Abstract
Background
Although renal replacement therapy prevents death from uremia, survival among patients with acute and chronic kidney diseases (CKD) remains an imperative concern. The expected life span of US dialysis patients 60–64 years of age is approximately 4.5 years; this is similar to that of patients with lung cancer. Despite substantial progress in many medical specialties over the past decades (e.g., notable reductions in myocardial infarction, stroke, and mortality rates in the general population), survival among dialysis patients has not improved significantly over the same period. A few decades ago, HIV infection and AIDS were pretty much a death sentence. Because of progress in HIV treatment, now it can be controlled with a daily pill, and ongoing research is pushing treatment even further and controls the virus with longer-acting treatment. A cure is no longer impossible for HIV and other viral infections such as hepatitis B and C and many malignancies, but so far there is no cure for CKD.
Summary
Billions of dollars have been spent on kidney disease research in the past decades, with no tangible progress in clinical practice. The challenges of improving the quantity and quality of trials in nephrology are enormous. The number of randomized controlled trials (RCTs) published in nephrology is lower than that in other medical subspecialties, and most of the big RCTs in nephrology yield negative results. Nephrology studies evaluating hard clinical endpoints or surrogate endpoints are scarce.
Key Message
Herein we discuss the slow progress in nephrology research that has impacted clinical practice over the last couple of decades and highlight the major obstacles, challenges, and potential solutions.
Key Words: Kidney diseases, Outcome studies, Randomized controlled trials, Nephrology research, Negative studies
Quantity and Proportion of Randomized Controlled Trials in Nephrology Compared to Other Subspecialties
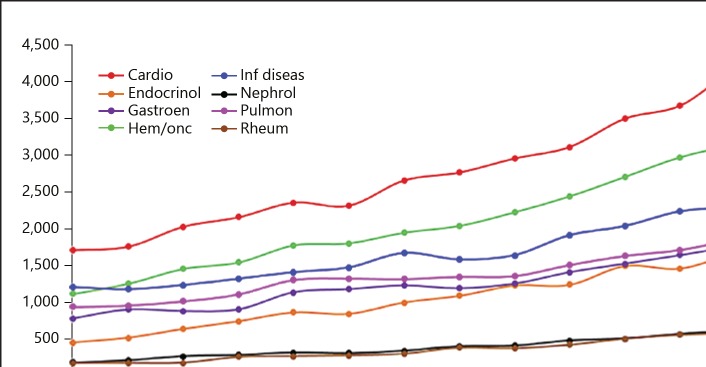
The PubMed Medical Subject Headings (MeSH) were used to identify subject headings for searching through eight primary subspecialties of internal medicine: cardiology, endocrinology, gastroenterology, hematology/oncology, infectious disease, nephrology, pulmonology, and rheumatology. The following MeSH terms were used: “cardiovascular diseases” or “cardiology”; “endocrine system diseases” or “endocrinology”; “digestive system diseases” or “gastroenterology”; “hemic and lymphatic diseases” or “hematology” or “neoplasms” or “medical oncology”; “bacterial infections and mycoses” or “virus diseases” or “parasitic diseases” or “infectious disease medicine”; “kidney diseases” or “nephrology”; “respiratory tract diseases” or “pulmonary medicine”; “rheumatic diseases” or “rheumatology.” The resulting number of total publications by subspecialty for each year from 2001 through 2015 was then limited to the PubMed publication type “randomized controlled trial.” Although we performed these searches looking at publication dates through 2017, we found from continued sampling of the data through 2018 that a significant number of articles are still being added with 2016 and 2017 publication dates. Rather than including data that might therefore be incomplete, we used 2015 as our data end date. The number of randomized controlled trials (RCTs) in nephrology in 2001 was 193 and has gradually increased to 601 by 2015. Most of the other subspecialties have also exhibited the same trend. However, nephrology as well as rheumatology have the lowest number of publications (Fig. 1).
Fig. 1.
Randomized controlled trials: published articles in PubMed by subspecialty from 2001 to 2015. Cardio, cardiology; Endocrinol, endocrinology; Gastroen, gastroenterology; Hem/onc, hematology/oncology; Inf diseas, infectious disease; Nephrol, nephrology; Pulmon, pulmonology; Rheum, rheumatology.
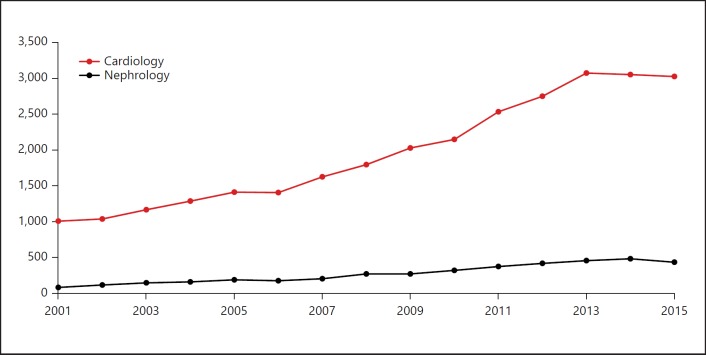
We also searched the number of publications resulting from grant-funded research and categorized them according to subspecialty using the same publication type terms identified above with the PubMed filters for grant support. We compared the grant-funded research between cardiology and nephrology for each year from 2001 through 2015. Cardiology had approximately 8 times more published articles from grant-funded research than nephrology. This difference was consistent over time (Fig. 2).
Fig. 2.
Numbers of grant-funded articles indexed in PubMed for cardiology and nephrology from 2001 to 2015.
