Precis:
T cells in the bone marrow from patients with acute myeloid leukemia have increased expression of several immune checkpoint molecules. Can this knowledge be translated to novel therapeutic strategies?
Keywords: Acute Myeloid Leukemia, Immunotherapy, Immune Checkpoint Blockade, AML, Bone Marrow
Although immune checkpoint blockade (ICB) has revolutionized therapy for many advanced solid tumors, reported early results from clinical trials have not suggested the same degree of impressive clinical benefits in myeloid malignancies, such as acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), using antibodies targeting CTLA4 or PD1/PD-L1 as single agents1–3. The underlying mechanisms for poor response to ICB remain to be fully elucidated. In solid cancers, there are two biomarkers approved by the United States Food and Drug Administration (FDA), namely PD-L1 expression level and microsatellite instability or mismatch repair deficiency. Amid the rush to try to replicate the clinical success of ICB in myeloid malignancies, many clinical trials were initiated using ICB. This was done despite the lack of comprehensive analyses of PD-L1 expression and T cell infiltration in human myeloid leukemia bone marrow microenvironment. Recently, studies characterizing T-cell dysfunction and their reversibility with intensive chemotherapy in AML have been published, but detailed understanding of the dynamic and serial changes in the immune checkpoint molecules remained lacking4, 5. In this issue of Cancer, Williams et al. explore bone marrow microenvironment including, but not limited to, PD-L1 expression and the quantity of infiltrated T cells in an exhaustive manner. They assessed the T cell quantity in healthy versus AML bone marrow, and demonstrated the expression pattern of a set of targetable coinhibitory and costimulatory receptors on T cells, and their respective ligands on the AML blasts, using multi-color flow cytometry.
A key result in the paper by Williams et al. is that neither the percentage or absolute number (adjusted for overall cellularity) of T cells are significantly different in bone marrows from patients with relapsed/refractory AML compared to age-matched healthy donors using immunohistochemistry (IHC) on bone marrow biopsies. Although this study was carried out on a smaller subset of the patients (n=13), the result bears promise for therapies targeting coinhibitory or costimulatory molecules on T cells as it indicates that T cells are present in the AML microenvironment and that reduced number of T cells, or T cell exclusion, is not a common phenomenon. Furthermore, assessment of bone marrow aspirate showed increased frequency of CD3+ T cells in the AML patients versus healthy donors, suggesting T cell immune response is indeed exerted in AML.
The bone marrow aspirates, from 39 newly diagnosed and 68 relapsed patients, were assessed for 17 surface markers using flow cytometry. The expression of these co-inhibitory and co-stimulatory molecules on the CD4+ T effector cells, regulatory T cells (Tregs), and CD8+ T cells was determined. In addition, the expression of the cognate ligands was assessed on the AML blasts. Key interactions and a schematic overview of T cell costimulation/coinhibition is illustrated in figure 1. In the end, the expression patterns of the various T cell receptors and ligands were compared with karyotype, patient age, and mutations in 28 myeloid associated genes.
Figure 1.
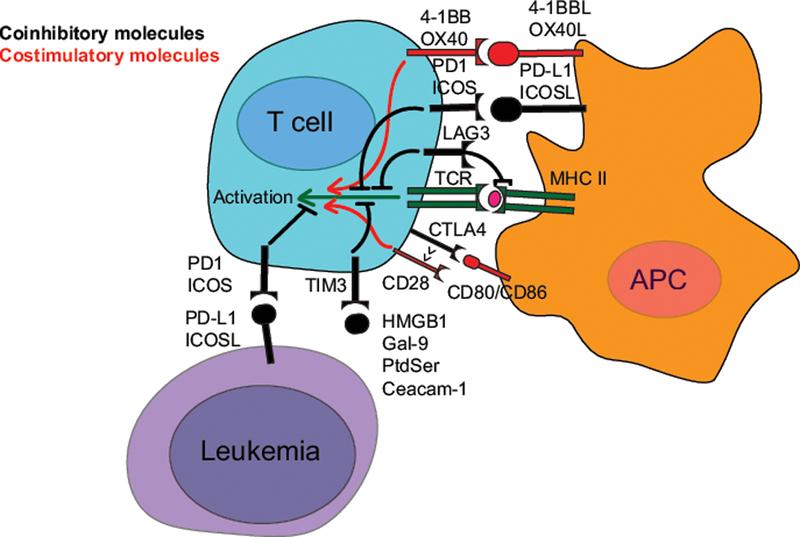
Key molecular mechanisms for a selection of immune checkpoint molecules. The initiation of T cell activation is dependent on the interaction of a T-Cell Receptor (TCR, dark green) on the T cell to a peptide antigen presented by the Major Histocompatibility Complex (MHC, dark green) on an Antigen Presenting Cell (APC). The coinhibitory (black) and costimulatory (red) receptors shape the response and fate of the T cell, and the subsequent immune response. For simplicity, the figure is focused on major interaction partners, and ignores the fact that these molecules are expressed on a variety of cells in distinct contexts.
