Recurrent genital herpes is one of the most common sexually transmitted diseases, with a global prevalence of HSV-2 infection predicted to be over 536 million worldwide. Despite the availability of many intervention strategies, such as sexual behavior education, barrier methods, and the costly antiviral drug treatments, eliminating or at least reducing recurrent genital herpes remains a challenge. Currently, no FDA-approved therapeutic vaccines are available. In this preclinical study, we investigated the immunogenicity and protective efficacy, in the guinea pig model of recurrent genital herpes, of subunit vaccine candidates that were based on eight recombinantly expressed herpes envelope and tegument proteins. We discovered that similar to the dl5-29 vaccine, based on a replication-defective HSV-2 mutant virus, which has been recently tested in clinical trials, the RR2 protein-based subunit vaccine elicited a significant reduction in virus shedding and a decrease in both the severity and frequency of recurrent genital herpes sores. This protection correlated with an increase in numbers of functional tissue-resident IFN-γ+ CRTAM+ CFSE+ CD4+ and IFN-γ+ CRTAM+ CFSE+ CD8+ TRM cells that infiltrate healed sites of the vaginal tissues. Our study sheds new light on the role of TRM cells in protection against recurrent genital herpes and promotes the RR2-based subunit therapeutic vaccine to be tested in the clinic.
KEYWORDS: Genital herpes, HSV-1, HSV-2, T cells, therapeutic vaccine, vaginal mucosa
ABSTRACT
Reactivation of herpes simplex virus 2 (HSV-2) from latency causes viral shedding that develops into recurrent genital lesions. The immune mechanisms of protection against recurrent genital herpes remain to be fully elucidated. In this preclinical study, we investigated the protective therapeutic efficacy, in the guinea pig model of recurrent genital herpes, of subunit vaccine candidates that were based on eight recombinantly expressed HSV-2 envelope and tegument proteins. These viral protein antigens (Ags) were rationally selected for their ability to recall strong CD4+ and CD8+ T-cell responses from naturally “protected” asymptomatic individuals, who, despite being infected, never develop any recurrent herpetic disease. Out of the eight HSV-2 proteins, the envelope glycoprotein D (gD), the tegument protein VP22 (encoded by the UL49 gene), and ribonucleotide reductase subunit 2 protein (RR2; encoded by the UL40 gene) produced significant protection against recurrent genital herpes. The RR2 protein, delivered either intramuscularly or intravaginally with CpG and alum adjuvants, (i) boosted the highest neutralizing antibodies, which appear to cross-react with both gB and gD, and (ii) enhanced the numbers of functional gamma interferon (IFN-γ)-producing CRTAM+ CFSE+ CD4+ and CRTAM+ CFSE+ CD8+ TRM cells, which express low levels of PD-1 and TIM-3 exhaustion markers and were localized to healed sites of the vaginal mucocutaneous (VM) tissues. The strong B- and T-cell immunogenicity of the RR2 protein was associated with a significant decrease in virus shedding and a reduction in both the severity and frequency of recurrent genital herpes lesions. In vivo depletion of either CD4+ or CD8+ T cells significantly abrogated the protection. Taken together, these preclinical results provide new insights into the immune mechanisms of protection against recurrent genital herpes and promote the tegument RR2 protein as a viable candidate Ag to be incorporated in future genital herpes therapeutic mucosal vaccines.
IMPORTANCE Recurrent genital herpes is one of the most common sexually transmitted diseases, with a global prevalence of HSV-2 infection predicted to be over 536 million worldwide. Despite the availability of many intervention strategies, such as sexual behavior education, barrier methods, and the costly antiviral drug treatments, eliminating or at least reducing recurrent genital herpes remains a challenge. Currently, no FDA-approved therapeutic vaccines are available. In this preclinical study, we investigated the immunogenicity and protective efficacy, in the guinea pig model of recurrent genital herpes, of subunit vaccine candidates that were based on eight recombinantly expressed herpes envelope and tegument proteins. We discovered that similar to the dl5-29 vaccine, based on a replication-defective HSV-2 mutant virus, which has been recently tested in clinical trials, the RR2 protein-based subunit vaccine elicited a significant reduction in virus shedding and a decrease in both the severity and frequency of recurrent genital herpes sores. This protection correlated with an increase in numbers of functional tissue-resident IFN-γ+ CRTAM+ CFSE+ CD4+ and IFN-γ+ CRTAM+ CFSE+ CD8+ TRM cells that infiltrate healed sites of the vaginal tissues. Our study sheds new light on the role of TRM cells in protection against recurrent genital herpes and promotes the RR2-based subunit therapeutic vaccine to be tested in the clinic.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) affects more women than men, with a staggering 315 million women worldwide 5 to 49 years old currently infected (1, 2). After vaginal mucosa (VM) exposure, HSV-2 replicates in the mucosal epithelial cells, thereby causing acute genital herpetic lesions (2–7). Once the acute primary infection is cleared, the virus enters the nerve termini innervating peripheral vaginal tissues and is subsequently transported to the nuclei of the sensory neurons of dorsal root ganglia (DRG), where it goes into a lifelong “steady-state” latency (6, 7). Roughly 80% of HSV-2-seropositive women are unaware of their infection, as they never develop any apparent recurrent symptoms (1). In contrast, in symptomatic women, the latent infection is often interrupted by sporadic reactivation that leads to recurrent genital lesions and painful blisters that can burst and form ulcers (6). Despite the availability of many intervention strategies, such as sexual behavior education, barrier methods, and antiviral drug therapies (e.g., acyclovir and derivatives), eliminating or at least reducing recurrent genital herpes remains a challenge (2, 8–11). In addition, the antiviral drugs can neither prevent de novo infection or reactivation nor clear the latent virus. Moreover, both symptomatic and asymptomatic women experience subclinical virus shedding and, hence, can transmit the virus, underscoring the need for an antiviral therapeutic vaccine to prevent or reduce virus reactivation and/or its shedding in the genital tract (instead of a therapeutic vaccine that would just reduce symptoms). Currently, the medical opinion is that an effective antiviral therapeutic vaccine would constitute the best approach to protect from recurrent genital herpetic disease (2, 4). It is becoming increasingly clear that the acquired immune responses that develop following first exposure to the virus are not sufficient for protection against recurrent genital herpes in symptomatic women (12–14). This implies that a successful therapeutic vaccine must be able to boost an immune response that is stronger and/or different than the acquired immunity induced by the virus itself (15). In animal models, studies have demonstrated that whole live attenuated vaccines induced B- and T-cell protective immunity against acute genital HSV-2 challenges (16). However, the same level of protection has yet to be achieved safely in clinical trials (17, 18). Proteins are proven to be excellent vaccine candidates due to their safety, cost effectiveness, and rapid preparation (19). Interestingly, over the last 2 decades, only a single subunit protein vaccine strategy, based on HSV-2 glycoprotein D (gD), delivered with or without gB, has been tested and retested in clinical trials (18, 20). This subunit vaccine strategy proved unsuccessful in clinical trials despite inducing strong neutralizing antibodies (18). Previous studies have identified other antigenic tegument proteins by screening HSV-2 open reading frames (ORFs) by antibodies and T cells from HSV-2-seropositive individuals (21). However, aside from three reports, first by our group in 2012 (10, 22) and later by Genocea Biosciences, Inc., in 2014 (23), comparison of the repertoire of proteins encoded by the 84+ ORFs of the HSV-2 152-kb genome, recognized by antibodies and T cells from HSV-2-seropositive symptomatic versus asymptomatic individuals, is largely incomplete. In the present study, we hypothesized that (i) HSV-2 proteins, other than gB and gD, that are frequently and highly recognized by antibodies and T cells from the naturally “protected” asymptomatic individuals would constitute a better therapeutic vaccine against recurrent genital herpes and (ii) besides neutralizing antibodies, boosting the number and function of antiviral tissue-resident memory CD4+ and CD8+ TRM cells, locally within both the DRG and vaginal mucocutaneous tissues, would lead to better protection against recurrent herpes.
Because of the ethical and practical limitations in obtaining fresh DRG and vaginal mucosa from herpes patients, a routine characterization of phenotype and function of human tissue-derived TRM cells remains a challenge (24–26). Thus, a reliable small-animal model that would mimic recurrent genital herpes as it occurs in humans would help speed up the characterization of tissue-resident HSV-specific TRM cells and compare the contribution of DRG- and vaginal mucosa-derived versus peripheral blood-derived CD4+ and CD8+ cells in protection against recurrent genital herpes. The most commonly used animal model for preclinical testing of therapeutic vaccine candidates against recurrent genital herpes has been the guinea pig (27–29). Unlike in mice, intravaginal HSV-2 infections in guinea pigs lead to latency followed by intermittent, spontaneous reactivation events resulting in recurrent virus shedding in the genital tract, as occurs in women, and this virus shedding can develop into clinical and pathological recurrent genital lesions in symptomatic guinea pigs, with self-limiting quantifiable vulvovaginitis, akin to those seen in symptomatic women (27–29).
We therefore opted to use the guinea pig model of recurrent herpes to test the safety, tissue-resident CD4+ and CD8+ T-cell immunogenicity, and protective efficacy of recombinantly expressed HSV-2 envelope and tegument HSV-2 proteins. Eight viral proteins were rationally selected as being highly and selectively recognized by effector CD4+ and CD8+ T cells from naturally “protected” asymptomatic women who, despite being infected, never develop any recurrent herpetic disease. Therapeutic immunization of latently infected guinea pigs with three out of the eight HSV-2 proteins—gD (encoded by the US6 gene), the tegument protein VP22 (encoded by the UL49 gene), and ribonucleotide reductase subunit 2 protein (RR2; encoded by the UL40 gene)—produced the most significant protection against recurrent genital herpes infection and disease. Four out of the eight HSV-2 proteins—the tegument protein VP16 (encoded by the UL48 gene), the tegument protein VP11/12 (encoded by the UL46 gene), the tegument protein VP13/14 (encoded by the UL47 gene), and gB (encoded by the UL27 gene)—produced moderate yet significant protection. In contrast, the UL25 protein did not produce any significant protection. Among the three most protective antigens (Ags), RR2 was the most immunogenic protein, boosting coordinated and broad-spectrum systemic and mucosal immune responses which included (i) serum neutralizing antibodies that appeared to cross-react with both gB and gD and (ii) increased numbers of functional tissue-resident PD-1low TIM-3low IFN-γ+ CRTAM+ CFSE+ CD4+ and PD-1low TIM-3low IFN-γ+ CRTAM+ CFSE+ CD8+ TRM cells, that were localized to healed sites of the vaginal mucocutaneous tissues. Similar to the live attenuated dl5-29 vaccine, based on a replication-defective HSV-2 mutant, which has been recently used in a phase I clinical trial, the RR2 protein-based subunit therapeutic vaccine elicited a significant reduction in virus shedding and a decrease in both the severity and frequency of recurrent genital herpes lesions. In light of the novel guinea pig-specific immunological reagents and assays reported in this study, we discuss the prospect of using this guinea pig model to characterize the role of tissue-resident TRM cells in the protection against recurrent herpes and to test novel herpes vaccine candidates in combination with blocking immune checkpoints.
RESULTS
Probing the HSV-2 ORFome by T cells from seropositive asymptomatic versus symptomatic individuals identifies eight immunodominant “asymptomatic” protein antigens.
The characteristics of HSV-2-seropositive symptomatic (SYMP) and HSV-2-seropositive asymptomatic (ASYMP) populations used in the present study, with respect to gender, age, HSV-1/HSV-2 seropositivity, and clinical status of genital herpetic disease, are summarized in Table 1 and detailed in Materials and Methods. The HSV-2-seropositive individuals were divided into two age- and sex-matched groups: (i) 10 ASYMP individuals who, despite being infected, never had any clinically detectable herpes disease and (ii) 10 SYMP individuals with a history of numerous episodes of clinically documented recurrent genital herpes. Ten age- and sex-matched HSV-1- and HSV-2-seronegative healthy subjects were enrolled as negative (NEG) controls.
TABLE 1.
