Abstract
Rare pain insensitive individuals offer unique insights into how pain circuits function, and have led to the development of new strategies for pain control. We investigated pain sensitivity in humans with WAGR (Wilms tumor, aniridia, genitourinary anomaly, range of intellectual disabilities) syndrome, who have variably-sized heterozygous deletion of the 11p13 region. The deletion region can be inclusive or exclusive of the brain-derived neurotrophic factor (BDNF) gene, a crucial trophic factor for nociceptive afferents. Nociceptive responses assessed by quantitative sensory testing (QST), demonstrated reduced pain sensitivity only in the WAGR subjects whose deletion boundaries included the BDNF gene. Corresponding behavioral assessments were made in heterozygous Bdnf knockout rats to examine the specific role of Bdnf. These analogous experiments revealed impairment of Aδ and C-fiber mediated heat nociception, determined by acute nociceptive thermal stimuli, and in aversive behaviors evoked when the rats were placed on a hot plate. Similar results were obtained for C-fiber mediated cold responses and cold avoidance on a cold plate device. Together, these results suggested a blunted responsiveness to aversive stimuli. Our parallel observations in humans and rats show that heterozygous deletion of the BDNF gene reduces pain sensitivity, and establish BDNF as a determinant of nociceptive sensitivity.
Keywords: BDNF, pain sensitivity, nociception, nerve growth factor, RNA-Seq
Summary:
Haploinsufficiency of BDNF in humans with WAGR syndrome, and in rats with a specific disruption of Bdnf on a single allele have reduced sensitivity to pain.
Introduction
The ability to sense and respond to potentially damaging stimuli is a fundamental role of the nervous system, conserved in all animals. Dedicated peripheral sensory neurons, called “nociceptors” respond to tissue damage, transducing the stimuli into neural impulses, and are the first neuron in the pain pathway. However, in the central nervous system the registration and interpretation of these sensory inputs involves complex circuitry some of which feeds back to modulate primary afferent nociceptive inputs in dorsal spinal cord[47]. Although there is variation in pain sensitivity across normal individuals, there are also rare outliers; at one extreme are people with congenital insensitivities to pain. Such people generally harbor mutations which inactivate or destroy the nociceptive apparatus in the peripheral nervous system, leading to profound loss of pain sensation[13; 50]. Such rare mutations have led to a greater understanding of pain transmission, development of pain circuits, and ultimately to new approaches to control pain[16; 25].
The present report focuses on patients with Wilms tumor-aniridia (WAGR) syndrome which is caused by a variable-length heterozygous deletion in 11p13, and is associated with a clinical heterogeneity and a large number of phenotypic presentations that include kidney tumors (nephroblastoma), aniridia, genitourinary anomalies (e.g., cryptorchidism), and intellectual disabilities. In part, clinical heterogeneity is driven by the variable genetic defect, that can be inclusive or exclusive of several genes, including the brain-derived neurotrophic factor (BDNF) gene. Based on parental reports of pain insensitivity in WAGR syndrome patients with BDNF deletion on one chromosome, as well as a large existing literature on the relationship between BDNF and pain [38; 40; 47], we systematically investigated pain sensitivity in individuals with WAGR syndrome. We show that haploinsufficiency of the BDNF gene is associated with a strong reduction in pain sensitivity in these individuals that was evident using quantitative sensory testing (QST) and via parental reports. Similarly, a rat model that specifically isolates the Bdnf haploinsufficiency also revealed impairment of cold and hot thermo-nociception. In our transcriptomic examinations of the first two elements of the nociceptive circuit, DRG and dorsal spinal cord, we see more genes differentially regulated at the level of the second order spinal neurons, suggestive of pain modulation, rather than complete abolition of the primary afferent nociceptive apparatus. Our observations in humans and rats establish corresponding phenotypic evidence in both species that BDNF haploinsufficiency is associated with altered nociceptive sensitivity, and have potential implications for future pharmacologic modulation of pain sensitivity.
Materials and Methods
Subjects.
Subjects with WAGR syndrome were recruited through the International WAGR Syndrome Association. All procedures were approved by the NICHD Institutional Review Board. Parents/legal guardians provided consent for minors and adults with intellectual disability. Testing was performed at the NIH Clinical Research Center in Bethesda, Maryland, USA. A detailed, standardized clinical neurological examination was performed by board-certified neurologists on all subjects.
Demographics, neurological examination, and genotyping of WAGR subjects.
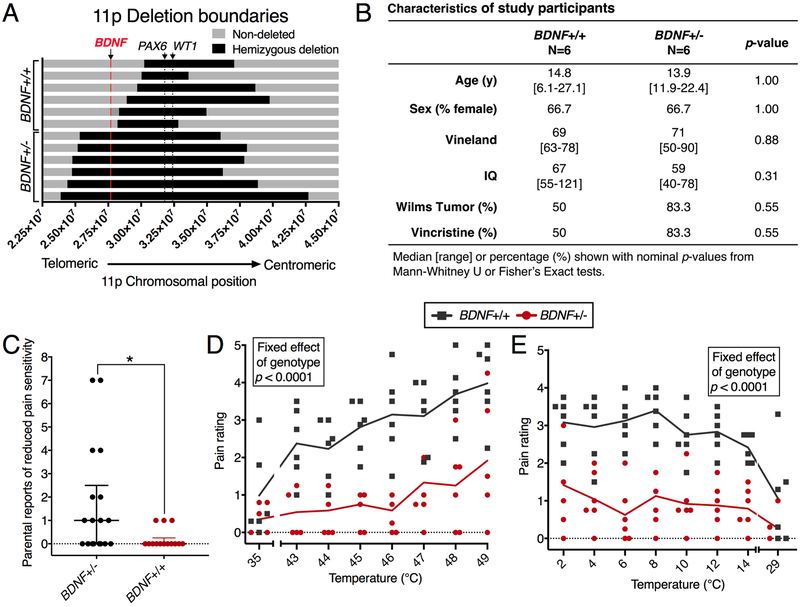
11p13 deletion boundaries for the WAGR subjects were determined by microarray comparative genomic hybridization[23]. The mapping of the WAGR hemideletion allowed the patient population to be split based on deletion boundaries. Out of the 12 patients in the present study, 6 harbored heterozygous deletion of BDNF, while 6 retained two copies of this gene (Figure 1A). These two groups of patients were not significantly different in intelligence, age, tumor status, chemotherapy treatment (Figure 1B), or in nerve conduction velocity (Supplementary Tables 1, 2). Both groups of patients were 2/3 female. Parental reports of these patients indicated a potential pain insensitivity phenotype, and were recorded formally (Figure 1C, further annotated in Supplementary Figure 1), and investigated with experimental thermal pain stimuli using QST (Figure 1D,E). Most WAGR syndrome subjects are intellectually impaired, and deletion of BDNF is associated with reduced general cognitive functioning[24]. Due to this impairment, criteria for eligibility included ability to rate thermal stimuli and complete QST. Sensitivity analysis was performed to rule out IQ as a confounding variable in the interpretation of QST results (Supplementary Figure 2). In addition, clinical neurological examination demonstrated normal peripheral motor and sensory findings consistent with the normal conduction velocity measurements on multiple peripheral nerves. Nerve conduction measurements are shown for the cohort in Supplementary tables 1 and 2.
Figure 1. Characteristics and thermal pain perception ratings of patients with WAGR syndrome. A cohort of 12 WAGR syndrome subjects were tested for thermal pain ratings.