Of the T cell subsets, only CD4+ Tregs were changed to a statistically significant degree, although all subsets (total CD4+, CD4+ T effector and CD8+) were increased. Thus, it is not unlikely that AML might lead to increase in other T cell subsets in the bone marrow of certain patients, as reflected in the large spread of the data. Further analyses showed that the quantity of OX40+ and PD1+ CD8 T cells were more abundant in AML patient samples than in healthy controls. Within the CD4+ effector population, the three subsets positive for either one of OX40+, PD1+ or ICOS+ were also increased. Among the CD4+ Tregs, OX40+ cells was the only subpopulation found in a statistically significant higher frequency in bone marrow from AML patients. Thus, this study highlights OX40 as an interesting costimulatory molecule expressed on several T cell populations in AML and raises questions regarding the implications of this finding for therapeutic interventions.
Another interesting observation in this paper is the high frequency of T cells expressing PD1 in addition to TIM3 or LAG3. These double positive cells are believed to represent more dysfunctional, but targetable, T cell populations than the populations only positive for either one of the molecules6, 7. This indicates that T cells recognize AML cells but fail to exert cytotoxicity. A subgroup of patients had much higher frequency of PD1+/TIM3+ or PD1+/LAG3+ T cells. This indicates that some patients probably have, whereas others might not have, more leukemia-antigen-experienced T cells in their bone marrow. It is unclear why only fraction of patients have higher expression of PD1+/TIM3+ or PD1+/LAG3+ T cells. Understanding the generation and function of various subsets of T cells in the AML bone marrow environments is important in the pursuit of immunotherapeutic strategies in AML.
A key challenge will be to reinvigorate exhausted T cells that are recognizing cancer antigens, possibly expressing multiple checkpoint molecules. In addition, T cell exhaustion in the setting of cancer is not that well defined, but it is reasonable to assume that the various cellular markers of exhaustion either are expressed on different subtypes of this dysfunctional state, or at different points along an “exhaustion scale”. Further exploration of the immune function in the patients seemingly without exhausted T cells will also prove important.
When focusing on the AML blasts, the authors found that samples with a mutation in the tumor suppressor gene TP53 had a higher percentage of PD-L1+ blasts, compared to blasts without this mutation. This is believed to be a result of an already documented mechanism, as loss of TP53 leads to decreased level of a microRNA that negatively regulates PD-L1 expression8. How this increase in PD-L1 influences the response of anti-PD1/PD-L1 therapy is not known. Further exploration of AML-blast-intrinsic mechanisms that might influence the immune milieu and response to immunotherapies such as ICB are underway. This knowledge, gained through next generation sequencing, might prove critical in designing therapies in the era of precision medicine.
Williams et al. showed that T cells are present and phenotypically changed in the AML bone marrow, and that the phenotype bears similarity to the exhausted phenotype seen in other cancers9. This indicates that the poor response to anti-PD therapy in AML might be associated with other immune evasion mechanisms, including other checkpoint molecules. Thus, mechanistic studies of the functional overlap and redundancy of the various checkpoint molecules will help guide the identification of the correct subset of exhausted T cells that can be therapeutically reinvigorated to eradicate the cancer. A detailed understanding of the immune defects in myeloid malignancies will hopefully allow for rational design of clinical trials and choice of specific agents and combinations. As the only curative treatment of AML is allogeneic hematopoietic stem cell transplantation (allo-HSCT), the benefit and safety of ICB (either anti-CTLA4, anti-PD1 or a combination both) after allo-HSCT to reduce relapse is under investigation (NCT02846376, table 1). A number of other combinations to increase efficacy are also under investigation, and some of these ongoing clinical trials are listed in table 1.
