Abstract
Background and Purpose
Sleep deprivation compromises learning and memory in both humans and animals, and can be reversed by administration of modafinil, a drug promoting wakefulness. Dysfunctional autophagy increases activation of apoptotic cascades, ultimately leading to increased neuronal death, which can be alleviated by autophagy inhibitors. This study aimed to investigate the alleviative effect and mechanism of modafinil on the excessive autophagy occurring in the hippocampus of mice with deficiency of learning and memory induced by sleep deprivation.
Experimental Approach
The Morris water maze was used to assess the effects of modafinil on male C57BL/6Slac mice after 48‐hr sleep deprivation. The HT‐22 hippocampal neuronal cell line was also used. Nissl staining, transmission electron microscope, immunofluorescence, Western blot, transient transfection, and autophagy inducer were used to study the effect and mechanism of modafinil on hippocampal neurons with excessive autophagy and apoptosis.
Key Results
Modafinil improved learning and memory in sleep‐deprived mice, associated with the inhibition of excessive autophage and apoptosis and an enhanced activation of the PI3K/Akt/mTOR/P70S6K signalling pathway in hippocampal neurons. These effects of modafinil were abolished by rapamycin. In addition, modafinil suppressed the aberrant autophagy and apoptosis induced by rapamycin and reactivated PI3K/Akt/mTOR/P70S6K signals in HT‐22 cells.
Conclusions and Implications
These results suggested that modafinil alleviated impaired learning and memory of sleep‐deprived mice potentially by suppressing excessive autophagy and apoptosis of hippocampal neurons. This novel mechanism may add to our knowledge of modafinil in the clinical treatment of impaired memory caused by sleep loss.
Abbreviations
- DG
dentate gyrus
- 3‐MA
3‐methyladenine
- mTOR
mammalian target of rapamycin
- MWM
Morris water maze
- p62
ubiquitin‐binding protein p62 or sequestosome‐1
- P70S6K
ribosomal protein S6 kinase
- p‐Akt
phosphorylated PKB
- p‐P70S6K
phosphorylated ribosomal protein S6 kinase
- p‐PI3K
phosphorylated PI3K
What is already known
Modafinil can reverse impairment of learning and memory caused by sleep deprivation
What this study adds
Modafinil suppresses excessive autophagy and apoptosis of hippocampal neurons induced by sleep deprivation.
What is the clinical significance
These results provide a novel mechanism for the clinical use of modafinil.
1. INTRODUCTION
Sleep is a restorative process that facilitates learning and memory consolidation (Doghramji, Lieberman, & Gordon, 2007). The hippocampus is one of the brain regions involved in higher nervous activities, including emotional integration, cognition, and memory. Disruption of sleep for a long period may have cumulative effects resulting in decreased hippocampal cell proliferation, cell survival, and neurogenesis, which may result in the deterioration of neurobehaviours such as mood, cognition, and memory (Kim, Mahmoud, & Grover, 2005; McCoy & Strecker, 2011; Meerlo, Mistlberger, Jacobs, Heller, & McGinty, 2009). Sleep loss or sleep deprivation may also compromise hippocampal function, probably through modification of synaptic plasticity at electrophysiological and molecular levels as well as at a structural level (Acosta‐Pena et al., 2015; Cirelli, 2013). Therefore, drugs improving learning and memory may benefit hippocampal impairment induced by sleep loss.
Autophagy is an intracellular degradation event in which a portion of cytoplasm is sequestered into autophagosomes, which degrade proteins and cellular structures upon fusing with lysosomes (Klionsky, 2005; Mizushima, 2007). It is a conserved catabolic process that plays a housekeeping role in eliminating protein aggregates and abnormal organelles. Basal autophagy is essential in the mammalian nervous system for the maintenance of normal function and homeostasis against neurodegeneration (Menzies et al., 2017). Dysfunctional autophagy disrupts neuronal intracellular homeostasis and predisposes individuals to neurodegenerative or neuropsychiatric disorders (Polajnar & Zerovnik, 2014). Learning and memory deficits have been closely associated with aberrant autophagy and modulation, and augmentation of autophagy may benefit impaired memory in rodents (Hylin et al., 2018; Wang, Du, et al., 2018; Wang, Ji, Liu, Li, & Zhang, 2018). Although sleep deprivation is thought to contribute to memory deficits, the relationships between sleep deprivation, hippocampal autophagy, and memory impairment remains elusive.
Autophagy is closely associated with apoptosis in neuronal cells. A number of reports have indicated that abnormal autophagy promotes the activation of apoptotic cascades, ultimately leading to neuronal death, which can be alleviated by autophagy inhibitors, such as 3‐methyladenine (3‐MA) and dl‐3‐n‐butylphthalide (Sun et al., 2018; Yu et al., 2018; Zhang, Wang, Li, Huang, & Sun, 2014). However, contradictory results also demonstrated that autophagy is likely to play a protective role in neuronal injury, and activation of autophagy inhibits neuronal apoptosis in neurodegenerative disorders (Liu et al., 2018; Qiao et al., 2018; Wu et al., 2018; Xiao et al., 2017). Therefore, autophagy in neurons appears to be delicately modulated and whether it is beneficial or harmful in neurological diseases depends on the balance between the amount of substrate and the capacity of the autophagy machinery (Yu et al., 2017).
Modafinil (2‐diphenyl‐methyl‐sulphinil‐2‐acetamide) is a wakefulness‐promoting drug approved for the treatment of narcolepsy, which improves memory, cognition, and attention in humans and animals (Sahakian & Morein‐Zamir, 2015). It can improve acquisition and reverse the memory impairment induced by sleep loss or sleep deprivation (Grady, Aeschbach, Wright, & Czeisler, 2010; Moreira et al., 2010; Pierard et al., 2007; Pierard et al., 2011; Ray et al., 2012; Shaffery, Sinton, Bissette, Roffwarg, & Marks, 2002). This effect may involve the induction of hippocampal neuronal proliferation and differentiation (Sahu et al., 2013). Additionally, modafinil may exert neuroprotective effect through directly interfering in the processes of energy metabolism, synthesis and release of neurotrophic factors, maintenance of calcium homeostasis, inhibition of the dopamine transporter and anti‐apoptosis (Raineri et al., 2012). However, so far, little is known about modafinil as a modulator of neuronal autophagy and apoptosis after sleep deprivation. In the present study, we found modafinil to attenuate excessive neuronal autophagy and apoptosis in the hippocampus of sleep‐deprived mice and in HT‐22 cells treated with rapamycin. These novel findings suggested that modafinil improved learning and memory deficits of sleep—deprived mice at least in part through counteracting excessive neuronal autophagy and apoptosis.
2. METHODS
2.1. Animals and treatment
All animal care and experimental procedures complied with the regulations of experimental animal administration issued by the State Committee of Science and Technology of People's Republic of China on November 14, 1988, and were approved by the Animal Care and Use Committee of SHUTC with an approval number (SZY20170626). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Male C57BL/6Slac mice (JAX number: 255709) aged 5 weeks were obtained from Animal Research Center of Shanghai University of Traditional Chinese Medicine (SHUTCM, Shanghai, China). All mice were housed in a controlled environment (21°C, 12‐hr light/dark cycle with light period from 7 a.m. to 7 p.m.) with free access to food and water. Animals were acclimatized for 1 week before the experiments.
To examine the effect of modafinil on learning and memory of sleep—deprived mice, 50 mice were randomly divided into five groups: control group, sleep—deprived group, modafinil (6.5 mg·kg−1) group, modafinil (13 mg·kg−1) group and modafinil (26 mg·kg−1) group (n = 10 per group). The mice in control and sleep‐‐deprived groups were given distilled water intragastrically (i.g.), whereas those in modafinil groups were treated i.g. with different doses of modafinil (6.5, 13, and 26 mg·kg−1) consecutively for 9 days.
To confirm modafinil's effect through inhibiting autophagy, 70 mice were randomly divided into seven groups: control, control + rapamycin, sleep‐‐deprived group, sleep‐‐deprived + rapamycin, modafinil (6.5 mg·kg−1) + rapamycin group, modafinil (13 mg·kg−1) + rapamycin group, and modafinil (26 mg·kg−1) + rapamycin (n = 10 per group). The mice in control and sleep‐‐deprived groups were given distilled water i.g., whereas those in the modafinil groups were given different doses of modafinil (6.5, 13, and 26 mg·kg−1, i.g.) consecutively for 9 days. The mice in rapamycin‐treated groups were given rapamycin (i.p., 2 mg·kg−1) consecutively for 9 days.
2.1. Morris water maze test and sleep deprivation procedure
The effect of modafinil on the learning and memory of sleep—deprived mice was evaluated by the Morris water maze (MWM) test as described previously (Liu et al., 2017). The time‐course of the experiment is illustrated in Figure 1. The MWM was filled with opaque water mixed with titanium dioxide (CAS number: 1317‐80‐2) at 22 ± 1°C.
Figure 1.
