Abstract
In this study, the concentration of fluoride and the associated health risks for infants, children, and adults were analyzed and compared for three drinking water sources in Yancheng City, Jiangsu Province, China. To analyze the relationship between the water quality parameters of pH, fluoride (F−), sulfate (SO42−), chloride (Cl−), total dissolved solids (TDS), total alkalinity (TAlk), sodium (Na+), and potassium (K+), statistical analyses including correlation analysis, R-mode cluster analysis and factor analysis were performed based on monthly data from the year 2010 to 2015. The results indicated: (1) Fluoride concentrations in the drinking water sources ranged from 0.38 to 1.00 mg L−1 (mean = 0.57 mg L−1) following the order of Tongyu River > Yanlong Lake > Mangshe River; (2) fluoride concentrations in 22.93% of the collected samples were lower than 0.5 mg L−1, which has the risk of tooth cavities, especially for the Mangshe River; (3) the fluoride exposure levels of infants were higher than children and adults, and 3.2% of the fluoride exposure levels of infants were higher than the recommended toxicity reference value of 122 μg kg−1 d−1 as referenced by Health Canada, which might cause dental fluorosis issues; (4) the physico-chemical characteristics are classified the into four groups reflecting F−- TAlk, Na+-K+, SO42−-Cl−, and pH-TDS, respectively, indicating that fluoride solubility in drinking water is TAlk dependent, which is also verified by R-mode cluster analysis and factor analysis. The results obtained supply useful information for the health department in Yancheng City, encouraging them to pay more attention to fluoride concentration and TAlk in drinking water sources.
Keywords: fluoride content, health risk assessment, correlation analysis, cluster analysis, factor analysis, physicochemical characteristics
1. Introduction
Fluoride (F−) enters the water body as a result of the geological composition of bedrock and soil, as well as dental products, food, combustion of fluoride-rich coal, pharmaceuticals, and pesticides. It is generally accepted that human beings consume fluoride largely from drinking water, and about 90% of fluoride that exists in drinking water is absorbed by the digestive system [1,2,3,4]. Fluoride deficiency or excess has an adverse effect on human health. Much work had been carried out on the effects of high fluoride concentration in drinking water, which may cause skeletal issues on both bones and teeth, especially for children and pregnant women [3,4,5,6,7,8]. Since fluoride concentration beyond 4 mg L−1 may induce skeletal fluorosis, and beyond 10 mg L−1 may cause crippling skeletal fluorosis, the U.S. Environmental Protection Agency (EPA) has set the maximum contaminant level (MCL) for fluoride in drinking water as 4 mg L−1, and the secondary maximum contaminant level (SMCL) as 2 mg L−1. According to Chinese sanitary standards, the fluoride content for drinking water sources is 1.0 mg L−1 (MHPRC, 2006). Although the lower limit of fluoride content in water sources is not specified, fluoride content of lower than 0.5 mg L−1 will increase the risk of dental caries, affect the formation of dental enamel, as well as decrease the mineralization of bones, especially for children who are in the body growth stage [9,10,11,12]. Since the 1940s, fluoridation schemes were introduced into the United States, England, and Wales with the objective of improving dental health, which decreased the proportion of decayed, missing and filled teeth in children significantly. Additionally, fluoridated toothpaste and fluoride-containing mouthwash can also contribute to fluoride intake in children [2,13]. The World Health Organization recommends F− concentrations in drinking water which range from 0.5 mg L−1 to 1.5 mg L−1 for tooth deterioration prevention. The US Public Health Service (USPHS) recently recommend optimal fluoride concentrations of 0.7 mg L−1 in community water systems, which can prevent dental caries but also lower the risk of dental fluorosis [12,14]. Since low and high contents of fluoride in drinking water both pose threats on human health, it is of great importance to evaluate the F− content in drinking water sources accurately [15,16].
This study aimed to analyze and compare fluoride content and the associated health risks for infants, children, and adults in three drinking water sources of Yancheng City: the Tongyu River, Yanlong Lake, and Mangshe River. Moreover, the relationship between the other physicochemical parameters of pH, fluoride (F−), sulfate (SO42−), chloride (Cl−), total dissolved solids (TDS), total alkalinity (TAlk), sodium (Na+), and potassium (K+) were investigated, summarized, and analyzed by multivariate statistical analysis method.
2. Materials and Methods
2.1. Study Area
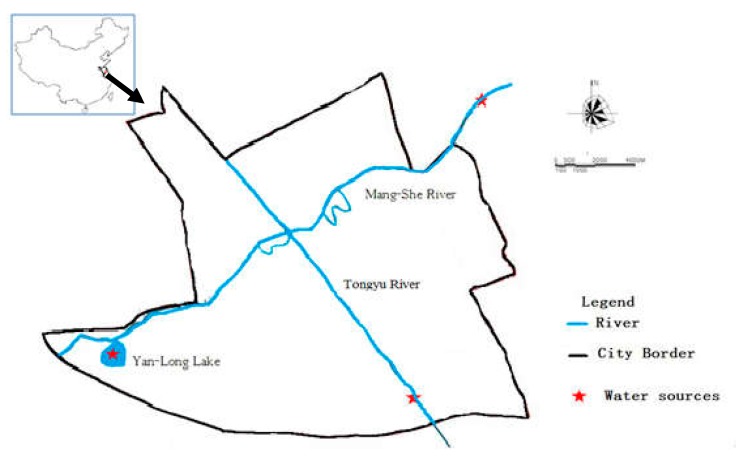
Yancheng (119°34′–120°27′ E, 32°51′–34°12′ N) is an eastern coastal city in the center of the Jiangsu province of China with a population of over 8 million. It faces the Yellow Sea on the east, and is to the west of Yangzhou City and Huai’an City, the north of Lianyungang City, and the south of Nantong and Taizhou cities. The research area is 48.54 km2, shown in Figure 1, with three drinking water sources: the Tongyu River, Mangshe River, and Yanlong Lake. The Tongyu River has a total length of 415 km, originating from the Changjiang River, and ends its course in Lianyungang City. The middle reach of the Tongyu River runs through Yancheng City with a length of 183.6 km, and has an average flow of approximately 100 m3/s. The Mangshe River originates from Dazong Lake and discharges into the East Sea with a total length of nearly 50 km. The Tongyu River and the Mangshe River serve the complex functions of drinking water sources, fairways, irrigation water sources, and flood drainage system. The Yanlong Lake is a man-made ecological lake built in 2012 occupying 2.23 km2 with pretreatment area, ecological wetland purification area, and deep purification area, which also serves as a drinking water source for Yancheng City [17]. Up to now, several water pollution events have occurred in the drinking water sources of Yancheng City. In 2009, sewage from chemical plants with volatile phenolic compounds were illegally drained upstream of water sources, resulting in the cutting off of the water supply for as long as 66 hours, which severely affected the life of nearly 200,000 inhabitants as well as industrial processes. Therefore, the water sources of Yancheng city should be monitored closely and paid more attention to, especially as regards the water quality parameters related to human health.
Figure 1.
The drinking water sources in Yancheng City.
