Abstract
A repeatable and sensitive method to evaluate the effect of three antiseptics and two disinfection techniques on viable micro-organisms on luer-activated catheter needleless connectors (NCs) was developed. NCs were inoculated with Staphylococcus epidermidis or Klebsiella pneumoniae and disinfected with 3·15% chlorhexidine gluconate + 70% isopropanol (CGI), 70% isopropanol (IPA) or 10% PVP povidone-iodine (PI) antiseptic pads using: (i) scrubbing the NC septum and threaded external surfaces or (ii) wiping only the surface of the septum. Treatments were also evaluated against NCs pretreated with human serum and exposed for 18 h to Staph. epidermidis prior to testing. Viable cells were quantified by plate count. The method for inoculation and recovery of luminal micro-organisms was repeatable (SD, 0·31; n = 28). IPA disinfection provided an approximate 3 log10 CFU reduction; CGI and PI provided 3–4 log10 reductions. PI and CGI were more effective than IPA (P < 0·05), but differences between CGI and PI were not significant for either disinfection method. IPA, but not CGI and PI was also less effective (P < 0·05) against NCs inoculated with Kl. pneumoniae than Staph. epidermidis. Pretreatment with serum and prolonged Staph. epidermidis inoculation removed the advantage seen with CGI and PI; log10 reductions were 1·80, 1·73 and 2·50 for CGI, PI and IPA, respectively. PI or CGI may be more effective than IPA for NC disinfection but effectiveness may be reduced on NCs contaminated with blood or serum.
Keywords: chlorhexidine gluconate plus isopropanol, disinfection, isopropanol, needleless connector, povidone-iodine
Introduction
The Centers for Disease Control and Prevention (CDC) estimates that there were 18 000 CLABSIs in U.S. intensive care units (ICUs) and 23 000 CLABSIs on inpatient hospital wards in 2009 (CDC 2011). These infections are associated with excess costs and increased lengths of stay in hospitals and have been associated with high crude mortality rates (Pittet et al. 1994; Blot et al. 2005).
Central line maintenance practices play an important role in preventing CLABSIs. Improper disinfection of central line hubs or needleless connectors (NCs) can result in contamination of the internal lumen of the catheter with bacteria resulting in the formation of biofilm and subsequent CLABSIs (O’Grady et al. 2011). Unfortunately, some catheter maintenance best practices remain undefined including the best antiseptic and technique for disinfecting catheter needleless connectors. Current guidelines from the CDC/Healthcare Infection Control Practices Advisory Committee (O’Grady et al. 2011) recommend the use of chlorhexidine, povidone-iodine, an iodophor, or 70% alcohol for disinfecting central line hubs; due to lack of evidence, no specific recommendation has been made about the technique used for this process.
In order to better define the impact of antiseptics and disinfection technique on the internal and external contamination of NCs, we designed this laboratory study to compare the efficacy of three different antiseptic pads and two disinfection techniques for reducing bacterial contamination on the external and internal surface of a single type of NC.
Results and discussion
Validation of the method
A primary goal of this study was to develop a sensitive and repeatable method that simulated contamination of the external and internal surfaces of a NC while in use in order to assess the effect of different commonly used disinfectant protocols, using Staph. epidermidis, the most common contaminant of these devices (Donlan et al. 2001). The total mean log10 CFU per untreated NC external surface for all experiments (n = 41) was 6·18 (standard deviation [SD], 0·32). The mean log10 CFU per untreated NC internal surfaces plus valve effluent for all experiments was 4·70 (n = 28; SD, 0·31). These results provided evidence that a single activation of the NC valve is sufficient to transfer viable cells from the external septum surface and threaded portions of the NC into the lumen of the device. It was estimated that the total time of cell contact with luminal surfaces, from inoculation through the valve to recovery of attached cells was no greater than 30 min, suggesting that viable cells can adhere rapidly to surfaces of device components. The method for recovering viable micro-organisms from NC surfaces was also repeatable. A review by Tilt and Hamilton (1999) suggested that repeatability standard deviations of standard antimicrobial assays generally range from 0·2 to 1·0. Our results were well within that range. Sterility controls of the sample processing procedure were performed. Two uninoculated, sterile NCs were immersed in sterile reverse osmosis water for 1 h and processed to recover and quantify any organisms on NC luminal surfaces or NC effluent, using the standard recovery protocol. No CFU/NC was detected in these samples.
