Abstract
Legionella pneumophila causes human lung infections resulting in severe pneumonia. High-resolution genotyping of L. pneumophila isolates can be achieved by multiple-locus variable-number tandem-repeat analysis (MLVA-8). Legionella infections in humans occur as a result of inhalation of bacteria-containing aerosols, thus, our aim was to study the antimicrobial susceptibilities of different MLVA-8 genotypes to ten commonly used antimicrobial agents in legionellosis therapy. Epidemiological cut-off values were determined for all antibiotics. Significant differences were found between the antimicrobial agents’ susceptibilities of the three studied environmental genotypes (Gt4, Gt6, and Gt15). Each genotype exhibited a significantly different susceptibility profile, with Gt4 strains (Sequence Type 1) significantly more resistant towards most studied antimicrobial agents. In contrast, Gt6 strains (also Sequence Type 1) were more susceptible to six of the ten studied antimicrobial agents compared to the other genotypes. Our findings show that environmental strains isolated from adjacent points of the same water system, exhibit distinct antimicrobial resistance profiles. These differences highlight the importance of susceptibility testing of Legionella strains. In Israel, the most extensively used macrolide for pneumonia is azithromycin. Our results point at the fact that clarithromycin (another macrolide) and trimethoprim with sulfamethoxazole (SXT) were the most effective antimicrobial agents towards L. pneumophila strains. Moreover, legionellosis can be caused by multiple L. pneumophila genotypes, thus, the treatment approach should be the use of combined antibiotic therapy. Further studies are needed to evaluate specific antimicrobial combinations for legionellosis therapy.
Introduction
Legionella pneumophila has been found worldwide to be a relatively common pulmonary pathogen of severe community-acquired or nosocomial pneumonia1–3. Legionella infections in humans occur via inhalation of bacteria-containing aerosols, thus, the source of Legionella infection in humans is the environmental strains. Because of the ability of Legionella spp. to survive and multiply in human macrophages, they are susceptible to intracellularly active antimicrobial agents4,5. Currently, fluoroquinolones, macrolides, and rifampicin are the most commonly used antimicrobials in the treatment of legionellosis4–7. However, mortality rates of 10–15% are usually reported in legionellosis patients and death may occur despite antimicrobial agent therapy6,8. Furthermore, the presence of antimicrobial agents in the environment may promote the evolution of microbial resistance mechanisms9. This is particularly important for Legionella spp. that colonize most man-made water systems, where they may be exposed to antimicrobial agents of various artificial origins, or even to those secreted by other microorganisms10.
Although 25 of the 59 described Legionella species have been implicated in human disease11–13, the vast majority of cases are caused by L. pneumophila strains, most of which belong to serogroup 114–16. Consequently, isolates of this common serogroup should be genotyped and further differentiated in order to evaluate the efficacy of antimicrobial agents in their treatment. Azithromycin is the most common macrolide used for treatment of community-acquired pneumonia in Israel. However, higher minimal inhibitory concentration (MIC) values have been reported for azithromycin compared with other macrolides for L. pneumophila serogroup 1. Thus, it is important to assess the susceptibility patterns of L. pneumophila in Israel as recommended in other countries17.
Multiple-locus variable-number tandem-repeat analysis (MLVA) was implemented by Pourcel et al.18,19 and approved by the European Centre for Disease Prevention and Control19. The method relies on the variability found in some tandemly repeated DNA sequences (VNTR) that represent sources of genetic polymorphism (Supplementary Fig. S1). This high-throughput typing method is used for epidemiological investigations of the origin of legionellosis cases since it allows rapid systematic typing of any new isolate and inclusion of data in shared databases19–21.
Recently, Rodríguez-Martínez et al.22 and Sharaby et al.23,24 showed that the level of genotypes (analyzed by MLVA-8) should be addressed in order to get insights into ecological traits of L. pneumophila strains inhabiting drinking water distribution systems (DWDSs). These studies showed that different sites of the same DWDS are dominated by different L. pneumophila MLVA genotypes. Analysis of the three dominant genotypes showed that they could be addressed as different ecotypes with a distinct temperature range, growth kinetics, virulence and abundances at their site of dominance22–24.
The aim of the current study was to analyze and compare the antimicrobial susceptibilities of different L. pneumophila genotypes to commonly used antimicrobial agents in legionellosis therapy. As far as we know, results from susceptibility testing of environmental and clinical L. pneumophila isolates have never been published in Israel. Since humans are infected with Legionella by inhaling Legionella-contaminated water aerosols, it is important to study the resistances of environmental strains to antibacterial agents and not only the clinical isolates. We determined the antimicrobial susceptibility profile for different L. pneumophila MLVA-8 genotypes. As each pneumonia patient can be infected by a mixture of L. pneumophila strains25,26, studying the antimicrobial susceptibility profiles of different environmental and clinical L. pneumophila strains is of great importance as it may shed light on the distribution of resistance to antimicrobial agents and assist in determining an accurate and efficient treatment for future legionellosis patients.
Methods
L. pneumophila strains
We studied the susceptibility of 93 environmental and 12 clinical strains to 10 antimicrobial compounds that are commonly used for legionellosis (Table 1). These strains were isolated from a drinking-water distribution system (DWDS) as part of a study conducted in northern Israel for two years (2013–2014, between coordinates 32°42′43.17″N, 35°6′28.66″E). During the sampling campaign, we sampled Legionella spp. seasonally from the drinking water systems of seven buildings. Legionella was isolated from water and biofilm samples according to ISO 11731:2004 and 11731:201727,28 as described by Rodriguez-Martinez et al.22. In addition, we studied the susceptibility to antimicrobial agents of twelve clinical strains that were isolated from sputum samples of hospitalized pneumonia patients at Poriya and Rambam hospitals in northern Israel, between April 2013 and September 201423.
Table 1.
