Abstract
Background
Severe bleeding and coagulopathy are serious clinical conditions that are associated with high mortality. Thromboelastography (TEG) and thromboelastometry (ROTEM) are increasingly used to guide transfusion strategy but their roles remain disputed. This review was first published in 2011 and updated in January 2016.
Objectives
We assessed the benefits and harms of thromboelastography (TEG)‐guided or thromboelastometry (ROTEM)‐guided transfusion in adults and children with bleeding. We looked at various outcomes, such as overall mortality and bleeding events, conducted subgroup and sensitivity analyses, examined the role of bias, and applied trial sequential analyses (TSAs) to examine the amount of evidence gathered so far.
Search methods
In this updated review we identified randomized controlled trials (RCTs) from the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1); MEDLINE; Embase; Science Citation Index Expanded; International Web of Science; CINAHL; LILACS; and the Chinese Biomedical Literature Database (up to 5 January 2016). We contacted trial authors, authors of previous reviews, and manufacturers in the field. The original search was run in October 2010.
Selection criteria
We included all RCTs, irrespective of blinding or language, that compared transfusion guided by TEG or ROTEM to transfusion guided by clinical judgement, guided by standard laboratory tests, or a combination. We also included interventional algorithms including both TEG or ROTEM in combination with standard laboratory tests or other devices. The primary analysis included trials on TEG or ROTEM versus any comparator.
Data collection and analysis
Two review authors independently abstracted data; we resolved any disagreements by discussion. We presented pooled estimates of the intervention effects on dichotomous outcomes as risk ratio (RR) with 95% confidence intervals (CIs). Due to skewed data, meta‐analysis was not provided for continuous outcome data. Our primary outcome measure was all‐cause mortality. We performed subgroup and sensitivity analyses to assess the effect based on the presence of coagulopathy of a TEG‐ or ROTEM‐guided algorithm, and in adults and children on various clinical and physiological outcomes. We assessed the risk of bias through assessment of trial methodological components and the risk of random error through TSA.
Main results
We included eight new studies (617 participants) in this updated review. In total we included 17 studies (1493 participants). A total of 15 trials provided data for the meta‐analyses. We judged only two trials as low risk of bias. The majority of studies included participants undergoing cardiac surgery.
We found six ongoing trials but were unable to retrieve any data from them. Compared with transfusion guided by any method, TEG or ROTEM seemed to reduce overall mortality (7.4% versus 3.9%; risk ratio (RR) 0.52, 95% CI 0.28 to 0.95; I2 = 0%, 8 studies, 717 participants, low quality of evidence) but only eight trials provided data on mortality, and two were zero event trials. Our analyses demonstrated a statistically significant effect of TEG or ROTEM compared to any comparison on the proportion of participants transfused with pooled red blood cells (PRBCs) (RR 0.86, 95% CI 0.79 to 0.94; I2 = 0%, 10 studies, 832 participants, low quality of evidence), fresh frozen plasma (FFP) (RR 0.57, 95% CI 0.33 to 0.96; I2 = 86%, 8 studies, 761 participants, low quality of evidence), platelets (RR 0.73, 95% CI 0.60 to 0.88; I2 = 0%, 10 studies, 832 participants, low quality of evidence), and overall haemostatic transfusion with FFP or platelets (low quality of evidence). Meta‐analyses also showed fewer participants with dialysis‐dependent renal failure.
We found no difference in the proportion needing surgical reinterventions (RR 0.75, 95% CI 0.50 to 1.10; I2 = 0%, 9 studies, 887 participants, low quality of evidence) and excessive bleeding events or massive transfusion (RR 0.38, 95% CI 0.38 to 1.77; I2 = 34%, 2 studies, 280 participants, low quality of evidence). The planned subgroup analyses failed to show any significant differences.
We graded the quality of evidence as low based on the high risk of bias in the studies, large heterogeneity, low number of events, imprecision, and indirectness. TSA indicates that only 54% of required information size has been reached so far in regards to mortality, while there may be evidence of benefit for transfusion outcomes. Overall, evaluated outcomes were consistent with a benefit in favour of a TEG‐ or ROTEM‐guided transfusion in bleeding patients.
Authors' conclusions
There is growing evidence that application of TEG‐ or ROTEM‐guided transfusion strategies may reduce the need for blood products, and improve morbidity in patients with bleeding. However, these results are primarily based on trials of elective cardiac surgery involving cardiopulmonary bypass, and the level of evidence remains low. Further evaluation of TEG‐ or ROTEM‐guided transfusion in acute settings and other patient categories in low risk of bias studies is needed.
Plain language summary
Blood clotting analysers (TEG or ROTEM) versus any comparison to guide the use of blood products in adults or children with bleeding
Background
The ability to make a sufficient blood clot is crucial in participants with bleeding. Clotting can be measured by various tests. TEG and ROTEM tests have the advantage of showing the total clotting capacity. These tests are performed at the bedside, and generally provide a rapid and useful result, guiding clinicians towards a more goal‐directed transfusion management.
Objective
In the present systematic review we set out to assess the benefits and harms of a TEG‐ or ROTEM‐guided use of blood products in comparison with standard tests, or doctors clinical judgement, in the treatment of bleeding patients. Evidence is current to January 2016.
Study characteristics
We identified 17 randomized controlled trials comparing TEG‐ or ROTEM‐guided use of blood transfusion to guidance from the clinical judgement of doctors or standard laboratory tests, or both. The included trials were conducted mainly in adults in need of cardiac surgery, and involved 1493 participants.
Key results
In terms of efficacy, the use of TEG or ROTEM tests seem to reduce the need for all types of blood transfusions. However, we could not find fewer participants in need of further operations due to continuous bleeding, or at risk of massive bleeding with transfusion. Despite signs of benefit in regards to survival, our findings are hampered by the overall low quality of included studies. Assessment of harms indicated a reduced risk of kidney failure, while no other significant adverse ‐events were found. However, the reported adverse event rates were very low. All included trials except two were marred by high risk of bias.
Quality of evidence
Due to few events and many poorly designed trials, we consider our overall findings to be of low quality evidence in favour of TEG and ROTEM use in the management of bleeding patients.
Summary of findings
Background
Description of the condition
Bleeding remains a serious condition related to surgery, invasive procedures, child birth, as well as trauma. Ongoing severe bleeding is associated with increased morbidity and mortality, and may prompt the need for additional surgery. Impaired haemostasis can be a contributing factor to postoperative bleeding (Hardy 2005), and may be caused by factors present preoperatively, such as, antithrombotic treatment or inherited deficiencies (Hartmann 2006). Antithrombotic treatment during surgery such as heparinization during cardiopulmonary bypass (Besser 2010; Paparella 2004), or challenges of liver surgery especially with the anhepatic phase may also cause impaired haemostasis in the postoperative period (Sabate 2012).
Dilutional coagulopathy from treatment with intravenous fluids and pooled red blood cell (PRBC) transfusions is frequent in cases of unbalanced multi‐transfusion, together with physiological consumption of haemostatic factors due to the ongoing bleeding (Johansson 2012). If bleeding becomes life‐threatening with development of hypovolaemic shock with acidosis, and if factors such as hypothermia and hypocalcaemia are not controlled (De Robertis 2015), the risk increases for developing severe consumptious coagulopathy, disseminated intravascular coagulation, and hyperfibrinolysis. This may complicate the situation even further, ultimately leading to increased mortality (Hardy 2005).
Coagulopathy, as a result of a massive transfusion and uncontrolled bleeding leads to defects in clot firmness due to fibrinogen, coagulation factor, and platelet deficiency; decreased clot stability due to hyperfibrinolysis and factor XIII deficiency (Brohi 2008; Levrat 2008; Rugeri 2007); and prolonged clot generation due to various coagulation factor deficiencies (Kozek‐Langenecker 2007). Coagulopathy as an isolated entity is just one cause of bleeding. However, despite the ability of various test systems to identify coagulopathy, the tests are unable to predict bleeding in a reliable fashion (Chee 2003; Segal 2005). Surgical bleeding or arterial injury is often the dominant reason for blood loss, resulting in a high transfusion requirement. Thus, identifying the cause of bleeding does not automatically resolve the problem.
Description of the intervention
The decision to transfuse PRBCs is usually guided by measures such as haemoglobin or haematocrit, or in severe cases, clinical signs of circulative instability. Transfusion of haemostatic blood products such as fresh frozen plasma (FFP), cryoprecipitate, platelet units, and various factor concentrates can be guided by clinical judgement, standard laboratory tests, thromboelastography (TEG) or thromboelastometry (ROTEM), or a combination of these, in a more or less fixed transfusion algorithm. Generally, standard laboratory tests include activated partial thromboplastin time, prothrombin time, international normalized ratio, platelet count, and plasma fibrinogen. However, none of these tests were developed to predict bleeding or to guide coagulation management in the surgical setting. They are of limited use in diagnosis and assessment of bleeding risk and in relation to algorithms used to guide the administration of blood products for surgical or critically ill patients (Benes 2015).
The limitations of these tests include a lack of real‐time monitoring; inability to identify singular or multiple coagulation factor deficiencies; no measurement of the effects of hypothermia on haemostasis; and no rapid assessment of fibrinolysis, platelet dysfunction, or haemostatic response to injury or surgery (Benes 2015; Hardy 2004). Additionally, all these tests are performed in plasma at 37 °C without the presence of platelets or other blood cells, and they seem unable to predict the role of the measured components in the context of haemostasis as a whole. Thus, none of these tests can estimate the risk of bleeding (Chee 2003), but they are being used to guide therapy in the presence of clinical bleeding.
TEG is a viscoelastic, haemostatic assay analyser invented by Hartert that imitates sluggish venous flow (Hartert 1948). It provides an evaluation of the kinetics of all stages of clot initiation, formation, stability, strength, and dissolution in whole blood (Benes 2015; Luddington 2005). In conventional TEG, a 0.36 mL blood sample is placed into a cup which is then rotated gently. When a sensor shaft is inserted into the sample a clot forms between the cup and the sensor. The speed and patterns of changes in strength and elasticity in the clot are measured in various ways by a computer and are depicted as a graph. In the reagent‐modified rotational thromboelastometry (ROTEM) analyser the sensor shaft rotates, rather than the cup rotating (Benes 2015; Lang 2005 ).
TEG and ROTEM have several advantages compared to routine coagulation tests. They are easy to use by non‐laboratory personnel as a point‐of‐care assay in the perioperative and emergency setting; produce rapid graphical and numerical results of the haemostatic status; are able to detect the anticoagulant effect of acidosis, hypo‐ or hyperthermia as they can be performed at between 22 °C and 42 °C; and are able to detect and quantify the underlying cause of coagulopathy, such as thrombocytopenia, factor deficiency, heparin effect, hypofibrinogenaemia, and hyperfibrinolysis (Luddington 2005). Treatment for such disorders may involve the transfusion of blood products (FFP, cryoprecipitate, and platelets) or specific drugs, and the effect can be evaluated in vitro (Benes 2015).
How the intervention might work
Clinical signs of coagulopathy, such as oozing, is a late sign, and therefore accurate management of massive transfusion is often challenged, since there is no simple, reliable, and rapid routine diagnostic coagulation test available (Chee 2003; Hardy 2004). Monitoring dynamic changes of haemostasis by repeatedly performing TEG or ROTEM is thought to enable clinicians to distinguish between a surgical cause of bleeding or coagulopathy, to diagnose the specific type of coagulopathic impairment, and to guide and evaluate the choice of haemostatic treatment. This may enable optimized and reduced use of blood products, while reducing bleeding, the need to reoperate, complications associated with hypovolaemic shock, and ultimately influence mortality positively.
Why it is important to do this review
A clinical method enabling a distinction between surgical bleeding and bleeding caused by coagulopathy, and at the same time providing guidance to drug administration, minimizing usage of blood products, and enabling real‐time monitoring of the patient's coagulation may be of great benefit. TEG or ROTEM may provide more complete diagnostic information more rapidly and perhaps at similar cost to the standard laboratory tests. A change in the clinical management of severe bleeding, as a consequence of this technology, might subsequently reduce transfusion‐related risks, and provide improved patient health outcomes, as well as optimizing the use of healthcare resources.
Randomized trials are needed to evaluate the potential effects of introducing a diagnostic test (Gluud 2005). The benefit and efficacy of TEG or ROTEM in patients with severe bleeding and coagulopathy is still debated. The test accuracy of TEG and ROTEM and the degree of their correlation to standard laboratory tests needs further evaluation, since introduction of yet another diagnostic test in the clinical evaluation of coagulation at the point‐of‐care may only add to the complexity of the problem, as well as lead to an increase in costs. The aim of this review was to assess the evidence as to whether TEG or ROTEM are beneficial or harmful for patients with bleeding.
Objectives
We assessed the benefits and harms of thromboelastography (TEG)‐guided or thromboelastometry (ROTEM)‐guided transfusion in adults and children with bleeding. We looked at various outcomes, such as overall mortality and bleeding events, conducted subgroup and sensitivity analyses, examined the role of bias, and applied trial sequential analyses (TSAs) to examine the amount of evidence gathered so far.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel group randomized controlled trials (RCTs) irrespective of quasi‐randomizations, publication status, blinding status, or language of the report. We contacted the investigators and the authors in order to retrieve the relevant data. We only included unpublished trials if trial data and methodological descriptions were either provided in written form or could be retrieved from the authors. We excluded observational studies. We did not include any studies with non‐standard designs, such as cross‐over trials or cluster‐randomized trials.
Types of participants
We included trials with adults and children who were bleeding. We did not exclude any subgroup of the patient population.
Types of interventions
We included trials comparing a TEG‐ or ROTEM‐guided transfusion algorithm. We also included interventional algorithms including both TEG or ROTEM in combination with standard laboratory tests or other devices.
The primary analysis included trials on thromboelastography (TEG) or thromboelastometry (ROTEM) versus any comparator.
We undertook separate subgroup analyses of trials in which a TEG‐ or ROTEM‐guided transfusion algorithm were compared with clinical judgement, usual treatment, or an algorithm based on standard laboratory tests.
Comparison 1: TEG‐ or ROTEM‐guided algorithm versus any comparison.
Comparison 2: TEG‐ or ROTEM‐guided algorithm versus clinical judgement or usual treatment.
Comparison 3: TEG‐ or ROTEM‐guided algorithm versus a predefined algorithm based on standard laboratory test‐guided transfusion.
Comparison 4: TEG or ROTEM in combination with standard laboratory tests or other devices in a guided algorithm versus clinical judgement or usual care.
Types of outcome measures
Primary outcomes
Overall mortality. We used the longest follow‐up data from each trial, regardless of the period of follow‐up.
Secondary outcomes
Bleeding events, blood loss, proportion of participants in need of transfusion, and amount of blood products transfused.
Complications probably related to the underlying condition, e.g. infections, thrombosis, allergic reactions, congestive cardiac failure, myocardial infarction, renal failure, and cerebrovascular accident.
Incidence of surgical interventions and reoperation due to bleeding.
Complications probably related to transfusion, e.g. infections and sepsis, haemolytic reactions, disseminated intravascular coagulation, and major immunological and allergic reactions.
Quality of life assessment, as defined by authors in included studies.
Duration of mechanical ventilation or improvement of respiratory failure (ventilator‐free days), or both.
Length of stay in the intensive care unit (ICU).
Number of days in hospital.
Cost‐benefit analyses.
Search methods for identification of studies
Electronic searches
In this updated review we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1); Ovid MEDLINE (WebSPIRS) (1950 to 5 January 2016); Ovid Embase (WebSPIRS) (1980 to January 2016); Ovid BIOSIS (WebSPIRS) (1993 to 5 January 2016); International Web of Science (1964 to 5 January 2016); Latin American and Caribbean Health Sciences Literature (LILACS) (via BIREME) (1982 to 5 January 2016); the Chinese Biomedical Literature Database; advanced Google; and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCO host) (1980 to 5 January 2016).
In the original review we searched until October 2010 (Afshari 2011).
We performed a systematic and sensitive search strategy to identify relevant RCTs with no language or date restrictions. For specific information regarding our search strategies please see Appendix 1. We reran the search on 5 January 2016.
Searching other resources
We handsearched the reference list of reviews, randomized and non‐randomized studies, and editorials for additional studies. We contacted the main authors of studies and experts in this field to ask for any missed, unreported, or ongoing studies. We contacted the manufacturers of TEG and ROTEM tests and pharmaceutical companies for any unpublished trials (5 January 2016).
We searched for ongoing clinical trials and unpublished studies on the following Internet sites (search date 6 January 2016).
ISRCTN registry.
Clinical trials registry.
Center Watch.
UMIN clinical trials registry.
Data collection and analysis
Selection of studies
Two review authors (AW and AA) independently evaluated all relevant trials and provided a detailed description of the included and excluded studies under the sections Characteristics of included studies and Characteristics of excluded studies. We also provided detailed descriptions of our search results (Figure 6), and resolved disagreements by discussion. We screened the titles and abstracts in order to identify eligible studies.
6.
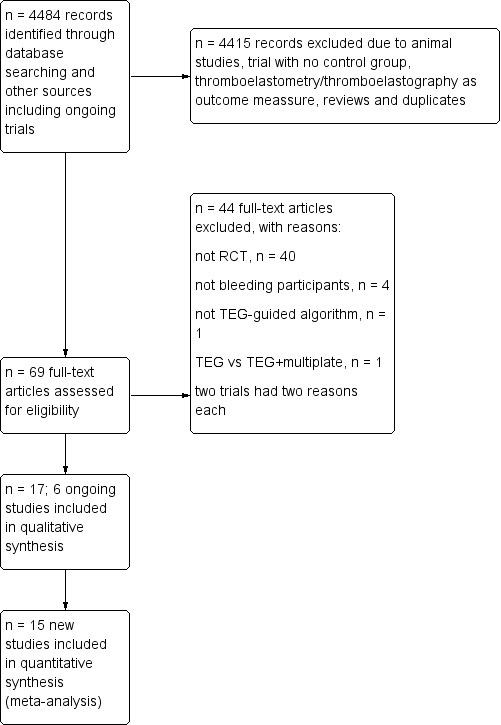
Updated flow diagram for selection of randomized controlled trials up to 5 January 2016.
Data extraction and management
AW and AA independently extracted and collected the data on a standardized paper form. We were not blinded to the author, source institution, or the publication source of trials. We resolved disagreements by discussion and approached all corresponding authors of the included trials for additional information on the review's outcome measures and risk of bias components. For more specific information, please see the section Contributions of authors.
Assessment of risk of bias in included studies
We addressed each question of validity systematically, as described by the following in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
1) Random sequence generation
Assessment of randomizations: the sufficiency of the method in producing two comparable groups prior to the intervention.
Grading: 'low risk' ‐ a truly random process, e.g. random computer number generator, coin tossing, or throwing dice; 'high risk' ‐ any non‐random process, e.g. date of birth, date of admission by hospital or clinic record number, or by availability of the intervention; or 'unclear risk' ‐ insufficient information.
2) Allocation concealment
Allocation method prevented the investigators or participants from foreseeing the assignment.
Grading: 'low risk' ‐ central allocation or sealed opaque envelopes; 'high risk' ‐ using open allocation schedule or other unconcealed procedure; or 'unclear risk' ‐ insufficient information.
3) Blinding
Assessment of appropriate blinding of the investigation team and participants: person responsible for participants' care, participants, and outcome assessor.
Grading: 'low risk' ‐ we consider blinding as adequate if participants and personnel were kept unaware of intervention allocations after inclusion of participants into the study, and the method of blinding involved a placebo indistinguishable from the intervention, since mortality is a robust outcome; 'high risk' not double‐blinded, categorized as an open‐label study, or without use of a placebo indistinguishable from the intervention; 'unclear risk' ‐ blinding not described.
4) Incomplete outcome data
Completeness of the outcome data including attritions and exclusions.
Grading: 'low risk' ‐ if the numbers and reasons for dropouts and withdrawals in the intervention groups were described or if it was specified that there were no dropouts or withdrawals; 'high risk' ‐ if no description of dropouts and withdrawals was provided; 'unclear risk' ‐ if the report gave the impression that there were no dropouts or withdrawals, but this was not specifically stated.
5) Selective reporting
The possibility of selective outcome reporting.
Grading: 'low risk' ‐ if the reported outcomes are those prespecified in an available study protocol or, if this is not available, the published report includes all expected outcomes; 'high risk' ‐ if not all prespecified outcomes have been reported, have been reported using non‐prespecified subscales, reported incompletely, or the report fails to include a key outcome that would have been expected for such a study); 'unclear risk' ‐ insufficient information.
6) Other bias
The assessment of any possible sources of bias not addressed in domains 1 to 5.
Grading: 'low risk' ‐ if the report appears to be free of such biases; 'high risk' ‐ if at least one important bias is present related to study design, early stopping due to some data‐dependent process, extreme baseline imbalance, academic bias, claimed fraudulence or other problems; or 'unclear risk' ‐ insufficient information, or evidence that an identified problem will introduce bias.
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (binary outcomes).
These included: overall mortality; bleeding events; proportion of participants in need of transfusion; complications probably related to the underlying condition, e.g. infections, thrombosis, allergic reactions, congestive cardiac failure, myocardial infarction, renal failure, cerebrovascular accident; incidence of surgical interventions and reoperation due to bleeding; complications probably related to transfusion, e.g. infections and sepsis, haemolytic reactions and disseminated intravascular coagulation, and major immunological and allergic reactions.
Continuous data
We planned to use the mean difference (MD) if data were continuous and measured in a similar way between trials. We used the standardized mean difference (SMD) to combine trials measuring the same outcome with different scales. Some of the trials provided their data as median values. The median value is very similar to the mean when the distribution of the data is symmetrical and so occasionally can be used directly in meta‐analyses (Higgins 2011). However, means and medians can be very different from each other if the data are skewed, and medians are often reported because the data are skewed.
Some of the included trials in this paper provided interquartile ranges, which describe where the central 50% of participants' outcomes lie. When sample sizes are large and the distribution of the outcome is similar to the normal distribution, the width of the interquartile range will be approximately 1.35 standard deviations (SDs) (Higgins 2011). When the distribution of outcomes is skewed, it is not possible to estimate a SD from an interquartile range. Application of interquartile ranges may thus be an indicator that the outcome distribution is skewed. When assessment of data from continuous outcomes showed an overall skewed tendency, we abstained from pooling data and performing meta‐analysis, and instead present the results as tables.
The continuous data included: blood loss; amount of blood transfused; quality of life assessment, as defined by authors in included studies; duration of mechanical ventilation or improvement of respiratory failure (ventilator‐free days), or both; mean length of stay in the ICU; number of days in hospital; and cost‐benefit analyses.
Unit of analysis issues
Cross‐over trials
We excluded cross‐over trials from our meta‐analyses because of the potential risk of carry‐over of treatment effect in the context of bleeding.
Studies with multiple intervention groups
In studies designed with multiple intervention groups, we combined groups to create a single pair‐wise comparison (Higgins 2011). In trials with two or more TEG or ROTEM groups, we combined data where possible, for the primary and secondary outcomes.
Dealing with missing data
We contacted all the first authors and contact persons of the trials with missing data in order to retrieve the relevant data.
For all included studies we noted levels of attrition and any exclusions. In case of missing data, we chose 'complete‐case analysis' for our primary outcomes, which excludes from the analysis all participants with the outcome missing. Selective outcome reporting occurs when non‐significant results are selectively withheld from publication (Chan 2004), and is defined as the selection, on the basis of the results, of a subset of the original variables recorded for inclusion in publication of trials (Hutton 2000). The most important types of selective outcome reporting are: selective omission of outcomes from reports; selective choice of data for an outcome; selective reporting of different analyses using the same data; selective reporting of subsets of the data; and selective under‐reporting of data (Higgins 2011). Statistical methods to detect within‐study selective reporting are still in their infancy. We tried to explore for selective outcome reporting by comparing publications with their protocols if the latter were available.
Assessment of heterogeneity
We explored heterogeneity using the I² statistic and Chi² test. An I² statistic above 50% represents substantial heterogeneity (Higgins 2003). In case of I² statistic > 0 (mortality outcome), we tried to determine the cause of heterogeneity by performing relevant subgroup analyses. We used the Chi² test to provide an indication of heterogeneity between studies, with P ≤ 0.1 considered significant.
Assessment of reporting biases
Publication bias occurs when the publication of research results depends on their nature and direction (Dickersin 1990). We examined this by providing a funnel plot in order to detect either publication bias or a difference between smaller and larger studies (small study effect), which is expressed by asymmetry (Higgins 2011).
Funding bias is related to the possible publication delay or discouragement of undesired results in trials sponsored by the industry (Higgins 2011). To explore the role of funding, we provide information on which studies were sponsored by industry.
Data synthesis
We used Review Manager 5 software (RevMan 2014) in order to perform meta‐analyses on pre‐stated outcomes from the included trials. If we performed the meta‐analyses and I² statistic = 0, we only reported the results from the fixed‐effect model; in the case of I² statistic > 0 we reported only the results from the random‐effects model, unless one or two trials contributed more than 60% of the total evidence provided, in which case the random‐effects model may be biased.
We believed there was little value in using a fixed‐effect model in cases of substantial heterogeneity, which we expected would be due to the various factors leading to massive bleeding. We pooled studies only in case of low clinical heterogeneity. When using meta‐analysis for combining results from several studies with binary outcomes (i.e. event or no event), adverse side effects may be rare but serious, and hence important (Sutton 2002). Most meta‐analytic software does not include trials with 'zero events' in both arms (intervention versus control) when calculating a RR. Exempting these trials from the calculation of a RR and 95% CI may lead to overestimation of a treatment effect. Cochrane recommends application of the Peto odds ratio (OR), which is the best method of estimating odds ratios when there are many trials with no events in one or both arms (Higgins 2011). However, the Peto method is generally less useful when the trials are small or when treatment effects are large. We planned to conduct a sensitivity analysis by applying the Peto OR if this appeared to be a valid option. However, the trials included did not fulfil criteria for Peto OR (Effects of interventions).
Trial sequential analysis (TSA)
TSA is a methodology that combines an information size calculation for meta‐analysis with a threshold of statistical significance. It is a tool for quantifying the statistical reliability of data in a cumulative meta‐analysis, adjusting significance levels for sparse data and repetitive testing on accumulating data. We conducted TSA at least on the primary outcomes (Brok 2009; Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2008), and on the secondary outcomes if the accrued information size was an acceptable fraction of the estimated required information size to allow meaningful analyses (greater than 20%). If the actual accrued information size was too low, we provided the required information size given the actual diversity (Wetterslev 2009), and a possible diversity of 25%.
Meta‐analysis may result in type I errors due to random errors arising from sparse data or repeated significance testing when updating the meta‐analysis with new trials (Brok 2009; Wetterslev 2008). Bias (systematic error) from trials with low methodological quality, outcome measure bias, publication bias, early stopping for benefit and small trial bias may also result in spurious P values (Brok 2009; Higgins 2011; Wetterslev 2008).
In a single trial, interim analysis increases the risk of type I errors. To avoid these, group sequential monitoring boundaries are applied to decide whether a trial could be terminated early because of a sufficiently small P value, i.e. the cumulative Z‐curve crosses the monitoring boundaries (Lan 1983). Sequential monitoring boundaries can also be applied to meta‐analysis, and are called trial sequential monitoring boundaries. In TSA, the addition of each trial in a cumulative meta‐analysis is regarded as an interim meta‐analysis and helps to clarify whether additional trials are needed.
The idea in TSA is that if the cumulative Z‐curve crosses the boundary, a sufficient level of evidence is reached and no further trials may be needed (firm evidence). If the Z‐curve does not cross the boundary, then there is insufficient evidence to reach a conclusion. To construct the trial sequential monitoring boundaries, the required information size is needed and is calculated as the least number of participants needed in a well‐powered single trial (Brok 2009; Pogue 1997; Pogue 1998; TSA 2010; Wetterslev 2008). We aimed to apply TSA as it prevents an increase in the risk of type I error with sparse data or multiple updating in a cumulative meta‐analysis. Hence, TSA provides us with important information in order to estimate the level of evidence of the experimental intervention. Additionally, TSA provides us with important information regarding the need for additional trials and their sample size. We used Trial Sequential Analysis software, version 0.8 (TSA 2010).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses to assess the benefits and harms of TEG and ROTEM based on:
the cause of the underlying condition (e.g. trauma, critically ill patients, surgery);
age group of children (aged less than 18 years) or adults;
the enrolment of the participants to TEG or ROTEM;
coagulopathy or severe postoperative bleeding as inclusion criteria.
If analyses of various subgroups were significant, we planned to perform a test of interaction by applying meta‐regression (Altman 2003; Higgins 2011 ‐ chapters 9.6.3.1 and 9.6.4). We considered P < 0.05 as indicating significant interaction between the TEG or ROTEM effect on mortality and the subgroup category (Higgins 2011 ‐ chapters 9.6.1 and 9.7).
Sensitivity analysis
We compared estimates of the pooled intervention effect in trials with low risk of bias to estimates from trials with high risk of bias (i.e. trials having at least one inadequate risk of bias component).
We calculated the RR with 95% CI and decided to apply complete case analysis, if possible, for our sensitivity and subgroup analyses based on our primary outcome measure (mortality).
Summary of findings
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2008). We constructed 'Summary of findings' tables for each comparison using the GRADE software (add ref). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of the effect estimates, and risk of publication bias. We included the following outcomes in 'Summary of findings' tables: overall mortality ‐ longest follow‐up (primary outcome); proportion of participants in need of transfusion; (need of PRBCs, FFP, and platelets); excessive bleeding events and massive transfusion; and incidence of reoperation due to bleeding.
Results
Description of studies
Results of the search
Through electronic searches and from the references of potentially relevant articles, we identified 4484 (1878 in update) publications. We excluded 4415 publications as they were either duplicates or were clearly irrelevant. We retrieved a total of 69 (46 in update) relevant publications for further assessment (Figure 6; Figure 7; Figure 8). From these, we included eight new trials to a total of 17 trials that randomized all together 1493 participants (Cui 2010; Kempfert 2011; Nakayama 2015; NCT00772239; Paniagua 2011; Schaden 2012; Rauter 2007; Weber 2012; see Included studies and Figure 6). Two trials provided no data in the meta‐analyses (NCT00772239; Rauter 2007). All together, we statistically evaluated the results of 15 trials and 1185 participants in the present systematic review. We found six ongoing trials but were unable to retrieve any data from the investigators at their current stage (NCT02352181; NCT02593877; NCT02461251; NCT01536496; NCT02416817; NCT01402739; see Characteristics of ongoing studies). The two review authors (AW, AFSH) completely agreed on the selection of included studies. We obtained additional information from six study authors as listed in the table Characteristics of included studies.
7.
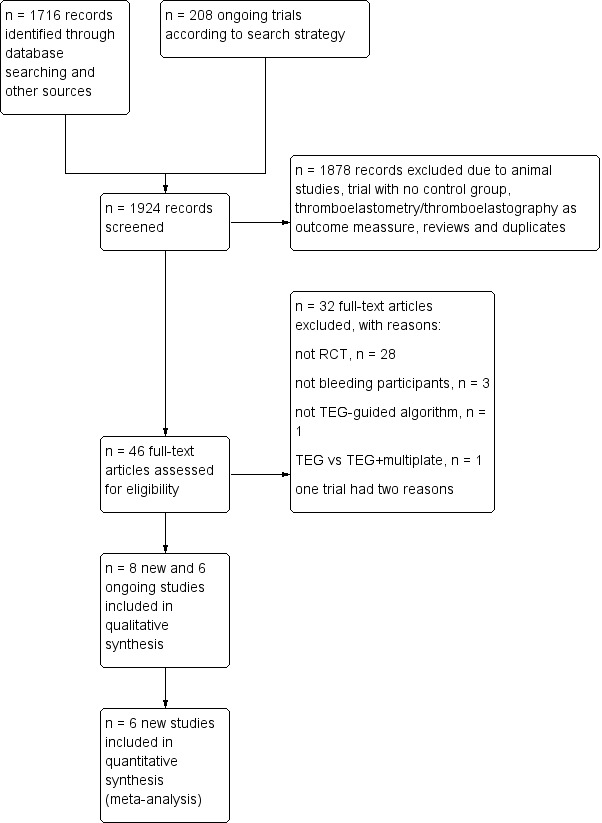
Updated flow diagram for selection of randomized controlled trials from 31 October 2010 to 5 January 5 2016.
8.
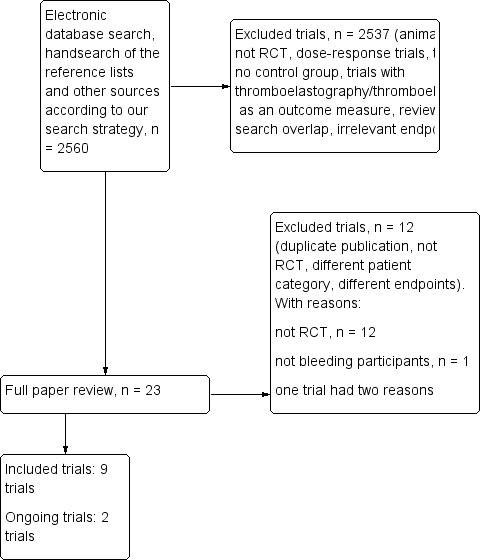
Flow diagram for selection of randomized controlled trials up to 31 October 31 according to last published version of this review (Afshari 2011).
Three of the included studies were published only as abstracts (Kempfert 2011; Paniagua 2011; Rauter 2007); and one study was terminated due to futile inclusion (NCT00772239), but with no published results. There were no duplicate reports. Mortality was reported in eight studies (Ak 2009; Girdauskas 2010; Nakayama 2015; Paniagua 2011; Royston 2001; Shore‐Lesserson 1999; Wang 2010; Weber 2012). For a more detailed description of the studies, see the table Characteristics of included studies, Table 10, and Table 11.
