Abstract
Cholesterol synthesis is a complex, coordinated process involving a series of enzymes. As of today, our understanding of subcellular localization of cholesterol biosynthesis enzymes is far from complete. Considering the complexity and intricacies of this pathway and the importance of functions of DHCR7, DHCR24 and EBP enzymes for human health, we undertook a study to determine their subcellular localization and co-localization.
Using expression constructs and antibody staining in cell cultures and transgenic mice, we found that all three enzymes are expressed in ER and nuclear envelope. However, their co-localization was considerably different across the cellular compartments. Furthermore, we observed that in the absence of DHCR7 protein, DHCR24 shows a compensatory upregulation in a Dhcr−/− transgenic mouse model.
The overall findings suggest that the sterol biosynthesis enzymes might not always work in a same functional complex, but that they potentially have different, multifunctional roles that go beyond the sterol biosynthesis pathway. Furthermore, the newly uncovered compensatory mechanism between DHCR7 and DHCR24 could be of importance for designing medications that would improve cholesterol production in patients with desmosterolosis and Smith-Lemli-Opitz syndrome.
Keywords: DHCR7, EBP, DHCR24, 7-dehydrocholesterol, 8-dehydrocholesterol, desmosterol
INTRODUCTION
Cholesterol is a critical component of cells, and is essential for life. Cholesterol synthesis is a complex, coordinated process involving a series of enzymes (Dietschy and Turley 2004; Mazein et al. 2013; Mitsche et al. 2015). Inherited metabolic disorders caused by mutations in sterol enzymes lead to prenatal demise or developmental disabilities such as Smith-Lemli-Opitz syndrome, desmosterolosis and chondrodysplasia punctata (Porter and Herman 2011).
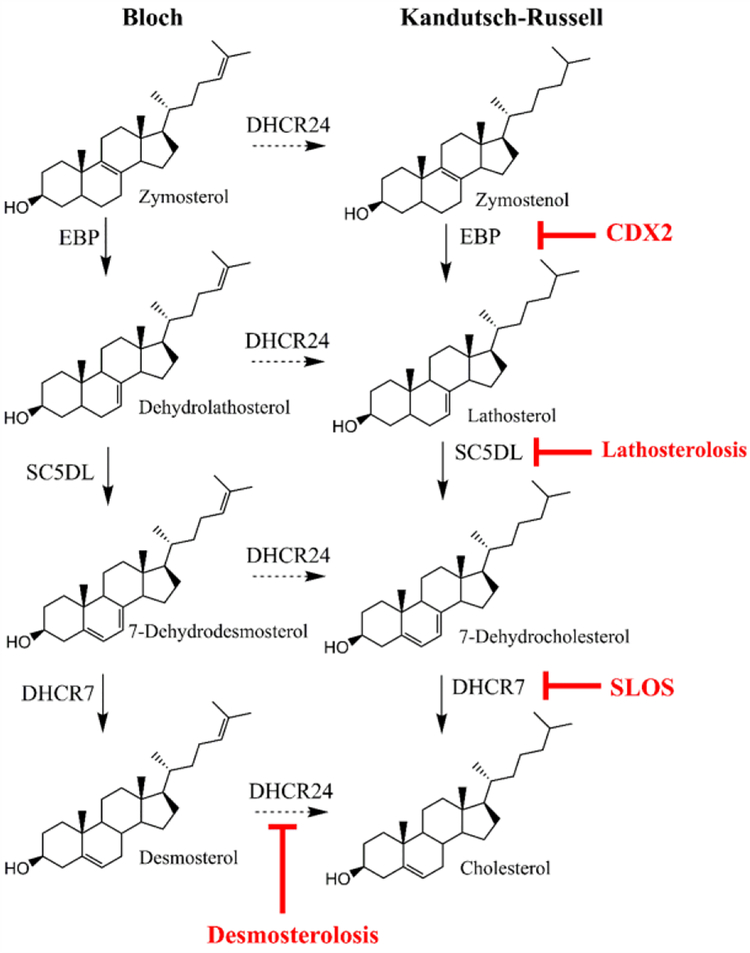
Cholesterol is synthesized by two distinct, yet interconnected pathways, known as the Bloch and Kandutsch-Russell pathways (Kandutsch and Russell 1960; Rittenberg et al. 1946). In the Kandutsch-Russell Pathway the last enzyme in the cholesterol biosynthesis pathway is 7-dehydrocholesterol reductase (DHCR7), while in the Bloch pathway the last step of cholesterol biosynthesis is catalyzed by 24-dehydrocholesterol reductase (DHCR24) (Figure 1).
Figure 1. Schematic drawing of the distal cholesterol biosynthesis pathway, with focus on DHCR7, DHCR24, SC5D and EBP.
Diseases associated with their insufficiency are in red lettering.
DHCR7 catalyzes the reduction of the C7–8 double bond in the B ring of 7-dehydrocholesterol (7-DHC) to form cholesterol, using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. Malfunction of DHCR7 enzyme leads to the accumulation of 7-DHC, a highly reactive and toxic compound, and a developmental disability known as Smith-Lemli-Opitz syndrome (SLOS, MIM 270400) (Smith et al. 1964).
In contrast, DHCR24 enzyme catalyzes the reduction of the C24 double bond, and converts desmosterol to cholesterol. DHCR24 activity is strictly dependent on flavine adenine dinucleotide (FAD) as the reducing agent, and mutations in DHCR24 give rise to a developmental disability known as desmosterolosis (MIM 602398) (Schaaf et al. 2011; Zolotushko et al. 2011).
