Abstract
Glioblastoma multiforme is one of the most invasive type of glial tumors, which rapidly grows and commonly spreads into nearby brain tissue. It is a devastating brain cancer that often results in death within approximately 12 to 15 months after diagnosis. In this work, optimal control theory was applied to regulate intracellular signaling pathways of miR-451–AMPK–mTOR–cell cycle dynamics via glucose and drug intravenous administration infusions. Glucose level is controlled to activate miR-451 in the up-stream pathway of the model. A potential drug blocking the inhibitory pathway of mTOR by AMPK complex is incorporated to explore regulation of the down-stream pathway to the cell cycle. Both miR-451 and mTOR levels are up-regulated inducing cell proliferation and reducing invasion in the neighboring tissues. Concomitant and alternating glucose and drug infusions are explored under various circumstances to predict best clinical outcomes with least administration costs.
Introduction
Glioblastoma multiforme (GBM) is the most common and the most aggressive type of brain cancer. The median length of survival time is approximately 12 to 15 months following diagnosis. GBM is characterized by anaplasia, nuclear atypia, cellular pleomorphism, mitotic activity, and more importantly, alternating phases of rapid proliferation and aggressive invasion into the surrounding brain tissue. This leads to an inevitably critical recurrence even after the surgical resection of the main tumor mass [1, 2]. The mainstay of treatment for GBM is surgery, followed by radiotherapy and chemotherapy. Despite advances in these approaches, glioma cells can still invade the neighboring tissues beyond detection leading to tumor recurrence. High probability of main treatment failure also encourages researchers to investigate the use of innovative treatments when the first line of therapy has failed, in order to improve clinical outcomes [3].
In the tumor microenvironment (TME), glioma cells encounter many challenges including hypoxia, acidity, and limited nutrient availability. To maintain rapid growth, tumor cells need to adapt to these biochemical changes and modify their metabolic activity by increasing glycolysis even in the presence of oxygen. This process is called the Warburg effect which requires consuming considerable amounts of glucose [4]. The tricarboxylic acid cycle, or Krebs cycle, plays an important role in the breakdown of organic fuel molecules and the survival in non-hypoxic normal differentiated cells. These molecules include glucose, fatty acids, and some amino acids. While differentiated cells favor this type of metabolism, which is very efficient in terms of ATP production, tumor cells adopt the inefficient aerobic glycolysis producing relatively large amounts of waste product in the form of lactic acid [5]. This may provide cancer cells the advantage of not having to depend on oxygen as an energy source especially in a hostile tumor microenvironment, thus leading to longer survival [6]. Inhibition of glycolysis may also prevent drug resistance thus a better understanding of this metabolic pathway may lead to better treatment options and clinical outcomes [7]. Developing strategies of metabolic adaptation, angiogenesis, and migration is critical for cancer cells in order to survive metabolic stress and ensure enough nutrient supply as tumor mass accumulates where glucose supply may fluctuate due to heterogeneous biochemical and biophysical conditions [8]. Therefore, adequate cellular responses to glucose withdrawal are critical for cancer cell survival. Cancer cells then activate the 5′-adenosine monophosphate activated protein kinase (AMPK) pathway under metabolic stress. It is the master cellular sensor of energy availability which enhances glucose uptake and conserve energy, thus avoiding cell death [9]. MicroRNAs, also abbreviated as miRNA, are approximately 22 nucleotide single-stranded non-coding ribonucleic acids (RNAs) that are known to regulate gene expression [10]. Dysregulation of microRNA expression has been linked to oncogenic and tumor suppressor activities in several types of cancer, including GBM [11, 12], where altered miRNA expression contributes to tumorigenesis [13, 14].
Godlewski et al. [8] identified an interesting mechanism of glioma cell migration and proliferation wherein a particular microRNA, miR-451, and its counterpart, AMPK complex (CAB39/ LKB1/STRAD/AMPK), determine whether the cell favors growth at the expense of invasion or conversely. Moreover, they also identified a potential feedback loop between LKB1 and miR-451 allowing for a sustained and robust response to glucose withdrawal [15]. It was found out that (i) under high (normal) glucose conditions, up-regulation of miR-451 leads to the down-regulation of AMPK complex, which then leads to elevated proliferation and decreased migration of glioma cells and (ii) glucose withdrawal induces down-regulation of miR-451 and up-regulation of AMPK, which promotes cell migration with reduced proliferation.
The mathematical models developed by Kim et al. [14, 16, 17] describe the effects of the miR-451–AMPK core control system on cell proliferation and migration in glioblastoma. It explains the response of miR-451 to high and low glucose levels as well as the mutual antagonism between miR-451 and AMPK complex concentrations. Kim et al. [18] then extended the model to include the dynamics of mammalian target of rapamycin (mTOR), which is a protein kinase that links with other proteins as well as to the cell cycle dynamics, to form the miR-451–AMPK–mTOR core control system. This mutual antagonism between miR-451 and AMPK, which was predicted in these mathematical models [14, 16–18], was confirmed by recent experiments. For example, Ansari et al. [19] recently found that (i) the miR-451 transcription in GBM cells is induced by unrestricted activity of its transcription factor OCT1 (official gene symbol POU2F1) in the presence of abundant glucose, resulting in AMPK inhibition through direct targeting of CAB39 in the LKB1 complex; and (ii) the miR-451 level is inhibited through the phosphorylation and inactivation of OCT1 at S335 by AMPK in response to glucose depletion-induced metabolic stress, leading to a reciprocal negative feedback loop between miR-451 and AMPK. In this case, suppression of miR-451 in turn leads to sustained AMPK activities and a robust response to glucose withdrawal in GBM cells. The multiscale mathematical models [14, 16–18] also predict the growth–invasion cycling patterns of glioma cells in response to fluctuating glucose uptake in the tumor microenvironment. The core control system predicts bistability and hysteresis bifurcation when delayed down-regulation of miR-451 activities along certain molecular pathways would induce glioma cells to stay longer in the proliferative phase despite relatively low glucose concentrations, making this mechanism a therapeutic target.
The cell cycle represents an integrated series of events that regulates complex processes including cell proliferation, cell division and DNA replication, regulated by a complex hierarchy of genetic and metabolic networks which involves several transition states of varied lengths and checkpoints [20]. The stages of the cell cycle are as follows: (i) synthesis phase (S), a period where DNA replication occurs; (ii) gap phase 2 (G2), during which proteins required for mitosis are produced; (iii) mitosis phase (M), a period where chromatin condensation, nuclear envelope breakdown (NEBD), chromatid separation, and cytokinesis happens; (iv) gap phase 1 (G1), in which genes necessary for DNA replication are activated and the protein agents of S phase progression are accumulated; and (v) resting phase (G0), a state in which cells can exit the cell cycle and enter a phase of quiescence or relative inactivity [21]. The progression of mammalian cell cycle is tightly regulated by coordinated activation of cyclin-dependent kinases (CDKs) family [22]. The CDKs are positively regulated by cyclins and negatively by CDK inhibitors (CDKIs) such as the proteins p15, p16, p21 and p27. In cancer cells, cyclins are over-expressed while CDKIs are under-expressed which results in the dysregulation of the cell cycle, and promoting uncontrolled cell growth [20]. Tyson and Novak [23, 24] identified that the transition between two stable steady states, G1 and S–G2–M cell-cycle phases, are described using the kinetic relations of the model that is controlled by changes in cell mass.
A standard GBM treatment is surgery followed by chemotherapy and radiotherapy. However, even under best circumstances, the mean survival of this disease is about a year. Poor outcomes of standard care treatments are due to the topographically diffuse nature of the disease [25]. By the time of diagnosis, typical GBM cells may have widely spread throughout the brain tissue [26–28], increasing the potential of recurrence. Thus, annihilation of distant tumor satellites is implausible despite surgically removing all the essential tumor seen on enhanced MRI scan [29]. Knowing the exact margins of a tumor mass in real patients is indeed a daunting task. In this study, it is assumed that a major tumour mass has been surgically removed and that the infiltrative tumor cells are near the surgical site. The objective is to prevent the glioma cells from further diffusing into the surrounding brain tissue. Localization approach will be utilized, that is, glioma cell invasion will be blocked keeping them in a proliferative phase while also attempting to limit excessive growth before a second surgery [17]. Our analytical tool is primarily based on the framework of optimal control theory, which has been successfully used to make informed decisions involving biological models such as optimal treatment strategies in human immunodeficiency virus (HIV) models [30–33], tuberculosis [34–36], and cardiopulmonary resuscitation (CPR) techniques [37, 38]. Optimal control theory is also applied to the miR-451–AMPK core control system to determine the intravenous glucose and/or drug infusion protocols with least possible cost under various circumstances in de los Reyes, et al. [39].
Recently, Kim et al. [40] developed an intracellular signaling pathway model that extends the miR-451–AMPK–mTOR core control system including the cell cycle dynamics. In this work, a potential control problem is formulated in order to maintain high levels of miR-451 and mTOR (low levels of AMPK) inducing cell proliferation prohibiting cell motility and invasion to the neighboring tissues. With glucose levels as a key regulator of miR-451 activity which also activates mTOR in the downstream signaling pathway, glucose intravenous infusion is considered to up-regulate miR-451 and mTOR concentrations above certain threshold values. In addition, a drug suppressing the inhibitory effect of mTOR by the AMPK complex is incorporated. This drug can be administered concomitantly or alternately with glucose as a secondary intravenous infusion. The controls are then given by dose rates of glucose and drug intravenous administrations regulating upstream and downstream signaling pathway, respectively. Solution of the optimal control problem aims to determine infusion protocols with minimal glucose and drug amount, and least administration costs. Hence, glucose and drug levels are regulated to prevent rapid tumour growth, hyperglycemia, and further drug complications. The results propose plausible glucose and drug intravenous infusion controls which indicate the time of administration, frequency, number of administrations, and dosages of glucose and drug per infusion.
In the current study, we will first present the miR-451–AMPK-mTOR–cell cycle intracellular signalling pathway developed by Kim et al. [40]. A drug module is incorporated to up-regulate mTOR activities inducing cell proliferation. Then an optimal control problem is formulated with the goal of activating miR-451 and mTOR levels through glucose and drug intravenous infusions. Two different infusion protocols will be explored, namely, concomitant and alternating glucose and drug intravenous administrations. Optimal solutions for two strategies are presented and results on frequency, dosage per infusion, total glucose and drug amount, and relative cost incurred in the administrations are compared. The conclusion section discusses and summarizes the optimal control results, and provides outlook for future research directions.
Materials and methods
Intracellular signaling dynamics model
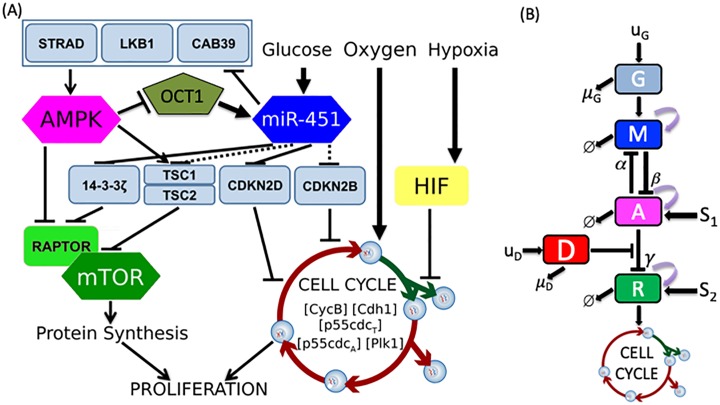
In this section, we present the basic components of intracellular signalling pathway of tumor cell containing the core control miR-451–AMPK–mTOR and cell cycle pathway developed in Kim et al. [40], incorporating a drug which blocks the inhibitory pathway of mTOR by AMPK complex. The core control model identifies a key mechanism which determines the molecular switches between the proliferative and migratory phases in response to fluctuating glucose and drug levels. The simplified signaling pathways consists of five key determinants of the intracellular structure, namely, glucose level G, miR-451 level M, AMPK complex activity A, mTOR concentration R, and drug level D. The intracellular cell cycle dynamics are developed by Tyson and Novak [23, 24] including only the essential interactions for its regulation and control. The model captures the kinetics of chemical processes within the cell such as production, destruction, and different molecule interactions. The transition between two main steady states, G1 and S–G2–M phases, of the cell cycle are described using the kinetic relations of the model that is controlled by changes in cell mass. Powathil et al. [41] modified the model by using equivalent mammalian proteins, namely the Cdk-cyclin B complex [CycB], the APC-Cdh1 complex [Cdh1], the active form of the p55cdc-APC complex [p55cdcA], the total p55cdc-APC complex [p55cdcT], the active form of Plk1 protein [Plk1] and the cell mass [mass]. Also included are the effects of the changes in oxygen dynamics at the macroscopic level through the activation and inactivation of HIF-1 α. This results in changes in cell cycle length. In addition, the cell cycle inhibitory effect of p21 or p27 genes of HIF-1 α is incorporated. Kim et al. [40] proposed to link both the miR-451–AMPK–mTOR control system and the cell cycle dynamics to provide a mechanism driving the cell cycle to undergo the quiescent stage G0-phase depending on the concentration level of mTOR. Fig 1A depicts the detailed schematic diagram of the intracellular signalling networks including miR-451, AMPK complex, mTOR, and key players in the cell cycle module (CycB, Cdh1, p55cdcT, p55cdcA, Plk1). Kinetic interpretation of arrows and hammerheads represent induction and inhibition in the signaling network, respectively. The dimensionless diagram of the core control miR-451, AMPK complex, and mTOR linking to the cell cycle is depicted in Fig 1B. It should be noted that uG and uD are the sources of glucose and drug with decay rates μG and μD, respectively, which can be controlled exogenously. S1 and S2 are signaling sources to AMPK complex and mTOR, respectively, while α, β and γ are inhibition strengths, and ϕ denotes decay.
