Summary:
Widespread antibiotic resistance has led to the re-appraisal of abandoned antibiotics including chloramphenicol. However, enzyme(s) underlying one form of chloramphenicol resistance, nitro reduction, have eluded identification. Here we demonstrate that expression of the H. influenzae nitroreductase gene nfsB confers chloramphenicol resistance in E. coli. We characterized the enzymatic product of H. influenzae NfsB acting on chloramphenicol and found it to be amino-chloramphenicol. Kinetic analysis revealed reduction of diverse substrates including the incomplete reduction of 5-nitro antibiotics metronidazole and nitrofurantoin, likely resulting in activation of these antibiotic pro-drugs to their cytotoxic forms. We observed that expression of the H. influenzae nfsB gene in E. coli results in significantly increased susceptibility to metronidazole. Finally, we found that in this strain metronidazole attenuates chloramphenicol resistance synergistically, and in vitro metronidazole weakly inhibits chloramphenicol reduction by NfsB. Our findings reveal the underpinnings of a chloramphenicol resistance mechanism nearly 70 years after its description.
Keywords: nfsB, nitro-reduction, nitroreductase, chloramphenicol, amphenicols, metronidazole, antibiotics, resistance, H. influenzae, E. cloacae, synergy
eTOC
Crofts et al. report the characterization of bacterial genes that confer chloramphenicol resistance by nitro-reduction. The most effective gene, H. influenzae nfsB, encodes an enzyme that efficiently reduces the chloramphenicol nitro group in vitro. The authors found that resistance via reduction could be countered using the anaerobic antibiotic metronidazole.
Introduction:
Antibiotic resistance is a growing threat to modern medicine and is expected to be responsible for an increasing number of deaths worldwide in the future (O’Neil, 2014). Compounding this threat is the ubiquity of cryptic resistance mechanisms present in the environment (Crofts et al., 2017) and declining development of new antibiotics (Kinch et al., 2014; Payne et al., 2007; Pye et al., 2017). One solution to this problem has been to re-consider the use of antibiotics which were previously abandoned due to compromised safety or efficacy profiles (Wright, 2017). In particular, the rise of resistance specifically to β-lactams, the most widely used class of antimicrobials world-wide, has changed the cost-benefit analysis for multiple other classes of antibiotics that were formerly concluded to be too toxic for general use. For example, colistin and daptomycin are now important clinical antibiotics of last resort despite prior abandonment due to toxicity issues. Their potential toxicity is outweighed by the lethality of otherwise virtually pan-resistant infections (Čivljak et al., 2014; Li et al., 2006; The Pew Charitable Trusts, 2016; Wright, 2017). It has been suggested that amphenicols, antibiotics such as chloramphenicol (figure 1A), thiamphenicol, and florfenicol, that have lost or failed to gain approval for human use due to potential toxicity, may follow this same path for re-adoption (Čivljak et al., 2014; Dinos et al., 2016; Rahim et al., 2015).
Figure 1. Structure of chloramphenicol and its bacterial modifications.
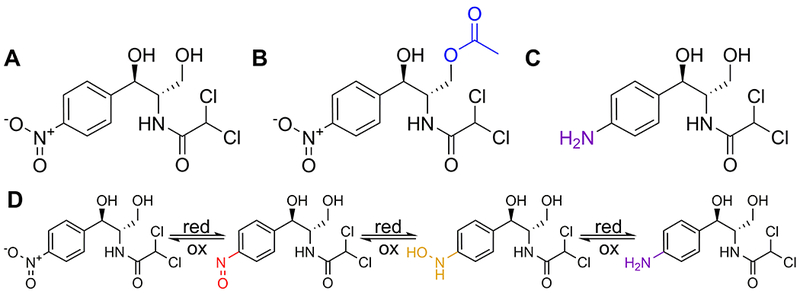
Chemical structure of (A) chloramphenicol, (B) chloramphenicol inactivated following acetylation, and (C) chloramphenicol inactivated following nitro reduction. (D) Products of step-wise chloramphenicol reduction, from left to right: chloramphenicol, nitroso-chloramphenicol, hydroxylamine-chloramphenicol, and amino-chloramphenicol.
Chloramphenicol (figure 1A), co-discovered and marketed by Parke-Davis beginning in the 1940s, is a natural product antibiotic produced by the bacterium Streptomyces venezuelae with antibiotic activity against Gram positive and Gram negative bacteria (Ehrlich et al., 1947; Smith et al., 1948). It is stable under a range of conditions (Ehrlich et al., 1947) and was the first natural product antibiotic to be economically produced synthetically (Ehrlich et al., 1947; Schwarz et al., 2004; Wright et al., 2014). Chloramphenicol is orally available and can cross the blood brain barrier and other difficult to reach sites, making it a potent therapy for bacterial meningitis and tissue infections (Dinos et al., 2016; Schwarz et al., 2004).
Despite their attractiveness as antibiotics, the clinical use of amphenicols has largely ceased in more economically developed countries as a result of chloramphenicol’s epidemiological linkage to hematological toxicity (Shu et al., 1987; Wallerstein et al., 1969). Potentially because of this, chloramphenicol remains active against a variety of bacterial pathogens. While chloramphenicol does not show greater efficacy against routine infections compared to current standard treatments, its use may be justifiable against multi-drug resistance (MDR) organisms (Eliakim-Raz et al., 2015) as chloramphenicol retains substantial activity against Gram positive organisms, especially methicillin resistant Staphylococcus aureus in more developed countries, and moderate activity against Gram negative organisms (Čivljak et al., 2014; Lim et al., 2016; Nitzan et al., 2010; Sood, 2016).
The most widespread chloramphenicol resistance mechanisms are via well annotated functions such as enzymatic inactivation (e.g. via chloramphenicol acetyltransferase, figure 1B), efflux pump removal, and ribosome protection (Dinos et al., 2016; Long et al., 2006; Schwarz et al., 2004). One of first reports of bacterial modification of chloramphenicol came only two years after its discovery. The authors described multiple species of bacteria, including E. coli, having the ability to fully reduce the nitro group of chloramphenicol and in this way resist its bacteriostatic effects (Smith and Worrel, 1949). The resulting compound, amino-chloramphenicol (figure 1C), lacked antibacterial activity, an observation borne out by studies demonstrating the importance of an electron withdrawing constituent at this position of the molecule (Dinos et al., 2016). Since this first observation, additional studies have replicated the finding that a variety of bacteria can reduce the nitro group of chloramphenicol, resulting in altered susceptibility (Egami et al., 1951; Merkel and Steers, 1953; O’Brien and Morris, 1971; Onderdonk et al., 1979; Smith and Worrel, 1950, 1953; Smith et al., 2007). While many of these bacteria demonstrated a susceptible phenotype in vitro, there is evidence that in a host this strategy leads to phenotypic resistance in the presence of serum chloramphenicol concentrations reaching almost 3-times the in vitro minimal inhibitory concentration (MIC) (Onderdonk et al., 1979). Furthermore, based on the phylogenetic distribution of these taxa, chloramphenicol nitro reduction is likely not limited to these individually studied organisms. Similarly, amino-chloramphenicol has been detected in the feces of conventional rats, but not in their germ-free counterparts (Glazko et al., 1952; Wal et al., 1983), and this metabolite has been documented in samples from dogs, non-human primates, and humans as well (Glazko, 1987a; Holt et al., 1995; Kunin et al., 1959). Despite its apparent ubiquity and potential medical importance, the mechanism underlying chloramphenicol reduction has never been described and no genes or enzymes having this activity have been documented.
We set out to identify bacterial genes that may be effectors of this activity. The oxygen-insensitive family of nitroreductases, such as those encoded by the nfsA or nfsB genes in E. coli, are known to be promiscuous reducers of aromatic nitro groups and homologs of these genes are found across diverse bacterial taxa, including in many members of the intestinal microbiome (Roldán et al., 2008). Nitro reduction via these enzymes occurs two electrons at a time, often stopping at the production of a hydroxylamino group after a four electron reduction (Roldán et al., 2008), though some enzymes, such as the NfsB homologs from E. coli, Enterobacter cloacae, and Salmonella enterica, have been demonstrated to reduce aromatic nitro groups completely to amines (LinWu et al., 2009; Yanto et al., 2010) (figure 1D). We found that expression of some of these genes in E. coli led to altered susceptibility/resistance to chloramphenicol. In vitro characterization of the enzyme corresponding to the most active gene, the Haemophilus influenzae nfsB homolog, revealed an ability to reduce chloramphenicol to amino-chloramphenicol, as well as an ability to at least partially reduce several other aromatic nitro substrates. Finally, we made initial strides into countering this cryptic resistance mechanism, showing that the commonly used antibiotic metronidazole both inhibits chloramphenicol reduction in vitro and synergistically kills chloramphenicol reducing E. coli in the presence of chloramphenicol. We report the isolation and characterization of genes/enzymes that confer chloramphenicol resistance via a reductive mechanism and consider the implications of our findings for future amphenicol use in the clinic and potential links to chloramphenicol toxicity.
Results:
Nitroreductase gene expression can alter E. coli chloramphenicol susceptibility.
In choosing which candidate genes to assay for chloramphenicol reduction activity we began our search by focusing (fortuitously) on homologs of the oxygen-insensitive Type I nitroreductase family. We choose to focus at first on oxygen insensitive enzymes due to the ability of this class to reduce aromatic nitro groups to a stable amino product, rather than to unstable intermediates as has been observed with many Type II oxygen-sensitive enzymes (Roldán et al., 2008). The most relevant members of this class of enzyme are the well-studied E. coli NfsA and NfsB enzymes (Zenno et al., 1996a, 1996b). Because a Lactococcus lactis homolog of E. coli NfsA (CinD) has been reported to be unable to reduce chloramphenicol in vitro (Mermod et al., 2010), we further chose to focus on predicted NfsB homologs originating from bacterial taxa that have been phenotypically associated with chloramphenicol reduction (supplementary table 1) including E. coli, S. enterica, E. cloacae, H. influenzae, Clostridium acetobutylicum, and Streptococcus pyogenes. We cloned a target gene from each organism into a constitutive expression vector for expression in E. coli. Because amino-chloramphenicol (figure 1C) does not show antibacterial properties (Smith and Worrel, 1949; Smith et al., 2007) we utilized microbroth dilution susceptibility testing to determine if any of the strains showed altered susceptibility to chloramphenicol as a result of reductase expression (figure 2A). Our negative control, E. coli carrying an empty vector, was susceptible to chloramphenicol, with a calculated 50% inhibitory concentration (IC50) of 2.12±0.19 μg/ml (figure 2B) and an observed minimal inhibitory concentration (MIC) of 8 μg/ml across three replicates. E. coli expressing a canonical chloramphenicol resistance gene, chloramphenicol acetyltransferase or cat (Schwarz et al., 2004), was highly resistant with an IC50/MIC greater than 256 μg/ml. Two of the cloned reductases imparted a significant increase in chloramphenicol resistance, as measured by IC50, when expressed in E. coli: the nfsB homologs from H. influenzae (IC50 21.5±1.1 μg/ml) and E. cloacae (IC50 6.24±1.1 μg/ml) (figure 2B). E. coli strains expressing these genes were also the only strains to show at least a two-step jump in MIC compared to vector control, with the H. influenzae nfsB expressing strain showing growth up to 64 μg/ml chloramphenicol and the E. cloacae nfsB expressing strain showing growth up to 32 μg/ml chloramphenicol.
