Abstract
Type 1 diabetes is an organ-specific autoimmune disease caused by chronic inflammation (insulitis), which damages the insulin producing β-cells of the pancreatic Islets of Langerhans. Dendritic cells (DCs) are generally the first cells of the immune system to process β-cell autoantigens and, by promoting autoreactivity, play a major role in the onset of insulitis. Although no cure for diabetes presently exists, the onset of insulitis can be diminished in the non-obese diabetic (NOD) mouse type 1 diabetes model by inoculation with endogenous β-cell autoantigens. These include the single peptide vaccines insulin, GAD65 (glutamic acid decarboxylase), and DiaPep277 (an immunogenic peptide from the 60-kDa heat shock protein). DiaPep277 is the only autoantigen so far to demonstrate positive results in human clinical trials. Diamyd (an alum adjuvant + recombinant GAD65 protein formulation) has shown great promise for suppressing β-cell autoreactivity in phase I and II clinical trials. While Diamyd preserved residual insulin secretion in early-onset type 1 diabetes patients, it did not reduce the amounts of insulin required to maintain euglycemia. Recently, multi-component vaccines composed of the anti-inflammatory cytokine (IL-10) and insulin or GAD55 linked to an immunostimulatory molecule, the cholera toxin B subunit, were shown to safely and completely inhibit diabetes onset in NOD mice. This result suggests that multi-component vaccine strategies are promising for prevention and reversal of diabetes autoimmunity in humans. Here we focus on the development of autoantigen vaccines for type 1 diabetes and demonstrate that multi-component vaccines are promising candidates for type 1 diabetes clinical studies.
Introduction
Insulin-dependent diabetes mellitus (type 1 diabetes) occurs predominantly during childhood or adolescence when the body’s immune system destroys the pancreatic islet β-cells. The β-cells are the only cells in the body that make insulin, the hormone responsible for regulating entry of blood glucose into cells. To date, there is no cure for type 1 diabetes. The only treatment is daily injection of recombinant human insulin to replace the loss of insulin production due to chronic inflammation of the pancreatic islets (insulitis). Current strategies for the inhibition of autoimmunity involve suppression of the entire immune system, but this could compromise normal immune responses to pathogens. A new therapeutic approach for type 1 diabetes is based on prevention of β-cell loss through vaccine restoration of normal immune system function. To restore normal blood sugar levels in type 1 diabetes patients, the goal is to (1) prevent the onset of autoreactivity to pancreatic islet antigens, (2) interrupt autoreactivity to pancreatic islet antigens through enhancement of immunological tolerance, and (3) use alternative therapies such as islet transplantation to restore lost β-cell function. Recently, several type 1 diabetes vaccine strategies that address these goals were developed. In this review, we present an overview of the development of autoantigen specific vaccines, existing problems, and assessments for a permanent mediated cure for type 1 diabetes.
The Role of Dendritic Cells in Inflammation
Dendritic cells (DCs), the body’s largest group of antigen presenting cells, are involved in the initiation of diabetes inflammation. DCs function by recognizing pathogenic and self antigens and by stimulating the development of antigen specific T lymphocytes. Normally, DCs will induce immune tolerance when they present β-cell autoantigens to T cells. However, in type 1 diabetes, DCs instruct naïve T helper cells to recognize β-cell proteins as foreign antigens. Consequently, DC-stimulated autoreactive T cells invade pancreatic tissues and destroy islet β-cells. This results in a progressive loss of insulin production and subsequent development of hyperglycemia (Figure 1). Because DCs are involved early in the initiation of insulitis, it is important to understand their role in the development of chronic inflammation responsible for the onset of type 1 diabetes. Much of our knowledge of DC function in type 1 diabetes comes from studies in the well characterized non-obese diabetic (NOD) mouse model. Based on immunofluorescent labeling of intact islets, normal pancreatic tissue contains from 8–10 DCs per islet (Calderon et al., 2008). Further, the pancreatic specific T-cell response detected in type 1 diabetes is due to major histocompatibility complex (MHC) class II presentation of islet antigens by DCs rather than MHC class I presentation by β-cells (Sarukhan et al., 1999). Not only was it demonstrated that intra-islet DCs expressed MHC II complexed with β-cell peptides, but it was also shown that intra-islet DCs were effective in β-cell antigen presentation to CD4+ T cells ex vivo (Calderon et al., 2008; Levisetti et al., 2008; Mohan et al., 2010). In addition to islet DCs, Hoglund et al. (1999) showed that DCs also reside in lymph nodes that drain the pancreas. The authors deduced that interactions between β-cell antigen presenting DCs and T cells occur in these lymph nodes and that the newly formed autoreactive T cells are then targeted to the islets. The observation that pancreatic lymph nodes in NOD mice are necessary for diabetes development further supports this notion (Gagnerault et al., 2002). Due to the presence of high levels of non-inflammatory chemokines in NOD mice, DCs gradually accumulate around the islets prior to invasion and the onset of insulitis (Bouma et al., 2005; Rosmalen et al., 2000). The ability of β-cell autoantigens to activate immature DCs emphasizes their significance as targets in the development of vaccine strategies for treatment of type 1 diabetes.
