Abstract
A growing body of literature suggests that there is a link between periodontitis and systemic diseases. These diseases include cardiovascular disease, gastrointestinal and colorectal cancer, diabetes and insulin resistance, and Alzheimer's disease, as well as respiratory tract infection and adverse pregnancy outcomes. The presence of periodontal pathogens and their metabolic by-products in the mouth may in fact modulate the immune response beyond the oral cavity, thus promoting the development of systemic conditions. A cause-and-effect relationship has not been established yet for most of the diseases, and the mediators of the association are still being identified. A better understanding of the systemic effects of oral microorganisms will contribute to the goal of using the oral cavity to diagnose and possibly treat non-oral systemic disease.
Keywords: Oral pathogens, Systemic disease, Periodontal disease, Chronic disease, Inflammation, Dentistry
Periodontal disease is one of the most common inflammatory diseases in adults. In 2010, 3.9 billion people worldwide were reported to have periodontal disease, with the prevalence of mild periodontitis being 35% and moderate to severe periodontitis, 11% [1]. As the global population ages, periodontal disease has become a significant public health concern and a mounting burden on the healthcare system [2]. According to the US Centers for Disease Control and Prevention, periodontal disease is considered to be a worldwide pandemic, causing disability, speech impairment, low self-esteem, and reduced quality of life [2].
The study of periodontal pathogens and inflammation has attracted the attention from researchers outside of dentistry due to the potential influence of periodontitis on initiation and/or progression of several systemic diseases. Over the years, evidence has accumulated that links oral diseases with many non-oral and systemic diseases, including cancer, cardiovascular disease, type 2 diabetes, respiratory tract infection, adverse pregnancy outcomes, and neurodegenerative disease [3], [4], [5].
For the most part, however, it remains to be established whether specific periodontal pathogens stimulate development of the systemic disease, or if the systemic disease causes the abundance of periodontal pathogens to change. If the pathogens cause non-oral disease, then they would represent obvious targets for therapeutic intervention. But as a minimum, the presence of periodontal pathogens could be used as diagnostic markers to predict susceptibility to non-oral disease.
Periodontal pathogens could promote development of non-oral disease directly or indirectly. For example, about 30 abundant species in the oral cavity, mainly Gram-negative anaerobic bacteria, are known to produce endotoxins, which could directly contribute to systemic disease [6]. Migration of oral pathogens to the blood stream could also occur in some cases, such as following surgical procedures. Bacterial accumulation on the teeth due to poor dental hygiene and/or environmental factors induces a host inflammatory response, which may result in periodontitis and bone loss [7] but could also be harmful to the host systemically.
Dental plaque, periodontal pathogens and bacteremia
The most common Gram-negative bacterial genera in the oral cavity include Treponema, Bacteroides, Porphyromonas, Prevotella, Capnocytophaga, Peptostreptococcus, Fusobacterium, Actinobacillus, and Eikenella [6], [8]. Early studies identified Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Tannerella forsythia as causative agents in periodontal disease [9], and much of the research on periodontal disease continues to focus on these microorganisms. However, more recent studies have determined that the oral cavity contains approximately 500–700 prevalent taxa; this microbial community is referred to as the oral microbiota, oral microflora, or oral microbiome [8], [10]. The oral microbiota is present in saliva, on gingival epithelium and other inner surfaces of the oral cavity and concentrated in dental plaque.
The dental plaque is an organized biofilm of microorganisms that are either attached to the tooth surface or to other microorganisms in a way that allows the microorganisms to survive and resist host defense mechanisms or antibiotic treatment [11]. As the biofilm matures, microbial dysbiosis occurs, causing a progressive shift from Gram-positive to predominantly Gram-negative anaerobic species, and resulting in biofilm formation under the gingival surface [12]. Additionally, sugar metabolism by the dental plaque biofilm leads to production of organic acids, which play a crucial role in pH reduction and demineralization of the tooth surface [13]. Thus, frequent sugar consumption is also known to induce dysbiosis of the supragingival microbiota, promoting development of carious lesions [12].
Tissue trauma, flossing, dental procedures, or even chewing food may induce breakage of blood vessels in close proximity to the dental plaque, which can introduce bacteria into the systemic bloodstream [14]. Bacteremia has in fact been observed following some dental or medical procedures, and some bacteria were isolated from the blood after endodontic treatment [15], [16].
Relationship between oral and non-oral systemic disease
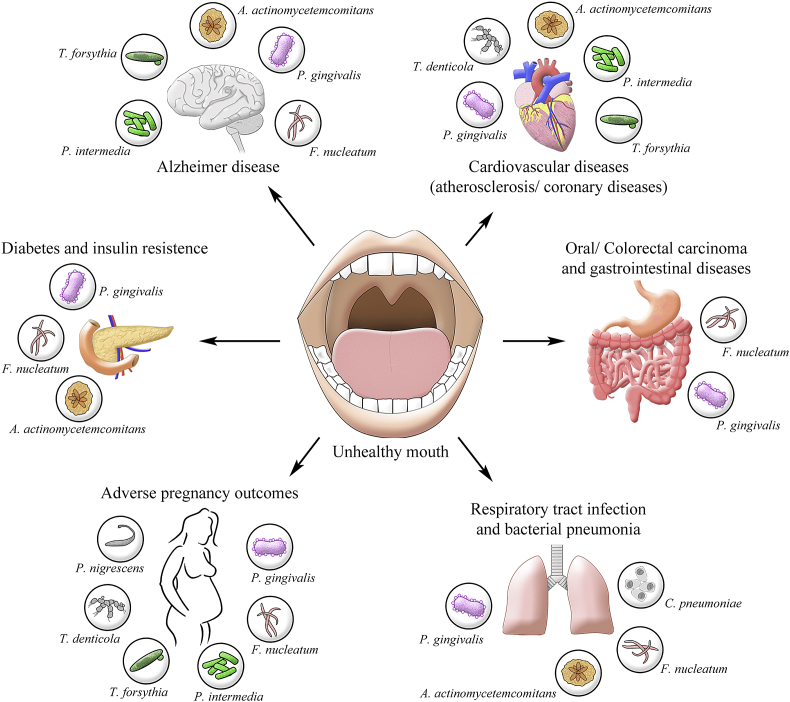
Many recent studies explore the interrelationship between oral health, inflammation, and systemic disease (Fig. 1). Oral microbiota can cause oral inflammation but may also directly contribute to systemic inflammation, increasing inflammation through the release of toxins or leakage of microbial products into the bloodstream. The association between oral inflammation and systemic inflammation is fundamental to understanding the detrimental effects of oral inflammation on several organ systems and the ability of oral disease to increase the risk of developing non-oral disease. We consider evidence linking oral disease with some major systemic non-oral diseases in the sections below.
