Abstract
Introduction
Including the patient perspective is important to achieve optimal outcomes in the treatment of rheumatoid arthritis (RA). Ample qualitative studies exist on patient outcomes in RA. A Belgian study recently unravelled what matters most to patients throughout the overwhelming and rapidly evolving early stage of RA. The present study, European Qualitative research project on Patient-preferred outcomes in Early Rheumatoid Arthritis (EQPERA) was created to contribute to a more universal understanding of patient-preferred health and treatment outcomes by integrating the perspectives of patients with early RA from three European countries.
Methods and analysis
In EQPERA, a qualitative, explorative, longitudinal study will be implemented in The Netherlands and Sweden, parallel to the methods applied in the previously conducted Belgian study. In each country, a purposive sample of patients with early RA will be individually interviewed 3–6 months after start of the initial RA treatment and subsequently, the same participants will be invited to take part in a focus group 12–18 months after RA treatment initiation. Data collection and analysis will be independently conducted by the local research teams in their native language. A meta-analysis of the local findings will be performed to explore and describe similarities, differences and patterns across countries.
Ethics and dissemination
Ethics approval was granted by the responsible local ethics committees. EQPERA follows the recommendations of the Declaration of Helsinki. Two main papers are foreseen (apart from the data reporting on the local findings) for peer-reviewed publication.
Keywords: rheumatoid arthritis, qualitative research, longitudinal study, patient preference
Strengths and limitations of this study.
The specific nature of the study, in which qualitative studies are carried out in different countries and languages using a uniform methodology, is novel, and we report in a transparent way about our approach and challenges.
As no formal meta-analysis method was present in literature applicable to our study, we developed a method based on established techniques for the synthesis of qualitative research which can guide other researchers interested in conducting this type of research.
Several quality enhancing strategies are applied to yield sound results in this multinational, multilingual, longitudinal qualitative study.
The participating countries might have rather similar cultural views and healthcare systems which would strengthen the Belgian findings; however, the study protocol offers a methodological framework for research in different parts of the world.
Introduction
In rheumatoid arthritis (RA), the outcome landscape dramatically changed over the past decades. RA is the most prevalent chronic, auto-immune inflammatory joint disease. It was typically described as an inevitably progressive disease with a destructive and disabling natural course. The continuous growth in effective pharmacological treatments contributed to this change, but the introduction of early therapy was one of the main drivers of transformed health outcomes of patients with RA.1 Nowadays, remission or at least low disease activity have become realistic treatment targets for a notable proportion of the population.2
Nevertheless, the burden of disease and unmet needs remain considerable.3 4 For example, most of the patients are at working age at time of diagnosis, but work disability rates remain high.5 Furthermore, patients with RA indicated the need for greater emotional support, and greater psychological support to manage the impact of disease on domains such as pain, fatigue, work and leisure.6 7 Hence, it seems that patient preferences are not sufficiently understood and met by health professionals. In a recent report, patient-centred care was identified as a recurrent unmet need across rheumatic diseases, including RA.8 Patient-centred care can be translated as care that is guided by the values and preferences of the patients,9 with patient preferences referring to the perspective, beliefs and expectations of patients regarding their health and life.10 As patient-centredness is acknowledged as one of the key dimensions of high-quality care,11 integrating the patient perspective in outcome assessment is increasingly advocated to achieve optimal outcomes in the treatment of RA.12 13
Qualitative studies shed light on the different views that patients with RA have on outcome compared with health professionals. These studies revealed the importance of fatigue and independence, among others,14–16 to consider in daily practice on top of the traditional measures of disease activity, that is, the swelling of joints and laboratory parameters of inflammation. Remarkably, limited attention has been given to the perspective of recently diagnosed patients. The early disease stage is probably the most daunting period for patients, indicating specific needs and preferences.17 18 The Belgian qualitative study of Van der Elst et al provided new insights into patient-preferred outcomes in early RA, concluding that returning to ‘normality’ as soon as possible was the core preferred outcome which related to aspects of disease control and participation, physical and mental aspects.19 However, understanding is lacking about the transferability of these local findings to other settings and cultures.
Despite recommendations for RA management, literature shows that there are differences in how rheumatology services are viewed and practised across countries.20 21 These differences may be attributable to characteristics of the national healthcare systems, local customs, practices and values. Such cultural differences may consequently influence how patients evaluate their disease. For example, the survey study of Van Tuyl et al demonstrated that the country in which patients were sampled resulted in slightly different key domains on how they perceived remission of disease.22 Hifinger et al showed that country of residence had an important influence on how patients with RA experienced fatigue.23 It can thus be questioned whether patients in other countries would bring out other preferred outcomes.
To examine the transferability of the Belgian findings and to contribute to a more universal understanding of patient-preferred outcomes, we initiated the European Qualitative research project on Patient-preferred outcomes in Early Rheumatoid Arthritis (EQPERA) consortium. It is a multicentre, multilingual, longitudinal qualitative study across Belgium, The Netherlands and Sweden. The present paper reports about the international study protocol, based on the Belgian study procedures.
Objectives
The overall research objective in EQPERA is to explore how local context influences patient-preferred health and treatment outcomes throughout the early disease course by integrating the perspectives of patients with early RA from three European countries.
The objective is twofold:
To describe patient-preferred outcomes in early RA and how they change throughout the early disease course (national objective).
To identify differences, similarities and patterns in patient-preferred outcomes across the three European countries (international objective).
Methods and analysis
The Belgian study was conducted during 2012–2013.19 Based on the lessons learnt and after multiple discussion rounds with the EQPERA steering group, an improved research protocol was written with the aim to implement a protocol as similar as possible in the other countries. Start of patient inclusion was 2016 in The Netherlands and 2017 in Sweden. We intend to publish the final results by the end of 2019.
