Abstract
Severe corneal injuries often result in permanent vision loss and remain a clinical challenge. Human bone marrow‐derived mesenchymal stem cells (MSCs) and their secreted factors (secretome) have been studied for their antiscarring, anti‐inflammatory, and antiangiogeneic properties. We aimed to deliver lyophilized MSC secretome (MSC‐S) within a viscoelastic gel composed of hyaluronic acid (HA) and chondroitin sulfate (CS) as a way to enhance corneal re‐epithelialization and reduce complications after mechanical and chemical injuries of the cornea. We hypothesized that delivering MSC‐S within HA/CS would have improved wound healing effects compared the with either MSC‐S or HA/CS alone. The results showed that a once‐daily application of MSC‐S in HA/CS enhances epithelial cell proliferation and wound healing after injury to the cornea. It also reduced scar formation, neovascularization, and hemorrhage after alkaline corneal burns. We found that combining MSC‐S and HA/CS increased the expression of CD44 receptors colocalized with HA, suggesting that the observed therapeutic effects between the MSC‐S and HA/CS are in part mediated by CD44 receptor upregulation and activation by HA. The results from this study demonstrate a reproducible and efficient approach for delivering the MSC‐S to the ocular surface for treatment of severe corneal injuries. stem cells translational medicine 2019;8:478–489
Keywords: Cellular proliferation, Chondroitin sulfate, Cornea, Mesenchymal stem cells
Significance Statement.
This study demonstrates that the lyophilized secretome of mesenchymal stem cells reconstituted within a viscoelastic gel of hyaluronic acid and chondroitin sulfate enhances corneal epithelial wound healing both in vitro and in vivo, and mitigates the development of stromal scarring and neovascularization after alkaline burns in vivo.
Introduction
The corneal epithelium protects the eye against pathogen invasion and plays an essential role in preserving corneal clarity. Thus, it is highly vulnerable to the blinding consequences of severe injury or disease. Following injury, the epithelium undergoes a highly coordinated repair process, involving migration, proliferation, and differentiation. This process is orchestrated in part by its interactions with the extracellular matrix, growth factor/cytokines, and the residents cells, including keratocytes and infiltrate leukocytes 1. In certain pathologic, highly inflammatory conditions such as chemical injury, the entire ocular surface is at risk for permanent scarring and visual loss. The management of corneal wounds has not changed significantly in the last few decades and consists mainly of supportive measures in the form of lubrication, antibiotics, steroids, patching, autologous serum eye, and in some cases, sutured lid closure or amniotic membrane grafting 2, 3. Thus, there is a definite need to develop effective clinical strategies to promote wound healing in patients with severe ocular surface injuries where the outcomes of current therapies are suboptimal.
Mesenchymal stem cells (MSCs) are known to play an important role in tissue repair and maintenance and have been extensively studied for regenerative therapies 4, 5. There is a wealth of evidence from animal studies that the plethora of cytokines and growth factors working in concert within the secreted factors (secretome) of MSCs are able to reduce scarring, neovascularization (NV), and inflammation while promoting epithelialization after injuries 6, 7, 8, 9, 10, 11, 12, 13. However, studies showing the application of harvested MSC secretome (MSC‐S) directly onto the cornea are limited. This may be due to the lack of effective delivery methods, thereby impacting MSC‐S bioavailability and consequently its biological role. Simple delivery of soluble therapeutic factors as an eye drop to the ocular surface is limited and cost‐inefficient due to fluid turnover and drainage which results in substantial volumetric losses. To address this problem, herein we delivered human bone marrow derived MSC‐S to both mechanical and chemical corneal wounds in rats using a viscoelastic gel as a carrier.
We aimed to answer (a) whether we can use a viscoelastic gel composed of hyaluronic acid (HA) and chondroitin sulfate (CS) as a carrier, and (b) whether combining MSC secretome (MSC‐S) and HA/CS (MSC‐S in HA/CS) improves the rate and extent of wound healing, and (c) by what mechanisms MSC‐S and HA/CS work together to enhance corneal wound healing.
Materials and Methods
Primary Human Corneal Epithelial Cell Culture
Primary human corneal epithelial cells (HCECs) were harvested from human cadaver corneas kindly provided by Eversight (Chicago, IL) as described elsewhere 14. Briefly, the iris, conjunctiva, and sclera were removed and the cornea was washed with phosphate‐buffered saline (PBS) at least three times. Then the cornea was treated with dispase (2 mg/ml; Gibco, Waltham, MA) at 37°C for 2 hours to separate the peripheral epithelium as sheets from the stroma. Epithelial sheets were gently scraped using a blunt scraper, collected, and then digested in 0.25% trypsin–EDTA for 5–10 minutes. Cells were washed and resuspended in keratinocyte serum‐free medium (KSFM; Thermo Fisher Scientific, Waltham, MA) fortified with 0.05 mg/ml of Bovine Pituitary Extract, 5 ng/ml of Epidermal Growth Factor, 0.005 mg/ml of insulin (Sigma‐Aldrich, St. Louis, MO), and 500 ng/ml of Hydrocortisone (Sigma‐Aldrich) and plated in collagen‐coated tissue culture plates. After being grown to confluence, cells from passages 3–5 were used for all experiments.
Primary HCEC Characterization
Harvested HCECs in complete KSFM with growth supplements were evaluated for the presence of CK3, p63, and ABCG2 using immunofluorescence. Later‐passage HCECs treated with HA/CS, MSC‐S, and MSC‐S in HA/CS, and complete KSFM were stained for CK3. The expression of CK3, p63, and ABCG2 was detected as follows. After 24 hours, the cells were fixed with 4% PFA for 15 minutes. After washing with PBS, the wells were blocked, and the cells were permeabilized for 30 minutes with 5% normal goat serum and 0.5% Triton‐X. Next, the cells were incubated overnight with primary antibody CK3 (Abcam, Cambridge, MA: 2Q1040, 1:100), p63 (GeneTex, Irvine, CA: C838X53, 1:50), and ABCG2 (Cell Signaling, Danvers, MA: 42078, 1:100). After washing three times with a secondary antibody (1:1,000), Alexa Fluor 488 was added for 2 hours. Finally, after washing with PBS, DAPI was added for 5 minutes in PBS solution (1:1,000). The cells were mounted, and the presence of cell markers was observed using confocal microscopy (ZEISS LSM 880, Carl Zeiss Ag, Oberkochen, Germany).
Treatment Formulations
HA/CS (DisCoVisc ophthalmic viscosurgical device [OVD], Alcon, Fort Worth, TX) was diluted with 1 ml of KSFM without supplements or PBS to achieve a concentration of 8.5 mg/ml of HA (1.7 MDa). Secretome was collected from MSCs as previously described 9, 10 and was lyophilized. Bone marrow (BM)‐derived MSCs were kindly provided to author ARD's research group at the University of Illinois at Chicago (UIC) by Dr. Peiman Hematti's group at the University of Wisconsin Hospital and Clinics. The protocol of BM MSC isolation was approved by the Health Sciences Institutional Review Board of University of Wisconsin‐Madison School of Medicine and Public Health; the BM MSCs were obtained from discarded BM filters of healthy donors after BM donation at the University of Wisconsin Hospital and Clinics. The BM‐MSCs were expanded and their secretome collected at UIC as follows. Briefly, MSCs were seeded onto 1% gelatin (Sigma‐Aldrich) coated wells of a six‐well tissue culture plate in alpha MEM media supplemented with 10% fetal bovine serum, 1x l‐glutamine (Corning, NY), and 1x NEAA (Corning). Culture media were changed every other day, and cells were subcultured by brief digestion with TrypLE Express (Thermo Fisher Scientific) when 90% confluent. Passages 3–6 of BM MSCs were used for all experiments. Upon reaching 100% confluency in a T175 flask, the MSCs were washed with 30 ml of prewarmed PBS three times. The media was then changed to phenol red‐free alpha MEM media supplemented with 1x l‐glutamine, and 1x NEAA. The conditioned media was collected after 48 hours. The cells were trypsinized and counted at the same time. The conditioned media was centrifuged at 500g for 15 minutes to remove any cells or debris. The supernatant was then transferred to a new tube and frozen with liquid nitrogen. The frozen secretome was then placed in a freeze dryer and lyophilized overnight under vacuum (70 mTorr). Lyophilized MSC‐S was then diluted in KSFM without growth supplements or PBS to a concentration of 100 mg/ml. Next, 100 μl of MSC‐S was added to 1.9 ml of diluted HA/CS, KSFM without growth supplements, or PBS to a concentration of 5.0 mg/ml. Finally, 50 μl of reconstituted MSC‐S in HA/CS was added to the cultured cells in 150 μl of KSFM without growth supplements. For cell culture assays final concentrations of MSC‐S and HA were 1.25 and 2.1 mg/ml, respectively. The no treatment group received complete KSFM with growth supplements (control).
