Abstract
Oligodendrocytes and their precursors are critical glial facilitators of neurophysiology, which is responsible for cognition and behavior. Devices that are used to interface with the brain allow for a more in-depth analysis of how neurons and these glia synergistically modulate brain activity. As projected by the BRAIN Initiative, technologies that acquire a high resolution, robust sampling of neural signals can provide a greater insight in both the healthy and diseased brain and support novel discoveries previously unobtainable with the current state of the art. However, a complex series of inflammatory events triggered during device insertion impede the potential applications of implanted biosensors. Characterizing the biological mechanisms responsible for the degradation of intracortical device performance will guide novel biomaterial and tissue regenerative approaches to rehabilitate the brain following injury. Glial subtypes which assist with neuronal survival and exchange of electrical signals, mainly oligodendrocytes, their precursors, and the insulating myelin membranes they produce, are sensitive to inflammation commonly induced from insults to the brain. This review explores essential physiological roles facilitated by oligodendroglia and their precursors and provides insight into their pathology following neurodegenerative injury and disease. From this knowledge, inferences can be made from the impact of device implantation on these supportive glia in order to engineer effective strategies that can attenuate their responses, enhance the efficacy of neural interfacing technology, and provide a greater understanding of challenges that impede wound healing and tissue regeneration during pathology.
Keywords: Glial pathology, Microelectrode array, Brain injury, Inflammation, Demyelination, Foreign body response
1. Introduction
Neural electrode devices that can be used to study and interface with the brain have the potential to advance the field of neuroscience and improve the treatment of nervous system-related disorders and disabilities. The multi-potential use of these devices depends on the ability to transmit and receive electrical signals to and from neural tissue in a stable and reliable manner. In order to do so, the electrode contacts must be minimally impacted by protein biofouling and cellular encapsulation as well as remain in close proximity to active neurons that are integrated into the functional neural network. This level of integration at the electrode-tissue interface is necessary to maximize detected signal and reduce impedances, which can increase noise. However, on average, electrode-tissue interfaces degrade over time. Neural degeneration and glial scar formation reduce the functional performance of chronically inserted microelectrodes. Recent efforts aim to understand the biological mechanisms behind the onset of tissue inflammation and apply that knowledge toward effective therapies to attenuate this response and achieve the full potential of neural interfaces.
1.1. Obstacles encountered during neural interface design
The capabilities of neural interfaces as assistive devices for disabled or neurologically impaired individuals are limited by the gradual degradation in recording or stimulating performances over time [1–4]. Failure modes of chronically implanted neural electrodes can be divided into three major categories: mechanical, material, and biological sources of device failure [5, 6]. Mechanical failures involve a perturbation in the external connectors of the device that are responsible for transmitting signal from the implanted electrode to an external computer. Examples include electrode displacement from the tissue or severing of connector cables. Material failures include the degradation of the conductive, insulating, or specially coated interface that impedes the exchange of information between the electrode and surrounding tissue. This type of failure manifests in the conductor and insulating material chosen, as well as the size, geometry, and mechanical characteristics of the device which have respective influences on the severity of tissue inflammation. Biological failures are the result of endogenous tissue reactions to the physical implantation or presence of the device. Failure in either of these categories is not exclusive; for example, while delamination of the insulation results in recording failures, subsequent exposure of the underlying electroactive material can also lead to electrode dissolution, which negatively impacts tissue viability. The persistence of a degenerative foreign body response afflicts both biological tissue as well as the material interface, reducing the long-term recording quality of chronically implanted devices. Consequently, the design of neural interfaces becomes a multi-faceted approach and must take into consideration a wide array of design parameters to functionally integrate electrical materials with biological tissue [7]. Efforts to solve issues of performance reliability and variability by engineering solutions of one aspect of device design prove insufficient when faced with multiple overwhelmingly complex and interconnected modes of failure. Furthermore, chronically implanted neural electrodes which avoid mechanical or material-induced mechanisms of failure ultimately succumb to the persistence of inflammation and degradation of neural tissue and lose quality in recording and stimulating performances [5].
A majority of investigations of the biological component of the electrode-tissue interface characterize the microglial and astrocyte reactivity around inserted devices and their impact on neural health (see review [8]). However, these studies neglect a variety of other cellular factors in the brain such as oligodendrocyte precursor cells, mature oligodendrocytes and their myelin components, mural cells such as pericytes and endothelial cells, and other immune system mediators such as monocytes, neutrophils, and leukocytes. Since each of these cells have their respective roles in maintaining brain health and may potentially deviate from their responsibility in homeostasis during disease and injury, careful characterization of their behavior around inserted neural devices is required to work toward effective device design. This review will detail the behavior of cells of the oligodendrocyte lineage as well as their myelin structures in both healthy and diseased states in an effort to predict the potential consequences of chronic microelectrode implantation on these cell types.
2. Current understanding of tissue response to injury
Normal physiology in the brain involves a variety of active signaling between neurons, glia, and vascular cells in order to perform functional activity and maintain tissue homeostasis. These sensitive connections are altered or compromised during injury, influencing neuronal output and tissue health [9]. The critical pathways that lead into the neurodegenerative insult induced by electrode implantation have been a subject of debate, however, major influences such as glial cell activation and blood-brain barrier permeability are widely accepted contributors to declining neuronal health [10, 11]. A brief review of the major constituents that exist within the parenchyma (neurons, glia, and the cells that support the blood-brain barrier) and their involvement in the foreign body response to inserted devices is required in order to contextualize the impact of implantation injury to other cell types within the brain.
2.1. Blood-brain barrier disruption on activation of the inflammatory tissue response
The blood-brain barrier actively regulates influx of nutrients and oxygen and efflux of waste products between CNS tissue and peripheral blood while providing protection to neuronal cells from toxins and pathogens that circulate throughout the body [12]. The insulating nature of this vascular membrane is the result of endothelial cells forming continuous intracellular tight junctions with one another, supported by astrocytes and pericytes that provide additional mechanical and trophic support to the blood-brain interface [13]. During brain injury, disrupting the endothelial cell-cell barrier can lead to vasogenic edema, loss of perfusion, activation of glia, and neurodegeneration [14]. Additionally, compromising the integrity of the BBB exposes the parenchyma to inflammatory plasma proteins and allows for the infiltration of inflammatory peripheral immune cells [14]. A review detailing the biochemical mechanisms behind the impacts of BBB disruption on degrading signal sensitivity of neural implants has been published previously [9]. Considering that microelectrode arrays constantly shift due to breathing and pulsating micromotions within the body, random tears to the BBB can occur at any time throughout the lifetime of the implant. As a result, maintaining BBB integrity is required to minimize the impact of glial cell activation and surrounding tissue inflammation during chronic implantation.
2.2. Glial cells on noise
Gliosis is commonly observed in the context of chronically implanted microelectrodes which hinders recording and stimulating performances over time [15–18]. Glial scarring can interfere with the exchange of ions and charged solutes between neurons and the conductive surface of the electrode which increases the noise and impedes signal detection, ultimately leading to a decreased signal-to-noise ratio (SNR). In vivo imaging has been used to characterize the acute and immediate activation and encapsulation of microglia cells in response to microelectrode insertion [19]. The astrocytic scarring response is commonly characterized by an upregulation in glial fibrillary acidic protein (GFAP) around the device-tissue interface. As a result, the compacted glial scar creates a membrane-bound barrier that inhibits transmission of signal across the electrode-tissue interface. Gliosis can persist at chronic time points in part due to the continual secretion of pro-inflammatory factors and repeated disruption of the BBB whose ability to heal may be impaired by the implant, negatively impacting the viability of the surrounding neurons.
2.3. Neuronal sources of signal loss
Blood-brain barrier disruption, glial cell activation, and inflammation influence neuronal physiology and, ultimately, neuronal function. Direct correlations between increased BBB leakage and decreased neuronal activity have been observed at chronic time points [20]. The presence of a glial scar prevents the transmission of electrical signals and displaces nearby neurons further from the electrode interface. This effectively increases the distance to the nearest neuron making it difficult for an electrode to record or discriminate between single units due to the voltage drop off from increasingly distant neurons [21]. Furthermore, neurons are particularly susceptible to oxidative stress events which may occur when the BBB is disrupted, creating an ischemic event, or production of reactive oxygen species (ROS) from activated glia. Finally, reactive oxygen species and proinflammatory cytokines secreted by activated immune cells are notably toxic to neurons and have been characterized in other neurodegenerative disorders [22, 23]. Therefore, implementation of successful therapeutic strategies or improved device design could mitigate the blood-brain barrier or glial-induced insult on neuronal loss, improving the performance of chronically implanted neural electrodes [24].
2.4. Summary and current limitations on an integrated tissue response
The influence of blood-brain barrier disruption and activated glia on neuronal health are not mutually exclusive; effects of one insult can trigger inflammatory events in the other. Potential strategies aimed at mitigating the currently known biological reactions in an attempt to improve the biocompatibility of neural interfaces have been suggested and published elsewhere [7, 9, 25]. However, recent studies have proven that increased neuronal viability, decreased glial cell activation, or reduced BBB leakage are not exclusively accurate predictors of functional electrode performance [26]. This implies that other endogenous tissue events which remain to be investigated could contribute to the degrading quality of neural microelectrodes, such as oligodendrocytes and their precursor cells, which share a more intimate relationship with neuronal cells compared to other glia.
3. Oligodendrocyte and NG2 glia physiology in the brain
As some of the most metabolically active cells in the brain, oligodendrocytes and their precursors serve a variety of different homeostatic functions and interact with neurons, glia, as well as the blood-brain barrier in order to facilitate normal brain activity. Under normal physiological conditions, cells from the oligodendrocyte lineage experience four distinct phases throughout their life cycle: origin, migration, and proliferation of oligodendrocyte precursor cells (OPCs); differentiation into mature myelinating oligodendrocytes; initiation of neuronal contact and myelination of axonal fibers; and provision of trophic and metabolic support to neurons. Oligodendrocyte precursors are identified based a number of criteria involving morphology, antigen expression, and physiological functions. OPCs are defined as cells displaying a stellate-shaped morphology, extending processes radially about their cell soma [27]. These cells are increasing and more commonly referred to as NG2 glia due to their constitutive expression of the neural-glia antigen 2 marker [28]. However, other parenchymal cells such as pericytes and macrophages express the NG2 antigen as well, requiring the need for co-localization of multiple identification markers [29]. Additionally, the inherent heterogeneity of oligodendrocyte precursor-specific NG2 glia further complicates precise identification since physiological as well as pathological function may by dependent on embryonic origins or the resident tissue environment of the cell [30].
3.1. NG2 origin, migration, proliferation, and differentiation in the developing CNS
NG2 glia are highly proliferative and widely abundant (compromises 5–8% of the total glial population in adult brain) within the CNS. The earliest observations of NG2 in the developing CNS are from the ventricular zones (VZ) of the spinal cord at embryonic day 12.5 (E12.5) for mice and E14 for rats [31, 32]. In the rodent brain, NG2 glia originate in the medial ganglionic eminence and anterior entopeduncular area of the ventral forebrain, populating areas of the telencephalon such as the cerebral cortex from E16 until birth (~E18) [33]. After the initial influx of oligodendrocyte precursors, a second and third wave emerge from the lateral ganglionic eminence and postnatal cortex, respectively, to replace the first wave, with NG2 glia reaching a maximum density before birth within the spinal cord and by postnatal day 3 (P3) in the cortex [34]. Despite originating from different regions, NG2 glia retain similar characteristics such as the ability to spread over non-overlapping domains and fill vacant spaces in the event of cell migration, differentiation, or death in vivo [35]. Likewise, studies of oligodendrocyte precursors from gray and white matter regions showed no differences in proliferation rates or cell cycle lengths [36]. However, functional properties such as those involving membrane channels and receptors differ between NG2 glia populations in the mouse gray and white matter [37].
After diverging from radial glia progenitors, NG2 glia travel along blood vessels towards other regions of the developing CNS [38]. Migration of NG2 glia during development is determined by two different mechanisms, one of which is contact-mediated migration involving extracellular matrix proteins (laminin, fibronectin) or cell surface proteins (cadherins, integrins) and the other involving the secretion of growth factors (PDGF, FGF-2, EGF, HGF), chemotactic molecules (netrins, semaphorins), or chemokines (CXCL1) [39]. More recently, it has been discovered that vascular and meningeal cells secrete growth factors (TGF-β and BMPs) that guide migration of NG2 glia into the cortex during development [40]. After migrating, NG2 glia evenly distribute throughout both gray and white matter and differentiate into mature myelinating oligodendrocytes. NG2 glia are commonly referred to as polydendrocytes due to their intrinsic ability to differentiate into neurons and astrocytes as well as oligodendrocytes (Figure 1) [41]. However, some NG2 glia do not differentiate into mature oligodendrocytes and maintain their precursor morphology throughout adulthood, consisting of ~4% of all cells in the adult cortex [42].
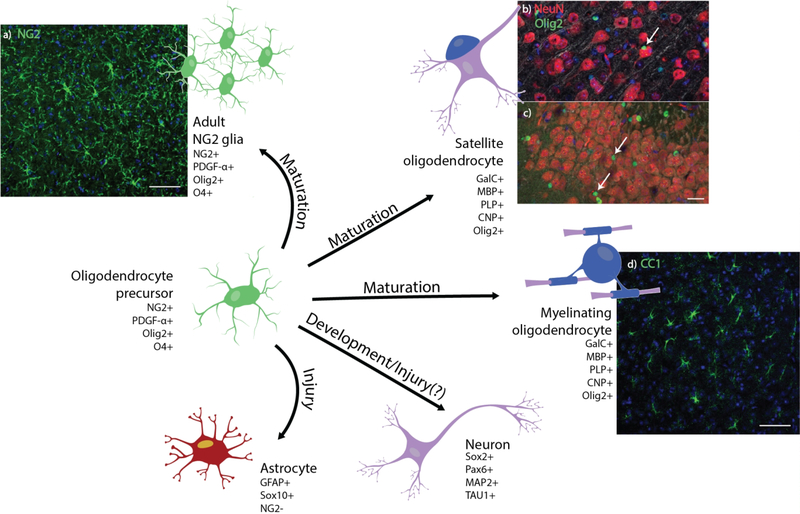
Figure 1. Development and cell fate of oligodendrocyte precursor cells.
Oligodendrocyte precursor cells maintain various paths of cell fate depending on tissue conditions. (a) Histological stain of NG2+ glia (green) and cell nuclei (blue) in horizontal sections of the adult mouse cortex. Scale bar = 100 μm. Olig2+ oligodendrocytes (green) can be perineuronal (white arrow), residing in close proximity to NeuN+ neuron cell bodies (red) in both the (b) cortex and (c) dentate gyrus of coronal sections of the adult mouse brain. Scale bar = 25 μm. (d) Confocal image of cortical CC1+ myelinating oligodendrocytes (green) and cell nuclei (blue) in horizontal sections of the adult mouse. Scale bar = 100 μm. Oligodendrocyte precursors have been observed in certain regions of the brain to transdifferentiate into neurons and, under specific injury conditions, into reactive astrocytes.
Through in vivo imaging of the mouse cortex, these cells are observed to be highly active exploring their surroundings through the extension and retraction of processes, maintaining a homeostatic distribution of cell bodies in a grid-like manner [35]. This local population of NG2 glia are thought to function as a reservoir of oligodendrocyte precursors in the event of demyelinating injury and as support cells for myelin maintenance and possible mediators of neuronal networks via formation of synaptic contacts [43].
