Abstract
To assess the contribution of the alternative pathway in complement activation and host defense and its possible role in the regulation of systemic energy balance in vivo, factor D-deficient mice were generated by gene targeting. The mutant mice have no apparent abnormality in development and their body weights are similar to those of factor D-sufficient littermates. Complement activation could not be initiated in the serum of deficient mice by the alternative pathway activators rabbit erythrocytes and zymosan. Surprisingly, injection of cobra venom factor (CVF) caused a profound and reproducible reduction in serum C3 levels, whereas, as expected, there was no C3 reduction in factor B-deficient mice treated similarly. Studies of C3 and factor B activation in vitro by CVF demonstrated that in factor D-deficient serum the α chain of C3 was cleaved gradually over a period of 60 min without detectable cleavage of factor B. CVF-dependent C3 cleavage in the deficient serum required the presence of Mg2+, whereas in normal mouse serum the presence of divalent cations was not required. These results suggest that in mouse proteolytic cleavage of factor B by factor D is not an absolute requirement for the zymogen to active enzyme conformational transition of CVF-bound factor B. Kinetics of opsonization of Streptococcus pneumoniae by C3 fragments was much slower in factor D-deficient serum, suggesting a significant contribution of the alternative pathway to antibacterial host defense early after infection.
The alternative pathway (AP) of complement activation is a self-amplifying mechanism important for pathogen recognition and elimination in the absence of specific antibodies. The AP also amplifies complement activation initiated by the other two pathways of complement activation, classical and lectin (1). The dual function (recognition and amplification) of the AP underlines its importance for host defense against pathogens. The proximal result of complement activation is the formation of convertases, enzymes that activate C3 and C5, generating biologically active protein fragments and complexes (1). In the AP, assembly of the C3/C5 convertase requires the initial attachment of the C3b fragment of C3 to the surface of a pathogen and proceeds through the formation of a complex with factor B (C3bB) and the subsequent cleavage of factor B by factor D to form C3bBb, the C3/C5 convertase of the AP.
Mouse factor D was initially recognized as a serine protease encoded by a differentiation-specific message present mainly in adipocytes and cells of the nervous system (2). Because its mRNA was significantly reduced in mouse and rat models of obesity, it was thought that the protein was involved in fat metabolism (3). These observations led to naming the protein adipsin, which soon was identified with mouse factor D (4). Subsequently it was demonstrated that adipocytes also synthesize and secrete C3 and factor B, leading to the formation of a C3 convertase in culture supernatants (5). Other results indicated that C3adesArg, the inactivated form of the complement anaphylatoxin C3a could act on adipocytes to regulate fatty acid metabolism (6, 7). Studies using C3-deficient mice failed to support a role of C3adesArg in lipid metabolism (8), but the possibility that factor D has a direct effect on adipocytes, independent of AP activation has never been ruled out. To evaluate the contribution of the AP in complement activation and host defense and its possible role in the fat metabolism, we targeted the factor D gene (Df) to generate factor D-deficient mice. The results indicate that factor D is indispensable for complement activation through the AP and that it plays a significant role in opsonization of bacteria by “natural” IgM antibody. It is also shown that factor D does not play a major role in fat metabolism. Interestingly, the data revealed a unique mode of factor B activation, which provides valuable insights into the mechanism of AP activation.
Materials and Methods
Generation of Factor D-Deficient Mice.
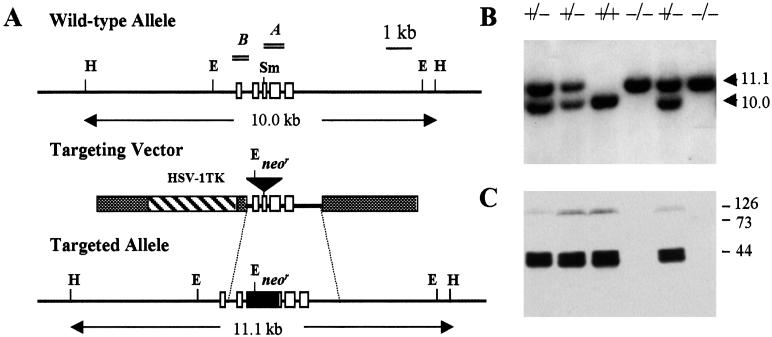
A gene targeting vector, Adn/TK, was constructed (9) on the backbone of pBluescript SK(+) by inserting a 1.1-kb neomycin-resistance (neor) gene cassette into a 2.6-kb Df genomic DNA fragment at a unique SmaI site within exon 3 (Fig. 1A). A 3.4-kb thymidine kinase (TK) gene fragment of herpes simplex virus 1 was included as a negative selection marker against random integration of the vector. Fifty micrograms of the linearized Adn/TK construct was transfected into embryonic day E14.1 embryonic stem (ES) cells by electroporation as described (10). Homologous recombinants were selected initially by culturing the transfected cells in complete medium containing 225 μg/ml G418. On day 5 of G418 selection, 2 μM gancyclovir was added to the media and double drug-resistant clones were selected for an additional 5 days. DNA from double drug-resistant ES clones was analyzed by Southern blotting to identify homologous recombinants.