Fig 1. A model of the miR-451–AMPK–mTOR–cell cycle signaling pathway.
(A) Detailed schematic diagram of cellular decision of proliferation and migration in glioblastoma [40]. (B) Block diagram of the theoretical model representing glucose (G) regulation on miR-451 (M), AMPK (A), mTOR (R) with the signaling pathway to the cell cycle dynamics and the drug (D) suppressing the inhibition of mTOR by AMPK.
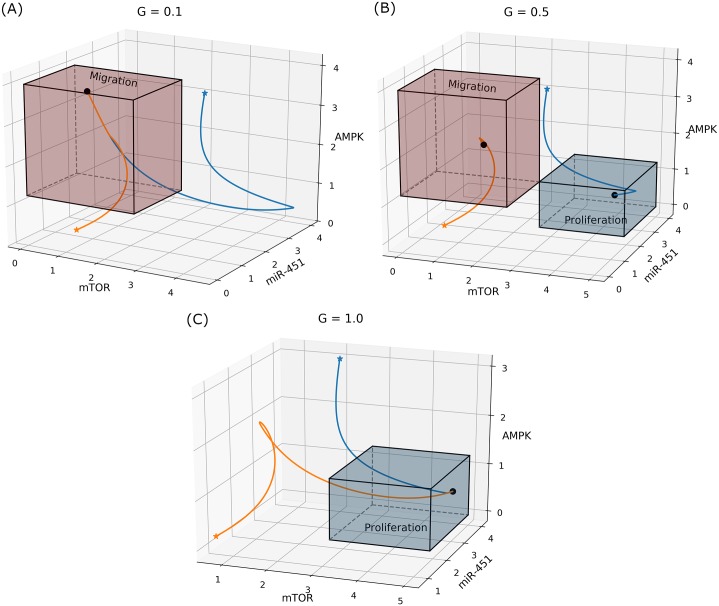
It has been shown that high (normal) glucose concentration yields over expression of miR-451 levels (down-regulation of AMPK complex and up-regulation of mTOR) leading to elevated cell proliferation and reduced migration, while low glucose levels leads to down-regulation of miR-451 activities (up-regulation of AMPK complex and down-regulation of mTOR) reducing cell proliferation and inducing migration into the surrounding brain tissue [8, 15, 18, 40]. The effect of various glucose levels in the regulation of the core control is depicted in Fig 2.
Fig 2. Effect of glucose on regulation of the core control system.
Two trajectories of core control control concentrations in miR-451–AMPK–mTOR space in response to (A) low (G = 0.1), (B) intermediate (G = 0.5), and (C) high (G = 1.0) glucose levels.
Kim et al. [14] developed a core control system of glioma cell migration and proliferation by using a regulatory network of key molecules (miR-451 (M), AMPK (A)) as follows:
| (1) |
As observed in experiments [8, 15, 19], the regulatory system in Eq (1) includes a mutually antagonistic loop between miR-451 and AMPK complex in response to high and low glucose levels (G). This genetic toggle switch induces a monostable and bistable system, characterizing proliferation and critical cell infiltration of GBM cells in brain [14]. In the follow-up studies [14, 16–18] including mTOR (R) and cell cycle modules, this mutual antagonism and bistable system played a critical role in developing anti-invasion strategies. A mutually antagonistic feedback loop has been well-studied for its bistable properties by use of mathematical models ([42–45] and other references in [45]). For instance, in a study on a genetic toggle switch in Escherichia coli [42], a mutually inhibitory loop between repressor 1 and repressor 2 was shown to induce bistability in addition to monostable status. In particular, Lu et al. [45] in a theoretical study on miR-based regulation showed that the regulatory network including a mutual inhibition feedback circuit between the miR-34/SNAIL and the miR-200/ZEB can induce a tristable circuit of epithelial-hybrid-mesenchymal fate differentiation. A detailed analysis on the self-activating, tristable state-inducing toggle switch or more general multistable genetic circuits can be found in [43, 44]. Other biological networks without mutual inhibition can also induce a bistable system. For instance, Aguda et al. [11] investigated the role of miRNAs in regulation of cell cycle and cancer zone by emerging bistable toggle switch in the feedback loops of miR-17-92, E2F, and Myc.
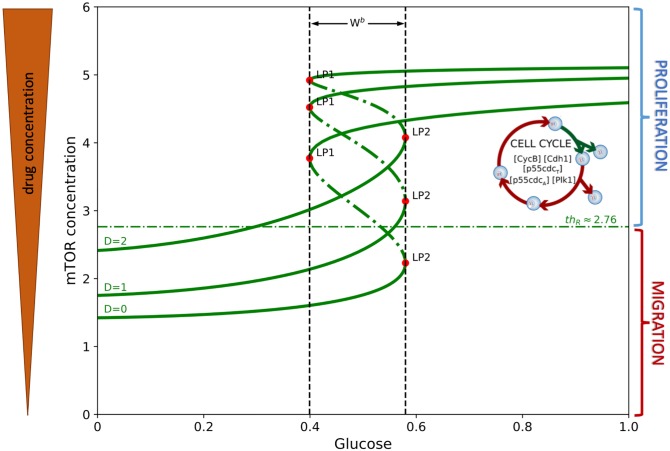
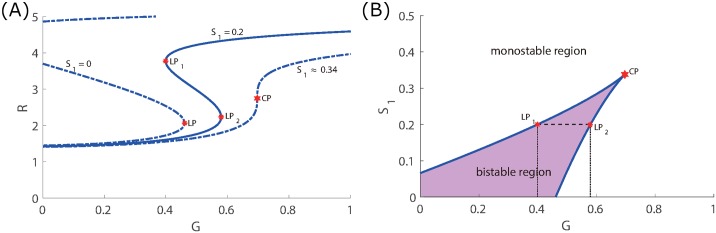
In this study, we consider a drug D suppressing the inhibitory effect of mTOR by AMPK complex where the inhibition strength is given by ζ(D) = e−D. When D is large, γe−D is small which makes dR/dt to be large. Thus, the presence of drug up-regulates mTOR activity that could eventually lead to cell proliferation. Fig 3 illustrates the mTOR bifurcation curve. Observe that increasing drug concentration shifts the hysteresis curve upwards keeping the same bistability window. Hence, with higher drug levels for the same glucose concentrations, mTOR is activated prompting elevated cell growth.
Fig 3. Bifurcation diagram of mTOR.
Hysteresis diagram of mTOR concentration over the range of glucose levels and the corresponding effect of different drug concentrations on its dynamics.
In the miR-451-AMPK-mTOR core control system developed by Kim and colleagues [14, 16–18, 40], the bistability regime of main variables emerges in response to glucose levels. As it was shown in [40], the existence and size of the bistability window (|Wb|) depend on other essential parameters and may disappear under perturbations of parameters. As it will be shown later (Figs 4 and 5), some of key parameters are sensitive in creation or destroying the bistability while other parameters are not. After achieving the equilibrium, continuation of the curve is computed by varying the glucose concentration G and bifurcation points are detected labeled LP and CP for limit and cusp points, respectively. Fig 6A illustrates the hysteresis diagram (G, R)−curves for different S1 values. In order to obtain the cusp point (CP), fold continuation is computed starting at a limit point where two parameters G and S1 are activated resulting to codim 2 bifurcations. Both parameters (G, S1) are varied along the curve where each point is a limit point for the equilibrium curve at the corresponding value of S1. This is depicted in Fig 6B. The cusp point gives the threshold values thM, thA, and thR. A Matlab software MatCont was used for numerical continuation and bifurcation study of continuous and discrete dynamical systems [46].
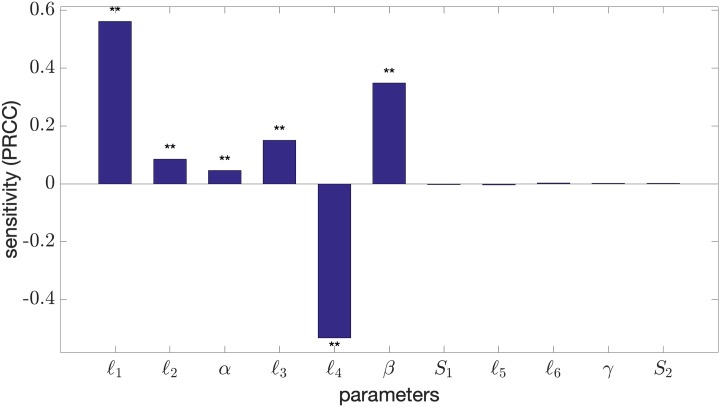
Fig 4. Sensitivity analysis on bistability of core control system.
PRCC values of the core control model parameters influencing the bistability of the (G, R) hysteresis curve. The double asterisk (**) indicates a p-value of less than 0.01. The sample size carried out in the method is N = 100, 000.
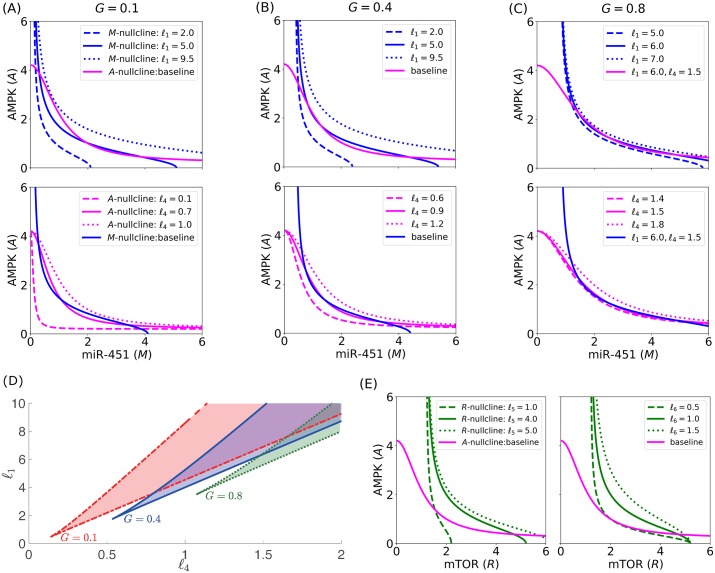
Fig 5. Effect of parameters ℓ1, ℓ4, ℓ5, ℓ6 on the core control nullclines and bistability.
M- and A-nullclines for different ℓ1 and ℓ4 values for specific glucose levels: (A) G = 0.1, (B) G = 0.4, and (C) G = 0.8, showing instances of bistability and monostability. (D) Bifurcation curves for ℓ1 and ℓ4 and shaded region of bistability. (E) R- and A-nullclines for different ℓ5 and ℓ6 values showing monostability.
Fig 6. Codimension 2 bifurcation.
(A) Hysteresis diagram of mTOR concentration over glucose level for three different values of S1 = 0, 0.2, 0.34. (B) Codimension 2 bifurcation varying G and S1 showing the equilibrium curves and cusp point (CP). Bistable and monostable regions are also depicted.