3.2. Oligodendrocyte maturation and myelination
At post-natal day 2 (P2) in rodents, most cells are in the pre-oligodendrocyte phase where markers of differentiated oligodendrocytes (O4) begin to be expressed, markers for NG2 glia (NG2, PDGFRα) are downregulated, and a morphology indicative of branched, multipolar oligodendrocytes begins to take shape. During this maturation period, pre-oligodendrocytes lose the ability to migrate or proliferate [44]. The differentiation of NG2 glia into myelinating oligodendrocytes involves signaling between the Notch 1 receptor and the axonal ligand Jagged 1 [45]. The earliest occurrence of myelination in the white matter is not observed until postnatal day 7 where oligodendrocytes only have a brief time period, between 12 to 18 hours after differentiation, to form myelin sheaths around axons [46, 47].
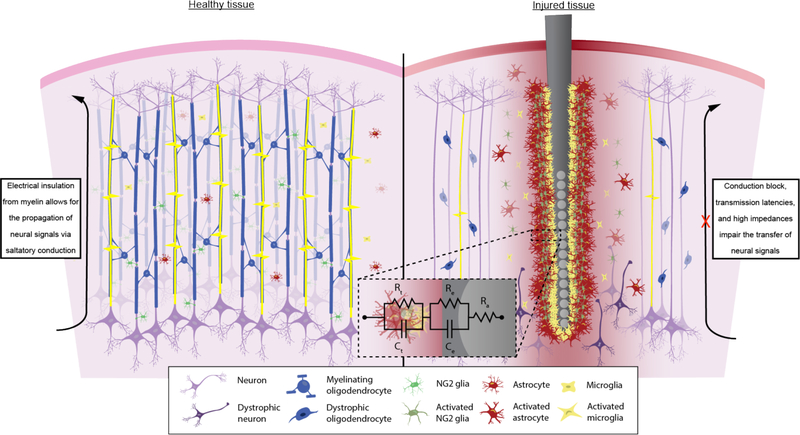
Myelin is effective at transversely reducing the capacitance and increasing the resistance of a neuronal membrane, allowing for the saltatory propagation of action potentials along an axon fiber. The minimum axonal diameter required to be myelinated by oligodendrocytes is 0.2 μm [48]. Myelination by oligodendrocytes is most notably mediated by the presence of electrical activity from neurons [49], however myelin formation can also be regulated by oligodendrocyte precursors [50], astrocytes [51], microglia [52], and oligodendrocytes themselves [53]. Inhibition of electrical activity using voltage gated Na+ channel blockers or increased concentration of extracellular K+ prevents myelination [54]. Oligodendrocytes cultured in vitro by themselves display intracellular proteolipid protein (PLP) with membrane bound myelin basic protein (MBP) and GalC myelin proteins; however, when cultured with neurons, PLP is produced and transported to the cell surface via lipid rafts where it is normally observed around myelinated axons [55]. Secretion of LINGO-1 by neurons and oligodendrocytes, which is a ligand inhibitory to neurite outgrowth [56], prevents differentiation and myelination of axons in vitro through the activation of RhoA [53], a protein that regulates the cytoskeletal and morphological changes during oligodendrocyte differentiation [57]. The presence of myelin solely on axonal fibers (and not dendrites or other cell structures) suggests cell surface markers specific to axons that regulate myelin, such as polysialic acid neural cell adhesion molecule (PSA-NCAM) [58, 59]. However, removal of negative PSA-NCAM modulation does not reverse the inhibition of myelination through use of voltage-gated Na+ channel blockers such as TTX in vitro, suggesting that both neuron cell surface moieties and electrical signaling are required to elicit the formation of myelin [59].
White matter consists of a high density of myelinated axons that are responsible for transferring information between the peripheral and central nervous system. Myelination in the gray matter was largely unknown until recent advances in high resolution neuroanatomy imaging have allowed researchers to visualize different myelination patterns of gray matter regions in the brain. Through the use of advanced electron microscopy (EM) techniques, it has been observed that patterns of gray matter myelination in the cortex are distinct from those commonly observed in white matter and myelination differs between different cortical layers, with a higher density of myelin covering deeper cortical layers (layers 5 and 6) compared to more superficial layers (layers 2 and 3) [60]. Interestingly, another study using EM combined with array tomography (AT) of the mouse neocortex observed a large portion of those myelinated cortical axons to be inhibitory interneurons (GABA-expressing neurons), specifically parvalbumin-positive (PV+) basket cells, comprising half of myelinated layer 2/3 axons and a quarter of myelinated layer 4 neurons [61]. The postsynaptic targets of these neurons are mainly the dendrites and neuronal soma of pyramidal cell neurons, which provide the large output of myelinated axons leading out of the CNS via white matter. Considering inhibitory neurons are only 10–20% of total neurons in the cortex, the myelination of these particular cells could play a critical function for neuronal viability and brain circuitry [62].
Lastly, there exists a different population of oligodendrocytes in the cortex, called satellite oligodendrocytes, distinct from their myelinating counterparts [63, 64]. These satellite oligodendrocytes, which reside in close proximity to the soma of neurons, are responsible for providing metabolic support, preventing cellular apoptosis, and assisting with remyelination following injury [65–67]. Whether there are heterogenous differences in the reaction of satellite and myelinating oligodendrocytes to injury induced during chronic microelectrode implantation remains to be seen.
3.3. NG2 glia and oligodendrocyte mediate neuronal physiology
Demyelinating diseases and oligodendropathies such as multiple sclerosis (MS) and amytrophic lateral sclerosis (ALS) are characterized by chronic axon degeneration, emphasizing the dependence of neurons for oligodendroglial support [68, 69]. Previously, it has been shown that NG2 glia and oligodendrocytes both promote neuronal viability through the secretion of insulin-like growth factor 1 (IGF-1) in vitro [70]. Although both oligodendrocytes and NG2 glia maintain direct contact with axons through the extension of myelin sheaths and formation of synaptic contacts, respectively, their purpose and function are distinctly different. However, both contribute to overall neuronal health and are essential in maintaining tissue homeostasis during injury.
Within the cortex, NG2 glia reside close to neuronal dendrites and soma where they receive synaptic input through glutamatergic and GABAergic activity [71, 72]. Depending on the cell fate of NG2 glia, some cells can possess voltage-gated sodium channels (NaV) [73]. NG2 glia that differentiate into myelinating oligodendrocytes exhibit a downregulation of both glutamate receptors and NaV channels, while undifferentiated glia maintain their excitability [74]. This is most likely due to the fact that neuronal activity can direct the migration, proliferation, and differentiation of mature NG2 glia, in which case differentiated oligodendrocytes would not require this excitability. Specifically, patterns of high frequency stimulation of callosum axons in the mouse brain promote an increased proliferation of NG2 glia while low frequency stimulation induces NG2 glia differentiation into myelinating oligodendrocytes [75]. NG2 glia have demonstrated neuronal modulation of network activity via cleavage of the NG2 proteoglycan, influencing alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) dependent activity in pyramidal cells [76], and secretion of known neuromodulatory factors [77]. The selective ablation of NG2 glia in the prefrontal cortex of mice induces altered glutamatergic signaling in neurons and subsequent expression of depressive-like behavior [78]. Another study involving the genetic ablation of adult NG2 glia in vivo observed induced neuroinflammation via activation of microglia, leading to neuronal cell death [29]. It was also found that hepatocyte-growth factor (HGF), which is an important anti-inflammatory factor that supports neuronal survival, is secreted by cultured NG2 glia [79]. During oligodendrocyte cell death, NG2 glia are responsible for the proliferation, differentiation, and initiation of myelination around demyelinated axons to preserve neuronal health. Thus, NG2 glia are critical for mediating neuronal communication and support through synaptic activity as well as providing precursors for oligodendrocyte differentiation and myelin development.
As well as providing electrical insulation via myelin ensheathment, oligodendrocytes provide neurotrophic support to neurons through the release of glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and IGF-1 [80–82]. Likewise, oligodendrocytes match the high metabolic demand of neurons through the provision of energy metabolites, glucose and lactate, via high expression of monocarboxylate transporter 1 (MCT1) [83]. MCT1 is predominantly localized to the myelin sheaths that surround axons and controls lactate export from oligodendrocytes [84]. Neurons also express their own form of monocarboxylate transporters (MCT2), allowing them to import energy metabolites secreted by astrocytes [85]. Additionally, through the release of glutamate that bind primarily to NMDA receptors, neurons instruct oligodendrocytes to transfer metabolite and antioxidant-loaded membrane vesicles, called exosomes, through the perineuronal space, which is the space between myelin and neuronal membranes [86]. As a result, in the condition in which oligodendrocytes are not able to establish contact with axons due to demyelination or via the physical blockade of trophic factors by an implanted device can have detrimental consequences to the health of neuronal tissue and the overall performance of neural interfaces in the brain.
3.4. NG2 and oligodendrocyte crosstalk with other glial cells
Astrocytes and NG2 glia are distinct types of glia that maintain differences in morphology and function despite originating from similar precursor cells during development [87]. However, both glia extend connections to nodes of Ranvier and synapses on neurons, indicating they may influence each other’s physiology [88]. Astrocytes are important mediators of the migration, proliferation, and differentiation of NG2 glia during CNS development. Ncadherins present on the surface of astrocytes influence NG2 glia migration in vitro by anchoring them to their cell surface [89]. Additionally, the same way neurons elicit a calcium Ca2+ transient current within NG2 glia following glutamate secretion, astrocytes elicit a similar NG2 glia calcium transient through the release of adenosine and ATP [90]. Both secreted factors regulate NG2 glia differentiation; however, adenosine acts directly on the NG2 glia while ATP acts indirectly by prompting astrocytes to express leukemia inhibitory factor (LIF) [91, 92]. Astrocytes also promote the proliferation and differentiation of NG2 glia by secretion of plateletderived growth factor AA (PDGF-AA). Likewise, different cytokines that are normally expressed during injury influence astrocyte-induced migration of NG2 glia [93]. Chemokine receptors CXCR1 and CXCR2 expressed by oligodendroglia during development are known targets for astrocyte-secreted CXCL1, which stimulates NG2 glia proliferation in vitro via a PDGF-AA signaling mechanism [94]. Astrocytes also secrete soluble factors that protect NG2 glia from oxidative stress via ERK and Akt signaling pathways [95]. However, during insertion injury reactive astrocytes may secrete alternative pro-inflammatory factors that supersede this protective effect. Lastly, it has been observed in vivo that NG2 glia can develop into protoplasmic astrocytes within gray matter regions of the brain [96]. However, NG2 glia also possess a limited ability to differentiate into scar-forming astrocytes during injury, prompting the question about their role during the development of an inflammatory glial scar and overall disease pathology.
Like NG2 glia, oligodendrocytes also share a close spatial relationship with astrocytes. Astrocytes create a “glial syncytium” or glial network with oligodendrocytes through the formation of gap junctions [97]. This glial syncytium is hypothesized to re-distribute potassium ions from axons following neuronal activity [98]. Astrocytes play a significant role in differentiation and myelination of oligodendrocytes throughout maturation and during injury. Through physical contact alone, astrocytes can promote oligodendrocyte maturation via interactions with integrins [99]. Additionally, a variety of astrocyte-secreted factors such as neuregulin-1, gamma-secretase, ciliary neurotrophic factor (CNTF), insulin-like growth factor 1 (IGF-1), osteopontin, and neurotrophin-3 (NT3) are known to influence myelination [100]. Bone morphogenetic proteins (BMPs) secreted by reactive astrocytes negatively regulate the differentiation of NG2 glia into mature oligodendrocytes in vitro, inhibiting the expression of myelin proteins during differentiation [101]. Hyaluronan, fibronectin, platelet-derived growth factor (PDGF), fibroblast-derived growth factor 2 (FGF-2), and tenanscin C also expressed by reactive astrocytes can impair remyelination [100]. This indicates that the pathological state of astrocytes is a strong determinant for the promotion or inhibition of myelination following injury.
Depending on the activation state of microglia, they can either secrete pro-inflammatory or anti-inflammatory molecules which differentially modulates NG2 glia and oligodendrocytes. During normal physiology, microglia are known to promote NG2 viability and oligodendrocyte maturation via upregulation of transcription factor NFκB as a result of PDGF-α receptor stimulation [102]. A reduction in tumor necrosis factor alpha (TNF-α) secretion has been shown to reduce NG2 glia proliferation and delay remyelination [103]. However, TNF-α also reduces NG2 glia differentiation into myelinating oligodendrocytes [104]. Binding of TNF-α to tumor necrosis factor receptor 2 (TNF-R2) as opposed to tumor necrosis factor receptor 1 (TNF-R1) induces this reparative effect following demyelination [105]. Microglia can modulate the differentiation of NG2 glia into oligodendrocytes via expression of galectin-3 but not galectin-1 [106]. However, galectin-1 attenuates the loss of myelin and neurodegeneration in animal models for MS [107]. Interestingly, non-reactive microglia and astrocytes influence NG2 glia differently [108]. Astrocyte-derived medium, which consists of increased PDGF-AA, FGF2, CNTF, and growth hormones, promotes proliferation of NG2 glia while microglia-derived medium, consisting more of IGF-1, CX3CL1, IL-2, and IL-5, promotes the differentiation of NG2 glia, indicating different fundamental roles in regulating the fate of oligodendroglia [100]. During injury, TGF-β instructs microglia to secrete hepatocyte growth factor (HGF), which is a chemotactic molecule that recruits NG2 glia for proliferation and differentiation into remyelinating oligodendrocytes [109]. Using two-photon microscopy to observe NG2 glia and microglia dynamics in response to probe insertion in vivo, there was a 12 hour delay in the activation of NG2 glia after microglia activation which may be representative of a temporal, chemotactic response between the two glial cells [110]. Similarly, activated microglia inhibit the proliferation of NG2 glia and promote their cell death [108, 111].
Microglia are a primary source of iron for oligodendrocytes. Prior to the differentiation of NG2 glia, microglia accumulate iron in preparation for myelination by mature oligodendrocytes [112]. Oligodendrocyte viability is dependent on the supplementation of ferritin by microglia. When microglia become activated during injury they produce many pro-inflammatory mediators such as glutamate, matrix metalloproteinases, and reactive oxygen and nitrogen species that make oligodendrocytes vulnerable to excitotoxic or oxidative stress [113]. Lipopolysaccharide-induced activation of microglia via the toll-like receptor 4 (TLR4) pathway has shown to induce oligodendrocyte cell death as well as hypomyelination [114]. However, activated microglia can still produce trophic factors such as insulin-like growth factor 2 (IGF-2), which supports oligodendrocyte viability [115]. Reverting microglia from a pro-inflammatory phenotype to an anti-inflammatory phenotype improves oligodendrogenesis and myelin formation during remyelination [116]. Likewise, oligodendrocytes under duress are known to produce several chemotactic factors (CXCL10, CCL2, and CCL3) that recruit microglia to areas of injury [117].
