Abstract
Introduction
Reducing cigarettes per day (CPD) aided by medication increases quit attempts (QA) among smokers not trying to quit. If this is due to reducing CPD per se, then a greater reduction should predict making a QA.
Aims and Methods
In this secondary analysis, 132 smokers completed nightly calls to report CPD, intention to quit tomorrow, and QAs over 12 weeks. We provided no treatment. We identified episodes of reduction and tested whether (1) percent reduction in CPD, (2) absolute reduction in CPD, (3) duration of reduction, or (4) CPD on the final day predicted a QA immediately after a reduction episode. We tested this separately among reduction episodes that began with and without an intention to quit.
Results
Among the 1179 episodes that began without intention to quit, all four measures of reduction predicted making a QA. Greater percent reduction, longer duration, and fewer CPD on the final day were retained in a multivariate model (all p < .05). Among the 85 episodes that began with intention to quit, greater percent reduction and greater absolute reduction predicted making a QA. Only mean percent reduction was retained in a multivariate model (p < .001).
Conclusions
Our results replicate and extend earlier studies by using fine-grained analyses and examining immediately proximal QAs in a sample of self-quitters. Findings suggest that reducing CPD per se increases the probability of a QA among smokers without intention to quit in a dose-related manner. Whether this is the case among smokers who intend to quit remains unclear.
Implications
Reducing CPD appears to be an effective strategy to increase the probability of making a QA for the majority of smokers who do not intend to quit in the near future. However, our findings are mixed regarding the effectiveness of reducing among smokers who intend to quit. Clinical interventions and policies that promote reducing CPD are likely to be an effective way to increase QAs. Reduction may be especially helpful for smokers who have not responded to traditional advice to stop abruptly.
Introduction
Increasing quit attempts (QA) appears to be one strategy to decrease the prevalence of smoking.1 Though retrospective studies found that a greater number of past QAs do not predict abstinence,2 our recent study using a true prospective design found that making multiple QAs predicted future abstinence.3 Furthermore, making an attempt to quit is a necessary prerequisite to cessation.
Reducing cigarettes per day (CPD) is common4 and, when aided by nicotine replacement therapy5 or other medications,6–8 is one way to increase QAs among smokers who do not intend to quit now. However, reduction may not increase quitting among those already motivated to quit now.9 Specifically, reducing CPD appears to delay quitting10 and is less effective than quitting abruptly11 when smokers already intend to quit. In contrast, reduction among smokers who do not intend to quit increases self-efficacy, intention to quit,12 and cessation.5 The effectiveness of medication-aided reduction in prior trials could be due to the reduction in CPD, precessation medication, or both. For example, precessation nicotine replacement therapy without instruction to reduce CPD increases quitting among smokers not trying to quit now in many, but not all studies.13–15 Also, whether reducing CPD without nicotine replacement therapy increases QAs is unclear. Two recent trials found a brief reduction intervention without medication increased QAs16 and abstinence16,17 more than no treatment. However, two others found that reduction did not increase QAs18 or abstinence18,19 more than a usual care intervention.
If reducing CPD per se is a mechanism responsible for increased quitting, then a dose–response relationship between the amount of reduction and quitting should occur. Our prior review of 11 studies found that a greater magnitude of reduction in CPD was associated with greater probability of cessation.20 Most of these studies tested whether percent or absolute magnitude of change in CPD over a period of 2 weeks to 6 years predicted quitting 6 weeks to 9 years later. Unfortunately, most of these studies examined abstinence only, and thus, it is unclear if reduction increased the likelihood of making a QA or success of a QA. More importantly, the studies could not describe proximal relationships, that is, if reduction increased the probability of making a QA soon thereafter. This would be a stronger case for causality than if reduction was associated with quitting at a more distant follow-up.
The current secondary analysis of one of our prior natural history studies adds to the existing literature in four ways. First, the study’s fine-grained data collection (ie, nightly reports of reduction and intentions to quit) allowed within-subject comparisons of changes in smoking as proximal predictors of quitting. Second, the study examined reduction separately among episodes that began with and without an intention to quit. Third, while prior studies only tested either percent or absolute reduction in CPD,20 the current study examined four different measures of reduction in CPD: percent magnitude, absolute magnitude, duration, and CPD on the final day of a reduction episode. Fourth, our analysis specifically focused on whether reduction per se increased QAs because this is the most likely way reduction increases later abstinence.