Study design
A qualitative, explorative, longitudinal research design will be applied within a European context. As we study a research domain still lacking evidence, the use of qualitative methods is justified because we will learn from the rich descriptions of participants being shaped in their local contexts.24 25 Longitudinal designs are relevant for studying complex phenomena and are specifically applicable in the context of a recent diagnosis since patients’ perceptions and expectations may change during the overwhelming and rapidly evolving early disease stage. Previous research also suggests that the way patients experience and evaluate their disease can differ depending on disease duration.15 26 27
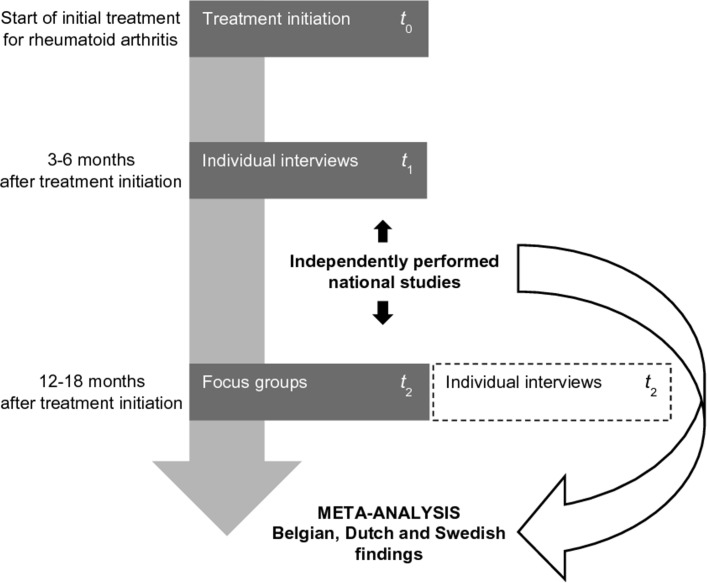
Patients with early RA will be invited to participate at two time points (figure 1). At t 1, participants will be individually interviewed 3–6 months after they have started their initial treatment for RA. At t 2, participants will be invited to take part in a focus group 12–18 months after RA treatment initiation. To address a potential dropout of participants at t 2, those who decline to participate in a focus group will be invited for a repeated individual interview instead. However, the preferred interview method at t 2 remains the focus group method to align with the original design of the Belgian study.
Figure 1.
Overview of the European, longitudinal, multimethod qualitative research design. t, time point.
The reason for selecting different interview methods at t 1 and t 2 is based on the input of patient research partners and aims to match with patient preference in the context of a recent diagnosis. At t 1, the individual interview method is chosen because adjusting to a recent diagnosis can be seen as a primarily individual matter. Consequently, sharing personal experiences and opinions in a group setting can be too confronting at that stage of disease. A timeframe of 3–6 months after initiation of the initial RA treatment is chosen to not interfere with the diagnostic and therapeutic procedures, however, still including patients’ earliest views on preferred outcomes. Furthermore, it is assumed that a few months of experience with the disease and treatment would help patients to communicate more easily about their outcome preferences.
At t 2, focus groups are chosen above the individual interview method for two reasons. First, compared with the first interview moment, participants may probably feel more comfortable in a group setting, because of a grown disease perspective and the potential interaction with other patients (eg, in the waiting room) by then. Second, group interactions potentially help participants to remember significant events and bring out personal thoughts which in turn may result in more and diverse data.25 28 It is reasoned that after 12–18 months of treatment experience, participants have had sufficient time to develop their view on the disease, with perhaps an observable change in their preferences accordingly.
Research context
EQPERA involves three countries in Northwest Europe: Belgium, The Netherlands and Sweden. These countries have a comparable organised healthcare system including a comprehensive social security system; however, differences exist in for example their reimbursement and referral system.
Participants will receive usual care according to local standards. Across countries, a comparable early RA management is implemented in respect of current international guidelines29 30: patients should be treated (1) early: as soon as the diagnosis is made; (2) intensively, with methotrexate in the first treatment if possible; (3) to target: treatment adjustments according to a predefined target of sustained remission or low disease activity. In addition, there is a common culture across the countries regarding interdisciplinary team care as key in disease management, but diversity can be expected concerning implementation aspects. For example, it has been shown that there is a wide variation in the role of nurses in the management of patients with chronic inflammatory arthritis,20 and in the composition of rheumatology multidisciplinary teams.31
In each country, an early RA cohort is available, the local teams include experienced qualitative researchers with a good command of the English language, and funding possibilities are available to work out their national project. The EQPERA steering group consists of team members with different disciplinary backgrounds: nurses (KVdE, IL, EGEM, YJLvE-H), physiotherapists (AB, ADG), a psychologist (JEV), a patient representative (ADG) and a rheumatologist (RW).
Level of collaboration between countries
Individual projects will be conducted in each country. The studies in Sweden and The Netherlands will be led by the local principal investigator (IL and EGEM, respectively) and supervised by the EQPERA project leader (KVdE), who designed and completed the Belgian qualitative study.19
Considering qualitative studies, potential language issues can be approached in two ways: either translate the transcripts and do the analysis in one place, or have the analysis done at each location and combine the data afterwards. After consideration, the project team decided that (1) data will be collected in the local settings by the local teams in their native language; (2) interviews will be transcribed in the original language and the transcripts will be analysed by the local teams; (3) only the results of the local analysis (ie, interpreted data) will be combined for EQPERA purposes and this after ending the analysis procedures and writing up the findings and conclusions in every country.
Original data will thus not be reviewed by the other teams (figure 1). Centralising data would mean translation of local transcripts to the common language in EQPERA (English). Translation holds the risk of losing the real meaning of words,32 and would be expensive and time consuming because of the mountains of words that will be produced in every country. Above and beyond translation issues, we assumed that local data should ideally be analysed by the people who are familiar with the local culture and context in order to get the most appropriate interpretations.
