Abstract
Background:
Physical function and strength decline with age and lead to limited mobility and independence in older adults. Alterations in mitochondrial function are thought to underlie numerous age-related changes, including declining physical ability. Recent studies suggest that systemic changes in bioenergetic capacity may be reported by analyzing mitochondrial function in circulating cells. The objective of this study was to determine whether the bioenergetic capacity of peripheral blood mononuclear cells (PBMCs) is related to differences in physical function among older, overweight/obese, adults. To address this, we tested the hypothesis that greater PBMC respirometric capacity would be associated with better physical function, muscular strength, leg lean mass, and muscle quality. Furthermore, we tested whether the respirometric capacity of PBMCs is related to cellular composition and inflammatory status reported by interleukin 6 (IL-6).
Methods:
Fasted PBMC respiration (pmol/min/500,000 cells), expanded short physical performance battery (Ex-SPPB), peak knee extensor (KE) strength (Nm), grip strength (kg), leg lean mass (kg, via dual energy X-ray absorptiometry [DXA]), muscle quality (Nm/kg), and plasma IL-6 (pg/mL) were analyzed in 15 well-functioning, community-dwelling, sedentary overweight/obese older men (n=9) and women (n=6) aged 65 to 78 (mean 68.3 ± 3.5 years). Pearson and partial correlations were calculated to determine associations between PBMC respiration and these variables.
Results:
Higher maximal respiration of PBMCs was associated with better Ex-SPPB (r = 0.58, p = 0.02), greater KE strength (r = 0.60, p = 0.02), greater grip strength (r = 0.52, p = 0.05) and lower IL-6 (r = −0.58, p = 0.04). Higher spare respiratory capacity was associated with better Ex-SPPB (r = 0.59, p = 0.02), greater KE strength (r = 0.60, p = 0.02), greater grip strength (r = 0.54, p = 0.04), greater leg muscle quality (r = 0.56, p = 0.04), and lower IL-6 (r = −0.55, p = 0.05). Monocyte and lymphocyte counts were not related to PBMC respiratory capacity.
Conclusions:
Our results indicate that respirometric profiles of readily obtainable blood cells are associated with physical function and strength. Future studies should be undertaken in order to determine whether blood-based bioenergetic profiling can provide an objective index of systemic mitochondrial health.
Keywords: blood, bioenergetics, physical function, inflammation, mitochondria
1. INTRODUCTION
The maintenance of physical function is a critical factor for mobility and independence in the growing population of adults 65 years and older (Katz et al., 1983; Avila et al., 2012). Measures of physical function, specifically involving the lower extremities, are strong predictors of morbidity and mortality (Guralnik et al., 1995), generalizable to older adults with a wide range of physical abilities, including those that are well-functioning (Simonsick et al., 2001). Physical function tests integrate multiple physiological systems including: nervous, musculoskeletal, and energy production/delivery, which likely underlies their ability to predict morbidity and mortality (Ferrucci et al., 2000). Our group has reported that chronic inflammation, such as elevated interleukin-6 (IL-6) levels in plasma, are associated with poorer physical function in older adults across a variety of diseases/health conditions, supporting the hypothesis that chronic inflammation represents a common mechanism underlying age-related functional decline (Brinkley et al., 2009). Indeed, studies focused on IL-6 have shown that blood levels and monocyte production of IL-6 are higher with advancing age (Ferrucci et al., 2005). In particular, there is a dramatic increase in the number of individuals with elevated IL-6 levels over the age of 70 years (Giuliani et al., 2001). In addition, chronic inflammation, along with other circulating factors, has been shown to detrimentally affect mitochondrial function in muscle tissue (Valerio et al., 2006).
Although measures of physical function are important indicators of health in geriatrics, the biological mechanisms that underlie age-related decline in physical function and associated health consequences are not fully understood. Aging and declining function are associated with bioenergetic decline. For example, lower maximal adenosine triphosphate (ATP) production in vastus lateralis skeletal muscle is associated with reduced physical function and aerobic capacity (Joseph et al., 2012; Tyrrell et al., 2014; Coen et al., 2013). Multiple lines of evidence, including these data, suggest that mitochondrial function is vitally important for physical function, particularly in the context of aging.
Bioenergetic profiling using readily obtainable blood-cells has been proposed as a way to assess systemic mitochondrial health (Chacko et al., 2014; Ravi et al., 2014). There is mounting evidence that circulating factors, including inflammatory cytokines, can mediate bioenergetic decline in multiple tissues throughout the body (Salminen et al., 2012). The effects of these factors on mitochondrial bioenergetics may be reflected in the respiratory profiles of circulating cells such as peripheral blood mononuclear cells (PBMCs). In fact, we recently showed that mitochondrial function measured in both skeletal muscle and PBMCs was associated with gait speed among older adults (Tyrrell et al., 2014). To expand on this work, the main objective of the current study was to determine whether physical and muscle function are related to bioenergetic capacity of PBMCs in well-functioning, community-dwelling, overweight/obese older adults. We also determined whether the heterogeneous composition of PBMCs underlies their bioenergetic capacity and whether the proinflamatory cytokine IL-6 is associated with PBMC respiratory capacity.