Live/Dead Cytotoxicity Assay
Primary HCECs were seeded on collagen‐coated 48‐well plates at a concentration of 2 × 104 cells per well, in KSFM complete with growth supplements. After 6 hours, the cells were washed and starved with medium without growth supplements for 12 hours. Then, the treatments were added to the cells for 24, 48, and 72 hours. After each time point, the medium was removed and labeling reagents from a Live/Dead cytotoxicity assay (Thermo Fisher Scientific) were added to the cells in KSFM without growth supplements, according to the manufacturer's instructions.
In Vitro HCEC Proliferation
Primary HCECs were seeded on collagen‐coated surfaces in complete growth medium, at a concentration of 5–8 × 103 cells per well. After 6 hours, the medium was removed and KSFM without growth supplements was added to the cells. The cells were starved overnight. The next day, 50 μl of the treatments were added to the cells, in 150 μl of medium without growth supplements. Treatments consisted of MSC‐S alone, HA/CS alone, MSC‐S in HA/CS, and KSFM complete with growth supplements. After 24, 48, and 72 hours, water‐soluble tetrazolium salt‐8 solution from Cell Counting Kit 8 (CCK‐8, Sigma‐Aldrich) was added to each well following manufacturer's protocol. The absorbance was measured 2 hours after incubation with the water‐soluble tetrazolium salt‐8 solution. In a separate, parallel set of experiment, plates with cultured and treated cells were frozen at −80°C for 7 days. After thawing the plates, CyQUANT (Thermo Fisher Scientific) solution was added to the wells following manufacturer's protocol, and the fluorescence was measured at 650 nm after 5 minutes.
In Vivo Animal Studies
All procedures involving animals conformed to the Association for Research in Vision and Ophthalmology Statement for the use of Animals in Ophthalmic and Vision Research. The study procedures were approved by the Administrative Panel on Laboratory Animal Care of Stanford University (rats) and UIC (mice). Mice experiments are discussed in further detail in the Supporting Information section. Female Sprague‐Dawley rats (7–8 weeks old, weighing approximately 200–250 g) were used in the present study. All surgical procedures were performed in the semipathogen free zone of our animal care facility. Prior to surgery and/or treatment, animals were placed individually into the anesthetic induction chamber. Inhaled anesthesia was induced with approximately 3%–4% isoflurane in oxygen (1 l/min) and maintained with 2% isoflurane in oxygen during the procedures. Then, 0.5% proparacaine (Alcaine, Alcon) ophthalmic solution drops were instilled in the left eyes of the animals. Postoperative analgesic with subcutaneous buprenorphine SR (ZooPharm, Windsor, CO) (0.5 mg/kg) was injected prior to the start of surgical procedure and every 72 hours until complete wound closure.
Corneal Mechanical Wound Model
A circular 5‐mm‐diameter epithelial wound was made over the center of the rat corneas as previously reported 15. Briefly, the corneal epithelium was demarcated with a biopsy punch (5 mm diameter, Mil‐Tec, Lehigh Acres, FL) centering on the visual axis and subsequently removed with a burr (Algerbrush II, Allomed, Milwaukee, WI) under an operating microscope (VistaVision, Champaign, IL). After the injury to the epithelium, animals were randomly divided into four groups: saline control (n = 5), MSC‐S‐only treatment (n = 5), HA/CS‐only treatment (n = 5), and MSC‐S in HA/CS treatment (n = 5). For all the experiments involving animals, the topical treatment was applied to the left eye of each rat and the treatment volume was 20 μl for all groups. The treatment was performed once daily, immediately after the injury and then continued each day until postoperative day 4.
Corneal Alkaline Burn Wound Model
Alkaline burns of the cornea were induced in the left eye of each rat. A filter paper disc (5 mm in diameter) soaked with 1 M NaOH was placed on the center of the corneal surface for 30 seconds, followed by irrigation with 200 ml of saline. After the corneal alkaline burn, animals were randomly divided into four groups: saline control (n = 7), MSC‐S only treatment (n = 7), HA/CS treatment (n = 7), and treatment with MSC‐S in HA/CS (n = 7). Starting immediately after the alkaline injury, the various treatments were applied once a day for 1 week. On day 14 postinjury, rats were sacrificed and corneas excised for histological examination. Photographs of fluorescein‐stained corneas were taken with a smartphone ophthalmic imaging adapter (Paxos Scope, by Digisight Technologies, now Verana Health, San Francisco, CA) equipped with a 15× magnifying lens and a blue LED immediately after the injury and every 24 hours until the corneal epithelium of all groups was completely healed 16. The area of the epithelial defects was measured using ImageJ software (version 1.52b, NIH). The percentage of wound closure (mean ± SD of the mean) was calculated at each time point using the following equation: wound closure (%) = [(initial defect area − remaining defect area)/initial defect area]. Corneal NV was quantified as previously described 17. Briefly, the area of NV was estimated by the length of radial penetration and extent in degrees using ImageJ. It was assumed that a mean rat corneal diameter was 2.5 mm, so that the maximal area of NV was no more than 2.52 mm2 (mean value of the radius of rat cornea) π (=19.95 mm2). Additionally, corneal NV was divided into nonsignificant or significant according to vessel involvement of the central 3 mm of cornea.
Immunofluorescent Staining of HCECs for CD44 and HA expression
HCECs were fixed with PFA 4% for 15 minutes, after 24 hours of incubation with the treatments. Next, cells were permeabilized for 30 minutes with 5% normal goat serum and 0.5% Triton‐X. Next, CD44 (Abcam: ab157107, 1:100) antibody and biotinylated HA‐binding protein (EMD Millipore, Burlington, MA, 1:100) were added to the cells overnight at 4°C. The next day, the cells were washed with PBS and secondary antibodies (Alexa Fluor 488) and Streptavidin‐Alexa Fluor 555 conjugate (1:1,000) was added for 2 hours. Finally, after washing with PBS, DAPI was added for 5 minutes in PBS solution (1:1,000). The cells were mounted and observed using confocal microscopy (ZEISS LSM 880, Carl Zeiss Ag). Quantitative analyses of the images were performed using ImageJ (version 1.52b, NIH). For these analyses three different sections were randomly acquired per sample. The average mean gray value of the samples normalized by the no treatment group was used for statistical analysis 18.
Immunohistochemical Staining of Treated Rat Corneas
Eyes from rats that had mechanical and chemical burn injuries with and without treatment were fixed with 4% PFA for 15 minutes. Then, the eyes were transferred to 15% sucrose for 2 hours and then left in 30% sucrose overnight at 4°C. The eyes were then immersed in Tissue‐Tek (Sakura Finetek, Torrance, CA). The eyes were sectioned and permeabilized for 30 minutes with 5% normal goat serum and 0.5% Triton‐X. Next, ZO‐1 (Thermo Fisher Scientist, 1:100), alpha muscle smooth actin (Abcam: ab7817, 1:100), CD31 (Abcam: ab64543, 1:100), Aldehyde Dehydrogenase 3 Family Member A1 (ALDH3A1‐Abcam: ab76976, 1:100), and CD44 were added to the cornea sections and incubated overnight in the same permeabilization solution. After washing three times with PBS, secondary antibodies (1:1000), Alexa Fluor 488, 555, and 546, were added for 2 hours. Finally, after washing with PBS, DAPI was added for 5 minutes in PBS solution (1:1,000). The corneas were mounted and observed using confocal microscopy (ZEISS LSM 880, Carl Zeiss Ag). Quantitative analyses of the images were performed using ImageJ (version 1.52b, NIH) For these analyses, a total of 10 pictures were taken for each eye sample. The average mean gray value of the samples normalized by the saline or normal group was used for statistical analysis.
