Abstract
Background
Child and adolescent obesity has increased globally, and can be associated with significant short‐ and long‐term health consequences.
Objectives
To assess the efficacy of drug interventions for the treatment of obesity in children and adolescents.
Search methods
We searched CENTRAL, MEDLINE, Embase, PubMed (subsets not available on Ovid), LILACS as well as the trial registers ICTRP (WHO) and ClinicalTrials.gov. Searches were undertaken from inception to March 2016. We checked references and applied no language restrictions.
Selection criteria
We selected randomised controlled trials (RCTs) of pharmacological interventions for treating obesity (licensed and unlicensed for this indication) in children and adolescents (mean age under 18 years) with or without support of family members, with a minimum of three months' pharmacological intervention and six months' follow‐up from baseline. We excluded interventions that specifically dealt with the treatment of eating disorders or type 2 diabetes, or included participants with a secondary or syndromic cause of obesity. In addition, we excluded trials which included growth hormone therapies and pregnant participants.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data following standard Cochrane methodology. Where necessary we contacted authors for additional information.
Main results
We included 21 trials and identified eight ongoing trials. The included trials evaluated metformin (11 trials), sibutramine (six trials), orlistat (four trials), and one trial arm investigated the combination of metformin and fluoxetine. The ongoing trials evaluated metformin (four trials), topiramate (two trials) and exenatide (two trials). A total of 2484 people participated in the included trials, 1478 participants were randomised to drug intervention and 904 to comparator groups (91 participants took part in two cross‐over trials; 11 participants not specified). Eighteen trials used a placebo in the comparator group. Two trials had a cross‐over design while the remaining 19 trials were parallel RCTs. The length of the intervention period ranged from 12 weeks to 48 weeks, and the length of follow‐up from baseline ranged from six months to 100 weeks.
Trials generally had a low risk of bias for random sequence generation, allocation concealment and blinding (participants, personnel and assessors) for subjective and objective outcomes. We judged approximately half of the trials as having a high risk of bias in one or more domain such as selective reporting.
The primary outcomes of this review were change in body mass index (BMI), change in weight and adverse events. All 21 trials measured these outcomes. The secondary outcomes were health‐related quality of life (only one trial reported results showing no marked differences; very low certainty evidence), body fat distribution (measured in 18 trials), behaviour change (measured in six trials), participants' views of the intervention (not reported), morbidity associated with the intervention (measured in one orlistat trial only reporting more new gallstones following the intervention; very low certainty evidence), all‐cause mortality (one suicide in the orlistat intervention group; low certainty evidence) and socioeconomic effects (not reported).
Intervention versus comparator for mean difference (MD) in BMI change was ‐1.3 kg/m2 (95% confidence interval (CI) ‐1.9 to ‐0.8; P < 0.00001; 16 trials; 1884 participants; low certainty evidence). When split by drug type, sibutramine, metformin and orlistat all showed reductions in BMI in favour of the intervention.
Intervention versus comparator for change in weight showed a MD of ‐3.9 kg (95% CI ‐5.9 to ‐1.9; P < 0.00001; 11 trials; 1180 participants; low certainty evidence). As with BMI, when the trials were split by drug type, sibutramine, metformin and orlistat all showed reductions in weight in favour of the intervention.
Five trials reported serious adverse events: 24/878 (2.7%) participants in the intervention groups versus 8/469 (1.7%) participants in the comparator groups (risk ratio (RR) 1.43, 95% CI 0.63 to 3.25; 1347 participants; low certainty evidence). A total 52/1043 (5.0%) participants in the intervention groups versus 17/621 (2.7%) in the comparator groups discontinued the trial because of adverse events (RR 1.45, 95% CI 0.83 to 2.52; 10 trials; 1664 participants; low certainty evidence). The most common adverse events in orlistat and metformin trials were gastrointestinal (such as diarrhoea, mild abdominal pain or discomfort, fatty stools). The most frequent adverse events in sibutramine trials included tachycardia, constipation and hypertension. The single fluoxetine trial reported dry mouth and loose stools. No trial investigated drug treatment for overweight children.
Authors' conclusions
This systematic review is part of a series of associated Cochrane reviews on interventions for obese children and adolescents and has shown that pharmacological interventions (metformin, sibutramine, orlistat and fluoxetine) may have small effects in reduction in BMI and bodyweight in obese children and adolescents. However, many of these drugs are not licensed for the treatment of obesity in children and adolescents, or have been withdrawn. Trials were generally of low quality with many having a short or no post‐intervention follow‐up period and high dropout rates (overall dropout of 25%). Future research should focus on conducting trials with sufficient power and long‐term follow‐up, to ensure the long‐term effects of any pharmacological intervention are comprehensively assessed. Adverse events should be reported in a more standardised manner specifying amongst other things the number of participants experiencing at least one adverse event. The requirement of regulatory authorities (US Food and Drug Administration and European Medicines Agency) for trials of all new medications to be used in children and adolescents should drive an increase in the number of high quality trials.
Plain language summary
Drug interventions for the treatment of obesity in children and adolescents
Review question
Do drug (medicine) interventions reduce weight in obese children and adolescents and are they safe?
Background
Across the world more children and adolescents are becoming overweight and obese. These children and adolescents are more likely to have health problems, both while as children or adolescents and in later life. More information is needed about what works best for treating this problem recognising that so‐called lifestyle changes (diet, exercise and counselling) have limited efficacy.
Study characteristics
We found 21 randomised controlled studies (clinical studies where people are randomly put into one of two or more treatment groups) comparing various drugs plus a behaviour changing intervention such as diet, exercise or both (= intervention groups) usually with placebo (a pretend drug) plus a behaviour changing intervention (= control groups). We also identified eight ongoing studies (studies which are currently running but not completed yet). A total of 2484 children and adolescents took part in the included studies. The length of the intervention period ranged from 12 weeks to 48 weeks, and the length of follow‐up ranged from six months to 100 weeks.
Key results
The included studies investigated metformin (10 studies), sibutramine (six studies), orlistat (four studies) and one study group evaluated the combination of metformin and fluoxetine. The ongoing studies are investigating metformin (four studies), topiramate (two studies) and exenatide (two studies).
Most studies reported on body mass index (BMI) and bodyweight: BMI is a measure of body fat and is calculated from weight and height measurements (kg/m2). In children, BMI is often measured in a way that takes into account sex, weight and height as children grow older (BMI z score). The average change in BMI across control groups was between a 1.8 kg/m2 reduction to a 0.9 kg/m2 increase, while across all intervention groups the average reduction was more pronounced (1.3 kg/m2 reduction). The same effect was observed for weight change: on average, children and adolescents in the intervention groups lost 3.9 kg more weight than the children and adolescents in the control groups. Study authors reported an average of serious side effects in 24 per 1000 participants in the intervention groups compared with an average of 17 per 1000 participants in the control groups. The numbers of participants dropping out of the study because of side effects were 40 per 1000 in the intervention groups and 27 per 1000 in the control groups. The most common side effects in the orlistat and metformin studies were gut (such as diarrhoea and mild tummy pain). Common side effects in the sibutramine trials included increased heart rate (tachycardia), constipation and high blood pressure. The fluoxetine study reported dry mouth and loose stools. One study reported health‐related quality of life (a measure of physical, mental, emotional and social functioning) and found no marked differences between intervention and control. No study reported the participants' views of the intervention or socioeconomic effects. Only one study reported on morbidity (how often a disease occurs in a specific area) associated with the intervention, where there were more gallstones after the orlistat treatment. Study authors reported one suicide in the orlistat intervention group. However, studies were not long enough to reliably investigate death from any cause. No study investigated drug treatment for children who were only overweight (obese children have a much higher weight, BMI or BMI z score than children being overweight).
This evidence is up to date to March 2016.
Quality of the evidence
The overall certainty of the evidence was low or very low, mainly because there were only a few studies per outcome measurement, the number of included children or adolescents was small, and due to variation in the results of the studies. In addition, many children or adolescents left the studies before the study had finished.
Summary of findings
Summary of findings for the main comparison. Drug interventions for the treatment of obesity in children and adolescents.
| Drug interventions for the treatment of obesity in children and adolescents | ||||||
|
Population: obese children and adolescents Settings: mainly outpatient settings Intervention: metformin, orlistat, sibutramine usually combined with behaviour changing interventions Comparison: placebo or no placebo usually with behaviour changing interventions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparator | Pharmacological intervention | |||||
|
a. BMI (kg/m2)
Follow‐up: 6 months (14 trials) ‐ 12 months (2 trials) b. Body weight (kg) Follow‐up: 6 months (10 trials) ‐ 12 months (1 trial) |
a. The mean reduction in BMI ranged across control groups from ‐1.8 to +0.9 b. The mean reduction in weight ranged across control groups from ‐3.8 kg to +4.9 kg |
a. The mean reduction in BMI in the intervention groups was ‐1.3 higher (‐1.9 to ‐0.8 higher) b. The mean reduction in weight in the intervention groups was ‐3.9 kg higher (‐5.9 kg to ‐1.9 kg higher) |
‐ | a. 1884 (16) b. 1180 (11) |
a. ⊕⊕⊝⊝ L owa b. ⊕⊕⊝⊝ Lowa |
‐ |
|
Adverse events a. Serious adverse events b. Discontinuation of trial because of adverse events Follow‐up: mostly 6 months, maximum 100 weeks (1 trial) |
a. 17 per 1000 b. 27 per 1000 |
a. 24 per 1000 (11 to 55) b. 40 per 1000 (23 to 69) |
a.RR 1.43 (0.63 to 3.25) b.RR 1.45 (0.83 to 2.52) |
a. 1347 (5) b. 1664 (10) |
a. ⊕⊕⊕⊝ L owb b. ⊕⊕⊕⊝ Lowb |
All trials reported if adverse events occurred; however, only 7/20 trials reported the number of participants who experienced at least 1 adverse event |
|
Health‐related quality of life 3 questionnaires (1 trial) and SF‐36 (1 trial) Follow‐up: 6 months |
See comment | See comment | See comment | 86 (2) | ⊕⊝⊝⊝ V ery lowc |
Results were only reported for SF‐36 (1 trial on sibutramine, 46 children), there were no marked differences between intervention and comparator groups |
|
All‐cause mortality Follow‐up: mostly 6 months, maximum 100 weeks (1 trial) |
See comment | See comment | See comment | 2176 (20) | ⊕⊕⊕⊝ L owd |
1 suicide in the orlistat intervention group |
| Morbidity | See comment | See comment | See comment | 533 (1) | ⊕⊝⊝⊝ V ery lowe |
Only 1 trial investigated morbidity defined as illness or harm associated with the intervention (Chanoine 2005). In the orlistat group 6/352 (1.7%) participants developed new gallstones compared with 1/181 (0.6%) in the placebo group |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; RR: risk ratio; SF‐36: Short‐Form Health Survey 36 items. | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups.
aDowngraded by two levels because of potential other risk of bias, inconsistency and imprecision (see Appendix 13). bDowngraded by two levels because of potential reporting bias, inconsistency and imprecision (see Appendix 13). cDowngraded by three levels because of one trial only with a small number of participants and imprecision (see Appendix 13). dDowngraded by two levels because of short follow‐up periods and no trial was powered to investigate mortality (see Appendix 13). eDowngraded by three levels because of one trial only and imprecision (see Appendix 13).
Background
The prevalence of overweight and obese children and adolescents has increased throughout the world, presenting a global public health crisis (Ng 2014; WHO 2015). It is not only a problem in high‐income countries, but a high prevalence has also been found in low‐ and middle‐income countries (Wang 2012). Evidence suggests that rates are slowing down or plateauing in high‐income countries; however, they are still rising in low‐ or middle‐income countries and prevalence continues to remain high in both (Olds 2011; Rokholm 2010). The Global Burden of Disease Study 2013 reported a mean of 24% of boys and 23% of girls from high‐income countries to be overweight or obese, whilst the estimated percentages of boys and girls in low‐ or middle‐income countries who are overweight or obese are 13% each (Ng 2014). This report used the International Obesity Task Force (IOTF) age and sex standardised cut points (Cole 2000). Furthermore, young children also have a high prevalence of being overweight or obese with an estimated 42 million overweight or obese children under five years of age in 2010 (approximately 35 million living in low‐ or middle‐income countries ‐ De Onis 2010); these statistics were based on the World Health Organization (WHO) growth standard (WHO 2006).
An additional concern in some high‐income countries, such as the USA (Kelly 2013; Skinner 2014) and England (CMO 2014; Ells 2015a), is the rise in severe paediatric obesity. In England during 2012/2013, 2.9% of girls and 3.9% of boys, aged 10 to 11 years, were classified as severely obese (body mass index (BMI) UK90 99.6th centile or greater ‐ Ells 2015a). In the USA from 2011 to 2012, 2.1% of youths (aged 2 to 19 years) were categorised as class 3 obese (Centers for Disease Control and Prevention growth charts: BMI 140% of greater of the 95th percentile or BMI 40 kg/m2 or greater ‐ Skinner 2014).
Whilst the IOTF published an international definition for paediatric severe (morbid) obesity in 2012 (Cole 2012), often severe obesity prevalence is reported using country‐specific cut points making international comparisons difficult. Data from the USA (Skinner 2014) and England (Ells 2015a) have shown that severe paediatric obesity prevalence varies by socioeconomic status and ethnicity, and may result in greater risk of adverse cardio‐metabolic events and severe obesity in adulthood (Kelly 2013).
The prevalence of overweight and obese children is influenced by inequalities, where rates are higher in children from areas of high deprivation in high‐income countries (Knai 2012; Shrewsbury 2008), and from more affluent areas in low‐ or middle‐income countries (Lobstein 2004; Wang 2012). Other variables are also likely to influence obesity prevalence including age, sex and ethnicity, with varying rates found in different groups in the USA (Freedman 2006; Skinner 2014), England (HSCIC 2014), and New Zealand (Rajput 2014).
Description of the condition
Being overweight or obese in childhood is associated with many conditions which may affect both physical and psychosocial health. Such conditions include hypertension, insulin resistance and hyperlipidaemia in obese children and adolescents, also including very young children (Bocca 2013; Freedman 1999; Reilly 2003; Weiss 2004). The prevalence of type 2 diabetes has continued to increase in children and adolescents, with recent projections in the USA suggesting a potential quadrupling from 2010 to 2050 in the number of youths (aged less than 20 years old) with type 2 diabetes (Imperatore 2012; Pinhas‐Hamiel 2005). Being overweight or obese in early childhood has also been linked to increased cardiovascular risk factors, such as high systolic blood pressure (Falaschetti 2010), with such risks factors also being present in people with type 2 diabetes (Maahs 2014). In addition medical conditions such as sleep apnoea, polycystic ovarian syndrome (PCOS) and poor pulmonary function have also been linked to childhood obesity (Dietz 1998; Ebbeling 2002; Lobstein 2004; Reilly 2003). Furthermore, childhood obesity has been shown to be strongly associated with nonalcoholic fatty liver disease (NAFLD), which is the most common cause of chronic liver disease in children and adolescents (Aggarwal 2014; Berardis 2014).
The condition can also affect the child's mental health and lead to early discrimination, low self‐esteem and depression (Dietz 1998; Puhl 2007; Tang‐Peronard 2008). There is also evidence that childhood obesity also tracks into adulthood (Parsons 1999; Singh 2008; Whitaker 1997), and hence is associated with an increased risk of ill health in later life (Reilly 2011).
Description of the intervention
Since childhood obesity can potentially have serious consequences on a child's health and well‐being, it is very important to identify interventions which can treat obesity in both the short‐ and long‐term. The purposes of such interventions are similar to treatment in adults whereby the primary aims are: to reduce energy intake, increase energy expenditure and decrease sedentary behaviour. However, the child's age and baseline degree of obesity should be taken into consideration before deciding the type, length and intensity of the intervention. This will allow the intervention to be more tailored to the target population and potentially increase the chances of success and reduce the likelihood of adverse events.
In recent years, only three drugs have been licensed for the treatment of adult obesity: rimonabant, sibutramine and orlistat. However, none of these were licensed for use in children (Petkar 2013). Rimonabant was withdrawn from the market due to psychiatric adverse events and sibutramine was suspended by the European Medicine Agency (EMA) and was withdrawn by the US Food and Drug Administration (FDA) in 2010 due to cardiovascular adverse effects; however, sibutramine is still licensed for treatment of obesity in Brazil. Orlistat has been approved by the FDA but only for people over the age of 12 years (Sherafat‐Kazemzadeh 2013). In England, National Institute for Health and Care Excellence (NICE) guidance recommends that orlistat should only be used in children under 12 years old in exceptional circumstances where severe comorbidities exist. Moreover, in children who are 12 years or older, treatment is only recommended if there are physical comorbidities such as sleep apnoea or severe psychological comorbidities (NICE 2014).
Metformin has been approved by the FDA to treat type 2 diabetes mellitus in both adults and children over the age of 10 years but does not have approval for treating obesity in children or adults (McDonagh 2014). However, an analysis of prescribing data in the UK in 2011 showed metformin has regularly been prescribed to treat childhood obesity, the main indication being PCOS (Hsia 2011). Other drugs which have also previously been used off‐licence to treat obesity in children and adolescents include antidepressants such as fluoxetine and bupropion (Petkar 2013).
While weight loss alone may be of clinical and psychological benefit, additional health benefits may be achieved by the amelioration of obesity‐related disorders, such as hyperglycaemia in type 2 diabetes (Pandey 2015), pain and mobility in osteoarthritis (Widhalm 2016), and improvement in obstructive sleep apnoea (Nespoli 2013). Weight loss may also reduce the risk factors for cardiovascular and metabolic disease (Halpern 2010), or even prevent the development of disease, for example type 2 diabetes (Power 2014). While registration of drugs usually does not require such clinical endpoints, people and health economic considerations increasingly demand evidence on more than just weight or BMI reduction, data that would be more difficult to establish in children and adolescents and have been poorly, if at all, studied.
Adverse effects of the intervention
One systematic review of pharmacological options for managing paediatric obesity stated that the most common adverse events when taking orlistat were gastrointestinal problems related to increased fat excretion (e.g. fatty or oily stools, increased defecation, soft stools, flatus, faecal leakage). Other adverse events included long‐term fat‐soluble deficiencies, decrease in vitamin D concentrations and asymptomatic gallstones (Boland 2015). The most frequent adverse events associated with metformin are gastrointestinal, some of which can be intolerable (McCreight 2016). A change in dose or duration may resolve these adverse effects (McDonagh 2014). Common adverse effects of sibutramine included dry mouth, headaches, constipation and insomnia (Cheung 2013). However, the drug has also been linked to increased risk of nonfatal stroke or myocardial infarction, as shown in the Sibutramine Cardiovascular Outcomes (SCOUT) trial (James 2010). Consequently, the drug was withdrawn from the market in numerous countries including the UK, USA and Australia.
How the intervention might work
Sibutramine is a serotonin and norepinephrine reuptake inhibitor. It works by reducing hunger and improving satiety leading to decreased food intake (Catoira 2010). Orlistat leads to the excretion of approximately 30% of ingested fat; it works by acting as a gastrointestinal lipase inhibitor (Yanovski 2014). Metformin is a biguanide derivative which activates adenosine monophosphate‐activated protein kinase leading to the reduction of glucose production and absorption in the intestines and increasing insulin sensitivity. It is thought to reduce bodyweight by inhibiting fat cell lipogenesis and potentially may decrease food intake by increasing glucagon‐like peptide (Matson 2012). Fluoxetine is an antidepressant which works by inhibiting serotonin re‐uptake. It can result in weight loss by decreasing appetite and therefore inhibiting energy intake (Ye 2011). Hence, it is important to recognise that any drug that produces aversive taste or gastrointestinal adverse effects could produce weight loss by such adverse effects (Halford 2010).
Why it is important to do this review
In 2003, a systematic Cochrane Review was published entitled "Interventions for treating obesity in children" which assessed the effects of lifestyle interventions (dietary, physical activity, behavioural, or a combination of these) and included the analysis of childhood obesity treatment trials published up to July 2001 (Summerbell 2003). The second version of this Cochrane Review was published in 2009 providing an update to the 2003 review, and assessing the effects of pharmacological and surgical interventions (Oude Luttikhuis 2009).
To reflect the rapid growth in this field, the third update to this review has been split across six reviews focusing on the following treatment approaches: "Surgery for the treatment of obesity in children and adolescents" (Ells 2015b); "Drug interventions for the treatment of obesity in children and adolescents"; "Parent‐only interventions for childhood overweight or obesity in children aged 5 to 11 years" (Loveman 2015); "Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years" (Colquitt 2016); "Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in school children from the age of 6 to 11 years"; and "Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in adolescents aged 12 to 17 years". This review in this series focuses on the efficacy of pharmacological interventions for obese children and adolescents. The review complements the Cochrane Review of "Long‐term pharmacotherapy for obesity and overweight" (Padwal 2003), which does not provide randomised controlled trial (RCT) data on pharmacological interventions for children and adolescents.
The results of this current review and other systematic reviews in this series will provide information on which to underpin clinical guidelines and health policy on the treatment of children and adolescents who are overweight or obese.
Objectives
To assess the effects of drug interventions for the treatment of obesity in children and adolescents.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs where the length of the intervention had to be at least three months and the length of follow‐up from baseline had to be a least six months.
Types of participants
We included trials evaluating obese children and adolescents with a mean age of less than 18 years at the commencement of the intervention. We excluded trials with pregnant or critically ill participants. We excluded interventions that specifically dealt with the treatment of eating disorders or type 2 diabetes, or included participants with a secondary or syndromic cause of obesity.
Types of interventions
We investigated any pharmacological intervention which aimed to treat paediatric obesity, using any of the following intervention versus control sequences, where the same letters indicate direct comparisons.
Intervention
(a) Pharmacological intervention.
(b) Pharmacological intervention plus other therapy.
Comparator
(a1) Placebo.
(a2) Usual care.
(b1) Placebo plus other therapy.
(b2) Usual care plus other therapy.
Concomitant therapies were required to be the same in both the intervention and comparator groups.
Summary of specific exclusion criteria
Trials which included a growth hormone therapy as treatment for obesity.
Trials which included pregnant participants.
Trials which included participants who were critically ill.
Trials where participants had a secondary or syndromic cause of obesity.
Interventions that specifically dealt with the treatment of eating disorders or type 2 diabetes.
Trials in which the aim was not to treat obesity in children or adolescents.
Duration of intervention less than three months.
Duration of follow‐up less than six months.
Types of outcome measures
Primary outcomes
Body mass index (BMI) and bodyweight.
Adverse events.
Secondary outcomes
Health‐related quality of life and self‐esteem.
Body fat distribution.
Behaviour change.
Participants' views of the intervention.
Morbidity.
All‐cause mortality.
Socioeconomic effects.
Timing of outcome measurement
BMI: defined as weight (kg) divided by height (m) squared, and bodyweight (kg): measured at baseline, 6, 12, 24 and more than 24 months.
Adverse events: defined as an adverse outcome that occurred during or after the intervention but was not necessarily caused by it, and measured at any time during the trial.
Health‐related quality of life and self‐esteem: evaluated by a validated instrument such as the Paediatric Quality of Life Inventory and measured at baseline, 6, 12, 24 and more than 24 months.
Body fat distribution: defined by validated tools such as dual energy X‐ray absorptiometry (DEXA), waist circumference, skin fold thickness, waist‐to‐hip ratio and bioelectrical impedance analysis and measured at baseline, 6, 12, 24 and more than 24 months.
Behaviour change: evaluated by a validated instrument and measured at baseline, 6, 12, 24 and more than 24 months.
Participants' views of the intervention: defined as documented accounts from participant feedback and measured at baseline, 6, 12, 24 and more than 24 months.
Morbidity: defined as illness or harm associated with the intervention and measured at baseline, 6, 12, 24 and more than 24 months.
All‐cause mortality: defined as any death that occurred during or after the intervention and measured at any time during the trial.
Socioeconomic effects: defined as a validated measure of socioeconomic status such as parental income or educational status and measured at baseline, 6, 12, 24 and more than 24 months.
'Summary of findings' table
We presented a 'Summary of findings' table to report the following outcomes, listed according to priority.
BMI and bodyweight.
Adverse events.
Health‐related quality of life.
All‐cause mortality.
Morbidity.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources on 15 March 2016 from inception to the specified date.
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Register of Studies Online (CRSO).
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) (1946 to 15 March 2016).
PubMed (subsets not available on Ovid) (15 March 2016).
Embase 1974 to 2016 Week 11.
LILACS (15 March 2016).
ClinicalTrials.gov (15 March 2016).
WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/) (15 March 2016).
For detailed search strategies, see Appendix 1. We continuously applied an email alert service for MEDLINE via OvidSP to identify newly published trials using the search strategy detailed in Appendix 1. If we detected additional relevant key words during any of the electronic or other searches, we modified the electronic search strategies to incorporate these terms and documented the changes. We placed no restrictions on the language of publication when searching the electronic databases or reviewing reference lists of identified trials.
Searching other resources
We attempted to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
To determine the trials to be assessed further, two review authors (of EM, LE, CO) independently scanned the abstract, title, or both, of every record retrieved by the searches. We obtained full‐text articles of all those trials deemed potentially relevant for inclusion. We resolved any differences in opinion by consultation of a third review author (of GA, EC, LE). If there was an outstanding issue with the trial, we added the article to those 'awaiting assessment' and we contacted trial authors for clarification. We presented an adapted PRISMA flow diagram of trial selection (Figure 1) (Liberati 2009).
1.
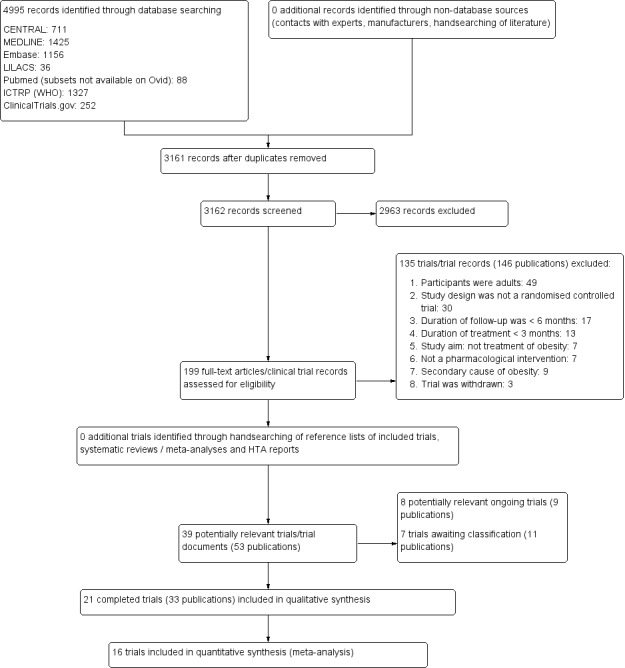
Trial flow diagram.
Data extraction and management
For trials that fulfilled the inclusion criteria, two review authors (of EM, LE, GA, NF, EC, LB, CO) independently extracted key participant and intervention characteristics and reported data on efficacy outcomes and adverse events using standard data extraction templates. We resolved any disagreements by discussion, or, if required, by consultation with a third review author (of NF, EC, LB, GA) (for details see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11).
1. Overview of trial populations.
| Trial | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | Safety (N) | ITT (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up timea |
| Atabek 2008b | I: metformin + diet and physical activity advice | ‐ | ‐ | 90 | 90 | ‐ | 90 | 100 | 6 months |
| C: placebo + diet and physical activity advice | 30 | 30 | ‐ | 30 | 100 | ||||
| total: | 120 | 120 | ‐ | 120 | 100 | ||||
| Berkowitz 2003 | I: behavioural programme + sibutramine | Powered to detect a 4% difference in % change in BMI between the 2 treatment groups with an SD of 5% (α = 0.05, β = 93%)c | 146 | 43 | 43 | 43 | 40 | 93.0 | 6 months (not including the 6‐month open‐label period where all participants received sibutramine) |
| C: behavioural programme + placebo | 39 | 39 | 39 | 34 | 87.2 | ||||
| total: | 82 | 82 | 82 | 62 | 75.6 | ||||
| Berkowitz 2006 | I: behavioural programme + sibutramine | "Planned sample size was approximately 400 participants with a 3:1 randomization ratio of sibutramine to placebo. On the basis of previous 12‐month adult trials, we determined that 300 participants in the sibutramine group would be adequate to assess safety and exposure, allowing an overall dropout rate of approximately 50% and a probability that approximately 50% of participants receiving 10 mg of sibutramine would lose 10% or more of initial BMI at 6 months" "Although the protocol did not document a formal sample size calculation for efficacy, approximately 132 adolescents (99 in the sibutramine group and 33 in the placebo group) would allow a between‐group difference in BMI of 2 kg/m2, with 90% power (2‐sided level of 0.05) to be statistically significant, assuming a common SD of 3 kg/m2)"d |
‐ | 368 | 368 | ‐ | 281 | 76.4 | 12 months |
| C: behavioural programme + placebo | 130 | 130 | ‐ | 80 | 61.5 | ||||
| total: | 498 | 498 | ‐ | 361 | 72.5 | ||||
| Chanoine 2005 | I: orlistat + diet + exercise + behaviour therapy | "We planned to enroll at least 450 individuals to provide more than 80% power to detect a difference of 1 BMI unit, assuming a 30% dropout rate" | 588 | 357 | 352 | 348 | 232 | 65.0 | 54 weeks |
| C: placebo + diet + exercise + behaviour therapy | 182 | 181 | 180 | 117 | 64.3 | ||||
| total: | 539 | 533 | 528 | 349 | 64.7 | ||||
| Clarson 2009 | I: metformin + lifestyle intervention | ‐ | 65 | 14 | ‐ | ‐ | 11 | 78.6 | 6 months |
| C: lifestyle intervention only | 17 | ‐ | ‐ | 14 | 82.4 | ||||
| total: | 31 | ‐ | ‐ | 25 | 80.6 | ||||
|
Franco 2014 (cross‐over trial) |
I: sibutramine + dietary guidance | ‐ | 73 | ‐ | ‐ | ‐ | ‐ | ‐ | 13 months |
| C: placebo + dietary guidance | ‐ | ‐ | ‐ | ‐ | ‐ | ||||
| total: | 63 | 63 | ‐ | 23 | 36.5 | ||||
| Freemark 2001 | I: metformin | ‐ | ‐ | 15 | ‐ | ‐ | 14 | 93.3 | 6 months |
| C: placebo | 17 | ‐ | ‐ | 15 | 88.2 | ||||
| total: | 32 | ‐ | ‐ | 29 | 90.6 | ||||
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 13 participants per group (expectations: mean loss of 7.5 kg (SD 5.3) in the sibutramine group vs 3.6 kg (SD 4.5) in the placebo group)e | 70 | 26 | 26 | 23 | 21 | 80.8 | 6 months |
| C: placebo + diet + exercise | 25 | 25 | 23 | 19 | 76.0 | ||||
| total: | 51 | 51 | 46 | 40 | 78.4 | ||||
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | ‐ | ‐ | 30 | 30 | 30 | 28 | 93.3 | 24 weeks |
| C: placebo + hypocaloric diet + exercise | 30 | 30 | 30 | 22 | 73.3 | ||||
| total: | 60 | 60 | 60 | 50 | 83.3 | ||||
| Kendall 2013 | I: metformin + healthy lifestyle advice | "The target recruitment was 140 patients, based on a power calculation using the results of a previous study. A standard power calculation was used to detect a reduction in BMI of 0.15 kg/m2 (SD 0.3). Sixty‐four participants in each group give a statistical power of 80% for a t test at the 5% significance level. This was rounded up to allow for some loss to follow‐up but recognizing that adjustment using multifactorial analysis would likely enhance the trial power by an unpredictable amount"f | 234 | ‐ | 74 | 74 | 55 | ‐ | 6 months |
| C: placebo + healthy lifestyle advice | ‐ | 77 | 77 | 55 | ‐ | ||||
| total: | 155 | 151 | 151 | 110 | 71.0 | ||||
| Maahs 2006 | I: orlistat + diet and exercise therapy | "We determined that a clinically important mean difference in decrease in BMI between the orlistat and placebo groups would be 2.0 kg/m2 at 6 months and used an SD of 1.8. On the basis of this approach, a sample size of 15 subjects per group would be adequate to detect a 2.0 kg/m2 difference in Student’s t test with 80% power and alpha = 0.05. In order to allow for a 25% dropout rate, 20 subjects were randomized to each group"g | 43 | 20 | ‐ | 20 | 18 | 90.0 | 6 months |
| C: placebo + diet and exercise therapy | 20 | ‐ | 20 | 16 | 80.0 | ||||
| total: | 40 | ‐ | 40 | 34 | 85.0 | ||||
| Mauras 2012 | I: metformin + diet/exercise intervention | "Differences in hsCRP and fibrinogen concentrations at 6 months were the primary outcomes. An n = 42 completed subjects provided > 90 % power to detect significant changes" | ‐ | 35 | 35 | ‐ | 23 | 65.7 | 6 months |
| C: diet/exercise intervention | 31 | 31 | ‐ | 19 | 61.3 | ||||
| total: | 66 | 66 | ‐ | 42 | 63.6 | ||||
| NCT00001723 | I: orlistat + behavioural weight loss programme | ‐ | ‐ | 100 | 100 | 100 | 87 | 87.0 | 6 months |
| C: placebo + behavioural weight loss programme | 100 | 100 | 100 | 84 | 84.0 | ||||
| 200 | 100 | 100 | 171 | 85.5 | |||||
| Ozkan 2004 | I: conventional treatment (nutritional and lifestyle modification programmes) + orlistat | ‐ | ‐ | 22 | ‐ | ‐ | 15 | 68.2 | 5 to 15 months |
| C: conventional treatment: nutritional and lifestyle modification programmes | 20 | ‐ | ‐ | 15 | 75.0 | ||||
| total: | 42 | ‐ | ‐ | 30 | 71.4 | ||||
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 8 participants were required per intervention group (SD 0.4; difference of 0.6, P < 0.05, power = 90%) | 41/26 | ‐ | 9 | ‐ | 7 | ‐ | 6 months |
| C: placebo + nutritional guide and exercise programme | ‐ | 10 | ‐ | 6 | ‐ | ||||
| total: | 26 | 19 | ‐ | 13 | 50 | ||||
| Rezvanian 2010 | I1: metformin + diet and physical activity advice | "By considering alpha = 0.05 and a power level of 0.8, the sample size was calculated as 160, and by considering the attrition during the follow‐up, we increased it to 180" | 180 | 45 | ‐ | ‐ | 41 | 91.1 | 24 weeks |
| I2: fluoxetine + diet and physical activity advice | 45 | ‐ | ‐ | 40 | 88.9 | ||||
| I3: metformin and fluoxetine + diet and physical activity advice | 45 | ‐ | ‐ | 41 | 91.1 | ||||
| C: placebo + diet and physical activity advice | 45 | ‐ | ‐ | 42 | 93.3 | ||||
| total: | 180 | ‐ | ‐ | 164 | 91.1 | ||||
|
Srinivasan 2006 (cross‐over trial) |
I: metformin + "standardised information on healthy eating and exercise" | ‐ | 34 | ‐ | ‐ | ‐ | ‐ | ‐ | 12 months |
| C: placebo + "standardised information on healthy eating and exercise" | ‐ | ‐ | ‐ | ‐ | ‐ | ||||
| total: | 28 | ‐ | ‐ | 22 | 78.6 | ||||
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | "The number of patients required per treatment group to detect a difference between treatment groups in mean change in BMI at endpoint intervention of 1.0 kg/m2, based on an estimate of variance (sd) of 0.65, an overall significance level of 5%, and a power of 90%, was nine. Allowing a drop‐out rate of 25%, the number of patients needed in each group was 12"h | ‐ | 12 | 12 | 12 | 11 | 91.7 | 24 weeks |
| C: placebo + energy‐restricted diet and exercise plan | 12 | 12 | 12 | 9 | 75.0 | ||||
| total: | 24 | 24 | 24 | 20 | 83.3 | ||||
| Wiegand 2010 | I: metformin + lifestyle intervention | "Since a clinically significant effect was defined as a decrease in HOMA‐IR by ‐1, two groups of 37 patients had to be included in the study to achieve a power of 0.9 with a α value of 0.05" | 278 | 36 | ‐ | ‐ | 34 | 94.4 | 6 months |
| C: placebo + lifestyle intervention | 34 | ‐ | ‐ | 29 | 85.3 | ||||
| total: | 70 | ‐ | ‐ | 63 | 90 | ||||
| Wilson 2010 | I: metformin + lifestyle intervention | "Assuming an SD of 1.9 for BMI change, an enrolled sample of 72 provided 80% power to detect a differential of 1.46 between treatment arms or between sexes and 1.75 between white subjects and others"i | 92 | 39 | 39 | 39 | 19 | 48.7 | 100 weeks |
| C: placebo + lifestyle intervention | 38 | 38 | 38 | 19 | 50.0 | ||||
| total: | 77 | 76 | 76 | 38 | 49.4 | ||||
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | "A total sample size of 60 participants would detect a between‐group difference of 0.09 BMI SD score units (approximately equivalent to a 2 kg/m2 difference) with 80% power. Participant accrual was set at 100 participants to allow as much as 40% loss to follow‐up"j | 278 | 53 | ‐ | 53 | 45 | 84.9 | 6 months (not including the 6‐month open‐label phase) |
| C: placebo + dietitian‐administered weight‐reduction programme | 47 | ‐ | 47 | 40 | 85.1 | ||||
| total: | 100 | ‐ | 100 | 85 | 85.0 | ||||
| Grand total | All interventionsk | 1395 | 1153 | ||||||
| All comparatorsk | 817 | 665 | |||||||
| All interventions and comparatorsk | 2484 | 1851 | |||||||
aDuration of intervention and follow‐up under randomised conditions until end of trial. bUnclear from the publication on the number which completed the trial and hence number of dropouts. cActual treatment difference between intervention groups was 4.5% reduction in BMI. dActual treatment difference between intervention groups at 12 months was 2.9 kg/m2. eActual weight loss was 7.3 kg in the sibutramine group vs 4.3 kg in the placebo group. fActual adjusted treatment difference at 6 months was ‐1.07 kg/m2. gActual treatment difference between intervention groups at 6 months was 0.5 kg/m2. hActual treatment difference between intervention groups at end of intervention (12 weeks) was 0.4 kg/m2 and at end of follow‐up (24 weeks) was 1.0 kg/m2. iActual treatment difference between intervention groups after 48 weeks was 1.1 kg/m2. jActual treatment difference between intervention groups at 6 months for BMI z score was 0.07. kNumbers for interventions and comparators do not add up to 'all interventions and comparators' because several trials did not provide information on randomised participants per intervention/comparator group but only the total number of randomised participants.
"‐" denotes not reported.
BMI: body mass index; C: comparator; hsCRP: high sensitivity C‐reactive protein; HOMA‐IR: homeostasis model assessment for insulin resistance index; I: intervention; ITT: intention‐to‐treat; n: number of participants; SD: standard deviation.
We provided information, including trial identifier, about potentially relevant ongoing trials in the Characteristics of ongoing studies table and in Appendix 5. We tried to obtain the protocol of each included trial, either in trial registers or in publications of trial designs, or both, and specified the data Appendix 5.
We sent an email to all authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 12 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we tried to maximise yield of information by collating all available data and used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (of EM, LE, GA, NF, EC, LB, CO) independently assessed the risk of bias of each included trial. We resolved possible disagreements by consensus, or with consultation of a third party. In cases of disagreement, the rest of the group were consulted and a judgement was made based on consensus.
We assessed risk of bias using Cochrane's 'Risk of bias' tool (Higgins 2011a; Higgins 2011b). We used the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We assessed outcome reporting bias by integrating the results of 'Examination of outcome reporting bias' (Appendix 6), 'Matrix of trial endpoints (publications trial documents)' (Appendix 5), and section 'Outcomes (outcomes reported in abstract of publication)' of the Characteristics of included studies table (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting (reporting bias).
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure.
We assessed the impact of individual bias domains on trial results at endpoint and trial levels.
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data), we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as self‐reported outcomes.
All self‐reported data such as a self‐reported health‐related quality of life questionnaires.
We defined the following endpoints as investigator‐assessed outcomes.
All measured data such as assessor measured height and weight.
Measures of treatment effect
We expressed continuous data as mean differences (MD) with 95% confidence intervals (CI). We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% CIs. We used Comprehensive Meta Analysis (CMA) version 3 and Review Manager 5 (RevMan 2014) to conduct the meta‐analyses.
Unit of analysis issues
We tried to consider the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from trial authors, if feasible, and evaluated important numerical data such as screened, eligible, randomised participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) populations. We investigated attrition rates, for example dropouts, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where standard deviations (SD) for outcomes were not reported, we imputed these values by assuming the SD of the missing outcome to be the mean of the SDs from those trials where this information was reported. We investigated the impact of imputation on meta‐analyses by means of sensitivity analyses.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report trial results as meta‐analytically pooled effect estimates. We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We examined heterogeneity using the I2 statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a). We also calculated Tau2, another statistic that provides information about heterogeneity.
When we found heterogeneity, we attempted to determine potential reasons for it by examining individual trial and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Differences in the age of trial population.
Differences in the trial population demographics.
Differences in the types of drugs.
Differences in BMI at baseline.
Assessment of reporting biases
If we included 10 trials or more for a given outcome, we used funnel plots to assess small‐trial effects. Due to several explanations for funnel plot asymmetry, we interpreted results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across trials, we primarily summarised low risk of bias data by means of a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We presented the overall certainty of the evidence for each outcome specified under 'Types of outcome measures: Summary of findings table' according to the GRADE approach which considers issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results. Two review authors (EM, GA) independently rated the certainty for each outcome. We presented a summary of the evidence in Table 1, which provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the Table 1 based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results on the outcomes as described in Types of outcome measures. If meta‐analysis was not possible, we presented results in a narrative form in Table 1.
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) to help with standardisation of 'Summary of findings' tables (Appendix 13).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses and investigated interactions.
Length of follow‐up.
Impact and nature of maintenance periods.
The impact of comparator/control: whether concomitant therapy or no treatment (true control).
The impact of population demographics.
Sensitivity analysis
We planned to performed sensitivity analyses to explore the influence of the following factors on effect size.
Restricting the analysis to published trials.
Restricting the analysis considering risk of bias, as specified in the Assessment of risk of bias in included studies section.
Restricting the analysis to very long or large trials (more than 300 participants in total) to establish how much they dominated the results.
Restricting the analysis to trials using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We also tested the robustness of the results by repeating the analysis using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of trials, see the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
Our comprehensive literature searches identified 4995 records; from these, 199 full‐text papers or clinical trial records were identified for further examination. We excluded trials based on their titles or abstracts because they did not meet the inclusion criteria or were not relevant to the question under trial (see Figure 1 for the amended PRISMA flow diagram). After screening the full text of the selected publications, 21 completed trials (33 publications) met the inclusion criteria and were included in the qualitative synthesis of this review. All trials were published in English apart from Franco 2014 (Portuguese) and Prado 2012 (Spanish). We contacted all trial authors of the included trials and received a reply from all but four authors (Atabek 2008; Berkowitz 2003; Berkowitz 2006; Ozkan 2004). We sought additional information from the authors of all 21 trials, 12 authors responded to these requests and provided further data (Chanoine 2005; Clarson 2009; Franco 2014; Freemark 2001; Godoy‐Matos 2005; Maahs 2006; Mauras 2012; NCT00001723; Prado 2012; Rezvanian 2010; Srinivasan 2006; Van Mil 2007). We also identified eight ongoing trials, and an additional seven trials were placed in the 'awaiting classification' section because we could not source the full publication, the trial was completed but there was not yet enough information to include it in this review or the publication was identified when a final draft of the review had been completed (NCT01487993).
Included studies
A detailed description of the characteristics of included trials is presented elsewhere (see Characteristics of included studies; Appendix 2; Appendix 3; Appendix 4). The following is a succinct overview.
Source of data
The literature search identified all 21 included trials in the review and all but one (NCT00001723) were published trials. Ten out of 21 trials were included in the previous review (Oude Luttikhuis 2009), and information relating to these 10 trials was extracted from the 2009 review ‐ two review authors extracted any missing information from the publication. All ongoing trials were found from searching online clinical trial registers.
Comparisons
Of the 21 included trials, 11 used metformin in their intervention arm; four of these trials gave metformin plus a behaviour changing programme to the intervention group and used a placebo plus a behaviour changing programme in the comparator group (Prado 2012; Wiegand 2010; Wilson 2010; Yanovski 2011). Two trials compared metformin plus a behaviour changing programme against a behaviour changing programme alone without using a placebo (Clarson 2009; Mauras 2012). Four trials compared metformin plus a behaviour changing intervention against placebo plus a behaviour changing intervention (Atabek 2008; Kendall 2013; Rezvanian 2010; Srinivasan 2006). Rezvanian 2010 also had two additional intervention arms: metformin plus fluoxetine plus healthy eating plus physical activity advice; and fluoxetine plus healthy eating plus physical activity advice. The remaining trial compared metformin with placebo; hence, there was no lifestyle component included in either arm (Freemark 2001).
Six trials used sibutramine as the pharmacological intervention; three trials compared sibutramine plus a behaviour changing programme with placebo plus a behaviour changing programme (Berkowitz 2003; Berkowitz 2006; Van Mil 2007). The other three trials compared sibutramine plus dietary/exercise advice with placebo plus dietary/exercise advice (Franco 2014; García‐Morales 2006; Godoy‐Matos 2005).
Four trials investigated orlistat. Chanoine 2005, Maahs 2006, and NCT00001723 examined orlistat plus a behaviour changing intervention versus placebo plus a behaviour changing intervention. Ozkan 2004 did not include a placebo in their comparator group; hence, they compared orlistat plus a behaviour changing intervention with a behaviour changing intervention only.
Overview of trial populations
A total of 2484 children and adolescents participated in the 21 included trials. A total of 1851 participants finished the trial (74.5%) and hence we measured at the study's endpoint. In 10 studies, the dropout rates were higher in the placebo group than the intervention group, potentially showing some dissatisfaction with the control condition. The individual trial sample size ranged from 24 to 539 participants.
The 11 metformin trials included 885 participants. The individual trial sample size ranged from 26 to 155 participants. One metformin trial also included two additional intervention arms of fluoxetine and fluoxetine plus metformin (45 randomised participants in each intervention arm).
The six sibutramine trials included 778 participants. The individual trial sample size ranged from 24 to 498 participants.
The four orlistat trials included 821 participants. The individual trial sample size ranged from 40 to 539 participants.
