Significance
The mRNA poly(A) tail controls gene expression at posttranscriptional levels, including mRNA degradation and translation. Here, we show that a hitherto unknown hepatic posttranscriptional network centered on the CCR4–NOT deadenylase plays a seminal role in regulating FGF21 expression and its effects on systemic metabolism. A genome-wide search for CNOT6L-associated mRNAs unveiled the mechanism whereby CNOT6L selectively degrades a subset of mRNAs encoding metabolic factors, including FGF21. Disruption of CCR4–NOT deadenylase activity, by targeting its catalytic subunit CNOT6L, leads to an increase in FGF21 levels, which is paralleled by a dramatic improvement of metabolic syndrome. Overall, our findings describe a new paradigm in regulation of whole-body metabolism, whereby a hepatic posttranscriptional network governs systemic metabolic regulation via FGF21.
Keywords: CCR4–NOT, deadenylase, FGF21, hepatokine, metabolic syndrome
Abstract
Whole-body metabolic homeostasis is tightly controlled by hormone-like factors with systemic or paracrine effects that are derived from nonendocrine organs, including adipose tissue (adipokines) and liver (hepatokines). Fibroblast growth factor 21 (FGF21) is a hormone-like protein, which is emerging as a major regulator of whole-body metabolism and has therapeutic potential for treating metabolic syndrome. However, the mechanisms that control FGF21 levels are not fully understood. Herein, we demonstrate that FGF21 production in the liver is regulated via a posttranscriptional network consisting of the CCR4–NOT deadenylase complex and RNA-binding protein tristetraprolin (TTP). In response to nutrient uptake, CCR4–NOT cooperates with TTP to degrade AU-rich mRNAs that encode pivotal metabolic regulators, including FGF21. Disruption of CCR4–NOT activity in the liver, by deletion of the catalytic subunit CNOT6L, increases serum FGF21 levels, which ameliorates diet-induced metabolic disorders and enhances energy expenditure without disrupting bone homeostasis. Taken together, our study describes a hepatic CCR4–NOT/FGF21 axis as a hitherto unrecognized systemic regulator of metabolism and suggests that hepatic CCR4–NOT may serve as a target for devising therapeutic strategies in metabolic syndrome and related morbidities.
The mRNA poly (A) tail plays an essential role in posttranscriptional regulation of gene expression by affecting mRNA decay and translation (1–3). Deadenylation is the rate-limiting step in mRNA degradation that, together with transcription, determines steady-state mRNA levels (4). mRNA deadenylation is primarily catalyzed by the CCR4–NOT complex, a multisubunit protein machinery composed of the CCR4 (CNOT6L/CNOT6) deadenylase, the CNOT1 scaffold protein, and several regulatory proteins (CNOT2–CNOT11) (5–7).
Direct recruitment of the CCR4–NOT complex to target mRNAs destined for deadenylation and decay is mediated by several RNA-binding proteins (RBPs), including tristetraprolin (TTP), Nanos2, and Roquin (8–13). In addition, posttranscriptional silencing by miRNAs occurs through association of the CCR4–NOT complex with the miRNA-induced silencing complex (miRISC) (14–16). The selectivity of mRNA deadenylation is controlled by cis-acting mRNA elements to which CCR4–NOT-associated RBPs and miRISC bind (17, 18). Previous structural and biochemical studies have provided mechanistic models for the selective CCR4–NOT-dependent deadenylation by RBPs and the miRISC (18). However, the composition and function of CCR4–NOT containing messenger ribonucleoprotein (mRNP) complexes in physiological and pathological states remain obscure (19).
The CCR4–NOT complex has been implicated in the development of metabolic diseases (20–24). These disorders, including diabetes, steatosis, hyperlipidemia, and obesity, are major worldwide health problems causally associated with dysregulation of metabolic homeostasis. Whole-body metabolic homeostasis is closely controlled in a systemic or paracrine manner by hormone-like factors secreted from nonendocrine organs, such as adipose tissue (adipokines) and liver (hepatokines) (25, 26). Hormone-like proteins can either enhance [e.g., fibroblast growth factor 21 (FGF21) and leptin] or impair (e.g., resistin and selenoprotein P) energy metabolism (26, 27). However, there are no studies that directly link the deadenylase activity of CCR4–NOT to hormone-like proteins and metabolic disorders.
Here, we identified target mRNAs associated with the CNOT6L deadenylase subunit of the CCR4–NOT complex in the liver by performing RNA immunoprecipitation followed by microarray analysis (RIP-CHIP). We demonstrate that, in response to feeding, the CCR4–NOT/TTP complex targets the AU-rich mRNA encoding the hepatokine FGF21, which alleviates diet-induced metabolic disorders (28–31). Deletion of the Cnot6l gene in mice decreased susceptibility to diet-induced metabolic disorders, such as obesity, steatosis, and hyperlipidemia in a deadenylase activity-dependent manner. We found that the observed metabolic disorders can largely be explained by CNOT6L-dependent control of Fgf21 mRNA decay. Thus, we conclude that the CNOT6L deadenylase targets a subset of mRNAs, including Fgf21, to control whole-body metabolism. Our findings show that CNOT6L plays a major role in regulation of FGF21 levels, thus providing unprecedented evidence that CNOT6L may serve as a therapeutic target to treat metabolic diseases.
Results
RIP-CHIP Identifies CNOT6L-Associated mRNAs That Contain AU-Rich Elements and Encode for Metabolic Regulators.