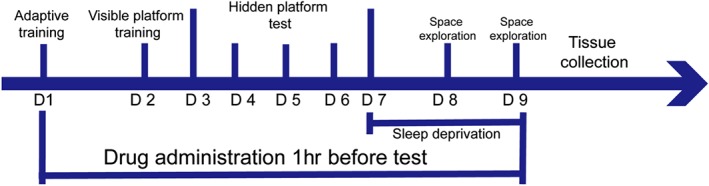
Timeline for the behavioural test
After the last hidden platform test, all the mice were given 48‐hr‐long sleep deprivation except the control group. Sleep deprivation was conducted using the modified multiple platform method (Suchecki & Tufik, 2000). In brief, groups of six animals were placed in polypropylene cages (41 × 34 × 16 cm). Each cage contained 12 platforms (3 cm diameter and 5 cm height) submerged into water up to 1 cm below the surface of the platforms. The animals could move freely, jumping from one platform to another one. Food and water were available during the entire procedure. To assess spatial memory, a space exploration test was performed 24 and 48 hr after the last training trial. In the tests, the platform was removed from the tank, and the mice were placed in a novel starting position of the maze, facing the tank wall, and allowed to swim freely for 60 s. The frequencies of an individual mouse passing the platform area and the time spent in the target quadrant were recorded as a measurement of spatial memory. Tracking of animal movement was achieved with a computerized tracking system (Mobiledatum Inc., Shanghai, China). After the last space exploration test, all the mice were killed (by injection of excessive 2% pelltobarbitalum natricum) for tissue collection.
2.2. Transmission electron microscopy
Immediately after killing, mice in each group were transcardially perfused with PBS followed by fixation with 2% glutaraldehyde (CAS number: 111‐30‐8). The brains were dissected, and the hippocampus (CA3 area) was diced (0.5 × 0.5 × 1.0 mm) and cut into ultrathin (50 nm) sections. After dehydration, ultrathin sections were stained with uranyl acetate (CAS number: 541‐09‐3) and lead citrate (CAS number: 126‐44‐3) for observation under an electron microscope. Images were taken using an H‐7650 electron microscope (Hitachi, Tokyo, Japan) in a blinded manner.
2.3. Immunohistochemistry
The antibody‐based procedures used comply with the recommendations made by the British Journal of Pharmacology. After anaesthesia with an overdose of 1% pentobarbital sodium (CAS number: 57‐33‐0), the mice were perfused intracardially with PBS followed by 4% paraformaldehyde (CAS number: 30525‐89‐4). Brains of the mice were dissected and fixed in 4% paraformaldehyde at 4°C overnight. Then they were dehydrated sequentially with 15% and 30% sucrose (CAS number: 57‐50‐1) solution (wt/vol) prepared in PBS, embedded with optimum cutting temperature (Sakura Finetek, USA, 3801480), and cut into 20‐μm‐thick sections. The sections were permeabilized and blocked with 5% donkey serum in PBS containing 0.3% Triton X‐100 (CAS number: 92046‐34‐9) for half an hour. Subsequently, they were incubated with primary antibodies against LC3B at 4°C overnight followed by secondary antibodies conjugated with Alexa fluorophore. Fluorescent images were taken with Olympus VS120 Virtual Slide Scanner.
2.4. Nissl staining
The brain sections (20μm) were hydrated in 95%, 85%, and 70% gradient of ethanol (CAS number: 64‐17‐5) for 5 min each. After washing with tap water three times for 5 min each, they were stained with 0.1% cresyl violet (CAS number: 10510‐54‐0) for 10 min. Then they were washed with distilled water, dehydrated in gradient alcohol, cleared with xylene (CAS number: 1330‐20‐7), and sealed with neutral gum (Song et al., 2016). The images were taken with Olympus VS120 Virtual Slide Scanner. Compared with the normal cells with regular shape and even staining, the injured neuronal cells in CA3 region were shrunken and sickle shaped and stained darkly with fragmented or no nucleus. The percentage of the injured cells within CA3 was calculated to evaluate the effect of sleep deprivation on the structure of hippocampal neurons. For each mouse, one of the representative coronal brain sections with the similar coordinate (bregma, −2.0 mm) was selected for statistical evaluation.
2.5. Cell culture and treatment
The mouse hippocampal cell line HT‐22 was purchased from Guangzhou Jennio Biotech Company (Guangzhou, China, RRID:CVCL_0321) and maintained in high‐glucose DMEM supplemented with 10% FBS. 3‐MA (CAS number: 5142‐23‐4) was packaged in stock solution as10 mmol·L−1 in 1 ml DMSO (CAS number: 67‐68‐5) upon arrival. Stock solutions of rapamycin (50 mg·ml−1) were prepared in DMSO. Stock solutions of modafinil were prepared in DMSO with a concentration of 100 mmol·L−1. The above‐mentioned reagents were diluted in cell culture medium before addition to cells. For drug treatment, the cells were subcultured at a density of 1.5 × 106 cells·ml−1 in 60‐mm cell culture dishes and allowed to grow to semi‐confluence. To induce autophagy, they were pretreated with rapamycin (50 μg·ml−1; Mei et al., 2014) for 4 hr. Then they were treated with 3‐MA (2.5 mmol·L−1) and modafinil (12.5, 25, and 50 μmol·L−1) in parallel for 48 hr. The cells were lysed in lysis reagent supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail for further analysis.
2.6. Plasmid transfection
The GFP‐LC3 and tf‐LC3 plasmids were kind gifts of Dr Cheng Huang (Drug Discovery Lab, School of Pharmacy, Shanghai University of Traditional Chinese Medicine) and were transfected into HT‐22 cells separately with Lipofectamine 2000 according to the manufacturer's protocol. In brief, HT‐22 cells (1 × 105 cells per well) cultured in 24‐well plates were transiently transfected with GFP‐LC3 plasmid or tf‐LC3 plasmid (0.5 μg per well) in opti‐medium for 6 hr. Afterwards, the cells were induced with rapamycin (50 μg·ml−1) for 4 hr and then treated with 3‐MA (2.5 mmol·L−1) or modafinil (12.5, 25, and 50 μmol·L−1) for 48 hr. Fluorescent images of the cells were visualized with an inverted fluorescence microscope.
2.7. Acridine orange staining
HT‐22 cells were cultured in 24‐well plates and induced with rapamycin (50 μg·ml−1) for 4 hr. Then the cells were treated with various concentrations of modafinil (12.5, 25, and 50 μmol·L−1) and 3‐MA (2.5 mmol·L−1) for 48 hr. After treatment, the media were discarded, and the cells were washed twice in PBS and stained with acridine orange (CAS number: 10127‐02‐3, 10 μg·ml−1 in PBS) for 15 min at 37°C in the dark. The fluorescent images were used to assess autophagy in HT‐22 cells.
2.8. Annexin V/propidium iodide staining
After induction with rapamycin (50 μg·ml−1) for 4 hr, HT‐22 cells were exposed to various concentrations of modafinil (12.5, 25, and 50 μmol·L−1) and 3‐MA (2.5 mmol·L−1) for 48 hr. Then cells were harvested by trypsinization, washed twice with PBS, incubated with propidium iodide and annexin V for 10 min, and subjected to flow cytometry analysis (Guava easyCyte HT, Millipore Company, Germany).
2.9. Immunocytochemistry
HT‐22 cells cultured on coverslips were induced with rapamycin (50 μg·ml−1) for 4 hr followed by treatment of various concentrations of modafinil (12.5, 25, and 50 μmol·L−1) and 3‐MA (2.5 mmol·L−1) for 48 hr. After washing once in PBS, cells were fixed with 3% paraformaldehyde in PBS, permeabilized with 0.3% Triton‐X‐100, and incubated with primary antibody against p62 (Cat. No. ab109012) overnight at 4°C. Consequently, the cells were washed with PBS and incubated with secondary antibody conjugated with Alexa‐488 fluorophore for 1 hr at room temperature. After washing with PBS twice, the coverslips were mounted on glass slides with mounting medium containing DAPI. Immunofluorescence images were acquired by an inverted fluorescence microscope.
2.10. Western blotting analysis
Tissues collected from mice were homogenized in CellLytic™ MT mammalian tissue lysis reagent supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail. HT‐22 cells were also added with the same lysis reagent mentioned above. The lysate was centrifuged at 16,099× g for 15 min at 4°C. Each sample (30 μg protein) was separated by SDS‐PAGE (10% or 12%) and transferred onto PVDF membranes by wet transfer. The blotted membranes were blocked with 5% non‐fat milk solution at room temperature for 1 hr and incubated with respective primary antibodies overnight at 4°C. After being washed with 1× PBS containing 0.1% Tween 20, the membranes were incubated with respective secondary antibodies. The protein bands were visualized by ECL Prime Kit and quantified with ImageJ 1.46r software (NIH, USA, RRID:SCR_003070).
2.11. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. All data are presented as mean ± SEM. Differences among groups were analysed by one‐way ANOVA with Dunnett's post hoc test or two‐way ANOVA with Bonferroni post tests using GraphPad Prism 5.0 (RRID:SCR_002798). If the variance was homogeneous, the data were further analysed by one‐way or two‐way ANOVA. The value of P < 0.05 was considered statistically significance.