Collaboration with patient research partners
As EQPERA aims to capture the patient perspective, the project would benefit from active collaboration with patient representatives, or those who have the lived experience of RA. Following the recommendations of the European League Against Rheumatism for the inclusion of patient representatives in scientific projects,33 each local team will preferably collaborate with two patient research partners.
The local principal investigators will be responsible for coordinating this research partnership, being guided by the Facilitate, Identify, Respect, Support and Train framework of Hewlett and colleagues.34 The exact level of the patient researchers’ contribution will depend on local agreements (feasibility). In general, they will help by reflecting on the methods, formulating clear and understandable interview questions, interpreting and explaining data, and providing feedback on the readability of the patient information leaflet and informed consent form.
Participants
Eligible patients will have to meet the following inclusion criteria: (1) confirmed diagnosis of RA, in accordance with the American College of Rheumatology/European League Against Rheumatism 2010 criteria35; (2) time between diagnosis and start of RA treatment of less or equal than 1 year; (3) minimum age of 18 years; (4) speak, read and write the local language; (5) started the initial RA treatment 3–6 months ago.
Sampling
Every country will strive to include a broad range of perspectives in their sample. To ensure this variation, participants will be purposively sampled based on their (1) age/life phase; (2) gender; and (3) treatment progress/treatment experience. Moreover, every country will apply a multicentre recruitment to account for possible variation in region.
Sampling in qualitative research corresponds to the assumption that collected data is of sufficient depth, that is, representing the various views and opinions of the population with no added value of including more participants for answering the research question.36 37 As there is no standardised definition of data saturation, we decided that data collection can be stopped if three consecutive interviews do not result in new themes or additional understanding (local team decision).
At t 1, we estimate that around 20 participants in every country will be needed to reach data saturation. At t 2, the sample sizes will foremost depend on the interest and willingness of participants to participate again. We aim for 4–8 participants in each focus group which seems an appropriate number to keep the discussions manageable and stimulate contribution of every group member.36 38 If possible, patient characteristics will be taken into account to create a mix of perspectives in the groups.
Recruitment
In each country, patients are recruited from multiple centres across different geographical locations, including academic and non-academic rheumatology centres. In Belgium, patients were sampled from nine centres across Flanders. The participating centres in The Netherlands are located in Nijmegen and Woerden, and in Sweden these are located in Lund, Malmö and Halmstad. A recruitment template will help the local teams to consider the main variables for creating heterogeneity in their samples.
Data collection
The interview guides
The semistructured interview guides include predefined topics, with open-ended questions and probing questions to reach a higher level of detail. All questions relate to the central interview question: ‘Which outcomes of your illness and antirheumatic treatment are important to you at this moment?’ In every country, the interview guides will have the same content at start, and main questions will be fixed across countries. Data collection and analysis will be performed simultaneously, making it possible to adapt the interview guides if necessary to increase participants’ understanding or to reach data saturation (local team decision). If adaptations are needed, these will be documented in the local research journal.
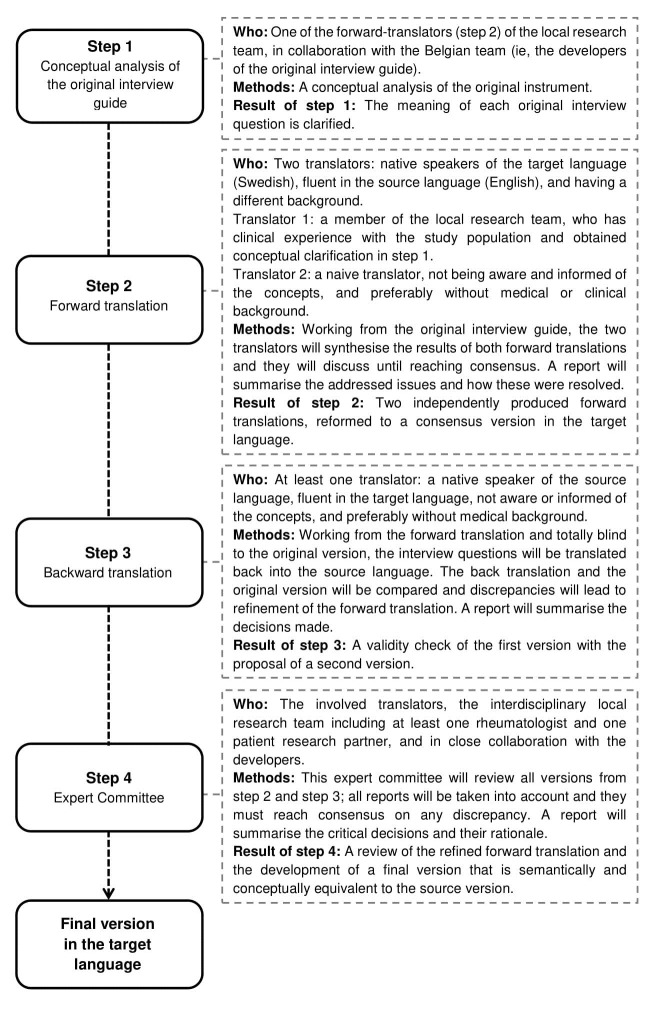
The content of the interview guides is inspired by previous qualitative studies on outcomes from the patient perspective.14 16 39 In EQPERA, Dutch and Swedish versions of the Belgian interview guides (Flemish language) will be prepared by the local teams. Given similarities between the Flemish and Dutch language, minor adaptations will be applied after discussion and consensus with the Belgian team. Forward and backward translation will be used to prepare translations into English which then will serve as a source to translate the interview guides into Swedish. The procedure of the translation from English into Swedish is presented in figure 2.40 41 The main interview questions and the interview procedures are elucidated in online supplementary file 1.
Figure 2.
