Abstract
Fluorescence resonance energy transfer (FRET) between fluorophores attached to single proteins provides a tool to study the conformation of proteins in solution and in cell culture. As a protein unfolds, nanometer-scale increases in distance between donor and acceptor fluorophores cause decreases in FRET. Here we demonstrate the application of FRET to imaging coexisting conformations of fibronectin (Fn) in cell culture. Fn is a flexible 440-kDa extracellular matrix protein, with functional sites that are regulated by unfolding events. Fn was labeled with multiple donor and acceptor fluorophores such that intramolecular FRET could be used to distinguish a range of Fn conformations. The sensitivity of FRET to unfolding was tested by progressively denaturing labeled Fn using guanidium chloride. To investigate Fn conformation changes during cell binding and matrix assembly, we added labeled Fn to the culture medium of NIH 3T3 fibroblasts. Coexisting conformations of Fn were visualized using fluorescence microscopy, and spectra from specific features were measured with an attached spectrometer. Using FRET as an indicator of Fn conformation, Fn diffusely bound to cells was in a compact state, whereas Fn in matrix fibrils was highly extended. Matrix fibrils exhibited a range of FRET that suggested some degree of unfolding of Fn's globular modules. Fn in cell-associated clusters that preceded fibril formation appeared more extended than diffuse cell-bound Fn but less extended than fibrillar Fn, suggesting that Fn undergoes extension after cell binding and before polymerization. FRET thus provides an approach to gain insight into the integrin-mediated pathway of Fn fibrillogenesis.
Imaging tools that discriminate between protein conformations in cell culture are essential for insight into how changes in protein structure modulate cell signaling. Fluorescence resonance energy transfer (FRET) has emerged to fill a void in techniques that provide high-resolution structural information on proteins adsorbed to surfaces or in cell culture. FRET is the nonradiative transfer of excitation energy from a fluorescent molecule, called the donor, to another molecule, called the acceptor (1, 2). It is generally detected as an increase in acceptor emission with a concomitant decrease in donor emission. Energy transfer from donor to acceptor fluorophore decays rapidly with increasing distance (the rate is inversely proportional to the sixth power of the separation), resulting in nanometer-scale sensitivity for distances up to 10 nm. By using fluorescently labeled proteins, FRET has been applied to detect protein–protein association on cell surfaces (3, 4) as well as unfolding transitions of proteins in solution (5, 6). Here we demonstrate FRET applied to imaging coexisting protein conformations in cell culture.
Fibronectin (Fn) was chosen as a model protein because it undergoes dramatic conformational changes that control its biological activity, yet the underlying molecular mechanisms are largely unknown. In blood and when secreted by cells such as fibroblasts, Fn is a soluble dimer. The dimer arms are connected at their carboxyl termini by a pair of disulfide bonds and consist of globular modules linearly connected by short linkers of variable flexibility. In soluble form, van der Waals and electrostatic interactions between modules stabilize Fn in a compact conformation, unreactive to other extracellular matrix proteins and to self-assembly. After binding cell integrins via its two arginine-glycine-aspartic acid sequences, Fn assembles into high molecular weight fibrils that constitute the primitive extracellular matrix before collagen deposition in wound healing and development (7). The mechanism controlling polymerization is unclear, although it is likely that cryptic sites required for self-assembly are exposed by integrin-mediated stretching of Fn (8–10). In fibrils, Fn is thought to exist with its dimer arms extended in an antiparallel orientation (11). Further extension by the unfolding of individual Fn modules has been proposed to account for the high degree of elasticity of Fn fibrils observed in cultures of Chinese hamster ovary cells expressing Fn chimeric with green fluorescent protein (12).
Our objective when labeling Fn was to place donor and acceptor fluorophores along the protein such that energy transfer between them would be sensitive to Fn unfolding. The Fn dimer is ≈160-nm long when linearly extended (13), whereas FRET occurs over a range of only 1–10 nm. As a result, a single donor-acceptor pair would give limited information about the unfolding of compact Fn to an extended state. We labeled Fn with multiple donor and acceptor fluorophores per protein such that a range of Fn conformations could be detected by gradual changes in the level of FRET.
