Abstract
Rationale: Exposure to particulates from burning biomass is an increasing global health issue. Burning biomass, including wood smoke, is associated with increased lower respiratory infections.
Objectives: To determine whether acute exposure to wood smoke modifies nasal inflammatory responses to influenza.
Methods: Healthy young adults (n = 39) were randomized to a 2-hour controlled chamber exposure to wood smoke, where exposure levels were controlled to particulate number (wood smoke particles [WSP]; 500 μg/cm3) or filtered air, followed by nasal inoculation with a vaccine dose of live attenuated influenza virus (LAIV). Nasal lavage was performed before exposure (Day 0) and on Days 1 and 2 after exposure. Nasal lavage fluid cells were analyzed for inflammatory gene expression profiles, and cell-free fluid was assayed for cytokines.
Measurements and Main Results: Only IP-10 protein levels were affected, suppressed, by WSP exposure in aggregate analysis. Subsequent analysis indicated an exposure × sex interaction, prompting additional analyses of WSP- and LAIV-induced changes in males and females. Inflammation-related gene expression profiles differed between the sexes, at baseline (males greater than females), after LAIV inoculation (females greater than males), and after WSP exposure (increase in males and decrease in females), demonstrating that WSP- and LAIV-induced changes in antiviral defense responses in the nasal mucosa occur in a sex-specific manner.
Conclusions: WSP exposure resulted in minimal modification of LAIV-induced responses in aggregate analysis. In contrast, analyzing WSP-induced modification of LAIV responses in the sexes separately unmasked sex-specific differences in response to exposure. These data highlight the need for additional studies to understand sex-specific pollutant-induced effects.
Clinical trial registered with www.clinicaltrials.gov (NCT02183753).
Keywords: air pollution, viral infection, sex difference, nasal mucosa
At a Glance Commentary
Scientific Knowledge on the Subject
Particulate matter has been linked to a variety of respiratory and cardiac diseases. Wood smoke particles (WSP) are an increasing source of particulate matter via wildfires and burning for heating or cooking. Epidemiologic and controlled exposure studies suggest that wood smoke and biomass exposure may contribute to increased risk of infection and reduced lung function. Emerging evidence suggests that responses to respiratory virus infection and immune response to WSP are sex specific. This is an important consideration because exposure to wood smoke varies greatly throughout the world by sex, with women primarily exposed to WSP and biomass in the home, while cooking or heating, and men as the majority of first responders to household fires and wildfires. The mechanistic link between WSP exposure and respiratory infections has not been well established.
What This Study Adds to the Field
This study shows that inflammation-related gene expression profiles significantly differed between males and females at baseline, after live attenuated influenza virus inoculation, and after WSP exposure, demonstrating that WSP-induced and live attenuated influenza virus–induced changes in antiviral defense responses in the nasal mucosa occur in a sex-specific manner.
Particulate matter (PM) has been linked to a variety of respiratory diseases (1–14). Wood smoke particles (WSP) are an increasing source of PM in the United States as the predicted prevalence of wildfire events rises (15). Another source of WSP is wood burning for heating or cooking, which is estimated to contribute up to 30% of ambient fine particles (PM2.5) in some areas of the United States during the winter (1). Throughout the world, more than 2 billion people rely on the burning of biomass, including wood, as their main source of energy for heating and cooking (14). The use of biomass as the primary source of fuel contributes to high concentrations of PM in the air (mg/m3 range), a major contributing source of air pollution (7), and is associated with increased lower respiratory infections, a chief cause of death in many populations (2, 14).
Epidemiologic studies suggest that wood smoke and biomass exposure may contribute to increased risk of infection and reduced lung function (3, 4, 6, 8–10). Several controlled exposure studies have also shown clear associations between exposure to WSP and respiratory dysfunction (16, 17). Other studies have found increased glutathione levels in BAL fluid along with upper respiratory symptoms after WSP exposure (18). Both systemic and respiratory proinflammatory effects, such as increased peripheral blood and BAL fluid neutrophil counts, have been shown in a WSP exposure model (5).
Emerging evidence also suggests that responses to respiratory virus infection are sex specific (19–21). This is an important consideration because exposure to WSP varies greatly throughout the world, with women primarily exposed to WSP and biomass in the home, while cooking or heating, and men as the majority of first responders to household fires and wildfires (22). Furthermore, early exposure to wildfire smoke has been shown to result in sex-specific alteration of immune response to infection by attenuating systemic TLR (toll-like receptor) responses (23).
Although controlled exposure studies link WSP exposure and respiratory proinflammatory effects, the mechanistic link between WSP exposure and respiratory infections has not been well established. Using our protocol of inoculation with live-attenuated influenza virus (LAIV) as a model for respiratory infection (24), we investigated the effect of controlled exposure to whole wood smoke in humans, in vivo, on viral infection and antiviral host defense responses. We hypothesized that short-term exposure to whole wood smoke enhances LAIV-induced nasal inflammation. To test this, we conducted a randomized, placebo-controlled study measuring the effect of a 2-hour wood smoke exposure on short-term nasal inflammatory responses to LAIV. Some of the results of these studies have been previously reported in conference abstracts (25, 26).
