Abstract
Background
The recent introduction of high‐sensitivity cardiac troponin (hs‐cTn) assays has allowed clinicians to measure hs‐cTn before and after cardiac stress testing, but the hs‐cTn release pattern and potential utility in identifying inducible myocardial ischemia are unclear. We thus conducted a systematic review and meta‐analysis to improve our understanding of hs‐cTn release associated with exercise and pharmacological stress testing.
Methods and Results
Studies published between January 2008 and July 2016 that reported hs‐cTn change values (high‐sensitivity cardiac troponin T [hs‐cTnT] or high‐sensitivity cardiac troponin I [hs‐cTnI]) in relation to cardiac stress testing were searched and reviewed by 2 independent screeners. Primary outcomes were pooled estimates of absolute and relative hs‐cTn changes after cardiac stress test, stratified by the presence of inducible myocardial ischemia. This meta‐analysis included 11 studies (n=2432 patients). After exercise stress testing, hs‐cTnT increased by 0.5 ng/L or 11% (6 studies, n=406) and hs‐cTnI by 2.4 ng/L or 41% (4 studies, n=365) in patients with inducible myocardial ischemia versus hs‐cTnT by 1.1 ng/L or 18% (8 studies, n=629; P=0.29) and hs‐cTnI by 1.8 ng/L or 72% (4 studies, n=831; P=0.61) in patients who did not develop inducible myocardial ischemia. After pharmacological stress test, hs‐cTnT changed by −0.1 ng/L or −0.4% (6 studies, n=251) and hs‐cTnI by 2.4 ng/L or 32% (2 studies, n=108) in patients with inducible myocardial ischemia versus hs‐cTnT by 0.7 ng/L or 11% (5 studies, n=443, P=0.44) and hs‐cTnI by 1.7 ng/L or 38% (2 studies, n=116; P=0.62) in patients who did not develop inducible myocardial ischemia.
Conclusions
hs‐cTn rising patterns after exercise and pharmacological stress testing appear inconsistent and comparably small, and do not appear to be correlated with inducible myocardial ischemia.
Keywords: myocardial ischemia, stress echocardiography, stress testing, troponin
Subject Categories: Diagnostic Testing, Exercise Testing, Biomarkers, Meta Analysis
Clinical Perspective
What Is New?
In this systematic review and meta‐analysis analyzing high‐sensitivity cardiac troponin kinetics in patients undergoing exercise or pharmacological stress testing, high‐sensitivity cardiac troponin rising patterns after exercise or pharmacological cardiac stress testing were inconsistent, comparably small, and did not correlate with inducible myocardial ischemia.
What Are the Clinical Implications?
We found little evidence to support the utility of high‐sensitivity cardiac troponin in improving the diagnostic utility in cardiac stress testing.
Introduction
High‐sensitivity cardiac troponin (hs‐cTn) assays have replaced contemporary cardiac troponin (cTn) assays throughout most of the world and continue to enhance our understanding of the pathophysiology of myocardial infarction, ischemia, and injury. In most adult patients, hs‐cTn assays have the ability to detect cTn at low concentrations and consequently to identify much smaller change values.1 For instance, multiple studies have found significant cTn increases following strenuous physical activity and cardiovascular stress.2, 3, 4, 5, 6
Of particular relevance to cardiovascular medicine are cardiac biomarker elevations associated with cardiac stress testing. Cardiac biomarkers have the potential to increase the clinical utility of cardiac stress testing if they are able to identify high‐risk patients who do not have an unequivocal stress test result. However, there is currently no consensus regarding the mechanism or diagnostic utility of hs‐cTn release during and after cardiac stress testing.7
To improve our understanding about hs‐cTn release associated with cardiac stress testing, we conducted a systematic review of the existing literature and performed a meta‐analysis.
