Abstract
Background
Epithelial–mesenchymal transition (emt) refers to the biologic process in which epithelial cells are transformed into interstitial phenotypes by specific pathways. This transition plays an important biologic role in the process by which epithelium-derived malignant tumour cells acquire the ability to migrate and invade. We explored the relationship between emt-associated molecules and patient-related clinical factors to determine whether any clinical characteristics could be used as biomarkers for emt-related protein alterations in lung cancer—especially lung adenocarcinoma.
Methods
Tumour specimens were collected from 80 patients with lung adenocarcinoma who underwent surgery or lung biopsy, with 4 patients being evaluated a 2nd time after re-biopsy. Expression of emt-related proteins, including E-cadherin and vimentin, was evaluated by immunohistochemistry. We analyzed the relationship between clinicopathologic characteristics and expression level of the emt markers.
Results
Positive expression of E-cadherin was observed in 63 patients (79%), and vimentin, in 46 patients (57.5%). No significant relationships between E-cadherin or vimentin expression and smoking history, sex, age, driving gene mutations, or cell differentiation were identified. A significant correlation was observed between vimentin expression and pathologic stage. Of the 4 patients who were evaluated a 2nd time after re-biopsy, 3 showed the same emt-related protein expression status as in the first analysis. In the remaining patient, E-cadherin had changed completely.
Conclusions
Clinicopathologic factors in cancer patients did not help to diagnose emt status in lung adenocarcinoma; however, TNM stage might be associated with vimentin expression.
Keywords: Epithelial–mesenchymal transition, lung adenocarcinoma, E-cadherin, vimentin, clinicopathologic factors
INTRODUCTION
Lung cancer is still one of the most common causes of cancer death worldwide and in China, although many efforts have been made to prolong survival for patients1,2. In non-small-cell lung cancer (nsclc), and especially adenocarcinoma, the major subtype of lung cancer3, overall survival is not optimistic. Molecular profiling studies have revealed that lung cancer is a complex disease with variable molecular mechanisms4,5. In patients with lung adenocarcinoma, infiltration and metastasis are the leading cause of death6.
E-Cadherin, a member of the cadherin superfamily, is classified as a calcium-dependent cell–cell adhesion molecule7. In addition, E-cadherin connects the extracellular environment with the cytoskeleton and interacts with catenins such as α-catenin and β-catenin through its conserved cytoplasmic domain. As a result, E-cadherin is crucial for the maintenance of structural and functional integrity8. Abnormal expression of E-cadherin has been proved to play an important role in invasion and metastasis in a variety of human malignancies9–12.
Vimentin is an intermediate filament protein in mesenchymal cells13. Vimentin regulates the cytoskeleton and cell adhesion molecules, and participates in adhesion, migration, invasion, and signal transduction for tumour cells and tumour-associated endothelial cells and macrophages14,15. Recent studies have shown that overexpression of vimentin in cancer correlates well with accelerated tumour growth, invasion, and poor prognosis16.
Epithelial–mesenchymal transition (emt) is an important biologic process that results in the loss of epithelial cell junction proteins such as E-cadherin and the gain of mesenchymal markers such as vimentin17. Research into emt has demonstrated that epithelium-derived malignant tumour cells use emt to acquire the ability to migrate and invade17,18.
Although the effect of the markers of emt has been described in recent years, the relationships between those markers and clinical factors in patients are still poorly understood. The aim of the present study was to evaluate the relationship between emt-related markers and clinical characteristics in patients with lung adenocarcinoma. We also determined consistency of the expression of emtrelated molecules in patients who were evaluated a 2nd time after re-biopsy.
METHODS
Patients
We collected data including clinical characteristics, tumour stage, and cell differentiation for 80 patients with lung adenocarcinoma who underwent biopsy or surgical resection from February 2013 to December 2017 at Drum Tower Hospital Medical School of Nanjing University. Tumour samples were also obtained for the 33 men (41%) and 47 women (59%) in that group, who had a mean age of 61 years. The group included 59 never-smokers and 21 past or current smokers. Tumour stage was determined using the TNM classification for lung cancer, version 8, and according to pathologic stage, 16 patients (20%) had stage i disease; 6 (7.5%), stage ii; 28 (35%), stage iii; and 30 (37.5%), stage iv. Tumours were poorly differentiated in 24 patients (30%) and well or moderately differentiated in 34 (42.5%), with no relevant information about cell differentiation being available for 22 patients (27.5%). Table I presents details.
