Abstract
Pancreatic β-cell regeneration, the therapeutic expansion of β-cell number to reverse diabetes, is an important goal. Replication of differentiated insulin-producing cells is the major source of new β-cells in adult mice and juvenile humans. Nucleoside analogs such as BrdU, which are incorporated into DNA during S-phase, have been widely used to quantify β-cell proliferation. However, reports of β-cell nuclei labeling with both BrdU and γ-phosphorylated H2A histone family member X (γH2AX), a DNA damage marker, have raised questions about the fidelity of BrdU to label S-phase, especially during conditions when DNA damage is present. We performed experiments to clarify the causes of BrdU-γH2AX double labeling in mouse and human β-cells. BrdU-γH2AX colabeling is neither an age-related phenomenon nor limited to human β-cells. DNA damage suppressed BrdU labeling and BrdU-γH2AX colabeling. In dispersed islet cells, but not in intact islets or in vivo, pro-proliferative conditions promoted both BrdU and γH2AX labeling, which could indicate DNA damage, DNA replication stress, or cell cycle–related intrinsic H2AX phosphorylation. Strategies to increase β-cell number must not only tackle the difficult challenge of enticing a quiescent cell to enter the cell cycle, but also achieve safe completion of the cell division process.
Introduction
β-Cell replication is the principal process generating new β-cells; therapeutic induction of β-cell replication is an important goal for diabetes research (1–3). The incorporation of nucleoside analogs has been a high-value tool in quantifying cell proliferation behavior for decades, allowing measurement of cumulative S-phase entry during a defined exposure period, in vitro and in vivo. Nucleoside analogs such as BrdU and 5-ethinyl-2′-deoxyuridine (EdU) (4) have been used not only in β-cell biology, but also broadly across developmental and cell biology.
Under certain conditions, BrdU incorporation in β-cells has been observed to colocalize with markers of DNA damage (5,6). In other cell types, BrdU exposure has been shown to activate a DNA damage response (7–9), but in β-cells the reasons for this colocalization are not well understood. The observation has been widely discussed and rapidly incorporated into the thinking in the field, with a range of impacts. In the most discussed work (5), the conclusion drawn by the originating authors was that some BrdU-labeled human β-cells, particularly the subset with atypical punctate BrdU staining, fail to complete S-phase, instead showing evidence of DNA damage, DNA rereplication, and failure to divide. In other words, BrdU did label cells that transitioned into S-phase, but BrdU-labeled cells could not be assumed to progress through successful mitosis. However, concern in the field has extended beyond this concept; in many quarters, the question has been raised as to whether BrdU labeling, counted as evidence of S-phase entry, could in fact be due to a completely unrelated process: nucleotide incorporation during DNA damage repair. If this hypothesis were true, then nuclei might label for BrdU in the absence of S-phase entry, invalidating some prior results and diminishing the value of nucleoside analogs in the study of cell replication.
The field of β-cell regeneration has been impacted by uncertainty of the correct interpretation of BrdU-labeled nuclei. There is an urgent need to clarify the reasons for co-occurrence of BrdU and DNA damage labeling in β-cells. The goals of this study were to explore the possible causes of BrdU and γ-phosphorylated H2A histone family member X (γH2AX) colocalization in nuclei of mouse and human β-cells, and to specifically test whether there are conditions in which BrdU labeling can be induced by DNA damage-related cellular processes rather than cell cycle entry.
Research Design and Methods
Mice for Islet Isolation
All mouse procedures were approved by the UMass Medical School Institutional Animal Care and Use Committee. Young (10- to 12-week-old) or old (50- to 60-week-old) C57BL/6J male and female mice, from an in-house breeding colony, had continuous access to normal mouse chow, on a 12-h light/dark cycle. Islets were isolated by pancreatic ductal collagenase injection and Ficoll (Histopaque-1077; Sigma-Aldrich) gradient (10). Islets from multiple mice were pooled and mixed before experiments. Whenever possible, all control and experimental comparisons were performed in parallel on islets from the same mice. Each combined pool of islets was considered one biological replicate.
In Vivo Mouse Experiments
To study proliferative conditions in vivo, pancreas sections were analyzed from experiments previously published on 10- to 12-week-old male mice fed a high-fat diet for 7 days (11), or 10- to 12-week-old male mice with continuous hyperglycemia achieved by intravenous infusion of glucose (10,12).
Mouse Islet Cell Culture
Whole islets were cultured overnight in islet medium (RPMI medium, 10% FBS, penicillin/streptomycin, 5.0 mmol/L glucose) after isolation. For dispersed-cell experiments, islets were hand picked and digested with single-use-apportioned 0.05% trypsin, and 50 islet equivalents per well were plated on uncoated glass coverslips (Fisherbrand; Thermo Fisher Scientific, Waltham, MA) in islet medium (12). Mouse islet cell experiments were 72 h in duration, starting the day after dispersion, with adenovirus, glucose and/or harmine (5 μmol/L) (catalog #286044; Sigma-Aldrich) exposure for 72 h, and BrdU (10 μg/mL) added 24 h prior to fixation. For whole-islet experiments, islets were cultured for 72 h in islet medium with additives including glucose and/or mitomycin C, with BrdU added for the final 24 h, fixed in 10% formalin for 30 min, washed with PBS, and embedded in paraffin for sectioning (13). For virus transduction, dispersed cells were transduced with adenovirus expressing bacterial Cre recombinase (control virus) or human cyclin D2 protein, at a multiplicity of infection of 5, as described previously (12). Mitomycin C (0.5 μmol/L) (Sigma-Aldrich) was added at the start of the 72-h experiment. Ultraviolet (UV) irradiation of islet cell cultures was administered by placing the culture dish for 10 min on a UVP Benchtop Transilluminator equipped with a 302-nm filter (Cole-Parmer) after 24 h of glucose treatment.
Human Islet Culture
Human islets received from the Integrated Islet Distribution Program (IIDP) at City of Hope (Duarte, CA) (Supplementary Table 1) were cultured in islet medium overnight after shipment, then dispersed and plated on coverslips, as described above for mouse islets. After 1 day of recovery from plating, human islet cell experiments were performed exactly as described above, except that the experiment duration was 96 h and BrdU was included for the entire 96 h of culture.
Immunostaining and Microscopy
Islet cell cultures were fixed in 4% paraformaldehyde for 10 min at room temperature. Whole islets (13) and pancreas sections were obtained from formalin-fixed, paraffin-embedded tissue blocks, as described in the publications where these samples originated (10–12). For BrdU, γH2AX, and pHH3 (phosphohistone H3) staining, fixed cells or rehydrated paraffin sections were submerged in 1 N HCl for 20 min at 37°C, blocked for 2 h in goat serum–based block with 0.1% Tween 20, labeled with primary antibodies then secondary antibodies (Invitrogen), and mounted on glass slides with Fluoroshield mounting media containing DAPI (Sigma-Aldrich). The primary antibodies used were guinea pig anti-insulin (catalog #A0564; Dako); rabbit anti-γH2AX (catalog #9718; Cell Signaling Technology); mouse anti-γH2AX (catalog #05–636; EMD Millipore); rat anti-BrdU (catalog Ab6326; Abcam); rabbit anti-pHH3 (catalog #3377; Cell Signaling Technology); and rabbit anti-Ki67 (catalog RM-9106; Thermo Fisher Scientific). Fluorescent images were acquired using a Nikon fluorescent microscope or a Solamere CSU10 Spinning Disk confocal system mounted on a Nikon TE2000-E2 Inverted Microscope. Image data were quantified by an unbiased laboratory member (B.G.) using Cell Profiler automated counting software from the Broad Institute (Cambridge, MA) (14,15) with manual image checking postquantification. A cell was considered to be γH2AX(+) if the nucleus was labeled with multiple intense nuclear foci. The number of cells counted for each experimental condition are included in Supplementary Table 2.