Methods
This is a secondary analysis of longitudinal data collected to study the natural history of smoking. A full description of the methodology and main findings are reported elsewhere.3 Briefly, we recruited adults who smoked at least 10 CPD and planned to quit at some point in the next 12 weeks. Participants answered questions about their smoking nightly for 12 weeks. We did not provide any treatment. The University of Vermont Committee on the Use of Human Participants approved the study, and we registered the study at www.clinicaltrials.gov (NCT00995644).
This secondary analysis tested whether, within a given participant, an episode with (1) greater absolute reduction in CPD, (2) greater percent reduction in CPD, (3) greater duration of reduction in CPD, or (4) fewer CPD on the final day of reduction prospectively predicted a greater probability of making a QA on the day after the reduction episode. We tested this separately among episodes that began with and without an intention to quit because prior studies suggest reduction interventions are effective among those who do not intend to quit5 but may not be effective among those who do intend to quit.9 Furthermore, reduction in CPD may occur for different reasons among smokers who do intend to quit versus who do not intend to quit now (ie, due to a failed QA vs. due to the cost of cigarettes). Importantly, our analysis tested whether, within a given participant, episodes of greater reduction in CPD predicted a greater probability of a QA than episodes with less reduction in CPD.
Recruitment
Major inclusion criteria were: 18 years of age or older, smoked at least 10 CPD for at least 1 year, intended to probably or definitely quit sometime in the next 3 months, and had a minimum of 7 days of regular smoking (see definition in Analysis). Of the 152 smokers recruited, we retained 132 for this secondary analysis.
Assessment
Participants completed questions about smoking via an interactive voice response system nightly for 12 weeks. The interactive voice response has many of the assets of computer-assisted telephone interviewing; for example, automatic skips, branching options, prohibition of illogical responses and outliers, standardized questioning, and direct data entry. The interactive voice response’s major assets are the increased confidentiality, the ability to prompt participants to call, and the ability of participants to determine when to call. Participants were asked the number of cigarettes they smoked and whether they planned to smoke tomorrow. We asked about plans to smoke tomorrow rather than plans to quit tomorrow because participants told us that asking about intentions to stop repeatedly (on 84 occasions) made them feel under pressure to try to quit. Days when participants planned to smoke were considered days with no intention to quit. Days when participants did not plan to smoke were considered days with intention to quit. If participants did not smoke any cigarettes on a given day, they were asked whether this was an attempt to stop smoking. To detect short QAs, at the end of each week, participants were also asked whether and when they made any QAs that lasted less than 1 day.
We did not use biochemical verification of abstinence because the Society for Research on Nicotine and Tobacco (SRNT) states that verification is usually not necessary when no treatment is provided and face-to-face contact is minimal.21
Analysis
To determine a mean CPD from which to calculate the amount of reduction, we calculated mean CPD on “regular smoking” days, that is, days in which participants reported no intentions to reduce or quit and were not within 1 week after a period of abstinence. We defined reduction episodes as a single day or consecutive days of at least 10% reduction in CPD. Most participants were not actively trying to quit or change their CPD, and thus, we decided to include small (ie, 10%) daily fluctuations in CPD that would have been missed with common cutoffs (ie, 50%) to increase the chances of detecting an effect. Sensitivity analyses where reduction episodes were defined as at least 50% were underpowered due to a small number of episodes of reduction at least 50%. However, analyses with episodes defined as at least 25% resulted in findings similar to the results when reduction was defined as at least 10%. We defined a QA as any day that the participant reported trying to quit, whether or not it lasted a full day.22
We used logistic regressions to examine four predictors of the likelihood of making a QA: mean absolute reduction in CPD during an episode, mean percent reduction in CPD during an episode, duration of a reduction episode, and CPD on the final day of an episode. We defined CPD on the final day of an episode as the CPD on the day prior to returning to at least 90% of the participant’s mean baseline CPD or to making a QA. We tested these predictors separately among reduction episodes that began on the day that participants had reported they intended to continue smoking (ie, no intention to quit) or stop smoking (ie, intention to quit). To make results comparable with prior studies,5,9–11,17,23,24 we examined episodes when participants intended to quit or not to quit separately. Our outcome was whether a QA of any length was initiated on the day following a reduction episode. We did not examine whether reduction increased the duration of abstinence because only 27 QAs lasted at least 24 hours (ie, 2% of episodes ended in a QA that lasted ≥24 hours).