TABLE I.
Characteristics of the 80 study patients
| Characteristic | Value [n (%)] |
|---|---|
| Sex | |
| Men | 33 (41) |
| Women | 47 (59) |
|
| |
| Age group | |
| >61 Years | 36 (45) |
| ≤61 Years | 44 (55) |
|
| |
| Smoking status | |
| Yes | 21 (26) |
| No | 59 (74) |
|
| |
| TNM stage | |
| I | 16 (20) |
| II | 6 (7.5) |
| III | 28 (35) |
| IV | 30 (37.5) |
|
| |
| Cell differentiation | |
| Poorly | 24 (30) |
| Well or moderately | 34 (42.5) |
| No information | 22 (27.5) |
|
| |
| Driver mutation | |
| EGFR | 43 (54) |
| ALK | 5 (6) |
| EGFR and ALK | 2 (2.5) |
| Wild type | 30 (37.5) |
Detection of Driver Mutations
Mutations in EGFR were examined using the amplification-refractory mutation system or next-generation sequencing in specimens of nsclc. Rearrangements in ALK were evaluated by immunohistochemistry or next-generation sequencing.
Staining of Tumour Specimens
Immunohistochemistry for E-cadherin and vimentin was performed. The pathology examination was carried out by the Department of Pathology, Drum Tower Hospital Medical School of Nanjing University. Tumour samples were formalin-fixed and, using standard histology practices, serially sectioned. Slides were placed in a 60°C oven for 30 minutes, deparaffinized, and rehydrated in xylenes and graded ethanol solutions to water. Antigen retrieval was performed by a steamer method in which the specimens were placed in a solution of 0.01 mol/L edta (pH 8) for 30 minutes at 94°C in a steamer. Primary antibodies were applied overnight at 4°C and then incubated at room temperature with horseradish peroxidase conjugated with anti-mouse secondary antibody for each primary antibody. Appropriate positive and negative controls were used during the immunohistochemical analysis. The slices stained for E-cadherin and vimentin then underwent optical microscopy. Positive expression of the proteins was determined in the cell membrane (E-cadherin) and cytoplasm (vimentin). Results are reported as an immunoreactivity score19:
where SI is the staining intensity (classified as 0, negative; 1, weak; 2, medium; or 3, strong), and PP is the percentage of positive cells (defined as 0, negative; 1, 1%–10% positive cells; 2, 11%–50% positive cells; 3, 51%–80% positive cells; or 4, >80% positive cells). A positive staining result was defined as score greater than or equal to 4. Table II shows the details of the immunohistochemistry analysis.
TABLE II.
Expression of epithelial–mesenchymal transition–related molecules in 80 patients
| E-Cadherin | Total | ||
|---|---|---|---|
|
|
|||
| Positive | Negative | ||
| Vimentin | |||
| Positive | 44 | 2 | 46 |
| Negative | 19 | 15 | 34 |
| Total | 63 | 17 | 80 |
Statistical Analyses
Statistical significance was evaluated using the chi-square or Fisher exact test, as appropriate. Differences were considered to be statistically significant at a p value less than 0.05. The data were analyzed using the IBM SPSS Statistics software application (version 19.0: IBM, Armonk, NY, U.S.A.).
RESULTS
Detection of Driver Mutations and EMT-Related Molecules
All patients were evaluated for driver mutations. Mutations in EGFR were present in 54% of patients (43 of 80), and ALK rearrangements were present in 6% (5 of 80). In addition, EGFR mutation and ALK arrangement were both present in 2 patients (Table I).
