Abstract
Background
Peritoneal dialysis (PD) is an important therapy for patients with end‐stage kidney disease and is used in more than 200,000 such patients globally. However, its value is often limited by the development of infections such as peritonitis and exit‐site and tunnel infections. Multiple strategies have been developed to reduce the risk of peritonitis including antibiotics, topical disinfectants to the exit site and antifungal agents. However, the effectiveness of these strategies has been variable and are based on a small number of randomised controlled trials (RCTs). The optimal preventive strategies to reduce the occurrence of peritonitis remain unclear.
This is an update of a Cochrane review first published in 2004.
Objectives
To evaluate the benefits and harms of antimicrobial strategies used to prevent peritonitis in PD patients.
Search methods
We searched the Cochrane Kidney and Transplant's Specialised Register to 4 October 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
RCTs or quasi‐RCTs in patients receiving chronic PD, which evaluated any antimicrobial agents used systemically or locally to prevent peritonitis or exit‐site/tunnel infection were included.
Data collection and analysis
Two authors independently assessed risk of bias and extracted data. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratio (RR) with 95% confidence intervals (CI).
Main results
Thirty‐nine studies, randomising 4435 patients, were included. Twenty additional studies have been included in this update. The risk of bias domains were often unclear or high; risk of bias was judged to be low in 19 (49%) studies for random sequence generation, 12 (31%) studies for allocation concealment, 22 (56%) studies for incomplete outcome reporting, and in 12 (31%) studies for selective outcome reporting. Blinding of participants and personnel was considered to be at low risk of bias in 8 (21%) and 10 studies (26%) for blinding of outcome assessors. It should be noted that blinding of participants and personnel was not possible in many of the studies because of the nature of the intervention or control treatment.
The use of oral or topical antibiotic compared with placebo/no treatment, had uncertain effects on the risk of exit‐site/tunnel infection (3 studies, 191 patients, low quality evidence: RR 0.45, 95% CI 0.19 to 1.04) and the risk of peritonitis (5 studies, 395 patients, low quality evidence: RR 0.82, 95% CI 0.57 to 1.19).
The use of nasal antibiotic compared with placebo/no treatment had uncertain effects on the risk of exit‐site/tunnel infection (3 studies, 338 patients, low quality evidence: RR 1.34, 95% CI 0.62 to 2.87) and the risk of peritonitis (3 studies, 338 patients, low quality evidence: RR 0.94, 95% CI 0.67 to 1.31).
Pre/perioperative intravenous vancomycin compared with no treatment may reduce the risk of early peritonitis (1 study, 177 patients, low quality evidence: RR 0.08, 95% CI 0.01 to 0.61) but has an uncertain effect on the risk of exit‐site/tunnel infection (1 study, 177 patients, low quality evidence: RR 0.36, 95% CI 0.10 to 1.32).
The use of topical disinfectant compared with standard care or other active treatment (antibiotic or other disinfectant) had uncertain effects on the risk of exit‐site/tunnel infection (8 studies, 973 patients, low quality evidence, RR 1.00, 95% CI 0.75 to 1.33) and the risk of peritonitis (6 studies, 853 patients, low quality evidence: RR 0.83, 95% CI 0.65 to 1.06).
Antifungal prophylaxis with oral nystatin/fluconazole compared with placebo/no treatment may reduce the risk of fungal peritonitis occurring after a patient has had an antibiotic course (2 studies, 817 patients, low quality evidence: RR 0.28, 95% CI 0.12 to 0.63).
No intervention reduced the risk of catheter removal or replacement. Most of the available studies were small and of suboptimal quality. Only six studies enrolled 200 or more patients.
Authors' conclusions
In this update, we identified limited data from RCTs and quasi‐RCTs which evaluated strategies to prevent peritonitis and exit‐site/tunnel infections. This review demonstrates that pre/peri‐operative intravenous vancomycin may reduce the risk of early peritonitis and that antifungal prophylaxis with oral nystatin or fluconazole reduces the risk of fungal peritonitis following an antibiotic course. However, no other antimicrobial interventions have proven efficacy. In particular, the use of nasal antibiotic to eradicate Staphylococcus aureus, had an uncertain effect on the risk of peritonitis and raises questions about the usefulness of this approach. Given the large number of patients on PD and the importance of peritonitis, the lack of adequately powered and high quality RCTs to inform decision making about strategies to prevent peritonitis is striking.
Plain language summary
Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients
What is the Issue?
People with kidney failure may be treated with peritoneal dialysis where a catheter is permanently inserted into the peritoneum (lining around abdominal contents) through the abdominal wall and sterile fluid is drained in and out a few times each day. The most common serious complication is infection of the peritoneum, which is called peritonitis. This may be caused by bacteria accidentally being transferred from the catheter.
What did we do?
We searched the literature up until 4 October 2016 and identified 39 studies randomising 4435 patients undergoing peritoneal dialysis that were evaluated in this review.
What did we find?
We found that antibiotics given when a peritoneal dialysis catheter is implanted may reduce the risk of early peritonitis but not of exit‐site/tunnel infection. Antifungal prophylaxis with oral nystatin or fluconazole reduces the risk of fungal peritonitis following an antibiotic course. The available studies are of low quality evidence and consequently, it is uncertain if there is any benefit from using nasal mupirocin or topical disinfectants or other interventions to reduce exit‐site/tunnel infection or peritonitis.
Summary of findings
Background
Peritoneal dialysis (PD) is one of the renal replacement therapies available to people with end‐stage kidney disease (ESKD). There is considerable variation in its use from country to country, with the proportion of total dialysis patients on PD in developed countries ranging from 3.3% (Japan), to 7.0% (USA), 8.3% (Greece), 17.0 % (UK), 36.3% (New Zealand), and up to 79.4% (Hong Kong) (Jain 2012). Because PD and haemodialysis have similar outcomes and patients feel that PD, compared with HD, allows them to live life more fully (Morton 2011), PD should be used more frequently than it is but the perceived risk of peritonitis may prevent this from occurring (Heaf 2004; Piraino 1998).
Description of the condition
Peritonitis due to various organisms(e.g.Staphylococcus aureus, Pseudomonas aeruginosa, coagulase‐negative staphylococci) is a leading complication of PD resulting in technique failure (Woodrow 1997), hospitalisation (Choi 2004; Churchill 1997), peritoneal membrane failure, switching to haemodialysis (Jaar 2009; Piraino 1989) and increased mortality (Annigeri 2001; Digenis 1990; Fried 1996; Piraino 2000). There has been a dramatic reduction in the rates of peritonitis from the start of continuous ambulatory PD (CAPD), but rates above the minimum acceptable peritonitis rate recommended by the International Society for Peritoneal Dialysis (ISPD) of one episode every 33 months (0.36 episodes/year at risk) are still common (Piraino 2011).
Risk factors for peritonitis include older age (Nessim 2009; Oxton 1994; Salusky 1997), depression (Troidle 2003), coexisting diseases such as diabetes (Chow 2005; Ghali 2011) and cardiovascular disease (McDonald 2004; Nolph 1987), obesity (McDonald 2004), connection methodology (Daly 2001), presence of a peritoneal catheter exit‐site infection (Lloyd 2013; van Diepen 2012), and the presence of nasal carriage of S. aureus (Golper 1996; Mupirocin Study 1996; Perez‐Fontan 1993; Schaefer 2003). Race is also an independent risk factor, with African‐American, native Canadian and indigenous Australian Aborigines on PD being shown to be at increased risk (Farias 1994; Fine 1994; Golper 1996; Holley 1993; Lim 2005).
Description of the intervention
Different antimicrobial interventions are used at PD catheter insertion and on an ongoing basis to prevent peritonitis. These include intravenous antibiotics, oral antibiotics, topical antibiotics (Thodis 2000), topical disinfectants, prophylactic treatment of S. aureus nasal carriage primarily with intranasal antibiotic ointment (Piraino 2002), different exit‐site dressing systems and antifungal prophylaxis. All of these strategies, particularly the use of antibiotic at catheter insertion and the cleansing and disinfection of the exit‐site, are widely accepted, but practice patterns are variable and it is not clear which practices have most benefit (Piraino 2011; Van Biesen 2014). Studies on preventing PD‐related infections are limited in number and quality (Piraino 2011). International guidelines differ in their recommendations on preventing PD‐related infections, with some countries not having relevant guidelines (Table 5).
1. Guidelines on antimicrobial interventions to prevent peritonitis in PD.
| Guideline | Country | Year | Recommendation |
| Kidney‐Disease Outcomes Quality Initiative | United States of America | NA | No guideline |
| The Renal Association | United Kingdom | April 2008 July 2010 |
Guideline 3.1 ‐ PD Access: Implantation Protocol
Guideline 5.1.4 ‐ PD Infectious Complications: Prevention Strategies
Guideline 5.1.5 ‐ PD Infectious Complications: Prevention Strategies
Guideline 5.1.6 ‐ PD Infectious Complications: Prevention Strategies
|
| Canadian Society of Nephrology | Canada | NA | No guideline |
| European Renal Best Practice | Europe | NA | No guideline |
| International Society for Peritoneal Dialysis | NA | July 2010 November 2011 |
Guideline 3.1: Implantation Protocol (1A)
Position Statement: Catheter Placement to Prevent Catheter Infections and the Related Peritonitis Episodes
Position Statement: Exit‐Site Care to Prevent Peritonitis
Position Statement: Prevention of Fungal Peritonitis
|
| Kidney Health Australia‐Caring for Australasians with Renal Impairment | Australia/ New Zealand | February 2014 |
Guideline 6. Prophylactic Antibiotics for Insertion of PD Catheters
Guideline 8. Treatment of Peritoneal Dialysis‐Associated Fungal Peritonitis
Guideline 10. Prophylaxis for Exit‐site/Tunnel Infections Using Mupirocin
|
MRSA ‐ methicillin‐resistant S. aureus; NA ‐ not applicable; PD ‐ peritoneal dialysis
None of these interventions are free of risks or without cost. Antibiotic prophylaxis carries the risk of gastrointestinal toxicity and may be a cause of antibiotic resistance (Annigeri 2001; Bernardini 1996); it may also be ineffective when patients already have resistance to some antibiotics. Care should be taken that any disinfectant used is at a concentration that is non‐cytotoxic (Piraino 2011).
How the intervention might work
For a patient to be able to successfully use PD as a dialysis therapy, PD‐related infections (exit‐site infections, tunnel infections and peritonitis) need to be avoided. The most important infection is peritonitis and a number of prophylactic strategies have been employed to limit its occurrence. Bacteria are known to be able to gain entry to the peritoneum in a variety of ways and hence, various strategies have been used to prevent this occurring (Campbell 2015).
Oral antibiotics
Oral antibiotics such as rifampin have been given as prophylaxis to PD patients to reduce catheter infections and peritonitis due to S. aureus (Bernardini 1996; Zimmerman 1991). This organism is a major cause of PD catheter infections which can result in S. aureus peritonitis and catheter removal. S. aureus nasal carriage is known to be a significant risk factor for S. aureus PD‐related infections (Bernardini 1996). Cyclic oral rifampin is superior to placebo in preventing S. aureus infections. Other oral antibiotics used include ofloxacin (Sesso 1994), cephalexin (Low 1980), and trimethoprim/sulphamethoxazole (Churchill 1988).
Topical antibiotics
Topical antibiotics such as mupirocin have been applied to the exit site once daily because this antibiotic has good activity against gram‐positive organisms such as staphylococci and streptococci, which are a common cause of exit‐site infection and peritonitis in PD patients (Keane 2000; Troidle 1998; Ward 1986). However, mupirocin is less active against most gram‐negative bacilli and anaerobes (Sutherland 1985). Sodium fusidate ointment (2%) has also been applied to the exit site at one‐month intervals and is known to have activity against staphylococci (Sesso 1994). Gentamicin cream is active against both gram‐positive and gram‐negative organisms and has been used long term on a once‐daily basis at the exit site as prophylaxis for exit‐site infection (Bernardini 2005; Chu 2008). Gentamicin is active against both S. aureus and P. aeruginosa, two important causes of exit‐site infection (Bernardini 2005). Polysporin triple ointment (P3) consists of bacitracin/gramicidin/polymyxin and has bacteriostatic activity against a wide range of skin flora and other organisms including gram‐negative bacteria (MP3 Study 2008).
Nasal antibiotic prophylaxis
Various antibiotic treatments have been trialled in attempts to eliminate S. aureus nasal carriage in PD patients. The nasal carriage of S. aureus is a well‐recognised risk factor for the development of S. aureus infections in CAPD patients (Davies 1989; Luzar 1990; Piraino 1990). Neomycin sulphate ointment has been used prophylactically. Mupirocin has also been used to eliminate nasal S. aureus. While mupirocin is effective at reducing S. aureus nasal carriage rates, re‐colonisation frequently occurs. Sodium fusidate ointment (2%) has also been used and is effective at reducing S. aureus nasal carriage rates (Sesso 1994).
Pre/peri‐operative intravenous antibiotic prophylaxis
The administration of intravenous antibiotics at catheter insertion has been trialled in order to determine if this practice reduces the risk of post‐operative peritonitis or exit‐site infection after PD catheter insertion. Although the insertion of a PD catheter involves "clean surgery involving the placement of a prosthesis or implant", there is the potential for contamination of the peritoneum with micro‐organisms from the patient's own body during surgery. Hence, the giving of a single dose of antibiotic prophylaxis intravenously on starting anaesthesia is recommended (Collier 2008).
Topical disinfectants of the exit site
Topical disinfectants have been applied to the exit site for many years, in an attempt to reduce the bacterial load around the exit site. It has been shown that PD patients with a history of an exit‐site infection have twice the risk of experiencing a peritonitis episode (Canadian CAPD Clinical Trials Group 1989) so it is important to keep the exit‐site infection‐free. Povidone iodine ointment is a broad spectrum antiseptic ointment that has been used and has minimal adverse events associated with its use (Waite 1997). Povidone iodine solution (20g/L) has also been used and shown to successfully reduce the number of exit‐site infections (Luzar 1990). Other antiseptic agents such as hydrogen peroxide, sodium hypochlorite and chlorhexidine have been used (Piraino 2011). The daily use of antibacterial honey at the exit site was trialled in the HONEYPOT Study 2009 This agent was used because it does not induce antimicrobial resistance and has been shown to be active against a broad range of bacteria and fungi (Cho 2014).
Dressing systems for exit sites
A number of exit‐site dressing systems have been devised, all with the aim of reducing exit‐site/tunnel infection and any subsequent peritonitis. The agents used include topical disinfectants and different dressing types and require more or less frequent removal. More frequent removal is seen to risk damaging the skin around the exit site and less frequent removal is felt to possibly encourage the growth of anaerobes. The concentration of topical disinfectants used need to be at non‐cytotoxic levels.
Silver ring system on catheter
The addition of a silver ring device mounted onto the PD catheter was trialled by German researchers in the 1990s (SIPROCE Study 1997). The silver ring was used because of the antimicrobial properties of silver. The use of silver‐coated catheters in animals had shown a reduction in infectious events (Dasgupta 1994; Fung 1996) and offered a non‐pharmaceutical approach to reducing PD catheter‐related infections.
Antistaphylococcal vaccine
An antistaphylococcal vaccine was trialled in the 1990s for the purpose of immunising patients with an anti‐staphylococcal agent. The expectation was that the vaccine would promote a significant increase in the dialysate level of specific antibodies against S. aureus and that this would lead to reduced peritonitis and exit‐site/tunnel infection rates (Poole‐Warren 1991).
Antifungal agents
Antifungal prophylaxis to prevent fungal peritonitis when a PD patient receives an antibiotic course is based on the fact that most episodes of fungal peritonitis are preceded by courses of antibiotics (Piraino 2011). Patients receiving prolonged or repeated antibiotic courses are at increased risk of fungal peritonitis, mostly due to Candida spp. The co‐administration of an oral antifungal agent with an antibiotic course has been trialled to determine if this practice reduces the risk of fungal peritonitis (Lo 1996; Restrepo 2010).
