Abstract
IL-15 is a critical cytokine for the maintenance of memory-phenotype CD8 cells in mice. Here, we investigated the role of IL-15 in the neurological disease termed human T cell lymphotropic virus I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The high number of viral-specific CD8 cells in these patients is associated with inflammatory responses in the central nervous system. Because IL-15 is overexpressed in these patients, we asked whether IL-15 contributes to the persistence of human T cell lymphotropic virus I viral-specific CD8 cells. Using ex vivo cultures of HAM/TSP peripheral blood mononuclear cells, we demonstrated that in the majority of patients examined here blocking IL-15 action resulted in a decrease in the number of viral-specific CD8 cells. This decrease was caused by both inhibition of proliferation and induction of apoptosis in these cells. The data indicate that IL-15 plays a major role in the maintenance of viral-specific CD8 cells in HAM/TSP.
Keywords: antigen-specific T lymphocytes
One of the hallmarks of the chronic neurological disease human T cell lymphotropic virus I (HTLV-I)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is the presence of an activated immune system (1, 2). This phenomenon is best demonstrated by the ability of patient T cells to spontaneously proliferate in an ex vivo culture (3, 4). This proliferation is attributed to two independent autocrine loops involving the IL-2/IL-2 receptor (IL-2R) and IL-15/IL-15 receptor (IL-15R) (5, 6). The production of these cytokines and their receptors has been shown to be up-regulated by an HTLV-I encoded protein named Tax (6–10). In addition to being a strong transactivator of viral and cellular genes, HTLV-I Tax is the dominant target antigen recognized by HTLV-I-specific cytotoxic T lymphocytes (CTLs) in most infected individuals (11, 12). It was shown previously that in the majority of HAM/TSP patients there is a high frequency of Tax-specific T lymphocytes, which are present in their peripheral blood and cerebrospinal fluid (11, 13, 14). Furthermore, it was demonstrated that these cells consist mainly of memory cells (more than 50%) as determined by phenotypic analysis (15). When these T cells encounter the HTLV-I-infected cells in the central nervous system and perform cytotoxic activity, they secret a number of proinflammatory cytokines such as tumor necrosis factor α and IFN-γ (16, 17). The localized accumulation of such cytokines may cause inflammation in the central nervous system and contribute to the disease progression (2, 18, 19).
It is not clear what factor(s) is responsible for the maintenance of such a high frequency of Tax-specific CTLs. Antigen stimulation theoretically could provide an adequate signal for the persistence of antigen-specific T lymphocytes. However, it has become clear recently that the persistence of the antigen-specific memory phenotype CD8 cells does not depend on the constant stimulation by antigen; cytokines can substitute for antigenic stimulation (20–23). There have been a number of reports on the critical role of IL-15 in the preferential stimulation of memory-phenotype CD8 cells (24–26). Both IL-15 and IL-15Rα knockout animals have impaired memory CD8 cells (27, 28). In contrast, mice carrying a transgene for IL-15 exhibit elevated levels of memory phenotype CD8 cells (29). Because IL-15 is overexpressed in HAM/TSP patients (5), we questioned whether it plays a role in the persistence of antigen-specific CD8 cells. If IL-15 is a factor that facilitates the long-term survival of these cells, it could contribute to the disease progression.
In this study, we demonstrated that addition of antibodies that block the action of IL-15 to ex vivo cultures of HAM/TSP peripheral blood mononuclear cells (PBMC) decreased the number of Tax-specific CD8 cells in the majority of patients studied. Furthermore, the cytotoxic activity of Tax-specific CD8 cells was reduced when the ex vivo cells were deprived of IL-15. These data are of importance because they show that in humans IL-15 is an important factor in the persistence of functional antigen-specific CD8 cells.
Methods
Ex Vivo Culture of HAM/TSP PBMC and Proliferation Assay.
The HAM/TSP patients studied here were determined to have HLA-A*0201 allele. The culture condition and proliferation assays using PBMC from HAM/TSP patients have been described (6).
Antibodies.
The antibodies used in this study include: UPC10, which is a control murine IgG2a Ig (Sigma), anti-IL-2 antibody (a neutralizing monoclonal anti-IL-2 antibody, R & D Systems), a neutralizing anti-IL-15 antibody (R & D Systems), an IL-2-blocking anti-IL-2Rα antibody, anti-Tac (Metabolism Branch, National Cancer Institute, National Institutes of Health) (30), or an anti-IL-2/15Rβ antibody, Mikβ1, which blocks IL-15 but not IL-2 action on T cells (a gift from Mitsuru Tsudo, Tokyo Metropolitan Institute of Medical Sciences) (31). The antibodies used in FACS analysis were anti-Ki-67-FITC, anti-CD122, anti-CD123 antibodies (PharMingen), anti-CD4-FITC, anti-CD8-TriColor, anti-CD25-FITC antibodies (Caltag, Burlingame, CA), and anti-IL-15Rα antibody (R & D Systems). Annexin V was purchased from PharMingen.