Emopamil binding protein (EBP) (also known as cholestenol delta-isomerase) catalyzes the conversion of delta(8)-sterols to their corresponding delta(7) isomers. This includes the conversion of zymostenol into lathosterol and the conversion of zymosterol into dehydrolathosterol (Mazein et al. 2013). However EBP may act on other sterol intermediates in specific situations (Batta et al. 1995; Paik et al. 1986). For instance, in the neurodevelopmental disorder SLOS, the DHCR7 enzyme is mutated, which results in higher levels of 7-DHC. As 7-DHC accumulates, it can be a substrate for EBP, where EBP catalyzes the isomerization of the 7–8 double bond in the opposite direction and as a result, SLOS cells and tissues have also elevated 8-DHC levels (Batta et al. 1995; Paik et al. 1986). Mutations in the EBP gene result in X-linked chondrodysplasia punctata 2 (MIM 302960), a developmental disorder characterized by bone, skin, and eye abnormalities (Derry et al. 1999).
Based on predicted protein structure, DHCR7 and EBP are multipass endoplasmic reticulum proteins whereas DHCR24 has an atypical membrane topology with peduncle or re-entrant loop (Zerenturk et al.). In addition, DHCR7 and DHCR24 are believed to form a functional complex (Luu et al. 2015). Similarly, DHCR7 and EBP are shown to form hetero-oligomeric complex that acts as microsomal antiestrogen binding site for various pharmaceuticals (Kedjouar et al. 2004). It is believed that cholesterol biosynthetic pathway is compartmentalized, with various steps taking place in cytosol, mitochondria, peroxisomes, and endoplasmic reticulum (Kovacs et al. 2002). The postsqualene part of the pathway is believed to take place in the ER (Zerenturk et al.). These conclusions are based mainly on subcellular fractionation studies, immunocytochemical studies using antisera of undefined specificity and/or overexpression studies with tagged proteins in various cell lines.
As of today, our understanding of subcellular localization of cholesterol biosynthesis enzymes is far from complete. Considering the complexity and intricacies of this pathway and the importance of DHCR7, DHCR24 and EBP function for human health, we undertook a study to determine the subcellular localization of these proteins. In addition to monitoring endogenous expression and co-localization under physiological conditions, we analyzed expression of fluorescently tagged DHCR7, DHCR24 and EBP in mouse cell line. Furthermore, we assessed a potential compensatory co-regulation of these enzymes.
MATERIAL and METHODS
Plasmids, antibodies and reagents.
Plasmids used in the study were constructed using human sequence for three sterol genes (DHCR7 reference sequence: NM_001360.2, DHCR24 reference sequence: NM_014762.3 and EBP reference sequence NM_006579.2). The two plasmids with N-terminal EGFP tag are: pEGFP-C1-DHCR7 (6198 bp), pEGFP-C1-DHCR24 (6321 bp) and were generated by Gene PASS Inc, Nashville, TN. Full length human DHCR7 and human EBP were generated by PCR using human fibroblasts cDNA as a template and then cloned by inserting PCR products into pEGFP-N1 vector (pEGFP-N1-DHCR7) and into ptdTomato-N1 (ptdTomato-N1-EBP) to generate the respective proteins fluorescently tagged at the C-terminus. All plasmids were sequence verified.
Cell line and transfections.
Neuro2a (ATCC CCL-131, mouse neuroblastoma) were purchased from ATCC (Manassas, Virginia). Neuro2a cells were grown in Eagle’s Minimum Essential Medium (EMEM, Quality Biological, Gaithersburgh, MD) with 10% fetal bovine serum, L-glutamine and penicillin/streptomycin. Neuro2a cells were incubated at 37°C incubator with 5% CO2. Transient transfections of Neuro2a were carried out using either Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA) or Lonza (Lonza, Basel, Switzerland) Nucleofection reagent following protocols described by the manufacturers.
Western blotting.
Whole cell lysates were prepared from control or transfected cells grown in 10 cm tissue culture dishes. Once confluent, or 48 hrs posttransfection, cultures were chilled on ice and washed two times in phosphate-buffered saline (PBS), scraped and transferred to a 15 ml centrifuge tube. Pelleted cells were lysed with 0.5 ml of RIPA lysis buffer (VWR International, Radnor, PA) plus phosphatase inhibitors (Sigma-Aldrich) and protease inhibitors (Thermo Fisher Scientific), briefly sonicated and incubated on ice for 30 min. To clear the lysates, the samples were spun at 14,000 X g at 4°C for 5 min to pellet the debris. The protein concentration of supernatant was quantified with BCA protein assay. Equal amounts of protein from each sample were mixed with reducing reagent and loading buffer and heated to 70°C for 10 min. Proteins were separated on NuPAGE™ 4–12% Bis-Tris Protein Gels, (Thermo Fisher Scientific). Prestained protein ladder was used to evaluate the molecular weight. The Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell was used for the electrophoretic transfer using the polyvinylidene difluoride membranes (Immobilon-P PVDF Membrane, Sigma-Aldrich) and transfer buffer (25 mM Tris, 192 mM glycine, and 20% (v/v) methanol (pH 8.3)). Following transfer, PVDF membranes were blocked in 5% milk in TBS (50 mM Tris-Cl, 150 mM NaCl, pH 7.5) with 0.05% Igepal (Spectrum Chemical, New Brunswick, NJ) and incubated in primary antibody overnight at +4°C and secondary antibodies at room temperature for 1 hr. Membranes were probed with the following primary antibodies: mouse anti-EBP at 1:1000 dilution (catalog no. sc-374267, Santa Cruz Biotechnology), rabbit anti-DHCR24/Seladin-1 at 1:1000 dilution (catalog no. 2033, Cell Signaling Technology), rabbit anti-DHCR7 at 1:1000 dilution (catalog no. PA5–48204, Thermo Fisher Scientific), rabbit anti-SC5DL at 1:2000 dilution (catalog no. ab221764, Abcam), and mouse anti-β-Actin at 1:20,000 dilution (catalog no. 3700, Cell Signaling Technology). As secondary antibodies, peroxidase-coupled anti-mouse and anti-rabbit antibodies were used (Sigma Aldrich). The blots were developed using enhanced chemiluminescence with Radiance Plus (Azure Biosystems, Dublin, CA) according to the manufacturers’ instructions. Chemiluminescent detection was carried out by Azure c300 system (Azure Biosystems). Quantification of protein levels was performed using the AzureSpot Analysis Software (Azure Biosystems), and data were normalized to β-actin. Validation of proper targeting and construct co-localization with antibody staining is shown in Supplemental Figure 1.
