Significance
In eukaryotes, the genomic DNA is organized in chromatin that consists of nucleosomal units formed by histone proteins. The accessibility of genes for RNA polymerase II transcription by the dynamic modification of histone tails during the transcript elongation phase is emerging as an important regulatory mechanism. Here, we show that RNA-binding proteins, together with the conserved HISTONE MONOUBIQUITINATION1–HISTONE MONOUBIQUITINATION2 complex that mediates histone H2B monoubiquitination at the circadian clock, and flowering time-regulatory genes during transcript elongation are required for the processing of their pre-mRNA and antisense RNA, respectively.
Keywords: H2Bub, HUB1 interactome, RNA-binding protein, RRM domain, KH domain
Abstract
HISTONE MONOUBIQUITINATION1 (HUB1) and its paralog HUB2 act in a conserved heterotetrameric complex in the chromatin-mediated transcriptional modulation of developmental programs, such as flowering time, dormancy, and the circadian clock. The KHD1 and SPEN3 proteins were identified as interactors of the HUB1 and HUB2 proteins with in vitro RNA-binding activity. Mutants in SPEN3 and KHD1 had reduced rosette and leaf areas. Strikingly, in spen3 mutants, the flowering time was slightly, but significantly, delayed, as opposed to the early flowering time in the hub1-4 mutant. The mutant phenotypes in biomass and flowering time suggested a deregulation of their respective regulatory genes CIRCADIAN CLOCK-ASSOCIATED1 (CCA1) and FLOWERING LOCUS C (FLC) that are known targets of the HUB1-mediated histone H2B monoubiquitination (H2Bub). Indeed, in the spen3-1 and hub1-4 mutants, the circadian clock period was shortened as observed by luciferase reporter assays, the levels of the CCA1α and CCA1β splice forms were altered, and the CCA1 expression and H2Bub levels were reduced. In the spen3-1 mutant, the delay in flowering time was correlated with an enhanced FLC expression, possibly due to an increased distal versus proximal ratio of its antisense COOLAIR transcript. Together with transcriptomic and double-mutant analyses, our data revealed that the HUB1 interaction with SPEN3 links H2Bub during transcript elongation with pre-mRNA processing at CCA1. Furthermore, the presence of an intact HUB1 at the FLC is required for SPEN3 function in the formation of the FLC-derived antisense COOLAIR transcripts.
In eukaryotic cells, the genomic DNA is organized in nucleosomes that consist of 146-bp-long DNA wrapped around an octamer of the “core” histone dimers of H2A, H2B, H3, and H4 (1), whereas linker DNA and histone H1 connect adjacent nucleosomes. The chromatin structure is highly dynamic, with nucleosomal histone tail modifications, such as methylation, acetylation, and ubiquitination that regulate the DNA availability to the RNA polymerase II (RNAPII) transcription. The major chromatin state for active genes in Arabidopsis thaliana is determined by histone H2B monoubiquitination (H2Bub), histone H3 acetylation, and methylation (2). H2Bub is absent from the Arabidopsis promoter regions, peaks at the gene bodies, and is required for maximal gene-expression levels, implying that this histone modification is specifically linked with transcript elongation (3–6). In yeast, the E3 ubiquitin ligase Bre1 and the E2-conjugating enzyme Rad6 form a complex that catalyzes the H2B-K123 monoubiquitination (7, 8). In Arabidopsis, the relatives HISTONE MONOUBIQUITINATION1 (HUB1) and HUB2 E3 ubiquitin ligases and the UBC1 and UBC2 E2-conjugating enzymes work also together in a complex to monoubiquitinate H2B (9–12). In hub1, hub2, and ubc1 ubc2 double mutants, H2Bub was reduced at the FLOWERING LOCUS C (FLC)/MADS AFFECTING FLOWERING (MAF) genes, resulting in reduced FLC/MAF gene-expression levels and early flowering (11–13). Monoubiquitinated H2B associated specifically with the gene body of the FLC clade genes and was necessary for enhancement of H3K4me3 and H3K36me2 and transcriptional activation in the FLC/MAF chromatin. Strikingly, the gene-expression levels of FLC and relatives were also reduced in the Arabidopsis sup32/ubp26 deubiquitination mutants that were early flowering. The accumulated H2Bub marks at the FLC led to a depletion of the activating H3K36me3 marks and an increase in repressive H3K27me3 marks, indicating that both H2B monoubiquitination and deubiquitylation and their steady state are critical in the deposition of other activating histone marks during transcript elongation and in the proper gene activation (14), as also demonstrated in the yeast Ubp8 deubiquitylation mutant (15).
Proteins interacting with the H2Bub machinery might represent regulators of H2Bub dynamics, transcript elongation efficiency, target gene specificity, or might identify a link to pre-mRNA processing or upstream signaling. In humans, H2B deubiquitylation enzymes are required for efficient cotranscriptional pre-mRNA splicing (16, 17), whereas in yeast, H2B monoubiquitination and deubiquitylation enzymes genetically interact with the Npl3 SR-like protein in splicing (18).
To further elucidate the H2Bub regulation and function in plants, we purified interacting proteins with HUB1 and HUB2 as baits and detected previously uncharacterized RNA-binding motif-containing proteins. SPEN3 and KHD1 bound RNA in an in vitro assay and their respective mutants showed circadian clock and flowering-time phenotypes, two pathways that are also targeted by the HUB1/HUB2 complex activity. SPEN3 functioned in pre-mRNA processing at the CCA1 regulatory gene in concert with HUB1/HUB2-mediated H2Bub and in antisense COOLAIR transcript formation at the FLC regulatory gene, independently of H2Bub.
Results and Discussion
KHD1 and SPEN3 Identified as Core Components of the HUB1/HUB2 Complex.
To detect HUB1-associated proteins that might represent upstream regulators, cofactors, or components of the core complex, several tandem-affinity purifications (TAPs) were carried out with Arabidopsis cell cultures overexpressing N-terminally tagged full-length HUB1 and HUB2 proteins and a modified HUB1, with two point mutations introduced into the RING domain by replacing two cysteines by serines (C826S and C829S), resulting in HUB1pm, similarly to the yBre1 mutations (19). With HUB1 as bait, only HUB2 and the RNA-binding domain-containing SPEN3 and KHD1 were retained in several TAPs (Table 1 and Dataset S1). The identified protein list was filtered for background proteins, according to standard procedures (20), and only the IDs represented by two significant peptides in at least two independent TAP purifications were kept.
Table 1.
Proteins identified by MS in TAP eluates of Arabidopsis cell cultures with tagged HUB1, HUB1pm (with mutated RING domain), HUB2, and SPEN3 as baits
| Bait | Tag | No.TAPs | No. of TAPs with identified protein | |||
| HUB1 | HUB2 | KHD1 | SPEN3 | |||
| At2G44950 | At1G55250 | At1G51580 | At1G27750 | |||
| HUB1 | N-TAP | 2 | 2 | 2 | 2 | 2 |
| HUB1 | N-GS | 4 | 4 | 4 | 3 | 2 |
| HUB1pm | N-TAP | 3 | 3 | 0 | 0 | 0 |
| HUB2 | N-GS | 2 | 2 | 2 | 2 | 2 |
| SPEN3 | C-GS | 4 | 2 | 2 | 3 | 4 |
Reverse TAP with either HUB2 or SPEN3 as bait purified HUB1, KHD1, and SPEN3, or HUB1, HUB2, and KHD1 proteins, respectively. Hence, the SPEN3 and KHD1 proteins are part of the HUB1–HUB2 complex interactome. Upon introduction of point mutations into the RING domain of HUB1 (HUB1pm), no HUB2, SPEN3, and KHD1 interactions could be detected (Table 1), suggesting that the RING domain could be essential for the heterodimerization and formation of the complex with other interactors. A pairwise yeast two-hybrid system (Y2H) corroborated strong HUB1/HUB1 and HUB1/HUB2, but not HUB2/HUB2 interactions, as already reported (13).
Interestingly, a strong pairwise interaction between HUB1 and SPEN3 was demonstrated at 3 mM and 10 mM 3-AT (SI Appendix, Fig. S1 A and B) and required both N and C termini of SPEN3 (SI Appendix, Fig. S1 B–D). The N-terminal SPEN3 fragment weakly interacted with HUB1 as shown at 3 mM 3-AT, whereas the C-terminal SPEN3 fragment did not at all interact with HUB1 (SI Appendix, Fig. S1 B–D). Interactions with KHD1 could not be tested due to self-activation of the fusion protein. In conclusion, two RNA-binding domain proteins, SPEN3 and KHD1, were identified by TAP as integral part of the HUB1/HUB2 core complex and a strong and direct interaction for SPEN3 with HUB1 was confirmed by Y2H.
