Abstract
Objective:
Error-processing and inhibitory control enable the adjustment of behaviors to meet task demands. Functional magnetic resonance imaging (fMRI) studies report brain activation abnormalities in patients with obsessive-compulsive disorder (OCD) during both processes. However, conclusions are limited by inconsistencies in the literature and small sample sizes. Therefore, the aim here was to perform a meta-analysis of the existing literature using unthresholded statistical maps from previous studies.
Method:
A voxel-wise Seed-based d Mapping meta-analysis was performed using t-maps from studies comparing patients with OCD and healthy controls (HC) during error-processing and inhibitory control. For the error-processing analysis, 239 patients with OCD (120 males; 79 medicated) and 229 HC (129 males) were included, while the inhibitory control analysis included 245 patients with OCD (120 males; 91 medicated) and 239 HC (135 males).
Results:
Patients with OCD, relative to HC, showed longer inhibitory control RT (SMD=0.2, p=0.03, 95% CI=(0.016, 0.393)) and more inhibitory control errors (SMD=0.22, p=0.02, 95% CI=(0.039, 0.399)). In the brain, patients showed hyperactivation in bilateral dorsal anterior cingulate cortex (dACC), supplementary motor area (SMA), pre-SMA, as well as right anterior insula/frontal operculum (aI/fO) and anterior lateral prefrontal cortex (aLPFC) during error-processing, but hypoactivation during inhibitory control in rostral and ventral anterior cingulate cortex (rACC/vACC) and bilateral thalamus/caudate, as well as in right aI/fO, supramarginal gyrus and medial orbitofrontal cortex (all SDM-Z value >2, p<0.001).
Conclusions:
An intact or hyperactive error-processing mechanism in conjunction with impairments in implementing inhibitory control may underlie deficits in stopping unwanted compulsive behaviors in the disorder.
Keywords: OCD, performance monitoring, inhibitory control, error-processing, fMRI, meta-analysis
Introduction
Obsessive-compulsive disorder (OCD) has a lifetime prevalence of 2–3% (1). The disorder is characterized by recurrent and intrusive obsessive thoughts, as well as by time consuming, ego-dystonic behavioral and mental compulsions (2).
Patients with OCD often show altered brain activation during erroneous and correct responses on inhibitory control tasks (3, 4). Relevant tasks include go/no-go and stop tasks, which measure the ability to inhibit responses to no-go stimuli among prepotent go stimuli, or to withdraw already triggered motor responses following stop-signals, respectively, as well as during tasks of interference inhibition such as anti-saccade, flanker, Simon, Stroop and multisource interference (MSIT) tasks which require participants to ignore interfering stimulus features and override prepotent responses in order to process relevant information and perform goal-directed actions (3, 5, 6). Impairments in the functioning of error-processing and inhibitory control brain networks may, in part, underlie poor control over obsessions and compulsions in OCD, with many patients showing good insight into their symptoms, but nonetheless continuing to carry out compulsive behaviors (3, 6–8).
Successful task performance involves the capacity to monitor for errors and to adjust behavioral responding accordingly (9). Error-processing is widely held to depend on the posterior medial frontal cortex (pMFC), incorporating dorsal anterior cingulate cortex (dACC), supplementary motor area (SMA) and posterior portions of pre-supplementary motor area (pre-SMA) (10). The pMFC, together with the anterior insula/frontal operculum (aI/fO), and rostral anterior cingulate (rACC), forms the cingulo-opercular network (4, 10, 11). During error-processing and inhibitory control, this cingulo-opercular network detects the demand for behavioral or attentional control and initiates recruitment of lateral fronto-parietal and fronto-striatal networks responsible for enacting top-down executive control (9, 10, 12–14).
Heightened error-processing, as indicated by an increased amplitude of a midline frontal electrophysiological potential, the error-related negativity (ERN), is arguably the most reliable neurocognitive biomarker of OCD (15–17). Consistent with this, several fMRI studies of OCD report cingulo-opercular hyperactivation during error-processing (4, 18–24). In contrast, during correct inhibitory control, patients with OCD often show decreased pMFC/rACC activation (21, 25–37) and altered striatal functioning (18, 19, 25, 28–31, 33–36, 38), as confirmed in recent meta-analyses (3, 5), although some studies report increased pMFC activation in patients relative to healthy controls (HC) (19, 20, 26, 35).
Given the reliability of heightened ERN findings in OCD, numerous theoretical accounts emphasize a role for cingulo-opercular hyperactivation as a key mechanism underlying OCD symptoms (11, 16, 39). However, most studies of error-processing in OCD have employed small samples, or focused on cingulo-opercular regions of interests, thereby limiting knowledge of potential group differences in other brain networks (4, 19–21). Moreover, some previous work has reported decreased activation or no differences in these regions in patients with OCD relative to HC during error-processing (28, 40, 41). Existing meta-analyses of inhibitory control in OCD did not consider error-processing and used coordinates from significant clusters, rather than unthresholded group maps, meaning that true group differences may have been lost (3, 5).
Therefore, the primary aim was to provide the first fMRI meta-analysis of error-processing in patients with OCD relative to HC based, where possible, on whole-brain unthresholded statistical maps (42). A second aim was to examine group differences in the same set of studies during inhibitory control. We anticipated heightened cingulo-opercular activation during error-processing, but decreased cingulo-opercular and altered striatal activation during inhibitory control, in patients with OCD relative to HC.
Methods and Materials
Search and Inclusion of Studies
The meta-analysis was conducted in line with meta-analysis of observational studies in epidemiology (MOOSE) guidelines (43). The study protocol was registered with PROSPERO (CRD42017062495) and is accessible from http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017062495.
A comprehensive literature search was performed using the PubMed, ScienceDirect, Web of Knowledge, and Scopus research databases through August 1, 2017. Reference lists of retrieved studies and recent meta-analyses (3, 5) were also hand-searched. Search syntax is provided in the Supplement. Included studies provided whole-brain pairwise voxel-based comparisons of OCD patient groups against HC using fMRI during errors on inhibitory control tasks (e.g., stop, go/no-go, Stroop, Simon, flanker, anti-saccade, MSIT tasks). Studies were excluded if they provided no case-control comparisons, were unable to provide findings from whole-brain analyses, had very high accuracy rates that precluded fMRI analysis of error-processing (See Supplement), or if they used subject data which overlapped with another, already included study. If the same patient group was used in multiple studies/tasks, then the study/task with the largest sample was included. For studies that used longitudinal/treatment designs, only baseline data were included. The meta-analysis examined both pediatric and adult patients with OCD diagnoses, regardless of medication status, gender, symptom subtype, or comorbidities. Details of current comorbid diagnoses were extracted for each included dataset, and are provided in Supplementary Table 1.
Authors of relevant papers were contacted and asked to provide whole-brain unthresholded t-maps for the pairwise group comparison OCD vs HC for the error contrast included in the original paper, as well as t-maps for the within-group error contrast separately for HC and OCD groups. Authors who did not report error contrasts in the original publication were contacted to ask for unpublished whole-brain data in the form of unthresholded t-maps, or else in the form of coordinates from a whole-brain analysis. For studies providing error contrast maps/coordinates, data were also requested for the inhibitory control contrast.
Meta-analyses
A random-effects meta-analysis of the standardized mean differences (SMD; Hedges’ g) between OCD and HC in task performance (reaction time (RT) measures of inhibitory control; inhibitory control errors; congruent/go errors) was performed in the Esc (44) and metafor packages (45) for R (http://www.r-project.org/). Details on the included measures and studies are provided in the Supplement.