The distributions of the reduction variables were nonlinear, skewed, and had influential outliers. Usual transformations did not solve these issues. For three of the variables, data were winsorized at the point where the plots plateaued. In the case of CPD on the final day of reduction, the values were categorized based on their frequencies. We checked for possible interactions among the reduction variables by starting with a model including all predictors and all two-way interactions and simplifying through backward selection. There were a smaller number of reduction episodes with intention to quit; thus, only one interaction was tested at a time. The software SAS v 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
Participants
Participants in this analysis were mostly middle aged, female, and Caucasian. At intake, participants reported smoking approximately 20 CPD and were moderately dependent (Table 1). Examples of transitions between reduction with and without intention to quit, QAs, and abstinence among a subset of light and heavy smokers are displayed in Figure 1.
Table 1.
Participant Characteristics
| Included participants | Average US daily smoker | |
|---|---|---|
| Sample size | 132 | — |
| Mean (SD) age | 45 (13) | 44a |
| % women | 70% | 45%a |
| % minorities | 21% | 29%a |
| Mean (SD) baseline CPD | 20 (10) | 16b |
| Mean (SD) FTCD | 5.4 (2.2) | 4.5c |
Figure 1.
Examples of transitions across reduction in cigarettes per day, intention to quit, quit attempts, and abstinence for eight light and eight heavy smoking participants. Participants were selected at random from subsets representing the distribution of reduction episodes in CPD and quit attempts. Rows represent individual participants. Columns represent days.  = days of 10%–24% reduction;
= days of 10%–24% reduction;  = days of 25%–49% reduction;
= days of 25%–49% reduction;  = days of at least 50% reduction;
= days of at least 50% reduction;  = reduction when participants intended to quit;
= reduction when participants intended to quit;  = quit attempts;
= quit attempts;  = days of abstinence. CPD = cigarettes per day.
= days of abstinence. CPD = cigarettes per day.
Reduction Episodes With No Intention to Quit
One hundred and thirty-one participants contributed 1179 reduction episodes that began without intention to quit. Participants contributed a median of nine reduction episodes, and 11% of episodes were followed by a QA. The median absolute reduction was 3.4 CPD (lower quartile = 2.3 CPD; upper quartile = 4.9 CPD), the median percent reduction in CPD was 21% (lower quartile = 15%; upper quartile = 30%), the median duration was 1 day (lower quartile = 1 day; upper quartile = 3 days), and the median CPD on the final day of reduction was 12 (lower quartile = 8 CPD; upper quartile = 16 CPD). Absolute reduction in CPD and percent reduction were highly positively correlated (r = 0.67, p < .001). All other reduction variables had small to medium correlations (see Supplementary Document A, Table 1).
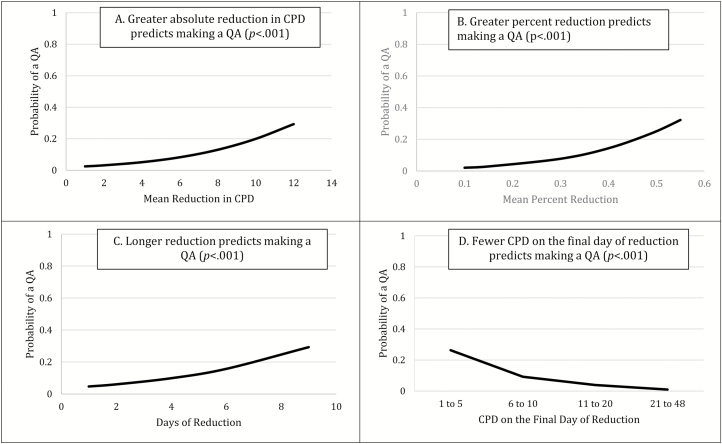
On days following participants’ report that they intended to continue smoking tomorrow (ie, no intention to quit), there was a greater probability of making a QA following reduction episodes than days of no reduction (7.9% vs. 2.9%; F = 47.2, p < .001). Greater absolute reduction in CPD, greater percent reduction, longer durations of reduction, and fewer CPD on the final day of an episode were univariate predictors of making a QA on the day following reduction episodes that began without intention to quit (Figure 2). When all four predictors were included in a multivariate model, percent reduction, duration of reduction, and CPD on the final day of an episode remained significant (Table 2). We tested all possible interactions among the four predictors, and none were significant. The probabilities displayed in Figures 1 and 2 are for a QA occurring on a single day (ie, the day following a reduction episode) and thus appear small. The effect sizes would probably appear larger if we examined the cumulative effect over multiple weeks or months.
