Abstract
A total of 18 matrine derivatives were designed, synthesized, and evaluated for their inhibitory effect against TGF-β1-induced total collagen accumulation in human fetal lung fibroblast MRC-5 cell lines. Among them, compound 3f displayed the most potent anti-fibrotic activity (IC50 = 3.3 ± 0.3 μM) which was 266-fold more potent than matrine. 3f significantly inhibited the fibroblast-to-myofibroblast transition and extracellular matrix production of MRC-5 cells. The TGF-β/small mothers against decapentaplegic homologs (Smad) signaling was also inhibited by 3f, as evidenced by inhibition of cytoplasm-to-nuclear translocation of Smad2/3 and suppression of TGF-β1-induced upregulation of TGF-β receptor type I (TGFβRI). Additionally, 3f exhibited potent inhibitory effects against TGF-β1-induced fibroblasts migration. These data suggested that 3f might be a potential agent for the treatment of idiopathic pulmonary fibrosis via repression of the TGFβ/Smad signaling pathway.
Keywords: idiopathic pulmonary fibrosis, matrine, indole, structure activity relationship, fibroblast-to-myofibroblast transition, TGF-β/Smad pathway
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible lung disease characterized by abnormally excessive accumulation of extracellular matrix (ECM) [1]. It is associated with high morbidity and mortality, with a median survival ranging from only three to five years from diagnosis [2]. Unfortunately, treatments for IPF were quite limited, and to date, only two small molecule drugs (Pirfenidone and Nintedanib) were approved by FDA for IPF treatment; nonetheless their treatment effect was not satisfactory [3,4]. Therefore, novel anti-IPF agents were urgently needed.
Matrine, a pyrrolizidine alkaloid isolated from traditional Chinese medicine Sophora flavescens, attracted our interest for its proved anti-fibrotic activities. Yu et al. [5] demonstrated that matrine exhibited significant inhibitory effect against liver fibrosis by reducing the expression of transforming growth factor-β1 (TGF-β1) and enhancing the activity of hepatocyte growth factor. The anti-fibrotic activities of matrine on cardiac fibrosis via inhibition of TGF-β/Smad pathway [6] or affection of ATF6 signaling pathway [7] had also been suggested by previous studies. In addition, two independent clinical studies [8,9] revealed that 150 mg/d matrine in combination of prednisone could significantly improve lung function and reduce symptom integral of the patients. Clinical study [10] also demonstrated that oxymatrine, the N-oxide analogue of matrine, could significantly reduce serum matrix metalloproteinase-9 (MMP-9) and TGF-β1 level in IPF patients. Collectively, matrine is of great potency for IPF treatment and worthy of further investigation.
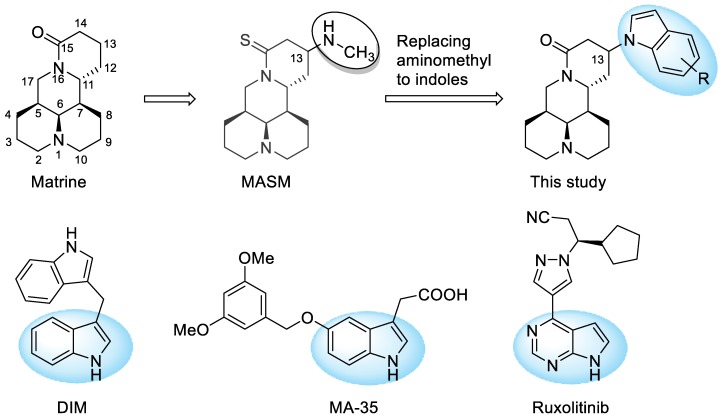
MASM (Figure 1) was a matrine derivative discovered by Xu et al. [11] which exhibited potent anti-fibrotic activities. Their research also suggested that the introduction of alkyl amine groups at the 13-position of matrine had a significant impact on anti-liver fibrosis [11] and anti-inflammatory activities [12]. Taking MASM as a lead, we changed the thioamide motif back to the original amide structure of matrine due to the water-instability of MASM [13], and performed structural optimization by varying the alkylamino substituent groups at the 13-position of matrine.
Figure 1.
Chemical structures of matrine, lead compound MASM, indole-containing anti-fibrotic drug or candidates, and the structure modification strategy.
Indole is a privileged scaffold in anti-fibrotic drug discovery. For instance, Xia and coworkers [14] demonstrated that indole derivative DIM could attenuate renal fibrosis via inhibiting fibroblast-to-myofibroblast transition and Smad2/3 phosphorylation. Additionally, in an anti-fibrotic screening of indole compound library, Shima et al. [15] observed that MA-35 displayed potent anti-fibrotic activities via inhibition of TGF-β1 signaling and suppression of ECM production. Moreover, indole scaffold could also be found in the chemical structure of anti-fibrotic drug Ruxolitinib [16]. Therefore, a series of 13-indolyl substituted derivatives was also designed and synthesized. Herein, we describe the synthesis of 18 matrine derivatives, SAR analysis, as well as the primary mechanism of action of the key compound.
2. Results and Discussion
2.1. Chemistry
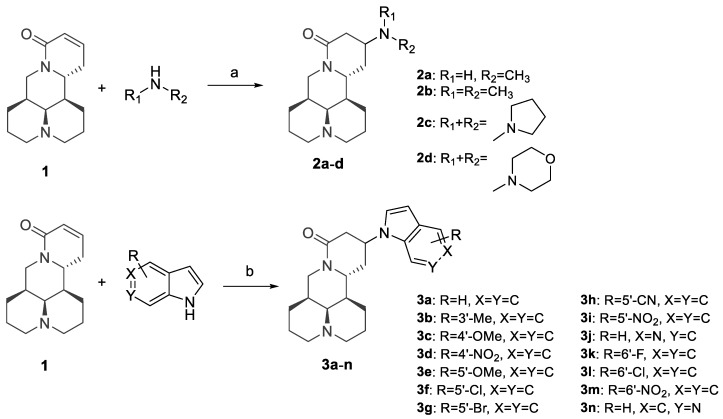
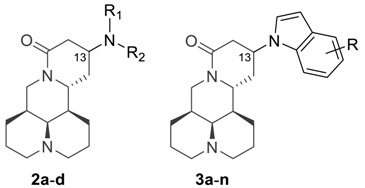
The synthetic routes of all target compounds were outlined in Scheme 1. The aliphatic amine substituted derivatives 2a–d were synthesized by microwave-assisted water-mediated Aza-Michael addition. Reactions of aliphatic amines and sophocarpine (1), a natural dehydrogenation analogue of matrine, proceeded smoothly under microwave irradiation to give desired products in yields of 90%–95%. Then, a series of indole substituted derivatives 3a–n were obtained by Michael addition of sophocarpine and indole in the presence of KHMDS in yields of 35–77%. All of the desired products were purified with silica gel chromatography using dichloromethane/methanol as eluents.
Scheme 1.
Reagents and conditions: (a) H2O, 100 W microwave irradiation, 30 min; (b) KHMDS, THF, −20 °C to rt, overnight.
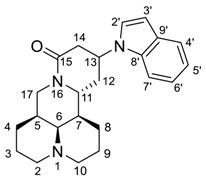
The Michael addition reaction introduced a new chiral center at the 13 position of matrine. Previous literature [12] determined the configuration of 13-position to be 13S, however, no experimental evidence supporting this determination was reported. In this study, we found that most of the synthesized compounds appeared in pairs of 13S/13R stereoisomers, of which the 13S-isomerswere the major stereoisomers. Nonetheless, four compounds (2a, 2b, 3f, 3j) appeared in pure 13S form, and we determined the configuration of 13 position based on NOE experiment of 3j (See Supplementary Materials). As illustrated in Figure 2, we observed NOE correlation between H-13 and H-7, indicating that these protons were in the same face. Thus, we confirmed that the major stereoisomers were 13S-isomers.
Figure 2.
Key NOE correlations of 3j.
2.2. Inhibition of TGF-β1-Induced Collagen Accumulation by Target Compounds
Excessive accumulation of ECM in the alveolar parenchyma and progressive scarring of lung tissue are major characteristics of IPF [17]. TGF-β1 is the most potent inducer of ECM production as well as the most significant fibrotic cytokines characterized so far [18]. It promotes IPF disease progression by inducing differentiation of fibroblast to myofibroblast and upregulating the secretion of ECM proteins [18,19], especially collagen, which is the most abundant protein in ECM [20]. Therefore, we established an in vitro anti-fibrotic screening model based on TGF-β1-induced collagen accumulation in human fetal lung fibroblasts (MRC-5 cell lines). The inhibitory effect against collagen accumulation of the synthesized compounds were quantitively determined by Sircol collagen assay, a colorimetric bioassay which was also adopted by Intermune incorporation (the innovator of Pirfenidone) for anti-fibrotic screening of Pirfenidone and its derivatives [21]. Cytotoxicity of all compounds were also determined against MRC-5 cells, so as to calculate selectivity index (SI, CC50/IC50). Higher SI values indicates better safety profile. The structures and activity data were summarized in Table 1. Pirfenidone was used as positive control, and its anti-fibrotic IC50 value determined in this study was 1320 ± 98 μM, which was consistent with Intermune’s results (23–69% inhibition of collagen accumulation at 1000 μM concentration for Pirfenidone derivatives [21]).
Table 1.