Cohorts of HLA-A*02:01-positive, HSV-seropositive symptomatic and asymptomatic individuals enrolled in this study
|
Characteristic |
Value for all subjects (n = 50) |
|---|---|
| Gender, no. (%) | |
| Female | 26 (52) |
| Male | 24 (48) |
| Race, no. (%) | |
| Caucasian | 21 (42) |
| Non-Caucasian | 29 (58) |
| Age, yrs, median (range) | 23 (22–66) |
| HSV status, no. (%) | |
| HSV-2 seropositive | 40 (80) |
| HSV-1 seropositive | 00 (0) |
| HSV-1 and -2 seropositive | 00 (0) |
| HSV seronegative | 10 (20) |
| HLA, no. (%) | |
| HLA-A*02:01 positive | 50 (100) |
| HLA-A*02:01 negative | 00 (0) |
| Herpes disease status, no. (%) | |
| Asymptomatic | 20 (40) |
| Symptomatic | 20 (40) |
Several open reading frames (ORFs) of HSV-2 were cloned and corresponding proteins expressed individually, as we previously reported (10, 22). We scanned this HSV-2 ORFome using peripheral blood-derived CD4+ and CD8+ T cells from the above-mentioned clinically defined SYMP and ASYMP individuals, which identified several “asymptomatic” ORFs whose corresponding proteins were specifically recognized by CD4+ and/or CD8+ T cells from ASYMP individuals (Fig. 1). To rank the symptomatic and asymptomatic ORFs (i.e., the corresponding proteins), the frequency and the intensity of CD4+ and CD8+ T-cell responses specific to each ORF product were determined. An ORF product is designated an asymptomatic Ag when it is highly and frequently recognized by CD4+ and CD8+ T cells from seropositive ASYMP individuals with high to medium intensity. Inversely, an ORF product is designated a symptomatic Ag when it is highly and frequently recognized by CD4+ and CD8+ T cells from seropositive SYMP individuals with high to medium intensity.
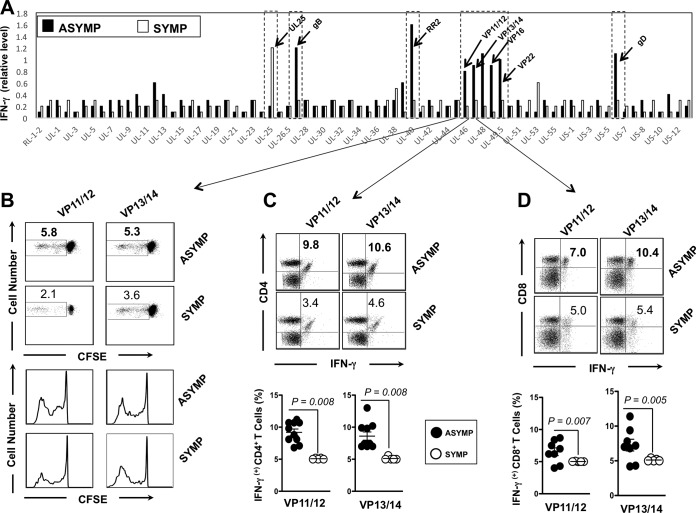
FIG 1.
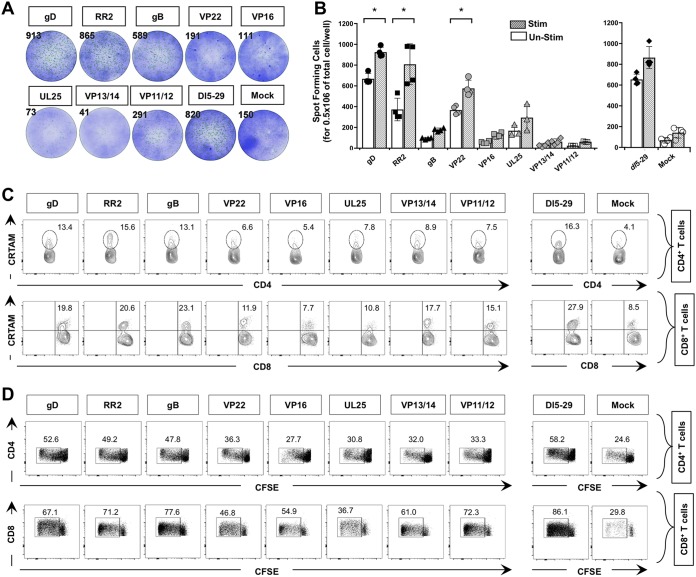
CD4+ and CD8+ T-cell responses against HSV-2 antigens detected from symptomatic and asymptomatic patients. (A) Average intensity of recognition of ORFs expressing HSV-2 proteins by T cells from HSV-2-seropositive symptomatic (n = 10) and asymptomatic individuals (n = 10), as tested by IFN-γ production. The names of HSV-2 ORFs driving two representative positive responses are indicated. (B) VP11/12- and VP13/14-specific CFSE+ proliferative T cells from symptomatic and asymptomatic individuals represented in panel A. (C and D) Representative analyses of HSV-2 VP11/12- and VP13/14-specific IFN-γ-producing CD4+ T cells (C) and CD8+ T cells (D). The top portion shows representative analysis from the one symptomatic and one asymptomatic individual, and the bottom portion shows averages from the 10 symptomatic and 10 asymptomatic individuals. Representative results are from three independent experiments. The P value compares the results from 10 symptomatic and 10 asymptomatic individuals. Values were calculated using the ANOVA two-tail test.
As shown in Fig. 1A, ORFs representing eight HSV-2 asymptomatic protein antigens (gD, RR1, RR2, VP22, gB, VP16, VP13/14, and VP11/12) were highly recognized by gamma interferon (IFN-γ)-producing CD4+ and CD8+ T cells from 10 out of 10 ASYMP patients but not by CD4+ and CD8+ T cells from 10 SYMP patients (P < 0.001; analysis of variance [ANOVA] posttest). The highest magnitudes of recognition of IFN-γ-producing T-cell responses from HSV-2-seropositive asymptomatic individuals were directed against gD, RR2, VP22, gB, VP13/14, and VP11/12 asymptomatic Ags (Fig. 1A). In contrast, CD4+ and CD8+ T cells from SYMP patients preferentially recognized UL25 antigen (P < 0.001 [Fig. 1A]). An elevated frequency of proliferative IFN-γ-producing T cells was also detected in ASYMP individuals against gD, RR2, VP22, gB, VP13/14, and VP11/12 Ags compared to SYMP individuals. An example of VP13/14 and VP11/12 is shown in Fig. 1B to D. Similar results were obtained with gD, RR2, VP22, and gB antigens (data not shown). Together, these results indicate that although CD4+ and CD8+ T cells from HSV-seropositive individuals recognize most of the HSV-2 ORF products, a set of eight ORF products were recognized mostly by T cells from ASYMP individuals, while the UL25 antigen was recognized mostly by T cells from SYMP individuals. Based on the above data, for the remainder of this study, we therefore selected the gD, RR2, VP22, gB, VP16, VP13/14, and VP11/12 asymptomatic protein Ags and the UL25 symptomatic protein Ag for preclinical therapeutic vaccine safety, immunogenicity, and protective efficacy testing in our guinea pig model of recurrent genital herpes.
Therapeutic immunization of HSV-2 infected guinea pigs with three out of eight asymptomatic HSV-2 protein-based subunit vaccines produced protection against recurrent genital herpes infection and disease.
We recombinantly expressed eight HSV-2 symptomatic and asymptomatic proteins selected above (gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25). As illustrated in Fig. 2A, guinea pigs (n = 90) were infected intravaginally on day 0 with 5 × 105 PFU of HSV-2 (strain MS). Once acute infection was resolved, the latently infected animals were randomly divided into 8 groups (n = 11) and then vaccinated twice intramuscularly, on day 15 and on day 25 postinfection, with 10 μg of the desired HSV-2 protein emulsified in alum plus CpG adjuvants. The replication-defective HSV-2 dl5-29 vaccine was used as a positive control. Mock-vaccinated guinea pigs, which received alum plus CpG adjuvants alone, were used as negative controls.
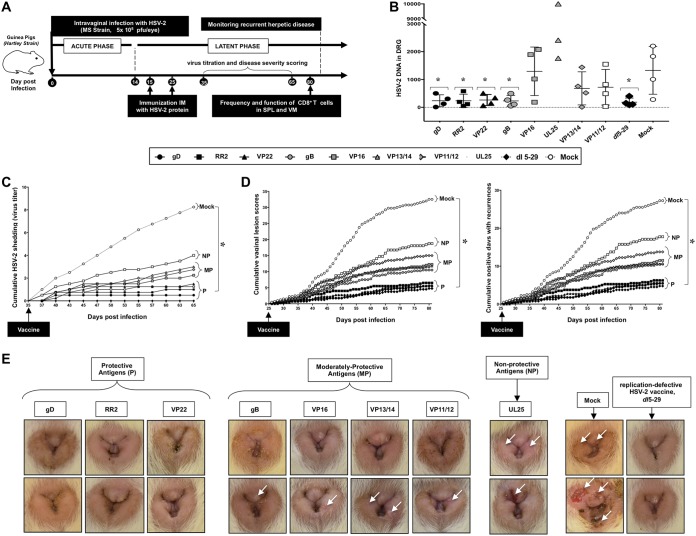
FIG 2.
Protection against recurrent genital herpes infection and disease in HSV-2-infected guinea pigs following therapeutic immunization with eight protein-based subunit vaccine candidates: (A) Timeline of HSV-2 infection, immunization, and immunological and virological analyses. Guinea pigs (n = 60) were infected intravaginally on day 0 with 5 × 105 PFU of HSV-2 (strain MS). Once acute infection was resolved, the remaining latently infected animals were randomly divided into 8 groups (n = 4) and then vaccinated intramuscularly twice, on day 15 and on day 25 postinfection, with 10 μg of the indicated HSV-2 protein-based subunit vaccines (gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25) emulsified in alum plus CpG adjuvants. The replication-defective HSV-2 dl5-29 mutant vaccine was used as a positive control. Mock-vaccinated guinea pigs which received alum plus CpG adjuvants alone were used as negative control. From day 35 to day 80 postinfection (i.e., day 10 to day 55 after the final immunization), vaccinated and nonvaccinated animals were observed daily for the severity of genital herpetic lesions, scored on a scale of 0 to 4, and pictures of genital areas were taken; vaginal swabs were collected daily from day 35 to day 65 postinfection (i.e., day 10 to day 40 after the final immunization) to detect virus shedding and to quantify HSV-2 DNA copy numbers. (B) HSV-2 DNA copy numbers detected in the DRG of each group of vaccinated and mock-vaccinated guinea pigs. (C) Cumulative vaginal shedding of HSV-2 during recurrent infection. (D) Cumulative vaginal lesions (left) and cumulative positive days with recurrent genital lesions (right). The severity of genital herpetic lesions was scored on a scale of 0 to 4, where 0 reflects no disease, 1 reflects redness, 2 reflects a single lesion, 3 reflects coalesced lesions, and 4 reflects ulcerated lesions. (E) Representative images of genital lesions in guinea pigs vaccinated with (i) protective antigens (gD, RR2, and VP22 proteins), (ii) moderately protective antigens (gB, VP16, VP13/14, and VP11/12), and (iii) nonprotective antigen (UL25); also shown are images for the mock-vaccinated control and the dl5-29 positive control. The indicated P values show statistical significance between the HSV-2-vaccinated and mock-vaccinated control groups. *, P < 0.05 (considered significant).
On day 80 postinfection (i.e., 55 days after the second and final immunization), the gD-, RR2-, and VP22-vaccinated guinea pigs exhibited lower HSV-2 DNA copy numbers in the DRG than did gB-, VP16-, VP13/14-, VP11/12-, and UL25-vaccinated guinea pigs and mock-vaccinated controls (Fig. 2B). As expected, the dl5-29 positive-control animals had the lowest DNA copy numbers. The reduction in HSV-2 DNA copy numbers in the DRG of gD-, RR2-, and VP22-vaccinated animals was associated with a significant reduction in cumulative virus vaginal shedding compared to that in the gB-, VP16-, VP13/14-, VP11/12-, and UL25-vaccinated animals and to mock-vaccinated controls (P < 0.05) (Fig. 2C). Moreover, the gD-, RR2-, and VP22-vaccinated animals exhibited significantly lower cumulative vaginal lesions (Fig. 2D, left graph) and a reduced number of days with recurrent genital lesions (Fig. 2D, right graph). The severity of genital herpetic lesions, scored on a scale of 0 to 4, also confirmed the cure of recurrent disease in gD-, RR2-, and VP22-vaccinated guinea pigs (Fig. 2E). From the representative images, the lowest genital lesions were evidenced in guinea pigs vaccinated with gD, RR2, and VP22 proteins (6 pictures in left group). This protection was similar to the protection provided by vaccination with live attenuated dl5-29 as a positive control (2 pictures in extreme right group). gB, VP16, VP13/14, and VP11/12 were moderately protective antigens against genital lesions (8 pictures in middle). In contrast, vaccination with UL25 protein produced no significant protection against recurrent genital herpes lesions, similar to that observed in the mock-vaccinated controls (P > 0.05) (Fig. 2E). Together these results therefore indicate that therapeutic immunization with gD, RR2, and VP22 antigens, selected for being highly recognized by T cells from HSV-2-seropositive but naturally protected ASYMP females (described above), also protected HSV-2-seropositive guinea pigs against recurrent genital herpes infection and disease.