Forward–backward translation framework applied to translate the interview questions and procedures.
bmjopen-2018-023606supp001.pdf (90.2KB, pdf)
Individual interviews (t 1)
At t 1, individual, face-to-face interviews will be conducted by maximum two interviewers per country, who are not involved in participants’ clinical care. As the patient research partners noted that patients are in general not used to talk about outcome preferences, they will be asked to prepare written keywords regarding the central interview question. The interviewer will start by elaborating on these keywords. It is anticipated that interviews will last no longer than 60 min.
Focus groups (t 2)
Focus groups will be facilitated by one of the interviewers of t 1 in assistance of at least one participating observer. The focus groups will consist of three rounds: round 1—preparatory phase; round 2—(1) round-robin listing, (2) developing a group list of patient-preferred outcomes, (3) eliciting preferred personal outcomes, (4) eliciting preferred outcomes in the actual stage of RA; round 3—exploring the view of participants on the evolution of their patient-preferred outcomes over the past year. The second round of the focus groups was inspired by the nominal group technique methodology (NGT).42 NGT is a consensus method that creates two types of data: (1) written ideas and prioritisation and (2) the wider discussion, generating and clarifying ideas.43 Our interest for using a prioritising methodology is first, to create discussion between participants about a potential inconvenient topic; and second, to capture participants’ underlying reasoning regarding preferences in outcomes. It is anticipated that focus groups will last about 60 min.
Individual interviews (t2)
If necessary, the interviewer of t 1 will conduct individual interviews at t 2. The interview guide for these interviews is slightly adapted compared with t 1 in order to question participants about their view on changes in their preferred outcomes over time.
Procedures at both time points
Both individual interviews and focus groups will be held at a neutral and convenient location, and will be audio-recorded and transcribed verbatim according to transcription guidelines.44
At both time points, the following information will be obtained. Prior to the (focus group) interview, participants will document socio-demographic information. They will report about their general health, level of pain and fatigue during the past week on a Visual Analogue Scale after the interviews to avoid influencing patient opinion in advance. Clinical information will be extracted from the medical records by the local health professionals and shared with the local principal investigator. A detailed overview of all collected variables can be found in online supplementary file 2.
bmjopen-2018-023606supp002.pdf (130.9KB, pdf)
Data analysis
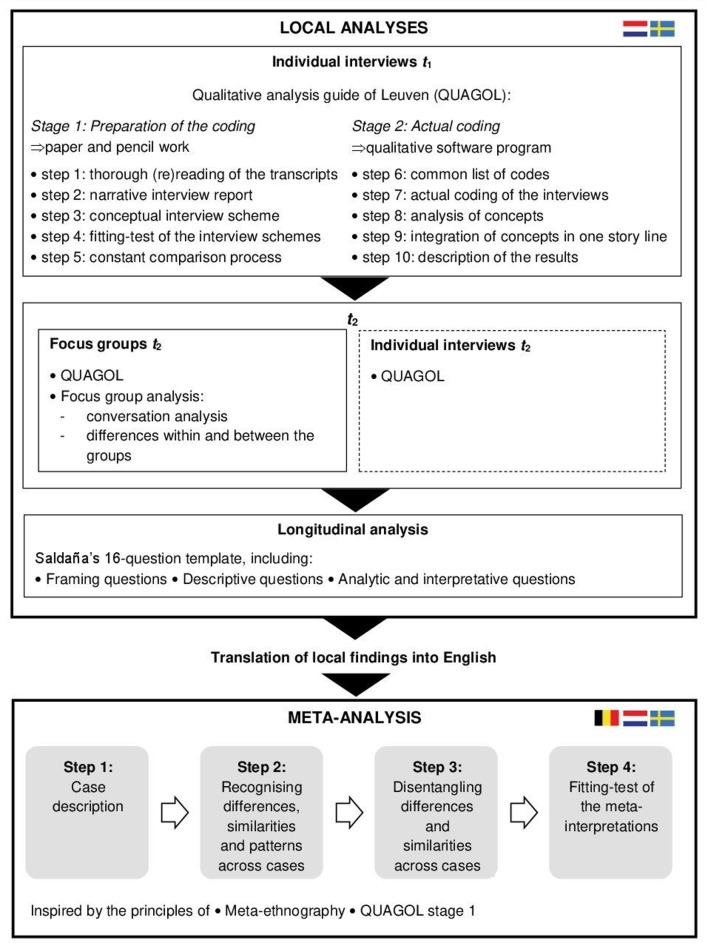
Data analysis will be conducted at two levels: (1) the local analyses of t 1 and t 2 data, followed by the longitudinal analysis; (2) the meta-analysis with locally interpreted local data. The process of data analysis was based on several frameworks which is summarised in figure 3.
Figure 3.
Simplified outline of the used frameworks,25 45–47 50 and the included steps in the local analyses and the meta-analysis.
Local analyses
In every country, the analysis process will be a team activity involving patient representatives. Preferably two researchers, including at least the local lead investigator, will independently code the interview transcripts. Data analysis will start after the first interview or focus group.
The local researchers will follow the steps that are presented in Qualitative Analysis Guide of Leuven (QUAGOL) to analyse the interview data of t 1 and t 2.45 The central activity in QUAGOL is the constant comparison process: between researchers’ interpretations and the actual participant story, as well as to check new ideas for their presence in previous interviews. QUAGOL divides data analysis into two phases.
The first phase suggests five steps of preparation, implying only paper and pencil work: (1) rereading of the transcript to get knowledge of what the interview is about, and highlighting the relevant fragments; (2) preparing a narrative summary by describing the key story lines close to participants’ words; (3) schematically describing the key ideas of the interview in a conceptual scheme; (4) fitting test and adaptation of the conceptual scheme by going back to the transcript; (5) looking for common ideas/concepts across conceptual schemes as a first comparison with the other interviews.