1. Details of included studies.
| Study | Year of publication | n | Population | Inclusion criteria |
Intervention algorithm (details in Table 11) |
Duration of intervention | Control group transfusion management | Follow‐up | Adequate blinding* |
| Ak 2009 | 2009 | 224 | Cardiac surgery | Elective first‐time CABG, with cardiopulmonary bypass | Fully TEG‐based transfusion algorithm | Intraoperative and until 24 hours post‐CPB | Clinical judgement and SLTs | Unclear; transfusion requirements recorded until discharge from hospital; mortality until 30 days | Yes |
| Avidan 2004 | 2004 | 102 | Cardiac surgery | Elective first‐time CABG, with cardiopulmonary bypass | Partly TEG‐based algorithm, included also the Hepcon and PFA‐100 platelet function analyser | Intraoperative and until 2 hours postsurgery | SLT‐guided transfusion management | 24 hours | No |
| Cui 2010 | 2010 | 31 | Cardiac surgery | Cyanotic paediatric patients undergoing arterial switch operation or double roots transplantation | Fully TEG‐based and fibrinogen concentrate part of algorithm | Unclear | Clinical judgement | Unclear, but at least until ICU discharge | Unclear |
| Girdauskas 2010 | 2010 | 56 | Cardiac surgery | High risk aortic surgery including urgent and emergency surgery (25 with acute type A dissection) with hypothermic circulatory arrest | Fully ROTEM‐based transfusion algorithm | Intra‐ and postoperative algorithm | Clinical judgement and SLTs | Hospital discharge | No |
| Kempfert 2011 | 2011 | 104 | Cardiac surgery | Adult patients with significant postoperative bleeding (> 200 mL/hour) following standard elective isolated or combined cardiac surgical procedures | Fully ROTEM‐based | Unclear | SLT‐guided transfusion management | Unclear | Unclear |
| Kultufan Turan 2006 | 2006 | 40 | Cardiac surgery | Either CABG or valve surgery | Fully ROTEG‐based transfusion algorithm | Intra‐ and postoperative algorithm | Clinical judgement and SLTs | 24 hours | Unclear |
| Nakayama 2015 | 2015 | 100 | Cardiac surgery | Elective cardiac surgery with CBP in children weighing less than 20 kg | Fully ROTEM‐based | Intraoperative algorithm | SLT‐guided transfusion management | Until discharge from PICU | Partly ‐ not blinded to staff attending the patient intraoperatively |
| NCT00772239 | 2010 | 100 | Cardiac Surgery | Cardiac surgery or heart transplantation with bleeding regardless of aetiology | ROTEM‐based | Unclear | Unclear | Unclear | Unclear |
| Nuttal 2001 | 2001 | 92 | Cardiac surgery | Abnormal microvascular bleeding after CPB, all types of elective open cardiac surgery requiring CPB | Partly TEG‐based algorithm included also point‐of‐care SLTs | Intraoperative algorithm | Clinical judgement and SLTs | Unclear; transfusion requirements recorded until discharge from hospital | No |
| Paniagua 2011 | 2011 | 44 | Cardiac surgery | Cardiac surgery with extracorporeal circulation and major postoperative bleeding (≥ 300 mL in the first postoperative hour) | Fully ROTEM‐based | Intraoperative and postoperative | SLT‐guided transfusion management | Until stopped bleeding or discharge from hospital | No |
| Rauter 2007 | 2007 | 208 | Cardiac Surgery | Elective on‐pump cardiac surgery | ROTEM‐based | Unclear | Clinical judgement and SLTs | The patients were observed intraoperatively and up to 48 hours postoperatively during their stay in the ICU | No |
| Royston 2001 | 2001 | 60 | Cardiac surgery | High risk of requiring haemostatic products (heart transplantation, revascularization bypass, Ross procedure, multiple valve and revascularization surgery) | Fully TEG‐based transfusion algorithm | Intraoperative algorithm | Clinical judgement and SLTs | Unclear; transfusion requirements and mortality were reported for 2 days postoperatively | Unclear |
| Schaden 2012 | 2012 | 30 | Excision of burn wounds | Surgical excision of burn wounds performed on the third day after burn trauma | Fully ROTEM‐based | Intraoperative and 24 hours postoperatively | Clinical judgement | Until discharge from ICU | No |
| Shore‐Lesserson 1999 | 1999 | 105 | Cardiac surgery | High risk cardiac procedures (single or multiple valve replacement, combined artery bypass plus valvular procedure, cardiac reoperations, thoracic aortic replacement) |
Fully TEG‐based transfusion algorithm instituted when microvascular bleeding occurred | Intraoperative algorithm | Clinical judgement and SLTs | Until hospital discharge, but transfusion requirements reported for 2 days | Yes |
| Wang 2010 | 2010 | 28 | Liver transplantation | Patients undergoing orthotopic liver transplantation | Fully TEG‐based transfusion algorithm | Intraoperative algorithm | SLT‐guided transfusion management | 3 years | No |
| Weber 2012 | 2012 | 100 | Cardiac surgery | Adult patients with significant postoperative bleeding (250 mL/hour or 50 mL/10 min) or diffuse coagulopathic bleeding following standard elective cardiac surgical procedures | Partly ROTEM‐based transfusion algorithm included also Platelet Mapping | Intraoperative and postoperative algorithm | SLT‐guided transfusion management | Until discharge from PICU | No |
| Westbrook 2009 | 2009 | 69 | Cardiac surgery | All types of procedures except lung transplantations | Partly TEG‐based transfusion algorithm included also Platelet Mapping | Intra‐ and postoperative algorithm | Clinical judgement and SLTs | Until hospital discharge, but transfusion requirements reported for 2 days | Unclear |
*Assessed as blinding to group allocation of physician in charge of the blood transfusion management. CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; ICU: intensive care unit; PICU: paediatric intensive care unit; ROTEG: rotational thromboelastography; ROTEM: rotational thromboelastometry; SLT: standard laboratory test; TEG: thromboelastography
2. Details of interventional algorithms.
| Author/year | Duration of intervention | Devices used | RBC trigger | FFP trigger | PLT trigger | Protamine trigger |
Cryo or fib.conc. trigger |
Antifibrinolytics trigger |
| Ak 2009 | Intraoperative and until 24 hours post‐CPB | TEG | Hct < 25% (18% accepted during CPB) |
TEG‐R > 14 min | TEG‐MA < 48 mm | h‐TEG‐R < 0.5 x TEG‐R | ‐ | LY30 > 7.5% |
| Avidan 2004* | Intraoperative and until 2 hours post‐CPB | TEG and PFA‐100 and Hepcon device | hb < 8g/dL | TEG‐R > 10 min (no heparin effect) and bleeding | Prolonged PFA‐100 channel closure time and persisting bleeding | Hepcon measurement | ‐ | LY30 > 7.5% and bleeding > 100 mL/hour |
| Cui 2010 | Unclear | TEG, kaolin activated and functional fibrinogen | No trigger stated, the Hct was higher than 54% before operation | Not described | Not described | Protamine at standard dose (4 mg/kg) | Fibrinogen concentrate to all in TEG group 500 mg to 1000 mg | Not described |
| Girdauskas 2010** | Intra‐ and postoperative | ROTEM (HEPTEM, APTEM, FIBTEM, INTEM) | Hct < 25% (hb < 8.5 g/dL) (20% (hb 6.8 g/dL) accepted during CPB) or severe haemodynamic instability |
HEPTEM‐CT > 260 sec | HEPTEM‐MCF = 35‐45 mm and FIBTEM‐MCF > 8 mm or HEPTEM‐MCF < 35 mm |
INTEM‐CT/HEPTEM‐CT > 1.5 | FIBTEM‐MCF < 8 mm | APTEM‐MCF /HEPTEM‐MCF > 1.5 (if needed beyond standard protocol) |
| Kempfert 2011 | Unclear | ROTEM (4 chamber) | Not stated | Not described | Not described | Not described | Not described | Not described |
| Kultufan Turan 2006 | Intra‐ and postoperative | ROTEG | Details not available | Details not available | Details not available | Details not available | ‐ | Details not available |
| Nakayama 2015 | Intraoperative | ROTEM (HEPTEM, APTEM, FIBTEM, INTEM, EXTEM) | Maintain the haematocrit at 25% to 30% during CPB | EXTEM‐A10 < 30 mm and FIBTEM‐A10 ≤ 5mm | EXTEM‐A10 ≤ 30 mm and FIBTEM‐A10 > 5mm | HEPTEM‐CT/INTEM‐CT < 0.8 | Not available | Not described |
| NCT00772239 | Unclear | ROTEM | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Nuttal 2001 | Intraoperative | TEG, Coaguchek Plus and Coulter‐MDII | Details not available | PT > 16.6 sec or aPTT > 57 sec | TEG‐MA < 48 mm or platelet count < 102,000/µL | ACT‐guided | p‐fibrinogen < 144 mg/dL | At the discretion of the anaesthesiologist |
| Paniagua 2011 | Intra‐ and postoperative | ROTEM (HEPTEM, FIBTEM, INTEM, EXTEM, APTEM) | Hb < 8 g/dL | First line: EXTEM‐CT > 80 S or HEPTEM‐CT > 280 S and INTEM‐CT > 240 S Second line: EXTEM‐MCF < 50 mm and FIBTEM‐MCF < 12 mm | EXTEM‐MCF < 50 mm and FIBTEM‐MCF > 12 mm* | INTEM‐CT > 240 sec and HEPTEM‐CT normal | One patient in the ROTEM group received fibrinogen concentrate. The drug was only available during the last three months of inclusions | EXTEM‐CT >80 S, CFT > 159, EXTEM‐MCF < 50 mm and/or Lysis at 1 hour > 15% |
| Rauter 2007 | Unclear | ROTEM | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Royston 2001 | Intraoperative | TEG and h‐TEG |
Details not available | h‐TEG‐R > 14 min | h‐TEG‐MA < 48 mm | Details not available | ‐ | LY30 > 7.5% |
| Schaden 2012 | Intra‐ and postoperative | ROTEM (APTEM, FIBTEM, EXTEM) | Hb < 8 g/dL | EXTEM‐CT > 100 S | EXTEM‐A10 < 45 mm and FIBTEM‐A10 > 12 mm |
Not relevant | EXTEM‐A10 < 45 mm and FIBTEM‐A10 < 12 mm | Spindle‐shaped trace Or APTEM‐A10 >> EXTEM‐A10 or EXTEM‐LY30 > 10 % |
| Shore‐Lesserson 1999 | Intraoperative | TEG and h‐TEG |
Hct < 25% (21% accepted during CPB) and bleeding (> 100 mL in 3 min or absence of visible clots) |
h‐TEG‐R > 20 min and bleeding (> 100 mL in 3 min or absence of visible clots) | TEG‐MA < 45 mm and platelet count < 100,000/µL and bleeding (> 100 mL in 3 min or absence of visible clots) | TEG‐R > 2 x h‐TEG‐R | p‐fibrinogen < 100 mg/dL and bleeding (> 100 mL in 3 min or absence of visible clots) | LY30 > 7.5% (if needed beyond standard protocol) |
| Wang 2010 | Unclear/most likely only intraoperative | TEG | hb < 8 g/dL | TEG‐R > 10 min | TEG‐MA < 55 mm | ‐ | TEG‐alpha‐angle < 45 degrees | LY30, no limit stated |
| Weber 2012 | Intra‐ and postoperative | ROTEM (HEPTEM, FIBTEM, INTEM, EXTEM) | Hb < 6 g/dL during CPB and < 8 g/dL after CPB | EXTEM‐CT > 80 S or HEPTEM‐CT > 240 S | EXTEM‐A10 < 40 mm and FIBTEM‐A10 > 10 mm or MULTIPLATE (TRAP < 50 AU and/or ASPI < 30 AU and/or ADP < 30 AU*) | ACT > 130 and INTEM‐CT > 240 sec and HEPTEM‐CT/INTEM‐CT < 0.8 | EXTEM‐A10 < 40 mm and FIBTEM –A10 < 10 mm | Antifibrinolytic therapy consisted of the application of 2 g tranexamic acid after the induction of anaesthesia, and another 2 g was added into the priming volume of the heart–lung machine and again during CPB |
| Westbrook 2009 | Intra‐ and postoperative | TEG, h‐TEG and Platelet Mapping |
hb < 7 g/dL | h‐TEG‐R > 11 min and persisting bleeding (> 60 mL in first 30 min after protamine or > 60 mL/hour in ICU) | h‐TEG‐MA ≤ 41 mm and persisting bleeding (> 60 mL in first 30 min after protamine or > 60 mL/hour in ICU) | TEG‐R –h‐TEG‐R ≥ 3 min | h‐TEG‐MA > 45 and ‐TEG‐alpha‐angle < 45 degrees | LY30 > 15% |
*In addition: prothrombin complex concentrate if APTEM‐CT > 120 sec; **in addition: desmopressin if persisting bleeding and prolonged PFA‐100 channel closure time; ***in addition: desmopressin singular therapy approach for first time confirmed platelet dysfunction. ‐A10: ROTEM value measured early at 10 min; ACT: activated clotting time; ADP: multiplate test; APTEM: aprotinin test of ROTEM; aPTT: activated partial thromboplastin time; ASPI: multiplate test; AU: arbitrary aggregation units; CPB: cardiopulmonary bypass; ‐CT: clot formation time; EXTEM: extrinsic system screen test; FFP: fresh frozen plasma; FIBTEM: fibrinogen test of ROTEM; h‐: heparin cup; hb: haemoglobin; Hct: haematocrit; HEPTEM: heparin test of ROTEM; ICU: intensive care unit; INTEM: intrinsic system screen test; LY30: Lysis time at 30 min; ‐MCF: maximum clot firmness; p‐: plasma; PLT: platelet transfusion data; PT: prothrombin time; RBC: red blood cell; ROTEG: rotational TEG; ROTEM: rotational thromboelastometry; TEG: thromboelastography; TEG‐R: thromboelastography reaction time; TEG‐MA: thromboelastography maximum amplitude; TRAP: thrombin receptor activating peptide multiplate test;
Included studies
We included 17 trials, of which two included only paediatric participants. The sample size varied from 28 participants to 224. One trial was conducted in a liver transplant setting (Wang 2010), another in wound excisions of burns patients (Schaden 2012), while the remaining trials (96% (1435) of included patients) were conducted in a cardiac surgery setting (see Table 10; Characteristics of included studies). The majority of trials applied the intervention algorithm intra‐ and postoperatively even if some only included the first two hours postoperatively. Fibrinogen substitution with fibrinogen concentrate or cryoprecipitate was described as part of eight interventional trial algorithms (Table 11). Follow‐up ranged from 24 hours to three years, but information on six trials was unclear or did not provide data on the length of follow‐up (see Table 10; Characteristics of included studies; Ak 2009; Cui 2010; Kempfert 2011; NCT00772239; Nuttal 2001; Royston 2001).
Four of the trials were stopped early either due to either futile inclusion (Kempfert 2011; NCT00772239; Paniagua 2011), or a positive interim analysis (Weber 2012). In ten trials, the transfusion strategy in the control group was at the clinicians' discretion in combination with standard laboratory tests (Ak 2009; Cui 2010; Girdauskas 2010; Kultufan Turan 2006; Nuttal 2001; Royston 2001; Schaden 2012; Shore‐Lesserson 1999; Rauter 2007; Westbrook 2009). Seven trials compared TEG or ROTEM versus a transfusion algorithm solely based on standard laboratory tests and without clinicians' discretion (Avidan 2004; Kempfert 2011; Nakayama 2015; NCT00772239; Paniagua 2011; Wang 2010; Weber 2012). Five trials used TEG or ROTEM in combination with other devices in the intervention group: Avidan 2004 also used PFA‐100 and Hepcon, Nuttal 2001 used Coagucheck Plus and Coulter‐MPIII, Westbrook 2009 used Platelet Mapping, Weber 2012 used Multiplate, and Shore‐Lesserson 1999 used platelet count and plasma fibrinogen concentration (Table 5).
10. Comparisons and interventional devices.
| Intervention device/comparison | TEG or ROTEM alone | TEG or ROTEM in combination with SLTs | TEG or ROTEM in combination with platelet function analysis |
| Clinical judgement or usual treatment |
Ak 2009; Cui 2010; Girdauskas 2010; Kultufan Turan 2006; Rauter 2007; Royston 2001; Schaden 2012 |
Nuttal 2001; Shore‐Lesserson 1999 |
Westbrook 2009 |
| SLT‐guided algorithm | Kempfert 2011; Nakayama 2015; Paniagua 2011; Wang 2010 | Avidan 2004; Weber 2012 |
ROTEM: rotational thromboelastometry; SLT: standard laboratory tests; TEG: thromboelastography In the NCT00772239 we did not have information on the comparison.
Half of the studies used ROTEM and half used TEG, with the majority of new trials using ROTEM (see Characteristics of included studies; Table 10; Table 11; Characteristics of ongoing studies).
Excluded studies
In this update we excluded 94% (282/300) of publications assessed as full‐text (Figure 7), with the 44 most relevant new publications described in Characteristics of excluded studies with reasons for exclusion.
Ongoing studies
We included a total of six ongoing studies in this review (NCT02352181; NCT02593877; NCT02461251; NCT01536496; NCT02416817; NCT01402739). In the first published version of this review we found two ongoing trials (Afshari 2011): one which is included as Weber 2012 while NCT00772239, despite being included, had no published data and was terminated due to futile inclusion. The six ongoing trials include three trauma trials using TEG (NCT01536496; NCT02416817) and TEG or ROTEM (NCT02593877), one obstetric (NCT02461251), and one cardiac surgery using ROTEM (NCT01402739), and finally one in a liver transplantation setting using ROTEM (NCT02352181).
Awaiting classification
No studies are awaiting classification.
Risk of bias in included studies
We evaluated the overall quality of trials based on the major sources of bias (domains) as described in Assessment of risk of bias in included studies. We classified only two trials at overall low risk of bias (Nakayama 2015; Shore‐Lesserson 1999; Figure 9). For a more detailed description of individual trial qualities see the table Characteristics of included studies. The various bias domains are presented in the 'Risk of bias' summary figure and a 'Risk of bias' graph (Figure 9; Figure 10).
9.
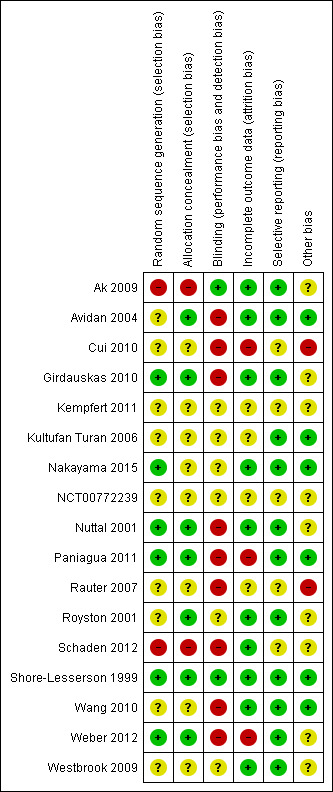
Updated risk of bias summary: review authors' judgements about each risk of bias item for each included study.
10.
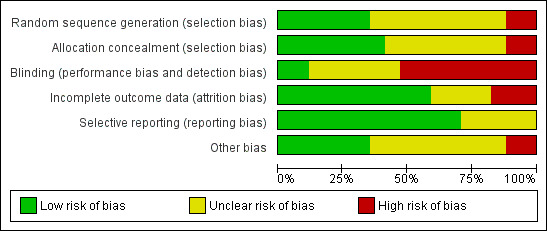
Updated risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation was adequately reported in six trials (35%) (Girdauskas 2010; Nakayama 2015; Nuttal 2001; Paniagua 2011; Shore‐Lesserson 1999; Weber 2012), while allocation concealment was adequately reported in seven trials (41%) (Avidan 2004; Girdauskas 2010; Nuttal 2001; Paniagua 2011; Royston 2001; Shore‐Lesserson 1999;Weber 2012).
Blinding
Adequate blinding in trials using transfusion strategy remains a challenge and many of the authors claimed to have adequate blinding. Indeed the trials did provide data, but on different levels of blinding. In our opinion, blinding of clinicians and participants in the operating theatre was the most important (performance bias). However,the masking of assessors of bleeding measurement and transfusion requirements (detection bias) is also very important. Lack of blinding or insufficient blinding in the operating theatre does raise doubts about the degree of critical information being passed on postoperatively to those responsible for the care and treatment of the patient.
Only two trials provided sufficient data to be categorized as blinded (12%) (Ak 2009; Shore‐Lesserson 1999). The remaining trials were either open‐label or did not provide sufficient data on how adequate blinding was achieved (Characteristics of included studies; Figure 9; Figure 10).
Incomplete outcome data
Ten (60%) of the trials performed their analysis according to the intention‐to‐treat (ITT) method or provided sufficient data to perform ITT analyses (Ak 2009; Avidan 2004; Girdauskas 2010; Nakayama 2015; Nuttal 2001; Royston 2001; Schaden 2012; Shore‐Lesserson 1999; Wang 2010; Westbrook 2009); the remaining studies were unclear about application of ITT. Additionally, some of the trials did not provide explicit information on the length of the longest follow‐up (Table 10). Many of our analyses were subject to limitations due to demonstration of therapeutic effect in graphic form and without numerical data in the publications.
Selective reporting
Twelve trials appeared to be free of selective reporting; judged in comparison to trial registration (Ak 2009; Avidan 2004; Girdauskas 2010; Kultufan Turan 2006; Nakayama 2015; Nuttal 2001; Paniagua 2011; Royston 2001; Shore‐Lesserson 1999; Wang 2010; Weber 2012; Westbrook 2009), protocol provided by authors (Paniagua 2011), or based on available information in the publication. Additionally, only eight trials provided data on our primary outcome, mortality (Ak 2009; Girdauskas 2010; Nakayama 2015; Paniagua 2011; Royston 2001; Shore‐Lesserson 1999; Wang 2010; Weber 2012), with two of them being zero event trials (Nakayama 2015; Royston 2001).
Other potential sources of bias
Eight trials disclosed the funding source and were defined as not for profit (Avidan 2004; Kultufan Turan 2006; Nakayama 2015; Paniagua 2011; Schaden 2012; Shore‐Lesserson 1999; Wang 2010Weber 2012), while the funding source for the rest was defined as unknown. Three of the independently funded trials have authors with relations to TEM innovations (Nakayama 2015; Schaden 2012; Weber 2012). Sample size calculation was reported in ten trials (Ak 2009; Avidan 2004; Girdauskas 2010; Nakayama 2015; Nuttal 2001; Paniagua 2011; Royston 2001; Schaden 2012; Shore‐Lesserson 1999; Weber 2012), but none were powered to show a statistically significant benefit in mortality. One trial was stopped early due to benefits (Weber 2012), and two due to slow enrolment or lack of funding (NCT00772239; Paniagua 2011).
Pooling trials with different follow‐up on mortality might bias the result caused by potential differences in the underlying mechanism. However, all included trials but one used hospital admission as the longest follow‐up (Wang 2010).
The funnel plot of standard error versus risk ratio for overall longest follow‐up mortality showed a symmetrical distribution that indicated no publication bias. Analyses of the impact of TEG and ROTEM on bleeding were hindered due to application of different indicators of bleeding and transfusion, different time points for measurement, and demonstration of therapeutic effect in graphic form without numerical data.
Other clinical outcome variables in line with our defined primary and secondary outcomes were inconsistently reported. In general, trials provided data with very skewed distribution of continuous outcomes such as bleeding, amount of blood products transfused, length of stay in ICU or hospital and time to extubation. Following statistical consultation with a Cochrane statistical editor and after re‐evaluation of the available data, we abstained from performing meta‐analyses, since the data were considered substantially skewed. As a consequence, the results of the included trials are summarized in tables demonstrating the distribution of each continuous outcome (Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18).
3. Continous outcome data: bleeding volume (mL), longest follow‐up.
| Study | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Mean (SD) | 481 (351) | 591 (339) | 0.087 |
| Avidan 2004 | Median (IQR) | 755 (606; 975) | 850 (688; 1095) | > 0.05 |
| Cui 2010* | Median (IQR) | 0.7 (0.6; 0.9) | 0.6 (0.4; 0.8) | 0.092 |
| Girdauskas 2010 | Median (IQR) | 890 (600; 1250) | 950 (650; 1400) | 0.50 |
| Kempfert 2011 | Mean (SD) | 1599 (834) | 1867 (827) | 0.066 |
| Kultufan Turan 2006 | Mean (SD) | 838 (494) | 711 (489) | 0.581 |
| Nakayama 2015** | Median (IQR) | 13 (9; 12) | 22 (13; 35) | 0.002 |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | Median (range) | 590 (240; 2335) | 850 (290; 10190) | 0.019 |
| Paniagua 2011 | Mean (SD) | 2408 (1771) | 2736 (1617) | ‐ |
| Rauter 2007 | ‐ | ‐ | ‐ | ‐ |
| Royston 2001 | Median (IQR) | 470 (295; 820) | 390 (240; 820) | ‐ |
| Schaden 2012 | ‐ | ‐ | ‐ | ‐ |
| Shore‐Lesserson 1999 | Mean (SD) | 720 (500) | 901 (847) | 0.27 |
| Wang 2010 | Mean (SD) | 4776 (4265) | 6348 (3704) | > 0.05 |
| Weber 2012 | Median (IQR) | 600 (263; 875) | 900 (600; 1288) | 0.021 |
| Westbrook 2009 | Median (IQR) | 875 (755; 1130) | 960 (820; 1200) | 0.437 |
*Data given in mL/kg/hour; **data given in mL/kg; ‐ indicates that data were not reported; significant result is highlighted with bold text. IQR: interquartile range; SD: standard deviation
4. Continous outcome data: total PRBC transfusion.
| Study | Reported unit | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Units | Median (IQR) | 1 (0; 1) | 1 (1; 2) | 0.599 |
| Avidan 2004 | mL | Median (IQR) | 500 (0; 678) | 495 (0; 612) | 0.03 |
| Cui 2010 | Units | Median (IQR) | 1 (1; 1) | 1 (0.7; 1.9) | > 0.05 |
| Girdauskas 2010 | Units | Median (IQR) | 6 (2; 13) | 9 (4; 14) | 0.20 |
| Kempfert 2011 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kultufan Turan 2006 | Units | Median (IQR) | 0 (0; 3) | 1 (0; 2) | 0.100 |
| Nakayama 2015 | mL/kg | Mean (IQR) | 22 (11; 34) | 30 (20; 39) | 0.02 |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | Units | Median (range) | 2 (0; 9) | 3 (0; 70) | 0.039 |
| Paniagua 2011 | mL | Mean (SD) | 1774 (1394) | 1604 (1366) | ‐ |
| Rauter 2007 | Units | Mean | 0.8 | 1.3 | * |
| Royston 2001 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Schaden 2012 | Units | Mean (SD) | 3.1 (2.1) | 4.8 (3.0) | 0.12 |
| Shore‐Lesserson 1999 | mL | Mean (SD) | 354 (487) | 475 (593) | 0.12 |
| Wang 2010 | Units | Mean (SD) | 14.2 (7.1) | 16.7 (12.8) | > 0.05 |
| Weber 2012 | Units | Median (IQR) | 3 (2; 6) | 5 (4; 9) | < 0.001 |
| Westbrook 2009 | Units | Total | 14 | 33 | ** |
*P value is reported as < 0.05, but seems to be calculated based on units given to each group instead of mean/median, thereby wrongly assuming that each of the units given are independent; **P value is reported as 0.12, but is calculated based on units given to each group instead of mean/median, thereby wrongly assuming that each of the units given are independent; ‐ indicates that data were not reported; significant result is highlighted with bold text. IQR: interquartile range; PRBC: pooled red blood cell; SD: standard deviation
5. Continous outcome data: total FFP transfusion.
| Study | Reported unit | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Units | Median (IQR) | 1 (1; 1) | 1(1; 2) | 0.001 |
| Avidan 2004 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cui 2010 | mL | Mean (SD) | 719 (216) | 883 (335) | < 0.05 |
| Girdauskas 2010 | Units | Median (IQR) | 3 (0; 12) | 8 (4; 18) | 0.01 |
| Kempfert 2011 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kultufan Turan 2006 | Units | Mean (SD) | 2.8 (0.95) | 2.7 (1.5) | 0.403 |
| Nakayama 2015 | mL/kg | Median (IQR) | 26 (16; 31) | 25 (12;41) | 0.87 |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | Units | Median (range) | 2 (0‐10) | 4 (0‐75) | 0.005 |
| Paniagua 2011 | mL | Mean (SD) | 799 (1188) | 707 (997) | ‐ |
| Rauter 2007 | Units | Total | 0 | 4 | ‐ |
| Royston 2001 | Units | Total | 5 | 16 | * |
| Schaden 2012 | Units | Median (IQR) | 0 (0; 0) | 5.0 (1.5‐7.5) | < 0.001 |
| Shore‐Lesserson 1999 | mL | Mean (SD) | 36 (142) | 217 (436) | < 0.04 |
| Wang 2010 | Units | Mean (SD) | 12.8 (7.0) | 21.5 (12.7) | < 0.05 |
| Weber 2012 | Units | Median (IQR) | 0 (0; 3) | 5 (3; 8) | < 0.001 |
| Westbrook 2009 | Units | Total | 22 | 18 | ‐ |
*P value is reported as < 0.05, but seems to be calculated based on units given to each group instead of mean/median, thereby wrongly assuming that each of the units given are independent; ‐ indicates that data were not reported; significant result is highlighted with bold text. FFP: fresh frozen plasma; IQR: interquartile range; SD: standard deviation
6. Continous outcome data: total platelet transfusion.
| Study | Reported unit | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Units | Median (IQR) | 1 (1; 1) | 1 (1; 2) | 0.001 |
| Avidan 2004 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cui 2010 | Units | Median (IQR) | 1 (1; 1) | 1 (0.7‐1.9) | > 0.05 |
| Girdauskas 2010 | Units | Median (IQR) | 2 (2; 3) | 2 (2;3) | 0.70 |
| Kempfert 2011 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kultufan Turan 2006 | Median (IQR) | 0 (0; 4) | 0 (0;0) | 0.411 | |
| Nakayama 2015 | mL/kg | Median (IQR) | 0 (0; 25) | 0 (0;17) | 0.28 |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | Units | Median (range) | 6 (0‐18) | 6 (0‐144) | 0.0001 |
| Paniagua 2011 | mL | Mean (SD) | 212 (307) | 331 (406) | ‐ |
| Rauter 2007 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Royston 2001 | Units | Total | 1 | 9 | * |
| Schaden 2012 | Units | Median (range) | 0 (0‐0) | 0 (0‐2) | 0.12 |
| Shore‐Lesserson 1999 | mL | Mean (SD) | 34 (94) | 83 (160) | 0.16 |
| Wang 2010 | Units | Mean (SD) | 27.5 (13.9) | 30.1 (18.5) | > 0.05 |
| Weber 2012 | Units | Median (IQR) | 2 (0; 2) | 2 (0; 5) | 0.010 |
| Westbrook 2009 | Units | Total | 5 | 15 | ‐ |
*P value is reported as < 0.05, but seems to be calculated based on units given to each group instead of mean/median, thereby wrongly assuming that each of the units given are independent; ‐ indicates that data were not reported; significant result is highlighted with bold text. IQR: interquartile range; SD: standard deviation
7. Continous outcome data: length of stay in hospital (days).
| Study | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Mean (SD) | 6.2 (1.1) | 6.3 (1.4) | 0.552 |
| Avidan 2004 | ‐ | ‐ | ‐ | ‐ |
| Cui 2010 | Median (IQR) | 21 (15.5; 30.0) | 32 (24.3; 40.3) | 0.006 |
| Girdauskas 2010 | Mean (SD) | 16.6 (16.4) | 17.0 (14.8) | 0.80 |
| Kempfert 2011 | ‐ | ‐ | ‐ | ‐ |
| Kultufan Turan 2006 | ‐ | ‐ | ‐ | ‐ |
| Nakayama 2015 | ‐ | ‐ | ‐ | ‐ |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | ‐ | ‐ | ‐ | ‐ |
| Paniagua 2011 | Mean (SD) | 13.6 (7.1) | 25.8 (19.2) | ‐ |
| Rauter 2007 | ‐ | ‐ | ‐ | ‐ |
| Royston 2001 | ‐ | ‐ | ‐ | ‐ |
| Schaden 2012 | ‐ | ‐ | ‐ | ‐ |
| Shore‐Lesserson 1999 | ‐ | ‐ | ‐ | ‐ |
| Wang 2010 | ‐ | ‐ | ‐ | ‐ |
| Weber 2012 | Median (IQR) | 12 (9;22) | 12 (9; 23) | 0.718 |
| Westbrook 2009 | Median (IQR) | 9 (7‐13) | 8 (7‐12) | > 0.05 |
‐ Indicates that data were not reported; significant result is highlighted with bold text. IQR: interquartile range; SD: standard deviation
8. Continous outcome data: length of stay in intensive care unit (hours).
| Study | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Mean (SD) | 23.3 (5.7) | 25.3 (11.2) | 0.099 |
| Avidan 2004 | ‐ | ‐ | ‐ | ‐ |
| Cui 2010 | Median (IQR) | 137 (106; 161) | 173 (138; 477) | 0.009 |
| Girdauskas 2010 | Mean (SD) | 175.2 (218.4) | 194.4 (201.6) | 0.6 |
| Kempfert 2011 | ‐ | ‐ | ‐ | ‐ |
| Kultufan Turan 2006 | ‐ | ‐ | ‐ | ‐ |
| Nakayama 2015 | Median (IQR) | 60 (35; 81) | 71 (60; 108) | 0.014 |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | ‐ | ‐ | ‐ | ‐ |
| Paniagua 2011 | Mean (SD) | 132 (120) | 236 (168) | ‐ |
| Rauter 2007 | ‐ | ‐ | ‐ | ‐ |
| Royston 2001 | ‐ | ‐ | ‐ | ‐ |
| Schaden 2012 | ‐ | ‐ | ‐ | ‐ |
| Shore‐Lesserson 1999 | ‐ | ‐ | ‐ | ‐ |
| Wang 2010 | ‐ | ‐ | ‐ | ‐ |
| Weber 2012 | Median (IQR) | 21 (18‐31) | 24 (20‐87) | 0.019 |
| Westbrook 2009 | Median (IQR) | 29.4 (14.3; 56.4) | 32.5 (22.0; 74.5) | 0.369 |
‐ Indicates that data was not reported; Significant result is highlighted with bold text. IQR: interquartile range; SD: standard deviation
9. Continous outcome data: time to extubation (hours).
| Study | Data available | Intervention results | Control results |
P value for difference between groups |
| Ak 2009 | Mean (SD) | 8.2 (2.1) | 7.9 (4.7) | 0.540 |
| Avidan 2004 | Median (IQR) | 4.4 (3.0; 6.2) | 4.3 (3.0; 5.9) | > 0.05 |
| Cui 2010 | Median (IQR) | 40.0 (25.5; 75.0) | 106.5 (54.8; 196.5) | 0.009 |
| Girdauskas 2010 | Mean (SD) | 144 (139) | 137 (172) | 0.8 |
| Kempfert 2011 | ‐ | ‐ | ‐ | ‐ |
| Kultufan Turan 2006 | ‐ | ‐ | ‐ | ‐ |
| Nakayama 2015 | Median (IQR) | 3 (2; 17) | 4 (2; 23) | 0.06 |
| NCT00772239 | ‐ | ‐ | ‐ | ‐ |
| Nuttal 2001 | ‐ | ‐ | ‐ | ‐ |
| Paniagua 2011 | Mean (SD) | 15.6 (12.3) | 32.0 (59.0) | ‐ |
| Rauter 2007 | ‐ | ‐ | ‐ | ‐ |
| Royston 2001 | ‐ | ‐ | ‐ | ‐ |
| Schaden 2012 | ‐ | ‐ | ‐ | ‐ |
| Shore‐Lesserson 1999 | ‐ | ‐ | ‐ | ‐ |
| Wang 2010 | ‐ | ‐ | ‐ | ‐ |
| Weber 2012 | Median (IQR) | 21 (18; 31) | 24 (20; 87) | 0.019 |
| Westbrook 2009 | Median (IQR) | 8 (5.3; 19.8) | 10.3 (5.8; 19.5) | ‐ |
‐ Indicates that data were not reported; significant result is highlighted with bold text. IQR: interquartile range; SD: standard deviation
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Thromboelastography (TEG) or thromboelastometry (ROTEM) versus any comparison.