2. METHODS
2.1. Participants
Fifteen participants were included in this study of older (65–79 years), overweight and obese (body mass index [BMI]: 28–35 kg/m2), sedentary men (n=9) and women (n=6) recruited to participate in a clinical trial of resistance training with or without dietary-induced weight loss. The assessments reported here were conducted at baseline, prior to randomization.
Eligible participants were in good health and had normal cognitive function, used no walking aids, and did not have uncontrolled diabetes or hypertension, cardiovascular disease, abnormal liver or kidney function, or cancer requiring treatment in the past 2 years. The study was approved by the Wake Forest School of Medicine Institutional Review Board and all participants provided written, informed consent.
2.2. Physical Function
2.2.1. Expanded Short Physical Performance Battery
The expanded short physical performance battery (Ex-SPPB) was used as previously reported to prevent ceiling effects when applying the measure to well-functioning, community-dwelling older adults (Simonsick et al., 2001). Briefly, the Ex-SPPB consists of a usual gait speed test, a usual gait speed test using a narrow course (20 cm), 5 repeated chair stands, and 30 second standing balance tests (semi-, full-tandem, and single leg). Scores and times are recorded for each and converted to ratios and summed to get a composite score on a continuous scale ranging from 0 to 4.
2.3. Muscle Strength
2.3.1. Peak Knee Extensor Strength
Peak knee extensor (KE) strength (in Newton meters; Nm) was measured on a dynamometer (Biodex Medical Systems, Inc., Shirley, NY) at 60° per second with the participant seated and the hips and knees flexed at 90°. To stabilize the hip joint and the trunk, participants were restrained with straps at the chest, hip and thigh. Seat height and depth, and the position of the lever arm ankle pad were adjusted to accommodate each participant. Participants were asked to extend the knee and push as hard as possible against the ankle pad. Strength of the right leg recorded as peak torque was used for analyses.
2.3.2. Grip Strength
Grip strength was measured twice on each hand to the nearest kg using an isometric Hydraulic Hand Dynamometer (Jamar, Bolingbrook, IL) and the maximal value from both hands was used in analyses.
2.4. Leg Lean Mass and Muscle Quality
Leg lean mass was measured by dual energy X-ray absorptiometry (DXA, Hologic Delphi QDR, Bedford, MA). Muscle quality was calculated as the ratio of knee extensor peak torque to lean mass of the right leg assessed by DXA (Nm/kg leg lean mass).
2.5. Blood Draw
Blood draws were performed in the morning after an overnight fast and were processed immediately after collection. 8 mL of whole blood was collected into cell preparation tubes (Vacutainer; Becton Dickinson, Franklin Lakes, NJ) for PBMC separation.
2.6. Respirometry of Peripheral Blood Mononuclear Cells
PBMCs were washed with phosphate-buffered saline and resuspended in extracellular flux (XF) assay buffer containing 1 mM Na+-pyruvate and 11 mM D-glucose (pH 7.4) for respirometry experiments. Using a defined series of inhibitors and uncoupler, the basal and maximal cellular respiration was quantified and used to calculate spare respiratory capacity (SRC) (Desler et al., 2012; Ferrick et al., 2008). A total of 500,000 cells were plated per well of the Seahorse microplate. Basal oxygen consumption rate (OCR) measures were followed by sequential addition of oligomycin (750 nM), Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP; 1 μM), and antimycin-A/rotenone (both 1 μM). Maximal OCR was calculated after addition of FCCP, a potent mitochondrial uncoupler. The use of FCCP as a chemical uncoupler allows us to estimate maximal respiration. SRC was calculated as the difference between Maximal OCR and the Basal OCR.
2.7. Inflammatory Cytokine Quantification
Blood from 12-hour fasted participants was collected, processed, divided into aliquots, stored at −80°C, and analyzed according to previously published methods (Beavers et al., 2010). Briefly, high-sensitivity IL-6 assays were run using Quantikine enzyme-linked immunosorbent assay (ELISA) kits from R&D systems (Minneapolis, MN). All samples were measured in duplicate, and the average of the two values was used for data analyses.
2.8. Statistical Analyses
Normality of distributions was assessed using Shapiro-Wilk tests. All variables were normally distributed except for monocyte cell count, which was square-root transformed to achieve a normal distribution. Pearson correlation coefficients and partial correlations (individually adjusted for age, BMI, or sex) were calculated using SPSS v.21 (Armonk, NY) to examine relationships between the dependent variables (physical function, muscle strength, leg lean mass, muscle quality, muscle mass, PBMC cell counts, and plasma IL-6 level) and the independent variables (SRC, maximal respiration, and basal respiration). An alpha level of 0.05 was used to assess statistical significance.
3. RESULTS
3.1. Participant Characteristics
The demographics and bioenergetic data are summarized in Table 1. Participants were older (mean age: 68.3 years), overweight or obese (mean BMI: 30.8 kg/m2), community-dwelling, sedentary men and women (40% females).