Understanding how these glial cells function during injury is obscured by the fact that multiple phenotypes, or “polarizations”, can arise under different circumstances. Based on simplified in vitro analysis, microglia have historically been categorized as either ‘M1’ pro-inflammatory or ‘M2’ anti-inflammatory phenotypes primarily on exposure to different cytokine stimuli [118]. However, these are isolated states and fail to account for the assortment of signaling molecules that are secreted during injury in vivo [119]. Additionally, these distinct inflammatory profiles are being refuted by investigators as further information is uncovered about the complexity of microglia polarization [119]. Microelectrode insertion injury induces both pro-inflammatory and antiinflammatory microglia states and administration of therapeutics can modulate microglia activation [120]. Similarly, astrocytes demonstrate varying phenotypes during inflammation (‘A1’/’A2’ polarizations) that complicate understanding of their roles following injury. For example, astrogliosis is traditionally viewed as neurodegenerative and detrimental to the surrounding tissue, however, evidence suggests that reactive astrocytes can provide neuronal protection via inhibition of apoptosis, neurotrophic support, and homeostatic balance [121]. Activation of microglia and astrocytes, while commonly seen as perpetuators of inflammation, are required for tissue repair and regeneration. Activated microglia are responsible for myelin debris clearance and promotion of oligodendrogenesis whereas reactive astrocytes secrete factors that modulate NG2 glial responses [122]. Thus, a more thorough understanding of these pro-inflammatory and anti-inflammatory glial phenotypes is required to determine the true nature of their functions and interactions with other cells during CNS injury.
3.5. Perivascular NG2 cells interact with the BBB
In the developing CNS, angiogenesis and foundation of the BBB occurs around E11, before the appearance of NG2 glia, where oligodendrocyte precursors use the vascular endothelium to migrate away from their sites of origin [38, 123]. From there, NG2 glia either distribute throughout the parenchyma (parenchymal NG2 glia) or reside in close proximity to endothelial-bound pericytes (perivascular NG2 glia). Previous studies suggest a bidirectional relationship between NG2 glia and pericytes, where exposing conditioned media of one cell type has a proliferative effect on the other [124]. Interactions between NG2 glia and endothelial cells also occur. NG2 glia prevent the permeability of the BBB by secreting transforming growth factor beta (TGF-β) which binds to TGF-β receptors on the surface of endothelial cells, upregulating the expression of tight junction proteins (claudin-5 and ZO-1) via MAPK/ERK pathways [125]. Likewise, endothelial cells secrete fibroblast growth factor-2 (FGF-2) and brain-derived neurotrophic factors (BDNF) which have an influence on the survivability and proliferation of NG2 glia via Akt and Src signaling pathways [126]. Endothelial cells also secrete vascular endothelial growth factor (VEGF-A) which promotes the migration of NG2 cells, but not their proliferation, through VEGF-receptor 2 (Flk-1) signaling [127]. VEGF induced migration of NG2 glia is mediated through cytoskeletal rearrangement in an ROS- and FAK-dependent manner [128]. In contrast, VEGF-C reportedly influences the proliferation of NG2 glia via VEGF-receptor 3 signaling cascades [129].
3.6. Summary of oligodendroglial role in brain homeostasis
Due to their inherent spatial relationships with neurons, oligodendrocytes and their precursor cells are critical regulators of brain physiology. Unlike other glia, oligodendrocytes and NG2 glia are direct sources of neurotrophic and metabolic support, and each cell type has specific roles in modulating neuronal activity. As a result, they must maintain high energy demands to keep up the constant metabolic activity of active neurons. This presents opportunities for severe trauma during injury such as microelectrode implantation, which induces oxidative stress and excitotoxic shock. Additionally, oligodendrocytes and NG2 glia share functional relationships with other constituents of the brain such as microglia, astrocytes, and mural cells that support the blood-brain barrier. Impairment in their normal physiological behavior could trigger a cascade of neuroinflammatory events involving different cell networks within the brain. Oligodendrocytes and NG2 glia may react similarly during injury, succumbing to inflammation-induced cell death, which would increase demyelination injury, impair the potential of remyelination repair, and deprive neurons of neurotrophic and metabolic support, ultimately impairing recording and stimulating capabilities using chronically implanted neural electrodes. However, NG2 glia may take on other roles such as possibly differentiating into scar-forming astrocytes and contributing to proinflammatory tissue events. As such, it is important to investigate these cells types during trauma and determine their influence in the foreign body reaction to implanted neural interfaces and their overall role in progressive neurodegenerative disease.
4. Role of oligodendroglia in acute tissue inflammation
The onset of acute inflammation following injury has a purpose to protect against foreign pathogens and repair damaged tissue. Immediate activation of phagocytotic microglia triggers removal of cellular debris and continual secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), chemokines (MCP-1, CXCL-1), reactive oxygen species (NADPH oxidase, MPO), and coagulation factors. Microglia also secrete neurotrophic factors such as BDNF upon activation, which indicates a parallel role in the reactive tissue response to repair tissue [130]. However, BDNF has been shown to increase the number of activated microglia in vivo, suggesting it may amplify microglia reactivity and persist in a chronic inflammatory response [131]. Reactive astrocytes increase expression of glial fibrillary acidic protein (GFAP), an intermediate filament involved in modulating the cell’s structure and mechanical strength, and encapsulate foreign bodies within a glial scar. Cells within this scar express axon growth inhibitory CSPGs that prevent the functional recovery of tissue [132]. Oxidative stress from microglia and astrocyte reactivity impairs function of tight junction proteins in endothelial cells, increasing BBB permeability [133, 134]. Endothelial cells express a variety of cell adhesion molecules (E-selectin, P-selectin, VCAM, ICAM) which promote the recruitment of leukocytes and neutrophils to the site of injury [135]. Pericytes, whose primary function is to help clear metabolic waste and maintain EC viability, are also known to promote entry of immunologic T cells and monocytes into the brain parenchyma [136]. These infiltrating cells produce harmful oxidative species, secrete BBB-degrading MMPs, and release pro-inflammatory cytokines such as IFN-γ which further exacerbates glial cell activation [137]. As a result, neuronal viability is compromised and the formation of a glial scar prevents the diffusion of neurotrophic factors, ions, and charged solutes, impairing tissue physiology.
The acute foreign body response to implanted neural devices is characterized by a mechanically-disrupted BBB, reduction in perfusion of oxygen and nutrients, impairment in waste removal, increase in mechanical strain due to tissue displacement and edema, and biofouling of the electrode surface [9]. Penetrating electrodes expose sensitive neural tissue to inflammatory pathogens through the disruption of the BBB [138, 139]. Due to the hydrophobicity of the electrode, plasma proteins adhere easily to the electrode surface and incite glial cell reactivity [24], which can persist over chronic time periods [140]. Chronic hypoxia as a result of occluded or damaged vasculature eventually promotes angiogenesis and the foundation of a new vascular network around sites of injury [141]. Indeed, vascular pericytes, which promote angiogenesis through the release of VEGFs and MMPs, have been observed near implanted electrodes [26, 142, 143]. Insertion causes dimpling of the surface of the brain, displacing and compressing cells and blood vessels, and can persist for hours after insertion [10, 19, 144–146]. Increased strain has been correlated with increased cell death in neurons and astrocytes as well as production of IL-1β via ERK signaling pathways in astrocytes [147–149]. Tissue movement due to stress and strain forces as well as biological micromotions are reflected in the performance of implanted electrodes, where detection of individual neurons come and go as tissue is displaced [150]. These initial tissue events following electrode insertion set the stage for a chronic inflammatory response and the severity of damage incurred during implantation is reflected at later time points in the form of exacerbated glial scar formation and neuronal loss. Thus, it is important to understand the consequences of acute inflammation during the implantation of neural interfaces into the brain.
4.1. Oligodendrocyte and NG2 glia susceptibility to oxidative stress
Oxidative stress is a primary effector of the acute inflammatory response to microelectrode failure, impacting neuronal viability and blood-brain barrier integrity [151, 152]. Oligodendrocytes and their precursors are particularly sensitive to oxidative stress after injury via multiple mechanisms of cell death (Figure 2). In order to meet the high-energy demands of myelin output oligodendrocytes must produce a substantial amount of myelinrelated proteins, requiring high metabolic rates [153]. While continuous myelin deposition does not occur in the adult brain, newly matured remyelinating oligodendrocytes could sustain increased susceptibility to oxidative insults following injury. As a result, potentially toxic byproducts of cellular metabolism are formed, such as hydrogen peroxide and reactive oxygen and nitrogen species, which can cause DNA damage, lipid peroxidation, and apoptosis of oligodendrocytes if not metabolized properly [154, 155]. Mitochondria of oligodendrocytes are targets for oxygen and nitrogen radicals, interfering with the essential proteins involved in cellular respiration. Indeed, toxic models of demyelination involve the use of cuprizone, a copper-chelator known to disrupt complex IV of the mitochondrial respiratory chain [156]. Both oligodendrocytes and NG2 glia contain low amounts of the antioxidant glutathione, which makes them even more vulnerable to oxidative damage than other glial cells in the brain when trying to cope with oxidative stress [157, 158].
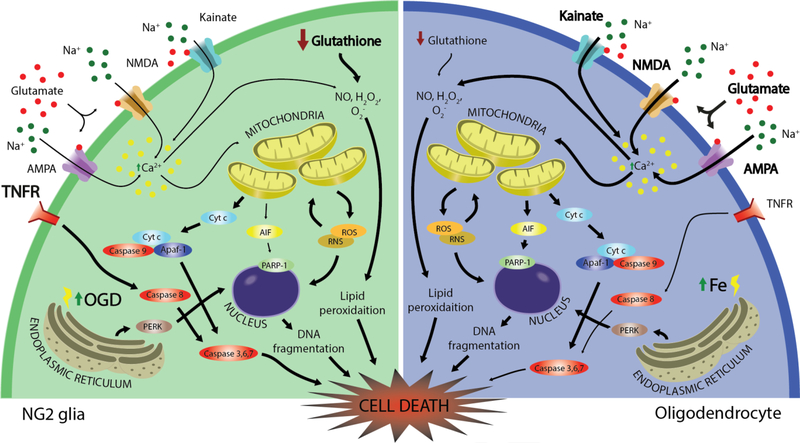
Figure 2. Molecular mechanisms of NG2 glia and oligodendrocyte cell death.
Both NG2 glia and oligodendrocytes are susceptible to oxidative and excitotoxic cell death. Low levels of glutathione render each cell vulnerable to reactive oxygen and nitrogen species that lead to mitochondrial-induced stress. Due to their high metabolic rates, oligodendrocytes have increased susceptibility to iron accumulation within their cell bodies and myelin membranes. Cell death pathways of greater severity are emphasized in bold. NMDA: N-methyl-Daspartate receptor, AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, TNFR: tumor necrosis factor receptor; NO: nitric oxide; Cyt c: cytochrome c; Apaf-1: apoptotic protease-activating factor 1; AIF: apoptosis-inducing factor, PARP-1: poly [ADP-ribose] polymerase 1, PERK: protein kinase R (PKR)-like endoplasmic reticulum kinase, ROS: reactive oxygen species; RNS: reactive nitrogen species; OGD: oxygen glucose deprivation.
Oligodendrocytes store high amounts of iron and ferritin subunits relative to other cells in the brain to account for the amount of oxygen consumed and ATP produced during myelin formation [159]. Binding of ferritin to iron occurs coincidentally with the formation of myelin in the developing brain and it has been suggested that oligodendrocytes are responsible for regulating iron throughout the CNS [160, 161]. Oxidative stress has been linked to iron dysfunction in oligodendrocytes and, when exposed to hypoxic conditions, iron accumulates inside the cell and induces endoplasmic reticulum (ER) stress and mitochondrial dysfunction resulting in cell death [162]. Likewise, excess levels of iron can result in the formation of hydroxyl radicals, a toxic reactive species implicated in DNA, lipid, and protein disruption [163]. Interfering with the ability for oligodendrocytes to maintain iron homeostasis in the brain can have detrimental effects on microelectrode performance. For example, transferrin, an iron transport protein, is synthesized by oligodendrocytes and is responsible for transporting iron into neurons [164]. Increased ferritin levels have been observed around microelectrodes with poor electrode performance [165]. Hemosiderin, an iron-binding molecule, is hypothesized to result from the interaction of iron and free-radicals. Hemosiderin-laden macrophages have been observed next to implanted electrodes, suggesting possible phagocytosis of iron-containing cells such as oligodendrocytes or iron-bound myelin debris [151]. Dysregulation of iron has been observed alongside neurodegeneration and demyelination in a variety of neurodegenerative diseases, which can allude to a role in the loss of neuronal signaling around chronically implanted electrodes [166–168].
4.2. Glutamate-mediated excitotoxicity of oligodendrocytes and their precursors
Oligodendroglial cells express glutamate-binding receptors AMPA, kainate, and NMDA [169, 170]. Both mature oligodendrocytes and NG2 glia have shown excitotoxicity during prolonged glutamate exposure in vitro [171, 172]. One mechanism of action leading to cell death via glutamate excitotoxicity involves increased production of free radical species in mitochondria responding to an increase in intracellular calcium [173]. It has been shown that varying the activation of kainate and AMPA receptors leads to apoptosis of oligodendrocytes in either caspase-dependent or –independent manners [173]. Likewise, oligodendrocyte cell death can occur indirectly through glutamate signaling via the release of pro-inflammatory cytokines TNF-α and IL-1β from activated microglia [174, 175]. Elevated expression of glutamate transporters has been observed acutely around inserted devices, which may precede NG2 glia and oligodendrocyte excitotoxic cell death, subsequently leading to demyelination and degeneration of axons around chronically implanted microelectrodes and impairing their recording and stimulating potential [176].
4.3. Oligodendrocytes and myelin susceptibility to BBB disruption
Although penetration of major blood vessels is preventable using vascular mapping techniques, damage to smaller vessels and capillaries can be difficult to avoid especially with multi-shank electrodes [10]. BBB disruption can persist throughout the lifetime of the implant in cases where microelectrode steric blockade prevents repair of the vasculature membrane or angiogenesis. Additionally, biological micromotion (i.e. breathing) or mechanical impact to the electrode can rupture vessels, resulting in secreted cytokines, which increase the permeability of endothelial cells and exposure of brain tissue to both inflammatory plasma proteins and coagulation factors that are known to contribute to neurodegeneration and demyelination [177]. Fibrin, the insoluble form of the plasma protein fibrinogen, is observed in active and chronic lesions of multiple sclerosis prior to the vasculature infiltration of T cell leukocytes and demyelination [177]. Direct correlation between the exposure of fibrin on the CNS and the recruitment of myelin-targeting Th1 cells has been demonstrated in vivo. In this study, fibrin promoted the secretion of chemokines CCL2 and CXCL10 via CD11b binding of activated microglia, which induced recruitment of T cells and macrophages, respectively [178]. Fibrin signaling also provokes T cell differentiation toward myelin antigen-specific Th1 cells via upregulation of IL-12 [178]. Thrombin, another inflammatory factor originating from vascular spillover, is responsible for converting fibrinogen into fibrin deposits. Thrombin acts as a suppressor of ERK1/2 and AKT-mediated CNS myelination via activation of protease-activated receptor 1 (PAR1) on oligodendrocyte precursors [179]. PAR1 is also expressed in T cells, endothelial cells, glial cells, and neurons [180]. PAR-1 activation by thrombin exerts direct neurophysiological effects at the nodes of Ranvier in vivo, inducing conduction block in rat sciatic nerves [181]. Thrombin can have both neuroprotective and neurodegenerative effects depending on its concentration, protecting hippocampal neurons and astrocytes at low concentrations yet mediating cell death at higher concentrations [182, 183]. As a result, disruption of the blood-brain barrier via microelectrode-induced mechanisms can have a negative impact on the myeloarchitecture which is responsible for the exchange of neuronal information to and from the implanted device, impairing performance output.