Methods
Study Protocol
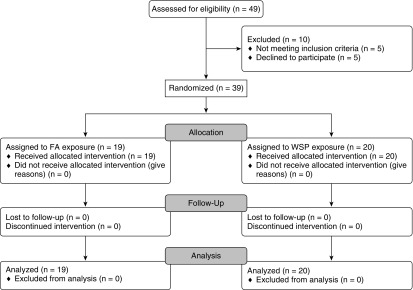
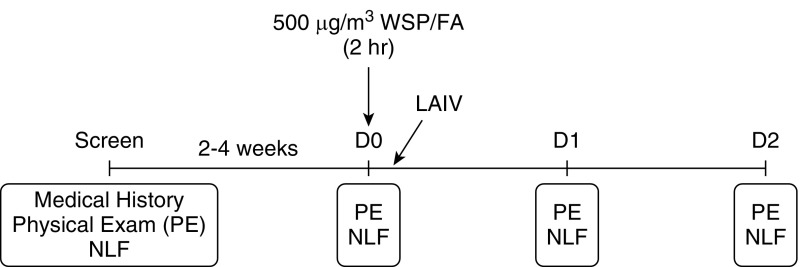
Healthy, young adults 18–40 years of age (Table 1) were recruited and entered into the protocol shown in Figures 1 and 2. Participants were either never-smokers or had been abstinent from smoking for at least 1 year. At baseline (Day 0), subjects underwent nasal lavage and were randomized to undergo exposure to either whole wood smoke, where exposure level was controlled by particulate number (WSP, 500 μg/m3) or filtered air (FA) for 2 hours, sitting at rest, in an exposure chamber controlling for levels of carbon monoxide and other gaseous pollutants (see Table E1 in the online supplement). The exposure level was based on prior studies demonstrating tolerance and similarity to levels found in homes heated by burning wood (24, 25). Following WSP or FA exposure, subjects were inoculated with a standard vaccine dose of 2014–2015 LAIV (FluMist, MedImmune Astra Zeneca). On Days 1 and 2 following inoculation, nasal lavage fluid (NLF) was collected as in our previous studies and stored at −80°C until analysis (23, 26–30). NLF cells were resuspended in RLT lysis buffer (Qiagen), at 50,000 cells per 7 μl of lysis buffer and stored at −80°C until analysis. Cell-free NLF supernatants were also stored at −80°C until analysis. Additional supplementary methodologic details can be found in the online supplement.
Table 1.
Subject Demographics
| FA (n = 19) |
WSP (n = 20) |
|||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| BMI | 22.87 ± 0.9 | 28.83 ± 1.6 | 27.82 ± 2.4 | 25.01 ± 0.9 |
| Age | 25.80 ± 1.7 | 27.33 ± 2.5 | 28.42 ± 1.7 | 27.25 ± 1.6 |
| Baseline FVC% | 104.7 ± 4.6 | 108.4 ± 2.9 | 107.1 ± 3.8 | 104.8 ± 4.0 |
| Baseline FEV1% | 99.9 ± 4.4 | 106 ± 3.9 | 106.8 ± 4.1 | 108.3 ± 3.0 |
| Sex, female/male | 10 | 9 | 12 | 8 |
| Race, white/African American/Asian | 7/3/0 | 8/1/0 | 6/5/1 | 8/0/0 |
| Former smokers, n | 2 | 1 | 1 | 0 |
Definition of abbreviations: BMI = body mass index; FA = filtered air; WSP = wood smoke particles.
Values are mean ± SE.
Figure 1.
Consolidated Standards of Reporting Trials diagram. Participant recruitment, screening, and randomization. FA = filtered air; WSP = wood smoke particles.
Figure 2.
Study design and sample collection timeline. FA = filtered air; LAIV = live attenuated influenza virus; NLF = nasal lavage fluid; PE = physical exam; WSP = wood smoke particles.
The protocol was approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board (number: 13–3076) and all methods were performed in accordance with relevant guidelines and regulations.
Sample Analysis
Cell-free NLF was analyzed using the V-PLEX Human Cytokine 30-Plex Kit from Meso Scale Diagnostics. IP-10 and IL-6 analysis was completed via single-plex ELISA (BD) and read using a CLARIOstar microplate reader (BMG Labtech Inc.).
NLF cells were analyzed for gene expression via Nanostring nCounter Human Inflammation code set (255 genes) with a 10-gene nCounter Panel-Plus add-in to include influenza genes from the 2014–2015 season of the LAIV FluMist (Table 2). Nanostring data were normalized per manufacturer recommendations.
Table 2.
Custom Add-in Probe Sequences to Nanostring nCounter Human Inflammation Code Set
| Gene Name | Accession Number | Target Region | Target Sequence |
|---|---|---|---|
| FluA_HA | FJ966952.1 | 735–834 | CTATTACTGGACACTAGTAGAGCCGGGAGACAAAATAACATTCGAAGCAACTGGAAATCTAGTGGTACCGAGATATGCATTCGCAATGGAAAGAAATGCT |
| FluA_NA | FJ966956.1 | 1134–1233 | CGGATGGACTGGGACAGACAATAACTTCTCAATAAAGCAAGATATCGTAGGAATAAATGAGTGGTCAGGATATAGCGGGAGTTTTGTTCAGCATCCAGAA |
| FluA_PB1 | KC866601.1 | 739–838 | AGGGCTATCGCAACACCTGGGATGCAGATTAGAGGTTTCGTATACTTTGTTGAAACTTTAGCTAGGAGCATTTGCGAAAAGCTTGAACAGTCTGGGCTCC |
| FluA_HA | KC892952.1 | 864–963 | ACCCATTGGCAAATGCAAGTCTGAATGCATCACTCCAAATGGAAGCATTCCCAATGACAAACCATTCCAAAATGTAAACAGGATCACATACGGGGCCTGT |
| FluA_M1-M2 | KC892233.1 | 288–387 | AGTTAAACTGTATAGGAAACTTAAGAGGGAGATAACGTTCCATGGGGCCAAAGAAATAGCTCTCAGTTATTCTGCTGGTGCACTTGCCAGTTGCATGGGC |
| FluA_NA | KC892281.1 | 289–388 | TTTGCACCTTTCTCTAAGGACAATTCGATTAGGCTTTCCGCTGGTGGGGACATCTGGGTGACAAGAGAACCTTATGTGTCATGCGATCCTGACAAGTGTT |
| FluA_NP | KJ942619.1 | 834–933 | ACTGATATTGAGAGGATCAGTTGCTCACAAATCTTGCCTACCTGCCTGTGCGTATGGACCTGCAGTATCCAGTGGGTACGACTTCGAAAAAGAGGGATAT |
| FluB_HA | CY115151.1 | 312–411 | CAGACCTGTTACATCTGGGTGCTTTCCTATAATGCACGACAGAACAAAAATTAGACAGCTGCCTAACCTTCTCCGAGGATACGAACATATCAGGTTATCA |
| FluB_M1-BM2 | KC866607.1 | 389–488 | CAGCGCTACTATACTGTCTCATGGTCATGTACCTGAATCCTGGAAATTATTCAATGCAAGTAAAACTAGGAACGCTCTGTGCTTTATGCGAGAAACAAGC |