Table 1:
Participant Demographics, Physical function, Strength, Blood Cell counts, and Bioenergetic Characteristics
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 68.3 ± 3.5 | 65–78 |
| BMI (kg/m2) | 30.8 ± 2.4 | 27.0–34.9 |
| Fast-Paced 400m Walking Speed (m/s) | 1.5 ± 0.2 | 1.1–1.8 |
| Expanded SPPB (range =0–4) | 2.5 ± 0.3 | 2.1–2.9 |
| Knee Extensor Strength (Nm) | 122.0 ± 36.0 | 57.9–179.5 |
| Leg Lean Mass (kg) | 8.3 ± 1.6 | 6.4–11.1 |
| Muscle Quality (Nm/kg) | 12.4 ± 2.5 | 7.6–17.3 |
| Grip Strength (kg) | 35.6 ± 10.7 | 20.0–52.0 |
| Interleukin-6 Level (pg/mL) | 2.2 ± 0.8 | 1.3–3.5 |
| Monocyte Cell Count (per mL whole blood)1 | 486667± 155226 | 300000–900000 |
| Lymphocyte Cell Count (per mL whole blood) | 1826667± 563746 | 500000–2600000 |
| Basal OCR (pmol/min/500,000 cells) | 146.3 ± 51.3 | 59.7–256.1 |
| Maximal OCR (pmol/min/500,000 cells) | 573.1 ± 212.0 | 285.5–980.2 |
| Spare Respiratory Capacity (pmol/min/500,000 cells) | 426.8 ± 167.8 | 183.7–779.2 |
Notes: N=13–15; SPPB = Short Physical Performance Battery; BMI = body mass index, OCR = oxygen consumption rate.
Monocyte cell count was square-root transformed to achieve normal distribution in subsequent figures.
3.2. Relationship Between Physical Function and Blood Bioenergetics
Correlations between PBMC SRC, maximal respiration, and basal respiration with physical function, muscle strength, lean mass, muscle quality, and IL-6 are summarized in Table 2
Table 2:
Correlations Between PBMC Bioenergetics (Spare Respiratory Capacity, Maximal Respiration, and Bas al Respiration) with Physical Function, Strength, Lean Mass, Muscle Quality, and Interleukin-6
| Spare Respiratory Capacity | Maximal Respiration | Basal Respiration | ||||
|---|---|---|---|---|---|---|
| Correlation | r | p-value | r | p-value | r | p-value |
| Expanded Short Physical Performance Battery | ||||||
| Pearson | 0.59 | 0.02* | 0.58 | 0.02* | 0.50 | 0.06^ |
| Partial (age) | 0.59 | 0.02* | 0.59 | 0.02* | 0.50 | 0.07^ |
| Partial (sex) | 0.55 | 0.04* | 0.55 | 0.04* | 0.46 | 0.10^ |
| Partial (BMI) | 0.59 | 0.02* | 0.59 | 0.03* | 0.49 | 0.07^ |
| Partial (IL-6) | 0.62 | 0.04* | 0.63 | 0.04* | 0.57 | 0.07^ |
| Knee Extensor Strength | ||||||
| Pearson | 0.60 | 0.02* | 0.60 | 0.02* | 0.51 | 0.05* |
| Partial (age) | 0.61 | 0.03* | 0.59 | 0.03* | 0.47 | 0.10^ |
| Partial (sex) | 0.66 | 0.01** | 0.65 | 0.01** | 0.55 | 0.05* |
| Partial (BMI) | 0.63 | 0.02* | 0.62 | 0.02* | 0.53 | 0.06^ |
| Partial (IL-6) | 0.55 | 0.08^ | 0.56 | 0.08^ | 0.50 | 0.11 |
| Grip Strength | ||||||
| Pearson | 0.54 | 0.04* | 0.52 | 0.05* | 0.40 | 0.13 |
| Partial (age) | 0.59 | 0.04* | 0.59 | 0.04* | 0.49 | 0.10^ |
| Partial (sex) | 0.53 | 0.05* | 0.51 | 0.06^ | 0.35 | 0.21 |
| Partial (BMI) | 0.57 | 0.03* | 0.57 | 0.03* | 0.52 | 0.06^ |
| Partial (IL-6) | 0.54 | 0.08^ | 0.54 | 0.09^ | 0.45 | 0.17 |
| Leg Lean Mass | ||||||
| Pearson | 0.23 | 0.42 | 0.18 | 0.53 | −0.02 | 0.96 |
| Partial (age) | 0.18 | 0.55 | 0.13 | 0.68 | −0.07 | 0.82 |
| Partial (sex) | 0.29 | 0.34 | 0.23 | 0.46 | 0.01 | 0.98 |
| Partial (BMI) | 0.20 | 0.51 | 0.17 | 0.57 | 0.05 | 0.86 |