Fig. 1.
Schematic representation of different systemic diseases and their association with oral pathogens. Periodontal diseases can predispose individuals to several systemic diseases such as cardiovascular disease, oral and colorectal cancer, gastrointestinal diseases, respiratory tract infection and pneumonia, adverse pregnancy outcomes, diabetes and insulin resistance, and Alzheimer's disease. The arrows show systemic diseases that can be affected by the oral cavity, and the periodontal pathogens associated with each systemic disease.
Cardiovascular disease
Cardiovascular disease is considered the leading cause of death in the U.S. and is a major cause of disability according to the CDC. Given its high economic and social impact, the correlation between cardiovascular and periodontal disease has attracted attention of many researchers. Although various epidemiological studies have suggested that there may be an association between periodontitis and cardiovascular disease, the impact of oral infection on cardiovascular diseases has remained unclear.
A meta-analysis that combined 5 cohort studies (86,092 patients) showed that individuals with periodontal disease had 1.14 times higher risk of developing coronary heart disease than the controls, independently of confounding factors [17]. The case-control studies (1423 patients) showed an even greater risk of developing coronary heart disease (2.22 times) [17]. This study showed that both prevalence and incidence of cardiovascular disease are significantly increased in patients with periodontitis. Moreover, an association between edentulousness and serum antibodies against P. gingivalis and A. actinomycetemcomitans with coronary heart disease was observed in a study with 1163 men [18]. An additional study confirmed the presence of bacterial DNA species in 42 atheromatous plaques retrieved by endarterectomy [19]. The bacterial species most commonly found in this study were P. gingivalis, followed by A. actinomycetemcomitans, T. forsythia, Eikenella corrodens, Fusobacterium nucleatum and Campylobacter rectus [19]. Along similar lines, DNA from periodontal pathogens, such as P. gingivalis, A. actinomycetemcomitans, Prevotella intermedia, and T. forsythia, was found in human atherosclerotic plaques, suggesting that these oral pathogens may migrate from the oral cavity to distant sites of the body [20], [21].
More recently, studies in an animal model of atherosclerosis using hyperlipidemic mice infected with P. gingivalis and Treponema denticola demonstrated that infection with these bacteria is associated with alveolar bone loss and aortic atherosclerosis [22], [23]. After oral infection, P. gingivalis and T. denticola induced a systemic immune response, and bacterial genomic DNA was found in the oral epithelium, aorta and within systemic organs [22], [23]. Additionally, P. gingivalis evades innate immune detection via Toll-like receptor (TLR)-4, facilitating chronic inflammation in the vasculature [24]. It was also demonstrated that P. gingivalis, by means of its secreted outer membrane vesicles, can induce platelet aggregation in human samples, which could be responsible for thrombus formation in vivo [25]. Interestingly, other oral pathogens such as A. actinomycetemcomitans, T. forsythia, C. rectus, F. nucleatum, P. intermedia and T. denticola failed to aggregate platelets when tested for aggregation activity [26], suggesting that only P. gingivalis expresses virulence factors that can induce platelet aggregation.
In summary, several oral pathogens are associated with a higher risk of cardiovascular disease in humans, and studies in mice support the possibility that infection with the oral pathogens may lead to the disease.
Respiratory tract infection and pneumonia
Pneumonia is a significant cause of morbidity and mortality in patients of all ages, especially in the old and immunocompromised. The lung infections can be caused by bacteria, fungi, viruses and parasites. Microorganisms can infect the lower respiratory tract by inhalation of infectious aerosols, spread of infection from contiguous sites, and spread from extrapulmonary sites. The oral cavity, especially saliva and dental plaque in patients with periodontal disease, seems to be a logical source for pathogens to accumulate and spread to the lower airways. Several oral pathogens have already been implicated in lung infections, including A. actinomycetemcomitans, Actinomyces israelii, Capnocytophaga spp, Chlamydia pneumoniae, E. corrodens, F. nucleatum, Fusobacterium necrophorum, P. gingivalis, P. intermedia and Streptococcus constellatus [27], [28], [29].
Respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from the same patients in the intensive care unit were shown to be genetically the same, which reinforces the view that dental plaque could serve as a significant reservoir for respiratory pathogens [30]. In fact, individuals with periodontitis are three times more likely to develop nosocomial pneumonia, compared with patients without periodontitis [31]. In an intratracheal mouse model of infection, P. gingivalis was responsible for persistent inflammatory responses in the lungs involving cell recruitment and proinflammatory cytokine production [27]. Interestingly, a study with 40 subjects undergoing orotracheal intubation showed large quantities of A. actinomycetemcomitans, P. gingivalis and T. forsythia in toothed and toothless patients, suggesting that the oral environment, even without teeth, presents favorable conditions for pathogenic bacterial accumulation [32].