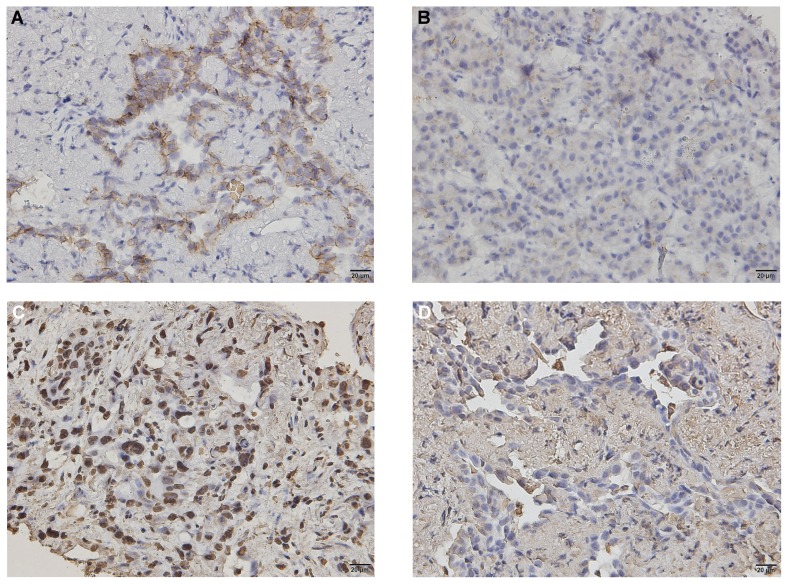
Figure 1 shows positive immunohistochemical expression of emt-related molecules (E-cadherin and vimentin) and negative expression in liver metastasis (E-cadherin) and in lung adenocarcinoma (vimentin). E-Cadherin positivity was observed in 63 patients (79%), and vimentin positivity, in 46 patients (57.5%). Positivity for both E-cadherin and vimentin was noted in 44 patients (55%, Table II).
FIGURE 1.
Positive immunohistochemical expression of epithelial–mesenchymal transition–related molecules in lung adenocarcinoma [(A) E-cadherin, (C) vimentin] and negative immunohistochemical expression of E-cadherin in liver metastasis and vimentin in lung adenocarcinoma [(B) E-cadherin, (D) vimentin].
Correlation of EMT-Related Molecules with Clinical Characteristics
In the present study, positive expression of vimentin was significantly correlated with tumour pathologic stage (p = 0.007, Table III). However, the clinical factors considered—such as sex, age, smoking history, cell differentiation, and driver mutations—showed no correlation with vimentin expression (p > 0.05, Table III). Similarly, positive expression of E-cadherin was not significantly associated with the clinicopathologic characteristics collected, nor with pathologic stage (all p > 0.05, Table III).
TABLE III.
Relationships of epithelial–mesenchymal transition–related molecules with clinical factors in lung adenocarcinoma
| Characteristic | Pts (n) | E-Cadherin | p Value | Vimentin | p Value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Positive | Negative | Positive | Negative | ||||
| Differentiation | 1.000 | 0.263 | |||||
| Poorly | 24 | 19 | 5 | 12 | 12 | ||
| Well or moderately | 34 | 27 | 7 | 22 | 12 | ||
| No information | 22 | 17 | 5 | 12 | 10 | ||
|
| |||||||
| TNM stage | 0.052 | 0.007 | |||||
| I–II | 22 | 21 | 1 | 18 | 4 | ||
| III–IV | 58 | 42 | 16 | 28 | 30 | ||
|
| |||||||
| Sex | 0.584 | 0.364 | |||||
| Men | 33 | 25 | 8 | 17 | 16 | ||
| Women | 47 | 38 | 9 | 29 | 18 | ||
|
| |||||||
| Age group | 0.066 | 0.892 | |||||
| ≤61 Years | 44 | 38 | 6 | 25 | 19 | ||
| >61 Years | 36 | 25 | 11 | 21 | 15 | ||
|
| |||||||
| Smoking status | 0.981 | 0.286 | |||||
| Yes | 21 | 16 | 5 | 10 | 11 | ||
| No | 59 | 47 | 12 | 36 | 23 | ||
|
| |||||||
| Driving gene mutation | 0.312 | 0.155 | |||||
| EGFR | 43 | 32 | 11 | 25 | 18 | ||
| ALK | 5 | 3 | 2 | 1 | 4 | ||
| EGFR and ALK | 2 | 2 | 0 | 2 | 0 | ||
| Wild type | 30 | 26 | 4 | 18 | 12 | ||
Pts = patients.
