Abstract
Ca2+ is a universal second messenger that plays a pivotal role in diverse signaling mechanisms in almost all life forms. Since the evolution of life from an aquatic to a terrestrial environment, Ca2+ signaling systems have expanded and diversified enormously. Although there are several Ca2+ sensing molecules found in a cell, EF-hand containing proteins play a principal role in calcium signaling event in plants. The major EF-hand containing proteins are calmodulins (CaMs), calmodulin like proteins (CMLs), calcineurin B-like (CBL) and calcium dependent protein kinases (CDPKs/CPKs). CaMs and CPKs contain calcium binding conserved D-x-D motifs in their EF-hands (one motif in each EF-hand) whereas CMLs contain a D-x3-D motif in the first and second EF-hands that bind the calcium ion. Calcium signaling proteins form a complex interactome network with their target proteins. The CMLs are the most primitive calcium binding proteins. During the course of evolution, CMLs are evolved into CaMs and subsequently the CaMs appear to have merged with protein kinase molecules to give rise to calcium dependent protein kinases with distinct and multiple new functions. Ca2+ signaling molecules have evolved in a lineage specific manner with several of the calcium signaling genes being lost in the monocot lineage.
Keywords: calmodulin, calmodulin-likes, calcium dependent protein kinases, calcineurin B-like, EF-hands
1. Introduction
Calcium is one of the most important plant nutrients that plays diverse roles in plant growth and development, as well as stress responses. It is a divalent cation that is obtained from the soil through the root system of the plants. Calcium is an essential macronutrient for plants [1]. The Ca2+ uptake from the soil occurs through the apoplast or symplast and is deliver to the above ground shoot through the xylem [2]. The Ca2+ is an essential nutrient for the normal development of the plant root and shoot tips and more specifically in cell division. It is also involved in the formation of microtubules, that play a critical role in the separation of chromosomes during the anaphase of the cell division [3,4,5,6]. Calcium pectate is found in the interface between the cell walls and provides structural stability to the cell and cell membrane [7]. Ca2+ accumulates extracellularly as calcium pectate and bind the neighboring cells together. Ca2+ is a versatile second messenger involved in signal transduction and regulates diverse physiological processes, such gene expression, ion balance, as well as carbohydrate, lipid and protein metabolism.
Several environmental stimuli and stress factors can modulate the free cytosolic Ca2+ concentration (100 nM–1000 nM) that has impact on the growth and development of the plants [8,9]. Different plant species vary in their Ca2+ requirement where monocot plants requiring less Ca2+ than the dicots for their growth and development [10]. In response to the external stimuli, the concentration of extracellular Ca2+ increases, leading to an increase in cytosolic Ca2+ whose distribution is balanced by the Ca2+ chelating proteins and sub-cellular organelles like vacuoles, mitochondria, endoplasmic reticulum (ER), as well as the cell wall. Cellular organelles enclosed with a double membrane also capable of generating a Ca2+ signal on their own [11]. The cytosolic concentration of free Ca2+ in a resting cell is approximately 10−7.5 M and can rise approximately ten-fold in response to an external stimulus. However, cytosolic concentration above 10-7 M is cytotoxic and deleterious to the cell function [12,13,14]. The Ca2+ level within the ER is maintained at a concentration that is at least 10–15 times higher than the level of cytosolic Ca2+ [15]. Plastids and mitochondria contain micromolar concentrations of Ca2+, which is comparatively less than the levels found in the ER.
However, studies have reported that the Ca2+ signaling event of sub-cellular organelles are independent of cytosolic Ca2+ levels [16,17]. The sieve tubes (cells lack nucleus at maturity) in vascular tissues contain 20–100-fold higher level of Ca2+ relative to other plant cells [18]. Environmental stimuli and hormonal signals can rapidly and strongly modulate cytosolic Ca2+ concentration in a transient manner. Thus, appropriate regulation of cytosolic Ca2+ levels is crucial for cell survival. Exorbitant cytosolic Ca2+ levels can be cytotoxic and lead to the cell death. The presence of chelating and buffering calcium-binding proteins in the cytosol used to moderate and balance free calcium level and facilitate the localization and spatial distribution of Ca2+ ions. Although external stimuli modulate cellular Ca2+ level, Ca2+ signals are also defined by a variety of kinetic parameters, such as origin, localization, time, duration, frequency and amplitude of the stimulus. The kinetics of Ca2+ signals are largely dependent on the strength of the external stimulus. Ca2+ signals are also cell-, tissue- and organ-specific. Ca2+ signatures regulate specific responses that allow plants to adapt to the prevailing conditions. Notably, elevations in Ca2+ concentration in cellular compartment may regulate different responses depending upon the nature of the stimulus.
Plants encode different types of calcium-binding proteins that are used to chelate and buffer the cytosolic Ca2+ to avoid the cytotoxicity. These proteins are classified as EF-hand-containing sensor protein/protein kinases, non-EF hand-containing Ca2+ binding proteins and transporter/pump proteins. The calcium transporter/pumps actively transport Ca2+ outside of the cytosol to maintain appropriate cytosolic Ca2+ levels. Ca2+-ATPase, P-type ATPase IIA family, P-type ATPase IIB family and Ca2+/H+ antiporters are the major Ca2+ transporter/pump proteins. The EF-hand-containing proteins actively bind to Ca2+ and chelate the cytosolic calcium to regulate calcium homeostasis. The EF-hand-containing calcium-binding proteins are calmodulins (CaMs) [19], calmodulin-like (CMLs) [19], calcineurin B-like (CBL) [20], calcium-dependent protein kinases (CDPK/CPKs) [21], CPK-related protein kinases (CRKs) and calcium and calmodulin dependent protein kinases (CCaMKs). There are also several proteins present in the cell that do not possess an EF-hand but still bind Ca2+. These proteins are phosphoenolpyruvate carboxylase kinase-related kinases (PEPRKs), annexins, calnexin, calreticulin, forisomes, pistil-expressed Ca2+-binding proteins and phospholipase D. Although functional studies of these proteins have been conducted, many detail and genomic aspects are still lacking. More specifically, the major signature sequences responsible for the binding Ca2+ has not been elucidated in Ca2+ transporter/pumps and non-EF-hand containing proteins. In addition, it is also important to understand the interacting network of calcium signaling proteins. This manuscript has reviewed the complex interactome network and genomic aspects of calcium binding signature sequences of EF-hand containing proteins, CaMs, CMLs, CBLs and CPKs. In the review, detailed genomic, evolutionary and complex interactome network of important EF-hand containing calcium binding proteins are presented and discussed.
2. Calmodulins (CaMs)
The genome of various plant species encodes 1 to 13 CaMs with the number of CaMs being independent of genome size (Table 1). For example, the genome size of the algal species, Osterococcus lucimarinus is 13.2 Mb and contains only two CaMs, whereas the genome size of Eucalyptus grandis is 691 Mb and encodes only one CaM (Table 1) [19]. The genomes of the algal species, Coccomyxa subellipsoidea and Chlamydomonas reinhardtii, encode three and six CaMs, respectively. The genomes of wild mustard, Brassica rapa and the common monkey flower, Mimulus guttatus contain 13 CaMs. The average number of CaM proteins encoded per plant species is 6.6 with most plant species encoding less than 10 CaMs in their genome [19]. The largest and smallest CaM genes reported to date in the plant kingdom are PvCaM1-9 (Panicum virgatum CaM, 1314 nucleotides CDS) and AcCaM5-3 (Aquilegia coerulea CaM, 399 nucleotides CDS), respectively. The majority of CaMs contain introns with only 5.16% of the CaMs are intronless [19]. A study of the CaM gene family in 41 plant species reported that 41.69%, 12.91%, 31.73%, 2.21%, 1.84% and 2.85% of the CaM genes contain one, two, three, four, five and six introns, respectively [19]. No CaM genes have been reported to contain seven or more introns.
Table 1.
Distribution of calcium binding protein CaMs (calmodulins), CMLs (calmodulin-like), CPKs (calcium dependent protein kinases) and CBLs (calcineurin B-like) in plants.
| Sl. No. | Name of the Species | Taxonomy | Genome Size | Total No. of Protein Coding Genes | No. of CaMs | No. of CMLs | No. of CPKs | No. of CBLs |
|---|---|---|---|---|---|---|---|---|
| 1 | Aquilegia coerulea | Dicot | 306.5 | 30023 | 5 | 21 | 16 | 5 |
| 2 | Arabidopsis thaliana | Dicot | 135 | 27416 | 9 | 47 | 34 | 10 |
| 3 | Brachypodium distachyon | Monocot | 272 | 34310 | 5 | 23 | 27 | 9 |
| 4 | Brassica rapa | Dicot | 283.8 | 40492 | 13 | 36 | 49 | 14 |
| 5 | Capsella rubella | Dicot | 134.8 | 26521 | 10 | 29 | 32 | 9 |
| 6 | Carica papaya | Dicot | 135 | 27332 | 5 | 15 | 15 | 4 |
| 7 | Chlamydomonas reinhardtii | Algae | 111.1 | 17741 | 6 | 3 | 14 | 2 |
| 8 | Citrus clementina | Dicot | 301.4 | 24533 | 8 | 19 | 26 | 7 |
| 9 | Citrus sinensis | Dicot | 319 | 25376 | 6 | 20 | 24 | 8 |
| 10 | Coccomyxa subellipsoidea | Algae | 49 | 9629 | 3 | 2 | 2 | 0 |
| 11 | Cucumis sativus | Dicot | 203 | 21491 | 6 | 21 | 18 | 7 |
| 12 | Eucalyptus grandis | Dicot | 691 | 36349 | 1 | 25 | 22 | 12 |
| 13 | Fragaria vesca | Dicot | 240 | 32831 | 5 | 19 | 14 | 6 |
| 14 | Glycine max | Dicot | 978 | 56044 | 6 | 27 | 41 | 9 |
| 15 | Gossypium raimondii | Dicot | 761.4 | 55294 | 6 | 30 | 40 | 13 |
| 16 | Linum usitatissimum | Dicot | 318.3 | 43471 | 11 | 21 | 47 | 12 |
| 17 | Malus domestica | Dicot | 881.3 | 63514 | 9 | 32 | 28 | 11 |
| 18 | Manihot esculenta | Dicot | 532.5 | 33033 | 9 | 22 | 26 | 9 |
| 19 | Medicago truncatula | Dicot | 360 | 50894 | 4 | 24 | 11 | 11 |
| 20 | Micromonas pusilla | Algae | 22 | 10660 | 5 | 8 | 2 | 3 |
| 21 | Mimulus guttatus | Dicot | 321.7 | 28140 | 13 | 19 | 25 | 9 |
| 22 | Oryza sativa | Monocot | 372 | 42189 | 5 | 33 | 30 | 11 |
| 23 | Ostreococcus lucimarinus | Algae | 13.2 | 7796 | 2 | 2 | 3 | 0 |
| 24 | Panicum virgatum | Monocot | 1358 | 102065 | 9 | 20 | 53 | 10 |
| 25 | Phaseolus vulgaris | Dicot | 537.2 | 27433 | 9 | 26 | 25 | 10 |
| 26 | Physcomitrella patens | Bryophyte | 480 | 32926 | 7 | 17 | 25 | 4 |
| 27 | Picea abies | Pinophyta | 1960 | 28354 | 9 | 15 | 11 | 13 |
| 28 | Populus trichocarpa | Dicot | 422.9 | 42950 | 8 | 26 | 28 | 11 |
| 29 | Prunus persica | Dicot | 225.7 | 26873 | 4 | 21 | 17 | 7 |
| 30 | Ricinus communis | Dicot | 400 | 31221 | 4 | 8 | 15 | 8 |
| 31 | Selaginella moellendorffii | Pteridophyte | 212.5 | 22273 | 6 | 11 | 9/11 | 4 |
| 32 | Setaria italica | Monocot | 405.7 | 34584 | 5 | 17 | 27 | 7 |
| 33 | Solanum lycopersicum | Dicot | 900 | 34727 | 9 | 27 | 28 | 11 |
| 34 | Solanum tuberosum | Dicot | 723 | 39028 | 5 | 27 | 21 | 12 |
| 35 | Sorghum bicolor | Monocot | 693.9 | 35490 | 8 | 22 | 28 | 8 |
| 36 | Thellungiella halophila | Dicot | 238.5 | 26351 | 10 | 27 | 31 | 9 |
| 37 | Theobroma cacao | Dicot | 346 | 29452 | 2 | 14 | 17 | 7 |
| 38 | Vitis vinifera | Dicot | 487 | 26346 | 5 | 13 | 17 | 9 |
| 39 | Volvox carteri | Algae | 131.2 | 14247 | 4 | 4 | 6 | 0 |
| 40 | Zea mays | Monocot | 2400 | 63540 | 8 | 21 | 47 | 9 |
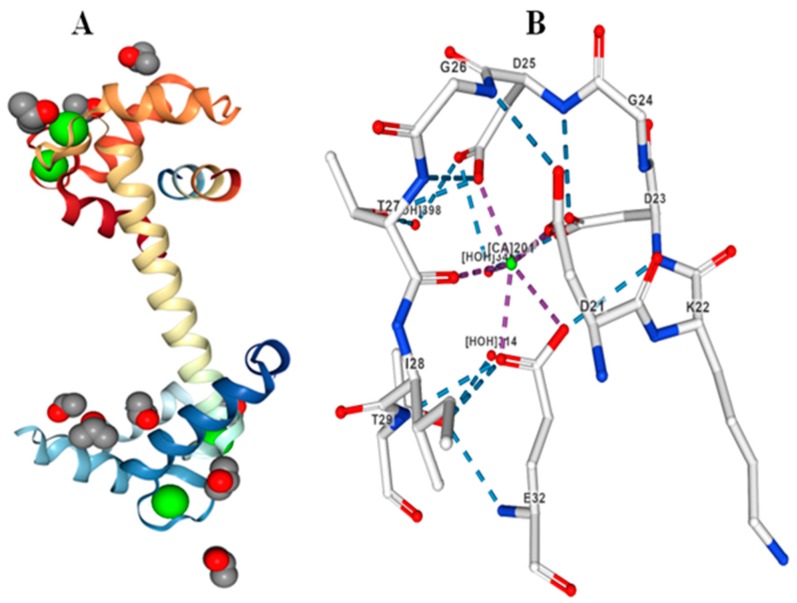
CaMs are small globular protein containing N- and C-terminal EF-hand pairs. The size of the CaM proteins are ranges from 124 (CsCaM4) to 438 amino acids (PvCaM1–9). The isoelectric points of CaM proteins are range from 3.95 (MgCaM9-2) to 6.279 (CsubCaM5-2) (Figure S1). Upon binding of Ca2+ to the EF-hands, the globular structure of the EF-hand is modified into an open conformation that allow the protein to interact with other proteins [22,23]. These interactions lead to the activation or repression of target proteins, thus translating Ca2+ signals into a biochemical response [24,25]. The EF-hands of CaMs contain 12 amino acids in a canonical helix-loop-helix structure. Therefore, each EF-hand contains 36 amino acids. Ca2+ bind to the EF-hands in a pentagonal bipyramidal geometry with seven coordination sites [21,26]. In the majority of cases, Ca2+ is coordinated through the side chain oxygen at X, Y and Z, by a water molecule at –X, by a backbone carbonyl at -Y and by a carboxylate molecule at –Z vertices of the EF-hands, making it a bidentate interaction with the metal ion [26,27]. In position X7, Ca2+ bind to the amino acids via a hydrogen-bonded water molecule (Figure 1). Positions X and Y are always occupied by either Asp (D) or Asn (N); Z is occupied by D, N or serine (S); -Y contains any amino acid; whereas –Z contribute two coordinate sites that contain a Glu (E) [26]. However, frequent substitution has been observed at position –Z where Glu can be substituted by Asp (D). The CaM proteins contain a conserved Ca2+ binding D-x-D signature signal sequence in the EF-hand domain (Table 2) [19]. The conserved Asp amino acid of D-x-D motifs are present coherently and each EF-hand contain one D-x-D motif [19]. Along with the side chain oxygen and back-bone carbonyl atom, the conserved Asp amino acids of D-x-D motifs are responsible for binding Ca2+ in the EF-hand (Figure 1).