| TEG or ROTEM versus any comparison for adults or children with bleeding | ||||||
| Patient or population: adults or children with bleeding Setting: majority of participants were undergoing cardiac surgery involving cardiopulmonary bypass in a high‐income hospital setting Intervention: TEG or ROTEM‐guided haemostatic transfusion Comparison: any comparison | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with any comparison | Risk with TEG or ROTEM | |||||
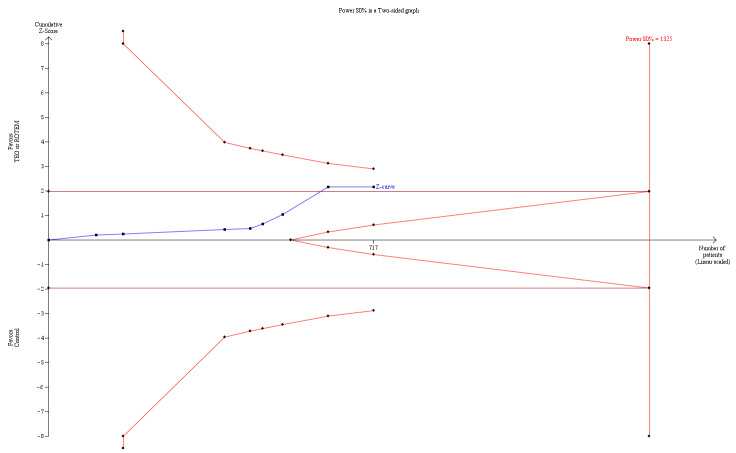
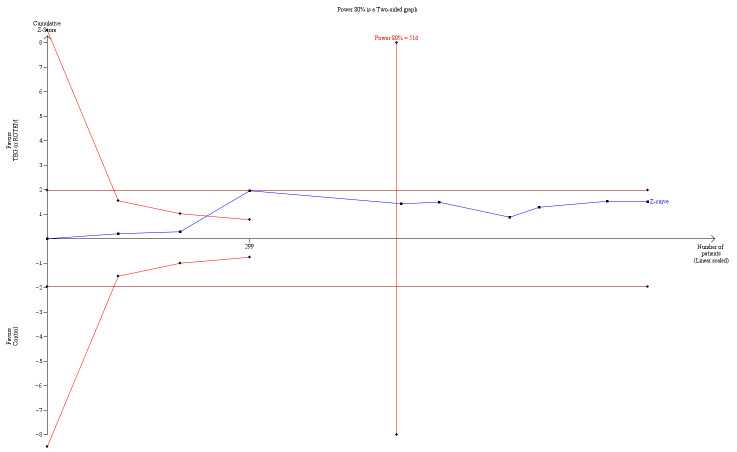
| Mortality longest follow‐up | Study population | RR 0.52 (0.28 to 0.95) | 717 (8 studies) | ⊕⊕⊝⊝ low | TSA shows that only 54% of the required information size (717 of 1325) has been reached (Effects of interventions, Figure 1). 1 |
|
| 74 per 1000 | 38 per 1000 (21 to 70) | |||||
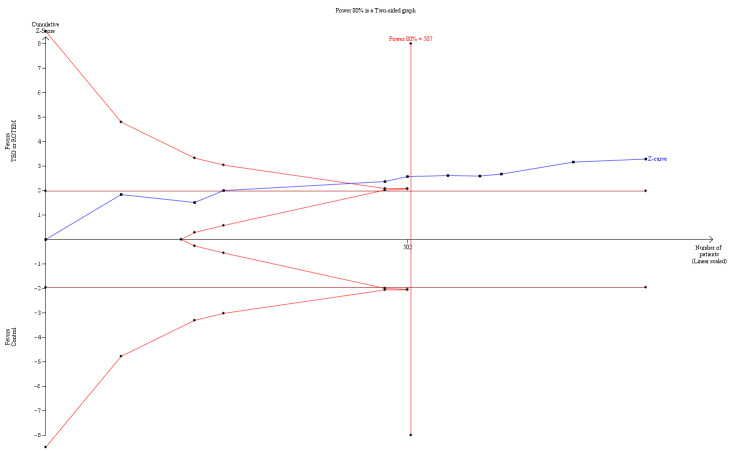
| Proportion of patients receiving PRBCs | Study population | RR 0.86 (0.79 to 0.94) | 832 (10 studies) | ⊕⊕⊝⊝ low | TSA indicates firm evidence (Effects of interventions; Figure 2).2 | |
| 720 per 1000 | 619 per 1000 (568 to 676) | |||||
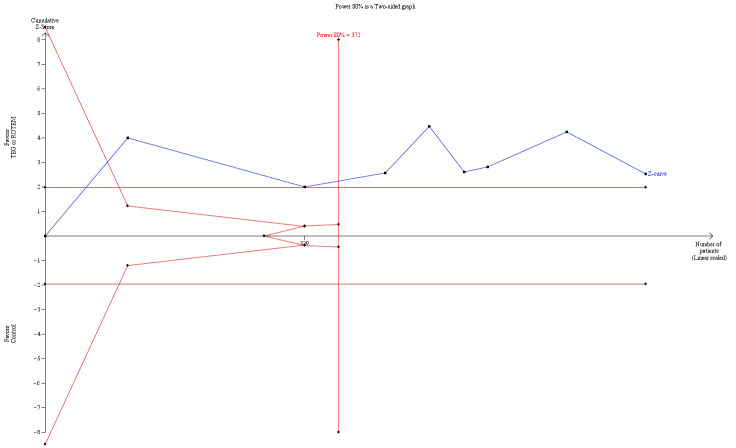
| Proportion of patients receiving FFP | Study population | RR 0.57 (0.33 to 0.96) | 761 (8 studies) | ⊕⊕⊝⊝ low | TSA indicates firm evidence (Effects of interventions; Figure 3)3 | |
| 471 per 1000 | 268 per 1000 (155 to 452) | |||||
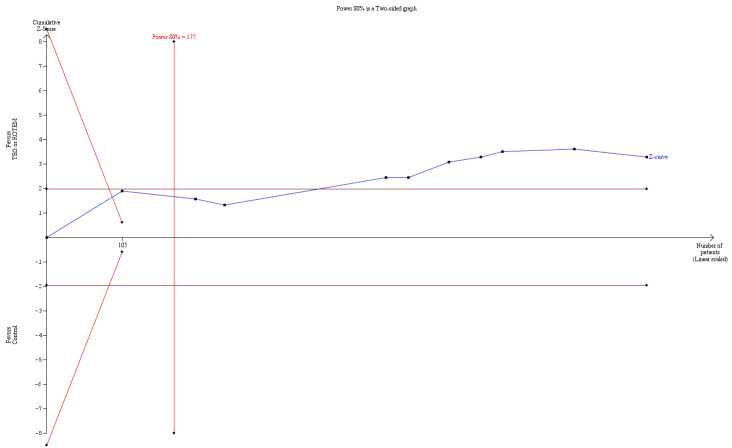
| Proportion of patients receiving platelets | Study population | RR 0.73 (0.60 to 0.88) | 832 (10 studies) | ⊕⊕⊝⊝ low | TSA indicates firm evidence, but the low risk of bias adjusted required information size has not been reached (Effects of interventions; Figure 4). 4 |
|
| 344 per 1000 | 251 per 1000 (206 to 303) | |||||
| Rate of surgical reintervention | Study population | RR 0.75 (0.50 to 1.10) | 887 (9 studies) | ⊕⊕⊝⊝ low | TSA showed a beneficial effect in favour of TEG/ROTEM‐guided transfusion management (Effects of interventions; Figure 5).5 | |
| 108 per 1000 | 81 per 1000 (54 to 119) | |||||
| Excessive bleeding events and massive transfusion | Study population | RR 0.82 (0.38 to 1.77) | 280 (2 studies) | ⊕⊕⊝⊝ low | Unable to carry out TSA because of the limited amount of data. | |
| 137 per 1000 | 112 per 1000 (52 to 242) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FFP: fresh frozen plasma; PRBC: pooled red blood cell; ROTEM: thromboelastometry; RR: risk ratio; TEG: thromboelastography; TSA: trial sequential analysis | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision.Two trials were zero event trials (Nakayama 2015; Royston 2001). Only two studies had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999), and none of the included trials in this analysis were powered to detect any difference for mortality. Changing from fixed‐effect model to random‐effects model changes the risk estimate to RR 0.57 (95% CI 0.30 to 1.07). The majority of patients are included in cardiac surgery setting, thus reducing generalizability and external validity of the finding.
2Quality of the evidence (GRADE) was adjusted due to high risk of bias and indirectness. Only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999). The direction of the effect estimate is consistent across the included trials and for the transfusion outcomes.
3Quality of the evidence (GRADE) was adjusted due to high risk of bias and imprecision. Only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999). The direction of the effect estimate is consistent across the included trials and for the transfusion outcomes.
4Quality of the evidence (GRADE) was adjusted due to high risk of bias and indirectness. Only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999). The direction of the effect estimate is consistent across the included trials and for the transfusion outcomes.
5Quality of the evidence (GRADE) was adjusted due to high risk of bias and imprecision. Only one trial had low risk of bias (Shore‐Lesserson 1999). Event rate of surgical reintervention was low overall. Inclusion of trials with coagulopathy or excessive bleeding as inclusion criteria might change this effect estimate.
6Quality of the evidence (GRADE) was adjusted due to high risk of bias, indirectness, and imprecision. Only two trials, both with high risk of bias, were included in this analysis (Ak 2009; Girdauskas 2010). Few events were reported, but the direction of the effect was consistent.
Summary of findings 2. Thromboelastography (TEG) or thromboelastometry (ROTEM) compared to clinical judgement or usual care in adults or children with bleeding.
| TEG or ROTEM compared to clinical judgement or usual care in adults or children with bleeding | ||||||
| Patient or population: adults or children with bleeding Setting: majority of participants were undergoing cardiac surgery involving cardiopulmonary bypass in a high‐income hospital setting Intervention: TEG or ROTEM Comparison: clinical judgement or usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with clinical judgement or usual care | Risk with TEG or ROTEM | |||||
| Mortality | Study population | RR 0.81 (0.32 to 2.01) | 445 (4 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 41 per 1000 | 33 per 1000 (13 to 82) | |||||
| Proportion of patients receiving PRBCs | Study population | RR 0.85 (0.73 to 1.00) | 486 (6 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 622 per 1000 | 529 per 1000 (454 to 622) | |||||
| Proportion of patients receiving FFP | Study population | RR 0.38 (0.21 to 0.68) | 415 (4 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 415 per 1000 | 158 per 1000 (87 to 283) | |||||
| Proportion of patients receiving platelets | Study population | RR 0.59 (0.43 to 0.80) | 486 (6 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 311 per 1000 | 184 per 1000 (134 to 249) | |||||
| Rate of surgical reintervention | Study population | RR 0.62 (0.32 to 1.20) | 537 (5 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 77 per 1000 | 48 per 1000 (25 to 93) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FFP: fresh frozen plasma; PRBC: pooled red blood cell; ROTEM: thromboelastometry; RR: risk ratio; TEG: thromboelastography. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 3. Thromboelastography (TEG) or thromboelastometry (ROTEM) compared to standard laboratory test (SLT)‐guided transfusion in adults or children with bleeding.
| TEG or ROTEM compared to SLT‐guided transfusion in adults or children with bleeding | ||||||
| Patient or population: adults or children with bleeding Setting: The majority of participants were undergoing cardiac surgery involving cardiopulmonary bypass in a high‐income hospital setting Intervention: TEG or ROTEM Comparison: SLT‐guided transfusion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with SLT‐guided transfusion | Risk with TEG or ROTEM | |||||
| Mortality | Study population | RR 0.36 (0.16 to 0.84) | 272 (4 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 129 per 1000 | 46 per 1000 (21 to 108) | |||||
| Proportion of patients receiving PRBCs | Study population | RR 0.91 (0.83 to 1.00) | 244 (3 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 932 per 1000 | 848 per 1000 (774 to 932) | |||||
| Proportion of patients receiving FFP | Study population | RR 0.83 (0.49 to 1.40) | 346 (4 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 538 per 1000 | 447 per 1000 (264 to 754) | |||||
| Proportion of patients receiving platelets | Study population | RR 0.87 (0.68 to 1.11) | 244 (3 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 551 per 1000 | 479 per 1000 (375 to 611) | |||||
| Rate of surgical reintervention | Study population | RR 0.82 (0.46 to 1.46) | 248 (3 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 217 per 1000 | 178 per 1000 (100 to 316) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FFP: fresh frozen plasma; PRBC: pooled red blood cell; ROTEM: thromboelastometry; RR: risk ratio; SLT: standard laboratory test; TEG: thromboelastography. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 4. Thromboelastography (TEG) or thromboelastometry (ROTEM) in combination with SLT or other devices compared to clinical judgement or usual care in adults or children with bleeding.
| TEG or ROTEM in combination with SLT or other devices compared to clinical judgement or usual care in adults or children with bleeding | ||||||
| Patient or population: adults or children with bleeding Setting: majority of participants were undergoing cardiac surgery involving cardiopulmonary bypass in a high‐income hospital setting Intervention: TEG or ROTEM in combination with SLT or other devices Comparison: clinical judgement or usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with clinical judgement or usual care | Risk with TEG or ROTEM in combination with SLT or other devices | |||||
| Mortality | Study population | RR 0.20 (0.05 to 0.75) | 205 (2 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 118 per 1000 | 24 per 1000 (6 to 88) | |||||
| Proportion of patients receiving PRBCs | Study population | RR 0.85 (0.74 to 0.98) | 307 (3 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 752 per 1000 | 639 per 1000 (556 to 737) | |||||
| Proportion of patients receiving FFP | Study population | RR 0.46 (0.20 to 1.08) | 307 (3 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 366 per 1000 | 168 per 1000 (73 to 395) | |||||
| Proportion of patients receiving platelets | Study population | RR 0.75 (0.47 to 1.20) | 307 (3 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 320 per 1000 | 240 per 1000 (151 to 384) | |||||
| Rate of surgical reintervention | Study population | RR 0.41 (0.17 to 0.96) | 400 (4 studies) | ⊕⊕⊝⊝ low | Quality of the evidence (GRADE) adjusted due to high risk of bias and imprecision. | |
| 83 per 1000 | 34 per 1000 (14 to 80) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FFP: fresh frozen plasma; PRBC: pooled red blood cell; ROTEM: thromboelastometry; RR: risk ratio; TEG: thromboelastography. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1. Thromboelastography (TEG)‐ or thromboelastometry (ROTEM)‐guided algorithm versus any comparison
See: Table 1
Primary outcome
Overall mortality
Combining data from eight trials (Ak 2009; Girdauskas 2010; Nakayama 2015; Paniagua 2011; Royston 2001; Shore‐Lesserson 1999; Wang 2010; Weber 2012), and applying complete‐case analysis showed a statistically significant effect of TEG‐ or ROTEM‐guided algorithms versus any comparison on longest follow‐up mortality: 14/364 deaths (3.9%) in the TEG/ROTEM group compared with 26/353 deaths (7.4%) in the control group (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.28 to 0.95; I2 = 0‐%, 8 studies, 717 participants, low quality of evidence) (Analysis 1.1). This corresponds to a 48% relative risk reduction favouring a TEG‐ or ROTEM‐guided transfusion. Two trials were zero event trials (Nakayama 2015; Royston 2001). All trials except Wang 2010 had point of hospital discharge as longest follow‐up.
1.1. Analysis.
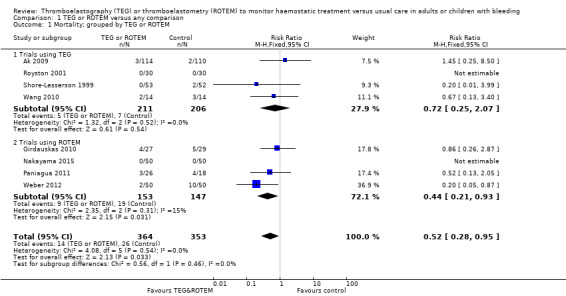
Comparison 1 TEG or ROTEM versus any comparison, Outcome 1 Mortality; grouped by TEG or ROTEM.
However, due to the large distribution of the CI, the substantial number of trials with high risk of bias and clinical heterogeneity involved, we carried out an exploratory analysis using a random‐effects model instead of a fixed‐effect model, by which the statistical significance was no longer present (RR 0.57, 95% CI 0.30 to 1.07; I2 = 0%).
Secondary outcomes
Transfusion requirements
The proportion of patients in need of pooled red blood cells (PRBCs) showed a significant reduction of 14% (RR 0.86, 95% CI 0.79 to 0.94; I2 = 0%, 10 studies, 832 participants, low quality of evidence) in favour of a TEG‐ or ROTEM‐guided transfusion (Analysis 1.3). In addition, we found a significant reduction in the proportion of patients in need of fresh frozen plasma (FFP) transfusion (RR 0.57, 95% CI 0.33 to 0.96; I2 = 86%, 8 studies, 761 participants, low quality of evidence; Analysis 1.6) and the proportion of patients in need of platelets (RR 0.73, 95% CI 0.60 to 0.88; I2 = 0‐%, 10 studies, 832 participants, low quality of evidence; Analysis 1.8) both in favour of a TEG/ROTEM‐based transfusion. We found a reduced risk of needing haemostatic treatment with FFP and platelets with 257 participants in the analysis and no new studies providing data (RR 0.44, 95% CI 0.28 to 0.81; I2= 0%; 2 studies, 257 participants, low quality of evidence; Analysis 1.11). Few trials used haemostatic treatment such as fibrinogen concentrate, prothrombin complex concentrate, or recombinant factor VIIa (Girdauskas 2010; Weber 2012), and no significant effect was identified in these outcomes (Table 11; Analysis 1.12; Analysis 1.13; Analysis 1.14).
1.3. Analysis.
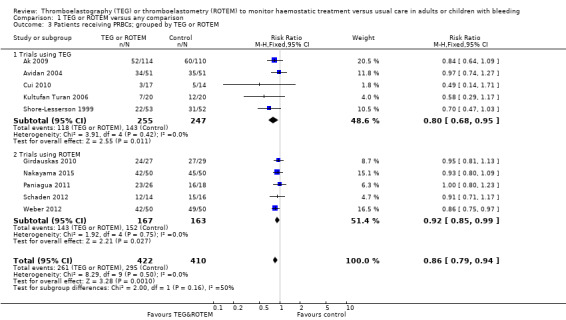
Comparison 1 TEG or ROTEM versus any comparison, Outcome 3 Patients receiving PRBCs; grouped by TEG or ROTEM.
1.6. Analysis.
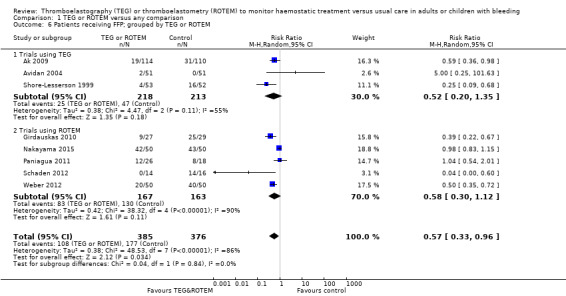
Comparison 1 TEG or ROTEM versus any comparison, Outcome 6 Patients receiving FFP; grouped by TEG or ROTEM.
1.8. Analysis.
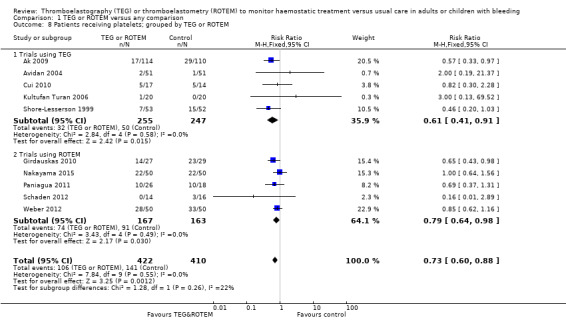
Comparison 1 TEG or ROTEM versus any comparison, Outcome 8 Patients receiving platelets; grouped by TEG or ROTEM.
1.11. Analysis.
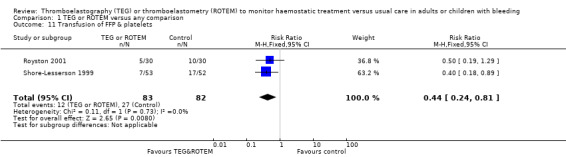
Comparison 1 TEG or ROTEM versus any comparison, Outcome 11 Transfusion of FFP & platelets.
1.12. Analysis.
Comparison 1 TEG or ROTEM versus any comparison, Outcome 12 Patients receiving fibrinogen concentrate.
1.13. Analysis.
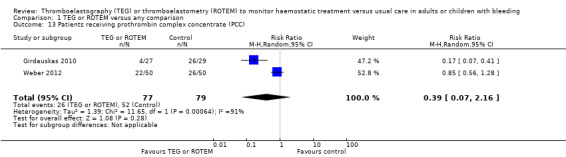
Comparison 1 TEG or ROTEM versus any comparison, Outcome 13 Patients receiving prothrombin complex concentrate (PCC).
1.14. Analysis.
Comparison 1 TEG or ROTEM versus any comparison, Outcome 14 Patients receiving factor VIIa.
Amount of blood products transfused
A total of 14 studies reported on the amount of PRBCs with variation in reporting of median and interquartile range (IQR), means and SD, and total number of units given to each group. Four trials (29%) had a significant result all favouring the use of TEG or ROTEM‐guided transfusion (Avidan 2004; Nakayama 2015; Nuttal 2001; Weber 2012). As illustrated in Table 13 there were substantial variations in the amount of blood transfused even when excluding the two paediatric trials (Cui 2010; Nakayama 2015). Mean or median of control groups varied between 1 to 17 units of PRBCs given. In addition, many trials report median and IQR and attempts to calculate mean and SD resulted in very skewed data. This was equally the case for volume of FFP and platelets transfused (Table 14; Table 15). More than half of the trials reporting on transfused amount of FFP showed a significant reduction favouring a TEG‐ or ROTEM‐based transfusion algorithm (Table 14). Two trials reported complete avoidance of FFP transfusion in the intervention group (Rauter 2007; Schaden 2012).
Platelet transfusion amount was reported in 13 trials, with three having a significant result indicating a reduced need of platelet transfusion favouring TEG‐ or ROTEM‐guided transfusion (Ak 2009; Nuttal 2001; Weber 2012). Schaden 2012 completely avoided platelet transfusion in the intervention group and Kultufan Turan 2006 avoided platelet transfusion in the comparison group guided by standard laboratory tests and clinical judgement.Only two studies transfused a mean/median amount of platelet of more than 2 units (Nuttal 2001; Wang 2010).
Surgical reintervention and bleeding events
Meta‐analysis of nine trials with a total of 90 events showed no reduction in surgical reinterventions (RR 0.75, 95% CI 0.50 to 1.10; I2 = 0%, 9 studies, 887 participants, low quality of evidence; Analysis 1.15). Only two trials reported on bleeding events such as excessive bleeding events or massive transfusion (Ak 2009; Girdauskas 2010), with no difference between groups (RR 0.82, 95% CI 0.38 to 1.77; I2 = 34%, 2 studies, 280 participants, low quality of evidence; Analysis 1.20). The outcome on estimated bleeding showed substantial variation and skewed data, hence we did not perform any meta‐analysis (Table 12). A total of 14 trials reported total bleeding volume, varying from 390 mL to 6348 mL in the control groups, with some trials reporting only perioperative bleeding, some postoperative, and some both. Three trials had a significant result all favouring a TEG‐ or ROTEM‐guided transfusion strategy (Nakayama 2015; Nuttal 2001; Weber 2012) . Only three studies had a mean/median blood loss in the control group exceeding 1000 mL (Kempfert 2011; Paniagua 2011; Wang 2010).
1.15. Analysis.
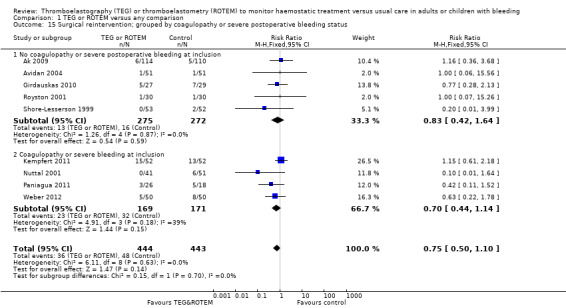
Comparison 1 TEG or ROTEM versus any comparison, Outcome 15 Surgical reintervention; grouped by coagulopathy or severe postoperative bleeding status.
1.20. Analysis.
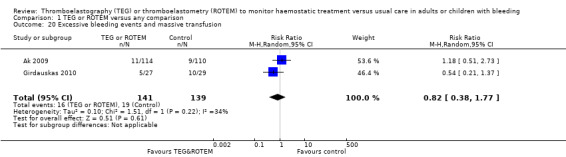
Comparison 1 TEG or ROTEM versus any comparison, Outcome 20 Excessive bleeding events and massive transfusion.
Adverse events and complications
These outcomes were variably reported. The pooled intervention effect from three studies (200 participants) reporting on dialysis‐dependent renal failure indicated a 54% relative risk reduction in favour of TEG‐ or ROTEM‐guided transfusion strategy (RR 0.46, 95% CI 0.28 to 0.76; I2 = 0%, 3 studies, 300 participants, low quality of evidence; Analysis 1.17). Events such as thrombotic events (Analysis 1.18), surgical wound infection, postoperative acute respiratory distress, postoperative confusion, and coagulopathy did not reach statistical significance (Appendix 3). We were unable to conduct our analyses on transfusion‐related complications (for example, sepsis, haemolytic reactions, disseminated intravascular coagulation, major immunological and allergic reactions) since none of the trials provided information on these outcomes. Continous outcomes such as time to extubation, length of stay in hospital or intensive care unit (ICU) showed skewed data, thus we did not perform a meta‐analysis (Table 16; Table 17; Table 18). One study reported significant reduced length of stay (Cui 2010), and three reported a significant reduced time in the ICU and time to extubation (Cui 2010; Nakayama 2015; Weber 2012).
1.17. Analysis.
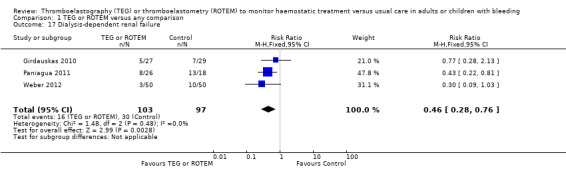
Comparison 1 TEG or ROTEM versus any comparison, Outcome 17 Dialysis‐dependent renal failure.
1.18. Analysis.
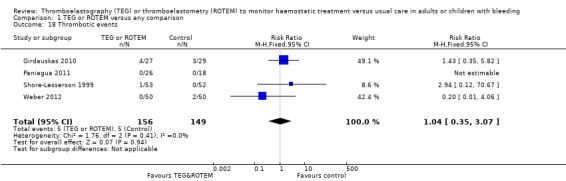
Comparison 1 TEG or ROTEM versus any comparison, Outcome 18 Thrombotic events.
One trial provided data on cost‐benefit (Weber 2012), but complete data were not available.
Finally, none of the trials provided data on quality of life assessment.
Subgroup analyses
Most of the included trials were conducted in populations undergoing cardiac surgery with the exception of one trial carried out in adult liver transplant surgery (Wang 2010), and one in excision of burn wounds in adults (Schaden 2012). Only two trials, with a total of 131 participants, involved children undergoing cardiac surgery (Cui 2010; Nakayama 2015; Table 10), and the majority had a body weight below 20 kg. We are currently unable to make any evidence‐based recommendations on the use of TEG or ROTEM in the paediatric or neonatal setting, or among the critically ill, obstetrical, and trauma populations. Subgroup analysis comparing trials using the TEG device with those using the ROTEM device showed no subgroup difference on mortality (Analysis 1.1), transfusion requirements (Analysis 1.3; Analysis 1.6; Analysis 1.8), or reoperations due to bleeding (Analysis 1.16). Four trials, with a total of 318 participants, included participants that were coagulopathic or had excessive/pathological bleeding at the time of inclusion (Kempfert 2011; Nuttal 2001; Paniagua 2011; Weber 2012). Subgroup analysis showed no difference in the interventional effect on mortality (Analysis 1.2), and the proportion of patients in need of transfusion had no subgroup differences (Analysis 1.5; Analysis 1.7; Analysis 1.10), as was the case with reoperations due to bleeding (Analysis 1.15).
1.16. Analysis.
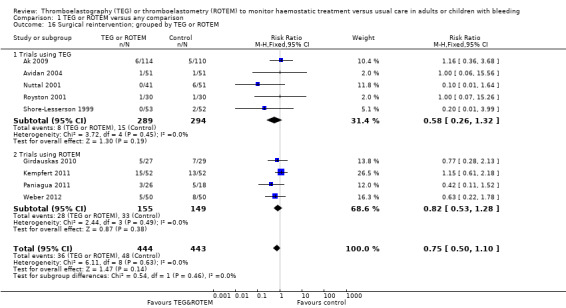
Comparison 1 TEG or ROTEM versus any comparison, Outcome 16 Surgical reintervention; grouped by TEG or ROTEM.
1.2. Analysis.
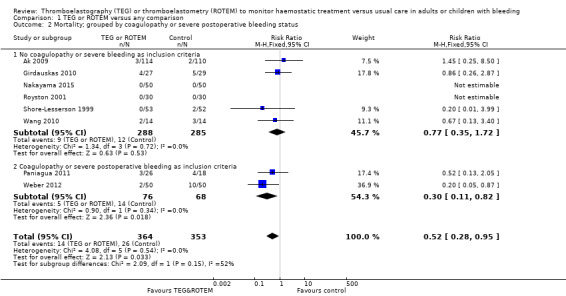
Comparison 1 TEG or ROTEM versus any comparison, Outcome 2 Mortality; grouped by coagulopathy or severe postoperative bleeding status.
1.5. Analysis.
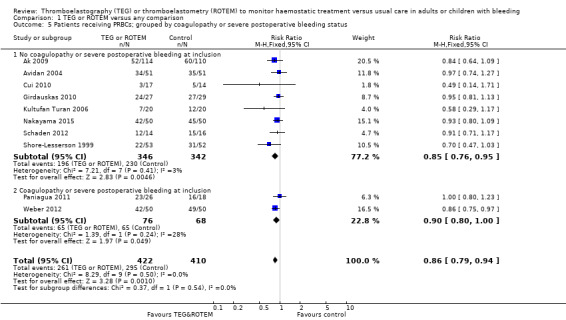
Comparison 1 TEG or ROTEM versus any comparison, Outcome 5 Patients receiving PRBCs; grouped by coagulopathy or severe postoperative bleeding status.
1.7. Analysis.
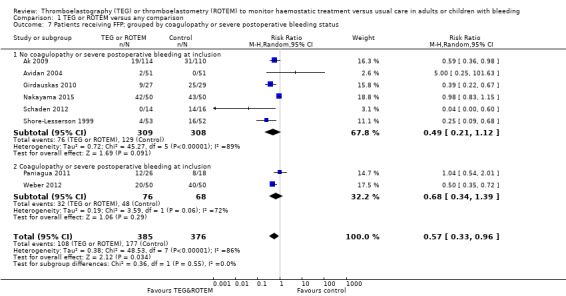
Comparison 1 TEG or ROTEM versus any comparison, Outcome 7 Patients receiving FFP; grouped by coagulopathy or severe postoperative bleeding status.
