Abstract
Background
Optimal management of patients with severe mitral stenosis (MS) and low transmitral gradient is incompletely understood.
Methods and Results
We examined 101 consecutive patients with severe rheumatic MS (mitral valve area ≤1.5 cm2) who underwent balloon valvuloplasty. Low gradient was defined as mean transmitral gradient <10 mm Hg and low flow as stroke volume index ≤35 mL/m2 by echocardiography. Symptoms and mortality data were collected. Systolic, diastolic, and arterial function were characterized by measuring left ventricular (LV) end‐systolic elastance, LV stiffness constant (β), diastolic capacitance (predicted LV end‐diastolic volume at a common LV filling pressure of 30 mm Hg), and effective arterial elastance. Low gradient (<10 mm Hg) was present in 55 patients, including low flow/low gradient in 11 and normal flow/low gradient in 44 patients, and high gradient was present in 46 patients. Participants with low‐flow/low‐gradient (LG) MS were older with higher rates of atrial fibrillation (64%) and subvalvular thickening, higher afterload, and decreased LV compliance with lower ejection fraction (57±10% versus 65±4% versus 63±6%, P=0.002) but similar end‐systolic elastance compared with patients with normal‐flow/LG and high‐gradient MS. The normal‐flow/LG group had larger mitral valve area and lower left atrial pressure by catheterization, as well as favorable long‐term outcomes compared with the low‐flow/LG and high‐gradient MS group. A total of 40% of patients with LG MS had no symptomatic benefit from valvuloplasty compared with 18% of patients with high‐gradient MS (P=0.02).
Conclusions
Presence of low gradient in patients with severe MS was associated with lesser symptomatic benefit from valvuloplasty. In the subset with low stroke volume index, this may be related to independent ventricular‐vascular uncoupling, decreased LV compliance, and high prevalence of atrial fibrillation in addition to intrinsic MS.
Keywords: mitral stenosis, valvuloplasty, echocardiography, heart valves, mitral valve
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Valvular Heart Disease, Echocardiography
Clinical Perspective
What Is New?
Among patients with severe mitral stenosis, a subset exists with low output and low transmitral gradient.
Patients with low‐flow, low‐gradient severe mitral stenosis had higher arterial afterload, greater prevalence of atrial fibrillation, more subvalvular thickening, and decreased left ventricular compliance compared with those with high‐gradient mitral stenosis.
What Are the Clinical Implications?
Patients with low‐flow, low‐gradient severe mitral stenosis had less symptomatic improvement after valvuloplasty; mitral valve gradient rather than area had the best predictive value for symptomatic improvement.
Mitral stenosis (MS) is characterized by an elevation in left atrial (LA) pressure as a result of impairments in mitral valve opening and LA emptying. Severe MS is defined by a mitral valve area (MVA) ≤1.5 cm2, but even with this degree of narrowing, mean mitral gradients (MGs) can vary considerably given their dependence on flow (stroke volume [SV]) and heart rate.1, 2, 3 This is relevant to patients with MS where SV is impacted by the fixed reduction in left ventricular (LV) preload from mitral valvular impedance to LV filling.4, 5, 6, 7, 8 SV can additionally be affected by direct extension of the inflammatory rheumatic process to the subvalvular apparatus and adjacent basal myocardium, impeding ventricular systolic and diastolic performance.8 Furthermore, the development of atrial fibrillation (AF) or pulmonary hypertension, secondary to MS or common age‐related comorbidities such as hypertension and coronary disease, can all independently affect cardiac performance lowering SV.9, 10 Whether patients with MS with a low gradient can also have functionally severe MS that responds to valvuloplasty is unclear, prompting this investigation.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. Sharing patient data is subject to the limitations of informed consent and approval by the Mayo Clinic's institutional review board.
Study Population
This study was approved by the Mayo Clinic's institutional review board and included consecutive patients with severe inflammatory MS who underwent mitral valvuloplasty between January 1, 2002, and December 31, 2011. Exclusion criteria included patients younger than 18 years, greater than moderate mitral/aortic regurgitation, greater than mild aortic stenosis, calcific MS, presence of prosthetic valves, ejection fraction (EF) <45%, complex congenital heart disease, constrictive pericarditis, and/or hypertrophic cardiomyopathy. Patients’ medical records were reviewed for baseline characteristics, symptoms, echocardiographic data, and cardiac catheterization data. Symptoms were assessed by chart review. Documentation included New York Heart Association class before valvuloplasty and at first follow‐up after the procedure in all patients; change in New York Heart Association class was used to document improvement. Mortality was assessed by reviewing medical records and from the Social Security Death Index.
Echocardiographic Data
Comprehensive 2‐dimensional and Doppler echocardiographic data were acquired before mitral balloon valvuloplasty in accordance with American Society of Echocardiography guidelines1 and interpreted by Mayo Clinic echocardiographers. For patients with AF, at least 5 cycles were averaged for all measurements. MVA was calculated using echocardiographic pressure half‐time (PHT) from the continuous‐wave Doppler signal across the mitral inflow, according to guideline recommendations3 and was considered severe if MVA was ≤1.5 cm2. MVA was also determined by planimetry when feasible and by continuity equation when left‐sided valvular regurgitation was mild or less. Patients were considered to have low gradient if their mean diastolic transmitral gradient from transthoracic echocardiography was <10 mm Hg. SV index (SVI) was calculated from the LV outflow tract diameter and tissue velocity integral and was considered to be low flow when SVI was ≤35 L/min per m2. LV end‐systolic and end‐diastolic volumes were determined by standard echocardiographic methods.
Cardiac Catheterization
All patients in this study underwent invasive hemodynamic assessment on their chronic medications following minimal sedation at the time of valvuloplasty. Right heart catheterization was performed using fluid‐filled balloon‐tipped catheters. Right atrial and pulmonary artery pressures were measured at end expiration (mean of ≥3 beats). Cardiac output (CO) was measured using thermodilution method. Left heart catheterization was performed by transseptal puncture from the right common femoral vein with an 8F Mullins sheath to measure direct LA pressure and a balloon wedge catheter through the Mullins sheath to measure LV pressure. Pulmonary vascular resistance was assessed by (mean pulmonary artery−LA pressure)/CO. MVA was calculated using the Gorlin formula. All hemodynamic measurements were repeated following valvuloplasty.