This cohort consisted of 6 BDNF+/− and 6 BDNF+/+ subjects with normal nerve conduction measurements (Supplementary Tables 1, 2), selected from a larger cohort of 32 patients with WAGR syndrome [23] based on capacity to understand and perform behavioral pain ratings. (A) 11p deletion boundaries, determined as previously described [23] are shown for each subject (N=6) with the deleted region indicated in black. These deletions can be inclusive or exclusive of the BDNF gene (shown in red), allowing us to divide the subjects by BDNF genotype. (B) BDNF+/− versus BDNF+/+ subjects were similar in age, sex distribution, adaptive behavior (Vineland), cognitive functioning (IQ), assessed as previously described [24], and history of vincristine treatment for Wilms tumor. (C) Parents of BDNF+/− individuals reported significantly more examples of pain insensitivity in their children in the optional comments section of a questionnaire about pain sensitivity. (D,E) Thermal pain perception was tested for hot (D) and cold (E) temperatures by application of a 1.6 × 1.6 cm Medoc thermode to the volar forearm followed by patient reports of pain intensity using a 6-point Wong-Baker FACES Pain Rating Scale [59]. Baseline measurements were also taken for non-noxious warm (35°C) and cool (29°C) stimuli. These measurements were used to create linear mixed-effects models for hot and cold pain ratings, using baseline rating and IQ as covariates in the model. For both hot and cold temperature ratings, genotype showed a significant effect, indicating that BDNF+/+ and BDNF+/− individuals rated both heat and cold stimuli as less painful (D, E). For hot temperatures, there was a significant effect of temperature on rating (D).
Quantitative sensory testing of human subjects with WAGR syndrome.
Testing was performed with the Medoc Thermal Sensory Analyzer (Medoc Ltd. Advanced Medical Systems, Durham, NC) using a 1.6 × 1.6 cm contact thermode placed on the volar forearm. This protocol was derived from a prior study conducted at the NIH in adults [33]. Subjects, who were masked with regard to the temperature of the stimulus, were asked to rate the thermal pain intensity of each target temperature using a 6-point Wong-Baker FACES Pain Rating Scale, which has been validated in children as young as age 3 years [59]. The experimenter administering the test to WAGR subjects was not informed of the patient’s BDNF deletion status, and efforts were made to blind the experimenter to BDNF deletion status. However, experimenters could have been cued to the subject’s genotype by phenotypic presentations [23], as well as familiarity with medical records associated with each patient, which contained information about deletion boundaries of the patients, but not BDNF deletion status directly. Experimenters were also involved in the clinical care of these subjects.
Genotyping of Bdnf+/+ and +/− rats.
Animal experiments were conducted under a protocol approved by the Clinical Center Animal Care and Use Committee. Rats were genotyped by two separate methods in an unbiased manner. Frozen liver samples were collected and sent to TransnetYX for genotyping to detect the 7 bp deletion (SD-Bdnftm1sage, Horizon Discovery, Cambridge, UK). Genotyping was performed using real-time PCR with a TaqMan reporter probe, and the following primers: Fwd GATGCCGCAAACATGTCTATGAG; Rev CCACTCGCTAATACTGTCACACA; Reporter CCCCGCCCGCCGTG. All animals corresponded to the expected genotype as determined by the vendor (SAGE Labs) before shipment of the animals. Two cohorts of N=5 per group male Bdnf+/+ and Bdnf+/− rats were assessed in the current study. Genotype was corroborated by RNA-Seq analyses performed on the DRG in 5/10 animals of each genotype, and on the dorsal spinal cord for 10 animals. Using a grep-based strategy (SPELL THIS acronym OUT OR explain a bit for the readers), reads were extracted surrounding the deletion, identifying all but one animal as the expected genotype based on the absence or presence of reads containing the deletion. This classification is imperfect due to the possibility that an mRNA containing the deletion may not be detected if the coverage is poor. For the one animal for which RNA-Seq did not detect reads containing the deletion, three samples of the animal’s liver were sent to TransnetYX, all three of which confirmed the correct genotype. This confirms that misclassification by the RNA-Seq method was due to lack of coverage at the deletion locus. Overall transcript levels of Bdnf did not differ between Bdnf+/+ and Bdnf+/− animals, suggesting that the mutant transcript is stable (Supplementary Figure 3).
Behavioral assessments of WT and Bdnf+/− rats.
Radiant thermal and noxious cold stimulation.
All behavioral assessments were performed by an experimenter who was blinded to genotype at the time of testing. Sample sizes were determined based on previous experience with similar testing paradigms. Rats were enclosed in individual boxes on a 2.5 mm thick elevated glass plate, and radiant heat stimulation was applied to the plantar surface of the hind paw using a focused infrared halogen bulb (Plantar Test, Ugo Basille, Monvalle VA, Italy) [28; 44]. Heating was terminated when the animal withdrew the hindlimb, and behavior was scored as the latency to withdraw. To determine temperature over time, the same radiant heat stimulus was delivered to awake animals while standing on a thermistor wire (type K) connected to a USB universal thermocouple connector (Omega Engineering Inc., Stamford, CT) to measure the temperature at the skin’s surface. Temperature over time curves were used to convert latency measurements into temperature of withdrawal. The temperature at which withdrawal occurred was calibrated by delivering the radiant heat stimulus in an awake behaving rat in a quadrupedal stance. A thin thermistor wire (type K, Omega Engineering Inc., Stamford CT) was placed under the stimulated hind paw in order to measure temperature at the skin’s surface. Temperature-over-time curves were used to convert latency measurements into temperature at which the animal withdraws.
Measurements of acute cold pain were based on a similar method published previously [7]. Powdered dry ice was compacted into the barrel of a 12 mm diameter plastic syringe and used to deliver a cold stimulus to the plantar surface [7]. Briefly, animals were enclosed in individual clear plastic boxes on an elevated glass platform that was 2.5 mm thick, and cold stimulation was delivered to the animal’s plantar hindpaw from below. Care was taken to ensure a smooth surface of the dry ice, and light pressure was applied to make contact between the dry ice and the glass. Withdrawal latency and thermistor measurements were made in the same manner as for radiant thermal stimulation.
Hot and cold plate behavioral assessment.
To determine behavioral response to noxious heat, animals were placed on a hot plate set to 48°C until they licked their hind paw twice and latency to first and second hind paw licks were recorded [1]. This temperature was chosen because it is at the low end of the noxious range usually used to perform this test, where we hypothesized the greatest likelihood of differentiating the two groups. To assess responses to noxious cold, animals were placed on the same plate and behaviors were recorded for 5 minutes using an iPhone 6 (Apple, Cupertino, CA). Behaviors were scored manually, and values were checked for agreement by three observers, one of whom was blinded to experimental conditions. During the cold plate assay, the rats displayed avoidance behaviors by lifting their tail off the surface of the plate. The sum of the duration of avoidance episodes was determined from the video recordings by observers blinded to genotype.
Aδ stimulation using 100 ms infrared diode laser pulse.