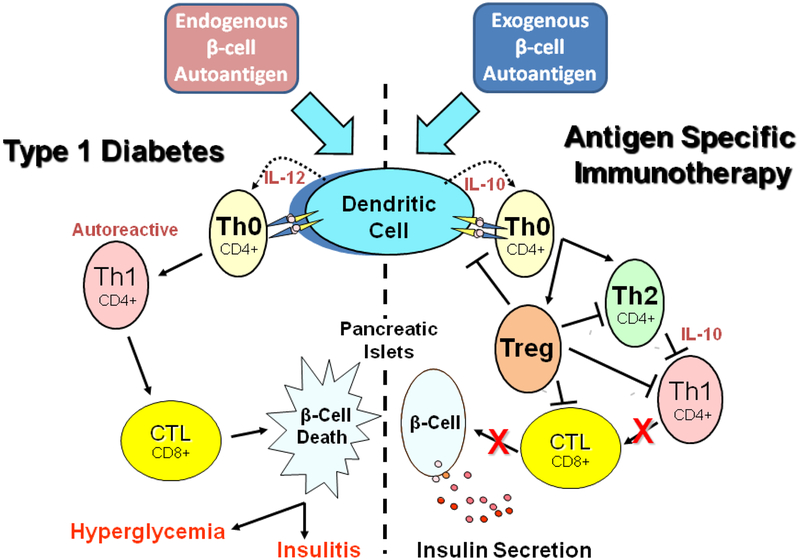
Figure 1.
Basic mechanism of type 1 diabetes inflammation and vaccine action. Antigen presenting cells, specifically dendritic cells, process endogenous pancreatic beta cell autoantigens (Insulin, GAD, etc.) in lysosomal vesicles and transfer peptide fragments of the autoantigen to MHC class II molecules that migrate to the plasma membrane and present the autoantigen to cognate T cell receptors on naïve T helper cells (Th0), left portion of the Figure. At the same time, dendritic cell processing of the autoantigen stimulates biosynthesis and secretion of the inflammatory cytokine interleukin 12 (IL-12) which stimulates the Th0 cells to undergo morphogenesis into autoreactive effector T helper cells (Th1). The autoreactive Th1 cells secrete inflammatory cytokines such as IFN-gamma and IL-2 that stimulate cytotoxic lymphocytes (CTL) to secrete nitric acid, peroxide, and several other inflammatory cytokines that stimulate pancreatic islet inflammation (insulitis). Chronic insulitis results in continual pancreatic beta cell death resulting in increasing insulin deficiency and a progressive increase in blood sugar (hyperglycemia). Oral delivery of small amounts of islet autoantigens (right portion of the Figure) exerts a protective antigen specific therapeutic effect by stimulating dendritic cells to secrete the anti-inflammatory cytokine IL-10 which stimulates naïve cognate Th0 lymphocytes to undergo morphogenesis into anti-inflammatory CD4+ Th2 helper cells that in turn secrete IL-10 which suppresses further development of autoreactive Th1 cells and decreases potential insulitis onset. Alternatively, naive Th0 cells may develop into one of several subclasses of regulatory T cells (Treg), which can block dendritic cell activation, Th2, Th1, and CTL development leading to prevention of insulitis, continued insulin secretion, and maintenance of immunological homeostasis.
Antigen Specific Immunotherapy
Traditionally, vaccination refers to prevention of an infectious disease by exposing the immune system to a weakened or dead infectious agent. Alternatively, “inverse vaccination” (the inhibition of an immune response) arrests autoimmunity through manipulation of the innate and adaptive arms of the immune system (Steinman, 2010). Current options for treatment of autoimmunity such as immunosuppressive drugs (e.g., cyclosporine) and anti-T cell antibodies (e.g., anti-CD3 antibodies) have shown varying degrees of success in suppression of β-cell autoimmunity in NOD mice. However, these strategies require repeated drug administration and may cause nonspecific harmful effects such as interference with normal immune system functions (Bougneres et al., 1990; Chatenoud et al., 1994). In contrast, antigen specific immunotherapy (ASI) makes use of inverse vaccination for a specific autoantigen. Application of ASI has a major advantage of permitting selective inactivation of autoreactive T cells without interfering with normal immune function (Wang and Tisch, 2008).
