Abstract
Background
Ovarian hyperstimulation syndrome (OHSS) in assisted reproductive technology (ART) cycles is a treatment‐induced disease that has an estimated prevalence of 20% to 33% in its mild form and 3% to 8% in its moderate or severe form. These numbers might even be higher for high‐risk women such as those with polycystic ovaries or a high oocyte yield from ovum pickup.
Objectives
The objective of this overview is to identify and summarise all evidence from Cochrane systematic reviews on interventions for prevention or treatment of moderate, severe and overall OHSS in couples with subfertility who are undergoing ART cycles.
Methods
Published Cochrane systematic reviews reporting on moderate, severe or overall OHSS as an outcome in ART cycles were eligible for inclusion in this overview. We also identified Cochrane submitted protocols and title registrations for future inclusion in the overview. The evidence is current to 12 December 2016. We identified reviews, protocols and titles by searching the Cochrane Gynaecology and Fertility Group Database of Systematic Reviews and Archie (the Cochrane information management system) in July 2016 on the effectiveness of interventions for outcomes of moderate, severe and overall OHSS. We undertook in duplicate selection of systematic reviews, data extraction and quality assessment. We used the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) tool to assess the quality of included reviews, and we used GRADE methods to assess the quality of the evidence for each outcome. We summarised the characteristics of included reviews in the text and in additional tables.
Main results
We included a total of 27 reviews in this overview. The reviews were generally of high quality according to AMSTAR ratings, and included studies provided evidence that ranged from very low to high in quality. Ten reviews had not been updated in the past three years. Seven reviews described interventions that provided a beneficial effect in reducing OHSS rates, and we categorised one additional review as 'promising'. Of the effective interventions, all except one had no detrimental effect on pregnancy outcomes.
Evidence of at least moderate quality indicates that clinicians should consider the following interventions in ART cycles to reduce OHSS rates.
• Metformin treatment before and during an ART cycle for women with PCOS (moderate‐quality evidence).
• Gonadotrophin‐releasing hormone (GnRH) antagonist protocol in ART cycles (moderate‐quality evidence).
• GnRH agonist (GnRHa) trigger in donor oocyte or 'freeze‐all' programmes (moderate‐quality evidence).
Evidence of low or very low quality suggests that clinicians should consider the following interventions in ART cycles to reduce OHSS rates.
• Clomiphene citrate for controlled ovarian stimulation in ART cycles (low‐quality evidence).
• Cabergoline around the time of human chorionic gonadotrophin (hCG) administration or oocyte pickup in ART cycles (low‐quality evidence).
• Intravenous fluids (plasma expanders) around the time of hCG administration or oocyte pickup in ART cycles (very low‐quality evidence).
• Progesterone for luteal phase support in ART cycles (low‐quality evidence).
• Coasting (withholding gonadotrophins) ‐ a promising intervention that needs to be researched further for reduction of OHSS.
On the basis of this overview, we must conclude that evidence is currently insufficient to support the widespread practice of embryo cryopreservation.
Authors' conclusions
Currently, 27 reviews in the Cochrane Library were conducted to report on or to try to report on OHSS in ART cycles. We identified four review protocols but no new registered titles that can potentially be included in this overview in the future. This overview provides the most up‐to‐date evidence on prevention of OHSS in ART cycles from all currently published Cochrane reviews on ART. Clinicians can use the evidence summarised in this overview to choose the best treatment regimen for individual patients ‐ a regimen that not only reduces the chance of developing OHSS but does not compromise other outcomes such as pregnancy or live birth rate. Review results, however, are limited by the lack of recent primary studies or updated reviews. Furthermore, this overview can be used by policymakers in developing local and regional protocols or guidelines and can reveal knowledge gaps for future research.
Plain language summary
Interventions for prevention of ovarian hyperstimulation syndrome in in vitro fertilisation cycles: an overview of Cochrane reviews
Overview question
This overview of Cochrane reviews aims to identify and summarise all evidence from Cochrane systematic reviews on interventions that could prevent or treat moderate, severe and overall ovarian hyperstimulation syndrome (OHSS) in couples with subfertility who are undergoing assisted reproductive technology (ART) cycles (i.e. in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI)).
Background
OHSS in ART cycles is an adverse event that follows ovarian stimulation for IVF. It is caused by a very high ovarian response to hormonal medication and results in enlarged ovaries and a fluid shift from blood vessels to the abdominal cavity, resulting in, for example, abdominal bloating, high risk of clots within the blood vessels (thrombosis) and decreased blood supply to important organs such as kidneys and liver. The mild form of OHSS is seen in almost 20% to 33% of cycles, whereas a moderate or severe form is found in approximately 3% to 8% of cycles and can lead to serious disease burden or even mortality if left untreated. It is therefore important to identify treatment regimens and interventions that can reduce the incidence of OHSS.
Study characteristics
We found a total of 27 Cochrane ART reviews of high quality that could be included for this overview. These reviews aimed to report on OHSS in cycles of IVF or ICSI. We did not include reviews of intrauterine insemination and ovulation induction. The evidence is current to 12 December 2016.
Key results
Of the 27 reviews included in this overview, 10 reviews had not been updated in the past three years.
Seven reviews described interventions that provided a beneficial effect in reducing OHSS rates, and we categorised one additional review as 'promising'. Of the effective interventions, all except one had no detrimental effect on pregnancy outcomes. Evidence of at least moderate quality evidence indicates that clinicians should consider the following interventions in ART cycles to reduce OHSS rates.
• Metformin treatment before and during an ART cycle in women with PCOS (moderate‐quality evidence).
• Gonadotrophin‐releasing hormone (GnRH) antagonist protocol in ART cycles (moderate‐quality evidence).
• GnRH agonist (GnRHa) trigger in donor oocyte or 'freeze‐all' programmes (moderate‐quality evidence).
Evidence of low or very low quality evidence suggests that clinicians should consider the following interventions in ART cycles to reduce OHSS rates.
• Clomiphene citrate for controlled ovarian stimulation in ART cycles (low‐quality evidence).
• Cabergoline around the time of human chorionic gonadotrophin (hCG) administration or oocyte pickup in ART cycles (low‐quality evidence).
• Intravenous fluids (blood plasma expanders) around the time of hCG administration or egg pickup in ART cycles (very low‐quality evidence).
• Progesterone for luteal phase support in ART cycles (low‐quality evidence).
A promising intervention that needs to be researched further is coasting (withholding gonadotrophins) for reduction of OHSS. On the basis of this overview, we must conclude that evidence is currently insufficient to support the widespread practice of freezing all embryos and replacing them at a later time when OHSS has dissolved.
Clinicians can use the evidence summarised in this overview to choose the best treatment regimen for individual patients ‐ a regimen that not only reduces the chance of developing OHSS but does not compromise pregnancy outcomes. However, results of this overview are limited by the lack of recent primary studies or updated reviews. Furthermore, this overview can be used by policymakers in developing local and regional protocols or guidelines and can reveal knowledge gaps for future research.
Background
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is a serious complication of controlled ovarian hyperstimulation cycles used in assisted reproductive technologies (ART). OHSS is clinically characterised by abdominal tenderness and swelling due to increased ovarian volume along with a sudden increase in vascular permeability, which results in a shift of fluid to the extravascular space. However, the exact pathophysiology of OHSS has not been completely elucidated. Cases of spontaneous OHSS have been reported and are suspected to be linked to follicle‐stimulating hormone (FSH) receptor gene mutations (Delbaere 2004). However, the development of OHSS during ART cycles is mainly an iatrogenic side effect of the high doses of gonadotropin used for ovarian stimulation, resulting in multi‐follicular growth. A key role is suspected for vascular endothelial growth factor (VEGF), which is produced by multiple follicles following ovarian stimulation (Agrawal 1999). Higher VEGF levels induce hyperpermeability of ovarian blood vessels, which leads to a fluid shift from the intravascular to the third space. Also, the administration of human chorionic gonadotrophin (hCG) as an ovulation trigger or luteal phase support in high‐risk women with extensive luteinisation and supraphysiological levels of oestradiol and progesterone in the presence of multiple corpora lutea can trigger OHSS (Delbaere 2005). Moreover, the extra hCG‐rise accompanying (multiple) pregnancy after ART can aggravate already existing OHSS or induce late‐onset OHSS.
Over the years, several criteria have been used to classify OHSS severity (Appendix 1; Aboulghar 2003; Golan 1989; Navot 1992; Schenker 1978). In general, when OHSS progresses to a moderate stage, women experience abdominal pain and nausea and vomiting, and ascites can be seen around the ovaries on vaginal ultrasonography. If the condition progresses to severe OHSS, extravascular fluid can be found in pleural and pericardial spaces, and several haemodynamic changes take place, such as intravascular volume depletion, haemoconcentration, hypoalbuminaemia and electrolyte imbalances. These changes can lead to severe morbidity associated with thromboembolic events (Stewart 1997), respiratory distress and liver or renal failure. If left untreated, OHSS demonstrates rapid progression, with potentially life‐threatening or lethal complications (Braat 2006).
The mild form of OHSS is common, is of less clinical importance and occurs in an estimated 20% to 33% of ART cycles. The more clinically relevant moderate and severe forms of OHSS occur in an estimated 3% to 8% of ART cycles (3% to 6% moderate and 0.5% to 5% severe forms) (Delvigne 2002; Golan 1989; Schenker 1994). These large differences in reported OHSS incidence occur mainly because most reports involve single‐centre data, use different definitions of OHSS, do not require that diagnosis must be ascertained by a formal classification system or must have adequate follow‐up and lack reporting of mild or moderate forms. A large European report on 2010 ART practice (Kupka 2014) provided OHSS data for 25 participating countries and revealed prevalence of 0.3% in 349,402 simulated ART cycles. However, this report lacked data for some countries with a high volume of ART cycles (e.g. France, Sweden, the Netherlands, UK) and for other countries reported extremely low rates of OHSS, possibly as the result of reporting bias. A large Swiss retrospective cohort study reported a decline in OHSS incidence from 3.6% to 1% from 2005 to 2009 (De Geyter 2015). Globally, the incidence of OHSS is declining; a steady decrease has been reported since its peak incidence in the 1990s, when the main goal of ART was to produce a high number of oocytes (Kol 2011), and the incidence of severe OHSS was considered to be around 0.2% to 1% (Abramov 1999). With the emergence of new treatment regimens, more judicious use of gonadotrophins, increased cycle monitoring and improved knowledge of OHSS risks, the incidence of this disorder fell gradually over subsequent decades.
Although it is relatively rare, OHSS in ART cycles is an iatrogenic disease, and women who are affected should be monitored carefully to avoid life‐threatening complications. Early recognition of risk factors for OHSS can help clinicians tailor treatment regimens. Women with a priori risk for development of OHSS are those with polycystic ovaries (PCOs) (with or without PCO syndrome (PCOS)) or a high antral follicle count (e.g. at a young age). During a controlled ovarian stimulation cycle, women can acquire 'high risk' status when they prove to have high oestradiol levels, excessive growth of follicles or a large number of retrieved oocytes. Besides the early OHSS type that develops during, or immediately after, ovarian stimulation, we can distinguish a late type, which appears after embryo implantation has been established. The presence of a multiple gestation can trigger or exacerbate this late type of OHSS (Delbaere 2005; Mathur 2000).
Description of interventions and how the interventions might work
Interventions that aim to reduce OHSS incidence can target diverse portions of stimulated ART cycles.
Selection or identification of 'high risk' populations for tailoring of stimulation regimens.
Prevention of recurrent OHSS by adjustment of the dose of gonadotrophins in the next cycle.
Prevention of large numbers of follicles by tailored ovarian stimulation for specific risk groups (e.g. use of different treatment regimens, use of adjuvant medication).
Prevention of a rise in VEGF levels (e.g. by prevention of development of large numbers of follicles, by targeting of VEGF receptors (e.g. by dopamine agonists)).
Dose reduction or withholding of hCG administration for ovulation trigger or luteal support.
Prevention of a rise in oestradiol by withholding of gonadotrophins (‘coasting’).
Prevention of a further rise in oestradiol and of ovulation triggering and pregnancy by cycle cancellation.
Prevention of intravascular volume depletion by administration of plasma‐expanding intravenous (IV) fluids.
Prevention of pregnancy by freezing of all embryos and transfer back during a subsequent cycle.
Moreover, trials of interventions within an ART cycle that are not specifically aimed at preventing OHSS may report on OHSS as an outcome. These interventions are of interest to this overview and might reveal new mechanisms for lowering risk of OHSS.
Why it is important to do this overview
OHSS is an iatrogenic disease with an estimated incidence of 3% to 6% in ART cycles of the clinically relevant moderate or severe form (Delvigne 2002). If left untreated, OHSS can lead to severe morbidity and can be life‐threatening. Multiple treatment options are available for prevention of OHSS in ART cycles; therefore, it is important to provide consumers, health professionals, policymakers and guideline developers with a summary of evidence on OHSS prevention obtained from the Cochrane Library. We will comment upon this evidence in light of the overall effectiveness of studied interventions in the separate reviews. By doing so, we will identify existing knowledge gaps or reporting flaws within the Cochrane systematic reviews published in the Cochrane Library on the topic of OHSS in ART cycles. This means that we can provide clear suggestions for future research.
Objectives
The objective of this overview is to identify and summarise all evidence from Cochrane systematic reviews on interventions for prevention or treatment of moderate, severe and overall OHSS in couples with subfertility who are undergoing ART cycles.
Methods
Criteria for considering reviews for inclusion
Types of reviews
For this overview of reviews, we included all published Cochrane systematic reviews of randomised controlled trials (RCTs) that examined:
interventions that aimed to prevent OHSS with reporting on the incidence of moderate, severe or overall OHSS as a primary outcome; and
other interventions in ART cycles with reporting on the incidence of moderate, severe or overall OHSS as a secondary outcome.