2.2. Samples and Measurements
One hundred and fifty-six samples, including 60 samples in the Tongyu River from January, 2010 to December, 2015, 54 samples in the Mangshe River from July, 2011 to Decemeber, 2015, and 42 samples in Yanlong Lake from July, 2012 to December, 2015, were collected during the study. The GPS coordination of sample sites are (120°02′6″ E, 33°32′1″ N), (120°04′39″ E, 33°21′56″ N), and (120°01′36″ E, 33°19′59″ N) for the Tongyu River, Mangshe River, and Yanlong Lake, respectively. Parameters of pH, fluoride (F−), sulfate (SO42−), chloride (Cl−), total dissolved solids (TDS), total alkalinity (TAlk), sodium (Na+) and potassium (K+) were analyzed using the standard water analysis methods (seen in Table 1). The selected parameters of pH, SO42−, Cl−, TDS, Na+, and K+ were reported to be related to fluoride concentration [5,18,19], and parameters of Ca2+ and Mg2+ had a minor relationship with fluoride concentration [19]. Moreover, TDS represents the sum of cations and anions as the principal inorganic constituents. The parameters were measured in accordance with the standard methods.
Table 1.
Limit specified of physicochemical parameters in drinking water sources (CJ3020-93).
| Parameters | Limit | Analytical Methods | Detection Criteria |
|---|---|---|---|
| Potassium (K+) | N/A | Flame photometer | (MHPRC, GB/T 5750.6-2006) |
| Sodium (Na+) | N/A | Flame photometer | (MHPRC, GB/T 5750.6-2006) |
| Fluoride (F−) | 1.0 | Ion selective electrode | (MHPRC, GB/T 5750.5-2006) |
| pH | 6.5–8.5 | pH-meter | (MHPRC, GB/T 5750.4-2006) |
| Chloride (Cl−) | 250 | Titrimetric | (MHPRC, GB/T 5750.5-2006) |
| Total Alkalinity (TAlk) | N/A | Titrimetric | (EPBPRC, 2002) |
| Sulfate (SO42−) | 250 | Spectrophotometric | (MHPRC, GB/T 5750.5-2006) |
| Total Dissolved Solids (TDS) | 1000 | Gravimetric | (MHPRC, GB/T 5750.4-2006) |
Note 1: Units for all parameters are mg L−1 except pH. Note 2: N/A: not available.
2.3. Health Risk Assessment
In order to measure the variation of the health risks caused by exposure to fluoride, the health risk assessment model was applied to simulate the health effects of fluoride as follows [20]. The population was divided into three age groups based on physiological and behavioral differences: infants, children, and adults.
| (1) |
where ADD is exposure to fluoride, expressed as μg kg−1 day−1, C is the concentration of fluoride in water (mg L−1), IR is water ingestion rate, which is set as 0.75, 1, and 2 L d−1 for infants, children, and adults, respectively, BW refers to body weight, which is set as 5, 10, and 60 kg for infants, children, and adults, respectively. A daily intake of 122 μg kg−1 d−1 of fluoride might cause fluorosis, while more than 200 μg kg−1 d−1 of fluoride might lead to skeletal fluorosis issues [15].
Hazard quotient is assessed by Equation (2) as follows:
| (2) |
where HQ is hazard quotient of fluoride, RfD is the oral reference dose of fluorine intake in drinking water, which is adopted to be 60 μg kg−1 d−1 [21]. When the value of HQ is larger than 1.00, the non-carcinogenic risk will exceed the acceptable level and a hazard is considered to occur.
2.4. Statistical Analysis
To gain insight into the chemical composition of drinking water sources, obtain more information about dominant indicators influencing the occurrence of fluoride, and analyze the relationships between fluoride and other physicochemical parameters, the multivariate statistical analysis was performed with the statistical package for social sciences (SPSS) version 19 (IBM Corporation, Chicago, USA).
Multivariate statistical analyses including correlation analysis, cluster analysis (CA), principal component analysis (PCA), and factor analysis (FA) are effective tools for analyzing water quality and obtaining meaningful results. The CA method is able to group objects into clusters with high internal (within cluster) homogeneity and high external (between clusters) heterogeneity, usually shown as a dendrogram (tree diagram), which is a visual display of the analysis results with Dlink/Dmax × 100 as the horizontal ordinate. Ward’s method with squared Euclidean distance is widely used in CA to determine the variability. The PCA method extracts principal components by combining original variables linearly to simplify data sets. FA further simplifies the data structure by rotating the axis defined by PCA [19]. In this paper, R-mode cluster analysis (R-CA) was applied to distinguish variables according to the distance between them, and R-mode factor analysis (R-FA) was applied to analyze and organize the large data sets. The comprehensive scores for drinking water sources were obtained by Equation (3) as follows:
| (3) |