Materials and Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. This systematic review and meta‐analysis was performed following the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analysis).8
Search Strategy
We searched the published literature using strategies created by a medical librarian for published evidence on hs‐cTn and exercise or pharmacological stress testing with a publication cutoff date of June 30, 2016. To exclude animal studies, the librarian used the human filter for Medline recommended in the Cochrane Handbook for Systematic Reviews of Interventions 9 and modified it to create similar filters for the other databases searched. The librarian established search strategies using a combination of standardized terms and key words, and implemented it in Ovid Medline, Embase, Scopus, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessments, and the NHS Economic Evaluation Database. We also conducted a search in clinicaltrials.gov. We searched for all studies in which high‐sensitivity cardiac troponin T (hs‐cTnT) or high‐sensitivity cardiac troponin I (hs‐cTnI) were evaluated before and after cardiac stress testing. Key words were “stress,” “adenosine,” “dobutamine,” “troponin T,” “troponin I,” and “high‐sensitivity troponin.” Two authors systematically screened titles and abstracts of studies identified in the search and excluded unrelated studies independently. Moreover, they verified the remaining full articles and reference lists.
Eligibility Criteria
The article or abstract was included in this meta‐analysis if it met the following criteria: (1) original article or abstract that evaluated the association between cardiac stress testing and hs‐cTn; and (2) hs‐cTn was measured before and after exposure cardiac stress testing. Since hs‐cTn assays have been introduced only during the past decade, we excluded all records before 2008. Articles or abstracts were excluded if the study population included patients who had active symptoms of myocardial ischemia or infarction, such as discomfort, pain, stress, or increased physical activity before the first hs‐cTn concentration was measured. Review articles, case reports, and studies without reference interval values for troponin were also excluded. If multiple publications overlapped or were duplicated, the most comprehensive study was used to extract the information needed.
Selection of Articles
Our literature search revealed 4221 articles and abstracts; 759 duplicates were accurately identified and removed for a total of 3462 unique citations. After screening titles and abstracts of articles, 57 records were reviewed with full texts. Finally, 11 studies were included in the meta‐analysis,1, 3, 5, 6, 10, 11, 12, 13, 14 and 46 were excluded because of incomplete or overlapping data. The Figure shows details of the selection process for studies. The collective methodological quality of included studies was good, using the National Heart, Lung, and Blood Institute (NHLBI) quality assessment tool for before–after (pre–post) studies with no control group.
Data Extraction and Quality Assessment
The authors extracted data from included articles independently and the results were cross‐checked. Authors, years, sample size, age, exercise or pharmacological stress test, duration of exercise, detection assay, and mean values of hs‐cTnT and hs‐cTnI levels measured before and after stress test were extracted in each individual article (Table 1). Authors were contacted by email if the required data were unavailable.
Table 1.
Baseline Characteristics of the Studies Included in the Meta‐Analysis
| Study | Country | Population | Type of Stress | Assay |
|---|---|---|---|---|
| Axelsson 20133 | Denmark | 12 patients with CAD and 12 healthy controls | Bicycle stress test | Roche Elecsys hs‐cTnT |
| Kurz 200810 | Germany | 144 patients with suspected CAD | Bicycle or dipyridamole stress test | Roche Elecsys hs‐cTnT |
| Lee 201612 | Switzerland | 819 patients with suspected myocardial ischemia | Bicycle SPECT | Singulex hs‐cTnI |
| Le Goff 201011 | Belgium | 50 patients with suspected CAD | Bicycle or dipyridamole stress test |
Roche Elecsys hs‐cTnT Abbott Diagnostics Architect STAT hs‐cTnI |
| Liebetrau 201513 | Germany | 383 patients with suspected or progressive CAD | Bicycle stress test | Roche Elecsys hs‐cTnT |
| Pastormerlo 201314 | Italy | 23 patients with CHF | Bicycle stress test | Roche Elecsys hs‐cTNT |
| Pastormerlo 20155 | Italy | 30 patients with systolic HF | Bicycle stress test | Roche Elecsys hs‐cTnT |
| Rosjo 201215 | Norway | 198 patients | Bicycle stress test | Roche Elecsys hs‐cTnT and Abbott Diagnostics Architect STAT hs‐cTnI |
| Sou 201616 | Switzerland | 229 patients with suspected CAD | Bicycle stress test | Roche Elecsys hs‐cTNT and Abbott Diagnostics Architect STAT hs‐cTnI |
| Wongpraparut 20116 | Thailand | 120 patients with suspected CAD | Pharmacologic stress MRI | Roche Elecsys hs‐cTNT |
| Wongpraparut 201517 | Thailand | 250 patients with suspected CAD | Pharmacologic stress MRI | Roche Elecsys hs‐cTnT |
CAD indicates coronary artery disease; CHF, chronic heart failure; HF, heart failure; hs‐cTnI, high‐sensitivity cardiac troponin I; hs‐cTnT, high‐sensitivity cardiac troponin T; MRI, magnetic resonance imaging; SPECT, single‐photon emission computed tomography.