Statistical Analysis
Data were analyzed and graphed using GraphPad Prism 7. In all column plots, each sample is represented by one data point. Venn diagrams were generated using EulerAPE 3.0.0 from the University of Kent (Canterbury, U.K.) (16). Data were compared using a two-tailed Student t test; P < 0.05 was considered to be significant.
Results
Possible Explanations for BrdU and DNA Damage Colabeling in the Same β-Cell
Consistent with the reports of human β-cell colabeling (5), we have observed BrdU-γH2AX colabeling in primary mouse islet cell cultures (17) (Fig. 1). We reasoned that these two markers could label the same nucleus if 1) DNA damage leads to BrdU incorporation, 2) BrdU incorporation leads to DNA damage, 3) an upstream process leads to both labels, or 4) BrdU incorporation and DNA damage occur stochastically and occasionally occur in the same cell but are unrelated (Supplementary Table 3).
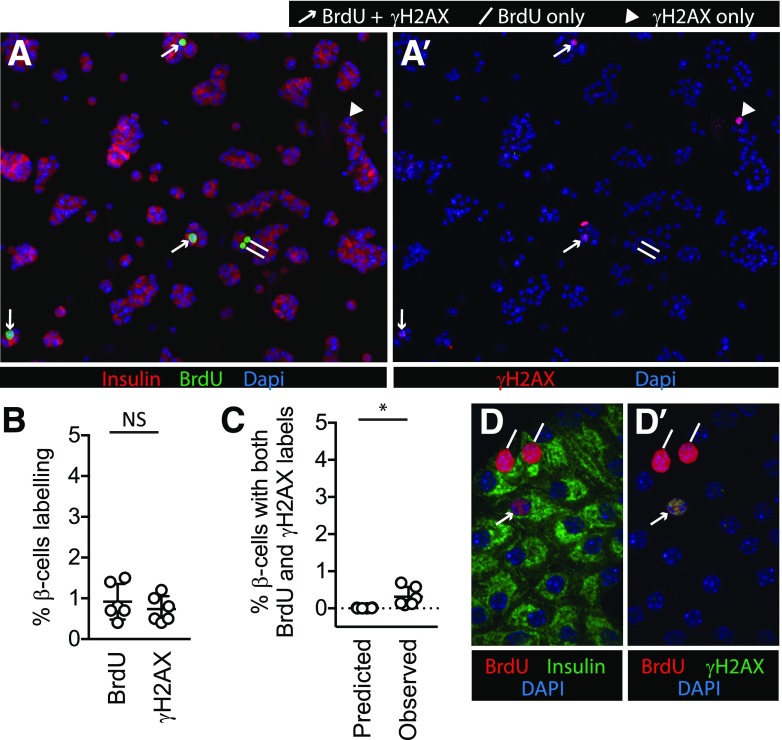
Figure 1.
BrdU and γH2AX labeling co-occur in some β-cells in mouse islet cell cultures. Dispersed mouse islet cells were cultured for 72 h in 5 mmol/L glucose (A, A′, B, and C) or 15 mmol/L glucose (D and D′), with BrdU added for the final 24 h. BrdU (A) and γH2AX (A′) each labeled a small fraction of β-cells under unstimulated culture conditions. In mouse islet cells cultured in unstimulated (5 mmol/L glucose) conditions, a small proportion of β-cells were labeled with BrdU or γH2AX (B). The observed fraction of β-cells colabeled with both BrdU and γH2AX was low, but higher than that predicted by random chance (the predicted fraction was defined by the product of the observed fractions of each single label) (C). D and D′: Confocal microscopy of mouse islet cells cultured in 15 mmol/L glucose confirmed both labels occur in the same nuclei. For A, A′, D, and D′, lines mark BrdU(+) γH2AX(−) nuclei, arrowheads mark BrdU(−) γH2AX(+) nuclei, and arrows mark BrdU(+) γH2AX(+) nuclei. *P < 0.05; NS, P > 0.1.
BrdU-γH2AX Colabeling in Mouse β-Cells Is Not Due to Random Chance
We first tested the simplest explanation for colocalization: that BrdU labeling and DNA damage each occur in a fraction of cultured primary β-cells, unrelated to one another, leading to occasional co-occurrence in the same cell. If this model were true, γH2AX and BrdU labels should both be detectable in standard culture conditions, and the presence of one label in a cell should not influence the likelihood of the presence of the other label. We cultured dispersed mouse islet cells under standard, unstimulated conditions. When the cells were fixed and immunostained for insulin, BrdU, and γH2AX, a small but consistent fraction of insulin-containing cells was labeled for each marker (Fig. 1A, A′, and B). To test whether colabeling could be due to random co-occurrence of two unrelated processes, we calculated the predicted likelihood of random colabeling based on the frequency of each individual label (predicted fraction colabeling was defined as the product of the observed fractions of each single label) and compared this predicted frequency with the actual observed frequency of colabeling. The observed frequency, although low, was higher than the predicted random co-occurrence frequency (Fig. 1C). Confocal microscopy confirmed nuclear colabeling in some β-cells (Fig. 1D and D′). As reported previously (5), in some BrdU-γH2AX–colabeled nuclei, the BrdU labeling was punctate in appearance. Thus, in unstimulated culture conditions, the colabeling of BrdU and γH2AX occurred more frequently in mouse β-cells than predicted by chance, suggesting that one process influences the other or that both are influenced by a common upstream process.
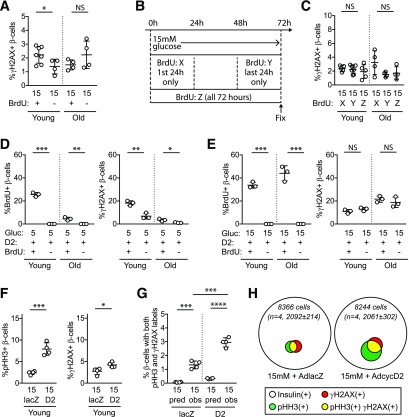
Proliferative Stimuli Increase BrdU-γH2AX Double-Labeling Frequency
Since the overexpression of HNFA8 or cyclin D3/Cdk6 in combination (5) increased the number of colabeled cells, we reasoned that proliferation drivers increase the frequency of double-labeled cells. Since the originating work was in human β-cells, which are considerably older than young-adult mouse β-cells, we also considered that β-cells from older mice might be more vulnerable. We tested whether the mitogenic stimuli frequently used in our laboratory, 15 mmol/L glucose and adenoviral-delivered cyclin D2 (12,17), altered the likelihood of BrdU and γH2AX colabeling in β-cells from younger (10- to 12-week-old) and older (50- to 60-week-old) mice. In 15 mmol/L glucose, both BrdU and γH2AX (Fig. 2A and B) labeling increased. Supporting the concept that BrdU labeling reflects S-phase entry, β-cells from younger mice had a higher frequency of BrdU incorporation than β-cells from older mice. Perhaps surprisingly, high-glucose β-cells from older mice did not label more frequently for γH2AX, or both markers, than β-cells from younger mice (Fig. 2C).