5. Oligodendroglia pathophysiology during the chronic foreign body response
The physiological functions of oligodendrocytes and NG2 glia are compromised during chronic disease. In MS, it is widely accepted that NG2 glia persist in chronic demyelinated lesions without differentiating into new, myelinating oligodendrocytes. However, the cause for this differentiation block is still unknown. Since NG2 glia are responsible for replenishing depleted oligodendrocytes after injury and oligodendrocytes themselves express membrane receptors for a variety of cytokines and chemokines produced by activated glia, their behavior in the context of chronically implanted electrodes can broaden the perspective of the foreign body response to inserted devices [184].
The damages sustained from chronic implantation of intracortical electrodes are most clearly demonstrated in stab wound studies. Immediately implanting and removing a probe results in a decreased activation of glial cells as well as a reduction in neuron loss over time, compared to a chronically implanted probe [185]. Following the physical removal of the implant, signs of neurogenesis in the lesion site begin to show owing to the limited capacity for the brain to regenerate after injury [186]. Long-term damage due to electrode implantation is thus characterized by persistent glial scarring and neurodegeneration leading to continual pro-inflammatory cytokine release and tissue degradation, which generates a “kill zone” radially around the implant [185]. Both gliosis and neurodegeneration are attributed to the tissue failure of chronically implanted microelectrodes via increases in noise and impedance and a loss of recordable signal [9]. Hypertrophic astrocytes and reactive microglia accumulate around the implant and displace neurons from the surface of the electrode, which reduces the ability to yield high SNRs [187]. These same activated microglia and astrocytes secrete a multitude of inflammatory cytokines that perpetuate BBB breakdown, further glial cell reactivity, and activate apoptotic signaling cascades in neurons [9]. Due to their influence on neuronal health, both NG2 glia and oligodendrocytes may contribute to degrading quality in performance on microelectrodes overtime and its apparent inability to recover from glial scarring and loss of neurons.
5.1. NG2-glia activation during glial scar formation
NG2 glia and astrocytes both divide from radial stem cells during development before migrating and proliferating throughout the CNS [188]. Use of NG2-Cre mice for genetic fate mapping of NG2 glia in vivo show their differentiation into protoplasmic astrocytes (as well as oligodendrocytes) during development [96]. During glial scar formation, astrocytes proliferate, become hypertrophied, release intermediate filaments GFAP and vimentin, and secrete cytokines, chemokines, and inhibitory CSPGs [189]. Similar to astrocytes, NG2 glia display a hypertrophied morphology and increase expression of inhibitory CSPGs within 24 hours after injury (Figure 3) [190]. Likewise, similar to microglia, NG2 glia extend processes and migrate towards the lesion site, however, at a slower rate [35, 110]. One of the characteristic features of the tissue response to electrode insertion into the brain is the gradual development of a glial scar that persists throughout the lifetime of the implant, impairing neuronal function and altering exchange of electrical signals [2, 185, 191]. NG2-Cre mice used to observe differential fate after cortical stab injury show only 8% of NG2 glia express astrocytic GFAP after 10 days, decreasing to 2% of NG2 glia that are GFAP-positive after 30 days post-lesion. However, in the case of spinal cord injury, 25% of NG2 glia are immunoreactive for GFAP at 7 days post injury, decreasing to 8% of NG2 glia that express GFAP by 4 weeks. It has been suggested, with respect to spinal cord injury where secondary damage is more likely to occur and gliosis is more prevalent, that the severity of injury modulates the astrocytic potential of differentiating NG2 glia under pathological conditions in the CNS [192]. Since the foreign body reaction to intracortical microelectrodes has been characterized as having acute and chronic inflammatory events persisting throughout the lifetime of the implant, the astrogliogenic potential of NG2 glia could be amplified under these conditions. However, that has yet to be observed.
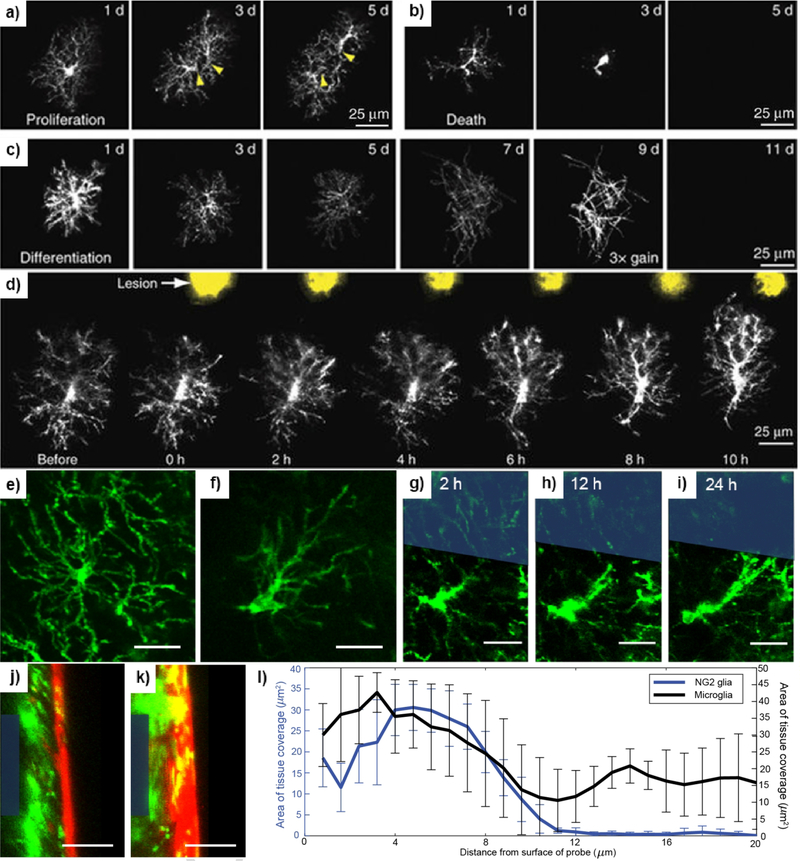
Figure 3: NG2 glia physiology and pathology.
(a) Proliferation of NG2 glia shown in vivo using two-photon microscopy on CSPG4-eGFP mice. Dividing cells can be identified by individual somas (yellow arrowheads) migrating in opposing directions. (b) NG2 glia turnover is identified as changes in cell morphology and decrease in fluorescence intensity. (c) NG2 glia can be observed differentiating into myelinating oligodendrocytes via distinct changes in the morphology of their cellular processes into more stratified myelin extensions and gradual loss of fluorescence intensity due to the downregulation of the NG2 promoter. (d) NG2 glia respond to photoablation injury via extension of processes and migration of cell somas. Reprinted by permission from Springer Nature: Nature Neuroscience [35], © 2013. (e) A ramified, or non-activated, NG2 glial cell identified by the extension of processes radially in equal directions around the cell body. (f) A transitional, or activated, NG2 glia cell in response to injury or perturbation in its surroundings. Activated NG2 glia are identified by preferential extension of processes in a particular direction relative to their cell soma. Scale bar = 25 μm. Reprinted from [110], with permission from Elsevier © 2018. NG2 glia become activated in response to electrode insertion (blue outline) and can be seen extending processes at 2 hours (g), 12 hours (h), and 24 hours (i) before making contact with the electrode surface. Scale bar = 25 μm. Reprinted with permission from [193]. © 2017 American Chemical Society. Visualization of the electrode surface via cross-sectional views of the probe (blue outline) implanted for 72 hours in mouse models labeling NG2 glia (j) and microglia (k) for comparison. A red fluorescent dye was used to label the vasculature. Scale bar = 25 μm. Adapted from [110], with permission from Elsevier © 2018. (l) Area of tissue coverage was measured as a function of distance from the probe surface showing the distribution of NG2 glia and microglia near the vicinity of the electrode. Counts were binned every 2 μm.
5.2. NG2-glia as neural growth inhibitors
Expression of CSPGs by NG2 glia is upregulated and localized around the lesion during CNS injury with notable inhibition of neuronal regeneration [189, 194, 195]. Indeed, expression of CSPGs was observed up to 135 μm around implanted microelectrodes at 1 week post-insertion, coinciding with the proliferation of astrocytes and generation of the glial scar [196]. During development, neurites actively avoid CSPG dense regions, implying CSPGs are “negative” regulators of axon growth [197]. In vitro neurite extension does not occur on substrates coated with purified NG2, a transmembrane CSPG expressed on the surface of oligodendrocyte precursors [198]. Use of antibodies to neutralize NG2 on oligodendrocyte precursors only partially reverses the inhibitory effect of the proteoglycan, due to the fact that NG2 glia express other growth-inhibitory molecules such as semaphorin 5A [199]. However, some studies suggest that NG2 expression promotes axon growth after injury [200, 201]. NG2 glia form synaptic connections with neurons, a feature unique amongst glia cells of the CNS. They express voltage-gated sodium channels as well as post-synaptic receptors for glutamate and GABA, demonstrating the ability to generate action potentials and sense activity from neurons [202]. NG2 glia also express fibronectin and laminin on their membrane surface, two ECM molecules that promote neurite outgrowth and are expressed in the lesion core after CNS injury [203]. After spinal cord injury, axons become dystrophic and “die back” from the lesion, associating with nearby NG2 glia [192]. In NG2 null mice, fewer synaptic connections are made after SCI and damaged axons retreated to farther distances than normal controls.
5.3. Demyelination and remyelination of axons during oligodendrocyte pathology
Changes to the myelin structure around implanted microelectrodes have only been briefly investigated previously (Figure 4). One study observed acute changes to the myelin membrane as early as 1 hour following microelectrode insertion via the formation of “myelinosome” perturbations [204]. Likewise, only one investigation observed potential demyelination around chronically implanted microelectrodes, visualizing demyelinated axons 12 weeks post-implantation in the rat cortex [11]. Usually demyelination occurs when the viability of the oligodendrocyte cell is compromised, referred to as primary demyelination, and is different from myelin degradation due to neuronal or axonal loss, referred to as secondary demyelination [205]. Following a demyelinating incident, remyelination does not occur from existing myelinating oligodendrocytes but from a reservoir of previously quiescent and mature NG2 glia which differentiate into new myelinating oligodendrocytes [206]. Activated microglia and astrocytes are responsible for prompting recruitment, proliferation, and transformation of these inert precursor cells into differentiating NG2 glia. Specifically, anti-inflammatory microglia promote remyelination after ethidium bromide induced demyelination via activation of TGFβ ligand receptors that are involved in GTPase, Akt, and mTOR signaling pathways implicated in NG2 glia differentiation and myelination [207, 208]. However, newly remyelinated sheaths are thinner than they were before injury as indicated by the g-ratio (the inner axon diameter divided by the total outer diameter) and the difference is more apparent in larger diameter axons than small diameter axons [207, 208]. Loss of oligodendrocytes and axonal demyelination lead to impairments in signal propagation and deprive neurons of essential neurotrophic factors for survival. Since signal conduction is dependent on the insulating structure of myelin, the consequence of demyelination around chronically implanted microelectrodes could result in an impairment in electrophysiological signals obtained from neurons. Additionally, the capacity for demyelinated axons to be remyelinated following injury could potentially be compromised due to the tissue response to implanted devices.
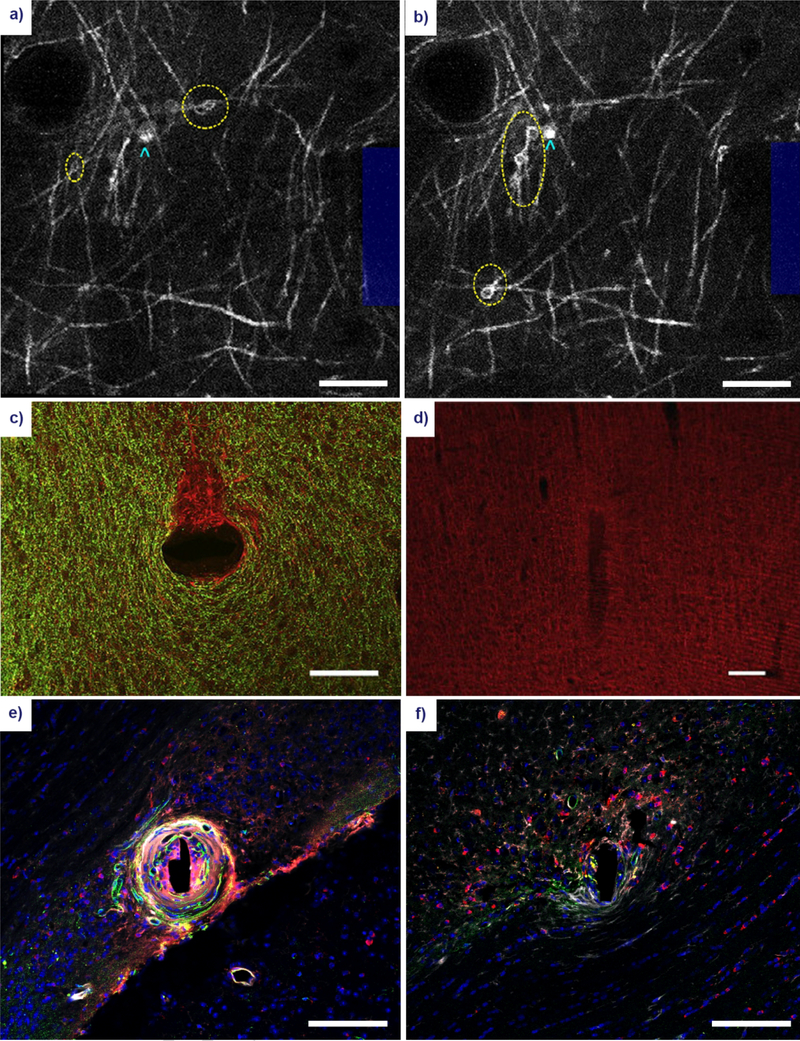
Figure 4. Gross morphological changes to oligodendrocyte and myelin structure following microelectrode implantation.
Acute myelin damage in the form of myelinosomes (yellow ellipses) can be observed within the vicinity of the electrode (blue outline) at 1 hour (a) and 6 hours (b) post-insertion using in vivo two-photon microscopy of CNP-eGFP mice. Oligodendrocyte cell bodies can be identified as adjacent somas (cyan arrowheads). Reproduced with permission from IOP Publishing [145]. Scale bar = 100 μm. Large scale damage to the myelin architecture has been observed at 12 weeks post-insertion of an electrode in the rat cortex. (c) A stain for myelin basic protein (green) shows demyelination in a discrete area of unmyelinated axons (red) in a horizontal section. Reprinted from [11], with permission from Elsevier. Scale bar = 100 μm. (d) A coronal section of the implanted electrode shows a similar characteristic reduction in myelin around the insertion site. Reprinted from [209], with permission from Elsevier. Scale bar = 100 μm. (e) A stain for APC/CC1 oligodendrocytes (red), platelet-derived growth factor β pericytes (PDGFβ, green), immunoglobulin IgG (white), and cell nuclei (blue) in white matter region CA1 of the adult mouse brain. Scale bar = 100 μm. (f) Similar stain, implant duration, and implant region as in (e) in an apoptosis-resistant Caspase-1 knockout mouse. Scale bar = 100 μm. Reprinted from [26], with permission from Elsevier.