The governing model equations for the dimensionless intracellular signaling dynamics are then described by the following ordinary differential equations
| (2) |
The cell cycle dynamics and the regulatory core control system are linked by a variable called pseudo-mass ([masss]) given by
| (3) |
The oxygen dynamics through HIF-1 α is described as
| (4) |
and the growth rate μ is expressed as
| (5) |
where is the probability density function with uniform distribution between −1 and 1. This growth rate formulation introduces cell cycle heterogeneity of length between 20 and 30 hrs to account for the natural variability between cell growth rates and to have a non-synchronous population [41]. The model parameters in system (2) are listed in Tables 1 and 2.
Table 1. Parameters in the core control (miR-451–AMPK–mTOR) model.
| Par | Description | Value | Ref |
|---|---|---|---|
| μG | glucose consumption rate | 0.5 | [17, 47] |
| ℓ1 | miR-451 autocatalytic production rate | 4.0 | [14, 17] |
| ℓ2 | Hill-type coefficient | 1.0 | [14, 17] |
| α | inhibition strength of miR-451 by AMPK complex | 1.6 | [14, 17] |
| thM | threshold of miR-451 for invasion/growth switch | 1.84 | computed* |
| ℓ3 | AMPK autocatalytic production rate | 4.0 | [14, 17] |
| ℓ4 | Hill-type coefficient | 1.0 | [14, 17] |
| β | inhibition strength of AMPK complex by miR-451 | 1.0 | [14, 17] |
| thA | threshold of AMPK for invasion/growth switch | 1.25 | computed* |
| S1 | signaling source of AMPK | 0.2 | [14, 17] |
| ϵ1 | scaling factor (slow dynamics) of AMPK complex | 0.02 | [11, 14, 17, 48, 49] |
| ℓ5 | AMPK autocatalytic production rate | 4.0 | [14, 17] |
| ℓ6 | Hill-type coefficient | 1.0 | [14, 17] |
| γ | inhibition strength of AMPK complex by miR-451 | 1.0 | [14, 17] |
| thR | threshold of mTOR for invasion/growth switch | 2.76 | computed* |
| S2 | signaling source of mTOR | 1.2 | [14, 17] |
| ϵ2 | scaling factor (slow dynamics) of mTOR | 0.02 | [11, 14, 17, 48, 49] |
| μD | drug decay rate | 1.316 | [41, 50] |
*The values are obtained using Matlab numerical bifurcation toolbox Matcont [46].
Table 2. Parameters in the cell cycle dynamics model.
| Par | Description | Value | Ref |
|---|---|---|---|
| k1 | production rate of [CycB] | 0.12(h−1) | [23, 24] |
| degradation rate of [CycB] | 0.12(h−1) | [23, 24] | |
| degradation rate of [CycB] by [Cdh1] | 4.5(h−1) | [23, 24] | |
| [p27/p21] | inhibitory effect of p21 or p27 genes | 1.05 | [41] |
| K | oxygen concentration threshold | 0.01 | [41] |
| [CycB]th | threshold for cell division | 0.1 | [23, 24] |
| activation rate of [Cdh1] | 3.0(h−1) | [23, 24] | |
| activation rate of [Cdh1] by [p55cdcA] | 30(h−1) | [23, 24] | |
| k4 | inactivation rate of [Cdh1] by [CycB] | 105(h−1) | [23, 24] |
| J3 | Michaelis-Menten activation constant | 0.04 | [23, 24] |
| J4 | Michaelis-Menten inactivation constant | 0.04 | [23, 24] |
| production rate of [p55cdcT] | 0.015(h−1) | [23, 24] | |
| transcription rate of [p55cdcT] by [CycB] | 0.6(h−1) | [23, 24] | |
| k6 | degradation rate of [p55cdcT] | 0.3(h−1) | [23, 24] |
| J5 | dissociation constant of [p55cdcT] | 0.3 | [23, 24] |
| n | Hill coefficient | 4.0 | [23, 24] |
| k7 | activation rate of [p55cdcA] by [Plk1] | 3.0(h−1) | [23, 24] |
| k8 | inactivation rate of [p55cdcA] by [Mad] | 1.5(h−1) | [23, 24] |
| J7 | Michaelis-Menten activation constant | 0.001 | [23, 24] |
| J8 | Michaelis-Menten inactivation constant | 0.001 | [23, 24] |
| [Mad] | spindle checkpoint genes concentration | 1.0 | [23, 24] |
| k9 | activation rate of [Plk1] by [CycB] | 0.3(h−1) | [23, 24] |
| k10 | degradation rate of [Plk1] | 0.06(h−1) | [23, 24] |
| μ+ | specific growth rate | 0.03 | [23, 24] |
| m* | maximum size to which a cell may grow | 10 | [23, 24] |
| ε | cell cycle heterogeneity growth rate parameter | 0.006 | [41] |
| ζ1 | Hill-type parameter in [masss] | 2.5 | [17] |
| n1 | Hill-type parameter in [masss] | 10 | [17] |
| Km | Hill-type parameter in [masss] | 0.5 | [17] |
| ζ2 | Hill-type parameter in [HIF] | 1.0 | [41] |
| n2 | Hill-type parameter in [HIF] | 10.0 | [41] |
| KH | Hill-type parameter in [HIF] | 10.0 | [41] |
| μD | Decay rate of drug | 1.316 | [41, 50] |
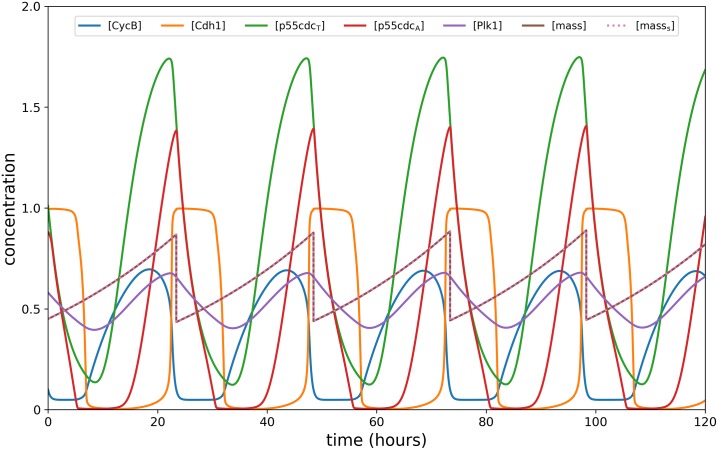
In the current model formulation, mTOR (R) is activated/inactivated in the same way as miR-451 (M). In addition, R links the core control system and the cell cycle dynamics via the pseudo-mass ([masss]) which influences the cell mass [mass] and the intracellular proteins (refer to Eq (3)). Therefore, when glucose supply is high, R is up-regulated (proliferative phase; up-regulated M, down-regulated A) and [masss] ≈ [mass] yielding a typical cell cycle (see Fig 7). On the other hand, when glucose supply is low, R is down-regulated (migratory phase; down-regulated M; up-regulated A) and [masss] exceeds [mass] influencing the cells to enter into resting phase G0 [40].
Fig 7. Typical cell cycle dynamics.
Dynamics of intracellular proteins, mass, and masss of the cell cycle model in response to constant (intermediate) high glucose level.
Let us assume that glucose (G) and drug (D) can be regulated through intravenous infusions and can be periodically administered. For illustrative purposes, consider 3h infusions every 12h with maximum dosage of 1 unit, and refer this as regular infusion. We then have
| (6) |
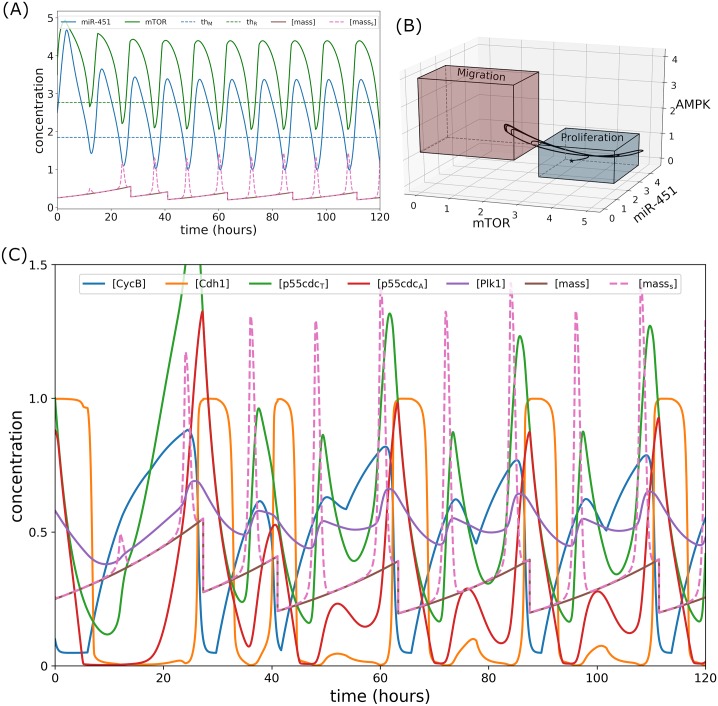
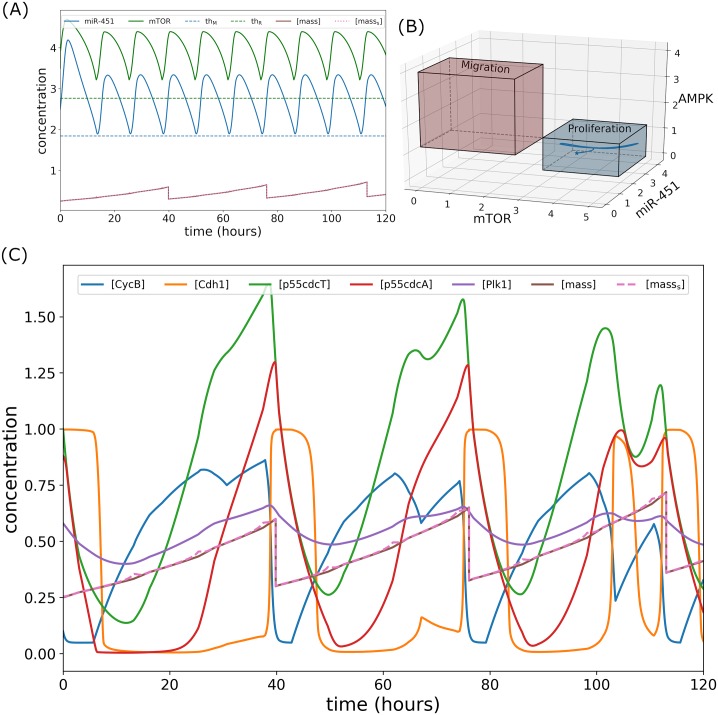
The intracellular dynamics under regular infusion can be seen in Fig 8. Since glucose and drug levels oscillate, it follows that M and R also periodically fluctuates around the threshold (thM ≈ 1.87, thR ≈ 2.76) as can be seen in Fig 8A. Note that when M and R crosses the threshold value from above, respectively, peak in pseudo-mass is generated. It can thus be inferred that these peaks indicate cell migration since M and R levels are below their respective threshold values. As shown in Fig 8B, a trajectory of core control concentrations in mTOR-miR-451–AMPK space switches between proliferation and migration region. A closer look at the intracellular cell cycle proteins, mass, and masss dynamics are illustrated in Fig 8C. Note that regular infusion significantly perturb the cell cycle dynamics stimulating several cell divisions (see mass profiles) and cell migration (see masss). Indeed, fluctuating glucose levels in the microenvironment leads to the dichotomy of grow and go dynamics of glioblastoma cells yielding bigger tumor mass as reported in [14], even in the presence of (fluctuating) drug concentrations to keep the cells in proliferation phase.
Fig 8. Intracellular dynamics under regular glucose and drug infusions.
(A) Concentration profiles of miR-451 (M) and mTOR (R) fluctuating around the threshold values under regular infusions. Peaks in pseudo-mass are generated when M and R crosses thM and thR, respectively. (B) Trajectory of mTOR–miR-451–AMPK concentration profiles switching between proliferation and migration mode. (C) Dynamics of intracellular proteins, mass, and masss of the cell cycle model in response to regular glucose and drug infusions.