Tetramers.
Analysis of antigen-specific CD8 cells was performed by using a phycoerythrin-conjugated HTLV-I Tax11–19 peptide (LLFGYPVYV) or HIV Gag77–85 peptide (SLYNTVATL)-loaded HLA-A*0201 tetramer (provided by National Institute of Allergy and Infectious Diseases MHC Tetramer Core Facility, Atlanta, and National Institutes of Health AIDS Research and Reference Reagent Program). Cytomegalovirus (CMV)-pp65 495–503 peptide (NLVPMVATV)-loaded HLA-A*0201 tetramer (kindly provided by Mats Engstrand, Uppsala University, Uppsala, Sweden) was also used.
CTL Assay.
The PBMC from HAM/TSP patients cultured for 6 days were used for the CTL assay. The CTL assay was performed by using Europium as described (32). The cultured PBMC (from the 6-day culture) were incubated with targets at varying effector-to-target ratios for 3 h. The target cells were the Hmy2.CIR cell line transfected with HLA-A2 genes (33). These cells were pulsed either with 100 nM of HIV-Gag77–85 peptide or HTLV-I Tax11–19 peptide (New England Peptide, Fitchburg, MA). The specific lysis was calculated as (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. The assay was performed in triplicate.
Results
Spontaneous Proliferation of ex Vivo PBMC of HAM/TSP Patients and Detection of Tax-Specific CD8 Cells.
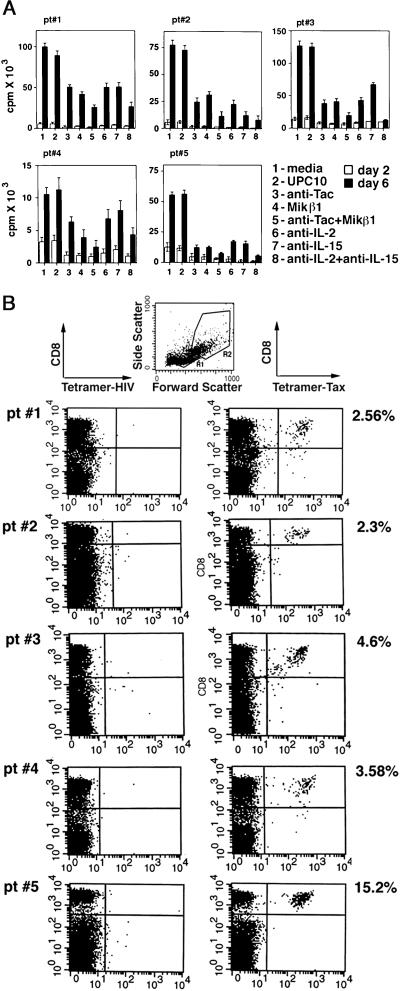
The goal of this study was to examine the impact of IL-15 on the survival of the Tax-specific CD8 cells in the ex vivo culture of HAM/TSP PBMC that undergo spontaneous proliferation. Therefore, we first examined selected HAM/TSP patients' PBMC for their IL-2- and IL-15-dependent spontaneous proliferation (Fig. 1A). The proliferation of all of the HAM/TSP PBMC was inhibited when cells were cultured with antibodies that blocked the actions of IL-2 (anti-IL-2 or anti-Tac that targets IL-2R α chain) or those of IL-15 (anti-IL-15 or MiKβ1 that recognizes the IL-2/15R β chain). Addition of a combination of antibodies against IL-2 or IL-15 markedly inhibited the spontaneous proliferation.
Figure 1.
(A) Proliferation assay of ex vivo cultures of five HAM/TSP PBMC. The PBMC from five HAM/TSP patients (pt#) were cultured for 2 and 6 days with media alone or with addition of 5 μg/ml of various antibodies as indicated. Each assay was performed in triplicate. The average cpm from each of the patients was plotted with standard errors shown for each entry. (B) Tetramer staining of PBMC of five HAM/TSP patients (pt#) at day 0. The uncultured PBMC (day 0) were used in a FACS analysis using tetramer-Tax or tetramer-HIV and an antibody against the CD8 molecule. The cells were analyzed in FACS analysis in gates R1 and R2. At day 0, the majority (>95%) of CD8 cells were in R1. The percentage of cells in gated area R1 + R2, which reacted with tetramer-Tax and anti-CD8 antibody, was considered as Tax-specific CD8 cells. These numbers are indicated on the right as the percentage of the Tax-specific CD8 cells relative to the total CD8 cells.