| Partial (IL-6) | −0.00 | 0.99 | −0.03 | 0.92 | −0.12 | 0.72 |
| Leg Muscle Quality | ||||||
| Pearson | 0.56 | 0.04* | 0.51 | 0.06^ | 0.29 | 0.31 |
| Partial (age) | 0.54 | 0.06^ | 0.48 | 0.10^ | 0.23 | 0.45 |
| Partial (sex) | 0.49 | 0.09^ | 0.44 | 0.13 | 0.21 | 0.48 |
| Partial (BMI) | 0.55 | 0.05* | 0.52 | 0.07^ | 0.33 | 0.26 |
| Partial (IL-6) | 0.50 | 0.12 | 0.47 | 0.14 | 0.33 | 0.32 |
| Plasma Interleukin-6 | ||||||
| Pearson | −0.55 | 0.05* | −0.58 | 0.04* | −0.61 | 0.03* |
| Partial (age) | −0.61 | 0.03* | −0.64 | 0.03* | −0.63 | 0.03* |
| Partial (sex) | −0.60 | 0.04* | −0.65 | 0.02* | −0.69 | 0.01* |
| Partial (BMI) | −0.53 | 0.08^ | −0.56 | 0.06^ | −0.59 | 0.04* |
Notes: N=13–15; SPPB = Short Physical Performance Battery; BMI = body mass index
p≤0.1,
p≤0.05,
p≤0.01
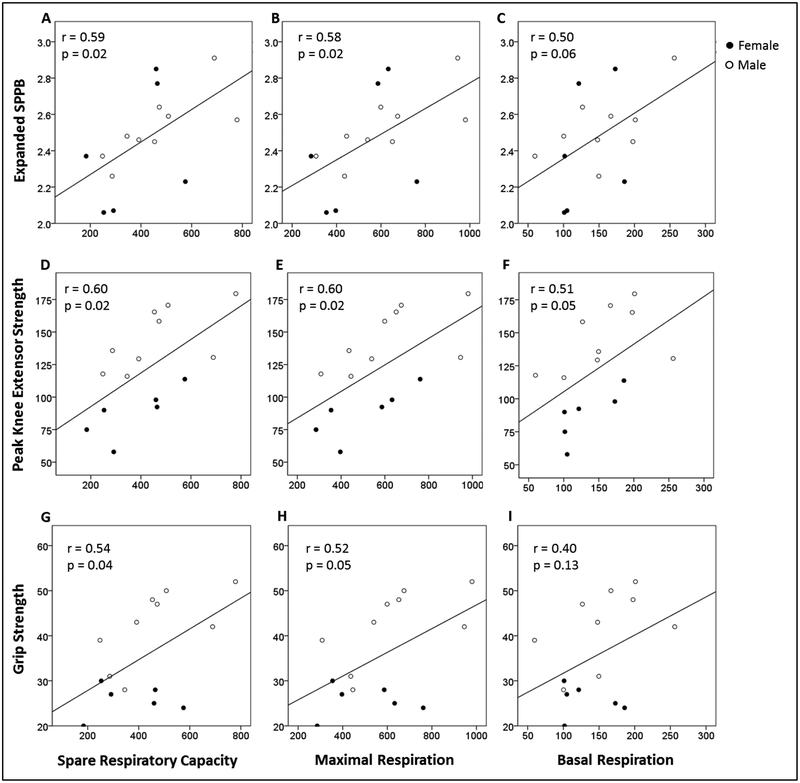
Ex-SPPB was plotted against SRC (pmol/min/500,000 cells), maximal respiration (pmol/min/500,000 cells), and basal respiration (pmol/min/500,000 cells) in Figure 1A–C. Higher SRC and maximal respiration were associated with greater Ex-SPPB scores. Positive correlations between SRC and maximal respiration with Ex-SPPB remained significant after independent adjustments for age, sex, and BMI (presented in Table 2).
Figure 1:
Expanded SPPB plotted against (A) SRC (Maximal Respiration minus Basal Respiration; pmol/min/500,000 cells), (B) Maximal Respiration (pmol/min/500,000 cells), and (C) Basal Respiration (pmol/min/500,000 cells) in peripheral blood mononuclear cells. Peak knee extensor strength (Nm) plotted against (D) SRC, (E) Maximal Respiration, and (F) Basal Respiration in peripheral blood mononuclear cells. Grip strength (kg) plotted against (G) SRC, (H) Maximal Respiration, and (I) Basal Respiration in peripheral blood mononuclear cells.
SPPB, short physical performance battery; SRC, spare respiratory capacity.
N=15.
3.3. Relationship Between Strength and Blood Bioenergetics
Peak KE strength (Nm) was plotted against SRC, maximal respiration, and basal respiration in Figure 1D–F. These results are summarized in Table 2. Higher SRC and maximal respiration were associated with greater peak KE strength. Positive correlations between SRC and maximal respiration remained significant after independent adjustments for age, sex, and BMI (Table 2). The positive association between basal respiration and peak KE strength also reached statistical significance when adjusting for sex. Grip strength (kg) was plotted against SRC, maximal respiration, and basal respiration in Figure 1G–I. Higher SRC and maximal respiration were associated with greater grip strength. These associations remained significant after independent adjustments for age and BMI. When adjusting for sex, SRC remained significantly positively associated with grip strength and the correlation between maximal respiration and grip strength had a trend toward significance.
3.4. Relationships Between Muscle Mass and Quality and Blood Bioenergetics
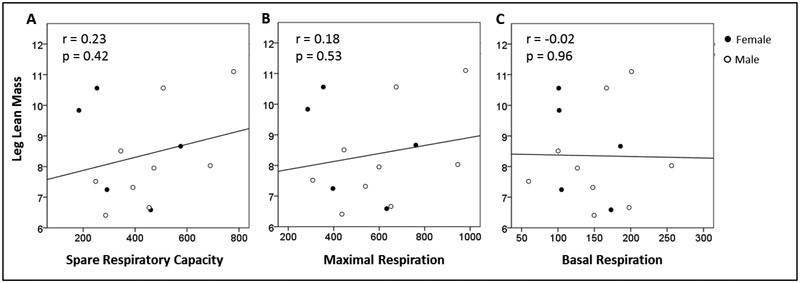
Leg lean mass (kg) was plotted against SRC, maximal respiration, and basal respiration in Figure 2A–C. These results are summarized in Table 2.