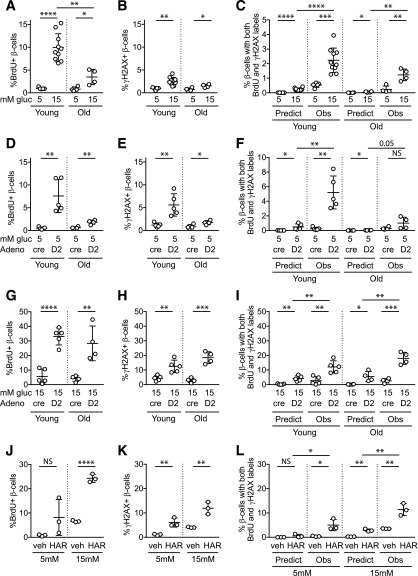
Figure 2.
Proliferative stimuli, especially in combination, increase BrdU-γH2AX colocalization frequency in mouse β-cells. Dispersed young (10- to 12-week-old) and old (50- to 60-week-old) mouse islet cells were cultured for 72 h in the indicated conditions, with BrdU added for the final 24 h. A 15 mmol/L glucose concentration markedly increased BrdU incorporation (A), especially in young islets, and modestly increased γH2AX labeling (B) and BrdU-γH2AX colabeling (C). Ad-cyclin D2 in 5 mmol/L glucose increased BrdU (D), γH2AX (E), and colabeled cells (F). Combined treatment with 15 mmol/L glucose and Ad-cyclin D2 markedly increased BrdU (G), γH2AX (H), and colabeled cells (I). Exposure to a different β-cell mitogen, harmine, also increased BrdU (J), γH2AX (K), and colabeled cells (L). Note the variable y-axis scale in A–L. C, F, I, and L: in all cases, the observed fraction of β-cells colabeled for both BrdU and γH2AX was greater than that predicted if colabeling occurred due to chance. Ad-cre was used as a control for Ad-cyclin D2. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Adeno, adenovirus; gluc, glucose; HAR, harmine; Obs, observed; Predict, predicted; veh, vehicle.
To test whether directly forcing cell cycle entry increases BrdU-γH2AX colabeling, we overexpressed cyclin D2. In normal 5 mmol/L glucose, Ad-cyclin D2 produced similar results to 15 mmol/L glucose stimulation (Fig. 2D–F): BrdU and γH2AX were individually modestly increased, the frequency of double-labeled cells increased, and older mouse age did not increase the frequency of β-cells with γH2AX or double labeling. However, when cyclin D2 overexpression was combined with 15 mmol/L glucose (Fig. 2G–I), BrdU, γH2AX, and BrdU-γH2AX–labeled β-cells were markedly increased over baseline. In all proliferation-stimulated conditions (Fig. 2C, F, and I), the observed frequency of colabeled cells was significantly higher than predicted if colabeling was due to chance. To test a third, unrelated proliferative stimulus, we treated the cultures with harmine, a Dyrk1a inhibitor that increases mouse and human β-cell proliferation (18). Harmine increased BrdU labeling synergistically with glucose and increased γH2AX labeling in both 5 and 15 mmol/L glucose, and, similar to the other stimuli, the observed frequency of colabeling was higher than predicted (Fig. 2J–L). Thus, the frequency of BrdU-γH2AX double labeling is increased by proliferative stimuli, especially when stimuli are combined in aging mouse β-cells.
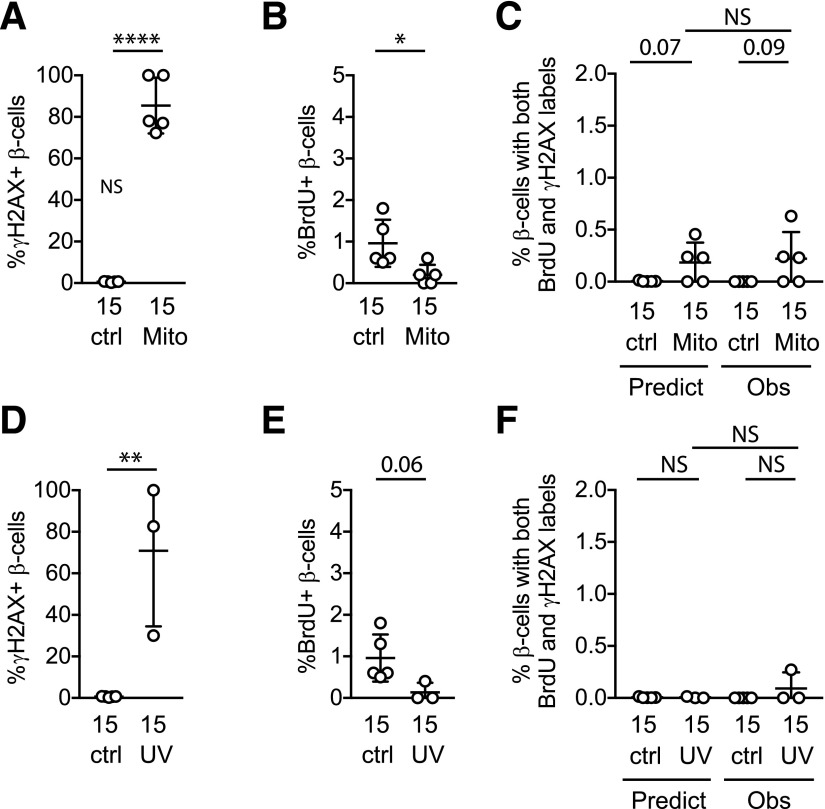
DNA Damage Does Not Lead to BrdU Incorporation
To explore whether DNA damage and BrdU incorporation influence each other, we next tested whether BrdU incorporation was increased during conditions that induce DNA damage. These were perhaps the most critical experiments in this study, because if BrdU is spuriously incorporated as part of a DNA damage response, then BrdU labeling cannot be used to quantify S-phase cell cycle entry. To address this question we cultured β-cells in DNA damage–inducing conditions and quantified BrdU- and double-labeled cells. In islet cell cultures exposed to a sublethal dose of mitomycin C, which induces DNA damage by cross-linking DNA (19–21), almost all β-cells had γH2AX labeling (Fig. 3A), confirming DNA damage. To test whether DNA damage caused spurious BrdU incorporation, we stained for BrdU. The results showed the opposite; mitomycin C strongly suppressed BrdU labeling frequency in both young and old β-cells, and the observed frequency of double-labeled cells was suppressed to the predicted frequency if colabeling were caused by random co-occurrence of unrelated processes (Fig. 3B and C). These results suggest that DNA damage repair following mitomycin C exposure did not cause spurious BrdU incorporation in mouse β-cells that could be misconstrued as proliferation.
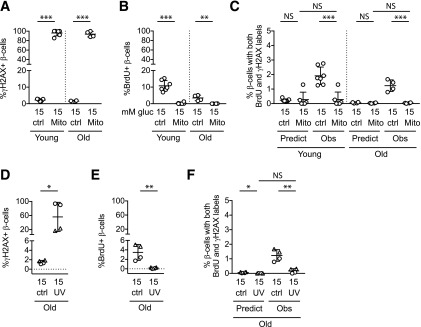
Figure 3.
