Abstract
Background
In soldiers with posttraumatic stress disorder, symptom provocation was found to induce increased connectivity within the salience network, as measured by functional magnetic resonance imaging and global brain connectivity with global signal regression (GBCr). However, it is unknown whether these GBCr disturbances would normalize following effective posttraumatic stress disorder treatment.
Methods
Sixty-nine US Army soldiers with (n = 42) and without posttraumatic stress disorder (n = 27) completed functional magnetic resonance imaging at rest and during symptom provocation using subject-specific script imagery. Then, participants with posttraumatic stress disorder received six weeks (12 sessions) of group cognitive processing therapy or present-centered therapy. At week 8, all participants repeated the functional magnetic resonance imaging scans. The primary analysis used a region-of-interest approach to determine the effect of treatment on salience GBCr. A secondary analysis was conducted to explore the pattern of GBCr alterations posttreatment in posttraumatic stress disorder participants compared to controls.
Results
Over the treatment period, present-centered therapy significantly reduced salience GBCr (p = .02). Compared to controls, salience GBCr was high pretreatment (present-centered therapy, p = .01; cognitive processing therapy, p = .03) and normalized post-present-centered therapy (p = .53) but not postcognitive processing therapy (p = .006). Whole-brain secondary analysis found high GBCr within the central executive network in posttraumatic stress disorder participants compared to controls. Post hoc exploratory analyses showed significant increases in executive GBCr following cognitive processing therapy treatment (p = .01).
Conclusion
The results support previous models relating cognitive processing therapy to central executive network and enhanced cognitive control while unraveling a previously unknown neurobiological mechanism of present-centered therapy treatment, demonstrating treatment-specific reduction in salience connectivity during trauma recollection.
Keywords: posttraumatic stress disorder, posttraumatic stress disorder, functional connectivity, functional magnetic resonance imaging, symptom provocation, salience network
Introduction
Advances in neuroimaging and connectomics have led to a shift in the clinical neuroscience field from an early focus on brain regions and localization to identifying neural circuits, and more recently, to establishing network functioning in health and disease.1 The investigation of neural correlates of posttraumatic stress disorder (PTSD) largely followed a comparable path. Early neuroimaging PTSD studies identified a number of regions of interest (ROIs), which were then integrated into circuitry-related hypotheses and more recently into network-based models.2–6 These network models suggested an association between PTSD and increased salience network but reduced default mode and central executive network connectivity.2,3,6 However, these models were primarily based on findings from seed analyses of resting-state functional connectivity magnetic resonance imaging (fcMRI) data in cross-sectional studies. Unfortunately, the seed-based approach does not fully interrogate the brain’s large-scale intrinsic connectivity networks (ICNs).7 In addition, the resting-state data may not necessarily generalize to functioning during provoked symptoms or other tasks.8 Moreover, the cross-sectional investigations are, by design, limited to association evidence without the ability to ascertain the network changes over the course of the illness. These limitations could be partially mitigated by employing graph-based measures and task fcMRI in longitudinal studies. Using a graph-based measure named global brain connectivity with global signal regression (GBCr), the current report complements previous literature by conducting a longitudinal fcMRI investigation at rest and during symptom provocation in active duty US Army soldiers with and without PTSD. The participants with PTSD were scanned pre- and postrandomized treatment with group cognitive processing therapy (CPT) or present-centered therapy (PCT).
Nodal strength (also known as nodal degree) is the amount of connections between a node and the nodes of the rest of the network. It is a fundamental measure in a graph-based network, as the majority of other network topology measures are ultimately related to it.9 Over the past decade, GBCr, a well-established measure of nodal strength, provided robust and reproducible evidence of network disturbances in several psychiatric disorders.10–18 GBCr was also found to be sensitive to treatment, with accumulating evidence of normalization of GBCr disturbances following ketamine treatment of depressed patients.10–12 In combat-exposed US military veterans, prefrontal GBCr did not correlate with PTSD total symptom severity.19 However, clusters of high prefrontal GBCr were found in those who reported high arousal over the past month.19 This raises the question whether the level of symptoms during the scan may have increased the GBCr values in this subpopulation. Recently, this hypothesis was supported by a data-driven cross-sectional analysis demonstrating increased GBCr within the salience network during symptom provocation, but not at rest, in PTSD compared to trauma and nontrauma control.20
In this report, we investigated the longitudinal effects of psychotherapy on the GBCr alterations in the salience network during symptom provocation. This was accomplished by conducting an ROI analysis examining the effects of CPT and PCT on GBCr compared to a nontreated combat control (CC) group without PTSD. Then, using a previously established approach,10 we conducted a data-driven whole-brain analysis comparing posttreatment GBCr, during symptom provocation, between the PTSD group and CC. The aim of this approach is to identify patterns of normalization (i.e., absence of pretreatment disturbances) and adaptation (i.e., evidence of new alterations). Follow-up ROI analyses examined whether the posttreatment alterations were differentially influenced by CPT and PCT. The study predictions were that psychotherapy will significantly reduce salience GBCr, leading to a normalization pattern posttreatment.
