Abstract
An alternative to human red blood cells (RBCs) for clinical transfusion would be advantageous, particularly in situations of massive acute blood loss (where availability and compatibility are limited) or chronic hematologic diseases requiring frequent transfusions (resulting in alloimmunization). Ideally, any alternative must be neither immunogenic nor pathogenic, but readily available, inexpensive, and physiologically effective. Pig RBCs (pRBCs) provide a promising alternative due to their several similarities with human RBCs, and our increasing ability to genetically-modify pigs to reduce cellular immunogenicity. We briefly summarize the history of xenotransfusion, the progress that has been made in recent years, and the remaining barriers. These barriers include prevention of (i) human natural antibody binding to pRBCs, (ii) their phagocytosis by macrophages, and (iii) the T cell adaptive immune response (in the absence of exogenous immunosuppressive therapy). Although techniques of genetic engineering have advanced in recent years, novel methods to introduce human transgenes into pRBCs (which do not have nuclei) will need to be developed before clinical trials can be initiated.
Keywords: Blood transfusion; Pig, genetically-engineered; Red blood cells; Sickle cell disease; Xenotransplantation; Xenotransfusion
1. Introduction
1.1. Blood product supply and demand
The World Health Organization estimates that approximately 112 million units of donated blood are collected each year, which is far from satisfying the global need [1]. In the United States, 12.6 million red blood cell (RBC) units were collected in 2015 with 11.3 million units transfused, highlighting a slim surplus of units on a national level, but with steadily decreasing rates of donation [2]. Transient critical blood shortages are well-recognized [2]. While blood type O availability is often scarce, supply and demand mismatch is not the only limitation to transfusion therapy [2,3].
1.2. Economic and safety burdens impacting human RBC transfusions
Although donor screening, leukocyte reduction, and nucleic acid viral screening help prevent deleterious consequences of transfusion, the risk is not zero [2,4]. Ensuring safety is requisite, but varying regulatory mandates, prohibitive costs, and strenuous screening encumber accessibility, excluding up to 15% of all donors prior to or after blood collection [2]. For example, because of concerns relating to new variant Creutzfeldt-Jakob disease in Europe, eligibility for blood donation has been made more restrictive [5]. In countries where the incidence of human immunodeficiency virus infection is much higher than in the US (e.g., sub-Saharan Africa), the shortage of acceptable RBCs and the risks of blood transfusion are, of course, significantly greater.
1.3. Limitations of repeated transfusion in chronic disease
Patients with diseases requiring repeated transfusions (e.g., sickle cell disease, thalassemia, hematologic cancers, etc.) are at risk of alloimmunization and the development of delayed hemolytic transfusion reactions, limiting their donor pool. Approximately 30% of transfused patients with sickle cell disease develop alloantibodies [6] and, over a 5-year period, 8% of patients develop a delayed hemolytic transfusion reaction following alloimmunization [7]. In some cases, the patient’s own RBCs are also lysed (hyper-hemolysis syndrome), characterized by macrophage activation, leading to the destruction of both donor and recipient RBCs [8].
Hematologic phenotypes are controlled by various loci that affect hematopoiesis, intrinsic RBC turnover, minor antigen expression, and recipient allosensitization [9,10]. Some individuals requiring chronic transfusions are predisposed to developing alloantibodies to rare donor antigens, and have a higher risk of future alloimmunization. Other groups have successfully implemented more extensive antigen matching for such vulnerable populations [10]. However, the practicality of cross-matching donors and recipients with precision to a single locus requires continued investigation. Complicating matters, this further diminishes an already limited donor pool, which should encourage the development of novel alternatives, including genetically-modified source (‘donor’) pigs with ‘immunologically inert’ RBCs for xenotransfusion.
1.4. Xenotransfusion as a novel alternative
In light of the aforementioned obstacles, innovative replacements to human RBC transfusion have garnered considerable attention, including perfluorocarbon hemoglobin, and stem cell-based therapies [11-13]. However, most substitutes have had relatively little clinical success. Important developments achieved in recent years offer hope for the eventual industrial production of in vitro-cultured human RBCs [13,14]. Still, technical challenges in mapping stem-cell fate and sub-optimal reticulocyte maturation pose significant hurdles to the promise of ex vivo human RBC production [14,15]. Transgenic manipulation of xenogeneic RBCs (to prevent immunologic responses) may be more tangible than programming allogeneic cell differentiation. Xenotransfusion (cross-species transfusion), using the pig as a source of RBCs, may provide a solution.
There have been significant advances in organ and tissue xenotransplantation, particularly when using genetically-modified pigs as the sources of organs and cells [16,17]. With recent technological advances, there have been dramatic improvements in the results in pig-to-nonhuman primate (NHP) transplantation models (See below: 2.4 Recent progress in pig-to-nonhuman primate organ transplantation). This progress in overcoming the pathobiological barriers to xenotransplantation can be applied to producing pigs whose RBCs might be suitable for clinical xenotransfusion.
There are a number of situations in which pig RBCs (pRBCs) might be particularly valuable, e.g., (i) in acute blood loss (hemorrhage, trauma) where sufficient human ABO-compatible blood is not available, and (ii) in patients with hematological and oncological disorders requiring frequent blood transfusions in whom sensitization to human RBCs has developed. Indeed, pRBCs may eventually provide a source of RBCs superior to that of human RBCs, and is the focus of this review.
1.5. History of xenotransfusion
The earliest documented evidence of human xenotransplantation begins with xenotransfusion, when in 1667 Jean Baptiste Denis and Paul Emmerez transfused the blood of a lamb into a 15-year-old feverish boy, and later that year transfused calf blood to a mentally ill man in an attempt to cure him [18,19]. Complications and politics ultimately led to a ban of transfusions by the French Parliament in 1678, and even denunciation by the Pope in 1679. Progress halted until 1749, when Andrew Cantwell, a member of the Faculty of Medicine in Paris, recognized the potential value of transfusion in emergencies with acute hemorrhage [20-23].
A series of allogeneic human transfusions were documented by James Blundell and colleagues beginning in 1819. In 1829, they described the first successful transfusion in a woman with post-partum hemorrhage [23]. Not only was Blundell one of the earliest proponents of transfusion, he demonstrated that cross-species xenotransfusion was more hazardous than allogeneic human transfusion. Despite his observations, xenotransfusion continued throughout the 19th century.
The modern era of transfusion was ushered in when Landsteiner published his landmark paper on the ABO blood groups in 1900 [24]. With this, debate was largely settled, and human RBC allogeneic transfusion became the focus of attention for nearly a century.
Similarly, after Harold Neuhof’s failed attempt at transplanting a lamb kidney into a man in 1923, it would be 40 years until xenotransplantation was attempted again [25,26]. In 1964, Keith Reemtsma began a small series of kidney transplants from chimpanzees into humans, marking the first use of immunosuppressive therapy in such a procedure. One patient survived a remarkable 9 months [26,27]. Transplantation and xenotransplantation experienced a renaissance, and the fields became more ambitious and successful with the aid of improving immunosuppressive therapy.
As a result of scientific advances in xenotransplantation over the past 30 years, particularly in the genetic engineering of pigs, attention has returned to the possibilities afforded by xenotransfusion.
2. Clinical application of xenotransfusion
2.1. Pig RBCs as a source for clinical transfusion
The potential for using genetically-modified pigs as sources of RBCs is considerable. pRBCs share a number of common characteristics with human RBCs (Table 1) with similar cell diameters and counts, although the average life-span of pRBCs is shorter than that of human RBCs [28,32,33]. Porcine hemoglobin shares only 85% sequence identity with its human counterpart [34]. Nevertheless, both pig and human hemoglobin have similar three-dimensional structures, and it is believed that most of the 22 and 21 amino acid substitutions on the alpha and beta subunits, respectively, have no significant functional effect. For example, human alpha-hemoglobin chains hybridize with pig beta-hemoglobin chains in vivo; however, the opposite (human beta-chains hybridizing with porcine alpha-chains) only occurs in vitro. It appears that despite subtle differences in structure affecting stability and function, the molecules are functionally competent [34].
Table 1: Pig RBCs share a number of common characteristics with human RBCs*.
| Pig | Human | |
|---|---|---|
| Blood groups | 27 | 30 |
| Hematocrit | 38-50% | 35-45% |
| Hemoglobin | 6-18 g/100ml | 12-18 g/100ml |
| Isotonicity | 0.85% NaCl | 0.9% NaCl |
| Lifespan | 86 days | 120 days |
| RBC count | 5.7-6.9 million/μl | 4.2-6.2 million/μl |
| RBC diameter | 4-8 μm | 6-8 μm |
| RBC volume | 56-95 ml/kg | 65-75 ml/kg |
The most closely studied pig blood group system is the A-O (H) system, which is loosely related to the human ABO system [35,36]. Pig herds have been developed that are uniformly of blood type O; thus, ABO compatibility between human recipients and ‘donor’ pigs can be assured. Furthermore, human hemoglobin has been expressed in transgenic pigs, with normal post-translational modifications and biological function [37].
The high breeding capacity of pigs and the ability to produce them in a designated pathogen-free environment obviates many of the potential infectious risks of human RBC transfusion. Moreover, pRBCs do not have nuclei, and therefore do not harbor porcine endogenous retroviruses (PERV), although this advantage would be reduced by contamination of the product by leukocytes [38]. Nonetheless, the use of white blood cell filters could feasibly ensure that leukocytes do not contaminate pRBCs, simultaneously preventing the theoretical risk of PERV transmission, as well as leukocyte immunogenicity. Most experts now agree that with the necessary established screening protocols, and requisite biosecured housing for source pig herds, the risk of porcine xenotransplantation spreading communicable diseases is minimal (when compared to human donors) [39-41].
