Abstract
The discovery of highly divergent lineages of hantaviruses (family Hantaviridae) in shrews, moles, and bats of multiple species raises the possibility that non-rodent hosts may have played a significant role in their evolutionary history. To further investigate this prospect, total RNA was extracted from RNAlater®-preserved lung tissues of 277 bats (representing five families, 14 genera and 40 species), captured in Myanmar and Vietnam during 2013–2016. Hantavirus RNA was detected in two of 15 black-bearded tomb bats (Taphozous melanopogon) and two of 26 Pomona roundleaf bats (Hipposideros pomona) in Myanmar, and in three of six ashy leaf-nosed bats (Hipposideros cineraceus) in Vietnam. Pair-wise alignment and comparison of coding regions of the S, M, and L segments of hantaviruses from Taphozous and Hipposideros bats revealed high nucleotide and amino acid sequence similarities to prototype Láibīn virus (LAIV) and Xuân Sơn virus (XSV), respectively. Phylogenetic analyses, generated by maximum-likelihood and Bayesian methods, showed a geographic clustering of LAIV strains from China and Myanmar, but not of XSV strains from China and Vietnam. These findings confirm that the black-bearded tomb bat is the natural reservoir of LAIV, and that more than one species of Hipposideros bats can host XSV.
Keywords: Hantaviridae, Mobatvirus, phylogeny
1. Introduction
Based on the phylogenetic analysis of the full-length S and M segments, members of the genus Hantavirus in the former family Bunyaviridae have been recently reclassified into a new family, designated Hantaviridae, of the order Bunyavirales [1,2]. In this revised taxonomic classification, hantaviruses have been assigned to four newly defined genera: Loanvirus, Mobatvirus, Orthohantavirus, and Thottimvirus [2]. All hantaviruses harbored by rodents, including those associated with hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome, belong to the genus Orthohantavirus, which also comprises nearly all of the genetically distinct hantaviruses recently detected in shrews and moles of multiple species (order Eulipotyphla, family Soricidae and Talpidae) from widely separated geographic regions in Asia, Europe, Africa and/or North America [2,3].
The genus Thottimvirus includes Thottapalayam virus (TPMV) in the Asian house shrew (Suncus murinus) [4,5,6], and Imjin virus (MJNV) in the Ussuri white-toothed shrew (Crocidura lasiura) [7]. Although sequence data are incomplete, Uluguru virus (ULUV) in the geata mouse shrew (Myosorex geata) [8] and Kilimanjaro virus (KMJV) in the Kilimanjaro mouse shrew (Myosorex zinki) [8] from Tanzania are also likely members of the genus Thottimvirus [3].
By contrast, hantaviruses hosted by bats (order Chiropera, suborder Yangochiroptera, and Yinpterochiroptera) have been assigned to the Loanvirus and Mobatvirus genera [2,3]. Members of the genus Loanvirus include Lóngquán virus (LQUV) in the intermediate horseshoe bat (Rhinolophus affinis), Formosan lesser horseshoe bat (Rhinolophus monoceros) and Chinese rufous horseshoe bat (Rhinolophus sinicus) [9] from China, and Brno virus (BRNV) in the common noctule (Nyctalus noctula) [10] from the Czech Republic. Other likely members include the Magboi virus (MGBV) in the hairy slit-faced bat (Nycteris hispida) [11] from Sierra Leone, Mouyassué virus (MOYV) in the banana pipistrelle (Neoromicia nanus) [12,13] from Côte d’Ivoire and in the cape serotine (Neoromicia capensis) [14] from Ethiopia, and Huángpí virus (HUPV) in the Japanese house bat (Pipistrellus abramus) [9] from China.
Members of the genus Mobatvirus include Láibīn virus (LAIV) in the black-bearded tomb bat (Taphozous melanopogon) [15] from China, and Quezon virus (QZNV) in the Geoffroy’s rousette (Rousettus amplexicaudatus) [16] from the Philippines. Other bat-borne hantaviruses which likely belong to the genus Mobatvirus include Xuân Sơn (XSV) virus in the Pomona roundleaf bat (Hipposideros pomona) [13,17] from Vietnam, Makokou virus (MAKV) in the Noack’s roundleaf bat (Hipposideros ruber) [18] from Gabon, and Dakrong virus (DKGV) in the Stoliczka’s Asian trident bat (Aselliscus stoliczkanus) [19] from Vietnam. Nova virus (NVAV), a highly divergent hantavirus harbored by the European mole (Talpa europaea) in Hungary [20], France [21], Poland [22], and Belgium [23], is the only mobatvirus not hosted by a bat species.
The realization that bats of multiple species harbor loanviruses and mobatviruses that are more genetically diverse than orthohantaviruses hosted by rodents and shrews suggests that ancestral bats may have served as the primordial hosts of hantaviruses [3,12,24,25]. Of the 10 bat-borne hantaviruses reported to date, full-length genomes are available for BRNV, DKGV, LAIV, QZNV, and XSV. That said, data about the genetic diversity and phylogeography of loanviruses and mobatviruses are largely unavailable. To address this gap in knowledge, we detected and analyzed hantavirus genomes in lung tissues from bats (representing five families, 14 genera and 40 species), captured in Myanmar and Vietnam.
2. Materials and Methods
2.1. Samples
RNAlater®-preserved lung tissues from 277 bats, of which 121 were captured in Myanmar and 156 in Vietnam during 2013–2016 (Table 1), were analyzed for hantavirus RNA by nested RT-PCR, using previously employed oligonucleotide primers [12,13,16,17,19]. Tested bats were from five families (Emballonuridae, Hipposideridae, Pteropodidae, Rhinolophidae, and Vespertilionidae), 14 genera, and 40 species.
Table 1.
Oligonucleotide primers used to amplify mobatvirus RNA from the lung tissues of bats.