Voxel-wise meta-analyses of regional brain differences were conducted using the anisotropic effect-size version of the Seed-based d Mapping (AES-SDM) software package (http://www.sdmproject.com). This method has been described in detail elsewhere (42, 46, 47), as well as in the Supplement. In brief, AES-SDM allows for a combination of peak coordinates and t-maps to create whole-brain effect size and variance maps, which are then used in voxel-wise random-effects meta-analyses (42, 46, 47). The SDM method has been empirically validated by comparing its results with a mega-analysis (47). While the control over the false positive rate is not formal but based on an empirical validation, this validation showed AES-SDM to have a good overlap with the mega-analysis, with an adequate sensitivity and an excellent control of false positives.
Assessment of statistical significance was performed using standard permutation testing, against the null hypothesis that BOLD response/group differences are the same throughout the brain (47). We used the default voxel p-value threshold of p<0.005 (uncorrected), which was shown to be equivalent to p<.05 FWE (47). In addition, a cluster extent threshold of 80 voxels and a peak SDM-Z value threshold of >2 were used to reduce the false positive rate. We first examined the brain regions showing activation or deactivation in the errors and inhibitory control contrasts separately within each group using the within-group maps (Supplementary Tables 2-5). We then performed a separate analysis using the between-group maps to examine regions showing reliable differences between groups. Voxelwise meta-regressions were used to examine the effects of age, gender, symptom severity, comorbid diagnosed anxiety and mood disorders, medication status, and error rates on brain activation differences between groups as well as on activation within the OCD and HC groups (46). Mood disorders were combined into a single category for this analysis, due to the limited details available from the original studies on the specific disorder sub-types. The relationship between group differences in task performance (as SMD) and group differences in brain activation was also examined.
Jackknife sensitivity analyses were performed to assess robustness of between-group findings (Supplementary Tables 6-9) (47). To illustrate the influence of each dataset on significant between-group clusters, cluster effect sizes for each dataset were extracted using the ‘extract’ function in AES-SDM and plotted in forest plots (see Supplement). Sensitivity analyses examined whether between-group differences remained when including only the adult datasets (See Supplement) (3). There were too few datasets for a pediatric sensitivity analysis to be performed.
The Egger test was used to examine potential publication bias in between-group findings (48), corrected for multiple comparisons using the Benjamini-Hochberg method (49). Heterogeneity was assessed using the Q statistic (47, 50).
Results
Included studies and characteristics
Nine datasets were available to be included as whole-brain t-maps in the current meta-analysis (4, 18, 23, 26, 35, 37, 41, 51, 52). Peak coordinate data from a whole-brain analysis were available for a tenth dataset for the between-group error contrast (40). Yücel and colleagues provided a new unpublished dataset, which partially overlapped with data included in their published study (35), and for the error contrast included only participants that made at least five errors. Details of each dataset are given in Table 1. See Supplement for details on excluded studies. Details on comorbidities are given in Supplementary Table 1.
Table 1.
Demographic and clinical characteristics of 10 OCD fMRI datasets.
| Patients | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Task | Adults/kids | Contrasts | N (% male) | Mean age, y (SD) | Mean (C)Y-BOCS (SD) | N Med1. (%) | Mean Performance (SD) | N (% male) | Mean age, y (SD) | Mean Performance (SD) |
| Agam2 | Anti-saccade | Adults | Errors > correct, Correct > fixation | 19 (42) | 33 (10) | 23 (5) | 6 (32) | Anti-saccade errors: 14% Anti-saccade RT: 267 ms |
15 (60) | 35 (12) | Anti-saccade errors: 12% Anti-saccade RT: 283 ms |
| de Wit | Stop | Adults | Failed stop > Successful stop, Successful stop > go | 41 (51) | 38.6 (9.8) | 21.9 (6.1) | 0 (0) | Stop errors: 45% (4) Go errors: 1% (1) SSRT: 204.9 ms (45) |
37 (57) | 39.7 (11.6) | Stop errors: 44% (4) Go errors: 1% (1) SSRT: 184.2 ms (43.2) |
| Fitzgerald | |||||||||||
| Errors | MSIT | Pediatric | Incongruent errors > incongruent correct | 51 (47) | 14.2 (2.8) | 17.8 (7.4) | 23 (45) | Incongruent errors: 13% (7) Congruent errors: 1% (2) Conflict RT: 324.33 ms (120.12) |
51 (55) | 14.1 (3.2) | Incongruent errors:12% (7) Congruent errors: 2% (2) Conflict RT: 322.78 ms (111.17) |
| Inhibitory | MSIT | Pediatric | Incongruent correct > congruent correct | 69 (45) | 13.9 (2.8) | 18.6 (7.7) | 34 (49) | Incongruent errors: 11% (7) Congruent errors: 1% (2) Conflict RT: 325.50 ms (118.08) |
72 (54) | 14 (3.5) | Incongruent errors: 9% (7) Congruent errors: 1% (2) Conflict RT: 306.63 ms (93.16) |
| Fitzgerald | Flanker | Adults | Errors > correct, Incongruent correct > congruent correct | 8 (75) | 27.4 (8.5) | 18 (3.9) | 5 (63) | Incongruent errors: 10% (11%) Congruent errors: 7% (9) Conflict RT: 27.78 ms (14.95) |
7 (86) | 30 (8.6) | Incongruent errors: 3% (3) Congruent errors: 2% (2) Conflict RT: 25.85 ms (12.72) |
| Grutzmann3 | Flanker | Adults | Incongruent errors > all correct | 20 (55) | 32.3 (8.6) | 19.8 (5.6) | 4 (20) | All errors: 15% (10) | 22 (50) | 30.8 (8.1) | All errors: 20% (10) |
| Hough | Go/no-go | Adults | No-Go > Go, No-Go errors > fixation | 17 (47) | 36.1 (10.4) | 27 (6.7) | 11 (65) | No-Go errors: 27% (18) | 22 (41) | 46.77 (16.77) | No-Go errors: 21% (15) |
| Huyser | Flanker | Pediatric | Errors > correct, Incongruent correct > congruent correct | 25 (36) | 13.95 (2.52) | 24.92 (5.08) | 0 (0) | Incongruent errors: 21% (12.31) Congruent errors: 8% (6) Conflict RT: 57.85 ms (22.47) |
25 (36) | 13.71 (2.85) | Incongruent errors: 25% (17) Congruent errors: 8% (10) Conflict RT: 54.51 ms (14.19) |
| Rubia | Stop | Pediatric | Failed stop > go, Successful stop > go | 10 (100) | 14.3 (1.7) | 11 | 8 (80) | Stop errors:52% (11) SSRT: 283 ms (193) |
20 (100) | 14.5 (1.1) | Stop errors:51% (7) SSRT: 256 ms (165) |
| Stern | Flanker | Adults | Errors > correct, Incongruent correct > congruent correct | 39 (43) | 27.73 (8.68) | 25.27 (3.84) | 19 (49) | Incongruent errors: 26% (13) Congruent errors: 11.39% (11) Conflict RT: 38.66 ms (19.29) |
20 (50) | 25.5 (7.7) | Incongruent errors: 26% (11) Congruent errors: 13% (8) Conflict RT: 31.25 ms (16.51) |
| Yücel | |||||||||||
| Errors | MSIT | Adults | Errors > correct | 9 (67) | 35.22 (12.08) | 17.44 (5.50) | 3 (33) | Incongruent errors: 12% (7) Congruent errors:3% (3) Conflict RT: 407.91 ms (100.90) |
10 (60) | 34.2 (8.83) | Incongruent errors: 10% (6) Congruent errors: 1% (1) Conflict RT: 384.83 ms (90.67) |
| Inhibitory | MSIT | Adults | Incongruent > congruent | 17 (59) | 33.65 (12.08) | 17.35 (5.12) | 8 (47) | Incongruent errors: 8% (8) Congruent errors: 2% (6) Conflict RT: 359.27 ms (102.03) |
21 (57) | 32.95 (8.73) | Incongruent errors: 6% (6) Congruent errors: 1% (8) Conflict RT: 342.12 ms (89.88) |
Abbreviations: (C)Y-BOCS, (Children’s) Yale-Brown Obsessive Compulsive Scale; ms, milliseconds; MSIT, Multisource Interference Task; RT, reaction time; SD, standard deviation; SSRT, stop signal reaction time; y, years.