Figure 2.
Reduction episodes without the intention to quit predict making a QA on the day after an episode in a dose-related manner. CPD = cigarettes per day; QA = quit attempt.
Table 2.
Reduction Episodes as Predictors of a QA Occurring on the Day After an Episode
| Episodes with no intention to quit | Episodes with intention to quit | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | aMultivariate | Univariate | bMultivariate | |||||
| F statistic | cOR (95% CI) | F statistic | cOR (95% CI) | F statistic | cOR (95% CI) | F statistic | cOR (95% CI) | |
| Absolute reduction | 27.5*** | 1.3 (1.2–1.4) | — | — | 4.2* | 1.3 (1.0–1.7) | — | — |
| Percent reduction | 64.4*** | 2.0 (1.7–2.4) | 20.1*** | 1.6 (1.3–2.0) | 15.1*** | 3.5 (1.8–6.8) | 15.1*** | 3.5 (1.8–6.8) |
| Duration of reduction | 36.2*** | 1.3 (1.2–1.4) | 20.4*** | 1.2 (1.1–1.3) | 3.6† | 1.3 (1.0–1.8) | — | — |
| CPD on final day | 14.2*** | 8.7 (4.3–18.9) | 2.7* | 6.67 (1.5–29.4) | 0.7 | 2.7 (0.6–12.3) | — | — |
OR = odds ratio; CI = confidence interval; CPD = cigarettes per day; QA = quit attempt.
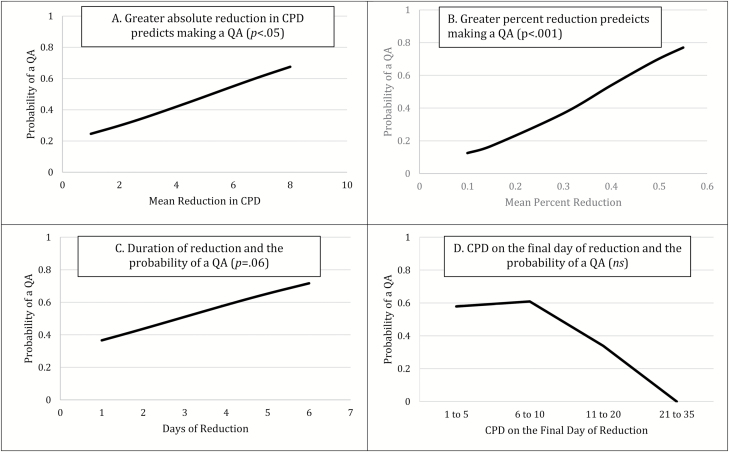
aUsing backward selection, mean percent reduction in CPD, duration of reduction episode, and CPD on the final day of an episode were retained in the final multivariate model.
bUsing backward selection, only mean percent reduction in CPD was retained in the final model.
cORs should be interpreted with caution because the unit is different for each reduction measure: absolute reduction unit = 1 CPD, percent reduction unit = 10% change in CPD; duration of reduction unit = 1 day of reduction, CPD on final day unit = 1–5 CPD versus 21–48 CPD.
† p = .06; *p < .05; **p < .01; ***p < .001.
Reduction Episodes With Intention to Quit
Forty-one participants contributed 85 reduction episodes that began with intention to quit. Participants contributed a median of two reduction episodes, and 47% of episodes were followed by a QA. The median absolute reduction was 4.5 CPD (lower quartile = 2.8 CPD; upper quartile = 6.6 CPD), the median percent reduction in CPD was 36% (lower quartile = 25%; upper quartile = 30%), the median duration was 2 days (lower quartile = 1 day; upper quartile = 3 days), and the median CPD on the final day of reduction was 7 (lower quartile = 5 CPD; upper quartile = 12 CPD). Mean absolute reduction in CPD and mean percent reduction were highly positively correlated (r = 0.63, p < .001). All other reduction variables had small to medium correlations (see Supplementary Document A, Table 2).