Figure 1.
Targeted disruption of the murine Df. (A) Schematic presentation of the Df allele, the targeting vector, and the targeted Df allele after recombination. Open boxes denote exons and thick lines intron sequences. The 1.1-kb neor gene cassette and the TK gene from herpes simplex virus 1 (HSV-1) are shown. Restriction fragments and probes used for Southern blotting are indicated. Restriction enzyme cleavage sites: H, HindIII; E, EcoRI; Sm, SmaI. (B) Southern blot analysis of HindIII-restricted tail DNA from offspring of F1 Df+/− intercross. (C) Western blot analysis of serum factor D of the same litter. Markers are on the right.
Northern and Western Blotting.
Northern blotting was carried out by using total RNA extracted from several organs (11) and 32P-labeled mouse factor D cDNA as probe. Factor D was detected on Western blots of mouse sera by biotinylated affinity-purified rabbit anti-mouse factor D followed by streptavidin-horseradish peroxidase conjugate (Southern Biotechnology Associates) and the ECL chemiluminescent detection system (Amersham Pharmacia). Rabbit anti-mouse factor D antibodies were raised by using recombinant profactor D as immunogen.
Functional Assays.
AP hemolytic assay was performed in 96-well U-bottomed microtiter plates using rabbit erythrocytes as described (12). Activation of the AP was also evaluated by measuring deposition of C3 on the surface of zymosan by using flow cytometry (13). Zymosan A (Sigma) was suspended at 0.1 mg (1 × 107)/ml in normal saline. One hundred microliters zymosan suspension was incubated with 10 μl pooled mouse serum for 15 min at 37°C and then washed with ice-cold PBS, pH 7.2 containing 1% BSA, and 5 mM EDTA. Bound C3 was detected by using FITC-conjugated F(ab)′2 fragments of goat anti-mouse C3 IgG (20 μg/ml; Cappel) and a FACScan flow cytometer (Becton Dickinson). In a reconstitution assay, purified human factor D (14) was added to the deficient serum at 0.1, 0.5, or 2.5 μg/ml. A reaction mixture of factor D-sufficient serum containing 10 mM EDTA was used as a negative control.
Treatment of Mice with Cobra Venom Factor (CVF).
Factor D-deficient mice of either sex, 9–26 weeks old, received i.p. 5, 10, 20, or 30 μg CVF (Quidel, San Diego). Controls included factor D-sufficient and factor B-deficient (13) mice, kindly donated by Harvey Colten (Washington Univ., St. Louis). Blood samples were collected at different time points after administration of CVF, and serum concentration of C3 was determined by ELISA as described (15).
CVF-Mediated C3 and Factor B Activation.
Equal volumes of mouse serum, CVF (75 μg/ml), and Veronal-buffered saline, pH 7.3, containing 7.5 mM CaCl2 and 5 mM MgCl2, or 15 mM MgCl2 and 24 mM EGTA, or 30 mM EDTA were incubated at 37°C. Aliquots were removed at 5, 10, 20, 30, and 60 min, mixed with SDS/PAGE buffer, and subjected to SDS/PAGE in duplicate. C3 and its cleavage products were detected on Western blots by using goat anti-mouse C3 IgG (Cappel) and factor B and its cleavage products by using goat anti-human factor B IgG (Quidel), which also reacts with mouse factor B. Proteins were visualized by using rabbit anti-goat IgG F(ab)′2 horseradish peroxidase conjugates (ICN) and the ECL chemiluminescent detection system.
C3 Opsonization of Streptococcus pneumoniae.
Pepsin-treated, heat-killed, nonencapsulated, type II S. pneumoniae (R36A) (16) was kindly provided by David E. Briles and John F. Kearney (Univ. of Alabama). R36A cells (1 × 107) were incubated with 10% mouse serum at 37°C for various time intervals, and deposition of C3 fragments was detected as described above. Surface-bound IgM and IgG were detected by using FITC-conjugated affinity-purified goat anti-mouse IgM and IgG antibodies (10 μg/ml, Southern Biotechnology Associates), respectively.
Results
Generation of Factor D-Deficient Mice.