The ASN Research Advocacy Committee estimated that the National Institutes of Health (NIH) spent only USD 30 on research annually for each chronic kidney disease (CKD) patient in the USA, while it spent over USD 500 for every patient with cancer and over USD 2,500 per individual with HIV infection. It is therefore not surprising that the cancer and HIV areas have experienced the greatest technological health care advances over the last few decades. In 2015, Medicare spent nearly USD 34 billion on end-stage renal disease (ESRD) patients. However, the NIH spent only USD 564 million on kidney disease research in the same year. Since only < 2% of the cost of care was spent on kidney research, several research initiatives have gained considerable momentum, focusing on increased federal and nonfederal research funding. The result hopefully will accelerate innovation toward the development of new technologies in managing kidney disease patients. Herein we focus on the recent nephrology studies that might have influenced our clinical practice.
CKD – Mineral and Bone Disorders
In 2003, the Kidney Disease Outcomes Quality Initiative (KDOQI) published its CKD – mineral and bone disorder (CKD-MBD) clinical practice guideline [1]. In 2009, the Kidney Disease: Improving Global Outcomes (KDIGO) issued their initial CKD-MBD guideline [2], and in 2017, a selective update was published [3]. The initial KDIGO guideline was based mostly on observational and expert opinion that led to universal changes in clinical practice. The updated 2017 KDIGO guideline recommended managing CKD-MBD with a more individualized approach in view of the lack of benefit regarding intermediate biochemical and cardiovascular endpoints. Moreover, they reported that overusing and/or misusing some therapies could potentially lead to harm such as hypercalcemia or unnecessary health care spending. Due to lack of evidence for many CKD-MBD management decisions, a large number of updated recommendations continue to be debated.
One of the major changes in the updated 2017 KDIGO guideline is using bone mineral density (BMD) testing to assess the fracture risk in CKD stage 3a–5D, as 4 prospective cohort studies demonstrated that BMD measurements predicted fractures in this patient population [4, 5, 6, 7]. However, this recommendation generates some uncertainty and does not provide clear advice on how to treat low BMD in CKD patients, as this patient population was excluded from most RCTs [8]. The language of the updated CKD-MBD guideline is often inconclusive, and all recommendations are at the level of “we suggest” (no level 1 “we recommend”) and the majority have “low” or “very low” levels of evidence. This challenges the nephrology community and underscores the need for better-quality research on this devastating disorder.
Block et al. [9] reported that the use of phosphate binders increased the progression of vascular calcification compared to placebo in patients with moderate CKD. Other RCTs showed a tendency toward increased morbidity and/or mortality among patients treated with calcium-based binders as compared with non-calcium-based binders [10, 11]. The largest 2 placebo-controlled RCTs on CKD-MBD management that have been published in the last decade were also disappointing [12, 13]. The EVOLVE trial studied the effect of cinacalcet on mortality, myocardial infarction, unstable angina, heart failure, and peripheral vascular disease [12]. The PRIMO trial examined the effect of paricalcitol on the left ventricular mass index and measures of diastolic dysfunction [13]. Neither of these trials found a significant effect of the study medicine on these primary endpoints. The results of these RCTs leave clinicians with a difficult choice. The ambiguity and lack of unequivocally actionable recommendations for CKD-MBD management highlight the potential challenges to their implementation and dissemination. In most circumstances, the clinical practice guidelines are to be used in conjunction with clinical judgement.
Anemia Management in CKD Patients
According to the 2012 KDIGO guideline for adult CKD patients prior to dialysis, the decision to initiate erythropoietin therapy for treating anemia should be individualized (2C recommendation) [14]. The CREATE trial studied the effect of early anemia management in CKD patients prior to dialysis. The authors reported that early complete correction of anemia does not reduce the risk of cardiovascular events [15]. Singh et al. [16] studied the effect of correction of anemia with epoetin alfa in 1,432 CKD patients (CHOIR trial). The patients were randomized to receive epoetin alfa to achieve a target hemoglobin level of either 13.5 or 11.3 g/dL. The composite events were higher and the hazard ratios for death and hospitalization for congestive heart failure had a strong trend toward a higher risk in the high-hemoglobin group. Moreover, the use of a target hemoglobin level of 13.5 g/dL (as compared with 11.3 g/dL) did not improve the quality of life [16].
TREAT is a large multicenter trial on CKD diabetic patients with moderate anemia who were not undergoing dialysis. The patients were randomly assigned to receive darbepoetin alfa or placebo. The use of darbepoetin did not reduce the risk of death or cardiovascular or renal events, and it was associated with an increased risk of stroke and thromboembolic events. Moreover, there was a sign that normalization of hemoglobin with darbepoetin may be harmful in patients with a history of malignancy [17].
Blood Pressure Control in CKD Patients
Papademetriou et al. [18] studied the effect of intensive blood pressure control in diabetic CKD patients. The benefit in cardiovascular risk reduction from intensive blood pressure control was lower in this patient population compared to patients with normal kidney function. The SPRINT trial studied the benefits of intensive systolic blood pressure lowering to a target < 120 mm Hg versus routine management with a target < 140 mm Hg in high-risk nondiabetic patients with hypertension. In the participants with baseline CKD, intensive systolic blood pressure lowering showed the same cardiovascular disease and mortality risk reductions as in the non-CKD patients. However, there were no specific renal benefits of intensive systolic blood pressure control [19]. In the African American Study of Kidney Disease and Hypertension (AASK), better blood pressure control was not associated with improvement of renal outcome [20]. Furthermore, Appel et al. [21] reported that intensive blood pressure control had no effect on kidney disease progression especially in nonproteinuric patients. The JNC 8 guidelines for the management of hypertension in adults report that the treatment threshold and target for blood pressure are the same in CKD patients and the general population, and there is no evidence that treating CKD patients to a lower blood pressure goal slows the progression of the disease [22].
