Abstract
Decades of research have emphasized the importance of dopamine (DA) D1 receptor (D1R) mechanisms to dorsolateral prefrontal cortex (dlPFC) working memory function, and the hope that D1R agonists could be used to treat cognitive disorders. However, existing D1R agonists all have had high affinity for D1R, and engage β-arrestin signaling, and these agonists have suppressed task-related neuronal firing. The current study provides the first physiological characterization of a novel D1R agonist, PF-3628, with low affinity for D1R –more similar to endogenous DA actions– as well as little engagement of β-arrestin signaling. PF-3628 was applied by iontophoresis directly onto dlPFC neurons in aged rhesus monkeys performing a delay-dependent working memory task. Aged monkeys have naturally-occurring loss of DA, and naturally-occurring reductions in dlPFC neuronal firing and working memory performance. We found the first evidence of excitatory actions of a D1R agonist on dlPFC task-related firing, and this PF-3628 beneficial response was blocked by co-application of a D1R antagonist. These D1R actions likely occur on pyramidal cells, based on previous immunoelectron microscopic studies showing expression of D1R on layer III spines, and current microarray experiments showing that D1R are four times more prevalent in pyramidal cells than in parvalbumin-containing interneurons laser-captured from layer III of the human dlPFC. These results encourage the translation of D1R mechanisms from monkey to human, with the hope PF-3628 and related, novel D1R agonists will be more appropriate for enhancing dlPFC cognitive functions in patients with mental disorders.
Keywords: schizophrenia, cognition, pyramidal cell, GABA interneuron, cognition, aging
1. INTRODUCTION
1.1. Overview
Decades of research have demonstrated that dopamine (DA) has an essential influence on the higher cognitive functions of the primate dorsolateral prefrontal cortex (dlPFC). Neuronal microcircuits in the dlPFC generate the mental representations that are the foundation of working memory and abstract thought (Goldman-Rakic, 1995), and which are the focus of schizophrenia neuropathology (Glantz and Lewis, 2000; Selemon and Goldman-Rakic, 1999). Depletion of catecholamines from the dlPFC is as devastating as removing the cortex itself (Brozoski et al., 1979; Collins et al., 1998), highlighting the importance of their neuromodulatory actions.
The DA innervation of the dlPFC likely arises from “salience DA neurons” in the midbrain, cells that increase their firing to aversive as well as rewarding events (Bromberg-Martin et al., 2010). This hypothesis is based on both their location in midbrain (Arnsten et al., 2015; Williams and Goldman-Rakic, 1998), and electrochemical data showing increased DA release to both aversive and rewarding events (Kodama et al., 2014). Thus, DA actions may be especially relevant in the context of salient events, such as a cue signaling future reward. DA acts at a variety of receptors in the primate dlPFC, but D1 DA receptors (D1R) are the most prominent, especially in superficial layers (Lidow et al., 1991; Smiley et al., 1994). Local blockade of D1R within the dlPFC impairs delay-dependent working memory performance (Sawaguchi and Goldman-Rakic, 1994), consistent with the DA depletion data. However, working memory is also impaired by excessive D1R stimulation, e.g. with local D1R agonist infusion into dlPFC (Gamo et al., 2015), or during stress exposure when high levels of DA are released in PFC (Murphy et al., 1996), indicating a powerful, inverted-U dose response.
1.2. Cellular basis- D1R inverted U dose response on Delay cell representation of space
Much has been learned about the cellular basis for the D1R inverted U dose-response. Neurons in primate dlPFC can represent information over the delay period in a delay-dependent working memory task (Delay cells), keeping information “in mind” to guide a subsequent response. For example, in a visual spatial delayed response task, Delay cells show sustained, persistent firing over the delay period if the cue had occurred in the neuron’s preferred location, maintaining firing in the absence of visual stimulation. These cells are spatially tuned, firing for their preferred location, but not nonpreferred locations. The circuit basis for spatial tuning includes lateral inhibition from fast-spiking, parvalbumin-containing GABAergic (PV) interneurons (Goldman-Rakic, 1995; González-Burgos et al., 2005). In contrast, the persistent firing is thought to arise from recurrent excitatory inputs on dendritic spines, enriched in deep layer III, where pyramidal cells with shared preferred locations excite each other to maintain firing across the delay period (Goldman-Rakic, 1995; González-Burgos et al., 2000). D1R in layer III are preferentially expressed on a subset of spines, some within the post-synaptic density (PSD), but most at extrasynaptic sites, often near HCN channels (Arnsten et al., 2015; Gamo et al., 2015; Paspalas et al., 2013; Smiley et al., 1994).
Iontophoretic application of pharmacological compounds during neuronal recordings allows local manipulation of D1R in monkeys performing a delay-dependent working memory task. Iontophoresis of D1R antagonists and agonists has revealed an inverted-U dose response on Delay cell firing, where there is a loss of persistent firing with either high dose D1R antagonist, or high dose D1R agonist application (Arnsten et al., 2015; Vijayraghavan et al., 2007; Williams and Goldman-Rakic, 1995). In contrast, moderate doses of D1R agonist preferentially sculpt away “noise”, enhancing the ratio of signal to noise (d’) (Vijayraghavan et al., 2017; Vijayraghavan et al., 2007). Similar effects have been seen as monkeys perform tasks requiring dynamic associative learning (Puig and Miller, 2012) or representation of rules (Ott et al., 2014; Vijayraghavan et al., 2016).
The loss of firing seen with high doses of D1R antagonist suggests that endogenous DA actions can have an important, excitatory component that has not been seen with available D1R agonists. A rare ex vivo study of primate dlPFC slices show that DA can be excitatory via actions at D1R (Henze et al., 2000), and it is these actions that may be most helpful for the treatment of cognitive disorders. DA itself has low affinity for D1R, while previously utilized D1R agonists such as SKF81297 have much higher affinity for D1R (Ryman-Rasmussen et al., 2005), which may preferentially engage D1R suppressive actions. Thus, there is a great need for a D1R agonist that can better mimic DA’s lower affinity, excitatory actions, both as a research tool and as a potential therapeutic (Arnsten et al., 2017).
1.3. Clinical needs-
There are many cognitive disorders associated with impaired dlPFC cognitive function and/or inadequate DA signaling that could benefit from a superior D1R agonist. Age-related cognitive disorders may be an appropriate target, as advancing age is associated with a large depletion of DA from the dlPFC (Goldman-Rakic and Brown, 1981; Wenk et al., 1989), as well as reduced neuronal firing and working memory deficits (Wang et al., 2011). DA depletion in PFC also occurs in Parkinson’s Disease, which afflicts cortically-projecting DA cells as well as those projecting to striatum (Narayanan et al., 2013). A D1R agonist may also be beneficial in younger patients, including those with Attention Deficit Hyperactivity Disorder (ADHD) who are helped by boosting DA actions (Arnsten and Pliszka, 2011; Schmeichel et al., 2013). Finally, a low affinity D1R agonist may be especially helpful in treating schizophrenia, where there are profound dlPFC cognitive deficits (Barch and Ceaser, 2012; Keefe and Harvey, 2012) and complex changes in the dlPFC DA system (Abi-Dargham et al., 2012; Akil et al., 1999; Slifstein et al., 2015).
1.4. Novel non-catechol D1R agonists
Pfizer has recently created a novel series of highly D1/D5 selective, potent non-catechol D1R agonists with excellent in vivo pharmacokinetic properties. Select non-catechol D1 agonists from this new series were shown to have a wide range of functional potencies, varying degrees of agonism, and favorable pharmacokinetics (Gray et al., 2018). These ligands stimulate adenylyl cyclase signaling, but exhibit distinct binding to the D1R orthosteric site, which leads to a novel functional profile compared to catechols, including minimal receptor desensitization, and reduced recruitment of β-arrestin (Gray et al., 2018), consistent with prolonged activation of motor function in preclinical studies (Gurrell et al., 2018).
The current study examined the effects of iontophoresing the novel, non-catechol D1R agonist, PF-3628, on dlPFC Delay cell firing in aged monkeys with naturally-occurring DA depletion and reduced persistent firing. PF-3628 was selected for this research, as it has modest in vitro functional activity and behaved as a near full agonist in a cAMP D1R assay (hD1R EC50 = 381 nM, Emax = 92% relative to dopamine), a profile that closely mimics that of endogenous DA in the same assay (hD1R EC50 = 115 nM, EMax = 100% by definition) and is selective for D1R and D5R vs. all of >50 other pharmacological targets tested (see Supplementary Materials for pharmacological characterization). Results were compared to ionotophoresis of the traditional, high affinity D1R agonist, SKF-81297 (hD1R EC50 = 4 nM, 96% relative to DA). Investigation of PF-3628 revealed the first evidence of excitatory actions with a selective D1R agonist. Examination of D1R microarray data from human dlPFC, in combination with immunoEM data from the rhesus monkey dlPFC, indicate that these actions may preferentially occur in the layer III pyramidal cells that subserve delay-dependent working memory.