Animals were set on the same glass platform as for noxious cold stimulation, and an infrared diode laser (LASS-10 M; Lasmed, Mountain View, CA, USA) with an output wavelength of 980 nm was used to deliver 100 ms thermal stimuli of approximately 1.6 mm diameter to the heel and plantar surface of the rat hind paw. This stimulus has previously been shown to selectively activate Aδ fiber-mediated nociceptors [3; 41; 56]. Laser stimulator was operated as described previously [41; 42] with an output between 3000 and 5500 mA to obtain a stimulus-response function. After delivering a stimulus, animals were scored for behavior according to the following rubric: 0 = no response, 1 = simple withdrawal, 2 = paw shake, 3 = orient towards paw, 4 = paw lick. Statistics were performed on average behavioral values using the Mann-Whitney U-test to account for ordinal rank-orders. Response ratios were calculated by considering 0’s vs all responses regardless of severity.
RNA extraction and sequencing.
RNA extraction was performed using a bead beating homogenizer (MP Biomedicals, Santa Ana, California) and the RNeasy Lipid Tissue Mini Kit as described previously [34; 46]. Sequencing was performed at the NIH Intramural Sequencing Center, as described previously [46]. Briefly, mRNA libraries were constructed starting from 1 μg total RNA using the Illumina TruSeq RNA Sample Prep Kits, version 2. The resulting cDNA was fragmented using a Covaris E210. Library amplification was performed for 10 cycles to minimize the risk of over-amplification. Unique barcode adapters were applied to each library. Libraries were pooled in equimolar ratio and sequenced together on a HiSeq 2500 with ver 4 flow cells and sequencing reagents. A minimum of 70 million 125-base read pairs were generated for each DRG library, and a minimum of 23 million 125-base read pairs were generated for each dorsal horn library.
Transcriptomic analyses.
Several datasets were mined from previous publications [48]. Mouse brain neural and non-neural cell population RNA expression data were mined from a previous database [62] and used to categorize significantly regulated genes [46]. Heatmap data were constructed from expression data mined from the human GTEx database and plotted as a ratio of expression per gene to identify tissue enrichment for genes of interest [8; 48]. Rat DRG and dorsal spinal cord datasets generated for this manuscript were aligned and quantified using MAGIC [34; 61] and a rat genome with annotations built based on rn6 [34]. Gene expression values are represented using significant fragments per kilobase of transcript per million aligned reads (sFPKM), a MAGIC-specific quantification method[34; 61]. Significantly differential genes were determined by controlling the false discovery rate at 5% after comparing against 80 orthogonally scrambled iterations of sample groupings to control for variance [34; 46]. In the dorsal horn dataset, which was collected after the DRG dataset, several candidate genes were examined using uncorrected Mann-Whitney U-tests based on previous observations in DRG. Gene expression and fold changes were analyzed for interactions using Ingenuity Pathway Analysis (Qiagen).
Data availability.
Rat sequencing data from the DRG and dorsal horn of Bdnf+/+ and Bdnf+/− animals has been deposited into the Sequence Read Archive under BioProject number (To be released upon publication of this manuscript).
Statistical analyses.
Linear mixed-effects models were constructed in SAS 9.4 (SAS Institute Inc., Cary, NC) for human sensory testing data (Figure 1D, E) using rating, temperature, baseline response, and IQ as fixed effects, with individual as a random effect. In these models, temperature was considered as a factor to account for nonlinearity in temperature ratings. Sensitivity analysis was performed to examine the effect of IQ on thermal ratings from QST (Supplementary Figure 2). A similar linear mixed-effects model was constructed for the cold plate assay (tail lifting) using temperature and genotype as fixed effects, and animal number as a random effect. For all models, least square means tests are reported for each measurement. Mann-Whitney U-tests and Student’s T-tests were performed in Prism 7 (GraphPad Software, LaJolla, CA). Full results from all statistical tests are reported in the supplementary data files. For RNA-Seq datasets, statistical analyses were performed using MAGIC [34]. GO terms and related statistics were calculated in DAVID 6.8 (https://david.ncifcrf.gov/).
Results
Parental reports.
Parents of BDNF+/− WAGR subjects were significantly more likely to provide descriptions of their child’s pain insensitivity in the optional comments section of the Non-Communicating Children’s Pain Checklist-Revised [6; 23] (Figure 1C, Supplementary Figure 1). These reports contained striking details of severely reduced pain sensitivity resembling those observed in cases of complete pain insensitivity syndromes: (a) “Got his toe caught in our gate and it ripped a big ½-inch gouge in it. It was bleeding pretty heavily and the skin was just hanging off. He also broke a bone in his hand while riding a bike, we didn’t notice the bruise until the next day.” (b) “I believe there are many times when I know nothing. She had a punctured ear drum once that I only discovered from the discharge coming out. Took her to the doctor and asked her if her ear hurt and she said, ‘not really.’ “(c) “When she complains of having pain or not feeling well, it is so rare for her to do so that now we always know to listen. Another time she was trying to sit on a lunch table bench at school, missed, and hit her collar bone: they examined her at school, but since she hardly complained they let her finish out the day. When she came home, we weren’t sure ourselves, but decided to have her checked out for reasons previously stated—sure enough: broken clavicle.” These reports are consistent with an impairment in nociception and/or increased pain tolerance, but not complete lack of pain sensation as is observed in patients with mutations in NGF[9; 11; 17], TRKA[30] or PRDM12[13] genes, all of which lead to developmental loss of entire subpopulations of primary afferent nociceptors and, consequently, cause a complete loss of painful sensations[10; 19; 35]. These parental reports prompted further investigation of pain insensitivity using quantitative sensory testing.
Quantitative sensory testing.
Based on the parental reports we tested the hypothesis that BDNF hemizygosity may lead to an impairment in nociceptive processing. We performed QST to determine the ability of BDNF+/− subjects to rate hot and cold stimuli. Subjects were asked to rate pain intensity of each temperature delivered using a 6-point Wong-Baker FACES Pain Rating Scale[59], which has been validated in children as young as 3 years of age; the youngest subject in our study was 6 years old (Figure 1B). The average rating of 4 replicates for each temperature (2 on the right forearm, 2 on the left forearm) was used for analyses. Figure 1D and E show the average rating for all subjects (black and red lines) and the average data for each patient (black and red circles) at each temperature. QST showed comparable ratings for non-noxious warm (35°C) and cool (29°C) temperatures (p=0.49 and p=0.18, respectively). However, when tested over a range of hot (43–49°C) and cold (14–2°C) stimuli, BDNF+/− WAGR subjects rated these stimuli as significantly less painful (Fig 1D, E).
Bioinformatic analysis of WAGR gene deletion locus.