2.2. Nonhuman primates as surrogate hosts
We have shown previously that Old World NHPs are satisfactory surrogates for humans in xenotransplant trials (See below: 2.4 Recent progress in pig-to-NHP organ transplantation) [42-44]. NHP recipients of blood group AB can be selected, simplifying the interpretation of the response to pRBCs from blood group O source pigs.
Like humans, NHPs hyperacutely reject transplanted wild-type (WT, genetically-unmodified) pig organs, largely due to antibody-antigen binding, complement activation, innate cell activation, and coagulation dysfunction [44]. An important antigen, galactose-α1,3-galactose (Gal), is expressed on the surface of many pig cells, including RBCs. The presence of anti-Gal antibodies in humans and Old World NHPs initiates much of this response (See below: 3.1 Antigen specific barriers in xenotransplantation). NHPs also develop similar elicited immune responses to humans, and demonstrate a similar inflammatory response.
2.3. Initial studies of pRBC xenotransfusion in NHPs
Scientific investigations into pRBCs for clinical transfusion began in the 1990s [45]. Initially, pRBCs from WT pigs were treated with the enzyme α-galactosidase to remove Gal epitopes [46,47]. In vitro binding of baboon or human antibodies to α-galactosidase-treated pRBCs was greatly reduced compared to untreated pRBCs. In vivo, however, whereas autologous baboon RBCs survived for >16 days, and WT pRBCs for <15 minutes, treating pRBCs with α-galactosidase increased pRBC survival to only two hours [30]. In complement-depleted baboons, pRBC survival increased to 24 hours, and to 72 hours when the baboon was depleted of both complement and anti-Gal antibodies, or of complement and macrophage phagocytes.
Although baboon recipients became sensitized to Gal, the lack of hemolysis of baboon RBCs, with no reduction in hematocrit and no increases in serum bilirubin or lactate dehydrogenase, suggested a lack of cross-reactive antibody-mediated destruction of baboon RBCs.
When large numbers of WT pRBCs were transfused into baboons depleted of anti-Gal antibodies and complement [48], pRBCs could be detected for 12 hours. At necropsy, the spleen was found to be congested and grossly enlarged, indicating that the pRBCs had been removed from the blood by splenic macrophages.
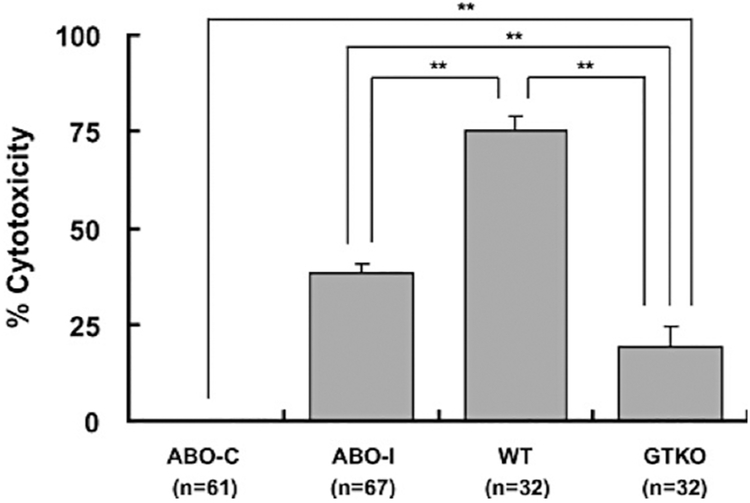
When α1,3-galactosyltransferase gene-knockout (GTKO) pigs (genetically-modified pigs whose cells do not express Gal) became available [49,50], in vitro binding of IgM from human or baboon sera was significantly less than to WT pRBCs. IgG binding to GTKO pRBCs was absent or minimal [51,52]. Sera had minimal cytotoxicity to GTKO pRBCs compared to WT pRBCs. Although antibody binding and serum cytotoxicity to GTKO pRBCs were significantly less than to ABO-incompatible human RBCs, they were not comparable to binding and cytotoxicity to ABO-compatible human RBCs (Figure 1) [52]. Nevertheless, GTKO pRBCs transfused into baboons could be detected in the blood for only 5 minutes, indicating that RBCs, even from GTKO pigs, are rapidly phagocytosed. While these studies were encouraging in some respects, they underscored the principle that rapid loss of GTKO pRBCs is associated with the presence of antigens other than Gal and/or to other heretofore unknown mechanisms.
Figure 1: Human serum complement-dependent cytoxicity (CDC) of ABO-compatible human RBCs (ABO-C), ABO-incompatible human RBCs (ABO-I), wild-type pRBCs (WT), and GTKO pRBCs (GTKO).
Human sera (50%) of blood types O (n=10), A (n=9), B (n=8), and AB (n=4) were tested for CDC of human ABO-C, human ABO-I, pig WT, and pig GTKO RBCs. There was significantly greater lysis of WT than of ABO-I and GTKO RBCs (p<0.01). ABO-I RBCs sustained significantly greater lysis than of GTKO RBCs (p<0.01), but there was significantly greater lysis of GTKO than of ABO-C RBCs (**p<0.01). (Reproduced with permission from reference [52])
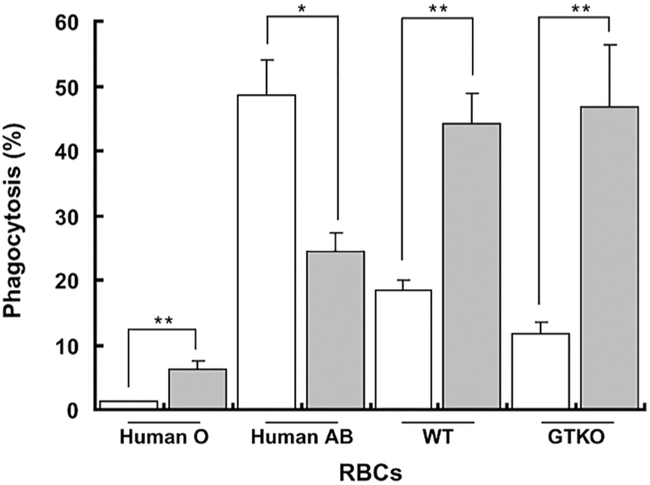
The rapid loss of pRBCs appeared to be related to two key factors – (i) antibody binding to the pRBCs (thus activating complement), and (ii) phagocytosis of the pRBCs by recipient macrophages through either antibody-dependent and/or antibody-independent mechanisms (Table 2). Similar responses have been recorded after the intravenous infusion of mobilized pig hematopoietic stem cells or bone marrow cells into baboons, and after human blood perfusion through pig livers [134]. Long et al. demonstrated that sensitization to pig antigens increased antibody-dependent phagocytosis of pRBCs (Figure 2), indicating that the adaptive immune response also has to be prevented (Table 2) [52].
Table 2: Immunological barriers to pRBC xenotransfusion.
| Barrier | References | Attempts to overcome barrier |
References |
|---|---|---|---|
| Xenoantigenic barriers:Gal, Neu5Gc, Sda |
Gal: Galili 1988 [53] Cooper 1992 [54] Good 1992 [55] Doucet 2004 [56] Neu5Gc: Asaoka 1994 [57] Bouhours 1996 [58] Zhu 2002 [59] Padler-Karavani 2011 [60] Sda: Byrne 2014 [61] Byrne 2018 [62] Zhao 2018 [63] |
Development of triple-knock out (TKO) pigs | Phelps 2003 [49] Kolber-Simonds 2004 [50] Long 2009 [52] Estrada 2015 [64] Butler 2016 [65] Lee 2016 [66] Gao 2017 [31] Wang 2017 [67] |
| Complement-mediated lysis |
CD55 Atkinson 1991 [68] Dalmasso 1991 [69] White 1995 [70] Morgan 2005 [71] CD46 Lublin 1988 [72] Perez de la Lastra 1991 [73] Thorley 1997 [74] CD59 Sugita 1988 [75] Rollins 1991 [76] |
Transgenic expression of human complement regulatory proteins, CD46 and CD55 | Cozzi 1994 [77] Fodor 1994 [78] Langford 1994 [79] McCurry 1995 [80] Diamond 1996 [81] Diamond 2001 [82] Schuurman 2002 [83] Loveland 2004 [84] Hara 2008 [85] Long 2009 [52] van der Windt 2009 [86] McGregor 2012 [87] Burdorf 2014 [88] Butler 2016 [65] Lee 2016 [66] |
| Macrophage phagocytosis | Qian 1999 [89] Leonard 2000 [90] Ide 2005 [91] Long 2009 [52] |
Transgenic expression of human CD47 | Ide 2007 [92] Yang 2010 [93] Navarro-Alvarez 2011 [94] Maeda 2013 [95] Tena 2014 [96] Tena 2017 [97] |
| Natural killer (NK) cells | Inverardi 1997 [98] Baumann 2004 [99] Kennett 2010 [100] |
Transgenic expression of HLA-G and/or HLA-E and/or Cw3 | Dorling 2000 [101] Matsunami 2001 [102] Forte 2005 [103] Crew 2007 [104] Weiss 2009 [105] Maeda 2013 [95] Esquivel 2015 [107] |
|
T-cell-mediated immune response |
Gill 1994 [107] Rollins 1994 [108] Elwood 1998 [109] Yamada 2005 [110] Davila 2006 [111] Koshika 2011 [112] Ezzelarab 2014 [113] Griesemer 2014 [114] Ezzelarab 2015 [115] |
CD154 and CD40 blockade-based immunosuppressive therapy Transgenic expression of CTLA4-Ig CIITA-knockdown SLA class 1-KO |
Buhler 2000 [116] Kuwaki 2004 [117] Tseng 2005 [118] Ezzelarab 2012 [119] Mohiuddin 2014 [120] Iwase 2015 [121, 122] Mohiuddin 2016 [123] Bottino 2017 [124] Iwase 2017 [125] Kim 2017 [126] Shin 2018 [127] Martin 2005 [128] Phelps 2009 [129] Bottino 2014 [130] Hara 2013 [131] Iwase 2015 [132] Reyes 2014 [133] |
Figure 2: Phagocytosis of pRBCs is increased in GTKO-sensitized baboons.