Therapeutic vaccination of HSV-2-infected guinea pigs with the RR2 protein-based subunit vaccine produced high neutralizing antibody titers that cross-reacted with gB and gD.
Guinea pigs were infected intravaginally with 5 × 105 PFU of HSV-2 (strain MS). Groups of HSV-2-infected animals (n = 11) were vaccinated twice, on day 15 and on day 25 postinfection, with the desired HSV-2 proteins, illustrated in Fig. 2. Fifty-five days after the second and final immunization, immune sera were collected and tested for Ag- and HSV-specific IgG by enzyme-linked immunosorbent assay (ELISA) and virus neutralization assay. Significantly higher levels of IgG specific to immunizing Ags were detected by ELISA in sera from the guinea pigs that were vaccinated with gD, RR2, VP22, VP16, VP11/12, and UL25 proteins than in those from the mock-vaccinated guinea pigs that received adjuvant alone (Fig. 3A). Sera from the dl5-29-vaccinated animals evaluated at a 1:1,000 dilution also recognized gD and RR2 as coating Ags (Fig. 3A). Immune sera from the guinea pigs that were vaccinated with gD, RR2, gB, VP13/14, VP11/12, and UL25, but not sera from the mock-vaccinated group, had IgG that bound with high affinity to native viral epitopes, as whole heat-inactivated HSV-2 used to coat ELISA plates (Fig. 3B). However, sera from mock-vaccinated guinea pigs that received adjuvant alone did not recognize heat-inactivated HSV-2, indicating the antigen specificity (Fig. 3B). Moreover, immune sera from gD- and RR2-vaccinated animals had the highest neutralizing antibody titers (Fig. 3C, left graph). The 50% neutralization titer in serum of mock-immunized animals was around 1:640, while immune sera of gD- and RR2-immunized animals had neutralization titers of over 1:1,280 and 1:2,560, respectively (Fig. 3C, left graph). As expected, sera from animals that were vaccinated with dl5-29 that delivered all the envelope glycoproteins produced high neutralizing antibody titers. However, immune sera from gB-, VP16-, VP13/14-, and VP11/12-vaccinated animals (Fig. 3C, middle graph) and UL25-vaccinated animals (Fig. 3C, right graph) had low to no neutralizing antibody titers.
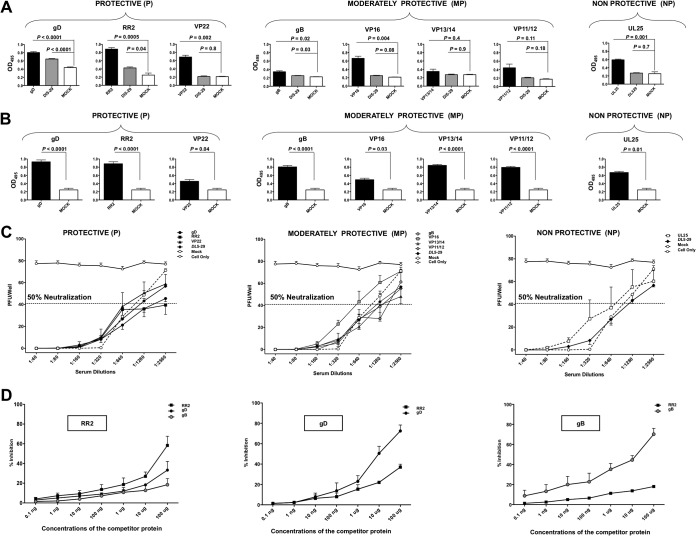
FIG 3.
Production of neutralizing antibodies in HSV-2-infected guinea pigs following therapeutic vaccination with gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25 proteins. (A) Levels of Ag-specific IgG detected by ELISA from the guinea pigs vaccinated with gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25, the mock-vaccinated group, and the DL5-29 vaccine positive-control group. All sera were evaluated at 1:1,000. (B) Binding affinity of IgG from the indicated HSV-2 antigen vaccinated groups compared to the mock-vaccinated group to native proteins on HSV-2 (MS strain) by ELISA. (C) Immune sera were tested for neutralizing antibody titers. The dotted line represents neutralizing antibody titers in mock-vaccinated guinea pigs. The neutralization point was defined as the serum dilution that reduced the number of plaques by 50% compared to the positive control (cells only). (D) ELISA plates were coated with 1 μg of RR2 protein (left), 1 μg of gD protein (middle), or 1 μg of gB protein (right). ELISA plates were then incubated with serum from RR2-, gD-, or gB-immunized guinea pigs and preincubated with different concentrations of the competitor protein (i.e., RR2, gB, or gD). The percent inhibition at each tested concentration of the competitor protein was calculated with respect to the OD value of the respective immune serum alone, incubated in the absence of the competitor protein. Statistical analysis was performed by 2-way ANOVA for repeated measures followed by Bonferroni’s posttest for significance. * P < 0.05 (considered significant).
Since the immune sera from animals vaccinated with RR2, a nonsurface protein, produced the highest neutralizing antibody titers, we next wanted to determine whether the RR2-induced antibodies cross-reacted with the immunodominant envelope proteins, such as gB and gD. As shown in Fig. 3D, we used competitive ELISAs, in which plates were first coated with 1 μg of RR2 protein, 1 μg of gD protein, or 1 μg of gB protein. ELISA plates were then incubated with immune sera from RR2-, gD-, or gB-immunized guinea pigs that had been preincubated with different concentrations of the competitor protein (i.e., RR2, gB, or gD). The percent inhibition at each tested concentration of the competitor protein was calculated with respect to the optical density at 495 nm (OD495) of the respective immune serum alone, without the competitor protein. When RR2 protein was used as the coating Ag, there was significant inhibition of IgG binding not only from immune sera preincubated with RR2 protein (control) but also from immune sera preincubated with gB or gD (Fig. 3D, left graph). Conversely, when gD was used as the coating Ag, there was a significant inhibition of IgG binding not only of immune sera preincubated with gD (control) but also of immune sera preincubated with RR2 protein, suggesting cross-reactivity of B-cell epitopes between RR2 and gD (Fig. 3D, middle graph). Similarly, when gB protein was used as the coating Ag, there was also significant inhibition of IgG binding of immune sera preincubated with RR2 protein, suggesting cross-reactive epitopes between RR2 and gB (Fig. 3D, right graph). These results indicate that therapeutic vaccination of HSV-2-infected guinea pigs with the RR2 protein-based subunit vaccine produced high neutralizing antibody titers that appeared to cross-react with surface glycoproteins, such as gB and gD.
Therapeutic vaccination of HSV-2-infected guinea pigs with RR2 protein induced frequent vaginal mucocutaneous tissue-resident CD4+ and CD8+ TRM cells that localized to healed sites of the vaginal mucocutaneous tissues.
We next infected guinea pigs (n = 60) intravaginally with 5 × 105 PFU of HSV-2 (strain MS). Once acute infection was resolved, latently infected animals were randomly divided into eight groups (n = 4) and then vaccinated intramuscularly twice, on day 15 and on day 25 postinfection, with individual gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25 antigens emulsified in alum plus CpG adjuvants. The replication-defective dl5-29 vaccine was used as a positive control. Mock-vaccinated guinea pigs, which received adjuvant alone, were used as a negative control. On day 55 after the second and final immunization, vaccinated and control animals were euthanized and the frequencies of vaginal mucosal (VM) tissue-resident CD4+ and CD8+ TRM cells were detected by fluorescence-activated cell sorting (FACS) and fluorescence microscopy.
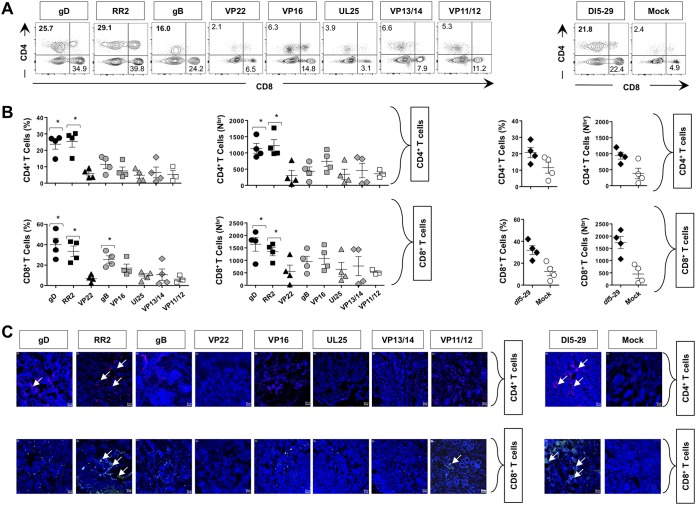
Overall high frequencies of CD4+ and CD8+ TRM cells were induced by gD-, RR2-, VP22-, gB-, VP16-, VP13/14-, VP11/12-, and UL25-based subunit vaccines compared to those with the mock vaccine (i.e., adjuvant alone) (Fig. 4A and B). The highest frequencies of CD4+ and CD8+ TRM cells were detected in the VM of guinea pigs that were vaccinated with gD and RR2 proteins (Fig. 4A and B, left two sets of graphs). These frequencies were similar to those detected in the VM of guinea pigs that were immunized with the dl5-29 vaccine (Fig. 4A and B, right two sets of graphs). Using fluorescence microscopy, we visualized more CD4+ and CD8+ TRM cell infiltrates that localized to healed sites of the vaginal mucocutaneous tissues of protected guinea pigs that were immunized with gD and RR2 Ags but not to the vaginal mucocutaneous tissues of nonprotected guinea pigs that were immunized with the remaining HSV-2 antigens or adjuvant alone (Fig. 4C).
FIG 4.
Frequency of CD4+ and CD8+ T cells in the vaginal mucosae of HSV-2-infected guinea pigs following therapeutic vaccination with gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25 proteins. (A) Representative FACS data for the frequencies of CD4+ and CD8+ T cells detected in the VM of vaccinated and mock-vaccinated animals. (B) Quantification of VM-resident CD4+ and CD8+ T cells following therapeutic vaccination with various HSV-2 Antigens. Average frequencies and absolute numbers of CD4+ T cells (top graphs) and CD8+ T cells (bottom graphs) were determined using FACS in the VM of vaccinated and mock-vaccinated animals. Cells were analyzed using a BD FACSCalibur flow cytometry system with a total of 4 × 105 events. Density plots showing the percentage of CD4+ T cells and CD8+ T cells are representative of results from two independent experiments. The indicated P values, obtained by 2-way ANOVA for repeated measures followed by Bonferroni’s posttest for significance, show statistical significance between HSV-2-vaccinated and mock-vaccinated control groups. *, P < 0.05 (considered significant). (C) Visualization of CD4+ and CD8+ T-cell infiltration, using fluorescence microscopy, within the vaginal mucosae of HSV-2-infected guinea pigs following therapeutic vaccination with various HSV-2 antigens. Sections of the vaginal mucosa from vaccinated and mock-vaccinated animals were costained using MAbs specific to CD4+ T cells (red), CD8+ T cells (green), and 4′,6-diamidino-2-phenylindole (DAPI; DNA stain) (blue).
Protection induced following therapeutic vaccination with RR2, gB, gD, and VP22 proteins is associated with more functional vaginal mucosa-resident IFN-γ+ CRTAM+ CD4+ and IFN-γ+ CRTAM+ CD8+ T cells.
We next compared the function and exhaustion of CD4+ and CD8+ TRM cells in the VM of HSV-2 infected guinea pigs following therapeutic vaccination with gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25 proteins. On day 55 after the second and final therapeutic immunization, guinea pigs were euthanized, single cell suspensions from the VM tissue were obtained, and the function or dysfunction of VM-resident CD4+ and CD8+ TRM cells was analyzed using both IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay and FACS. Significantly high numbers of IFN-γ-producing CD4+ and CD8+ T cells, enumerated by ex vivo ELISpot assay in the VM of HSV-2 infected guinea pigs following vaccination with gB, gD, RR2, and VP22 proteins, were observed compared to those following vaccination with VP16, VP13/14, VP11/12, and UL25 proteins (P < 0.05) (Fig. 5A and B). The number of IFN-γ-producing CD4+ and CD8+ T cells detected in the VM of the gD- and RR2-vaccinated groups equaled the number of IFN-γ-producing CD4+ and CD8+ T cells detected in the VM of the dl5-29-vaccinated group (positive control). Following a 48-h stimulation with 10 μg of immunizing HSV-2 proteins, there was a boost in the number of IFN-γ-producing CD4+ and CD8+ T cells in the VM cells compared to that in ex vivo nonstimulated VM cells from the gD, RR2, and VP22 groups but not from the remaining five groups, gB, VP16, VP13/14, VP11/12, and UL25 (P < 0.05) (Fig. 5B). Similarly, as shown in Fig. 5C, significantly higher frequencies of functional CRTAM+ CD8+ TRM cells (top row) and of CRTAM+CD4+ TRM cells (bottom row) were detected in VM of guinea pigs following vaccination with gD, RR2, and gB compared to VP22, VP16, VP13/14, VP11/12, and UL25 (P < 0.05). As shown in Fig. 5D, significantly high proliferative responses of CFSE+ CD4+ TRM cells (top panels) and CFSE+ CD8+ TRM cells (bottom panels) were detected in the VM of the gB, gD, RR2, and VP22 groups compared to the VP16, gB, VP13/14, VP11/12, and UL25 groups, confirming that more functional VM-resident CD4+ and CD8+ TRM cells are associated with protection (P < 0.05). As expected, high numbers of functional CD4+ and CD8+ TRM cells were detected in the VM of the dl5-29-vaccinated group (positive control) (Fig. 5). Together, these results indicate that therapeutic vaccination of HSV-2-infected guinea pigs with gB, gD, RR2, or VP22 protein induced more IFN-γ+ CRTAM+ CFSE+ CD4+ and IFN-γ+ CRTAM+ CFSE+ CD8+ TRM cells within the vaginal mucocutaneous tissue associated with significant protection against recurrent genital herpes.