We applied the NHLBI quality assessment tool for before–after (pre–post) studies with no control group18 to assess the quality of eligible studies. Methodological quality of eligible studies was assessed independently. Any disagreements were resolved by discussions or by consensus including the senior author. All studies were separated into groups according to type of cardiac stress test (exercise or pharmacological stress test) and troponin type (hs‐cTnT or hs‐cTnI). Furthermore, patients who developed myocardial ischemia (“positive stress test”) and those who did not were analyzed separately. The only exception was for the studies by LeGoff et al11 and Kurz et al10 which did not distinguish between hs‐cTn values of exercise and pharmacological stress tests (we thus added the data to both exercise and pharmacological stress test analyses).
Statistical Analysis
If an hs‐cTn value was not exactly reported, we used the closest lowest/highest value (eg, 4.9 instead of <5). Ranges or interquartile ranges were converted into SDs as described by Wan et al19 to be able to compute pooled estimates in the meta‐analysis. The mean/median hs‐cTn change from baseline was computed as the difference between peak values after exercise or stress test and baseline values, as well as the standard error, using the Comprehensive Meta‐Analysis software package (version 3.3, Biostat). If missing, a correlation coefficient between pre‐ and post‐values of 0.3 was assumed.20 The CIs for relative change from baseline values were calculated using GraphPad software (GraphPad Software Inc). Random‐effects meta‐analyses of the absolute and relative changes from baseline were computed. We used the I 2 and Cochran Q statistics to assess the heterogeneity of results across studies. A subgroup analysis was conducted by systematically excluding each study at a time and rerunning the analysis to assess any change in effect size. We compared results between patients who developed myocardial ischemia versus those who did not (“positive” versus “negative” stress test) using a Q test based on analysis of variance applying random‐effects weights (mixed effects analysis) in the Comprehensive Meta‐Analysis software package.
Results
This meta‐analysis included 11 studies with a total of 2432 participants, 11 studies (n=1729) evaluating hs‐cTnT, and 4 studies (n=1420) evaluating hs‐cTnI (Figure). Baseline characteristics of the included studies are shown in Table 1. Table 2 provides an overview of the study results, and Table S1 provides an assessment of the quality of the included studies. Peak hs‐cTn values were uniformly obtained between 3 and 4 hours after stress test.
Figure 1.
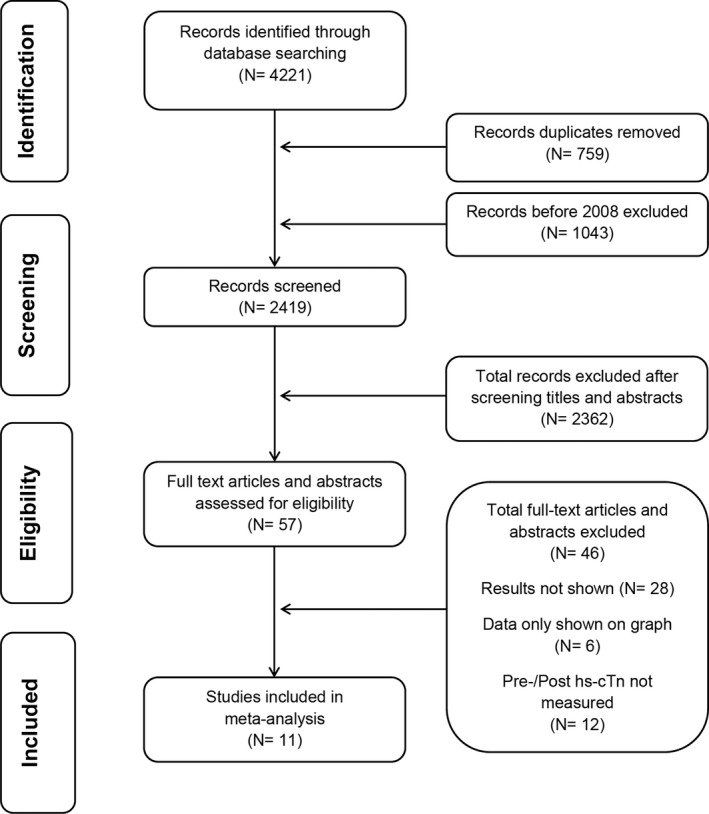
Flow diagram summarizing study identification and selection. hs‐cTn indicates high‐sensitivity cardiac troponin.