Number receiving antidepressant medication at time of scan.
Performance and Y-BOCS data were available only for a larger sample of n=21 OCD and n=20 HC, some of whom were excluded from the final analysis.
Performance data were available for only a subset (n=16 OCD and n=16 HC) of subjects who completed both the fMRI and electroencephalographic tasks.
Data from 239 patients with OCD (120 males; 79 medicated) and 229 HC (129 males) were included for the error contrast. Patient and control datasets did not differ on sample-size weighted mean age (t(1,18)=0.06, p=0.95) or percentage of males and females (t(1,18)=0.68, p=0.51) (3). Seven datasets included adult patients and controls (n=286), while 3 focused on adolescent/child samples (n=182).
For the inhibitory control contrast, data from 245 patients with OCD (120 males; 91 medicated) and 239 HC (135 males) were included. This included 6 adult datasets (n=263) and 3 adolescent/child datasets (n=221). Groups did not differ on age (t(1,16)=0.06, p=0.95) or gender (t(1, 16)=1.04, p=0.31).
All studies reported event-related designs, except for the study by Yucel and colleagues that used a block design. However, for inclusion in the error contrast in the current meta-analysis, this dataset was re-analyzed as an event-related design with separate regressors for correct incongruent, erroneous incongruent, correct congruent and erroneous congruent trials.
Task Performance
Patients showed impaired inhibitory control relative to HC, as determined by RT measures (SMD=0.2, p=0.03, 95% CI=(0.016, 0.393)) (Supplementary Figure 2). Tests for heterogeneity (Q(7)=4.64, p= 0.7, I2=0%) and publication bias (z=0.52, p=0.6) were non-significant.
Patients also made significantly more inhibitory control errors (SMD=0.22, p=0.02, 95% CI=(0.039, 0.399)), but groups did not differ on the number of congruent/go errors (SMD=0.02, p=0.9, 95% CI=(−0.21, 0.24)) (Supplementary Figures 3 & 4). Tests for heterogeneity (incongruent: Q(8)=6, p= 0.65, 12=0%; congruent: Q(5)=2.36, p=0.8, I2=0%) and publication bias (incongruent: z=0.38, p=0.7; congruent: z=0.71, p=0.48) were also non-significant.
Within-group brain findings
A summary of within-group findings can be found in the Supplement and Figures 1 and 2.
Figure 1. Findings from a meta-analysis of differences in brain activation during error-processing in patients with OCD and HC.
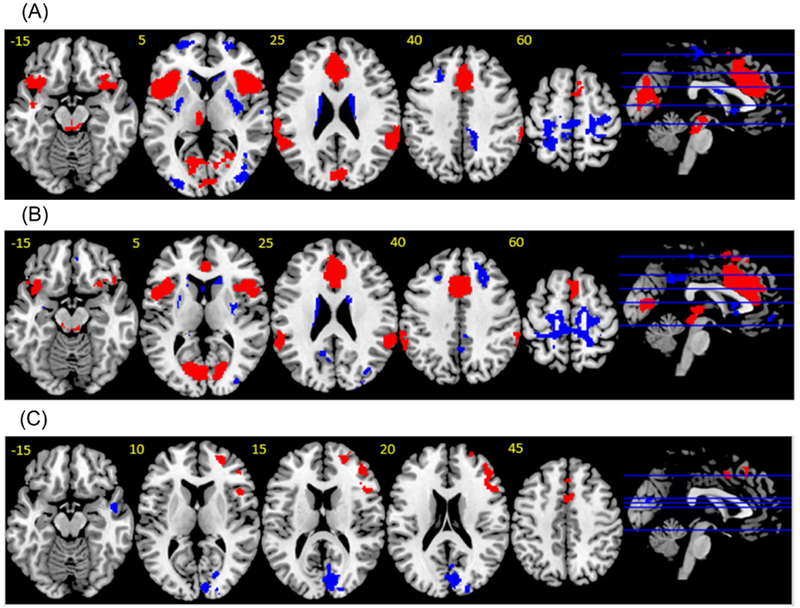
(a) Error-processing in HC. Red indicates regions showing activation. Blue indicates regions showing deactivation. (b) Error-processing in OCD. Red indicates regions showing activation. Blue indicates regions showing deactivation. (c) Group differences during error processing. Red indicates regions OCD>HC. Blue indicates regions HC>OCD. Thresholded at p<0.005, SDM z-value >2, >80 voxels.
Figure 2. Findings from a meta-analysis of differences in brain activation during inhibitory control in patients with OCD and HC.

(a) Inhibitory control in HC. Red indicates regions showing activation. Blue indicates regions showing deactivation. (b) Inhibitory control in OCD. Red indicates regions showing activation. Blue indicates regions showing deactivation. (c) Group differences during error processing. Red indicates regions OCD>HC. Blue indicates regions HC>OCD. Thresholded at p<0.005, SDM z-value >2, >80 voxels.
Between-group brain findings
OCD versus HC errors
Patients with OCD showed greater activation than HC during error-processing in bilateral dACC/SMA, pre-SMA, as well as right aI/fO and anterior lateral prefrontal cortex (aLPFC).
Patients with OCD showed decreased activation relative to HC in bilateral occipital lobe and right middle temporal lobe (MTL) (Table 2., Figure 1a., Supplementary Figures 5-10).
Table 2.
Meta-analysis results for fMRI studies of error-processing and inhibitory control in OCD and HC.