| Genomic Segment | Primer Name | Sequences (5′ to 3′) | Polarity |
|---|---|---|---|
| S | HTS-3R | TAGTAGTAIGCTCCYT | + |
| XSS-147F | CYTWGGRCCTGAACCTGATGA | + | |
| XSS-467R | GCCTTYARSAGGATRACWACAGG | - | |
| S-437F | SWGGTCARACTGCHRAYTGG | + | |
| Cro2R(1126R) | AIGAYTGRTARAAIGAIGAYTTYTT | - | |
| Cro2F(685F) | AGYCCIGTIATGRGWGTIRTYGG | + | |
| XSV-S6R | AGITCIGGRTCCATRTCRTCICC | - | |
| DGS-453R | GTARAAGGRAATGTCASCAGGT | - | |
| DGS-596F | TGTGTCACTTCCTACTGGTCAG | + | |
| DGS-704R | GAGCCTTAGTCTCWGCAGCRT | - | |
| XSS-729R | CCWATIACYCCCATKACWGGRCT | - | |
| SMGS-1079F | ATIATGGCWTCTAAGCTTGTYGG | + | |
| XSS-1245F | CTTGGTGATGAYATGGAYTCWGA | + | |
| XSS-1709R | GCRACTAGTACGTACCTAWAGCGA | - | |
| M | XM-3endR | TAGTAGTAKRCTCCGCARGA | + |
| XSM-435R | TTGCCCAGGTCTGCTCAGCA | - | |
| MKWM-917R | TRTCATGATCTTCICCATRTGG | - | |
| T-M1199F | TAAVTTCAMCAACATGTCT | + | |
| T-M1485R | CCAGCCAAARCARAATGT | - | |
| HTM-1490F | TGTGTICCWGGITTYCATGGIT | + | |
| XDM-2017R | ACICCRTGWGCTGTRTCYTGCCA | - | |
| HTM-2409R | CCACAIGCWGTRCAICCWGT | - | |
| MKWM-2631R | CATGATRTCICCAGGRTCICC | - | |
| XDM-2841F | TIATGTGGKCTGAYCCWGATGG | + | |
| XSM-2959R | CTGAACCCCAWGMICCTTCAAT | - | |
| XDM-3360F | GKWTRTTYCAYGGMAACTGGTGG | + | |
| L | HL-3endR | TAGTAGTAKRCTCCGGA | + |
| PHL-173F | GATWAAGCATGAYTGGTCTGA | + | |
| XAL-948F | CAATMTGAGTATTCMCCWKCTAC | + | |
| XAL-1534F | CAAARTWYTGGTCTGTYCATGC | + | |
| XAL-2137F | AGGWGCIAGTGGWGTKTATCC | + | |
| XSL-2227R | TGCTTCTTCTGTCATWGTICCAYG | - | |
| HAI-L-F1 | ATGTAYGTBAGTGCWGATGC | + | |
| HAI-L-R1 | AACCADTCWGTYCCRTCATC | - | |
| HAI-L-F2 | TGCWGATGCHACIAARTGGTC | + | |
| HAI-L-R2 | GCRTCRTCWGARTGRTGDGCAA | - | |
| DGL-3225F | TACGTGGIAATTGGTTGCARGG | + | |
| XSL-3719F | TRGCTGCTKCWCARASTMGKTGTG | + | |
| XSL-4183R | GTCATAKRCAGGATGCTCWTSTG | - | |
| XSL-4720F | GATATYAGTGACAGRCARGTTATG | + | |
| PHL-5167R | CATAYTGYTTHCCTGAATAWGC | - | |
| HTL-5278F | GTGCAAGSYTAGARATITYYTGGG | + | |
| XDL-5809R | GCAYTAGGRGGRATWGATGCAGG | - | |
| XDL-6088R | GTAGRAAATGCTCWATGTCATC | - |
Abbreviations: A, Adenine; B, C or G or T; C, cytosine; D, A or G or T; G, guanine; H, or C or T; I, inosine; K, G or T; M, A or C; R, A or G; S, G or C; T, thymine; V, A or C or G; W, A or T; Y, C or T.
2.2. Genome Detection and Sequencing
Total RNA was extracted from lung tissues, using the MagDEA RNA 100 Kit (Precision System Science, Matsudo, Japan), and complementary DNA (cDNA) was synthesized, using the PrimeScript II First strand cDNA Synthesis Kit (Takara Bio, Inc., Otsu, Japan) with oligonucleotide primer (OSM55F, 5′–TAGTAGTAGACTCC–3′), designed from the conserved 5′-ends of the S-, M-, and L-segments of hantaviruses [20]. Oligonucleotide primers used to amplify S-, M-, and L-genomic segments of bat-borne hantaviruses are listed on Table 1. First- and second-round PCR reactions were performed in 20 μL reaction mixtures, containing 250 μM dNTPs, 2.5 mM MgCl2, 1 U of Takara LA Taq polymerase Host Start version (Takara Bio, Inc., Ohtsu, Japan), and 0.25 μM of each primer [26]. Initial denaturation at 94 °C for 2 min was followed by two cycles each of denaturation at 94 °C for 30 s, two-degree step-down annealing from 46 °C to 38 °C for 40 s, and elongation at 72 °C for 1 min, then 30 cycles of denaturation at 94 °C for 30 s, annealing at 42 °C for 40 s, and elongation at 72 °C for 1 min, in a Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA) [16,26,27,28,29,30]. PCR products, treated with ExoSAP-IT (Thermo Fisher Science, Waltham, MA, USA) according to the manufacturer’s instruction, were sequenced directly, using an ABI 3730xl DNA Analyzer (Applied Biosystems) [24,25].
2.3. Phylogenetic Analysis
Maximum-likelihood and Bayesian methods, implemented in MrBayes 3.1 [31], under the best-fit GTR + I + Γ model of evolution [32] and jModelTest [33], were used to generate phylogenetic trees. Two replicate Bayesian Metropolis–Hastings Markov chain Monte Carlo runs, each consisting of six chains of 10 million generations sampled every 100 generations with a burn-in of 25,000 (25%), resulted in 150,000 trees overall. The S, M, and L segments were treated separately in phylogenetic analyses. Topologies were evaluated by a bootstrap analysis of 1000 iterations, and posterior node probabilities were based on two million generations, and sample sizes were estimated to be over 100 (implemented in MrBayes). Parameters were re-estimated during successive rounds of maximum-likelihood heuristic searches using the tree bisection reconnection and subtree-pruning–regrafting algorithms implemented in PAUP*.
2.4. Host Identification and Phylogeny
Total DNA was extracted from the lung tissues of bats, using MagDEA DNA 200 Kit (Precision System Science, Matsudo, Japan), and PCR amplification of the cytochrome b (Cyt b) and cytochrome oxidase I (COI) genes of selected bats was performed using primer sets: Cy-14724F (5′–GACYARTRRCATGAAAAAYCAYCGTTGT–3′)/Cy-15909R (5′–CYYCWTYIYTGGTTTACAAGAC YAG–3′) [34] and KOD multi-enzyme (Toyobo, Osaka, Japan), and MammMt-5533F (5′–CYCTGTSYTTRRATTTACAGTYYAA–3′)/MammMt-7159R (5′–GRGGTTCRAWWCCTYCCTYTCT T–3′) and Phusion enzyme (New England Biolabs, Ipswitch, MA, USA), respectively. Initial denaturation was at 95 °C for 2 min, and PCR was performed, using two cycles each of denaturation at 95 °C for 15 s, step-down annealing from 60 °C to 50 °C every two degrees for 30 s, and elongation at 68 °C for 1 min 30 s, then 30 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and elongation at 68 °C for 1 min 30 s, in a Veriti thermal cycler. PCR products were purified by a Mobispin S-400 (Molecular Biotechnology, Lotzzestrasse, Germany), and were sequenced directly. The newly generated sequences were then edited, assembled using ATGC bundled in Genetyx (v. 13) (Genetyx, Shibuya, Tokyo, Japan), and deposited in GenBank under the accession numbers MK410312–MK410432, MK430027–MK430032, and MK462230–MK462234 (Table S4).