Statistical Analyses
All data are expressed as the mean ± SD. Statistical evaluation of cell proliferation and in vivo wound healing data was performed using a two‐way ANOVA followed by a Dunnett's multiple comparisons test. Immunostaining and NV data were statistically evaluated using one‐way ANOVA followed by a Dunnett's multiple comparisons test. A value of p < .05 was considered statistically significant. The statistical analysis was performed by using GraphPad Prism 7.
Results
HCECs Remain Viable After Treatment With Combined MSC‐S and HA/CS
Untreated HCECs were found to express p63 and ABCG2 in addition to CK3, indicating that the cultures contained a mixed population of limbal and central epithelial cells. Primary cultured HCECs stained positively for CK3 after treatment with MSC‐S, HA/CS, and MSC‐S in HA/CS (Fig. 1A), indicating epithelial differentiation. The HCECs also remained viable under all conditions compared to complete KSFM out to 72 hours after treatment as determined through a Live/Dead cytotoxicity assay (Fig. 1B). Of note, cell agglomeration with the formation of 3D structures was observed in MSC‐S at 72 hours, and earlier (at 48 hours) in the MSC‐S in HA/CS group (Fig. 1B).
Figure 1.
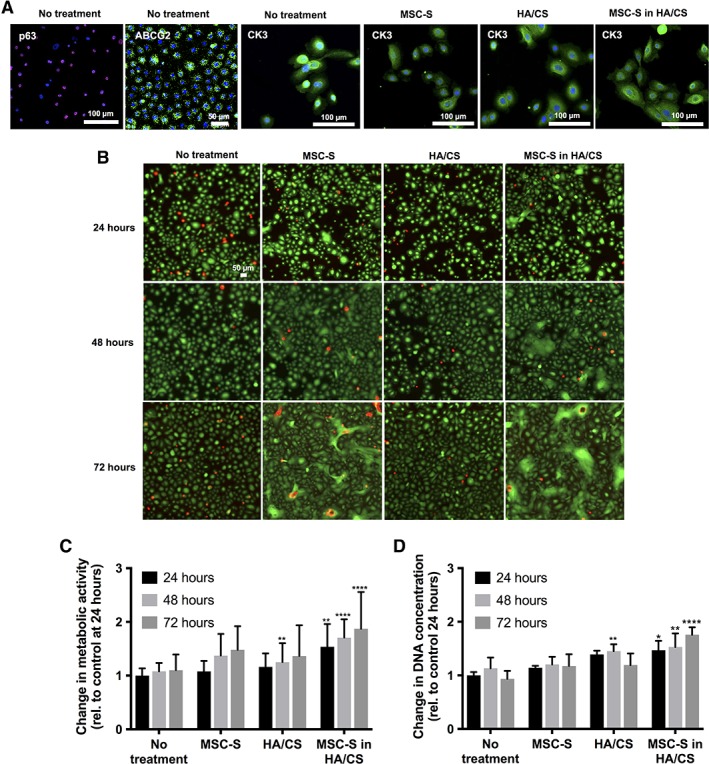
(A): P63, ABCG2, and CK3 staining were observed in the harvested primary human corneal epithelial cells (HCECs) used in this study, indicating a mixed population of limbal and central stem cells. CK3 was expressed in these cells 24 hours after treating with complete keratinocyte serum‐free medium (KSFM) with growth factors (no treatment, control), MSC‐S, HA/CS, MSC‐S in HA/CS. (B): Live/dead cytotoxicity assay after treatment with complete KSFM with growth factors, MSC‐S, HA/CS, and MSC‐S in HA/CS over 72 hours in primary corneal epithelial cells. (C): Effect of MSC‐S, HA/CS, and MSC‐S in HA/CS on primary HCECs proliferation. Cell proliferation was determined using a cell metabolic activity assay, and (D): DNA concentration, at 24, 48, and 72 hours. The data were normalized to no treatment at 24 hours. In both assays, proliferation was statistically significantly greater when MSC‐S was delivered in HA/CS compared to no treatment (n = 4; *, p < .05; **, p < .01; ****, p < .0001). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
MSC‐S in HA/CS Increases HCEC Proliferation
An increase in HCEC proliferation was observed when the cells were treated with MSC‐S in HA/CS, compared to the no treatment group (Fig. 1C, 1D). The increased cell proliferation was determined by an increase in cell metabolic activity (Fig. 1C) and DNA concentration (Fig. 1D). The increase in cell metabolic activity was statistically different at 24 hours (**, p < .01), 48 hours (****, p < .0001), and 72 hours (****, p < .0001) compared to the no treatment group. It was also higher at 48 and 72 hours compared to 24 hours. MSC‐S alone did not statistically increase cell metabolic activity compared to the no treatment group. HA/CS did increase cell proliferation compared to no treatment, but only at the 48 hour time point (**, p < .01). In addition, cells treated with MSC‐S in HA/CS exhibited increased proliferation compared to HA/CS and MSC‐S alone, at 24, 48, and 72 hours (**, p < .01). The increase in DNA concentration was also statistically different at 24 hours (*, p < .05), 48 hours (**, p < .01), and 72 hours (****, p < .0001) compared to the no treatment group. The increase in DNA concentration (Fig. 1D) was also higher at 48 and 72 hours compared to 24 hours within the MSC‐S in HA/CS group. In both assays, MSC‐S did not significantly increase cell proliferation compared to the no treatment group while HA/CS increased DNA concentration at the 48 hours (**, p < .01) time point but not at 24 or 72 hours.
MSC‐S in HA/CS Increases the Rate of Re‐Epithelialization in a Mechanical Wound Model in Rat Corneas
We next aimed to evaluate if MSC‐S in HA/CS would increase the epithelial wound healing rate in a rat model of mechanical cornea injury (Fig. 2A). After creating 5‐mm diameter epithelial defects on rat corneas, we compared the area of the wound, in all groups, over 72 hours using fluorescein staining. After 24 hours, the percent wound closure for the group that received MSC‐S in HA/CS was 84.3% ± 6.7%, compared to 51.3% ± 12.3% for HA/CS, 52.7% ± 8.1% for MSC‐S and 58.5% ± 17.5% for the saline group (**, p < .01) (Fig. 2B). The percent wound closure in the MSC‐S in HA/CS group was statistically significant over the saline group (**, p < .01). To confirm corneal epithelial phenotype after healing, the treated corneas were stained with ZO‐1. Figure 2C shows that this marker was present 7 days after treating the cornea with MSC‐S in HA/CS. In addition, a multilayered epithelium was observed in all the groups as shown by light microscopy (Fig. 2D).
Figure 2.
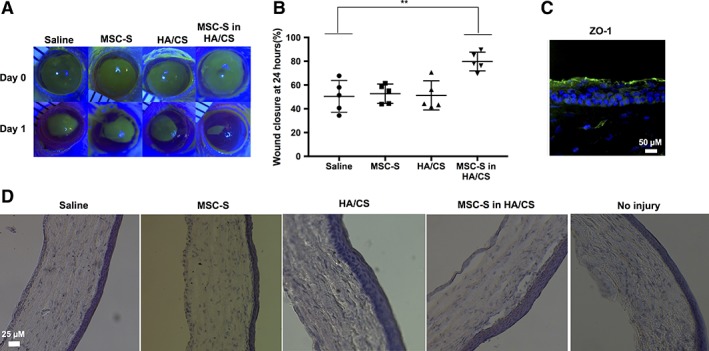
Results of MSC‐S treatments in a mechanical corneal wound model in rats in vivo. The epithelial layer from rat corneas was debrided and the MSC‐S was applied with and without HA/CS, compared to HA/CS alone and saline alone, using 1 drop of each daily. (A): Fluorescein staining of the treated corneas was used to quantify the size of the epithelial defect on a daily basis for each of the treatment groups. Shown are representative photos under blue light illumination for each of the treatment groups (Saline, MSC‐S, HA/CS, and MSC‐S in HA/CS). (B): After 24 hours, the group that received MSC‐S in HA/CS had smaller wound sizes compared to saline group (**, p < .01), whereas HA/CS and MSC‐S treatments alone did not. (C): Immunostaining of rat corneas 7 days after MSC‐S in HA/CS treatment showing that the epithelial layer was able to form ZO‐1—scale bar 50 μm. (D): Hematoxylin and eosin staining of the cornea from saline, MSC‐S, HA/CS, MSC‐S in HA/CS and no injury. A stratified epithelium formed in all groups by day 7—scale bar 25 μm. Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
To test whether increased viscosity of a gel vehicle alone, or the presence of inert protein alone could be responsible for the observed wound healing effects of MSC‐S in HA/CS, we substituted HA/CS with an over‐the‐counter Systane ophthalmic lubricant gel (0.3% Hypromellose), and the MSC‐S with bovine serum albumin (BSA) (Fig. 3A). We applied saline, MSC‐S in Systane, BSA in Systane, and MSC‐S in HA/CS. Only the eyes that received MSC‐S in HA/CS showed faster wound healing compared to the saline group (*, p < .05) at 24 hours (Fig. 3B).