Figure 1.
Molecular structure of CaM protein. (A) Three-dimensional structure of CaM protein with Ca2+ ligands bind to the EF-hands. (B) CaM protein binding to the Ca2+ ion in the D-x-D motif. Three water molecules (vertices -X) are present adjacent to the Ca2+ ion with strong hydrogen bonding. The molecular structure of CaM protein was elucidated using calmodulin protein 1UP5 as reported by Rupp et al., (1996) [28].
Table 2.
Conserved motifs of calcium binding protein CaMs, CMLs, CBLs and CPKs. In CaMs and CPKs D-x-D motifs are conserved at 14th and 16th position in each EF-hand.
| EF-Hands | CaMs | CMLs | CBLs | CPKs |
|---|---|---|---|---|
| 1st | D-x-D, E-x2-E | D-x3-D, F-x2-F | V-F-H-P-N | D-x-D, D-E-E-L, E-E-I, E-M-F |
| 2nd | D-x-D, D-F-x-E-F | D-x3-D | D & E | D-x-D, D-E-E-L, E-x-E |
| 3rd | D-x-D | D & E | E-E-x-D, D-D-x2-E | D-x-D, D-E-E-L, x-E-D |
| 4th | D-x-D | D-x-D-x-D, F-x-E-F | D-x-D, D-E-E-L, D/E |
The D-x-D motifs are conserved at the 14th and 16th position of each EF-hand whereas the 15th position is occupied by any amino acid [19]. In addition to the D-x-D motifs, the first EF-hand contains conserved an E-x2-E motif at the 5th and 8th position and an E amino acid at the 25th position [19]. The E amino acid in the 2nd EF-hand is conserved at 5th and 12th position, at the 22nd, 23rd, 25th and 26th position of the D-F-x-E-F motif and also has a conserved D amino acid at the 36th position [19]. The third EF-hand contains a conserved D amino acid at the 1st position and an E amino acid at 8th, 25th and 36th position [19]. The 4th EF-hand contains conserved E amino acid at the 4th, 5th, 12th and 25th position [19]. Conservation of the E amino acid is observed at the 5th position of the 1st, 2nd and 4th EF-hand, while conservation of E-amino acids is observed at the 25th position in all of the four EF-hands [19]. The CaMs bind to their target proteins in CaM binding domains (a stretch of 16–35 amino acids in the target protein) present in the target protein [29,30]. In few instances, CaM bind to its target protein via an IQ motif, in a Ca2+ dependent manner. The IQ motif contains a conserved consensus sequence of I-Q-x3-K/R-G-x3-R amino acids where the I amino acid can sometimes be substituted by a F/L/V amino acid [22,30]. The sequence alignment analysis indicate that CaMs are highly conserved throughout their protein sequence [19]. The presence of multiple genes encoding identical or nearly identical proteins represent an interesting aspect of evolution. Duplicated genes gradually acquire mutations that often lead to a divergence in the coded sequence and closely-related paralogs lead to the functional divergence. Duplicated genes that do not undergo any mutations, remain under selective pressure to maintain the original protein sequence and function. Interestingly, CaMs are believed to be under strong selection pressure to maintain the original protein sequence intact [26].
Post-translational events play a crucial role in protein signaling, trafficking, sub-cellular localization, metabolism and regulation. Palmitoylation and myristoylation are two post-translational processes that occur in calcium signaling proteins where they direct the sub-cellular localization of the protein. CaM proteins do not contain any signaling sequences for palmitoylation or myristoylation event [19]. Therefore, CaM proteins are always found in the cytosol and function in binding Ca2+. The major reason for the high level of sequence conservation in CaMs may be because CaMs are required at levels that exceed single gene output. In this case, concurrent production from multiple genes would be an imperative. Alternatively, different proteins may have evolved specific regulatory responses or expression patterns. Such divergence and specialization would favor the selection and retention of paralogs.
3. Calmodulin-Like (CMLs)
A genome-wide analysis of the CML gene family across 40 plant species indicated the presence of a variable number of CML genes in each genome. The number of CML genes present in a plant genome is independent of the genome size. The number of CML genes in plant genome ranges from 2 to 47 till the date studied so far (Table 1) [19]. Coccomyxa subellipsoidea and Ostreococcus lucimarinus possess only two CML genes, whereas A. thaliana possesses forty-seven CMLs. The monocot plants, Brachypodium distachyon, Oryza sativa, Panicum virgatum, Setaria italica, Sorghum bicolor and Zea mays contain 23, 33, 20, 17, 22 and 21 CMLs, respectively (Table 1) [19]. The dicot plants, Brassica rapa, Eucalyptus grandis, Gossypium raimondii, Solanum tuberosum and Vitis vinifera contain 36, 25, 30, 27 and 13 CMLs, respectively (Table 1) [19]. Analysis of the individual CML genes indicates that the majority of CMLs are intronless, suggesting their ancient origin. Approximately, 71.72% of the CMLs are intronless whereas 9.5% have one, 2.88% have two, 5.29% have three, 3.48% have four and 1.8% have five introns [19]. Only a small number of CMLs contain six, seven, eight or nine introns, where no CMLs contain more than ten introns [19]. Although the majority of CMLs are intronless, the presence of introns in a few CML genes suggest that these introns have evolved recently. The observation that 71.72% CML genes are intronless suggests that CML genes are highly conserved orthologous genes and have evolved from a common ancestor.
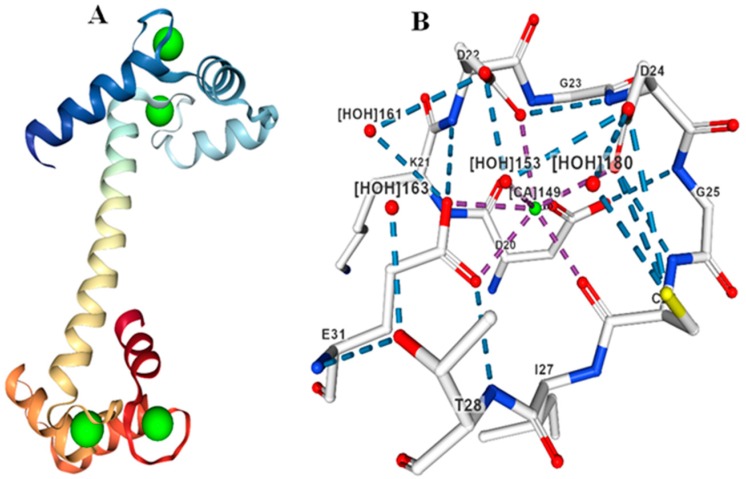
CML proteins are also contain four calcium-binding EF-hands (Figure 2) that share at least 16% sequence similarity with each other [31]. The majority of CMLs, however, share less than 50% sequence similarity with CaMs [32]. Most of the CMLs contain 100–150 more amino acids in their proteins relative to the CaMs. Although there is considerable sequence divergence exist between CaMs and CMLs, still CMLs have the capacity to bind Ca2+. The size of CML proteins range from 115 (AcCML25-3) to 703 amino acids (RcCML23). The isoelectric points of CML proteins are range from 3.263 (PtMCL25-3) to 9.703 (PhCML11) (Figure S2). The CMLs exhibit a shift in electrophoretic mobility in the presence of Ca2+, suggesting that they function as important Ca2+ sensors [33,34]. Except for the presence of EF-hands, CMLs do not possess any other functional domains and therefore do not possess any biochemical or enzymatic activity. Like CaMs, CMLs are also contain conserved Ca2+ binding signature sequences in their EF-hand domain. Sequence alignment showed the presence of a conserved D-x-D-x-D signature motif in the fourth EF-hand (Table 2) [19]. The conserved D-x-D-x-D motif amino acids of the EF-hand bind Ca2+ (Figure 2). The amino acids at the 14th, 16th and 18th position of the fourth EF-hand are conserved [19]. CaM proteins contain four conserved D-x-D motifs, one in each EF-hand, whereas CMLs contain only one D-x-D-x-D motif in the fourth EF-hand. This is the major molecular difference between the CaMs and CMLs. With the exception of the fourth EF-hand, none of the other three EF-hands in CMLs contain a conserved D-x-D motif [19]. Instead, the first EF-hand contains a conserved F-x2-F motif at the 5th and 8th position, a D-x3-D motif at the 9th and 13th position, a G at the 14th and an E at the 20th position [19]. The second EF-hand in CMLs also contain a conserved D-x3-D motif at the 13th and 17th position, a G at the 18th and E and F amino acids are conserved at the 24th and 25th position, respectively [19]. The third EF-hand contains conserved F, D and E amino acids at the 10th, 14th and 25th position, respectively [19]. In addition to the presence of a conserved D-x-D-x-D signature motif, the fourth EF-hand also contains an F-x-E-F conserved signature motif at the 23rd, 25th and 26th position [19]. The CMLs, relative to CaMs, contain an extended conserved signature motif of D amino acids. This suggests that the D-x-D motifs in CaMs have been substituted for D-x-D-x-D motifs in CMLs. The position of the conserved D amino acid in CaMs is different from CMLs.
Figure 2.
Molecular structure of CML protein. (A) Three-dimensional structure of CML protein with Ca2+ ligand binding to the EF-hands. (B) D amino acids binding with the Ca2+ ion in the EF-hand of CML protein. Four water molecules (vertices -X) are present adjacent to the Ca2+ ion with hydrogen bonding providing strong structural stability. The molecular structure of CML protein was created according to CML from protein 1UP5 from databank as reported by Rupp et al., (1996) [28].
Approximately 7.58% of the CML protein contain a conserved G amino acid at the 2nd position in the N-terminal region [19]. Co-translational and irreversible addition of myristic acid occurs in the N-terminal G amino acid leading to the myristoylation of proteins. Therefore, the presence of a conserved G amino acid at the 2nd position of the protein most likely results in the myristoylation of CMLs. The conserved myristoylation motifs present in the N-terminal end of CMLs are M-G-F, M-G-G and M-G-x (Table 3) [19]. This indicates that CMLs contain a signal sequence whereas CaMs do not. Myristoylation and palmitoylation events sometimes occur together and the absence of myristoylation may negate a palmitoylation event. Myristoylation event, however, can occur independently as well.
Table 3.
N-terminal myristoylation and palmitoylation sites of calcium binding proteins CaM, CML, CBL and CPKs. The presence of palmitoylation and myristoylation sites were studied using CSS palm2.0 software.
| Calcium Binding Proteins | Myristoylation Sites | Palmitoylation Sites |
|---|---|---|
| CaM | Not present | Not present |
| CML | M-G-F, M-G-G, M-G-A | Not present |
| CBL | M-G-C | M-G-C, M-L-Q-C |
| CPK | MGN, MGC, QFG, MGL, MGS, MGI, MGQ, MGV, | M-G-N-C, M-G-N-C-C, M-G-C, M-G-N-T-C-V, Q-F-G-T-T-Y-L-C, M-G-N-C-C-R, M-G-L-C, M-G-G-C, M-G-N-N-C, M-G-S-C, M-G-N-S-C, Q-F-G-T-T-F-L-C, M-G-I-C, M-G-N-C-N-A-C, M-G-Q-C, M-G-N-A-C, Q-F-G-T-T-Y-Q-C, M-G-N-V-C, M-G-V-C, M-G-N-Q-C, Q-F-G-I-T-Y-L-C, M-G-N-C-N-T-C, M-E-L-C |
4. Calcineurin B-like (CBL)
Like CaMs and CMLs, plants possess a CBL gene family as well. An analysis of 38 plant species encompassing diverse genera indicates that plant genomes encode 2 to 14 CBL genes per genome [20]. The algal species, Chlamydomonas reinhardtii possesses the lowest number of CBL genes, whereas Brassica rapa contains the highest number of CBL genes among the plant species that have been thus far studied [20]. The CBL genes were not found in the algal species, Coccomyxa subellipsoidea, Ostreococcus lucimarinus and Volvox carteri [20]. The majority of CBL genes contain either six, seven, eight or nine introns, whereas only a few CBL genes are intronless [20]. The intronless CBL genes are present in Micromonas pusilla (MpCBL2) (algae) Physcomitrella patens (PpCBL3-3) (bryophytes) and Selaginella moellendorffii (SmCBL5) (pteridophytes) [20].