Figure 2. Reductase expression can alter susceptibility of E. coli to chloramphenicol.
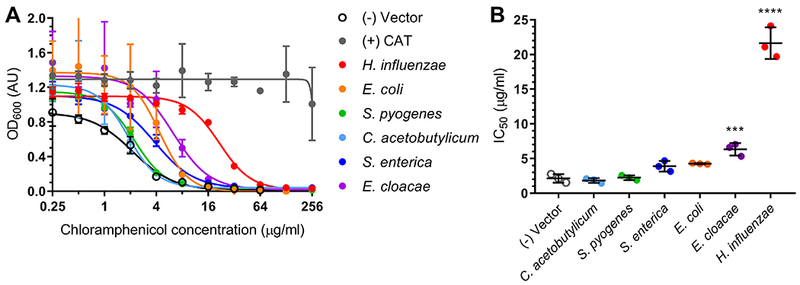
(A) Dose-response curves of microbroth dilution assays for E. coli expressing predicted reductase gene homologs in the presence of chloramphenicol with (B) corresponding 50% inhibitory concentrations (IC50) calculated from the curve fit. All points are averages of triplicate experiments with standard deviation error bars. Statistical significance was calculated with respect to the vector control by ordinary one-way ANOVA with Dunnett’s correction for multiple comparisons. Adjusted p-value displayed as p≤0.0001 (****), p<0.001 (***). Abbreviation: CAT, chloramphenicol acetyltransferase.
Because we observed variation in the ability of nfsB expression to confer resistance to chloramphenicol, we set out to determine if activity could be predicted by amino acid sequence. We focused on the amino acid sequences of the six reductases tested in E. coli (figure 2), two other well-studied nitroreductases, (E. coli NfsA (Zenno et al., 1996a) and L. lactis CinD (Mermod et al., 2010)), and nine sequences corresponding to nfsB homologs from bacterial taxa previously tested for their ability to reduce chloramphenicol in culture (Smith et al., 2007). For a broader comparison, a selection of bacterial proteins annotated in UniProt as NfsB homologs were compared as well. The resulting maximum likelihood tree (figure 3) is organized largely by phylogeny but with evidence for clustering by function as well.
Figure 3. Phylogenetic and amino acid analysis of chloramphenicol reducing and non-reducing NfsB homologs.
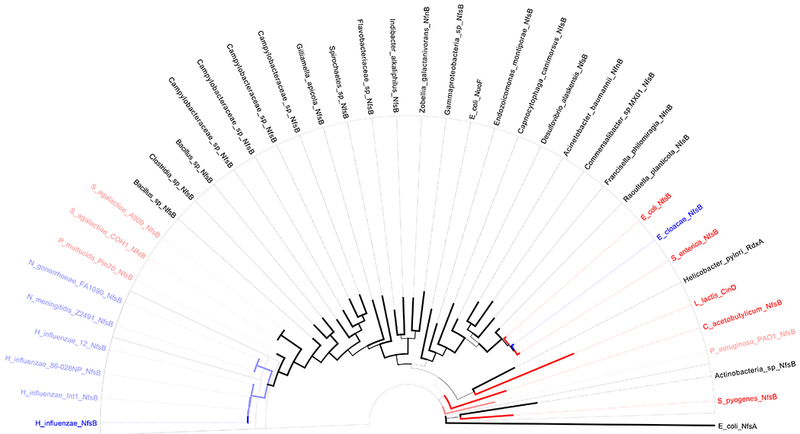
Maximum likelihood tree with bootstrap support (100 tests, indicated by branch thickness) of predicted NfsB homologs. Solid blue branches/taxa are those with direct evidence of chloramphenicol reduction (this paper). Light blue and light red branches/taxa are those with indirect evidence for or against, respectively, chloramphenicol reduction (reduction observed or not observed in native host, Smith et al. 2007). Solid red branches/taxa are those with direct evidence against chloramphenicol reduction (this paper and L. lactis CinD from Mermod et al. 2010).
This is evident in the clustering of the Pasteurellales family, where Haemophilus, Neisseria, and Pasteurella genera appear to cluster as a group by phylogeny. Within this group, the Neisseria NfsB homologs cluster with Haemophilus NfsB homologs while the Pasteurella homolog is further removed despite its closer phylogenetic relatedness to Haemophilus. This sub-grouping instead matches predicted ability to reduce chloramphenicol based on previous phenotypic studies (Smith et al., 2007). On the other hand, the E. cloacae NfsB clusters with its phylogenetic relatives, E. coli and S. enterica, though it appears to have significantly greater chloramphenicol reduction activity (figure 2). Because our analyses did not establish any sequence markers for predicting chloramphenicol reduction we chose to focus on further characterizing the H. influenzae NfsB homolog, the reductase with greatest apparent activity.
Chloramphenicol reducing E. coli cultures can degrade chloramphenicol to non-inhibitory levels and rescue susceptible strains.
One report of chloramphenicol reduction occurring in the context of a rat infection model noted the survival of susceptible bacteria in spite of chloramphenicol treatment with confirmed serum levels well above their MIC (Onderdonk et al., 1979). To test our system for this phenotype, we performed a series of experiments on the E. coli strain expressing the H. influenzae nfsB gene. Our first approach used a chloramphenicol challenge assay where we measured culture lag phase duration in the presence of increasing concentrations of chloramphenicol. The utility of this lag-phase assay has previously been demonstrated for chloramphenicol (Tao et al., 2012). We observed that while our control E. coli strain did not show substantial growth in the presence of any concentration of chloramphenicol (figure 4A), the H. influenzae nfsB expressing strain grew at several concentrations (figure 4B), in line with previous observations of chloramphenicol reduction. We found a clear correlation between the time it took the resistant strain to reach half-maximal growth and the chloramphenicol concentration in the media (figure 4C). In a second assay, we cultured E. coli containing an empty vector or a vector expressing H. influenzae nfsB in inhibitory concentrations of chloramphenicol. After removing cells, this pre-conditioned media was assayed for its ability to support the growth of a chloramphenicol-susceptible E. coli strain. Both pre-conditioned media from the empty vector E. coli strain and fresh media containing chloramphenicol inhibited growth of the susceptible E. coli strain, while pre-conditioned media lacking chloramphenicol supported robust growth (figure 4D). Media with chloramphenicol but pre-conditioned by the H. influenzae nfsB expressing E. coli strain was capable of supporting significant growth, reaching approximately half the cell density as chloramphenicol-free media, suggesting chloramphenicol levels had been reduced from 8 μg/ml to a concentration near 2 μg/ml, the approximate IC50 of the susceptible strain.
Figure 4. Chloramphenicol resistance in E. coli expressing H. influenzae nfsB occurs via a drug-modification mechanism.
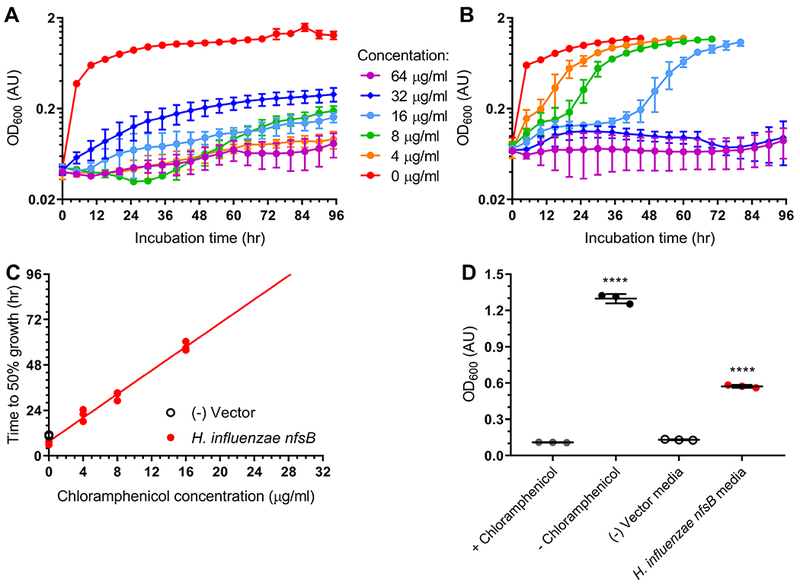
Lag-phase assays of E. coli expressing (A) empty vector or (B) H. influenzae nfsB grown in the presence of increasing concentrations of chloramphenicol ranging from 0 μg/ml to 64 μg/ml. (C) Linear regression of incubation time required to reach half-maximal OD600 plotted against chloramphenicol concentration. The slope of the fit line is 3.12±0.11 with an R2 of 0.998. (D) Growth of chloramphenicol-susceptible E. coli in chloramphenicol media pre-conditioned by empty vector or reductase-expressing E. coli strains. All experiments were performed in triplicate with error bars representing standard deviation when present. Significance at p≤0.0001 (****) was calculated based on triplicate experiments using a one-way ANOVA test with Dunnett’s correction for multiple comparisons and reflect a comparison to vector control.