Anti-fibrotic activity, cytotoxicity, and selectivity index of matrine derivatives.
| Code | R | IC50 (μM) a | CC50 (μM) b | SI c | 13S/13R ratio d | ClogP e |
|---|---|---|---|---|---|---|
| 2a | R1 = Me, R2 = H | 65.3 ± 4.2 | 609.3 ± 29.8 | 9.3 | pure 13S | 0.75 |
| 2b | R1 = R2 = Me | 92.1 ± 1.9 | 624.5 ± 13.2 | 6.8 | pure 13S | 1.33 |
| 2c |
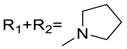
|
255.8 ± 22.1 | 675.8 ± 49.1 | 2.6 | 2.6:1 | 1.89 |
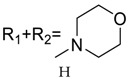
| 2d |
|
138.4 ± 9.8 | 507.2 ± 25.3 | 3.7 | 2.4:1 | 0.92 |
| 3a | H | 28.3 ± 3.9 | 106.6 ± 5.9 | 3.8 | 8.1:1 | 3.28 |
| 3b | 3′-Me | 39.0 ± 0.6 | 95.1 ± 6.5 | 2.4 | 5.7:1 | 3.78 |
| 3c | 4′-OMe | 44.7 ± 3.6 | 56.7 ± 3.9 | 1.3 | 4.9:1 | 3.34 |
| 3d | 4′-NO2 | 4.3 ± 0.4 | 32.8 ± 2.7 | 7.6 | 10.1:1 | 3.11 |
| 3e | 5′-OMe | 68.1 ± 5.5 | 127.5 ± 1.4 | 1.9 | 7.3:1 | 3.34 |
| 3f | 5′-Cl | 3.3 ± 0.3 | 26.7 ± 0.7 | 8.0 | pure 13S | 4.03 |
| 3g | 5′-Br | 6.5 ± 1.2 | 18.2 ± 0.6 | 2.8 | 4.9:1 | 4.18 |
| 3h | 5′-CN | 72.1 ± 4.0 | 153.6 ± 11.0 | 2.1 | 1.6:1 | 2.81 |
| 3i | 5′-NO2 | 14.5 ± 1.0 | 65.4 ± 3.1 | 4.5 | 3.3:1 | 3.11 |
| 3j | 5′-aza | 16.7 ± 3.5 | 114.0 ± 5.9 | 6.8 | pure 13S | 1.98 |
| 3k | 6′-F | 23.1 ± 5.3 | 78.1 ± 3.4 | 3.4 | 1.9:1 | 3.46 |
| 3l | 6′-Cl | 27.0 ± 1.4 | 45.8 ± 1.1 | 1.7 | 4.9:1 | 4.03 |
| 3m | 6′-NO2 | 19.3 ± 2.2 | 53.3 ± 1.9 | 2.8 | 11.5:1 | 3.11 |
| 3n | 6′-aza | 69.5 ± 8.2 | 84.2 ± 2.2 | 1.2 | 1.5:1 | 1.98 |
| Matrine | - | 878 ± 68 | 2466 ± 103 | 2.8 | - | 1.36 |
| Sophocarpine | - | 1186 ± 135 | 3160 ± 355 | 2.7 | - | 1.36 |
| Pirfenidone | - | 1320 ± 98 | 4159 ± 239 | 3.1 | - | 2.40 |
a. Inhibition of TGF-β1 induced total collagen accumulation in MRC-5 cells; b. 50% cytotoxic concentration; c. Selectivity index (SI = CC50/IC50); d. the ratio of 13S isomer vs. 13R isomer, determined by 1H-NMR spectra; e. ClogP (Calculated log partition coefficient) calculated using ChemDraw Professional.
The SAR investigation was initiated with variation of the alkylamino substituent groups at the 13 position of matrine, by which four derivatives were obtained (2a–d). As shown in Table 1, the IC50 values of 2a–d were 65.3 μM to 255.8 μM, which improved the potency of matrine by 3- to 13-folds, corroborating that 13 position substitution was beneficial for anti-pulmonary fibrosis activity. However, it seemed that the anti-fibrotic potency was largely influenced by steric effect of alkylamino substituents, and smaller substituent was favored. Introduction of bulky substituents such as cycloalkylamino group (2c–d) or dimethylamino group (2b) resulted in 1.4- to 3.9-fold decrease in anti-fibrotic potency compared to methylamino substituted derivative (2a). As a consequence, the steric influence in alkylamino substituted derivatives limited the scope for further improvement.
Next, we sought to replace the alkylamino substituents of the 13 position to indolyl group, and 14 derivatives were obtained (3a–n). To our delight, most of the compounds displayed more significant improvement in anti-fibrotic potency than matrine. Among them, compounds 3d, 3f, and 3g gave inspiring IC50 values of 4.3 μM, 3.3 μM, and 6.5 μM, respectively, which was 204-fold, 266-fold, and 135-fold more potent than matrine; or 307-fold, 400-fold, and 203-fold more potent than Pirfenidone. Compounds 3d and 3f also showed marked improvement in SI over matrine (from 2.8 to 7.6–8.0).
Further analysis of 3a–n suggested a remarkable impact of electronic properties of indolyl substitution on anti-fibrotic potency. Compounds with strong electron withdrawing groups such as halogen (3f–g, 3k–l) or nitro group (3d, 3i, 3m) gave more potent IC50 values (3.3–27.0 μM), an exception being compound 3h with cyano group whose IC50 was 72.1 μM. Contrarily, the introduction of electron donating groups resulted in significant loss of anti-fibrotic potency, and the IC50 values of methyl (3b) or methoxy (3c, 3e) substituted compounds were around 39.0 to 68.1 μM.
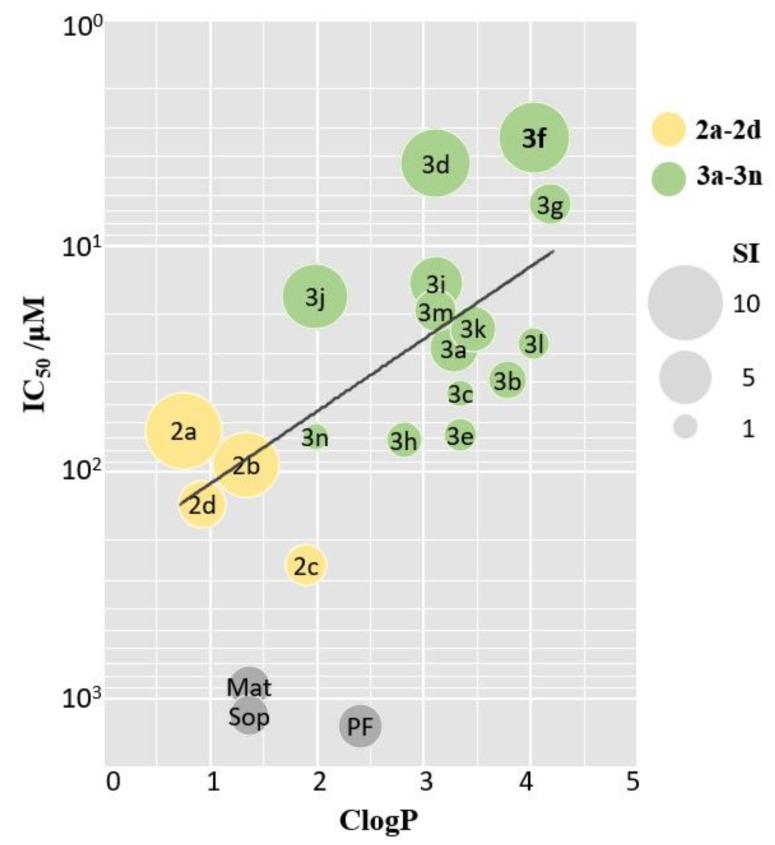
Additionally, we found an interesting phenomenon that the anti-fibrotic potency was positively correlated with lipophilicity of the corresponding compounds, and a bubble chart based on calculated log partition coefficients (ClogP) and IC50 values was established. As depicted in Figure 3, compounds exhibiting better anti-fibrotic activities tended to possess higher ClogP values. The introduction of indoles significantly increased the ClogP values of matrine (from 1.36 to 1.98–4.18), which might permit better membrane penetration and consequently more potent activities.
Figure 3.
Bubble chart of ClogP-IC50. X-axis and Y-axis represents ClogP and IC50 values, respectively. Bubble size reflects SI value. Yellow, green, and grey bubbles represent derivatives 2a–d, 3a–n, and positive control, respectively. Mat, Sop, PF was abbreviation for Matrine, Sophocarpine, and Pirfenidone, respectively.
2.3. Inhibition Effects of Expression Levels of ECM Proteins by Key Compound 3f
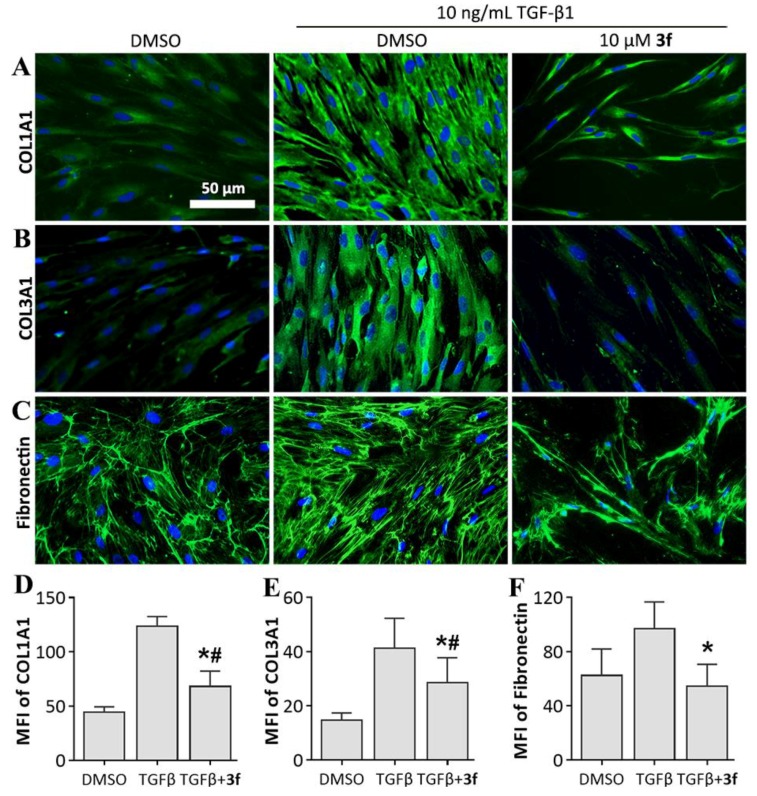
Due to the significance of collagen in IPF pathogenesis, we first evaluated the inhibitory effect of key compound 3f against collagen accumulation. Immunofluorescence staining was employed to investigate collagen protein expression. As indicated in Figure 4A,B,D,E, collagen synthesis was significantly elevated upon TGF-β1 stimulation, and the expression of Collagen type I alpha-1 chain (COL1A1) and Collagen type III alpha-1 chain (COL3A1) was upregulated by 178.2% and 183.1%, respectively. 10 μM 3f treatment resulted in remarkable decrease in expression level of COL1A1 and COL3A1 relative to TGF-β1 stimulation group (46.2% and 31.1%, respectively), indicating a potent inhibitory effect of 3f against TGF-β1-induced collagen accumulation.
Figure 4.
Effects of compound 3f on inhibiting extracellular matrix (ECM) protein levels in MRC-5 cells. (A–C) Immunofluorescence staining of each protein; (D–F) Mean fluorescence intensity (MFI) of each protein, presented as mean ± SD of three individual experiments. * p < 0.05 vs TGFβ group, # p < 0.05 vs. DMSO group.