2.12. Materials
Modafinil (CAS: 112111‐47‐4) was provided by Dr Rifang Yang at the Academy of Military Medical Sciences (Beijing, China). The purity of modafinil was determined to be more than 99% by HPLC analysis. Antibodies against Beclin‐1 (Cell Signaling Technology Cat# 3495, RRID:AB_1903911), LC3B (Cell Signaling Technology Cat# 3868, RRID:AB_2137707), phospho‐PI3K p85 (Cell Signaling Technology Cat# 4228, RRID:AB_659940), phospho‐Akt (Cell Signaling Technology Cat# 9271, RRID:AB_329825), PI3K p85 (Cell Signaling Technology Cat# 4257, RRID:AB_659889), Akt (Cell Signaling Technology Cat# 9272, RRID:AB_329827), Bcl‐2 (Cell Signaling Technology Cat# 2870, RRID:AB_2290370), Bax (Cell Signaling Technology Cat# 2772, RRID:AB_10695870), cleaved caspase‐3 (Cell Signaling Technology Cat# 9664, RRID:AB_2070042), phospho‐P70S6 kinase (Cell Signaling Technology Cat# 9234, RRID:AB_2269803), and P70S6 kinase (Cell Signaling Technology Cat# 2708, RRID:AB_390722) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against p62 (ab109012), p‐mTOR (Abcam Cat# ab84400, RRID:AB_10973187), mTOR (Abcam Cat# ab87540, RRID:AB_10710016), and β‐actin (Abcam Cat# ab8227, RRID:AB_2305186) were obtained from Abcam (Cambridge, MA, USA). Rapamycin and 3‐MA were purchased from Selleckchem (Boston, MA, USA). All the other reagents were of analytical grade unless mentioned otherwise.
2.13 Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017)
3. RESULTS
3.1. Modafinil attenuated the cognitive impairments of sleep—deprived mice
All mice were trained in the MWM for spatial learning assessment to an equivalent level of performance with probe trial and memory test before sleep deprivation. As shown in Figure 2a, in the hidden platform test, modafinil treatment gradually improved the memory of mice. Accordingly, mice in the higher dose modafinil groups (13 and 26 mg·kg−1) displayed shorter escape latency compared with the control group in the fourth day and the fifth day of hidden platform test (Figure 2b,c,). Sleep deprivation impaired the spatial memory of mice strikingly. As shown in Figure 2d–g, mice in the sleep—deprived group after 24 or 48 hr sleep deprivation crossed the hidden platform less times and stayed in target quadrant for a shorter time in contrast with that in the control group. However, modafinil, when administered at higher doses (13 and 26 mg·kg−1), alleviated the impaired memory of sleep—deprived mice significantly. As shown in Figure 2d–g, mice in modafinil (13 and 26 mg·kg−1) groups passed through the hidden platform more times and stayed in the target quadrant for a longer time. The representative swimming tracks of mice in each group are illustrated in Figure 2h to show the difference among the groups.
Figure 2.
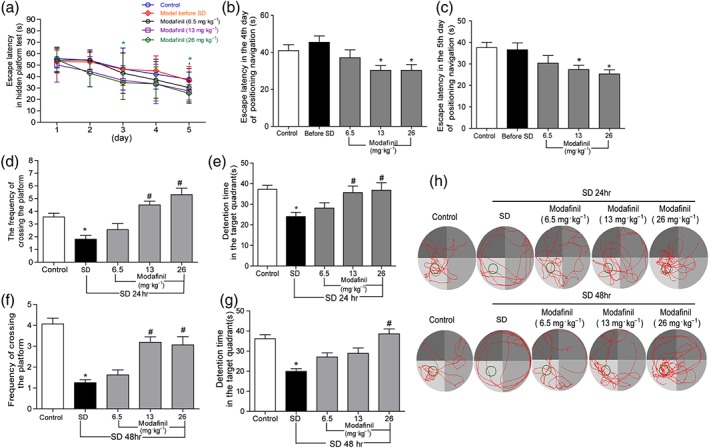
The effect of modafinil on learning and memory of sleep deprived (SD) mice tested in the Morris water maze. (a) Escape latency of mice in hidden platform test for five consecutive days. (b) Escape latency of mice in the fourth day of hidden platform test. (c) Escape latency of mice in the fifth day of hidden platform test. (d) Frequency of crossing the platform of mice after 24‐hr sleep deprivation. (e) Time spent in target quadrant after 24‐hr sleep deprivation. (f) Frequency of crossing the platform of mice after 48‐hr sleep deprivation. (g) Time spent in target quadrant after 48‐hr sleep deprivation. (h) Representative swimming tracks of mice after sleep deprivation for 48 hr. All data presented are means ± SEM; N = 10 mice per group. *P < 0.05, significantly different from control; # P < 0.05, significantly different from SD group
3.2. Modafinil prevented the morphological change of hippocampal neurons in sleep—deprived mice
Sleep deprivation induced marked injury to the neuronal structure in the CA and dentate gyrus (DG) regions of mouse hippocampus. As shown in Figure 3a,b, many neurons in CA3 and DG regions of hippocampus in mice of the sleep—deprived group became shrunken with darker Nissl staining, compared with that in the control group. Modafinil treatment at all three doses (6.5, 13, and 26 mg·kg−1) reduced the morphological changes in these regions. Most of the neuronal cells in mice of modafinil treatment groups were evenly stained light blue and had regularly shaped cell bodies. Moreover, as shown in Figure 3c, the percentage of injured cells in CA3 region was significantly reduced by modafinil treatment. These data suggested that administration of modafinil could suppress structural damage on neurons in hippocampal regions of mice induced by sleep deprivation.
Figure 3.
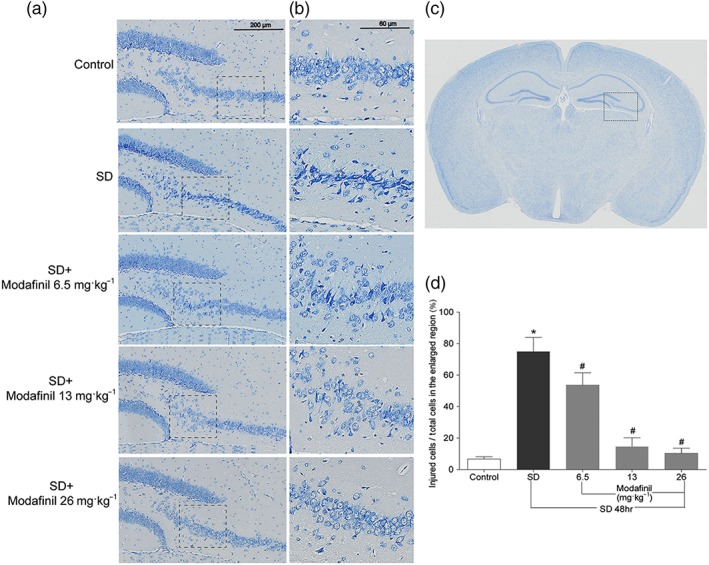
The effect of modafinil on hippocampal neuronal morphology of mice after sleep deprivation (SD) for 48 hr. (a) Nissl staining of the dentate gyrus and CA3 of hippocampus. (b) The enlarged regions within respective rectangular boxes of (a). (c) An illustration of mouse brain section. The region in the rectangular box was observed for Nissl staining. (d) The percentage of injured cells in the enlarged CA3 region of hippocampus. All data presented are means ± SEM; N = 5 mice per group. *P < 0.05; significantly different from control. # P < 0.05, significantly different from SD group
3.3. Modafinil suppressed the neuronal autophagy in hippocampus of sleep—deprived mice
To explore if modafinil had some effect on ultrastructure of neurons in hippocampus of sleep—deprived mice, the ultrathin brain sections were examined by transmission electron microscopy. As shown in Figure 4a, sleep deprivation induced a marked accumulation of autophagosomes with double membranes in neurons. However, modafinil administration decreased the number of autophagosomes significantly. To confirm the effect of modafinil on autophagy, brain sections of mice were subjected to LC3B immunostaining. As shown in Figure 4b, in CA and DG regions of sleep—deprived mice, the expression of LC3B in neurons was enhanced markedly compared with that in the control mice. Modafinil administered at higher doses (13 and 26 mg·kg−1) decreased the expression of LC3B in the same regions. These results suggested that modafinil had inhibitory effects on the formation of autophagosomes in the hippocampus of sleep—deprived mice.
Figure 4.