We tested the sensitivity of intramolecular FRET to conformational change by unfolding labeled Fn in solution using guanidium chloride (GdnHCl). Under denaturing conditions sufficient to disrupt interactions between modules but too mild to unfold them (1–2 M GdnHCl), Fn undergoes a transition to a more extended conformation thought to be similar to that observed by electron microscopy when Fn is adsorbed to surfaces with its arms extended (13). Higher concentrations of denaturant (>2 M GdnHCl) result in progressive unfolding of Fn's globular modules (14). We used the known unfolding behavior of Fn in solution to correlate the level of FRET to the degree of Fn unfolding. We then added labeled Fn to the culture medium of fibroblasts and allowed the cells to incorporate it into their fibrillar matrix. In this report we describe the use of epifluorescence microscopy and spectroscopy to study the conformational transitions of Fn as it is bound to cells from solution, clustered by cell membrane integrins, and assembled into fibrils.
Materials and Methods
Fn Labeling.
Human plasma Fn (>95% purity, Life Technologies, Rockville, MD) at 2 mg/ml in PBS (0.02 M sodium phosphate buffer/0.15 M NaCl, pH 7.2) was denatured with 4 M GdnHCl (Sigma) for 15 min to expose the four free sulfhydryl groups per Fn dimer (15). The sulfhydryl-reactive fluorescent probe, tetramethylrhodamine-5-maleimide (TMR, Molecular Probes), was added at a 20-fold molar excess and incubated for 2 h with gentle mixing. The resulting Fn-fluorophore conjugate was separated from unbound fluorophores and GdnHCl by size-exclusion chromatography using a Sephadex G-25 gel filtration column (Amersham Pharmacia). The labeling ratio (average number of fluorophores per Fn dimer) was calculated from the solution absorbance at 280 and 556 nm. In the second labeling step, the amine-reactive fluorescent probe, Oregon green 488 carboxylic acid-succinimidyl ester-6-isomer (OG, Molecular Probes), was reacted to the Fn-TMR conjugate according to standard amine-labeling protocol (16). The resulting Fn labeled with donors and acceptors (Fn-D/A) was separated from unbound fluorophores as described before, and labeling ratios were calculated from the solution absorbance at 280, 496, and 556 nm. Extinction coefficients for TMR and OG were measured in PBS buffer, whereas a literature value of 1.28 cm⋅mg−1⋅ml−1 was used for Fn (17). The two-step labeling process resulted in an average of 7.2 ± 0.8 OG donors and 3.2 ± 0.5 TMR acceptors per Fn dimer (n = 5 batches). A single batch of Fn labeled with an average of 7.0 OG donors and 3.7 TMR acceptors per dimer was used in the experiments presented here.
Fluorescence Spectroscopy of Labeled Fn in Solution.
FRET associated with labeled Fn in PBS was measured using a standard fluorescence spectrophotometer (Hitachi F-4500) with excitation at 488 nm. The sensitivity of FRET to unfolding was evaluated by progressively denaturing Fn-D/A in PBS using a series of GdnHCl concentrations from 0 to 8 M. A mixture of unbound donor and acceptor fluorophores in solution was used as a control for direct excitation of the acceptor. Dilution of Fn-D/A with PBS had no effect on the ratio of donor and acceptor emission, indicating the absence of energy transfer between adjacent proteins in solution. Measurement of FRET over a range of denaturant concentrations was repeated using an epifluorescence microscope with an attached spectrometer (see below). The resulting curve allowed correlation of FRET efficiency and degree of Fn unfolding.
Incorporation of Labeled Fn into Cell Matrix Fibrils.