Trial design
Trials were RCTs. Nineteen trials adopted a parallel group superiority design and two were cross‐over trials (Franco 2014; Srinivasan 2006). All but three trials used a placebo comparator (Clarson 2009; Mauras 2012; Ozkan 2004). Five trials were multicentred (Berkowitz 2006; Chanoine 2005; Kendall 2013; Wiegand 2010; Wilson 2010), with the number of centres ranging from two (Wiegand 2010) to 33 (Berkowitz 2006). In terms of blinding, 14 trials were double‐blinded for participants and personnel (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Franco 2014; Freemark 2001; Godoy‐Matos 2005; Maahs 2006; NCT00001723; Prado 2012; Rezvanian 2010; Srinivasan 2006; Van Mil 2007; Wilson 2010; Yanovski 2011), no trials were single‐blinded for participants, and four trials did not define blinding (Atabek 2008; García‐Morales 2006; Kendall 2013; Ozkan 2004). Thirteen trials blinded outcome assessors (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Franco 2014; Freemark 2001; Godoy‐Matos 2005; Maahs 2006; NCT00001723; Rezvanian 2010; Srinivasan 2006; Van Mil 2007; Wiegand 2010; Wilson 2010; Yanovski 2011). Trials were published between the 2001 and 2014; all but one sibutramine trial were published before the drug was withdrawn by the FDA ‐ Franco 2014 was conducted in Brazil where the drug is still licensed. All metformin trials were published between 2006 and 2012 apart from Freemark 2001. Orlistat trials were published between 2004 and 2006, but one trial did not have any publications available and only posted results on a clinical trial website and in a conference abstract (NCT00001723).
The duration of interventions ranged from 12 weeks to 17 months, with a mean duration of 28 weeks. The duration of follow‐up (from end of intervention) ranged from 0 to 52 weeks, with a mean follow‐up period of 12 weeks. Participants in nine trials received the intervention/comparator for six months with no additional follow‐up; in three trials, participants received the intervention/comparator for six months, which was then followed by an open‐label period for six months (Berkowitz 2003; NCT00001723; Yanovski 2011); two trials received the intervention for 12 months with no additional follow‐up (Berkowitz 2006; Chanoine 2005); two cross‐over trials included a six‐month intervention or control condition followed by a washout period, then each participant crossed over into the alternative condition for an additional six months (Franco 2014; Srinivasan 2006); three trials included an intervention/comparator period for three months (or 12 weeks) then a follow‐up period for an additional three months (or 12 weeks) (Prado 2012Rezvanian 2010; Van Mil 2007); one trial gave the intervention or comparator condition for 48 weeks, then included an additional follow‐up period for another 48 weeks (Wilson 2010); and finally in one trial the length of the intervention and follow‐up varied across participants (Ozkan 2004).
Five trials had a run‐in period, of which three included a placebo run‐in phase (Chanoine 2005; Godoy‐Matos 2005; Wilson 2010), with a duration varying from two to four weeks; Freemark 2001 included 48‐hour inpatient tests as their run‐in period; two trial gave dietetic advice/counselling (García‐Morales 2006; Godoy‐Matos 2005); Wilson 2010 also included a lifestyle modification programme in their run‐in period. Outcomes were not assessed in these run‐in periods. Furthermore, three trials included an open‐label phase six months after randomisation where both groups received the drug intervention (Berkowitz 2003; NCT00001723; Yanovski 2011); these open‐label phases were not included in our analyses. Participants in one of these trials were also followed up for two years after the open‐label phase (NCT00001723). None of the included trials were terminated before regular end; however, two trials that we identified from ClinicalTrials.gov were terminated before enrolment and have been placed in the excluded trials section (see Characteristics of excluded studies table).
Settings
Nine of the 21 trials were conducted in the USA (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Freemark 2001; Maahs 2006; Mauras 2012; NCT00001723; Wilson 2010; Yanovski 2011). The other trials were completed in Turkey (Atabek 2008; Ozkan 2004), Canada (Chanoine 2005; Clarson 2009), Brazil (Franco 2014; Godoy‐Matos 2005), Mexico (García‐Morales 2006), the UK (Kendall 2013), Australia (Srinivasan 2006), Chile (Prado 2012), Iran (Rezvanian 2010), the Netherlands (Van Mil 2007), Germany (Wiegand 2010), and Switzerland (Wiegand 2010). All trials were performed in an outpatient setting apart from three trials which had both an inpatient and outpatient setting (Freemark 2001; Maahs 2006; Yanovski 2011).
Participants
The participating population consisted of the following: mainly obese children or adolescents (Maahs 2006 also included overweight participants). The mean age of the participants in the trials ranged from 10.1 to 16.3 years with only two trials having a mean age less than 12 years old (Atabek 2008; Yanovski 2011). Two studies required all participants to be postmenarchal (Berkowitz 2003; Prado 2012), while Yanovski 2011 only included children who were prepubertal or early pubertal. Fifteen trials included participants from high‐income countries, and six recruited participants from middle‐income countries (Atabek 2008; Franco 2014; García‐Morales 2006; Godoy‐Matos 2005; Ozkan 2004; Rezvanian 2010) ‐ based on the World Bank list of economies July 2015 (World Bank 2015). Ethnic groups were distributed as follows: six trials did not report on ethnic groups (Atabek 2008; Franco 2014; Ozkan 2004; Prado 2012; Rezvanian 2010; Van Mil 2007); one trial reported all their participants were white (Clarson 2009), three trials reported approximately 75% of their population were white (Chanoine 2005; Kendall 2013; Wiegand 2010); five trials reported approximately half of their population were white (Berkowitz 2003; Berkowitz 2006; Freemark 2001; Mauras 2012; Wilson 2010); one trial reported that approximately 60% of their population were Hispanic (Maahs 2006); one trial reported approximately 50% of their population were non‐Hispanic (Yanovski 2011); 63% of participants in one trial were non‐Hispanic black people while the remaining were non‐Hispanic white people (NCT00001723); and one trial reported that 64% of their participants came from ethnic backgrounds with a high prevalence of insulin resistance and metabolic syndrome (Srinivasan 2006). Participants' sex was not distributed evenly in 11 trials (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Freemark 2001; Godoy‐Matos 2005; Kendall 2013; Maahs 2006; NCT00001723; Prado 2012; Wiegand 2010; Wilson 2010). Three trials reported glycosylated haemoglobin A1c (HbA1c) at baseline and the mean HbA1c ranged from 5.3% to 5.6% (Freemark 2001; Maahs 2006; Wilson 2010). The mean BMI at baseline for the interventions groups ranged from 26.5 kg/m2 to 41.5 kg/m2. The BMI at baseline for the comparator groups ranged from 26.2 kg/m2 to 41.7 kg/m2. Thirteen trials reported comorbidities of participants at baseline (Atabek 2008; Berkowitz 2006; Chanoine 2005; Clarson 2009; Freemark 2001; García‐Morales 2006; Kendall 2013; Mauras 2012; NCT00001723; Prado 2012; Srinivasan 2006; Wiegand 2010; Yanovski 2011), all but one trial (Freemark 2001) reported cointerventions in participants, and four trials had comedications used by participants (NCT00001723; Ozkan 2004; Wilson 2010; Yanovski 2011). Criteria for entry into the individual trials are outlined in the Characteristics of included studies table. Major trial exclusion criteria were major illnesses such as type 1 or 2 diabetes mellitus or cardiovascular disease; pregnancy; major psychiatric disorders; taking or previously taken medication known to influence body composition or contradiction to the drug therapy; cigarette smoking or alcohol use; obesity associated with genetic disorders; and eating disorders such as bulimia. Adherence/compliance with the intervention was reported in most trials as good (70% or more) and was usually assessed by pill counts.
Diagnosis
All trials included participants who were defined as obese at baseline according to the growth reference they used, apart from one trial (Maahs 2006), which also included overweight children in their inclusion criteria. Seven trials define obesity using the 95th percentile or greater cut‐off on the Centers for Disease Control and Prevention (CDC; Kuczmarski 2000) charts (Atabek 2008; Clarson 2009; García‐Morales 2006; Mauras 2012; Rezvanian 2010; Wilson 2010; Yanovski 2011), but Wilson 2010 also required their participants to weigh less than 136 kg. One trial used greater than 85th percentile (to include also overweight participants) (Maahs 2006), while Van Mil 2007 used the 97th percentile or greater but also further selected for triceps skinfold thickness 97th percentile or greater for age and sex. NCT00001723 defined obesity by BMI for age and triceps skinfold above the 95th percentile (determined by National Health and Nutrition Examination Survey (NHANES) I age‐, sex‐ and race‐specific data) and all participants were required to be over 60 kg in bodyweight. Alternatively two trials used the definition of obesity given by Rosner 1998 of two units more than the US weighted mean of the 95th percentile but no greater than 44 kg/m2 (Berkowitz 2006; Chanoine 2005). One trial used the IOTF (Cole 2000) definitions for obesity (Srinivasan 2006), while another used the WHO (WHO 1995) growth standards cut‐off (Franco 2014). Kendall 2013 used the UK BMI growth charts (Cole 1995), and used the 98th centile as the cut‐off for obesity. One trial used German references (Kromeyer‐Hausschild 2001) to define obesity using greater than 97th percentile (Wiegand 2010). Three trials used raw BMI to define obesity: BMI greater than 30 kg/m2 (Freemark 2001); BMI 32 kg/m2 to 44 kg/m2 (Berkowitz 2003); and BMI 30 kg/m2 to 45 kg/m² (Godoy‐Matos 2005). In two trials, it was unclear which growth reference charts they were referring to (Ozkan 2004; Prado 2012). Participants were diagnosed with type 1 or 2 diabetes mellitus in none of our included trials. However, some trials included additional inclusion criteria other than age and obesity: Atabek 2008 required all participants to have hyperinsulinaemia; Clarson 2009 only included participants who were insulin resistant (defined by homeostasis model assessment (HOMA) for insulin resistance values greater than 3); Godoy‐Matos 2005 required all participants to have an adult bone age determined by left hand radiography (Greulich‐Pyle method); Kendall 2013 only included participants who had impaired glucose tolerance or hyperinsulinaemia; NCT00001723 only recruited participants who had comorbidities at baseline and these included hypertension, hyperinsulinaemia and hepatic steatosis; Srinivasan 2006 only included participants where there was a suspicion of insulin resistance (fasting insulin to glucose ratio greater than 4.5 or presence of acanthosis nigricans); Prado 2012 required all participants to present with at least one risk factor for type 2 diabetes (e.g. first‐ or second‐degree relative with history of type 2 diabetes); Mauras 2012 only included participants who had normal glucose tolerance but also had elevated highly sensitive C‐reactive protein (hsCRP), fibrinogen concentrations or both; Freemark 2001 inclusion criteria included a fasting insulin concentration exceeding 15 IU/mL and at least one first‐ or second‐degree relative with type 2 diabetes; and Yanovski 2011 required all participants to have hyperinsulinaemia (defined as fasting insulin 15 IU/mL or greater). All participants in Wiegand 2010 presented with comorbidities at baseline (features of the metabolic syndrome); however, this did not appear to be an inclusion criterion.
Interventions
Eleven trials used metformin as their pharmacological intervention (Atabek 2008; Clarson 2009; Freemark 2001; Kendall 2013; Mauras 2012; Prado 2012; Rezvanian 2010; Srinivasan 2006; Wiegand 2010; Wilson 2010; Yanovski 2011). The intervention was administered orally and varied between one and four times per day. Between trials, the daily dosage of metformin varied between 500 mg and 2000 mg, with a mean daily dosage of 1364 mg. Four metformin trials reported treatment before the start of the trial (Kendall 2013; Rezvanian 2010; Wiegand 2010; Wilson 2010); this included a healthy 'lifestyle' advice sheet, lifestyle modification treatment and a six‐month multiprofessional lifestyle intervention. Seven trials had a titration period, consisting of increasing the number of tablets taken over a period of weeks until the maximum dosage was tolerated (Clarson 2009; Kendall 2013; Mauras 2012; Rezvanian 2010; Srinivasan 2006; Wilson 2010; Yanovski 2011). Two trials did not have a matching placebo in the comparator group ‐ participants received a lifestyle intervention only (Clarson 2009; Mauras 2012). The duration of treatment ranged from 12 weeks/three months to six months with a mean treatment duration of 5.5 months.
Six trials used sibutramine as their intervention (Berkowitz 2003; Berkowitz 2006; Franco 2014; García‐Morales 2006; Godoy‐Matos 2005; Van Mil 2007). In all six trials, the drug was administered orally once daily. The daily dosage of sibutramine varied between 5 mg and 15 mg, with a mean daily dose of 11 mg. Three trials reported that participants received treatment before the start of the trial (Franco 2014; García‐Morales 2006; Godoy‐Matos 2005); this included dietetic advice/counselling and a six‐month lifestyle intervention. Two trials had a titration period (Berkowitz 2003; Van Mil 2007). All trials had a matching placebo as the comparator intervention. The duration of treatment ranged from 12 weeks to 12 months, with a mean treatment duration of 6.5 months.
Four trials gave orlistat to their intervention group (Chanoine 2005; Maahs 2006; NCT00001723; Ozkan 2004). The drug was administered orally three times per day and the daily dosage of orlistat was 360 mg in all four trials. No trials gave participants any treatment before the trial. One trial did not give a matching placebo to the comparator group ‐ participants received a lifestyle intervention only (Ozkan 2004). The duration of treatment ranged from six months to 12 months, with a mean treatment duration of 8.9 months.
One trial also included two additional intervention arms: metformin plus fluoxetine and fluoxetine only (Rezvanian 2010). The drugs were given by the oral route once daily. The daily dose of fluoxetine was 20 mg. Participants were also given lifestyle modification treatment before the start of the trial. They also had a titration period. The comparator group received a matching placebo. The duration of treatment was 12 weeks.
Outcomes
Fourteen trials explicitly stated a primary endpoint in the publication (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Clarson 2009; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Maahs 2006; Mauras 2012; Prado 2012; Van Mil 2007; Wiegand 2010; Wilson 2010; Yanovski 2011), 10 trials reported 'secondary' endpoints (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Maahs 2006; Wiegand 2010; Wilson 2010; Yanovski 2011). NCT00001723 had no publication attached; however, the trial authors reported both a primary and secondary endpoint on the clinical trials website. The most commonly defined primary outcomes in publications were change in absolute BMI, change in BMI z score/standard deviation score (SDS) and change in bodyweight. The most commonly defined primary outcomes in trial protocols were change in BMI from baseline and per cent change in BMI.
Reporting of endpoints
Twenty‐one trials collected a mean of 14 (range four to 25) outcomes. All 21 trials measured raw BMI. Ten trials reported change in BMI z score/SDS (Berkowitz 2003; Clarson 2009; Freemark 2001; Kendall 2013; NCT00001723; Srinivasan 2006; Van Mil 2007; Wiegand 2010; Wilson 2010; Yanovski 2011). All 21 trials reported on whether adverse events occurred. Of those trials which reported adverse events, some reported the total number of adverse events whilst others reported the total number of participants who experienced at least one adverse event. We asked all authors to provide further details on adverse events, such as how many participants experienced severe adverse events and if so, whether they were hospitalised. Two trials measured health‐related quality of life with validated questionnaires (García‐Morales 2006; Maahs 2006). Seventeen trials reported that they measured body fat distribution. Fifteen trials measured waist circumference, hip circumference, or both (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Clarson 2009; Franco 2014; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Mauras 2012; Prado 2012; Rezvanian 2010; Srinivasan 2006; Wiegand 2010; Wilson 2010; Yanovski 2011). Seven trials measured body fat mass by DEXA (Chanoine 2005; Mauras 2012; NCT00001723; Srinivasan 2006; Van Mil 2007; Wilson 2010; Yanovski 2011). Two trials also measured body fat mass by bioelectrical impedance (Maahs 2006; Wiegand 2010). Six trials measured behaviour change (Atabek 2008; Berkowitz 2003; García‐Morales 2006; Kendall 2013; Maahs 2006; Van Mil 2007). Five trials measured food consumption through dietary records or questionnaires (Atabek 2008; García‐Morales 2006; Kendall 2013; Maahs 2006; Van Mil 2007), and one trial measured the feeling of hunger (Berkowitz 2003). Two trials measure changes in physical activity: Kendall 2013 used a physical activity questionnaire and Van Mil 2007 measured total energy expenditure which accounts for level of physical activity. Only one trial investigated morbidity defined as illness or harm associated with the intervention (Chanoine 2005). One trial reported a death from suicide (Maahs 2006). Berkowitz 2006 reported two suicide attempts which did not result in death.
No trials assessed participants' views or socioeconomic effects as outcomes. For a summary of all outcomes assessed in each trial, see Appendix 5.
Excluded studies
We excluded 135 trials or trial records after careful evaluation of the full publication. The main reasons for exclusion were the participants were adults or had a mean age of more than 18 years, the trial design was not an RCT, the duration of treatment was less than three months or the duration of follow‐up was less than six months. For further details, see Characteristics of excluded studies table.
Risk of bias in included studies
For details on risk of bias of included trials see Characteristics of included studies table. For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials, see Figure 2 and Figure 3. We investigated performance bias, detection bias and attrition bias separately for objective and subjective outcome measures.
2.
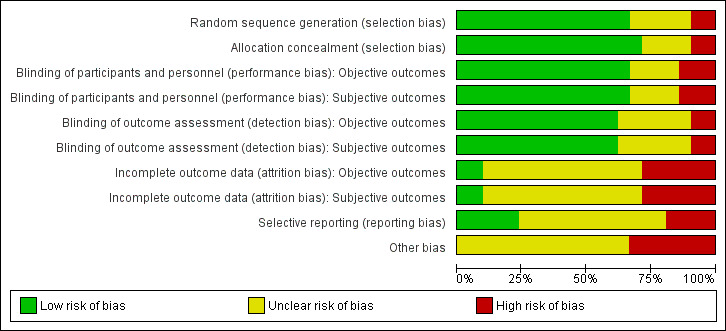
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.
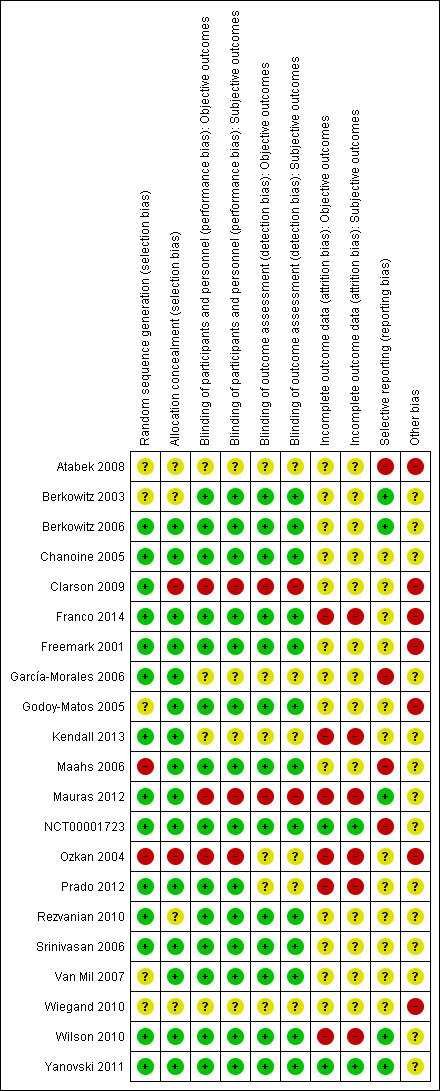
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Fifteen trials reported allocation was concealed (Berkowitz 2006; Chanoine 2005; Franco 2014;Freemark 2001; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Mauras 2012; NCT00001723; Prado 2012; Rezvanian 2010; Srinivasan 2006; Wiegand 2010; Wilson 2010; Yanovski 2011); two trials did not conceal allocation (Clarson 2009; Ozkan 2004). It was unclear whether four trials concealed allocation (Atabek 2008; Berkowitz 2003; Rezvanian 2010;Wiegand 2010) ). Fourteen trials reported an adequate random sequence generation (Berkowitz 2006; Chanoine 2005; Clarson 2009; Franco 2014; Freemark 2001; García‐Morales 2006; Kendall 2013; Mauras 2012; NCT00001723; Prado 2012; Rezvanian 2010; Srinivasan 2006; Wilson 2010; Yanovski 2011). Two trials reported random sequence generation was inadequate; hence, would have likely of introduced bias (Maahs 2006; Ozkan 2004).Five trials did not describe the randomisation process (Atabek 2008; Berkowitz 2003; Godoy‐Matos 2005; Van Mil 2007; Wiegand 2010).
Blinding
All 21 trials reported both objective and subjective outcomes. The main objectives outcomes were BMI, weight, waist or hip circumference, blood pressure, cholesterol insulin, glucose and triglycerides, whilst the main subjective outcomes were adverse events, food consumption and health‐related quality of life. Subjective outcomes tended to be self‐reported (e.g. quality of life and dietary questionnaires), while objective measures usually were investigator‐assessed (e.g. BMI, waist circumference). Adverse events could be either self‐reported or investigator assessed.
Ten trials explicitly stated that blinding of the participants, personnel and outcome assessors was undertaken (Berkowitz 2003; Berkowitz 2006; Franco 2014; Maahs 2006; NCT00001723; Rezvanian 2010; Srinivasan 2006; Van Mil 2007; Wilson 2010; Yanovski 2011). Seven trials reported that double blinding took place (Atabek 2008; Chanoine 2005; Freemark 2001; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Wiegand 2010), but only three of the trials' authors confirmed this meant blinding was undertaken of participants, personnel and outcomes assessors (Chanoine 2005; Freemark 2001; Godoy‐Matos 2005). No trials reported that single blinding was undertaken. Six trials did not provide sufficient information about blinding procedures (Atabek 2008; García‐Morales 2006; Kendall 2013; Ozkan 2004; Prado 2012; Wiegand 2010).
Incomplete outcome data
Twenty trials that had losses to follow‐up described the number of trial withdrawals (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; Clarson 2009; Franco 2014; Freemark 2001; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Maahs 2006; Mauras 2012; NCT00001723; Ozkan 2004; Prado 2012; Rezvanian 2010; Srinivasan 2006; Van Mil 2007; Wiegand 2010; Wilson 2010; Yanovski 2011). Twelve trials used ITT analyses (Berkowitz 2003; Berkowitz 2006; Chanoine 2005; García‐Morales 2006; Godoy‐Matos 2005; Kendall 2013; Maahs 2006; NCT00001723; Rezvanian 2010; Van Mil 2007; Wilson 2010; Yanovski 2011). One trial did not report whether there were any losses to follow‐up (Atabek 2008). Five trials did not provide detailed descriptions of participants' withdrawals and reasons underpinning them (Atabek 2008; Franco 2014; Freemark 2001; García‐Morales 2006; Mauras 2012). Four trials had attrition rates greater than 30% with possible impact on the outcomes(Chanoine 2005; Franco 2014; Mauras 2012; Prado 2012;Wilson 2010).
Selective reporting
Only nine trials provided a clinical trial identifier or reference to a protocol (Berkowitz 2003; Berkowitz 2006; Kendall 2013; Mauras 2012; NCT00001723; Srinivasan 2006; Wiegand 2010; Wilson 2010; Yanovski 2011); however, we were unable to source the clinical trial entry of one trial (Wiegand 2010). Three trials had a high risk of reporting bias after failure to report results for one or more outcomes they described as having measured (Atabek 2008; García‐Morales 2006; Maahs 2006), and a further trial had a high risk due to differences in results reported on the clinical trial website and in a conference abstract (NCT00001723). The remaining trials had unclear risk of reporting bias due to no protocol being available.
Other potential sources of bias
Seven trials were at high risk of other biases. These biases included: the trial not including a power calculation (Atabek 2008; Clarson 2009; Franco 2014; Freemark 2001; Godoy‐Matos 2005; Ozkan 2004), the trial lacking methodological detail (Atabek 2008; Franco 2014) and the trial not adjusting for baseline differences (Freemark 2001; Ozkan 2004), The remaining 14 trials were at unclear risk of other potential sources of bias. It is important to note that the trials which do not include a power calculation may not be powered to detect differences in their primary outcome. BMI or weight was the primary outcome in all but two trials (Mauras 2012; Wiegand 2010) that included a power calculation. Mauras 2012 and Wiegand 2010 may not have been adequately powered to detect differences in BMI or weight. With regards to adverse events and the review's secondary outcomes (e.g. morbidity), it is likely that most trials would not have been powered to detect differences in these outcomes. Hence, these results should be interpreted with caution.
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics, see Appendix 3 and Appendix 4.
Pharmacological intervention versus comparators
We performed the meta‐analyses with CMA software version 3 and aligned with the data in the Review Manager 5 (RevMan 2014) meta‐analyses. Because the cross‐over design did not appear suitable for our research question due to inadequate washout periods and noncomparable baseline measures in the two cross‐over periods, we excluded Franco 2014 and Srinivasan 2006 from all meta‐analyses. We also excluded Rezvanian 2010 from the meta‐analyses because the reported SDs were unreliably small in comparison to all other published SDs of included trials and probably denoted standard errors. We excluded two further trials because of substantial methodological concerns (Ozkan 2004; Prado 2012). In addition, Prado 2012 did not report change in BMI from baseline to follow‐up and Ozkan 2004 did not have a consistent follow‐up time frame across all participants.
Primary outcomes
Body mass index and bodyweight
We included 16 trials in the meta‐analysis of BMI. Most of the BMI data were from the publications, except for Chanoine 2005 and Freemark 2001, where raw BMI, SDs or both were not available; hence, we obtained additional data from the trial authors. We extracted data for NCT00001723 from the ClinicalTrials.gov website. In the meta‐analysis, we included trials which had either a six‐month or 12‐month follow‐up from baseline (Berkowitz 2006; Wilson 2010), which was the endpoint in most of the trials. However, even though Chanoine 2005 had a 12‐month follow‐up, we only had data available at six months from baseline. Wilson 2010 provided data at 100 weeks' follow‐up but we did not include these in the meta‐analysis.
The summary estimate across all pharmacological interventions versus all comparators (metformin, orlistat or sibutramine mostly versus placebo ‐ usually combined with behaviour changing interventions) showed a MD in BMI change of ‐1.3 kg/m2 (95% CI ‐1.9 to ‐0.8; P < 0.00001; 16 trials; 1884 participants; low certainty evidence ‐ Analysis 1.1) in favour of the drug interventions. Heterogeneity was considerable (I2 = 77%).
1.1. Analysis.
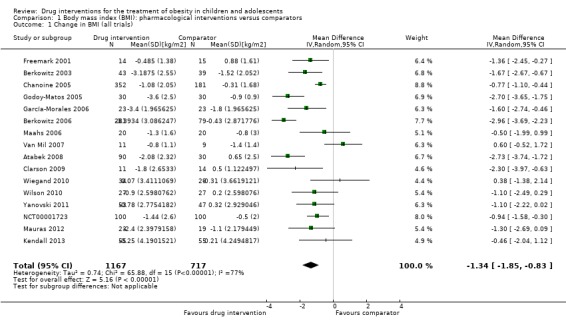
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 1 Change in BMI (all trials).
In Wilson 2010, which reported a BMI change at 100 weeks from baseline (48 weeks of metformin or placebo treatment, then a 48‐week drug‐free period), the metformin group increased their BMI during the drug‐free period (+0.5) while the placebo group decreased their BMI (‐0.8), measured as the difference between 52 and 100 weeks from baseline. In the metformin plus fluoxetine trial, the fluoxetine only group had a decrease in BMI of ‐0.6 (SD 0.1) and the metformin plus fluoxetine group had a decrease in BMI of ‐0.9 (SD 0.02), compared to an increase of 0.2 (SD 0.04) in the placebo group at 24 weeks from baseline.
Only 11 trials reported weight data at baseline or follow‐up (or change from baseline) in their publications; hence, we only included these trials in the meta‐analysis. Data were reported at six months from baseline apart from one trial (Berkowitz 2006), which reported the change in weight at 12 months from baseline. The summary estimate across all pharmacological interventions versus comparators (metformin, orlistat or sibutramine mostly versus placebo ‐ usually combined with behaviour changing interventions) showed an MD in change in weight of ‐3.9 kg (95% CI ‐5.9 to ‐1.9; P < 0.00001; 11 trials; 1180 participants; low certainty evidence ‐ Analysis 2.1) in favour of the drug interventions. Heterogeneity was considerable (I2 = 79%).
2.1. Analysis.
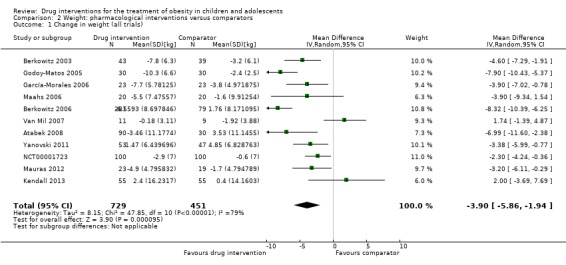
Comparison 2 Weight: pharmacological interventions versus comparators, Outcome 1 Change in weight (all trials).
Adverse events
Only three trials had sufficiently long exposure times to evaluate adverse events possibly associated with drug interventions for obesity in children and adolescents: one trial with 39 participants randomised to metformin treatment for 100 weeks (Wilson 2010), one trial with 368 participants randomised to sibutramine treatment for 12 months (Berkowitz 2006), and one trial with 357 participants randomised to orlistat treatment for 54 weeks (Chanoine 2005).
Adverse events were reported to have occurred in all 11 metformin trials except from Clarson 2009, which reported that metformin was well tolerated, and the author clarified no adverse events occurred. Gastrointestinal adverse events were most commonly reported with one metformin trial reporting that gastrointestinal adverse events were statistically more prevalent in the intervention group compared to the control group (Yanovski 2011). However, Wiegand 2010 reported such events occurred more frequently in the placebo group. Kendall 2013 reported adverse events were more common in the metformin group and were mainly gastrointestinal. Atabek 2008 reported that two metformin‐treated participants experienced diarrhoea, mild abdominal pain/discomfort, or both. Freemark 2001 also reported three participants experienced transient abdominal discomfort or diarrhoea, however so did one placebo participant. Wilson 2010 reported that the most common adverse events included headache, nausea, vomiting, upper respiratory tract infection and musculoskeletal complaints; however, none were statistically different between the metformin and placebo groups. One trial showed the fatigue was more common in the metformin‐treated children (Yanovski 2011). Furthermore, Freemark 2001 reported one case of an exacerbation of migraine and one case of transient nausea in the metformin arm. Nausea was reported in the Srinivasan 2006 trial where two participants were unable to tolerate a higher dose of metformin (1 g); however, they tolerated a lower dose and continued in the trial. Yanovski 2011 also reported that levels of serum vitamin B12 were reduced in the metformin group compared with an increase in the placebo group ‐ this difference was statistically significant. Rezvanian 2010 reported two cases of headache, two cases of abdominal pain and three cases of loose stools in the metformin arm but they were all minor and tolerable. Mauras 2012 reported metformin was well tolerated and safe, and the author added that the adverse effects between groups were comparable. Prado 2012 reported metformin was well tolerated by participants and both groups showed a significant increase in alanine transaminase (ALT) and aspartate transaminase (AST), and a reduction in haemoglobin levels, but these were within the normal ranges.
Three of six trials on sibutramine therapy reported on adverse events: one large trial showed tachycardia, dry mouth, constipation, dizziness, insomnia and hypertension were all reported more frequently by sibutramine participants than by placebo participants (Berkowitz 2006). Sibutramine‐treated participants also had a higher blood pressure and pulse rate at 12 months' follow‐up compared to the placebo‐treated participants (Berkowitz 2006). However, another trial reported that there was no statistically significant difference between changes in heart rate or blood pressure between the sibutramine and placebo groups, although abdominal cramps were significantly higher in the sibutramine group (Van Mil 2007). Godoy‐Matos 2005 showed constipation was significantly higher in the sibutramine group compared to the placebo group.
All four orlistat trials reported on adverse events: gastrointestinal problems such as fatty stools, oily spotting and fecal urgency, along with headaches and upper respiratory tract infections, were the most common adverse effects. In the NCT00001723 trial, the prevalence of some gastrointestinal problems was higher in the orlistat group compared to the placebo group and this included: fatty‐appearing stools, bloating/gas, frequent urge for bowel movement and uncontrolled passage of stool or oil. Chanoine 2005 reported that gastrointestinal tract‐related adverse events were more common in the orlistat group compared to the placebo group; however, most were classed as mild to moderate intensity. Maahs 2006 also reported that the orlistat group had significantly increased gastrointestinal adverse events (e.g. soft stools, oily spotting) compared to the placebo group. Mild gastrointestinal complaints (frequent stools) were experienced by all orlistat‐receiving participants in the Ozkan 2004 trial. Chanoine 2005 also reported that 10 orlistat and one placebo participant showed abnormalities detected on electrocardiograms; however, an independent cardiologist concluded that none were connected to the treatment; in addition, levels of oestradiol in girls decreased in the orlistat group versus a slight increase in the placebo group (P = 0.05). Symptomatic gallstones were also seen in six orlistat participants which were not seen at baseline (five of these participants had lost large amounts of weight).
In the trial which included a fluoxetine arm, there were five adverse events with regards to the drug which included three cases of dry mouth and two cases of loose stool; these were all considered as minor and tolerable, and reported as transient (Rezvanian 2010).
Serious/severe adverse events were also investigated: most trials did not report how they defined a serious/severe adverse event. It was also unclear in four trials whether a serious/severe adverse event actually occurred (Berkowitz 2003; Ozkan 2004; Van Mil 2007; Wiegand 2010). Only five trials reported that a serious or severe adverse event occurred (Berkowitz 2006; Chanoine 2005; Maahs 2006; NCT00001723; Wilson 2010); the remaining 12 trials reported that there were no serious or severe adverse events.
Across all trials the RR for serious adverse events comparing drug interventions with comparators was 1.43 (95% CI 0.63 to 3.25; P = 0.39; 5 trials; 1347 participants; low certainty evidence ‐ Analysis 3.1). Absolute numbers experiencing a serious adverse event were 24/878 (2.7%) participants in the drug intervention groups versus 8/469 (1.7%) participants in the comparator groups.
3.1. Analysis.
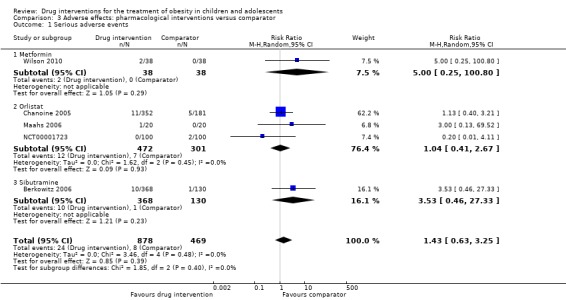
Comparison 3 Adverse effects: pharmacological interventions versus comparator, Outcome 1 Serious adverse events.
In the metformin trials, only one trial reported that there were serious adverse events and these included one case of appendectomy and one case of leg vein thrombosis in the metformin group, but these were both seen as unrelated to the drug (Wilson 2010). One sibutramine trial reported that 2.7% of sibutramine‐treated participants experienced serious adverse events which included one case of excessive nausea and vomiting, one suicide attempt and five depression cases (Berkowitz 2006). The placebo group had one case of suicide attempt and one case of depression. Chanoine 2005 reported 3% of participants experienced at least one serious adverse event: the five events in the placebo group included acute demyelinating encephalomyelitis, facial palsy, pneumonia, worsening of asthma and pain in the right side; and the 11 events in the orlistat group included pilonidal abscess, depression, asthma attack, seizure, admission for repair of deviated nasal septum, appendicitis, cholelithiasis, gallbladder disorder followed by cholecystectomy, adenoidal hypertrophy and aseptic meningitis. It was only the case of cholelithiasis in the orlistat participant which was seen to be possibly related to the trial medication potentially due to rapid weight loss. Another orlistat trial reported two serious adverse events in the placebo group and these were one case of hypoglycaemia and one case of left lower quadrant pain and vomiting (NCT00001723).
In the sibutramine trials, 32 participants (24 in the intervention groups and eight in the control groups) left the trial because of adverse events. Berkowitz 2006 reported that withdrawals due to tachycardia were similar in both groups but hypertension led to the withdrawal of five participants in the sibutramine group versus none in the placebo group. Two cases of attempted suicide (one intervention and one placebo) also led to discontinuation but were considered unlikely to be related to the trial drug; one case of excessive nausea and vomiting in the sibutramine group also led to withdrawal and may have been related to the drug. Van Mil 2007 had one withdrawal from the sibutramine group due to symptoms of clinical depression and Berkowitz 2003 had one withdrawal from the placebo group.
In the metformin trials, nine participants withdrew due to adverse events (five in intervention group and four in placebo group). Wilson 2010 reported one participant from the metformin group withdrew due to nausea which was probably related to the drug, and a further two metformin and one placebo participants dropped out of the trial due to elevated levels of ALT. Gastrointestinal symptoms caused 6% of participants (one in metformin group and three in placebo group) to drop out of the Wiegand 2010 trial. In addition, Yanovski 2011 reported one participant dropped out of the metformin group due to medication intolerance.
Across all trials the RR for discontinuing the trial because of adverse events comparing drug interventions with comparators was 1.45 (95% CI 0.83 to 2.52; P = 0.19; 10 trials; 1664 participants; low certainty evidence ‐ Analysis 3.2). Absolute numbers discontinuing the trial because of an adverse event were 52/1043 (5.0%) participants in the drug intervention groups versus 17/621 (2.7%) participants in the comparator groups.
3.2. Analysis.
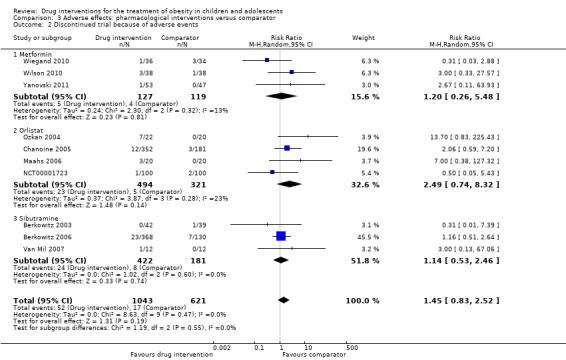
Comparison 3 Adverse effects: pharmacological interventions versus comparator, Outcome 2 Discontinued trial because of adverse events.
All four orlistat trials had dropouts due to adverse events; 28 participants (23 in the intervention group and five in the placebo group). Chanoine 2005 reported 12 dropouts (3%) in the orlistat group and three dropouts (2%) in the placebo group, mainly due to gastrointestinal adverse events. Ozkan 2004 reported seven participants (32%) dropped out of the orlistat group due to gastrointestinal complaints. Maahs 2006 reported two participants in the orlistat group discontinued due to adverse events (assumed to be gastrointestinal) and one participant in the orlistat group committed suicide. NCT00001723 reported one participant in the orlistat group and two participants in the placebo group dropped out of the trial due to medication intolerance.
For further details, see Appendix 9, Appendix 10, and Appendix 11.
Secondary outcomes
Health‐related quality of life
Two trials measured health‐related quality of life; the certainty of the evidence was very low. García‐Morales 2006 used the 36‐Item Short‐Form Health Survey (SF‐36) questionnaire and found changes in the total score were slightly higher in the sibutramine group compared to the placebo group, but this difference was not statistically significant. Maahs 2006 used three questionnaires to assess health‐related quality of life, but found no statistically significant differences between the orlistat and placebo group from baseline to six months. For further details on the health‐related quality of life measurements, see Appendix 14.
Body fat distribution
Eighteen trials reported outcomes which measured body fat distribution. Fifteen of these trials measured waist, hip, or both circumferences at baseline and follow‐up. In the metformin trials, Mauras 2012 found greater decreases in waist circumference in the metformin plus diet plus exercise group compared with the diet plus exercise group at six months' follow‐up. However, this trial was not placebo controlled. In addition, Srinivasan 2006, a cross‐over trial, reported a beneficial treatment effect on waist circumference in participants taking metformin for six months, when compared to six months of placebo. However, there was no statistically significant difference in waist circumference between the drug and control groups in Clarson 2009 and Prado 2012 trials at six months' follow‐up. Wilson 2010 measured waist circumference but did not report results. Two metformin trials also measured waist‐to‐hip ratio and found no statistically significant difference between groups at six months' follow‐up (Kendall 2013; Wiegand 2010). Yanovski 2011 also measured abdominal and hip circumference at six months' follow‐up, and found a statistically significant difference between metformin and placebo, in favour of the intervention. In the metformin plus fluoxetine trial, only the metformin plus fluoxetine arm had a statistically significant between‐group difference in waist circumference at 24 weeks from baseline (Rezvanian 2010). In the sibutramine trials, there was a statistically significant difference in waist circumference in favour of the intervention in five trials (Berkowitz 2003; Berkowitz 2006; Franco 2014; García‐Morales 2006; Godoy‐Matos 2005). Godoy‐Matos 2005 also reported a statistically significant reduction in hip circumference in the sibutramine group compared to the placebo group; however, there was no statistically significant difference for waist‐to‐hip ratio at six months. Only one orlistat trial measured waist circumference and found it increased in the placebo group but decreased in the orlistat group at one year' follow‐up (difference statistically significant); this was also seen for hip circumference (Chanoine 2005).
Seven trials measured body composition by DEXA. Four metformin trials and two orlistat trials measured body fat using DEXA. Three metformin trials found no statistically significant difference between groups in the percentage of body fat lost (Mauras 2012; Srinivasan 2006; Wilson 2010). However, one trial observed a statistically significant difference of 1.4 kg between the metformin and placebo groups, in favour of the intervention group at six months' follow‐up (Yanovski 2011). One sibutramine trial assessed body composition using underwater weighing and DEXA; however, there was no statistically significant difference in percentage of fat mass between groups. Chanoine 2005 reported they measured fat mass by DEXA in a subgroup of participants as a safety measure and the orlistat group lost more fat mass compared to the placebo group (P = 0.03). NCT00001723 found a slightly greater decrease in body fat (kg) in the orlistat group compared to the placebo group. Two trials estimated fat mass from bioimpedence analysis: one orlistat trial (Maahs 2006) and one metformin trial (Wiegand 2010), but they reported no statistically significant difference between intervention and placebo groups. Yanovski 2011 measured fat mass by air displacement plethysmography and found metformin participants had statistically significant decreases in their fat mass compared to placebo participants; they also measured intra‐abdominal fat by magnetic resonance imaging but found no statistically significant difference between groups. Srinivasan 2006 also used magnetic resonance imaging and found a beneficial treatment effect of metformin over placebo for subcutaneous abdominal adipose tissue but not visceral abdominal adipose tissue; Mauras 2012 also used this technique and found that intrahepatic fat only decreased in the nonplacebo control group. Wiegand 2010 used abdominal computer tomography (CT) scans to evaluate abdominal fat content but also found no statistically significant difference between metformin and placebo participants in the results.
Behaviour change
Six trials measured behaviour change; however, only two trials reported the results. Participants in three trials all completed a food frequency questionnaire at the beginning and end of the trial; however, no results were presented (Atabek 2008; García‐Morales 2006; Maahs 2006). Kendall 2013 assessed dietary habits and exercise levels through three previously validated questionnaires but were unable to analyse the data due to insufficient resources. Van Mil 2007 measured total energy expenditure, using the Maastricht protocol and included data from a seven‐day dietary record; however, the difference between the sibutramine and control groups after 12 weeks of intervention was not statistically significant. Physical activity level was also measured using an activity questionnaire, but there was no statistically significant difference between groups at 12 weeks. Changes in total energy expenditure and physical activity levels were not measured at 24‐week follow‐up due to unavailability of equipment.
Participants' views of the intervention
No trials investigated participants' views of the intervention.
Morbidity
Only one trial investigated morbidity defined as illness or harm associated with the intervention (Chanoine 2005). In the orlistat group, 6/352 (1.7%) participants developed new gallstones compared with 1/181 (0.6%) in the placebo group. The certainty of the evidence was very low.
Some trials investigated various risk indicators, mainly insulin resistance or insulin sensitivity and hyperinsulinaemia (Atabek 2008; Clarson 2009; Freemark 2001; Kendall 2013; Srinivasan 2006; Wiegand 2010; Yanovski 2011). García‐Morales 2006 investigated changes in blood pressure, glucose and triglycerides. Prado 2012 investigated glycaemia, insulin resistance and lipid profiles. Mauras 2012 investigated changes in hsCRP and fibrogen concentrations.
All‐cause mortality
One trial reported a death from suicide (Maahs 2006); the certainty of evidence was low. The authors reported that quality of life factors were screened extensively and the participant gave negative responses to quality of life questions specific to suicide and was also under the care of a psychiatrist for depression at the time of the trial. Berkowitz 2006 reported two suicide attempts (one in the intervention group and one in the placebo group).
Socioeconomic effects
No trials investigated socioeconomic effects.
For a summary of all outcomes assessed in each trial, see Appendix 5. For further explanation on how trial outcomes were defined, see Appendix 7 and Appendix 8.
Subgroup analyses
We performed subgroup analyses on our primary outcomes of BMI and weight. In our protocol, we specified we would analyse length of follow‐up; however, only two trials provided data at a time point greater than six months. There was too much heterogeneity to analyse the maintenance periods and most trials ended on completion of the intervention. In addition, there were only two trials which did not use a placebo; hence, we did not perform subgroup analyses based on type of control given. However, we performed subgroup analyses on BMI for the following factors: drug type (Analysis 1.2), dropout rates (Analysis 1.3), ITT analysis (Analysis 1.4), funding source (Analysis 1.5), publication date (Analysis 1.6), quality of trial (Analysis 1.7), country income (Analysis 1.8), and mean age of participants (Analysis 1.9).
1.2. Analysis.
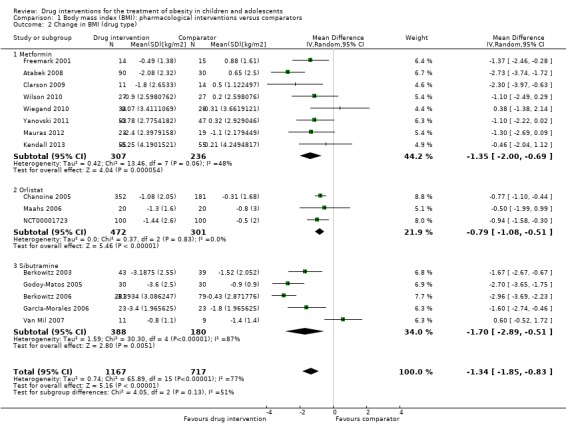
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 2 Change in BMI (drug type).
1.3. Analysis.
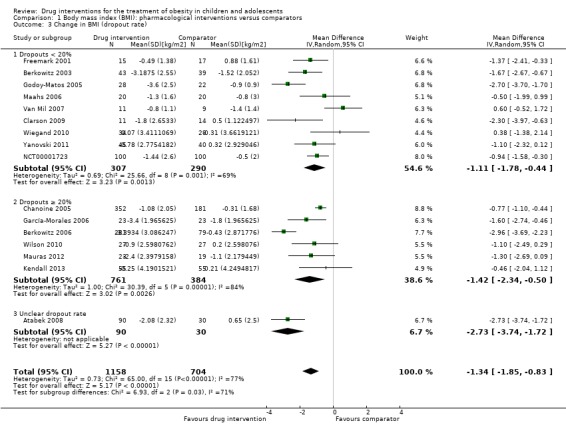
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 3 Change in BMI (dropout rate).