1.10. Analysis.
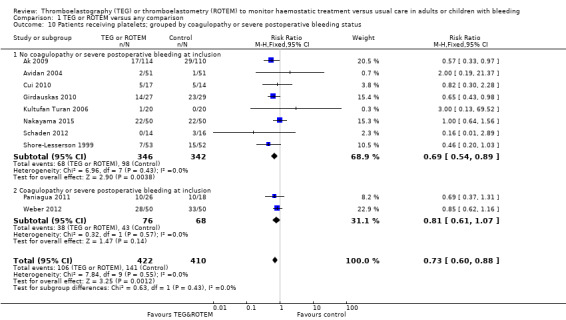
Comparison 1 TEG or ROTEM versus any comparison, Outcome 10 Patients receiving platelets; grouped by coagulopathy or severe postoperative bleeding status.
Sensitivity analyses
Bias assessment
Comparing estimates of the pooled intervention effect based on random sequence generation, allocation concealment, blinding, follow‐up, sample size calculation, early stopping, and the overall risk of bias did not result in any statistically significant finding in any of the subgroups examined. We identified two trials with a low risk of bias with no statistical significance for our primary endpoint (Nakayama 2015; Shore‐Lesserson 1999).
Post hoc sensitivity
Wang 2010 was the only study with a long (3 years) follow‐up on mortality. Our mortality analyses includes mortality at longest follow‐up, but long‐term and short‐term follow‐up might not reflect the same causes (Roth 2016). We performed an analysis on mortality excluding the Wang 2010 trial and using a random‐effects model (I2 = 1.0%) showed RR 0.55 (95% CI 0.28 to 1.10). In order to further evaluate the impact of zero event trials on mortality, we carried out a sensitivity analysis choosing risk difference (RD) as the statistical approach for our primary outcome. The two trials with zero events contributed with 29% of the included population (Nakayama 2015; Royston 2001): RD was ‐0.04 (95% CI ‐0.07 to ‐0.00; I2 = 51.0%) before exclusion of zero event trials and ‐0.05 (95% CI ‐0.09 to ‐0.01; I2 = 57.0%) after. Thus, zero event trials do not affect our overall findings and this is a further indication of robustness for our primary analyses.
Post hoc exploration of the comparison
Comparing a predefined algorithm with doctors' clinical judgement might show a difference merely reflecting the effect of having a treatment algorithm in itself rather than the effect owing to one type of coagulation measurement than another. We assessed if trials that compared TEG/ROTEM with clinical judgement had a different treatment effect than those compared with standard laboratory test‐guided algorithms in respect to mortality. No difference across the subgroups was found (P = 0.21, I2 = 37.1%; Analysis 1.21). Equally, exploring the same question in regards to RBC transfusion showed no difference across the subgroups (P = 0.21, I2 = 59.3%; Analysis 1.22). However, in regards to the proportion transfused with FFP we did see an increased treatment effect comparing TEG/ROTEM with clinical judgement (subgroup RR 0.38, 95% CI 0.21 to 0.68) than with standard laboratory test‐guided algorithm (subgroup RR 0.83, 95% CI 0.49 to 1.40) P = 0.05, I2 = 74.0%; Analysis 1.23).
1.21. Analysis.
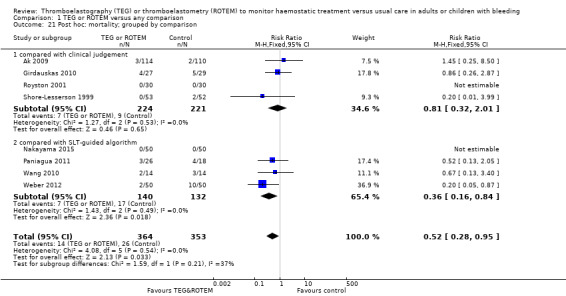
Comparison 1 TEG or ROTEM versus any comparison, Outcome 21 Post hoc: mortality; grouped by comparison.
1.22. Analysis.

Comparison 1 TEG or ROTEM versus any comparison, Outcome 22 Post hoc: patients receiving PRBCs; grouped by comparisons.
1.23. Analysis.
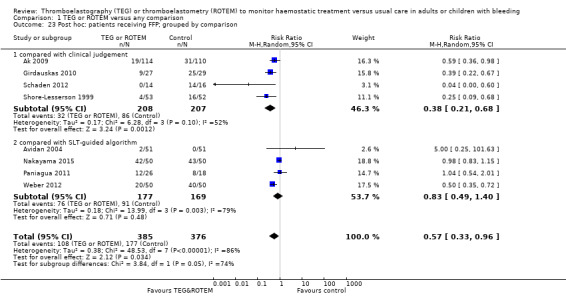
Comparison 1 TEG or ROTEM versus any comparison, Outcome 23 Post hoc: patients receiving FFP; grouped by comparison.
Trial sequential analysis (TSA)
We applied trial sequential analysis (TSA) on all outcome data described in Table 1 except the outcome of excessive bleeding events and massive transfusion due to low numbers of trials and participants (Analysis 1.20). A TSA of all trials on the effect of haemostatic transfusion guided by TEG or ROTEM on mortality showed that only 54% of the required information size (717 of 1325) had been reached in a fixed‐effect model, with continuity adjustment for zero event trials (0.001 in each arm) resulting in a non‐statistically significant TSA alfa‐boundary adjusted RR of 0.51 (95% CI 0.21 to 1.26; diversity (D2) = 0%, I2 = 0%, fixed‐effect model; Figure 1), with a control event proportion of 7.4%. Cumulative Z‐curve does not cross the monitoring boundary constructed for a required information size of 1325 participants corresponding to a relative risk reduction (RRR) of 49% with 80% power and alpha of 0.05. However, only two trials had low risk of bias, with an insufficient event rate to carry out a separate meta‐analysis and more importantly, TSA is ideally designed for trials with low risk of bias and is unable to adjust for risk of bias. Additionally, when carrying out the TSA by using a random‐effects model instead of a fixed‐effect model, the RR is 0.59 (95% CI 0.23 to 1.54; D2 = 0%, I2 = 0%).
1.
Trial sequential analysis (TSA) of mortality shows that only 54% of the required information size (717 of 1325) for a 49% relative risk reduction (RRR) has been reached in a fixed‐effect model with continuity adjustment for zero event trials (0.001 in each arm) resulting in a TSA alfa‐boundary adjusted RR of 0.51 (95% CI 0.21 to 1.26, Diversity (D2) = 0%, I2 = 0%, fixed‐effect model) with a control event proportion of 7.4%. Cumulative Z‐curve does not cross the monitoring boundary constructed for a required information size of 1325 participants corresponding to a RRR of 49% with 80% power and alpha of 0.05. However, only two trials had low risk of bias, with insufficient event rate to carry out a separate meta‐analysis for low risk of bias trials. When carrying out the TSA by using random‐effects model instead of fixed‐effect model, the RR is 0.59 (95% CI 0.23 to 1.54, Diversity (D2) = 0%, I2 = 0%).
A TSA of dichotomous transfusion outcomes all supports the use of a TEG‐ or ROTEM‐guided algorithm: TSA of all trials on the effect of haemostatic transfusion guided by TEG or ROTEM on the need for PRBCs resulted in a TSA alfa‐spending boundary adjusted RR of 0.86 (95% CI 0.79 to 0.95; D2 = 0%, I2 = 0%, fixed‐effect model; Figure 2), with a control event proportion of 93.3% with continuity adjustment for zero event trials (0.001 in each arm). Cumulative Z‐curve in blue crosses the monitoring boundary constructed for a required information size of 507 participants corresponding to a RRR of 14% with 80% power and alpha of 0.05.
2.
Trial Sequential Analysis (TSA) of all trials on the effect of haemostatic transfusion guided by TEG or ROTEM on the need for PRBCs resulted in a TSA alfa‐spending boundary adjusted RR of 0.86 (95% CI 0.79 to 0.95, D2= 0%, I2= 0%, fixed‐effect model) with a control event proportion of 93.3% with continuity adjustment for zero event trials (0.001 in each arm). Cumulative Z‐curve in blue crosses the monitoring boundary constructed for an adjusted information size of 507 participants corresponding to a RRR of 14% with 80% power and alpha of 0.05.
A TSA of all trials for the effect of haemostatic transfusion guided by TEG or ROTEM on proportion of patients in need of FFP resulted in a TSA alfa‐spending boundary adjusted RR of 0.6 (95% CI 0.55 to 0.65; Figure 3), with the cumulative Z‐curve crossing the boundary constructed for an information size of 372 in the meta‐analysis with a RRR of 40% (alfa = 0.05) and a power of 80% (beta = 0.20) in a random‐effects model with high heterogeneity (I2 = 73%) and diversity (D2= 88%) and control group event rate of 47.1% with continuity correction for zero event trials (0.001 in each arm). However, one has to exert caution when interpreting signs of firm evidence for this outcome, since only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999), and the required information size based on these two trials is 2921, and the cumulative Z‐curve does not cross the boundary.
3.
TSA of the effect of haemostatic transfusion guided by TEG or ROTEM on proportion of patients in need of FFP resulted in a TSA alfa‐spending boundary adjusted RR of 0.6 (95% CI 0.55 to 0.65) with the cumulative Z‐curve crossing the boundary constructed for an information size of 372 in the meta‐analysis with a RRR of 40% (alfa = 0.05) and a power of 80% (beta = 0.20) in a random‐effects model with high heterogeneity (I2 = 73%) and diversity (D2= 88%) and control group event rate of 47.1% with continuity adjustment for zero event trials (0.001 in each arm). However, one has to exert caution when interpreting indications of firm evidence for this outcome, since only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999) and the required information size based on these two trials is 2921 and the cumulative Z‐curve does not cross the boundary.
A TSA of all trials for the effect of haemostatic transfusion guided by TEG or ROTEM on the need for platelets indicates firm evidence and resulted in a TSA alfa‐spending boundary adjusted RR of 0.73 (95% CI 0.70 to 0.76; D2 = 0%, I2 = 0%, fixed‐effect model; Figure 4), with a control event proportion of 34.4% and with continuity adjustment for zero event trials (0.001 in each arm). Cumulative Z‐curve crosses the monitoring boundary constructed for a required information size of 177 participants corresponding to a RRR of 27% with 90% power and alpha of 0.05. However, as with previous analysis, only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999), and the low risk of bias adjusted required information size is 1090 participants.
4.
TSA of all trials for the effect of haemostatic transfusion guided by TEG or ROTEM on the need for platelets indicates firm evidence and resulted in a TSA alfa‐spending boundary adjusted RR of 0.73 (95% CI 0.70 to 0.76, Diversity (D2) = 0%, I2 = 0%, fixed‐effect model) with a control event proportion of 34.4% and with continuity adjustment for zero event trials (0.001 in each arm). Cumulative Z‐curve crosses the monitoring boundary constructed for an adjusted information size of 177 participants corresponding to a RRR of 27% with 90% power and alpha of 0.05. However, as with previous analysis, only two trials had low risk of bias (Nakayama 2015; Shore‐Lesserson 1999) and the low‐risk of bias adjusted required information size is 1090 participants.
Concerning the incidence of reoperations (Figure 5), in contrast to the conventional meta‐analysis (Analysis 1.15), TSA showed a beneficial effect in favour of TEG/ROTEM‐guided transfusion management with a TSA alfa‐spending boundary adjusted RR of 0.74 (95% CI 0.63 to 0.86; D2 = 0%, I2 = 0%, fixed‐effect model) but the cumulative Z‐curve did not cross the monitoring boundary constructed for a required information size of 516 participants corresponding to a RRR of 26% with 80% power and alpha of 0.05, and a control event proportion of 10.8%, with continuity correction for zero event trials (0.001 in each arm). Additionally, we were unable to calculate the low risk of bias adjusted required information size, and as such we do not have firm evidence at this stage.
5.
TSA of all trials for the effect of haemostatic transfusion guided by TEG or ROTEM on the need for re operations results in a TSA alfa‐spending boundary adjusted RR of 0.74 (CI 0.63 to 0.86, D2= 0%, I2 = 0%, fixed‐effect model) but the cumulative Z‐curve does not cross the monitoring boundary constructed for an adjusted information size of 516 participants corresponding to a RRR of 26% with 80% power and alpha of 0.05 and a control event proportion of 10.8% with continuity adjustment for zero event trials (0.001 in each arm). However, only trial was with low risk of bias.
Finally, due to the overall high risk of bias, imprecision, and indirectness involved in assessment of GRADE for the above analysis, one could easily argue that the required power should be 90% and not 80%, by which the required information size would be substantially increased.
2. TEG‐ or ROTEM‐guided algorithm versus clinical judgement or usual treatment
See: Table 2.
Primary outcome
Overall mortality
Out of the nine trials comparing TEG or ROTEM with clinical judgement or usual treatment, only four provided data on mortality (Ak 2009; Girdauskas 2010; Royston 2001; Shore‐Lesserson 1999), with Royston 2001, being a zero event trial. We found no difference with a total of 7/224 deaths in the TEG/ROTEM group compared with 9/221 deaths in the control group (RR 0.81, 95% CI 0.32 to 2.01; I2 = 0%; low quality of evidence).
Secondary outcomes
Transfusion requirements and surgical reinterventions
However, a reduced need for FFP and for platelets was found, indicating a reduction of 62% and 1%, respectively (Analysis 2.3; Analysis 2.4), but there was no reduction in the need for PRBCs (RR 0.85, 95% CI 0.73 to 1.00; I2 = 31%; low quality of evidence; Analysis 2.2), or surgical reintervention (Analysis 2.5).
2.3. Analysis.
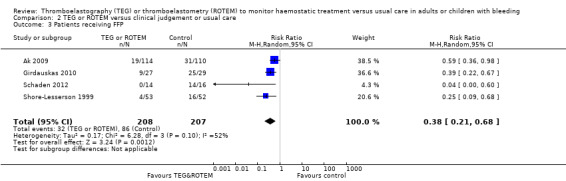
Comparison 2 TEG or ROTEM versus clinical judgement or usual care, Outcome 3 Patients receiving FFP.
2.4. Analysis.
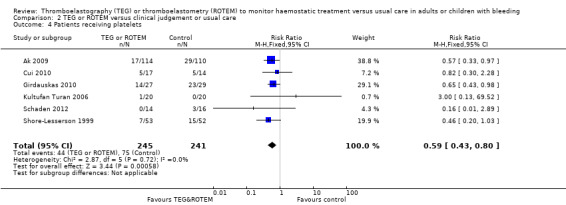
Comparison 2 TEG or ROTEM versus clinical judgement or usual care, Outcome 4 Patients receiving platelets.
2.2. Analysis.
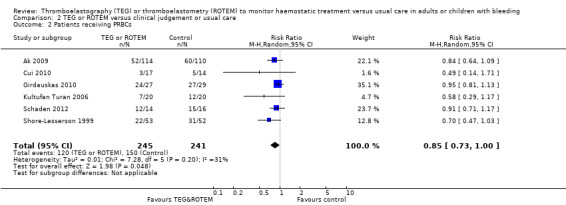
Comparison 2 TEG or ROTEM versus clinical judgement or usual care, Outcome 2 Patients receiving PRBCs.
2.5. Analysis.
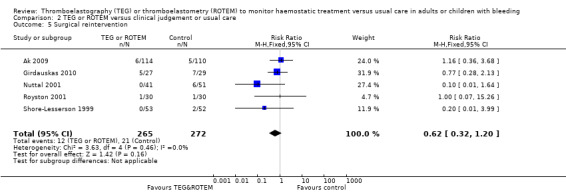
Comparison 2 TEG or ROTEM versus clinical judgement or usual care, Outcome 5 Surgical reintervention.
3. TEG‐ or ROTEM‐guided algorithm versus a predefined algorithm based on standard laboratory test‐guided transfusion
See: Table 3.
Primary outcome
Overall mortality
Out of seven trials comparing TEG or ROTEM with standard laboratory test‐guided transfusion, a total of four provided data on mortality (Nakayama 2015; Paniagua 2011; Wang 2010; Weber 2012), with one trial being a zero event trial (Nakayama 2015). We found a total of 7/140 deaths in the TEG or ROTEM group compared with 9/132 in the control group (RR 0.36, 95% CI 0.16 to 0.84; I2 = 0%; low quality of evidence).
Secondary outcomes
Transfusion requirements and surgical reinterventions
Applying this comparison we found no significant reduction in the proportion of patients in need of PRBC transfusion (RR 0.91, 95% CI 0.83 to 1.00, I2 = 0%; low quality evidence; Analysis 3.2), and no difference in need of FFP or platelets (Analysis 3.3; Analysis 3.4), or the need for surgical reintervention (Analysis 3.5).
3.2. Analysis.
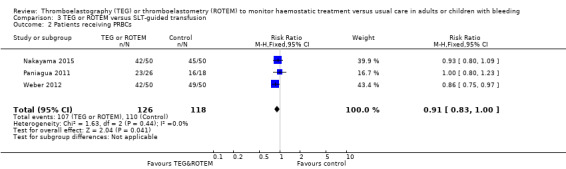
Comparison 3 TEG or ROTEM versus SLT‐guided transfusion, Outcome 2 Patients receiving PRBCs.
3.3. Analysis.
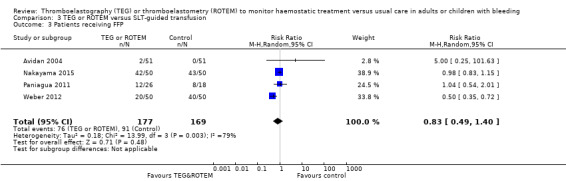
Comparison 3 TEG or ROTEM versus SLT‐guided transfusion, Outcome 3 Patients receiving FFP.
3.4. Analysis.
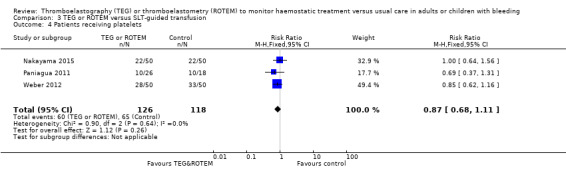
Comparison 3 TEG or ROTEM versus SLT‐guided transfusion, Outcome 4 Patients receiving platelets.
3.5. Analysis.
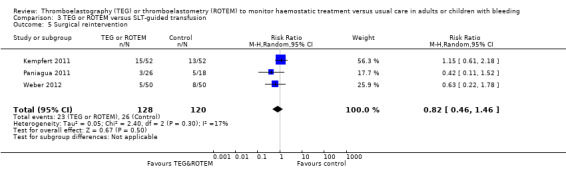
Comparison 3 TEG or ROTEM versus SLT‐guided transfusion, Outcome 5 Surgical reintervention.
4. TEG or ROTEM in combination with standard laboratory tests or other devices in a guided algorithm versus clinical judgement or usual care
See: Table 4.
Primary outcome
Overall mortality
Five trials used TEG or ROTEM in combination with other devices such as: PFA‐100 and Hepcon (Avidan 2004), Coagucheck Plus and Coulter‐MPII (Nuttal 2001), platelet count and p‐fibrinogen (Shore‐Lesserson 1999), Multiplate (Weber 2012), and Platelet Mapping (Westbrook 2009) (see Table 11 for complete description of intervention algorithms and trigger levels). Two trials with 205 participants reported on mortality showing a reduction of 80% (RR 0.20, 95% CI 0.05 to 0.75; I2 = 0%; low quality evidence; Analysis 4.1).
4.1. Analysis.
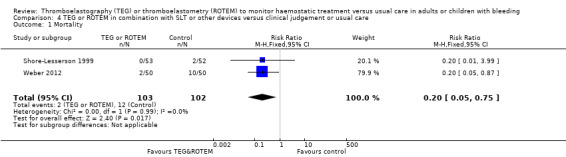
Comparison 4 TEG or ROTEM in combination with SLT or other devices versus clinical judgement or usual care, Outcome 1 Mortality.
Secondary outcomes
Transfusion requirements and surgical reinterventions
Two trials with 205 participants reported a reduction in the need for PRBC transfusion (Analysis 4.2): Meta‐analysis of three trials with 207 participants showed a significant risk reduction in need of PRBC transfusion, favouring an addition of TEG or ROTEM to the algorithm used to guide transfusion (RR 0.85, 95% CI 0.74 to 0.98; I2 = 0%; low quality of evidence). The need for FFP and platelet transfusion was reported in three trials with no significant pooled effect estimate (Analysis 4.3; Analysis 4.4).
4.2. Analysis.
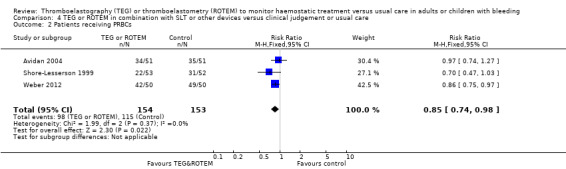
Comparison 4 TEG or ROTEM in combination with SLT or other devices versus clinical judgement or usual care, Outcome 2 Patients receiving PRBCs.
4.3. Analysis.
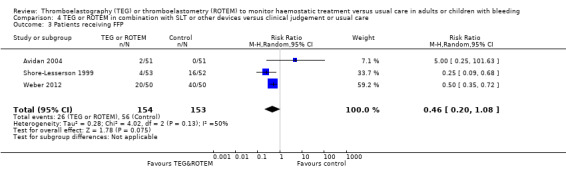
Comparison 4 TEG or ROTEM in combination with SLT or other devices versus clinical judgement or usual care, Outcome 3 Patients receiving FFP.
4.4. Analysis.
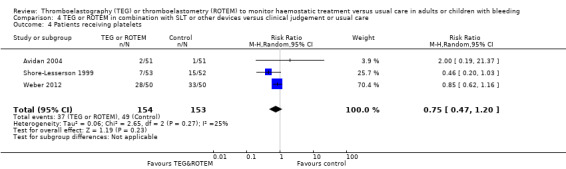
Comparison 4 TEG or ROTEM in combination with SLT or other devices versus clinical judgement or usual care, Outcome 4 Patients receiving platelets.
Surgical reinterventions reported by four trials were significantly reduced by 59% in this subgroup of trials (RR 0.41, 95% CI 0.17 to 0.96; I2 = 0%; low quality of evidence; Analysis 4.5).
4.5. Analysis.
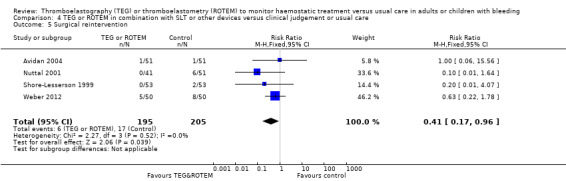
Comparison 4 TEG or ROTEM in combination with SLT or other devices versus clinical judgement or usual care, Outcome 5 Surgical reintervention.
Discussion
Summary of main results
In this systematic review of 17 randomized trials involving 1493 participants with bleeding due to elective cardiac surgery, excision of burn wounds, and liver transplantation, we found an indication of reduced mortality by 48% favouring a thromboelastography (TEG)‐ or thromboelastometry (ROTEM)‐guided blood transfusion. However, only eight trials provided data on mortality (Ak 2009; Girdauskas 2010; Nakayama 2015; Paniagua 2011; Royston 2001; Shore‐Lesserson 1999; Wang 2010; Weber 2012), including two zero event trials (Nakayama 2015; Royston 2001); when changing from a fixed‐effect model to a random‐effects model, the statistical significance was no longer present, e.g. from risk ratio (RR) 0.52 (95% CI 0.28 to 0.95; I2 = 0%) to RR 0.57 (95% CI 0.30 to 1.07; I2 = 0%). Additionally, the trial sequential analysis (TSA) indicates that only 54% of the required information size had been reached (Figure 1).
Results on secondary outcomes showed a reduction in the need for PRBCs of 14% (RR 0.86, 95% CI 0.79 to 0.94; I2 = 0%), reduced need for fresh frozen plasma (FFP) of 43% (RR 0.57, 95% CI 0.33 to 0.96; I2 = 80%), reduced need for platelet transfusion of 25% (RR 0.73, 95% CI 0.60 to 0.88; I2 = 0%), and reduced need for combined treatment with FFP and platelets of 56% (RR 0.44, 95% CI 0.28 to 0.81; I2 = 0%) (Table 1).
We did not assess the amount of blood products transfused in meta‐analyses due to very skewed data. However, the most convincing treatment effect across trials was seen in the amount of FFP transfused, with half of the trials having a significant reduction (Table 14). In general, the studies included participants with low to moderate bleeding (Table 12), thus only three studies had a mean/median blood loss in the control group exceeding 1000 mL (Kempfert 2011; Paniagua 2011; Wang 2010). Three trials (Nakayama 2015; Nuttal 2001; Weber 2012), all with moderate bleeding volume, showed a significant reduction in bleeding volume in favour of TEG or ROTEM (Table 12). Two of these trials included participants with pathological or excessive postoperative bleeding (Nuttal 2001; Weber 2012). Our meta‐analysis found no significant effect on the need for surgical reintervention due to bleeding (Table 1).
All together, all analyses with or without significance, point towards a benefit of using a TEG‐ or ROTEM‐guided transfusion. We abstained from performing a meta‐analysis on skewed continuous data, including bleeding volume, transfusion volume, duration of stay and intensive care unit (ICU) admittance, and time to extubation. The total number of adverse events were few (Analysis 1.17; Analysis 1.18; Analysis 1.20, Appendix 3), and this limits our ability to rule out any adverse effects. We found no significant adverse events except for a 54% reduced risk of dialysis‐dependent renal failure (RR 0.46, 95% CI 0.28 to 0.76; I2 = 0%). Only one study with unclear blinding found reduced length of stay (Cui 2010; Table 16), and three found a significant reduced time in the ICU (Cui 2010; Nakayama 2015; Weber 2012; Table 17), and reduced time to extubation (Table 18).
We found six ongoing trials in an adult population (estimated to be 962) of trauma, liver transplantation, and elective cardiac surgery with bleeding, but we were unable to retrieve any information from investigators of most of the trials at the current stage (NCT02352181; NCT02593877; NCT02461251; NCT01536496; NCT02416817; NCT01402739).
Our subgroup analysis, comparing trials using TEG versus ROTEM did not identify any difference between the two devices, and large differences between trial algorithms were present (Table 11). We were able to assess the subgroups comparing paediatric studies with adults, and those using coagulopathy or excessive bleeding as inclusion criteria against those without, but found no significant subgroup effect.
A significant reduction in mortality was also found in all comparisons except when compared with clinical judgement or usual care (Analysis 2.1). The need for pooled red blood cell (PRBC) transfusions was significant compared with any comparison (Analysis 1.3), and that of a guided transfusion based on TEG or ROTEM in combination with standard laboratory tests or other devices (Analysis 4.2), but compared to clinical judgement (Analysis 2.2), or a standard laboratory test‐based algorithm (Analysis 3.2), the upper confidence interval was 1.00 in both analyses. The need for FFP was significantly reduced when compared with clinical judgement or usual treatment, but not with a standard laboratory test‐based algorithm (Analysis 3.3), or when combining TEG or ROTEM with other devices (Analysis 4.3); the same was the case with platelets (Analysis 2.4; Analysis 3.4; Analysis 4.4). Only when combining TEG or ROTEM with standard laboratory tests or other devices such as platelet function analysis did we find a significant reduction in reoperations due to bleeding (Analysis 4.5). Only 205 to 537 participants were part of these smaller analyses, and as a result confidence intervals are very wide, and therefore, we have abstained from attempting to interpret any differences in results between comparisons (Table 2; Table 3; Table 4).
2.1. Analysis.
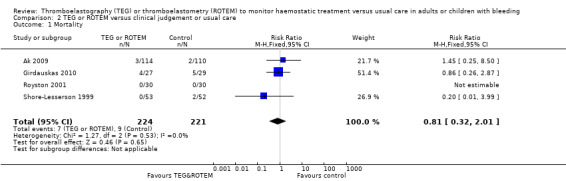
Comparison 2 TEG or ROTEM versus clinical judgement or usual care, Outcome 1 Mortality.
Overall completeness and applicability of evidence
Some may argue that technologies such as TEG and ROTEM were not designed as life‐saving instruments, but rather as qualitative point‐of‐care tools, assisting clinicians in the interpretation of whether the transfusion or substitution strategy is adequate to ensure optimal fibrin formation in a patient's blood. In this review, we evaluate the use of viscoelastic haemostatic measuring devices in an interventional review ‐ not in a review of diagnostic accuracy. The pooling of trials with different algorithms based on different triggers of haemostatic treatment including FFP, platelets, fibrinogen concentrate, cryoprecipitate, protamine, and antifibrinolytics (Table 11), renders us with results that we believe should be interpreted as a 'proof‐of‐treatment concept' ‐ simply answering whether or not the algorithm approach is reasonable or inappropriate. Unfortunately this higher‐level approach causes us to lose the ability to evaluate specific trigger details, or ultimately conclude which of the tested algorithms seems to be the very best. Therefore, other reviews aiming specifically at, for instance, cardiac surgery or trauma, will have to evaluate and differentiate these questions.
Our primary outcome measure (overall mortality) may be contested by many. However, the choice of overall mortality as the primary outcome measure summarizes ultimate harms and benefits simultaneously. We acknowledge, of course, that other outcomes may also have great clinical importance and, accordingly, we have analysed the effects of the intervention on several of these. However, it should be equally recognized that as long as none of the surrogate outcomes have been evaluated thoroughly, or proven to be relevant (Gluud 2005), we should be careful not to incorporate evidence of benefit based solely on these as arguments for using the intervention.
Rapid point‐of‐care assessment of alterations in haemostasis using TEG or ROTEM could result in appropriate therapy through the systematic evaluation of haemostatic functionality. The evaluation potentially enables clinicians to administer relevant pharmacological and blood products in patients with excessive bleeding. As supported by our results, TEG and ROTEM may have the potential to reduce mortality and the proportion of patients receiving transfusion, and the development of dialysis‐dependent renal failure. TSA showed firm evidence in support of a reduced need for blood products, but only 54% of the required information size had been reached in regards to the suggestion of reduced mortality. It is important to notice that TSA software is unable to directly adjust for risk of bias, and as such, is ideally designed for trials with low risk of bias. However, the majority of trials included in these TSA analyses are of high risk of bias. As a consequence, the true required information size may be much higher than the estimated in our TSA analyses, and that would certainly be the case if the required power was set at 90% and not 80%, as is the case for most of the outcomes.
The majority of trial participants included in this review (1435/1493, 96%) were elective cardiac surgery patients, of whom the majority were adults (1304/1435, 91%). This limits the external validity, and a direct translation to other clinical settings should therefore be made with great caution. In addition, our estimates of the required information size are not static, and inclusion of trials with populations at high risk of bleeding and critically ill patients in settings such as sepsis, disseminated intravascular coagulation, trauma, obstetrics, and transplantation may indeed alter our conclusions and estimates. Several trials in settings of non‐cardiac surgery are ongoing (Characteristics of ongoing studies).
The elective surgical setting of the included trials is far from the scenario of uncontrolled, and sometimes undiagnosed, life‐threatening haemorrhage which usually characterizes trauma, upper gastrointestinal bleeding, or postpartum haemorrhage setting. No trials included in this review compare a TEG‐ or ROTEM‐guided treatment with a ratio based 1:1:1 transfusion strategy in cases of severe life‐threatening haemorrhage (Johansson 2014), and therefore, we are unable to make any conclusions as to the optimal strategy in these situations. One ongoing trauma study compares TEG‐ or ROTEM‐guided treatment with an algorithm based on standard laboratory tests (NCT01536496), and one with a ratio based transfusion of 1:1:1 between blood products (PRBCs: FFP: platelets) (NCT02416817). Results from these and future studies will hopefully shed some light on this discussion. Fibrinogen is the first coagulation factor to be depleted during ongoing bleeding (Hiippala 1995), and three sources provide fibrinogen for substitution therapy: FFP, cryoprecipitate, and fibrinogen concentrate. Ultimately, an algorithm's ability to reduce FFP use might be caused by the preferred use of one of the other sources. Few trials reported cryoprecipitate or fibrinogen concentrate as an outcome (Analysis 1.12; Characteristics of included studies).
The use of massive transfusion protocols is generally advocated as a tool for the team, as well as the organization, to improve (AAGBI 2010), standardize treatment, and rehearse situations of ongoing severe bleeding. A team performs better with a clearly defined plan or treatment strategy. Therefore, one could argue that the effect of an unblinded comparison between a TEG‐ or ROTEM‐guided treatment with loosely defined 'clinical decision‐based treatment' might just reflect the effect of having a clearly defined plan known by everyone in the team. In a post hoc sensitivity analysis, we compared the included studies that compared TEG or ROTEM with a clinical decision and those studies compared it with another well‐defined algorithm based on standard laboratory tests. We did not identify any significant difference between these two groups for our primary outcome, mortality (Analysis 1.21), and the need for PRBCs (Analysis 1.22), but we found a P value of 0.05 for subgroup difference in the need for FFP, with indication of a greater efficacy in trials comparing TEG/ROTEM with clinical judgement or usual treatment as compared to those with standard laboratory test‐guided transfusion (Analysis 1.23). Post hoc analyses and multiple testing holds in itself a high risk of bias, and should therefore be interpreted with great caution. All together, we believe that the effect of a TEG‐ or ROTEM‐based treatment identified in this review is not just explained by the use of well‐defined protocols.