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial did not find any renal or cardiovascular benefit of renal artery stenting in advanced hypertensive CKD patients with more than 60% renal artery stenosis [23]. The SYMPLICITY HTN-2 trial showed large reductions in blood pressure 6 months after catheter-based radiofrequency denervation [24]. However, the SYMPLICITY HTN-3 trial did not show any benefit of renal artery denervation; particularly for patients with a glomerular filtration rate (GFR) < 60 mL/min there was no benefit from the intervention. Furthermore, there was no renal benefit from renal artery denervation [25]. The Rheos Pivotal Trial, a large-scale double-blinded placebo-controlled RCT evaluating baroreflex activation therapy, showed a sustained efficacy benefit. However, it did not meet either the primary endpoint of lowering blood pressure or procedural safety requirements [26].
Impact of Tight Blood Sugar Control on Renal Outcome in Type 2 Diabetic Patients
Tight glycemic control has been shown to alter the outcome in diabetic patients with early CKD. However, data supporting the benefits of intensive glycemic control for advanced CKD patients are scarce. The United Kingdom Prospective Diabetes Study (UKPDS) investigated the effect of intensive blood glucose control on the risk of microvascular and macrovascular complications in patients with type 2 diabetes [27]. The tighter blood sugar control group had a decreased risk of microvascular disease. However, there was a higher risk of hypoglycemia and weight gain, and there was no effect on macrovascular complications. Aggressive blood sugar control by targeting HbA1c < 6% increased mortality and did not significantly decrease major cardiovascular events in the ACCORD trial [28]. Moreover, in patients with mild and moderate CKD, intensive glycemic control significantly increased the cardiovascular risk and all-cause mortality [29]. The VADT studied the effect of tight blood sugar control on vascular complications in veterans with type 2 diabetes [30]. There was no significant effect on the rates of major cardiovascular events, death, or microvascular complications, with the exception of progression of albuminuria.
ADVANCE is the most optimistic trial so far demonstrating a benefit from tight blood sugar control [31]. The chance of developing microalbuminuria as well as the progression of macroalbuminuria and the ESRD risk decreased in the intensively blood sugar-controlled group. However, the number of patients who developed ESRD was exceedingly low (0.24%), which might reduce the confidence in their findings [31]. Moreover, there was no significant effect on serum creatinine over time and there was a nonsignificant trend toward more frequent doubling of serum creatinine in the intensively treated group. To date, no RCT has convincingly demonstrated a beneficial effect of intensive therapy on macrovascular outcomes in individuals with longstanding type 2 diabetes.
Bardoxolone methyl improved the kidney function in multiple RCTs on patients with diabetic kidney disease. In a phase II study, the BEAM trial showed that bardoxolone had improved the estimated GFR (eGFR) in type 2 diabetic patients with advanced CKD [32]. However, the BEACON trial reported that bardoxolone did not reduce the risk of ESRD or death in stage 4 CKD type 2 diabetic patients in a phase III study. Moreover, they had to terminate the trial prematurely because of a higher rate of volume overload in the bardoxolone group [33]. More recently, the TSUBAKI study group presented an abstract at the last American Society of Nephrology's annual meeting, reporting that bardoxolone improved renal function as assessed by inulin clearance in diabetic stage 3 and 4 CKD patients in a preselected cohort without identified risk factors for fluid overload [34].
Advancements in Glomerulonephritis Management
Glomerulonephritis (GN) diagnosis and treatment is one of the fastest-growing fields in nephrology. Herein we are going to discuss the progress in GN management, focusing on the foremost RCTs.
Idiopathic membranous nephropathy (IMN) is diagnosed primarily by the presence of the glomerular subepithelial antigen-antibody immune complex. This complex was identified as a composite of IgG4 antibody that binds to the phospholipase A2 receptor (PLA2R) in podocytes [35]. Circulating PLA2R antibody in IMN linearly correlates with disease activity and plays an important role in better understanding the disease and the response to immunosuppressive therapy [36]. Rituximab as first- or second-line therapy for IMN induced remission. Moreover, it significantly improved GFRs in patients who achieved complete remission. The adverse effects with rituximab administration were transient, well tolerated, and not serious [37]. In a nonblinded phase Ιb/ΙΙ study, H.P. Acthar gel injection twice weekly (structurally related corticotropin peptide) safely showed significant improvement of proteinuria in IMN at the 1-year follow-up [38]. Combination of Acthar and tacrolimus significantly improved the rate of complete or partial remission in IMN and focal segmental glomerulosclerosis (FSGS) patients who were resistant to two or more immunosuppressive agents [39]. Rituximab effectively improved the clinical and immunological remission rates in severe IMN patients in the French GEMRITUX multicenter RCT [40]. The nephrology community is excited to hear the results of the ongoing MENTOR and STARMEN multicenter RCTs on IMN. The MENTOR trial is a noninferiority study comparing rituximab with cyclosporin [41], while the STARMEN trial is studying the efficacy of sequential treatment with tacrolimus-rituximab versus steroids plus cyclophosphamide [42].
In a phase II RCT, fresolimumab (a monoclonal anti-transforming growth factor-β antibody) did not achieve the primary or secondary endpoint in patients with steroid-resistant FSGS [43]. Abatacept (a costimulatory inhibitor that targets B7-1) successfully induced complete or partial remission in 5 patients with primary FSGS [44]. These promising results encouraged investigators to study the effect of abatacept in patients with resistant FSGS or minimal change disease in an ongoing phase ΙΙ RCT [45].
The Aspreva Lupus Management Study (ALMAS) investigated the effect of induction and maintenance therapy for proliferative lupus nephritis. The patients had similar rates of remission using either cyclophosphamide or mycophenolate mofetil (MMF) in addition to corticosteroids as induction therapy [46]. MMF was superior to azathioprine in maintaining remission and time to treatment failure. Furthermore, the patients who received MMF had longer times until needing rescue therapy [47]. Moreover, in the MAINTAIN study, an insignificantly lower number of renal flares occurred with MMF as a maintenance therapy in comparison to azathioprine, which had a significantly higher number of hematological adverse effects [48]. These results support the use of MMF as the first choice for maintenance therapy of lupus nephritis. The LUNAR study failed to demonstrate the superiority of adding rituximab to the standard MMF-plus-corticosteroid therapy in patients with active proliferative lupus nephritis [49]. In a recent double-blinded phase III trial, belimumab (a monoclonal antibody against B lymphocyte stimulator) significantly reduced proteinuria in moderate-to-severe systemic lupus erythematosus patients [50]. Furthermore, belimumab was well tolerated and its efficacy was maintained during the extension phase [51].