1.4. Analysis.
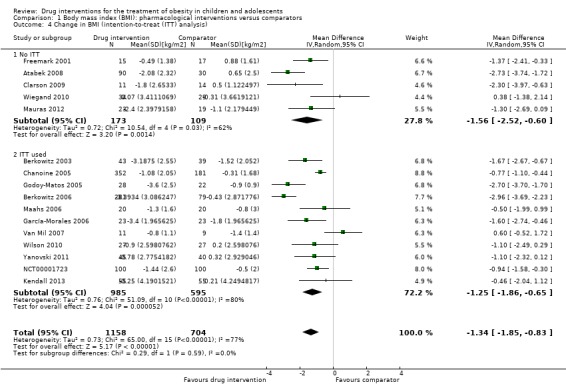
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 4 Change in BMI (intention‐to‐treat (ITT) analysis).
1.5. Analysis.
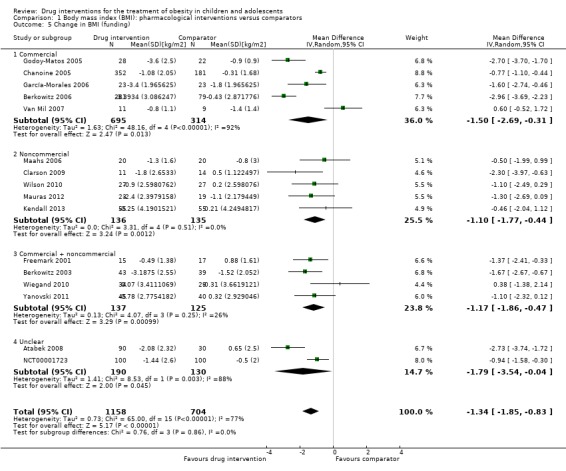
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 5 Change in BMI (funding).
1.6. Analysis.
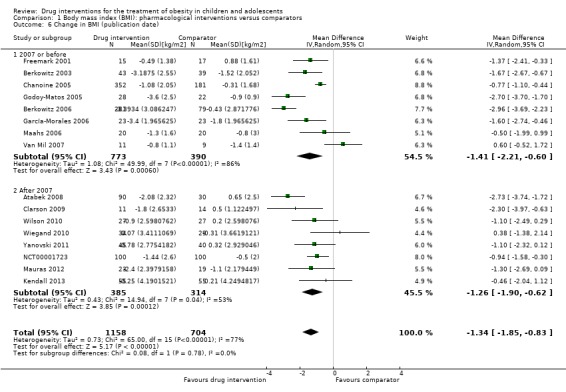
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 6 Change in BMI (publication date).
1.7. Analysis.
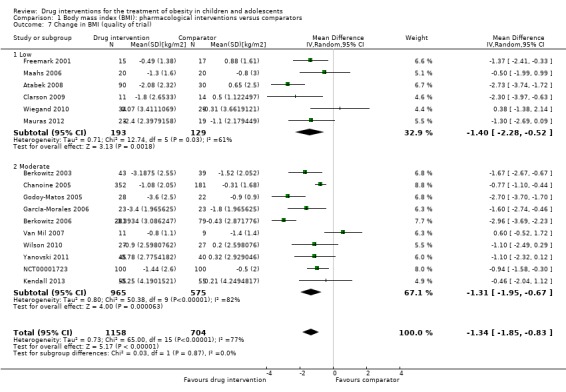
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 7 Change in BMI (quality of trial).
1.8. Analysis.
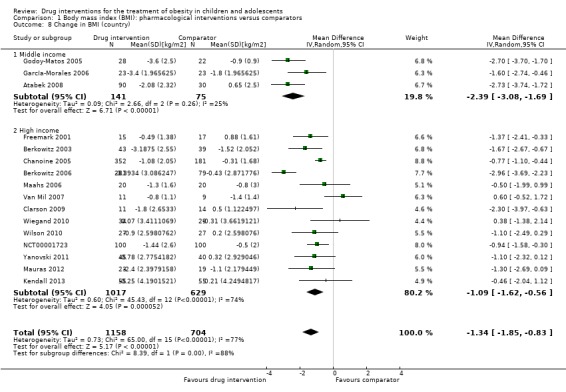
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 8 Change in BMI (country).
1.9. Analysis.
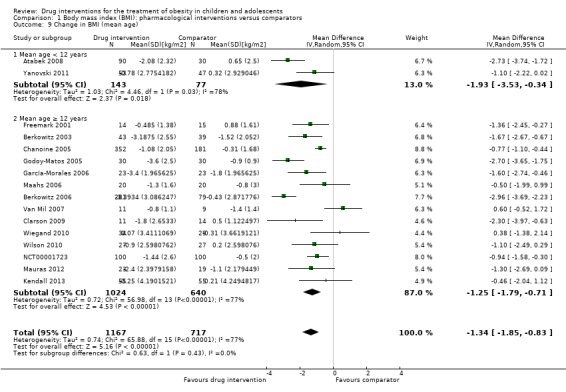
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 9 Change in BMI (mean age).
Only two interaction tests for subgroup differences indicated statistically significant differences.
Comparing dropout rates less than 20% showed an MD in BMI change of ‐1.1 kg/m2 (95% CI ‐1.8 to ‐0.4; 9 trials), with dropout rates 20% or greater showed an MD in BMI change of ‐1.4 kg/m2 (95% CI ‐2.3 to ‐0.5; 6 trials), and with unclear dropout rates showed an MD in BMI change of ‐2.7 kg/m2 (95% CI ‐3.7 to ‐1.7; 1 trial). The P value for interaction was 0.03 and heterogeneity was substantial (I2 = 71%).
Comparing middle‐income countries with high‐income countries showed an MD in BMI change of ‐2.4 kg/m2 (95% CI ‐3.1 to ‐1.7; 3 trials) versus ‐1.1 kg/m2 (95% CI ‐1.6 to ‐0.6; 13 trials). The P value for interaction was 0.004 and heterogeneity was considerable (I2 = 88%).
For the outcome measure change in weight, only drug type could be used for a subgroup analysis and the interaction test for subgroup differences was not statistically significant (P = 0.52, I2 = 0%).
We also explored the effects of participant sex on the BMI point estimate, using a meta‐regression model in CMA. The proportion of boys at follow‐up (or baseline if not reported) in each study was selected as a covariate. We found that the coefficient of determination (r2) from this model was zero. The 95% CI for the meta‐regression slope was extremely wide either side of zero (‐6.3 to 4.1). We noted that some study authors only reported the percentage of boys and girls in the total sample at baseline and not at follow‐up.
Sensitivity analyses
Table 3 shows the sensitivity analyses on BMI change. Our first analysis removed the trials which only reported pre‐ and post‐BMI (not change scores) (Kendall 2013; Wiegand 2010), and hence required the use of a correlation coefficient of 0.78 (Bayer 2011) to predict the point estimates. This made very little difference in the point estimate. Only two trials had larger sample sizes (Berkowitz 2006; Chanoine 2005); however, when we removed these trials from the meta‐analysis the point estimate did not change. Furthermore, all trials in the meta‐analysis were published and were in English; hence, we could not perform a sensitivity analysis on these criteria. However, we performed a sensitivity analysis with allocation bias, blinding bias (participant and trial personnel, and assessor) and attrition bias by removing the high risk or unclear risk trials, and did not find substantial differences. This was also the case when we removed trials with higher drug dose and 12 months' follow‐up, as well as when we removed the trials with an active lifestyle intervention.
2. Sensitivity analyses: BMI.
| Trials with data on mean change only | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐ 1.5 (‐2.0 to ‐0.9) favouring drug intervention |
| Trials with concealment of allocation | |
| Number of trials | 12 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.8 to ‐0.7) favouring drug interventions |
| Trials with blinding of participants/personnel | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.9 to ‐0.7) favouring drug interventions |
| Trials with blinding of outcome assessors | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.9 to ‐0.7) favouring drug interventions |
| Trials without large sample size trials | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.8 to ‐0.7) favouring drug interventions |
| Trials with trials with 6 months' follow‐up only | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐1.2 (‐1.7 to ‐0.7) favouring drug interventions |
| Trials without trials with higher drug dose | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐1.2 (‐1.7 to ‐0.7) favouring drug interventions |
| Trials with trials with a high dose/active lifestyle intervention | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.9 to ‐0.7) favouring drug interventions |
| Trials without trials with high attrition | |
| Number of trials | 13 |
| Point estimate (95% CI) (kg/m2) | ‐1.4 (‐2.0 to ‐0.8) favouring drug interventions |
BMI: body mass index; CI: confidence interval.
We performed similar analyses for weight change (Table 4). We removed trials which did not report change in weight (Atabek 2008; Kendall 2013; Maahs 2006), and this resulted in a slightly greater reduction in the point estimate. We used the same correlation coefficient to calculate the mean change in weight (in the intervention and comparator groups) as we did for the BMI outcome (r = 0.78), although we were able to calculate an exact correlation coefficient in one trial (Maahs 2006) by using their reported BMI at baseline, follow‐up and change score (r = 0.975). Reductions in point estimates increased slightly when we removed trials with blinding (participant and trial personnel, and assessor) and attrition bias. This also occurred when we restricted the analysis to trials which also included a high‐dose behaviour change intervention. In the sensitivity analyses where we removed trials with high allocation concealment bias, high drug dose, large sample size or follow‐up greater than six months, point estimate reductions were slightly less than in the original analysis.
3. Sensitivity analyses: weight.
| Trials with data on mean change only | |
| Number of trials | 8 |
| Point estimate (95% CI) (kg) | ‐ 4.1 (‐6.3 to ‐1.8) favouring drug intervention |
| Trials with concealment of allocation | |
| Number of trials | 9 |
| Point estimate (95% CI) (kg) | ‐3.5 (‐5.8 to ‐1.2) favouring drug interventions |
| Trials with blinding of participants/personnel | |
| Number of trials | 7 |
| Point estimate (95% CI) (kg) | ‐4.2 (‐6.8 to ‐1.5) favouring drug interventions |
| Trials with blinding of outcome assessors | |
| Number of trials | 7 |
| Point estimate (95% CI) (kg) | ‐4.2 (‐6.8 to ‐1.5) favouring drug interventions |
| Trials without large sample size trials | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg) | ‐3.4 (‐5.2 to ‐1.6) favouring drug interventions |
| Trials with 6 months' follow‐up only | |
| Number of trials | 9 |
| Point estimate (95% CI) (kg) | ‐3.5 (‐5.6 to ‐1.4) favouring drug interventions |
| Trials without trials with higher drug dose | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg) | ‐3.4 (‐5.2 to ‐1.6) favouring drug interventions |
| Trials with trials with a high dose/active lifestyle intervention | |
| Number of trials | 6 |
| Point estimate (95% CI) (kg) | ‐4.3 (‐6.5 to ‐2.2) favouring drug interventions |
| Trials without trials with high attrition | |
| Number of trials | 9 |
| Point estimate (95% CI) (kg) | ‐4.4 (‐6.6 to ‐2.2) favouring drug interventions |
CI: confidence interval.
Assessment of reporting bias
We drew a funnel plot in CMA version 3 and Review Manager 5 for change in BMI as there was a sufficient number of trials (16) (Figure 4). The Egger's regression intercept was ‐0.75 (95% CI ‐3.2 to 1.7; P = 0.52); this suggests there was no evidence of reporting bias.
4.
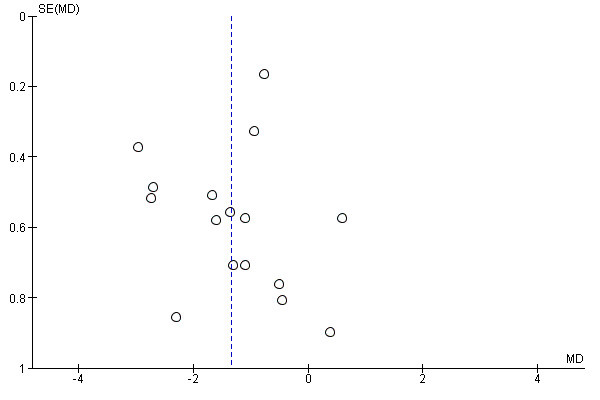
Funnel plot of comparison: 1 Body mass index (BMI): pharmacological interventions versus comparators, outcome: 1.1 Change in BMI (all trials) (kg/m2).
There was a similar finding when we drew a funnel plot for change in weight (11 trials) (Egger's regression intercept 1.7, 95% CI ‐3.5 to 6.8; P = 0.48) (Figure 5).
5.
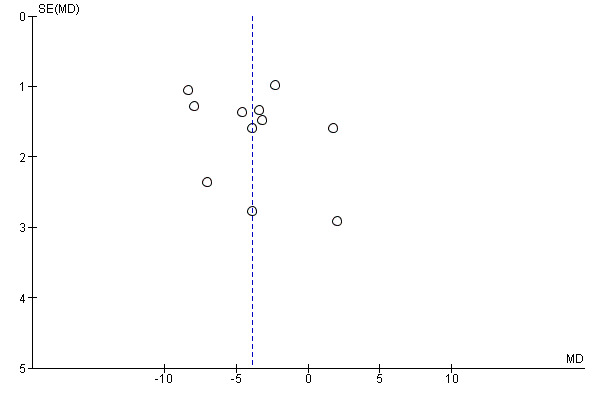
Funnel plot of comparison: 2 Weight: pharmacological interventions versus comparators, outcome: 2.1 Change in weight (all trials) (kg).
Ongoing trials
We found eight ongoing RCTs with four receiving metformin in the intervention group (EUCTR2010‐023061‐21; NCT00889876; NCT01677923; NCT02274948), two receiving topiramate (NCT01859013; NCT02273804), and two receiving exenatide (EUCTR2015‐001628‐45‐SE; NCT02496611). Three trials, originally identified as ongoing, have been moved into the 'awaiting classification' section because the trial has been completed but no results are available on the clinical trial website or through a publication (ISRCTN08063839; NCT00934570; NCT00940628). In addition, one trial which was originally classified as ongoing was moved to the 'awaiting classification' section because during the final stages of conducting the review we identified a new publication (via the MEDLINE email alert service), which included results from 18‐month follow‐up (van der Aa 2016, see NCT01487993 for a summary of the results). Results from this trial will be incorporated in the next update of the review. For two trials, we were unable to locate the source (Golebiowska 1981; Linquette 1971). In addition, we identified one conference abstract (Pastor 2014a, see EUCTR2010‐023061‐21) and one conference poster (Smetanina 2015). We attempted to contact both authors but only received a reply from Smetanina 2015, who confirmed the trial had been completed but these data were still being analysed.
Discussion
Summary of main results
We included 21 published RCTs and eight ongoing RCTs in this review. The included trials evaluated metformin (11 trials), sibutramine (six trials), orlistat (four trials), and one trial arm investigating the combination of metformin plus fluoxetine. The ongoing trials included four metformin, two topiramate and two exenatide trials. There were 2484 participants in the included trials, 1478 participants were randomised to drug intervention groups and 904 to comparator groups. All trials but three used a placebo in the comparator group. Two trials had a cross‐over design while the remaining 19 trials were parallel RCTs. The length of the intervention period ranged from 12 weeks to 48 weeks, and the length of follow‐up from baseline ranged from six months to 100 weeks. Overall there were small reductions in BMI (MD ‐1.3, 95% CI ‐1.9 to ‐0.8) and bodyweight change (MD ‐3.9 kg. 95% CI ‐5.9 to ‐1.9) in favour of the drug interventions. Five trials reported serious adverse events (24/878 (2.7%) participants in the intervention groups versus 8/469 (1.7%) participants in the comparator groups; RR 1.43, 95% CI 0.63 to 3.25; 1347 participants; low certainty evidence). A total of 52/1043 (5.0%) participants in the intervention groups versus 17/621 (2.7%) in the comparator groups discontinued the trial because of adverse events (RR 1.45, 95% CI 0.83 to 2.52; 10 trials; 1664 participants; low certainty evidence). The most common adverse events in orlistat and metformin trials were gastrointestinal. Common adverse effects in sibutramine trials included tachycardia, constipation and hypertension. The fluoxetine trial reported dry mouth and loose stools. One trials reported health‐related quality of life showing no marked differences between intervention and comparator. No trial reported the participants' views of the intervention or socioeconomic effects. Only one trial reported on morbidity associated with the intervention where there were more gallstones after the orlistat treatment. Trial authors reported one suicide in one of the orlistat intervention groups. However, the trials were not sufficiently long to investigate all‐cause mortality reliably. No trial investigated drug treatment for overweight children.
Overall completeness and applicability of evidence
We faced problems in meta‐analysing BMI as some trials did not report the raw data we required; therefore, we had to try and obtain this from the trial authors. In addition, age and sex are usually taken into account when measuring the weight status of a child because they are growing. However, in this review, we only assessed changes in raw BMI because previous research has shown short‐term changes in adiposity are best represented by changes in raw BMI units compared to BMI z scores or BMI centiles (Cole 2005; Kakinami 2014). Furthermore, only 10 trials reported changes in BMI z scores; therefore, we thought it was more appropriate to only meta‐analyse raw BMI, then this change could be converted into change in BMI z score (using the desired growth reference) ‐ which we have done in the conclusion section of this review.
All 21 trials measured adverse events; however, some trials reported the total number of participants who experienced at least one adverse event whilst others only reported the number of specific adverse events. Hence, we also had to attempt to obtain this information from the trial authors. Of the six trials which measured behaviour change, only two trials reported the results at follow‐up. Only two trials reported health‐related quality of life and they used different methods. Hence, more trials are needed to investigate how drugs used to treat obesity affect the participants' health‐related quality of life. No trials reported differences in participant views or socioeconomic effects.
Quality of the evidence
Based on the GRADE criteria, we rated the outcomes BMI, body weight, all‐cause mortality and adverse events as low. We downgraded the levels of evidence because of potential other risk of bias or reporting bias, inconsistency and imprecision. We rated health‐related quality of life and morbidity as very low certainty evidence, mainly because of the small number of participants, one trial only and imprecision.
Potential biases in the review process
We decided to perform unplanned subgroup analyses looking at funding and country as there were enough trials to divide them into groups. We were unable to analyse length of follow‐up, impact of maintenance periods and type of control group in the subgroup analyses as there were too few trials or too much heterogeneity. The meta‐analyses for BMI and weight included trials which differed in follow‐up length, behavioural interventions and drug dose. However, when sensitivity analyses were performed, the changes in point estimates were small.
We did not restrict our search strategy to any date, hence we have sifted through trials ranging back to the 1960s. However, we did not undertake any searches of the grey literature. We had some correspondence with most of trial authors, and some have supplied us with additional information including raw BMI data. Only seven trials gave clinical trial identifiers or protocols available; hence, it was difficult to assess whether reporting bias occurred. However, from the trials where a protocol/clinical trial entry was available, there was little evidence to suggest a source of reporting bias. We excluded a large number of trials because of follow‐up times less than six months, especially older trials, and this may have impacted on the overall findings from this review. In addition, we had difficulties calculating the number of adverse events in each trial due to different reporting metrics; for example, some trials reported the total number of adverse events in each group whilst others reported the total number of participants who suffered at least one adverse event. Despite our attempts to contact authors for additional information, we were still unable to meta‐analyse most of these findings.
Agreements and disagreements with other studies or reviews
Since the previous review (Oude Luttikhuis 2009), we identified one new orlistat trial; however, the reduction in BMI was similar to what was found previously. The reduction in BMI for the sibutramine trials found in this review was smaller than the change reported in the previous review; however, these data still favour the intervention. This difference is likely to be due to the inclusion of three extra sibutramine trials in the meta‐analysis. The previous review did not include a meta‐analysis of metformin trials.
Another review and meta‐analysis of the effect of orlistat and sibutramine on adolescent weight loss derived a difference in BMI of ‐2.3 kg/m2 for sibutramine (95% CI ‐2.9 to ‐1.8; 5 trials; 770 participants) and ‐1.7 kg/m2 for orlistat (95% CI ‐3.5 to 0.2; 3 trials; 621 participants) (Czernichow 2010). These point estimates reductions are greater than the ones derived in our review. However, the orlistat point estimate in the Czernichow 2010 review includes the Ozkan 2004 trial with a large BMI weight reduction which we excluded from the meta‐analysis in our review for not having a common follow‐up time across participants. In addition, the sibutramine analysis included data from a secondary analysis of white and African‐American participants from Berkowitz 2003 which may explain why the point estimate was different. In an earlier review, there was an MD of ‐0.7 kg/m2 (95% CI ‐1.2 to ‐0.3) in orlistat participants compared to placebo, which is consistent with our findings (McGovern 2008). There was a reduction of ‐2.4 kg/m2 (95% CI ‐3.1 to ‐1.8) in sibutramine trials, which is higher than the reduction we found (McGovern 2008). There were similar findings also found in another meta‐analysis where the reduction for sibutramine was an MD of ‐2.2 kg/m2 (95% CI ‐2.8 to ‐1.6; 4 trials; 686 participants) and the reduction for orlistat was an MD of ‐0.8 (95% CI ‐1.2 to ‐0.5; 2 trials; 573 participants) (Viner 2010).
In metformin trials, McDonagh 2014 determined an effect size of an MD of ‐1.2 kg/m2 (95% CI ‐1.6 to ‐0.7; 13 trials), which is similar to the point estimate found in this review. In addition, Bouza 2012 found a reduction of a MD of ‐1.2 kg/m2 (95% CI ‐1.8 to ‐0.5; 7 trials), in favour of metformin. An earlier review found a reduction of ‐1.4 kg/m2 (95% CI ‐2 to ‐0.8; 5 trials; 320 participants) in metformin trials (Park 2009); however, the review only included five trials and we excluded one of the trials in this review (Love‐Osborne 2008).
Overall findings from meta‐analyses of metformin, sibutramine and orlistat trials are similar to the ones presented in this review, and reasons for any differences are likely to derive from different inclusion criteria and our inclusion of more recent trials.
Authors' conclusions
Implications for practice.
This systematic review highlights the paucity of both the availability of reliable pharmacotherapy options for the treatment of obese children and adolescents, and the clinical trial evidence to support efficacy and safety. Trial quality and reporting overall was poor, with high dropout and discontinuation rates. Many of the trials assessed the efficacy of drugs which have now been withdrawn (sibutramine) or are not recommended for obesity treatment (metformin) in many countries.
In this review, we found an overall reduction in body mass index (BMI) of 1.3 kg/m2 in favour of the drug interventions. Using the International Obesity Task Force (IOTF) BMI cut‐offs for overweight and obesity (Cole 2012), a 12‐year‐old boy would have a cut‐off of 21.2 kg/m2 for being overweight, 26.02 kg/m2 for being obese and 31.21 kg/m2 for being morbidly obesity. Therefore, it would be possible for a 12‐year‐old boy who reduces his BMI by 1.3 kg/m2 to move down a weight status category ‐ but only if they happen to lie just above the cut‐off points. This is also similar for girls and older children. In terms of a standardised mean difference (SMD), the reduction in BMI found in this review would equate to a reduction of 0.28 between‐individuals standard deviation scores.
Whilst this finding suggests that drug interventions can result in a small BMI and weight reduction over the short term, it is not known whether this is:
sustainable over the longer term, which is an important consideration given evidence from the pharmacological management of adult obesity demonstrating a need for continued medication to maintain weight loss (Yanovski 2014), that is, drug withdrawal is followed by weight regain, which occurred in Rezvanian 2010, Van Mil 2007, and Wilson 2010 during the drug‐free follow‐up.
has any impact on existing or future clinical risk factors or disease. Additionally, though all trials reported adverse events, quantitative data were only available in the minority of the included trials. This is particularly important as none of the included trials collected data on participants' views.
Implications for research.
As new pharmacotherapies for the treatment of adult obesity become available (phentermine plus topiramate extended release; liraglutide 3.0 mg; bupropion plus naltrexone; lorcaserin), there may be a demand for an evaluation of their efficacy within an obese paediatric population. The requirement of regulatory authorities (US Food and Drug Administration (FDA) and European Medicines Agency (EMA)) for trials of all new medications to be used in children and adolescents should drive more and better trials. Hence, any future trials should ensure they are evaluated over the longer term (i.e. longer than one year) and collect data on cardiovascular and metabolic parameters, morbidities, health‐related quality of life, social and psychological well‐being, diet and physical activity behaviours, participant views and socioeconomic effects. It is also important that new trials' protocols reduce all possible sources of bias and provide accurate interpretation of findings, by ensuring power calculations and intention‐to‐treat analyses are described and conducted, and robust sequence allocation, allocation concealment methods and blinding measures are used and comprehensively described. All new trial protocols should also be registered and published to ensure reporting bias can be assessed. There should also be standardisation in reporting to ensure all trials report a raw BMI score and adverse events per participant. As evidence from adult weight management indicates the intensity of adjunctive lifestyle interventions can impact on weight loss and associated outcomes, future trials should aim to ensure they maximise and adequately report any concomitant behaviour changing programme. Participant retention is also an issue that needs addressing with improved and novel mechanisms to reduce dropout rates and ensure treatment concordance. Since overweight and obesity is developing at an increasingly early age, future evaluation and trials may need to consider recruiting young, prepubertal participants in whom clearly high levels of safety will need to be established.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2020 | Amended | Clarification message added to the Declarations of interest statement about the review's compliance with the Cochrane Commercial Sponsorship Policy. |
History
Review first published: Issue 11, 2016
| Date | Event | Description |
|---|---|---|
| 1 September 2016 | New search has been performed | This is an update of the former Cochrane review 'Interventions for treating obesity in children and adolescents'. |
| 1 September 2016 | New citation required and conclusions have changed | Given the rapid growth in the treatment of child and adolescent obesity, we have split the original review ('Interventions for treating obesity in children and adolescents') into six separate reviews, with a specific intervention and age focus. (1) Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in adolescents aged 12 to 17 years. (2) Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in schoolchildren from the age of 6 to 11 years. (3) Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. (4) Drug interventions for the treatment of obesity in children and adolescents. (5) Parent‐only interventions for childhood overweight or obesity in children aged 5 to 11 years. (6) Surgery for the treatment of obesity in children and adolescents. |
Notes
Portions of the methods sections, the appendices, additional tables and figures 1 to 3 of this review are based on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
We would like to thank Liane Azevedo (Teesside University), Leanne Mohan (Teesside University), Sarah Smith (Durham University), Katherine Roberts (Public Health England) and Giulia Mainardi (University of Sao Paulo) for their assistance with the data extractions. Also, we would like to thank all of the study authors who provided additional information about their trials, and we also acknowledge the editorial contributions by Gudrun Paletta (Cochrane Metabolic and Endocrine Disorders Group).
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies) |
|
Part I: Obesity 1. MESH DESCRIPTOR Obesity 2. MESH DESCRIPTOR Obesity, Morbid 3. MESH DESCRIPTOR Obesity, Abdominal 4. MESH DESCRIPTOR Pediatric Obesity 5. MESH DESCRIPTOR Overweight 6. MESH DESCRIPTOR Weight Loss 7. (adipos* or obes*):TI,AB,KY 8. (overweight* or over weight*):TI,AB,KY 9. (weight adj2 (reduc* or los* or control* or gain*)):TI,AB,KY 10. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 Part II: Anti‐obesity drugs 11. MESH DESCRIPTOR Anti‐Obesity Agents 12. MESH DESCRIPTOR Appetite Depressants 13. ((anti obes* or antiobes* or weight loss) adj3 (agent* or drug* or medicine* or pharmac*)):TI,AB,KY 14. (appetite adj3 (suppress* or depress*)):TI,AB,KY 15. ((anorexi* or anorectic*) adj3 (agent* or drug*)):TI,AB,KY 16. anorectics:TI,AB,KY 17. metformin*:TI,AB,KY 18. exenatide*:TI,AB,KY 19. liraglutid*:TI,AB,KY 20. dulaglutid*:TI,AB,KY 21. albiglutid*:TI,AB,KY 22. taspoglutid*:TI,AB,KY 23. lixisenatid*:TI,AB,KY 24. semaglutid*:TI,AB,KY 25. orlistat*:TI,AB,KY 26. cetilistat*:TI,AB,KY 27. sibutramin*:TI,AB,KY 28. fluoxetin*:TI,AB,KY 29. rimonabant*:TI,AB,KY 30. lorcaserin*:TI,AB,KY 31. benzphetamin*:TI,AB,KY 32. diethylpropion*:TI,AB,KY 33. phendimetrazin*:TI,AB,KY 34. mazindol*:TI,AB,KY 35. (phentermin* or chlorphentermin* or mephentermin*):TI,AB,KY 36. (phentermin* adj3 topiramat*):TI,AB,KY 37. (bupropion* adj3 naltrexon*):TI,AB,KY 38. (bupropion* adj3 zonisamid*):TI,AB,KY 39. beloranib*:TI,AB,KY 40. velneperit*:TI,AB,KY 41. tesofensin*:TI,AB,KY 42. #11 or #12 or #13 or #14 or #15 or#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 Part III: Part I + Part II 43. #10 and #42 44. MESH DESCRIPTOR Obesity WITH QUALIFIERS DT 45. MESH DESCRIPTOR Obesity, Morbid WITH QUALIFIERS DT 46. MESH DESCRIPTOR Weight Loss WITH QUALIFIERS DT 47. MESH DESCRIPTOR Overweight WITH QUALIFIERS DT 48. #43 or #44 or #45 or #46 or #47 Part IV: Population 49. MESH DESCRIPTOR Adolescent 50. MESH DESCRIPTOR Child 51. MESH DESCRIPTOR Pediatrics 52. minors:TI,AB,KY 53. (boy or boys or boyhood):TI,AB,KY 54. girl*:TI,AB,KY 55. (kid or kids):TI,AB,KY 56. (child* or schoolchild*):TI,AB,KY 57. adolescen*:TI,AB,KY 58. juvenil*:TI,AB,KY 59. youth*:TI,AB,KY 60. (teen* or preteen*):TI,AB,KY 61. (underage* or under age*):TI,AB,KY 62. pubescen*:TI,AB,KY 63. p?ediatric*:TI,AB,KY 64. #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 Part V: Part III AND IV 65. #48 and #64 66. MESH DESCRIPTOR Pediatric Obesity WITH QUALIFIERS DT 67. #65 or #66 |
| MEDLINE (OvidSP) |
|
Part I: Obesity 1 Obesity/ 2 Obesity, Morbid/ 3 Obesity, Abdominal/ 4 Pediatric Obesity/ 5 Overweight/ 6 Weight Loss/ 7 (adipos* or obes*).tw. 8 (overweight* or over weight*).tw. 9 (weight adj2 (reduc* or los* or control* or gain*)).tw. 10 or/1‐9 Part II: Anti‐obesity drugs 11 Anti‐Obesity Agents/ 12 Appetite Depressants/ 13 ((anti obes* or antiobes* or weight loss) adj3 (agent* or drug* or medicine* or pharmac*)).tw. 14 (appetite adj3 (suppress* or depress*)).mp. 15 ((anorexi* or anorectic*) adj (agent* or drug*)).tw. 16 anorectics.tw. 17 metformin*.mp. 18 exenatide*.mp. 19 liraglutid*.mp. 20 dulaglutid*.mp. 21 albiglutid*.mp. 22 taspoglutid*.mp. 23 lixisenatid*.mp. 24 semaglutid*.mp. 25 orlistat*.mp. 26 cetilistat*.mp. 27 sibutramin*.mp. 28 fluoxetin*.mp. 29 rimonabant*.mp. 30 lorcaserin*.mp. 31 benzphetamin*.mp. 32 diethylpropion*.mp. 33 phendimetrazin*.mp. 34 mazindol*.mp. 35 (phentermin* or chlorphentermin* or mephentermin*).mp. 36 ((phentermin* adj3 topiramat*) or phentermine?topiramat*).mp. 37 ((bupropion* adj3 naltrexon*) or bupropion?naltrexon*).mp. 38 ((bupropion* adj3 zonisamid*) or bupropion?zonisamid*).mp. 39 beloranib*.mp. 40 velneperit*.mp. 41 tesofensin*.mp. 42 or/11‐41 Part III: Part I + Part II and additional MeSH/subheading combination 43 10 and 42 44 Obesity/dt [drug therapy] 45 Obesity, Morbid/dt [drug therapy] 46 Weight Loss/dt [drug therapy] 47 Overweight/dt [drug therapy] 48 or/43‐47 Part IV: Population [based onLeclercq 2013] 49 Adolescent/ 50 Child/ 51 Pediatrics/ 52 minors.tw. 53 (boy or boys or boyhood).tw. 54 girl*.tw. 55 (kid or kids).tw. 56 (child* or schoolchild*).tw. 57 adolescen*.tw. 58 juvenil*.tw. 59 youth*.tw. 60 (teen* or preteen*).tw. 61 (underage* or under age*).tw. 62 pubescen*.tw. 63 p?ediatric*.tw. 64 or/49‐63 Part V: Part III AND IV and additional MeSH/subheading combination 65 48 and 64 66 Pediatric Obesity/dt 67 65 or 66 Part VI: Study filter [Cochrane Handbook 2008 RCT filter ‐ sensitivity max. version] 68 randomized controlled trial.pt. 69 controlled clinical trial.pt. 70 randomi?ed.ab. 71 placebo.ab. 72 drug therapy.fs. 73 randomly.ab. 74 trial.ab. 75 groups.ab. 76 or/68‐75 77 exp animals/ not humans/ 78 76 not 77 Part VII: Part V + Part VI 79 67 and 78 |
| Embase (OvidSP) |
|
Part I: Obesity 1 obesity/ 2 morbid obesity/ 3 abdominal obesity/ 4 childhood obesity/ 5 weight reduction/ 6 (adipos* or obes*).tw. 7 (overweight* or over weight*).tw. 8 (weight adj2 (reduc* or los* or control* or gain*)).tw. 9 or/1‐8 Part II: Anti‐obesity drugs 10 antiobesity agent/ 11 anorexigenic agent/ 12 ((anti obes* or antiobes* or weight loss) adj3 (agent* or drug* or medicine* or pharmac*)).tw. 13 (appetite adj3 (suppress* or depress*)).tw. 14 ((anorexi* or anorectic*) adj (agent* or drug*)).tw. 15 anorectics.tw. 16 metformin*.mp. 17 exenatide*.mp. 18 liraglutid*.mp. 19 dulaglutid*.mp. 20 albiglutid*.mp. 21 taspoglutid*.mp. 22 lixisenatid*.mp. 23 semaglutid*.mp. 24 orlistat*.mp. 25 cetilistat*.mp. 26 sibutramin*.mp. 27 fluoxetin*.mp. 28 rimonabant*.mp. 29 lorcaserin*.mp. 30 benzphetamin*.mp. 31 diethylpropion*.mp. 32 phendimetrazin*.mp. 33 mazindol*.mp. 34 (phentermin* or chlorphentermin* or mephentermin*).mp. 35 ((phentermin* adj3 topiramat*) or phentermine?topiramat*).mp. 36 ((bupropion* adj3 naltrexon*) or bupropion?naltrexon*).mp. 37 ((bupropion* adj3 zonisamid*) or bupropion?zonisamid*).mp. 38 beloranib*.mp. 39 velneperit*.mp. 40 tesofensin*.mp. 41 or/10‐40 Part III: Part I + Part II and additional MeSH/subheading combination 42 9 and 41 43 obesity/dt [drug therapy] 44 morbid obesity/dt [drug therapy] 45 weight reduction/dt [drug therapy] 46 or/42‐45 Part IV: Population [adapted fromLeclercq 2013] 47 juvenile/ 48 adolescent/ 49 child/ 50 preschool child/ 51 schoolchild/ 52 pediatrics/ 53 minors.tw. 54 (boy or boys or boyhood).tw. 55 girl*.tw. 56 (kid or kids).tw. 57 (child* or schoolchild*).tw. 58 adolescen*.tw. 59 juvenil*.tw. 60 youth*.tw. 61 (teen* or preteen*).tw. 62 (underage* or under age*).tw. 63 pubescen*.tw. 64 p?ediatric*.tw. 65 or/47‐64 Part V: Part III AND IV and additional MeSH/subheading combination 66 46 and 65 67 childhood obesity/dt 68 66 or 67 Part VI: Study filter [Wong 2006afilter – BS version] 69 random*.tw. or clinical trial*.mp. or exp health care quality/ Part VII: Part V + Part VI 70 68 and 69 71 limit 70 to embase |
| LILACS (IAHx) |
| ((MH:"Obesity" OR MH:"Obesity, Morbid" OR MH:"Obesity, Abdominal" OR MH:"Pediatric Obesity" OR MH:"Overweight" OR MH:"Weight Loss" OR adipos$ OR obes$ OR overweight$ OR "over weight" OR sobrepes$ OR "exceso de peso" OR "excesso de peso" OR "weight reduction" OR "weight loss" OR "weight control") AND (MH:"Obesity/drug therapy" OR MH:"Obesity, Morbid/drug therapy" OR MH:"Overweight/drug therapy" OR MH:"Weight Loss/drug therapy" OR MH:"Anti‐Obesity Agents" OR MH:"Appetite Depressants" OR "farmacos antiobesidad" OR "farmacos antiobesidade" OR "depresores del apetito" OR "depressores do apetite" OR metformin$ OR exenatide$ OR liraglutid$ OR dulaglutid$ OR albiglutid$ OR taspoglutid$ OR lixisenatid$ OR semaglutid$ OR orlistat$ OR cetilistat$ OR sibutramin$ OR fluoxetin$ OR rimonabant$ OR lorcaserin$ OR benzphetamin$ OR diethylpropion$ OR phendimetrazin$ OR mazindol$ OR phentermin$ or chlorphentermin$ or mephentermin$ OR (phentermin$ AND topiramat$) OR (bupropion$ AND (naltrexon$ OR zonisamid$)) OR beloranib$ OR velneperit$ OR tesofensin$) AND (MH:"Adolescent" OR MH:"Child" OR MH:"Pediatrics" OR minors OR boy OR boys OR girl$ OR kid OR kids OR child$ OR schoolchild$ OR escolar$ OR adolescen$ OR preadolescen$ OR juvenil$ OR juventud$ OR youth$ OR teen$ OR preteen$ OR underage$ OR pubescen$ OR paediatri$ OR pediatri$ OR joven$ OR jovem$ OR niños OR niñas OR crianca$ OR menin$ OR "menor de edad" OR "menores de edad" OR "menor de idade" OR "menores de idade") OR MH:"Pediatric Obesity/drug therapy") + Controlled Clinical Trial |
| PubMed (only subsets not available on Ovid) |
|
#1 Part I: Obesity adipos*[tw] OR obes*[tw] OR overweight*[tw] OR over weight*[tw] OR weight reduc*[tw] OR weight los*[tw] OR weight control*[tw] OR weight gain*[tw] #2 Part II: Antiobesity drugs anti obesity agent*[tw] OR antiobesity agent*[tw] OR anti obesity drug*[tw] OR antiobesity drug*[tw] OR weight loss agent[tw] OR weight loss drug[tw] OR appetite suppress*[tw] OR appetite depress*[tw] OR anorexigenic agent*[tw] OR anorexigenic drug*[tw] OR anorectics[tw] OR metformin*[tw] OR exenatide*[tw] OR liraglutid*[tw] OR dulaglutid*[tw] OR albiglutid*[tw] OR taspoglutid*[tw] OR lixisenatid*[tw] OR semaglutid*[tw] OR orlistat*[tw] OR cetilistat*[tw] OR sibutramin*[tw] OR fluoxetin*[tw] OR rimonabant*[tw] OR lorcaserin*[tw] OR benzphetamin*[tw] OR diethylpropion*[tw] OR phendimetrazin*[tw] OR mazindol*[tw] OR phentermin*[tw] OR chlorphentermin*[tw] OR mephentermin*[tw] OR topiramat*[tw] OR bupropion*[tw] OR naltrexon*[tw] OR zonisamid*[tw] OR beloranib*[tw] OR velneperit*[tw] OR tesofensin*[tw] #3 Part III: Part I + Part II #1 AND #2 #4 Part IV: Population minors[tw] OR boy[tw] OR boys[tw] OR boyhood[tw] OR girl*[tw] OR kid[tw] OR kids[tw] OR child*[tw] OR schoolchild*[tw] OR adolescen*[tw] OR juvenil*[tw] OR youth*[tw] OR teen*[tw] OR preteen*[tw] OR underage*[tw] OR under age*[tw] OR pubescen*[tw] OR paediatric*[tw] OR pediatric*[tw] #5 Part V: Part III AND IV #3 AND #4 #6 Part VI: Limiting to subsets not available on Ovid #5 not medline[sb] not pmcbook |
| ICTRP Search Portal (Standard search) |
| obes* AND child* OR obes* AND schoolchild* OR obes* AND adolesc* OR obes* AND young* OR obes* AND pediatric* OR obes* AND teen* OR obes* AND preteen* OR obes* AND juvenil* OR obes* AND minors OR obes* AND boy* OR obes* AND girl* OR obes* AND kids OR obes* AND youth* OR obes* AND underage* OR obes* AND pube* OR overweight* AND child* OR overweight* AND schoolchild* OR overweight* AND adolesc* OR overweight* AND young* OR overweight* AND pediatric* OR overweight* AND teen* OR overweight* AND preteen* OR overweight* AND juvenil* OR overweight* AND minors OR overweight* AND boy* OR overweight* AND girl* OR overweight* AND kids OR overweight* AND youth* OR overweight* AND underage* OR overweight* AND pube* |
| ClinicalTrials.gov (Expert search) |
| ( obese OR overweight OR obesity ) [DISEASE] AND ( drug or drugs OR agent OR agents OR appetite OR metformin OR exenatide OR liraglutide OR dulaglutide OR albiglutide OR taspoglutide OR lixisenatide OR semaglutide OR orlistat OR cetilistat OR sibutramine OR fluoxetine OR rimonabant OR lorcaserin OR benzphetamine OR diethylpropion OR phendimetrazine OR mazindol OR phentermine OR chlorphentermine OR mephentermine OR topiramate OR bupropion OR naltrexone OR zonisamide OR beloranib OR velneperit OR tesofensine ) [TREATMENT] AND INFLECT EXACT "Child" [AGE‐GROUP] |
Appendix 2. Description of interventions
| Trial | Intervention(s): drug component (route, frequency, total dose/day), behaviour changing component | Comparator(s): drug component (route, frequency, total dose/day), behaviour changing component |
| Atabek 2008 |
Metformin: oral, twice daily, 500 mg x 2 (1 g)/d, 6 months Diet and physical activity advice: individual consultation sessions with a nutritionist, completed food diary at beginning and end of trial, advised to perform 30 min of aerobic physical activity per day, 6 months |
Placebo: oral, twice daily, 2 tablets/d, 6 months Diet and physical activity advice: same as the intervention group |
| Berkowitz 2003 |
Sibutramine: oral, 1 dose per day, placebo (week 1) 5 mg/d sibutramine (week 2) 10 mg/d (weeks 3 to 6) 15 mg/d (week 7 to month 6), length = 6 months (plus an open‐label phase for additional 6 months) Behavioural programme: in phase 1 (drug‐placebo phase) participants attended 13 weekly group sessions while in phase 2 (drug, open label) group sessions were held biweekly then monthly. Parents met separately from participants. Instructed to consume 1200 kcal/d to 1500 kcal/d and to engage in 120 min of walking or similar activity per week. Eating and activity logs kept daily. Length = 12 months |
Placebo: oral, 1 dose per day, (months 1 to 6), 6 months Behavioural programme: same as intervention group |
| Berkowitz 2006 |
Sibutramine: oral, 1 dose per day, 10 mg/d (baseline to month 6), 15 mg/d from month 6 in participants who had not lost more than 10% of their initial BMI, 12 months Behavioural therapy programme: each individual centre implemented flexible lifestyle modification approaches that were specific to participants' needs. This included self‐monitoring of eating habits and physical activity, stress management, stimulus control, problem solving, contingency management, cognitive restructuring and social support. Participants were given counselling at each visit and nutritional counselling. Length = 12 months |
Placebo: oral, 1 dose per day, placebo (baseline to month 6), uptitrated after 6 months in participants who had not lost more than 10% of their initial BMI, 12 months Behavioural therapy programme: same as intervention group |
| Chanoine 2005 |
Orlistat: oral, dose 3 times per day, 120 mg x 3 (360 mg)/d, 1 year Behavioural therapy: participants were prescribed a nutritionally balanced, hypocaloric diet and at each trial visit the dietitian spoke about compliance. Behavioural modification involved techniques to limit calorie and fat intake, eating more slowly, avoiding snacks and avoiding overeating. Guidelines were given to encourage regular physical activity and reduce sedentary behaviour; compliance was monitored by a behavioural psychologist at each visit. Length = 54 weeks |
Placebo: oral, dose 3 times per day, 1 year Behavioural therapy: same as intervention group |
| Clarson 2009 |
Metformin: oral, 3 times daily, 500 mg x 3 (1.5 g), 6 months Behaviour changing intervention: monthly individual visits and 2 group sessions. Fitness specialist supervised participants in an individual 30‐min exercise sessions every 2 months. Diet advice and physical activity advice given. Progress monitored by weekly telephone calls and monthly visits. Length = 6 months |
No placebo (N/A) Behaviour changing intervention: same as intervention group |
| Franco 2014 |
Sibutramine: oral, once daily, 10 mg, 6 months Dietary guidance: the dietary guideline proposal was of a low‐calorie diet with restriction of 25% of the total recommended calories for a teenager |
Placebo: oral, once daily, 10 mg, 6 months Dietary guidance: same as intervention group |
| Freemark 2001 |
Metformin: oral, 2 doses per day, 500 mg x 2 (1g)/d, 6 months No behaviour changing intervention |
Placebo: oral, 2 doses per day, 6 months No behaviour changing intervention |
| Garcia‐Morales 2006 |
Sibutramine: oral, 1 dose per day, 10 mg/d, 6 months Diet + exercise: diet and exercise advice was tailored to each participant. Advice was given on recommended food portions and possible combinations, and all participants were advised to perform at least 30 min of aerobic physical activity per day. Each participant also attended individual consultation sessions with a registered paediatric nutritionist. A detailed food consumption questionnaire was completed at the beginning and end of trial medication period. Length = 6 months |
Placebo: oral, 1 dose per day, 6 months Diet + exercise: same as intervention group |
| Godoy‐Matos 2005 |
Sibutramine: oral, 1 dose per day, 10 mg/d, 6 months Hypocaloric diet + exercise: participants were given dietary counselling to achieve an energy deficit of 500 kcal/d at the start of the run‐in phase (no further visits after). Physical activity instructions were delivered by the attendant doctors in a brief written protocol aimed to obtain mainly aerobic moderate exercises for at least 30 min/d. A lifestyle intervention was not given during 6‐month trial |
Placebo: oral, 1 dose per day, 6 months Hypocaloric diet + exercise: same as intervention group |
| Kendall 2013 |
Metformin: oral, twice daily, 500 mg x 2 + 500 mg (1.5 g), 6 months Healthy lifestyle advice: participants provided with a standardised healthy lifestyle advice at the start in a 1‐to‐1 sessions, including a healthy diet advice sheet and increased levels of exercise (available upon request). A lifestyle intervention was not given during the 6‐month trial |
Placebo: oral, twice daily, 2 + 1 (3) tablets/d, 6 months Healthy lifestyle advice : same as intervention group |
| Maahs 2006 |
Orlistat: oral, 3 doses per day, 120 mg x 3 (360 mg)/d, 6 months Diet + exercise therapy: the goal caloric intake was calculated using the Harris‐Benedict equation with ambulating activity factor (500 calories was subtracted from the final number to obtain daily calorie level). Participants were instructed to increase activity using a paediatric activity pyramid and encouraged to exercise for at least 30 min, 3 times per week. Monthly follow‐up visits with a dietitian reinforced this advice. Log sheets and diet records were also completed. Length = 6 months |
Placebo: oral, 3 doses per day, 6 months Diet + exercise therapy: same as intervention group |
| Mauras 2012 |
Metformin: oral, twice daily, 500 mg or 1000 mg (dependent on age), 6 months Diet + exercise intervention: dietary counselling provided with recommended decrease of 250 calories/d to 500 calories/d. Intense follow‐up provided by dietitian. Participants given free membership to YMCA or gym. Encouraged to exercise at least 3 times per week for 30 min per sessions. Activity diary kept and pedometer worn. Length = 6 months |
No placebo Diet + exercise intervention: same as intervention group |
| NCT00001723 |
Orlistat: 120 mg 3 times daily for 6 months Behavioural weight loss programme: 12‐week intensive programme |
Placebo: 120 mg 3 times daily for 6 months Behavioural weight loss programme: same as intervention group |
| Ozkan 2004 |
Orlistat: oral, 3 doses per day, 120 mg x 3 (360 mg)/d, mean 11.7 months ‐ length of treatment was not consistent across participants Conventional treatment: the lifestyle modification programme included reducing daily calories. Was administered by a team comprising of a paediatric endocrinologist, paediatrician and a dietitian. Participants seen by dietitian monthly and in the outpatient clinic every 2 months. Length = between 6 and 17 months |
No placebo Conventional treatment: same as intervention group; length between 6 and 17 months |
| Prado 2012 |
Metformin: oral, once daily, 500 mg, 3 months Nutritional guide and exercise programme: according to pattern 1500 kcal/d. Exercise classes once per week and exercise guide to be practiced twice per week. Length = 3 months |
Placebo: oral, once daily, 3 months Nutritional guide and exercise programme: same as intervention group |
| Rezvanian 2010 |
Metformin: oral, once daily, 1500 mg/d, 12 weeks Healthy eating and physical activity advice: physical activity advice included reducing sedentary time and taking part in 30 min of enjoyable, moderate‐intensity physical activity per day. A registered dietitian conducted a nutrition education session with recommendations on diet such as increasing consumption of fruit and vegetables and not using hydrogenated fat |
Placebo: oral, once daily, 12 weeks Healthy eating and physical activity advice: same as intervention group |
|
Fluoxetine: oral, once daily, 20 mg/d, 12 weeks Healthy eating and physical activity advice: same as the other intervention groups | ||
|
Metformin + fluoxetine: oral, once daily, dosage not given, 12 weeks Healthy eating and physical activity advice: same as the other intervention groups | ||
| Srinivasan 2006 |
Metformin: oral, 2 doses per day, dose gradually built up to 1g x 2 (2 g)/d, 6 months "Standardised information on healthy eating and exercise": no further information given |
