Abstract
The oral mucosa is a minimally invasive and immunologically rich site that has been underutilized for vaccination due to physiological and immunological barriers. To develop effective oral mucosal vaccines, key questions regarding vaccine residence time, uptake, adjuvant formulation, dose, and delivery location must be answered. However, currently available dosage forms are insufficient to address all of these questions. An ideal oral mucosal vaccine delivery system would improve both residence time and epithelial permeation while enabling efficient delivery of physicochemically diverse vaccine formulations. Microneedles have demonstrated these capabilities for dermal vaccine delivery. Additionally, microneedles enable precise control over delivery properties like depth, uniformity, and dosing, making them an ideal tool to study oral mucosal vaccination. Select studies have demonstrated the feasibility of microneedle mediated oral mucosal vaccination, but they have only begun to explore the broad functionality of microneedles. This review describes the physiological and immunological challenges related to oral mucosal vaccine delivery and provides specific examples of how microneedles can be used to address these challenges. It summarizes and compares the few existing oral mucosal microneedle vaccine studies and offers a perspective for the future of the field.
Keywords: mucosal immunization, dosage form, immune tolerance, sustained release
Graphical Abstract
Microneedles possess broad capabilities that could be used to address challenges in oral mucosal vaccine delivery. This review highlights the advantages and challenges of oral mucosal vaccine delivery and the rationale for using microneedles in the oral cavity. Previous studies in the field are limited, but motivate further exploration of microneedle mediated oral mucosal vaccine delivery.
1. Introduction
A vast majority of pathogens infect humans via mucosal surfaces, where local immune responses serve as the first line of defense to prevent infection. Mucosal responses that are particularly key in providing protection include innate immune factors like γT cells, and adaptive immune factors like mucosal IgA antibodies and antigen specific cytotoxic T cells.[1–3] Intraepithelial lymphocytes like γδ T cells perform multiple functions, including maintaining the epithelial barrier and killing infected cells.[4] Secretory IgA antibodies bind pathogens to inhibit their entry into the mucosa, induce phagocytosis through CD89 binding, and activate the complement cascade through the lectin pathway and the alternative pathway.[5] Upon pathogen entry, cytotoxic T cell responses can restrict infection by selectively killing infected cells.[6] Vaccine studies in mice deficient in TLR signaling and IgA antibody secretion have confirmed the importance of both innate and adaptive immunity in protection from infection.[7] Collectively, these local responses can prevent pathogen entry and infection at mucosal sites and are the primary effector mechanisms desired for effective mucosal vaccines.
Evidence strongly suggests that mucosal immune responses are strongest when elicited from mucosal rather than parenteral routes of administration.[1, 8] Studies directly comparing mucosal or parenteral vaccination show that mucosal vaccine delivery generates a higher quantity of mucosal IgA antibodies and a higher frequency of mucosal cytotoxic T lymphocytes (Table 1). Data also suggests that mucosal vaccination generates more sustained mucosal immune responses than those from parenteral vaccination.[9–12] However, parenteral and mucosal administration will likely need to be combined to elicit both robust systemic and mucosal immune responses.[9, 13–15] For example, compared to intramuscular prime and boost alone, sublingual prime with a whole inactivated flu virus combined with an intramuscular boost generated significantly higher hemagglutinin inhibition titers and nasal IgA titers.[13] More research is needed to determine the optimal prime-boost schedule, delivery methods, and vaccine formulations depending on the specific pathogen, but a body of data support the continued development of mucosal vaccines for the prevention of pathogens that are otherwise challenging to combat with parenteral vaccination alone.
Table 1.
Systemic and mucosal responses elicited from parenteral or mucosal vaccines.
| Vaccine type | Routes Tested | Systemic Response | Mucosal Response | Source | |||||
|---|---|---|---|---|---|---|---|---|---|
| Protein (cholera toxin) | IgG | IgA | IgGa) | IgAa) | [17] | ||||
| GI b) | + | + | ++ | ++ | |||||
| IR c) | + | ++ | − | − | |||||
| I vag d) | ++ | ++ | +++ | +++ | |||||
| TD e) | +++ | + | − | − | |||||
| Protein (HPV 16 L1) | IgG | Vaginal IgA | Saliva IgA | [12] | |||||
| IN f) | + | +++ | + | ||||||
| SL g) | +++ | +++ | +++ | ||||||
| I vag | − | − | − | ||||||
| TD | ++ | + | − | ||||||
| IM h) | + | + | − | ||||||
| Adenoviral vector (GagPolSIV, EnvSIV) | CD4 | CD8 | Bronchoalveolar lavage CD4 |
Bronchoalveolar lavage CD8 | [122] | ||||
| IN | + | + | ++ | ++ | |||||
| Aerosol | ++ | − | +++ | +++ | |||||
| IM | +++ | + | + | − | |||||
| Adenoviral vector (Ag85A) | IFN-γ | Lung CD4 | Lung CD8 | [9] | |||||
| IN | − | ++ | ++ | ||||||
| IM | +++ | − | ++ | ||||||
| Adenoviral vector (glycoprotein B of herpes simplex virus) | CTL memory |
Mesenteric lymph node CTL memory |
[123] | ||||||
| IN | + | +++ | |||||||
| IM | +++ | − | |||||||
| Adenoviral vector (spike protein of SARS-CoV) | IgG | CD8 | Bronchoalveolar lavage IgA |
Lung CD8 | [18] | ||||
| SL | ++ | + | ++ | +++ | |||||
| IN | ++ | + | ++ | +++ | |||||
| IM | +++ | +++ | n.d.i) | + | |||||
| Live attenuated (Bacillus Calmette-Guerin) | T cell (CD4, CD8) |
T cell (CD4, CD8) |
[124] | ||||||
| IN | ++ | ++ | |||||||
| TD | ++ | − | |||||||
| Baculovirus nanovector (trivalent human papillomavirus-16L1, −18L1, −58L1) | IgG | CD4 | CD8 | Vaginal IgA | [24] | ||||
| SL | ++ | ++ | +++ | +++ | |||||
| IM | +++ | +++ | +++ | ++ | |||||
| Salmonella vector (glycoprotein D of herpes simplex virus 2) | IgG | CD4 | CD8 | Peyer’s Patch CD8 | Vaginal CD8 | [125] | |||
| GI | + | + | +++ | +++ | + | ||||
| IM | +++ | + | + | − | − | ||||
| Plasmid DNA (tat, gp160, gag) | IgG | IgA | Lung IgA | Lung IgG | GI IgA | GI IgG | [19] | ||
| IN | +++ | +++ | ++ | +++ | + | + | |||
| Buccal | ++ | ++ | +++ | +++ | ++ | − | |||
| Oral gene gun | + | + | − | + | ++ | + | |||
| IM | ++ | ++ | ++ | ++ | + | − | |||
Location of mucosal antibody responses was not specified;
Abbreviations:
gastrointestinal;
intrarectal;
intravaginal;
transdermal;
intranasal;
sublingual;
intramuscular;
not determined.
A promising attribute of mucosal vaccination is that immune responses can be elicited at distal locations from the site of induction (Table 1). Compartmentalization of mucosal immune mechanisms occur in specific patterns and have been reviewed in detail by others, but a brief overview will be given here for context.[1, 8, 16] Following mucosal vaccine administration, antigens are taken up by mucosal tissue resident antigen presenting cells, which then migrate to the draining lymph node through chemokine signaling. During T-cell priming in the lymph node, these antigen presentign cells induce expression of cellular homing receptors such as CCR10 that recognize ligands like CCL28 expressed in the gut, oral mucosa, respiratory tract, and mammary glands. This receptor-ligand recognition and generation of chemokines in these tissues provide cues for effector cells to traffic back to a variety of mucosal surfaces.[8] Because ligands for homing receptors are often expressed in a variety of mucosal tissues, T cells primed by antigen presenting cells from one mucosal tissue can home to other mucosal tissues. This is not true for delivery at all mucosal sites. For example, vaccines delivered intravaginally or rectally often produce effector responses only at that site. In general, there is strong evidence that intranasal, oral mucosal (OM), and gastrointestinal vaccine delivery are able to generate effector responses at distal mucosal sites.[12, 17–19]
Compared to parenteral vaccines, development of effective mucosal vaccines has been slow due to unique physical and immunological barriers present in mucosal compartments. For example, delivery via the gastrointestinal route requires protection of the vaccine components in the harsh gastric enviroment and effective absorption and activation in the gut. To survive the low pH of the stomach, most oral vaccines are live attenuated formulations, which are known to cause some adverse events.[20] The intranasal route carries a risk of severe neurological adverse effects, despite its demonstrated ability to elicit highly effective immune responses.[21] Other mucosal routes like intravaginal and intrarectal vaccination may induce strong immune responses locally in the genital tract, but systemic responses and mucosal immune responses in distal locations are modest. Currently, there are licensed mucosal vaccines for five different pathogens: rotavirus, poliovirus, Salmonella Typhi, Vibrio cholera, and influenza. Most of these vaccines are delivered orally to the gastrointestial associated lymphoid tissue with the exception of the influenza mucosal vaccine, which is delivered intranasally.[22] The currently licensed gastrointestinal vaccines require up to four doses, show variable efficacy, and there are some safety concerns due to their live attenuated formulation.[20] Additionally, while the intranasal flu vaccine is highly effective in children, its efficacy in adults is highly variable for reasons that are not well understood.[23] Development of more effective mucosal vaccines will require a better understanding of both the physiological and immunological barriers specific to different mucosal compartments.
One mucosal site that warrants further study is vaccination to the oral cavity, which includes the buccal and sublingual mucosa and the palatine and lingual tonsils in Waldeyer’s Tonsillar Ring (WTR). These tissues are rich in immune cells, but access to and activation of these cells requires overcoming barriers like saliva, stratified squamous epithelium, and a predisposition for immunological tolerance. Existing dosing strategies to the oral cavity have a limited ability to address both physiological and immunological barriers. For example, while aqueous suspensions allow formulation of many vaccine compositions, they have low oral residence time and are primarily limited to sublingual delivery. Oral dosing strategies that can decouple and overcome these barriers will promote our understanding of oral immunity and enable development of optimized vaccines for oral mucosal delivery. Microneedles have been studied extensively for intradermal vaccine delivery and possess characteristics like tunable geometry, dimension, and materials design to address questions about oral mucosal immunity. Very few studies have applied microneedles to the oral cavity, and there is significant room for innovation in this field. Ultimately, this work could lead to innovative oral mucosal microneedle vaccines and also a better understanding of oral mucosal immunity.
2. The oral mucosa is a promising and underutilized route of vaccine delivery
The oral cavity is an attractive route for mucosal vaccination, but it has been underutilized in previous studies compared to intranasal and gastrointestinal routes. The oral cavity is easily accessible, minimally invasive, contains rich lymphoid tissue, and it has a pH that is more favorable for biologics compared to the gastrointestinal tract. Additionally, evidence suggests that oral mucosal vaccine delivery elicits systemic humoral and cellular immune responses comparable to intramuscular vaccination, and enhances levels of mucosal antibody responses locally and at distal sites such as the nasal cavity and female reproductive tract.[12, 24, 25] However, vaccine delivery to the oral cavity has been limited by a lack of appropriate dosage forms to address physical barriers such as salivary flow and barriers to immune activation such as the predisposition for oral mucosal immune cells to be tolerogenic.
2.1. Immunization in the WTR, buccal, and sublingual mucosa of the oral cavity
The oral mucosa is a particularly promising route for vaccination due to direct access to the rich lymphoid tissue of the Waldeyer’s tonsillar ring (WTR), specifically the palatine and lingual tonsils.[26–28] Like Peyer’s patches in the gut, tonsils are not fully encapsulated by connective tissue, allowing direct antigen uptake. While the outer surface of the tonsils consists of a non-keratinized stratified squamous epithelium, the tonsils contain large crypts lined by a lymphoepithelium (Figure 1a). This non-uniform network of reticulated epithelial cells, antigen presenting cells, and lymphocytes function as transporters for secreted IgG, IgA, and IgM antibodies and provides a direct method of antigen transport.[29] The subepithelial lymphoid tissue consists of B-cell rich secondary lymphoid follicles surrounded by T-cell rich interfollicular regions, and it contains a variety of dendritic cell (DC) subsets capable of inducing immunogenic or tolerogenic responses.[30] Lymphocytes infiltrate the follicular and interfollicular regions via capillaries, high endothelial venules, or through interruptions in the basement membrane.[31]
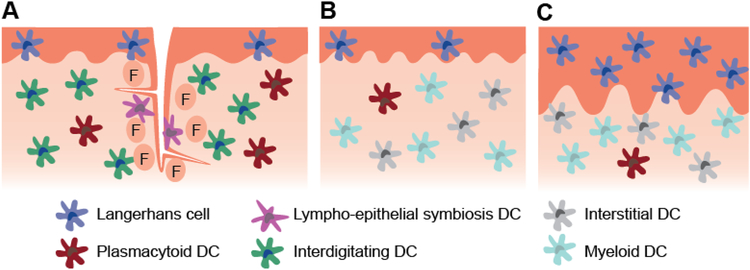
Figure 1.