Next, we evaluated the inhibitory effect of 3f against fibronectin expression. Fibronectin is another major constituent of ECM and plays a critical role in fibrogenesis by promoting the migration [22], proliferation [23], and adhesion [24] of fibroblasts. We observed that 3f treatment reduced the expression level of fibronectin by 40.0% comparing to TGF-β1 stimulation group, which resulted in even lower expression level than vehicle group (Figure 4C,F). These results suggested that 3f could revert the increase of ECM production induced by TGF-β1 stimulation.
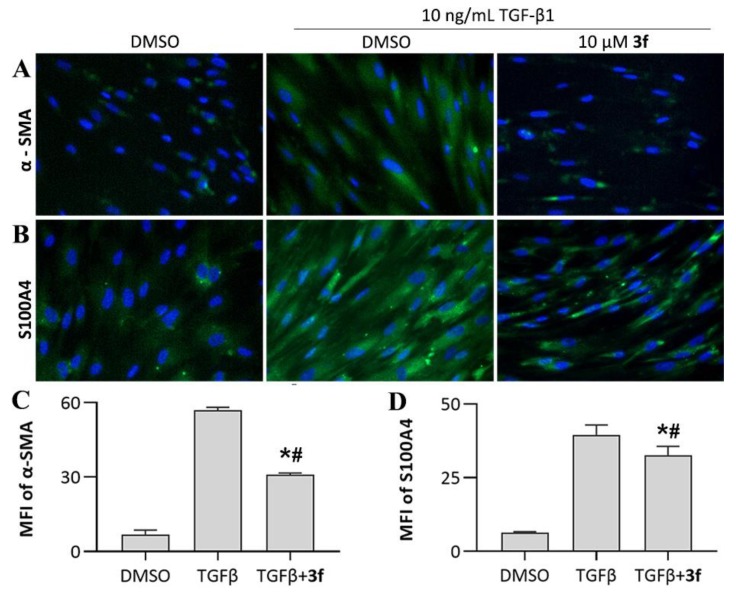
2.4. Inhibition Effects against Fibroblast-to-Myfibroblast Transition by 3f
Myofibroblasts, a further differentiated subset of fibroblasts, are primary effector cells for collagen synthesis in fibrotic disorders. Inhibition of fibroblast differentiation is an important therapeutic target for IPF treatment. To examine whether 3f could inhibit TGF-β1-induced fibroblast-to-myofibroblast transition, we investigated the expression level of alpha-smooth muscle actin (α-SMA) and S100A4, the key markers which distinguish myofibroblasts from their precursor fibroblasts [25]. We observed dramatic upregulation of myofibroblast markers in response to TGF-β1 stimulation (774.9% and 537.7% for α-SMA and S100A4, respectively), characterizing a strong expression of myofibroblast phenotype. 3f treatment resulted in disruption of fibroblast-to-myofibroblast transition, as evidenced by 45.8% and 17.7% downregulation in expression levels of α-SMA and S100A4, respectively, compared to TGF-β1 stimulation group (Figure 5).
Figure 5.
Effects of compound 3f on inhibiting fibroblast-to-myofibroblast transition. (A,B) Immunofluorescence staining of myofibroblast markers; (C,D) Mean fluorescence intensity of myofibroblast markers, presented as mean ± SD of three individual experiments. * p < 0.05 vs. TGFβ group, # p < 0.05 vs. DMSO group.
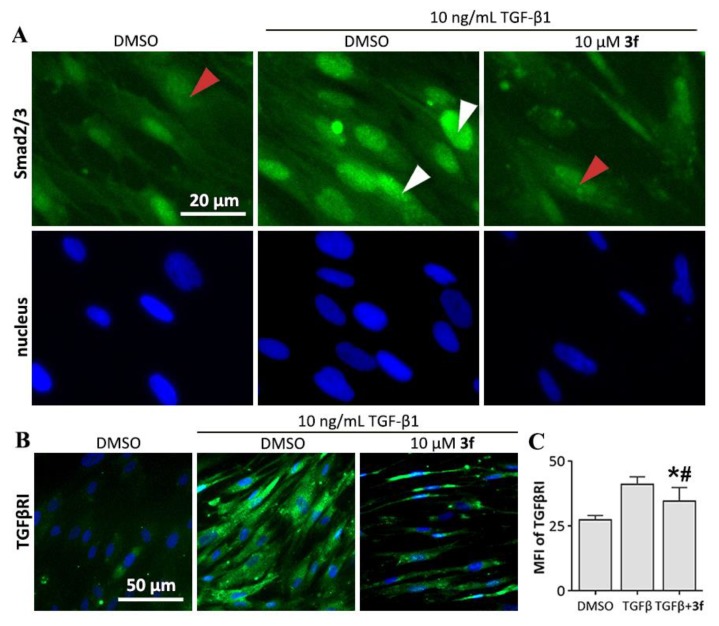
2.5. Inhibition Effects against Smad-Mediated Signaling by 3f
Small mothers against decapentaplegic homologs (Smad) are key mediators and regulators of TGFβ signaling. The binding of TGF-β1 to TGFβ receptors leads to the phosphorylation of Smad2/3 complex, which then translocate into nucleus to enhance gene transcription by cooperating with DNA transcription factors [26]. Inhibition of the nuclear translocation of Smad2/3 could attenuate the transcription of profibrotic genes such as collagens and α-SMA. Therefore, we investigated the cellular localization of Smad2/3 by immunofluorescence assay. As depicted in Figure 6A, Smad2/3 predominately localize in cytoplasm under normal conditions, but translocate into nucleus upon TGF-β1 stimulation. We observed that 3f could significantly inhibit the cytoplasm-to-nuclear translocation of Smad2/3.
Figure 6.
Effect of compound 3f against small mothers against decapentaplegic homologs (Smad) signaling. (A) 3f inhibited cytoplasm to nuclear translocation of Smad2/3 in MRC-5 cells. Immunofluorescence staining using an antibody recognizing total Smad2 and Smad3. Red arrows indicate the localization of Smad2/3 in cytoplasm, and white arrows indicate the nuclear localization of Smad2/3; (B) 3f inhibited TGF-β1-induced upregulation of expression level of TGF-β receptor type I (TGFβRI); (C) mean fluorescence intensity (MFI) of TGFβRI, presented as mean ± SD of three individual experiments. * p < 0.05 vs. TGFβ group, # p < 0.05 vs. DMSO group.
Another emerging effect of Smad which acts as the amplifier of TGF-β signaling also contribute to fibrotic diseases. The nuclear translocation of Smad3 leads to the activation of the miR-433 promotor region. miR-433 promotes the decrease of antizyme inhibitor I (Azin1) and eventually results in depletion of polyamine, which causes the upregulation of expression level of TGF-β receptor type I (TGFβRI) and TGF-β1. This series of signaling creates a positive-feedback loop which amplify the TGFβ signaling [27]. TGF-β1-induced upregulation of expression level of TGFβRI was also observed in this study (Figure 6B), which was reduced by 46.7% under 3f treatment (Figure 6C), suggesting an inhibitory effect of 3f against the positive-feedback loop of TGFβ signaling.
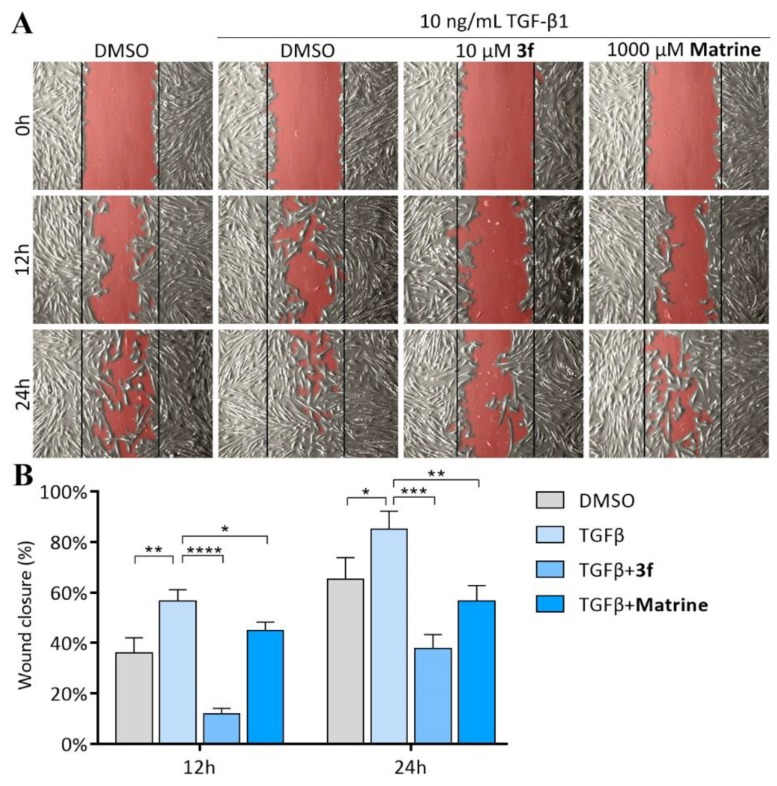
2.6. Inhibition Effects against TGF-β1 Induced Migration of MRC-5 Cells by 3f
Fibroblast migration is the key event in pulmonary fibrogenesis. Lung fibroblasts migrate across basement membranes and deposit excessive collagen which resulted in loss of function and eventually destruction of alveoli [28]. Inhibition of lung fibroblasts migration is a promising potential therapeutic target for IPF. We performed wound healing assay to investigate the inhibitory effect of 3f and matrine against fibroblast migration (Figure 7). The results indicated that MRC-5 fibroblasts displayed limited migration capacity under normal conditions, with 65.2% closure of wound area over 24 h period. However, wound closure increased to 85.0% over 24 h period upon TGF-β1 stimulation, indicating a significant enhancement in migration capacity, whilst treatment of 3f resulted in potent inhibition of TGF-β1-induced fibroblast migration, with only 37.7% closure of wound area over 24 h period. It is also worth mentioning that the inhibitory effect of 10 μM 3f was more potent than that of 1000 μM matrine (56.6% closure of wound area over 24 h period).
Figure 7.
Effects of compound 3f and matrine on inhibiting TGF-β1-induced MRC-5 fibroblasts migration. (A) Microscopic picture taken on 0 h, 12 h, and 24 h, the open area of wound region was highlighted in red; (B) Percentage of closure of wound area, analyzed by ImageJ software and MRI would healing tool plugin. Data are presented as mean ± SD of three individual experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs. TGFβ group.