Why it is important to do this review
The aim of this update was to include any new studies of antimicrobial interventions designed to prevent peritonitis in PD patients that have been published since the original review was published in 2004. We also aim to provide a critical appraisal of the current available evidence. As peritonitis is a significant problem for patients using PD, frequently leading to morbidity and technique failure and sometimes to mortality, we have updated the review.
Objectives
To evaluate the benefits and harms of antimicrobial strategies used to prevent peritonitis in PD patients.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) in which antimicrobial interventions designed to prevent peritonitis were compared in patients on PD.
Types of participants
We included adults and children with ESKD who were undergoing PD treatment.
Types of interventions
We included studies involving the use of any antimicrobial agent, whether the interventions were tested between themselves (head‐to‐head) or against placebo/no treatment. The inclusion criteria have been expanded in this update, with the intervention "oral antibiotics" becoming "oral or topical or intraperitoneal antibiotics" and with the interventions "dressing systems for exit sites" and "silver ring system on catheter" being added.
Specifically, the following antimicrobial interventions were analysed.
Oral or topical or intraperitoneal antibiotics
Nasal antibiotic prophylaxis (mupirocin, rifampicin, other)
Pre/peri‐operative intravenous antibiotic prophylaxis
Topical disinfectants of the exit‐site (povidone‐iodine, chlorhexidine, triclosan, soap and water, other)
Germicidal systems for connection devices
Dressing systems for exit sites
Silver ring system on catheter
Antistaphylococcal vaccine
Antifungal agents
Types of outcome measures
Peritonitis‐number of patients with peritonitis and peritonitis rate (peritonitis defined as dialysate count of > 100 cells/mm3 with > 50% being polymorphonuclear leukocytes; peritonitis rate defined as number of episodes of peritonitis over total patient months on PD)
Peritonitis relapse (reoccurrence of peritonitis due to the same organism within two to four weeks)
Death due to peritonitis
All‐cause mortality
Exit‐site and tunnel infection‐number of patients with exit‐site and tunnel infections and exit‐site and tunnel infection rate
Catheter removal/catheter replacement
Technique failure (transfer from PD to haemodialysis/transplant due to peritonitis)
Toxicity of antimicrobial treatments (nasal irritation, sneezing, generalised pruritus, headache, diarrhoea, nausea, vomiting, jaundice, local irritation, rash)
Time to first peritonitis episode
Primary outcomes
Peritonitis
Exit‐site infection/tunnel infection
Catheter removal/catheter replacement
Secondary outcomes
Peritonitis relapse
Death due to peritonitis
All‐cause mortality
Technique failure
Toxicity of antimicrobial treatments
Time to first peritonitis episode
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 4 October 2016 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals & the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal & ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were potentially relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and where necessary, the full text of these studies, to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction and assessment of the risk of bias were performed independently by the same authors using standardised data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancy between published versions was highlighted. Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (peritonitis (number), peritonitis (rate), death due to peritonitis, all‐cause mortality, exit‐site/tunnel infection (number), exit‐site/tunnel infection (rate), catheter removal/replacement, technique failure, toxicity of antimicrobial treatments) results were expressed as risk ratios (RR) with 95% confidence intervals (CI). No continuous outcomes were identified.
Unit of analysis issues
Where data on the number of subjects with events (e.g. number of subjects with one or more episodes of peritonitis) were available, the RR was calculated as the ratio of the incidence of the event (one or more episodes) in the experimental treatment group over the incidence in the control group. Where data on the number of episodes were available the RR was calculated as the ratio of the rate of the outcome (e.g. the peritonitis rate) in the experimental treatment group (given by number of episodes of the outcome over total patient months on PD) over the rate in the control group.
Dealing with missing data
Where necessary, we contacted triallists to request missing patient data due to loss to follow‐up and exclusion from study analyses in an effort to conduct intention‐to‐treat analyses. With the update, four authors responded to our requests. Where missing dichotomous data were few, and unlikely to affect the overall results, we analysed available data.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
The search strategy included searching major databases, conference proceedings and prospective trial registers without language restriction in an attempt to reduce publication bias related to failure of authors to publish negative results or inability to publish negative results in journals indexed in major databases. Insufficient studies were available to assess for publication bias using funnel plots. Where multiple publications of the same study were identified, data were included from the most recent publication, and preferably, the definitive publication. However, all publications were reviewed to identify outcomes not reported in the index publication in an attempt to reduce outcome reporting bias.
Data synthesis
Data were pooled using the random‐effects model for dichotomous data.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned to explore potential sources of variability in observed treatment effect where possible (paediatric versus adult population, diabetic versus non‐diabetic, time on PD before beginning of antimicrobial treatment). However, no subgroup analyses were performed due to lack of available data from the included studies.
Sensitivity analysis
Sensitivity analysis was planned to investigate the effect of year of study and study performance. However, there were insufficient studies to do this.
Summarising and interpreting results
We used the GRADE approach to assess the quality of evidence for each of the key outcomes (Guyatt 2008). We used the GRADE profiler to create 'Summary of Findings' tables (Schünemann 2011a).
For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs, we downgraded the evidence from 'high quality' by one level for serious study limitations and by two levels for very serious study limitations. The evidence was appraised using the five GRADE considerations: risk of bias, imprecision of effect estimates, inconsistency, indirectness and potential publication bias. None were upgraded to moderate or high quality as most pooled estimates did not reveal a large magnitude of effect, there was potential for impact by confounders, and most did not show a strong dose‐response gradient (Schünemann 2011b). The exception was the pooled estimate obtained for the comparison of the use of an antifungal agent versus placebo/no treatment for preventing fungal peritonitis, but the evidence was not upgraded from 'low' because only two studies contributed data for the outcome of fungal peritonitis and one of the studies had a high risk of bias. We used these assessments and the evidence for absolute benefit or harm of the interventions and the sum of available data on all important outcomes from each study included for each comparison, to arrive at conclusions about the effectiveness of antimicrobial agents at preventing catheter‐related infection or the need for catheter removal/replacement in PD patients.
'Summary of Findings' tables consisted of the following clinically important outcomes identified in the selected studies:
Peritonitis (number of patients with one or more episodes)
Exit‐site/tunnel infection (number of patients with one or more episodes)
Catheter removal or replacement (number of patients)
Fungal peritonitis (number of patients with one or more episodes).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
For this update we searched the Specialised Register to 4 October 2016 and identified 48 new reports. After full‐text assessment 31 new studies were identified. Twenty new studies (33 reports) were included, 10 were excluded (14 reports), and one ongoing study was identified. We also identified four new reports of four existing included studies. Search results are shown in Figure 1.
1.
Study flow diagram.
Included studies
We included 39 studies in the review, 19 of which had been included in the original review. Of the 20 new studies, 10 had been published since the search was done for the previous review and 10 (Axelrod 1973; Cheng 1999a; Cocksedge 1993; Fuchs 1990; Moore 1989; Ryckelynck 1987; Sharma 1971; SIPROCE Study 1997; Wadhwa 1995; Wadhwa 1997) had not been identified in the previous search. There was one four‐arm study (Swartz 1991), three three‐arm studies (Fuchs 1990; Gadallah 2000c; Sesso 1994), and the remaining studies were two‐arm studies. No cross‐over studies were identified.
Of the 39 studies included (4435 randomised participants), all were parallel group studies. All participants were chronic PD patients treated in‐centre or in satellite facilities. Two studies (Axelrod 1973; Sharma 1971) reported the number of dialyses but not the number of participants in each group and hence, the data from these studies could not be added to the meta‐analyses. Most studies included only adult patients; two studies (Blowey 1994; Mendoza‐Guevara 2007) included only children and young adults on PD. Twenty‐six studies (Bernardini 2005; Bernardini 1996; Chu 2008; Cocksedge 1993; Fuchs 1990; Gadallah 2000c; HONEYPOT Study 2009; Lo 1996; Luzar 1990; Lye 1992; MP3 Study 2008; Mupirocin Study 1996; Nolph 1985; Nunez‐Moral 2014; Perez‐Fontan 1992; Poole‐Warren 1991; Restrepo 2010; Sesso 1994; SIPROCE Study 1997; Swartz 1991; Wadhwa 1995; Wadhwa 1997; Waite 1997; Wikdahl 1997; Wong 2003; Zimmerman 1991) identified the proportion of patients who had diabetes mellitus.
Most studies reported only some of the primary outcomes of interest to this review. The primary outcomes reported in the studies were as follows: peritonitis ‐ number of patients (22 studies), peritonitis rate (14 studies), exit‐site/tunnel infection ‐ number of patients (22 studies), exit‐site/tunnel infection ‐ rate (12 studies) and catheter removal/replacement (15 studies). Other outcomes reported included death due to peritonitis (2 studies), all‐cause mortality (13 studies), technique failure (3 studies) and toxicity of antimicrobial treatments (5 studies). No studies had data on peritonitis relapse and only two had time to first peritonitis episode (HONEYPOT Study 2009; MP3 Study 2008).
Three study authors responded to queries about study methods and/or requests for additional unpublished information (Chu 2008; Danguilan 2003; HONEYPOT Study 2009).
Oral or topical antibiotics versus placebo/no treatment
In seven studies (469 participants), patients were randomised to oral, or topical (exit site, nasal) or intraperitoneal prophylactic antibiotics versus placebo or no treatment (Blowey 1994; Churchill 1988; Low 1980; Sesso 1994; Swartz 1991; Wong 2003; Zimmerman 1991). The duration of follow‐up ranged from one to 12 months.
Oral or topical antibiotics versus other antibiotic
Seven studies (640 participants) randomised patients to oral, or topical (exit site, nasal) or intraperitoneal antibiotics versus other antibiotics (Bernardini 1996; Bernardini 2005; Chu 2008; Danguilan 2003; MP3 Study 2008; Perez‐Fontan 1992; Sesso 1994) with follow‐up ranging from 7.8 to 18 months.
Nasal antibiotic prophylaxis versus placebo/no treatment
Three studies (338 participants) compared the use of nasal prophylactic antibiotics with placebo (Mupirocin Study 1996; Sesso 1994; Sit 2007). The duration of follow‐up ranged from 7.8 to 18 months.
Pre/peri‐operative antibiotic prophylaxis versus placebo/no treatment or other antibiotic
One study (178 participants) assessed the use of vancomycin with cefazolin as perioperative intravenous prophylaxis head‐to‐head (Gadallah 2000c), and four studies (379 patients) compared the use of perioperative intravenous antibiotic prophylaxis against no antibiotic treatment (Bennet‐Jones 1988; Gadallah 2000c; Lye 1992; Wikdahl 1997). Follow‐up periods ranged from 10 to 28 days.
Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant)
Nine studies (1039 participants) evaluated the effect of topical disinfectants versus standard care or other intervention at the exit site on a range of outcomes (Cheng 1999a; HONEYPOT Study 2009; Luzar 1990; Mendoza‐Guevara 2007; Nunez‐Moral 2014; Wadhwa 1995; Wadhwa 1997; Waite 1997; Wilson 1997). The duration of follow‐up ranged from 6 to 24 months.
Other interventions
Other interventions included one study (167 participants) which compared the use of an ultraviolet germicidal chamber to disinfect the spike and the solution bag outlet port versus no treatment (Nolph 1985) while another study (50 participants) directed one group to soak their connectors in antiseptic before performing a bag exchange while the control group did not use antiseptic (Ryckelynck 1987). Three studies (140 participants) compared different dressing systems (Cocksedge 1993; Fuchs 1990; Moore 1989) and one study (195 participants) compared the addition of a silver ring device on the catheter versus no ring (SIPROCE Study 1997). One study (124 participants) compared the antistaphylococcal vaccine Staphypan Berna against placebo (Poole‐Warren 1991).
Antifungal prophylaxis versus placebo/no treatment interventions
Two studies (817 participants) compared the administration of an antifungal agent with an antibiotic course against no treatment (Lo 1996; Restrepo 2010). Follow‐up periods ranged from 1 to 18 months.
See Table 6 for comparisons included in Strippoli 2004a and this 2017 update.
2. Comparisons in original review and updated review.
| Comparisons in 2004 review | Comparisons in 2017 review |
| Oral antibiotics versus none | Oral or topical antibiotics versus placebo/no treatment |
| Nasal antibiotics versus none | Oral or topical antibiotics versus other antibiotic |
| Peri‐operative IV prophylaxis versus none | Nasal antibiotics versus no treatment |
| Peri‐operative IV prophylaxis head‐to‐head | Pre/peri‐operative IV prophylaxis versus none or head‐to‐head |
| Topical disinfectants versus none | Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant) |
| Germicidal chamber versus none | Germicidal chamber versus none |
| Antistaphylococcal vaccine (Staphypan) versus placebo | Dressing systems (any) |
| Antibiotic prophylaxis head‐to‐head agents | Silver ring system on catheter versus none |
| ‐‐ | Antistaphylococcal vaccine (Staphypan) versus placebo |
| ‐‐ | Antifungal versus placebo/no treatment |
Excluded studies
Twelve studies (17 reports) were excluded after full text review. The characteristics of the excluded studies are shown in "Characteristics of excluded studies". Reasons for excluding studies included focus of study was about treatment of PD‐related infection not prevention, report was of a pharmacokinetics study, agent used in intervention was not an antimicrobial, and PD‐related infection data was not readily available in the published report.
Risk of bias in included studies
The assessment of risk of bias is shown in Figure 2 and Figure 3. Figure 2 shows relative proportional rankings of studies for each risk of bias indicator. Figure 3 shows the risk of bias items for individual studies.
2.
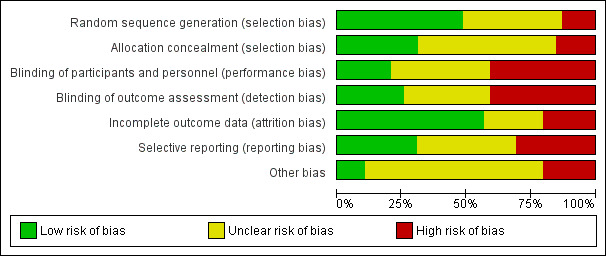
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
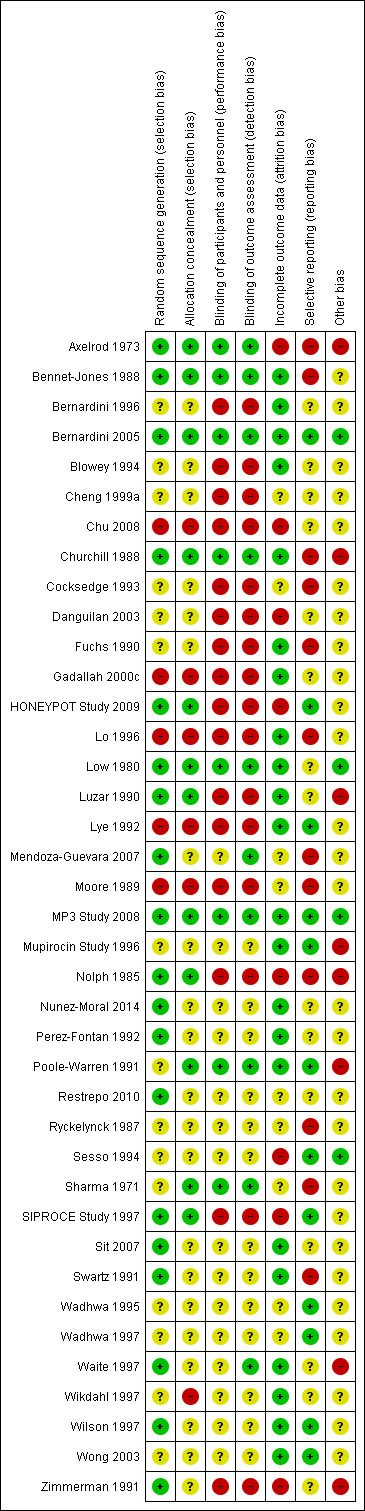
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation of sequence generation was judged to be at low risk of bias in 19 studies (Axelrod 1973; Bennet‐Jones 1988; Bernardini 2005; Churchill 1988; HONEYPOT Study 2009; Low 1980; Luzar 1990; Mendoza‐Guevara 2007; MP3 Study 2008; Nolph 1985; Nunez‐Moral 2014; Perez‐Fontan 1992; Restrepo 2010; SIPROCE Study 1997; Sit 2007; Swartz 1991; Waite 1997; Wilson 1997; Zimmerman 1991). Randomisation method was unclear in 15 studies and was judged to be at high risk of bias in five studies (Chu 2008; Gadallah 2000c; Lo 1996; Lye 1992; Moore 1989).