| Contrast | MNI x, y, z Coordinates | Peak SDM-Z | Peak SMD | Mean SMD | P Value | No. of Voxels | Brodmann areas |
|---|---|---|---|---|---|---|---|
| Errors OCD > HC | |||||||
| R aI/fO | 44,42,18 | 2.80 | 0.27 | 0.22 | 0.0005 | 302 | 45,44 |
| R aLPFC | 24,50,12 | 3.714 | 0.35 | 0.24 | 0.000005 | 123 | 10,46 |
| L & R pre-SMA, R premotor cortex | 20,12,48 | 2.797 | 0.26 | 0.21 | 0.0005 | 126 | 8 |
| L & R dACC/SMA | 4,10,46 | 2.527 | 0.25 | 0.21 | 0.001 | 111 | 32,24,6 |
| Errors HC > OCD | |||||||
| L & R occipital lobe | 10,−82,16 | −2.630 | −0.25 | −0.17 | 0.00005 | 545 | 18,17,19 |
| R MTL | 50,−4,−20 | −2.224 | −0.21 | −0.17 | 0.0005 | 97 | 21 |
| Inhibitory control OCD > HC | |||||||
| L premotor cortex | −26,0,60 | 2.5 | 0.23 | 0.16 | 0.00005 | 283 | 6 |
| R premotor cortex | 30,−6,52 | 2.349 | 0.22 | 0.15 | 0.00005 | 134 | 6 |
| R ITL/occipital lobe | 48,−54,−10 | 2.237 | 0.21 | 0.15 | 0.0001 | 102 | 37,19,20 |
| R SPL | 28,−52,56 | 2.096 | 0.19 | 0.14 | 0.0005 | 100 | 7 |
| Inhibitory control HC > OCD | |||||||
| L & R thalamus, L caudate | −16,6,22 | −3.68 | −0.34 | −0.27 | 0.00005 | 437 | |
| L & R dACC/rACC/vACC | 14,42,12 | −3.789 | −0.35 | −0.27 | 0.00005 | 410 | 32,24,11,25 |
| R occipital lobe | 26,−54,2 | −4.205 | −0.4 | −0.31 | 0.000005 | 310 | 19,17 |
| R SMG/angular gyrus | 56,−44,30 | −3.483 | −0.32 | −0.25 | 0.0001 | 347 | 40,39 |
| L occipital lobe/cerebellum | −28,−46,−8 | −2.813 | −0.26 | −0.24 | 0.005 | 135 | 37,18,19 |
| R mOFC | 20,36,−18 | −3.514 | −0.33 | −0.26 | 0.0001 | 101 | 11 |
| R caudate | 18,−12,22 | −3.399 | −0.32 | −0.27 | 0.0005 | 90 | |
| R aI/fO/STL | 48,18,4 | −3.131 | −0.29 | −0.25 | 0.0005 | 88 | 45,44,38 |
Abbreviations: ai, anterior insula; aLPFC, anterior lateral prefrontal cortex; dACC, dorsal anterior cingulate cortex; fO, frontal operculum; HC, healthy controls; ITL, inferior temporal lobe; IPL, inferior parietal lobe; mOFC, medial orbitofrontal cortex; MNI, Montreal Neurological Institute; MTL, middle temporal lobe; pre-SMA, pre-supplementary motor area; OCD, obsessive-compulsive disorder; rACC, rostral anterior cingulate cortex; SDM, a Seed-based d Mapping; SMD, standardized mean difference (Hedges’ g); SMA, supplementary motor area; SMG, supramarginal gyrus; SPL, superior parietal lobe; STL, superior temporal lobe; vACC, ventral anterior cingulate cortex.
OCD versus HC inhibitory control
Patients with OCD showed greater activation than HC during inhibitory control in bilateral premotor cortex and right inferior temporal lobe (ITL)/occipital lobe and superior parietal lobule (SPL). Patients with OCD showed decreased activation relative to HC in bilateral rostral/ventral anterior cingulate cortex (rACC/vACC) and thalamus/caudate and right supramarginal gyrus (SMG)/angular gyrus, aI/fO/superior temporal lobe (STL), medial orbitofrontal cortex (mOFC), and occipital lobe/cerebellum (Table 2., Figure 1b., Supplementary Figures 10-22).
Adult subgroup analysis
See Supplement.
Meta-regressions
There were no significant effects of age, gender, symptom severity, comorbid diagnosed anxiety and mood disorders, medication status and error-rates or group performance differences on brain activation during errors or inhibitory control except that comorbid specific phobia was associated with greater occipital lobe activation (left: MNI x,y,z =; −16,−66,−24, p<0.001, voxels=681; right: MNI x,y,z = 18,−64,4, p<0.001, voxels=88) within patients with OCD during errors.
Publication bias and heterogeneity tests
The results of the Egger tests were non-significant (p>.05, corrected), suggesting that there was no publication bias. No regions from the between-group analysis showed significant heterogeneity in the voxel-wise analysis.
Discussion
Error-processing and inhibitory control enable adaptive behavioral regulation, and are hypothesized to be abnormal in OCD (3, 53). In this meta-analysis, patients with OCD showed impaired task performance relative to HC during tasks of inhibitory control. In addition, patients showed hyperactivation relative to HC during error-processing in cingulo-opercular regions including dACC/SMA, pre-SMA, and right aI/fO as well as in right aLPFC. In contrast, patients primarily showed hypoactivation relative to HC both within the cingulo-opercular network (in rACC/vACC and right aI/fO), and outside this network in caudate, thalamus, SMG, mOFC and cerebellum, during inhibitory control.
Some smaller studies have reported cingulo-opercular hyperactivation in patients with OCD during error-processing (4, 18–23, 51). We confirm here in a meta-analytic sample that patients with OCD showed increased activation in key dACC, SMA, pre-SMA and aI/fO cingulo-opercular regions relative to HC during error-processing. Such findings are in line with previously reported robust differences in ERN in OCD (16, 17), as well as theoretical accounts proposing important roles for error-related hyperactivation in driving OCD symptoms (11, 16, 39).
Outside of cingulo-opercular regions we also found that a cluster in aLPFC was more activated in patients with OCD relative to HC. To investigate this unexpected cluster, we extracted the SDM-Z values for the cluster peak from the within-group error contrast maps, finding that while HC deactivated aLPFC in response to errors (SDM-Z=−2.33), patients with OCD had a positive SDM-Z value (SDM-Z=1.68), suggesting relatively greater activation during errors compared with during correct trials. While not typically emphasized in OCD, previous research has found altered activity in anterior prefrontal regions during resting-state (54), decision-making (55) and symptom provocation studies (56). Moreover, treatment with cognitive behavioral therapy (57), antidepressants (58) and repetitive transcranial magnetic stimulation (59) modulates aLPFC cortex activity in OCD, and targeting this region with neurofeedback training decreases OCD symptoms (60, 61). In patients with OCD, activation to errors might represent additional neural resources that are assigned to error-processing outside of the cingulo-opercular network due to compensatory efforts at engaging corrective behavioral adjustments.
In addition to finding cingulo-opercular hyperactivation during errors relative to HC, we found cingulo-opercular hypoactivation in patients with OCD during inhibitory control within rACC/vACC and right aI/fO. Hypoactivation was also observed in patients during inhibitory control within the thalamus, caudate, SMG, mOFC, and cerebellum, while hyperactivation was found in bilateral premotor cortex, and right ITL/occipital lobe and SPL. Hypoactivation within rACC/vACC and caudate and hyperactivation in premotor cortex replicates our previous meta analyses in OCD (3, 5). Novel findings may result from the inclusion of t-maps in the current analysis (47).
It is interesting to note that the rACC/vACC cluster overlaps with an area of deactivation in the OCD group during inhibitory control, indicating that group differences in this region are driven by greater deactivation in patients with OCD, as reported elsewhere (29, 35). Importantly, this shows that the previous findings of reduced rACC/vACC deactivation in patients with OCD during tasks of “hot” executive functions, such as emotional Stroop, emotion regulation and decision-making tasks (62–64), do not extend to “cool” executive function tasks such as those measuring inhibitory control. Nonetheless, the current findings are consistent with the notion that patients with OCD show perturbations in the pattern of rACC activations/deactivations.
During inhibitory control, patients with OCD also showed bilateral dorsal premotor cortex hyperactivation relative to HC. Findings of decreased right aI/fO, but increased premotor cortex activation, in patients with OCD during inhibitory control is in line with a previous report using a stop task (included in the meta-analysis), which reported that premotor cortex hyperactivation was shared with unaffected siblings and predicted better task performance (26). Similar findings were also reported during an n-back task in the same sample, where premotor cortex was also more activated in unaffected siblings than in patients (65). Together, this evidence suggests that increased dorsal premotor cortex activation may be compensatory in OCD, and also may be protective in unaffected siblings (26, 65).