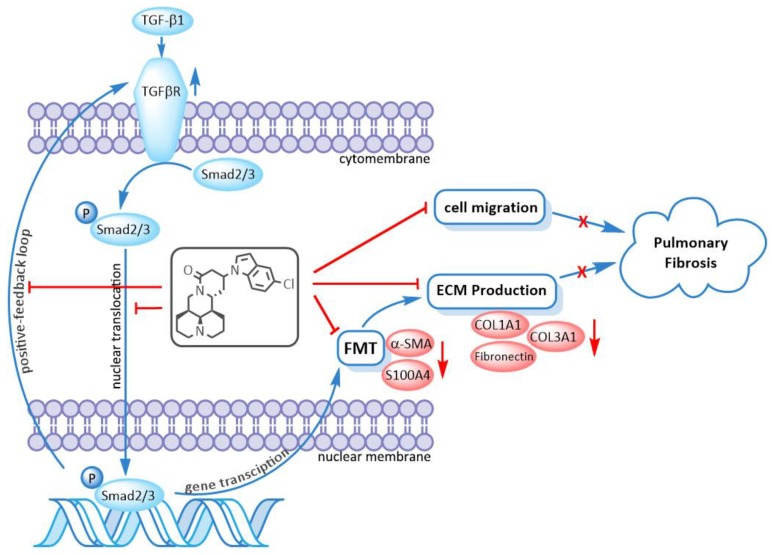
2.7. Action on TGFβ/Smad Pathway of 3f
As illustrated earlier, 3f could significantly inhibit fibroblast-to-myofibroblast transition induced by TGF-β1, which led to the downregulation of ECM production. 3f could also inhibit Smad signaling by inhibiting cytoplasm-to-nuclear translocation of Smad2/3. The inhibition of positive-feedback loop of TGFβ signaling was evidenced by suppression of TGF-β1-induced upregulation of TGFβRI by 3f as well. Therefore, it was speculated that 3f might exert anti-pulmonary fibrosis activity via repression of the TGF-β/Smad signaling pathway, as depicted in Figure 8.
Figure 8.
Compound 3f exerted anti-pulmonary fibrosis activity via repression of the TGFβ/Smad signaling pathway. FMT is abbreviation for fibroblast-to-myofibroblast transition.
3. Experimental Section
3.1. Apparatus, Materials, and Analysis Reagents
Matrine (98%) and sophocarpine (95%) were obtained from Yanchi Dushun biotechnology limited company (Ningxia, China). Pirfenidone (98%) were obtained from TCI development limited company (Shanghai, China). All reagents were used without further purification as received from commercial sources. Reactions were monitored by Thin Layer Chromatography on plates (GF254) supplied by Yantai Chemicals (China). If not specially mentioned, flash column chromatography was performed on silica gel (200–300 mesh) supplied by Tsingtao Haiyang Chemicals (China).1H-, 13C-NMR, and NOE spectra were taken in Methanol-d4 or CDCl3 at room temperature on Bruker Avance III 600 MHz instruments (Bruker, Rheinstetten, Germany) using the solvent signals (Methanol-d4: δH 3.31 ppm/δC 49.0 ppm; CDCl3: δH 7.26 ppm/δC 77.4 ppm) as references. ESI high-resolution mass spectra (ESI-HRMS) data were acquired using Q-TOF analyzer in SYNAPT HDMS system (Waters, Milford, MA, USA). Microwave irradiation experiments were carried out in a Discover SP microwave synthesizer (CEM, Matthews, NC, USA).
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Compounds 2a–d
To a 10 mL microwave vial was added sophocarpine (0.3 mmol) and aliphatic amine (1.5 mmol), then water (1 mL) was added as solvent. The solution was irradiated under the microwave power of 100 W for 30 min. After irradiation, the reaction solution was poured into saturated aqueous ammonium chloride (20 mL), and then extracted with dichloromethane (20 mL) twice. The organic layer was washed by saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel with dichloromethane/methanol as the eluent. Compounds 2a–d were mostly presented in pairs of 13S/13R stereoisomers, of which 13S-isomers were the major stereoisomers. The NMR peaks recorded below were signals of the major stereoisomers.
13-methylamino-matrine (2a). Sophocarpine (73.9 mg, 0.3 mmol) was treated with methylamine (1.5 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 2a as a light-yellow solid, yield: 95.2%; 1H-NMR (600 MHz, MeOD-d4) δ 4.25 (dd, J = 12.7, 4.4 Hz, 1H), 3.98 (dt, J = 11.7, 6.2 Hz, 1H), 3.13 (t, J = 12.7 Hz, 1H), 2.95–2.92 (m, 1H), 2.85 (d, J = 11.5 Hz, 1H), 2.81 (d, J = 11.5 Hz, 1H), 2.57 (dd, J = 17.1, 4.6 Hz, 1H), 2.38 (s, 3H), 2.27 (dd, J = 17.2, 6.5 Hz, 1H), 2.21 (brs, 1H), 2.05–1.93 (m, 4H), 1.89 (ddd, J = 13.8, 6.6, 2.8 Hz, 1H), 1.78–1.57 (m, 6H), 1.49–1.44 (m, 3H). 13C-NMR (150 MHz, MeOD-d4) δ 169.93, 65.21, 58.33, 58.26, 52.01, 51.06, 43.54, 43.21, 38.44, 37.16, 33.43, 30.69, 28.81, 27.46, 22.15, 21.64; ESI-HRMS m/z calcd for C16H28N3O [M + H]+, 278.2227; found 278.2216.
13-dimethylamino-matrine (2b). Sophocarpine (73.9 mg, 0.3 mmol) was treated with dimethylamine (1.5 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 2b as a white solid, yield: 93.8%; 1H-NMR (600 MHz, MeOD-d4) δ 4.23 (dd, J = 12.6, 4.4 Hz, 1H), 4.04 (dt, J = 11.0, 5.5 Hz, 1H), 3.13 (t, J = 12.7 Hz, 1H), 2.87 (d, J = 11.3 Hz, 1H), 2.82 (d, J = 11.4 Hz, 1H), 2.69–2.64 (m, 1H), 2.53 (dd, J = 17.0, 3.6 Hz, 1H), 2.41 (dd, J = 16.9, 8.4 Hz, 1H), 2.31 (s, 6H), 2.26 (brs, 1H), 2.07–1.95 (m, 5H), 1.78–1.57 (m, 6H), 1.49–1.45 (m, 3H). 13C-NMR (150 MHz, MeOD-d4) δ 169.86, 65.38, 58.29, 58.23, 56.39, 52.57, 43.56, 42.66, 42.27 (2), 37.33, 36.24, 28.70, 28.31, 27.49, 22.12, 21.66; ESI-HRMS m/z calcd for C17H30N3O [M + H]+, 292.2383; found 292.2371.
13-(pyrrolidine-1-yl)-matrine (2c). Sophocarpine (73.9 mg, 0.3 mmol) was treated with pyrrolidine (1.5 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 2c as a light-yellow solid, yield: 93.1%; 1H-NMR (600 MHz, MeOD-d4) δ 4.32 (dd, J = 12.7, 4.5 Hz, 1H), 4.01 (dt, J = 11.3, 5.8 Hz, 1H), 3.09 (t, J = 13.0 Hz, 1H), 2.86–2.79 (m, 2H), 2.64–2.55 (m, 5H), 2.50–2.38 (m, 2H), 2.14 (brs, 1H), 2.00–1.87 (m, 4H), 1.83–1.39 (m, 14H); 13C-NMR (150 MHz, MeOD-d4) δ 167.80, 64.08, 57.28, 57.23, 55.48, 51.92 (2), 50.84, 42.00, 41.87, 38.54, 35.74, 30.32, 27.70, 26.76, 23.36 (2), 21.18, 20.78; ESI-HRMS m/z calcd for C19H32N3O [M + H]+, 318.2540; found 318.2549.
13-morpholinocyclohexane-matrine (2d). Sophocarpine (73.9 mg, 0.3 mmol) was treated with morpholine (1.5 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 2d as a white solid, yield: 94.5%; 1H-NMR (600 MHz, MeOD-d4) δ 4.31–4.25 (m, 1H), 4.00 (dt, J = 11.9, 6.1 Hz, 1H), 3.70 (brs, 4H), 3.11–3.04 (m, 1H), 2.89 (brs, 2H), 2.70–1.93 (m, 13H), 1.78–1.50 (m, 9H); 13C-NMR (150 MHz, MeOD-d4) δ 170.16, 67.94 (2), 65.30, 58.19, 58.12, 56.00, 51.45 (2), 50.54, 43.16, 42.86, 37.02, 36.20, 31.63, 28.36, 27.26, 21.99, 21.58; ESI-HRMS m/z calcd for C19H32N3O2 [M + H]+, 334.2489; found 334.2493.
3.2.2. General Procedure for the Synthesis of Compounds 3a–n
 |
To a solution of the corresponding substituted indole (0.36 mmol) in THF (2 mL) was added 2 mol/L KHDMS (0.36 mmol) at −20 °C; the resulting solution was stirred at the same temperature for 20 min, and then sophocarpine (0.3 mmol) was added. The reaction temperature was allowed to raise to r.t. and stirred overnight. After the completion of the reaction, the reaction mixture was diluted with dichloromethane (50 mL). The organic layer was washed with water and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel with dichloromethane/methanol as the eluent. Compounds 3a–n were mostly presented in pairs of 13S/13R stereoisomers, of which 13S-isomers were the major stereoisomers. The NMR peaks recorded below were signals of the major stereoisomers.
13-(1H-indol-1-yl)-matrine (3a). Sophocarpine (73.9 mg, 0.3 mmol) was treated with indole (42.2 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3a as a white solid, yield: 65.1%; 1H-NMR (600 MHz, MeOD-d4) δ 7.56 (d, J = 7.8 Hz, 1H, 4’-CH), 7.45 (d, J = 8.3 Hz, 1H, 7’-CH), 7.18–7.16 (m, 2H, 6’-CH, 3’-CH), 7.05 (t, J = 7.4 Hz, 1H, 5’-CH), 6.50 (d, J = 3.3 Hz, 1H, 2’-CH), 4.97–4.93 (m, 1H, 13-CH), 4.35 (dd, J = 12.7, 4.4 Hz, 1H, 17α-CH2), 3.85 (dt, J = 11.4, 5.8 Hz, 1H, 11-CH), 3.13 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.97 (dd, J = 17.3, 5.4 Hz, 1H, 14α-CH2), 2.87–2.74 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.53–2.48 (m, 1H, 12α-CH2), 2.24 (brs, 1H, 6-CH), 2.15–2.12 (m, 1H, 12β-CH2), 2.04–1.95 (m, 2H, 2β-CH2, 10β-CH2), 1.91–1.31 (m, 10H, 5-CH, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.48 (15-C), 137.16 (8’-C), 130.17 (9’-C), 124.77 (2’-CH), 122.67 (6’-CH), 121.93 (4’-CH), 120.66 (5’-CH), 110.31 (7’-CH), 103.14 (3’-CH), 65.14 (6-CH), 58.28 (2-CH2, 10-CH2), 52.44 (11-CH), 47.56 (13-CH), 43.44 (17-CH2), 42.82 (7-CH), 38.66 (14-CH2), 37.07 (5-CH), 31.48 (12-CH2), 28.71 (4-CH2), 27.44 (8-CH2), 22.08 (3-CH2), 21.42 (9-CH2); ESI-HRMS m/z Calcd for C23H30N3O [M + H]+, 364.2383; found 364.2382.