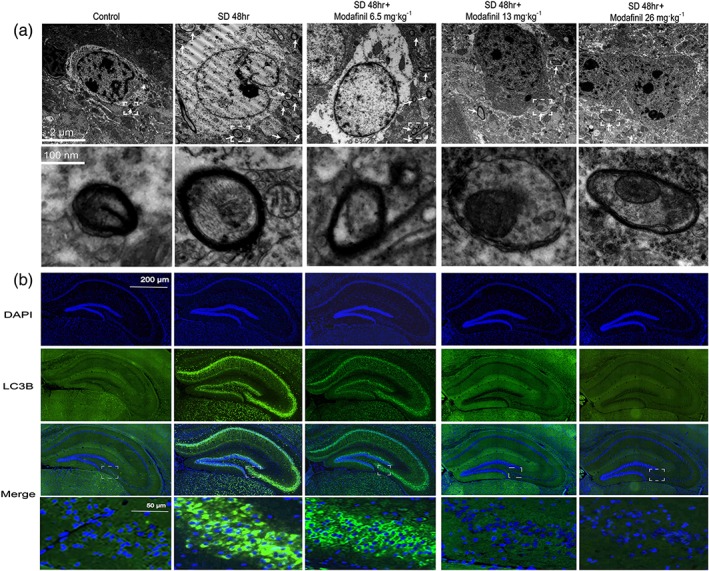
The effect of modafinil on the formation of autophagosomes and the expression of LC3B in hippocampus of mice after sleep deprivation (SD) for 48 hr. (a) Images of hippocampus of sleep—deprived mice obtained from transmission electron microscopy. The arrows denote the autophagosomes. The pictures in the lower panel were the enlarged regions in the rectangular boxes of that in the upper panel respectively. (b) Expression of LC3B in hippocampus of sleep—deprived mice. The images shown were one of the representative results of immunohistochemical staining from each group
3.4. Modafinil regulated the expression of apoptotic and autophagic proteins in hippocampus of sleep—deprived mice
In the hippocampus of sleep—deprived mice, many proteins involved in autophagy were changed. As shown in Figure 5, sleep deprivation decreased the phosphorylation of Akt, PI3K, mTOR, and P70S6K but increased the expression of Beclin‐1, LC3B, and p62 in hippocampus, compared with the control. Moreover, sleep deprivation changed the expression of apoptotic proteins, such as Bcl‐2, Bax, and cleaved caspase‐3. However, modafinil, particularly at 26 mg·kg−1, could counteract the effect of sleep deprivation on the expression of apoptotic and autophagic proteins. These results indicated that modafinil could attenuate neuronal autophagy and apoptosis in hippocampus of sleep—deprived mice.
Figure 5.
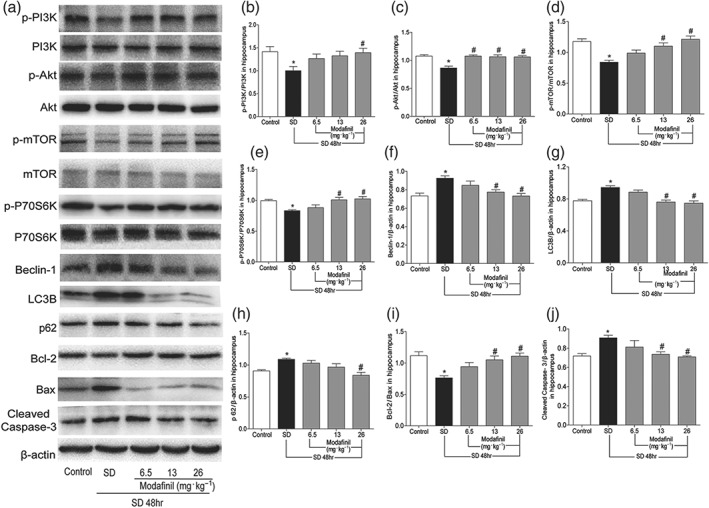
The effect of modafinil on the proteins related to autophagy and apoptosis in hippocampus of mice after sleep deprivation (SD) for 48 hr. (a) Western blotting bands of respective proteins in hippocampus of mice. (b–j) Grey intensity analysis of p‐PI3K/PI3K, p‐Akt/Akt, p‐mTOR/mTOR, p‐P70S6K/P70S6K, Beclin‐1/β‐actin, LC3B/β‐actin, p62/β‐actin, Bcl‐2/Bax, and cleaved caspase‐3/β‐actin. All data presented are means ± SEM; N = 5 per group. *P < 0.05, significantly different from control; # P < 0.05, significantly different from SD group
3.5. Modafinil suppressed the autophagy and apoptosis of HT‐22 cells induced by rapamycin
Because modafinil showed neuroprotective effect on the hippocampus of sleep—deprived mice, we next investigated its direct effect on hippocampal neuronal cells . As shown in Figure 6a, in the hippocampal neuronal cell line HT‐22, transiently transfected with GFP‐LC3 plasmid and induced by rapamycin, many autophagosomes indicated by GFP puncta were found in the cytoplasm. 3‐MA, the autophagy inhibitor, and modafinil treatment significantly reduced the number and intensity of GFP, suggesting their anti‐autophagy effect.
Figure 6.
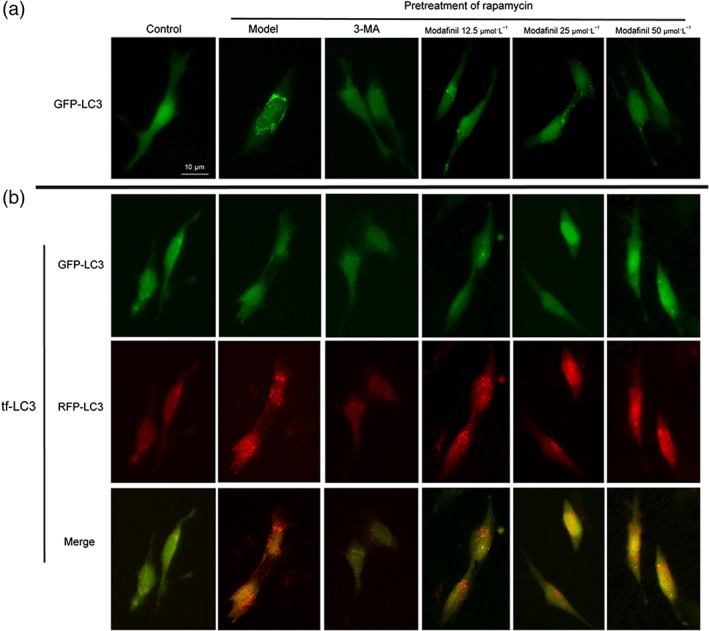
The effect of modafinil on HT‐22 cells transfected with GFP‐LC3 and tf‐LC3. (a) The effect of modafinil on HT‐22 cell transfected with GFP‐LC3. (b) The effect of modafinil on HT‐22 transfected with tf‐LC3. HT‐22 cells were transfected with GFP‐LC3 or tf‐LC3 plasmid for 6 hr. After pretreatment with rapamycin (50 μg·ml−1) for 4 hr, the cells were exposed to different concentrations (12.5, 25, and 50 μmol·L−1) of modafinil for 48 hr. The fluorescence of the treated cells was visualized with an inverted fluorescence microscope
To further evaluate the effect of modafinil on the autophagy process, the tandem fluorescent‐tagged LC3 reporter, tf‐LC3, was transfected into HT‐22 cells. As shown in Figure 6b, when induced by rapamycin, the transfected cells had increased autophagosomes indicated by GFP puncta and autolysosomes labelled by RFP as well as yellow puncta.
However, modafinil treatment at different concentrations could reduce the number of both green and red fluorescent puncta, suggesting that modafinil could inhibit the formation of autophagosome and, therefore, reduce the formation of autolysosomes. Similarly, acridine orange staining showed that there were many more acidic vesicular organelles appeared in bright red in the cytoplasm of rapamycin‐induced HT‐22 cells, compared with the controls (Figure 7a). When treated with modafinil, especially at higher concentrations (25 and 50 μmol·L−1), less acidic vesicular organelles were found.
Figure 7.