Optimal control problem
In the current investigation, we aim to regulate the amount of glucose and drug infusions to up-regulate miR-451 and mTOR above its threshold values inducing cell proliferation avoiding migration to neighboring tissues. The modeling approach utilizes optimal control theory to identify infusion administration protocols. Let uG(t) and uD(t) be the controls of the system representing dose rates of glucose and drug intravenous administrations, respectively. Two different administration protocols are examined, namely, (1) concomitant, and (2) alternating glucose and drug infusions, with the following objective functionals
| (7) |
respectively. Here, M(t) and R(t) denote the level of miR-451 and mTOR concentrations, respectively. Parameters B1 and B2 are weight factors measuring the relative cost based on maximizing miR-451 M(t) and mTOR R(t), and administering glucose and drug intravenous infusions over a specified time interval, respectively. The control costs are modeled by the linear combination of quadratic terms and . Our objective is to find optimal infusion regimen for glucose and drug administrations, denoted by and , such that the objective functionals are satisfied, that is,
| (8) |
where
| (9) |
The bounds for the controls represent the limits on dose rates for glucose and drug administrations. Assuming that miR-451, AMPK, and mTOR responses are regulated by glucose levels and influenced by drug levels, our control strategies deal with finding optimal control regimens for both glucose and drug intravenous infusions. We also note that the existence of optimal controls is guaranteed by standard results in control theory [51]. In this maximization problem, the necessary convexity of the integrand in the objective functional holds. Therefore, we can proceed with applying Pontryagin’s Maximum Principle [52]. An iterative method is used for solving the optimality system which is a two-point boundary value problem having initial conditions for the state variables and terminal conditions for the adjoints. Numerical simulations are obtained using a fourth-order iterative Runge-Kutta method. Given the initial conditions and guess for the controls, state equations are solved using the forward scheme while the corresponding adjoint equations are solved using the backward scheme with the transversality conditions. The controls are updated by using a convex combination of the previous controls and the value from the characterizations. This is commonly referred as the Forward–Backward Sweep Method (FBSM) which is shown to be convergent [53]. Further details on the optimal control under study can be found in the S1 Appendix.
Results and discussion
Sensitivity analysis on bistability of the miR-451-AMPK-mTOR system
In Kim et al. [40] (see Fig S5 in Supplementary Appendix File in [40]), sensitivity analysis of the core control miR-451-AMPK-mTOR model (equations for M, A, R in Eq (2)) was performed in order to determine which parameters have the most/least influence on the reference output (main variables). All the core control parameters were considered to assess their corresponding influence on the miR-451, AMPK, and mTOR activity. It was inferred that miR-451 activity will be enhanced by increasing glucose signal (G) and autocatalytic production rates (ℓ1, ℓ2) of miR-451. These parameters were negatively correlated with AMPK activity due to the mutual antagonistic mechanism between miR-451 and AMPK complex. In addition, AMPK activity will be up-regulated by an increase in signaling source S1 and autocatalytic rates (ℓ3, ℓ4) of the AMPK complex in the model. It has been also noted that increasing S1 and the inhibition strength of mTOR by AMPK (γ) down-regulates mTOR level. In a similar fashion, S2 is strongly positively correlated with mTOR levels but little correlated with G, ℓ1, ℓ2, ℓ3, ℓ4, α, β, ϵ1, ϵ2 at time 100.
In this work sensitivity analysis is carried out to determine which model parameters have consequential effect in achieving or inhibiting bistability in the (G, R)−curve. As in [40], a method from [54] is adapted where a range for each parameter is selected and divided into N intervals of uniform length. For each parameter of interest, a partial rank correlation coefficient (PRCC) value is computed. In order to obtain the PRCC values, Latin Hypercube Sampling (LHS), a stratified sampling without replacement technique, is chosen. The PRCC values range between –1 and 1 with the sign determining whether a change in the parameter value will promote (+) or suppress (−) bistability. The following algorithm is performed:
Assign a probability distribution to each parameter μj and let N be the number of samples to be selected. Divide the interval [μj,min, μj,max] into N equiprobable subintervals, and draw an independent sample from each subinterval.
Assemble the LHS matrix L, wherein each row of L represents a unique combination of parameters sampled without replacement.
For each row of the LHS matrix L, solve for mTOR R in terms of glucose G and check for bistability window Wb. If bistability exists assign 1 to output variable Wb in matrix Y, else assign −1.
Rank-transform the matrices L and Y to obtain LR and YR. By rank-transform, we mean to replace the value by its rank when the data are sorted from lowest to highest, e.g. the smallest value is assigned a rank 1. Tied values are assigned an average rank.
- Fix a parameter μj, which is encoded in the jth column in the matrix LR. Form the following linear regression models using the data matrices LR and YR for μj and y, respectively:
(10)
Compute and , the residuals in the input parameter and the output after removing the linear effects of the other input parameters.(11) - Obtain the PRCC of μj using
(12) Repeat Steps 5 and 6 for the remaining parameters.
Fig 4 shows the sensitivity (PRCC value) of the model parameters in promoting (+) or destroying (−) bistability. It illustrates that a change in the miR-451 autocatalytic production rate (ℓ1) or inhibition strength of AMPK complex by miR-451 (β) enhances bistability. On the contrary, the Hill-type coefficient ℓ4 in the regulation of AMPK is responsible for losing bistability. It should be noted that due to the model’s structure, ℓ1 up-regulates miR-451 which in turn increases mTOR and suppress AMPK activity. The parameter ℓ4 promotes AMPK complex and down-regulates miR-451 and mTOR. However, other parameters ℓ2, α, ℓ3, S1, ℓ5, ℓ6, γ, S2 are little sensitive in emergence or destruction of the bistability.
The bistability of miR-451–AMPK–mTOR core control system depends on the geometric structure of its nullclines. In particular, bistability arises when M- and A-nullclines (i.e., and ) intersect at three distinct points, producing one unstable and two stable steady states. The nullclines intersect three times due to their sigmoidicity influenced by catalytic rates ℓ1 and ℓ4. These rates must be proportionate for a given glucose G level. Otherwise, the nullclines will intersect only once. This bistability condition has been shown for a genetic toggle switch in E. coli [42]. Fig 5A–5C depict the M- and A-nullclines under different G levels with various ℓ1 and ℓ4 values. The bifurcation curves for ℓ1, ℓ4 and region of bistability for specific G levels are depicted in Fig 5D. Under given circumstances, ℓ1 and ℓ4 showed significant sensitivities in the bistability of the system. On the contrary, both ℓ5 and ℓ6 affect the R-nullcline only. It is illustrated in Fig 5E that for several ℓ5 and ℓ6 values, R- and A-nullclines intersect at only one point, leading to a single steady state. In effect, M- and A-nullclines will also intersect once, producing monostability. Hence, ℓ5 and ℓ6 show insignificant consequence in achieving bistability (see Fig 4).
In the following numerical experiments, it is assumed that initially glioma cells are in growth phase (a probable occurrence of post primary tumour surgery) with M > thM, A < thA, and R > thR. It is also considered that glucose and drug can be administered exogenously as intravenous infusions. Further, the weight parameters B1 = 1 and B2 = 1 are used as default values unless specified.
Concomitant infusion control
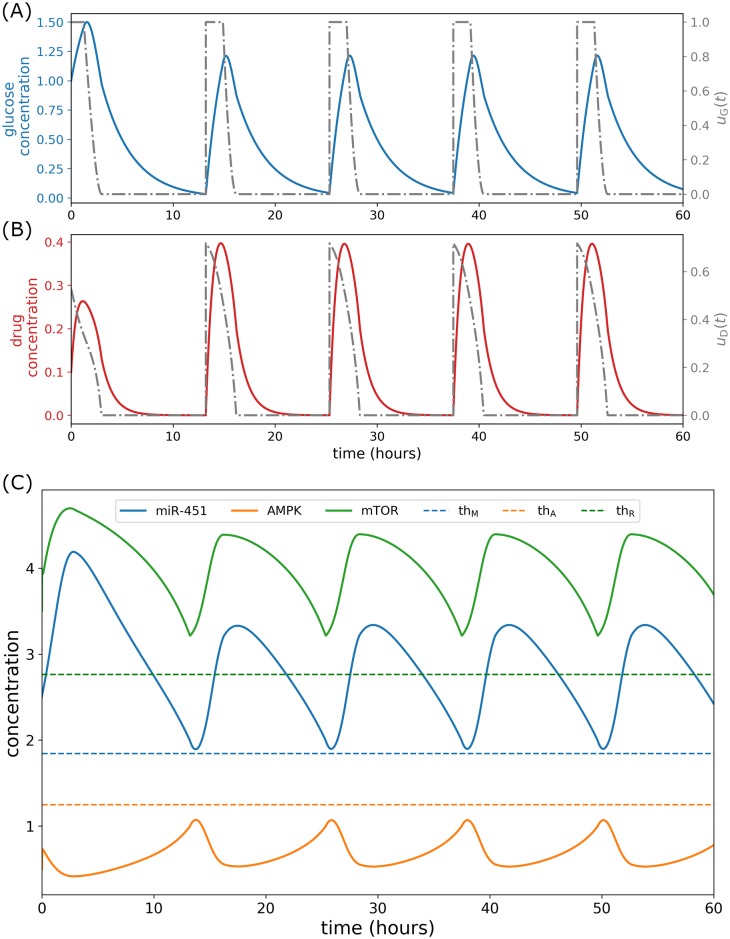
In this strategy, glucose and drug infusions are administered simultaneously, in particular, both controls uG(t) and uD(t) are infused at the same. Thus, this is referred to as concomitant control. Here, it is assumed that the drug in consideration which blocks the inhibitory pathway of mTOR by AMPK complex had negligible side effects and had inconsequential chemical reactions with glucose. In order to determine an efficient strategy of concomitant infusion protocol, glucose and drug are administered concurrently at initial time t = 0 for 3 hours using the numerical scheme FBSM. Infusion spontaneously increases glucose and drug concentrations as depicted in Fig 9(A) and 9(B). Consequently, miR-451 and mTOR levels are up-regulated while AMPK complex is down-regulated as shown in Fig 9C. A closer look at the control curves shows that both uG(t) and uD(t) decrease from 0 < ti < 3 suggesting that both glucose and drug dose rates should be decreased from time ti. This leads to the decrease of glucose and drug concentrations due to consumption and decay. Accordingly, miR-451 and mTOR levels decrease while AMPK complex increases. Before miR-451 crosses the threshold value thM, FBSM is again applied for the next 3 hours. This suggests a time for the next concomitant administrations in which miR-451 profiles are monitored subsequently. The procedures in tracking miR-451 profiles and applying FBSM for 3 hours are repeated over a specified time duration. Thus, the number of concomitant administrations are determined. It is important to note that keeping miR-451 level above its threshold value consequently confine AMPK and mTOR levels, below and above its corresponding threshold values, respectively (see Fig 9C). Fig 10 depicts the intracellular dynamics under concomitant glucose and drug control. In Fig 10A, it can be observed that both miR-451 and mTOR levels are above their threshold values, and [masss] ≈ [mass]. Under concomitant control, the trajectory of mTOR–miR-451–AMPK concentration profiles are restricted only in the proliferation region as shown in Fig 10B. The time courses of cell cycle variables under concomitant control is illustrated in Fig 10C. It can be seen that concomitant control induces fewer (only 3) mass divisions over 120h period as compared to regular infusions with 5 mass divisions, see Fig 8C. It should be noted as well that in concomitant control, mass concentration is increased before division.
Fig 9. Concomitant glucose and drug control.
(A) Glucose control and concentration levels, (B) drug control and concentration levels, and (C) concentration profiles of miR-451, AMPK complex, and mTOR under concomitant control.
Fig 10. Intracellular dynamics under concomitant glucose and drug control.
(A) Concentration profiles of miR-451 (M) and mTOR (R) above the threshold values under concomitant infusions. (B) Trajectory of mTOR–miR-451–AMPK concentration profiles restrained in the proliferation region. (C) Dynamics of intracellular proteins, mass, and masss of the cell cycle model in response to concomitant glucose and drug infusions.