| FluB_NB-NA | FJ766839.1 | 482–581 | CAATGGAACAAGAGGAGACAGAAACAAGCTGAGGCATCTAATTTCAGTCAAATTGGGCAAAATCCCAACAGTAGAAAACTCCATTTTCCACATGGCAGCA |
Statistical Analysis
To complete the a priori analysis, we defined the dependent variable as the LAIV response measures calculated from the area under the curve (https://cran.r-project.org/web/packages/AUC/AUC.pdf) over the days of the study. This measure has been used in previous studies with similar experimental design of exposure followed by LAIV (24, 27). We used multiple regression analysis to evaluate the relationship between LAIV response and the independent exposure variable, WSP exposure. Following this, we included demographic factors in the regression as a secondary analysis. The inclusion of sex was particularly of interest based on rodent studies suggesting sex-dependent modifications of responses to influenza virus (19, 28). Because of significant interaction between sex and exposure, we conducted a sex-specific subgroup analysis. All analyses were conducted using R (http://www.R-project.org/). In our subgroup analysis, we were also interested in differential response patterns in males and females to LAIV because of acute response pattern differences noted in previous studies (19, 28). We conducted a Wilcoxon paired signed rank test after Shapiro-Wilk normality testing, where the independent variable was defined as postexposure − preexposure LAIV gene expression. Next, effect of sex at baseline was evaluated using a two-sample Student’s t test for unequal variance because no biologic variables influence both sex of the individual and gene expression profiles. Lastly, to determine the functional outcome of gene expression profile changes, we assessed concomitant changes in protein expression through regression analyses. For all analyses, statistical significance was set at P less than 0.05 and |fold change (FC)| greater than 1.5.
Results
Effect of WSP and LAIV on Aggregate Data
No effect of WSP exposure on response to LAIV in aggregate gene expression data was found; none of the genes examined reached statistical significance (see Table E2). Because WSP exposure induced no significant effects on gene expression changes, we confirmed that LAIV inoculation indeed resulted in gene expression changes consistent with an antiviral host defense response. Similar to previous studies (19, 28, 29), we saw an upregulation of genes that are canonical responses to viral inoculation and the presence of the season-specific influenza strain subunit genes (influenza B NA gene; Infl_B_NA) in the FA group, confirming infection with LAIV (data not shown).
Area Under the Curve Effect Measure Modification of Exposure × Sex on LAIV Response
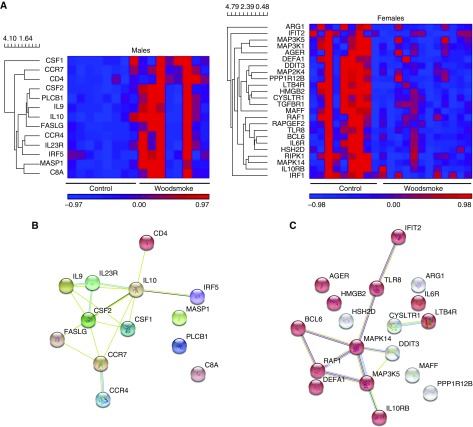
Considering the known effects of sex as a biologic variable in the response to influenza virus (19, 20, 28, 30, 31), we examined whether there was an interaction between WSP and sex. Twenty-five genes displayed a sex-modified effect of WSP exposure in response to LAIV (see Table E2). These genes included complement factors (C1S, C4A, C6, and C8A), cell surface markers (CD4 and CD55), and inflammatory signaling genes (TGFBR1, TGFB1, CCL16, IL21, and IL12B). Because of the contribution of sex as an interactive factor, we also completed statistical testing within strata. In males, 13 genes were differentially affected after WSP exposure (see Table E3). All of these were upregulated more than twofold when WSP-exposed subjects were compared with FA. Most of the upregulated genes were proinflammatory (Figure 3). The top five genes differentially affected in males were: CD4, CCR7, CSF2, PLCB1, and MASP1. In females, 18 genes were differentially expressed after WSP exposure (see Table E3). All of these were moderately downregulated, with most changes being less than twofold (Figure 3). The top five genes differentially affected in females were: HMGB2, LTB4R, DEFA1, MAFF, and CYSLTR1.
Figure 3.
Stratum-specific effects of wood smoke particles (WSP) on live attenuated influenza virus response in males and females. (A) Unbiased clustering of differentially expressed genes in male (left) and female (right) control (filtered air) and WSP-exposed subjects. (B) Male WSP response was primarily upregulation of inflammatory genes, whereas (C) female effects seem to be a milder suppression of defense responses per enrichment for the associated Gene Ontology (GO) pathway (GO:0006952, shown in red). Inclusion in heat map was determined by significance P < 0.05 and |fold change| >1.5. Clustering maps of genes differentially expressed in (B) males and (C) females after WSP compared with filtered air are shown.