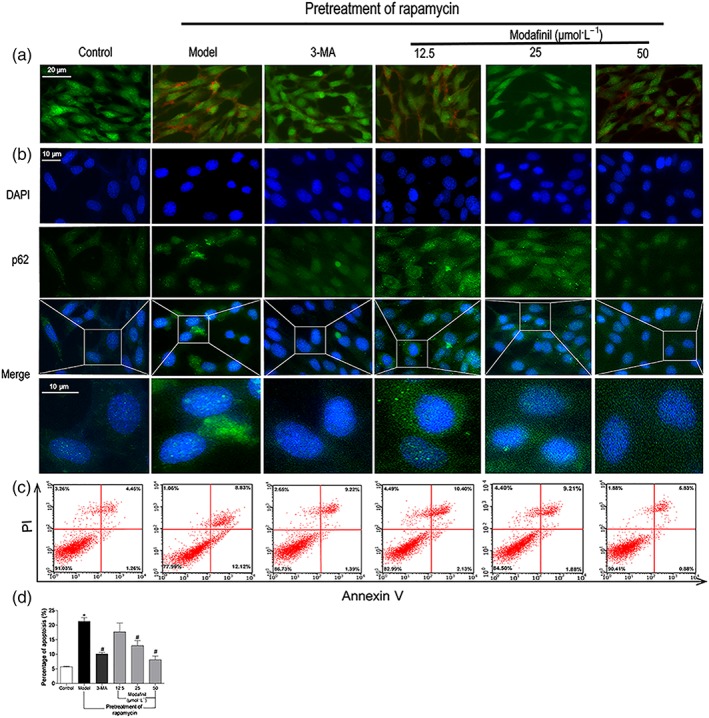
The effect of modafinil on autophagy and apoptosis of HT‐22 cells. (a) Acridine orange staining showed that modafinil could reduce acidic vesicular organelles in bright red colour in HT‐22 cells induced by rapamycin (Model). (b) Immunostaining of p62 exposed that modafinil could decrease p62 expression in HT‐22 cells induced by rapamycin. (c) AnnexinV/propidium iodide staining showed that modafinil could reduce the percentage of apoptotic HT‐22 cells induced by rapamycin. The right upper and lower quadrants of flow cytometry plot depicted the late apoptosis and early apoptosis respectively. (d) The total percentage of the early and late apoptotic cells. Generally, the total percentage of the early and late apoptotic cells was used to represent total apoptotic cells. All data presented are means ± SEM; N=3. *P < 0.05, significantly different from control; # P < 0.05, significantly different from Model group
p62, known as ubiquitin‐binding protein p62 or sequestosome‐1, is an adaptor protein involved in the selective degradation of ubiquitinated autophagy substrates. The appearance of p62 in abnormal autophagy is a reliable marker for autophagy progress. As shown in Figure 7b, rapamycin induced the accumulation of p62 within HT‐22 cells, which was counteracted by either 3‐MA or modafinil treatments.
In addition, rapamycin seemed to increase the apoptosis of HT‐22 cells. As shown in Figure 7c, compared with the control cells, the percentage of apoptotic cells was significantly higher after rapamycin induction. Modafinil treatment could prevent the increase of apoptotic cells induced by rapamycin. These results implied that modafinil suppressed the autophagy and apoptosis of hippocampal neuronal cells.
3.6. Modafinil prevented the abnormal change of apoptotic and autophagic proteins in HT‐22 cells induced by rapamycin
As shown in Figure 8, compared with the control, rapamycin reduced the phosphorylation of PI3K, Akt, mTOR, and P70S6K and enhanced the expression of Beclin‐1, LC3B, and p62 in HT‐22 cells, suggesting its induction of autophagy. Meanwhile, it decreased the ratio of Bcl‐2/Bax but increased the expression of cleaved caspase‐3 within the cells, suggesting an induction of apoptosis. However, these changes were reversed by modafinil treatment, suggesting anti‐autophagic and anti‐apoptotic effects of this compound on hippocampal neuronal cells.
Figure 8.
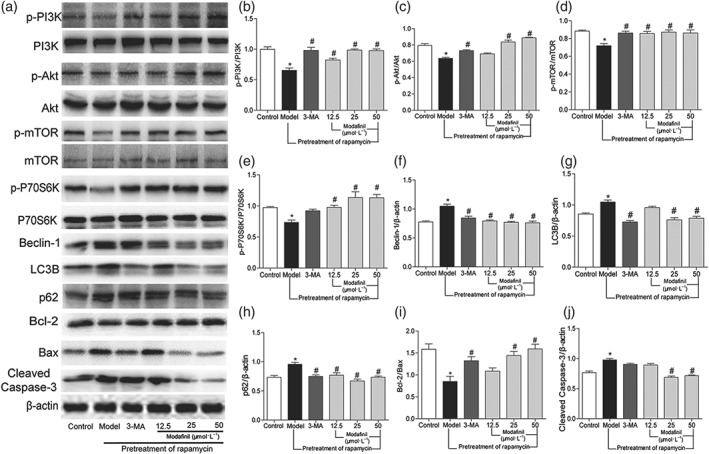
The effect of modafinil on the proteins related with autophagy and apoptosis in HT‐22 cells induced by rapamycin (Model). (a) Western blotting bands of respective proteins. (b–j) Grey intensity analysis of p‐PI3K/PI3K, p‐Akt/Akt, p‐mTOR/mTOR, p‐P70S6K/P70S6K, Beclin‐1/β‐actin, LC3B/β‐actin, p62/β‐actin, Bcl‐2/Bax, and cleaved caspase‐3/β‐actin. All data presented are means ± SEM; N = 5 per group. *P < 0.05, significantly different from control; # P < 0.05, significantly different from Model group
3.7. Rapamycin abolished the protective effect of modafinil on sleep—deprived mice
To confirm the protective effect of modafinil through inhibiting excessive autophagy, rapamycin was administered together with modafinil in mice. As shown in Figure 9, sleep deprivation for 24 or 48 hr, significantly impaired the spatial memory of mice . Rapamycin alone did not change the spatial memory of normal or sleep—deprived mice. However, when used together with modafinil, rapamycin blocked the memory improvement effect of the latter. The sleep—deprived mice treated with both modafinil and rapamycin did not cross the platform differently from those without any treatment. As shown in Figure 10, when rapamycin was used, the alleviative effects of modafinil on autophagy‐related proteins, such as Beclin‐1, LC3B, and p62, were blocked. Meanwhile, the suppression by modafinil on the apoptosis‐related proteins, including Bcl‐2, Bax, and cleaved caspase‐3, was more or less absent. These results indicated that modafinil improved the memory of sleep—deprived mice through counteracting excessive autophagy.
Figure 9.
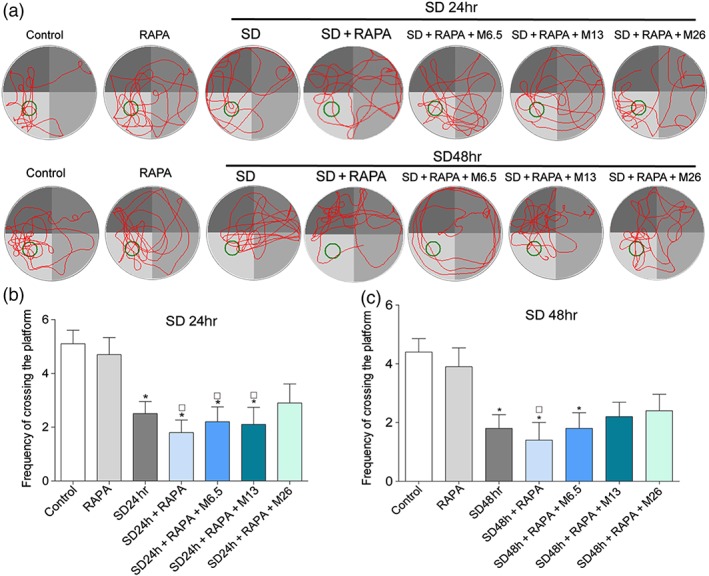
Rapamycin (RAPA) abolished the protective effect of modafinil on spatial memory of sleep deprivation (SD) mice. (a) Representative swimming tracks of sleep—deprived mice in Morris water maze test. (b) Frequency of crossing the platform of mice after sleep deprivation for 24 hr. (c) Frequency of crossing the platform of mice after sleep deprivation for 48 hr. All data presented are means ± SEM; N = 10 per group. *P < 0.05, significantly different from control; # P < 0.05, significantly different from control + rapamycin group
Figure 10.