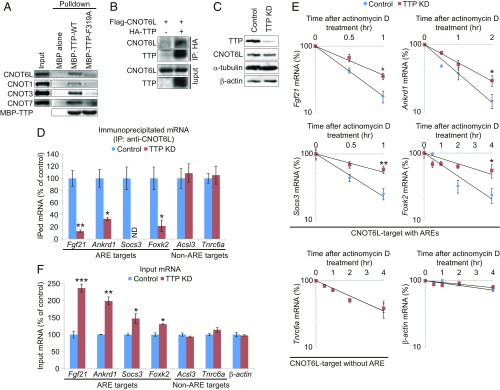
The CCR4–NOT complex is a multisubunit protein machinery composed of the CCR4 (CNOT6L/CNOT6) deadenylase, the CNOT1 scaffold protein, and several regulatory proteins (CNOT2–CNOT11) (5), and has been implicated in metabolic disorders (20–23). Mammals have two paralogs of the CCR4 deadenylase gene, Cnot6 and Cnot6l (32, 33). CNOT6L is highly expressed in metabolically active tissues, such as the liver and adipose tissue, whereas CNOT6 is predominantly expressed in testis and thymus (SI Appendix, Fig. S1 A and B). To investigate the role of CCR4–NOT in regulation of metabolism, we first identified bona fide endogenous mRNA targets of the CCR4–NOT deadenylase in the liver. This was achieved by immunopurifying CCR4–NOT-associated mRNP complexes and analyzing their mRNA content on a transcriptome-wide scale using microarrays (Fig. 1A). Hepatocytes derived from mice lacking Cnot6l (Cnot6l−/−) were used as a control (SI Appendix, Fig. S1 C–F). We identified 195 CNOT6L-associated mRNAs (Dataset S1), then searched consensus sequences among 3′UTRs of 195 mRNAs (Dataset S2). These mRNAs were significantly enriched for AU-rich elements (AREs) within their 3′UTR (Fig. 1B, SI Appendix, Fig. S2A, and Dataset S2). AREs generally destabilize mRNAs and are found within the 3′UTR of mRNAs, such as cytokines and growth factors that respond to acute external stimuli (34, 35). We classified 195 mRNAs according to their biological functions (Fig. 1C). CNOT6L-associated mRNAs exhibited enrichment for those encoding metabolic factors (27% of 195 genes, P = 2.0 × 10−3) (Fig. 1C). We selected 12 mRNAs among those mRNAs encoding metabolism-related proteins according to the P values and those that were associated with known metabolic functions, and validated the interactions between CNOT6L and 12 of these mRNAs using qRT-PCR (SI Appendix, Fig. S2 B and C). We found that of the 12 selected mRNAs, 5 contained AREs, and that the stability and steady-state levels of these mRNAs, such as Fgf21, Ankrd1, Socs3, and Foxk2, were increased in Cnot6l−/− hepatocytes (Fig. 1 D and E and SI Appendix, Fig. S2D). These results suggest that CNOT6L decreases the stability of mRNAs that contain AREs and are enriched in those encoding metabolic regulators.
Fig. 1.
RIP-CHIP identified CNOT6L-associated mRNAs, many of which contain AREs and encode metabolic regulators. (A) Schematic diagram of RIP-CHIP in wild-type and Cnot6l−/− primary hepatocytes. Primary hepatocytes were isolated from wild-type and Cnot6l−/− livers. Each sample was divided into two for total RNA extraction (upper arrows) and anti-CNOT6L immunoprecipitation followed by RNA extraction (lower arrows). Input and immunoprecipitated (IPed) RNA were hybridized to separate microarrays. Cnot6l−/− hepatocytes were used as a negative control. (B) Percentage of nontarget mRNAs containing AREs (blue bar) and CNOT6L-target mRNAs containing AREs (red bar). The bar graph was generated from Dataset S1. (C) Functional classification of 195 CNOT6L-associated mRNAs using the Panther Classification System. (D) Stability of the indicated mRNAs in wild-type and Cnot6l−/− hepatocytes. Hepatocytes were incubated with actinomycin D for the indicated times. Levels of the indicated mRNAs were determined by RT-qPCR, normalized to the level of Hprt mRNA, expressed as percent change of the initial mRNA level, and plotted semilogarithmically. n = 3 per group. (E) Input levels of the indicated mRNAs in wild-type and Cnot6l−/− hepatocytes were determined by RT-qPCR. n = 3 per group. Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t test. A representative experiment of two independent experiments (each carried out in triplicate) is presented.
TTP Recruits the CCR4–NOT Complex to ARE-Containing Target mRNAs Destined for Degradation.
Because the CCR4–NOT complex directly interacts with the ARE-binding protein, TTP (9, 11, 12, 36), we investigated whether TTP promotes CCR4–NOT-dependent degradation of endogenous target mRNAs in hepatocytes. The CCR4–NOT complex subunits (CNOT6L, CNOT1, CNOT3, and CNOT7) were precipitated with TTP from a hepatocyte extract (Fig. 2A). TTP directly binds to CNOT1 via a conserved phenylalanine (F319) (9). Mutation of F319 to alanine (F319A) dramatically reduced the interaction between TTP and the CCR4–NOT complex subunits (Fig. 2A). Moreover, CNOT6L coimmunoprecipitated with TTP in hepatocytes (Fig. 2B). Depletion of TTP (Fig. 2C and SI Appendix, Fig. S3A), but not another CCR4–NOT-associated protein, Roquin (SI Appendix, Fig. S3 B–D), impaired the binding of CNOT6L to ARE-containing mRNAs (Fig. 2D), and enhanced their stability and steady-state levels (Fig. 2 E and F). These results demonstrate that TTP mediates the interaction of the CCR4–NOT complex with ARE-containing mRNAs encoding several pivotal metabolic regulators to promote their degradation.