Moreover, we listed the protocols of reviews and title registrations on OHSS prevention in a table included in the overview. Thus we will be able to identify and add new reviews, once published, at the time of the next overview update. We excluded reviews on non‐ART cycles and reviews on ART cycles that did not report on OHSS as an outcome.
Types of participants
We included reviews that enrolled women who underwent fresh ART cycles, including those who acted as oocyte donors. We considered Cochrane systematic reviews that reported on ‘high risk’ subgroups (e.g. minimum number of follicles, minimum number of oocytes retrieved, minimum oestradiol level, women with PCOS) and those that reported on unselected populations. We excluded reviews of non‐ART cycles, such as ovulation induction or intrauterine insemination cycles.
Types of interventions
We considered for inclusion reviews on two types of interventions.
Interventions specifically aimed at prevention of OHSS for which OHSS was reported as a primary outcome.
Any interventions in ART cycles for which OHSS was reported as a secondary outcome.
Search methods for identification of reviews
We searched for reviews within the Cochrane Database of Systematic Reviews and Archie (the Cochrane information management system) for the following keywords: in vitro fertilisation (IVF), intracytoplasmic sperm injection (ICSI), ART, adverse events and OHSS (search dates 18/11/2015, 24/7/2016 and 12/12/2016). The overview 'Assisted reproductive technology: an overview of Cochrane Reviews' by Farquhar 2015 identified all current reviews on ART that reported on OHSS as a primary or secondary outcome, and we used this as complementary guidance.
Data collection and analysis
We based the methods used for data collection and analysis for this overview on Chapter 22 of the Cochrane Handbook for Systematic Reviews of Interventions (Becker 2011; Higgins 2011).
Primary outcomes
The primary outcome measure is the incidence of moderate, severe and overall OHSS per woman randomised.
The OHSS subgroups of moderate and severe are defined by the criteria set forth by Aboulghar 2003, Golan 1989, Navot 1992, Rabau 1967, Rizk 1999 and Schenker 1978, or by any other classification used in the included reviews (Appendix 1).
Secondary outcomes
Secondary outcomes studied were live birth rate, clinical pregnancy rate, miscarriage rate, multiple pregnancy rate and any reported adverse effects that derived from the interventions studied (as reported by separate reviews, e.g. side effects of medication, admission to the hospital).
Selection of reviews
Two overview authors independently selected reviews for inclusion according to the criteria stated. A third overview author acted as a referee and discussed disagreements that arose. We added the following to the overview for future overview updates: protocols of reviews and title registrations on prevention of OHSS submitted to the Cochrane Library, and reviews on ART interventions that will report on OHSS as a secondary outcome.
Data extraction and management
Two overview authors (SM and JB) performed data extraction using a Microsoft Excel spreadsheet. If data from the reviews were unclear or seemed to be missing, we contacted review authors for clarification, searched primary RCTs or contacted primary study authors for details. A third overview author (CF) acted as a referee and discussed discrepancies or disputes that arose.
We extracted and summarised the following data for the additional tables.
Population demographics: participant characteristics, definition of high‐risk groups when applicable.
Review characteristics: number of included trials, number of participants, date the review was assessed as up‐to‐date (date of search), interventions and comparisons, all primary and secondary outcomes and limitations of the review.
Timing of intervention: e.g. pretreatment selection of participants, pretreatment adjuvant therapy, stimulation phase, stimulation phase adjuvant treatment, ovulation trigger, embryo transfer phase, luteal support phase.
Statistical summary: summary effects from relevant comparisons on our primary outcome of moderate, severe or overall OHSS.
We used the same summary effect measures as were used in the original reviews, in most cases odds ratios.
Assessment of methodological quality of included reviews
Two overview authors independently assessed the quality of the evidence derived from included systematic reviews. We resolved discrepancies by discussion, and a third overview author acted as an arbiter.
Quality of evidence from primary studies in included reviews
Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method, we summarised the quality of the evidence from primary studies in the included reviews (Guyatt 2008; Schünemann 2013). We prepared 'Summary of findings' tables using GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT) for overview outcomes for each comparison by taking ratings from the original review, or we appraised the review ourselves if the review had not yet been assessed through the GRADE approach.
Risk of bias of included trials.
Directness of the evidence.
Precision of the evidence.
Heterogeneity.
Risk of publication bias.
We summarised the evidence for each of the selected clinical outcomes in a 'Summary of findings' table, to which we added the summary risk estimate and 95% confidence intervals. We allocated the quality of evidence for the clinical outcome with a score for strength of the evidence, ranging from 'high' to 'very low'.
Quality of included reviews
We assessed the methodological quality of included reviews using the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) instrument (Shea 2007). This instrument evaluates methods used in systematic reviews and the degree to which reviews are biased by comparing them on the basis of distinct criteria. Ratings used in AMSTAR include 'yes' (clearly done), 'no' (clearly not done), 'cannot answer' and 'not applicable' (Appendix 2).
Data synthesis
We undertook a narrative description of the included trials. We included an ‘Overview of reviews’ table, which shows the characteristics of included reviews. Moreover, we displayed a summary of the quality of evidence within individual reviews that was based on GRADE judgements, and we provided an AMSTAR rating for each included review.
We summarised the main results of the included systematic reviews and the effect on OHSS rates of their individual comparisons using the following framework.
Effective interventions: indicates that the review found evidence of effectiveness for an intervention.
Promising interventions (more evidence needed): indicates that the review found some evidence of effectiveness for an intervention, but more evidence is needed.
Ineffective interventions: indicates that the review found evidence of lack of effectiveness for an intervention.
Probably ineffective interventions (more evidence needed): indicates that the review found evidence suggesting lack of effectiveness for an intervention, but more evidence is needed.
No conclusions possible due to lack of evidence: indicates that the review found insufficient evidence for review authors to comment on the effectiveness of an intervention.
The choice of category to be allocated reflects the conclusions stated by authors of the individual reviews and our judgement as overview authors. We resolved disagreements by discussion.
We based our approach to summarising the evidence on the framework used for the ART overview (Farquhar 2015).
Results
Upon screening the ART overview (Farquhar 2015), we identified a total of 20 reviews reporting on OHSS as an outcome. We subsequently screened full texts for remaining reviews in the ART overview reporting on OHSS or adverse events that could include OHSS as an outcome. By doing this, we identified an additional seven reviews (see flow diagram of included reviews, Figure 1). We excluded 33 reviews from the ART overview for not reporting on OHSS. From the Cochrane Gynaecology and Fertility (CGF) Database of registered titles, we identified no titles that were expected to report on OHSS in ART cycles as an outcome. From the CGF database of submitted protocols, we identified four protocols that potentially would report on OHSS in ART cycles as an outcome (Appendix 3). Most often, we excluded titles and protocols because they did not concern ART cycles or because they concerned laboratory interventions.
1.
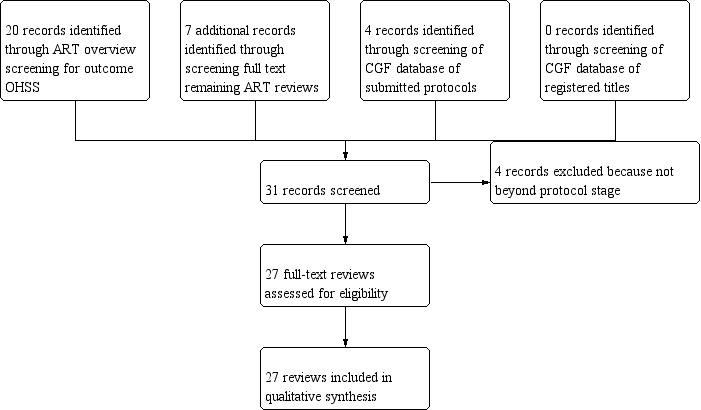
Flow diagram of included reviews.
Description of included reviews
In total, we included 27 Cochrane systematic reviews that reported on OHSS (85,497 participants). See Table 1 for a summary of the characteristics of these reviews (review title and author, numbers of randomised controlled trials and participants included, interventions and comparisons, outcomes, main limitations of each review). Of the 27 included reviews, two were empty reviews (reviews with no included studies), with last search dates in 2009 and 2011, respectively (Siristatidis 2009; Yossry 2006). We deemed two reviews to be stable, meaning that searches would be repeated only when review authors became aware of newly published evidence (D'Angelo 2007; Yossry 2006).
1. Review characteristics.
| Review ID | Number of included trials |
Population Definition of high risk for OHSS (where applicable) |
Intervention | Comparison intervention/control | Primary outcomesa | Review limitations |
| ADA563 D'Angelo 2011 Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome |
4 RCTs | 340 women with PCOS downregulated by GnRHa, undergoing superovulation in IVF or ICSI cycles High risk: women with PCOS |
Coasting when oestradiol levels were > 2500 pg/mL or > 9000 pmol/L |
Early unilateral follicular aspiration No coasting or other interventions |
OHSSa Live birtha Clinical pregnancy Number of oocytes retrieved Multiple pregnancy Miscarriage |
Comparisons based on limited trial data Live birth reported in only 1 trial Trials lacked blinding, and half the trials lacked details on allocation concealment and incomplete outcome assessment |
| ADA561 D'Angelo 2007 Embryo freezing for preventing ovarian hyperstimulation syndrome |
2 RCTs | 151 women downregulated by GnRHa, undergoing superovulation in IVF or ICSI cycles. High risk: as defined by included studies |
Cryopreservation | Fresh embryo transfer Intravenous albumin |
OHSSa Clinical pregnancya Live birth Admissions |
Evidence based on 2 trials, 1 for each comparison Live birth reported in only 1 trial Issues around methodological quality of both trials |
| TH1338 Tang 2016 Dopamine agonists for preventing ovarian hyperstimulation syndrome |
16 RCTs | 2091 women at high risk of developing OHSS undergoing ART High risk: as defined by included studies |
Cabergoline quinagolide, bromocriptine, cabergoline + albumin, cabergoline + HES |
Placebo/no treatment/other treatment: Albumin alone HES Coasting Prednisolone |
OHSSa Live birtha Clinical pregnancy Adverse effects Miscarriage Multiple pregnancy |
Allocation concealment and blinding not adequately reported. One study used a co‐intervention of albumin IV and 1 of HES Different regimens of cabergoline administration between included studies Live birth rate reported in only 2 studies Incomplete reporting of multiple pregnancy rate, adverse effects and miscarriage rate |
| PMA481 Youssef 2016b Volume expanders for prevention of OHSS |
9 RCTs | 1660 (albumin) + 487 (HES) women at high risk of developing OHSS undergoing ART cycles High risk: determined as number of follicles or oestradiol levels on day of hCG, as defined by included studies |
Human albumin Hydroxyethyl starch (HES) |
Placebo/no treatment | OHSSa Clinical pregnancy Number of oocytes retrieved Multiple pregnancy Miscarriage Live birth |
No reporting of live birth rate Limited by incomplete data reporting and lack of (details on) blinding |
| HA413 Youssef 2016 Recombinant vs urinary hCG for final oocyte maturation triggering in IVF and ICSI cycles |
18 RCTs | 2952 women undergoing ART | Recombinant hCG Recombinant LH |
Urinary hCG | OHSSa Clinical pregnancy Miscarriage Oocytes retrieved Tolerance Live birth |
Review authors combined ongoing pregnancy and live births together Only 7 trials reported on live birth Trials lacked details on allocation concealment, randomisation and blinding |
| MM1690 Youssef 2014 GnRHa vs hCG for oocyte triggering in antagonist‐assisted reproductive technology |
17 RCTs | 1847 women undergoing ART | GnRH agonist | hCG | OHSSa Live birth ratea Ongoing pregnancy Clinical pregnancy Multiple pregnancy Miscarriage rate |
Risk of bias in included studies. Limitations included premature termination, failure to clearly report methods and substantial heterogeneity Adverse events such as multiple pregnancy rate were not well reported |
| AWP1710 Pouwer 2015 Long‐acting FSH vs daily FSH for women undergoing assisted reproduction |
6 RCTs | 3753 women with subfertility | Long‐acting FSH | Daily FSH | OHSSa Live birth ratea Ongoing pregnancy rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Adverse events Satisfaction |
Limited by risk of attrition bias in some primary studies and by serious imprecision |
| LDT120 Tso 2014 Metformin treatment before and during IVF or ICSI in women with PCOS |
9 RCTs | 816 women with PCOS | Metformin | Placebo No treatment |
OHSSa Live birtha Clinical pregnancya Miscarriage Adverse events Number of oocytes retrieved Total dose FSH (IU) Number of days gonadotrophin treatment Cycle cancellation rate Serum E2 level (nmol/L) |
Half the trials were not blinded and lacked details on allocation concealment and randomisation |