2. MATERIALS AND METHODS
2.1. The non-catechol D1R agonist PF-3628
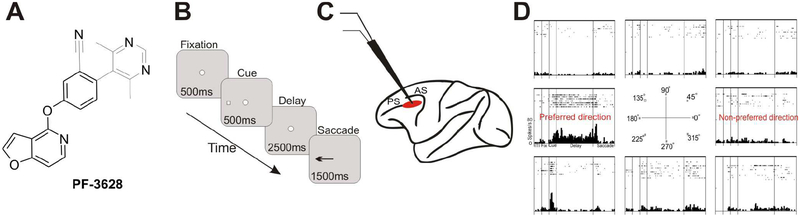
The structure of the non-catechol D1R agonist, PF-3628, is shown in Figure 1A. The synthesis of this compound is described in the Supplementary Materials.
Figure 1-. Experimental paradigm.
A. The molecular structure of PF-3628. B. The ODR task for testing delay-dependent visuo-spatial working memory. C. The recording site (red) in the rhesus monkey dlPFC. PS=principal sulcal; AS=arcuate sulcus D. Example of a dlPFC Delay cell. Rasters and histograms are arranged to indicate the location of the corresponding cue. Only the preferred direction and one non-preferred direction will be shown in subsequent figures in order to conserve space.
2.2. Physiological recordings in cognitively-engaged monkeys
2.2.1. Subjects:
Two aged rhesus monkeys (one female, 22-yr old, one male, 20-yr old, Macaca mulatta) were used for the current study, and cared for under the guidelines of the National Institutes of Health and the Yale IACUC.
2.2.2. Oculomotor delayed response (ODR) task:
The monkeys were seated in primate chairs with their heads fixed, and faced a 27 inch computer monitor 30 inch in front of them. The monkeys’ eye positions were monitored with the ISCAN Eye Movement Monitoring System (ISCAN, Burlington, MA). The monkeys were trained in the visuospatial ODR task, which required the subject to make a memory-guided saccade to a remembered visuo-spatial target. Patients with schizophrenia have been shown to be impaired on a human version of this task (Keedy et al., 2006). The ODR task was generated by the PICTO system (custom-designed Windows-based data-acquisition software). The task is illustrated in Figure 1B. A central small white circle was illuminated on the computer monitor, which served as the fixation target. To initiate a trial, the animal fixated this central target and maintained fixation for 0.5 seconds (fixation period), whereupon a cue (the same sized white circle) was illuminated for a period of 0.5 seconds (cue period) at one of eight peripheral targets located at an eccentricity of 13° with respect to the fixation spot. After the cue was extinguished, a 2.5-second delay period followed. The subject was required to maintain central fixation throughout both the cue presentation and the delay period. At the end of the delay, the fixation spot was extinguished which instructed the monkey to make a memory guided saccade to the location where the cue had been shown prior to the delay period. A trial was considered successful if the subject’s response was completed within 0.5 seconds of the offset of the fixation spot and was within 2° around the correct cue location. The subject was rewarded with fruit juice immediately after every successful response. The position of the stimulus was randomized over trials such that it had to be remembered on a trial-by-trial basis. The inter-trial intervals (ITI) were at least 3 sec. The subject performed 1000–1500 trials per session.
2.2.3. Recording Locus:
Prior to recording, the animals underwent a magnetic resonance image (MRI) scan in order to obtain exact anatomical co-ordinates of brain structures, which guided placement of the chronic recording chambers. MRI-compatible materials were used for the implant so that another MRI could be performed after implantation to confirm the position of the recording chambers. The recording wells were placed over the caudal principal sulcus as illustrated in Figure 1C.
2.2.4. In vivo single unit recordings and iontophoresis:
PF-3628 from Pfizer was dissolved in a solution of 5% DMSO, 5% cremaphor and 90% saline, and acidified with 1M HCL. SKF81297 from Sigma was dissolved in the same solution for comparison. Iontophoretic electrodes were constructed with a 20-μm-pitch carbon fiber (ELSI, San Diego, CA) inserted in the central barrel of a seven-barrel non-filamented capillary glass (Friedrich and Dimmock, Millville, NJ). The assembly was pulled using a multipipette electrode puller (PMP-107L, Microdata Instrument Inc., South Plainfield, NJ) and the tip was beveled to obtain the finished electrode. Finished electrodes had impedances of 0.3–1.5 MΩ (at 1 kHz) and tip sizes of 30–40μm. The outer barrels of the electrode were then filled with 3 drug solutions (two consecutive barrels each) and the solutions were pushed to the tip of the electrode using compressed air. A Neurophore BH2 iontophoretic system (Medical Systems Corp., Greenvale, NY) was used to control the delivery of the drugs. The drug was ejected at currents that varied from 10–100 nA. Note that the PF-3628 molecule has only a small electric charge, and thus the vehicle was acidified to a pH of about 3 to create a mixture of charged (+1, +2) as well as neutral conformations. Given the small electric charge, relatively higher electric currents were needed to eject compound from the pipette. Retaining currents of −5 to −10 nA were used in a cycled manner (1sec on, 1 sec off) when not applying drugs. Drug ejection did not create noise in the recording, and there was no systematic change in either spike amplitude or time course at any ejection current.
The electrode was mounted on a MO-95 micromanipulator (Narishige, East Meadow, NY) in a 25-gauge stainless steel guide tube. The dura was then punctured using the guide tube to facilitate access of the electrode to cortex. Extra-cellular voltage was amplified using a AC/DC differential preamplifier (Model 3000, A-M SYSTEMS) and band-pass filtered (180Hz-6kHz, 20dB gain, 4-pole Butterworth; Kron-Hite, Avon, MA). Signals were digitized (15 kHz, micro 1401, Cambridge Electronics Design, Cambridge, UK) and acquired using the Spike2 software (CED, Cambridge, UK). Neural activity was analyzed using waveform sorting by a template-matching algorithm. Post-stimulus time histograms (PSTHs) and rastergrams were constructed online to determine the relationship of unit activity to the task. Unit activity was measured in spikes per second. We classified four different kinds of ODR task-related cells: Fixation cells, Cue cells, Delay cells, and Response cells. If the rastergrams showed that a neuron displayed task-related activity, recording continued and pharmacological testing was performed. A total of 34 Delay cells were recorded and tested with agents (25 from Monkey C, 9 from Monkey A).
Neuronal activities were first collected from the cell under a control condition in which at least eight trials at each of 8 cue locations was obtained. A typical Delay cell is shown in Figure 1D. Upon establishing the stability of the cells’ activity, this control condition was followed by iontophoretic application of drug(s). Dose-dependent effects of the drug were tested in two or more consecutive conditions, which then was followed by a Recovery condition or a Reversal condition. Drugs were continuously applied at a relevant current throughout a given condition. Each condition had ~8 (6–12) trials at each location to allow for statistical analyses of drug effects.
2.2.5. Data analyses:
For purposes of data analysis, each trial in the ODR task was divided into four epochs – initial Fixation, Cue, Delay and Response (Saccade). The initial Fixation epoch lasted for 0.5 sec. The Cue epoch lasted for 0.5 sec and corresponds to the stimulus presentation phase of the task. The Delay lasted for 2.5 sec and reflects the mnemonic component of the task. The Response phase started immediately after the Delay epoch and lasted ~1.5 sec. Data analysis was performed in MATLAB and SPSS. Spike density functions were constructed in 50ms windows. Two-way analysis of variance (ANOVA) was used to examine the spatial tuned task-related activity with regard to: (1) different periods of the task (fixation, cue, delay, and response vs ITI) and (2) different cue locations. This study mainly focused on Delay cells that represent delay-dependent working memory. Many Delay cells fire during the cue and/or response epochs as well as the delay epoch; given their variable responding to the cue and response epochs, data analyses focused on the delay epoch. One-way ANOVA or two tailed paired t-test were employed to assess the effects of drug application on task-related activity. In the interest of brevity, figures often show the neurons’ preferred direction in comparison to just one non-preferred direction, the “anti-preferred” direction directly opposed to the neurons’ preferred direction. For Delay cells, spatial tuning was assessed by comparing firing levels for the neuron’s preferred direction vs. its non-preferred directions. Quantification of spatial tuning was performed by calculating a measure of d’ using the formula:
2.3. Microarray analysis of D1R in human dlPFC
2.3.1. Human subjects:
Brain specimens (n=7) of healthy control subjects were obtained during routine autopsies conducted at the Alleghany County Office of the Medical Examiner (Pittsburgh, PA, USA) after consent was obtained from the next-of-kin. For details pertaining to age, post-mortem interval, RNA integrity number, brain pH, tissue storage time or race for subjects assessed, please refer to previously published papers (Arion et al., 2015; Enwright et al., 2017). All procedures were approved by the University of Pittsburgh Committee for the Oversight of Research Involving the Dead, and the University of Pittsburgh Institutional Review Board for Biomedical Research.