The second phase comprises another five steps, representing the actual coding process: (6) creating a common code list, without hierarchical structure and based on the insights from the refined conceptual schemes; (7) coding of each significant passage in a qualitative software program, while critically reviewing and refining the introduced code list; (8) defining the concepts by looking across-cases and reviewing all citations connected to a concept; (9) integration of all concepts in one story line that answers the research question, followed by verification of this overarching framework against all interviews and interview schemes; (10) describing the results.
QUAGOL is not specifically developed for focus group analysis. Therefore, the group process will also be analysed (ie, how the conversation in the group is organised, developing and changing), as well as the differences within and between the groups will be taken into account.25
For the longitudinal analysis, the local teams will merge their data of t 1 and t 2, in which meaningful individual statements will be extracted and compared between time points. There are no universal frameworks for analysing longitudinal qualitative data. The local teams will be guided by the method described by Saldaña,46 47 who developed a 16-question template including (1) framing questions to help focusing on the context and conditions that influence changes over time; (2) descriptive questions to describe what kinds of changes occur; and (3) analytic and interpretive questions to reach richer levels of analysis.
Meta-analysis
The findings of the three independently performed qualitative studies will be combined in a meta-analysis. Several methods for synthesising qualitative studies have been developed,48 with some studies also using a combination of methods.49 The methodology developed for EQPERA is inspired by the principles of meta-ethnography as practised by Britten et al,50 and by the coding process of QUAGOL (preparatory phase) that is based on grounded theory principles.45 We combined key methodological elements of both approaches and summarised these into four steps: (1) describing each case; (2) recognising differences, similarities and patterns across cases; (3) disentangling differences and similarities across cases; (4) fitting-test of the meta-interpretations.
The findings of the participating countries will be integrated by face to face interaction between the different local teams about their data in a consensus meeting. Local findings will be translated into English. The local teams of Belgium, The Netherlands and Sweden will at least consist of the principal investigator, a patient research partner and a rheumatologist to achieve an interdisciplinary view and prevent bias due to solo interpretations. A senior researcher of the EQPERA team (YJLvE-H), who is not linked to the local teams and data, will moderate the meeting. Below, we describe our stepwise approach.
Step 1: describing each case
In step 1, the aim is to understand the course and results of each study on its own. Each country will be viewed as a case, with each case reflecting the overarching story of all local participants.
The lead investigators (KVdE, IL, EGEM) will present their findings (including quotes) and conclusions, covering: (1) the name and description of the patient-preferred outcomes; (2) when, where, why and in which circumstances they were put forward by the participants; (3) the change through time of the description participants attached to the different outcomes. Furthermore, they will report about study details, using three short reports45: (1) a descriptive report, including what is specific to the participants, the treatment strategy, the research group and the healthcare system; (2) a methodological report, including deviations from the protocol, such as modifications to the interview guide, recruitment problems and level of data saturation; (3) a content report, including the main message derived from the data. A standard form will be used to enhance uniformity across presentations. The three cases will be presented one by one without immediate cross-comparison. After the case description, local teams will have familiarised with the other team’s data and the particular context in each country.
In preparation of step 2, each team will individually reflect on the following questions to stimulate the across-case analysis: ‘What do I hear in every case?’, ‘What do I only hear in our case?’, ‘What do I not hear in our case?’ Furthermore, they will write down the patient-preferred outcomes they identified (codes and concepts) on colour-coded sticky notes, each country representing another colour, to support visually the comparison of the local findings in step 2.
Step 2: recognising differences, similarities and patterns across cases
In step 2, the aim is to translate concepts from one study to another,50 to determine how studies are related (ie, what emerges across cases) and to recognise what is typical for each case.
An affinity diagram will be created to organise the multinational data.51 The patient-preferred outcomes of the three studies will be displayed side by side (using the colour-coded sticky notes). Their meaning will constantly be compared from one country to another in order to identify common and recurring, as well as conceptually different outcomes. We will start with a small set of concepts including the higher-level concepts of each study, after which we will refine our first interpretations by discussing the lower-level codes.45 During this process similar outcomes will be grouped if possible (by replacing the sticky notes), and we will look specifically for subtle differences between grouped outcomes.
After reaching consensus on similarities and differences, a ‘saturation grid’ will be completed in preparation of step 3. This is a technique used in qualitative studies to identify covered (sub)themes in each interview and decide on data saturation.52 However, we will use a prespecified grid to identify the coverage of outcomes across the three studies.50 First, the grouped outcomes will be renamed. Second, all outcomes will be listed, meaning that each outcome of each local study is encompassed by one of the renamed outcomes in the grid. The main explanation of each outcome will be added. Thirdly, each country will represent a column and their sticky notes will be pasted next to the outcome in the grid that fits best the description on the sticky note. Hence, the empty cells will represent the outcomes that do not emerge across countries. By completing the grid, an overview will be developed of differences and similarities across cases.
Step 3: disentangling differences and similarities across cases
In step 3, the aim is to explain the recognised differences and similarities by discussing why (or why not) certain outcomes emerge in a particular country or across countries.
Starting from the saturation grid (step 2), we will first go back to the methodological considerations and contextual features (step 1), before looking for possible cultural explanations. The group discussion will be an essential element in this step. For this reason we will view this discussion as a focus group, producing data that will be audio-recorded and transcribed verbatim. After step 3, we will have obtained consensus on cross-cultural variation in patient-preferred outcomes in early RA.
In preparation of step 4, the local teams will separately draft a written summary of the discussion immediately after the focus group and with special attention to how their case was similar or different to the other cases.
Step 4: fitting-test of the meta-interpretations
In step 4, the aim is to verify the appropriateness of the interpretations made during the focus group (step 3) regarding similarities and differences across countries.