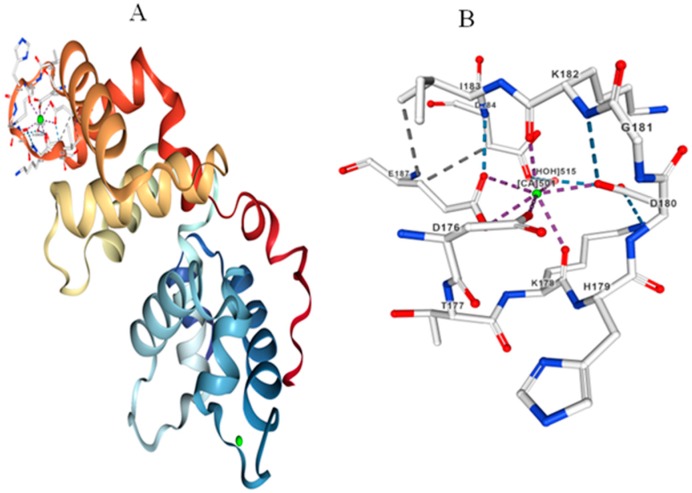
In contrast to the four EF-hands present in CaM and CML proteins, the CBL proteins contain only three EF-hands. The size of CBL proteins range from 119 amino acids (StCBL5) to 1015 amino acids (FvCBL4). The isoelectric point of CBL proteins are ranges from 3.94 (CreinCBL8) to 8.83 (MpCBL1) (Figure S3). Multiple sequence alignments of CBL proteins showed the presence of conserved sequence motifs. The first EF-hand contains conserved E amino acids at the 1st, 23rd and 24th position whereas a D amino acid is conserved at the 6th, 10th and 13th position. The Ca2+ binding D and E amino acids are conserved at the 3rd, 4th, 7th and 14th position [20]. The E amino acids are conserved at the 22nd, 25th and 36th position. The third EF-hand of CBL proteins contain a conserved D-D-x2-E motif at the 7th, 8th and 11th position and a E-E-x-D motif at the 19th, 20th and 22nd position [20]. The third EF-hand also contain a conserved D-x-E-E motif at 30th, 32nd and 33rd position [20]. The D and E amino acids in CBL proteins are responsible for binding Ca2+ ion (Figure 3). The conservation of calcium binding D and E amino acids in CBL proteins is more prominent in the 3rd EF-hand than in the 1st and 2nd EF-hands.
Figure 3.
Molecular structure of CBL protein. (A) Three-dimensional structure of CBL protein with Ca2+ ligands binding to the EF-hands. (B) D amino acids binding to the to the Ca2+ ion in the EF-hand of CBL protein. One water molecule (vertices -X) provides structural stability to the Ca2+ ion in the EF-hand region of the CBL protein. The molecular structure of CPK protein was created according to A. thaliana CBL from protein databank as reported by Akaboshi et al., (2008) [35].
The positions of the conserved amino acids in CBL proteins are different from those found in CaMs and CMLs. The CBL proteins are classified into four groups namely group A, B, C and D [20]. Group D CBLs contain conserved D and E amino acids at the 16th, 17th and 18th (E-E-/D-P) positions at the N-terminal end of the protein (not in the EF-hand region). A conserved D/E-x-E/D motif is present upstream from the first EF-hand of group A CBL proteins [20]. In addition to these conserved regions, the N-terminal region of the CBL proteins contain a less conserved E/D-D-P-E-x4-E-x6-E motif and the C-terminal region contain a conserved P-S-F-V-F-x-S-E-V-D-E motif [20].
The N-terminal region of CBL proteins contain a conserved G amino acid at the 2nd position that is a requisite for protein myristoylation (Table 3) [20]. In a few cases, the N-terminal G amino acid is conserved at the 7th position [20]. Additionally, the presence of a Cys (C) amino acid in the N-terminal region of CBL proteins may lead to protein palmitoylation. The C amino acid is conserved at the 4th position in group A CBLs, while it is conserved at the 3rd position in the N-terminal region in group D CBL proteins [20]. Group B CBLs do not contain an N-terminal C amino acid, suggesting that group B CBLs do not undergo protein myristoylation or palmitoylation [20]. The presence and absence of palmitoylation and myristoylation sites in different CBL proteins show, CBLs are involved in diverse cellular functions.
5. Calcium Dependent Protein Kinases (CPKs)
Calcium-dependent protein kinases are novel calcium sensors that are members of the serine/threonine protein kinase family. The number of CPKs genes in the plant genomes ranged from 2 (Coccomyxa subellipsoidea and Micromonas pusilla) to 53 (Panicum virgatum) [21]. CreinCPK17-5 of Chlamydomonas reinhardtii is the largest reported CPK gene containing an ORF of 5940 nucleotides whereas CpCPK2 of Carica papaya is the smallest CPK gene with an ORF of 693 nucleotides [21]. The presence of larger CPK genes in Chlamydomonas reinhardtii and Physcomitrella patens and the presence of smaller CPK genes in higher eukaryotes suggests, the evolution of eukaryotic CPKs genes are associated with loss of gene size [21]. The majority of CPK genes contain either 6, 7 or 8 introns and at least one CPK gene contains 11 introns from all of the species studied thus far, except for Carica papaya, Ostreococcus lucimarinus and Ricinus communis [21]. The phylogenetic analysis indicates the presence of four groups of plant CPKs that are designated as group A, B, C and D [21]. Furthermore, it appears that CPK genes originated from a common ancestor and that they have evolved very recently via gene duplication in which the genes possess similar or overlapping functions [21]. The presence of only four groups in the phylogeny of CPK genes indicates they have arisen through duplication.
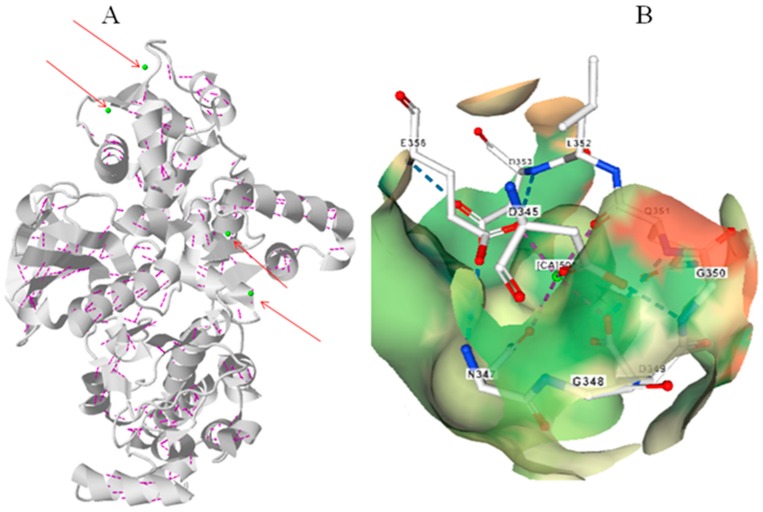
The CPKs are evolutionarily conserved calcium binding proteins whose isoelectric ranges from 4.333 (OlCPK3) to 8.447 (SlCPK16) (Figure S4). Sequence alignments of CPKs indicate the presence of conserved D-x-D and D/E-E-L motifs in the EF-hands in both monocot and dicot plants [21]. Each of the four EF-hands contain at least one D-x-D motif. The species of algae, bryophytes and pteridophytes possess only two D-x-D motifs and only one D-x-x-D motif in the EF-hand [21]. The D-x-D motif in algae, bryophyte and pteridophyte present only in 3rd and 4th EF-hand [21]. The D-x-D motif is conserved at the 14th and 16th position of each EF-hand, whereas a D/E-E-L motif is conserved at the 24th, 25th and 26th position [21]. The D/E amino acids of the D-x-D and D/E-E-L motifs in CPKs are responsible for binding of Ca2+ ion (Figure 4). In addition to the D-x-D motif, the EF-hands in both monocot and dicot plants contain conserved E-E-I/x, D/E-E-L, D-Y-x-E-F, F-D-x-D, E-E-L, D-G-x-I and Y-x-E-F-x2-M-M motifs [21]. A conserved E-D-x4-A-F consensus sequence is found only in the CPKs of monocot plants [21]. In some cases, the conserved D-x-D motif is substituted by either x-D/E-L or Q-E-L (BdCPK30, OsCPK9, PvCPK30-1, PvCPK30-2, SbCPK30 and SiCPK30) or E-E-F-M (BdCPK7-2, BdCPK16-2, OsCPK3, OsCPK4, OsCPK8, PvCPK7-1, PvCPK8-2, PvCPK16-2, SbCPK8-1, SbCPK28, SiCPK7-1, SiCPK16, ZmCPK8-1, ZmCPK16-1/2 and ZmCPK32-1) motif [21]. In addition to the presence of conserved D-x-D and D/E-E-L motifs, the 1st EF-hand in CPKs also contain a conserved E-E-I/x motif at the 1st, 2nd and 3rd position and a E-M-F motif at the 8th, 9th and 10th position [21]. The second EF-hand also contains a conserved E-x-E motif at the 3rd, 4th and 5th position, while the 3rd EF-hand contains a conserved x-E-D motif at the 3rd and 4th position. The 4th EF-hand in CPKs do not contain any conserved signature sequences at the same position [21]. Therefore, the N-terminal EF-hand pair (1st and 2nd EF-hands) possess the characteristic conserved signature sequences, while the C-terminal EF-hand pair (3rd and 4th EF-hands) do not contain any conserved signature sequences [21]. Instead, the 4th EF-hand contains conserved D/E amino acids at the 11th and 12th positions, respectively [21]. The presence of a conserved E-E-I/x motif at the 1st, 2nd and 3rd positions and an E-M-F motif at the 8th, 9th and 10th positions is specific to the 1st EF-hand. An E-x-E motif at the 3rd, 4th and 5th positions is specific to the 2nd EF-hand. An x-E-D motif at the 3rd and 4th positions is specific to the 3rd EF-hand and a conserved D/E amino acid at the 11th and 12th position is specific to the 4th EF-hand [21].
Figure 4.
Molecular structure of CPK protein. (A) Three-dimensional structure of CBL protein with Ca2+ ligands marked in arrow. (B) D-x-D motif binding Ca2+ ion in the EF-hand of CPK protein. No water molecule was found to be associated with the Ca2+ ion in the EF-hand domain of CPK protein. The molecular structure of CPK protein was created according to A. thaliana CPK from protein databank as reported by Chandran et al., (2005) [36].
The first EF-hand in algae, bryophyte and pteridophyte contains a E-E-I/x motif at the 1st, 2nd and 3rd position whereas the first and fourth EF-hands contain a conserved D amino acid at the 14th position [21]. The second EF-hand in algae, bryophyte and pteridophyte possess conserved D and E amino acids at the 22nd and 25th position, respectively [21]. Differences in the number and position of conserved amino acids are the basis for variations in the allosteric properties of the Ca2+ binding and activation threshold of CPKs [37].
The sequence of the N-terminal region of CPKs is highly variable and dynamic while the kinase domain of CPKs contains conserved consensus sequences. The kinase domain in monocot and dicot plants contain the conserved consensus sequences C-x-G-G-E-L-x-D-R-I, H-R-D-L-K-P-E-N-F-L, D-x-V-G-S-x-Y-Y, A-P-E-V-L, D-V/I-W-S, G-V-I-x-Y-I-L-L, G-x-P-P-F-W, P-W-P-x-I-S, A-K-D-L-V and H-P-W. In contrast, the kinase domain in Chlamydomonas reinhardtii, Volvox carteri, Micromonas pusilla, Ostreococcus lucimarinus and Physcomitrella patens contain M-E-L-C-x-G-G-E-L-F, H-R-D-L-K-P-E-N-F-L, D-F-G-L-S-V/x, D-I-W-S-x-G-V and P-F-W conserved amino acid motifs [21]. The conserved consensus sequences D-x-V-G-S-x-Y-Y, G-V-I-x-Y-I-L-L, G-x-P-P-F-W, P-W-P-x-I-S, A-K-D-L-V and H-P-W are absent in algae, bryophyte and pteridophyte species [21]. The auto-inhibitory domain in CPKs is also highly conserved. The conserved consensus sequences present in the auto-inhibitory domain of monocot and dicot plants are K-P-L-D, F-S-A-M-N-K-L and A-L-x2-I-A, whereas in algae, bryophyte and pteridophyte the conserved domain contain A-M-N-K-L consensus sequence [21].
The CPKs contain putative myristoylation and palmitoylation sites [21,38]. Although the N-terminal domain in CPKs is variable, it does contain a conserved G amino acid at the second position and a C amino acid at either the 3rd, 4th, 5th or 6th position [21,39,40,41]. The N-terminal myristoylation supports protein-membrane attachment and protein-protein interactions [42]. Mutations in the N-terminal G amino acid suppress myristoylation and inhibit membrane association [41,43]. Sequence analysis indicates the presence of conserved myristoylation and palmitoylation sites in CPK [21]. The major myristoylation sites present in CPKs are M-G-N, M-G-C, M-G-L, M-G-G, M-G-S, M-G-I, M-G-Q, M-G-V and Q-F-G, while the major palmitoylation sites in CPKs are M-G-N-C, M-G-N-C-C, M-G-C, M-G-N-T-C-V, Q-F-G-T-T-F/Y-L/Q-C, M-G-N-C-C-R, M-G-L-C, M-G-G-C, M-G-N-N-C, M-G-S-C, M-G-N-S-C, M-G-I-C, M-G-N-C-N-A-C, M-G-Q-C, M-G-N-A-C, M-G-N-V-C, M-G-V-C, M-G-N-Q-C, M-G-N-C-N-T-C and M-E-L-C (Table 3) [21]. The N-terminal myristoylation of the G residue at the 2nd position and palmitoylation of the C residue at the 4th and 5th position has been experimentally validated for OsCPK2, a membrane bound CPK [41].