where is the rotated factor loadings matrix on all the parameters, which is obtained by the rotated factor analysis method, is the normalized data matrix, which is obtained from the measured data in drinking water sources, is the eigenvalue of the rotated component matrix, and n is the primary component number obtained by R-FA.
3. Results and Discussion
3.1. Temporal and Spatial Variations of Fluoride Concentrations
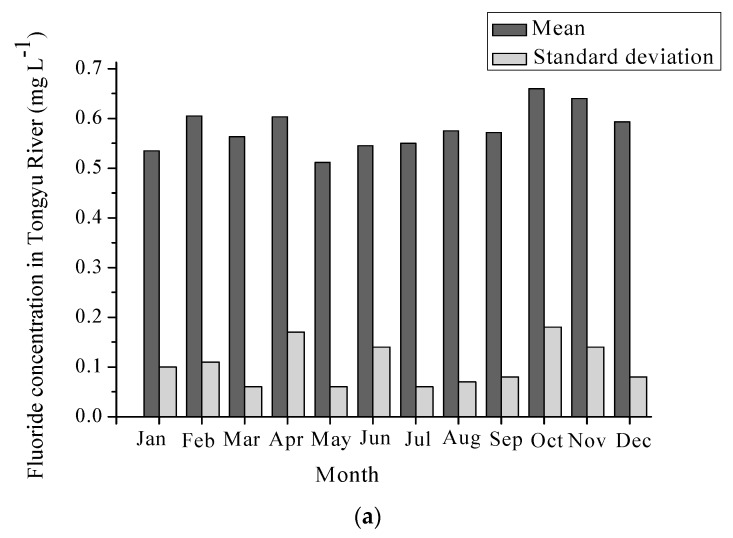
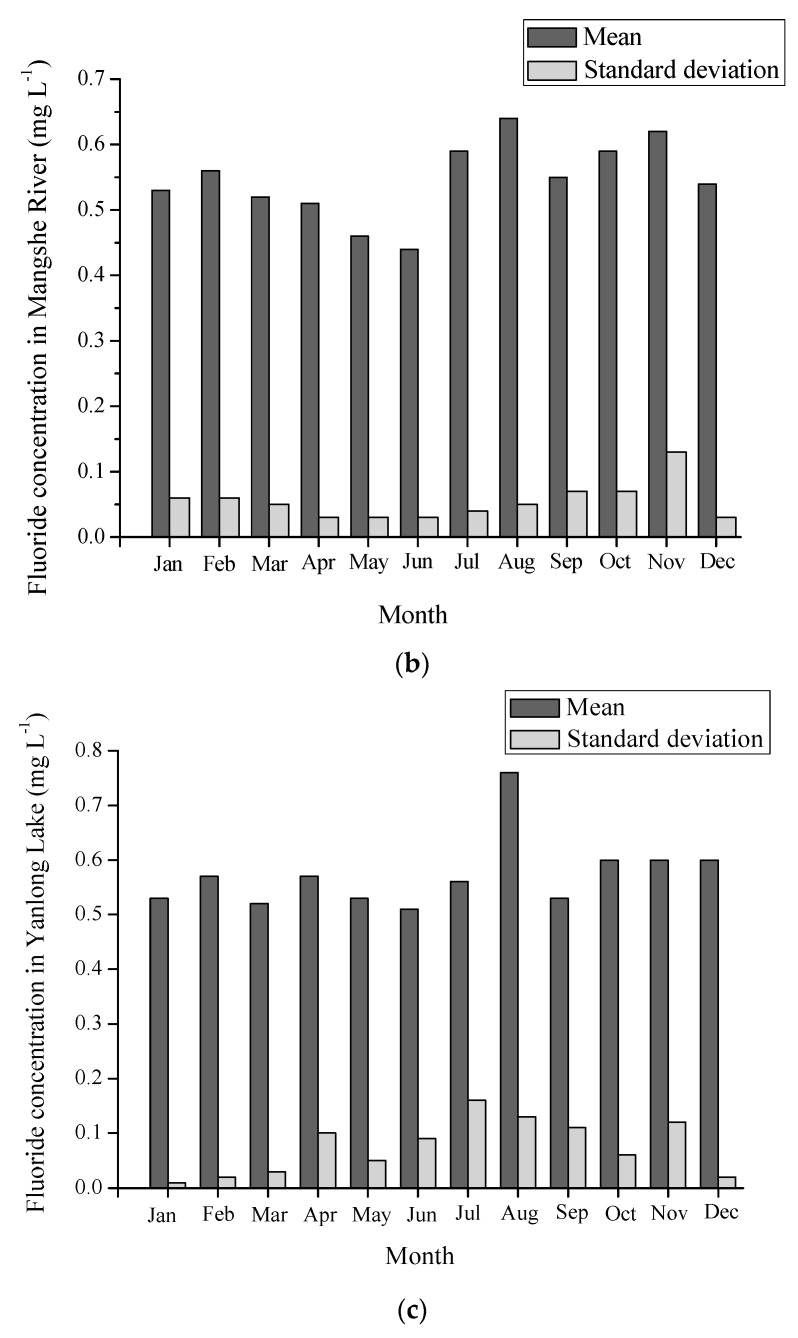
The temporal variations of fluoride concentrations in three drinking water sources are shown in Figure 2. The mean fluoride concentrations were 0.58, 0.55, and 0.57 mg L−1 in the Tongyu River, the Mangshe River, and the Yanlong Lake, respectively. In the Tongyu River, the variation amplitude was slight, with monthly mean fluoride concentrations ranging from 0.51 to 0.66 mg L−1. However, the minimum fluoride concentrations were less than 0.5 mg L−1 in almost all of the months except 0.53 mg L−1 which occurred in October, 2015 and 0.51 mg L−1 which occurred in November, 2013. Furthermore, the fluoride concentration dropped to as low as 0.38 mg L−1 in January, 2010. The low concentration of fluoride may induce dental caries. Similarly, the maximum and mean fluoride concentrations also reached higher values in October and November, 2010, which means that fluoride contents may be associated with groundwater with high fluoride concentration since the river is supplied by groundwater in dry seasons (October and November). The fluoride of shallow groundwater in Yancheng City was reported to exceed the standard level of 1.0 mg L−1, which led to dental and bone fluorosis [22]. Additionally, a rising number of fluorine-related chemical plants were constructed in the Yancheng districts, which may increase the fluoride content and corresponding health risks through irregular discharge of wastewater into surrounding drinking water sources. As for the Mangshe River, the variety range of maximal, minimal, and mean fluoride content remained almost invariable, which suggests that fluoride in the Mangshe River may come from steady groundwater. However, it is worth noting that the maximal fluorides were lowest (<0.5 mg L−1) in May, 2012 and June, 2015, and minimal fluorides were also lowest (<0.5 mg L−1) in May, 2015 and June, 2013. The mean fluoride content reached its maximum value of 0.64 mg L−1 in August. This supported the claim that large quantities of upstream agricultural nonpoint sources and domestic pollution sources were drained into the Mangshe River, especially in flood seasons. The differences between the Tongyu River and the Mangshe River could be due to the fluoride in the Mangshe River being affected by anthropogenic activity more seriously than the Tongyu River. As for the Yanlong Lake, the maximum fluoride content in July, 2012 and August, 2014 reached 0.80 and 0.95 mg L−1, respectively. Since July and August are in flood seasons, large quantities of wastewater probably gave rise to fluoride in the Yanlong Lake.
Figure 2.
Temporal variations of fluoride concentrations in three drinking water sources (a) Monthly fluoride concentration in the Tongyu River. (b) Monthly fluoride concentration in the Mangshe River. (c) Monthly fluoride concentration in the Yanlong Lake.
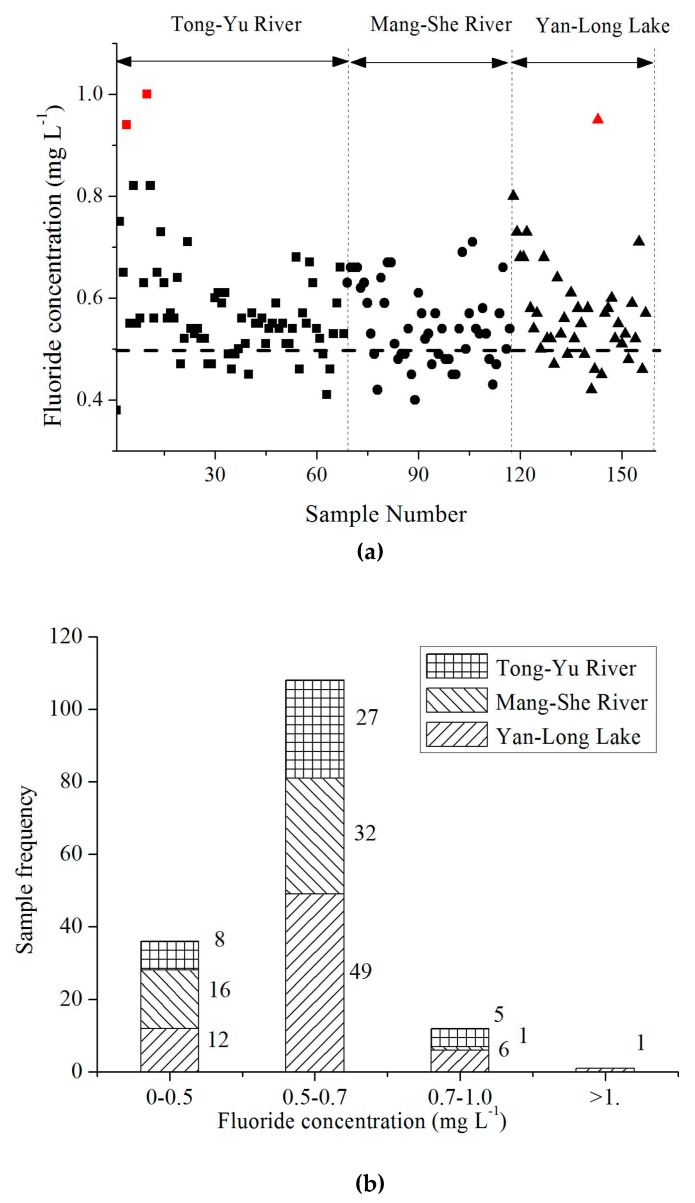
The distributions of fluoride concentration in drinking water sources are presented in Figure 3. In 157 representative water samples, including 68 samples in the Tongyu River, 49 samples in the Mangshe River, and 40 samples in the Yanlong Lake (Figure 3a), the fluoride contents were less than the Chinese sanitary standard for drinking water (1.0 mg L−1), the WHO drinking water quality guideline (1.50 mg L−1) and U.S. standard (2.0 mg L−1). However, in moderate samples (22.9%, n = 157) including 12 samples in the Tongyu River, 16 in the Mangshe River, and 8 in the Yanlong Lake, the fluoride concentrations were less than 0.5 mg L−1 recommended by the WHO to prevent tooth cavity (seen in Figure 3b). This may pose an elevated risk of dental decay, especially for children. Moreover, the data for fluoride concentration refer to drinking water sources, not treated finished water from drinking water treatment plants, where fluoride may be removed by coagulation–sedimentation in the traditional purification process. The removal efficiency depends on the type of coagulants, dosage, and sedimentation time. The removal of fluoride increased the risk of dental decay for children. For preventing dental decay, adding fluoride to drinking water is an effective measure. In the U.S., the prevalence rate of dental caries has dropped by 50%–60% in the last 30 years as a result of fluoridation in drinking water. Similar operations could be found in other countries such as England, Russia, Brazil, and Cuba. Therefore, local governments should take measures to prevent dental decay, such as promoting the use of toothpaste with fluoride.