| AM1335 Gibreel 2012 Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing IVF |
14 RCTs, 12 for meta‐analysis |
2536 (12 trials) Subfertile women undergoing ART |
Clomiphene citrate ± additional treatments |
Alternative treatments for COH |
OHSSa Live birth ratea Miscarriage rate Ectopic pregnancy Foetal abnormality Ongoing pregnancy rate Cancellation rate |
Live birth reported in only 5 trials Most studies suffered from suboptimal methods and information on some outcomes was insufficient |
| TA1860 Allersma 2013 Natural cycle IVF for subfertile couples |
5 RCTs | 382 subfertile women and couples undertaking IVF treatment | Natural cycle IVF Modified natural cycle IVF |
COH IVF | OHSSa Live birtha Pregnancy Ongoing pregnancy Number of oocytes retrieved Time to live birth Number of cycles required to conceive Cumulative pregnancy/live birth rate Multiple pregnancy Lack of embryos for cryopreservation Cycle cancellation Gestational abnormalities Cancellation of treatment Cost‐effectiveness |
Few studies, live birth reported in only 1 very small trial Inclusion criteria differed |
| MV263 van der Linden 2015 Luteal phase support for ART cycles |
94 RCTs | 26,198 women with any cause of subfertility undergoing ART | Progesterone hCG |
Placebo or no treatment hCG Progesterone + oestrogen Progesterone + GnRHa |
Live birtha Clinical pregnancy Ongoing pregnancy Miscarriage OHSS Multiple pregnancy |
Poor reporting of study methods and imprecision due to small sample sizes |
| HA412 Al‐Inany 2016 Gonadotrophin‐releasing hormone antagonists for ART |
73 RCTs | 12,212 women undergoing ART | GnRH antagonist | Long‐course GnRHa | OHSSa Live birtha Ongoing pregnancy Clinical pregnancy Miscarriage Cycle cancellation |
Only 12 trials reported live birth Trial methods limited by lack of blinding Poor reporting of study methods for OHSS |
| AMY731 Yossry 2006 IVF vs tubal re‐anastomosis (sterilisation reversal) for subfertility after tubal sterilisation |
No RCTs | NA | IVF | Tubal re‐anastomosis | Live birtha Clinical pregnancy Multiple pregnancy Other serious maternal morbidity, (incl OHSS) |
Empty review with no trials No longer being updated |
| ZP672 Pandian 2015 IVF for unexplained subfertility |
6 RCTs | 733 couples with unexplained subfertility | IVF | Expectant management Intrauterine insemination Intrauterine insemination + ovarian stimulation Clomiphene citrate |
Live birtha OHSS Clinical pregnancy Multiple pregnancy |
Some evidence was based on a single trial Limitations included imprecision and heterogeneity for some outcomes |
| LA541 Albuquerque 2013 Depot vs daily administration of GnRHa protocols for pituitary desensitisation in assisted reproduction cycles |
16 RCTs, 12 for meta‐analysis |
1811 couples with any cause of subfertility undergoing IVF with COH with hFSH, hMG or rFSH | Pituitary downregulation with depot administration of GnRHa | Daily administration of GnRHa | OHSSa Live birtha Clinical pregnancya Miscarriage Multiple pregnancy |
S tudy quality unclear due to poor reporting. O nly four stu dies reported live birth an d only five described adequate methods for allocation concealment . |
| IOK973 van Wely 2011 Recombinant vs urinary gonadotrophin for ovarian stimulation in ART cycles |
42 RCTs | 9606 normogonadotrophic women undergoing fresh and/or frozen thawed IVF or ICSI cycles | Recombinant FSH | Urinary FSH | OHSSa Live birtha Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects |
No difference reported in moderate/severe OHSS |
| WPM1780 Martins 2013 FSH replaced by low‐dose hCG in the late follicular phase vs continued FSH for ART |
5 RCTs | 351 women undergoing COH for ART | Low‐dose hCG instead of FSH in late follicular phase | Continued FSH in late follicular phase | OHSSa Live birtha Clinical pregnancy Miscarriage |
Small studies and low event rate Total OHSS incidence reported |
| DHH752 Smulders 2010 Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing ART |
23 RCTs | 2596 women of any age with subfertility regardless of cause, undergoing ART | Pretreatment with combined oral contraceptive pills Pretreatment with progestogens |
No pretreatment Placebo Progestogens Oestrogens |
Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects |
Only 3/23 studies reported on OHSS 2 of these 3 studies did not define how they diagnosed the condition |
| IOK972 Kwan 2014 Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) |
6 RCTs | 781 women undergoing COH in an IVF/ICSI cycle | Transvaginal ultrasonography + Oestradiol measurement | Transvaginal ultrasonography | Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects |
Only total OHSS reported, including mild OHSS |
| CMB1261 Boomsma 2012 Peri‐implantation glucocorticoid administration for ART cycles |
14 RCTs | 1879 subfertile patients undergoing IVF/ICSI, regardless of cause of infertility | Glucocorticoids in the peri‐implantation phase | No glucocorticoids in the peri‐implantation phase | Live birtha Multiple pregnancya OHSS Clinical pregnancy Miscarriage Adverse effects |
Only 2 studies, pooled total OHSS |
| VJP951 Siristatidis 2016 Aspirin for IVF |
13 RCTs | 2653 women undergoing IVF/ICSI and their partners | Aspirin | No treatment Placebo |
Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects |
Only 1 of 13 studies reported on OHSS and without exact numbers or explanation for numerators/denominators |
| CS1400 Siristatidis 2009 In vitro maturation in subfertile women with PCOS undergoing assisted reproduction |
None | 0 women with PCOS and subfertility | In vitro maturation + IVF/ICSI in women with PCOS | Conventional IVF/ICSI in women with PCOS | Live birtha OHSS Effectiveness Clinical pregnancy Miscarriage Adverse effects |
Empty review |
| IRS911 Cheong 2013 Acupuncture and ART |
20 RCTs | 4544 women undergoing ART, any type of acupuncture at any time point before, after or during ART, intended to improve ART outcome | Acupuncture of men, women or both during COH Acupuncture + ART Acupuncture alone |
No treatment Placebo Sham acupuncture Acupuncture + ART |
Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects |
No trials reported on OHSS |
| MHM931 Mochtar 2007 Recombinant luteinising hormone (rLH) for COH in assisted reproductive cycles |
14 RCTs | 2612 subfertile ovulatory women undergoing IVF or ICSI High risk: NA |
Combination of rLH and rFSH for COH in IVF/ICSI followed by ET in GnRHa and GnRH antagonist protocols | rFSH alone for COH in IVF/ICSI followed by ET in GnRHa and GnRH antagonist protocols | OHSSa Live birtha Clinical pregnancy Miscarriage |
Only 4/14 trials reported on OHSS Pooled OHSS No GRADE assessment in old version |
| KH291 Duffy 2010 Growth hormone for IVF |
10 RCTs | 440 women part of a subfertile couple undergoing IVF | Adjuvant growth hormone during conventional IVF | Conventional IVF | Live birtha OHSS Clinical pregnancy Adverse effects |
Only 4 of 10 RCTs reported on adverse events (which could include OHSS) 1 study actually mentioned OHSS (however, no cases); pooled OHSS |
| SD265 Siristatidis 2015 GnRHa protocols for pituitary suppression in assisted reproduction |
37 RCTs | 3872 women/couples with all types of infertility undergoing ART and using GnRHa for pituitary downregulation | Long protocol Long luteal protocol Short protocol Dose continued Dose continued after hCG administration Pretreatment 2 weeks |
Short protocol Ultrashort protocol Long follicular phase protocol Ultrashort protocol Dose stopped Dose reduced Dose discontinued after hCG administration Pretreatment 3 weeks |
Live birtha OHSS Clinical pregnancy Adverse effects |
Only 2 of 37 included RCTs reported on OHSS for 2 of 9 compared regimens |
| JC1630 Showell 2013 Antioxidants for female subfertility |
28 RCTs | 3548 subfertile women referred to fertility clinic who might or might not undergo ART (IVF, ICSI or IUI) | Adjuvant antioxidants in females | No treatment Placebo Another antioxidant |
Live birtha Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects (incl OHSS) |
Only 3 studies reported: 1 no data and 2 no cases |
aPrimary review outcome.
ART: artifical reproductive technology.
COH: controlled ovarian hyperstimulation.
ET: embryo transfer.
FSH: follicle‐stimulating hormone.
GnRHa: gonadotrophin‐releasing hormone agonist.
hCG: human chorionic gonadotrophin.
HES: hydroxyethyl starch.
hFSH: human follicle‐stimulating hormone.
hMG: human menopausal gonadotrophin.
ICSI: intracytoplasmic sperm injection.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
LH: luteinising hormone.
NA: not applicable.
OHSS: ovarian hyperstimulation syndrome.
PCOS: polycystic ovary syndrome.
RCT: randomised controlled trial.
rFSH: recombinant follicle‐stimulating hormone.
rLH: recombinant luteinising hormone.
Pandian 2015 (IVF for unexplained subfertility) compared IVF versus IUI (we did not formally consider IUI a treatment for inclusion in this overview). As all studies also included a IVF/ICSI comparator group, we decided that we should include this review in the overview. Also, Cheong 2013 ('Acupuncture and assisted reproductive technology') could theoretically include studies on IUI or ovulation induction; however, all current comparisons in this review involve acupuncture around the time of oocyte retrieval and/or embryo transfer, which means that the current version of the review reports only on IVF/ICSI treatments.
Reporting on OHSS
A total of 15 reviews reported on OHSS as a primary outcome, and 12 reported on OHSS as a secondary outcome. The number of included primary studies per review ranged from zero to 94. We also noted large variation in the number of included primary studies that actually reported data on OHSS, which ranged from zero to 32 studies. For example, in the review that included 94 primary studies, only one study actually reported on OHSS (van der Linden 2015).
Four reviews focused specifically on prevention of OHSS and compared the following interventions: coasting versus no/other treatment (D'Angelo 2011), embryo freezing versus fresh transfer or intravenous albumin plus fresh transfer (D'Angelo 2007), volume expanders versus placebo or no treatment (Youssef 2016b) and dopamine agonists versus placebo or no/other treatment (Tang 2016). These reviews included studies that identified high‐risk groups on the basis of oestradiol levels, a minimum number of follicles of a certain size, a minimum number of retrieved oocytes or a diagnosis of PCOS. Some primary studies excluded extremely high risk groups on the basis of oestradiol levels.
Three reviews (D'Angelo 2007; D'Angelo 2011; Tang 2016) reported separately on the subgroups 'moderate' and 'severe' OHSS, and two reported only on 'severe OHSS' (Al‐Inany 2016; Youssef 2016). The other 22 reviews were described as reporting 'total OHSS' with or without defining this as inclusion of mild, moderate or severe cases.
Timing of intervention
Timing of interventions in the included reviews differed (see Table 1) as follows.
No reviews: interventions regarding pretreatment selection of participants.
Five reviews: interventions regarding pretreatment adjuvant therapy (Duffy 2010; Showell 2013; Siristatidis 2009; Smulders 2010; Tso 2014).
One review: the pituitary downregulation phase (Albuquerque 2013).
No reviews: interventions regarding adjuvants during the stimulation phase.
11 reviews: interventions regarding the stimulation phase (Al‐Inany 2016; Allersma 2013; Cheong 2013; D'Angelo 2011; Gibreel 2012; Kwan 2014; Martins 2013; Mochtar 2007; Pouwer 2015; Siristatidis 2015; van Wely 2011).
Three reviews: the ovulation trigger phase (Tang 2016; Youssef 2014; Youssef 2016).
Three reviews: the embryo transfer phase (Boomsma 2012; D'Angelo 2007; Youssef 2016b).
One review: the luteal support phase (van der Linden 2015).
We could not classify the Yossry 2006 and Pandian 2015 reviews according to this framework because they studied IVF versus other strategies.
Main limitations of the reviews
The major and most frequent limitations of included reviews were the mere reporting of 'total OHSS', as opposed to reporting separately on the more clinically relevant subgroups 'moderate' and 'severe'; failure to include any or inclusion of only a few studies per comparison; and a generally low proportion of primary studies reporting data on OHSS.
The 12 reviews that did report on OHSS as a secondary outcome often described lack of statistical power for the outcome 'OHSS' due to the low incidence of the condition in general and more specifically in populations not selected for risk of developing OHSS. For example, given a population size of 2000 women undergoing ART, as well as a 5% margin of error and a 95% confidence interval, the required sample size would be 323 women. In light of the fact that the incidence of moderate to severe OHSS in this population would be set at 5% (range from literature 3% to 8%), at least 6460 women should be included in the study for enough women to develop OHSS that data would show differences in OHSS rates. For most countries and settings, this inclusion number is not realistically attainable for any study.
Last search date of the reviews
Table 2 shows the last search date per review. Only 17 of the 27 included reviews conducted a literature search within the past three years (to 12 December 2016), and overview authors deemed an additional two reviews (D'Angelo 2007; Yossry 2006) with an older literature search to be stable. At our third search date (12 December 2016), we became aware of four reviews that were in the process of being updated (D'Angelo 2011; Duffy 2010; Gibreel 2012; Mochtar 2007). Progress of these updates at the date of the search varied widely, from just starting the literature search to completing the final editorial phase.