Single‐Beat Pressure Volume Relationships
The LV end‐diastolic pressure, end‐diastolic volume relationship (end‐diastolic pressure=αend‐diastolic volumeβ) was assessed using invasive LV end‐diastolic pressure and echocardiographic LV volumes according to the single‐beat method of Klotz et al.11 This analysis yields the LV stiffness constant β (which is directly proportional to LV chamber stiffness) and scaling constant α. From these parameters, predicted LV end‐diastolic volume at a common LV filling pressure of 30 mm Hg was calculated, which provides a measure of diastolic chamber capacitance (predicted LV end‐diastolic volume at a common LV filling pressure of 30 mm Hg decreases as diastolic LV chamber stiffness increases). The LV end‐systolic elastance (Ees), which provides a load‐independent measure of LV contractility, was calculated using the single‐beat method of Chen.12 Effective arterial elastance, a composite measure of steady state and pulsatile load was calculated as end‐systolic pressure/SV.13 Ventricular vascular coupling was calculated as the ratio of effective arterial elastance to Ees.14
Statistical Analysis
Normally distributed variables are reported as mean±SD. Continuous normally distributed variables were compared using t test. For non‐normally distributed variables, the Wilcoxon rank sum test was used. Categorical variables between groups were compared using chi‐square or Fisher exact tests and continuous variables between groups were compared using Tukey test. Paired t test was used to compare hemodynamics before and after valvuloplasty. Linear regression and Pearson correlation were used to determine linear relationships between variables of interest. Survival after valvuloplasty was determined by Kaplan–Meier curves and the log‐rank test. Statistical analysis was performed with JMP version 13.0. Statistical significance was assumed at P<0.05.
Results
Baseline Characteristics
A total of 101 consecutive patients with severe MS who underwent mitral balloon valvuloplasty were included. Low gradient (<10 mm Hg) was present in 55 (54%) patients, including low flow /low gradient in 11 (11%) and normal flow/low gradient in 44 (44%), and high gradient was present in 46 (46%). The baseline characteristics and echocardiographic data of low‐gradient (LG) MS compared with high‐gradient (HG) MS are summarized in Table 1, and the catheterization data are summarized in Table 2. Additionally, 68 of the patients had MVA by continuity, and 46 had MVA by planimetry. MVA using PHT demonstrated significant correlation with catheterization‐derived MVA by Gorlin (r=+0.5, P<0.0001), and echocardiographic measurements by planimetry (r=+0.4, P=0.002) and continuity (r=+0.4, P=0.0005).
Table 1.
LG Versus HG MS
| LG MS (n=55) | HG MS (n=46) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 65±10 | 56±13 | 0.0001 |
| Women, % | 93 | 92 | 0.8 |
| BMI, kg/m2 | 29.7±6.2 | 28.6±5.5 | 0.4 |
| BSA, m2 | 1.85±0.21 | 1.82±0.16 | 0.4 |
| Smoker, % | 9 | 19 | 0.1 |
| Rheumatic heart disease, % | 98 | 98 | 0.3 |
| Methysergide, % | 2 | 0 | |
| Systemic lupus, % | 0 | 2 | |
| Symptoms | |||
| NYHA class II, % | 30 | 13 | 0.03 |
| NYHA class III, % | 70 | 87 | |
| Permanent AF, % | 26 | 9 | 0.02 |
| Paroxysmal/ permanent AF, % | 58 | 26 | 0.0008 |
| Comorbidities | |||
| Hypertension, % | 46 | 43 | 0.8 |
| Diabetes mellitus, % | 16 | 11 | 0.4 |
| Obstructive sleep apnea, % | 7 | 9 | 0.8 |
| COPD, % | 7 | 13 | 0.3 |
| Stroke, % | 12 | 4 | 0.1 |
| Coronary artery disease, % | 7 | 11 | 0.5 |
| Medication | |||
| β‐Blocker, % | 68 | 40 | 0.004 |
| ACEI/ARB, % | 35 | 32 | 0.7 |
| CCB, % | 16 | 8 | 0.3 |
| Digoxin, % | 24 | 19 | 0.6 |
| Diuretic, % | 53 | 50 | 0.8 |
| Echocardiography | |||
| EF, % | 63±7 | 63±6 | 0.8 |
| Heart rate, bpm | 68±10 | 77±11 | <0.0001 |
| SV, mL | 84±22 | 73±12 | 0.005 |
| SVI, mL/m2 | 46±12 | 40±7 | 0.005 |
| CO, L/min | 4.7±1.0 | 4.9±1.2 | 0.3 |
| Cardiac index, L/min per m2 | 3.0±0.7 | 3.1±0.5 | 0.6 |
| MAP, mm Hg | 107±14 | 103±15 | 0.2 |
| RVSP | 46±15 | 63±22 | 0.03 |
| Abascal score | 8±2 | 7±2 | 0.3 |
| Mean gradient, mm Hg | 7±2 | 14±4 | <0.0001 |
| PHT, ms | 177±26 | 190±44 | 0.05 |
| MVA (PHT), cm2 | 1.27±0.18 | 1.21±0.24 | 0.1 |
| MVA (planimetry), cm2 (n=46) | 1.42±0.52 | 1.34±0.48 | 0.6 |
| MVA (continuity), cm2 (n=68) | 1.26±0.36 | 1.03±0.23 | 0.002 |
| Mitral regurgitation, % | |||
| None/trivial | 24 | 26 | 0.9 |
| Mild | 53 | 54 | |
| Mild‐moderate | 23 | 20 | |
ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; BSA, body surface area; CCB, calcium channel blocker; CO, cardiac output; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; HG, high‐gradient; LG, low‐gradient; MAP, mean arterial pressure; MS, mitral stenosis; MVA, mitral valve area; NYHA, New York Heart Association; PHT, pressure half‐time; RVSP, right ventricular systolic pressure; SV, stroke volume; SVI, stroke volume index.
Table 2.