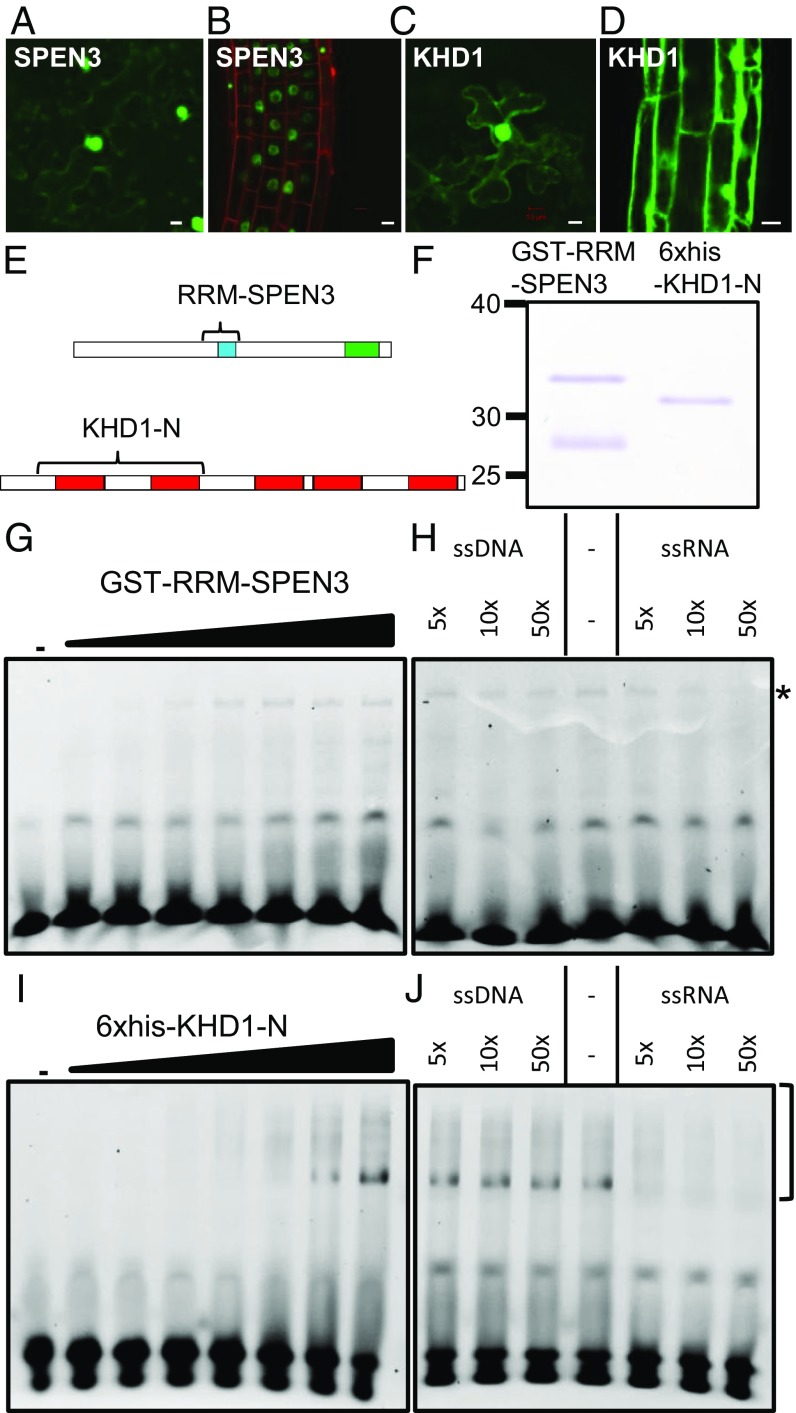
The fusion constructs GFP–SPEN3 and GFP–KHD1 were transiently expressed upon infiltration of Nicotiana benthamiana (tobacco) leaves with agrobacteria and stably expressed in Arabidopsis lines obtained by floral dip. The GFP–SPEN3 fluorescence was located exclusively in the nucleus, excluding the nucleolus, of leaf (Fig. 1A) and primary root (Fig. 1B) epidermis cells, whereas the GFP–KHD1 fluorescence occurred in the nucleus, with the exclusion of the nucleolus, in the leaf (Fig. 1C) and root (Fig. 1D) epidermis, in the cytoplasm around the nucleus, and near the plasmalemma, similar to the GFP–HUB1 and GFP–HUB2 localization (10).
Fig. 1.
SPEN3 and KHD1 localization and RNA binding. (A–D) Detection of GFP::SPEN3 and GFP::KHD1 upon infiltration of N. benthamiana leaves (A and C) and stable transgenic A. thaliana roots (B and D). (Scale bars, 10 µm.) (E) Schemes of the SPEN3 and KHD1 proteins with their functional domains, RRM (blue), SPOC (green), and KH (red). Bracket indicates the part that was expressed in E. coli and purified for EMSA. (F) SDS/PAGE analysis of purified GST-RRM-SPEN3 and 6×His-KHD1-N proteins, stained with Coomassie brilliant blue. GST-RRM-SPEN3 is partially cleaved in E. coli and the band migrating below the fusion protein is free GST, as determined by MS. (G–J) Comparison of the binding of GST-RRM-SPEN3 (G) or 6×His-KHD1-N (I) to ssRNA, and competition assay with unlabeled ssRNA or ssDNA for analysis of GST-RRM-SPEN3 (H) or 6×His-KHD1-N (J). For titrations (G and I), the Cy3-labeled 25-nucleotide probe was incubated either in the absence (lane 1) or in the presence of increasing protein concentrations (0.1, 0.2, 0.5, 1, 2, 3, and 5 µM) (lanes 2–8, respectively). Samples were analyzed by PAGE. For competition assays, the Cy3-ssRNA 25-nucleotide probe was incubated with 3 µM of protein in all samples and binding to the labeled probe was competed (as indicated) with increasing concentrations of 25-bp ssDNA or ssRNA (5×, 10×, and 50× excess over Cy3-ssRNA). The bottom bands correspond to the unbound RNA and the asterisk or brackets indicate the protein–RNA complexes.
The SPEN3 and KHD1 Proteins Contain RNA-Binding Domains.
The SPEN3 and KHD1 interactors of the HUB1/HUB2 complex contain widely spread protein domains involved in RNA binding in eukaryotes, i.e., an RNA recognition motif (RRM) and the K homology (KH) motif, respectively (21). The SPEN3 gene (At1g27750) is 4,326 nucleotides long, contains eight exons, and encodes a 117.47-kDa protein. Domain search performed with the InterProScan tool (www.ebi.ac.uk/interpro) revealed the presence of a conserved RRM and a Spen Paralog and Ortholog C-terminal (SPOC) protein-binding domain (SI Appendix, Fig. S2A), similarly to the mammalian Split Ends (Spen) proteins that function in transcriptional regulation, posttranscriptional processing, and nuclear export of mRNA (22). In humans, Spen proteins interact with histone modification enzymes such as histone deacetylases (23, 24) and histone methyltransferases (25). Some of the mammalian Spen proteins regulate gene expression via the control of splicing activity (26–28). In the Arabidopsis genome, only three Spen proteins were retrieved that combined SPOC with one or more RRM motifs: (i) the SPEN3 protein studied in this work with one RRM, (ii) the SPEN2 (AT4G12640) protein with two RRMs with an unknown function [because the knockout and overexpressing lines had no apparent phenotype (29)], and (iii) the flowering-time regulator FPA with three RRMs that controls alternative splicing and polyadenylation of antisense transcripts of the floral repressor FLC (30). The role in flowering-time regulation has also been suggested in rice (Oryza sativa) for the Spen protein encoded by the OsRRMh gene with two RRM motifs, as indicated by the flowering-time delay in the knockdown line (31). Spen proteins with one, two, or three RRM domains occur throughout eudicots. The Spen protein cladogram of five selected eudicots created with the MEGAX bioinformatics tool showed clustering of all single-RRM domain Spen proteins, including SPEN3 (SI Appendix, Fig. S2A).
The KHD1 gene (At1g51580) is 2,491 nucleotides long, consists of seven exons, and encodes a 67.12-kDa protein. The InterProScan analysis identified five KH domains in the KHD1 protein, ranging from 70 to 77 amino acids (SI Appendix, Fig. S2B). KH motifs were found in proteins of archeae, bacteria, and eukaryota with five conserved KH domains ranging from 70 to 77 amino acids (SI Appendix, Fig. S2B). In Arabidopsis, 37 unique AGI codes of proteins containing 1–5 KH domains were present. All proteins with three or more KH motifs (including KHD1) had no other domains. Proteins containing only one or two KH motifs frequently combined up to eight different domains. A cladogram was generated of 12 proteins with three, four, or five KH domains (SI Appendix, Fig. S2B). KHD1 grouped with HUA ENHANCER4 (HEN4; At5g64390) that facilitates the processing of AGAMOUS pre-mRNA (32) and REGULATOR OF CBF GENE EXPRESSION 3/SHINY1/HIGH OSMOTIC STRESS GENE EXPRESSION 5 (RCF3/SHI1/HOS5; At5g53060) that is involved in pre-mRNA processing (33). KH proteins with three KH motifs clustered together, among which FLOWERING LOCUS KH DOMAIN (FLK) and PEPPER (PEP), an FLC repressor and activator, respectively (34, 35).
The in planta mRNA-binding proteome datasets (21, 36–38) that represent RNA interactors in a number of tissues and developmental stages contained KHD1 (21, 37), suggesting a role in poly(A) RNA-related processes. These datasets do not cover all RNA interactors, possibly accounting for the absence of SPEN3 or hinting at pre-mRNA or noncoding RNA rather than poly(A) RNA binding of SPEN3.
SPEN3 and KHD1 Bind RNA.
To test the in vitro RNA-binding capabilities of the SPEN3 protein, its RRM-containing region was selected because the production of the full-length protein in Escherichia coli proved unsuccessful. A GST-RRM-SPEN3 fusion was expressed and purified by affinity chromatography (Fig. 1 E and F). The RNA binding of the purified GST-RRM-SPEN3 was examined by EMSAs. Incubating increasing concentrations of the recombinant protein with the fluorescently labeled single-stranded (ss)RNA, followed by EMSA analysis, revealed a dose-dependent interaction of GST-RRM-SPEN3 with the RNA probe, starting at a protein concentration of 0.2 µM (Fig. 1G). To test the preference of the protein for RNA, competition experiments with increasing amounts of unlabeled ssRNA or ssDNA were carried out (Fig. 1H). The protein–RNA complex formed at a fixed concentration of GST-RRM-SPEN3 (3 µM) and a constant amount of labeled RNA probe is competed by the addition of a 50-fold excess of unlabeled ssRNA. In contrast, protein–RNA complex formation was hardly affected by the addition of a 50-fold excess of unlabeled ssDNA, demonstrating that the GST-RRM-SPEN3 displays a preference for ssRNA over ssDNA.