DNA damage does not increase β-cell BrdU incorporation or BrdU-γH2AX colabeling. Dispersed mouse islet cells were cultured for 72 h in the indicated conditions, with BrdU added for the final 24 h. A–C: Mitomycin C treatment resulted in γH2AX labeling in the majority of β-cells, confirming DNA damage. B: Contrary to the hypothesis that BrdU labeling might spuriously occur during DNA damage repair, mitomycin C treatment decreased, rather than increased, the percentage of β-cells labeling for BrdU. C: Double-labeled cells were not increased under conditions of DNA damage; mitomycin C treatment decreased the percentage of β-cells colabeling with both γH2AX and BrdU. In fact, the observed fraction with mitomycin C treatment was suppressed to the fraction predicted if colabeling was due to chance. Mitomycin treatment was performed on the same biological samples and at the same time as the experiments shown in Fig. 2; control data are repeated from Fig. 2. To test the mitomycin result in a different system, UV irradiation treatment (only performed on islets from old mice) increased the percentage of β-cells labeling with γH2AX (D) but suppressed BrdU incorporation (E) and double-labeled cells (F). In D–F, biological replicates with triangle labels and circle labels were treated identically, but in the triangle samples a lower fraction labeled for γH2AX. The different labels are used to allow identification of the samples with lower DNA damage across panels D–G. *P < 0.05, **P < 0.01, ***P < 0.001. ctrl, control; gluc, glucose; Mito, mitomycin; Obs, observed.
To thoroughly explore this important question, we repeated the experiment using a second DNA damage insult: UV irradiation, which damages DNA by joining adjacent thymine nucleotides into pyrimidine dimers (22–24). We selected a UV dose that caused moderate DNA damage based on trial experiments (data not shown). Cellular response to UV irradiation was variable, with two experiments showing a high proportion of cells labeling for γH2AX (Fig. 3D–F, circles), and two experiments, despite being performed identically, showing fewer cells labeling for γH2AX (Fig. 3D–F, triangles). Similar to the mitomycin C result, UV irradiation–induced DNA damage suppressed BrdU incorporation rather than increasing it, and reduced the observed frequency of double-labeled β-cells to that predicted if colabeling was due to random co-occurrence of unrelated events (Fig. 3E). Reduced BrdU incorporation even in the cultures with only modest γH2AX labeling frequency (Fig. 3E, triangles) suggests that the suppression of BrdU label was not due to the overwhelming toxicity of the DNA damage insult. Taken together, these data suggest that BrdU labeling in cultured mouse β-cells is not increased by DNA damage.
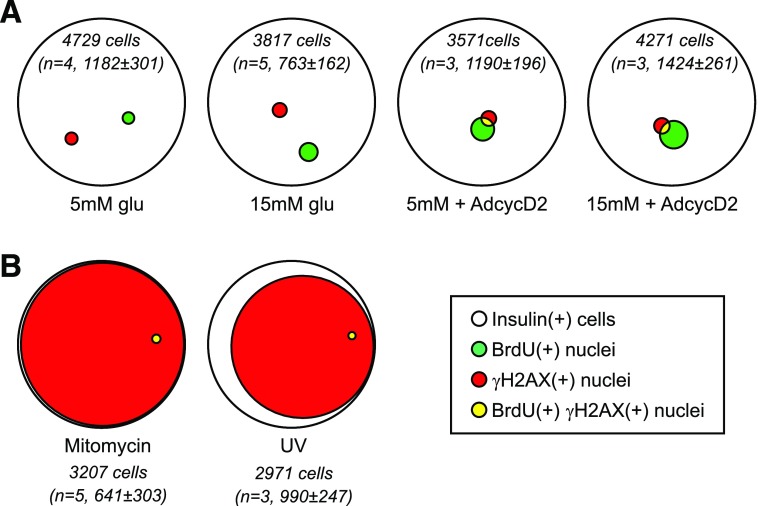
γH2AX Labels a Subset of BrdU-Labeled β-Cells in Ex Vivo Culture
Under most conditions, we noticed that although many BrdU-labeled cells were not γH2AX labeled, the converse was not true: most γH2AX-labeled cells were BrdU labeled. Stated another way, the population of γH2AX-labeling β-cells seemed to be a subset of the BrdU-labeled population. Expressing the data using quantitative Venn diagrams confirmed this observation; especially under proliferation-stimulated conditions, the majority of γH2AX-labeled β-cells were within the BrdU-labeled population (Fig. 4A and B). In contrast, under DNA-damaging conditions, Venn analysis showed that the vast majority of γH2AX-labeling β-cells did not label with BrdU (Fig. 4C). We used confocal microscopy to identify the pattern of BrdU labeling in double-labeled cells. We specifically asked whether BrdU labeled nuclei uniformly, as is characteristic of S-phase DNA replication, or in punctate fashion overlapping with foci of the γH2AX DNA damage label. Images showed that many of the double-labeled nuclei were uniformly labeled with BrdU (Fig. 4D and E), suggesting the possibility that γH2AX labeling occurred after DNA replication. Some nuclei had punctate BrdU, as described previously (Fig. 4F) (5). Consistent with the notion that DNA damage prevents successful cell division, the few mitotic nuclei visualized had no γH2AX label (Fig. 4G). On the other hand, mitotic figures did label for BrdU (Fig. 4G), and many BrdU-labeled nuclei costained for the mitotic marker pHH3 or appeared as doublets (Supplementary Fig. 1), confirming that some BrdU-labeled β-cells do enter mitosis. We tested whether γH2AX-BrdU colabeling of β-cells occurs in vivo under conditions in which β-cell mass increases, such as high-fat feeding (11) and hyperglycemia (10,12). Careful examination of many islets in sections from multiple mice with elevated BrdU incorporation revealed very rare γH2AX labeling in either condition: in eight pancreata from the high-fat diet experiment, only four islet-related nuclei were found that stained for γH2AX (Supplementary Fig. 2), and in four pancreata from the hyperglycemia experiment only four islet-related nuclei stained for γH2AX (Supplementary Fig. 3). Of the eight islet nuclei labeling for γH2AX across both experiments, six were β-cell nuclei, but only two costained for BrdU; interestingly, these two were found in the same islet (Supplementary Fig. 2B). To assess whether γH2AX labeling is induced by ex vivo islet culture in general, or by dispersed islet cell cultures specifically, whole intact islets were cultured under proliferative conditions and assessed for BrdU and γH2AX colabeling (Supplementary Fig. 4). β-Cells colabeling for both markers were very rare in intact islets (Supplementary Fig. 4D). Taken together, these results suggest that in ex vivo dispersed islet cell culture, but not intact islet culture or in vivo pancreas, conditions that promote cell cycle entry or BrdU incorporation increase β-cell risk of γH2AX labeling, whereas conditions that promote DNA damage do not increase the likelihood of BrdU incorporation.