Alternating infusion control
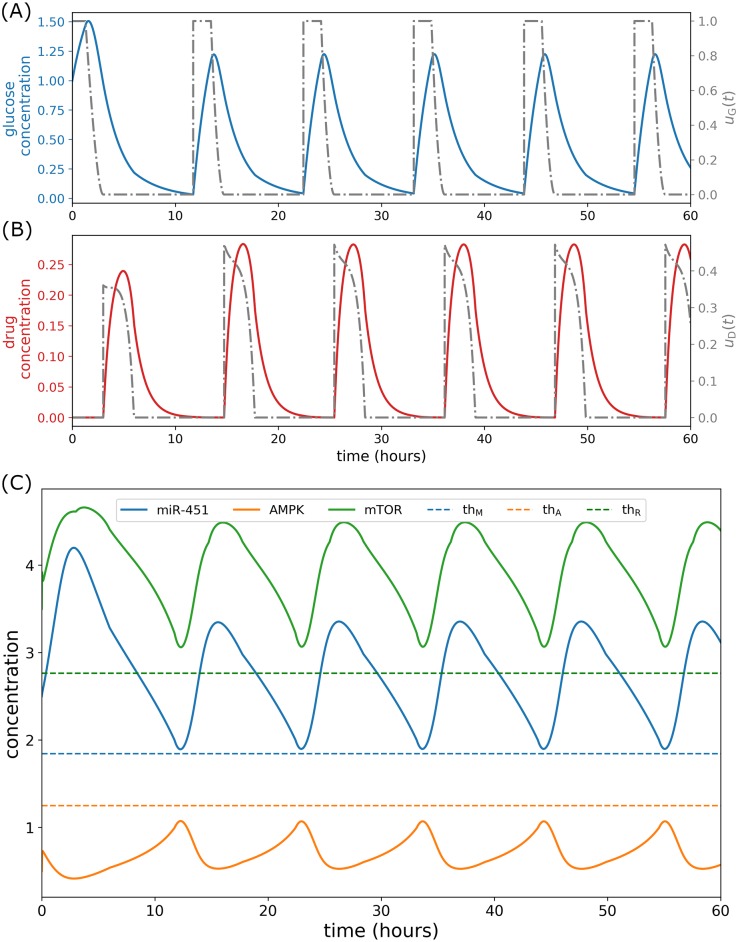
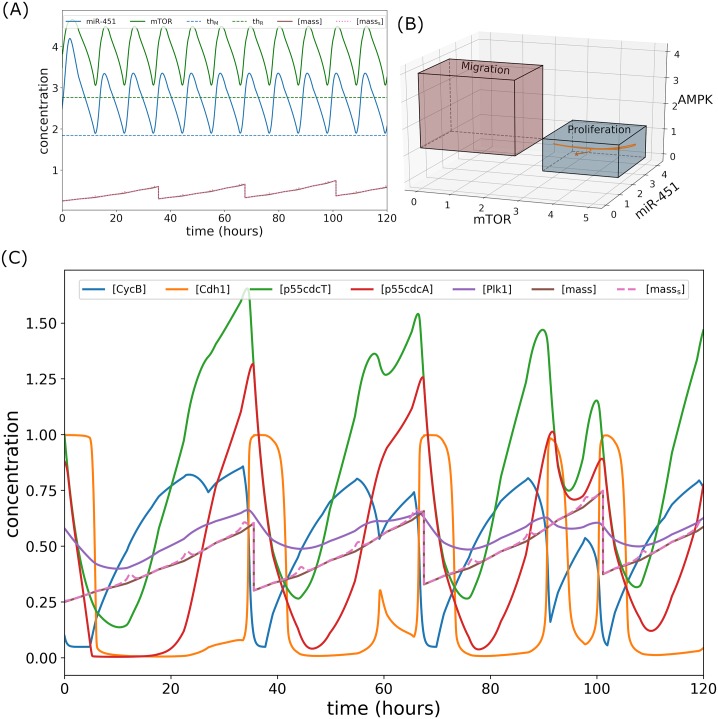
Suppose that concurrent glucose and drug administrations is not plausible due to unwanted chemical reactions. We propose another control strategy that administers glucose uG(t) and drug uD(t) infusions alternately. At initial time t = 0, glucose infusion is obtained by solving the optimal control problem using the numerical scheme FBSM for 3 hours. Next, drug infusion is attained for the next 3 hours in a similar manner. Hence, the controls uG(t) and uD(t) are applied one after the other. Subsequently, miR-451 levels are then tracked and before it crosses the threshold value, glucose infusion uG(t) and drug infusion uD(t) are again administered alternately in a similar fashion above, over the specified duration of administration. Thus, the time for the next alternating infusion is determined by tracking miR-451 levels. This strategy is referred simply as alternating control. This infusion protocol is shown in Fig 11(A) and 11(B). Note that glucose infusion increases miR-451 levels and drug infusion activates mTOR activities keeping AMPK down-regulated as depicted in Fig 11C. The intracellular dynamics under alternating control is illustrated in Fig 12. As shown in Fig 12A, miR-451 and mTOR levels stay above the threshold values, and [masss] ≈ [mass]. Fig 12B depicts that mTOR–miR-451–AMPK trajectory are confined in proliferation region. Fig 12C exhibits the time courses of cell cycle variables under alternating control.
Fig 11. Alternating glucose and drug control.
(A) Glucose control and concentration levels, (B) drug control and concentration levels, and (C) concentration profiles of miR-451, AMPK complex, and mTOR under alternating control.
Fig 12. Intracellular dynamics under alternating glucose and drug control.
(A) Concentration profiles of miR-451 (M) and mTOR (R) above the threshold values under concomitant infusions. (B) Trajectory of mTOR–miR-451–AMPK concentration profiles restrained in the proliferation region. (C) Dynamics of intracellular proteins, mass, and masss of the cell cycle model in response to alternating glucose and drug infusions.
Comparison between control strategies
Both concomitant and alternating control strategies are able to sustain elevated miR-451 and mTOR levels above their threshold values and AMPK levels below its threshold value. It was also shown that mTOR–miR-451–AMPK trajectory is restrained in the proliferation region prohibiting cell migration. Further, number of cell divisions is reduced with slightly higher mass concentration before division compared to regular infusions but lower compared to constant (intermediate) high glucose concentrations. In this section, we compare the cost efficiency of the proposed strategies in terms of frequency of administration, dose per infusion, total glucose and drug amount, relative cost per infusion, and total cost incurred in the administrations. For the following results, the time for simulation duration is considered to be 168 hours (7days).
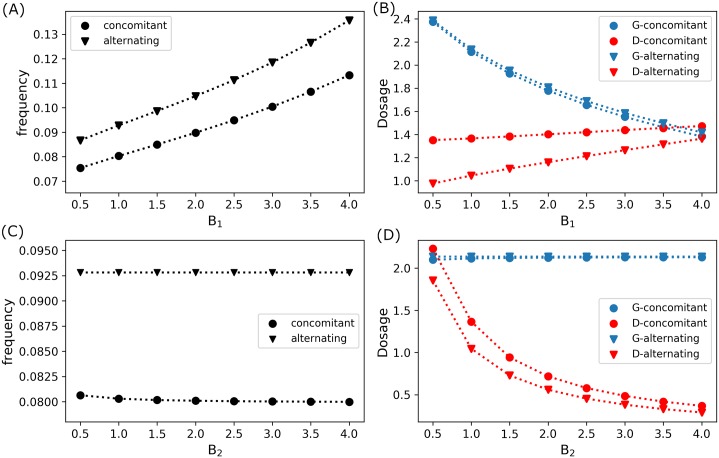
Recall that parameters B1 and B2 are the weight factors associated in our objective functional which represent the measure of costs involved in the administration of glucose uG(t) and drug uD(t) infusions, respectively, which also includes dosage, type, brand, medical fee for administration, etc. Fig 13A shows that as the cost of glucose administration becomes expensive (increasing B1 values) with fixed drug administration cost (B2 = 1.0), frequency of concomitant and alternating infusion increases. However, it should be observed that as frequency of administration increases, the optimal glucose dose per infusion decreases with drug dose per infusion increases, see Fig 13B. Increasing drug dosage compensates the decreasing glucose dosage in order to keep mTOR activities up-regulated leading to cell proliferation. On the contrary, if drug administration cost increases (increasing B2 values) with fixed glucose administration cost (B1 = 1.0), frequency of administration remains almost constant. This is depicted in Fig 13C. The glucose dose per infusion is almost the same but the drug dose per infusion decreases with increasing B2 as illustrated in Fig 13D. Further, note that frequency of concomitant infusion control is always lower than that of alternating infusion control. In addition, drug (glucose) dose per infusion of concomitant control is always more (slightly less) than that of alternating control.
Fig 13. Frequency and dosage of optimal infusions.
(A) Frequency and (B) dose per optimal infusion of concomitant (circle) and alternating (triangle) controls with fixed drug administration cost B2 = 1.0 and varying glucose administration cost B1. (C) Frequency and (D) dose per optimal infusion of concomitant (circle) and alternating (triangle) controls with fixed glucose administration cost B1 = 1.0 and varying drug administration cost B2.
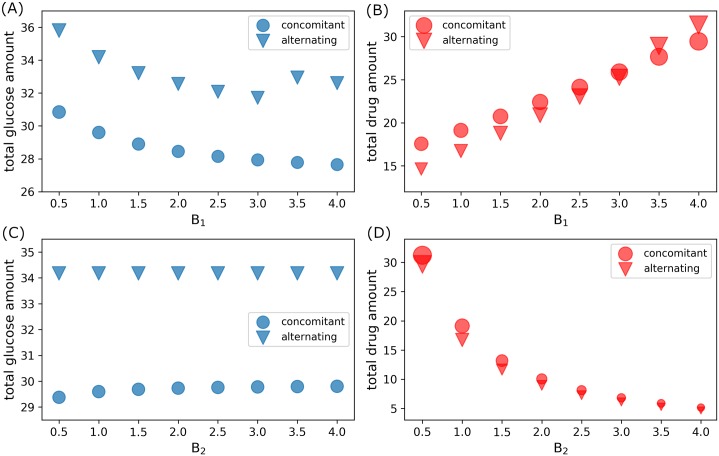
For increasing glucose administration cost (increasing B1) with fixed drug administration cost (B2 = 1.0), total amount of glucose used for both control strategies generally decrease (except for high B1 > 3.0 values), as depicted in Fig 14A, while total amount of drug increases, see Fig 14B. On the other hand, when drug administration becomes expensive (increasing B2 values) with fixed glucose administration cost (B1 = 1.0), the total amount of glucose dosage is almost constant (just slightly increasing for concomitant control) as shown in Fig 14C, but the total drug amount is decreasing as can be seen in Fig 14D. As illustrated in Fig 14, concomitant control use less total amount of glucose than that of alternating control. Contrarily, concomitant administration consumes more total drug amount as compared to that of alternating control (except possibly for high glucose administration cost, B1 > 3.0, see Fig 14B).
Fig 14. Total glucose and drug amount used in concomitant and alternating control infusions.
(A) Total glucose and (B) drug amount used in concomitant (circle) and alternating (triangle) controls with fixed drug administration cost B2 = 1.0 and varying glucose administration cost B1. (C) Total glucose and (D) drug amount used in concomitant (circle) and alternating (triangle) controls with fixed glucose administration cost B1 = 1.0 and varying drug administration cost B2.
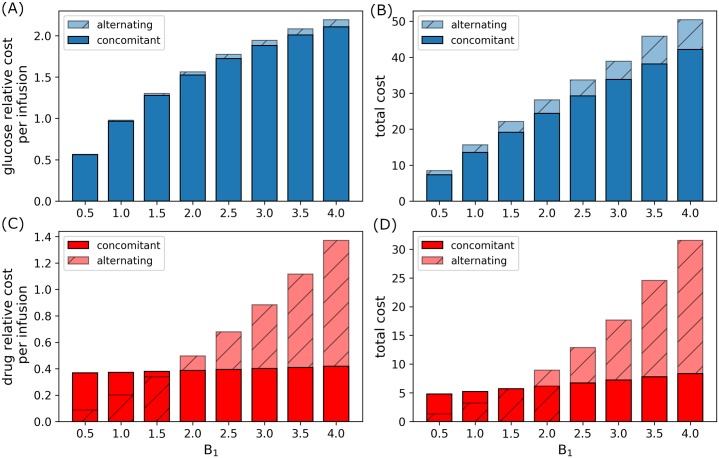
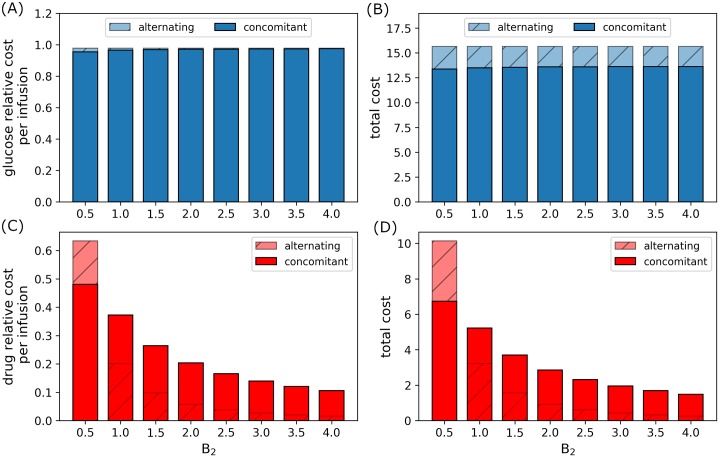
Figs 15 and 16 reflect the relative cost per infusion and total administration cost of glucose and drug infusions incurred under concomitant and alternating controls. With fixed drug administration cost (B2 = 1.0), relative glucose cost per infusion and total administration cost increases as B1 increases. Note that alternating control cost for glucose is always higher than concomitant infusions, see Fig 15(A) and 15(B). The relative and total administration cost for concomitant infusion slightly increase as compared to alternating control as B1 increases. Again, alternating control incur more cost for higher glucose administration cost as illustrated in Fig 15(C) and 15(D). On the other hand, when B1 = 1.0 and drug administration cost (B2) increases, the relative glucose cost per infusion and total administration cost is almost constant. This can be seen in Fig 16(A) and 16(B). Again, relative total cost for alternating control is higher than that of concomitant control. Further, as B2 increases, relative drug cost per infusion and total administration cost decreases (Fig 16(C) and 16(D)), since total drug dosage also decreases as shown in Fig 14D. In this case, it can be seen that drug administration cost for alternating control is generally lower than that of concomitant control.