Next, we identified the Tax-specific CD8 cells in the HAM/TSP PBMC by using tetramer technology (34). Two types of tetramers were used in these experiments: tetramer-HIV Gag specific for the Gag peptide 77–85 (hereafter referred to as tetramer-HIV) as a negative control and tetramer-Tax specific for Tax peptide 11–19. The PBMC obtained from these patients (which were determined to be HLA-A2) were stained with fluorochrome-conjugated tetramer-Tax or tetramer-HIV and an antibody against the CD8 molecule. The cells were analyzed subsequently by FACS analysis in the gated area R1 that contained the majority of lymphocytes (Fig. 1B). The frequency of Tax-specific CD8 cells for each patient at day 0, before culture, is indicated in Fig. 1B as the percentage of the Tax-specific CD8 cells relative to the total number of CD8 cells. No staining was observed with tetramer-Tax when five HLA-A2 normal donor PBMC were analyzed in similar experiments (data not shown).
Antibodies That Inhibit the Action of IL-15 Target Tax-Specific CD8+ Cells.
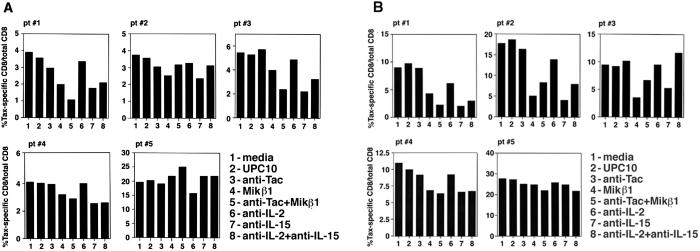
Next, we questioned whether addition of antibodies that block the action of IL-2 or IL-15 can affect the number of Tax-specific CD8 cells. After 2 and 6 days in culture conditions similar to those described above, the PBMC were stained with an anti-CD8 antibody and tetramer-Tax or tetramer-HIV and examined subsequently by FACS analysis in gate 1 (R1) and gate 2 (R2) (Fig. 2). The number of the Tax-specific CD8 cells reported in Fig. 2 was calculated as the percentage of the Tax-specific CD8 cells (the sum of these cells in R1 and R2) relative to the total number of CD8 cells (the sum of these cells in R1 and R2). In all of the patients, the percentage of the Tax-specific CD8 cells increased, in particular, by day 6 when the PBMC were cultured with media or UPC10 control antibody (compare the values in Fig. 1B for each patient to those shown in Fig. 2). Addition of antibodies that blocked the action of IL-2, namely anti-IL-2 or anti-Tac, did not alter the percentage of the Tax-specific CD8 cells. However, when antibodies that inhibited the action of IL-15, namely MiKβ1 and anti-IL-15, were added to the culture media the percentage of the Tax-specific CD8 cells in four of five patients was reduced, an observation that was more prominent at day 6. In case of patient 5, neither anti-IL-15 nor MiKβ1 influenced the percentage of Tax-specific CD8 cells. Addition of the combination of antibodies against IL-2 or IL-15 or their receptors had a mixed impact on this number (see Discussion).
Figure 2.
The tetramer staining of HAM/TSP PBMC cultured with various antibodies. The PBMC from five HAM/TSP patients (pt#) were cultured for 2 (A) or 6 (B) days. The cells were stained with tetramer-Tax or tetramer-HIV and anti-CD8 antibody and analyzed by FACS. The CD8 cells proliferated and were detected in both R1 and R2. The percentage of the Tax-specific CD8 cells is reported as the percentage of the total number of Tax-specific CD8 cells in gates R1 + R2 relative to the total number of CD8 cells in gates R1 + R2.
IL-15 Is Important in the Survival of Tax-Specific CD8 Cells in ex Vivo Culture of HAM/TSP PBMC.