Overall, activation abnormalities within cingulo-opercular and orbito-striato-thalamic regions are consistent with previous findings of alterations in these regions at rest (54, 66, 67), in gray matter structure (3, 5, 68–70), during symptom provocation (64, 71, 72), and across multiple cognitive and decision-making tasks in OCD (3–5, 73, 74). Moreover, many resting-state, structural and functional abnormalities within these regions are shared with unaffected relatives of patients with OCD (26, 65, 66, 69, 72, 75, 76), and are OCD-specific relative to disorders such as attention deficit/hyperactivity disorder, autism spectrum disorders and anxiety disorders (3, 42, 55, 73, 74, 77). The current findings provide further evidence for cross-modal abnormalities in cingulo-opercular and orbito-striato-thalamic brain networks in OCD (3, 5), which may be endophenotypes for the disorder (69, 78).
The current results are also interesting when considering that existing neurosurgical treatments for severe refractory OCD target cingulo-opercular and orbito-striato-thalamic networks (79–81). For instance, dorsal anterior cingulotomy involves making small stereotactic lesions to a region of pMFC similar to the one found to be hyperactive to errors in the current metaanalysis, and treatment response following this surgery is predicted by pMFC gray matter volume and pMFC-striatal structural connectivity (80). In subcortical regions, anterior capsulotomy (stereotactic lesioning of the white matter between caudate and putamen, targeting thalamo-cortical projections) normalizes heightened resting-state pMFC-striatal connectivity (81), while deep-brain stimulation of the ventral striatum or sub-thalamic nucleus normalizes heightened rACC-striatal connectivity and pMFC, rACC, mOFC and striatum hyperactivation at rest (54, 82, 83), as well as normalizing hypoactivation in right AI/fO and striatum during inhibitory control (79). The current meta-analytic findings provide further support for these network regions as potential targets for surgical treatments in the disorder. However, findings of cingulo-opercular hyperactivation during error-processing but cingulo-opercular and orbito-striato-thalamic hypoactivation during inhibitory control demonstrate that future developments of such treatments must be guided by theoretical accounts which recognize the context-specificity of neurofunctional abnormalities in OCD.
Historically, heightened error-processing in OCD has been interpreted as generating context inappropriate feelings that “something is wrong”, which trigger hypercorrective OCD behaviors (39), although this account does not explain the hypoactivation observed in aI/fO, caudate, thalamus and SMG during inhibitory control. In healthy participants, error-processing is hypothesized to be an adaptive process associated with subsequent changes in behavioral strategies and neural functioning that improve ongoing task performance (9, 10, 13, 84), and the magnitude of cingulo-opercular activation during error-processing has been found to predict the degree of post-error adjustment (14, 85). These post-error adjustments include behavioral adjustments such as correcting the original incorrect response, recalibrating speed-accuracy tradeoffs (e.g., post-error slowing), and enhancing task-focused attention and interference resolution, as well as neural adaptations including the up-regulation of task-relevant brain activation on subsequent trials (9, 14, 85, 86). However, patients with OCD typically show either no performance differences relative to controls or poorer performance and impaired post-error adjustments (41, 87, 88), perhaps suggesting that the mechanism linking cingulo-opercular activation during errors and subsequent corrective recruitment of inhibitory control brain networks may be inefficient in OCD, or else suggesting that cingulo-opercular hyperactivation to errors during error-processing is unable to correct pre-existing deficits in inhibitory control related brain activation in the disorder.
As with inhibitory control errors, OCD compulsions likely result, in part, from impaired top-down control over bottom-up stimulus driven actions (3, 6, 8, 89). We propose that impairments in implementing corrective inhibitory control following the detection of goal-incongruent behaviors is a key mechanism in OCD, which leads to patients becoming stuck in compulsive “loops”. While existing research in OCD has concentrated on inhibitory control tasks, the wider literature shows that cingulo-opercular regions respond strongly when participants detect or regulate behaviors resulting from “urges” (90), supporting a broader role outside of standard cognitive tasks. Moreover, error-processing is aversive and anxiety-provoking (91, 92), and is potentially heightened and continuously reactivated in patients with OCD as compulsive behaviors persist. Detecting that performed actions do not align with beliefs and goals leads to the aversive state of “cognitive dissonance”, which others have proposed to drive or worsen some instances of obsessions (89, 93) (although see (94) for an excellent critique), and found to be associated with cingulo-opercular activation (95, 96). In other words, the unease caused by prolonged and heightened error-processing during compulsions may motivate rationalizations of OCD behaviors (“e.g., I continue to check the stove, therefore it must be important that the stove is checked and re-checked”). In addition, the resultant anxiety may further bias behavior towards bottom-up stimulus generated responses (e.g., compulsions). An overview of our proposed model is given in Figure 3. In order to test aspects of this model, future studies should use paradigms specially designed to examine trial-to-trial modulations in task-related activation following error-processing (14, 97), with the hypothesis that cingulo-opercular activation to errors is less efficient in OCD than in HC at bringing about post-error adjustments in brain activation.
Figure 3: Error-processing and inhibitory control in OCD.
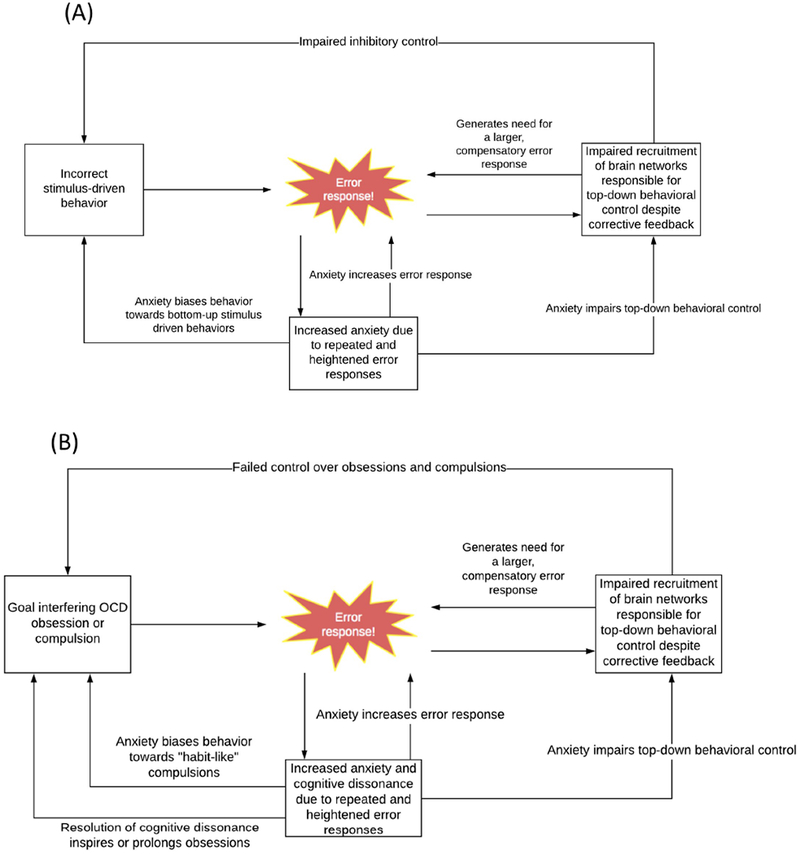
(a) During errors on inhibitory control tasks, error responses in the cingulo-opercular network signal a need for behavioral correction. In patients with OCD, this error signal does not efficiently increase activation within underactive brain networks responsible for inhibitory control. Due to continued under recruitment of these brain networks, error-processing signals are increased as a compensatory attempt at correction. Heightened and repeated error signaling increases anxiety in the disorder, which further interferes with top-down behavioral control, biases behavior towards bottom-up stimulus driven responses (errors), and feeds back to further increase error signaling. (b) During obsessions and compulsions, error responses are generated to signal the need to stop goal-incongruent or goal-irrelevant behaviors. This error signal does not appropriately recruit activation in brain networks responsible for behavioral control in OCD. This means that patients with OCD continue to experience obsessive and compulsive symptoms, with these generating repeated error signals, and these signals are increased in the disorder as a compensatory attempt at generating behavioral control. Heightened, repeated and aversive error signaling increases anxiety, which further interferes with top-down behavioral control in the disorder and biases behavior towards bottom-up stimulus driven responses (compulsions). Anxiety caused by continued performance and poor perceived control over of interfering OCD compulsions also further increases cingulo-opercular activation, and creates a feeling of cognitive dissonance that is resolved through rationalization of compulsive behaviors (e.g., through reinforcement of obsessions).
