on l-arginine metabolism: differential regulation of the human and murine iNOS gene. Biol Chem. 2005;386:1055–1063. doi: 10.1515/BC.2005.121. [DOI] [PubMed] [Google Scholar]
- Kandasamy P, Zarini S, Chan ED, Leslie CC, Murphy RC, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae-stimulated eicosanoid production from human and mouse macrophages. J Biol Chem. 2011;286:7841–7853. doi: 10.1074/jbc.M110.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss DA, Summerfield TL. Discovery of HeLa cell contamination in HES cells: call for cell line authentication in reproductive biology research. Reprod Sci. 2014;21:1015–1019. doi: 10.1177/1933719114522518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W, Park JS, Kim KW, Kim J, Kim YC, Oh H. Nardosinone-type sesquiterpenes from the hexane fraction of Nardostachys jatamansi attenuate NF-kappaB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 microglial cells. Inflammation. 2018 doi: 10.1007/s10753-018-0768-9. [DOI] [PubMed] [Google Scholar]
- Latta CH, Sudduth TL, Weekman EM, Brothers HM, Abner EL, Popa GJ, Mendenhall MD, Gonzalez-Oregon F, Braun K, Wilcock DM. Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-beta using BV2 microglial cells and APP/PS1 transgenic mice. J Neuroinflamm. 2015;12:41. doi: 10.1186/s12974-015-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Hwang MA, Han JH, Cho EH, Lee JB, Park SY, Song CS, Choi IS, Lee SW. Reduction of mycoplasmal lesions and clinical signs by vaccination against Mycoplasma hyorhinis. Vet Immunol Immunopathol. 2018;196:14–17. doi: 10.1016/j.vetimm.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Leigh SA, Evans JD, Collier SD, Branton SL. The impact of vaccination route on Mycoplasma gallisepticum vaccine efficacy. Poult Sci. 2018 doi: 10.3382/ps/pey188. [DOI] [PubMed] [Google Scholar]
- Li X, Peng H, Wu J, Xu Y. Brain natriuretic peptide-regulated expression of inflammatory cytokines in lipopolysaccharide (LPS)-activated macrophages via NF-kappaB and mitogen activated protein kinase (MAPK) pathways. Med Sci Monit. 2018;24:3119–3126. doi: 10.12659/MSM.905580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirjalili A, Parmoor E, Moradi BS, Sarkari B. Microbial contamination of cell cultures: a 2 years study. Biologicals. 2005;33:81–85. doi: 10.1016/j.biologicals.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Nikfarjam L, Farzaneh P. Prevention and detection of mycoplasma contamination in cell culture. Cell J. 2012;13:203–212. [PMC free article] [PubMed] [Google Scholar]
- Nolan TJ, Gadsby NJ, Hellyer TP, Templeton KE, Mcmullan R, Mckenna JP, Rennie J, Robb CT, Walsh TS, Rossi AG, Conway MA, Simpson AJ. Low-pathogenicity Mycoplasma spp. alter human monocyte and macrophage function and are highly prevalent among patients with ventilator-acquired pneumonia. Thorax. 2016;71:594–600. doi: 10.1136/thoraxjnl-2015-208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WJ, Jung U, Eom HS, Shin HJ, Park HR. Inhibition of lipopolysaccharide-induced proinflammatory responses by Buddleja officinalis extract in BV-2 microglial cells via negative regulation of NF-kB and ERK1/2 signaling. Molecules. 2013;18:9195–9206. doi: 10.3390/molecules18089195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Ito S, Mizutani K, Sugawara T, Seike K, Tsuchiya T, Yokoi S, Nakano M, Yasuda M, Deguchi T. Bacterial loads of Ureaplasma urealyticum contribute to development of urethritis in men. Int J STD AIDS. 2014;25:294–298. doi: 10.1177/0956462413504556. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Williams M. Stroke neuroprotection: oestrogen and insulin-like growth factor-1 interactions and the role of microglia. J Neuroendocrinol. 2013;25:1173–1181. doi: 10.1111/jne.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphoff CC, Lange S, Denkmann SA, Garritsen HS, Drexler HG. Prevalence and characterization of murine leukemia virus contamination in human cell lines. PLoS ONE. 2015;10:e125622. doi: 10.1371/journal.pone.0125622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bi C, Wang Y, Sun J, Meng X, Li J. Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-kappaB and MAPK signaling pathways. BMC Vet Res. 2018;14:197. doi: 10.1186/s12917-018-1508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Sun J, Yang T, Fang X, Cheng J, Xiong YQ, Liu YH. Pharmacokinetic/pharmacodynamic profiles of tiamulin in an experimental intratracheal infection model of Mycoplasma gallisepticum. Front Vet Sci. 2016;3:75. doi: 10.3389/fvets.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Wang Y, Zhang H, Johnson CH, Jiang Y, Li X, Wu Z, Liu T, Krausz KW, Yu A, Gonzalez FJ, Huang M, Bi H. Metabolomics reveals mycoplasma contamination interferes with the metabolism of PANC-1 cells. Anal Bioanal Chem. 2016;408:4267–4273. doi: 10.1007/s00216-016-9525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wang Q, Cheng X, Li X, Li N, Liu T, Li J, Yang Q, Dong R, Zhang Y, Zhang L. Inhibitive effect of resveratrol on the inflammation in cultured astrocytes and microglia induced by Abeta1-42. Neuroscience. 2018;379:390–404. doi: 10.1016/j.neuroscience.2018.03.047. [DOI] [PubMed] [Google Scholar]
- Zinocker S, Wang MY, Gaustad P, Kvalheim G, Rolstad B, Vaage JT. Mycoplasma contamination revisited: mesenchymal stromal cells harboring Mycoplasma hyorhinis potently inhibit lymphocyte proliferation in vitro. PLoS ONE. 2011;6:e16005. doi: 10.1371/journal.pone.0016005. [DOI] [PMC free article] [PubMed] [Google Scholar]