H. influenzae NfsB reduction of chloramphenicol produces amino-chloramphenicol.
Because reduction of aromatic nitro groups can result in formation of fully reduced or partially reduced products, we next sought to characterize the degree of chloramphenicol reduction by H. influenzae NfsB. The ability of E. coli expressing H. influenzae nfsB to resist chloramphenicol (figure 2) and rescue susceptible strains (figure 4D), suggests complete reduction of the nitro group to an amine as partially reduced intermediates could potentially spontaneously oxidize back to chloramphenicol in a futile cycle (Dingsdag and Hunter, 2017; Roldán et al., 2008). We therefore set out to characterize the H. influenzae NfsB chloramphenicol product in vitro. We cloned the H. influenzae nfsB gene into an inducible system for heterologous expression and purification in E. coli. After optimization (supplemental figure 1A) we obtained an enzyme of the expected denatured size of ~25 kDa (supplemental figure 1B). We found that H. influenzae NfsB (UniProt Q57431) requires NADPH as a cofactor and does not utilize NADH (supplemental figure 2A) and tightly binds a flavin cofactor, most likely flavin mononucleotide (FMN) (supplemental figure 2B). The enzyme shows activity across a wide range of pH values, with highest activity recorded between pH 6.5 and pH 9 (supplemental figure 2C) and shows activity in our assay at temperatures between 25°C and 55°C (supplemental figure 2D). Based on these results assays were performed at 25°C in 50 mM Tris-HCl buffer at pH 8 with NADPH and FMN.
To determine if chloramphenicol is reduced completely to amino-chloramphenicol we utilized liquid chromatography coupled to mass spectrometry (LCMS). We first verified enzymatic activity by following chloramphenicol degradation over the course of time. Two reactions were initiated by adding enzyme or buffer to solutions containing chloramphenicol and NADPH, following which paired aliquots were quenched every two and a half minutes for quantitative analysis of chloramphenicol concentrations. LCMS analysis confirmed loss of chloramphenicol in the enzyme reactions but not in the control reaction (figures 5A and 5B).
Figure 5. Analytical characterization of H. influenzae NfsB chloramphenicol reduction products.
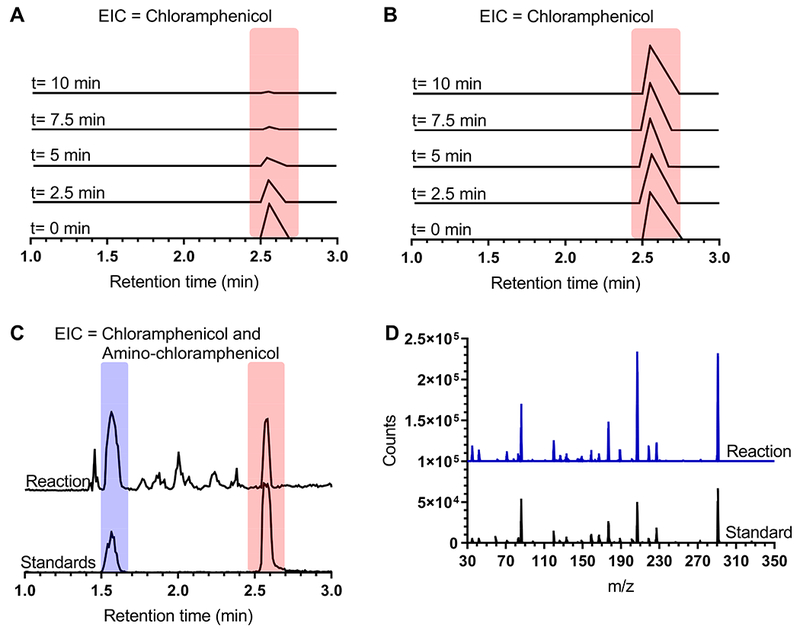
LCMS monitoring of loss of chloramphenicol (323.132 m/z, monitored at 321 m/z) over ten minutes in the presence (A) and absence (B) of enzyme. (C-D) LCMS analysis of products extracted from overnight enzymatic reaction with chloramphenicol and NADPH regeneration system. (C) Extracted ion counts corresponding to amino-chloramphenicol (291 m/z, blue bar) and chloramphenicol (321 m/z, red bar) of reaction products (top traces) compared to co-mixed amino-chloramphenicol and chloramphenicol standards (bottom traces). (D) Mass spectra of 1.6-minute retention time product peak (blue trace) compared to amino-chloramphenicol standard (black trace).
To confirm that the product of this reaction was amino-chloramphenicol, a preparative scale reaction with an NADPH regeneration system was incubated for 22.5 hours, extracted with ethyl acetate, and the resulting products were analyzed by LCMS. Extraction of ion m/z corresponding to amino-chloramphenicol and chloramphenicol revealed peaks at the same retention time as standards (figures 5C and supplemental figure 3A), with the first peak exhibiting a mass spectra and retention time consistent with amino-chloramphenicol (figure 5D). Verification of amino-chloramphenicol as a product of H. influenzae NfsB reduction of chloramphenicol was completed by comparison of product to standard by multiple-reaction monitoring (MRM) mass spectrometry fragment analysis (supplemental figures 3B and 3C).
Nitrated compounds and chloramphenicol, but not other amphenicols, are H. influenzae NfsB substrates.
Nitroreductase enzymes generally show wide substrate specificity (Akiva et al., 2017; Pitsawong et al., 2014; Roldán et al., 2008) so we next set out to more thoroughly characterize the substrate range of the H. influenzae NfsB enzyme and its kinetics. We first attempted to measure crude chloramphenicol reduction kinetics via the Bratton-Marshall derivatization test (Bratton and Marshall, EK, 1939), using time quenched reaction samples as inputs. The Bratton-Marshall assay is sensitive and specific for aromatic amines, but not other aromatic nitrogenous substituents, and is especially apt for detecting amino-chloramphenicol (Bratton and Marshall, EK, 1939; Glazko, 1987b; Smith et al., 2007). Reaction progress curves of Bratton-Marshall positive products at different concentrations of chloramphenicol indicated enzymatic production of amino-chloramphenicol and suggested that the H. influenzae NfsB enzyme follows Michaelis-Menten kinetics (supplemental figure 4A). Nitroreductase reactions can be followed in real time spectrophotometrically by measuring the oxidation of NAD(P)H to NAD(P) (Mermod et al., 2010). To confirm that H. influenzae NfsB oxidation of NADPH corresponds to chloramphenicol reduction we followed the same reaction wells at a wavelength specific to NADPH (beyond the absorbance range of chloramphenicol, supplemental figure 4B) and at a wavelength specific to the chloramphenicol nitro group (at the isosbestic point of NADPH/NADP), as determined by comparison of UV-Vis spectra of chloramphenicol and the nitro-group free amphenicols thiamphenicol and florfenicol (supplemental figure 4B). The resulting progress curves (supplemental figures 4C and 4D) match closely, confirming NADPH monitoring as an appropriate proxy for nitro reduction.
We next measured the kinetics of H. influenzae NfsB reduction of chloramphenicol, as well as other potential aromatic nitro substrates. We began by comparing reduction of chloramphenicol to reduction of thiamphenicol and florfenicol. Because thiamphenicol and florfenicol do not contain nitro groups we expected to see no activity with these substrates. Of the three amphenicols, only the reaction containing chloramphenicol showed evidence of enzymatic activity, demonstrating classic Michaelis-Menten kinetics (figure 6A). NfsB and other nitroreductase enzymes are the targets of the 5-nitro class of antibiotics, including metronidazole and nitrofurantoin. These antibiotics are pro-drugs and become bactericidal when reductases act on their aromatic nitrogen groups leading to the production of highly reactive hydroxylamines (Dingsdag and Hunter, 2017). We assayed the activity of H. influenzae NfsB against these two compounds and confirmed that both are substrates, with nitrofurantoin in particular showing rapid turn-over rates (figure 6B). Finally, we also assayed 3- and 4-nitrophenol as well as menadione, a quinone lacking nitro groups that is often nonetheless reduced by NfsB nitroreductases (Zenno et al., 1996b). All three of these compounds also acted as substrates, with menadione showing relatively high activity and 4-nitrophenol showing relatively low activity (figure 6C). Michaelis-Menten kinetics for each substrate are reported in supplemental table 2.
Figure 6. H. influenzae NfsB shows Michaelis-Menten kinetics with chloramphenicol, menadione and nitro- substrates.
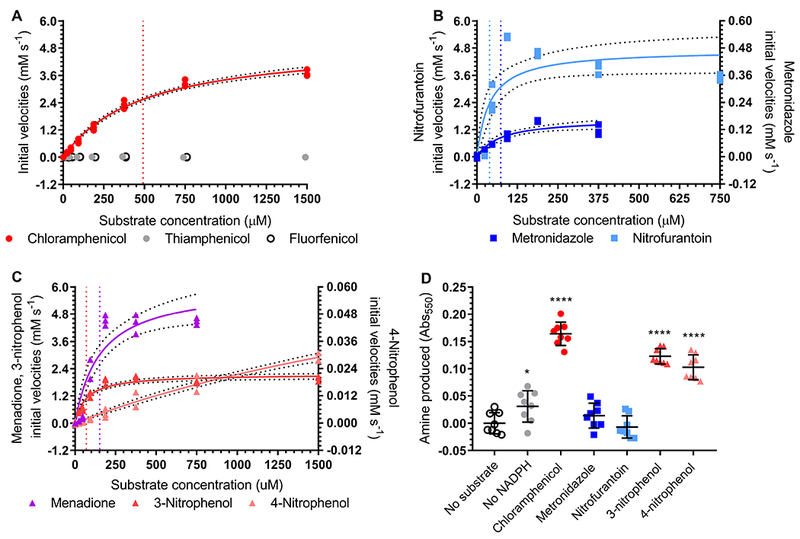
Graphs of Michaelis-Menten kinetics for H. influenzae NfsB with kcat/KM (mM−1 s−1) for the following substrate groups (nd not determined): (A) amphenicols (chloramphenicol 20.71, thiamphenicol nd, and florfenicol nd), (B) 5-nitro antibiotics (metronidazole 4.57 and nitrofurantoin 242.89), and (C) other substrates (menadione 79.34, 3-nitrophenol 61.80, and 4-nitrophenol nd). All reactions were performed in triplicate and the best-fit Michaelis-Menten kinetics curves (solid lines) and corresponding 95% confidence intervals (dotted lines) are shown. Vertical dotted lines correspond to the calculated KM values and are color-coded by substrate within each panel. In (A) florfenicol and thiamphenicol points are offset for clarity. (D) Bratton-Marshall analysis of aryl amine formation in reactions containing H. influenzae NfsB. Reactions contained enzyme, FMN, NADPH, and substrate unless otherwise noted. Reactions were performed in 8 technical replicates with mean and standard deviation shown. Significance compared to no substrate (at p≤0.0001 ****, p≤0.05 *) was calculated using a one-way ANOVA test with Dunnett’s correction for multiple comparisons.