Fig 15. Relative administration cost for varying B1.
Relative glucose administration (A) cost per infusion and (B) total cost, and relative drug administration (C) cost per infusion and (D) total cost incurred for a period of 168h (7d) for concomitant and alternating controls with increasing B1.
Fig 16. Relative administration cost for varying B2.
Relative glucose administration (A) cost per infusion and (B) total cost, and relative drug administration (C) cost per infusion and (D) total cost incurred for a period of 168h (7d) for concomitant and alternating controls with increasing B2.
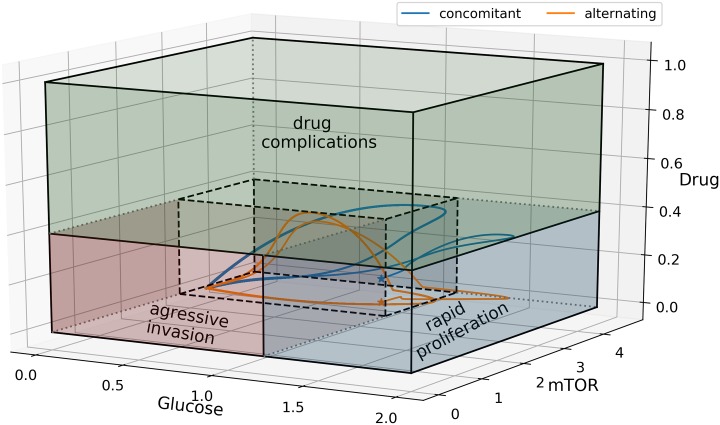
Observe that in Fig 17, both concomitant (blue) and alternating (orange) control trajectories in glucose–mTOR–drug space are restricted in a smaller region avoiding aggressive invasion, rapid proliferation, and unwanted drug complications. Both strategies achieved the goal of keeping mTOR (miR-451) up-regulated inducing cell proliferation and thus avoiding aggressive cell migration. It is important to note that under these proposed optimal control infusions, glucose and drug levels are regulated to prevent excessive cell division and tumor growth. As a consequence, these strategies suggest safer infusion administration preventing hyperglycemia for diabetic patients and risk of drug complications.
Fig 17. Glucose–mTOR–drug dynamics for concomitant and alternating controls.
Concomitant (blue) and alternating (orange) control trajectories are confined in a smaller region avoiding aggressive invasion, rapid proliferation, and unwanted drug complications.
Table 3 provides the average frequency, dosage and relative cost of concomitant and alternating control strategies where B1 = B2 = 1.0 and simulation time is 168h (7d).
Table 3. Summary for concomitant and alternating controls.
| Concomitant | Alternating | ||
|---|---|---|---|
| frequency | glucose infusion | 0.080297 | 0.092806 |
| drug infusion | 0.080297 | 0.092806 | |
| glucose dose | per infusion | 2.114161 | 2.135903 |
| total | 29.598253 | 34.174444 | |
| drug dose | per infusion | 1.365346 | 1.044772 |
| total | 19.114839 | 16.716352 | |
| glucose relative cost | per infusion | 0.964683 | 0.978384 |
| total | 13.505558 | 15.65414 | |
| drug relative cost | per infusion | 0.372771 | 0.201441 |
| total | 19.114839 | 16.716352 |
Conclusion
The periodic switching behavior of glioblastoma cells between proliferation and invasion phases is highly influenced by fluctuating glucose levels [14, 17]. In response to high glucose supply, miR-451 and mTOR are up-regulated and AMPK complex is down-regulated inducing cell growth. On the contrary, low glucose level up-regulates AMPK complex, down-regulating miR-451 and mTOR, promoting cell migration [15]. The mutual antagonistic mechanism between miR-451 (mTOR) and AMPK complex and the cell’s strategic metabolic adaptation support the survival of cancer cells even in a nutrient-deprived microenvironment [14, 55].
In addition to rapid proliferation of glioblastoma cells, aggressive invasion to the surrounding tissue is a major cause of treatment failure. Despite advances in medical imaging technology such as MRI and PET, glioblastoma cells can spread beyond detection leading to tumor recurrence within 2 to 3 cm of the resection cavity even after surgical removal of a malignant glioblastoma [29]. These glioma cells are capable to deform cell membrane and nucleus for cell infiltration through a narrow gap between normal glial cells in brain tissue by upregulation of myosin II along with actin bundles [26, 56]. While exact migratory patterns are still poorly understood, these invasive glioma cells prefer white matter and blood vessels [26, 57] with a wide range of speeds in the range of 5-80 μm/h [58–61] showing sometimes saltatory patterns in the migration direction in brain [58]. Prediction of tumour invasion directions in nearby tissue may help define exact boundaries of focal treatments (surgery or radiosurgery), preventing future growth and recurrence [57]. For example, medical doctors could determine specific locations for radiation target volumes, not just using a rough estimate of 2-3 cm in all directions as a guidance as commonly done today [57].
Assuming that migratory cells are localized near the surgery site [16], one possible approach is to keep the cells in its proliferative phase preventing them from invading brain tissue in a combination with transport of therapeutic drugs near blood vessels [18]. As a result, the tumor mass will be visible for a succeeding surgery while killing proliferative tumor cells at the blood sites.
In this study, we considered the intracellular dynamics of the miR-451–AMPK-mTOR-cell cycle signaling pathway model developed recently by Kim et al. [40]. Incorporated in the model is a drug component which blocks the inhibitory pathway of mTOR by AMPK complex. This drug targets up-regulation of mTOR activities enhancing cell proliferation. The focus of the current work is to regulate up-stream signaling pathway via glucose infusion activating miR-451, and control the down-stream pathway to cell cycle via drug infusion enhancing mTOR activities. Optimal control problem is formulated with the goal of keeping high levels of miR-451 and mTOR to induce cell growth and reduce invasion to the surrounding tissues. In the framework of optimal control theory, two administration strategies are explored to achieve the goal with minimal cost incurred in glucose and drug administrations. The control strategies investigated in this study are (1) concomitant infusion control and, and (2) alternating glucose and drug infusion control. Both strategies are able to switch on the proliferative mode of glioblastoma cells and turn off its migratory mode. Cell cycle is regulated with fewer mass divisions restricting rapid growth. Numerical results show that concomitant control had fewer infusions, lesser glucose dosage and cheaper administration cost. However, when glucose and drug poses unwanted chemical reactions during concurrent administration, alternating control would be beneficial with lower drug amount usage.
The mathematical models of the miR-451-AMPK-mTOR core control [14, 16–18, 40] are based on a ‘go-or-grow’ hypothesis and supporting experiments in GBM cells [8, 15, 19]. However, the range of bistable behavior indicates a ‘go-and-grow’ program which has been proposed for other cancers too [62]. In the glioma cases, ‘go-or-grow’ hypothesis has been long suggested [29, 55, 63–66] in addition to miR-451-induced ‘go-or-grow’ mechanism [8, 15, 19]. Glioma consists of a bulky, proliferative core in the center and highly invasive individual cells in the outer rim [55, 63, 64, 67] and sequential transition between proliferative and invasive phenotypes characterizes tumor progression and may lead to faster growth [14]. At least at the microscopic level, these migration/proliferation phenotypes appear to be mutually exclusive characteristics at different time frames [64], as suggested by in vivo imaging data of glioma cells migrating in a saltatory fashion [58]. Glioma cells was shown to pause for a short period of time (∼an hour) for cell division before the daughter cells begin to move again [58]. Gal et al. [68] further experimentally observed that this phenotypic switch can happen under different extracellular environment: (i) proliferative type in soft agar via activation of Myc signaling pathways in response to hepatocyte growth factor (HGF); and (ii) infiltrative type in Matrigel through Ras signaling pathways in response to the same HGF. Recently, Dhruv et al. [69] also affirmed the role of activation of key molecules in dichotomy between proliferation and invasion: c-Myc and NFκB in the proliferative core and radially dispersed, invasive region of GBM tumors, respectively. Glial cells can also interact with GBM tumor cells for phenotypic switch of GBM cells between cell migration and active proliferation [66]. Although these experiment and mathematical modeling support the ‘go-or-grow’ hypothesis, it is not certain if alternative mechanism such as ‘go-and-grow’ occurs in such a heterogenous TME in real patients [64]. Mansury et al. [70] for instance illustrated that individual glioma cell in a mixed group of proliferative and invasive phenotypes can depend on genotype of counter part and tumor microenvironment. In a similar conceptual studies on epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET) [45] can give a hint on the complexities of this complex regulation of those two phenotypes and glioma in TME might not posses the simple ‘go-or-grow’ dogma. Increasing number of evidences now suggest that the ‘go-or-grow’ model has similar molecular basis with EMT [64]. For instance, HGF and TGFβ are major regulators of EMT, and these also provide strong stimuli of GBM invasion [71, 72] and, upregulation of MET and CD44 activities, as well as an activated NFκB signaling pathway, was reported in both mesenchymal GBM and metastatic cancer [73–76]. All these experimental observations suggest that metastatic epithelial cancers and mesenchymal GBM drive common mechanisms that regulate the phenotypic transition between invasion and proliferation, suggesting the possibility of the ‘go-and-grow’ mechanism. The EMT paradigm in GBM has not been extensively studied as relevant to the progression of the disease due to different origin and rare metastasis of glioma [77, 78]. Further studies and experimentation need to be done for better understanding of the fundamental principles.
In this paper, we did not take into account key microenvironmental factors such as endogenous immune dynamics including NK cells [79] and M1/M2 macrophages [72], other major signaling networks [80, 81] such as E2F and Myc [11, 82], angiogenesis [80, 83], biophysical interaction between tumor cells and blood vessels [80], ECM remodeling for therapy [81, 84–86], or growth factors [87, 88] such as epidermal growth factors [72, 89, 90], fibroblast growth factors [91], transforming growth factor-β [72, 92], and CSF-1 [72, 93, 94], that may play critical roles in proliferation, progress, aggressive invasion of gliomas and development of anti-cancer strategies [95]. For example, endogenous NK cells may interfere oncolytic virus combination therapy in GBM while exogenous NK cells increase anti-tumor efficacy [79], illustrating the complex nature of tumor microenvironment. Recently, mTOR was considered to be a master regulator of cell growth and recognized as a good therapeutic target for therapies in glioblastoma [96]. A recent study found that withaferin A and temozolomide can induce apoptosis and reduce drug resistance by G2/M cell cycle arrest through intrinsic and extrinsic apoptotic signaling pathways [97]. More detailed analysis and experiments on Akt/mTOR/PI3K are necessary. Interestingly, radiation was shown to indirectly promote the export of lactate into the extracellular space and inhibition of AMPK/NFκB signaling pathways were involved in radiation-induced invasion of cancer cells [98]. On the other hand, M2 microglia/macrophages induce matrix remodeling and glioma cell invasion [72, 99–101]. Since PI3K signaling was shown to contribute to M2-polarization of these tumor associated macrophages (TAMs) [102] and PI3K binding to CSF1R was shown to enhance spreading of macrophages, thus promoting glioma cell invasion [103]. Therefore, better understanding of these PI3K-mTOR-CSF1R signaling networks in macrophages would lead to development of blocking aggressive infiltration of cancer cells. Signaling pathways of apoptosis and necroptosis are important parts of oncolytic virus (OVs) therapy [104, 105]. Tumor extracellular matrix (ECM) plays a significant role in regulation of glioma invasion in brain tissue as well as OV spread [106]. For example, CSPGs, one of major tumor ECM in brain can characterize the invasive and non-invasive gliomas in a complex TME where microglia and astrocytes mechanically interact with CSPGs and tumor cells [40, 107–109]. Hybrid models [56, 110–112] and its associated optimal control strategies can be adapted to take into account this important intracellular signaling as well as cell population dynamics and tissue dynamics. Better understanding of various roles of these components in tumor microenvironment may provide better anti-invasion strategies of glioma cells.