Figure 3.
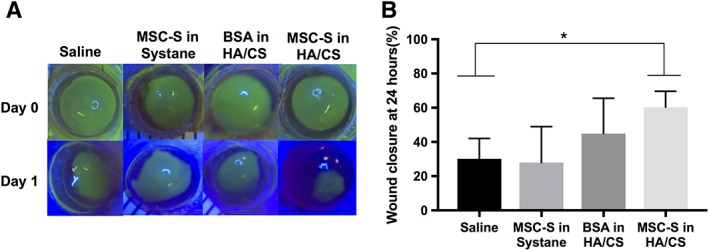
Results of different carrier gel treatments in a mechanical corneal wound model in rats in vivo. (A): Fluorescein staining of corneas examined under blue light revealed that MSC‐S delivered in HA/CS yielded smaller wound areas 1 day after treatment compared to saline, MSC‐S in Systane gel (0.4% polyethylene glycol 400% and 0.3% propylene glycol) and BSA in HA/CS. Each treatment was applied right after the injury on day 0. (B): Quantification of fluorescently stained wound areas showed that the MSC‐S in HA/CS treatment led to a statistically significantly smaller wound area at 24 hours compared to all other treatments (*, p < .05). Abbreviations: BSA, bovine serum albumin; CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
MSC‐S in HA/CS Enhances Re‐Epithelialization and Reduces Scar Formation and Hemorrhage in a Corneal Alkali Burn Model
Next, we aimed to evaluate if MSC‐S in HA/CS would enhance wound closure in a severe alkaline burn model (Fig. 4). All treatments in this model were given once daily out to 7 days, and eyes were monitored out to 14 days. For epithelial wound healing after alkaline burns, the results were consistent with those seen in the mechanical wound model. After 24 hours, wound closure for the group that received MSC‐S in HA/CS was 90.5% ± 5.9%, for those that received HA/CS alone was 66% ± 21.8%, for those that received MSC‐S alone was 62.4% ± 25.9% and for saline group it was 63.6% ± 11.12% (*, p < .05). The wound area at 24 hours in the MSC‐S in HA/CS group was significantly smaller than the saline group (*, p < .05) (Fig. 3B). At day 2, the wound was completely closed only in the MSC‐S in HA/CS group. By day 3, the wound had completely closed in the eyes that received HA/CS and MSC‐S alone, but not in the eyes that received saline. These results are summarized in Figure 4A. After wound closure, we continued monitoring the corneas for ongoing tissue damage after the alkaline burn, such as scar formation, NV, and hemorrhage (Fig. 4A). At day 4, scar formation is observed in the saline group and at day 5 NV with hemorrhage was present in the HA/CS group. The scar and hemorrhage worsened each day for these groups over the 14 days. The differences in the overall sizes of the neovascular areas between the saline group and the MSC‐S in HA/CS group was found to be statistically significant at 14 days (Fig. 4D, *, p < .05). In summary, the corneas that received MSC‐S and MSC‐S in HA/CS daily for 7 days exhibited reduced overall scar formation and hemorrhage compared to the saline group, with the MSC‐S in HA/CS group exhibiting the fastest closure of the epithelial defect induced by the initial alkali burn injury. Representative images of the saline, HA/CS, MSC‐S, and MSC‐S in the HA/CS group corneas at 14 days are shown in Figure 4C).
Figure 4.
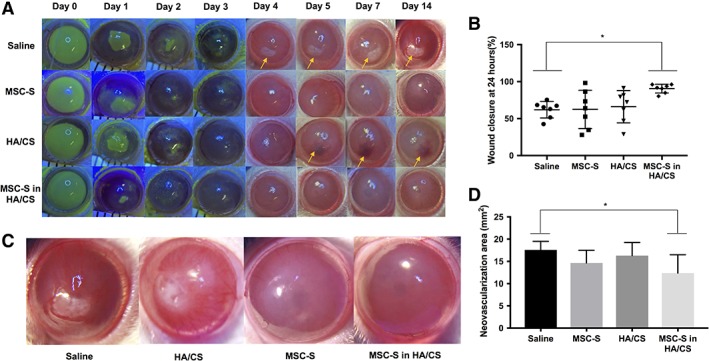
Results from corneal alkaline burn model experiments in rats in vivo. Sodium hydroxide (NaOH) was applied to the epithelium followed by copious irrigation with balanced salt solution, followed by treatments (saline, MSC‐S, HA/CS, MSC‐S in HA/CS) 1 drop daily for 7 days and the corneas were photographed with and without fluorescein staining out to day 14. (A): Representative photographs for each of the study arms. Yellow arrows show areas of scar formation in the saline group and intrastromal hemorrhage in the HA/CS group (B): Percent (%) wound closure 24 hours after treatment. Eyes treated with MSC‐S in HA/CS yielded significantly smaller wound areas compared to the saline group (*, p < .05), while the HA/CS and MSC‐S treatments alone did not. (C): High‐magnification photographs of representative photos of alkaline burned corneas 14 days after saline, HA/CS, MSC‐S, MSC‐S in HA/CS. (D): Area of corneal neovascularization (NV) 14 days after treatments. The corneas treated with MSC‐S in HA/CS resulted in a lower NV area compared to the saline group (*, p < .05), whereas the HA/CS and MSC‐S groups did not (representative photographs taken at 15× magnification). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
The treated rat corneas were excised, fixed, sectioned, and stained with alpha smooth muscle actin, CD31 and ALDH3A1 (Fig. 5A, 5B). Alpha smooth muscle actin stain was increased in the stromal layer of corneas treated with HA/CS and saline compared to MSC‐S groups. Similarly, CD31 was also increased in the stromal layer of corneas treated with HA/CS alone and saline, compared to MSC‐S alone and MSC‐S in HA/CS groups. Additionally, we observed a higher number of nuclei stained with DAPI in the stromal layer of corneas treated with HA/CS alone and saline. Next, we evaluated the expression of ALDH3A1, a corneal crystallin known to be involved in maintaining corneal transparency 19. We observed that only in those groups that received MSC‐S was the expression of this enzyme maintained compared to the no treatment group. The corneas treated with HA/CS alone and saline had a decreased expression of this protein (Fig. 5A, 5B).
Figure 5.