Mouse Df comprises five exons (17). The serine protease active center residues His57/Asp102/Ser195 (chymotrypsinogen numbering) are encoded separately by exons 2, 3, and 5, respectively. The Df in E14.1 cells was disrupted by insertion of a 1.1-kb neor gene cassette into exon 3 (Fig. 1A). Two of 122 double-drug resistant ES clones were identified as homologous recombinants by the presence in Southern blots of a single 11-kb HindIII fragment as opposed to 10 kb of the wild-type allele. One of the two targeted ES clones was used for microinjection of C57BL/6J blastocysts to generate ES chimeras. Germ-line transmission of the targeted allele was identified in F1 offspring of four chimeric founder mice bred with C57BL/6J breeding partners. Southern blot analysis (Fig. 1B) of tail DNA isolated from offspring of F1 heterozygous brother × sister intercrosses yielded the expected frequency of genotype +/+/+/−/−/− = 41 (27.2%):68 (45.0%):42 (27.8%). Homozygous Df-targeted mice were maintained under pathogen-free conditions and showed no apparent abnormality in development. Northern blots of RNA isolated from fat, muscle, lung, and liver of deficient mice showed complete absence of factor D message, whereas abundant message was detected in all these tissues except for liver of sufficient littermates (data not shown). Factor D protein was not detectable by Western blotting in serum of deficient mice (Fig. 1C). In contrast, in the serum of factor D-sufficient littermates the expected amount of factor D was present in the form of two major protein bands of apparent molecular masses of about 42 and 45 kDa. Similar dimorphism of mouse factor D was also observed previously and was attributed to differential glycosylation (4).
In mice, factor D was originally termed adipsin because of its abundant synthesis by adipocytes (2). Based on early observations that in certain acquired and genetic rodent models of obesity its mRNA levels were reduced by 96–99% (3, 5), a possible role for factor D in fat metabolism and/or systemic energy balance was proposed. To test this possibility, we monitored body mass and serum lipid profiles of factor D-deficient mice in comparison to their sufficient littermates. At 4, 8, and 26 weeks of age the body weight of factor D-deficient mice fed a normal diet was not different from that of factor D-sufficient littermates. Also, serum concentrations of triglycerides, cholesterol, and free fatty acids of the deficient mice were similar to those of control littermates (data not shown).
Activation of the AP by Rabbit Erythrocytes and Zymosan.
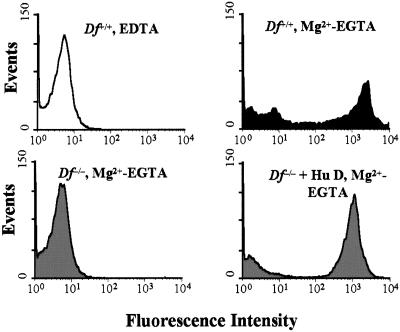
To confirm elimination of AP activity, pooled deficient serum was assayed for cytolytic activity against rabbit erythrocytes and for ability to support deposition of C3 fragments on zymosan particles. As expected from the absence of factor D protein, no hemolytic activity was detected in factor D-deficient serum. Reconstitution of the deficient serum with purified human factor D at a final concentration of 5 μg/ml restored hemolytic activity to a level even higher than that of factor D-sufficient littermates (Table 1). This result indicates that factor D is the only AP component missing from the deficient serum. The higher hemolytic activity of the reconstituted serum could perhaps be attributed to the higher concentration of C3 in factor D-deficient (928 μg/ml) than sufficient (569 μg/ml) pooled serum, which may have resulted from lack of C3 “tick-over.” We also examined activation of the AP by zymosan. Zymosan particles incubated with pooled normal serum in Mg2+/EGTA buffer showed very strong surface staining for C3, which was inhibited completely by 10 mM EDTA (Fig. 2). In contrast, no detectable C3 was present on the surface of zymosan particles incubated with pooled factor D-deficient serum, but full reconstitution was achieved by adding purified human factor D. C3 deposition from reconstituted deficient serum was Mg2+-dependent (data not shown). The combined results indicate that targeting Df resulted in complete inhibition of in vitro complement activation by the well-characterized AP activators, rabbit erythrocytes, and zymosan.
Table 1.
Alternative pathway hemolytic activity of serum from factor D-deficient mice
| Mouse serum | AP50, units/ml |
|---|---|
| +/+ (n = 8) | 76.1 |
| +/− (n = 7) | 74.5 |
| −/− (n = 11) | Undetectable |
| −/−, + Hu D, 5 μg/ml | 140.4 |
The microassay of complement activity was carried out by incubation of 75 μl of pooled mouse serum dilutions with 25 μl rabbit erythrocytes (7.5 × 106) for 1 h at 39°C. Heat-inactivated serum was used as control. Percent lysis was measured in an ELISA reader at 405 nm and hemolytic activity was calculated in units/ml.
Figure 2.
Flow cytometric analysis of C3 deposition on zymosan. Zymosan particles (1 × 106) were incubated with 10% wild-type (black) or factor D-deficient (gray) serum in Mg2+-EGTA or EDTA buffer. C3 deposition was allowed to occur at 37°C for 15 min and detected by FITC-conjugated F(ab)′2 fragments of goat anti-mouse C3 IgG. In the reconstitution assay, purified human factor D was supplemented at 0.5 μg/ml of deficient serum.