Twelve studies reported allocation concealment adequately (Axelrod 1973; Bennet‐Jones 1988; Bernardini 2005; Churchill 1988; HONEYPOT Study 2009; Low 1980; Luzar 1990; MP3 Study 2008; Nolph 1985; Poole‐Warren 1991; Sharma 1971; SIPROCE Study 1997). Allocation concealment was unclear in 21 studies and six studies were judged to be at high risk of bias (Chu 2008; Gadallah 2000c; Lo 1996; Lye 1992; Moore 1989; Wikdahl 1997).
Blinding
Performance bias (blinding of participants and investigators) was judged to be at low risk of bias in eight studies (Axelrod 1973; Bennet‐Jones 1988; Bernardini 2005; Churchill 1988; Low 1980; MP3 Study 2008; Poole‐Warren 1991; Sharma 1971), was unclear in 15 studies, and was judged to be a high risk of bias in 16 studies (Bernardini 1996; Blowey 1994; Cheng 1999a; Chu 2008; Cocksedge 1993; Danguilan 2003; Fuchs 1990; Gadallah 2000c; HONEYPOT Study 2009; Lo 1996; Luzar 1990; Lye 1992; Moore 1989; Nolph 1985; SIPROCE Study 1997; Zimmerman 1991).
Detection bias (blinding of outcome assessors) was judged to be at low risk of bias in 10 studies (Axelrod 1973; Bennet‐Jones 1988; Bernardini 2005; Churchill 1988; Low 1980; Mendoza‐Guevara 2007; MP3 Study 2008; Poole‐Warren 1991; Sharma 1971; Waite 1997), was unclear in 13 studies, and was judged to be at high risk of bias in 16 studies (Bernardini 1996; Blowey 1994; Cheng 1999a; Chu 2008; Cocksedge 1993; Danguilan 2003; Fuchs 1990; Gadallah 2000c; HONEYPOT Study 2009; Lo 1996; Luzar 1990; Lye 1992; Moore 1989; Nolph 1985; SIPROCE Study 1997; Zimmerman 1991).
Incomplete outcome data
Outcomes data reporting was considered to be complete with a low risk of bias in 22 studies (Bennet‐Jones 1988; Bernardini 1996; Bernardini 2005; Blowey 1994; Churchill 1988; Fuchs 1990; Gadallah 2000c; Lo 1996; Low 1980; Luzar 1990; Lye 1992; MP3 Study 2008; Mupirocin Study 1996; Nunez‐Moral 2014; Perez‐Fontan 1992; Poole‐Warren 1991; Sit 2007; Swartz 1991; Waite 1997; Wikdahl 1997; Wilson 1997; Wong 2003). Eight studies (Axelrod 1973; Chu 2008; Danguilan 2003; HONEYPOT Study 2009; Nolph 1985; Sesso 1994; SIPROCE Study 1997; Zimmerman 1991) reported that from 9.2% to 77.7% of patients were excluded from analyses, so were considered to be at high risk of bias. The risk of bias was unclear in nine studies because there was insufficient information provided to determine if data from all patients who entered the study were included in the analysis.
Selective reporting
We identified 12 studies (Bernardini 2005; HONEYPOT Study 2009; Lye 1992; MP3 Study 2008; Mupirocin Study 1996; Poole‐Warren 1991; Sesso 1994; SIPROCE Study 1997; Wadhwa 1995; Wadhwa 1997; Wilson 1997; Wong 2003) and reported all outcomes based on the protocols described in the study methods and could be meta‐analysed. Twelve studies were judged to be at high risk of bias of reporting bias (Axelrod 1973; Bennet‐Jones 1988; Churchill 1988; Cocksedge 1993; Fuchs 1990; Lo 1996; Mendoza‐Guevara 2007; Moore 1989; Nolph 1985; Ryckelynck 1987; Sharma 1971; Swartz 1991) because only one our primary outcomes cold be meta‐analysed; two studies reported outcomes incompletely so they could not be included in any of our meta‐analyses (Axelrod 1973; Sharma 1971). Reporting bias was unclear for 15 studies because only two (of our three) primary outcomes were reported or could be meta‐analysed.
Other potential sources of bias
Eight studies (Axelrod 1973; Churchill 1988; Luzar 1990; Mupirocin Study 1996; Nolph 1985; Poole‐Warren 1991; SIPROCE Study 1997; Waite 1997; Zimmerman 1991) reported receiving monetary support from pharmaceutical companies; one study received combined funding from industry and government (HONEYPOT Study 2009) and was judged to at unclear risk of bias. Four studies were judged to be at low risk bias (Bernardini 2005; Low 1980; MP3 Study 2008; Sesso 1994) and the remaining 26 studies were judged unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Oral or topical or intraperitoneal antibiotics versus placebo/no treatment for preventing peritonitis in peritoneal dialysis patients.
| Oral or topical or intraperitoneal antibiotics versus placebo/no treatment for preventing peritonitis in peritoneal dialysis patients | ||||||
| Patient or population: patients with CKD on peritoneal dialysis Settings: tertiary settings Intervention: oral or topical or intraperitoneal antibiotics versus placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral or topical or intraperitoneal antibiotics versus placebo/no treatment | |||||
| Peritonitis (number of patients with one or more episodes) | Study population | RR 0.82 (0.57 to 1.19) | 395 (5) | ⊕⊕⊝⊝ low1,2 | ||
| 360 per 1000 | 295 per 1000 (205 to 428) | |||||
| Moderate | ||||||
| 385 per 1000 | 316 per 1000 (219 to 458) | |||||
| Exit‐site/tunnel infection (number of patients with one or more episodes) | Study population | RR 0.45 (0.19 to 1.04) | 191 (3) | ⊕⊕⊝⊝ low2 | ||
| 176 per 1000 | 79 per 1000 (34 to 184) | |||||
| Moderate | ||||||
| 231 per 1000 | 104 per 1000 (44 to 240) | |||||
| Catheter removal or replacement (number of patients) | Study population | RR 0.82 (0.46 to 1.46) | 395 (5) | ⊕⊕⊝⊝ low1,2 | ||
| 115 per 1000 | 94 per 1000 (53 to 168) | |||||
| Moderate | ||||||
| 156 per 1000 | 128 per 1000 (72 to 228) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear or high risk of bias in 3 of 5 studies 2 Wide confidence intervals due to small patient numbers
Abbreviations: CKD ‐ chronic kidney disease; GRADE ‐ Grading of Recommendations Assessment, Development and Evaluation
Summary of findings 2. Nasal antibiotics versus placebo/no treatment for preventing peritonitis in peritoneal dialysis patients.
| Nasal antibiotics versus no treatment for preventing peritonitis in peritoneal dialysis patients | ||||||
| Patient or population: patients with CKD on peritoneal dialysis Settings: tertiary settings Intervention: nasal antibiotics versus placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nasal antibiotics versus placebo/no treatment | |||||
| Peritonitis (number of patients with one or more episodes) | Study population | RR 0.94 (0.67 to 1.31) | 338 (3) | ⊕⊕⊝⊝ low¹,² | ||
| 294 per 1000 | 276 per 1000 (197 to 385) | |||||
| Moderate | ||||||
| 331 per 1000 | 311 per 1000 (222 to 434) | |||||
| Exit‐site/ tunnel infection (number of patients with one or more episodes) | Study population | RR 1.34 (0.62 to 2.87) | 338 (3) | ⊕⊕⊝⊝ low¹,² | ||
| 165 per 1000 | 221 per 1000 (102 to 473) | |||||
| Moderate | ||||||
| 188 per 1000 | 252 per 1000 (117 to 540) | |||||
| Catheter removal or replacement (number of patients) | Study population | RR 0.92 (0.48 to 1.78) | 289 (2) | ⊕⊕⊝⊝ low¹,² | ||
| 103 per 1000 | 95 per 1000 (49 to 183) | |||||
| Moderate | ||||||
| 265 per 1000 | 244 per 1000 (127 to 472) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ Unclear risk of bias for allocation concealment in largest study (Mupirocin Study 1996) ² Wide confidence intervals due to small patient numbers
Abbreviations: CKD ‐ chronic kidney disease; GRADE ‐ Grading of Recommendations Assessment, Development and Evaluation
Summary of findings 3. Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant) for preventing peritonitis in peritoneal dialysis patients.
| Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant) for preventing peritonitis in peritoneal dialysis patients | ||||||
| Patient or population: patients with CKD on peritoneal dialysis Settings: tertiary settings Intervention: topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant) | |||||
| Peritonitis (number of patients with one or more episodes) | Study population | RR 0.83 (0.65 to 1.06) | 853 (6) | ⊕⊕⊝⊝ low1,2 | ||
| 235 per 1000 | 195 per 1000 (153 to 250) | |||||
| Moderate | ||||||
| 152 per 1000 | 126 per 1000 (99 to 161) | |||||
| Exit‐site/tunnel infection (number of patients with one or more episodes) | Study population | RR 0.97 (0.74 to 1.27) | 913 (7) | ⊕⊕⊝⊝ low1,2 | ||
| 238 per 1000 | 230 per 1000 (176 to 302) | |||||
| Moderate | ||||||
| 222 per 1000 | 215 per 1000 (164 to 282) | |||||
| Catheter removal or replacement (number of patients) | Study population | RR 0.89 (0.57 to 1.38) | 792 (6) | ⊕⊕⊝⊝ low1,2 | ||
| 97 per 1000 | 86 per 1000 (55 to 134) | |||||
| Moderate | ||||||
| 93 per 1000 | 83 per 1000 (53 to 128) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 Unclear allocation in several studies 2 Imprecision due to small number of patients and events in several studies
Abbreviations: CKD ‐ chronic kidney disease; GRADE ‐ Grading of Recommendations Assessment, Development and Evaluation
Summary of findings 4. Antifungal versus placebo/no treatment for preventing peritonitis in peritoneal dialysis patients.
| Antifungal versus placebo/no treatment for preventing fungal peritonitis in peritoneal dialysis patients | ||||||
| Patient or population: patients with CKD on peritoneal dialysis Settings: tertiary settings Intervention: antifungal versus placebo/no treatment during antibiotic course | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antifungal versus placebo/no treatment | |||||
| Fungal peritonitis (number of patients with one or more episodes) | Study population | RR 0.28 (0.12 to 0.63) | 817 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 64 per 1000 | 18 per 1000 (8 to 40) | |||||
| Moderate | ||||||
| 64 per 1000 | 18 per 1000 (8 to 40) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk of bias in one study (Lo 1996) 2 Imprecision due to small number of events and studies
Abbreviations: CKD ‐ chronic kidney disease; GRADE ‐ Grading of Recommendations Assessment, Development and Evaluation
See: Table 1: Oral or topical or intraperitoneal antibiotics versus placebo/no treatment for preventing peritonitis in PD patients; Table 2: Nasal antibiotics versus no treatment for preventing peritonitis in PD patients; Table 3: Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant) for preventing peritonitis in PD patients; Table 4: Antifungal versus placebo/no treatment for preventing fungal peritonitis in PD patients.
In most studies, the primary outcomes were peritonitis (number of patients), peritonitis rate, exit‐site/tunnel infection (number of patients), exit‐site/tunnel infection rate, and catheter removal or replacement (number). Many studies only included one or two of these outcomes. Other outcomes included all‐cause mortality, time to first catheter‐related infection, hospitalisation, death due to catheter‐related infection, technique failure, local pruritus/rash, and toxicity.
Oral or topical antibiotics versus placebo or no treatment
The oral antibiotic used was ofloxacin, cephalexin, rifampin or cotrimoxazole, and the topical antibiotic used was mupirocin ointment (exit site, nasal).
The use of oral or topical antibiotic prophylaxis had uncertain effects on the risk of peritonitis (Analysis 1.1 (5 studies, 395 participants): RR 0.82, 95% CI 0.57 to 1.19). There was low to moderate heterogeneity across these studies (I2 = 33%). The risk of peritonitis outcome was assessed as low quality because of unclear or high risk of bias in 3 of 5 studies and because of wide confidence intervals in all 5 studies due to small patient numbers.
1.1. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 1 Peritonitis (number of patients with one or more episodes).
The two interventions also had uncertain effects on the peritonitis rate (Analysis 1.2.1 (3 studies, 1440 patient‐months): RR 0.68, 95% CI 0.40 to 1.14), the risk of exit‐site and tunnel infection (Analysis 1.3.1 (3 studies, 191 participants): RR 0.45, 95% CI 0.19 to 1.04), exit‐site/tunnel infection rate (Analysis 1.4.1 (2 studies, 939 patient‐months): RR 0.42, 95% CI 0.17 to 1.05), risk of catheter removal or replacement (Analysis 1.5.1 (5 studies, 395 participants): RR 0.82, 95% CI 0.46 to 1.46), and all‐cause mortality (Analysis 1.6.1 (4 studies, 201 participants): RR 0.88, 95% CI 0.41 to 1.89), with no significant heterogeneity across studies for any of these analyses (I2 = 0%).
1.2. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 2 Peritonitis rate (episodes/total patient‐months on PD).
1.3. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 3 Exit‐site/tunnel infection (number of patients with one or more episodes).
1.4. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 4 Exit‐site/tunnel infection rate (episodes/total patient‐months on PD).
1.5. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 5 Catheter removal or replacement (number of patients).
1.6. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 6 Mortality (all‐cause).
The risk of exit‐site/tunnel infection outcome was assessed as low quality because of unclear or high risk of bias in all 3 studies and because of wide confidence intervals in all 3 studies due to small patient numbers. The risk of catheter removal/replacement outcome was also assessed as low quality because of unclear or high risk of bias in 3 of 5 studies and because of wide confidence intervals in all 5 studies due to small patient numbers.
Oral or topical antibiotics versus other antibiotic
The use of antibiotic ointment prophylaxis (either sodium fusidate (exit site plus nasal) or mupirocin (exit site)) was compared with another antibiotic (oral ofloxacin, oral rifampin or gentamicin cream (exit site)) in four studies.
The interventions had uncertain effects on the risk of peritonitis (Analysis 2.1 (4 studies, 314 participants): RR 1.28, 95% CI 0.89 to 1.84). There was low heterogeneity across these studies (I2 = 9%). Similarly, topical antibiotic prophylaxis (either mupirocin ointment (exit site), sodium fusidate ointment (exit site plus nasal) or mupirocin cream (exit site)) compared with other antibiotic (either sodium fusidate ointment (exit site), oral ofloxacin or gentamicin cream (exit site)) had uncertain effects on the risk of exit‐site and tunnel infection (Analysis 2.3 (4 studies, 336 participants): RR 1.28, 95% CI 0.71 to 2.31). There was medium heterogeneity across these studies (I2 = 56%).
2.1. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 1 Peritonitis (number of patients with one or more episodes).
2.3. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 3 Exit‐site/tunnel infection (number of patients with one or more episodes).