Figure 3.
Fluoride contents and frequency distribution in drinking water sources of Yancheng City (a) Fluoride contents in drinking water sources of Yancheng City. Note: Dashed line refers to fluoride concentration of 0.5 mg L−1, which is the limit to prevent tooth cavity recommended by the WHO. (b) Frequency distributions of fluoride concentration in the studied area.
3.2. Health Risk Assessment of Fluoride
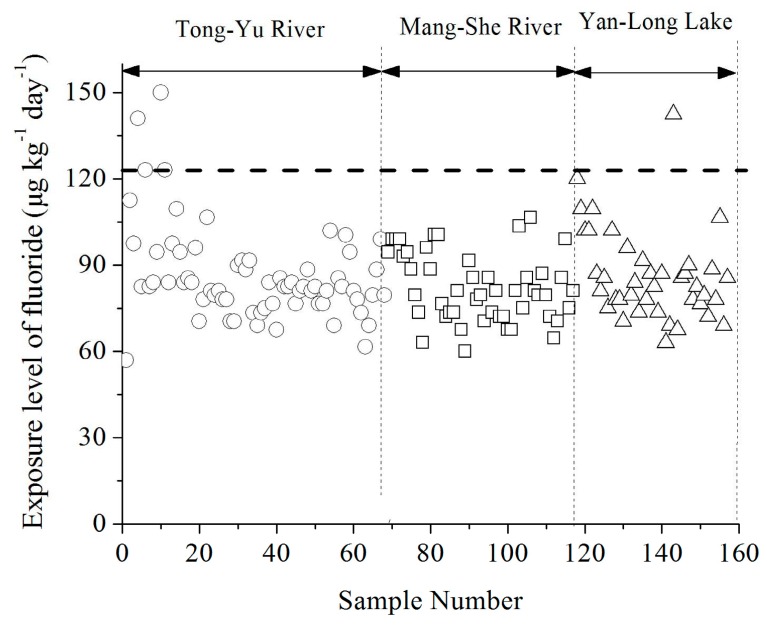
The exposure levels of fluoride (ADD) were calculated using Equation (1), and shown in Table S1 (Supporting information). The fluoride exposure level for infants in all the samples were analyzed and shown in Figure 4. The infants of 4 of 68 (5.9%), 0 of 49 (0%), and 1 of 40 (2.5%) for the Tongyu River, Mangshe River, and Yanlong Lake, respectively, had fluoride exposure levels higher than the toxicity reference value (TRVs) as recommended by Health Canada (122 μg kg−1 d−1), which can cause dental fluorosis issues.
Figure 4.
Exposure of fluoride for infants in the three drinking water sources of Yancheng City. Note: Dashed line in Figure 4 refers to the toxicity reference value of 122 μg kg−1 d−1, which is recommended by Health Canada.
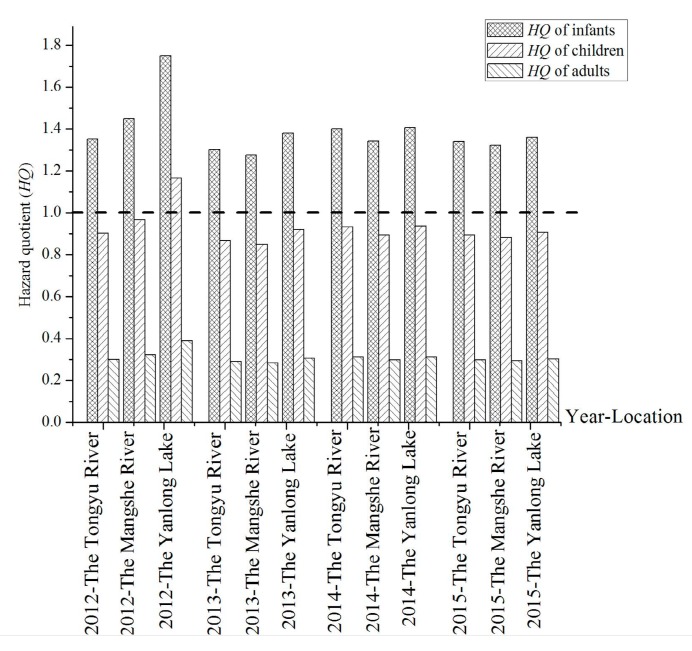
The hazard quotients were calculated using Equation (2) and shown in Figure 5. All the hazard quotients of infants were higher than the recommended value of 1.0 by United States Environmental Protection Agency (USEPA). The results suggested that the infants in Yancheng City suffer from the health risks associated with fluoride. Compared with the Tongyu River and Mangshe River, the hazard quotients were the biggest in Yanlong Lake in 2012 and 2013. In 2012, the hazard quotients showed a significant difference among the three drinking water sources. However, in 2015, the hazard quotients of the three drinking water sources were similar. In addition, the hazard quotients of children and adults were almost always lower than 1.0 except in Yanlong Lake in 2012, which means that the children and adults would not suffer from the harmful effects associated with daily fluoride intake.
Figure 5.
Hazard quotient of fluoride in the three drinking water sources from 2012 to 2015. Note: Dashed line in Figure 5 refers to the hazard quotient (HQ) of 1.0, which is the limit recommended by the WHO.