6. Interactome map of CaMs, CMLs, CBLs and CDPKs
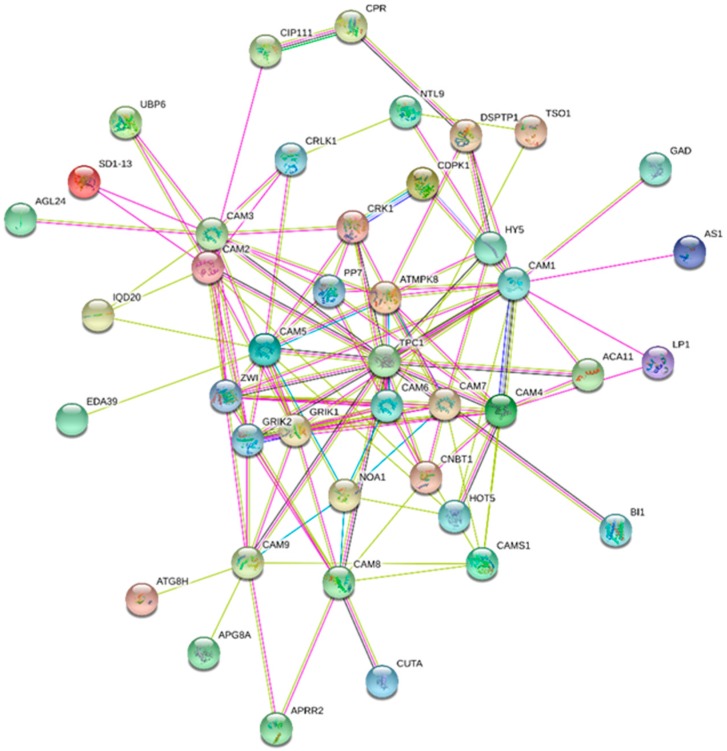
All of the EF-hand containing protein performs the basic and common function of calcium binding to regulate calcium signaling event. However, it is indeed important to know whether all the calcium binding protein possess similar or diverse interacting partners. The cell should maintain the specificity of individual Ca2+ binding proteins to avoid unwanted crosstalk of calcium signaling event. The CaM protein interacts with diverse sets of proteins involved in calmodulin-dependent protein kinase activity. They include CREB (cAMP-response-element-binding protein) phosphorylation through the activation of CaMKK, ion channel transport, opioid signaling, CaM induced events, signal transduction, signaling by GPCR (Figure 5, Table 4 and Table 5) [44,45]. CREB is an important transcription factor involved in the activation of early genes and is phosphorylated by mitogen and stress activated kinase in response to activation of MAPK (mitogen activated protein kinase) [46]. The CaM1, CaM4, CaM5, CaM6 and CaM7 interacts with MPK8 (Figure 5). MAPK8 is involved in phosphorylation of diverse transcription factors. CaM3 interacts with CRLK1 (calcium/calmodulin-regulated receptor-like kinase 1) and AGL24 (MADS box protein) whereas CaM1 interacts with GAD (glutamate decarboxylase 1), LP1 (lipid transfer protein), AK1 (aspartokinase) and HY5 (basic leucine zipper) protein (Figure 5) [44,45]. The GAD protein catalyzes the α-decarboxylation of γ-aminobutyrate (GABA) contain a CaM binding domain at its C-terminal end [47]. Transient elevation of cytosolic Ca2+ due to various stresses lead to activation of GAD via CaM [47]. The LP1 protein act as anti-microbial, anti-fungal, anti-viral and enzymatic inhibitors, suggesting their role in pathogen defense mechanism [48]. Aspartokinase is involved in phosphorylation of amino acid aspartate that involved in the biosynthesis of amino acid methionine, lysine and threonine suggesting the involvement of CaM in biosynthesis of amino acids [49]. The HY5 transcription factor promotes photomorphogenesis in the presence of light. Pfam pathway show the involvement of CaM proteins in calcium and calmodulin binding, protein tyrosine kinase, ubiquitin-like autophagy and lipopolysaccharide pathway (Table 5).
Figure 5.
Interactome network of CaM proteins. The major interacting partners of CaM proteins are MPK8, TPC1, CDPK1, NOA1, UBP, HOT5 and others. The interactome network was constructed using the CaM proteins of model plant A. thaliana. String database was used to construct the interactome network [44,45].
Table 4.
Table showing the interacting partners of CaMs, CMLs, CBLs and CPKs. CBL protein majorly interacts with CIPKs (CBL-interacting protein kinases) whereas majority of CPKs are interacts with CPKs themselves.
| Gene Name | Interacting Proteins |
|---|---|
| CaM | |
| CaM8 | APRR2, TPC1, At2g01210, NOA1, At2g20050, At1g73440, CUTA, GRIK2, GRIK1, CAMS1 |
| CaM1 | ZW1, CDPK1, NTL9, At2g01210, DSPTP1, CRCK, GAD, At5g62570, At5g28300, AS1 |
| CaM2 | UBP6, ZWI, At3g07670, At5g14260, CIP111, At1g24610, At5g53920, IQD20, SD1-13 |
| CaM3 | PP7, ZWI, UBP6, MPK8, TPC1, CRK1, SD1-13, AGL24, At3g07670, At5g14260 |
| CaM5 | MPK8, At2g19750, TPC1, EDA39, CRK1, NOA1, PP7, CRLK1, At2g02970 |
| CaM4 | HOT5, CNBT1, At4g33080, ACA11, TPC1, PP7, MPK8, At2g01820, LP1, CaM1 |
| CaM7 | At5g57110, MPK8, HY5, CNBT1, BI1, TPC1, At2g18750, NOA1, TSO1-like |
| CaM8 | APRR2, TPC1, At2g01210, NOA1, At2g20050, At1g73440, CUTA, GRIK2, GRIK1, CAMS1 |
| CaM9 | APRR2, At1g73440, TPC1, ATG8H, APG8A, At2g27480, TSO1-like |
| CML | |
| CML15: AT1G18530 | ACOS5, TPC1, LAP6, At2g27480, LAP5, GDSL, TKPR1, NHX1, CYP704B1 |
| CML25: AT1G24620 | CCP3, HSP81-3, ARP2, HSP81-4, DEK1, PHS2, LOS1, PGSIP4, HSP90.1 |
| CML14: AT1G62820 | CML43, CML42, TPC1, LRR, ZWI, NOA1, BIP2 |
| CML23: AT1G66400 | TCH2, MSS3, ARP2, DEK1, PHS2, LOS1, HSP81.3, HSP81.4, HSP90.1 |
| CML39: AT1G76640 | At1g54850, IDL3, TPC1, NOA1 |
| CML38: AT1G76650 | WRKY40, SZF1, STZ, BAP1, TPC1, RHL41, |
| CML30: AT2G15680 | ARP2, DEK1, PHS2, LOS1, HSP81, ROC1 |
| CML10: AT2G41090 | At3g19100, CPK30, CDPK6, CPK31, CPK29, CPK16, CPK18, CPK4, CPK28, CPK15 |
| CML5: AT2G43290 | TCH2, CML23, ARP2, DEK1, PHS2, LOS1, At2g41410, At1g07940 |
| CML3: AT3G07490 | HSP70, ARP2, DEK1, PHS2, LOS1, PGSIP4, HSP81 |
| CML36: AT3G10190 | At1g62820, ARP2, DEK1, LOS1, HSP81, HSP90, CML30 |
| CML16: AT3G25600 | At4g27280, At3g10300, TPC1, At4g26470, SYP122, GILP, NHL3 |
| CML20: AT3G50360 | RAD4, RAD23B, RAD23A, RAD23D, RAD23C, DDB1B, DDB1A, GTF2H2, SAC3B |
| CML41: AT3G50770 | At5g43260, TPC1, BGLU28, TI1, MDHAR, BT2, NOA1, ST2A |
| CML9: AT3G51920 | APRR2, TPC1, ATG8H, APG8A, CDPK19, |
| CML4: AT3G59440 | F5M15.5, ARP2, DEK1, PHS2, LOS1, HSP81, HSP90, ROC1 |
| CML42: AT4G20780 | TPC1, PPI3A, CYP86A |
| CML19: AT4G37010 | RAD4, RAD23B, RAD23A, RAD23D, RAD23C, DDB2, SAC3B, At5g16090, CUL4, GTF2H2 |
| CML37: AT5G42380 | WRKY46, RHL41, BAP1, WRKY40, TPC1, GILP |
| CML43: AT5G44460 | TPC1, CYP86A1, WRKY27, NOA1, CIPK26, CIPK3 |
| CBL | |
| CBL1 | CIPK23, AKT1, SOS2, CIPK1, CIPK7, At2g20050, CIPK15, CIPK8, CIPK26 |
| CBL2 | CIPK14, SIP4, At2g20050, CIPK23, At3g51390, SOS2, SIP3, CIPK12, CIPK18, MTN1 |
| CBL3 | CIPK23, At2g20050, SIP3, CIPK9, MTN1, CIPK1, MTN2, At3g51390, SOS2, CIPK14 |
| CBL4 | SOS2, SOS1, SIP3, NHX1, AKT1, At2g20050, At1g61575, CIPK8, CIPK14 |
| CBL5 | At2g20050, FKBP12, AKT1, CIPK1, CIPK23, SOS2, SIP4, CIPK14, TPC1, At3g59440 |
| CBL6 | VSR2, MTN1, FKBP12, CIPK9, CIPK1, TPC1, At4g10170 |
| CBL7 | GLR2.8, SOS2, CIPK3, CIPK26, CIPK9, FIBP12 |
| CBL8 | CIPK23, FKBP12, CIPK14, HAK5, TPC1, CIPK9, AKT1, SOS2, CIPK12 |
| CBL9 | CIPK23, AKT1, CIPK1, SIP3, CIPK8, SOS2, CIPK9, CIPK3, CIPK14 |
| CBL10 | SOS2, AKT1, CIPK23, SIP3, SOS1, FKBP12, CIPK8, TPC1, AVP1 |
| CPK | |
| At5g24430 | CPK1, At2g45300, CDPK1, CPK6, CPK9, At2g41090, CPK19, CPK24, CPK28, CPK32 |
| CDPK1 | CPK1, CPK4, CPK5, CPK6, CPK9, CPK28, CPK29, At5g34430, CPK32, At2g41090 |
| CPK30 | At2g41090, CRK1, CPK6, CPK9, CPK16, CPK18, CPK28, CDPK2, At5g24430, CPK23 |
| CPK9 | At2g41090, ABI2, CRK1, CPK4, At3g49370, At5g24430, CPK23, CPK28, CPK31, CPK32 |
| CPK19 | At2g41090, At5g24430, CRK1, CPK5, CPK9, CPK16, CPK21, CPK28, F5M15.5, |
| CPK7 | CPK1, CPK4, CPK6, CPK9, CPK16, CPK18, CPK28, CPK29, CDPK1, At2g41090 |
| CPK1 | CDPK1, CDPK6, CPK28, CPK6, CPK4, CPK9, At2g41090, CPK32, CPK7, At5g24430 |
| CPK18 | At2g41090, CPK14, CPK19, CPK7, CDPK1, CPK5, CPK13, CPK32, CPK24, CPK30 |
| CDPK6 | At2g41090, ORP2A, CPK1, CPK9, CPK6, CDPK1, OZS1, CPK28, CPK18, CPK16 |
| CPK15 | At2g41090, CPK18, CPK16, CPK28, CPK24, CPK14, At3g49370, At5g24430, CPK30, CPK7 |
| CPK4 | ABF1, CPK28, CPK29, At2g41090, CPK1, CPK32, CPK6, CPK9, RBOHD |
| CPK31 | At2g41090, CPK27, CRK1, At3g49370, CPK16, CPK18, CPK32, CPK25, CPK14, CPK13 |
| CPK32 | CPK28, ABF4, CPK6, CPK4, CDPK1, CPK9, CPK1, At2g41090, CPK9, CPK16 |
| CPK2 | At2g41090, CPK16, CPK19, CPK18, CPK5, At3g49370, At5g24430, CRK1, CPK1, At3g56760 |
| CPK14 | CPK24, CPK16, At2g41090, CPK20, CPK28, CPK18, CPK31, CPK29, CPK15, CPK19 |
| CPK6 | OZS1, CDPK1, CPK9, CPK28, CPK32, At5g24430, CPK29, CPK1, CPK4, CPK5 |
Table 5.