To test the RNA-binding properties of KHD1, we expressed the region comprising the two N-terminal KH domains, as a 6×His-tagged fusion protein, designated 6×His-KHD1-N, in E. coli (Fig. 1 E and F), because the production of the full-length protein was also unsuccessful. The recombinant protein was purified by metal-chelate affinity chromatography (Fig. 1F). In EMSAs, a dose-dependent interaction with ssRNA was observed, starting at a protein concentration of 0.5 µM (Fig. 1I). In a competition assay (Fig. 1J), the complex formed by a fixed amount of 6×His-KHD1-N (3 µM) and the labeled ssRNA probe was efficiently competed by the addition of excess amounts of unlabeled ssRNA, whereas the addition of ssDNA did not affect the detected protein–RNA complex. Therefore, 6×His-KHD1-N preferentially interacts with the ssRNA probe.
The protein–nucleic acid interactions were additionally analyzed by means of microscale thermophoresis (MST). MST is an in-solution method to detect molecular interactions, due to the changes in thermophoretic mobility or fluorescence of interacting molecules, in a microscopic temperature gradient (39). Increasing concentrations of GST-RRM-SPEN3 resulted in enhanced binding to ssRNA, visualized as bound fraction (SI Appendix, Fig. S3A), whereas no binding occurred to ssDNA. Due to the weak RNA-binding activity, no complete binding of the SPEN3 protein to the nucleic acids was observed at the highest protein concentration used. Because of the variance in the three biological replicates and the lack of binding saturation, no exact KD affinity values could be derived, but the preferential binding of SPEN3 to ssRNA was evident from the sloping curve for ssRNA (SI Appendix, Fig. S3A) compared with the absence of slope for ssDNA, hence confirming the EMSA data. The qualitative analysis of 6×His-KHD1-N binding to ssRNA and ssDNA by MST revealed a complex pattern of changes in the thermophoretic mobility of the interacting molecules (for details, see SI Appendix, Fig. S3), but the experiment supports the EMSA results, showing an enhanced binding to ssRNA relative to ssDNA (SI Appendix, Fig. S3B).
Thus, EMSA and MST experiments showed that SPEN3 and KHD1 interact preferentially with ssRNA. RNA-binding proteins frequently contain more than one RNA-binding region (i.e., additional KH domains in the case of KHD1), possibly increasing the RNA-binding affinity (40). Hence, the relatively low affinity for ssRNA observed in our assays relates most probably to the use for technical reasons of truncated SPEN3 and KHD1 proteins (Fig. 1E).
Growth and Flowering Time in spen3, khd1, and hub1/2 Mutants.
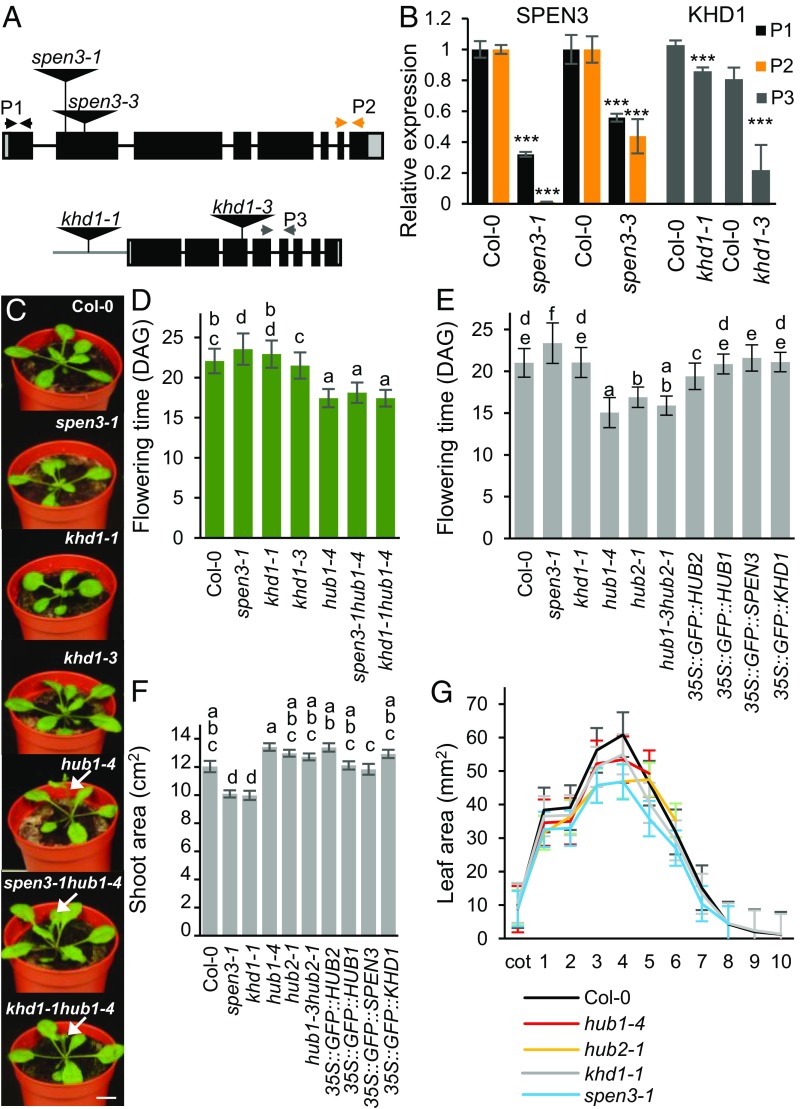
The expressions of HUB1, KHD1, and SPEN3 in the shoot apex and the root apical meristem were analyzed with whole-mount, multiprobe in situ hybridizations of 4-d-old seedlings grown in vitro with sequence-specific probes for SPEN3, KHD1, and HUB1 genes. A red fluorochrome label was used for SPEN3, a green one for KHD1, and a blue one for HUB1. Interestingly, HUB1, SPEN3, and KHD1 coexpressed strongly in the shoot apical meristem, visible as the white-pink complementary color of green, red, and blue (SI Appendix, Fig. S4A). The SPEN3 and HUB1 genes were coexpressed in cotelydons and KHD1 and HUB1 in leaf primordia and vascular tissues. In primary roots, the three genes did not coexpress, but SPEN3 and KHD1 coexpressed in the cortex, stele, and root apical meristem and SPEN3 with HUB1 in the epidermal cell layers (SI Appendix, Fig. S4B). The whole-mount in situ expression patterns of HUB1 correlated with previously described phenotypes in hub1 leaf, root, and flowering time (9, 13). Hence, flowering time and leaf and root growth were measured in spen3, khd1, and hub1 single and double mutants and in overexpression lines. The SALK_025388 and GABI_626H01 lines with T-DNA insertions into exon 2 of At1g27750 (SPEN3) with severely reduced SPEN3 transcript levels were designated spen3-1 and spen3-3, respectively (Fig. 2 A and B). The SALK_046957 line with T-DNA insertion into the promoter, next to the 5′UTR region of At1g51580 (KHD1) with slightly but significantly reduced KHD1 transcript levels, was designated khd1-1 (Fig. 2 A and B); the SAIL_1285_H03C1 line, with T-DNA insertion into exon 3 of At1g51580 with severely reduced KHD1 transcript levels, was designated khd1-3 (Fig. 2 A and B).
Fig. 2.
Phenotypes of single and double mutants and of overexpression lines. (A) Schematic view of the SPEN3 and KHD1 T-DNA insertion lines, spen3-1 (SALK_025388), spen3-3 (GABI_626H01), khd1-1 (SALK_046957), and khd1-3 (SAIL_1285_H03C1). (B) Relative expression of KHD1 and SPEN3 in Col-0 and mutant lines estimated with the qPCR assay in six biological replicates. Asterisks indicate statistically significant differences by Student’s t test (***P < 0.001). (C) Representative seedlings at 19 DAG grown in jiffy containers. Arrows mark early-emerging inflorescences. (Scale bar, 1 cm.) (D) Flowering time of lines germinated in jiffy pots (n = 51). (E and F) Flowering time and projected rosette area of seedlings at 23 DAS (n = 24) respectively, grown on the WIWAM phenotyping platform. Error bars represent SEs. Ordinary one-way ANOVA with 95% confidence shows a significant difference between the genotypes, represented by letters (D–F). (G) Individual leaf areas of seedlings at 21 DAS of mutant lines, in vitro grown on the IGIS platform.