When GTKO-sensitized baboon serum (gray) was added to human and pig RBCs, there was significantly increased phagocytosis of WT and GTKO pRBCs, but decreased phagocytosis of human AB RBCs. When pooled human O serum (white) was added, human ABO-incompatible (AB) RBCs underwent greater phagocytosis than pRBCs. The small increase in phagocytosis of human group O RBCs likely reflects binding of baboon anti-human antibodies to the RBCs. *P<0.05, **P<0.01. (Modified with permission from reference [52])
2.4. Recent progress in pig-to-NHP organ transplantation
Recent technological advances have dramatically improved the results of pre-clinical models of organ xenotransplantation aimed at overcoming the shortage of human organs [16,17,44]. Using an immunosuppressive regimen that prevents a T cell-dependent elicited antibody response, survival of pig heterotopic (non-life-supporting) hearts and life-supporting kidneys is now being measured in months or even years [120-122,125,136,137], rather than minutes as originally reported [42,43].
These encouraging results have been obtained by two key genetic approaches—(i) deletion of pig xenoantigens against which humans (and NHPs) have natural (preformed) antibodies, and (ii) introduction into the pig cells of transgenes for human complement- and/or coagulation-regulatory proteins [17,44,138]. We suggest that the ability to delete key pig antigens by genetic manipulation, and novel approaches to inhibit phagocytosis, will eventually overcome the barriers to pRBC xenotransfusion (detailed below). On the basis of this progress, we suggest it is timely to reconsider pRBCs for clinical transfusion.
3. Overcoming the remaining barriers to pRBC xenotransfusion
3.1. Antigen-specific barriers in xenotransplantation
Pigs express antigens that correlate with human A or O blood group antigens, but only blood group O pigs are used in the field of xenotransplantation [36]. Pigs have a single Rh gene that does not appear to represent a blood group antigen [139]. The many ‘minor’ blood group antigens (e.g., Kell) that have been investigated do not appear to be expressed on pRBCs (Gregory Martens, personal communication June 2018). Still, investigation of additional variant antigens may need to be explored to prevent potential rejection or immunization from developing, as seen in allogeneic transfusion [10].
The presence of antibodies to human leukocyte antigens (HLA) is not uncommon in patients who have been exposed to blood transfusion, organ allotransplantation, or pregnancy. Increasing evidence suggests that some humans have anti-HLA antibodies that cross-react with swine leukocyte antigens (SLA). However, SLA are not expressed on pRBCs and so will not be problematic [140].
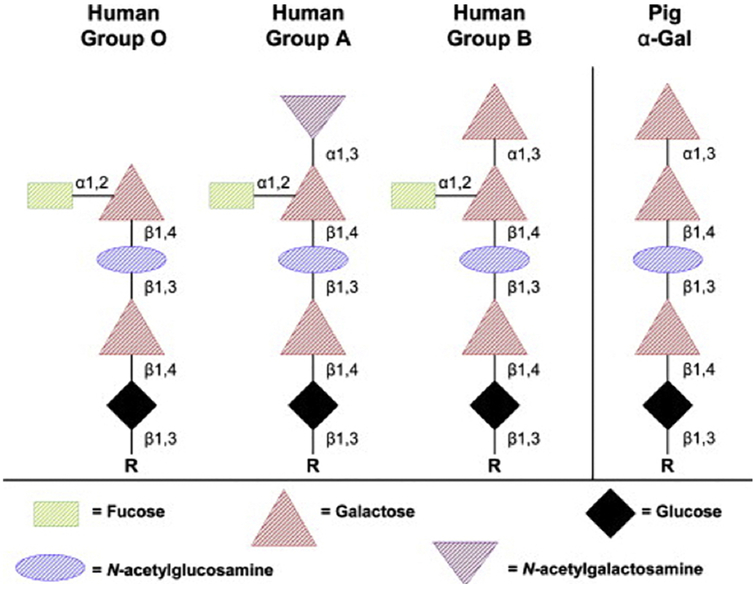
Three major carbohydrate antigens are expressed on pRBCs against which humans have natural (preformed) antibodies, namely Gal, Neu5Gc (N-glycolylneuraminic acid), and Sda (Table 3). The major target antigen for primate natural antibodies is Gal, a terminal oligosaccharide similar to the human blood group A, B, and O saccharides (Figure 3) [45]. Like anti-A/B antibodies, anti-Gal antibodies are believed to develop during infancy as a response to colonization of the gastro-intestinal tract by various bacterial and viral flora [143].
Table 3: Carbohydrates known to be expressed on pRBCs against which humans have natural (preformed) antibodies.
| Carbohydrate | Enzyme | References |
|---|---|---|
| Gal (Galactose-α1,3-galactose) |
GT (α1,3-galactosyltransferase) |
Cooper 1992 [54] Good 1992 [55] Phelps 2003 [49] Rouhani 2004 [51] Long 2009 [52] |
| Neu5Gc (N-glycolylneuraminic acid) |
CMAH (cytidine monophosphate-N-acetylneuraminic acid hydroxylase) |
Zhu 2002 [59] Padler-Karavani 2011 [60] Burlak 2014 [141] Wang 2014 [142] Lee 2016 [66] |
| Sda | β4GalNT2 (β1,4N-acetylgalactosaminyltransferase) |
Byrne 2014 [61] Estrada 2015 [64] Byrne 2018 [62] Zhao 2018 [63] |
Figure 3: Structures of human ABO and pig Gal glycans.
Pig RBCs express Gal epitopes on oligosaccharides that are similar in structure to the human blood type B oligosaccharide, except for the fucose side-arm. (Reproduced with permission from reference [45])
When WT pig organs are transplanted or pRBCs are transfused into humans or Old World NHPs, expression of Gal results in almost uniform hyperacute rejection or cell lysis, similar to that seen after ABO-incompatible organ allotransplantation or blood transfusion. Pigs homozygous for GTKO do not express Gal, thereby overcoming this immediate cause of cell destruction [49,50].
Human natural antibodies to two other carbohydrate epitopes have been identified (Tables 2 and 3) [59,61], although the cytotoxicity associated with these antibodies is relatively reduced compared to anti-Gal [85,144,145]. Nevertheless, they can initiate lysis of pig cells in vitro and cause rejection of pig organs in NHPs. Therefore, if pRBCs are to be transfused successfully into humans, RBCs from triple-knockout (TKO) pigs will be required, in which all three of these antigens have been deleted (Figure 4) [67]. The current evidence is that many patients awaiting kidney allografts (who do not express anti-HLA antibodies) have natural anti-pig antibodies directed only to these three known pig antigens [146], although there may be other minor, unidentified carbohydrate xenoantigens in the remaining members of the population.
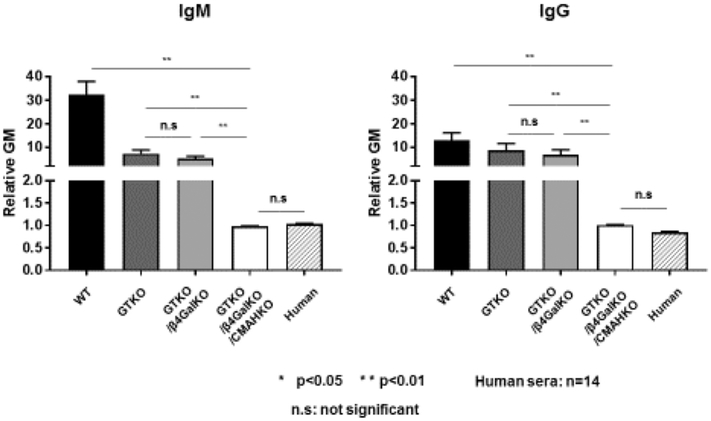
Figure 4: Flow cytometric comparison of human IgM and IgG antibody binding to pig and human RBCs.
Human IgM (left) and IgG (right) binding to WT, GTKO, GTKO/βGalNT2-KO (DKO), and GTKO/βGalNT2-KO/CMAHKO (TKO) pig RBCs and to human RBCs. The significant differences in human IgM and IgG binding to the various RBCs are indicated (*p<0.05, **p<0.01; ‡<0.05). There was no IgM/IgG binding to TKO pig or human RBCs (ns = not significant). (A relative Mean Fluorescence Intensity [MFI] <1 indicates no significant binding of IgM or IgG).