Placebo: oral, 2 doses per day, dose gradually built up to 1 g x 2 (2 g)/d, 6 months "Standardised information on healthy eating and exercise": same as intervention group |
| Van Mil 2007 |
Sibutramine: oral, once daily, 5 mg/d, 12 weeks Energy‐restricted diet and exercise plan: the energy prescription calculated from measured basal metabolic rate multiplied by an estimated physical activity level minus 500 kcal. Physical activity prescribed based on individual preferences and information obtained by physical activity questionnaire. It contained a daily bout of exercise of at least 30 min. Length = 12 weeks |
Placebo: oral, once daily, 5 mg/d, 12 weeks Energy‐restricted diet and exercise plan: same as intervention group |
| Wiegand 2010 |
Metformin: oral, twice daily, 2 x 500 mg (1 g)/d, 6 months Multiprofessional behaviour changing intervention: an interview was performed before randomisation to determine 1 to 3 individually chosen tasks (goals). Multiprofessional reinforcement sessions took place every 4 to 8 weeks. Regarding physical activity, participants and their families attended specialised sport classes (2 sport classes per week, 45 min each, was recommended) in addition to regular sport classes at school. Length = 6 months |
Placebo: oral, twice daily, 6 months Multiprofessional behaviour changing intervention: same as intervention group |
| Wilson 2010 |
Metformin: oral, 4 times daily, 4 x 500 mg (2 g)/d, 48 weeks Behaviour changing intervention: used the Weigh of Life LITE programme developed at Texas Children's Hospital, Houston. There were 10 individualised "intensive" sessions at weekly intervals and monthly follow‐up sessions for the reminder of the trial. Sessions led by trained health specialist and parent/guardians were invited. Length = 48 weeks |
Placebo: oral, 4 times daily, 48 weeks Behaviour changing intervention: same as intervention group |
| Yanovski 2011 |
Metformin: oral, twice daily, 2 x 1000 mg (2000 mg)/d, 6 months Dietitian‐administered weight‐reduction programme: each participant and parent/guardian met with a dietitian monthly, who promoted a reduced‐energy diet, increased physical activity and decreased inactivity. Participants trained to completed a 7‐day food diary which was used to prescribe a "traffic light" style 500 kcal/d deficit diet, and exercise was encouraged for 30 min/d, monitored by pedometers readings. Length = 6 months |
Placebo: oral, twice daily, 6 months Dietitian‐administered weight‐reduction programme: same as intervention group |
| "‐" denotes not reported. BMI: body mass index; /d: per day; kcal: kilocalories; min: minute; N/A: not applicable; YMCA: Young Men's Christian Association. | ||
Appendix 3. Baseline characteristics (I)
| Trial | Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) | Duration of obesity (mean years (SD)) |
| Atabek 2008 | I: metformin + diet and physical activity advice | 6 months (6 months) | Obese adolescents with hyperinsulinaemia | ‐ | Turkey | Hospital in/outpatient clinic/other based in University School of Medicine | ‐ | ‐ |
| C: placebo + diet and physical activity advice | ||||||||
| Berkowitz 2003 | I: behavioural programme + sibutramine | 6 months (6 months) | Obese adolescent boys and postmenarchal girls | March 1999 to August 2002 | USA | University of Pennsylvania School of Medicine | White 49, black 49, other 2 | ‐ |
| C: behavioural programme + placebo | White 62, black 33, other 5 | |||||||
| Berkowitz 2006 | I: behavioural therapy programme + sibutramine | 12 months (12 months) | Obese adolescents | July 2000 to February 2002 | USA | 33 weight loss clinics and outpatient clinic based in a University School of Medicine | White 56, African‐American 22, Hispanic or Mexican American 16, other 6 | ‐ |
| C: behavioural therapy programme + placebo | White 59, African‐American 19, Hispanic or Mexican‐American 14, other 9 | |||||||
| Chanoine 2005 | I: orlistat + diet + exercise + behavioural therapy | 54 weeks (54 weeks) | Obese adolescents | August 2000 to October 2002 | USA and Canada | 32 clinical centres | White 75, black 19, other 6 | ‐ |
| C: placebo + diet + exercise + behavioural therapy | White 78, black 14, other 8 | |||||||
| Clarson 2009 | I: metformin + lifestyle intervention | 6 months (6 months) | Obese adolescents with insulin resistance | Enrolled 2005 to 2007 | Canada | Participants assessed in community clinic and there were monthly visits to clinic during intervention. Intervention carried out in community ‐ at adolescent's home ‐ unclear where group sessions took place | ‐ | ‐ |
| C: lifestyle intervention only | ||||||||
| Franco 2014 | I: sibutramine + dietary guidance | 6 months (6 months) | Obese adolescents | ‐ | Brazil | Paediatric endocrinology outpatient clinic in childhood obesity group of the Instituto da Crianca do Hospital das Clinicas de Faculdade de Medicina de Universidade de Sao Paulo | ‐ | ‐ |
| C: placebo + dietary guidance | ||||||||
| Freemark 2001 | I: metformin | 6 months (6 months) | Obese adolescents with fasting hyperinsulinaemia and a family history of type 2 diabetes | ‐ | USA | Inpatient and outpatient clinic of a university | White 64, black 36 | ‐ |
| C: placebo | White 47, black 53 | |||||||
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 6 months (6 months) | Obese Mexican adolescents | August 2001 to August 2003 | Mexico | Outpatients attending the endocrinology department of the Federico Gomez Children's Hospital of Mexico | ‐ | ‐ |
| C: placebo + diet + exercise | ||||||||
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | 7 months (7 months) | Obese adolescents | January 2002 to April 2003 | Brazil | Regular clinical setting | ‐ | ‐ |
| C: placebo + hypocaloric diet + exercise | ||||||||
| Kendall 2013 | I: metformin + healthy lifestyle advice | 6 months (6 months) | Obese children and adolescents with hyperinsulinaemia or impaired fasting glucose or impaired glucose tolerance (or both) | ‐ | UK | UK paediatric endocrine centres | White 80, Asian 19, Afro‐Caribbean 11 | ‐ |
| C: placebo + healthy lifestyle advice | White 72, Asian 26, Afro‐Caribbean 1 | |||||||
| Maahs 2006 | I: orlistat + diet and exercise therapy | 26 weeks (26 weeks) | Obese adolescents | December 2002 to September 2003 | USA | General clinical research centre at University of New Mexico Hospital | Hispanic 60 | ‐ |
| C: placebo + diet and exercise therapy | Hispanic 65 | |||||||
| Mauras 2012 | I: metformin + diet/exercise intervention | 6 months (6 months) | Obese children with normal glucose tolerance but elevated hsCRP or fibrinogen concentrations (or both) | ‐ | USA | ‐ | White 51, African‐American 37, other 11 | Uncomplicated (exogenous) obesity for < 5 years |
| C: diet/exercise intervention | White 39, African‐American 42, other 19 | |||||||
| NCT00001723 | I: orlistat + behavioural weight loss programme | 6 months (6 months) | Obese children and adolescents with obesity‐related diseases | RCT began in 1999 and ended in 2008 | USA | National Institutes of Health Clinical Center | Non‐Hispanic black 63, non‐Hispanic white 37 |
‐ |
| C: placebo + behavioural weight loss programme | Non‐Hispanic black 60, non‐Hispanic whites 40 |
|||||||
| Ozkan 2004 | I: conventional treatment + orlistat | Intervention group was followed for 5 to 15 months (mean duration of treatment 11.7, SD 3.7 months) | Adolescents with severe exogenous obesity | ‐ | Turkey | Outpatient clinic | ‐ | ‐ |
| C: conventional treatment | Control group was followed for 6 to 17 months (mean 10.2, SD 3.7 months) | |||||||
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 3 months (6 months) | Obese female adolescents at risk of developing type 2 diabetes | June 2009 to July 2010 | Chile | Conducted at Center of Adolescent Health Serjoven | ‐ | ‐ |
| C: placebo + nutritional guide and exercise programme | ||||||||
| Rezvanian 2010 | I1: metformin + healthy eating and physical activity advice | 12 weeks (24 weeks) | Obese children and adolescents | ‐ | Iran | Pediatric Obesity and Metabolic Syndrome Research Clinic of the Pediatric Preventive Cardiology Department, Isfahan Cardiovascular Research Center | ‐ | ‐ |
| I2: fluoxetine + healthy eating and physical activity advice | ||||||||
| I3: metformin and fluoxetine + healthy eating and physical activity advice | ||||||||
| C: placebo + healthy eating and physical activity advice | ||||||||
| Srinivasan 2006 | I: metformin first then placebo + standardised information on healthy eating and exercise | 6 months (12 months) | Obese children and adolescents (aged 9 to 18) with suspected insulin resistance | ‐ | Australia | Outpatient clinic of a tertiary paediatric hospital (university teaching hospital) | 64% were from ethnic backgrounds with high prevalence of insulin resistance and the metabolic syndrome (e.g. Indian subcontinent, Pacific Islands), 25% were from a northern European background, and 11% were from a mixed background | ‐ |
| C: placebo first then metformin + standardised information on healthy eating and exercise | ||||||||
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | 12 weeks (24 weeks) | Obese adolescents | ‐ | The Netherlands | Outpatient clinic | ‐ | ‐ |
| C: placebo + energy‐restricted diet and exercise plan | ||||||||
| Wiegand 2010 | I: metformin + multiprofessional lifestyle intervention | 6 months (6 months) | Obese insulin‐resistant adolescents | May 2006 to December 2006 | Germany and Switzerland | Paediatric obesity centre | White 87, other 13 | ‐ |
| C: placebo + multiprofessional lifestyle intervention | White 92, other 9 | |||||||
| Wilson 2010 | I: metformin + lifestyle intervention programme | 52 weeks (100 weeks) | Obese adolescents | October 2003 to August 2007 | USA | 6 Glaser paediatric research centres | White 56, African‐American 21, Asian 8, other 15, Hispanic ethnicity 18 | ‐ |
| C: placebo + lifestyle intervention programme | White 71, African‐American 16, Asian 0, other 13, Hispanic ethnicity 29 | |||||||
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | 6 months (12 months) | Obese insulin‐resistant children | September 2000 to August 2008 | USA | Trial took place at the NIH clinical research centre | Non‐Hispanic white 42, Non‐Hispanic black 42, Hispanic white 11, other 5 | ‐ ‐ |
| C: placebo + dietitian‐administered weight‐reduction programme | Non‐Hispanic white 49, Non‐Hispanic black 38, Hispanic white 11, other 2 | |||||||
| "‐" denotes not reported. C: comparator; hsCRP: high sensitivity C‐reactive protein; I: intervention; RCT: randomised controlled trial; SD: standard deviation. | ||||||||
Appendix 4. Baseline characteristics (II)
| Trial | Intervention(s) and comparator(s) | Sex (female %) | Age (mean years (SD)) | HbA1c (mean % (SD)) | BMI (mean kg/m² (SD)) | Bodyweight (mean kg (SD)) | Comedications/cointerventions | Comorbidities |
| Atabek 2008 | I: metformin + diet and physical activity advice | 50 | 11.8 (2.8) | ‐ | 28.5 (3.4) | 67.16 (16.8) | Diet and physical activity advice. Individual consultation sessions with a registered paediatric nutritionist | All participants had hyperinsulinaemia |
| C: placebo + diet and physical activity advice | 50 | 11.6 (2.7) | ‐ | 28.0 (3.4) | 66.27 (16.9) | |||
| Berkowitz 2003 | I: behavioural programme + sibutramine | 72 | 14.1 (1.3) | ‐ | 37.5 (4.0) | 102 (14.7) | Behavioural therapy | ‐ |
| C: behavioural programme + placebo | 62 | 14.1 (1.2) | ‐ | 38.0 (3.6) | 105.3 (16.2) | |||
| Berkowitz 2006 | I: behavioural therapy programme + sibutramine | 66 | 13.6 (1.3) | ‐ | 35.9 (4.1) | 97.9 (14.7) | Behavioural therapy | 50.5% had dyslipidaemia, 1.4% had hypertension |
| C: behavioural therapy programme + placebo | 62 | 13.7 (1.3) | ‐ | 36.1 (3.8) | 97.8 (14.6) | 57.4% had dyslipidaemia, 2.3% had hypertension | ||
| Chanoine 2005 | I: orlistat + diet + exercise + behavioural therapy | 65 | 13.6 (1.3) | ‐ | 35.7 (4.2) | 97.7 (15.0) | Behavioural modification + diet + exercise counselling | In the orlistat group, 14 participants had a baseline abnormality revealed by gallbladder ultrasound, including 8 participants with fatty liver infiltration or hepatomegaly and 3 participants with gallstones; 25.3% of participants had the metabolic syndrome at baseline |
| C: placebo + diet + exercise + behavioural therapy | 71 | 13.5 (1.2) | ‐ | 35.4 (4.1) | 95.1 (14.2) | ‐ | ||
| Clarson 2009 | I: metformin + lifestyle intervention | ‐ | 13.1 | ‐ | 36.4 (1.8) | ‐ | Lifestyle intervention | All participants insulin resistant. 15 participants had acanthosis nigricans |
| C: lifestyle intervention only | ‐ | 13.1 | ‐ | 33.9 (1.1) | ‐ | |||
| Franco 2014 | I: sibutramine + dietary guidance | 56 | 13.3 (1.8) | ‐ | 33.9 (7.2) | 85.5 (23.2) | Dietary guidance | ‐ |
| C: placebo + dietary guidance | 12.3 (1.7) | ‐ | 32.8 (5.8) | 83.1 (19.6) | ||||
| Freemark 2001 | I: metformin | 79 | 14.4 (0.6) | 5.6 (0.1) | 41.5 (0.9) | ‐ | ‐ | All participants had fasting hyperinsulinaemia. 8 participants had acanthosis nigricans |
| C: placebo | 46 | 15.4 (0.5) | 5.5 (0.1) | 38.7 (1.3) | ‐ | |||
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 61 | 15.2 (1.3) | ‐ | 35.1 (5.3) | 92.6 (14.6) | Diet and exercise advice | 8.7% high blood pressure, 8.7% glucose, 43.5% high triglycerides, 8.7% high cholesterol, 4.3% high LDL, 13% high HDL |
| C: placebo + diet + exercise | 52 | 14.7 (1.1) | ‐ | 36.6 (5.2) | 98.9 (22.7) | 30.4% high blood pressure, 8.7% glucose, 52.2% high triglycerides, 34.8% high cholesterol, 17.4% high LDL | ||
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | 83 | Females: 15.9 (1.1) Males: 16.7 (0.6) | ‐ | Females: 37.5 (3.8) Males: 37.6 (4.3) |
Females: 100.5 (14.2) Males: 117.1 (11.7) |
Exercise advice | ‐ |
| C: placebo + hypocaloric diet + exercise | 80 | Females: 16.3 (1.2) Males: 16.7 (0.6) | ‐ | Females: 35.8 (4.2) Males: 37.4 (1.9) |
Females: 94.0 (13.6) Males: 113.4 (10.0) | ‐ | ||
| Kendall 2013 | I: metformin + healthy lifestyle advice | 66 | 13.7 (2.3) | ‐ | 37.1 (6.4) | 100.3 (24.1) | Standardised healthy lifestyle advice | All participants had hyperinsulinaemia or impaired fasting glucose or impaired glucose tolerance (or both) |
| C: placebo + healthy lifestyle advice | 69 | 13.6 (2.2) | ‐ | 36 (6.3) | 96.4 (21.8) | |||
| Maahs 2006 | I: orlistat + diet and exercise therapy | 60 | 15.8 (1.5) | 5.4 (0.1) | 39.2 (5.3) | 111.1 (22.9) | Dietary and exercise counselling | ‐ |
| C: placebo + diet and exercise therapy | 75 | 15.8 (1.4) | 5.4 (0.1) | 41.7 (11.7) | 114.3 (38.4) | ‐ | ||
| Mauras 2012 | I: metformin + diet/exercise intervention | 57 | 12.3 (0.5) | ‐ | 32 (1) | ‐ | Dietary counselling and free membership to a sports club/gym | Elevated hsCRP or fibrinogen (or both) concentrations |
| C: diet/exercise intervention | 52 | 12.0 (0.4) | ‐ | 33.2 (0.7) | ‐ | |||
| NCT00001723 | I: orlistat + behavioural weight loss programme | 65 | 14.65 (1.38) | ‐ | 41.7 (0.6) | ‐ | Behavioural therapy and a multivitamin for 6 months | All participants had at least 1 of the following: systolic or diastolic hypertension (determined by age‐specific charts); frank type 2 diabetes, impaired glucose tolerance assessed by oral glucose tolerance testing; hyperinsulinaemia (defined as a fasting insulin > 15 IU/mL); significant hyperlipidaemia (total cholesterol > 200 mg/dL, LDL cholesterol > 129 mg/dL or fasting triglycerides > 200 mg/dL); hepatic steatosis (ALT or AST above normal range with negative hepatitis studies) or sleep apnoea documented by a sleep trial |
| C: placebo + behavioural weight loss programme | 66 | 14.52 (1.46) | ‐ | ‐ | ||||
| Ozkan 2004 | I: conventional treatment + orlistat | 67 | 12.9 (2.4) | ‐ | 32.5 | 82.1 (20.9) | Daily oral multivitamin preparation, lifestyle modification programme | ‐ |
| C: conventional treatment | 12.5 (2.2) | ‐ | 31.2 | 73.9 (15.3) | Lifestyle modification programme | ‐ | ||
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 100 | 15.6 (1.9) | ‐ | 33.6 | ‐ | Nutritional guide and exercise programme | 30% of participants had psychiatric comorbidities |
| C: placebo + nutritional guide and exercise programme | 100 | ‐ | 33.3 | ‐ | 11.1% of participants had psychiatric comorbidities | |||
| Rezvanian 2010 | I1: metformin + healthy eating and physical activity advice | ‐ | 13.1 (1.4) | ‐ | 26.4 (0.5) | ‐ | Physical activity advice; nutritional education session and dietary advice | ‐ |
| I2: fluoxetine + healthy eating and physical activity advice | ‐ | 13.5 (1.2) | ‐ | 26.5 (0.7) | ‐ | ‐ | ||
| I3: metformin and fluoxetine + healthy eating and physical activity advice | ‐ | 13.7 (1.1) | ‐ | 26.6 (0.8) | ‐ | ‐ | ||
| C: placebo + healthy eating and physical activity advice | ‐ | 13.4 (1.4) | ‐ | 26.2 (0.6) | ‐ | ‐ | ||
| Srinivasan 2006 | I: metformin first then placebo + standardised information on healthy eating and exercise | 54 | 12.5 (2.2) | ‐ | ‐ | ‐ | Information on healthy eating and exercise | Suspicion of insulin resistance; 89% participants had acanthosis nigricans |
| C: placebo first then metformin + standardised information on healthy eating and exercise | ‐ | ‐ | ‐ | |||||
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | 45 | 14.1 (1.0) | ‐ | 30.1 (4.5) | 80.8 (15.6) | Diet and exercise plan | ‐ |
| C: placebo + energy‐restricted diet and exercise plan | 58 | 13.8 (1.5) | ‐ | 33.3 (5.0) | 89.2 (16.4) | ‐ | ||
| Wiegand 2010 | I: metformin + multiprofessional lifestyle intervention | 72 | 15.1 | ‐ | 34.3 (5) | ‐ | Lifestyle intervention | All had risk factors for developing type 2 diabetes: acanthosis nigricans, signs of the metabolic syndrome, impaired fasting glucose, and positive family history of type 2 diabetes, or with impaired glucose tolerance |
| C: placebo + multiprofessional lifestyle intervention | 62 | 15 | ‐ | 35.5 (5.8) | ‐ | |||
| Wilson 2010 | I: metformin + lifestyle intervention programme | 67 | 14.8 (1.3) | 5.4 (0.3) | 35.9 (5.7) | 95.9 (16.6) | Lifestyle intervention given during run‐in period and follow‐up sessions provided monthly for the remainder of the trial; a multivitamin tablet and calcium carbonate 1000 mg was taken daily | ‐ |
| C: placebo + lifestyle intervention programme | 66 | 15.0 (1.5) | 5.3 (0.3) | 35.9 (4.7) | 101.8 (15.7) | ‐ | ||
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | 57 | 10.1 (1.6) | ‐ | 34.2 (6.8) | 76.4 (23.1) | A monthly dietitian administered weight‐reduction programme; a daily chewable multivitamin containing cyanocobalamin 6 mg was also prescribed | 26.4% had paediatric metabolic syndrome. 64% showed a presence of acanthosis nigricans; all participants had fasting hyperinsulinaemia. |
| C: placebo + dietitian‐administered weight‐reduction programme | 64 | 10.4 (1.4) | ‐ | 34.6 (6.2) | 80.1 (20.5) | 31.9% had paediatric metabolic syndrome. 68% showed a presence of acanthosis nigricans; all participants had fasting hyperinsulinaemia | ||
| "‐" denotes not reported. ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HsCRP: high sensitivity C‐reactive protein; I: intervention; LDL: low‐density lipoprotein; SD: standard deviation. | ||||||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Trial | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Endpoints quoted in publication(s)b | Time of measurement | |
| Atabek 2008 | N/T | Primary outcome measures: ‐ | 6 months | |
| Secondary outcome measures : ‐ | ||||
| Other outcome measures: % change in BMI, DBP, SBP, pulse rate, lipids, triglycerides, serum insulin, serum glucose, HOMA, HDL, BMI z score, LDL, total cholesterol, weight change, adverse events | ||||
| Berkowitz 2003 |
Source:NCT00212173 (added 13 September 2005) Primary outcome measure(s): BMI, weight |
No trial results posted. No link to Berkowitz 2003 publication but links to 2 additional publications. A second protocol for an adolescent lifestyle intervention also included | Primary outcome measure: % change in BMI | 3, 6, 9, 12 months |
| Secondary outcome measure(s): BP, lipids, glucose, insulin | Secondary outcome measures: BP, pulse, hunger | |||
| Other outcome measure(s): ‐ | Other outcome measures: lipids, triglycerides, serum insulin, serum glucose, HOMA, HDL, BMI z score, LDL, total cholesterol, weight change, waist circumference, adverse events | |||
| Berkowitz 2006 |
Source:NCT00261911 Primary outcome measure(s): absolute change in BMI from baseline to endpoint (12 months) |
No trial results posted, publications specified |
Primary outcome measures: absolute change from baseline in BMI |
0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 months |
| Secondary outcome measure(s): % change from baseline in BMI, proportions of participants achieving ≥ 5% and ≥ 10% BMI and bodyweight reduction, absolute and % change from baseline in waist circumference, body composition (DEXA), lipid and glycaemic variables (all: 12 months) | Secondary outcome measures: % change in BMI, proportion of participants achieving reductions in BMI of ≥ 5% or ≥ 10%, absolute and % changes in bodyweight and lipid and glycaemic variables, absolute change in waist circumference | |||
| Other outcome measure(s): ‐ | Other outcome measures: DBP, SBP, pulse rate, QTc interval, maturation (Tanner staging), adverse events | |||
| Chanoine 2005 | N/T | Primary outcome measures: change in BMI from baseline to trial end (or trial exit) | ‐0.5, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, 10, 11, 12 months | |
| Secondary outcome measures: change in bodyweight, levels of total, HDL and LDL cholesterol, LDL‐to‐HDL cholesterol ratio, triglyceride levels, SBP and DBP, waist and hip circumference, glucose and insulin responses to an oral glucose challenge, and changes in body composition | ||||
| Other outcome measures: beta carotene, vitamin A, 25‐hydroxyvitamin D, vitamin E, Tanner staging, adverse events | ||||
| Clarson 2009 | N/T | Primary outcome measures: change in BMI and modification of metabolic risk factors, including insulin resistance, plasma lipids and adipocytokines, assessment of metformin on the attainment of a target metabolic profile | 6 months | |
| Secondary outcome measures: ‐ | ||||
| Other outcome measures: BMI z score, BP, adverse events, waist circumference | ||||
| Franco 2014 | N/T | Primary outcome measures: ‐ | On average every 40 days for 13 months | |
| Secondary outcome measures: ‐ | ||||
|
Other outcome measures: % of participants loosing 10% of their initial weight, weight, BMI, SBP, DBP, cholesterol, LDL, HDL, triglycerides, leptin, CRP, transaminases, blood glucose, insulin, adverse events, waist circumference | ||||
| Freemark 2001 | N/T | Primary outcome measures: ‐ | 6 months | |
| Secondary outcome measures: ‐ | ||||
|
Other outcome measures: BMI SDS, insulin, glucose tolerance, leptin, serum lipids, HbA1c, IGF‐1, lactate, cholesterol, LDL, HDL, LDL/HDL, triglycerides, adverse events, ALT, AST | ||||
| Garcia‐Morales 2006 | N/T |
Primary outcome measures: baseline versus endpoint absolute values for bodyweight, BMI, and % of the initial BMI (%BMI) |
‐15, 30, 60, 90, 120, 150, 180 days | |
|
Secondary outcome measures: waist circumference and % of the initial waist circumference (%waist) | ||||
| Other outcome measures: health‐related quality of life, white blood cells, monocytes, eosinophils, glucose, uric acid, creatinine, albumin, chloride, total cholesterol, LDL, AST, alkaline phosphatase, SBP, DBP, heart rate, ST segment, adverse events | ||||
| Godoy‐Matos 2005 | N/T | Primary outcome measures: change in weight and BMI | ‐4, 4, 8, 12, 16, 20, 24 weeks | |
| Secondary outcome measures: change in waist, hip, and waist‐to‐hip ratio | ||||
| Other outcome measures: SBP, DBP, heart rate, glucose, total cholesterol, triglycerides, HDL, LDL, VLDL, insulin, total cholesterol/HDL cholesterol, left atrium diameter, left ventricular mass, adverse events, satiety score | ||||
| Kendall 2013 |
Source:ISRCTN19517475 Primary outcome measure: reduction in BMI SDS |
Prior to 16 December 2008: 80 participants aged 9 to 18 years As of 16 December 2008:
|
Primary outcome measure: reduction of BMI SDS | 3, 6 months |
| Secondary outcome measures: Added 16 December 2008: fasting and 2‐hour insulin and glucose levels on OGTT, measures of insulin resistance, fasting lipids, CRP, adiponectin, leptin, resistin, BP | Secondary outcome measures: BMI and waist‐to‐hip ratio, fasting and postprandial insulin and glucose levels, metabolic risk factors, adipokines | |||
| Other outcome measure(s): ‐ | Other outcome measures: weight, height, SBP, DBP, cholesterol, HDL, LDL, triglycerides, bilirubin, CRP, lactate, resistin, adverse events | |||
| Maahs 2006 | N/T | Primary outcome measures: change in BMI from baseline to 6 months | 1, 2, 3, 4, 5, 6 months | |
| Secondary outcome measures: changes in weight, lean body mass, results of blood chemistry studies | ||||
| Other outcome measures: health‐related quality of life, all‐cause mortality, vitamin A, vitamin D, vitamin E, adverse events | ||||
| Mauras 2012 |
Source:NCT00139477 Primary outcome measures: change from baseline in hsCRP at 6 months, change from baseline in fibrinogen at 6 months, change from baseline in IL‐6 at 6 months, change from baseline in PAI‐1 at 6 months |
Trial results posted, publications specified | Primary outcome measures: hsCRP and fibrinogen concentrations at 6 months | 3, 6 months |
| Secondary outcome measure(s): ‐ | Secondary outcome measures: ‐ | |||
| Other outcome measure(s): ‐ | Other outcome measures: weight, BMI percentile, systolic BP, diastolic BP, IL‐6, PAI‐1, adiponectin, IGF‐1, insulin, total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides, free fatty acids, glucose tolerance, resting energy expenditure rates, adverse events, waist circumference | |||
| NCT00001723 |
Source:NCT00001723 Primary outcome measure: change in BMI SDS (time frame: baseline to 6 months) |
Trial results posted, linked to pilot trial but no link to publication | No publication available | 6 months |
| Secondary outcome measures: change in bodyweight (time frame: baseline to 6 months), weight, change in BMI (time frame: baseline to 6 months), change in body fat (time frame: baseline to 6 months), body fat distribution measures obtained DEXA, effect of race on change in weight (time frame: baseline to 6 months), difference in change of weight according to race (non‐Hispanic white versus non‐Hispanic black) | ||||
| Other outcome measure(s): ‐ | ||||
| Ozkan 2004 | N/T | Primary outcome measures: ‐ | 1 to 15 months | |
| Secondary outcome measures: ‐ | ||||
| Other outcome measures: weight change, % weight change, BMI, adverse events | ||||
| Prado 2012 | N/T | Primary outcome measures: weight | 1, 2, 3, 4, 5, 6 months | |
| Secondary outcome measures: ‐ | ||||
| Other outcome measures: BMI, motivational survey results, glycaemia, afterload glucose, HDL, adverse events, waist circumference | ||||
| Rezvanian 2010 | N/T | Primary outcome measures: ‐ | 12, 24 weeks | |
| Secondary outcome measures: ‐ | ||||
| Other outcome measures: BMI, BMI SDS, waist circumference, waist‐to‐height ratio, adverse events | ||||
| Srinivasan 2006 |
Source:ISRCTN43267711 Primary outcome measures: ‐ |
No results posted or links to publication Retrospectively registered |
Primary outcome measures: ‐ | 6, 12 months |
| Secondary outcome measures: ‐ | Secondary outcome measures: ‐ | |||
| Other outcome measures: ‐ | Other outcome measures: BMI, waist circumference z score, fasting insulin, fasting glucose, glucose effectiveness, acute insulin response, disposition index, glucose disposal, acanthosis nigricans neck score, Tanner staging, weight loss, weight z score, BMI z score, adverse events | |||
| Van Mil 2007 | N/T | Primary outcome measure: change in BMI between the 2 periods (12 weeks' randomised treatment period and 12 weeks' follow‐up) | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, 18, 20, 22, 24 weeks | |
| Secondary outcome measures: ‐ | ||||
| Other outcome measures: height, weight, sleeping metabolic rate, basal metabolic rate, total energy expenditure, physical activity level, basal metabolic rate adjusted, total energy expenditure residuals, adverse events | ||||
| Wiegand 2010 | Found in the references of included trials section: EudraCT Nr. 2004‐003816‐47 (but currently not available at EU CTR. We contacted EMA and received the following answer "Note that this trial is not in the public domain due to missing information from Ethics Committee therefore we recommend you to contact the National Competent Authority concerned by this application."; in the dissertation by Hübel it is specified "Vor Beginn der Studie wurde die Zustimmung der jeweils zuständigen Ethikkommissionen eingeholt (Charité Berlin, Deutschland; St. Gallen, Schweiz)" ‐ "before start of the study approval of the appropriate ethics committees was obtained (Charité Berlin, Germany; St. Gallen, Switzerland)) | Primary outcome measures: HOMA‐IR | ‐6, 3, 6 months | |
| Secondary outcome measures: anthropometric measurements (BMI and waist‐to‐hip ratio), cardiovascular risk parameters (SBP and DBP), lipid profile (total, LDL, HDL cholesterol and triglycerides), and other metabolic parameters (glucose tolerance and fasting insulin) | ||||
| Other outcome measures: adverse events | ||||
| Wilson 2010 |
Source:NCT00209482 and NCT00120146 Primary outcome measures: NCT00209482: mean change from baseline in individual BMIs between the 2 groups (compared at 2 time points: at week 52 and week 100) NCT00120146: change in BMI, BMI |
No trial results posted, publications specified | Primary outcome measures: BMI change, BMI z score | ‐4, 12, 24, 36, 48, 60, 72, 84, 96 weeks |
| Secondary outcome measures: NCT00209482: ‐ NCT00120146: change in insulin sensitivity; fasting insulin concentrations; characterisation of insulin dynamics and insulin sensitivity; characterisation of fat distribution and fatty infiltration of the liver; use of CT to characterise abdominal fat distribution; use of CT and ALT levels to assess fatty infiltration of the liver; characterisation of body composition; characterisation of dietary amino acids; characterisation of the insulin‐to‐glucagon ratio; characterisation of the impact of sex on response to metformin XR; characterisation of the impact of race/ethnicity on response to metformin XR; characterisation of health‐related quality of life | Secondary outcome measures: fat mass, lean mass, fat area, HOMA‐IR, area under insulin curve, area under glucose curve, corrected insulin release at glucose peak, LDL cholesterol, HDL cholesterol, triglycerides, triglyceride‐to‐HDL cholesterol ratio, adverse events | |||
| Other outcome measures: NCT00209482: ‐ NCT00120146: ‐ | Other outcome measures: waist circumference | |||
| Yanovski 2011 |
Source:NCT00005669 Primary outcome measures: changes in bodyweight as determined by BMI SDS (6 months) |
Trial results posted, publications specified | Primary outcome measures: change in BMI SD score (BMI z score), as determined at the end of the 6‐month randomised treatment phase | 1, 2, 3, 4, 5, 6 months |
| Secondary outcome measures: change in bodyweight as determined by BMI (6 months), change in bodyweight (6 months), change in body fat by DEXA (6 months), change in body fat by Bod Pod (6 months) | Secondary outcome measures: changes in BMI, bodyweight and fat mass at the conclusion of the randomised phase | |||
| Other outcome measures: ‐ | Other outcome measures: changes in skinfold thickness, body circumferences, visceral adipose tissue, insulin resistance and laboratory components of the metabolic syndrome ‐ SBP, DBP, serum insulin, plasma glucose, total cholesterol, HDL cholesterol, LDL cholesterol, LDL‐to‐HDL cholesterol ratio, triglycerides, ALT, AST, hsCRP, vitamin B12, adverse events | |||
| ‐ denotes not reported. aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial). ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; BMI SDS: body mass index standardised deviation score; BP: blood pressure; CDC: Centers for Disease Control and Prevention; CRP: C‐reactive protein; CT: computed tomography; DBP: diastolic blood pressure; DEXA: dual‐energy X‐ray absorptiometry; EMA: European Medicines Agency; EU CTR: European Clinical Trials Register; FDA: Food and Drug Administration (US); HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HOMA(‐IR): homeostasis model assessment (insulin resistance); hsCRP: high sensitivity C‐reactive protein; IGF‐1: insulin‐like growth factor 1; IL‐6: interleukin‐6; LDL: low‐density lipoprotein; N/T: no trial document available; OGTT: oral glucose tolerance test; PAI‐1: plasminogen activator inhibitor‐1; QTc: heart‐rate corrected QT interval; SBP: systolic blood pressure; VLDL: very low density lipoprotein; XR: extended release. | ||||
Appendix 6. Examination of outcome reporting bias
| Trial | Outcome | Clear that outcome was measured and analyseda (trial report states that outcome was analysed but only reports that result was not significant) | Clear that outcome was measured and analysedb (trial report states that outcome was analysed but no results reported) | Clear that outcome was measuredc (clear that outcome was measured but not necessarily analysed (judgement says likely to have been analysed but not reported because of nonsignificant results)) | Unclear whether the outcome was measuredd (not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of nonsignificant results) |
| Atabek 2008 | Behaviour change | ‐ | ‐ | Yes | ‐ |
| Berkowitz 2003 | N/A | ||||
| Berkowitz 2006 | N/A | ||||
| Chanoine 2005 | N/A | ||||
| Clarson 2009 | Body fat distribution | Yes | ‐ | ‐ | ‐ |
| Franco 2014 | Body fat distribution | ‐ | ‐ | Yes | ‐ |
| Freemark 2001 | N/A | ||||
| Garcia‐Morales 2006 | Behaviour change | ‐ | ‐ | Yes | ‐ |
| Godoy‐Matos 2005 | N/A | ||||
| Kendall 2013 | Behaviour change | ‐ | ‐ | Yes | ‐ |
| Maahs 2006 | Behaviour change | ‐ | ‐ | Yes | ‐ |
| Health‐related quality of life and self esteem | Yes | ‐ | ‐ | ‐ | |
| Mauras 2012 | N/A | ||||
| NCT00001723 | N/A | ||||
| Ozkan 2004 | N/A | ||||
| Prado 2012 | Measured BMI | Yes | ‐ | ‐ | ‐ |
| Body fat distribution | Yes | ‐ | ‐ | ‐ | |
| Rezvanian 2010 | N/A | ||||
| Srinivasan 2006 | Measured BMI | ‐ | Yes | ‐ | ‐ |
| Body fat distribution | ‐ | Yes | ‐ | ‐ | |
| Van Mil 2007 | N/A | ||||
| Wiegand 2010 | Body fat distribution | Yes | ‐ | ‐ | ‐ |
| Wilson 2010 | Body fat distribution | ‐ | Yes | ‐ | ‐ |
| Yanovski 2011 | N/A | ||||
| BMI: body mass index; N/A: not applicable. | |||||
Appendix 7. Definition of endpoint measurementa (I)
| Trial | Measured BMI | Adverse events | Health‐related quality of life and self‐esteem | All‐cause mortality | Morbidity |
| Atabek 2008 | Expressed as BMI (kg/m2). Obesity defined as ≥ the 95th percentile for age and sex based on the standards of the CDC (IO) | Participants were asked to report any adverse effects every month. Serious adverse events defined as vomiting or lactic acidosis (SO) | N/I | N/I | Hyperinsulinaemia was defined from norms for pubertal stages 2 to 4: mid‐puberty > 30 mU/L, and postpubertal hyperinsulinism was defined by adult WHO criteria (> 20 mU/L). Insulin sensitivity was estimated using FGIR, HOMA‐IR and QUICKI (IO) |
| Berkowitz 2003 | The change in raw BMI is not given. Instead it is expressed as % reduction in BMI (kg/m2). BMI also used to calculate BMI z score (calculated using CDC standards) Obesity defined as BMI 32 to 44 kg/m2 (IO) |
Adverse events were recorded at each medical visit. In addition, blood pressure and heart rate were monitored closely, and any abnormalities were considered as adverse events. A serious adverse event was not defined (AO, IO, SO) | N/I | N/I | N/I |
| Berkowitz 2006 | Expressed as BMI (kg/m2) in graphical format and % change in BMI in text and tabular format. Used Rosner 1998 to define obesity (IO) | The investigator recorded all adverse events, both observed and volunteered. The only serious event defined as excessive nausea and vomiting. Unclear whether suicide attempt and depression were defined as serious (AO, IO, SO) | N/I | 2 suicide attempts ‐ did not result in mortality (AO, IO) | N/I |
| Chanoine 2005 | Expressed as BMI (kg/m2). Used Barlow 1998 to define obesity (IO) | Gastrointestinal tract adverse effects assessed at each visit by a specially designed dictionary of standard terms for defecation patterns for reproducibility and consistency of reporting. Other adverse events were noted and followed by questioning. Serious adverse events included acute demyelinating encephalomyelitis, facial palsy, pneumonia, worsening of asthma, pain in the right side, pilonidal abscess, depression, asthma attack, seizure, admission for repair of deviated nasal septum, appendicitis, cholelithiasis, gallbladder disorder followed by cholecystectomy, adenoidal hypertrophy and aseptic meningitis. The trial also used electrocardiograms to detect abnormalities and measured gallbladder ultrasounds to detect gallstones (AO, IO, SO) | N/I | N/I | Gallstones and fatty liver infiltration or hepatomegaly identified by gallbladder ultrasound (IO) |
| Clarson 2009 | Expressed as BMI (kg/m2). Obesity defined as BMI > 95th percentile for age and sex (no reference). BMI z scores calculated using the CDC reference data (IO) | No adverse events reported, trial highlights that metformin was well tolerated by all participants ‐ unclear how this was assessed | N/I | N/I | Insulin resistance was defined using HOMA > 3 (IO) |
| Franco 2014 | Weight and height used to calculate BMI. Obesity defined by WHO classification (IO) | Adverse effects were investigated on a preset questionnaire and described voluntarily by the participant at each consultation (on average every 40 days). A serious adverse event was not defined (AO, SO) | N/I | N/I | N/I |
| Freemark 2001 | Expressed change in BMI. Also expressed as BMI SDS. Used Rosner 1998 to adjusting for age, sex and race (IO) | Unclear how and when adverse events were assessed | N/I | N/I | Hyperinsulinaemia defined as fasting insulin concentration exceeding 15 μU/mL. Insulin sensitivity assessed by fasting insulin‐to‐glucose concentration ratio, QUICKI and HOMA‐IR (IO) |
| Garcia‐Morales 2006 | Expressed as BMI (kg/m2). Used CDC growth charts (Kuczmarski 2000) (IO) | Adverse events were reported as they were detected by the participant or investigator. They were also assessed during visits. Severe adverse events defined as life‐threatening or those resulting in hospitalisation or producing long‐term disabilities (AO, IO, SO) | Health‐related quality of life assessed by a 36‐item Short‐Form Health Survey (SF‐36) questionnaire (Alonso 1995) (SO) | N/I | Comorbidities were accessed at baseline and follow‐up. These included high blood pressure, high glucose, high triglycerides, high cholesterol, high LDL and high HDL (IO) |
| Godoy‐Matos 2005 | Expressed as BMI (kg/m2). Obesity defined as BMI between 30 and 45 (no reference) (IO) | Adverse events were assessed and recorded at each visit. A significant event was defined as a serious or rare event ‐ serious event not defined (AO, IO, SO) | N/I | N/I | N/I |
| Kendall 2013 | Expressed as BMI (kg/m2). Obesity defined by UK BMI centile charts. No reference for how BMI SDS was calculated (IO) | How and when trial authors assessed adverse events was not described. No explanation to how they defined a severe/series event | N/I | N/I | Participants had hyperinsulinaemia, impaired fasting glucose or impaired glucose tolerance Insulin resistance/sensitivity was assessed using: HOMA‐IR, QUICKI, whole‐body insulin sensitivity index, adiponectin‐to‐leptin ratio (IO) |
| Maahs 2006 | Expressed as BMI (kg/m2). Obesity defined as BMI that exceeded the 85th percentile for age and sex (assume this from the CDC standards) (IO) | Adverse events assessed at each monthly visit. Serious/severe adverse events not defined (AO, IO, SO) | Health‐related quality of life assessed by 4 questionnaires: Brief Symptom Inventory (Derogatis 1983), Parents and Children's KINDL (Ravens‐Sieberer 2001), IWQOL‐Kids (Kolotkin 1997; Kolotkin 2001), and a global ratings scale (SO) | Defined as suicide ‐ 1 participant in the orlistat group (AO, IO) | N/I |
| Mauras 2012 | Expressed as BMI (kg/m2). BMI % determined using CDC standards (Kuczmarski 2000) (IO) | Trial authors did not describe how and when adverse events were assessed. Did not report number or type of adverse events | N/I | N/I | Elevated hsCRP and fibrogen concentrations were measured by immuno‐nephelometry (IO) |
| NCT00001723 | BMI SDS calculated for age and sex according to CDC standards (IO) | Events were collected by systematic assessment. Trial authors did not define what a serious adverse event was (IO, SO) | N/I | N/I | N/I |
| Ozkan 2004 | Expressed as BMI (kg/m2). Severe obesity defined as weight for height index > 140% (no reference) (IO) | Unclear how and when adverse events were assessed. All with mild gastrointestinal complaints apart from 2 (mild diffuse hair loss and another with muscle cramps). A serious/severe event was not defined | N/I | N/I | N/I |
| Prado 2012 | Expressed as BMI (kg/m2). Obesity defined as BMI > 95th percentile for age and sex (no reference) (IO) | Adverse events monitored by ALT, AST and haemoglobin levels (IO) | N/I | N/I | Risk factors for diabetes mellitus type 2: high glycaemia fasting, high postload glucose or high insulin sensitivity. Insulin sensitivity accessed by HOMA |
| Rezvanian 2010 | Expressed as BMI (kg/m2). Gave baseline BMI SDS (calculated using revised CDC growth charts: Kuczmarski 2000 but did not give follow‐up measurements (IO) | Participants and parents educated on possible signs of symptoms of hypoglycaemia. They were also given a 24‐hour mobile phone number to call if any adverse events occurred. No definition of severe/serious adverse events (SO) | N/I | N/I | N/I |
| Srinivasan 2006 | Raw BMI (kg/m2) data not provided BMI z score (presented only on a graph) was calculated from the CDC reference data 2000. Obesity defined by the International Obesity Task Force (Cole 2000) (IO) | Unclear how and when adverse events were assessed. No definition for serious/severe events | N/I | N/I | Clinical suspicion of insulin resistance defined by fasting insulin‐to‐glucose ratio or presence of acanthosis nigricans (assessed by severity at the neck by a validated scale) Insulin sensitivity was accessed by SI clamp (minimal model), fasting insulin and fasting glucose (IO) |
| Van Mil 2007 | Expressed as BMI (kg/m2). BMI SDS was determined using the Dutch age‐ and sex‐adjusted BMI curves (Hansen 1998). Obesity defined as ≥ 97th percentile (no reference) (IO) | Adverse events were determined at each visit. Heart rate, DBP, SBP were monitored throughout the trial. No definition of a serious/severe event (AO, IO, SO) | N/I | N/I | N/I |
| Wiegand 2010 | Expressed as BMI (kg/m2) ‐ reference: Park 2009. Also provided BMI SDS, no reference (IO) | Adverse events determined at the 3‐month and 6‐month visit by a clinical and biochemical assessment. No definition of a serious/severe event (AO, IO, SO) | N/I | N/I | Risk factors for type 2 diabetes: acanthosis nigricans, signs of metabolic syndrome and impaired fasting glucose Insulin sensitivity was assessed by HOMA‐IR and insulin sensitivity index (IO) |
| Wilson 2010 | Expressed as BMI (kg/m2). Used CDC charts to convert BMI to BMI z score (Kuczmarski 2000) (IO) | Adverse events assessed at each visit. An appendectomy was defined as a serious/severe event (AO, IO, SO) | N/I | N/I | N/I |
| Yanovski 2011 | Expressed as BMI (kg/m2). Also expressed as BMI SDS (no reference) (IO) | Adverse events accessed at each visit and by laboratory analysis. A serious/severe event was not defined (AO, IO, SO) | N/I | N/I | Insulin sensitivity was calculated (from a SI clamp) using the metabolic rate‐to‐steady‐state insulin ratio. Insulin resistance estimated using HOMA‐IR (IO) |
|
aIn addition to definition of endpoint measurement, description who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement). ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; BMI SDS: body mass index standard deviation score; CDC: Centers for Disease Control and Prevention; DBP: diastolic blood pressure; FGIR: fasting insulin concentration/fasting glucose concentration; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HOMA(‐IR): homeostasis model assessment (insulin resistance); hsCRP: high sensitivity C‐reactive protein; IWQOL: Impact of Weight on Quality of Life questionnaire; LDL: low‐density lipoprotein; N/I: not investigated; OGTT: oral glucose tolerance test; QUICKI: quantitative insulin check index; SBP: systolic blood pressure; SI clamp: insulin sensitivity clamp; WHO: World Health Organization. | |||||
Appendix 8. Definition of endpoint measurementa (II)
| Trial | Body fat distribution | Behaviour change | Participants views of the intervention | Socioeconomic effects |
| Atabek 2008 | N/I | Food consumption was assessed by the completion of a detailed questionnaire at the beginning and end of the trial (no reference or results given) (SO) | N/I | N/I |
| Berkowitz 2003 | Waist circumference measured using reference: Calloway 1988 (IO) | Hunger was evaluated by the Eating Inventory (range 0 to 14) (Stunkard 1985) (SO) | N/I | N/I |