Legionella pneumophila genotypes used in the current study.
| Sampling point | MLVA-8 Genotypes (n) | Sequence type (ST), Serogroup (Sg) | MLVA-8 genotype (Lpms) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (3) | (13) | (17) | (19) | (33) | (34) | (35) | |||
| Environmental strains | ||||||||||
| A | Gt15 (11) | NA, Sg3 | 9 | 8 | 8 | 2 | 5 | 2 | 2 | 21 |
| C, D | Gt4 (64) | ST1, Sg1 | 7 | 7 | 10 | 2 | 4 | 4 | 2 | 17 |
| E, F, G | Gt6 (16) | ST1, Sg1 | 7 | 7 | 10 | 2 | 4 | 4 | 2 | 18 |
| D | Gt18 (1) | ST1, Sg1 | 7 | 7 | 7 | 2 | 4 | 4 | 2 | 17 |
| E | Gt3 (1) | NA, Sg1 | 7 | 7 | 10 | 2 | 4 | 4 | 2 | 0 |
| Clinical strains | ||||||||||
| Hospital | Gt4 (4) | ST1, Sg1 | 7 | 7 | 10 | 2 | 4 | 4 | 2 | 17 |
| Hospital | Gt6 (2) | ST1, Sg1 | 7 | 7 | 10 | 2 | 4 | 4 | 2 | 18 |
| Hospital | Gt19 (1) | ST1, Sg1 | 7 | 7 | 10 | 1 | 4 | 4 | 2 | 17 |
| Hospital | Gt20 (1) | ST1, Sg1 | 7 | 7 | 10 | 2 | 4 | 4 | 3 | 17 |
| Hospital | Gt22 (2) | ST59, Sg1 | 8 | 8 | 10 | 2 | 5 | 4 | 1 | 13 |
| Hospital | Gt24 (2) | ST93, Sg1 | 8 | 8 | 11 | 2 | 0 | 1 | 1 | 3 |
| Reference strain | ||||||||||
| Philadelphia-1 | Gt64 | ST36, Sg1 | 8 | 8 | 11 | 2 | 4 | 1 | 1 | 3 |
Overview of the studied genotypes and their MLVA-8 allelic profiles; number of tandem repeats observed for each L. pneumophila minisatellite locus (Lpms). The indicated sampling points in the drinking-water network were representative for the whole network. The water flow direction was from sampling point A to G. For more details regarding the sampling points please see Rodríguez-Martínez et al.22.
Allelic repeats profiles for the reference strain were obtained from Pourcel et al.19. Highlighted in bold are differences in tandem repeats for each genotype compared to the type strain L. pneumophila Philadelphia-1.
Reference strains
L. pneumophila subsp. Pneumophila sg. 1 of the American Type Culture Collection (ATCC 33152) was used as the reference strain. In addition, Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922) were also selected for validation of susceptibility testing results (Table 2). The selected strains were kept frozen at −80 °C prior to analysis.
Table 2.
Minimal inhibitory concentrations (µg/ml) of each antimicrobial agent, towards the reference strains.
| L. pneumophila (ATCC 33152) | E. coli (ATCC 25922) | S. aureus (ATCC 29213) | |
|---|---|---|---|
| Ciprofloxacin | 0.032 | 0.25 | N.D. |
| Moxifloxacin | 0.032 | 0.25 | N.D. |
| Levofloxacin | 0.032 | 0.064 | 0.25 |
| Tigecycline | 0.064 | 0.25 | N.D. |
| Doxycycline | 0.032 | N.D. | 0.023 |
| Azithromycin | 0.032 | N.D. | 0.023 |
| Erythromycin | 0.047 | N.D. | 0.25 |
| Clarithromycin | 0.047 | N.D. | 0.032 |
| Rifampicin | 0.023 | N.D. | 0.25 |
| SXTa | 0.023 | 0.19 | 2.0 |
*SXT, Trimethoprim and sulfamethoxazole; N.D., not determined.
L. pneumophila molecular typing
Genotyping of the strains was achieved by Multi Locus Variable number of tandem repeat Analysis using eight loci (MLVA-8) as described by Pourcel et al.21,22, Kahlisch et al.29 and Pecellin30 (Fig. 1). Briefly, 1 × 10−2 ng of DNA template was used in 25 µl PCR reactions containing 1 Multiplex PCR Master Mix (Qiagen, Hilden, Germany) and 1.25 pmol of each primer (VIC®-, NED®-, FAM-, and NET-labeled forward primers from Applied Biosystems, Foster City, CA). Primers sequences, position, and repeat sizes at each variable number of tandem repeats (VNTR) locus are listed in Supplementary Table S1. After amplification, PCR products were pooled and denatured. Amplicons were then separated by size using fluorescent capillary electrophoresis, a powerful separation technique based on the differential size-dependent migration of DNA molecules in an electric field. Fluorescent capillary electrophoresis of the multiplex PCR products was performed with a 3730 × L sequencer (Applied Biosystems) as described in Nederbragt et al.21. We used a pre-run voltage of 8.0 kV, run voltage of 8 kV, injection voltage of 1.8 kV and injection time of 15 sec. Each L. pneumophila mini-microsatellite locus (Lpms) was identified by color and assigned a size by GeneMapper software, version 3.7 (Applied Biosystems), using settings for VNTR analysis as shown in Fig. 1. The final repeat profile was then compared with the MLVA-8 database for Legionella (http://microbesgenotyping.i2bc.paris-saclay.fr/databases/view/887). The genotypes of 63 environmental strains were reported in detail by Rodríguez-Martínez et al.22. Additionally, in this study we genotyped 30 more environmental strains isolated from the same sampling campaign. The genotypes for the 12 clinical isolates were reported in Sharaby et al.23. The details of the studied strains regarding their isolation source, their serogroup (sg), sequence type (ST), and their genotypes (Gt) are listed in Table 1.
Figure 1.
Representative electropherograms of MLVA-8 PCR products of multiplex PCR, separated by capillary electrophoresis and identified according to their sizes and colors. Electropherograms correspond to MLVA-8 PCR products of panel 1 and panel 2 of (A) Genotype 64 (Gt64) L. pneumophila Philadelphia-1 and (B) Genotype 4 (Gt4) an environmental strain. Repeats number at each Lpms locus was identified by color and peak size by GeneMapper software, version 3.7 (Applied Biosystems). Repeat profiles were then compared with the MLVA-8 database for Legionella (http://microbesgenotyping.i2bc.paris-saclay.fr/databases/view/887). The workflow chart of the MLVA-8 analysis is explained in Supplementary Fig. S1 and in the Methods section 2.3.