To explore the phylogenetic relationships of the hosts of mobatviruses, out-group species were selected, including Macroscelidea (genus Elephantulus), as well as small mammals of the orders Eulipotyphla (families Soricidae and Talpidae) and Rodentia (families Muridae and Cricetidae), which are known to serve as reservoir hosts of hantaviruses (Table S4). Phylogenetic relationships were inferred from sequencing analysis of the entire 1140-nucleotide Cyt b (54 taxa), and the 1545-nucleotide COI (53 taxa) genes of mitochondrial DNA, using respective models of sequence evolution (GTR+I+Γ for Cyt b and HYK+I+Γ for COI), selected with jModelTest v2.1.6 [35]. Posterior probabilities were calculated by using two replicate Markov chain Monte Carlo runs, consisting of six chains of 10 million generations, each sampled every 100 generations, with a burn-in of 25,000 (25%).
3. Results and Discussion
3.1. Virus Detection
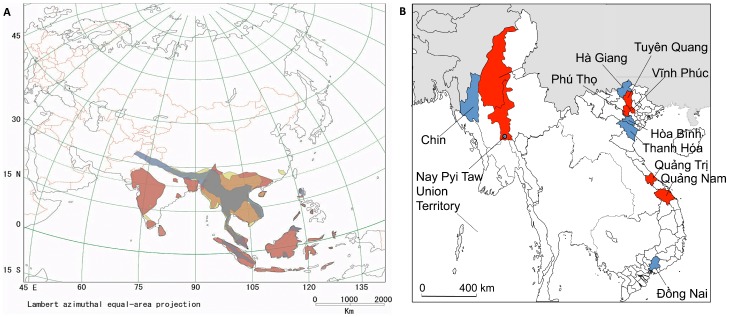
LAIV RNA was detected by nested RT-PCR in two of 15 black-bearded tomb bats captured in Shwe Ba Hill Cave (22.05680555 N, 94.97880555 E) in the Sagaing Region of Myanmar (Table 2 and Figure 1). In addition, XSV RNA was found in one of 25 Pomona roundleaf bats captured in a nearby forest (22.056788 N, 94.978702 E) in the Sagaing Region, and in a single Pomona roundleaf bat trapped near a hotel (19.864542 N, 96.158342 E) in Nay Pyi Taw Union Territory in Myanmar, as well as in three of six ashy leaf-nosed bats (Hipposideros cineraceus) captured in Vietnam: one XSV strain each in Bắc Hướng Hóa Nature Reserve (16.8891 N 106.5705 E) in Hướng Hóa District, Quảng Trị Province, in Xuân Sơn National Park (21.123103 N, 104.960002 E) in Tân Sơn District, Phú Thọ Province, and in Me Linh Station for Biodiversity (21.123103 N, 104.960002 E) in Phúc Yên District, Vĩnh Phúc Province (Table 2 and Figure 2).
Table 2.
RT-PCR detection of mobatvirus RNA in the lung tissues of bats captured in Myanmar and Vietnam.
| Country | Species | Capture Site * | Trap Year | Number Tested | Number Positive † | Mobatvirus Identity § |
|---|---|---|---|---|---|---|
| Myanmar | Taphozous melanopogon | Shwe Ba Hill Cave, Sagaing Region | 2015 | 15 | 2 | LAIV |
| Hipposideros pomona | Nearby forest, Sagaing Region | 2015 | 25 | 1 | XSV | |
| Nay Pyi Taw Union Territory | 2015 | 1 | 1 | XSV | ||
| Vietnam | Hipposideros cineraceus | Bắc Hướng Hóa Nature Reserve, Hướng Hóa District, Quảng Trị Province | 2013 | 2 | 1 | XSV |
| Xuân Sơn National Park, Tân Sơn District, Phú Thọ Province | 2015 | 3 | 1 | XSV | ||
| Me Linh Station, Phúc Yên District, Vĩnh Phúc Province | 2016 | 1 | 1 | XSV |
* Tissues from other bat species captured during the same period were negative for hantavirus RNA by RT-PCR. In Myanmar: 11 greater short-nosed fruit bat (Cynopterus sphinx), two Horsfield’s leaf-nosed bat (Hipposideros larvatus), one great evening bat (Ia io), one painted woolly bat (Kerivoula picta), 22 bent-winged bat (Miniopterus sp.), five intermediate horseshoe bat (Rhinolophus affinis), two woolly horseshoe bat (Rhinolophus luctus), 10 Thomas’s horseshoe bat (Rhinolophus thomasi), 23 lesser Asiatic yellow bat (Scotophilus kuhlii), three long-winged tomb bat (Taphozous longimanus). In Vietnam: four Stoliczka’s Asian trident bat (Aselliscus stoliczkanus), five greater short-nosed fruit bat (Cynopterus sphinx), 13 Horsfield’s leaf-nosed bat (Hipposideros larvatus), two Cantor’s roundleaf bat (Hipposideros galeritus), 13 Pomona roundleaf bat (Hipposideros pomona), one Chinese pipistrelle (Hypsugo pulveratus), one black woolly bat (Kerivoula furva), one painted bat (Kerivoula picta), one long-tongued fruit bat (Macroglossus sorbinus), three Western bent-winged bat (Miniopterus magnater), four tube-nosed bats (Murina feae), two Hutton’s tube-nosed bat (Murina huttoni), seven Scully’s tube-nosed bat (Murina tubinaris), three Himalayan whiskered bat (Myotis siligorensis), one Indochinese mouse-eared bat (Myotis indochinensis), three Chinese water myotis (Myotis laniger), three wall-roosting mouse-eared bat (Myotis muricola), 10 Japanese house bat (Pipistrellus abramus), three Indian pipistrelle (Pipistrellus coromandra), six Java pipistrelle (Pipistrellus javanicus), two least pipistrelle (Pipistrellus tenuis), 15 intermediate horseshoe bat (Rhinolophus affinis), one Con Dao horseshoe bat (Rhinolophus chaseni), 10 least horseshoe bat (Rhinolophus pusillus), one Marshall’s horseshoe bat (Rhinolophus marshalli), three Indo-Chinese lesser brown horseshoe bat (Rhinolophus microglobosus), five Pearson’s horseshoe bat (Rhinolophus pearsonii), six Thai horseshoe bat (Rhinolophus siamensis), seven Chinese rufous horseshoe bat (Rhinolophus sinicus), one lesser brown horseshoe bat (Rhinolophus stheno), three Dobson’s horseshoe bat (Rhinolophus yunanensis), four lesser Asiatic yellow bat (Scotophilus kuhlii), three greater Asiatic yellow bat (Scotophilus heathii), and three bamboo bats (Tylonycteris fulvida). † RT-PCR amplicons were confirmed as mobatvirus by DNA sequencing. § Mobatvirus: LAIV, Láibīn virus; XSV, Xuân Sơn virus.
Figure 1.
Insectivorous bats harboring mobatviruses in Myanmar and Vietnam. The black-bearded tomb bat (Taphozous melanopogon) (family Emballonuridae) hosts Láibīn virus, and the Pomona roundleaf bat (Hipposideros pomona) and ashy leaf-nosed bat (Hipposideros cineraceus) (family Hipposideridae) hosts Xuân Sơn virus.