| Berkowitz 2006 | Waist circumference ‐ no description (IO) | N/I | N/I | N/I |
| Chanoine 2005 | Waist circumference ‐ no description on how it was measured. Body composition measured by whole body DEXA (IO) | N/I | N/I | N/I |
| Clarson 2009 | Waist circumference was measured in the standing position at the level of the umbilicus to the nearest 0.1 cm using a constant tension tape (no reference) (IO) | N/I | N/I | N/I |
| Franco 2014 | Waist circumference and hip circumference measured at the smallest and largest diameter. Arm circumference at the middle third of the left arm (no reference) (IO) | N/I | N/I | N/I |
| Freemark 2001 | N/I | N/I | N/I | N/I |
| Garcia‐Morales 2006 | Waist circumference ‐ measured with a flexible tape between the highest point of the iliac crest and the lowest part of the costal margin at the midaxillary line (no reference) (IO) | A detailed questionnaire on food consumption was completed at the beginning and end of the trial (no reference) (SO) | N/I | N/I |
| Godoy‐Matos 2005 | Waist circumference ‐ measured at the minimal circumference between iliac crest and last rib edge. Hip circumference assessed at the greatest circumference through the major trochanters (no reference) (IO) | N/I | N/I | N/I |
| Kendall 2013 | Waist‐to‐hip ratio (no reference) (IO) | 3 previously validated questionnaires (food frequency, diet and eating behaviour, and physical activity) were completed by each child at the start and end of the trial. No results presented (SO) | N/I | N/I |
| Maahs 2006 | BIA | Diet records recorded before enrolment and at 3 and 6 months. No reference or results provided (SO) | N/I | N/I |
| Mauras 2012 | Waist circumference measured at umbilicus (no reference). Intrahepatic fat content measured using fast MRI. References: Fishbein 2001; Fishbein 2003. Body composition was measured DEXA. Also measured waist‐to‐height ratio (no reference) (IO) | N/I | N/I | N/I |
| NCT00001723 | N/I | N/I | N/I | N/I |
| Ozkan 2004 | N/I | N/I | N/I | N/I |
| Prado 2012 | Waist circumference ‐ measured with a central flexible tape, corresponding to the perimeter less between the iliac crest and the bottom edge last rib, then exhale with arms relaxed on both sides (no reference) (IO) | N/I | N/I | N/I |
| Rezvanian 2010 | Waist circumference ‐ measured at a point midway between the lower border of the rib cage and the iliac crest at the end of normal expiration (no reference) (IO) | N/I | N/I | N/I |
| Srinivasan 2006 | Raw waist circumference data not reported. Waist circumference was calculated from the mean of 3 measures at the level of the umbilicus (no reference). Waist circumference z scores calculated from recent multiracial American reference data (Fernandez 2004). Raw body composition data were not reported. DEXA scans also used. MRI whole‐body scans (IO) | N/I | N/I | N/I |
| Van Mil 2007 | Body composition was assessed using a 4‐component reference model: total bodyweight = fat mass + total body water + total bone mineral content and remaining fat‐free mass (references: Fuller 1992; Van Marken Lichtenbelt 1999). To calculate this they used densitometry, deuterium dilution (Maastricht protocol) with labelled water test and DEXA (IO) | Physical activity level was estimated using an activity questionnaire. A 7‐day dietary record was given to each participant. Only the food quotient used in the assessment (respiratory exchange ratio to calculate TEE). Rest of the data not presented (IO, SO) | N/I | N/I |
| Wiegand 2010 | Waist‐to‐hip ratio and body composition (BIA) were measured but no explanation to how and no results given (apart from saying they were not significant) (IO) | N/I | N/I | N/I |
| Wilson 2010 | Abdominal CT scans evaluated abdominal fat content and distribution (Borkan 1982). Whole body DEXA used to measure % body fat and lean body mass (von Scheven 2006). Waist circumference measured at the smallest circumference below the rib cage and above the umbilicus (Wang 2003) (IO) | N/I | N/I | N/I |
| Yanovski 2011 | Abdominal and hip circumferences (assessed in triplicate) and triceps skinfold thickness. Whole‐body fat mass by DEXA and by air displacement plethysmography; and intra‐abdominal and subcutaneous abdominal adipose tissue by MRI at L2 to L3 and L4 to L5 (IO) | N/I | N/I | N/I |
|
aIn addition to definition of endpoint measurement, description who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement). BIA: bioelectrical impedance analysis; CT: computed tomography; DEXA: dual‐energy X‐ray absorptiometry; MRI: magnetic resonance imaging; n: number of participants; N/I: not investigated; TEE: total energy expenditure. | ||||
Appendix 9. Adverse events (I)
| Trial | Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths (% of participants) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with at least one severe/serious adverse event (N) | Participants with at least one severe/serious adverse event (%) |
| Atabek 2008 | I: metformin + diet and physical activity advice | 90 | 0 | 0 | 2 | 2.2 | 0 | 0 |
| C: placebo + diet and physical activity advice | 30 | 0 | 0 | 0 | 0.0 | 0 | 0 | |
| Berkowitz 2003 | I: behavioural programme + sibutramine | 43 | 0 | 0 | 6 | 14.0 | ‐ | ‐ |
| C: behavioural programme + placebo | 39 | 0 | 0 | 3 | 7.7 | ‐ | ‐ | |
| Berkowitz 2006 | I: behavioural therapy programme + sibutramine | 368 | 0 | 0 | 327 | 88.9 | 10 | 2.7 |
| C: behavioural therapy programme + placebo | 130 | 0 | 0 | 111 | 85.4 | 1 | 0.8 | |
| Chanoine 2005 | I: orlistat + diet + exercise + behavioural therapy | 352 | 0 | 0 | 341 | 97 | 11 | 3 |
| C: placebo + diet + exercise + behavioural therapy | 181 | 0 | 0 | 170 | 94 | 5 | 3 | |
| Clarson 2009 | I: metformin + lifestyle intervention | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: lifestyle intervention only | 17 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Franco 2014 | I: sibutramine + dietary guidance | 63 | ‐ | ‐ | 8 | 13.4 | 0 | 0 |
| C: placebo + dietary guidance | 63 | ‐ | ‐ | 3 | 4.9 | 0 | 0 | |
| Freemark 2001 | I: metformin | 15 | 0 | 0 | 4 | 26.7 | 0 | 0 |
| C: placebo | 17 | 0 | 0 | 1 | 5.9 | 0 | 0 | |
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 23 | 0 | 0 | 10 | 43.5 | 0 | 0 |
| C: placebo + diet + exercise | 23 | 0 | 0 | 10 | 43.5‐ | 0 | 0 | |
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | 30 | 0 | 0 | ‐a | ‐ | 0 | 0 |
| C: placebo + hypocaloric diet + exercise | 30 | 0 | 0 | ‐a | ‐ | 0 | 0 | |
| Kendall 2013 | I: metformin + healthy lifestyle advice | 74 | 0 | 0 | 20 | 27.0 | 0 | 0 |
| C: control + healthy lifestyle advice | 77 | 0 | 0 | 8 | 10.4 | 0 | 0 | |
| Maahs 2006 | I: orlistat + diet and exercise therapy | 20 | 1 | 5.0 | ‐ | ‐ | 1 | 5.0 |
| C: placebo + diet and exercise therapy | 20 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| Mauras 2012 | I: metformin + diet/exercise intervention | 35 | 0 | 0 | ‐ | ‐ | 0 | 0 |
| C: diet/exercise intervention | 31 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| NCT00001723 | I: orlistat + behavioural weight loss programme | 100 | 0 | 0 | 95 | 95 | 0 | 0 |
| C: placebo + behavioural weight loss programme | 100 | 0 | 0 | 94 | 94 | 2 | 2 | |
| Ozkan 2004 | I: conventional treatment + orlistat | 22 | 0 | 0 | 22 | 100 | ‐ | ‐ |
| C: conventional treatment | 20 | 0 | 0 | 0 | 0 | ‐ | ‐ | |
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 10 | 0 | 0 | ‐ | ‐ | 0 | 0 |
| C: placebo + nutritional guide and exercise programme | 9 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| Rezvanian 2010 | I1: metformin + healthy eating and physical activity advice | 45 | 0 | 0 | 7 | 15.6 | 0 | 0 |
| I2: fluoxetine + healthy eating and physical activity advice | 45 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| I3: metformin and fluoxetine + healthy eating and physical activity advice | 45 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| C: placebo + healthy eating and physical activity advice | 45 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| Srinivasan 2006 | I: metformin first then placebo + "standardised information on healthy eating and exercise" | 13 | 0 | 0 | ‐b | ‐ | 0 | 0 |
| C: placebo first then metformin + "standardised information on healthy eating and exercise" | 15 | 0 | 0 | ‐b | ‐ | 0 | 0 | |
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | 12 | 0 | 0 | 12 | 100 | ‐ | ‐ |
| C: placebo + energy‐restricted diet and exercise plan | 12 | 0 | 0 | 9 | 75 | ‐ | ‐ | |
| Wiegand 2010 | I: metformin + multiprofessional lifestyle intervention | 36 | 0 | 0 | 8 | 22.2 | ‐ | ‐ |
| C: placebo + multiprofessional lifestyle intervention | 34 | 0 | 0 | 13 | 38.2 | ‐ | ‐ | |
| Wilson 2010 | I: metformin + lifestyle intervention | 38 | 0 | 0 | ‐c | ‐ | 2 | 5.3 |
| C: placebo + lifestyle intervention | 38 | 0 | 0 | ‐c | ‐ | 0 | 0 | |
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | 53 | 0 | 0 | ‐d | ‐ | 0 | 0 |
| C: placebo + dietitian‐administered weight‐reduction programme | 47 | 0 | 0 | ‐d | ‐ | 0 | 0 | |
| "‐" denotes not reported. aNumber of participants with one or multiple adverse events: sibutramine group 47 events in 30 participants; placebo group 45 events in 30 participants. bTwo participants were unable to tolerate metformin 1000 mg twice daily because of nausea and were switched to metformin 750 mg twice daily with slower dose increments. cNumber of participants with one or multiple adverse events: metformin group: 52 events in 38 participants; placebo group: 43 events in 38 participants. dA total of 9/53 (17%) metformin‐treated children were unable to take the highest dose of 2000 mg/d and were prescribed doses ranging from 500 mg/d to 1500 mg/d; number of participants with one or multiple adverse events: metformin group: 64 events in 53 participants; placebo group: 25 events in 47 participants. C: comparator; I: intervention; n: number pf participants. | ||||||||
Appendix 10. Adverse events (II)
| Trial | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) | Participants with at least one hospitalisation (N) | Participants with at least one hospitalisation (%) | Participants with at least one outpatient treatment (N) | Participants with at least one outpatient treatment (%) |
| Atabek 2008 | I: metformin + diet and physical activity advice | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo + diet and physical activity advice | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Berkowitz 2003 | I: behavioural programme + sibutramine | 42 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: behavioural programme + placebo | 39 | 1 | 2.5 | ‐ | ‐ | ‐ | ‐ | |
| Berkowitz 2006 | I: behavioural therapy programme + sibutramine | 368 | 23 | 6 | ‐ | ‐ | ‐ | ‐ |
| C: behavioural therapy programme + placebo | 130 | 7 | 5 | ‐ | ‐ | ‐ | ‐ | |
| Chanoine 2005 | I: orlistat + diet + exercise + behavioural therapy | 352 | 12 | 3 | 10 | 2.8 | 0 | 0 |
| C: placebo + diet + exercise + behavioural therapy | 181 | 3 | 2 | 5 | 2.8 | 0 | 0 | |
| Clarson 2009 | I: metformin + lifestyle intervention | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: lifestyle intervention only | 17 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Franco 2014 | I: sibutramine + dietary guidance | 63 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo + dietary guidance | 63 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Freemark 2001 | I: metformin | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 17 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 23 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo + diet + exercise | 23 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo + hypocaloric diet + exercise | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Kendall 2013 | I: metformin + healthy lifestyle advice | 74 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: control + healthy lifestyle advice | 77 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Maahs 2006 | I: orlistat + diet and exercise therapy | 20 | 3 | 15 | ‐ | ‐ | ‐ | ‐ |
| C: placebo + diet and exercise therapy | 20 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Mauras 2012 | I: metformin + diet/exercise intervention | 35 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: diet/exercise intervention | 31 | 0 | 0 | 0 | 0 | 0 | 0 | |
| NCT00001723 | I: orlistat + behavioural weight loss programme | 100 | 1 | 1 | ‐ | ‐ | ‐ | ‐ |
| C: placebo + behavioural weight loss programme | 100 | 2 | 2 | ‐ | ‐ | ‐ | ‐ | |
| Ozkan 2004 | I: conventional treatment + orlistat | 22 | 7 | 32 | ‐ | ‐ | ‐ | ‐ |
| C: conventional treatment | 20 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo + nutritional guide and exercise programme | 9 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rezvanian 2010 | I1: metformin + healthy eating and physical activity advice | 45 | 0 | 0 | 0 | 0 | 0 | 0 |
| I2: fluoxetine + healthy eating and physical activity advice | 45 | 0 | 0 | 0 | 0 | 0 | 0 | |
| I3: metformin and fluoxetine + healthy eating and physical activity advice | 45 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C: placebo + healthy eating and physical activity advice | 45 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Srinivasan 2006 | I: metformin first then placebo + "standardised information on healthy eating and exercise" | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo first then metformin + "standardised information on healthy eating and exercise" | 15 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | 12 | 1 | 8 | 0 | 0 | 0 | 0 |
| C: placebo + energy‐restricted diet and exercise plan | 12 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Wiegand 2010 | I: metformin + multiprofessional lifestyle intervention | 36 | 3 | 8.3 | ‐ | ‐ | ‐ | ‐ |
| C: placebo + multiprofessional lifestyle intervention | 34 | 1 | 2.9 | ‐ | ‐ | ‐ | ‐ | |
| Wilson 2010 | I: metformin + lifestyle intervention | 38 | 3 | 7.9 | ‐ | ‐ | ‐ | ‐ |
| C: placebo + lifestyle intervention | 38 | 1 | 2.6 | ‐ | ‐ | ‐ | ‐ | |
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | 53 | 1 | 1.9 | ‐ | ‐ | ‐ | ‐ |
| C: placebo + dietitian‐administered weight‐reduction programme | 47 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported. C: comparator; I: intervention; n: number of participants. | ||||||||
Appendix 11. Adverse events (III)
| Trial | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Atabek 2008 | I: metformin + diet and physical activity advice | 90 |
1. Diarrhoea and mild abdominal pain 2. Mild discomfort from the abdomen |
1. 1 2. 1 |
1. 1.1 2. 1.1 |
| C: placebo + diet and physical activity advice | 30 | ‐ | ‐ | ‐ | |
| Berkowitz 2003 | I: behavioural programme + sibutramine | 42 |
1. High blood pressure and pulse rate 2. High blood pressure only 3. High pulse rate only 4. Knee surgery 5. Ventricular premature beats 6. Cholelithiasis/cholecystectomy 7. Rash, viral |
1. 3 2. 1 3. 1 4. 1 5. 1 6. 1 7. 1 |
1. 7.1 2. 2.4 3. 2.4 4. 2.4 5. 2.4 6. 2.4 7. 2.4 |
| C: behavioural programme + placebo | 39 |
1. Elevated blood pressure and pulse rate* 2. Elevated pulse rate only* 3. Atrial premature beats 4. Tonsillectomy 5. Ventricular premature beats* 6. Ecchymoses* |
1. 1 2. 1 3. 1 4. 2 5. 1 6. 2 |
1. 2.6 2. 2.6 3. 2.6 4. 5.1 5. 2.6 6. 5.1 |
|
| Berkowitz 2006 | I: behavioural therapy programme + sibutramine | 368 |
1. Infection 2. Headache 3. Pharyngitis 4. Tachycardia 5. Accidental injury 6. Dry mouth 7. Pain 8. Hypertension 9. Rhinitis 10. Abdominal pain 11. Dysmenorrhoea 12. Vomiting 13. Cough increased 14. Nausea 15. Dizziness 16. Rash 17. Sinusitis 18. Constipation 19. Flu syndrome 20. Insomnia 21. Viral infection 22. Allergic reaction 23. Suicide attempt 24. Depression 25. Syncope 26. Chest pain 27. Arrhythmia 28. Extra systoles |
1. 167 2. 113 3. 49 4. 46 5. 41 6. 41 7. 42 8. 39 9. 41 10. 37 11. 21 12. 32 13. 28 14. 31 15. 28 16. 25 17. 24 18. 24 19. 23 20. 23 21. 20 22. 18 23. 1 24. 5 25. ‐ 26. ‐ 27. ‐ 28. ‐ |
1. 45.3 2. 30.7 3. 13.3 4. 12.5 5.11.1 6.11.1 7. 11.4 8. 10.6 9. 11.1 10. 10.1 11. 5.7 12. 8.7 13. 7.6 14. 8.4 15. 7.6 16. 6.8 17. 6.5 18. 6.5 19. 6.3 20. 6.3 21. 5.4 22. 4.9 23. 0.3 24. 1.4 25. ≤ 1.5 26. ≤ 1.5 27. ≤ 1.5 28. ≤ 1.5 |
| C: behavioural therapy programme + placebo | 130 |
1. Infection 2. Headache 3. Pharyngitis 4. Tachycardia 5. Accidental injury 6. Dry mouth 7. Pain 8. Hypertension 9. Rhinitis 10. Abdominal pain 11. Dysmenorrhoea 12. Vomiting 13. Cough increased 14. Nausea 15. Dizziness 16. Rash 17. Sinusitis 18. Constipation 19. Flu syndrome 20. Insomnia 21. Viral infection 22. Allergic reaction 23. Suicide attempt 24. Depression 25. Syncope 26. Chest pain 27. Arrhythmia 28. Extra systoles |
1. 53 2. 39 3. 23 4. 8 5. 8 6. 8 7. 12 8. 11 9. 17 10. 12 11. 13 12. 7 13. 12 14. 12 15. 5 16. 7 17. 6 18. 3 19. 7 20. 4 21. 2 22. 7 23. 1 24. 1 25. ‐ 26. ‐ 27. ‐ 28. ‐ |
1. 41 2. 30 3. 18 4. 6.2 5. 6.2 6. 6.2 7. 9.2 8. 8.5 9. 13.1 10. 9.2 11. 10 12. 5.4 13. 9.2 14. 9.2 15. 3.8 16. 5.4 17. 4.6 18. 2.3 19. 5.4 20. 3.1 21. 1.5 22. 5.4 23. 0.8 24. 0.8 25. ≤ 1.5 26. ≤ 1.5 27. ≤ 1.5 28. ≤ 1.5 |
|
| Chanoine 2005 | I: orlistat + diet + exercise + behavioural therapy | 352 |
1. Fatty/oily stool 2. Oily spotting 3. Oily evacuation 4. Abdominal pain 5. Fecal urgency 6. Flatus with discharge 7. Soft stool 8. Nausea 9. Increased defecation 10. Flatulence 11. Fecal incontinence 12. Headache 13. Upper respiratory tract infection 14. Nasopharyngitis 15. Sore throat 16. Sinusitis 17. Joint sprain 18. Nasal congestion 19. Back pain 20. Gastroenteritis 21. Seasonal rhinitis 22. Limb injury 23. Asymptomatic gallstones 24. Pilonidal abscess 25. Depression 26. Asthma attack 27. Seizure 28. Admission for repair of deviated nasal septum 29. Appendicitis 30. Cholelithiasis 31. Gallbladder disorder followed by cholecystectomy 32. Adenoidal hypertrophy 33. Aseptic meningitis 34. Electrocardiogram abnormalities |
1. 177 2. 102 3. 82 4. 77 5. 73 6. 70 7. 53 8. 52 9. 48 10. 32 11. 31 12. 134 13. 114 14. 99 15. 59 16. 40 17. 35 18. 31 19. 28 20. 23 21. 21 22. 18 23. 6 24. 1 25. 2 26. 1 27. 1 28. 1 29. 1 30. 1 31. 1 32. 1 33. 1 34. 10 |
1. 50.3 2. 29.0 3. 23.3 4. 21.9 5. 20.7 6. 19.9 7. 15.1 8. 14.8 9. 13.6 10. 9.1 11. 8.8 12. 38.1 13. 32.4 14. 28.1 15. 16.8 16. 11.4 17. 9.9 18. 8.8 19. 8.0 20. 6.5 21. 6.0 22. 5.1 23. 1.7 24. 0.3 25. 0.6 26. 0.3 27. 0.3 28. 0.3 29. 0.3 30. 0.3 31. 0.3 32. 0.3 33. 0.3 34. 2.8 |
| C: placebo + diet + exercise + behavioural therapy | 181 |
1. Fatty/oily stool 2. Oily spotting 3. Oily evacuation 4. Abdominal pain 5. Fecal urgency 6. Flatus with discharge 7. Soft stool 8. Nausea 9. Increased defecation 10. Flatulence 11. Fecal incontinence 12. Headache 13. Upper respiratory tract infection 14. Nasopharyngitis 15. Sore throat 16. Sinusitis 17. Joint sprain 18. Nasal congestion 19. Back pain 20. Gastroenteritis 21. Seasonal rhinitis 22. Limb injury 23. Acute demyelinating encephalomyelitis 24. Facial palsy 25. Pneumonia 26. Worsening of asthma 27. Pain in the right side 28. Electrocardiogram abnormalities |
1.15 2. 7 3. 3 4. 20 5. 20 6. 5 7. 19 8. 23 9. 16 10. 8 11. 1 12. 56 13. 48 14. 46 15. 29 16. 19 17. 17 18. 11 19. 11 20. 8 21. 9 22. 5 23. 1 24. 1 25. 1 26. 1 27. 1 28. 1 |
1. 8.3 2. 3.9 3. 1.7 4. 11.0 5. 11.0 6. 2.8 7. 10.5 8. 12.7 9. 8.8 10. 4.4 11. 0.6 12. 30.9 13. 26.5 14. 25.4 15. 16.0 16. 10.5 17. 9.4 18. 6.1 19. 6.1 20. 4.4 21. 5.0 22. 2.8 23. 0.6 24. 0.6 25. 0.6 26. 0.6 27. 0.6 28. 0.6 |
|
| Clarson 2009 | I: metformin + lifestyle intervention | 14 | ‐ | ‐ | ‐ |
| C: lifestyle intervention only | 17 | ‐ | ‐ | ‐ | |
| Franco 2014 | I: sibutramine + dietary guidance | 63 |
1. Anorexia 2. Dry mouth 3. Headache 4. Constipation 5. Changing the mood 6. Dyspnoea 7. Epigastralgia 8. Hypertension 9. Insomnia 10. Nausea 11. Tachycardia 12. Dizziness 13. Tremors 14. Vomiting |
‐ |
1. 0.9 2. 1.7 3. 6.8 4. 3.8 5. 1.3 6. 0.4 7. 0.9 8. 0.9 9. 1.3 10. 2.1 11. 1.3 12. 3.4 13. 0.4 14. 0.4 |
| C: placebo + dietary guidance | 63 |
1. Change in taste 2. Headache 3. Diarrhoea 4. Hypertension 5. Irritability 6. Tachycardia 7. Dizziness |
‐ |
1. 0.9 2. 3.3 3. 2.8 4. 0.5 5. 1.4 6. 0.5 7. 0.9 |
|
| Freemark 2001 | I: metformin | 15 |
1. Transient abdominal discomfort or diarrhoea 2. Intermittent nausea |
1. 3 2. 1 |
1. 20 2. 6.7 |
| C: placebo | 17 | 1. Transient abdominal discomfort or diarrhoea | 1. 1 | 1. 5.9 | |
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 23 |
1. Headache 2. Dry mouth 3. Headache with nausea 4. Headache with weakness 5. High DBP 6. High heart rate 7. High blood pressure (baseline) 8. High blood pressure (end of trial) |
1. 1 2. 1 3. 1 4. 1 5. 1 6. 3 7. 2 8. 2 |
1. 4.3 2. 4.3 3. 4.3 4. 4.3 5. 4.3 6. 13.0 7. 8.7 8. 8.7 |
| C: placebo + diet + exercise | 23 |
1. Headache 2. Headache with somnolence 3. Headache with dry mouth 4. High DBP 5. High heart rate 6. High blood pressure (baseline) 7. High blood pressure (end of trial) |
1. 2 2. 1 3. 1 4. 2 5. 2 6. 7 7. 2 |
1. 8.7 2. 4.3 3. 4.3 4. 8.7 5. 8.7 6. 30.4 7. 8.7 |
|
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | 30 |
1. Dry mouth 2. Headache 3. Constipation 4. Abdominal pain 5. Cold 6. Dizzy 7. Tonsillitis 8. Menstrual cramp 9. Nausea 10. Toothache 11. Otitis 12. Hair loss 13. Rhinitis 14. Sinusitis 15. Sleepiness 16. Dry cough 17. Myalgia 18. Viral infection 19. Lumbago |
1. 7 2. 13 3. 12 4. 3 5. 9 6. 3 7. 2 8. 8 9. 3 10. 3 11. 3 12. 2 13. 1 14. 1 15. 1 16. 1 17. 1 18. 2 19. 2 |
1. 23.3 2. 43.3 3. 40.0 4. 10.0 5. 30.0 6. 10.0 7. 6.7 8. 26.7 9. 10.0 10. 10.0 11. 10.0 12. 6.7 13. 3.3 14. 3.3 15. 3.3 16. 3.3 17. 3.3 18. 6.7 19. 6.7 |
| C: placebo + hypocaloric diet + exercise | 30 |
1. Dry mouth 2. Headache 3. Constipation 4. Abdominal pain 5. Cold 6. Dizzy 7. Tonsillitis 8. Menstrual cramp 9. Nausea 10. Toothache 11. Otitis 12. Hair loss 13. Rhinitis 14. Sinusitis 15. Sleepiness 16. Dry cough 17. Myalgia 18. Bronchitis 19. Inguinal dermatitis 20. Fever |
1. 3 2. 21 3. 4 4. 4 5. 11 6. 2 7. 2 8. 6 9. 1 10. 1 11. 1 12. 1 13. 2 14. 2 15. 2 16. 2 17. 2 18. 2 19. 2 20. 2 |
1. 10.0 2. 70.0 3. 13.3 4. 13.3 5. 36.7 6. 6.7 7. 6.7 8. 20.0 9. 3.3 10. 3.3 11. 3.3 12. 3.3 13. 6.7 14. 6.7 15. 6.7 16. 6.7 17. 6.7 18. 6.7 19. 6.7 20. 6.7 |
|
| Kendall 2013 | I: metformin + healthy lifestyle advice | 74 | ‐ | ‐ | ‐ |
| C: control + healthy lifestyle advice | 77 | ‐ | ‐ | ‐ | |
| Maahs 2006 | I: orlistat + diet and exercise therapy | 20 | ‐ | ‐ | ‐ |
| C: placebo + diet and exercise therapy | 20 | ‐ | ‐ | ‐ | |
| All: | 40 |
1. Soft stools 2. Oily spotting 3. Fatty or oily stools 4. Oily evacuation 5. Liquid stools 6. Cramping 7. Flatus with discharge 8. Fecal incontinence |
‐ | ‐ | |
| Mauras 2012 | I: metformin + diet/exercise intervention | 35 | ‐ | ‐ | ‐ |
| C: diet/exercise intervention | 31 | ‐ | ‐ | ‐ | |
| NCT00001723 | I: orlistat + behavioural weight loss programme | 100 |
1. Hypoglycaemia 2. Left lower quadrant pain and vomiting 3. Ear disorders (otitis, earache, ear pain) 4. Eye disorders (change in vision, conjunctivitis, styes) 5. Abdominal pain or cramping 6. Bloating or gas 7. Borborygmi 8. Constipation 9. Controlled discharge of oil without stool 10. Decreased frequency of bowel movements 11. Diarrhoea 12. Fatty‐appearing stools 13. Flatulence (passage of gas) 14. Flatus with discharge 15. Frequent urge for bowel movement 16. Hiccups 17. Increased frequency of bowel movements 18. Nausea 19. Oily spotting 20. Rectal bleeding ‐ haemorrhoids 21. Soft or deliquescent stools 22. Stomach pain or cramps 23. Stools almost all liquid with very few solid parts 24. Stools hard and in the shape of small pellets 25. Stools mixed with fat or with a separate oily layer 26. Uncontrolled passage of stool or oil 27. Urgent, but controlled, need to produce stools 28. Vomiting 29. Dizziness 30. Epistaxis 31. Feeling cold 32. Headache 33. Increased sweating 34. Increased thirst 35. Sinusitis, postnasal drip or nasal stuffiness 36. Unusual tiredness or weakness (fatigue) 37. Pharyngitis 38. Sinusitis, postnasal drip or nasal stuffiness 39. Decrease in appetite 40. Muscle pain, stiffness, cramps or ache 41. Migraine headaches 42. Mental depression 43. Dysuria or UTI 44. Nocturia 45. Asthma symptoms 46. Cough 47. Upper respiratory infection 48. Skin rash |
1. 0 2. 0 3. 7 4. 8 5.16 6. 18 7. 6 8. 1 9. 56 10. 25 11. 21 12. 61 13. 60 14. 43 15. 19 16. 1 17. 68 18. 10 19. 6 20. 4 21. 68 22. 8 23. 64 24. 11 25. 83 26. 60 27. 44 28. 7 29. 4 30. 5 31. 5 32. 14 33. 3 34. 5 35. 2 36. 1 37. 6 38. 1 39. 11 40. 16 41. 3 42. 1 43. 1 44. 0 45. 5 46. 0 47. 14 48. 5 |
1. 0 2. 0 3. 7 4. 8 5.16 6. 18 7. 6 8. 1 9. 56 10. 25 11. 21 12. 61 13. 60 14. 43 15. 19 16. 1 17. 68 18. 10 19. 6 20. 4 21. 68 22. 8 23. 64 24. 11 25. 83 26. 60 27. 44 28. 7 29. 4 30. 5 31. 5 32. 14 33. 3 34. 5 35. 2 36. 1 37. 6 38. 1 39. 11 40. 16 41. 3 42. 1 43. 1 44. 0 45. 5 46. 0 47. 14 48. 5 |
| C: placebo + behavioural weight loss programme | 100 |
1. Hypoglycaemia 2. Left lower quadrant pain and vomiting 3. Ear disorders (otitis, earache, ear pain) 4. Eye disorders (change in vision, conjunctivitis, styes) 5. Abdominal pain or cramping 6. Bloating or gas 7. Borborygmi 8. Constipation 9. Controlled discharge of oil without stool 10. Decreased frequency of bowel movements 11. Diarrhoea 12. Fatty‐appearing stools 13. Flatulence (passage of gas) 14. Flatus with discharge 15. Frequent urge for bowel movement 16. Hiccups 17. Increased frequency of bowel movements 18. Nausea 19. Oily spotting 20. Rectal bleeding ‐ haemorrhoids 21. Soft or deliquescent stools 22. Stomach pain or cramps 23. Stools almost all liquid with very few solid parts 24. Stools hard and in the shape of small pellets 25. Stools mixed with fat or with a separate oily layer 26. Uncontrolled passage of stool or oil 27. Urgent, but controlled, need to produce stools 28. Vomiting 29. Dizziness 30. Epistaxis 31. Feeling cold 32. Headache 33. Increased sweating 34. Increased thirst 35. Sinusitis, postnasal drip or nasal stuffiness 36. Unusual tiredness or weakness (fatigue) 37. Pharyngitis 38. Sinusitis, postnasal drip or nasal stuffiness 39. Decrease in appetite 40. Muscle pain, stiffness, cramps, or ache 41. Migraine headaches 42. Mental depression 43. Dysuria or UTI 44. Nocturia 45. Asthma symptoms 46. Cough 47. Upper respiratory infection 48. Skin rash |
1. 1 2. 1 3. 7 4. 9 5. 21 6. 5 7. 2 8. 7 9. 11 10. 22 11. 8 12. 6 13. 47 14. 11 15. 3 16. 3 17. 45 18. 9 19. 0 20. 2 21. 42 22. 9 23. 34 24. 10 25. 18 26. 11 27. 18 28. 7 29. 4 30. 2 31.2 32. 17 33. 4 34. 4 35. 5 36. 5 37. 12 38. 3 39. 9 40. 12 41. 0 42. 3 43. 5 44. 3 45. 3 46. 7 47. 17 48. 2 |
1. 1 2. 1 3. 7 4. 9 5. 21 6. 5 7. 2 8. 7 9. 11 10. 22 11. 8 12. 6 13. 47 14. 11 15. 3 16. 3 17. 45 18. 9 19. 0 20. 2 21. 42 22. 9 23. 34 24. 10 25. 18 26. 11 27. 18 28. 7 29. 4 30. 2 31.2 32. 17 33. 4 34. 4 35. 5 36. 5 37. 12 38. 3 39. 9 40. 12 41. 0 42. 3 43. 5 44. 3 45. 3 46. 7 47. 17 48. 2 |
|
| Ozkan 2004 | I: conventional treatment + orlistat | 22 |
1. Frequent stools 2. Soiling, frequent defecation 3. Mild hair loss 4. Reported muscle cramps |
1. 22 2. 5 3. 1 4. 1 |
1. 100 2. 22.7 3. 4.5 4. 4.5 |
| C: conventional treatment | 20 | ‐ | ‐ | ‐ | |
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 10 |
1. Increase levels of ALT 2. Increase levels of AST 3. Reduction in haemoglobin |
‐ | ‐ |
| C: placebo + nutritional guide and exercise programme | 9 |
1. Increase levels of ALT 2. Increase levels of AST 3. Reduction in haemoglobin |
‐ | ‐ | |
| Rezvanian 2010 | I1: metformin + healthy eating and physical activity advice | 45 |
1. Headache 2. Abdominal pain 3. Loose stool |
1. 2 2. 2 3. 3 |
1. 4.4 2. 4.4 3. 6.6 |
| I2: fluoxetine + healthy eating and physical activity advice | 45 |
1. Dry mouth 2. Loose stool |
1. 3 2. 2 |
1. 6.6 2. 4.4 |
|
| I3: metformin and fluoxetine + healthy eating and physical activity advice | 45 | ‐ | ‐ | ‐ | |
| C: placebo + healthy eating and physical activity advice | 45 | ‐ | ‐ | ‐ | |
| Srinivasan 2006 | I: metformin first then placebo + "standardised information on healthy eating and exercise" | 13 | ‐ | ‐ | ‐ |
| C: placebo first then metformin + "standardised information on healthy eating and exercise" | 15 | ‐ | ‐ | ‐ | |
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | 12 |
1. Clinical depression 2. Flu syndrome 3. Headache 4. Abdominal complaints 5. Agitation 6. Increased appetite 7. Rash 8. Dizziness 9. Dysmenorrhoea 10. Joint problem 11. Heart rate > 100 bpm on 2 occasions 12. DBP > 85 mmHg on 2 occasions |
1. 1 2. 6 3. 2 4. 7 5. 3 6. 4 7. 2 8. 3 9. 3 10. 2 11. 4 12. 1 |
1. 8.3 2. 50 3. 16.6 4. 58.3 5. 25 6. 33.3 7. 16.6 8. 25 9. 25 10. 16.6 11. 33.3 12. 8.3 |
| C: placebo + energy‐restricted diet and exercise plan | 12 |
1. Flu syndrome 2. Headache 3. Agitation 4. Increased appetite 5. Dizziness 6. Joint problem 7. DBP > 85 mmHg on 2 occasions |
1. 6 2. 3 3. 1 4. 2 5. 1 6. 2 7. 1 |
1. 50 2. 25 3. 8.3 4. 16.6 5. 8.3 6. 16.6 7. 8.3 |
|
| Wiegand 2010 | I: metformin + multiprofessional lifestyle intervention | 36 |
1. Gastrointestinal symptoms 2. Unspecific (e.g. weakness or fatigue) |
1. 5 2. 3 |
1. 13.9 2. 8.3 |
| C: placebo + multiprofessional lifestyle intervention | 34 |
1. Gastrointestinal symptoms 2. Unspecific (e.g. weakness or fatigue) |
1. 9 2. 4 |
1. 26.5 2. 11.8 |
|
| Wilson 2010 | I: metformin + lifestyle intervention | 38 |
1. Headache 2. Nausea 3. Vomiting 4. Upper respiratory tract infection 5. Musculoskeletal complaints 6. Elevated ALT levels 7. Appendectomy 8. Leg vein thrombosis |
1. 18 2. 9 3. 6 4. 18 5. 5 6. 2 7. 1 8. 1 |
1. 47 2. 24 3. 16 4. 47 5. 13 6. 5 7. 3 8. 3 |
| C: placebo + lifestyle intervention | 38 |
1. Headache 2. Nausea 3. Vomiting 4. Upper respiratory tract infection 5. Musculoskeletal complaints 6. Elevated ALT levels |
1. 13 2. 3 3. 1 4. 23 5. 7 6. 1 |
1. 34 2. 8 3. 3 4. 61 5. 18 6. 3 |
|
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | 53 |
1. Liquid or loose stools 2. Vomiting 3. Fatigue 4. Lost interest in usual pleasurable activities |
1. 22 2. 22 3. 20 4. 1 |
1. 41.5 2. 41.5 3. 37.7 4. 1.9 |
| C: placebo + dietitian‐administered weight‐reduction programme | 47 |
1. Liquid or loose stools 2. Vomiting 3. Fatigue |
1. 8 2. 10 3. 7 |
1. 17 2. 21.3 3. 14.9 |
|
| *Berkowitz 2003: these adverse events occurred during the open‐label phase where all participants received sibutramine ALT: alanine transaminase; AST: aspartate transaminase; bpm: beats per minute; DBP: diastolic blood pressure; n: number of participants; UTI: urinary tract infection | |||||
Appendix 12. Survey of authors providing information on included trials
| Trial | Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Atabek 2008 | 24 January 2014 15 May 2014 |
No | 24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details about the trial including funding, allocation concealment, randomisation method, dropout rates and adverse events |
N/A |
| Berkowitz 2003 | 20 January 2014 15 May 2014 |
No | 20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details about the trial including randomisation method, allocation concealment and adverse events |
N/A |
| Berkowitz 2006 | 20 January 2014 15 May 2014 |
No | 20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial's adverse events |
N/A |
| Chanoine 2005 | 20 January 2014 25 March 2014 15 May 2014 |
20 January 2014 25 March 2014 15 May 2014 03/06/2014 |
20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 25 March 2014 ‐ asked for raw BMI and SD values at 6 months' follow‐up 15 May 2014 ‐ asked for further details about the trial including blinding and adverse events |
20 January 2014 ‐ author replied with confirmation the data of the trial was correct and attached an addition paper for the trial 25 March 2014 ‐ author provided the raw data at 6 months 15 May 2014 ‐ provided further details on blinding 3 June 2014 ‐ gave additional results on adverse events |
| Clarson 2009 | 24 January 2014 15 May 2014 |
31 January 2014 19 May 2014 |
24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details about the trial including allocation concealment, blinding and adverse events |
31 January 2014 ‐ author confirmed there was no further data for the trial and provided a protocol for an ongoing trial 19 May 2014 ‐ author provided further details about the trial |
| Franco 2014 | 24 February 2015 | 9 March 2015 | 24 February 2015 | 9 March 2015 ‐ authors replied with further details on the trial such as funding source, randomisation method and blinding |
| Freemark 2001 | 20 January 2014 25 March 2014 15 May 2014 |
16 May 2014 | 20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 25 March 2014 ‐ asked for BMI raw data and associated SDs 15 May 2014 ‐ asked for further details about the trial including allocation concealment, blinding and adverse events |
16 May 2014 ‐ author provided further details about the trial |
| Garcia‐Morales 2006 | 21 January 2014 15 May 2014 |
No | 21 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details about the trial including the run‐in period, blinding and adverse events |
N/A |
| Godoy‐Matos 2005 | 20 January 2014 15 April 2014 |
17 May 2014 | 20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 April 2014 ‐ asked for further details about the trial including allocation concealment, randomisation method, blinding and adverse events |
17 May 2014 ‐ author provided further details about the trial |
| Kendall 2013 | 24 January 2014 15 May 2014 |
29 January 2014 | 24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details about the trial including blinding and adverse events |
29 January 2014 ‐ author confirmed no additional data were available for the trial Author did not reply to the follow‐up email (15 May 2014) |
| Maahs 2006 | 20 January 2014 09 May 2014 15 May 2014 |
20 January 2014 09 May 2014 15 May 2014 |
20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 09 May 2014 ‐ asked to confirmed if the data presented were SDs or SEs 15 May 2014 ‐ asked for further details on the trial's adverse events |
20 January 2014 ‐ author confirmed no further data were available for the trial 09 May 2014 ‐ author confirmed the data were SDs 15 May 2014 ‐ author could not provide further information on the adverse events |
| Mauras 2012 | 24 January 2014 15 May 2014 16 May 2014 |
15 May 2014 27 May 2014 |
24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including allocation concealment, randomisation method, number of trial centres, blinding and adverse events 16 May 2014 ‐ asked for further information on adverse events |
15 May 2014 ‐ author provided further details on the trial 27 May 2014 ‐ author said she would try to obtain the data; however, we received no further emails |
| NCT00001723 | 30 October 2015 | 30 October 2015 | 30 October 2015 ‐ asked for further details on the trial: blinding, allocation concealment, randomisation process, funding, publications and lifestyle programme | 30 October 2015 ‐ author replied and gave further details |
| Ozkan 2004 | No ‐ was unable to send emails to the address given in the publication | N/A | N/A | N/A |
| Prado 2012 | 17 January 2014 24 January 2014 15 May 2014 |
28 January 2014 18 May 2014 |
17 January 2014 ‐ asked for raw BMI data 24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including blinding, allocation concealment and adverse events |
28 January 2014 ‐ author was unable to provide any unpublished data 18 May 2014 ‐ author provided further information about the trial |
| Rezvanian 2010 | 24 January 2014 15 May 2014 |
24 January 2014 15 May 2014 |
24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including allocation concealment and adverse events |
24 January 2014 ‐ author confirmed there were no further details to give on the trial and provided references to other potentially relevant trials 15 May 2014 ‐ author provided further details about the trial |
| Srinivasan 2006 | 20 January 2014 15 May 2014 |
15 May 2014 | 20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including allocation concealment and adverse events |
15 May 2014 ‐ author provided further details about the trial |
| Van Mil 2007 | 20 January 2014 15 May 2014 |
20 January 2014 30 May 2014 |
20 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including randomisation, blinding and adverse events |
20 January 2014 ‐ author confirmed findings were correct and highlighted the main finding of their trial 30 May 2014 ‐ author provided further details about the trial |
| Wiegand 2010 | 24 January 2014 15/04/2014 |
27 January 2014 | 24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including allocation concealment, randomisation, blinding and adverse events |
27 January 2014 ‐ author confirmed there was no further data available for the trial Author did not reply to the follow‐up email |
| Wilson 2010 | 24 January 2014 15 May 2014 |
24 January 2014 15 May 2014 |
24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial including dropouts and adverse events |
24 January 2014 ‐ author confirmed there was no unpublished data on the main outcomes of the trial 15 May 2014 ‐ author said he would try to obtain the data; however, I did not receive any further emails |
| Yanovski 2011 | 24 January 2014 15 May 2014 |
24 January 2014 | 24 January 2014 ‐ asked for additional unpublished data and other ongoing trials 15 May 2014 ‐ asked for further details on the trial's adverse events |
24 January 2014 ‐ author confirmed the trial was over and there is no further information available Author did not reply to the follow‐up email |
| BMI: body mass index; N/A: not applicable; SD: standard deviation; SE: standard error. | ||||
Appendix 13. Checklist to aid consistency and reproducibility of GRADE assessments
| Questions | BMI | Weight | Adverse events (serious adverse events / adverse events causing discontinuation of trial) | Health‐related quality of life | All‐cause mortality | Morbidity | Socioeconomic effects | |
| Trial limitations (risk of bias)a | 1. Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes / Yes | N/A | N/A | N/A | N/A |
| 2. Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | Yes / Yes | |||||
| 3. Was there blinding of participants and personnel (i.e. no potential for performance bias)? | Yes | Yes | Yes / Yes | |||||
| 4. Was there blinding of outcome assessment (i.e. no potential for detection bias)? | Yes | Yes | Yes / Yes | |||||
| 5. Was an objective outcome used? | Yes | Yes | Yes / Yes | |||||
| 6. Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes / No (↓) | |||||
| 7. Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | No (↓) / No (↓) | |||||
| 8. No other biases reported (i.e. no potential of other bias)? | No (↓) | No (↓) | Unclear / Unclear | |||||
| 9. Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes / Yes | |||||
| Inconsistencyb | 1. Point estimates did not vary widely? | Yes | Yes | Yes / Yes | ||||
| 2. To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Some | Some | Substantial / Substantial | |||||
| 3. Was the direction of effect consistent? | No (↓) | No (↓) | No (↓) / No (↓) | |||||
| 4. What was the magnitude of statistical heterogeneity (as measured by I2) ‐ low (I2 < 40%), moderate (I2 = 40% to 60%), high (I2 > 60%)? | High (↓) | High (↓) | Low / Low | |||||
| 5. Was the test for heterogeneity statistically significant (P < 0.1)? | Statistically significant (↓) | Statistically significant (↓) | Not statistically significant / Not statistically significant | |||||
| Indirectnessa | 1. Were the populations in included studies applicable to the decision context? | Applicable | Applicable | Applicable / Applicable | ||||
| 2. Were the interventions in the included studies applicable to the decision context? | Applicable | Applicable | Applicable / Applicable | |||||
| 3. Was the included outcome not a surrogate outcome? | Yes | Yes | Yes / Yes | |||||
| 4. Was the outcome time frame sufficient? | Sufficient | Sufficient | Sufficient / Sufficient | |||||
| 5. Were the conclusions based on direct comparisons? | Yes | Yes | Yes / Yes | |||||
| Imprecisionc | 1. Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | Yes | No (↓) / No (↓) | ||||
| 2. What is the magnitude of the median sample size (high: > 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | Intermediate / Low (↓) | |||||
| 3. What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Large | Large | Moderate / Moderate | |||||
| 4. Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | N/A | Yes / Yes | |||||
| Publication biasd | 1. Was a comprehensive search conducted? | Yes | Yes | Yes / Yes | ||||
| 2. Was grey literature searched? | No (↓) | No (↓) | No (↓) / No (↓) | |||||
| 3. Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes / Yes | |||||
| 4. There was no industry influence on studies included in the review? | No (↓) | No (↓) | No (↓) / No (↓) | |||||
| 5. There was no evidence of funnel plot asymmetry? | No (↓) | No (↓) | Unclear / Unclear | |||||
| 6. There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear / Unclear | |||||
|
aQuestions on risk of bias are answered in relation to most of the aggregated evidence in the meta‐analysis rather than to individual studies.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I2 statistic. cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the certainty of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table. BMI: body mass index; N/A: not applicable. | ||||||||
Appendix 14. Health‐related quality of life: instruments
| Instrument |
| Short‐Form health survey (SF‐36, generic questionnaire) ‐ employed in García‐Morales 2006. |
| Brief Symptom Inventory (BSI, generic questionnaire), parent and children's KINDL (generic questionnaire), Impact of Weight on Quality of Life ‐ Kids (IWQOL‐Kids, specific questionnaire) and global ratings scale ‐ all employed in Maahs 2006. |
Data and analyses
Comparison 1. Body mass index (BMI): pharmacological interventions versus comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in BMI (all trials) | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 2 Change in BMI (drug type) | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 2.1 Metformin | 8 | 543 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [0.00, ‐0.69] |
| 2.2 Orlistat | 3 | 773 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.08, ‐0.51] |
| 2.3 Sibutramine | 5 | 568 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.89, ‐0.51] |
| 3 Change in BMI (dropout rate) | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 3.1 Dropouts < 20% | 9 | 597 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.78, ‐0.44] |
| 3.2 Dropouts ≥ 20% | 6 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.34, ‐0.50] |
| 3.3 Unclear dropout rate | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐3.74, ‐1.72] |
| 4 Change in BMI (intention‐to‐treat (ITT) analysis) | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 4.1 No ITT | 5 | 282 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐2.52, ‐0.60] |
| 4.2 ITT used | 11 | 1580 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.86, ‐0.65] |
| 5 Change in BMI (funding) | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 5.1 Commercial | 5 | 1009 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.69, ‐0.31] |
| 5.2 Noncommercial | 5 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.77, ‐0.44] |
| 5.3 Commercial + noncommercial | 4 | 262 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.86, ‐0.47] |
| 5.4 Unclear | 2 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐3.54, ‐0.04] |
| 6 Change in BMI (publication date) | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 6.1 2007 or before | 8 | 1163 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.21, ‐0.60] |
| 6.2 After 2007 | 8 | 699 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.90, ‐0.62] |
| 7 Change in BMI (quality of trial) | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 7.1 Low | 6 | 322 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.28, ‐0.52] |
| 7.2 Moderate | 10 | 1540 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.95, ‐0.67] |
| 8 Change in BMI (country) | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 8.1 Middle income | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐3.08, ‐1.69] |
| 8.2 High income | 13 | 1646 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.62, ‐0.56] |
| 9 Change in BMI (mean age) | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 9.1 Mean age < 12 years | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.53, ‐0.34] |
| 9.2 Mean age ≥ 12 years | 14 | 1664 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.79, ‐0.71] |
Comparison 2. Weight: pharmacological interventions versus comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight (all trials) | 11 | 1180 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.86, ‐1.94] |
| 2 Change in weight (drug type) | 11 | 1180 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.86, ‐1.94] |
| 2.1 Metformin | 4 | 372 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐5.79, ‐0.69] |
| 2.2 Sibutramine | 5 | 568 | Mean Difference (IV, Random, 95% CI) | ‐4.71 [‐8.10, ‐1.32] |
| 2.3 Orlistat | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐4.31, ‐0.65] |