Methods
The behavioral and imaging data were provided by the STRONG STAR data repository (https://tango.uthscsa.edu/strongstar/subs/rpinfo.asp?prj=12). The clinical trial results for the PTSD treatment study were previously reported21 (NCT01286415). The pretreatment GBCr data were reported elsewhere.20 The posttreatment data and analyses are new and have not been reported previously.
Study Population
PTSD (n = 42) and CC (n = 27) active military participants with successful scans were investigated (Table 1) as a subset of a larger randomized controlled clinical trial.21 The patients with PTSD completed pretreatment scans and received CPT (cognitive only22) or PCT23 group therapy (90-min sessions, twice per week for six weeks). Posttreatment scans were repeated two weeks after the end of treatment (i.e., a total of approximately eight weeks between scans). Similarly, the CC group completed repeated scans, eight weeks apart, without receiving any intervention.
Table 1.
Demographics and clinical characteristics.
| PCT (n = 23) | CPT (n = 19) | Combat control (n = 27) | |
|---|---|---|---|
| Mean (SEM) or N (%) | Mean (SEM) or N (%) | Mean (SEM) or N (%) | |
| Age | 32.7 (1.9) | 32.3 (1.5) | 31.8 (1.1) |
| Body mass index | 29.6 (1.0) | 28.4 (1.0) | 28.2 (0.6) |
| Intelligence quotient | 97 (1.8) | 102 (2.6) | 99 (2.1) |
| Sex (Male) | 22 (96%) | 17 (90%) | 25 (93%) |
| Race (White) | 16 (70%) | 13 (68%) | 17 (63%) |
| Race (Black) | 4 (17%) | 3 (16%) | 6 (22%) |
| Ethnicity (Hispanic) | 6 (26%) | 3 (16%) | 9 (33%) |
| Baseline BDI-II | 28.0 (2.7) | 28.4 (2.5) | |
| Delta BDI-II | 7.0 (1.9) | 7.3 (2.8) | |
| Baseline BAI | 27.0 (3.0) | 24.0 (2.5) | |
| Delta BAI | 8.3 (2.0) | 8.6 (2.1) | |
| Baseline PCL | 56.7 (2.3) | 56.4 (2.5) | |
| Delta PCL | 11.7 (2.7) | 9.7 (3.8) | |
| Baseline PSS-I | 27.2 (1.5) | 28.3 (1.7) | |
| Delta PSS-I | 6.1 (2.6) | 6.2 (2.7) |
Note: No significant differences between subgroups. SEM: standard error of means; PCT: present-centered therapy; CPT: cognitive processing therapy; BDI-II: Beck Depression Inventory-II; BAI: Beck Anxiety Inventory; PCL: PTSD Checklist; PSS-I: PTSD Symptom Scale—Interview Version.
All participants completed informed consent prior to participation. Study procedures were approved by institutional review boards. All participants had no MR contraindication and had a negative drug screen on the day of the scan. The clinical trial criteria were previously reported.21 Briefly, patients with PTSD were active duty US Army soldiers, following deployment to or near Iraq or Afghanistan, who were 18 years or older with DSM-IV PTSD diagnoses and were stable on or off psychotropic medications for at least six weeks; they did not have imminent suicide or homicide risk, psychosis, or more than mild traumatic brain injury. The CC group endorsed a Criterion A traumatic event during deployment but did not have current PTSD. Severity of symptoms pretreatment and posttreatment (week 8) was assessed using the PTSD Symptom Scale—Interview Version (PSS-I),24 PTSD Checklist (PCL) for DSM-IV,25 the Beck Depression Inventory-II (BDI-II),26 and the Beck Anxiety Inventory (BAI).27
FcMRI Acquisition and Processing
The acquisition parameters were previously reported.20 Briefly, each functional magnetic resonance imaging scan (voxel size = 2 × 2 × 3 mm; TR = 3000 ms; TE = 30 ms) included 10 min at rest and 12 min during symptom provocation—that is, script imagery during which participants listened to recorded retelling of a personal event (alternating between trauma and neutral) over a 1-min period, followed by a 1-min period of thinking about the event and then a 1-min break. The Human Connectome Pipeline was adapted to conduct surface-based preprocessing and optimize registration.28 Details of our image processing pipeline were previously reported12 and are provided in the Supplemental Information. Following our previous reports,10–12,19 GBCr values were computed as the average of the correlations between each vertex/voxel and all other vertices/voxels in the brain gray matter (see Supplemental Information).