Table 2.
Absolute and Relative Change Values
| No Ischemia | Ischemia | P Value | |
|---|---|---|---|
| Exercise stress test—hs‐cTnT | |||
| Absolute change, ng/L | 1.1 (0–2.2) | 0.5 (0–0.9) | 0.29 |
| Relative change, % | 18 (3–34) | 11 (−0.3 to 23) | 0.48 |
| Exercise stress test—hs‐cTnI | |||
| Absolute change, ng/L | 1.8 (0.6–3) | 2.4 (0.2–4.7) | 0.61 |
| Relative change, % | 72 (31–113) | 41 (3–79) | 0.28 |
| Pharmacologic stress test—hs‐cTnT | |||
| Absolute change, ng/L | 0.7 (−0.5 to 1.9) | −0.1 (−1.7 to 1.5) | 0.44 |
| Relative change, % | 11 (−6 to 28) | 5 (−8 to 18) | 0.59 |
| Pharmacologic stress test—hs‐cTnI | |||
| Absolute change, ng/L | 1.7 (0.6–2.9) | 2.4 (0.2–4.5) | 0.62 |
| Relative change, % | 38 (4–71) | 32 (3–66) | 0.81 |
Values are expressed as pooled estimates from the meta‐analysis and corresponding 95% CIs.
hs‐cTnI indicates high‐sensitivity cardiac troponin I; hs‐cTnT, high‐sensitivity cardiac troponin T.
Exercise Stress Test
High‐sensitivity cTnT
The pooled estimated absolute hs‐cTnT change in patients who developed inducible myocardial ischemia after exercise stress test (“positive test”) was 0.5 ng/L (95% CI, 0.0–0.9 ng/L), or a relative change of 11% (95% CI, 0–23%; 6 studies, n=406), compared with 1.1 ng/L (95% CI, 0.0–2.2 ng/L), or a relative change of 18% (95% CI, 3–34%), in patients who did not develop inducible myocardial ischemia after exercise stress test (8 studies, n=629; P=0.29) (Figure S1).
High‐sensitivity cTnI
The pooled estimated absolute hs‐cTnI change in patients who developed inducible myocardial ischemia after exercise stress test was 2.4 ng/L (95% CI, 0.2–4.7 ng/L), or a relative change of 41% (95% CI, 4–79%; 4 studies, n=365), compared with 1.8 ng/L (95% CI, 1–3 ng/L), which corresponded to a relative change of 72% (95% CI, 31–113%) in patients who did not develop inducible myocardial ischemia after exercise stress test (4 studies, n=831; P=0.61) (Figure S2A).
Pharmacological Stress Test
High‐sensitivity cTnT
The pooled estimated absolute hs‐cTnT change in patients who developed inducible myocardial ischemia after pharmacological stress test was −0.1 ng/L (95% CI, −1.7 to 1.5 ng/L), which corresponded to a relative change of 5% (95% CI, −8 to 18%; 6 studies, n=251), compared with 0.7 ng/L (95% CI, −0.5, 2 ng/L), which corresponded to a relative change of 11% (95% CI, −6 to 28%) in patients who did not develop inducible myocardial ischemia (5 studies, n=443; P=0.44) (Figure S3).
High‐sensitivity cTnI
The pooled estimated absolute hs‐cTnI change in patients who developed inducible myocardial ischemia after pharmacological stress was 2.4 ng/L (95% CI, 0.2–4.5 ng/L), which represented a relative change of 32% (95% CI, −3 to 66%; 2 studies, n=108), compared with 1.7 ng/L (95% CI, 0.6–2.9 ng/L), which represented a relative change of 38% (95% CI, 4–71%), in patients who did not develop inducible myocardial ischemia after exercise stress testing (2 studies, n=116; P=0.62) (Figure S4).