Schematic representation of the mucosal structure and select dendritic cell subsets present in the a) tonsils of the WTR, b) sublingual mucosa, c) buccal mucosa. The lingual and palatine tonsils are covered with ~100 μm thick stratified squamous epithelium and contain crypts lined by a non-uniform lymphoepithelium. The outer epithelium primarily contains Langerhans cells, while the crypt epithelium contains lympho-epithelial symbiosis DCs along with macrophages and lymphocytes (not depicted). Other DC populations including plasmacytoid DCs and interdigitating DCs densely populate the follicular region (F). Germinal center DCs are also present within germinal centers (not depicted). The sublingual epithelium ranges from 100–200 μm thick, while the buccal epithelium ranges from 500–800 μm thick. The buccal epithelium contains approximately 2.5 times the number of Langerhans cells in the sublingual epithelium. Submucosal DC subsets are not well characterized in humans, but evidence suggests that interstitial and myeloid DCs are present and plasmacytoid DCs may infiltrate during inflammation.
Because of its specialized lymphoid function, the WTR is an attractive site for immunization that may be accessed through intranasal or oral mucosal routes. Previous studies have accessed the WTR primarily through intranasal aerosol vaccine delivery, which has demonstrated potent immune responses.[32, 33] However, adverse neurological effects such as Bell’s palsy may occur due to vaccine entry into the olfactory bulb.[21, 27, 34] While continued studies of intranasal vaccine delivery are identifying new adjuvants and new delivery systems to improve the safety profile of these vaccines,[35] alternative strategies to access the WTR are also warranted. A select number of studies have vaccinated animals by applying the vaccine directly to the palatine or lingual tonsils (Table 2).[15, 36–38] In one study, tonsillar delivery of a vaccinia vector expressing HIV envelope and SIV gag-pol proteins generated neutralizing antibodies and protected primates from SIV challenge when combined with an intramuscular protein boost.[15] Another study delivered an adenoviral vector to the tonsils and observed systemic antigen specific cellular and humoral immune responses that led to a 83-fold reduction in peak viral RNA during challenge compared to intramuscular delivery.[36] Finally, in a study of tonsillar delivery of a live attenuated SIV vaccine, researchers found no significant increases of viral RNA in vaccinated primates, and they found that a tonsillar SIV challenge was controlled locally in the tonsils, primarily by γδ T cells and mature DCs.[38] Therefore, the oral mucosal route has demonstrated success as a relatively simple route for vaccination of the rich WTR lymphoid tissue.
Table 2.
Systemic and mucosal immune responses induced by vaccination at different regions of the oral mucosa
| Delivery location | Vaccine type | Systemic Response | Mucosal Response | Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buccal | Measles virus nucleoprotein | IgG | CD8 | No IgG in lung | [126] | |||||||
| Killed whole cell Streptococcus pneumonae | IL-17A responses |
n.d. a) | [127] | |||||||||
| Synthetic HPV long peptideb) | CD8 | Buccal CD8 | [47] | |||||||||
| Plasmid DNA (HA) b) | CD8 | n.d. | [128] | |||||||||
| Protein (OVA)c) | IgG | Buccal IgA | Lymph node IgA | Peyer’s Patch IgA |
[48] | |||||||
| Plasmid DNA (HIV- tat, gp160, gag) d) | IgG | IgA | Lung IgA | Lung IgG | No GI IgG | [19] | ||||||
| Sublingual | Adenoviral vector (HIV-gag) | CD8 | Submandibular Lymph node CD8 |
[129] | ||||||||
| RSV glycoprotein | IgG | BAL IgA |
CD4 | [130] | ||||||||
| Adenoviral vector (RSV glycoprotein) | IgG | CD8 | BAL IgA |
CD8 | [131] | |||||||
| Protein (HPV 16 L1) | IgG | CD8 | Vaginal IgA |
Saliva IgA |
[12] | |||||||
| Adenoviral vector (HIV-1 Env) | IgG | IgA | Vaginal IgG |
Vaginal IgA |
[132] | |||||||
| Adenoviral vector (spike protein of SARS-CoV) | IgG | CD8 | BAL IgA |
Lung CD8 |
[18] | |||||||
| Tetanus toxoid | IgG | Saliva IgA | Vaginal IgA | Rectal IgA |
[78] | |||||||
| Inactivated influenza | IgG | IgA | CD4 | CD8 | BAL IgG | BAL IgA | Nasal IgA |
Saliva IgA | Rectal IgA |
[7] | ||
| Live influenza | IgG | BAL IgG | BAL IgA | Nasal IgA |
Saliva IgA | Rectal IgA |
[7] | |||||
| Protein (OVA) | IgG | CD8 | Saliva IgG | Saliva IgA | Nasal IgG |
Nasal IgA | BAL IgG |
BAL IgA |
[52] | |||
| WTR | Single cycle immunodeficiency virus vaccine e) | IgG | CD8 | n.d. | [36] | |||||||
| Recombinant modified vaccinia virus Ankara d) | IgG | CD8 | n.d. | [37] | ||||||||
| Attenuated recombinant vaccinia vector (HIV SF162 Env, SIV gag-pol) | IgG | IgA | CD8 | Saliva IgG | Rectal IgG |
[15] | ||||||
not determined;
Vaccine injected in buccal mucosa using hypodermic needle;
Vaccine delivered to buccal mucosa using Microjet technology;
Vaccine administered using Syrijet Mark II needleless injector;
Vaccine administered by oral spray;
bronchoalveolar lavage.
Vaccines have also been delivered to the sublingual and buccal mucosa in the oral cavity, and has been reviewed in full by Kraan et al.[39] Both sites contain a stratified squamous epithelium, but the epithelium of the buccal mucosa is approximately four times thicker than the sublingual mucosa.[40] While both sites lack the organized mucosal lymphoid tissue present in the tonsils, they contain diverse populations of antigen presenting cells (Figure 1b, 1c).[41, 42] Both contain Langerhans cells within the mucosal epithelium, which are the primary antigen sampling cells, although in humans the frequency of Langerhans cells in the buccal mucosa is approximately 2.5-fold greater than the sublingual mucosa.[43] Although not well characterized in humans, there are significant differences between the submucosal DC population of the buccal and sublingual mucosa in mice. For example, the murine buccal submucosa contains a much higher frequency of langerin+CD103+ DCs and langerin-CD103+ DCs compared to the sublingual mucosa. These DCs are capable of priming CD4 and CD8 T cells, making them a potential vaccine target. However, only the sublingual mucosa contains plasmacytoid DCs, another potential vaccine target that are unresponsive to bacterial TLRs, but respond strongly to single stranded RNA through TLR7 and TLR9.[44] Plasmacytoid DCs are able to enter the sublingual submucosa from circulation, produce type 1 interferon, and induce T cell activation in the draining lymph nodes.[45]
Vaccine delivery to the sublingual and buccal mucosa can generate mucosal immune responses at distal sites including the urogenital tract, the respiratory tract, and the intestinal tract.[24, 33, 46] A wide variety of systemic and mucosal immune responses have been induced by vaccine delivery to these sites (Table 2). Despite the differences in immune cell populations between different regions of the oral mucosa, it is challenging to identify patterns in immune outcomes from existing vaccine studies potentially because immune responses are confounded by differences in vaccine bioavailability at different mucosal locations (reviewed further in section 2.3). For example, topical aqueous vaccination in the sublingual mucosa is generally more common and has more reliably generated humoral and cellular mucosal immune responses compared to aqueous formulations in the buccal mucosa (Table 2). Notably, studies that have observed mucosal immune responses after buccal vaccination used specialized delivery systems such as hypodermic needles or various needleless injection systems.[19, 47, 48] Despite these apparent limitations of buccal delivery, existing studies establish a strong rationale for the ability of various oral mucosal locations to induce broad, potent immune responses.
2.2. Oral mucosal vaccination compared to dermal vaccination
Dermal vaccine delivery has many of the same advantages of oral mucosal vaccine delivery such as ease of delivery, patient acceptability, and generation of mucosal and systemic immune reponses. Although some studies have suggested oral mucosal and dermal delivery are analogous, others suggest that immune cell populations in the oral mucosa have different phenotype and function that make it a potentially more effective route of vaccination.[49, 50] For example, one study found that the population of oral mucosal Langerhans cells highly expressing MHC and co-stimulatory molecules was larger than dermal Langerhans cells. In the same study, oral mucosal Langerhans cells induced a stronger mixed lymphocyte response compared to skin Langerhans cells due in part to a lack of secreted suppressive soluble factors.[50] Furthermore, the buccal mucosa contains 37-fold higher levels of T lymphocytes in the epithelium compared to the epithelium of dermal tissue.[49] Immune responses resulting from dermal delivery are sometimes more analogous to parenteral delivery routes like intramuscular delivery, while delivery to the oral mucosa reliably results in immune responses analogous to other mucosal delivery routes (Table 1). Dermal vaccination fits into a unique category as a vaccine delivery route as it shares some properties with mucosal delivery and some with parenteral delivery. However, evidence suggests that oral mucosal vaccine delivery could result in more robust mucosal immune responses.
2.3. Challenges of oral mucosal vaccine delivery
The oral mucosa contains many barriers to vaccine delivery, including the physical mucosal barrier and the tolerogenic immune barrier. Oral tissues are subject to daily salivary flow of one to 1.5L at an unstimulated rate of 0.1 mL/min.[51] This flow can dilute and completely remove vaccines before they can be taken up by antigen presenting cells in the tissue. As a result, most studies using the sublingual route require small volumes (<10 μL) and animal sedation for efficient vaccine uptake.[52] Apart from the physical barrier that saliva presents, it also contains a range of enzymes that may alter the vaccine prior to antigen presenting cell uptake. The salivary flow rate and the composition of saliva can vary with factors like age, gender, diet, and location in the mouth, further complicating oral mucosal delivery.[53, 54] Additionally, the mucosal tissue itself is literally a physical barrier to vaccine delivery. To protect underlying tissues from the mechanical forces of chewing, the oral mucosa is structured as a multilayered stratified squamous epithelium. In regions where masticatory forces are especially strong, like the hard palate and the gingiva, a tough keratinized layer also protects the tissue. The thickness of the human oral epithelium varies greatly depending on specific location, ranging from 500–800 μm in the buccal region and 100–200 μm sublingually.[40] Studies have indicated that large molecules move through these epithelial layers primarily by intercellular transport. However, the intercellular space of the oral epithelium contains lipids and molecules like ceramides and glycosylceramides that limit the intercellular transport of nonpolar molecules.[55] This barrier mechanism limits the depth that large molecules may penetrate into the tissue, meaning that uptake of topically applied vaccines is limited to Langerhans cells located superficially within the epithelium.