A comparative analysis of human antibody binding to pRBCs isolated from GTKO, double-knockout (GTKO/CMAH-KO), and TKO pigs, as well as autologous and allogeneic (blood type O donor) human RBCs, has been carried out [67,142]. Human antibody bound less to TKO pRBCs than to allogeneic human RBCs in 43% (36/87) of samples, with varying but minimal binding in the remaining specimens (Figure 5) [67]. This demonstrated that TKO dramatically reduces or eliminates the xenoantigenicity of these pRBCs.
Figure 5: Flow cytometric comparison of human IgG and IgM antibody binding to pig and human RBCs.
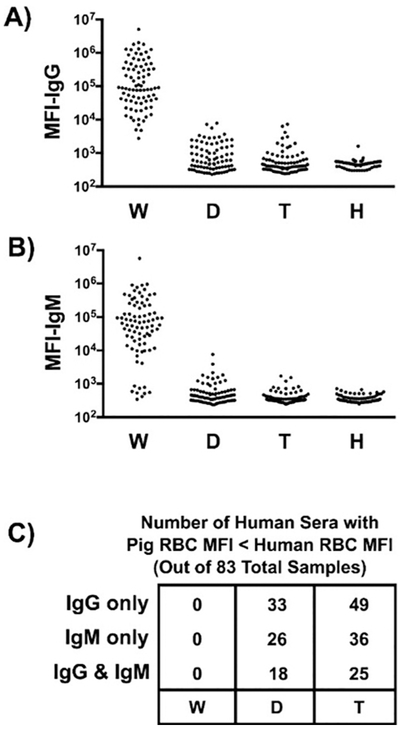
Human sera (n=83) were incubated with RBCs isolated from WT pigs (W), or from pigs lacking Gal (i.e., GTKO) and Neu5Gc (i.e., CMAH-KO) (double-knockout, D) or lacking Gal, Neu5Gc, and Sda (i.e., β4GalNT2-KO) (triple-knockout, T). These sera were also mixed with human (allogeneic) RBCs (H) expressing blood group O. Panels A and B show a summary of IgG and IgM binding to various RBCs, respectively. The data represent median fluorescent intensity (MFI). Panel C summarizes the number of samples from panels A and B where the indicated pRBC MFI is less than the MFI for the human blood group O RBCs. (Reproduced with permission from reference [67])
In the absence of human serum antibody binding to TKO pRBCs, survival of the pRBCs after transfusion would likely be prolonged. This would be a result of (i) reduced antibody-mediated cell loss, and (ii) reduced phagocytosis (resulting from reduced or absent antibody binding) [67,142]. Therefore, in some patients, transfusion of TKO pRBCs may be sufficient to obtain clinically-relevant prolonged survival of the pRBCs.
Baboons and other Old World monkeys have been established as reliable surrogate hosts in xenotransplantation models because they express comparable proteins to humans. Perhaps more importantly, they lack certain carbohydrate antigens as do humans, and therefore develop similar anti-pig antibodies. For example, neither expresses Gal or Sda, and therefore produce anti-Gal and anti-Sda antibodies (which bind genetically-unmodified pRBCs) [147]. For these species, therefore, double-knockout pigs lacking Gal and Sda (GTKO/Sda-KO) will be required for in vivo experimental studies of pRBC transfusion. However, Old World nonhuman primates differ from humans in one important respect: they do express Neu5Gc, and therefore do not make anti-Neu5Gc antibodies (in contrast to humans) [60]. Alternatively, the capuchin monkey (a New World monkey) mimics humans exactly with respect to the antibodies developed against Gal, Sda, and Neu5Gc expressed on pRBCs. Therefore, New World monkeys may make a preferable experimental model for studying the comparable human antibody response to TKO pRBCs in vivo [147].
3.1.1. Complement regulation
If antibody binding to TKO pRBCs is present, e.g., because of expression of other (hitherto unidentified) glycan antigens, the cells may be destroyed by complement activation. Based on experience with pig organ and cell transplantation in NHPs, antibody-mediated rejection can be prevented or significantly reduced by the expression of a human complement-regulatory protein, such as hCD55 (decay-accelerating factor), hCD46 (membrane cofactor protein), or hCD59 (membrane attack complex-inhibiting protein) [17,44,85,139].
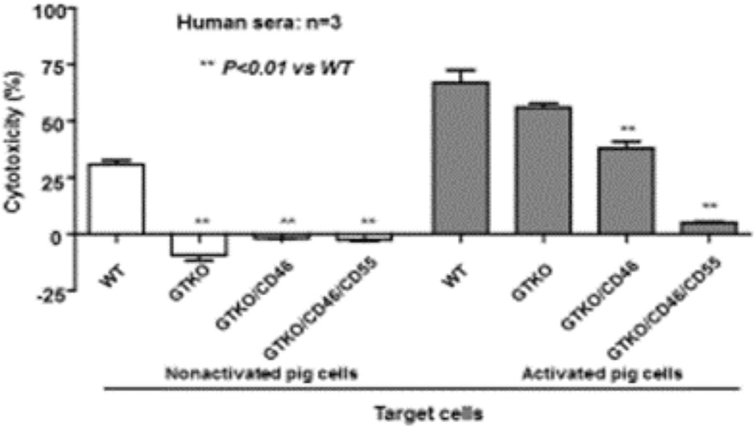
Pigs expressing high levels of one or more human complement-regulatory protein on vascular endothelial cells have been available for many years, and demonstrate resistance to complement-mediated injury (Figure 6). However, using current technology, it has not proved possible to express these proteins on pRBCs, as mature RBCs lack nuclei. hCD46 is expressed on almost all human cells (sparing RBCs), including peripheral blood mononuclear cells (PBMCs), and has been expressed on porcine PBMCs [66]. If expressed on pRBCs, human complement-regulatory proteins would undoubtedly protect the pRBCs from lysis. To achieve human transgene expression will be challenging (discussed below), and represents the major novel contribution needed to advance this field.
Figure 6: Protection from serum cytotoxicity provided by transgenic expression of human complement-regulatory proteins on nonactivated (left) and TNF-α-activated (right) pig corneal endothelial cells.
Before activation, there was no serum cytotoxicity to GTKO, GTKO/CD46, or GTKO/CD46/CD55 pig cells. After activation, serum cytotoxicity was significantly increased, but the expression of two human complement-regulatory proteins (CD46 and CD55) almost completely prevented cytotoxicity.
3.2. Phagocytosis of pRBCs
Human macrophages present a unique immunological challenge to xenotransfusion [92,93]. Xenografts activate host immunity not only by expressing immunogenic antigens that initiate rejection, but also by lacking antigenic inhibitory signals that normally prevent host immune responses [94].
One potential mechanism of macrophage activation is associated with species incompatibility of CD47/SIRP-α (signal-regulatory protein-α) signaling. Normally, cells expressing human (h)CD47 interact with human macrophage SIRP-α to inhibit phagocytosis [91-97,106,148,149]. Expression of pig CD47 does not inhibit the activation of human macrophages [92], indicating that hCD47 will need to be transgenically expressed in pRBCs to prevent phagocytic responses. Transfection of cells with the gene for CD47 from the same species as the recipient macrophages prevents phagocytosis by activation of SIRP-α in mice [150] and humans [92].
Expression of human CD47 on pRBCs should, therefore, inhibit human macrophage activity through its inhibitory effect on human SIRP-α. The generation of human CD47-expressing pigs (and the recent production of viable human SIRP-α-expressing mice) increased engraftment in a murine model of pig-to-human hematopoietic progenitor cell transplantation [96,97]. There was a substantial protective effect of hCD47 expression on engraftment, associated with prolonged survival of porcine hematopoietic cells, presumably by modulation of macrophage phagocytosis. The generation of human CD47-expressing pigs indicates the potential that CD47/SIRP-α signaling can be manipulated. We anticipate that the transfusion of TKO/CD55 pRBCs that are additionally transgenic for hCD47 will successfully inhibit phagocytosis. Expression of hCD47 may also have a beneficial effect by modulating the monocyte and T cell responses [96].
As with transgenic expression of a human complement-regulatory protein, however, expression of hCD47 on pRBCs will be difficult, and novel techniques of transgenesis will be required.
3.2.1. Potential methods for expressing human regulatory proteins in pRBCs
From extensive studies on pig hematopoietic cell transplantation [134], we anticipate that the problem of phagocytosis can be resolved by genetic engineering of the pig. However, one of the major challenges facing xenotransfusion is to induce expression of human transgenes in pRBCs (which lack a nucleus).