To determine whether the two emt-related biomarkers, when expressed simultaneously, behaved differently, we classified the expression of E-cadherin and vimentin into various levels. Expression levels of the proteins were significantly correlated with tumour pathologic stage (p = 0.007, Table IV). However, such expression was never found to be associated with driver mutations (p > 0.05).
TABLE IV.
Relationships of the expression levels of epithelial– mesenchymal transition–related molecules with clinical factors in lung adenocarcinoma
| Characteristic | Expression type | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| EC+, V+ | EC+, V− | EC−, V+ | EC−, V− | ||
| TNM stage | 0.007 | ||||
|
| |||||
| I | 14 | 1 | 0 | 1 | |
|
| |||||
| II | 4 | 2 | 0 | 0 | |
|
| |||||
| III | 16 | 7 | 2 | 3 | |
|
| |||||
| IV | 10 | 9 | 0 | 11 | |
| Driver mutation | 0.47 | ||||
|
| |||||
| EGFR | 23 | 9 | 2 | 9 | |
|
| |||||
| ALK | 1 | 2 | 0 | 2 | |
|
| |||||
| EGFR and ALK | 2 | 0 | 0 | 0 | |
|
| |||||
| Wild type | 18 | 8 | 0 | 4 | |
EC = E-cadherin (+, positive; −, negative); V = vimentin (+, positive; −, negative).
Multiple Detections of EMT-Related Molecules
In 4 patients, the emt-related molecules were evaluated a 2nd time after re-biopsy, with 2 of the samples being obtained from metastases. In 3 patients, expression results for both E-cadherin and vimentin were similar in the first and second samples. However, in 1 patient, E-cadherin was negative in liver at re-biopsy, but still positive in lung. Table V shows details of the analyses.
TABLE V.
Multiple detections of epithelial–mesenchymal transition–related molecules in the study patients
| Pt ID | Sample sitea | Sex | Age (years) | Smoking status | EGFR mutation | Biopsy status | TNM stage | Cell differentiation | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| E-Cadherin | Vimentin | ||||||||
| 1 | Lung primary | Female | 62 | No | Exon 21 | Positive | Positive | IB | Well or moderately |
| Lung metastasis | Positive | Positive | |||||||
|
| |||||||||
| 2 | Lung primary | Female | 54 | No | Exon 19 | Positive | Negative | IV | Poorly |
| Liver metastasis | Positive | Negative | |||||||
|
| |||||||||
| 3 | Lung primary | Male | 70 | Yes | Exon 19 | Positive | Negative | IV | Well or moderately |
| Lung metastasis | Positive | Negative | |||||||
|
| |||||||||
| 4 | Lung primary | Male | 66 | Yes | Exon 19 | Positive | Negative | IV | Poorly |
| Liver metastasis | Negative | Negative | |||||||
For all 4 patients, the first biopsy site was the primary lung lesion. The re-biopsy site depended on circumstances. For patients 1 and 3, the sample came from an intrapulmonary metastasis resulting from local progression; for patients 2 and 4, the sample came from liver because of distant metastasis.
DISCUSSION
In recent decades, substantial progress has been made in all aspects of lung cancer, including screening, diagnostic evaluation, surgery, radiation therapy, and chemotherapy, as well as in therapies targeting the epidermal growth factor receptor and anaplastic lymphoma kinase, among others. Tyrosine kinase inhibitors are used in patients with EGFR mutations and ALK rearrangements. In addition, immunotherapy has also recently become a major therapeutic modality in nsclc20–22. Despite that progress, 5-year survival in lung adenocarcinoma is less than 12%–15%23.