Figure 2.
(A) Geographic distribution of the black-bearded tomb bat (Taphozous melanopogon) (suborder Yangochiroptera, family Emballonuridae) (colored rust), Pomona roundleaf bat (Hipposideros pomona) (suborder Yinpterochiroptera, family Hipposideridae) (maize), and ashy leaf-nosed bat (Hipposideros cineraceus) (suborder Yinpterochiroptera, family Hipposideridae) (grey). Areas of overlap between the bat species are colored brown. (B) Bats were captured in seven provinces in Vietnam, and three districts in Myanmar (colored red and blue). Capture sites in Vietnam and Myanmar yielding bats infected with Láibīn virus and Xuân Sơn virus are shown in red.
The overly simplistic view that each genetically distinct hantavirus is harbored by a single reservoir host species (with which it co-evolved) is no longer tenable, as evidenced by multiple examples of host sharing, or the hosting of hantaviruses of the same species by more than one closely-related reservoir species [3,24,25]. The demonstration that XSV is harbored by two species of Hipposideros bats serves as another example of host sharing, similar to earlier reported examples, such as MOYV in Neoromicia nanus [12,13] and Neoromicia capensis [14], and LQUV in Rhinolophus affinis, Rhinolophus monoceros and Rhinolophus sinicus [9]. Multiple other examples of host sharing have also been reported for orthohantaviruses harbored by rodents, shrews and moles [3].
Despite employing oligonucleotide primers and PCR cycling conditions used previously to detect MOYV [12,13], XSV [13,17] and QZNV [16], repeated attempts failed to uncover hantavirus RNA in all other bat samples, including lung tissues from bat species previously reported to harbor loanviruses and mobatviruses. That is, tissues from 10 Japanese house bats, 15 intermediate horseshoe bats, seven Chinese rufous horseshoe bats, and four Stoliczka’s Asian trident bats were negative. The reasons for the overall low success rates of detecting mobatvirus or loanvirus RNA in bat tissues may be the highly divergent nature of their genomes, the very focal or localized nature of infection in bats, the small sample sizes, the suboptimal tissue preservation with degraded RNA, and the low virus load. Another possibility may be that bats are less susceptible to mobatvirus or loanvirus infection, or that bats have immune mechanisms to curtail viral replication and/or persistence.
3.2. Sequence Analysis
Pair-wise alignments of the partial and full-length sequences of S, M, and L segments of mobatviruses from Taphozous and Hipposideros bats were compared with prototype LAIV and XSV strains, as well as other LAIV and XSV strains available in GenBank (Table 3). The near-full-length S (1774 nucleotides) and M (3881 nucleotides) segment sequences, and the full-length L (6531 nucleotides) segment sequences of LAIV strains MM4377M17 and MM4378M18 from Myanmar exhibited 96.4–97.2% nucleotide and 99.0–99.7% amino acid sequence similarities to the prototype LAIV strain BT20 from Guǎngxī, China (Tables S1–S3).
Table 3.
Láibīn virus (LAIV) and Xuân Sơn virus (XSV) in insectivorous bats in Myanmar and Vietnam.
| Virus | Strain | Bat Species | Country | Province/Region | S | M | L |
|---|---|---|---|---|---|---|---|
| LAIV | BT20 | Taphozous melanopogon | China | Guǎngxī | 1935 bp | 3908 bp | 6531 bp |
| KM102247 | KM102248 | KM102249 | |||||
| BT33 | Guǎngxī | 1935 bp | 3908 bp | 6531 bp | |||
| KY662264 | KY662265 | KY662266 | |||||
| MM4377M17 * | Myanmar | Sagaing | 1776 bp | 3881 bp | 6531 bp | ||
| MK064114 | MK064115 | MK064116 | |||||
| MM4378M18 * | Sagaing | 1798 bp | 3707 bp | 6531 bp | |||
| MK393932 | MK393933 | MK393934 | |||||
| XSV | VN1982B4 | Hipposideros pomona | Vietnam | Phú Thọ | 1748 bp | 3756 bp | 6520 bp |
| KC688335 | KU976427 | JX912953 | |||||
| F42640 | Tuyên Quang | 516 bp | 567 bp | ||||
| KF704708 | KF704713 | ||||||
| F42682 | Tuyên Quang | 1752 bp | 663 bp | 1160 bp | |||
| KF704709 | KJ000538 | KF704714 | |||||
| F44580 | Quảng Nam | 1728 bp | 804 bp | ||||
| KF704710 | KF704715 | ||||||
| F44583 | Quảng Nam | 1728 bp | 1160 bp | ||||
| KF704711 | KF704716 | ||||||
| F44601 | Quảng Nam | 1728 bp | 663 bp | 1160 bp | |||
| KF704712 | KJ000539 | KF704717 | |||||
| PR15 | China | Yúnnán | 1743 bp | 3583 bp | 6522 bp | ||
| KY662273 | KY662274 | KY662275 | |||||
| Dode puerP36 | Shāndōng | 1702 bp | 2730 bp | 4581 bp | |||
| MG37438 | MG637437 | MG637436 | |||||
| MM4398M38 * | Myanmar | Sagaing | 356 bp | ||||
| MK393935 | |||||||
| MM4425M65 * | Nay Pyi Taw | 356 bp | |||||
| MK393936 | |||||||
| XSV | AR18 | Hipposideros cineraceus | China | Guǎngxī | 1752 bp | 3753 bp | 6521 bp |
| KY662267 | KY662268 | KY662269 | |||||
| AR23 | Guǎngxī | 1753 bp | 3751 bp | 6521 bp | |||
| KY662270 | KY662271 | KY662272 | |||||
| VN2829B3 * | Vietnam | Quảng Trị | 1660 bp | 1754 bp | 6521 bp | ||
| MK393927 | MK393928 | LC406451 | |||||
| VN4201B87 * | Phú Thọ | 1714 bp | 3704 bp | 6521 bp | |||
| MK393929 | MK393930 | MK393931 | |||||
| VN6169VN16-003 * | Vĩnh Phúc | 1740 bp | 782 bp | 3117 bp | |||
| MK393937 | MK393938 | MK393939 |
* Mobatvirus strains from this study. bp, base pairs.
On the other hand, compared to the prototype XSV strain VN1982B4 from Hipposideros pomona in Phú Thọ Province [13,17], the nucleotide sequence similarity among the 14 XSV strains (five new strains and nine previously reported strains) amplified from Hipposideros bats (Table 3) ranged from 79.2–87.6%, 79.7–86.8% and 77.6–85.8% for the S-, M- and L-genomic segments, respectively (Tables S1–S3). At the deduced amino acid levels, the sequence similarity was considerably higher, ranging from 93.6–99.5% for the nucleocapsid protein, 92.9–97.3% for the envelope glycoproteins, and 91.2–99.5% for the RNA-dependent RNA polymerase (Tables S1–S3).