2.3.2. Laser microdissection (LMD):
The right hemisphere of each brain was blocked coronally, immediately frozen and stored at −80°C. Tissue sections (12 μm) containing dlPFC area 9 were cut on a cryostat, mounted on glass polyethylene napthalate membrane slides (Leica Microsystems, Bannockburn, IL, USA). For pyramidal cell microdissections, slides were stained with thionin for Nissl substance (Arion et al. 2015) and pyramidal neuron (n=100 cells per sample) cell bodies with a characteristic triangular shape and prominent apical dendrite were identified and dissected from dlPFC layer III using the Leica microdissection system (LMD 6500). For dlPFC layer III parvalbumin-positive GABA interneuron (n=150 cells per sample) dissection, sections were immunolabeled using antibody raised against aggrecan (Enwright et al., 2017), a component of perineuronal nets (PNNs). PNNs are a condensed form of the extracellular matrix around most PV interneurons (Härtig et al., 1992). For each subject, two replicates were processed independently and replicate samples were averaged for data analysis.
2.3.3. Microarray profiling for cell-type specific transcript enrichment:
For each sample, RNA was extracted using the QIAGEN Micro RNeasy kit Plus (Qiagen, Valencia, CA, USA). The Ovation Pico WTA System (San Carlos, CA, USA) was used for synthesis and amplification of cDNA and samples were profiled using the Affymetrix GeneChip U219. Data from all 14 samples were normalized together.
2.3.4. Statistical Analyses:
Following normalization, average log2 expression values were determined for each probe for a cell type. P-values from paired t-tests were corrected using the Benjamini-Hochberg procedure, and differential-expression determined using a false discovery rate of q<0.05.
3. RESULTS
3.1. Physiological recordings in cognitively engaged monkeys
We examined the effects of iontophoretic application of PF-3628, a novel D1R agonist, directly onto memory-related neurons in the primate dlPFC. iontophoresis releases very small amounts of drug, which likely influence a mini-column of nearby neurons, but are inadequate to influence behavioral performance. Behavioral performance was maintained at optimal levels during the drug applications.
3.1.1. Iontophoresis of lower doses of PF-3628 enhance the delay-related firing and spatial tuning of dlPFC Delay cells
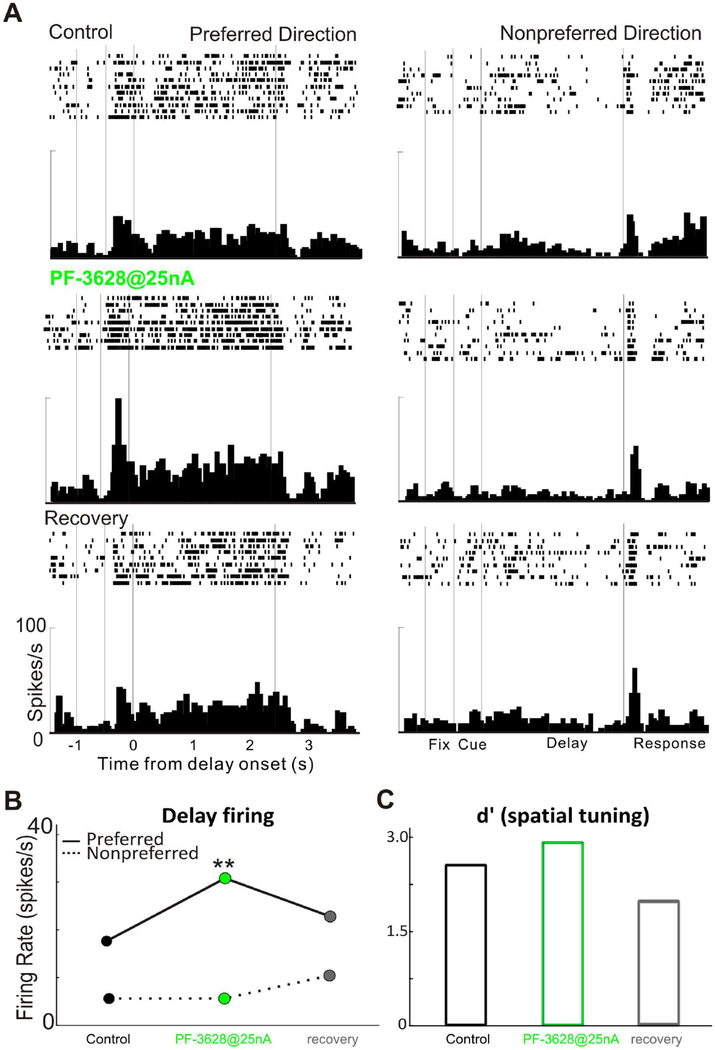
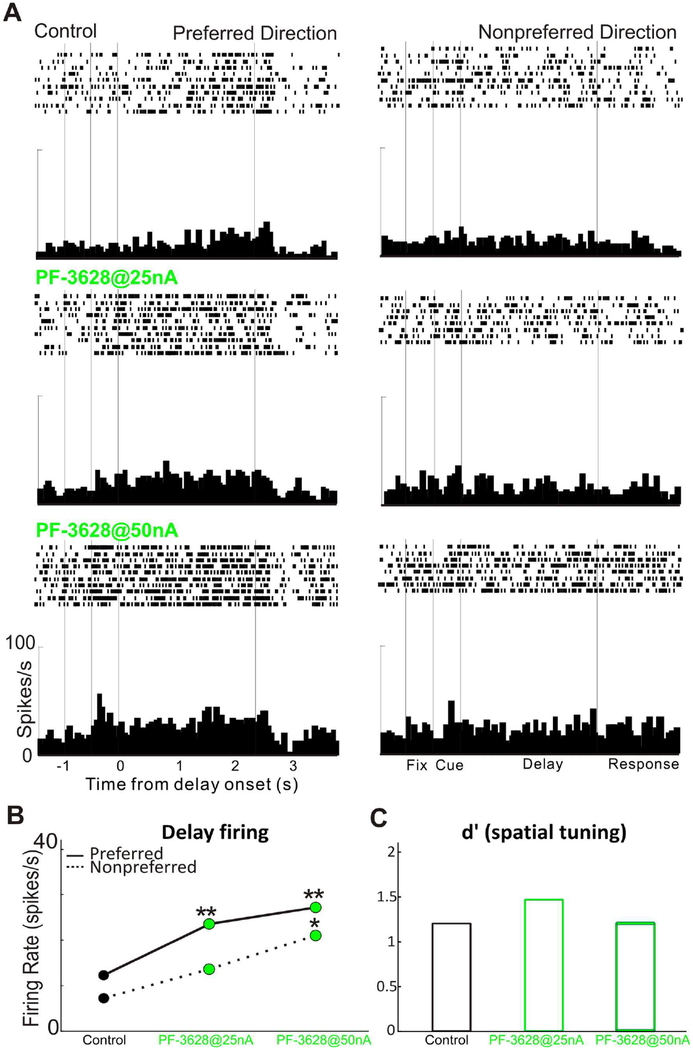
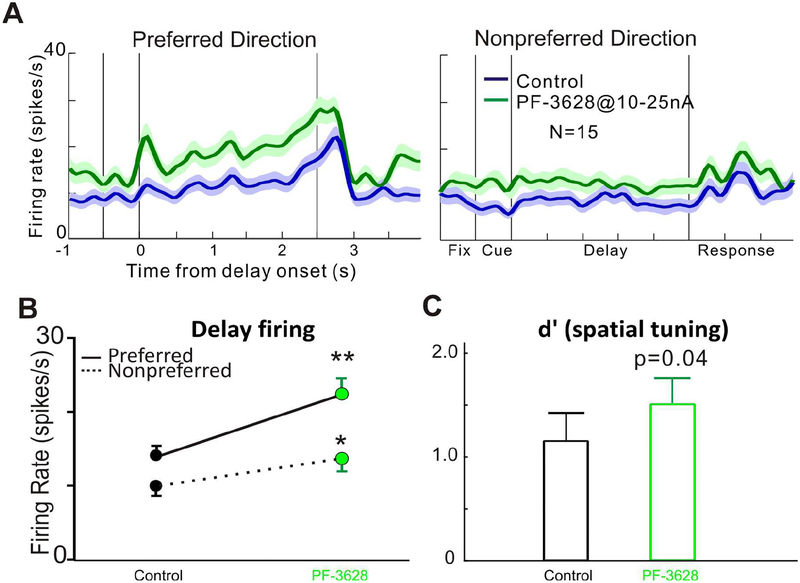
We first examined the effect of PF-3628 on the delay-related firing and spatial tuning of dlPFC Delay cells in monkeys performing the ODR task. We found that iontophoresis of PF-3628 significantly enhanced the delay-related firing and spatial tuning of Delay cells. As seen in a single-cell example in Figure 2, iontophoretic application of PF-3628 significantly enhanced the delay activity for the neuron’s preferred direction (p<0.01), but not for its non-preferred direction (p>0.05; figure 2A and 2B), enhancing the spatial tuning (figure 2C). Delay firing returned to control levels when the drug was no longer applied (recovery condition). In another single-cell example in figure 3, iontophoretic application of the PF-3628, produced a dose-related enhancement in delay-related firing and spatial tuning. PF-3628 at dose of 25nA significantly enhanced the delay activity for the neuron’s preferred direction (p<0.01), but not for its non-preferred direction (p>0.05; figure 3A and 3B), enhancing the spatial tuning (figure 3C), while PF-3628 at a high dose of 50nA further increased the delay activity for both preferred and non-preferred directions significantly (p<0.01 for preferred direction and p<0.05 for non-preferred direction; figure 3A and 3B), therefore, bringing the spatial tuning down (figure 3C). The enhancing effects of PF-3628 at lower doses were consistent in the population level. Figure 4 shows the averaged effect of PF-3628 on Delay cell firing in 15 Delay cells. PF-3628 at doses of 10–25nA significantly enhanced Delay cell firing during the delay period. Statistical analysis showed that PF3628 application significantly increased neuronal firing during the delay epoch for both the neurons’ preferred direction (figure 4B; p=0.0002, Wilcoxon matched-pairs signed rank test), and the non-preferred direction (figure 4B; p=0.003, Wilcoxon matched-pairs signed rank test). To evaluate the direction selectivity of PF-3628, we calculate a measure of d’ to examined whether PF3628 enhance the spatial tuning of Delay cells. This measure captures how well a Delay cell can represent a spatial position over the delay epoch in the absence of sensory stimulation, and thus is particularly important to the strength of working memory. A greater d’ value indicates greater directional selectivity, i.e, greater spatial tuning. A total of 12 out of 15 Delay cells showed higher d’ in the PF-3628 condition compared with the control condition. Overall, iontophoresis of PF-3628 significantly enhanced the spatial tuning of Delay cell firing during the delay epoch by increasing d’ (figure 4C; p=0.012, Wilcoxon matched-pairs signed rank test).