Fig. 2.
TTP recruits the CCR4–NOT complex to ARE-containing target mRNAs destined for degradation. (A) Interaction of the CCR4–NOT complex with WT or mutant (F319A) TTP protein in hepatocyte extracts. Western blots of the indicated proteins from recombinant maltose binding protein (MBP)-tagged TTP proteins immobilized on amylose beads and incubated with hepatocyte extracts. (B) Interaction of Flag-CNOT6L with HA-TTP in hepatocytes examined by coimmunoprecipitation with anti-HA antibody, followed by Western blot with anti-Flag antibody. (C) Western blots of the indicated proteins in control and TTP knockdown (KD) hepatocytes. α-Tubulin and β-actin were used as loading controls. (D) Association of CNOT6L with the indicated mRNAs in the lysates from control and TTP KD hepatocytes. Immunoprecipitated (IPed) and input RNAs were isolated from anti-CNOT6L immunoprecipitates and total cell lysates, respectively. Levels of IPed and input mRNAs were determined by RT-qPCR. IPed mRNA levels were normalized to those of input mRNA. n = 3 per group. (E) Stability of the indicated mRNAs in control and TTP KD hepatocytes. Hepatocytes were incubated with actinomycin D for the indicated times. Levels of the indicated mRNAs were determined by RT-qPCR, normalized to the level of Hprt mRNA, expressed as percent change of the initial mRNA level, and plotted semilogarithmically. n = 3 per group. (F) Input levels of the indicated mRNAs in the lysates from control and TTP KD hepatocytes. n = 3 per group. Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ND, not determined; Student’s t test. For D–F, a representative experiment of two independent experiments (each carried out in triplicate) is presented.
The CCR4–NOT/TTP Complex Degrades the Hepatokine Fgf21 mRNA in Response to Feeding.
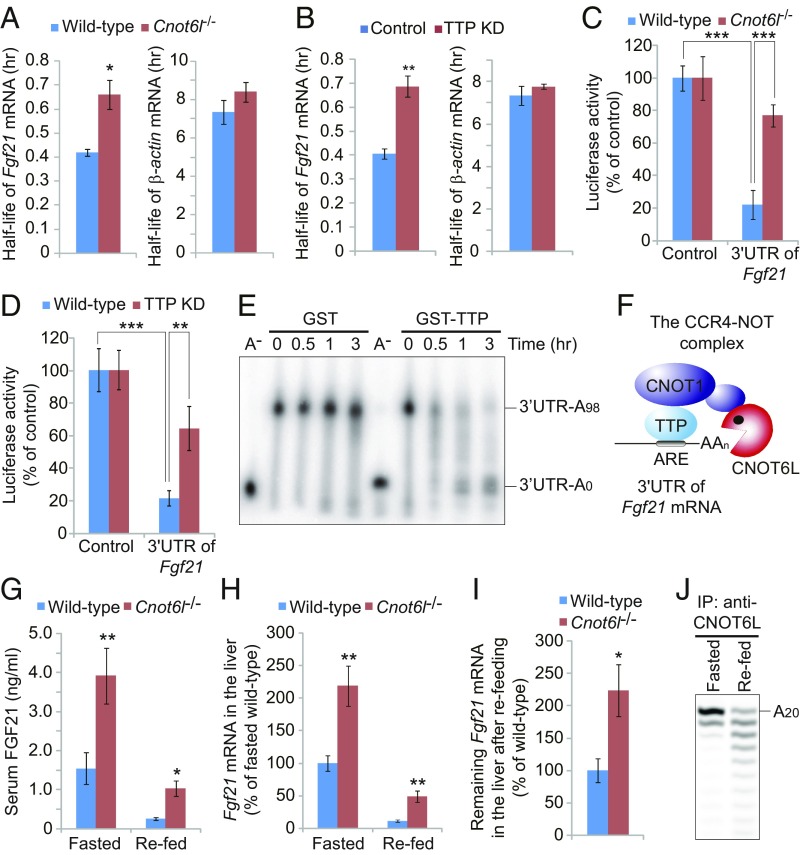
Fgf21 mRNA contains canonical AREs in its 3′UTR (SI Appendix, Fig. S3 E and F) and was dramatically enriched (P = 4.7 × 10−5) in CNOT6L-immunoprecipitated material (Dataset S1). Fgf21 mRNA levels were increased in Cnot6l−/− (Fig. 1E) and TTP-depleted hepatocytes (Fig. 2F). This correlated with longer Fgf21 mRNA half-life in Cnot6l−/− and TTP-depleted hepatocytes (T1/2, ∼40 min) vs. wild-type and control (T1/2, ∼25 min), respectively (Fig. 3 A and B). Luciferase reporter assays confirmed that the 3′UTR is required for CCR4–NOT/TTP-mediated degradation of Fgf21 mRNA (Fig. 3 C and D). The CCR4–NOT/TTP complex also induced deadenylation of an Fgf21 3′UTR reporter mRNA (Fig. 3E). These data demonstrate that the CCR4–NOT/TTP complex promotes deadenylation and degradation of Fgf21 mRNA via interaction with its 3′UTR (Fig. 3F).
Fig. 3.