In type 1 diabetes, vaccination with β-cell autoantigens was shown to induce a partial state of immunological tolerance in NOD mice (Peakman and von Herrath, 2010). The precise mechanisms by which DC presentation of an autoantigen stimulates immune tolerance or autoreactivity are incompletely understood. Beta-cell self antigens can induce tolerance through three possible DC mediated mechanisms: (1) induction of T cell deletion/anergy, (2) induction of anti-inflammatory T helper 2 (Th2) cells, and (3) stimulation of regulatory T cell proliferation (Figure 1) (Falb et al., 1996; Maldonado and von Andrian, 2010). Because autoanti-bodies to insulin, glutamic acid decarboxylase (GAD), and tyrosine phosphatase-like insulinoma antigen (IA-2) are clinical predictors of type 1 diabetes onset, these β-cell antigens can serve as peptides for constructing ASI vaccines against diabetes onset or progression (Harrison, 2001). The goal of this review is to outline novel diabetes vaccine strategies based on β-cell autoantigens suppression of diabetes onset and progression in NOD mice and translation of these vaccines as safe and effective therapies for type 1 diabetes in humans.
Single Peptide Vaccines
Insulin, GAD, and glial cell autoantigens are considered to be the first pancreatic autoantigens detected early during diabetes onset in both humans and NOD mice. However, the primary β-cell antigen responsible for triggering autoimmunity in type 1 diabetes remains under dispute (Harrison, 2008; Wang and Tisch, 2008). Due to this uncertainty, several pancreatic autoantigens have been selected for development into type 1 diabetes vaccines. Initial vaccine strategies have centered on using the entire autoantigen or selected portions (epitopes) of the autoantigen determined to be most immunogenic (Table 1).
Table 1.
Summary of Current Type 1 Diabetes Antigen Specific Vaccines
| Type | Vaccine | Pre-clinical Trials | Clinical Trials |
|---|---|---|---|
| Single autoantigen | Insulin | • Partial suppression of diabetes onset | Unsuccessful phase III Currently in new phase III |
| GAD65 | • Partial suppression diabetes of onset | Successful Phase I | |
| DiaPep277 (Hsp60p277) | • Complete suppression of diabetes onset • Stop/reverse progression |
Currently in Phase III | |
| Adjuvant + autoantigen | Diamyd (alum formulated GAD65) | • Partial suppression of diabetes onset | Currently in Phase III |
| Adjuvant linked to autoantigen | CTB-INS | • Enhanced suppression of diabetes onset | N/A |
| CTB-GAD | • Enhanced suppression of diabetes onset • Partial inhibition of progression |
N/A | |
| Adjuvant linked to autoantigen + cytokine | CTB-GAD+IL-10 | • Complete suppression of diabetes onset | N/A |
Note: N/A = not applicable.
Insulin
Historically, insulin, the major β-cell product, was selected as the primary autoantigen to be considered for its therapeutic potential. Zhang et al. (1991) was among the first to report protection from diabetes in NOD mice following oral administration of porcine insulin. A number of studies followed that showed proinsulin/insulin or epitopes from insulin could partially protect NOD mice from developing diabetes when administered orally. Subsequent experiments have shown that oral administration of human insulin to NOD mice can induce the proliferation of CD4+ T regulatory cells that protect pre-diabetic mice from diabetes onset (Bergerot et al., 1994). Expansion of the Th2 cell population was thought to be based on the ability of insulin to promote an anti-inflammatory state in DCs, ultimately leading to immunological suppression of T cell function (Odumosu et al., 2010). In addition, insulin can inhibit diabetes onset when administered by different routes of entry into the body. Proinsulin inoculated intraperitoneally or intranasally, or insulin B chain peptide B:9–23 delivered subcutaneously were shown to be effective in partially suppressing diabetes onset in NOD mice (Chen et al., 2001; Hanninen and Harrison, 2000; Liu et al., 2002). These promising experimental outcomes were responsible for initiation of human clinical trials for assessment of insulin as a safe and effective vaccine for prevention of type 1 diabetes.
Initial human clinical studies recruited 14 individuals with a high genetic probability for development of type 1 diabetes. Intravenous insulin administration was shown to delay diabetes onset in the treatment group (Fuchtenbusch et al., 1998). These positive results served as the basis for a larger phase III clinical trial, referred to as the Diabetes Prevention Trial-Type 1 (DPT-1). Patients considered eligible for the trial were relatives of type 1 diabetics with detectable serum islet cell antibodies (ICA) and glucose tolerance in the non-diabetic level (Yu et al., 2001). These newly diagnosed type 1 diabetics were administered 5.0 mg of oral insulin daily along with subcutaneous insulin therapy for 12 months. Unfortunately, the results for both prevention of diabetes onset and intervention in diabetes progression were negative. No significant differences in insulin C peptide levels (a marker of insulin secretion) were detected between the treated and placebo groups (Pozzilli et al., 2000; Skyler et al., 2005). Although the insulin therapy studies failed to demonstrate efficacy in preventing diabetes onset, much information was gathered for application to future clinical trials. Present clinical studies for assessment of type 1 diabetes vaccines are now more practical due to proven prediction tools (e.g., insulin and GAD autoantigen antibody titers) for assessment of diabetes risk in the preclinical state (Pozzilli, 2002). To date, no clinical trials for insulin efficacy have been successful. Additional phase III clinical trials with insulin as a vaccine, such as TrialNet (an extension of DPT-1), are currently ongoing, but the experimental results have not yet been determined (Grose, 2007).