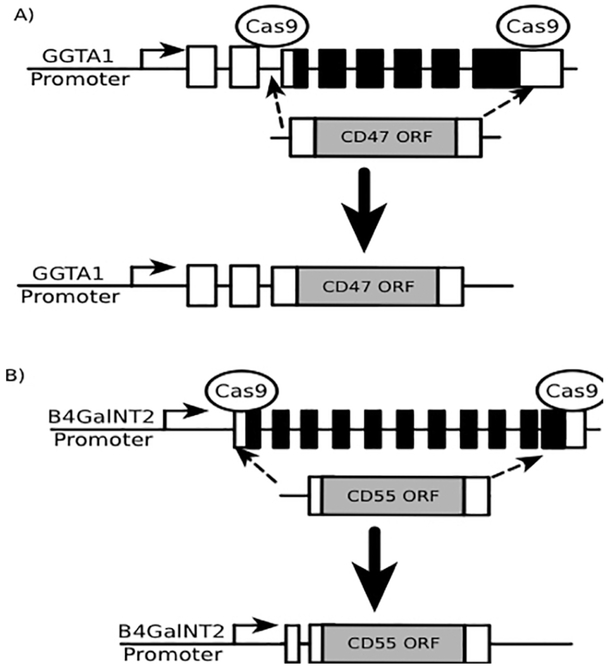
Novel gene manipulations in fetal fibroblasts, somatic cell nuclear transfer, and embryo implantation may possibly resolve this biological barrier (Figure 7) [64]. Exons and introns spanning the protein coding sequences of the pig α1,3-galactosyltransferase (Gal) and β4GalNT2 (Sda) genes could be replaced with genes for hCD47 and/or a human complement-regulatory transgene (e.g., CD55) open reading frames. α1,3-galactosyltransferase and β4GalNT2 promoters could drive expression of these transgenes. The CRISPR/Cas9 gene editing system has been used to insert transgenes at specific genomic locations [151] and a guide RNA could be designed to direct Cas9 to cleave specific genomic locations flanking the open reading frames of α1,3-galactosyltransferase and β4GalNT2. This would increase the efficiency of gene replacement mediated by homologous recombination. Two α1,3-galactosyltransferase-specific Cas9/gRNA complexes would need to be electroporated into cells along with the transgene template (Figure 7).
Figure 7: Potential novel techniques for transgenic expression in pRBCs lacking nuclei.
(A) CRISPR/Cas9 could be used to cleave genomic regions of GGTA1 (i.e., Gal) at sites flanking exons that contain the open reading frame (ORF). A replacement construct containing the ORF of CD47 flanked by GGTA1 sequences could be simultaneously introduced to facilitate the replacement of GGTA1 protein coding sequences with CD47. (B) The identical approach to that used in panel A could be repeated to replace codons encoding the CMAH gene with the CD55 ORF.
If the described gene-swaps failed to appropriately express CD47 and/or CD55 in pRBCs, an alternate approach could be to express the transgenes as a cassette using an exogenous promoter. The human elongation factor 1-alpha promoter has been used to express CD47 in pRBCs when the entire construct was inserted into the α1,3-galactosyltransferase gene [96,97]. Hemoglobin and glycophorin promoters could also be explored.
3.2.2. Natural killer (NK) cells
Natural killer cells have also been demonstrated by in vitro studies to participate in rejection following porcine xenotransplantation (although this has been difficult to detect in vivo) [98-100]. Transgenic expression of HLA-G and/or E and/or Cw3 may inhibit the natural killer cell response that contributes to rejection [101-104,152]. Pigs expressing HLA-E or HLA-G have been produced, but not yet fully tested on the preferable GTKO (or TKO)/human complement-regulatory protein background [95,105,106]. Importantly, studies by Miyagawa’s group indicate that expression of these transgenes also inhibits macrophage activity [95,106], and would be an additional approach that could be explored to prevent phagocytosis.
3.3. The adaptive immune response
Inhibition of the T cell-mediated immune response is a necessary component of preventing rejection following organ xenotransplantation. Blockade of the CD154-CD40 T cell costimulation pathway has been shown to successfully block the adaptive immune response to a GTKO pig organ transplant [116,117,120-125], whereas conventional pharmacologic immunosuppressive regimens have proved less successful.
Regardless, overcoming the adaptive immune response without administering exogenous immunosuppressive therapy remains another significant challenge.Certainly, some acutely-ill patients could tolerate short-term immunosuppression for limited transfusions. However, requiring immunosuppression to tolerate repeated pRBC xenotransfusions would effectively, and unacceptably, substitute the morbidities of chronic hematologic conditions (and blood transfusions) for those of lifelong immunosuppression. Moreover, there would be considerable contraindications in some chronically- or critically-ill patients requiring immunocompetence (i.e. cancers, HIV, etc.), or those at risk for microbial infection (i.e. requiring parenteral nutrition, indwelling catheters, or requiring mechanical ventilation, etc.).
Without exogenous immunosuppressive therapy, sensitization to pRBCs (or contaminating WBCs) is likely to develop, but may not be inevitable. (i) Many human sera do not have natural antibodies that bind TKO pRBCs, suggesting that there are no additional glycan or protein targets on pRBCs for human natural antibodies. The absence of expression of pig glycans on pRBCs will abrogate the development of elicited antibodies to these antigens. (ii) The absence of expression of SLA on pRBCs will negate the development of elicited antibodies to these antigens. (iii) The absence of Gal expression alone [153] and the expression of a human complement-regulatory protein [115] have both been demonstrated to reduce the T cell response to pig cells, thus reducing the risk of the development of elicited antibodies. The proliferative T cell response to TKO pig cells has not yet been tested, but may be reduced further.
Nevertheless, some humans demonstrate low levels of antibody binding to TKO pRBCs, and these pRBCs almost certainly express low levels of hitherto-unidentified glycan or protein antigens to which those subjects (and maybe others) may develop elicited antibodies. One approach to this potential problem might be to develop transgenic, ‘enzymatically-converted’ group O red blood cells, or use polymer derivatives to ‘mask’ any unknown pig antigens [154-157].
Several other genetic modifications could be considered, such as transgenic expression on pRBCs of the immunosuppressive agent, CTLA4-Ig, which would provide local suppression of T cell activation [128,129]. If monitoring indicates that there is a significant inflammatory response to the pRBCs (which would, in turn, augment the adaptive immune response), then pigs could be engineered to express one or more human ‘anti-inflammatory’ transgenes/proteins (e.g., hemeoxygenase-1 or A20) [158-161]. There are several genetic-engineering techniques to reduce or delete expression of SLA [131-133], but, as SLA is not expressed on pRBCs, these approaches will not help.
Recently, in a rat model, autologous mesenchymal stromal cells (and even allogeneic MSCs to some extent) were demonstrated to prevent transfusion-elicited sensitization (even when transfused several days after the blood transfusion), and so, in the future, this form of therapy might become another possible method of preventing xenosensitization [162].
Future Considerations
Prior to considering clinical trials of pRBC transfusion, the pathobiologic barriers must be better understood and overcome through both in vitro and in vivo investigations. We must characterize the survival of pRBCs after xenotransfusion in NHPs, and determine to what extent pRBC loss is related to antibody binding, complement activation, phagocytosis, and/or the adaptive immune response. Significant advances in genetic engineering can be applied to pRBCs that may overcome or diminish these barriers. Importantly, for the majority of cases, this must be done in the absence of exogenous immunosuppressive therapy.
As sensitization to pig antigens does not appear to be detrimental to the outcome of a subsequent allograft, pRBC transfusions could be followed successfully by a transfusion of ABO-compatible human RBCs [163,164]. This would allow a pRBC transfusion to be employed in an emergency before human blood becomes available. It is not known whether sensitization to human RBCs (as opposed to sensitization to HLA) results in sensitization to pRBCs, though this seems unlikely.
We anticipate that, within the next decade, pRBCs will prove to be satisfactory alternatives for human RBCs in clinical transfusion for acute blood loss and for conditions necessitating frequent transfusions, such as sickle cell disease. In these latter patients, if they are highly sensitized to human RBCs (a potentially life-threatening situation), then consideration could even be given to pRBC transfusion under exogenous immunosuppressive therapy.
Practice Points.
Early success in xenotransplantation has demonstrated survival of tissue and whole-organs for months to years. These principles can be applied to pig RBC xenotransfusion.
Triple-knockout source pigs (lacking Gal, Neu5Gc, and Sda) drastically reduce xenoantigen expression on pig cells, and provides a foundation for xenotransfusion.
Sensitization to pig antigens does not appear to be detrimental to the outcome of subsequent human ABO-compatible RBC transfusion.
Significant advances in genetic engineering may be applied to pig RBCs, which could overcome or diminish remaining barriers to xenotransfusion, including complement-mediated rejection and phagocytosis.
Research Agenda.
Future investigations (prior to clinical trials) must characterize (1) the survival of porcine RBCs after xenotransfusion in NHPs, and (2) determine to what extent porcine RBC loss is related to antibody binding, complement activation, phagocytosis, and/or the adaptive immune response.
Novel methods to introduce human transgenes into pig RBCs (which do not have nuclei) will need to be developed before clinical trials can be initiated.
Acknowledgments
Funding
Work on xenotransplantation at UAB is supported in part by NIH grant #U19 AI090959/08.