In addition to these physical barriers of the oral cavity, the tolerogenic environment presents a significant challenge for oral vaccination. A key goal of mucosal immunization is to induce inflammatory responses that will overcome tolerance and induce protective adaptive immunity without abrogating natural mucosal barriers. In contrast to the systemic immune system, which responds vigorously to pathogens that enter the sterile circulatory system or internal organs, mucosal administration of immunogens in the absence of inflammatory stimuli is widely recognized to induce a state of active unresponsiveness.[41, 56, 57] This tolerance is important to maintain the balance of commensal bacteria in the oral cavity and to prevent uncontrolled immune responses to innocuous ingested food particles. In fact, the predisposition for tolerance has been harnessed to treat allergies through sublingual immunotherapy. Oral mucosal tolerance is not fully understood, but it is thought to be mediated through a variety of different mechanisms that includes activation of DCs by TLR2 or TLR4, antigen uptake by CD206 or ICAM-3-grabbing non-integrin binding, and antigen uptake by CD11b+ CD11c− macrophage-like cells.[41, 58, 59]
Induction of tolerance versus immunogenicity will vary based on the site of delivery in the oral cavity due to differences in antigen presenting cell populations (Figure 1). While Langerhans cells are most often the target of oral mucosal vaccines due to their superficial location and high rates of sampling at the surface of the oral epithelium, these cells are also thought to be the key mediators of regulatory T cell responses and may not be the ideal cell population for vaccine uptake.[41] Oral mucosal Langerhans cells constitutively express the FCεR1 receptor, which is important for immune regulation and homeostasis.[60, 61] Langerhans cells have also been found to migrate slowly after activation and to express low levels of co-stimulatory molecules CD86, CD273, and CD274 compared to other oral mucosal DC populations.[62] Submucosal DCs, which are thought to be more immunogenic than Langerhans cells, vary in frequency and phenotype between regions of the oral mucosa (described in Section 2.1), responding to different pathogen-associated molecular patterns and resulting in different immune responses. Furthermore, submucosal DC populations can vary greatly depending on age, presence of oral pathology, and composition of the oral microbiome. A full description of these complexities is outside the scope of this review but has been reviewed elsewhere.[3, 57, 58, 63] These properties of oral mucosal Langerhans cells and distinct DC populations necessitate careful selection of antigen and adjuvant formulations depending on the dosing location to ensure stimulation of the desired immune response.
In addition to vaccine composition and delivery site, delivery kinetics must be considered as a potential factor in the immunogenicity of oral mucosal vaccines. Evidence strongly suggests that sustained delivery of vaccines via transdermal, subcutaneous, or intra-lymph node injection can enhance humoral immune responses up to 19-fold and cellular immune responses up to 10-fold.[64–69] In these studies, vaccine release is sustained for 4 days for intra-lymph node delivery and up to 4 weeks for intradermal delivery.[65, 68] Furthermore, sustained vaccine delivery has been shown to improve the persistence of immune responses, demonstrating enhanced serum IgG titers compared to boosted bolus vaccines at an 80 day time point.[69] Due to limitations of currently available dosage forms (reviewed in Section 2.4), the effect of sustained vaccine delivery in the oral mucosa on resulting immune responses is not well understood. While the majority of existing data on oral mucosal vaccination have delivered a bolus of vaccine, one study delivered low doses of antigen at narrow time intervals to achieve tolerance.[70] With repeated dosing, DC morphology changed to a more rounded structure and had reduced capacity for migration to the draining lymph node. Interestingly, this effect was only observed in the sublingual mucosa, not in the buccal mucosa. Therefore, while sustained vaccine delivery at other anatomical sites has increased vaccine immunogenicity, more experiments are needed to understand how the interplay of vaccine delivery kinetics and delivery location can affect immune outcomes from vaccine delivery to the oral mucosa.
2.4. Strategies to overcome physical and immunological barriers
Physical barriers may be overcome through the development of dosage forms with high viscosity or mucoadhesion to increase oral mucosal residence time, therefore improving the likelihood of uptake by antigen presenting cells (Figure 2). Existing reviews summarize the use of various dosage forms for oral mucosal delivery.[71] Technologies such as mucoadhesive gels, films, and tablets have been developed that can reduce the effects of salivary washout and allow more time for antigen uptake without dilution (Table 4). These semi-solid and solid dosage forms can theoretically overcome select limitations of aqueous topical delivery including the small dosage volume and need for sedation. However, implementation of these dosage forms is still highly complex. In a study of a thermoresponsive gel for polio vaccination in mice, anesthetization was still necessary to generate any immune responses.[72] Therefore, this gel offers little improvement in comparison with topical aqueous formulations. Tablet formulations have faced similar problems, with one study demonstrating that a fast release tablet offered no advantage over aqueous formulation and an extended release tablet formulation produced very low immune responses.[73] This result is potentially due to an inability to overcome epithelial barriers to transport despite an improvement in residence time. Because these topical dosage forms are unable to breach epithelial barriers, vaccine uptake is likely limited to Langerhans cells. While targeting Langerhans cells has been successful in past studies, this delivery limitation hinders the use of mucoadhesive topical delivery systems to address key questions about oral mucosal immunity and the function of other DC populations residing deeper in the mucosa. An exception to this is films that deliver nanoparticles, which are able to permeate deeper in the mucosa via paracellular transport and eventually enter the lymphatics.[74] However, a lack of control over these trafficking mechanisms still precludes their use as a tool to probe the function of specific oral DC populations.
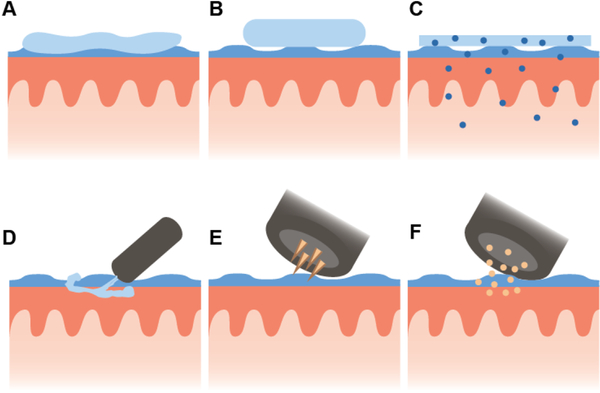
Figure 2.
Schematics of delivery for selected oral mucosal vaccine dosage forms depicting a) a thermo-responsive gel, b) a tablet, c) a film containing nanoparticles, d) MucoJet, e) electroporation, and f) a gene gun.
Table 4.
Features of various oral mucosal vaccine dosage forms.
| Dosage form | Features of Delivery | Sources | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retention Time |
Mucus Penetration |
Vaccine stability |
Specific delivery site |
Specific delivery depth |
Delivery depth versatility |
Controlled release |
Delivery at multiple sites |
Vaccine formulation versatility |
||
| Thermo-responsive gel | + | − | − | + | − | − | − | ++ | ++ | [72] |
| Tablet | ++ | − | ++ | + | − | − | + | ++ | ++ | [73, 134] |
| Nanoparticles | +++ | + | ++ | + | − | − | + | ++ | +++ | [74, 135] |
| MucoJet | +++ | +++ | +++ | +++ | + | − | − | + | ++ | [48] |
| Electroporation | +++ | +++ | +++ | +++ | +++ | − | − | + | + | [10] |
| Gene gun | +++ | +++ | +++ | +++ | +++ | − | − | + | + | [19, 75] |
Alternative technologies for the oral cavity that have also shown promise rely on high velocity or high pressure to increase the permeability of the oral mucosal tissue to enhance uptake (Figure 2). These involve products like MucoJet and technologies like electroporation and gene gun.[10, 48, 75] The MucoJet system is a capsule containing a carbon dioxide-generating propellant. When the capsule is assembled, the propellant contacts water within the capsule, generating carbon dioxide gas and increasing the pressure within the capsule. This increase in pressure creates a high velocity jet of vaccine that can penetrate through the epithelial layer of the mucosa to access antigen presenting cells. The proof of concept studies using this device to deliver the model antigen ovalbumin (OVA) suggest vastly improved systemic IgG and mucosal IgA antibody responses for the MucoJet compared to topical delivery. In contrast to mechanical forces, electroporation has also been used to deliver a 30-volt electric pulse that temporarily creates pores in buccal epithelial cells to permit entry of a DNA vaccine.[10] Electroporation combined with mucosal injection via hypodermic needle resulted in increased mucosal IgA and serum IgG responses compared to mucosal injection alone. While technologies like these can effectively overcome the physical barriers for oral mucosal vaccine delivery, these delivery systems primarily deliver vaccine to the superficial epithelium (Table 4). Therefore, these technologies still are not able to specifically address questions about the role of different immune cells in oral immunity. Additionally, it is unclear how these systems could be adapted to probe questions about vaccine delivery kinetics.
Because of the plethora of mechanisms to induce oral tolerance, oral mucosal vaccines must be carefully designed to overcome this immunological barrier with the proper adjuvants and delivery vectors to instead activate pro-inflammatory pathways. It is important to note that there is conflicting evidence with regards to the proper adjuvants, adjuvant doses, and delivery vectors (Table 3). For example, cholera toxin has been used as an adjuvant to induce both oral tolerance and oral immunogenicity.[52, 76] In these studies, the conformation of the antigen and adjuvant appear to be significant. The study that induced tolerogenic responses delivered OVA conjugated to the cholera toxin B subunit while the study inducing immunity delivered OVA admixed with cholera toxin. However, this structural comparison was not made in either study, and factors such as the OVA dose differed between the two studies, making it difficult to discern precisely what aspect of the vaccine formulation caused the induction of immunity versus tolerance. The mechanism of action of different adjuvants must also be taken into account. Evidence suggests that the ADP-ribosylating activity present in cholera toxin and cholera toxin A subunit, but not cholera toxin B subunit, is the primary mechanism of inducing immunity.[77] However, select studies have used the B unit of cholera toxin in the oral mucosa as an immunogenic adjuvant. Clearly the mechanisms for adjuvant activity of cholera toxin and its subunits are not completely understood, particularly in the context of the oral mucosa. For further detail on adjuvant mechanisms of action, readers are referred to a review by Lycke, 2005.[77] Similar conflicting evidence exists for the use of the heat-labile toxin of Escherichia coli and its mutants as adjuvants for oral mucosal vaccination.[78, 79] Additionally, lipopolysaccharide derivatives have been successfully used in the oral cavity to induce antigen specific immune responses via the TLR4 pathway, despite evidence suggesting the TLR4 pathway stimulates tolerance in oral mucosal Langerhans cells.[60, 80] In fact, another study investigating various adjuvants for oral mucosal delivery of HIV gp140 subunit vaccine and tetanus toxoid vaccine found that sublingually delivered monophosporyl lipid A, a TLR4 agonist, suppressed systemic immunogenicity.[81] Reasons for these seemingly conflicting results are unclear, but differences in vaccine application protocols or dosing regimens could be responsible.
Table 3.
Controversies surrounding the selection and formulation of adjuvants for oral mucosal vaccination.
| Antigen | Adjuvant | Dose | Conformation | Resulta) | Source |
|---|---|---|---|---|---|
| Bacillus subtilis vector (Tetanus toxoid) | mLTb) | 5 μg | Mixed | Less effective than w/o adjuvant | [78] |
| Tetanus toxoid | LTc) | 1 μg | Mixed | Improved immune responses with LT | [79] |
| Formalin inactivated influenza | mCTA-LTBd) | 5 μg | Mixed | Improved CD4 and CD8 T cell responses, increased antibody secreting cells in spleen | [7] |
| RSV glycoprotein | CTe) | 2 μg | Mixed | No adjuvant effect of CT | [130] |
| OVA | CT | 2 μg | Mixed | CT increased mucosal antibody responses, systemic Th1 and Th2 cytokine responses, systemic CD8 | [52] |
| HPV 16 L1 protein | CTBf) | 10 μg | Mixed | CTB improved mucosal and systemic antibody responses, increased CD4 T cell cytokine secretion | [12] |
| Pol463–495 peptide | CTB, CT | 1 μg, 25 μg CTB-pol | Conjugated and mixed | MIR with CTB-pol mixed with CT, but not CTB-pol or Pol mixed with CT | [133] |
| OVA | CTB | 60 μg CTB-OVA | Conjugated | Depletion of OVA-specific T effector cells, induction of regulatory T cells | [76] |
| HA split vaccine | LPSpag) | 1 μg | Mixed | Significant increase in serum IgG and nasal IgA with adjuvant | [80] |
| HIV gp140, Tetanus toxoid | MPLAh) | 20 μg | Mixed | Dampened serum and mucosal IgA responses compared to unvaccinated controls | [81] |
All data in this table are from aqueous sublingual delivery;
Mutant heat-labile enterotoxin from Escherichia coli;
heat-labile enterotoxin from Escherichia coli;
mutant CT A subunit conjugated to LT B subunit;
heat-labile enterotoxin from Vibrio cholerae;
B subunit of CT;
lipopolysaccharide from Pantoea agglomerans;
monophosphoryl lipid A.