Sterile glass coverslips were coated with unlabeled Fn by adsorption from a 25 μg/ml Fn solution in PBS for 1 h at 25°C. NIH 3T3 fibroblasts (American Type Culture Collection) were plated at a density of 5 × 103 cells per cm2 in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. Unbound cells were removed by rinsing at 1 h, and a mixture of Fn-D/A with a 10-fold excess of unlabeled Fn was added to the culture medium to give a final Fn concentration of 100 μg/ml. Excess unlabeled Fn was used to prevent energy transfer between adjacent proteins. Samples were incubated for 24 h to allow cells to incorporate labeled Fn into well developed matrix fibrils, or 1–4 h to examine earlier stages of matrix assembly. Cells were fixed with 4% paraformaldehyde in PBS for 30 min and mounted on glass slides with Pro-Long anti-fade reagent (Molecular Probes).
Fluorescence Microscopy and Spectroscopy of Labeled Fn in Cell Culture.
Fluorescence imaging and spectroscopy were performed using an inverted epifluorescence microscope (Nikon TE 200) with a 512 × 512 pixel, back-illuminated, liquid nitrogen-cooled charge-coupled device (CCD) camera (TEK512, Princeton Instruments, Trenton, NJ). Spectroscopy was accomplished by placing a spectrometer (Acton 150, Acton Research Corporation, Acton, MA) in the emitted light path between microscope and camera. For direct visualization (through the oculars), we selected filters that allowed donor excitation and transmitted both donor and acceptor emission: an FITC filter cube [460–500-nm (blue) excitation filter and 510-nm cutoff dichroic mirror] modified with a 510-nm high-pass emission filter. This filter combination resulted in samples with features that appeared green, yellow, and red. For collection of emission spectra from isolated features in the samples, we used the same filter combination but inserted a slit and a diffraction grating into the emitted light path. The slit restricted the emitted light to a thin section of the sample, and the grating (300 lines per mm, 500-nm blaze) separated the emitted light into a range of wavelengths before reaching the CCD chip. METAMORPH software (Universal Imaging, Media, PA) was used to convert imaged light into spectral intensities at each wavelength, from 500 to 700 nm along the image horizontal axis, and at the region of interest along the vertical axis. Background spectra were taken from a region in the same image without features and subtracted. IGOR PRO (WaveMetrics, Lake Oswego, OR) was used for normalization of spectra and for graphing.
For imaging using a black and white CCD (higher sensitivity than color CCDs), it was necessary to collect donor and acceptor images separately, assign false colors to each (green and red, respectively), and overlay the images. Donor fluorescence was collected with a standard fluorescein filter set: 460–500-nm (blue) excitation filter, 510-nm cutoff dichroic mirror, and 510–560-nm (green) emission filter. Acceptor fluorescence caused by FRET was collected with the same excitation filter and dichroic mirror but with a 590–650-nm (red) emission filter. Acceptor fluorescence caused by direct acceptor excitation was collected as well, using a standard rhodamine filter set: 500–550-nm (green) excitation filter, 560-nm cutoff dichroic mirror, and 590–650-nm (red) emission filter. Images and spectra were collected with an ×100 oil immersion objective (Plan Fluor, 1.30 numerical aperture, Nikon).
Results
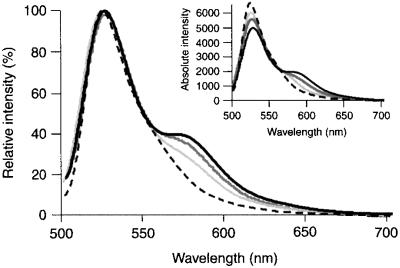
Fn was labeled with donor and acceptor fluorophores, and the sensitivity of intramolecular FRET to changes in Fn conformation was confirmed. Labeling was performed using two simple reaction chemistries: the conjugation of fluorophores to cysteine and amine residues. There are two free cysteines on each arm of the Fn dimer, one on the FnIII7 module and the other on FnIII15, and they become accessible after denaturing with 4 M GdnHCl (15). However, when only the two cysteine residues were labeled, FRET between donor-acceptor pairs was sensitive only to initial Fn unfolding at a low denaturant concentration (<2 M GdnHCl) and insensitive to further unfolding at a higher concentration (2–8 M GdnHCl). This behavior is consistent with changes in Fn conformation after denaturing shown by Khan et al. (14). When we labeled the cysteines with acceptors and amines with donors, FRET varied over the entire range of GdnHCl concentrations, indicating sensitivity to a larger range of unfolding events. Fig. 1 shows the decrease in FRET after denaturation of Fn in 1 and 4 M GdnHCl in PBS. Energy transfer yields an increase in acceptor emission accompanied by a decrease in donor emission (Fig. 1, Inset); spectra have been normalized to the donor peak. Thus, changes in energy transfer are reflected only by changes in the acceptor emission peak. The energy transfer remaining at high denaturant concentration (4 M GdnHCl) was likely caused by close proximity of donors and acceptors along the protein or to bending of labeled Fn (Fn-D/A) in solution bringing two fluorophores close together.