However, our mathematical model in this work is a first step toward further experimental/clinical investigation and more optimal anti-invasion strategies of GBM by incorporating these microenvironmental components. We will address these issues in future work.
Supporting information
Formulation of the optimal control problem for concomitant and alternating glucose and drug infusion.
(PDF)
Acknowledgments
This work is resulted from the Konkuk University research support program (E.J.). This paper is also supported by the Korea National Research Foundation (NRF) grant funded by the Korean government (MEST): NRF- 2017R1A2B2004651 (E.J.) and the Basic Science Research Program through National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning: (2018R1A2B6007288) (Y.K.). This work was funded by the University of the Philippines System Enhanced Creative Work and Research Grant (ECWRG 2016-1-030) (A.d.l.R). K.J.A.P. is supported by the University of the Philippines-Office of International Linkages-Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement (UP-OIL-COOPERATE) grant and the Department of Science and Technology-Accelerated Science and Technology Human Resource Development Program-National Science Consortium (DOST-ASTHRDP-NSC) Scholarship.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work is resulted from the Konkuk University research support program (E.J.). This paper is also supported by the Korea National Research Foundation (NRF) grant funded by the Korean government (MEST): NRF- 2017R1A2B2004651 (E.J.) and the Basic Science Research Program through National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning: (2018R1A2B6007288) (Y.K.). This work was funded by the University of the Philippines System Enhanced Creative Work and Research Grant (ECWRG 2016-1-030). K.J.A.P. is supported by the University of the Philippines-Office of International Linkages-Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement (UP-OIL-COOPERATE) grant and the Department of Science and Technology-Accelerated Science and Technology Human Resource Development Program-National Science Consortium (DOST-ASTHRDP-NSC) Scholarship.
References
- 1. Chintala SK, Tonn JC, Rao JS. Matrix metalloproteinases and their biological function in human gliomas. Int J Dev Neurosci. 1999;17(5-6):495–502. 10.1016/S0736-5748(99)00010-6 [DOI] [PubMed] [Google Scholar]
- 2. Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–710. 10.1101/gad.1596707 [DOI] [PubMed] [Google Scholar]
- 3. Davis FG, McCarthy BJ. Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther. 2001;1(3):395–401. 10.1586/14737140.1.3.395 [DOI] [PubMed] [Google Scholar]
- 4. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 5. Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66(18):8927–30. 10.1158/0008-5472.CAN-06-1501 [DOI] [PubMed] [Google Scholar]
- 7. Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65(2):613–21. [PubMed] [Google Scholar]
- 8. Godlewski J, Nowicki MO, Bronisz A, Palatini GNJ, Lay MD, Brocklyn JV, et al. MircroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Molecular Cell. 2010;37:620–632. 10.1016/j.molcel.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–85. 10.1038/nrm2249 [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB. MicroRNA regulation of a cancer network: Consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proceedings of the National Academy of Sciences. 2008;105(50):19678–19683. 10.1073/pnas.0811166106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esquela-Kerscher A, Slack FJ. OncomiRs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69. 10.1038/nrc1840 [DOI] [PubMed] [Google Scholar]
- 13. Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, et al. Targeting of the BMI-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68(22):9125–30. 10.1158/0008-5472.CAN-08-2629 [DOI] [PubMed] [Google Scholar]
- 14. Kim Y, Roh S, Lawler S, Friedman A. miR451 and AMPK Mutual Antagonism in Glioma Cell Migration and Proliferation: A Mathematical Model. PLoS One. 2011;6(12):1–10. 10.1371/journal.pone.0028293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Godlewski J, Bronisz A, Nowicki MO, Chiocca EA, Lawler S. microRNA-451: A conditional switch controlling glioma cell proliferation and migration. Cell Cycle. 2010;9(14):2742–8. 10.4161/cc.9.14.12248 [DOI] [PubMed] [Google Scholar]
- 16. Kim Y. Regulation of Cell Proliferation and Migration in Glioblastoma: New Therapeutic Approach. Frontiers in Oncology. 2013;3:53 10.3389/fonc.2013.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim Y, Roh S. A hybrid model for cell proliferation and migration in glioblastoma. Discrete & Continuous Dynamical Systems—B. 2013;18:969–1015. 10.3934/dcdsb.2013.18.969 [DOI] [Google Scholar]
- 18. Kim Y, Powathil G, Kang H, Trucu D, Kim H, Lawler S, et al. Strategies of Eradicating Glioma Cells: A Multi-Scale Mathematical Model with MiR-451-AMPK-mTOR Control. PLoS One. 2015;10(1):1–30. 10.1371/journal.pone.0114370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ansari KI, Ogawa D, Rooj AK, Lawler SE, Krichevsky AM, Johnson MD, et al. Glucose-based regulation of miR-451/AMPK signaling depends on the OCT1 transcription factor. Cell Rep. 2015;11(6):902–909. 10.1016/j.celrep.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. 10.1200/JCO.2005.01.5594 [DOI] [PubMed] [Google Scholar]
- 21. Douglas RM, Haddad GG. Genetic models in applied physiology: invited review: effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J Appl Physiol. 2003;94:2068–2083. [DOI] [PubMed] [Google Scholar]
- 22. Garrett MD, Fattaey A. CDK inhibition and cancer therapy. Curr Opin Genet Dev. 1999;9:104–111. 10.1016/S0959-437X(99)80015-X [DOI] [PubMed] [Google Scholar]
- 23. Tyson JJ, Novak B. Regulation of the eukaryotic cell cycle: molecular antagonism, hysteresis, and irreversible transitions. J Theor Biol. 2001;210:249–263. 10.1006/jtbi.2001.2293 [DOI] [PubMed] [Google Scholar]
- 24. Novak B, Tyson JJ. Modelling the controls of the eukaryotic cell cycle. Biochem Soc Trans. 2003;31:1526–1529. [DOI] [PubMed] [Google Scholar]
- 25. Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242–4. 10.1073/pnas.97.12.6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfield SS, Canoll P. The role of myosin II in glioma invasion of the brain. Mol Biol Cell. 2008;19:3357–3368. 10.1091/mbc.E08-03-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathologica. 2007;114(5):443–458. 10.1007/s00401-007-0293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wesseling P, Kros JM, Jeuken JWM. The pathological diagnosis of diffuse gliomas: towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagnostic Histopathology. 2011;17(11):486–494. 10.1016/j.mpdhp.2011.08.005 [DOI] [Google Scholar]
- 29. Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–36. 10.1200/JCO.2003.05.063 [DOI] [PubMed] [Google Scholar]
- 30. Joshi HR. Optimal control of an HIV immunology model. Optimal Control Applications and Methods. 2002;23(4):199–213. 10.1002/oca.710 [DOI] [Google Scholar]
- 31. Kwon HD. Optimal treatment strategies derived from a HIV model with drug-resistant mutants. Applied Mathematics and Computation. 2007;188(2):1193–1204. 10.1016/j.amc.2006.10.071 [DOI] [Google Scholar]
- 32. Okosun KO, Makinde OD, Takaidza I. Impact of optimal control on the treatment of HIV/AIDS and screening of unaware infectives. Applied Mathematical Modelling. 2013;37(6):3802–3820. 10.1016/j.apm.2012.08.004 [DOI] [Google Scholar]
- 33. Silva CJ, Torres DFM. Modeling and optimal control of HIV/AIDS prevention through PrEP. Discrete & Continuous Dynamical Systems—S. 2018;11(1):119–141. 10.3934/dcdss.2018008 [DOI] [Google Scholar]
- 34. Jung E, Lenhart S, Feng Z. Optimal control of treatments in a two-strain tuberculosis model. Discrete & Continuous Dynamical Systems—B. 2002;2(4):473–482. 10.3934/dcdsb.2002.2.473 [DOI] [Google Scholar]
- 35. Whang S, Choi S, Jung E. A dynamic model for tuberculosis transmission and optimal treatment strategies in South Korea. Journal of Theoretical Biology. 2011;279(1):120–131. 10.1016/j.jtbi.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 36. Kim S, de los Reyes AA, Jung E. Mathematical model and intervention strategies for mitigating tuberculosis in the Philippines. Journal of Theoretical Biology. 2018;443:100–112. 10.1016/j.jtbi.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 37. Jung E, Babbs CF, Lenhart S, Protopopescu VA. Optimal Strategy for Cardiopulmonary Resuscitation with Continuous Chest Compression. Academic Emergency Medicine. 2006;13(7):715–721. 10.1197/j.aem.2006.03.550 [DOI] [PubMed] [Google Scholar]
- 38. Jung E, Lenhart S, Protopopescu V, Babbs C. Optimal control applied to a thoraco-abdominal CPR model. Mathematical Medicine and Biology: A Journal of the IMA. 2008;25(2):157–170. 10.1093/imammb/dqn009 [DOI] [PubMed] [Google Scholar]
- 39. de los Reyes V AA, Jung E, Kim Y. Optimal control strategies of eradicating invisible glioblastoma cells after conventional surgery. Journal of The Royal Society Interface. 2015;12(106). 10.1098/rsif.2014.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim Y, Kang H, Powathil G, Kim H, Trucu D, Lee W, et al. Role of extracellular matrix and microenvironment in regulation of tumor growth and LAR-mediated invasion in glioblastoma. PLoS One. 2018;13(10):1–40. 10.1371/journal.pone.0204865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powathil GG, Gordon KE, Hill LA, Chaplain MA. Modelling the effects of cell-cycle heterogeneity on the response of a solid tumour to chemotherapy: Biological insights from a hybrid multiscale cellular automaton model. J Theor Biol. 2012;308:1–19. 10.1016/j.jtbi.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 42. Gardner T, Cantor C, Collins J. Construction of a genetic toggle switch in E. coli. Nature. 2000;403:339–342. 10.1038/35002131 [DOI] [PubMed] [Google Scholar]
- 43. Macia J, Widder S, Sole R. Why are cellular switches Boolean? General conditions for multistable genetic circuits. J Theor Biol. 2009;261(1):126–35. 10.1016/j.jtbi.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 44. Jia D, Jolly MK, Harrison W, Boareto M, Ben-Jacob E, Levine H. Operating principles of tristable circuits regulating cellular differentiation. Phys Biol. 2017;14(3):035007 10.1088/1478-3975/aa6f90 [DOI] [PubMed] [Google Scholar]
- 45. Lu M, Jolly MK, Levine H, Onuchic JN, Ben-Jacob E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc Natl Acad Sci USA. 2013;110(45):18144–9. 10.1073/pnas.1318192110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dhooge A, Govaerts W, Kuznetsov YA. MATCONT: A Matlab Package for Numerical Bifurcation Analysis of ODEs. SIGSAM Bull. 2004;38(1):21–22. 10.1145/980175.980184 [DOI] [Google Scholar]
- 47. Gaetano AD, Arino O. Mathematical modelling of the intravenous glucose tolerance test. J Math Biol. 2000;40:136–168. 10.1007/s002850050007 [DOI] [PubMed] [Google Scholar]
- 48. Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273(52):35347–54. 10.1074/jbc.273.52.35347 [DOI] [PubMed] [Google Scholar]
- 49. Gantier MP, McCoy CE, Rusinova I, Saulep D, Wang D, Xu D, et al. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res. 2011;39(13):5692–703. 10.1093/nar/gkr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon K. Mathematical modelling of cell-cycle-dependent chemotherapy drugs-implications for cancer treatment. PhD Thesis, University of Dundee, Dundee. 2006.
- 51. Fleming WH, Rishel RW. Deterministic and Stochastic Optimal Control vol. 1 of Stochastic Modelling and Applied Probability. 1st ed Springer-Verlag; New York; 1975. [Google Scholar]
- 52.Pontryagin LS. Mathematical Theory of Optimal Processes. Classics of Soviet Mathematics. Taylor & Francis; 1987. Available from: https://books.google.co.kr/books?id=kwzq0F4cBVAC.