A review of in vitro and clinical studies of NCs indicates that most investigators recovered viable cells from the luminal surfaces by flushing saline or a microbiological medium through the valve. Our approach was to recover cells from NC luminal surfaces by flushing the device with a neutralizing solution and exposing the inner surfaces of the device to mechanical forces (vortexing and water bath sonication) to dislodge microbial cells that were not removed by flushing. The mean log10 CFU/NC internal surface was 4·48, and the mean log10 CFU/NC effluent was 4·17, demonstrating that a substantial number of viable cells (>104) were not recovered solely by flushing but required the sonication and vortexing treatment. This suggests that methods incorporating mechanical forces such as sonication and vortexing will provide greater sensitivity.
Effect of antiseptics and disinfection technique
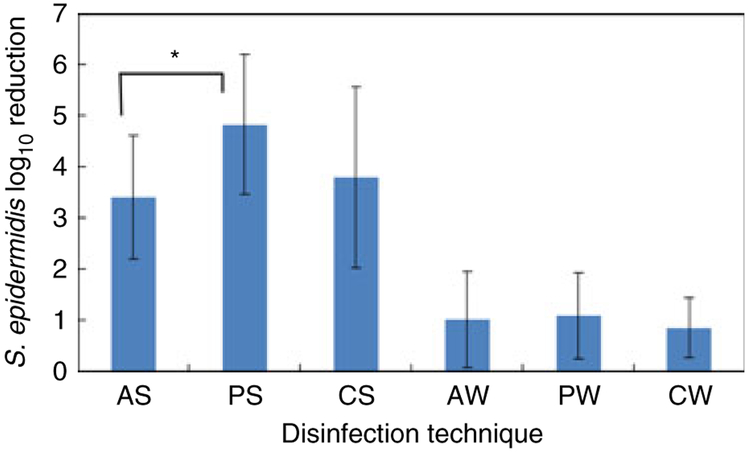
The effect of antiseptics on NC external surfaces using each disinfection technique is shown in Fig. 1. Although alcohol has been shown to reduce NC contamination (Salzman et al. 1993; Rupp et al. 2012; Tarrand et al. 2012), one limitation is its reduced contact time due to rapid evaporation from surfaces. Our results lend support for the limitation of alcohol as a surface disinfectant of NCs. However, there were no significant differences in log10 reductions for NCs treated with CGI or PI for either disinfection technique. In vitro and clinical studies comparing these antiseptics have provided mixed results. Povidone-iodine was more effective in in vitro quantitative suspension tests (McLure and Gordon 1992) but was equivalent to chlorhexidine on steel (Block et al. 2000) in a clinical study (Casey et al. 2003). Chlorhexidine was more effective on human skin or other tissue surfaces (Marchetti et al. 2003) and in clinical studies for reducing catheter colonization and CRBSI (Maki et al. 1991). An advantage of chlorhexidine is that it provides persistent antimicrobial activity, whereas iodophors (povidone-iodine) have limited residual activity (Widmer and Frei 2011).
Figure 1.
Effect of disinfection technique on log10 reduction of viable organisms on NC external surface. AS, alcohol scrubbing; PS, povidone-iodine scrubbing; CS, chlorhexidine gluconate + isopropanol scrubbing; AW, alcohol wiping; PW, povidone-iodine wiping; CW, chlorhexidine gluconate + isopropanol wiping. Error bars, ±1 standard deviation. AS, PS, CS, n = 20; PW, CW, n = 19; AW, n = 18. Untreated Mean Log10 CFU/NC = 6·18. *, P < 0·05.