FIG 5.
Function of vaginal mucosa-resident CD4+ and CD8+ T cells from HSV-2-infected guinea pigs following therapeutic vaccination with gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25 proteins. (A) Enumeration of IFN-γ spot-forming units from guinea pigs immunized with various antigens. Representative wells of IFN-γ-producing cells measured ex vivo by ELISpot assay from VM cells (5 × 105 cells/well) and stimulated for 48 h with 10 μg of various HSV-2 proteins are depicted. The number at the top of each image of representative ELISpot wells represents the number of IFN-γ-producing spot-forming T cells per one million total vaginal mucosal cells. (B) Average data for IFN-γ-producing cells measured by ELISpot assay from the VM of four guinea pigs/per group, either ex vivo or after 48 h of in vitro stimulation with the indicated immunizing HSV-2 protein. Vaginal mucosa cell suspensions plated 105 cells/well and either left unstimulated (Un-Stim) or stimulated with 10 μg (Stim) with the indicated HSV-2 proteins. IFN-γ production is depicted by the number of spot-forming cells per one million VM cells of HSV-2 infected vaccinated and mock-vaccinated control groups. (C) Frequencies of vaginal mucosa-resident CRTAM+ CD4+ T cells (top row) and of CRTAM+ CD8+ T cells (bottom row). VM cell suspensions were stained with a MAb specific to the activation marker CRTAM, CD4, or CD8 and analyzed by FACS. The numbers at the top of each dot plot indicate the percentages of CRTAM+ CD4+ T cells (top row) and CRTAM+ CD8+ T cells (bottom row) per vaginal mucosal total cells, as determined by FACS. (D) Proliferative responses of vaginal mucosa-resident CFSE+ CD4+ T cells (top row) and CFSE+ CD8+ T cells (bottom row). VM cell suspensions were labeled with CFSE (2.5 × 106 cells/well) either alone (mock) or in the presence of the indicated immunizing HSV-2 protein, and proliferative cells were quantified by FACS. The indicated P values show statistical significance between HSV-2-infected vaccinated and mock-vaccinated control groups. *, P < 0.05 (considered significant).
The reduction in virus shedding and severity and frequency of recurrent genital herpes lesions in RR2-vaccinated guinea pigs is associated with increased numbers of functional VM tissue-resident CD4+ and CD8+ TRM cells.
We next sought to determine the association of various parameters of protection (i.e., virus shedding and severity and frequency of recurrent genital herpes lesions) with the number, function, and exhaustion of CD4+ and CD8+ TRM cells that reside at the vaginal mucocutaneous tissue of HSV-2-infected RR2-vaccinated guinea pigs.
As illustrated in Fig. 6A, guinea pigs (n = 40) were infected intravaginally with 5 × 105 PFU of HSV-2 (strain MS). Once acute infection was resolved, latently infected animals were randomly divided into 3 groups (n = 11) and then vaccinated intramuscularly twice, on day 15 and on day 25 postinfection, with either RR2 protein emulsified in alum plus CpG adjuvants (group 1) or the replication-defective dl5-29 vaccine (positive control; group 2). Mock-vaccinated guinea pigs, which received adjuvant alone, were used as negative controls (group 3).
FIG 6.
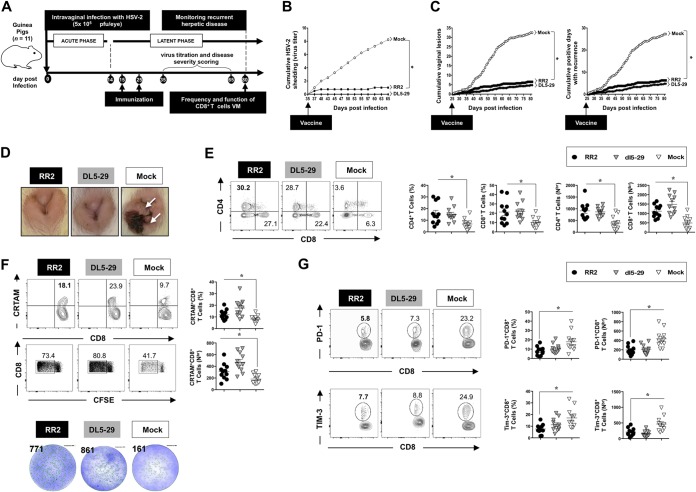
Correlates of protection with function and exhaustion of local vaginal mucosa-resident CD8+ T cells following RR2 protein therapeutic vaccination of HSV-2-infected guinea pigs. (A) Timeline of HSV-2 infection, IVAG and intramuscular (IM) immunization, and immunological and virological analyses. Once acute infection was resolved, latently infected animals were randomly divided into three groups (n = 11) and then vaccinated twice intramuscularly, on days 15 and 25 postinfection, with either RR2 protein emulsified in alum plus CpG adjuvants (group 1) or the replication-defective dl5-29 vaccine (positive control; group 2). Mock-vaccinated guinea pigs, which received adjuvant alone, were used as a negative control (group 3). (B) Cumulative virus shedding detected RR2-vaccinated, dl5-29-vaccinated and mock-vaccinated controls is shown. (C) Cumulative vaginal lesions (left) and cumulative number of days with recurrent genital lesions (right) detected for RR2-vaccinated, dl5-29-vaccinated, and mock-vaccinated controls are shown. (D) Representative images of genital lesions in guinea pigs vaccinated with RR2 protein or dl5-29 vaccine and mock-vaccinated animals. (E) Frequencies of CD4+ and CD8+ T cells detected by FACS in the VM tissue of RR2-vaccinated animals. (F) Frequencies of functional CRTAM+ CD8+ T cells (top row of plots and graphs) and of CFSE+ CD8+ T cells (bottom row of plots and graphs) and IFN-γ-producing cells, enumerated ex vivo by ELISpot assay (bottom portion) in the VM of RR2-vaccinated and dl5-29-vaccinated animals compared to those in mock-vaccinated animals. (G) Frequencies of exhausted PD-1+ CD8+ T cells (top row of plots and graphs) and TIM-3+ CD8+ T cells (bottom row of plots and graphs) detected in the VM of RR2-vaccinated and dl5-29-vaccinated animals compared to those in mock-vaccinated animals. The indicated P values show statistical significance between RR2-vaccinated and mock-vaccinated control groups. *, P < 0.05 (considered significant).
A significant reduction in cumulative virus shedding was detected in the vaginal swabs from RR2-vaccinated animals compared to those from mock-vaccinated controls (P < 0.05) (Fig. 6B). This was associated with a significant reduction in cumulative vaginal lesions (Fig. 6C, left graph) and a reduced number of days with recurrent genital lesions (Fig. 6C, right graph). The level of protection induced with RR2 was similar to the level in the dl5-29 positive-control group. As expected, mock-vaccinated animals developed no protection against recurrent genital herpes infection and disease (P > 0.05) (Fig. 6C). Neither the dl5-29- nor the RR2-vaccinated animals had any apparent genital herpetic lesions, in contrast to severe ulcerative vaginitis detected in all mock-vaccinated animals (Fig. 6D).
On day 55 after the second and final immunization, significantly higher percentages of both CD4+ and CD8+ TRM cells were detected by FACS in the VM tissue of RR2-vaccinated animals than in mock-vaccinated animals (P < 0.05) (Fig. 6E). These frequencies were similar to those detected in the VM of guinea pigs that were immunized with the whole replication-defective dl5-29 virus (Fig. 6E, left two panels).
Significantly higher frequencies of functional CRTAM+ CD8+ TRM cells (top row) and of CFSE+ CD8+ TRM cells (middle row) were detected in the VM of RR2 protein-vaccinated animals than in mock-vaccinated animals (P < 0.05) (Fig. 6F). Significantly higher numbers of IFN-γ-producing cells were enumerated ex vivo by the ELISpot assay in the VM of RR2-vaccinated animals than of mock-vaccinated animals (Fig. 6F, middle row). In contrast, lower frequencies of exhausted PD-1+ CD8+ T cells (Fig. 6G, top row) and TIM-3+ CD8+ T cells (Fig. 6G, bottom row) were detected in the VM of the RR2 group than of the mock-vaccinated group, confirming less exhausted and more functional VM-resident T cells following vaccination with the RR2 protein (P < 0.05). As expected, high numbers of functional CD4+ and CD8+ TRM cells were detected in the VM of the dl5-29-vaccinated group (positive control), while high numbers of dysfunctional CD4+ and CD8+ TRM cells were detected in the mock-vaccinated group (negative control) (Fig. 6F and G).
Together, these results confirmed that therapeutic vaccination of HSV-2-infected guinea pigs with RR2 proteins induced more functional and less exhausted vaginal mucosa-resident CD4+ and CD8+ TRM cells associated with a significant reduction in both virus shedding and recurrent genital herpes lesions.
Intravaginal immunization of HSV-2-infected guinea pigs with RR2 protein is superior to intramuscular immunization.
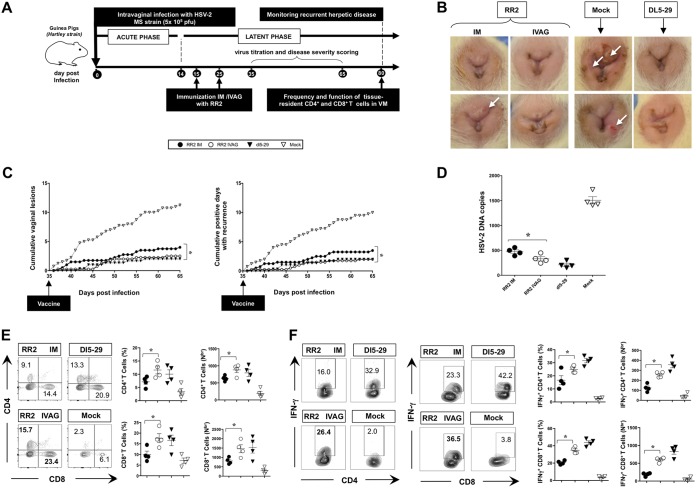
Genital herpes infections and lesions are initiated at mucocutaneous surfaces, yet intramuscular vaccinations are often used and usually provide only minimal protection at vaginal mucosal sites of infection, likely because of a suboptimal activation of protective local tissue-resident CD4+ and CD8+ TRM cells. To optimize the number and function of local CD4+ and CD8+ TRM cells that would localize to healed sites of the vaginal mucocutaneous tissues, we compared side by side the intravaginal (IVAG) and intramuscular routes of vaccination in HSV-2-infected guinea pigs using the RR2 vaccinogen formulated in CpG and alum adjuvants. We found that protection induced following immunization through the intravaginal route was significantly higher than with the intramuscular route (Fig. 7B to D). Moreover, significant increases in the numbers of vaginal mucosa-resident IFN-γ-producing CD4+ and CD8+ TRM cells were induced following intravaginal (RR2 IVAG) immunization compared to intramuscular immunization (RR2 IM) with the RR2 protein (Fig. 7E and F).
FIG 7.