3.3. Phylogenetic Analysis
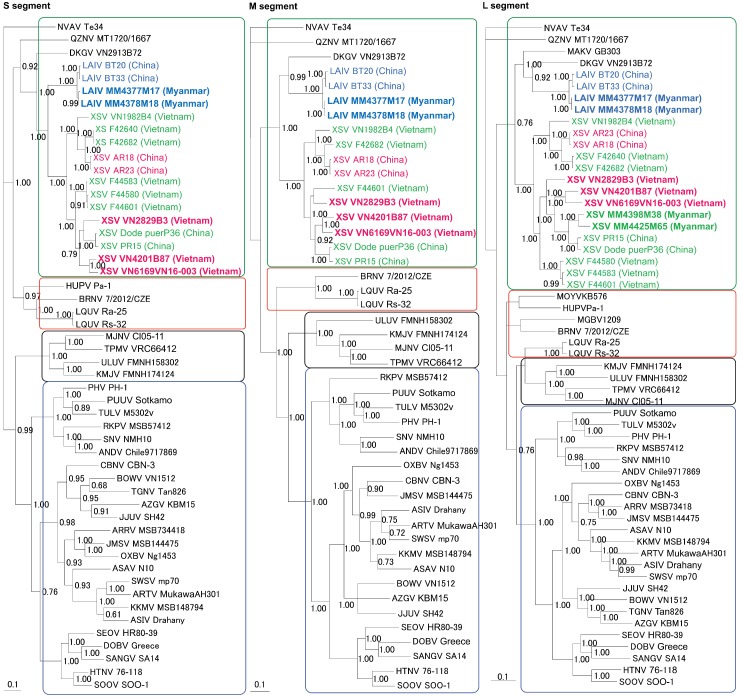
Nearly identical tree topologies, well-supported by posterior node probabilities (>0.70), were generated from an analysis of the S-, M-, and L-segment sequences (Figure 3). Moreover, phylogenetic analyses, using maximum-likelihood and Bayesian methods indicated that LAIV and XSV shared a common ancestry with other mobatviruses (MAKV and DKGV) (Figure 3). The LAIV strains BT20 and BT33 from China, and the LAIV strains MM4377M17 and MM4378M18 from Myanmar formed a monophyletic group, despite the more than 1000-km distance between the trap sites of black bearded tomb bats in China and Myanmar. Nevertheless, LAIV strains from China and Myanmar exhibited geographic clustering, whereas XSV strains from China and Vietnam failed to segregate according to geography or to bat host species.
Figure 3.
Phylogenetic trees, based sequences of the S-, M-, and L-genomic segments, respectively, generated by the Bayesian Markov chain Monte Carlo estimation method, under the GTR + I + Γ model of evolution. Láibīn virus (LAIV) strains BT20 (S: KM102247; M: KM102248; L: KM102249), BT30 (S: KY662264; M: KY662265; L: KY662266), MM4377M17 (S: MK064114; M: MK064115; L: MK064116), and MM4378M18 (S: MK393932; M: MK393933; L: MK393934) from Taphozous melanopogon are shown in blue. Xuân Sơn virus (XSV) strains VN1982B4 (S: KC688335; M: KU976427; L: JX912953), F42640 (S: KF704708; L: KF704713), F42682 (S: KF704709; M: KJ000538; L: KF704714), F44580 (S: KF704710; L: KF704715), F44583 (S: KF704711; L: KF704716), F44601 (S: KF704712; M: KJ000539; L: KF704717), PR15 (S: KY662273; M: KY662274; L: KY662275), Dode puerP36 (S: MG37438; M: MG637437; L: MG637436), MM4398M38 (L: MK393935) and MM4425M65 (L: MK393936) from Hipposideros pomona are shown in green. XSV strains VN2829B3 (S: MK393927; M: MK393928; L: LC406451), VN4201B87 (S: MK393929; M: MK393930; L: MK393931), VN6169VN16-003 (S: MK393937; M: MK393938; L: MK393939), AR18 (S: KY662267; M: KY662268; L: KY662269) and AR23 (S: KY662270; M: KY662271; L: KY662272) from Hipposideros cineraceus are shown in red. LAIV and XSV strains reported in this study are shown in bold text. Also shown are the phylogenetic positions of other bat-borne hantaviruses, including Dakrong virus (DKGV) strain VN2913B72 (S: MG663536; M: MG663535; L: MG663534) from Aselliscus stoliczkanus, Magboi virus (MGBV) strain 1209 (L: JN037851) from Nycteris hispida, Mouyassué virus (MOYV) strains KB576 (L: JQ287716) and KB577 (L: KJ000540) from Neoromicia nanus, Huángpí virus (HUPV) strain Pa-1 (S: JX473273; L: JX465369) from Pipistrellus abramus, Brno virus (BRNV) strains 7/2012 (S: KX845678; M: KX845679; L: KX845680) and 11/2013 (L: KR920360) from Nyctalus noctula, Lóngquán virus (LQUV) strains Ra25 (S: JX465415; M: JX465397) from Rhinolophus affinis, and LQUV Rs32 (S: JX465422; M: JX465402; L: JX465388) from Rhinolophus sinicus, Makokou virus (MAKV) strain GB303 (L: KT316176) from Hipposideros ruber, and Quezon virus (QZNV) strain MT1720/1657 (S: KU950713; M: KU950714; L: KU950715) from Rousettus amplexicaudatus. Shrew-borne hantaviruses include Cao Bằng virus (CBNV) strain CBN-3 (S: EF543524; M: EF543526; L: EF543525) from Anourosorex squamipes, Ash River virus (ARRV) strain MSB734418 (S: EF650086; L: EF619961) from Sorex cinereus, Jemez Springs virus (JMSV) strain MSB144475 (S: FJ593499; M: FJ593500; L: FJ593501) from Sorex monticolus, Seewis virus (SWSV) strain mp70 (S: EF636024; M: EF636025; L: EF636026) from Sorex araneus, Artybash virus (ARTV) strain AH301 (S: KF974360; M: KF974359; L: KF974361) from Sorex caecutiens, Kenkeme virus (KKMV) strain MSB148794 (S: GQ306148; M: GQ306149; L: GQ306150) from Sorex roboratus, and Asikkala virus (ASIV) strain Drahany (S: KC880342; M: KC880345; L: KC880348) from Sorex minutus, as well as Thottapalayam virus (TPMV) strain VRC66412 (S: AY526097; M: NC_010708; L: EU001330) from Suncus murinus, Imjin virus (MJNV) strain Cl05-11 (S: EF641804; M: EF641798; L: EF641806) from Crocidura lasiura, Azagny virus (AZGV) strain KBM15 (S: JF276226; M: JF276227; L: JF276228) from Crocidura obscurior, Tanganya virus (TGNV) strain Tan826 (S: EF050455; L: EF050454) from Crocidura theresea, Bowé virus (BOWV) strain VN1512 (S: KC631782; M: KC631783; L: KC631784) from Crocidura douceti, Jeju virus (JJUV) strain SH42 (S: HQ663933; M: HQ663934; L: HQ663935) from Crocidura shantungensis, Uluguru virus (ULUV) strain FMNH158302 (S: JX193695; M: JX193696; L: JX193697) from Myosorex geata, and Kilimanjaro virus (KMJV) strain FMNH174124 (S: JX193698; M: JX193699; L: JX193700) from Myosorex zinki. Mole-borne orthohantaviruses include Asama virus (ASAV) strain N10 (S: EU929072; M: EU929075; L: EU929078) from Urotrichus talpoides, Oxbow virus (OXBV) strain Ng1453 (S: FJ5339166; M: FJ539167; L: FJ593497) from Neurotrichus gibbsii, and Rockport virus (RKPV) strain MSB57412 (S: HM015223; M: HM015222; L: HM015221) from Scalopus aquaticus. The single non-bat-borne mobatvirus, Nova virus (NVAV) strain Te34 (S: KR072621; M: KR072622; L: KR072623) from Talpa europaea, is also included. Rodent-borne orthohantaviruses include Sin Nombre virus (SNV) strain NMH10 (S: NC_005216; M: NC_005215; L: NC_005217), Andes virus (ANDV) strain Chile9717869 (S: AF291702; M: AF291703; L: AF291704), Prospect Hill virus (PHV) stain PH-1 (S: Z49098; M: X55129; L: EF646763), Tula virus (TULV) strain M5302v (S: NC_005227; M: NC_005228; L: NC_005226), Puumala virus (PUUV) strain Sotkamo (S: NC_005224; M: NC_005223; L: NC_005225), Dobrava virus (DOBV) strain Greece (S: NC_005233; M: NC_005234; L: NC_005235), Hantaan virus (HTNV) strain 76-118 (S: NC_005218; M: NC_005219; L: NC_005222), Soochong virus (SOOV) strain SOO-1 (S: AY675349; M: AY675353; L: DQ056292), Sangassou virus (SANGV) strain SA14 (S: JQ082300; M: JQ082301; L: JQ082302), Tigray virus (TIGV) strain ET2121 (S: KU934010; M: KU934009; L: KU934008), and Seoul virus (SEOV) strain 80-39 (S: NC_005236; M: NC_005237; L: NC_005238). The numbers at each node are Bayesian posterior probabilities (>0.7) based on 150,000 trees: two replicate Markov chain Monte Carlo runs, consisting of six chains of 10 million generations, each sampled every 100 generations with a burn-in of 25,000 (25%). The scale bars indicate nucleotide substitutions per site.
Also, NVAV from the European mole segregated with the bat-associated mobatviruses, which is reminiscent of trees based on the complete mitochondrial genomes of the European mole and bats [36,37]. The basal position of chiropteran-borne mobatviruses in phylogenetic trees suggests that bats, rather than rodents, may have been the primordial mammalian hosts of ancestral hantaviruses (Figure 3).
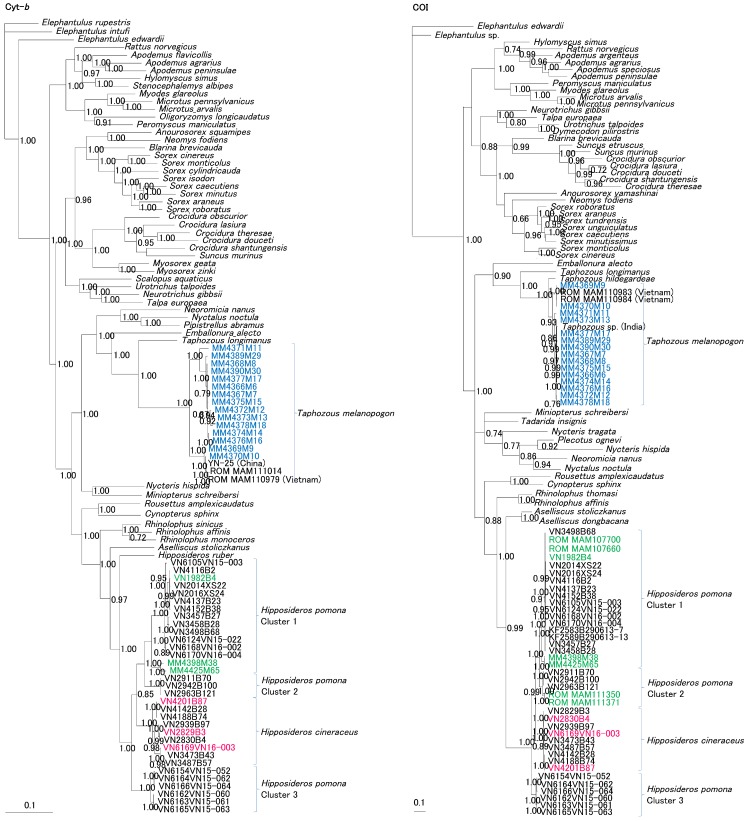
The molecular identification of LAIV- and XSV-infected bats was confirmed by the amplification and sequencing of the Cyt b and COI genes of mitochondrial DNA. By phylogenetic analysis, Pomona roundleaf bats and ashy leaf-nosed bats formed a species complex comprising four closely related clusters, suggestive of local co-circulation in Vietnam (Figure 4).
Figure 4.
Bayesian phylogenetic trees of the host organisms of mobatviruses reconstructed from the alignments of 1140-nucleotide cytochrome b (Cyt b) and 1545-nucleotide cytochrome oxidase I (COI) gene sequences. Taphozous melanopogon collected in Myanmar (blue), XSV-positive Hipposideros pomona (green) in Myanmar and XSV-positive Hipposideros cineraceus (red) in Vietnam are shown. Numbers at the nodes indicate posterior probability values (>0.7) based on 150,000 trees: two replicate Markov chain Monte Carlo runs, consisting of six chains of 10 million generations, each sampled every 100 generations, with a burn-in of 25,000 (25%). Scale bars indicate nucleotide substitutions per site. Gene accession numbers are listed in Table S4.
4. Conclusions
Second only to rodents (order Rodentia), bats (order Chiroptera) represent the most species-rich mammalian order, with nearly 1400 species distributed worldwide, except in the frigid polar regions [38,39]. Bats are notorious for hosting many medically important microbial pathogens, and their ability of self-powered flight, longevity and social structures contribute to their role in the transmission of zoonotic diseases [40,41,42].
Formerly divided into the Megachiroptera and Microchiroptera suborders, a new taxonomic nomenclature has been proposed, in which the suborder Yinpterochiroptera comprises megabats or flying foxes (family Pteropodidae), and bats of five microbat families (Craseonycteridae, Hipposideridae, Megadermatidae, Rhinolophidae and Rhinopomatidae), and the suborder Yangochiroptera comprises the remaining microbat families [36].