The CCR4–NOT/TTP complex degrades the ARE mRNA for hepatokine FGF21 in response to feeding. (A and B) Half-lives of Fgf21 and β-actin mRNAs in wild-type and Cnot6l−/− hepatocytes (A) or in control and TTP KD hepatocytes (B). Half-lives of A and B were calculated from Figs. 1D and 2E, respectively. n = 3 per group. (C and D) Relative luciferase activities of luciferase construct with or without 3′UTR of Fgf21 mRNA in Cnot6l−/− (C) or TTP-depleted (D) hepatocytes. n = 3 per group. (E) Deadenylation assay of Fgf21-3′UTR-A98 RNA in Krebs ascites extract in the presence of recombinant GST or GST-tagged TTP. Polyadenylated and deadenylated RNAs are marked on the right. (F) Model for structural organization of Fgf21 mRNA-bound TTP in the complex with CNOT6L deadenylase (9). (G and H) Serum FGF21 protein (G) and hepatic Fgf21 mRNA levels (H) of wild-type and Cnot6l−/− mice following 4-h refeeding after 24-h fasting. n = 8 per group. (I) Remining Fgf21 mRNA in the liver of refed wild-type and Cnot6l−/− mice was calculated from Fig. 3H. n = 8 per group. (J) Deadenylation assay of 20-mer poly(A) RNA (A20) in anti-CNOT6L immunoprecipitates from livers of fasted or refed mice. The 20 mer poly(A) RNA is marked on the right. Validation of the immunoprecipitation is shown in SI Appendix, Fig. S3G. Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t test for A, B, and G–I, and two-way ANOVA with Tukey’s post hoc test for C and D.
Fgf21 transcription is stimulated by peroxisome proliferator-activated receptor-α (PPAR-α) under fasting conditions, leading to hepatic lipid oxidation, triglyceride clearance, and ketogenesis (37–39). In contrast, Fgf21 mRNA is rapidly suppressed 2 h after refeeding by an unknown mechanism (37, 39). Serum FGF21 and hepatic Fgf21 mRNA levels were ∼2.5-fold higher under fasting and refeeding conditions in Cnot6l−/− compared with wild-type mice (Fig. 3 G and H). CNOT6L deficiency partially rescued Fgf21 mRNA degradation by refeeding (Fig. 3I), indicating that reduction in serum FGF21 levels upon refeeding is mediated at least in part by the CCR4–NOT complex. Consistently, refeeding stimulated CNOT6L deadenylase activity in the liver (Fig. 3J and SI Appendix, Fig. S3G). These results demonstrate that the CCR4–NOT/TTP complex controls hepatic FGF21 production by inducing degradation of Fgf21 mRNA after feeding.
Resistance to Diet-Induced Obesity, Enhanced Energy Expenditure, and Improved Insulin Sensitivity in Cnot6l−/− Mice.
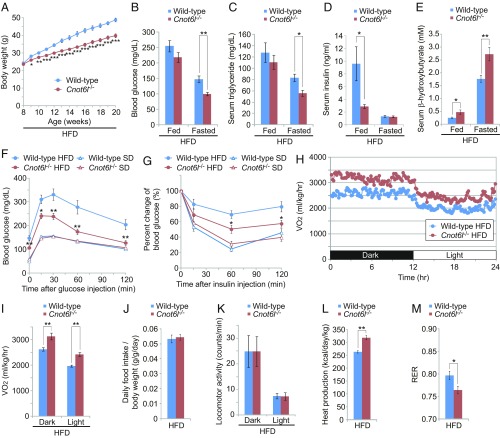
FGF21 promotes weight and lipid reduction, and delays development of diabetes (29, 31). To examine the impact of hepatic CCR4–NOT on FGF21 levels in a physiological context pertinent to the development of metabolic disorders, we analyzed Cnot6l−/− mice, which are viable and fertile and age without gross abnormalities (SI Appendix, Fig. S4A). The mice are leaner and protected from high-fat diet (HFD)-induced obesity compared with wild-type mice (Fig. 4A and SI Appendix, Fig. S4 A and B). Cnot6l deficiency ameliorated HFD-induced hyperglycemia, hyperlipidemia, and hyperinsulinemia (Fig. 4 B–D and SI Appendix, Fig. S4 C–E). Furthermore, serum levels of ketone body β-hydroxybutyrate were increased in Cnot6l−/− mice (Fig. 4E and SI Appendix, Fig. S4F), suggesting enhancement in fatty-acid oxidation. Finally, Cnot6l deletion significantly improved HFD-induced glucose tolerance and alleviated insulin resistance compared with control wild-type mice that exhibited typical metabolic syndrome phenotypes (Fig. 4 F and G). We next measured whole-body energy metabolism and found that loss of Cnot6l resulted in a significant increase in oxygen consumption (Fig. 4 H and I and SI Appendix, Fig. S4 G and H). There were no differences in food intake or locomotor activity, but increased heat production and decreased respiratory exchange ratio were observed (Fig. 4 J–M and SI Appendix, Fig. S4 I–L).
Fig. 4.
Protection from diet-induced metabolic disorders and enhanced energy expenditure in Cnot6l−/− mice. (A) Growth curve of wild-type and Cnot6l−/− mice fed on an HFD. HFD feeding started at 8 wk of age. n = 14–17 per group. (B–E) Levels of blood glucose (B), serum triglycerides (C), serum insulin (D), and serum β-hydroxybutyrates (E) in wild-type or Cnot6l−/− mice fed on an HFD during ad libitum feeding or fasting. n = 7–10 per group. (F and G) Intraperitoneal glucose tolerance tests (GTTs) (F) and insulin tolerance tests (ITTs) (G) in wild-type and Cnot6l−/− mice fed on an HFD or standard diet (SD). Blood glucose levels were measured at the indicated time points following intraperitoneal injection of glucose or insulin. n = 7–10 per group. *P < 0.05, **P < 0.01 for Cnot6l−/− on HFD versus wild-type on HFD. (H and I) Oxygen consumption (VO2) over 24 h (H) and average VO2 (I) in wild-type and Cnot6l−/− mice fed on HFD. VO2 were normalized to body weight. n = 5 per group. (J–M) Daily food intake per body weight (J), locomotor activity (K), calculated heat production (L), and respiratory exchange ratio (M) in wild-type and Cnot6l−/− mice fed on an HFD. n = 5 per group. Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; two-way ANOVA with Tukey’s post hoc test for A–G and I and Student’s t test for J–M.