In the MEPEX (Methylprednisolone versus Plasma Exchange) trial, the rate of renal recovery was higher in the plasma exchange group than in the intravenous methylprednisolone group among patients with severe antineutrophil cytoplasmic autoantibody (ANCA)-associated renal vasculitis. However, the patients’ survival and severe adverse effects were not significantly different between the two modalities [52]. The RITUXIVAS trial did not demonstrate any superiority in attaining remission with the combination of rituximab and reduced-dose cyclophosphamide over a traditional cyclophosphamide regimen, nor did it demonstrate a safety benefit of rituximab [53]. However, in the RAVE study, rituximab was more effective in relapsing ANCA-associated vasculitis and it was not inferior to cyclophosphamide in newly diagnosed severe cases with no significant difference in adverse effects [54]. The MAINRITSAN trial showed that rituximab is a more effective and safer option than azathioprine as a maintenance therapy in ANCA-associated vasculitis [55]. In a phase II trial, the CLEAR study showed that an orally administered C5a receptor inhibitor (avacopan) can safely and effectively replace high-dose oral glucocorticoids in patients with ANCA-associated vasculitis without any significant difference in adverse effects [56]. These results supported the advancement of avacopan into an ongoing phase III study with a larger number of patients [57].
Major advances have been achieved in the pathophysiological understanding of GN which demands new treatment strategies in the past years but lack of larger RCTs in this field is striking and more RCTs become a pressing need.
Metabolic Acidosis
Metabolic acidosis is a common and well-known problem in patients with advanced CKD [58, 59]. However, only a few RCTs have been published studying the effect of the correction of metabolic acidosis in a relatively small number of patients. The KDIGO guideline suggests that to people with CKD and serum bicarbonate concentrations < 22 mmol/L, treatment with oral bicarbonate supplementation be given to maintain serum bicarbonate within the normal range, unless contraindicated [60]. This KDIGO guideline was only a suggestion and was not set at the recommendation level because of lack of evidence (Level 2B). In a single-blind controlled trial on 46 patients, Mathur et al. [61] found that correction of metabolic acidosis was associated with attenuation of a rise in blood urea and parathyroid hormone levels but did not affect other CKD-MBD metabolic parameters. De Brito-Ashurst et al. [62] found that correction of metabolic acidosis can slow the progression of CKD and the need for dialysis. Although this was the largest published RCT on correction of metabolic acidosis in CKD patients, it was a single-center open-label study with only 134 patients. Now the question is why there are no well-designed RCTs with large numbers of patients for such a common problem as metabolic acidosis in CKD patients.
Homocystinemia
Plasma homocysteine levels increase with a decreasing GFR [63]. Elevated plasma homocysteine has been proposed as a risk factor for cardiovascular morbidity and mortality among CKD patients [64]. However, Jamison et al. [65] did not find any benefit in risk reduction either regarding mortality or regarding cardiovascular disease by lowering homocysteine levels in patients with advanced CKD. Moreover, other RCTs showed similar results using folic acid, vitamin B12, and vitamin B6 in CKD and ESRD patients, respectively [66, 67].
Hyperuricemia
Hyperuricemia is a known risk factor for the development and progression of CKD [68]. However, according to the KDIGO guideline there is insufficient evidence to support or refute the use of agents to lower serum uric acid concentrations in people with CKD and either symptomatic or asymptomatic hyperuricemia in order to delay the progression of CKD. Goicoechea et al. [69] treated 113 CKD patients with either allopurinol or usual therapy. After 24 months, the decline in eGFR was lower in the allopurinol group than in the controls. Moreover, the authors concluded that allopurinol decreases C-reactive protein and slows down the progression of renal disease and also reduces the cardiovascular and hospitalization risk in these subjects. Sircar et al. [70] reported that febuxostat slowed the decline in eGFR in asymptomatic hyperuricemic CKD stages 3 and 4 compared to placebo. However, in a more recent study, Saag et al. [71] did not find significant differences in the change in serum creatinine or eGFR levels from baseline between a febuxostat group and a placebo group.
Autosomal Dominant Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease (ADPKD) is responsible for about 10% of ESRD cases. Tolvaptan is an antidiuretic hormone antagonist which was studied for probable benefits on ADPKD. Torres et al. [72] showed that tolvaptan retarded the increase in total kidney volume and slowed the progression of kidney disease in patients with ADPKD, but it was associated with high aquaresis-related adverse effects and higher discontinuation rates. Moreover, in a more recent study, tolvaptan slowed the decline in kidney function in patients with later-stage ADPKD [73]. Gansevoort et al. [74] showed that tolvaptan decreased albuminuria without affecting blood pressure in patients with ADPKD. This beneficial effect remained after withdrawal of the drug. Casteleijn et al. [75] showed that tolvaptan significantly lowered kidney pain. This effect was explained by a decrease in incidence of urinary tract infections, kidney stones, and hematuria. Tolvaptan was approved in 2014 in Japan for slowing the progression of ADPKD in patients with rapid increases in total kidney volume. Recently, tolvaptan has been approved by the FDA to slow the decline in kidney function in adults at risk of rapidly progressing ADPKD [76].