Discussion
The goal of this systematic review and meta‐analysis was to obtain and quantify the available evidence regarding hs‐cTn release after cardiac stress testing. We distinguished between exercise and pharmacological stress tests. Additionally, we sought to determine whether hs‐cTn release is more pronounced among patients with inducible myocardial ischemia during stress test compared with patients who do not develop myocardial ischemia, which could have diagnostic utility.
The results of this study indicate that hs‐cTnT and hs‐cTnI release after cardiac stress testing is modest in magnitude and on average ranges from 0 to 2 ng/L for absolute change values. Relative change values appear to be smaller for hs‐cTnT (median range 5–18%) compared with hs‐cTnI (median range, 32–72%). There was no statistically significant difference in hs‐cTn release between patients who developed inducible myocardial ischemia versus those who did not. In fact, given the low baseline values in most patients, these values do not exceed conjoint biological and analytical variation.21
The concept that the addition of cardiac biomarker data to cardiac stress testing may improve diagnostic utility is not new and has been tested in several studies. In general, studies have found that low baseline values are highly predictive of a negative stress test. For example, Lee et al12 showed that a baseline hs‐cTnI value <1.5 ng/L had an 87% negative predictive value for inducible myocardial ischemia after stress test. Furthermore, patients with inducible myocardial ischemia were found to have higher baseline hs‐cTn values in most,7, 12, 13, 15, 16 but not all, studies.10 Interestingly, our analysis showed smaller relative changes in hs‐cTn levels in patients with inducible ischemia when compared with those without inducible ischemia.
Difference Between hs‐cTnT and hs‐cTnI
The results of this study suggest that the release of hs‐cTnT after stress testing may be substantially smaller compared with hs‐cTnI. While it is possible that cardiac stress releases different quantities of hs‐cTnI and hs‐cTnT, it appears unlikely and biologically somewhat implausible. Stress that damages the cardiomyocyte cell membrane should result in simultaneous release of cTnI and cTnT, the quantities of which should be tightly correlated. A more logical explanation for the apparent discrepancy between hs‐cTnT and hs‐cTnI results may be related to the calibration of the assays and their sensitivity and precision at low values. Another possible explanation may be related to the rhythmic diurnal variation of hs‐cTnT, which shows higher levels in the morning and at nighttime and lower levels during the day.22, 23, 24 While rhythmic diurnal variation is characteristic for hs‐cTnT, it has not been observed in hs‐cTnI.
cTn Release During Stress Test
cTn is highly specific for myocardial tissue. Any process that causes injury to cardiomyocytes including myocardial infarction will cause an elevation of cTn in the blood stream. Until recently—before the introduction of hs‐cTn assays—it was widely assumed that cTn is only released during myocardial cell necrosis. Recent hs‐cTn data, however, have strongly questioned this assumption. Data obtained from young, healthy athletes have shown that hs‐cTn levels may rise several‐fold after strenuous exercise. Likewise, an intravenous infusion of dobutamine or rapid atrial pacing will lead to a rise in hs‐cTn concentrations. In experimental models, hs‐cTnI release has been documented with transient ischemia and caused by volume loading.25 In these models, the myocardial cells die as a result of apoptosis.26 Thus, although speculative, during cardiac stress testing, several potential mechanisms may contribute to hs‐cTn increase other than myocardial cell necrosis such as inducible myocardial ischemia, transient increases in cardiomyocyte permeability,27, 28, 29 ischemic‐induced membranous blebs that rupture without necrosis,30 free radical overload,31 increased turnover of troponins,32 and direct toxic effects of catecholamines. However, this topic is controversially discussed.
Limitations
This study focused on hs‐cTn change values and did not investigate the ability of baseline values (low or high) to predict inducible myocardial ischemia during stress test. Second, we did not distinguish between individual hs‐cTn assays and platforms that were used in each study. Third, cardiac biomarker research and development in industry is currently occurring at a fast pace, and thus there may have been temporal trends influencing the study (older versus newer assays, reagents, and platforms). From a statistical standpoint, we had to convert median and range values to means and SDs, which may have influenced some results.