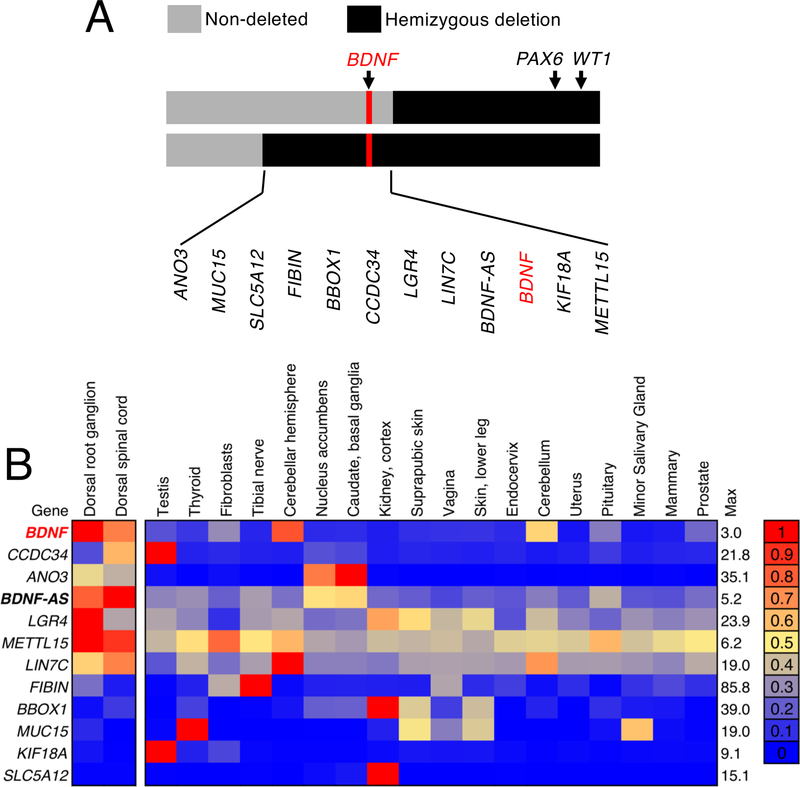
Several genes are located at the deletion boundary that separates the two groups of individuals in the present study (Figure 2A), and several of these genes are expressed in pain circuit tissues (Supplementary Figure 4). Despite the large number of genes in the WAGR locus, a substantially smaller subset of genes is located in the sub-region of the locus that differentiates the two groups of WAGR subjects. Of this subset of genes, BDNF is the most highly enriched in pain circuit tissues such as the dorsal root ganglion (Figure 2B) as compared to other regions of the human body.
Figure 2. Enrichment analysis of selected WAGR locus genes compared to 53 human tissues in the GTEx database.
(A,B) Many of the genes deleted in patients in the present study are expressed in pain circuit tissues in humans. (A) We examined the subset of genes within the deletion boundaries shared by the BDNF+/− individuals but not the BDNF+/+ individuals. (B) To address whether these genes are enriched in these tissues, expression values (sFPKM, MAGIC) were compared with reported median RPKM gene expression values in the GTEx database v6, and ordered by degree of enrichment. The top 20 human tissues enriched for these genes are shown out of a total of 55 tissues examined. Of the genes within this locus, BDNF is the most highly enriched gene in human dorsal root ganglia and dorsal spinal cord samples relative to other tissues. It shows expression in other neural tissues, such as the cerebellum. Notably, the ANO3 gene, which is also among the most enriched genes has been implicated in nociceptive sensitivity in rats, where knockout studies of the orthologous rat gene caused increased thermal and mechanical pain sensitivity[27]. These alterations in pain sensitivity have not been reported in humans with ANO3 mutations to date, who present with cervical dystonia[12].
Heterozygous knockout rat.
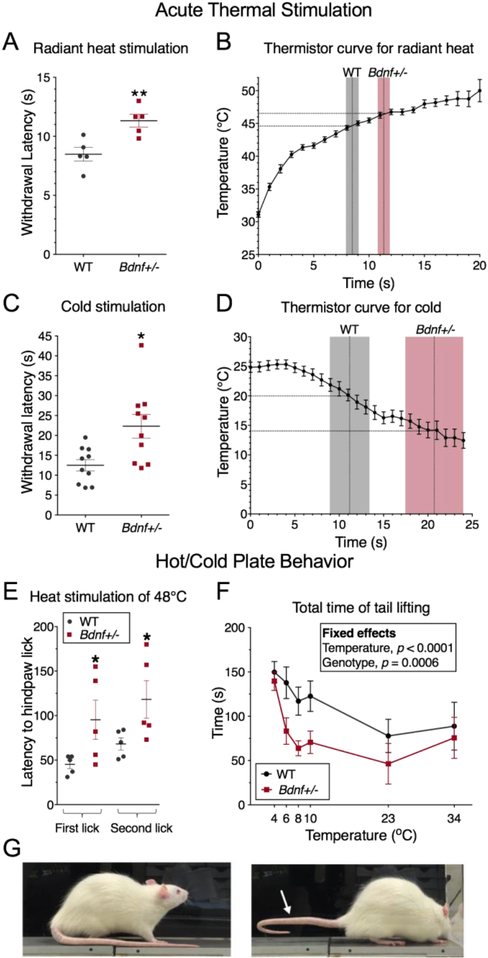
In order to assess the role of BDNF haploinsufficiency specifically, we performed nociceptive testing on rats harboring a loss of function frameshift mutation within the Bdnf gene on one allele (Bdnf+/−; SD-Bdnftm1sage, Horizon Discovery, Cambridge, UK)[54]. Expression from both alleles, and in wildtype rats, was assessed by RNA-Seq, revealing equal levels of Bdnf transcripts, however, approximately half of the transcript from the +/− animals contained the deletion which introduced a frameshift and stop codon shortly after the propeptide cleavage site (Supplementary Figure 3). Previous studies in the same strain have showed that serum levels of BDNF are reduced by approximately 50%[54]. All behavioral assessments in rats were performed by an individual blinded to genotype. Heat was delivered to the hind paw by a focused halogen bulb, terminating when the animal withdrew its hind limb[28; 44] (Plantar Test, Ugo Basille, Monvalle VA, Italy) and calibrated using the thermistor wire (see Methods). Bdnf+/− rats exhibited a significantly increased latency to withdrawal corresponding to a higher temperature of withdrawal (44.6°C for +/+ vs. 46.5°C for +/−; Figure 3A, B). Sensitivity to noxious cold was tested by measuring withdrawal latency upon application of an aversive cold stimulus to the hind paw as described by Brenner et al.[7] using a compacted dry ice stimulus. Relative to Bdnf+/+ rats, Bdnf+/− rats exhibited a significantly longer latency to withdraw from the cold stimulus, corresponding to a colder temperature of withdrawal (18.5°C for +/+ vs. 12.9°C for +/−; Figure 3C, D).
Figure 3. Thermal pain testing in Bdnf+/− rats.
Behavioral assessment of thermal nociception was performed in rats by application of thermal stimuli to the plantar surface of the hind paw. (A) Radiant heat stimulation was applied and behavior was rated as withdrawal latency Bdnf+/− rats (N=5) withdrew significantly later than WT animals. (B) A thermistor probe was placed under the paw of awake behaving Bdnf+/− and WT rats to measure the temperature ramp over time occurring at the dermal surface (N=21). Withdrawal latency data is superimposed as gray (WT) or red (Bdnf+/−) bars showing that WT animals withdraw at approximately 44.8°C, while Bdnf+/− animals withdraw at approximately 46.5°C. (C) Noxious cold stimulation was performed by indirect contact with dry ice. Bdnf+/− animals (N=10). withdrew later in response to noxious cold than WT (N=10) (D) Behavioral response was elicited at approximately 20.0°C plantar dermal temperature in WT, and at approximately 14.0°C for Bdnf+/− rats. (E) When placed on a 48°C hot plate both groups responded by licking their hind paw. In both groups the first lick was followed by a second lick, which was also quantified (E; N=5 for each group). The latency to both the first and second hind paw lick was longer in Bdnf+/− rats. (F) When placed on a cold plate, both groups of animals displayed avoidance behavior to the cold surface evidenced by raising the tail to reduce contact area, with WT exhibiting significantly more tail lifting than Bdnf+/− animals (N=5; Genotype effect p = 0.0006). An example of an animal lifting his tail in response to noxious cold is shown (G). (A) Two-tailed Student’s T-test; C, Two-tailed Mann-Whitney test; (E) Fisher’s Exact test; (F) linear mixed-effects model. Error bars represent SEM; *, p < 0.05; **, p < 0.01.