Figure 2-.
A single neuron example of the enhancing effects of PF-3628 on delay-related firing. A: Iontophoresis of PF-3628@25nA significantly enhanced delay-related firing for the neuron’s preferred direction, not for the non-preferred direction. Activity was reduced to control levels after drug application was terminated during the recovery condition (third panel; control vs recovery: p>0.05, one-way ANOVA). Rasters and histograms for preferred direction and non-preferred direction during control condition and PF-3628 application condition and recovery are shown. B: Statistical analysis of the neuronal firing rates across the delay epoch for the Delay cell shown above, during control, PF-3628, and recovery conditions. C: Spatial tuning changes for this Delay cell during control, PF-3628, and recovery conditions.
Figure 3-.
A single neuron example of the dose-dependent, beneficial effects of PF-3628 on delay-related firing. A: Iontophoresis of PF-3628@25nA significantly enhanced delay-related firing for the neuron’s preferred direction, but not for the non-preferred direction. Increasing the PF-3628 dose to 50nA significantly increased delay-related firing for both the preferred and nonpreferred directions. B: Statistical analysis of neuronal firing rates during the depay epoch for this Delay cell during control and PF-3628 conditions. C: Spatial tuning change for the above Delay cell during control and PF-3628 conditions.
Figure 4-.
The enhancing effects of PF-3628 on delay-related firing at the population level. Top panel A shows population spike density functions for the average of 15 Delay cells, showing firing for their preferred vs. their non-preferred directions under control conditions (blue) and following iontophoresis of PF-3628 conditions (green). B: Statistical analysis showed that PF-3628 application significantly increased neuronal firing during the delay epoch for both the neurons’ preferred and the non-preferred direction, with higher increase for the preferred direction. C: Iontophoresis of PF-3628 significantly enhanced the spatial tuning of Delay cells by increasing d’.
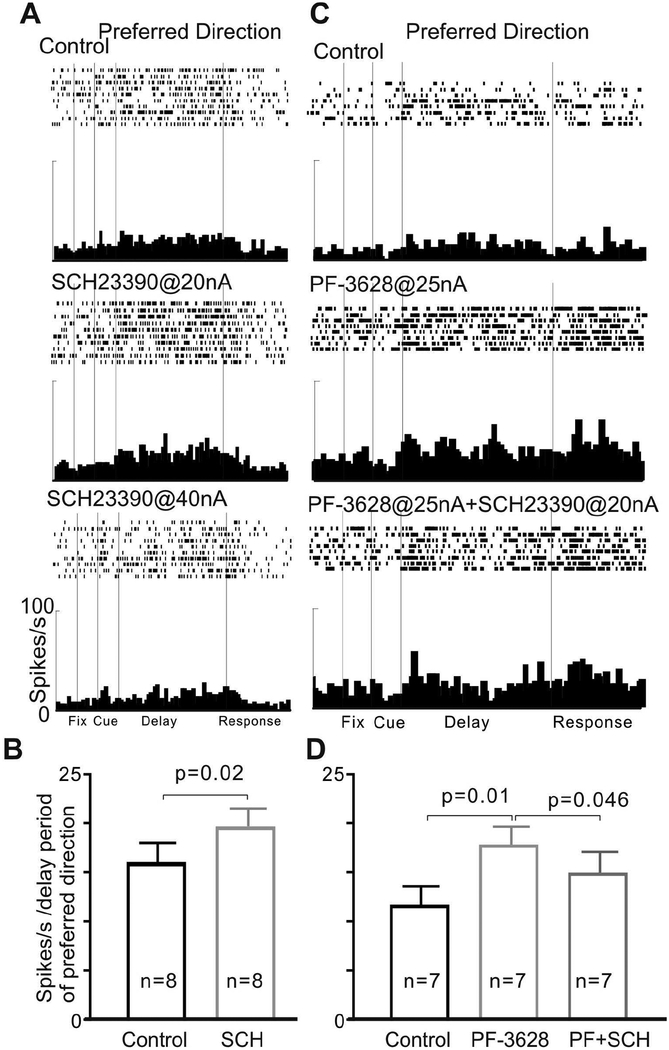
To test whether the enhancing effects of PF3628 occurred through actions at D1Rs, we co-applied the D1R antagonist, SCH23390 with PF-3628 to examine whether SCH23390 would block the enhancing effects of PF-3628. Iontophoresis of SCH23390 at 20nA caused a significant increase in delay firing (Figure 5A and Figure 5B, p=0.02, Wilcoxon matched-pairs signed rank test), consistent with previous studies (Williams and Goldman-Rakic, 1995; Vijayraghavan et al., 2007), while we found that co-iontophoresis of SCH23390 reversed the enhancing effects of PF-3628 on delay-related firing. As shown in Figure 5, PF3628 @ 25nA significantly increased delay-related firing for the neurons’ preferred direction, and these enhancing effects of PF3628 were partially but significantly reversed by co-iontophoresis of SCH23390 (Figure 5; overall: p=0.008, Friedman test; pair-wise comparisons: Control vs. PF-3628: p=0.01; PF-3628 vs. PF-3628+SCH: p=0.046, control vs. PF-3628+SCH: p=0.031; Wilcoxon signed rank test). As both agents increased firing on their own, the results cannot be explained by simple, additive effects, but instead are consistent with PF-3628 acting at D1R.
Figure 5-.
The DA D1R antagonist, SCH23390, reversed the enhancing effects of PF-3628 on delay-related firing of dlPFC neurons in aged monkeys. A: iontophoresis of SCH alone had an inverted-U effect on delay-related firing: SCH23390 at a lower dose (20nA) increased delay firing, but decreased firing at a higher dose (40nA). B: The mean ± SEM firing rate (delay period of preferred direction) of 8 Delay cells showing that low dose SCH23390 (20nA) increased delay-related firing. C: PF-3628 at 25 nA significantly enhanced delay-related firing for the neuron’s preferred direction, and the enhancement was reversed by co-iontophoresis of the D1 antagonist, SCH23390, consistent with PF-3628 actions at DA D1R. D: The mean ± SEM firing rate (delay period of preferred direction) of 7 Delay cells showing SCH23390 reversed the enhancing effect of PF-3628.