The common oral pathogens F. nucleatum and F. necrophorum were found to cause a distinct condition beginning with pharyngitis and leading to respiratory tract infection called Lemierre's syndrome [28], [29]. Interestingly, a cross-sectional study of students presenting acute sore throat detected F. necrophorum in 20.5% of subjects and 9.4% of asymptomatic individuals, which was approximately twice as often as the usually investigated group A β-hemolytic streptococcus (10.3% of patients and 1.1% of asymptomatic subjects) [33]. These studies suggest that Fusobacterium is a potential pathogen of the lungs and should be taken into account when investigating airway complications.
Conversely, C. pneumoniae is well-studied as a respiratory pathogen and has been associated with asthma, bronchitis and chronic obstructive pulmonary disease [34]. This pathogen has also been found in the oral cavity [35], [36] and could probably translocate from the oral cavity to the lower airways, from where it could disseminate systemically to other sites such as spleen, heart, and aorta via monocytes through blood circulation, as suggested in a mouse model [37]. Furthermore, C. pneumoniae infection has been associated with an increased risk of atherosclerosis development [38], which could provide another mechanism whereby pathogens in the oral cavity contribute to atherosclerosis.
Together, these studies demonstrate that unhealthy oral cavities can predispose to respiratory infections and suggest that oral or non-oral pathogens present in the oral cavity could contribute to respiratory disease.
Oral and colorectal cancer
Cancer accounts for one out of four deaths every year, costing the US approximately $100 billion in healthcare besides the emotional and medical burden that it represents to families and society. In the early 1990s, Helicobacter pylori was recognized as a causative agent of human gastric cancer [39], becoming the first major bacterial pathogen to be associated with human cancer [40], [41]. In this review, we will focus on the relationship between periodontal bacterial pathogens and cancer.
A meta-analysis study including 3183 subjects showed that patients with periodontal disease have an increased susceptibility to oral cancer [42]. More recent studies found a positive correlation between periodontal disease and pancreatic, head and neck, and lung cancers [40]. Another study examined one million randomly selected insurance cases in Taiwan, and found that patients in the periodontitis cohort exhibited a higher risk of developing cancer than those in the gingivitis cohort [43].
Moreover, the periodontal pathogen P. gingivalis was found at significantly elevated levels in oral squamous cell carcinoma (OSCC) [44] and esophagus squamous cell carcinoma (ESCC) patients, but not in healthy mucosa [41]. The potential role for periodontal pathogens in the induction of oral cancer was confirmed in an oral-specific chemical carcinogenesis animal model [45]. The study showed that the periodontal pathogens P. gingivalis and F. nucleatum stimulate tumorigenesis via direct interaction with oral epithelial cells, and that the effect is mediated by the host innate immune system [45]. In the case of OSCC, it was demonstrated that P. gingivalis, but not F. nucleatum, promotes invasion and metastasis of oral squamous cells by inducing matrix metalloproteinase 9 (pro-MMP9) expression [44]. Another study showed that prolonged and repetitive exposure to P. gingivalis increases aggressiveness of oral cancer cells via epithelial-mesenchymal transition-like changes in the cells [46]. Therefore, oral pathogens, specifically P. gingivalis and F. nucleatum, have been shown to positively correlate with development of oral cancer, suggesting that they could be biomarkers for early stages of the disease, or even targets for prevention of oral cancers in humans.
Colorectal carcinoma (CRC) is the fourth leading cause of cancer deaths worldwide, and has been associated with a high abundance of F. nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients [47]. A polymicrobial signature of Gram-negative anaerobic bacteria was associated with colorectal carcinoma in 130 tissues analyzed [48]. Gram-negative anaerobic oral pathogens such as Fusobacterium, Leptotrichia and Campylobacter species were identified in individuals with tumors [48]. It is thought that oral F. nucleatum could migrate to and colonize the human intestinal tract, to cause deleterious inflammatory infections [47], [49]. Furthermore, F. nucleatum was found in higher numbers in human colonic adenomas relative to surrounding tissues and in stool samples from patients with CRC, compared with subjects without CRC [50]. Additionally, in a mouse model of intestinal tumorigenesis, F. nucleatum increased tumor multiplicity and selectively recruited tumor-infiltrating myeloid cells, which can promote tumor progression [50]. Another mechanistic study showed that levels of F. nucleatum gradually increased during the colorectal adenoma-carcinoma sequence in human fecal and mucosal samples [51]. F. nucleatum's ability to alter the composition of the lumen microbiota as well as its ability to mediate secretion of cytokines and activate tumorigenesis-related pathways was demonstrated in mouse models of CRC [51]. In summary, these data indicate that F. nucleatum promotes a pro-inflammatory microenvironment that may contribute to neoplasia progression in colorectal adenoma-carcinoma.
Diabetes mellitus
Diabetes mellitus is a chronic metabolic disorder characterized by hyperglycemia due to a defect in insulin production by pancreatic β cells (type 1 diabetes), a decrease in insulin sensitivity (type 2 diabetes), or a combination of both [52] that can affect adults, teenagers and children. Noteworthy, diabetes mellitus and periodontitis present a “two-way” association in which one affects the other. Chronic infection during periodontitis can lead to exacerbated and dysregulated inflammatory responses, which may result in poor metabolic control of blood sugar and increased insulin requirements [53]. In fact, individuals with acute bacterial and viral infection demonstrated severe and long-lasting insulin resistance [54]. This idea was confirmed by a study with 124 middle-aged men showing that the burden of enteroviruses and C. pneumoniae associated strongly with insulin resistance, probably because of the chronic low-grade inflammation resulting from these infections [55]. Regarding P. gingivalis infection, a decrease in gingival vascular function and increased insulin resistance was observed in a diabetes model in rats [56]. Interestingly, results from a meta-analysis study suggested that periodontal treatment leads to an improvement of glycemic control in type 2 diabetic patients, for at least 3 months [57]. In general, even though the mechanisms underlying this association are not completed understood, periodontitis seems to increase the risk of diabetes due to infection and/or inflammatory responses [53], [58].