Table 1.
A selection of ongoing trials investigating the effect of the two clinically approved ICBs, anti-PD1 and anti-CTLA4, in acute myeloid leukemia.
| Selected ongoing trials investigating Immune Checkpoint Blockade in AML | |||
| Study | Intervention | Design/phase | NCT |
| Lymphodepletion and anti-PD-1 blockade to Reduce Relapse in AML patients not eligble for transplant | Fludarabin/melphalan and prembolizumab | Phase II | NCT02771197 |
| Nivolumab in AML in remission at High Risk for Relapse | Nivolumab | Phase II | NCT02532231 |
| Single Agent and Combined Inhibition after Allogenic Stem Cell Transplant | Nivolumab, ipilumab or both, after aIIo-HSCT in AML or MDS | Phase I | NCT02846376 |
| Study of PDR001 and/or MBG453 in Combination with Decitabine in Patients with AML or High Risk MDS | Anti-PD1 and/or anti-TlM3 together with decitabine | Phase I | NCT03066648 |
| Ipilimumab and decitabine in treating patients with relapsed or refractory MDS or AML | Ipilimumab together with decitabine | Phase I | NCT02890329 |
| Nivolumab and Azacitidine with or without Ipilimumab in treating patients with Refractory/Relapsed or Newly diagnosed Acute Myeloid Leukemia | 5-azacytidine and nivolumab, or nivolumab and ipilimumab and 5-azacytidine | Phase II | NCT02397720 |
| Ipilimumab or Nivolumab in Treating Patients With Relapsed Hematologic Malignancies After Donor Stem Cell Transplant | Ipilimumab or nivolumab | Phase I | NCT01822509 |
The study published by Williams et al. indicates that T cells are recognizing antigen and become activated, even though AML is a cancer with relatively low mutational rate. It also suggests that T cells are not able to eradicate the leukemia cells due to inhibitory mechanisms, and become dysfunctional/exhausted instead. This implies that given identification of proper molecular targets and cell subsets, reinvigorating these dysfunctional T cells through ICB might be a viable therapeutic strategy in AML. In addition to the well-known immune checkpoint receptor PD1, the study by Williams et al. suggests that OX40 should be explored closer in future studies, due to consistent upregulation on multiple T cell subsets. It remains to be seen if preclinical studies will justify specific combinations that will synergize and increase the response to ICB in AML, or if useful combinations will come out of the many iterations that are being tested in clinical trials, at times without any mechanistic insight. This stands in sharp contrast to how ICB was discovered in the first place.
Acknowledgement/funding:
Amer M. Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA).
Tae Kon Kim is supported by Seery Foundation Clinical Investigator Award for Cancer Research (William O. Seery Foundation), American Society of Hematology Scholar Award, EvansMDS Young Investigator Award (Edward P. Evans Foundation), and American Society of Clinical Oncology, Career Development Grant (Conquer Cancer Foundation).
Esten N. Vandsemb is supported by the Central Norway Regional Health Authority.
Footnotes
Conflicts of interest: A.M.Z. had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Ariad, Agios, Novartis, Acceleron, Astellas, Daiichi Sankyo and Takeda; and received honoraria from and was a speaker for Takeda (past). None of these relationships were related to the development of this manuscript. TKK and ENV declare that they have no conflicts of interest.
References
- 1.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clinical Cancer Research. 2008;14: 3044–3051. [DOI] [PubMed] [Google Scholar]
- 2.Zeidan AM, Knaus HA, Robinson TM, et al. A Multi-center Phase I Trial of Ipilimumab in Patients with Myelodysplastic Syndromes following Hypomethylating Agent Failure. Clinical Cancer Research. 2018;24: 3519–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gbolahan OB, Zeidan AM, Stahl M, et al. Immunotherapeutic Concepts to Target Acute Myeloid Leukemia: Focusing on the Role of Monoclonal Antibodies, Hypomethylating Agents and the Leukemic Microenvironment. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knaus HA, Berglund S, Hackl H, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanakry CG, Hess AD, Gocke CD, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117: 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117: 4501–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez MA, Ivan C, Valdecanas D, et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature Reviews Immunology. 2015;15: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]