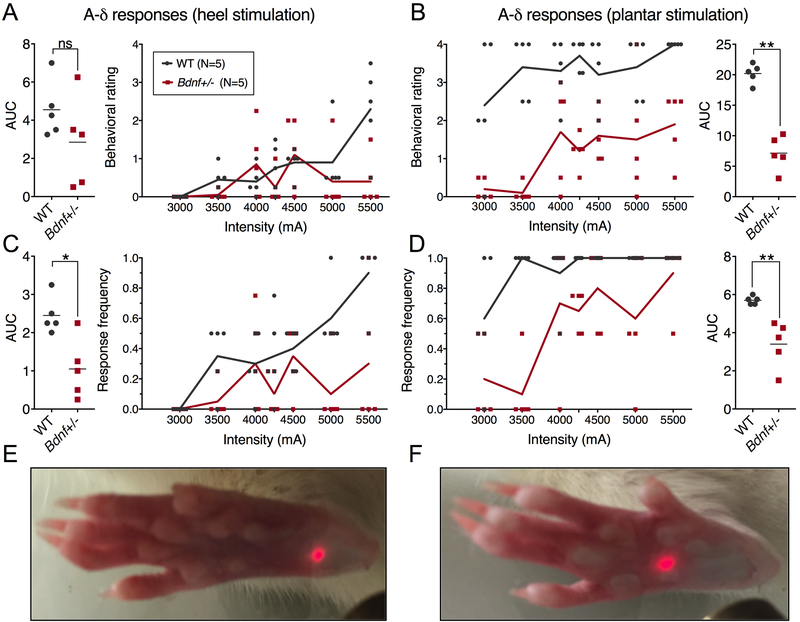
Heat sensitivity also was examined using the hot plate test (48°C) and measuring the latency to hind paw licking behavior [1]. The lower temperature was chosen because it is at the low end of the noxious range commonly used, where we hypothesized the greatest chance of seeing a behavioral difference between groups. Bdnf+/− rats exhibited significantly longer latencies to lick their hind paws on the hot plate compared to the Bdnf+/+ littermates (for Bdnf+/− compared to WT, respectively: 95.4 ± 49.3 versus 45.2 ± 10.6 sec for the first lick, and 118.2 ± 47.2 versus 68.2 ± 15.3 sec for the second lick; Figure 3E). Cold sensitivity was assessed further by recording spontaneous cold avoidance behaviors using a cold plate. Animals were tested at plate temperatures of 34°C, 23°, 10°, 8°, 6°, and 4°C (Figure 3F). From blinded evaluation of video recordings of this test we observed that, in response to noxious cold temperatures, WT rats raised their tail to avoid contact with the cold surface. Quantification of this endpoint showed that Bdnf+/− animals exhibited significantly less total duration of tail lifting (genotype effect, p=0.0006, Figure 3G), indicating a reduced, but not abolished, avoidance of noxious cold temperatures. In addition to the hot plate and C-fiber noxious heat-mediated responses, Bdnf+/− rats were tested for responsiveness to Aδ-mediated[3] hind limb withdrawal to short (100 ms) heat pulses delivered by an infrared diode laser. Animals were tested over a range of laser intensities on both the heel and plantar surfaces of the hind paw[42; 46](Figure 4). Relative to Bdnf+/+ rats, Bdnf+/− animals showed a reduction in responsiveness to the short thermal Aδ stimuli relative to WT littermates. While their responses are reduced, the rats approach 100% response rates at the highest level of intensity on the more sensitive mid-plantar area of skin (Figure 4D) indicating an ability to respond to sufficiently strong stimulation. As a non-thermal behavioral assay, both groups of rats were tested using von Frey filaments, with the Bdnf+/− rats requiring larger filaments (more force) to evoke a withdrawal response (Supplementary Figure 5).
Figure 4. Short-pulse A-δ laser stimulation of WT and Bdnf+/− heel and plantar surface of the hind paw.
A 100ms pulse was delivered to the heel (A, C, E) or plantar surface (B, D, F) across a range of stimulus intensities, and behavioral assessments were scored by a blinded observer (N=5). Ratings of behavioral responses were made using a previously validated scale [41; 42], with significant differences observed on the plantar (B), but not the heel (A). The plantar area is more sensitive to thermal stimulation than the heel, resulting in lower overall responsiveness to the same stimulus intensity on the heel (C,D) Response ratios to hind paw stimulation were significantly different (P<0.01)for both the heel and plantar stimulation paradigms. Differences in average area under the curve (AUC) measurements were calculated using a Wilcoxon Mann-Whitney U-test; *, p < 0.05; **, p < 0.01.
DRG and Dorsal Spinal Cord Transcriptomic Analyses.
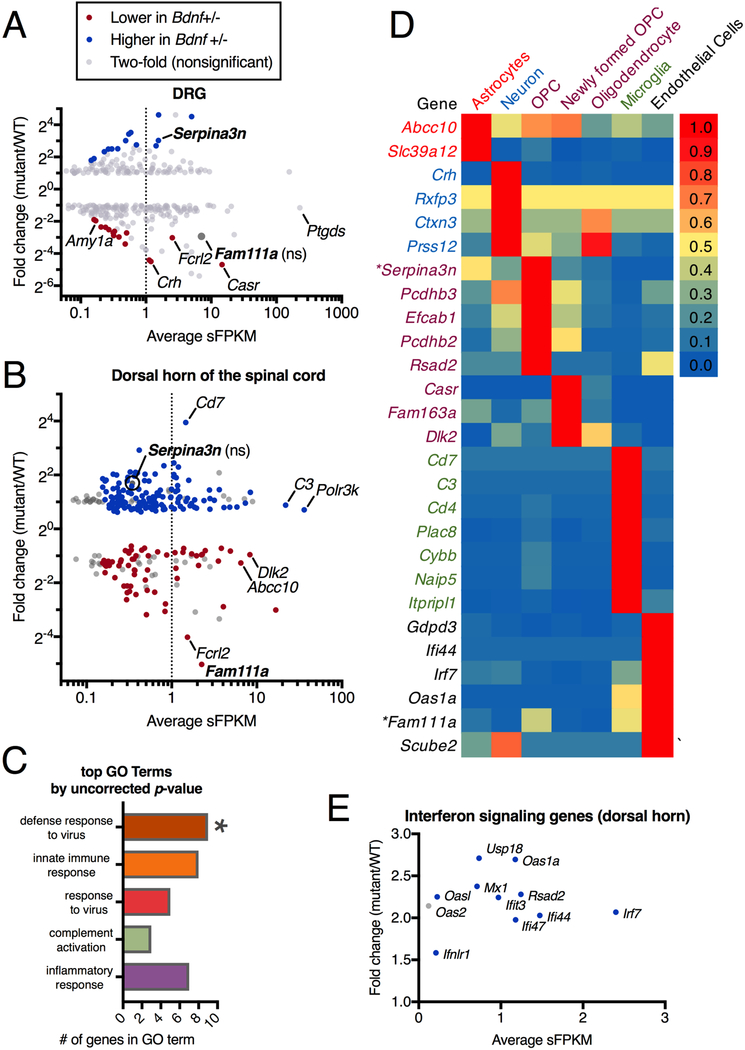
Using RNA-Seq we examined gene expression in Bdnf+/− and Bdnf+/+ rats in both DRG and dorsal spinal cord. In the DRG, our results indicated no reduction in molecular markers for any specific sensory cell type or functional modality. We previously validated this method to detect deletion vs. preservation of specific neuronal subtypes within the DRG[49]. Our quantification includes examination of genes encoding ion channels such as Trpv1 and Trpm8, which transduce hot and cold thermal stimuli (Figure 5A, Supplementary Figure 6), which were not differential in the DRG. In the dorsal horn of the spinal cord, the overall number of significantly differential genes was greater than in the DRG (Figure 5B). We did not observe alterations in known markers of spinal second order nociceptive neurons. Markers of these cells were examined because deletion of cells expressing the substance P/neurokinin1 receptor (Tacr1) is known to cause profound pain insensitivity[29; 39]. Of the genes that are altered, we see some evidence that neuropeptide signaling may be modified in Bdnf+/− rats (Supplementary Figure 6, 7), with the gene encoding corticotrophin-releasing hormone (Crh) strongly reduced in the deleted animals in both tissues examined (Supplementary Figure 8). This is consistent with other reports where decreased Bdnf expression, or that of its receptor, was associated with decreased Crh expression[31]. However, this gene is not highly expressed, and it is unclear to what extent Crh signaling contributes to the Bdnf+/− phenotype. Based on sequencing experiments of glia and other non-neural nervous system cells [62], many of the significantly differential genes are expressed in astrocytes and microglia (Figure 5C, D), pointing to changes in gene expression programs within glial cells, perhaps responding to, or driven by altered neuronal signaling in the spinal cord (Supplementary Figure 9). Ingenuity Pathway Analysis was performed to identify common hub genes based on the observed gene changes. Based on these analyses, many of the genes observed to be altered in Bdnf+/− animals are related to interferon signaling, and all of them are upregulated (Figure 5E, Supplementary Figure 10).