Thus far, only one hantavirus (QZNV) has been found in a flying fox [16], and four hantaviruses (DKGV, LQUV, MAKV, XSV) have been detected hitherto in bats belonging to the Hipposideridae and Rhinolophidae families [9,13,17,18,19] of the suborder Yinpterochiroptera, and five hantaviruses (BRNV, HUPV, LAIV, MGBV, MOYV) have been detected to date in bats belonging to the Emballonuridae, Nycteridae, and Vespertilionidae families [9,10,11,12,13,14,15] of the suborder Yangochiroptera. Thus, irrespective of the classification, bat species in both suborders have been found to host viruses in the newly created genera of Loanvirus and Mobatvirus, suggesting that primordial hantaviruses may have emerged in an early common ancestor of bats or other members of the Laurasiatheria superorder, such as shrews and moles [3,12,13,16,20]. A similar conclusion was reached recently from an ancestral state reconstruction analysis of MAKV in the Noack’s roundleaf bat in Gabon [18].
Phylogeographic studies of LAIV and XSV throughout the vast geographic range of the black-bearded tomb bat, Pomona roundleaf bat, and ashy leaf-nosed bat are necessary to obtain additional insights into the biogeographic origin and radiation of mobatviruses and their chiropteran hosts, as well as to clarify whether other bat species harbor LAIV- or XSV-related mobatviruses in Asia.
Acknowledgments
We thank Hitoshi Suzuki, Shinichiro Kawada, and Dai Fukui for supporting field investigations, and for helpful suggestions. We also thank Yu Ikeyama for technical assistance. This work was supported in part by a grant-in-aid on Research Program on Emerging and Re-emerging Infectious Diseases, Japan Agency for Medical Research and Development (AMED) (JP15fk0108005, JP16fk0108117, JP17fk0108217 and JP18fk0108017), a grant-in aid from the Japan Society for the Promotion of Science 24405045 (Scientific Research grant B), a grant-in-aid on NAFOSTED (106-NN.05-2016.14), and VAST-JSPS (QTJP01.02/18-20), and a grant (P30GM114737) from the U.S. National Institutes of Health.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/11/3/228/s1, Table S1: Nucleotide and amino acid sequence similarities of the S segment of newfound mobatviruses in Myanmar and Vietnam, Table S2: Nucleotide and amino acid sequence similarities of the M segment of newfound mobatviruses in Myanmar and Vietnam, Table S3: Nucleotide and amino acid sequence similarities of the L segment of newfound mobatviruses in Myanmar and Vietnam, Table S4: Gene accession numbers of cytochrome b (Cyt b) and cytochrome oxidase subunit 1 (COI) sequences.
Author Contributions
Conceived and designed the experiments: S.A., K.T.-T., S.M., K.O., R.Y., Collected samples: S.A., S.B., N.T.S., K.S.L., V.T.T., K.T., Contributed reagents/materials/analysis tools: S.A., F.K., V.T.T., K.A., R.Y., Performed the experiments: S.A., F.K., K.A., Analyzed the data: S.A., F.K., V.T.T., R.Y., Wrote the paper: S.A., F.K., S.B., N.T.S., K.S.L., V.T.T., K.A., K.T.-T., S.M., K.O., R.Y.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Adams M.J., Lefkowitz E.J., King A.M.Q., Harrach B., Harrison R.L., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Mushegian A.R., et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2017;162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 2.Maes P., Adkins S., Alkhovsky S.V., Avšič-Županc T., Ballinger M.J., Bente D.A., Beer M., Bergeron E., Blair C.D., Briese T., et al. Taxonomy of the order Bunyavirales: Second update 2018. Arch. Virol. 2019 doi: 10.1007/s00705-018-04127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai S., Yanagihara R. Genetic diversity and geographic distribution of bat-borne hantaviruses. In: Corrales-Aguilar E., Schwemmle M., editors. Bat-Borne Viruses. Caister Academic Press; Poole, UK: 2019. in press. [DOI] [PubMed] [Google Scholar]
- 4.Carey D.E., Reuben R., Panicker K.N., Shope R.E., Myers R.M. Thottapalayam virus: A presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 1971;59:1758–1760. [PubMed] [Google Scholar]
- 5.Song J.-W., Baek L.J., Schmaljohn C.S., Yanagihara R. Thottapalayam virus, a prototype shrewborne hantavirus. Emerg. Infect. Dis. 2007;13:980–985. doi: 10.3201/eid1307.070031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang H.J., Kosoy M.Y., Shrestha S.K., Shrestha M.P., Pavlin J.A., Gibbons R.V., Yanagihara R. Genetic diversity of Thottapalayam virus, a hantavirus harbored by the Asian house shrew (Suncus murinus) in Nepal. Am. J. Trop. Med. Hyg. 2011;85:540–545. doi: 10.4269/ajtmh.2011.11-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J.-W., Kang H.J., Gu S.H., Moon S.S., Bennett S.N., Song K.-J., Baek L.J., Kim H.C., O’Guinn M.L., Chong S.T., et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura) J. Virol. 2009;83:6184–6191. doi: 10.1128/JVI.00371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang H.J., Stanley W.T., Esselstyn J.A., Gu S.H., Yanagihara R. Expanded host diversity and geographic distribution of hantaviruses in sub-Saharan Africa. J. Virol. 2014;88:7663–7667. doi: 10.1128/JVI.00285-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W.P., Lin X.D., Wang W., Tian J.H., Cong M.L., Zhang H.L., Wang M.R., Zhou R.H., Wang J.B., Li M.H., et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013;9:e1003159. doi: 10.1371/journal.ppat.1003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straková P., Dufkova L., Širmarová J., Salát J., Bartonička T., Klempa B., Pfaff F., Höper D., Hoffmann B., Ulrich R.G., et al. Novel hantavirus identified in European bat species Nyctalus noctula. Infect. Genet. Evol. 2017;48:127–130. doi: 10.1016/j.meegid.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss S., Witkowski P.T., Auste B., Nowak K., Weber N., Fahr J., Mombouli J.V., Wolfe N.D., Drexler J.F., Drosten C., et al. Hantavirus in bat, Sierra Leone. Emerg. Infect. Dis. 2012;18:159–161. doi: 10.3201/eid1801.111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumibcay L., Kadjo B., Gu S.H., Kang H.J., Lim B.K., Cook J.A., Song J.-W., Yanagihara R. Divergent lineage of a novel hantavirus in the banana pipistrelle (Neoromicia nanus) in Côte d’Ivoire. Virol. J. 2012;9:34. doi: 10.1186/1743-422X-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu S.H., Lim B.K., Kadjo B., Arai S., Kim J.A., Nicolas V., Lalis A., Denys C., Cook J.A., Dominguez S.R. Molecular phylogeny of hantaviruses harbored by insectivorous bats in Côte d’Ivoire and Vietnam. Viruses. 2014;6:1897–1910. doi: 10.3390/v6051897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Těšíková J., Bryjová A., Bryja J., Lavrenchenko L.A., Goüy de Bellocq J. Hantavirus strains in East Africa related to Western African hantaviruses. Vector Borne Zoonotic Dis. 2017;17:278–280. doi: 10.1089/vbz.2016.2022. [DOI] [PubMed] [Google Scholar]