Seedlings, 19 d after germination (DAG), grown in soil, showed slightly reduced rosette growth in spen3-1, khd1-1, and khd1-3, and early flowering in hub1-4 and the double mutants, spen3-1 hub1-4 and khd1-1 hub1-4 compared with the wild type [Columbia-0 accession (Col-0)] (Fig. 2C); reduced rosette growth was also observed in the spen3-3 allele (SI Appendix, Fig. S4C). Flowering time was determined in four independent biological repeats in a randomization experimental set up in soil under standardized growth room conditions. The genotypes were grouped by statistical ANOVA. Strikingly, in spen3-1, the flowering time was significantly delayed by 2 d (Fig. 2D), which is an opposite phenotype compared with hub1-4 that flowers 4 d earlier than the wild type (Fig. 2D), as shown previously (13); the khd1-1 and khd1-3 mutants had a wild-type flowering time. Also in the spen3-3 allele, a small but significant delay in flowering time was observed in two independent biological repeat experiments under similar growth room conditions (SI Appendix, Fig. S4D). A second T-DNA insertion in the spen3-3 mutant did not affect the gene-expression levels of its adjacent genes, At1g77920 and At1g77930 (SI Appendix, Fig. S5), thus, the delay in flowering time in spen3-3 was caused by the T-DNA insertion in exon 2 of the SPEN3 gene. Interestingly, the spen3-1 hub1-4 and khd1-1 hub1-4 double mutants were flowering as early as the hub1-4 parental (Fig. 2D), suggesting that HUB1 is epistatic to SPEN3 and KHD1 in flowering-time regulation. The small but significant delay in flowering time in spen3-1, the normal flowering time in khd1-1, and the earlier flowering in hub1-4 were also shown in an automated weighing, imaging, and watering phenotyping platform (WIWAM XY; https://www.wiwam.be) (Fig. 2E). The overexpression lines of HUB1, SPEN3, and KHD1 included in this experiment had normal flowering times (Fig. 2E). The number of leaves at bolting, which is tightly correlated with flowering time (41, 42), was lower in the early-flowering genotypes and slightly higher in spen3-1 and spen3-3 than that in the wild type, as expected (SI Appendix, Fig. S4 E–G). In conclusion, both HUB1/HUB2 and SPEN3 regulate flowering time, but in an opposite manner, suggesting that SPEN3 might also function in the transcriptional control of the flowering-time regulator FLC, which is targeted by HUB1/HUB2 for H2Bub activity (13).
Seedling growth of the mutant and overexpression lines was monitored in soil with the automated WIWAM platform. At 23 d after sowing (DAS), the projected rosette area (all green area from photographed rosette) was measured (Fig. 2F) and stockiness (leaf shape indicator) and compactness (projected rosette area/area of convex hull) were calculated (SI Appendix, Fig. S4 H and I). The projected rosette area was reduced in spen3-1 and khd1-1 by 16% and 17%, respectively, whereas it was similar to that of the wild type in hub1-4 and hub2-1 (Fig. 2F). In hub1-4 and hub2-1, stockiness was reduced (SI Appendix, Fig. S4H) and in spen3-1 compactness was reduced (SI Appendix, Fig. S4I). In 21-d-old in vitro-grown plants, the individual leaf area was reduced in the hub1-4, hub2-1, khd1-1, and spen3-1 mutants (Fig. 2G), corresponding with the data obtained in soil for spen3-1 and khd1-1, but differing for those for hub1-4 and hub2-1 (Fig. 2F and SI Appendix, Fig. S4 H and I), possibly hinting at a sensitivity of the hub mutants to the more stressful in vitro conditions. The leaf number decreased in the in vitro-grown hub1-4 and hub2-1 seedlings, but was normal in spen3-1 and khd1-1 (Fig. 2G), correlating with their flowering time measured in soil (Fig. 2 D and E). Leaf series of 26 DAG seedlings of the randomized jiffy-grown experiment at bolting (for flowering time and leaf number, see Fig. 2D and SI Appendix, Fig. S4E) showed that the spen3-1 hub1-4 and khd1-1 hub1-4 double mutants had a smaller leaf size and a narrower shape than those of the hub1-4 parental under these conditions (SI Appendix, Fig. S4J), suggesting that HUB1 is also epistatic to SPEN3 and KHD1 for these parameters. The primary root length was reduced in all genotypes, except in hub1-4 (SI Appendix, Fig. S6A), correlating with a reduced primary root meristem size (SI Appendix, Fig. S6 B and C), hence, indicating that mutation and overexpression of SPEN3 and KHD1 affect root growth by cell proliferation.
Transcriptomes of spen3, khd1, and hub1 Mutants.
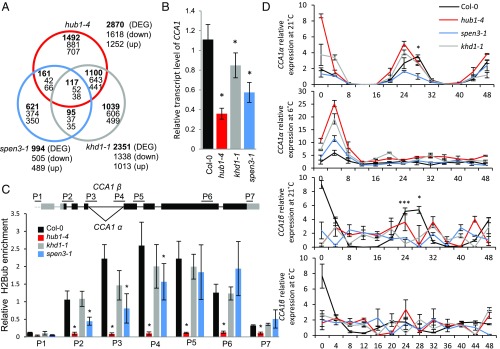
RNA deep-sequencing was done on hub1-4, khd1-1, spen3-1, and Col-0 total RNA prepared from shoot apices. The dataset (ArrayExpress ID E-MTAB-6780) (43) was analyzed for differentially expressed genes (DEGs), down-regulated with log2 fold-change ≤ −0.5 and up-regulated with log2 fold-change ≥ 0.5 (P < 0.05) (Fig. 3A). More than 40% and almost 50% of the DEGs in hub1-4 and khd1-1, respectively, were common, suggesting that KHD1 might act together with HUB1 in the transcriptional regulation of a large number of genes.
Fig. 3.
Transcriptomes, CCA1 transcripts, and H2Bub in mutants. (A) Venn diagram of the transcriptomes of the hub1-4, spen3-1, and khd1-1 expression profiles compared with Col-0. (B) Relative expression of CCA1 by qPCR in Col-0 and mutants in six biological replicates. (C) Scheme of the CCA1 gene structure with the splice forms CCA1α and CCA1β at intron 4 and position of the primers used in ChIP-qPCR (Upper). Relative enrichment of H2Bub at the CCA1 gene established with antibodies against H2Bub and four biological replicates used for ChIP assay (Lower). Results were normalized versus input samples. Error bars represent SDs (B and C) or SEs (D). Asterisks indicate statistically significant differences to Col-0 with the Student’s t test (*P < 0.05, B and C) or from spen3-1 to Col-0 (*P < 0.05, ***P < 0.001, D). (D) Relative expression in a 48-h time course experiment of the CCA1 splice forms, CCA1α and CCA1β, by qPCR at 21 °C and 6 °C in three biological replicates.
In contrast, only ∼5.5% and 16% of the DEGs in hub1-4 and spen3-1 were common, indicating that SPEN3 and HUB1 coregulate limited but specific classes of genes, possibly related to functions of the tissue used for RNA sequencing (such as shoot apices), and that SPEN3 controls the expression of a number of other genes via a mechanism that is unrelated to HUB1-mediated H2Bub. Very few DEGs occurred in all three mutants, hinting at a limited combined activity of KHD1, SPEN3, and HUB1 in transcriptional regulation (SI Appendix, Table S1). Substantial portions of the DEGs were unique to each mutant (i.e., 44% in khd1-1, 52% in hub1-4, and 63% in spen3-1), implying additional specific roles for HUB1, KHD1, and SPEN1.
Next, gene ontology (GO) classes were identified among down- or up-regulated genes common for all three or two mutants, or unique for only one mutant, to define the molecular functions and biological processes affected (SI Appendix, Table S2). Genes down-regulated in all three mutants and in the two mutants hub1-4 and spen3-1 fall into the same GO classes coding for cell cycle proteins, histone kinases, and ribosomal proteins. The detailed analysis of cell cycle-related genes commonly down-regulated in hub1-4 and spen3-1 revealed several genes crucial for cell division—KNOLLE, HINKEL, AURORA2, AURORA3, CYCB1;4, CDKB1;2, RSW7, BUBR1, and POK2—confirming specialized function for the HUB1–SPEN3 interaction. Genes down-regulated in hub1-4 and khd1-1 encode proteins related to cell cycle, DNA replication, response to stimuli, or involved in secondary metabolism. The hub1-4–specific down-regulated genes grouped mainly into the GO classes related to defense and stress response or cell wall organization or biosynthesis, but included also the GO flower development, containing the flowering repressors FLC, FLM, SMZ, and BOP2. Many khd1-1 unique down-regulated genes clustered into the classes organ morphogenesis, shoot and leaf development, reproductive structure development (growth regulator GRF9, phase transition regulator SPL15, and RBR1 and VIM3 repressors of flowering activator FWA), or were involved in cell cycle or nucleic acid metabolism. The spen3-1 uniquely down-regulated genes related to the circadian clock and flowering time (PRR3, PRR5, ELF3, FKF1, and SRR1). In summary, analysis of down-regulated genes showed that HUB1, KHD1, and SPEN3 are involved in common pathways, but might only occasionally regulate the same target genes in a complex.
Because the number of genes commonly up-regulated in all three mutants was too low for a reliable GO analysis (i.e., 38 genes), we compared the hub1-4 mutant individually with khd1-1 or spen3-1 (SI Appendix, Table S2). Both comparisons identified genes involved mainly in programmed cell death, regulatory processes, and response to different stimuli. Among the overlapping genes between the hub1-4 and khd1-1 transcriptomes, additional categories were detected, such as tropism, growth, cell wall, transmembrane transport, and response to hormones. Genes up-regulated only in individual mutants clustered predominantly to the response to stimulus or signaling classes, but GO categories specific for only one mutant and highly enriched were also identified: circadian clock-related genes in hub1-4 (log2 enrichment 2.53) and growth-related genes in khd1-1 (log2 enrichment 1.77).
SPEN3 and HUB1 Regulate CCA1 Gene Expression Through H2Bub and Pre-mRNA Processing.