5.4. Lack of oligodendrocyte support leads to axonal loss
Oligodendrocyte dysfunction is observed not just in demyelinating diseases such as MS, but in neurodegenerative disorders such as Alzheimer’s and ALS as well, implicating their involvement in the degradation of neurons. For example, aggregates of mutated insoluble tau protein have been observed in oligodendrocytes alongside axon degeneration [210]. Additionally, MCT1, which is responsible for transport of metabolic factors into axons, was markedly reduced in mouse models of ALS [211]. Specific ablation of oligodendrocytes, which exclude any influence of other activated glial cells or infiltrating immune cells, has demonstrated secondary axonal damage. Studies in which myelin proteins such as PLP, are overexpressed or depleted via genetic mutation are complemented by severe degeneration of neurons [212–215]. However, PLP is a protein primarily localized to the myelin sheath while CNP is exclusive to the surface of oligodendrocyte cell bodies. Studies involving the mutation of CNP demonstrated functional motor deficits alongside neuron pathology, while the amount and structure of other myelin proteins remained intact between experimental and wild-type controls [213]. This indicates a myelin-independent support of neurons by oligodendrocytes, such as through the production and secretion of neurotrophic and metabolic products.
6. Potential strategies to mitigate oligodendrocyte/NG2 glia pathophysiology
NG2 glia, oligodendrocytes, and myelin are all negatively susceptible to both acute and chronic injury events. Mediating the response of oligodendroglia during injury is the primary focus of oligodendrocyte and myelinaffected diseases such as MS, ALS, Alzheimer’s and more. Therapeutic strategies are either designed to be preventative, attempting to prevent oligodendrocyte/oligodendrocyte precursor cell loss and demyelination, or reparative, rehabilitating injured tissue following injury through the promotion of oligodendrocyte differentiation and remyelination. Additionally, a materials-based approach at influencing oligodendrocyte and oligodendrocyte precursor responses during injury is observed.
6.1. Preventing oligodendrocyte cell death and demyelination
As metabolically demanding cells, oligodendrocytes are easily susceptible to oxidative stress and excitotoxicity during injury-induced inflammation. Antioxidants such as NADPH help prevent oxidative stress-induced death in both oligodendrocytes and their precursors. For example, administration of dehydroepiandrosterone (DHEA) protects NG2 glia lacking NADPH during metabolic stress [216]. As an endogenous neurosteroid, DHEA is also known to protect against NMDA-mediated excitotoxicity and hydrogen peroxide-mediated cytotoxicity [217, 218]. Additionally, DHEA promotes neuronal survival in stroke models [219]. The upregulation of reactive oxygen and nitrogen species during inflammation due to activation of reactive glia poses a threat to oligodendrocytes by impacting mitochondrial metabolism. An anti-inflammatory agent such as methotrexate (ITMTX), which is commonly used to treat MS patients, can mitigate the immune response and alleviate the insult to oligodendrocytes and myelin. Methotrexate is a immunosuppressant that has demonstrated effective results in MS patients [220]. However, in cuprizone-treated animals, ITMTX mitigates the formation of gliosis and inhibits demyelination, demonstrating that it acts more specifically to preventing demyelination without systemic immunosuppression [221]. Recently, we have shown a novel role of GSK3b in cuprizone induced oligodendrocyte apoptosis [222]. Conditional depletion of oligodendrocytic GSK3b protects oligodendrocytes from caspase-dependent apoptosis and leads to significant attenuation of demyelination with preservation of myelin thickness and has broad effects on the surrounding glia, resulting in decreased glial activation. Inhibitors of GSK3b may be exploited to preserve oligodendrocyte viability and myelin integrity. Lastly, recent work has demonstrated a protective effect of the antioxidant resveratrol on oxidative stress-induced cell death of oligodendrocytes and NG2 glia [223, 224]. Resveratrol has previously been used to improve neuronal density surrounding chronically implanted neural electrodes and the apparent effect on neuronal viability could be a consequence of preserved oligodendrocyte and NG2 glia health. However, their viability around inserted microelectrodes remains to be seen.
6.2. Promoting oligodendrogenesis of NG2-glia and remyelination
The natural response following demyelination is for NG2 glia to migrate, proliferate, and differentiate into myelinforming oligodendrocytes around the lesion site. However, when the demyelinating injury is severe and chronic, as is the case in MS, remyelination is impaired leading to progressive neurodegenerative symptoms. Deficiencies in axon remyelination are less attributed to the reduction of NG2 glia and more a result of an impairment in the proliferation, migration, and differentiation that allows NG2 glia to transform into myelinating oligodendrocytes [225]. Region-dependent differences were found to influence oligodendrocyte differentiation; with oligodendrogenesis more likely to occur in a white matter-derived environment compared to gray matter [226]. This presents different possibilities for potential therapies, whether it is promoting differentiation by implantation of cells into the white matter or utilizing the specialized environmental or molecular cues that promote differentiation for gray matter cells. A common way to promote oligodendrogenesis is to manipulate the mammalian target of rapamycin, also known as mTOR, which is a downstream mediator of NG2 glia differentiation and oligodendrocyte myelination and acts through the phosphatidylinositol-3-phosphate kinase (PI3K/Akt) signaling pathway [227]. Rapamycin is an inhibitor of mTOR, and is known to impair myelination via reduced Akt signaling [228]. The mTOR kinase also has the ability to reduce T-cell activation and modulate other glial functions making it an emerging therapeutic target in MS research [229]. Another important pathway responsible for myelin growth involves extracellular signal regulated protein kinases 1 and 2 (ERK1/ERK2), which are downstream mediators of mitogen-activated protein kinases (MAPK) [230]. This pathway is responsible for regulating myelin thickness and mutant mice deficient in this pathway display normal NG2 glia differentiation and initiation of myelin formation, yet no observable increase in myelin thickness that normally follows axonal contact. Whether the PI3K/Akt pathway and ERK1/ERK2 pathway are independent or dependent pathways of one another remain to be seen. Acting independently of these two growth factor pathways is a third mechanism which controls oligodendrogenesis of NG2 glia via canonical Wnt-signaling, which is negatively regulated by endogenous GSK3β [231]. Application of inhibitors of GSK3β have successfully improved oligodendrocyte regeneration and remyelination within demyelinating lesions [232]. Due to their anti-apoptotic influence on cell death pathways, GSK3β inhibitors could have a dual advantage of enhancing the differentiation of oligodendrocyte precursors and protecting mature oligodendrocytes. Finally, recent work to promote cortical repair following injury involves converting other cells into functional neurons due to their inherent neurogenic potential. NG2 glia maintain the genetic machinery to be reprogrammed through the induced expression of specific transcription factors (Sox2, Ascl1) into functional neurons in the context of injury [233]. Regenerating lost neurons following injury may be an effective first step in tissue repair before NG2 differentiation into oligodendrocytes and initiation of remyelination, promoting a more innervated neural network to restore brain circuitry and function. However, if demyelination is a primary mode of failure for chronically implanted neural interfaces, restoring oligodendrocytes, their precursors, and the myelin structure may be essential to support neuronal physiology and improve device performances.
6.3. A materials-based modulation of oligodendrocyte and oligodendrocyte progenitor responses
While biomaterials have had a widespread use in an attempt to promote neural regeneration within the injured central and peripheral nervous system, oligodendrocyte regeneration using similar materials-based approaches are not as common or understood. The chemical composition of the substrate can have distinct modulating effects on NG2 glial maturation and differentiation. For example, chitosan-based biomaterials have been used in the past to promote oligodendrocyte survival and attachment [234], as well as differentiation into oligodendrocytes [234–237]. Materials that influence oligodendrogenesis from oligodendrocyte precursors include fibrin, HA-methylcellulose, poly(lactic-co-glycolic) acid (PLGA), and methacrylimde, while materials that promote myelination from mature oligodendrocytes include fibrin, PLGA, chitosan, and HA-gelatin [238]. The topographical cues and fiber diameters of varying materials also influence oligodendrocyte generation [239, 240]. Random or unaligned polycaprolactone fibers favored oligodendrocyte adherence and survival compared to aligned fibers [241], as well as promote oligodendrogenesis from NG2 glia [242]. Topographical cues provided by nanofiber alignment of varying biomaterials also positively support myelin production and remyelination of axons in vitro [243–245].
Previous studies demonstrated that substrate stiffness modulated oligodendrocyte and NG2 glial responses. Substrates of lower stiffness contributed more to NG2 glial sprouting of processes and proliferation [246, 247], while substrates of higher stiffness contributed more to NG2 glia-mediated oligodendrogenesis [247, 248].
Migration of oligodendrocyte progenitors is also dependent on the modulus of the substrate, with the optimal range for migration of NG2 glia appearing on gels with an elastic modulus around 0.7 kPa, near the modulus for soft brain tissue [248]. Many in vitro studies demonstrated that the potential application for neural progenitor cells (NPCs) to differentiate into oligodendrocytes also depends on the mechanical properties of the environment; however, the results are more material-dependent. NPCs cultured on softer hyaluronic acid (HA)-methacrylate hydrogels differentiated into oligodendrocytes, whereas stiffer HA-methacrylate gels generated more NPC-derived astrocytes [249]. Alternatively, NPC differentiation into oligodendrocytes favored stiffer methacrylamide chitosan substrates [235]. The implications of these results can have significant effects on the oligodendroglial response considering the field of neural interface engineering is moving toward softer, more flexible biomaterials [250, 251]. As a result, the increased compliancy of these materials may be favoring increased NG2 glia recruitment and proliferation, whereas stiffer substrates would promote oligodendrocyte generation from neuronal and oligodendrocyte progenitors alike [248].
Finally, use of nanoparticles (NPs) for delivery of modulating drugs are also being investigated as potential therapeutic approaches to improving oligodendroglial function. NPs targeted toward oligodendrocyte progenitors specifically (for example, via surface-coated antibodies against NG2 antigen) can be used to deliver promyelination factors such as leukaemia inhibitory factor (LIF) or oligodendrocyte-generating transcription factors Olig1 and Olig2 [252, 253]. Similar methods can be applied to the potential delivery of pro-oligodendrocyte and pro-myelination factors, such as BDNF, neuregulin, and/or neurotrophin-3 [254–257]. Surface functionalization or hydrogel encapsulation of nanoparticles around devices could help facilitate this method of therapy.
7. Attenuating reactive tissue response of implanted devices: new insights
A variety of approaches to intervene during the inflammatory reaction of neural interfaces have been investigated to improve their long-term stability and functionality. Historically, these approaches are evaluated on their ability to mitigate the activation of glial cells and reduce chronic neurodegeneration in an attempt to improve recording performances following chronic implantation. Given the newly acquired knowledge of oligodendrocyte and NG2 glia susceptibility to inflammation-induced injury, alternative and novel approaches should be considered to account for their potential role in the biological failure mode of implanted devices.
7.1. Reducing the accumulation of oxidative stress following probe insertion
Hypoxic and ischemic insults due to BBB disruption and the accumulation of reactive oxgen species due to glial cell activation contribute to the oxidative stressors that mediate some of neuroinflammatory responses of implanted microelectrode arrays [258]. Previously, anti-oxidants have been used to reduce the build-up of ROS through either localized drug delivery, systemic administration, or surface modification [152, 259, 260]. Oligodendrocytes and oligodendrocyte precursors are particularly susceptible to these stressors in an ERmediated manner, which is a common method of oligodendroglial loss in neurodegenerative diseases such as MS, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, and Parkinson’s disease [261, 262]. Using guanabenz for pharmacological inhibition of IFN-γ which phosphorylates PERK, a common ER-stress associated protein, has demonstrated increased oligodendrocyte viability both in vitro and in vivo, reducing clinical symptoms in chronic MS models [263]. Additionally, protecting oligodendrocytes from ER-stress directly with salubrinal rescued hypomyelination and oligodendrocyte cell death in oligodendrocyte cultures exposed to IFN-γ directly [264]. Further methods to reduce the onset and build-up of oxidative species production would be to minimize damage to the blood-brain barrier, reducing hypoxic and ischemic inflammatory events, as well as reducing glial cell activation, which can lead to increased ROS and free radical production.
7.2. Attenuating glutamate-mediated excitotoxicity around neural probes
Insertion of a microelectrode array into the brain induces cortical spreading depression in neurons, a global calcium activating event that can lead to neuronal injury and cell death [265]. Although the impact of CSDs on oligodendrocytes and their precursors are unknown, spreading depolarizations in the cortex are accompanied by glutamate-induced excitotoxic events that can be detrimental to oligodendrocyte viability [266]. Reducing or attenuating glutamate-induced excitotoxicity in oligodendrocytes and NG2 glia may protect against myelin loss and inflammation following CNS injury. Ionotropic glutamate receptors, AMPA and kainite, on the surface of oligodendrocytes and their precursors mediate glutamate-induced excitotoxicity. Antagonists of these ionotropic receptors have attenuated neurodegeneration in models of MS [267, 268]. On the other hand, activation of metabotropic glutamate receptor, attenuates hypoxic/ischemic-induced excitotoxicity in NG2 glia in vitro via protein kinase C alpha (PKCα) activation, which reduces intracellular ROS and maintains antioxidant glutathione levels [269]. Use of agonists to activate metabotropic glutamate receptor pathways or antagonists to inhibit AMPA/kainite-mediated excitotoxicity such as with TNF-α inhibitors could protect cells of the oligodendrocyte lineage from excitotoxic consequences of CNS injury that can occur during microelectrode implantation.
7.3. Minimizing the insult to the blood-brain barrier
Severe permeability of the blood-brain barrier has been correlated to the decreasing recording performance of chronically implanted neural electrodes [20]. Due to the micromotions of a fixed, mechanically stiff device within the brain, disruptions to the BBB can persist throughout the lifetime of the implant. Vascular mapping using twophoton microscopy prior to microelectrode implantation allows for insertion in regions devoid of large penetrating vessels, reducing the amount of BBB disrupted [10]. Minimizing the severity of blood-brain barrier leakage can provide multiple benefits to the viability and integrity of oligodendrocytes, their precursors, and the myelin structures they form. First, reducing the exposure of pro-inflammatory plasma proteins to the parenchyma will mitigate glial cell activation, decreasing the amount of reactive species and harmful cytokines produced. This includes the activation of NG2 glia and their potential differentiation into scar-forming astrocytes. Secondly, less BBB permeability will result in reduced infiltration of peripheral immune cells, some of which are programmed to target myelin specifically.
7.4. Probe surface functionalization to reduce glial cell activation
Activation of microglia and astrocytes is at the center of many neuroinflammatory pathways either directly or indirectly due to their secretion of pro-inflammatory cytokines, upregulation of BBB disrupting proteinases, and formation of an inhibitory glial scar. Engineering the surface of the probe to reduce glial cell activation or promote anti-inflammatory glial cell phenotypes is an important first step toward designing biocompatible neural interfaces. One way of mitigating glial activation is by modifying the surface of the probe to reduce the absorption of microglia and astrocyte activating plasma proteins following rupture of the vasculature. This can be done by decreasing the hydrophobicity of the device through chemical surface coatings in order to prevent biofouling. A recent strategy is to coat the probe in a bioactive compound, the cell adhesion molecule L1, which reduces the pro-inflammatory activation of microglia in vivo [270]. The additional benefit of promoting neuronal integration and reducing microglia activation is the possibility of supporting oligodendrocyte viability and myelin integrity around implanted microelectrodes. Extracellular matrix-derived proteins functionalized on the surface of the probe is also another strategy to modulate glial cell phenotype [271]. ROS scavengers to minimize oxidative damage, extracellular proteins to promote neuronal cell adhesion, and glial cell modulating proteins are also being explored. Lastly, functionalizing the surface of the probe in compounds that manipulate the pathways influencing NG2 glia and oligodendrocyte cell death, differentiation, or myelination discussed in the previous section are potentially new approaches to improving the tissue response to chronically implanted devices as well.