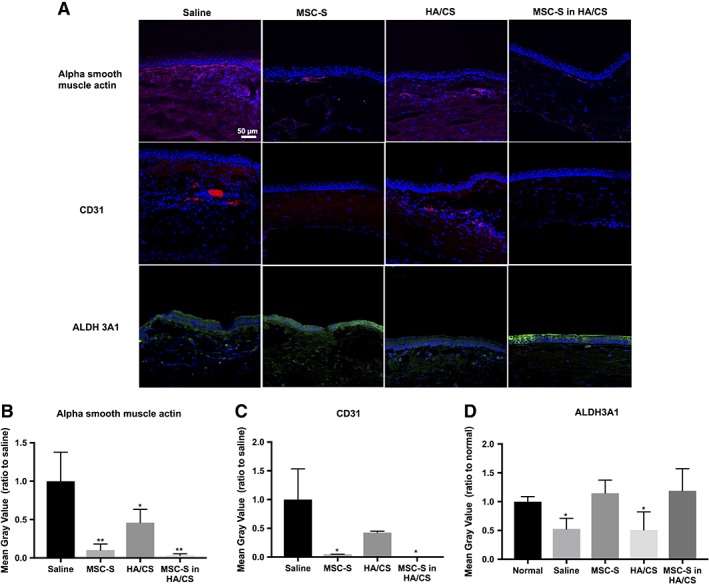
(A): Immunofluorescence images of rat cornea sections 14 days after treatment with saline, MSC‐S, HA/CS, and MSC‐S in HA/CS—scale bar 50 μm. Alpha smooth muscle actin and CD31 staining were greater in the HA/CS and saline groups compared to both MSC‐S‐containing groups (with and without the HA/CS carrier). Expression of Aldehyde Dehydrogenase 3 Family Member A1 (ALDH3A1) was maintained for both group containing MSC‐S. Decreased ALDH3A1 expression was observed in the corneas that received saline and HA/CS. (B): Quantification of the fluorescence intensities using ImageJ revealed that the alpha smooth muscle actin expression was significantly lower in all treatment groups compared the saline group, and lower in the MSC‐S‐containing groups compared to the HA/CS group, while (C): CD31 expression was significantly lower in the MSC‐S‐containing groups compared to the saline group, and (D): ALDH3A1 expression was similar to normal corneas in MSC‐S containing groups but was significantly lower in the HA/CS and saline groups (*, p < .05; **, p < .01). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
Upregulation of CD44 Receptors in HCECs In Vitro and Corneas In Vivo After Treatment with MSC‐S in HA/CS
Next, we explored whether the MSC‐S and HA/CS have any effect on CD44 expression. Figure 6A and 6B shows CD44 expression on HCECs, 24 hours after applying the treatments. Cells in the no treatment group showed weak expression of CD44 receptor and the receptor was not homogeneously distributed in all the cells. After applying MSC‐S and MSC‐S in HA/CS, HCECs exhibited an upregulation of CD44 receptor compared to all other groups (**, p < .01 and ***, p < .001, respectively).
Figure 6.
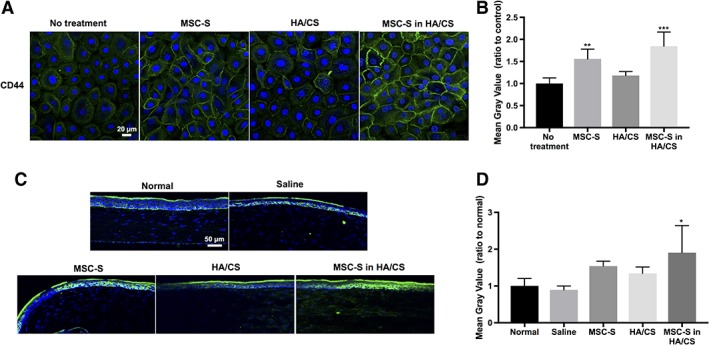
(A): CD44 expression (green fluorescence) in human corneal epithelial cells after treatment with complete keratinocyte serum free medium with growth factors (no treatment, control), MSC‐S, HA/CS, MSC‐S in HA/CS—scale bar 20 μm. (B): Quantification of the fluorescence intensities using ImageJ revealed that CD44 receptors are upregulated in the cells that received MSC‐S (**, p < .01) and MSC‐S in HA/CS (***, p < .001). (C): CD44 expression in rat corneas in vivo 7 days after treatment with saline, MSC‐S, HA/CS, MSC‐S in HA/CS, and uninjured/untreated corneas—scale bar 50 μm. (D): Quantification of the fluorescence intensities using ImageJ showed that CD44 receptors are upregulated in the cornea treated with MSC‐S in HA/CS (*, p < .05). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
CD44 expression was also evaluated in vivo, 7 days after mechanical wounding in rats (Fig. 6C, 6D). In normal corneas without injury, the CD44 receptor is expressed in both the basal and apical epithelial layers. After injury, the group that received MSC‐S in HA/CS exhibited an upregulation of CD44 receptors compared to all other groups (*, p < .05).
Next, we aimed to evaluate for the colocalization of HA and CD44 in cultured HCECs, 8 and 24 hours after applying the treatments. Figure 7 shows that 8 hours after treating cells with MSC‐S in HA/CS and MSC‐S, HA is highly concentrated around cell membranes. In the no treatment group, HA is distributed mostly intracellularly at 24 hours, and without colocalization with CD44. In the MSC‐S treatment group, some colocalization of HA and CD44 occurs at 8 hours, but not at 24 hours. Likewise, in the HA/CS treatment group, some colocalization occurs at 8 hours but not at 24 hours. When treated with MSC‐S in HA/CS, HCECs exhibited strong colocalization of HA and CD44 around the cell membrane at 8 hours, and maintains this colocalization at 24 hours to the greatest extent compared to the other treatment groups.
Figure 7.
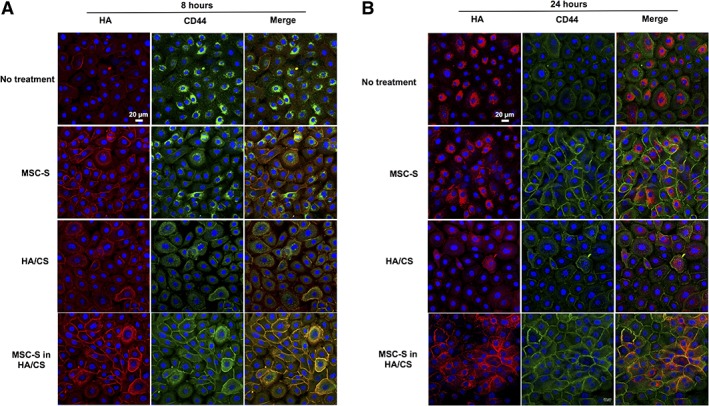
Localization of HA and CD44 receptors on confluent monolayers of primary human corneal epithelial cells after treatment with complete keratinocyte serum free medium with growth factors (no treatment, control), MSC‐S, HA/CS, MSC‐S in HA/CS after (A): 8 hours and (B): 24 hours. The localization of HA at the cell membranes suggests binding to CD44 (scale bar 20 μm). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
Discussion
In this study, we applied MSC‐S to primary cultured HCECs and in both a mechanical and alkaline corneal burn model in vivo using an HA/CS gel as a carrier. The delivery of MSCs and their secreted factors to enhance corneal wound healing has been explored in various ways in preclinical studies 20, 21. Subconjunctival application of MSCs has been successfully reported 13, 22, 23, 24. However, cellular therapy presents many challenges such as maintaining cell viability, phenotypic stability, potentially high costs, and certain regulatory issues specific to the application of living, allogeneic cells to the eye. An acellular, molecular therapy composed of factors secreted by MSCs may overcome some of these limitations. Studies have shown increased epithelial cell proliferation and wound healing after treatments with MSC‐S in culture models 25, 26. We chose to test the performance of MSC‐S in a carrier gel because we were working with lyophilized MSC‐S. Lyophilized MSC‐S presents certain advantages as well as disadvantages over “fresh” collected secretome (also known as conditioned media). Although lyophilization has the potential for reduced biological activity, it is widely used with protein‐based therapeutics and enables shelf‐stability. In the case of MSC‐S, it provides a way to control concentration upon reconstitution, and also enables consistency between treatments: we were able to use lyophilized MSC‐S over many experiments over many months with very consistent results—and to also enable future clinical studies with the same batch of lyophilized material. We chose to use HA/CS as an alternative carrier to saline for MSC‐S because it is biocompatible, already FDA‐approved in ophthalmic surgery, and its constituents have known beneficial effects on corneal wound healing 27, 28. The combination of HA and CS has been used in ophthalmic viscosurgical devices and HA has been used in topical ophthalmic eye drops 29. We hypothesized that reconstituting MSC‐S in HA/CS may provide certain advantages over reconstituting it in saline alone while providing a highly consistent treatment formulation. In particular, we posited that using a viscous gel could reduce the number of required topical applications per day, thus making the treatment more efficient in terms of the volumetric losses that are typical of standard eye drops.