Conversely, diabetes can also lead to different complications such as poor wound healing, retinopathy, nephropathy, neuropathy, macrovascular disease and periodontitis [52], [58]. In fact, diabetic individuals have a threefold increase in the risk of periodontitis, compared with non-diabetics subjects [59]. In another study, periodontitis was found in 58% of type 1 diabetes patients and in 15% of non-diabetic controls [60]. When periodontal status in children and adolescents with type 1 diabetes mellitus was examined, a prevalence of 21% of gingivitis and 6% of periodontitis was detected; also, patients having lived more than five years with diabetes mellitus type 1 showed more affected sites on periodontal disease parameters [61].
In summary, a bidirectional association between diabetes mellitus and periodontal disease has been shown, in which diabetes enhances the risk for periodontitis, and conversely, periodontal inflammation negatively affects glycemic control. Additionally, periodontal treatment improves diabetes symptoms, confirming their association and the importance of oral health for the overall organism.
Alzheimer's disease
Alzheimer's disease is a progressive neurodegenerative disease characterized by a progressive and irreversible impairment in memory, thinking, and language and learning capacity, which ultimately ends in death [62]. The cognitive decline has been related to the formation of synaptotoxic β-amyloid plaques and hyperphosphorylated tau proteins in the regions of the brain associated with advanced cognitive functions [62], [63]. As shown for diabetes mellitus, Alzheimer's disease and periodontitis also present a bidirectional relationship that will be discussed in this section.
A recent comprehensive oral-health study showed that individuals with brain injury had a higher prevalence of poor oral health parameters and chronic generalized periodontitis [64]. The brain, which was thought to have no or diminished immune responses because of its “immunologic privilege” status, can undergo different inflammatory processes that contribute to development of Alzheimer's disease, such as complement activation and cytokine and chemokine expression [65]. In fact, inflammation is viewed as the link between periodontitis and Alzheimer's disease. The presence of activated glial cells that produce significant levels of inflammatory cytokines is a hallmark of Alzheimer's disease [66]. Besides the direct damage caused by β-amyloid plaques and tau aggregates, the innate immune response attempts to purge these aggregates from the brain, but instead aggravates neurodegeneration [62], [63]. Thus, an increase in pro-inflammatory cytokines is detected in elderly patients with Alzheimer's disease and periodontitis [67]. Studies using different anti-inflammatory drugs and cytokines reinforce the hypothesis that inflammation is a major driver of neurodegeneration in Alzheimer's disease, suggesting that nasal nonsteroidal anti-inflammatory drugs (NSAIDs) might be effective in slowing the onset of Alzheimer's disease [68]. In addition, the IL-1 receptor antagonist and immunosuppressive cytokines can protect the brain from further damage and decrease the rate of Alzheimer's disease progression [69].
The host synthesizes pro-inflammatory cytokines systemically in response to oral bacterial infection, suggesting that periodontal disease may contribute to the brain inflammation that characterizes Alzheimer's disease [65], [70]. Interestingly, lipopolysaccharide (LPS) from periodontal pathogens such as P. gingivalis and T. denticola was isolated from short-term postmortem Alzheimer's disease human brains, suggesting that virulence factors from these pathogens could play a role in development of brain inflammation and Alzheimer's disease [71]. Moreover, bacteria such as the periodontal pathogen T. denticola [72] and C. pneumoniae [73], [74] were detected in postmortem Alzheimer's disease brains, suggesting that besides inflammatory mediators, some periodontal pathogens may invade the brain by crossing the brain-blood barrier. This was confirmed in animal studies, which showed the presence of P. gingivalis in mouse brains [75]. Moreover, higher levels of antibodies against A. actinomycetemcomitans, P. gingivalis, T. forsythia [70], F. nucleatum and P. intermedia [76] were observed in elderly patients with Alzheimer's disease, compared with healthy controls. Although these periodontal pathogens seem to be associated with symptoms of Alzheimer's disease, further longitudinal studies will be needed to directly link periodontal pathogens (and antibodies against them) with neurodegeneration in Alzheimer's disease.
In summary, poor oral hygiene contributes to chronic periodontitis and may indirectly increase the risk for Alzheimer's disease. Conversely, patients with Alzheimer's disease present impairments in the ability to maintain proper oral hygiene or even visit a dentist for professional care, which enhances the risk for periodontitis. In this sense, it is tempting to speculate that maintaining good oral health could become a prophylactic measure against Alzheimer's disease.
Adverse pregnancy outcomes
Maternal infections are associated with adverse pregnancy outcomes, including preterm labor, preterm premature rupture of the membranes, pre-eclampsia, miscarriage, intra-uterine growth retardation, low birthweight, stillbirth, and neonatal sepsis [77]. Due to hormonal changes in pregnant women, they are more susceptible to gingivitis and periodontitis than non-pregnant women [78]. Indeed, approximately 40% of pregnant women demonstrate clinical evidence of periodontal disease [78].
Two different mechanisms have been proposed to explain how oral health is associated with adverse pregnancy outcomes. The first proposes that oral pathogens themselves can translocate from an unhealthy oral cavity and cross the placenta, reaching the intra-amniotic fluid and fetal circulation [79]. The second hypothesizes that the systemic dissemination of endotoxins or inflammatory mediators derived from periodontal disease could affect development of the fetus or spontaneous abortion [80].