Abbreviations
- Gal
galactoseα1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- HLA
human leukocyte antigens
- Neu5Gc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- pRBCs
pig red blood cells
- SIRP-α
signal-regulatory protein-α
- SLA
swine leukocyte antigens
- TKO
triple-knockout (i.e., pigs that express none of the three known pig antigens against which humans have natural antibodies)
- WT
wild-type
Footnotes
Conflicts of Interest Statement
The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global status report on blood safety and availability 2016. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.Ellingson KD, Sapiano MRP, Haass KA, Savinkina AA, Baker ML, Chung KW, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion 2017;57:1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Red Cross. Blood needs and blood supply. RedCrossBlood.Org 2018. Accessed Apr 28, 2018. [Google Scholar]
- 4.Center for Biologics Evaluation and Research. Donating blood questions and answers. U S Food and Drug Administration Home Page. https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/QuestionsaboutBlood/DonatingBlood/default.htm 2018. Accessed Apr 28, 2018. [Google Scholar]
- 5.Revised preventative measures to reduce the possible risk of transmission of Creutzfeldt-Jakob disease and variant Creutzfeld-Jakob disease by blood and blood products. US FDA-US Department of Health and Human Services; https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM307137.pdf 2018. Accessed Jun 24, 2018. [Google Scholar]
- 6.Wahl S, Quirolo KC. Current issues in blood transfusion for sickle cell disease. Curr Opin Pediatr 2009;21:15–21. [DOI] [PubMed] [Google Scholar]
- 7.Vidler JB, Gardner K, Amenyah K, Mijovic A, Thein SL. Delayed haemolytic transfusion reaction in adults with sickle cell disease: a 5-year experience. Br J Haematol 2015;169:746–53 [DOI] [PubMed] [Google Scholar]
- 8.Win N, Doughty H, Telfer P, Wild BJ, Pearson TC. Hyperhemolytic transfusion reaction in sickle cell disease. Transfusion 2001;41:323–8. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Tang H, Qayyum R, Schick UM, Nalls MA, Handsaker R, et al. Genomewide association analysis of red blood cell traits in African Americans: the COGENT Network. Hum Mol Genet 2013;22(12):2529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee MEM, Josephson CD, Winkler AM, Webb J, Luban NLC, Leong T, et al. Red blood cell minor antigen mismatches during chronic transfusion therapy for sickle cell anemia. Transfusion 2017;57:2738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahr JS, Walker V, Manoochehri K. Blood substitutes as pharmacotherapies in clinical practice. Curr Opin Anaesthesiol 2007;20:325–30. [DOI] [PubMed] [Google Scholar]
- 12.Darghouth D, Giarratana MC, Oliveira L, Jolly S, Marie T, Boudah S, et al. Bioengineered and native red blood cells from cord blood exhibit the same metabolomic profile. Hematologica 2016;101:220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trakarnsanga K, Griffiths RE, Wilson MC, Blair A, Satchwell TJ, Meinders M, et al. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional cells. Nature Commun 2017;8:14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douay L Why industrial production of red blood cells from stem cells is essential for tomorrow’s transfusion. Regen Med 2018;13:627–32. [DOI] [PubMed] [Google Scholar]
- 15.Ovchynnikova E, Aglialoro F, von Lindern M, van den Akker E. The shape shifting role of reticulocyte maturation. Front Physiol 2018;9:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler JR, Ladowski JM, Martens GR, Tector M, Tector AJ. Recent advances in genome editing and creation of genetically modified pigs. Internat J Surg 2015;23:217–22. [DOI] [PubMed] [Google Scholar]
- 17.Cooper DKC, Gaston R, Eckhoff D, Ladowski J, Yamamoto T, Wang L, et al. Xenotransplantation-current status and prospects. Br Med Bull 2018;125:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keynes G Tercentenary of Blood Transfusion. Brit Med J 1967;4:410–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt PJ, Leacock AG. Forgotten transfusion history: John Leacock of Barbados. BMJ 2002;325:1485–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roux FA, Sai P, Deschamps JY. Xenotransfusions, past and present. Xenotransplantation 2007;14:208–16. [DOI] [PubMed] [Google Scholar]
- 21.Roux FA, Sai P, Deschamps JY. Some ethical issues regarding xenotransfusion. Xenotransplantation 2007;14:217–21. [DOI] [PubMed] [Google Scholar]
- 22.Learoyd P The history of blood transfusion prior to the 20th century--part 1. Transfus Med 2012;22:308–14. [DOI] [PubMed] [Google Scholar]
- 23.Learoyd P The history of blood transfusion prior to the 20th century--part 2. Transfus Med 2012;22:372–6. [DOI] [PubMed] [Google Scholar]
- 24.Landsteiner K Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe. Zentralblatt für Bakteriologie 1900;28:357–62. [Google Scholar]
- 25.Neuhof H The Transplantation of Tissues. New York, NY, USA: Appleton; 1923. [Google Scholar]
- 26.Deschamps JY, Roux FA, Sai P, Gouin E. History of xenotransplantation. Xenotransplantation 2005;12:91–109. [DOI] [PubMed] [Google Scholar]
- 27.Reemtsma K, McCracken BH, Schlegel JU, Pearl M. Heterotransplantation of the Kidney: Two Clinical Experiences. Science 1964;143:700–2. [DOI] [PubMed] [Google Scholar]
- 28.Zhu A Introduction to porcine red blood cells: implications for xenotransfusion. Semin Hematol 2000;37:143–9. [DOI] [PubMed] [Google Scholar]
- 29.Cooper DKC. Porcine red blood cells as a source of blood transfusion in humans. Xenotransplantation 2003;10:384–6. [DOI] [PubMed] [Google Scholar]
- 30.Eckermann JM, Bühler LH, Zhu A, Dor FJ, Awwad M, Cooper DK. Initial investigation of the potential of modified porcine erythrocytes for transfusion in primates. Xenotransplantation 2004;11:18–26. [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Long C, Lee W, Zhang Z, Gao X, Landsittel D, et al. Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLoS One 2017;12:e0180768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pond WG, Houpt KA. The Biology of the Pig. Ithaca: Comstock Pub. Associates, 1978. [Google Scholar]
- 33.Jandl JH. Blood: Textbook of Hematology. Boston: Little Brown, 1996. [Google Scholar]
- 34.Katz DS, White SP, Huang W, Kumar R, Christianson DW. Structure determination of aquomet porcine hemoglobin at 2.8A resolution. J Mol Biol 1994;244:541–3. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto F, Yamamoto M. Molecular genetic basis of porcine histo-blood group AO system. Blood 2001;97:3308–10. [DOI] [PubMed] [Google Scholar]
- 36.Smith DM, Newhouse M, Naziruddin B, Kresie L. Blood groups and transfusions in pigs. Xenotransplantation 2006;13:186–94. [DOI] [PubMed] [Google Scholar]
- 37.Rao MJ, Schneider K, Chait BT, Chao TL, Keller H, Anderson S, et al. Recombinant hemoglobin A produced in transgenic swine: structural equivalence with human hemoglobin A. Artif Cells Blood Substit Immobil Biotechnol 1994;22:695–700. [DOI] [PubMed] [Google Scholar]
- 38.Blusch JH, Patience C, Martin U. Pig endogenous retroviruses and xenotransplantation. Xenotransplantation 2002;9:242–51. [DOI] [PubMed] [Google Scholar]
- 39.Onions D, Cooper DKC, Alexander TJL, Brown C, Claassen E, Foweraker JE, et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation 2000;7:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishman J, Patience C. Xenotransplantation: infectious risk revisited. Am J Transplant 2004;4:1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fishman JA, Infectious disease risks in xenotransplantation. Am J Transplant 2018;18:1857–64. [DOI] [PubMed] [Google Scholar]
- 42.Lexer G, Cooper DKC, Rose AG, Wicomb WN, Rees J, Keraan M, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant 1986;5:411–8. [PubMed] [Google Scholar]
- 43.Cooper DKC, Human PA, Lexer G, Rose AG, Rees J, Keraan M, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant 1988;7:238–46. [PubMed] [Google Scholar]
- 44.Cooper DKC, Ezzelarab MB, Hara H, Iwase H, Lee W, Wijkstrom M, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation 2016;23:83–105. [DOI] [PubMed] [Google Scholar]
- 45.Cooper DKC, Hara H, Yazer M. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clin Lab Med 2010;30:365–80. [DOI] [PubMed] [Google Scholar]
- 46.LaVecchio JA, Dunne AD, Edge AS. Enzymatic removal of alpha-galactosyl epitopes from porcine endothelial cells diminishes the cytotoxic effect of natural antibodies. Transplantation 1995;60:841–7. [PubMed] [Google Scholar]
- 47.Luo Y, Wen J, Luo C, Cummings RD, Cooper DK. Pig xenogeneic antigen modification with green coffee bean α-galactosidase: working conditions and potential application in xenotransplantation. Xenotransplantation 1999;6:238–48. [DOI] [PubMed] [Google Scholar]
- 48.Dor FJ, Rouhani FJ, Cooper DKC. Transfusion of pig red cells into baboons. Xenotransplantation 2004;11:295–7. [DOI] [PubMed] [Google Scholar]
- 49.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003;299:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA 2004;101:7335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouhani FJ, Dor FJ, Cooper DKC. Investigation of red blood cells from α1,3-galactosyltransferase-knockout pigs for human blood transfusion. Transfusion 2004;44:1004–12. [DOI] [PubMed] [Google Scholar]
- 52.Long C, Hara H, Pawlikowski Z, Koike N, d’Arville T, Yeh P, et al. Genetically-engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion 2009;49:2418–29. [DOI] [PubMed] [Google Scholar]
- 53.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988;263:17755–62. [PubMed] [Google Scholar]
- 54.Cooper DKC. Depletion of natural antibodies in nonhuman primates–a step towards successful discordant xenografting in man. Clin Transplant 1992;6:178–83. [PubMed] [Google Scholar]
- 55.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc 1992;24:559–62. [PubMed] [Google Scholar]
- 56.Doucet J, Gao ZH, MacLaren LA, McAlister VC. Modification of xenoantigens on porcine erythrocytes for xenotransfusion. Surgery 2004;135:178–86. [DOI] [PubMed] [Google Scholar]