It is also important to note that the effect sizes for between group differences in performance and brain activation were small, indicating substantial overlap between patients and HC on these measures. Crucially, even large, reliable differences between groups would not have necessarily indicated a causal mechanistic relationship. For instance, it is also plausible that observed neurocognitive abnormalities in OCD are secondary to the OCD-specific symptoms of the disorder, and it has been proposed that obsessive or worrying thoughts in OCD patients may occur at the expense of task engagement/attention, resulting in non-optimal performance and altered brain activation during cognitive tasks (the ‘overload’ model of neuropsychological impairment in OCD) (98). Alternatively, observed neurocognitive abnormalities may be driven by trans-diagnostic phenotypes that are closely associated with OCD such as heightened anxiety, which has also been associated with heightened error-processing and impaired inhibitory control (17). Finally, heightened error-processing and impaired inhibitory control may share genetic risk and co-occur with OCD without there being a direct causal relationship between these phenotypes. With a few exceptions (23, 26), most fMRI studies on the topic have focused on simple case-control comparisons. Now that reliable differences between OCD and HC have been determined, future work should utilize sophisticated imaging genetics, longitudinal and treatment designs to further elucidate whether heightened error-processing and impaired inhibitory control do indeed have mechanistic roles in the etiology and treatment of OCD, or whether they are instead secondary to OCD symptoms or otherwise linked in a noncausal way to the disorder.
Limitations of the meta-analysis include a reliance on meta-regressions to test for relationships between brain activation and age, gender, symptom severity, comorbid anxiety and mood disorders, medication status and error-rates. In particular, many patients were medicated with antidepressants and this may have exacerbated between-group findings (24, 99). A more sensitive approach would be to test for relationships between these variables using large samples and subject-level individual differences. In addition, we combined data from different inhibitory control tasks with varying levels of difficulty and error rates. Degree of error-related brain activation varies according to task error rates, and these rates varied widely in the current meta-analysis (100, 101). Moreover, while there is substantial overlap in the neural underpinnings observed across different inhibitory control tasks (102), the specific cognitive demands and underlying neural bases of each task also vary between tasks (103). The aim here was to investigate the most consistent abnormalities in OCD regardless of task type. As the field grows, future meta-analyses will be better placed to test for task-specific effects. Finally, we combined data from both pediatric and adult samples, and although the primary between-group findings were also present in the adult sensitivity analysis, there are likely developmental changes in brain activation that we were unable to investigate here (24, 97).
To summarize, in a large meta-analytic sample, patients with OCD relative to HC showed impaired task performance as well as hyperactivation in dACC/SMA, pre-SMA, right aI/fO and right aLPFC during error-processing, and hypoactivation in rACC/vACC and right aI/fO, striatum, SMG, mOFC and cerebellum during inhibitory control. These findings may support a model wherein patients become stuck in compulsive “loops”, because detected erroneous OCD behaviors remain uncorrected by hypoactive inhibitory control networks. However, more work is needed to further our understanding of how these performance and brain function abnormalities relate to OCD symptoms.
Supplementary Material
Acknowledgments.
Luke J. Norman was supported as a postdoctoral fellow funded by a R01 MH102242 grant awarded to Kate Fitzgerald and Stephan F. Taylor. This work was presented as a poster presentation at the American College of Neuropsychopharmacology (ACNP) 56th Annual Meeting, Palm Springs, California, USA as well as at the 73rd Society of Biological Psychiatry (SOBP) Annual Meeting, New York City, New York.
Disclosures
Stephan F. Taylor has received research support from Neuronetics, St. Jude Medical (now Abbott), Boehringer-Ingelheim and Otsuka Pharmaceutical. Katya Rubia has received speaker’s honoraria and grants for other projects from Lilly and Shire. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruscio AM, Stein DJ, Chiu WT, Kessler RC (2010): The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 15: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed Arlington, VA: American Psychiatric Association. [Google Scholar]
- 3.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, Rubia K (2016): Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: A comparative meta-analysis. JAMA Psychiatry. 73: 815–825. [DOI] [PubMed] [Google Scholar]
- 4.Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle JA, et al. (2011): Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol Psychiatry. 69: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlisi CO, Norman LJ, Lukito SS, Radua J, Mataix-Cols D, Rubia K (2017): Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry. 82: 83–102. [DOI] [PubMed] [Google Scholar]
- 6.van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA (2014): Response Inhibition and Interference Control in Obsessive-Compulsive Spectrum Disorders. Front Hum Neurosci 8: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlosser RGM, Wagner G, Schachtzabel C, Peikert G, Koch K, Reichenbach JR, Sauer H (2010): Fronto-cingulate effective connectivity in obsessive compulsive disorder: a study with fMRI and dynamic causal modeling. Hum Brain Mapp 31: 1834–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ (2005): The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev 29: 399–419. [DOI] [PubMed] [Google Scholar]
- 9.Ullsperger M, Danielmeier C, Jocham G (2014): Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev 94: 35–79. [DOI] [PubMed] [Google Scholar]
- 10.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science. 306: 443–447. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KD, Taylor SF (2015): Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS Spectr 20: 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (2004): Anterior cingulate conflict monitoring and adjustments in control. Science. 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- 13.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- 14.King JA, Korb FM, von Cramon DY, Ullsperger M (2010): Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. J Neurosci Off J Soc Neurosci 30: 12759–12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews CA, Perez VB, Delucchi KL, Mathalon DH (2012): Error-related negativity in individuals with obsessive-compulsive symptoms: Toward an understanding of hoarding behaviors. Biol Psychol 89: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillan CM, Fineberg NA, Robbins TW (2017): A trans-diagnostic perspective on obsessive-compulsive disorder. Psychol Med 47: 1528–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endrass T, Ullsperger M (2014): Specificity of performance monitoring changes in obsessive-compulsive disorder. Micro-Macro-Perspect Cogn Confl Control. 46: 124–138. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF (2005): Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 57: 287–294. [DOI] [PubMed] [Google Scholar]
- 19.Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA (2005): Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 24: 495–503. [DOI] [PubMed] [Google Scholar]
- 20.Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS (2003): Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci 14: 347–353. [DOI] [PubMed] [Google Scholar]