A systematic evaluation of cost‐benefit commissioned by the UK National Health System identified a potential cost‐effectiveness of TEG, ROTEM or a third device (SonoClot) in cardiac surgery if more than 326 tests were performed per machine (Whiting 2015). We planned to include data from trials reporting on cost‐benefit, but found only one trial with insufficient data for potential meta‐analysis (Weber 2012). Standard laboratory tests, including platelet count, international normalized ratio (INR), activated partial thromboplastin time (aPTT), and plasma fibrinogen are the traditional and most widespread tests used for assessing haemostasis. Implementation of TEG or ROTEM into clinical practice based on results favouring its use, will most likely result in the addition of TEG or ROTEM to the traditionally used standard laboratory tests. In such case, the cost‐benefit will naturally be limited. The standard assays of TEG and ROTEM cannot assess platelet function (Luddington 2005), and especially in cardiac surgery with cardiopulmonary bypass, platelet function might be suppressed perioperatively through drugs or through shear stress of the pump (Besser 2010; Paparella 2004). As a consequence, three trials incorporated platelet function assays in their TEG or ROTEM algorithms (Avidan 2004; Weber 2012; Westbrook 2009). Comparison 4, assessing the effect of TEG or ROTEM in combination with standard laboratory tests or other devices (Table 4), thus includes both the potential effect of adding a platelet function analysis and adding TEG or ROTEM to standard laboratory tests. Table 5 illustrates the multiplicity of interventions in combination with comparisons, which ultimately reduces our ability to assess the actual effect of adding a platelet function analysis or adding TEG or ROTEM to standard laboratory tests. In fact, no trial compared TEG or ROTEM as add‐on to a standard laboratory test‐guided algorithm with a group guided solely by standard laboratory test. The potential additional effect of co‐operating platelet function analysers is not directly assessed in the present review, but an ongoing study directly addresses this issue (NCT01218074, described in Characteristics of excluded studies). Furthermore, a recently published systematic review on the subject came to a conclusion in favour of adding platelet function tests to the TEG‐ or ROTEM‐guided algorithm (Corredor 2015), but the statistical interpretation of their subgroup analyses may be questioned (see Agreements and disagreements with other studies or reviews).
Quality of the evidence
We applied several statistical methods in order to explore and reduce bias, such as complete case analysis, TSA, overall methodological bias assessment, and analyses of various relevant clinical and physiological outcomes. Although there was moderate statistical heterogeneity among trial results, we are aware that we may have pooled heterogeneous trials in terms of age, patients, settings, and treatment regimens. Thus, the validity of our meta‐analysis may be criticised. However, all trials included patients with bleeding and, with the exception of two trials (Schaden 2012; Wang 2010), were all conducted in a cardiac surgery setting. Therefore, we think there is good biologic reason to perform a broad meta‐analysis, which also considerably increases the generalizability and usefulness of the review. Further, a broad meta‐analysis increases power, reduces the risk of erroneous conclusions, and facilitates exploratory analyses that can generate hypotheses for future research (Gotzsche 2000).
Our systematic review has several potential limitations and our findings and interpretations are limited by the quality and quantity of available evidence. The risk of bias of the included trials was mainly assessed using the published data, which ultimately may not reflect the truth. All trial authors were contacted, but only a few responded to provide further information. The fact that only a small number of trials contributed to our subgroup and sensitivity analyses does limit the value of these analyses. We chose to abstain from performing a meta‐analysis on all continuous outcomes due to considerable skewedness of data and the need for estimations regarding volumes of reported transfusion units. This may be a conservative approach that ultimately may lead to underestimation of intervention benefits since we found reduced bleeding and volume of PRBCs in the first version of this review (Afshari 2011). However, the contrary approach is advocated and supported by other review groups (Whiting 2015). Three trials reported results from analyses comparing the total amount of blood products given to each treatment group instead of mean/median (Rauter 2007; Royston 2001; Westbrook 2009; Table 13; Table 14; Table 15). By doing so, the authors assume that each unit of blood is given independently, but this is far from the truth, since the risk of receiving another unit will have been increased when the first is given.
We judged only two trials to have low risk of bias (Nakayama 2015; Shore‐Lesserson 1999; Figure 9), and one was quasi‐randomized. Blinding of a treatment guided by algorithms, especially during ongoing severe bleeding, is troublesome and may claim additional man power and resources in the process. However, with the very short periods of follow‐up and the lack of blinding used in the majority of the included trials, this may have an impact, even if our sensibility analysis were unable to detect it.
Application of TSA to our primary outcome indicates, that at this stage we lack firm evidence in regards to survival benefits by applying a TEG/ROTEM‐guided transfusion strategy. TSA did however indicate firm evidence in favour of the proportion of patients receiving PRBCs, FFP, and platelets. However, very few of the included trials were at low risk of bias, and since TSA is unable to adjust for the risk of bias, as a consequence the low risk of bias adjusted information size has not been reached for the analyses of FFP and platelet proportions.
Nevertheless, evaluated outcomes were consistently in favour of a TEG‐ or ROTEM‐guided transfusion in bleeding patients. However, we graded the quality of evidence as low based on the high proportion of trials at high risk of bias, large clinical and statistical heterogeneity, small and inadequate information size (as indicated by TSA analyses), low number of events, imprecision, and wide confidence intervals for many of the meta‐analyses in this review.
Potential biases in the review process
Inspired by the first published version of this review (Afshari 2011), and our knowledge of the field and of new trials, we made a decision (before the analysis stage of the review) to change some of the methods.We decided to undertake the post hoc analyses after the primary data analyses. The decision to change the definition of participants from "potentially requiring massive transfusion" to "adults or children with bleeding" served mainly to clarify the clinical context since "potentially requiring" seems very imprecise. The latest included trials did not differ in terms of bleeding or transfusion compared to the ones included in the first version of this review (Afshari 2011). We made the decision to include quasi‐randomized trials during the screening phase in order to increase the generalizability of our findings since we found fewer than expected eligible trials, with most being at high risk of bias.
Agreements and disagreements with other studies or reviews
In general, our review reaches the same conclusions as many of the included RCTs, and many of the excluded trials and reviews.
The UK National Health Service (NHS)‐funded Health Technology Assessment (HTA) report updated in 2015 (Whiting 2015), and first published in Craig 2007, focuses on three groups of patients, namely cardiac surgery, trauma, and postpartum haemorrhage (obstetric bleeding). By including both RCTs and observational studies it aims to assess the effectiveness and cost‐benefit of TEG/ROTEM. Our update includes another six RCTs not mentioned in the HTA report: Wang 2010 and Schaden 2012 include patients not covered by the report and NCT00772239 has no published results. However, three studies had data on cardiac surgery patients (Cui 2010; Kempfert 2011; Paniagua 2011). They were published before the publication of the HTA report (Whiting 2015), but two are only published as abstracts (Kempfert 2011; Paniagua 2011), and the latter seems to be a fibrinogen concentrate study, but when assessed closer it fulfils the inclusion criteria of being a trial assessing a TEG algorithm with fibrinogen concentrate as part of treatment in this algorithm. Our updated review finds the same indications of a reduced need for blood transfusion (PRBCs, FFP or platelets), but in addition we had more data on mortality. The HTA report uses random‐effects models in all analyses independent of I2 statistics. The NHS review also concludes that TEG or ROTEM as an 'add‐on' to a standard laboratory test‐based protocol seems to be unsupported, and therefore not recommendable from a cost‐benefit point of view (Whiting 2015). However, we did not identify any trial comparing TEG or ROTEM as add‐on to a standard laboratory test‐guided algorithm with a group guided solely by standard laboratory tests (Table 5).
A systematic review included 6835 participants across 12 studies (Bolliger 2013), but most of the data were derived from one retrospective study and only 11% (749) from RCTs. Analyses were all together supportive of a TEG‐ or ROTEM‐guided transfusion. This review did not provide additional information on side effects or adverse events derived from the included observational studies.
One additional Cochrane Review involving TEG or ROTEM examines viscoelastic whole blood assays as a predictor of coagulopathy in trauma patients (Hunt 2015). Coagulopathy was defined using a reference standard of prothrombin time ratio and/or the international normalized ratio of 1.2 or greater, or 1.5 or greater. The aim was to evaluate the diagnostic test accuracy of TEG and ROTEM in trauma patients with clinically‐suspected coagulopathy and included cross‐sectional studies and case‐control studies. No evidence on the accuracy of TEG and very little evidence on the accuracy of ROTEM was found, but this was undermined by a small number of included studies and risk of bias. Results of the present review assessing the interventional effect of haemostatic treatment guided by TEG or ROTEM is difficult to interpret in the context of the accuracy of which TEG or ROTEM predicts values of standard laboratory tests.
A recently published systematic review with meta‐analysis addressed the possible beneficial effect of adding a platelet function test to the TEG‐ or ROTEM‐guided algorithm, specifically in cardiac surgery (Corredor 2015). The review authors concluded that a significant increased effect of adding platelet function tests was found in regards to a decrease in blood loss and a reduction in the use of PRBCs and FFP, but with the opposite being the case for platelet transfusions. However, interpretation of the published meta‐analyses reveals that this conclusion has been reached from comparing mean differences and relative risks of each subgroup, and without addressing the fact that CIs overlap between subgroup results, and the published test for subgroup differences was insignificant with no test of interaction carried out. Based on this and the results of the present review, we believe that there is currently no evidence to support the routine and systematic addition of a platelet function analyser to a TEG‐ or ROTEM‐guided transfusion in cardiac surgery or any other type of surgery.
Authors' conclusions
Implications for practice.
There is growing, but weak, evidence in support of the use of thromboelastography (TEG) or thromboelastometry (ROTEM), and mainly in the elective cardiac surgery setting. This updated systematic review with meta‐analysis finds indications of reduced mortality and a reduction in the need for pooled red blood cells (PRBCs), platelets, and dialysis‐dependent renal failure. However, one has to exert great caution in interpreting benefits of TEG/ROTEM in regards to mortality due to imprecision, inadequate information size (power), and the high proportion of trials at risk of bias. We did not find a significant effect on need for reoperations, and few adverse effects were reported in general. However, we were challenged by the skewed character of data for continuous outcomes. In terms of paediatric, neonatal, obstetric, critically ill, trauma, and other surgical patients with high risk of bleeding, there are currently no data to support or refute the routine use of TEG and ROTEM. No data were available to evaluate a TEG‐ or ROTEM‐guided transfusion compared to a ratio‐based transfusion strategy (1:1:1).
Implications for research.
There is an urgent need for several large RCTs with low risk of bias to evaluate the use of TEG and ROTEM in different clinical settings, such as in paediatric and neonatal, septic, trauma, obstetrical, critically ill patients, and other surgical populations with massive transfusion following aneurism repair and liver surgery. These trials ought to have large sample sizes before the intervention definitely can be either rejected or accepted. Further trials need to focus on other relevant outcomes such as long‐term survival, adverse events, and cost‐benefit. The impact associated with the presence of coagulopathy or excessive bleeding needs further exploration. Finally we are disappointed to find that four (24%) trials with approximately 456 participants, and with relevant data, remain unpublished. In order to avoid publication bias, it is crucial to publish positive as well as negative studies, and it is unethical not to publish data from the enrolled participants.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 3, 2011
| Date | Event | Description |
|---|---|---|
| 5 January 2017 | Amended | Co‐pubished in Anaesthesia (Wikkelsø 2017) |
| 26 February 2016 | New search has been performed | Updated search was updated on 5 January 2016. Eight new studies were included. This review now includes 17 trials, 44 excluded, 0 awaiting classification, and 6 ongoing studies. |
| 5 January 2016 | New citation required but conclusions have not changed | Conclusions have not changed. Jesper Brok has left the author group. Changes in methods: ‐ Type of participants was changed from "potentially requiring massive transfusion" to "adults or children with bleeding" in order to simplify. ‐ Type of studies was changed to include trials using quasi‐randomization. ‐ "Improvement of oxygen delivery and decrease of tissue anoxia" was removed as a secondary outcome in order to reduce the number of analyses. ‐ Subgroup of "Coagulopathy or severe postoperative bleeding at inclusion" has been added. ‐ The comparisons were changed from "TEG or ROTEM versus usual care" to four different comparisons; please see Types of interventions (Table 1; Table 2; Table 3; Table 4). ‐ Appendix tables have been changed and updated (Table 10; Table 11). ‐ Skewed data on continuous outcomes of bleeding volume, transfusion volume, length of stay in hospital and ICU, and time to extubation was omitted from the meta‐analyses following statistical consultation and are now presented in Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18. ‐ The numbers of sensitivity analyses have been reduced, please see Sensitivity analysis. Other corrections: Title changed in accordance with changes in type of participants. The specific criticisms and errors pointed out by Whiting 2015 have been corrected accordingly (e.g. reoperation data (Westbrook 2009) and fresh frozen plasma and platelet transfusion data (Nuttal 2001) have been removed from the meta‐analyses). Continous outcomes are not included in meta‐analyses. Please also see Agreements and disagreements with other studies or reviews. |
| 17 April 2012 | Amended | Contact details updated. |
Acknowledgements
We thank Harald Herkner, and especially Marialena Trivella for patience and clear feedback. In addition we would like to thank Prof Harald Herkner (Content Editor), Vibeke E Horstmann (statistical editor), Wulf Dietrich, Edoardo De Robertis, Aamer B Ahmed (peer reviewers) and Prof Nathan Pace for their help and editorial advice during the preparation of this updated systematic review. We also thank Dr Karen Hovhannisyan for his assistance in providing our different search strategies.
We also thank Prof Pär Johansson and Prof Benny Sørensen for their advice and critical reading of our previously published protocol. Special thanks to Lisete Costa and Pentapharm Germany for providing us with ROTEM illustrations and to Peter Fischer and Hemoscope Corp, US for providing us with TEG illustrations. We thank Dr Bülent Uslu for translation of the included Turkish study report (Kultufan Turan 2006). Related to the preparation of the protocol (Afshari 2009), and the previous version of this review (Afshari 2011) we thank Dr Harald Herkner (Content Editor), Dr Nathan Pace (Statistical Editor), Prof Jørgen Ingerslev, Dr Wulf Dietrich, and Prof Benny Sørensen (Peer Reviewers), and Janet Wale and Clare Jeffrey (Cochrane Consumer Network) for their help and editorial advice
Finally, we thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care Group) for her valuable assistance during the entire process.
Appendices
Appendix 1. Search strategy
| Biosis (Ovid SP) | 1. (thromboelastogra* or thrombelastogra* or (thromb* adj2 elastogra*) or thromboelastom* or ROTEG or ROTEM or TEG).ti,ab. 2. (random* or (clinical adj4 trial*) or placebo* or multicenter* or prospective* or ((double or single or triple) and (mask* or blind*))).mp. 3. 1 and 2 |
| CENTRAL, the Cochrane Library | #1 MeSH descriptor Thrombelastography explode all trees #2 thromboelastogra* or thrombelastogra* or thromboelastom*ROTEG or ROTEM or TEG #3 (#1 OR #2) |
| OVID Embase | 1. Thromboelastography/ or (thromboelastogra$ or thrombelastogra$ or thrombo elastogra$ orthromboelastom$ or thrombo elastom$).ti,ab. or (ROTEM or ROTEG or TEG).mp. 2. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 3. 1 and 2 |
| ISI Web of Science | #1 TS=(thromboelastogra* or thrombelastogra* or thrombo elastogra* orthromboelastom* or thrombo elastom*) or TS=(ROTEM or ROTEG) or TS=(TEG) #2 TS=(random* or (controlled SAME trial*) or placebo* or multicenter* or prospective*) or TS=((single or double or triple or treble) and (mask* or blind*)) #3 #2 AND #1 |
| LILACS (via BIREME) | "THROMBOELASTOGRAPHY/" or "thromboelastogra$" or "thrombelastogra$" or "thrombo elastogra$" or "thromboelastom$" or "thrombo elastom$" or "ROTEM" or "ROTEG" or "TEG" |
| OVID MEDLINE | 1. exp Thrombelastography/ or Thromb?elastograph*.mp.or (ROTEM or TEG or ROTEG).mp. or Thromboelastometry.mp. 2. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. (2177961) 3. 1 and 2 |
| CINAHL (EBSCOhost) | S1. (MM "Thrombelastography") S2. TX thromboelastogra* or thrombelastogra* or thrombo elastogra* orthromboelastom* or thrombo elastom* or ROTEM or ROTEG or TEG S3. S1 or S2 |
Appendix 2. Transfusion requirements: TEG or ROTEM versus control, single study analyses
| Subgroup analysis | Study | Participants | Statistical method | Effect estimate |
| Autologus reinfusion volume (mL) | Shore‐Lesserson 1999 | 105 | Mean difference (IV, fixed, 95% CI) | ‐13.00 (‐100.96 to 74.96) |
| Cryoprecipitate exposure (units) | Wang 2010 | 28 | Mean difference (IV, fixed, 95% CI) | ‐2.60 (‐9.94 to 4.74) |
| Proportion of participants transfused with allogenic blood | Girdauskas 2010 | 56 | Risk ratio (M‐H, fixed, 95% CI) | 0.89 (0.77 to 1.03) |
Appendix 3. Complications and outcomes during patient in‐hospital stay: TEG or ROTEM versus control, single study analyses
| Subgroup analysis | Study | Participants | Statistical method | Effect estimate |
| Postoperative confusion | Girdauskas 2010 | 56 | Risk ratio (M‐H, fixed, 95% CI) | 0.61 (0.20 to 1.86) |
| Postoperative acute respiratory distress | Ak 2009 | 224 | Risk ratio (M‐H, fixed, 95% CI) | 0.75 (0.29 to 1.95) |
| Coagulopathy | Nuttal 2001 | 92 | Risk ratio (M‐H, fixed, 95% CI) | 0.41 (0.02 to 9.87) |
| Rate of reintubation | Girdauskas 2010 | 56 | Risk ratio (M‐H, fixed, 95% CI) | 1.50 (0.54 to 4.17) |
Appendix 4. Abbreviations
ACT: activated coagulation time; ADP: adenosine diphosphate; APTEM: aprotinin inhibated thromboelastometry; aPTT: activated partial thromboplastin time; ARDS: acute respiratory distress syndrome; CABG: coronary artery bypass grafting; CCHD: complex congenital heart disease; CI: confidence interval; cm: centimetre; CINAHL: Cumulative Index to Nursing and Allied Health Literature; CPB: cardiopulmonary bypass; DIC: disseminated intravascular coagulation; EXTEM: extrinsic system screen test; FIBTEM: fibrinogen test of ROTEM; FFP: fresh frozen plasma; Hb: haemoglobin; HEPTEM: heparinase modified thromboelastometry; ICU: intensive care unit; INR: international normalized ratio; INTEM: intrinsic system screen test; ITT: intention‐to‐treat analysis; IV: intravenous; LILACS: Latin American Caribbean Health Sciences Literature; LY30: lysis of clot at 30 minutes; MCF: maximum clot firmness; MD: mean difference; mg/dL: miligram per decilitre; mL/kg: millilitre per kilogram; min: minutes; n: count numbers; PCC: prothrombin complex concentrate; PFA: platelet function analyser; PICU: paediatric intensive care unit; PLT: unit of pooled platelets; POC: point‐of‐ care; PRBC: pooled red blood cell; PT: prothrombin time; R: reaction time; RBC: red blood cell; RCT: randomized controlled trial; RD: risk difference; rFVIIa: recombinant factor VIIa; ROTEG: rotative thrombelastography; ROTEM: rotative thromboelastometry; r‐TEG: rapid thromboelastography; SLT: standard laboratory tests; TEG: thromboelastography; VHA: viscoelastic haemostatic assay.
Data and analyses
Comparison 1. TEG or ROTEM versus any comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality; grouped by TEG or ROTEM | 8 | 717 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.95] |
| 1.1 Trials using TEG | 4 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.07] |
| 1.2 Trials using ROTEM | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.93] |
| 2 Mortality; grouped by coagulopathy or severe postoperative bleeding status | 8 | 717 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.95] |
| 2.1 No coagulopathy or severe bleeding as inclusion criteria | 6 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.72] |
| 2.2 Coagulopathy or severe postoperative bleeding as inclusion criteria | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.11, 0.82] |
| 3 Patients receiving PRBCs; grouped by TEG or ROTEM | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 3.1 Trials using TEG | 5 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.68, 0.95] |
| 3.2 Trials using ROTEM | 5 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 4 Patients receiving PRBCs; grouped by adult or paediatric patients | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 4.1 Trials with adults | 8 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.95] |
| 4.2 Trials with children (age less than 18) | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.05] |
| 5 Patients receiving PRBCs; grouped by coagulopathy or severe postoperative bleeding status | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 5.1 No coagulopathy or severe postoperative bleeding at inclusion | 8 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.95] |
| 5.2 Coagulopathy or severe postoperative bleeding at inclusion | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.00] |
| 6 Patients receiving FFP; grouped by TEG or ROTEM | 8 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.96] |
| 6.1 Trials using TEG | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.20, 1.35] |
| 6.2 Trials using ROTEM | 5 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.30, 1.12] |
| 7 Patients receiving FFP; grouped by coagulopathy or severe postoperative bleeding status | 8 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.96] |
| 7.1 No coagulopathy or severe postoperative bleeding at inclusion | 6 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.12] |
| 7.2 Coagulopathy or severe postoperative bleeding at inclusion | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.39] |
| 8 Patients receiving platelets; grouped by TEG or ROTEM | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.88] |
| 8.1 Trials using TEG | 5 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.41, 0.91] |
| 8.2 Trials using ROTEM | 5 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.98] |
| 9 Patients receiving platelets; grouped by adult or paediatric patients | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.88] |
| 9.1 Trials with adults | 8 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.84] |
| 9.2 Trials with children (age less than 18) | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.64, 1.45] |
| 10 Patients receiving platelets; grouped by coagulopathy or severe postoperative bleeding status | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.88] |
| 10.1 No coagulopathy or severe postoperative bleeding at inclusion | 8 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.54, 0.89] |
| 10.2 Coagulopathy or severe postoperative bleeding at inclusion | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.07] |
| 11 Transfusion of FFP & platelets | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.24, 0.81] |
| 12 Patients receiving fibrinogen concentrate | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.17] |
| 13 Patients receiving prothrombin complex concentrate (PCC) | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.07, 2.16] |
| 14 Patients receiving factor VIIa | 3 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.24] |
| 15 Surgical reintervention; grouped by coagulopathy or severe postoperative bleeding status | 9 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.10] |
| 15.1 No coagulopathy or severe postoperative bleeding at inclusion | 5 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.64] |
| 15.2 Coagulopathy or severe bleeding at inclusion | 4 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.14] |
| 16 Surgical reintervention; grouped by TEG or ROTEM | 9 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.10] |
| 16.1 Trials using TEG | 5 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.32] |
| 16.2 Trials using ROTEM | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.28] |
| 17 Dialysis‐dependent renal failure | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.28, 0.76] |
| 18 Thrombotic events | 4 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.35, 3.07] |
| 19 Surgical source of re‐bleeding | 4 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.42, 2.57] |
| 20 Excessive bleeding events and massive transfusion | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.38, 1.77] |
| 21 Post hoc: mortality; grouped by comparison | 8 | 717 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.95] |
| 21.1 compared with clinical judgement | 4 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.01] |
| 21.2 compared with SLT‐guided algorithm | 4 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.84] |
| 22 Post hoc: patients receiving PRBCs; grouped by comparisons | 10 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 22.1 compared with clinical judgement | 6 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.93] |
| 22.2 compared with SLT‐guided algorithm | 4 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.02] |
| 23 Post hoc: patients receiving FFP; grouped by comparison | 8 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.96] |
| 23.1 compared with clinical judgement | 4 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.68] |
| 23.2 compared with SLT‐guided algorithm | 4 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.49, 1.40] |
1.4. Analysis.
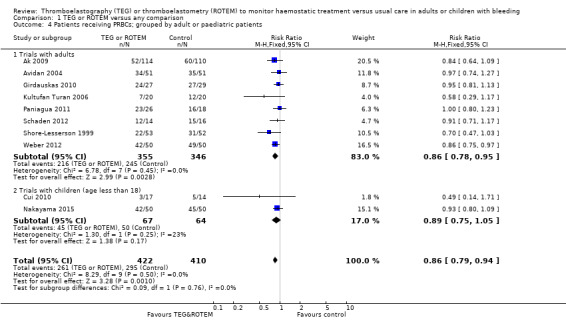
Comparison 1 TEG or ROTEM versus any comparison, Outcome 4 Patients receiving PRBCs; grouped by adult or paediatric patients.
1.9. Analysis.
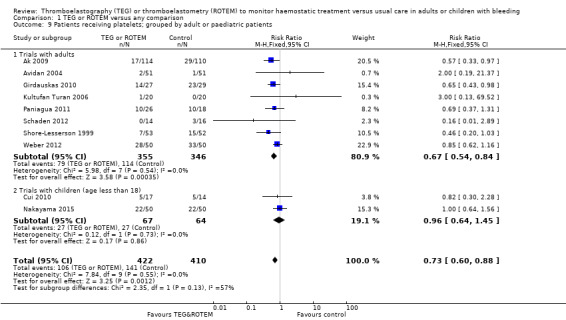
Comparison 1 TEG or ROTEM versus any comparison, Outcome 9 Patients receiving platelets; grouped by adult or paediatric patients.
1.19. Analysis.
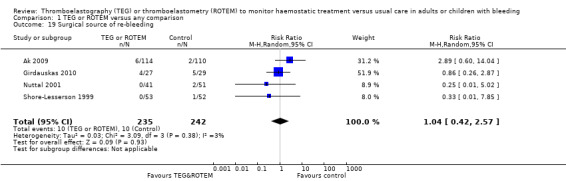
Comparison 1 TEG or ROTEM versus any comparison, Outcome 19 Surgical source of re‐bleeding.
Comparison 2. TEG or ROTEM versus clinical judgement or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.01] |
| 2 Patients receiving PRBCs | 6 | 486 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 1.00] |
| 3 Patients receiving FFP | 4 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.68] |
| 4 Patients receiving platelets | 6 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 5 Surgical reintervention | 5 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.20] |
Comparison 3. TEG or ROTEM versus SLT‐guided transfusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.84] |
| 2 Patients receiving PRBCs | 3 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 3 Patients receiving FFP | 4 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.49, 1.40] |
| 4 Patients receiving platelets | 3 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.11] |
| 5 Surgical reintervention | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.46] |
3.1. Analysis.
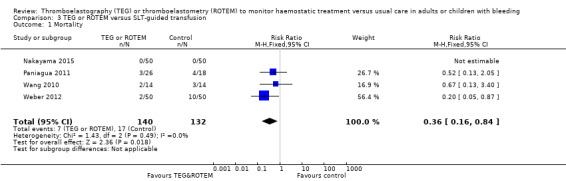
Comparison 3 TEG or ROTEM versus SLT‐guided transfusion, Outcome 1 Mortality.
Comparison 4. TEG or ROTEM in combination with SLT or other devices versus clinical judgement or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.75] |
| 2 Patients receiving PRBCs | 3 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 3 Patients receiving FFP | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.08] |
| 4 Patients receiving platelets | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.47, 1.20] |
| 5 Surgical reintervention | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.17, 0.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ak 2009.
| Methods | Two‐group parallel RCT, one centre
ITT: unclear Funding: unclear Overall study quality: high risk of bias Sample size calculation was reported |
|
| Participants |
Inclusion criteria: 224 consecutive adult patients undergoing elective first‐time CABG with cardiopulmonary bypass Exclusion criteria: preoperative haemodynamic instability, malignancies, history of bleeding diathesis, use of low molecular weight heparin molecules until the day of operation, recent treatment (< 5 days) with a glycoprotein IIb/IIIa antagonist or clopidogrel, impaired renal function (creatinine > 2 mg/dL) and any liver disease resulting in elevated liver function tests 64.5% of the patients in the control group and 58.7% of patients in the TEG group were on aspirin therapy until the day before the operation |
|
| Interventions |
TEG algorithm group: (n = 114) kaolin‐activated TEG‐based algorithm‐guided perioperative transfusion management. Transfusion algorithm was fully based on TEG Control group: clinician‐directed transfusion (n = 110), need for blood transfusion was based on clinician’s discretion and standard coagulation tests. The decision for blood product was determined by using the criteria obtained from abnormal conventional laboratory tests, absence of visible clots, and presence of generalized oozing‐type bleeding in the surgical field Duration of intervention: algorithms were used intraoperatively and until 24 hours post‐CPB Concomitant treatment: tranexamic acid was administered in 10.3% of patients in the TEG group compared with 19% in the control group (P = 0.007). Perioperative anticoagulation was performed with standard heparin and monitored with repeated ACT analyses |
|
| Outcomes |
Primary: incidence of blood transfusion, blood loss Secondary: amount of blood and blood products consumed perioperatively, blood loss mediastinal chest tube drainage, need for additional protamine, need of tranexamic acid infusion, mortality, risk of surgical cause of reoperation for bleeding and clinical complications outcome after CABG (superficial soft tissue infection, major respiratory complications, postoperative renal dysfunction) and haematological variables (haematocrit and platelets) |
|
| Notes |
Country: Turkey. Language: English Letter sent to authors in April and June 2010. No reply received Follow‐up: unclear, but transfusion requirements were recorded until discharge from hospital and mortality until 30 days "Excessive bleeding was defined as mediastinal blood loss over 400 mL in the first hour after surgery or over 100 mL/hour for 4 consecutive hours. Early mortality was defined as any dearth occurring within 30 days after operation" The authors provided bleeding data and transfusion requirements as median values with quartile values Authors conclusion:"Our results show that routine use of a kTEG‐guided algorithm reduces the consumption of blood products in patients undergoing elective CABG. Adopting such an algorithm into routine management of these patients may help to improve clinical outcome and reduce the potential risks of transfusion‐related complications and total costs after CABG" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization based on clinic record number. |
| Allocation concealment (selection bias) | High risk | Unconcealed procedure based on clinic record number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Transfusions were performed by the anaesthesiologist who was blinded to the patient’s group assignment. However, we are uncertain who was in charge of interpretation and performing of the TEG/SLTs and how the results were passed to the anaesthesiologist. Thus a potential risk for blinding to have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be complete follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Unclear risk | No data on funding, but otherwise appears free of other biases. |
Avidan 2004.
| Methods | Two‐group parallel RCT, one centre. In this study the data are compared with a third group based on retrospective data (n = 108)
ITT: yes, complete follow‐up Funding: not for profit Overall study quality: high risk of bias Sample size calculation was reported |
|
| Participants |
Inclusion criteria: routine elective first‐time CABG surgery with cardiopulmonary bypass, managed according to standard clinical practice at local institution treated by the same surgical, intensivist and anaesthetic teams Exclusion criteria: patients with preoperative abnormal clotting tests, including INR > 1.5, aPTT ratio > 1.5, platelet count < 150 X 109 litre‐1, any medication affecting coagulation within 72 hours of surgery, including warfarin, heparin, low molecular weight heparin, aspirin and clopidogrel |
|
| Interventions |
Intervention group: management algorithm based on near‐patient tests based on information provided by three devices, the Hepcon (in order to identify the dose of heparin required to achieve adequate anticoagulation), TEG and the PFA‐100 platelet function analyser, (n = 51). Thus, transfusion algorithm was partly based on TEG. Type of TEG: standard tissue factor initiated and heparinase‐coated cups Control group: algorithm based on routine laboratory assays (n = 51), management depended on rapidly available laboratory clotting tests. Transfusion of haemostatic blood components was performed only if specific criteria were met, thus not guided by clinical discretion Duration of intervention: algorithms were used during surgery and until 2 hours postsurgery. No information regarding transfusions in the ICU postsurgery. Staff in special recovery unit were not aware of study group allocation Concomitant treatment: all patients were given prophylactic antifibrinolytic therapy (tranexamic acid) before surgery. Aprotinin was administered to ten patients in the control group and two in the intervention group. Anticoagulation for CPB was accomplished with heparin. Three patients in the control group and six in the intervention group were treated with postoperative desmopressin |
|
| Outcomes |
Primary: Blood loss and transfusion, postoperative 24‐hour blood loss Secondary: INR, aPTT, TEG variables, haemoglobin and platelet values, coagulation values |
|
| Notes |
Country: United Kingdom Blood loss and transfusion were compared with a retrospective case‐control group (n = 108), in which management of bleeding had been performed according to the clinician's discretion. Data from this group has been excluded from our analyses Excessive bleeding was defined as any patient who continued to bleed excessively (> 100 mL/hour), had no evidence of a haemostatic abnormality or had failed to respond to the treatment. These patients underwent surgical re‐exploration Total amount of PRBCs transfused: 99 units in the TEG group versus 93 in the control group. Total amount of FFP transfused: 6 units in TEG group versus 0 in control group. Total amount of platelets transfused: 3 units in the TEG group versus 2 in the control group Letter sent to authors in April and June 2010. No reply received Follow‐up: 24 hours for blood loss into chest tube drains and fluids and blood components administered Authors conclusion:"Following algorithms based on point of care tests or on structured clinical practice with standard laboratory tests does not decrease blood loss, but reduces the transfusion of PRBCs and blood components after routine cardiac surgery, when compared with clinician discretion. Cardiac surgery services should use transfusion guidelines based on laboratory guided algorithms, and the possible benefits of point of care testing should be tested against this standard" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate. Investigators were not blinded to group allocation. Those measuring and documenting postoperative bleeding and thus in charge of outcome assessment were blinded to group allocation. Point‐of‐care haemostatic tests were also run for the control group, but investigators were blinded to the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be complete follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Low risk | Appears free of other biases. |
Cui 2010.