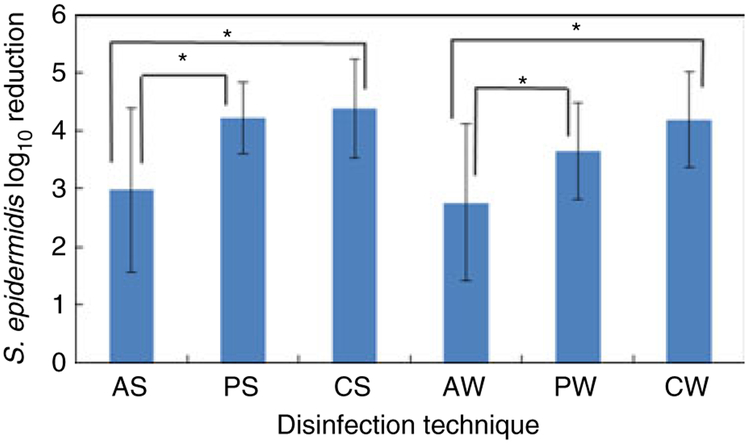
The effect of disinfection technique on the number of viable organisms contaminating the device lumen following valve activation is shown in Fig. 2. Although CGI and PI were significantly more effective than IPA for both disinfection techniques, there were no significant differences between disinfection techniques (wiping compared with scrubbing) for each antiseptic. As expected, scrubbing the septum and entire threaded area of the NC was significantly more effective for reducing the number of viable cells on the NC external surface than simply wiping the septum surface (Fig. 1), because the latter technique did not disinfect the contaminated threaded portions of the device. However, the results suggest that this more extensive disinfection technique might not provide any added benefit, because luminal viable cell counts using both the scrubbing and wiping techniques for all disinfectants were not significantly different. This also suggests that viable cells contaminating the internal lumen of the NC during valve activation originate primarily from the septum external surface. However, this study only evaluated a single activation of the valve. Multiple activations of a contaminated NC could result in a greater potential for luminal contamination in the event that threaded portions of the device were not cleaned and disinfected properly.
Figure 2.
Effect of disinfection technique on log10 reduction of viable organisms on NC internal surfaces + valve effluent following valve activation. AS, alcohol scrubbing; PS, povidone-iodine scrubbing; CS, chlorhexidine gluconate + isopropanol scrubbing; AW, alcohol wiping; PW, povidone-iodine wiping; CW, chlorhexidine gluconate + isopropanol wiping. Error bars, ±1 standard deviation. AS, PS, PW, n = 10; CS, AW, n = 9; CW, n = 12. Untreated Mean Log10 CFU/NC = 4·71 *, P < 0·05.
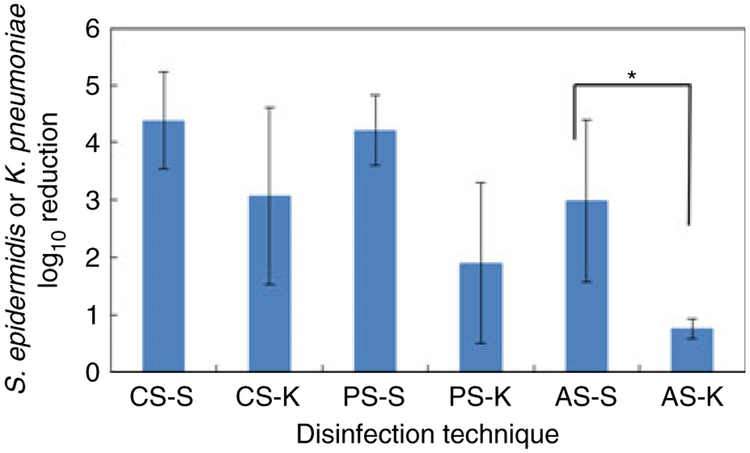
To evaluate these antiseptics on another clinically relevant organism, NCs were inoculated with Klebsiella pneumoniae and disinfected. IPA was significantly less effective against Kl. pneumoniae (Fig. 3), consistent with the observation that Gram-negative bacteria may exhibit greater resistance towards biocides than Gram-positive organisms, due to their outer membrane (Russell 1998).
Figure 3.