Figure 1.
Fluorescence emission spectra of Fn-D/A in PBS solution measured with a standard fluorescence spectrophotometer. The sensitivity of energy transfer to Fn unfolding was tested by denaturing Fn-D/A with GdnHCl at 0 (solid black), 1 (dark gray), and 4 M (light gray) in PBS. A 50–50 mixture of Fn labeled with either donor or acceptor fluorophore at the same total Fn concentration is shown for comparison (dashes). Spectra are normalized to the donor emission peak (520 nm) such that changes in energy transfer are reflected only by changes in the acceptor peak (570 nm). (Inset) Spectra before normalization.
When Fn-D/A was diluted 50-fold with PBS, from 0.5 to 0.01 mg/ml, there was no effect on the relative intensity of the donor and acceptor peaks. A second control using a 50–50 mixture of Fn labeled with either all donors or all acceptors showed no energy transfer. These results confirmed that FRET was caused by intra- rather than intermolecular interactions.
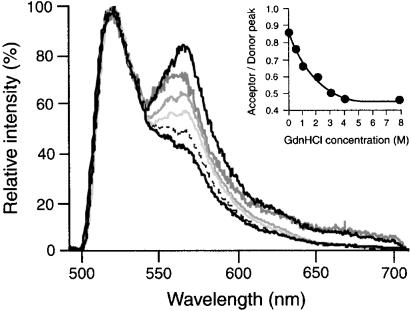
For an accurate comparison of FRET between Fn-D/A in solution and in cell culture, solution spectra were measured again, this time using an epifluorescence microscope with an attached spectrometer (see Materials and Methods). Changes in FRET associated with Fn-D/A unfolding were measured over a range of GdnHCl concentrations (0–8 M) in PBS (Fig. 2). The change in spectral shape from Fig. 1 to Fig. 2 is caused by a reduction of the low-wavelength portion of the 520-nm donor emission peak by the dichroic and emission filters in the microscope, a tradeoff for minimizing direct excitation of the acceptor fluorophore. The dramatic decrease in FRET from 0 to 2 M GdnHCl was caused by extension of Fn due to disruption of ionic interactions that stabilize the globular state. At higher denaturant concentrations (>2 M GdnHCl) Fn modules progressively unfold (14, 18). Thus, the decreases in FRET observed in >2 M GdnHCl were likely the result of increasing donor-acceptor separations caused by module unfolding. Fn-D/A in 4 and 8 M GdnHCl exhibited spectra indistinguishable from that of unbound fluorophores in solution, indicating that complete denaturing of Fn-D/A eliminated FRET. The fluorescence remaining at 570 nm even at 8 M GdnHCl was caused by the spectral tail of the donor emission and by direct excitation of the acceptor.
Figure 2.
Fluorescence emission spectra of Fn-D/A in PBS solution measured with a spectrometer attached to an epifluorescence microscope. The difference in spectral shape (compare with Fig. 1) is caused by a reduction of the low-wavelength fraction of donor emission by the microscope dichroic mirror (510-nm cutoff), a tradeoff for minimizing direct excitation of the acceptor fluorophore. FRET was measured over a range of GdnHCl concentrations: 0 (black, upper curve), 0.5 (dark gray), 1 (gray), 2 (light gray), 3 (dashed), 4 (black, lower curve), and 8 M (indistinguishable from 4 M). A mixture of unbound donor and acceptor fluorophores in PBS is indistinguishable from 4 and 8 M GdnHCl spectra. (Inset) FRET (acceptor peak intensity divided by donor peak) as a function of GdnHCl concentration.