13-(3-methyl-1H-indol-1-yl)-matrine (3b). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 3-methylindole (47.2 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3b as a white solid, yield: 35.2%; 1H-NMR (600 MHz, MeOD-d4) δ 7.50 (d, J = 7.9 Hz, 1H, 4’-CH), 7.38 (d, J = 8.4 Hz, 1H, 7’-CH), 7.16 (t, J = 8.2 Hz, 1H, 6’-CH), 7.04 (t, J = 7.9 Hz, 1H, 5’-CH), 6.92 (d, J = 1.1 Hz, 1H, 2’-CH), 4.90–4.86 (m, 1H, 13-CH), 4.35 (dd, J = 12.7, 4.8 Hz, 1H, 17α-CH2), 3.81 (dt, J = 11.7, 6.0 Hz, 1H, 11-CH), 3.10 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.94–2.73 (m, 4H, 14-CH2, 10α-CH2, 2α-CH2), 2.45 (ddd, J = 14.1, 8.1, 5.5 Hz, 1H, 12α-CH2), 2.29 (d, J = 1.02 Hz, 3H, Ph-Me), 2.28–2.19 (m, 1H, 6-CH), 2.09–1.87 (m, 4H, 12β-CH2, 2β-CH2, 10β-CH2, 5-CH), 1.82–1.29 (m, 9H, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.63 (15-C), 137.51 (8’-C), 130.20 (9’-C), 122.68 (2’-CH), 122.38 (6’-CH), 120.04 (5’-CH), 119.91 (4’-CH), 112.21 (3’-C), 110.10 (7’-CH), 65.11 (6-CH), 58.11 (2-CH2, 10-CH2), 52.38 (11-CH), 47.24 (13-CH), 43.32 (17-CH2), 42.75 (7-CH), 38.64 (14-CH2), 36.96 (5-CH), 31.56 (12-CH2), 28.61 (4-CH2), 27.33 (8-CH2), 22.00 (3-CH2), 21.35 (9-CH2), 9.82 (Ph-CH3); ESI-HRMS m/z Calcd for C24H32N3O [M + H]+, 378.2540; found 378.2551.
13-(4-methoxy-1H-indol-1-yl)-matrine (3c). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 4-methoxyindole (53.0 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3c as a white solid, yield: 41.7%; 1H-NMR (600 MHz, MeOD-d4) δ 7.11–7.02 (m, 3H, 6’-CH, 7’-CH, 2’-CH), 6.57–6.52 (m, 2H, 5’-CH, 3’-CH), 4.34 (dd, J = 12.7, 4.4 Hz, 1H), 4.84–4.80 (m, 1H, 13-CH), 4.31 (dd, J = 12.6, 4.4 Hz, 1H, 17α-CH2), 3.90 (s, 3H, Ph-OMe), 3.80 (dt, J = 11.3, 5.8 Hz, 1H, 11-CH), 3.09 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.91 (dd, J = 17.3, 5.4 Hz, 1H, 14α-CH2), 2.81–2.70 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.43–2.37 (m, 1H, 12α-CH2), 2.16 (brs, 1H, 6-CH), 2.06–2.04 (m, 1H, 12β-CH2), 1.99–1.96 (m, 2H, 2β-CH2, 10β-CH2), 1.83–1.79 (m, 1H, 5-CH), 1.74–1.26 (m, 9H, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.31 (15-C), 154.86 (4’-C), 138.57 (8’-C), 123.73 (2’-CH), 123.12 (6’-CH), 120.49 (9’-C), 103.82 (5’-CH), 100.59 (3’-CH), 100.47 (7’-CH), 65.06 (6-CH), 58.21 (2-CH2), 58.11 (10-CH2), 55.64 (Ph-OMe), 52.39 (11-CH), 47.74 (13-CH), 43.42 (17-CH2), 42.65 (7-CH), 38.66 (14-CH2), 37.01 (5-CH), 31.39 (12-CH2), 28.67 (4-CH2), 27.41 (8-CH2), 22.05 (3-CH2), 21.37 (9-CH2); ESI-HRMS m/z Calcd for C24H32N3O2 [M + H]+, 394.2489; found 394.2481.
13-(4-nitro-1H-indol-1-yl)-matrine (3d). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 4-nitroindole (58.4 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3d as a yellow solid, yield: 68.3%; 1H-NMR (600 MHz, MeOD-d4) δ 8.09 (d, J = 8.0 Hz, 1H, 5’-CH), 7.96 (d, J = 8.2 Hz, 1H, 7’-CH), 7.56 (d, J = 3.3 Hz, 1H, 2’-CH), 7.33 (t, J = 8.1 Hz, 1H, 6’-CH), 7.19 (d, J = 3.2 Hz, 1H, 3’-CH), 5.09 (ddd, J = 9.2, 8.6, 4.2 Hz, 1H, 13-CH), 4.34 (dd, J = 12.7, 4.4 Hz, 1H, 17α-CH2), 3.94–3.90 (m, 1H, 11-CH), 3.14 (t, J = 12.7 Hz, 1H, 17β-CH2), 3.00 (dd, J = 17.3, 5.5 Hz, 1H, 14α-CH2), 2.87 (dd, J = 17.2, 7.7 Hz, 1H, 14β-CH2), 2.78–2.74 (m, 2H, 10α-CH2, 2α-CH2), 2.56–2.51 (m, 1H, 12α-CH2), 2.24–2.19 (m, 2H, 6-CH, 12β-CH2), 2.01–1.95 (m, 2H, 2β-CH2, 10β-CH2), 1.91–1.87 (m, 2H, 5-CH, 7-CH), 1.79–1.32 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 168.88 (15-C), 141.48 (4’-C), 139.40 (8’-C), 130.01 (2’-CH), 123.78 (9’-C), 121.74 (6’-CH), 118.50 (5’-CH), 117.79 (7’-CH), 103.53 (3’-CH), 65.17 (6-CH), 58.16 (2-CH2, 10-CH2), 52.51 (11-CH), 48.27 (13-CH), 43.57 (17-CH2), 42.55 (7-CH), 38.79 (14-CH2), 37.13 (5-CH), 31.26 (12-CH2), 28.67 (4-CH2), 27.45 (8-CH2), 22.05 (3-CH2), 21.40 (9-CH2); ESI-HRMS m/z Calcd for C23H29N4O [M + H]+, 409.2234; found 409.2222.
13-(5-methoxy-1H-indol-1-yl)-matrine (3e). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 5-methoxyindole (53.0 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3e as a white solid, yield: 55.1%; 1H-NMR (600 MHz, MeOD-d4) δ 7.31 (d, J = 8.8 Hz, 1H, 7’-CH), 7.11 (s, 1H, 2’-CH), 7.06 (s, 1H, 4’-CH), 6.82 (d, J = 7.5 Hz, 1H, 6’-CH), 6.41 (s, 1H, 3’-CH), 4.85–4.81 (m, 1H, 13-CH), 4.32 (d, J = 13.2 Hz, 1H, 17α-CH2), 3.82–3.80 (m, 1H, 11-CH), 3.79 (s, 3H, Ph-OMe), 3.10 (t, J = 12.9 Hz, 1H, 17β-CH2), 2.94–2.90 (m, 1H, 14α-CH2), 2.81–2.72 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.46–2.41 (m, 1H, 12α-CH2), 2.19 (brs, 1H, 6-CH), 2.08 (d, J = 13.3 Hz, 1H, 12β-CH2), 1.98–1.93 (m, 2H, 2β-CH2, 10β-CH2), 1.85 (d, J = 13.8 Hz, 1H, 5-CH), 1.78–1.28 (m, 9H, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.44 (15-C), 155.61 (5’-C), 132.47 (9’-C), 130.61 (8’-C), 125.35 (2’-CH), 112.89 (6’-CH), 111.03 (4’-CH), 103.70 (7’-CH), 102.86 (3’-CH), 65.12 (6-CH), 58.18 (2-CH2, 10-CH2), 56.19 (Ph-OMe), 52.41 (11-CH), 47.70 (13-CH), 43.44 (17-CH2), 42.79 (7-CH), 38.71 (14-CH2), 37.07 (5-CH), 31.54 (12-CH2), 28.73 (4-CH2), 27.46 (8-CH2), 22.09 (3-CH2), 21.43 (9-CH2); ESI-HRMS m/z Calcd for C24H32N3O2 [M + H]+, 394.2489; found 394.2483.
13-(5-chloro-1H-indol-1-yl)-matrine (3f). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 5-chloroindole (54.6 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3f as a white solid, yield: 77.2%; 1H-NMR (600 MHz, MeOD-d4) δ 7.54 (d, J = 1.9 Hz, 1H, 4’-CH), 7.43 (d, J = 8.8 Hz, 1H, 7’-CH), 7.24 (d, J = 3.3 Hz, 1H, 2’-CH), 7.13 (d, J = 8.8, 2.0 Hz, 1H, 6’-CH), 6.48 (d, J = 3.0 Hz, 1H, 3’-CH), 4.94–4.90 (m, 1H, 13-CH), 4.33 (dd, J = 12.6, 4.4 Hz, 1H, 17α-CH2), 3.85 (dt, J = 11.3, 5.8 Hz, 1H, 11-CH), 3.12 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.95 (dd, J = 16.9, 5.1 Hz, 1H, 14α-CH2), 2.82 (dd, J = 17.3, 7.3 Hz, 1H, 14β-CH2), 2.77–2.73 (m, 2H, 10α-CH2, 2α-CH2), 2.48 (ddd, J = 14.3, 8.9, 5.8 Hz, 1H, 12α-CH2), 2.22 (brs, 1H, 6-CH), 2.13 (dd, J = 13.8, 7.2 Hz, 1H, 12β-CH2), 2.00 –1.82 (m, 4H, 2β-CH2, 10β-CH2, 5-CH, 7-CH), 1.77–1.30 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.20 (15-C), 135.57 (8’-C), 131.16 (9’-C), 126.53 (2’-CH), 126.39 (5’-C), 122.78 (6’-CH), 121.18 (4’-CH), 111.65 (7’-CH), 102.89 (3-CH), 65.12 (6-CH), 58.16 (2-CH2,10-CH2), 52.42 (11-CH), 47.88 (13-CH), 43.46 (17-CH2), 42.72 (7-CH), 38.66 (14-CH2), 37.08 (5-CH), 31.37 (12-CH2), 28.69 (4-CH2), 27.44 (8-CH2), 22.07 (3-CH2), 21.41 (9-CH2); ESI-HRMS m/z Calcd for C23H29ClN3O [M + H]+, 398.1994; found 398.1999.