In Vivo Activation of the AP by CVF.
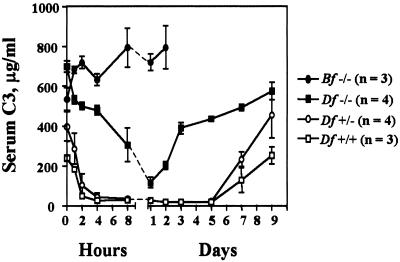
To confirm the elimination of AP activity in vivo, factor D-deficient mice were given a single i.p. injection of 30 μg CVF. Surprisingly, this treatment caused a profound and reproducible reduction of serum C3. As shown in Fig. 3, C3 concentration decreased slowly during the first 4 h and reached a nadir of about 10% of preinjection levels 24 h after injection. Levels of C3 started rising by day 2 and reached normal values by day 3. As expected, in control factor D-sufficient littermates, C3 levels declined much faster reaching the lowest point 4 h after CVF administration (Fig. 3). During the next 5 days, C3 remained at less than 5% of normal and preinjection levels were not recovered until day 8 or 9. Similar C3 consumption was also observed when lower doses (5, 10, and 20 μg) of CVF were used. In contrast to factor D-deficient mice, even the high dose of CVF (30 μg) failed to cause C3 consumption in factor B-deficient mice. Therefore, CVF-induced C3 consumption in factor D-deficient mice could not be attributed to nonspecific proteolysis of C3 by a contaminating protease. However, the possibility that a factor D-like activity, e.g., cobra factor D, was contaminating the preparation of CVF could not be ruled out.
Figure 3.
Consumption of serum C3 by CVF. Factor D-sufficient or -deficient and factor B-deficient mice were treated with single doses (30 μg) of CVF. Mouse sera were collected at the indicated time intervals for 9 days. Concentration of serum C3 was measured by ELISA.
Kinetic Analysis of CVF-Mediated Cleavage of C3 and Factor B.
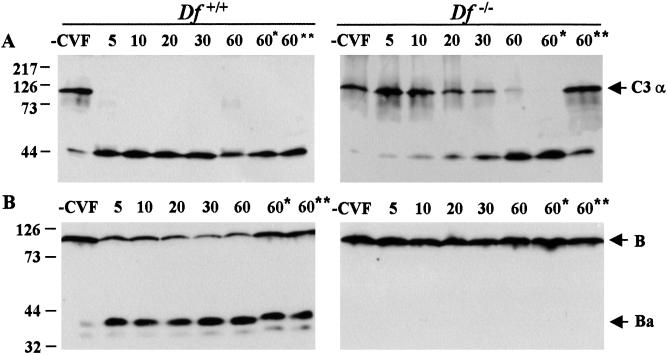
To elucidate the mechanism of CVF-induced activation of C3 in factor D-deficient serum, we investigated the kinetics of C3 and factor B cleavage in vitro in the presence or absence of divalent cations. Pooled mouse sera were incubated with CVF at 37°C in the presence of Ca2+ and Mg2+, and aliquots were withdrawn at the indicated time intervals and analyzed for C3 and factor B cleavage by Western blotting (Fig. 4). In normal serum, the α chain of C3 was cleaved completely in less than 5 min, detected as about 40-kDa fragments. In contrast, in the factor D-deficient serum the α chain of C3 was cleaved gradually over the 60-min incubation (Fig. 4A). The much slower kinetics of C3 cleavage in the deficient serum is consistent with the slower rate of depletion and faster rate of recovery of serum C3 levels we observed in the deficient mice after CVF injection. In vitro C3 cleavage was also examined in Mg2+/EGTA- or EDTA-containing buffers. As expected, chelating Ca2+ only did not affect CVF-mediated cleavage of C3 in either sufficient or deficient serum. Interestingly, chelating all divalent cations with EDTA did not inhibit cleavage of C3 in sufficient serum, whereas in the deficient serum significant, although not complete inhibition was observed (Fig. 4A). The data suggest differential requirement of Mg2+ for expression of C3 convertase activity in the presence or absence of factor D. Because CVF-induced C3 activation requires the participation of factor B as demonstrated by the in vivo study (Fig. 3), we examined whether or not factor B was cleaved in factor D-deficient serum. Western blots of duplicate samples from the same experiment clearly showed that in the absence of factor D factor B was not cleaved in any of the three buffers used, containing Ca2+/Mg2+, Mg2+/EGTA, or EDTA (Fig. 4B). This result ruled out the possibility of a “factor D-like” protease contaminating the CVF preparation. In factor D-sufficient serum incubated with CVF in the presence of Ca2+ and Mg2+, the majority of factor B was cleaved after 5 min and slow further cleavage was observed over the next 55 min. This result is consistent with initial rapid formation of stable CVFBb convertase and subsequent slow formation of unstable C3bBb convertase. On the Western blots, a trace amount of factor B and C3 cleavage was detected in control normal serum in the absence of CVF, which perhaps is caused by C3 tick-over, in the absence of an activator. Such low-level activation of the AP was not observed in factor D deficient serum (Fig. 4), indicating a requirement for factor D for the formation of the “initiation” C3 convertase (C3H2OBb) of the AP.