On days following participants’ report that they did not intend to smoke tomorrow (ie, intention to quit), there was a greater probability of making a QA following reduction episodes than days of no reduction (46.3% vs. 8.5%; F = 27.2, p < .001). Greater absolute reduction in CPD and greater percent reduction were univariate predictors of making a QA on the day following a reduction episode that began with intention to quit (Figure 3). When all four predictors were entered into a multivariate model, only percent reduction was retained and remained a significant predictor of making a QA (Table 2). We tested all possible interactions among the four predictors, and none were significant.
Figure 3.
Reduction episodes with the intention to quit as a predictor of making a QA on the day after an episode. CPD = cigarettes per day; ns = nonsignificant; QA = quit attempt.
Moderators of Reduction Episodes
Post hoc analyses tested interactions between intention to quit and absolute reduction in CPD, percent reduction, duration of reduction, and CPD on the final day of a reduction episode when all episodes were included in the same model. None were significant. We also examined the following variables as moderators of the effect of reduction episodes that began without intention to quit and with intention to quit: (1) whether or not participants used a smoking cessation medication (eg, nicotine replacement therapy) during the study, (2) whether participants smoked regular, light, or ultralight cigarettes, (3) participants’ opinion on whether adults should smoke cigarettes, (4) perceived community norms, (5) whether smoking is allowed in the participant’s home, (6) perceived importance of making a commitment to quit smoking,25 (7) confidence in their ability to quit smoking,25 (8) whether the reduction in CPD was intentional or unintentional, (9) whether they set a quit date in the next 3 months, (10) whether they had reduced CPD as a part of prior QAs, (11) whether they had reduced CPD not as a part of prior QAs, and (12) score on the Fagerström Test for Cigarette Dependence.26 There was only one statistically significant interaction: Among episodes without an intention to quit, greater mean percent reduction increased the probability of a QA more among episodes from participants who sometimes allowed smoking in the home than among reduction episodes from those who either always or never allowed smoking in the home (F = 4.8, p < .01; see Supplementary Document B, Figure 1). This finding was not readily interpretable and was not replicated in other measures of reduction nor among episodes when participants intended to quit. We conducted 96 moderator analyses, and thus, it is possible that this finding is the result of a Type I error.
Discussion
Findings from this fine-grained secondary analysis indicate that greater reduction in CPD predicts making a QA. Reduction was assessed separately among episodes that began the day after participants reported that they either intended to smoke tomorrow (ie, no intention to quit) or did not intend to smoke tomorrow (ie, intention to quit). This decision was made to be consistent with prior literature.5,9–11,17,23,24 In episodes without intention to quit, greater reduction predicted a greater probability of a QA in all four tests of reduction in a dose-related manner and three of these predictors remained significant in a multivariate model. In episodes with intention to quit, two of the four measures predicted making a QA in a dose-related manner (and there was a similar trend in the other two measures) and one measure remained significant in a multivariate model.
Our findings indicate that reducing CPD per se could be partly responsible for increased quitting in prior trials of medication-aided reduction. Though mechanisms were not measured, reducing CPD could be effective because it increases intention to quit by increasing smokers’ exposure to the beneficial effects of not smoking (eg, less cost or less stigma). Reduction could also increase self-efficacy to quit by providing opportunities for smokers to experience their own control over smoking cigarettes. Reducing CPD could decrease dependence by reducing the smoker’s nicotine level and by disrupting conditioned nicotine effects via increasing instances of not smoking in the presence of cues to smoke. Finally, the fact that use of smoking cessation medication did not moderate reduction’s influence on QAs suggests that reduction per se could be an effective component of prior medication-aided reduction interventions.
Smokers in this study self-selected whether, when, and how much to reduce CPD. Reduction in CPD without intention to quit could have occurred to decrease the harm from continued smoking, to prepare to quit later, or in response to increased taxation, tobacco control policies, or social norms (ie, stigma). Reduction with intention to quit could have occurred because participants changed their mind about quitting. Also, reduction with intent to quit could have been due to failed QAs that were not reported as such on study questionnaires.