The H2Bub at the clock regulator CCA1, a known HUB1 target (4), was determined in the khd1-1 and spen3-1 mutants to investigate whether it could explain the reduced CCA1 expression in mutant seedlings (Fig. 3B) and whether the HUB1/HUB2-mediated histone H2B monoubiquitylase activity could be affected by KHD1 and SPEN3 during transcript elongation. Chromatin immunoprecipitation (ChIP) with H2Bub antibodies followed by a qPCR with primers annealing to the promoter and coding regions of the CCA1 gene (Fig. 3C) revealed that H2Bub was absent from the promoter and peaked centrally in the gene body in the wild type (Fig. 3C), but was very low over the whole gene in the hub1-4 mutant (Fig. 3C), characteristically for HUB1/HUB2 target genes (4). In the spen3-1 mutant, the reduced gene expression correlated with a significant decrease in H2Bub at the 5′ and central part of the CCA1 gene, suggesting that SPEN3 affects the HUB1-mediated H2Bub activity at the CCA1 locus (Fig. 3C). In the knockdown allele, khd1-1, the H2Bub at the CCA1 gene is reduced, but not significantly (Fig. 3C); hence, it is unclear whether KHD1 functions in HUB1-mediated H2Bub at the CCA1 locus.
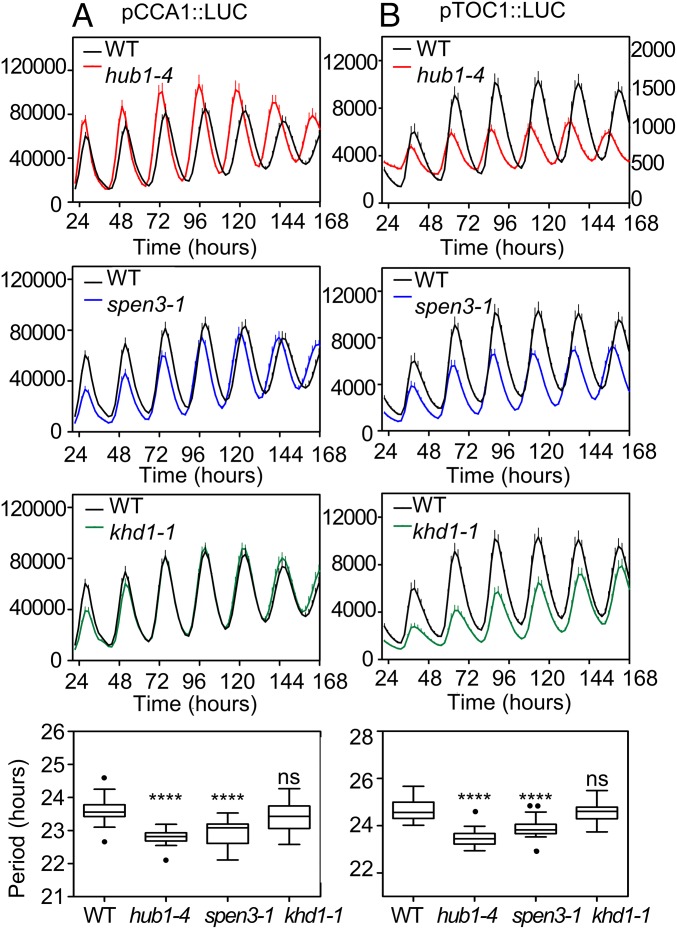
The down-regulation of the CCA1 gene expression in spen3-1 and khd1-1, as well as in hub1-4, prompted us to investigate circadian rhythms by means of reporter lines expressing the LUCIFERASE (LUC) fused to the promoters of CCA1 and TIMING OF CHLOROPHYLL A/B (CAB) EXPRESSION 1 (TOC1) (pCCA1::LUC and pTOC1::LUC) that were introgressed into the spen3-1, khd1-1, and hub1-4 mutants. Bioluminescence analysis showed that for both reporters and in hub1-4 and spen3-1, the amplitude of the rhythms was not altered, but the circadian period was significantly shorter than that of wild-type plants (Fig. 4). In hub1-4 mutant plants, the advanced phase and short period were already detected from the second day under constant light, whereas in the spen3-1 mutant plants these phenotypes were visible from the third day. The observed period shortening was not very severe (∼1 h in hub1-4 and spen3-1), but it was reproducible in the biological triplicates (with 12 independent seedlings per genotype and replicate) and statistically significant. The results were fully consistent among the triplicates in terms of period, phase, and amplitude, confirming the reliability of the conclusions. In khd1-1, the circadian period was not affected, but the amplitude of pTOC1::LUC was significantly reduced (Fig. 4). The short circadian period in hub1-4 and spen3-1 suggests that the loss of the HUB1 and SPEN3 activities makes the clock run faster than in wild-type plants.
Fig. 4.
Circadian waveforms and circadian period in wild type and mutants by means of LUC reporter lines. Luminescence in hub1-4, spen3-1, and khd1-1 mutants containing pCCA1::LUC (A) or pTOC1::LUC (B) reporter genes was recorded under constant white-light conditions, following synchronization under 12-h light/12-h dark cycles; y axes represent counts per seedling per 5 s; lower scale in right-handed y axis visualizes the pTOC1::LUC amplitude and period in the hub1-4 graph. Data are means + SEM of at least 12 individual seedlings. Statistical significance was calculated with an ordinary one-way ANOVA with 95% confidence value: ns, not significant, ****P ≤ 0.0001. The dots represent outliers.
Subsequently, the alternative splicing of CCA1 was analyzed in the hub1-4, spen3-1, and khd1-1 mutants by qPCR measurements of the relative transcript levels of the CCA1α and CCA1β splice forms over a time course of 48 h under continuous light and under control (21 °C) and cold (6 °C) conditions (Fig. 3D) (44, 45). In CCA1α, the fourth intron was spliced, whereas in CCA1β the fourth intron was retained (Fig. 3C). The Col-0 wild type showed rhythmic patterns for both splice forms at the control temperature with a peak at the circadian time point CT28 (Fig. 3D). In the cold, Col-0 loses the CT28 peak and the relative levels of CCA1α and CCA1β are reduced. In the hub1-4, khd1-1, and spen3-1 mutants, under control conditions, the CCA1α splice variant peak shifts toward the time point CT24 (Fig. 3D), which correlates with the shorter circadian period observed with the LUC reporter lines for hub1-4 and spen3-1 (Fig. 4). Interestingly, under control conditions, spen3-1 has reduced CCA1α transcript levels, which might reveal a positive role for SPEN3 in the processing of the CCA1 pre-mRNA at the fourth intron. In contrast, in hub1-4, the CCA1α levels had increased, suggesting a regulatory effect of H2Bub on transcript processing. No peak for the CCA1β splice variant was observed in any of the mutants, in contrast to the Col-0 control with a clear peak, indicating that the intron retention mechanism necessary for the formation of the β transcript is affected in the mutants. Under cold conditions, Col-0 and the three mutants do not differ, because the rhythmic patterns of the CCA1α and CCA1β transcript levels were absent and all peaks were lost. The alternative splicing is regulated by RNA-binding proteins, chromatin structure, histone modifications, and the RNA polymerase II elongation rate (46). Slow transcript elongation expands and fast transcript elongation compresses the “window of opportunity” for recognition of upstream splice sites, thereby decreasing or increasing intron retention (47). Moreover, alternatively spliced introns are removed more slowly than constitutive introns and, therefore, their splicing requires a longer transcript elongation time.
In summary, at control temperature, the general CCA1 transcript level is reduced in hub1-4, but the level of the CCA1α splice form is increased and that of the CCA1β splice form is reduced, indicating that the transcript elongation rate might be slowed down by the reduced H2Bub, enhancing splicing of intron 4 and shifting the CCA1α/CCA1β balance toward CCA1α. In spen3-1, the H2Bub at CCA1 is reduced in the first part of the coding region until intron 4, where the intron retention/splicing occurs, suggesting that SPEN3 plays a role in the establishment of a H2Bub maximum at the splice site that might function as a signal for splice site selection. Consequently, in spen3-1, a decrease in H2Bub at the intron 4 splice site might slow down the transcript elongation rate and retard the splicing, which might explain the reduced total CCA1, CCA1α, and CCA1β transcript levels without shift in the CCA1α/CCA1β balance. In yeast, H2Bub facilitates the early spliceosome assembly at certain genes (48). Our data suggest that SPEN3 might provide an important link between the splicing machinery and the HUB1-mediated H2Bub and that SPEN3 might be an adaptor protein between the histone mark H2Bub and the splicing factors. Moreover, the RRM domain of SPEN3 might bind the nascent ssRNA and its SPOC domain might associate with proteins of the spliceosome or the transcript elongation complex. Indeed, the RNA polymerase II transcript elongation complex interacts with the mRNA-splicing factors (49). We hypothesize that the interaction between the HUB1-mediated H2Bub and SPEN3 regulates the coupling of transcript elongation and the pre-mRNA processing at CCA1, identifying an until now unknown transcription regulation mechanism in plants.
SPEN3 Function in FLC-Derived Antisense COOLAIR Transcript Formation.