Bacterial pathogens, antigens, endotoxins, and pro-inflammatory cytokines produced during periodontal disease can cross the placental barrier, resulting in disturbances in the maternal-fetal unit that could contribute to adverse pregnancy outcomes [81], [82]. F. nucleatum is the most common oral pathogen found in a variety of placental and fetal tissues [77]. A case report of term stillbirth suggested that F. nucleatum could translocate from the mother's mouth to the uterus when her immune response was weakened during a respiratory infection [83]. Consistent with its high invasiveness, F. nucleatum is repeatedly isolated from amniotic fluid and cord blood in cases of preterm birth and neonatal sepsis [84], [85]. Furthermore, F. nucleatum is often detected along with other oral subspecies in intrauterine infections, which are likely from the same infectious origin, implying co-translocation from the oral cavity [77], [85]. Other oral pathogens, such as P. gingivalis (and its endotoxins), were also found in the placenta of preterm delivery patients [86], [87]. Studies in animal models demonstrate the ability of P. gingivalis to impact negatively on pregnancy: LPS from P. gingivalis induced placental and fetal growth restriction and resorption in rats [88]; and antibodies raised against P. gingivalis caused fetal loss when passively administered into mice [89].
The maternal-fetal interface represents an immunologically unique site that must promote immune tolerance to the fetus while at the same time maintaining a robust host defense against possible infections. Even though little is known about the role of innate immune receptors during pregnancy, it was known that the placenta expresses Toll-like receptors (TLRs) during normal pregnancy [90]. Periodontal disease or the presence of periodontal pathogens such as T. denticola and P. gingivalis have been shown to increase the expression of TLRs [82], [91], suggesting increased innate immune responses.
Although more studies will be required to establish conclusively that there is a cause-and-effect relationship between periodontal disease and adverse pregnancy outcomes, the results so far suggest that preventive measures against periodontal disease in pregnant women are warranted.
The oral cavity as a diagnostic tool
The realization that oral health is linked to systemic disease and can affect the progression or development of diverse diseases has led to the search for biomarkers in the oral cavity that could detect systemic disease. The oral cavity is easily accessible, allowing for non-invasive tests in most cases; and patients usually visit dentists more often than general practitioners. Thus, use of the oral cavity for early diagnosis of systemic disease should increase the likelihood of successful treatment of many non-oral diseases.
The saliva is becoming an attractive diagnostic tool for systemic disease, including cancer, bowel disease, diabetes, neurodegenerative disease, and muscle and joint disease [92], [93] since the collection of saliva is quick, simple, and non-invasive [94]. The analysis of multiple biomarkers in saliva could help to detect the presence of several diseases simultaneously, and electrochemical sensor systems could quickly detect salivary protein and genetic markers for diagnosis with high specificity and sensitivity, allowing health care providers to screen for systemic diseases easily and quickly [95].
Current progress has been made with the development of “omics”-based markers for some diseases, such as OSCC [94]. Microbiomics, methylomics, and metabolomics, among other high-throughput approaches, have shown promising potential for detection of some diseases, but further research is needed in many cases to confirm the specific type of disease [96], [97], [98], [99]. Although this field is in its infancy, we are confident that dental practitioners will soon be diagnosing many non-oral systemic diseases in their clinic.
Concluding remarks
A growing body of evidence in the literature shows the direct and indirect impact of periodontal pathogens on overall health. Recent epidemiological, clinical and experimental studies support the relationship between bacteremia or inflammation due to periodontal disease and systemic disease. More studies are needed to elucidate the mechanisms whereby periodontal pathogens or the ensuing inflammation cause or contribute to systemic disease. Nonetheless, it is already clear that management of periodontal disease and proper oral care can positively impact the morbidity, mortality, and health care costs associated with non-oral systemic diseases.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by grants from the University of the Pacific.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Richards D. Oral diseases affect some 3.9 billion people. Evid Based Dent. 2013;14:35. doi: 10.1038/sj.ebd.6400925. [DOI] [PubMed] [Google Scholar]
- 2.CDC researchers find close to half of American adults have periodontitis. J Can Dent Assoc. 2012;78:c136. [PubMed] [Google Scholar]
- 3.Whitmore S.E., Lamont R.J. Oral bacteria and cancer. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo B.K., Lim L.P., Paquette D.W., Williams R.C. Periodontal disease -- the emergence of a risk for systemic conditions: pre-term low birth weight. Ann Acad Med Singapore. 2005;34:111–116. [PubMed] [Google Scholar]