Statistical Analyses
We used the Statistical Package for the Social Sciences (version 24) for the behavioral and ROI analyses. The normal distribution of outcome measures was confirmed using probability plots and test statistics. The standard error of means was provided as estimates of variation. Significance was set at p ≤ .05, with two-tailed tests. Analysis of variance and chi square test were used to compare behavioral data across groups.
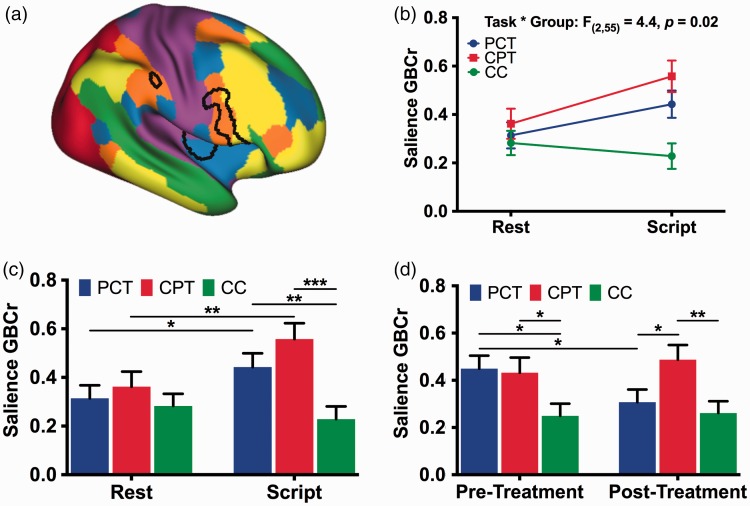
To investigate the salience ROI (Figure 1(a); based on the study by Abdallah et al.20), we constructed a general linear model (GLM) to determine the main effects of group (PCT vs. CPT vs. CC), task (rest vs. scripts), and time (pretreatment vs. posttreatment), as well as the interactions between the main effects, followed by post hoc pairwise comparisons.
Figure 1.
The effects of psychotherapy on salience connectivity. (a) The Akiki-Abdallah29 map of 6 intrinsic connectivity networks: ventral salience (blue), dorsal salience (orange), central executive (yellow), default mode (green), visual (red), and sensorimotor (purple). The black lines mark the salience clusters based on previous cross-sectional findings. (b) There was a significant group by task interaction effects on salience global brain connectivity with GBCr. (c) There was significant increase in GBCr during trauma recollection (i.e., script imagery) compared to during resting state in PTSD patients treated with PCT or CPT, but not in CC. The higher GBCr values in PTSD compared to CC were significant only during trauma recollection, but not a rest. (d) PCT, but not CPT, significantly reduced salience GBCr. *p ≤ .05; **p ≤ .01; ***p ≤ .001. PCT: present-centered therapy; CPT: cognitive processing therapy; CC: combat control.
To determine the pattern of GBCr alterations following treatment, we conducted a vertex-/voxel-wise fcMRI nonparametric analysis using FSL Permutation Analysis of Linear Models (PALM), with tail approximation and cluster mass threshold of 1.96 for Type I error correction (corrected α = .05).30 This data-driven whole-brain analysis used independent t tests to identify posttreatment clusters with altered GBCr during symptom provocation in the PTSD group compared to CC. To facilitate the interpretation of the whole-brain findings (i.e., increase in executive GBCr), the identified clusters (vertex/voxel p < .005; corrected α = .05) were extracted to conduct follow-up post hoc ROI analyses to better characterize the executive GBCr changes across time, tasks, and subgroups. This was accomplished by conducting a GLM comparable to the one used for investigating the salience ROI.
Finally, we conducted exploratory analyses examining the correlation in the PTSD group between salience/executive GBCr and improvement/severity measures (BDI-II, BAI, PCL, PSS).
Results
Participants were well matched for age, sex, body mass index, intelligence quotient, race, and ethnicity (Table 1). Pretreatment PSS-I, PCL, BDI-II, and BAI did not differ between treatment groups. In the clinical trial participants, both CPT and PCT significantly reduced clinical symptoms on the PSS-I, PCL, BDI-II, and BAI at week 8 (all p values < .05), but there were no significant differences between treatments (all p values > .6).