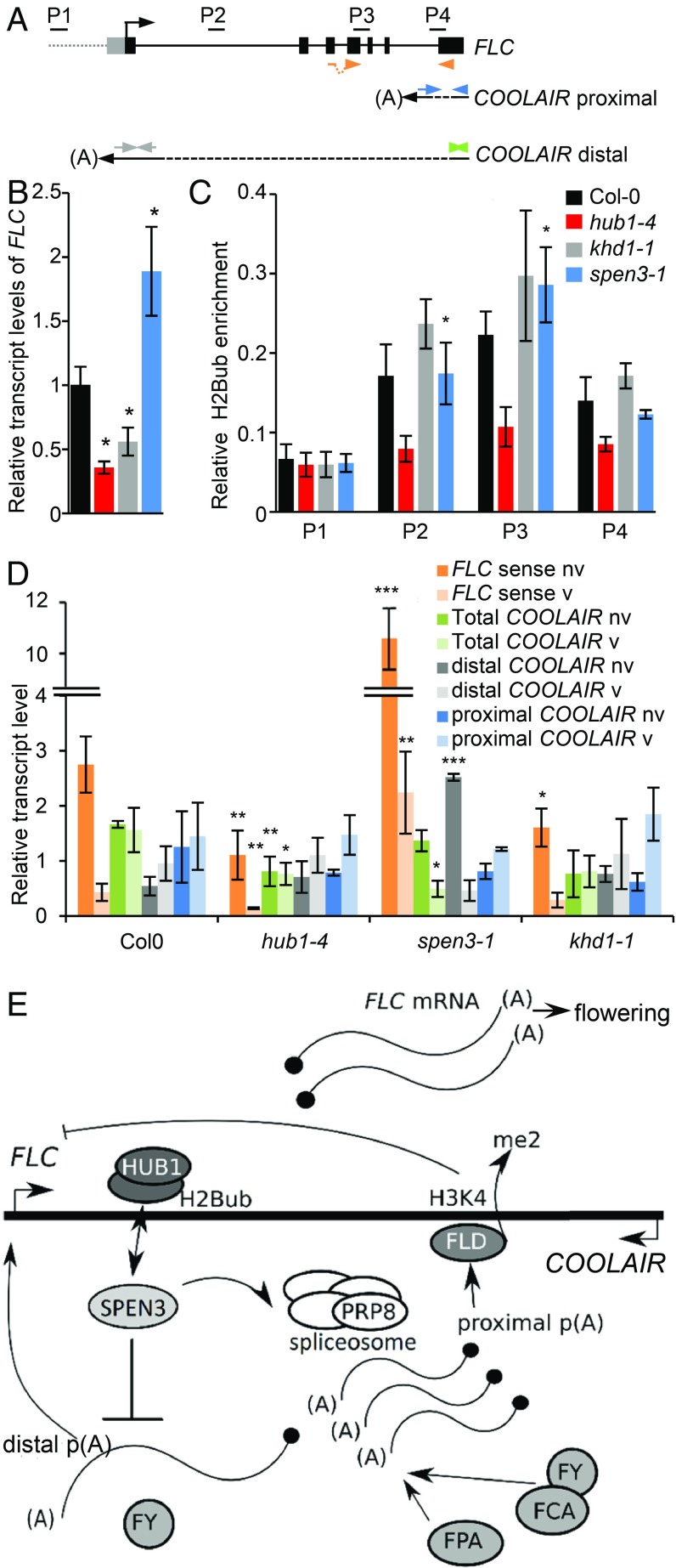
The flowering-time repressor gene FLC (Fig. 5A) is also a known target of the HUB1-mediated H2bub that promotes FLC transcription (13, 50). In the hub1-4 mutant, the down-regulation of the FLC gene expression correlated with reduced H2Bub levels (Fig. 5 B and C) and early flowering time (Fig. 2 C–E). In khd1-1, FLC was down-regulated and in spen3-1, FLC was up-regulated (Fig. 5B); however, the H2Bub level at FLC was normal in both mutants (Fig. 5C). Thus, SPEN3 and KHD1 affect FLC gene expression through a mechanism that is not related to HUB1-mediated activity in H2Bub.
Fig. 5.
FLC and COOLAIR transcripts and H2Bub in mutants. (A) Schematic representation of the FLC gene structure and antisense COOLAIR with primers (P2–P4) used in ChIP-qPCR and in qPCR for spliced FLC (orange), total COOLAIR (green), distal COOLAIR (gray), and proximal COOLAIR (blue). (B) Relative expression of FLC by qPCR in Col-0 and mutants in six biological replicates. (C) Relative enrichment of H2Bub at the FLC gene established with antibodies against H2Bub and four biological replicates used for ChIP assay. Results were normalized versus input samples. (D) qRT-PCR showing the relative expression of the FLC and COOLAIR forms before (bv) and after (av) vernalization in four biological replicates. (E) Hypothetical position and function of SPEN3 in FLC/COOLAIR regulation under normal conditions (model modified from ref. 60). (B–D) Error bars represent SEs. Asterisks indicate statistically significant differences to Col-0 with the Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001).
The FLC transcription factor prevents flowering by repression of the floral pathway integrator genes that activate the transition to flowering and the FLC expression level quantitatively correlates with the time to flower (51, 52). Several regulatory pathways regulate the FLC expression at ambient temperatures, namely the FRIGIDA (FRI) pathway that triggers the FLC expression through recruitment of activating chromatin-modifying complexes, promotion of its 5′ cap cotranscriptional processing, and the autonomous pathway that down-regulates the FLC expression through an antisense RNA-mediated chromatin-silencing mechanism. Indeed, noncoding FLC antisense transcripts, designated COOLAIR, might fully encompass the FLC gene or be shorter depending on the use of distal or proximal splice and polyadenylation sites at the FLC (Fig. 5A) (30, 53, 54). Splicing and 3′ processing at the proximal site are promoted by the core spliceosome component and pre-mRNA–processing splicing factor 8 (PRP8), respectively, and by components of the autonomous pathway, including the RNA-binding proteins, FCA and FPA and the 3′ processing factors that trigger histone demethylation by FLOWERING LOCUS D (FLD) at the FLC gene body, which suppresses the FLC expression (see model in Fig. 5E) (54, 55).
In the spen3 alleles, the FLC expression is enhanced and the flowering time is delayed, which are phenotypes similar to those in the autonomous pathway mutants or the prp8 spliceosome-defective mutant affecting the COOLAIR proximal site selection/processing. Hence, we investigated the FLC antisense COOLAIR transcripts by using total RNA of spen3-1, hub1-4, and khd1-1 mutant seedlings by means of qPCR in four biological replicates (Fig. 5D). In ambient temperature, the FLC expression was reduced in hub1-4 and khd1-1 and increased in spen3-1, confirming the results shown in Fig. 5B. In the hub1-4 and khd1-1 mutants, the proximal and distal COOLAIR transcript levels and their ratios were comparable to those in the Col-0 control. However, in spen3-1, significantly increased distal and reduced proximal transcript levels and altered distal/proximal ratios were observed (Fig. 5D). Thus, SPEN3 might inhibit the COOLAIR distal or promote the proximal transcript formation. Distal transcript secondary structure correlated with the FLC expression and flowering time in natural accessions (56, 57). Hence, it would be interesting to investigate whether SPEN3 affects the secondary structure or stability of distal transcripts in addition to a postulated role in distal/proximal site selection.
In our qPCR experiment (Fig. 5D), the 7-d cold treatment only slightly increased the proximal and distal COOLAIR transcripts in all genotypes, including the Col-0 control, and might have been too short for mimicking vernalization, usually 30 d of cold. Hence, as a consequence, no COOLAIR transcripts were induced under the cold conditions used in contrast to the vernalization conditions reported previously (58). However, the FLC expression was significantly reduced in all mutants upon the 7-d cold treatment (Fig. 5D), indicating that the applied cold conditions did induce the FLC repression. The Col-0 background contains a mutated fri allele that is responsible for a weak FLC expression (59) and for the attenuation of the late flowering-time phenotype in prp8 or autonomous pathway mutants (54, 55), a possible reason for the mild but significant delay in flowering time in the spen3-1 allele.
In conclusion, our data link the increase in FLC transcript in spen3-1 to a function in distal or proximal antisense COOLAIR formation without effect on the H2Bub level at the FLC, whereas the decrease in the FLC transcript in hub1-4 is only associated with a reduced H2Bub at the FLC. The reduced FLC expression in khd1-1 does not result in a flowering-time phenotype and does not correspond to altered COOLAIR splice variant ratios or H2Bub levels.
The delayed flowering time in spen3-1 and earlier flowering in the hub1 mutants were correlated with increased and decreased FLC expression levels, respectively. In the spen3-1 mutant, an increased distal antisense COOLAIR transcript and a distal/proximal COOLAIR ratio indicate that SPEN3 plays a role in COOLAIR polyadenylation or splicing to control the sense FLC transcript level and that it acts antagonistically to HUB1 in the FLC regulation. A number of flowering-time regulators with RNA-binding capacity, such as FPA and FCA (RRM domain), and FLK and PEP (KH domain) have been identified and are part of a regulatory loop in which FPA and FCA independently regulate 3′ end formation of antisense COOLAIR RNA at the FLC locus, triggering the FLD demethylation of H3K4me2, with a repressed chromatin state as a consequence (60). FLK and PEP have an antagonistic effect on the FLC expression, but their action mechanisms are unknown (35). They interact with the KH proteins HEN4 and HUA1 to form a complex that assists in transcript elongation and facilitates correct splicing (61). The SPOC domain in SPEN3 is important for its copurification with HUB1 and KHD1; hence, SPEN3 might function together with these proteins in the RNA-mediated control of FLC and might represent an antagonistic regulatory loop for the HUB1-mediated H2Bub transcriptional regulation. Both activities might cross-talk with environmental and developmental cues. The early flowering phenotype of the spen3-1 hub1-4 double mutant, together with the molecular data, suggest that at FLC the presence of the intact HUB1 complex is required for SPEN3 to function in antisense COOLAIR transcript formation. Thus, SPEN3 represents an, until now, unidentified player requiring HUB1 to regulate FLC expression and flowering time.