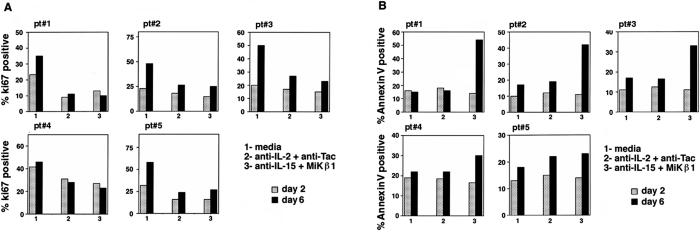
Depriving the culture of endogenously made IL-15 might have had two different impacts on the fate of the Tax-specific CD8 cells: it either inhibited the proliferation of Tax-specific CD8 cells or promoted the death process in these cells. To examine each possibility, the PBMC obtained from the same five HAM/TSP patients were cultured in media alone, anti-IL-2 and anti-Tac, or anti-IL-15 and MiKβ1 for 2 or 6 days. A combination of antibodies against the cytokine and its receptor was added to completely block the actions of the respective cytokine. The cells were collected and stained with tetramer-Tax, anti-CD8 antibody, and either Ki-67 or Annexin V [Ki-67 as an indicator of proliferation (35) and Annexin V as a marker for cell death]. In each assay, the Tax-specific CD8 cells that reacted with tetramer-Tax were gated and the percentage of this population that was positive for Ki-67 or Annexin V was determined. The data are presented in Fig. 3. The percentages of the proliferating Tax-specific CD8 cells in all of the patients dropped when cells were cultured with anti-IL-2 or anti-IL-15 antibodies in particular when assayed at day 6 (Fig. 3A). However, it was only after culturing the PBMC with anti-IL-15 antibodies for 6 days that a percentage of the Tax-specific CD8 cells stained positively for Annexin V (Fig. 3B). The exception was patient 5 in whom the Annexin V value did not change when antibodies against IL-15 and its receptor were added. Together, these data suggest that the endogenously made IL-2 and IL-15 play major roles in proliferation of Tax-specific CD8 cells. However, only blockade of IL-15 action, but not that of IL-2, resulted in induction of death in Tax-specific CD8 cells. This finding suggests that IL-15 is contributing to the survival of Tax-specific CD8 cells in the majority of HAM/TSP patients.
Figure 3.
The Ki-67 and Annexin V staining of HAM/TSP PBMC after 2- and 6-day cultures. The PBMC from HAM/TSP patients (pt#) were cultured for 2 or 6 days with media alone, anti-IL-2 + anti-Tac, or anti-IL-15 + Mikβ1. The cells were collected and stained with tetramer-Tax, anti-CD8 antibody, and either Ki-67 or Annexin V. The Tax-specific CD8 cells were gated and analyzed for Annexin V or Ki-67 staining. (A) The Ki-67 staining of Tax-specific CD8 cells in day 2 and day 6 is reported as the percentage of the Tax-specific CD8 cells that were positive for Ki-67 relative to the total Tax-specific CD8 cells. (B) The Annexin V staining of Tax-specific CD8 cells in day 2 and day 6 is reported as the percentage of the Tax-specific CD8 cells that were positive for Annexin V staining at day 2 or day 6 relative to the total number of Tax-specific CD8 cells.
Cytotoxic Activity of Tax-Specific CD8 Cells from Selected HAM/TSP Patients PBMC Decreased When IL-15 Action Was Inhibited.
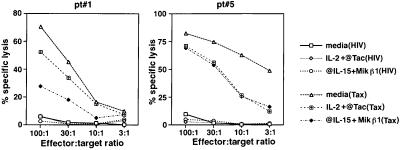
Next, we examined whether IL-15 has any impact on the cytotoxic activity of these cells. The Tax-specific CD8 cells circulating in the PBMC obtained from HAM/TSP patients are capable of killing HTLV-I-infected cells in an HLA class I-restricted manner without further stimulation by exogenously added antigen (11, 36, 37). The PBMC obtained from two HAM/TSP patients (patients 1 and 5 as representatives) were cultured for 6 days with media alone, anti-IL-2 and anti-Tac, or anti-IL-15 and MiKβ1 and were used subsequently in a CTL assay with a progressive reduction in effector-to-target-cell ratio (Fig. 4). The target cells were HLA-A2-restricted cells pulsed either with HIV-Gag (for nonspecific lysis) or HTLV-I Tax peptides. Both patient 1 and patient 5 showed strong CTL activity against their HTLV-Tax-loaded target cells and not against HIV-Gag-loaded cells. However, it was only in patient 1 that a significant reduction in CTL activity (more than 70%) was observed when effector cells from anti-IL-15/Mikβ1-added cultures were used. In this patient, effector cells from the anti-IL-2/anti-Tac culture showed only about 20% reduction in their CTL activity. In contrast to patient 1, in the case of patient 5, effector cells from either anti-IL-15/Mikβ1 or anti-IL-2/anti-Tac-added cultures showed similar cytotoxic activities against their Tax-loaded target cells. This finding indicates that in patient 5 IL-15 does not have a preferential effect on Tax-specific CD8 cells. These data correlate with those obtained from the tetramer staining experiments that were shown in Fig. 2 and demonstrate that IL-15 is an important factor in the maintenance of both the number and function of Tax-specific CD8 cells in the majority of HAM/TSP patients.
Figure 4.