Sex-Specific Effects of LAIV
Because our analysis indicated an interactive response of LAIV by WSP exposure and sex, we also evaluated the potential for sex-specific responses to LAIV, which have not previously been reported to our knowledge (28). Ten genes were differentially expressed in males and 78 genes in females, when comparing before inoculation (Day 0) with after inoculation (Day 1) (see Table E4). When comparing Day 0 with Day 2, a total of 16 genes were differentially expressed in males and 78 genes in females (see Table E4). Interestingly, in addition to fewer genes responding to LAIV in males compared with females, influenza subunit (Infl_B_NA) was upregulated in females on Day 1, whereas in males it was not upregulated until Day 2.
Baseline Sex Differences
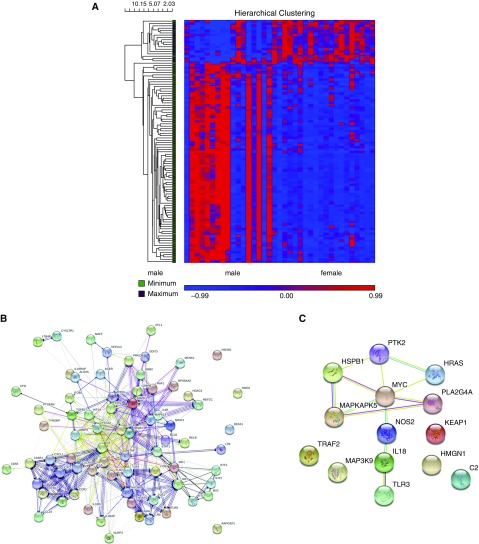
Based on the sex-dependent gene expression changes in response to WSP and LAIV, we examined whether NLF cell gene expression differed at baseline in males and females. Eighty-eight genes were differentially expressed at baseline when comparing males with females (see Table E5). Seventy-four of 88 genes were upregulated in males compared with females, whereas the remaining 14 of 88 were downregulated in males compared with females (Figure 4). The significant upregulation of IL-8 in males compared with females recapitulates a sex difference of this gene in the nasal mucosa we reported in our previous study (32). Genes upregulated and downregulated in males compared with females were clustered and analyzed for functional enrichment using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in STRING version 10.5 (33). The top five genes upregulated in males compared with females were: RHOA, CXCL1, PRKCB, IL-8, and RELA. The top five genes downregulated in males compared with females were: NOS2, HRAS, IL18, PTK2, and PLA2G4A. Additionally, we compared genes within-sex between exposure groups at baseline to ensure that at baseline groups were not different. We found 1 of 255 genes in females and 5 of 255 genes in males were different between exposure groups at baseline (data not shown). Based on a small number of significant genes and that only two of the six differentially expressed genes appear in other results, we do not believe that our baseline groups differed substantially.
Figure 4.
Baseline (Day 0) sex difference. (A) Unbiased clustering of differentially expressed genes in males and females from nasal lavage fluid cells at baseline (Day 0). Inclusion in heat map was determined by significance P < 0.05 and |fold change| >1.5. (B and C) Cluster maps of genes upregulated (B) and downregulated (C) in males compared with females.
Cytokine Levels in NLF and Correlation with Gene Expression Changes
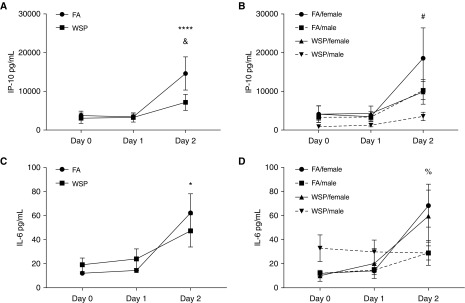
IP-10 and IL-6 increased after LAIV (Figures 5A and 5C). WSP exposure suppressed IP-10 on Day 2 as compared with FA exposed (Figure 5A) in both males and females. Exposure × sex differences were observed in levels of IL-6 and IP-10 (Figures 5B and 5D). The remainder of the cytokine data are shown in Table E6.
Figure 5.
Protein data in aggregate, by sex, and at baseline. Graphs depict protein levels of (A and B) IP-10 and (C and D) IL-6 on Days 0, 1, and 2. Data are shown as mean ± SE and are Benjamini and Hochberg false discovery rate corrected, *q ≤ 0.05 and ****q ≤ 0.001 (vs. Day 0); &q ≤ 0.05 (woodsmoke particles vs. filtered air); #q ≤ 0.1 (female, wood smoke particles vs. filtered air); %q ≤ 0.1 (filtered air, males vs. females). FA = filtered air; WSP = wood smoke particles.
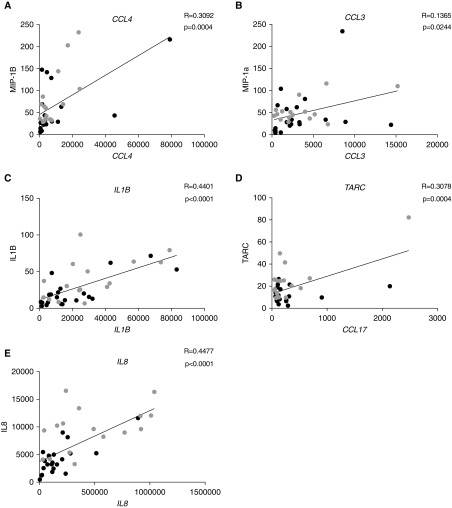
We also examined whether cytokine levels in NLF correlated with gene expression changes in NLF cells. Protein data that significantly correlated with gene expression data for genes of interest included IL-8, MIP-1β, MIP-1α, TARC, and IL-1β (Figure 6). Five analytes indicated positive concordance between gene and protein data, thus changes in gene expression data likely resulted in similar changes in protein data.
Figure 6.
Gene and protein data correlation. Correlation of associated gene and protein data is shown, demonstrating that functional protein data correlates with gene expression data. Representative data for proteins (y-axis) with matching gene expression data (x-axis) are shown. (A) CCL4, (B) CCL3, (C) IL1B, (D) TARC, and (E) IL8.