- 53. McAsey M, Mou L, Han W. Convergence of the forward-backward sweep method in optimal control. Computational Optimization and Applications. 2012;53(1):207–226. 10.1007/s10589-011-9454-7 [DOI] [Google Scholar]
- 54. Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254(1):178–196. 10.1016/j.jtbi.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim Y, Lawler S, Nowicki MO, Chiocca EA, Friedman A. A mathematical model of Brain tumor: pattern formation of glioma cells outside the tumor spheroid core. J Theor Biol. 2009;260:359–371. 10.1016/j.jtbi.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 56. Lee W, Lim S, Kim Y. The role of myosin II in glioma invasion: A mathematical model. PLoS One. 2017;12(2):e0171312 10.1371/journal.pone.0171312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Esmaeili M, Stensjoen AL, Berntsen EM, Solheim O, Reinertsen I. The Direction of Tumour Growth in Glioblastoma Patients. Sci Rep. 2018;8(1):1199 10.1038/s41598-018-19420-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53(8):799–808. 10.1002/glia.20334 [DOI] [PubMed] [Google Scholar]
- 59. Kim HD, Guo TW, Wu AP, Wells A, Gertler FB, Lauffenburger DA. Epidermal growth factor-induced enhancement of glioblastoma cell migration in 3D arises from an intrinsic increase in speed but an extrinsic matrix and proteolysis-dependent increase in persistence. Mol Biol Cell. 2008;19:4249–4259. 10.1091/mbc.E08-05-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sen S, Dong M, Kumar S. Isoform-specific contributions of a-cctinin to glioma cell mechanobiology. PLoS One. 2009;4(12):e8427 10.1371/journal.pone.0008427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaufman LJ, Brangwynne CP, Kasza KE, Filippidi E, Gordon VD, Deisboeck TS, et al. Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys J BioFAST. 2005;89:635–650. 10.1529/biophysj.105.061994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017;11(7):755–769. 10.1002/1878-0261.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stein AM, Demuth T, Mobley D, Berens M, Sander LM. A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys J. 2007;92(1):356–65. 10.1529/biophysj.106.093468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol. 2014;16(12):1575–84. 10.1093/neuonc/nou147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lamszus K, MD, Kathagen A, Holz M, Schulte A, Westphal M. GO OR GROW—LINKS BETWEEN CELLULAR FUNCTION, GLUCOSE METABOLISM AND GLIOMA MICROENVIRONMENT. Neuro Oncol. 2014;16(Suppl 3):iii6 10.1093/neuonc/nou206.19 [DOI] [Google Scholar]
- 66. Oliveira AI, Anjo SI, de Castro JV, Serra SC, Salgado AJ, Manadas B, et al. Crosstalk between glial and glioblastoma cells triggers the go-or-grow phenotype of tumor cells. Cell Commun Signal. 2017;15(1):37 10.1186/s12964-017-0194-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Khain E, Sander LM. Dynamics and pattern formation in invasive tumor growth. Phys Rev Lett. 2006;96(18):188103 10.1103/PhysRevLett.96.188103 [DOI] [PubMed] [Google Scholar]
- 68. Gao CF, Xie Q, Su YL, Koeman J, Khoo SK, Gustafson M, et al. Proliferation and invasion: plasticity in tumor cells. Proc Natl Acad Sci USA. 2005;102(30):10528–10533. 10.1073/pnas.0504367102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dhruv HD, Winslow WSM, Armstrong B, Tuncali S, Eschbacher J, Kislin K, et al. Reciprocal activation of transcription factors underlies the dichotomy between proliferation and invasion of glioma cells. PLoS One. 2013;8(8):e72134 10.1371/journal.pone.0072134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mansury Y, Diggory M, Deisboeck TS. Evolutionary game theory in an agent-based brain tumor model: exploring the Genotype-Phenotype link. J Theor Biol. 2006;238:146–156. 10.1016/j.jtbi.2005.05.027 [DOI] [PubMed] [Google Scholar]
- 71. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews Cancer. 2009;9(4):265–273. 10.1038/nrc2620 [DOI] [PubMed] [Google Scholar]
- 72. Kim Y, Jeon H, Othmer HG. The role of the tumor microenvironment in glioblastoma: A mathematical model. IEEE Trans Biomed Eng. 2017;64(3):519–527. 10.1109/TBME.2016.2637828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res. 2010;70(19):7360–4. 10.1158/0008-5472.CAN-10-1208 [DOI] [PubMed] [Google Scholar]
- 74. Knudsen BS, Woude GV. Showering c-MET-dependent cancers with drugs. Curr Opin Genet Dev. 2008;18(1):87–96. 10.1016/j.gde.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 75. Raychaudhuri B, Han Y, Lu T, Vogelbaum MA. Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drivesinvasive phenotype. J Neurooncol. 2007;85(1):39–47. 10.1007/s11060-007-9390-7 [DOI] [PubMed] [Google Scholar]
- 76. Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108(19):7950–5. 10.1073/pnas.1102454108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Krol I, Castro-Giner F, Maurer M, Gkountela S, Szczerba BM, Scherrer R, et al. Detection of circulating tumour cell clusters in human glioblastoma. Br J Cancer. 2018;119(4):487–491. 10.1038/s41416-018-0186-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goodwin CR, Liang L, Abu-Bonsrah N, Hdeib A, Elder BD, Kosztowski T, et al. Extraneural Glioblastoma Multiforme Vertebral Metastasis. World Neurosurg. 2016;89:578–582. 10.1016/j.wneu.2015.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim Y, Yoo JY, Lee TJ, Liu J, Yu J, Caligiuri MA, et al. Complex role of NK cells in regulation of oncolytic virus-bortezomib therapy. Proc Natl Acad Sci USA. 2018;115(19):4927–4932. 10.1073/pnas.1715295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, et al. A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell. 2018;online:pii: S1535–6108(18)30125–9. 10.1016/j.ccell.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rajesh Y, Biswas A, Mandal M. Glioma progression through the prism of heat shock protein mediated extracellular matrix remodeling and epithelial to mesenchymal transition. Exp Cell Res. 2017;359(2):299–311. 10.1016/j.yexcr.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 82. Aguda BD, Kim Y, Kim HS, Friedman A, Fine H. Qualitative network modeling of the MYC-p53 control system of cell proliferation and differentiation. Biophysical Journal. 2011;101(9):2082–2091. 10.1016/j.bpj.2011.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kobayashi T, Kakizaki I, Nozaka H, Nakamura T. Chondroitin sulfate proteoglycans from salmon nasal cartilage inhibit angiogenesis. Biochem Biophys Rep. 2016;9:72–78. 10.1016/j.bbrep.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koh I, Cha J, Park J, Choi J, Kang SG, Kim P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci Rep. 2018;8(1):4608 10.1038/s41598-018-22681-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee S, Han H, Koo H, Na JH, Yoon HY, Lee KE, et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J Control Release. 2017;263:68–78. 10.1016/j.jconrel.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 86. Rape A, Ananthanarayanan B, Kumar S. Engineering strategies to mimic the glioblastoma microenvironment. Adv Drug Deliv Rev. 2014;79–80:172–83. 10.1016/j.addr.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 2008;99(7):1319–25. 10.1111/j.1349-7006.2008.00840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim J, Kim PH, Kim SW, Yun CO. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials. 2012;33(6):1838–50. 10.1016/j.biomaterials.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Black PC, Agarwal PK, Dinney CP. Targeted therapies in bladder cancer–an update. Urol Oncol. 2007;25(5):433–8. 10.1016/j.urolonc.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 90. Morrison J, Briggs SS, Green N, Fisher K, Subr V, Ulbrich K, et al. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16(2):244–51. 10.1038/sj.mt.6300363 [DOI] [PubMed] [Google Scholar]
- 91. Green NK, Morrison J, Hale S, Briggs SS, Stevenson M, Subr V, et al. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J Gene Med. 2008;10(3):280–9. 10.1002/jgm.1121 [DOI] [PubMed] [Google Scholar]
- 92. Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-beta signaling and its targeting for glioma treatment. Am J Cancer Res. 2015;5(3):945–55. [PMC free article] [PubMed] [Google Scholar]
- 93. Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Molecular medicine. 2012;18(3):519 10.2119/molmed.2011.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72. 10.1038/nm.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Verheije MH, Lamfers ML, Wurdinger T, Grinwis GC, Gerritsen WR, van Beusechem VW, et al. Coronavirus genetically redirected to the epidermal growth factor receptor exhibits effective antitumor activity against a malignant glioblastoma. J Virol. 2009;83(15):7507–16. 10.1128/JVI.00495-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duzgun Z, Eroglu Z, Avci CB. Role of mTOR in glioblastoma. Gene. 2016;575(2 Pt 1):187–90. 10.1016/j.gene.2015.08.060 [DOI] [PubMed] [Google Scholar]
- 97. Grogan PT, Sarkaria JN, Timmermann BN, Cohen MS. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation. Invest New Drugs. 2014;32(4):604–17. 10.1007/s10637-014-0084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liao EC, Hsu YT, Chuah QY, Lee YJ, Hu JY, Huang TC, et al. Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis. 2014;5:e1255 10.1038/cddis.2014.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kettenmann H, Hanisch U, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. 10.1152/physrev.00011.2010 [DOI] [PubMed] [Google Scholar]
- 100. Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol. 2013;2013:285246 10.1155/2013/285246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA. 2009;106:12530–12535. 10.1073/pnas.0804273106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Crespo S, Kind M, Arcaro A. The role of the PI3K/AKT/mTOR pathway in brain tumor metastasis. J Cancer Metastasis Treat. 2016;2:80–9. 10.20517/2394-4722.2015.72 [DOI] [Google Scholar]
- 103. Sampaio NG, Yu W, Cox D, Wyckoff J, Condeelis J, Stanley ER, et al. Phosphorylation of CSF-1R Y721 mediates its association with PI3K to regulate macrophage motility and enhancement of tumor cell invasion. J Cell Sci. 2011;124:2021–31. 10.1242/jcs.075309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yoo JY, Hurwitz BS, Bolyard C, Yu J, Zhang J, Selvendiran K, et al. Bortezomib-Induced Unfolded Protein Response Increases Oncolytic HSV-1 Replication Resulting in Synergistic Antitumor Effects. Clin Cancer Res. 2014;20(14):3787–3798. 10.1158/1078-0432.CCR-14-0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yoo JY, Jaime-Ramirez AC, Bolyard C, Dai H, Nallanagulagari T, Wojton J, et al. Bortezomib treatment sensitizes oncolytic HSV-1 treated tumors to NK cell immunotherapy. Clin Cancer Res. 2016;pii: clincanres.:1003.2016. 10.1158/1078-0432.CCR-16-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim Y, Lee HG, Dmitrieva N, Kim J, Kaur B, Friedman A. Choindroitinase ABC I-mediated enhancement of oncolytic virus spread and anti-tumor efficacy: A mathematical model. PLoS One. 2014;9(7):e102499 10.1371/journal.pone.0102499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Silver DJ, Siebzehnrubl FA, Schildts MJ, Yachnis AT, Smith GM, Smith AA, et al. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. The Journal of Neuroscience. 2013;33(39):15603–15617. 10.1523/JNEUROSCI.3004-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Silver DJ, Silver J. Contributions of chondroitin sulfate proteoglycans to neurodevelopment, injury, and cancer. Curr Opin Neurobiol. 2014;27:171–8. 10.1016/j.conb.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kundu S, Forsberg-Nilsson K. Glycosaminoglycans and Glioma Invasion. Eur Assoc NeuroOncol Mag. 2014;4(2):75–80. [Google Scholar]
- 110. Kim Y, Stolarska M, Othmer HG. A hybrid model for tumor spheroid growth in vitro I: Theoretical development and early results. Math Models Methods in Appl Scis. 2007;17:1773–1798. 10.1142/S0218202507002479 [DOI] [Google Scholar]
- 111. Stolarska M, Kim Y, Othmer HG. Multiscale Models of Cell and Tissue Dynamics. Phil Trans Roy Soc A. 2009;367:3525–3553. 10.1098/rsta.2009.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim Y, Stolarska M, Othmer HG. The role of the microenvironment in tumor growth and invasion. Prog Biophys Mol Biol. 2011;106:353–379. 10.1016/j.pbiomolbio.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Formulation of the optimal control problem for concomitant and alternating glucose and drug infusion.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.