Normalization: PCT Reduced Salience Functional Connectivity
Investigating the salience ROI (Figure 1(a)), the GLM revealed significant effects of group (F(2,55) = 4.8, p = .01), task (F(1,55) = 6.1, p = 0.02), and task × group interaction (F(2,55) = 4.4, p = .02; Figure 1(b)), with increased salience GBCr during symptom provocation compared to resting state in the PCT and CPT groups but not in CC (Figure 1(c)). In addition, salience GBCr values were higher in the PCT and CPT groups compared to CC during symptom provocation but not at rest (Figure 1(c)). We also found trends for time × task (F(1,55) = 3.4, p = .07) and time × group interaction (F(2,55) = 2.8, p = .07; Figure 1(d)), with significant reduction of salience GBCr following PCT (p = .02). Compared to CC, salience GBCr was high pretreatment for the 2 PTSD groups (PCT, p = .01; CPT, p = .03) and normalized post-PCT treatment (p = .53) but not post-CPT treatment (p = .006). There were no main time effects (p = .48) or time × task × group interaction (p = .75).
Adaptation: CPT-Enhanced Central Executive Functional Connectivity
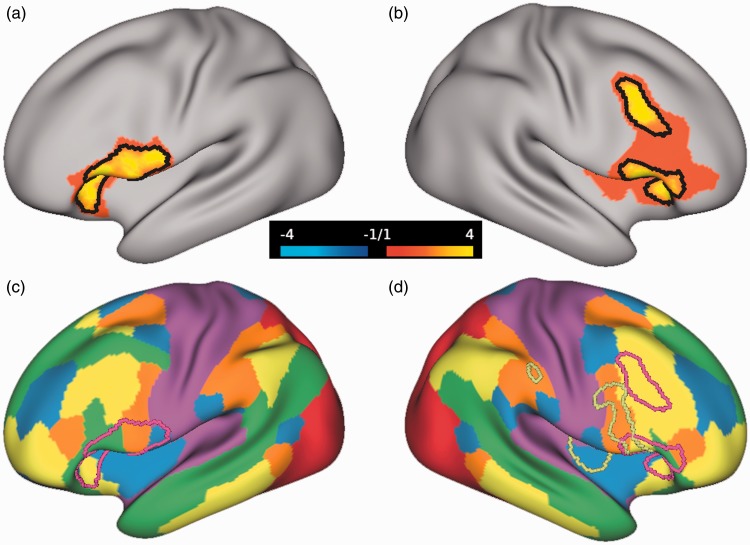
In the participants with PTSD compared to the CC group, posttreatment whole-brain analysis revealed a significantly high GBCr in areas within the left ventrolateral prefrontal, right rostral-ventrolateral, and dorsolateral prefrontal cortices (Figure 2(a) and (b)). We also found significant clusters of low GBCr in the rostral-ventral areas of the cerebellum in the treated participants compared to the CC group (Figure S1). Notably, the salience cluster, which showed high GBCr in the two treated groups compared to controls in the cross-sectional study,20 appears to normalize following treatment with adaptation shift toward higher GBCr within the central executive network (Figure 2(c) and (d)). Hence, to facilitate the interpretation of the whole-brain findings, we conducted post hoc analyses by extracting average GBCr from each subject within this executive ROI, which included areas that showed significantly high GBCr in PTSD (Figure 2).
Figure 2.
Cortical global connectivity posttreatment. (a and b) The red-yellow clusters mark the vertices with increased global brain connectivity with global signal regression (GBCr) in treated posttraumatic stress disorder (PTSD) compared to controls during symptom provocation. The black lines mark the vertices with p < .005 and corrected α = .05. (c and d) The Akiki-Abdallah29 map of six intrinsic connectivity networks: ventral salience (blue), dorsal salience (orange), central executive (yellow), default mode (green), visual (red), and sensorimotor (purple). The dark yellow lines mark the salience cluster and the red lines mirror the black lines in (a) and (b), marking the executive cluster.