Discussion
The goal of this study was to test the hypotheses that WSP exposure alters antiviral host defense responses in human volunteers. To probe this hypothesis, we chose nasal LAIV inoculation following a single exposure to WSP as a model for community-acquired infection with circulating influenza in the context of a wildfire. Overall, our study demonstrated that WSP exposure did not significantly alter markers of viral replication, such as Infl_B_NA expression, but resulted in reduced NLF IP-10 levels (Figure 5). In our previous studies, LAIV-induced responses, including IP-10, were blunted in individuals exposed to inhaled toxicants, such as cigarette smoke and diesel exhaust (22, 29–31). Suppression of IP-10 could impede antiviral defense, because IP-10 is essential for the recruitment of cytotoxic lymphocytes, such as T cells and natural killer cells, to infected tissue (32).
Although our data in aggregate did not show significant effects of WSP on LAIV-induced inflammatory gene expression, there was a significant exposure × sex interaction, prompting separate analysis of WSP-induced changes in males and females. Inflammation-related gene expression profiles differed in several ways between males and females, suggesting that WSP-induced changes in antiviral defense responses in the nasal mucosa occur in a sex-specific manner.
We conducted a formal test of effect measure modification with reporting of interaction terms, and sex-stratified analysis in our gene expression data, to look for the effects of WSP and sex on immune response to LAIV. For example, genes that tested positive for an interaction of exposure × sex and were also found in the male stratum data include CD4, MASP1, and FASLG. When male and female data are combined in the interaction dataset, the |FC| for each of these genes is less than 1.5. When analyzed separately in males, the FCs are FC = 2.23, FC = 4.02, and FC = 3.76, respectively, in subjects exposed to WSP as compared with FA. Similarly in females, WSP-induced genes that overlap in both interaction and stratum-specific analysis include HMGB2, LTB4R, MAFF, ARG1, and MAP3K5. In the combined interaction analysis exposure, |FC| ranges from 1.01 to 1.18, which was below our threshold of 1.5-fold. However, in the female-specific stratum analysis, FC increased to FC = −1.91, FC = −2.06, FC = −1.82, FC = −2.63, and FC = −1.55, respectively. Thus, if the data were analyzed in aggregate (males and females together), and sex was not considered as a biologic variable, significant results would have been minimal and would have appeared as though the WSP exposure did not substantially affect LAIV-induced antiviral host defense responses. Moreover, when sex is included in the analysis as a factor, sex-specific effects in opposite directions emerge, which has been observed in multiple studies (34).
Our sex-specific analysis demonstrated that in males, WSP significantly increased expression of 13 genes as compared with FA. The upregulation of these genes likely indicates increased inflammation in response to WSP, which could increase cellular damage and the duration and severity of infection or impede recovery, potentially worsening previously observed sex differences in response to infection (30). In contrast, we observed downregulation of inflammatory genes in females exposed to WSP before inoculation with LAIV, although to a moderate degree. The inflammatory effects seen in males replicate more closely the effects seen in previous controlled WSP exposure studies (1, 2, 5, 16), whereas female effects seem to be a milder suppression of defense responses per enrichment for the associated GO pathway (GO:0006952, shown in red in Figure 3C) (Figures 3B and 3C).
Any sex-dependent modification in vaccine response, including LAIV, could present an alteration in vaccine efficacy. Vaccine efficacy is normally assessed by comparing the percentage of reduction in disease incidence in a vaccinated versus unvaccinated population or vaccine-specific antibody production. Previous studies have uncovered potential sex bias of vaccine efficacy and antibody production following influenza vaccination (28, 35, 36). Although we cannot make direct inferences about the impact of WSP on vaccine efficacy, increases in such genes as IL10 in males after WSP and LAIV may indicate an impaired vaccine response, because IL-10 has been associated with decreased pulmonary-specific antibody protection against influenza virus (37). Further studies are needed to better understand potential implications of WSP on vaccine efficacy of LAIV or other vaccines.
Although our study was designed to test WSP effects, data in the control group (FA) also allowed us to analyze sex-specific responses to LAIV alone (see Table E4). Females responded more robustly to LAIV than males on Day 1 and Day 2 after inoculation with 79 and 78 genes differentially expressed, respectively, compared with 10 and 16 genes in males, respectively. The genes found to be upregulated in women include interferon and TLR genes, which have previously been shown to be higher in females and may promote a more robust response to infection, compared with males (reviewed in Reference 31). In addition, influenza gene subunit Infl_B_NA (a marker of viral replication) increased in females on Day 1 (ninefold) and on Day 2 (sixfold) compared with males. Furthermore, there were many more immune and inflammatory genes that were upregulated in females in response to the LAIV inoculation, supporting previous findings in studies of sex-specific response to viral infections and vaccines and demonstrating, for the first time, sex-specific responses to LAIV. These observations contribute to the growing body of evidence that virus-induced host defense responses differ between males and females (19–21, 28, 30, 31, 38).
We also compared baseline sex differences in our data. We have previously reported that nasal mucosal IL-8 levels are significantly greater in males as compared with females (32). Similarly, in this study we observed 88 genes that were sex-biased at baseline, with most of the sex-different genes upregulated in males as compared with females (Figure 4). The 74 genes that were upregulated in males include genes involved in NF-κB (nuclear factor-κB) signaling pathway, such as IL-8 and their receptors and TLRs (Figure 4B). The functional origin and significance of these differences are not well understood. Others have hypothesized that sex-differences in disease incidence and severity and responses to pathogens may be genetic (X chromosome), hormonal (estrogen and testosterone), evolutionary, or developmental in origin (20, 39–41). In addition, we hypothesize that sex differences in gene and protein expression at baseline could explain sex-biased responses to pathogens. Increased levels of inflammatory mediators in males may represent an increased response threshold to pathogens, needing more of the pathogen to trigger influx and activation of neutrophils and other innate immune cells. The increased threshold would allow for more pathogen replication before being recognized by the immune system as invasive and would thus explain the increased intensity and duration of infection seen in males compared with females. The concept of immune signaling thresholds has been introduced and modeled in the microbiome literature with macrophage activation; however, the model presented only investigated cells from male human subjects and the sex of the rodents used was not identified (42, 43). Therefore, the potential for threshold response to pathogens to differ in females needs further investigation to affirm this hypothesis.