Figure 2:
Leg Lean Mass (Kg) plotted against: (A) SRC (Maximal Respiration minus Basal Respiration; pmol/min/500,000 cells), (B) Maximal Respiration (pmol/min/500,000 cells), and (C) Basal Respiration (pmol/min/500,000 cells) in peripheral mononuclear cells.
SRC, spare respiratory capacity.
N=14.
There were no significant correlations between leg lean mass and PBMC bioenergetic parameters. Muscle quality (Nm/kg) was plotted against SRC, maximal respiration, and basal respiration in Figure 3A–C. These results are summarized in Table 2 and show a significant positive association between muscle quality and SRC, as well as a trend for a significant association between muscle quality and maximal respiration. The significant association between muscle quality and SRC as well as the trend for significance between muscle quality and maximal respiration remained after independently adjusting for BMI. The associations were no longer statistically significant after adjusting for age and sex; however, a trend toward a statistically significant p-value remained.
Figure 3:
Muscle Quality (peak knee extensor strength divided by leg lean mass; Nm/kg) plotted against: (A) SRC (pmol/min/500,000 cells), (B) Maximal Respiration (pmol/min/500,000 cells), and (C) Basal Respiration (pmol/min/500,000 cells) in peripheral mononuclear cells.
SRC, spare respiratory capacity.
N=14.
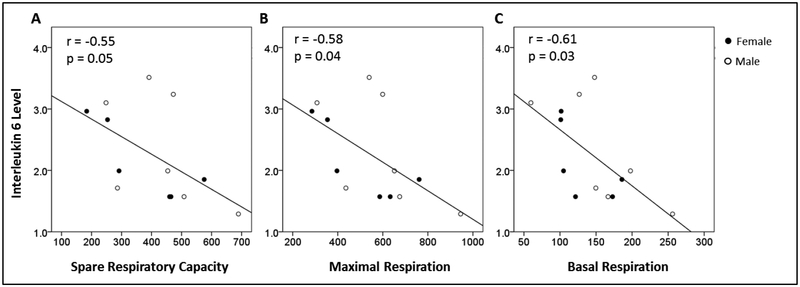
3.5. Relationship Between Interleukin 6 and Blood Bioenergetics
Plasma level of IL-6 was plotted against SRC, maximal respiration, and basal respiration in Figure 4A–C. These results are summarized in Table 2 and show a statistically significant negative correlation, indicating that greater levels of IL-6 were associated with reduced bioenergetic function. These associations remained statistically significant when independently adjusting for age and sex. When adjusted for BMI, only the association between basal respiration and plasma IL-6 remained significant; however the p-values for correlations between SRC and maximal respiration and IL-6 level had trends toward statistical significance. These results suggest a relationship between systemic inflammatory status and systemic mitochondrial bioenergetic function measured in peripheral blood cells.
Figure 4:
Interleukin-6 level (pg/mL) plotted against: (A) SRC (Maximal Respiration minus Basal Respiration; pmol/min/500,000 cells), (B) Maximal Respiration (pmol/min/500,000 cells), and (C) Basal Respiration (pmol/min/500,000 cells) in peripheral mononuclear cells.
SRC, spare respiratory capacity.
N=13.
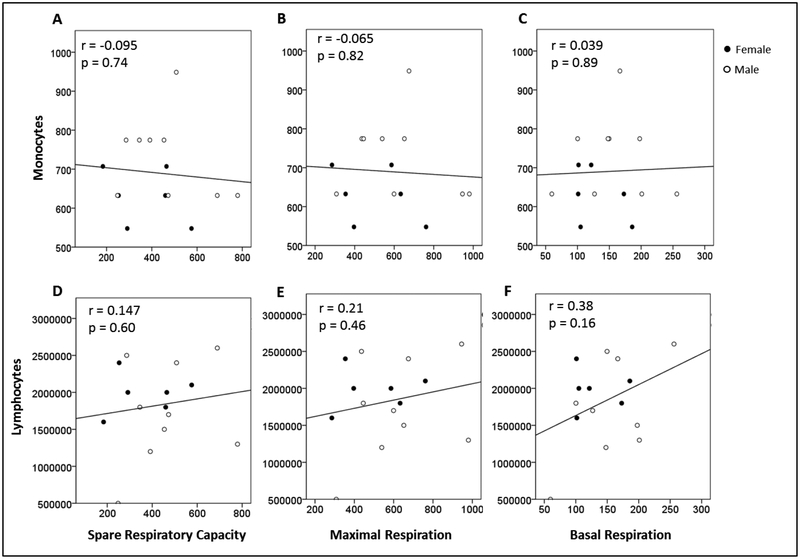
3.6. Relationships Between Blood-cell Counts and Blood Bioenergetics
Monocyte cell count (cells/mL whole blood) was plotted against SRC, maximal respiration, and basal respiration in Figure 5A–C, and lymphocyte cell count (cells/mL whole blood) was plotted against SRC, maximal respiration, and basal respiration in Figure 5D–F. Our results show no significant associations between monocyte or lymphocyte cell counts and SRC, maximal respiration, or basal respiration, indicating that the absolute number of lymphocytes or monocytes was not associated with bioenergetic function measured in PBMCs.