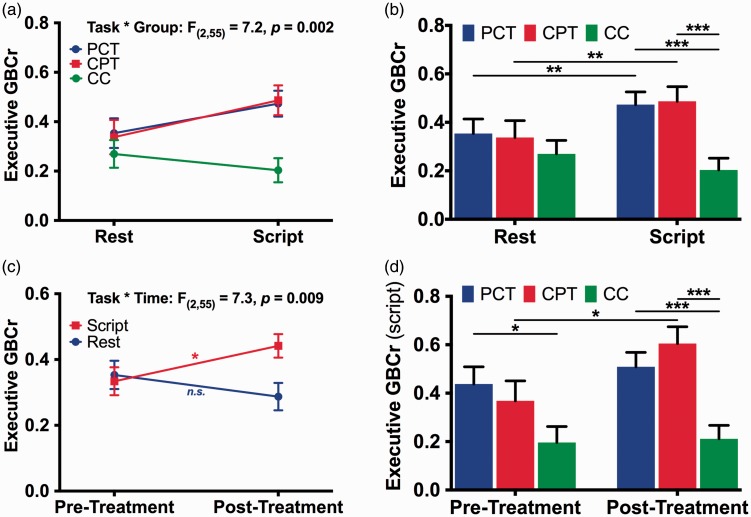
Investigating the executive ROI, the GLM revealed significant effects of group (F(2,55) = 4.0, p = .02), task (F(1,55) = 6.6, p = .01), and task × group (F(2,55) = 7.1, p = .002; Figure 3(a) and (b)) and task × time interaction (F(1,55) = 7.3, p = .009; Figure 3(c)), with increased executive GBCr during symptom provocation compared to resting state in the CPT and PCT groups but not in CC (Figure 1(c)). In addition, executive GBCr values were higher in the PCT and CPT groups compared to CC during symptom provocation but not at rest (Figure 3(b)). We also found significant increases in executive GBCr following CPT (p = .01) during symptom provocation (Figure 3(d)). There were no main time effects (p = .54), time × group interaction (p = .30), or time × task × group interaction (p = .22). Additional analyses of the cerebellar ROI are provided in the Supplemental Information (Figure S2).
Figure 3.
The effects of psychotherapy on executive connectivity. (a) There was a significant group by task interaction effects on executive global brain connectivity with GBCr. (b) There was significant increase in GBCr during trauma recollection (i.e., script imagery) compared to during resting state in PTSD patients treated with PCT or CPT, but not in CC. The higher GBCr values in PTSD compared to CC were significant only during trauma recollection, but not a rest. (c) There was a significant time by task interaction effects on executive GBCr. (d) CPT, but not PCT, significantly increased executive GBCr. n.s.: not significant; *p ≤ .05; **p ≤ .01; ***p ≤ .001. PCT: present-centered therapy; CPT: cognitive processing therapy; CC: combat control.
Exploring the Relationship Between GBCr and Symptoms
Pretreatment executive GBCr during symptom provocation was associated with improvement in PCL scores (i.e., pre- minus posttreatment) over the treatment period (r = .36, p = .027). In addition, pretreatment executive GBCr at rest was negatively associated with pretreatment BAI (r = –.42, p = .008), PCL (r = –.43, p = .006), and PSS-I scores (r = –.45, p = .004). No other correlations between executive GBCr and symptoms severity or improvement were found. We found no correlations between salience GBCr and improvement of symptoms. Finally, the readers should cautiously interpret these exploratory findings, considering that they do not survive correction for multiple comparisons.
Discussion
Overall, we found a pattern of salience network normalization (i.e., reduction) and executive network adaptation (i.e., increase) following evidence-based psychotherapy in PTSD patients treated twice per week for six weeks. There were no significant cortical connectivity changes in the CC group. Post hoc analyses showed that CPT induced a significant increase in executive connectivity leading to adaptation changes with higher salience and executive connectivity values post-CPT in PTSD compared to CC. In contrast, PCT induced a significant reduction in salience connectivity leading to normalization and salience connectivity values comparable to CC. Finally, the data-driven analysis posttreatment showed reduced global connectivity in PTSD in areas within the cerebellum, including both the spinocerebellum and cerebrocerebellum (Figure S1). However, there was no significant treatment effect compared to changes in CC (Figure S2).
CPT is a cognitive therapy in which patients examine their thinking and emotions about the traumatic event. The patients are systematically taught how to change their thinking to more balanced beliefs with the use of Socratic questioning by the therapist.21,22 The findings of CPT-related increases in global connectivity within the executive network are consistent with the cognitive model wherein executive control improves the processing of trauma-related stimuli, resulting in moderated expression of emotion in response to trauma-related cues. Consistent with this hypothesis, a previous study using seed-based analysis showed CPT-induced increases in central executive functional connectivity, which were interpreted as indicative of top-down cognitive control of affective processes that are disrupted in PTSD.31 Moreover, systemic reviews and meta-analyses of neuroimaging research have reported an association between cognitive therapies and increased activity in brain regions within the executive network.32,33 To further advance this hypothesis, future studies should investigate GBCr during a cognitive task to determine the extent of pretreatment executive abnormalities in PTSD and whether the connectivity of a cognitively engaged central executive network could predict response to psychotherapy or whether it is affected by treatment.