Epithelial–mesenchymal transition is described as downregulation of the expression of E-cadherin (the epithelial cell marker) and upregulation of the expression of vimentin (the mesenchymal marker)24. Some research has suggested that emt could be an important mechanism of tumour invasion25,26. In the present study, we collected data for 80 patients and analyzed relationships between the clinical characteristics of those patients and the emt-related molecules to identify whether the emt molecules have any special diagnostic value in lung adenocarcinoma. Deletions or insertions in exon 19 and point mutations in exons 18 and 21 in EGFR are common in patients with advanced-stage nsclc and might contribute to metastasis to specific organs27–29. We therefore classified the study patients into groups with and without driver mutations, hypothesizing that emt status might be associated with the driver mutations in lung adenocarcinoma. In addition, we evaluated multiple detections of emt status to assess consistency.
All samples from our patients were evaluated for driver mutation status, and 54% were positive for an EGFR mutation. About 20% of patients with lung adenocarcinoma are reported to have an EGFR mutation; however, a prospective molecular epidemiology study demonstrated that the rate of EGFR mutation rose to more than 60% in non-smoking and Asian populations30, and thus the EGFR mutation results in our study appear reasonable. We can do more to investigate the reasons for those results in future.
The incidence of ALK-positive nsclc has been reported to be approximately 3%–7%, with no significant difference between Eastern and Western populations31,32. In our study, 5 of 80 patients (6%) were positive for ALK rearrangement, which corresponds with those prior reports.
Given our results, we have three main findings. First, positive expression of E-cadherin, a biomarker of epithelial cells, was approximately 79% in our patients, which corresponds to rates published in other reports16,33; however, we observed no associations between E-cadherin and any of the disease characteristics that we collected in our research, including pathologic stage, cell differentiation, and driver gene mutations. A previous report showed that downregulation of E-cadherin was more common in patients with advanced-stage disease than in those with early-stage disease (iii/iv vs. i/ii; odds ratio: 1.87; 95% confidence interval: 1.27 to 2.76; p = 0.002)34, a result that seemed reasonable because E-cadherin has been used as a representative epithelial marker. Positive expression of E-cadherin was lower in tissue from poorly differentiated tumours than in tissue from well and moderately differentiated tumours (p < 0.0001)35. Our study might suggest that, although emt is one of the most important causes of tumour metastasis, other factors could play crucial role in lung adenocarcinoma. Bulk tumour-cell migration has been reported to potentially be more common than emt in lung carcinoma, and tumour cells were found to be able to migrate without changing to the mesenchymal phenotype called “epithelial migration type”36.
Some studies have shown that reduction in E-cadherin expression is related to a decrease in sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor and to EGFR status37,38, results that are not consistent with the present study. No matter the kind of driver mutation, we found little association of such mutations with E-cadherin expression. We believe that it might be too simple to classify the biomarker as either positive or negative; further study into expression levels should be done.
Vimentin is reported to play an important role in emt, being involved in the complex process of tumour metastasis and migration39–41. Although positive expression of vimentin was 57.5% in the present study, no significant relationship of vimentin with clinical factors such as sex, age, smoking history, driver gene mutations, or cell differentiation were observed in the study. But vimentin expression is supposed to be associated with tumour pathologic stage, a result supported by some studies42,43. We noted a higher trend of positive vimentin expression in early-stage disease (p = 0.007), which is contrary to the report by Chikaishi et al.16. But looking at specific tumour stages, we find that vimentin is downregulated and E-cadherin is upregulated in early-stage nsclc. Our study results suggest that a mesenchymal–epithelial transition might take place in the early stages of tumour development. Results from the present study indicate that increased vimentin expression correlates positively with poorly differentiated tumours (odds ratio: 2.133; 95% confidence interval: 1.664 to 2.735; p < 0.001)44, although we observed no particular relevance of that finding. Studies have shown that vimentin is abnormally expressed in tumour cytoplasm14,45, but in the present study, we found that nuclear vimentin was expressed in 27 patients [Figure 1(C)], and Luo et al.46 reported positive expression of vimentin in cell nuclei in nasopharyngeal carcinoma. Thus, we suggest that abnormal localization of vimentin expression might be important in tumour metastasis. Still, it is hard to say that vimentin plays a key role in tumour migration and metastasis; further research is required to elucidate that hypothesis.