Figure 5. RNA-Seq analysis of dorsal root ganglia and dorsal spinal cord samples from Bdnf+/− and Bdnf+/+ rats.
DRG (N=5) and dorsal spinal cord (N= 10) were collected from Bdnf+/− and Bdnf+/+ rats and RNA-Seq was performed. Significantly differentially-expressed genes between these two groups were plotted according to level of expression (average sFPKM) and fold difference (mutant/WT). The dotted line at 1 sFPKM highlights genes which are highly expressed. Compared to (A) DRG, a larger number of genes are differentially expressed in (B) the dorsal horn and these genes are also more highly differential. (C) GO analysis was performed, with one term “defense response to virus” significantly enriched, suggesting the involvement of non-neural immune cells. (D) Highly expressed significantly differential genes were queried against a database sampling mouse cerebral cortex cell populations [62], showing that most of the differential genes are expressed in glial and other supportive non-neural cells. (E) A large percent of the most upregulated genes are involved in interferon signaling. This relationship was also examined further by IPA analysis in Supplementary Figure 9.
Discussion
The majority of human genetic pain insensitivity syndromes described to date affect primary afferent neurons, leading to inability to transduce noxious stimuli[50]. The present study supports the finding that Bdnf haploinsufficiency leads to modulation of pain sensitivity without loss of primary afferent neurons, a result consistent with previous studies showing unaltered DRG nociceptor populations in homozygous Bdnf knockout mice[26; 63]. Behavioral results from thermal testing of both human WAGR subjects and Bdnf+/− rats point to a model where BDNF haploinsufficiency produces an elevated pain threshold that can be superseded by sufficiently strong stimulation. Our transcriptomic evidence points to a broader alteration in gene expression from Bdnf haploinsufficiency in dorsal spinal cord than in DRG (Figure 5). In concert with our behavioral battery, this suggests a system where transmission in peripheral afferent nociceptors is intact, but the synaptic transmission of these signals to spinal second order neurons, or transmission of the signals by second order neurons to higher CNS regions, is potentially disrupted, requiring abnormally strong stimuli to elicit responses[40]. In part, this may be attributable to altered synaptic efficacy between components of the nociceptive circuitry, which has been reported at a number of synapses throughout the CNS[5; 52]. Given the lack of gene signatures indicative of DRG neuronal disruption, the results are suggestive of congenital indifference to pain[45], although parsing indifference vs. insensitivity in WAGR subjects is challenging due to their rarity, young age, and intellectual disabilities.
Previous experiments using Bdnf knockout mice to examine basal pain sensitivity have yielded conflicting results, with at least one study showing sensitization to some forms of thermal stimulation in DRG-specific Bdnf−/− mice[63]. At least one other study using Bdnf+/− mice showed no difference in baseline thermal pain sensitivity[60]. To add to these apparently conflicting results, reduced sensitivity to thermal sensations has been observed using a hotplate test in both Bdnf+/− mice, and in DRG-specific Bdnf−/− mice[36; 51] consistent with our results in the rat. Overall, strain and species differences as well as technical elements may have contributed to some differences in findings. However, based on mouse studies, it has generally been accepted that BDNF plays a role in the transition from acute to chronic pain, and not in the determination of pain sensitivity[51]. The Bdnf gene is strongly upregulated by persistent inflammatory pain[38](Supplementary Figure 11), supporting its role in the plasticities that occur in the transition between acute and chronic pain[47]. Our results point to actions of Bdnf in spinal cord or other pain modulatory circuit regions, such as the brainstem[47] and are consistent with earlier studies in rat which indicated a CNS site of action[32]. It has also been suggested that spinal BDNF contributes to hyperalgesia by sensitizing second order dorsal horn neurons[4; 20]. BDNF secretion from glial cells has been implicated in regulating many functions related to learning and memory[53], and has been implicated in modulation of pain states[14]. Based on these aggregated findings, BDNF most likely act at a variety of different sites throughout the neuraxis to modulate pain sensitivity.
However, it seems likely that BDNF does modulate the activity of glial cells in the spinal cord, based on the relatively large number of regulated genes in the spinal cord that are differentially expressed in response to Bdnf haploinsufficiency. Within this gene set, the most prominent changes are related to interferon signaling, which is known to participate in neuron-glia interactions, and which has been shown to regulate pain states[55]. In addition to this, it is notable that two of the most highly regulated, most highly expressed genes in our dataset (Serpina3n and Fam111a) are the only two genes identified by a microarray-based study examining high and low pain-sensitive animals after a sciatic nerve ligation model of neuropathic pain[57]. Of particular interest, Serpina3n, a secreted serine protease inhibitor, was posited by the authors of that study to participate in the intercellular communication between neurons and immune cells. Relatedly, treatment of leukocytes with Serpina3n prevented neuronal killing in a neurodegeneration context[22]. In the context of the present paper it is perhaps more likely that the biological process engaged has to do with the formation of appropriate synapses and/or removal or pruning of synapses, as this process is known to involve Bdnf, neuron-glia interactions, and interferon signaling[2].