| Methods | Two‐group parallel RCT, single centre Overall study quality: high risk of bias Sample size calculation: none reported ITT: No Funding: not stated |
|
| Participants | 40 participants randomly assigned, of which 31 received intervention (17 in fibrinogen group) Inclusion criteria: cyanotic paediatric patients diagnosed with transposition of the great arteries or double‐outlet right ventricle; the operation that the patients underwent was arterial switch operation or double roots transplantation. Haematocrit higher than 54% before operation Exclusion criteria: history of blood disease; anticoagulation treatment before surgery; medication that affects haemostasis (such as prostaglandin E1); difficult sternal closure caused by anatomical or surgical reasons |
|
| Interventions |
Intervention: transfusion guided by TEG, Haemoscope Corp. combined with fibrinogen administration (0.5 to 1 gram) Control: traditional transfusion guided by clinical experience |
|
| Outcomes | No primary outcome is stated Closure time, transfusion at closure (FFP/PLT), transfusion requirements at ICU (FFP/PLT/RBC), chest tube drainage (1, 6, 24 hours) and total transfusion requirements (FFP/PLT/RBC*) *RBC at closure time was not assessed because residuals of blood were present in the CPB machine |
|
| Notes |
Country: China We used mean body weight for each group to calculate transfused amount, when outcome was reported in mL/kg Letter to author 3 April 2015. No reply received Authors' conclusion:"The present study suggests that fibrinogen might be a better haemostatic agent for paediatric patients with severely cyanotic complex congenital heart diseasethan FFP. This new therapy method could reduce the use of allogeneic blood products and shorten the operative recovery period. In addition, TEG is effective for blood protection" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The intervention would be difficult to blind, so we expect this to be provided without blinding of personnel. However, participants could potentially have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22.5% excluded (9/40), not accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Unable to compare with trial registration or protocol and unable to assess the degree of follow‐up and missing outcomes. |
| Other bias | High risk | Sample size not stated, funding not stated, baseline parameters are provided, but it is unclear if they include the excluded patients, and if the exclusions influence baseline balance. A small study and operative recovery data show very large differences between small trial groups, suggesting that the intervention group might have consisted of healthier individuals overall. |
Girdauskas 2010.
| Methods | Two‐group parallel RCT, one centre ITT: yes Funding: unclear Overall study quality: high risk of bias Sample size calculation was reported based on 40% reduction in the use of allogeneic blood products |
|
| Participants |
Inclusion criteria: 56 adult patients (> 18 years) undergoing high risk aortic surgery including urgent and emergency surgery (25 with acute type A dissection) with hypothermic circulatory arrest Exclusion criteria: pregnant, known (inherited) coagulation disorders (haemophilia A or B, activated protein C resistance, etc), inability to give informed consent Patients receiving preoperative antiplatelet or anticoagulant therapy were eligible to participate. However, none of the included patients were receiving preoperative clopidogrel or heparin and there was no major differences in the use of aspirin and warfarin before surgery. A total of 79% of patients in the control group and 82% of patients in the ROTEM group (P = 0.8) were classified as having high‐risk score for massive perioperative transfusion |
|
| Interventions |
Intervention group: ROTEM‐guided intraoperative and postoperative transfusion algorithm (n = 27) Control group: routine transfusion practices (clinical judgment‐guided transfusion followed by transfusion according to coagulation test results), (n = 29) |
|
| Outcomes |
Primary outcome: cumulative transfusion of allogeneic blood units (PRBCs, FFP, and platelets) Secondary outcome: use of prothrombin complex concentrate, fibrinogen concentrate, and recombinant factor VIIa (NovoSeven), blood losses in the first 12 and 24 postoperative hours, risk of surgical re‐exploration for bleeding, time to extubation, neurologic and renal complications, length of stay in ICU |
|
| Notes |
Country: Germany. Language: English Letter sent to authors in December and January 2010. No reply received Follow‐up: hospital discharge Divergences from the treatment algorithm were required in 4 patients (15%) in the ROTEM group and 2 patients (7%) in the control group. The results from all 6 patients were included on an ITT basis Authors conclusion:"Thromboelastometrically guided transfusion is associated with a decreased use of allogeneic blood units and reduced risk of massive transfusion in patients undergoing aortic surgery with circulatory arrest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list, random computer number generator. |
| Allocation concealment (selection bias) | Low risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes. |
| Selective reporting (reporting bias) | Low risk | Yes. Unable to compare with protocol or trial registration, but appears to be free of selective reporting. |
| Other bias | Unclear risk | No information on funding, but otherwise appears free of other biases. |
Kempfert 2011.
| Methods | Only published as conference abstract Apparently two‐group parallel RCT in one centre N = 104, but no information on how many were allocated to each group. Data from this study have been used in the meta‐analysis with the assumption that the two groups were equal in size (e.g. 52 patients in each) ITT: unclear Funding: unclear Overall study quality: high risk Sample size calculation was not reported |
|
| Participants |
Inclusion criteria: adult patients were included only in the case of significant postoperative bleeding (> 200 mL/hour) following standard elective isolated or combined cardiac surgical procedures Exclusion criteria: Not described 13.1% were re‐do operations and 3.7% required circulatory arrest |
|
| Interventions | Intervention group: ROTEM‐guided (4‐chamber ROTEM) blood component transfusion protocol Control group: transfusion protocol based on standard coagulation testing | |
| Outcomes | No primary was stated. Transfusion requirements, re‐thoracotomy and 24‐hour drainage blood loss | |
| Notes |
Country: Germany Letter send to authors in January 2015 and February 2015. Reply from Dr. Girdauskas received in March 2015 but we were unable to retrieve any additional information Follow‐up: unclear Subgroup analysis was performed on patients with long CPB time (> 115 min, n = 55) Authors conclusion: "In cases of postoperative bleeding following cardiac surgical procedures, a treatment algorithm based on ‘point‐of‐care’ 4‐chamber TEG seems to be at least as effective as standard coagulation testing protocols In patients with long CPB times TEG‐guided treatment resulted in significantly less bleeding" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. We were not able to locate public trial registration. |
| Other bias | Unclear risk | Unable to assess because of insufficient information, no information on funding. |
Kultufan Turan 2006.
| Methods | Two‐group parallel RCT, one centre ITT: unclear Funding: not for profit Overall study quality: high risk of bias No sample size calculation was reported |
|
| Participants |
Inclusion criteria: Cardiac surgery either CABG or valve surgery Exclusion criteria: None stated |
|
| Interventions |
Intervention group: TEG‐guided transfusion algorithm group for intervention after open heart surgery Transfusion algorithm was fully based on TEG (ROTEG), (n = 20) Control group: routine transfusion therapy for intervention after cardiopulmonary bypass, standard laboratory coagulation testing, (n = 20) Duration of intervention: peri‐ and postoperative algorithm. Postoperative transfusion was indicated if bleeding was > 400 mL within an hour or > 1000 mL within 4 hours. ROTEG was performed perioperatively and 1 hour postoperation |
|
| Outcomes |
Primary: incidence of blood transfusion (whole blood, RBCs, FFP, and platelets) Secondary: unclear. No data on adverse events |
|
| Notes |
Country: Turkey. Language: Turkish Letter sent to authors in April and June 2010. Reply and supplemental data received in April and June 2010 Follow‐up: 24 hours. Authors conclusion:"We conclude that utilization of ROTEG does not alter the transfusion management significantly in open heart surgery" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The doctor in charge of performing the ROTEG and the medical doctor in the ICU were blinded. However, it is unclear if the anaesthesiologist in charge of transfusion perioperatively were blinded to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Low risk | Yes. Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Low risk | Appears adequate and free of other biases. |
Nakayama 2015.
| Methods | Two‐group parallel RCT of 100 patients, one centre trial with initial algorithm validation phase involving 78 patients ITT: yes Funding: independent funds. But one co‐author has served at advisory board for TEM international Overall study quality: low risk of bias Sample size calculation was reported and based on the primary phase of algorithm development |
|
| Participants |
Inclusion criteria: elective cardiac surgery with CBP in children weighing less than 20 kg The algorithm defines "diffuse bleeding patients" as entry criteria for the algorithm, but some of the included patients did not fulfil this criteria. No data available on how many patients presented with diffuse bleeding in each group Exclusion criteria: known coagulation defect, liver dysfunction, or under anticoagulants or if they required a second run of CPB for additional surgical repair(s) after the initial CPB during surgery Aspirin, if used, was discontinued 7 days before surgery, and warfarin, if used, was discontinued 5 days before surgery and replaced with heparin |
|
| Interventions |
Intervention group: ROTEM‐based algorithm of post‐CPB blood transfusion, (n =50) Control group: routine transfusion therapy for post‐CPB blood transfusion based on standard laboratory coagulation testing (platelet count and ACT), (n = 50). When chest tube drainage exceeded 1.0 mL/kg/hour with haemodynamic perturbation (decreased arterial pressure, decreased pulse pressure, increased heart rate by 20% from baseline, urine blood volume, 1.0 mL/kg/hour), we performed coagulation tests. ACT ≥150 S for FFP, and platelet count ≤ 80×103 /mL for platelet concentrates were used as transfusion trigger in PICU Duration of intervention: intraoperative algorithm Blood transfusion after paediatric intensive care unit (PICU) admission was managed without ROTEM guidance by paediatric cardiac surgeons and intensivists who were blinded to group assignment and intraoperative ROTEM results Neither cryoprecipitate nor fibrinogen concentrate was available at this institution |
|
| Outcomes |
Primary: total amount of chest tube drainage at 12 hours after PICU admission Secondary: postoperative red cell transfusion requirements over the initial 12 hours after paediatric cardiac surgery Bleeding and transfusion requirements during the initial 24 hours after surgery, mechanical ventilation time in the PICU, and duration of PICU stay |
|
| Notes |
Country: Japan. Language: English Letter sent to authors 7 April 2015. Reply received 16 April 2015 Follow‐up: until discharge from PICU Authors conclusion:"Haemostatic therapy for paediatric patients based on post‐CPB thromboelastometric measurements reduced postoperative blood loss and led to less postoperative blood transfusion and shorter intensive care stay" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted blocks without stratification |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Allocation was revealed only before the induction of anaesthesia. So staff at operation room were not blinded. Blood transfusion after PICU admission was managed by paediatric cardiac surgeons and intensivists who were blinded to group assignment and intraoperative ROTEM results. Clinicians making discharge decisions were blinded to the randomizations. Patients were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Trial registration was UMIN Clinical Trials Registry: UMIN000006832. Comparing article and registration we found no indications of selective reporting. |
| Other bias | Low risk | Independent funding. But one co‐author has served at advisory board for TEM international. |
NCT00772239.
| Methods | Randomized open‐label parallel assigned clinical trial Trial was terminated due to futile inclusion ITT: unclear Funding: unclear Overall study quality: high risk of bias |
|
| Participants |
Inclusion Criteria: adults > 18 years; cardiac surgery or heart transplantation with abnormal bleeding (regardless the etiology); given informed consent
Exclusion criteria: patient supported by a pre‐ or postoperative circulatory technical assistance Estimated enrolment:100 participants (50 in each arm) |
|
| Interventions |
Intervention group: a therapeutic algorithm based on the use of ROTEM Control group: coagulation management based solely on standard laboratory tests Duration of intervention: unclear |
|
| Outcomes | Primary outcome measures: quantity of different blood transfusion during cardiac surgery management | |
| Notes | Investigators contacted in May 2015. Reply received but no additional data was provided ClinicalTrials.gov identifier: NCT00772239 Country: France Follow‐up: unclear Authors conclusion: unclear No published description of the study was available so information is based only on trial registration. No data are available so this trial is not part of meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data available. |
| Allocation concealment (selection bias) | Unclear risk | No data available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No data available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study was terminated before completion. |
| Selective reporting (reporting bias) | Unclear risk | No data available. |
| Other bias | Unclear risk | Insufficient information to assess other types of bias. |
Nuttal 2001.
| Methods | Two‐group parallel RCT, one centre
ITT: Yes Funding: unclear Overall study quality: high risk of bias Sample size calculation was reported |
|
| Participants |
Inclusion criteria: adult men and nonpregnant adult women with abnormal microvascular bleeding after CPB, all types of elective open cardiac surgery requiring CPB "Abnormal microvascular bleeding" was defined as diffuse oozing with no visible clot at inspection of the operative field performed by the surgeon and the anaesthetist after CBP Exclusion criteria: patients were not excluded if they received preoperative aspirin or antiplatelet therapy "The two groups were similar with regard to preoperative characteristics, except that 34.2% of patients in the algorithm group and 15.7% in the control group were receiving preoperative warfarin therapy" |
|
| Interventions |
Intervention group: a transfusion algorithm guided by coagulation tests, (n = 41). Transfusion algorithm was only partly based on TEG (only platelet transfusion and desmopressin acetate administration). No information on the type of TEG Control group: following individual anaesthesiologist’s transfusion practices, based solely on clinical judgment with or without laboratory tests (n = 51) The transfusion algorithm did not guide erythrocyte transfusions in the operating room. Algorithms were used as long as the patient was still in the operating room. No transfusion algorithm was used in the ICU. Intraoperative blood salvage and reinfusion of shed mediastinal blood was used in all cases Concomitant treatment: prophylactic antifibrinolytic therapy, aprotinin, tranexamic acid, and epsilon aminocaproic acid were used at the discretion of the attending anaesthesiologist. Anticoagulation for CPB was accomplished with porcine heparin |
|
| Outcomes |
Primary: need for allogenic blood products during the entire stay in hospital Secondary: platelet count, TEG variables, PT, aPTT, mediastinal drainage in the ICU, risk of reoperation due to bleeding |
|
| Notes |
Country: USA; Language: English Letter sent to authors in April and June 2010. No reply received Follow‐up: unclear, but transfusion requirements were reported for the entire hospital stay. However, no data were provided on the length of stay in ICU or hospital Cross‐over: four patients were moved from the algorithm to the control group because the study personnel were not available Authors conclusion:"Use of a coagulation test–based transfusion algorithm in cardiac surgery patients with abnormal bleeding after CPB reduced non‐erythrocyte allogeneic transfusions in the operating room and ICU blood loss" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list with a block size of four to one of two groups. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The surgeons and anaesthesiologists were not made aware of which group the patients were placed in until after they decided that the patient had abnormal bleeding after CPB and they felt the patient needed to have transfusion of non‐erythrocyte components. Therefore, the people making the transfusion decisions were blinded to group designation of the patients until after the determination of abnormal bleeding after CPB." Thus, persons responsible for participants care were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unable to compare with protocol or trial registration, but appears to be free of selective reporting. |
| Other bias | Unclear risk | No information on funding, but otherwise appears free of other biases. |
Paniagua 2011.
| Methods | Only published as conference abstract, stopped before completion due to slow inclusion. Have unpublished data on 44 patients (planned for 100 patients) Two‐group parallel RCT, one centre ITT: no. 52 patients were randomized: 24 patients in control group and 28 in the intervention group. But 6 patients in control group and 2 patients in the intervention were excluded from analysis due to patients having received off‐pump surgery or did not fulfil the criteria of excessive bleeding Funding: not for profit Overall study quality: high risk of bias Sample size calculation was reported in protocol (additional information from authors) |
|
| Participants |
Inclusion criteria: adult patients undergoing cardiac surgery and with trial consent given before surgery were randomized if: • they presented diffuse bleeding after protamine and/or • they bled excessively after surgery. With criteria for excessive bleeding: the mediastinal chest tube drainage ≥ 300 mL in the first hour after surgery; ≥ 250 mL in the second hour or ≥ 150 mL at any later time. "Bleeding stopped" was defined as mediastinal chest tube drainage < 150 mL/hour Exclusion criteria: • patients ≤ 18 years • surgery without cardiopulmonary bypass • surgery with mini extracorporeal circulation • refuse to participate in the study |
|
| Interventions |
Intervention group: ROTEM‐guided transfusion algorithm, (n = 26). Hypofibrinogenemia was diagnosed if MCF in EXTEM < 50 and in FIBTEM < 9 and thrombocytopenia if MCF in EXTEM < 50 and in FIBTEM ≥ 9 Control group: routine transfusion therapy based on standard laboratory coagulation testing, (n = 18). Hypofibrinogenaemia was diagnosed if fibrinogen (Clauss method) < 1 g/L and thrombocytopenia if platelet count is below 80x109/L Duration of intervention: peri‐ and postoperative algorithm (until patient stops bleeding (mediastinal chest tube drainage bellow 150 mL/hour)) |
|
| Outcomes |
Primary: the number of transfused units of packed red blood cells during the period between inclusion into the study and after mediastinal chest tube drainage was < 150 mL/hour (stops bleeding) Secondary: the number of transfused units of FFP, platelet concentrates and any other administered haemostatic therapy during the period between inclusion into the study and after mediastinal chest tube drainage was < 150 mL/hour ( stops bleeding) Postoperative chest tube blood loss (until chest tube withdrawal, during acute bleeding and at 24 hours after ICU admission) Mortality during hospital stay. No data on adverse events |
|
| Notes |
Country: Spain. Language: English Letter sent to authors on 18 December 2015. Reply received 19 December 2015. Additional information provided by author. A total of 13 in control group and 8 in ROTEM group had chronic kidney disease at baseline, defined as a creatinine clearance of less than 60 mL/min/m2before the operation. Ten patients had pre‐existing thrombocytopenia Follow‐up: until stopped bleeding for primary outcome and until discharge on mortality. No patients lost to follow‐up Authors conclusion:"Our objective is to include 100pts in order to archive statistical significance, but in this preliminary analysis we have already seen a clear tendency towards reduction in need of RBC and FFP transfusion.The reduction of platelet transfusion and total bleeding time reached statistical significant levels" Wound infection as outcome was insignificant with one case in each group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No patients were lost to follow‐up but no ITT analysis ‐ Authors description: "We actually randomized 52 patients in both groups: 24 patients in control group and 28 in the intervention group. Unfortunatelly when we start to analyze data we realized that 6 patients in control group and 2 patients in the intervention group didn’t meet inclusion criteria. Reasons for exclusion were: patients had received off‐pump surgery or didn’t fulfil the criteria of excessive bleeding laid down in the protocol" Terminated before time due to slow inclusion. |
| Selective reporting (reporting bias) | Low risk | Clinical trials registration: NCT01919840. Appears free of selection bias. |
| Other bias | Low risk | Funding: not for profit. Apears free of other biases. |
Rauter 2007.
| Methods | Only published as conference abstract. Identified by Whiting 2015 and not included in our original search and first published version of this review (Afshari 2011) Randomized, controlled and unblinded trial, single centre ITT: Not performed. About 5/213 patients had to be excluded due to protocol violations (ROTEM group: 2, control group: 3). Funding: unclear Overall study quality: high risk of bias Sample size calculation was not reported in protocol |
|
| Participants | Elective on‐pump cardiac surgery, 208 patients | |
| Interventions |
Intervention group: transfusion guided by ROTEM plus clinical signs Control group: routine transfusion management (aPTT, Quick, fibrinogen, haemoglobin, clinical signs of anaemia) Duration of intervention: Not described |
|
| Outcomes | Perioperative use of blood products | |
| Notes |
Country: Austria. Language: English Follow‐up: The patients were observed intraoperatively and up to 48 hours postoperatively during their stay in the ICU Stastitical issues: results are based on number of blood units given to each group instead of each patient, hereby wrongly assuming that each unit of blood is independently given Letter sent to authors on 10 November 2015. No reply received Authors conclusion:"ROTEM guided coagulation therapy lead to a significant reduction in the use of RBC units. This reduction was not matched with a concomitant rise in the use of coagulation factors and thus was possibly caused by the more appropriate use of coagulation factors according to immediately available test results" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described in detail and about 5/213 patients was excluded due to protocol violations (ROTEM group: 2, control group: 3). |
| Selective reporting (reporting bias) | Unclear risk | Not described. No access to trial description or registration, only published as abstract. |
| Other bias | High risk | Funding unclear. Statistical issues (see above), no information on sample size calculation. |
Royston 2001.
| Methods | Two‐group parallel RCT, one centre
ITT: unclear Funding: unclear Overall study quality: high risk of bias Sample size calculation was reported |
|
| Participants |
Inclusion criteria: adult patients (> 21 years), high risk of requiring haemostatic products, cardiac surgery (heart transplantation, revascularization, bypass, Ross procedure, multiple valve or valve and revascularization surgery) No patient was a repeat operation. 10% of all the patients in each group had a heart transplantation and were taking aspirin and/or warfarin immediately before surgery. About 50% in each group had revascularization and were taking aspirin Exclusion criteria: if reoperation due to bleeding was performed or early death of the patient, the data were excluded and replaced by measurements from an additional patient allocated to the same group |
|
| Interventions |
Intervention: TEG‐guided transfusion algorithm group for intervention during cardiac surgery (n = 30). Transfusion algorithm was fully based on TEG. Type of TEG: Celite TEG and heparinase‐modified Celite‐activated TEG Control group: clinical criteria and laboratory‐based test, (n = 30), treatment at clinicians' discretion Duration of intervention: algorithms were used as long as the patient was in the operating room. No information on transfusions in the ICU in the postoperative period Concomitant treatment: No information on perioperative anticoagulation for CPB. No patient received any type of antifibrinolytic treatment |
|
| Outcomes |
Primary: reduced total exposure to haemostatic component therapies Secondary: mortality, TEG variables, PT, aPTT, platelet count, fibrinogen concentration, mediastinal tube drainage at 6 and 12 hours |
|
| Notes |
Country: United Kingdom. Language: English Letter sent to authors in April and June 2010. No reply received Follow‐up: unclear, but transfusion requirements and mortality data were reported for 2 days postoperatively This study was carried out in two series. In the first series, 60 patients were examined in a non‐randomized fashion using a simple algorithm predicting a possible 60% to 80% decrease in the use of haemostatic components. The second stage consisted of 60 randomized patients. We have only included the latter stage patients Total amount of FFP transfused: 5 units in the TEG group versus 16 in the control group. Total amount of platelets transfused: 1 unit in the TEG group versus 9 in the control group Authors conclusion:"Intraoperative monitoring of coagulation in the anti‐coagulated patient can be used to guide treatment" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, no information provided. |
| Allocation concealment (selection bias) | Low risk | Series of sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear, no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate follow‐up. |
| Selective reporting (reporting bias) | Low risk | Yes. Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Unclear risk | No data on funding, but otherwise appears free of other types of bias. |
Schaden 2012.
| Methods | Two‐group parallel quasi RCT, one centre. n = 30 ITT: no. "Owing to technical problems, ROTEM could not be used in three patients of the algorithm group; these patients were treated like patients allocated to the control group" Funding: funded by independent funds, but one author declared relation to TEM innovations Overall study quality: high risk of bias Sample size reported and based on primary outcome |
|
| Participants |
Inclusion criteria: surgical excision of burn wounds performed on the third day after burn trauma Exclusion criteria: none |
|
| Interventions |
Intervention group: treatment in the algorithm group was ROTEM‐based and standardized based on the recommendations for bleeding management in trauma‐induced coagulopathy by the Austrian Task Force of Perioperative Coagulation. The treatment algorithm was based on three commercially available tests (EXTEM, FIBTEM, and APTEM), (n = 14) Transfusion algorithm starting criteria was "Clinically bleeding patient, Diffuse bleeding, no visible clot in the operation site, no apparent vascular injury; haemodynamically relevant blood loss requiring additional volume therapy". All patients in ROTEM group fulfilled these criteria but unclear how many in control group Control group: coagulation management was performed according to the clinician’s discretion and included administration of FFP, platelet concentrate, fibrinogen concentrate, PCC, and tranexamic acid according to clinical judgement based on expertise, impression of diffuse bleeding in the surgical field, and/or routine coagulation tests if deemed necessary, (n = 16) Duration of intervention: until the morning after surgery (approximately 24 hours) |
|
| Outcomes |
Primary: total number of blood transfusions Secondary: use of PRBCs alone, FFP alone, platelet concentrate alone, fibrinogen concentrate, PCC, and tranexamic acid |
|
| Notes |
Country: Austria. Language: English Letter sent to authors in January 2015. Reply received in January 2015. Additional information provided Follow‐up: until discharge from ICU Authors conclusion:"..showed a significant reduction in allogeneic blood product requirements in burn victims allocated to a ROTEM‐guided treatment algorithm during surgical burn wound excision" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | By date of admission. |
| Allocation concealment (selection bias) | High risk | None. |
| Blinding (performance bias and detection bias) All outcomes | High risk | None. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration available, unable to assess selective outcome reporting because of the overall quality of the trial and publication. |
| Other bias | Unclear risk | Funded by independent funds, but one author declare relation to TEM innovations: "travel reimbursement and honoraria for consulting at a Biotest advisory board; and an unrestricted educational grant for the e‐learning platform www.perioperativebleeding.org from CSL Behring and TEM Innovations." |
Shore‐Lesserson 1999.
| Methods | Two‐group parallel RCT, one centre
ITT: yes Funding: not for profit Overall study quality: low risk of bias Sample size calculation was reported |
|
| Participants |
Inclusion criteria: adult cardiac surgical patients at moderate to high risk of microvascular bleeding and thus had a moderate to high risk for requiring a transfusion. Included patients underwent single valve replacement, multiple valve replacement, combined coronary artery bypass plus valvular procedure, cardiac reoperation, or thoracic aortic replacement. Patients receiving preoperative heparin infusion and those who had taken aspirin within the past 7 days were included Exclusion criteria: significant preexisting hepatic disease (transaminase levels > 2 times control) or renal disease requiring dialysis, or if they required preoperative inotropic support |
|
| Interventions |
Intervention group: TEG‐guided transfusion algorithm group for intervention after cardiopulmonary bypass (n = 53) Transfusion algorithm was fully based on TEG. Type of TEG: Celite and tissue factor‐activated TEG, heparinase‐modified Celite‐activated TEG Control group: routine transfusion therapy for intervention after cardiopulmonary bypass, standard laboratory coagulation testing (n = 52) Duration of intervention: algorithms were used as long as the patient was still in the operating room Concomitant treatment: all patients were given prophylactic antifibrinolytic therapy (e‐aminocaproic acid). Anticoagulation for CPB was accomplished with bovine lung heparin. PRBCs were transfused when the haematocrit was < 25%. During cardiopulmonary by‐pass (CPB), a haematocrit of 21% was accepted |
|
| Outcomes |
Primary: reduction in transfusion requirements Secondary: Coagulation tests, TEG variables, postoperative blood loss into mediastinal drainage at 6‐hour intervals for 2 days postoperatively, platelet count, PT, aPTT, fibrinogen level, TEG variables |
|
| Notes |
Country: USA, Language: English Letter sent to authors in April and June 2010. Reply received in April 2010 Follow‐up: until hospital discharge, but transfusion requirements were reported for 2 days postoperatively "One patient in the control group who received numerous transfusions of PRBC and non‐PRBC components was excluded from analysis because a surgical source of bleeding was present on reexploration. Had this patient’s data been included, the difference in transfusions between the two groups would have been even greater, merely strengthening the results" "Significant bleeding was defined objectively as >100 mL in a 3‐min period or subjectively as the absence of visible clots in the surgical field" Lost to follow‐up:"One patient enrolled but not studied was undergoing cardiac reoperation and was placed emergently on CPB because of massive haemorrhage during sternotomy. The patient was excluded from the study at this time. The other patient who did not complete the protocol was excluded due to a severe protamine reaction that required immediate reinstitution of CPB. Both of these patients were in the TEG group" Authors conclusion:"We conclude that the reduction in transfusions may have been due to improved haemostasis in these patients who had earlier and specific identification of the haemostasis abnormality and thus received more appropriate intraoperative transfusion therapy. These data support the use of TEG in an algorithm to guide transfusion therapy in complex cardiac surgery. Implications: Transfusion of allogeneic blood products is common during complex cardiac surgical procedures. In a prospective, randomised trial, we compared a transfusion algorithm using point‐of‐care coagulation testing with routine laboratory testing, and found the algorithm to be effective in reducing transfusion requirements" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central generation of table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
"The anaesthesiologist and surgeon caring for the patient were blinded to the patient’s group assignment. All intraoperative results of the TEG and laboratory coagulation tests were interpreted by an anaesthesiologist investigator not directly involved with the patient’s care. The recommended therapy according to the patient’s group assignment was communicated to the anaesthesiologist and surgeon by this investigator, as appropriate." Data entry person was blinded to group assignment. Transfusions in the ICU after the first postoperative hour were performed at the discretion of the ICU physician, who was blinded to the patient’s group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate follow‐up. |
| Selective reporting (reporting bias) | Low risk | Yes. Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Low risk | Adequate. |
Wang 2010.
| Methods | Two‐group parallel RCT, one centre ITT: yes Funding: supported by grant from Taipei Veterans General Hospital Overall study quality: high risk of bias No sample size calculation was reported |
|
| Participants | 28 adult patients undergoing orthotopic liver transplantation. No exclusion criteria stated | |
| Interventions |
Intervention group: monitored during surgery using point‐of‐care TEG analysis Control Group: monitored using standard laboratory measures of blood coagulation Specific trigger points for transfusion were established in each group with corresponding transfusion algorithms |
|
| Outcomes | Outcome measures: 3 years mortality, transfusion requirements, total amount of IV fluids (fluid total, hydroxyethyl starch, albumin), blood loss, urine output | |
| Notes |
Country: Taiwan. Language: English Letter sent to authors in November and December 2010. No reply received. Follow‐up: 3 years. No cross‐over Authors conclusion:"Thromboelastography‐guided transfusion decreases transfusion of fresh frozen plasma in patients undergoing orthotopic liver transplantation, but does not affect 3‐year survival" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, no information provided. |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No data provided on blinding, but adequate blinding appears highly unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to have adequate follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Low risk | Appears to be free of other types of bias. |
Weber 2012.
| Methods | Two‐group parallel RCT, one centre, n = 100 ITT: yes Funding: support was provided solely from institutional and/or departmental sources. But two authors declare relation to TEM innovations Overall study quality: high risk of bias Sample size calculation was reported. An interim analysis of the primary outcome variable was planned after inclusion of 50% (n = 100) of the study population. The study was planned to be terminated early if group differences in the number of transfused packed erythrocytes exceeded a level of significance defined as P < 0.01. Terminated at interim analysis n = 100. Original plan was to include 200 patients |
|
| Participants |
Inclusion criteria: patients were suitable for this trial after two inclusion steps Step 1: Patients (>= 18 years) scheduled for elective, complex cardiothoracic surgery (combined CABG and valve surgery, double or triple valve procedures, aortic surgery or redo surgery) with CPB were preoperatively screened for eligibility, and written consent was obtained Step 2: Patients were enrolled in the study after heparin reversal following CPB if at least one of the two inclusion criteria were fulfilled: (1) diffuse bleeding from capillary beds at wound surfaces requiring haemostatic therapy as assessed by the anaesthesiologist and surgeon by inspecting the operative field and/or (2) intraoperative or postoperative (during the first 24 postoperative hours) blood loss exceeding 250 mL/hour or 50 mL/10 min Exclusion criteria: pregnancy |
|
| Interventions |
Intervention group: point‐of‐care testing guided algorithm based on ROTEM and whole blood impedance aggregometry (Multiplate) An algorithm for the perioperative setting and one for the postoperative ICU setting, but the therapeutic options (protamine, tranexamic acid, desmopressin, fibrinogen concentrate, PCC, FFP, and platelet concentrates) were the same Control group: algorithm based on standard laboratory tests (ACT, INR, aPTT, platelet count and fibrinogen) Duration of intervention: until discharge from ICU Concomitant treatment: the therapeutic options (protamine, tranexamic acid, desmopressin, fibrinogen concentrate, PCC, FFP, and platelet concentrates) were the same in both groups. In cases of ongoing bleeding despite algorithm‐conforming therapy, both algorithms suggested the administration of coagulation factor XIII or rFVIIa concentrates Packed erythrocytes were transfused to maintain a haemoglobin concentration above 6 g/dL during CPB and 8 g/dL after CPB |
|
| Outcomes |
Primary: the number of transfused units of packed erythrocytes during the period between inclusion into the study and 24 hours after ICU admission Secondary: •The number of transfused units of FFP, platelet concentrates and any other administered haemostatic therapy during the period between inclusion into the study and 24 hours after ICU admission • Volume of intraoperatively and up to 24 hours postoperatively retransfused salvaged washed erythrocytes • Postoperative chest tube blood loss 6, 12, and 24 hours after ICU admission • Lowest haemoglobin concentration between inclusion into the study and 24 hours after ICU admission • Number of re‐thoracotomies during the first 24 postoperative hours • PaO2/FiO2 indices at 2, 4, 12, and 24 hours after ICU admission • Postoperative time of mechanical ventilation • Length of ICU stay and hospital stay • Incidence of acute renal failure, sepsis, thromboembolism, and allergic complications • Mortality during a 6‐month follow‐up • Costs of haemostatic therapy as prescribed by local pharmacy and blood bank |
|
| Notes |
Country: Germany. Language: English Follow‐up: 24 hours primary outcome, but 6 month on mortality Authors contacted June 2015 and reply received with additional information Authors conclusion:"haemostatic therapy algorithms in conjunction with POC testing reduced the number of transfused units of packed erythrocytes when compared with conventional laboratory coagulation testing. Moreover, POC‐guided therapy was associated with lower FFP and PC usage and costs as well as an improved clinical outcome in this prospective randomised single‐cent er study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated using a balanced (allocation ratio 1:1) block wise (20x10) randomizations. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | After patient randomizations to the conventional or POC group, coagulation analyses and algorithm‐based haemostatic therapy were performed in a non‐blinded fashion. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete data, but terminated early due to an interim analysis at 50% of the planned sample size. |
| Selective reporting (reporting bias) | Low risk | Clinical trial NCT00997841. Comparing with trial registration we found no indications of selective reporting. |
| Other bias | Unclear risk | Funded by independent funds, but two authors declared to have received speakers’ honoraria from TEM innovations. |
Westbrook 2009.