Immunofluorescence microscopy.
For the confocal imaging, cells were plated on 12 mm round glass coverslips coated with either poly-L-lysine hydrobromide (Sigma-Aldrich, Saint Louis, MO) or poly-D-lysine (Corning, Corning, NY) and grown in defined medium without cholesterol (Neuro2a in EMEM plus N2 supplement). Cells were fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature. For EBP staining an additional permeabilization step was performed with ice-cold methanol for 10 minutes at −20°C. Specimens were blocked in blocking buffer (PBS with 10% FBS and 0.1% saponin (Alfa Aesar, Haverhill, MA) for 60 minutes at room temperature. The following primary antibodies were used for immunofluorescence microscopic analysis: rabbit anti-PDI at 1:100 dilution (catalog no. 3501, Cell Signaling Technology, Danvers, MA), rabbit anti-RCAS1 at 1:200 dilution (catalog no. 12290, Cell Signaling Technology), rabbit anti-emerin at 1:400 dilution (catalog no. 30853, Cell Signaling Technology), mouse anti-EBP at 1:50 dilution (catalog no. sc-374267, Santa Cruz Biotechnology), rabbit anti-DHCR24/Seladin-1 at 1:50 dilution (catalog no. 2033, Cell Signaling Technology), rabbit anti-SC5D at 1:50 dilution (catalog no. ab221764, Abcam), and rabbit anti-DHCR7 at 1:10 dilution (PA5–48204, Thermo Fisher Scientific, Waltham, MA). The secondary antibodies (Cy3-conjugated, DyLight488-conjugated or Alexa Fluor 488- conjugated) were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and used at 1:2,000, 1:500 and 1:500 dilutions, respectively. Nuclei were counterstained with Hoechst dye (0.5 μg/mL final concentration, Thermo Fisher Scientific, Waltham, MA). Slides were imaged on a Nikon Eclipse FM inverted confocal laser-scanning microscope. Images were acquired using a 60X (1.4 oil Plan APO OFN25 DIC, 0.17 WD, 0.13) oil objective and LU-NV laser unit measuring wavelengths: 405 nm, 488 nm, 561 nm, and 640 nm.
Antibody to DHCR7.
We found a commercially available anti-DHCR7 antibody (catalog no. PA5–48204, Thermo Fisher Scientific), which, based on our data, seems to recognize correctly endogenous DHCR7 protein in whole brain lysates of transgenic mice in western blot experiments (Figure 6). In addition the antibody performed well in immunocytochemistry studies as shown in Supplemental Figure 1 and recognizing both recombinant and endogenous DHCR7 protein (not shown), respectively. Western blotting experiments also showed that DHCR7 protein migrates at ~44 kDa, which is less than its predicted molecular weight, 54.5 kDa (similar observation was reported previously (Moebius et al. 1998)). Aberrant migration pattern has been shown for the structurally related lamin B receptor as well, in the case of which a similar discrepancy between the calculated and observed molecular mass was observed (Worman et al. 1990). This phenomenon (“gel shifting”) is not uncommon among membrane proteins (Rath et al. 2009).
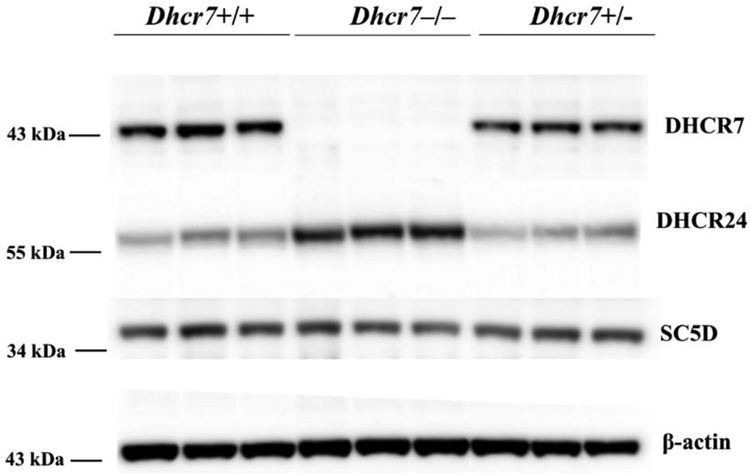
Figure 6. DHCR24 expression compensates for the lack of DHCR7 in Dhcr7−/− newborn mice brains.