Intravaginal versus intramuscular immunization of HSV-2-infected guinea pigs with RR2 protein. (A) Timeline of HSV-2 infection, intravaginal (IVAG) and intramuscular (IM) immunization, and immunological and virological analyses. Guinea pigs (n = 30) were infected intravaginally with 5 × 105 PFU of HSV-2 (MS strain). Once acute infection was resolved, latently infected animals were randomly divided into four groups (n = 10) and then vaccinated with the RR2 protein emulsified in alum plus CpG adjuvant either IVAG or IM on days 15 and 25. Replication-defective HSV-2 dl5-29 mutant vaccine delivered IVAG was used as a positive control. Mock-vaccinated guinea pigs, which received IVAG alum plus CpG adjuvants alone, were used as a negative control. (B) Representative images of genital lesions in guinea pigs vaccinated with RR2 protein either IVAG or IM are shown, along with representative images of dl5-29-vaccinated and mock-vaccinated animals. (C) Cumulative vaginal lesions (left panel) and cumulative positive days with recurrences (right) following IVAG versus IM vaccinations. (D) HSV-2 DNA copy numbers detected in DRG on day 80 p.i. following vaccination by either the IVAG or IM route are shown. (E) Representative FACS data (left panels) and average frequencies and numbers (right panels) of CD4+ T cells and CD8+ T cells in guinea pigs vaccinated with RR2 protein IVAG or IM are shown. (F) Representative FACS data (left plots) and average frequencies and numbers (right graphs) of IFN-γ+ CD4+ T cells and IFN-γ+ CD8+ T cells vaccinated IVAG or IM. The indicated P values show statistical significance between the groups of animals vaccinated IVAG versus IM. *, P < 0.05 (considered significant).
The protective immunity against recurrent genital herpes infection and disease induced following therapeutic immunization with RR2 protein is CD4+ and CD8+ T-cell dependent.
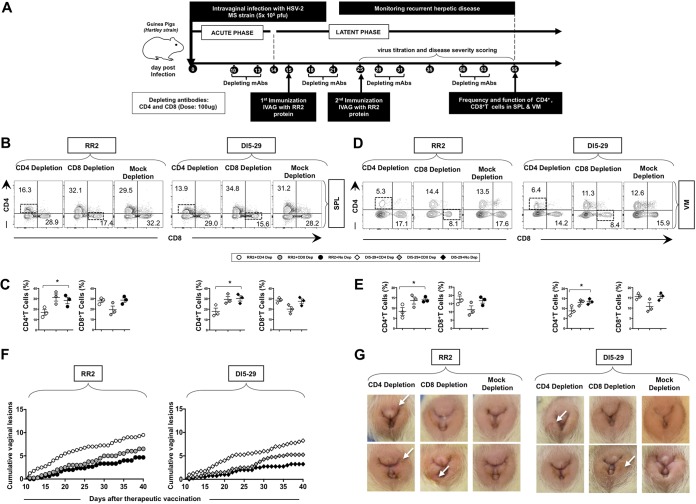
As illustrated in Fig. 8A, to assess the involvement of CD4+ and CD8+ T-cell subsets in the protection induced by IVAG immunization with the RR2 protein, in vivo depletion of either CD4+ or CD8+ T cells was performed in infected guinea pigs before and after each IVAG immunization. Depletion of either CD4+ or CD8+ T cells significantly reduced the number of respective cells in both the spleen (Fig. 8B and C) and vaginal mucosal tissues (Fig. 8D and E). Such depletion of CD4+ or CD8+ T cells was associated with a significant abrogation in the protection induced following IVAG immunization with the RR2 protein or dl5-29 (P < 0.05) (Fig. 8F and G). This suggests that in this system, both CD4+ and CD8+ T cells are required for protection against recurrent genital herpes. Together, these results indicate that IVAG therapeutic immunization with the RR2 protein decreased overt signs of recurrent genital herpes disease and that this decrease is CD4+ and CD8+ T-cell dependent.
FIG 8.
Effect of CD4 and CD8 depletion on RR2 induced protection against recurrent genital disease. (A) Timeline of HSV-2 infection, IVAG and IM immunization, and CD4+ and CD8+ T-cell depletion. (B to E) Representative FACS data for the frequencies of CD4+ and CD8+ T cells detected in the spleen (SPL) (B and C) and VM (D and E) are shown. (F) Cumulative vaginal lesions in the RR2- and dl5-29-vaccinated groups following CD4+ or CD8+ T-cell depletion. (G) Representative images of genital lesions in guinea pigs vaccinated with either the RR2 protein or dl5-29 and depleted from either CD4+ or CD8+ cells. The indicated P values show statistical significance between CD4+ and CD8+ T-cell-depleted groups and the mock-depleted group. *, P < 0.05 (considered significant).
DISCUSSION
The morbidity and socioeconomic and economic burden associated with recurrent genital herpes underscore the need for developing a therapeutic herpes vaccine (1, 30). Despite its toll, herpes has generally been seen as a “marginal” disease. Only a few pharmaceutical companies and academic institutions have invested in herpes vaccine research over the past few decades due to the failure of many vaccines designed to protect against genital herpes, leading many to give up in developing a novel herpes vaccine strategies (3, 31, 32). In the present study, lessons learned from the failure of past clinical trials using gB/gD-based vaccines led us to hypothesize that a therapeutic subunit vaccine incorporating non-gB/gD asymptomatic HSV-2 proteins that selectively induce CD4+ and CD8+ TRM cells from naturally protected asymptomatic women would halt or at least decrease the frequency and severity of recurrent herpetic disease.
A reliable small-animal model is the ultimate key factor for preclinical testing of the efficacy of the growing numbers of therapeutic herpes vaccine candidates against spontaneous herpes reactivation and recurrent disease, yet its relevance has been often overlooked. The use of mouse models of genital herpes to test therapeutic vaccine candidates is precluded by the lack of spontaneous reactivation and shedding of reactivated virus in the genital tract and hence absence of recurrent genital herpetic disease. In contrast, spontaneous reactivation occurs in HSV-2-infected guinea pigs, which develop clinical and pathological features of recurrent genital herpes that are similar to those seen in human infections. The appearance and evolution of genital lesions, histologic changes in the genital epithelium and nerve tissues, and establishment of latent virus infection in the DRG with the potential for reactivation all mimic human disease. Since analyses of human blood-derived T cells may not always reflect tissue-resident T cells, the guinea pig represents the gold standard model to determine the contribution of the vaginal mucosal-tissue-resident CD4+ and CD8+ cells versus blood-derived CD8+ T cells in protection against recurrent genital herpes. The guinea pig model allowed us to speed up testing whether therapeutic vaccine candidates, encompassing asymptomatic protein Ags (e.g., RR2 and UL49), decrease the level of latency in DRG, spontaneous reactivation from sensory neurons of DRG, virus shedding in genital tract, and recurrent genital herpetic disease. It is worth emphasizing that similar to the case with dl5-29 vaccine, we found that the RR2 subunit vaccine significantly boosted the numbers of functional IFN-γ+ CRTAM+ CFSE+ CD4+ and IFN-γ+ CRTAM+ CFSE+ CD8+ T cells that were localized to healed sites of the vaginal mucocutaneous tissues and that this was associated with a significant decrease in virus shedding and a reduction in the severity and frequency of recurrent genital herpes lesions. To our knowledge, this is the first study that demonstrated in the guinea pig model a correlation between, on one hand, a reduction in virus shedding and a decrease in recurrent genital herpes lesions induced by RR2 and, on the other hand, an increase in the number and function of tissue-resident memory CD4+ and CD8+ T cells in the vaginal mucosa. A study of the phenotype memory CD4+ and CD8+ T cells in the vaginal mucosa as to their expression of CD103, CD69, CCR7, and CD44 molecules is currently ongoing, and we plan to report the results in a future publication.
Stress, following immunosuppression or exposure to a variety of physical or psychological/emotional stimuli, triggers HSV to reactivate from latency, and virus shedding in the genital tract causes recurrent herpetic diseases (27). Asymptomatic HSV-1 and HSV-2 infections are common, pointing to the ability of the immune system to control the symptoms, despite failure to clear the virus (33). Most genital herpetic disease is due to viral reactivations from latency, rather than to primary acute infection (18). Over two-thirds of women (80%) who are seropositive for HSV-2 are asymptomatic (ASYMP) and unaware of their infection (18). They shed and can transmit the reactivated virus but have no history of recurrent herpetic disease. In contrast, a small proportion of seropositive women are symptomatic (SYMP). They have a history of frequent and lifelong episodes of recurrent herpetic disease, often requiring doctor visits and continuous antiviral therapy (18). It is becoming increasingly clear that the acquired herpes immunity in SYMP individuals is not sufficient for protection against virus reactivation and virus replication and disease at the genital tract (18). This implies that a successful herpes vaccine must be able to either boost the natural immune responses or initiate a protective immune mechanism that does not occur following natural exposure to the virus (13, 34, 35). Such protective immune responses might be directed against different Ags than those by the immune system from SYMP individuals. The correct response to the recent “failures” of clinical HSV vaccine trials using gB and gD is to encourage the testing of novel herpes tegument, capsid, and regulatory proteins as candidate vaccines.
Historically, it has been harder to develop therapeutic vaccines than prophylactic vaccines because of the many strategies employed by HSV-1 and HSV-2 to evade control by the host’s immune system, which are in place in already infected individuals (36–38). Thus, considerable effort has been applied to developing a less challenging prophylactic herpes vaccine to prevent herpes infection of seronegative patients. However, given the staggering number of already infected HSV-1 and HSV-2 individuals, we contend that developing a therapeutic herpes vaccine to reduce herpes shedding and alleviate herpetic disease in symptomatic patients with recurrent outbreaks would be highly desired. Recurrent genital herpetic disease is one of the most common sexually transmitted diseases, with a worldwide prevalence of infection predicted to be over 3.5 billion individuals for HSV-1 and over 536 million for HSV-2 (1). Moreover, HSV-1/HSV-2 recombinant strains are reported to widely circulating (39). The majority of HSV-1- and HSV-2-seropositive individuals are asymptomatic, i.e., do not show any apparent recurrent symptoms, implying that many seropositive individuals can readily and quietly transmit the virus to their partners. This gloomy picture of global herpes prevalence stresses the urgency of developing a therapeutic vaccine. In the present study, we rationally selected HSV proteins that were highly recognized by the immune system from naturally protected asymptomatic individuals as therapeutic vaccine candidates. The safety, immunogenicity, and protective efficacy of these eight HSV-2 proteins was assessed in a preclinical therapeutic vaccine trial using the guinea pig model of recurrent genital herpes. Compared to the dl5-29, a whole HSV-2 live vaccine that was recently tested in clinical trials (40), we found that therapeutic immunization with gD, VP22, or RR2 protein produced significant protection against recurrent genital herpes infection and disease. While RR2 has been previously reported as a major target of HSV-2-specific CD8+ T cells in humans (23), its antibody, CD4+ and CD8+ T-cell immunogenicity, and protective efficacy against recurrent genital herpes have never been reported. Recently, a recombinant protein vaccine for herpes zoster (Shingrix) was shown to be highly effective, with >90% efficacy, even in subjects >80 years of age (41, 42). Similar to the herpes simplex vaccine presented in this study, the herpes zoster vaccine contains a single varicella-zoster virus (VZV) adjuvanted protein that stimulates VZV-specific CD4+ T cells (and low-level CD8+ memory T cells) and humoral responses (43). Thus, very high levels of protection can be induced against both herpes simplex (this study) and herpes zoster (41–43) by a single recombinant viral protein combined with an adjuvant that induces the appropriate B- and T-cell responses. The results presented in this report are a strong improvement over the response induced by live attenuated vaccines (40).
To the best of our knowledge, the present study is first to demonstrate that intravaginal delivery of HSV-2 RR2 protein antigen (i) boosted both neutralizing antibodies, which appear to cross-react with gB and gD, glycoproteins, (ii) increased the number of functional tissue-resident IFN-γ-producing CRTAM+ CFSE+ CD4+ and CRTAM+ CFSE+ CD8+ TRM cells in the vaginal mucosa, and (iii) protected against spontaneous recurrent genital herpes in the infected guinea pigs. The HSV-2 RR2 protein, therefore, constitutes a viable B- and T-cell candidate antigen to be incorporated in future genital herpes therapeutic mucosal vaccines. In a recent clinical trial, a candidate therapeutic HSV-2 vaccine formulation designated GEN-003, made by a mixture of a fragment of infected cell protein 4 (ICP4.2), a deletion mutant of gD2 (gD2ΔTMR), and Matrix-M2 adjuvant, appeared to (i) boost HSV-2-specific antibody and T-cell responses, as measured in the peripheral blood, and (ii) decrease rates of viral shedding and severity of lesions (44–46). The same level of protective immunity has yet to be reproduced in a larger phase III clinical trial.