Figure 5:
Monocyte cell count (cells/mL blood; square root transformed) plotted against: (A) SRC (Maximal Respiration minus Basal Respiration; pmol/min/500,000 cells), (B) Maximal Respiration (pmol/min/500,000 cells), and (C) Basal Respiration (pmol/min/500,000 cells). Lymphocyte cell count (cells/mL blood) plotted against: (D) SRC (pmol/min/500,000 cells), (E) Maximal Respiration (pmol/min/500,000 cells), and (F) Basal Respiration (pmol/min/500,000 cells) in peripheral blood mononuclear cells.
SRC, spare respiratory capacity.
N=15.
4. DISCUSSION
The findings presented in this manuscript indicate that, in a cohort of well-functioning, overweight/obese community-dwelling older adults aged 65 to 78 years, without overt aging-related disease, higher maximal respiration and SRC of PBMCs were associated with better Ex-SPPB, greater peak KE strength, grip strength, greater leg muscle quality, and lower plasma IL-6 level. As a measure of bioenergetic capacity, SRC is indicative of the maximum amount of ATP that can be generated in response to increased energy demand above resting cellular energy requirements (Brand & Nicholls, 2011). Similar to the age-dependent decline in physical function and muscular strength, SRC declines with aging in multiple tissues including neurons (Nicholls, 2002), cardiac (Gong et al., 2003; Hill et al., 2009), and skeletal muscle (Kayo et al., 2001; Short et al., 2005; Hiona et al., 2010). An age-related decline in SRC is even evident in well-functioning, older adults who engage in exercise (Waters et al., 2003); however, exercise partially ablates this decline (Menshikova et al., 2006). Moreover, in our study, bioenergetic capacity was negatively correlated with circulating IL-6, an inflammatory cytokine that increases with age and is related to an array of geriatric syndromes and chronic diseases (Taaffe et al., 2000; Nicklas et al., 2008). Previous studies support functional relationships between parameters of mitochondrial bioenergetic capacity in blood cells with aerobic activities (Tyrrell et al., 2014); however, our data further indicate that the relationship of bioenergetic capacity with measures of peak muscle strength which are primarily anaerobic. Circulating inflammatory cytokines may be one link mediating relationships between blood-cell bioenergetics and peak muscle strength. Cytokines are known to damage mitochondrial DNA and protein as well as induce redox stress which may result in dysfunction of the more anaerobic phosphocreatine system in mitochondria as well as the ETC (Sweet et al., 2009; Zhou et al., 2011; Valerio et al., 2006). Exposure to higher levels of IL-6 was associated with reduced muscle function in a study of age-matched older adults (Bautmans et al., 2005), and IL-6 overexpression has been shown to regulate muscle protein breakdown (Tsujinaka et al., 1995). This would reduce both aerobic and anaerobic power, and cytokines may also cause diminished blood-cell bioenergetic capacity. Taken together, our results show a relationship between reduced PBMC mitochondrial function with reduced muscle strength, muscle quality, and overall physical function, with age-related chronic inflammation as a possible mediating factor for both physical and bioenergetic decline. Further studies are required to determine whether reduced blood cell bioenergetic capacity precedes age-dependent declines in muscular and physical function and whether or not they are a product of chronic inflammation.
We and others have proposed that measures of mitochondrial function in circulating cells may provide a biomarker of systemic mitochondrial health that may be related to numerous disorders linked to mitochondrial dysfunction (Tyrrell et al., 2014; Ravi et al., 2014; Chacko et al., 2014). We propose that bioenergetic decline associated with age-related disorders may be diagnosed using blood cell reporters. Indeed, aging-related diseases such as atherosclerosis and diabetes also present with components of mitochondrial dysfunction which can be detected in skeletal muscle (Larsen et al., 2011) as well as peripheral blood cells (Japiassu et al., 2011; Hartman et al., 2014; Avila et al., 2012). Here we extend these observations by providing evidence that phenotypic profiling of PBMCs is associated with key physical markers of aging, including reduced physical function, muscle strength and quality, and higher inflammatory status.
Bioenergetic capacity across multiple tissues declines with age (Short et al., 2005; Hansford, 1983). Although it is unknown how mitochondrial function or content are altered in peripheral blood cells with age, transcriptomic studies in human monocytes show a marked downregulation of mitochondrial electron transport chain (ETC) gene expression with increasing age as well as distinct changes in DNA methylation patterns (Reynolds et al., 2014; Reynolds et al., 2015). These data suggest that systemic cellular changes associated with aging, including bioenergetic decline, is evident in blood cells. Further support for this idea comes from a recent publication from our group that demonstrated an association between PBMC bioenergetic capacity with 400m gait speed (Tyrrell et al., 2014). Gait speed is a predictor of morbidity and mortality among older adults (Studenski et al., 2011). It is hypothesized that this prognostic ability stems from the integration of multiple physiological systems that contribute to gait speed. In this manuscript, we focus on specific measures that can directly contribute to gait speed including lower body physical function, muscle strength, and muscle quality; and measures that may indirectly contribute by impacting muscle function, such as inflammation. Importantly, the statistically significant partial correlations between SRC and maximal respiration with Ex-SPPB, and both KE and grip strength show these associations are independent of age, sex, and BMI. Our data indicate that PBMC bioenergetic function may be more indicative of muscle function and strength than overall muscle mass because associations with muscle mass were weak in comparison to muscle strength and quality.