Western blot analysis of DHCR7, DHCR24 and SC5D proteins in whole brain tissue samples of Dhcr7+/+, Dhcr7–/–, Dhcr7+/− newborn mice. 40 μg total protein was loaded per lane, 3 biological replicates are shown. Data were normalized to beta-actin. DHCR24 protein expression was greatly increased (2.25-fold, p<0.05) in Dhcr7–/– (knockout) whole brain tissue lysates when compared to Dhcr7+/+ (wild type). There was no difference between Dhcr7+/− (heterozygous) and Dhcr7+/+ (wild type) samples for DHCR24. SC5D protein expression level did not show any difference in knockout or heterozygous samples in comparison to the wild type samples.
Mouse studies:
Adult mice, B6.129P2(Cg)-Dhcr7tm1Gst/J, were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were housed under a 12-h light-dark cycle at constant temperature (25°C) and humidity with ad libitum access to food and water in Comparative Medicine at the UNMC, Omaha, NE. Newborn mice from Het (Dhcr7+/−) × Het (Dhcr7+/−) mating were used for the study. Genotyping was done on tail samples to determine the phenotype of samples. Whole brains were dissected, frozen in pre-chilled methyl butane on dry ice, and stored at −80°C. The brain samples of Dhcr7+/+, Dhcr7−/−, and Dhcr7+/− newborn mice were homogenized in ice-cold RIPA lysis buffer (VWR International, Radnor, PA) by sonication. The rest of procedure for protein extraction, electrophoresis and western blotting was identical to the procedure outlined above for the cell cultures. All procedures were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals. The use of mice in this study was approved by the Institutional Animal Care and Use Committee of UNMC.
RESULTS
DHCR7, DHCR24 and EBP proteins localize to the endoplasmic reticulum (ER)
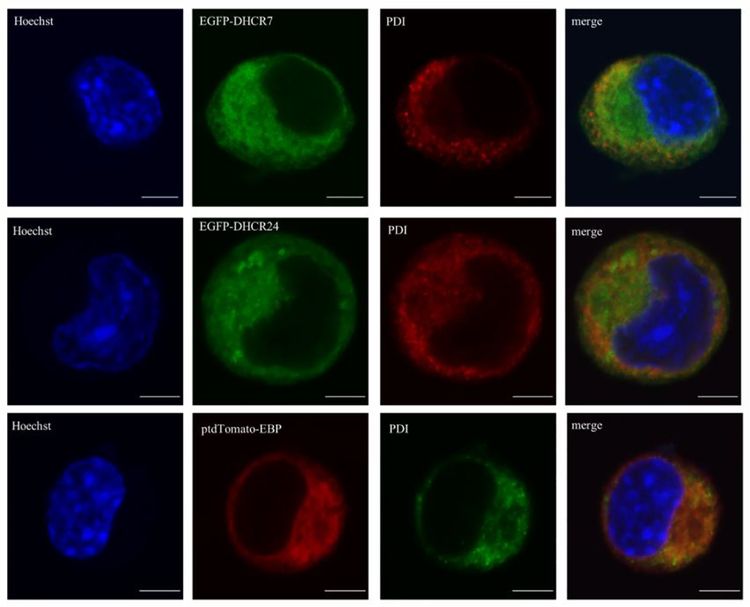
Sterol synthesis takes place in the endoplasmic reticulum (Dietschy and Turley 2004). Using confocal imaging, we compared the subcellular localization of transfected EGFP-tagged DHCR7 with an ER resident protein using Neuro2a cells. These cell lines were chosen based on their previously established cholesterol production (Korade et al. 2009). Protein disulfide isomerase (PDI), a luminal ER protein, was used as a marker of ER for establishing co-localization. Nuclei were visualized by Hoechst dye.
Our experiments revealed that all three sterol biosynthesis proteins (DHCR7, DHCR24 and EBP) were localized in the ER (Figure 2). The ER localization was similar regardless of the fluorescent tag position at the C- or N-terminal of the DHCR7 protein (data not shown). However, we noticed that the transient transfection was more effective with N-terminal tag in our experiments. It is also important to note that the co-localization was not complete with PDI staining in the case of DHCR7 and DHCR24, suggesting that these two proteins localize to other cell compartments as well, while EBP was shown to be mainly localized in the ER.
Figure 2. DHCR7, DHCR24 and EBP proteins localize to the endoplasmic reticulum.
Neuro2a cells were transiently transfected with EGFP-DHCR7, EGFPDHCR24 and ptdTomato-EBP fusion constructs. Cells were labeled with anti-PDI (luminal ER protein) antibody and analyzed by confocal microscopy. Nuclei were visualized by Hoechst dye. Original images were collected with a 60× oil objective. Calibration bar, 5 μm. Note that all three proteins of interest co-localize with PDI, suggesting ER expression.