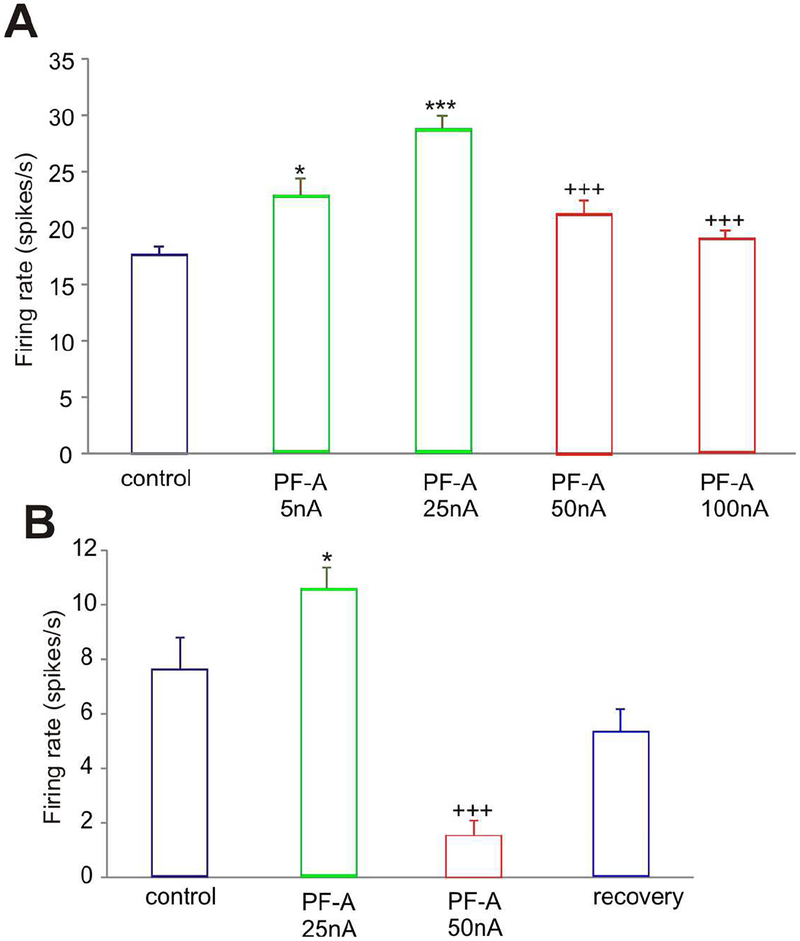
3.1.2. Higher doses of PF3628 have a mixed effect on the delay-related firing of dlPFC Delay cells
In contrast to the consistent enhancing effects of PF-3628 at lower doses (10–25nA), PF-3628 had mixed effects at higher doses (50–100nA). Figure 3 had shown an example where PF-3628 increased delay-related firing, but did so for both preferred and non-preferred directions (figure 3A and 3B), and thus eroded spatial tuning (figure 3C). However, Figure 6 shows examples of neurons where PF-3628 produced an inverted U dose response, where the enhancement of firing was eroded at higher doses (Figure 6A), or a pronounced decrease in firing with 50nA (Figure 6B). This neuron recovered to more normal levels of firing when the drug was withdrawn, indicating that the loss of firing at 50nA was due to drug actions.
Figure 6-.
Higher doses of PF-3628 had mixed effects on delay-related firing of dlPFC neurons in aged monkeys. A: The mean ± SEM firing rate (delay period of the neuron’s preferred direction) of a Delay cell showing that PF-3628 produced an inverted U dose-response. The enhancement in firing following lower doses was eroded at higher doses. B: Another neuronal example where PF-3628 produced an inverted U dose-response, with a low dose enhancing, but a higher dose decreasing, delay-related neuronal firing.
3.1.3. Vehicle has no effect, while SKF 81297 reduces dlPFC Delay cell firing
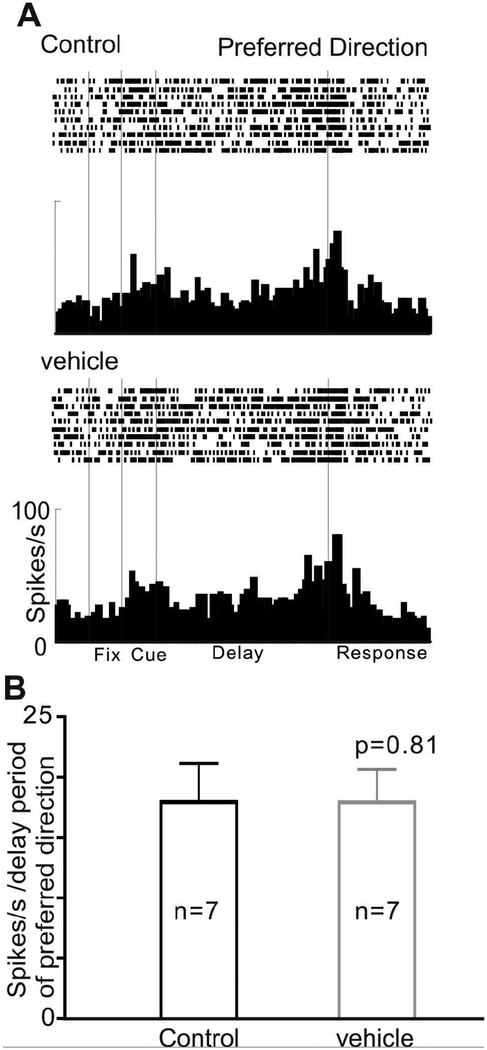
We examined whether the vehicle used to dissolve PF-3628 (5% DMSO, 5% cremaphor and 90% saline) had any effect on delay-related firing of dlPFC Delay cells on its own. As shown in Figure 7, the iontophoresis of vehicle had no effect on delay-related firing (figure 7; p=0.81, Wilcoxon matched-pairs signed rank test). This is similar to results with our standard vehicle of distilled water, which similarly had no effect neuronal firing (Vijayraghavan et al., 2007).
Figure 7-.
The vehicle used to dissolve PF-3628 had no effect on delay-related firing of dlPFC neurons in aged monkeys. A: An example neuron shows that the vehicle (25nA) produced no change in delay-related firing for the neuron’s preferred direction. B: The mean ± SEM firing rate (delay period of preferred direction) of 7 Delay cells, showing the vehicle had no effect on delay-related firing.
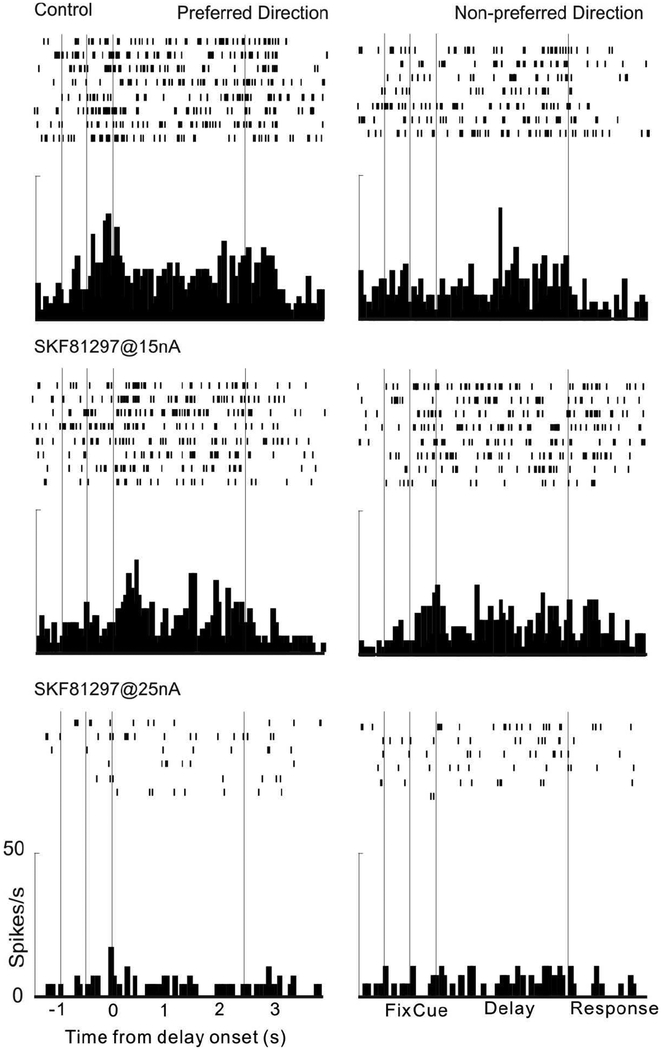
Finally, we tested the effects of the traditional D1R agonist, SKF81297, on delay-related firing of dlPFC Delay cells in the same aged animals used to test PF-3628. SKF81279 was dissolved in the same vehicle as used for PF3628. As shown in Figure 8, SKF81297 significantly reduced delay-related firing, replicating our previous findings with this compound in young adult monkeys (Vijayraghavan et al., 2007).
Figure 8-.
A single neuron example of the suppressing effects of SKF81297 on delay-related firing in a dose-dependent manner. Iontophoresis of SKF81297@15nA suppressed delay-related firing for both the neuron’s preferred and the non-preferred direction; firing was further suppressed when the dose was Increased to 25nA. These results are similar to previously published findings with this compound.