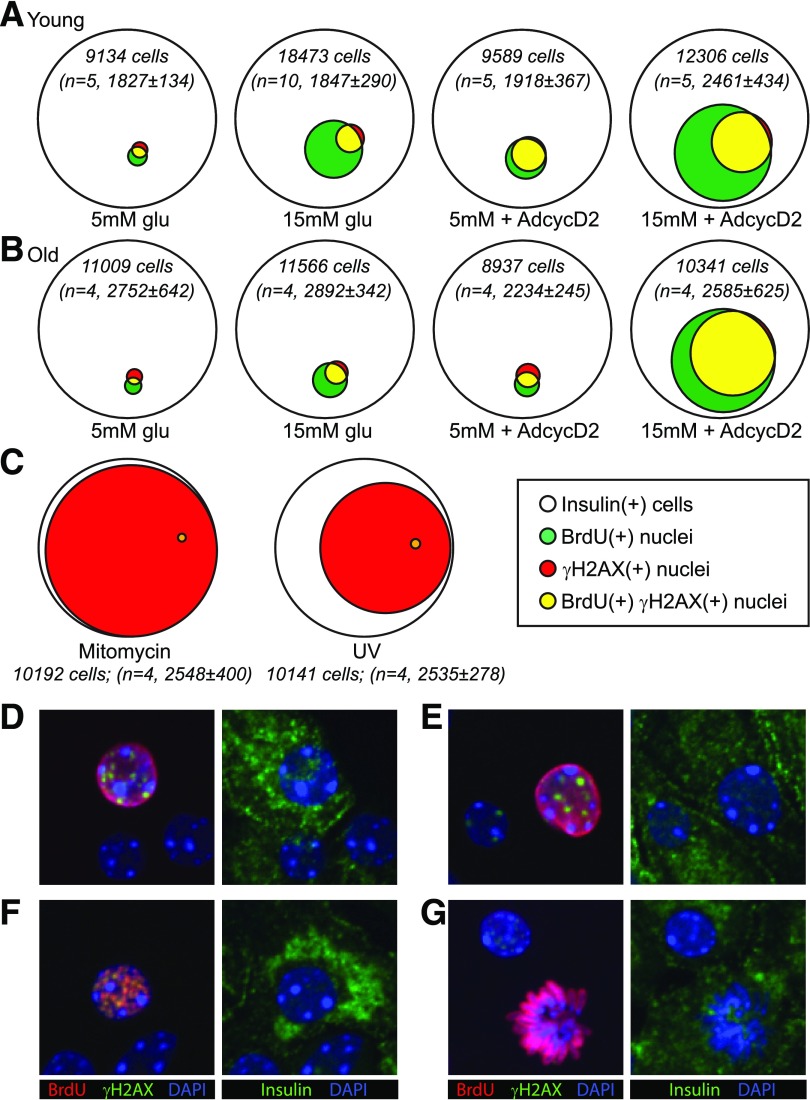
Figure 4.
γH2AX mostly labels a subset of BrdU-labeled cells. Quantitative Venn diagrams were used to generate a visual representation of the data shown in Figs. 2 and 3. Diagrams represent the total number (sum of all replicates) of insulin(+) cells (white circles) that labeled with BrdU (green), γH2AX (red), and both labels (yellow) under different culture conditions. The total number of insulin(+) cells counted and the number of biological replicates for each condition are included for each diagram (in italics). A: In young islet cells, 15 mmol/L glucose and Ad-cyclin D2 increased both the BrdU(+) and γH2AX fractions. Insulin(+) nuclei labeled for γH2AX were mostly a subset of the BrdU-labeled nuclei, in both basal and stimulated conditions. B: β-Cells from older mice behaved similarly to young β-cells, except that a higher fraction of BrdU(+) cells were also γH2AX(+) under 15 mmol/L glucose stimulation both with and without Ad-cyclin D2, and older β-cells required both glucose and Ad-cyclin D2 to meaningfully increase the fraction of BrdU-labeled cells. C: Venn depiction of the data in Fig. 3 demonstrates visually that DNA damage exposure did not increase, and in fact decreased, the frequency of BrdU(+) and colabeled nuclei. Confocal microscopy of cultures (15 mmol/L glucose) showed many examples of smoothly labeled BrdU(+) nuclei that also had γH2AX puncta (D and E) and some examples of nuclei with punctate labeling of both BrdU and γH2AX (F). Active mitoses generally had no γH2AX label (G). AdcycD2, Ad-cyclin D2; glu, glucose.
BrdU Incorporation Itself Is a Minor Cause of β-Cell DNA Damage
Since the data suggested that cell cycle entry, or possibly BrdU incorporation itself, increased the likelihood of γH2AX labeling, and nucleoside analogs are known to induce a DNA damage response in other cell types (7–9), we next asked whether BrdU exposure leads to DNA damage in β-cells. To test this, we cultured mouse islet cells with or without BrdU and assessed the insulin(+) fraction labeling for γH2AX. Omitting BrdU from the culture medium eliminated all BrdU labeling in the cultures (data not shown). In the absence of BrdU exposure, we observed a subtle reduction in γH2AX labeling in β-cells from younger mice that was not observed in β-cells from older mice (Fig. 5A). Hypothesizing that γH2AX labeling might increase over time following BrdU exposure, we compared cells exposed to BrdU during the first 24 h (condition X), the last 24 h (condition Y), or the entire 72-h glucose exposure (condition Z) (Fig. 5B). γH2AX labeling was not different among these conditions (Fig. 5C). To test whether BrdU exposure increased DNA damage under more pronounced proliferative stimuli, we cultured islet cells with or without BrdU in the presence of Ad-cyclin D2 in low-glucose (Fig. 5D) or high-glucose (Fig. 5E) conditions. Intriguingly, BrdU exposure increased γH2AX labeling only in the low-glucose condition. To test whether γH2AX labeling was more related to cycling β-cells or to BrdU incorporation itself, we stained proliferation-stimulated cultures with pHH3 and γH2AX, predicting that if γH2AX labeling was due to BrdU toxicity, there would not be colabeling of γH2AX with pHH3 in the absence of BrdU exposure (Fig. 5F–H). In fact, β-cell nuclei frequently colabeled with both pHH3 and γH2AX, at levels much higher than expected if colabeling was due to random chance (Fig. 5G and H). Similar results were observed with experiments in which costaining was performed for Ki67 and γH2AX (data not shown). These data suggest that although BrdU exposure or BrdU incorporation into DNA under certain conditions can cause double-strand DNA breaks or nucleotide excision/repair that label with γH2AX (23), this toxicity is not the major cause of BrdU-γH2AX double-labeled cells in islet cell cultures.
Figure 5.
BrdU-induced DNA damage does not explain most β-cell γH2AX labeling. Dispersed mouse islet cells were cultured with and without BrdU added to the culture medium, with 15 mmol/L glucose (A–C), Ad-cyclin D2 (D), or Ad-cyclin D2 +15 mmol/L glucose (E). A: In 15 mmol/L glucose, the presence of BrdU caused a subtle increase in γH2AX labeling in young islets. B and C: Shifting the timing of the BrdU exposure earlier in the culture to the first 24 h of glucose exposure with BrdU washed out after 24 h (exposure X), or for the entire 72 h (exposure Z) did not increase the proportion of cells showing evidence of DNA damage compared with the standard exposure during the final 24 h of the 72-h culture (exposure Y). The time course of exposures is diagrammed in B, and the γH2AX quantification is shown in C. Experiments in A–C were performed on the same biological samples; the Y data in C are the same as the BrdU(+) data in A. D: With Ad-cyclin D2 stimulation, BrdU exposure (final 24 h, similar to the rest of the experiments throughout this study) increased γH2AX labeling in 5 mmol/L glucose (B) but not in 15 mmol/L glucose (C). For D and E, the BrdU labeling fraction in these experiments is shown for context, since these cultures had higher levels of stimulated proliferation than the experiments shown in Fig. 2. F–H: dispersed mouse islet cells cultured without BrdU in 15 mmol/L glucose with control or Ad-cyclin D2 had substantial colabeling of γH2AX and pHH3, suggesting that γH2AX is associated with cycling β-cells rather than BrdU incorporation itself. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Gluc, glucose; obs, observed; pred, predicted.