2.2. Analysis.
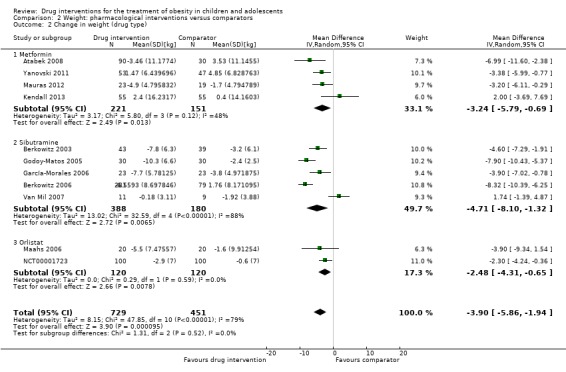
Comparison 2 Weight: pharmacological interventions versus comparators, Outcome 2 Change in weight (drug type).
Comparison 3. Adverse effects: pharmacological interventions versus comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 5 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.63, 3.25] |
| 1.1 Metformin | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.80] |
| 1.2 Orlistat | 3 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.41, 2.67] |
| 1.3 Sibutramine | 1 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.46, 27.33] |
| 2 Discontinued trial because of adverse events | 10 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.83, 2.52] |
| 2.1 Metformin | 3 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.26, 5.48] |
| 2.2 Orlistat | 4 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.74, 8.32] |
| 2.3 Sibutramine | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.53, 2.46] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Atabek 2008.
| Methods | Parallel randomised controlled trial, randomisation ratio 3:1 (intervention:control), superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + diet and physical activity advice Comparator: placebo + diet and physical activity advice Number of trial centres: 1 Treatment before trial: none Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, fasting insulin, 120‐min insulin levels, FGIR, HOMA‐IR, QUICKI | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: no information given Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine whether metformin treatment for 6 months is effective in reducing body weight and hyperinsulinaemia and also ameliorating insulin sensitivity indices in obese adolescents with hyperinsulinaemia" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of how allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk |
Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial" Comment: unsure who was blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk |
Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial" Comment: unsure who was blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk |
Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial." Comment: unsure who was blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial" Comment: unsure who was blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: the trial did not report the number of dropouts, or clarify there were no dropouts |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: the trial did not report the number of dropouts, or clarify there were no dropouts |
| Selective reporting (reporting bias) | High risk |
Quote: "A detailed questionnaire on food consumption was completed at the beginning and at the end of the trial medication period" Comment: no results shown for food consumption data. Also, very unclear on the number lost to follow‐up and what type of analyses were conducted |
| Other bias | High risk | Comment: there was uncertainty to whether this was a randomised controlled trial or a matched controlled trial. Concern arose over a lack of description about randomisation, blinding and allocation. No rationale for the size of intervention group and no calculation of power. They also do not declare who funded the trial |
Berkowitz 2003.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: behavioural programme + sibutramine Comparator: behavioural programme + placebo Number of trial centres: 1 Treatment before trial: none Titration period: in medication‐treated participants, sibutramine was increased to 10 mg/day at week 3, and to 15 mg/day at week 7 |
|
| Outcomes | Outcomes reported in abstract of publication: weight (kg), BMI (kg/m2), reductions in hunger, number of participants who reduced dose or discontinued | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding and noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To increase weight loss in obese adolescents by combining a comprehensive behavioral program with pharmacotherapy" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: trial did not describe how allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of detection bias likely to be low due to blinding of all trial personnel |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of detection bias likely to be low due to blinding of all trial personnel |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: whilst dropout numbers were small, a more appropriate imputation method could have been used to strengthen data analysis. Imputation method only used for primary outcome measures (weight and waist circumference, which were objectively measured) |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: whilst dropout numbers were small, a more appropriate imputation method could have been used to strengthen data analysis. Imputation method only used for primary outcome measures (weight and waist circumference, which were objectively measured) |
| Selective reporting (reporting bias) | Low risk | Comment: no differences found between clinical trial entry and publication |
| Other bias | Unclear risk | Comment: trial was partly funded by 2 pharmaceutical companies. The trial declared these companies had no involvement in the design, analysis or interpretation of the data; however, still could have influenced the reporting of results in some way |
Berkowitz 2006.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 3:1 (sibutramine:placebo), superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: behaviour therapy programme + sibutramine Comparator: behaviour therapy programme + placebo Number of trial centres: 33 Treatment before trial: no Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, weight, triglyceride levels, high‐density lipoprotein cholesterol levels, insulin levels, insulin sensitivity, rate of tachycardia, completion rate | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To see whether sibutramine reduced weight more than placebo in obese adolescents who were receiving a behavior therapy program" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The randomization schedule was stratified by center and baseline BMI (≤37 kg/m2 or >37 kg/m2) and was computer‐generated in blocks of 4 by the sponsor. Each site was responsible for assigning sequential treatments within each stratum" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "The sponsor kept allocation codes sealed and secure until the database was locked before analysis" Comment: allocation concealment was sufficient to protect against bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: LOCF was only used to replace BMI missing data; other objective outcome data were expressed for completers only. Dropout rate was fairly moderate and higher in the placebo group compared to the drug group. Difficult to access level of attrition bias based on these factors |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: LOCF was only used to replace BMI missing data only |
| Selective reporting (reporting bias) | Low risk | Comment: same outcomes reported in both clinical trial register and publication |
| Other bias | Unclear risk |
Quote: "the Statistics Department of Abbott Global Pharmaceutical Research and Development (including Ms. Hewkin) was responsible for data management and statistical analysis" Comment: potential influence of the funding body (Abbot Global Pharmaceuticals) |
Chanoine 2005.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 2:1 (orlistat: placebo), superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: orlistat + diet + exercise + behaviour therapy Comparator: placebo + diet + exercise + behaviour therapy Number of trial centres: 32 Treatment before trial: no Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, weight, fat mass (DEXA), waist circumference, adverse events | |
| Study details |
Run‐in period: placebo was given for 2 weeks before treatment began in the intervention group Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine the efficacy and safety of orlistat in weight management of adolescents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were randomized centrally according to a computer‐generated randomization schedule prepared by the study’s sponsor, with stratification by body weight (<80 kg or ≥80 kg) on day 1 and by weight loss during the lead‐in period (<1 kg or ≥1 kg)" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "The allocation process was triple‐blind; the allotted treatment group was obtained through an automated telephone system" Comment: allocation concealment was sufficient to protect against bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: even though an imputation method was used (LOCF), dropout rates were high. Effect on objective outcomes unclear |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: even though an imputation method was used (LOCF), dropout rates were high. Effect on subjective outcomes unclear |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to assess if all outcomes were reported due to the trial protocol not previously been published |
| Other bias | Unclear risk |
Quote: "Hoffmann‐La Roche was involved in the study design and conduct and in the analysis and interpretation of the data. All data were independently reanalyzed by an academic statistician" Comment: potential influence from the funding body (Hoffmann‐La Roche). No rationale to explain the imbalance in the number of participants in the 2 groups |
Clarson 2009.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + lifestyle intervention Comparator: lifestyle intervention Number of trial centres: 1 Treatment before trial: no Titration period: started metformin therapy at 500 mg/day, increasing by 500 mg/day every 7 days to a maximum tolerated dose of 500 mg x 3 per day |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, HOMA, adiponectin‐to‐leptin ratio, dyslipidaemic profiles, metabolic risk factors e.g. plasma lipids and adipocytokines | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To access the efficacy of adding metformin to a structured lifestyle intervention in reducing BMI in obese adolescents with insulin resistance" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "subjects were randomized using computer random number generation to lifestyle intervention alone or lifestyle in combination with metformin" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | High risk | Comment: author confirmed allocation was not concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote: "limitations to this study include the relatively small sample size and the absence of a placebo control group" Comment: the absence of a placebo in the control group meant participant and personnel blinding could not have been achieved. Author confirmed participants and personnel were not blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote: "limitations to this study include the relatively small sample size and the absence of a placebo control group" Comment: the absence of a placebo in the control group meant participant and personnel blinding could not have been achieved. Author confirmed participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: outcomes assessment was not blinded as confirmed by the author |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: outcomes assessment was not blinded as confirmed by the author |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: trial dropouts were fairly low; however, no imputation method was used |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: trial dropouts were fairly low; however, no imputation method was used |
| Selective reporting (reporting bias) | Unclear risk | Comment: no previously published protocol; therefore, unable to access reporting bias |
| Other bias | High risk |
Quote: "limitations to this study include the relatively small sample size and the absence of a placebo control group" Comment: a power calculation was not performed, therefore likely the trial was underpowered. No placebo given to the control group. Unclear whether there were baseline differences |
Franco 2014.
| Methods | Cross‐over randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: sibutramine + dietary guidance Comparator: placebo + dietary guidance Number of trial centres: 1 Treatment before trial: all participants had to have under gone at least 6 months of lifestyle intervention prior to recruitment Titration period: none |
|
| Outcomes | Outcomes reported in abstract of publication: % of participants who lost 10% of initial weight, weight, BMI | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: Portuguese Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The aim of this study was to evaluate the efficacy and safety of sibutramine in association with a multidisciplinary program for treatment of obesity and check its influence on metabolic laboratory changes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
From author: "patients were distributed according to a table of random numbers" Comment: randomisation process assessed as low risk |
| Allocation concealment (selection bias) | Low risk |
From author: "the study was double‐blind placebo‐controlled. Patients received placebo or sibutramine for 6 months, 1 month washout and in the next six months who received placebo began receiving sibutramine and vice verse. The researchers had no knowledge who was getting the drug and who was getting the placebo" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk |
Quote: "of the 63 patients who initiated the study only 23 patients completed the study" Comment: high attrition rate likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk |
Quote: "of the 63 patients who initiated the study only 23 patients completed the study" Comment: high attrition rate likely to affect subjective outcome (i.e. adverse effects) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available so risk was unclear |
| Other bias | High risk | Comment: lacked appropriate methodological detail and the failed to present the results in a meaningful and balanced manner. The cross‐over nature of the trial added to the difficulty in deciphering the results with such a high attrition rate. No power calculation performed |
Freemark 2001.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria: ‐ Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin Comparator: placebo Number of trial centres: 1 Treatment before trial: none Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, serum leptin, fasting blood glucose, fasting insulin levels, insulin sensitivity, glucose effectiveness, haemoglobin A1c, serum lipids, serum lactate, adverse events | |
| Study details |
Run‐in period: 48 hours' inpatient tests Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding and noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "We reasoned that drugs that increase glucose tolerance in diabetic patients might prove useful in preventing the progression to glucose intolerance in high‐risk patients" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "patients were randomized to the metformin and placebo groups by a research pharmacist using computer‐generated randomization tables" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "the allocation was made by the research pharmacist at the first medication visit. The pill bottles were coded ‐ thus the pharmacist was blinded to the medication" Comment: author confirmed allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: a missing data method was not used; however, dropout rates were fairly low |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: a missing data method was not used; however, dropout rates were fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: since no protocol was published before trial was completed, it is unclear whether all outcomes were reported |
| Other bias | High risk |
Quote: "the study involved a small number of patients and the results must be confirmed in a larger sample" Comment: the trial did not perform a power calculation and the sample size was small. It is likely the trial was underpowered. Potential influence of a commercial funding source. Baseline differences identified and not adjusted for in the analysis |
García‐Morales 2006.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: sibutramine + diet + exercise Comparator: placebo + diet + exercise Number of trial centres: 1 Treatment before trial: participants received dietetic advice 15 days before the beginning of the medications. In addition, clinical control visits also occurred before the start of the trial Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: mean weight loss, net weight loss, waist circumference, % BMI loss, SBP, DBP, heart rate, adverse events | |
| Study details |
Run‐in period: yes ‐ dietetic advice Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The goal of this article was to assess the efficacy and safety of sibutramine in obese Mexican adolescents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were block‐randomized by using a computer generated list" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "Patients were block‐randomized by using a computer generated list. All the materials for a patient were identified by the patient number. The placebo and drug capsules were identical in appearance and smell. The trial medications were prepared by one author (A.B.), who did not know the identity of the patients. Another author (L.M.G.‐M.) received the trial materials without any knowledge of the procedures or order in the random number list" Comment: allocation was appropriately concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk |
Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk |
Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk |
Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk |
Quote: "the last observation replaced the missing values" Comment: LOCF and modified intention‐to‐treat analysis was used to replace missing data for the primary outcomes. However, the 5 participants who dropped out before the first month were not included |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: baseline and follow‐up data for subjective outcomes were not reported in the publication |
| Selective reporting (reporting bias) | High risk |
Quote: "A detailed questionnaire on food consumption was completed at the beginning and end of the trial" Comment: data on food consumption were not provided in the publication |
| Other bias | Unclear risk |
Quote: "This trial was supported by Abbott Laboratories de Mexico, S.A. de C.V., Mexico City, D.F, Mexico. Dr. Berber was the medical manager of sibutramine in Mexico from 1995 to April 2004. The protocol was designed by all the authors; the study was conducted by the non industry authors; and analysis and publication formalities were performed by Drs. Garcia‐Morales, Del‐Rio‐Navarro, and Berber. The non industry authors had access to all the data generated" Comment: the trial sponsor (Abbot Laboratories) may have influenced the trial's results |
Godoy‐Matos 2005.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: sibutramine + hypocaloric diet + exercise Comparator: placebo + hypocaloric diet + exercise Number of trial centres: 1 Treatment before trial: during the run‐in period all participants received dietary counselling to achieve an energy deficit of 500 kcal/day. They also all received placebo capsules Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, mean BMI reduction, adverse events | |
| Study details |
Run‐in period: a single‐blind, 4‐week, placebo run‐in period Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The aim of this study was to determine the efficacy and safety of sibutramine in obese adolescents" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were allocated in a random block fashion to placebo or sibutramine" Comment: details of the randomisation process was provided by the author ‐ process seems adequate |
| Allocation concealment (selection bias) | Low risk |
Quote: "By means of a sealed envelope with a coded number. A container with boxes for each patient displaying the code number were provided. Each box had blisters for each visit with 40 capsules (similar for placebo or active drug). Patients were supplied in each visit with a new box. Adherence was judged by counting used capsules" Comment: allocation was concealed as confirmed by the author |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: dropout fairly low; however, was higher in the placebo group. Only completers results shown |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: dropout fairly low; however, was higher in the placebo group. Only completers results shown |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol was not published before the trial was completed, therefore it is unclear whether all outcomes were reported |
| Other bias | High risk |
Quote: "this work was supported by a grant from Abbott Laboratories" Comment: the trial did not highlight how involved Abbott Laboratories were the trial design, analysis and interpretation of the results Quote: "Conclusions regarding treatment group differences are somewhat limited by the small sample size" Comment: the trial did not perform a power calculation. Likely the trial was underpowered |
Kendall 2013.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + healthy lifestyle advice Comparator: placebo + healthy lifestyle advice Number of trial centres: 6 Treatment before trial: all participants were provided with standardised healthy lifestyle advice at the start in a 1‐to‐1 session, including a healthy diet advice sheet and increased levels of exercise (available upon request) Titration period: participants were instructed to gradually increase the dose by taking 1 pill with breakfast for 1 week and then 1 pill with breakfast and the evening meal the next week and then 2 pills with breakfast and 1 pill with the evening meal thereafter (1.5 g/day) |
|
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, fasting glucose, ALT, ALR | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The objective of the study was to assess the effect of metformin on body mass index SD score (BMI‐SDS), metabolic risk factors, and adipokines" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Independent pharmacists dispensed either metformin or placebo according to a computer‐generated randomization list for each stratification group" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "The third party, concealed allocation process ensured that participants and all investigators were unaware of the allocated treatment" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk |
Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk |
Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk |
Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk |
Quote: "There were a number of limitations to the MOCA [Metformin in Obese Children and Adolescents] trial including the dropout rate" Comment: dropout rate was high and no imputation method was used to replace missing data |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk |
Quote: "There were a number of limitations to the MOCA trial including the dropout rate" Comment: dropout rate was high and no imputation method was used to replace missing data |
| Selective reporting (reporting bias) | Unclear risk |
Quote: "In the MOCA trial, three previously validated questionnaires (food frequency, diet and eating behavior, and physical activity) were completed by each child at the start and end of the trial. This amounted to a large amount of data, and resources were unfortunately insufficient to allow analysis of these data for inclusion in this paper" Comment: behaviour change results were not reported; however, the publication did give a valid reason to why |
| Other bias | Unclear risk | Comment: insufficient information to assess whether an important risk of bias exists |
Maahs 2006.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: orlistat + diet and exercise therapy Control: placebo + diet and exercise therapy Number of trial centres: 1 Treatment before trial: none Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: BMI reduction, adverse effects, laboratory measurements | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To evaluate the efficacy of orlistat to enhance weight loss in obese adolescents" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "The GCRC [General Clinical Research Center] statistician generated the randomization sequence before the start of the study" "Two sets of subjects (a sister‐sister pair and a girlfriend‐boyfriend pair) were assigned to the same cohort, as determined by the order of entry of the first member of the pair; the next paired subject was blocked into the same cohort and given the next available number in that cohort" Comment: not all participants were randomised |
| Allocation concealment (selection bias) | Low risk |
Quote: "The list of randomization assignments was sealed and sent to the study pharmacist, who had no contact with study subjects" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout was fairly low |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: unable to access effect on subjective outcomes as quality of life results were not reported |
| Selective reporting (reporting bias) | High risk | Comment: results from the quality of life questionnaires were not reported |
| Other bias | Unclear risk | Comment: unclear if any other bias exists |
Mauras 2012.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + diet/exercise intervention Comparator: diet/exercise intervention Number of trial centres: 1 Treatment before trial: no Titration period: metformin was started at 250 mg orally, twice daily, before meals titrating up to 500 mg twice daily in children < 12 years old and 1000 mg twice daily as tolerated in older children |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, hsCRP, fibrinogen, intrahepatic fat | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine if metformin improves markers of inflammation, thrombosis, and intrahepatic fat contents in children with uncomplicated obesity" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote (from the author): "randomisation assignments were balanced for pubertal status. We used sealed envelopes with equal amount of labels organized at random for pubertal and pre‐pubertal kids to choose from at their CRC visit (baseline)" Comment: adequate randomisation process |
| Allocation concealment (selection bias) | Low risk | Comment: the author of the trial confirmed allocation was concealed via the sealed envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: no placebo was given to the control group, therefore the participants would not have been blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: no placebo was given to the control group, therefore the participants would not have been blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: author confirmed the outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: author confirmed the outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: there was a high number of dropouts and no imputation method was used to replace missing data |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: there was a high number of dropouts and no imputation method was used to replace missing data |
| Selective reporting (reporting bias) | Low risk |
Quote: "The study was registered at http://www.clinicaltrials.gov (NCT00139477)" Comment: all outcomes reported on the clinical trial register page were reported in the publication |
| Other bias | Unclear risk | Comment: unable to access if any other bias were present |
NCT00001723.
| Methods |
Type of trial: interventional, randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
|
| Participants |
Condition:
Enrolment: 200 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: orlistat Comparator: placebo |
|
| Outcomes |
Primary outcome: change in BMI SDS (baseline to 6 months) Secondary outcomes:
|
|
| Study details |
NCT number: NCT00001723 Other trial ID numbers: 980111, 98‐CH‐0111 |
|
| Publication details | "Safety and Efficacy of Orlistat (Xenical, Hoffmann LaRoche) in African American and Caucasian Children and Adolescents with Obesity‐Related Comorbid Conditions" | |
| Stated aim for study | Quote: "Researchers propose to determine the safety, tolerability, and efficacy of Xenical [orlistat] in 12‐17 year old severely obese African American and Caucasian children and adolescents who have one or more obesity‐related disease (hypertension, hyperlipidemia, sleep apnea, hepatic steatosis, insulin resistance, impaired glucose tolerance, or Type 2 diabetes)" | |
| Notes | The trial was completed when identified. Trial collaborators:
Results presented on the clinicaltrials.gov website and in a conference abstract. Results from ClinicalTrials.gov Results Database: change in BMI SDS orlistat: ‐0.12 ± 0.02 and placebo: ‐0.06 ± 0.02. ANCOVA differences between groups P value = 0.007. Change in bodyweight orlistat: ‐2.9 ± 0.7 and placebo: ‐0.6 ± 0.7. No statistical analysis provided. Change in BMI orlistat: ‐1.44 ± 0.26 and placebo: ‐0.50 ± 0.20. No statistical analysis provided. 95/100 participants in orlistat and 94/100 in placebo group experienced adverse events with the most common being gastrointestinal disorders. No serious adverse events in orlistat group. In placebo group, 1 participant had hypoglycaemia and 1 participant had left lower quadrant pain and vomiting, and was admitted to hospital overnight |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from author (via email): "We randomized participants in a 1:1 fashion to orlistat 120 mg or identical appearing placebo thrice daily with meals plus a daily multivitamin (Centrum, Whitehall‐Robins Healthcare, Madison, NJ) containing 5000 IU vitamin A (80% as retinol, 20% as beta carotene), 400 IU vitamin D as ergocalciferol, 30 IU vitamin E (as di‐α tocopheryl acetate), and 25 mcg vitamin K (as phytonadione). Investigators assigned consecutive code numbers to participants from pre‐specified lists that were stratified by race (Caucasian versus African American), sex (Male, Female), and degree of pubertal development (3 strata for boys: testes <15ml, testes 15‐20mL, and testes >20mL; for girls: Breast Tanner stage I‐III; Tanner stage IV, and Tanner stage V). The NIH CRC Pharmaceutical Development Section used permuted blocks with stratification to generate allocations that translated code numbers into trial group assignments by using a pseudo‐random number program" Comment: randomisation process described |
| Allocation concealment (selection bias) | Low risk |
Quote from author (via email): "Pharmacy personnel not involved with the conduct of the study, dispensed identical‐appearing study capsules in containers that differed only by participant code number. During the trial, no participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: assessors were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: assessors were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: according to ClinicalTrials.gov, 87% of orlistat participants completed the trial, 84% completed placebo arm |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: according to ClinicalTrials.gov, 87% of orlistat participants completed the trial, 84% completed placebo arm |
| Selective reporting (reporting bias) | High risk | Comment: there are differences in the results reported on the ClinicalTrial.gov website and in the conference abstract |
| Other bias | Unclear risk | Comment: unclear as limited information available |
Ozkan 2004.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria: ‐ Diagnostic criteria: see above |
|
| Interventions |
Intervention: conventional treatment + orlistat Control: conventional treatment Number of trial centres: 1 Treatment before trial: no Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: adverse effects, bodyweight loss, % bodyweight lost, BMI | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To investigate the efficacy and tolerability of orlistat in obese adolescents, a prospective, open‐label, randomised, controlled pilot trial was performed" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "Randomisation was done by alternation of successive patients, who met the inclusion criteria, to receive conventional treatment alone or orlistat in addition to conventional treatment" Comment: an inappropriate randomisation method was used |
| Allocation concealment (selection bias) | High risk | Comment: allocation was likely not concealed due to the randomisation method used |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote: "the true benefit of orlistat versus conventional therapy remains to be determined in a larger placebo‐controlled study" Comment: the control group did not receive a placebo therefore could not have been blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote: "the true benefit of orlistat versus conventional therapy remains to be determined in a larger placebo‐controlled study" Comment: the control group did not receive a placebo therefore could not have been blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: unclear if outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: an imputation method to replace missing data were not performed, and dropout rate was moderate |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: an imputation method to replace missing data were not performed, and dropout rate was moderate |
| Selective reporting (reporting bias) | Unclear risk | Comment: BMI was reported in different formats; median BMI at baseline and mean BMI at follow‐up. No protocol published |
| Other bias | High risk | Comment: there were significant differences in baseline BMI between groups which were not accounted for. A power calculation was not performed, therefore trial may have been underpowered |
Prado 2012.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: obesity defined as BMI > 95th percentile for age and sex. Risk factors for type 2 diabetes include first‐ or second‐degree relative with a history of type 2 diabetes, or alteration in the results of the following examinations within the past 6 months: glycaemia fasting ≥ 100 mg/dL, postload glucose ≥ 140 mg/dL or HOMA > 3.0 |
|
| Interventions |
Intervention: metformin + nutritional guide + exercise programme Comparator: placebo + nutritional guide + exercise programme Number of trial centres: 1 Treatment before trial: none Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: weight, BMI, metabolic risk profile | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: Spanish Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To analyze the anthropometric and metabolic impact of metformin in obese adolescents at risk for type 2 diabetes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Recruited adolescents were randomly assigned into two groups (A and B) through a sequence computational randomization" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "An external laboratory was in charge of packing and labelling bottles, keeping content knowledge in confidence until the study ended" Comment: there was allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: author confirmed participants and trial personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: author confirmed participants and trial personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: unclear if outcome assessment was blinded and if this would have results in detection bias for the objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: unclear if outcome assessment was blinded and if this would have results in detection bias for the subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: there was no imputation method to replace missing data and dropout rates were fairly high |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: there was no imputation method to replace missing data and dropout rates were fairly high |
| Selective reporting (reporting bias) | Unclear risk | Comment: do not give follow‐up data for some outcomes such as blood pressure |
| Other bias | Unclear risk | Comment: unable to make an assessment on other bias due to lack of information |
Rezvanian 2010.
| Methods | Parallel randomised controlled trial, randomisation ratio: 1:1:1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention 1: metformin + healthy eating + physical activity advice Intervention 2: fluoxetine + healthy eating + physical activity advice Intervention 3: metformin + fluoxetine + healthy eating + physical activity advice Comparator: placebo + healthy eating + physical activity advice Number of trial centres: 1 Treatment before trial: 3 months of nonpharmacological treatment (by lifestyle modification advised in study author's clinic) Titration period: metformin dosage increased weekly from 500 mg/day to 1500 mg/day. Fluoxetine dosage of 10 mg/day increased to 20 mg/day after 3 weeks |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, waist circumference, adverse effects | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "We aimed to compare the effects of three types of drug regimens and placebo on generalized and abdominal obesity among obese children and adolescents who did not succeed to lose weight 3 months after lifestyle modification (diet and exercise)" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Sequence was generated by computer generated random number table" Comment: randomisation was an adequate method |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "triple‐masked randomized clinical trial" Comment: participants and personnel would have been blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "triple‐masked randomized clinical trial" Comment: participants and personnel would have been blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "triple‐masked randomized clinical trial" Comment: outcomes assessors would have been blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "triple‐masked randomized clinical trial" Comment: outcomes assessors would have been blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rate was fairly low |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rate was fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to assess if all outcomes were reported due to the unavailability of a protocol |
| Other bias | Unclear risk | Comment: unable to access if any other bias was present |
Srinivasan 2006.
| Methods | Cross‐over randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + "standardised information on healthy eating and exercise" Comparator: placebo + "standardised information on healthy eating and exercise" Number of trial centres: 1 Treatment before trial: no Titration period: both metformin and placebo doses were gradually built up over a 3‐week period to a final dose of 1 g twice daily |
|
| Outcomes | Outcomes reported in abstract of publication: mean age, median BMI z score, weight, BMI, waist circumference, subcutaneous abdominal adipose tissue, fasting insulin | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "We assessed the effect of metformin on body composition and insulin sensitivity in pediatric subjects with exogenous obesity" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Block randomization (blocks of four) with stratification by pubertal stage (Tanner 1‐2 or Tanner 3‐5) was performed by computer generated random number allocation" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote (from the author): "randomisation was performed in the hospital pharmacy by random number generation and only revealed for data analysis" Comment: allocation was likely concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rates were fairly low |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rates were fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: the publication did not report raw data for some of the outcomes, but a clinical trial entry was available and there were no differences |
| Other bias | Unclear risk | Comment: no power calculation was performed; therefore, the trial may have been underpowered |
Van Mil 2007.
| Methods | Parallel controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: sibutramine + energy‐restricted diet and exercise plan Comparator: placebo + energy‐restricted diet and exercise plan Number of trial centres: 1 Treatment before trial: no Titration period: 5 mg placebo or sibutramine, taken once daily in the morning. After 2 weeks, the dose was increased to 10 mg/day |
|
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, BMI, % fat mass, BMRadj, total energy expenditure | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The objective of this trial was to examine the effect of treatment with sibutramine (10 mg) on body composition and energy expenditure in obese adolescents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomisation was performed by Knoll Pharmaceuticals. Boxes with medication for each visit were numbered for each subject. Subjects received their number and the boxes with medication that belonged to that number. The numbers/medication was handed out in order of inclusion in the study" Comment: author clarified randomisation process; however, it was unclear if the process would have introduced selection bias |
| Allocation concealment (selection bias) | Low risk |
Quote: "Knoll Pharmaceuticals BV [currently Abbott Laboratories (Hoofddorp, The Netherlands)], manufactured and provided code‐numbered placebo and sibutramine capsules. Subjects received their trial and medication code according to order of entrance into the study, without stratification" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: author confirmed participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: author confirmed participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: author confirmed outcome assessment was blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: author confirmed outcome assessment was blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: an imputation method was used; however, results only shown for completers. Dropout rates fairly low |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: an imputation method was used; however, results only shown for completers. Dropout rates fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear whether all outcomes were reported due to no previously published protocol |
| Other bias | Unclear risk |
Quote: "E.G.A.H.V.M. was previously employed by Maastricht University, partly on a research grant from Knoll, currently Abbott Pharmaceuticals, The Netherlands" Comment: potential influence of funding source |
Wiegand 2010.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: obesity (not defined) |
|
| Interventions |
Intervention: metformin + multiprofessional lifestyle intervention Comparator: placebo + multiprofessional lifestyle intervention Number of trial centres: 2 Treatment before trial: 6‐month multiprofessional lifestyle intervention Titration period: no |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, HOMA‐IR, fasting insulin, insulin sensitivity index, metabolic syndrome | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial and noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To study whether metformin reduces obesity, homeostasis model assessment for insulin resistance index (HOMA‐IR), and the metabolic syndrome (MtS) in obese European adolescents in addition to previous unsuccessful lifestyle intervention" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk |
Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk |
Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk |
Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: no imputation method was used to replace missing data; however, dropout was fairly low |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: no imputation method was used to replace missing data; however, dropout was fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to find the clinical trial entry; hence, it is unclear whether selective reporting occurred |
| Other bias | High risk |
Quote: "The study was supported in part by BMBF Research grant 01 GS 0825 and by MERCK SANTE S.A.S, Lyon, France (10’000,‐ Euro)" Comment: trial was partly funded by a pharmaceutical company. The authors do not declare their involvement in the design, analysis and interpretation of the results |
Wilson 2010.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + lifestyle intervention programme Comparator: placebo + lifestyle intervention programme Number of trial centres: 6 Treatment before trial: 4‐week placebo run‐in phase, during which participants were required to attend at least 2 of 3 scheduled lifestyle modification sessions and demonstrate 80% compliance with daily placebo treatment (pill count) for subsequent randomisation Titration period: participants either given metformin XR or identical placebo tablets and instructed to take 1 tablet/day (metformin hydrochloride XR 500 mg or placebo) orally before dinner for 2 weeks, then 2 tablets/day for 2 weeks, then 4 tablets/day from week 8 to week 52 |
|
| Outcomes | Outcomes reported in abstract of publication: mean adjusted BMI, body compositions, abdominal fat, insulin indices | |
| Study details |
Run‐in period: 4‐week placebo run‐in phase (see above) Trial terminated early: no |
|
| Publication details |
Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to test the hypothesis that 48 weeks of daily metformin hydrochloride extended release (EX) will reduce body mass index in obese adolescents, as compared with placebo" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Subjects who successfully completed the run‐in period were randomized to metformin XR or placebo treatment according to random sequences constructed at the Data Coordinating Center. To ensure balance across major factors, the randomization was stratified by site and sex" "To ensure nonpredictability of assignment, the randomization sequence was grouped in randomly permuted blocks of 2 and 4, and assignments were randomly permuted within block" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "Subjects who successfully completed the run‐in period were randomized to metformin XR or placebo treatment according to random sequences constructed at the Data Coordinating Center" Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "Subjects and study personnel were blinded to assignment throughout the entire study" Comment: performance bias likely to be reduced by blinding participants and trial personnel |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "Subjects and study personnel were blinded to assignment throughout the entire study" "Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician" Comment: performance bias likely to be reduced by blinding participants and trial personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "Subjects and study personnel were blinded to assignment throughout the entire study" "Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician" Comment: outcomes assessors blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "Subjects and trial personnel were blinded to assignment throughout the entire study" "Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician" Comment: outcomes assessors blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk |
Quote: "Ninety‐two subjects were screened and 77 were randomized, 39 to metformin XR, 38 to placebo; 27 and 19 in each group were measured at weeks 52 and 100, respectively" Comment: dropout fairly high in each group and no imputation method was performed to replace missing data |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk |
Quote: "Ninety‐two subjects were screened and 77 were randomized, 39 to metformin XR, 38 to placebo; 27 and 19 in each group were measured at weeks 52 and 100, respectively" Comment: dropout fairly high in each group and no imputation method was performed to replace missing data |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: baseline means seemed to be adjusted |
Yanovski 2011.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above |
|
| Interventions |
Intervention: metformin + dietitian‐administered weight‐reduction programme Comparator: placebo + dietitian‐administered weight‐reduction programme Number of trial centres: 1 Treatment before trial: no Titration period: once baseline assessments were completed, participant's trial medication dose was progressively increased according to a prespecified algorithm over a 3‐week period, starting with 500 mg twice daily and increasing to a maximum dose of 1000 mg twice daily |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, bodyweight, BMI z score, fat mass, fasting plasma glucose, HOMA‐IR, adverse events | |
| Study details |
Run‐in period: no Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial and noncommercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine whether metformin treatment causes weight loss and improves obesity related comorbidities in obese children, who are insulin resistant" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "We randomly assigned participants in a 1:1 randomization ratio to receive metformin hydrochloride or placebo, twice daily with meals. Investigators assigned consecutive code numbers to participants from prespecified lists stratified by race/ethnicity, sex, and degree of pubertal development" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk |
Quote: "The CRC Pharmaceutical Development Section used permuted blocks with stratification to generate allocations that translated code numbers into study group assignments by using a pseudo‐random number program and prepared identically appearing placebo and metformin capsules" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk |
Quote: "We assessed efficacy in the intention‐to‐treat sample of all randomly assigned participants using a multiple imputation model for missing data under a missing‐at‐random assumption" Comment: low risk of attrition bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk |
Quote: "We assessed efficacy in the intention‐to‐treat sample of all randomly assigned participants using a multiple imputation model for missing data under a missing‐at‐random assumption" Comment: low risk of attrition bias for subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported from protocol |
| Other bias | Unclear risk | Comment: unclear if any other bias was present |
"‐" denotes not reported.
ALR: adiponectin‐to‐leptin ratio; ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; BMIadj: adjusted body mass index: BMI‐SDS: body mass index standardised score; BMRadj: adjusted basal metabolic rate; CDC: Centers for Disease Control and Prevention; DBP: diastolic blood pressure; DEXA: dual energy X‐ray absorptiometry; FGIR: fasting glucose insulin ratio; HbA1c: glycosylated haemoglobin A1c; HOMA‐IR: homeostasis model assessment for insulin resistance index; hsCRP: highly sensitive C‐reactive protein; LOCF: last observation carried forward; min: minute; OGTT: oral glucose tolerance test; QUICKI: quantitative insulin check index; SBP: systolic blood pressure; SD: standard deviation; SDS: standard deviation score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andelman 1967 | Duration of treatment only 11 weeks |
| Ardizzi 1996 | Duration of drug treatment only 2 months |
| Arman 2008 | Treatment of schizophrenia or schizoaffective disorder |
| Bacon 1967 | Duration of treatment only 2 months |
| Beyer 1980 | Adults |
| Burgert 2008 | Duration of follow‐up < 6 months |
| Canlorbe 1976 | Duration of follow‐up only 12 weeks |
| Cannella 1968 | Adults |
| Casteels 2010 | Children had neurogenic or myogenic motor deficit |
| Cayir 2015 | Not an RCT |
| CTRI/2011/10/002081 2011 | Duration of follow‐up < 6 months |
| Danielsson 2007 | Aim was to treat hypothalamic obesity |
| Danilovich 2014 | Duration of follow‐up < 6 months |
| De Bock 2012 | Duration of drug treatment only 6 weeks |
| Delitala 1977 | Adults |
| Di Natale 1973 | Not an RCT |
| Diaz 2013 | Study aim, not all obese at baseline |
| Doggrell 2006 | Not an RCT |
| EUCTR2009‐016921‐32‐ES | A dietary therapy, intervention not relevant for this review |
| EUCTR2012‐000038‐20‐DE | Duration of treatment only 6 weeks |
| Fanghänel 2001 | Adults |
| Faria 2002 | Adults |
| Ferguson 1986 | Adults |
| Ferrara 2013 | Duration of follow‐up < 6 months |
| Fox 2015 | Not an RCT |
| Freemark 2007 | Not an RCT |
| Galloway 1975 | Adults |
| Gamski 1968 | Adults |
| Garnett 2010 | Not a pharmacological intervention |
| Genova 1967 | Study aim not to treat to obesity |
| Gill 1977 | Adults |
| Giovannini 1990 | Duration of treatment only 90 days |
| Godefroy 1968 | Adults |
| Goldrick 1973 | Adults |
| Goldstein 1993 | Adults |
| González Barranco 1974 | Adults |
| Griboff 1975 | Adults |
| Grube 2014 | Adults |
| Guazzelli 1987 | Adults |
| Gwinup 1967 | Duration of follow‐up in the placebo group only 13 weeks |
| Halpern 2006 | Adults |
| Hamilton 2003 | Duration of follow‐up only 3 months |
| Hansen 2001 | Adults |
| Haug 1973 | Adults |
| Hawkins 2012 | Not an RCT |
| Honzak 1976 | Adults |
| Hooper 1972 | Adults |
| Huston 1966 | Adults |
| IRCT2013021012421N1 | Aim of trial to treat fatty liver disease |
| IRCT2014020116435N1 | Not an RCT |
| Israsena 1980 | Duration of follow‐up only 4 months |
| James 2000 | Adults |
| Kasa‐Vubu 2008 | Not an RCT |
| Kay 2001 | Duration of treatment only 10 weeks |
| Kelly 2012 | 1 arm of the cross‐over trial was only followed up for 3 months after receiving the drug |
| Kelly 2013a | Not an RCT |
| Kelly 2013b | Not an RCT |
| Kendall 2014 | Not an RCT |
| Klein 2006 | Duration of follow‐up < 6 months |
| Kneebone 1968 | Adults |
| Knoll 1975 | Not an RCT |
| Komarnicka 1975 | Adults |
| Komorowski 1982 | Duration of treatment only 8 weeks |
| Kreze 1967 | Adults |
| Lamberto 1993 | Not an RCT |
| Leite 1971 | Adults |
| Lewis 1978 | Adults |
| Libman 2015 | Participants had type 1 diabetes ‐ secondary cause of obesity |
| Liebermeister 1969 | Adults |
| Liu 2013 | Adults |
| Lorber 1966 | Duration of treatment only 4 weeks |
| Love‐Osborne 2008 | The aim of the study was to treat insulin resistance, not all participants were obese |
| Maclay 1977 | Adults |
| Malchow‐Møller 1980 | Duration of follow‐up only 12 weeks |
| Marques 2016 | Not an RCT |
| McDuffie 2002 | Not an RCT |
| Molnár 2000 | Duration of follow‐up only 20 weeks |
| Muls 2001 | Adults |
| Nadeau 2015 | Participants had type 1 diabetes ‐ secondary cause of obesity |
| Nathan 2016 | A description paper of 2 trials which do not meet the inclusion criteria of this review |
| NCT00076362 | Aim of trial to treat hypothalamic obesity |
| NCT00284557 | Not a pharmacological intervention |
| NCT00775164 | Withdrawn prior to enrolment ‐ inadequate enrolment |
| NCT00845559 | Withdrawn prior to enrolment ‐ no reason provided |
| NCT01023139 | Not a pharmacological RCT ‐ all participants were given drugs then randomised to lifestyle intervention or control |
| NCT01061775 | Aim to treat hypothalamic obesity |
| NCT01107808 | Withdrawn prior to enrolment ‐ poor recruitment to the study |
| NCT01169103 | Intervention was a growth hormone therapy |
| NCT01242241 | Aim of the study: not treatment of obesity |
| NCT01329367 | Not a pharmacological intervention |
| NCT01332448 | Not an RCT |
| NCT01410604 | Duration of follow‐up only 3 months |
| NCT01456221 | Not a pharmacological intervention |
| NCT01910246 | Not an RCT |
| NCT02022956 | Not an RCT |
| NCT02063802 | Duration of follow‐up only 4 months |
| NCT02186652 | Not an RCT |
| NCT02378259 | Surgery intervention |
| NCT02398669 | No control group |
| NCT02438020 | Duration of follow‐up < 6 months |
| NCT02515773 | Participants had bipolar disorder and were critically ill. They were all treated with anti‐psychotics which can cause obesity (potential secondary cause of obesity) |
| Nwosu 2015 | Participants had type 1 diabetes ‐ secondary cause of obesity |
| O'connor 1995 | Adults |
| Park 2010 | Not an RCT |
| Pedrinola 1994 | Not an RCT |
| Persson 1973 | Adults |
| Plauchu 1967a | Adults |
| Plauchu 1967b | Adults |
| Plauchu 1972 | Adults |
| Pugnoli 1978 | Adults |
| Rauh 1968 | Duration of follow‐up only 12 weeks |
| Resnick 1967 | Adults |
| Rodos 1969 | Adults |
| Rodriguez 2007 | Not an RCT |
| Roed 1980 | Adults |
| Roginsky 1966 | Adults |
| Sabuncu 2004 | Adults |
| Sainani 1973 | Mainly adults |
| Scavo 1976 | Not an RCT |
| Shutter 1966 | Duration of treatment only 6 weeks |
| Spence 1966 | Not an RCT |
| Spranger 1963 | Duration of treatment only 4 weeks |
| Spranger 1965 | Duration of treatment only 4 weeks |
| Sproule 1969 | Adults |
| Stewart 1970 | Duration of follow‐up only 16 weeks |
| Sukkari 2010 | Not an RCT |
| TODAY study group 2013 | The aim of the study was to treat diabetes, not obesity |
| Tong 2005 | Adults |
| Toubro 2001 | Adults |
| Tsai 2006 | Not an RCT |
| Van Seters 1982 | Adults |
| Warren‐Ulanch 2008 | Not an RCT |
| Weintraub 1984 | Adults |
| Yanovski 2003 | Not an RCT |
| Yu 2013 | Duration of drug treatment only 10 weeks |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Golebiowska 1981.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study identifier | |
| Official title | |
| Stated purpose of study | |
| Notes | Unable to source |
ISRCTN08063839.