Effect of disinfectant on log10 reduction of viable Staphylococcus epidermidis and Klebsiella pneumoniae on NC internal surfaces + valve effluent following valve activation. AS-S, alcohol scrubbing, Staph. epidermidis-contaminated NC; AS-K, alcohol scrubbing, Kl. pneumoniae-con-taminated NC; PS-S, povidone-iodine scrubbing, Staph. epidermidis-contaminated NC; PS-K, povidone-iodine scrubbing, Kl. pneumoniae-contaminated NC; CS-S, chlorhexidine gluconate + isopropanol scrubbing, Staph. epidermidis-contaminated NC; CS-K, chlorhexidine gluconate + isopropanol scrubbing, Kl. pneumoniae-contaminated NC. Error bars, ±1 standard deviation. AS-S, PS-S, n = 10; CS-S, n = 9; AS-K, PS-K, CS-K, n = 3. *, P < 0·05, T-test only.
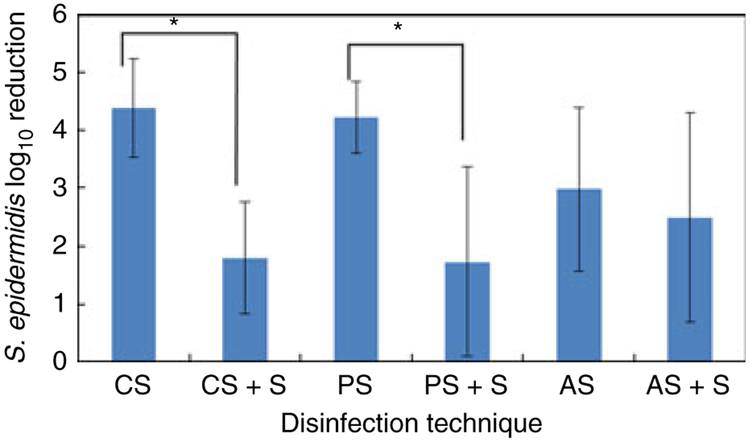
The presence of blood or organic materials has been shown to reduce the effectiveness of surface disinfectants (Larson and Bobo 1992; Widmer and Frei 2011). We pretreated NCs with human serum, and the bacterial inoculum was allowed to remain on the serum-pretreated surface for 18 h prior to treatment, to provide a more robust challenge for each disinfectant. These conditions significantly reduced the efficacy of CGI and PI but not IPA (Fig. 4). Hare et al. (1963) reported that different concentrations of ethanol were more effective against dried bacterial films than chlorhexidine solutions. Larson and Bobo (1992) found isopropanol to be significantly more effective than povidone-iodine or a detergent containing 4% chlorhexidine gluconate on human skin containing blood. It is possible that NC surfaces will become contaminated with blood during normal use and that contaminating organisms on septum surfaces may persist and dry. Although we did not dissect the effect of each factor individually (exposure to serum or prolonged inoculation), our results support the conclusions of earlier studies that disinfectant activity will be reduced, and IPA may be equally as effective as CGI and PI when these conditions are present.
Figure 4.
Effect of disinfectant on log10 reduction of viable organisms on NC internal surfaces + valve effluent following valve activation, using standard inoculation protocol or NCs pretreated with complement-inactivated human serum and exposed to Staphylococcus epidermidis for 18 h prior to disinfection treatment. AS, alcohol scrubbing, standard protocol; AS + S, alcohol scrubbing, serum pretreatment/extended inoculation; PS, povidone-iodine scrubbing, standard protocol; PS + S, povidone-iodine scrubbing, serum pretreatment/extended inoculation; CS, chlorhexidine gluconate + isopropanol scrubbing, standard protocol; CS + S, chlorhexidine gluconate + isopropanol scrubbing, serum pretreatment/extended inoculation. Error bars, ±1 standard deviation. AS, PS, n = 10; CS, AS + S, n = 9; PS + S, CS + S, n = 6. *, P < 0·05, T-test only.
In summary, this study suggests that the internal surfaces of NCs may become rapidly colonized following valve activation by micro-organisms contaminating external surfaces of these devices. Wiping or scrubbing the device external surfaces using IPA, PI or CGI antiseptic pads can reduce the number of viable microbial cells that contaminate the internal surfaces. PI and CGI may provide similar reductions in internal contamination, and it is expected that both may be significantly more effective than IPA. However, contamination of NCs with high levels of serum or exposure to bacterial cells for extended periods will remove the benefit seen with CGI and PI. Future studies should evaluate the effect of different antiseptic pads and disinfection techniques on other types of NCs in laboratory and clinical studies, using methods that accurately measure organisms associated with the internal surfaces of these devices.