- 21.Tolin DF, Witt ST, Stevens MC (2014): Hoarding disorder and obsessive-compulsive disorder show different patterns of neural activity during response inhibition. Psychiatry Res 221: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth K, Maynor M, Welsh RC, et al. (2010): Altered function and connectivity of the medial frontal cortex in pediatric obsessive compulsive disorder. Biol Psychiatry. 68: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huyser C, Veltman DJ, Wolters LH, de Haan E, Boer F (2011): Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a Flanker task before and after CBT. J Child Psychol Psychiatry. 52: 1251–1260. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald KD, Liu Y, Johnson T, Moser J, Marsh R, Hanna GL, Taylor SF (In Press): Development of posterior medial frontal cortex function in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang D-H, Jang JH, Han JY, Kim J-H, Jung WH, Choi J-S, et al. (2013): Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 40: 340–346. [DOI] [PubMed] [Google Scholar]
- 26.de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, et al. (2012): Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 169: 1100–1108. [DOI] [PubMed] [Google Scholar]
- 27.Rubia K, Cubillo A, Woolley J, Brammer MJ, Smith A (2011): Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum Brain Mapp 32: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K (2008): Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br J Psychiatry J Ment Sci 192: 25–31. [DOI] [PubMed] [Google Scholar]
- 29.Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB (2014): Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry. 75: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix-Cols D, et al. (2009): A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res 174: 202–209. [DOI] [PubMed] [Google Scholar]
- 31.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. (2005): A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res 139: 101–114. [DOI] [PubMed] [Google Scholar]
- 32.Nabeyama M, Nakagawa A, Yoshiura T, Nakao T, Nakatani E, Togao O, et al. (2008): Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res 163: 236–247. [DOI] [PubMed] [Google Scholar]
- 33.Hou JM, Li HT, Wu WJ, Qu W, Ran JF, Chen Y (2011): Functional MRI of brain dysfunction during Stroop task in obsessive compulsive disorder patients. Chin J Med Imaging Technol 27: 1977–1980. [Google Scholar]
- 34.Pena-Garijo J, Barros-Loscertales A, Ventura-Campos N, Ruiperez-Rodriguez MA, Edo-Villamon S, Avila C (2011): [Involvement of the thalamic-cortical-striatal circuit in patients with obsessive-compulsive disorder during an inhibitory control task with reward and punishment contingencies]. Rev Neurol 53: 77–86. [PubMed] [Google Scholar]
- 35.Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. (2007): Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 64: 946–955. [DOI] [PubMed] [Google Scholar]
- 36.Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC (2007): Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. 62: 901–909. [DOI] [PubMed] [Google Scholar]
- 37.Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ (2010): Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp 31: 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morein-Zamir S, Voon V, Dodds CM, Sule A, van Niekerk J, Sahakian BJ, Robbins TW (2016): Divergent subcortical activity for distinct executive functions: stopping and shifting in obsessive compulsive disorder. Psychol Med 46: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitman RK (1987): A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 28: 334–343. [DOI] [PubMed] [Google Scholar]
- 40.Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N (2016): Presupplementary Motor Area Contributes to Altered Error Monitoring in Obsessive-Compulsive Disorder. Biol Psychiatry. 80: 562–571. [DOI] [PubMed] [Google Scholar]
- 41.Agam Y, Greenberg JL, Isom M, Falkenstein MJ, Jenike E, Wilhelm S, Manoach DS (2014): Aberrant error processing in relation to symptom severity in obsessive-compulsive disorder: A multimodal neuroimaging study. NeuroImage Clin 5: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D (2010): Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 67: 701–711. [DOI] [PubMed] [Google Scholar]
- 43.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. (2000): Metaanalysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 44.Lüdecke D (2018): esc: Effect Size Computation for Meta Analysis. Retrieved from https://CRAN.R-project.org/package=esc.
- 45.Viechtbauer W (2010): Conducting meta-analyses in R with the metafor package. J Stat Softw 36. [Google Scholar]
- 46.Radua J, Mataix-Cols D (2009): Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry J Ment Sci 195: 393–402. [DOI] [PubMed] [Google Scholar]
- 47.Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S (2012): A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry J Assoc Eur Psychiatr 27: 605–611. [DOI] [PubMed] [Google Scholar]
- 48.Egger M, Smith G Davey, Schneider M, Minder C (1997): Bias in meta-analysis detected by a simple, graphical test. BMJ. 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol 57: 289–300. [Google Scholar]
- 50.Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. (2016): Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 22: 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitzgerald K, Norman L, Liu Y, Hanna G, Taylor S (n.d.): Cognitive control networks in pediatric obsessive compulsive disorder: Target for treatment response? Biol Psychiatry. 81: S316–S317. [Google Scholar]
- 52.Hough CM, Luks TL, Lai K, Vigil O, Guillory S, Nongpiur A, et al. (2016): Comparison of brain activation patterns during executive function tasks in hoarding disorder and nonhoarding OCD. Psychiatry Res 255: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ (2007): Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 164: 335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Jeune F, Verin M, N’Diaye K, Drapier D, Leray E, Du Montcel ST, et al. (2010): Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 68: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 55.Norman LJ, Carlisi CO, Christakou A, Chantiluke K, Murphy C, Simmons A, et al. (2017): Neural dysfunction during temporal discounting in paediatric Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder. Psychiatry Res 269: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotge J-Y, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. (2008): Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci JPN. 33: 405–412. [PMC free article] [PubMed] [Google Scholar]
- 57.Yamanishi T, Nakaaki S, Omori IM, Hashimoto N, Shinagawa Y, Hongo J, et al. (2009): Changes after behavior therapy among responsive and nonresponsive patients with obsessive-compulsive disorder. Psychiatry Res 172: 242–250. [DOI] [PubMed] [Google Scholar]
- 58.Carey PD, Warwick J, Niehaus DJH, van der Linden G, van Heerden BB, Harvey BH, et al. (2004): Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry. 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nauczyciel C, Le Jeune F, Naudet F, Douabin S, Esquevin A, Verin M, et al. (2014): Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: a double-blind, crossover study. Transl Psychiatry. 4: e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheinost D, Stoica T, Saksa J, Papademetris X, Constable RT, Pittenger C, Hampson M (2013): Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl Psychiatry. 3: e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheinost D, Stoica T, Wasylink S, Gruner P, Saksa J, Pittenger C, Hampson M (2014): Resting state functional connectivity predicts neurofeedback response. Front Behav Neurosci 8: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlisi CO, Norman L, Murphy CM, Christakou A, Chantiluke K, Giampietro V, et al. (2017): Comparison of neural substrates of temporal discounting between youth with autism spectrum disorder and with obsessive-compulsive disorder. Psychol Med 47: 2513–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan BP, Tkachenko O, Schwab ZJ, Juelich RJ, Ryan EM, Athey AJ, et al. (2015): An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 40: 1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Wit SJ, van der Werf YD, Mataix-Cols D, Trujillo JP, van Oppen P, Veltman DJ, van den Heuvel OA (2015): Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychol Med 45: 3059–3073. [DOI] [PubMed] [Google Scholar]
- 65.de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, et al. (2014): Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 76: 878–887. [DOI] [PubMed] [Google Scholar]
- 66.de Vries FE, de Wit SJ, van den Heuvel OA, Veltman DJ, Cath DC, van Balkom AJLM, van der Werf YD (2017): Cognitive control networks in OCD: A resting-state connectivity study in unmedicated patients with obsessive-compulsive disorder and their unaffected relatives. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 1–13. [DOI] [PubMed] [Google Scholar]