Assets and Limitations
One asset is that this study used prospective and fine-grained measurements of smoking to make within-subject comparisons of reduction in CPD as a very proximal predictor of making a QA. Thus, our analysis was asking whether episodes of greater reduction in CPD within the same participant predict a greater probability of a QA than episodes of less reduction. Most prior reduction studies made between-subject comparisons and measured the effects of reduction at a distant follow-up.20 These studies could not determine the duration or proximity of reduction in CPD to the follow-up assessment. More importantly, our finding that greater reduction increases the probability of a QA immediately following a reduction episode makes a stronger case for causality than showing reduction is associated with quitting at a follow-up several months later. Prior studies also did not discriminate between reduction’s effects on increasing the likelihood versus success of a QA. This study focused on the former as we thought this a more likely effect of reduction. Finally, the daily assessment in our study is important because longer-term retrospective recall of smoking is poor.27–30
One limitation of our study is that completing nightly surveys may have artificially increased the reduction in CPD or QAs via reactivity.31 The fact that smokers self-selected if, when, and how much to reduce CPD is another limitation discussed earlier. Our study did not collect data on exactly when or how much cessation medication was used. We did not assess when QAs occurred within a given day and thus could not determine whether QAs occurred before a participant’s first cigarette or later in the day. We assessed intention to quit by asking participants whether they planned to smoke tomorrow to minimize experimenter demand that may have occurred by repeatedly asking about plans to quit. However, plans to not smoke may differ from plans to quit. Thus, our findings may not be comparable with studies that assess intention to quit with different wording. We used percentage to define episodes of reduction in CPD because this is commonly used in the literature and incorporates baseline CPD. However, our decision to use percentage to define reduction episodes may have contributed to the fact that percent reduction had a larger effect on QAs than other measures (Table 2). Our lack of significant findings among moderator analyses and reduction episodes when participants did not intend to smoke (ie, intention to quit) may have been due to the relatively small sample size and insufficient power to detect an effect. Importantly, all QAs were self-reported, and thus, the distinction between QAs lasting less than 1 day versus a day of reduced smoking was subject to participants’ interpretation. However, a prior study using this dataset found that reporting a QA (including those lasting <1 day) predicted making more and longer QAs.3 We included four predictors in our main analyses, which increase the probability of false-positive findings. Finally, due to the small number of longer QAs, the study could not report on the effect of reduction on prolonged abstinence. While evidence is mixed regarding the extent to which increasing the number of QAs predicts cessation,2 a prior analysis of the dataset used for this article suggested that duration of abstinence increased with a greater number of QAs.3
Conclusion
This secondary analysis tested four measures of reduction in CPD and found that greater reduction in CPD when smokers do not intend to quit increases the probability of making a QA. Findings were mixed regarding smokers who intend to quit. A randomized controlled trial of reduction to prompt QAs is necessary to determine the extent to which our findings are due to reducing CPD per se or participants’ choice to reduce. Nonetheless, this study and others12,16,17,20 suggest that clinical interventions and policies that promote reducing CPD are likely to be an effective way to increase QAs among the majority of smokers who do not intend to quit in the near future. Reduction may be especially helpful for smokers who have not responded to traditional advice to stop abruptly.
Funding
This study was supported by research grant DA-025089 and training grant T32 DA 07242 from the National Institute on Drug Abuse.
Declaration of Interests
EMK and SN have nothing to disclose. JRH has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several nonprofit organizations that promote tobacco control. He also consults for Swedish Match on their snus product and Phillip Morris on their harm reduction products.
Supplementary Material
References
- 1. Abrams DB, Graham AL, Levy DT, Mabry PL, Orleans CT. Boosting population quits through evidence-based cessation treatment and policy. Am J Prev Med. 2010;38(3 suppl):S351–S363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Partos TR, Borland R, Yong HH, Hyland A, Cummings KM. The quitting rollercoaster: how recent quitting history affects future cessation outcomes (data from the International Tobacco Control 4-country cohort study). Nicotine Tob Res. 2013;15(9):1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, Callas PW. Natural history of attempts to stop smoking. Nicotine Tob Res. 2014;16(9):1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. PROPEL Centre for Population Health Impact. Tobacco use in Canada: patterns and trends, 2015 edition 2015. www.tobaccoreport.ca. Accessed September 15, 2016.