Subsequently, we performed Bratton-Marshall end-point assays on reactions with each nitro-containing substrate (substrates listed above except thiamphenicol, florfenicol, and menadione), as well as enzyme and cofactor controls. As expected, the control lacking NADPH showed very little production of Bratton-Marshall derivatized material, while the full chloramphenicol reaction showed a significant amount. In contrast, the 5-nitro antibiotics showed no significant production compared to the substrate control reaction, while the two nitrophenol compounds showed intermediate levels (figure 6D).
Metronidazole inhibits chloramphenicol reduction by H. influenzae NfsB in vitro and synergizes with chloramphenicol to inhibit E. coli expressing nfsB.
During the course of our in vitro experiments we were surprised to see that the H. influenzae NfsB enzyme reduces chloramphenicol to amino-chloramphenicol (figure 5 and supplemental figure 3) and also uses metronidazole as a substrate (figure 6C) but does not reduce the metronidazole nitro group all the way to an amino group (figure 6D). Because partial reduction of the aromatic nitro group underlies the bactericidal activity of 5-nitro class of antibiotics like metronidazole (Dingsdag and Hunter, 2017), we next assayed E. coli expressing nfsB homologs from H. influenzae and E. cloacae, as well as from S. enterica and E. coli, for altered susceptibility to metronidazole. Metronidazole susceptibility is usually limited to anaerobic bacteria (Dingsdag and Hunter, 2017), as illustrated by the high IC50 (23.7±2.0 μg/ml) of our vector control. All four E. coli strains expressing nfsB homologs showed significantly increased susceptibility to metronidazole compared to the vector control, with the strains expressing the H. influenzae and E. cloacae nfsB genes showing the most sensitivity (supplemental figures 5A and 5B).
Based on the antibiotic susceptibility evidence, H. influenzae nfsB expression is advantageous in the presence of chloramphenicol, providing resistance through reduction (figure 2). However, in the presence of metronidazole nfsB expression is disadvantageous, as E. coli does not normally activate metronidazole to its toxic form but appears to do so under these conditions (supplemental figures 5A and 5B). Our kinetics data (supplementary table 2) also suggests that, based on KM measures, metronidazole should be able to compete with chloramphenicol substrate binding. We therefore hypothesized that chloramphenicol and metronidazole might act synergistically when used in combination against chloramphenicol reducing bacteria. We found that by increasing the concentration of metronidazole, the chloramphenicol susceptibility of the H. influenzae nfsB expression E. coli strain increased markedly (figure 7A).
Figure 7. Inhibition of chloramphenicol reduction and synergistic killing by metronidazole.
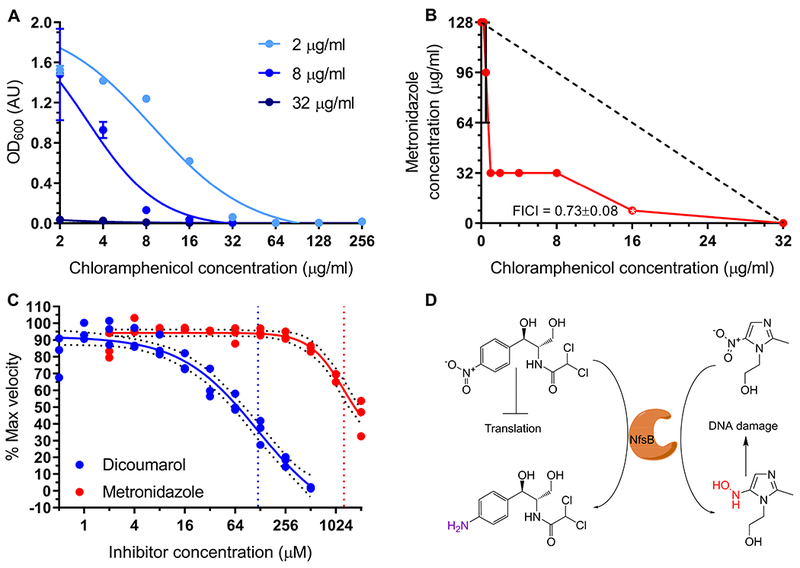
(A) Dose-response curves of E. coli expressing H. influenzae nfsB at various concentrations of chloramphenicol and the indicated concentrations of metronidazole (2 μg/ml, 8 μg/ml, and 32 μg/ml metronidazole). (B) FICI analysis of metronidazole vs chloramphenicol 2D checkerboard assay. The lowest FICI score of 0.73±0.08 was found at 16 μg/ml chloramphenicol and 8 μg/ml metronidazole. Dashed black line represents theoretical line of additivity. (C) In vitro assay of chloramphenicol reduction inhibition by dicoumarol (blue curve) or metronidazole (red curve). 95% confidence intervals of curve fits of triplicate data points are shown (black dotted lines) and fit IC50 (vertical colored lines) are indicated (dicoumarol 119.7 μM; metronidazole 1274 μM). Panels (A-B) report mean of three replicate experiments with standard deviation error bars. (D) Schematic representation role of NfsB in synergy experiment: (left) Chloramphenicol resistance and (right) metronidazole toxic activation, both via nitro-reduction.
Our follow-up analysis using a checkboard-type assay to measure interactions between the antibiotics demonstrated that chloramphenicol and metronidazole do indeed act synergistically in E. coli expressing H. influenzae nfsB, with the lowest FICI score reaching 0.727±0.080 (figure 7B), significantly lower than E. coli with empty vector (FICI = 2.30±0.73). We further studied this interaction in vitro, by measuring the rate of chloramphenicol reduction in the presence of increasing concentrations of metronidazole or dicoumarol, a known inhibitor of NfsB enzymes (Roldán et al., 2008). In vitro we observed that both substrates have the potential to inhibit chloramphenicol reduction, though with metronidazole showing an IC50 of ~1 mM (figure 7C). The hypothesized interplay between resistance via reduction and pro-drug activation via reduction is summarized in figure 7D.
Discussion:
We present results from a series of cross-validating experiments that demonstrate the ability of H. influenzae NfsB to reduce the nitro group of chloramphenicol. We provide the identification and characterization of an enzyme with chloramphenicol reductase activity, beginning with its ability to confer resistance to chloramphenicol when expressed in E. coli (figure 2). While resistance wasn’t as thorough as that seen in E. coli expressing the cat gene, resistance conferred by H. influenzae nfsB allowed for growth well beyond the EUCAST clinical breakpoint for E. coli of 8 μg/ml. Similarly, expression of the nfsB homolog from E. cloacae was sufficient to confer increased resistance to chloramphenicol in E. coli, again above the clinical breakpoint for defining resistance.
We were curious as to whether we could detect a signal in the amino acid sequence of the H. influenzae NfsB enzyme that could predict its heightened ability to resist chloramphenicol but were unable to do so (figure 3), suggesting that structural studies may be warranted. Nevertheless, the phylogenetic distance between H. influenzae and E. cloacae NfsB proteins suggests to us that chloramphenicol reduction may be relatively widely spread. Chloramphenicol is a natural product antibiotic that has been recovered from across the globe (Ehrlich et al., 1947; Gottlieb and Bhattacharyya, 1948) and is detectable in the soil at low concentrations (Berendsen et al., 2013), suggesting that nitro reduction may be a feasible resistance mechanism against environmental levels of the drug.
We also found compelling evidence for utility of this resistance mechanism in the face of clinical therapy. In addition to growing in the presence of chloramphenicol concentrations above resistance breakpoints (figure 2) we found in a different assay that E. coli expressing H. influenzae nfsB appears capable of de-toxifying increasing levels of chloramphenicol given enough time (figure 4). This finding is in agreement with a literature report (Onderdonk et al., 1979) that during infection, Bacteroides fragilis strains that showed in vitro susceptibility to chloramphenicol were capable of resisting treatment through reduction. It is also therefore possible that during treatment with chloramphenicol commensal or pathogenic bacteria may find a selective advantage in reducing the nitro group of chloramphenicol.
In vitro, the H. influenzae NfsB enzyme is capable of reducing the chloramphenicol nitro group completely to an amine, as well as potentially the nitro groups of other nitrophenols (figures 5 and 6). Complete reduction of aromatic nitro groups is a rare feature among described NfsB homologs (Pitsawong et al., 2014; Roldán et al., 2008) that may have further industrial or medical applications (Copp et al., 2017). The kinetics of chloramphenicol reduction (supplemental table 2), with a kcat/KM of 20.71 mM−1 sec−1, are slower than many canonical antibiotic resistance enzymes, such as diffusion limited β-lactamases, but fall within the range of kinetics observed for average enzymes (Bar-Even et al., 2011). The NfsB enzyme from H. influenzae appears to have promiscuous substrate binding, based on the variety of substrates it is capable of turning-over and their KM’s, ranging from roughly 40 μM to 500 μM. In this respect it is similar to other nitroreductase enzymes which, as a class, show substrate promiscuity and relatively weak substrate binding (Akiva et al., 2017; Pitsawong et al., 2014; Race et al., 2005; Roldán et al., 2008). Of the substrates tested, H. influenzae NfsB showed the greatest activity with nitrofurantoin and lowest reliably measured activity with metronidazole, with kcat/KM measures approximately an order of magnitude above and below that measured for chloramphenicol, respectively. Despite their wide difference in kinetics, reactions containing neither nitrofurantoin nor metronidazole showed evidence for the production of aromatic amino groups (figure 6D). Partial reduction of these compounds is key to their antibiotic activity, but full reduction can provide resistance (Roldán et al., 2008). In the case of H. influenzae NfsB, reduction is sufficient to provide resistance to chloramphenicol, but might only serve to more fully activate the antibacterial activity of metronidazole and nitrofurantoin.