Outcomes of Valvuloplasty in LG Versus HG MS
| LG MS (n=55) | HG MS(n=46) | P Value | |
|---|---|---|---|
| Catheterization prevalvuloplasty | |||
| Cardiac index, L/min per m2 | 2.5±0.5 | 2.7±0.6 | 0.2 |
| SVI, mL/m2 | 37±9 | 35±7 | 0.3 |
| LA pressure, mm Hg | 20±5 | 24±7 | 0.0002 |
| LVEDP, mm Hg | 15±5 | 13±4 | 0.04 |
| MG, mm Hg | 8±2 | 12±4 | <0.0001 |
| MVA, cm2 | 1.5±0.4 | 1.3±0.4 | 0.005 |
| Mean PAP, mm Hg | 31±9 | 42±12 | <0.0001 |
| PVR, WU | 2.6±1.4 | 3.6±2.0 | 0.008 |
| Postvalvuloplasty | |||
| Cardiac index, L/min per m2 | 2.7±0.6 | 2.9±0.6 | 0.2 |
| SVI, mL/m2 | 39±10 | 37±9 | 0.4 |
| LA pressure, mm Hg | 18±5 | 19±6 | 0.2 |
| LVEDP, mm Hg | 16±5 | 15±5 | 0.3 |
| MG, mm Hg | 4±2 | 6±2 | <0.0001 |
| MVA, cm2 | 2.2±0.6 | 2.0±0.6 | 0.3 |
| ≥Moderate MR, % | 16 | 13 | 0.7 |
| Mean PAP, mm Hg | 29±8 | 36±12 | 0.002 |
| PVR, WU | 2.6±1.2 | 3.2±1.5 | 0.1 |
| Change | |||
| ∆ Cardiac index, L/min per m2 | +0.1±0.4 | +0.3±0.4 | 0.2 |
| ∆ SVI, mL/m2 | +3.0±7.3 | +2.9±6.9 | 0.9 |
| ∆ LA pressure, mm Hg | −2±4 | −6±7 | 0.003 |
| ∆ LVEDP, mm Hg | +1±5 | +1±4 | 0.4 |
| ∆ MG, mm Hg | −3±2 | −6±4 | 0.0001 |
| ∆ MVA, cm2 | +0.7±0.5 | +0.8±0.5 | 0.2 |
| ∆ Mean PAP, mm Hg | −3±6 | −5±9 | 0.3 |
| ∆ PVR, WU | −0.1±0.8 | +0.0±1.3 | 0.8 |
| ∆ NYHA class | −0.9±0.9 | −1.3±0.8 | 0.02 |
| No symptom improvement, % | 40 | 18 | 0.02 |
LA indicates left atrial; LG, low‐gradient; HG, high‐gradient; LVEDP, left ventricular end‐diastolic pressure; MG, mean gradient; MR, mitral regurgitation; MS, mitral stenosis; MVA, mitral valve area; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; SVI, stroke volume index; Δ, change.
When compared with HG MS, patients with LG MS were older (P=0.0001) and had higher prevalence of AF (P=0.0008), higher use of β‐blockers, and lower baseline heart rate (P<0.0001) compared with patients with HG MS. LG MS was associated with lower mean LA pressure but higher LV end‐diastolic pressure compared with HG MS. Mean pulmonary artery pressure, pulmonary vascular resistance, and MVA were all less abnormal in patients with LG MS.
Response to Valvuloplasty
Both LG MS and HG MS groups responded to valvuloplasty on average, with an improvement in mean MVA by +0.7±0.5 versus +0.8±0.5 cm2 (P=0.2), SVI by +3.0±7.3 versus +2.9±6.9 mL/m2 (P=0.9), and forward cardiac index by +0.1±0.4 versus +0.3±0.4 L/min per m2 (P=0.2), with no differences between both groups (Table 2). The MG and LA pressure decreased less in the LG groups (∆MG −3±2 versus −6±4 mm Hg [P=0.0001], ∆LA −2±4 versus −6±7 mm Hg [P=0.003]) following valvuloplasty. There was no difference in postvalvuloplasty ≥ moderate mitral regurgitation between the 2 groups (16% versus 13%, P=0.7). At a median follow‐up of 30 months for symptom reassessment (interquartile range, 5–52), a preprocedure low gradient was coupled with less improvement in New York Heart Association class, with 40% of LG MS having no symptom improvement following valvuloplasty compared with only 18% of those with HG MS (P=0.02) (Figure 1).
Figure 1.
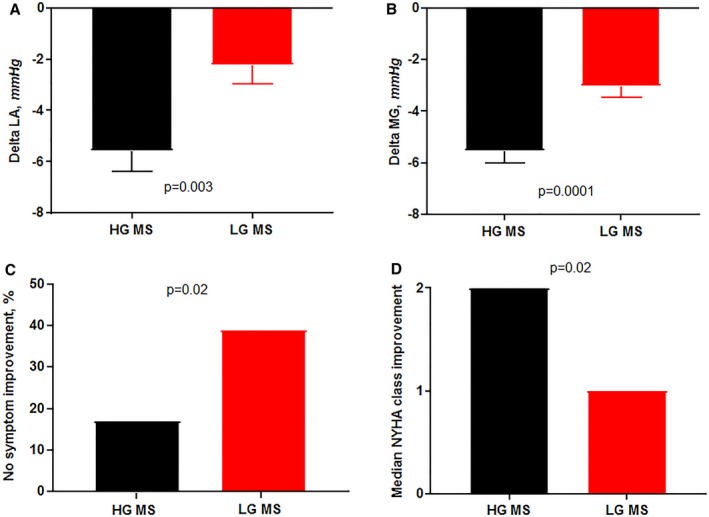
Response to valvuloplasty by preprocedure mitral gradient. Low‐gradient (LG) mitral stenosis (MS) was associated with a smaller drop in left atrial (LA) pressure (A) and mean gradient (MG) (B). These patients also had a higher proportion of nonresponders (C) and worse functional class improvement (D). HG indicates high‐gradient; LG, low‐gradient; NYHA, New York Heart Association.
Hemodynamic Subsets of LG MS
Normal‐flow, LG MS
Compared with low‐flow (LF)/LG and HG MS, the 44 patients with normal‐flow (NF)/LG MS had the highest catheterization‐derived MVA (1.5±0.4 versus 1.2±0.3 cm2 versus 1.3±0.4 cm2, P=0.002) and lowest LA pressure (20±6 versus 21±4 versus 24±7 mm Hg, P=0.001) at baseline during catheterization, as well as the lowest mortality consistent with less severe MS (Figure 2).
Figure 2.