- 57.Asaoka H, Matsuda H. Detection of N-glycolylnemaminic acid-containing glycoproteins from various animal erythrocytes by chicken monoclonal antibody against Hanganutziu-Deicher antigens. J Vet Med Sci 1994;56:375–7. [DOI] [PubMed] [Google Scholar]
- 58.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1–3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J 1996;13:947–53. [DOI] [PubMed] [Google Scholar]
- 59.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation 2002;9:376–81. [DOI] [PubMed] [Google Scholar]
- 60.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid n-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation 2011;18:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation 2014;21:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrne G, Ahmad-Villers S, Du Z, McGregor C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation 2018:e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C, Cooper DKC, Dai Y, Hara H, Cai Z, Mou L. The Sda and Cad glycan antigens and their glycosyltransferase, β1,4GalNAcT-II, in xenotransplantation. Xenotransplantation 2018;25:e12386. [DOI] [PubMed] [Google Scholar]
- 64.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GALNT2 genes. Xenotransplantation 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butler JR, Martens GR, Estrada JL, Reyes LM, Ladowski JM, Galli C, et al. Silencing porcine genes significantly reduces human-anti-pig cytotoxicity profiles: an alternative to direct complement regulation. Transgenic Res 2016;25:751–9. [DOI] [PubMed] [Google Scholar]
- 66.Lee W, Hara H, Ezzelarab MB, Iwase H, Bottino R, Long C, et al. Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs. Xenotransplantation 2016;23:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang ZY, Martens GR, Blankenship RL, Sidner RA, Li P, Estrada JL, et al. Eliminating xenoantigen expression on swine RBC. Transplantation 2017;101:517–23. [DOI] [PubMed] [Google Scholar]
- 68.Atkinson JP, Oglesby TJ, White D, Adams EA, Liszewski MK. Separation of self from non-self in the complement system: a role for membrane cofactor protein and decay accelerating factor. Clin Exp Immunol 1991;86:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalmasso AP, Vercellotti GM, Platt JL, Bach FH. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation 1991;52:530–3. [DOI] [PubMed] [Google Scholar]
- 70.White DJG, Langford GA, Cozzi E, Young VK. Production of pigs transgenic for human DAF: a strategy for xenotransplantation. Xenotransplantation 1995;2:213–7. [Google Scholar]
- 71.Morgan BP, Berg CW, Harris CL. “Homologous restriction” in complement lysis: roles of membrane complement regulators. Xenotransplantation 2005;12:258–65. [DOI] [PubMed] [Google Scholar]
- 72.Lublin DM, Liszewski MK, Post TW, Arce MA, Le Beau MM, Rebentisch MB, et al. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med 1988;168:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez de la Lastra JM, Hanna SM, Morgan BP. Distribution of membrane cofactor protein (MCP/CD46) on pig tissues. Relevance to xenotransplantation. Immunology 1999;98:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorley BR, Milland J, Christiansen D, Lanteri MB, McInnes B, Moeller I, et al. Transgenic expression of a CD46 (membrane cofactor protein) minigene: studies of xenotransplantation and measles virus infection. Eur J Immunol 1997;27:726–34. [DOI] [PubMed] [Google Scholar]
- 75.Sugita Y, Nakano Y, Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem 1988;104:633–7. [DOI] [PubMed] [Google Scholar]
- 76.Rollins SA, Zhao J, Ninomiya H, Sims PJ. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol 1991; 146:2345–51. [PubMed] [Google Scholar]
- 77.Cozzi E, Langford E, Richards GA, Elsome K, Lancaster R, Chen P, et al. Expression of human decay accelerating factor in transgenic pigs. Transplant Proc 1994;26:1402–3. [PubMed] [Google Scholar]
- 78.Fodor WL, Williams BL, Matis LA, Madri JA, Rollins SA, Knight JW, et al. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci USA 1994;91:11153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langford GA, Yannoutsos N, Cozzi E, Lancaster R, Elsome K, Chen P, et al. Production of pigs transgenic for human decay accelerating factor. Transplant Proc 1994;26:1400–1. [PubMed] [Google Scholar]
- 80.McCurry KR, Kooyman DL, Alvarado CG, Cotterell AH, Martin MJ, Logan JS, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med 1995;1:423–7. [DOI] [PubMed] [Google Scholar]
- 81.Diamond LE, McCurry KR, Martin MJ, McClellan SB, Oldham ER, Platt JL, et al. Characterization of transgenic pigs expressing functionally active human CD59 on cardiac endothelium. Transplantation 1996;61:1241–9. [DOI] [PubMed] [Google Scholar]
- 82.Diamond LE, Quinn CM, Martin MJ, Lawson J, Platt JL, Logan JS. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation 2001;71:132–42. [DOI] [PubMed] [Google Scholar]
- 83.Schuurman HJ, Pino-Chavez G, Phillips MJ, Thomas L, White DJ, Cozzi E. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation 2002;73:1146–51. [DOI] [PubMed] [Google Scholar]
- 84.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in nonimmunosuppressed baboons. Xenotransplantation 2004;11:171–83. [DOI] [PubMed] [Google Scholar]
- 85.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transplant Int 2008;21:1163–74. [DOI] [PubMed] [Google Scholar]
- 86.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant 2009;9:2716–26. [DOI] [PubMed] [Google Scholar]
- 87.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation 2012;93:666–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burdorf L, Stoddard T, Zhang T, Rybak E, Riner A, Avon C, et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft Injury. Am Transplant 2014;14:1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qian Z, Wasowska Ba, Behrens E, Cangello DL, Brody JR, Kadkol SS, et al. C6 produced by macrophages contributes to cardiac allograft rejection. Am J Pathol 1999;155:1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leonard CT, Soccal PM, Singer L, Berry GJ, Theodore J, Holt PG, et al. Dendritic cells and macrophages in lung allografts: A role in chronic rejection? Am J Respir Crit Care Med 2000;161:1349–54. [DOI] [PubMed] [Google Scholar]
- 91.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation 2005;12:81–8. [DOI] [PubMed] [Google Scholar]
- 92.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci 2007;104:5062–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation 2010;17:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Navarro-Alvarez N, Yang YG. CD47: a new player in phagocytosis and xenograft rejection. Cell Mol Immunol 2011;8:285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maeda A, Kawamura T, Ueno T, Usui N, Eguchi H, Miyagawa S. The suppression of inflammatory macrophage-mediated cytotoxicity and proinflammatory cytokine production by transgenic expression of HLA-E. Transpl Immunol 2013;29:76–81. [DOI] [PubMed] [Google Scholar]
- 96.Tena A, Kurtz J, Leonard DA, Dobrinsky JR, Terlouw SL, Mtango N, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant 2014;14:2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, et al. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 2017;101:316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inverardi L, Clissi B, Stolzer AL, Bender JR, Sandrin MS, Pardi R. Human natural killer lymphocytes directly recognize evolutionarily conserved oligosaccharide ligands expressed by xenogeneic tissues. Transplantation 1997;63:1318–30. [DOI] [PubMed] [Google Scholar]
- 99.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-alpha-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol 2004;172:6460–7. [DOI] [PubMed] [Google Scholar]
- 100.Kennett SB, Porter CM, Horvath-Arcidiacono JA, Bloom ET. Characterization of baboon NK cells and their xenogeneic activity. Xenotransplantation 2010;17:288–99. [DOI] [PubMed] [Google Scholar]
- 101.Dorling A, Monk N, Lechler R. HLA-G inhibits the transendothelial cell migration of human NK cells: A strategy for inhibiting xenograft rejection. Transplant Proc 2000;32:938. [DOI] [PubMed] [Google Scholar]
- 102.Matsunami K, Miyagawa S, Nakai R, Murase A, Shirakura R. The possible use of HLA-G1 and G3 in the inhibition of NK cell-mediated swine endothelial cell lysis. Clin Exp Immunol 2001;126:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: Protection from human NK cytotoxicity depends on peptide loading. Am J Transplant 2005;5:2085–93. [DOI] [PubMed] [Google Scholar]
- 104.Crew MD. Play it in E or G: Utilization of HLA-E and -G in xenotransplantation. Xenotransplantation 2007;14:198–207. [DOI] [PubMed] [Google Scholar]
- 105.Weiss EH, Lilienfeld BG, Muller S, Muller E, Herbach N, Kessler B, et al. HLA-E/human beta2-microglobulin transgenic pigs: Protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 2009;87:35–43. [DOI] [PubMed] [Google Scholar]
- 106.Esquivel EL, Maeda A, Eguchi H, Asada M, Sugiyama M, Manabe C, et al. Suppression of human macrophage-mediated cytotoxicity by transgenic swine endothelial cell expression of HLA-G. Transpl Immunol 2015;32:109–15. [DOI] [PubMed] [Google Scholar]
- 107.Gill RG, Wolf L, Daniel D, Coulombe M. CD4+ T cells are both necessary and sufficient for islet xenograft rejection. Transplant Proc 1994;26:1203. [PubMed] [Google Scholar]
- 108.Rollins SA, Kennedy SP, Chodera AJ, Elliott EA, Zavoico GB, Matis LA. Evidence that activation of human T cells by porcine endothelium involves direct recognition of porcine SLA and costimulation by porcine ligands for LFA-1 and CD2. Transplantation 1994;57:1709–16. [PubMed] [Google Scholar]
- 109.Elwood ET, Larsen CP, Cho HR, Corbascio M, Ritchie SC, Alexander DZ, et al. Prolonged acceptance of concordant and discordant xenografts with combined CD40 and CD28 pathway blockade. Transplantation 1998;65:1422–8. [DOI] [PubMed] [Google Scholar]
- 110.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 2005;11:32–4. [DOI] [PubMed] [Google Scholar]
- 111.Davila E, Byrne GW, LaBreche PT, McGregor HC, Schwab AK, Davies WR, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation 2006;13:31–40. [DOI] [PubMed] [Google Scholar]