3.2. Microarray analyses of human dlPFC
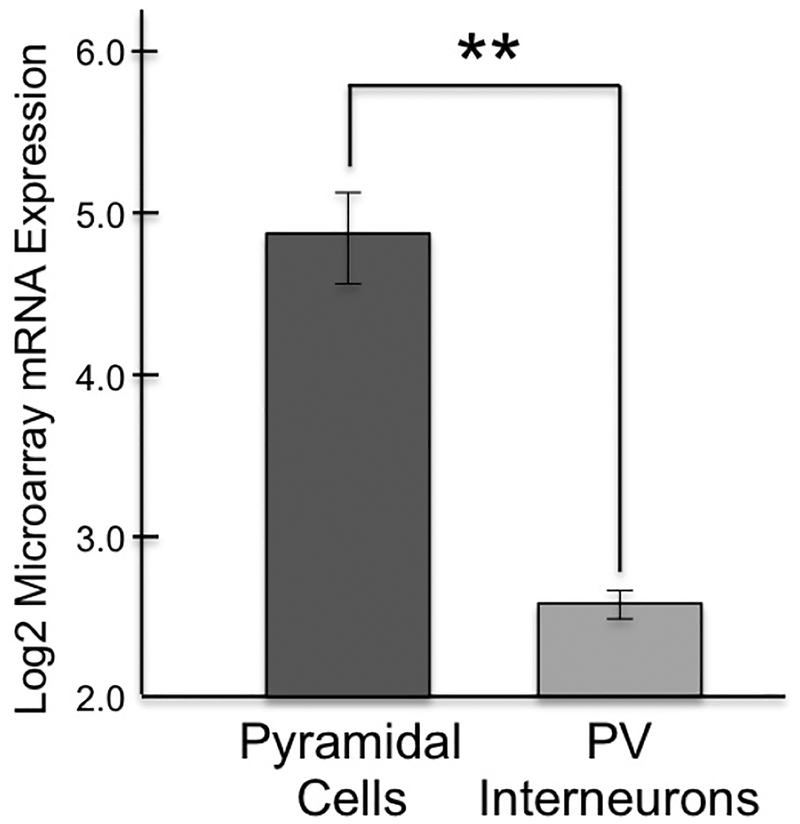
Microarray analyses compared the expression of DRD1 message in pyramidal cells vs. PV interneurons in layer III of the healthy human dlPFC. Gene expression profiles of pyramidal cells compared to PV interneurons demonstrated 4.78-fold enrichment for DRD1 mRNA specific to pyramidal cells compared to PV cells. The mean log2 microarray signal for DRD1 mRNA in pyramidal cells was 4.85, compared to 2.60 in PV cells, indicating significantly greater expression of DRD1 mRNA in pyramidal cells from the same subjects ((p<0.001; q=0.001; Fig. 9). These results are consistent with the rhesus monkey data, where DRD1 are especially prominent on spines.
Figure 9-. Human microarray data showing greater DRD1 expression in pyramidal cells vs. PV interneurons in layer III dlPFC.
Log2-transformed microarray signal of DRD1 mRNA in dlPFC layer III pyramidal and PV cells within healthy human subjects (n=7). The log2 microarray DRD1 mRNA expression in pyramidal cells (4.85 ± 0.718) was significantly 4-fold higher (p<0.001; q=0.001) compared to PV interneurons (2.60 ± 0.210) in human dlPFC layer III.
4.0. DISCUSSION
4.1. Overview
The current data provide the first evidence of excitatory effects of a selective D1R agonist in the primate dlPFC, and confirm an exciting breakthrough in D1R pharmacology. The noncatechol D1R agonist, PF-3628, produced an inverted-U dose response curve on the firing of dlPFC Delay cells in aged monkeys, increasing persistent firing at low to moderate doses, but with an erosion of efficacy as the dose was raised. The excitatory effects of PF-3628 were reversed by the D1R antagonist, SCH23390, consistent with drug actions at the D1 family of receptors (D1/D5). The effects were particularly evident in the oldest monkey, who had naturally-occurring, marked reductions in persistent firing (Wang et al., 2011). As DA depletes from the dlPFC with advancing age (Goldman-Rakic and Brown, 1981; Wenk et al., 1989), it is possible that PF-3628 helped to restore lost, endogenous excitatory D1R actions on aging neurons.
High doses of PF-3628 were less effective than lower doses in exciting neuronal firing, but did not suppress firing below baseline, as was seen with the traditional D1R agonist, SKF81297. This is likely due to the chemical properties of PF-3628, which has only a small electric charge, and thus it is difficult to eject high concentrations via iontophoresis, even with the application of large electric currents. However, the loss of firing as the dose was raised would be consistent with the engagement of suppressive mechanisms seen so readily with higher affinity, traditional D1R agonists.
The in vivo physiological recordings in this study were performed “blind” to laminar location or neuronal type. Although a fast-spiking signature can be used to suggest a PV-containing interneuron (González-Burgos et al., 2005), this method is not definitive. The monkey electron microscopy data suggests that most D1R in dlPFC are on spines (Smiley et al., 1994), indicating expression on pyramidal cells. The human microarray data examined in the current study are consistent with these findings, showing more prominent D1R expression in pyramidal cells than in PV interneurons. These data increase confidence that the results in monkeys are relevant to dlPFC in humans.
4.2. D1R actions in primate dlPFC
Until now, all research with D1R agonists has been restricted to agonists with high affinity for the receptor. These studies have all observed a reduction in firing with D1R agonist application, often with a preferential reduction of “noise” at lower doses, thus benefiting information processing. This profile has been seen with dlPFC neurons in monkeys performing tasks that require representation of spatial position, numerical information or changing rules (Ott et al., 2018; Vijayraghavan et al., 2017; Vijayraghavan et al., 2007), and in vlPFC neurons involved in flexible, associative learning (Puig and Miller, 2012). This reduction in firing is not simply due to internalization of D1R following intensive, high affinity stimulation, as excitatory effects are seen when low doses of D1R antagonists are applied (Vijayraghavan et al., 2007; Williams and Goldman-Rakic, 1995), indicating that endogenous DA, as well as high affinity agonists, can suppress dlPFC firing. Excitatory effects of D1R antagonist application were also seen in the nearby frontal eye field (FEF), where local infusion of SCH23390 into the FEF increased neuronal firing and enhanced FEF top down control of attentional responding in V4 neurons (Noudoost and Moore, 2011). Thus, the suppressive effects of D1R stimulation by endogenous or exogenous ligands in primate lateral frontal lobe are well-established.
However, there has also been the suggestion of excitatory D1R actions when DA itself has been applied to neurons. In particular, there was strong evidence from ex vivo studies of dlPFC slices, where DA application induced EPSPs that were reversed by a D1R antagonist (Henze et al., 2000). There has also been suggestive data from in vivo recordings in monkeys where DA application was excitatory (Jacob et al., 2013; Sawaguchi et al., 1988; Sawaguchi et al., 1990), but these studies did not utilize a D1R antagonist to test for receptor actions. This is essential, as DA can have excitatory actions via D2R (Ott et al., 2014), or via uptake and rerelease by NE terminals (Lewis et al., 2001; Mazei et al., 2002), e.g. onto postsynaptic alpha-2A-AR (Wang et al., 2007). In vivo recordings have shown a marked reduction in the firing of dlPFC neurons following iontophoresis of high dose D1R antagonist (Williams and Goldman-Rakic, 1995), consistent with endogenous excitatory actions of DA at D1R. However, the very high doses needed to reduce firing raise the possibility of off-target drug actions at very high concentrations. Thus, the field has been awaiting in vivo evidence that a D1R selective agonist could excite dlPFC neuronal firing, and the current data provide these data for the first time.
4.3. Speculations on cellular and subcellular basis for drug actions
The microarray analysis of human dlPFC indicate that D1R message is most concentrated in pyramidal cells, consistent with previous immunoEM studies showing a primary location on spines (Smiley et al., 1994). The subcellular locations of D1R in spines within the PSD, as well as at extra-synaptic locations near HCN channels, suggest hypotheses about the potential mechanisms underlying D1R excitatory vs. suppressive actions (Arnsten et al., 2015). The excitatory effects of PF-3628 in the current study might arise from D1R-PKA/PKC phosphorylation of NMDAR, maintaining them within the PSD. There is direct evidence for this in rat medial PFC, (Li et al., 2010), and the presence of D1R within or near the PSD of glutamate-like synapses in monkey layer III dlPFC suggests it could also occur in primate dlPFC (Fig. 10; (Arnsten et al., 2015; Gamo et al., 2015; Paspalas et al., 2013). Increased numbers of NMDAR in the PSD would be consistent with PF-3628 increasing firing for Delay cells’ nonpreferred, as well as preferred directions. D1R excitatory effects might also include D1R inhibition of GABAergic interneurons, but these effects appear subtle and complex when studied ex vivo (Gonzalez-Burgos et al., 2005).