Determination of MICs
Isolates from buffered charcoal yeast extract agar supplemented with α-ketoglutarate (BCYE-α) plates (BD Diagnostics Sparks, MD) were suspended in 0.85% NaCl solution to a 0.5 McFarland standard and subjected to MIC test strip (Liofilchem s.r.l., Italy) on BCYE-α plates. A sterile cotton swab was soaked in the inoculum suspension of each isolate. Each swab was then streaked over the entire BCYE-α agar plate surface. Plates were left to dry for 10 minutes so that the surface was completely dry before applying the Epsilometer test gradient strip. The following antimicrobial agents were used: Azithromycin, Clarithromycin, Ciprofloxacin, Moxifloxacin, Rifampicin, Tigecycline, Doxycycline, Levofloxacin, Erythromycin, and Trimethoprim-sulfamethoxazole. The MICs were read after 48 hours of incubation at 35 ± 1 °C at 2.5% CO2. The MIC of each antimicrobial agent was taken as the lowest concentration of the antimicrobial agent at which the zone of inhibition intersected the strip. MIC tests for L. pneumophila isolates were repeated in triplicates. Epidemiological Cut-off values (ECOFFs) were determined according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines for Legionella pneumophila31. Briefly, MIC values were fitted to the cumulative log-normal distribution using non-linear least squares regression in order to determine the ECOFF for each antimicrobial agent31.
Statistical analysis
All statistical analyses were performed using IBM SPSS 22® and Primer7 software (Primer-e, Auckland, New Zealand). All tests were applied at a 95% level of confidence. Repeated-measures analysis of variance (ANOVA) was applied to study the differences between the MICs of different antimicrobials. Data sphericity was not violated (Mauchly’s test: p > 0.05). T-tests were applied in order to compare the antimicrobials’ MICs for strains isolated from environmental versus clinical sources, water versus biofilm and hot versus cold water. In addition, analysis of similarities (ANOSIM) was performed32 in order to compare the antimicrobial agent resistance profiles of environmental genotypes and clinical isolates taking into account all studied antimicrobial agents’ MIC values. The resemblance matrix was calculated using the Bray-Curtis index of association (Primer7 software). One-way ANOVA was used to determine whether significant differences exist in antimicrobial agents’ MICs between different MLVA-8 genotypes (Gt4, Gt6, and Gt15). All groups were normally distributed according to Shapiro-Wilk test (p > 0.05) and variances were equal between groups (Levene’s test: p > 0.05).
Results
The susceptibilities of 93 environmental and 12 clinical L. pneumophila strains to 10 antimicrobial agents commonly used in legionellosis therapy were analyzed. The environmental strains that were studied here represent a subset of the strains belonging to three MLVA-8 genotypes (Gt) 4, 6, and 1522 that dominated a water network in northern Israel (Table 1). The clinical strains belonged to Gt4 and Gt6, Gt19, Gt20, Gt22, and Gt24 (Table 1). All strains except Gt15 were classified as serogroup 1. Gt15 strains were classified as serogroup 3 (Table 1). We used L. pneumophila subsp. Pneumophila sg 1 (ATCC 33152) as the reference strain. In addition, Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922) were also selected for validation of susceptibility testing results (Table 2). The MICs obtained for both S. aureus and E. coli were generally lower but within an order of magnitude compared to the findings of previous studies33,34.
Overall, significant differences were observed in L. pneumophila sensitivities to different antimicrobial agents (Repeated measures ANOVA: F9,792 = 15.27, p < 0.001). Minimal inhibitory concentrations (MICs) of antimicrobial agents from the fluoroquinolone family were significantly higher compared to those of the macrolides, doxycycline, rifampicin, and trimethoprim & sulfamethoxazole (SXT) (Fig. 2, Table 3). The lowest MICs were observed after exposure to SXT, yet no significant differences were found between the MICs of SXT, erythromycin, Clarithromycin, and Rifampicin (Fig. 2). The highest MIC was found for ciprofloxacin (0.74 ± 0.06 µg/ml) and it was significantly higher than the MICs of moxifloxacin and levofloxacin, which are fluoroquinolones (0.52 ± 0.04 and 0.37 ± 0.04 µg/ml, respectively). MIC50 values yielded similar results with the highest MIC50 found for ciprofloxacin (0.75 µg/ml) and the lowest for SXT with 0.023 µg/ml (Tables 3, 4).
Figure 2.

Minimal inhibitory concentrations (average ± standard error) of each studied antimicrobial agent towards L. pneumophila strains isolated from both clinical and environmental sources (n = 105). Ciprofloxacin – CIP, moxifloxacin – MXF, levofloxacin – LEV, tigecycline – TGC, doxycycline – DXT, azithromycin – AMZ, erythromycin – E, clarithromycin – CLR, rifampicin – RD, trimethoprim & sulfamethoxazole – SXT. Bars connected by different letters are significantly different by repeated-measures ANOVA with Tukey’s HSD post-hoc test with a confidence interval of 95%.
Table 3.
The accumulated percentages (%) of all the tested strains (93 environmental and 12 clinical L. pneumophila isolates), that were inhibited at each concentration of the different antimicrobial agents (µg/ml).
| µg/ml | 0.016 | 0.023 | 0.032 | 0.064 | 0.094 | 0.125 | 0.19 | 0.25 | 0.38 | 0.5 | 0.75 | 1 | 1.5 | 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | 2 | 2 | 18 | 18 | 21 | 21 | 28 | 39 | 40 | 47 | 53 | 81 | 92 | 100 |
| Moxifloxacin | 27 | 27 | 28 | 30 | 30 | 42 | 42 | 62 | 68 | 99 | 100 | |||
| Levofloxacin | 9 | 43 | 50 | 51 | 51 | 51 | 53 | 56 | 73 | 88 | 96 | 100 | ||
| Tigecycline | 2 | 40 | 43 | 43 | 43 | 43 | 43 | 43 | 66 | 74 | 88 | 94 | 100 | |
| Doxycycline | 10 | 69 | 73 | 73 | 73 | 82 | 87 | 87 | 100 | |||||
| Azithromycin | 13 | 19 | 26 | 31 | 36 | 49 | 59 | 87 | 96 | 100 | ||||
| Erythromycin | 5 | 42 | 49 | 50 | 54 | 72 | 79 | 81 | 96 | 98 | 100 | |||
| Clarithromycin | 1 | 5 | 49 | 72 | 74 | 75 | 81 | 93 | 93 | 100 | ||||
| Rifampicin | 48 | 51 | 77 | 87 | 89 | 89 | 89 | 89 | 89 | 95 | 98 | 100 | ||
| SXT* | 42 | 53 | 72 | 80 | 81 | 83 | 86 | 90 | 92 | 99 | 100 |
*SXT, Trimethoprim and sulfamethoxazole.