Second, to identify any interaction between the expression of E-cadherin and vimentin, we analyzed our results using various combinations of expression types. We observed a significant association between the various biomarker expression types and tumour stage (p = 0.007, Table IV). The various biomarker expression combinations were seen to be different in tumours of various stages, potentially suggesting that interactions between the emt-related proteins might play a key role in tumour progression. Notably, we observed no significant difference in the combination of expression types for patients having driver mutations.
Third, 4 of our 80 patients were evaluated multiple times because of cancer progression. The first biopsy site in all 4 patients was the primary lung lesion. The rebiopsy sites depended on circumstances: in patients 1 and 3, the rebiopsy came from an intrapulmonary metastasis resulting from local progression; in patients 2 and 4, the rebiopsy came from a distant metastasis to the liver. Just 1 patient showed a change in E-cadherin expression—a result that seemed to be reasonable, because emt occurs during tumour metastasis. That finding might suggest that tumour progression was related to emt and that the downregulation of E-cadherin might lead to tumour metastasis. However, we could not draw a conclusion because the evidence was too weak. Although emt happens during tumour progression and metastasis, research has not demonstrated a significant difference in emt characteristics between primary cancers, lymphatic metastases, and cancer emboli47. Hence, results from the analysis of primary and metastatic lesions remain a matter of debate. Further studies of emt-related proteins in primary and metastatic lesions are therefore required.
CONCLUSIONS
Our study showed that clinical characteristics do not have any significant relationship with the expression of E-cadherin in patients with lung adenocarcinoma. However, alteration in vimentin expression was associated with TNM stage in patients with lung adenocarcinoma. Some combination of expression types might be also associated with TNM stage. Although E-cadherin was downregulated in our study, the evidence was too weak to support any definitive conclusions. Further study about primary and metastatic lesions should be done to evaluate expression consistency.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (nos. 81672398 and 81472216), the 5th group of the “333 Talent Project” of Jiangsu Province (level 3), and the 14th group of the “Six Talent Peak Project” in Jiangsu province (YY-068).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hensing T, Chawla A, Batra R, Salgia R. A personalized treatment for lung cancer: molecular pathways, targeted therapies, and genomic characterization. Adv Exp Med Biol. 2014;799:85–117. doi: 10.1007/978-1-4614-8778-4_5. [DOI] [PubMed] [Google Scholar]
- 3.Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11:533–8. doi: 10.1111/j.1440-1843.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 4.Feng J, Wei X, Li C, et al. Mechanism of EGFR over-expression and mutations leading to biological characteristics changes of human lung adenocarcinoma cells through cxcr4/cxcl12 signaling pathway [Chinese] Zhongguo Fei Ai Za Zhi. 2018;21:503–12. doi: 10.3779/j.issn.1009-3419.2018.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan GF, Zhou XF, Zhao JP. Correlation between expression of long non-coding rna zxf1 and prognosis of lung adenocarcinoma and its potential molecular mechanism [Chinese] Zhonghua Zhong Liu Za Zhi. 2017;39:102–8. doi: 10.3760/cma.j.issn.0253-3766.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Zagryazhskaya A, Gyuraszova K, Zhivotovsky B. Cell death in cancer therapy of lung adenocarcinoma. Int J Dev Biol. 2015;59:119–29. doi: 10.1387/ijdb.150044bz. [DOI] [PubMed] [Google Scholar]
- 7.Su CY, Li YS, Han Y, Zhou SJ, Liu ZD. Correlation between expression of cell adhesion molecules CD44 v6 and Ecadherin and lymphatic metastasis in non–small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:2221–4. doi: 10.7314/APJCP.2014.15.5.2221. [DOI] [PubMed] [Google Scholar]