The CTL activity of the PBMC from HAM/TSP patients (pt#) cultured for 6 days. The cultured PBMC from two HAM/TSP patients were incubated with target cells at the various effector-to-target ratios indicated. The target cells were the Hmy2.CIR cell line transfected with HLA-A2 genes and pulsed either with 100 nM HIV-Gag77–85 peptide or HTLV-I Tax11–19 peptide. The description on the right indicates the culture condition of patient PBMC and the HIV or Tax target peptide, which are shown in parentheses. The specific lysis was calculated as (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. The assay was performed in triplicate.
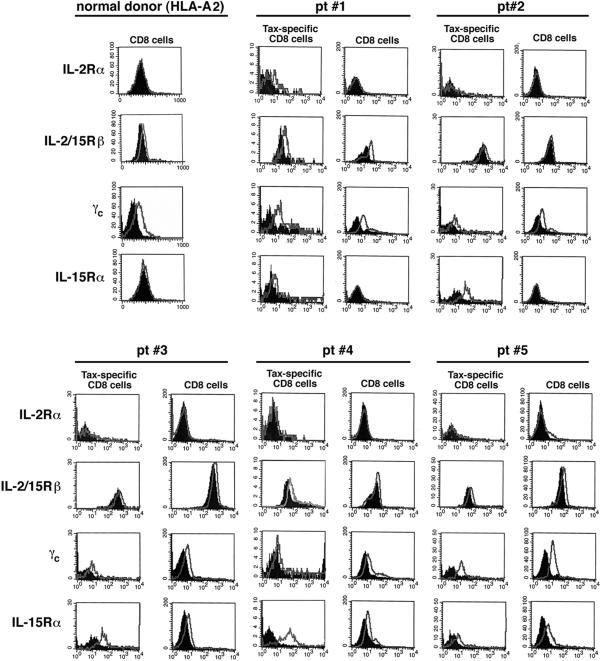
IL-15R Subunits Are Expressed on the Tax-Specific CD8 Cells.
One question that emerged from this study is why not all of the patients examined showed a decline in the proportion of the Tax-specific CD8 cells when IL-15 actions were blocked. We considered the possibility that this may be caused by a differential expression of IL-15Rs on the Tax-specific CD8 cells vs. total CD8 cells. To test this hypothesis, we examined both Tax-specific and total CD8 cell populations for their expression of IL-15R and IL-2R subunits. IL-15 and IL-2 share two receptor subunits namely CD122 (β chain) and CD132 (γc chain). In addition, each of the cytokines has its own private α chain, namely IL-15Rα and CD25 (IL-2Rα) (38). The PBMC obtained from these five HAM/TSP patients were stained with a tetramer-Tax, antibody against CD8, as well as with antibodies against different chains of the IL-15R and IL-2R systems or with an isotype control. In addition to five HAM/TSP patients, the PBMC from five HLA-A2 normal donors were stained with antibodies against CD8 and each receptor subunit. The cells subsequently were subjected to flow cytometric analysis. Two populations were examined for expression of IL-2R and IL-15R: the Tax-specific CD8 cells and total CD8 cells (Fig. 5). The CD8 cell staining data from one normal donor are shown as a representative. The Tax-specific CD8 and total CD8 cells from all of the HAM/TSP patients and normal donors expressed IL-2/15Rβ and γc chains (Fig. 5). At the sensitivity of FACS analysis, almost all of the other patient samples were negative for IL-2Rα expression on their Tax-specific CD8 or total CD8 cells. Expression of IL-15Rα was variable among patient samples. In the cases of patients 1 and 2, IL-15Rα was exclusively expressed on the Tax-specific CD8 cells. The expression of this receptor chain in the cases of patients 3 and 4 was higher in Tax-specific CD8 cells than that in total CD8 cells as determined by the magnitude of the shift out of background. In the case of patient 5, both Tax-specific CD8 and total CD8 cells expressed IL-15Rα to a similar extent. The distribution of the IL-15R complex may explain the variation in response among the patients when antibodies against IL-15 were added (see Discussion).
Figure 5.
Analysis of the IL-2 and IL-15R complex in the Tax-specific CD8 cells and total CD8 cells. Uncultured PBMC (day 0) of five normal donors and five HAM/TSP patients (pt#) studied here were stained with tetramer-Tax, an anti-CD8 antibody, and antibodies against different chains of the IL-2Rs or IL-15Rs (CD25 or IL-2Rα, CD122 or IL-2/15Rβ, CD132 or γc, and IL-15Rα), and an appropriate isotype control antibody. The Tax-specific CD8 cells were gated, and expression of each receptor subunit by these cells was examined. In addition, the expression of IL-2Rs and IL-15Rs by total CD8 cells was analyzed. The data presented for the normal donor are representative of five different normal donors examined.