Materials and methods
Organisms
Staphylococcus epidermidis 520-07 and Klebsiella pneumoniae 6-11 were clinical isolates obtained from the Clinical and Environmental Microbiology Branch culture collections. Cultures were stored at −71°C, and a fresh subculture, grown overnight on trypticase soy agar (TSA) at 35°C, was used for each experiment.
Needleless connector device
The Clear Link™ device, a luer-activated mechanical valve needless connector manufactured by Baxter Healthcare (Deerfield, IL, USA), was used in all evaluations.
Protocol for pretreatment of needleless connectors with human serum
Human blood from nonsmokers who were not using any medications was poured into 50-ml tubes, allowed to clot at 37°C for a few hours and centrifuged at 4000 g for 20 min at 4°C. The serum was removed and filter-sterilized (0·2 μ), heated in a water bath at 56°C for 30 min to inactivate the complement and stored in 5 ml aliquots at −20°C. Serum was thawed at 4°C no more than 24 h prior to use. For certain experiments, unused NCs were first exposed to sterile serum by immersing the NC septum and threaded surface in 5 ml sterile serum for 2 h under static conditions in a Class II biological safety cabinet (BSC) immediately prior to exposure to the bacterial inoculum.
Protocol for inoculation of device
A standardized 1 × 108 CFU (colony forming units)/ml cell suspension (OD600 0·12) in butterfield buffer (pH 7·2) was prepared on the day of use. Twelve ml of this cell suspension was added to a 30-ml sterile beaker containing a magnetic stirring bar in a BSC. The actual inoculum cell concentration used for each experiment was determined by spread plating onto TSA (24 h, 35°C). The beaker containing the cell suspension was placed onto a digital stirring plate (Barnstead/Thermolyne, Dubuque, Iowa) in the BSC. An unused NC was removed from its packaging using a sterile hemostat and suspended over the beaker containing the cell suspension using the hemostat, such that the external threaded portion of the device and the septum were immersed in the cell suspension. The cell suspension was mixed at 120 rpm for 1 h. Following exposure to the cell suspension, the NC was removed and placed into a sterile Petri dish in the BSC and air-dried for 1 h. In certain experiments, NCs that were pre-exposed to serum prior to the bacterial culture were exposed to the bacterial culture for 1 h, placed into a sterile, sealed Petri dish and held in the BSC for 18 h prior to disinfection and processing.
Experimental protocol to determine the effect of disinfection on NC external surfaces
Disinfection
Following air drying of the inoculated NC, it was disinfected using one of three different antiseptic pads: Chlorascrub Swab (3·15% chlorhexidine gluconate (w/v) + 70% isopropanol (v/v) (PDI; Orangeburg, NY, USA); Alcohol Prep Pad (70% isopropyl alcohol, (PDI); and Povidone-Iodine Prep Pad (10% PVP Povidone-Iodine), (PDI). Two disinfection techniques were used for each antiseptic wipe: (i) covering the septum and threaded area of the NC with the antiseptic pad, then applying pressure using the thumb and index finger to wipe the entire threaded area and the septum of the NC in a back and forth rotational motion continuously for 15 s (scrubbing), or (ii) placing the antiseptic pad on the NC septum and applying pressure while wiping only the septum back and forth continuously for 10 s (wiping). Following the disinfection, the device was placed into a sterile Petri dish and allowed to dry for 10 min in the BSC. All treated devices and an untreated device were placed into 50-ml centrifuge tubes containing 10 ml D/E broth (Difco Laboratories, Becton Dickinson and Co., Sparks, MD, USA) for processing to recover and quantify viable cells attached to the device surface.