- 15.Xu L., Wu J., He B., Qin S., Xia L., Qin M., Li N., Tu C. Novel hantavirus identified in black-bearded tomb bats, China. Infect. Genet. Evol. 2015;31:158–160. doi: 10.1016/j.meegid.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai S., Taniguchi S., Aoki K., Yoshikawa Y., Kyuwa S., Tanaka-Taya K., Masangkay J.S., Omatsu T., Puentespina R., Jr., Watanabe S., et al. Molecular phylogeny of a genetically divergent hantavirus harbored by the Geoffroy’s rousette (Rousettus amplexicaudatus), a frugivorous bat species in the Philippines. Infect. Genet. Evol. 2016;45:26–32. doi: 10.1016/j.meegid.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Arai S., Nguyễn S.T., Boldgiv B., Fukui D., Araki K., Dang C.N., Ohdachi S.D., Nguyễn N.X., Pham T.D., Boldbaatar B., et al. Novel bat-borne hantavirus, Vietnam. Emerg. Infect. Dis. 2013;19:1159–1161. doi: 10.3201/eid1907.121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witkowski P.T., Drexler J.F., Kallies R., Lickova M., Bokorova S., Mananga G.D., Szemes T., Leroy E.M., Krüger D.H., Drosten C., et al. Phylogenetic analysis of a newfound bat-borne hantavirus supports a laurasiatherian host association for ancestral mammalian hantaviruses. Infect. Genet. Evol. 2016;41:113–119. doi: 10.1016/j.meegid.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Arai S., Aoki K., Nguyễn S.T., Vương T.T., Kikuchi F., Kinoshita G., Fukui D., Hoàng T.T., Gu S.H., Yoshikawa Y., et al. Dakrong virus, a novel hantavirus harbored by the Stoliczka’s Asian trident bat (Aselliscus stoliczkanus) in Vietnam. Sci. Rep. 2019 doi: 10.1038/s41598-019-46697-5. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang H.J., Bennett S.N., Sumibcay L., Arai S., Hope A.G., Mocz G., Song J.-W., Cook J.A., Yanagihara R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea) PLoS ONE. 2009;4:e6149. doi: 10.1371/journal.pone.0006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu S.H., Dormion J., Hugot J.-P., Yanagihara R. High prevalence of Nova hantavirus infection in the European mole (Talpa europaea) in France. Epidemiol. Infect. 2014;142:1167–1171. doi: 10.1017/S0950268813002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu S.H., Hejduk J., Markowski J., Kang H.J., Markowski M., Połatyńska M., Sikorska B., Liberski P.P., Yanagihara R. Co-circulation of soricid- and talpid-borne hantaviruses in Poland. Infect. Genet. Evol. 2014;28:296–303. doi: 10.1016/j.meegid.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laenen L., Vergote V., Kafetzopoulou L.E., Bokalinga T.W., Vassou D., Cook J.A., Hugot J.P., Deboutte W., Kang H.J., Witkowski P.T., et al. A novel hantavirus of the European mole, Bruges virus, is involved in frequent Nova virus co-infections. Genome Biol. Evol. 2018;10:45–55. doi: 10.1093/gbe/evx268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett S.N., Gu S.H., Kang H.J., Arai S., Yanagihara R. Reconstructing the evolutionary origins and phylogeography of hantaviruses. Trends Microbiol. 2014;22:473–482. doi: 10.1016/j.tim.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagihara R., Gu S.H., Arai S., Kang H.J., Song J.-W. Hantaviruses: Rediscovery and new beginnings. Virus Res. 2014;187:6–14. doi: 10.1016/j.virusres.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu S.H., Nicolas V., Lalis A., Sathirapongsasuti N., Yanagihara R. Complete genome sequence and molecular phylogeny of a newfound hantavirus harbored by the Doucet’s musk shrew (Crocidura douceti) in Guinea. Infect. Genet. Evol. 2013;20:118–123. doi: 10.1016/j.meegid.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J.-W., Kang H.J., Song K.-J., Truong T.T., Bennett S.N., Arai S., Truong N.U., Yanagihara R. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg. Infect. Dis. 2007;13:1784–1787. doi: 10.3201/eid1311.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai S., Gu S.H., Baek L.J., Tabara K., Bennett S.N., Oh H.S., Takada N., Kang H.J., Tanaka-Taya K., Morikawa S., et al. Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology. 2012;424:99–105. doi: 10.1016/j.virol.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai S., Ohdachi S.D., Asakawa M., Kang H.J., Mocz G., Arikawa J., Okabe N., Yanagihara R. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides) Proc. Natl. Acad. Sci. USA. 2008;105:16296–16301. doi: 10.1073/pnas.0808942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang H.J., Bennett S.N., Dizney L., Sumibcay L., Arai S., Ruedas L.A., Song J.-W., Yanagihara R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii) Virology. 2009;388:8–14. doi: 10.1016/j.virol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 32.Posada D., Crandall K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 33.Posada D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 34.Arai S., Kang H.J., Gu S.H., Ohdachi S.D., Cook J.A., Yashina L.N., Tanaka-Taya K., Abramov S.A., Morikawa S., Okabe N., et al. Genetic diversity of Artybash virus in the Laxmann’s shrew (Sorex caecutiens) Vector Borne Zoonotic Dis. 2016;16:468–475. doi: 10.1089/vbz.2015.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouchaty S.K., Gullberg A., Janke A., Arnason U. The phylogenetic position of the Talpidae within eutheria based on analysis of complete mitochondrial sequences. Mol. Biol. Evol. 2000;17:60–67. doi: 10.1093/oxfordjournals.molbev.a026238. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y.H., Penny D. Implications for bat evolution from two new complete mitochondrial genomes. Mol. Biol. Evol. 2001;18:684–688. doi: 10.1093/oxfordjournals.molbev.a003850. [DOI] [PubMed] [Google Scholar]
- 38.Teeling E.C. Bats (Chiroptera) In: Hedges S.B., Kumar S., editors. The Timetree of Life. Oxford University Press; Oxford, UK: 2009. pp. 499–503. [Google Scholar]
- 39.Burgin C.J., Colella J.P., Kahn P.L., Upham N.S. How many species of mammals are there? J. Mammal. 2018;99:1–14. doi: 10.1093/jmammal/gyx147. [DOI] [Google Scholar]
- 40.Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett A.J., Bushmaker T., Cameron K., Ondzie A., Niama F.R., Parra H.J., Mombouli J.V., Olson S.H., Munster V.J., Goldberg T.L. Diverse RNA viruses of arthropod origin in the blood of fruit bats suggest a link between bat and arthropod viromes. Virology. 2018;528:64–72. doi: 10.1016/j.virol.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L.F., Anderson D.E. Viruses in bats and potential spillover to animals and humans. Curr. Opin. Virol. 2019;34:79–89. doi: 10.1016/j.coviro.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.