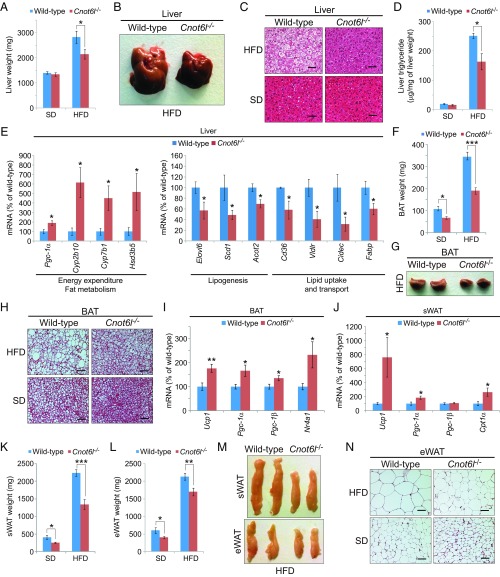
Cnot6l−/− mice on an HFD exhibited significantly reduced weight of liver, white adipose tissue (WAT), and brown adipose tissue (BAT) compared with wild-type mice (SI Appendix, Fig. S5A). While an HFD-induced hepatic steatosis in wild-type mice, Cnot6l−/− liver appeared smaller (∼25%) than control (Fig. 5 A and B). Smaller lipid droplets and lower hepatic triglyceride accumulation were observed in Cnot6l−/− liver on an HFD compared with control (Fig. 5 C and D). Moreover, Cnot6l deletion led to alterations in expression of genes relevant to energy expenditure and fat metabolism, such as Pgc-1α, Scd1, and Cd36 mRNAs in the liver (Fig. 5E and SI Appendix, Fig. S5B). In agreement with the enhanced energy expenditure in Cnot6l−/− mice, less fat accumulation and smaller adipocytes were observed in Cnot6l−/− BATs (Fig. 5 F–H). Cnot6l ablation engendered expression of Ucp1 and Pgc-1α, which are induced by FGF21 treatment (40–43), in BAT and subcutaneous WAT (sWAT) (Fig. 5 I and J and SI Appendix, Fig. S5 C and D) (40–43). Consequently, the weight and adipocyte size of sWAT and epididymal WAT (eWAT) were significantly decreased in Cnot6l−/− compared with control mice (Fig. 5 K–N). These results indicate that loss of CNOT6L activity dramatically improves HFD-induced metabolic disorders.
Fig. 5.
Cnot6l deletion leads to a resistance to lipid accumulation and an increase in energy expenditure in the liver, BAT, and WAT. (A–D) Weight (A), representative image (B), representative H&E staining (C), and triglyceride contents (D) of livers from wild-type and Cnot6l−/− mice fed on a SD or HFD. n = 8–12 per group. (Scale bars, 50 μm.) Liver triglyceride contents were normalized to liver weight. (E) Expression of genes involved in energy expenditure, fatty acid oxidation, lipogenesis, and lipid uptake and transport in the liver of wild-type and Cnot6l−/− mice fed on an HFD. n = 4–6 per group. (F–H) Weight (F), representative image (G), and representative H&E staining (H) of BAT of wild-type and Cnot6l−/− mice fed on an SD or HFD. n = 8–12 per group. (I–J) Expression of the indicated mRNAs in BAT (I) and sWAT (J) of wild-type and Cnot6l−/− mice fed on an HFD. n = 5–7 per group. (K–N) Weight of sWAT (K) and eWAT (L), representative sWAT and eWAT (M), and representative H&E staining of eWAT (N) from wild-type and Cnot6l−/− mice fed on SD or HFD. n = 8–12 per group. Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t test for E, I, and J and two-way ANOVA with Tukey’s post hoc test for A, D, F, K, and L. (Scale bars, H and N, 50 μm.)
Hepatic CNOT6L Deadenylase Activity Systemically Controls Lipid Metabolism, Steatosis, and Whole-Body Metabolism.
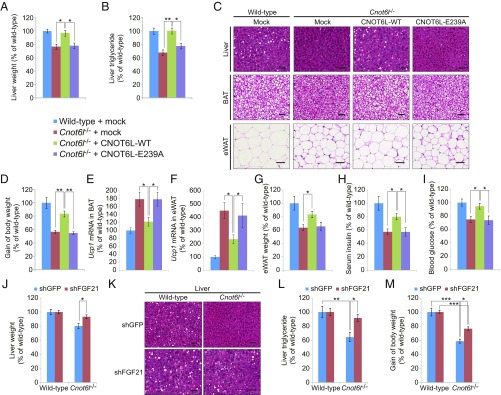
To support our observation that hepatic CNOT6L deadenylase activity plays an important role in metabolic regulation, we reintroduced a wild-type or an inactive (E239A) CNOT6L mutant (33, 44) into Cnot6l−/− liver by adenoviral-mediated gene transduction (SI Appendix, Fig. S6A) and maintained the mice on an HFD for 2 wk. Liver-specific expression of wild-type CNOT6L significantly increased liver weight and triglyceride content in Cnot6l−/− mice (Fig. 6 A–C). Notably, CNOT6L partially reversed the resistance of Cnot6l−/− mice to HFD-induced obesity (Fig. 6D and SI Appendix, Fig. S6B). This demonstrates that the reduced weight phenotype in Cnot6l−/− mice is attributed by the systemic effects caused by the lack of CCR4–NOT liver activity. Restoring CNOT6L levels in the liver significantly decreased Ucp1 levels in BATs and eWAT (Fig. 6 E and F), increased the size of lipid droplets in adipose tissues and eWAT weight (Fig. 6 C and G), and reversed serum insulin and blood glucose levels (Fig. 6 H and I). In striking contrast, the E239A mutant of CNOT6L failed to reverse the phenotypes of Cnot6l−/− mice (Fig. 6 A–I and SI Appendix, Fig. S6 A and B). Taken together, these data highlight the contribution of hepatic CNOT6L deadenylase activity in metabolic disorders and whole-body energy homeostasis.