To confirm the validity of our LAIV infection model, we evaluated the acute effects of LAIV on nasal inflammatory gene expression. Similar to previous studies (44), we found that genes and proteins canonically associated with response to viral infection were upregulated after infection, indicative of host defense response against viral insult (Figure 5). Largely, the genes and proteins affected are part of the IFN antiviral response pathway, essential for host-defense and development of immune memory (29). For example, in our protein data we observed increases in levels of IL-6 and IP-10 after LAIV on Day 2 (Figure 5). Overall, we recapitulated previously observed increases in the expression of genes associated with antiviral host defense responses, indicating our model appropriately mimics responses to community-acquired influenza infections.
The findings presented here are important for future controlled human exposure studies beyond those examining the health effects of exposure to WSP or biomass, because exposure and response differs by sex. Women primarily experience a subchronic exposure, especially in the developing world, to indoor WSP and biomass while cooking or heating the household (9, 12). In contrast, men are the vast majority of firefighters and first responders and have the potential to be exposed to WSP and biomass in multiple large acute doses (22, 45). Clinical studies on either group would thus only describe half of the effects in either males or females, but together these data may better inform on the effects on naturally occurring exposures in the sexes. The few studies that have completed controlled WSP exposures in humans vary in the type of wood used, combustion method, and exposure concentration, but have investigated the potential for altered inflammatory profiles (18, 46–50). Interestingly, these studies included both sexes, but did not analyze by sex. Therefore, the finding of no or few inflammatory effects on aggregate data in these studies, similar to our findings, is not surprising.
The study that replicates the wood, combustion, and exposure concentration used in our study found an influx of neutrophils in bronchial and BAL after WSP exposure, but no change in cytokines; however, they did not disclose the sex of their 10 subjects, so a thorough comparison cannot be completed (5). Similarly, rodent WSP exposure studies have been largely completed in just one sex (e.g., males only [51–55], females only [13, 56–58]) or with rodents of undisclosed sex (59, 60). Although these studies have provided important information on the effects of WSP exposure, determining whether differential responses between males and females exist becomes difficult with a one-sided experimental design. A limited number of animal studies have included both sexes and reported differential effects of WSP exposure by sex (11, 23). Thus, divergent effects of WSP exposure on the sexes should be taken into account when designing exposure studies in the future.
In summary, our study identifies several baseline sex differences in gene expression and protein levels in the airway, which are further altered in a sex-dependent manner after WSP exposure and LAIV inoculation. These findings add to the growing literature on physiologic differences in immune function or regulation between males and females, and emphasize the importance of inclusion of both sexes in controlled exposure studies and analyzing data with sex as a biologic factor. Further investigation is needed to better understand the origin and functional significance of respiratory immune sex differences and its impact on susceptibility to air pollutants and pathogens.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Center for Environmental Medicine, Asthma and Lung Biology study coordinators and recruiters, TRC Pollutant Control System operators, and participants for their contributions to this study.
Footnotes
Supported by the NIH (R01ES01361, T32ES00712634, T32ES007126, and P30ES010126), Environmental Protection Agency (EPA) Cooperative Agreement (CR 83578501), and Leon and Bertha Golberg Postdoctoral Fellowship. This research was funded in part by U.S. EPA Cooperative Agreement CR83578501, but has not been subjected to review and does not necessarily reflect EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author Contributions: T.L.N. and I.J. conceptualized and designed the work. E.A.P., C.R., and E.G.-B. completed sample acquisition. M.E.R., A.M.S., E.M.M., and K.A.A. completed sample analysis. M.E.R., A.M.S., H.Z., T.L.N., and I.J. completed data interpretation. M.E.R. and I.J. drafted the manuscript. M.E.R., A.M.S., E.M.M., K.A.A., E.A.P., E.G.-B., C.R., H.Z., T.L.N., and I.J. critically revised the manuscript, approved the final version, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201807-1287OC on October 26, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Croft DP, Cameron SJ, Morrell CN, Lowenstein CJ, Ling F, Zareba W, et al. Associations between ambient wood smoke and other particulate pollutants and biomarkers of systemic inflammation, coagulation and thrombosis in cardiac patients. Environ Res. 2017;154:352–361. doi: 10.1016/j.envres.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358:619–624. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- 3.Fullerton DG, Suseno A, Semple S, Kalambo F, Malamba R, White S, et al. Wood smoke exposure, poverty and impaired lung function in Malawian adults. Int J Tuberc Lung Dis. 2011;15:391–398. [PubMed] [Google Scholar]
- 4.Garcia-Sancho MC, Garcia-Garcia L, Baez-Saldana R, Ponce-De-Leon A, Sifuentes-Osornio J, Bobadilla-Del-valle M, et al. Indoor pollution as an occupational risk factor for tuberculosis among women: a population-based, gender oriented, case-control study in Southern Mexico. Rev Invest Clin. 2009;61:392–398. [PubMed] [Google Scholar]