3.3. Statistical Analysis
3.3.1. Concentrations of Other Physic-Chemical Parameters
The concentration ranges of the other physicochemical parameters are shown in Table 2. The pH values ranged from 7.2 to 8.4 with an average of 7.6 in the Tongyu River, from 7.3 to 8.7 with an average of 7.7 in the Mangshe River, and from 7.4 to 8.5 with an average of 8.1 in Yanlong Lake. Compared with the WHO recommended limits of pH 6.5–8.5, the drinking water sources of Yancheng City are alkaline. The TDS levels of the three drinking water sources ranged from 122 to 594 mg L−1, less than the maximum contamination limit (MCL) of 2000 mg L−1 recommended by the WHO (shown in Table 1). However, some of them exceeded the TDS levels of 500 mg L−1, which may be unacceptable for consumers because of its taste. High intake of sodium (Na+) is harmful for patients suffering from renal, cardiac, and circulatory disease. In Table 2, the concentrations of Na+ are less than 200 mg L−1, and amounts of K+ are less than 250 mg L−1, which is listed as potential complaint for taste. The concentrations of Cl− ranged from 35 to 194 mg L−1, less than the limit value of 250 mg L−1 to prevent heart and kidney diseases from a health perspective. The SO42− concentration in drinking water sources ranged from 23 to 81 mg L−1, and high concentration of SO42− may contribute to the taste and the corrosion of water supply distribution systems [5]. The relationship between pH and dissolution of fluoride can be obtained from the Mangshe River and Yanlong Lake. In the Mangshe River, the highest pH value of 8.72 occurred in February, 2012, while the concentration of fluoride was also at high level of 0.63 mg L−1. The lowest pH value of 7.28 occurred in September, 2015, while the concentration of fluoride was also at lower level of 0.50 mg L−1. Similarly, in Yanlong Lake, the highest pH value of 8.54 occurred in August, 2012, while the concentration of fluoride reached 0.73 mg L−1. The lowest pH value of 7.42 occurred in November, 2013, while the concentration of fluoride was also at lower level of 0.49 mg L−1. However, in the Tongyu River, the highest pH value of 8.40 occurred in February, 2012, while the concentration of fluoride was only 0.53 mg L−1. The lowest pH value of 7.20 occurred in February, 2013, while the concentration of fluoride was also at lower level of 0.49 mg L−1. Generally, the results comply with the conclusion that F− contents are associated with the pH values, which is obtained from other literature [23]. The concentrations of Na+ in the Tongyu River are higher than that in the Mangshe River and Yanlong Lake, which is the same result obtained from fluoride. The results indicate that higher Na+ concentration increases the dissolution of fluoride, which is similar to the findings from other literature [18].
Table 2.
Other physicochemical parameters in three drinking water sources of Yancheng City.
| Drinking Water Sources | pH | SO42− | Cl− | TDS | TAlk | Na+ | K+ | |
|---|---|---|---|---|---|---|---|---|
| Tongyu River | Max. | 8.40 | 81 | 194 | 594 | 218 | 85 | 12 |
| Min. | 7.20 | 23 | 59 | 269 | 117 | 44 | 1.2 | |
| Mean | 7.57 | 49 | 114 | 411 | 167 | 65 | 6.9 | |
| Mangshe River | Max. | 8.72 | 64 | 116 | 523 | 187 | 90 | 7.4 |
| Min. | 7.28 | 25 | 35 | 122 | 123 | 34 | 1.6 | |
| Mean | 7.65 | 43 | 69 | 380 | 158 | 60 | 5.7 | |
| Yanlong Lake | Max. | 8.54 | 61 | 97 | 512 | 183 | 89 | 9 |
| Min. | 7.42 | 24 | 46 | 231 | 116 | 34 | 1.6 | |
| Mean | 8.12 | 44 | 71 | 369 | 156 | 60 | 6.4 |
Note: Units for all parameters are mg L−1 except pH.
3.3.2. Correlation Analysis
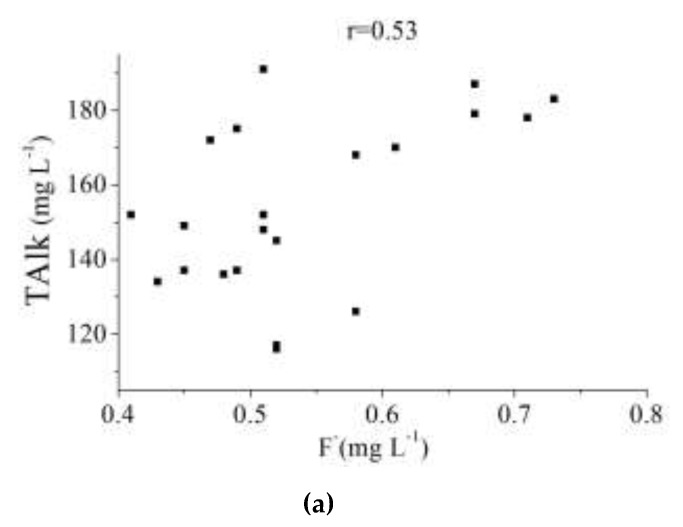
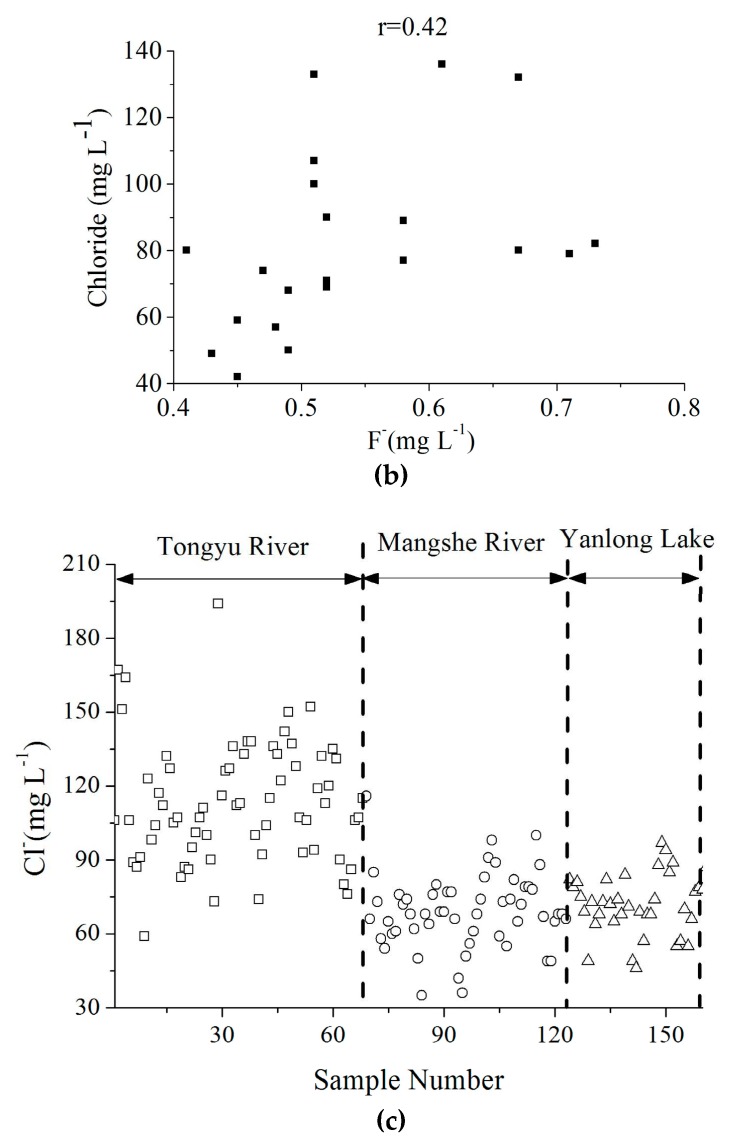
Pearson correlation coefficients for the possible relationships between fluoride and the other monitored physicochemical parameters are summarized in Table 3. There is a positive relationship between fluoride and total alkalinity (TAlk) (r = 0.53), suggesting that fluoride content is associated with the increase of alkalinity in surface water, which is similar to results from other literature [24]. The concentrations of TAlk in the Tongyu River are higher than the Mangshe River and Yanlong Lake, where the concentration of fluoride is also the highest. The correlation between fluoride and TAlk is shown in Figure 6a. Besides TAlk, the statistical relationship between fluoride concentration and Cl− is shown in Figure 6b with positive correlation coefficients of 0.42. The chlorine contents in three drinking water sources are shown in Figure 6c. Similar to fluoride, the chlorine contents in the Tongyu River are obviously higher than those in the Mangshe River and Yanlong Lake. As for the other parameters, the relationship between fluoride and pH, SO42−, and TDS are positive with correlation coefficients of 0.39, 0.22, and 0.11, respectively. Moreover, the relationship between fluoride and Na+ and K+ are negative with correlation coefficients of −0.15 and −0.47, respectively.