The HSV-2 ribonucleotide reductase (RR) consists of two heterologous protein subunits. The small subunit (RR2) is a 38-kDa protein encoded by the UL40 gene, and the large subunit (RR1), designated ICP10, is a 140-kDa protein encoded by the UL39 gene (47). RR2 is a major target of HSV-2-specific CD8+ T cells in humans (23). Hensel et al. recently reported that a preventive prophylactic vaccine based on a combination of UL40 and the extracellular portion of the gD (gDt) protected noninfected guinea pigs from an intravaginal HSV2 challenge (48). It is unclear whether the observed prophylactic protection was due to UL40 or to gD, since immunizations with the single Ags that formed that particular combination were not compared in that study. The present investigation extended those studies by demonstrating that a therapeutic vaccination of HSV-2-infected guinea pigs with RR2 protein alone, boosted gB- and gD-cross-reactive neutralizing antibodies, and enhanced the numbers of functional IFN- γ+ CRTAM+ CFSE+ CD4+ and IFN-γ+ CRTAM+ CFSE+ CD8+ T cells within the VM tissues. These robust local B- and T-cell responses were associated with a significant reduction in both virus shedding and the severity and frequency of recurrent genital herpes lesions. Interestingly, therapeutic immunization with RR1 protein or a combination of the RR1 and RR2 proteins did not result in a more robust protection than with RR2 alone (data not shown). These preclinical findings suggest the HSV-2 RR2 protein alone (without combination with gB, gD, or RR1 antigen) as a viable candidate to be incorporated in the next genital herpes therapeutic mucosal vaccine to be clinically tested. The intravaginal route proved to be far better than the intramuscular route in boosting tissue-resident HSV-specific CD4+ and CD8+ T cells detected at the genital epithelium. The mechanisms that lead to superiority of intravaginal and intranasal routes remain to be elucidated. Nevertheless, it is surprising that the protective efficacy of RR2 protein-based subunit vaccine delivered intravaginally in HSV-2-infected guinea pigs was comparable to that induced with the dl5-29, a whole HSV-2 live vaccine that was recently tested in a phase I clinical trial (40). The dl5-29 live vaccine completes only part of the herpes lytic cycle, without spreading to other cells, while expressing many HSV-2 Ags (40).
Acyclovir reduces or suppresses recurrent symptomatic disease, asymptomatic shedding, and lateral transmission to some extent; however, it does not clear the infection or stop recurrent disease (30, 49). This emphasizes the need for a therapeutic vaccine (more than a prophylactic vaccine), which can boost the immune system to decrease virus shedding and reduce or eliminate herpes pathology. At present, no FDA-approved therapeutic vaccines are available. Historically, developing therapeutic vaccines has been more difficult than developing prophylactic vaccines. Besides, prophylactic herpes vaccines have primarily focused on eliciting virus neutralizing antibodies before viral particles infect the vaginal epithelial cells (49). In contrast, therapeutic vaccines require induction of both antibodies and potent local CD4+ and CD8+ T-cell responses at the sites of infections (i.e., vaginal mucocutaneous tissue and DRG) as shown in this study. Developing a T-cell-based therapeutic vaccines is often confronted with multiple immune evasion strategies successfully developed by herpesviruses, immune escape artists, to persist in their hosts (50–53). Among the immune evasion mechanisms is the exhaustion of antiviral memory and effector CD4+ and CD8+ T cells (38, 54, 55). Total or partial loss of T-cell function (dysfunction) occurs following repetitive HSV latency and reactivation cycles. When T-cell dysfunction develops under conditions of repetitive exposure to antigens, it is called exhaustion (as reviewed in reference 56) and is usually linked with expression of T-cell coinhibitory receptors: PD-1, TIM-3, LAG-3 (CD223), TIGIT, PSGL-1, 2B4 (CD244), GITR, CTLA-4 (CD152), CD160, and BTLA (CD272). In humans, tissue-resident CD8+ T cells express PD-1 and appear to be dysfunctional and simultaneously express high levels of 2 or 3 immune checkpoint receptors (e.g., PD-1, TIM-3, and LAG-3). To the best of our knowledge, this is the first report demonstrating the differential expression of exhaustion markers PD-1 and TIM-3 on guinea pig memory CD4+ and CD8+ T cells associated with a lack of protective immunity against recurrent genital herpes. The mechanisms that lead to an increased expression of PD-1 and TIM-3 on vaginal mucosa-resident CD4+ and CD8+ T cells remain to be elucidated. It is likely that repetitive/sporadic virus reactivations in nonprotected animals lead to persistent antigenic stimulations of T cells at the vaginal mucosa, causing the observed phenotypic and functional exhaustion of tissue-resident antiviral CD4+ and CD8+ T cells. Nevertheless, our results suggest that a single or combined immune checkpoint blockade using anti-PD-1 and/or anti-TIM-3 monoclonal antibodies (MAbs) would reverse the exhaustion of antiviral tissue-resident CD4+ and CD8+ T cells at the vaginal mucosa and hence restore their protective function. The prospect of a novel RR1 and RR2-based therapeutic herpes vaccine combined with the immune checkpoint blockade would likely increase the efficacy and longevity of protection against recurrent herpes. Therapeutic subunit vaccination, using three of the moderately protective Ags tested in this study (i.e., VP16, VP11/12, and VP13/14) combined with an immune checkpoint blockade (i.e., PD-1 and/or TIM-3), is currently being tested in our laboratory using the guinea pig model of recurrent genital herpes.
Considering the wealth of data addressing the antibody responses to herpes infections/immunizations in the guinea pig model, it is surprising how few reports exist characterizing the phenotype, function, and exhaustion of CD4+ and CD8+ T cells in this model. One limitation is the unavailability of MAbs specific to guinea pigs immune cell surface CD, cytokines, chemokines, and receptors. Compared to those for mice, relatively few immunological reagents are available to characterize CD4+ and CD8+ T cells in guinea pigs. Here we describe new immunological reagents and novel approaches that quantitatively and qualitatively help characterize the phenotype, function, and exhaustion of CD4+ and CD8+ T-cell responses both systemically and as they occur in the female reproductive tract of the infected and vaccinated guinea pigs. IFN-γ ELISpot, carboxyfluorescein succinimidyl ester (CFSE)-based proliferation, surface markers of T-cell activation (CRTAM), and T-cell exhaustion (PD-1 and TIM-3), intracellular cytokines are investigated in this report. Moreover, quantitative PCR (qPCR) techniques to detect and quantify recurrent HSV-2 shedding have been optimized in the guinea pig model. These immunological reagents and novel approaches are useful to study infection, immunity, and immunopathogenesis of many infectious and allergic diseases in the guinea pig model (57–59), besides genital herpes infection and disease.
In summary, our results represent the first in-depth evidence that intravaginal immunization of HSV-2-infected guinea pigs with a recombinantly expressed HSV-2 RR2 protein in CpG and alum adjuvants safely boosted a coordinated and broad spectrum of B- and T-cell responses, including gB- and gD-cross-reactive neutralizing antibodies and tissue-resident IFN-γ-producing CD4+ and CD8+ T cells that localized to healed sites of the vaginal mucocutaneous tissues. The strong B- and T-cell immunogenicity of the RR2-based therapeutic vaccine was associated with significant protection against recurrent genital herpes. In the context of limited success of recent herpes clinical vaccines delivered parenterally, this is a rather interesting finding, since antiviral local CD4+ and CD8+ T-cell responses within the infected vaginal mucocutaneous tissue might be suboptimal due to the unique vaginal immune environment. The present findings are encouraging because they offer relatively low-cost mucosal routes to provide a T-cell-based vaccine for clinical trials. Which T-cell epitopes from RR2 will be protective in clinical trials remains to be determined. Nevertheless, these findings support the Ag selection strategy and suggest that vaccination with asymptomatic protein Ags will likely boost the number and function of CD4+ and CD8+ T cells preventing (or at least reducing) virus reactivation and, hence, protecting against recurrent genital herpes. The findings lay the groundwork for an accessible mucosal subunit therapeutic vaccine approach to decrease virus shedding and reduce or eliminate genital herpes pathology, and presumably other sexually transmitted infectious diseases.
MATERIALS AND METHODS
Human study population.
During the last 15 years (i.e., January 2003 to October 2018), we have screened 925 individuals for HSV-1 and HSV-2 seropositivity. Five hundred eighty-seven were white, 338 were nonwhite (African, Asian, Hispanic, and other), 467 were female, and 458 were male. Among this sample, a cohort of 308 immunocompetent individuals, ranging from 21 to 69 years old (median, 45 years), were seropositive for HSV-2. All patients were negative for HIV and hepatitis B virus (HBV), with no history of immunodeficiency. Two hundred seventy-eight patients were healthy and defined as asymptomatic (ASYMP). These patients never had any herpes disease (genital, orofacial, dermal, or ocular) based on self-reporting and clinical examination. Even a single episode of any herpetic disease excluded the individual from this group. The remaining 30 patients were defined as HSV-seropositive symptomatic (SYMP) individuals who suffered from frequent and severe recurrent genital lesions. Patients were also excluded if they (i) had an active genital (or elsewhere) herpetic lesion or had had one within the past 30 days, (ii) were pregnant or breastfeeding, or (iii) were on acyclovir and other related antiviral drugs or any other immunosuppressive drugs at the time of blood draw. SYMP and ASYMP groups were matched for age, sex, serological status, and race. Among this large cohort, 20 SYMP and 20 ASYMP women were enrolled in the current study (Table 1). We also enrolled 10 age- and sex-matched healthy controls seronegative for both HSV-1 and HSV-2 who had no history of herpes diseases. All clinical investigations in this study were conducted according to the Declaration of Helsinki. All subjects were enrolled at the University of California, Irvine, under approved Institutional Review Board-approved protocols (IRB no. 2003-3111 and IRB no. 2009-6963). Written informed consent was received from all participants prior to inclusion in the study.
HSV-specific serotyping.
The sera collected from random donors were tested for anti-HSV antibodies. ELISA was performed on sterile 96-well flat-bottom microplates coated with the HSV-2 antigen in coating buffer overnight at 4°C. The next day, plates were washed with phosphate-buffered saline (PBS)–1% Tween 20 (PBST) five times, and nonspecific binding was blocked by incubation with a 5% solution of skimmed milk in PBS (200 μl/well) at 4°C for 1 h at room temperature (RT). The microplates were washed three times with PBS-Tween and incubated with various sera at 37°C for 2 h. Following five washes, biotinylated rabbit anti-human IgG, diluted 1: 20,000 with PBST, was incubated at 37°C for 2 h. After five washes, streptavidin-peroxidase was added at a 1: 5,000 dilution and incubated for 30 min at RT. After five additional washes, the color was developed by adding 100 μl of tetramethylbenzidene (TMB) substrate. The mixture was incubated for 5 to 15 min at RT in the dark. The reaction was terminated by adding 1 M H2SO4. The absorbance was measured at 450 nm.
Peripheral blood mononuclear cell isolation.
Individuals (negative for HIV and HBV and with or without any HSV infection history) were recruited at the UC Irvine Institute for Clinical and Translational Science (ICTS). One hundred milliliters of blood was drawn into yellow-top Vacutainer tubes (Becton, Dickinson). The serum was isolated and stored at −80°C for the detection of anti-HSV-1 and anti-HSV-2 antibodies, as we have previously described (60). Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation using leukocyte separation medium (Life Sciences, Tewksbury, MA). The cells were then washed in PBS and suspended in complete culture medium consisting of RPMI 1640, 10% fetal bovine serum (FBS; Bio-Products, Woodland, CA) supplemented with 1× penicillin–streptomycin–l-glutamine, 1× sodium pyruvate, 1× nonessential amino acids, and 50 μM 2-mercaptoethanol (Life Technologies, Rockville, MD).
Flow cytometry assays.
Peripheral blood mononuclear cells were analyzed by flow cytometry after staining with fluorochrome-conjugated human-specific MAbs. The following anti-human antibodies were used for flow cytometry assays: CD3 (clone SK7) phycoerythrin (PE)-Cy7, CD4 (clone DE54), CD8 (clone SK1), CD45RA FITC, CD62L allophycocyanin, IFN-γ Alexa Fluor 647, granzyme B (clone GB11) A647, CD107a (clone H4A3) fluorescein isothiocyanate (FITC), CD107b (clone H4B4) FITC (BioLegend, San Diego, CA), and granzyme K (clone G3H69). Surface-staining MAbs against various cell markers were added to a total of 1 × 106 PBMC in PBS containing 1% FBS and 0.1% sodium azide (FACS buffer) for 45 min at 4°C. After the cells were washed with FACS buffer, they were permeabilized for 20 min on ice using a Cytofix/Cytoperm kit (BD Biosciences) and then washed twice with Perm/Wash buffer (BD Biosciences). Intracellular cytokine-staining MAbs were added to the cells, and the mixture was incubated for 45 min on ice in the dark. The cells were washed with Perm/Wash buffer and FACS buffer and subsequently fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For each sample, 100,000 total events were acquired on the BD LSRII. Ab capture beads (BD Biosciences) were used as individual compensation tubes for each fluorophore in the experiment. To define positive and negative populations, we used isotype control MAbs for each fluorophore and further optimized gating by examining known negative cell populations for background expression levels. The gating strategy, similar to that used in our previous work (61), is shown in Fig. S1 in the supplemental material. Briefly, we gated single cells, viable cells (Aqua blue), lymphocytes, CD3+ CD4+ cells, and CD3+CD8+ cells. Data analysis was performed using FlowJo software (version 10.4.2; TreeStar, Ashland, OR). Statistical analyses were done using GraphPad Prism (version 5; La Jolla, CA).