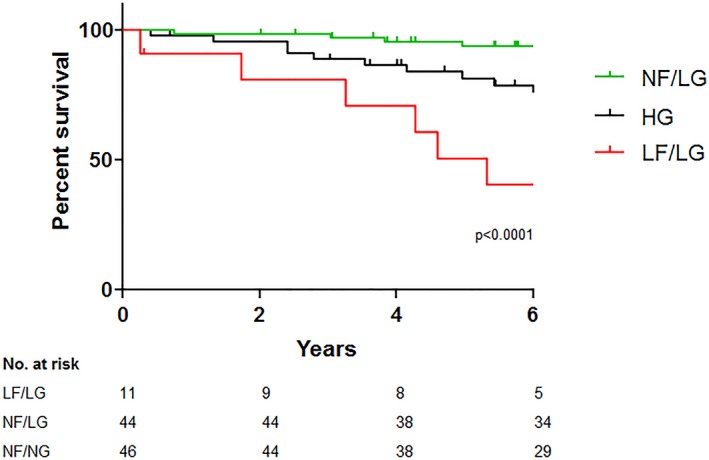
Survival of low‐gradient (LG) subtypes vs high‐gradient (HG) mitral stenosis (MS). Low‐flow (LF), LG MS was associated with the poorest survival, normal‐flow (NF), LG MS with the best survival, with HG MS having intermediate survival between the 2 LG groups.
Low‐flow, LG MS
Compared with other groups, the 11 patients in the LF/LG group were older (P<0.0001) with less female predominance. AF (91%, P=0.0002) and previous stroke (55%, P<0.0001) were more prevalent in the LF/LG group (Table 3). The heart rates (73±12 versus 78±12, P=0.2) at baseline were similar between the LF/LG MS and HG MS groups. The LF/LG group also had the highest prevalence of grade ≥2 subvalvular thickening based on the Abascal‐Wilkins scoring system (100%). Catheterization‐derived SVI and MG differences among groups (Table 3) were similar to those defined by echocardiography. Moreover, the LF/LG group had lower catheterization‐derived SVI with a low MG of 8±3 mm Hg despite a catheterization‐derived MVA in the severe range (1.2±0.3 cm2) (Table 3).
Table 3.
LF/LG Versus HG MS
| LF/LG MS (n=11) | NF/LG MS (n=44) | HG MS (n=46) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 73±7‡ | 63±10‡ | 56±13‡ | <0.0001 |
| Women, % | 73 | 98 | 91 | 0.04 |
| BMI, kg/m2 | 28.2±4.9 | 30.2±6.6 | 28.6±5.5 | 0.4 |
| Paroxysmal/permanent AF, % | 91 | 50 | 26 | 0.0002 |
| Hypertension, % | 64 | 43 | 43 | 0.4 |
| Diabetes mellitus, % | 36 | 11 | 11 | 0.4 |
| Stroke, % | 55 | 2 | 4 | <0.0001 |
| Coronary artery disease, % | 18 | 5 | 11 | 0.2 |
| NYHA class (II/III), % | 18/82 | 34/66 | 11/89 | 0.02 |
| Echocardiography | ||||
| Heart rate, bpm | 73±12 | 71±12† | 78±12 | 0.03 |
| LV end‐diastolic diameter, cm | 43±4 | 48±5‡ | 45±5 | 0.001 |
| LV end‐systolic diameter, cm | 30±5 | 30±4 | 28±4 | 0.09 |
| EF, % | 57±10‡ | 65±4 | 63±6 | 0.002 |
| LV mass index, g/m2 | 81±22 | 90±21 | 85±22 | 0.3 |
| Relative wall thickness, cm | 0.47±0.09 | 0.41±0.05 | 0.43±0.09 | 0.06 |
| LA volume index, mL/m2 | 60±13 | 56±17 | 59±17 | 0.6 |
| Abnormal RV function, % | 18 | 7 | 24 | 0.06 |
| Abnormal RV size, % | 27 | 9 | 24 | 0.09 |
| SVI, mL/m2 | 30±3‡ | 50±10‡ | 40±7‡ | <0.0001 |
| Cardiac index, L/min per m2 | 2.3±0.3‡ | 3.2±0.6 | 3.1±0.5 | <0.0001 |
| E wave, m/s | 2.1±0.3 | 1.9±0.3† | 2.4±0.4 | <0.0001 |
| Medial e′, cm/s | 4±2 | 5±1 | 5±2 | 0.6 |
| RV systolic pressure, mm Hg | 46±15 | 45±9 | 63±23‡ | <0.0001 |
| Abascal score | 9±2‡ | 7±1 | 7±2 | 0.02 |
| Subvalvular thickening | 100 | 82 | 44 | 0.03 |
| MG, mm Hg | 8±1 | 7±2 | 14±4‡ | <0.0001 |
| PHT, ms | 195±30 | 172±23 | 190±44 | 0.04 |
| MVA (PHT), cm2 | 1.16±0.17 | 1.30±0.17 | 1.21±0.24 | 0.06 |
| MR, % | ||||
| None/trivial | 27 | 23 | 26 | 0.7 |
| Mild | 36 | 57 | 54 | |
| Mild‐moderate | 37 | 20 | 20 | |
| Catheterization | ||||
| Mean atrial pressure, mm Hg | 110±10 | 106±15 | 103±15 | 0.4 |
| Cardiac index, L/min per m2 | 2.0±0.4‡ | 2.6±0.5 | 2.7±0.6 | 0.003 |
| SVI, mL/m2 | 27±5‡ | 39±8 | 35±7 | 0.0004 |
| LA pressure, mm Hg | 21±4 | 20±6† | 24±7 | 0.001 |
| LVEDP, mm Hg | 15±3 | 16±5 | 13±4 | 0.08 |
| MG, mm Hg | 8±3 | 8±2 | 12±4‡ | <0.0001 |
| MVA, cm2 | 1.2±0.3 | 1.5±0.4† | 1.3±0.4 | 0.002 |
| Mean PAP, mm Hg | 35±7 | 31±9† | 42±12 | <0.0001 |
| PVR, WU | 3.6±1.7 | 2.4±1.3† | 3.7±2.0 | 0.006 |
| Ventricular vascular coupling | ||||
| Ea, mm Hg/mL | 2.1±0.5‡ | 1.3±0.3‡ | 1.5±0.3‡ | <0.0001 |
| Ees, mm Hg/mL | 1.9±0.5 | 2.1±0.7 | 1.9±0.6 | 0.3 |
| Ea/Ees | 1.12±0.22* | 0.63±0.11* | 0.84±0.24* | <0.0001 |
| Stiffness constant β | 0.61±0.13* | 0.48±0.13 | 0.540.14 | 0.007 |
| V30, mL | 93±21 | 121±27‡ | 106±25 | 0.003 |
AF indicates atrial fibrillation; BMI, body mass index; bpm, beats per minute; Ea, effective arterial elastance; Ea/Ees, effective arterial elastance/end‐systolic elastance ventricular vascular coupling; Ees, end‐systolic elastance; EF, ejection fraction; LA, left atrial; LF, low‐flow; LV, left ventricular; LVEDP, left ventricular end‐diastolic pressure; MG, mean gradient; MR, mitral regurgitation; MVA, mitral valve area; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PHT, pressure half‐time; PVR, pulmonary vascular resistance; RV, right ventricular; SVI, stroke volume index; V30, predicted left ventricular end‐diastolic volume at a common left ventricular filling pressure of 30 mm Hg.