Having tested the sensitivity of intramolecular FRET to known Fn unfolding behavior, we applied the technique to study Fn conformation in cell culture. Fn-D/A mixed with a 10-fold excess of unlabeled Fn was added to the growth medium of newly adherent NIH 3T3 fibroblasts on glass. Cells were allowed to incorporate the exogenous Fn into their extracellular matrix for 1, 2, 4, or 24 h. By using epifluorescence microscopy to excite donor fluorophores and transmit both donor and acceptor emission, we were able to directly visualize coexisting protein conformations. Regions of the sample at which Fn-D/A exhibited high levels of FRET appeared red, and regions with low levels of FRET appeared green. Because high FRET is caused by a smaller average separation of donors and acceptors, red indicated Fn in a more compact state, and green indicated Fn in a more extended state.
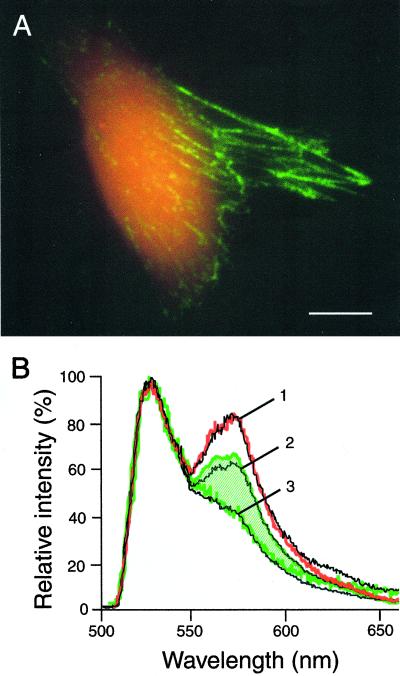
Fig. 3 shows the variation in FRET associated with Fn-D/A in fibroblast cultures after 24 h. Dramatic differences between populations of labeled Fn are apparent immediately. The most striking difference was observed between Fn-D/A diffusely bound to cells, which appeared red, and Fn-D/A in the fibrillar matrix, which appeared green (Fig. 3A). A 10-fold excess of unlabeled Fn was used to prevent intermolecular energy transfer in these experiments; when a 50-fold excess of unlabeled Fn was used as a control, no significant differences in the shape of FRET spectra were observed.
Figure 3.
Coexisting conformations of Fn in cell culture. (A) Superposition of donor and acceptor emission images after donor excitation. Fn-D/A diffusely bound to the cell appears red because of a high level of FRET, indicating a compact Fn conformation. Fn-D/A in the fibrillar matrix appears green because of low FRET, which suggests that Fn is in a more extended conformation. (Scale bar, 10 μm.) (B) Fluorescence emission spectra from Fn-D/A in cell culture. Diffuse cell-bound Fn-D/A (red) shows higher FRET than Fn-D/A in the fibrillar matrix (green). Fibrillar Fn-D/A exhibits a range of FRET (indicated by the hatched region). Shown in black are solution spectra of Fn, indicated by numbered lines: 1, 0 M GdnHCl; 2, 1 M GdnHCl; 3, 4 M GdnHCl.
For spatially resolved FRET measurements, we switched from an imaging to a spectroscopic mode (see Materials and Methods). A microscopic feature of interest was centered on the CCD, and a slit was inserted to block fluorescence from the rest of the sample. Fig. 3B shows typical emission spectra from different cellular features. Fn-D/A diffusely bound to cells exhibited higher FRET than Fn-D/A in matrix fibrils, as was visible in the images. Fn-D/A bound to cells showed an emission spectrum nearly identical to Fn-D/A in PBS with no denaturant, whereas matrix fibrils exhibited FRET that ranged between that of Fn-D/A in 1 and 4 M GdnHCl solutions (hatched region in Fig. 3B). All spectra were obtained from fibrils that were distinctly separate from cell membranes to prevent superposition of spectra from the two protein populations.