GAD65
Glutamic acid decarboxylase (GAD), an enzyme synthesized in the brain and in the pancreatic islets, is responsible for conversion of glutamate into gamma-aminobutyric acid (GABA) and CO2. In the brain, GABA is an important neurotransmitter while in the pancreas GABA appears to regulate glucagon action and insulin secretion in response to serum glucose levels (Bjork et al., 1992). Two separate GAD genes encode the 65 kDa and 67 kDa enzyme isoforms. The 65 kDa isoform is expressed in human pancreatic β-cells and the 67 kDa isoform is synthesized in the brain (Okada et al., 1976). The role of GAD65 and its product GABA in pancreatic islets remains incompletely understood. However, it has recently been shown that insulin secretion may be dependent on GABA metabolism (Pizarro-Delgado et al., 2010). While the role of GAD65 in β-cell function remains understudied, it is another major β-cell antigen to which type 1 diabetics develop autoantibodies. For this reason, GAD65 is considered an important target for ASI therapy.
The administration of exogenous GAD65 to female NOD mice was shown to prevent diabetes onset in some mice in addition to reducing the frequency of insulitis (Tisch et al., 1994). These observations were further supported by the result that intravenously injected GAD65 prevented the onset of type 1 diabetes in mice (Kaufman et al., 1993). Glutamic acid decarboxylase (GAD65) knockout NOD mice develop diabetes, although at a reduced rate (Kanazawa et al., 2009). This observation suggests GAD65 is not the only autoantigen responsible for initiating insulitis, while also demonstrating its importance in the inflammatory process. The mechanism underlying GAD vaccine induction of immunological tolerance is presently attributed to GAD mediated expansion of T regulatory cell and anti-inflammatory Th2 cell populations (Tian et al., 1996). In 1999, phase I clinical trials demonstrated the safety of GAD65 as a treatment option for the prevention of type 1 diabetes (Ludvigsson, 2009).
DiaPep277
Heat shock proteins (Hsp) are a group of highly conserved proteins that serve as intracellular chaperones that are induced under stressful conditions. Recent research implicates Hsp60 as an autoantigen involved in type 1 diabetes pathogenesis. Prediabetic NOD mice were shown to have T cells that are autoreactive to epitopes of Hsp60 (Elias et al., 1990). Further NOD mouse studies identified Hsp60 as a β-cell target antigen (Birk et al., 1996). Cloning and sequencing of mouse Hsp60 led to the identification of a 24-amino-acid portion of the protein (p277) that is strongly immunogenic (Birk et al., 1996). When the Hsp60p277 protein fragment was inoculated into normal mice, it was sufficient to induce β-cell autoimmunity (Elias et al., 1995). Evidence that Hsp60 is a type 1 diabetes β-cell autoantigen was also found in humans (Horvath et al., 2002; Sobel and Creswell, 2006). Children with type 1 diabetes develop autoantibodies to different epitopes of Hsp60 (Horvath et al., 2002). Further, T cells autoreactive to Hsp60 in adults with type 1 diabetes were shown to be associated with β-cell cytotoxicity (Sobel and Creswell, 2006). Although Hsp60 autoanti-bodies are not yet considered a diagnostic tool for type 1 diabetes, the evidence suggests this autoantigen may be important in disease development. Therefore, vaccine strategies that are based on Hsp60 as a diabetes autoantigen have been selected for further development.
DiaPep277 (Andromeda Biotech) is a stable peptide isolated from heat shock protein 60 (Hsp60). This heat shock peptide can block the onset of type 1 diabetes in NOD mice as well as arrest the progression of insulitis in mice that have already become hyperglycemic (Elias and Cohen, 1994). These promising experimental results led to clinical trials with DiaPep277 formulated for subcutaneous injection. The first phase I trial demonstrated that the vaccine was safe for humans. Since that time, seven phase II clinical trials have been completed in different countries (Eldor et al., 2009). DiaPep277 treatment of adults newly diagnosed with type 1 diabetes resulted in the preservation of residual C peptide levels. However, no reduction could be made in the amount of exogenous insulin delivery required for patients to maintain normal blood glucose levels (Huurman et al., 2007; Raz et al., 2001). Unfortunately, phase II trials for immune suppression in children were less successful. The vaccine appears to have delivered no therapeutic effect as young patient C-peptide levels continued to decline during the course of the trial (Schloot et al., 2007). While DiaPep277 was unable to deliver significant therapeutic improvements in newly diagnosed adult type 1 diabetic patients, the continued maintenance of C-peptide levels in these patients have enabled the vaccine to enter phase III trials. The goal of the current phase III trial is to assess maintenance of insulin secretion in adults based on measurement of C-peptide levels (Eldor et al., 2009).