Recovery and quantification of attached microbial cells
Centrifuge tubes containing NCs were removed from the BSC and subjected to three alternating 30-s cycles of vortexing and sonication (42 kHz, Bransonic Ultrasonic Cleaner, Branson, MO, USA). This method was shown previously to recover 97% of attached viable organisms from plastic surfaces (Donlan et al. 2001). The liquid was pipetted into a sterile centrifuge tube and centrifuged at 9384 g for 15 min at 4°C (Beckman Coulter, Indianapolis, Indiana). The supernatant was discarded, and the pellet suspended in phosphate-buffered saline. After vortexing, the resulting cell suspension was serially diluted in butterfield buffer and spread plated onto TSA. 1 and 5 ml portions of the cell suspension were also passed through a 0·22-μm filter (Pall, Ann Arbor, MI, USA), and filters were placed onto TSA plates. All plates were incubated at 35°C for 24 h and counted. The limit of detection for this method was 2 CFU/NC. For purposes of statistical analysis, a value of 1 CFU/NC was assigned to all samples reported as nondetectable.
Experimental protocol to determine the effect of disinfection on NC Internal surfaces
Disinfection
Following air drying of the inoculated NC, it was disinfected using one of three antiseptic pads and one of two disinfection techniques, as already described.
Procedure to assess the effectiveness of disinfection on contamination of internal NC surfaces
A 5 cc syringe (injecting syringe) containing 5 ml of D/E broth was attached to the male-threaded end of each treated and untreated NC. A second empty 5 cc syringe (receiving syringe) was attached to the other end of the device, and approximately 2·5 ml of D/E broth was passed through the device into this second syringe (simulating activation of the NC valve). While connected with both syringes, the device external surfaces were treated to inactivate microbial cells using a modification of a published method (Donlan et al. 2001). The receiving syringe was removed from the NC, and the 2·5 ml of D/E broth remaining in this syringe was discharged into a 50-ml centrifuge tube containing 5 ml of D/E broth. The D/E broth remaining in the injecting syringe still attached to the NC was flushed through the NC into the 50-ml tube (designated NC effluent). The total volume of D/E broth in the effluent tube was approximately 10 ml. A flame-sterilized pipe cutter was used to cut the NC into two sections. All components of the valve were dissected using flame-sterilized blades and placed into a 50-ml centrifuge tube containing 10 ml of D/E broth (designated NC internal surfaces).
Recovery and quantification of microbial cells
Centrifuge tubes containing NC internal surfaces or NC effluent were removed from the BSC and subjected to three alternating 30-s cycles of vortexing and sonication, and the liquid was pipetted into a separate tube, centrifuged, diluted and plated on TSA, as already described. All plates were incubated at 35°C for 24 h and counted. The limit of detection for this method was 2 CFU/NC or NC effluent. For purposes of statistical analysis, a value of 1 CFU/NC or NC effluent was assigned to all samples reported as nondetectable.
Experimental approach
For each of the four categories: (i) external inoculation + scrubbing, (ii) external inoculation + wiping, (iii) external inoculation + scrubbing + valve activation and (iv) external inoculation + wiping + valve activation, a single-cell suspension was used to inoculate each of four devices. A single experiment consisted of treating and testing four devices (untreated, IPA, PI and CGI) using either the scrubbing or wiping disinfection technique. Number of organisms for each treatment category was measured by viable count. For experiments involving external inoculation followed by valve activation, the number of organisms recovered was the sum of cells recovered from NC internal surfaces and cells in the NC effluent (as already described).
Statistical analysis
Plate count results (CFU/NC) were log10-transformed. To compare mean log10 CFU reductions among each of the four categories where each disinfectant-treated device was paired with an untreated device as a control, we initially used a paired T-test. These test results were further supported through the use of generalized estimating equations to assess mean paired log10 CFU reductions accounting for clustering within the replicates generated across the three treatments used to disinfect the NCs. A compound symmetrical correlation structure was used to estimate a single common correlation between log10 CFU reductions across the disinfection treatments within a given replicate. The P-values from these tests were considered significant if P ≤ 0·05 and were two-sided. Data were analysed using SAS 9.2.
Significance and Impact of the Study: A sensitive and repeatable protocol was developed to evaluate antiseptics for disinfecting catheter needleless connectors (NCs). Povidone-iodine (PI) and chlorhexidine gluconate plus isopropanol (CGI) were more effective than isopropanol (IPA) for reducing Staphylococcus epidermidis contamination of NCs. The effectiveness of PI and CGI was reduced on NCs pre-exposed to human serum and prolonged bacterial inoculation. IPA was also less effective against NCs contaminated with Klebsiella pneumoniae.