*P<0.05 vs normal‐flow (NF), low‐gradient (LG) mitral stenosis (MS), † P<0.05 vs high‐gradient (HG) MS, ‡ P<0.05 vs all.
Arterial afterload as assessed by effective arterial elastance was highest in patients with LF/LG MS (2.1±0.5 versus 1.3±0.3 versus 1.5±0.3, P<0.0001) (Table 3, Figure 3). Moreover, SVI was inversely related to effective arterial elastance and lower in AF (Figure 4). Patients with LF/LG MS had the lowest EF (57±10% versus 65±4% versus 63±6%, P=0.002). Despite the high afterload in patients with LF/LG MS, Ees was similar across groups indicating that reduced systolic performance was related to afterload mismatch and ventricular‐vascular uncoupling rather than intrinsic contractile dysfunction. Patients with LF/LG MS had reduced ventricular capacitance (predicted LV end‐diastolic volume at a common LV filling pressure of 30 mm Hg) (93±21 versus 121±27 versus 106±25, P=0.003) and increased chamber stiffness (β) (0.61±0.13 versus 0.48±0.13 versus 0.540.14, P=0.007). All groups had similar LV end‐diastolic pressure (Table 3). During median follow‐up of 7.1 years (interquartile range, 4.2–10.0), patients with LF/LG MS had the worst survival of all groups (Figure 2).
Figure 3.
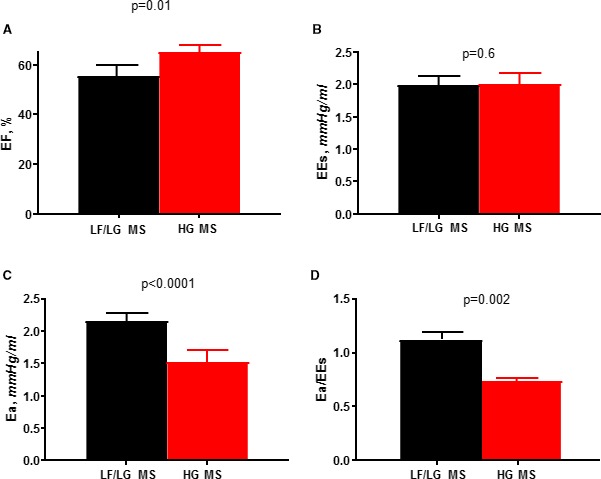
Loading conditions and systolic performance in low‐flow (LF), low‐gradient (LG) severe mitral stenosis (MS). LF/LG MS was associated with a decrease in myocardial performance as assessed by ejection fraction (EF) (A). However, load‐independent systolic function as assessed by end‐systolic elastance (Ees) (B) was preserved, suggesting that the decrease in EF was load dependent with a higher afterload (effective arterial elastance [Ea]) (C) and ventricular‐vascular uncoupling (D). HG indicates high‐gradient.
Figure 4.
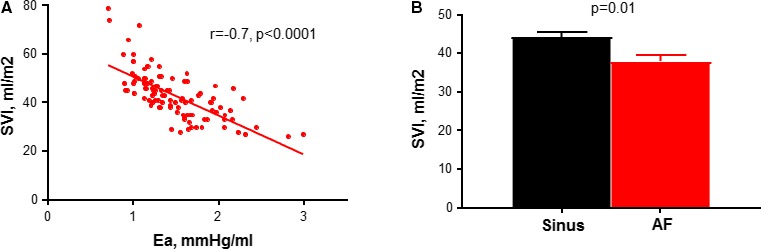
Determinants of stroke volume in severe mitral stenosis. Stroke volume index (SVI) decreased with higher effective arterial elastance (afterload) (A) and atrial fibrillation (AF) (B). Ea indicates effective arterial elastance.
Predictors of symptom response
On logistic regression to identify predictors of poor symptom improvement with valvuloplasty among all patients with severe MS, a gradient <10 mm Hg by Doppler echocardiography best identified poor responders (area under the curve 0.641, P=0.01) (Table 4). In addition, an elevated LV end‐diastolic pressure >15 mm Hg and a higher β stiffness coefficient were predictive of worse symptom response. Notably, MVA by catheterization or echocardiography and baseline LA pressure did not predict symptom response. Although mean gradient was significantly higher in responders than nonresponders, there was significant overlap between groups (Figure 5). MVA was similar between responders and nonresponders by both echocardiography (1.2±0.2 versus 1.3±0.2 cm2, P=0.3) and catheterization (1.4±0.4 versus 1.3±0.4, P=0.5). All patients with a mean gradient ≥15 mm Hg derived symptomatic benefit from valvuloplasty (Figure 5).
Table 4.
Univariate Preprocedure Predictors of Symptom Improvement With Valvuloplasty
| Predictor | OR (95% CI) | AUC | Optimal Cut Point | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 0.97 (0.93–1.01) | 0.593 | 50 | 0.07 |
| BMI | 0.91 (0.84–0.99) | 0.638 | 32.1 | 0.02 |
| AF | 0.33 (0.12–0.96) | 0.590 | ··· | 0.04 |
| Echocardiography | ||||
| MG | 1.18 (1.02–1.36) | 0.641 | 10 | 0.01 |
| Low gradient <10 mm Hg | 0.33 (0.13–0.85) | 0.630 | ··· | 0.02 |
| MVA | 0.32 (0.04–2.57) | 0.564 | ··· | 0.3 |
| SVI | 0.99 (0.95–1.04) | 0.521 | ··· | 0.7 |
| LA volume index, mL/m2 | 1.02 (0.99–1.05) | 0.576 | ··· | 0.2 |
| RV systolic pressure | 0.99 (0.98–1.02) | 0.495 | ··· | 0.9 |
| LV mass index | 0.99 (0.97–1.01) | 0.525 | ··· | 0.4 |
| EF | 1.01 (0.94–1.08) | 0.493 | ··· | 0.8 |
| Catheterization | ||||
| Ees | 0.83 (0.63–1.10) | 0.589 | ··· | 0.2 |
| Ea | 0.72 (0.27–1.92) | 0.551 | ··· | 0.5 |
| Ea/Ees | 1.27 (0.11–14.12) | 0.569 | ··· | 0.9 |
| Stiffness constant β | 0.40 (0.16–0.97) | 0.735 | 6 | 0.02 |
| MVA | 1.45 (0.48–4.41) | 0.545 | ··· | 0.5 |
| MG | 1.13 (0.99–1.28) | 0.610 | 9 | 0.05 |
| LA pressure | 0.97 (0.90–1.04) | 0.572 | ··· | 0.3 |
| LV end‐diastolic pressure | 0.84 (0.75–0.93) | 0.735 | 15 | 0.0005 |
AF indicates atrial fibrillation; AUC, area under the curve; BMI, body mass index; Ea, effective arterial elastance; Ea/Ees, effective arterial elastance/end‐systolic elastance ventricular vascular coupling; Ees, end‐systolic elastance; EF, ejection fraction; LA, left atrial; LV, left ventricular; MG, mean gradient; MVA, mitral valve area; OR, odds ratio; RV, right ventricular; SVI, stroke volume index.