- 67.Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF (2011): Developmental alterations of frontal-striatal-thalamic connectivity in obsessive compulsive disorder. J Am Acad Child Adolesc Psychiatry. 50: 938–948.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boedhoe PSW, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. (2017): Distinct subcortical volume alterations in pediatric and adult OCD: A worldwide meta- and mega-analysis. Am J Psychiatry. 174: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen C-H, del Campo N, et al. (2007): Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain J Neurol 130: 3223–3236. [DOI] [PubMed] [Google Scholar]
- 70.de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchon JM, et al. (2014): Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 171: 340–349. [DOI] [PubMed] [Google Scholar]
- 71.Thorsen AL, Hagland P, Radua J, Mataix-Cols D, Kvale G, Hansen B, van den Heuvel OA (In Press): Emotional Processing in Obsessive-Compulsive Disorder: A Systematic Review and Meta-analysis of 25 Functional Neuroimaging Studies. Biol Psychiatry Cogn Neurosci Neuroimaging. doi: 10.1016/j.bpsc.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorsen AL, de Wit SJ, de Vries FE, Cath DC, Veltman DJ, van der Werf YD, et al. (2018): Emotion regulation in obsessive-compulsive disorder, unaffected siblings and unrelated healthy controls. Biol Psychiatry Cogn Neurosci Neuroimaging. doi: 10.1016/j.bpsc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Norman LJ, Carlisi CO, Christakou A, Cubillo A, Murphy CM, Chantiluke K, et al. (2017): Shared and disorder-specific task-positive and default mode network dysfunctions during sustained attention in paediatric Attention-Deficit/Hyperactivity Disorder and obsessive/compulsive disorder. NeuroImage Clin 15: 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norman L, Carlisi CO, Christakou A, Murphy C, Chantiluke K, Giampietro V, et al. (In Press): Fronto-striatal dysfunction during decision-making in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaghi MM, Hampshire A, Fineberg NA, Kaser M, Brühl AB, Sahakian BJ, et al. (2017): Hypoactivation and Dysconnectivity of a Frontostriatal Circuit During Goal-Directed Planning as an Endophenotype for Obsessive-Compulsive Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Velzen LS, de Wit SJ, Curcic-Blake B, Cath DC, de Vries FE, Veltman DJ, et al. (2015): Altered inhibition-related frontolimbic connectivity in obsessive-compulsive disorder. Hum Brain Mapp 36: 4064–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlisi CO, Norman L, Murphy CM, Christakou A, Chantiluke K, Giampietro V, et al. (2017): Disorder-Specific and Shared Brain Abnormalities During Vigilance in Autism and Obsessive-Compulsive Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manoach DS, Agam Y (2013): Neural markers of errors as endophenotypes in neuropsychiatric disorders. Front Hum Neurosci 7: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Figee M, Vink M, Luigjes J, van Wingen G, Denys D (2017): Deep Brain Stimulation Modulates Frontostriatal Inhibitory Control in Obsessive-Compulsive Disorder. 72nd Annu Sci Conv Meet. 81: S96–S97. [Google Scholar]
- 80.Banks GP, Mikell CB, Youngerman BE, Henriques B, Kelly KM, Chan AK, et al. (2015): Neuroanatomical characteristics associated with response to dorsal anterior cingulotomy for obsessive-compulsive disorder. JAMA Psychiatry. 72: 127–135. [DOI] [PubMed] [Google Scholar]
- 81.Yin D, Zhang C, Lv Q, Chen X, Zeljic K, Gong H, et al. (2018): Dissociable Frontostriatal Connectivity: Mechanism and Predictor of the Clinical Efficacy of Capsulotomy in Obsessive-Compulsive Disorder. Biol Psychiatry. doi: 10.1016/j.biopsych.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Suetens K, Nuttin B, Gabriels L, Van Laere K (2014): Differences in metabolic network modulation between capsulotomy and deep-brain stimulation for refractory obsessive-compulsive disorder. J Nucl Med Off Publ Soc Nucl Med 55: 951–959. [DOI] [PubMed] [Google Scholar]
- 83.Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, van Wingen G, de Kwaasteniet B, et al. (2013): Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 16: 386–387. [DOI] [PubMed] [Google Scholar]
- 84.Holroyd CB, Coles MGH (2002): The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109: 679–709. [DOI] [PubMed] [Google Scholar]
- 85.Kerns JG (2006): Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. NeuroImage. 33: 399–405. [DOI] [PubMed] [Google Scholar]
- 86.Danielmeier C, Allen EA, Jocham G, Onur OA, Eichele T, Ullsperger M (2015): Acetylcholine mediates behavioral and neural post-error control. Curr Biol CB. 25: 1461–1468. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Gehring WJ, Weissman DH, Taylor SF, Fitzgerald KD (2012): Trial-by-trial adjustments of cognitive control following errors and response conflict are altered in pediatric obsessive compulsive disorder. Front Psychiatry. 3: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Modirrousta M, Meek BP, Sareen J, Enns MW (2015): Impaired trial-by-trial adjustment of cognitive control in obsessive compulsive disorder improves after deep repetitive transcranial magnetic stimulation. BMC Neurosci. 16: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gillan CM, Robbins TW (2014): Goal-directed learning and obsessive-compulsive disorder. Philos Trans R Soc Lond B Biol Sci 369. doi: 10.1098/rstb.2013.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, et al. (2009): Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex N Y N 1991. 19: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hajcak G, Foti D (2008): Errors are aversive: defensive motivation and the error-related negativity. Psychol Sci 19: 103–108. [DOI] [PubMed] [Google Scholar]
- 92.Spunt RP, Lieberman MD, Cohen JR, Eisenberger NI (2012): The phenomenology of error processing: the dorsal ACC response to stop-signal errors tracks reports of negative affect. J Cogn Neurosci 24: 1753–1765. [DOI] [PubMed] [Google Scholar]
- 93.Gillan CM, Sahakian BJ (2015): Which is the driver, the obsessions or the compulsions, in OCD? Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 40: 247–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalanthroff E, Abramovitch A, Steinman SA, Abramowitz JS, Simpson HB (2016): The chicken or the egg: What drives OCD? J Obsessive-Compuls Relat Disord. 11: 9–12. [Google Scholar]
- 95.van Veen V, Krug MK, Schooler JW, Carter CS (2009): Neural activity predicts attitude change in cognitive dissonance. Nat Neurosci 12: 1469–1474. [DOI] [PubMed] [Google Scholar]
- 96.de Vries J, Byrne M, Kehoe E (2015): Cognitive dissonance induction in everyday life: An fMRI study. Soc Neurosci 10: 268–281. [DOI] [PubMed] [Google Scholar]
- 97.Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M (2011): Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci Off J Soc Neurosci 31: 1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abramovitch A, Dar R, Hermesh H, Schweiger A (2012): Comparative neuropsychology of adult obsessive-compulsive disorder and attention deficit/hyperactivity disorder: implications for a novel executive overload model of OCD. J Neuropsychol 6: 161–191. [DOI] [PubMed] [Google Scholar]
- 99.Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. (2018): Cortical Abnormalities Associated With Pediatric and Adult Obsessive-Compulsive Disorder: Findings From the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry. 175: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ullsperger M (2006): Performance monitoring in neurological and psychiatric patients. Int J Psychophysiol 59: 59–69. [DOI] [PubMed] [Google Scholar]
- 101.de Bruijn ERA, Ullsperger M (2011): Pathological Changes in Performance Monitoring (Mars RB, Sallet J, Rushworth MFS, & Yeung N, editors) Neural Basis Motiv Cogn Control. 263–280. [Google Scholar]
- 102.Zhang R, Geng X, Lee TMC (2017): Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct Funct . doi: 10.1007/s00429-017-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sebastian A, Pohl MF, Kloppel S, Feige B, Lange T, Stahl C, et al. (2013): Disentangling common and specific neural subprocesses of response inhibition. NeuroImage. 64: 601–615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