Conclusions
Results from this meta‐analysis suggest that hs‐cTn rising patterns after exercise and pharmacological stress testing appear inconsistent and comparably small and do not appear to be correlated with inducible myocardial ischemia. These results cast doubt on the idea that rising patterns of hs‐cTn may be used to stratify patients after cardiac stress testing.
Sources of Funding
This study was conducted with departmental funds only. Dr Nagele is currently supported by the National Institutes of Health/NHLBI (R01HL126892).
Disclosures
Dr Nagele reports research support from Abbott Diagnostics. Dr Scott reports research support from Siemens Healthcare Diagnostic, Abbott Diagnostics, and Instrumentation Laboratories, and consulting for Instrumentation Laboratories and Roche Diagnostics. Dr Jaffe reports consulting for Beckman, Siemens, Abbott, Alere, ET Healthcare, Becton Dickinson, Shingotec, Singulex, and Novartis. The remaining authors have no disclosures to report.
Supporting information
Table S1. Quality Assessment of Included Studies
Figure S1. Pooled estimates of the absolute and relative high‐sensitivity cardiac troponin T (hs‐cTnT) change from baseline after exercise stress testing in patients without (A) and with (B) inducible myocardial ischemia.
Figure S2. Pooled estimates of the absolute high‐sensitivity cardiac troponin I (hs‐cTnI) change from baseline after exercise stress testing in patients without (A) and with (B) inducible myocardial ischemia.
Figure S3. Forest plot showing pooled estimate of the absolute high‐sensitivity cardiac troponin T (hs‐cTnT) change from baseline after pharmacological stress testing in patients without (A) and with (B) inducible myocardial ischemia.
Figure S4. Forest plot showing pooled estimate of the absolute high‐sensitivity cardiac troponin I (hs‐cTnI) change from baseline after pharmacological stress testing in patients without (A) and with (B) inducible myocardial ischemia. No funnel plots to assess publication bias could be produced as only 2 studies were available (and a minimum of 3 are needed for a funnel plot).
Acknowledgments
We would like to thank Susan A. Fowler, MLIS, Washington University in St. Louis School of Medicine, Bernard Becker Medical Library, for the literature search.
(J Am Heart Assoc. 2019;8:e008626 DOI: 10.1161/JAHA.118.008626)
References
- 1. Sherwood MW, Newby KL. High‐sensitivity troponin assays: evidence, indications, and reasonable use. J Am Heart Assoc. 2014;3:e000403 DOI: 10.1161/JAHA.113.000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aagaard P, Sahlen A, Bergfeldt L, Braunschweig F. Heart rate and its variability in response to running‐associations with troponin. Med Sci Sports Exerc. 2014;46:1624–1630. [DOI] [PubMed] [Google Scholar]
- 3. Axelsson A, Ruwald MH, Dalsgaard M, Rossing K, Steffensen R, Iversen K. Serial measurements of high‐sensitivity cardiac troponin T after exercise stress test in stable coronary artery disease. Biomarkers. 2013;18:304–309. [DOI] [PubMed] [Google Scholar]
- 4. Baker P, Davies SL, Larkin J, Moult D, Benton S, Roberts A, Harris T. Changes to the cardiac biomarkers of non‐elite athletes completing the 2009 London Marathon. Emerg Med J. 2014;31:374–379. [DOI] [PubMed] [Google Scholar]
- 5. Pastormerlo LE, Agazio A, Benelli E, Gabutti A, Poletti R, Prontera C, Clerico A, Emdin M, Passino C. Usefulness of high‐sensitive troponin elevation after effort stress to unveil vulnerable myocardium in patients with heart failure. Am J Cardiol. 2015;116:567–572. [DOI] [PubMed] [Google Scholar]