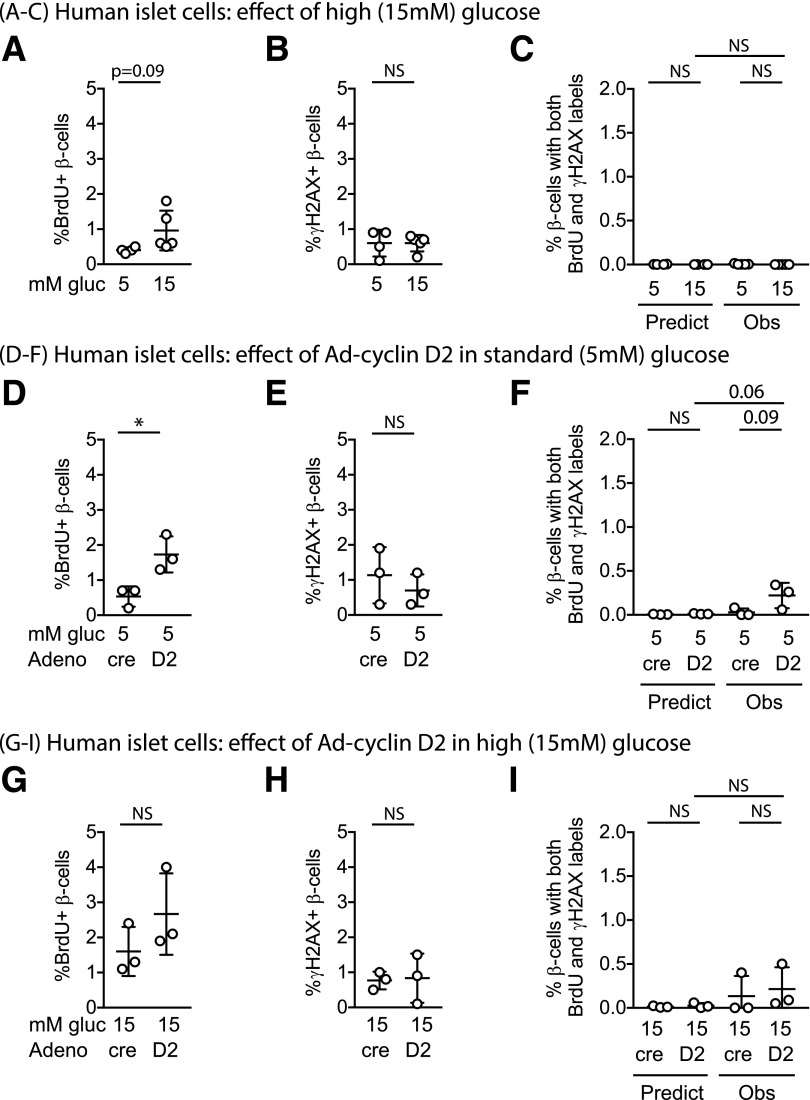
Human β-Cells, Resistant to Proliferative Stimuli, Were Less Likely to Colabel for BrdU and γH2AX Than Mouse β-Cells
The goal of studies in β-cell regeneration is to find approaches to expand human β-cell numbers (25). To learn more about BrdU-γH2AX colabeling in human β-cells (5,6), dispersed human islet cells were cultured under the same conditions as in the mouse experiments, and tested for BrdU incorporation, γH2AX labeling, and colabeling for both markers. Under basal 5 mmol/L glucose conditions, the frequency of human β-cells labeling for BrdU was low, and the frequency of γH2AX labeling was also rare (Fig. 6A and B). To determine whether DNA damage increased in human β-cells during conditions of forced proliferation, we compared basal conditions with pro-proliferative stimuli 15 mmol/L glucose without or with Ad-cyclin D2. Similar to prior published results, insulin(+) cells in human islet cultures were relatively resistant to proliferative stimuli, showing only a marginal increase in the fraction labeling with BrdU when exposed to 15 mmol/L glucose (Fig. 6A–C), Ad-cyclin D2 (Fig. 6D–F), or a combination of the two (Fig. 6G–I). Surprisingly, only a small fraction of human β-cells labeled for γH2AX under basal or stimulated conditions (Fig. 6B, E, and H). The fraction of human β-cells labeling for both labels remained low and was not significantly higher than predicted by the random chance of co-occurrence of two rare events (Fig. 6C, F, and I). These data suggest that under these growth conditions, human β-cells do not frequently colabel for γH2AX and BrdU.
Figure 6.
Human β-cells did not increase DNA damage labeling after proliferative stimulation. Dispersed human islet cells were cultured for 96 h in the described conditions, with BrdU included in the culture media for the entire 96 h. Human insulin(+) cells cultured in 15 mmol/L glucose showed a trend toward modestly increased BrdU incorporation (A) but did not increase γH2AX labeling (B) or BrdU-γH2AX double labeling (C). Adenoviral overexpression of human cyclin D2 in 5 mmol/L glucose increased the BrdU labeling fraction (D) but not the γH2AX-labeling fraction (E); double-labeled cells trended upward (F). Combining glucose and cyclin D2 proliferative stimuli did not further increase BrdU (G), γH2AX (H), or double-labeled nuclei (I). Adeno-cre was used as a control for Adeno-cyclin D2, at the same multiplicity of infection. *P < 0.05. Adeno, adenovirus; gluc, glucose; Obs, observed; Predict, predicted.
DNA Damage Did Not Increase BrdU Labeling in Human β-Cells
To test whether human β-cells incorporate BrdU during DNA damage repair, DNA-damaging conditions were applied to human islet cultures. Mitomycin C exposure caused γH2AX labeling in the majority of β-cells (Fig. 7A). Despite widespread DNA damage in the cultures, the percentage of insulin(+) cells labeling for BrdU decreased (Fig. 7B) and the observed fraction of double-labeled cells was similar to predicted if colabeling was by random chance. When human islet cultures were exposed to UV irradiation (Fig. 7D–F), the DNA damage response was variable (Fig. 7D), but BrdU incorporation was suppressed (Fig. 7E) and there was no increase in the proportion of β-cells labeling for both markers (Fig. 7F). In sum, human β-cells behaved similarly to mouse β-cells: DNA damage did not cause a spurious increase in BrdU labeling.
Figure 7.
DNA damage does not cause spurious BrdU incorporation in human β-cells. Dispersed human islet cells were cultured for 96 h in the described conditions, with BrdU included in the culture media for the entire 96 h. Human insulin(+) cells exposed to mitomycin C were nearly all labeled for γH2AX (A) but had suppressed BrdU labeling fraction (B) and no excess double-labeled cells beyond the fraction predicted by random chance (C). Human β-cells exposed to UV irradiation showed variable induction of the DNA damage label γH2AX (D), suppression of BrdU labeling (E), and no excess double-labeled cells (F). Note the scale differences in y-axes from left to right. *P < 0.05, **P < 0.01, ****P < 0.0001. ctrl, control; Mito, mitomycin; Obs, observed; Predict, predicted.