The significance of BDNF as a pain modulatory gene is evident based on the magnitude and penetrance of the observed reduction in pain sensitivity phenotype. Pain drives individuals to seek medical intervention. In the specific case of WAGR syndrome, these patients are at risk for developing pancreatitis[18], which is frequently accompanied by severe abdominal pain. Diagnosis and treatment of painful medical emergencies can be delayed in patients with pain insensitivity[37] such as that observed in WAGR syndrome, emphasizing a need for close monitoring of patient symptoms. In terms of translational potential for analgesia, our data suggest that BDNF may be part of a larger gene network that modulates the complex trait of pain sensitivity in the general population[50]. Additional studies may further elucidate the relationship between BDNF and nociceptive sensitivity, a topic which has been investigated in a limited manner with regard to the relatively common Val66Met polymorphism[15; 58]. Additionally, while this study did not specifically examine a potential role of proBDNF in pain, recent work has investigated its role in hippocampal plasticity[21; 43]. Ultimately, a greater understanding of these pain regulatory circuits may aid in the development of new analgesic approaches.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of NICHD and the NIH Clinical Center with supplemental funding from the NIH Bench-to-Bedside Program to J.C.H. and to M.J.I. from NCCIH and the Office of Behavioral and Social Sciences. We thank Matthew Tsang, Miriya Tune, Kyra Jefferson-George, and Jamila Crossman for technical assistance. We also thank the families of the International WAGR Syndrome Association for promotion and participation in the clinical studies. The authors thank Xiaobai Li for assistance with statistical analyses.
Abbreviations
- WAGR
Wilms tumor, Aniridia, Genitorinary anomalies, and mental Retardation
- BDNF
Brain-derived neurotrophic factor
- QST
Quantitative sensory testing
Footnotes
The authors have no conflicts of interest at the time this manuscript was written.
References
- [1].Barrot M. Tests and models of nociception and pain in rodents. Neuroscience 2012;211:39–50. [DOI] [PubMed] [Google Scholar]
- [2].Bialas AR, Presumey J, Das A, van der Poel CE, Lapchak PH, Mesin L, Victora G, Tsokos GC, Mawrin C, Herbst R, Carroll MC. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature 2017;546(7659):539–543. [DOI] [PubMed] [Google Scholar]
- [3].Blivis D, Haspel G, Mannes PZ, O’Donovan MJ, Iadarola MJ. Identification of a novel spinal nociceptive-motor gate control for Adelta pain stimuli in rats. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. The European journal of neuroscience 2012;35(2):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in neurobiology 2005;76(2):99–125. [DOI] [PubMed] [Google Scholar]
- [6].Breau LM, McGrath PJ, Camfield CS, Finley GA. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain 2002;99(1–2):349–357. [DOI] [PubMed] [Google Scholar]
- [7].Brenner DS, Golden JP, Gereau RWt. A novel behavioral assay for measuring cold sensation in mice. PloS one 2012;7(6):e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burbelo PD, Iadarola MJ, Alevizos I, Sapio MR. Transcriptomic Segregation of Human Autoantigens Useful for the Diagnosis of Autoimmune Diseases. Mol Diagn Ther 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Capsoni S, Covaceuszach S, Marinelli S, Ceci M, Bernardo A, Minghetti L, Ugolini G, Pavone F, Cattaneo A. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PloS one 2011;6(2):e17321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron 1992;9(4):779–788. [DOI] [PubMed] [Google Scholar]
- [11].Carvalho OP, Thornton GK, Hertecant J, Houlden H, Nicholas AK, Cox JJ, Rielly M, Al-Gazali L, Woods CG. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet 2011;48(2):131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Charlesworth G, Plagnol V, Holmstrom KM, Bras J, Sheerin UM, Preza E, Rubio-Agusti I, Ryten M, Schneider SA, Stamelou M, Trabzuni D, Abramov AY, Bhatia KP, Wood NW. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. American journal of human genetics 2012;91(6):1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen YC, Auer-Grumbach M, Matsukawa S, Zitzelsberger M, Themistocleous AC, Strom TM, Samara C, Moore AW, Cho LT, Young GT, Weiss C, Schabhuttl M, Stucka R, Schmid AB, Parman Y, Graul-Neumann L, Heinritz W, Passarge E, Watson RM, Hertz JM, Moog U, Baumgartner M, Valente EM, Pereira D, Restrepo CM, Katona I, Dusl M, Stendel C, Wieland T, Stafford F, Reimann F, von Au K, Finke C, Willems PJ, Nahorski MS, Shaikh SS, Carvalho OP, Nicholas AK, Karbani G, McAleer MA, Cilio MR, McHugh JC, Murphy SM, Irvine AD, Jensen UB, Windhager R, Weis J, Bergmann C, Rautenstrauss B, Baets J, De Jonghe P, Reilly MM, Kropatsch R, Kurth I, Chrast R, Michiue T, Bennett DL, Woods CG, Senderek J. Transcriptional regulator PRDM12 is essential for human pain perception. Nature genetics 2015;47(7):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005;438(7070):1017–1021. [DOI] [PubMed] [Google Scholar]
- [15].Di Lorenzo C, Di Lorenzo G, Daverio A, Pasqualetti P, Coppola G, Giannoudas I, Barone Y, Grieco GS, Niolu C, Pascale E, Santorelli FM, Nicoletti F, Pierelli F, Siracusano A, Seri S. The Val66Met polymorphism of the BDNF gene influences trigeminal pain-related evoked responses. The journal of pain : official journal of the American Pain Society 2012;13(9):866–873. [DOI] [PubMed] [Google Scholar]
- [16].Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na v 1.7 and human pain disorders. Trends in neurosciences 2007;30(11):555–563. [DOI] [PubMed] [Google Scholar]
- [17].Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Human molecular genetics 2004;13(8):799–805. [DOI] [PubMed] [Google Scholar]
- [18].Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M. WAGR syndrome: a clinical review of 54 cases. Pediatrics 2005;116(4):984–988. [DOI] [PubMed] [Google Scholar]
- [19].Fitzgerald M The development of nociceptive circuits. Nat Rev Neurosci 2005;6(7):507–520. [DOI] [PubMed] [Google Scholar]
- [20].Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. The European journal of neuroscience 2003;18(9):2467–2476. [DOI] [PubMed] [Google Scholar]
- [21].Giza JI, Kim J, Meyer HC, Anastasia A, Dincheva I, Zheng CI, Lopez K, Bains H, Yang J, Bracken C, Liston C, Jing D, Hempstead BL, Lee FS. The BDNF Val66Met Prodomain Disassembles Dendritic Spines Altering Fear Extinction Circuitry and Behavior. Neuron 2018;99(1):163–178 e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haile Y, Carmine-Simmen K, Olechowski C, Kerr B, Bleackley RC, Giuliani F. Granzyme B-inhibitor serpina3n induces neuroprotection in vitro and in vivo. J Neuroinflammation 2015;12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med 2008;359(9):918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Han JC, Thurm A, Golden Williams C, Joseph LA, Zein WM, Brooks BP, Butman JA, Brady SM, Fuhr SR, Hicks MD, Huey AE, Hanish AE, Danley KM, Raygada MJ, Rennert OM, Martinowich K, Sharp SJ, Tsao JW, Swedo SE. Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behaviour and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex 2013;49(10):2700–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, Davies AM. Novel class of pain drugs based on antagonism of NGF. Trends in pharmacological sciences 2006;27(2):85–91. [DOI] [PubMed] [Google Scholar]