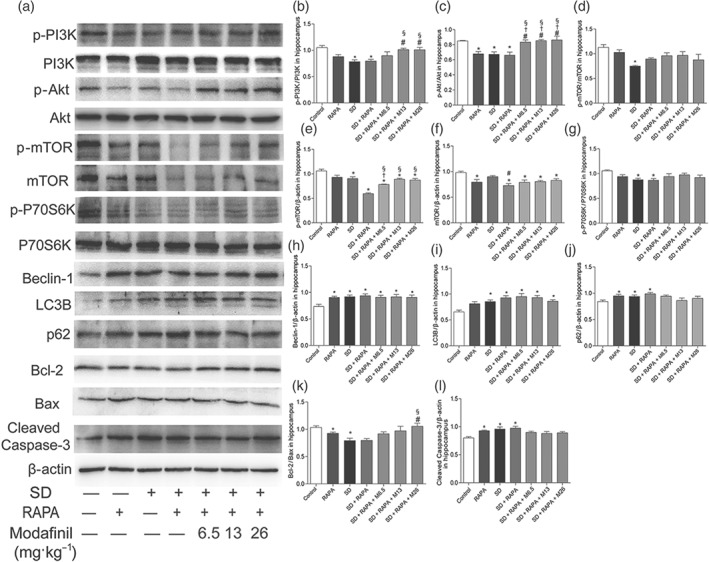
Rapamycin (RAPA) reversed the anti‐autophagic effect of modafinil on hippocampus of sleep deprivation (SD) mice. (a) Western blotting bands of respective proteins in hippocampus of mice. (b–l) Grey intensity analysis of p‐PI3K/PI3K, p‐Akt/Akt, p‐mTOR/mTOR, p‐mTOR/β‐actin, mTOR/β‐actin, p‐P70S6K/P70S6K, Beclin‐1/β‐actin, LC3B/β‐actin, p62/β‐actin, Bcl‐2/Bax, and cleaved caspase‐3/β‐actin. All data presented are means ± SEM; N = 5 per group. *P < 0.05, significantly different from control; # P < 0.05, significantly different from SD group; †P < 0.05, significantly different from control + RAPA group; § P < 0.05, significantly different from SD + RAPA group
4. DISCUSSION
Basal autophagy has been shown to be important for the prevention of the accumulation of abnormal cytosolic proteins in neurons, whereas impairment of basal autophagy may cause neurodegeneration shown by extensive neuronal loss (Hara et al., 2006; Komatsu et al., 2006). Meanwhile, excessive autophagy is detrimental to neuronal survival, and inhibition of autophagy delays the neurodegenerative progression (Lee & Gao, 2009). Sleep deprivation is known to impair the learning and memory by modification of neuronal network at physiological, molecular, and synaptic levels. But whether sleep deprivation can modulate or disrupt the process of autophagy in neurons has not been clearly demonstrated. In agreement with earlier reports, in our present study, sleep deprivation for 24 and 48 hr significantly impaired the spatial memory of mice. Furthermore, the hippocampal neurons in sleep—deprived mice became shrunken with condensed Nissl staining, accompanied by excessive autophagy, as shown by increased autophagosomes as well as LC3B, Beclin‐1, and p62. At the same time, aggravated apoptosis shown by decreased Bcl‐2/Bax ratio and increased cleaved caspase‐3, found within the hippocampus of sleep—deprived mice. These results implied that hippocampal neuronal injury caused by excessive autophagy might be one of the factors that contributed to the memory deficits of sleep—deprived mice. As a wakefulness‐promoting drug, modafinil is known to rescue memory deficits induced by sleep deprivation in rodents (He, Peng, Zhao, Zhou, & Zhao, 2011; Moreira et al., 2010; Pierard et al., 2007). Consistent with these reports, in the present study, modafinil restored the spatial memory of sleep—deprived mice. Moreover, modafinil attenuated excessive autophagy and apoptosis in hippocampal neurons both in vitro and in vivo. These findings indicated that modafinil prevented the deficits of memory induced by sleep deprivation, through inhibiting excessive autophagy.
Autophagy is the main cellular catabolic process for the degradation of protein aggregates and damaged organelles and is mainly involved in neural function and neuron survival other than protein degradation in the brain (Yamamoto & Yue, 2014). It is controlled by the main autophagy repressor, mTOR, and its upstream modulator PI3K/Akt (Yang & Klionsky, 2010). Evidence suggested that autophagy plays a neuroprotective role through the PI3K/Akt/mTOR pathway (Chen, Xiong, Tong, & Mao, 2013). Activation of the serine/threonine protein kinase mTOR inhibits the formation of autophagosomes, whereas suppression of mTOR pathway signalling causes cell death related with apoptosis and autophagy (Degtyarev et al., 2008). PI3K/Akt modulates the phosphorylation of mTOR and, therefore, plays a crucial role in regulating cell autophagy (Yano et al., 2001). P70S6K also contributes to the induction of autophagy directly by activating autophagic mechanisms or indirectly by inducing the protein synthesis needed for normal expansion and maturation of autophagosomes (Abeliovich, Dunn, Kim, & Klionsky, 2000; Chang et al., 2009; Zeng & Kinsella, 2008). The ribosomal protein P70S6K is one of the best characterized downstream effectors of mTORC1, which is activated by mTOR. It is generally recognized that the mTORC1–P70S6K axis controls the fundamental cellular processes, including transcription, translation, protein and lipid synthesis, cell growth, and cell metabolism (Fenton & Gout, 2011). In the process of autophagosome maturation, members of the LC3 family are cleaved by ATG4 proteases, lipidated, and incorporated into maturing autophagosomes, in which LC3B is the predominant form (Vidoni, Secomandi, Castiglioni, Melone, & Isidoro, 2018). Beclin‐1 (known as autophagy‐related gene Atg6) was regarded as a key regulator of autophagosome formation (Wirawan et al., 2012). p62 has been extensively studied as a substrate of autophagy and continuously degraded (Pankiv et al., 2007). In the present study, modafinil enhanced the phosphorylation of PI3K, Akt, mTOR, and P70S6K and suppressed the expression of LC3B, Beclin‐1, and p62 in hippocampus of sleep—deprived mice and rapamycin‐induced HT‐22 cells. These results implied that modafinil mitigated excessive autophagy in hippocampal neurons probably through activation of the PI3K/Akt/mTOR/P70S6K signalling pathway.
Both autophagy and apoptosis play important roles in the functional development of the nervous system (Nikoletopoulou, Markaki, Palikaras, & Tavernarakis, 2013). Apoptosis is widely appreciated as a major mechanism in the regulation of cell death initiated not only upon cell damage or stress but also during normal development and morphogenesis (Nikoletopoulou et al., 2013). Autophagy is a self‐digesting process that is morphologically characterized by the formation of double‐membrane autophagosomes, which sequester impaired organelles and dysfunctional cellular components and deliver them to lysosomes for degradation and recycling (Eskelinen & Saftig, 2009). However, when the formation of autophagosomes exceeds the degradation capacity of lysosome, autophagy will induce apoptosis (Ma, Guo, Yu, Zhang, & Ren, 2011). In the present study, significantly increased autophagic markers, such as Beclin‐1, LC3B, and p62, as well as autophagosomes, were elevated in hippocampal neurons of sleep‐‐deprived mice, and were accompanied with the increased apoptotic signals, such as Bcl‐2/Bax and cleaved caspase‐3, suggesting neuronal injury resulted from excessive autophage‐induced apoptosis. Modafinil significantly reduced the production of autophagic proteins and mitigate apoptotic signals in hippocampus of sleep‐‐deprived mice. Moreover, it suppressed autophagic process, as well as apoptotic signals in HT‐22 cells induced by rapamycin. These results robustly implicated that modafinil improved memory, impaired by sleep deprivation, probably through suppressing hippocampal neuronal apoptosis induced by excessive autophagy. To confirm this hypothesis, rapamycin, the autophagy inducer, was employed together with modafinil on sleep‐‐deprived mice. As shown in Figure 9, additional administration of rapamycin abolished the memory improvement by modafinil in sleep—deprived mice and suppressed its alleviation of excessive autophagy in the hippocampus, suggesting that modafinil improved the impaired learning and memory of mice through anti‐autophagic actions.
Rapamycin impairs synaptic plasticity (Casadio et al., 1999; Gong, Park, Abbassi, & Tang, 2006; Tang et al., 2002), contextual fear memory (Bekinschtein et al., 2007; Blundell, Kouser, & Powell, 2008), and long‐term memory (Lana, Di Russo, Mello, Wenk, & Giovannini, 2017). It is worth mentioning that although in Figure 9, statistical results showed that co‐administration of rapamycin affected the beneficial effect of modafinil on sleep—deprived mice in MWM to some degree, as the lower doses of modafinil (6.5 and 13 mg·kg−1) and rapamycin treated mice, sleep‐‐deprived for 24 hr, showed no difference with the rapamycin mice. However, when sleep—deprived for 48 hr, only the lowest dosage of modafinil (6.5 mg·kg−1) and rapamycin‐treated mice performed worse than the rapamycin mice. These results implied that higher doses of modafinil (26 mg·kg−1) could partly counteract the autophagy‐inductive effect of short‐term sleep deprivation even with the current dosage of rapamycin (2 mg·kg−1) in mice.
In addition, currently, the underlying mechanisms for the wakefulness‐promoting properties or cognitive‐enhancing abilities of modafinil have not been elucidated. This drug may inhibit dopamine reuptake as a dopamine transporter inhibitor (Greenblatt & Adams, 2018; Loland et al., 2012), thereby increasing synaptic dopamine levels exciting dopamine receptors and adrenoceptors (Wisor, 2013). Meanwhile, dopamine receptor subtypes have been demonstrated to differentially regulate autophagy (Wang, Du, et al., 2018; Wang, Ji, et al., 2018) as D1‐like receptors act as negative regulators, whereas D2‐like receptors work as positive regulators. However, in the present study, we do not know which receptors in hippocampus were modulated by modafinil in the process of the alleviation of excessive autophagy induced by sleep deprivation. Further experiments should be carried out to elucidate the potential molecular targets of modafinil in counteracting neuronal autophagy in sleep—deprived mice.
In summary, our present study clearly demonstrated that modafinil could counteract excessive neuronal autophagy and apoptosis in sleep—deprived mice and, thus, improved the impaired memory of mice, probably through activating PI3K/Akt/mTOR/P70S6K signalling pathway.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
X.W., Z.W., Q.L., and Y.C. designed the study. Y.C., L.L., H.W., F.H., F.Z., F.X., Q.Z., and Q.L. performed the experiment. Y.C., Y.L., and C.W. completed the statistical analysis. The whole experiment was conducted in X.W.'s lab and under the supervision of X.W. and Z.W. Y.C. prepared the draft, which was further revised and edited by X.W.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was financially supported by the National Natural Science Foundation of China (81530096 and 81673626), Shanghai Eastern Scholar Program (2013‐59), and Shanghai E‐Research Institute of Bioactive Constituent in TCM Plan.