Figure 4.
Kinetics of CVF-mediated activation of the alternative pathway. Pooled mouse serum from wild-type (Left) or factor D-deficient (Right) mice was incubated with an equal volume of CVF (75 μg/ml) in Ca2+-Mg2+, Mg2+-EGTA (*) or EDTA (**) buffer at 37°C for the indicated time periods. Duplicate aliquots of the mixtures were subjected to 10% SDS/PAGE under reducing conditions. C3 (A) and factor B (B) and their activation fragments were detected by goat anti-C3 and anti-factor B IgG, respectively. Markers are on the left.
Antibody-Induced C3 Opsonization.
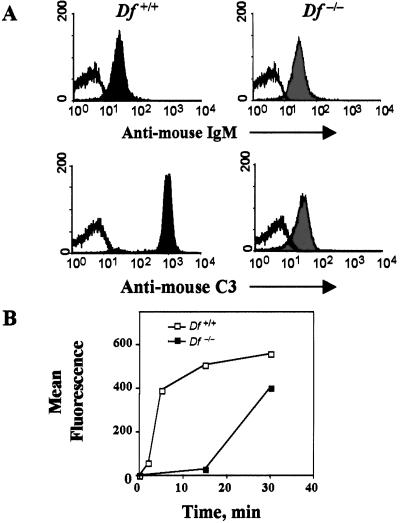
We next evaluated the contribution of the AP in opsonization of bacteria with C3 fragments in sera of nonimmunized mice. Unencapsulated S. pneumoniae, type II, was incubated with naive mouse serum at 37°C for 15 min, and bound antibody and deposited C3 fragments were analyzed by flow cytometry. Equivalent amounts of IgM antibody bound to bacteria incubated with factor D-sufficient or -deficient serum (Fig. 5A), whereas no IgG was detected on the bacterial surface (not shown). In contrast to the about equal amounts of bound IgM, the amount of C3 deposited on bacteria incubated with the deficient serum was about 2 logs less than that on bacteria incubated with sufficient serum. Furthermore, no C3 deposition was observed on bacteria incubated with sufficient serum containing Mg2+/EGTA(data not shown), indicating a requirement for Ca2+ and therefore, initiation of complement activation through the classical pathway. Kinetic comparison of C3 fragment deposition on the bacteria indicated opsonization by sufficient pooled serum occurred quickly with the majority of C3 fragments deposited within the first 5 min and maximum levels reached by 15 min (Fig. 5B). In contrast, C3 opsonization of bacteria by factor D-deficient serum proceeded slowly with a latent period of more than 15 min. By 30 min the amount of C3 deposited on bacteria incubated with deficient serum was equivalent to that on bacteria incubated with normal serum for 5 min. Thus, classical pathway activation is necessary for IgM-mediated C3 opsonization of bacteria, but the kinetics of C3 fragments deposition depends critically on amplification by the AP.
Figure 5.
C3 opsonization of S. pneumoniae. Heat-killed S. pneumoniae R36A (1 × 107) were incubated with 10% wild-type (black) or factor D-deficient (gray) serum at 37°C for 15 min (A) or for indicated time intervals (B). Surface-bound IgM (A Upper) and C3 (A Lower and B) were stained by FITC-conjugated goat IgG anti-mouse μ-chain and C3, respectively, and analyzed by flow cytometry.
Discussion
The present studies confirm that factor D is indispensable for the formation of the intrinsic alternative pathway initiation and amplification C3 convertases. The results also indicate that factor D-deficient serum supports the formation of a CVF-dependent C3 convertase containing intact factor B. Thus, factor B upon binding to CVF can undergo the zymogen to active protease conformational transition without the need for its cleavage by factor D. In addition, our data demonstrate that the efficiency of opsonization of S. pneumoniae by C3 fragments, a process initiated by means of activation of the classical pathway by IgM antibodies, depends on an intact alternative pathway amplification loop.