- [26].Heppenstall PA, Lewin GR. BDNF but not NT-4 is required for normal flexion reflex plasticity and function. Proceedings of the National Academy of Sciences of the United States of America 2001;98(14):8107–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang F, Wang X, Ostertag EM, Nuwal T, Huang B, Jan YN, Basbaum AI, Jan LY. TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nature neuroscience 2013;16(9):1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain research 1988;455(2):205–212. [DOI] [PubMed] [Google Scholar]
- [29].Iadarola MJ, Sapio MR, Wang X, Carrero H, Virata-Theimer ML, Sarnovsky R, Mannes AJ, FitzGerald DJ. [EXPRESS]Analgesia by Deletion of Spinal Neurokinin 1 Receptor Expressing Neurons Using a Bioengineered Substance P-Pseudomonas Exotoxin Conjugate. Molecular pain 2017;13:1744806917727657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature genetics 1996;13(4):485–488. [DOI] [PubMed] [Google Scholar]
- [31].Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proceedings of the National Academy of Sciences of the United States of America 2012;109(4):1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 1999;19(12):5138–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim H, Neubert JK, Rowan JS, Brahim JS, Iadarola MJ, Dionne RA. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain 2004;5(7):377–384. [DOI] [PubMed] [Google Scholar]
- [34].LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, Ramsden CE, Iadarola MJ, Mannes AJ. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 2018;38(5):912–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends in neurosciences 1993;16(9):353–359. [DOI] [PubMed] [Google Scholar]
- [36].MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci 2001;115(5):1145–1153. [DOI] [PubMed] [Google Scholar]
- [37].Mannes A, Iadarola M. Potential downsides of perfect pain relief. Nature 2007;446(7131):24. [DOI] [PubMed] [Google Scholar]
- [38].Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proceedings of the National Academy of Sciences of the United States of America 1999;96(16):9385–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 1997;278(5336):275–279. [DOI] [PubMed] [Google Scholar]
- [40].Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Progress in neurobiology 2008;85(3):297–317. [DOI] [PubMed] [Google Scholar]
- [41].Mitchell K, Bates BD, Keller JM, Lopez M, Scholl L, Navarro J, Madian N, Haspel G, Nemenov MI, Iadarola MJ. Ablation of rat TRPV1-expressing Adelta/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Molecular pain 2010;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mitchell K, Lebovitz EE, Keller JM, Mannes AJ, Nemenov MI, Iadarola MJ. Nociception and inflammatory hyperalgesia evaluated in rodents using infrared laser stimulation after Trpv1 gene knockout or resiniferatoxin lesion. Pain 2014;155(4):733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T, Yamawaki S, Takahashi M, Shiosaka S, Itami C, Uegaki K, Saarma M, Kojima M. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proceedings of the National Academy of Sciences of the United States of America 2015;112(23):E3067–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Montagne-Clavel J, Oliveras JL. The “plantar test” apparatus (Ugo Basile Biological Apparatus), a controlled infrared noxious radiant heat stimulus for precise withdrawal latency measurement in the rat, as a tool for humans? Somatosens Mot Res 1996;13(3–4):215–223. [DOI] [PubMed] [Google Scholar]
- [45].Nagasako EM, Oaklander AL, Dworkin RH. Congenital insensitivity to pain: an update. Pain 2003;101(3):213–219. [DOI] [PubMed] [Google Scholar]
- [46].Raithel SJ, Sapio MR, LaPaglia DM, Iadarola MJ, Mannes AJ. Transcriptional Changes in Dorsal Spinal Cord Persist after Surgical Incision Despite Preemptive Analgesia with Peripheral Resiniferatoxin. Anesthesiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ren K, Dubner R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: role of BDNF-TrkB signaling and NMDA receptors. Molecular neurobiology 2007;35(3):224–235. [DOI] [PubMed] [Google Scholar]
- [48].Sapio MR, Goswami SC, Gross JR, Mannes AJ, Iadarola MJ. Transcriptomic analyses of genes and tissues in inherited sensory neuropathies. Experimental neurology 2016;283(Pt A):375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sapio MR, Neubert JK, LaPaglia DM, Maric D, Keller JM, Raithel SJ, Rohrs EL, Anderson EM, Butman JA, Caudle RM, Brown DC, Heiss JD, Mannes AJ, Iadarola MJ. Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. The Journal of clinical investigation 2018;128(4):1657–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sexton JE, Cox JJ, Zhao J, Wood JN. The Genetics of Pain: Implications for Therapeutics. Annu Rev Pharmacol Toxicol 2018;58:123–142. [DOI] [PubMed] [Google Scholar]
- [51].Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, Zhao J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain : a journal of neurology 2018;141(4):1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 1994;77(5):627–638. [DOI] [PubMed] [Google Scholar]
- [53].Song M, Martinowich K, Lee FS. BDNF at the synapse: why location matters. Mol Psychiatry 2017;22(10):1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].St Laurent R, Helm SR, Glenn MJ. Reduced cocaine-seeking behavior in heterozygous BDNF knockout rats. Neuroscience letters 2013;544:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America 2009;106(19):8032–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tzabazis A, Klyukinov M, Manering N, Nemenov MI, Shafer SL, Yeomans DC. Differential activation of trigeminal C or Adelta nociceptors by infrared diode laser in rats: behavioral evidence. Brain research 2005;1037(1–2):148–156. [DOI] [PubMed] [Google Scholar]
- [57].Vicuna L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA, Arnold B, Devor M, Woolf CJ, Liberles SD, Costigan M, Kuner R. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med 2015;21(5):518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wei SY, Chao HT, Tu CH, Lin MW, Li WC, Low I, Shen HD, Chen LF, Hsieh JC. The BDNF Val66Met polymorphism is associated with the functional connectivity dynamics of pain modulatory systems in primary dysmenorrhea. Scientific reports 2016;6:23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wong DL, Baker CM. Smiling faces as anchor for pain intensity scales. Pain 2001;89(2–3):295–300. [DOI] [PubMed] [Google Scholar]
- [60].Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. Journal of neurochemistry 2005;93(3):584–594. [DOI] [PubMed] [Google Scholar]
- [61].Zhang W, Yu Y, Hertwig F, Thierry-Mieg J, Zhang W, Thierry-Mieg D, Wang J, Furlanello C, Devanarayan V, Cheng J, Deng Y, Hero B, Hong H, Jia M, Li L, Lin SM, Nikolsky Y, Oberthuer A, Qing T, Su Z, Volland R, Wang C, Wang MD, Ai J, Albanese D, Asgharzadeh S, Avigad S, Bao W, Bessarabova M, Brilliant MH, Brors B, Chierici M, Chu TM, Zhang J, Grundy RG, He MM, Hebbring S, Kaufman HL, Lababidi S, Lancashire LJ, Li Y, Lu XX, Luo H, Ma X, Ning B, Noguera R, Peifer M, Phan JH, Roels F, Rosswog C, Shao S, Shen J, Theissen J, Tonini GP, Vandesompele J, Wu PY, Xiao W, Xu J, Xu W, Xuan J, Yang Y, Ye Z, Dong Z, Zhang KK, Yin Y, Zhao C, Zheng Y, Wolfinger RD, Shi T, Malkas LH, Berthold F, Wang J, Tong W, Shi L, Peng Z, Fischer M. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome biology 2015;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014;34(36):11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN, London Pain C. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Molecular and cellular neurosciences 2006;31(3):539–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Rat sequencing data from the DRG and dorsal horn of Bdnf+/+ and Bdnf+/− animals has been deposited into the Sequence Read Archive under BioProject number (To be released upon publication of this manuscript).