Acute Kidney Injury and Critical Care Nephrology
Acute kidney injury (AKI) is a complex syndrome associated with high morbidity, mortality, health care resource utilization, and risk of long-term consequences [77, 78]. The occurrence of AKI has been linked to a higher risk of incident or progressive CKD [79, 80], ESRD [81], and cardiovascular disease [82, 83, 84]. The incidence of AKI among critically ill patients has been described as high as 50%, and up to 10% of these patients require acute renal replacement therapy (RRT) [77, 85, 86, 87]. Over the last two decades, clinical critical care nephrology research has significantly evolved; however, clinical practice still lags behind. A few examples are briefly described below.
Discovery and Validation of Biomarkers for the Early Prediction of AKI
In September 2014, the FDA released a press note announcing marketing of the NephroCheck test to help identify critically ill patients in the intensive care unit (ICU) who are at risk of developing AKI within 12 h. The NephroCheck test combines assessments of urinary levels of tissue inhibitor of metalloproteinases 2 (TIMP2) and insulin-like growth factor-binding protein 7 (IGFBP7), which are markers of cell cycle arrest. Kashani et al. [88] conducted a prospective multicenter cohort study (SAPPHIRE) to assess a panel of 340 candidate urinary and plasma biomarkers for the early detection of AKI in a mixed ICU population. They found that the product of urinary TIMP2 and IGFBP7 provided the best performance in predicting the occurrence of moderate-to- severe AKI in critically ill patients. These findings were further validated in a separate prospective cohort study (TOPAZ) using clinical assessment of AKI [89]. Since then, several other studies have utilized these biomarkers to predict AKI in distinct settings such as cardiac surgery [90] or for enrichment of enrollment in RCTs of AKI [91]. Although the disillusionment in AKI biomarker research was halted with this discovery, the implementation of these or other biomarkers in the routine care of patients at high risk of AKI remains behind and continues to be an area of intense investigation.
Development of Clinical AKI Risk Prediction Models in the ICU
In recent years, several investigators have developed and validated clinical risk prediction models for AKI. In critically ill children, Basu et al. [92] combined risk criteria (solid organ or stem-cell transplantation, mechanical ventilation, or vasoactive drug support) and injury criteria (change in serum creatinine relative to baseline or fluid overload percentage) assessed 12 h after ICU admission into a score named the renal angina index (RAI). They showed that a RAI ≥8 was independently associated with the occurrence of severe AKI at 72 h. In critically ill adults, Malhotra et al. [93] developed a risk score including 10 clinical parameters for the prediction of AKI in the first 7 days. The AUC for the discovery and external validation cohorts was 0.79 and 0.81, respectively, and the positive predictive value was 23% for a risk score ≥5. Similarly, Flechet et al. [94] developed a tool named AKIpredictor using random forest machine-learning schemes and correlation-based ranking algorithms. This tool incorporates clinical parameters before, at the time of, and during the first day of ICU admission and was developed to predict AKI within the first 7 days following ICU admission. The authors reported that their risk model demonstrated good calibration and it could help clinicians to stratify patients for primary prevention, surveillance, and early therapeutic intervention. However, overall, the proposed clinical models are heterogeneous and need to be further validated in multicenter and multiethnic cohorts. Furthermore, the addition of novel biomarkers, functional testing of renal reserve, and functional imaging studies may improve the performance of these prediction models.
Accelerated versus Standard Initiation of RRT in the ICU
Initial reports from small RCTs showed no difference in mortality when accelerated versus standard RRT initiation strategies were tested [95, 96]. In 2016, reports on two larger RCTs were published and offered contradictory results at first glance. The AKIKI study [97] showed no difference in 60-day mortality and that 49% of the patients did not require RRT in the standard arm. In contrast, the ELAIN study [98] demonstrated a significant difference in 90-day mortality (39% with the accelerated vs. 55% with the standard RRT initiation strategy) and described that only 9% of the patients in the standard arm did not require RRT. Important differences between these studies need to be noted: (1) AKIKI was a multicenter and ELAIN a single-center study; (2) AKIKI enrolled patients with AKI stage 3 and ELAIN enrolled patients with AKI stage 2 + neutrophil gelatinase-associated lipocalin > 150 ng/mL; (3) AKIKI used continuous RRT or intermittent hemodialysis and ELAIN used continuous RRT only; and (4) AKIKI included 80% medical ICU patients and ELAIN included 95% surgical ICU patients. Therefore, one might argue that the study populations and criteria for enrollment were different and no conclusive evidence (or comparison) can be derived from these two studies. Ongoing larger RCTs such as the STARRT-AKI trial will hopefully provide more evidence to support the development of individualized strategies for RRT initiation for patients with AKI in the ICU [99]. Importantly, quality metrics of RRT delivery should also be considered in interventional RRT studies [100]. Furthermore, the implementation of quality management systems for RRT in the ICU is an innovative concept that is rapidly emerging and may affect patient-centered outcomes [101].
Prevention of AKI and Development of AKI Therapeutics
Specific therapies to prevent or attenuate AKI are not available. AKI therapeutics is challenging because interventions sometimes may be only provided after a significant elevation in serum creatinine or deterioration of urine output has been detected. Therefore, AKI therapeutics also involves promoting AKI recovery and halting post-AKI multiorgan complications. Importantly, renoprotective recommendations such as avoidance of nephrotoxic drugs and hyperglycemia, as well as optimization of the hemodynamic status (KDIGO bundle) [102], can prevent AKI in high-risk groups. This was demonstrated in the PrevAKI trial [92], in which patients undergoing cardiac surgery with TIMP2 × IGFBP7 > 0.3 (measured 4 h after cardiopulmonary bypass) were randomized to receive the KDIGO bundle versus standard of care. The investigators showed a significant decrease in the incidence of postoperative AKI when the KDIGO bundle was implemented. More recently, angiotensin II, a newly approved drug for septic or distributive shock (ATHOS-3 trial) [103], showed improved liberation from RRT at day 7 in a post hoc analysis of AKI patients [104]. These findings open an interesting prospect for further research focusing on AKI recovery in critically ill patients with shock that require RRT.