Animals.
Female guinea pigs (Hartley strain; Charles River Laboratories, San Diego, CA) weighing 275 to 350 g (5 to 6 weeks old) were housed at the University of California, Irvine, vivarium. The number of animals required per group for each experiment was calculated based on our experience using animal models in spontaneous and induced recurrent herpes infection and disease. A group size of 10 had 90% power to detect a difference 2-fold or higher between experimental group means, with a significance level of 0.05. The Institutional Animal Care and Use Committee of the University of California, Irvine, reviewed and approved the protocol for these studies (IACUC no. 2002-2372).
Vaccine candidates.
We used eight recombinantly expressed protein antigens from HSV-2: gD, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25, selected as highly recognized by T cells from naturally protected asymptomatic individuals (Table 1). The replication-defective HSV-2 dl5-29 mutant (i.e., dl5-29 vaccine) was used as a positive control (62). The dl5-29 mutant has deletions in the UL5 and UL29 genes and has been shown to protect mice and guinea pigs against challenge with wild-type (wt) HSV-2 (62) and was recently tested in clinical trials (40).
Infection and immunization of guinea pigs.
Throughout this study, we used the MS strain of HSV-2, generously gifted by David Bernstein (Cincinnati Children’s Hospital Medical Center, University of Cincinnati, OH). Guinea pigs (n = 11) were infected intravaginally with 5 × 105 PFU of HSV-2 (strain MS). Once acute infection was resolved, latently infected animals were vaccinated intramuscularly twice, in the right hind calf muscle on day 15 and on day 25 postinfection. Animals were immunized on day 15 with 20 μg and on day 25 with 10 μg of various HSV-2 proteins (gD, RR1, RR2, VP22, gB, VP16, VP13/14, VP11/12, and UL25) mixed with 100 μg of CpG/guinea pig and 150 μg of alum. Animals were mock immunized with the CpG oligonucleotide (5′-TCGTCGTTGTCGTTTTGTCGTT-3′) (Trilink Inc., Santa Fe Springs, CA) using 100 μg of CpG/guinea pig and 150 μg of alum (Alhydrogel; Accurate Chemical and Scientific Corp., Westbury, NY).
Monitoring of primary or recurrent HSV-2 disease in guinea pigs.
Guinea pigs were examined for vaginal lesions, which were recorded for each individual animal on a daily basis starting right after the second immunization on a scale of 0 to 4, where 0 reflects no disease, 1 reflects redness, 2 reflects a single lesion, 3 reflects coalesced lesions, and 4 reflects ulcerated lesions.
Real-time qPCR for HSV-2 quantification from vaginal swabs and dorsal root ganglia.
Vaginal swabs were collected daily using a Dacron swab (type 1; Spectrum Laboratories, Los Angeles, CA) starting from day 35 until day 65 postchallenge. Individual swabs were transferred to a 2-ml sterile cryogenic vial containing 1 ml of culture medium and stored at −80°C until use. On day 65 postchallenge, 12 lower lumbar and sacral dorsal root ganglia (DRG) per guinea pig were collected by cutting through the lumbar end of the spine. DNA was isolated from the collected vaginal swab and DRG of guinea pigs by using DNeasy blood and tissue kits (Qiagen). The presence of HSV-2 DNA was quantified by real-time PCR (StepOnePlus real-time PCR system) with 50 to 100 ng of vaginal swab DNA or 250 ng of DRG DNA. HSV-2 DNA copy number was determined using purified HSV-2 DNA (Advanced Biotechnologies, Columbia, MD) and based on a standard curve of the threshold cycle (CT) values that was generated with 50,000, 5,000, 500, 50, and 5 copies of DNA and run in triplicates. The CT values from unknown samples were plotted on the purified HSV-2 DNA standard curves, and the number of HSV-2 DNA copies per assay was calculated. Each guinea pig sample was analyzed in duplicate. Samples with <150 copies/ml by 40 cycles or positive in only one of two wells were reported as negative. Primer and probe sequences for HSV-2 Us9 were as follows: primer forward, 5′-GGCAGAAGCCTACTACTCGGAAA-3′; primer reverse, 5′-CCATGCGCACGAGGAAGT-3′; and probe with reporter dye, 5′-6-carboxyfluorescein (FAM)-CGAGGCCGCCAAC-MGBNFQ-3′. All reactions were performed using TaqMan gene expression master mix (Applied Biosystems), and data were collected and analyzed on StepOnePlus real-time PCR system.
ELISA and neutralizing antibodies.
Blood (5 ml) was drawn from each guinea pig into yellow-top Vacutainer tubes (Becton, Dickinson). Sera were isolated by centrifugation for 10 min at 800 × g. For measuring antigen-specific antibody titers in the sera of immunized guinea pigs, ELISA plates were coated with 50 ng of individual HSV-2 proteins and incubated with guinea pig serum at a 1:1,000 dilution, followed by horseradish peroxidase (HRP)-conjugated anti-guinea pig IgG. For measuring the affinity of binding of antigen-specific antibodies to the HSV-2 MS strain, ELISA plates were coated overnight at 4°C with 1 × 104 PFU/well of heat-inactivated MS strain and incubated with respective guinea pig serum at a 1:1,000 dilution, followed by HRP-conjugated anti-guinea pig IgG. For competitive inhibition assays, antigen-specific sera, diluted 1:100, were incubated overnight at 4°C with test inhibitory peptides at concentrations ranging from 10−1 to 10−5 ng/ml. These samples were then processed as standard ELSA assays, using 1 μg of respective antigen-coated plates. Results for each peptide tested at different concentrations were expressed as percent inhibition, calculated as follows: percent inhibition = [OD450 (immune serum incubated with test peptide at test concentration) − OD450 (background, serum dilution buffer)/(OD450 (immune serum incubated in the absence of peptide) − OD450 (background, serum dilution buffer)] × 100. Neutralizing antibody titers were determined by incubating 100 PFU of HSV-2 strain MS with serial dilutions of serum starting at 1:40 for 1 h at 37°C. The endpoint neutralization titer was determined by the plaque assay on RS cells and was calculated as the serum dilution that reduced the number of plaques by 50% compared with PBS controls.
Splenocyte isolation.
Spleens were harvested from guinea pigs at 80 days postinfection. Spleens were placed in 10 ml of cold PBS with 10% FBS and 2× antibiotic-antimycotic (Life Technologies, Carlsbad, CA). Spleens were minced finely and sequentially passed through a 100-μm mesh and a 70-μm mesh (BD Biosciences, San Jose, CA). Cells were then pelleted via centrifugation at 400 × g for 10 min at 4°C. Red blood cells were lysed using a lysis buffer (ammonium chloride) and washed again. Isolated splenocytes were diluted to 1 × 106 viable cells per ml in RPMI medium with 10% (vol/vol) FBS and 2× antibiotic-antimycotic. Viability was determined by trypan blue staining.
Isolation of lymphocytes from guinea pig vaginal mucosa.
Vaginal mucosae were removed from the guinea pigs. The genital tract was minced into fine pieces; the tissue pieces were then transferred into a new tube with fresh RPMI-10 containing collagenase and digested at 37°C for 2 h on a rocker set to vigorously stir the tissue. The digested tissue suspension was then passed through a 100-μm cell strainer on ice, followed by centrifugation. Lymphocytes in the cell pellets were separated using Percoll gradients by centrifugation at 900 × g, at room temperature, for 20 min with the brake off. The lymphocytes at the interface layer between 40% and 70% Percoll layers were harvested, washed with RPMI medium (1:3), and spun down at 740 × g.
Flow cytometry analysis.
Vaginal mucosa cells and splenocytes were analyzed by flow cytometry. The following antibodies were used: mouse anti-guinea pig CD8 (clone MCA752F; Bio-Rad Laboratories, Hercules, CA), mouse anti-guinea pig CD4 (clone MCA749PE; Bio-Rad Laboratories), anti-mouse CRTAM (clone 11-5; Biolegend), hamster anti-mouse PD-1 (clone J43; BD Biosciences, San Jose, CA), and human TIM-3 (clone F38-2E2; BD Biosciences, San Jose, CA). For surface staining, MAbs against various cell markers were added to a total of 1 × 106 cells in phosphate-buffered saline containing 1% FBS and 0.1% sodium azide (FACS buffer) and left for 45 min at 4°C. At the end of the incubation period, the cells were washed twice with FACS buffer. A total of 100,000 events were acquired by the LSRII (Becton, Dickinson, Mountain View, CA), followed by analysis using FlowJo software (TreeStar, Ashland, OR).
T-cell proliferation assay.
CD8+ T-cell proliferation was measured using a CFSE assay. Briefly, VM cells were labeled with CFSE (2.5 μM; Molecular Probes) in 1× PBS at room temperature. Cold fetal calf serum (FCS) was added, and cells were washed extensively with RPMI 1640 plus 10% FCS. CFSE-labeled cells were either incubated or not with various individual HSV-2 proteins (10 μg/ml) for 6 days. Cells were then washed and stained with PE-conjugated MAbs specific to guinea pig CD8 molecules. The numbers of dividing CD8+ T cells per 300,000 total cells were analyzed by FACS.
ELISpot assay.
All reagents used were filtered through a 0.22-μm filter. Wells of 96-well Multiscreen HTS plates (Millipore, Billerica, MA) were prewet with 70% methanol and then coated with 100 μl of primary anti-IFN-γ antibody solution (10 μg/ml in PBS [pH 7.4]; V-E4) OVN at 4°C (63). After a washing, nonspecific binding was blocked with 200 μl of RPMI medium with 10% (vol/vol) FBS for 2 h at room temperature. Following the blockade, 5 × 105 vaginal mucosa cells and splenocytes in 100 μl of RPMI were mixed with 50 μl of stimulant (no stimulation or with individual HSV-2 proteins at a final concentration of 10 μg/ml) in duplicate. After incubation in humidified 5% CO2 at 37°C for 48 h, cells were removed by washing (using PBS and PBS–0.02% Tween solution) and 100 μl of biotinylated secondary anti-IFN-γ antibody (2 μg/ml, N-G3) in blocking buffer (PBS, 0.5% bovine serum albumin [BSA]) was added to each well. Following a 2-h incubation and washing, alkaline phosphatase-conjugated streptavidin (SEL002, R&D Systems Inc., Minneapolis, MN) was diluted 1:100 and wells were incubated with 100 μl for 1 h at room temperature. Following a washing, wells were incubated for 1 h at room temperature with 100 μl of 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium (NBT) detection reagent (SEL002; R&D Systems, Inc.) and spots counted with an automated ELISpot reader system (ImmunoSpot reader; Cellular Technology).
Statistical analyses.
Data for each assay were compared by analysis of variance (ANOVA) and Student’s t test using GraphPad Prism version 5 (La Jolla, CA). Differences between the groups were identified by ANOVA and multiple-comparison procedures, as we previously described (64). Data are expressed as means ± standard deviations (SD). Results were considered statistically significant at a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sita Awasthi from the Infectious Disease Division, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, for her help with the initial experiments of this project, including assessing the genital herpes infection and disease and the neutralization antibody assays in the guinea pig model.
This work was supported by Public Health Service research grants EY026103, EY019896 and EY024618 from the National Eye Institute (NEI) and grants AI143326, AI138764, AI124911, and AI110902 from the National Institutes of Allergy and Infectious Diseases (NIAID) and in part by the Discovery Center for Eye Research (DCER) and a Research to Prevent Blindness (RPB) grant.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.02309-18.