The values for each antimicrobial agent are the percentage of the strains that were inhibited in the above mentioned antimicrobial agent concentration, thus, MIC50 and MIC90 values can be read directly from this table. For example, SXT has a MIC50 of between 0.016–0.023 µg/ml and a MIC90 of 0.25 µg/ml.
Table 4.
MIC50, MIC90, MIC range and ECOFF values (µg/ml) of the 10 tested antimicrobial agents for all L. pneumophila strains (n = 105).
| Drug | MIC50 | MIC90 | Range | ECOFF* |
|---|---|---|---|---|
| Ciprofloxacin | 0.75 | 1.5 | 0.019–2.0 | 4.0 |
| Moxifloxacin | 0.5 | 1.0 | 0.032–1.5 | 4.0 |
| Levofloxacin | 0.075 | 1.0 | 0.023–1.5 | 1.0 |
| Tigecycline | 0.5 | 1.5 | 0.023–2.0 | 0.5 |
| Doxycyline | 0.032 | 0.5 | 0.023–0.5 | 0.5 |
| Azithromycin | 0.38 | 0.75 | 0.032–1.0 | 2.0 |
| Erythromycin | 0.094 | 0.5 | 0.023–1.0 | 0.5 |
| clarithromycin | 0.064 | 0.25 | 0.025–0.5 | 0.5 |
| Rifampicin | 0.023 | 0.5 | 0.003–1.0 | 0.063 |
| SXT** | 0.023 | 0.25 | 0.003–0.75 | 0.5 |
MIC50, MIC90; Lowest concentration of the antimicrobial agents at which 50% and 90% of the isolates were inhibited, respectively. *ECOFF, epidemiological cut-off values. **SXT, Trimethoprim and sulfamethoxazole.
No significant differences were detected between the susceptibilities of environmental strains isolated from bulk water (n = 58) vs. biofilms (n = 35) (t-tests: df = 91, p > 0.1, for all studied antimicrobial agents). Moreover, t-tests did not detect any significant differences in antimicrobial agent resistances of strains isolated from cold (n = 32) vs. hot water (n = 26) (t-tests: df = 56, p > 0.1, for all studied antimicrobial agents). For additional details, see Supplementary Table S2. In contrast, t-tests revealed significant differences in antimicrobial agent susceptibilities of environmental vs. clinical (e.g., isolated from patients’ sputum) strains. Environmental strains were significantly more resistant towards five of the 10 studied antimicrobial agents compared to L. pneumophila strains from clinical sources (Table 5). The largest difference was found after exposure to ciprofloxacin; MIC50 of ciprofloxacin was 1.0 µg/ml for the environmental strains and only 0.22 µg/ml for the clinical strains (Table 5). In addition, the MICs of tigecycline, clarithromycin, rifampicin, and SXT were also significantly higher for the environmental strains compared to the clinical L. pneumophila strains (Table 5). In contrast, doxycycline was the only studied antimicrobial agent for which the clinical strains were more resistant, with MIC50 and MIC90 of 0.19 and 0.5 µg/ml compared to 0.25 and 0.032 µg/ml for the environmental strains, respectively (Table 5). In addition, analysis of similarities (ANOSIM) revealed significant differences between the antimicrobial agent resistance profiles of clinical and environmental L. pneumophila isolates (R = 0.62, p < 0.001). However, a comparison of Gt4 strains from clinical vs. environmental sources showed no significant differences in antimicrobial agent resistances (t-tests: df = 62, p > 0.05). This may be due to low sample size of the clinical Gt4 strains (n = 4).
Table 5.
Antimicrobial MICs (µg/ml) for the clinical and the environmental L. pneumophila strains and for each of the environmental genotypes.
| Gt4 (n = 64) | Gt6 (n = 16) | Gt15 (n = 11) | *Environmental (n = 93) | Clinical (n = 12) | |
|---|---|---|---|---|---|
| Ciprofloxacin | 2.0 (1) | 0.875 (0.22) | 1.0 (0.5) | 1.5 (1) | 0.475 (0.22) |
| Moxifloxacin | 1.0 (0.75) | 0.25 (0.0395) | 1.0 (0.5) | 1.0 (0.5) | 1.0 (0.5) |
| Levofloxacin | 1.0 (0.5) | 0.157 (0.032) | 0.75 (0.032) | 1.0 (0.064) | 0.75 (0.288) |
| Tigecycline | 1.5 (0.75) | 0.056 (0.032) | 0.5 (0.047) | 1.5 (0.5) | 0.5 (0.047) |
| Doxycycline | 0.25 (0.032) | 0.19 (0.032) | 0.5 (0.064) | 0.25 (0.032) | 0.5 (0.19) |
| Azithromycin | 0.75 (0.44) | 0.19 (0.0555) | 0.5 (0.25) | 0.75 (0.38) | 0.725 (0.375) |
| Erythromycin | 0.5 (0.079) | 0.25 (0.142) | 0.5 (0.19) | 0.5 (0.125) | 0.19 (0.032) |
| Clarithromycin | 0.25 (0.056) | 0.5 (0.064) | 0.5 (0.25) | 0.25 (0.064) | 0.064 (0.047) |
| Rifampicin | 0.5 (0.032) | 0.012 (0.006) | 1.0 (0.032) | 0.5 (0.032) | 0.006 (0.004) |
| SXT** | 0.25 (0.023) | 0.253 (0.032) | 0.5 (0.032) | 0.354 (0.032) | 0.023 (0.006) |
*Environmental strains included two strains designated Gt3 and Gt18 in addition to the listed Gt4, Gt6, Gt15 strains. **SXT, Trimethoprim and sulfamethoxazole.
MIC90 values are in bold and MIC50 values are presented in brackets.