2. Last search date assessment.
| Review no. | First review author | Review title | Date last assessed up to date | < 3 years since last assessed up to date or deemed stable |
| ADA561 | D'Angelo 2007 | Embryo freezing for preventing OHSS | 26/11/2010 | Stable |
| ADA 563 | D'Angelo 2011 | Coasting (withholding of gonadotrophins) for preventing OHSS | 19/07/2010 | X |
| TH1338 | Tang 2016 | Dopamine agonists for preventing OHSS | 15/08/2016 | ✔ |
| PMA481 | Youssef 2016b | Volume expanders for prevention of OHSS | 21/09/2016 | ✔ |
| HA413 | Youssef 2016 | Recombinant vs urinary hCG for final oocyte maturation triggering in IVF and ICSI cycles | 23/04/2015 | ✔ |
| MM1690 | Youssef 2014 | GnRHa vs hCG for oocyte triggering in antagonist‐assisted reproductive technology | 08/09/2014 | X |
| LDT1201 | Tso 2014 | Metformin treatment before and during IVF or ICSI in women with PCOS | 15/10/2014 | ✔ |
| AWP1710 | Pouwer 2015 | Long‐acting FSH vs daily FSH for women undergoing assisted reproduction | 8/06/2015 | ✔ |
| AM1335 | Gibreel 2012 | Clomiphene citrate in combination with gonadotrophins for controlled ovarian stimulation in women undergoing IVF | 23/03/2012 | X |
| TA1860 | Allersma 2013 | Natural cycle IVF for subfertile couples | 5/03/2013 | ✔ |
| MV263 | van der Linden2015 | Luteal phase support for ART cycles | 25/11/2014 | ✔ |
| HA412 | Al‐Inany 2016 | Gonadotrophin‐releasing hormone antagonists for ART | 28/04/2016 | ✔ |
| AMY731 | Yossry 2006 | IVF vs tubal re‐anastomosis (sterilisation reversal) for subfertility after tubal sterilisation | 15/05/2009 | Empty, stable |
| ZP672 | Pandian 2015 | IVF for unexplained subfertility | 4/05/2015 | ✔ |
| LA541 | Albuquerque 2013 | Depot vs daily administration of GnRHa protocols for pituitary desensitisation in assisted reproduction cycles | 3/07/2012 | ✔ |
| IOK973 | van Wely 2011 | Recombinant vs urinary gonadotrophin for ovarian stimulation in ART cycles | 20/10/2010 | X |
| WPM1780 | Martins 2013 | FSH replaced by low‐dose hCG in late follicular phase vs continued FSH for ART | 5/02/2013 | ✔ |
| DHH752 | Smulders 2010 | Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing ART | 16/11/2008 | X |
| IOK972 | Kwan 2014 | Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) | 30/05/2014 | ✔ |
| CMB1261 | Boomsma 2012 | Peri‐implantation glucocorticoid administration for ART cycles | 20/09/2011 | X |
| VJP951 | Siristatidis 2016 | Aspirin for IVF | 9/05/2016 | ✔ |
| CS1400 | Siristatidis 2009 | In vitro maturation in subfertile women with PCOS undergoing assisted reproduction | 17/02/2011 | Empty |
| IRS911 | Cheong 2013 | Acupuncture and ART | 22/07/2013 | ✔ |
| MHM931 | Mochtar 2007 | Recombinant luteinising hormone (rLH) for COH in assisted reproductive cycles | 25/01/2007 | X |
| KH291 | Duffy 2010 | Growth hormone for IVF | 20/07/2009 | X |
| SD265 | Siristatidis 2015 | GnRHa protocols for pituitary suppression in assisted reproduction | 23/04/2015 | ✔ |
| JC1630 | Showell 2013 | Antioxidants for female subfertility | 15/04/2014 | ✔ |
ART: artifical reproductive technology.
COH: controlled ovarian hyperstimulation.
FSH: follicle‐stimulating hormone.
GnRHa: gonadotrophin‐releasing hormone agonist.
hCG: human chorionic gonadotrophin.
ICSI: intracytoplasmic sperm injection.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
OHSS: ovarian hyperstimulation syndrome.
PCOS: polycystic ovary syndrome.
rLH: recombinant luteinising hormone.
✔ under 3 years since last assessed as up to date
X over 3 years since last assessed as up to date
Statistical summary
Quality of evidence from primary studies in included reviews
The quality of the evidence reported by primary studies in the included reviews assessed by the GRADE approach ranged from very low to high for individual comparisons. The main reasons for downgrading of reviews for quality included inadequate reporting of allocation concealment and randomisation methods, lack of blinding and imprecision. Eleven of the 27 reviews included fewer than 10 primary studies.
Methodological quality of included reviews
Quality of systematic reviews
We rated the quality of the included reviews using the AMSTAR tool (Shea 2007) and listed the domains per review in Table 3.
3. AMSTAR assessment per review.
| Review no. | First review author + year | Review title | AMSTAR criteria | |||||||||
| Prespecified question and inclusion criteria | Duplicate study selection and data extraction | Comprehensive literature search | Grey literature included | Lists included and excluded studies | Describes characteristics of included studies | Study quality assessed | Studies combined using appropriate methods | Likelihood of publication bias considered/tested | Potential for conflict of interest addressed | |||
| ADA561 | D'Angelo 2007 | Embryo freezing for preventing ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| ADA 563 | D'Angelo 2011 | Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| TH1338 | Tang 2016 | Dopamine agonists for preventing ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| PMA481 | Youssef 2016b | Volume expanders for the prevention of ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| HA413 | Youssef 2016 | Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| MM1690 | Youssef 2014 | Gonadotropin‐releasing hormone agonist versus hCG for oocyte triggering in antagonist‐assisted reproductive technology | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| LDT1201 | Tso 2014 | Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| AWP1710 | Pouwer 2015 | Long‐acting FSH versus daily FSH for women undergoing assisted reproduction | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| AM1335 | Gibreel 2012 | Clomiphene citrate in combination with gonadotrophins for controlled ovarian stimulation in women undergoing in vitro fertilisation | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| TA1860 | Allersma 2013 | Natural cycle IVF for subfertile couples | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| MV263 | van der Linden 2015 | Luteal phase support for ART cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| HA412 | Al‐Inany 2016 | Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| AMY731 | Yossry 2006 | In vitro fertilisation versus tubal re‐anastomosis (sterilisation reversal) for subfertility after tubal sterilisation | ✔ | ✔ | ✔ | ✔ | ✔ | NA | NA | NA | NA | ✔ |
| ZP672 | Pandian 2015 | In vitro fertilisation for unexplained subfertility | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| LA541 | Albuquerque 2013 | Depot versus daily administration of gonadotrophin‐releasing hormone agonist protocols for pituitary desensitisation in assisted reproduction cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| IOK973 | van Wely 2011 | Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| WPM1780 | Martins 2013 | FSH replaced by low‐dose hCG in the late follicular phase versus continued FSH for assisted reproductive techniques | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| DHH752 | Smulders 2010 | Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| IOK972 | Kwan 2014 | Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| CMB1261 | Boomsma 2012 | Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| VJP951 | Siristatidis 2016 | Aspirin for in vitro fertilisation | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| CS1400 | Siristatidis 2009 | In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction | ✔ | ✔ | ✔ | ✔ | ✔ | NA | NA | NA | NA | ✔ |
| IRS911 | Cheong 2013 | Acupuncture and assisted reproductive technology | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| MHM931 | Mochtar 2007 | Recombinant luteinising hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | X | ✔ |
| KH291 | Duffy 2010 | Growth hormone for in vitro fertilisation | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | X | ✔ |
| SD265 | Siristatidis 2015 | Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| JC1630 | Showell 2013 | Antioxidants for female subfertility | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
Search date: 24/07/2016.
ART: artifical reproductive technology.
FSH: follicle‐stimulating hormone.
hCG: human chorionic gonadotrophin.
ICSI: intracytoplasmic sperm injection.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
NA: not applicable.
rLH: recombinant luteinising hormone.
All reviews had prespecified their clinical question and inclusion criteria.
All reviews conducted study selection and data extraction in duplicate.
All reviews conducted a comprehensive literature search.
All reviews included searches of grey literature.
All reviews listed included and excluded studies.
All reviews described the characteristics of included studies.
All reviews assessed study quality.
All reviews combined studies using appropriate methods.
A total of 25/27 reviews addressed the risk of reporting bias by using a statistical test when appropriate.
All reviews addressed the potential for conflict of interest.
Effect of interventions
We categorised all included intervention reviews by effectiveness for reduction of OHSS and by effectiveness for the primary pregnancy outcome stated in the review. In total, with regard to reduction of OHSS rates, seven reviews showed a beneficial effect of the intervention on the incidence of OHSS, one was promising,13 were ineffective and six remained inconclusive. Of the effective interventions, one intervention did reduce OHSS rates but had a detrimental effect on pregnancy outcomes (Youssef 2014).
We listed effects of the interventions on the incidence of OHSS in the 'Summary of findings' table (Table 4). Most reviews did not report the incidence of OHSS as the (sole) primary outcome. This implies that the effectiveness of studied interventions can very well be different for the main primary outcome and for reduction of OHSS. Among the 25 non‐empty reviews, the effect of the intervention on OHSS rates was beneficial in eight reviews, and the intervention had no effect on OHSS rates in 14 reviews. One review reported that the control group had lower OHSS rates than the intervention group; however, the only primary study in this review reporting on OHSS did not provide exact numbers, so we could not calculate the effect size (Siristatidis 2016). For three reviews, we could not calculate effect size because they included insufficient primary studies reporting on OHSS (Cheong 2013; Duffy 2010; Showell 2013).
4. Summary of findings for OHSS: per review and/or per intervention.
|
Review title and comparison intervention/control |
Assumed risk with comparator |
Corresponding risk with intervention |
Relative effect (95% CI) |
Number of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| D'Angelo 2007 | Embryo freezing for preventing ovarian hyperstimulation syndrome (Embryo freezing vs fresh transfer) |
Overall OHSS: 60 per 1000 | Overall OHSS: 125 per 1000 (62 to 240) |
OR 1.12 (0.01 to 2.29) |
125 (1 study) |
Low | Imprecision, number of events < 300 Evidence based on a single open‐label study with insufficient methodological details provided |
| D'Angelo 2007 | Embryo freezing for preventing ovarian hyperstimulation syndrome (Embryo freezing vs intravenous albumin) |
Moderate or severe OHSS: 77 per 1000 |
Moderate or severe OHSS: 308 per 1000 (41 to 824) |
OR 5.33 (0.51 to 56.24) |
26 (1 study) |
Very low | Imprecision, number of events < 300 Evidence based on a single open‐label trial |
| D'Angelo 2011 | Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome | Moderate or severe OHSS: 265 per 1000 |
Moderate or severe OHSS: 58 per 1000 (11 to 241) |
OR 0.17 (0.03 to 0.88) |
68 (1 study) |
Very low | Imprecision, number of events < 300 Evidence based on a single conference abstract Insufficient methodological details provided |
| Tang 2016 | Dopamine agonists for preventing ovarian hyperstimulation syndrome | Moderate or severe OHSS: 286 per 1000 | Moderate or severe OHSS: 97 per 1000 (71 to 135) |
OR 0.27 (0.19 to 0.39) |
2091 (16 studies)) |
Moderate | Imprecision, number of events < 300 Lack of details for allocation concealment and blinding, selective reporting |
| Youssef 2016b | Volume expanders for the prevention of ovarian hyperstimulation syndrome (human albumin vs placebo/no treatment) |
Moderate or severe OHSS: 122 per 1000 |
Moderate or severe OHSS: 85 per 1000 (61 to 177) | OR 0,67 (0.47 to 0.95) |
1452 (7 studies) |
Very low | Imprecision, number of events < 300 Lack of details on allocation concealment and selective reporting |
| Youssef 2016b | Volume expanders for the prevention of ovarian hyperstimulation syndrome (HES vs placebo) |
Moderate or severe OHSS: 164 per 1000 |
Moderate or severe OHSS: 50 per 1000 (23 to 104) |
OR 0.27 (0.12 to 0.59 |
272 (2 studies) |
Very low | Imprecision, number of events < 300 Lack of details on allocation concealment and selective reporting |
| Youssef 2016b | Volume expanders for the prevention of ovarian hyperstimulation syndrome (mannitol vs placebo) |
Moderate or severe OHSS: 517 per 1000 |
Moderate or severe OHSS: 289 per 1000 (191 to 407) |
OR 0.38 (0.22 to 0.64) |
226 (1 study) |
Low | Imprecision, number of events < 300 Lack of details on allocation concealment and selective reporting |
| Youssef 2016 | Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles (r‐hCG vs u‐hCG) | Overall OHSS: 27 per 1000 | Overall OHSS:40 per 1000 (15 to 102) |
OR 0.39 (0.25 to 0.61) |
374 (3 studies) |
Moderate | Imprecision, number of events < 300 One of the trials lacked methodological details on randomisation, allocation concealment and blinding |
| Youssef 2016 | Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles (r‐LH vs u‐hCG) | Overall OHSS: 10 per 1000 | Overall OHSS: 17 per 1000 (11 to 84) |
OR 1.76 (0.37 to 8.45) |
417 (3 studies) |
Low | Imprecision, number of events < 300 One of the trials lacked adequate methodological details |
| Youssef 2014 | Gonadotropin‐releasing hormone agonist versus hCG for oocyte triggering in antagonist‐assisted reproductive technology | Overall OHSS: 5 per 1000 | Overall OHSS: 1 per 1000 (0 to 2) |
OR 0.15 (0.05 to 0.47) |
989 (9 studies) |