PCT was originally developed as an active comparator to trauma-focused cognitive therapy.23 Hence, PCT includes common components of efficacious psychotherapy without focusing on the trauma or using cognitive or supportive frameworks. PCT focuses on managing PTSD symptoms using psychoeducation and problem-solving strategies to generate possible solutions to current problems or PTSD symptoms that the patient can practice off-sessions. Although neuroimaging studies examining the effects of PCT are scarce, one pilot study reported PCT-induced reduction in the activation of the insula during the presentation of traumatic images and sounds.34 Another study reported increased resting-state functional connectivity between clusters within the default mode network following PCT treatment.35 PTSD is associated with reduced default mode connectivity,7 an abnormality that is believed to be the result of an overactive salience network failing to effectively arbitrate between default mode and central executive networks.2,3 In this context, the previously reported PCT-induced reduction in insula activity during trauma cues and increase in default mode connectivity during resting state may reflect a pattern of normalization of the salience network following PCT treatment. This study results further support this model by demonstrating significant reduction in trauma-induced global brain connectivity within the salience network following PCT. Although the data of this study do not allow us to distinguish which components of PCT are responsible for the reduction in salience connectivity, we speculate that perhaps the out-of-sessions, repeated practice of the symptom reduction solutions generated through problem-solving strategies during the sessions may have led to enhanced utilization of habitual rather than cognitive reactions to trauma cues. However, it is important to underscore the speculative nature of this hypothesis and the need to fully test it in future studies.
Finally, accumulating evidence repeatedly demonstrates functional and structural abnormalities in the cerebellum of PTSD patients.36–41 Moreover, two recent studies have shown reduced functional nodal strength in the cerebellum in PTSD.20,42 In this study, PTSD patients continued to show reduction in cerebellar connectivity posttreatment compared to controls (Figure S1). Follow-up analyses showed persistently lower cerebellar connectivity in PTSD during trauma recollection, regardless of treatment modality, with no significant treatment effects compared to changes in CC (Figure S2).
Limitations and Strengths
Considering that both interventions were active, efficacious treatments, the study design cannot confirm that the observed connectivity changes posttreatment are due to the specific intervention rather than generalized, nonspecific changes due to reduction in PTSD symptoms. However, the differential changes in connectivity patterns per treatment suggest a direct relationship between CPT and PCT with executive and salience global connectivity, respectively. Another limitation is that the executive ROI analyses are dependent on the vertex-wise results. Therefore, these data should be interpreted within the context of better understanding data-driven findings, rather than fully independent test results. Additional limitations include the majority of participants were males, thus it was not possible to investigate sex differences. Moreover, we did not exclude mild traumatic brain injury or patients on stable medications to enhance the generalizability of the findings to the target of population of combat PTSD. Therefore, it possible that these factors may have contributed to the results. Similarly, the control subjects were combat exposed; hence, it is not possible to ascertain whether these findings would hold compared to nontrauma-exposed control. However, previous work suggests that the global brain connectivity alterations we observe following trauma recall are specific to PTSD patients compared to both nontrauma-exposed and trauma-exposed control.20 Finally, the measure of nodal strength is not limited to a specific ICN but rather measures the role of each node within the whole-brain network. Therefore, the salience and executive connectivity alternations may either indicate increased internal (i.e., within network) and/or external connectivity (i.e., between networks). Future studies could use network-restricted topology approaches7 to further delineate the role of each ICN as well as the interaction of ICNs.
This study has many strengths including: (a) a longitudinal design in an adequate sample with randomization to two evidence-based efficacious treatments for these purposes; (b) the inclusion of repeated scans in the control group to account for nonspecific test-retest changes; (c) the use of symptom provocation paradigm to identify trauma-specific dynamic shift in ICNs; (d) the use of a well-validated measure of nodal strength. GBCr has been repeatedly associated with psychopathology and successful treatment.10–12 In addition, GBCr does not require a priori selection of seed or ROI, which here permitted the posttreatment data-driven analysis. In this study, the lack of significant cortical GBCr changes in the control group underscores the robustness of the measure and the specificity of the study paradigm; (e) the use of state-of-the-art neuroimaging methods based on the Human Connectome Pipeline, including enhanced registration, surface-based analysis, and nonparametric correction for the vertex/voxel-wise multiple comparisons.