To determine the best concentration of each substance individually, we performed a titration assay for HA/CS and MSC‐S (Supporting Information Fig. S1) in primary rabbit corneal epithelial cells (not human cells). Higher concentrations of HA/CS caused an increase in rabbit corneal epithelial cell proliferation, compared to no treatment in a dose‐dependent manner. However, we found that MSC‐S at the highest concentration (15 mg/ml) appeared to be toxic to the cells, probably due to the pH (9.0) after diluting in medium. It is possible that if the pH was balanced to neutral for the highest concentration of MSC‐S in HA/CS, no toxicity would have been observed. MSC‐S increased cell proliferation in a dose‐dependent response. Based on this data, we chose to work with a final MSC‐S concentration of 1.25 mg/ml as this concentration significantly increased cell proliferation and required lower quantities of lyophilized MSC‐S.
Having identified concentrations of MSC‐S and HA/CS, we then turned our attention to testing these both individually and in combination on primary HCECs. The cultured HCECs—which contained a mixed population of central and limbal cells based on their expression of p63, ABCG2, and CK3—were found to stain positively for CK3 at later passages both in complete KSFM and after treatment with MSC‐S, HA/CS, and MSC‐S in HA/CS (Fig. 1A), indicating epithelial differentiation under all conditions.
To assess cell toxicity, we stained the cells with calcein and propidium iodide after treating with MSC‐S, HA/CS, and MSC‐S in HA/CS (Fig. 1B). We observed that there was no significant cell death in any of the treatment groups as compared to the no treatment group. We then performed cell proliferation assays based on both cell metabolic activity and DNA concentration, and found that the combination of MSC‐S in HA/CS led to greater cell proliferation in HCECs compared to the control group (Fig. 1C, 1D). HA/CS did show an improvement in cell proliferation at 48 hours compared to control. No such improvement was shown in the MSC‐S alone treatment group.
To evaluate if this increased cell proliferation could be translated to preclinical animal models, we first performed a mechanical corneal injury in rodents, applying the various treatments as a single drop daily, for 4 days. Of note, we began our experiments in a mouse model but then quickly transitioned to a rat model due to the larger diameter of their corneas. After 24 hours in rats (Fig. 2A), we observed that the eyes that received saline, MSC‐S alone, and HA/CS alone exhibited similar rates of wound closure of around 60%. In contrast, approximately 80% of the wound was closed for the group that received MSC‐S in HA/CS. We were also able to show similar results in mice, in which significant wound closure was observed for the group that received MSC‐S in HA/CS compared to saline (*, p < .05) (Supporting Information Fig. S2). Thus, in two different species, an increased rate of corneal epithelial wound healing was observed after mechanical injury, when treated with the combination of MSC‐S and HA/CS. Interestingly, Ke and collaborators showed that combining MSCs and another polysaccharide extracted from the Hardy Orchid resulted in significantly better recovery of the corneal epithelial layer compared to each of the substances alone 24. In the present study, the group that received MSC‐S alone did not show improvement in the rate of re‐epithelialization compared to the saline group. This does not necessarily mean that MSC‐S alone does not have wound healing effects, but rather that topical MSC‐S alone may require more than just a once‐daily application to produce any beneficial effects.
Next, we evaluated the treated and healed tissue to see if MSC‐S in HA/CS treatment could facilitate normal re‐epithelialization. We stained corneal tissue sections after various treatments with ZO‐1 (Fig. 2C). ZO‐1 is a marker of epithelial tight junctions. We observed that the group treated with MSC‐S in HA/CS expressed ZO‐1 with normal cellular morphology.
We further aimed to evaluate if the same enhanced wound healing effects would occur in a more severe corneal injury: an alkaline burn (Fig. 4). In contrast to simple mechanical injuries, alkaline burns create a profound and often devastating inflammatory response leading to NV and scar formation in the normally clear cornea. We sought to determine whether the MSC‐S in HA/CS treatment could attenuate these effects after alkaline burn injury. Some studies have shown that MSC can reduce NV and inflammation, as well as increase corneal transparency 13, 22. However, these effects have not been reported with the application of just the secretome (without cells) to the cornea. In our study, we applied MSC‐S with and without HA/CS as a once‐daily treatment for 7 days after alkaline burn. Fluorescein staining showed that, similar to the results seen in the mechanical wound model, 90% of the wound was closed in the MSC‐S in HA/CS group, compared to around 60% for the other groups (each after a single treatment) (Fig. 4A, 4B). By day 2, the wound was completely closed only for the group that received MSC‐S in HA/CS. At day 4, all eyes in the saline group and some eyes in the HA/CS treated group started to show subepithelial scarring and fibrosis of the central cornea. However, these findings were not observed in the MSC‐S and MSC‐S in HA/CS groups (Fig. 4A–4C). Corneal NV developed in all groups starting from day 4. Hemorrhage as a result of the NV was found in the saline and HA/CS groups. By day 14, the quantified area of NV was largest in the saline control group (17.6 ± 1.9 mm2) followed by HA/CS treated group (16.3 ± 3.0 mm2), MSC‐S group (14.6 ± 2.9 mm2), and MSC‐S in HA/CS group (12.4 ± 4.1 mm2) (Fig. 4D).
After sacrificing the animals, we also evaluated the treated tissues for smooth muscle actin alpha, CD31, and ALDH3A1 (Fig. 5). Smooth muscle actin alpha is associated with the transformation of keratocytes to myofibroblasts and was clearly present in corneas treated with saline. This result correlates with our macroscopic observation of fibrotic changes in these eyes over 14 days. Myofibroblastic transformation is associated with scarring due to abnormal remodeling and extracellular matrix deposition by these cells 30. CD31 staining was present in eyes treated with HA/CS and saline alone but not for MSC‐S and MSC‐S in HA/CS. Interestingly, after staining for ALDH3A1, decreased expression was observed for the saline and HA/CS group compared to the MSC‐S in HA/CS and MSC‐S groups. ALDH3A1 is a corneal crystallin responsible for preventing the detrimental effects of toxic aldhehydes; reduced expression of this protein can compromise cornea transparency 19. In this study, we found that ALDH3A1 expression was correlated positively with eyes that maintained transparency after alkaline burn injury through topical treatment with MSC‐S in HA/CS, suggesting that MSC‐S may help to preserve corneal clarity by maintaining ALDH3A1 expression. Further work is merited to understand the role of ALDH3A1 in corneal injuries treated with MSC‐S.
Finally, we aimed to understand the reason behind the observed enhanced effects of treating with MSC‐S in combination with HA/CS. To determine whether it is simply due to increased viscosity of the reconstituted MSC‐S, we also dissolved the MSC‐S in an over‐the‐counter ophthalmic lubricant gel, Systane (0.3% Hypromellose). Systane was chosen because it is viscous, water‐soluble, commonly used by patients, and generally thought to be biologically inert. We also tested HA/CS with dissolved BSA rather than MSC‐S, to evaluate the opposite situation of the HA/CS with a biologically inert protein. The results, shown in Figure 2, showed that the MSC‐S in HA/CS treatment led to faster wound healing compared to MSC‐S in Systane and BSA in HA/CS. Specifically, after 24 hours, the increased wound healing observed with MSC‐S in HA/CS was not observed for MSC‐S in Systane gel. This suggests that the observed healing effect is specific to HA/CS and is not simply due to the increase in viscosity of the MSC‐S solution on the corneal surface. To confirm that MSC‐S is necessary for wound healing, we replaced it with BSA in the HA/CS gel. We observed that delivering BSA in HA/CS did not enhance corneal wound healing. These results led us to conclude that both MSC‐S and HA/CS are essential to the observed corneal wound healing effects of the combined treatment under study. This suggests that there is a mutually enhancing effect of delivering MSC‐S in HA/CS, and that viscosity alone or the presence of protein alone is not what is responsible for the observed healing effects of the combination treatment under study. Of note, the authors considered and tested the fibrin gel (Tiseel glue) as an alternative vehicle to HA/CS (data not shown). However, as it is designed to do, fibrin gel forms a solid matrix upon mixing, and does not remain a viscous, flowable liquid like HA/CS does. This creates a much different release profile compared to a viscous agent such as HA/CS, where the MSC‐S must escape from a solid matrix. Therefore, we chose a flowable gel material such as Systane which is not a solid, can be self‐administered as an eye drop, and is also regarded to be physically, biologically, and chemically inert.