Environmental genotypes
One-way ANOVA revealed significant differences in the resistance of the co-localized environmental genotypes (F2,88 = 128.73, p < 0.001). Gt4 strains were found to be significantly more resistant towards ciprofloxacin, moxifloxacin, levofloxacin, tigecycline, and azithromycin compared to strains belonging to Gt6 and Gt15 (Fig. 3 and Table 5). The highest MIC90 values were obtained for Gt4 strains after exposure to ciprofloxacin and tigecycline (2 µg/ml and 1.5 µg/ml, respectively). The MIC90 values of Gt6 strains were significantly lower compared to other genotypes after exposure to six out of the ten studied antimicrobial agents. The lowest MIC90 values for Gt6 strains were obtained with tigecycline and rifampicin (0.056 and 0.012 µg/ml, respectively); an order of magnitude lower compared to the MICs of Gt4 and Gt15 strains (Fig. 3 and Table 5). Gt15 strains were significantly more resistant to clarithromycin, rifampicin, and SXT (with MIC90 of 0.5, 1, and 0.5 µg/ml, respectively). In addition, analysis of similarities showed that different genotypes possess significantly different resistance profiles (ANOSIM: R = 0.287, p = 0.001).
Figure 3.
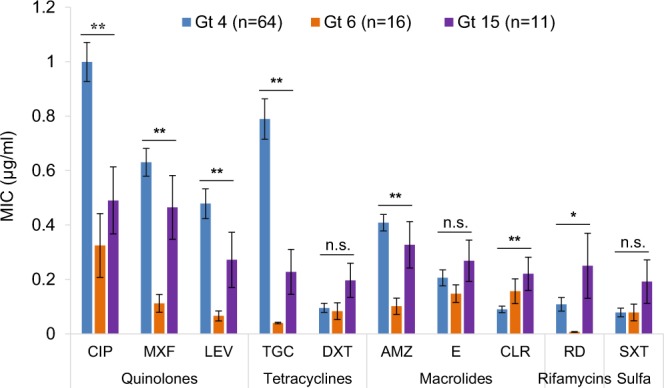
Minimal inhibitory concentrations (average ± standard error) of each studied antimicrobial agent for different MLVA-8 genotypes. Ciprofloxacin – CIP, moxifloxacin – MXF, levofloxacin – LEV, tigecycline – TGC, doxycycline – DXT, azithromycin – AMZ, erythromycin – E, clarithromycin – CLR, rifampicin – RD, trimethoprim & sulfamethoxazole – SXT. Asterisks represent significant differences by one-way ANOVA with Tukey’s post-hoc tests between genotypes at the 0.05* and 0.001** levels of confidence. n.s., not significant.
Discussion
MLVA is a useful genotyping method as it allows a good resolution within the highly health-relevant and abundant Sequence Type 1 (ST1) strains (Table 1). For example, genotypes 4 and 6 are both classified as ST1, and cannot be differentiated by the sequence-based typing method. Moreover, genotype 4 comprises the reference strain L. pneumophila Paris, which belongs to ST119. Mercante and Winchell12 and McDade35 have suggested that the level of genotypes should be addressed in order to assess the health risks posed by the presence of different L. pneumophila strains in DWDSs. As far as we know this is the first study that compares susceptibilities of environmental L. pneumophila MLVA-8 genotypes to antimicrobial agents.
Recently, we have demonstrated that L. pneumophila dominated different sites of a small Israeli drinking water network, with MLVA-8 genotype related abundance regime22. These genotypes demonstrated different temperature-dependent growth kinetics and different cytotoxicity towards amoebae, macrophages and red blood cells23,24. Hence, here we show that these same isolates differed also in their susceptibilities to antimicrobial agents (Tables 1 and 5). MLVA-8 genotypes 4 and 6 strains exhibited distinct growth characteristics despite the fact that both are classified as ST1 by sequence-based typing. Gt4 strains were able to proliferate more rapidly in temperatures of 25–37 °C compared to genotypes Gt6 and Gt15 strains23. In addition, Gt4 strains were significantly more cytotoxic towards amoebae and macrophages under in vitro experimental conditions24. In the current study, Gt4 strains were significantly more resistant towards five out of the 10 antimicrobial agents that were studied, compared to Gt6 strains (Fig. 3). These findings suggest that ST1 strains belonging to Gt4 genotypes may pose a much more severe health risk compared to ST1 strains belonging to Gt6 (Tables 1 and 5). Our current findings indicate that these environmental genotypes, although colonizing the same niche in the drinking water system, should be addressed as different ecotypes since a high variability exists even among ST1 strains in terms of their antimicrobial resistance profiles.
Coscollá et al.25 observed mixed infections of L. pneumophila strains in outbreak patients. They analyzed sequence based typing profiles of uncultured respiratory samples and found evidence of a mixture of Legionella ST profiles in patients. They concluded that patients might be infected from the environment by more than one L. pneumophila strain. Recently, Mizrahi et al.26 also reported that a mix of L. pneumophila strains were identified from sputum samples of pneumonia patients. These findings, along with the results described here regarding the high variability of L. pneumophila genotypes’ antimicrobial agent resistances, emphasize the importance of high-resolution identification of different genotypes and their antimicrobial agent susceptibility profiles, especially in pneumonia patients. In such cases of mixed lung infections caused by multiple L. pneumophila genotypes, the application of combination of antibiotic therapy should be considered since it might provide better treatment outcomes. Dual combination antibiotic therapy was shown to improve treatment outcomes and survival in patients with severe community-acquired pneumonia caused by Legionella and other pathogenic bacteria8,36. Adding a macrolide or fluoroquinolone to a β-lactam was already recommended by the Infectious Diseases Society of America/American Thoracic Society guidelines37. For example, the combination of rifampicin with clarithromycin showed decreased mortality rates in patients8. In our study, both rifampicin and clarithromycin, were found to be very effective towards the three compared genotypes (Fig. 3 and Table 5). Therefore, their combination in treating mixed infections caused by several L. pneumophila genotypes may improve treatment outcomes compared to monotherapy. Further research with emphasis on different MLVA genotyping will allow more accurate assessments of the different antimicrobials’ efficacies in treatment of human infections.