γH2AX and BrdU Labeling Were Mostly Independent in Human β-Cells
Quantitative Venn analysis of the human β-cell BrdU- and γH2AX-labeling populations revealed the interesting result that under basal and proliferation-stimulated conditions, insulin(+) cells labeling for BrdU were mostly not the same cells that labeled with γH2AX (Fig. 8A). In fact, without Ad-cyclin D2, no double-labeled cells were identified. Similar to mouse islet cultures, however, DNA damage stimuli caused the majority of human β-cells to label for γH2AX, and only a tiny fraction of those cells also labeled for BrdU (Fig. 8B). Overall, fewer human β-cells labeled for γH2AX than mouse. As in mouse β-cells, DNA damage conditions did not induce spurious BrdU incorporation in human β-cells. Neither BrdU nor γH2AX were meaningfully increased by proliferative stimuli in human β-cells, and in these conditions, the observed double-labeled fraction was not increased over that predicted by random chance.
Figure 8.
In human β-cells, proliferation was infrequent even under stimulated conditions, and γH2AX labeling was mostly independent of BrdU-labeled cells. The data in Figs. 6 and 7 are shown using quantitative Venn diagrams to illustrate the relationship between γH2AX labeling and BrdU labeling in these cultures. Diagrams represent the total number (sum of all replicates) of insulin(+) cells (white) that labeled with BrdU (green), γH2AX (red), and both labels (yellow) under different culture conditions. The total number of insulin(+) cells counted, summed for all biological replicates (n = 3–5), is included for each diagram (in italics). A: Although BrdU labeling increased somewhat in proliferative conditions, the γH2AX-labeling index did not, and most γH2AX-labeled cells were not BrdU labeled. B: Mitomycin C and UV irradiation caused DNA damage in the majority of β-cells, but did not increase, in fact decreased, the BrdU-labeling fraction.
Discussion
These studies explore the frequency and causes of mouse and human β-cell colabeling for BrdU and the γH2AX DNA damage marker. The results suggest that in mouse β-cells, colabeling occurs more frequently than predicted if the association is due to random chance. Evidence does not support the hypothesis that colabeling is due to BrdU incorporation during DNA repair. Instead, the results suggest that γH2AX labeling can be triggered by something related to the proliferative process, either by an upstream proliferation stimulus or by cellular events associated with the cell cycle itself.
These experiments are important, because the uncertainty of the fidelity of BrdU as a label of S-phase in β-cells has impacted the field. For some, conflating the concepts of cell cycle and DNA damage has rekindled long-standing doubts that β-cells can replicate at all, despite a large quantity of evidence to the contrary (3,14–16). Investigators have been at times required to use multiple duplicative tools to measure the same outcome, slowing progress. The results of these current studies are reassuring. Labeling for γH2AX in human β-cells was rare, even under proliferation-stimulating conditions (25), and most BrdU(+) human β-cells were not γH2AX(+). In vivo mouse pancreata rarely labeled with γH2AX, even under conditions when many β-cells were BrdU(+). Overall, the results are consistent with the conclusion that, even in mouse islets, ex vivo BrdU authentically labels β-cells that enter S-phase.
The data show that DNA damage does not cause BrdU labeling that could be misconstrued as S-phase entry, in either mouse or human β-cells. Using two different DNA damage–inducing conditions, one of which caused widespread damage in essentially all β-cells (mitomycin C), and the other of which caused more moderate damage (UV irradiation), BrdU incorporation was reduced rather than increased. Furthermore, the observed frequency of double-labeled nuclei was suppressed in DNA damage conditions to that expected if the occurrence was due to random chance. Thus, β-cell colabeling for BrdU and γH2AX in these cultures was not caused by spurious BrdU incorporation as part of the cellular response to DNA damage.
The data show a clear association between γH2AX labeling and proliferative stimuli in mouse β-cells. γH2AX labeling, and γH2AX-BrdU or γH2AX-pHH3 colabeling, occurred occasionally under unstimulated conditions but were markedly increased in 15 mmol/L glucose with or without the overexpression of cyclin D2 or the addition of harmine. Intriguingly, the γH2AX-labeled cells were mostly a subset of the BrdU-labeled population. Also, many γH2AX-labeled nuclei were evenly and completely BrdU labeled, giving the impression that S-phase had occurred smoothly. Taken together with the observations that DNA damage suppressed new S-phase entry and that γH2AX foci were absent from the few mitotic spindles observed, we speculate that the γH2AX foci may mostly occur in a cell cycle window from late S-phase through G2 phase or in postmitotic daughter cells. γH2AX is best known for labeling double-strand breaks or nucleotide excision repair, recruiting DNA repair effectors (23,24). γH2AX also labels DNA replication stress, such as replication fork collapse, defects in chromatin assembly, cell cycle arrest, or prolonged mitosis (26–29). This raises the possibility that β-cells forced into the cell cycle may have weakened replication forks or other defects in completing mitosis. In some cell types, γ-phosphorylation of H2AX may occur intrinsically during S- or G2-M phases of the cell cycle (30,31). It is possible that chromatin handling during DNA replication or G2 may predispose β-cells to γH2AX phosphorylation; whether this is a sign of damaged DNA or another process remains uncertain.
A toxic effect of nucleoside analogs, including both BrdU and EdU, has been reported (7–9). In the β-cell field, EdU is also commonly used, especially for high-throughput screening; whether EdU performs similarly to BrdU has not been carefully tested. The final concentration of BrdU used in our study (33 μmol/L) was similar to those in other published studies (10–100 μmol/L) (5,7,8). BrdU exposure may or may not induce sister chromatid exchanges, a marker of genome instability (32). Triphosphate nucleoside analogs such as 5-fluorouracil act as replication fork blocks; however, these induce γH2AX not at the time of initial incorporation, but rather during S-phase of a subsequent cell cycle event (33). Extensive colabeling of γH2AX and pHH3 in the absence of BrdU exposure argue against a primary role for BrdU toxicity in the observed γH2AX population, but rather that γH2AX staining is associated with cycling cells. β-Cells with smooth BrdU label and γH2AX foci may be cells that have re-entered the cell cycle following a successful initial cell division.
Surprisingly, β-cells from older mice were not spontaneously more likely to label for γH2AX. However, when both high-glucose conditions and cyclin D2 overexpression were combined, β-cells from older mice had a higher frequency of γH2AX labeling, and a lower fraction of BrdU-labeled β-cells that did not show evidence of DNA damage. This may be related to the fact that β-cells from older mice required a combination of both glucose and cyclin D2 to enter the cell cycle, whereas β-cells from younger mice responded to either stimulus. The human islet donors used for this study were from a narrow range of ages, 41–56 years, precluding assessment of the impact of age on DNA damage markers in human β-cells.
Also contrary to expectations, in these studies human β-cells did not have a high frequency of γH2AX labeling. If anything, human β-cells were less likely than mouse β-cells to label for γH2AX, especially in the presence of proliferative stimuli. However, the pro-proliferation stimuli used in these studies did not result in a high rate of cell cycle entry in human β-cells. As such, the low rate of human γH2AX labeling is consistent with the hypothesis that DNA damage occurs in later stages of the cell cycle, or during cell cycle re-entry following successful mitosis. We have not tested conditions that induce rapid proliferation in human β-cells.