Each local team will perform a fitting-test of common and own meta-interpretations with their local data. The local researchers will go back to their data, after rereading the focus group transcript and with their written summary in mind. Two questions will need to be answered: (1) Do the contextual interpretations actually reflect what is seen in our data? Is certain context information overlooked in the focus group? (2) Can we support the meta-interpretations with quotes that typically describe the perspective of our participants? During conference call meetings, the meta-interpretations will be adapted, completed or refined based on the fitting-test in each country.45
Patient and public involvement
Patients were involved in every step of the research project, as described throughout the paper. Research findings will be disseminated at Patient and Public Engagement events where appropriate.
Enhancing data quality and methodological rigour
Quality assurance
EQPERA is a large, multicountry, multicentre, multilingual, longitudinal qualitative research project. To yield sound results, several strategies are applied to ensure trustworthiness. These are: (1) recruitment of a qualified and motivated team; (2) use of forward-backward translation procedures; (3) uniformity in recruitment, conducting the interviews and focus groups, transcription of audio files, data coding, data storing and reporting; (4) interdisciplinary team analysis and (5) training of local staff to the protocol and hands-on guidance by the project leader. In table 1, a detailed description is provided of the used strategies according to four quality criteria (ie, credibility, dependability, confirmability and transferability).53 54
Table 1.
Applied quality assurance strategies in EQPERA, described for each research stage, according to Lincoln and Guba’s framework for evaluating trustworthiness53
| Research stage | Employed strategies for supporting trustworthiness | Assessing quality: (1) How congruent are the findings with reality? (2) Would the research findings be the same if the study would be replicated in essentially the same way? (3) Do the research findings emerge from the context and the respondents and not solely from the minds of the researchers? (4) Can the research be applied in other contexts? |
|||
| (1) Credibility (internal validity) |
(2) Dependability (reliability) |
(3) Confirmability (objectivity) |
(4) Transferability (generalisability) |
||
| Study design |
|
● | |||
|
● | ||||
|
● | ||||
| Establishment of the EQPERA team |
|
● | ● | ● | ● |
| Protocol development and implementation |
|
● | ● | ||
|
● | ● | |||
|
● | ● | |||
|
● | ● | |||
|
● | ● | ● | ● | |
| Sampling and recruitment |
|
● | ● | ||
|
● | ● | |||
|
● | ● | |||
|
● | ||||
| Data collection |
|
● | ● | ● | |
|
● | ||||
|
● | ||||
|
● | ||||
|
● | ||||
| t1 |
|
● | |||
| t2 |
|
● | |||
| Data analysis Local level |
|
● | ● | ||
|
● | ||||
|
● | ||||
|
● | ● | |||
|
● | ● | |||
|
● | ||||
|
● | ● | |||
|
● | ● | |||
|
● | ● | |||
| International level |
|
● | |||
| Reporting |
|
● | |||
EQPERA, European Qualitative research project on Patient-preferred outcomes in Early Rheumatoid Arthritis; t 1, time point 1=3–6 months after start of the initial treatment for early rheumatoid arthritis; t 2, time point 2=at least 1 year after start of the initial treatment for early rheumatoid arthritis.
Quality appraisal
As the findings of independently performed primary studies will be combined, quality is an important aspect to consider requiring a formal system for appraisal. The local teams will use a quality reporting tool to support a consistent use of methods and documentation across studies. Johnson et al provided a useful template,51 based on the consolidated criteria for reporting qualitative research,55 and the quality criteria suggested by Mays and Pope.56 In EQPERA, several items were added regarding data management and quality appraisal in qualitative research.32 44 57–59 Our tool comprises 50 items regarding four domains: (1) research team and reflexivity, (2) study design, (3) analysis and findings, (4) data management strategies (online supplementary file 3).
bmjopen-2018-023606supp003.pdf (176.5KB, pdf)
Ethics and dissemination
Ethical considerations
EQPERA will apply the principles established in the Declaration of Helsinki.60 Participants will provide written informed consent before data collection of t 1 and t 2. Only coded and interpreted data will be shared between the local teams for the meta-analysis. Ethics approval for the original studies were granted by the responsible institutional review boards.
Dissemination of results
Every country will prepare a publication on their national findings. Two EQPERA main papers are foreseen: (1) the present paper describes the rationale, design and methods of EQPERA; (2) a publication on the results of the meta-analysis. Next to peer-reviewed publications, we will also disseminate our findings in (inter)national research presentations, and also patient organisations will be updated about the study findings.
Conclusion
In EQPERA, the aim is to confirm the Belgian findings on patient-preferred outcomes in early RA in a European context, and provide a study protocol that has the potential to offer a methodological framework for further exploration of transferability in other contexts. Ultimately, study findings will be used to inform and optimise current care initiatives in early RA in order to address the unmet need of patient-centred care in RA.
Supplementary Material
Acknowledgments
We wish to thank Patrick Verschueren and Bernadette Dierckx de Casterlé for sharing their methodological advice on the meta-analysis approach.
Footnotes
Contributors: The following authors were involved in this study: KVdE, AB, ADG, IL, EGEM, JEV, RW, YJLvE-H. KVdE and RW had the main idea of the study. KVdE, AB, ADG, IL, EGEM, JEV, RW and YJLvE-H contributed to the design of the study. KVdE, YJLvE-H and RW drafted the manuscript. KVdE, AB, ADG, IL, EGEM, JEV, RW and YJLvE-H were involved in the editing of the manuscript. All authors read and approved the final version of the manuscript. Apart from the first and last authors, the other authors are listed in alphabetical order.