DHCR7 protein is localized in the Golgi apparatus (GA)
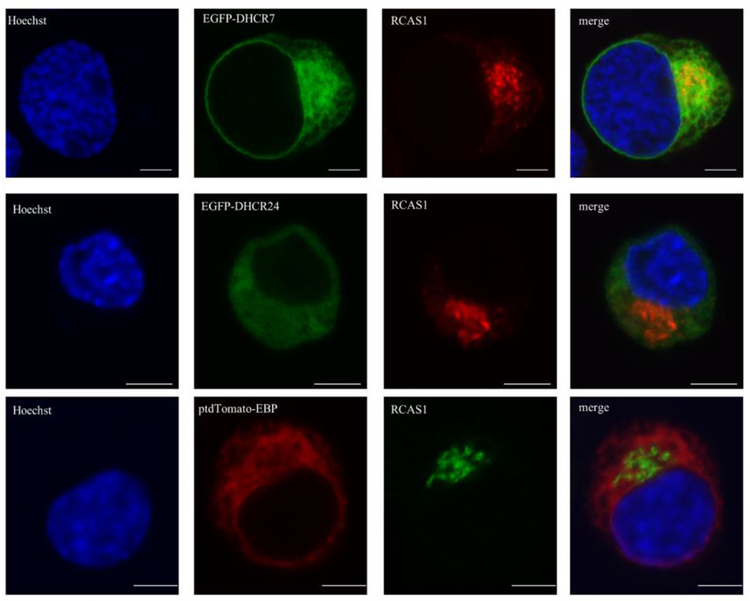
Golgi is a place where proteins undergo extensive post-translational modification, such as addition of carbohydrates (Deng et al. 2018). To test the localization of the three recombinant proteins of interest, we analyzed their expression in Golgi apparatus, using antibody staining for receptor binding cancer antigen expressed on Siso cells (RCAS1), a transmembrane Golgi resident protein (Figure 3). From the three assessed proteins, DHCR7 showed staining in Golgi apparatus, while we observed no staining in Golgi with DHCR24 and EBP compared to that observed in ER.
Figure 3. DHCR7 protein localizes to the Golgi apparatus.
Neuro2a cells were transiently transfected with EGFP-DHCR7, EGFP-DHCR24 and ptdTomato-EBP fusion constructs. Cells were labeled with anti-RCAS1 (Golgi resident protein) antibody and analyzed by confocal microscopy. Nuclei were visualized by Hoechst dye. Original images were collected with a 60× oil objective. Calibration bar, 5 μm. Note that EGFPDHCR7 localized to the Golgi apparatus while EGFP-DHCR24 and ptdTomato-EBP showed no expression in the Golgi.
DHCR7, DHCR24 and EBP proteins are expressed in the nuclear envelope (NE)
The nuclear envelope is a double membrane that provides compartmentalization between the cytoplasm and the nuclear material (Hetzer 2010). It serves as an active trafficking route for ribonucleotides and is comprised of diverse groups of proteins that are typically not enriched in the ER (Hetzer 2010).
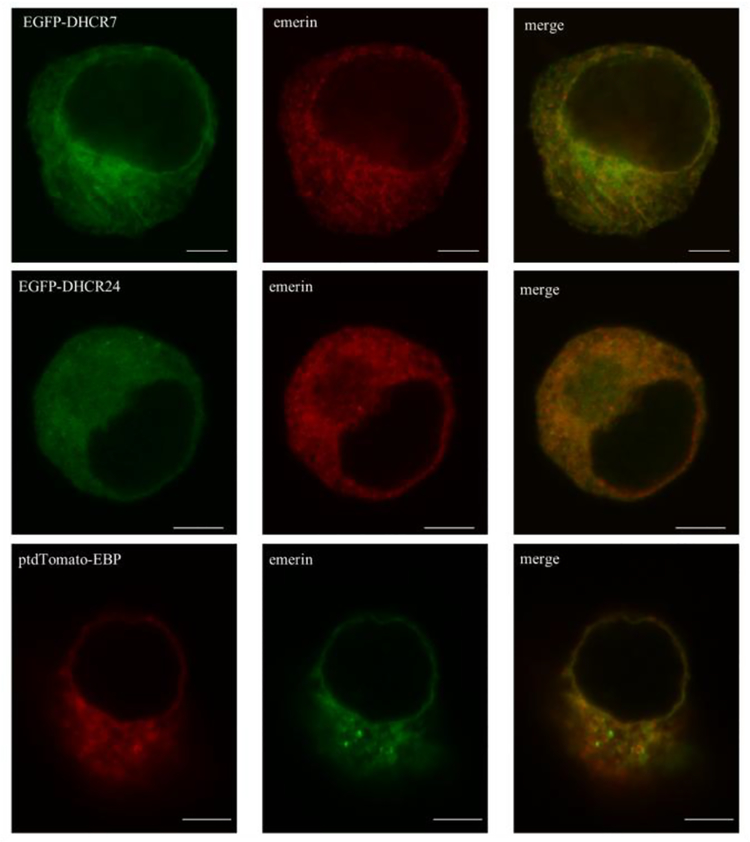
To test the expression of the three proteins of our interest, we stained the cells for emerin, a protein known to localize to the ER and NE inner and outer membranes. Confocal imaging revealed localization of DHCR7, DHCR24 and EBP to the NE (Figure 4). All three proteins showed nearly identical staining in NE, showing a full co-localization at this subcellular site.
Figure 4. DHCR7, DHCR24 and EBP proteins show comparable expression in the nuclear envelope.
Neuro2a cells were transiently transfected with EGFP-DHCR7, EGFP-DHCR24 and ptdTomato-EBP fusion constructs. Cells were labeled with anti-emerin (localizing to the ER and NE inner and outer membranes) antibody and analyzed by confocal microscopy. Nuclei were visualized by Hoechst dye. Original images were collected with a 60× oil objective. Calibration bar, 5 μm. Note that all three recombinant proteins co-localized with emerin in the nuclear envelope, and ER localization for all three proteins was similar to that observed previously with anti-PDI labeling.