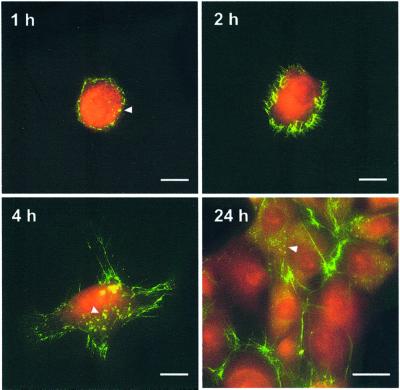
One of the outstanding questions regarding Fn–cell interactions concerns how soluble Fn dimers are polymerized into a multimeric fibrillar matrix. Although the molecular pathway is unknown, conformation changes in Fn are thought to play an integral role (see Discussion). To examine Fn conformational changes during matrix assembly, we analyzed samples fixed at 1, 2, 4, or 24 h after plating fibroblasts with Fn-D/A. Typical results are shown in Fig. 4.
Figure 4.
Conformational transitions of Fn-D/A during matrix assembly. The transition of Fn-D/A from a more compact (red, high FRET) state to a more extended (green, low FRET) state is visible around the cell periphery before fibril formation (1 and 2 h). Yellow clusters of Fn-D/A were observed on regions of cells where fibrils did not yet exist (1, 4, and 24 h; arrows). (Scale bars, 10 μm in the 1-, 2-, and 4-h images and 25 μm in the 24-h image.)
At 1 h, most of the cell-bound Fn-D/A shows red emission while a bright green ring begins to form around the cell. Fn-D/A in this ring exhibits sharply reduced FRET comparable to that of Fn-D/A in 3–4 M GdnHCl solutions, indicating conversion to a more extended state. At 2 h the green ring becomes filamentous with small fibrils radiating from the cell edge. By 4 h, long fibrils are associated with cell lamellopodia, and by 24 h many cells are confluent and coexist in various states of matrix assembly. In many samples we observed clusters of Fn-D/A on the surfaces of cells (Fig. 4, arrows). The clusters were brighter than the diffuse, cell surface-bound Fn-D/A and appeared yellow. FRET from the clusters was comparable to Fn-D/A in 1–2 M GdnHCl, suggesting that Fn in clusters is on average extended relative to the diffuse, cell-bound Fn but not as extended as Fn in the fibrillar matrix. The clusters were not caused by precipitation of Fn aggregates because they were absent from the sample background.
Discussion
In this study we used FRET to identify different Fn conformations in solution and in cell culture, illustrating that intramolecular energy transfer can be used as an indicator of protein conformation. Labeling Fn with multiple donor and acceptor fluorophores rather than a single donor-acceptor pair resulted in sensitivity to a range of Fn conformations. Fn unfolding from globular to fully denatured led to an increase in the average donor-acceptor separation and thus a decrease in energy transfer. Differences in FRET between populations of labeled Fn in cell culture were apparent immediately in fluorescence microscopy by the color of fluorescence emission. Images provide a conformational map in which red indicated Fn in a compact state, green a more extended state, and yellow an intermediate degree of unfolding. Spectroscopy allowed comparison of more subtle differences in FRET within and between samples. If conformational changes of Fn-D/A produce consistent changes in the donor-acceptor separation, then FRET can be used to provide a spectral “fingerprint” that identifies a particular protein conformation.
The experimental method was validated by comparing FRET results to the known behavior of Fn in solution. Based on sedimentation velocities (18) and electron microscopy (19), Fn is thought to assume a compact, globular conformation in solution at physiological pH and ionic strength. By disrupting electrostatic interactions between Fn domains, denaturants such as GdnHCl have been used to progressively extend and unfold Fn in solution (14). We applied this technique to calibrate the level of intramolecular FRET to Fn unfolding (Fig. 2).