Funding: This work was supported by an unrestricted educational grant of Bristol-Myers Squibb, by a travel grant from Fonds voor Wetenschappelijk Reuma Onderzoek (fund for Scientific Rheumatism Research) (Belgium) and by Southern Health Care Region (Sweden).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2. Sokka T, Hetland ML, Mäkinen H, et al. Remission and rheumatoid arthritis: data on patients receiving usual care in twenty-four countries. Arthritis Rheum 2008;58:2642–51. 10.1002/art.23794 [DOI] [PubMed] [Google Scholar]
- 3. Andersson MLE, Forslind K, Hafström I, et al. Patients with early rheumatoid arthritis in the 2000s have equal disability and pain despite less disease activity compared with the 1990s: data from the BARFOT Study over 8 Years. J Rheumatol 2017;44:723–31. 10.3899/jrheum.161235 [DOI] [PubMed] [Google Scholar]
- 4. Taylor PC, Moore A, Vasilescu R, et al. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int 2016;36:685–95. 10.1007/s00296-015-3415-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokka T, Kautiainen H, Pincus T, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther 2010;12:R42 10.1186/ar2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dures E, Almeida C, Caesley J, et al. Patient preferences for psychological support in inflammatory arthritis: a multicentre survey. Ann Rheum Dis 2016;75:142–7. 10.1136/annrheumdis-2014-205636 [DOI] [PubMed] [Google Scholar]
- 7. Zuidema RM, Repping-Wuts H, Evers AW, et al. What do we know about rheumatoid arthritis patients’ support needs for self-management? A scoping review. Int J Nurs Stud 2015;52:1617–24. 10.1016/j.ijnurstu.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 8. Winthrop KL, Strand V, van der Heijde D, et al. The unmet need in rheumatology: reports from the targeted therapies meeting 2017. Clin Immunol 2018;186:87–93. 10.1016/j.clim.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 9. Washburn ER. Fast forward: a blueprint for the future from the Institute of Medicine. Physician Exec 2001;27:8–14. [PubMed] [Google Scholar]
- 10. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA 2013;310:2503–4. 10.1001/jama.2013.281422 [DOI] [PubMed] [Google Scholar]
- 11. Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med 2000;51:1087–110. 10.1016/S0277-9536(00)00098-8 [DOI] [PubMed] [Google Scholar]
- 12. Hsiao B, Fraenkel L. Incorporating the patient’s perspective in outcomes research. Curr Opin Rheumatol 2017;29:144–9. 10.1097/BOR.0000000000000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor PC. The importance of the patients’ experience of RA compared with clinical measures of disease activity. Clin Exp Rheumatol 2010;28:S28–31. [PubMed] [Google Scholar]
- 14. Carr A, Hewlett S, Hughes R, et al. Rheumatology outcomes: the patient’s perspective. J Rheumatol 2003;30:880–3. [PubMed] [Google Scholar]
- 15. Sanderson T, Morris M, Calnan M, et al. Patient perspective of measuring treatment efficacy: the rheumatoid arthritis patient priorities for pharmacologic interventions outcomes. Arthritis Care Res 2010;62:647–56. 10.1002/acr.20151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanderson T, Morris M, Calnan M, et al. What outcomes from pharmacologic treatments are important to people with rheumatoid arthritis? Creating the basis of a patient core set. Arthritis Care Res 2010;62:640–6. 10.1002/acr.20034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hehir M, Carr M, Davis B, et al. Nursing support at the onset of rheumatoid arthritis: Time and space for emotions, practicalities and self-management. Musculoskeletal Care 2008;6:124–34. 10.1002/msc.115 [DOI] [PubMed] [Google Scholar]
- 18. Griffith J, Carr A. What is the impact of early rheumatoid arthritis on the individual? Best Pract Res Clin Rheumatol 2001;15:77–90. 10.1053/berh.2000.0127 [DOI] [PubMed] [Google Scholar]
- 19. van der Elst K, Meyfroidt S, De Cock D, et al. Unraveling patient-preferred health and treatment outcomes in early rheumatoid arthritis: a longitudinal qualitative study. Arthritis Care Res 2016;68:1278–87. 10.1002/acr.22824 [DOI] [PubMed] [Google Scholar]
- 20. van Eijk-Hustings Y, Ndosi M, Buss B, et al. Dissemination and evaluation of the european league against rheumatism recommendations for the role of the nurse in the management of chronic inflammatory arthritis: results of a multinational survey among nurses, rheumatologists and patients. Rheumatology 2014;53:1491–6. 10.1093/rheumatology/keu134 [DOI] [PubMed] [Google Scholar]
- 21. Stamm T, Hill J. Extended roles of non-physician health professionals and innovative models of care within Europe: results from a web-based survey. Musculoskeletal Care 2011;9:93–101. 10.1002/msc.201 [DOI] [PubMed] [Google Scholar]
- 22. van Tuyl LH, Sadlonova M, Hewlett S, et al. The patient perspective on absence of disease activity in rheumatoid arthritis: a survey to identify key domains of patient-perceived remission. Ann Rheum Dis 2017;76:855–61. 10.1136/annrheumdis-2016-209835 [DOI] [PubMed] [Google Scholar]
- 23. Hifinger M, Putrik P, Ramiro S, et al. In rheumatoid arthritis, country of residence has an important influence on fatigue: results from the multinational COMORA study. Rheumatology 2016;55:735–44. 10.1093/rheumatology/kev395 [DOI] [PubMed] [Google Scholar]
- 24. Sofaer S. Qualitative methods: what are they and why use them? Health Serv Res 1999;34:1101–18. [PMC free article] [PubMed] [Google Scholar]
- 25. Flick U. An introduction to qualitative research. London: Sage Publications, 2014. [Google Scholar]
- 26. van Tuyl LH, Hewlett S, Sadlonova M, et al. The patient perspective on remission in rheumatoid arthritis: ‘You’ve got limits, but you’re back to being you again’. Ann Rheum Dis 2015;74:1004–10. 10.1136/annrheumdis-2013-204798 [DOI] [PubMed] [Google Scholar]