In exploring this idea further, we found that H. influenzae nfsB and other nfsB homologs in E. coli leads to significantly increased susceptibility to metronidazole, a drug that is usually only used to treat anaerobes (supplemental figures 5A and 5B). We further observed that metronidazole potentiates chloramphenicol resistance in the H. influenzae nfsB expressing strain and that in fact the two antibiotics synergistically inhibit the growth of this strain (figures 7A and 7B). We hypothesized that this synergy may be the result of two activities. The first of these relates to our observation that metronidazole is not fully reduced to form an amino group, but it does act as an NfsB substrate. Therefore it is likely that while NfsB protect the cell against chloramphenicol it also activates metronidazole to its lethal form, counteracting any survival benefit. The second activity that we believe might be relevant is substrate inhibition. While metronidazole is a poor substrate compared to chloramphenicol as measured by kcat (0.337 sec−1 vs 10.17 sec−1) it is much better when measured by KM (74 μM vs ~500 μM) (supplemental table 2). We hypothesized that metronidazole may therefore be able to compete with chloramphenicol as a substrate, a hypothesis borne out by our measurements of chloramphenicol reduction in the presence of titrated metronidazole (figure 7C). A schematic of chloramphenicol/metronidazole synergy is represented in figure 7D, wherein metronidazole directly kills H. influenzae expressing E. coli via its reduction-mediated activation to a toxic form and indirectly inhibits growth of this strain by competing with chloramphenicol, resulting in diminished resistance to it.
Finally, it has been hypothesized that chloramphenicol nitro reduction by host-associated bacteria underlies the development of a rare, serious side-effect of chloramphenicol: aplastic anemia (Holt, 1967). The lack of mechanistic understanding of chloramphenicol-induced aplastic anemia is largely responsible for the failure of many developed countries to utilize amphenicols in the clinic, including potentially superior second and third generation compounds. For example, florfenicol, which lacks a nitro group, is immune to inactivation by chloramphenicol acetyltransferases (Dinos et al., 2016) (supplemental figures 5C and 5D) but is not used clinically due to fears that use in humans may result in development of aplastic anemia. The identification of a bacterial enzyme capable of chloramphenicol reduction provides the prerequisite for testing the aplastic anemia hypothesis and potentially shedding light on the safety of nitro group free amphenicols.
Star*Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Gautam Dantas (dantas@wustl.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Escherichia coli (DH10B and BL21(DE3)), Haemophilus influenzae KW20 (ATCC 51907), Salmonella enterica LT2 (ATCC 700720), Enterobacter cloacae 96-3 (ATCC 13047), and Clostridium acetobutylicum NCIB 8052 (ATCC 824) were cultured respectively (1) aerobically at 37°C in LB, (2) aerobically at 37°C in DSMZ Haemophilus medium (per liter: 21 g Mueller-Hinton broth, 5 g yeast extract, supplemented with 15 mg each of NAD and hemine), (3) aerobically at 37°C in Mueller-Hinton broth, (4) aerobically at 37°C in Mueller-Hinton broth, or (5) anaerobically at 37°C in ATCC modified reinforced Clostridial broth (per liter: 10 g tryptose, 10 g beef extract, 3 g yeast extract, 5 g dextrose, 5 g NaCl, 1 g soluble starch, 0.5 g L-cysteine HCl, 3 g sodium acetate, and 4 ml of 0.025% resazurin). For routine growth, E. coli strains were incubated aerobically at 37°C in LB media or in Mueller-Hinton media for antibiotic susceptibility tests with kanamycin sulfate at 50 μg/ml when appropriate. Strain identities were verified through successful amplification of target reductase genes confirmed by Sanger sequencing (see methods details: Gene Amplification and Cloning).
METHODS DETAILS
Gene amplification and cloning
Genomic DNA from grown cultures was extracted and purified using a Wizard Genomic DNA purification kit (Promega, No. A1120) according to manufacturer’s instructions. Streptococcus pyogenes HSC5 genomic DNA was kindly provided to us by the Caparon lab at Washington University in St. Louis. Reductase and cat genes were amplified from genomic DNA, or the pZE31 vector (Lutz and Bujard, 1997) for cat, with primer pairs specified in supplemental table 1 using Platinum Pfx polymerase (ThermoFisher Scientific, No. 11708039) or Q5 hotstart 2X master mix (New England Biosciences, No. M0494L) according to manufacturer’s guidelines. Inserts were purified by QIAquick PCR purification kit (Qiagen, No. 28104) and ligated into modified pZE21(Lutz and Bujard, 1997) or pET-28b(+) (with N-terminal 6X-his tag) vectors via blunt-ended ligation using Fast-Link DNA ligation kit (Epicentre, No. LK0750H) or 2X Gibson Assembly master mix (New England Biosciences, No. E2611S). Constructs were next transformed into chemically competent E. coli DH10B cells (pZE21 vectors) or E. coli BL21(DE3) (pET-28b(+) vectors) by heat shock at 42°C followed by recovery for 30 minutes at 37°C in SOC media (Invitrogen, No. 46-0821) and plating on to LB agar with 50 μg/ml kanamycin. Colonies were picked into LB with kanamycin, grown overnight at 37°C with aeration and plasmids extracted by QIAprep Spin Miniprep kit (Qiagen, No. 27104). Insert integrity was finally verified by Sanger sequencing (Genewiz). All strains were stored long-term at −80°C as stocks in 15% glycerol in LB.
Microbroth serial dilution assays
Microbroth serial dilution assays were used to determine IC50 values for amphenicols and metronidazole in a modification of EUCAST standard protocol. 96-well plates (COSTAR, 3595) were prepared with 100 μl volumes of antibiotics in Mueller-Hinton broth with kanamycin arranged in two-fold dilutions by column, with concentrations ranging from 512 μg/ml to 0.5 μg/ml for chloramphenicol, 512 μg/ml to 0.25 μg/ml for metronidazole and florfenicol, or 1024 μg/ml to 0.5 μg/ml for thiamphenicol (concentrations are two-fold greater than final concentrations).
Overnight Mueller-Hinton with kanamycin starter cultures of E. coli DH10B strains expressing reductase genes on pZE21 vectors were inoculated into fresh Mueller-Hinton with kanamycin at a density of 0.1 AU measured at 600 nm (OD600). Diluted cultures were added to the prepared antibiotic-containing 96-well plates in 100 μl aliquots for an initial OD600 and volume of 0.05 AU and 200 μl respectively, with each strain/antibiotic combination prepared in triplicate. After inoculation, 96-well plates were sealed with Breathe-Easy membranes (Sigma-Aldrich, Z380059) and incubated with shaking at 220 rpm at 37°C for 18 to 24 hours. Growth was measured at 600 nm in a Powerwave HT microplate spectrophotometer (Biotek, Inc.). Dose-response curves were plotted and analyzed using GraphPad Prism version 7.01 for Windows (GraphPad Software, La Jolla California, USA). Triplicate data were fit to the following four parameter Hill equation:
IC50 p-values were calculated by One-way ANOVA with Dunnett’s correction for multiple comparisons. MIC was determined by taking the lowest concentration that resulted in culture growth measuring less than 0.1 AU at 600 nm. Chloramphenicol resistant/susceptible breakpoint of 8 μg/ml is according to EUCAST standards for E. coli.
Phylogenetic analysis of reductase proteins
Amino acid sequences were downloaded and aligned using ClustalW (Thompson et al., 1994) in Mega7 (Kumar et al., 2016) using default parameters with manual trimming. Un-targeted NfsB homologs were downloaded from the UniProt database (UniProt Consortium, 2015). The resulting alignment was used to construct a Maximum Likelihood tree with default parameters and 100 rounds of bootstrapping in Mega7. The tree was visualized in the tree viewing program FigTree (Rambaut, 2008).
Lag-phase chloramphenicol modification assay
Triplicate 100 μl aliquots of LB with kanamycin and 0 μg/ml, 8 μg/ml, 16 μg/ml, 32 μg/ml, 64 μg/ml, or 128 μg/ml chloramphenicol were prepared in a 96-well plate (COSTAR, 3595). Overnight triplicate cultures of empty vector or H. influenzae nfsB-expressing E. coli cultures were grown in LB with kanamycin and diluted 10-fold into fresh LB with kanamycin and incubated for 3 hours at 37°C in order to enter exponential phase. Exponentially growing cultures were diluted to an OD600 of 0.1 AU and added in 100 μl volumes to the 96-well plate. Plates were sealed as before and incubated in a Powerwave HT microplate spectrophotometer (Biotek, Inc.) at 37°C with constant shaking (medium setting, rpm not available) for 96 hours with monitoring every 30 minutes. Growth data were fit to the Hill equation as above to determine how many hours of incubation were required to reach half-maximal growth. A plot of chloramphenicol concentration against time to reach 50% growth was prepared and projected to 96 hours.
Pre-conditioned media assay
E. coli containing empty pZE21 vector or vector-expressed H. influenzae nfsB were inoculated into LB with kanamycin and 8 μg/ml chloramphenicol and incubated overnight at 37°C alongside a cell-free (+) chloramphenicol control and an empty vector (−) chloramphenicol control. Following overnight growth cells were removed from cultures by first pelleting at 16,000 rcf for 10 minutes, followed by filtration of the supernatant through a 0.22 μm filter. Cell-free supernatants were split into three aliquots as technical replicates and inoculated with E. coli containing empty vector (to provide resistance to residual kanamycin) prior to incubating overnight at 37°C. Following incubation growth was measured as before and OD600 values were compared using a one-way ANOVA test with Dunnett’s correction for multiple comparisons to determine level of chloramphenicol removal in the pre-conditioned media.
H. influenzae NfsB expression and purification
E. coli BL21(DE3) containing pET-28b(+) with H. influenzae nfsB open reading frame downstream of a 6-histidine tag was inoculated into 3 ml Studier auto-inducing media ZYM-5052 (Studier, 2005) with kanamycin and grown overnight with aeration at 37°C. 750 μl aliquots of grown culture were added a 750 ml culture of ZYM-5052 with 100 μg/ml kanamycin and to three 750 ml cultures of Terrific broth (TB, Fisher Scientific, No. BP9729-600) supplemented with 2 mM MgSO4 in 2.8 L Fernbach flasks. Cultures were shaken at 350 rpm at 37°C in a MaxQ 5000 (Thermo Scientific) temperature-controlled incubator until OD600 of ~1 was reached (~3 hour incubation). The incubator temperature was reduced to 25°C and the TB flasks were induced with 0 μM, 100 μM, or 250 μM IPTG with shaking for 48 hours with aliquots taken at 24 hours as well. Cell aliquots taken at 24 hours and 48 hours were pelleted at 8,000 rcf, resuspended in phosphate buffered saline (PBS), and 100 ul aliquots were lysed by sonication using a Bioruptor Pico (Diagenode) at 4°C for 10 minutes with 30 seconds on and 30 seconds off. Lysates were centrifuged at 21,000 rcf to separate soluble and insoluble fractions and analyzed for soluble enzyme production via SDS-PAGE analysis with Sypro Ruby gel stain (ThermoFisher, No. S12001). While 24 hour incubation in ZYM-5052 gave the best results, the 48 hour incubations for all four flasks were combined and cells pelleted at 8,000 rcf for 20 minutes using a Sorvall Legends XTR centrifuge (Thermo Scientific) in a Fiberlite F14-6×250 LE rotor (Thermo Scientific) before removal of supernatants and freezing of wet cell pellets overnight at −80°C.