In normal serum formation of the AP C3 convertase is initiated by the Mg2+-dependent binding of factor B to C3b (reviewed in ref. 18). Factor B comprises three distinct globular regions (19) consisting of a cluster of three complement control protein modules, a von Willebrand factor type A (VWFA) module, and a serine protease domain. Many types of experiments have indicated that initial binding to C3b is mediated by two binding sites, one contributed by the three complement control protein modules and the other by the VWFA module of factor B (20–23). The assembly of the C3 convertase is concluded by the factor D-catalyzed cleavage of factor B into Ba, which consists of the three complement control protein modules and is released in the fluid phase, and Bb, which remains bound to C3b through the VWFA module binding site. It is generally accepted that cleavage by factor D is the key step in the formation of the convertase as it leads to conformational changes in both the C3b-binding site of the VWFA module and the active center of the serine protease domain of factor B. The former change is indicated by an increase in binding avidity for C3b and sequestration of the Mg2+ and the latter by expression of C3-cleaving activity. The necessity for structural rearrangement of the active center of factor B is indicated by the zymogen-like conformation of its oxyanion hole, which precludes expression of proteolytic activity (24).
The present data indicate that when CVF substitutes for C3b, the factor D-catalyzed cleavage of mouse factor B is not necessary for the conformational change of the serine protease domain. Instead, the findings of CVF-dependent C3 consumption in Df−/− mice (Fig. 3) and of cleavage of the α-chain of C3 in factor D-deficient serum without cleavage of factor B (Fig. 4) indicate that the structural rearrangement of the active center is induced by the binding of intact factor B to CVF. CVF is a functional analog of C3b and has been shown to form a C3 convertase by a similar process except that the binding site on the VWFA module of factor B has greater avidity for CVF than C3b. In normal serum, the increased avidity results in a C3-convertase (CVFBb) much more stable than the C3bBb one (25). As indicated by the kinetics of C3 consumption in vivo and of C3 cleavage in vitro (Figs. 3 and 4), the CVFB convertase formed in the absence of factor D is unstable or its catalytic efficiency is lower than that of the CVFBb convertase. It is also relevant that formation of C3 convertase in the deficient serum requires the presence of Mg2+, whereas in the sufficient serum the CVFBb convertase is formed even in the presence of EDTA (Fig. 4). This latter result is consistent with a previous report indicating activation of the alternative complement pathway by CVF in EDTA-treated mouse and rat sera (26).
In addition to VWF, factor B, and its classical pathway homolog C2, VWFA modules are found in a large variety of other proteins, including integrin α subunits, and some matrix proteins (27). High-resolution crystal structures have been reported for VWFA modules from several proteins. They are all very similar to each other, but probably more relevant to factor B is the structure of the VWFA module of the α chain of complement receptor type 3 (CR3) or αM (CD11b), which has Mg2+-dependent binding affinity for a C3 fragment, iC3b. The structure of the αM VWFA module has been solved from two different crystal forms and conforms to the typical “Rossmann” or α/β open sheet fold (28, 29). The Mg2+ binding site of αM is located at the top of the β-sheet on the surface of the module. Residues whose side chains coordinate the Mg ion are completely conserved in all cation-binding VWFA integrin modules. They have been termed the MIDAS (metal ion-dependent adhesion site) motif and reside in three surface loops (28, 29). It has been shown that the two crystal forms, termed closed and open, correspond to low and high affinity states for ligand binding, respectively (29–31). The affinity for ligand is regulated by conformational changes involving cation coordination and a significant downward movement of the C-terminal α helix, which propagates the signal to the opposite face of the module (29–31).
The VWFA module of factor B has a MIDAS-like motif and mutational studies have indicated that similarly to integrins ligand binding is through the surface loops surrounding the metal ion (22). It seems reasonable to suggest that affinity regulation of the C3-binding site of the VWFA module of factor B involves similar structural rearrangements as those documented for integrins. The present data indicate that up-regulation of the affinity of the factor B binding site for CVF is mediated by two events, Mg2+ binding and factor D-catalyzed cleavage. The effects of these two events apparently are additive and independent of each other, as indicated by the formation of a CVFB(Mg2+) convertase in the absence of factor D and a CVFBb one in the absence of Mg2+. In both cases affinity maturation, albeit short-lived, is associated with expression of protease activity, indicating a structural rearrangement of the active center of the serine protease domain. We have proposed (18) that the latter is induced by the downward shift of the C-terminal α helix of the VWFA module, which is linked to the N terminus of the serine protease domain. The present data indicate that the key signal for this structural rearrangement is provided by the binding of the VWFA module to C3b. This interpretation is consistent with a previous report (32) demonstrating the expression of C3 convertase activity by a complex of C3b, C3 nephritic factor, and intact factor B. C3 nephritic factor is an autoantibody that binds to and stabilizes the C3bB complex. Thus again, stable binding of factor B to C3b is sufficient for expression of proteolytic activity.