- 5.Kim J., Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 7.Larjava H., Koivisto L., Hakkinen L., Heino J. Epithelial integrins with special reference to oral epithelia. J Dent Res. 2011;90:1367–1376. doi: 10.1177/0022034511402207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paster B.J., Boches S.K., Galvin J.L., Ericson R.E., Lau C.N., Levanos V.A. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambon J.J. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 10.Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann E.E., Wozniak D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudney J.D., Jagtap P.D., Reilly C.S., Chen R., Markowski T.W., Higgins L. Protein relative abundance patterns associated with sucrose-induced dysbiosis are conserved across taxonomically diverse oral microcosm biofilm models of dental caries. Microbiome. 2015;3:69. doi: 10.1186/s40168-015-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paes Leme A.F., Koo H., Bellato C.M., Bedi G., Cury J.A. The role of sucrose in cariogenic dental biofilm formation--new insight. J Dent Res. 2006;85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baltch A.L., Pressman H.L., Schaffer C., Smith R.P., Hammer M.C., Shayegani M. Bacteremia in patients undergoing oral procedures. Study following parenteral antimicrobial prophylaxis as recommended by the American Heart Association, 1977. Arch Intern Med. 1988;148:1084–1088. doi: 10.1001/archinte.148.5.1084. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa M., Prada-Lopez I., Alvarez M., Amaral B., de los Angeles C.D., Tomas I. Post-tooth extraction bacteraemia: a randomized clinical trial on the efficacy of chlorhexidine prophylaxis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morozumi T., Kubota T., Abe D., Shimizu T., Komatsu Y., Yoshie H. Effects of irrigation with an antiseptic and oral administration of azithromycin on bacteremia caused by scaling and root planing. J Periodontol. 2010;81:1555–1563. doi: 10.1902/jop.2010.100163. [DOI] [PubMed] [Google Scholar]
- 17.Bahekar A.A., Singh S., Saha S., Molnar J., Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Pussinen P.J., Jousilahti P., Alfthan G., Palosuo T., Asikainen S., Salomaa V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:1250–1254. doi: 10.1161/01.ATV.0000072969.71452.87. [DOI] [PubMed] [Google Scholar]
- 19.Figuero E., Sanchez-Beltran M., Cuesta-Frechoso S., Tejerina J.M., del Castro J.A., Gutierrez J.M. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82:1469–1477. doi: 10.1902/jop.2011.100719. [DOI] [PubMed] [Google Scholar]
- 20.Haraszthy V.I., Zambon J.J., Trevisan M., Zeid M., Genco R.J. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 21.Nakano K., Inaba H., Nomura R., Nemoto H., Takeda M., Yoshioka H. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol. 2006;44:3313–3317. doi: 10.1128/JCM.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chukkapalli S.S., Rivera M.F., Velsko I.M., Lee J.Y., Chen H., Zheng D. Invasion of oral and aortic tissues by oral spirochete Treponema denticola in ApoE(-/-) mice causally links periodontal disease and atherosclerosis. Infect Immun. 2014;82:1959–1967. doi: 10.1128/IAI.01511-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velsko I.M., Chukkapalli S.S., Rivera M.F., Lee J.Y., Chen H., Zheng D. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slocum C., Coats S.R., Hua N., Kramer C., Papadopoulos G., Weinberg E.O. Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzberg M.C., Meyer M.W. Effects of oral flora on platelets: possible consequences in cardiovascular disease. J Periodontol. 1996;67:1138–1142. doi: 10.1902/jop.1996.67.10s.1138. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A., Novak E.K., Sojar H.T., Swank R.T., Kuramitsu H.K., Genco R.J. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol Immunol. 2000;15:393–396. doi: 10.1034/j.1399-302x.2000.150610.x. [DOI] [PubMed] [Google Scholar]
- 27.Hajishengallis G., Wang M., Bagby G.J., Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J Immunol. 2008;181:4141–4149. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonti R., Fleury C. Fusobacterium necrophorum presenting as isolated lung nodules. Respir Med Case Rep. 2015;15:80–82. doi: 10.1016/j.rmcr.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams M.D., Kerber C.A., Tergin H.F. Unusual presentation of Lemierre's syndrome due to Fusobacterium nucleatum. J Clin Microbiol. 2003;41:3445–3448. doi: 10.1128/JCM.41.7.3445-3448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heo S.M., Sung R.S., Scannapieco F.A., Haase E.M. Genetic relationships between Candida albicans strains isolated from dental plaque, trachea, and bronchoalveolar lavage fluid from mechanically ventilated intensive care unit patients. J Oral Microbiol. 2011;3:6362. doi: 10.3402/jom.v3i0.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes-Filho I.S., de Oliveira T.F., da Cruz S.S., Passos-Soares Jde S., Trindade S.C., Oliveira M.T. Influence of periodontitis in the development of nosocomial pneumonia: a case control study. J Periodontol. 2014;85:e82–e90. doi: 10.1902/jop.2013.130369. [DOI] [PubMed] [Google Scholar]
- 32.Porto A.N., Borges A.H., Rocatto G., Matos F.Z., Borba A.M., Pedro F.L. Periodontal and microbiological profile of intensive care unit inpatients. J Contemp Dent Pract. 2016;17:807–814. doi: 10.5005/jp-journals-10024-1935. [DOI] [PubMed] [Google Scholar]
- 33.Centor R.M., Atkinson T.P., Ratliff A.E., Xiao L., Crabb D.M., Estrada C.A. The clinical presentation of Fusobacterium-positive and streptococcal-positive pharyngitis in a university health clinic: a cross-sectional study. Ann Intern Med. 2015;162:241–247. doi: 10.7326/M14-1305. [DOI] [PubMed] [Google Scholar]
- 34.Roulis E., Polkinghorne A., Timms P. Chlamydia pneumoniae: modern insights into an ancient pathogen. Trends Microbiol. 2013;21:120–128. doi: 10.1016/j.tim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Mantyla P., Stenman M., Paldanius M., Saikku P., Sorsa T., Meurman J.H. Chlamydia pneumoniae together with collagenase-2 (MMP-8) in periodontal lesions. Oral Dis. 2004;10:32–35. doi: 10.1046/j.1354-523x.2003.00980.x. [DOI] [PubMed] [Google Scholar]