Cell pellets were weighed and allowed to thaw on ice. All following steps were performed at 4°C or on ice. The cell pellets were resuspended to 20% w/v in ice-cold loading buffer consisting of (per liter) 8.709 g K2HPO4 (50 mM), 29.22 g NaCl (500 mM), 351 μl β-mercaptoethanol (5 mM), 0.68 g imidazole (10 mM), and 100 ml glycerol (10% v/v) at pH 8 with addition of lysozyme at 1 mg lysozyme/g wet cell mass. Suspensions were incubated on ice for 30 minutes then lysed by sonication on a Branson Sonifier 250 instrument (Branson Ultrasonics) with microtip adjusted to reach ca. 30% output on 50% duty until suspension viscosity and color were reduced. Insoluble matter was clarified from the suspensions by centrifugation for 30 min at 24,446 rcf at 4°C in a Sorvall Legends XTR centrifuge (Thermo Scientific) in a Fiberlite F15-8×50 cy rotor (Thermo Scientific). Supernatants were collected and stored at 4°C until purification.
Purification of the His-tagged enzyme was performed using an Ni-NTA nickel resin (BioRad, No. 7800800) and a low-pressure chromatography system (BioRad, Econo system) coupled to a fraction collector (BioRad, No. 7318122) as previously described (Crofts et al., 2018). Fractions containing purified enzyme were identified by their yellow color and verified by SDS-PAGE analysis and staining with Bio-Safe Coomassie stain (BioRad, No. 1610796) according to manufacturer’s instructions. Fractions containing pure recombinant H. influenzae NfsB were pooled and concentrated via centrifugation through a 10 kDa molecular weight cutoff filter (Amicon, No. UFC901024) according to manufacturer’s instructions. The ca. 1 ml concentrate was washed three times as above with storage buffer consisting of (per liter) 8.709 g K2HPO4 (50 mM), 8.766 g NaCl (250 mM), 1 ml of 1 M dithiothreitol (1 mM) and 50 ml of glycerol (5% v/v) at pH of 7.5. Purified enzyme concentration was determined by Qubit Protein Assay kit (Thermo Scientific, No. Q33211) and the mass concentration was converted to molar concentration using molecular weights predicted by EXPASY (Gasteiger et al., 2003). Aliquots of 55 μl were stored at −80°C.
Chloramphenicol reduction assay optimization
A preliminary in vitro assay based on reported reductase conditions (Mermod et al., 2010; Smith et al., 2007) demonstrated that the H. influenzae NfsB enzyme could reduce chloramphenicol (see below for optimized conditions). We used these baseline conditions to determine the effect of reduced cofactor on activity. A pre-reaction mixture was prepared containing 50 mM Tris-HCl pH 8, 2 mM chloramphenicol, 10 μM flavin mononucleotide (FMN), and 1 μM H. influenzae NfsB. Six 100 μl aliquots were transferred to a 96-well plate (COSTAR, 3595) to which was added 100 μl of 2 mM NADH (Grainger, No. 31GA86) or NADPH (Cayman chemical, No. 9000743) in 50 mM Tris-HCl pH 8. Oxidation of NAD(P)H to NAD(P)+ was monitored at 340 nm (Mermod et al., 2010) for 30 min at 25°C in a Powerwave HT microplate spectrophotometer (Biotek, Inc.) with minimal kinetic interval.
In order to determine the identity of the native flavin cofactor, a 50 μl aliquot of enzyme was heated at 70°C for 20 minutes to denature the enzyme and release the tightly bound flavin (Zenno et al., 1996b). The yellow supernatant was removed following centrifugation at 21,000 rcf for 20 min and analyzed via thin layer chromatography using silica gel 60 F254 (Millipore Sigma, No. 105794) alongside standards and extract/standard co-spots in a solvent system consisting of 5:2:3 1-butanol:acetic acid:H2O (2003). The plate was visualized and photographed under UV-vis at 366 nm in a Gel-Doc XR+ (BioRad). The observed retention factors of the standards were consistent with the literature.
To determine optimal pH for H. influenzae NfsB activity 100 mM Tris/citrate buffer with 10 μM FMN and 2 mM chloramphenicol was prepared. 400 μl aliquots of buffer were prepared at pH values ranging from 3 to 9 and aliquoted as seven technical replicates in 100 μl volumes in a 96-well plate (COSTAR, 3595). A second solution containing 1 μM enzyme and 6 mM NADPH was prepared and added in 100 μl volume to all buffer wells. Reactions were monitored at 340 nm as before and the progress curve slopes were compared to determine relative activity compared to the most active samples in GraphPad Prism (GraphPad Software, La Jolla California, USA). Due to the highly variable nature of the assay, Grubbs test with α=0.2 for outlier detection was used to remove potentially inaccurate samples.
To determine appropriate temperature ranges for enzyme activity, substrate mixes of 50 mM Tris-HCl pH 8, 1.25 mM NADPH, 1.25 mM chloramphenicol, and 6.25 μM FMN were aliquoted 40 μl at a time into PCR tubes (four replicates for each temperature to be tested). Substrate mixes were pre-incubated in a thermocycler at the test temperature for 1 minute following which 10 μl of enzyme solution (2.5 μM in 50 mM Tris-HCl pH 8) was added. Reactions were allowed to incubate for 4 minutes at which point they were quenched by addition of 50 μl of 20% trichloroacetic acid (TCA) and transfer to ice. Reaction progress was measured in quenched samples by derivatization of amino-chloramphenicol by the Bratton-Marshall method (Bratton and Marshall, EK, 1939; Smith et al., 2007). Briefly, 12.5 μl of the following solutions were added sequentially with 10 minute incubations at room temperature: 0.1% sodium nitrite, 0.5% ammonium sulfamate, and 0.05% Bratton-Marshall reagent (N-(1-Naphthyl)ethylenediamine dihydrochloride, 20 minutes). The reactions were then transferred to half-area 96-well plates (Corning, No. 3696) and Abs550 measurements were taken using a Powerwave HT microplate spectrophotometer (Biotek, Inc.) to quantify production of aryl-amines. Absorbance at 550 nm was normalized as percent maximum for each reaction and outliers were excluded using a Grubbs test with α=0.2. All following reactions were performed in 50 mM Tris-HCl at pH 8 and at 25°C unless otherwise stated.
Chloramphenicol modification LCMS assay
Enzymatic loss of chloramphenicol during NfsB reduction was monitored by liquid chromatography tandem mass spectrometry (LCMS) as follows. Two 700 μl aliquots of substrate buffer consisting of 2 mM NADPH, 10 μM FMN, and 600 μM chloramphenicol were prepared. An equal volume of Tris buffer or 1 μM enzyme in Tris buffer was added to each aliquot at t=0 and 250 μl was immediately removed and quenched with 12 μl of 88% formic acid. Additional 250 μl aliquots were quenched at 2.5 min, 5 min, 7.5 min, and 10 min.
Samples were sent to the Metabolomics Facility at Washington University for LCMS analysis where they were diluted 5-fold in methanol. An equal volume of 10 μg/ml thiamphenicol as internal standard was added to each sample before loading onto an ACE Excel Super C18 column (3 μm, 50 × 4.6 mm) on an Applied Biosystems Sciex 4000QTRAP tandem mass spectrometer. Samples were run with multiple reaction monitoring (MRM) in negative mode. Chloramphenicol standards were used to prepare calibration curves in duplicate for quantification.
Amino-chloramphenicol detection assay
The product of H. influenzae NfsB reduction of chloramphenicol in the presence of excess reducing equivalence was analyzed by targeted LCMS. Amino-chloramphenicol HCl salt was purchased from Toronto Research Chemicals (No. A622670) as a standard. An NADPH regeneration system (Xenotech, No. K5000) was used according to the manufacturer’s protocols to provide the equivalent of 700 μM to 900 μM NADPH concentrations during an overnight reaction. The reaction consisted of the regeneration system, 2 mM chloramphenicol, 15 μM FMN, and ca. 1.5 μM enzyme dissolved in 35 ml of 50 mM Tris-HCl buffer. Following incubation for 22.5 hours at room temperature the reaction was extracted three times with 10 ml of ethyl acetate. The combined organic layers were extracted three times with 2.5 ml of 0.1 M aqueous HCl. The aqueous extracts were combined, frozen at −80°C, and lyophilized to dryness. The dried yellow product was dissolved in 750 μl water and checked for potential presence of amino-chloramphenicol by Bratton-Marshall derivatization and TLC analysis against an amino-chloramphenicol standard (silica, 8:2 dichloromethane:methanol, visualized with iodine vapour).
LCMS analyses were performed at the Metabolomics Facility at Washington University on an Applied Biosystems Sciex 4000QTRAP tandem mass spectrometer coupled to a Shimadzu 20AD HPLC system. Metabolites were separated using an ACE Excel 3 super C18 column (3 μm, 50 × 4.6 mm) running 0.03% diethylamine and 20 mM hexafluoro-2-propanol in water, pH 8.5 (solvent A) against methanol (solvent B). The full mass scan (Q3 scan) and MS2 scans of m/z 291 (amino-chloramphenicol) and m/z 321 (chloramphenicol) were acquired for the samples as well as amino-chloramphenicol and chloramphenicol standards. Data processing was conducted with Analyst 1.5.2 (Applied Biosystems).