Cao Y, Li Q, Liu L, et al. Modafinil protects hippocampal neurons by suppressing excessive autophagy and apoptosis in mice with sleep deprivation. Br J Pharmacol. 2019;176:1282–1297. 10.1111/bph.14626
Contributor Information
Zhengtao Wang, Email: ztwang@shutcm.edu.cn.
Xiaojun Wu, Email: xiaojunwu320@126.com.
REFERENCES
- Abeliovich, H. , Dunn, W. J. , Kim, J. , & Klionsky, D. J. (2000). Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. The Journal of Cell Biology, 151(5), 1025–1034. 10.1083/jcb.151.5.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta‐Pena, E. , Camacho‐Abrego, I. , Melgarejo‐Gutierrez, M. , Flores, G. , Drucker‐Colin, R. , & Garcia‐Garcia, F. (2015). Sleep deprivation induces differential morphological changes in the hippocampus and prefrontal cortex in young and old rats. Synapse, 69(1), 15–25. 10.1002/syn.21779 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein, P. , Cammarota, M. , Igaz, L. M. , Bevilaqua, L. R. , Izquierdo, I. , & Medina, J. H. (2007). Persistence of long‐term memory storage requires a late protein synthesis‐ and BDNF‐dependent phase in the hippocampus. Neuron, 53(2), 261–277. 10.1016/j.neuron.2006.11.025 [DOI] [PubMed] [Google Scholar]
- Blundell, J. , Kouser, M. , & Powell, C. M. (2008). Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiology of Learning and Memory, 90(1), 28–35. 10.1016/j.nlm.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio, A. , Martin, K. C. , Giustetto, M. , Zhu, H. , Chen, M. , Bartsch, D. , … Kandel, E. R. (1999). A transient, neuron‐wide form of CREB‐mediated long‐term facilitation can be stabilized at specific synapses by local protein synthesis. Cell, 99(2), 221–237. 10.1016/S0092-8674(00)81653-0 [DOI] [PubMed] [Google Scholar]
- Chang, Y. Y. , Juhasz, G. , Goraksha‐Hicks, P. , Arsham, A. M. , Mallin, D. R. , Muller, L. K. , et al. (2009). Nutrient‐dependent regulation of autophagy through the target of rapamycin pathway. Biochemical Society Transactions, 37(Pt 1), 232–236. 10.1042/BST0370232 [DOI] [PubMed] [Google Scholar]
- Chen, A. , Xiong, L. J. , Tong, Y. , & Mao, M. (2013). Neuroprotective effect of brain‐derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Molecular Medicine Reports, 8(4), 1011–1016. 10.3892/mmr.2013.1628 [DOI] [PubMed] [Google Scholar]
- Cirelli, C. (2013). Sleep and synaptic changes. Current Opinion in Neurobiology, 23(5), 841–846. 10.1016/j.conb.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev, M. , De Maziere, A. , Orr, C. , Lin, J. , Lee, B. B. , Tien, J. Y. , … Lin, K. (2008). Akt inhibition promotes autophagy and sensitizes PTEN‐null tumors to lysosomotropic agents. The Journal of Cell Biology, 183(1), 101–116. 10.1083/jcb.200801099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doghramji, P. P. , Lieberman, J. R. , & Gordon, M. L. (2007). Stay awake! Understanding, diagnosing, and successfully managing narcolepsy. The Journal of Family Practice, 56(11 Suppl Stay), S17–S31. S32 [PubMed] [Google Scholar]
- Eskelinen, E. L. , & Saftig, P. (2009). Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochimica et Biophysica Acta, 1793(4), 664–673. 10.1016/j.bbamcr.2008.07.014 [DOI] [PubMed] [Google Scholar]
- Fenton, T. R. , & Gout, I. T. (2011). Functions and regulation of the 70 kDa ribosomal S6 kinases. The International Journal of Biochemistry & Cell Biology, 43(1), 47–59. 10.1016/j.biocel.2010.09.018 [DOI] [PubMed] [Google Scholar]
- Gong, R. , Park, C. S. , Abbassi, N. R. , & Tang, S. J. (2006). Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity‐dependent dendritic protein synthesis in hippocampal neurons. The Journal of Biological Chemistry, 281(27), 18802–18815. 10.1074/jbc.M512524200 [DOI] [PubMed] [Google Scholar]
- Grady, S. , Aeschbach, D. , Wright, K. J. , & Czeisler, C. A. (2010). Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology, 35(9), 1910–1920. 10.1038/npp.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt, K , Adams, N . (2018). Modafinil. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan‐. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531476/. Accessed March 15, 2019.
- Hara, T. , Nakamura, K. , Matsui, M. , Yamamoto, A. , Nakahara, Y. , Suzuki‐Migishima, R. , … Mizushima, N. (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature, 441(7095), 885–889. 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B. , Peng, H. , Zhao, Y. , Zhou, H. , & Zhao, Z. (2011). Modafinil treatment prevents REM sleep deprivation‐induced brain function impairment by increasing MMP‐9 expression. Brain Research, 1426, 38–42. 10.1016/j.brainres.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Hylin, M. J. , Zhao, J. , Tangavelou, K. , Rozas, N. S. , Hood, K. N. , MacGowan, J. S. , … Dash, P. K. (2018). A role for autophagy in long‐term spatial memory formation in male rodents. Journal of Neuroscience Research, 96(3), 416–426. 10.1002/jnr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. Y. , Mahmoud, G. S. , & Grover, L. M. (2005). REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neuroscience Letters, 388(3), 163–167. 10.1016/j.neulet.2005.06.057 [DOI] [PubMed] [Google Scholar]
- Klionsky, D. J. (2005). The molecular machinery of autophagy: Unanswered questions. Journal of Cell Science, 118(Pt 1), 7–18. 10.1242/jcs.01620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu, M. , Waguri, S. , Chiba, T. , Murata, S. , Iwata, J. , Tanida, I. , … Tanaka, K. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature, 441(7095), 880–884. 10.1038/nature04723 [DOI] [PubMed] [Google Scholar]
- Lana, D. , Di Russo, J. , Mello, T. , Wenk, G. L. , & Giovannini, M. G. (2017). Rapamycin inhibits mTOR/p70S6K activation in CA3 region of the hippocampus of the rat and impairs long term memory. Neurobiology of Learning and Memory, 137, 15–26. 10.1016/j.nlm.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. A. , & Gao, F. B. (2009). Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT‐III in frontotemporal dementia. The Journal of Neuroscience, 29(26), 8506–8511. 10.1523/JNEUROSCI.0924-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Liu, W. , Lu, Y. , Tian, H. , Duan, C. , Lu, L. , … Yang, H. (2018). Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone‐induced Parkinson disease models. Autophagy, 14(5), 845–861. 10.1080/15548627.2017.1390636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Zhu, Y. , Wang, Y. , Qi, S. , Wang, Y. , Ma, C. , … Wang, C. (2017). Anti‐amnesic effect of extract and alkaloid fraction from aerial parts of Peganum harmala on scopolamine‐induced memory deficits in mice. Journal of Ethnopharmacology, 204, 95–106. 10.1016/j.jep.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Loland, C. J. , Mereu, M. , Okunola, O. M. , Cao, J. , Prisinzano, T. E. , Mazier, S. , … Newman, A. H. (2012). R‐modafinil (armodafinil): A unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biological Psychiatry, 72(5), 405–413. 10.1016/j.biopsych.2012.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H. , Guo, R. , Yu, L. , Zhang, Y. , & Ren, J. (2011). Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: Role of autophagy paradox and toxic aldehyde. European Heart Journal, 32(8), 1025–1038. 10.1093/eurheartj/ehq253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy, J. G. , & Strecker, R. E. (2011). The cognitive cost of sleep lost. Neurobiology of Learning and Memory, 96(4), 564–582. 10.1016/j.nlm.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo, P. , Mistlberger, R. E. , Jacobs, B. L. , Heller, H. C. , & McGinty, D. (2009). New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Medicine Reviews, 13(3), 187–194. 10.1016/j.smrv.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, W. , Dong, C. , Hui, C. , Bin, L. , Fenggen, Y. , Jingjing, S. , … Qinglin, L. (2014). Gambogenic acid kills lung cancer cells through aberrant autophagy. PLoS ONE, 9(1), e83604 10.1371/journal.pone.0083604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies, F. M. , Fleming, A. , Caricasole, A. , Bento, C. F. , Andrews, S. P. , Ashkenazi, A. , … Rubinsztein, D. C. (2017). Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron, 93(5), 1015–1034. 10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Mizushima, N. (2007). Autophagy: Process and function. Genes & Development, 21(22), 2861–2873. 10.1101/gad.1599207 [DOI] [PubMed] [Google Scholar]