Nasal antibiotic prophylaxis versus placebo or no treatment
The use of nasal antibiotic prophylaxis had uncertain effects on the risk of peritonitis (Analysis 3.1 (3 studies, 338 participants): RR 0.94, 95% CI 0.67 to 1.31), the peritonitis rate (Analysis 3.2 (2 studies, 2797 patient‐months): RR 0.67, 95% CI 0.16 to 2.77), the risk of exit‐site and tunnel infection (Analysis 3.3 (3 studies, 338 participants): RR 1.34, 95% CI 0.62 to 2.87), the exit‐site and tunnel infection rate (Analysis 3.4 (2 studies, 2796 patient‐months): RR 0.91, 95% CI 0.29 to 2.92), and the number of patients with catheter removal or replacement (Analysis 3.5 (2 studies, 289 participants): RR 0.92, 95% CI 0.48 to 1.78). There was no significant heterogeneity across the studies for any of these analyses. Although in 1 study, there was a significant reduction in the exit‐site/tunnel infection rate when CAPD patients identified as S. aureus carriers (nasal) were treated with mupirocin ointment (nasal application, twice/day for 5 days, every 1 month), there were no significant differences with any of the other primary outcomes of interest (Mupirocin Study 1996).
3.1. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 1 Peritonitis (number of patients with one or more episodes).
3.2. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 2 Peritonitis rate (episodes/total patient‐months on PD).
3.3. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 3 Exit site and tunnel infection (number of patients with one or more episodes).
3.4. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 4 Exit site and tunnel infection rate (episodes/total patient‐months on PD).
3.5. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 5 Catheter removal or replacement (number of patients).
The risk of peritonitis and the risk of exit‐site/tunnel infection outcomes were assessed as low quality because of unclear or high risk of bias in all 3 studies and because of wide confidence intervals in all 3 studies due to small patient numbers. The risk of catheter removal/replacement was assessed as low quality because of unclear to high risk of bias in the 2 studies and because of wide confidence intervals in the 2 studies due to small patient numbers.
Pre‐ or peri‐operative antibiotic prophylaxis versus placebo or no treatment or other antibiotic
Pre‐ or peri‐operative intravenous antibiotic prophylaxis compared with no treatment may reduce the risk of early peritonitis (less than one month from catheter insertion) in one study (Gadallah 2000c) but there was no difference between the interventions in three other studies using different antibiotics (Analysis 4.1 (4 studies, 379 participants). The single 3‐arm study (Gadallah 2000c) compared vancomycin with placebo, cefazolin with placebo and vancomycin with cefazolin and found the risk of peritonitis was reduced by vancomycin compared with placebo (Analysis 4.1.1 (1 study, 177 participants): RR 0.08. 95% CI 0.01 to 0.61) and by vancomycin compared with cefazolin (Analysis 4.1.6 (1 study, 178 participants): RR 0.11, 95% CI 0.01 to 0.84); there was no difference between cefazolin compared with placebo. None of the antibiotic interventions made a difference to the risk of exit‐site and tunnel infection (Analysis 4.2 (4 studies, 379 participants). When outcomes at more than one month after catheter insertion were considered, there was no difference between the interventions for the risk of peritonitis or exit‐site/tunnel infection.
4.1. Analysis.
Comparison 4 Pre/peri‐operative prophylaxis versus placebo/no treatment or other antibiotic, Outcome 1 Peritonitis (number of patients with one or more episodes).
4.2. Analysis.
Comparison 4 Pre/peri‐operative prophylaxis versus placebo/no treatment or other antibiotic, Outcome 2 Exit site/tunnel infection (number of patients with one or more episodes).
Because each study used a different antibiotic intervention, it is not possible to comment on heterogeneity across the studies.
Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant)
Eight studies reported on the use of disinfectant at the exit site versus standard care or other active treatment. As the test for subgroup differences was not significant for any of our outcomes the total summary estimates are reported here.
Overall topical disinfectants versus standard care or other active treatment had uncertain effects on the risk of peritonitis (Analysis 5.1 (6 studies, 853 participants): RR 0.83, 95% CI 0.65 to 1.06). exit‐site/tunnel infection (Analysis 5.2 (8 studies, 973 participants): RR 1.00, 95% CI 0.75 to 1.33), catheter removal or replacement (Analysis 5.4 (7 studies, 852 participants): RR 0.89, 95% CI 0.57 to 1.38), and all‐cause mortality (Analysis 5.5 (4 studies, 697 participants): RR 0.88, 95% CI 0.53 to 1.44), with no significant heterogeneity across studies for any of these analyses.
5.1. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 1 Peritonitis (number of patients with one or more episodes).
5.2. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 2 Exit site/tunnel infection (number of patients with one or more episodes).
5.4. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 4 Catheter removal or replacement (number of patients).
5.5. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 5 Mortality (all‐cause).
The risk of peritonitis outcome was assessed as low quality because of unclear allocation concealment and blinding in four of six studies and imprecision due to the small number of patients and events in five of six studies. The risk of exit‐site/tunnel infection outcome was assessed as low quality because of unclear allocation concealment and blinding in six of eight studies and imprecision due to the small number of patients and events in seven of eight studies. The risk of catheter removal/replacement outcome was assessed as low quality because of unclear allocation concealment and blinding in five of seven studies and imprecision due to the small number of patients and events in six of seven studies.
Other interventions
Seven studies reported on other interventions designed to reduce PD‐related infections. There was no difference in the peritonitis rate with other interventions.
Germicidal chamber for connection devices or soaking of the connector in antiseptic prior to bag exchange versus none (Analysis 6.1 (2 studies, 1855 patient‐months): RR 1.05, 95% CI 0.74 to 1.51)
Staphypan Berna antistaphylococcal vaccine (Analysis 9.1 (1 study, 1099 patient‐months): RR 1.11, 95% CI 0.77 to 1.59). Staphypan Berna compared with placebo was also shown to make no difference to the exit‐site and tunnel infection rate (Analysis 9.2 (1 study, 1107 patient‐months): RR 0.98, 95% CI 0.65 to 1.48).
6.1. Analysis.
Comparison 6 Germicidal chamber versus none, Outcome 1 Peritonitis rate (episodes/total patient‐months on PD).
9.1. Analysis.
Comparison 9 Antistaphylococcal vaccine (Staphypan) versus placebo, Outcome 1 Peritonitis rate (episodes/total patient‐months on PD).
9.2. Analysis.
Comparison 9 Antistaphylococcal vaccine (Staphypan) versus placebo, Outcome 2 Exit site/tunnel infection rate (episodes/total patient‐months on PD).
Three studies (140 participants) reported on the use of different dressing systems. There was no difference between the comparisons for the number of patients with one or more episodes of exit‐site/tunnel infection (Analysis 7.1) or the exit‐site/tunnel infection rate (Analysis 7.2 (1 study, 679 patient‐months).
7.1. Analysis.
Comparison 7 Dressing systems (any), Outcome 1 Exit site/tunnel infection (number of patients with one or more episodes).
7.2. Analysis.
Comparison 7 Dressing systems (any), Outcome 2 Exit site/tunnel infection rate (episodes/total patient‐months on PD).
There was no difference between use of a silver ring on the PD catheter versus none for the risk of peritonitis (Analysis 8.1 (1 study, 195 participants): RR 0.90, 95% CI 0.49 to 1.66), risk of exit‐site/tunnel infection (Analysis 8.2 (1 study, 195 participants): RR 1.26, 95% CI 0.84 to 1.90) or risk of catheter removal/replacement (Analysis 8.3 (1 study, 195 participants): RR 1.26, 95% CI 0.35 to 4.56).
8.1. Analysis.
Comparison 8 Silver ring system on catheter versus none, Outcome 1 Peritonitis (number of patients with one or more episodes).
8.2. Analysis.
Comparison 8 Silver ring system on catheter versus none, Outcome 2 Exit site/tunnel infection (number of patients with one or more episodes).
8.3. Analysis.
Comparison 8 Silver ring system on catheter versus none, Outcome 3 Catheter removal or replacement (number of patients).
Antifungal prophylaxis versus placebo or no treatment
The use of antifungal agents (oral fluconazole or oral nystatin) compared with no antifungal agent being given when a patient receives a course of antibiotics for bacterial peritonitis were reported in 2 studies. The antifungal intervention may reduce the risk of fungal peritonitis (Analysis 10.1 (2 studies, 817 participants): RR 0.28, 95% CI 0.12 to 0.63). There was low heterogeneity across the two studies for this analysis. The risk of fungal peritonitis outcome was assessed as low quality because of unclear risk of bias in 1 study and high risk of bias in 1 study and imprecision due to the small number of events and patient numbers. One study of oral nystatin in PD patients who were receiving treatment for bacterial peritonitis showed a significant reduction in the rate of fungal peritonitis due to Candida spp. with nystatin prophylaxis (Analysis 10.2 (1 study, 6864 patient‐months): RR 0.31, 95% CI 0.10 to 0.95).
10.1. Analysis.
Comparison 10 Antifungal versus placebo/no treatment, Outcome 1 Fungal peritonitis (number of patients with one or more episodes).
10.2. Analysis.
Comparison 10 Antifungal versus placebo/no treatment, Outcome 2 Fungal peritonitis rate (episodes/total patient‐months on PD).
Adverse effects
For the comparisons which included oral or topical antibiotics versus placebo/no treatment, two studies (86 participants) provided some information on adverse effects of therapy. They were reported in relation to the use of oral rifampin and sodium fusidate ointment (nasal and exit site). More patients reported adverse effects with oral rifampin therapy but the results did not achieve significance. Heterogeneity could not be determined (Analysis 1.8).
1.8. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 8 Adverse effects.
For the studies which included oral or topical antibiotics versus other antibiotic, three studies (419 participants) reported on adverse effects of therapy. The antibiotics used were applied daily/routinely to the exit site and included Polysporin triple ointment, gentamicin cream and cyclic oral rifampin against mupirocin ointment or cream. There were fewer patients who reported adverse effects with mupirocin but the result was not significantly different; nausea (Analysis 2.8.1 (1 study, 82 participants): RR 0.09, 95% CI 0.01 to 1.59); pruritus (Analysis 2.8.2 (2 studies, 337 participants): RR 0.65, 95% CI 0.29 to 1.49).
2.8. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 8 Adverse effects.
Three studies (289 participants) compared nasal antibiotics against placebo/no treatment and two of them reported information on adverse effects of therapy. The antibiotics used included mupirocin ointment (nasal) and sodium fusidate ointment (nasal and exit site) versus placebo ointment (nasal) or placebo tablets. More patients reported adverse effects with the antibiotic treatments (headache, diarrhoea, nausea, vomiting, pruritus, nasal irritation/rhinitis) but the results did not achieve significance. Heterogeneity could not be determined (Analysis 3.7).
3.7. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 7 Adverse effects.
For the studies which included topical disinfectant versus standard care or other active treatment at the exit site, four studies reported on adverse effects of therapy. The interventions that these reports related to were sodium hypochlorite solution, antibacterial honey and povidone iodine dry powder spray against povidone iodine solution, mupirocin ointment (nasal) or alcohol wipes. More patients reported adverse effects with use of the former agents and a statistically significant increase in pruritus occurred with topical disinfectants versus standard care (Analysis 5.7 (4 studies, 609 participants): RR 2.80, 95% CI 1.21 to 6.48; I2 = 44%). There was low heterogeneity of results.
5.7. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 7 Pruritus (local).
Antibiotic resistance was not adequately reported in the included studies (Table 7).
3. Other outcomes analysed.
| Outcome analysed | Number of studies | Number of patients | RR (95% CI) |
| Oral antibiotic prophylaxis | |||
| Pruritus | 1 | 64 | 3.00 (0.13 to 71.00) |
| Diarrhoea | 1 | 64 | 0.09 (0.01 to 1.58) |
| Nausea | 1 | 64 | 9.00 (0.50 to 160.59) |
| Allergy | 1 | 64 | 5.00 (0.25 to 100.20) |
| Nasal antibiotic prophylaxis | |||
| Nasal irritation | 1 | 15 | 2.10 (0.10 to 44.40) |
| Rhinitis | 1 | 267 | 0.74 (0.27 to 2.09) |
| Headache | 1 | 267 | 0.99 (0.14 to 6.94) |
| Diarrhoea | 1 | 267 | 1.65 (0.40 to 6.78) |
| Nausea | 1 | 267 | 0.99 (0.14 to 6.94) |
| Vomiting | 1 | 267 | 2.98 (0.61 to 14.94) |
| Pruritus | 1 | 267 | 1.49 (0.25 to 8.77) |
| Topical disinfectants | |||
| Technique failure | 1 | 149 | 0.19 (0.01 to 3.83) |
| Pruritus | 1 | 149 | 10.29 (0.58 to 182.92) |
Outcomes sought but not reported
Very few studies reported on peritonitis relapse, development of antibiotic resistance (topical use), hospitalisation due to PD‐related infections or peritonitis, time to first peritonitis episode, technique failure (transfer from PD to haemodialysis/transplant due to peritonitis), or death due to peritonitis.
Discussion
Summary of main results
We identified 39 studies that compared antimicrobial agents with placebo/no treatment or other antimicrobial agent or standard care in CKD patients on PD. A range of antimicrobial agents were found and studies using antibiotic prophylaxis showed wide variability regarding the dose and duration of the interventions trialled. The duration of studies ranged from 1 month to 8 years. The quality of the evidence for all of the findings listed below was low.
Key findings are as follows.
The use of oral or topical antibiotic had uncertain effects on the risk of exit‐site/tunnel infection and the risk of peritonitis.
The topical administration of antibiotic ointment to the anterior nares of PD patients (sodium fusidate or mupirocin ointment) had uncertain effects on the risk of exit‐site/tunnel infection and the risk of peritonitis.
Pre/peri‐operative intravenous vancomycin may reduce the risk of early peritonitis in the first few weeks (< 1 month) following Tenckhoff catheter insertion but has an uncertain effect on the risk of exit‐site/tunnel infection. The comparisons using other antibiotics (i.e. IV gentamicin; IV cefazolin plus gentamicin; IV cefuroxime plus cefuroxime intraperitoneal) did not reduce the risk of peritonitis or exit‐site/tunnel infection.
The use of topical disinfectant had uncertain effects on the risk of exit‐site/tunnel infection and the risk of peritonitis.
Oral antifungal prophylaxis (fluconazole or nystatin) with each antibiotic course given to a PD patient may reduce the risk of fungal peritonitis.
No intervention reduced the risk of catheter removal or replacement.
Oral or topical antibiotics versus placebo/no treatment, oral or topical antibiotics versus other antibiotic, nasal antibiotics versus placebo/no treatment, pre/peri‐operative prophylaxis versus placebo/no treatment or other antibiotic, topical disinfectants versus standard care or other active treatment, germicidal chamber versus none, or silver ring system on catheter versus none had an effect on all‐cause mortality. Neither oral or topical antibiotics versus other antibiotic nor topical disinfectants versus standard care or other active treatment had an effect on the risk of technique failure.
Heterogeneity among the studies was low except for the interventions oral or topical antibiotics versus placebo/no treatment and oral or topical antibiotics versus other antibiotic. Heterogeneity in the former comparison for the risk of peritonitis was 33% and was likely related to the variety of antibiotics used, the frequency of administration (daily, monthly, every three months), the route of administration (oral, topical) and the population studied (adults in Brazil, Canada, USA, Hong Kong). Heterogeneity in the latter comparison for the risk of exit‐site/tunnel infection was 56% and was probably related to the range of antibiotics used, the frequency of administration (twice daily, daily, every 2 days, weekly), the route of administration (oral, topical) and the population studied (adults in the Philippines, Brazil, Hong Kong, USA).