- 27. Corbin JM, Strauss A. A nursing model for chronic illness management based upon the Trajectory Framework. Sch Inq Nurs Pract 1991;5:155–74. [PubMed] [Google Scholar]
- 28. Coenen M, Stamm TA, Stucki G, et al. Individual interviews and focus groups in patients with rheumatoid arthritis: a comparison of two qualitative methods. Qual Life Res 2012;21:359–70. 10.1007/s11136-011-9943-2 [DOI] [PubMed] [Google Scholar]
- 29. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 30. Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017;76:948–59. 10.1136/annrheumdis-2016-210602 [DOI] [PubMed] [Google Scholar]
- 31. Ndosi M, Ferguson R, Backhouse MR, et al. National variation in the composition of rheumatology multidisciplinary teams: a cross-sectional study. Rheumatol Int 2017;37:1453–9. 10.1007/s00296-017-3751-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White DE, Oelke ND, Friesen S. Management of a large qualitative data set: establishing trustworthiness of the data. Int J Qual Methods 2012;11:244–58. 10.1177/160940691201100305 [DOI] [Google Scholar]
- 33. de Wit MP, Berlo SE, Aanerud GJ, et al. European league against rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis 2011;70:722–6. 10.1136/ard.2010.135129 [DOI] [PubMed] [Google Scholar]
- 34. Hewlett S, Wit M, Richards P, et al. Patients and professionals as research partners: challenges, practicalities, and benefits. Arthritis Rheum 2006;55:676–80. 10.1002/art.22091 [DOI] [PubMed] [Google Scholar]
- 35. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rheumatism 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 36. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229–45. 10.1080/08870440903194015 [DOI] [PubMed] [Google Scholar]
- 37. Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep 2015;20:1408–16. [Google Scholar]
- 38. Carlsen B, Glenton C. What about N? A methodological study of sample-size reporting in focus group studies. BMC Med Res Methodol 2011;11:26 10.1186/1471-2288-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marshall NJ, Wilson G, Lapworth K, et al. Patients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: a qualitative study. Rheumatology 2004;43:1034–8. 10.1093/rheumatology/keh237 [DOI] [PubMed] [Google Scholar]
- 40. Acquadro C, Kopp Z, Coyne KS, et al. Translating overactive bladder questionnaires in 14 languages. Urology 2006;67:536–40. 10.1016/j.urology.2005.09.035 [DOI] [PubMed] [Google Scholar]
- 41. Beaton DE, Bombardier C, Guillemin F, et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000;25:3186–91. 10.1097/00007632-200012150-00014 [DOI] [PubMed] [Google Scholar]
- 42. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drennan V, Walters K, Lenihan P, et al. Priorities in identifying unmet need in older people attending general practice: a nominal group technique study. Fam Pract 2007;24:454–60. 10.1093/fampra/cmm034 [DOI] [PubMed] [Google Scholar]
- 44. Bailey J. First steps in qualitative data analysis: transcribing. Fam Pract 2008;25:127–31. 10.1093/fampra/cmn003 [DOI] [PubMed] [Google Scholar]
- 45. Dierckx de Casterlé B, Gastmans C, Bryon E, et al. QUAGOL: a guide for qualitative data analysis. Int J Nurs Stud 2012;49:360–71. 10.1016/j.ijnurstu.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 46. Saldaña J. Longitudinal qualitative research: analyzing change through time. Walnut Creek, CA: AltaMira Press, 2003. [Google Scholar]
- 47. Saldaña J. Analyzing Change in Longitudinal Qualitative Data. Youth Theatre Journal 2002;16:1–17. 10.1080/08929092.2002.10012536 [DOI] [Google Scholar]
- 48. Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9:59 10.1186/1471-2288-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mohammed MA, Moles RJ, Chen TF. Meta-synthesis of qualitative research: the challenges and opportunities. Int J Clin Pharm 2016;38:695–704. 10.1007/s11096-016-0289-2 [DOI] [PubMed] [Google Scholar]
- 50. Britten N, Campbell R, Pope C, et al. Using meta ethnography to synthesise qualitative research: a worked example. J Health Serv Res Policy 2002;7:209–15. 10.1258/135581902320432732 [DOI] [PubMed] [Google Scholar]
- 51. Johnson JK, Barach P, Vernooij-Dassen M, et al. Conducting a multicentre and multinational qualitative study on patient transitions. BMJ Qual Saf 2012;21:i22–8. 10.1136/bmjqs-2012-001197 [DOI] [PubMed] [Google Scholar]
- 52. Morse JM. The significance of saturation. Qual Health Res 1995;5:147–9. 10.1177/104973239500500201 [DOI] [Google Scholar]
- 53. Lincoln YS, Guba EG, Pilotta JJ. Naturalistic inquiry. 9 Beverly Hills, CA: Sage Publications, 1985:438–9. [Google Scholar]
- 54. Polit DF, Beck CT. Nursing research: generating and assessing evidence for nursing practice. Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins, 2008. [Google Scholar]
- 55. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 56. Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50–2. 10.1136/bmj.320.7226.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson C. Presenting and evaluating qualitative research. Am J Pharm Educ 2010;74:141 10.5688/aj7408141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Johnson BD, Dunlap E, Benoit E. Organizing “mountains of words” for data analysis, both qualitative and quantitative. Subst Use Misuse 2010;45:648–70. 10.3109/10826081003594757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shenton AK. Strategies for ensuring trustworthiness in qualitative research projects. Education for Information 2004;22:63–75. 10.3233/EFI-2004-22201 [DOI] [Google Scholar]
- 60. World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 61. Tong A, Flemming K, McInnes E, et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol 2012;12:181 10.1186/1471-2288-12-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023606supp001.pdf (90.2KB, pdf)
bmjopen-2018-023606supp002.pdf (130.9KB, pdf)
bmjopen-2018-023606supp003.pdf (176.5KB, pdf)