13-(5-bromo-1H-indol-1-yl)-matrine (3g). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 5-bromoindole (70.6 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3g as a white solid, yield: 62.1%; 1H-NMR (600 MHz, MeOD-d4) δ 7.70 (d, J = 1.9 Hz, 1H, 4’-CH), 7.39 (d, J = 8.9 Hz, 1H, 7’-CH), 7.25 (dd, J = 8.8, 1.9 Hz, 1H, 6’-CH), 7.22 (d, J = 3.3 Hz, 1H, 2’-CH), 6.48 (d, J = 3.3 Hz, 1H, 3’-CH), 4.94–4.90 (m, 1H, 13-CH), 4.32 (dd, J = 12.6, 4.4 Hz, 1H, 17α-CH2), 3.84 (dt, J = 11.3, 5.7 Hz, 1H, 11-CH), 3.11 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.94 (dd, J = 17.3, 5.5 Hz, 1H, 14α-CH2), 2.84–2.72 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.46 (ddd, J = 15.1, 10.0, 6.1 Hz, 1H, 12α-CH2), 2.20 (brs, 1H, 6-CH), 2.14–2.10 (m, 1H, 12β-CH2), 2.04–1.93 (m, 2H, 2β-CH2, 10β-CH2), 1.85 (d, J = 14.4 Hz, 1H, 5-CH), 1.82–1.29 (m, 9H, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.17 (15-C), 135.84 (8’-C), 131.84 (9’-C), 126.42 (2’-CH), 125.41 (6’-CH), 124.37 (4’-CH), 113.85 (5’-C), 112.12 (7’-CH), 102.85 (3’-CH), 65.13 (6-CH), 58.17 (2-CH2, 10-CH2), 52.43 (11-CH), 47.88 (13-CH), 43.49 (17-CH2), 42.72 (7-CH), 38.67 (14-CH2), 37.10 (5-CH), 31.36 (12-CH2), 28.71 (4-CH2), 27.47 (8-CH2), 22.11 (3-CH2), 21.44 (9-CH2); ESI-HRMS m/z Calcd for C23H29BrN3O [M + H]+, 442.1489; found 442.1474.
13-(5-cyano-1H-indol-1-yl)-matrine(3h). Sophocarpine (73.9 mg, 0.3 mmol) was treated with indole-5-carbonitrile (51.2 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3h as a white solid, yield: 68.9%; 1H-NMR (600 MHz, MeOD-d4) δ 8.01 (d, J = 1.6 Hz, 1H, 4’-CH), 7.66 (d, J = 8.6 Hz, 1H, 7’-CH), 7.45 (dd, J = 8.5, 1.6 Hz, 1H, 6’-CH), 7.43 (d, J = 3.4 Hz, 1H, 2’-CH), 6.68 (d, J = 3.4 Hz, 1H, 3’-CH), 5.04 (tdd, J = 8.6, 5.4, 2.9 Hz, 1H, 13-CH), 4.34 (dd, J = 12.6, 4.4 Hz, 1H, 17α-CH2), 3.91 (dt, J = 11.1, 5.6 Hz, 1H, 11-CH), 3.14 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.97 (dd, J = 17.3, 5.6 Hz, 1H, 14α-CH2), 2.87–2.76 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.54–2.49 (m, 1H, 12α-CH2), 2.25 (brs, 1H, 6-CH), 2.22–2.18 (m, 1H, 12β-CH2), 2.06–1.96 (m, 2H, 2β-CH2, 10β-CH2), 1.91–1.88 (m, 1H, 5-CH), 1.80–1.32 (m, 9H, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 168.93 (15-C), 138.76 (8’-C), 129.87 (9’-C), 127.82 (2’-CH), 127.67 (4’-CH), 125.46 (6’-CH), 121.50 (Ph-CN), 111.79 (7’-CH), 104.36 (3’-CH), 103.52 (5’-CH), 65.15 (6-CH), 58.24 (2-CH2, 10-CH2), 52.50 (11-CH), 48.05 (13-CH), 43.54 (17-CH2), 42.55 (7-CH), 38.67 (14-CH2), 37.11 (5-CH), 31.19 (12-CH2), 28.66 (4-CH2), 27.44 (8-CH2), 22.05 (3-CH2), 21.40 (9-CH2); ESI-HRMS m/z Calcd for C24H29N4O [M + H]+, 389.2336; found 389.2345.
13-(5-nitro-1H-indol-1-yl)-matrine (3i). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 5-nitroindole (58.4 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3i as a yellow solid, yield: 68.0%; 1H-NMR (600 MHz, MeOD-d4) δ 8.55 (d, J = 2.2 Hz, 1H, 4’-CH), 8.09 (dd, J = 9.1, 2.2 Hz, 1H, 6’-CH), 7.64 (d, J = 9.1 Hz, 1H, 7’-CH), 7.47 (d, J = 3.4 Hz, 1H, 2’-CH), 6.78 (d, J = 3.3 Hz, 1H, 3’-CH), 5.07 (tdd, J = 8.6, 5.4, 3.0 Hz, 1H, 13-CH), 4.34 (dd, J = 12.7, 4.4 Hz, 1H, 17α-CH2), 3.94 (dt, J = 11.1, 5.6 Hz, 1H, 11-CH), 3.15 (t, J = 12.6 Hz, 1H, 17β-CH2), 2.98 (dd, J = 17.4, 5.4 Hz, 1H, 14α-CH2), 2.89–2.77 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.54 (ddd, J = 14.5, 9.3, 5.7 Hz, 1H, 12α-CH2), 2.26–2.21 (m, 2H, 6-CH, 12β-CH2), 2.05–1.89 (m, 3H, 2β-CH2, 10β-CH2, 5-CH), 1.81–1.33 (m, 9H, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 168.91 (15-C), 143.07 (8’-C), 139.97 (9’-C), 129.31 (5’-C), 128.79 (2’-CH), 118.89 (6’-CH), 118.04 (4’-CH), 110.77 (7’-CH), 105.91 (3’-CH), 65.22 (6-CH), 58.22 (2-CH2, 10-CH2), 52.57 (11-CH), 48.41 (13-CH), 43.62 (17-CH2), 42.63 (7-CH), 38.71 (14-CH2), 37.19 (5-CH), 31.23 (12-CH2), 28.73 (4-CH2), 27.51 (8-CH2), 22.11 (3-CH2), 21.46 (9-CH2); ESI-HRMS m/z Calcd for C23H29N4O [M + H]+, 409.2234; found 409.2221.
13-(1H-pyrrolo[3,2-c]pyridin-1-yl)-matrine (3j). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 5-azaindole (42.5 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3j as a yellow solid, yield: 37.6%; 1H-NMR (600 MHz, MeOD-d4) δ 8.80 (s, 1H, 4’-CH), 8.20 (d, J = 6.0 Hz, 1H, 6’-CH), 7.58 (d, J = 6.0 Hz, 1H, 7’-CH), 7.40 (d, J = 3.3 Hz, 1H, 2’-CH), 6.74 (d, J = 3.1 Hz, 1H, 3’-CH), 5.06–5.01 (m, 1H, 11-CH), 4.34 (dd, J = 12.6, 4.1 Hz, 1H, 17α-CH2), 3.94 (dt, J = 10.9, 5.4 Hz, 1H, 11-CH), 3.15 (t, J = 12.6 Hz, 1H, 17β-CH2), 2.97 (dd, J = 17.3, 5.4 Hz, 1H, 14α-CH2), 2.88 (dd, J = 17.3, 7.7 Hz, 1H, 14β-CH2), 2.77 (d, J = 11.3 Hz, 2H, 10α-CH2, 2α-CH2), 2.54 (tt, J = 9.2, 5.6 Hz, 1H, 12α-CH2), 2.25 (brs, 1H, 6-CH), 2.21 (d, J = 15.0 Hz, 1H, 12β-CH2), 2.00–1.97 (m, 2H, 2β-CH2, 10β-CH2), 1.92– 1.88 (m, 2H, 5-CH, 7-CH), 1.80 –1.33 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 168.85 (15-C), 144.09 (4’-CH), 140.98 (8’-C), 140.52 (6’-CH), 127.37 (2’-CH), 127.01 (9’-C), 106.61 (7’-CH), 103.23 (3’-CH), 65.17 (6-CH), 58.18 (2-CH2, 10-CH2), 52.52 (11-CH), 48.14 (13-CH), 43.58 (17-CH2), 42.59 (7-CH), 38.63 (14-CH2), 37.16 (5-CH), 31.18 (12-CH2), 28.70 (4-CH2), 27.47 (8-CH2), 22.08 (3-CH2), 21.43 (9-CH2); ESI-HRMS m/z Calcd for C24H29N4O [M + H]+, 365.2336; found 365.2330.