- 36.Filardo S., Di Pietro M., Schiavoni G., Minniti G., Ortolani E., Romano S. Chlamydia pneumoniae clinical isolate from gingival crevicular fluid: a potential atherogenic strain. Front Cell Infect Microbiol. 2015;5:86. doi: 10.3389/fcimb.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi H., Oshio I., Osaki T., Kurata S., Yamamoto Y., Kamiya S. Development of diabetes in non-obese diabetic mice promotes Chlamydia pneumoniae dissemination from lung to peripheral blood. Int J Exp Pathol. 2006;87:121–129. doi: 10.1111/j.0959-9673.2006.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell L.A., Rosenfeld M.E. Infection and atherosclerosis development. Arch Med Res. 2015;46:339–350. doi: 10.1016/j.arcmed.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S.S., Ruiz V.E., Carroll J.D., Moss S.F. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305:228–238. doi: 10.1016/j.canlet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michaud D.S., Fu Z., Shi J., Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39:49–58. doi: 10.1093/epirev/mxx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao S., Li S., Ma Z., Liang S., Shan T., Zhang M. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer. 2016;11:3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Q.W., Zhou D.S., Peng H.J., Ji P., Liu D.S. Association of periodontal disease with oral cancer: a meta-analysis. Tumour Biol. 2014;35:7073–7077. doi: 10.1007/s13277-014-1951-8. [DOI] [PubMed] [Google Scholar]
- 43.Wen B.W., Tsai C.S., Lin C.L., Chang Y.J., Lee C.F., Hsu C.H. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 2014;107:283–290. doi: 10.1093/qjmed/hct248. [DOI] [PubMed] [Google Scholar]
- 44.Inaba H., Sugita H., Kuboniwa M., Iwai S., Hamada M., Noda T. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16:131–145. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binder Gallimidi A., Fischman S., Revach B., Bulvik R., Maliutina A., Rubinstein A.M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6:22613–22623. doi: 10.18632/oncotarget.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ha N.H., Woo B.H., Kim D.J., Ha E.S., Choi J.I., Kim S.J. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015;36:9947–9960. doi: 10.1007/s13277-015-3764-9. [DOI] [PubMed] [Google Scholar]
- 47.Fukugaiti M.H., Ignacio A., Fernandes M.R., Ribeiro Junior U., Nakano V., Avila-Campos M.J. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz J Microbiol. 2015;46:1135–1140. doi: 10.1590/S1517-838246420140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren R.L., Freeman D.J., Pleasance S., Watson P., Moore R.A., Cochrane K. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1:16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellarin M., Warren R.L., Freeman J.D., Dreolini L., Krzywinski M., Strauss J. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kostic A.D., Chun E., Robertson L., Glickman J.N., Gallini C.A., Michaud M. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu Y.N., Yu T.C., Zhao H.J., Sun T.T., Chen H.M., Chen H.Y. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget. 2015;6:32013–32026. doi: 10.18632/oncotarget.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanko P., Izakovicova Holla L. Bidirectional association between diabetes mellitus and inflammatory periodontal disease. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:35–38. doi: 10.5507/bp.2014.005. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura F., Iwamoto Y., Mineshiba J., Shimizu A., Soga Y., Murayama Y. Periodontal disease and diabetes mellitus: the role of tumor necrosis factor-alpha in a 2-way relationship. J Periodontol. 2003;74:97–102. doi: 10.1902/jop.2003.74.1.97. [DOI] [PubMed] [Google Scholar]
- 54.Yki-Jarvinen H., Sammalkorpi K., Koivisto V.A., Nikkila E.A. Severity, duration, and mechanisms of insulin resistance during acute infections. J Clin Endocrinol Metab. 1989;69:317–323. doi: 10.1210/jcem-69-2-317. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Real J.M., Lopez-Bermejo A., Vendrell J., Ferri M.J., Recasens M., Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 56.Sugiyama S., Takahashi S.S., Tokutomi F.A., Yoshida A., Kobayashi K., Yoshino F. Gingival vascular functions are altered in type 2 diabetes mellitus model and/or periodontitis model. J Clin Biochem Nutr. 2012;51:108–113. doi: 10.3164/jcbn.11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teeuw W.J., Gerdes V.E., Loos B.G. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Preshaw P.M., Alba A.L., Herrera D., Jepsen S., Konstantinidis A., Makrilakis K. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mealey B.L., Ocampo G.L. Diabetes mellitus and periodontal disease. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 60.Poplawska-Kita A., Siewko K., Szpak P., Krol B., Telejko B., Klimiuk P.A. Association between type 1 diabetes and periodontal health. Adv Med Sci. 2014;59:126–131. doi: 10.1016/j.advms.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Xavier A.C., Silva I.N., Costa Fde O., Correa D.S. Periodontal status in children and adolescents with type 1 diabetes mellitus. Arq Bras Endocrinol Metabol. 2009;53:348–354. doi: 10.1590/s0004-27302009000300009. [DOI] [PubMed] [Google Scholar]
- 62.Gaur S., Agnihotri R. Alzheimer's disease and chronic periodontitis: is there an association? Geriatr Gerontol Int. 2015;15:391–404. doi: 10.1111/ggi.12425. [DOI] [PubMed] [Google Scholar]
- 63.McGeer P.L., McGeer E.G. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 64.Kothari M., Spin-Neto R., Nielsen J.F. Comprehensive oral-health assessment of individuals with acquired brain-injury in neuro-rehabilitation setting. Brain Inj. 2016;30:1103–1108. doi: 10.3109/02699052.2016.1167244. [DOI] [PubMed] [Google Scholar]
- 65.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamer A.R., Craig R.G., Dasanayake A.P., Brys M., Glodzik-Sobanska L., de Leon M.J. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4:242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Cestari J.A., Fabri G.M., Kalil J., Nitrini R., Jacob-Filho W., de Siqueira J.T. Oral infections and cytokine levels in patients with Alzheimer's disease and mild cognitive impairment compared with controls. J Alzheimers Dis. 2016;52:1479–1485. doi: 10.3233/JAD-160212. [DOI] [PubMed] [Google Scholar]