It has been previously reported that performing E-test on BCYE-α agar may yield elevated MICs38. Nonetheless, it still provides a simple yet accurate method for routine and comparative susceptibility testing of Legionella spp. However, the MIC value itself, should not be directly translated to serum concentrations of these antimicrobial agents. Thus, it can be used for detecting antimicrobial resistances. Sufficient data to establish ECOFFs are currently not available31. In the current study, ECOFFs were determined according to the EUCAST guidelines for L. pneumophila susceptibility testing (Table 4)31. Our findings can be used in the future in the process of setting epidemiological cut off values33.
Antimicrobial agent susceptibility of Legionella strains isolated from drinking water sources was studied previously. Xiong et al.39 found that levofloxacin was the most effective drug against different L. pneumophila serogroups. Minocycline and doxycycline were also found to be effective. Torre et al.34 and Sikora et al.40 found that ciprofloxacin and rifampicin have good activity against environmental L. pneumophila sg 1 and sg 2–14. For the overall set of strains tested in the current study, we found that the most effective drugs towards L. pneumophila strains were doxycycline, clarithromycin, rifampicin, and SXT (Fig. 2). Moreover, the strains in the current study were found to be relatively resistant towards levofloxacin and ciprofloxacin (the most effective drugs according to Xiong et al.39 and Sikora et al.40, respectively).
Azithromycin (macrolides) and respiratory fluoroquinolones are the most commonly used antimicrobial agent treatments for community-acquired pneumonia37,41–44. Numerous public health agencies such as the Infectious Diseases Society of America (IDSA), the British Thoracic Society (BTS) and the Dutch Association of Chest Physicians recommend using fluoroquinolones (ciprofloxacin in particular), or azithromycin, as a preferred antimicrobial therapy for legionellosis cases6,37,45. Thus, it is of major importance to verify high susceptibility rates of L. pneumophila to these antimicrobial agents. We found significantly higher MIC values to fluoroquinolones compared with macrolides, which might justify empiric and definitive treatment with macrolides as first line treatment of L. pneumophila pneumonia in Israel (Fig. 2, Table 3). In contrast, other studies reported that quinolones have greater activity toward L. pneumophila compared with macrolides, with a reduced length of stay, and reduced time to clinical resolution43,46. Nevertheless, these differences probably resulted from differences in the susceptibility testing methods used43,44,46,47. In Israel, azithromycin is the most extensively used macrolide for treatment of community-acquired pneumonia as well as L. pneumophila pneumonia. In the current study, we found higher MIC values for azithromycin versus clarithromycin (Fig. 2 and Table 4). This is similar to the findings of studies conducted in southern Italy on the susceptibilities of L. pneumophila strains17,34.
Recently, Massip et al.48 showed that lpeAB genes encode components of a tripartite efflux pump implicated in resistance to azithromycin among other macrolides in L. pneumophila. In addition, Vandewalle-Capo et al.49 demonstrated that the reduced azithromycin susceptibility of ST1 strains was linked to the presence of these lpeAB genes. In our study, we found significant differences in resistance to azithromycin between co-localized genotypes, especially Gt4 and Gt6 strains, both belonging to ST1 (Fig. 3 and Table 5). This finding might justify further studies of antimicrobial agents’ clinical efficacy towards different genotypes and possibly a switch to treatment with clarithromycin. Moreover, in our study, the lowest MIC values were observed after exposure to trimethoprim – SXT (Fig. 2 and Tables 3, 4). These antimicrobial agents are not regularly used to treat L. pneumophila infections and thus, further research is needed to evaluate the efficiency of SXT for treating legionellosis.
Legionella patients are infected with the bacteria by inhaling water droplets containing Legionella. Thus, the source of the clinical strains is the environmental strains and it is important and useful to predict the onset of antimicrobial agent resistance in the environment before it is evidenced in clinical specimens33,50. In the current study, environmental strains were significantly more resistant towards five (ciprofloxacin, tigecycline, clarithromycin, rifampicin, and SXT) out of the 10 studied antibacterial agents, compared to strains of clinical source. Clinical strains were significantly more resistant only to Doxycycline compared to the environmental strains (Table 5). In addition, the antimicrobial resistance profiles of clinical and environmental strains differed significantly (Table 5). Earlier studies suggested that the presence of antimicrobial agents in the environment, and especially in man-made drinking-water distribution systems (DWDSs), might promote the evolution of microbial resistance mechanisms9,10.
Since only one case of person-to-person transmission of Legionella has been reported so far51, the human body is considered to be a “dead-end” for the evolution of this pathogen. Therefore, clinical strains probably do not transfer antimicrobial resistances to the environment and the environmental strains are the source for the clinical cases of L. pneumophila pneumonia infections. It is of great importance to adjust the antibacterial therapy for legionellosis patients to fit the susceptibilities of environmental strains that are present in DWDSs. Our results show that a considerable amount of variability exists in terms of antimicrobial resistances of environmental strains (Fig. 3 and Table 5). A rapid and reliable method for distinguishing between strains is necessary in order to determine the specific susceptibilities of environmental L. pneumophila genotypes.
Routine monitoring and susceptibility testing of environmental strains from DWDSs can allow detection of antimicrobial resistances acquisition. However, as reported in previous studies, there are difficulties in determining MICs for Legionella (for example, inactivation of some antibiotics by charcoal)34,40. Consequently, it is difficult to compare results obtained from different methods and establish ECOFF values. Therefore, highly efficient techniques are needed in order to isolate environmental Legionella strains from the environment and then test and monitor the acquisition of resistance in the environmental context of the network.
In conclusion
We determined the antimicrobial agent susceptibility profiles for different L. pneumophila MLVA-8 genotypes. Gt4 strains belonging to ST1 were significantly more resistant towards Ciprofloxacin, Moxifloxacin, Levofloxacin, Tigecycline, and Azithromycin compared to strains belonging to Gt6 (also belonging to ST1), and Gt15 genotypes (Fig. 3). Our results demonstrate that although these environmental strains were isolated from adjacent points of the same drinking water system, they are distinct in terms of their antimicrobial agent susceptibilities as was also observed for their other physiological traits23,24. Evidence pointed out that pneumonia patients may acquire a mixture of L. pneumophila strains25,26. These, along with the results regarding the high variability of L. pneumophila genotypes’ antimicrobial resistance profiles, emphasize the importance of studying antimicrobial resistances of different L. pneumophila genotypes. Moreover, since the human body is considered a “dead-end” for the evolution of Legionella, it is important to study the antimicrobial resistances not only for clinical isolates, but also for the environmental strains that are the source of the clinical infections.