We also investigated whether the CD44 receptor plays a role in the observed biological effects of the MSC‐S and HA/CS treatments. CD44 is a well‐characterized transmembrane protein that serves as the main surface receptor both HA and CS 31. The binding of CD44 to HA is known to induce cell proliferation and changes in cell cytoskeleton 32. Interestingly, HA does not bind to CD44 receptor under normal conditions. Instead, CD44 is activated and upregulated by cytokines and growth factors and this activation further induces HA to bind to the CD44 receptor 33. We found that under normal conditions, corneal epithelial cells express CD44 on their surfaces. However, this expression is not homogeneously distributed to all cells. The addition of MSC‐S in HA/CS and MSC‐S alone both substantially upregulated the CD44 compared to the no treatment group after 24 hours (Fig. 6A, 6B). It has been shown CD44 expression is more prominent at the basal and apical layers of the normal cornea and increases after injury 34, 35. Several investigators have evaluated and reported on the role of CD44 receptors in corneal wound healing. For instance, Yu et al. showed that the expression of CD44 receptors increased 3 hours after wounding, with a peak at 18 hours 35. The authors suggested that the expression of CD44 receptors correlates with corneal re‐epithelialization. Zhong et al. showed that CD44 receptors were increased in corneal epithelial cells treated with HA, at 48 hours of incubation 36. They also showed that these cells had enhanced migration and decreased inflammatory proteins. Gomes et al. showed that migration phase in corneal wound healing is influenced by the synthesis of CD44 37. Zhu et al. suggested that CD44 may play an important role in corneal cell–cell and cell–matrix interactions 34. Yang et al. showed that a crosslinked hyaluronan gel accelerated wound healing in rabbit eyes, after chemical and mechanical injury 38. Moreover, the presence of high molecular weight HA has been shown to facilitate binding to CD44 32. CS, also known to bind to CD44, may play a role in the wound healing effects of the combined MSC‐S and HA/CS treatment.
Our findings are in line with the aforementioned reports from the literature. We found that after applying MSC‐S in HA/CS, CD44 expression increases in all layers of the cornea compared to all other groups (Fig. 6D). In the presence of MSC‐S, CD44 is upregulated and activated which increases HA binding to the cells (Fig. 6C) 39, 40. Of particular interest is the pronounced colocalization of HA and CD44 at the cell membranes of the cultured HCECs in the MSC‐S in HA/CS treatment group (Fig. 7) compared to what is seen in the MSC‐S, HA/CS, and no treatment groups. This colocalization is most apparent at the 8 hour time point but still persists at 24 hours. On the other hand, this colocalization of both HA and CD44 is less evident in the single agent or no treatment groups at both 8 and 24 hours. These results are consistent with previous reports, and indicate that CD44 having an integral role in the interplay between the MSC‐S and HA/CS and on the enhanced wound healing effects that they exert together, as observed in this study.
There are some limitations to this study. Although both MSC‐S and HA/CS have been shown to have independent effects on corneal would healing by others (as well as in our rabbit corneal epithelial cell data shown in Supporting Information Fig. S1), they did not have such an effect in the human HCECs culture system we used at the concentration and dosing regimen we selected. Another limitation is that the MSC‐S in our study was lyophilized. As mentioned earlier, lyophilization provides certain batch‐to‐batch consistency advantages, but may not be as biologically active as “fresh” MSC‐S. The potential differences between fresh and lyophilized secretome are outside the scope of this study but do merit further investigation. The goal of our study was to ascertain whether the reconstitution medium (HA/CS vs. saline) would have any impact on the wound healing effects of MSC‐S. Another limitation is that the topical treatments in the alkaline burn study was only carried out to 7 days, and the eyes were monitored out to 14 days. The intention of this study was to observe the effects of the various treatment groups with once‐daily topical administration on the time to wound closure as the primary endpoint, and maintenance of corneal scarring and NV as a secondary endpoint. In future work, we plan to treat daily out to longer time points and also evaluate the impact of a more frequent dosing regimen and/or a subconjunctival injection. In spite of this limitation, our alkaline burn experiments demonstrate the beneficial effects of the MSC‐S in HA/CS treatment on both epithelial wound closure and on scarring and NV formation compared to MSC‐S, HA/CS, and saline alone.
Previous work has shown that secreted factors from MSCs can reduce corneal inflammation and decrease epithelial cells apoptosis and maintain cornea transparency after injury 21, 22, 25. Another study showed that MSCs maintain corneal transparency after injury 13. We showed that, with just a once‐daily topical administration, MSC‐S mixed with HA/CS can accelerate wound closure in both mechanical and chemical injuries and can reduce scar formation, NV, and hemorrhage after chemical injury. This work presents a strategy for harnessing the beneficial effects of the MSC‐S with a highly reproducible and efficient approach to its storage and topical delivery. Further work is merited to continue elucidating the molecular mechanisms behind the effects seen in this study, such as what factors predominate the interaction between MSC‐S and HA/CS and what the relative dose‐dependent effects are between these factors and the HA and/or CS matrix elements.
Conclusion
The study reveals that the combination of the MSC‐S and HA/CS gel can promote enhanced corneal wound healing in vitro on HCECs and in both a mechanical and chemical corneal burn wound model in rats. The MSC‐S in HA/CS accelerated epithelial wound closure and reduced corneal NV, scar formation, and hemorrhage to a greater extent compared to saline alone, MSC‐S alone, or HA/CS alone. Our results suggest that this effect is not simply due to an increase in solution viscosity, and that the HA‐binding CD44 receptor may play an important role in the interplay between MSC‐S and HA/CS during wound healing.
Author Contributions
G.M.F.‐C., K.‐S.N., D.M.: conceptualization, final approval of manuscript; I.P., G.M.F.‐C.: manuscript writing, collection and/or assembly of data, data analysis, data interpretation; K.‐S.N:manuscript writing, collection and/or assembly of data, data analysis; I.P., HJ.L, Y‐C.C., M.E., S.H., I.J.B.: collection of the data and data analysis; S.H., D.M., M.E., A.R.D.: writing—review and editing; D.M., M.E., A.R.D.: funding acquisition.
Disclosure of Potential Conflicts of Interest
G.F.‐C., H.J.L., A.D., and D.M. are co‐inventors on a patent application related to the technology described in this article. The other authors indicated no potential conflicts of interest.
Supporting information
Appendix S1: Supporting Information
Supplemental Figure S1. (A): Effect of the MSC‐S concentration on primary corneal epithelial cell proliferation as measured by metabolic activity over 72 hours. (B): Effect of the HA/CS concentration on primary corneal epithelial cell proliferation as measured by metabolic activity over 72 hours (**, p < .01; ***, p < .001; ****, p < .0001). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
Supplemental Figure S2. Results of MSC‐S treatments in a mechanical corneal wound model in mice in vivo. The epithelial layer from mice corneas was debrided and the MSC‐S was applied with and without HA/CS, compared to HA/CS alone and saline alone, using 1 drop of each daily. (A): Fluorescein staining of the treated corneas was used to quantify the size of the epithelial defect on a daily basis for each of the treatment groups. Shown are representative photos under blue light illumination for each of the treatment groups (Saline, MSC‐S, HA/CS, and MSC‐S in HA/CS). (B): After 24 hours, the group that received MSC‐S in HA/CS had smaller wound sizes compared to saline group (*, p < .05), whereas HA/CS and MSC‐S treatments alone did not. Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
Acknowledgments
This work was supported by the National Institutes of Health (National Eye Institute K08EY028176 (D.M.) and a Departmental P30‐EY026877 core grant), the Stanford SPARK Translational Research Grant (D.M.), a core grant from the Research to Prevent Blindness (RPB) Foundation, and the Byers Eye Institute at Stanford. This work was also supported by Clinical Scientist Development Program Award K12EY021475 (M.E.) and R01 EY024349‐01A1 (ARD) and Core grant EY01792 from NEI/NIH; MR130543 (A.R.D.) from US Department of Defense, U.S. ARMY, Vision for Tomorrow (A.R.D.), unrestricted grant to the department from RPB; and Eversight (providing both seed funding and human corneal research tissue). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We like to acknowledge Dr. Hematti's group at the University of Wisconsin for kindly providing the BM MSCs that were used in this study. We also thank Dr. Daria Mochly‐Rosen and Dr. Che‐Hong Chen from the Department of Chemical and Systems Biology at Stanford University for their support of this work.