Previously, it was not known if the heterogeneous composition of PBMCs influenced the bioenergetic profiles of these cells. The major cell types represented in PBMCs, lymphocytes and monocytes, have unique and distinct bioenergetic profiles (Kramer et al., 2014). Our results suggest that, although there is heterogeneity in the composition of PBMCs, this variability is not correlated with the basal, maximal, and spare respiratory capacity of PBMCs as a whole. This suggests that the cellular composition of PBMCs does not impact the associations with measures of physical function, muscle strength, or muscle quality. Nevertheless, because specific PBMC types go on to mediate specific stress signals and responses, future studies should investigate whether stronger correlations with measures of physical function or strength might be found if homogenous populations of cells were profiled.
It is hypothesized that mitochondria may provide a robust indicator of bioenergetic crisis because they are sensitive to redox status (Bonnard et al., 2008), acute and chronic inflammation (Sun et al., 2014; Quoilin et al., 2014; Zapelini et al., 2008), and metabolic stress (Russell et al., 2002; Widlansky et al., 2010; Schulz et al., 2007), which are common features among many of the complex, chronic diseases associated with aging (Chacko et al., 2014; Kramer et al., 2014). Peripheral blood cells may recapitulate bioenergetic signatures found in skeletal muscle and other vascular tissues. Indeed, a number of papers have reported that skeletal muscle mitochondrial metabolism is related to aging and physical function decline (Tyrrell et al., 2014; Coen et al., 2013; Joseph et al., 2012). Stress signals may be released from cells in affected tissues, and adjuncts or metabolites of those signals can be secreted into blood where they exert their effect on peripheral blood-cell mitochondria (Ravi et al., 2014; Kramer et al., 2014). Alternatively, systemic circulating stress signals known to affect various tissues may simultaneously exert detrimental effects on blood-cell bioenergetics, as is likely the case with hyperglycemia and insulin resistance (Rolo & Palmeira, 2006; Widlansky et al., 2010). Similarly, our data shows that SRC, maximal respiration, and basal respiration in peripheral blood cells negatively correlates with plasma IL-6 level, independently of age, sex, and BMI. In either scenario, blood-based bioenergetic profiling may be able to report on systemic mitochondrial changes related to disease progression or functional status.
This cross-sectional analysis revealed associations between peripheral blood-cell bioenergetics with physical function, strength, muscle quality, and inflammation in well-functioning, overweight/obese community-dwelling older adults. To date, no single assessment provides a reliable readout of systemic age-related bioenergetic decline. Multiple lines of evidence suggest that systemic mitochondrial health, assessed using readily obtainable blood cells may provide this index. As we further understand the role of mitochondria in aging, this assay may provide a powerful and objective diagnostic and prognostic tool to detect and track bioenergetic health with age, which can be implemented in large-scale clinical trials, and potentially, geriatric practice.
Highlights:
This study reports on respirometric profiling of peripheral blood mononuclear cells (PBMCs) from overweight/obese community dwelling older adults.
PBMC mitochondrial function is positively correlated with physical function reported by the expanded short physical performance battery score.
PBMC mitochondrial function is positively correlated with muscle strength and quality.
PBMC mitochondrial function is negatively correlated with inflammation reported by plasma interleukin-6 concentration.
ACKNOWLEDGMENTS
We thank the men and women who volunteered for this study as well as the research staff who conducted the recruitment and assessments.