Reactome, functional and Pfam pathway of calcium binding CaM, CML, CBL and CPK proteins. These proteins are involved in diverse cellular events. More specifically CMLs are involved in excision and DNA damage, CaM are involved in CaM induced signaling and CPKs are involved in abscisic acid signaling, oxidative burst and reactive oxygen species homeostasis. No reactome pathway was found for CBL proteins.
| Reactome Pathway | Functional Pathway | Pfam Pathway | GO Component |
|---|---|---|---|
| CML | |||
| DNA Repair | Calcium ion binding | EF-hand domain | GO:0005623→cell |
| DNA Damage Recognition in GG-NER | Binding | EF-hand domain pair | GO:0005622→intracellular |
| Formation of Incision Complex in GG-NER | Metal ion binding | EF-hand domain pair | GO:0044464→cell part |
| Nucleotide Excision Repair | Ion binding | EF hand | GO:0044424→intracellular part |
| Calmodulin induced events | Calmodulin-dependent protein kinase activity | EF hand | GO:0005634→nucleus |
| Ca-dependent events | Calcium-dependent protein serine/threonine kinase activity | XPC-binding domain | GO:0043229→intracellular organelle |
| CaM pathway | Damaged DNA binding | WD domain, G-beta repeat | GO:0043227→membrane-bounded organelle |
| DAG and IP3 signaling | Calmodulin binding | UBA/TS-N domain | GO:0005737→cytoplasm |
| Activation of CaMK IV | Protein binding | WRKY DNA -binding domain | GO:0043231→intracellular membrane-bounded organelle |
| Opioid Signaling | Hsp90 protein binding | TPR repeat | GO:0080008→Cul4-RING E3 ubiquitin ligase complex |
| G-protein mediated events | Organic cyclic compound binding | Kinase-like | GO:0031461→cullin-RING ubiquitin ligase complex |
| PLC beta mediated events | Heterocyclic compound binding | Tetratricopeptide repeat | GO:1990234→transferase complex |
| Intracellular signaling by second messengers | Proteasome binding | Sulfotransferase family | GO:0005829→cytosol |
| G2/M DNA damage checkpoint | Polyubiquitin modification-dependent protein binding | Ubiquitin family | GO:0005886→plasma membrane |
| G alpha (i) signaling events | Preprotein binding | Hsp90 protein | GO:0044444→cytoplasmic part |
| Signaling by GPCR | Ubiquitin binding | CPSF A subunit region | GO:0071944→cell periphery |
| GPCR downstream signaling | Protein serine/threonine kinase activity | 3-Oxoacyl-[acyl-carrier-protein (ACP)] synthase III | GO:0005773→vacuole |
| Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks | Tetraketide alpha-pyrone synthase activity | Mono-functional DNA-alkylating methyl methanesulfonate N-term | GO:0016020→membrane |
| Neutrophil degranulation | Nucleic acid binding | Secreted protein acidic and rich in cysteine Ca binding region | GO:0032991→protein-containing complex |
| Immune System | Protein-containing complex binding | Tetratricopeptide repeat | GO:0034399→nuclear periphery |
| Post-translational protein modification | DNA binding | Protein kinase domain | GO:0098805→whole membrane |
| Josephin domain DUBs | Anion binding | Protein tyrosine kinase | GO:0005777→peroxisome |
| Metabolism of proteins | Purine ribonucleotide triphosphate binding | Chalcone and stilbene synthases, N-terminal domain | GO:0012505→endomembrane system |
| Dual Incision in GG-NER | Purine ribonucleotide binding | Tetratricopeptide repeat | |
| Signal Transduction | Serine-type endopeptidase inhibitor activity | Histidine kinase-, DNA gyrase B- and HSP90-like ATPase | |
| Neddylation | Drug binding | Sulfotransferase domain | |
| Formation of TC-NER Pre-Incision Complex | Small molecule binding | Tetratricopeptide repeat | |
| Dual incision in TC-NER | Nucleotide binding | Chalcone and stilbene synthases, C-terminal domain | |
| Gap-filling DNA repair synthesis and ligation in TC-NER | ATP binding | NAF domain | |
| HSP90 chaperone cycle for steroid hormone receptors (SHR) | Sulfotransferase activity | Histidine kinase-, DNA gyrase B- and HSP90-like ATPase | |
| N-glycan trimming in the ER and Calnexin/Calreticulin cycle | Transferase activity | Ubiquitin-2 like Rad60 SUMO-like | |
| Recognition of DNA damage by PCNA-containing replication complex | Enzyme binding | Lipopolysaccharide kinase (Kdo/WaaP) family | |
| Cellular responses to external stimuli | Sequence-specific DNA binding | C2H2-type zinc finger | |
| Cellular responses to stress | Transcription regulator activity | ||
| Glycogen breakdown (glycogenolysis) | Catalytic activity, acting on a protein | ||
| Protein methylation | Ras GTPase binding | ||
| Cytosolic sulfonation of small molecules | |||
| Asparagine N-linked glycosylation | |||
| CPK | |||
| Calmodulin induced events | Calmodulin-dependent protein kinase activity | EF-hand domain pair | GO:0005634 nucleus |
| Ca-dependent events | Calcium-dependent protein serine/threonine kinase activity | EF-hand domain pair | GO:0005886 Plasma membrane |
| CaM pathway | Calmodulin binding | EF hand | GO:0016020 membrane |
| DAG and IP3 signaling | Calcium ion binding | EF-hand domain | GO:0043231 Intracellular membrane bound organelle |
| Activation of CaMK IV | Protein binding | EF hand | GO:0005737 cytoplasm |
| G2/M DNA damage checkpoint | ATP binding | Protein kinase domain | GO:0071944 cell periphery |
| Opioid Signaling | Metal ion binding | Protein tyrosine kinase | GO:0005622 intracellular |
| G-protein mediated events | Heterocyclic compound binding | Lipopolysaccharide kinase (Kdo/WaaP) family | GO:0005623 cell |
| PLC beta mediated events | Organic cyclic compound binding | Secreted protein acidic and rich in cysteine Ca binding region | GO:0044464 cell part |
| Intracellular signaling by second messengers | Binding | Kinase-like | GO:0005829 cytosol |
| G alpha (i) signaling events | Catalytic activity | bZIP transcription factor | |
| Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks | Protein phosphatase binding | Basic region leucine zipper | |
| Signaling by GPCR | Peroxidase activity | ||
| GPCR downstream signaling | |||
| Signal Transduction | |||
| CaM | |||
| Neurotransmitter receptors and postsynaptic signal transmission | Calmodulin binding | Cytoskeletal-regulatory complex EF hand | GO:0005622 Intracellular |
| Post NMDA receptor activation events | Calcium ion binding | EF hand | GO:0005623 Cell |
| CREB phosphorylation through the activation of CaMKK | Binding | EF-hand domain pair | GO:0044464 Cell Part |
| Activation of NMDA receptors and postsynaptic events | Protein binding | EF-hand domain pair | GO:0044424 Intracellular part |
| Transmission across Chemical Synapses | Ion binding | EF hand | GO:0043229 Intracellular organelle |
| Neuronal System | Metal ion binding | EF-hand domain | GO:0005737 Cytoplasm |
| Ion channel transport | Protein serine/threonine kinase activity | EF-hand domain | GO:0043227 Membrane bound organelle |
| Opioid Signaling | Purine ribonucleotide binding | Lipopolysaccharide kinase (Kdo/WaaP) family | GO:0005874 Microtubule |
| Calmodulin induced events | Adenyl ribonucleotide binding | Autophagy protein Atg8 ubiquitin like | GO:0015630 Microtubule cytoskeleton |
| Ca-dependent events | Small molecule binding | Ubiquitin-like autophagy protein Apg12 | GO:0043231 Intracellular membrane bound organelle |
| CaM pathway | Anion binding | Protein kinase domain | GO:0005634 Nucleus |
| G-protein mediated events | Drug binding | Protein tyrosine kinase | GO:0044446 Intracellular organelle part |
| PLC beta mediated events | Purine ribonucleoside triphosphate binding | Ion transport protein | GO:0000421 Autophagosome membrane |
| DAG and IP3 signaling | Organic cyclic compound binding | Kinase-like | GO:0005886 Plasma membrane |
| Signal Transduction | Heterocyclic compound binding | GO:0071944 Cell periphery | |
| Activation of CaMK IV | ATP binding | GO:0005776 Autophagosome | |
| G2/M DNA damage checkpoint | Catalytic activity, acting on a protein | GO:0043232 Intracellular non-membrane bound organelle | |
| Intracellular signaling by second messengers | Voltage-gated cation channel activity | GO:0016020 Membrane | |
| G alpha (i) signaling events | Calmodulin-dependent protein kinase activity | GO:0098805 Whole membrane | |
| Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks | Calcium-dependent protein serine/threonine kinase activity | GO:0005774 Vacuolar membrane | |
| Signaling by GPCR | Transcription regulatory region DNA binding | GO:0005773 Vauole | |
| GPCR downstream signaling | Calcium ion transmembrane transporter activity | ||
| Metal ion transmembrane transporter activity | |||
| Identical protein binding | |||
| RNA polymerase II regulatory region sequence-specific DNA binding | |||
| CBL | |||
| NA | Calcium ion binding | NAF domain | GO:0005623 Cell |
| Protein serine/threonine kinase activity | Kinase-like | GO:0044464 Cell part | |
| Ion binding | EF hand | GO:0005886 Plasma membrane | |
| Binding | Protein kinase domain | GO:0005622 Intracellular | |
| Catalytic activity, acting on a protein | Protein tyrosine kinase | GO:0000325 Plant-type vacuole | |
| Drug binding | EF-hand domain pair | GO:0071944 Cell periphery | |
| ATP binding | EF-hand domain pair | GO:0005737 Cytoplasm | |
| Metal ion transmembrane transporter activity | Phosphorylase superfamily | GO:0005774 Vacuolar membrane | |
| Potassium ion transmembrane transporter activity | Ion transport protein | GO:0016020 Membrane | |
| Cation channel activity | EF hand | GO:0005773 Vacuole | |
| Adenosylhomocysteine nucleosidase activity | GO:0009705 Plant-type vacuole membrane | ||
| Methylthioadenosine nucleosidase activity | GO:0043231 Intracellular membrane bound organelle | ||
| Metal ion binding | GO:0005634 Nucleus | ||
| Ion gated channel activity | GO:0044444 Cytoplasmic part | ||
| Sodium ion transmembrane transporter activity | |||
| Catalytic activity | |||
| Transmembrane transporter activity | |||
| Ligand-gated ion channel activity | |||
| Voltage-gated cation channel activity | |||
| Calcium channel activity | |||
| Potassium channel activity | |||
| Organic cyclic compound binding | |||
| Heterocyclic compound binding | |||
| Active transmembrane transporter activity | |||
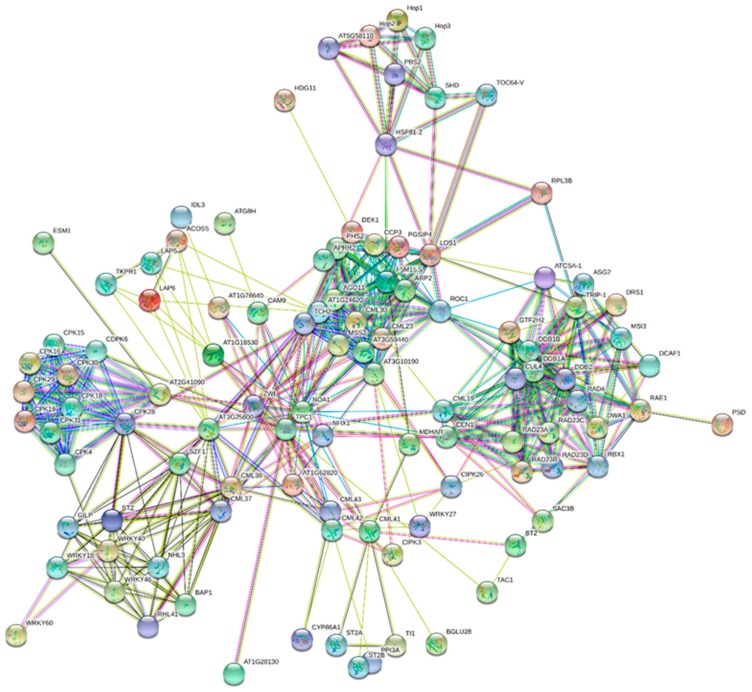
CMLs were are majorly interact with ACOS5 (Acyl-CoA synthetase 5), TPC1 (two pore calcium channel 1), LAP6 (chalcone and stilbene synthase), TKPR1 (tetraketide alpha-pyrone reductase), NHX1 (sodium hydrogen exchanger 1), CCP3 (serine protease inhibitor 3), HSP (heat shock protein), DEK1 (calpain type cysteine protease), PHS2 (alpha glucan phosphorylase 2), LOS1 (ribosomal protein S5/elongation factor G/III/V family 1), WRKY (WRKY transcription factor), BAP1 (BON associated protein), SZF (zinc finger CCCH domain), RAD (UV excision repair protein), CIPK (CBL interacting protein kinase), ARP (DNA lyase) and other (Figure 6, Table 4) [44,45]. The 4-coumarate CoA-ligase-like and LAP6 are involved in phenylpropanoid metabolism and phenylpropanoid metabolism have diverse role in plant biotic and abiotic stress responses [50,51]. The TPC1 found in acidic organelle such as lysosome and vacuoles involved in transport of Ca2+ ion and regulate germination and stomatal movement [52,53]. The NHX1 protein is involved in regulation of cellular pH though the accumulation of Na+ in vacuoles [54]. Heat shock proteins act as molecular chaperons of plant immunity [55]. DEK1 is involved in development of epidermis and maintain adaxial/abaxial axis formation in developing leaves through the regulation of cell proliferation. PHS2 play important role in tolerance to abiotic stress in A. thaliana [56]. The RAD proteins are associated with chromatin in response to UV mediated DNA damage during S the phase [57]. WRKY40 is involved in plant biotic and abiotic stress responses through the induction of abscisic acid. WRKY46 is involved in brassinosteroid metabolism and modulate plant growth and drought tolerance [58,59]. BAP1 gene in A. thaliana act as general inhibitor of programmed cell death. The BON1 negatively regulate defense response gene SNC1 [60]. Overall, the interacting proteins are involved in nucleotide excision repair, neddylation, transport, cellular development and cellular response to biotic and abiotic stresses (Table 5) [44,45]. Pfam pathway also shows their involvement in WRKY DNA binding, EF-hand domain pair, TPR-repeats, Hsp90, ubiquitin pathway, chalcone and stilbene synthesis (Table 5) [44,45].
Figure 6.
Interactome network of CML proteins. The major interacting partners of CML proteins are CLU4, MDHAR, WRKY transcription factor, CPKs, NHX, DDB1, RAD23A, RAD23C, RAD23D and others. The interactome network was constructed using the CML proteins of model plant A. thaliana. String database was used to construct the interactome network [44,45].