7.5. Drug delivery
Drug delivery around microelectrode arrays has yielded beneficial tissue responses in the past. Administration of anti-inflammatory agents such as dexamethasone and minocycline has reduced the reactive glial response elicited around inserted devices. Using retrodialysis techniques, dexamethasone was shown to attenuate acute microglial activation in vivo [272]. Additionally, dexamethasone attenuates glial scar formation as well as reduces ischemic insult around inserted electrodes [273, 274]. Minocycline administered orally was shown to significantly increased the recording performance of chronically implanted microelectrodes compared to controls as well as significantly attenuate the reactivity of astrocytes around the implant [275]. In experimental autoimmune encephalomyelitis (EAE), animals treated with minocycline experience reduced inflammation and demyelination [276]. Minocycline is also known to inhibit matrix metalloproteinases (MMPs), which are responsible for tissue inflammation, neurodegeneration, and demyelination [277]. A reduction of microglia activation has the potential to reduce pro-inflammatory insults on oligodendrocytes, myelin, and oligodendrocyte precursors by reducing the secretion of harmful cytokines, chemokines, and reactive oxygen and nitrogen species. However, this approach is a double-edged sword since activated glia are responsible for the clearance of myelin debris and tissue remodeling that are necessary for wound repair and regeneration [278]. Finally, using neutralizing antibodies against Lingo-1, to direct modulation of oligodendrocytes and oligodendrocyte precursors, or myelin protein Nogo-A, to improve neuronal growth inhibition, hold promising potential to regenerate lesions following CNS injury [279, 280]. Specifically, Lingo-1 neutralizing antibodies are beginning to undergo clinical trials to treat MS patients [281, 282]. Before microelectrode-directed pharmacological interventions can be evaluated on the response of oligodendroglia, however, a time course of microelectrode-induced insult to myelinating oligodendrocytes and their precursors should be investigated in order to optimize potential therapeutic approaches.
8. Conclusion
The role of oligodendrocytes and their precursors are key components missing in the series of events that constitutes the tissue response to chronically implanted neural interfaces (Figure 5). The myelin produced from mature, differentiated oligodendrocytes is responsible for the electrical conduction of neuronal signals, the critical component of information transfer between neurons and implanted microelectrode technology. Oligodendrocytes and the NG2 glia responsible for replacing myelinating oligodendrocytes are highly susceptible to oxidative and excitotoxic injury, key characteristic events in the foreign body reaction to inserted devices. The dependence that neurons have on these two cells types for their survivability and physiology prompt the question of their individual fates during implantation injury and to what extent, if any, are they responsible for the characteristic increase in neurodegeneration around chronically implanted devices. Defining a spatial and temporal time course of the viability of oligodendrocytes, their precursors, and their myelin structures around chronically implanted neural electrodes will reveal a clearer understanding of the biological mechanisms that result in the reduced recording and stimulating performance of these devices. This knowledge can then be applied to alternative therapies to increase the longevity and stability of chronic neural interfaces implanted in the body and increase their lifespan of usability for the patients afflicted with neurological deficits.
Figure 5. Schematic representation of the physiological consequences of microelectrode implantation in the brain.
The presence of the microelectrode has the potential to alter critical cellular functions that help maintain normal tissue homeostasis. NG2 glia are responsible for supporting neuronal health and modulating neuronal activity while maintaining a reservoir of oligodendrocyte precursors in the event of demyelination injury. Oligodendrocytes also support neuronal viability and produce insulating myelin sheaths that are necessary for signal propagation between neurons throughout the brain. Following electrode insertion, NG2 glia, along with microglia and astrocytes, become activated, proliferating and migrating toward the surface of the device. NG2 glia maintain the potential to differentiate into reactive astrocytes and participate in the encapsulation of the electrode via a glial scar. Scarring, which increases electrical impedances within the tissue (resistance and capacitance of the tissue, Rt and Ct), coupled with the impedance formed by the electrode-electrolyte layer (Re and Ce) as well as the electrode access resistance (Ra) impair ion exchange and reduce transfer of signal. Oligodendrocytes, which are easily susceptible to oxidative and excitotoxic injuries, succumb to inflammation and perish. The myelin structures they established begin to degrade leaving axons demyelinated, which can result in reduced signal amplitudes and latency delays in the exchange of electrical signals. The simultaneous increase in impedance due to glial scar formation and reduced neuronal viability or function due to lack of oligodendrocyte or myelin support can affect the recording and stimulating potential of the tissue surrounding the electrode. Chronic injuries will leave tissue unrepairable as remyelination or regeneration of axons fails to occur.
Acknowledgements:
This work was supported by NIH NINDS R01NS094396 and a diversity supplement to this parent grant as well as NIH NINDS R01NS089688, NIH NINDS R01NS062019, and VA BLRD I21BX003237.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collinger JL, et al. , High-performance neuroprosthetic control by an individual with tetraplegia. Lancet, 2013. 381(9866): p. 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polikov VS, Tresco PA, and Reichert WM, Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods, 2005. 148(1): p. 1–18. [DOI] [PubMed] [Google Scholar]
- 3.Ward MP, et al. , Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res, 2009. 1282: p. 183–200. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg LR, et al. , Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature, 2012. 485(7398): p. 372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrese JC, et al. , Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng, 2013. 10(6): p. 066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozai TDY, et al. , Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials, 2015. 37: p. 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellman SM, et al. , A Materials Roadmap to Functional Neural Interface Design. Advanced Functional Materials, 2017: p. 1701269–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salatino JW, et al. , Glial responses to implanted electrodes in the brain. Nature BME, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozai TDY, et al. , Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chemical Neuroscience, 2015. 6(1): p. 48–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozai TDY, et al. , Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J Neural Eng, 2010. 7(4): p. 046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winslow BD, et al. , A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Serlin Y, et al. , Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol, 2015. 38: p. 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muoio V, Persson PB, and Sendeski MM, The neurovascular unit - concept review. Acta physiologica (Oxford, England), 2014. 210(4): p. 790–8. [DOI] [PubMed] [Google Scholar]
- 14.Venkat P, Chopp M, and Chen J, Blood-Brain Barrier Disruption, Vascular Impairment, and Ischemia/Reperfusion Damage in Diabetic Stroke. J Am Heart Assoc, 2017. 6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JC, et al. , Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng, 2007. 4(4): p. 410–23. [DOI] [PubMed] [Google Scholar]
- 16.McConnell GC, Butera RJ, and Bellamkonda RV, Bioimpedance modeling to monitor astrocytic response to chronically implanted electrodes. J Neural Eng, 2009. 6(5): p. 055005. [DOI] [PubMed] [Google Scholar]
- 17.Szarowski DH, et al. , Brain responses to micro-machined silicon devices. Brain Res, 2003. 983(1–2): p. 23–35. [DOI] [PubMed] [Google Scholar]
- 18.Marin C and Fernandez E, Biocompatibility of intracortical microelectrodes: current status and future prospects. Front Neuroeng, 2010. 3: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozai TDY, Vazquez AL, and Weaver CL, In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through. Journal of neural engineering, 2012. 9: p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena T, et al. , The impact of chronic blood-brain barrier breach on intracortical electrode function. Biomaterials, 2013. 34(20): p. 4703–13. [DOI] [PubMed] [Google Scholar]
- 21.Mechler F and Victor JD, Dipole characterization of single neurons from their extracellular action potentials. Journal of computational neuroscience, 2012. 32(1): p. 73–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang WY, et al. , Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med, 2015. 3(10): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis CA, et al. , The Neuroinflammatory Response in ALS: The Roles of Microglia and T Cells. Neurol Res Int, 2012. 2012: p. 803701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozai TD, et al. , Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater, 2012. 11(12): p. 1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehdi J, et al. , Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. Journal of Neural Engineering, 2015. 12(1): p. 011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozai TDY, et al. , Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials, 2014. 35(36): p. 9620–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigley R, et al. , Morphological and physiological interactions of NG2-glia with astrocytes and neurons. Journal of Anatomy, 2007. 210(6): p. 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eugenín-von Bernhardi J and Dimou L, NG2-glia, more than progenitor cells, in Glial Cells in Health and Disease of the CNS. 2016, Springer; p. 27–45. [DOI] [PubMed] [Google Scholar]
- 29.Nakano M, et al. , NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci Rep, 2017. 7: p. 42041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viganò F and Dimou L, The heterogeneous nature of NG2-glia. Brain Research, 2016. 1638: p. 129–137. [DOI] [PubMed] [Google Scholar]
- 31.Timsit S, et al. , Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J Neurosci, 1995. 15(2): p. 1012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pringle NP and Richardson WD, A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development (Cambridge, England), 1993. 117(2): p. 525–33. [DOI] [PubMed] [Google Scholar]
- 33.Kessaris N, et al. , Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci, 2006. 9(2): p. 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishiyama A, et al. , Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res, 1996. 43(3): p. 299–314. [DOI] [PubMed] [Google Scholar]
- 35.Hughes EG, et al. , Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci, 2013. 16(6): p. 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Psachoulia K, et al. , Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biology, 2010. 5(3–4): p. 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chittajallu R, Aguirre A, and Gallo V, NG2-positive cells in the mouse white and grey matter display distinct physiological properties. The Journal of Physiology, 2004. 561(Pt 1): p. 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai HH, et al. , Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science, 2016. 351(6271): p. 379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Castro F and Bribian A, The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Brain Res Rev, 2005. 49(2): p. 227–41. [DOI] [PubMed] [Google Scholar]
- 40.Choe Y, Huynh T, and Pleasure SJ, Migration of oligodendrocyte progenitor cells is controlled by transforming growth factor beta family proteins during corticogenesis. J Neurosci, 2014. 34(45): p. 14973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiyama A, et al. , Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci, 2009. 10(1): p. 9–22. [DOI] [PubMed] [Google Scholar]
- 42.Dawson MRL, et al. , NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Molecular and Cellular Neuroscience, 2003. 24(2): p. 476–488. [DOI] [PubMed] [Google Scholar]
- 43.Rivers LE, et al. , PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci, 2008. 11(12): p. 1392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barateiro A and Fernandes A, Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta, 2014. 1843(9): p. 1917–29. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. , Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proceedings of the National Academy of Sciences of the United States of America, 2009. 106(45): p. 19162–19167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean JM, et al. , Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci, 2011. 33(3–4): p. 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins TA, et al. , Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron, 2008. 60(4): p. 555–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S, et al. , A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods, 2012. 9(9): p. 917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson EM, et al. , Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science, 2014. 344(6183): p. 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg SS, et al. , The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A, 2008. 105(38): p. 14662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Back SA, et al. , Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med, 2005. 11(9): p. 966–72. [DOI] [PubMed] [Google Scholar]
- 52.Miron VE, et al. , M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci, 2013. 16(9): p. 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mi S, et al. , LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci, 2005. 8(6): p. 745–751. [DOI] [PubMed] [Google Scholar]
- 54.Demerens C, et al. , Induction of myelination in the central nervous system by electrical activity. Proceedings of the National Academy of Sciences of the United States of America, 1996. 93(18): p. 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trajkovic K, et al. , Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. The Journal of Cell Biology, 2006. 172(6): p. 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mi S, et al. , LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature neuroscience, 2004. 7(3): p. 221–8. [DOI] [PubMed] [Google Scholar]
- 57.Liang X, Draghi NA, and Resh MD, Signaling from Integrins to Fyn to Rho Family GTPases Regulates Morphologic Differentiation of Oligodendrocytes. The Journal of Neuroscience, 2004. 24(32): p. 7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lubetzki C, et al. , Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci U S A, 1993. 90(14): p. 6820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charles P, et al. , Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci U S A, 2000. 97(13): p. 7585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomassy GS, et al. , Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science, 2014. 344(6181): p. 319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micheva KD, et al. , A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gulyás AI, et al. , Total Number and Ratio of Excitatory and Inhibitory Synapses Converging onto Single Interneurons of Different Types in the CA1 Area of the Rat Hippocampus. The Journal of Neuroscience, 1999. 19(22): p. 10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeVine SM and Torres MV, Satellite oligodendrocytes and myelin are displaced in the cortex of the reeler mouse. Developmental Brain Research, 1993. 75(2): p. 279–284. [DOI] [PubMed] [Google Scholar]
- 64.Battefeld A, Klooster J, and Kole MHP, Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nature Communications, 2016. 7: p. 11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludwin SK, The perineuronal satellite oligodendrocyte. A role in remyelination. Acta Neuropathol, 1979. 47(1): p. 49–53. [DOI] [PubMed] [Google Scholar]
- 66.Takasaki C, et al. , Cytochemical and cytological properties of perineuronal oligodendrocytes in the mouse cortex. Eur J Neurosci, 2010. 32(8): p. 1326–36. [DOI] [PubMed] [Google Scholar]
- 67.Taniike M, et al. , Perineuronal oligodendrocytes protect against neuronal apoptosis through the production of lipocalin-type prostaglandin D synthase in a genetic demyelinating model. J Neurosci, 2002. 22(12): p. 4885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garbern JY, et al. , Patients lacking the major CNS myelin protein, proteolipid protein 1, develop lengthdependent axonal degeneration in the absence of demyelination and inflammation. Brain, 2002. 125(Pt 3): p. 551–61. [DOI] [PubMed] [Google Scholar]
- 69.Trapp BD, et al. , Axonal transection in the lesions of multiple sclerosis. N Engl J Med, 1998. 338(5): p. 278–85. [DOI] [PubMed] [Google Scholar]
- 70.Wilkins A, Chandran S, and Compston A, A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia, 2001. 36(1): p. 48–57. [DOI] [PubMed] [Google Scholar]
- 71.Kataoka Y, et al. , Perineuronal germinal cells in the rat cerebral cortex. Med Mol Morphol, 2006. 39(1): p. 2832. [DOI] [PubMed] [Google Scholar]
- 72.Lin S-C, et al. , Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron, 2005. 46(5): p. 773–85. [DOI] [PubMed] [Google Scholar]