Overall completeness and applicability of evidence
Twelve studies reported all three primary outcomes of interest and could be meta‐analysed (peritonitis, exit‐site/tunnel infection, catheter removal/replacement), 15 studies reported two primary outcomes of interest, and 12 studies reported on one primary outcome of interest; two studies reported all primary outcomes in a way that could not be meta‐analysed (Axelrod 1973; Sharma 1971). Our meta‐analyses identified that use of oral or topical antibiotics had uncertain effects on the risk of exit‐site/tunnel infection and the risk of peritonitis and did not appear to affect the exit‐site/tunnel infection rate, peritonitis rate, or the risk of catheter removal/replacement. It is unclear if the use of nasal mupirocin in identified nasal carriers of S. aureus reduces the risk of exit‐site/tunnel infection or peritonitis. The use of pre/peri‐operative IV antibiotics at PD catheter insertion may reduce the occurrence of early peritonitis (within 1 month of insertion) with vancomycin being the most effective antibiotic to use. The RR of 0.08 for the outcome of early peritonitis could be classified as clinically important, should it be confirmed with future studies. The use of topical disinfectant had uncertain effects on the risk of exit‐site/tunnel infection and the risk of peritonitis. The co‐administration of antifungal agents with an antibiotic course appears to reduce the risk of fungal peritonitis developing in a PD patient. The risk ratio of 0.28 for the outcome of fungal peritonitis could prove to be clinically important, should it be confirmed with future studies.
No RCT was found which had the comparison of routine courses of intranasal mupirocin versus daily exit‐site mupirocin. Likewise, no RCT was found which compared S. aureus nasal carriage eradication at the time of PD catheter insertion versus no eradication of S. aureus nasal carriage. In addition, some outcomes were either not addressed (development of antibiotic resistance with topical use) or not often addressed (peritonitis relapse, hospitalisation rates due to PD‐related infections or peritonitis, technique failure due to peritonitis). It should also be mentioned that for most comparisons there are only a few studies and small numbers of patients.
Quality of the evidence
Our review included 39 studies that involved 4374 patients; all were either on PD (CAPD, CCPD or APD) or were having surgery to insert the Tenckhoff catheter prior to commencing PD. Two studies had paediatric populations, two studies had a mix of adults and children, with the remainder having only adult patients. We found the quality of evidence for all outcomes to be of low quality mainly due to unclear or high risk of bias in a majority of studies and imprecise results because of small patient numbers and events. This means that further research is likely to have an important impact on our confidence in the estimates of effect and is likely to change those estimates.
Of the 39 included studies, four were available only as abstracts; 19 reported adequate sequence generation, and 12 had adequate allocation concealment. Hence, allocation concealment was either unclear or inadequate in two‐thirds of the studies. Studies that do not have adequate allocation concealment are felt to be at increased risk of bias (Moher 1998; Schulz 1995). Eight studies reported adequate blinding of patients and personnel, and 10 studies reported adequate blinding of outcome assessment. Therefore, blinding methodology was either unclear or inadequate in three‐quarters of the studies. We found that 22 studies provided complete data reporting, and 12 reported all primary outcomes. Seven studies reported receiving some form of sponsorship from pharmaceutical companies, four studies reported complete or partial funding from an institute or government organisation, one study received funding from both pharmaceutical and a government organisation, and 27 studies did not report any funding source. In this review, we did not observe a difference between studies that were sponsored by pharmaceutical companies and those that were not. Of the eight pharma‐sponsored/pharma plus government funded studies, five had adequate allocation concealment; of the four studies with partial or full funding from an institute/government organisation, three reported adequate allocation concealment; and of the 27 studies that did not report a funding source, only four demonstrated adequate allocation concealment. Likewise, in terms of selective outcome reporting, two of the eight pharma‐sponsored/pharma plus government funded studies reported all of our primary outcomes; three of the four institute/ government‐sponsored studies reported all our primary outcomes; and seven of the 27 studies without a declared funding source included all our primary outcomes.
For the comparison of oral or topical antibiotics versus placebo/no treatment, the variable quality of relevant studies and the small patient numbers, meant the quality of evidence was rated as low for the outcomes of peritonitis, exit‐site/tunnel infection and catheter removal/replacement (Table 1). For the comparison of nasal antibiotics versus placebo/no treatment, the variable quality of relevant studies and the small patient numbers, reduced the quality of evidence to low for the outcomes of peritonitis, exit‐site/tunnel infection and catheter removal/replacement (Table 2). With the comparison of topical disinfectants versus standard care or other active treatment, the unclear allocation in several studies and imprecision due to small patient numbers and events in several studies, meant the quality of evidence was rated as low for the outcomes of peritonitis, exit‐site/tunnel infection and catheter removal/replacement (Table 3).
With the comparison of antifungal prophylaxis versus placebo/no treatment, the quality of evidence for the outcome of fungal peritonitis was considered to be low because of high risk of bias in one study and modest patient numbers and the limited number of studies reporting this outcome (Table 4).
Potential biases in the review process
Four of the 39 included studies were available only as abstracts but this was not considered a major source of bias. Since the original version of this review was published, the literature search has been run several times (up to 4 October 2016), to increase the chance that all eligible studies published before that time have been included. Although the Cochrane Kidney and Transplant Specialised Register includes references of reports of studies identified by handsearching resources including conference proceedings, it is a possibility that relevant studies may have been added since our last search of the register. Some outcomes were reported in only a few studies, which increased the risk of the non‐randomised selection of patients for the intervention or control group in a study. For example, the outcome of fungal peritonitis was reported in two studies (817 patients), with one study finding a significant difference between the fungal prophylaxis and control groups, while the second study did not have this finding. In addition, adverse effects were reported in only five studies.
Agreements and disagreements with other studies or reviews
A systematic review was performed as part of the HONEYPOT Study 2009 and was published in 2014 (Johnson 2014). The authors systematically reviewed studies of topical antimicrobial prophylaxis for prevention of infections in PD. Nine studies were identified using a search strategy that included electronic searches of MEDLINE (through Ovid) and the Cochrane Central Register of Controlled Trials. Our review included all of the studies included by Johnson 2014, as well as two studies not reported in that review (Chu 2008; Danguilan 2003). This review concluded that the evidence from the nine studies was inconclusive for nasal mupirocin, exit‐site mupirocin and exit‐site gentamicin prophylaxis. In the present review, we reached a similar conclusion, with some individual studies making a significant difference to the risk of exit‐site/tunnel infection or the exit‐site/tunnel infection rate but not having an effect on the other outcomes of peritonitis, catheter removal/replacement and technique failure.
The Renal Association (UK) guidelines currently recommend that "topical antibiotic administration be used to reduce the frequency of S. aureus and Gram‐negative exit‐site infection and peritonitis" (Woodrow 2010). The suggested antibiotics are mupirocin ointment or gentamicin cream (the latter for patients with a known history of Pseudomonas infections). The ISPD position statement on exit‐site care to prevent peritonitis (Piraino 2011) states that "antibiotic protocols against S. aureus are effective in reducing the risk of S. aureus catheter infections" and that "all PD patients should use topical antibiotic either at the catheter exit‐site or intranasally or both". The Kidney Health Australia‐Caring for Australasians with Renal Impairment (KHA‐CARI) guidelines (Walker 2014) recommend that "prophylactic therapy using mupirocin ointment be used, especially for S. aureus carriage (intranasally or at the exit site) to decrease the risk of S. aureus catheter exit‐site/tunnel infections and peritonitis" and suggest that the "PD catheter exit site be cleaned daily and a topical antimicrobial agent (either mupirocin or gentamicin) be applied". This review found that the use of oral antibiotic or mupirocin ointment (at the exit site) may reduce the risk of exit‐site/tunnel infection and the risk of peritonitis but was not seen to reduce the exit‐site/tunnel infection rate, the peritonitis rate or the number of patients with catheter removal/replacement. The head‐to‐head comparison of application of mupirocin ointment or cream against gentamicin cream is based on two studies and shows that there is no difference between the effectiveness of mupirocin and gentamicin in terms of preventing exit‐site/tunnel infection and peritonitis. It is unclear if the nasal application of mupirocin reduces the risk of exit‐site/tunnel infection or the risk of peritonitis.
The Renal Association (UK) guidelines state it is "recommended that initial catheter insertion be accompanied by antibiotic prophylaxis" and refer to the RCT evidence supporting the use of vancomycin (Figueiredo 2010; Woodrow 2010). The ISPD position statement says that "prophylactic antibiotics administered at the time of insertion decrease the infection risk. A first‐generation cephalosporin or vancomycin can be used, but suggested each program should weigh the potential benefit against the risk of vancomycin use (development of resistant organisms)" (Piraino 2011). The KHA‐CARI guidelines say it is "recommended that intravenous antibiotic prophylaxis be used prior to PD catheter insertion to reduce the risk of early peritonitis" and "vancomycin, cephalosporins and gentamicin have demonstrated effectiveness in reducing the risk of peritonitis" (Walker 2014). The inclusion of first generation cephalosporins is based on extrapolations from the results of pre‐operative antibiotic studies in patients without chronic kidney disease. However, our study indicates that the evidence supporting the use of first generation cephalosporins in PD patients undergoing Tenckhoff catheter insertion is scant. In the present review, we identified four RCTs of different pre‐operative antibiotic prophylaxis regimens, including parenteral gentamicin, vancomycin, cephazolin and cefuroxime, with only two evaluating a first generation cephalosporin. One small study involving 50 PD patients found that cephazolin and gentamicin were no better than no treatment (Lye 1992). The largest of the studies (265 patients) showed that cephazolin was inferior to vancomycin in preventing post‐operative catheter‐associated infections (7% versus 1%, respectively; P < 0.05) (Gadallah 2000c). However, the recommendation to use a first generation cephalosporin or vancomycin is understandable because of the risk of selecting for resistant organisms such as vancomycin‐resistant enterococci and S. aureus (HICPAC 1995) and the development of Clostridium difficile colitis (Figueiredo 2010). The postoperative incidence of peritonitis in the control arms of three of the evaluated studies were high, ranging from 14% to 46% (Bennet‐Jones 1988; Gadallah 2000c; Wikdahl 1997) and the applicability of these data to PD units with lower infection rates following PD catheter insertion is unclear.
The ISPD position statement suggests "most episodes of fungal peritonitis are preceded by courses of antibiotics" and "fungal prophylaxis during antibiotic therapy may prevent some cases of Candida peritonitis in programs that have high rates of fungal peritonitis" (Piraino 2011). The KHA‐CARI guidelines recommend "oral antifungal prophylaxis should be considered when antibiotics are administered to patients undergoing PD to reduce the risk of developing fungal peritonitis" (Walker 2014). This review indicates that fluconazole reduced the risk of fungal peritonitis following antibiotic treatment and that nystatin reduced the rate of Candida peritonitis in PD patients. The authors of the fluconazole study (Restrepo 2010) noted that a growing number of Candida strains were resistant to fluconazole during their study, and this would limit its use.
The ISPD no longer recommends that the exit site be regularly disinfected with antibacterial soap or a medical antiseptic to keep the exit site clean and reduce the numbers of resident bacteria. The current position statement states that "water and antibacterial soap are recommended by many centres. Use of an antiseptic to clean the exit site is preferred in some programs, but the agent must be non‐cytotoxic" (Piraino 2011). The four studies in this review which compared the use of disinfectant against standard care did not show any benefit with the use of disinfectant (povidone‐iodine 10% ointment; povidone‐iodine 2.5% dry powder spray; povidone‐iodine 20g/L solution; sodium hypochlorite 10% solution) compared with standard care (povidone‐iodine 10% solution; alcohol chlorhexidine hand wash and use of alcohol wipes; non‐disinfectant soap and water; pH neutral soap and water). Three of the studies did not report on adverse effects of the interventions and one study observed that skin rashes/pruritus occurred in 6% of patients following use of the povidone‐iodine dry powder spray (Wilson 1997). The three studies in this review which looked at the use of disinfectant versus antibiotic or other disinfectant also did not show any benefit with the use of disinfectant (sodium hypochlorite 10% solution; sodium hypochlorite 5% solution; antibacterial honey 10 mg) compared with antibiotic (2% mupirocin ointment) or other disinfectant (povidone iodine 10% solution). Adverse effects were reported in each of these studies. Sodium hypochlorite solution was associated with more irritation around the exit site than povidone iodine solution (Wadhwa 1995; Wadhwa 1997) and 5.9% of patients using antibacterial honey at the exit site in HONEYPOT Study 2009 reported local reaction as the reason for withdrawing from the study whereas no patients in the control group reported this adverse effect.
Authors' conclusions
Implications for practice.
This update of a systematic review identified low quality evidence for the outcomes under consideration. Our findings are as follows.
The use of oral or topical antibiotic had uncertain effects on the risk of exit‐site/tunnel infection and the risk of peritonitis
It is uncertain whether the use of nasal antibiotic reduces the risk of exit‐site/tunnel infection or the risk of peritonitis
The use of pre/perioperative intravenous vancomycin may reduce the risk of early peritonitis but has an uncertain effect on the risk of exit‐site/tunnel infection
The use of topical disinfectant has an uncertain effect on the risk of exit‐site/tunnel infection and the risk of peritonitis
Antifungal prophylaxis with oral nystatin/fluconazole may reduce the risk of fungal peritonitis occurring after a PD patient has had an antibiotic course.
Implications for research.
Many of the studies included in this review have significant methodological limitations, including lack of statistical power, and potential for bias. Further large randomised studies with sufficiently long follow‐up periods are required. These need to assess patient‐important outcomes such as adverse effects of the interventions given as well as quality of life. Studies need to be designed so they yield useful data on the key outcomes of exit‐site/tunnel infection, peritonitis, catheter loss/replacement, and technique failure due to infection.
These studies should be large enough to enable subgroup analyses to determine which patients would benefit most from a prophylactic intervention and to clearly identify any harms associated with an intervention. There is a pressing need for more well‐designed RCTs in this area, which adequately assess safety, as well as efficacy.
What's new
| Date | Event | Description |
|---|---|---|
| 6 June 2017 | Amended | Corrections made to numbering in MEDLINE & CENTRAL search strategies |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 12 January 2017 | New citation required and conclusions have changed | New studies and comparisons added |
| 12 January 2017 | New search has been performed | 20 new studies added |
| 30 April 2014 | Amended | Minor copy‐edit made |
| 18 March 2010 | Amended | Contact details updated. |
| 13 May 2009 | Amended | Contact details updated. |
| 16 September 2008 | Amended | Converted to new review format. |
| 18 December 2007 | Amended | New trials sought but none found |
Acknowledgements
2004 review
We acknowledge the contribution of Drs Peter Wilson, Ignatius Fong, Gerald Coles and Cliff Holmes of the Mupirocin Study Group, David Churchill and Judy Bernardini, who responded to our queries about their studies. We are indebted to Dr R Russo and Dr R Curciulo of the University of Bari, Italy, who commented on the original project and provided useful background information. Particular thanks to Dr Paolo Strippoli, Head of the Nephrology Unit of Ospedale "A. Perrino", Brindisi, Italy, for his intellectual input in this manuscript with comments on the original project and final manuscript and providing abundant background information and advice. We acknowledge the contribution of Narelle Willis, Coordinator of the Cochrane Renal Group, who coordinated our activities throughout the project and edited the latest draft of this review.
2017 review
We would like to thank Drs Chu, Danguilan, Johnson, Jassal, Ayliffe and Selgas for their responses to our queries about their studies.
We would like to thank Dr E Hodson for her comments and feedback during the preparation of this updated review.