Moderate | Imprecision, number of events < 300 All studies at high risk of bias in 1 or more domains None clearly reported blinded outcome assessment |
| Tso 2014 | Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. | Overall OHSS: 270 per 1000 | Overall OHSS: 97 per 1000 (62 to 153) |
OR 0.29 (0.18 to 0.49) |
798 (8 studies) |
Moderate | Imprecision, number of events < 300 |
| Pouwer 2015 | Long‐acting FSH versus daily FSH for women undergoing assisted reproduction (low dose) |
Overall OHSS: 47 per 1000 | Overall OHSS: 57 per 1000 (26 to 125) |
RR 1.22 (0.56 to 2.66) |
645 (3 studies) |
Moderate | Imprecision, number of events < 300 |
| Pouwer 2015 | Long‐acting FSH versus daily FSH for women undergoing assisted reproduction (medium dose) |
Overall OHSS: 63 per 1000 | Overall OHSS: 60 per 1000 (45 to 85) | RR 0.96 (0.68 to 1.35) |
3075 (5 studies) |
Low | Imprecision, number of events < 300 Confidence intervals compatible with clinically meaningful benefit in either arm or with no effect, plus high risk of attrition bias in 2 studies |
| Pouwer 2015 | Long‐acting FSH versus daily FSH for women undergoing assisted reproduction (high dose) |
Overall OHSS: 0 per 1000 | Overall OHSS: 0 per 1000 (0 to 0) |
RR 1.73 (0.09 to 32.75) |
33 (1 study) |
Very low | Imprecision, number of events < 300 High risk of attrition bias |
| Gibreel 2012 | Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation (clomiphene + gonadotropins vs gonadotropins) |
Overall OHSS: 50 per 1000 | Overall OHSS:12 per 1000 (5 to 27) |
OR 0.23 (0.1 to 0.52) |
1559 (5 studies) |
Low | Imprecision, number of events < 300 Very wide 95% confidence interval crossing the threshold points of appreciable benefit or harm, which is 25% |
| Allersma 2013 | Natural cycle IVF for subfertile couples (natural cycle vs conventional IVF) |
Overall OHSS: 67 per 1000 | Overall OHSS: 13 per 1000 (1 to 393) |
OR 0.10 (0.01 to 4.06) |
60 (1 study) |
Very low | Imprecision, number of events < 300 Only 1 study reporting on OHSS Allocation concealment method not reported |
| van der Linden 2015 | Luteal phase support for ART cycles (hCG versus placebo/no treatment) |
Overall OHSS: 41 per 1000 | Overall OHSS: 155 per 100 (76 to 292) |
OR 4.28 (1.191 to 9.6) |
387 (1 study) |
Low | Imprecision, number of events < 300 Poor reporting of study methods |
| van der Linden 2015 | Luteal phase support for ART cycles (progesterone vs hCG regimens) |
Overall OHSS: 126 per 1000 | Overall OHSS: 72 per 1000 (31 to 162) |
OR 0.54 (0.22 to 1.34) |
615 (4 studies) |
Low | Imprecision, number of events < 300 Poor reporting of study methods |
| van der Linden 2015 | Luteal phase support for ART cycles (progesterone + GnRH agonist) |
Overall OHSS: 50 per 1000 | Overall OHSS: 50 per 1000 (17 to 137) |
OR 1.00 (0.33 to 3.01) |
300 (1 study) |
Very low | Imprecision, number of events < 300 Poor reporting of study methods |
| van der Linden 2015 | Luteal phase support for ART cycles (progesterone vs progesterone + oestrogens) |
Overall OHSS: 39 per 1000 | Overall OHSS: 22 per 1000 (8 to 62) |
OR 0.56 (0.2 to 1.63) |
461 (2 studies) |
Low | Imprecision, number of events < 300 Poor reporting of study methods |
| Al‐Inany 2016 | Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology (GnRH antagonist vs GnRH agonist) |
Overall OHSS: 114 per 1000 | Overall OHSS: 73 per 1000 (62 to 85) |
OR 0.61 (0.51 to 0.72) | 7944 (36 studies) |
Moderate | Methodological limitations including poor allocation concealment and lack of blinding |
| Yossry 2006 | In vitro fertilisation versus tubal reanastomosis (sterilisation reversal) for subfertility after tubal sterilisation (IVF vs tubal reanastomosis) |
NA | NA | NA | NA | NA | Empty review |
| Pandian 2015 | In vitro fertilisation for unexplained subfertility (IVF vs IUI + gonadotropins/clomiphene citrate) |
Overall OHSS: 58 per 1000 | Overall OHSS: 66 per 1000 (26 to 158) |
OR 1.15 (0.43 to 3.06) | 324 (2 studies) |
Low | Imprecision, number of events < 300 Only 2 studies on OHSS reported |
| Albuquerque 2013 | Depot versus daily administration of gonadotrophin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles (depot vs daily gonadotropins) |
Overall OHSS: 3 per 100 | Overall OHSS: 2 per 100 (1 to 6) | OR 0.84 (0.29 to 2.42) | 570 (5 studies) |
Low | Most studies were classified as at unclear risk of bias for all domains Imprecision, number of events < 300 Studies were insufficient to assess publication bias |
| van Wely 2011 | Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rFSH vs HMG/HMG‐HP) |
Overall OHSS: 17 per 1000 | Overall OHSS: 17 per 1000 (10 to 28) |
OR 1.00 (0.58 to 1.71) |
3197 (11 studies) |
High | Imprecision, number of events < 300 |
| van Wely 2011 | Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rFSH vs FSH‐P) |
Overall OHSS: 28 per 1000 | Overall OHSS:49 per 1000 (25 to 95) |
OR 1.79 (0.89 to 3.62) |
1490 (6 studies) |
Higha | Imprecision, number of events < 300 |
| van Wely 2011 | Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rFSH vs FSH‐HP) |
Overall OHSS: 28 per 1000 | Overall OHSS: 31 per 1000 (20 to 48) |
OR 1.11 (0.70 vs 1.75) |
3053 (14 studies) |
Higha | Two additional trials excluded in sensitivity analyses because it was unclear if data were reported according to ITT analysis (those were included for "Overall OHSS") |
| van Wely 2011 | Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rec‐hCG vs u‐hCG) |
Overall OHSS: 19 per 1000 | Overall OHSS: 22 per 1000 (16 to 30) |
OR 1.18 (0.86 vs 1.61) | 7740 (32 studies) |
Higha | Imprecision number of events < 300 |
| Martins 2013 | FSH replaced by low‐dose hCG in the late follicular phase versus continued FSH for assisted reproductive techniques (low‐dose hCG vs FSH in late follicular phase) |
Overall OHSS: 3 per 100 | Overall OHSS: 1 per 100 (0 to 4) |
OR 0.30 (0.06 to 1.59) | 351 (5 studies) |
Very low | Imprecision, number of events < 300 Inconsistency, high risk of bias |
| Smulders 2010 | Oral contraceptive pill, progestogen or oestrogen pre‐ treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques (OAC plus antagonist vs antagonist) |
Overall OHSS: 17 per 1000 | Overall OHSS: 25 per 1000 (5 to 133) |
OR 1.5 (0.26 to 8.8) | 234 (1 study) |
Very low | Single study reporting on OHSS Imprecision, number of events < 300 Wide confidence intervals that cross line of no effect High risk of attrition bias |
| Smulders 2010 | Oral contraceptive pill, progestogen or oestrogen pre‐ treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques (OAC plus antagonist vs agonist) |
Overall OHSS: 55 per 1000 | Overall OHSS: 35 per 1000 (12 to 100) |
OR 0.63 (0.21 to 1.92) |
290 (2 studies) | Very low | Imprecision, number of events < 300 One study at high risk of attrition bias |
| Kwan 2014 | Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) (transvaginal ultrasound + estradiol vs transvaginal ultrasound) |
Overall OHSS: 36 per 1000 | Overall OHSS: 36 per 1000 (18 to 75) |
OR 1.03 (0.48 to 2.20) |
781 (6 studies) |
Low | Imprecision, number of events < 300 with wide confidence intervals Methods of randomisation inadequately described in 3 of 6 trials, allocation concealment inadequately described in all 6 trials and blinding inadequately described in 5 of 6 trials No definition of OHSS provided by authors of these 6 studies |
| Boomsma 2012 | Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles (adjuvant glucocorticoids vs no glucocorticoids) |
Overall OHSS: 194 per 1000 | Overall OHSS: 159 per 1000 (64 to 392) |
OR 0.82 (0.33 to 2.02) | 151 (2 studies) |
Low | Imprecision, number of events < 300 |
| Siristatidis 2016 | Aspirin for in vitro fertilisation (aspirin vs no treatment/placebo) |
NA | NA | NA | NA | NA | Only 1 study reported on OHSS; no exact numbers or explanation of numerators/denominators given |
| Siristatidis 2009 | In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction (IVM vs conventional IVF) |
NA | NA | NA | NA | NA | Empty review |
| Cheong 2013 | Acupuncture and assisted reproductive technology (acupuncture vs no acupuncture/sham acupuncture) |
NA | NA | NA | NA | NA | No studies reported on OHSS |
| Mochtar 2007 | Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles (combined rLH + FSH vs FSH ) |
Overall OHSS: 20 per 1000 | Overall OHSS: 27 per 1000 (12 to 59) |
OR 1.34 (0.58 to 3.09) |
986 (7 studies) |
Low | Imprecision, number of events < 300 Some methodological details unclear |
| Duffy 2010 | Growth hormone for in vitro fertilization (growth hormone vs no treatment/placebo) |
NA | NA | NA | NA | NA | Only 1 study reported on OHSS; however, no cases of OHSS were reported |
| Siristatidis 2015 | Gonadotropin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction (different protocols vs other protocol) |
Overall OHSS: 20 per 1000 | Overall OHSS: 27 per 1000 (12 to 59) |
OR 1.34 (0.58 to 3.09) |
986 (7 studies) |
Low | Imprecision, number of events < 300 Some methodological details unclear |
| Showell 2013 | Antioxidants for female subfertility (antioxidants vs no treatment/placebo/other antioxidant) |
NA | NA | NA | NA | NA | Although 3 studies reported on OHSS, no numbers were given, so effect size could not be calculated |
aReview authors GRADED these outcomes as 'high quality'; however, the total event rate < 300 would justify downgrading for this to moderate‐quality evidence.
ART: artifical reproductive technology.
FSH: follicle‐stimulating hormone.
FSH‐HP: highly purified FSH.
hCG: human chorionic gonadotrophin.
HES: hydroxyethyl starch.
ICSI: intracytoplasmic sperm injection.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
IVM: in vitro maturation.
NA: not applicable.
OAC: oral anticoagulant.
OHSS: ovarian hyperstimulation syndrome.
OR: odds ratio.
rFSH: recombinant follicle‐stimulating hormone.
r‐hCG: recombinant human chorionic gonadotrophin.
rLH: recombinant luteinising hormone.
Total: any grade of OHSS.
u‐hCG: urinary human chorionic gonadotrophin.
We summarise here the effectiveness of interventions for both reduction of OHSS and pregnancy outcomes, ranked by timing of the intervention within an ART cycle.
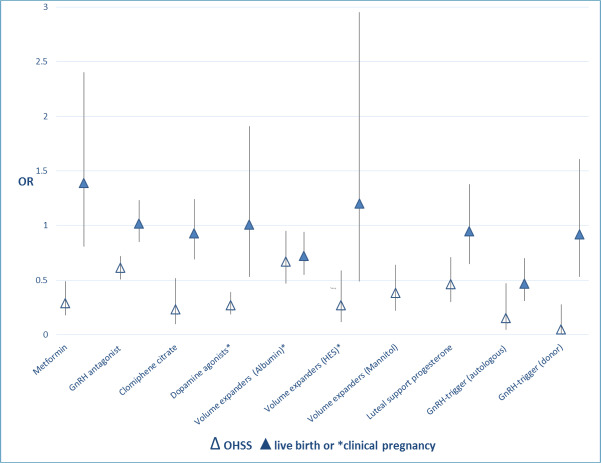
Moreover, for interventions that had a beneficial effect on OHSS rate, Figure 2 shows the extent of this effect in relation to the effect on live birth rate (when reported) or clinical pregnancy.
2.
Extent of effect of interventions on OHSS rate and live birth rate (when reported) or clinical pregnancy: OR and 95% CI.
Effective interventions for reduction of OHSS with no impact on nor improvement in pregnancy outcomes
Pretreatment adjuvant therapy
Metformin treatment before and during IVF or ICSI in women with PCOS: No conclusive evidence suggests that metformin treatment before or during ART cycles improved live birth rates (low‐quality evidence). However, use of this insulin‐sensitising agent increased clinical pregnancy rates and decreased the risk of OHSS (moderate‐quality evidence) (Tso 2014). Evidence showed a beneficial effect of the intervention on OHSS rates.
Pituitary downregulation phase
Gonadotrophin‐releasing hormone (GnRH) antagonists for ART: Use of antagonists compared with long GnRHa protocols was associated with a large reduction in OHSS, and no evidence suggested a difference in live birth rates (moderate‐quality evidence) (Al‐Inany 2016). Evidence showed a beneficial effect of the intervention on OHSS rates.
Stimulation phase
Clomiphene citrate for controlled ovarian stimulation in women undergoing IVF: This review suggested that regimens with clomiphene could be used in controlled ovarian stimulation for IVF treatment without a reduction in pregnancy rates. However, further evidence is required before these regimens can be recommended with confidence as alternatives to gonadotrophins alone in GnRH long or short protocols (low‐quality evidence) (Gibreel 2012). Evidence showed a beneficial effect of the intervention on OHSS rates.
Ovulation trigger phase
Dopamine agonists for preventing OHSS: Dopamine agonists appeared to reduce the risk of OHSS in high‐risk women, especially for moderate OHSS. Use of dopamine agonists did not appear to affect clinical pregnancy rates or miscarriage rates, nor did they increase the risk of other adverse events (moderate‐quality evidence) (Tang 2016). Evidence showed a beneficial effect of the intervention on moderate or severe OHSS rates.
Volume expanders for prevention of OHSS: The volume expanders hydroxyethyl starch and mannitol decreased the incidence of moderate or severe OHSS without affecting pregnancy rates (very low‐quality evidence) (Youssef 2016b). Evidence showed a beneficial effect of the intervention on moderate or severe OHSS rates.
Luteal support phase
Luteal support phase in ART cycles: This review concluded that progesterone appears to provide the best method of providing luteal phase support, as it is associated with higher rates of live birth or ongoing pregnancy than placebo, and lower rates of OHSS than hCG. Addition of one or more doses of GnRH agonists to progesterone was associated with higher live birth and ongoing pregnancy rates than progesterone alone. Overall, addition of other substances such as oestrogen or hCG did not seem to improve outcomes, and hCG was associated with higher risk of OHSS. The route of progesterone administration did not seem to matter (quality of evidence was low for most comparisons) (van der Linden 2015). Evidence showed a beneficial effect of the intervention on OHSS rates for the comparison hCG versus placebo/no treatment. For the other comparisons, no evidence showed an effect on OHSS rates.
Effective interventions for reduction of OHSS with negative impact on pregnancy outcomes
Ovulation trigger phase
GnRHa versus hCG for oocyte triggering in antagonist ART cycles: Evidence suggested a lower live birth rate, a reduced ongoing pregnancy rate and a higher miscarriage rate among women who received a GnRHa. However, OHSS rates were reduced with GnRHa triggering; therefore, clinicians should consider the tradeoff between benefits and harms (moderate‐quality evidence) (Youssef 2014). Evidence showed a beneficial effect of the intervention on OHSS rates.