Fig. 6.
Systemic regulation of whole-body metabolism by the CCR4–NOT/FGF21 axis. (A–I) Liver weight (A), liver triglyceride contents (B), representative H&E staining of the liver, BAT, and eWAT (C), gain of body weight (D), Ucp1 mRNA level in BAT (E) and eWAT (F), eWAT weight (G), serum insulin (H), and blood glucose levels (I) of wild-type and Cnot6l−/− mice injected with adenovirus expressing EGFP, CNOT6L-WT, or CNOT6L-E239A. Ten-week-old mice were administered with adenovirus and fed on HFD for 2 wk. (Scale bars, 50 μm.) Liver triglyceride contents were normalized to liver weight. n = 7–8 per group. (J–M) Liver weight (J), representative liver H&E staining (K), liver triglyceride contents (L), and gain of body weight (M) of wild-type and Cnot6l−/− mice injected with adenovirus expressing control (shGFP) or Fgf21 shRNA (shFGF21). Ten-week-old mice were injected with adenovirus and fed on HFD for 2 wk. (Scale bars, 50 μm.) Liver triglyceride contents were normalized to liver weight. n = 10 per group. Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; two-way ANOVA with Tukey’s post hoc test.
FGF21 Is the Mediator of CNOT6L-Dependent Hepatic Steatosis and Obesity.
To determine whether FGF21 mediates the effects of the hepatic CCR4–NOT/TTP complex on whole-body metabolism, we depleted FGF21 (SI Appendix, Fig. S6C) using adenovirus-delivered short-hairpin RNA (shRNA) (39, 45). Fgf21 shRNA reversed the beneficial effects of CNOT6L loss on liver weight, hepatic lipid droplet content, and the resistance to HFD-induced triglyceride accumulation (Fig. 6 J–L). In addition to partial restoration of Ucp1 expression in BAT and insulin levels in serum (SI Appendix, Fig. S6 D and E), Fgf21 knockdown in Cnot6l−/− mice resulted in increased body weight (Fig. 6M and SI Appendix, Fig. S6F). These findings further support the tenet that FGF21 is a major mediator of the systemic metabolic effects of CCR4–NOT and that the hepatic CCR4–NOT/TTP/FGF21 axis plays an essential role in whole-body energy homeostasis.
FGF21-based therapies have shown promise for the treatment of metabolic disorders in humans; however, concerns have been raised due to side effects reported in mice, including changes in bone development and homeostasis (29, 46). Strikingly, Cnot6l−/− mice did not display any defects in bone homeostasis (SI Appendix, Fig. S6 G–J), suggesting that induction of FGF21 via inhibition of CNOT6L could circumvent bone homeostasis issues associated with administration of FGF21. In summary, our data establish a strong link between CNOT6L deadenylase-mediated regulation of the hormone-like protein FGF21 and downstream systemic metabolic control (SI Appendix, Fig. S6K). Thus, targeting CNOT6L could potentially provide better options for the treatment of metabolic disorders with fewer side effects than FGF21-based therapies.
Discussion
Selective deadenylation by the CCR4–NOT complex contributes significantly to the wide range of mRNA half-lives and is mediated by specific RBPs that recruit the complex to target mRNAs, as has been described for TTP (9), Roquin (10), and miRISC (14–16). RIP-CHIP analysis of CNOT6L-associated mRNAs in the liver revealed a TTP-dependent posttranscriptional program that systemically alters mammalian metabolism. CNOT6L targets a subset of metabolism-related mRNAs, such as Fgf21 mRNA, whose expression is rapidly altered in response to changes in feeding conditions (37, 39, 47). In accordance with previous reports (9, 11, 12), the association between the CCR4–NOT complex and ARE mRNAs depends on TTP expression (Figs. 2 and 3). Together, these data demonstrate that CCR4–NOT selectively controls TTP-specific ARE mRNAs encoding metabolic factors in hepatocytes. Although the impact of TTP on immune regulation in mammals is well documented (36), these results ascribe a function for TTP in organismal metabolism.