The Effect of IL-15 on CMV-Specific CD8 Cells.
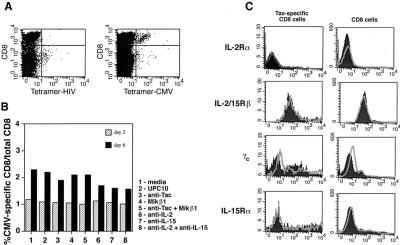
The next question was whether the IL-15 effect on Tax-specific CD8 cells was restricted to these cells or was a universal phenomenon impacting every kind of antigen-specific CD8 cell regardless of its antigenic target. This question prompted us to study the CMV-specific CD8 cells in experiments similar to those performed above. Among the five patients studied, only patient 2 had detectable levels of CMV-specific CD8 cells, which were about 1.3% of the total CD8 cells (Fig. 6A). Therefore, an experiment was set up in which PBMC from patient 2 were cultured for 2 and 6 days in the absence or presence of antibodies that blocked IL-2 or IL-15 actions. The CMV-specific CD8 cell population was monitored by using tetramer-CMV or tetramer-HIV as a negative control. The data presented in Fig. 6B demonstrate the percentage of the CMV-specific CD8 cells relative to the total number of CD8 cells at 2 and 6 days of culture at various conditions. As indicated in Fig. 6, the percentage of CMV-specific CD8 cells increased only modestly after 6 days in culture as compared with the previously discussed increase in the percentage of Tax-specific CD8 cells (Fig. 2). Addition of IL-15 blocking antibodies (anti-IL-15 or MiKβ1) to the culture media did not lower the percentage of CMV-specific CD8 cells meaningfully when compared with media alone nor was there an effect of IL-2 blocking antibodies (anti-IL-2 or anti-Tac) or a combination of anti-IL-2 and anti-IL-15 antibodies on the proportion of CMV-specific CD8 cells.
Figure 6.
Staining of the PBMC obtained from patient 2 with tetramer-CMV. (A) The uncultured PBMC from patient 2 were stained with tetramer-CMV or tetramer-HIV as a negative control as well as with an antibody against CD8 cells. The specific reactivity of tetramer-CMV with a population of CD8 cells identified the CMV-specific CD8 cells. (B) The PBMC were cultured for 2 or 6 days with media alone or with the addition of the various antibodies as indicated. The cells were stained with tetramer-CMV or tetramer-HIV as a negative control and an antibody against CD8 cells. The data are presented as the percentage of the CMV-specific CD8 cells relative to the total number of CD8 cells in both R1 and R2 gates. (C) The uncultured PBMC were examined for expression of IL-2Rs and IL-15Rs by CMV-specific CD8 cells. The cells were stained with tetramer-CMV, an anti-CD8 antibody, and antibodies against different chains of the IL-2R or IL-15R or an appropriate isotype control antibody. Two populations of CMV-specific CD8 cells and total CD8 cells were gated, and the expression of IL-2R and IL-15R by these cells was examined.
Next, we examined the CMV-specific CD8 cells for expression of IL-15R and IL-2R. The uncultured PBMC from patient 2 was stained with antibodies against CD8 and each component of the IL-2R or IL-15R, and with either tetramer-CMV or tetramer-HIV. The cells were analyzed by FACS analysis. The data presented in Fig. 6C demonstrate the distribution of IL-2R and IL-15R components on CMV-specific CD8 cells and total CD8 cells. Comparison of these data with those of patient 2 in Fig. 5 reveals that unlike the Tax-specific CD8 cells, the CMV-specific CD8 cells do not express the IL-15Rα chain at levels detectable by this assay. However, both of these cell populations express CD122 (IL-2/15Rβ) and CD132 (γc) chains.
Discussion
In this study, we examined the PBMC in the ex vivo culture of five HAM/TSP patients with an HLA-A2 background to define the role of IL-15 in the persistence of Tax-specific CD8 cells. The fact that HAM/TSP PBMC undergo spontaneous proliferation has enabled us to avoid adding exogenous cytokines, growth factors, or antigen. Therefore, the ex vivo system used in this study presumably reflects the events that occur naturally in vivo in HAM/TSP patients. Using such a working model, we demonstrated that IL-15 plays a major role in the survival and function of Tax-specific CD8 cells in the majority of patients studied. The data suggest that IL-15 contributes to the survival of Tax-specific CD8 cells by supporting their proliferation and inhibiting their apoptosis. This observation is in accord with previous reports that IL-15 has an antiapoptotic effect (39, 40). Furthermore, it has been suggested that IL-2 and IL-15 may have opposing effects in maintaining the homeostasis of lymphocytes (41, 42). The observation that in some of the patients blocking IL-15 actions resulted in a decrease in the percentage of the Tax-specific CD8 cells, whereas blocking both IL-2 and IL-15 actions did not have such effect (Fig. 2), may reflect the contrasting roles of these two cytokines in determining the fate of the T cells.