Conclusions
The results provide strong neurobiological evidence supporting the role of the central executive network in the mechanism of CPT treatment to engage cognitive control and ultimately reduce PTSD symptoms. Intriguingly, the study findings may have unraveled a previously unknown neurobiological mechanism of PCT treatment, demonstrating treatment-specific reduction in salience connectivity during trauma recollection. It remains to be seen whether the normalized salience connectivity is primarily driven by the habitual reactions established through off-session practicing of symptom-reduction solutions devised during therapy sessions. In summary, evidence-based psychotherapy exerted a pattern of normalization within the salience network and adaptation in the executive network. Although the adaptational changes favored CPT, the normalization was mostly limited to PCT. The used biomarkers are well validated and have previously shown notable reproducibility following pharmacotherapeutic interventions.10–12 Therefore, future studies may capitalize on current findings to determine the clinical utility of these biomarkers in predicting or optimizing treatment for millions of patients suffering from PTSD.
Supplemental Material
Supplemental Material for Reduced Salience and Enhanced Central Executive Connectivity Following PTSD Treatment by Chadi G. Abdallah, Christopher L. Averill, Amy E. Ramage, Lynnette A. Averill, Evelyn Alkin, Samaneh Nemati, John H. Krystal, John D. Roache, Patricia A. Resick, Stacey Young-McCaughan, Alan L. Peterson, Peter Fox and the STRONG STAR Consortium in Chronic Stress
Acknowledgments
STRONG STAR Consortium group authors include (listed alphabetically): Elisa V. Borah (Department of Psychiatry, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA; now at School of Social Work, University of Texas at Austin, Austin, Texas); Katherine A. Dondanville (Department of Psychiatry, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA); Mitchell Kok (Carl R. Darnall Army Medical Center, Fort Hood, Texas; now at South Sound Radiology, Olympia, WA, USA); Brett Litz (Massachusetts Veterans Epidemiological Research and Information Center, VA Boston Healthcare System, Boston, MA, USA; Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA; and Department of Psychological and Brain Sciences, Boston University, Boston, MA, USA); Jim Mintz (Department of Psychiatry and Department of Epidemiology and Biostatistics, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA); Paul C. Robinson (Carl R. Darnall Army Medical Center, Fort Hood, Texas; now at Department of Radiology & Biomedical Imaging, University of California, San Francisco, CA, USA); Mary Woolsey (Research Imaging Institute, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA); and Jeffrey Yarvis (Carl R. Darnall Army Medical Center, Fort Hood, Texas; now at 21st Combat Support Hospital, Fort Hood, TX, USA). The authors would like to thank the individuals who participated in these studies for their invaluable contribution.
Footnotes
*Details of the STRONG STAR Consortium are given at the end of the article.
Authors’ Note
STRONG STAR Consortium contact person and PI: Dr. Alan Peterson, petersona3@uthscsa.edu
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C. G. A. has served as a consultant and/or on advisory boards for FSV7, Genentech, and Janssen, and editor of Chronic Stress for Sage Publications, Inc.; he has filed a patent for using mTOR inhibitors to augment the effects of antidepressants (filed on August 20, 2018). J. H. K is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; is a stockholder in Biohaven Pharmaceuticals; holds stock options in Mnemosyne Pharmaceuticals, Inc.; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, US Patent No. 5,447,948 (issued September 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued July 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on March 5, 2014); US application or Patent Cooperation Treaty international application No. 14/306,382 (filed on June 17, 2014); filed a patent for using mTOR inhibitors to augment the effects of antidepressants (filed on August 20, 2018). All other coauthors declare no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this work was made possible by grants to the STRONG STAR Consortium by the US Department of Defense through the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, Psychological Health and Traumatic Brain Injury Research Program awards W81XWH-08-02-109 (Alan Peterson), W81XWH-08-02-0112 (Peter Fox), W81XWH-08-02-0114 (Brett Litz), and W81XWH-08-02-0116 (Patricia Resick). Some of the investigators also had additional support from the National Institute of Mental Health (K23MH101498) and the VA National Center for PTSD. The views expressed in this article are solely those of the authors and do not represent and endorsement by or the official policy or position of the Department of Defense, the Department of Veterans Affairs, the National Institutes of Health, or the US Government.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Sporns O, Bassett DS. Editorial: new trends in connectomics. Netw Neurosci 2018; 2: 125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdallah CG, Averill LA, Akiki TJ, et al. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu Rev Pharmacol Toxicol 2019; 59: 171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiki TJ, Averill CL, Abdallah CG. A Network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep 2017; 19: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negreira AM, Abdallah CG. A review of fMRI affective processing paradigms used in the neurobiological study of Posttraumatic Stress Disorder. Chronic Stress 2018; 3: 2470547019829035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheynin J, Liberzon I. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett 2017; 649: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage 2018; 176: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014; 83: 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10: 186–198. [DOI] [PubMed] [Google Scholar]