Adjuvant Stimulated Diabetes Vaccines
The partial success of individual peptide vaccines for immunological suppression of diabetes onset has provided renewed hope for curing the disease (Table 1). The NOD mouse model of type 1 diabetes has established “proof-of-concept” for adjuvant modulation of the immune system for improved β-cell tolerance of autoantigens. The question remains why single peptide vaccines have not generally been able to completely suppress human diabetes symptoms. Part of this problem may be that a single autoantigen may not be solely responsible for the onset of type 1 diabetes as evidenced in patients expressing antibodies to a progressively increasing number of β-cell autoantigens, e.g., GAD65, insulin, Hsp60, and IA-2. Confirmation of this concept was later obtained when an additive effect on suppression of diabetes onset was obtained when NOD mice were co-inoculated with two autoantigens insulin and GAD (Arakawa et al., 1999). One way to overcome the problem of multiple diabetes autoantigens may be to couple the autoantigen with an adjuvant to boost the level of autoantigen mediated immune suppression. For purposes of this review, an adjuvant can be defined as any molecule that increases an immune response to the antigen it is delivered without stimulating a significant immune response to itself. It is understood that delivery of an adjuvant with a diabetes autoantigen can increase the immunosuppressive effects of the vaccine while reducing the amount of autoantigen required for generating the desired immune response.
Diamyd
A vaccine composed of GAD65 protein formulated in combination with the well accepted adjuvant alum (a suspension of aluminum and magnesium hydroxide) known as “Diamyd” (Diamyd Medical, Stockholm, Sweden) was constructed for phase I and II human clinical studies following positive results in preclinical trials. Because, aluminum salts preferentially induce a type 2 immune response rather than cell mediated immunity, it was used to minimize the potential of exacerbating T cell mediated β-cell destruction (Ludvigsson, 2009). Although poorly understood, the mechanism by which aluminum adjuvants selectively enhance the immune response is attributed to activation of complement, induction of cytokine release, and direct or indirect DC stimulation (HogenEsch, 2002).
The initial phase II clinical trial determined that two 20 μg subcutaneous injections of Diamyd one month apart was the most effective dose in adults with recent-onset type 1 diabetes. This same trial showed that Diamyd could preserve residual C-peptide levels and did not compromise β-cell function (Agardh et al., 2005). In addition, antibody levels against GAD were not increased except at the 500 μg dose level (Bekris et al., 2007). A 5-year follow-up study showed persistent effects of the vaccine as the overall drop in C-peptide levels in the treatment group was less in comparison with that detected in the placebo group (Agardh et al., 2009).
Successful Diamyd therapy in adults has led to phase II trials in children and adolescents. Treatment with Diamyd in these two experimental groups with recent onset type 1 diabetes showed continued maintenance of C-peptide levels even after 4 years (Ludvigsson et al., 2008; 2010). The mechanism of Diamyd action is attributed to induction of a Th2 immune response. In vaccinated children, an increase in GAD IgG4 antibodies, which is characteristic of a Th2 immune response, was observed (Cheramy et al., 2010; Lundgren et al., 1989). In addition, Diamyd induced the expansion of T regulatory cell populations as measured by an increase in Foxp3+ T cells and secretion of anti-inflammatory cytokines IL-5, IL-10, and IL-13 (Axelsson et al., 2010; Hjorth et al., 2011). Currently, phase III trials for Diamyd are being conducted in Europe and in the United States (Ludvigsson, 2009). Although Diamyd has been successful in preserving residual insulin secretion, it is not sufficiently effective to restore euglycemia in type 1 diabetes patients. Therefore, new vaccine strategies must be explored.
CTB-insulin and CTB-GAD + IL-10 vaccines
The promise identified by oral administration of autoantigens such as insulin for prevention of autoimmune diabetes (Trentham et al., 1993; Zhang et al., 1991) has not been without limitations. This form of immunotherapy is complicated by the fact that relatively low immune suppression levels of diabetes onset can be established by oral administration of insulin or GAD to NOD mice (Arakawa et al., 1998). However, previous experiments have shown that this problem can be largely overcome through the linkage of the islet autoantigen to the non-toxic B subunit of the cholera enterotoxin (CTB) from Vibrio cholerae (Arakawa et al., 1998; Arakawa et al., 1999; Bergerot et al., 1997). Suppression of hyperglycemia and pancreatic inflammation can be significantly enhanced if islet autoanti gens are linked to CTB (Arakawa et al., 1999). Several researchers have shown CTB to be an efficient and safe adjuvant for stimulating autoantigen induction of peripheral tolerance (Shreedhar et al., 2003; Sun et al., 1994). Additional microbial and plant AB enterotoxins were shown to stimulate autoantigen mediated immune tolerance leading to inhibition of type 1 diabetes onset, suggesting a common mechanism underlying pathogen suppression of innate immunity (Carter et al., 2006).