REFERENCES
- 1.Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, Spencer D, Chentoufi AA, Lemonnier FA, BenMohamed L. 2014. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol 75:715–729. doi: 10.1016/j.humimm.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava R, Hernandez-Ruiz M, Khan AA, Fouladi MA, Kim GJ, Ly VT, Yamada T, Lam C, Sarain SAB, Boldbaatar U, Zlotnik A, Bahraoui E, BenMohamed L. 2018. CXCL17 chemokine-dependent mobilization of CXCR8(+)CD8(+) effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J Immunol 200:2915–2926. doi: 10.4049/jimmunol.1701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. 2012. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chentoufi AA, BenMohamed L, Van De Perre P, Ashkar AA. 2012. Immunity to ocular and genital herpes simplex viruses infections. Clin Dev Immunol 2012:732546. doi: 10.1155/2012/732546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chentoufi AA, Benmohamed L. 2012. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin Dev Immunol 2012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettahi I, Zhang X, Afifi RE, BenMohamed L. 2006. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol 19:220–236. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. 2010. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol 5:1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. 2008. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol 15:1436–1449. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta G, Chentoufi AA, Kalantari-Dehaghi M, Falatoonzadeh P, Chun S, H LC, Felgner PL, H HD, BenMohamed L. 2012. Immunodominant “asymptomatic” herpes simplex virus type 1 and type 2 protein antigens identified by probing whole ORFome microarrays by serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol 86:4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chentoufi AA, Kritzer E, D YM, Nesburn AB, BenMohamed L. 2012. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol 2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava R, Coulon PG, Roy S, Chilukuri S, Garg S, BenMohamed L. 2018. Phenotypic and functional signatures of herpes simplex virus-specific effector memory CD73(+)CD45RA(high)CCR7(low)CD8(+) TEMRA and CD73(+)CD45RA(low)CCR7(low)CD8(+) TEM cells are associated with asymptomatic ocular herpes. J Immunol 201:2315–2330. doi: 10.4049/jimmunol.1800725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AA, Srivastava R, Vahed H, Roy S, Walia SS, Kim GJ, Fouladi MA, Yamada T, Ly VT, Lam C, Lou A, Nguyen V, Boldbaatar U, Geertsema R, Fraser NW, BenMohamed L. 2018. Human asymptomatic epitope peptide/CXCL10-based prime/pull vaccine induces herpes simplex virus-specific gamma interferon-positive CD107+ CD8+ T cells that infiltrate the corneas and trigeminal ganglia of humanized HLA transgenic rabbits and protect against ocular herpes challenge. J Virol 92:e00535-18. doi: 10.1128/JVI.00535-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan AA, Srivastava R, Spencer D, Garg S, Fremgen D, Vahed H, Lopes PP, Pham TT, Hewett C, Kuang J, Ong N, Huang L, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes. J Virol 89:3776–3792. doi: 10.1128/JVI.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, Nesburn AB, Wechsler SL, Benmohamed L. 2012. Future of an “asymptomatic” T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol 7:371–378. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dropulic LK, Cohen JI. 2012. The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev Vaccines 11:1429–1440. doi: 10.1586/erv.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanberry LR. 2004. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11:161A–169A. [PubMed] [Google Scholar]
- 18.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, Flechtner JB, Long D. 2013. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J Virol 87:3930–3942. doi: 10.1128/JVI.02745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston C, Koelle DM, Wald A. 2011. HSV-2: in pursuit of a vaccine. J Clin Invest 121:4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, Koelle DM, Wald A, Corey L. 2010. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol 30:703–722. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantari-Dehaghi M, Chun S, Chentoufi AA, Pablo J, Liang L, Dasgupta G, Molina DM, Jasinskas A, Nakajima-Sasaki R, Felgner J, Hermanson G, BenMohamed L, Felgner PL, Davies DH. 2012. Discovery of potential diagnostic and vaccine antigens in herpes simplex virus 1 and 2 by proteome-wide antibody profiling. J Virol 86:4328–4339. doi: 10.1128/JVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long D, Skoberne M, Gierahn TM, Larson S, Price JA, Clemens V, Baccari AE, Cohane KP, Garvie D, Siber GR, Flechtner JB. 2014. Identification of novel virus-specific antigens by CD4(+) and CD8(+) T cells from asymptomatic HSV-2 seropositive and seronegative donors. Virology 464-465:296–311. doi: 10.1016/j.virol.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. 2012. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol 86:10587–10596. doi: 10.1128/JVI.01237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L. 2013. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awasthi S, Huang J, Shaw C, Friedman HM. 2014. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol 88:8421–8432. doi: 10.1128/JVI.01130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, Cai M, Brown M, Smith JF, Kowalski R, Swoyer R, Galli J, Copeland V, Rios S, Davidson RC, Salnikova M, Kingsley S, Bryan J, Casimiro DR, Friedman HM. 2014. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol 88:2000–2010. doi: 10.1128/JVI.03163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol 85:10472–10486. doi: 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. 2014. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine 32:6733–6745. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hook LM, Awasthi S, Dubin J, Flechtner J, Long D, Friedman HM. 2019. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine 37:664–669. doi: 10.1016/j.vaccine.2018.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein DI, Pullum DA, Cardin RD, Bravo FJ, Dixon DA, Kousoulas KG. 2019. The HSV-1 live attenuated VC2 vaccine provides protection against HSV-2 genital infection in the guinea pig model of genital herpes. Vaccine 37:61–68. doi: 10.1016/j.vaccine.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 33.Lekstrom-Himes JA, Hohman P, Warren T, Wald A, Nam JM, Simonis T, Corey L, Straus SE. 1999. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J Infect Dis 179:1077–1085. doi: 10.1086/314729. [DOI] [PubMed] [Google Scholar]
- 34.Roy S, Fouladi MA, Kim GJ, Ly VT, Yamada T, Lam C, Sarain SAB, BenMohamed L. 2018. Blockade of LAG-3 immune checkpoint combined with therapeutic vaccination restore the function of tissue-resident anti-viral CD8+ T cells and protect against recurrent ocular herpes simplex infection and disease. Frontiers in Immunol 9:2922. doi: 10.3389/fimmu.2018.02922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes PP, Todorov G, Pham TT, Nesburn AB, Bahraoui E, BenMohamed L. 2018. Laser adjuvant-assisted peptide vaccine promotes skin mobilization of dendritic cells and enhances protective CD8(+) TEM and TRM cell responses against herpesvirus infection and disease. J Virol 92:e02156-17. doi: 10.1128/JVI.02156-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava R, Dervillez X, Khan AA, Chentoufi AA, Chilukuri S, Shukr N, Fazli Y, Ong NN, Afifi RE, Osorio N, Geertsema R, Nesburn AB, Wechsler SL, BenMohamed L. 2016. The herpes simplex virus latency-associated transcript gene is associated with a broader repertoire of virus-specific exhausted CD8+ T cells retained within the trigeminal ganglia of latently infected HLA transgenic rabbits. J Virol 90:3913–3928. doi: 10.1128/JVI.02450-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara KW, Jiang X, Nesburn AB, Wechsler SL, Benmohamed L. 2012. The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol 25:204–215. doi: 10.1089/vim.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L. 2011. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol 85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koelle DM, Norberg P, Fitzgibbon MP, Russell RM, Greninger AL, Huang ML, Stensland L, Jing L, Magaret AS, Diem K, Selke S, Xie H, Celum C, Lingappa JR, Jerome KR, Wald A, Johnston C. 2017. Worldwide circulation of HSV-2 x HSV-1 recombinant strains. Sci Rep 7:44084. doi: 10.1038/srep44084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz F, Gregory S, Nakashima H, Viapiano MS, Knipe DM. 2018. Intramuscular delivery of replication-defective herpes simplex virus gives antigen expression in muscle syncytia and improved protection against pathogenic HSV-2 strains. Virology 513:129–135. doi: 10.1016/j.virol.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lal H, Cunningham AL, Heineman TC. 2015. Adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 373:1576–1577. doi: 10.1056/NEJMc1508392. [DOI] [PubMed] [Google Scholar]
- 42.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC, ZO-50 Study Group . 2015. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 43.Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapelière P, Vassilev V, Ledent E, Heineman TC. 2012. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis 206:1280–1290. doi: 10.1093/infdis/jis497. [DOI] [PubMed] [Google Scholar]
- 44.Van Wagoner N, Fife K, Leone PA, Bernstein DI, Warren T, Panther L, Novak RM, Beigi R, Kriesel J, Tyring S, Koltun W, Lucksinger G, Morris A, Zhang B, McNeil LK, Tasker S, Hetherington S, Wald A. 2018. Effects of different doses of GEN-003, a therapeutic vaccine for genital herpes simplex virus-2, on viral shedding and lesions: results of a randomized placebo-controlled trial. J Infect Dis 218:1890–1899. doi: 10.1093/infdis/jiy415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernstein DI, Wald A, Warren T, Fife K, Tyring S, Lee P, Van Wagoner N, Magaret A, Flechtner JB, Tasker S, Chan J, Morris A, Hetherington S. 2017. Therapeutic vaccine for genital herpes simplex virus-2 infection: findings from a randomized trial. J Infect Dis 215:856–864. doi: 10.1093/infdis/jix004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flechtner JB, Long D, Larson S, Clemens V, Baccari A, Kien L, Chan J, Skoberne M, Brudner M, Hetherington S. 2016. Immune responses elicited by the GEN-003 candidate HSV-2 therapeutic vaccine in a randomized controlled dose-ranging phase 1/2a trial. Vaccine 34:5314–5320. doi: 10.1016/j.vaccine.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Smith CC, Peng T, Kulka M, Aurelian L. 1998. The PK domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is required for immediate-early gene expression and virus growth. J Virol 72:9131–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hensel MT, Marshall JD, Dorwart MR, Heeke DS, Rao E, Tummala P, Yu L, Cohen GH, Eisenberg RJ, Sloan DD. 2017. Prophylactic herpes simplex virus 2 (HSV-2) vaccines adjuvanted with stable emulsion and Toll-like receptor 9 agonist induce a robust HSV-2-specific cell-mediated immune response, protect against symptomatic disease, and reduce the latent viral reservoir. J Virol 91:e02257-16. doi: 10.1128/JVI.02257-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. 2009. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines 8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks TA, Rouse BT. 1992. Herpesviruses—immune escape artists? Clin Infect Dis 14:933–941. doi: 10.1093/clinids/14.4.933. [DOI] [PubMed] [Google Scholar]
- 51.Sharma S, Rajasagi NK, Veiga-Parga T, Rouse BT. 2014. Herpes virus entry mediator (HVEM) modulates proliferation and activation of regulatory T cells following HSV-1 infection. Microbes Infect 16:648–660. doi: 10.1016/j.micinf.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. 2011. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol 187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. 2008. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol 82:6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol 85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virol 83:2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wherry EJ. 2011. T cell exhaustion. Nat Immunol 12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 57.Wodal W, Schwendinger MG, Savidis-Dacho H, Crowe BA, Hohenadl C, Fritz R, Bruhl P, Portsmouth D, Karner-Pichl A, Balta D, Grillberger L, Kistner O, Barrett PN, Howard MK. 2015. Immunogenicity and protective efficacy of a Vero cell culture-derived whole-virus H7N9 vaccine in mice and guinea pigs. PLoS One 10:e0113963. doi: 10.1371/journal.pone.0113963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanson EC, Gillis P, Hernandez-Alvarado N, Fernandez-Alarcon C, Schmit M, Zabeli JC, Wussow F, Diamond DJ, Schleiss MR. 2015. Comparison of monovalent glycoprotein B with bivalent gB/pp65 (GP83) vaccine for congenital cytomegalovirus infection in a guinea pig model: inclusion of GP83 reduces gB antibody response but both vaccine approaches provide equivalent protection against pup mortality. Vaccine 33:4013–4018. doi: 10.1016/j.vaccine.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillis PA, Hernandez-Alvarado N, Gnanandarajah JS, Wussow F, Diamond DJ, Schleiss MR. 2014. Development of a novel, guinea pig-specific IFN-gamma ELISPOT assay and characterization of guinea pig cytomegalovirus GP83-specific cellular immune responses following immunization with a modified vaccinia virus Ankara (MVA)-vectored GP83 vaccine. Vaccine 32:3963–3970. doi: 10.1016/j.vaccine.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. 2008. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol 82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 62.Reszka NJ, Dudek T, Knipe DM. 2010. Construction and properties of a herpes simplex virus 2 dl5-29 vaccine candidate strain encoding an HSV-1 virion host shutoff protein. Vaccine 28:2754–2762. doi: 10.1016/j.vaccine.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultheis K, Schaefer H, Yung BS, Oh J, Muthumani K, Humeau L, Broderick KE, Smith TR. 2017. Characterization of guinea pig T cell responses elicited after EP-assisted delivery of DNA vaccines to the skin. Vaccine 35:61–70. doi: 10.1016/j.vaccine.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan AA, Srivastava R, Chentoufi AA, Kritzer E, Chilukuri S, Garg S, Yu DC, Vahed H, Huang L, Syed SA, Furness JN, Tran TT, Anthony NB, McLaren CE, Sidney J, Sette A, Noelle RJ, BenMohamed L. 2017. Bolstering the number and function of HSV-1-specific CD8+ effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J Immunol 199:186–203. doi: 10.4049/jimmunol.1700145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.