13-(6-fluoro-1H-indol-1-yl)-matrine (3k). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 6-fluoroindole (48.7 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3k as a yellow solid, yield: 69.1%; 1H-NMR (600 MHz, MeOD-d4) δ 7.50 (dd, J = 8.8, 5.4 Hz, 1H, 4’-CH), 7.22 (dd, J = 10.3, 2.1 Hz, 1H, 5’-CH), 7.17 (d, J = 3.4 Hz, 1H, 2’-CH), 6.84 (td, J = 8.7, 2.2 Hz, 1H, 7’-CH), 6.50 (t, J = 3.1 Hz, 1H, 3’-CH), 4.88–4.84 (m, 1H, 13-CH), 4.33 (dd, J = 14.1, 3.3 Hz, 1H, 17α-CH2), 3.85 (dt, J = 11.3, 5.8 Hz, 1H, 11-CH), 3.12 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.94 (dd, J = 18.3, 5.4 Hz, 1H, 14α-CH2), 2.86–2.73 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.51–2.44 (m, 1H, 12α-CH2), 2.20 (brs, 1H, 6-CH), 2.12–1.91 (m, 3H, 12β-CH2, 2β-CH2, 10β-CH2), 1.88–1.30 (m, 10H, 5-CH, 7-CH, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.26 (15-C), 161.24 (d, J = 236.2 Hz, 6’-C), 137.16 (d, J = 12.1 Hz, 8’-C), 126.59 (2’-CH), 125.53 (d, J = 3.7 Hz, 9’-C), 122.76 (d, J = 10.1 Hz, 4’-CH), 109.14 (d, J = 24.7 Hz, 7’-CH), 103.48 (3’-CH), 96.78 (d, J = 27.0 Hz, 5’-CH), 65.13 (6-CH), 58.19 (10-CH2), 58.17 (10-CH2), 52.43 (11-CH), 47.81 (13-CH), 43.47 (17-CH2), 42.72 (7-CH), 38.63 (14-CH2), 37.07 (5-CH), 31.28 (12-CH2), 28.73 (4-CH2), 27.44 (8-CH2), 22.10 (3-CH2), 21.43 (9-CH2); ESI-HRMS m/z Calcd for C23H29N3FO [M + H]+, 382.2289; found 382.2276.
13-(6-chloro-1H-indol-1-yl)-matrine (3l). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 6-chloroindole (54.6 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3l as a white solid, yield: 64.2%; 1H-NMR (600 MHz, MeOD-d4) δ 7.54–7.49 (m, 2H, 4’-CH, 5’-CH), 7.21 (d, J = 3.3 Hz, 1H, 2’-CH), 7.03 (d, J = 9.1 Hz, 1H, 7’-CH), 6.51 (d, J = 6.5 Hz, 1H, 3’-CH), 4.94–4.91 (m, 1H, 13-CH), 4.34 (dd, J = 12.8, 4.4 Hz, 1H, 17α-CH2), 3.86 (dt, J = 11.3, 5.6 Hz, 1H, 11-CH), 3.12 (t, J = 12.7 Hz, 1H, 17β-CH2), 2.94 (dd, J = 17.3, 5.4 Hz, 1H, 14α-CH2), 2.87–2.76 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.49–2.44 (m, 1H, 12α-CH2), 2.24 (brs, 1H, 6-CH), 2.15–2.12 (m, 1H, 12β-CH2), 2.01–1.96 (m, 2H2β-CH2, 10β-CH2), 1.90–1.83 (m, 2H, 5-CH, 7-CH), 1.79–1.33 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 169.24 (15-C), 137.58 (8’-C), 128.72 (6’-C), 128.68 (9’-C), 125.87 (2’-CH), 122.94 (4’-CH), 121.24 (5’-CH), 110.50 (7’-CH), 103.48 (3’-CH), 65.13 (6-CH), 58.16 (2-CH2, 10-CH2), 52.45 (11-CH), 47.72 (13-CH), 43.47 (17-CH2), 42.68 (7-CH), 38.65 (14-CH2), 37.04 (5-CH), 31.32 (12-CH2), 28.68 (4-CH2), 27.42 (8-CH2), 22.06 (3-CH2), 21.42 (9-CH2); ESI-HRMS m/z Calcd for C23H29ClN3O [M + H]+, 398.1994; found 398.1993.
13-(6-nitro-1H-indol-1-yl)-matrine (3m). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 6-nitroindole (58.4 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3m as a yellow solid, yield: 61.3%; 1H-NMR (600 MHz, MeOD-d4) δ 8.52 (s, 1H, 7’-CH), 7.93 (d, J = 8.8 Hz, 1H, 4’-CH), 7.67 (dd, J = 8.9, 2.4 Hz, 1H, 5’-CH), 7.60 (t, J = 3.1 Hz, 1H, 2’-CH), 6.78 (t, J = 3.0 Hz, 1H, 3’-CH), 5.13–5.10 (m, 1H, 13-CH), 4.33 (dd, J = 9.4, 6.4 Hz, 1H, 17α-CH2), 3.94–3.90 (m, 1H, 11-CH), 3.12 (t, J = 12.5 Hz, 1H, 17β-CH2), 2.99 (dd, J = 17.4, 5.3 Hz, 1H, 14α-CH2), 2.86–2.73 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.51–2.46 (m, 1H, 12α-CH2), 2.25–2.23 (m, 2H, 6-CH, 12β-CH2), 1.98–1.90 (m, 4H, 2β-CH2, 10β-CH2, 5-CH, 7-CH), 1.77–1.31 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 168.77 (15-C), 144.27 (6’-C), 135.68 (8’-C), 134.79 (9’-C), 131.37 (2’-CH), 121.96 (4’-CH), 115.92 (5’-CH), 107.49 (7’-CH), 104.31 (3’-CH), 65.17 (6-CH), 58.15 (2-CH2, 10-CH2), 52.59 (11-CH), 48.15 (13-CH), 43.57 (17-CH2), 42.39 (7-CH), 38.85 (14-CH2), 37.09 (5-CH), 31.28 (12-CH2), 28.65 (4-CH2), 27.44 (8-CH2), 22.05 (3-CH2), 21.41 (9-CH2); ESI-HRMS m/z Calcd for C23H29N4O [M + H]+, 409.2234; found 409.2225.
13-(1H-pyrrolo[2,3-c]pyridin-1-yl)-matrine (3n). Sophocarpine (73.9 mg, 0.3 mmol) was treated with 6-azaindole (42.5 mg, 0.36 mmol) according to the general procedure, then purified by silica gel column chromatography with dichloromethane/methanol as the eluents to give the desired product 3n as a yellow solid, yield: 33.4%; 1H-NMR (600 MHz, MeOD-d4) δ 8.29 (d, J = 5.9 Hz, 1H, 7’-CH), 7.98 (d, J = 8.9 Hz, 1H, 4’-CH), 7.56 (d, J = 3.4 Hz, 1H, 2’-CH), 7.19 (t, J = 5.4 Hz, 1H, 5’-CH), 6.64 (d, J = 3.2 Hz, 1H, 3’-CH), 5.00 (ddd, J = 9.2, 8.6, 4.3 Hz, 1H, 11-CH), 4.31 (dd, J = 12.6, 4.5 Hz, 1H, 17α-CH2), 3.89 (dt, J = 11.3, 5.6 Hz, 1H, 11-CH), 3.11 (t, J = 11.8 Hz, 1H, 17β-CH2), 2.94 (dd, J = 17.3, 5.5 Hz, 1H, 14α-CH2), 2.88–2.72 (m, 3H, 14β-CH2, 10α-CH2, 2α-CH2), 2.53–2.49 (m, 1H, 12α-CH2), 2.23–2.17 (m, 2H, 6-CH, 12β-CH2), 2.03–1.94 (m, 2H, 2β-CH2, 10β-CH2), 1.89–1.84 (m, 2H, 5-CH, 7-CH), 1.76–1.30 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2); 13C-NMR (151 MHz, MeOD-d4) δ 168.97 (15-C), 147.24 (5’-CH), 143.47 (7’-CH), 130.56 (8’-C), 129.61 (2’-CH), 119.17 (4’-CH), 117.71 (9’-C), 103.06 (3’-CH), 65.19 (6-CH), 58.18 (2-CH2, 10-CH2), 52.54 (11-CH), 48.15 (13-CH), 43.57 (17-CH2), 42.58 (7-CH), 38.66 (14-CH2), 37.16 (5-CH), 31.21 (12-CH2), 28.69 (4-CH2), 27.47 (8-CH2), 22.08 (3-CH2), 21.42 (9-CH2); ESI-HRMS m/z Calcd for C24H29N4O [M + H]+, 365.2336; found 365.2331.
3.3. BiologyAassay
3.3.1. Cell Culture
Human fetal lung fibroblasts (MRC-5 cells) were purchased from Chinese National Infrastructure of Cell Line Resource and cultured in MEM-NEAA medium containing 10% fetal bovine serum (FBS) in 5% CO2 atmosphere at 37 °C. MRC-5 cells in passage between 21 to 26 were used for experiments.
3.3.2. Sircol Collagen Assay
MRC-5 cells in 1 × 105 cells/mL density were seeded in TC treated 96-well plates and allowed to reach confluence for 48 h. The cells were then starved using MEM-NEAA containing 0.2% BSA for 24 h. After starvation, the medium was changed to MEM-NEAA supplemented with 0.2% BSA, 100 μM L-Proline, 100 μM Ascorbic acid, with or without 10 ng/mL TGF-β1 (Peprotech) in the presence of vehicle or different concentrations of compounds. After 48 h incubation, the MRC-5 cells were washed with phosphate buffered saline (PBS) and fixed in −20 °C precooled methanol for 10 min. The cells were than washed with PBS before incubation in 0.1% PSR staining solution (0.1% Sirus red in saturated water solution of picric acid) for 1 h. The staining solution was then removed and the cells were washed with 0.1% acetic acid for three times. 200 μL of 0.1 mol/L sodium hydroxide solution were added to each well and the plates were placed on a rocking platform for 1 h. The optical density (OD) values at 550 nm were determined with an Infinite 2000 spectrophotometer.
3.3.3. Cytotoxicity Assay
MRC-5 cells were incubated with different concentrations of compounds for 48 h, and then 10 μL of CCK-8 solution were added to each well. The cells were incubated at 37 °C for another 3 h before determining the OD values at 492 nm. DMSO at 0.1% v/v final concentration was used to solubilize the test compounds, except for compound 3b, 3c, and 3e, where 1% v/v DMSO was used to ensure complete solubilization. This concentration was based on our preliminary findings and reference [29] which suggested that exposure of human dermal fibroblast cultures for 48 h to concentrations reaching 1% v/v DMSO had no significant effect on cell viability.