Hormone-like proteins, whose expression is tightly controlled in response to nutrients, systemically control whole-body metabolism (25, 26). Most studies have focused on transcriptional regulators of hormone-like proteins. For example, Fgf21 transcription is stimulated by the transcription factor PPAR-α under fasting conditions, leading to hepatic lipid oxidation, triglyceride clearance, and ketogenesis (37–39, 47). In contrast, Fgf21 mRNA is rapidly suppressed 2 h after refeeding by an unknown mechanism (37, 39). We show that this suppression is less pronounced in Cnot6l−/− liver (Fig. 3I), which demonstrates that feeding-induced suppression of Fgf21 mRNA is at least in part controlled by the CCR4–NOT deadenylase. Accordingly, the CCR4–NOT complex is activated following feeding (Fig. 3J). Thus, posttranscriptional regulation of Fgf21 mRNA by the CCR4–NOT deadenylase is critical for the repression of triglyceride clearance and fatty acid oxidation following feeding. Consistent with the increased FGF21 serum level in Cnot6l−/− mice, CNOT6L ablation leads to an increase in serum ketone bodies, oxygen consumption, and expression of genes involved in energy expenditure and fatty acid oxidation (Figs. 4 and 5). Thus, our data show that Fgf21 mRNA stability, in addition to transcription, plays an important role in balancing serum FGF21 levels to maintain metabolic homeostasis in response to nutrients.
The obesity-resistant phenotype of Cnot6l−/− mice is largely, but not completely, reversed by reexpression of CNOT6L in the liver (Fig. 6), demonstrating a major role for hepatic CNOT6L in regulating systemic metabolism. The partial rescue in metabolic sensitivity suggests that CNOT6L activity in other metabolic tissues, such as BAT and WAT, contributes to the regulation of whole-body metabolism. Furthermore, knockdown of Fgf21 in the liver of Cnot6l−/− mice restores sensitivity to nutrient excess and reverses the decrease seen in diet-induced weight gain (Fig. 6). It is evident that there is a significant rescue of liver-specific CNOT6L function by Fgf21 knockdown by comparing the extent of rescue by CNOT6L reexpression versus Fgf21 knockdown on body weight. These results provide compelling evidence that FGF21 is the major effector of CNOT6L function in the liver, but also indicate that CNOT6L controls some Fgf21-unrelated pathways relevant to systemic metabolism. Our RIP-CHIP data show that ∼27% of mRNAs associated with CNOT6L encode metabolism-related factors. Characterization of these CNOT6L targets should provide additional insight into metabolic control by CCR4–NOT.
FGF21 is a bona fide therapeutic target that has been explored in the clinic (29). Treatment with FGF21 ameliorated several metabolic disorders, such as obesity, hyperlipidemia, and hyperglycemia in a variety of species, including rodents, monkeys, and humans (41, 42, 48–54). However, the development of FGF21 as a drug is challenging due to its short half-life in blood (T1/2 = 0.5 ∼ 2 h), and the aggregation of its recombinant form (55). An FGF21 analog, LY2405319, whose efficacy was validated in humans, was developed to address these issues (54). Unfortunately, its half-life remains relatively short (T1/2 = 1.5 ∼ 3 h), which motivated a search for other strategies to increase FGF21 levels in vivo (29). Additionally, exogenous administration of FGF21 has been used at a concentration of 5- to 10-fold higher than endogenous levels (29). We found that CNOT6L ablation alleviated metabolic disorders with only a 2.5-fold increase in serum FGF21 levels (Fig. 3G). These findings demonstrate that a modest increase in basal FGF21 levels through mRNA stabilization is sufficient to ameliorate hepatic steatosis, and result in more sustained effects compared with transient administration of FGF21. Importantly, the 2.5-fold increase we observed did not cause deleterious effects on bone density, unlike exogenous FGF21 (SI Appendix, Fig. S6). Coupled with the observation in humans, which show a common variant in the locus of CNOT6L correlating with altered blood cholesterol levels (NCBI PheGenI) (56), posttranscriptional control of FGF21 by CNOT6L underscores the therapeutic potential of targeting CNOT6L for metabolic disorders.
Methods
All animal experiments were conducted according to the guidelines for animal use issued by the Committee of Animal Experiments, McGill University, and Institute of Medical Science, University of Tokyo. Molecular studies were performed according to routine protocol previously published by our group (57). Differences among groups were compared using two-way ANOVA followed by between-group comparison with Tukey’s post hoc test, one-way ANOVA with Bonferroni’s post hoc test, or Student’s t test (two-tailed, unpaired) when there were only two groups. All statistical analyses were performed using IBM SPSS Statistics v22 software, and the differences were considered significant when P < 0.05. For detailed in vivo and in vitro experimental methods, see SI Appendix.
Supplementary Material
Acknowledgments
We thank I. Saito for the pAxCAwtit vector; M. Fisher for providing the pAd-shFGF21 vector; J. St-Pierre, D. Pearl, C. Chapat, and S. Tahmasebi for text proofreading; A. Sylvestre, K. Kitazawa, and H. Adachi for technical assistance; the Animal Facility and the Histology Facility at the Goodman Cancer Research Centre for mouse work and tissue processing; and C. Lister, I. Harvey, C. Sgherri, and S. Perreault for assistance. This work was supported by Canadian Institutes of Health Research Grants CIHR MOP-93607 (to N. Sonenberg) and MOP-125885 (to V.G.); Terry Fox Research Institute Grant TFF-116128 (to V.G., I.T., and N. Sonenberg); and the Ministry of Education, Culture, Sports, Science and Technology, Japan Grant-in-Aid for Scientific Research 19390070 (to T.Y.) and 18K07237 (to M.M.). M.M. is supported by the UT Rising Stars Award from the University of Texas System. O.L. is supported by the Wallenberg Academy Fellows Program and the Swedish Research Council. I.T. is a Junior 2 Research Scholar of the Fonds de Recherche du Québec–Santé.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE62365).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816023116/-/DCSupplemental.