C3b generated by activation of any pathway can activate the AP amplification loop, provided it is attached to an appropriate cell or protein surface. The present studies demonstrate that although classical pathway complement activation is necessary for IgM-initiated C3 opsonization of S. pneumoniae, the kinetics of C3 deposition depends critically on amplification by the alternative pathway (Fig. 5). Because the mice were maintained under pathogen-free conditions and had not been immunized, bound IgM probably represents “natural” antibody. This interpretation is supported by the absence of IgG deposition on the S. pneumoniae. Natural antibodies are spontaneously occurring immunoglobulins in naïve animals and in normal individuals in the absence of apparent antigen simulation (33, 34). The bulk of natural antibodies belongs to IgM isotype, exhibits polyreactivity with a variety of self or foreign antigens (35), and is considered to be part of the innate immune system (36–38). Complement, another component of innate immunity, has a special relationship with natural antibodies depending on them for recognition of important pathogens and in turn providing signals for clonal selection and expansion of CD5+ B1 cells that produce them (39). It has been shown that antiphosphocholine natural antibodies are protective against i.v. infection of mice with S. pneumoniae (40). Here we present evidence that activation of the alternative pathway exerts a great impact on the kinetics of deposition of C3 opsonins on the phosphocholine-rich surface of the bacteria, which probably promotes their uptake by phagocytes and amplifies natural resistance to S. pneumoniae. Our findings are consistent with a recent report of pneumococcal sepsis and meningitis in a factor D-deficient neonate (41). Factor D deficiency has also been reported in adult patients (42, 43). In the most recent case (43), a factor D-deficient individual had a severe Neisseria meningitidis infection and his serum showed a very low capacity in opsonization of Escherichia coli and N. meningitidis for phagocytosis by normal granulocytes. Taken together with these clinical observations, our results suggest a significant contribution of the alternative pathway amplification loop to natural antibody-dependent antibacterial host defense, which is important early after infection before induced antibodies reach effective levels in blood.
In conclusion, the present studies demonstrate that affinity up-regulation of the C3b-binding site on the VWFA module of factor B provides the signal for the conformational transition of the serine protease domain from zymogen to active protease. In addition, a critical role of the alternative pathway amplification loop in natural antibody-mediated opsonization of S. pneumoniae is strongly indicated by our results.
Acknowledgments
We thank Mrs. Yuling Dai for technical assistance, Drs. R. S. Johnson and B. M. Spiegelman for generously providing the targeting vector, Adn/TK, Dr. A. J. Szalai for assistance in maintenance and propagation of the “knockout” line, Dr. H. Colten for the gift of Bf−/− mice, Dr. C. Walkey for performing serum lipid profile, and Drs. D. E. Briles and J. F. Kearney for providing R36A streptococci. This work was supported by U.S. Public Health Service Grants P60 AR20614 (to Y.X.) and AR44505 (to J.E.V.).
Abbreviations
- CVF
cobra venom factor
- AP
alternative pathway
- Df
factor D gene
- ES
embryonic stem
- VWFA
von Willebrand factor type A
- TK
thymidine kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Volanakis J E. In: The Human Complement System in Health and Disease. Volanakis J E, Frank M M, editors. New York: Dekker; 1998. pp. 9–32. [Google Scholar]
- 2.Cook K S, Groves D L, Min H Y, Spiegelman B M. Proc Natl Acad Sci USA. 1985;82:6480–6484. doi: 10.1073/pnas.82.19.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flier J S, Cook K S, Usher P, Spiegelman B M. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- 4.Rosen B S, Cook K S, Yaglom J, Groves D L, Volanakis J E, Damm D, White T, Spiegelman B M. Science. 1989;244:1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- 5.Choy L N, Rosen B S, Spiegelman B M. J Biol Chem. 1992;267:12736–12741. [PubMed] [Google Scholar]
- 6.Cianflone K, Vu H, Walsh M, Baldo A, Sniderman A. J Lipid Res. 1989;30:1727–1733. [PubMed] [Google Scholar]
- 7.Baldo A, Sniderman A D, St-Luce S. J Clin Invest. 1993;92:1543–1547. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetsel R A, Kildsgaard J, Zsigmond E, Liao W, Chan L. J Biol Chem. 1999;274:19429–19433. doi: 10.1074/jbc.274.27.19429. [DOI] [PubMed] [Google Scholar]