As demonstrated here the survival of Tax-specific CD8 cells depended on IL-15 in the majority of the patients. This may be explained by the distribution pattern of IL-15Rs on their CD8 cells. The IL-15R system in T and NK cells consists of three chains: IL-15Rα, IL-2/15Rβ (CD122), and γc (CD132) (38). As demonstrated in this study, the β and γc chains were expressed on both Tax-specific CD8 and total CD8 cells. However, the IL-15Rα expression pattern was different. In four of five patients studied here, the expression of IL-15Rα was more abundant (patients 3 and 4) or was limited (patients 1 and 2) to the Tax-specific CD8 cells compared with its distribution on total CD8 cells. However, in the case of patient 5, both Tax-specific CD8 and total CD8 cells expressed IL-15Rα to a similar extent. The expression pattern of IL-15Rα by Tax-specific CD8 cells might have had an effect on the fate of these cells when cultured with blocking antibodies against IL-15. IL-15 binds to IL-15Rα with an extremely high affinity of 10−11 M (43). Therefore, cells that express IL-15Rα can compete successfully for IL-15 protein, which is not abundantly secreted because of numerous posttranscriptional impediments to its expression (44). The patients (nos. 1–4) who expressed IL-15Rα preferentially on their Tax-specific CD8 cells were those in whom the percentages of Tax-specific CD8 cells were reduced when blocking antibodies against IL-15 or its receptor were added to the culture. One explanation for the expression of IL-15Rα by Tax-specific CD8 cells may be the recent finding that these cells are preferentially infected with HTLV-I (45), which in turn can up-regulate transcription of the IL-15Rα gene (10).
Although IL-15 was shown to play a major role in the survival of Tax-specific CD8 cells, the CMV-specific CD8 cells examined in patient 2 were not affected by IL-15 blockade. Unlike Tax-specific CD8 cells that proliferated an average of 3- to 4-fold after 6 days in culture over that at day 0 the CMV-specific CD8 cells did not proliferate. Interestingly, in contrast to the Tax-specific CD8 cells, the CMV-specific CD8 cells did not express IL-15Rα, which may have prevented them from proliferating in response to endogenously made IL-15. Lack of proliferation, therefore, may have masked the impact of IL-15 withdrawal on the proportion of CMV-specific CD8 cells as related to the total CD8 cells. Overall, this study is of importance because it demonstrates that in HAM/TSP patients IL-15 plays a major role in survival of Tax-specific CD8 cells, as well as in their function as antigen-specific cytotoxic cells.
Abbreviations
- CMV
cytomegalovirus
- HTLV-I
human T cell lymphotropic virus I
- HAM
HTLV-I-associated myelopathy
- TSP
tropical spastic paraparesis
- PBMC
peripheral blood mononuclear cells
- CTL
cytotoxic T lymphocyte
- IL-2R
IL-2 receptor
- IL-15R
IL-15 receptor
References
- 1.Bangham C R. Curr Opin Immunol. 2000;12:397–402. doi: 10.1016/s0952-7915(00)00107-2. [DOI] [PubMed] [Google Scholar]
- 2.Kubota R, Osame M, Jacobson S. Retroviruses: Human T Cell Lymphotropic Virus Type I-Associated Diseases and Immune Dysfunction. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 349–379. [Google Scholar]
- 3.Ijichi S, Eiraku N, Osame M, Izumo S, Kubota R, Maruyama I, Matsumoto M, Niimura T, Sonada S. J Neuroimmunol. 1989;25:251–254. doi: 10.1016/0165-5728(89)90143-4. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson S, Zaninovic V, Mora C, Rodgers-Johnson P, Sheremata W A, Gibbs C J, Gajusek D C, McFarland H E. Ann Neurol. 1988;23:196–200. doi: 10.1002/ana.410230744. [DOI] [PubMed] [Google Scholar]
- 5.Azimi N, Jacobson S, Leist T, Waldmann T A. J Immunol. 1999;163:4064–4072. [PubMed] [Google Scholar]
- 6.Tendler C L, Greenberg S J, Blattner W A, Manns A, Murphy E, Fleisher T, Hanchard B, Morgan O, Burton J D, Nelson D L, Waldmann T A. Proc Natl Acad Sci USA. 1990;87:5218–5222. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azimi N, Brown K, Bamford R N, Tagaya Y, Siebenlist U, Waldmann T A. Proc Natl Acad Sci USA. 1998;95:2452–2457. doi: 10.1073/pnas.95.5.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross S L, Feinberg M B, Wolf J B, Holbrook N J, Wong-Staal F, Leonard W J. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 9.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. EMBO. 1986;5:2883–2889. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariner J M, Lantz V, Waldmann T A, Azimi N. J Immunol. 2001;166:2602–2609. doi: 10.4049/jimmunol.166.4.2602. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Nature (London) 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 12.Parker C E, Daenke S, Nightlingale S, Bangham C R. Virology. 1992;188:628–636. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson S, McFarlin D E, Robinson S, Voskuhl R, Martin R, Brewah A, Newell A J, Koenig S. Ann Neurol. 1992;32:651–657. doi: 10.1002/ana.410320508. [DOI] [PubMed] [Google Scholar]