Taken together, analysis of previous studies focused on developing new dosage forms and evaluating various adjuvants for oral mucosal delivery reveals key gaps and questions, relating mainly to dosing practicalities, dosing location, function of oral DC populations in immunity, and vaccine delivery kinetics. We propose that innovative microneedle designs could be used as dosage forms for oral mucosal delivery to address these outstanding questions.
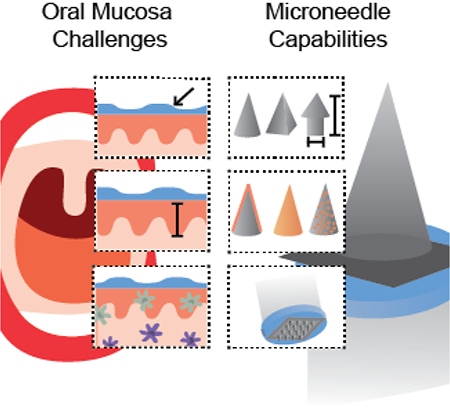
3. Microneedles have broad capabilities that could improve oral mucosal vaccine delivery
Given the limitations of currently available dosage forms for vaccine delivery to the oral cavity and the large number of questions surrounding oral immunity, there is a strong rationale for developing more effective delivery technologies. An ideal vaccine delivery system for the oral cavity would enable the delivery of physicochemically diverse vaccine compositions (e.g., antigen and adjuvant) while also possessing design attributes that would overcome local physical barriers (e.g., residence time, penetration depth) (Table 4). Decoupling of these properties would allow specific determination of the role that different vaccine compositions delivered to different sites and immune targets cells would have on the resulting immune response. Microneedles have been widely utilized as vaccine delivery systems, primarily intradermally,[65, 82–88] and have broad functionality that makes them an ideal strategy to overcome numerous challenges in oral mucosal delivery (Figure 3). Microneedles can be fabricated from a variety of materials into different geometries and different sizes to reach specific depths in tissue. They have also been used to deliver a broad range of antigen and adjuvant combinations. Although specific studies of microneedle mediated vaccine delivery to the oral mucosa are limited, several recent studies suggest that microneedles could overcome the limitations of previous dosage forms to improve our understanding of oral mucosal immunity and ultimately improve vaccine delivery to the oral cavity.[89–91]
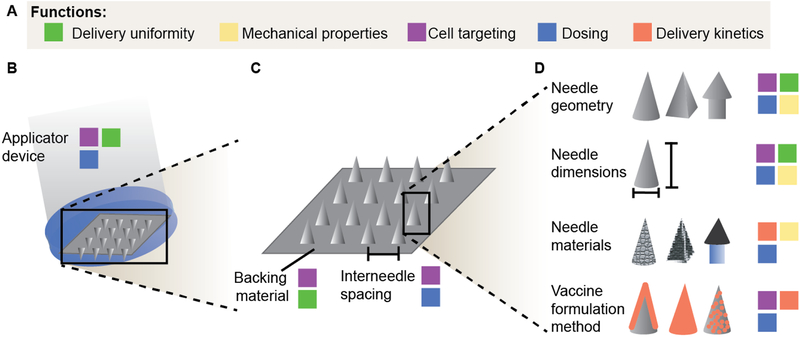
Figure 3.
Schematic of the microneedle design space and its potential to control various functions related to oral mucosal vaccine delivery. a) Relevant functions include: delivery uniformity, or equal tissue puncture by all needles in an array and equal delivery of vaccine components from each needle; mechanical properties, including needle strength and sharpness which are critical to easily puncture tissue; cell targeting, either actively or passively by designing the system to access a specific depth in tissue; dosing, specifically dosing efficiency, dose delivered per area of tissue, and dose per needle; and delivery kinetics, whether through design of the needle materials or integration of the vaccine with the needle. b) At the complete device scale, applicator devices may be designed to apply specific, repeatable forces across the microneedle array. c) Within a microneedle array, the backing material selection is an important consideration in ensuring close contact with the entire tissue, and interneedle spacing can affect the microneedle-tissue mechanical interactions. d) At the single needle level, needle geometry, dimensions, and materials all influence mechanical properties of the needles and resulting dose delivered after tissue puncture. Vaccines may be formulated as a coating, within the needle matrix, or encapsulated within a secondary delivery system like nanoparticles to achieve the desired dose and delivery kinetics.
3.1. Microneedle geometry design
Microneedles can be fabricated by many different strategies, resulting in a wide range of possible dimensions and geometries (Figure 3c, 3d). Most studies have used microneedles with a standard pyramidal geometry and height range from 70 μm to 1.5 mm. However, with the wide range of fabrication techniques available, needles with virtually any dimensions are feasible to fabricate.[83, 92] Conical and pyramidal needles may be fabricated using silicon based manufacturing, specialized photopolymerization processing of polymer resist, laser micromachining, and polymer drawing.[93] These methods for microneedle fabrication have been reviewed in detail elsewhere.[94] More complex structures with hollow geometry, serrations, and arrowheads are also possible through more complex microfabrication processes.[95]
The wide variety of microneedle geometries and dimensions that can be created allow for highly reproducible needle application and optimization of delivery. Multiple studies have already been conducted to study the effect of microneedle shape, height, and tip diameter on insertion depth and delivery of biologics into dermal tissue.[96–98] The effect of microneedle tip diameter on skin penetration properties has shown that needles with a tip diameter of 5 μm entered the skin with approximately 25 mN of force, while a larger tip diameter of 37 μm required over 150 mN of force.[97] In general, for microneedle tip radii less than 40 μm, the force required for skin penetration is linearly related to the tip radius.[97] The tip diameter also had a significant effect on penetration depth, with a 10 μm increase in tip diameter resulting in approximately a 50 μm decrease in penetration depth.[97] Microneedle geometry is also an important factor that can affect delivery of agents into tissue. A study of the effect of microneedle geometry on penetration of a fluorescent dye found that the needles with the sharpest tip and a pitch angle of 15 degrees resulted in the largest area of fluorescence deposited into the skin. However, needles with a higher aspect ratio resulted in fluorescent signal deeper in the skin.[96] Together, these results provide a rationale to control microneedle geometry and dimensions to ensure microneedles can effectively penetrate skin and efficiently deliver agents.
A particular application for microneedle design is to access specific cell populations that reside at different depths in the tissue. Aqueous formulations are primarily taken up by Langerhans cells in the superficial layers of the mucosal epithelium, while alternative DC populations that may be more immunogenic like interstitial DCs and plasmacytoid DCs reside deeper in the tissue at the mucosa/submucosa interface or in the submucosa (Figure 1).[41, 42] In studies of dermal vaccination, significantly different serum IgG titers have been generated from microneedle arrays with different needle geometry and interneedle spacing that deliver agents at different depths in the skin.[99] Given these promising results in dermal tissue, there is a strong rationale to evaluate the effect of microneedle design parameters such as shape, dimension, and interneedle spacing on penetration of different oral mucosal tissues and the resulting immune responses.
3.2. Applicator devices for microneedle array delivery
Multiple applicator devices have been designed for dermal microneedle insertion to improve the uniformity and the percent of the microneedle height that penetrates into the tissue (Figure 3b). Microneedle applicator devices can be used in conjunction with standard microneedle patch designs, or they can act to support individual needles and enable patchless microneedle array delivery.[99–101] Applicator designs include simple buttons, rollers, syringe-inspired devices, and spring loaded devices.[102] Details of each of these designs and additional designs have been reviewed elsewhere.[102] These devices are particularly important to improve the penetration of short (~300 μm) microneedles that may otherwise incompletely penetrate tissue. Verbaan et al. found that without an electric device to apply needles at high velocity (3 m/s), dermal tissue simply folded around the microneedles without penetration. However, with an applicator, needles were delivered into tissue with efficiency of approximately 83%.[101] Even higher delivery efficiency up to 97% was observed for a patchless microneedle array design in which individual microneedles supported by micropillars were applied to skin using a spring loaded device.[100] Applicators can also improve the reproducibility of microneedle insertion, which is especially significant considering the potential of microneedles to be self-administered. In a study using a simple manual device to apply a static force, van der Maaden et al. found that the applicator improved delivery efficiency and reduced the variance between insertions by non-skilled users.[99]
While existing applicator devices for dermal microneedles are unlikely to be directly translated to oral mucosal microneedle delivery, these studies suggest the promise of designing applicators to improve delivery uniformity, efficiency, and reproducibility. The improvements for oral mucosal delivery will likely be even more dramatic than dermal delivery because of the anatomy of the oral cavity. While dermal tissues are typically supported by muscle or bone, much of the oral mucosa is not. Without a firm backing to provide support, tissue may be more likely to bend around needles rather than puncture.[103] Existing oral mucosal microneedle vaccine studies have demonstrated variable needle penetration and dosing efficiency.[89–91] Design of applicators for oral mucosal microneedle vaccination could improve the delivery efficiency and uniformity to previously evaluated tissues such as the buccal region and the tongue. Well-designed applicators could also enable delivery of microneedles to regions that are difficult to access like the lingual or palatine tonsils. Therefore, design of applicators for oral mucosal microneedle delivery is likely to improve the quality of future oral mucosal vaccine studies and also enable delivery of microneedles to previously unexplored oral tissues.
3.3. Microneedle tip implantation
Several previous studies have been able to implant depots of biodegradable polymers into dermal tissue using microneedles. This tip implantation has been shown to improve delivery efficiency in dermal tissue, and could also be particularly advantageous for the moist environment of the oral mucosa.
Silk hydrogels have been loaded into microneedle tips for implantation into skin and serve as a sustained release depot. DeMuth et al. observed that when crosslinked by methanol treatment, silk hydrogel implants maintained the model antigen OVA at detectable levels in the skin up to 16 days after initial microneedle application.[67] Other microneedle designs have incorporated biodegradable chitosan tips on top of polylactic acid posts.[66, 104] Upon application to dermal tissue of rats, the chitosan tips were implanted 600–850 μm below the tissue surface. After 60 minutes, the skin sealed over the implanted chitosan tips, resulting in depots for long term drug release. Compared to non-implantable chitosan microneedle tips fabricated by the same group that released BSA for only 4 days, tip-implantable chitosan microneedles released BSA up to 2 weeks after delivery.
Designing microneedles to achieve implantation of biodegradable tips could be useful in the oral cavity to improve dosing efficiency and to prevent salivary washout effects. With this design, there is greater confidence in the vaccine dose actually delivered into the tissue compared to topical delivery or standard microneedle delivery. Future studies to design these systems would need to consider the kinetics of tissue healing concurrently with vaccine diffusion kinetics to ensure that the tips and the encapsulated biologics are retained in the tissue. If needed, skin resealing kinetics could be optimized by changing features of the needle design such as the length, number of needles, and aspect ratio.[105]
3.4. Vaccine formulations incorporated in microneedles
Many different vaccine biologics can be formulated into microneedles due to the abundance of materials and designs that are available. Vaccine compositions have included virus-like particles, DNA polyplexes, protein subunit, and viral vectors.[65, 84, 88, 106, 107] Vaccine components can be formulated onto solid microneedles as coatings through a variety of dry and wet coating techniques, and these coatings can be further layered to incorporate both antigen and adjuvant.[86] Dissolving microneedles may contain antigen distributed throughout the polymer matrix forming the needle or encapsulated within nano- or microparticles in the needle (Figure 3d).[88]
The ability to deliver essentially any formulation of vaccine would be a leap forward for oral mucosal delivery. Previous preclinical studies of oral mucosal vaccine delivery have primarily been limited to inactivated vaccines, protein subunit vaccines, and virus-like particle vaccines. While some promising results have been observed with these vaccines, another key type of vaccine that has not been fully explored for oral mucosal delivery is DNA vaccines. In one study, DNA-PEI polyplexes were delivered to the sublingual mucosa as a prime followed by a sublingual protein boost.[108] Sublingual delivery resulted in 10-fold lower serum IgG and IgA responses compared to intranasal delivery and insignificant mucosal IgA titers in the vaginal mucosa. In another study, plasmid DNA was incorporated into a non-replicating baculovirus nanovector for sublingual delivery.[24] This delivery strategy generated comparable serum IgG titers, CD4+ and CD8+ T cell responses to intramuscular delivery. Mucosal IgA antibody titers were also very comparable to intramuscular delivery, meaning that this delivery system was not able to enhance mucosal immune responses. Other strategies for oral mucosal DNA vaccination such as electroporation have been able to elicit IgA and cellular mucosal immune responses, but the bulky and cumbersome device limits its use to specific regions of the oral mucosa.[10]
Many studies have evaluated microneedles for DNA vaccine delivery to dermal tissue.[86, 87, 89, 109] The majority of these studies have been performed in mice, where microneedle mediated DNA vaccination has induced antigen specific cytotoxic T cell responses comparable to electroporation and antigen specific serum IgG responses superior to subcutaneous and intramuscular delivery.[86, 87, 109] Microneedles have also been found to enhance gene expression in non-human primates by 140-fold compared to intradermal injection, which suggests that microneedle delivery of DNA will enhance immune responses in non-human primates.[86] Microneedles have also been used to deliver a DNA vaccine in the oral mucosa of a rabbit, one of very few studies evaluating oral mucosal microneedle vaccination, which will be reviewed further in Section 4.[89] Based on the demonstrated success of dermal microneedle mediated DNA delivery and the limitations of DNA delivery to the oral mucosa in previous studies, application of microneedles could overcome a major hurdle in oral mucosal DNA vaccination.