- 10.Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 2017; 42: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdallah CG, Averill CL, Salas R, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress 2018; 2: 2470547018796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anticevic A, Brumbaugh MS, Winkler AM, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 2013; 73: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry 2014; 75: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anticevic A, Corlett PR, Cole MW, et al. N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 2015; 77: 569–580. [DOI] [PubMed] [Google Scholar]
- 16.Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry 2011; 70: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murrough JW, Abdallah CG, Anticevic A, et al. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp 2016; 37: 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Dai Z, Peng H, et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp 2014; 35: 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdallah CG, Wrocklage KM, Averill CL, et al. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Translational psychiatry 2017; 7: e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdallah C, Averill C, Ramage A, et al. Salience network disruption in US army soldiers with PTSD. Chronic Stress 2018; In press. [DOI] [PMC free article] [PubMed]
- 21.Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol 2015; 83: 1058–1068. [DOI] [PubMed] [Google Scholar]
- 22.Resick PA, Galovski TE, Uhlmansiek MO, Scher CD, Clum GA, Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J Consult Clin Psychol 2008; 76: 243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnurr PP, Friedman MJ, Lavori PW, Hsieh FY. Design of Department of Veterans Affairs Cooperative Study no. 420: group treatment of posttraumatic stress disorder. Control Clin Trials 2001; 22: 74–88. [DOI] [PubMed] [Google Scholar]
- 24.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Traum Stress 1993; 6: 459–473. [Google Scholar]
- 25.Weathers FW, Huska J, Keane TM. The PTSD Checklist Military Version (PCL-M), Boston, MA: VA National Center for PTSD, 1991. [Google Scholar]
- 26.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory – II, San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 27.Beck AT, Steer RA. Beck Anxiety Inventory Manual, San Antonio, TX: Psychological Corporation, 1993. [Google Scholar]
- 28.Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013; 80: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiki TJ, Abdallah CG. Determining the hierarchical architecture of the human brain using subject-level clustering of functional networks. BioRxiv 2018; DOI: 10.1101/350462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin 2017; 14: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks SJ, Stein DJ. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin Neurosci 2015; 17: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomaes K, Dorrepaal E, Draijer N, Jansma EP, Veltman DJ, van Balkom AJ. Can pharmacological and psychological treatment change brain structure and function in PTSD? A systematic review. J Psychiatr Res 2014; 50: 1–15. [DOI] [PubMed] [Google Scholar]
- 34.Bremner JD, Mishra S, Campanella C, et al. A pilot study of the effects of mindfulness-based stress reduction on post-traumatic stress disorder symptoms and brain response to traumatic reminders of combat in operation enduring freedom/Operation Iraqi Freedom combat veterans with post-traumatic stress disorder. Front Psychiatry 2017; 8: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King AP, Block SR, Sripada RK, et al. Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety 2016; 33: 289–299. [DOI] [PubMed] [Google Scholar]
- 36.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2012; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety 2016; 33: 592–605. [DOI] [PubMed] [Google Scholar]
- 38.Baldacara L, Borgio JGF, Araujo C, et al. Relationship between structural abnormalities in the cerebellum and dementia, posttraumatic stress disorder and bipolar disorder. Dement Neuropsychol 2012; 6: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldacara L, Jackowski AP, Schoedl A, et al. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiatr Res 2011; 45: 1627–1633. [DOI] [PubMed] [Google Scholar]
- 40.De Bellis MD, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2006; 60: 697–703. [DOI] [PubMed] [Google Scholar]
- 41.Rabellino D, Densmore M, Theberge J, McKinnon MC, Lanius RA. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum Brain Mapp 2018; 39: 3354–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes SE, Scheinost D, DellaGioia N, et al. Cerebellar and prefrontal cortical alterations in PTSD: structural and functional evidence. Chronic Stress (Thousand Oaks) 2018; 2: DOI: 10.1177/2470547018786390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Reduced Salience and Enhanced Central Executive Connectivity Following PTSD Treatment by Chadi G. Abdallah, Christopher L. Averill, Amy E. Ramage, Lynnette A. Averill, Evelyn Alkin, Samaneh Nemati, John H. Krystal, John D. Roache, Patricia A. Resick, Stacey Young-McCaughan, Alan L. Peterson, Peter Fox and the STRONG STAR Consortium in Chronic Stress