Recently, it was shown that the recombinant vaccinia virus biosynthesis of CTB protein fused to GAD (CTBGAD) in NOD mice provided effective and durable immunotherapy against type 1 diabetes when delivered in combination with genes encoding the immunosuppressive cytokine IL-10 (Denes et al., 2010). This result supports the idea that combinatorial vaccination strategies based on co-delivery of immunosuppressive cytokines such as IL-10 and adjuvant linked autoantigens hold promise for suppression of diabetes onset and for restoration of durable immunologic homeostasis in patients with type 1 diabetes.
Conclusions
While inhibition of autoantigen specific immunity is an attractive vaccination strategy for type 1 diabetes therapy, vaccine development for this organ specific autoimmune disease has remained an ongoing challenge. Impediments to the development of effective vaccine therapeutic strategies are, in large part, due to our lack of knowledge of the role that β-cell autoantigens play in islet inflammation and restoration of immunological tolerance. Improvements in vaccine construction will come with increased characterization of mechanisms underlying DC recognition of autoantigens and adjuvants, as either foreign or self, and how vaccine inoculated DCs guide T cell development into immunosuppressive or autoreactive T cell subsets.
Single diabetes autoantigen based vaccines, while partially effective in the suppression of diabetes onset in animals, were found to be ineffective when tested in human clinical trials. However, major improvements in autoantigen based vaccination strategies came with linkage of the autoantigen to adjuvants. Experiments have shown that multi-component vaccines that combine β-cell autoantigens with enterotoxin B subunit adjuvants enhance DC mechanisms responsible for suppression of inflammation. This vaccine strategy has shown increasing promise for effective, safe, and persistent suppression of β-cell specific autoimmunity. Although multi-component vaccines have been shown to be highly effective in animals, clinical trials for suppression of β-cell autoimmunity in human diabetes patients remain to be accomplished. Thus, achievement of full suppression of diabetes autoimmunity onset and progression may well rest on the application of multi-component vaccine strategies that deliver adjuvantautoantigen complexes and immunosuppressive cytokines. Achievement of this goal will serve as a therapeutic autoantigen vaccine model for the ever growing list of organ specific autoimmune diseases as well as to establish a firm basis for restorative therapies including pancreatic islet and β stem cell transplantation as means for restoration of diabetic patients to a permanent normoglycemic state.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, Harris RA, Robertson JA, Lernmark A. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications 19(4):238–246, 2005. [DOI] [PubMed] [Google Scholar]
- Agardh CD, Lynch KF, Palmer M, Link K, Lernmark A. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 52(7):1363–1368, 2009. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol 16(10):934–938, 1998. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Yu J, Langridge WH. Food plant-delivered cholera toxin B subunit for vaccination and immunotolerization. Adv Exp Med Biol 464:161–178, 1999. [DOI] [PubMed] [Google Scholar]
- Axelsson S, Hjorth M, Akerman L, Ludvigsson J, Casas R. Early induction of GAD(65)-reactive Th2 response in type 1 diabetic children treated with alum-formulated GAD(65). Diabetes Metab Res Rev 26(7):559–568, 2010. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Jensen RA, Lagerquist E, Hall TR, Agardh CD, Cilio CM, Lethagen AL, Lernmark A, Robertson JA, Hampe CS. GAD65 autoantibody epitopes in adult patients with latent autoimmune diabetes following GAD65 vaccination. Diabet Med 24(5):521–526, 2007. [DOI] [PubMed] [Google Scholar]
- Bergerot I, Fabien N, Maguer V, Thivolet C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J Autoimmun 7(5):655–663, 1994. [DOI] [PubMed] [Google Scholar]
- Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci U S A 94(9):4610–4614, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Elias D, Weiss AS, Rosen A, Van-Der Zee R, Walker MD, Cohen IR. NOD mouse diabetes: the ubiquitous mouse hsp60 is a beta-cell target antigen of autoimmune T cells. J Autoimmun 9(2):159–166, 1996. [DOI] [PubMed] [Google Scholar]
- Bjork E, Kampe O, Andersson A, Karlsson FA. Expression of the 64 kDa/glutamic acid decarboxylase rat islet cell autoantigen is influenced by the rate of insulin secretion. Diabetologia 35(5):490–493, 1992. [DOI] [PubMed] [Google Scholar]
- Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes 39(10):1264–1272, 1990. [DOI] [PubMed] [Google Scholar]