- 5.Ghio AJ, Soukup JM, Case M, Dailey LA, Richards J, Berntsen J, et al. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup Environ Med. 2012;69:170–175. doi: 10.1136/oem.2011.065276. [DOI] [PubMed] [Google Scholar]
- 6.Mishra V. Indoor air pollution from biomass combustion and acute respiratory illness in preschool age children in Zimbabwe. Int J Epidemiol. 2003;32:847–853. doi: 10.1093/ije/dyg240. [DOI] [PubMed] [Google Scholar]
- 7.Molnár P, Gustafson P, Johannesson S, Boman J, Barregård L, Sällsten G. Domestic wood burning and PM2.5 trace elements: personal exposures, indoor and outdoor levels. Atmos Environ. 2005;39:2643–2653. [Google Scholar]
- 8.Oloyede IP, Ekrikpo UE, Ekanem EE. Lung function indices of children exposed to wood smoke in a fishing port in South-South Nigeria. J Trop Pediatr. 2013;59:399–402. doi: 10.1093/tropej/fmt033. [DOI] [PubMed] [Google Scholar]
- 9.Opotowsky AR, Vedanthan R, Mamlin JJ. A case report of cor pulmonale in a woman without exposure to tobacco smoke: an example of the risks of indoor wood burning. Medscape J Med. 2008;10:22. [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Padilla R, Perez-Guzman C, Baez-Saldana R, Torres-Cruz A. Cooking with biomass stoves and tuberculosis: a case control study. Int J Tuberc Lung Dis. 2001;5:441–447. [PubMed] [Google Scholar]
- 11.Reed MD, Campen MJ, Gigliotti AP, Harrod KS, McDonald JD, Seagrave JC, et al. Health effects of subchronic exposure to environmental levels of hardwood smoke. Inhal Toxicol. 2006;18:523–539. doi: 10.1080/08958370600685707. [DOI] [PubMed] [Google Scholar]
- 12.Riojas-Rodríguez H, Romano-Riquer P, Santos-Burgoa C, Smith KR. Household firewood use and the health of children and women of Indian communities in Chiapas, Mexico. Int J Occup Environ Health. 2001;7:44–53. doi: 10.1179/107735201800339650. [DOI] [PubMed] [Google Scholar]
- 13.Samuelsen M, Cecilie Nygaard U, Løvik M. Particles from wood smoke and road traffic differently affect the innate immune system of the lung. Inhal Toxicol. 2009;21:943–951. doi: 10.1080/08958370802590499. [DOI] [PubMed] [Google Scholar]
- 14.Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55:518–532. doi: 10.1136/thorax.55.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JC, Mickley LJ, Sulprizio MP, Dominici F, Yue X, Ebisu K, et al. particulate air pollution from wildfires in the western US under climate change. Clim Change. 2016;138:655–666. doi: 10.1007/s10584-016-1762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barregard L, Sällsten G, Gustafson P, Andersson L, Johansson L, Basu S, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol. 2006;18:845–853. doi: 10.1080/08958370600685798. [DOI] [PubMed] [Google Scholar]
- 17.Sällsten G, Gustafson P, Johansson L, Johannesson S, Molnár P, Strandberg B, et al. Experimental wood smoke exposure in humans. Inhal Toxicol. 2006;18:855–864. doi: 10.1080/08958370600822391. [DOI] [PubMed] [Google Scholar]
- 18.Sehlstedt M, Dove R, Boman C, Pagels J, Swietlicki E, Löndahl J, et al. Antioxidant airway responses following experimental exposure to wood smoke in man. Part Fibre Toxicol. 2010;7:21. doi: 10.1186/1743-8977-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 20.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 21.vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomoda S, Programme ILOSA. Public emergency services: social dialogue in a changing environment: report for discussion at the joint meeting on public emergency services: social dialogue in a changing environment. Geneva: International Labour Office; 2003. [Google Scholar]
- 23.Black C, Gerriets JE, Fontaine JH, Harper RW, Kenyon NJ, Tablin F, et al. Early life wildfire smoke exposure is associated with immune dysregulation and lung function decrements in adolescence. Am J Respir Cell Mol Biol. 2017;56:657–666. doi: 10.1165/rcmb.2016-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noah TL, Zhou H, Zhang H, Horvath K, Robinette C, Kesic M, et al. Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers. Am J Respir Crit Care Med. 2012;185:179–185. doi: 10.1164/rccm.201103-0465OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaspers I, Pawlak EA, Addo K, Diamond C, Speen AM, Robinette C, et al. Effects of wood smoke exposure on nasal mucosal host defense responses after infection with live-attenuated influenza virus [abstract] Am J Respir Crit Care Med. 2016;193:A2872. [Google Scholar]
- 26.Noah TL, Rebuli ME, Speen AM, Robinette C, Jaspers I. Effect of woodsmoke particle exposure on antiviral host defense in human volunteers: nasal inflammatory mediators and influence of gender on responses [abstract] Am J Respir Crit Care Med. 2018;197:A4165. [Google Scholar]
- 27.Noah TL, Zhang H, Zhou H, Glista-Baker E, Müller L, Bauer RN, et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One. 2014;9:e98671. doi: 10.1371/journal.pone.0098671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis. 2014;209:S114–S119. doi: 10.1093/infdis/jiu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forero A, Fenstermacher K, Wohlgemuth N, Nishida A, Carter V, Smith EA, et al. Evaluation of the innate immune responses to influenza and live-attenuated influenza vaccine infection in primary differentiated human nasal epithelial cells. Vaccine. 2017;35:6112–6121. doi: 10.1016/j.vaccine.2017.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sue K. The science behind “man flu”. BMJ. 2017;359:j5560. doi: 10.1136/bmj.j5560. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, Klein RS. Sex drives dimorphic immune responses to viral infections. J Immunol. 2017;198:1782–1790. doi: 10.4049/jimmunol.1601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebuli ME, Speen AM, Clapp PW, Jaspers I. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol. 2017;312:L288–L296. doi: 10.1152/ajplung.00476.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton JA. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav. 2018;187:2–5. doi: 10.1016/j.physbeh.