- 68.Lehrer S. Nasal NSAIDs for Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2014;29:401–403. doi: 10.1177/1533317513518658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubio-Perez J.M., Morillas-Ruiz J.M. A review: inflammatory process in Alzheimer's disease, role of cytokines. Sci World J. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamer A.R., Craig R.G., Pirraglia E., Dasanayake A.P., Norman R.G., Boylan R.J. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poole S., Singhrao S.K., Kesavalu L., Curtis M.A., Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer's disease brain tissue. J Alzheimers Dis. 2013;36:665–677. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- 72.Ellen R.P., Galimanas V.B. Spirochetes at the forefront of periodontal infections. Periodontol 2000. 2005;38:13–32. doi: 10.1111/j.1600-0757.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 73.Roulis E., Bachmann N.L., Myers G.S., Huston W., Summersgill J., Hudson A. Comparative genomic analysis of human Chlamydia pneumoniae isolates from respiratory, brain and cardiac tissues. Genomics. 2015;106:373–383. doi: 10.1016/j.ygeno.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Hammond C.J., Hallock L.R., Howanski R.J., Appelt D.M., Little C.S., Balin B.J. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer's disease brain. BMC Neurosci. 2010;11:121. doi: 10.1186/1471-2202-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poole S., Singhrao S.K., Chukkapalli S., Rivera M., Velsko I., Kesavalu L. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE-/- mice brains. J Alzheimers Dis. 2015;43:67–80. doi: 10.3233/JAD-140315. [DOI] [PubMed] [Google Scholar]
- 76.Sparks Stein P., Steffen M.J., Smith C., Jicha G., Ebersole J.L., Abner E. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer's disease. Alzheimers Dement. 2012;8:196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Y.W., Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vamos C.A., Thompson E.L., Avendano M., Daley E.M., Quinonez R.B., Boggess K. Oral health promotion interventions during pregnancy: a systematic review. Community Dent Oral Epidemiol. 2015;43:385–396. doi: 10.1111/cdoe.12167. [DOI] [PubMed] [Google Scholar]
- 79.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebersole J.L., Stevens J., Steffen M.J., Dawson D., Iii, Novak M.J. Systemic endotoxin levels in chronic indolent periodontal infections. J Periodontal Res. 2010;45:1–7. doi: 10.1111/j.1600-0765.2008.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaur M., Geisinger M.L., Geurs N.C., Griffin R., Vassilopoulos P.J., Vermeulen L. Effect of intensive oral hygiene regimen during pregnancy on periodontal health, cytokine levels, and pregnancy outcomes: a pilot study. J Periodontol. 2014;85:1684–1692. doi: 10.1902/jop.2014.140248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin D., Moss K., Beck J.D., Hefti A., Offenbacher S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007;78:833–841. doi: 10.1902/jop.2007.060201. [DOI] [PubMed] [Google Scholar]
- 83.Han Y.W., Fardini Y., Chen C., Iacampo K.G., Peraino V.A., Shamonki J.M. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol. 2010;115:442–445. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han Y.W. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gauthier S., Tetu A., Himaya E., Morand M., Chandad F., Rallu F. The origin of Fusobacterium nucleatum involved in intra-amniotic infection and preterm birth. J Matern Fetal Neonatal Med. 2011;24:1329–1332. doi: 10.3109/14767058.2010.550977. [DOI] [PubMed] [Google Scholar]
- 86.Katz J., Chegini N., Shiverick K.T., Lamont R.J. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. 2009;88:575–578. doi: 10.1177/0022034509338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reyes L., Phillips P., Wolfe B., Golos T.G., Walkenhorst M., Progulske-Fox A. Porphyromonas gingivalis and adverse pregnancy outcome. J Oral Microbiol. 2018;10:1374153. doi: 10.1080/20002297.2017.1374153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kunnen A., van Pampus M.G., Aarnoudse J.G., van der Schans C.P., Abbas F., Faas M.M. The effect of Porphyromonas gingivalis lipopolysaccharide on pregnancy in the rat. Oral Dis. 2014;20:591–601. doi: 10.1111/odi.12177. [DOI] [PubMed] [Google Scholar]
- 89.Schenkein H.A., Bradley J.L., Purkall D.B. Anticardiolipin in Porphyromonas gingivalis antisera causes fetal loss in mice. J Dent Res. 2013;92:814–818. doi: 10.1177/0022034513497959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abrahams V.M., Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 91.Chaparro A., Blanlot C., Ramirez V., Sanz A., Quintero A., Inostroza C. Porphyromonas gingivalis, Treponema denticola and Toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case-control study. J Periodontal Res. 2013;48:802–809. doi: 10.1111/jre.12074. [DOI] [PubMed] [Google Scholar]
- 92.Rathnayake N., Akerman S., Klinge B., Lundegren N., Jansson H., Tryselius Y. Salivary biomarkers for detection of systemic diseases. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farah R., Haraty H., Salame Z., Fares Y., Ojcius D.M., Said Sadier N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed J. 2018;41:63–87. doi: 10.1016/j.bj.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yakob M., Fuentes L., Wang M.B., Abemayor E., Wong D.T. Salivary biomarkers for detection of oral squamous cell carcinoma - current state and recent advances. Curr Oral Health Rep. 2014;1:133–141. doi: 10.1007/s40496-014-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gau V., Wong D. Oral fluid nanosensor test (OFNASET) with advanced electrochemical-based molecular analysis platform. Ann N Y Acad Sci. 2007;1098:401–410. doi: 10.1196/annals.1384.005. [DOI] [PubMed] [Google Scholar]
- 96.Lee L.T., Wong Y.K., Hsiao H.Y., Wang Y.W., Chan M.Y., Chang K.W. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2018;47:699–707. doi: 10.1016/j.ijom.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 97.Cuevas-Cordoba B., Santiago-Garcia J. Saliva: a fluid of study for OMICS. OMICS. 2014;18:87–97. doi: 10.1089/omi.2013.0064. [DOI] [PubMed] [Google Scholar]
- 98.Galloway J.W., Keijser B.J., Williams D.M. Saliva in studies of epidemiology of human disease: the UK Biobank project. Periodontol 2000. 2016;70:184–195. doi: 10.1111/prd.12108. [DOI] [PubMed] [Google Scholar]
- 99.Taylor J.J., Preshaw P.M. Gingival crevicular fluid and saliva. Periodontol 2000. 2016;70:7–10. doi: 10.1111/prd.12118. [DOI] [PubMed] [Google Scholar]