DHCR7, DHCR24, EBP proteins co-localize with each other
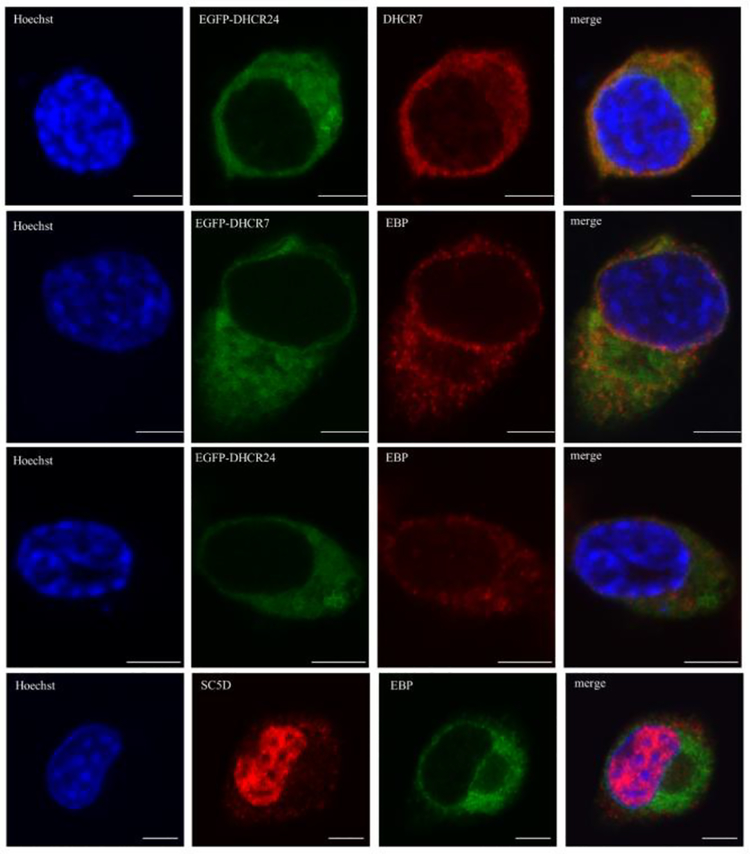
As cholesterol biosynthesis is a step-wise process, initially we expected all three proteins will show identical subcellular expression pattern. However, based on the staining patterns observed in Figures 2–3, this did not seem to be the case. Thus, we decided to test the subcellular co-localization of the three proteins of interest.
For the co-localization experiments, we used commercially available antibodies against DHCR7and EBP. We also used antibody against sterol-C5-desaturase (SC5D), another upstream cholesterol biosynthesis enzyme, which catalyzes the conversion of lathosterol into 7-dehydrocholesterol (Anderson et al. 2018; Jiang et al. 2010; Rossi et al. 2007). We performed three different sets of co-localization experiments: 1) recombinant DHCR7 and recombinant DHCR24 with endogenous EBP; 2) recombinant DHCR24 with endogenous DHCR7, and; 3) endogenous SC5D with endogenous EBP (Figure 5). Although our experiments confirmed that DHCR7 and DHCR24 both localize to the ER these two proteins did not show a full overlap with each other.
Figure 5. DHCR7, DHCR24, EBP and SC5D cholesterol biosynthesis enzymes show only a partial co-localization.
Neuro2a cells were transiently transfected with EGFP-DHCR7 and EGFP-DHCR24 fusion constructs (rows 1–3) and labeled with anti-EBP and anti-DHCR7. Control Neuro2a cells were double stained with anti-SC5D and anti-EBP antibodies (bottom row). Cells were analyzed by confocal microscopy. Nuclei were visualized by Hoechst dye. Original images were collected with a 60× oil objective. Calibration bar, 5 μm. Note that DHCR7 and DHCR24 showed the most prominent co-localization throughout the cellular compartments, while the more proximal sterol biosynthesis enzyme SC5D in addition to overlap with DHCR7, DHCR24 and EBP within ER is also present in the nucleus.
SC5D was localized to ER in close proximity to other enzymes. Surprisingly, SC5D showed very strong expression in the nucleus, suggesting that it may have another role not related to cholesterol synthesis.
Compensatory co-regulation of DHCR7 and DHCR24 expression in vivo
Based on our observation that DHCR7 and DHCR24 showed highest subcellular co-localization (whereas SC5D showed overlapping and a distinct localization from both), we hypothesized that there could be compensatory co-regulation between DHCR7 and DHCR24, but not SC5D.
To test this in an in vivo model, we performed a Western blot analysis of DHCR7, DHCR24 and SC5D proteins in whole brain tissue samples of Dhcr7–/–, Dhcr7+/− and wild type (Dhcr7+/+) mice at postnatal day 0 (Figure 6). We found that DHCR24 protein expression was greatly increased (2.25-fold; p<0.01) in Dhcr7−/− knockout whole brain tissue lysates when compared to Dhcr7+/+ (wild type) samples. However, there was no difference between Dhcr7+/− (heterozygous) and Dhcr7+/+ (wild type) samples for DHCR24 protein levels. As expected, SC5D protein expressions were unaltered in all three genotypes.