Appendices
Appendix 1. Electronic search strategies
| Database searched | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Oral or topical antibiotics versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis (number of patients with one or more episodes) | 5 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.57, 1.19] |
| 1.1 Oral antibiotic versus placebo | 4 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.32] |
| 1.2 Mupirocin ointment versus standard care | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.40] |
| 2 Peritonitis rate (episodes/total patient‐months on PD) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any systemic antibiotic versus placebo/no treatment (excluding nystatin) | 3 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.40, 1.14] |
| 3 Exit‐site/tunnel infection (number of patients with one or more episodes) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Any systemic antibiotic versus placebo/no treatment | 3 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.04] |
| 4 Exit‐site/tunnel infection rate (episodes/total patient‐months on PD) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Any systemic antibiotic versus placebo/no treatment | 2 | 939 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.05] |
| 5 Catheter removal or replacement (number of patients) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any systemic antibiotic versus placebo/no treatment | 5 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.46] |
| 6 Mortality (all‐cause) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any systemic antibiotic versus placebo/no treatment | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.41, 1.89] |
| 7 Mortality due to peritonitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Oral antibiotic versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Pruritus (generalised) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Nasal irritation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Allergy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.7. Analysis.
Comparison 1 Oral or topical antibiotics versus placebo/no treatment, Outcome 7 Mortality due to peritonitis.
Comparison 2. Oral or topical antibiotics versus other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis (number of patients with one or more episodes) | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.89, 1.84] |
| 1.1 Sodium fusidate ointment versus ofloxacin (oral) | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 1.82] |
| 1.2 Mupirocin ointment versus rifampin (oral) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.67, 2.33] |
| 1.3 Mupirocin ointment/cream versus gentamicin cream (topical) | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.93, 2.07] |
| 2 Peritonitis rate (episodes/total patient‐months on PD) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Mupirocin ointment versus polysporin triple ointment (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sodium fusidate ointment versus ofloxacin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Mupirocin ointment versus neomycin sulphate ointment (nasal) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mupirocin ointment versus rifampin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Mupirocin ointment versus gentamicin cream (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Exit‐site/tunnel infection (number of patients with one or more episodes) | 4 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.71, 2.31] |
| 3.1 Mupirocin ointment versus sodium fusidate ointment (topical) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.42, 1.95] |
| 3.2 Sodium fusidate ointment versus ofloxacin (oral) | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [0.76, 7.62] |
| 3.3 Mupirocin ointment/cream versus gentamicin cream (topical) | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.41, 3.46] |
| 4 Exit‐site/tunnel infection rate (episodes/total patient‐months on PD) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Mupirocin ointment versus polysporin triple ointment (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Mupirocin ointment versus gentamicin cream (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Sodium fusidate ointment versus ofloxacin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Catheter removal or replacement (number of patients) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Mupirocin ointment versus polysporin triple ointment (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Sodium fusidate ointment versus ofloxacin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Mupirocin ointment (exit site) versus rifampin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Mupirocin cream versus gentamicin cream (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Mortality (all‐cause) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Mupirocin ointment versus polysporin triple ointment (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Sodium fusidate ointment versus ofloxacin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Mupirocin ointment versus rifampin (oral) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Mupirocin ointment versus gentamicin cream (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Technique failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Mupirocin ointment versus polysporin triple ointment (exit site) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Nausea | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.59] |
| 8.2 Pruritus (local) | 2 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.29, 1.49] |
2.2. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 2 Peritonitis rate (episodes/total patient‐months on PD).
2.4. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 4 Exit‐site/tunnel infection rate (episodes/total patient‐months on PD).
2.5. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 5 Catheter removal or replacement (number of patients).
2.6. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 6 Mortality (all‐cause).
2.7. Analysis.
Comparison 2 Oral or topical antibiotics versus other antibiotic, Outcome 7 Technique failure.
Comparison 3. Nasal antibiotics versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis (number of patients with one or more episodes) | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.31] |
| 2 Peritonitis rate (episodes/total patient‐months on PD) | 2 | 2797 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.16, 2.77] |
| 3 Exit site and tunnel infection (number of patients with one or more episodes) | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.62, 2.87] |
| 4 Exit site and tunnel infection rate (episodes/total patient‐months on PD) | 2 | 2796 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.29, 2.92] |
| 5 Catheter removal or replacement (number of patients) | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.48, 1.78] |
| 6 Mortality (all‐cause) | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.47] |
| 7 Adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Headache | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.94] |
| 7.2 Diarrhoea | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.40, 6.78] |
| 7.3 Nausea | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.94] |
| 7.4 Vomiting | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [0.61, 14.49] |
| 7.5 Pruritus | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.25, 8.77] |
| 7.6 Nasal irritation/rhinitis | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.30, 2.94] |
3.6. Analysis.
Comparison 3 Nasal antibiotics versus placebo/no treatment, Outcome 6 Mortality (all‐cause).
Comparison 4. Pre/peri‐operative prophylaxis versus placebo/no treatment or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis (number of patients with one or more episodes) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Vancomycin versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Cefazolin versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 IV gentamicin versus no antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 IV cefazolin + gentamicin versus no antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 IV cefuroxime + cefuroxime (intraperitoneal) versus no antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Vancomycin versus cefazolin | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exit site/tunnel infection (number of patients with one or more episodes) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Vancomycin versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Cefazolin versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 IV gentamicin versus no antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 IV cefazolin + gentamicin versus no antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 IV cefuroxime + cefuroxime (intraperitoneal) versus no antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Vancomycin versus cefazolin | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Catheter removal or replacement (number of patients) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Mortality (all‐cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.3. Analysis.
Comparison 4 Pre/peri‐operative prophylaxis versus placebo/no treatment or other antibiotic, Outcome 3 Catheter removal or replacement (number of patients).
4.4. Analysis.
Comparison 4 Pre/peri‐operative prophylaxis versus placebo/no treatment or other antibiotic, Outcome 4 Mortality (all‐cause).
Comparison 5. Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis (number of patients with one or more episodes) | 6 | 853 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.06] |
| 1.1 Disinfectant versus standard care | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.52, 1.26] |
| 1.2 Disinfectant versus other active treatment (antibiotics, other disinfectant) | 3 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.13] |
| 2 Exit site/tunnel infection (number of patients with one or more episodes) | 8 | 973 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.33] |
| 2.1 Disinfectant versus standard care | 4 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| 2.2 Disinfectant versus other active treatment (antibiotics, other disinfectant) | 4 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.89, 1.60] |
| 3 Exit site/tunnel infection rate (episodes/total patient‐months on PD) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Disinfectant versus other active treatment (antibiotics, other disinfectant) | 2 | 1752 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.31, 4.93] |
| 4 Catheter removal or replacement (number of patients) | 7 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.38] |
| 4.1 Disinfectant versus standard care | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.34, 1.55] |
| 4.2 Disinfectant versus other active treatment (antibiotics, other disinfectant) | 5 | 586 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.57, 1.69] |
| 5 Mortality (all‐cause) | 4 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.53, 1.44] |
| 5.1 Disinfectant versus standard care | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.54, 2.84] |
| 5.2 Disinfectant versus other active treatment (antibiotics, other disinfectant) | 2 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.39, 1.35] |
| 6 Technique failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Pruritus (local) | 4 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [1.21, 6.48] |
5.3. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 3 Exit site/tunnel infection rate (episodes/total patient‐months on PD).
5.6. Analysis.
Comparison 5 Topical disinfectants versus standard care or other active treatment (antibiotic or other disinfectant), Outcome 6 Technique failure.
Comparison 6. Germicidal chamber versus none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis rate (episodes/total patient‐months on PD) | 2 | 1855 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.74, 1.51] |
| 2 Mortality (all‐cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
6.2. Analysis.
Comparison 6 Germicidal chamber versus none, Outcome 2 Mortality (all‐cause).
Comparison 7. Dressing systems (any).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exit site/tunnel infection (number of patients with one or more episodes) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Chlorhexidine gluconate + water versus povidone‐iodine solution | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sodium hypochlorite solution versus povidone‐iodine solution | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Shower + gauze versus dressing pack + fixomull | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Blisterfilm versus gauze | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exit site/tunnel infection rate (episodes/total patient‐months on PD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Shower + gauze versus dressing pack + fixomull | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Silver ring system on catheter versus none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis (number of patients with one or more episodes) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Exit site/tunnel infection (number of patients with one or more episodes) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Catheter removal or replacement (number of patients) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Mortality (all‐cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
8.4. Analysis.
Comparison 8 Silver ring system on catheter versus none, Outcome 4 Mortality (all‐cause).
Comparison 9. Antistaphylococcal vaccine (Staphypan) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peritonitis rate (episodes/total patient‐months on PD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Exit site/tunnel infection rate (episodes/total patient‐months on PD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Antifungal versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fungal peritonitis (number of patients with one or more episodes) | 2 | 817 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.12, 0.63] |
| 2 Fungal peritonitis rate (episodes/total patient‐months on PD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Axelrod 1973.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table "Patients were selected to receive placebo or antibiotic according to a random number list kept by the pharmacy..." |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken. "We conducted a random double‐blind trial of cephalothin sodium as the prophylactic agent." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/105 (9.5%) dialyses excluded from analysis because pre‐dialysis serum showed antibiotic activity (9) and antibiotic had not been added to dialysate fluid (1). Data reported as no. episodes peritonitis/no. dialyses not no. episodes peritonitis/total patient‐months on PD |
| Selective reporting (reporting bias) | High risk | Outcomes not reported as expected. Also, only 1 of 3 expected primary outcomes reported (peritonitis) |
| Other bias | High risk | Partly funded by Eli Lilly & Company, Indianapolis |
Bennet‐Jones 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutively numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised by being assigned consecutively numbered sealed envelopes, which contained either a prescription for gentamicin to be administered with the anaesthetic, or an instruction to the anaesthetist to give no antibiotic." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither the surgeon nor physician knew whether or not the patient had received the antibiotic." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Neither the surgeon nor physician knew whether or not the patient had received the antibiotic." Physician assessing outcomes did not know whether patient had received antibiotic or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/27 (3.7%) patients not included in the analysis |
| Selective reporting (reporting bias) | High risk | 2 of 3 primary outcomes of interest reported (exit‐site infection, peritonitis). No report of adverse effects of intervention |
| Other bias | Unclear risk | No information provided about funding source |
Bernardini 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study said to be randomised but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Study said to be randomised but no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible ‐ topical antibiotic ointment vs oral antibiotic therapy. The outcome could be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Catheter infections were defined as ... and were diagnosed by the peritoneal dialysis nurse and physician, who were not blinded to the patient's treatment arm." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in analysis including patients who ceased therapy |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes for this review were reported, however unable to meta‐analyse exit‐site infections (reported as infection rate/dialysis‐year) |
| Other bias | Unclear risk | No information on funding provided |
Bernardini 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using a random number generator "Randomization lists were computer generated using a random number generator." |
| Allocation concealment (selection bias) | Low risk | "The sequence of allocation was known only by the investigators at the coordinating center." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators and patients were blinded to the cream used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to the cream used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who received intervention included in analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes for this review were reported |
| Other bias | Low risk | Supported by National Kidney Foundations of Western Pennsylvania and Upstate New York and by Paul Teschan Fund of Dialysis Clinic Inc |
Blowey 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients said to be randomised but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients said to be randomised but no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not done ‐ oral antibiotic + topical antibiotic ointment vs no therapy. The outcome could be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical assessment of outcome could be influenced by knowledge of treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | Only 2 of 3 primary outcomes of interest for this review were reported (exit‐site/tunnel infection, peritonitis) |
| Other bias | Unclear risk | No information on funding provided |
Cheng 1999a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study said to be randomised but no information on method provided |
| Allocation concealment (selection bias) | Unclear risk | Study said to be randomised but no information on method provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement; abstract only available |
| Selective reporting (reporting bias) | Unclear risk | Only 2 of 3 expected primary outcomes were reported (exit‐site infection, catheter removal) |
| Other bias | Unclear risk | No information on funding provided |
Chu 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation "The patients were assigned to either drug on a one‐to‐one alternate basis." |
| Allocation concealment (selection bias) | High risk | Alternate allocation "The patients were assigned to either drug on a one‐to‐one alternate basis." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients were not informed of which cream/ointment they were using. However the cream/ointment were not covered or blinded." (email from author) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/95 (15%) withdrew from the study and were excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Only 2 of 3 expected primary outcomes were reported (peritonitis, exit‐site/tunnel infection) |
| Other bias | Unclear risk | No information on funding provided |
Churchill 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified or block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis for primary outcome; loss to follow‐up: 20 in cotrimoxazole group (35.7%); 9 in placebo group (18.4%) |
| Selective reporting (reporting bias) | High risk | 2 of 3 primary outcomes not reported (exit‐site/tunnel infection, catheter removal/replacement) |
| Other bias | High risk | Hoffman La Roche supplied the antibiotic (cotrimoxazole) and placebo tablets |
Cocksedge 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes "New patients to the program were asked to select a sealed envelope from a pack. Each envelope contained a card allocating the patient to either Method One or Method Two." |