During development, wound healing, and other processes involving extracellular matrix remodeling, fibroblasts secrete soluble Fn and assemble it into insoluble fibrils. The process involves Fn binding to cells, mechanical coupling of Fn to the cytoskeleton by integrins, exposure of self-assembly sites via contractile cell forces, and elongation of fibrils by Fn polymerization (20). The initial cell attachment, exposure of the integrin-binding arginine-glycine-aspartic acid loop, and availability of self-assembly sites are all likely regulated by Fn conformation (10, 11, 21). To study the conformation changes during cell binding and matrix assembly, we allowed NIH 3T3 fibroblasts to incorporate Fn-D/A into their extracellular matrices.
Several significant observations were made using FRET to study Fn conformation during matrix assembly. First, Fn-D/A diffusely bound to cell surfaces showed FRET that was indistinguishable from Fn-D/A in PBS with no denaturant (Fig. 3). This cell-bound population probably represents the initial interaction of soluble Fn with the cell surface, because it could be displaced by exchanging medium containing Fn-D/A with normal growth medium for 1 h. The initial interaction of Fn with cells may involve integrins or other receptors or the insertion of hydrophobic domains of Fn into the hydrophobic chains of the cell's phospholipid bilayer. Our results suggest that soluble Fn anchored to the cell is highly compact compared with fibrillar Fn.
Fig. 3 suggests that Fn-D/A in cell matrix fibrils is significantly more extended than Fn-D/A in physiological solution or diffusely bound to cells. This result supports the idea that Fn in fibrils exists with its two arms extended in an anti-parallel orientation (12). Fn fibrils are subject to tension generated by cytoskeletal contractility, and an ≈4-fold elasticity of fibrils has been observed by using Chinese hamster ovary cells that secrete Fn-green fluorescent protein (13). One mechanism proposed to explain fibrillar elasticity is the unraveling and refolding of individual modules (10, 22). The fact that FRET from many fibrils was reduced to the level found in completely denatured Fn-D/A implies that some of the type III domains that surround the acceptor-labeled cysteine residues on FaIII7, and FnIII15 were unfolded. Further work such as introducing fluorophore-labeling sites at the edges of the least stable Fn modules could reveal the specific location of module unraveling via changes in energy transfer.
A second observation was the development of cell-associated clusters of Fn-D/A that appeared yellow and were brighter than the diffuse surface-bound protein (Fig. 4, arrows). Clusters were found on cells that were not spread and had not formed fibrils and on spread cells where fibrils were absent. FRET levels in clusters were intermediate between those of Fn-D/A diffusely bound to the cell surface and in fibrils, suggesting that Fn in clusters was extended relative to the diffuse, cell-bound Fn but not as extended as Fn in fibrils.
In the earliest visible stages of matrix assembly there was a dramatic conversion of diffusely bound Fn-D/A from a compact high-FRET state to an extended state in which FRET was nearly eliminated (Fig. 4, 1 h). This conversion occurred in a narrow ring around many cells within 1 h after plating. The ring evolved into small fibrils radiating from the cell periphery and by 4 h developed into longer and thicker fibrils associated with cell lamellopodia. The intermediate FRET levels observed in the clusters, comparable to Fn-D/A in 1–2 M GdnHCl, suggest that, before formation of mature fibrils, cell-bound Fn-D/A is converted to a state of intermediate extension.
These results support a model of Fn fibril assembly in which the binding and clustering of Fn by integrins cause extension of the protein, which exposes self-assembly sites leading to Fn polymerization and fibril formation (20). The causes for the initial extension of Fn are unknown, although it is possible that a Fn dimer bound to two integrins experiences a force from small movements of the integrins or from tension applied by the actin cytoskeleton transmitted by the integrins. The results also support a mechanism of fibril elasticity involving the unfolding and refolding of type III modules.
Conclusions
By using FRET, we have observed several distinct degrees of Fn extension associated with the following states (in order of increasing extension): in physiological buffer, diffusely bound to cells, in cell-associated clusters, in matrix fibrils, and denatured with GdnHCl. The observed range of Fn conformations opposes the bimodal model, “globular” versus “extended” or biologically “active” versus “inactive,” commonly applied to Fn behavior. In this study we used FRET to obtain insight into Fn's unfolding transitions in cell culture. Using FRET between multiple donors and acceptors as an indicator of conformation is a promising method to study the mechanism by which mechanical forces alter Fn's conformation, control the exposure of its cryptic sites, and ultimately regulate cell signaling. Although we demonstrate the use of FRET to study Fn in cell culture, a similar approach can be used to investigate other proteins. FRET thus may serve as a versatile tool to investigate the processes by which cells control the conformation and activity of extracellular matrix proteins and assemble them into a complex and ordered fabric.