3.5. Microneedle materials design used to control vaccine delivery kinetics
Dissolving and solid microneedles can be designed to sustain release of antigens and adjuvants over time periods of days to weeks. These sustained release depots have a demonstrated ability to increase the magnitude and persistence of immune responses through dermal administration. Dissolving microneedles can achieve sustained release through incorporation of materials like silk, chitosan, or biodegradable polyesters, while solid microneedles can be coated with materials that will slowly dissociate and degrade in vivo (Figure 3d). As described above, silk release kinetics can be tuned through methanol treatment, which has been shown to decrease the release rate by approximately two-fold. [67, 110] In another study, microneedle tips were fabricated out of chitosan to create depots for sustained release of OVA over 28 days.[65] Microneedles can also be designed to deliver depots of biodegradable polyester microparticles that persist in dermal tissue up to 18 days and in the draining lymph node 10 days after delivery.[111] Sustained release has also been achieved by deposition of polymer multilayer films containing DNA antigen, RNA adjuvant, and polymer cations into dermal tissue through solid microneedles.[86] Depending on the design of the polymers in the film, duration of cell transfection from a reporter plasmid DNA antigen varied from 10 days to 22 days.
Slow release formulations including methanol treated silk, polyester microparticles, and biodegradable films have demonstrated more IFN-γ and TNF-α expressing antigen specific CD8+ T cells than rapid releasing microneedles or intramuscular bolus vaccination.[67, 86, 111] Release kinetics also have a significant effect on antibody responses, with the methanol treated silk microneedle formulation eliciting lower serum IgG titers and lower IgG avidity compared to fast release microneedles, but chitosan microneedles eliciting more robust serum IgG levels than bolus intramuscular delivery. [65] These immune responses were consistent even for the different antigens and materials used in each experiment.
Collectively, literature describing sustained vaccine delivery to dermal tissue by microneedles suggests that sustained vaccine release enhances primarily CD8+ effector and central memory T cells. Vaccine release kinetics have not previously been studied in the oral cavity due to limitations of currently available delivery methods. Existing microneedle technology for sustained vaccine delivery in dermal tissue could overcome these limitations to enable a more detailed evaluation of oral mucosal immunity. It is reasonable to infer that similar improvements in the quantity and quality of cellular immune response could be observed in the oral cavity compared to the dermal tissue given some similarities in cell phenotype and function. However, vaccine release kinetics in the oral mucosa needs to be specifically studied because the dermal and oral mucosal tissue are not directly analogous, particularly with regard to immune cell infiltration and migration kinetics.[41] It would also be interesting to evalulate the effects of sustained release on mucosal cellular and humoral immunity, as previous studies in dermal tissue have primarily focused on systemic cellular immunity.
4. Microneedles for oral vaccine delivery
Recently, three groups have reported on microneedle mediated delivery of vaccines to the oral mucosa (Table 5). Two of these technologies were based on solid coated microneedles, while the third used a dissolvable microneedle system. These studies have demonstrated the promise of microneedles for delivery to the oral mucosa and have also revealed new questions and challenges for future studies.
Table 5.
Summary of existing studies of microneedle mediated oral mucosal vaccine delivery.
| MN Technology | Animal Model | Delivery location | Puncture depth | Delivery efficiency | Vaccine composition | Immune response | Other notes |
|---|---|---|---|---|---|---|---|
| Solid coated microneedles | Rabbit | Inner lip | ~400 μm | 63.9% | OVA | IgA, IgG | -Statistical significance of IgA titers in saliva for intramuscular vs. oral mucosal not evaluated -OVA specific IgA and IgG responses induced only after second dose |
| Virus-like particle, plasmid | IgA, IgG | ||||||
| Dorsal tongue | ~400 μm | 91.2% | OVA | IgA, IgG | |||
| Virus-like particle, plasmid | IgA, IgG | ||||||
| Microprojection array | Mouse | Buccal | 47.8 μm | 30% | Fluvax (inactivated split virion) | IgG | -IgG titer similar to intramuscular delivery, lower than dermal |
| Liposome containing dissolving MNs | Mouse | n.d.a) | n.d. | n.d. | Liposomes containing lipid A+ Hepatitis B surface antigen or bovine serum albumin | IgG, IgA, CD8+ T cells |
-Specific site of oral mucosal delivery not reported -IgG titer lower than subcutaneous delivery -CD8+ T cell numbers greater than subcutaneous |
Not determined.
4.1. Solid coated microneedles for vaccine delivery in a rabbit model
The first study of microneedle delivery of vaccines to the oral mucosa was published in 2014 by Ma et al.[89] The study focused primarily on a single row of coated stainless steel microneedles applied to either the inner lip or the dorsal tongue of rabbits. Using sulforhodamine as a model, the researchers demonstrated delivery into both the lip and tongue, with efficiency of 63.9% and 91.2% respectively. Needles penetrated approximately 400 μm into the tissue, or 57% of the needle height, while penetrations around 30% of the needle height are more typical in other studies.[112] This improvement in penetration depth could be caused by several factors, including the design of the needle array as a 1D rather than 2D array, and the way the tissue was held taut during the needle insertion.
In this study, adaptive immune responses resulting from delivery of 125 μg OVA to the lip were compared to responses from delivery in the dorsal tongue. To achieve this dose, five microneedle arrays were each coated with 25 μg OVA. Similar antigen-specific serum IgG titers were detected after delivery at both sites, and these titers were only significant after two vaccine doses. Meanwhile, all IgA titers were low and variable. Based on these studies, it was concluded that there was no significant difference between delivery to either the lip or the tongue. Immune responses elicited by delivery of a virus like particle and plasmid DNA HIV antigens resulted in similar results as OVA.
This group has also conducted studies with the same microneedle technology to evaluate the effect of salivary washout on microneedle delivery to the oral cavity.[113] Although microneedles deliver biologics directly into the tissue, they do create holes that saliva could enter and still dilute and wash out the biologic. To evaluate the significance of this effect, a series of in vitro studies were conducted delivering sulforhodamine dye into porcine buccal mucosa mounted in a modified Franz diffusion cell. Unsurprisingly, the dynamic flow conditions resulted in approximately 3-fold increased diffusion out of the tissue compared to static flow conditions. It is unclear how directly these results translate to delivery of vaccine components like proteins and DNA that possess very different diffusion behavior compared to small molecule dyes. Nevertheless, this finding is an important consideration in the dosing of microneedles for oral mucosal delivery, and suggests that the use of mucoadhesive films as coverings to prevent this diffusion may help delivery efficiency. It would also be interesting to study how this diffusion varies with microneedle penetration depth, and how it varies in vivo with different delivery locations since salivary flow can vary greatly in different areas of the mouth.[51, 53]
4.2. Silicon microprojection array for flu vaccination in a mouse model
McNeilly et al. have investigated microprojection arrays for vaccine delivery in the mouse buccal mucosa. The microprojections consisted of 110 μm -long conical structures of silicon sputter-coated with gold and then dry coated with fluorescent nanoparticles or 37 ng of the Fluvax commercial influenza vaccine, an inactivated split virion vaccine. The projections were designed to be long enough for accessing the lamina propria, which is approximately 50 μm below the buccal tissue surface in mice. This study used a clip applicator device to help ensure uniform delivery, but only about two-thirds of the needles penetrated the tissue. The device delivered the Fluvax vaccine to an average depth of 47.8 μm into the murine buccal tissue, but the depth varied from 20 μm to ~100 μm. Meanwhile, the delivery efficiency of the microprojection arrays was around 30%, with about 10% of the vaccine delivered on the tissue surface and the other 60% remaining on the projections.
In the vaccination experiment, immune responses induced by the microprojection array were compared head-to-head with intramuscular injection, oral gavage delivery, and dermal delivery at t= 0 and at t= 4 weeks. The serum IgG titers were highest for dermal delivery, and buccal and intramuscular delivery had similarly lower titers. The only significant IgA titer was detected in the intestine from mice that received the vaccine dermally through the microprojection array. Hemagglutinin inhibition was measured to predict protective efficacy of these different vaccine delivery methods, and protective levels were detected only for dermal and buccal microprojection but not intramuscular delivery. This is a surprising result considering that the Fluvax commercial vaccine is designed for intramuscular injection.[114] The authors attributed the improved immune responses for dermal compared to buccal delivery to the increased penetration of ~90% in the dermal ear skin. It is not possible to directly compare the immune responses in this study to the 1D stainless steel arrays used by Ma et al. due to differences in animal model, delivery location, vaccine composition, delivery efficiency and method of reporting antibody titers. However, similar trends were observed in both studies, with oral mucosal microneedle vaccine delivery producing similar systemic IgG titers to intramuscular delivery and oral mucosal microneedle delivery resulting in modest and variable mucosal IgA titers.
4.3. Dissolving microneedles containing liposomes for vaccine delivery
Dissolving microneedle arrays have also been reported for oral mucosal delivery. Poly(vinyl-pyrrolidone) matrix microneedles contained liposomes incorporating lipid-A adjuvant and either Hepatitis B surface antigen (HBs) or bovine serum albumin (BSA) antigen.[91] The needles had a base diameter of 250 μm and a height of 660 μm. The microneedles were used to deliver either 4 μg of BSA or 0.5 μg of HBs to the murine oral mucosa, but no data was published regarding the penetration depth in oral mucosal tissue or the delivery efficiency of this system.
Regardless of antigen, microneedle delivery of liposomes to the oral mucosa produced higher levels of serum IgG antibodies than topical oral mucosal delivery but lower levels of serum IgG antibodies compared to subcutaneous and dermal delivery. Oral mucosal microneedle delivery elicited significantly higher levels of CD8+ T cells in the spleen and IgA antibodies in saliva, the intestines, and the vagina compared to any other evaluated delivery method. Out of all three reported technologies, the dissolving microneedles seemingly elicit the most robust mucosal IgA responses. There are several factors that could be responsible for this, including an approximately 100-fold increased dose compared to the microprojection array or the presence of an adjuvant. Additionally, of the three microneedle technologies evaluated for oral mucosal vaccination, this is the only device for which tissue penetration and delivery efficiency was not reported. This is a particularly important question for this dissolving microneedle system because small amounts of wetting in the moist environment of the mouth could significantly reduce the microneedle tip sharpness and stiffness. While previous studies indicate that dissolving microneedle penetration of moist surfaces such as the cornea and sclera is possible, the microneedle material must be carefully designed to ensure needle penetration before needle dissolution.[115]
5. Perspectives and outlook
There is a strong rationale for further evaluation of microneedles for oral mucosal vaccine delivery. These studies may focus on device optimization for generating high quality mucosal immune responses and the use of microneedles as a tool to answer key questions about oral mucosal immunity.