- 73.Káradóttir R, et al. , Spiking and non-spiking classes of oligodendrocyte precursor glia in CNS white matter. Nature neuroscience, 2008. 11(4): p. 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Biase LM, Nishiyama A, and Bergles DE, Excitability and synaptic communication within the oligodendrocyte lineage. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2010. 30(10): p. 3600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagy B, et al. , Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLOS Biology, 2017. 15(8): p. e2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakry D, et al. , Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol, 2014. 12(11): p. e1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakry D, et al. , Oligodendrocyte precursor cells synthesize neuromodulatory factors. PLoS One, 2015. 10(5): p. e0127222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birey F, et al. , Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron, 2015. 88(5): p. 941–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan H and Rivkees Scott A, Hepatocyte growth factor stimulates the proliferation and migration of oligodendrocyte precursor cells. Journal of Neuroscience Research, 2002. 69(5): p. 597–606. [DOI] [PubMed] [Google Scholar]
- 80.Wilkins A, et al. , Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci, 2003. 23(12): p. 4967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dougherty KD, Dreyfus CF, and Black IB, Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis, 2000. 7(6 Pt B): p. 574–85. [DOI] [PubMed] [Google Scholar]
- 82.Du Y and Dreyfus CF, Oligodendrocytes as providers of growth factors. J Neurosci Res, 2002. 68(6): p. 647–54. [DOI] [PubMed] [Google Scholar]
- 83.Pierre K and Pellerin L, Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem, 2005. 94(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 84.Rinholm JE, et al. , Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci, 2011. 31(2): p. 538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pellerin L, et al. , Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci, 1998. 20(4–5): p. 291–9. [DOI] [PubMed] [Google Scholar]
- 86.Fruhbeis C, et al. , Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol, 2013. 11(7): p. e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nishiyama A, Yang Z, and Butt A, Astrocytes and NG2-glia: what’s in a name? J Anat, 2005. 207(6): p. 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serwanski DR, Jukkola P, and Nishiyama A, Heterogeneity of astrocyte and NG2 cell insertion at the node of Ranvier. The Journal of comparative neurology, 2017. 525(3): p. 535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fok-Seang J, et al. , Migration of oligodendrocyte precursors on astrocytes and meningeal cells. Developmental biology, 1995. 171(1): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 90.Wigley R, et al. , Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat, 2007. 210(6): p. 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishibashi T, et al. , Astrocytes promote myelination in response to electrical impulses. Neuron, 2006. 49(6): p. 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevens B, et al. , Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron, 2002. 36(5): p. 855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fok-Seang J, et al. , Cytokine-induced changes in the ability of astrocytes to support migration of oligodendrocyte precursors and axon growth. The European journal of neuroscience, 1998. 10(7): p. 2400–15. [DOI] [PubMed] [Google Scholar]
- 94.Filipovic R and Zecevic N, The effect of CXCL1 on human fetal oligodendrocyte progenitor cells. Glia, 2008. 56(1): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 95.Arai K and Lo EH, Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. Journal of neuroscience research, 2010. 88(4): p. 758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu X, Bergles DE, and Nishiyama A, NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development (Cambridge, England), 2008. 135(1): p. 145–57. [DOI] [PubMed] [Google Scholar]
- 97.Orthmann-Murphy JL, Abrams CK, and Scherer SS, Gap Junctions Couple Astrocytes and Oligodendrocytes. Journal of molecular neuroscience : MN, 2008. 35(1): p. 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamasawa N, et al. , Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: Implications for ionic homeostasis and potassium siphoning. Neuroscience, 2005. 136(1): p. 65–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sakurai Y, et al. , Differentiation of oligodendrocyte occurs in contact with astrocyte. J Neurosci Res, 1998. 52(1): p. 17–26. [DOI] [PubMed] [Google Scholar]
- 100.Domingues HS, et al. , Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Frontiers in cell and developmental biology, 2016. 4: p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu J-G, et al. , BMP Signaling Mediates Astrocyte Differentiation of Oligodendrocyte Progenitor Cells. The Tohoku Journal of Experimental Medicine, 2010. 222(3): p. 195–200. [DOI] [PubMed] [Google Scholar]
- 102.Nicholas RSTJ, Wing MG, and Compston A, Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-κB. European Journal of Neuroscience, 2001. 13(5): p. 959–967. [DOI] [PubMed] [Google Scholar]
- 103.Arnett HA, et al. , TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nature neuroscience, 2001. 4(11): p. 1116–22. [DOI] [PubMed] [Google Scholar]
- 104.Bonora M, et al. , Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondriadependent process. Cell Death and Differentiation, 2014. 21(8): p. 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Voss EV, et al. , Characterisation of microglia during de- and remyelination: can they create a repair promoting environment? Neurobiology of disease, 2012. 45(1): p. 519–28. [DOI] [PubMed] [Google Scholar]
- 106.Pasquini LA, et al. , Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death And Differentiation, 2011. 18: p. 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Starossom Sarah C., et al. , Galectin-1 Deactivates Classically Activated Microglia and Protects from Inflammation-Induced Neurodegeneration. Immunity, 2012. 37(2): p. 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pang Y, et al. , Differential roles of astrocyte and microglia in supporting oligodendrocyte development and myelination in vitro. Brain and Behavior, 2013. 3(5): p. 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lalive PH, et al. , TGF-β-treated microglia induce oligodendrocyte precursor cell chemotaxis through the HGF-cMet pathway. European Journal of Immunology, 2005. 35(3): p. 727–737. [DOI] [PubMed] [Google Scholar]
- 110.Wellman SM and Kozai TDY, In vivo spatiotemporal dynamics of NG2 glia activity caused by neural electrode implantation. Biomaterials, 2018. 164: p. 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J, et al. , Tumor necrosis factor alpha mediates lipopolysaccharide-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2008. 28(20): p. 5321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang X, et al. , Cellular iron status influences the functional relationship between microglia and oligodendrocytes. Glia, 2006. 54(8): p. 795–804. [DOI] [PubMed] [Google Scholar]
- 113.Pang Y, Cai Z, and Rhodes PG, Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res, 2003. 140(2): p. 205–14. [DOI] [PubMed] [Google Scholar]
- 114.Lehnardt S, et al. , The Toll-Like Receptor TLR4 Is Necessary for Lipopolysaccharide-Induced Oligodendrocyte Injury in the CNS. The Journal of Neuroscience, 2002. 22(7): p. 2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicholas RS, et al. , Microglia-derived IGF-2 prevents TNFalpha induced death of mature oligodendrocytes in vitro. Journal of Neuroimmunology, 2002. 124(1): p. 36–44. [DOI] [PubMed] [Google Scholar]
- 116.Miron VE, et al. , M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature Neuroscience, 2013. 16: p. 1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balabanov R, et al. , Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2007. 27(8): p. 2013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hellwig S, Heinrich A, and Biber K, The brain’s best friend: microglial neurotoxicity revisited. Frontiers in cellular neuroscience, 2013. 7: p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ransohoff RM, A polarizing question: do M1 and M2 microglia exist? Nature Neuroscience, 2016. 19: p. 987. [DOI] [PubMed] [Google Scholar]
- 120.Golabchi A, et al. , Melatonin improves quality and longevity of chronic neural recording. Biomaterials, 2018. 180: p. 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teo L and Bourne J, Current opinion on a role of the astrocytes in neuroprotection. Neural Regeneration Research, 2018. 13(5): p. 797–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bradl M and Lassmann H, Oligodendrocytes: biology and pathology. Acta Neuropathologica, 2010. 119(1): p. 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Daneman R, et al. , Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proceedings of the National Academy of Sciences, 2009. 106(2): p. 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maki T, et al. , Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett, 2015. 597: p. 164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seo JH, et al. , Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One, 2014. 9(7): p. e103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arai K and Lo EH, An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci, 2009. 29(14): p. 4351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hayakawa K, et al. , Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neurosci Lett, 2012. 513(1): p. 42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayakawa K, et al. , Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci, 2011. 31(29): p. 10666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le Bras B, et al. , VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci, 2006. 9(3): p. 340–8. [DOI] [PubMed] [Google Scholar]
- 130.Gomes C, et al. , Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. Journal of Neuroinflammation, 2013. 10(1): p. 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang Y, et al. , Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience, 2011. 172: p. 398–405. [DOI] [PubMed] [Google Scholar]
- 132.Fitzgerald M, The complex contribution of the astrocyte scar. Neural Regeneration Research, 2016. 11(7): p. 1052–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rigor RR, et al. , Myosin Light Chain Kinase Signaling in Endothelial Barrier Dysfunction. Medicinal research reviews, 2013. 33(5): p. 911–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.da Fonseca ACC, et al. , The impact of microglial activation on blood-brain barrier in brain diseases. Frontiers in Cellular Neuroscience, 2014. 8: p. 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kadl A and Leitinger N, The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal, 2005. 7(11–12): p. 1744–54. [DOI] [PubMed] [Google Scholar]
- 136.Verbeek MM, et al. , T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J Immunol, 1995. 154(11): p. 5876–84. [PubMed] [Google Scholar]
- 137.Larochelle C, Alvarez JI, and Prat A, How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS letters, 2011. 585(23): p. 3770–80. [DOI] [PubMed] [Google Scholar]
- 138.Mitala CM, et al. , Impact of microdialysis probes on vasculature and dopamine in the rat striatum: a combined fluorescence and voltammetric study. J Neurosci Methods, 2008. 174(2): p. 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jaquins-Gerstl A and Michael AC, Comparison of the brain penetration injury associated with microdialysis and voltammetry. J Neurosci Methods, 2009. 183(2): p. 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leung BK, et al. , Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials, 2008. 29(23): p. 3289–3297. [DOI] [PubMed] [Google Scholar]
- 141.Masamoto K, et al. , Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience, 2012. 212: p. 190–200. [DOI] [PubMed] [Google Scholar]
- 142.Arihiro S, et al. , Vascular smooth muscle cells and pericytes express MMP-1, MMP-9, TIMP-1 and type I procollagen in inflammatory bowel disease. Histopathology, 2001. 39(1): p. 50–59. [DOI] [PubMed] [Google Scholar]
- 143.Darland DC, et al. , Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Developmental Biology, 2003. 264(1): p. 275–288. [DOI] [PubMed] [Google Scholar]
- 144.Eles JR, et al. , In vivo imaging of neuronal calcium during electrode implantation: spatial and temporal mapping of damage and recovery. Biomaterials, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Michelson NJ, et al. , Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissueimplant neural interface: New Emphasis on the Biological Interface. Journal of Neural Engineering, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bjornsson CS, et al. , Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng, 2006. 3(3): p. 196–207. [DOI] [PubMed] [Google Scholar]
- 147.LaPlaca MC, et al. , High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. Journal of Biomechanics, 2005. 38(5): p. 1093–1105. [DOI] [PubMed] [Google Scholar]
- 148.Neary JT, et al. , Activation of Extracellular Signal-Regulated Kinase by Stretch-Induced Injury in Astrocytes Involves Extracellular ATP and P2 Purinergic Receptors. The Journal of Neuroscience, 2003. 23(6): p. 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ranaivo HR and Wainwright MS, Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain research, 2010. 1313C: p. 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parker RA, et al. , The functional consequences of chronic, physiologically effective intracortical microstimulation. Progress in Brain Research, 2011. 194: p. 145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McConnell GC, et al. , Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng, 2009. 6(5): p. 056003. [DOI] [PubMed] [Google Scholar]
- 152.Potter KA, et al. , The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials, 2013. 34(29): p. 7001–15. [DOI] [PubMed] [Google Scholar]
- 153.McTigue DM and Tripathi RB, The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem, 2008. 107(1): p. 1–19. [DOI] [PubMed] [Google Scholar]
- 154.Mouzannar R, et al. , Hydrogen peroxide induces rapid digestion of oligodendrocyte chromatin into high molecular weight fragments. Neurochem Int, 2001. 38(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 155.Dewar D, Underhill SM, and Goldberg MP, Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab, 2003. 23(3): p. 263–74. [DOI] [PubMed] [Google Scholar]
- 156.Torkildsen O, et al. , The cuprizone model for demyelination. Acta Neurol Scand Suppl, 2008. 188: p. 72–6. [DOI] [PubMed] [Google Scholar]
- 157.Thorburne SK and Juurlink BH, Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem, 1996. 67(3): p. 1014–22. [DOI] [PubMed] [Google Scholar]
- 158.Juurlink BH, Response of glial cells to ischemia: roles of reactive oxygen species and glutathione. Neurosci Biobehav Rev, 1997. 21(2): p. 151–66. [DOI] [PubMed] [Google Scholar]
- 159.Connor JR and Menzies SL, Relationship of iron to oligodendrocytes and myelination. Glia, 1996. 17(2): p. 8393. [DOI] [PubMed] [Google Scholar]
- 160.Hulet SW, Menzies S, and Connor JR, Ferritin binding in the developing mouse brain follows a pattern similar to myelination and is unaffected by the jimpy mutation. Dev Neurosci, 2002. 24(2–3): p. 208–13. [DOI] [PubMed] [Google Scholar]
- 161.Gerber MR and Connor JR, Do oligodendrocytes mediate iron regulation in the human brain? Ann Neurol, 1989. 26(1): p. 95–8. [DOI] [PubMed] [Google Scholar]
- 162.Rathnasamy G, et al. , Hypoxia-Induced Iron Accumulation in Oligodendrocytes Mediates Apoptosis by Eliciting Endoplasmic Reticulum Stress. Mol Neurobiol, 2016. 53(7): p. 4713–27. [DOI] [PubMed] [Google Scholar]
- 163.Evans MD, Dizdaroglu M, and Cooke MS, Oxidative DNA damage and disease: induction, repair and significance. Mutat Res, 2004. 567(1): p. 1–61. [DOI] [PubMed] [Google Scholar]
- 164.Leitner DF and Connor JR, Functional roles of transferrin in the brain. Biochim Biophys Acta, 2012. 1820(3): p. 393–402. [DOI] [PubMed] [Google Scholar]
- 165.Prasad A, et al. , Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front Neuroeng, 2014. 7: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bartzokis G, et al. , Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci, 2004. 1012: p. 224–36. [DOI] [PubMed] [Google Scholar]
- 167.Connor JR, Myelin breakdown in Alzheimer’s disease: a commentary. Neurobiol Aging, 2004. 25(1): p. 45–7. [DOI] [PubMed] [Google Scholar]
- 168.Todorich B, et al. , Oligodendrocytes and myelination: the role of iron. Glia, 2009. 57(5): p. 467–78. [DOI] [PubMed] [Google Scholar]
- 169.Karadottir R, et al. , NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature, 2005. 438(7071): p. 1162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Deng W, et al. , Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci U S A, 2004. 101(20): p. 7751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yoshioka A, Bacskai B, and Pleasure D, Pathophysiology of oligodendroglial excitotoxicity. J Neurosci Res, 1996. 46(4): p. 427–37. [DOI] [PubMed] [Google Scholar]
- 172.Matute C, et al. , Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A, 1997. 94(16): p. 8830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sanchez-Gomez MV, et al. , Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci, 2003. 23(29): p. 9519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Taylor DL, et al. , Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci, 2005. 25(11): p. 2952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takahashi JL, et al. , Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol, 2003. 53(5): p. 588–95. [DOI] [PubMed] [Google Scholar]
- 176.Salatino JW, et al. , Functional remodeling of subtype-specific markers surrounding implanted neuroprostheses. J Neurophysiol, 2017. 118(1): p. 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marik C, et al. , Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain, 2007. 130(Pt 11): p. 2800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ryu JK, et al. , Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun, 2015. 6: p. 8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoon H, et al. , The thrombin receptor is a critical extracellular switch controlling myelination. Glia, 2015. 63(5): p. 846–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dery O, et al. , Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol, 1998. 274(6 Pt 1): p. C1429–52. [DOI] [PubMed] [Google Scholar]
- 181.Shavit E, et al. , Thrombin receptor PAR-1 on myelin at the node of Ranvier: a new anatomy and physiology of conduction block. Brain : a journal of neurology, 2008. 131(Pt 4): p. 1113–22. [DOI] [PubMed] [Google Scholar]
- 182.Pike CJ, et al. , Thrombin attenuates neuronal cell death and modulates astrocyte reactivity induced by betaamyloid in vitro. J Neurochem, 1996. 66(4): p. 1374–82. [DOI] [PubMed] [Google Scholar]