| Methods | Two‐group parallel RCT, one centre ITT: unclear Funding: unclear Overall study quality: high risk of bias No sample size calculation was reported |
|
| Participants | All patients presenting for cardiac surgery with the exception of lung transplantation. 10% of the control group and 9.38% of the TEG group were patients with urgent presentation | |
| Interventions |
Intervention group: TEG (plain and heparinase coated cups) before bypass in the re‐warming phase (core body temperature > 36.5 °C), and 15 min after protamine administration (dose matching total heparin dose) at the end of bypass. Platelet Mapping in patients taking aspirin or clopidogrel immediately prior to induction of anaesthesia. Transfusion of blood products, administration of protamine and/or procoagulant blood products strictly according to predefined protocols based on several TEG measurements alone. In ICU transfusion strategy and treatment (additional administration of protamine) strictly according to protocols and TEG analyses. In ICU protocols for: postop ICU monitoring, ICU surgical intervention, Novo 7 administration and ICU red blood cell replacement Control group: transfusion strategy at the attending clinician's discretion based on previous experience and standard coagulation tests (e.g. aPTT, INR, fibrinogen level, platelet count). The timing of these tests were also at clinician's discretion. In case of blood loss > 200 mL over 15 minutes, activated factor 7 was considered Re‐sternotomy was performed when sustained bleeding > 100 mL/hour in the presence of a normal TEG. Timing of a re‐sternotomy was at clinician's discretion Concomitant treatment: aprotinin was used in 41.6% of control group during surgery versus 40.6 in the TEG group |
|
| Outcomes | Blood loss, intubation time (hours), minimum Hb (g/L), ICU stay, hospital stay (days) | |
| Notes |
Country: Australia. Language: English Letter sent to authors in June 2010. No reply received Follow‐up: until hospital discharge Statistical issues: results are based on number of blood units given to each group instead of each patient, hereby wrongly assuming that each unit of blood is independently given Authors conclusion:"This pilot study suggests that a strict protocol for blood product replacement based on the TEG might be highly effective in reducing usage without impairing short‐term outcome" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, no information provided. |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear to which extend the blinding took place but the surgeons were blinded as to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to have adequate follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unable to compare with protocol or trial registration but appears to be free of selective reporting. |
| Other bias | Unclear risk | No information on funding but otherwise appears free of other types of bias. |
Please see Appendix 4 for abbreviations.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2006 | Not RCT Retrospective comparative study. (n = 990, 502 in intervention group). Cardiac surgery patients with excessive bleeding or suspected coagulopathy. Intervention: postoperative ROTEM‐based transfusion algorithm. Outcome: amount transfused, risk of transfusion. Excluded due to the design |
| Andreasen 2011 | Not RCT A prospective, descriptive study in 60 children undergoing congenital cardiac surgery Objective: to compare the performance of ROTEM with that of conventional coagulation tests in children |
| Aoki 2012 | Not RCT This study included patients who underwent cardiovascular surgery using CPB In the first 50 patients PT was guided by experience‐based guidelines In the next 50 patients PT was controlled by a TEG‐guided protocol that was based on a combination of platelet count and maximum amplitude Authors conclusion: "Use of a TEG‐guided transfusion protocol dramatically reduced PT after CPB, particularly in patients undergoing aortic arch aneurysm repair" |
| Blasi 2012 | Not RCT This study was aimed at assessing the value of TEM in monitoring blood coagulation and guide transfusion support in orthotopic liver transplantation (OLT) N = 236. Prospective observational design Author conclusion: "A10(EXTEM) is an adequate TEM variable to guide therapeutic decisions during OLT. Patients with A10(EXTEM) of greater than 35 mm are unlikely to bleed because of coagulation deficiencies, but using A10(EXTEM) of not more than 35 mm as the sole transfusion criterion can lead to unnecessary utilization of PLTs and fibrinogen‐rich products" |
| Coakley 2006 | Not RCT Prospective observational study, (n = 20), orthotopic liver transplantation. Objective: comparing the agreement between TEG, ROTEM and standard laboratory tests concerning the indication of blood transfusion. Excluded due to the design |
| Cui 2009 | Not RCT This study investigated features and treatments of perioperative coagulopathies in cyanotic infants with CCHD. Thirty‐six infants with cyanotic CCHD were involved and divided into two groups: In group H (n = 20), haematocrit > 54%, and in group L (n = 16), haematocrit < 54%. After surgery, group H was treated with fibrinogen‐combined platelets (PLT), while group L was treated with PLT only. TEG was used to evaluate the haemostatic changes |
| De Pietri 2014 | Not bleeding patients Aim of this study was to evaluate the efficacy of TEG before invasive procedure as a guide for haemoderivates transfusion in cirrhotics. Patients with cirrhosis and coagulation disorders (INR > 1.8 and/or PLTs < 50x10^3/mmc) undergoing invasive procedures were eligible. Exclusion criteria were: ongoing bleeding, thrombotic events, anticoagulant or antiaggregant medications, sepsis and renal replacement therapy. Patients were randomly allocated either to TEG group, receiving FFP 10 mL/kg in case of R > 40 mm and/or platelets (PLTs 1 unit/10 kg) for maximum amplitude < 30 mm before procedure, or to per‐ protocol group, receiving PLTs and/or FFP before procedure according to internal guidelines. Transfusion requirement, side effects, and related costs were recorded. The four publications represents preliminary results of the trial when 30, 40, and 50 patients were included. The goal is to include 60 patients |
| Dirkmann 2013 | Not RCT Results of 437 ROTEM assays (EXTEM , INTEM , FIBTEM , and HEPTEM ) from 84 patients undergoing CPB surgery were analysed. Measurements were performed prior to and after heparin administration, as well as after protamine administration Authors conclusion: "...early values of CF (A5‐A15) reliably predict maximum CF under all conditions and, therefore, allow for marked time savings in the interpretation of ROTEM measurements. This may guide earlier and more specific treatment of CPB‐related coagulation disorders" |
| Doran 2010 | Not RCT A prospective observational field study was performed in a deployed military setting to determine the feasibility of using TEM to assess the coagulation status of patients admitted to the emergency department and who subsequently received a massive transfusion. N = 31 Auhtors conclusion: "It is feasible to use TEM in a deployed military setting. We have shown that rotational thromboelastometry significantly detects more abnormalities in the coagulation status than the standard laboratory tests (prothrombin time, and activated partial thromboplastin time)" |
| Dua 2005 | Not RCT Prospective intervention study with historical control group, (n = 100). Participants: off‐pump CAB patients. Intervention: TEG‐guided postoperative blood transfusion algorithm versus standard algorithm using standard laboratory tests and clinical judgement. Outcomes: amount of blood transfused postoperatively, risk of postoperative transfusions and 24 hours blood loss (chest tube drainage). Excluded due to design |
| El 2009 | Not RCT Evaluation of the perioperative coagulation profile using both standard laboratory work and TEG in paediatric patients undergoing craniotomy for primary brain tumours. N = 40 Authors conclusion: "Thromboelastography may be useful in the perioperative assessment and monitoring of coagulation in paediatric neurosurgical patients and helps in identifying patients at increased risk of bleeding or thromboembolic events" |
| Faraoni 2013 | Not RCT Review discussing the possibilities of using TEG‐guided algorithms |
| Gorlinger 2011a | Not RCT Development and implementation of an algorithm for coagulation management in cardiovascular surgery based on first‐line administration of coagulation factor concentrates combined with point‐of‐care TEG/impedance aggregometry |
| Gorlinger 2011b | Not RCT Review discussing ratio based transfusion (1.1:1) and TEG‐guided algorithms |
| Gronchi 2014 | Not RCT Prospective observational study. Twenty patients undergoing coronary artery bypass grafting Authors conclusion: "HEPTEM and EXTEM measurements are valid in the presence of very high heparin concentrations and can be performed before protamine administration in patients undergoing cardiac surgery with CPB" |
| Haas 2015 | Not TEG‐guided algorithm versus comparison RCT randomizing children (n = 49) with craniosynostosis or scoliosis surgery to a ROTEM ‐ FIBTEM‐guided use of fibrinogen concentrate: 30 mg/kg if FIBTEM MCF < 8 mm versus 30 mg/kg if FIBTEM MCF < 13 mm (named "early substitution") |
| Hanke 2012 | Not RCT After 5 cases of acute type A aortic dissection and aortic arch replacement had been treated based on ROTEM findings, 5 cases without ROTEM were matched as control group. Control Group treatment was based on conventional tests and clinical findings. Blood component and coagulation factor requirements, ventilation time, duration of stay at ICU, hospitalizations, and thrombotic or bleeding incidents as well as transfusion‐associated costs were compared. Two publications |
| Hill 2012 | Not RCT Prospective observational study of healthy patients N = 57. Healthy, term‐parturients provided pre‐caesarean whole blood specimens for TEG‐analyses. Aims to establish reference ranges for treatment algorithm during haemorrhage |
| Howland 1974 | Not RCT Prospective observational uncontrolled study, (n = 158). A comparison of native‐TEG with standard laboratory tests, with the aim of diagnosis of hypo‐ and hypercoagulability. Excluded due to design |
| Hvas 2012 | Not RCT Observational prospective study using a historic control. Aimed to monitor the use of blood products and haemostatic intervention after implementation of ROTEM in patients undergoing cardiac surgery. Excluded due to design |
| Jambor 2009 | Not RCT Editorial. Describing a clinical transfusion algorithm for cardiac surgery based on measures of fibrinogen level, aPTT, platelets count and ROTEM and/or Multiplate. Excluded due to design |
| Johansson 2007 | Not RCT Retrospective comparative study (n = 148; 55 received intervention). Participants: patients with ruptured abdominal aorta aneurisms (vascular surgery). Intervention: the Blood Bank as partner in treatment, providing feedback to clinicians regarding on‐going transfusion strategy and TEG‐monitored haemostatic evaluation. Outcome: mortality, amount of blood transfusion, haemostatic laboratory values and hospital/ICU stay. Excluded due to design |
| Johansson 2009 | Not RCT Retrospective comparative study, (n = 832; 442 received intervention). Participants: surgical patients receiving multi‐transfusion (> 10 units of RBCs within 24 hours). Intervention: "haemostatic control resuscitation"‐concept: comprising primary resuscitation with ratio 5:5:2 (RBCs: FFP: platelets) and TEG‐guided peri‐ and postoperative transfusion. Outcome: amount of transfusion, haemostatic lab values and mortality (30 days and 90 days). Excluded due to design |
| Kunio 2012 | Not RCT Aimed at determining the relationship between coagulopathy and outcome after traumatic brain injury. N = 69 Authors conclusion: "Hypocoagulability as shown by thromboelastography after traumatic brain injury is associated with worse outcomes and an increased incidence of neurosurgical intervention" |
| Mendeloff 2009 | Not RCT Retrospective comparative study, (n = 182; 112 received intervention). Participants: infants (< 6 months of age) undergoing open heart surgery. Subgroups comprising categories of "acyanotic" and "cyanotic" patients. Intervention: pre‐ and postoperative TEG‐guided transfusion algorithm. Outcome: amount transfused, chest‐tube drainage and haemostatic laboratory variables. Excluded due to design |
| Naik 2015 | Not RCT Retrospective cohort with historical comparison between ROTEM group and previous standard treatment Correctional spine surgery |
| NCT01218074 | Ongoing study Not TEG/ROTEM versus other treatment TEG versus TEG + Multiplate‐guided algorithm, ongoing study Platelets Antiaggregation Control Enhancement (PACE) Study Randomized parallel assignment, double‐blind Estimated enrolment: 400 Inclusion criteria: All patients undergoing surgical myocardial revascularization Experimental: aggregometry+tromboelastography Patients undergo standard TEG and subsequent aggregometry to test effectiveness of residual antiaggregation drugs. Patients found to have altered value undergo optimization with desmopressin Control: TEG alone Patients undergo standard of care TEG to evaluate overall coagulation performances Primary outcome measures: Bleeding volume (12 hours after end of operation) Total amount of bleeding in the first 12 hours after cardiac surgery expressed as millilitres of blood in the chest drains reservoir Secondary outcome measures: use of allogenic blood transfusions. (In hospital stay (usually 5 to 8 days after operation)) Number of allogenic blood units transfused per patients during the full hospital stay, usually 5 to 8 days after operation Start date December 2010, estimated completion date September 2016 NCT01218074, Luca P Weltert, MD lweltert@gmail.com |
| Ogawa 2012 | Not RCT The assessment of whole blood coagulation using rotation ROTEM was compared to coagulation tests routinely performed during cardiac surgery Blood was obtained from 26 patients undergoing CPB surgery. Prospective observational design Authors conclusion: "ROTEM variables demonstrated clinically relevant correlations with PLT counts and fibrinogen levels. In particular, decreasing levels of fibrinogen can be quickly determined (<15‐20 min) using FIBTEM" |
| Ploppa 2010 | Not bleeding patients Not RCT A case of a patient who suffered a massive pulmonary embolism with cardiac arrest on postoperative day 4 after a Whipple operation Authors suggest that ROTEM may be useful for early dose adjustment when standard dosing regimens fail |
| Rafiq 2012 | Not bleeding patients Ongoing study Objective: to evaluate whether TEG‐hypercoagulable CABG patients will benefit from intensified antiplatelet therapy after surgery. Monitoring of platelet inhibition from instituted antithrombotic therapy will elucidate platelet resistance patterns after CABG surgery. Clinicaltrials.gov Identifier NCT01046942 |
| Rahe‐Meyer 2009a | Not RCT Investigates the addition of fibrinogen concentrate to the algorithm First a retrospective group of 42 participants undergoing elective thoracoabdominal aortic aneurysm surgery, clinically relevant diffuse bleeding after weaning from cardiopulmonary bypass was treated with allogeneic blood products (platelet concentrates, followed by fresh frozen plasma) according to a predetermined algorithm. Afterwards a prospective group of 15 participants having a first therapy step with fibrinogen concentrate was added to the algorithm. The dose of fibrinogen concentrate was estimated by using thromboelastometric data (ROTEM) |
| Rahe‐Meyer 2009b | Not RCT Prospective intervention group with historical retrospective controls, (n = 18; 6 received intervention). Participants: elective thoracoabdominal aortic aneurism surgery with a postoperative bleeding of 60‐250 gram. Intervention: postoperative ROTEM (FIBTEM) guided dosage of fibrinogen‐concentrate given initially. Blood components not guided with ROTEM. Outcome: amount and risk of transfusion, drainage volume and haemostatic laboratory variables. Excluded due to design |
| Roullet 2014 | Not RCT Objective: to assess the use of a ROTEM‐based transfusion algorithm during ortotopic liver transplantation would lead to transfusing more fibrinogen and to decreasing bleeding and blood transfusion. Sixty adult patients were consecutively included in a prospective without‐with study: 30 in the group without ROTEM results and 30 in the group with ROTEM‐based algorithm Authors conclusion: "It was not associated with a decrease in blood transfusion or in the number of patients exposed to blood products" |
| Schochl 2010 | Not RCT This retrospective analysis included trauma patients who received > or = 5 units of red blood cell concentrate within 24 hours. Coagulation management was guided by ROTEM. Fibrinogen concentrate was given as first‐line haemostatic therapy when MCF measured by FibTEM (fibrin‐based test) was < 10 mm. PCC was given in case of recent coumarin intake or clotting time measured by extrinsic activation test (EXTEM) > 1.5 times normal. Lack of improvement in EXTEM MCF after fibrinogen concentrate administration was an indication for platelet concentrate. The observed mortality was compared with the mortality predicted by the trauma injury severity score and by the revised injury severity classification score |
| Schochl 2011 | Not RCT Aim to assess ROTEM‐guided haemostatic therapy, with fibrinogen concentrate as first‐line haemostatic therapy and additional PCC This retrospective analysis compared patients treated with fibrinogen concentrate and/or PCC, but no FFP (n = 80), and patients from the trauma register receiving ≥ 2 units of FFP, but no fibrinogen concentrate/PCC (n = 601) |
| Schochl 2014 | Not RCT Review explaining ROTEM‐guided algorithms |
| Smart 2015 | Not RCT Conference abstract Retrospective study comparing ROTEM‐guided transfusion with historical control group in liver transplantation |
| Solomon 2012 | Not RCT This prospective, randomized, placebo‐controlled trial of 61 patients investigated fibrinogen concentrate as a first‐line haemostatic therapy in adults undergoing elective aortic replacement surgery. ROTEM‐guided transfusion in both intervention and control group |
| Spalding 2007 | Not RCT Retrospective comparative study, (n = 1422, 174 received intervention). Participants: cardiac surgery patients. Intervention: a postoperative ROTEM‐based transfusion algorithm used if mediastinal drain loss > 200 mL/hour. Outcomes: annual treatment costs, early mortality and reoperations due to bleeding. Excluded due to design |
| Spiess 1995 | Not RCT Retrospective comparative study. Participants: cardiac surgery patients. Intervention: un‐protocolled transfusion guided by TEG peri‐ and postoperative. Outcomes: transfusion need, risk of multi‐transfusion, mediastinal re‐exploration for bleeding and overall donor exposure. Excluded due to design |
| Tapia 2013 | Not RCT Study hypothesis: TEG‐directed resuscitation is equivalent to multitransfusion (MTP) protocol resuscitation (1:1:1). Retrospective chart evaluation Authors conclusion: "TEG‐directed resuscitation is equivalent to standardized MTP for patients receiving 6 Units or more RBC and for blunt trauma patients receiving 10 Units or more RBC. MTP therapy worsened mortality in penetrating trauma patients receiving 10 Units or more RBC, indicating a continued need for TEG‐directed therapy. A 1:1:1 strategy may not be adequate in all patients" |
| Urwyler 2012 | Not RCT Observational pilot study assessing whether fibrinogen measured by point‐of‐care (ROTEM, fibtem‐test) may lead to a similar therapeutical decision concerning the administration of fibrinogen concentrate when compared to the standard method (Clauss). N = 36 |
| Vaidya 2007 | Not RCT Not patients with bleeding Retrospective uncontrolled trial (n = 74). Objective: the use of TEG as a tool to individualize anticoagulation treatment in pancreatic transplant patients. Excluded due to design and clinical problem not being bleeding |
| Wang 2012 | Not RCT Before and after study: Thirty‐eight patients received coagulation products when standard TEG cut‐off values were exceeded, afterwards another 39 patients received coagulation products when the TEG values were 35% greater than normal Authors conclusion: "In conclusion, the use of higher critical TEG values to initiate the transfusion of plasma‐containing products is not associated with increased blood loss. Further testing is necessary to identify what TEG value predicts bleeding due to a deficit in coagulation factors" |
Please see Appendix 4 for abbreviations.
Characteristics of ongoing studies [ordered by study ID]
NCT01402739.
| Trial name or title | Algorithm‐guided transfusions in cardiac surgery patients for reduction of drainage blood losses (HEART‐PoC) |
| Methods | Randomized parallel assignment open‐label Estimated enrolment: 116 |
| Participants |
Inclusion criteria: 1. elective cardiac surgery patient requiring cardiopulmonary bypass 2. moderate or high transfusion risk 3. signed informed consent Exclusion criteria: 1. age < 18 or > 80 years 2. known haemophilia 3. known thrombophilia 4. known thrombocytopathy 5. hereditary or acquired coagulation disorder 6. active endocarditis 7. ejection fraction < 30% 8. body surface area (BSA) < 1.8 sqm 9. planned aortic arch surgery 10. preoperative thrombocytopenia < 150/nL 11. underlying haemostaseological disease 12. preoperative anaemia 13. liver cirrhosis Child B or higher 14. preoperative creatinine > 2 mg/dL 15. terminal renal insufficiency requiring dialysis 16. vitamin K antagonists during 5 days prior to surgery 17. pregnant or breastfeeding women 18. known allergy against allogeneic blood products or coagulation factors 19. refusal of blood transfusions 20. any concomitant investigational agent or participation in another trial |
| Interventions |
Experimental: point‐of‐care algorithm‐guided transfusions Point‐of‐care coagulation monitoring‐guided transfusion algorithm (ROTEM, aggregometry, blood gas analysis); other name: ROTEM delta, Multiplate, ABL 725 Control: standard of care transfusions standard coagulation monitoring‐guided transfusion algorithm aPTT, ACT, platelet count, haemoglobin, fibrinogen |
| Outcomes |
Primary outcome measures 1. chest tube output (24 hours) Secondary outcome measures 1. need for allogeneic blood transfusions (24 hours) 2. course of conventional coagulation parameters (aPTT, PT, fibrinogen, FXIII, ACT) (24 hours) 3. duration of mechanical ventilation (hours (average)) 4. incidence of RRT (during 30 days or until hospital discharge, whatever is earlier) |
| Starting date | Start date August 2011. Estimated completion date December 2013 |
| Contact information | Michael Sander, MD, michael.sander@charite.de Christian von Heymann, MD, christian.heymann@vivantes.de Department of Anesthesiology CCM/CVK Charité Universitätsmedizin Berlin Authors contacted 5 May 2015, reply received 6 May 2015 |
| Notes | NCT01402739 |
NCT01536496.
| Trial name or title | Comparison of rapid thromboelastography and conventional coagulation testing for haemostatic resuscitation in trauma |
| Methods | Randomized parallel assignment open‐label Estimated enrolment: 114 |
| Participants |
Inclusion criteria: 1. male or female, age > 18 years admitted to Denver Health Medical Center 2. blunt or penetrating trauma sustained < 6 hours before admission, with injury severity score > 15 (ISS > 15), likely to require transfusion of RBC within 6 hours from admission as indicated by clinical assessment Exclusion criteria: 1. age < 18 years 2. documented chronic liver disease (total bilirubin > 2.0 mg/dL). Advanced cirrhosis discovered on laparotomy will be a criterion for study withdrawal and exclusion of conventional coagulation or r‐TEG/TEG data from the analysis) 3. known inherited defects of coagulation function (e.g. haemophilia, Von Willebrand's disease) 4. prisoner 5. pregnancy |
| Interventions |
Intervention group: blood product transfusion based on rapid thromboelastography (r‐TEG) results Patients randomized to the r‐TEG‐guided haemostatic resuscitation group (test group) will receive blood component therapy per usual clinical practice. The test arm involves the use of rapid‐TEG to diagnose and describe postinjury coagulopathy and to guide blood product replacement per institutional algorithm. In the test group, blood for r‐TEG will be collected on admission, or upon entering the operating room, depending on the acuity of the injury (baseline), and this will be followed by two additional r‐TEG analyses during the first six hours at the discretion of the treating team (attending surgeon, anaesthesiologist) and then two further r‐TEG analyses at 12 hours and at 24 hours postinjury, respectively. The current institutional massive transfusion protocol will be followed. Only the results pertinent to the group to which randomized will be released to the treating team, unless otherwise requested Control group: blood product transfusion based on conventional coagulation tests Patients randomized to the control group will receive blood component therapy guided by conventional coagulation tests per usual clinical practice. The control arm involves the use of conventional coagulation tests (aPTT, INR, fibrinogen level, D‐dimer) to diagnose and describe postinjury coagulopathy and to guide blood product replacement. In the control group, blood will be drawn for conventional coagulation testing (aPTT, INR, platelet count, fibrinogen level, D‐dimer) at baseline (as defined above), then twice more during the first six hours at the discretion of the treating team, then again at 12 hours and at 24 hours postinjury. The current institutional massive transfusion protocol will be followed. Only the results pertinent to the group to which randomized will be released to the treating team, unless otherwise requested |
| Outcomes |
Primary outcome measures: 1. change in r‐TEG parameters (TEG‐ACT, alpha angle, K value, maximum amplitude, G value (clot strength), and fibrinolysis (EPL = estimated percent lysis)). (On hospital admission (usually within an hour), twice within first 6 hours postinjury, 12, and 24 hours postinjury) 2. change in conventional coagulation test results (aPTT, INR, platelet count, fibrinogen level, D‐dimer). (On hospital admission (usually within an hour), twice within first 6 hours postinjury, 12, and 24 hours postinjury) 3. Quality and quantity of blood products transfused (within 24 hours postinjury). Quantities of blood products transfused (PRBCs, FFP, cryoprecipitate, and apheresis platelets) in the first 24 hours postinjury) 4. patterns of transfusion ratios of RBC: FFP: platelets in the first 24 hours postinjury 5. haemorrhage‐related deaths specified as very early mortality (< 2 hours postinjury), early mortality (2 < 6 hours postinjury) 6. delayed mortality (6‐24 hours postinjury) ‐ incidence, cause and hours since injury (within 24 hours postinjury) 7. late mortality (> 24 hours postinjury through day 30) ‐ incidence, cause and days since injury (up to 30 days postinjury) Secondary outcome measures: 1. cessation of coagulopathic bleeding based upon clinical impressions of the treating surgeons and review of operative records and outcome (hours since injury ‐ up to 24 hours postinjury) 2. timeframe of all transfusions during the first 24 hours postinjury (stratified by: 0 < 2 hours, 2 < 4 hours, 4 < 6 hours, 6 < 12 hours, and 12‐24 hours postinjury) 3. number of participants with multiple organ failure during this hospitalizations (Up to 30 days postinjury) 4. multiple organ failure score (Denver method) will be calculated 5. length of stay (days) in the surgical ICU and number of ventilator‐free days in the surgical ICU (within 28 days) |
| Starting date | Start date September 2010; estimated completion date July 2014 |
| Contact information | Ernest Moore, Director, Surgery/Trauma Service, Denver Health and Hospital Authority, Colorado, United States, ernest.moore@dhha.org |
| Notes | NCT01536496 Authors had been contacted on 5 May 2015, and September 2015, but with no response |
NCT02352181.
| Trial name or title | Management of coagulopathy during orthotopic liver transplantation. Comparison between ROTEM‐based management and standard biological assessment |
| Methods | Randomized parallel assignment, open‐label Estimated enrolment: 80 |
| Participants |
Inclusion criteria: 1. patients >= 18 years of age 2. patients undergoing orthotopic liver transplantation in the Croix‐Rousse Hospital within 24 months after inclusion and who have received clear information and who are not opposed to participation in the study 3. patients affiliated to a social security system or similar 4. patients not subject to a measure of legal protection Exclusion criteria: 1. opposition to participation in the study 2. patients < 18 years of age 3. patients who participated in the previous month in another study protocol 4. pregnant women or breastfeeding 5. not affiliated with a social security system 6. patients with haemostasis pathology (haemophilia) |
| Interventions |
Experimental: R group The R group will consist of patients transfused according to an algorithm based on the data of the coagulation ROTEM analysis Transfusional protocol for ROTEM group. RBC concentrate if haemoglobin < 9 gram per litre; fibrinogen 3 gram, if A10 FIBTEM < 8 mm Platelet concentrate: 1. if MCF EXTEM < 40 mm or A10 < 35 mm and MCF or A10 FIBTEM > 8 mm 2. if platelets < 30 gram per litre at vascular unclamping time at the end of intervention or without bleeding. 2 FFP if CT EXTEM >100s Bolus tranexamic acid 1 g and 3 g every 24 hours: 1. if fibrinolysis in EXTEM 2. reduction of 15% of clotting time or clot formation time and increase of MCF in APTEM compared to EXTEM, or maximal lysis at 60 minutes > 15% Analyses in R group only: blood sampling on citrated tube for ROTEM analysis (EXTEM, INTEM, FIBTEM, APTEM +/‐ HEPTEM), coagulation profile (same as that of the S Group, for emergency procedure) Placebo comparator: S group S Group: will be transfused patients according to standard management based on conventional coagulation profile of the laboratory Procedure: conventional coagulation profile analysis Transfusional protocol for standard group RBC concentrate if haemoglobin < 9 gram per litre; fibrinogen 3 gram, if fibrinogen < 1 gram per litre Platelet concentrate: 1. if platelets < 50 gram per litre before transfusion, at anhepatic phase, or in case of bleeding 2. if platelets < 30 gram per litre at vascular unclamping time at the end of intervention or without bleeding; 2 FFP if:
Analyses in S group only: coagulation profile (PT, aPTT, INR, fibrinogen, platelet count, soluble complexes, PDF) Analyses common to both groups: NFS, chemistry panel with ionised serum calcium, blood gas with lactates, HemoCue, capillary blood glucose |
| Outcomes | Primary outcome measures: amount of blood product (in millilitre) transfused during liver transplantation, during time of liver transplantation an average of 9 hours Secondary outcome measures: (within first 48 hours after liver transplantation). Occurrence of serious respiratory complication, reintubation, acute pulmonary oedema, occurrence of thrombotic complication, hepatic artery thrombosis, portal thrombosis Occurrence of serious infectious complication, septic shock; serious sepsis, intubation necessity for sepsis |
| Starting date | Starting December 2014 and estimated study completion October 2017 |
| Contact information | Contact: Aurélie Bonnet, aurelie.bonnet@chu‐lyon.fr, Isabelle Delfour isabelle.delfour@chu‐lyon.fr, Hôpital de la Croix Rousse, Lyon, France |
| Notes | NCT02352181 |
NCT02416817.
| Trial name or title | Strategy of transfusion in trauma patients ‐ STATA Trial |
| Methods | Randomized, parallel assignment, open‐label Estimated enrolment: 200 |
| Participants | To be included, patients must meet the following inclusion criteria. 1. Trauma victims 2. Adults between 18 ‐ 80 years old 3. Injury severity score (ISS) between 15 and 45 4. Assessment of blood consumption (ABC) score ≥ 3 points 5. Shock index ≥ 1.4 6. Acute haemorrhage of more than 50% estimated blood volume in 3 hours or more than 1.5 mL/kg/min of blood during 20 minutes Exclusion criteria: 1. early cardiac arrest 2. pregnancy 3. injury severity score (ISS) > 45 4. patient transferred from another hospital 5. drug abuse history 6. known coagulation impairment 7. known use of anticoagulants, or platelet antiaggregants |
| Interventions | 1. FFP, platelets concentrate and PRBCs in 1:1:1 ratio 2. RBC based on haemoglobin measurements and will receive either Beriplex P/N (CSL Behring GmbH, Marburg, Germany) or Haemocomplettan P (CSL Behring, Marburg, Germany), or platelets based on ROTEM |
| Outcomes | Primary outcome measures: SOFA score ‐ 5 days |
| Starting date | July 2014 (current status: recruiting) estimated completion date January 2017 |
| Contact information | Roseny R Rodrigues, Ph.D +5511987187880 ny_rodrigues@yahoo.com.br, University of Sao Paulo General Hospital, Brazil |
| Notes | NCT02416817 |
NCT02461251.
| Trial name or title | Thromboelastometry‐guided treatment protocol versus standard care of major haemorrhage in obstetric patients (ROTEM‐PPH) |
| Methods | Parallel open‐label randomized clinical trial Estimated enrolment: 60 |
| Participants |
Inclusion criteria: 1. age over 18 2. severe postpartum haemorrhage i.e. active bleeding of more than 1000 mL within 24 hours after vaginal delivery or cesarean section 3. informed consent (after randomizations) Exclusion criteria: 1. known haemophilia or von Willebrand's disease 2. unacceptance of allogeneic blood products (Jehovah's witnesses) |
| Interventions | A comparison of two different treatment protocols will be made in patients suffering major obstetric haemorrhage: those who after a normal delivery are bleeding more than 1000 mL and are in need of surgical intervention to control the bleeding and those in cesarean section with ongoing bleeding of more than 1000 mL. Patients are randomized into two groups: 1. control group (n = 30) will be treated according to a protocol based on clinical decision making, standard coagulation tests and massive transfusion packages of blood products(1:1:1), if needed. This is referred as 'standard care' in this hospital 2. intervention group (n = 30) will be treated according to a ROTEM‐guided protocol, and massive transfusion packages, if needed. The study is powered to detect a reduction of one unit in RBC transfusion. Blood product, fibrinogen concentrate, prothrombin complex concentrate usage and total amount of blood loss will be compared, and the number of transfusion‐related side effects and thromboembolic events 30 days after the bleeding will be recorded |
| Outcomes |
Primary outcome measures: 1. reduction in blood transfusions within 24 hours Secondary outcome measures: 1. reduction of transfusion‐related side effects within 30 days 2. number of thromboembolic events within 30 days |
| Starting date | Not yet recruiting |
| Contact information | Samuli Jokinen, MD samuli.jokinen@phsp.fi |
| Notes | NCT02461251 |
NCT02593877.
| Trial name or title | Implementing treatment algorithms for the correction of trauma induced coagulopathy (iTACTIC) |
| Methods | Multicentre, parallel, patient‐blinded randomized clinical trial Estimated enrolment: 392 |
| Participants |
Inclusion criteria: adult trauma patients (according to local definitions) will be enrolled if they: 1. present with haemorrhagic shock at any time from the time of injury until admission to the emergency department (where shock is defined byheart rate > 100 b/min and/or systolic blood pressure < 90 mmHg) and activate the local massive transfusion protocol 2. randomized within 3 hours of injury and 1 hour of admission to the emergency department 3. agreement is provided on behalf of incapacitated patients by personal consultee or nominated consultee (e.g. trauma team leader) |
| Interventions |
Experimental VHA algorithm group: massive transfusion protocol resuscitation aiming at ratio 1:1:1 of blood components (RBC 1: plasma 1: platelets 1) and viscoelastic haemostatic assay (VHA)‐guiding further resuscitation with blood products and procoagulant factors Control group: massive transfusion protocol resuscitation aiming at ratio 1:1:1 of blood components (RBC 1: plasma 1: platelets 1) and conventional coagulation tests guiding further resuscitation with blood products and procoagulant factors |
| Outcomes |
Primary outcome measures: 1. proportion of subjects alive and free of massive transfusion within 24 hours 2. proportion of subjects at 24 hours postadmission who are alive and free of massive transfusion (i.e. received 10 or more units of RBC within 24 hours) Secondary outcome measures: 1. 6‐hour mortality 2. 24‐hour mortality 3. 28 days mortality 4. 90 days mortality 5. duration of coagulopathy within 28 days: the time spent in coagulopathic state, as defined by PT/International Ratio (PTr) PTr > 1.2) from admission until the point of haemostasis (itself defined as having occurred at the end of the first hour free of red cell transfusions and the treating clinicians believe primary haemostasis has been achieved) 6. severity of coagulopathy within 28 days: defined by the area under the PT/ International Ratio (PTr) curve from admission to the point of haemostasis (where time of haemostasis is defined as having occurred at the end of the first hour free of red cell transfusions and the treating clinicians believe primary haemostasis has been achieved) 7. proportion of patients with corrected coagulopathy after first 8 units of PRBCs within 28 days 8. time to haemostasis within 28 days: time from admission to the point of haemostasis (where time of haemostasis is defined as having occurred at the end of the first hour free of red cell transfusions and the treating clinicians believe primary haemostasis has been achieved) 9. time spent in coagulopathic condition until haemostasis within 28 days: time of haemostasis is defined as the period from admission to the point as having occurred at the end of the first hour free of red cell transfusions and the treating clinicians believe primary haemostasis has been achieved. Coagulopathy defined as PTr > 1.2 10. 6‐hour blood products transfused: total blood products (RBC, plasma, platelets alone and in total) transfused in first 6 hours after admission 11. 24‐hour blood products transfused: total blood products (RBC, plasma, platelets alone and in total) transfused in first 24 hours after admission 12. 28 days ventilator‐free days: calculated by subtracting the number of days spent on mechanical ventilation from 28 13. 28 days ICU‐free days: calculated by subtracting the number of days spent in ICU from 28 14. length of stay: length of stay will be recorded in days, for the total number spent in ICU and in hospital. If the patient is in the hospital at any time point during a day, this day will be considered a hospital day 15. symptomatic thromboembolic events within 28 days: symptomatic venous thromboembolic events shall be recorded, as confirmed by radiology. Other thromboembolic events such as myocardial infarction and/or stroke shall be identified by standard clinical diagnostic investigation(s) 16. transfusion‐related complications within 28 days: incidence, category, and severity of acute transfusion reactions will be defined according to UK SHOT (United Kingdom Serious Hazards of Transfusion) 17. organ dysfunction within 28 days: organ dysfunction shall be measured as SOFA score from admission to day 28 or discharge 18. 28 days discharge quality of life: health‐related quality of life will be measured at 28 days postadmission or upon discharge, if sooner 19. 90 days quality of life: health‐related quality of life will be measured at 90 days postadmission |
| Starting date | Not yet recruiting |
| Contact information | Claire Rourke, +442035940731 claire.rourke@bartshealth.nhs.uk Lewis Gall , +442035940731 lewisgall@nhs.net |
| Notes | NCT02593877 |
Please see Appendix 4 for abbreviations.