- Bouma G, Coppens JM, Mourits S, Nikolic T, Sozzani S, Drexhage HA, Versnel MA. Evidence for an enhanced adhesion of DC to fibronectin and a role of CCL19 and CCL21 in the accumulation of DC around the pre-diabetic islets in NOD mice. Eur J Immunol 35(8):2386–2396, 2005. [DOI] [PubMed] [Google Scholar]
- Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci U S A 105(16):6121–6126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JE 3rd, Yu J, Choi NW, Hough J, Henderson D, He D, Langridge WH. Bacterial and plant enterotoxin B subunit-autoantigen fusion proteins suppress diabetes insulitis. Mol Biotechnol 32(1):1–15, 2006. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 91(1):123–127, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL. Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol 167(9):4926–4935, 2001. [DOI] [PubMed] [Google Scholar]
- Cheramy M, Skoglund C, Johansson I, Ludvigsson J, Hampe CS, Casas R. GAD-alum treatment in patients with type 1 diabetes and the subsequent effect on GADA IgG subclass distribution, GAD65 enzyme activity and humoral response. Clin Immunol 137(1):31–40, 2010. [DOI] [PubMed] [Google Scholar]
- Denes B, Fodor I, Langridge WH. Autoantigens plus interleukin-10 suppress diabetes autoimmunity. Diabetes Technol Ther 12(8):649–661, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldor R, Kassem S, Raz I. Immune modulation in type 1 diabetes mellitus using DiaPep277: a short review and update of recent clinical trial results. Diabetes Metab Res Rev 25(4):316–320, 2009. [DOI] [PubMed] [Google Scholar]
- Elias D, Cohen IR. Peptide therapy for diabetes in NOD mice. Lancet 343(8899):704–706, 1994. [DOI] [PubMed] [Google Scholar]
- Elias D, Marcus H, Reshef T, Ablamunits V, Cohen IR. Induction of diabetes in standard mice by immunization with the p277 peptide of a 60-kDa heat shock protein. Eur J Immunol 25(10):2851–2857, 1995. [DOI] [PubMed] [Google Scholar]
- Elias D, Markovits D, Reshef T, Van Der Zee R, Cohen IR. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A 87(4):1576–1580, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falb D, Briner TJ, Sunshine GH, Bourque CR, Luqman M, Gefter ML, Kamradt T. Peripheral tolerance in T cell receptor-transgenic mice: evidence for T cell anergy. Eur J Immunol 26(1):130–135, 1996. [DOI] [PubMed] [Google Scholar]
- Fuchtenbusch M, Rabl W, Grassl B, Bachmann W, Standl E, Ziegler AG. Delay of type I diabetes in high risk, first degree relatives by parenteral antigen administration: the Schwabing Insulin Prophylaxis Pilot Trial. Diabetologia 41(5):536–541, 1998. [DOI] [PubMed] [Google Scholar]
- Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med 196(3):369–377, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose S Australia set to test insulin ‘vaccine’ for juvenile diabetes. Nat Med 13(2):111, 2007. [DOI] [PubMed] [Google Scholar]
- Hanninen A, Harrison LC. Gamma delta T cells as mediators of mucosal tolerance: the autoimmune diabetes model. Immunol Rev 173:109–119, 2000. [DOI] [PubMed] [Google Scholar]
- Harrison LC. Risk assessment, prediction and prevention of type 1 diabetes. Pediatr Diabetes 2(2):71–82, 2001. [DOI] [PubMed] [Google Scholar]
- Harrison LC. Vaccination against self to prevent autoimmune disease: the type 1 diabetes model. Immunol Cell Biol 86(2):139–145, 2008. [DOI] [PubMed] [Google Scholar]
- Hjorth M, Axelsson S, Ryden A, Faresjo M, Ludvigsson J, Casas R. GAD-alum treatment induces GAD65-specific CD4+CD25highFOXP3+ cells in type 1 diabetic patients. Clin Immunol 138(1):117–126, 2011. [DOI] [PubMed] [Google Scholar]
- HogenEsch H Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 20:S34–S39, 2002. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 189(2):331–339, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath L, Cervenak L, Oroszlan M, Prohaszka Z, Uray K, Hudecz F, Baranyi E, Madacsy L, Singh M, Romics L, Fust G, Panczel P. Antibodies against different epitopes of heat-shock protein 60 in children with type 1 diabetes mellitus. Immunol Lett 80(3):155–162, 2002. [DOI] [PubMed] [Google Scholar]
- Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev 23(4):269–275, 2007. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y, Shimada A, Oikawa Y, Okubo Y, Tada A, Imai T, Miyazaki J, Itoh H. Induction of anti-whole GAD65 reactivity in vivo results in disease suppression in type 1 diabetes. J Autoimmun 32(2):104–109, 2009. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366(6450):69–72, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisetti MG, Lewis DM, Suri A, Unanue ER. Weak proinsulin peptide-major histocompatibility complexes are targeted in autoimmune diabetes in mice. Diabetes 57(7):1852–1860, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Abiru N, Moriyama H, Miao D, Eisenbarth GS. Induction of insulin autoantibodies and protection from diabetes with subcutaneous insulin B:9–23 peptide without adjuvant. Ann N Y Acad Sci 958:224–227, 2002. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J Therapy with GAD in diabetes. Diabetes Metab Res Rev 25(4):307–315, 2009. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 359(18):1909–1920, 2008. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J, Hjorth M, Cheramy M, Axelsson S, Pihl M, Forsander G, Nilsson NO, Samuelsson BO, Wood T, Aman J, Ortqvist E, Casas R. Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trial. Diabetologia 54(3):634–640, 2011. [DOI] [PubMed] [Google Scholar]