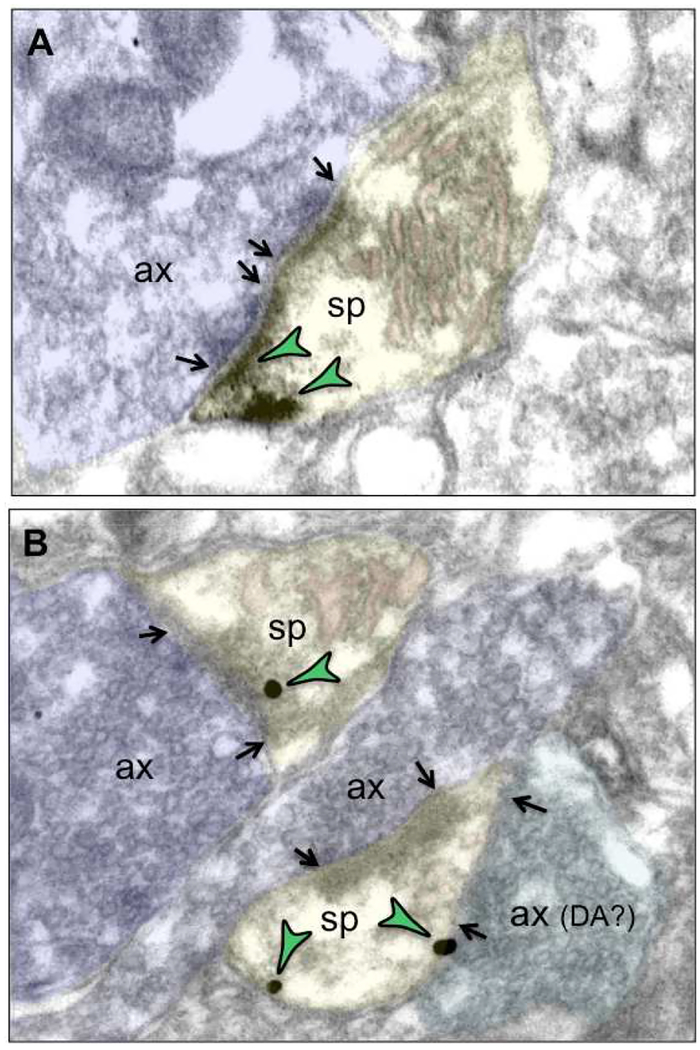
Figure 10-. Immunoelectron microscopy showing D1R labeling (green arrowheads) in or near the glutamate PSD on spines in layer III of the rhesus monkey dlPFC.
A. DAB (3,3-diaminobenzidine) peroxidase labeling indicates that D1R are localized within and next to a spine synapse receiving a glutamate-like axon terminal. This synapse is “perforated”, a sign of synaptic maturity that is a common feature in monkey dlPFC. B. Immunogold labeling shows D1R in two spines. The top spine is receiving a glutamate-like asymmetric synapse, with D1R label within the elaborated PSD. The bottom synapse forms a synaptic triad, with both an asymmetric glutamate-like synapse, and a symmetric, possibly DAergic synapse. Double-labeling, e.g. with tyrosine hydroxylase, would be needed to determine whether it is actually a DA terminal, but DA-glutamatergic synaptic triads are long-established in the primate dlPFC (Goldman-Rakic et al., 1989). In this spine, D1R can be seen at a distance from the synapse, a common site for interaction with HCN channels (Arnsten et al., 2015; Paspalas et al., 2013), as well as near the symmetric synapse, possibly mediating DA actions. Spines (sp) are pseudocolored in yellow, axon terminals (ax) in blue, and the calcium-containing spine apparatus in pink; synapses are denoted by arrows.
In contrast, the suppressive effects of D1R stimulation at higher levels likely involve cAMP-opening of HCN channels on spines, reducing network connectivity (Arnsten et al., 2015; Gamo et al., 2015; Paspalas et al., 2013), but also may involve decreased glutamate release from axon terminals (Gao et al., 2001; Paspalas and Goldman-Rakic, 2005). Finally, all current D1R agonists, including PF-3628, additionally stimulate D5R, which are found on the plasma membrane of layer III and V pyramidal cells near the SER and mitochondria (Paspalas and Goldman-Rakic, 2004). The functional contributions of D5R in primate dlPFC are not known, but nonetheless should be considered when speculating about cellular actions.
4.4. Clinical relevance
The current suggest that PF-3628, or related, low affinity D1R agonists, may be beneficial for humans with reduced DA levels in PFC and/or PFC cognitive deficits. Age-related cognitive changes may be aided by low affinity D1R therapy. PFC executive abilities begin to decline as early as middle age in both monkeys (Moore et al., 2006) and humans (Adólfsdóttir et al., 2017; Park and Bischof, 2013), and there is loss of DA from primate dlPFC early in the aging process (Goldman-Rakic and Brown, 1981). Thus, low dose D1R stimulation may be helpful in maintaining dlPFC functions needed to thrive in the Information Age.
Low affinity D1R agonists may also be helpful in treating the PFC cognitive deficits associated with schizophrenia. Schizophrenia is associated with profound dlPFC cognitive deficits, not helped by current medications, which impede societal integration and overall outcome (Barch and Ceaser, 2012; Keefe and Harvey, 2012). Thus, there is a great need for medications that can restore cognitive function (Arnsten and Wang, 2016). Schizophrenia involves multiple changes in brain, including atrophy of pyramidal cells in layer III dlPFC (reviewed in (Datta and Arnsten, 2018)), and complex changes in the DA system. Neuroimaging studies have shown increased D1R expression in PFC early in the course of illness in drug naïve patients (Abi-Dargham et al., 2012), which may be compensation for reduced DA (Abi-Dargham et al., 2012; Akil et al., 1999; Slifstein et al., 2015), or alternatively, part of a hyperdopaminergic response during adolescence (Abi-Dargham et al., 2012; Rosenberg and Lewis, 1994). However, established disease appears associated with reduced DA release in cortex (Slifstein et al., 2015) and weaker TH expression in layer VI of PFC (Akil et al., 1999), in concert with increased DA release in caudate (Kegeles et al., 2010; Laruelle et al., 1996) which may magnify cortical errors (Datta and Arnsten, 2018). Post-mortem studies have also shown that the rs5326-A allele in the promoter region of the D1R was associated with poorer cognition and lower cortical D1R gene expression in patients with schizophrenia (Tsang et al., 2015), suggesting that D1R agonist therapy, using a strategy that strengthens layer III pyramidal cell synaptic connectivity, should be helpful.
Initial trials of D1R agonist treatments have been problematic, but were necessarily performed with traditional, high affinity agonists (Arnsten et al., 2017). Dihydrexidine was the first synthesized D1R agonist, and has high affinity for D1R (Brewster et al., 1990; Lovenberg et al., 1989). There were hints that dihydrexidine may help working memory in schizotypical patients (Rosell et al., 2015), but it did not benefit patients with schizophrenia in a small, proof-of-concept trial (Girgis et al., 2016). The current data in monkeys caution that the dose of D1R agonist must be low, even with a low affinity D1R agonist, or the beneficial effects on PFC neuronal physiology will erode. It will also be important to test patients relatively early in the disease process, before spine loss (Glantz and Lewis, 2000) removes the substrate for therapeutic drug actions. Small dose-finding phase II studies may be essential for finding an effective dose range, and focus should be on very low affinity D1R agonists. A related compound in this new class of noncatechol D1R agonists has begun to show promise in treating movement disorder in Parkinson’s Disease (Gurrell et al., 2018). However, smaller doses of D1R agonist would be needed for schizophrenia, as the dlPFC has a much lower level of DA innervation than striatum (Berger et al., 1991; Lewis et al., 1987), and a nonlinear dose/response. The current data show that DRD1 message is enriched in pyramidal cells in layer III of the healthy human dlPFC, and thus a D1R treatment that can excite and strengthen these synapses early in the course of schizophrenia may be beneficial in protecting these important cognitive circuits.
In summary, the availability of novel D1R agonists that can excite PFC delay-dependent working memory circuits is an important step forward in providing pharmacological tools that better mimic the beneficial influence of endogenous DA. It is hoped that future studies may find that low doses of these compounds can benefit patients with PFC cognitive dysfunction.
Supplementary Material
HIGHLIGHTS.
There’s great need for a low-affinity D1R agonist that mimics DA excitatory actions
We tested a new noncatechol, low affinity D1R agonist, PF-3628, in aged monkey cortex
PF-3628 excited the dlPFC neurons that maintain information in working memory
D1R are concentrated in human dlPFC layer III pyramidal cells compared to PV cells
PF-3628 and related agonists may be a new path for treating human cognitive disorders
Acknowledgements-
The D1R physiological experiments were supported by funds from Pfizer, Inc. to AFTA and MW. The human microarray data were funded by NIH Grants MH103204 and MH043784, and a grant from Bristol-Myers Squibb to DAL. The immunoEM data were funded by NIH Pioneer Award DP1AG047744–01 to AFTA. We thank Steven O’Neil for the synthesis of PF-3628.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing interests.