Funding Source: This work was supported by National Institutes of Health grants 5R01-AG020583 and 3R01-AG020583–09S1, and the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Funding Sources: 5R01-AG020583 and 3R01-AG020583–09S1, and the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332)
Abbreviations:
- ATP
adenosine triphosphate
- BMI
body mass index
- DXA
dual energy X-ray absorptiometry
- ELISA
enzyme-linked immunosorbent assay
- ETC
electron transport chain
- Ex-SPPB
expanded short physical performance battery
- FCCP
Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone
- IL-6
interleukin 6
- KE
knee extensor
- Nm
Newton meters
- OCR
oxygen consumption rate
- PBMC
peripheral blood mononuclear cell
- SRC
spare respiratory capacity
- XF
extracellular flux
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY et al. (2012). Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp.Clin.Endocrinol.Diabetes, 120, 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautmans I, Njemini R, Lambert M, Demanet C, & Mets T (2005). Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J.Gerontol.A Biol.Sci.Med.Sci, 60, 361–367. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Hsu FC, Isom S, Kritchevsky SB, Church T, Goodpaster B et al. (2010). Long-term physical activity and inflammatory biomarkers in older adults. Med.Sci.Sports Exerc, 42, 2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B et al. (2008). Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J.Clin.Invest, 118, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD & Nicholls DG (2011). Assessing mitochondrial dysfunction in cells. The Biochemical Journal, 435, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ et al. (2009). Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP et al. (2014). The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin.Sci.(Lond), 127, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW et al. (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J.Gerontol.A Biol.Sci.Med.Sci, 68, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, & Juel RL (2012). Is There a Link between Mitochondrial Reserve Respiratory Capacity and Aging? J.Aging Res, 2012, 192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, & Beeson C (2008). Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov.Today, 13, 268–274. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di IA, Macchi C, Harris TB et al. (2000). Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J.Am.Geriatr.Soc, 48, 1618–1625. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD et al. (2005). The origins of age-related proinflammatory state. Blood, 105, 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N, Sansoni P, Girasole G, Vescovini R, Passeri G, Passeri M et al. (2001). Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp.Gerontol, 36, 547–557. [DOI] [PubMed] [Google Scholar]
- Gong G, Liu J, Liang P, Guo T, Hu Q, Ochiai K et al. (2003). Oxidative capacity in failing hearts. Am.J.Physiol Heart Circ.Physiol, 285, H541–H548. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, & Wallace RB (1995). Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N.Engl.J.Med, 332, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford RG (1983). Bioenergetics in aging. Biochim.Biophys.Acta, 726, 41–80. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Shirihai OS, Holbrook M, Xu G, Kocherla M, Shah A et al. (2014). Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc.Med, 19, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Dranka BP, Zou L, Chatham JC, & Darley-Usmar VM (2009). Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem.J, 424, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T et al. (2010). Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS.One., 5, e11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japiassu AM, Santiago AP, d’Avila JC, Garcia-Souza LF, Galina A, Castro Faria-Neto HC et al. (2011). Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5’-triphosphate synthase activity. Crit Care Med, 39, 1056–1063. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM et al. (2012). The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell, 11, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, & Greer DS (1983). Active life expectancy. N.Engl.J.Med, 309, 1218–1224. [DOI] [PubMed] [Google Scholar]
- Kayo T, Allison DB, Weindruch R, & Prolla TA (2001). Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc.Natl.Acad.Sci.U.S.A, 98, 5093–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, & Darley-Usmar VM (2014). Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J.Vis.Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PA, Ravi S, Chacko B, Johnson MS, & Darley-Usmar VM (2014). A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox.Biol, 2, 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Andersen JL, Madsbad S et al. (2011). Increased mitochondrial substrate sensitivity in skeletal muscle of patients with type 2 diabetes. Diabetologia, 54, 1427–1436. [DOI] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, & Goodpaster BH (2006). Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J.Gerontol.A Biol.Sci.Med.Sci, 61, 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG (2002). Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int.J.Biochem.Cell Biol, 34, 1372–1381. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB et al. (2008). Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J.Am.Geriatr.Soc, 56, 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Mouithys-Mickalad A, Lecart S, Fontaine-Aupart MP, & Hoebeke M (2014). Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim.Biophys.Acta, 1837, 1790–1800. [DOI] [PubMed] [Google Scholar]
- Ravi S, Mitchell T, Kramer PA, Chacko B, & Darley-Usmar VM (2014). Mitochondria in monocytes and macrophages-implications for translational and basic research. Int.J.Biochem.Cell Biol, 53C, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Ding J, Taylor JR, Lohman K, Soranzo N, de la Fuente A et al. (2015). Transcriptomic profiles of aging in purified human immune cells. BMC.Genomics, 16, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Taylor JR, Ding J, Lohman K, Johnson C, Siscovick D et al. (2014). Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat.Commun, 5, 5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo AP & Palmeira CM (2006). Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol.Appl.Pharmacol, 212, 167–178. [DOI] [PubMed] [Google Scholar]
- Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A et al. (2002). High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J, 16, 1738–1748. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, & Kauppinen A (2012). Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany.NY), 4, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, & Ristow M (2007). Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab, 6, 280–293. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S et al. (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proc.Natl.Acad.Sci.U.S.A, 102, 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM et al. (2001). Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J.Gerontol.A Biol.Sci.Med.Sci, 56, M644–M649. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M et al. (2011). Gait speed and survival in older adults. JAMA, 305, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Zheng Y, Lu Z, Wang H, Feng Z, Wang J et al. (2014). LL-37 attenuates inflammatory impairment via mTOR signaling-dependent mitochondrial protection. Int.J.Biochem.Cell Biol, 54, 26–35. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, & Kim F (2009). Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia, 52, 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, & Seeman TE (2000). Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J.Gerontol.A Biol.Sci.Med.Sci, 55, M709–M715. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A et al. (1995). Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem.Biophys.Res.Commun, 207, 168–174. [DOI] [PubMed] [Google Scholar]
- Tyrrell DJ, Bharadwaj MS, Van Horn CS, Kritchevsky SB, Nicklas BJ, & Molina AJA (2014). Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. J.Gerontol.A Biol.Sci.Med.Sci, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A et al. (2006). TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J.Clin.Invest, 116, 2791–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters DL, Brooks WM, Qualls CR, & Baumgartner RN (2003). Skeletal muscle mitochondrial function and lean body mass in healthy exercising elderly. Mech.Ageing Dev, 124, 301–309. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ et al. (2010). Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl.Res, 156, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapelini PH, Rezin GT, Cardoso MR, Ritter C, Klamt F, Moreira JC et al. (2008). Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion, 8, 211–218. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, & Tschopp J (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature, 469, 221–225. [DOI] [PubMed] [Google Scholar]