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: not reported Primary purpose: treatment |
| Participants |
Condition: adolescent obesity Enrolment: target 48 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: metformin + lifestyle intervention Comparator: placebo + lifestyle intervention |
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier |
ISRCTN number: ISRCTN08063839 Trial start date: 1 July 2010 Trial completion date: 30 June 2014 |
| Official title | Investigating the use of pharmacotherapy in adolescents for weight loss maintenance: the role of appetite: a randomised, placebo controlled trial |
| Stated purpose of study | Quote: "Eat Smart is a novel research study in which 2 dietary approaches to treat childhood obesity are being tested." |
| Notes |
Trial completed in 2014, no publication available and page not found on website Trial sponsor: Royal Children's Hospital (Australia) Ethics approved by the Human Research Ethics Committee (HREC) of the Royal Children's Hospital (ref: HREC/10/QRCH/53) Sources of funding are:
Further information obtained from trial website: www2.som.uq.edu.au/som/Research/ResearchCentres/cnrc/Pages/CNRCHome.aspx |
Linquette 1971.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study identifier | |
| Official title | |
| Stated purpose of study | |
| Notes | Unable to source |
NCT00934570.
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: participants are randomised to metformin medication or placebo, and then randomised to engage in a moderate or vigorous intensity exercise programme for the first 12 weeks of the 2‐year programme Intervention model: parallel assignment Masking: single blind (participant) Primary purpose: prevention |
| Participants |
Condition: obesity, type 2 diabetes Enrolment: estimated 72 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Interventions:
Comparators:
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier |
NCT number: NCT00934570 Other trial ID numbers: R‐08‐259, 15590 Trial start date: April 2009 Trial completion date: May 2012 |
| Official title | Reduction of Adolescent Risk Factors for Type 2 Diabetes and Cardiovascular Disease |
| Stated purpose of study | Quote: "Assess the sustainability of a two‐year intervention aimed at improving body mass index (BMI) and metabolic and vascular health in obese youth." |
| Notes |
No full publication Results were presented in a poster (Clarson et al 2013) ‐ "In the MXR [metformin] group, there were significant differences in BMI z score at baseline (2.22 ± 037) and 6 months (2.08 ± 0.48, P < .001), 12 months (2.05 ± 0.49, P = .002) and 24 months (2.10 ± 0.46, P = 0.04)" Author asked for additional results but none were provided Sponsored by: Lawson Health Research Institute and Canadian Institutes of Health Research (CIHR) Protocol: Wilson et al 2009 Further trial details are provided by Lawson Health Research Institute The health authority associated with this trial: "Canada: Health Canada" |
NCT00940628.
| Methods |
Type of trial: interventional; randomised control trial Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: orlistat (Xenical) + diet and exercise programme Comparator: diet and exercise programme |
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier |
NCT number: NCT00940628 Other trial ID number: ML19569 Trial start date: April 2008 Trial completion date: September 2010 |
| Official title | Open‐label Comparative Randomized Study of the Efficacy and Safety of Orlistat (Xenical) in Complex Therapy of Obesity and Metabolic Disorders in Adolescents |
| Stated purpose of study | Quote: "This 2 arm study will assess the effect of Xenical on body mass index (BMI) in obese or overweight adolescents" |
| Notes |
Trial was completed in 2010, no publication is available The health authority associated with this trial is "Russia: Federal Service on Surveillance in Healthcare and Social Development of RF" |
NCT01487993.
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator) Primary purpose: treatment |
| Participants |
Condition: obesity; insulin resistance Enrolment: 127 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: metformin + lifestyle intervention Comparator: placebo + lifestyle intervention |
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier |
NCT number: NCT01487993 Other trial ID numbers: metformin 2011‐6, 2010‐023980‐17 |
| Official title | An Efficacy, Safety and Pharmacokinetic Study on the Short‐term and Long‐term Use of Metformin in Obese Children and Adolescents |
| Stated purpose of study | Quote: "The purpose of this study is to determine whether metformin is effective in reducing BMI and insulin resistance in obese children and adolescents" |
| Notes | The trial was sponsored by St. Antonius Hospital; Jeroen Bosch Ziekenhuis is a collaborator on the trial; the health authority associated to this trial is "Netherlands: The Central Committee on Research Involving Human Subjects (CCMO)" Results of trial are now published (see Van der Aa 2016 ‐ NCT01487993): 62 participants randomised (32 metformin, 30 placebo), 42 analysed (23 metformin, 19 placebo); 18 months' intervention; median change in BMI was +0.2 kg/m2 (95% CI ‐2.9 to 1.3) (metformin) versus +1.2 kg/m2 (95% CI ‐0.3 to 2.4) kg/m2 (placebo) (P = 0.02). No serious adverse events reported. 2 out of 9 participants lost to follow‐up in the metformin group discontinued treatment because of adverse events. No placebo participants dropped out due to adverse events (13 participants lost to follow‐up) |
Smetanina 2015.
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: unclear Primary purpose: treatment |
| Participants |
Condition: overweight and obesity in children and adolescents Enrolment: 145 Inclusion criteria:
Exclusion criteria: ‐ |
| Interventions |
Interventions:
Comparators:
|
| Outcomes |
Primary outcomes:
Secondary outcomes: none given Other outcomes: none given |
| Study identifier | ‐ |
| Official title | ‐ |
| Stated purpose of study | "To assess the efficacy and safety of Metformin use in combination with lifestyle changes or alone for weight management in OW and OB children and adolescents" |
| Notes | Project supported by Research Council of Lithuania (grant Nr MIP‐039/2013) and Research Foundation of Lithuanian University of Health Sciences (grants 2012 and 2013) Results: reduction in BMI, waist circumference and waist circumference SDS adjusted by sex and puberty stages was significantly greater in the metformin + lifestyle changes group compared to the controls no treatment group. Change in BMI after 12 months' intervention: controls = +0.18 kg/m2, lifestyle changes only = +0.43 kg/m2, metformin only = ‐0.59 kg/m2, metformin + lifestyle changes = ‐1.07 kg/m2. Change in waist circumference after 12 months' intervention: controls = ‐1.8 cm, lifestyle changes only = ‐2.8 cm, metformin only = ‐2.3 cm, metformin + lifestyle changes = ‐4.5 cm. Change in waist circumference SDS after 12 months' intervention: controls = ‐0.38, lifestyle changes only = ‐0.58, metformin only = ‐0.49, metformin + lifestyle changes = ‐0.85. Initially, there were mild adverse effects with metformin (nausea, diarrhoea) in 21.6% of participants from metformin only group and metformin + lifestyle changes group, which disappeared within 1 week of metformin administration. Adjusted by sex and puberty status, lean mass was significantly increased in lifestyle only group compared to controls no treatment and metformin only groups. Change in lean mass after 12 months' intervention: controls = +1.6 kg, lifestyle changes only = +3.98 kg, metformin only = ‐0.36 kg, metformin + lifestyle changes = ‐0.37 kg. 12 months' metformin treatment with lifestyle modification was effective and safe method reducing BMI and waist circumference in overweight/obese children and adolescents, superior to that of lifestyle changes alone Correspondence with author: the results presented in the poster are only partial results of a larger trial, where these data are currently being analysed. They aim to publish the results in a publication and as part of a PhD thesis |
"‐" denotes not reported.
ACE: angiotensin converting enzyme; ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; GFR: glomerular filtration rate; HbA1c: haemoglobin A1c; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HOMA‐IR: homeostasis model assessment for insulin resistance; IOTF: International Obesity Task Force; min: minute; SD: standard deviation; SDS: standard deviation score.
Characteristics of ongoing studies [ordered by study ID]
EUCTR2010‐023061‐21.
| Trial name or title | Efectos de la metformina en la obesidad infantil: "Effects of metformin on childhood obesity" |
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind Primary purpose: treatment |
| Participants |
Condition: obesity in prepubertal and pubertal children Enrolment: target 160 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: metformin Comparator: placebo |
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes: not given |
| Starting date |
Trial start date: not given Trial completion date: not given |
| Contact information |
Trial sponsor: Ramón Cañete Estrada Name of organisation: Instituto de Salud Carlos III Country: Spain Contact details: Avda Menendez Pidal s/n, Córdoba, 14004, Spain. Tel: 34957011227. Email: cetico.hrs.sspa@juntadeandalucia.es |
| Study identifier | EU clinical trials register number: EUCTR2010‐023061‐21 |
| Official title |
Original title: Ensayo clínico sobre efectos de la metformina en la obesidad pediátrica: efectos en el peso corporal, perfil de biomarcadores inflamatorios y de riesgo cardiovascular, e impacto en factores relacionados con el síndrome metabólico English title: Clinical trial on the effect of metformin in pediatric obesity: effects on bodyweight, profile and inflammatory biomarkers of cardiovascular risk, and impact on factors related to metabolic syndrome |
| Stated purpose of study | To study the clinical and biochemical impact of metformin along with changing lifestyle (diet and exercise) in obese children |
| Notes | Majority of this online entry is in Spanish; sponsor status: noncommercial; trial was ongoing when identified |
EUCTR2015‐001628‐45‐SE.
| Trial name or title | A study with lifestyle intervention and study medication once weekly or lifestyle intervention and placebo in adolescents with obesity to explore differences between groups with regard to change in BMI |
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind Primary purpose: treatment |
| Participants |
Condition: obesity in adolescents Enrolment: 44 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: exenatide + lifestyle intervention Comparator: placebo + lifestyle intervention |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date |
Trial start date: not given Trial completion date: not given |
| Contact information | Responsible party/principal investigator: Peter Bergsten, Department of Medical Cell Biology Uppsala University |
| Study identifier | EudraCT Number: 2015‐001628‐45 |
| Official title | A parallel, double‐blinded, randomized, 6 months, two arms trial with lifestyle intervention and exenatide 2 mg once weekly or lifestyle intervention and placebo in adolescents with obesity to explore differences between groups with regard to change in BMI SDS (according to WHO) |
| Stated purpose of study | Quote: "To compare the change from baseline to the 6 months visit at the end of treatment, between lifestyle intervention + exenatide 2 mg once weekly and lifestyle intervention + placebo, in BMI SDS (according to WHO) for adolescents with obesity" |
| Notes | Trial registered on 27 July 2015. Trial status: ongoing (when identified). Trial sponsor: Department of Medical Cell Biology Uppsala University. Monetary or material support provided by: European Commission's Seventh Framework Programme (FP7) project Beta_JUDO (grant 279153). Country: Sweden |
NCT00889876.
| Trial name or title | Effect of exercise or metformin on nocturnal blood pressure and other risk factors for CVD among obese adolescents |
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: factorial assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: CVDs Enrolment: 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: metformin Comparator: exercise |
| Outcomes |
Primary outcome:
Secondary outcome:
|
| Starting date |
Trial start date: February 2009 Trial completion date: December 2012 (estimated) |
| Contact information | Responsible party/principal investigator: Professor Claude Marcus, Karolinska Institutet, Karolinska Institute |
| Study identifier |
NCT number: NCT00889876 Other trial ID numbers: 2008‐000461‐28 |
| Official title | Effect of Exercise or Metformin on Nocturnal Blood Pressure and Other Risk Factors for Cardiovascular Disease (CVD) Among Obese Adolescents |
| Stated purpose of study | Quote: "The objective is to, among obese adolescents, study impact of regular physical activity or metformin therapy on nocturnal blood pressure and related cardiovascular disease risk factors" |
| Notes | This trial has not been verified on the clinicaltrials.gov website since February 2011. We have attempted to contact the principal investigator via email; however, have not received a response. Trial sponsor: Karolinska Institutet |
NCT01677923.
| Trial name or title | Obesity in children and adolescents: associated risks and early intervention (OCA) |
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrolment: 400 (estimated) Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Interventions:
Comparators:
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes:
|
| Starting date |
Trial start date: May 2013 Trial completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Rasa Verkauskiene, Lithuanian University of Health Sciences. rasa.verkauskiene@kaunoklinikos.lt. 00370‐37‐327097 |
| Study identifier |
NCT number: NCT01677923 Other trial ID numbers: BE‐2‐1 |
| Official title | Phase 3: Effect of Diet, Physical Activity and Insulin Sensitizer Metformin on Obesity and Associated Risks in Children and Adolescents |
| Stated purpose of study | Quote: "The investigators hypothesize that Metformin decreases weight, normalizes lipid profile and increases insulin sensitivity; the study team hope to get better effect of weight decrease and metabolic processes repair in the intensive treatment group with intervention of physical activity, diet correction and Metformin use" |
| Notes | The health authorities associated with this trial are Lithuania: Bioethics Committee and Lithuania: State Medicine Control Agency ‐ Ministry of Health; the trial is sponsored by Lithuanian University of Health Sciences; this trial was recruiting participants when identified |
NCT01859013.
| Trial name or title | Topiramate in Adolescents with Severe Obesity |
| Methods |
Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: obesity, morbid obesity, weight loss Enrolment: estimated 36 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: topiramate Comparator: placebo |
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Starting date |
Trial start date: June 2013 Trial completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Aaron S Kelly, PhD University of Minnesota ‐ Clinical and Translational Science Institute. Tel: 612‐626‐3492. Email: kelly105@umn.edu |
| Study identifier |
NCT number: NCT01859013 Other trial ID number: 1304M31241 |
| Official title | BMI Reduction with Meal Replacements + Topiramate in Adolescents with Severe Obesity |
| Stated purpose of study | Quote: "the goal of this pilot study is to evaluate the safety and efficacy of 24 weeks of topiramate therapy with a 4‐week run‐in of meal replacement therapy in adolescents with severe obesity" |
| Notes | The health authority associated with this trial: "United States: Institutional Review Board"; the trial is sponsored by University of Minnesota ‐ Clinical and Translational Science Institute; the trial was recruiting participants when identified; publication identified for retrospective analysis of participants who received topiramate: Fox et al 2015 |
NCT02273804.
| Trial name or title | Topiramate and Severe Obesity (TOBI) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: obese children and adolescents Enrolment: estimated 160 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: topiramate Comparator: placebo |
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes: none given |
| Starting date |
Trial start date: June 2015 Trial completion date: December 2020 |
| Contact information |
Responsible party: Assistance Publique ‐ Hôpitaux de Paris Principal investigator: Marie‐Laure Frelut, MD |
| Study identifier | NCT number: NCT02273804 |
| Official title | Topiramate and Severe Obesity in Children and Adolescents |
| Stated purpose of study | The purpose of this trial is to evaluate the efficacy of topiramate on the decrease of BMI compared to placebo at 9 months |
| Notes | The trial is sponsored by Assistance Publique ‐ Hôpitaux de Paris; recruitment status when identified: not yet recruiting |
NCT02274948.
| Trial name or title | Use of Metformin in Treatment of Childhood Obesity |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: paediatric obesity Enrolment: estimated 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: metformin Comparator: placebo |
| Outcomes |
Primary outcomes:
Secondary outcome:
Other outcomes: none given |
| Starting date |
Trial start date: July 2014 Trial completion date: February 2016 |
| Contact information | Responsible party/principal investigator: Pujitha Wickramasinghe, University of Colombo |
| Study identifier | NCT number: NCT02274948 |
| Official title | Effects of Metformin on Body Weight, Composition and Metabolic Derangements in Obese Children. A Randomized Clinical Trial |
| Stated purpose of study | This study expects to evaluate the use of metformin in the management of obese children. Insulin resistance among obese Sri Lankan children (south Asian origin) is high, which had been shown in the investigators previous work. This study will look at the effect of metformin on changes in insulin resistance, fatty liver state, body fat content, BMI and other metabolic derangement |
| Notes | Trial sponsored by University of Colombo; recruitment status when identified: this trial is currently recruiting participants; location: Sri Lanka |
NCT02496611.
| Trial name or title | Enhancing Weight Loss Maintenance With GLP‐1RA (BYDUREON™) in Adolescents with Severe Obesity |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: severe obesity Enrolment: estimated 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: exenatide extended‐release for injectable suspension (BYDUREON™) Comparator: placebo |
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes: none given |
| Starting date |
Trial start date: December 2015 Trial completion date: July 2020 |
| Contact information | Responsible party/principal investigator: University of Minnesota ‐ Clinical and Translational Science Institute |
| Study identifier | NCT number: NCT02496611 |
| Official title | Enhancing Weight Loss Maintenance with GLP‐1RA (BYDUREON™) in Adolescents with Severe Obesity |
| Stated purpose of study | Primary objective: evaluate the effect of GLP‐1RA treatment on the maintenance of weight loss and durability of cardiometabolic risk factor improvements among adolescents with severe obesity following a meal replacement induction period Secondary objectives: investigate the mechanisms by which glucagon‐like peptide‐1 receptor agonists treatment facilitates weight loss maintenance and identify predictors of response to treatment |
| Notes | Trial sponsored by University of Minnesota ‐ Clinical and Translational Science Institute; recruitment status when identified: this trial is currently recruiting participants; location: USA |
ACE: angiotensin‐converting enzyme; ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; CVD: cardiovascular disease; HbA1c: glycated haemoglobin; HOMA‐IR: homeostasis model assessment‐insulin resistance; IOTF: International Obesity Task Force; SD: standard deviation; GFR: glomerular filtration rate; min: minute; MRI: magnetic resonance imaging; PK: pharmacokinetics; PCOS: polycystic ovary syndrome; SDS: standard deviation score; WHO: World Health Organization.
Differences between protocol and review
Given the rapid growth in the treatment of child and adolescent obesity, the original review has now been split into six separate reviews, with a specific intervention and age focus. Whilst the other reviews in this series utilised an updated version of the original search strategy, we developed a new search strategy (see Appendix 1) to reflect advances in pharmacological therapies that may not have been adequately captured in the original search strategy. We decided to exclude trials which included growth hormone therapies to avoid including trials which treated conditions such as Cushing's syndrome. In addition, some subgroup analyses were not possible due to a limited number of trials.
We included only randomised controlled trials that were specifically designed to treat obesity in children and observed participants for a minimum of six months. The rationale for introducing this criterion arose from the belief that many interventions appear to be effective in the short term (up to three months), but not in the long term (Glenny 1997). It seemed to be more important to evaluate the longer‐term effects of treatments, as this would provide a more valuable indication of effectiveness, given the chronic nature of obesity.
Contributions of authors
EM: search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and future review updates. GA: data extraction, data analysis, data interpretation, review draft and future review updates. BR: data analysis, data interpretation and review draft. MIM: search strategy development and review draft. LB: data extraction, data interpretation, review draft and future review updates. NF: data extraction, data interpretation, review draft and future review updates. EC: data extraction, data interpretation, review draft and future review updates. CO: acquiring trial reports, trial selection, data extraction, data interpretation, review draft and future review updates. LE: search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and future review updates.
Sources of support
Internal sources
University Medical Center, Groningen, Netherlands.
The Children's Hospital at Westmead, Sydney, Australia.
Centre for Food Physical Activity and Obesity Research, University of Teesside, UK.
The Wolfson Research Institute, University of Durham, UK.
-
Australian National Health & Medical Research Council, Australia.
Postgraduate Research Scholarship for Ms Shrewsbury
External sources
No sources of support supplied
Declarations of interest
EM: none known. GA: none known. BR: none known. MIM: none known. LB: none known. NF: has provided medical consultancy to several pharmaceutical companies developing and marketing (outside of the UK at present) treatments for obesity. Since March 2016 he is employed by Novo Nordisk, Denmark in Global Medical Affairs. The review was submitted for publication in November 2015, pre‐dating an offer of employment by Novo Nordisk A/S made in December 2015. NF made no further contributions to the review after this date. NF’s employment by NovoNordisk during review production violated Cochrane’s Commercial Sponsorship Policy, the review group assured NF did not contribute to the review after this employment started, and the Funding Arbiters reviewed and approved an exception for this case. EC: none known. CO: none known. LE: none known.
Please see Authors’ Declarations of Interest for further details.
Edited (no change to conclusions)
References
References to studies included in this review
Atabek 2008 {published data only}
- Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6‐month, randomized, double‐blind, placebo‐controlled clinical trial. Journal of Pediatric Endocrinology and Metabolism 2008;21(4):339‐48. [DOI] [PubMed] [Google Scholar]
Berkowitz 2003 {published data only}
- Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA 2003;289(14):1805‐12. [DOI] [PubMed] [Google Scholar]
- Bishop‐Gilyard CT, Berkowitz RI, Wadden TA, Gehrman CA, Cronquist JL, Moore RH. Weight reduction in obese adolescents with and without binge eating. Obesity (Silver Spring) 2011;19(5):982‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd GM, Hayman LL, Crump E, Pollydore C, Hawley K, Cronquist JL, et al. Weight loss in obese African American and Caucasian adolescents. Journal of Cardiovascular Nursing 2007;22(4):288‐96. [DOI] [PubMed] [Google Scholar]
- Parks EP, Zemel B, Moore RH, Berkowitz RI. Change in body composition during a weight loss trial in obese adolescents. Pediatric Obesity 2014;9:26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkowitz 2006 {published data only}
- Berkowitz RI, Fujioka K, Daniels SR, Hoppin AG, Owen S, Perry AC, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Annals of Internal Medicine 2006;145(2):81‐90. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Long B, Crow S, Styne D, Sothern M, Vargas‐Rodriguez I, et al. Cardiovascular effects of sibutramine in the treatment of obese adolescents: results of a randomized, double‐blind, placebo‐controlled study. Pediatrics 2007;120:e147‐57. [DOI: 10.1542/peds.2006-2137] [DOI] [PubMed] [Google Scholar]
Chanoine 2005 {published and unpublished data}
- Chanoine J, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 2005;293(23):2873‐83. [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Richard M. Early weight loss and outcome at one year in obese adolescents treated with orlistat or placebo. International Journal of Pediatric Obesity 2010;6(2):95‐101. [DOI: 10.3109/17477166.2010.519387] [DOI] [PubMed] [Google Scholar]
Clarson 2009 {published data only}
- Clarson CL, Mahmud FH, Baker JE, Clark HE, Mckay WM, Schauteet VD, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine 2009;36(1):141‐6. [DOI: 10.1007/s12020-009-9196-9] [DOI] [PubMed] [Google Scholar]
Franco 2014 {published data only}
- Franco RR, Cominato L, Damiani D. The effect of sibutramine on weight loss in obese adolescents [O efeito da sibutramina na perda de peso de adolescentes obesos]. Arquivos Brasileiros de Endocrinologia e Metabologia 2014;58:243‐50. [DOI] [PubMed] [Google Scholar]
Freemark 2001 {published and unpublished data}
- Freemark M. Liver dysfunction in paediatric obesity: a randomized, controlled trial of metformin. Acta Paediatrica 2007;96:1326‐32. [DOI: 10.1111/j.1651-2227.2007.00429.x] [DOI] [PubMed] [Google Scholar]
- Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics 2001;107(4):e55. [DOI] [PubMed] [Google Scholar]
García‐Morales 2006 {published data only}
- García‐Morales LM, Berber A, Macias‐Lara CC, Lucio‐Ortiz C, Del‐Rio‐Navarro BE, Dorantes‐Alvárez LM. Use of sibutramine in obese Mexican adolescents: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group trial. Clinical Therapeutics 2006;28(5):770‐82. [DOI: 10.1016/j.clinthera.2006.05.008] [DOI] [PubMed] [Google Scholar]
Godoy‐Matos 2005 {published data only}
- Correa LL, Platt MW, Carraro L, Moreira RO, Junior RF, Godoy‐Matos AF, et al. Evaluation of the sibutramine effect on satiety with a visual analogue scale in obese adolescents [Avaliação do efeito da sibutramina sobre a saciedade por escala visual analógica em adolescentes obesos]. Arquivos Brasileiros de Endocrinologia & Metabologia 2005;49(2):286‐90. [DOI] [PubMed] [Google Scholar]
- Godoy‐Matos A, Carraro L, Vieira A, Oliveira J, Guedes EP, Mattos L, et al. Treatment of obese adolescents with sibutramine: a randomized, double‐blind, controlled study. Journal of Clinical Endocrinology & Metabolism 2005;90(3):1460‐5. [DOI] [PubMed] [Google Scholar]
Kendall 2013 {published data only}
- Kendall D, Vail A, Amin R, Barrett T, Dimitri P, Ivison F, et al. Metformin in obese children and adolescents: the MOCA trial. Journal of Clinical Endocrinology & Metabolism 2013;98(1):322‐9. [DOI: 10.1210/jc.2012-2710] [DOI] [PubMed] [Google Scholar]
Maahs 2006 {published data only}
- Maahs D, Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, et al. Randomized, double‐blind, placebo‐controlled trial of orlistat for weight loss in adolescents. Endocrine Practice 2006;12(1):18‐28. [DOI] [PubMed] [Google Scholar]
Mauras 2012 {published data only}
- Benson M, Hossain J, Caulfield MP, Damaso L, Gidding S, Mauras N. Lipoprotein subfractions by ion mobility in lean and obese children. Journal of Pediatrics 2012;161:997‐1003. [DOI] [PubMed] [Google Scholar]
- Mauras N, DelGiorno C, Hossain J, Bird K, Killen K, Merinbaum D, et al. Metformin use in children with obesity and normal glucose tolerance ‐ effects on cardiovascular markers and intrahepatic fat. Journal of Pediatric Endocrinology and Metabolism 2012;25(1‐2):33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynders C, Weltman A, Delgiorno C, Balagopal P, Damaso L, Killen K, et al. Lifestyle intervention improves fitness independent of metformin in obese adolescents. Medicine & Science in Sports & Exercise 2012;44(5):786‐92. [DOI: 10.1249/MSS.0b013e31823cef5e] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00001723 {unpublished data only}
- Condarco TA, Sherafat‐Kazemzadeh R, McDuffie JR, Brady S, Salaita C, Sebring NG, et al. Long‐term follow‐up of a randomized, placebo‐controlled trial of orlistat in African‐American and Caucasian adolescents with obesity‐related comorbid conditions. Endocrine Reviews 2013.
- Radin RM, Tanofsky‐Kraff M, Shomaker LB, Kelly NR, Pickworth CK, Shank LM, et al. Metabolic characteristics of youth with loss of control eating. Eating Behaviors 2015;19:86‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozkan 2004 {published data only}
- Ozkan B, Bereket A, Turan S, Keskin S. Addition of orlistat to conventional treatment in adolescents with severe obesity. European Journal of Pediatrics 2004;163(12):738‐41. [DOI] [PubMed] [Google Scholar]
Prado 2012 {published data only (unpublished sought but not used)}
- Prado B, Gaete V, Corona F, Peralta E, Donoso P, Raimann X. Metabolic effects of metformin in obese adolescents at risk for type 2 diabetes mellitus [Efecto metabólico de la metformina en adolescentes obesas con riesgo de diabetes mellitus tipo 2]. Revista Chilena de Pediatría 2012;83(1):48‐57. [Google Scholar]
Rezvanian 2010 {published data only}
- Rezvanian H, Hashemipour M, Kelishadi R, Tavakoli N, Poursafa P. A randomized, triple masked, placebo‐controlled clinical trial for controlling childhood obesity. World Journal of Pediatrics 2010;6(4):317‐22. [DOI: 10.1007/s12519-010-0232-x] [DOI] [PubMed] [Google Scholar]
Srinivasan 2006 {published data only}
- Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. Journal of Clinical Endocrinology & Metabolism 2006;91(6):2074‐80. [DOI: 10.1210/jc.2006-0241] [DOI] [PubMed] [Google Scholar]
Van Mil 2007 {published data only}
- Mil EG, Westerterp KR, Kester AD, Delemarre‐van de Waal HA, Gerver WJ, Saris WH. The effect of sibutramine on energy expenditure and body composition in obese adolescents. Journal of Clinical Endocrinology & Metabolism 2007;92(4):1409‐14. [DOI] [PubMed] [Google Scholar]
Wiegand 2010 {published data only}
- Hübel H. Prospective randomised controlled study for the prevention of type 2 diabetes mellitus in obese children and adolescents, 2012 [Prospektive, randomisierte, kontrollierte Studie zur Prävention des Typ 2 Diabetes mellitus bei adipösen Kindern und Jugendlichen (Dissertation)]. www.diss.fu‐berlin.de/diss/servlets/MCRFileNodeServlet/FUDISS_derivate_000000010572/2012.01.01.HHuebel.e_version_diss.pdf (last accessed 20 May 2016).
- Wiegand S, l'Allemand D, Hübel H, Krude H, Bürmann M, Martus P, et al. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin‐resistant adolescents: a prospective, placebo‐controlled, randomized study. European Journal of Endocrinology 2010;163(4):585‐92. [DOI] [PubMed] [Google Scholar]
Wilson 2010 {published data only}
- Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, et al. Metformin extended release treatment of adolescent obesity: a 48‐week randomized, double‐blind, placebo‐controlled trial with 48‐week follow‐up. Archives of Pediatrics & Adolescent Medicine 2010;164(2):116‐23. [DOI: 10.1001/archpediatrics.2009.264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yanovski 2011 {published data only}
- Adeyemo MA, McDuffie JR, Kozlosky M, Krakoff J, Calis KA, Brady SM, et al. Effects of metformin on energy intake and satiety in obese children. Diabetes, Obesity and Metabolism 2015;17(4):363‐70. [DOI: 10.1111/dom.12426] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, et al. Effects of metformin on body weight and body composition in obese insulin‐resistant children a randomized clinical trial. Diabetes 2011;60(2):477‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andelman 1967 {published data only}
- Andelman MB, Jones C, Nathan S. Treatment of obesity in underprivileged adolescents. Comparison of diethylpropion hydrochloride with placebo in a double‐blind study. Clinical Pediatrics 1967;6:327‐30. [DOI] [PubMed] [Google Scholar]
Ardizzi 1996 {published data only}
- Ardizzi A, Grugni G, Guzzaloni G, Moro D, Morabito F. Effects of protracted rhGH administration on body composition and intermediate metabolism in juvenile obesity. Acta Medica Auxologica 1996;28:103‐9. [Google Scholar]
Arman 2008 {published data only}
- Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double‐blind, placebo‐controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Medical Journal 2008;29:1130‐4. [PubMed] [Google Scholar]
Bacon 1967 {published data only}
- Bacon GE, Lowrey GH. A clinical trial of fenfluramine in obese children. Current therapeutic research. Clinical and Experimental 1967;9:626‐30. [PubMed] [Google Scholar]
Beyer 1980 {published data only}
- Beyer G, Huth K, Muller GM, Niemoller H, Raisp I, Vorberg G. The treatment of obesity with the appetite curbing agent mefenorex [Zur Behandlung Von Übergewicht mit dem Appetitzügler Mefenorex]. Medizinische Welt 1980;31:306‐9. [PubMed] [Google Scholar]
Burgert 2008 {published data only}
- Burgert TS, Duran EJ, Goldberg‐Gell R, Dziura J, Yeckel CW, Katz S, et al. Short‐term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatric Diabetes 2008;9:567‐76. [DOI] [PubMed] [Google Scholar]
Canlorbe 1976 {published data only}
- Canlorbe P, Borniche P, Toublanc JE. Controlled trial of an anorectic (An 448) in the treatment of childhood obesity [Essai controle d'un anorexigene (An 448) dans le traitment de l'obesite de l'enfant]. La Nouvelle Presse Médicale 1976;5:1061‐2. [PubMed] [Google Scholar]
Cannella 1968 {published data only}
- Cannella M, Scarpelli PT, Vailati G. The role of anorexic drugs in the treatment of obesity. Clinical data on the use of a new anorexic drug (Ro 6‐1343) [Il ruolo dei farmaci antioressici nel trattamento delle obesità. Rilievi clinici sull'impiego di un nuovo antioressico (Ro 6‐1343)]. Minerva Medica 1968;59:3472‐83. [PubMed] [Google Scholar]
Casteels 2010 {published data only}
- Casteels K, Fieuws S, Helvoirt M, Verpoorten C, Goemans N, Coudyzer W, et al. Metformin therapy to reduce weight gain and visceral adiposity in children and adolescents with neurogenic or myogenic motor deficit. Pediatric Diabetes 2010;11:61‐9. [DOI] [PubMed] [Google Scholar]
Cayir 2015 {published data only}
- Cayir A, Turan MI, Gurbuz F, Kurt N, Yildirim A. The effect of lifestyle change and metformin therapy on serum arylesterase and paraoxonase activity in obese children. Journal of Pediatric Endocrinology and Metabolism 2015;28(5‐6):551‐6. [DOI] [PubMed] [Google Scholar]
CTRI/2011/10/002081 2011 {published data only}
- Biju KR, Patel KS. A clinical trial to study the effect of an Ayurvedic drug, Thriphalaguduchyadi Vati, in children with obesity. Clinical Trial Registry ‐ India 2011.
Danielsson 2007 {published data only}
- Danielsson P, Janson A, Norgren S, Marcus C. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. Journal of Clinical Endocrinology and Metabolism 2007;92:4101‐6. [DOI] [PubMed] [Google Scholar]
Danilovich 2014 {published data only}
- Danilovich N, Mastrandrea LD, Cataldi L, Quattrin T. Methylphenidate decreases fat and carbohydrate intake in obese teenagers. Obesity (Silver Spring, Md.) 2014;22:781‐5. [DOI] [PubMed] [Google Scholar]
De Bock 2012 {published data only}
- Bock M, Derraik JGB, Brennan CM, Biggs JB, Smith GC, Cameron‐Smith D, et al. Psyllium supplementation in adolescents improves fat distribution & lipid profile: a randomized, participant‐blinded, placebo‐controlled, crossover trial. PLoS One 2012;7(7):e41735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delitala 1977 {published data only}
- Delitala G, Alagna S, Masala A. Mazindol (AN‐448): a new non‐amphetamine anorectic in the treatment of exogenous obesity [Il mazindolo (AN‐448): un nuovo anoressante non amfetaminico nel trattamento dell'obesita esogena]. Clinica Terapeutica 1977;81:547‐54. [PubMed] [Google Scholar]
Diaz 2013 {published data only}
- Diaz M, Bassols J, Lopez‐Bermejo A, Zegher F, Ibanez L. Metformin treatment to reduce central adiposity after prenatal growth restraint: a placebo‐controlled pilot study in prepubertal children. Pediatric Diabetes. 2015;16(7):538‐45. [DOI] [PubMed] [Google Scholar]
- Diaz M, Ibanez L, Lopez‐Bermejo A, Sanchez‐Infantes D, Bassols J, Zegher F. Growth restraint before birth, weight catch‐up in infancy, and central adiposity in childhood: a placebo‐controlled pilot study of early metformin intervention. Hormone Research in Paediatrics 2013;80:116. [Google Scholar]
Di Natale 1973 {published data only}
- Natale B, Devetta M, Rossi L, Garlaschi C, Caccamo A, Guercia MJ, et al. Arginine infusion in obese children. Helvetica Paediatrica Acta 1973;28:331‐9. [PubMed] [Google Scholar]
Doggrell 2006 {published data only}
- Doggrell SA. Sibutramine for obesity in adolescents. Expert Opinion on Pharmacotherapy 2006;7:2435‐8. [DOI] [PubMed] [Google Scholar]
EUCTR2009‐016921‐32‐ES {unpublished data only}
- Fundació Sant Joan de Déu. A phase II, randomized, double‐blind, placebo‐controlled, in parallel groups clinical trial to assess the safety and efficacy of dietary supplementation with tryptophan to achieve weight loss, and its neuropsychological effects in adolescents with obesity. clinicaltrials.gov/ct2/show/NCT02612259 Date first received: 17 November 2015. [NCT02612259]
EUCTR2012‐000038‐20‐DE {unpublished data only}
- Novo Nordisk A/S. A randomised, double‐blind, placebo‐controlled trial to assess safety, tolerability and pharmacokinetics of liraglutide in obese adolescent subjects aged 12 to 17 years. clinicaltrials.gov/ct2/show/NCT01789086 Date first received: 8 February 2013.
Fanghänel 2001 {published data only}
- Fanghänel G, Cortinas L, Sánchez‐Reyes L, Berber A. Second phase of a double‐blind study clinical trial on sibutramine for the treatment of patients suffering essential obesity: 6 months after treatment cross‐over. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2001;25:741‐7. [DOI] [PubMed] [Google Scholar]
Faria 2002 {published data only}
- Faria AN, Ribeiro Filho FF, Lerário DDG, Kohlmann N, Ferreira SRG, Zanella MT. Effects of sibutramine on the treatment of obesity in patients with arterial hypertension [Efeitos da sibutramina no tratamento da obesidade em pacientes com hipertensão arterial]. Arquivos Brasileiros de Cardiologia 2002;78:172‐80. [DOI] [PubMed] [Google Scholar]
Ferguson 1986 {published data only}
- Ferguson JM. Fluoxetine‐induced weight loss in overweight, nondepressed subjects. American Journal of Psychiatry 1986;143:1496. [DOI] [PubMed] [Google Scholar]
Ferrara 2013 {published data only}
- Ferrara P, Bufalo F, Ianniello F, Franceschini A, Paolini Paoletti F, Massart F, et al. Diet and physical activity "defeated" Tuberil in treatment of childhood obesity. Minerva Endocrinologica 2013;38:181‐5. [PubMed] [Google Scholar]
Fox 2015 {published data only}
- Fox CK, Marlatt KL, Rudser KD, Kelly AS. Topiramate for weight reduction in adolescents with severe obesity. Clinical Pediatrics 2015;54:19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freemark 2007 {published data only}
- Freemark M. Pharmacotherapy of childhood obesity: an evidence‐based, conceptual approach. Diabetes Care 2007;30:395‐402. [DOI] [PubMed] [Google Scholar]
Galloway 1975 {published data only}
- Galloway DB, Logie AW, Petrie JC. Prolonged‐action fenfluramine in non‐diabetic patients with refractory obesity. Postgraduate Medical Journal 1975;51(Suppl 1):155‐7. [PubMed] [Google Scholar]
Gamski 1968 {published data only}
- Gamski M, Komarnicka R. Regulation of appetite in obese subjects by means of neutrotropic drugs [Regulacja łaknienia u osób otyłych za pomoca leków neutrotropowych]. Polski Tygodnik Lekarski 1968;23:1688‐90. [PubMed] [Google Scholar]
Garnett 2010 {published data only}
- Garnett SP, Baur LA, Noakes M, Steinbeck K, Woodhead HJ, Burrell S, et al. Researching effective strategies to improve insulin sensitivity in children and teenagers ‐ RESIST. A randomised control trial investigating the effects of two different diets on insulin sensitivity in young people with insulin resistance and/or pre‐diabetes. BMC Public Health 2010;10:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett SP, Dunkley M, Ho M, Baur LA, Noakes M, Cowell CT. Optimum macronutrient content of the diet for adolescents with pre‐diabetes: RESIST, a randomised control trial (ACTRN12608000416392). Pediatric Diabetes 2012;13:23‐4. [Google Scholar]
- Garnett SP, Gow M, Ho M, Baur LA, Noakes M, Woodhead HJ, et al. Improved insulin sensitivity and body composition, irrespective of macronutrient intake, after a 12 month intervention in adolescents with pre‐diabetes; RESIST a randomised control trial. BMC Pediatrics 2014;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C, Lu J, Gow M, Ho M, Cowell C, Baur L, et al. Changes in systemic inflammation in obese children with prediabetes after a 12 month lifestyle intervention; RESIST, a randomised control trial. Obesity Research and Clinical Practice 2013;7:e80. [Google Scholar]
Genova 1967 {published data only}
- Genova R, Massolo F, Venturelli, D. The lipolytic action of ACTH. 3. comparison of the lipolytic and corticoadrenal response to extrative and synthetic corticotropin in obese children [L'azione lipopolitica dell'ACTH. 3. Confronto tra la riposta lipopolitica e corticosurrenalica alla corticotropina estrattiva ed a quella sintetica nei bambini obesi]. Clinica Pediatrica 1967;49(6):277‐87. [PubMed] [Google Scholar]
Gill 1977 {published data only}
- Gill E. Long‐term treatment of obesity with the anorectic agent tenuate as an adjuvant to reducing diet [Langzeitbehandlung der Adipositas mit dem anorektikum tenuate als adjuvans der reduktionsdiät]. Medizinische Welt 1977;28:2080‐1. [PubMed] [Google Scholar]
Giovannini 1990 {published data only}
- Giovannini C, Ciucci E, Facchinetti F. Plasma levels of beta‐endorphin, ACTH and cortisol in obese patients subjected to several weight‐loss treatments [Livelli Plasmatici Di Beta‐Endorfinemia, Acth E Cortisolo In Pazienti Obesi Sottoposti A Differenti Trattamenti Dimagranti]. Recenti Progressi In Medicina 1990;81:301‐5. [PubMed] [Google Scholar]
Godefroy 1968 {published data only}
- Godefroy G, Napolier A, Andriambao‐Damasy S. Study of the action of concentrated lucofen with prolonged effect [Etude de l'action du lucofène fort à effet prolongé]. Marseille Medical 1968;105:289‐93. [PubMed] [Google Scholar]
Goldrick 1973 {published data only}
- Goldrick RB, Havenstein N, Whyte HM. Effects of caloric restriction and fenfluramine on weight loss and personality profiles of patients with long standing obesity. Australian And New Zealand Journal of Medicine 1973;3:131‐41. [DOI] [PubMed] [Google Scholar]
Goldstein 1993 {published data only}
- Goldstein DJ, Rampey AH, Dornseif BE, Levine LR, Potvin JH, Fludzinski LA. Fluoxetine: a randomized clinical trial in the maintenance of weight loss. Obesity Research 1993;1:92‐8. [DOI] [PubMed] [Google Scholar]
González Barranco 1974 {published data only}
- González Barranco J, Rull JA, Lozano Castañeda O. L‐Thyroxine propylhexedrine in the treatment of obesity. Crossed double blind study [Propilhexedrina L‐tiroxina en el tratamiento de la obesidad. Estudio doble ciego gruzado]. La Prensa Médica Mexicana 1974;39:298‐9. [PubMed] [Google Scholar]
Griboff 1975 {published data only}
- Griboff SI, Berman R. A double blind clinical evaluation of a phenylpropanolamine‐caffeine‐vitamine combination and a placebo in the treatment of exogenous obesity. Current Therapeutic Research, Clinical and Experimental 1975;17:535‐43. [PubMed] [Google Scholar]
Grube 2014 {published data only}
- Grube B, Chong WF, Chong PW, Riede L. Weight reduction and maintenance with Iqp‐Pv‐101: a 12‐week randomized controlled study with a 24‐week open label period. Obesity 2014;22:645‐51. [DOI] [PubMed] [Google Scholar]
Guazzelli 1987 {published data only}
- Guazzelli R, Piazzini M, Conti C, Papi L, Strazzulla G, Matassi L. Clinical use of mazindol in the treatment of essential obesity [Impiego clinico del mazindolo nel trattamento dell'obesità essenziale]. Clinica Terapeutica 1987;120:385‐91. [PubMed] [Google Scholar]
Gwinup 1967 {published data only}
- Gwinup G, Poucher R. A controlled study of thyroid analogs in the therapy of obesity. American Journal of the Medical Sciences 1967;254:416‐20. [DOI] [PubMed] [Google Scholar]
Halpern 2006 {published data only}
- Halpern A, Mancini MC, Cercato C, Villares SMF, Costa AP. Effects of growth hormone on anthropometric and metabolic parameters in android obesity [Efeito Do Hormônio De Crescimento Sobre Parâmetros Antropométricos E Metabólicos Na Obesidade Andróide]. Arquivos Brasileiros de Endocrinologia & Metabologia 2006;50:68‐73. [DOI] [PubMed] [Google Scholar]
Hamilton 2003 {published data only}
- Hamilton J, Cummings E, Zdravkovic V, Finegood D, Daneman D. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance: a randomized controlled trial. Diabetes Care 2003;26:138‐43. [DOI] [PubMed] [Google Scholar]
Hansen 2001 {published data only}
- Hansen D, Astrup A, Toubro S, Finer N, Kopelman P, Hilsted J, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi‐centre Storm trial (Sibutramine Trial of Obesity Reduction and Maintenance). International Journal of Obesity and Related Metabolic Disorders : Journal of the International Association for the Study of Obesity 2001;25:496‐501. [DOI] [PubMed] [Google Scholar]
Haug 1973 {published data only}
- Haug E, Myklebust R. The therapeutic effect of fenfluramine on body weight in overweight patients. A controlled clinical study [Den terapeutiske effekt av fenfluramin på kroppsvekten hos overvektige. En kontrollert klinisk undersøkelse]. Tidsskrift For Den Norske Lægeforening : Tidsskrift For Praktisk Medicin, Ny Række 1973;93(35):2545‐9. [PubMed] [Google Scholar]
Hawkins 2012 {published data only}
- Hawkins FP. Metformin and placebo therapy in adjunct with lifestyle intervention both improve weight loss and insulin resistance in obese adolescents. Archives of Disease in Childhood ‐ Education and Practice 2012;97:79‐80. [DOI] [PubMed] [Google Scholar]
Honzak 1976 {published data only}
- Honzak R, Rath R, Vondra K. Effect of cafilon in the treatment of obesity (author's translation). Ceskoslovenska Gastroenterologie A Vyziva 1976;30:155‐61. [PubMed] [Google Scholar]
Hooper 1972 {published data only}
- Hooper AC. Comparison of fenfluramine (with ad libitum food intake) with 1,000 calorie diet in obesity. Journal of the Irish Medical Association 1972;65:35‐7. [PubMed] [Google Scholar]
Huston 1966 {published data only}
- Huston JR. Obesity: phenmetrazine effect without dietary restriction. Ohio State Medical Journal 1966;62:805‐7. [PubMed] [Google Scholar]
IRCT2013021012421N1 {published data only}
- Shiasi Arani K, Taghavi Ardakani A, Moazami Goudarzi R, Talari HR, Hami K, Akbari H, et al. Effect of vitamin E and metformin on fatty liver disease in obese children ‐ randomized clinical trial. Iranian Journal of Public Health 2014;43(10):1417‐23. [PMC free article] [PubMed] [Google Scholar]
IRCT2014020116435N1 {unpublished data only}
- Shahrekord University of Medical Sciences. The effect of folic acid on homocysteine and plasma insulin levels in weight gain and obesity in children and adolescents. en.search.irct.ir/view/16869 Date first received: 23 May 2014.