- 14.Umehara F, Izumo S, Nakagawa M, Ronquillo A T, Takahashi K, Matsumuro K, Sato E, Osame M. Neuropathol Exp Neurol. 1993;52:4244–4249. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Nagai M, Kubota R, Greten T F, Schneck J P, Leist T P, Jacobson S. J Infect Dis. 2001;183:197–205. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- 16.Cowan E P, Alexander R K, Daniel S, Kashanchi F, Brady J N. J Virol. 1997;71:6982–6989. doi: 10.1128/jvi.71.9.6982-6989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroda Y, Matsui M. J Neuroimmunol. 1993;42:223–226. doi: 10.1016/0165-5728(93)90014-p. [DOI] [PubMed] [Google Scholar]
- 18.Ijichi S, Izumo S, Eiraku N. Med Hypotheses. 1993;41:542–547. doi: 10.1016/0306-9877(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 19.Hollsberg P, Hafler D A. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 20.Lau L L, Jamieson B D, Somasundaram R, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 21.Mullbacher A. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprent J, Surh C D. Curr Opin Immunol. 2001;13:248–254. doi: 10.1016/s0952-7915(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 23.Tough D F, Borrow P, Sprent J. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 24.Marks-Konczalik J, Dubois S, Losi J M, Sabzevari H, Yamada N, Feigenbaum L, Waldmann T A, Tagaya Y. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. . (First Published October 3, 2000; 10.1073/pnas.200363097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura H, Yajima T, Naiki Y, Tsunobuchi H, Umemura M, Itano K, Matsuguchi T, Suzuki M, Ohashi P S, Yoshikai Y. J Exp Med. 2000;191:157–170. doi: 10.1084/jem.191.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Siquan S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 29.Fehniger T A, Suzuki K, Ponnappan A, VanDeusen J B, Cooper M A, Florea S M, Freud A G, Robinson M L, Durbin J, Caligiuri M A. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchiyama T, Border S, Waldmann T A. J Immunol. 1981;126:1393–1397. [PubMed] [Google Scholar]
- 31.Tsudo M, Kitamura F, Miyasaka M. Proc Natl Acad Sci USA. 1989;86:1982–1986. doi: 10.1073/pnas.86.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blomberg K, Hautala R, Lovgren J, Mukkala V-M, Lindqvist C, Akerman K. J Immunol Methods. 1996;193:199–206. doi: 10.1016/0022-1759(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 33.Storkus W J, Alexander J, Payne J A, Dawson J R, Cresswell P. Proc Natl Acad Sci USA. 1989;86:2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altman J D, Moss P A, Goulder P J, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 35.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 36.Kannagi M, Matsushita S, Shida H, Harada S. Leukemia. 1994;8, Suppl. 1:S54–S59. [PubMed] [Google Scholar]
- 37.Koenig S, Woods R M, Brewah Y A, Newell A J, Jones G M, Boone E, Adelsberger J W, Baseler M W, Robinson S M, Jacobson S. J Immunol. 1993;156:3874–3879. [PubMed] [Google Scholar]
- 38.Waldmann T, Tagaya Y, Bamford R. Int Rev Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- 39.Dooms H, Desmedt M, Vancaeneghem S, Rottiers P, Goossens V, Fiers W, Grooten J. J Immunol. 1998;161:2141–2150. [PubMed] [Google Scholar]
- 40.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Nat Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 41.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrak P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 42.Waldmann T A, Dubois S, Tagaya Y. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 43.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D. EMBO J. 1995;14:3654–3661. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bamford R N, DeFilippis A P, Azimi N, Kurys G, Waldmann T A. J Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- 45.Hanon E, Stinchcombe J C, Saito M, Asquith B E, Taylor G P, Tanaka Y, Weber J N, Griffiths G M, Bangham C R. Immunity. 2000;13:657–664. doi: 10.1016/s1074-7613(00)00065-0. [DOI] [PubMed] [Google Scholar]