- 8.Margineanu E, Cotrutz CE, Cotrutz C. Correlation between E-cadherin abnormal expressions in different types of cancer and the process of metastasis. Rev Med Chir Soc Med Nat Iasi. 2008;112:432–6. [PubMed] [Google Scholar]
- 9.Fan C, Miao Y, Zhang X, et al. Btbd7 contributes to reduced E-cadherin expression and predicts poor prognosis in non–small cell lung cancer. BMC Cancer. 2014;14:704. doi: 10.1186/1471-2407-14-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buda A, Pignatelli M. E-Cadherin and the cytoskeletal network in colorectal cancer development and metastasis. Cell Commun Adhes. 2011;18:133–43. doi: 10.3109/15419061.2011.636465. [DOI] [PubMed] [Google Scholar]
- 11.Iwanami T, Uramoto H, Nakagawa M, et al. Clinical significance of epithelial–mesenchymal transition–associated markers in malignant pleural mesothelioma. Oncology. 2014;86:109–16. doi: 10.1159/000356874. [DOI] [PubMed] [Google Scholar]
- 12.Dong S, Khoo A, Wei J, et al. Serum starvation regulates E-cadherin upregulation via activation of c-Src in non-small-cell lung cancer A549 cells. Am J Physiol Cell Physiol. 2014;307:C893–9. doi: 10.1152/ajpcell.00132.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CY, Lin HH, Tang MJ, Wang YK. Vimentin contributes to epithelial–mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015;6:15966–83. doi: 10.18632/oncotarget.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell SA, Cherry EM, Bayless KJ. Akt, 14-3-3zeta, and vimentin mediate a drug-resistant invasive phenotype in diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52:849–64. doi: 10.3109/10428194.2010.551793. [DOI] [PubMed] [Google Scholar]
- 16.Chikaishi Y, Uramoto H, Tanaka F. The emt status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res. 2011;31:4451–6. [PubMed] [Google Scholar]
- 17.Ding XM. Micrornas: regulators of cancer metastasis and epithelial–mesenchymal transition (emt) Chin J Cancer. 2014;33:140–7. doi: 10.5732/cjc.013.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurzu S, Silveanu C, Fetyko A, Butiurca V, Kovacs Z, Jung I. Systematic review of the old and new concepts in the epithelial–mesenchymal transition of colorectal cancer. World J Gastroenterol. 2016;22:6764–75. doi: 10.3748/wjg.v22.i30.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engels K, Knauer SK, Metzler D, et al. Dynamic intracellular survivin in oral squamous cell carcinoma: underlying molecular mechanism and potential as an early prognostic marker. J Pathol. 2007;211:532–40. doi: 10.1002/path.2134. [DOI] [PubMed] [Google Scholar]
- 20.Wakelee H, Kelly K, Edelman MJ. 50 Years of progress in the systemic therapy of non–small cell lung cancer. Am Soc Clin Oncol Educ Book. 2014:177–89. doi: 10.14694/EdBook_AM.2014.34.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito S, Espinoza-Mercado F, Liu H, Sata N, Cui X, Soukiasian HJ. Current status of research and treatment for non–small cell lung cancer in never-smoking females. Cancer Biol Ther. 2017;18:359–68. doi: 10.1080/15384047.2017.1323580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non–small cell lung cancer (nsclc) Respirology. 2015;20:370–8. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 23.Myers DJ, Wallen JM. Cancer, Lung Adenocarcinoma. Treasure Island, FL: StatPearls Publishing; 2018. [Available online at: https://www.ncbi.nlm.nih.gov/books/NBK519578/; cited 1 March 2019] [PubMed] [Google Scholar]
- 24.Tian L, Shen D, Li X, et al. Ginsenoside Rg3 inhibits epithelial–mesenchymal transition (emt) and invasion of lung cancer by down-regulating fut4. Oncotarget. 2016;7:1619–32. doi: 10.18632/oncotarget.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Wang J, Kong J, et al. gdf15 promotes emt and metastasis in colorectal cancer. Oncotarget. 2016;7:860–72. doi: 10.18632/oncotarget.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soltermann A. Epithelial–mesenchymal transition in non–small cell lung cancer [German] Pathologe. 2012;33(suppl 2):311–17. doi: 10.1007/s00292-012-1635-3. [DOI] [PubMed] [Google Scholar]