Conclusions
Although there were ground-breaking advancements in the field of clinical nephrology in the 1970s and 1980s, little innovation has occurred in the last few decades, especially when compared with the revolutionary high-tech advancements in other subspecialties. The unsatisfactory progress is mainly due to a lack of therapeutic and technological advancement. The broader kidney research community is united in advocating the CKD Improvement in Research and Treatment Act (H.R. 2644), which would have a positive influence on research in our field. The federal government has also engaged with the private sector in unique partnerships to create innovation and with the American Society of Nephrology (ASN) to accelerate pharmaceutical and nonpharmaceutical developments. Nonetheless, planting these seeds will not be enough, and further cultivation combining collaboration and creativity is required to win the fight against kidney diseases. Still, after this stagnant era, there is hope on the horizon.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 Oct;42((4 Suppl 3)):S1–201. [PubMed] [Google Scholar]
- 2.Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosisevaluation, prevention and treatment of chronic kidney disease-mineral and bone disorders (CKD-MBD) Kidney Int Suppl. 2009;113:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosisevaluation, prevention and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7((1)):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012 Jan;27((1)):345–51. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- 5.Naylor KL, Garg AX, Zou G, Langsetmo L, Leslie WD, Fraser LA, et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol. 2015 Apr;10((4)):646–53. doi: 10.2215/CJN.06040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015 May;30((5)):913–9. doi: 10.1002/jbmr.2406. [DOI] [PubMed] [Google Scholar]
- 7.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, et al. Health, Aging and Body Composition Study Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012 Jul;7((7)):1130–6. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the DiagnosisEvaluation, Prevention and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Am J Kidney Dis. 2017 Dec;70((6)):737–51. doi: 10.1053/j.ajkd.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012 Aug;23((8)):1407–15. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Iorio B, Bellasi A, Russo D, INDEPENDENT Study Investigators Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012 Mar;7((3)):487–93. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 11.Di Iorio B, Molony D, Bell C, Cucciniello E, Bellizzi V, Russo D, et al. INDEPENDENT Study Investigators Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis. 2013 Oct;62((4)):771–8. doi: 10.1053/j.ajkd.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Block GA, Correa-Rotter R, et al. EVOLVE Trial Investigators Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;27((26)):2482–2494. doi: 10.1056/NEJMoa1205624. 367. [DOI] [PubMed] [Google Scholar]
- 13.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;15((7)):674–684. doi: 10.1001/jama.2012.120. 307. [DOI] [PubMed] [Google Scholar]
- 14.Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s) Kidney Int. 2012 Nov;82((9)):952–60. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]
- 15.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. CREATE Investigators Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov;355((20)):2071–84. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 16.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. CHOIR Investigators Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov;355((20)):2085–98. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. TREAT Investigators A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov;361((21)):2019–32. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 18.Papademetriou V, Zaheer M, Doumas M, Lovato L, Applegate WB, Tsioufis C, et al. ACCORD Study Group Cardiovascular Outcomes in Action to Control Cardiovascular Risk in Diabetes: Impact of Blood Pressure Level and Presence of Kidney Disease. Am J Nephrol. 2016;43((4)):271–80. doi: 10.1159/000446122. [DOI] [PubMed] [Google Scholar]
- 19.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al. SPRINT Research Group Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017 Sep;28((9)):2812–23. doi: 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. African American Study of Kidney Disease and Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002 Nov;288((19)):2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. AASK Collaborative Research Group Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010 Sep;363((10)):918–29. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014 Feb;311((5)):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 23.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. CORAL Investigators Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014 Jan;370((1)):13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M, Symplicity HTN-2 Investigators Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010 Dec;376((9756)):1903–9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al. SYMPLICITY HTN-3 Investigators A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014 Apr;370((15)):1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 26.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomizedplacebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011 Aug;58((7)):765–73. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 27.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998 Sep;352((9131)):837–53. [PubMed] [Google Scholar]
- 28.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun;358((24)):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papademetriou V, Lovato L, Doumas M, Nylen E, Mottl A, Cohen RM, et al. ACCORD Study Group Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015 Mar;87((3)):649–59. doi: 10.1038/ki.2014.296. [DOI] [PubMed] [Google Scholar]
- 30.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan;360((2)):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 31.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, et al. ADVANCE Collaborative Group Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013 Mar;83((3)):517–23. doi: 10.1038/ki.2012.401. [DOI] [PubMed] [Google Scholar]
- 32.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. BEAM Study Investigators Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011 Jul;365((4)):327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 33.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. BEACON Trial Investigators Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013 Dec;369((26)):2492–503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nangaku M, Shimazaki R, Akizawa T. Bardoxolone Methyl Improved GFR Measured by Standard Inulin Clearance: The TSUBAKI Study. Abstract from the. 2017 American Society of Nephrology Annual Conference: SA-OR122. https://www.asn-online.org/education/kidneyweek/2017/program-abstract.aspx?controlId=2826315. [Google Scholar]