Figure 5.
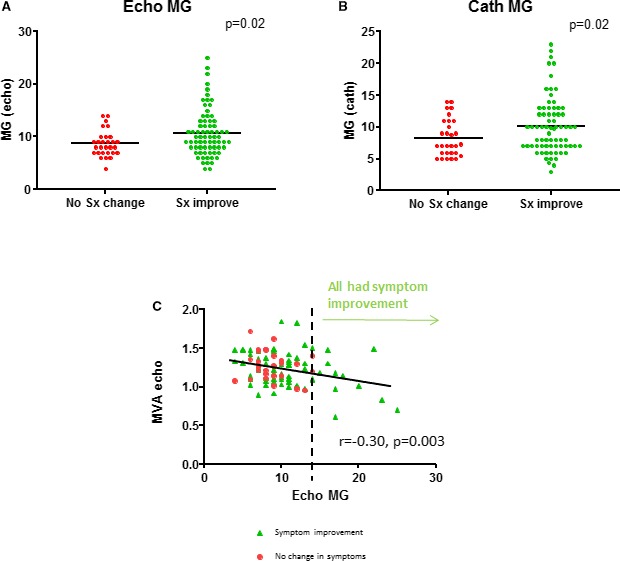
In both echocardiography (A) and catheterization (B), the mean gradient (MG) was significantly higher in responders than nonresponders, with significant scatter and overlap. The mitral valve area (MVA) and gradient demonstrated poor correlation, with an MG ≥15 mm Hg associated with most symptomatic benefit from valvuloplasty (C). Sx indicates symptom.
Discussion
The presence of LG MS was associated with lesser symptomatic improvement after valvuloplasty compared with HG MS. LF/LG was associated with a distinct constellation of findings, similar to what is seen in paradoxical LF/LG aortic stenosis,15 including high arterial afterload with ventricular‐vascular uncoupling, high prevalence of AF, and decreased LV compliance with subvalvular thickening (Figure 6). Although the EF was lower in LF/LG, this did not reflect a reduction in intrinsic contractility (Ees) but was related to loading conditions, which is associated with decreased SV and mean gradient. This raises the possibility that these patients could have pseudosevere MS with symptoms driven by arterial stiffness and ventricular‐vascular uncoupling, AF, and decreased LV compliance rather than intrinsic true severe MS, which are not addressed by valvuloplasty. This could explain the decreased symptomatic benefit in these patients. On the other hand, patients with NF/LG MS had higher catheterization‐derived MVA and lower baseline LA pressure, suggesting that this entity represents less than severe MS that may not benefit from valvuloplasty. These hemodynamic phenotypes (LF/LG MS and NF/LG MS) provide new insight into why some patients with MS and a low gradient extract smaller benefits from valvuloplasty. In addition, in patients with MS overall, we were unable to demonstrate a predictive value to MVA whether by catheterization or echocardiography to predict symptomatic improvement following valvuloplasty. The mitral gradient best identified patients likely to respond, suggesting that the gradient should be the key determinant of symptomatic severe MS that is likely to respond to therapy.
Figure 6.
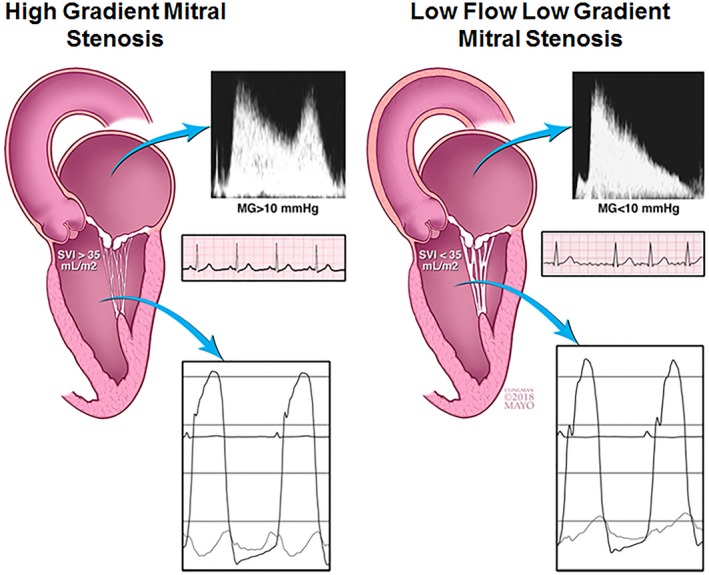
Stroke volume determinants in patients with low‐flow (LF), low‐gradient (LG) (right) vs high‐gradient mitral stenosis (MS) (left). Patients with LF/LG MS have prevalent atrial fibrillation, subvalvular thickening, and higher afterload caused by increased arterial elastance and decreased ventricular compliance. MG indicates mean gradient; SVI, stroke volume index.
Low‐Flow, LG Severe MS
The hemodynamic manifestation of MS has typically been described as an elevated LA pressure along with reduced CO as a result of the obstruction across the stenotic valve.5, 16, 17 However, there has been a shift in the demographic characteristics of MS in the Western world.9 The mean age of patients with LF/LG MS was 73 years compared with 58 years in older epidemiologic studies of patients with rheumatic MS.18 Age‐related cardiovascular changes including aortic stiffening and LV noncompliance, similar to what is seen in heart failure with preserved EF, may confound the presentation of rheumatic MS. Despite the long‐held theory that rheumatic MS is an isolated disease of the mitral valve, a number of preliminary studies have identified LV myocardial abnormalities and an increased afterload in a subset of patients with MS,7, 19 as well as a low gradient in some patients with pathologically confirmed severe MS.20, 21 However, the intersection of these abnormalities and relationship with SV has not been well described.