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zivkovic I, Petrovic R, Arsenovic-Ranin N, Petrusic V, Minic R, Bufan B, et al. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals. 2018;52:18–24. doi: 10.1016/j.biologicals.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Sun K, Torres L, Metzger DW. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 2010;84:5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casimir GJ, Lefèvre N, Corazza F, Duchateau J. Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol Sex Differ. 2013;4:16. doi: 10.1186/2042-6410-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen T, Liu HX, Yan HY, Wu DM, Ping J. Developmental origins of inflammatory and immune diseases. Mol Hum Reprod. 2016;22:858–865. doi: 10.1093/molehr/gaw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roved J, Westerdahl H, Hasselquist D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. 2017;88:95–105. doi: 10.1016/j.yhbeh.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Gottschalk RA, Martins AJ, Angermann BR, Dutta B, Ng CE, Uderhardt S, et al. Distinct NF-κB and MAPK activation thresholds uncouple steady-state microbe sensing from anti-pathogen inflammatory responses. Cell Syst. 2016;2:378–390. doi: 10.1016/j.cels.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller-Jensen K. Distinct signaling thresholds distinguish friend from foe. Cell Syst. 2016;2:360–361. doi: 10.1016/j.cels.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Tarabichi Y, Li K, Hu S, Nguyen C, Wang X, Elashoff D, et al. The administration of intranasal live attenuated influenza vaccine induces changes in the nasal microbiota and nasal epithelium gene expression profiles. Microbiome. 2015;3:74. doi: 10.1186/s40168-015-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Fire Protection Association. Firefighting occupations by women and race. 2017 [accessed 2017 Dec 21]. Available from: http://www.nfpa.org/News-and-Research/Fire-statistics-and-reports/Fire-statistics/The-fire-service/Administration/Firefighting-occupations-by-women-and-race.
- 46.Bønløkke JH, Riddervold IS, Grønborg TK, Skogstrand K, Hougaard DM, Barregard L, et al. Systemic effects of wood smoke in a short-term experimental exposure study of atopic volunteers. J Occup Environ Med. 2014;56:177–183. doi: 10.1097/JOM.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forchhammer L, Møller P, Riddervold IS, Bønløkke J, Massling A, Sigsgaard T, et al. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 2012;9:7. doi: 10.1186/1743-8977-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riddervold IS, Bønløkke JH, Mølhave L, Massling A, Jensen B, Grønborg TK, et al. Wood smoke in a controlled exposure experiment with human volunteers. Inhal Toxicol. 2011;23:277–288. doi: 10.3109/08958378.2011.567401. [DOI] [PubMed] [Google Scholar]
- 49.Stockfelt L, Sallsten G, Olin AC, Almerud P, Samuelsson L, Johannesson S, et al. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal Toxicol. 2012;24:47–59. doi: 10.3109/08958378.2011.633281. [DOI] [PubMed] [Google Scholar]
- 50.Unosson J, Blomberg A, Sandström T, Muala A, Boman C, Nyström R, et al. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol. 2013;10:20. doi: 10.1186/1743-8977-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awji EG, Chand H, Bruse S, Smith KR, Colby JK, Mebratu Y, et al. Wood smoke enhances cigarette smoke-induced inflammation by inducing the aryl hydrocarbon receptor repressor in airway epithelial cells. Am J Respir Cell Mol Biol. 2015;52:377–386. doi: 10.1165/rcmb.2014-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett EG, Henson RD, Seilkop SK, McDonald JD, Reed MD. Effects of hardwood smoke exposure on allergic airway inflammation in mice. Inhal Toxicol. 2006;18:33–43. doi: 10.1080/08958370500282340. [DOI] [PubMed] [Google Scholar]
- 53.Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat JL, et al. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol Sci. 2010;118:574–585. doi: 10.1093/toxsci/kfq290. [DOI] [PubMed] [Google Scholar]
- 54.Perng DW, Chang TM, Wang JY, Lee CC, Lu SH, Shyue SK, et al. Inflammatory role of AMP-activated protein kinase signaling in an experimental model of toxic smoke inhalation injury. Crit Care Med. 2013;41:120–132. doi: 10.1097/CCM.0b013e318265f653. [DOI] [PubMed] [Google Scholar]
- 55.Seilkop SK, Campen MJ, Lund AK, McDonald JD, Mauderly JL. Identification of chemical components of combustion emissions that affect pro-atherosclerotic vascular responses in mice. Inhal Toxicol. 2012;24:270–287. doi: 10.3109/08958378.2012.667455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burchiel SW, Lauer FT, Dunaway SL, Zawadzki J, McDonald JD, Reed MD. Hardwood smoke alters murine splenic T cell responses to mitogens following a 6-month whole body inhalation exposure. Toxicol Appl Pharmacol. 2005;202:229–236. doi: 10.1016/j.taap.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 57.Matthew E, Warden G, Dedman J. A murine model of smoke inhalation. Am J Physiol Lung Cell Mol Physiol. 2001;280:L716–L723. doi: 10.1152/ajplung.2001.280.4.L716. [DOI] [PubMed] [Google Scholar]
- 58.Kim YH, Warren SH, Krantz QT, King C, Jaskot R, Preston WT, et al. Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: implications for health effects from wildland fires. Environ Health Perspect. 2018;126:017011. doi: 10.1289/EHP2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Migliaccio CT, Bergauff MA, Palmer CP, Jessop F, Noonan CW, Ward TJ. Urinary levoglucosan as a biomarker of wood smoke exposure: observations in a mouse model and in children. Environ Health Perspect. 2009;117:74–79. doi: 10.1289/ehp.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. Adverse effects of wood smoke PM(2.5) exposure on macrophage functions. Inhal Toxicol. 2013;25:67–76. doi: 10.3109/08958378.2012.756086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.