- 35.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009 Jul;361((1)):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radice A, Trezzi B, Maggiore U, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in PMN. Autoimmun Rev. 2016;15:146–54. doi: 10.1016/j.autrev.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012 Aug;23((8)):1416–25. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hladunewich MA, Cattran D, Beck LH, Odutayo A, Sethi S, Ayalon R, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014 Aug;29((8)):1570–7. doi: 10.1093/ndt/gfu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tumlin J, Galphin C, Santos R, Rovin B. Safety and Efficacy of Combination ACTHar Gel and Tacrolimus in Treatment-Resistant Focal Segmental Glomerulosclerosis and Membranous Glomerulopathy. Kidney Int Rep. 2017 Jun;2((5)):924–32. doi: 10.1016/j.ekir.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al. GEMRITUX Study Group Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J Am Soc Nephrol. 2017 Jan;28((1)):348–58. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fervenza FC, Canetta PA, Barbour SJ, Lafayette RA, Rovin BH, Aslam N, et al. Mentor Consortium group A multicenter randomized controlled trial of rituximab versus cyclosporine in the treatment of idiopathic membranous nephropathy (MENTOR) Nephron. 2015;130((3)):159–68. doi: 10.1159/000430849. [DOI] [PubMed] [Google Scholar]
- 42.Rojas-Rivera J, Fernández-Juárez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, et al. A European multicentre and open-label controlled randomized trial to evaluate the efficacy of Sequential treatment with TAcrolimus-Rituximab versus steroids plus cyclophosphamide in patients with primary MEmbranous Nephropathy: the STARMEN study. Clin Kidney J. 2015 Oct;8((5)):503–10. doi: 10.1093/ckj/sfv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincenti F, Fervenza FC, Campbell KN, Diaz M, Gesualdo L, Nelson P, et al. Focal Segmental Glomerulosclerosis Study Group A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis. Kidney Int Rep. 2017 Apr;2((5)):800–10. doi: 10.1016/j.ekir.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alachkar N, Carter-Monroe N, Reiser J, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;27((13)):1263–1264. doi: 10.1056/NEJMc1400502. 370. [DOI] [PubMed] [Google Scholar]
- 45.Trachtman H, Gipson DS, Somers M, Spino C, Adler S, Holzman L, et al. Randomized Clinical Trial Design to Assess Abatacept in Resistant Nephrotic Syndrome. Kidney Int Rep. 2017 Aug;3((1)):115–21. doi: 10.1016/j.ekir.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. ALMS Group Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011 Nov;365((20)):1886–95. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 47.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Aspreva Lupus Management Study Group Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009 May;20((5)):1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. MAINTAIN Nephritis Trial Group Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010 Dec;69((12)):2083–9. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. LUNAR Investigator Group Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012 Apr;64((4)):1215–26. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 50.Doria A, Stohl W, Schwarting A, Okada M, Scheinberg M, van Vollenhoven R, et al. Efficacy and Safety of Subcutaneous Belimumab in Anti-Double-Stranded DNA-Positive, Hypocomplementemic Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018 Aug;70((8)):1256–64. doi: 10.1002/art.40511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doria A, Bass D, Schwarting A, Hammer A, Gordon D, Scheinberg M, et al. A 6-month open-label extension study of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus. Lupus. 2018 Aug;27((9)):1489–98. doi: 10.1177/0961203318777634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. European Vasculitis Study Group Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007 Jul;18((7)):2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 53.Jones RB, Tervaert JW, Hauser T, et al. European Vasculitis Study Group. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;15((3)):211–220. doi: 10.1056/NEJMoa0909169. 363. [DOI] [PubMed] [Google Scholar]
- 54.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. RAVE-ITN Research Group Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010 Jul;363((3)):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. French Vasculitis Study Group Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014 Nov;371((19)):1771–80. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 56.Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. CLEAR Study Group Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis. J Am Soc Nephrol. 2017 Sep;28((9)):2756–67. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ChemoCentryx A Randomized Double-Blind, Active-Controlled, Phase 3 Study to Evaluate the Safety and Efficacy of CCX168 (Avacopan) in Patients With ANCA-Associated Vasculitis Treated Concomitantly with Rituximab or Cyclophosphamide/ Azathioprine. Available from: https://clinicaltrials.gov/ct2/show/NCT02994927. [Google Scholar]
- 58.Lyon D, Dunlop D, Stewart C. The alkaline treatment of chronic nephritis. Lancet. 1931;218((5645)):1009–13. [Google Scholar]
- 59.Rustom R, Grime JS, Costigan M, Maltby P, Hughes A, Taylor W, et al. Oral sodium bicarbonate reduces proximal renal tubular peptide catabolismammoniogenesis, and tubular damage in renal patients. Ren Fail. 1998 Mar;20((2)):371–82. doi: 10.3109/08860229809045124. [DOI] [PubMed] [Google Scholar]
- 60.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(suppl 1):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 61.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006;28((1)):1–5. doi: 10.1080/08860220500461187. [DOI] [PubMed] [Google Scholar]
- 62.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009 Sep;20((9)):2075–84. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wollesen F, Brattstrom B, Refsum H, et al. Plasma total homocysteine and cystein in relation to glomerular filtration rate. Kidney Int. 1999;55((3)):1028–35. doi: 10.1046/j.1523-1755.1999.0550031028.x. [DOI] [PubMed] [Google Scholar]
- 64.Bayés B, Pastor MC, Bonal J, Romero R. “New” cardiovascular risk factors in patients with chronic kidney disease: role of folic acid treatment. Kidney Int Suppl. 2005 Jan;67((93)):S39–43. doi: 10.1111/j.1523-1755.2005.09309.x. [DOI] [PubMed] [Google Scholar]
- 65.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Veterans Affairs Site Investigators Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007 Sep;298((10)):1163–70. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 66.Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al. HOPE-2 investigators Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease—results of the renal Hope-2 study. Nephrol Dial Transplant. 2008 Feb;23((2)):645–53. doi: 10.1093/ndt/gfm485. [DOI] [PubMed] [Google Scholar]
- 67.Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, Luley C, et al. B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: results of a randomized controlled trial. Circulation. 2010 Mar;121((12)):1432–8. doi: 10.1161/CIRCULATIONAHA.109.904672. [DOI] [PubMed] [Google Scholar]