- 27.Gao G, Deng L. Association between EGFR, ALK and KRAS gene status and synchronous distant organ metastasis in non–small cell lung cancer [Chinese] Zhongguo Fei Ai Za Zhi. 2018;21:536–42. doi: 10.3779/j.issn.1009-3419.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suda K, Murakami I, Yu H, et al. CD44 facilitates epithelial to mesenchymal transition phenotypic change at acquisition of resistance to egfr kinase inhibitors in lung cancer. Mol Cancer Ther. 2018;17:2257–65. doi: 10.1158/1535-7163.MCT-17-1279. [DOI] [PubMed] [Google Scholar]
- 29.Sesumi Y, Suda K, Mizuuchi H, et al. Effect of dasatinib on emt-mediated-mechanism of resistance against egfr inhibitors in lung cancer cells. Lung Cancer. 2017;104:85–90. doi: 10.1016/j.lungcan.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (pioneer) J Thorac Oncol. 2014;9:154–62. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Wu H, Zhang M, Ding L, Meng F, Fan X. Expression of the epithelial–mesenchymal transition–related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol. 2013;8:89. doi: 10.1186/1746-1596-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang YL, Chen MW, Xian L. Prognostic and clinicopathological significance of downregulated E-cadherin expression in patients with non–small cell lung cancer (nsclc): a meta-analysis. PLoS One. 2014;9:e99763. doi: 10.1371/journal.pone.0099763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagaki E, Patsouris E, Gonidi M, et al. The value of E-cadherin/beta-catenin expression in imprints of nsclc: relationship with clinicopathological factors. Diagn Cytopathol. 2010;38:419–24. doi: 10.1002/dc.21188. [DOI] [PubMed] [Google Scholar]
- 36.Zacharias M, Brcic L, Eidenhammer S, Popper H. Bulk tumour cell migration in lung carcinomas might be more common than epithelial–mesenchymal transition and be differently regulated. BMC Cancer. 2018;18:717. doi: 10.1186/s12885-018-4640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Wang J, Zeng Y, et al. Implication of epithelial–mesenchymal transition in igf1r-induced resistance to egfr-tkis in advanced non–small cell lung cancer. Oncotarget. 2015;6:44332–45. doi: 10.18632/oncotarget.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suda K, Tomizawa K, Fujii M, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor–mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–61. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 39.Richardson AM, Havel LS, Koyen AE, et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin Cancer Res. 2018;24:420–32. doi: 10.1158/1078-0432.CCR-17-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate filaments play a pivotal role in regulating cell architecture and function. J Biol Chem. 2015;290:17145–53. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol. 2014;50:1–6. doi: 10.1165/rcmb.2013-0314TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dauphin M, Barbe C, Lemaire S, et al. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer. 2013;81:117–22. doi: 10.1016/j.lungcan.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Kim SH, Kim JM, Shin MH, et al. Correlation of epithelial–mesenchymal transition markers with clinicopathologic parameters in adenocarcinomas and squamous cell carcinoma of the lung. Histol Histopathol. 2012;27:581–91. doi: 10.14670/HH-27.581. [DOI] [PubMed] [Google Scholar]
- 44.Ye Z, Zhang X, Luo Y, et al. Prognostic values of vimentin expression and its clinicopathological significance in non–small cell lung cancer: a meta-analysis of observational studies with 4118 cases. PLoS One. 2016;11:e163162. doi: 10.1371/journal.pone.0163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costigliola N, Ding L, Burckhardt CJ, et al. Vimentin fibers orient traction stress. Proc Natl Acad Sci U S A. 2017;114:5195–200. doi: 10.1073/pnas.1614610114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo W, Fang W, Li S, Yao K. Aberrant expression of nuclear vimentin and related epithelial–mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer. 2012;131:1863–73. doi: 10.1002/ijc.27467. [DOI] [PubMed] [Google Scholar]
- 47.He N, Wu GF, Zhao HY, Han HX. Comparison of epithelial–mesenchymal transition–related markers between cancer tissue and tumor emboli [Chinese] Zhonghua Bing Li Xue Za Zhi. 2011;40:758–61. [PubMed] [Google Scholar]