This study does not address the extent to which BrdU, marking S-phase entry, predicts completion of the cell cycle and production of two daughter cells. Evidence supporting the conclusion that some BrdU-labeled β-cells do successfully complete cell division include the many studies showing that BrdU labeling correlates with other proliferation markers such as Ki67, PCNA (proliferating cell nuclear antigen), and pHH3 (17,34,35), the documented increase in β-cell number under conditions when BrdU labeling is increased in vitro (17,36,37) and in vivo (33), and our current data showing BrdU(+) nuclei in mitosis (Fig. 4G) and colabeling with pHH3 (Supplementary Fig. 1). We have not formally tested for β-cell endoreduplication, but, similar to prior observations (38), we did not detect systematically increased nuclear size in double-labeled cells. On the other hand, the fate of β-cells labeled for both BrdU and γH2AX remains unknown. Although getting β-cells to traverse the G1-S transition is an important and challenging goal, studies are also needed to find ways to increase the frequency of successful mitosis in β-cells that do enter the cell cycle. Taken together, the results of the current studies support the use of BrdU labeling as a faithful measure of S-phase entry and a useful tool in the overarching goal of the pursuit of β-cell regeneration strategies as a therapy for diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the Beta Cell Biology Group at the University of Massachusetts Medical School for helpful discussions.
Funding. Human pancreatic islets were provided by the IIDP at City of Hope, which was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This work was supported by National Institutes of Health/NIDDK grants R01-DK-114686 (L.C.A.), R01-DK-113300 (L.C.A.), R01-DK-095140 (L.C.A.), U2C-D-093000 (National Mouse Metabolic Phenotyping Center Analytical and Functional Core; L.C.A.), and 2UC4-DK-098085 (IIDP); American Diabetes Association grant 1-18-IBS-233 (L.C.A.) in collaboration with the Order of the Amaranth; and the George F. and Sybil H. Fuller Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.B.S. performed and analyzed the experiments, with assistance from C.D. and X.Z. (immunostaining and microscopy) as well as B.G. (unbiased image analysis). R.B.S. and L.C.A. devised and planned the project. L.C.A. wrote the manuscript. All authors had the opportunity to edit and approve of the manuscript. L.C.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0761/-/DC1.
References
- 1.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004;114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 4.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 2008;105:2415–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rieck S, Zhang J, Li Z, et al. Overexpression of hepatocyte nuclear factor-4α initiates cell cycle entry, but is not sufficient to promote β-cell expansion in human islets. Mol Endocrinol 2012;26:1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S-H, Hao E, Levine F, Itkin-Ansari P. Id3 upregulates BrdU incorporation associated with a DNA damage response, not replication, in human pancreatic β-cells. Islets 2011;3:358–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masterson JC, O’Dea S. 5-Bromo-2-deoxyuridine activates DNA damage signalling responses and induces a senescence-like phenotype in p16-null lung cancer cells. Anticancer Drugs 2007;18:1053–1068 [DOI] [PubMed] [Google Scholar]
- 8.Kohlmeier F, Maya-Mendoza A, Jackson DA. EdU induces DNA damage response and cell death in mESC in culture. Chromosome Res 2013;21:87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anda S, Boye E, Grallert B. Cell-cycle analyses using thymidine analogues in fission yeast. PLoS One 2014;9:e88629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab 2013;305:E149–E159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamateris RE, Sharma RB, Kong Y, et al. Glucose induces mouse β-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes 2016;65:981–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong Y, Ebrahimpour P, Liu Y, Yang C, Alonso LC. Pancreatic islet embedding for paraffin sections. J Vis Exp 2018;(136):e57931. [DOI] [PMC free article] [PubMed]
- 14.Carpenter AE, Jones TR, Lamprecht MR, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 2006;7:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques 2007;42:71–75 [DOI] [PubMed] [Google Scholar]
- 16.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One 2014;9:e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma RB, O’Donnell AC, Stamateris RE, et al. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest 2015;125:3831–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P, Alvarez-Perez J-C, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dusre L, Covey JM, Collins C, Sinha BK. DNA damage, cytotoxicity and free radical formation by mitomycin C in human cells. Chem Biol Interact 1989;71:63–78 [DOI] [PubMed] [Google Scholar]
- 20.Mladenov E, Tsaneva I, Anachkova B. Activation of the S phase DNA damage checkpoint by mitomycin C. J Cell Physiol 2007;211:468–476 [DOI] [PubMed] [Google Scholar]
- 21.Lee Y-J, Park S-J, Ciccone SLM, Kim C-R, Lee S-H. An in vivo analysis of MMC-induced DNA damage and its repair. Carcinogenesis 2006;27:446–453 [DOI] [PubMed] [Google Scholar]
- 22.Hanasoge S, Ljungman M. H2AX phosphorylation after UV irradiation is triggered by DNA repair intermediates and is mediated by the ATR kinase. Carcinogenesis 2007;28:2298–2304 [DOI] [PubMed] [Google Scholar]
- 23.Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci U S A 2006;103:9891–9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh K-S, Bustin M, Mazur SJ, Appella E, Kraemer KH. UV-induced histone H2AX phosphorylation and DNA damage related proteins accumulate and persist in nucleotide excision repair-deficient XP-B cells. DNA Repair (Amst) 2011;10:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 2011;54:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagou ME, Zuazua-Villar P, Meuth M. Enhanced H2AX phosphorylation, DNA replication fork arrest, and cell death in the absence of Chk1. Mol Biol Cell 2010;21:739–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye X, Franco AA, Santos H, Nelson DM, Kaufman PD, Adams PD. Defective S phase chromatin assembly causes DNA damage, activation of the S phase checkpoint, and S phase arrest. Mol Cell 2003;11:341–351 [DOI] [PubMed] [Google Scholar]
- 28.Colin DJ, Hain KO, Allan LA, Clarke PR. Cellular responses to a prolonged delay in mitosis are determined by a DNA damage response controlled by Bcl-2 family proteins. Open Biol 2015;5:140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szüts D, Krude T. Cell cycle arrest at the initiation step of human chromosomal DNA replication causes DNA damage. J Cell Sci 2004;117:4897–4908 [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif 2005;38:223–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacPhail SH, Banáth JP, Yu Y, Chu E, Olive PL. Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat Res 2003;159:759–767 [DOI] [PubMed] [Google Scholar]
- 32.van Wietmarschen N, Lansdorp PM. Bromodeoxyuridine does not contribute to sister chromatid exchange events in normal or Bloom syndrome cells. Nucleic Acids Res 2016;44:6787–6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuda M, Terada K, Ooka M, et al. The dominant role of proofreading exonuclease activity of replicative polymerase ε in cellular tolerance to cytarabine (Ara-C). Oncotarget 2017;8:33457–33474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascoe J, Hollern D, Stamateris R, et al. Free fatty acids block glucose-induced β-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes 2012;61:632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, et al. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human β-cell replication: a revised model of human β-cell G1/S control. Diabetes 2013;62:2460–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiwari S, Roel C, Wills R, et al. Early and late G1/S cyclins and Cdks act complementarily to enhance authentic human β-cell proliferation and expansion. Diabetes 2015;64:3485–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P, Karakose E, Liu H, et al. Combined inhibition of DYRK1A, SMAD, and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metab 2019;29:638–652.e5. DOI: 10.1016/j.cmet.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pechhold K, Koczwara K, Zhu X, et al. Blood glucose levels regulate pancreatic beta-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS One 2009;4:e4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.