References
- 1.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 2.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY, Shyu AB. Mechanisms of deadenylation-dependent decay. Wiley Interdiscip Rev RNA. 2011;2:167–183. doi: 10.1002/wrna.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collart MA, Panasenko OO. The Ccr4–Not complex. Gene. 2012;492:42–53. doi: 10.1016/j.gene.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Shirai YT, Suzuki T, Morita M, Takahashi A, Yamamoto T. Multifunctional roles of the mammalian CCR4-NOT complex in physiological phenomena. Front Genet. 2014;5:286. doi: 10.3389/fgene.2014.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler GS, Balacco DL. Heterogeneity and complexity within the nuclease module of the Ccr4-Not complex. Front Genet. 2013;4:296. doi: 10.3389/fgene.2013.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhandari D, Raisch T, Weichenrieder O, Jonas S, Izaurralde E. Structural basis for the Nanos-mediated recruitment of the CCR4-NOT complex and translational repression. Genes Dev. 2014;28:888–901. doi: 10.1101/gad.237289.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabian MR, et al. Structural basis for the recruitment of the human CCR4-NOT deadenylase complex by tristetraprolin. Nat Struct Mol Biol. 2013;20:735–739. doi: 10.1038/nsmb.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leppek K, et al. Roquin promotes constitutive mRNA decay via a conserved class of stem-loop recognition motifs. Cell. 2013;153:869–881. doi: 10.1016/j.cell.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351–361. doi: 10.1101/gad.1282305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler H, Kreth J, Timmers HT, Stoecklin G. Not1 mediates recruitment of the deadenylase Caf1 to mRNAs targeted for degradation by tristetraprolin. Nucleic Acids Res. 2011;39:4373–4386. doi: 10.1093/nar/gkr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc Natl Acad Sci USA. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun JE, Huntzinger E, Fauser M, Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell. 2011;44:120–133. doi: 10.1016/j.molcel.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Chekulaeva M, et al. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol. 2011;18:1218–1226. doi: 10.1038/nsmb.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabian MR, et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol. 2011;18:1211–1217. doi: 10.1038/nsmb.2149. [DOI] [PubMed] [Google Scholar]
- 17.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell SF, Parker R. Principles and properties of eukaryotic mRNPs. Mol Cell. 2014;54:547–558. doi: 10.1016/j.molcel.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol. 2008;9:337–344. doi: 10.1038/nrm2370. [DOI] [PubMed] [Google Scholar]
- 20.Morita M, et al. Obesity resistance and increased hepatic expression of catabolism-related mRNAs in Cnot3+/- mice. EMBO J. 2011;30:4678–4691. doi: 10.1038/emboj.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely GG, et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell. 2010;141:142–153. doi: 10.1016/j.cell.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe C, et al. Stability of mRNA influences osteoporotic bone mass via CNOT3. Proc Natl Acad Sci USA. 2014;111:2692–2697. doi: 10.1073/pnas.1316932111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, et al. Adipocyte-specific disruption of mouse Cnot3 causes lipodystrophy. FEBS Lett. 2017;591:358–368. doi: 10.1002/1873-3468.12550. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi A, et al. Post-transcriptional stabilization of Ucp1 mRNA protects mice from diet-induced obesity. Cell Rep. 2015;13:2756–2767. doi: 10.1016/j.celrep.2015.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefan N, Häring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9:144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domouzoglou EM, Maratos-Flier E. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am J Clin Nutr. 2011;93:901S–905S. doi: 10.3945/ajcn.110.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimeno RE, Moller DE. FGF21-based pharmacotherapy—Potential utility for metabolic disorders. Trends Endocrinol Metab. 2014;25:303–311. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Kharitonenkov A, Larsen P. FGF21 reloaded: Challenges of a rapidly growing field. Trends Endocrinol Metab. 2011;22:81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita A, et al. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat Struct Mol Biol. 2005;12:1054–1063. doi: 10.1038/nsmb1016. [DOI] [PubMed] [Google Scholar]
- 33.Morita M, et al. Depletion of mammalian CCR4b deadenylase triggers elevation of the p27Kip1 mRNA level and impairs cell growth. Mol Cell Biol. 2007;27:4980–4990. doi: 10.1128/MCB.02304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol. 2010;10:24–35. doi: 10.1038/nri2685. [DOI] [PubMed] [Google Scholar]
- 35.von Roretz C, Di Marco S, Mazroui R, Gallouzi IE. Turnover of AU-rich-containing mRNAs during stress: A matter of survival. Wiley Interdiscip Rev RNA. 2011;2:336–347. doi: 10.1002/wrna.55. [DOI] [PubMed] [Google Scholar]
- 36.Brooks SA, Blackshear PJ. Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829:666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Dutchak PA, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Fisher FM, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samms RJ, et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 2015;11:991–999. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 42.Véniant MM, et al. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 2015;21:731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 43.Hondares E, et al. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, et al. Crystal structure of the human CNOT6L nuclease domain reveals strict poly(A) substrate specificity. EMBO J. 2010;29:2566–2576. doi: 10.1038/emboj.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornu M, et al. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc Natl Acad Sci USA. 2014;111:11592–11599. doi: 10.1073/pnas.1412047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei W, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci USA. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inagaki T, et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecht R, et al. Rationale-based engineering of a potent long-acting FGF21 analog for the treatment of type 2 diabetes. PLoS One. 2012;7:e49345. doi: 10.1371/journal.pone.0049345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Véniant MM, et al. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153:4192–4203. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—Association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 54.Gaich G, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Kharitonenkov A, et al. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS One. 2013;8:e58575. doi: 10.1371/journal.pone.0058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kathiresan S, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita M, et al. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol cell. 2017;67:922–935. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.