Volume expanders for prevention of OHSS: Evidence suggested that human albumin decreased the incidence of moderate or severe OHSS. However, contrary to the (very low‐quality) evidence found with hydroxyethyl starch (HES) and mannitol, human albumin appeared to have a detrimental effect on pregnancy rates (moderate‐quality evidence) (Youssef 2016b).
Promising interventions for reduction of OHSS with no impact on or improvement in pregnancy outcomes (more evidence needed)
Ovulation trigger phase
Coasting (withholding gonadotrophins) for preventing OHSS: Evidence was insufficient to show benefit derived from coasting done to prevent OHSS compared with no coasting or other interventions (very low‐quality evidence) (D'Angelo 2011). Evidence showed a beneficial effect of the intervention on OHSS rates, but this was reported only in a single abstract on an RCT that provided insufficient methodological details.
Ineffective interventions for reduction of OHSS with no impact on or improvement in pregnancy outcomes
Pretreatment adjuvant therapy
Oral contraceptive pill (OCP), progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing ARTs: Evidence suggested improved pregnancy outcomes with progestogen pretreatment and poorer pregnancy outcomes with combined OCP pretreatment (Smulders 2010). No evidence showed an effect of the intervention on OHSS rates.
Pituitary downregulation phase
GnRHa protocols for pituitary suppression in ART cycles: The pregnancy rate was higher when GnRHa was used in a long protocol as compared with a short or ultra‐short protocol (low‐quality evidence) (Siristatidis 2015). No evidence showed an effect of the intervention on OHSS rates.
Depot versus daily administration of GnRHa protocols for pituitary desensitisation in assisted reproduction cycles: No evidence suggested a significant difference in live birth or pregnancy outcomes between depot and daily GnRHa use for pituitary downregulation in IVF cycles using the long protocol, but substantial differences could not be ruled out (moderate‐quality evidence) (Albuquerque 2013). No evidence showed an effect of the intervention on OHSS rates.
Stimulation phase
FSH replaced by low‐dose hCG in the late follicular phase versus FSH alone for ARTs: Review authors were very uncertain about effects on live birth, OHSS and miscarriage, but evidence suggested that this intervention did not reduce the chances of ongoing and clinical pregnancy, and that it was likely to result in retrieval of an equivalent number of oocytes with less FSH expended (very low‐quality evidence) (Martins 2013). No evidence showed an effect of the intervention on OHSS rate.
Recombinant versus urinary gonadotrophin for ovarian stimulation in ART cycles: It appeared that all available gonadotrophins were equally effective and safe. The choice of one or the other product would depend upon the availability of the product, the convenience of its use and associated costs. Any specific differences are likely to be too small to justify further research (high‐quality evidence) (van Wely 2011). No evidence showed an effect of the intervention on OHSS rates.
Long‐acting FSH versus daily FSH for women undergoing assisted reproduction: A medium dose (150 to 180 μg) of long‐acting FSH appeared to offer a safe treatment option that was as effective as daily FSH in women with unexplained subfertility. Evidence showed a reduced live birth rate among women receiving a low dose (60 to 120 μg) of long‐acting FSH compared with daily FSH (moderate‐quality evidence) (Pouwer 2015). No evidence showed an effect of the intervention on OHSS rates.
Natural cycle IVF for subfertile couples: No evidence showed a significant difference between natural cycle and standard IVF for outcomes including live birth, OHSS, clinical pregnancy and multiple pregnancy (very low‐quality evidence) (Allersma 2013). No evidence showed an effect of the intervention on OHSS rates.
Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI): RCTs provided no evidence to support cycle monitoring by ultrasonography plus serum oestradiol as more efficacious than cycle monitoring by ultrasonography only for the outcomes of live birth and pregnancy. A large well‐designed RCT is needed (low‐quality evidence) (Kwan 2014). No evidence showed an effect of the intervention on OHSS rates.
Ovulation trigger phase
Recombinant versus urinary hCG for final oocyte maturation triggering in IVF and ICSI cycles: Review authors concluded that urinary hCG remains the best choice for final oocyte maturation triggering in IVF and ICSI treatment cycles owing to availability and cost and no difference in live birth rates (moderate‐quality evidence) (Youssef 2016). No evidence showed an effect of the intervention on OHSS rates.
Embryo transfer phase
Peri‐implantation glucocorticoid administration for ART cycles: Overall, no clear evidence suggests that administration of peri‐implantation glucocorticoids in ART cycles significantly improved clinical outcomes (low‐quality evidence) (Boomsma 2012). No evidence showed an effect of the intervention on OHSS rates.
Embryo freezing for prevention of OHSS: Evidence was insufficient to show benefit for routine cryopreservation and the relative merits of intravenous albumin versus cryopreservation (low‐quality evidence) (D'Angelo 2007). No evidence showed an effect of the intervention on OHSS rates.
Luteal support phase
Recombinant luteinising hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles: No evidence suggested that coadministration of rLH and recombinant follicle‐stimulating hormone (rFSH) in GnRHa‐downregulated women resulted in more live births than were reported with controlled ovarian hyperstimulation (COH) with rFSH alone. Nevertheless, all pooled pregnancy estimates, although not significantly different, pointed towards a beneficial effect of cotreatment with rLH, in particular with respect to pregnancy loss (low‐quality evidence) (Mochtar 2007). No evidence showed an effect of the intervention on OHSS rates.
ART versus other interventions
In vitro fertilisation for unexplained subfertility: IVF may be more effective than IUI plus ovarian stimulation (low‐quality evidence) (Pandian 2015). No evidence showed an effect of the intervention on OHSS rates.
Possibly ineffective interventions for reduction of OHSS with no impact on or improvement in pregnancy outcomes (more evidence needed)
None were reported.
No conclusions possible on effectiveness for reduction of OHSS (lack of evidence)
For six reviews, review authors could provide no conclusions on the effects of interventions on OHSS rates.
Pretreatment adjuvant therapy
Aspirin for IVF: Evidence from adequately powered RCTs was insufficient for review authors to reach a conclusion (Siristatidis 2016). No evidence showed an effect of the intervention on OHSS rates. No numbers were provided, so we could not calculate effect size.
Antioxidants for female subfertility: Antioxidants were not associated with increased live birth or clinical pregnancy rates, although more evidence is needed (low‐quality evidence) (Showell 2013). No trials reported actual numbers of cases of OHSS.
Acupuncture and ART: No evidence suggested benefit for acupuncture in improving live birth or clinical pregnancy rates in assisted conception (low‐quality evidence) (Cheong 2013). No trials reported on OHSS.
Stimulation phase
Growth hormone for IVF: We could not calculate the effect of the intervention on OHSS rates. Use of growth hormone in poor responders was associated with significant improvement in live birth rates (moderate‐quality evidence) (Duffy 2010).
In vitro maturation in subfertile women with polycystic ovarian syndrome (PCOS) undergoing assisted reproduction: This is an empty review (Siristatidis 2009).
ART versus other interventions
IVF versus tubal reanastomosis (sterilisation reversal) for subfertility after tubal sterilisation: This is an empty review (Yossry 2006).
Discussion
Summary of main results
We found seven interventions that were effective in reducing the occurrence of ovarian hyperstimulation syndrome (OHSS) while not influencing or even improving pregnancy outcomes: metformin pretreatment in women with polycystic ovary syndrome (PCOS), use of a gonadotrophin‐releasing hormone (GnRH) antagonist protocol for pituitary suppression, use of clomiphene citrate for controlled ovarian stimulation, use of dopamine agonists and the volume expanders hydroxyethyl starch (HES) and mannitol around the time of oocyte triggering, coasting before oocyte triggering and use of progesterone for luteal phase support (Al‐Inany 2016; D'Angelo 2011; Gibreel 2012; Tang 2016; Tso 2014; van der Linden 2015; Youssef 2016b).
Two additional interventions ‐ gonadotrophin‐releasing agonist (GnRHa) versus human chorionic gonadotrophin (hCG) for oocyte triggering, and use of the volume expander human albumin around the time of ovulation triggering ‐ proved effective in reducing OHSS but negatively impacted pregnancy outcomes in autologous cycles (Youssef 2014; Youssef 2016b).
Concerning the GnRHa trigger, this detrimental effect on pregnancy outcomes was not found in oocyte donation cycles. This would make the use of GnRHa for ovulation triggering suitable for oocyte donation programmes, as it would largely eradicate the chance of OHSS in the donor, without negatively influencing pregnancy outcomes in the recipient. GnRHa could also be useful in preventing OHSS in "freeze‐all" programmes (i.e. embryo transfer is not performed in the fresh autologous cycle) ‐ a regimen that is currently the topic of numerous research projects and new randomised controlled trials (RCTs).
Overall completeness and applicability of evidence
This overview of Cochrane reviews is complete and up‐to‐date as of December 2016. Only four reviews specifically addressed prevention of OHSS; by reporting all reviews on assisted reproduction technology (ART) that include OHSS as a primary or secondary outcome, we aimed to provide the most up‐to‐date overview of strategies to prevent OHSS currently included within the Cochrane Library. In keeping with the nature of a Cochrane overview, this body of work does not cover non‐Cochrane reviews on OHSS. Moreover, alternative or emerging strategies for prevention of OHSS may not yet have been covered in a Cochrane review and therefore cannot be found in this overview, for example, use of calcium gluconate infusion or the dual GnRHa and hCG trigger. Once such strategies have been assessed in new reviews, we can and will update this overview accordingly.
Clinically relevant moderate OHSS and severe OHSS are still rare conditions; moreover, inclusion of 'high risk' groups was not based on a variety of criteria and was not a prerequisite at all for many of the included reviews. In combination with the fact that most studies included OHSS as a secondary outcome, this means that most primary studies lacked statistical power to report on OHSS. However, as OHSS is an undesirable outcome in ART, it is unlikely that the prevalence will change, and it is considered unethical by many institutional ethics review boards to randomise very high‐risk groups as a control in current and future studies. Unfortunately, this means that it will be difficult to nearly impossible for researchers to perform well‐powered studies that address these shortcomings.
A major limitation of many of the included reviews is reporting of 'total OHSS' only. A total of three reviews reported separately on the subgroups 'moderate' and 'severe' OHSS. Three additional reviews reported only 'severe' OHSS; the remaining 21 reviews described that they reported on total 'OHSS' and provided no further explanation (e.g. this could have included moderate + severe, mild + moderate + severe, severe only). As almost all hyperstimulation cycles for in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) are accompanied by some form of discomfort and ovarian enlargement, the subgroup 'mild OHSS' does not seem to represent a very significant clinical outcome (for a description of subgroups, see Appendix 1). Reporting only on the total incidence of OHSS with no further specification of cases per subgroup biases the comparability of studies and actual reporting on clinically significant OHSS rates. For example, some studies could have found mainly, or only, mild cases of OHSS, whereas other studies might not have even included or assessed mild cases and would have reported only moderate and/or severe cases of OHSS. This would mean that the former study would overestimate the number of OHSS cases for which the latter finding is more precise. Also, a study that reports only on hospitalised cases might underestimate moderate cases, which we consider clinically significant too. Moreover, reviews reporting on interventions that aim to reduce the incidence of OHSS included only 'high risk' populations, whereas most of the other reviews did not select their population specifically for this criterion, which makes it difficult to compare the effectiveness of different interventions within this overview. This heterogeneous method of reporting fails to acknowledge the incidence of moderate and severe cases, resulting in an evidence gap for this important adverse outcome of ART cycles.
Currently, many ART clinics apply preventive strategies such as natural cycle/mild stimulation IVF or cryopreservation of all embryos (the 'freeze‐all' approach) that have not been proven to have a beneficial effect on OHSS on the basis of low‐quality evidence (Allersma 2013; D'Angelo 2007; D'Angelo 2011). However, theoretical considerations suggest that these strategies are probably effective, as they eliminate certain steps of an ART cycle (preventing multiple follicle growth and implantation, thereby preventing an hCG surge); additional RCTs are needed to provide a robust evidence base for these practices.
Quality of the evidence
All included systematic reviews were prepared according to Cochrane guidelines and were of high quality in most respects, although only 17 of 27 had conducted a literature search within the past three years. Moreover, the included primary studies might be significantly older than the review publication date, thus sometimes reflecting outdated clinical practice or stimulation regimens. This cannot be avoided in an overview, as we summarise available evidence from existing reviews, and sometimes few or no recent RCTs are available.
This also has an impact on the quality of the evidence reported by primary studies in the included reviews. Using GRADE methods, we rated this quality level as very low to high. The main reasons for downgrading the quality of evidence included bias in the primary studies (inadequate reporting of allocation concealment and randomisation methods, lack of blinding) and imprecision. Evidence was frequently restricted to that provided by only a few included trials per comparison. Because clinically relevant OHSS is still a relatively rare outcome in ART cycles, and given that the primary study size and the number of studies per comparison have been limited for most reviews, the event rate of reported OHSS will remain low, as mild OHSS frequently is not reported. This implies that the quality of the evidence should be downgraded by one level (according to GRADE rules for downgrading dichotomous outcomes by one level for imprecision), and that the event rate < 300 and the total cumulative sample size were lower than the calculated optimal information size (OIS) (Schünemann 2013). As a result, the quality of the evidence on effectiveness of interventions for the outcome of OHSS remains 'very low' or 'low' for most interventions and comparisons.
Potential biases in the overview process
We identified no biases during the overview process.
Agreements and disagreements with other studies or reviews
Over the years, as new evidence from RCTs continues to emerge, a steady stream of publications aims to provide a comprehensive overview on the pathophysiology, prevention and treatment of OHSS. For example, in 2016 alone, Guo 2016 and Kwik 2016 were published, and recently, the American Society for Reproductive Medicine provided practice guidelines (ASRM 2016). In addition to this, an abundance of reviews have examined particular interventions covered in this overview, such as use of dopamine agonists (Baumgarten 2013; Kalampokas 2013; Kasum 2015; Leitao 2014).