This study suggests that the hemodynamic and symptomatic benefit from valvuloplasty may be limited in some patients with a mean gradient <10 mm Hg. The mechanism of this lack of response to valvuloplasty can be explained according to the hemodynamic profile with unique characteristics present in patient with LF/LG MS. An increase in arterial afterload was demonstrated in the LF/LG group similar to what has been described in patients with paradoxical LF/LG aortic stenosis.13, 22, 23 This was associated with lower EF, suggesting myocardial dysfunction, which could be related to either intrinsic contractile dysfunction or afterload mismatch. Several previous studies have postulated mechanisms for the decreased myocardial performance in rheumatic MS including chronic myocardial inflammation and basal tethering by subvalvular remodeling.4, 6, 24, 25, 26, 27, 28 In our study, however, since intrinsic contractility, as defined by the gold‐standard Ees, was not decreased and since EF is load dependent,22, 29 the increased afterload likely accounts for the apparent decrease in systolic performance and SV.
We are unable to determine from our cross‐sectional study whether the increase in afterload is the cause or consequence of the low CO and SV, since a low CO can result in neurohormonal and sympathetic activation resulting in peripheral vasoconstriction similar to patients with systolic heart failure.30 The older age of the LF/LG population is also consistent with greater age‐related vascular stiffening. Several smaller studies have previously explored the determinants of myocardial performance in severe MS but have not systematically correlated this with flow and gradient patterns. Gash et al7 proposed that patients with severe MS had high afterload with reduced preload caused by the transmitral obstruction, offsetting adequate preload‐dependent Frank‐Starling compensation. Others showed that the elevated afterload persisted even after valvuloplasty despite the increase in preload.21
Moreover, our study showed that patients with severe LF/LG MS had a higher prevalence of AF, suggesting that the loss of atrial contribution to filling could be a factor in their reduced SV. AF in MS has been shown to impact LV preload and CO as a result of inefficient ventricular filling from the irregular cycle length and the loss of the atrial kick.31, 32 Our study also showed that patients with LF/LG MS have decreased LV compliance (increased β and decreased predicted LV end‐diastolic volume at a common LV filling pressure of 30 mm Hg). This could be related to age‐related changes that can occur in patients with LF/LG MS, but can also be associated with the prevalent subvalvular thickening seen in that group. Contrary to prior studies,33 high pulmonary vascular resistance and right ventricular dysfunction did not appear to be a major cause of low SV in the patients with LF/LG MS. Older age, AF, prior stroke, arterial stiffness, and decreased LV compliance remain unaltered by the valvuloplasty procedure and likely contributed to worse long‐term outcomes in patients with LG MS.
Normal‐Flow LG MS
The NF/LG group had higher MVA and lower LA pressure by catheterization compared with the HG MS group. This group had a lesser degree of reduction in MG and LA pressure after valvuloplasty, and had the most favorable long‐term outcomes. This suggests that this group represents a less severe form of MS. Such patients may benefit from exercise testing to better assess the functional response and mean gradient of the valve before proceeding with invasive valvuloplasty.
Clinical Implications
Among these patients with MS overall, the MVA, whether measured by echocardiography or by catheterization, did not predict symptom improvement. This, in addition to a recent study on aortic stenosis,34 suggests that the assumptions underlying the valve area calculations may be less accurate in predicting functional significance compared with gradient measurements.
Given the decreased hemodynamic benefit afforded by valvuloplasty in patients with LG MS, functional assessment with pharmacological manipulation of arterial afterload with vasodilator testing may help determine which of the patients with LF/LG MS have true versus pseudosevere MS and enable better selection of candidates who are likely to benefit from valvuloplasty.22, 23 For patients with pseudosevere MS, simple afterload reduction to improve vascular stiffness may be all that is indicated to improve filling pressures and symptoms. This requires prospective study and future investigation. In addition, the high prevalence of AF in the LF/LG MS group suggests that AF may be a viable target for intervention with cardioversion or rhythm control in these patients, with the goal being improvement in LV preload, myocardial performance and exercise capacity.
Limitations
There is selection bias in our study population since only patients undergoing valvuloplasty procedures were included. Echocardiographic and catheterization measurements were not simultaneous, but, with a median time difference of only 6 days and with both studies performed in the fasting state and without active diuresis or medication changes, this is unlikely to have significantly altered our results. MVA using PHT was the only method feasible in all patients in this study and was therefore used for standardized measurement of valve area. PHT can be shortened by LV noncompliance, thereby falsely elevating MVA.35 However, this would only have been expected to overestimate MVA in our patients with LF MS, but, since all of them had an MVA in the severe area at baseline, this should not have affected their inclusion in the study sample. Although there is no true gold standard for MVA measurement, PHT valve area correlated well with all other methods of valve area assessment. This study included patients with predominantly rheumatic MS. Future studies should seek to establish whether similar hemodynamics of LG MS with ventricular‐vascular uncoupling and LV diastolic dysfunction occur in calcific degenerative MS.
Conclusions
Patients with LG MS display less benefit from valvuloplasty despite valve intervention. This could be related to the presence of less‐than‐severe MS (NF/LG) or pseudosevere MS in the LF/LG subset, which was characterized by high arterial afterload, prevalent AF, and decreased LV compliance. Future studies using exercise testing are needed to determine whether we can better differentiate which patients with LG MS may be better responders to mitral valve intervention.
Disclosures
Dr. Pellikka is the Betty Knight Scripps Professor in Cardiovascular Diseases Clinical Research honoring George M. Gura, MD. She also receives research support from GE Healthcare, OxThera, and Lantheus Medical Imaging, with money paid to her employer, Mayo Clinic. She is on the Advisory Board for Bracco Diagnostics, Inc., and is a section editor for UpToDate.
(J Am Heart Assoc. 2019;8:e010736 DOI: 10.1161/JAHA.118.010736.)