- 112.Koshika T, Phelps C, Fang J, Lee SE, Fujita M, Ayares D, et al. Relative efficiency of porcine and human cytotoxic T-lymphocyte antigen 4 immunoglobulin in inhibiting human CD4+ T-cell responses co-stimulated by porcine and human B7 molecules. Immunology 2011;134:386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ezzelarab MB, Ekser B, Isse K, Iwase H, Morelli AE, Ayares D, et al. Increased soluble CD154 (CD40 ligand) levels in xenograft recipients correlate with the development of de novo anti-pig IgG antibodies. Transplantation 2014;97:502–8. [DOI] [PubMed] [Google Scholar]
- 114.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev 2014;258:241–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ezzelarab MB, Ayares D, Cooper DKC. Transgenic expression of human CD46: does it reduce the primate T-cell response to pig endothelial cells? Xenotransplantation 2015;22:487–9. [DOI] [PubMed] [Google Scholar]
- 116.Bühler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation 2000;69:2296–304. [DOI] [PubMed] [Google Scholar]
- 117.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb based regimen. Am J Transplant 2004;4:363–72. [DOI] [PubMed] [Google Scholar]
- 118.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, et al. Alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation 2005;80:1493–500. [DOI] [PubMed] [Google Scholar]
- 119.Ezzelarab MB, Ekser B, Echeverri G, Hara H, Ezzelarab C, Long C, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation 2012;9:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML 3rd, Lewis BG, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant 2014;14:488–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iwase H, Esker B, Satyananda V, Bhama J, Hara H, Ezzelarab M, et al. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015;22:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 2015;22:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML 3rd, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bottino R, Knoll MF, Graeme-Wilson J, Klein EC, Ayares D, Trucco M, et al. Safe use of anti-CD154 monoclonal antibody in pig islet xenotransplantation in monkeys. Xenotransplantation 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. Immunologic and physiologic observations in baboons with life-supporting genetically-engineered pig kidney grafts. Xenotransplantation 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J, Kim DH, Choi HJ, Lee HJ, Kang HJ, Park CG, et al. Anti-CD40 antibody-mediated costimulation blockade promotes long-term survival of deep-lamellar porcine corneal grafts in non-human primates. Xenotransplantation 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin JS, Kim JM, Min BH, Yoon IH, Kim HJ, Kim HS, et al. Pre-clinical results in pig-to-non-human primate islet xenotransplantation using anti-CD40 antibody (2C10R4)-based immunosuppression. Xenotransplantation 2018;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin C, Plat M, Nerriere-Daguin V, Coulon F, Uzbekova S, Venturi E, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res 2005;14:373–84. [DOI] [PubMed] [Google Scholar]
- 129.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation 2009;16:477–85. [DOI] [PubMed] [Google Scholar]
- 130.Bottino R, Wijkstrom M, van der Windt DJ, Hara H, Ezzelarab M, Murase N, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant 2014;14:2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, et al. Human dominantnegative class II transactivator transgenic pigs–effect on the human anti-pig T cell immune response and immune status. Immunology 2013;140:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iwase H, Ekser B, Satyananda V, Zhou H, Hara H, Bajona P, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol 2015;32:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reyes LM, Estrada JL, Wang ZY, Blosser RJ, Smith RF, Sidner RF, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol 2014;193:5751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tseng YL, Sachs DH, Cooper DKC. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation 2005;79:1–9. [DOI] [PubMed] [Google Scholar]
- 135.Rees MA, Butler AJ, Brons IG, Negus MC, Skepper JN, Friend PJ. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation 2005;12:13–9. [DOI] [PubMed] [Google Scholar]
- 136.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB 3rd, Larsen CP, et al. Pre-transplant antibody screening and anti-cd154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015;22:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adams AB, Kim SC, Martens GR, Ladowski JM, Estrada JM, Reyes LM, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg 268:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cooper DKC, Hara H, Ezzelarab M, Bottino R, Trucco M, Phelps C, et al. The potential of genetically-engineered pigs in providing an alternative source of organs and cells for transplantation. J Biomed Res 2013;27:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Omi T, Vogeli P, Hagger C, Schelling C, Spilar S, Kajii E, et al. cDNA cloning, mapping and polymorphism of the porcine Rhesus (RH) gene. Anim Genet 2003;34:176–82. [DOI] [PubMed] [Google Scholar]
- 140.Oostingh GJ, Davies HF, Tang KC, Bradley JA, Taylor CJ. Sensitization to swine leukocyte antigens in patients with broadly reactive HLA specific antibodies. Am J Transplant 2002;2:267–73. [DOI] [PubMed] [Google Scholar]
- 141.Burlak C, Paris LL, Lutz AJ, Sidner RA, Estrada J, Li P, et al. Reduced binding of human antibodies to cells from GGTA/CMAH KO pigs. Am J Transplant 2014;14:1895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation 2014;21:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rood PP, Tai HC, Hara H, Long C, Ezzelarab M, Lin YJ, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int 2007;20:1050–8. [DOI] [PubMed] [Google Scholar]
- 144.Rood PP, Hara H, Busch JL, Ezzelarab M, Zhu X, Ball S, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transplant Int 2006;19:158–65. [DOI] [PubMed] [Google Scholar]
- 145.Ezzelarab M, Hara H, Busch J, Rood PP, Zhu X, Ibrahim Z, et al. Antibodies directed to pig non-Gal antigens in naive and sensitized baboons. Xenotransplantation 2006;13:400–7. [DOI] [PubMed] [Google Scholar]
- 146.Martens GR, Reyes LM, Li P, Butler JR, Ladowski JM, Estrada JL, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA Class I knockout pigs. Transplantation 2017;101:e86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Q, Shaikh S, Iwase H, Long C, Lee W, Zhang Z, et al. Carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys: relevance to studies of xenotransplantation. Xenotransplantation 2018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000;288:2051–4. [DOI] [PubMed] [Google Scholar]
- 149.Vernon-Wilson EF, Kee WJ, Willis AC, Barclay AN, Simmons DL, Brown MH. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol 2000;30:2130–7. [DOI] [PubMed] [Google Scholar]
- 150.Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood 2007;109:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee JS, Kallehauge TB, Pedersen LE, Kildegaard HF. Site-specific integration in CHO cells mediated by CRISPR/Cas9 and homology-directed DNA repair pathway. Sci Rep 2015;5:8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Seebach JD, Comrack C, Germana S, LeGuern C, Sachs DH, DerSimion H. HLA-CW3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J immunol 1997;159:3655–61. [PubMed] [Google Scholar]
- 153.Wilhite T, Ezzelarab C, Hara H, Long C, Ayares D, Cooper DK, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation 2012;19:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramsoondar JJ, Machaty Z, Costa C, Williams BL, Fodor WL, Bondioli KR. Production of alpha 1,3- galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod 2003;69:437–45. [DOI] [PubMed] [Google Scholar]
- 155.Liu QP, Yuan H, Bennett EP, Levery SB, Nudelman E, Spence J, et al. Identification of a GH110 subfamily of alpha 1,3-galactosidases: novel enzymes for removal of the alpha 3Gal xenotransplantation antigen. J Biol Chem 2008;283:8545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bagnis C, Chiaroni J, Bailly P. Elimination of blood group antigens: hope and reality. Br J Haematol 2011;152:392–400. [DOI] [PubMed] [Google Scholar]
- 157.Gao HW, Zhuo HL, Zhang X, Ji SP, Tan YZ, Li SB, et al. Evaluation of group A1B erythrocytes converted to type as group O: studies of markers of function and compatibility. Blood Transfus 2016;14:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oropeza M, Petersen B, Carnwath JW, Lucas-Hahn A, Lemme E, Hassel P, et al. Transgenic expression of the human a20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation 2009;16:522–34. [DOI] [PubMed] [Google Scholar]
- 159.Petersen B, Lucas-Hahn A, Lemme E, Quiesser AL, Oropeza M, Herrmann D, et al. Generation and characterization of pigs transgenic for human hemeoxygenase-1 (hho-1). Xenotransplantation 2010;17:102–3. [Google Scholar]
- 160.Petersen B, Ramackers W, Lucas-Hahn A, Lemme E, Hassel P, Quiesser AL, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation 2011;18:355–68. [DOI] [PubMed] [Google Scholar]
- 161.Fischer K, Kraner-Schiber, Peterson B, Rieblinger B, Buermann A, Flisikowska T, et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci Rep 2016;6:29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Z, Wilson NA, Chinnaadurai R, Panzer SE, Redfield RR 3rd, Reese SR, et al. Autologous mesenchymal stromal cells prevent transfusion-elicited sensitization and upregulate transitional and regulatory b cells. Transplant Direct 2018;4:e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cooper DKC, Tseng YL, Saidman SL. Alloantibody and xenoantibody crossreactivity in transplantation. Transplantation 2004;77:1–5. [DOI] [PubMed] [Google Scholar]
- 164.Li Q, Hara H, Zhang Z, Breimer ME, Wang Y, Cooper DKC. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation 2018:e12393. [DOI] [PMC free article] [PubMed] [Google Scholar]