Such publications encompass, for example, regional or national clinical practice guidelines or an overview of the literature. However, most also include data derived from retrospective and population‐based longitudinal studies or non‐randomised controlled trials. To our knowledge, this overview is the first to include solely systematic reviews on ART conducted according to rigorous Cochrane standards, thus showing high methodological quality. Moreover, we included not only reviews of interventions directly aiming to prevent OHSS, but also reviews of other ART interventions reporting on OHSS as an adverse effect, thus presenting a more complete overview of the literature currently available in the Cochrane Library.
Authors' conclusions
This overview provides the most up‐to‐date evidence on prevention of OHSS in ART cycles from all currently published Cochrane reviews on ART. Clinicians can use the evidence summarised in this overview to choose the best treatment regimen for individual patients: a regimen that not only reduces the chance of developing OHSS but does not compromise other outcomes such as pregnancy or live birth rate. Furthermore, policymakers can use this overview when developing local and regional protocols or guidelines, and investigators can use it to identify knowledge gaps for future research.
Implications for practice.
Evidence of at least moderate quality shows that clinicians should consider the following interventions to reduce OHSS rates in ART cycles.
Metformin treatment before and during an ART cycle for women with PCOS (moderate‐quality evidence) (Tso 2014).
Dopamine agonists around the time of hCG administration or oocyte pickup in ART cycles (moderate‐quality evidence) (Tang 2016).
GnRH antagonist protocol in ART cycles (moderate‐quality evidence) (Al‐Inany 2016).
GnRHa trigger in donor oocyte or 'freeze‐all' programmes, as it reduces OHSS and leads to lower pregnancy rates when embryo transfer is performed in the same cycle (moderate‐quality evidence) (Youssef 2014).
All of the above mentioned interventions are preventive measures used to reduce OHSS rates.
Evidence of low or very low quality indicates that clinicians can consider the following interventions to reduce OHSS rates in ART cycles.
Clomiphene citrate for controlled ovarian stimulation in ART cycles (low‐quality evidence) (Gibreel 2012).
Intravenous fluids (plasma expanders) around the time of hCG administration or oocyte pickup in ART cycles (very low‐quality evidence) (Youssef 2016b).
Progesterone for luteal phase support in ART cycles (low‐quality evidence) (van der Linden 2015).
Among the interventions mentioned above, clomiphene for ovarian stimulation and progesterone for luteal support are preventive measures used to reduce OHSS rates; dopamine agonists and plasma expanders are considered treatments for women with OHSS.
On the basis of this overview, we must conclude that evidence is currently insufficient to support the widespread practice of embryo cryopreservation and coasting (withholding of gonadotrophins) for reduction of OHSS (D'Angelo 2007; D'Angelo 2011).
Implications for research.
This overview clearly identifies ways in which current evidence on effectiveness of interventions for prevention of OHSS is lacking. First, it highlights the need for review authors to update existing ART reviews to decrease knowledge gaps on this topic. Second, it should motivate clinicians and researchers to generate larger RCTs of higher quality to perform comparisons of new and existing interventions intended to reduce the incidence of OHSS.
The following three interventions have been shown to reduce OHSS on the basis of low‐quality or very low‐quality evidence. The fourth intervention listed here has shown a promising effect on reduction of OHSS and should be prioritised for examination by researchers in new high‐quality RCTs.
Clomiphene citrate for controlled ovarian stimulation in ART cycles (low‐quality evidence) (Gibreel 2012).
Intravenous fluids (plasma expanders) around the time of hCG administration or oocyte pickup in ART cycles (very low‐quality evidence) (Youssef 2016b).
Progesterone for luteal phase support in ART cycles (low‐quality evidence) (van der Linden 2015).
Coasting (withholding gonadotrophins) before hCG triggering in ART cycles (very low‐quality evidence) (D'Angelo 2011).
The uptake of subgroups 'moderate' and 'severe' OHSS in outcome reporting would be very useful for future studies and reviews, as these categories are the most clinically significant subgroups of OHSS. Reporting these subgroups separately provides clinicians and policymakers with a far more balanced reflection of treatment risks than is provided by mere use of the outcome 'total OHSS', which also includes mild OHSS and might not be as clinically important. Furthermore, clinicians would benefit if new RCTs would distinguish early and late types of OHSS, as the time of development of OHSS could influence the choice of therapy.
Large, well‐conducted RCTs are urgently needed to support the current evidence base for interventions described in this overview, including dose‐finding studies and research to determine the optimal timing of interventions. These trials should first examine interventions that seem to be effective but for which only very low‐quality, low‐quality or moderate‐quality evidence is available (Table 4).
Acknowledgements
Managing editor of the CGF Group Helen Nagels for helping with identification of registered titles and submitted protocols, and editor Jane Marjoribanks for reviewing and editing the protocol.
Appendices
Appendix 1. Clinical classification of ovarian hyperstimulation syndrome (OHSS) (from Aboulghar and Mansour 2003)
| Study | Mild | Moderate | Severe | |
| Rabau et al (1967) | Grade 1: oestrogen > 150 µg and pregnanediol > 10 mg/24 h Grade 2: + enlarged ovaries and possibly palpable cysts Grades 1 and 2 were not included under the title of mild OHSS |
Grade 3: grade 2 + confirmed palpable cysts and distended abdomen Grade 4: grade 3 + vomiting and possibly diarrhoea |
Grade 5: grade 4 + ascites and possibly hydrothorax | Grade 6: grade 5 + changes in blood volume, viscosity and coagulation, time |
| Schenker and Weinstein (1978) | Grade 1: oestrogen > 150 µg/24 h and pregnanediol > 10 mg/24 h Grade 2: grade 1+ enlarged ovaries, sometimes small cysts |
Grade 3: grade 2 + abdominal distension Grade 4: grade 3 + nausea, vomiting and/or diarrhoea |
Grade 5: grade 4 + large ovarian cysts, ascites and/or hydrothorax | Grade 6: marked haemoconcentration + increased blood viscosity and possibly coagulation abnormalities |
| Golan et al (1989) | Grade 1: abdominal distension and discomfort Grade 2: grade 1 + nausea, vomiting and/or diarrhoea, enlarged ovaries 5‐12 cm |
Grade 3: grade 2 + ultrasound evidence of ascites | Grade 4: grade 3 + clinical evidence of ascites and/or hydrothorax and breathing difficulties | Grade 5: grade 4 + haemoconcentration, increased blood viscosity, coagulation abnormality and diminished renal perfusion |
| Navot et al (1992) | Severe OHSS: variable enlarged ovary; massive ascites ± hydrothorax; Hct > 45%; WBC > 15 000; oliguria; creatinine 1.0‐1.5; creatinine clearance ≥ 50 mL/min; liver dysfunction; anasarca | Critical OHSS: variable enlarged ovary; tense ascites ± hydrothorax; Hct > 55%; WBC > 25 000; oliguria; creatinine > 1.6; creatinine clearance < 50 mL/min; renal failure; thromboembolic phenomena; ARDS | ||
| Rizk and Aboulghar (1999) | Discomfort, pain, nausea, distension, ultrasonic evidence of ascites and enlarged ovaries, normal haematological and biological profiles | Grade A: dyspnoea, oliguria, nausea, vomiting, diarrhoea, abdominal pain, clinical evidence of ascites, marked distension of abdomen or hydrothorax, US showing large ovaries and marked ascites, normal biochemical profile Grade B: grade A plus massive tension ascites, markedly enlarged ovaries, severe dyspnoea and marked oliguria, increased hematocrit, elevated serum creatinine and liver dysfunction |
Grade C: complications such as respiratory distress syndrome, renal shut‐down or venous thrombosis |
Footnotes
ARDS: acute respiratory distress syndrome. Hct: hematocrit. OHSS: ovarian hyperstimulation syndrome. US: ultrasonography. WBC: white blood cell count.
Appendix 2. AMSTAR ratings
| 1. | Was an ’a priori’ design provided? (Yes: the research question and inclusion criteria were established before conducting the review.) |
| 2. | Was there duplicate study selection and data extraction? (Yes: at least two people working independently extracted the data and the method was reported for reaching consensus if disagreements arose.) |
| 3. | Was a comprehensive literature search performed? (Yes: at least two electronic sources were searched; details of the databases, years searched and search strategy were provided; the search was supplemented by searching reference lists of included studies and specialised registers, and by contacting experts.) |
| 4. | Was status of publication used as an exclusion criterion? (Yes: the authors stated that they excluded studies from the review based on publication status. No: authors searched for reports irrespective of publication type. They did not exclude reports based on publication from the systematic review.) |
| 5. | Was a list of studies (included and excluded provided)? (Yes: a list was provided.) |
| 6. | Were the characteristics of the included studies provided? (Yes: data on participants, interventions and outcomes were provided, and the range of relevant characteristics reported.) |
| 7. | Was the scientific quality of the included studies assessed and reported? (Yes: predetermined methods of assessing quality were reported.) |
| 8. | Was the scientific quality of the included studies used appropriately in formulating conclusions? (Yes: the quality, and limitations, of included studies were used in the analysis, conclusions and recommendations of the review.) |
| 9. | Were the methods used to combine the findings of studies appropriate? (Yes: if results were pooled statistically, heterogeneity was assessed and used to inform the decision of the statistical model to be used. If heterogeneity was present, the appropriateness of combining studies was considered by review authors.) |
| 10. | Was the likelihood of publication bias assessed? (Yes: publication bias was explicitly considered and assessed.) |
| 11. | Was the conflict of interest stated? (Yes: sources of support were clearly acknowledged.) |
Footnotes
Appendix 3. ART protocols and titles for potential future inclusion
(date of search 24 July 2016)
No titles were registered that were expected to potentially list OHSS as an outcome.
Four registered protocols, upon title screening, were judged as potentially reporting on OHSS as an outcome. When these reviews are published as a full review, they can be assessed for the future update of this overview.
| Review registration number | Lead review author | Review title |
| IDG1973 | Gallos | Controlled ovarian stimulation protocols for assisted reproduction: a network meta‐analysis |
| MGS1974 | Showell | Inositol for subfertile women with polycystic ovary syndrome |
| SHJ 881 | Jaafar | Long‐term GnRH agonist therapy before in vitro fertilization (IVF) for improving fertility outcomes in women with endometriosis |
| LC1971 | Craciunas | Oxytocin antagonists for assisted reproduction |
Contributions of authors
SM drafted first versions of the protocol and overview manuscripts. All three overview authors (SM, JB, CF) contributed to preparation of the protocol, data extraction and analysis of reviews for this overview. JB and CF contributed to the definitive version of the manuscript.
Sources of support
Internal sources
-
Department of Obstetrics and Gynaecology, University of Auckland, New Zealand.
This department provided infrastructure support.
External sources
None, Other.
Declarations of interest
All three overview authors (SM, JB,CF) were co‐review authors on several of the included reviews. CF is a director/shareholder of a small day stay surgical unit and gynaecology clinic and undertakes private practice within these facilities. She has received travel/accommodation/meeting expenses from ESHRE or ASRM for attendance at scientific meetings. She does not receive any industry or commercial payments for research or travel. SM and JB report no conflicts of interest regarding industry.