Conclusion
We demonstrated that the HUB1 and HUB2 subunits of the H2B monoubiquitination complex and the RNA-binding proteins, SPEN3 and KHD1, strongly interact. Moreover, SPEN3 is involved in the processing of the pre-mRNA of CCA1 and in the formation of antisense COOLAIR at FLC, key regulators of the circadian clock and flowering time, respectively. Consequently, these pathways were affected in the spen3 knockout mutants. Our data on CCA1 indicate that during transcript elongation the HUB1/HUB2 complex is a platform for integration of H2Bub deposition activity with pre-mRNA processing and that these processes influence each other reciprocally. Indeed, in the spen3-1 mutant, a reduced H2Bub level at the CCA1 intron 4 correlated with a decrease in CCA1α and CCA1β splice forms, a lowered CCA1 expression, and a shortened circadian clock period. These results are in line with the absence of SPEN3 in the mRNA-binding proteome datasets (21) and with the composition of the RNAPII transcript elongation complex that contains transcript elongation factors (such as PAF and FACT), histone modification enzymes (such as HUB1, Elongator, and histone methyltransferases), but also spliceosome and polyadenylation factors involved in pre-mRNA processing (49). In yeast, Npl3, an arginine-serine–like protein with an RRM motif and a domain enriched in arginine-serine dipeptides, is involved in pre-mRNA processing of predominantly ribosomal proteins in close cooperation with Bre1 (the yeast HUB1 ortholog) and its activity in H2B monoubiquitination during transcription (18). We showed that HUB1 is an anchor point for the SPEN3 RNA-binding protein that functions in transcript formation at specific genes, thus providing insight into the mechanism, players, and target genes in plants. Hence, the integration of transcript elongation and transcript processing occurs through a conserved mechanism in yeast and plants, in which HUB1/Bre1 acts as anchor point for RNA-binding proteins, such as SPEN3 with a function in transcript formation. Importantly, SPEN3 is not a homolog of the yeast Npl3 and, moreover, both proteins differ in their protein-interacting domain, which might explain the difference in target proteins between plants and yeast. The particular function of SPEN3 related to the formation of the noncoding antisense COOLAIR transcript might be facilitated by the presence of HUB1 at the FLC gene during transcript elongation, whereas the SPOC domain of SPEN3 might promote interaction with RNA-processing factors.
The KHD1 interactor of HUB1 localizes in the nucleus and cytoplasm, binds to ssRNA; its mutant, khd1-1, has decreased leaf and root growth, and a transcriptome that overlapped substantially with that in hub1-4. However, flowering time and circadian clock period were normal in the khd1-1 mutant, coinciding with a normal H2Bub at the FLC and CCA1, a normal splicing at the CCA1 and FLC-derived antisense COOLAIR, despite the reduced expression of FLC and CCA1. The recently studied knockout allele khd1-3 has a normal flowering time as well, indicating that the complete loss-of-function of KHD1 might not affect the FLC biological function and that FLC might not be a target of the KHD1 activity. We postulate that KHD1 might function in mRNA stability, export, or translation rather than in transcript formation, which is supported by its presence in an mRNA-binding interactome (21, 37).
Materials and Methods
Plant Material and Growth Conditions.
The mutant lines and growth conditions are described in SI Appendix, Materials and Methods.
Tandem Affinity Purification and Y2H.
Cloning, tandem affinity purification of protein complexes, mass spectrometry, and Y2H are described in SI Appendix, Materials and Methods.
RNA-Binding Assays.
Cloning into the E. coli expression vector, protein production in E. coli, EMSA, and MST RNA-binding assays are described in SI Appendix, Materials and Methods.
Confocal Microscopy and Multiprobe in Situ Hybridization.
Primary roots of 5-d-old transgenic Arabidopsis seedlings, transformed with 35S::GFP::SPEN3 and 35S::GFP::KHD1, were grown vertically under continuous light and analyzed by confocal microscopy (Olympus, FV10 ASW) for fusion protein localization. Multiprobe in situ hybridization is described in SI Appendix, Materials and Methods.
Flowering-Time Determination and Shoot and Root Growth Analyses.
Flowering time was determined in several biological repeats in soil as the number of days between germination and the initiation of the floral stem elongation at 2-mm height. The number of rosette leaves produced by the apical meristem was recorded at that time (n ≥ 28). Image acquisition and data analyses on growth parameters are described in SI Appendix, Materials and Methods. Leaf series were prepared from in vitro-grown plants (IGIS platform) by aligning all of the rosette leaves on 1% (wt/vol) agar plates (n = 10) at day 19. Leaves were photographed and scanned to measure the leaf area by ImageJ 1.41 (https://imagej.nih.gov/ij/). Root growth and root meristem analyses are described in SI Appendix, Materials and Methods.
RNA Methods.
RNA isolation, real-time PCR, and transcriptome analysis are described in SI Appendix, Materials and Methods.
ChIP-qPCR.
ChIP-qPCR was done as described previously (4) with 2-wk-old seedlings. For details, see SI Appendix, Materials and Methods.
Detection and Quantification of Polyadenylated COOLAIR.
For nonvernalized samples, seedlings were grown under long-day (16-h light/8-h darkness) conditions for 10 d at 21 °C. For vernalization, seedlings were grown until 10 DAG under control conditions (long day, 21 °C), then transferred for 7 d to cold [short day (8-h light, 16-h darkness) at 7 °C], and finally allowed to recover for 7 d under control conditions (long day, 21 °C). Primer pairs used (Fig. 5A) are given in SI Appendix, Table S3.
Detection and Quantification of Alternatively Spliced CCA1 over a 48-h Time Course.
Circadian time is defined under free-running conditions (constant light) based on the previous synchronization under light/dark cycles. By convention, the onset of activity of diurnal organisms (lights on under entraining conditions) is defined as circadian time 0 (CT0). Seedlings were grown under long-day conditions for 15 d at 21 °C, then transferred at the circadian time point CT0, under continuous light conditions at 21 °C or 6 °C. Seedling pools were harvested in triplicate every 4 h from time point CT0 until CT48. Primer pairs were used to identify the two splice variants, CCA1α and CCA1β (44) (Fig. 3D and SI Appendix, Table S3).
Supplementary Material
Acknowledgments
The work was supported by the European Commission Marie Curie Initial Research Training network (FP7-PEOPLE-2013-ITN-607880) (to P.M., K.D.G., and M.V.L.); the Spanish Ministry of Economy and Competitiveness, by the Generalitat de Catalunya and by the Spanish Ministry of Economy and Competitiveness through the “Severo Ochoa Program for Centers of Excellence in R&D” 2016–2019 (to P.M.); and Deutsche Forschungsgemeinschaft Grant SFB960 (to K.D.G.). M.W. was the recipient of a Marie Curie Intra-European fellowship (FP7-PEOPLE-2010-IEF-273068; acronym, LightEr) and S.D. was a postdoctoral fellow of the Research Foundation-Flanders.
Footnotes
The authors declare no conflict of interest.
Data deposition: RNA sequencing data are publicly accessible in the ArrayExpress database, https://www.ebi.ac.uk/arrayexpress/ (accession no. E-MTAB-6780).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806541116/-/DCSupplemental.