- 5. Wu L, Sun S, He Y, Zeng J. Effect of smoking reduction therapy on smoking cessation for smokers without an intention to quit: an updated systematic review and meta-analysis of randomized controlled. Int J Environ Res Public Health. 2015;12(9):10235–10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171(8):770–777. [DOI] [PubMed] [Google Scholar]
- 7. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687–694. doi:10.1001/jama.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hawk LW Jr, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, Mahoney MC. Does extended pre quit bupropion aid in extinguishing smoking behavior?Nicotine Tob Res. 2015;17(11):1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033. doi:10.1002/14651858.CD008033.pub3 [DOI] [PubMed] [Google Scholar]
- 10. Hughes JR, Solomon LJ, Livingston AE, Callas PW, Peters EN. A randomized, controlled trial of NRT-aided gradual vs. abrupt cessation in smokers actively trying to quit. Drug Alcohol Depend. 2010;111(1–2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindson-Hawley N, Banting M, West R, Michie S, Shinkins B, Aveyard P. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164(9):585–592. [DOI] [PubMed] [Google Scholar]
- 12. Klemperer EM, Hughes JR, Callas PW, Solomon LJ. A mediation analysis of motivational, reduction, and usual care interventions for smokers who are not ready to quit. Nicotine Tob Res. 2017;19(8):916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carpenter MJ, Jardin BF, Burris JL, et al. Clinical strategies to enhance the efficacy of nicotine replacement therapy for smoking cessation: a review of the literature. Drugs. 2013;73(5):407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Arch Intern Med. 2009;169(11):1028–1034. [DOI] [PubMed] [Google Scholar]
- 15. Bullen C, Howe C, Lin RB, et al. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction. 2010;105(8):1474–1483. [DOI] [PubMed] [Google Scholar]
- 16. Meyer C, Ulbricht S, Haug S, et al. Motivating smokers to quit using computer-generated letters that target either reduction or cessation: a population-based randomized controlled trial among smokers who do not intend to quit. Drug Alcohol Depend. 2016;166:177–186. [DOI] [PubMed] [Google Scholar]
- 17. Wu L, He Y, Jiang B, et al. Very brief physician advice and supplemental proactive telephone calls to promote smoking reduction and cessation in Chinese male smokers with no intention to quit: a randomized trial. Addiction. 2017; 112(11):2032–2040. doi:10.1111/add.13908. [DOI] [PubMed] [Google Scholar]
- 18. Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction, and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction. 2017; 112(1): 146–155 doi:10.1111/add.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glasgow RE, Gaglio B, Estabrooks PA, et al. Long-term results of a smoking reduction program. Med Care. 2009;47(1):115–120. [DOI] [PubMed] [Google Scholar]
- 20. Klemperer EM, Hughes JR. Does the magnitude of reduction in cigarettes per day predict smoking cessation? A qualitative review. Nicotine Tob Res. 2016;18(1):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 22. Carpenter MJ, Hughes JR. Defining quit attempts: what difference does a day make?Addiction. 2005;100(2):257–258. [DOI] [PubMed] [Google Scholar]
- 23. Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371–381. [DOI] [PubMed] [Google Scholar]
- 24. Klemperer EM, Hughes JR. Commentary on Wu et al. (2017): do very brief reduction interventions increase quitting among smokers not ready to quit?Addiction. 2017;112(11):2041–2042. [DOI] [PubMed] [Google Scholar]
- 25. Balmford J, Borland R. What does it mean to want to quit?Drug Alcohol Rev. 2008;27(1):21–27. [DOI] [PubMed] [Google Scholar]
- 26. Fagerstrom KO. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. doi:10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 27. Berg CJ, An LC, Kirch M, et al. Failure to report attempts to quit smoking. Addict Behav. 2010;35(10):900–904. [DOI] [PubMed] [Google Scholar]
- 28. Borland R, Partos TR, Yong HH, Cummings KM, Hyland A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four country cohort survey. Addiction. 2012;107(3):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borland R, Partos TR, Cummings KM. Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res. 2012;14(12):1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gilpin E, Pierce JP. Measuring smoking cessation: problems with recall in the 1990 California Tobacco Survey. Cancer Epidemiol Biomarkers Prev. 1994;3(7):613–617. [PubMed] [Google Scholar]
- 31. McCarthy DE, Minami H, Yeh VM, Bold KW. An experimental investigation of reactivity to ecological momentary assessment frequency among adults trying to quit smoking. Addiction. 2015;110(10):1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cha S, Erar B, Niaura RS, Graham AL. Baseline characteristics and generalizability of participants in an internet smoking cessation randomized trial. Ann Behav Med. 2016;50(5):751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hughes JR, Callas PW. Data to assess the generalizability of samples from studies of adult smokers. Nicotine Tob Res. 2010;12(1):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fagerström K, Furberg H. A comparison of the Fagerström Test for Nicotine Dependence and smoking prevalence across countries. Addiction. 2008;103(5):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.