5.0 REFERENCES
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban NB, Narendran R, Hwang DR, Laruelle M, Slifstein M, 2012. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 26, 794–805. [DOI] [PubMed] [Google Scholar]
- Adólfsdóttir S, Wollschlaeger D, Wehling E, Lundervold AJ, 2017. Inhibition and Switching in Healthy Aging: A Longitudinal Study. J Int Neuropsychol Soc. 23, 90–97. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA, 1999. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 156, 1580–1589. [DOI] [PubMed] [Google Scholar]
- Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, Cacace AM, Zaczek R, Albright CF, Tseng GF, Lewis DA, 2015. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry 20, 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Girgis RR, Gray DL, Mailman RB, 2017. Novel Dopamine Therapeutics for Cognitive Deficits in Schizophrenia. Biol Psychiatry 81, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR, 2011. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 99, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Wang M, 2016. Targeting Prefrontal Cortical Systems for Drug Development: Potential Therapies for Cognitive Disorders. Annual Rev Pharmacology Toxicology 56, 339–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Wang M, Paspalas CD, 2015. Dopamine’s actions in primate prefrontal cortex: Challenges for treating cognitive disorders. Pharmacological Rev 67, 681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Ceaser A, 2012. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 16, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C, 1991. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neuroscience 14, 21–27. [DOI] [PubMed] [Google Scholar]
- Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, Mailman RB, 1990. trans-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine: A highly potent selective dopamine D1 full agonist. J. Med. Chem 33, 1756. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O, 2010. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T, Brown RM, Rosvold HE, Goldman PS, 1979. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205, 929–931. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW, 1998. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J. Cognitive Neuroscience 10, 332–354. [DOI] [PubMed] [Google Scholar]
- Datta D, Arnsten AFT, 2018. Unique molecular regulation of higher-order prefrontal cortical circuits: Insights into the neurobiology of schizophrenia. ACS Chem Neurosci. March 1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwright JF, Huo Z, Arion D, Corradi JP, Tseng G, Lewis DA, 2017. Transcriptome alterations of prefrontal cortical parvalbumin neurons in schizophrenia. Mol Psychiatry November 7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Lur G, Higley MJ, Wang M, Paspalas CD, Vijayraghavan S, Yang Y, Ramos BP, Peng K, Kata A, Boven L, Lin F, Roman L, Lee D, Arnsten AFT, 2015. Stress impairs prefrontal cortical function via D1 dopamine receptor interactions with HCN channels. Biol. Psychiatry 78, 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS, 2001. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 98, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Van Snellenberg JX, Glass A, Kegeles LS, Thompson JL, Wall M, Cho RY, Carter CS, Slifstein M, Abi-Dargham A, Lieberman JA, 2016. A proof-of-concept, randomized controlled trial of DAR-0100A, a dopamine-1 receptor agonist, for cognitive enhancement in schizophrenia. J Psychopharmacol. 30, 428–435. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA, 2000. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives General Psychiatry 57, 65–73. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, 1995. Cellular basis of working memory. Neuron 14, 477–485. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM, 1981. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience 6, 177–187. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M, 1989. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA 86, 9015–9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos G, Barrionuevo G, Lewis DA, 2000. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 10, 82–92. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA, 2005. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 93, 942–953. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos GR, Kroener S, Seamans JK, Lewis DA, Barrionuevo G, 2005. Dopaminergic modulation of short-term synaptic plasticity in fast-spiking interneurons of primate dorsolateral prefrontal cortex. J Neurophysiol 94, 4168–4177. [DOI] [PubMed] [Google Scholar]
- Gray DL, Allen JA, Mente S, O’Connor RE, DeMarco GJ, Efremov I, Tierney P, Volfson D, Davoren J, Guilmette E, Salafia M, Kozak R, Ehlers MD, 2018. Impaired β-arrestin recruitment and reduced desensitization by non-catechol agonists of the D1 dopamine receptor. Nat Commun. 9, 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurrell R, Duvvuri S, Sun P, DeMartinis N, 2018. A Phase I Study of the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Novel Dopamine D1 Receptor Partial Agonist, PF-06669571, in Subjects with Idiopathic Parkinson’s Disease. Clin Drug Investig. 38, 509–517. [DOI] [PubMed] [Google Scholar]
- Härtig W, Brauer K, Brückner G, 1992. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport 3, 869–872. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G, 2000. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 84, 2799–2809. [DOI] [PubMed] [Google Scholar]
- Jacob SN, Ott T, Nieder A, 2013. Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J Neurosci. 33, 13724–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA, 2006. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 146, 199–211. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, 2012. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 213, 11–37. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M, 2010. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67, 231–239. [DOI] [PubMed] [Google Scholar]
- Kodama T, Hikosaka K, Honda Y, Kojima T, Watanabe M, 2014. Higher dopamine release induced by less rather than more preferred reward during a working memory task in the primate prefrontal cortex. Behav Brain Res. 266, 104–107. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB, 1996. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 93, 9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cambell MJ, Foote SL, Goldstein M, Morrison JH, 1987. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J. Neurosci 282, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DA, Sesack SR, Whitehead RE, Auh S, Sampson A, 2001. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol 432, 119–136. [DOI] [PubMed] [Google Scholar]
- Li YC, Liu G, Hu JL, Gao WJ, Huang YQ, 2010. Dopamine D(1) receptor-mediated enhancement of NMDA receptor trafficking requires rapid PKC-dependent synaptic insertion in the prefrontal neurons. J Neurochem. 114, 62–73. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P, 1991. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone, and [3H]SCH 23390. Neurosci 40, 657–671. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Brewster WK, Mottola DM, Lee RC, Riggs RM, Nichols DE, Lewis MH, Mailman RB, 1989. Dihydrexidine, a novel selective high potency full D-1 receptor agonist. Eur. J. Pharmacol 166, 111. [DOI] [PubMed] [Google Scholar]
- Mazei MS, Pluto CP, Kirkbride B, Pehek EA, 2002. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 936, 58–67. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB, 2006. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging 27, 1484–1493. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH, 1996. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Nat. Acad. Sci. U.S.A 93, 1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY, 2013. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci. 24, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T, 2011. Control of visual cortical signals by prefrontal dopamine. Nature 474, 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott T, Jacob SN, Nieder A, 2014. Dopamine receptors differentially enhance rule coding in primate prefrontal cortex neurons. Neuron 84, 1317–1328. [DOI] [PubMed] [Google Scholar]
- Ott T, Westendorff S, Nieder A, 2018. Dopamine Receptors Influence Internally Generated Oscillations during Rule Processing in Primate Prefrontal Cortex. J Cogn Neurosci. 30, 770–784. [DOI] [PubMed] [Google Scholar]
- Park DC, Bischof GN, 2013. The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin Neurosci. 15, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS, 2004. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J. Neurosci 24, 5292–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS, 2005. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J. Neurosci 25, 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Min Wang M, Arnsten AFT, 2013. Constellation of HCN Channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex – Potential substrate for working memory deficits in schizophrenia. Cereb Cortex 23, 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Miller EK, 2012. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron 74, 874–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell DR, Zaluda LC, McClure MM, Perez-Rodriguez MM, Strike KS, Barch DM, Harvey PD, Girgis RR, Hazlett EA, Mailman RB, Abi-Dargham A, Lieberman JA, Siever LJ, 2015. Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology 40, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA, 1994. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyosine hydroxylase immunohistochemical study. Biol. Psychiat 36, 272–277. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Nichols DE, Mailman RB, 2005. Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol Pharmacol. 68, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS, 1994. The role of D1-dopamine receptors in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed response task. J. Neurophysiol 71, 515–528. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K, 1988. Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci. Res 5, 465–473. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K, 1990. Catecholaminergic effects on neuronal activity related to delayed response task in monkey prefrontal cortex. J. Neurophys 63, 1385–1400. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Zemlan FP, Berridge CW, 2013. A selective dopamine reuptake inhibitor improves prefrontal cortex-dependent cognitive function: potential relevance to attention deficit hyperactivity disorder. Neuropharmacology 64, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS, 1999. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45, 17–25. [DOI] [PubMed] [Google Scholar]
- Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, Hackett E, Girgis R, Ojeil N, Moore H, D’Souza D, Malison RT, Huang Y, Lim K, Nabulsi N, Carson RE, Lieberman JA, Abi-Dargham A, 2015. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS, 1994. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A 91, 5720–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J, Fullard JF, Giakoumaki SG, Katsel P, Karagiorga VE, Greenwood TA, Braff DL, Siever LJ, Bitsios P, Haroutunian V, Roussos P, 2015. The relationship between dopamine receptor D1 and cognitive performance. NPJ Schizophr. 1, 14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Major AJ, Everling S, 2016. Dopamine D1 and D2 Receptors Make Dissociable Contributions to Dorsolateral Prefrontal Cortical Regulation of Rule-Guided Oculomotor Behavior. Cell Rep. 16, 805–816. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Major AJ, Everling S, 2017. Neuromodulation of Prefrontal Cortex in Non-Human Primates by Dopaminergic Receptors during Rule-Guided Flexible Behavior and Cognitive Control. Front Neural Circuits. 11, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT, 2007. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience 10, 376–384. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AFT, 2011. Neuronal basis of age-related working memory decline. Nature 476, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley AG, Nou E, Mazer JA, McCormick DA, Arnsten AFT, 2007. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129, 397–410. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC, 1989. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobio. Aging 10, 11–19. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS, 1995. Blockade of dopamine D1 receptors enhances memory fields of prefrontal neurons in primate cerebral cortex. Nature 376, 572–575. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, 1998. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex 8, 321–345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.