Table 3.
Correlation matrix of parameters in the studied area.
| Parameters | pH | F− | SO42− | Cl− | TDS | TAlk | Na+ | K+ |
|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | |||||||
| F− | 0.39 | 1.00 | ||||||
| SO42− | 0.19 | 0.22 | 1.00 | |||||
| Cl− | −0.23 | 0.42 | 0.52 * | 1.00 | ||||
| TDS | 0.09 | 0.11 | 0.22 | −0.06 | 1.00 | |||
| TAlk | −0.17 | 0.53 * | −0.12 | 0.44 | −0.22 | 1.00 | ||
| Na+ | −0.46 | −0.15 | −0.05 | 0.24 | −0.31 | 0.32 | 1.00 | |
| K+ | −0.32 | −0.47 | −0.18 | 0.00 | −0.07 | −0.10 | 0.68 * | 1.00 |
Note: “*” refers to relative coefficients higher than 0.5.
Figure 6.
Relationships between F− and TAlk, F− and chloride, and chlorine contents in drinking water sources of Yancheng City (a) Correlation between F− and TAlk (b) Correlation between F− and chloride (c) Chlorine contents in drinking water sources of Yancheng City.
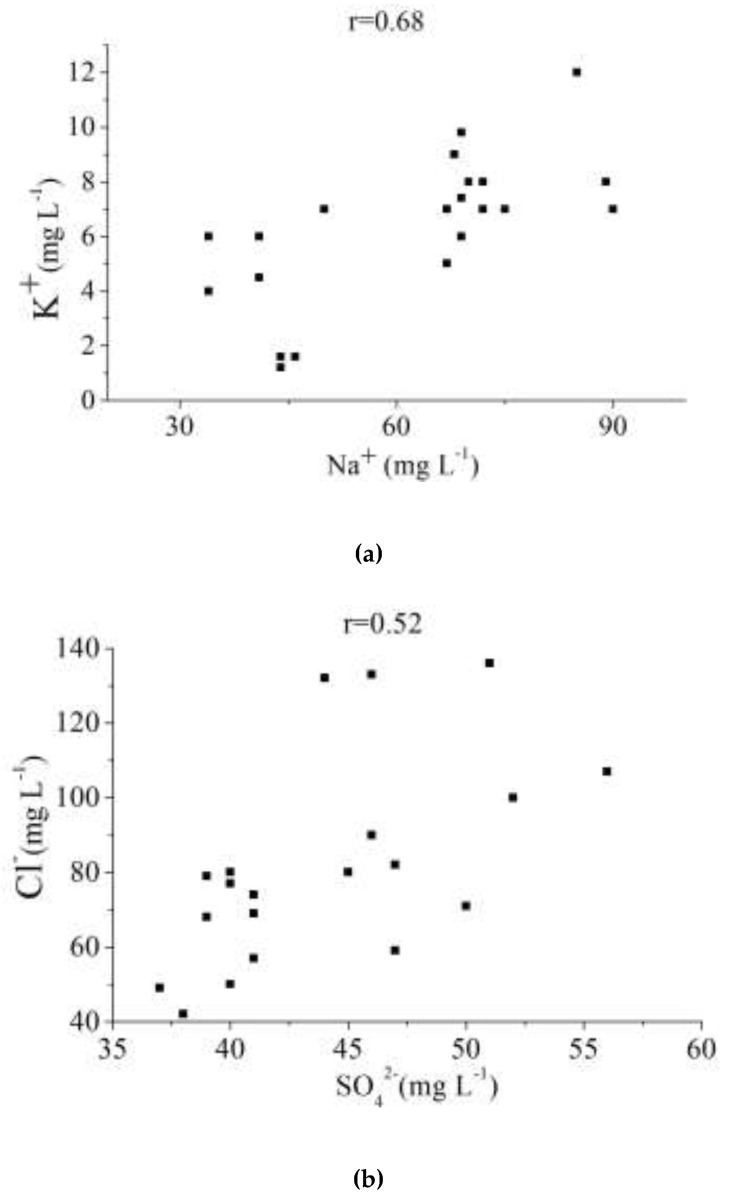
Besides the relationship between fluoride and alkalinity, the correlation analyses of other parameters are described as follows: (1) Chloride is related to sulfate with a correlation coefficient of 0.52; (2) sodium (Na+) is observed to have a close relationship with potassium (K+) (r = 0.68). The correlation was further interpreted through R-mode cluster analysis and factor analysis.
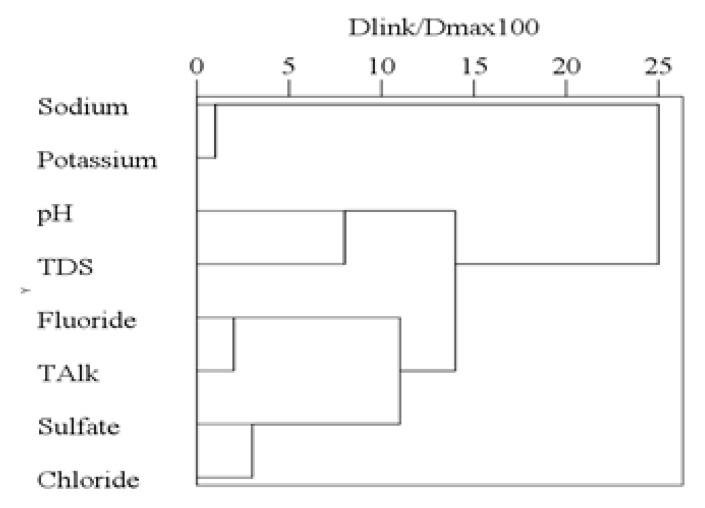
3.3.3. R-mode Cluster Analysis and Factor Analysis
The R-mode cluster analysis was carried out for different physicochemical characters, and the results are shown in a Figure 7. All the variables are grouped into four clusters in a convincing way of Dlink/Dmax × 100 < 10. Fluoride (F−) and total alkalinity (TAlk) were grouped into a cluster. The correlation between F− and TAlk is also shown in Table 3 and Figure 6a with a correlation coefficient of 0.53. In addition, the correlation between sodium (Na+) and potassium (K+) is shown in Table 3 and Figure 8a with a correlation coefficient of 0.68. Similarly, the correlation between sulfate (SO42−) and chloride (Cl−) is shown in Table 3 and Figure 8b with a correlation coefficient of 0.52.