- 6. Wongpraparut N, Piyophirapong S, Maneesai A, Sribhen K, Pongasira R, Komoltri C. Highly sensitive cardiac troponin T level and the degree of myocardial ischemia during cardiac pharmacological stress MRI. Eur Heart J. 2011;32:1033–1034. [Google Scholar]
- 7. Roysland R, Kravdal G, Hoiseth AD, Nygard S, Badr P, Hagve TA, Omland T, Rosjo H. Cardiac troponin T levels and exercise stress testing in patients with suspected coronary artery disease: the Akershus Cardiac Examination (ACE) 1 study. Clin Sci (Lond). 2012;122:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods G, Cochrane Statistical Methods G . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurz K, Giannitsis E, Zehelein J, Katus HA. Highly sensitive cardiac troponin T values remain constant after brief exercise‐ or pharmacologic‐induced reversible myocardial ischemia. Clin Chem. 2008;54:1234–1238. [DOI] [PubMed] [Google Scholar]
- 11. Le Goff CL, Laurent T, Garweg C, Kaux J, Deroyer C, Fillet M, Lancellotti P, Pierard L, Chapelle J. Does echocardiographic stress test induced release of hsTnT and TnI II? Clin Chem. 2010;56:A128. [Google Scholar]
- 12. Lee G, Twerenbold R, Tanglay Y, Reichlin T, Honegger U, Wagener M, Jaeger C, Rubini Gimenez M, Hochgruber T, Puelacher C, Radosavac M, Kreutzinger P, Stallone F, Hillinger P, Krivoshei L, Herrmann T, Mayr R, Freese M, Wild D, Rentsch KM, Todd J, Osswald S, Zellweger MJ, Mueller C. Clinical benefit of high‐sensitivity cardiac troponin I in the detection of exercise‐induced myocardial ischemia. Am Heart J. 2016;173:8–17. [DOI] [PubMed] [Google Scholar]
- 13. Liebetrau C, Gaede L, Dorr O, Hoffmann J, Wolter JS, Weber M, Rolf A, Hamm CW, Nef HM, Mollmann H. High‐sensitivity cardiac troponin T and copeptin assays to improve diagnostic accuracy of exercise stress test in patients with suspected coronary artery disease. Eur J Prev Cardiol. 2015;22:684–692. [DOI] [PubMed] [Google Scholar]
- 14. Pastormerlo LE, Prontera C, Agazio A, Benelli E, Gabutti A, Mammini C, Poletti R, Clerico A, Passino C, Emdin M. Prediction of ongoing myocardial damage by noradrenergic response and haemodynamic impairment during exercise in systolic heart failure. Non invasive estimation of frank‐starling curve. Eur J Heart Fail. 2013;12:S246–S246. [Google Scholar]
- 15. Rosjo H, Kravdal G, Hoiseth AD, Jorgensen M, Badr P, Roysland R, Omland T. Troponin I measured by a high‐sensitivity assay in patients with suspected reversible myocardial ischemia: data from the Akershus Cardiac Examination (ACE) 1 study. Clin Chem. 2012;58:1565–1573. [DOI] [PubMed] [Google Scholar]
- 16. Sou SM, Puelacher C, Twerenbold R, Wagener M, Honegger U, Reichlin T, Schaerli N, Pretre G, Abacherli R, Jaeger C, Rubini Gimenez M, Wild D, Rentsch KM, Zellweger MJ, Mueller C. Direct comparison of cardiac troponin I and cardiac troponin T in the detection of exercise‐induced myocardial ischemia. Clin Biochem. 2016;49:421–432. [DOI] [PubMed] [Google Scholar]
- 17. Wongpraparut N, Piyophirapong S, Maneesai A, Sribhen K, Krittayaphong R, Pongakasira R, White HD. High‐sensitivity cardiac troponin T in stable patients undergoing pharmacological stress testing. Clin Cardiol. 2015;38:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Heart, Lung, and Blood Institute . Quality assessment tool for before‐after (pre‐post) studies with no control group, 2014. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed March 1, 2019.