Differences between protocol and review
As indicated in the text, we carried out several new analyses examining thromboelastography (TEG) or thromboelastometry (ROTEM) versus clinical judgement or usual care (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5); TEG or ROTEM versus standard laboratory test‐guided transfusion (Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5), and TEG or ROTEM in combination with standard laboratory tests or other devices versus clinical judgement or usual care (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5) after consultation with the Cochrane Statistical Editor (Marialena Trivella). We provided 'Summary of findings' tables ‐‐for each comparison.
Contributions of authors
Conceiving the review: Anne Wikkelsø (AW), Arash Afshari (AFSH), Ann Merete Møller (AMM), Jørn Wetterslev (JW)
Co‐ordinating the review: AW
Undertaking manual searches: AW
Screening search results: AW, AFSH
Organizing retrieval of papers: AW
Screening retrieved papers against inclusion criteria: AW, AFSH
Appraising quality of papers: AW, AFSH
Abstracting data from papers: AW, AFSH
Writing to authors of papers for additional information: AW
Providing additional data about papers: AW
Obtaining and screening data on unpublished studies: AW
Data management for the review: AW, AFSH, JW
Entering data into Review Manager (RevMan 5.3): AW, AFSH
RevMan statistical data: JW, AFSH, AW
Other statistical analysis not using RevMan: JW, AFSH
Double entry of data: (data entered by person one: AW; data entered by person two: AFSH)
Interpretation of data: AW, AFSH, JW, AMM
Statistical analysis: JW, AFSH, AW
Writing the review: AW, AFSH, JW, AMM
Securing funding for the review: AA, AW
Performing previous work that was the foundation of the present study: AFSH, AW, JW, AMM
Guarantor for the review (one author): AW
Person responsible for reading and checking review before submission: AW
Sources of support
Internal sources
-
Cochrane Anaesthesia, Critical and Emergency Care Review Group (ACE), Denmark.
Technical support and search strategy design provided by Karen Hovhannisyan
External sources
No sources of support supplied
Declarations of interest
Anne Wikkelsø: Declares to have received thromboelastography (TEG) assays from Haemonetics Corp. but no financial support for a randomized controlled trial (RCT) investigating fibrinogen concentrate in postpartum haemorrhage with TEG used as haemostatic monitoring (trial not part of this review).
Arash Afshari: Declares to have received TEG assays from Haemonetics Corp. but no financial support for an RCT investigating fibrinogen concentrate in postpartum haemorrhage with TEG used as haemostatic monitoring (trial not part of this review).
Ann Merete Møller: Declares to have received TEG assays from Haemonetics Corp. but no financial support for an RCT investigating fibrinogen concentrate in postpartum haemorrhage with TEG used as haemostatic monitoring (trial not part of this review).
Jørn Wetterslev: Declares that he is a member of Trial Sequential Analysis (TSA) at Copenhagen Trial Unit developing and programming TSA.
Edited (no change to conclusions)
References
References to studies included in this review
Ak 2009 {published data only (unpublished sought but not used)}
- Ak K, Isbir CS, Tetik S, Atalan N, Tekeli A, Aljodi M, et al. Thromboelastography‐based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. Journal of Cardiac Surgery 2009;24(4):404‐10. [PUBMED: 19583608] [DOI] [PubMed] [Google Scholar]
Avidan 2004 {published data only (unpublished sought but not used)}
- Avidan MS, Alcock EL, Fonseca J, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near‐patient assessment with clinical judgement in the management of bleeding after cardiac surgery. British Journal of Anaesthesia 2004;92(2):178‐86. [PUBMED: 14722166 ] [DOI] [PubMed] [Google Scholar]
Cui 2010 {published data only (unpublished sought but not used)}
- Cui Y, Hei F, Long C, Feng Z, Zhao J, Yan F, et al. Perioperative monitoring of thromboelastograph on blood protection and recovery for severely cyanotic patients undergoing complex cardiac surgery. Artificial Organs 2010;34(11):955‐60. [PUBMED: 21092037] [DOI] [PubMed] [Google Scholar]
Girdauskas 2010 {published data only (unpublished sought but not used)}
- Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. Journal of Thoracic and Cardiovascular Surgery 2010;140(5):1117‐24. [PUBMED: 20951260] [DOI] [PubMed] [Google Scholar]
Kempfert 2011 {published data only (unpublished sought but not used)}
- Kempfert J. Thromboelastography‐guided blood component therapy after cardiac surgery: A randomised study. Interactive Cardiovascular and Thoracic Surgery 2011;13:S106‐7. [Google Scholar]
Kultufan Turan 2006 {published and unpublished data}
- Kultufan Turan S, Aydinli B, Ayik H, Yaar S, Kazanci D, Karadenz Ü, et al. The role of rotational thromboelastgraphy on decision of blood transfusion in open heart surgery [Açik Kalp Cerrahisinde TromboelastografininTransfüzyon Karari Üzerine Etkisi]. Göğüs‐Kalp‐Damar Anestezi ve Yoğun Bakım Derneği Dergisi 2006;12(4):154‐9. [Google Scholar]
Nakayama 2015 {published and unpublished data}
- Nakayama Y, Nakajima Y, Tanaka KA, Sessler DI, Maeda S, Iida J, et al. Thromboelastometry‐guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. British Journal of Anaesthesia 2015;114(1):91‐102. [PUBMED: 25303988] [DOI] [PubMed] [Google Scholar]
NCT00772239 {unpublished data only}
- NCT00772239. Perioperative coagulation management in cardiac surgery. (ROTEM). clinicaltrials.gov/ct2/show/NCT00772239 (first received 13 October 2008).
Nuttal 2001 {published data only (unpublished sought but not used)}
- Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology 2001;94(5):773‐81. [PUBMED: 11388527] [DOI] [PubMed] [Google Scholar]
Paniagua 2011 {published and unpublished data}
- Paniagua P, Koller T, Requena T, Gil JM, Campos JM, Galan J. Randomized controlled trial to evaluate postoperative coagulation management with bed‐side trombelastometry (Rotem) compared with a transfusion protocol based on laboratory measurements in bleeding patients after cardiac surgery: Preliminary data. European Journal of Anaesthesiology 2011;28:94. [Google Scholar]
Rauter 2007 {published data only (unpublished sought but not used)}
- Rauter M, Kästenbauer T, Schwarz S, Fitzgerald R. Reduced number of red blood cell transfusions in cardiac surgery due to perioperative coagulation testing by thromboelastometry. Acta Anaesthesiologica Scandinavica. 2007; Vol. 51 (Suppl 118):40.
Royston 2001 {published data only (unpublished sought but not used)}
- Royston D, Kier S. Reduced haemostatic factor transfusion using heparinase‐modified thrombelastography during cardiopulmonary bypass. British Journal of Anaesthesia 2001;86(4):575‐8. [PUBMED: 11573637] [DOI] [PubMed] [Google Scholar]
Schaden 2012 {published and unpublished data}
- Schaden E, Kimberger O, Kraincuk P, Baron DM, Metnitz PG, Kozek‐Langenecker S. Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. British Journal of Anaesthesia 2012;109(3):376‐81. [PUBMED: 22719014] [DOI] [PubMed] [Google Scholar]
Shore‐Lesserson 1999 {published and unpublished data}
- Shore‐Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela‐Cantos F, Ergin MA. Thromboelastography‐guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesthesia and Analgesia 1999;88(2):312‐9. [PUBMED: 9972747] [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only (unpublished sought but not used)}
- Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC. Thromboelastography‐guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proceedings 2010;42(7):2590‐3. [PUBMED: 20832550] [DOI] [PubMed] [Google Scholar]
Weber 2012 {published and unpublished data}
- Weber CF, Goerlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point‐of‐care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012;117(3):531‐47. [ClinicalTrials.gov: NCT00997841; PUBMED: 22914710] [DOI] [PubMed] [Google Scholar]
Westbrook 2009 {published data only (unpublished sought but not used)}
- Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out‐performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung and Circulation 2009;18(4):277‐88. [PUBMED: 19117801] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2006 {published data only}
- Anderson L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An audit of red cell and blood product use after the institution of thrombelastometry in cardiac intensive care unit. Transfusion Medicine 2006;16:31‐9. [PUBMED: 16480437 ] [DOI] [PubMed] [Google Scholar]
- Anderson L, Quasim I, Steven M, Soutar R, Macfie A. An audit of red cell and blood products use after institution of thrombelastography in cardiac intensive care unit. British Journal of Haematology 2003;121(Suppl 1):80. [Google Scholar]
Andreasen 2011 {published data only}
- Andreasen JB, Hvas AM, Christiansen K, Ravn HB. Can RoTEM analysis be applied for haemostatic monitoring in paediatric congenital heart surgery?. Cardiology in the Young 2011;21(06):684‐91. [PUBMED: 21729518] [DOI] [PubMed] [Google Scholar]
Aoki 2012 {published data only}
- Aoki K, Sugimoto A, Nagasawa A, Saito M, Ohzeki H. Optimization of thromboelastography‐guided platelet transfusion in cardiovascular surgery. General Thoracic and Cardiovascular Surgery 2012;60(7):411‐6. [PUBMED: 22566265] [DOI] [PubMed] [Google Scholar]
Blasi 2012 {published data only}
- Blasi A, Beltran J, Pereira A, Martinez‐Palli G, Torrents A, Balust J, et al. An assessment of thromboelastometry to monitor blood coagulation and guide transfusion support in liver transplantation. Transfusion 2012;52(9):1989‐98. [PUBMED: 22304465] [DOI] [PubMed] [Google Scholar]
Coakley 2006 {published data only}
- Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. Journal of Cardiothoracic and Vascular Anesthesia 2006;20(4):548‐53. [PUBMED: 16884987] [DOI] [PubMed] [Google Scholar]
Cui 2009 {published data only}
- Cui Y, Hei F, Long C, Feng Z, Zhao J, Yan F, et al. Perioperative monitoring of thromboelastograph on hemostasis and therapy for cyanotic infants undergoing complex cardiac surgery. Artificial Organs 2009;33(11):909‐14. [PUBMED: 20021469] [DOI] [PubMed] [Google Scholar]
De Pietri 2014 {published data only}
- Bianchini M, Pietri L, Maira T, Maria N, Begliomini B, Gerunda GE, et al. Thrombelastography decreases hemoderivates requirement before invasive procedures in cirrhotic patients with coagulation disorders. A randomized controlled trial. Hepatology. John Wiley and Sons Inc., 2013, issue 4 Suppl 1:292A‐3A.
- Pietri L, Bianchini M, Montalti R, Maria N, Maira T, Begliomini B, et al. Thrombelastography (TEG) decreases blood products requirement before invasive procedures in cirrhotic patients with coagulation tests derangement. A randomized controlled trial. Digestive and Liver Disease. 2014; Vol. 46:e5‐6.
- Pietri L, Bianchini M, Montalti R, Maira T, Maria N, Begliomini B, et al. 194 Thrombelastography before invasive procedures reduces hemoderivates requirements in cirrhotic patients with coagulation disorders. a randomized controlled trial. Journal of Hepatology 2013;58:S85‐6. [Google Scholar]
Dirkmann 2013 {published data only}
- Dirkmann D, Gorlinger K, Dusse F, Kottenberg E, Peters J. Early thromboelastometric variables reliably predict maximum clot firmness in patients undergoing cardiac surgery: A step towards earlier decision making. Acta Anaesthesiologica Scandinavica 2013;5:594‐603. [PUBMED: 23240733] [DOI] [PubMed] [Google Scholar]
Doran 2010 {published data only}
- Doran CM, Woolley T, Midwinter MJ. Feasibility of using rotational thromboelastometry to assess coagulation status of combat casualties in a deployed setting. Journal of Trauma 2010;69:S40‐8. [PUBMED: 20622618 ] [DOI] [PubMed] [Google Scholar]
Dua 2005 {published data only}
- Dua K, Singh J, Chatterji C, Makroo R, Das BS. Blood component therapy in postoperative OBCAB (off pump coronary artery bypass surgery) patients: Our experiences with thromboelastography (TEG). Vox Sanguinis 2005;89 Suppl 2:5P‐144. [Google Scholar]
El 2009 {published data only}
- Kady N, Khedr H, Yosry M, Mekawi S. Perioperative assessment of coagulation in paediatric neurosurgical patients using thromboelastography. European Journal of Anaesthesiology 2009;26(4):293‐7. [PUBMED: 19401660] [DOI] [PubMed] [Google Scholar]
Faraoni 2013 {published data only}
- Faraoni D, Savan V, Levy JH, Theusinger OM. Goal‐directed coagulation management in the perioperative period of cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(6):1347‐54. [PUBMED: 24103717] [DOI] [PubMed] [Google Scholar]
Gorlinger 2011a {published data only}
- Gorlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, et al. First‐line therapy with coagulation factor concentrates combined with point‐of‐care coagulation testing Is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single‐center cohort study. Anesthesiology 2011;115(6):1179‐91. [PUBMED: 21970887] [DOI] [PubMed] [Google Scholar]
Gorlinger 2011b {published data only}
- Gorlinger K, Dirkmann D, Rahe‐Meyer N, Weber CF, Hanke AA. Algorithms for transfusion and coagulation management in massive haemorrhage. Anasthesiologie und Intensivmedizin 2011;52(2):145‐59. [Google Scholar]
Gronchi 2014 {published data only}
- Gronchi F, Perret A, Ferrari E, Marcucci CM, Fleche J, Crosset M, et al. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in‐vivo study. European Journal of Anaesthesiology 2014;31(2):68‐75. [DOI] [PubMed] [Google Scholar]
Haas 2015 {published data only}
- Haas T, Spielmann N, Restin T, Seifert B, Henze G, Obwegeser J, et al. Higher fibrinogen concentrations for reduction of transfusion requirements during major paediatric surgery: A prospective randomised controlled trial. British Journal of Anaesthesia 2015;115(2):234‐43. [PUBMED: 25982134] [DOI] [PubMed] [Google Scholar]
Hanke 2012 {published data only}
- Hanke AA, Herold U, Dirkmann D, Tsagakis K, Jakob H, Gorlinger K. Thromboelastometry based early goal‐directed coagulation management reduces blood transfusion requirements, adverse events, and costs in acute Type A aortic dissection: A pilot study. Transfusion Medicine and Hemotherapy 2012;39(2):121‐8. [PUBMED: 22670130] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke AA, Herold U, Dirkmann D, Tsagakis K, Jakob H, Gorlinger K. Thromboelastometry based early goal‐directed coagulation management reduces blood transfusion requirements, adverse events, and costs in acute type A aortic dissection: A pilot study. Transfusion Medicine and Hemotherapy 2012;39(2):121‐8. [PUBMED: 22670130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2012 {published data only}
- Hill JS, Devenie G, Powell M. Point‐of‐care testing of coagulation and fibrinolytic status during postpartum haemorrhage: developing a thrombelastography‐guided transfusion algorithm. Anaesthesia and Intensive Care 2012;40(6):1007‐15. [PUBMED: 23194210] [DOI] [PubMed] [Google Scholar]
Howland 1974 {published data only}
- Howland WS, Schweizer O, Gould P. A comparison of intraoperative measurements of coagulation. Anesthesia and Analgesia 1974;53(5):657‐63. [PUBMED: 4606102] [PubMed] [Google Scholar]
Hvas 2012 {published data only}
Jambor 2009 {published data only}
- Jambor C, Spannagl M, Zwissler B. Perioperative management of patients with coronary stents in non‐cardiac surgery. Anaesthesist 2009;58(10):971‐85. [PUBMED: 19823781] [DOI] [PubMed] [Google Scholar]
Johansson 2007 {published data only}
- Johansson PIM. The blood bank: From provider to partner in treatment of massively bleeding patients. Transfusion 2007;47 Suppl(2):176‐81. [PUBMED: 17651347] [DOI] [PubMed] [Google Scholar]
Johansson 2009 {published data only}
- Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: A before and after study. Vox Sanguinis 2009;96(2):111‐8. [PUBMED: 19152603] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunio 2012 {published data only}
- Kunio NR, Differding JA, Watson KM, Stucke RS, Schreiber MA. Thrombelastography‐identified coagulopathy Is associated with increased morbidity and mortality after traumatic brain injury. American Journal of Surgery 2012;203(5):584‐8. [PUBMED: 22425448] [DOI] [PubMed] [Google Scholar]
Mendeloff 2009 {published data only}
- Mendeloff EN, Glenn GF, Tavakolian P, Lin E, Leonard A, Prince SL, et al. The role of thromboelastography in directing blood product usage in infant open heart surgery. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery 2009;4(5):282‐90. [DOI: 10.1097/IMI.0b013e3181bbd4ff] [DOI] [PubMed] [Google Scholar]
Naik 2015 {published data only}
- Naik BI, Pajewski TN, Bogdonoff DI, Zuo Z, Clark P, Terkawi Abdullah S, et al. Rotational thromboelastometry‐guided blood product management in major spine surgery. Journal of Neurosurgery. Spine 2015;23(2):239‐49. [PUBMED: 26053893] [DOI] [PubMed] [Google Scholar]
NCT01218074 {published data only}
- NCT01218074. Platelets antiaggregation control enhancement (PACE) study. clinicaltrials.gov/ct2/show/NCT01218074 (first received 5 October 2010).
Ogawa 2012 {published data only}
- Ogawa S, Szlam F, Chen EP, Nishimura T, Kim H, Roback JD, et al. A comparative evaluation of rotation thromboelastometry and standard coagulation tests in hemodilution‐induced coagulation changes after cardiac surgery. Transfusion 2012;52(1):14‐22. [PUBMED: 21756263] [DOI] [PubMed] [Google Scholar]
Ploppa 2010 {published data only}
- Ploppa A, Unertl KE, Nohe B. Thrombelastometry‐guided thrombolytic therapy in massive pulmonary artery embolism. Acta Anaesthesiologica Scandinavica 2010;54(9):1145‐8. [PUBMED: 20670312] [DOI] [PubMed] [Google Scholar]
Rafiq 2012 {published data only}
- Rafiq S, Johansson PI, Zacho M, Stissing T, Kofoed K, Lilleor NB, et al. Thrombelastographic haemostatic status and antiplatelet therapy after coronary artery bypass surgery (TEG‐CABG trial): assessing and monitoring the antithrombotic effect of clopidogrel and aspirin versus aspirin alone in hypercoagulable patients. Trials. BioMed Central Ltd. (Floor 6, 236 Gray's Inn Road, London WC1X 8HB, United Kingdom), 2012; Vol. 13:48. [Clinical trials NCT01046942.; PUBMED: 22540524] [DOI] [PMC free article] [PubMed]
Rahe‐Meyer 2009a {published data only}
- Rahe‐Meyer N, Pichlmaier M, Haverich A, Solomon C, Winterhalter M, Piepenbrock S, et al. Bleeding management with fibrinogen concentrate targeting a high‐normal plasma fibrinogen level: a pilot study. British Journal of Anaesthesia 2009;102(6):785‐92. [PUBMED: 19698858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahe‐Meyer 2009b {published data only}
- Rahe‐Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, et al. Thromboelastometry‐guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. Journal of Thoracic and Cardiovascular Surgery 2009;138(3):694‐702. [PUBMED: 19698858] [DOI] [PubMed] [Google Scholar]
Roullet 2014 {published data only}
- Roullet S, Freyburger G, Cruc M, Quinart A, Stecken L, Audy M, et al. Management of bleeding and transfusion during liver transplantation before and after the introduction of a ROTEM‐based algorithm. Liver Transplantation 2014;21(2):169‐79. [PUBMED: 25331016] [DOI] [PubMed] [Google Scholar]
Schochl 2010 {published data only}
- Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, et al. Goal‐directed coagulation management of major trauma patients using thromboelastometry (ROTEM)‐guided administration of fibrinogen concentrate and prothrombin complex concentrate. Critical Care 2010;14(2):R55. [PUBMED: 20374650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schochl 2011 {published data only}
- Scochl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, et al. Transfusion in trauma: thromboelastometry‐guided coagulation factor concentrate‐based therapy versus standard fresh frozen plasma‐based therapy. Critical Care 2011;15(2):R83. [PUBMED: 21375741] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schochl 2014 {published data only}
- Schochl H, Schlimp CJ, Voelckel W. Perioperative coagulation management in multiple trauma patients based on viscoelastic test results. Unfallchirurg 2014;117(2):111‐7. [PUBMED: 24482057] [DOI] [PubMed] [Google Scholar]
Smart 2015 {published data only}
- Smart L, Scharpf DT, Gray NO, Traetow D, Black S, Michaels A, et al. Rotational thromboelastometry (ROTEM) versus conventional coagulation tests during orthotopic liver transplantation: Comparison of intra‐operative blood loss, transfusion requirements, and cost. Hepatology. 2015; Vol. 62 Suppl 1:832A.
Solomon 2012 {published data only}
- Solomon C, Schoechl H, Hagl C, Rahe‐Meyer N. Fibrinogen concentrate as first‐line haemostatic therapy during aortic replacement surgery. A randomised trial. Journal of Thrombosis and Haemostasis 2012, issue 6:e7.
Spalding 2007 {published data only}
- Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of 'bedside' thrombelastography (ROTEM). European Journal of Cardio‐thoracic Surgery 2007;31(6):1052‐7. [PUBMED: 17516038] [DOI] [PubMed] [Google Scholar]
Spiess 1995 {published data only}
- Spiess BD, Gillies BS, Chandler W, Verrier E. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. Journal of Cardiothoracic and Vascular Anesthesia 1995;9(2):168‐73. [PUBMED: 7780073 ] [DOI] [PubMed] [Google Scholar]
Tapia 2013 {published data only}
- Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, et al. TEG‐guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. Journal of Trauma and Acute Care Surgery 2013;74(2):378‐86. [DOI] [PubMed] [Google Scholar]
Urwyler 2012 {published data only}
- Urwyler N, Theiler L, Hirschberg M, Kleine‐Brueggeney M, Colucci G, Greif R. Standard vs. point‐of‐care measurement of fibrinogen: Potential impact on clinical decisions. Minerva Anestesiologica 2012; Vol. 78, issue 5:550‐5. [PubMed]
Vaidya 2007 {published data only}
- Vaidya A, Muthusamy AS, Hadjianastassiou VG, Roy D, Elker DE, Moustafellos P, et al. Simultaneous pancreas‐kidney transplantation: To anticoagulate or not? Is that a question?. Clinical Transplantation 2007;21(4):554‐7. [PUBMED: 17645719] [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang SC, Lin HT, Chang KY, Mandell MS, Ting CK, Chu YC, et al. Use of higher thromboelastogram transfusion values is not associated with greater blood loss in liver transplant surgery. Liver Transplantation 2012;18(10):1254‐8. [PUBMED: 22730210] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01402739 {published data only}
- NCT01402739. Algorithm‐guided transfusions in cardiac surgery patients for reduction of drainage blood losses (HEART‐PoC). clinicaltrials.gov/ct2/show/NCT01402739 (first received 25 July 2011).
NCT01536496 {published data only}
- NCT01536496. Comparison of rapid thrombelastography and conventional coagulation testing for haemostatic resuscitation in trauma. clinicaltrials.gov/ct2/show/NCT01536496 (first received 19 July 2011).
NCT02352181 {published data only}
- NCT02352181. Management of coagulopathy during orthotopic liver transplantation. comparison between ROTEM‐based management and standard biological assessment. clinicaltrials.gov/ct2/show/NCT02352181 (first received 18 December 2014).
NCT02416817 {published data only}
- NCT02416817. Strategy of transfusion in trauma patients ‐ STATA trial (STATA). clinicaltrials.gov/ct2/show/NCT02416817 (first received 28 August 2014).
NCT02461251 {published data only}
- NCT02461251. Thromboelastometry‐guided treatment protocol versus standard care of major haemorrhage in obstetric patients (ROTEM‐PPH). clinicaltrials.gov/ct2/show/NCT02461251 (first received 29 May 2015).
NCT02593877 {published data only}
- NCT02593877. Implementing treatment algorithms for the correction of trauma induced coagulopathy (iTACTIC). clinicaltrials.gov/ct2/show/NCT02593877 (first received 15 October 2015).
Additional references
AAGBI 2010
- Thomas D, Wee M, Clyburn P, Walker I, Brohi K, Collins P, et al. AAGBI (Association of Anaesthetists of Great Britain and Ireland). Blood transfusion and the anaesthetist: management of massive haemorrhage. Anaesthesia 2010;65(11):1153‐61. [PUBMED: 20963925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benes 2015
- Benes J, Zatloukal J, Kletecka J. Viscoelastic methods of blood clotting assessment ‐ a multidisciplinary review. Frontiers in Medicine 2015;2:62. [PUBMED: 26442265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Besser 2010
- Besser MW, Klein AA. The coagulopathy of cardiopulmonary bypass. Critical Reviews in Clinical Laboratory Sciences 2010;47(5‐6):197‐212. [PUBMED: 21391830] [DOI] [PubMed] [Google Scholar]
Bolliger 2013
- Bolliger D, Tanaka KA. Roles of thrombelastography and thromboelastometry for patient blood management in cardiac surgery. Transfusion Medicine Reviews 2013;27(4):213‐20. [PUBMED: 24075802] [DOI] [PubMed] [Google Scholar]
Brohi 2008
- Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. The Journal of Trauma 2008;64(5):1211‐7. [PUBMED: 18469643] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Journal of Clinical Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
Chee 2003
- Chee YL, Greaves M. Role of coagulation testing in predicting bleeding risk. The Hematology Journal 2003;4(6):373‐8. [PUBMED: 14671609] [DOI] [PubMed] [Google Scholar]
Corredor 2015
- Corredor C, Wasowicz M, Karkouti K, Sharma V. The role of point‐of‐care platelet function testing in predicting postoperative bleeding following cardiac surgery: a systematic review and meta‐analysis. Anaesthesia 2015;70(6):715‐31. [PUBMED: 25916344] [DOI] [PubMed] [Google Scholar]
Craig 2007
- Craig J, Aguiar‐Ibanez R, Bhattacharya S, Downie S, Duffy S, Kohli H, et al. The clinical and cost effectiveness of thromboelastography/thromboelastometry. Health Technology Assessment, Report 11. www.nhshealthquality.org (accessed January 2016).
De Robertis 2015
- Robertis E, Kozek‐Langenecker SA, Tufano R, Romano GM, Piazza O, Zito Marinosci G. Coagulopathy induced by acidosis, hypothermia and hypocalcaemia in severe bleeding. Minerva Anestesiologica 2015;81(1):65‐75. [PUBMED: 24608516] [PubMed] [Google Scholar]
Dickersin 1990
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990;263(10):1359‐85. [PUBMED: 2406472] [PubMed] [Google Scholar]
Gluud 2005
- Gluud C, Gluud LL. Evidence based diagnostics. BMJ 2005;330(7493):724‐6. [PUBMED: 15790646] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gotzsche 2000
- Gotzsche PC. Why we need a broad perspective on meta‐analysis. It may be crucially important for patients. BMJ 2000;321(7261):585–6. [PUBMED: 10977820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 2004
- Hardy JF, Moerloose P, Samama M, Groupe d'intérêt en Hémostase Périopératoire. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Canadian Journal of Anaesthesia 2004;51(4):293‐310. [PUBMED: 15064258] [DOI] [PubMed] [Google Scholar]
Hardy 2005
- Hardy JF, Moerloose P, Samama CM. The coagulopathy of massive transfusion. Vox Sanguinis 2005;89(3):123‐7. [PUBMED: 16146503] [DOI] [PubMed] [Google Scholar]
Hartert 1948
- Hartert. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klinische Wochenschrift 1948;26(37‐38):577‐83. [PUBMED: 18101974] [DOI] [PubMed] [Google Scholar]
Hartmann 2006
- Hartmann M, Sucker C, Boehm O, Koch A, Loer S, Zacharowski K. Effects of cardiac surgery on hemostasis. Transfusion Medicine Reviews 2006;20(3):230‐41. [PUBMED: 16787830] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hiippala 1995
- Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma‐poor red cell concentrates. Anesthesia and Analgesia 1995;81(2):360‐5. [PUBMED: 7542432] [DOI] [PubMed] [Google Scholar]
Hunt 2015
- Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma‐induced coagulopathy in adult trauma patients with bleeding. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD010438.pub2; PUBMED: 25686465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series C 2000;49:359‐70. [PUBMED: 16248351] 16248351 [Google Scholar]
Johansson 2012
- Johansson PI, Stensballe J, Ostrowski SR. Current management of massive hemorrhage in trauma. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2012;20(1):47. [PUBMED: 22776724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansson 2014
- Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood 2014;124(20):3052‐8. [PUBMED: 25293771] [DOI] [PubMed] [Google Scholar]
Kozek‐Langenecker 2007
- Kozek‐Langenecker S. Management of massive operative blood loss. Minerva Anestesiologica 2007;73(7‐8):401‐15. [PUBMED: 17380100] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63. [Google Scholar]
Lang 2005
- Lang T, Bauters A, Braun SL, Pötzsch B, Pape KW, Kolde HJ, et al. Multi‐centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagulation & Fibrinolysis 2005;16(4):301‐10. [PUBMED: 15870552] [DOI] [PubMed] [Google Scholar]
Levrat 2008
- Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. British Journal of Anaesthesia 2008;100(6):792‐7. [PUBMED: 18440953] [DOI] [PubMed] [Google Scholar]
Luddington 2005
- Luddington RJ. Thrombelastography/thromboelastometry. Clinical and Laboratory Haematology 2005;27(2):81‐90. [PUBMED: 15784122] [DOI] [PubMed] [Google Scholar]
Paparella 2004
- Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Medicine 2004;30(10):1873‐81. [PUBMED: 15278267] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: Utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomized controlled trials. Lancet 1998;351(9095):45‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2016
- Roth D, Heidinger B, Havel C, Herkner H. Different mortality time points in critical care trials: current practice and influence on effect estimates in meta‐analyses. Critical Care Medicine 2016;44(8):e737‐41. [PUBMED: 26963325] [DOI] [PubMed] [Google Scholar]
Rugeri 2007
- Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. Journal of Thrombosis and Haemostasis 2007;5(2):289‐95. [PUBMED: 17109736] [DOI] [PubMed] [Google Scholar]
Sabate 2012
- Sabate A, Dalmau A, Koo M, Aparicio I, Costa M, Contreras L. Coagulopathy management in liver transplantation. Transplantation Proceedings 2012;44(6):1523‐5. [PUBMED: 22841202] [DOI] [PubMed] [Google Scholar]
Segal 2005
- Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence‐based review. Transfusion 2005;45(9):1413‐25. [PUBMED: 16131373] [DOI] [PubMed] [Google Scholar]
Sutton 2002
- Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta‐analysis of rare and adverse event data. Expert Review of Pharmacoeconomics and Outcomes Research 2002;2(4):367‐79. [PUBMED: 19807443] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Clinical Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
TSA 2010 [Computer program]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis. Version 0.8. Copenhagen: Copenhagn Trial Unit, 2010.
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. International Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. Bio Medical Central: Medical Research Methodology 2009;30(9):86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2015
- Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point‐of‐care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost‐effectiveness analysis. Health Technology Assessment 2015;19(58):1‐228. [PUBMED: 26215747] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Afshari 2009
- Afshari A, Wikkelsø A, Brok J, Møller AM, Wetterslev J. Thrombelastography (TEG) or Thrombelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007871] [DOI] [PubMed] [Google Scholar]
Afshari 2011
- Afshari A, Wikkelsø A, Brok J, Møller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007871.pub2] [DOI] [PubMed] [Google Scholar]
Wikkelsø 2017
- Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta‐analysis and trial sequential analysis. Anaesthesia 2017;epub ahead of print. [DOI: 10.1111/anae.13765] [DOI] [PubMed] [Google Scholar]