Figure 7.
R-Cluster analysis results.
Figure 8.
Relationships between Na+ and K+, and SO42− and Cl− (a) Correlation between Na+ and K+. (b) Correlation between SO42− and Cl−.
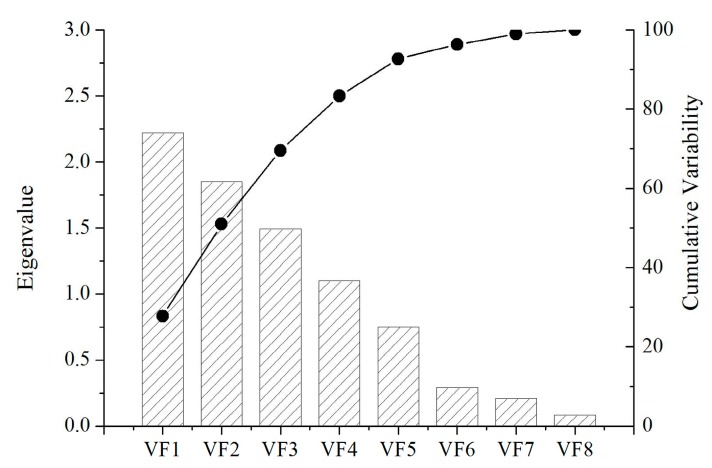
The qualitative information about clustering behaviors was extracted by R-mode factor analysis. The eigenvalues and cumulative variability of all the eight factors are plotted in Figure 9. The first four factors, having a total variance of 83.31% from all the parameters, correspond to four clusters obtained from the cluster analysis.
Figure 9.
Scree plot of varimax rotated factors.
In addition, the rotated factor loadings matrix on all the parameters is expressed in Table 4, including eigenvalues, percentage of variance, and cumulative percentage.
Table 4.
Varimax rotated R-mode factor loadings on all the parameters.
| Parameters | VF1 | VF2 | VF3 | VF4 |
|---|---|---|---|---|
| pH | −0.80 * | −0.14 | 0.15 | −0.19 |
| F− | −0.45 | 0.75 * | 0.25 | 0.04 |
| SO42− | −0.15 | −0.06 | 0.94 * | 0.13 |
| Cl− | 0.24 | 0.55 | 0.70 * | −0.01 |
| TDS | −0.09 | −0.09 | 0.12 | 0.94 * |
| TAlk | 0.16 | 0.91 * | −0.07 | −0.16 |
| Na+ | 0.81 * | 0.14 | 0.12 | −0.35 |
| K+ | 0.78 * | −0.32 | 0.01 | −0.15 |
| Eigenvalue | 2.22 | 1.85 | 1.49 | 1.10 |
| % of the total variance | 27.77 | 23.17 | 18.60 | 13.77 |
| % of cumulative | 27.77 | 50.94 | 69.54 | 83.31 |
Note: “*” refers to absolute values higher than 0.7.
In Table 4, Factor 1 accounted for 27.77% of the total variance and had high loadings of pH (−0.80), Na+ (0.81) and K+ (0.78), which indicate that the presence of Na+ and K+ decreased the values of pH. Factor 2, accounting for 23.17% of the total variance, was mainly associated with significantly higher loading of TAlk (0.91) and moderate loading of F− (0.75), which illustrated that F− solubility is TAlk dependent. Factor 3 was an index of the main anions, including SO42− (0.94) and Cl− (0.70), and counted for 18.6% of the total variability, which indicated that the SO42− values of those samples are higher when there is an elevated amount of Cl−. Factor 4, counting for 13.77% of the total variability, had a strong relationship with TDS (0.94), which reflected the amount of cations and anions.
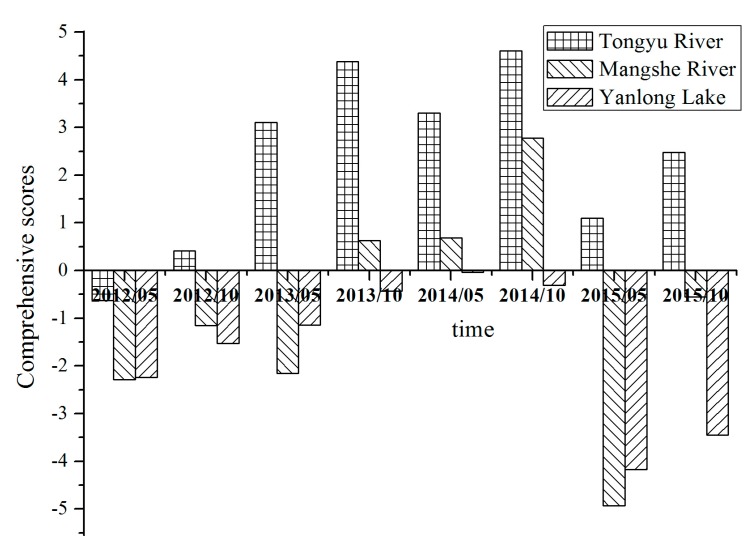
The comprehensive scores of water sources were calculated using Equation (3), and shown in Figure 10. It is evident that the comprehensive scores of the Tongyu River are significantly greater than those of the Mangshe River and Yanlong Lake, which is due to the fact that the hydrogeological conditions in the Tongyu River were quite different from that in the Mangshe River and Yanlong Lake.
Figure 10.
Comprehensive score of drinking water sources.
4. Conclusions
This study provided a systematic analysis of fluoride contents in the three drinking water sources of Yancheng City in the Jiangsu province of China. The fluoride concentrations in the drinking water sources were between 0.38 and 1.0 mg L−1, which were lower than the recommended value in either the local or international drinking water sources quality guidelines, which means that local people would not suffer from dental fluorosis. The mean fluoride concentrations decreased in the order of Tongyu River > Yanlong Lake > Mangshe River. The results also indicated that the health risks associated with fluoride in infants are higher than children and adults, which might cause dental fluorosis issues, especially for the Tongyu River and Yanlong Lake. However, the fluoride contents less than 0.50 mg L−1 unexpectedly occurred in 22.93% of the samples, including 12 samples in the Tongyu River, 16 in the Mangshe River and 8 in Yanlong Lake, which increase the risk of tooth cavities. Therefore, continuous and closer monitoring work should be performed by the local water management department. In addition, to prevent dental caries, corresponding measures should be taken to increase fluoride content through tea, fluoridated toothpaste, and fluoridated salt, etc., especially for the Mangshe River.