Acknowledgments
We gratefully acknowledge Ms. Lichia Feng and Mr. William Little for their contributions and Dr. Jean Schwarzbauer for fruitful discussions regarding Fn matrix assembly. This work was supported by National Institutes of Health Research Grant 5R01GM49063.
Abbreviations
- FRET
fluorescence resonance energy transfer
- Fn
fibronectin
- GdnHCl
guanidium chloride
- TMR
tetramethylrhodamine-5-maleimide
- Fn-D/A
Fn labeled with donors and acceptors
- CCD
charge-coupled device
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stryer L. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- 2.Lakowicz J R. Principles of Fluorescence Spectroscopy. New York: Plenum; 1986. pp. 305–316. [Google Scholar]
- 3.Sako Y, Minoghchi S, Yanagida T. Nat Cell Biol. 2000;2:168–172. doi: 10.1038/35004044. [DOI] [PubMed] [Google Scholar]
- 4.Rye H S. Methods. 2001;24:278–288. doi: 10.1006/meth.2001.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deniz A A, Laurence T A, Beligere G S, Dahan M, Martin A B, Chemla D S, Dawson P E, Schultz P G, Weiss S. Proc Natl Acad Sci USA. 2000;97:5179–5184. doi: 10.1073/pnas.090104997. . (First Published May 2, 2000; 10.1073/pnas.090104997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lillo M P, Szpikowska B K, Mas M T, Sutin J D, Beechem J M. Biochemistry. 1997;36:11273–11281. doi: 10.1021/bi970789z. [DOI] [PubMed] [Google Scholar]
- 7.Hynes R O. Fibronectins. New York: Springer; 1990. pp. 349–364. [Google Scholar]
- 8.Hocking D C, Smith R K, McKeown-Longo P L. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin A M, Burridge K. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel V, Thomas W E, Craig D W, Krammer A, Baneyx G. Trends Biotechnol. 2001;19:416–423. doi: 10.1016/S0167-7799(01)01737-1. [DOI] [PubMed] [Google Scholar]
- 11.An S S A, Jimenez-Barbero J, Petersen T E, Llinas M. Biochemistry. 1992;31:9927–9933. doi: 10.1021/bi00156a010. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi T, Kiehart D P, Erickson H P. Proc Natl Acad Sci USA. 1999;96:2153–2158. doi: 10.1073/pnas.96.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson H P, Carrell N, McDonagh J. J Cell Biol. 1981;91:267–269. doi: 10.1083/jcb.91.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M Y, Medow M S, Newman S A. Biochem J. 1990;270:33–38. doi: 10.1042/bj2700033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff C, Lai C S. Biochemistry. 1990;29:3354–3361. doi: 10.1021/bi00465a030. [DOI] [PubMed] [Google Scholar]
- 16.Haugland R P. In: Handbook of Fluorescent Probes and Research Chemicals. Spence M Z, editor. Eugene, OR: Molecular Probes; 1996. pp. 8–13. [Google Scholar]
- 17.Mosesson M W. Biochim Biophys Acta. 1975;386:509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- 18.Erickson H P, Carrell N A. J Biol Chem. 1983;254:14539–14544. [PubMed] [Google Scholar]
- 19.Zenhausern F, Adrian M, Descouts P. J Electron Microsc (Tokyo) 1993;42:378–388. [PubMed] [Google Scholar]
- 20.Schwarzbauer J E, Sechler J L. Curr Opin Cell Biol. 1999;11:622–627. doi: 10.1016/s0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 21.Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V. Proc Natl Acad Sci USA. 1999;96:1351–1356. doi: 10.1073/pnas.96.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson H P. Proc Natl Acad Sci USA. 1994;91:10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]