DISCUSSION
Cholesterol is an essential component of life, and participates in hundreds of processes in the cell (Dietschy and Turley 2004; Mitsche et al. 2015). Mutations in cholesterol biosynthesis enzymes lead to about a dozen of well-described genetic syndromes, with various clinical presentations (Porter and Herman 2011). However, the role of cholesterol precursors and the roles of synthesizing enzymes is not well understood, and it is unclear how does their improper function give rise to the disease-associated pathophysiology. Importantly, beyond the well-defined genetic syndromes, expression changes of both DHCR7 and DHCR24 has been observed in multiple non-genetic diseases, ranging from Alzheimer’s disease to adrenal cancer (Luu et al. 2015; Sharpe et al.; Zerenturk et al.). Thus, understanding their intracellular localization and co-localization is an important avenue of investigation, which might give us clues about the functioning of this complex protein network.
In this study we set out to understand the subcellular localization of DHCR7, DHCR24 and EBP proteins. The main conclusions of this study can be summarized as follows: 1) DHCR7, DHCR24 and EBP are all expressed in ER. 2) The sterol enzyme DHCR7 is expressed in Golgi. 3) All three proteins show nearly identical expression level in the nuclear envelope. 4) Co-localization with upstream cholesterol biosynthesis enzymes, such as SC5D, is minimal. 5) In the absence of DHCR7 protein, DHCR24 shows a compensatory upregulation in an in vivo transgenic mouse model.
ER is a network of membranes distributed throughout the cytoplasm and contiguous with the nuclear envelope membrane, and it is a site of protein and lipid biosynthesis (Krisans 1996). Therefore, it is not surprising that critical sterol synthesizing enzymes are co-localized in the ER and may act on multiple substrates. EBP converts delta(8)-sterols to their corresponding delta(7) isomers but under certain conditions EBP can act on 7-DHC to convert it into 8-DHC (Batta et al. 1995; Paik et al. 1986). Another example is DHCR24 enzyme which reduces the double bond at the C24 position. This particular enzyme can act on different sterols in the cholesterol biosynthesis pathway, working as a bridge between the Kandutsch-Russell and Bloch pathways. In addition to acting on different substrates, sterol biosynthesis enzymes, just like many other proteins, are multifunctional, and participate in physiological processes that are unrelated to sterol biosynthesis. These additional roles might be tissue specific, which is suggested by the different clinical manifestations of various genetic disorders associated with sterol synthesis enzyme dysfunctions. Clearly, this is a very interesting hypothesis, and should be assessed in follow-up studies.
The in vivo upregulation of DHCR24 in the absence of DHCR7 is also noteworthy. This is a yet unknown compensatory mechanism, potentially shifting cholesterol biosynthesis from the Kandutsch-Russell pathway to the Bloch pathway (Mitsche et al. 2015). It is also possible that post-transcriptional regulation may yield differential activity of specific sterol enzymes as shown by Robertson et al (Robertson and Ghazal 2016; Robertson et al. 2016). Their study shows that mir-342 preferentially targets DHCR7 over DHCR24. However, knowing the severe phenotype of the Dhcr7−/− mice, it is clear that this compensatory mechanism is not sufficient to maintain regular cholesterol production. Regardless of this insufficient adaptation process, this newly uncovered compensatory mechanism could be of importance for designing medications that would improve cholesterol production in patients with desmosterolosis and Smith-Lemi-Opitz syndrome.
Supplementary Material
In this validation study, Neuro2a cells were transiently transfected with EGFP-DHCR7, EGFP-DHCR24 and ptdTomato-EBP fusion constructs. Cells were labeled with anti-DHCR7, anti-DHCR24 and anti-EBP antibodies, respectively, in order to verify antibody specificity and analyzed by confocal microscopy. Nuclei were visualized by Hoechst dye. Original images were collected with a 60× oil objective. Calibration bar, 5 μm. Note that the recombinant proteins and antibody staining fully co-localize.
Acknowledgements
This work was supported by The National Institutes of Health, NIMH MH110636 (KM) and MN067234 (KM). Katalin Koczok was the Rosztoczy Foundation scholar.