| Allocation concealment (selection bias) | Unclear risk | Do not know if the envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given re loss to follow‐up or any patient withdrawals |
| Selective reporting (reporting bias) | High risk | Only 1 of 3 expected primary outcomes are reported (exit‐site infection). No report of adverse effects of either intervention |
| Other bias | Unclear risk | No report of funding source |
Danguilan 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated. "One hundred patients were enrolled in the study... 50 patients were randomly assigned to each treatment group." |
| Allocation concealment (selection bias) | Unclear risk | No details given re concealment of patient allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment. "Exit sites were monitored weekly during regular follow up." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total of 22/100 dropouts from the study (22%). Proportion missing enough to have a clinically relevant effect |
| Selective reporting (reporting bias) | Unclear risk | Only 2 of 3 expected primary outcomes are reported (exit‐site infection, peritonitis) |
| Other bias | Unclear risk | No report of funding source |
Fuchs 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "Fifty‐one patients were randomly assigned to one of three catheter exit site care regimens." |
| Allocation concealment (selection bias) | Unclear risk | No details given re concealment of patient allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion missing not enough to have a clinically relevant effect. 2/13 (15.4%) in sodium hypochlorite group withdrew from the study |
| Selective reporting (reporting bias) | High risk | Only 1 of 3 expected primary outcomes reported (exit‐site infection) |
| Other bias | Unclear risk | No report of funding source |
Gadallah 2000c.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive allocation of intervention "first patient received vancomycin; second, cefazolin; third, neither; fourth, vancomycin; and so on." |
| Allocation concealment (selection bias) | High risk | Non‐random, predictable sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data re peritonitis outcome; data for exit‐site/tunnel infection excluded from analysis (vancomycin (3); cefazolin (6); no antibiotic (8)) |
| Selective reporting (reporting bias) | Unclear risk | 2 of 3 expected outcomes of interest are reported |
| Other bias | Unclear risk | No report of funding source |
HONEYPOT Study 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization method "Participants were randomly assigned in a 1:1 ratio by use of an adaptive allocation algorithm designed to minimise imbalance in treatment groups for the three variables." |
| Allocation concealment (selection bias) | Low risk | Central allocation (web). "To ensure adequate concealment of allocation, the randomisation was done with a password‐protected internet‐based system." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions. "Blinding of investigators and patients is not possible because of the completely different characteristics of Medihoney and mupirocin ointment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of the interventions could influence outcome assessment. "The trial was open label, but microbiology staff at the local laboratories were not informed of the treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data not balanced between groups. 17/185 (9.2%) withdrew from control group; 54/186 (29%) withdrew from honey group Loss to follow‐up: 1 in honey group (0.5%); 3 in mupirocin group (1.6%) |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes are reported |
| Other bias | Unclear risk | The study appears to be free of other sources of risk. Although 3 of 4 funders are pharmaceutical companies, there is an explicit statement about their role on page 26 of the paper |
Lo 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomised according to odd or even identity numbers |
| Allocation concealment (selection bias) | High risk | A non‐random, predictable sequence was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of the interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis for primary outcome |
| Selective reporting (reporting bias) | High risk | The expected primary outcome is reported (peritonitis), however catheter removal and exit‐site infection not reported |
| Other bias | Unclear risk | No report of funding source |
Low 1980.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation method used |
| Allocation concealment (selection bias) | Low risk | Allocation done by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | 1 of 3 primary outcomes not reported (exit‐site/tunnel infection) |
| Other bias | Low risk | Study appears to be free of other sources of risk. Funding was from a National Institutes of Health contract |
Luzar 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion missing not enough to have a clinically relevant effect; loss to follow‐up: 8 of 127 (6.3%) |
| Selective reporting (reporting bias) | Unclear risk | 3 of 3 primary outcomes of interest are reported, however unable to meta‐analyse catheter removal |
| Other bias | High risk | Funding source not specified but seems to be Baxter Healthcare Corporation |
Lye 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation |
| Allocation concealment (selection bias) | High risk | Non‐random, predictable sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups, and reasons similar; 4 (16%) excluded from analysis in treatment group due to lack of effect of study antibiotics on MRSA bacteria; 3 (12%) excluded from analysis in control group for the same reason |
| Selective reporting (reporting bias) | Low risk | 3 of 3 primary outcomes of interest are reported |
| Other bias | Unclear risk | No report of funding source |
Mendoza‐Guevara 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables "Patients were assigned 1:1 in two groups, with only one treatment; the Rand Corporation tables were used for randomization." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patient cleans own exit site ‐ impossible to conceal intervention allocation. "The study was blind for the investigators and laboratory personnel." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken. "The study was blind for the investigators and laboratory personnel." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Only 1 of 3 expected primary outcomes reported (exit‐site infection) |
| Other bias | Unclear risk | No report of funding source |
Moore 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation "The numbering was consecutive so all participants were given an equal chance of being admitted to either group." "Odd numbers were admitted to the Blisterfilm group and even numbers admitted to the gauze group." |
| Allocation concealment (selection bias) | High risk | Non‐random, predictable sequence. However, allocation concealment not possible ‐ the two dressings are of different sizes and types |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of the interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The published report states percentage of patients in each group that experienced exit‐site infection but does not state actual patient numbers. No report of loss to follow‐up or withdrawals |
| Selective reporting (reporting bias) | High risk | Only 1 of 3 expected primary outcomes of interest reported (exit‐site infection) |
| Other bias | Unclear risk | No report of funding source |
MP3 Study 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator "All randomization is determined by a computer‐generated random number list..." "...201 patients from two centers were randomly assigned to either mupirocin or P3 using stratified block randomization as per protocol." |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy) "Randomization occurs centrally in coordination with the central clinical trials pharmacy... The ointments are placed in containers that are labeled only with the site investigator, study number, and expiry date." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken "The treatments resemble each other in odor, color, and consistency to allow for a double blinded controlled trial." "Neither the healthcare workers not the participants know which intervention the participant will receive." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups, and reasons similar (2 from each group lost to follow‐up); data for 3 patients from 1 site were excluded |
| Selective reporting (reporting bias) | Low risk | Protocol is available and all pre‐specified outcomes of interest to the review are reported in the pre‐specified way |
| Other bias | Low risk | Funded by the Kidney Foundation of Canada. Study appears to be free of other sources of risk |
Mupirocin Study 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding, and unlikely patients were aware of treatment group. Unclear if personnel were aware of patient treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given re who did the outcome assessment and if they were blind to patient treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups, and reasons similar; ITT analysis used |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes of interest are reported |
| Other bias | High risk | Funding source: SmithKline Beecham, UK, and Baxter Healthcare, USA |
Nolph 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Central allocation (Travenol Laboratories) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of the interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12.9% withdrew from control group; 24.3% withdrew from intervention group. Proportion missing enough to have a clinically relevant effect |
| Selective reporting (reporting bias) | High risk | Only 1 of 3 primary expected outcomes of interest is reported (peritonitis) |
| Other bias | High risk | Funding source: Travenol Laboratories Inc., USA |
Nunez‐Moral 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table "Randomization was performed by means of a randomization code via random number table..." |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Only 2 of 3 expected primary outcomes of interest reported fully |
| Other bias | Unclear risk | Disclosure states that "Part of these data belong to Baxter S. L. funds as we received the Nephrological Nursing Investigation Baxter award 2010" |
Perez‐Fontan 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation method used "Staph. aureus nasal carriers were assigned to one of two groups, randomized for age, time on CAPD and prevalence of diabetes." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing data not related to outcome "Patients of Group 2 in whom eradication was not obtained after two neomycin cycles were treated with mupirocin." |
| Selective reporting (reporting bias) | Unclear risk | 2 of 3 expected primary outcomes of interest are reported |
| Other bias | Unclear risk | No report of funding source |
Poole‐Warren 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "Patients were randomly assigned by an independent third party to either the vaccinated group or the saline solution (SS) placebo administered group." |
| Allocation concealment (selection bias) | Low risk | Central allocation (independent third party) "The assigned injection group was not known to either patient or staff immediately connected with the patient's care at any time during the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups, and reasons similar |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes of interest are reported |
| Other bias | High risk | Funding source: Baxter Healthcare Corporation, USA |
Restrepo 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots "The randomization procedure was performed by drawing from a bag cards indicating whether the patient would or would not receive this treatment." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of missing data given |
| Selective reporting (reporting bias) | Unclear risk | The expected primary outcome is reported. However, adverse effects of antifungal use are not reported |
| Other bias | Unclear risk | No report of funding source |
Ryckelynck 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "24 patients using a single use Y‐set and 26 using a reusable Y‐set (O‐set) were separately randomized into two groups." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of missing data given |
| Selective reporting (reporting bias) | High risk | Only 1 of 3 expected primary outcomes is reported |
| Other bias | Unclear risk | No report of funding source; abstract‐only publication |
Sesso 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "Each carrier was then randomly assigned to one of the three groups". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44.4% withdrew from sodium fusidate group; 77.7% withdrew from ofloxacin group; 53.8% withdrew from control group. Proportion missing enough to have a clinically relevant effect |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes of interest are reported |
| Other bias | Low risk | Supported by a grant from Instituto Paulista de Estudos e Pesquisas em Nefrologia e Hipertensao. Study appears to be free of other sources of risk |
Sharma 1971.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of missing data given on a patient basis; 6 dialyses excluded from analysis |
| Selective reporting (reporting bias) | High risk | Outcomes not reported as expected ‐ number of episodes peritonitis/number of dialyses not number of episodes peritonitis/total patient‐months on dialysis. Also, only 1 of 3 expected primary outcomes reported (peritonitis) |
| Other bias | Unclear risk | No report of funding source |
SIPROCE Study 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation "After informed consent had been obtained, the patients were stratified by diabetes mellitus status (types I and II) and randomly assigned by the coordinating study center (Berlin) to either the silver ring or the control group." |
| Allocation concealment (selection bias) | Low risk | Central allocation (coordinating study centre) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of the interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion missing enough to have a clinically relevant effect. Dropouts: 29/97 (29.9%) in silver ring group; 30/98 (30.6%) in control group. Withdrawals: 6/97 (6.2%) in silver ring group; 0/98 (0%) in control group |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes are reported |
| Other bias | Unclear risk | "Supported in part by Baxter Deutschland GmbH, Ettlingen, Germany." |
Sit 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss "Randomization was guided by the flip of a coin..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion missing not enough to have a clinically relevant effect; 2 (8%) excluded from analysis in mupirocin group due to kidney transplantation (1) and death (1) |
| Selective reporting (reporting bias) | Unclear risk | 2 of 3 expected primary outcomes are reported (exit‐site/tunnel infection, peritonitis) |
| Other bias | Unclear risk | No report of funding source |
Swartz 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups, and reasons similar; loss to follow‐up: 2 of 29 in antibiotic prophylaxis group (6.9%) |
| Selective reporting (reporting bias) | High risk | 3 of 3 expected primary outcomes of interest are reported, however peritonitis, exit‐site infection and catheter removal could not be meta‐analysed |
| Other bias | Unclear risk | No report of funding source |
Wadhwa 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "Fifty PD patients were prospectively randomized to perform daily exit‐site care with soap and water followed by Amuchina 10% or Povidone Iodine 10% solution." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement; no details re missing data provided |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes are reported (exit‐site infection, peritonitis, catheter loss) |
| Other bias | Unclear risk | No report of funding source |
Wadhwa 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "Thirty nine PD patients were prospectively randomized to perform daily exit‐site care with soap and water followed by Amuchina 5% or povidone iodine 10% solution." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. No details re missing data provided |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes are reported (exit‐site infection, peritonitis, catheter loss) |
| Other bias | Unclear risk | No report of funding source |
Waite 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators assessing response (presence or absence of infection) were blinded to the treatment received by the individual patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion missing not enough to have a clinically relevant effect; 3 (2.5%) excluded from analysis due to withdrawal (2) and failure to have PD catheter inserted (1) ‐ group allocation not reported |
| Selective reporting (reporting bias) | Unclear risk | 2 of 3 expected primary outcomes of interest are reported |
| Other bias | High risk | Funding source: Purdue‐Frederick |
Wikdahl 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | High risk | Sealed envelopes without all safeguards |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | 3 of 3 expected primary outcomes of interest are reported, however unable to meta‐analyse catheter removal |
| Other bias | Unclear risk | No report of funding source |
Wilson 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion missing not enough to have a clinically relevant effect; loss to follow‐up: 1 in spray group (1.3%), 3 in control group (4.2%) Withdrawals: 5 in spray group (6.5%) (adverse events) 1 (1.3%) excluded from analysis in povidone iodine spray group due to missing results |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes of interest are reported |
| Other bias | Unclear risk | No report of funding source |
Wong 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated "Patients not excluded were randomized into two groups." No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups, and reasons similar. Outcome data for tunnel infection not reported ‐ this is ok as this infection is the least frequent one in PD patients; 1 withdrawal (not stated which intervention group) Excluded from analysis: 5 (6.4%) from mupirocin group; 7 (8.0%) from control group |
| Selective reporting (reporting bias) | Low risk | 3 of 3 expected primary outcomes are reported (exit‐site infection, peritonitis, catheter loss) |
| Other bias | Unclear risk | No report of funding source |
Zimmerman 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcomes are likely to be influenced by lack of blinding and knowledge of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and knowledge of the interventions could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12.5% in rifampin group withdrew; 0% in control group withdrew. Proportion missing enough to have a clinically relevant effect |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available but all expected outcomes of interest are reported, however unable to meta‐analyse exit‐site infection |
| Other bias | High risk | Funding source: Baxter Healthcare |
AKI ‐ acute kidney injury; APD ‐ automated peritoneal dialysis; CAPD ‐ continuous ambulatory peritoneal dialysis; CCPD ‐ continuous cycling peritoneal dialysis; CKD ‐ chronic kidney disease; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; IP ‐ intraperitoneal; ITT ‐ intention‐to‐treat; IV ‐ intravenous; M/F ‐ male/female; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial; SD ‐ standard deviation; SE ‐ standard error; SEM ‐ standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cavdar 2004 | Not an intervention of interest |
| Churchill 1989 | Not an intervention of interest |
| Crabtree 2003 | Not an intervention of interest |
| de Fijter 1992a | Pharmacokinetics study not prevention |
| Gadallah 2000 | Urokinase is not an antimicrobial agent; treatment study not prevention |
| Maiorca 1983 | Not an intervention of interest |
| Naylor 1997 | Small pilot study |
| Oh 2000 | It is an RCT but peritonitis data is not readily available; no reply from authors to query email |
| Plum 1997a | Treatment study not prevention |
| Rodriguez‐Perez 1989 | Not an intervention of interest |
| Thomae 1982 | Study only went for 84 hours; of the 7 patients, 3 had previously had peritonitis |
| Trooskin 1990 | Not an intervention of interest |
RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02547103.