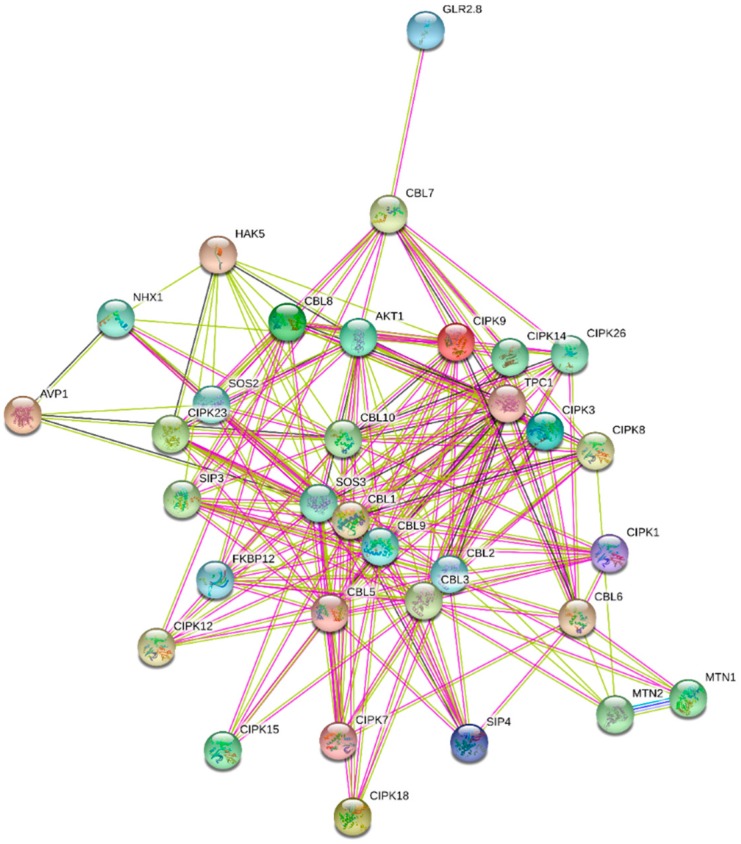
In the majority of cases CBLs are interact with CIPKs (CBL-interacting protein kinases) and regulate diverse functions including abscisic acid signaling and salt tolerance and [61,62]. The CIPKs which are the major interacting partner of CBLs are involved in immune response, response to salt, osmotic and abscisic acid stress, plant growth and development [61,62,63]. Wheat CIPK 25 is negatively regulate salt tolerance in the transgenic wheat [64]. In addition to interaction with CIPKs, CBLs are also interact with AKT1 (potassium channel 1), MTN1 (5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase 1), SOS2 (CBL-interacting serine/threonine protein kinase 2), NHX1 (sodium hydrogen exchanger 1), SIP3 (CBL-interacting serine/threonine protein kinase 6), FKBP12 (peptidyl-prolyl cis trans isomerase), VSR2 (vacuolar sorting receptor 2), GLR2.8 (glutamate receptor 2.8), HAK5 (high affinity K+ transporter 5), TPC1 (two pore calcium channel protein 1), AVP1 (pyrophosphate-energized vacuolar membrane proton pump 1) (Figure 7, Table 4). MTN1 catalyzes irreversible breakage of glycosydic bond in 5-methylthioadenosine and S-adenosylhomocysteine to adenine, 5-methylthioribose and s-ribosylhomocysteine, thus maintaining AdoMet homeostasis and ethylene biosynthesis [65]. A. thaliana SOS2 is essential for intracellular Na+ and K+ homeostasis and regulate important role toward low K+ and high Na+ concentration [66]. FKBP12 protein is an important gene involved in physiologic regulator of cell cycle [67]. FKBPs are molecular chaperon and regulate diverse cellular process including adaptation to photosynthetic adaptations [68,69]. Glutamate receptor in plants is involved in light signal transduction and two pore calcium channel is actively involved in calcium signaling [60,70]. Vacuolar sorting receptors are involved in targeting of soluble cargo proteins to their respective destination [71]. Functional pathway shows the involvement of CBLs in calcium ion binding, potassium ion transmembrane transporter activity, ion gated channel activity and others (Table 5) [44,45].
Figure 7.
Interactome network of CBL proteins. The major interacting partners of CBL proteins are CIPKs. In addition, CBLs also interact with NHX1, MTN1, MTN2, SOS2 and other proteins. The interactome network was constructed using the CBL proteins of model plant A. thaliana. String database was used to construct the interactome network [44,45].
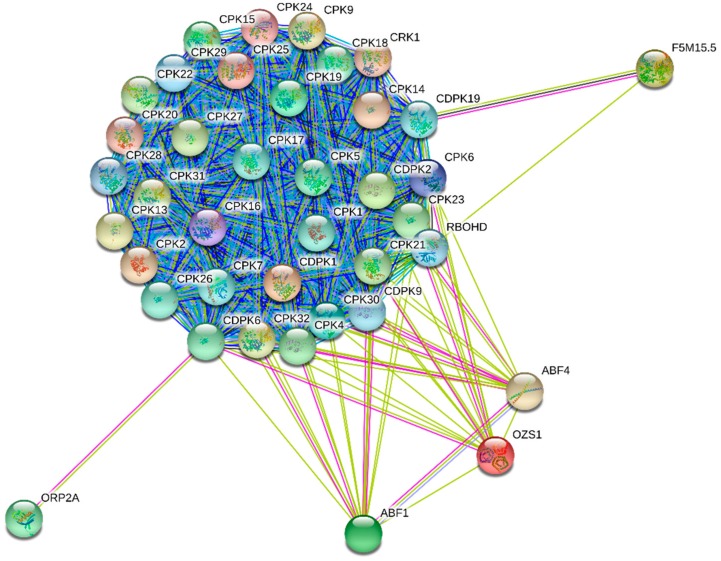
CPKs are involved in diverse cellular function starting from cell signaling to stress tolerance in plants. They act as a hub of plant stress signaling and development [72]. The CPK genes are predominantly expressed in pollen where AtCPK17/AtCPK34, AtCPK11/AtCPK24 play role in growth of pollen tube [72]. AtCPK4, AtCPK5, AtCPK6 and AtCPK11 modulate pathogen associated molecular pattern [73]. AtCPK5 and AtCPK6 are involved in reactive oxygen species mediated cell death [74]. Interactome map generated from string database show the majorities of CPKs undergo cross interactions through the network of events (Figure 8, Table 4). However, in addition to the interaction with other CPKs, CPK4 interacts with ABF1 (abscisic acid responsive element-binding factor 1), RBOHD (respiratory burst oxidase homology D); CPK6 interact with OZS1 (C4-dicarboxylate transporter/malic acid transport protein) and ORP2A (oxysterol-binding protein-related protein 2A), CPK9 interacts with CRK1 (CDPK related kinase 1) and CPK19 interacts with F5M15.5 (catalase) (Figure 8, Table 4). The interactome map of CPK revealed that CPKs are involved in diverse cellular events involved in abscisic acid signaling, oxidative burst (early response to stress) and homeostasis of cellular oxidation through action of catalase [8,75].
Figure 8.
Interactome map of CPK proteins. The interactome map shows CPK proteins are interact with other CPKs proteins of the same protein family. However, CPKs are also interact with other proteins including ORP2A, RBOHD, ABF1, ABF4, OZS1 and F5M15.5. The interactome network was constructed using the CPK proteins of model plant A. thaliana. String database was used to construct the interactome network [44,45].
7. Evolution of Calcium Signaling Events
The evolution of multi-cellular life forms from a single-celled ancestor is one of the most remarkable transitions that occurred during the evolution of life. Calcium ions may have a role as a “promoter” in the evolution of early forms of life [13]. Although, several different ionic molecules are present and available in the cytosol and on extracellular surfaces, Ca2+ dominated as the major cation for use as a universal intracellular signaling molecule [76,77]. This was most likely due to the properties of Ca2+ which possesses flexible coordination and rapid binding kinetics with biological molecules [78,79]. Additionally, when life arose in the ocean environment, sustaining cells in this oceanic environment would have been problematic since it contains high amount of Ca2+ and other minerals. Ca2+ ion enter into cells through different pumps and ion channels present in the cell membrane [80,81,82,83]. High concentrations of Ca2+ are cytotoxic [84,85,86]. Therefore, cells developed “tool kits” consisting of calcium sensing molecules that are capable of binding Ca2+ to maintain cellular homeostasis of calcium levels [9,87,88,89]. In addition to mitochondria and the endoplasmic reticulum, vacuoles in plant cells serve as storehouses for calcium ions [90,91,92,93,94,95]. Calcium sensing molecules, along with organelles to sequester calcium ions, provide a variety of sophisticated, mechanistic possibilities for regulating calcium homeostasis in cells [96].
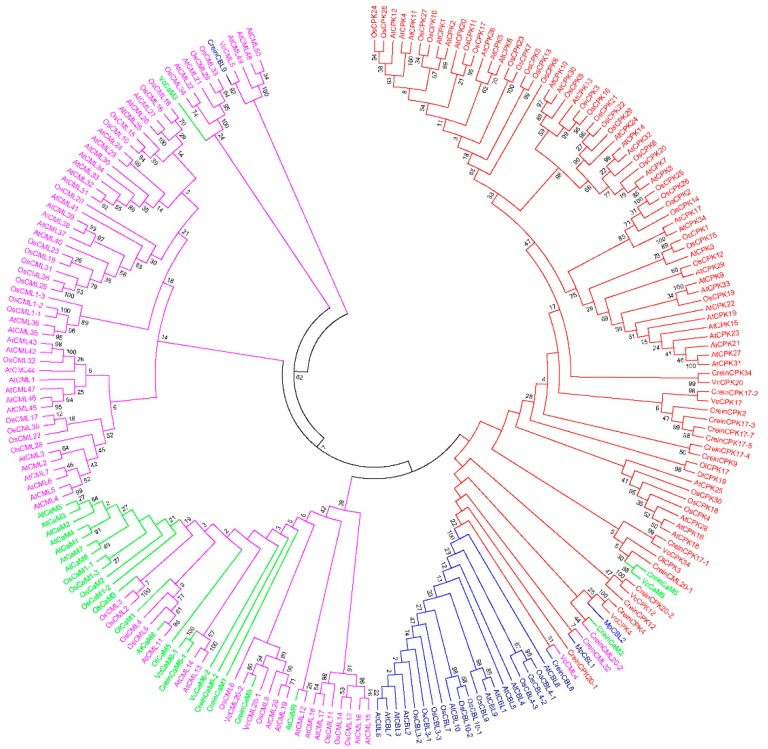
The major EF-hand-containing calcium sensing molecules are calcium-dependent protein kinases, calmodulins, calmodulin-like and calcineurin B-likes proteins. The intra-genomic diversity of Ca2+ tool kit components has been reported to have emerged at bigger rate as compared to the evolution of other proteins [12]. Therefore, we constructed a phylogenetic tree in order to better understand various evolutionary aspects of EF-hand containing calcium signaling proteins (Figure 9). An analysis of the phylogenetic tree indicates that calcium sensing molecules have a polyphyletic origin. CMLs appear to be the most primitive calcium binding proteins followed by the evolution of CaMs, CBLs and CPKs. The phylogenetic tree of CPKs, CMLs, CaMs and CBLs is divided into five clusters. Cluster I contain CPKs (red) and CBLs (navy blue) and is divided into two sub-groups. Cluster I, in addition to CPKs and CBLs, also contains a few CaMs and CMLs. Cluster II contains CaMs (aqua) and CMLs (fuchsia) but no CPKs or CBLs. Cluster III contains only CMLs (fuchsia), while cluster IV contains CMLs and a CaM (VcCaM3). Cluster V contains CMLs and a CBL (CreinCBL9) (Figure 9). The presence of CMLs in all of the clusters and the absence of CBLs and CPKs in cluster II, III and IV indicates that CaMs, CBLs and CPKs evolved from CMLs and more specifically that CPK and CBLs evolved most recently.
Figure 9.
Phylogenetic tree of calcium sensor proteins. Phylogenetic tree of CaMs, CMLs, CBLs and CPKs resulted into five clusters. Cluster I contain CPKs (red) and CBLs (navy), cluster II contain CMLs (fuchsia) and CaMs (aqua), cluster III contain CMLs (fuchsia), cluster IV contain CaM (aqua) and CML (fuchsia) and cluster V contain CBL (aqua) and CML (fuchsia). Phylogenetic tress shows that CMLs are the primitive EF-hand containing calcium sensing proteins and evolved from monophyletic common ancestor [19]. Evolutions of CMLs were followed by the evolution of CaMs, CBLs and CPKs through gene duplication and subsequent expansion. Phylogenetic tree was constructed by maximum likelihood method and LG (Le & Gascuel) model using 1000 bootstrap replicates. Phylogenetic tree was constructed by MEGA6 software using the amino acid sequences of calcium sensor proteins.
A species tree was constructed to discern the duplication and loss events that had occurred in the evolution of EF-hands containing calcium-sensing molecules. Results indicated that the calcium-sensing molecules underwent duplication events during their evolution (Figure 10). Further duplication and loss analysis indicated that duplication of calcium-sensing genes preceded the loss events. Only a few calcium sensing genes that had been duplicated in the A. thaliana genome were had been lost in the Oryza sativa genome (Figure 10). These findings suggest that calcium sensing genes evolved in a lineage-specific manner. Lineage-specific evolution leads to a diversification in organismal complexity. The existence of a high number of CMLs and CPKs indicates that Ca2+ binding proteins associated with calcium signaling have expanded enormously during evolution [12,19,20,21]. The potential for diverse Ca2+ decoding mechanisms may have fostered the early evolution of calcium signaling molecules.
Figure 10.