REFERENCES
- Anderson R, Rust S, Ashworth J, Clayton-Smith J, Taylor RL, Clayton PT, Morris AAM (2018) Lathosterolosis: A Relatively Mild Case with Cataracts and Learning Difficulties JIMD Rep doi: 10.1007/8904_2018_127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batta AK, Tint GS, Shefer S, Abuelo D, Salen G (1995) Identification of 8-dehydrocholesterol (cholesta-5,8-dien-3 beta-ol) in patients with Smith-Lemli-Opitz syndrome J Lipid Res 36:705–713 [PubMed] [Google Scholar]
- Deng S, Liu H, Qiu K, You H, Lei Q, Lu W (2018) Role of the Golgi Apparatus in the Blood-Brain Barrier: Golgi Protection May Be a Targeted Therapy for Neurological Diseases Mol Neurobiol 55:4788–4801 doi: 10.1007/s12035-017-0691-3 [DOI] [PubMed] [Google Scholar]
- Derry JM et al. (1999) Mutations in a delta 8-delta 7 sterol isomerase in the tattered mouse and X-linked dominant chondrodysplasia punctata. jderry@immunex.com Nat Genet 22:286–290 doi: 10.1038/10350 [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD (2004) Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal J Lipid Res 45:1375–1397 doi: 10.1194/jlr.R400004-JLR200 [DOI] [PubMed] [Google Scholar]
- Hetzer MW (2010) The nuclear envelope Cold Spring Harb Perspect Biol 2:a000539 doi: 10.1101/cshperspect.a000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XS, Backlund PS, Wassif CA, Yergey AL, Porter FD (2010) Quantitative proteomics analysis of inborn errors of cholesterol synthesis: identification of altered metabolic pathways in DHCR7 and SC5D deficiency Mol Cell Proteomics 9:1461–1475 doi: 10.1074/mcp.M900548-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandutsch AA, Russell AE (1960) Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol J Biol Chem 235:2256–2261 [PubMed] [Google Scholar]
- Kedjouar B et al. (2004) Molecular characterization of the microsomal tamoxifen binding site J Biol Chem 279:34048–34061 doi: 10.1074/jbc.M405230200 M405230200 [pii] [DOI] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK, Mirnics K (2009) Molecular consequences of altered neuronal cholesterol biosynthesis J Neurosci Res 87:866–875 doi: 10.1002/jnr.21917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs WJ, Olivier LM, Krisans SK (2002) Central role of peroxisomes in isoprenoid biosynthesis Prog Lipid Res 41:369–391 [DOI] [PubMed] [Google Scholar]
- Krisans SK (1996) Cell compartmentalization of cholesterol biosynthesis Ann N Y Acad Sci 804:142–164 [DOI] [PubMed] [Google Scholar]
- Luu W, Hart-Smith G, Sharpe LJ, Brown AJ (2015) The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally J Lipid Res 56:888–897 doi: 10.1194/jlr.M056986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazein A, Watterson S, Hsieh WY, Griffiths WJ, Ghazal P (2013) A comprehensive machine-readable view of the mammalian cholesterol biosynthesis pathway Biochem Pharmacol 86:56–66 doi: 10.1016/j.bcp.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsche MA, McDonald JG, Hobbs HH, Cohen JC (2015) Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways Elife 4:e07999 doi: 10.7554/eLife.07999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius FF, Fitzky BU, Lee JN, Paik YK, Glossmann H (1998) Molecular cloning and expression of the human delta7-sterol reductase Proc Natl Acad Sci U S A 95:1899–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik YK, Billheimer JT, Magolda RL, Gaylor JL (1986) Microsomal enzymes of cholesterol biosynthesis from lanosterol. Solubilization and purification of steroid 8-isomerase J Biol Chem 261:6470–6477 [PubMed] [Google Scholar]
- Porter FD, Herman GE (2011) Malformation syndromes caused by disorders of cholesterol synthesis J Lipid Res 52:6–34 doi: 10.1194/jlr.R009548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM (2009) Detergent binding explains anomalous SDS-PAGE migration of membrane proteins Proc Natl Acad Sci U S A 106:1760–1765 doi: 10.1073/pnas.0813167106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg D, Borek E, Bloch K (1946) Synthesis of cholesterol in liver slices Fed Proc 5:151. [PubMed] [Google Scholar]
- Robertson KA, Ghazal P (2016) Interferon Control of the Sterol Metabolic Network: Bidirectional Molecular Circuitry-Mediating Host Protection Front Immunol 7:634 doi: 10.3389/fimmu.2016.00634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KA et al. (2016) An Interferon Regulated MicroRNA Provides Broad Cell-Intrinsic Antiviral Immunity through Multihit Host-Directed Targeting of the Sterol Pathway PLoS Biol 14:e1002364 doi: 10.1371/journal.pbio.1002364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M et al. (2007) Clinical phenotype of lathosterolosis Am J Med Genet A 143A:2371–2381 doi: 10.1002/ajmg.a.31929 [DOI] [PubMed] [Google Scholar]
- Schaaf CP et al. (2011) Desmosterolosis-phenotypic and molecular characterization of a third case and review of the literature Am J Med Genet A 155A:1597–1604 doi: 10.1002/ajmg.a.34040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LJ, Wong J, Garner B, Halliday GM, Brown AJ Is seladin-1 really a selective Alzheimer’s disease indicator? J Alzheimers Dis 30:35–39 doi:UP771303357T5205 [pii] 10.3233/JAD-2012-111955 [DOI] [PubMed] [Google Scholar]
- Smith DW, Lemli L, Opitz JM (1964) A Newly Recognized Syndrome of Multiple Congenital Anomalies J Pediatr 64:210–217 [DOI] [PubMed] [Google Scholar]
- Worman HJ, Evans CD, Blobel G (1990) The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains J Cell Biol 111:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerenturk EJ, Sharpe LJ, Brown AJ DHCR24 associates strongly with the endoplasmic reticulum beyond predicted membrane domains: implications for the activities of this multi-functional enzyme Biosci Rep 34 doi:BSR20130127 [pii] 10.1042/BSR20130127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerenturk EJ, Sharpe LJ, Ikonen E, Brown AJ Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis Prog Lipid Res 52:666–680 doi:S0163–7827(13)00055–6 [pii] 10.1016/j.plipres.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Zolotushko J et al. (2011) The desmosterolosis phenotype: spasticity, microcephaly and micrognathia with agenesis of corpus callosum and loss of white matter Eur J Hum Genet 19:942–946 doi: 10.1038/ejhg.2011.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this validation study, Neuro2a cells were transiently transfected with EGFP-DHCR7, EGFP-DHCR24 and ptdTomato-EBP fusion constructs. Cells were labeled with anti-DHCR7, anti-DHCR24 and anti-EBP antibodies, respectively, in order to verify antibody specificity and analyzed by confocal microscopy. Nuclei were visualized by Hoechst dye. Original images were collected with a 60× oil objective. Calibration bar, 5 μm. Note that the recombinant proteins and antibody staining fully co-localize.