Enzyme substrate specificity and activity
Reactions to examine substrate activity and specificity were set-up by preparing substrate mixes containing 2 mM NADPH and 10 μM FMN and each substrate in triplicate. Concentrations (at twice the final reaction concentration) ranged from 3 mM to 46.875 μM (chloramphenicol. thiamphenicol, florfenicol, 3-nitrophenol, and 4-nitrophenol), 750 μM to 46.875 μM (metronidazole), and 1.5 mM to 46.875 μM (menadione) by 2-fold steps and were prepared as 50 μl aliquots in a half-area 96-well plate (Corning, No. 3696). Enzyme solution was prepared at 1 μM, and 50 μl of enzyme solution was added to all wells with mixing, followed by monitoring of the reaction progression in a Powerwave HT microplate spectrophotometer (Biotek, Inc.) with minimal kinetic interval at 340 nm for NADPH oxidation (chloramphenicol, thiamphenicol, florfenicol, metronidazole, menadione, and 3-nitrophenol), or at other wavelengths when spectra interfered substantially with measurements at 340 nm (420 nm for nitrofurantoin, 450 nm for 4-nitrophenol). In some cases (metronidazole and menadione) an optimal wavelength could not be found resulting in the exclusion of high substrate concentration data points where spectral overlap was too high.
Initial rate velocities in absorbance change, found by taking the slope during the linear stage of the reactions, were converted to μM s−1 using automatic pathlength correction and the following extinction coefficients for the substrate being monitored (determined empirically in triplicate if needed): NADPH ε340 = 6220 M−1 cm−1 (Mermod et al., 2010), nitrofurantoin ε420 = 7970 M−1 cm−1, and 4-nitrophenol ε450 = 3211 M−1 cm−1. Triplicate data points were plotted in GraphPad Prism (GraphPad Software, La Jolla California, USA) and fit to Michaelis-Menten curves for kcat and KM determination using the following equation:
Where Et corresponds to enzyme concentration (0.5 μM). For each fit the estimated 95% confidence interval as well as KM were plotted on the graph while kcat values were recorded elsewhere.
In order to monitor chloramphenicol nitro group reduction three assays were performed. In the first assay, 100 μl reactions arrayed in 8 replicates containing 50 mM Tris-HCl buffer at pH 8, 1 mM NADPH, 50 μM FMN, 5 μM enzyme, and 250 μM substrate were allowed to react for 30 minutes at room temperature along with control reactions lacking substrate or NADPH. Substrates included chloramphenicol, metronidazole, nitrofurantoin, 3-nitrophenol, and 4-nitrophenol. Following incubation, reactions were quenched with an equal volume of 20% TCA and analyzed by Bratton-Marshall assay (see previous section for method). In the second assay, four reactions in triplicate containing ca. 2 mM NADPH, 5 μM FMN, 0.5 μM enzyme, and 1 mM, 333 μM, 111 μM, or 37 μM chloramphenicol were allowed to proceed with an aliquot being removed at t=0, 1, 2, 3, and 4 minutes into an equal volume of 20% TCA for Bratton-Marshall derivatization as performed earlier. The absorbance at 550 nm was plotted to demonstrate accumulation of amino-chloramphenicol over time, with increased accumulation at higher chloramphenicol concentrations. In the third assay, 200 μl reactions in a UV transparent 96-well plate (Corning, No. 3635) containing 0.5 mM NADPH, 0.5 mM chloramphenicol, and 0.25 μM enzyme, or with each ingredient missing, were monitored at 340 nm for NADPH oxidation to NADP+ and at 282 nm (the isosbestic point of NADPH/NADP+) for loss of the chloramphenicol nitro group with minimal interval on a Powerwave HT microplate spectrophotometer (Biotek, Inc.).
Checkerboard assay to detect synergy
Triplicate 96-well plates (COSTAR, 3595) were prepared containing 100 μl of Mueller-Hinton broth with kanamycin and mixtures of chloramphenicol and metronidazole. Chloramphenicol ranged in concentrations from 512 μg/ml to 0.25 μg/ml by 2-fold jumps and metronidazole ranged in concentrations from 1024 μg/ml to 0.125 μg/ml by 4-fold jumps, with each chloramphenicol concentration existing as a mixture with each metronidazole concentration. E. coli expressing H. influenzae nfsB or containing empty vector were inoculated into Mueller-Hinton broth with kanamycin and incubated overnight with aeration at 37°C. The resulting cultures were diluted in Mueller-Hinton broth with kanamycin to and OD600 of 0.1 AU and added as 100 μl aliquots to the wells of the prepared antibiotics plates. Plates were sealed as before and incubated at 37°C with shaking for 20 to 24 hours before measuring OD600 as above. Chloramphenicol growth inhibition at each metronidazole concentration was analyzed using GraphPad Prism (GraphPad Software, La Jolla California, USA) as before. Fractional inhibitory concentration indices (FICI scores) were calculated following growth by scoring wells with OD600 less than 0.1 AU as inhibited. FICI scores of <1 were taken as evidence for synergistic relationships, near 1 as additive, and >1 as antagonistic.
In vitro inhibition of chloramphenicol reduction
Substrate buffer was prepared with 2 mM NADPH, 10 μM FN, and 982 μM (twice the measured KM). Inhibitors metronidazole and dicoumarol were prepared in substrate buffer and added in triplicate to a UV transparent 96-well plate (Corning, No. 3635) in 50 μl aliquots with metronidazole ranging in concentration from 4096 μg/ml to 2 μg/ml and dicoumarol ranging in concentration from 1024 μg/ml to 1 μg/ml by 2-fold jumps. H. influenzae NfsB was prepared at 1 μM in Tris buffer and 50 μl aliquots were added with mixing prior to monitoring of chloramphenicol reduction at 281 nm on a Powerwave HT microplate spectrophotometer (Biotek, Inc.) with minimal interval. Velocities were normalized against wells containing no inhibitor, and inhibition dose-response curves were determined as before using GraphPad Prism (GraphPad Software, La Jolla California, USA) to find IC50 concentrations.
QUANTIFICATION AND STATISTICAL ANALYSIS
For all assays, replicate descriptions are available in the corresponding methods section as well as in the specific figure legend. Statistical analyses were carried out using GraphPad Prism with experiment-specific details available in the corresponding methods section and figure legend.
Supplementary Material
Significance:
Chloramphenicol was first identified as a potent antibiotic more than 70 years ago and since then a number of bacterial resistance mechanisms against it have been identified. However, the enzyme underling the first reported mechanism of chloramphenicol resistance, reduction of the nitro group to an amine, has remained elusive despite numerous reports of bacteria showing this activity. We describe an example of an enzyme with this activity, the oxygen insensitive nitroreductase NfsB from H. influenzae. Expression of the gene for this enzyme, or its homolog from E. cloacae, is sufficient to confer resistance to chloramphenicol in E. coli. In vitro characterization of the purified enzyme revealed its ability to fully reduce aromatic nitro groups on a variety of substrates and partially reduce the nitro groups of metronidazole and nitrofurantoin, two antibiotics that gain their ability to kill bacteria following reduction. We found that expression of the H. influenzae nitroreductase gene in E. coli augments the killing power of metronidazole and potentiates the efficacy of chloramphenicol, likely through both direct and indirect mechanisms. Finally, identification of this enzyme closes a long open chapter in chloramphenicol resistance and potentially provides a clue to the mechanistic basis of one of chloramphenicol’s serious side effects, aplastic anemia, that has prevented adoption of promising chloramphenicol analogs in the clinic.
Highlights.