Israsena 1980 {published data only}
- Israsena T, Israngkura M, Srivuthana S. Treatment of childhood obesity. Journal of the Medical Association of Thailand 1980;63:433‐7. [PubMed] [Google Scholar]
James 2000 {published data only}
- James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rössner S, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Storm study group. Sibutramine trial of obesity reduction and maintenance. Lancet 2000;356:2119‐25. [DOI] [PubMed] [Google Scholar]
Kasa‐Vubu 2008 {published data only}
- Kasa‐Vubu JZ. Metformin as a weight‐loss tool in "at‐risk" obese adolescents: a magic bullet?. Journal of Pediatrics 2008;152(6):750‐2. [DOI] [PubMed] [Google Scholar]
Kay 2001 {published data only}
- Kay JP, Alemzadeh R, Langley G, D'angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism: Clinical and Experimental 2001;50:1457‐61. [DOI] [PubMed] [Google Scholar]
Kelly 2012 {published data only}
- Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, et al. Exenatide as a weight‐loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity 2012;20:364‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2013a {published data only}
- Kelly AS. The role of glucagon‐like peptide‐1 receptor agonists for the treatment of adolescent obesity. Expert Review of Endocrinology and Metabolism 2013;8:315‐7. [DOI] [PubMed] [Google Scholar]
Kelly 2013b {published data only}
- Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of glucagon‐like peptide‐1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo‐controlled, clinical trial. JAMA Pediatrics 2013;167:355‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2014 {published data only}
- Kendall DL, Amin R, Clayton PE. Metformin in the treatment of obese children and adolescents at risk of type 2 diabetes. Paediatric Drugs 2014;16:13‐20. [DOI] [PubMed] [Google Scholar]
Klein 2006 {published data only}
- Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double‐blind, placebo‐controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. American Journal of Psychiatry 2006;163:2072‐9. [DOI] [PubMed] [Google Scholar]
Kneebone 1968 {published data only}
- Kneebone GM. Fenfluramine in the treatment of obesity. Medical Journal of Australia 1968;2:833‐5. [DOI] [PubMed] [Google Scholar]
Knoll 1975 {published data only}
- Knoll G, Spahn U, Plenert W. Therapy of obesity in childhood. Kinderarztliche Praxis 1975;43:412‐26. [PubMed] [Google Scholar]
Komarnicka 1975 {published data only}
- Komarnicka R, Gorzko M. Results of treatment of obesity with low calorie diet and additional drugs (author's translation) [Wyniki leczenia otyłości dieta niskokaloryczna i lekami wspomagajacymi]. Przeglad Lekarski 1975;32:814‐7. [PubMed] [Google Scholar]
Komorowski 1982 {published data only}
- Komorowski JM, Zwaigzne‐Raczynska J, Owczarczyk I, Golebiowska M, Zarzycki J. Effect of mazindol (Teronac) on various hormonal indicators in children with simple obesity. Pediatria Polska 1982;57:241‐6. [PubMed] [Google Scholar]
Kreze 1967 {published data only}
- Kreze A, Kiselá J. Biguanides and the treatment of obesity [Biguanidy a terapia obezity]. Bratislavské Lekárske Listy 1967;48:184‐9. [PubMed] [Google Scholar]
Lamberto 1993 {published data only}
- Lamberto M, Novi RF, Mantovan M. Fluoxetine and obesity [Fluoxetina ed obesità]. Minerva Endocrinologica 1993;18:41‐3.. [PubMed] [Google Scholar]
Leite 1971 {published data only}
- Leite AC, Liepen LL, Costa VP. Clinical trial of stimsem thozalinone in the treatment of obese patients [Ensaio clĩnico com o stimsem thozalinone no tratamento de pacientes obesos]. Revista Brasileira De Medicina 1971;28:475‐8. [PubMed] [Google Scholar]
Lewis 1978 {published data only}
- Lewis EA, Adeleye GI, Ukponmwan OO. Comparison of fenfluramine and placebo on obese Nigerians. Nigerian Medical Journal 1978;8:104‐7. [PubMed] [Google Scholar]
Libman 2015 {published data only}
- Libman IM, Miller KM, Dimeglio LA, Bethin KE, Katz ML, Shah A, et al. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes a randomized clinical trial. JAMA 2015;314:2241‐50. [DOI] [PubMed] [Google Scholar]
Liebermeister 1969 {published data only}
- Liebermeister H, Probst G, Jahnke K. Experience with the appetite depressant, fenfluramine hydrochloride, in adiposity [Erfahrungen mit dem Appetithemmer Fenfluraminhydrochlorid bei der Adipositas]. Medizinische Klinik 1969;64:1201‐7. [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu AG, Smith SR, Fujioka K, Greenway FL. The effect of leptin, caffeine/ephedrine, and their combination upon visceral fat mass and weight loss. Obesity 2013;21:1991‐6. [DOI] [PubMed] [Google Scholar]
Lorber 1966 {published data only}
- Lorber J. Obesity in childhood. A controlled trial of anorectic drugs. Archives of Disease in Childhood 1966;41:309‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Love‐Osborne 2008 {published data only}
- Love‐Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. Journal of Pediatrics 2008;152:817‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maclay 1977 {published data only}
- Maclay WP, Wallace MG. A multi‐centre general practice trial of mazindol in the treatment of obesity. Practitioner 1977;218:431‐4. [PubMed] [Google Scholar]
Malchow‐Møller 1980 {published data only}
- Malchow‐Møller A, Larsen S, Hey H, Stokholm KH, Juhl E, Quaade F. Effect of elsinore tablets in the treatment of obesity. A controlled clinical trial. Ugeskrift For Laeger 1980;142:1496‐9. [PubMed] [Google Scholar]
Marques 2016 {published data only}
- Marques P, Limbert C, Oliveira L, Santos MI, Lopes L. Metformin effectiveness and safety in the management of overweight/obese nondiabetic children and adolescents: metabolic benefits of the continuous exposure to metformin at 12 and 24 months. International Journal of Adolescent Medicine and Health 2016 Feb 19;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
McDuffie 2002 {published data only}
- McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Hubbard VS, et al. Three‐month tolerability of orlistat in adolescents with obesity‐related comorbid conditions. Obesity Research 2002;10:642‐50. [DOI] [PubMed] [Google Scholar]
Molnár 2000 {published data only}
- Molnár D, Török K, Erhardt E, Jeges S. Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double‐blind placebo‐controlled pilot study in adolescents. International Journal of Obesity and Related Metabolic Disorders 2000;24:1573‐8. [DOI] [PubMed] [Google Scholar]
Muls 2001 {published data only}
- Muls E, Kolanowski J, Scheen A, Gaal L, Obelhyx Study Group. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: a randomized, double‐blind, placebo‐controlled, multicentre study. International Journal of Obesity and Related Metabolic Disorders 2001;25:1713‐21. [DOI] [PubMed] [Google Scholar]
Nadeau 2015 {published data only}
- Nadeau KJ, Chow K, Alam S, Lindquist K, Campbell S, Mcfann K, et al. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double‐blinded placebo‐controlled study. Pediatric Diabetes 2015;16:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2016 {published data only}
- Nathan BM, Rudser KD, Abuzzahab MJ, Fox CK, Coombes BJ, Bomberg EM, et al. Predictors of weight‐loss response with glucagon‐like peptide‐1 receptor agonist treatment among adolescents with severe obesity. Clinical Obesity 2016;6:73‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00076362 {unpublished data only}
- Novartis Pharmaceuticals. Pediatric hypothalamic obesity. clinicaltrials.gov/ct2/show/NCT00076362 Date first received: 21 January 2004.
NCT00284557 {unpublished data only}
- Emory University. A primary care behavioral approach for addressing childhood overweight. clinicaltrials.gov/ct2/show/NCT00284557 Date first received: 30 January 2006.
NCT00775164 {unpublished data only}
- University of Minnesota ‐ Clinical and Translational Science Institute. Pioglitazone therapy in obese children with insulin resistance: a randomized, controlled pilot study. clinicaltrials.gov/ct2/show/NCT00775164 Date first received: 16 October 2008.
NCT00845559 {unpublished data only}
- Yale University. The effects of exenatide on post‐meal sugar peaks and vascular health in obese/pre‐diabetic young adults. clinicaltrials.gov/ct2/show/NCT00845559 Date first received: 16 February 2009.
NCT01023139 {unpublished data only}
- Brooke Army Medical Center. "Efficacy in adolescents of continued behavior modification following a six month sibutramine‐based weight management intervention" (AOS). clinicaltrials.gov/ct2/show/NCT01023139 Date first received: 1 December 2009.
NCT01061775 {unpublished data only}
- Children's Hospitals and Clinics of Minnesota. Effects of exenatide on hypothalamic obesity. clinicaltrials.gov/ct2/show/NCT01061775 Date first received: 2 February 2010.
NCT01107808 {published data only}
- University of Alabama at Birmingham. Calcium, vitamin D and metformin to treat insulin resistance in obese African American adolescent females. clinicaltrials.gov/ct2/show/NCT01107808 Date first received: 1 April 2010.
NCT01169103 {published data only}
- NCT01169103. Effect of recombinant human growth hormone (rhGH) on abdominal fat and cardiovascular risk in obese girls. clinicaltrials.gov/ct2/show/NCT01169103 Date first received: 22 July 2010.
NCT01242241 {unpublished data only}
- Baylor College of Medicine. Propofol in obese children. clinicaltrials.gov/ct2/show/NCT01242241 Date first received: 15 November 2010.
NCT01329367 {unpublished data only}
- Clinica Universidad de Navarra, Universidad de Navarra. Nutrigenomics and children obesity: a moderate weight loss intervention study (NUGENOI). clinicaltrials.gov/ct2/show/NCT01329367 Date first received: 20 January 2011.
NCT01332448 {unpublished data only}
- GlaxoSmithKline. Meta‐analysis of orlistat laboratory data from placebo‐controlled clinical trials. clinicaltrials.gov/ct2/show/NCT01332448 Date first received: 7 April 2011.
NCT01410604 {unpublished data only}
- Hospital Regional de Alta Especialidad del Bajio. Adipokines in obese adolescents with insulin resistance. clinicaltrials.gov/ct2/show/NCT01410604 Date first received: 4 August 2011.
NCT01456221 {unpublished data only}
- Coordinación de Investigación en Salud, Mexico. Intervention study with omega‐3 fatty acids for weight loss and insulin resistance in adolescents (O3WLIRADOL). clinicaltrials.gov/ct2/show/NCT01456221 Date first received: 12 October 2011.
NCT01910246 {unpublished data only}
- University of California, San Francisco. Cardiovascular effects of metformin on obesity. clinicaltrials.gov/ct2/show/NCT01910246 Date first received: 22 April 2013.
NCT02022956 {unpublished data only}
- Arena Pharmaceuticals. Single dose study to determine the safety, tolerability, and pharmacokinetic properties of lorcaserin hydrochloride (BELVIQ) in obese adolescents from 12 to 17 years of age. clinicaltrials.gov/ct2/show/NCT02022956 Date first received: 23 December 2013.
NCT02063802 {published data only}
- Laboratorios Silanes S.A. de C.V. Metformin vs conjugated linoleic acid and an intervention program with healthy habits in obese children. clinicaltrials.gov/ct2/show/NCT02063802 Date first received: 12 February 2014.
NCT02186652 {unpublished data only}
- Duke University. PK study with pantoprazole in obese children and adolescents (PAN01). clinicaltrials.gov/ct2/show/NCT02186652 Date first received: 3 July 2014.
NCT02378259 {published data only}
- NCT02378259. Randomized clinical trial; medical vs bariatric surgery for adolescents (13‐16 y) with severe obesity. clinicaltrials.gov/ct2/show/NCT02378259 Date first received: 26 August 2014.
NCT02398669 {published data only}
- Eisai Inc. A single dose pharmacokinetic study of lorcaserin hydrochloride in obese pediatric subjects 6 to 11 years of age. clinicaltrials.gov/ct2/show/NCT02398669 Date first received: 20 March 2015.
NCT02438020 {unpublished data only}
- Juan Pablo, C.‐C, Hospital Central "Dr. Ignacio Morones, P, Universidad Autonoma De San Luis. P. Study of efficacy of metformin in the treatment of acanthosis nigricans in children with obesity. clinicaltrials.gov/ct2/show/NCT02438020 Date first received: 3 May 2015.
NCT02515773 {unpublished data only}
- NCT02515773. Metformin for overweight & obese children and adolescents with BDS treated with SGAs. clinicaltrials.gov/ct2/show/NCT02515773 Date first received: 31 July 2015.
Nwosu 2015 {published data only}
- Nwosu BU, Maranda L, Cullen K, Greenman L, Fleshman J, Mcshea N, et al. A randomized, double‐blind, placebo‐controlled trial of adjunctive metformin therapy in overweight/obese youth with type 1 diabetes. PLoS One 2015;10:e0137525. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'connor 1995 {published data only}
- O'connor HT, Richman RM, Steinbeck KS, Caterson ID. Dexfenfluramine treatment of obesity: a double blind trial with post trial follow up. International Journal of Obesity and Related Metabolic Disorders : Journal of the International Association for the Study of Obesity 1995;19:181‐9. [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park MH, Kinra S. Metformin treatment for adolescent obesity has limited long‐term benefits. Journal of Pediatrics 2010;157:172. [DOI] [PubMed] [Google Scholar]
Pedrinola 1994 {published data only}
- Pedrinola F, Cavaliere H, Lima N, Medeiros‐Neto G. Is Dl‐Fenfluramine a potentially helpful drug therapy in overweight adolescent subjects?. Obesity Research 1994;2:1‐4. [DOI] [PubMed] [Google Scholar]
Persson 1973 {published data only}
- Persson I, Andersen U, Deckert T. Treatment of obesity with fenfluramine. European Journal of Clinical Pharmacology 1973;6:93‐7. [DOI] [PubMed] [Google Scholar]
Plauchu 1967a {published data only}
- Plauchu M, Montgolfier R, Noel P. Clinical study on a new appetite depressant Mg 559 [Etude clinique d'un anorexigène récent: le MG 559]. Lyon Medical 1967;217(15):1105‐16. [PubMed] [Google Scholar]
Plauchu 1967b {published data only}
- Plauchu M, Kofman J, Chapuy H. Clinical study of a new anorexigen: RD 354 (Hepatrol) [Etude clinique d'un nouvel anorexigène: le RD 354]. Lyon Medical 1967;217(17):1247‐61. [PubMed] [Google Scholar]
Plauchu 1972 {published data only}
- Plauchu M, Paffoy JC, Philippe LP, Nove‐Josserand G, Chollet A, Valancogne C. Clinical study of a current anorexigenic agent: T.D [Etude clinique d'un anorexigène récent: le T.D]. Lyon Medical 1972;228(15):291‐4. [PubMed] [Google Scholar]
Pugnoli 1978 {published data only}
- Pugnoli C, Bandini L, Caronna S, Ciarlini E, Magnati G, Strata A, et al. Clinical trial of a new long‐acting fenfluramine in the treatment of obesity [Ricerca Clinica Controllata Su Un Nuovo Preparato Di Fenfluramina 'A Lenta Cessione' Nel Trattamento Dell'obesita]. Giornale Di Clinica Medica 1978;59:486‐510. [PubMed] [Google Scholar]
Rauh 1968 {published data only}
- Rauh JL, Lipp R. Chlorphentermine as an anorexigenic agent in adolescent obesity. Report of its efficacy in a double‐blind study of 30 teen‐agers. Clinical Pediatrics 1968;7:138‐40. [DOI] [PubMed] [Google Scholar]
Resnick 1967 {published data only}
- Resnick M, Joubert L. A double‐blind evaluation of an anorexiant, a placebo and diet alone in obese subjects. Canadian Medical Association Journal 1967;97:1011‐5. [PMC free article] [PubMed] [Google Scholar]
Rodos 1969 {published data only}
- Rodos JJ. The treatment of obesity with voranil (Su‐10568), a new non‐amphetamine anorexigenic agent. Journal of the American Osteopathic Association 1969;69:161‐4. [PubMed] [Google Scholar]
Rodriguez 2007 {published data only}
- Rodriguez JE, Shearer B, Slawson DC. Metformin therapy and diabetes prevention in adolescents who are obese. American Family Physician 2007;76:1357‐9. [PubMed] [Google Scholar]
Roed 1980 {published data only}
- Roed P, Hansen PW, Bidstrup B, Kaern M, Helles A, Petersen KP. Elsinore banting tablets. A controlled clinical trial in general practice [Helsingor‐slankepiller: En kontrolleret klinisk undersogelse i almen praksis]. Ugeskrift For Laeger 1980;142:1491‐5. [PubMed] [Google Scholar]
Roginsky 1966 {published data only}
- Roginsky MS, Barnett J. Double‐blind study of phenethyldiguanide in weight control of obese nondiabetic subjects. American Journal of Clinical Nutrition 1966;19:223‐6. [DOI] [PubMed] [Google Scholar]
Sabuncu 2004 {published data only}
- Sabuncu T, Ucar E, Birden F, Yasar O. The effect of 1‐yr sibutramine treatment on glucose tolerance, insulin sensitivity and serum lipid profiles in obese subjects. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 2004;17:103‐7. [PubMed] [Google Scholar]
Sainani 1973 {published data only}
- Sainani GS, Fulambarkar AM, Khurana BK. A double‐blind clinical trial of fenfluramine in the treatment of obesity. British Journal of Clinical Practice 1973;27:136‐8. [PubMed] [Google Scholar]
Scavo 1976 {published data only}
- Scavo D, Giovannini C, Sellini M, Fierro A. Therapy of obesity in childhood and adolescence. Comparison of results obtained with dietary and drug treatment [La terapia dell'obesita nell'infanzia e nell'adolescenza]. Clinica Terapeutica 1976;79:205‐18. [PubMed] [Google Scholar]
Shutter 1966 {published data only}
- Shutter L, Garell DC. Obesity in children and adolescents: a double‐blind study with cross‐over. Journal of School Health 1966;36:273‐5. [DOI] [PubMed] [Google Scholar]
Spence 1966 {published data only}
- Spence AW, Medvei VC. Fenfluramine in the treatment of obesity. British Journal of Clinical Practice 1966;20:643‐4. [PubMed] [Google Scholar]
Spranger 1963 {published data only}
- Spranger J. Anorexigenic drugs in the treatment of obesity in children. An enlarged double‐blind study with chlorphentermin. Munchener Medizinische Wochenschrift (1950) 1963;105:1338‐41. [PubMed] [Google Scholar]
Spranger 1965 {published data only}
- Spranger J. Phentermine resinate in obesity. Clinical trial of Mirapront in adipose children [Phentermin‐Resinat bei Fettsucht. Klinische Prüfung von Mirapront bei adipösen Kindern]. Münchener medizinische Wochenschrift (1950) 1965;107:1833‐4. [PubMed] [Google Scholar]
Sproule 1969 {published data only}
- Sproule BC. Double‐blind trial of anorectic agents. Medical Journal of Australia 1969;1:394‐5. [PubMed] [Google Scholar]
Stewart 1970 {published data only}
- Stewart DA, Bailey JD, Patell H. Tenuate dospan as an appetite suppressant in the treatment of obese children. Applied Therapeutics 1970;12:34‐6. [PubMed] [Google Scholar]
Sukkari 2010 {published data only}
- Sukkari SR, Sasich LD, Humaidan AS, Burikan O. Analysis of metformin treatment for adolescent obesity at 48 rather than 24 weeks after treatment cessation. Archives of Pediatrics & Adolescent Medicine 2010;164:678‐9 (author reply). [DOI] [PubMed] [Google Scholar]
TODAY study group 2013 {published data only}
- Arslanian SA, Pyle L, White NH, Bacha F, Caprio S, Haymond MW, et al. Racial disparity in maintenance of glycemic control in the TODAY trial: does insulin sensitivity, beta‐cell function, or both play a role?. Diabetes 2013;62:A338. [Google Scholar]
- Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of adolescents and youth with recent‐onset type 2 diabetes: the TODAY cohort at baseline. Journal of Clinical Endocrinology and Metabolism 2011;96:159‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffel L, Chang N, Grey M, Hale D, Higgins L, Hirst K, et al. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run‐in phase of the TODAY study. Pediatric Diabetes 2012;13:369‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY Study Group. Treatment effects on measures of body composition in the TODAY clinical trial. Diabetes Care 2013;36(6):1742‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY study group. Design of a family‐based lifestyle intervention for youth with type 2 diabetes: the TODAY study. International Journal of Obesity 2010;34:217‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY study group. Treatment effects on measures of body composition in the TODAY clinical trial. Diabetes Care 2013;36:1742‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY study group, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. New England Journal of Medicine 2012;366:2247‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2005 {published data only}
- Tong NW, Ran XW, Li QF, Tang BD, Li R, Yang FY, et al. Effects of sibutramine on blood glucose and lipids, body fat mass and insulin resistance in obese patients: a multi‐center clinical trial. Zhonghua Nei Ke Za Zhi 2005;44:659‐63. [PubMed] [Google Scholar]
Toubro 2001 {published data only}
- Toubro S, Hansen DL, Hilsted JC, Porsborg PA, Astrup AV, James WPT, et al. The effect of sibutramine for the maintenance of weight loss: a randomised, clinical, controlled study [Effekt af sibutramin til vægttabsvedligeholdelse: en randomiseret klinisk kontrolleret undersøgelse]. Ugeskrift for Laeger 2001;163(21):2935‐40. [PubMed] [Google Scholar]
Tsai 2006 {published data only}
- Tsai AG, Wadden TA, Berkowitz RI. Pharmacotherapy for overweight adolescents. Obesity Management 2006;2:98‐102. [Google Scholar]
Van Seters 1982 {published data only}
- Seters AP, Bouwhuis‐Hoogerwerf ML, Goslings BM, Nieuwkoop L, Slooten H, Struijk‐Wielinga T. Long‐term treatment of patients with obesity using mazindol and a reducing diet. Nederlands Tijdschrift Voor Geneeskunde 1982;126:990‐4. [PubMed] [Google Scholar]
Warren‐Ulanch 2008 {published data only}
- Warren‐Ulanch J. Metformin may aid weight loss in overweight teenage girls. Journal of Pediatrics 2008;153:725‐6. [DOI] [PubMed] [Google Scholar]
Weintraub 1984 {published data only}
- Weintraub M, Hasday JD, Mushlin AI, Lockwood DH. Double‐blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Archives of Internal Medicine 1984;144:1143‐8. [PubMed] [Google Scholar]
Yanovski 2003 {published data only}
- Adeyemo MA, McDuffie JR, Kozlosky M, Krakoff J, Calis KA, Brady SM, et al. Effects of metformin on energy intake and satiety in obese children. Diabetes, Obesity & Metabolism 2015;17(4):363‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski JA, Yanovski SZ. Treatment of pediatric and adolescent obesity. JAMA 2003;289:1851‐3. [DOI] [PubMed] [Google Scholar]
Yu 2013 {published data only}
- Yu CC, Li AM, Chan KO, Chook P, Kam JT, Au CT, et al. Orlistat improves endothelial function in obese adolescents: a randomised trial. Journal of Paediatrics and Child Health 2013;49:969‐72. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Golebiowska 1981 {published data only}
- Golebiowska M, Chlebna‐Sokol D, Kobierska I, Konopinska A, Malek M, Mastalska A, et al. Clinical evaluation of teronac (mazindol) in the treatment of obesity in children. Part II. Anorectic properties and side effects (author's translation). Przeglad Lekarski 1981;38:355‐8. [PubMed] [Google Scholar]
ISRCTN08063839 {unpublished data only}
Linquette 1971 {published data only}
- Linquette A, Fossati, P. Hunger control with benzphetamine hydrochloride in the treatment of obesity [Le contrôle de la faim par le chlorydrate de benzphétamine dans le traitement de l'obésité]. Lille Medical 1971;16(Suppl 2):620‐4. [PubMed] [Google Scholar]
NCT00934570 {unpublished data only}
- Clarson CL, Brown H, Dejesus S, Jackman M, Mahmud FH, Prapavessis H, et al. Structured lifestyle intervention with metformin extended release or placebo in obese adolescents. Diabetes. 2013; Vol. 62:A337‐8.
- Wilson AJ, Prapavessis H, Jung ME, Cramp AG, Vascotto J, Lenhardt L, et al. Lifestyle modification and metformin as long‐term treatment options for obese adolescents: study protocol. BMC Public Health 2009;9:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00940628 {unpublished data only}
NCT01487993 {unpublished data only}
- Aa MP, Elst MA, Mil EG, Knibbe CA, Vorst MM. Metformin: an efficacy, safety and pharmacokinetic study on the short‐term and long‐term use in obese children and adolescents ‐ study protocol of a randomized controlled study. Trials 2014;15:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aa MP, Elst MA, Garde EM, Mil EG, Knibbe CA, Vorst MM. Long‐term treatment with metformin in obese, insulin‐resistant adolescents: results of a randomized double‐blinded placebo‐controlled trial. Nutrition & Diabetes 2016;6(8):e228. [DOI: 10.1038/nutd.2016.37] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smetanina 2015 {published data only}
- Smetanina N, Seibokaite A, Valickas R, Kuprionis G, Grigoniene JJ, Rokaite R, et. al. Metformin in combination with lifestyle changes effectively reduces BMI and waist circumference in overweight/obese children and adolescents. Hormone Research in Paediatrics 2015;84:229. [Google Scholar]
References to ongoing studies
EUCTR2010‐023061‐21 {unpublished data only}
- Pastor MB, Canete MD, Hoyos R, Latorre M, Vazquez‐Cobela R, Rangel‐Huerta OD, et al. Metformin in the treatment of obese children shows differential response according to pubertal stage. Acta Physiologica. 2014; Vol. 212:36‐7.
EUCTR2015‐001628‐45‐SE {unpublished data only}
- A study with lifestyle intervention and study medication once weekly or lifestyle intervention and placebo in adolescents with obesity to explore differences between groups with regard to change in BMI. Ongoing study Trial start date: not givenTrial completion date: not given.
NCT00889876 {unpublished data only}
- Effect of exercise or metformin on nocturnal blood pressure and other risk factors for CVD among obese adolescents. Ongoing study Trial start date: February 2009Trial completion date: December 2012 (estimated).
NCT01677923 {unpublished data only}
- Obesity in children and adolescents: associated risks and early intervention (OCA). Ongoing study Trial start date: May 2013Trial completion date: December 2015.
NCT01859013 {unpublished data only}
- Topiramate in Adolescents with Severe Obesity. Ongoing study Trial start date: June 2013Trial completion date: December 2015.
NCT02273804 {unpublished data only}
- Topiramate and Severe Obesity (TOBI). Ongoing study Trial start date: June 2015Trial completion date: December 2020.
NCT02274948 {unpublished data only}
- Use of Metformin in Treatment of Childhood Obesity. Ongoing study Trial start date: July 2014Trial completion date: February 2016.
NCT02496611 {unpublished data only}
- Enhancing Weight Loss Maintenance With GLP‐1RA (BYDUREON™) in Adolescents with Severe Obesity. Ongoing study Trial start date: December 2015Trial completion date: July 2020.
Additional references
Aggarwal 2014
- Aggarwal A, Puri K, Thangada S, Zein N, Alkhouri N. Nonalcoholic fatty liver disease in children: recent practice guidelines, where do they take us?. Current Pediatric Reviews 2014;10(2):151‐61. [DOI] [PubMed] [Google Scholar]
Alonso 1995
- Alonso J, Prieto L, Anto JM. The Spanish version of the SF 36 Health Survey (the SF 36 health questionnaire): an instrument for measuring clinical results. Medicina Clinica 1995;104:771‐6. [PubMed] [Google Scholar]
Barlow 1998
- Barlow SE, Dietz WH. Maternal and Child Health Resources and Services Administration and Department of Health and Human Services. Obesity evaluation and treatment: expert committee recommendations. Pediatrics 1998;102:E29. [DOI] [PubMed] [Google Scholar]
Bayer 2011
- Bayer O, Krüger H, Kries R, Toschke AM. Factors associated with tracking of BMI: a meta‐regression analysis on BMI tracking. Obesity 2011;19(5):1069‐76. [DOI] [PubMed] [Google Scholar]
Berardis 2014
- Berardis S, Sokal E. Pediatric non‐alcoholic fatty liver disease: an increasing public health issue. European Journal of Pediatrics 2014;173(2):131‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bocca 2013
- Bocca G, Ongering EC, Stolk RP, Sauer PJ. Insulin resistance and cardiovascular risk factors in 3‐ to 5‐year‐old overweight or obese children. Hormone Research in Paediatrics 2013;80(3):201‐6. [DOI] [PubMed] [Google Scholar]
Boland 2015
- Boland CL, Brock Harris J, Harris, KB. Pharmacological management of obesity in pediatric patients. Annals of Pharmacotherapy 2015;49(2):220‐32. [DOI: 10.1177/1060028014557859] [DOI] [PubMed] [Google Scholar]
Borkan 1982
- Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. American Journal of Clinical Nutrition 1982;36(1):172‐7. [DOI] [PubMed] [Google Scholar]
Bouza 2012
- Bouza C, Lopez‐Cuadrado T, Gutierrez‐Torres LF, Amate J. Efficacy and safety of metformin for treatment of overweight and obesity in adolescents: an updated systematic review and meta‐analysis. Obesity Facts 2012;5:753‐65. [DOI] [PubMed] [Google Scholar]
Calloway 1988
- Calloway CW, Chumlea WC, Bouchard C. Circumferences. In: Lohman TC, Roche AR, Martorell R editor(s). Anthropometric Standardization Manual. Campaign, III. Champaign, IL: Human Kinetics Publishers, 1988:39‐64. [Google Scholar]
Catoira 2010
- Catoira N, Nagel M, Girolamo G, Gonzalez CD. Pharmacological treatment of obesity in children and adolescents: current status and perspectives. Expert Opinion on Pharmacotherapy 2010;11(18):2973‐83. [DOI] [PubMed] [Google Scholar]
Cheung 2013
- Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Therapeutic Advances in Drug Safety 2013;4(4):171‐81. [DOI: 10.1177/2042098613489721] [DOI] [PMC free article] [PubMed] [Google Scholar]
CMO 2014
- Chief Medical Officer. Chief Medical Officer annual report: surveillance volume 2012. www.gov.uk/government/publications/chief‐medicalofficer‐annual‐report‐surveillance‐volume‐2012 27 March 2014.
Cole 1995
- Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Archives of Disease in Childhood 1995;73:25‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2000
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2005
- Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z‐score or BMI centile?. European Journal of Clinical Nutrition 2005;59:419‐25. [DOI] [PubMed] [Google Scholar]
Cole 2012
- Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut offs for thinness, overweight and obesity. Pediatric Obesity 2012;7:284‐94. [DOI] [PubMed] [Google Scholar]
Colquitt 2016
- Colquitt JL, Loveman E, O'Malley C, Azevedo LB, Mead E, Al‐Khudairy L, et al. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD012105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czernichow 2010
- Czernichow S, Lee CM, Barzi F, Green F. Efficacy of weight loss drugs on obesity and cardiovascular risk factors in obese adolescents: a meta‐analysis of randomized controlled trials. Obesity Reviews 2010;11(2):150‐8. [DOI] [PubMed] [Google Scholar]
De Onis 2010
- Onis M, Blossner M. Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition 2010;92:1257‐64. [DOI] [PubMed] [Google Scholar]
Derogatis 1983
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine 1983;13:595‐605. [PubMed] [Google Scholar]
Dietz 1998
- Dietz, WH. Health consequences of obesity in youth: childhood predictors of adult disease. Paediatrics 1998;101(3S):518‐25. [PubMed] [Google Scholar]
Ebbeling 2002
- Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public‐health crisis, common sense cure. Lancet 2002;360(9331):473‐82. [DOI] [PubMed] [Google Scholar]
Ells 2015a
- Ells L, Hancock C, Copley VR, Mead E, Dinsdale H, Kinra S, et al. Prevalence of severe childhood obesity in England: 2006‐2013. Archives of Disease in Childhood 2015;100(7):631‐6. [DOI: 10.1136/archdischild-2014-307036] [DOI] [PubMed] [Google Scholar]
Ells 2015b
- Ells LJ, Mead E, Atkinson G, Corpeleijn E, Roberts K, Viner R, et al. Surgery for the treatment of obesity in children and adolescents. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011740] [DOI] [PubMed] [Google Scholar]
Falaschetti 2010
- Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre‐pubertal children. European Heart Journal 2010;173(2):131‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez 2004
- Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African‐American, European‐American, and Mexican‐American children and adolescent. Journal of Pediatrics 2004;145:439‐44. [DOI] [PubMed] [Google Scholar]
Fishbein 2001
- Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non‐invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatric Radiology 2001;31(11):806‐9. [DOI] [PubMed] [Google Scholar]
Fishbein 2003
- Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. Journal of Pediatric Gastroenterology and Nutrition 2003;36(1):54‐61. [DOI] [PubMed] [Google Scholar]
Freedman 1999
- Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103(6 Pt 1):1175‐82. [DOI] [PubMed] [Google Scholar]
Freedman 2006
- Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity 2006;14:301‐8. [DOI] [PubMed] [Google Scholar]
Fuller 1992
- Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four‐component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat‐free mass. Clinical Science (London) 1992;82:687‐93. [DOI] [PubMed] [Google Scholar]
Glenny 1997
- Glenny A M. O'Meara S, Melville A, sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 1997;21(9):715‐37. [DOI] [PubMed] [Google Scholar]
Halford 2010
- Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nature Reviews. Endocrinology 2010;6(5):255‐69. [DOI] [PubMed] [Google Scholar]
Halpern 2010
- Halpern A, Mancini MC, Magalhães ME, Fisberg M, Radominski R, Bertolami MC, et al. Metabolic syndrome, dyslipidemia, hypertension and type 2 diabetes in youth: from diagnosis to treatment. Diabetology & Metabolic Syndrome 2010;2:55. [PUBMED: 20718958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 1998
- Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A. Thermogenic effects of sibutramine in humans. American Journal of Clinical Nutrition 1998;68:1180‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
HSCIC 2014
- Health and Social Care Information Centre. National Child Measurement Programme ‐ England, 2013‐14 school year. www.hscic.gov.uk/ (22 October 2016).
Hsia 2011
- Hsia Y, Dawoud D, Sutcliffe AG, Viner RM, Kinra S, Wong ICK. Unlicensed use of metformin in children and adolescents in the UK. British Journal of Clinical Pharmacology 2011;73(1):135‐9. [DOI: 10.1111/j.1365-2125.2011.04063.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imperatore 2012
- Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35(12):2515‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2010
- James WP, Caterson ID, Coutinho W, Finer N, Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. New England Journal of Medicine 2010;363(10):905‐17. [DOI] [PubMed] [Google Scholar]
Kakinami 2014
- Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Archives of Disease in Childhood 2014;99:1020‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2013
- Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement From the American Heart Association. Circulation 2013;128:1689‐712. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Knai 2012
- Knai C, Lobstein T, Darmon N, Rutter H, McKee M. Socioeconomic patterning of childhood overweight status in Europe. International Journal of Environmental Research and Public Health 2012;9(4):1472‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolotkin 1997
- Kolotkin RL, Head S, Brookhart A. Construct validity of the Impact of Weight on Quality of Life Questionnaire. Obesity Research 1997;5(5):434‐41. [DOI] [PubMed] [Google Scholar]
Kolotkin 2001
- Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health‐related quality of life and weight loss. Obesity Research 2001;9(9):564‐71. [DOI] [PubMed] [Google Scholar]
Kromeyer‐Hausschild 2001
- Kromeyer‐Hausschild K, Wabitsch M, Kunze D. Perzentile fur den Body‐mass‐Index fűr das Kindes‐und Jugendalterunter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001;149:807‐18. [DOI: 10.1007/s001120170107] [DOI] [Google Scholar]
Kuczmarski 2000
- Kuczmarski RJ, Ogden CL, Grummer‐Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Advance Data 2000;314:1‐27. [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobstein 2004
- Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obesity Review 2004;5(Suppl 1):4‐104. [DOI] [PubMed] [Google Scholar]
Loveman 2015
- Loveman E, Al‐Khudairy L, Johnson RE, Robertson W, Colquitt JL, Mead EL, et al. Parent‐only interventions for childhood overweight or obesity in children aged 5 to 11 years. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD012008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maahs 2014
- Maahs DM, Daniels SR, Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2014;130(17):1532‐58. [DOI] [PubMed] [Google Scholar]
Matson 2012
- Matson KL, Fallon RM. Treatment of obesity in children and adolescents. Journal of Pediatric Pharmacology and Therapeutics 2012;17(1):45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCreight 2016
- McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia 2016;59(3):426‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonagh 2014
- McDonagh M, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatrics 2014;168(2):178‐84. [DOI: 10.1001/jamapediatrics.2013.4200] [DOI] [PubMed] [Google Scholar]
McGovern 2008
- McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Treatment of pediatric obesity: a systematic review and meta‐analysis of randomized trials. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4600‐5. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nespoli 2013
- Nespoli L, Caprioglio A, Brunetti L, Nosetti L. Obstructive sleep apnea syndrome in childhood. Early Human Development 2013;89(Suppl 3):S33‐7. [DOI] [PubMed] [Google Scholar]
Ng 2014
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis of the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence. Obesity: identification, assessment and management of overweight and obesity in children, young people and adults. www.nice.org.uk/guidance/cg189/chapter/1‐recommendations#pharmacological‐interventions (accessed 5 August 2015). [PubMed]
Olds 2011
- Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. International Journal of Pediatric Obesity 2011;6:342‐60. [DOI: 10.3109/17477166.2011.605895] [DOI] [PubMed] [Google Scholar]
Padwal 2003
- Padwal RS, Rucker D, Li SK, Curioni C, Lau DCW. Long‐term pharmacotherapy for obesity and overweight. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004094.pub2] [DOI] [PubMed] [Google Scholar]
Pandey 2015
- Pandey A, Chawla S, Guchhait P. Type‐2 diabetes: current understanding and future perspectives. IUBMB Life 2015;67(7):506‐13. [DOI] [PubMed] [Google Scholar]
Park 2009
- Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32(9):1743‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsons 1999
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International Journal of Obesity 1999;23(Suppl 8):S1‐107. [PubMed] [Google Scholar]
Petkar 2013
- Petkar R, Wright N. Pharmacological management of obese child. Archives of Disease in Childhood ‐ Education and Practice 2013;98:108‐12. [DOI: 10.1136/archdischild-2011-301127] [DOI] [PubMed] [Google Scholar]
Pinhas‐Hamiel 2005
- Pinhas‐Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. Journal of Pediatrics 2005;146:693‐700. [DOI] [PubMed] [Google Scholar]
Power 2014
- Power C, Pinto Pereira SM, Law C, Ki M. Obesity and risk factors for cardiovascular disease and type 2 diabetes: investigating the role of physical activity and sedentary behaviour in mid‐life in the 1958 British cohort. Atherosclerosis. 2014;233(2):363‐9. [DOI] [PubMed] [Google Scholar]
Puhl 2007
- Puhl RM, Latner JD. Stigma, obesity, and the health of the nation's children. Psychology Bulletin 2007;133(4):557‐80. [DOI] [PubMed] [Google Scholar]
Rajput 2014
- Rajput N, Tuohy P, Mishra S, Smith A, Taylor B. Overweight and obesity in 4‐5‐year‐old children in New Zealand: results from the first 4 years (2009‐2012) of the B4School Check programme. Journal of Paediatrics and Child Health 2014;51(3):334‐43. [DOI: 10.1111/jpc.12716] [DOI] [PubMed] [Google Scholar]
Ravens‐Sieberer 2001
- Ravens‐Sieberer U, Redegeld M, Bullinger M. Quality of life after in‐patient rehabilitation in children with obesity. International Journal of Obesity and Related Metabolic Disorders 2001;25 Suppl 1:S63‐5. [DOI] [PubMed] [Google Scholar]
Reilly 2003
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Archives of Disease in Childhood 2003;88(9):748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reilly 2011
- Reilly JJ, Kelly J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International Journal of Obesity 2011;35:891‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rokholm 2010
- Rokholm B, Baker JL, Sørenson TIA. The levelling off of the obesity epidemic since the year 1999 ‐ a review of evidence and perspectives. Obesity Reviews 2010;11:835‐46. [DOI] [PubMed] [Google Scholar]
Rosner 1998
- Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S. children 5 to 17 years of age. Journal of Pediatrics 1998;132:211‐22. [DOI] [PubMed] [Google Scholar]
Sherafat‐Kazemzadeh 2013
- Sherafat‐Kazemzadeh R, Yanovski SZ, Yanovski JA. Pharmacotherapy for childhood obesity: present and future prospects. International Journal of Obesity (London) 2013;37(1):1‐15. [DOI: 10.1038/ijo.2012.144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrewsbury 2008
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross‐sectional studies 1990‐2005. Obesity (Silver Spring) 2008;16(2):275‐84. [DOI] [PubMed] [Google Scholar]
Singh 2008
- Singh AS, Mulder C, Twisk JW, vanMechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Reviews 2008;9(5):474‐88. [DOI] [PubMed] [Google Scholar]
Skinner 2014
- Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999‐2012. JAMA Pediatrics 2014;168(6):561‐6. [DOI: 10.1001/jamapediatrics.2014.21] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stunkard 1985
- Stunkard AJ, Messick S. The three‐factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research 1985;29:71‐83. [DOI] [PubMed] [Google Scholar]
Tang‐Peronard 2008
- Tang‐Peronard JL, Heitmann BL. Stigmatization of obese children and adolescents, the importance of gender. Obesity Reviews 2008;9:1467‐89. [DOI] [PubMed] [Google Scholar]
Van Marken Lichtenbelt 1999
- Marken Lichtenbelt W, Fogelholm M. Body composition. In: Westerterp‐Plantenga MS, Steffens AB, Tremblay A, eds editor(s). Regulation of Food Intake and Energy Expenditure. Milan: EDRA, 1999:383‐404. [Google Scholar]
Viner 2010
- Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti‐obesity drugs in children and adolescents: systematic review and meta‐analysis. Obesity Reviews 2010;11(8):593‐602. [DOI] [PubMed] [Google Scholar]
von Scheven 2006
- Scheven E, Gordon CM, Wypij D, Wertz M, Gallagher KT, Bachrach L. Variable deficits of bone mineral despite chronic glucocorticoid therapy in pediatric patients with inflammatory diseases: a Glaser Pediatric Research Network study. Journal of Pediatric Endocrinology & Metabolism 2006;19(6):821‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2003
- Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. American Journal of Clinical Nutrition 2003;77(2):379‐84. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang Y, Lim H. The global childhood obesity epidemic and the association between socio‐economic status and childhood obesity. International Review of Psychiatry 2012;24(3):176‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2004
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. New England Journal of Medicine 2004;350(23):2362‐74. [DOI] [PubMed] [Google Scholar]
Whitaker 1997
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine 1997;337(13):869‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WHO 1995
- World Health Organization. The Use and Interpretation of Antropometry. Geneva: WHO 1995.
WHO 2006
- World Health Organization. The WHO Child Growth Standards. www.who.int/childgrowth/standards/Technical_report.pdf 2006 (accessed 08/11/2016).
WHO 2015
- World Health Organization. Fact sheet on overweight and obesity. www.who.int/mediacentre/factsheets/fs311/en/ (accessed 24 February 2015).
Widhalm 2016
- Widhalm HK, Seemann R, Hamboeck M, Mittlboeck M, Neuhold A, Friedrich K, et al. Osteoarthritis in morbidly obese children and adolescents, an age‐matched controlled study. Knee Surgery, Sports Traumatology, Arthroscopy 2016;24(3):644‐52. [DOI] [PubMed] [Google Scholar]
Wong 2006a
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2015
- The World Bank. Country and Lending Groups. data.worldbank.org/about/country‐and‐lending‐groups (accessed 5 August 2015).
Yanovski 2014
- Yanovski SZ, Yanovski JA. Long‐term drug treatment of obesity: a systematic and clinical review. JAMA 2014;311(1):74‐86. [DOI: 10.1001/jama.2013.281361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2011
- Ye Z, Chen L, Yang Z, Li Q, Huang Y, He M, et al. Metabolic effects of fluoxetine in adults with type 2 diabetes mellitus: a meta‐analysis of randomized placebo‐controlled trials. PLoS One 2011;6(7):e21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Oude Luttikhuis 2009
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001872.pub2] [DOI] [PubMed] [Google Scholar]
Summerbell 2003
- Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001872] [DOI] [PubMed] [Google Scholar]