One key gap in oral mucosal microneedle vaccine delivery is the unclear role of dosing location. Microneedles have unique capabilities that enable precise vaccine delivery to locations that may be challenging with other dosage forms. However, existing studies have not fully utilized this function of microneedles for oral mucosal vaccine delivery. Although Ma et al. found that delivery in the lip and sublingually resulted in similar immune responses, this may be due to the specific measurement outputs used in their studies. Ma et al. used IgA and IgG titers to determine the difference between dosing location. However, it is possible that measurements like DC activation markers or cytokine expression could provide more insight into the immune functions of different oral mucosal dosing locations. Therefore, there is still a rationale for vaccine delivery in different oral mucosal locations.
The effect of microneedle mediated vaccine delivery on innate and cellular immunity in the oral mucosa is another key gap in existing studies. In all of the completed studies, the primary immune output was serum IgG or mucosal IgA antibodies, and cellular responses were only evaluated for the dissolving microneedle technology. Meanwhile, none of these studies evaluated innate immune responses. While humoral immune responses are one key goal of vaccination, studies suggest that cellular immune responses are critical for clearing initial infection, particularly at mucosal surfaces where many pathogens enter and establish infection.[6] Additionally, previous studies using microneedles for dermal vaccination have demonstrated robust cellular immune responses.[85, 88, 106] The increased CD8+ T cell responses for the dissolving microneedles suggests that analogous responses in the oral mucosa can be induced by microneedle-mediated vaccine delivery. In future studies, cellular immune responses should be included as a measure of the vaccine immunogenicity and ultimately should be optimized. Future studies should also evaluate innate immune responses to better understand the type, magnitude, and quality of innate response that correlates with lasting adaptive immunity and protection from infection.
There is also clear room for improvement and innovation in the incorporation of adjuvants into oral mucosal microneedle systems. Only one microneedle formulation used for oral mucosal vaccination incorporated an adjuvant, specifically lipid A, a TLR4 agonist.[91] However, it is unclear what effect the adjuvant had on the resulting immune responses since an unadjuvanted control was not included. Even so, the minimal immune responses generated by the microneedle system compared to subcutaneous delivery suggest further exploration of different adjuvants that could improve efficacy. Given that responses comparable to intramuscular delivery have been induced without any adjuvant,[89, 90] it is plausible that incorporation of adjuvants in oral mucosal microneedle systems could greatly improve the quality and quantity of mucosal and systemic immune responses, as has been observed for topical dosage forms. The delivery capabilities of microneedles could also improve our understanding of adjuvant behavior in the oral mucosa. Because immune cell populations vary greatly with mucosal depth and with specific mucosal region, the unique ability of microneedles to target adjuvants to specific tissue regions containing target cells could lead to a better understanding of oral mucosal immunity.
In addition to studying new factors related to oral mucosal vaccine delivery, research should also pursue the use of more representative animal models. Existing studies of microneedle mediated vaccine delivery to the oral mucosa are limited to mice and rabbits. In these studies, the small animal model was a major constraint that limited the type and number of mucosal sites that could be accessed. The use of an animal model that permits delivery of vaccines at multiple oral mucosal sites would enable more specific studies of the effect of delivery location on immune responses. The anatomy of the lymphoid tissues also varies greatly between rodents and humans. Mice lack palatine tonsils, and rabbit palatine tonsils are monocryptic rather than branched. Animal models that are representative of humans with regards to anatomy of the WTR include primates and pigs.[28] Additionally, mice have a keratinized oral mucosal epithelium that possesses very different properties than the non-keratinized squamous epithelium of humans, and they are known to be poor predictors of human innate immunity because they lack receptors for certain TLRs.[116] Expansion of microneedle mediated oral mucosal vaccine delivery to the non-human primate model would enable a more accurate assessment of the potential of this vaccine delivery method for generating immunogenicity in humans.
Clinical translation of the microneedle fabrication process and applicator design must be considered in the design of microneedles for oral mucosal vaccination. As described in detail within other reviews, key considerations for translation of microneedle products include sterile and scalable manufacture, procedures for quality control, design of applicators and instructions for appropriate use, and careful market analysis.[117] Despite investments in microneedle technology by large companies, commercially available products are currently limited to cosmetic microneedle rollers that do not contain any active ingredients, and Fluzone,™ a trivalent inactivated influenza vaccine injected intradermally using a microneedle system called Soluvia™ manufactured by Becton Dickinson.[118, 119] Microneedle translation is an active area of research, with recent papers reporting scalable microneedle manufacturing processes, new methods for assessing microneedle performance, and applicators for easy, reproducible application by non-skilled users.[99, 102, 120] Additionally, completed clinical trials have demonstrated the promise of microneedle technology for improving vaccination. Fluzone™ exhibited dose sparing in clinical trials, with 9 μg Fluzone™ generating comparable antibody responses to 15 μg of the same trivalent vaccine delivered intramuscularly, and more recently, another clinical trial demonstrated the safety and immunogenicity of a dissolving microneedle patch for influenza vaccination.[119, 121]
6. Conclusion
Although the oral cavity is a promising site for mucosal vaccination, the current limited understanding of oral mucosal immunity precludes rational development and optimization of the next generation of oral mucosal vaccines. Currently available vaccine delivery systems for the oral mucosa are insufficient to specifically study the role of factors like vaccine composition, dose, and release kinetics on antigen presenting cell uptake and innate and adaptive immune responses. Microneedles possess broad capabilities that have been thoroughly demonstrated for dermal delivery, but demonstrations of these capabilities in the oral mucosa are extremely limited. There are many opportunities for advancement in this field, including the incorporation of adjuvants, the study of innate immune responses, and direct comparison of oral mucosal delivery sites. Future studies should not only optimize microneedle mediated oral mucosal vaccination but also use microneedles as a tool to address gaps in the current understanding of oral mucosal immunity.
Acknowledgements
This work was supported by NIH/NIHCD grant DP2HD075703 to KAW. RC was supported by an NSF Graduate Research Fellowship under Grant number DGE-1256082.
Biographies
Rachel L. Creighton graduated with a B.S. in Materials Engineering from Iowa State University in 2015. She is currently a Ph.D. student in the Department of Bioengineering at the University of Washington under the supervision of Dr. Kim Woodrow. Her current research is focused on the use of integrated fiber microneedles for oral mucosal DNA vaccination.

Kim A. Woodrow is an Associate Professor at the University of Washington in the Department of Bioengineering. She earned a B.A. in Biochemistry and Molecular Biology from Wells College, and a M.S. and Ph.D. in Chemical Engineering from Stanford University. Her research is focused on applications of engineered biomaterials in mucosal infections and mucosal immunity.

References
- [1].Neutra MR, Kozlowski PA, Nat Rev Immunol 2006, 6, 148. [DOI] [PubMed] [Google Scholar]
- [2].Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Blé C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi ML, Tofani N, Biasin M, Villa ML, Mazzotta F, Clerici M, Nat Med 1997, 3, 1250. [DOI] [PubMed] [Google Scholar]
- [3].Moutsopoulos NM, Konkel JE, Trends Immunol 2018, 39, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R, Proc Natl Acad Sci U S A 2002, 99, 14338; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB, J Exp Med 2000, 191, 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Lamm ME, Annu Rev Microbiol 1997, 51, 311; [DOI] [PubMed] [Google Scholar]; b) Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR, J Immunol 2001, 167, 2861. [DOI] [PubMed] [Google Scholar]
- [6].a) Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald KS, Bwayo JJ, McMichael AJ, Rowland-Jones SL, J Immunol 2000, 164, 1602; [DOI] [PubMed] [Google Scholar]; b) Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Lifson JD, Nelson JA, Jarvis MA, Picker LJ, Nat Med 2009, 15, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN, Proc Natl Acad Sci U S A 2008, 105, 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Holmgren J, Czerkinsky C, Nat Med 2005, 11, S45. [DOI] [PubMed] [Google Scholar]
- [9].Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z, J Immunol 2004, 173, 6357. [DOI] [PubMed] [Google Scholar]
- [10].Kichaev G, Mendoza J, Amante D, Smith T, McCoy J, Sardesai N, Broderick K, Human Vaccines & Immunotherapeutics 2013, 9, 2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hayden CA, Fischer ME, Andrews BL, Chilton HC, Turner DD, Walker JH, Tizard IR, Howard JA, Vaccine 2015, 33, 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, Oh YK, Vaccine 2010, 28, 2598. [DOI] [PubMed] [Google Scholar]
- [13].Murugappan S, Patil HP, Frijlink HW, Huckriede A, Hinrichs WL, AAPS J 2014, 16, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Barnetta SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, Ferrai MG, Weiss DE, Letvin NL, Montefiori D, Pal R, Vajdy M, Aids 2008, 22, 339; [DOI] [PubMed] [Google Scholar]; b) Pakkanen SH, Kantele JM, Savolainen LE, Rombo L, Kantele A, Vaccine 2015, 33, 451; [DOI] [PubMed] [Google Scholar]; c) Saeed MI, Omar AR, Hussein MZ, Elkhidir IM, Sekawi Z, Hum Vaccin Immunother 2015, 11, 2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thippeshappa R, Tian B, Cleveland B, Guo W, Polacino P, Hu SL, Clin Vaccine Immunol 2015, 23, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lycke N, Nat Rev Immunol 2012, 12, 592. [DOI] [PubMed] [Google Scholar]
- [17].Eriksson K, Quiding-Järbrink M, Osek J, Möller A, Björk S, Holmgren J, Czerkinsky C, Infect Immun 1998, 66, 5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shim BS, Stadler K, Nguyen HH, Yun CH, Kim DW, Chang J, Czerkinsky C, Song MK, Virol J 2012, 9, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lundholm P, Asakura Y, Hinkula J, Lucht E, Wahren B, Vaccine 1999, 17, 2036. [DOI] [PubMed] [Google Scholar]