Acknowledgements
We thank Renee Maciejewski for assistance with the figures and Wayne Kirby, Elizabeth Perez and Judith Noble-Wang for helpful suggestions.
Footnotes
Conflict of interest
M. M., A. K. and J. E. report no conflict of interest relevant to this article. R. D. participated in an earlier unrelated study in collaboration with a university that was funded by Baxter Healthcare, the manufacturer of the needleless connector evaluated in this study. Baxter Healthcare had no input on the present study. Otherwise, R.D. reports no conflict of interest relevant to this article. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDC.
References
- Block C, Robenshtok E, Simhon A and Shapiro M (2000) Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J Hosp Infect 46, 147–152. [DOI] [PubMed] [Google Scholar]
- Blot SI, Depuydt P, Annemans L, Benoit D, Hoste E, De Waele JJ, Decruyenaere J, Vogelaers D et al. (2005) Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis 41, 1591–1598. [DOI] [PubMed] [Google Scholar]
- Casey AL, Worthington T, Lambert PA, Quinn D, Faroqui MH and Elliott TSJ (2003) A randomized, prospective clinical trial to assess the potential infection risk associated with the PosiFlow needleless connector. J Hosp Infect 54, 288–293. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011) Vital Signs: central line associated bloodstream infections, United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rpt 60, 243–248. [PubMed] [Google Scholar]
- Donlan RM, Murga R, Bell M, Toscano CM, Carr JH, Novicki TJ, Zuckerman C, Corey LC et al. (2001) Protocol for detection of biofilms on needleless connectors attached to central venous catheters. J Clin Microbiol 39, 750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R, Raik E and Gash S (1963) Efficiency of antiseptics when acting on dried organisms. Br Med J 1, 496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E and Bobo L (1992) Effective hand degerming in the presence of blood. J Emerg Med 10, 7–11. [DOI] [PubMed] [Google Scholar]
- Maki DG, Ringer M and Alvarado CJ (1991) Prospective randomized trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338, 339–343. [DOI] [PubMed] [Google Scholar]
- Marchetti MG, Kampf G, Finzi G and Salvatorelli G (2003) Evaluation of the bactericidal effect of five products for surgical hand disinfection according to prEN 12054 and prEN 12791. J Hosp Infect 54, 63–67. [DOI] [PubMed] [Google Scholar]
- McLure AR and Gordon J (1992) In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect 21, 291–299. [DOI] [PubMed] [Google Scholar]
- O’Grady NP, Alexander M, Burns LM, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H et al. (2011) Guideline for the prevention of intravascular catheter-related infections. Clin Infect Dis 52, e162–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D, Tarava D and Wenzel RP (1994) Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA 271, 1598–1601. [DOI] [PubMed] [Google Scholar]
- Rupp ME, Yu S, Huerta T, Cavalieri RJ, Alter R, Fey PD, Van Schooneveld T and Anderson JR (2012) Adequate disinfection of a split-septum needleless intravascular connector with a 5-second alcohol scrub. Infect Control Hosp Epidemiol 33, 661–665. [DOI] [PubMed] [Google Scholar]
- Russell AD (1998) Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect 43 (suppl), S57–S68. [DOI] [PubMed] [Google Scholar]
- Salzman MB, Isenberg HD and Rubin LG (1993) Use of disinfectants to reduce microbial contamination of hubs of vascular catheters. J Clin Microbiol 31, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrand JJ, LaSala PR, Han X-Y, Rolston KV and Kontoyiannis DP (2012) Dimethyl sulfoxide enhances effectiveness of skin antiseptics and reduces contamination rates of blood cultures. J Clin Microbiol 50, 1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilt N and Hamilton MA (1999) Repeatability and reproducibility of germicide tests: a literature review. J AOAC Int 82, 384–389. [PubMed] [Google Scholar]
- Widmer AF and Frei R (2011) Decontamination, disinfection, and sterilization In Manual of Clinical Microbiology, 10th edn, ed. Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML and Warnock DW pp. 143–173. Washington, DC: ASM Press. [Google Scholar]