Supplementary information
Acknowledgements
This study was supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft DFG grant GZ: HO 930/5-2) and by the joint Indian University Grant Commission and Israel Science Foundation (UGC-ISF grant: 2728/17). Special thanks to Marina Pecellin and Leila Kahlisch for assistance and fruitful discussions regarding the MLVA analysis.
Author Contributions
Y.S., A.P. and M.H. conceived and designed the experiments. Y.S. and A.P. performed the experiments. A.P., I.B., M.G.H. and M.H. contributed reagents, materials, and analysis tools. Y.S. analyzed the data. Y.S. and M.H. wrote the paper. Y.S., O.N., I.B., M.G.H., A.P. and M.H. discussed, reviewed and commented the draft manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Avi Peretz and Malka Halpern contributed equally.
Contributor Information
Avi Peretz, Email: APeretz@poria.health.gov.il.
Malka Halpern, Email: mhalpern@research.haifa.ac.il.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42425-1.
References
- 1.Adams D, et al. Summary of Notifiable Infectious Diseases and Conditions — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1–119. doi: 10.15585/mmwr.mm6253a1. [DOI] [PubMed] [Google Scholar]
- 2.Lee HK, Shim JI, Kim HE, Yu JY, Kang YH. Distribution of Legionella species from environmental water sources of public facilities and genetic diversity of L. pneumophila serogroup 1 in South Korea. Appl Environ Microbiol. 2010;76:6547–54. doi: 10.1128/AEM.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin T, et al. Distribution of sequence-based types of Legionella pneumophila serogroup 1 strains isolated from cooling towers, hot springs, and potable water systems in China. Appl Environ Microbiol. 2014;80:2150–2157. doi: 10.1128/AEM.03844-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postma DF, et al. Antibiotic Treatment Strategies for Community-Acquired Pneumonia in Adults. N Engl J Med. 2015;372:1312–1323. doi: 10.1056/NEJMoa1406330. [DOI] [PubMed] [Google Scholar]
- 5.Cunha BA, Burillo A, Bouza E. Legionnaires’ disease. Lancet. 2016;387:376–385. doi: 10.1016/S0140-6736(15)60078-2. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, et al. Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT) Neth J Med. 2018;76:4–13. [PubMed] [Google Scholar]
- 7.Edelstein, P. H. & Roy, C. R. Legionnaires’ Disease and Pontiac Fever. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 10.1016/B978-1-4557-4801-3.00234-4 (2014).
- 8.Rello J., Gattarello S., Souto J., Sole-Violan J., Valles J., Peredo R., Zaragoza R., Vidaur L., Parra A., Roig J. Community-acquired Legionella Pneumonia in the intensive care unit: Impact on survival of combined antibiotic therapy. Medicina Intensiva. 2013;37(5):320–326. doi: 10.1016/j.medin.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 9.D’Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the Antibiotic Resistome. Science (80-) 2006;311:374–377. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- 10.Almahmoud I, Kay E, Schneider D, Maurin M. Mutational paths towards increased fluoroquinolone resistance in Legionella pneumophila. J Antimicrob Chemother. 2009;64:284–293. doi: 10.1093/jac/dkp173. [DOI] [PubMed] [Google Scholar]
- 11.Diederen BMW. Legionella spp. and Legionnaires’ disease. J Infect. 2008;56:1–12. doi: 10.1016/j.jinf.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28:95–133. doi: 10.1128/CMR.00029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein, P. H., Luck, C. & LÜCK, C. Legionella. in Manual of Clinical Microbiology, 11th Edition 887–904 American Society of Microbiology, 10.1128/9781555817381.ch49 (2011).
- 14.Yu VL, et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis. 2002;186:127–128. doi: 10.1086/341087. [DOI] [PubMed] [Google Scholar]
- 15.Cao B, Yao F, Liu X, Feng L, Wang L. Development of a DNA microarray method for detection and identification of all 15 distinct O-antigen forms of Legionella pneumophila. Appl Environ Microbiol. 2013;79:6647–54. doi: 10.1128/AEM.01957-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marston BJ, et al. Incidence of Community-Acquired Pneumonia Requiring Hospitalization. Results of a population-based active surveillance study in Ohio. Ann Intern Med. 1997;157:1709–1718. doi: 10.1001/archinte.1997.00440360129015. [DOI] [PubMed] [Google Scholar]
- 17.De Giglio O, et al. Antibiotic susceptibility of Legionella pneumophila strains isolated from hospital water systems in Southern Italy. Environ Res. 2015;142:586–590. doi: 10.1016/j.envres.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Pourcel C, Vidgop Y, Ramisse F, Vergnaud G, Tram C. Characterization of a Tandem Repeat Polymorphism in Legionella pneumophila and Its Use for Genotyping. J Clin Microbiol. 2003;41:1819–1826. doi: 10.1128/JCM.41.5.1819-1826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourcel C, et al. Identification of Variable-Number Tandem-Repeat (VNTR) Sequences in Legionella pneumophila and Development of an Optimized Multiple-Locus VNTR Analysis Typing Scheme. J Clin Microbiol. 2007;45:1190–1199. doi: 10.1128/JCM.02078-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobral D, et al. High-Throughput Typing Method To Identify a Non-Outbreak-Involved Legionella pneumophila Strain Colonizing the Entire Water Supply System in the Town of Rennes, France. Appl Environ Microbiol. 2011;77:6899–6907. doi: 10.1128/AEM.05556-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nederbragt AJ, et al. Multiple-locus variable-number tandem repeat analysis of Legionella pneumophila using multi-colored capillary electrophoresis. J Microbiol Methods. 2008;73:111–7. doi: 10.1016/j.mimet.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Martínez S, et al. Spatial distribution of Legionella pneumophila MLVA-genotypes in a drinking water system. Water Res. 2015;77:119–132. doi: 10.1016/j.watres.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Sharaby Y, et al. Temperature-Dependent Growth Modeling of Environmental and Clinical Legionella pneumophila Multilocus Variable-Number Tandem-Repeat Analysis (MLVA) Genotypes. Appl Environ Microbiol. 2017;83:e03295–16. doi: 10.1128/AEM.03295-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharaby Y, et al. Virulence Traits of Environmental and Clinical Legionella pneumophila Multilocus Variable-Number Tandem-Repeat Analysis (MLVA) Genotypes. Appl Environ Microbiol. 2018;84:AEM.00429–18. doi: 10.1128/AEM.00429-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coscollá M, Fernández C, Colomina J, Sánchez-Busó L, González-Candelas F. Mixed infection by Legionella pneumophila in outbreak patients. Int J Med Microbiol. 2014;304:307–313. doi: 10.1016/j.ijmm.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Mizrahi, H. et al. Comparison of sputum microbiome of legionellosis-associated patients and other pneumonia patients: indications for polybacterial infections. Sci Rep7 (2017). [DOI] [PMC free article] [PubMed]
- 27.International Organization for Standardization. ISO 11731-2:2004: water quality — detection and enumeration of Legionella — part 2: direct membrane filtration method for waters with low bacterial count (2004).