The correlation analysis among other physicochemical parameters indicated that relationships existed between F− and TAlk (r = 0.53), SO42− and Cl− (r = 0.52), and Na+ and K+ (r = 0.68), which was also verified by R-mode cluster analysis and factor analysis. Four factors were extracted by R-mode factor analysis, which represent cation, fluoride content, anion, and TDS, respectively. The concentrations of TAlk in the Tongyu River were higher than Yanlong Lake and the Mangshe River, which is in accordance with the fluoride level in three drinking water sources. The results of comprehensive assessment illustrated that physicochemical character of the Tongyu River was significantly different from that of the Mangshe River and Yanlong Lake, which is primarily due to different hydrogeological conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/16/6/1030/s1.
Author Contributions
Writing-Review & Editing, Y.W.; Investigation, R.Y.; Supervision, G.Z.
Funding
This work was funded by the Special S&T Project on Treatment and Control of Water Pollution from Bureau of Housing and Urban-rural Development of Jiangsu Province numbered as 2014ZX07405002.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Abouleish M.Y. Evaluation of fluoride levels in bottled water and their contribution to health and teeth problems in the United Arab Emirates. Saudi Dent. J. 2016;28:194–202. doi: 10.1016/j.sdentj.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuah C.J., Lye H.R., Ziegler A.D., Wood S.H., Kongpun C., Rajchagool S. Fluoride: A naturally-occurring health hazard in drinking-water resources of Northern Thailand. Sci. Total Environ. 2016;545–546:266–279. doi: 10.1016/j.scitotenv.2015.12.069. [DOI] [PubMed] [Google Scholar]
- 3.Gautam R., Bhardwaj N., Saini Y. Dental fluorosis—A case study from Nawa tehsil in Nagaur district, Rajasthan (India) Environmentalist. 2011;31:401–406. doi: 10.1007/s10669-011-9354-5. [DOI] [Google Scholar]
- 4.Yousefi M., Ghoochani M., Mahvi A.H. Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol. Environ. Saf. 2018;148:426–430. doi: 10.1016/j.ecoenv.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 5.Chandio T.A., Khan M.N., Sarwar A. Fluoride estimation and its correlation with other physicochemical parameters in drinking water of some areas of Balochistan, Pakistan. Environ. Monit. Assess. 2015;187:531–539. doi: 10.1007/s10661-015-4753-6. [DOI] [PubMed] [Google Scholar]
- 6.Fang J., Wu X., Xu J., Yang X., Song X., Wang G., Yan M., Yan M., Wang D. Water management challenges in the context of agricultural intensification and endemic fluorosis: The case of Yuanmou County. Ecohealth. 2011;8:444–455. doi: 10.1007/s10393-011-0730-x. [DOI] [PubMed] [Google Scholar]
- 7.Jianmin B., Yu W., Juan Z. Arsenic and fluorine in groundwater in western Jilin Province, China: Occurrence and health risk assessment. Nat. Hazards. 2015;77:1903–1914. doi: 10.1007/s11069-015-1682-1. [DOI] [Google Scholar]
- 8.McGrady M.G., Ellwood R.P. Patcharawan Srisilapanan, Narumanas Korwanich, Helen V. Worthington & Lain Pretty. Dental fluorosis in populations from Chiang Mai, Thailand with different fluoride exposures—Paper 1: Assessing fluorosis risk, predictors of fluorosis and the potential role of food preparation. BMC Oral Health. 2012;12:16–27. doi: 10.1186/1472-6831-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehbandi R., Moore F., Keshavarzi B. Geochemical sources, hydrogeochemical behavior, and health risk assessment of fluoride in an endemic fluorosis area, central Iran. Chemosphere. 2018;193:763–776. doi: 10.1016/j.chemosphere.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Ozsvath D.L. Fluoride and environmental health: A review. Rev. Environ. Sci. Biotechnol. 2008;8:59–79. doi: 10.1007/s11157-008-9136-9. [DOI] [Google Scholar]
- 11.Thivya C., Chidambaram S., Rao M.S., Thilagavathi R., Prasanna M.V., Manikandan S. Assessment of fluoride contaminations in groundwater of hard rock aquifers in Madurai district, Tamil Nadu (India) Appl. Water Sci. 2017;7:1011–1023. doi: 10.1007/s13201-015-0312-0. [DOI] [Google Scholar]
- 12.Walia T., Abu Fanas S., Akbar M., Eddin J., Adnan M. Estimation of fluoride concentration in drinking water and common beverages in United Arab Emirates (UAE) Saudi Dent. J. 2017;29:117–122. doi: 10.1016/j.sdentj.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmunds W.M., Smedley P.L. Fluoride in natural waters. In: Selinus O., editor. Essentials of Medical Geology—Impacts of the Natural Environment on Public Health. Elsevier Academic Press; Burlington, MA, USA: 2005. [Google Scholar]
- 14.United States Public Health Service (USPHS) U.S. Public health service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Rep. Recomm. 2015;130:318–331. doi: 10.1177/003335491513000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guissouma W., Hakami O., Al-Rajab A.J., Tarhouni J. Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere. 2017;177:102–108. doi: 10.1016/j.chemosphere.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Samal A.C., Bhattacharya P., Mallick A., Ali M.M., Pyne J., Santra S.C. A study to investigate fluoride contamination and fluoride exposure dose assessment in lateritic zones of West Bengal, India. Environ. Sci. Pollut. Res. Int. 2015;22:6220–6229. doi: 10.1007/s11356-014-3817-4. [DOI] [PubMed] [Google Scholar]
- 17.Huang G., Gu X. Water environment status analysis and policy research of drinking water sources in Yancheng. Zhihuai. 2009;12:19–21. (In Chinese) [Google Scholar]
- 18.Chae G., Yun S., Mayer B., Kim K., Kim S., Kwon J., Kim K., Koh Y. Fluorine geochemistry in bedrock groundwater of South Korea. Sci. Total Environ. 2007;385:272–283. doi: 10.1016/j.scitotenv.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Salifu A., Petrusevski B., Ghebremichael K., Buamah R., Amy G. Multivariate statistical analysis for fluoride occurrence in groundwater in the Northern region of Ghana. J. Contam. Hydrol. 2012;140–141:34–44. doi: 10.1016/j.jconhyd.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Sultana J., Farooqi A., Ali U. Arsenic concentration variability, health risk assessment, and source identification using multivariate analysis in selected villages of public water system, Lahore, Pakistan. Environ. Monit. Assess. 2014;186:1241–1251. doi: 10.1007/s10661-013-3453-3. [DOI] [PubMed] [Google Scholar]
- 21.US Environmental Protection Agency (EPA) Guidelines for developmental toxicity risk assessment. Fed. Regist. 1991;56:63798–63826. [Google Scholar]
- 22.Gu T., Wang C., Lian S., Fan W., Bo X., Zhao S., Zhang Y. Discussion of fluorosis area distribution and controp measures in Jiangsu Province. Prev. Control Endem. Dis. China. 1991;6:304. (In Chinese) [Google Scholar]
- 23.Karro E., Indermitte E., Saava A., Haamer K., Marandi A. Fluoride occurrence in publicly supplied drinking water in Estonia. Environ. Geol. 2006;50:389–396. doi: 10.1007/s00254-006-0217-1. [DOI] [Google Scholar]
- 24.Saxena V.K., Ahmed S. Dissolution of fluoride in groundwater: A water-rock interaction study. Environ. Geol. 2001;40:1084–1087. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.