New
References
References to included reviews
Al‐Inany 2016
- Al‐Inany HG, Youssef MAFM, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD001750.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albuquerque 2013
- Albuquerque LET, Tso LO, Saconato H, Albuquerque MCRM, Macedo CR. Depot versus daily administration of gonadotrophin‐releasing hormone agonist protocols for pituitary down regulation in assisted reproduction cycles. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD002808.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allersma 2013
- Allersma T, Farquhar C, Cantineau AEP. Natural cycle in vitro fertilisation (IVF) for subfertile couples. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010550.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boomsma 2012
- Boomsma CM, Keay SD, Macklon NS. Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005996.pub3] [DOI] [PubMed] [Google Scholar]
Cheong 2013
- Cheong YC, Dix S, Hung Yu Ng E, Ledger WL, Farquhar C. Acupuncture and assisted reproductive technology. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Angelo 2007
- D'Angelo A, Amso NN. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002806.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Angelo 2011
- D'Angelo A, Brown J, Amso NN. Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD002811.pub3] [DOI] [PubMed] [Google Scholar]
Duffy 2010
- Duffy JMN, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000099.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibreel 2012
- Gibreel A, Maheshwari A, Bhattacharya S. Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilization. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008528.pub2] [DOI] [PubMed] [Google Scholar]
Kwan 2014
- Kwan I, Bhattacharya S, Kang A, Woolner A. Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI). Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD005289.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martins 2013
- Martins WP, Vieira ADD, Figueiredo JBP, Nastri CO. FSH replaced by low‐dose hCG in the late follicular phase versus continued FSH for assisted reproductive techniques. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010042.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mochtar 2007
- Mochtar MH, Veen F, Ziech M, Wely M, Musters A. Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005070.pub2] [DOI] [PubMed] [Google Scholar]
Pandian 2015
- Pandian Z, Gibreel A, Bhattacharya S. In vitro fertilisation for unexplained subfertility. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD003357.pub3] [DOI] [PubMed] [Google Scholar]
Pouwer 2015
- Pouwer AW, Farquhar C, Kremer JAM. Long‐acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD009577.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Showell 2013
- Showell MG, Brown J, Clarke J, Hart RJ. Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD007807.pub2] [DOI] [PubMed] [Google Scholar]
Siristatidis 2009
- Siristatidis CS, Maheshwari A, Bhattacharya S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006606.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siristatidis 2015
- Siristatidis Charalampos S, Gibreel A, Basios G, Maheshwari A, Bhattacharya S. Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2015, issue 11. [DOI: 10.1002/14651858.CD006919.pub4; CD006919] [DOI] [PMC free article] [PubMed]
Siristatidis 2016
- Siristatidis CS, Basios G, Pergialiotis V, Vogiatzi P. Aspirin for in vitro fertilisation. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD004832.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smulders 2010
- Smulders B, Oirschot SM, Farquhar C, Rombauts L, Kremer JAM. Oral contraceptive pill, progestogen or estrogen pre‐treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006109.pub2] [DOI] [PubMed] [Google Scholar]
Tang 2016
- Tang H, Mourad S, Zhai SD, Hart RJ. Dopamine agonists for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD008605.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tso 2014
- Tso LO, Costello MF, Albuquerque LET, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD006105.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Linden 2015
- Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD009154.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Wely 2011
- Wely M, Kwan I, Burt AL, Thomas J, Vail A, Veen F, Al‐Inany HG. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD005354.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yossry 2006
- Yossry M, Aboulghar M, D'Angelo A, Gillett W. In vitro fertilisation versus tubal reanastomosis (sterilisation reversal) for subfertility after tubal sterilisation. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004144.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Youssef 2014
- Youssef MAFM, Veen F, Al‐Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, et al. Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist‐assisted reproductive technology. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008046.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Youssef 2016
- Youssef MA, Abou‐Setta AM, Lam WS. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD003719.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Youssef 2016b
- Youssef MA, Mourad S. Volume expanders for the prevention of ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD001302.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Abou‐Setta 2014
- Abou‐Setta AM, Peters LR, D'Angelo A, Sallam HN, Hart RJ, Al‐Inany HG. Post‐embryo transfer interventions for assisted reproduction technology cycles. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD006567.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akhtar 2013
- Akhtar M, Sur S, Raine‐Fenning N, Jayaprakasan K, Thornton JG, Quenby S. Heparin for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009452.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2010
- Anderson K, Norman RJ, Middleton P. Preconception lifestyle advice for people with subfertility. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008189.pub2] [DOI] [PubMed] [Google Scholar]
Armstrong 2015
- Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011320.pub2] [DOI] [PubMed] [Google Scholar]
Benschop 2010
- Benschop L, Farquhar C, Poel N, Heineman MJ. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008571.pub2] [DOI] [PubMed] [Google Scholar]
Bontekoe 2012
- Bontekoe S, Mantikou E, Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD008950.pub2] [DOI] [PubMed] [Google Scholar]
Bontekoe 2014
- Bontekoe S, Heineman MJ, Johnson N, Blake D. Adherence compounds in embryo transfer media for assisted reproductive technologies. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD007421.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2010
- Brown J, Buckingham K, Abou‐Setta AM, Buckett W. Ultrasound versus 'clinical touch' for catheter guidance during embryo transfer in women. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006107.pub3] [DOI] [PubMed] [Google Scholar]
Carney 2012
- Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on assisted conception (in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI)). Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD001894.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derks 2009
- Derks RS, Farquhar C, Mol BWJ, Buckingham K, Heineman MJ. Techniques for preparation prior to embryo transfer. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007682.pub2] [DOI] [PubMed] [Google Scholar]
Ghobara 2008
- Ghobara T, Vanderkerchove P. Cycle regimens for frozen‐thawed embryo transfer. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003414.pub2] [DOI] [PubMed] [Google Scholar]
Glujovsky 2010
- Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006359.pub2] [DOI] [PubMed] [Google Scholar]
Glujovsky 2012
- Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002118.pub4] [DOI] [PubMed] [Google Scholar]
Glujovsky 2014
- Glujovsky D, Riestra B, Sueldo C, Fiszbajn G, Repping S, Nodar F, et al. Vitrification versus slow freezing for women undergoing oocyte cryopreservation. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010047.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunby 2004
- Gunby JL, Daya S, Olive D, Brown J. Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004378.pub2] [DOI] [PubMed] [Google Scholar]
Gutarra‐Vilchez 2014
- Gutarra‐Vilchez RB, Urrútia G, Glujovsky D, Coscia A, Bonfill Cosp X. Vasodilators for women undergoing fertility treatment. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD010001.pub2] [DOI] [PubMed] [Google Scholar]
Huang 2013
- Huang Z, Li J, Wang L, Yan J, Shi Y, Li S. Brief co‐incubation of sperm and oocytes for in vitro fertilization techniques. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD009391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2010
- Johnson N, Voorst S, Sowter MC, Strandell A, Mol BWJ. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002125.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroon 2012
- Kroon B, Hart RJ, Wong BMS, Ford E, Yazdani A. Antibiotics prior to embryo transfer in ART. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008995.pub2] [DOI] [PubMed] [Google Scholar]
Kwan 2013
- Kwan I, Bhattacharya S, Knox F, McNeil A. Pain relief for women undergoing oocyte retrieval for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004829.pub3] [DOI] [PubMed] [Google Scholar]
Maheshwari 2011
- Maheshwari A, Gibreel A, Siristatidis CS, Bhattacharya S. Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD006919.pub3] [DOI] [PubMed] [Google Scholar]
McDonnell 2014
- McDonnell R, Marjoribanks J, Hart RJ. Ovarian cyst aspiration prior to in vitro fertilization treatment for subfertility. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD005999.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDowell 2014
- McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD010461.pub2] [DOI] [PubMed] [Google Scholar]
Nastri 2015
- Nastri CO, Lensen SF, Gibreel A, Raine‐Fenning N, Ferriani RA, Bhattacharya S, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009517] [DOI] [PubMed] [Google Scholar]
Pandian 2010
- Pandian Z, McTavish AR, Aucott L, Hamilton MPR, Bhattacharya S. Interventions for 'poor responders' to controlled ovarian hyperstimulation (COH) in in‐vitro fertilisation (IVF). Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD004379.pub3] [DOI] [PubMed] [Google Scholar]
Pandian 2013
- Pandian Z, Marjoribanks J, Ozturk O, Serour G, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra‐cytoplasmic sperm injection. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003416.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2008
- Proctor M, Johnson N, Peperstraten AM, Phillipson G. Techniques for surgical retrieval of sperm prior to intra‐cytoplasmic sperm injection (ICSI) for azoospermia. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002807.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sallam 2006
- Sallam HN, Garcia‐Velasco JA, Dias S, Arici A, Abou‐Setta AM. Long‐term pituitary down‐regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004635.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Showell 2014
- Showell MG, Mackenzie‐Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD007411.pub3] [DOI] [PubMed] [Google Scholar]
Teixeira 2013
- Teixeira DM, Barbosa MAP, Ferriani RA, Navarro PA, Raine‐Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra‐high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010167.pub2] [DOI] [PubMed] [Google Scholar]
Twisk 2006
- Twisk M, Mastenbroek S, Wely M, Heineman MJ, Veen F, Repping S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005291.pub2] [DOI] [PubMed] [Google Scholar]
Van Rumste 2003
- Rumste MME, Evers JLH, Farquhar C. Intra‐cytoplasmic sperm injection versus conventional techniques for oocyte insemination during in vitro fertilisation in couples with non‐male subfertility. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD001301] [DOI] [PubMed] [Google Scholar]
Wongtra‐ngan 2010
- Wongtra‐ngan S, Vutyavanich T, Brown J. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD004634.pub2] [DOI] [PubMed] [Google Scholar]
Additional references
Aboulghar 2003
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update 2003;9(3):275‐89. [DOI] [PubMed] [Google Scholar]
Abramov 1999
- Abramov Y, Elchalal U, Schenker JG. Severe OHSS: an 'epidemic' of severe OHSS: a price we have to pay?. Human Reproduction 1999;14(9):2181‐3. [DOI] [PubMed] [Google Scholar]
Agrawal 1999
- Agrawal R, Tan SL, Wild S, Sladkevicius P, Engmann L, Payne N, et al. Serum vascular endothelial growth factor concentrations in in vitro fertilization cycles predict the risk of ovarian hyperstimulation syndrome. Fertility and Sterility 1999;71(2):287–93. [DOI] [PubMed] [Google Scholar]
ASRM 2016
- Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertility and Sterility 2016;106(7):1634‐47. [DOI] [PubMed] [Google Scholar]
Baumgarten 2013
- Baumgarten M, Polanski L, Campbell B, Raine‐Fenning N. Do dopamine agonists prevent or reduce the severity of ovarian hyperstimulation syndrome in women undergoing assisted reproduction? A systematic review and meta‐analysis. Human Fertility (Cambridge) 2013;16(3):168‐74. [DOI] [PubMed] [Google Scholar]
Becker 2011
- Becker LA, Oxman AD. Chapter 22: Overviews of reviews. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Braat 2006
- Braat D, Bernardus R, Gerris J. Anonymous reports of lethal cases of ovarian hyperstimulation syndrome. In: Gerris J, Olivennes F, Delvigne A editor(s). Ovarian Hyperstimulation Syndrome. London: Informa UK Limited, 2006:59‐66. [Google Scholar]
De Geyter 2015
- Geyter C, Fehr P, Moffat R, Gruber IM, Wolff M. Twenty years' experience with the Swiss data registry for assisted reproductive medicine: outcomes, key trends and recommendations for improved practice. Swiss Medical Weekly 2015;145:w14087. [DOI: 10.4414/smw.2015.14087] [DOI] [PubMed] [Google Scholar]
Delbaere 2004
- Delbaere A, Smits G, Olatunbosun O, Pierson R, Vassart G, Costagliola S. New insights into the pathophysiology of ovarian hyperstimulation syndrome. What makes the difference between spontaneous and iatrogenic syndrome?. Human Reproduction 2004;19(3):486‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delbaere 2005
- Delbaere A, Smits G, Leener A, Costagliola S, Vassart G. Understanding ovarian hyperstimulation syndrome. Endocrine 2005;26(3):285‐90. [DOI] [PubMed] [Google Scholar]
Delvigne 2002
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update 2002;8(6):559‐77. [DOI] [PubMed] [Google Scholar]
Farquhar 2015
- Farquhar C, Rishworth JR, Brown J, Nelen WLDM, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD010537.pub4] [DOI] [PubMed] [Google Scholar]
Golan 1989
- Golan A, Ron‐El R, Herman A, Soffer Y, Wainraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstetrical and Gynecological Survey 1989;44(6):430‐40. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime Inc.). www.gradepro.org.
Guo 2016
- Guo JL, Zhang DD, Zhao Y, Zhang D, Zhang XM, Zhou CQ, et al. Pharmacologic interventions in preventing ovarian hyperstimulation syndrome: a systematic review and network meta‐analysis. Scientific Reports 2016;11(6):19093.doi:10.1038/srep19093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. Rating quality of evidence and strength of recommendations: what is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kalampokas 2013
- Kalampokas T, Creatsas G, Kalampokas E. Cabergoline as treatment of ovarian hyperstimulation syndrome: a review. Gynecological Endocrinology 2013;29(2):98‐100. [DOI] [PubMed] [Google Scholar]
Kasum 2015
- Kasum M, Vrcic H, Stanic P, Jezek D, Oreskovic S, Beketic‐Oreskovic L, et al. Dopamine agonists in prevention of ovarian hyperstimulation syndrome. Gynecological Endocrinology 2014;30(12):845‐9. [DOI] [PubMed] [Google Scholar]
Kol 2011
- Kol S. Ovarian hyperstimulation syndrome prevention. Global Library of Women's Medicine, 2011. [DOI: 10.3843/GLOWM.10466; ISSN:1756‐2228] [DOI]
Kupka 2014
- Kupka MS, Ferraretti AP, Mouzon J, Erb K, D'Hooghe T, Castilla JA, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Human Reproduction 2014;29(10):2099‐113. [DOI] [PubMed] [Google Scholar]
Kwik 2016
- Kwik M, Maxwell E. Pathophysiology, treatment and prevention of ovarian hyperstimulation syndrome. Current Opinion in Obstetrics and Gynecology 2016;28(4):236‐41. [DOI] [PubMed] [Google Scholar]
Leitao 2014
- Leitao VM, Moroni RM, Seko LM, Nastri Co, Martins WP. Cabergoline for the prevention of ovarian hyperstimulation syndrome: systematic review and meta‐analysis of randomized controlled trials. Fertility and Sterility 2014;101(3):664‐75. [DOI] [PubMed] [Google Scholar]
Mathur 2000
- Mathur RS, Akande AV, Keay SD, Hunt LP, Jenkins JM. Distinction between early and late ovarian hyperstimulation syndrome. Fertility and Sterility 2000;73(5):901‐7. [DOI] [PubMed] [Google Scholar]
Navot 1992
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertility and Sterility 1992;58(2):249‐61. [DOI] [PubMed] [Google Scholar]
Rabau 1967
- Rabau E, Serr DM, David A, Maschiac S, Lunenfield B. Human menopausal gonadotropins for anovulation and sterility. American Journal of Obstetrics and Gynaecology 1967;98:92‐8. [DOI] [PubMed] [Google Scholar]
Rizk 1999
- Rizk B, Aboulghar MA. Classification, pathophysiology and management of ovarian hyperstimulation syndrome. In: Brinsden P editor(s). In‐Vitro Fertilization and Assisted Reproduction. New York, London: The Parthenon Publishing Group, 1999:131‐55. [Google Scholar]
Schenker 1978
- Schenker JG, Weinstein D. Ovarian hyperstimulation syndrome: a current survey. Fertility and Sterility 1978;30(3):255‐68. [DOI] [PubMed] [Google Scholar]
Schenker 1994
- Schenker JG, Ezra Y. Complications of assisted reproductive techniques. Fertility and Sterility 1994;61(3):411‐22. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated March 2013. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed 15 November 2015).
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1997
- Stewart JA, Hamilton PJ, Murdoch AP. Thromboembolic disease associated with ovarian stimulation and assisted conception techniques. Human Reproduction 1997;12(10):2167‐73. [DOI] [PubMed] [Google Scholar]