- 28.International Organization for Standardization. ISO 11731:2017 water quality — enumeration of Legionella (2017).
- 29.Kahlisch, L., Henne, K., Draheim, J., Brettar, I. & Höfle, M. G. High-resolution in situ genotyping of Legionella pneumophila populations in drinking water by multiple-locus variable-number tandem-repeat analysis using environmental DNA. Appl Environ Microbiol76, 6186–95 (2010). [DOI] [PMC free article] [PubMed]
- 30.Pecellin, M. Structure and virulence of Legionella pneumophila populations from freshwater systems in Germany and Middle East. (Technical University of Braunschweig, Germany, 2016).
- 31.Turnidge, J., Kahlmeter, G. & Kronvall, G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clinical Microbiology and Infection12(5), 418–425 (2006). [DOI] [PubMed]
- 32.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol. 1993;18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 33.Bruin JP, Ijzerman EPF, den Boer JW, Mouton JW, Diederen BMW. Wild-type MIC distribution and epidemiological cut-off values in clinical Legionella pneumophila serogroup 1 isolates. Diagn Microbiol Infect Dis. 2012;72:103–108. doi: 10.1016/j.diagmicrobio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Torre I, et al. Environmental surveillance and in vitro activity of antimicrobial agents against Legionella pneumophila isolated from hospital water systems in Campania, South Italy: a 5-year study. Environ Res. 2018;164:574–579. doi: 10.1016/j.envres.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 35.McDade JE. Legionella and the Prevention of Legionellosis. Emerg Infect Dis. 2008;14:1006a–1006. doi: 10.3201/eid1406.080345. [DOI] [Google Scholar]
- 36.Nie W, Li B, Xiu Q. β-Lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1441–1446. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 37.Mandell LA, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia MT, Pelaz C, Gimenez MJ, Aguilar L. In Vitro Activities of Gemifloxacin versus Five Quinolones and Two Macrolides against 271 Spanish Isolates of Legionella pneumophila: Influence of Charcoal on Susceptibility Test Results. Antimicrob Agents Chemother. 2000;44:2176–2178. doi: 10.1128/AAC.44.8.2176-2178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong L, Yan H, Shi L, Mo Z. Antibiotic susceptibility of Legionella strains isolated from public water sources in Macau and Guangzhou. J Water Health. 2016;14:1041–1046. doi: 10.2166/wh.2016.056. [DOI] [PubMed] [Google Scholar]
- 40.Sikora A, et al. Assessment of antibiotic susceptibility of Legionella pneumophila isolated from water systems in Poland. Ann Agric Environ Med. 2017;24:66–69. doi: 10.5604/12321966.1234048. [DOI] [PubMed] [Google Scholar]
- 41.Griffin AT, Peyrani P, Wiemken T, Arnold F. Macrolides versus quinolones in Legionella pneumonia: Results from the Community-Acquired Pneumonia Organization international study. Int J Tuberc Lung Dis. 2010;14:495–499. [PubMed] [Google Scholar]
- 42.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. J Am Med Assoc. 2009;302:758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabrià M, et al. Fluoroquinolones vs macrolides in the treatment of Legionnaires disease. Chest. 2005;128:1401–1405. doi: 10.1378/chest.128.3.1401. [DOI] [PubMed] [Google Scholar]
- 44.Pedro-Botet ML, Yu VL. Treatment strategies for Legionella infection. Expert Opin Pharmacother. 2009;10:1109–1121. doi: 10.1517/14656560902900820. [DOI] [PubMed] [Google Scholar]
- 45.Harris M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66:ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 46.Blázquez Garrido RM, et al. Antimicrobial Chemotherapy for Legionnaires Disease: Levofloxacin versus Macrolides. Clin Infect Dis. 2005;40:800–806. doi: 10.1086/428049. [DOI] [PubMed] [Google Scholar]
- 47.Dunbar LM, Farrell DJ. Activity of telithromycin and comparators against isolates of Legionella pneumophila collected from patients with community-acquired respiratory tract infections: PROTEKT Years 1–5. Clin Microbiol Infect. 2007;13:743–746. doi: 10.1111/j.1469-0691.2007.01717.x. [DOI] [PubMed] [Google Scholar]
- 48.Massip, C. et al. Macrolide resistance in Legionella pneumophila: the role of LpeAB efflux pump. J Antimicrob Chemother dkw594, 10.1093/jac/dkw594 (2017). [DOI] [PubMed]
- 49.Vandewalle-Capo M, et al. Minimum inhibitory concentration (MIC) distribution among wild-type strains of Legionella pneumophila identifies a subpopulation with reduced susceptibility to macrolides owing to efflux pump genes. Int J Antimicrob Agents. 2017;50:684–689. doi: 10.1016/j.ijantimicag.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Sandalakis V, Chochlakis D, Goniotakis I, Tselentis Y, Psaroulaki A. Minimum inhibitory concentration distribution in environmental Legionella spp. isolates. J Water Health. 2014;12:678–685. doi: 10.2166/wh.2014.217. [DOI] [PubMed] [Google Scholar]
- 51.Correia AM, et al. Probable Person-to-Person Transmission of Legionnaires’ Disease. N Engl J Med. 2016;374:497–498. doi: 10.1056/NEJMc1505356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