- Lundgren M, Persson U, Larsson P, Magnusson C, Smith CI, Hammarstrom L, Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol 19(7):1311–1315, 1989. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, Von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol 108:111–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol 11(4):350–354, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumosu O, Payne K, Baez I, Jutzy J, Wall N, Langridge W. Suppression of dendritic cell activation by diabetes autoantigens linked to the cholera toxin B subunit. Immunobiology 216(4):447–456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Taniguchi H, Schimada C. High concentration of GABA and high glutamate decarboxylase activity in rat pancreatic islets and human insulinoma. Science 194:620–622, 1976. [DOI] [PubMed] [Google Scholar]
- Peakman M, Von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes 59(9):2087–2093, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Delgado J, Braun M, Hernandez-Fisac I, Martin-Del-Rio R, Tamarit-Rodriguez J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in beta-cells. Biochem J 431(3):381–389, 2010. [DOI] [PubMed] [Google Scholar]
- Pozzilli P The DPT-1 trial: a negative result with lessons for future type 1 diabetes prevention. Diabetes Metab Res Rev 18(4):257–259, 2002. [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, Manca Bitti ML, Matteoli MC, Marietti G, Ferrazzoli F, Cassone Faldetta MR, Giordano C, Sbriglia M, Sarugeri E, Ghirlanda G. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia 43(8):1000–1004, 2000. [DOI] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 358(9295):1749–1753, 2001. [DOI] [PubMed] [Google Scholar]
- Rosmalen JG, Homo-Delarche F, Durant S, Kap M, Leenen PJ, Drexhage HA. Islet abnormalities associated with an early influx of dendritic cells and macrophages in NOD and NODscid mice. Lab Invest 80(5):769–777, 2000. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Lechner O, Von Boehmer H. Autoimmune insulitis and diabetes in the absence of antigen-specific contact between T cells and islet beta-cells. Eur J Immunol 29(10):3410–3416, 1999. [DOI] [PubMed] [Google Scholar]
- Schloot NC, Meierhoff G, Lengyel C, Vandorfi G, Takacs J, Panczel P, Barkai L, Madacsy L, Oroszlan T, Kovacs P, Suto G, Battelino T, Hosszufalusi N, Jermendy G. Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev 23(4):276–285, 2007. [DOI] [PubMed] [Google Scholar]
- Shreedhar VK, Kelsall BL, Neutra MR. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T-and B-cell areas of Peyer’s patches. Infect Immun 71(1):504–509, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care 28(5):1068–1076, 2005. [DOI] [PubMed] [Google Scholar]
- Sobel DO, Creswell K. Characterization of anti-islet cytotoxic human T-cell clones from patients with type 1 diabetes mellitus. Autoimmunity 39(4):323–332, 2006. [DOI] [PubMed] [Google Scholar]
- Steinman L Inverse vaccination, the opposite of Jenner’s concept, for therapy of autoimmunity. J Intern Med 267(5):441–451, 2010. [DOI] [PubMed] [Google Scholar]
- Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci U S A 91(23):10795–10799, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med 183(4):1561–1567, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R, Yang XD, Liblau RS, Mcdevitt HO. Administering glutamic acid decarboxylase to NOD mice prevents diabetes. J Autoimmun 7(6):845–850, 1994. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 261(5129):1727–1730, 1993. [DOI] [PubMed] [Google Scholar]
- Wang B, Tisch R. Parameters influencing antigen-specific immunotherapy for Type 1 diabetes. Immunol Res 42(1–3):246–258, 2008. [DOI] [PubMed] [Google Scholar]
- Yu L, Cuthbertson DD, Maclaren N, Jackson R, Palmer JP, Orban T, Eisenbarth GS, Krischer JP. Expression of GAD65 and islet cell antibody (ICA512) autoantibodies among cytoplasmic ICA+ relatives is associated with eligibility for the Diabetes Prevention Trial-Type 1. Diabetes 50(8):1735–1740, 2001. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A 88(22):10252–10256, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]