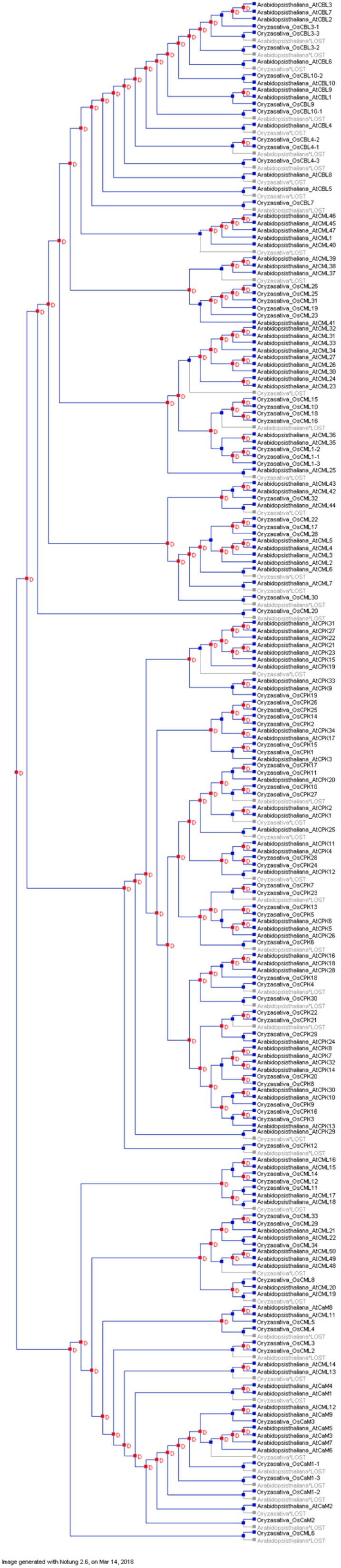
Duplication and loss event of calcium sensor proteins. A species tree was constructed to study the duplication and loss events of calcium binding sensor proteins. Result shows that, duplication event predominates the loss event during the evolution. Gene duplication events confirm the evolution of calcium sensing proteins through expansion. Duplicated genes in dicot plants are sometimes lost in the monocot lineage, suggesting the lineage specific evolution of calcium sensing genes. No conditional duplication was found during this study. The study was conducted using Notung software 2.9.
Ca2+-decoding protein families have greatly expanded and diversified in plants. An example of this is the existence of the large and diverse CML, CaM, CPK and CBL gene families. The presence of only one and three CaMs in Eucalyptus grandis (dicot) and Coccomyxa subellipsoidea (algae), respectively, expanded up to 13 in Brassica rapa (dicot) and Mimulus guttatus (eudicot), whereas the presence of two CMLs in Coccomyxa subellipsoidea (algae) and Ostreococcus lucimarinus (algae) expanded up to 47 in A. thaliana (dicot) (Table 1). Similarly, the absence of CBLs in Coccomyxa subellipsoidea (algae) contrasts with a total of 14 CBLs in Brassica rapa and the two CPKs in Coccomyxa subellipsoidea (algae) and Ostreococcus lucimarinus (algae) have expanded to 53 CPKs in Panicum virgatum (monocot). These examples reflect the evolutionary expansion and diversity of calcium-decoding molecules. Most unicellular eukaryotes, including algae, have a low calcium requirement for normal growth and development [12]. Therefore, they contain only a few numbers of CaMs, CMLs and CPKs in their genomes, while CBLs are absent in Coccomyxa subellipsoidea. Although several other EF-hand containing proteins are exist in plant cells, CMLs, CaMs, CBLs and CPKs account for more than one-third of all the EF-hand containing proteins present in plant genomes [12]. This suggests that, despite the hypothetical bottleneck in evolutionary events that occurs in complex, multicellular plants, CMLs, CaMs, CPKs and CBLs became diversified and gained multiple distinct functions. The complexity of Ca2+ signaling systems in plants is congruent with the increasing cellular and morphological complexity that has evolved in plants along with their ability to adapt to and live in a fluctuating and often harsh environment. The CPKs are quite abundant in algae and higher plants and they cluster with CBLs in phylogenetic trees. This suggests that CPKs and CBLs played prominent functional roles in the evolution of land plants. It seems that plants expanded the number of proteins within specific protein families during the evolution to foster functional diversification and specialization or that new functions evolved within existing protein families. The number of Ca2+-decoding protein families and the number of proteins within each family, was originally very low and subsequently expanded during evolution. The CMLs and CPKs families expanded dramatically and the increase in the number of family members is correlated with the increase in biochemical complexity that arose during plant evolution. The increase in organismal complexity was a driving force in the expansion and diversification of plant Ca2+ signaling systems where new functions had to be regulated by a limited number of sensors. This led to their expansion and functional diversification. The presence of conserved D-x-D motifs in the EF-hands of CaMs and CPKs suggest that the four primitive EF-hands of CaMs merged with protein kinase molecules to give rise to calcium-dependent protein kinases. The increase in CaM and CMLs gene numbers are correlated with the transition of plants from aquatic to terrestrial life forms [19]. The CaM proteins are highly conserved across all eukaryotes and CMLs are absent in the Unikonta super-group [19]. The acquisition of novel functions may have contributed to the ability of plants to adapt to the colonization of land habitats and the expansion and diversification of calcium-decoding proteins was certainly driven by selective pressures. The increase in the number of CaM and CML genes is correlated with the plant adaptation to a terrestrial environment (Physcomitrella patens, Bryophytes) and the subsequent organismal complexity of gymnosperms and angiosperms.
8. Conclusions
The genomic features of calcium binding proteins are quite conserved and interactome network reflect the involvement of CaMs, CMLs, CBLs and CPKs in diverse cellular events. More specifically CBLs are interacts with CIPKs and CPKs interacts with themselves. A complex set of evolutionary events have played an important role in shaping the Ca2+ signaling systems in plants. A genomic analysis of Ca2+ signaling system genes in plants provides a clearer picture of the evolution of the increased number of Ca2+ signaling proteins and their diverse roles in plant metabolism, physiology and morphology. The mapping of the duplication and loss events that have occurred in Ca2+ signaling sensor molecules provides clarification of the evolution of these gene families. The interactions of CPKs with other CPKs suggests, CPKs are most possibly co-regulated together and the presence of conserved molecular feature of D-x-D, D/E-E-LD motif as well as the presence of conserved D and E amino acid at different position across the EF-hand containing protein reflect their conserved evolutionary perspectives. Differential kinetic studies of calcium signaling associated enzymes can also be very useful for understanding the functional diversification of Ca2+ signaling cascades. Additionally, more research is needed to clarify the role of the chloroplast and mitochondria in the evolution of calcium-decoding molecules, as these organelles also play a critical role in Ca2+signaling. Functional characterization of conserved calcium binding motifs and their binding kinetics will be an excellent approach to better understand calcium signaling events in detail.
Acknowledgments
The authors would like to extend their sincere appreciation to the chair, University of Nizwa for the support to conduct this study. Authors also like to extend their sincere thanks to the Deanship of Scientific Research at King Saud University for its funding to the Research Group number (RG-1435-014).
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/6/1476/s1.
Author Contributions
T.K.M.: Conceived the idea, surveyed the literature, drafted the manuscript, D.Y.: Revised the manuscript, A.H. and E.F.A._A.: revised and edited the manuscript, A.L.K. and A.A.-H.: revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Burstrom H.G. Calcium and plant growth. Biol. Rev. 1968;43:287–316. doi: 10.1111/j.1469-185X.1968.tb00962.x. [DOI] [Google Scholar]
- 2.Tuteja N., Mahajan S. Calcium Signaling Network in Plants. Plant Signal. Behav. 2007:79–85. doi: 10.4161/psb.2.2.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin T., Li J., Yuan M., Mao T. Characterization of the role of calcium in regulating the microtubule-destabilizing activity of MDP25. Plant Signal. Behav. 2012;7:708–710. doi: 10.4161/psb.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hepler P.K. The Cytoskeleton and Its Regulation by Calcium and Protons. Plant Physiol. 2016;170:3. doi: 10.1104/pp.15.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandoval I.V., Weber K. Calcium-Induced Inactivation of Microtubule Formation in Brain Extracts: Presence of a Calcium-Dependent Protease Acting on Polymerization-Stimulating Microtubule-Associated Proteins. Eur. J. Biochem. 1978;92:463–470. doi: 10.1111/j.1432-1033.1978.tb12768.x. [DOI] [PubMed] [Google Scholar]
- 6.Vater W., Bohm K.J., Unger E. Tubulin assembly in the presence of calcium ions and taxol: Microtubule bundling and formation of macrotubule—Ring complexes. Cell Motil. 1998;36:76–83. doi: 10.1002/(SICI)1097-0169(1997)36:1<76::AID-CM7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Dematry M., Morvan C., Thellier M. Calcium and the cell wall. Plant. Cell Environ. 2006;7:441–448. doi: 10.1111/j.1365-3040.1984.tb01434.x. [DOI] [Google Scholar]
- 8.Mohanta T.K., Bashir T., Hashem A., Abd_Allah E.F., Khan A.L., Al-Harrasi A.S. Early Events in Plant Abiotic Stress Signaling: Interplay Between Calcium, Reactive Oxygen Species and Phytohormones. J. Plant Growth Regul. 2018 doi: 10.1007/s00344-018-9833-8. [DOI] [Google Scholar]
- 9.Bootman M. Calcium Signaling. Cold Cold Spring Harb Perspect Biol. 2012;4:a011171. doi: 10.1101/cshperspect.a011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuteja N. Integrated Calcium Signaling in Plants. In: Mancuso S., Balu¿ka F., editors. Signaling in Plants. Springer; Berlin/Heidelberg, Germany: 2009. pp. 29–49. [Google Scholar]
- 11.Xiong T.-C., Bourque S., Lecourieux D., Amelot N., Grat S., Brière C., Mazars C., Pugin A., Ranjeva R. Calcium signaling in plant cell organelles delimited by a double membrane. Biochim. Biophys. Acta Mol. Cell Res. 2006;1763:1209–1215. doi: 10.1016/j.bbamcr.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Edel K.H., Marchadier E., Brownlee C., Kudla J., Hetherington A.M. The Evolution of Calcium-Based Signalling in Plants. Curr. Biol. 2017;27:R667–R679. doi: 10.1016/j.cub.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Kazmierczak J., Kempe S., Kremer B. Calcium in the Early Evolution of Living Systems: A Biohistorical Approach. Curr. Org. Chem. 2013;17:1738–1750. doi: 10.2174/13852728113179990081. [DOI] [Google Scholar]
- 14.Clapham D.E. Calcium Signaling. Cell. 2014;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Bush D.S., Biswas A.K., Jones R.L. Gibberellic-acid-stimulated Ca2+ accumulation in endoplasmic reticulum of barley aleurone: Ca2+ transport and steady-state levels. Planta. 1989;178:411–420. doi: 10.1007/BF00391870. [DOI] [PubMed] [Google Scholar]
- 16.Logan D.C., Knight M.R. Mitochondrial and Cytosolic Calcium Dynamics Are Differentially Regulated in Plants. Plant Physiol. 2003;133:21–24. doi: 10.1104/pp.103.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauly N., Knight M.R., Thuleau P., van der Luit A.H., Moreau M., Trewavas A.J., Ranjeva R., Mazars C. Control of free calcium in plant cell nuclei. Nature. 2000;405:754. doi: 10.1038/35015671. [DOI] [PubMed] [Google Scholar]
- 18.Brauer M., Zhong W.-J., Jelitto T., Schobert C., Sanders D., Komor E. Free calcium ion concentration in the sieve-tube sap of Ricinus communis L. seedlings. Planta. 1998;206:103–107. doi: 10.1007/s004250050379. [DOI] [Google Scholar]
- 19.Mohanta T.K., Kumar P., Bae H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol. 2017;17:38. doi: 10.1186/s12870-017-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohanta T.K., Mohanta N., Mohanta Y.K., Parida P., Bae H. Genome-wide Identification of Calcineurin B-Like (CBL) Gene Family Of Plants Reveals Novel Conserved Motifs and Evolutionary Aspects In Calcium Signaling Events. BMC Plant Biol. 2015;15:189. doi: 10.1186/s12870-015-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohanta T., Mohanta N., Mohanta Y., Bae H. Genome-Wide Identification of Calcium Dependent Protein Kinase Gene Family in Plant Lineage Shows Presence of Novel D-x-D and D-E-L Motifs in EF-Hand Domain. Front. Plant Sci. 2015;6:1146. doi: 10.3389/fpls.2015.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamniuk A.P., Vogel H.J. Structural Investigation into the Differential Target Enzyme Regulation Displayed by Plant Calmodulin Isoforms. Biochemistry. 2005;44:3101–3111. doi: 10.1021/bi047770y. [DOI] [PubMed] [Google Scholar]
- 23.Luan S., Kudla J., Rodriguez-concepcion M., Yalovsky S., Gruissem W. Calmodulins and Calcineurin B –like Proteins: Calcium Sensors for Specific Signal Response Coupling in Plants. Plant Cell. 2002;14:389–400. doi: 10.1105/tpc.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S.H., Johnson J.D., Walsh M.P., van Lierop J.E., Sutherland C., Xu A., Snedden W.A., Kosk-Kosicka D., Fromm H., Narayanan N., Cho M.J. Differential regulation of Ca2+/calmodulin-dependent enzymes by plant calmodulin isoforms and free Ca2+ concentration. Biochem. J. 2000;350:299–306. doi: 10.1042/bj3500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo J.H., Park C.Y., Kim J.C., Do Heo W., Cheong M.S., Park H.C., Kim M.C., Moon B.C., Choi M.S., Kang Y.H., et al. Direct Interaction of a Divergent CaM Isoform and the Transcription Factor, MYB2, Enhances Salt Tolerance in Arabidopsis. J. Biol. Chem. 2005;280:3697–3706. doi: 10.1074/jbc.M408237200. [DOI] [PubMed] [Google Scholar]
- 26.McCormack E., Tsai Y.C., Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–389. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Busch E., Hohenester E., Timpl R., Paulsson M., Maurer P. Calcium affinity, cooperativity, and domain interactions of extracellular EF-hands present in BM-40. J. Biol. Chem. 2000;275:25508–25515. doi: 10.1074/jbc.M001770200. [DOI] [PubMed] [Google Scholar]
- 28.Rupp B., Marshak D.R., Parkin S. Crystallization and preliminary X-ray analysis of two new crystal forms of calmodulin. Acta Crystallogr. Sect. D. 1996;52:411–413. doi: 10.1107/S0907444995011826. [DOI] [PubMed] [Google Scholar]
- 29.Rhoads R., Friedberg F. Sequence motifs for Calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 30.Hoeflich K.P., Ikura M. Calmodulin in Action: Diversity in Target Recognition and Activation Mechanisms. Cell. 2002;108:739–742. doi: 10.1016/S0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 31.McCormack E., Braam J. Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol. 2003;159:585–598. doi: 10.1046/j.1469-8137.2003.00845.x. [DOI] [PubMed] [Google Scholar]