- Moreira, K. M. , Ferreira, T. L. , Hipolide, D. C. , Fornari, R. V. , Tufik, S. , & Oliveira, M. G. (2010). Modafinil prevents inhibitory avoidance memory deficit induced by sleep deprivation in rats. Sleep, 33(7), 990–993. 10.1093/sleep/33.7.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou, V. , Markaki, M. , Palikaras, K. , & Tavernarakis, N. (2013). Crosstalk between apoptosis, necrosis and autophagy. Biochimica et Biophysica Acta, 1833(12), 3448–3459. 10.1016/j.bbamcr.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Pankiv, S. , Clausen, T. H. , Lamark, T. , Brech, A. , Bruun, J. A. , Outzen, H. , … Johansen, T. (2007). p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. The Journal of Biological Chemistry, 282(33), 24131–24145. 10.1074/jbc.M702824200 [DOI] [PubMed] [Google Scholar]
- Pierard, C. , Liscia, P. , Chauveau, F. , Coutan, M. , Corio, M. , Krazem, A. , & Beracochea, D. (2011). Differential effects of total sleep deprivation on contextual and spatial memory: Modulatory effects of modafinil. Pharmacology, Biochemistry, and Behavior, 97(3), 399–405. 10.1016/j.pbb.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Pierard, C. , Liscia, P. , Philippin, J. N. , Mons, N. , Lafon, T. , Chauveau, F. , … Béracochéa, D. (2007). Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacology, Biochemistry, and Behavior, 88(1), 55–63. 10.1016/j.pbb.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Polajnar, M. , & Zerovnik, E. (2014). Impaired autophagy: A link between neurodegenerative and neuropsychiatric diseases. Journal of Cellular and Molecular Medicine, 18(9), 1705–1711. 10.1111/jcmm.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, L. , Fu, J. , Xue, X. , Shi, Y. , Yao, L. , Huang, W. , … Pan, Y. (2018). Neuronalinjury and roles of apoptosis and autophagy in a neonatal rat model of hypoxia–ischemia‐induced periventricular leukomalacia. Molecular Medicine Reports, 17(4), 5940–5949. 10.3892/mmr.2018.8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri, M. , Gonzalez, B. , Goitia, B. , Garcia‐Rill, E. , Krasnova, I. N. , Cadet, J. L. , … Bisagno, V. (2012). Modafinil abrogates methamphetamine‐induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS ONE, 7(10), e46599 10.1371/journal.pone.0046599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, K. , Chatterjee, A. , Panjwani, U. , Kumar, S. , Sahu, S. , Ghosh, S. , … Anand, J. P. (2012). Modafinil improves event related potentials P300 and contingent negative variation after 24 h sleep deprivation. Life Sciences, 91(3–4), 94–99. 10.1016/j.lfs.2012.06.012 [DOI] [PubMed] [Google Scholar]
- Sahakian, B. J. , & Morein‐Zamir, S. (2015). Pharmacological cognitive enhancement: Treatment of neuropsychiatric disorders and lifestyle use by healthy people. Lancet Psychiat, 2(4), 357–362. 10.1016/S2215-0366(15)00004-8 [DOI] [PubMed] [Google Scholar]
- Sahu, S. , Kauser, H. , Ray, K. , Kishore, K. , Kumar, S. , & Panjwani, U. (2013). Caffeine and modafinil promote adult neuronal cell proliferation during 48 h of total sleep deprivation in rat dentate gyrus. Experimental Neurology, 248, 470–481. 10.1016/j.expneurol.2013.07.021 [DOI] [PubMed] [Google Scholar]
- Shaffery, J. P. , Sinton, C. M. , Bissette, G. , Roffwarg, H. P. , & Marks, G. A. (2002). Rapid eye movement sleep deprivation modifies expression of long‐term potentiation in visual cortex of immature rats. Neuroscience, 110(3), 431–443. 10.1016/S0306-4522(01)00589-9 [DOI] [PubMed] [Google Scholar]
- Song, X. , Zhou, B. , Zhang, P. , Lei, D. , Wang, Y. , Yao, G. , … Ikejima, T. (2016). Protective effect of silibinin on learning and memory impairment in LPS‐treated rats via ROS–BDNF–TrkB Pathway. Neurochemical Research, 41(7), 1662–1672. 10.1007/s11064-016-1881-5 [DOI] [PubMed] [Google Scholar]
- Suchecki, D. , & Tufik, S. (2000). Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiology & Behavior, 68(3), 309–316. 10.1016/S0031-9384(99)00181-X [DOI] [PubMed] [Google Scholar]
- Sun, D. , Sun, X. D. , Zhao, L. , Lee, D. H. , Hu, J. X. , Tang, F. L. , … Xiong, W. C. (2018). Neogenin, a regulator of adult hippocampal neurogenesis, prevents depressive‐like behavior. Cell Death & Disease, 9(1), 8 10.1038/s41419-017-0019-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S. J. , Reis, G. , Kang, H. , Gingras, A. C. , Sonenberg, N. , & Schuman, E. M. (2002). A rapamycin‐sensitive signaling pathway contributes to long‐term synaptic plasticity in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 99(1), 467–472. 10.1073/pnas.012605299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni, C. , Secomandi, E. , Castiglioni, A. , Melone, M. , & Isidoro, C. (2018). Resveratrol protects neuronal‐like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4‐mediated autophagosome formation. Neurochemistry International, 117, 174–187. 10.1016/j.neuint.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Wang, D. , Ji, X. , Liu, J. , Li, Z. , & Zhang, X. (2018). Dopamine receptor subtypes differentially regulate autophagy. International Journal of Molecular Sciences, 19(5), E1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Du, J. , Zhao, F. , Chen, Z. , Chang, J. , Qin, F. , … Chen, N. (2018). Trillium tschonoskii maxim saponin mitigates d‐galactose‐induced brain aging of rats through rescuing dysfunctional autophagy mediated by Rheb–mTOR signal pathway. Biomedicine & Pharmacotherapy, 98, 516–522. 10.1016/j.biopha.2017.12.046 [DOI] [PubMed] [Google Scholar]
- Wirawan, E. , Lippens, S. , Vanden Berghe, T. , Romagnoli, A. , Fimia, G. M. , Piacentini, M. , & Vandenabeele, P. (2012). Beclin1: A role in membrane dynamics and beyond. Autophagy, 8(1), 6–17. 10.4161/auto.8.1.16645 [DOI] [PubMed] [Google Scholar]
- Wisor, J. (2013). Modafinil as a catecholaminergic agent: Empirical evidence and unanswered questions. Frontiers in Neurology, 4, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Zhang, H. , Kai, J. , Zhu, F. , Dong, J. , Xu, Z. , … Zeng, L. H. (2018). Rapamycin prevents cerebral stroke by modulating apoptosis and autophagy in penumbra in rats. Annals of Clinical Translational Neurology, 5(2), 138–146. 10.1002/acn3.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X. , Zhu, Y. , Bu, J. , Li, G. , Liang, Z. , Yang, L. , & Hou, B. (2017). The autophagy inhibitor 3‐methyladenine restores sevoflurane anesthesiainduced cognitive dysfunction and neurons apoptosis. Pharmazie, 72(4), 214–218. 10.1691/ph.2017.6872 [DOI] [PubMed] [Google Scholar]
- Yamamoto, A. , & Yue, Z. (2014). Autophagy and its normal and pathogenic states in the brain. Annual Review of Neuroscience, 37, 55–78. 10.1146/annurev-neuro-071013-014149 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , & Klionsky, D. J. (2010). Mammalian autophagy: Core molecular machinery and signaling regulation. Current Opinion in Cell Biology, 22(2), 124–131. 10.1016/j.ceb.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, S. , Morioka, M. , Fukunaga, K. , Kawano, T. , Hara, T. , Kai, Y. , … Ushio, Y. (2001). Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. Journal of Cerebral Blood Flow and Metabolism, 21(4), 351–360. 10.1097/00004647-200104000-00004 [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Feng, L. , Li, J. , Lan, X. , A, L. , Lv, X. , … Chen, L. (2017). The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging‐dependent cognitive deficits. Behavioural Brain Research, 334, 155–162. 10.1016/j.bbr.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Wu, X. , Pu, J. , Luo, P. , Ma, W. , Wang, J. , … Fei, Z. (2018). Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation‐induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochemical and Biophysical Research Communications, 495(1), 1187–1194. 10.1016/j.bbrc.2017.11.165 [DOI] [PubMed] [Google Scholar]
- Zeng, X. , & Kinsella, T. J. (2008). Mammalian target of rapamycin and S6 kinase 1 positively regulate 6‐thioguanine‐induced autophagy. Cancer Research, 68(7), 2384–2390. 10.1158/0008-5472.CAN-07-6163 [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Wang, H. , Li, Q. , Huang, J. , & Sun, X. (2014). Modulating autophagy affects neuroamyloidogenesis in an in vitro ischemic stroke model. Neuroscience, 263, 130–137. 10.1016/j.neuroscience.2014.01.012 [DOI] [PubMed] [Google Scholar]