- 68.Eleftheriadis T, Golphinopoulos S, Pissas G, Stefanidis I. Asymptomatic hyperuricemia and chronic kidney disease: narrative review of a treatment controversial. J Adv Res. 2017 Sep;8((5)):555–60. doi: 10.1016/j.jare.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010 Aug;5((8)):1388–93. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, et al. Efficacy of febuxostat for slowing the gfr decline in patients with ckd and asymptomatic hyperuricemia: A 6-month, double-blind, randomizedplacebo-controlled trial. Am J Kidney Dis. 2015 Dec;66((6)):945–50. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 71.Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of Febuxostat on Renal Function in Gout Patients With Moderate-to-Severe Renal Impairment. Arthritis Rheumatol. 2016 Aug;68((8)):2035–43. doi: 10.1002/art.39654. [DOI] [PubMed] [Google Scholar]
- 72.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. TEMPO 3:4 Trial Investigators Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012 Dec;367((25)):2407–18. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torres VE, Gansevoort RT, Czerwiec FS, et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2018;1((5)):489–490. doi: 10.1056/NEJMc1716478. 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gansevoort RT, Meijer E, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, et al. TEMPO 3:4 Investigators Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 Trial. Nephrol Dial Transplant. 2016 Nov;31((11)):1887–94. doi: 10.1093/ndt/gfv422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casteleijn NF, Blais JD, Chapman AB, Czerwiec FS, Devuyst O, Higashihara E, et al. TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes) 3:4 Trial Investigators Tolvaptan and Kidney Pain in Patients With Autosomal Dominant Polycystic Kidney Disease: Secondary Analysis From a Randomized Controlled Trial. Am J Kidney Dis. 2017 Feb;69((2)):210–9. doi: 10.1053/j.ajkd.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jynarque (tolvaptan) [package insert]. Otsuka Pharmaceutical Development & Commercialization Inc. FDA Application Number 204441. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/204441Orig1s000TOC.cfm
- 77.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008 May;3((3)):844–61. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 78.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010 Feb;21((2)):345–52. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011 Jun;79((12)):1361–9. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012 Mar;81((5)):442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wald R, Quinn RR, Adhikari NK, Burns KE, Friedrich JO, Garg AX, et al. University of Toronto Acute Kidney Injury Research Group Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012 Jun;125((6)):585–93. doi: 10.1016/j.amjmed.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, et al. NSARF Group Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014 Mar;25((3)):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bansal N, Matheny ME, Greevy RA, Jr, Eden SK, Perkins AM, Parr SK, et al. Acute Kidney Injury and Risk of Incident Heart Failure Among US Veterans. Am J Kidney Dis. 2018 Feb;71((2)):236–45. doi: 10.1053/j.ajkd.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 84.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS. Elevated BP after AKI. J Am Soc Nephrol. 2016 Mar;27((3)):914–23. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011 Dec;39((12)):2659–64. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neyra JA, Manllo J, Li X, Jacobsen G, Yee J, Yessayan L, AKICI Study Group Association of de novo dipstick albuminuria with severe acute kidney injury in critically ill septic patients. Nephron Clin Pract. 2014;128((3-4)):373–80. doi: 10.1159/000368902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015 Aug;41((8)):1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 88.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013 Feb;17((1)):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014 Apr;189((8)):932–9. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 90.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014 Mar;9((3)):e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017 Nov;43((11)):1551–61. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Basu RK, Kaddourah A, Goldstein SL, Akcan-Arikan A, Arnold M, Cruz C, et al. AWARE Study Investigators Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentremultinational, prospective observational study. Lancet Child Adolesc Health. 2018 Feb;2((2)):112–20. doi: 10.1016/S2352-4642(17)30181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malhotra R, Kashani KB, Macedo E, Kim J, Bouchard J, Wynn S, et al. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2017 May;32((5)):814–22. doi: 10.1093/ndt/gfx026. [DOI] [PubMed] [Google Scholar]
- 94.Flechet M, Güiza F, Schetz M, Wouters P, Vanhorebeek I, Derese I, et al. AKIpredictor, an online prognostic calculator for acute kidney injury in adult critically ill patients: developmentvalidation and comparison to serum neutrophil gelatinase-associated lipocalin. Intensive Care Med. 2017 Jun;43((6)):764–73. doi: 10.1007/s00134-017-4678-3. [DOI] [PubMed] [Google Scholar]
- 95.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospectiverandomized trial. Crit Care Med. 2002 Oct;30((10)):2205–11. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Wald R, Adhikari NK, Smith OM, Weir MA, Pope K, Cohen A, et al. Canadian Critical Care Trials Group Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015 Oct;88((4)):897–904. doi: 10.1038/ki.2015.184. [DOI] [PubMed] [Google Scholar]
- 97.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. AKIKI Study Group Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016 Jul;375((2)):122–33. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 98.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016 May;315((20)):2190–9. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 99.St. Michael's Hospital Toronto. Standard vs. Accelerated Initiation of RRT in Acute Kidney Injury (STARRT-AKI: Principal Trial) Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02568722&cntry=&state=&city=&dist=.NLM. [Google Scholar]
- 100.Heung M, Bagshaw SM, House AA, Juncos LA, Piazza R, Goldstein SL. CRRTnet: a prospectivemulti-national, observational study of continuous renal replacement therapy practices. BMC Nephrol. 2017 Jul;18((1)):222. doi: 10.1186/s12882-017-0650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neyra JA, Goldstein SL. Optimizing renal replacement therapy deliverables through multidisciplinary work in the intensive care unit. Clin Nephrol. 2018 Jul;90((1)):1–5. doi: 10.5414/CN109447. [DOI] [PubMed] [Google Scholar]
- 102.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 103.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. ATHOS-3 Investigators Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017 Aug;377((5)):419–30. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 104.Tumlin JA, Murugan R, Deane AM, Ostermann M, Busse LW, Ham KR, et al. Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3) Investigators Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II. Crit Care Med. 2018 Jun;46((6)):949–57. doi: 10.1097/CCM.0000000000003092. [DOI] [PMC free article] [PubMed] [Google Scholar]