3.3.4. Immunofluorescence Assay
MRC-5 cells were seeded on 0.2% gelatin-coated glass coverslips and treated with or without 10 ng/mL TGF-β1 in the presence of vehicle or 10 μM 3f for 48 h. The cells were washed with PBS and fixed in freshly prepared 4% paraformaldehyde (PFA) at room temperature for 10 min. The cells were then permeabilized with 0.25% Triton X-100 for 10 min before 30 min of blocking by PBS solution supplemented with 1% BSA and 22.5 mg/mL glycine (the permeabilization was omitted for S100A4 and TGFβRI staining). The cells were incubated with primary antibody overnight at 4 °C and labeled with AlexaFluor 488-conjugated or Dylight 488-conjugated secondary antibody. The antibodies used in this study were listed as follows: anti-COL1A1 (1:100 dilution; Boster Biological Technology, Wuhan, China; #BA0326); anti-COL3A1 (1:100 dilution; Boster Biological Technology, Wuhan, China; #BM1625); anti-Fibronectin (1:400 dilution; Boster Biological Technology, Wuhan, China; #BA1771); anti-αsma (1:100 dilution; Boster Biological Technology, Wuhan, China; #BM0002); anti-S100A4 (1:50 dilution; Boster Biological Technology, Wuhan, China; # BM4636); anti-TGFβRI (1:100 dilution; Boster Biological Technology, Wuhan, China; #BA0294); anti-Smad2/3 (1:100 dilution; Boster Biological Technology, Wuhan, China; #BA1395); AlexaFluor 488-conjugated goat anti-rabbit IgG preabsorbed antibody (1:200 dilution; Abcam, Cambridge, UK; #ab15065); Dylight 488-conjugated goat anti-mouse IgG antibody (1:200 dilution; Boster Biological Technology, Wuhan, China; #BA1126). Nuclei were stained with Prolong diamond antifade mountant with DAPI (Invitrogen, Carlsbad, CA, USA; P36966). The photos were taken on a Zeiss observer D1 epifluorescence microscope. The mean fluorescence intensity (MFI) of each photo was calculated by ImageJ. Student’s t-test was used to evaluate difference between groups. Differences were considered as statistically significant at p-values less than 0.05.
3.3.5. Scratch Assay
MRC-5 cells were seeded on 6-well plates and allowed to reach confluence for 48 h. The cell monolayer was gently scratched with a 1 mL pipette tip across the center of the well. After scratching, the cells were washed twice with medium to remove detached cells. The cells were then treated with or without 10 ng/mL TGF-β1 in the presence of vehicle or 10 μM 3f for 24 h. The gap area in wound region was quantitatively evaluated using ImageJ [30] and MRI wound healing tool plugin [31].
4. Conclusions
In summary, a total of 18 matrine derivatives were designed, synthesized, and evaluated for their anti-fibrotic activities. Among the new derivatives, compound 3f exhibited the most potent inhibitory effect against TGF-β1-induced total collagen accumulation with the IC50 value of 3.3 μM, which was 266-fold more potent than matrine or 400-fold more potent than Pirfenidone. Anti-fibrotic mechanism study suggested that 3f could significantly inhibit the fibroblast-to-myofibroblast transition and extracellular matrix production in MRC-5 cells. The TGF-β/Smad signaling was also repressed by 3f, as evidenced by inhibition of cytoplasm-to-nuclear translocation of Smad2/3 and suppression of TGF-β1-induced upregulation of TGFβRI. Additionally, 3f exhibited potent inhibitory effect against TGF-β1-induced fibroblasts migration. Overall, this work provided useful information for further structural modification and development of matrine-based anti-pulmonary fibrosis agents.
Supplementary Materials
The following are available online, 1H, 13C-NMR spectra of the synthesized matrine derivatives and NOE spectrum of compound 3j.
Author Contributions
Investigation, L.L. and L.M.; methodology, D.W., H.J., and M.Y.; resources, Y.G.; supervision, Z.Z.; writing—original draft, L.L.; writing—review and editing, L.M., H.S., and Z.Z.
Funding
This research was funded by National Natural Science Foundation of China (81502929), the Drug Innovation Major Project (2018ZX09711-001-005-008), CAMS Innovation Fund for Medical Sciences (2016-I2M-3-015) and PUMC Youth Fund (2017350025).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2a–d and 3a–n are available from the authors.
References
- 1.Raghu G., Rochwerg B., Zhang Y., Garcia C.A.C., Azuma A., Behr J., Brozek J.L., Collard H.R., Cunningham W., Homma S., et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care. Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 2.Cottin V., Schmidt A., Catella L., Porte F., Fernandez-Montoya C., Le Lay K., Bénard S. Burden of Idiopathic Pulmonary Fibrosis Progression: A 5-Year Longitudinal Follow-Up Study. PLoS ONE. 2017;12:e0166462. doi: 10.1371/journal.pone.0166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathai S., Polito A. The questionable efficacy of pirfenidone in IPF. Am. J. Respir. Crit. Care. Med. 2015;172:1228. doi: 10.1164/ajrccm.172.9.950. [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Yu J.L., Li J.H., Chengz R.G., Ma Y.M., Wang X.J., Liu J.C. Effect of matrine on transforming growth factor β1 and hepatocyte growth factor in rat liver fibrosis model. Asian Pac. J. Trop. Med. 2014;7:390–393. doi: 10.1016/S1995-7645(14)60062-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Cui L., Guan G.C., Wang J.K., Qiu C., Yang T.L., Guo Y., Liu Z.W. Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad pathway in experimental diabetic cardiomyopathy. Mol. Med. Rep. 2018;17:1775–1781. doi: 10.3892/mmr.2017.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z.W., Zhang Y., Tang Z.G., Xu J., Ma M.J., Pan S., Qiu C., Guan G.C., Wang J.K. Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur. J. Pharmacol. 2017;804:21–30. doi: 10.1016/j.ejphar.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 8.Li Q.F. Clinical study on matrine intervention in idiopathic pulmonary fibrosis. J. Shandong Univ. Tradit. Chin. Med. 2011;5:40–41. [Google Scholar]
- 9.Jia L. Clinical observation of matrine combined with prednisone in the treatment of idiopathic pulmonary fibrosis. Med. Inf. 2011;24:1067–1068. [Google Scholar]
- 10.Zhang W.H., Yuan X.M. Effect of oxymatrine on serum matrix metalloproteinase-9 and transforming growth factor-β1 in patients with idiopathic pulmonary fibrosis. Chin. J. Postgrad. Med. 2013;36:6–8. [Google Scholar]
- 11.Xu W.H., Hu H.G., Tian Y., Wang S.Z., Li J., Li J.Z., Deng X., Qian H., Qiu L., Hu Z.L., et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology. 2014;60:648–660. doi: 10.1002/hep.27138. [DOI] [PubMed] [Google Scholar]
- 12.Hu H.G., Wang S.Z., Zhang C.M., Wang L., Ding L., Zhang J.P., Wu Q.Y. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines. Bioorg. Med. Chem. Lett. 2010;20:7537–7539. doi: 10.1016/j.bmcl.2010.09.075. [DOI] [PubMed] [Google Scholar]
- 13.Fu B., Wu M.C., Ding L., Wu Q.Y., Zhao Q.J., Guo Z.W. Synthesis and in vitro antifibrosis activities of 13-acetylmethylaminomatrine derivatives. Pharm. Care. Res. 2017;17:102–104. [Google Scholar]
- 14.Xia Z.E., Xi J.L., Shi L. 3,3′-Diindolylmethane ameliorates renal fibrosis through the inhibition of renal fibroblast activation in vivo and in vitro. Renal Fail. 2018;40:447–454. doi: 10.1080/0886022X.2018.1490322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima H., Sasaki K., Suzuki T., Mukawa C., Obara T., Oba Y., Matsuo A., Kobayashi T., Mishima E., Watanabe S., et al. A novel indole compound MA-35 attenuates renal fibrosis by inhibiting both TNF-α and TGF-β1 pathways. Sci. Rep. 2017;7:1884. doi: 10.1038/s41598-017-01702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesa R.A., Yasothan U., Kirkpatrick P. Ruxolitinib. Nat. Rev. Drug Discov. 2012;11:103. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- 17.Lars K., Clemens R., Matthias O. Tissue remodelling in pulmonary fibrosis. Cell Tissue Res. 2017;367:607–626. doi: 10.1007/s00441-016-2543-2. [DOI] [PubMed] [Google Scholar]
- 18.Yue X.P., Shan B., Lasky J.A. TGF-β: Titan of Lung Fibrogenesis. Curr. Enzym. Inhib. 2010;6:67. doi: 10.2174/157340810791233033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scotton C.J., Chambers R.C. Molecular targets in pulmonary fibrosis: The myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 20.Laurent G.J. Collagen in Normal Lung and During Pulmonary Fibrosis. In: Cumming G., Bonsignore G., editors. Cellular Biology of the Lung. Springer US; Boston, MA, USA: 1982. pp. 311–325. [Google Scholar]
- 21.Blatt L.M., Seiwert S.D., Beigelman L., Radhakrishnan R., Kossen K., Serebryany V. Method of Modulating Stress-Activated Protein Kinase System. 7720813. U.S. Patent. 2010 Jun 1;
- 22.White E.S., Thannickal V.J., Carskadon S.L., Dickie E.G., Livant D.L., Markwart S., Toews G.B., Arenberg D.A. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: A role for phosphatase and tensin homologue deleted on chromosome 10. Am. J. Respir. Crit Care Med. 2003;168:436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitterman P.B., Rennard S.I., Adelberg S., Crystal R.G. Role of fibronectin as a growth factor for fibroblasts. J. Cell Biol. 1983;97:1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao C.T., Cheng H.W., Huang M.T., Li J.Z., Ou M.H., Huang J.R., Khoo K.H., Yu H.W., Chen Y.Q., Wang J.K., et al. Fibronectin in cell adhesion and migration via N-glycosylation. Oncotarget. 2017;8:70653–70668. doi: 10.18632/oncotarget.19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.-L., Gabbiani G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu P.L., Liu J.M., Derynck R. Post-translational regulation of TGF-β receptor and Smad signaling. FEBS Lett. 2012;586:1871–1884. doi: 10.1016/j.febslet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R., Chung A.C.K., Dong Y., Yang W., Zhong X., Lan H.Y. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int. 2013;84:1129–1144. doi: 10.1038/ki.2013.272. [DOI] [PubMed] [Google Scholar]
- 28.Selman M., Pardo A. Idiopathic pulmonary fibrosis: An epithelial/fibroblastic cross-talk disorder. Respir. Res. 2002;3:3. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskot M., Jakóbkiewicz-Banecka J., Kloska A., Piotrowska E., Narajczyk M., Gabig-Cimińska M. The Role of Dimethyl Sulfoxide (DMSO) in Gene Expression Modulation and Glycosaminoglycan Metabolism in Lysosomal Storage Disorders on an Example of Mucopolysaccharidosis. Int. J. Mol. Sci. 2019;20:304. doi: 10.3390/ijms20020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rueden C.T., Schindelin J., Hiner M.C., DeZonia B.E., Walter A.E., Arena E.T., Eliceiri K.W. ImageJ2: ImageJ for the next generation of scientific image data. J. BMC Bioinform. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecker V. ImageJ Macro Tool Sets for Biological Image Analysis; Proceedings of the ImageJ User and Developer Conference; Luxembourg. 24–26 October 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.