Bacteria can resist chloramphenicol via reduction, but the causal genes are unknown
nfsB nitroreductase expression can confer chloramphenicol resistance in E. coli
H. influenza NfsB quickly reduces chloramphenicol to amino-chloramphenicol in vitro
Metronidazole potentiates chloramphenicol activity against nitro reducing E. coli
Acknowledgements
Support for this work came in part by awards to G.D. through the Edward Mallinckrodt, Jr. Foundation (Scholar Award), the NIH Director’s New Innovator Award (http://commonfund.nih.gov/newinnovator/), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: http://www.niddk.nih.gov/), the National Institute of General Medical Sciences (NIGMS: http://www.nigms.nih.gov/), and the National Institute of Allergy and Infectious Diseases (NIAID: https://www.niaid.nih.gov/) of the National Institutes of Health (NIH) under award numbers DP2DK098089, R01GM099538, and R01AI123394, respectively. T.S.C. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Training Grant through award number T32 DK077653 (Phillip I. Tarr, Principal Investigator) and the National Institute of Child Health and Development Training Grant through award number T32 HD049305 (Kelle H. Moley, Principal Investigator). Mass spectrometry was performed in the Metabolomics Facility at Washington University (P30 DK020579). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. We are thankful to the Caparon lab for supplying S. pyogenes genomic DNA, to Dr. Tim Wencewicz for his insightful discussions regarding enzymology and biochemistry, and to Drew Gasparrini and the rest of the Dantas lab for many helpful discussions of the work and manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References:
- Akiva E, Copp JN, Tokuriki N, and Babbitt PC (2017). Evolutionary and molecular foundations of multiple contemporary functions of the nitroreductase superfamily. Proc. Natl. Acad. Sci. U. S. A 114, E9549–E9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Even A, Noor E, Savir Y, Liebermeister W, Davidi D, Tawfik DS, and Milo R (2011). The moderately efficient enzyme: Evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50, 4402–4410. [DOI] [PubMed] [Google Scholar]
- Berendsen B, Pikkemaat M, Römkens P, Wegh R, van Sisseren M, Stolker L, and Nielen M (2013). Occurrence of chloramphenicol in crops through natural production by bacteria in soil. J. Agric. Food Chem 61, 4004–4010. [DOI] [PubMed] [Google Scholar]
- Bratton A, and Marshall EK, J. (1939). A new coupling component for sulfanilamide determination. J. Biol. Chem 128, 537–550. [Google Scholar]
- Bryant C, and DeLuca M (1991). Purification and characterization of an oxygen-insensitive NAD(P)H nitroreductase from Enterobacter cloacae. J. Biol. Chem 266, 4119–4125. [PubMed] [Google Scholar]
- Čivljak R, Giannella M, Di Bella S, and Petrosillo N (2014). Could chloramphenicol be used against ESKAPE pathogens? A review of in vitro data in the literature from the 21st century. Expert Rev. Anti. Infect. Ther 12, 249–264. [DOI] [PubMed] [Google Scholar]
- Copp JN, Mowday AM, Williams EM, Guise CP, Ashoorzadeh A, Sharrock AV, Flanagan JU, Smaill JB, Patterson AV, and Ackerley DF (2017). Engineering a Multifunctional Nitroreductase for Improved Activation of Prodrugs and PET Probes for Cancer Gene Therapy. Cell Chem. Biol 24, 391–403. [DOI] [PubMed] [Google Scholar]
- Crofts TS, Gasparrini AJ, and Dantas G (2017). Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol 15, 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts TS, Wang B, Spivak A, Gianoulis TA, Forsberg KJ, Gibson MK, Johnsky LA, Broomall SM, Rosenzweig CN, Skowronski EW, et al. (2018). Shared strategies for β-lactam catabolism in the soil microbiome. Nat. Chem. Biol 14, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingsdag SA, and Hunter N (2017). Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother 1–15. [DOI] [PubMed] [Google Scholar]
- Dinos G, Athanassopoulos C, Missiri D, Giannopoulou P, Vlachogiannis I, Papadopoulos G, Papaioannou D, and Kalpaxis D (2016). Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami F, Ebata M, and Sato R (1951). Reduction of chloromycetin by a cell-free bacterial extract and its relation to nitrite reduction. Nature 167, 118–119. [DOI] [PubMed] [Google Scholar]
- Ehrlich J, Bartz QR, Smith RM, Joslyn DA, and Burkholder PR (1947). Chloromycetin, a New Antibiotic From a Soil Actinomycete. Science 106, 417. [DOI] [PubMed] [Google Scholar]
- Eliakim-Raz N, Lador a., Leibovici-Weissman Y, Elbaz M, Paul M, and Leibovici L (2015). Efficacy and safety of chloramphenicol: joining the revival of old antibiotics? Systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, and Bairoch A (2003). ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazko AJ (1987a). Early adventures in drug metabolism. 3. Chloramphenicol. Ther. Drug Monit 9, 320–330. [DOI] [PubMed] [Google Scholar]
- Glazko AJ (1987b). Early adventures in drug metabolism: 1. Role of the Bratton-Marshall reagent. Ther. Drug Monit 9, 53–60. [PubMed] [Google Scholar]
- Glazko AJ, Dill WA, and Wolf LM (1952). Observations on the metabolic disposition of chloramphenicol (chloromycetin) in the rat. J. Pharmacol. Exp. Ther 104, 452–458. [PubMed] [Google Scholar]
- Gottlieb D, and Bhattacharyya PK (1948). Some properties of an antibiotic obtained from a species of streptomyces. J. Bacteriol 55, 409–417. [DOI] [PubMed] [Google Scholar]
- Holt R (1967). The bacterial degradation of chloramphenicol. Lancet 1, 1259–1260. [DOI] [PubMed] [Google Scholar]
- Holt DE, Hurley R, and Harvey D (1995). A reappraisal of chloramphenicol metabolism: Detection and quantification of metabolites in the sera of children. J. Antimicrob. Chemother 35, 115–127. [DOI] [PubMed] [Google Scholar]
- Kinch MS, Patridge E, Plummer M, and Hoyer D (2014). An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov. Today 19, 1283–1287. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, and Tamura K (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol 33, msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin CM, Glazko AJ, and Finland M (1959). Persistence of antibiotics in blood of patients with acute renal failure. II. Chloramphenicol and its metabolic products in the blood of patients with severe renal disease or hepatic cirrhosis. J. Clin. Invest 38, 1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, and Paterson DL (2006). Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis 6, 589–601. [DOI] [PubMed] [Google Scholar]
- Lim C, Takahashi E, Hongsuwan M, Wuthiekanun V, Thamlikitkul V, Hinjoy S, Day NP, Peacock SJ, and Limmathurotsakul D (2016). Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. Elife 5, e18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LinWu SW, Syu CJ, Chen YL, Wang AHJ, and Peng FC (2009). Characterization of Escherichia coli nitroreductase NfsB in the metabolism of nitrobenzodiazepines. Biochem. Pharmacol 78, 96–103. [DOI] [PubMed] [Google Scholar]
- Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, and Vester B (2006). The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob. Agents Chemother 50, 2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz R, and Bujard H (1997). Independent and tight regulation of transcriptional units in escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel JR, and Steers E (1953). Relationship between chloramphenicol reductase activity and chloramphenicol resistance in Escherichia coli. J. Bacteriol 66, 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermod M, Mourlane F, Waltersperger S, Oberholzer AE, Baumann U, and Solioz M (2010). Structure and function of CinD (YtjD) of Lactococcus lactis, a copper-induced nitroreductase involved in defense against oxidative stress. J. Bacteriol 192, 4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan O, Suponitzky U, Kennes Y, Chazan B, Raz R, and Colodner R (2010). Is chloramphenicol making a comeback? Isr. Med. Assoc. J 12, 371–374. [PubMed] [Google Scholar]
- O’Brien RW, and Morris JG (1971). The Ferredoxin-dependent reduction of chloramphenicol by clostridium acetobutylicum. J. Gen. Microbiol 67, 265–271. [DOI] [PubMed] [Google Scholar]
- O’Neil J (2014). The Review on Antimicrobial Resistance.
- Onderdonk AB, Kasper DL, Mansheim BJ, Louie TJ, Gorbach SL, and Bartlett JG (1979). Experimental animal models for anaerobic infections. Rev. Infect. Dis 1, 291–301. [DOI] [PubMed] [Google Scholar]
- Payne DJ, Gwynn MN, Holmes DJ, and Pompliano DL (2007). Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov 6, 29–40. [DOI] [PubMed] [Google Scholar]
- Pitsawong W, Hoben JP, and Miller AF (2014). Understanding the broad substrate repertoire of nitroreductase based on its kinetic mechanism. J. Biol. Chem 289, 15203–15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye CR, Bertin MJ, Lokey RS, Gerwick WH, and Linington RG (2017). Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. U. S. A 114, 5601–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race PR, Lovering AL, Green RM, Ossor A, White S. a, Searle PF, Wrighton CJ, and Hyde EI (2005). Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone. Reversed binding orientations in different redox states of the enzyme. J. Biol. Chem 280, 13256–13264. [DOI] [PubMed] [Google Scholar]
- Rahim NA, Cheah SE, Johnson MD, Yu H, Sidjabat HE, Boyce J, Butler MS, Cooper MA, Fu J, Paterson DL, et al. (2015). Synergistic killing of NDM-producing MDR Klebsiella pneumoniae by two “old” antibiotics-polymyxin B and chloramphenicol. J. Antimicrob. Chemother 70, 2589–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A (2008). FigTree v1.1.1: Tree figure drawing tool.
- Roldán MD, Pérez-Reinado E, Castillo F, and Moreno-Vivián C (2008). Reduction of polynitroaromatic compounds: The bacterial nitroreductases. FEMS Microbiol. Rev 32, 474–500. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Kehrenberg C, Doublet B, and Cloeckaert A (2004). Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev 28, 519–542. [DOI] [PubMed] [Google Scholar]
- Shu XO, Gao YT, Linet MS, Brinton LA, Gao RN, Jin F, and Fraumeni JF (1987). Chloramphenicol use and childhood leukaemia in Shanghai. Lancet (London, England) 2, 934–937. [DOI] [PubMed] [Google Scholar]
- Smith GN, and Worrel CS (1949). Enzymatic reduction of chloramphenicol. Arch. Biochem 24, 216–223. [PubMed] [Google Scholar]
- Smith GN, and Worrel CS (1950). The decomposition of chloromycetin (chloramphenicol) by microorganisms. Arch. Biochem 28, 232–241. [PubMed] [Google Scholar]
- Smith GN, and Worrel CS (1953). Reduction of chloromycetin and related compounds by Escherichia coli. J. Bacteriol 65, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Erwin AL, Kline T, Unrath WCT, Nelson K, Weber A, and Howald WN (2007). Chloramphenicol is a substrate for a novel nitroreductase pathway in Haemophilus influenzae. Antimicrob. Agents Chemother 51, 2820–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Joslyn DA, Gruhzit OM, McLean IW, Penner MA, and Ehrlich J (1948). Chloromycetin: Biological Studies. J. Bacteriol 55, 425–448. [PMC free article] [PubMed] [Google Scholar]
- Sood S (2016). Chloramphenicol – A potent armament against multi-drug resistant (MDR) gram negative bacilli? J. Clin. Diagnostic Res 10, DC01–DC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW (2005). Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif 41, 207–234. [DOI] [PubMed] [Google Scholar]
- Tao W, Lee MH, Wu J, Kim NH, Kim JC, Chung E, Hwang EC, and Lee SW (2012). Inactivation of chloramphenicol and florfenicol by a novel chloramphenicol hydrolase. Appl. Environ. Microbiol 78, 6295–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Pew Charitable Trusts (2016). A Scientific Roadmap for Antibiotic Discovery.
- Thompson JD, Higgins DG, and Gibson TJ (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium (2015). UniProt: a hub for protein information. Nucleic Acids Res. 43, D204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wal JM, Corpet DE, Peleran JC, and Bories GF (1983). Comparative metabolism of chloramphenicol in germfree and conventional rats. Antimicrob. Agents Chemother 24, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein RO, Condit PK, Kasper CK, Brown JW, and Morrison FR (1969). Statewide study of chloramphenicol therapy and fatal aplastic anemia. JAMA 208, 2045–2050. [PubMed] [Google Scholar]
- Wright GD (2017). Opportunities for natural products in 21 st century antibiotic discovery. Nat. Prod. Rep 00, 1–8. [DOI] [PubMed] [Google Scholar]
- Wright PM, Seiple IB, and Myers AG (2014). The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. Engl 53, 8840–8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanto Y, Hall M, and Bommarius AS (2010). Nitroreductase from Salmonella typhimurium: characterization and catalytic activity. Org. Biomol. Chem 8, 1826–1832. [DOI] [PubMed] [Google Scholar]
- Zenno S, Koike H, Kumar AN, Jayaraman R, Tanokura M, and Saigo K (1996a). Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J. Bacteriol 178, 4508–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenno S, Koike H, Tanokura M, and Saigo K (1996b). Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri. J. Biochem 120, 736–744. [DOI] [PubMed] [Google Scholar]
- (2003). Handbook of Thin-Layer Chromatography (Bosa Roca, United States: Taylor & Francis Inc; ). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