- 32.Perochon A., Aldon D., Galaud J.-P., Ranty B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie. 2011;93:2048–2053. doi: 10.1016/j.biochi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Zielinski R.E. Characterization of three new members of the Arabidopsis thaliana calmodulin gene family: conserved and highly diverged members of the gene family functionally complement a yeast calmodulin null. Planta. 2002;214:446–455. doi: 10.1007/s004250100636. [DOI] [PubMed] [Google Scholar]
- 34.Vanderbeld B., Snedden W.A. Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol. Biol. 2007;64:683–697. doi: 10.1007/s11103-007-9189-0. [DOI] [PubMed] [Google Scholar]
- 35.Akaboshi M., Hashimoto H., Ishida H., Saijo S., Koizumi N., Sato M., Shimizu T. The Crystal Structure of Plant-Specific Calcium-Binding Protein AtCBL2 in Complex with the Regulatory Domain of AtCIPK14. J. Mol. Biol. 2008;377:246–257. doi: 10.1016/j.jmb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Chandran V., Stollar E.J., Lindorff-Larsen K., Harper J.F., Chazin W.J., Dobson C.M., Luisi B.F., Christodoulou J. Structure of the Regulatory Apparatus of a Calcium-dependent Protein Kinase (CDPK): A Novel Mode of Calmodulin-target Recognition. J. Mol. Biol. 2006;357:400–410. doi: 10.1016/j.jmb.2005.11.093. [DOI] [PubMed] [Google Scholar]
- 37.Hrabak E., Chan C., Gribskov M. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003;132:666–680. doi: 10.1104/pp.102.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos-Soriano L., Gómez-Ariza J., Bonfante P., San Segundo B. A rice calcium-dependent protein kinase is expressed in cortical root cells during the presymbiotic phase of the arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2011;11:90. doi: 10.1186/1471-2229-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren D., Liu Y., Yang K.-Y., Han L., Mao G., Glazebrook J., Zhang S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2008;105:5638–5643. doi: 10.1073/pnas.0711301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smotrys J.E., Linder M.E. Palmitoylation of intracellular signaling proteins: Regulation and function. Annu. Rev. Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 41.Martín M.L., Busconi L. A rice membrane-bound calcium-dependent protein kinase is activated in response to low temperature. Plant Physiol. 2001;125:1442–1449. doi: 10.1104/pp.125.3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng S., Willmann M., Chen H., Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002;129:469–485. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaandrager A.B., Ehlert E.M., Jarchu T., Lohmann S.M., Jonge H.R. N-terminal myristoylation is required for membrane localization of cGMP-dependent protein kinase type II. J. Biol. Chem. 1996;271:7025–7029. doi: 10.1074/jbc.271.12.7025. [DOI] [PubMed] [Google Scholar]
- 44.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., Tsafou K.P., et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naqvi S., Martin K.J., Arthur J.S.C. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem. J. 2014;458:469–479. doi: 10.1042/BJ20131115. [DOI] [PubMed] [Google Scholar]
- 47.Astegno A., Capitani G., Dominici P. Functional roles of the hexamer organization of plant glutamate decarboxylase. Biochim. Biophys. Acta Proteins Proteomics. 2015;1854:1229–1237. doi: 10.1016/j.bbapap.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Finkina E.I., Melnikova D.N., Bogdanov I.V., Ovchinnikova T.V. Lipid Transfer Proteins As Components of the Plant Innate Immune System: Structure, Functions, and Applications. Acta Naturae. 2016;8:47–61. [PMC free article] [PubMed] [Google Scholar]
- 49.Viola R.E. The Central Enzymes of the Aspartate Family of Amino Acid Biosynthesis. Acc. Chem. Res. 2001;34:339–349. doi: 10.1021/ar000057q. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., Kim J.I., Pysh L., Chapple C. Four Isoforms of Arabidopsis 4-Coumarate: CoA Ligase Have Overlapping yet Distinct Roles in Phenylpropanoid Metabolism. Plant Physiol. 2015;169:2409–2421. doi: 10.1104/pp.15.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohanta T.K., Occhipinti A., Zebelo S., Foti M., Fliegmann J., Bossi S., Maffei M.E., Bertea C.M. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0032822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peiter E., Maathuis F.J.M., Mills L.N., Knight H., Pelloux J., Hetherington A.M., Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 53.Patel S., Kilpatrick B.S. Two-pore channels and disease. Biochim. Biophys. Acta. Mol. cell Res. 1865:1678–1686. doi: 10.1016/j.bbamcr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Rosales M.P., Gálvez F.J., Huertas R., Aranda M.N., Baghour M., Cagnac O., Venema K. Plant NHX cation/proton antiporters. Plant Signal. Behav. 2009;4:265–276. doi: 10.4161/psb.4.4.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park C.-J., Seo Y.-S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015;31:323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeeman S.C., Thorneycroft D., Schupp N., Chapple A., Weck M., Dunstan H., Haldimann P., Bechtold N., Smith A.M., Smith S.M. Plastidial α-Glucan Phosphorylase Is Not Required for Starch Degradation in Arabidopsis Leaves But Has a Role in the Tolerance of Abiotic Stress. Plant Physiol. 2004;135:849–858. doi: 10.1104/pp.103.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Post S.M., Tomkinson A.E., Lee E.Y.-H.P. The human checkpoint Rad protein Rad17 is chromatin-associated throughout the cell cycle, localizes to DNA replication sites, and interacts with DNA polymerase epsilon. Nucleic Acids Res. 2003;31:5568–5575. doi: 10.1093/nar/gkg765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J., Nolan T.M., Ye H., Zhang M., Tong H., Xin P., Chu J., Chu C., Li Z., Yin Y. Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Responses. Plant Cell. 2017;29:1425–1439. doi: 10.1105/tpc.17.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen H., Lai Z., Shi J., Xiao Y., Chen Z., Xu X. Roles of arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010;10:281. doi: 10.1186/1471-2229-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H., Yang S., Li Y., Hua J. The Arabidopsis BAP1 and BAP2 genes are general inhibitors of programmed cell death. Plant Physiol. 2007;145:135–146. doi: 10.1104/pp.107.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tripathi V., Parasuraman B., Laxmi A., Chattopadhyay D. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. Plant J. 2009;58:778–790. doi: 10.1111/j.1365-313X.2009.03812.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen L., Wang Q.-Q., Zhou L., Ren F., Li D.-D., Li X.-B. Arabidopsis CBL-interacting protein kinase (CIPK6) is involved in plant response to salt/osmotic stress and ABA. Mol. Biol. Rep. 2013;40:4759–4767. doi: 10.1007/s11033-013-2572-9. [DOI] [PubMed] [Google Scholar]
- 63.Sardar A., Chattopadhyay D., Nandi A.K. CBL-interacting protein kinase 6 negatively regulates immune response to Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 2017;68:3573–3584. doi: 10.1093/jxb/erx170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin X., Sun T., Wang X., Su P., Ma J., He G., Yang G. Wheat CBL-interacting protein kinase 25 negatively regulates salt tolerance in transgenic wheat. Sci. Rep. 2016;6:28884. doi: 10.1038/srep28884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bürstenbinder K., Rzewuski G., Wirtz M., Hell R., Sauter M. The role of methionine recycling for ethylene synthesis in Arabidopsis. Plant J. 2007;49:238–249. doi: 10.1111/j.1365-313X.2006.02942.x. [DOI] [PubMed] [Google Scholar]
- 66.Halfter U., Ishitani M., Zhu J.-K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA. 2000;97:3735–3740. doi: 10.1073/pnas.97.7.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aghdasi B., Ye K., Resnick A., Huang A., Ha H.C., Guo X., Dawson T.M., Dawson V.L., Snyder S.H. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc. Natl. Acad. Sci. USA. 2001;98:2425–2430. doi: 10.1073/pnas.041614198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gollan P.J., Bhave M., Aro E.-M. The FKBP families of higher plants: Exploring the structures and functions of protein interaction specialists. FEBS Lett. 2012;586:3539–3547. doi: 10.1016/j.febslet.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Lam H.-M., Chiu J., Hsieh M.-H., Meisel L., Oliveira I.C., Shin M., Coruzzi G. Glutamate-receptor genes in plants. Nature. 1998;396:125. doi: 10.1038/24066. [DOI] [PubMed] [Google Scholar]
- 70.Zhu M.X., Evans A.M., Ma J., Parrington J., Galione A. Two-pore channels for integrative Ca signaling. Commun. Integr. Biol. 2010;3:12–17. doi: 10.4161/cib.3.1.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avila E.L., Surpin M., Brown M., Pan S., Girke T., Raikhel N.V., Desikan R., Neill S.J. Expression analysis of Arabidopsis vacuolar sorting receptor 3 reveals a putative function in guard cells. J. Exp. Bot. 2008;59:1149–1161. doi: 10.1093/jxb/ern025. [DOI] [PubMed] [Google Scholar]
- 72.Schulz P., Herde M., Romeis T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013;163:523–530. doi: 10.1104/pp.113.222539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boudsocq M., Willmann M.R., McCormack M., Lee H., Shan L., He P., Bush J., Cheng S.-H., Sheen J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 2010;464:418. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubiella U., Seybold H., Durian G., Komander E., Lassig R., Witte C.-P., Schulze W.X., Romeis T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA. 2013;110:8744–8749. doi: 10.1073/pnas.1221294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franz S., Ehlert B., Liese A., Kurth J., Cazale A.-C., Romies T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant. 2015;4:83–96. doi: 10.1093/mp/ssq064. [DOI] [PubMed] [Google Scholar]
- 76.Bouschet T., Henley J.M. Calcium as an extracellular signalling molecule: Perspectives on the Calcium Sensing Receptor in the brain. C. R. Biol. 2005;328:691–700. doi: 10.1016/j.crvi.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carafoli E., Krebs J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naraghi M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium. 1997;22:255–268. doi: 10.1016/S0143-4160(97)90064-6. [DOI] [PubMed] [Google Scholar]
- 79.Lee J.-Y., Yoo B.-C., Harmon A.C. Kinetic and Calcium-Binding Properties of Three Calcium-Dependent Protein Kinase Isoenzymes from Soybean. Biochemistry. 1998;37:6801–6809. doi: 10.1021/bi980062q. [DOI] [PubMed] [Google Scholar]
- 80.Hammond C. Cellular and Molecular Neurophysiology. 4th ed. Academic Press; Boston, MA, USA: 2015. Ionic gradients, membrane potential and ionic currents, Chapter 3; pp. 39–54. [Google Scholar]
- 81.Carafoli E. The Calcium Pumping ATPase of the Plasma Membrane. Annu. Rev. Physiol. 1991;53:531–547. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- 82.Stokes D.L., Green N.M. Structure and Function of the Calcium Pump. Annu. Rev. Biophys. Biomol. Struct. 2003;32:445–468. doi: 10.1146/annurev.biophys.32.110601.142433. [DOI] [PubMed] [Google Scholar]
- 83.Brini M., Carafoli E. The Plasma Membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium. Cold Spring Harb. Perspect. Biol. 2011;3:a004168. doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhivotovsky B., Orrenius S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Celsi F., Pizzo P., Brini M., Leo S., Fotino C., Pinton P., Rizzuto R. Mitochondria, calcium and cell death: A deadly triad in neurodegeneration. Biochim. Biophys. Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dubois C., Prevarskaya N., Vanden Abeele F. The calcium-signaling toolkit: Updates needed. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:1337–1343. doi: 10.1016/j.bbamcr.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 88.Edel K.H., Kudla J. Increasing complexity and versatility: How the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium. 2015;57:231–246. doi: 10.1016/j.ceca.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Marchadier E., Oates M.E., Fang H., Donoghue P.C.J., Hetherington A.M., Gough J. Evolution of the Calcium-Based Intracellular Signaling System. Genome Biol. Evol. 2016;8:2118–2132. doi: 10.1093/gbe/evw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Contreras L., Drago I., Zampese E., Pozzan T. Mitochondria: The calcium connection. Biochim. Biophys. Acta Bioenerg. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Duchen M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stael S., Wurzinger B., Mair A., Mehlmer N., Vothknecht U.C., Teige M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2012;63:1525–1542. doi: 10.1093/jxb/err394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schönknecht G. Calcium Signals from the Vacuole. Plants. 2013;2:589–614. doi: 10.3390/plants2040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sello S., Moscatiello R., Mehlmer N., Leonardelli M., Carraretto L., Cortese E., Zanella F.G., Baldan B., Szabò I., Vothknecht U.C., Navazio L. Chloroplast Ca2+ fluxes into and across thylakoids revealed by thylakoid-targeted aequorin probes. Plant Physiol. 2018;177:38–51. doi: 10.1104/pp.18.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peiter E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium. 2011;50:120–128. doi: 10.1016/j.ceca.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 96.Ranty B., Aldon D., Cotelle V., Galaud J.-P., Thuleau P., Mazars C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016;7:327. doi: 10.3389/fpls.2016.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.