References
- 1.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Roudier F, et al. Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J. 2011;30:1928–1938. doi: 10.1038/emboj.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourbousse C, et al. Histone H2B monoubiquitination facilitates the rapid modulation of gene expression during Arabidopsis photomorphogenesis. PLoS Genet. 2012;8:e1002825. doi: 10.1371/journal.pgen.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himanen K, et al. Histone H2B monoubiquitination is required to reach maximal transcript levels of circadian clock genes in Arabidopsis. Plant J. 2012;72:249–260. doi: 10.1111/j.1365-313X.2012.05071.x. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Shen W-H. Dynamic regulation and function of histone monoubiquitination in plants. Front Plant Sci. 2014;5:83. doi: 10.3389/fpls.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Lijsebettens M, Grasser KD. Transcript elongation factors: Shaping transcriptomes after transcript initiation. Trends Plant Sci. 2014;19:717–726. doi: 10.1016/j.tplants.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Robzyk K, Recht J, Osley MA. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287:501–504. doi: 10.1126/science.287.5452.501. [DOI] [PubMed] [Google Scholar]
- 8.Wood A, et al. Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol Cell. 2003;11:267–274. doi: 10.1016/s1097-2765(02)00802-x. [DOI] [PubMed] [Google Scholar]
- 9.Fleury D, et al. The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell. 2007;19:417–432. doi: 10.1105/tpc.106.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Koornneef M, Soppe WJJ. The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell. 2007;19:433–444. doi: 10.1105/tpc.106.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu X, Jiang D, Wang Y, Bachmair A, He Y. Repression of the floral transition via histone H2B monoubiquitination. Plant J. 2009;57:522–533. doi: 10.1111/j.1365-313X.2008.03709.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, et al. The E2 ubiquitin-conjugating enzymes, AtUBC1 and AtUBC2, play redundant roles and are involved in activation of FLC expression and repression of flowering in Arabidopsis thaliana. Plant J. 2009;57:279–288. doi: 10.1111/j.1365-313X.2008.03684.x. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Dai Y, Cui S, Ma L. Histone H2B monoubiquitination in the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell. 2008;20:2586–2602. doi: 10.1105/tpc.108.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz RJ, Tamada Y, Doyle MR, Zhang X, Amasino RM. Histone H2B deubiquitination is required for transcriptional activation of FLOWERING LOCUS C and for proper control of flowering in Arabidopsis. Plant Physiol. 2009;149:1196–1204. doi: 10.1104/pp.108.131508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry KW, et al. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 2003;17:2648–2663. doi: 10.1101/gad.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. USP49 deubiquitinates histone H2B and regulates cotranscriptional pre-mRNA splicing. Genes Dev. 2013;27:1581–1595. doi: 10.1101/gad.211037.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long L, et al. The U4/U6 recycling factor SART3 has histone chaperone activity and associates with USP15 to regulate H2B deubiquitination. J Biol Chem. 2014;289:8916–8930. doi: 10.1074/jbc.M114.551754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moehle EA, Ryan CJ, Krogan NJ, Kress TL, Guthrie C. The yeast SR-like protein Npl3 links chromatin modification to mRNA processing. PLoS Genet. 2012;8:e1003101. doi: 10.1371/journal.pgen.1003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Roeder RG. Direct Bre1-Paf1 complex interactions and RING finger-independent Bre1-Rad6 interactions mediate histone H2B ubiquitylation in yeast. J Biol Chem. 2009;284:20582–20592. doi: 10.1074/jbc.M109.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Leene J, et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol. 2010;6:397. doi: 10.1038/msb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köster T, Marondedze C, Meyer K, Staiger D. RNA-binding proteins revisited—The emerging Arabidopsis mRNA interactome. Trends Plant Sci. 2017;22:512–526. doi: 10.1016/j.tplants.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Ariyoshi M, Schwabe JWR. A conserved structural motif reveals the essential transcriptional repression function of Spen proteins and their role in developmental signaling. Genes Dev. 2003;17:1909–1920. doi: 10.1101/gad.266203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, et al. Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev. 2001;15:1140–1151. doi: 10.1101/gad.871201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawada T, et al. Fusion of OTT to BSAC results in aberrant up-regulation of transcriptional activity. J Biol Chem. 2008;283:26820–26828. doi: 10.1074/jbc.M802315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J-H, Skalnik DG. Rbm15-Mkl1 interacts with the Setd1b histone H3-Lys4 methyltransferase via a SPOC domain that is required for cytokine-independent proliferation. PLoS One. 2012;7:e42965. doi: 10.1371/journal.pone.0042965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majerciak V, Lu M, Li X, Zheng Z-M. Attenuation of the suppressive activity of cellular splicing factor SRSF3 by Kaposi sarcoma-associated herpesvirus ORF57 protein is required for RNA splicing. RNA. 2014;20:1747–1758. doi: 10.1261/rna.045500.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiriart E, et al. Interaction of the Epstein-Barr virus mRNA export factor EB2 with human Spen proteins SHARP, OTT1, and a novel member of the family, OTT3, links Spen proteins with splicing regulation and mRNA export. J Biol Chem. 2005;280:36935–36945. doi: 10.1074/jbc.M501725200. [DOI] [PubMed] [Google Scholar]
- 28.Yan D, Perrimon N. spenito is required for sex determination in Drosophila melanogaster. Proc Natl Acad Sci USA. 2015;112:11606–11611. doi: 10.1073/pnas.1515891112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solís-Guzmán MG, et al. Expression analysis of the Arabidopsis thaliana AtSpen2 gene, and its relationship with other plant genes encoding Spen proteins. Genet Mol Biol. 2017;40:643–655. doi: 10.1590/1678-4685-GMB-2016-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornyik C, Terzi LC, Simpson GG. The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell. 2010;18:203–213. doi: 10.1016/j.devcel.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Cai X. OsRRMh, a Spen-like gene, plays an important role during the vegetative to reproductive transition in rice. J Integr Plant Biol. 2013;55:876–887. doi: 10.1111/jipb.12056. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y, Kato N, Wang W, Li J, Chen X. Two RNA binding proteins, HEN4 and HUA1, act in the processing of AGAMOUS pre-mRNA in Arabidopsis thaliana. Dev Cell. 2003;4:53–66. doi: 10.1016/s1534-5807(02)00399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, et al. A KH-domain RNA-binding protein interacts with FIERY2/CTD phosphatase-like 1 and splicing factors and is important for pre-mRNA splicing in Arabidopsis. PLoS Genet. 2013;9:e1003875. doi: 10.1371/journal.pgen.1003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mockler TC, et al. Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc Natl Acad Sci USA. 2004;101:12759–12764. doi: 10.1073/pnas.0404552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ripoll JJ, et al. Antagonistic interactions between Arabidopsis K-homology domain genes uncover PEPPER as a positive regulator of the central floral repressor FLOWERING LOCUS C. Dev Biol. 2009;333:251–262. doi: 10.1016/j.ydbio.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, et al. UV crosslinked mRNA-binding proteins captured from leaf mesophyll protoplasts. Plant Methods. 2016;12:42. doi: 10.1186/s13007-016-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichel M, et al. In planta determination of the mRNA-binding proteome of Arabidopsis etiolated seedlings. Plant Cell. 2016;28:2435–2452. doi: 10.1105/tpc.16.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marondedze C, Thomas L, Serrano NL, Lilley KS, Gehring C. The RNA-binding protein repertoire of Arabidopsis thaliana. Sci Rep. 2016;6:29766. doi: 10.1038/srep29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baaske P, Wienken CJ, Reineck P, Duhr S, Braun D. Optical thermophoresis for quantifying the buffer dependence of aptamer binding. Angew Chem Int Ed Engl. 2010;49:2238–2241. doi: 10.1002/anie.200903998. [DOI] [PubMed] [Google Scholar]
- 40.Helder S, Blythe AJ, Bond CS, Mackay JP. Determinants of affinity and specificity in RNA-binding proteins. Curr Opin Struct Biol. 2016;38:83–91. doi: 10.1016/j.sbi.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- 42.Alonso-Blanco C, El-Din El-Assal S, Coupland G, Koornneef M. Analysis of natural allelic variation at flowering time loci in the Landsberg erecta and Cape Verde Islands ecotypes of Arabidopsis thaliana. Genetics. 1998;149:749–764. doi: 10.1093/genetics/149.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Lijsebettens M. 2018 Transcriptome analysis of khd1-1, spen3-1 and hub1-4 Arabidopsis mutants. ArrayExpress. Available at https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-6780/. Deposited November 1, 2014.
- 44.Seo PJ, et al. A self-regulatory circuit of CIRCADIAN CLOCK-ASSOCIATED1 underlies the circadian clock regulation of temperature responses in Arabidopsis. Plant Cell. 2012;24:2427–2442. doi: 10.1105/tpc.112.098723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui Z, Xu Q, Wang X. Regulation of the circadian clock through pre-mRNA splicing in Arabidopsis. J Exp Bot. 2014;65:1973–1980. doi: 10.1093/jxb/eru085. [DOI] [PubMed] [Google Scholar]
- 46.Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144:16–26. doi: 10.1016/j.cell.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fong N, et al. Pre-mRNA splicing is facilitated by an optimal RNA polymerase II elongation rate. Genes Dev. 2014;28:2663–2676. doi: 10.1101/gad.252106.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hérissant L, et al. H2B ubiquitylation modulates spliceosome assembly and function in budding yeast. Biol Cell. 2014;106:126–138. doi: 10.1111/boc.201400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antosz W, et al. The composition of the Arabidopsis RNA polymerase II transcript elongation complex reveals the interplay between elongation and mRNA processing factors. Plant Cell. 2017;29:854–870. doi: 10.1105/tpc.16.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crevillén P, Dean C. Regulation of the floral repressor gene FLC: The complexity of transcription in a chromatin context. Curr Opin Plant Biol. 2011;14:38–44. doi: 10.1016/j.pbi.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whittaker C, Dean C. The FLC locus: A platform for discoveries in epigenetics and adaptation. Annu Rev Cell Dev Biol. 2017;33:555–575. doi: 10.1146/annurev-cellbio-100616-060546. [DOI] [PubMed] [Google Scholar]
- 53.Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009;462:799–802. doi: 10.1038/nature08618. [DOI] [PubMed] [Google Scholar]
- 54.Liu F, Marquardt S, Lister C, Swiezewski S, Dean C. Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science. 2010;327:94–97. doi: 10.1126/science.1180278. [DOI] [PubMed] [Google Scholar]
- 55.Marquardt S, et al. Functional consequences of splicing of the antisense transcript COOLAIR on FLC transcription. Mol Cell. 2014;54:156–165. doi: 10.1016/j.molcel.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li P, Tao Z, Dean C. Phenotypic evolution through variation in splicing of the noncoding RNA COOLAIR. Genes Dev. 2015;29:696–701. doi: 10.1101/gad.258814.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hawkes EJ, et al. COOLAIR antisense RNAs form evolutionarily conserved elaborate secondary structures. Cell Rep. 2016;16:3087–3096. doi: 10.1016/j.celrep.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csorba T, Questa JI, Sun Q, Dean C. Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc Natl Acad Sci USA. 2014;111:16160–16165. doi: 10.1073/pnas.1419030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johanson U, et al. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science. 2000;290:344–347. doi: 10.1126/science.290.5490.344. [DOI] [PubMed] [Google Scholar]
- 60.Ietswaart R, Wu Z, Dean C. Flowering time control: Another window to the connection between antisense RNA and chromatin. Trends Genet. 2012;28:445–453. doi: 10.1016/j.tig.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez-Cazorla E, et al. K-homology nuclear ribonucleoproteins regulate floral organ identity and determinacy in Arabidopsis. PLoS Genet. 2015;11:e1004983. doi: 10.1371/journal.pgen.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.