- 9.Johnson R S, Sheng M, Greenberg M E, Kolodner R D, Papaioannou V E, Spiegelman B M. Science. 1989;245:1234–1236. doi: 10.1126/science.2506639. [DOI] [PubMed] [Google Scholar]
- 10.Torres R M, Kühn R, Torres P M. In: Laboratory Protocols for Conditional Gene Targeting. Torres R M, Kühn R, editors. Oxford: Oxford Univ. Press; 1997. pp. 73–81. [Google Scholar]
- 11.Garnier G, Ault B, Kramer M, Colten H R. J Exp Med. 1992;175:471–479. doi: 10.1084/jem.175.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klerx J P, Beukelman C J, Van Dijk H, Willers J M N. J Immunol Methods. 1983;63:215–220. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin D D, Colten H R. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volanakis J E, Macon K J. Anal Biochem. 1987;163:242–246. doi: 10.1016/0003-2697(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 15.Taktak Y S, Stenning B. Horm Metab Res. 1992;243:71–374. doi: 10.1055/s-2007-1003338. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z Q, Vos Q, Shen Y, Lees A, Wilson S R, Briles D E, Gause W C, Mond J J, Snapper C M. J Immunol. 1999;163:659–667. [PubMed] [Google Scholar]
- 17.Min H Y, Spiegelman B M. Nucleic Acids Res. 1986;14:8879–8892. doi: 10.1093/nar/14.22.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Narayana S V L, Volanakis J E. Immunol Rev. 2001;180:123–135. doi: 10.1034/j.1600-065x.2001.1800111.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith C A, Vogel C-W, Müller-Eberhard H J. J Biol Chem. 1982;257:9879–9882. [PubMed] [Google Scholar]
- 20.Ueda A, Kearney J F, Roux K H, Volanakis J E. J Immunol. 1987;138:1143–1149. [PubMed] [Google Scholar]
- 21.Hourcade D E, Mitchell L M, Oglesby T J. J Biol Chem. 1995;270:19716–19722. doi: 10.1074/jbc.270.34.19716. [DOI] [PubMed] [Google Scholar]
- 22.Tuckwell D S, Xu Y, Newham P, Humphries M J, Volanakis J E. Biochemistry. 1997;36:6605–6613. doi: 10.1021/bi963155l. [DOI] [PubMed] [Google Scholar]
- 23.Hourcade D E, Mitchell L M, Oglesby T J. J Immunol. 1999;162:2906–2911. [PubMed] [Google Scholar]
- 24.Jing H, Xu Y, Carson M, Moore D, Macon K J, Volanakis J E, Narayana S V L. EMBO J. 2000;19:164–173. doi: 10.1093/emboj/19.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensley P, O'Keefe M C, Spangler C J, Osborne J C, Jr, Vogel C-W. J Biol Chem. 1986;261:11038–11044. [PubMed] [Google Scholar]
- 26.Goldman J N, Bangalore S, Goldman M B. J Immunol. 1979;123:2421–2427. [PubMed] [Google Scholar]
- 27.Perkins S J, Smith K F, William S C, Haris P I, Chapman D, Sim R B. J Mol Biol. 1994;238:104–119. doi: 10.1006/jmbi.1994.1271. [DOI] [PubMed] [Google Scholar]
- 28.Lee J-O, Rieu P, Arnaout M A, Liddington R. Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 29.Lee J-O, Bankston L A, Arnaout M A, Liddington R. Structure (London) 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 30.Emsley J, Knight C G, Farndale R W, Barnes M J, Liddington R C. Cell. 2000;100:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Shimaoka M, Ferzly M, Oxvig C, Takagi J, Springer T A. Proc Natl Acad Sci USA. 2001;98:2387–2392. doi: 10.1073/pnas.041606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daha M R, Fearon D T, Austen K F. J Immunol. 1976;116:568–570. [PubMed] [Google Scholar]
- 33.Casali P, Notkins A L. Immunol Today. 1989;10:364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 34.Herzenberg L A, Kantor A B. Immunol Today. 1993;14:79–83. doi: 10.1016/0167-5699(93)90063-Q. [DOI] [PubMed] [Google Scholar]
- 35.Kantor A B, Herzenberg L A. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 36.Baumgarth N, Herman O C, Jager G C, Brown L, Herzenberg L A. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumgarth N, Herman O C, Jager G C, Brown L E, Herzenberg L A, Chen J. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boes M, Prodeus A P, Schmidt T, Carroll M C, Chen J. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll M C, Prodeus A P. Curr Opin Immunol. 1998;10:36–40. doi: 10.1016/s0952-7915(98)80028-9. [DOI] [PubMed] [Google Scholar]
- 40.Briles D E, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss S J, Ahmed A E, Bonagura V R. J Allergy Clin Immunol. 1998;102:1043–1044. doi: 10.1016/s0091-6749(98)70346-x. [DOI] [PubMed] [Google Scholar]
- 42.Hiemstra P S, Langeler E, Compier B, Keepers Y, Leijh P C, van den Barselaar M T, Overbosch D, Daha M R. J Clin Invest. 1989;84:1957–1961. doi: 10.1172/JCI114384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biesnma D H, Hannema A J, van Velzen-Blad H, Mulder L, van Zwieten R, Kluijt I, Roos D. J Clin Invest. 2001;108:233–240. doi: 10.1172/JCI12023. [DOI] [PMC free article] [PubMed] [Google Scholar]