- 183.Striggow F, et al. , The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proceedings of the National Academy of Sciences of the United States of America, 2000. 97(5): p. 2264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peferoen L, et al. , Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology, 2014. 141(3): p. 302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Biran R, Martin DC, and Tresco PA, Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol, 2005. 195(1): p. 115–26. [DOI] [PubMed] [Google Scholar]
- 186.Kozai TD, et al. , Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultrasmall neural probes. Biomaterials, 2014. 35(34): p. 9255–68. [DOI] [PubMed] [Google Scholar]
- 187.Edell DJ, et al. , Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng, 1992. 39(6): p. 635–43. [DOI] [PubMed] [Google Scholar]
- 188.Bergles DE and Richardson WD, Oligodendrocyte Development and Plasticity. Cold Spring Harb Perspect Biol, 2015. 8(2): p. a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silver J and Miller JH, Regeneration beyond the glial scar. Nat Rev Neurosci, 2004. 5(2): p. 146–56. [DOI] [PubMed] [Google Scholar]
- 190.Levine JM, Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci, 1994. 14(8): p. 4716–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Turner JN, et al. , Cerebral astrocyte response to micromachined silicon implants. Exp Neurol, 1999. 156(1): p. 33–49. [DOI] [PubMed] [Google Scholar]
- 192.Hackett AR and Lee JK, Understanding the NG2 Glial Scar after Spinal Cord Injury. Front Neurol, 2016. 7: p. 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wellman SM and Kozai TDY, Understanding the Inflammatory Tissue Reaction to Brain Implants To Improve Neurochemical Sensing Performance. ACS Chemical Neuroscience, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yiu G and He Z, Glial inhibition of CNS axon regeneration. Nat Rev Neurosci, 2006. 7(8): p. 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jones LL, et al. , NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2002. 22(7): p. 2792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhong Y and Bellamkonda RV, Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res, 2007. 1148: p. 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carulli D, et al. , Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol, 2005. 15(1): p. 116–20. [DOI] [PubMed] [Google Scholar]
- 198.Dou CL and Levine JM, Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci, 1994. 14(12): p. 7616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goldberg JL, et al. , An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci, 2004. 24(21): p. 4989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jones LL, Sajed D, and Tuszynski MH, Axonal Regeneration through Regions of Chondroitin Sulfate Proteoglycan Deposition after Spinal Cord Injury: A Balance of Permissiveness and Inhibition. The Journal of Neuroscience, 2003. 23(28): p. 9276–9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang Z, et al. , NG2 Glial Cells Provide a Favorable Substrate for Growing Axons. The Journal of Neuroscience, 2006. 26(14): p. 3829–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Karadottir R, et al. , Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci, 2008. 11(4): p. 450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Busch SA, et al. , Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci, 2010. 30(1): p. 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Michelson NJ, et al. , Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissueimplant neural interface: new emphasis on the biological interface. Journal of neural engineering, 2017. 15(3): p. 033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Franklin RJ and Ffrench-Constant C, Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci, 2008. 9(11): p. 839–55. [DOI] [PubMed] [Google Scholar]
- 206.Watanabe M, Toyama Y, and Nishiyama A, Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res, 2002. 69(6): p. 826–36. [DOI] [PubMed] [Google Scholar]
- 207.Prineas JW and Connell F, Remyelination in multiple sclerosis. Ann Neurol, 1979. 5(1): p. 22–31. [DOI] [PubMed] [Google Scholar]
- 208.Stidworthy MF, et al. , Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol, 2003. 13(3): p. 329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Winslow BD and Tresco PA, Quantitative analysis of the tissue response to chronically implanted microwire electrodes in rat cortex. Biomaterials, 2010. 31(7): p. 1558–1567. [DOI] [PubMed] [Google Scholar]
- 210.Higuchi M, et al. , Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J Neurosci, 2005. 25(41): p. 9434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee Y, et al. , Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature, 2012. 487(7408): p. 443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Anderson TJ, et al. , Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. The Journal of comparative neurology, 1998. 394(4): p. 506–19. [DOI] [PubMed] [Google Scholar]
- 213.Lappe-Siefke C, et al. , Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet, 2003. 33(3): p. 366–74. [DOI] [PubMed] [Google Scholar]
- 214.Wang E, et al. , Deletion of a splicing enhancer disrupts PLP1/DM20 ratio and myelin stability. Experimental Neurology, 2008. 214(2): p. 322–330. [DOI] [PubMed] [Google Scholar]
- 215.Bachstetter AD, et al. , Clinically relevant intronic splicing enhancer mutation in myelin proteolipid protein leads to progressive microglia and astrocyte activation in white and gray matter regions of the brain. Journal of Neuroinflammation, 2013. 10: p. 146–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kilanczyk E, et al. , Antioxidant Protection of NADPH-Depleted Oligodendrocyte Precursor Cells Is Dependent on Supply of Reduced Glutathione. ASN Neuro, 2016. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Leskiewicz M, et al. , Effects of neurosteroids on hydrogen peroxide- and staurosporine-induced damage of human neuroblastoma SH-SY5Y cells. J Neurosci Res, 2008. 86(6): p. 1361–70. [DOI] [PubMed] [Google Scholar]
- 218.Kimonides VG, et al. , Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A, 1998. 95(4): p. 1852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li Z, et al. , DHEA-neuroprotection and -neurotoxicity after transient cerebral ischemia in rats. J Cereb Blood Flow Metab, 2009. 29(2): p. 287–96. [DOI] [PubMed] [Google Scholar]
- 220.Lugaresi A, et al. , Low-dose oral methotrexate treatment in chronic progressive multiple sclerosis. Neurol Sci, 2001. 22(2): p. 209–10. [DOI] [PubMed] [Google Scholar]
- 221.Mueller AM, et al. , Effects of intraventricular methotrexate administration on Cuprizone-induced demyelination in mice. Front Mol Neurosci, 2013. 6: p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xing B, et al. , Conditional depletion of GSK3b protects oligodendrocytes from apoptosis and lessens demyelination GLIA, 2018. [DOI] [PubMed] [Google Scholar]
- 223.Rosa PM, et al. , Glioprotective Effect of Resveratrol: an Emerging Therapeutic Role for Oligodendroglial Cells. Mol Neurobiol, 2017. [DOI] [PubMed] [Google Scholar]
- 224.Ghaiad HR, et al. , Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Molecular neurobiology, 2017. 54(5): p. 3219–3229. [DOI] [PubMed] [Google Scholar]
- 225.Michailidou I, et al. , Activation of endogenous neural stem cells for multiple sclerosis therapy. Front Neurosci, 2014. 8: p. 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vigano F, et al. , Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci, 2013. 16(10): p. 1370–2. [DOI] [PubMed] [Google Scholar]
- 227.Wahl SE, et al. , Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J Neurosci, 2014. 34(13): p. 4453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Narayanan SP, et al. , Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci, 2009. 29(21): p. 6860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pollizzi KN and Powell JD, Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol, 2015. 36(1): p. 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ishii A, et al. , ERK1/ERK2 MAPK Signaling is Required to Increase Myelin Thickness Independent of Oligodendrocyte Differentiation and Initiation of Myelination. The Journal of Neuroscience, 2012. 32(26): p. 8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Azim K, et al. , GSK3β regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-β-catenin signaling. Glia, 2014. 62(5): p. 778–789. [DOI] [PubMed] [Google Scholar]
- 232.Azim K and Butt AM, GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia, 2011. 59(4): p. 540–553. [DOI] [PubMed] [Google Scholar]
- 233.Heinrich C, et al. , Sox2-Mediated Conversion of NG2 Glia into Induced Neurons in the Injured Adult Cerebral Cortex. Stem Cell Reports, 2014. 3(6): p. 1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mekhail M, Almazan G, and Tabrizian M, Purine-crosslinked injectable chitosan sponges promote oligodendrocyte progenitor cells’ attachment and differentiation. Biomaterials Science, 2015. 3(2): p. 279–287. [DOI] [PubMed] [Google Scholar]
- 235.Leipzig ND and Shoichet MS, The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials, 2009. 30(36): p. 6867–6878. [DOI] [PubMed] [Google Scholar]
- 236.Chedly J, et al. , Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials, 2017. 138: p. 91–107. [DOI] [PubMed] [Google Scholar]
- 237.Nomura H, et al. , Extramedullary Chitosan Channels Promote Survival of Transplanted Neural Stem and Progenitor Cells and Create a Tissue Bridge After Complete Spinal Cord Transection. Tissue Engineering Part A, 2008. 14(5): p. 649–665. [DOI] [PubMed] [Google Scholar]
- 238.Russell T and Shelly S-E, Using biomaterials to promote pro-regenerative glial phenotypes after nervous system injuries. Biomedical Materials, 2018. 13(2): p. 024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Christopherson GT, Song H, and Mao H-Q, The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials, 2009. 30(4): p. 556–564. [DOI] [PubMed] [Google Scholar]
- 240.Chen Y-C, et al. , The effect of ultra-nanocrystalline diamond films on the proliferation and differentiation of neural stem cells. Biomaterials, 2009. 30(20): p. 3428–3435. [DOI] [PubMed] [Google Scholar]
- 241.Lim SH, et al. , The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials, 2010. 31(34): p. 9031–9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Diao HJ, et al. , Topographical effects on fiber-mediated microRNA delivery to control oligodendroglial precursor cells development. Biomaterials, 2015. 70: p. 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nguyen LH, et al. , Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Scientific Reports, 2017. 7: p. 42212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Donoghue PS, et al. , The Development of a ɛ-Polycaprolactone Scaffold for Central Nervous System Repair. Tissue Engineering Part A, 2012. 19(3–4): p. 497–507. [DOI] [PubMed] [Google Scholar]
- 245.Li Y, et al. , Nanofibers Support Oligodendrocyte Precursor Cell Growth and Function as a Neuron-Free Model for Myelination Study. Biomacromolecules, 2014. 15(1): p. 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li X, et al. , Engineering an in situ crosslinkable hydrogel for enhanced remyelination. The FASEB Journal, 2013. 27(3): p. 1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Russell LN and Lampe KJ, Oligodendrocyte Precursor Cell Viability, Proliferation, and Morphology is Dependent on Mesh Size and Storage Modulus in 3D Poly(ethylene glycol)-Based Hydrogels. ACS Biomaterials Science & Engineering, 2017. 3(12): p. 3459–3468. [DOI] [PubMed] [Google Scholar]
- 248.Jagielska A, et al. , Mechanical Environment Modulates Biological Properties of Oligodendrocyte Progenitor Cells. Stem Cells and Development, 2012. 21(16): p. 2905–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Seidlits SK, et al. , The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials, 2010. 31(14): p. 3930–3940. [DOI] [PubMed] [Google Scholar]
- 250.Harris JP, et al. , Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. Journal of Neural Engineering, 2011. 8(6): p. 066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Luan L, et al. , Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Science Advances, 2017. 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rittchen S, et al. , Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials, 2015. 56: p. 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li X, et al. , Enhancing Oligodendrocyte Differentiation by Transient Transcription Activation via DNA Nanoparticle-Mediated Transfection. Acta biomaterialia, 2017. 54: p. 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lundgaard I, et al. , Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLOS Biology, 2014. 11(12): p. e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Calaora V, et al. , Neuregulin Signaling Regulates Neural Precursor Growth and the Generation of Oligodendrocytes In Vitro. The Journal of Neuroscience, 2001. 21(13): p. 4740–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.VonDran MW, et al. , Levels of BDNF Impact Oligodendrocyte Lineage Cells Following a Cuprizone Lesion. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2011. 31(40): p. 14182–14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thomas AM, et al. , Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integrative biology, 2014. 6(7): p. 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ereifej ES, et al. , Implantation of Neural Probes in the Brain Elicits Oxidative Stress. Frontiers in Bioengineering and Biotechnology, 2018. 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Potter-Baker KA, et al. , Development of superoxide dismutase mimetic surfaces to reduce accumulation of reactive oxygen species for neural interfacing applications. Journal of Materials Chemistry B, 2014. 2(16): p. 2248–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Potter KA, et al. , Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood–brain barrier stability. Acta Biomaterialia, 2014. 10(5): p. 2209–2222. [DOI] [PubMed] [Google Scholar]
- 261.Haile Y, et al. , Rab32 connects ER stress to mitochondrial defects in multiple sclerosis. Journal of Neuroinflammation, 2017. 14: p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Roussel BD, et al. , Endoplasmic reticulum dysfunction in neurological disease. The Lancet Neurology, 2013. 12(1): p. 105–118. [DOI] [PubMed] [Google Scholar]
- 263.Way SW, et al. , Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nature Communications, 2015. 6: p. 6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lin W, et al. , Enhanced Integrated Stress Response Promotes Myelinating Oligodendrocyte Survival in Response to Interferon-γ. The American Journal of Pathology, 2008. 173(5): p. 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Eles JR, et al. , In vivo imaging of neuronal calcium during electrode implantation: Spatial and temporal mapping of damage and recovery. Biomaterials, 2018. 174: p. 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hinzman JM, et al. , Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Experimental Neurology, 2015. 267: p. 243–253. [DOI] [PubMed] [Google Scholar]
- 267.Smith T, et al. , Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nature Medicine, 2000. 6: p. 62. [DOI] [PubMed] [Google Scholar]
- 268.Wallström E, et al. , Memantine abrogates neurological deficits, but not CNS inflammation, in Lewis rat experimental autoimmune encephalomyelitis. Journal of the Neurological Sciences, 1996. 137(2): p. 89–96. [DOI] [PubMed] [Google Scholar]
- 269.Deng W, et al. , Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proceedings of the National Academy of Sciences of the United States of America, 2004. 101(20): p. 7751–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Eles JR, et al. , Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy. Biomaterials, 2017. 113: p. 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Oakes RS, et al. , An astrocyte derived extracellular matrix coating reduces astrogliosis surrounding chronically implanted microelectrode arrays in rat cortex. Biomaterials, 2018. 154(Supplement C): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 272.Kozai TDY, et al. , Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials, 2016. 87: p. 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nesbitt KM, et al. , Pharmacological mitigation of tissue damage during brain microdialysis. Anal Chem, 2013. 85(17): p. 8173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nesbitt KM, et al. , Microdialysis in the rat striatum: effects of 24 h dexamethasone retrodialysis on evoked dopamine release and penetration injury. ACS Chem Neurosci, 2015. 6(1): p. 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rennaker RL, et al. , Minocycline increases quality and longevity of chronic neural recordings. J Neural Eng, 2007. 4(2): p. L1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Brundula V, et al. , Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain : a journal of neurology, 2002. 125(Pt 6): p. 1297–308. [DOI] [PubMed] [Google Scholar]
- 277.Yong VW, et al. , Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci, 2001. 2(7): p. 502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lampron A, et al. , Inefficient clearance of myelin debris by microglia impairs remyelinating processes. The Journal of Experimental Medicine, 2015. 212(4): p. 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Guzik-Kornacka AM, Vajda F, and Schwab ME, Blocking the Nogo-A Signaling Pathway to Promote Regeneration and Plasticity After Spinal Cord Injury and Stroke, in Translational Neuroscience: Fundamental Approaches for Neurological Disorders, Tuszynski MH, Editor. 2016, Springer US: Boston, MA: p. 369–397. [Google Scholar]
- 280.Sun J-J, et al. , LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in experimental autoimmune encephalomyelitis mice. Scientific Reports, 2015. 5: p. 14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tran JQ, et al. , Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurology - Neuroimmunology Neuroinflammation, 2014. 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ranger A, et al. , Anti-LINGO-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies. Neurology - Neuroimmunology Neuroinflammation, 2018. 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]