- 19. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sedaghat‐Hamedani F, Kayvanpour E, Frankenstein L, Mereles D, Amr A, Buss S, Keller A, Giannitsis E, Jensen K, Katus HA, Meder B. Biomarker changes after strenuous exercise can mimic pulmonary embolism and cardiac injury—a metaanalysis of 45 studies. Clin Chem. 2015;61:1246–1255. [DOI] [PubMed] [Google Scholar]
- 21. Apple FS, Sandoval Y, Jaffe AS, Ordonez‐Llanos J; Bio‐Markers; IFCC Task Force on Clinical Applications of Cardiac Bio‐Markers . Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63:73–81. [DOI] [PubMed] [Google Scholar]
- 22. Klinkenberg LJ, van Dijk JW, Tan FE, van Loon LJ, van Dieijen‐Visser MP, Meex SJ. Circulating cardiac troponin T exhibits a diurnal rhythm. J Am Coll Cardiol. 2014;63:1788–1795. [DOI] [PubMed] [Google Scholar]
- 23. Fournier S, Iten L, Marques‐Vidal P, Boulat O, Bardy D, Beggah A, Calderara R, Morawiec B, Lauriers N, Monney P, Iglesias JF, Pascale P, Harbaoui B, Eeckhout E, Muller O. Circadian rhythm of blood cardiac troponin T concentration. Clin Res Cardiol. 2017;106:1026–1032. [DOI] [PubMed] [Google Scholar]
- 24. Klinkenberg LJ, Wildi K, van der Linden N, Kouw IW, Niens M, Twerenbold R, Rubini Gimenez M, Puelacher C, Daniel Neuhaus J, Hillinger P, Nestelberger T, Boeddinghaus J, Grimm K, Sabti Z, Bons JA, van Suijlen JD, Tan FE, Ten Kate J, Bekers O, van Loon LJ, van Dieijen‐Visser MP, Mueller C, Meex SJ. Diurnal rhythm of cardiac troponin: consequences for the diagnosis of acute myocardial infarction. Clin Chem. 2016;62:1602–1611. [DOI] [PubMed] [Google Scholar]
- 25. Feng J, Schaus BJ, Fallavollita JA, Lee TC, Canty JM Jr. Preload induces troponin I degradation independently of myocardial ischemia. Circulation. 2001;103:2035–2037. [DOI] [PubMed] [Google Scholar]
- 26. Weil BR, Young RF, Shen X, Suzuki G, Qu J, Malhotra S, Canty JM. Brief myocardial ischemia produces cardiac troponin I release and focal myocyte apoptosis in the absence of pathological infarction in swine. JACC Basic Transl Sci. 2017;2:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D, Thompson PD. Exercise‐induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56:169–176. [DOI] [PubMed] [Google Scholar]
- 28. Wu AH, Ford L. Release of cardiac troponin in acute coronary syndromes: ischemia or necrosis? Clin Chim Acta. 1999;284:161–174. [DOI] [PubMed] [Google Scholar]
- 29. Lippi G, Schena F, Salvagno GL, Tarperi C, Aloe R, Guidi GC. Comparison of conventional and highly‐sensitive troponin I measurement in ultra‐marathon runners. J Thromb Thrombolysis. 2012;33:338–342. [DOI] [PubMed] [Google Scholar]
- 30. Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, Roberts MS. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta. 2010;411:318–323. [DOI] [PubMed] [Google Scholar]
- 31. Neumayr G, Pfister R, Mitterbauer G, Maurer A, Gaenzer H, Sturm W, Hoertnagl H. Effect of the “Race Across The Alps” in elite cyclists on plasma cardiac troponins I and T. Am J Cardiol. 2002;89:484–486. [DOI] [PubMed] [Google Scholar]
- 32. Middleton N, George K, Whyte G, Gaze D, Collinson P, Shave R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J Am Coll Cardiol. 2008;52:1813–1814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quality Assessment of Included Studies
Figure S1. Pooled estimates of the absolute and relative high‐sensitivity cardiac troponin T (hs‐cTnT) change from baseline after exercise stress testing in patients without (A) and with (B) inducible myocardial ischemia.
Figure S2. Pooled estimates of the absolute high‐sensitivity cardiac troponin I (hs‐cTnI) change from baseline after exercise stress testing in patients without (A) and with (B) inducible myocardial ischemia.
Figure S3. Forest plot showing pooled estimate of the absolute high‐sensitivity cardiac troponin T (hs‐cTnT) change from baseline after pharmacological stress testing in patients without (A) and with (B) inducible myocardial ischemia.
Figure S4. Forest plot showing pooled estimate of the absolute high‐sensitivity cardiac troponin I (hs‐cTnI) change from baseline after pharmacological stress testing in patients without (A) and with (B) inducible myocardial ischemia. No funnel plots to assess publication bias could be produced as only 2 studies were available (and a minimum of 3 are needed for a funnel plot).