References
- 1. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M; American Society of E and European Association of E . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101‐2. [DOI] [PubMed] [Google Scholar]
- 2. Mohan JC, Patel AR, Passey R, Gupta D, Kumar M, Arora R, Pandian NG. Is the mitral valve area flow‐dependent in mitral stenosis? A dobutamine stress echocardiographic study. J Am Coll Cardiol. 2002;40:1809–1815. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 4. Bolen JL, Lopes MG, Harrison DC, Alderman EL. Analysis of left ventricular function in response to afterload changes in patients with mitral stenosis. Circulation. 1975;52:894–900. [DOI] [PubMed] [Google Scholar]
- 5. Braunwald E, Moscovitz HL, Amram SS, Lasser RP, Sapin SO, Himmelstein A, Ravitch MM, Gordon AJ. The hemodynamics of the left side of the heart as studied by simultaneous left atrial, left ventricular, and aortic pressures; particular reference to mitral stenosis. Circulation. 1955;12:69–81. [DOI] [PubMed] [Google Scholar]
- 6. Fleming HA, Wood P. The myocardial factor in mitral valve disease. Br Heart J. 1959;21:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gash AK, Carabello BA, Cepin D, Spann JF. Left ventricular ejection performance and systolic muscle function in patients with mitral stenosis. Circulation. 1983;67:148–154. [DOI] [PubMed] [Google Scholar]
- 8. Klein AJ, Carroll JD. Left ventricular dysfunction and mitral stenosis. Heart Fail Clin. 2006;2:443–452. [DOI] [PubMed] [Google Scholar]
- 9. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 10. Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al‐Kebsi MM, Hugo‐Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode‐Thomas F, Okeahialam BN, Ige O, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani M, Ogah OS, Olunuga T, Elhassan HH, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Characteristics, complications, and gaps in evidence‐based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36:1115–1122a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klotz S, Dickstein ML, Burkhoff D. A computational method of prediction of the end‐diastolic pressure‐volume relationship by single beat. Nat Protoc. 2007;2:2152–2158. [DOI] [PubMed] [Google Scholar]
- 12. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. [DOI] [PubMed] [Google Scholar]
- 13. Borlaug BA, Kass DA. Ventricular‐vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. [DOI] [PubMed] [Google Scholar]
- 15. Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow‐gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carman GH, Lange RL. Variant hemodynamic patterns in mitral stenosis. Circulation. 1961;24:712–719. [DOI] [PubMed] [Google Scholar]
- 17. Hugenholtz PG, Ryan TJ, Stein SW, Abelmann WH. The spectrum of pure mitral stenosis. Hemodynamic studies in relation to clinical disability. Am J Cardiol. 1962;10:773–784. [DOI] [PubMed] [Google Scholar]
- 18. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke‐Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 19. Dutrey DE, Drake EH. Preoperative diagnosis of acquired valvular disease. Evaluation of different diagnostic methods with special emphasis on left heart catheterization and comparison with surgical findings. Am J Cardiol. 1961;8:319–327. [DOI] [PubMed] [Google Scholar]
- 20. Rayburn BK, Fortuin NJ. Severely symptomatic mitral stenosis with a low gradient: a case for low‐technology medicine. Am Heart J. 1996;132:628–632. [DOI] [PubMed] [Google Scholar]
- 21. Wisenbaugh T, Essop R, Middlemost S, Skoularigis J, Sareli P. Excessive vasoconstriction in rheumatic mitral stenosis with modestly reduced ejection fraction. J Am Coll Cardiol. 1992;20:1339–1344. [DOI] [PubMed] [Google Scholar]
- 22. Eleid MF, Nishimura RA, Sorajja P, Borlaug BA. Systemic hypertension in low‐gradient severe aortic stenosis with preserved ejection fraction. Circulation. 2013;128:1349–1353. [DOI] [PubMed] [Google Scholar]
- 23. Eleid MF, Nishimura RA, Borlaug BA, Sorajja P. Invasive measures of afterload in low gradient severe aortic stenosis with preserved ejection fraction. Circ Heart Fail. 2013;6:703–710. [DOI] [PubMed] [Google Scholar]
- 24. Gaasch WH, Folland ED. Left ventricular function in rheumatic mitral stenosis. Eur Heart J. 1991;12(suppl B):66–69. [DOI] [PubMed] [Google Scholar]
- 25. Harvey RM, Ferrer I, Samet P, Bader RA, Bader ME, Cournand A, Richards DW. Mechanical and myocardial factors in rheumatic heart disease with mitral stenosis. Circulation. 1955;11:531–551. [DOI] [PubMed] [Google Scholar]
- 26. Heller SJ, Carleton RA. Abnormal left ventricular contraction in patients with mitral stenosis. Circulation. 1970;42:1099–1110. [DOI] [PubMed] [Google Scholar]
- 27. Holzer JA, Karliner JS, O'Rourke RA, Peterson KL. Quantitative angiographic analysis of the left ventricle in patients with isolated rheumatic mitral stenosis. Br Heart J. 1973;35:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horwitz LD, Mullins CB, Payne RM, Curry GC. Left ventricular function in mitral stenosis. Chest. 1973;64:609–614. [DOI] [PubMed] [Google Scholar]
- 29. Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, Veulemans P, Pellens M, La Gerche A, Rademakers F, Herijgers P, D'Hooge J. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol. 2012;302:H188–H195. [DOI] [PubMed] [Google Scholar]
- 30. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Selzer A. Effects of atrial fibrillation upon the circulation in patients with mitral stenosis. Am Heart J. 1960;59:518–526. [DOI] [PubMed] [Google Scholar]
- 32. Ha JW, Chung N, Jang Y, Kang WC, Kang SM, Rim SJ, Shim WH, Cho SY, Kim SS. Is the left atrial v. wave the determinant of peak pulmonary artery pressure in patients with pure mitral stenosis? Am J Cardiol. 2000;85:986–991. [DOI] [PubMed] [Google Scholar]
- 33. Sagie A, Freitas N, Padial LR, Leavitt M, Morris E, Weyman AE, Levine RA. Doppler echocardiographic assessment of long‐term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol. 1996;28:472–479. [DOI] [PubMed] [Google Scholar]
- 34. Johnson NP, Zelis JM, Tonino PAL, Houthuizen P, Bouwman RA, Brueren GRG, Johnson DT, Koolen JJ, Korsten HHM, Wijnbergen IF, Zimmermann FM, Kirkeeide RL, Pijls NHJ, Gould KL. Pressure gradient vs. flow relationships to characterize the physiology of a severely stenotic aortic valve before and after transcatheter valve implantation. Eur Heart J. 2018;39:2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karp K, Teien D, Bjerle P, Eriksson P. Reassessment of valve area determinations in mitral stenosis by the pressure half‐time method: impact of left ventricular stiffness and peak diastolic pressure difference. J Am Coll Cardiol. 1989;13:594–599. [DOI] [PubMed] [Google Scholar]