Contributor Information
Ali R. Djalilian, Email: adjalili@uic.edu
David Myung, Email: david.myung@stanford.edu.
References
- 1. Eslani M, Movahedan A, Afsharkhamseh N et al. The role of toll‐like receptor 4 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci 2014;55:6108–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baradaran‐Rafii A, Eslani M, Haq Z et al. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf 2017;15:48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziaei M, Greene C, Green CR. Wound healing in the eye: Therapeutic prospects. Adv Drug Deliv Rev 2018;126:162–176. [DOI] [PubMed] [Google Scholar]
- 4. Park S‐R, Kim J‐W, Jun H‐S et al. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther 2018;26:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saghizadeh M, Kramerov AA, Svendsen CN et al. Concise review: Stem cells for corneal wound healing. Stem Cells 2017;35:2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bray LJ, Heazlewood CF, Munster DJ et al. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy 2014;16:64–73. [DOI] [PubMed] [Google Scholar]
- 7. Yanling M, Yongsheng X, Zhifeng X et al. Reconstruction of chemically burned rat corneal surface by bone marrow–derived human mesenchymal stem cells. Stem Cells 2006;24:315–321. [DOI] [PubMed] [Google Scholar]
- 8. Song HB, Park SY, Ko JH et al. Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the cornea by suppressing macrophage in a TSG‐6‐dependent manner. Mol Ther 2018;26:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eslani M, Putra I, Shen X et al. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT‐1. Invest Ophthalmol Vis Sci 2017;58:5507–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eslani M, Putra I, Shen X et al. Cornea‐derived mesenchymal stromal cells therapeutically modulate macrophage immunophenotype and angiogenic function. Stem Cells 2018;36:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajendran V, Netuková M, Griffith M et al. Mesenchymal stem cell therapy for retro‐corneal membrane—A clinical challenge in full‐thickness transplantation of biosynthetic corneal equivalents. Acta Biomater 2017;64:346–356. [DOI] [PubMed] [Google Scholar]
- 12. Di G, Du X, Qi X et al. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG‐6–dependent stem cell activation and macrophage switch. Invest Ophthalmol Vis Sci 2017;58:4344–4354. [DOI] [PubMed] [Google Scholar]
- 13. Mittal Sharad K, Omoto M, Amouzegar A et al. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Reports 2016;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gidfar S, Milani FY, Milani BY et al. Rapamycin prolongs the survival of corneal epithelial cells in culture. Sci Rep 2017;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saika S, Shiraishi A, Saika S et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem 2000;275:2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludwig CA, Newsom MR, Jais A et al. Training time and quality of smartphone‐based anterior segment screening in rural India. Clin Ophthalmol (Auckland, NZ) 2017;11:1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soiberman U, Kambhampati SP, Wu T et al. Subconjunctival injectable dendrimer‐dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017;125:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badner A, Vawda R, Laliberte A et al. Early intravenous delivery of human brain stromal cells modulates systemic inflammation and leads to vasoprotection in traumatic spinal cord injury. Stem Cells Translational Medicine 2016;5:991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Thompson DC, Koppaka V et al. Ocular aldehyde dehydrogenases: Protection against ultraviolet damage and maintenance of transparency for vision. Prog Retin Eye Res 2013;33:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh JY, Lee RH, Yu JM et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther 2012;20:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee MJ, Ko AY, Ko JH et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther 2015;23:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Youn OJ, Kum KM, Sun SM et al. The anti‐inflammatory and anti‐angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 2008;26:1047–1055. [DOI] [PubMed] [Google Scholar]
- 23. Gipson IK. The ocular surface: The challenge to enable and protect vision:The Friedenwald lecture. Invest Ophthalmol Vis Sci 2007;48:4391–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ke Y, Wu Y, Cui X et al. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS One 2015;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oh JY, Ko JH, Kim MK et al. Effects of mesenchymal stem/stromal cells on cultures of corneal epithelial progenitor cells with ethanol injury. Invest Ophthalmol Vis Sci 2014;55:7628–7635. [DOI] [PubMed] [Google Scholar]
- 26. Wright B, Hopkinson A, Leyland M et al. The secretome of alginate‐encapsulated limbal epithelial stem cells modulates corneal epithelial cell proliferation. PLoS One 2013;8:e70860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eric C, Kao WWY, Abayomi O. Impact of hyaluronic acid‐containing artificial tear products on reepithelialization in an in vivo corneal wound model. J Ocul Pharmacol Ther 2018;34:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ledbetter EC, Munger RJ, Ring RD et al. Efficacy of two chondroitin sulfate ophthalmic solutions in the therapy of spontaneous chronic corneal epithelial defects and ulcerative keratitis associated with bullous keratopathy in dogs. Vet Ophthalmol 2006;9:77–87. [DOI] [PubMed] [Google Scholar]
- 29. Limberg MB, McCaa C, Kissling GE et al. Topical application of hyaluronic acid and chondroitin sulfate in the treatment of dry eyes. Am J Ophthalmol 1987;103:194–197. [DOI] [PubMed] [Google Scholar]
- 30. Torricelli AAM, Santhanam A, Wu J et al. The corneal fibrosis response to epithelial‐stromal injury. Exp Eye Res 2016;142:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esford LE, Maiti A, Bader SA et al. Analysis of CD44 interactions with hyaluronan in murine L cell fibroblasts deficient in glycosaminoglycan synthesis: A role for chondroitin sulfate. J Cell Sci 1998;111:1021–1029. [DOI] [PubMed] [Google Scholar]
- 32. Knudson W, Peterson RS. Chapter 5 ‐ The hyaluronan receptor: CD44 In: Hari GG, Charles AH, eds. Chemistry and Biology of Hyaluronan. Oxford: Elsevier Science Ltd, 2004:83–123. [Google Scholar]
- 33. Puré E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med 2001;7:213–221. [DOI] [PubMed] [Google Scholar]
- 34. Zhu S, Nolle B, Duncker G. Expression of adhesion molecule CD44 on human corneas. Br J Ophthalmol 1997;81:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu FX, Guo J, Zhang Q. Expression and distribution of adhesion molecule CD44 in healing corneal epithelia. Invest Ophthalmol Vis Sci 1998;39:710–717. [PubMed] [Google Scholar]
- 36. Zhong J, Deng Y, Tian B et al. Hyaluronate acid‐dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J Ophthalmol 2016;2016:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gomes JAP, Amankwah R, Powell‐Richards A et al. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol 2004;88:821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang G, Espandar L, Mamalis N et al. A cross‐linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet Ophthalmol 2010;13:144–150. [DOI] [PubMed] [Google Scholar]
- 39. Foster LC, Arkonac BM, Sibinga NES et al. Regulation of CD44 gene expression by the proinflammatory cytokine interleukin‐1β in vascular smooth muscle cells. J Biol Chem 1998;273:20341–20346. [DOI] [PubMed] [Google Scholar]
- 40. Harada H, Takahashi M. CD44‐dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase‐1 and ‐2. J Biol Chem 2007;282:5597–5607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Supplemental Figure S1. (A): Effect of the MSC‐S concentration on primary corneal epithelial cell proliferation as measured by metabolic activity over 72 hours. (B): Effect of the HA/CS concentration on primary corneal epithelial cell proliferation as measured by metabolic activity over 72 hours (**, p < .01; ***, p < .001; ****, p < .0001). Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.
Supplemental Figure S2. Results of MSC‐S treatments in a mechanical corneal wound model in mice in vivo. The epithelial layer from mice corneas was debrided and the MSC‐S was applied with and without HA/CS, compared to HA/CS alone and saline alone, using 1 drop of each daily. (A): Fluorescein staining of the treated corneas was used to quantify the size of the epithelial defect on a daily basis for each of the treatment groups. Shown are representative photos under blue light illumination for each of the treatment groups (Saline, MSC‐S, HA/CS, and MSC‐S in HA/CS). (B): After 24 hours, the group that received MSC‐S in HA/CS had smaller wound sizes compared to saline group (*, p < .05), whereas HA/CS and MSC‐S treatments alone did not. Abbreviations: CS, chondroitin sulfate; HA, hyaluronic acid; MSC‐S, mesenchymal stem cells secretome.