- [20].a) WHO, Vaccine 2014, 32, 4117; [DOI] [PubMed] [Google Scholar]; b) Wkly Epidemiol Rec 2013, 88, 49.23424730 [Google Scholar]
- [21].Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R, N Engl J Med 2004, 350, 896. [DOI] [PubMed] [Google Scholar]
- [22].Shakya AK, Chowdhury MYE, Tao W, Gill HS, J Control Release 2016, 240, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].a) Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, Glezen WP, Wittes J, JAMA 1999, 282, 137; [DOI] [PubMed] [Google Scholar]; b) Osterholm MT, Kelley NS, Sommer A, Belongia EA, Lancet Infect Dis 2012, 12, 36; [DOI] [PubMed] [Google Scholar]; c) Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, Iacuzio D, Wolff M, N Engl J Med 1998, 338, 1405. [DOI] [PubMed] [Google Scholar]
- [24].Lee HJ, Cho H, Kim MG, Heo YK, Cho Y, Gwon YD, Park KH, Jin H, Kim J, Oh YK, Kim YB, Plos One 2015, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) Gallorini S, Taccone M, Bonci A, Nardelli F, Casini D, Bonificio A, Kommareddy S, Bertholet S, O’Hagan DT, Baudner BC, Vaccine 2014, 32, 2382; [DOI] [PubMed] [Google Scholar]; b) Pedersen GK, Ebensen T, Gjeraker IH, Svindland S, Bredholt G, Guzmán CA, Cox RJ, PLoS One 2011, 6, e26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brandtzaeg P, Adv Otorhinolaryngol 2011, 72, 20. [DOI] [PubMed] [Google Scholar]
- [27].Illum L, J Control Release 2003, 87, 187. [DOI] [PubMed] [Google Scholar]
- [28].Perry M, Whyte A, Immunol Today 1998, 19, 414. [DOI] [PubMed] [Google Scholar]
- [29].a) Brandtzaeg P, Korsrud FR, Clin Exp Immunol 1984, 58, 709; [PMC free article] [PubMed] [Google Scholar]; b) Perry ME, J Anat 1994, 185 (Pt 1), 111; [PMC free article] [PubMed] [Google Scholar]; c) Takahashi K, Nishikawa Y, Sato H, Oka T, Yoshino T, Miyatani K, Virchows Arch 2006, 448, 623. [DOI] [PubMed] [Google Scholar]
- [30].Summers KL, Hock BD, McKenzie JL, Hart DN, Am J Pathol 2001, 159, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fujihara K, Kuki K, Kimura T, Tabata T, Auris Nasus Larynx 1988, 15, 191. [DOI] [PubMed] [Google Scholar]
- [32].a) Jiang HL, Kang ML, Quan JS, Kang SG, Akaike T, Yoo HS, Cho CS, Biomaterials 2008, 29, 1931; [DOI] [PubMed] [Google Scholar]; b) Khatri K, Goyal AK, Gupta PN, Mishra N, Vyas SP, Int J Pharm 2008, 354, 235. [DOI] [PubMed] [Google Scholar]
- [33].Kraan H, Soema P, Amorij JP, Kersten G, Vaccine 2017, 35, 2647. [DOI] [PubMed] [Google Scholar]
- [34].van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR, J Immunol 2000, 165, 4778. [DOI] [PubMed] [Google Scholar]
- [35].a) Smith A, Perelman M, Hinchcliffe M, Hum Vaccin Immunother 2014, 10, 797; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ren ST, Zhang XM, Sun PF, Sun LJ, Guo X, Tian T, Zhang J, Guo QY, Li X, Guo LJ, Che J, Wang B, Zhang H, PLoS One 2017, 12, e0169501; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yoshino N, Endo M, Kanno H, Matsukawa N, Tsutsumi R, Takeshita R, Sato S, PLoS One 2013, 8, e61643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stahl-Hennig C, Kuate S, Franz M, Suh YS, Stoiber H, Sauermann U, Tenner-Racz K, Norley S, Park KS, Sung YC, Steinman R, Racz P, Uberla K, J Virol 2007, 81, 13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Earl PL, Americo JL, Wyatt LS, Eller LA, Montefiori DC, Byrum R, Piatak M, Lifson JD, Amara RR, Robinson HL, Huggins JW, Moss B, Virology 2007, 366, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tenner-Racz K, Stahl Hennig C, Uberla K, Stoiber H, Ignatius R, Heeney J, Steinman RM, Racz P, Proc Natl Acad Sci U S A 2004, 101, 3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij J-P, Journal of Controlled Release 2014, 190, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shojaei AH, J Pharm Pharm Sci 1998, 1, 15. [PubMed] [Google Scholar]
- [41].Hovav AH, Mucosal Immunol 2014, 7, 27. [DOI] [PubMed] [Google Scholar]
- [42].a) Lukić A, Vasilijić S, Majstorović I, Vucević D, Mojsilović S, Gazivoda D, Danilović V, Petrović R, Colić M, Int Endod J 2006, 39, 626; [DOI] [PubMed] [Google Scholar]; b) Jotwani R, Cutler CW, J Dent Res 2003, 82, 736. [DOI] [PubMed] [Google Scholar]
- [43].Allam JP, Stojanovski G, Friedrichs N, Peng W, Bieber T, Wenzel J, Novak N, Allergy 2008, 63, 720. [DOI] [PubMed] [Google Scholar]
- [44].a) Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, Betbeder D, Balazuc AM, Van Overtvelt L, Moingeon P, J Allergy Clin Immunol 2008, 122, 603; [DOI] [PubMed] [Google Scholar]; b) Ito T, Wang YH, Liu YJ, Springer Semin Immunopathol 2005, 26, 221. [DOI] [PubMed] [Google Scholar]
- [45].Villadangos JA, Young L, Immunity 2008, 29, 352. [DOI] [PubMed] [Google Scholar]
- [46].a) Benito-Villalvilla C, Cirauqui C, Diez-Rivero CM, Casanovas M, Subiza JL, Palomares O, Mucosal Immunology 2017, 10, 924; [DOI] [PubMed] [Google Scholar]; b) Khan A, Singh S, Galvan G, Jagannath C, Sastry KJ, Vaccines 2017, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang MC, Yang A, Qiu J, Yang B, He L, Tsai YC, Jeang J, Wu TC, Hung CF, Cell Biosci 2016, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aran K, Chooljian M, Paredes J, Rafi M, Lee K, Kim AY, An J, Yau JF, Chum H, Conboy I, Murthy N, Liepmann D, Science Translational Medicine 2017, 9. [DOI] [PubMed] [Google Scholar]
- [49].van Loon LA, Krieg SR, Davidson CL, Bos JD, J Oral Pathol Med 1989, 18, 197. [DOI] [PubMed] [Google Scholar]
- [50].Hasséus B, Jontell M, Bergenholtz G, Dahlgren UI, Eur J Oral Sci 2004, 112, 48. [DOI] [PubMed] [Google Scholar]
- [51].Humphrey SP, Williamson RT, J Prosthet Dent 2001, 85, 162. [DOI] [PubMed] [Google Scholar]
- [52].Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuère F, Czerkinsky C, Vaccine 2007, 25, 8598. [DOI] [PubMed] [Google Scholar]
- [53].Percival RS, Challacombe SJ, Marsh PD, J Dent Res 1994, 73, 1416. [DOI] [PubMed] [Google Scholar]
- [54].Veerman EC, van den Keybus PA, Vissink A, Nieuw Amerongen AV, Eur J Oral Sci 1996, 104, 346. [DOI] [PubMed] [Google Scholar]
- [55].a) Law S, Wertz PW, Swartzendruber DC, Squier CA, Arch Oral Biol 1995, 40, 1085; [DOI] [PubMed] [Google Scholar]; b) Squier CA, Cox P, Wertz PW, J Invest Dermatol 1991, 96, 123. [DOI] [PubMed] [Google Scholar]
- [56].Iwasaki A, Annu Rev Immunol 2007, 25, 381. [DOI] [PubMed] [Google Scholar]
- [57].Novak N, Haberstok J, Bieber T, Allam JP, Trends Mol Med 2008, 14, 191. [DOI] [PubMed] [Google Scholar]
- [58].Cutler CW, Jotwani R, J Dent Res 2006, 85, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Soria I, Lopez-Relano J, Vinuela M, Tudela JI, Angelina A, Benito-Villalvilla C, Diez-Rivero CM, Cases B, Manzano AI, Fernandez-Caldas E, Casanovas M, Palomares O, Subiza JL, Allergy 2018, 73, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Allam JP, Peng WM, Appel T, Wenghoefer M, Niederhagen B, Bieber T, Bergé S, Novak N, J Allergy Clin Immunol 2008, 121, 368. [DOI] [PubMed] [Google Scholar]
- [61].Shin JS, Greer AM, Cell Mol Life Sci 2015, 72, 2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Aramaki O, Chalermsarp N, Otsuki M, Tagami J, Azuma M, Biochem Biophys Res Commun 2011, 413, 407. [DOI] [PubMed] [Google Scholar]
- [63].Hajishengallis G, Mol Oral Microbiol 2010, 25, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].a) Adams JR, Haughney SL, Mallapragada SK, Acta Biomater 2015, 14, 104; [DOI] [PubMed] [Google Scholar]; b) Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, Proceedings of the National Academy of Sciences 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chen MC, Lai KY, Ling MH, Lin CW, Acta Biomaterialia 2018, 65, 66. [DOI] [PubMed] [Google Scholar]
- [66].Chen MC, Huang SF, Lai KY, Ling MH, Biomaterials 2013, 34, 3077. [DOI] [PubMed] [Google Scholar]
- [67].DeMuth PC, Min Y, Irvine DJ, Hammond PT, Advanced Healthcare Materials 2014, 3, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jewell CM, López SC, Irvine DJ, Proc Natl Acad Sci U S A 2011, 108, 15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kim J, Li W, Choi Y, Lewin S, Verbeke C, Dranoff G, Mooney D, Nature Biotechnology 2015, 33, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang C, Ohno T, Kang S, Takai T, Azuma M, Vaccine 2014, 32, 5669. [DOI] [PubMed] [Google Scholar]
- [71].a) Chinna Reddy P, Chaitanya KS, Madhusudan Rao Y, Daru 2011, 19, 385; [PMC free article] [PubMed] [Google Scholar]; b) Silva BMA, Borges AF, Silva C, Coelho JFJ, Simoes S, International Journal of Pharmaceutics 2015, 494, 537; [DOI] [PubMed] [Google Scholar]; c) Borges AF, Silva C, Coelho JFJ, Simoes S, Journal of Controlled Release 2015, 206, 108. [DOI] [PubMed] [Google Scholar]
- [72].White JA, Blum JS, Hosken NA, Marshak JO, Duncan L, Zhu C, Norton EB, Clements JD, Koelle DM, Chen D, Weldon WC, Oberste MS, Lal M, Hum Vaccin Immunother 2014, 10, 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Borde A, Ekman A, Holmgren J, Larsson A, Eur J Pharm Sci 2012, 47, 695. [DOI] [PubMed] [Google Scholar]
- [74].Mašek J, Lubasová D, Lukáč R, Turánek-Knotigová P, Kulich P, Plocková J, Mašková E, Procházka L, Koudelka Š, Sasithorn N, Gombos J, Bartheldyová E, Hubatka F, Raška M, Miller AD, Turánek J, J Control Release 2017, 249, 183. [DOI] [PubMed] [Google Scholar]
- [75].Mitchell TJ, Kendall MAF, Bellhouse BJ, International Journal of Impact Engineering 2003, 28, 581. [Google Scholar]
- [76].Sun JB, Czerkinsky C, Holmgren J, Scand J Immunol 2007, 66, 278. [DOI] [PubMed] [Google Scholar]
- [77].Lycke N, Immunol Lett 2005, 97, 193. [DOI] [PubMed] [Google Scholar]
- [78].Amuguni JH, Lee S, Kerstein KO, Brown DW, Belitsky BR, Herrmann JE, Keusch GT, Sonenshein AL, Tzipori S, Vaccine 2011, 29, 4778. [DOI] [PubMed] [Google Scholar]
- [79].Negri DR, Riccomi A, Pinto D, Vendetti S, Rossi A, Cicconi R, Ruggiero P, Del Giudice G, De Magistris MT, Vaccine 2010, 28, 4175. [DOI] [PubMed] [Google Scholar]
- [80].Fukasaka M, Asari D, Kiyotoh E, Okazaki A, Gomi Y, Tanimoto T, Takeuchi O, Akira S, Hori M, Plos One 2015, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Buffa V, Klein K, Fischetti L, Shattock RJ, PLoS One 2012, 7, e50529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].a) Vrdoljak A, McGrath MG, Carey JB, Draper SJ, Hill AV, O’Mahony C, Crean AM, Moore AC, J Control Release 2012, 159, 34; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim YC, Yoo DG, Compans RW, Kang SM, Prausnitz MR, J Control Release 2013, 172, 579; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Weldon WC, Zarnitsyn VG, Esser ES, Taherbhai MT, Koutsonanos DG, Vassilieva EV, Skountzou I, Prausnitz MR, Compans RW, PLoS One 2012, 7, e41501; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR, J Infect Dis 2010, 201, 190; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Pearton M, Pirri D, Kang SM, Compans RW, Birchall JC, Adv Healthc Mater 2013, 2, 1401; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kommareddy S, Baudner BC, Bonificio A, Gallorini S, Palladino G, Determan AS, Dohmeier DM, Kroells KD, Sternjohn JR, Singh M, Dormitzer PR, Hansen KJ, O’Hagan DT, Vaccine 2013, 31, 3435; [DOI] [PubMed] [Google Scholar]; g) Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK, Current topics in microbiology and immunology 2009, 333, 369; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Kim YC, Lee SH, Choi WH, Choi HJ, Goo TW, Lee JH, Quan FS, J Drug Target 2016, 24, 943; [DOI] [PubMed] [Google Scholar]; i) Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, Malcolm RK, Shattock RJ, McCarthy HO, Donnelly RF, J Control Release 2012, 162, 529; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Arya J, Prausnitz M, Journal of Controlled Release 2016, 240, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Laurent P, Bonnet S, Alchas P, Regolini P, Mikszta J, Pettis R, Harvey N, Vaccine 2007, 25, 8833. [DOI] [PubMed] [Google Scholar]
- [84].a) Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR, Vaccine 2015, 33, 4683; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, Koster AJ, Slütter B, Kros A, Jiskoot W, Bouwstra JA, Mönkäre J, J Control Release 2017, 266, 109; [DOI] [PubMed] [Google Scholar]; c) Carey JB, Vrdoljak A, O’Mahony C, Hill AV, Draper SJ, Moore AC, Sci Rep 2014, 4, 6154; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Alarcon JB, Hartley AW, Harvey NG, Mikszta JA, Clin Vaccine Immunol 2007, 14, 375; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ, ACS Nano 2012, 6, 8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pearson FE, O’Mahony C, Moore AC, Hill AV, Vaccine 2015, 33, 3248. [DOI] [PubMed] [Google Scholar]
- [86].DeMuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, Hammond PT, Irvine DJ, Nat Mater 2013, 12, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim NW, Lee MS, Kim KR, Lee JE, Lee K, Park JS, Matsumoto Y, Jo DG, Lee H, Lee DS, Jeong JH, J Control Release 2014, 179, 11. [DOI] [PubMed] [Google Scholar]
- [88].Becker PD, Hervouet C, Mason GM, Kwon SY, Klavinskis LS, Vaccine 2015, 33, 4691. [DOI] [PubMed] [Google Scholar]
- [89].Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS, Pharm Res 2014, 31, 2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].McNeilly CL, Crichton ML, Primiero CA, Frazer IH, Roberts MS, Kendall MA, J Control Release 2014, 196, 252. [DOI] [PubMed] [Google Scholar]
- [91].a) Wang T, Zhen Y, Ma X, Wei B, Li S, Wang N, Colloids Surf B Biointerfaces 2015, 126, 520; [DOI] [PubMed] [Google Scholar]; b) Zhen YY, Wang N, Gao ZB, Ma XY, Wei BA, Deng YH, Wang T, Vaccine 2015, 33, 4330. [DOI] [PubMed] [Google Scholar]
- [92].Wei-Ze L, Mei-Rong H, Jian-Ping Z, Yong-Qiang Z, Bao-Hua H, Ting L, Yong Z, International Journal of Pharmaceutics 2010, 389, 122.20096759 [Google Scholar]
- [93].a) Donnelly RF, Majithiya R, Singh TRR, Morrow DIJ, Garland MJ, Demir YK, Migalska K, Ryan E, Gillen D, Scott CJ, Woolfson AD, Pharmaceutical Research 2011, 28, 41; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Martin CJ, Allender CJ, Brain KR, Morrissey A, Birchall JC, Journal of Controlled Release 2012, 158, 93; [DOI] [PubMed] [Google Scholar]; c) Moon S, Lee S, Lee H, Kwon T, Microsystem Technologies-Micro-and Nanosystems-Information Storage and Processing Systems 2005, 11, 311; [Google Scholar]; d) Park JH, Yoon YK, Choi SO, Prausnitz MR, Allen MG, IEEE Trans Biomed Eng 2007, 54, 903; [DOI] [PubMed] [Google Scholar]; e) Park JH, Allen MG, Prausnitz MR, Journal of Controlled Release 2005, 104, 51. [DOI] [PubMed] [Google Scholar]
- [94].Kim YC, Park JH, Prausnitz MR, Adv Drug Deliv Rev 2012, 64, 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].a) van der Maaden K, Trietsch SJ, Kraan H, Varypataki EM, Romeijn S, Zwier R, van der Linden HJ, Kersten G, Hankemeier T, Jiskoot W, Bouwstra J, Pharm Res 2014, 31, 1846; [DOI] [PubMed] [Google Scholar]; b) Han M, Kim DK, Kang SH, Yoon HR, Kim BY, Lee SS, Kim KD, Lee HG, Sensors and Actuators B-Chemical 2009, 137, 274; [Google Scholar]; c) Gill HS, Prausnitz MR, J Control Release 2007, 117, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bal SM, Kruithof AC, Zwier R, Dietz E, Bouwstra JA, Lademann J, Meinke MC, J Control Release 2010, 147, 218. [DOI] [PubMed] [Google Scholar]
- [97].Römgens AM, Bader DL, Bouwstra JA, Baaijens FPT, Oomens CWJ, J Mech Behav Biomed Mater 2014, 40, 397. [DOI] [PubMed] [Google Scholar]
- [98].Donnelly RF, Garland MJ, Morrow DI, Migalska K, Singh TR, Majithiya R, Woolfson AD, J Control Release 2010, 147, 333. [DOI] [PubMed] [Google Scholar]
- [99].van der Maaden K, Sekerdag E, Jiskoot W, Bouwstra J, AAPS J 2014, 16, 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lahiji SF, Dangol M, Jung H, Sci Rep 2015, 5, 7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Verbaan FJ, Bal SM, van den Berg DJ, Dijksman JA, van Hecke M, Verpoorten H, van den Berg A, Luttge R, Bouwstra JA, J Control Release 2008, 128, 80. [DOI] [PubMed] [Google Scholar]
- [102].a) Donnelly RF, Singh TRR, Morrow DIJ, Woolfson AD, in Microneedle-mediated Transdermal and Intradermal Drug Delivery, John Wiley & Sons, Ltd., 2012, 57; [Google Scholar]; b) Singh TR, Dunne NJ, Cunningham E, Donnelly RF, Recent Pat Drug Deliv Formul 2011, 5, 11. [DOI] [PubMed] [Google Scholar]
- [103].a) Moronkeji K, Todd S, Dawidowska I, Barrett SD, Akhtar R, J Control Release 2017, 265, 102; [DOI] [PubMed] [Google Scholar]; b) Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR, Journal of Biomechanics 2004, 37, 1155. [DOI] [PubMed] [Google Scholar]
- [104].Chen MC, Ling MH, Lai KY, Pramudityo E, Biomacromolecules 2012, 13, 4022. [DOI] [PubMed] [Google Scholar]
- [105].Gupta J, Gill HS, Andrews SN, Prausnitz MR, Journal of Controlled Release 2011, 154, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bachy V, Hervouet C, Becker PD, Chorro L, Carlin LM, Herath S, Papagatsias T, Barbaroux JB, Oh SJ, Benlahrech A, Athanasopoulos T, Dickson G, Patterson S, Kwon SY, Geissmann F, Klavinskis LS, Proc Natl Acad Sci U S A 2013, 110, 3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].a) Cole G, McCaffrey J, Ali AA, McBride JW, McCrudden CM, Vincente-Perez EM, Donnelly RF, McCarthy HO, Hum Vaccin Immunother 2017, 13, 50; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B, Vaccine 2013, 31, 3396; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR, Vaccine 2013, 31, 3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mann JFS, McKay PF, Arokiasamy S, Patel RK, Tregoning JS, Shattock RJ, Plos One 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kim YC, Song JM, Lipatov AS, Choi SO, Lee JW, Donis RO, Compans RW, Kang SM, Prausnitz MR, Eur J Pharm Biopharm 2012, 81, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tsioris K, Raja W, Pritchard E, Panilaitis B, Kaplan D, Omenetto F, Advanced Functional Materials 2012, 22, 330. [Google Scholar]
- [111].DeMuth PC, Garcia-Beltran WF, Ai-Ling ML, Hammond PT, Irvine DJ, Advanced Functional Materials 2013, 23, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].a) Lee JW, Park JH, Prausnitz MR, Biomaterials 2008, 29, 2113; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sullivan SP, Koutsonanos DG, del Pilar Martin M, Lee JW, Zarnitsyn V, Choi S-O, Murthy N, Compans RW, Skountzou I, Prausnitz MR, Nat Med 2010, 16, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Serpe L, Jain A, de Macedo CG, Volpato MC, Groppo FC, Gill HS, Franz-Montan M, Eur J Pharm Sci 2016, 93, 215. [DOI] [PubMed] [Google Scholar]
- [114].Nolan T, Richmond PC, McVernon J, Skeljo MV, Hartel GF, Bennet J, Basser RL, Influenza Other Respir Viruses 2009, 3, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Thakur RR, Tekko IA, Al-Shammari F, Ali AA, McCarthy H, Donnelly RF, Drug Deliv Transl Res 2016, 6, 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Mestas J, Hughes CC, J Immunol 2004, 172, 2731. [DOI] [PubMed] [Google Scholar]
- [117].a) Watkinson AC, Kearney MC, Quinn HL, Courtenay AJ, Donnelly RF, Expert Opin Drug Deliv 2016, 13, 523; [DOI] [PubMed] [Google Scholar]; b) Quinn HL, Kearney MC, Courtenay AJ, McCrudden MT, Donnelly RF, Expert Opin Drug Deliv 2014, 11, 1769. [DOI] [PubMed] [Google Scholar]
- [118].a) McCrudden MT, McAlister E, Courtenay AJ, González-Vázquez P, Singh TR, Donnelly RF, Exp Dermatol 2015, 24, 561; [DOI] [PubMed] [Google Scholar]; b) Richter-Johnson J, Kumar P, Choonara YE, du Toit LC, Pillay V, Expert Rev Pharmacoecon Outcomes Res 2018, 18, 359; [DOI] [PubMed] [Google Scholar]; c) Ansaldi F, de Florentiis D, Durando P, Icardi G, Expert Rev Vaccines 2012, 11, 17. [DOI] [PubMed] [Google Scholar]
- [119].Frenck RW, Belshe R, Brady RC, Winokur PL, Campbell JD, Treanor J, Hay CM, Dekker CL, Walter EB, Cate TR, Edwards KM, Hill H, Wolff M, Leduc T, Tornieporth N, Vaccine 2011, 29, 5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].a) Lutton RE, Larrañeta E, Kearney MC, Boyd P, Woolfson AD, Donnelly RF, Int J Pharm 2015, 494, 417; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Larrañeta E, Stewart S, Fallows SJ, Birkhäuer LL, McCrudden MT, Woolfson AD, Donnelly RF, Int J Pharm 2016, 497, 62; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ripolin A, Quinn J, Larrañeta E, Vicente-Perez EM, Barry J, Donnelly RF, Int J Pharm 2017, 521, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR, Group T-MS, Lancet 2017, 390, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bolton DL, Song K, Tomaras GD, Rao S, Roederer M, Vaccine 2017, 35, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Gallichan WS, Rosenthal KL, J Exp Med 1996, 184, 1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV, J Immunol 2003, 171, 1602. [DOI] [PubMed] [Google Scholar]
- [125].Fló J, Tisminetzky S, Baralle F, Vaccine 2001, 19, 1772. [DOI] [PubMed] [Google Scholar]
- [126].Etchart N, Desmoulins PO, Chemin K, Maliszewski C, Dubois B, Wild F, Kaiserlian D, J Immunol 2001, 167, 384. [DOI] [PubMed] [Google Scholar]
- [127].Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, Seid R, Look J, Alderson M, Tate A, Maisonneuve JF, Robertson G, Anderson PW, Malley R, Clin Vaccine Immunol 2010, 17, 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Etchart N, Buckland R, Liu MA, Wild TF, Kaiserlian D, J Gen Virol 1997, 78 (Pt 7), 1577. [DOI] [PubMed] [Google Scholar]
- [129].Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A, Clin Vaccine Immunol 2011, 18, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, Song HH, Kim KH, Song MK, Chang J, PLoS One 2012, 7, e32226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Fu YH, Jiao YY, He JS, Giang GY, Zhang W, Yan YF, Ma Y, Hua Y, Zhang Y, Peng XL, Shi CX, Hong T, Antiviral Res 2014, 105, 72. [DOI] [PubMed] [Google Scholar]
- [132].Domm W, Brooks L, Chung HL, Feng C, Bowers WJ, Watson G, McGrath JL, Dewhurst S, Vaccine 2011, 29, 7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hervouet C, Luci C, Cuburu N, Cremel M, Bekri S, Vimeux L, Marañon C, Czerkinsky C, Hosmalin A, Anjuère F, Vaccine 2010, 28, 5582. [DOI] [PubMed] [Google Scholar]
- [134].Busignies V, Simon G, Mollereau G, Bourry O, Mazel V, Rosa-Calatrava M, Tchoreloff P, International Journal of Pharmaceutics 2018, 538, 87. [DOI] [PubMed] [Google Scholar]
- [135].a) Deng L, Chang TZ, Wang Y, Li S, Wang S, Matsuyama S, Yu G, Compans RW, Li JD, Prausnitz MR, Champion JA, Wang BZ, Proc Natl Acad Sci U S A 2018, 115, E7758; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Saint-Lu N, Tourdot S, Razafindratsita A, Mascarell L, Berjont N, Chabre H, Louise A, Van Overtvelt L, Moingeon P, Allergy 2009, 64, 1003. [DOI] [PubMed] [Google Scholar]