| Trial name or title | Efficacy and safety of local application of chlorhexidine gluconate versus mupirocin ointment in the prevention of peritoneal dialysis‐related infection: a pilot study, double‐ blind, stratified randomized controlled trial |
| Methods | Allocation: randomised, parallel RCT Double blind (subject, caregiver, investigator, outcomes assessor) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | Surapon Nochaiwong Chidchanok Ruengorn Maharaj Nakorn Chiang Mai Hospital Chiang Mai, Thailand, 50200 |
| Notes | Sponsors and Collaborators Chiang Mai University, Health Systems Research Institute, Thailand |
APD ‐ automated peritoneal dialysis; CAPD ‐ continuous ambulatory peritoneal dialysis; ESKD ‐ end‐stage kidney disease; PD ‐ peritoneal dialysis
Differences between protocol and review
The risk of bias assessment tool has replaced the quality assessment checklist used in the original review
Summary of findings tables have been incorporated into this update
Contributions of authors
Designing the review: GS, DJ, JC
Coordinating the update of the review: DC, GS, JC
Data collection for the update of the review: DC and GS, independently
Developing search strategy: DC and GS, independently
Undertaking searches: DC and GS, independently
Screening search results: DC and GS, independently
Organising retrieval of papers: DC and GS, independently
Screening retrieved papers against inclusion criteria; DC and GS, independently
Appraising quality of papers: DC and GS, independently
Abstracting data from papers (modified Cochrane Kidney and Transplant's data extraction form): DC and GS, independently
Searching for additional data in unpublished studies: DC and GS, independently
Entering data into RevMan: DC
Analysis of data: DC, GS, JC
Interpretation of data: DC, GS, JC
Providing a methodological perspective: GS, JC
Providing a clinical perspective: GS, DJ, JC
Providing a policy perspective: GS, DJ, JC
Providing a consumer perspective: GS, DJ, JC
Writing the review: DC, GS, DJ, JC
Providing general advice on the review: JC, GS
Declarations of interest
Denise Campbell: none
David W Mudge has received consultancy fees, speakers' honoraria and travel assistance from Baxter Healthcare for activities unrelated to this review
Jonathan C Craig: none
David W Johnson has received consultancy fees, speakers' honoraria, travel sponsorships and research funding from Fresenius Medical Care and Baxter Healthcare for activities unrelated to this review
Allison Tong: none
Giovanni FM Strippoli: none
Edited (no change to conclusions)
References
References to studies included in this review
Axelrod 1973 {published data only}
- Axelrod J, Meyers BR, Hirschman SZ, Stein R. Prophylaxis with cephalothin in peritoneal dialysis. Archives of Internal Medicine 1973;132(3):368‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Bennet‐Jones 1988 {published data only}
- Bennet‐Jones D, Martin J, Barratt AJ, Duffy TJ, Naish PF, Aber GM. Prophylactic gentamicin in the prevention of early exit‐site infections and peritonitis in CAPD. Advances in Peritoneal Dialysis 1988;4:147‐50. [CENTRAL: CN‐00775909] [Google Scholar]
Bernardini 1996 {published data only}
- Bernardini J, Piraino B, Holley J, Johnston JR, Lutes R. A randomized trial of staphylococcus aureus prophylaxis in peritoneal dialysis patients: mupirocin calcium ointment 2% applied to the exit site versus cyclic oral rifampin. American Journal of Kidney Diseases 1996;27(5):695‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernardini 2005 {published data only}
- Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomized, double‐blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. Journal of the American Society of Nephrology 2005;16(2):539‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blowey 1994 {published data only}
- Blowey DL, Warady BA, McFarland KS. Treatment of staphylococcus aureus nasal carriage in pediatric peritoneal dialysis patients. Advances in Peritoneal Dialysis 1994;10:297‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Cheng 1999a {published data only}
- Cheng YY, Ho YW, Chiu SW. Exit site care, an open labelled randomized prospective study comparing the use of povidone‐iodine and chlorhexidine soap [abstract no: 6]. Hong Kong Journal of Nephrology 1999;1(1):A3. [Google Scholar]
Chu 2008 {published data only}
- Chu KH, Choy WY, Cheung CC, Fung KS, Tang HL, Lee W, et al. A prospective study of the efficacy of local application of gentamicin versus mupirocin in the prevention of peritoneal dialysis catheter‐related infections. Peritoneal Dialysis International 2008;28(5):505‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Churchill 1988 {published data only}
- Churchill DN, Oreopoulos DG, Taylor DW, Vas SI, Manuel MA, Wu G. Peritonitis in CAPD patients ‐ a randomized clinical trial (RCT) of trimethoprim‐sulfamethoxazole (TMP/SMX) prophylaxis [abstract]. Kidney International 1988;33(1):244. [CENTRAL: CN‐00636126] [Google Scholar]
- Churchill DN, Taylor DW, Vas SI, Singer J, Beecroft ML, Wu G, et al. Peritonitis in continuous ambulatory peritoneal dialysis (CAPD) patients: a randomized clinical trial of cotrimoxazole prophylaxis. Peritoneal Dialysis International 1988;8(2):125‐8. [EMBASE: 18252568] [Google Scholar]
Cocksedge 1993 {published data only}
- Cocksedge B, Hunt D, Westerholm W, Heathcote K, Pollock C. Peritoneal dialysis catheter exit site care for the maintenance continuous ambulatory peritoneal dialysis (CAPD) patient: report of a randomised prospective study. Renal Educator 1993;13(1):4‐6. [CENTRAL: CN‐00626103] [Google Scholar]
Danguilan 2003 {published data only}
- Danguilan RA, Evangelista LP, Abrenica MS, Rondilla SM. Comparative study of mupirocin and sodium fucidate in the prophylaxis of exit‐site infections in CAPD patients. Peritoneal Dialysis International 2003;23(6):593‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Fuchs 1990 {published data only}
- Fuchs J, Gallagher ME, Jackson‐Bey D, Krawtz D, Schreiber J. A prospective randomized study of peritoneal catheter exit‐site care. Dialysis & Transplantation 1990;19(2):81‐4. [EMBASE: 20066020] [Google Scholar]
Gadallah 2000c {published data only}
- Gadallah MF, Ramdeen G, Mignone J, Patel D, Mitchell L, Tatro S. Role of preoperative antibiotic prophylaxis in preventing postoperative peritonitis in newly placed peritoneal dialysis catheters. American Journal of Kidney Diseases 2000;36(5):1014‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gadallah MF, Ramdeen G, Torres C, Mignone J, Patel D, Mitchell L, et al. Preoperative vancomycin prophylaxis for newly placed peritoneal dialysis catheters prevents postoperative peritonitis. Advances in Peritoneal Dialysis 2000;16:199‐203. [MEDLINE: ] [PubMed] [Google Scholar]
HONEYPOT Study 2009 {published data only}
- Johnson DW, Badve SV, Pascoe EM, Beller E, Cass A, Clark C, et al. Antibacterial honey for the prevention of peritoneal‐dialysis‐related infections (HONEYPOT): a randomised trial. Lancet Infectious Diseases 2014;14(1):23‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson DW, Clark C, Isbel NM, Hawley CM, Beller E, Cass A, et al. The Honeypot study protocol: a randomized controlled trial of exit‐site application of medihoney antibacterial wound gel for the prevention of catheter‐associated infections in peritoneal dialysis patients. Peritoneal Dialysis International 2009;29(3):303‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Pascoe EM, Lo S, Scaria A, Badve SV, Beller EM, Cass A, et al. The HONEYPOT randomized controlled trial statistical analysis plan. Peritoneal Dialysis International 2013;33(4):426‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Badve SV, Pascoe EM, Beller E, Cass A, Clark C, et al. Microbiological results of the Honeypot study‐secondary analysis of a randomised, controlled trial of exit site application of MEDIHONEY for the prevention of catheter‐associated infections in PD patients [abstract]. Nephrology 2014;19(Suppl 4):30. [EMBASE: 71587829] [Google Scholar]
- Zhang L, Badve SV, Pascoe EM, Beller E, Cass A, Clark C, et al. The effect of exit‐site antibacterial honey versus nasal mupirocin prophylaxis on the microbiology and outcomes of peritoneal dialysis‐associated peritonitis and exit‐site infections: a sub‐study of the Honeypot Trial. Peritoneal Dialysis International 2015;35(7):712‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 1996 {published data only}
- Lo WK, Chan CY, Cheng SW, Poon JF, Chan DT, Cheng IK. A prospective randomized control study of oral nystatin prophylaxis for Candida peritonitis complicating continuous ambulatory peritoneal dialysis. American Journal of Kidney Diseases 1996;28(4):549‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Low 1980 {published data only}
- Low DE, Vas S, Oreopoulos DG, Manuel A, Saiphoo C, Finer C, et al. Randomized clinical trial of cephalexin in preventing peritonitis in patients on continuous ambulatory peritoneal dialysis [abstract]. Kidney International 1981;19(2):392. [Google Scholar]
- Low DE, Vas SI, Oreopoulos DG, Manuel MA, Saiphoo MM, Finer C, et al. Prophylactic cephalexin ineffective in chronic ambulatory peritoneal dialysis. Lancet 1980;2(8197):753‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Luzar 1990 {published data only}
- Luzar MA, Brown CB, Balf D, Hill L, Issad B, Monnier B, et al. Exit‐site care and exit‐site infection in continuous ambulatory peritoneal dialysis (CAPD): results of a randomized multicenter trial. Peritoneal Dialysis International 1990;10(1):25‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lye 1992 {published data only}
- Lye WC, Lee EJ, Tan CC. Prophylactic antibiotics in the insertion of Tenckhoff catheters. Scandinavian Journal of Urology & Nephrology 1992;26(2):177‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lye WC, Straaten JC, Lee EJ. A prospective study on the use of prophylactic antibiotics for the implantation of tenckhoff catheters in CAPD patients [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:343. [CENTRAL: CN‐00626018]
Mendoza‐Guevara 2007 {published data only}
- Mendoza‐Guevara L, Castro‐Vazquez F, Aguilar‐Kitsu A, Morales‐Nava A, Rodriguez‐Leyva F, Sanchez‐Barbosa JL. Amuchina 10% solution, safe antiseptic for preventing infections of exit‐site of Tenckhoff catheters, in the pediatric population of a dialysis program. Contributions to Nephrology 2007;154:139‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moore 1989 {published data only}
- Moore C. A clinical study comparing a polyurethane dressing, blisterfilm, to standard gauze dressing on the tenckhoff catheter [abstract]. ANNA Journal 1989;16(2):105. [Google Scholar]
- Moore CG. Comparison of Blisterfilm and gauze for peritoneal catheter exit site care. ANNA Journal 1989;16(7):475‐8. [MEDLINE: ] [PubMed] [Google Scholar]
MP3 Study 2008 {published data only}
- Jassal SV, Lok CE, MP3 Study Group. A randomized controlled trial comparing mupirocin versus polysporin triple for the prevention of catheter‐related infections in peritoneal dialysis patients (the MP3 study). Peritoneal Dialysis International 2008;28(1):67‐72. [MEDLINE: ] [PubMed] [Google Scholar]
- McQuillan RF, Chiu E, Nessim S, Lok CE, Roscoe JM, Tam P, et al. A randomized controlled trial comparing mupirocin and polysporin triple ointments in peritoneal dialysis patients: the MP3 Study. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(2):297‐303. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepen AT, Tomlinson GA, Jassal SV. The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(8):1266‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mupirocin Study 1996 {published data only}
- Anonymous. Nasal mupirocin prevents staphylococcus aureus exit‐site infection during peritoneal dialysis. Mupirocin Study Group. Journal of the American Society of Nephrology 1996;7(11):2403‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coles GA, Mupirocin Study Group. The effect of intranasal mupirocin on CAPD exit site infection (ESI) [abstract]. Journal of the American Society of Nephrology 1994;5(3):439. [CENTRAL: CN‐00550592] [Google Scholar]
- Davey P, Craig AM, Hau C, Malek M. Cost‐effectiveness of prophylactic nasal mupirocin in patients undergoing peritoneal dialysis based on a randomized placebo‐controlled trial. Journal of Antimicrobial Chemotherapy 1999;43(1):105‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nolph 1985 {published data only}
- Nolph KD, Prowant B, Serkes KD, Morgan LM. A randomized multicenter clinical trial to evaluate the effects of an ultraviolet germicidal system on peritonitis rate in continuous ambulatory peritoneal dialysis. Peritoneal Dialysis Bulletin 1985;5(1):19‐24. [EMBASE: 15143789] [Google Scholar]
Nunez‐Moral 2014 {published data only}
- Nunez‐Moral M, Sanchez‐Alvarez E, Gonzalez‐Diaz I, Pelaez‐Requejo B, Fernandez‐Vina A, Quintana‐Fernandez A, et al. Exit‐site infection of peritoneal catheter is reduced by the use of polyhexanide. results of a prospective randomized trial. Peritoneal Dialysis International 2014;34(3):271‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Fontan 1992 {published data only}
- Perez‐Fontan M, Rosales M, Rodriguez‐Carmona A, Moncalian J, Femindez‐Rivera C, Cao M, et al. Treatment of staphylococcus aureus nasal carriers in CAPD with mupirocin. Advances in Peritoneal Dialysis 1992;8:242‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Poole‐Warren 1991 {published data only}
- Poole‐Warren LA, Hallett MD, Hone PW, Burden SH, Farrell PC. Vaccination for prevention of CAPD associated staphylococcal infection: results of a prospective multicentre clinical trial. Clinical Nephrology 1991;35(5):198‐206. [MEDLINE: ] [PubMed] [Google Scholar]
Restrepo 2010 {published data only}
- Restrepo C, Chacon J, Manjarres G. Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial.[Erratum appears in Perit Dial Int. 2011 Mar‐Apr;31(2):120]. Peritoneal Dialysis International 2010;30(6):619‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ryckelynck 1987 {published data only}
- Ryckelynck JP, Verger C, Cam G, Faller B, Pierre D. A prospective study to evaluate the role of antiseptics in CAPD Y‐line systems [abstract]. 24th Annual Congress. EDTA‐ERA; 1987 Oct 25‐29; West Berlin, Germany. 1987:161. [CENTRAL: CN‐00644147]
Sesso 1994 {published data only}
- Sesso R, Parisio K, Dalboni A, Rabelo T, Barbosa D, Cendoroglo M, et al. Effect of sodium fusidate and ofloxacin on staphylococcus aureus colonization and infection in patients on continuous ambulatory peritoneal dialysis. Clinical Nephrology 1994;41(6):370‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Sesso R, Parisio K, Draibe S, Ajzen H. Effect of sodium fusidate and ofloxacin on staphylococcus aureus carriage and infection in patients on peritoneal dialysis (CAPD) [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):417. [CENTRAL: CN‐00485819] [PubMed] [Google Scholar]
Sharma 1971 {published data only}
- Sharma BK, Rodriguez H, Ghandi VC, Smith EC, Pillay VK, Dunea G. Trial of oral neomycin during peritoneal dialysis. American Journal of the Medical Sciences 1971;262(3):175‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SIPROCE Study 1997 {published data only}
- Pommer W. The efficiency of a silver ring to prevent exit‐site and other catheter‐related infections in PD‐patients‐final results of the SIPROCE study [abstract]. Journal of the American Society of Nephrology 1997;8:182A. [CENTRAL: CN‐00447255] [Google Scholar]
- Pommer W, Brauner M, Westphale HJ, Brunkhorst R, Kramer R, Bundschu D, et al. Effect of a silver device in preventing catheter‐related infections in peritoneal dialysis patients: silver ring prophylaxis at the catheter exit study. American Journal of Kidney Diseases 1998;32(5):752‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- SIPROCE Study. Efficiency of a silver ring in preventing exit‐site infections in adult PD patients: results of the SIPROCE Study. Silver ring Prophylaxis of the Catheter Exit Site. Advances in Peritoneal Dialysis 1997;13:227‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Sit 2007 {published data only}
- Sit D, Kadiroglu AK, Kayabasi H, Yilmaz ME. Prophylactic intranasal mupirocin ointment in the treatment of peritonitis in continuous ambulatory peritoneal dialysis patients. Advances in Therapy 2007;24(2):387‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Swartz 1991 {published data only}
- Swartz R, Messana J, Starmann B, Weber M, Reynolds J. Preventing Staphylococcus aureus infection during chronic peritoneal dialysis. Journal of the American Society of Nephrology 1991;2(6):1085‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wadhwa 1995 {published data only}
- Wadhwa NK, Suh H, Cabralda T, Stratos J, Cascio C, Irwin C, et al. A randomized trial of amuchina 10% versus povidone‐iodine 10% solution for exit‐site care/infection in peritoneal dialysis patients [abstract]. Peritoneal Dialysis International 1995;15(Suppl 1):S64. [Google Scholar]
Wadhwa 1997 {published data only}
- Wadhwa NK, Suh H, Cabralda T. Amuchina 5% versus povidone‐iodine 10% solution for exit‐site care/infection in peritoneal dialysis patients [abstract]. Peritoneal Dialysis International 1997;17(Suppl 1):S46. [Google Scholar]
Waite 1997 {published data only}
- Waite N, Webster N, Laurel M, Johnson M, Fong IW. The efficacy of exit site povidone‐iodine ointment in the prevention of early peritoneal dialysis‐related infections. American Journal of Kidney Diseases 1997;29(5):763‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wikdahl 1997 {published data only}
- Wikdahl AM, Engman U, Stegmayr BJ, Sorenson JG. One‐dose cefuroxime i.v. and i.p. reduces microbial growth in PD patients after catheter insertion. Nephrology Dialysis Transplantation 1997;12(1):157‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilson 1997 {published data only}
- Wilson AP, Lewis C, O'Sullivan HO, Shetty N, Neild GH, Mansell M. The use of povidone iodine in exit site care for patients undergoing continuous peritoneal dialysis (CAPD). Journal of Hospital Infection 1997;35(4):287‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2003 {published data only}
- Wong SS, Chu KH, Cheuk A, Tsang WK, Fung SK, Chan HW, et al. Prophylaxis against gram‐positive organisms causing exit‐site infection and peritonitis in continuous ambulatory peritoneal dialysis patients by applying mupirocin ointment at the catheter exit site. Peritoneal Dialysis International 2003;23 Suppl 2:S153‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Zimmerman 1991 {published data only}
- Zimmerman SW, Ahrens E, Johnson CA, Craig W, Leggett J, O'Brien M, et al. Randomized controlled trial of prophylactic rifampin for peritoneal dialysis‐related infections. American Journal of Kidney Diseases 1991;18(2):225‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zimmerman SW, Ahrens E, Johnson CA, Craig W, Leggett J, O'Brien M, et al. Randomized, controlled trial of prophylactic rifampin (RIF) for PD catheter‐related infections (CRI) and peritonitis (P) [abstract]. Kidney International 1990;37(1):335. [Google Scholar]
References to studies excluded from this review
Cavdar 2004 {published data only}
- Cavdar C, Saglam F, Sifil A, Celik A, Atay T, Gungor O, et al. Effect of once‐a‐week vs thrice‐a‐week application of mupirocin on methicillin and mupirocin resistance in peritoneal dialysis patients: three years of experience. Renal Failure 2008;30(4):417‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cavdar C, Zeybel M, Atay T, Sifil A, Sanlidag C, Gulay Z, et al. The effects of once‐ or thrice‐weekly mupirocin application on mupirocin resistance in patients on continuous ambulatory peritoneal dialysis‐‐first 6 months' experience. Advances in Peritoneal Dialysis 2004;20:62‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Churchill 1989 {published data only}
- Churchill DN, Canadian CAPD Clinical Trials Group. Randomized clinical trial (RCT) comparing peritonitis rates among new CAPD patients using the Y set disinfectant system (Y) to standard systems (S) [abstract]. Kidney International 1989;35(1):268. [CENTRAL: CN‐00602155] [Google Scholar]
- Churchill DN, Taylor DW, Vas SI, Oreopoulos DG, Bettcher KB, Fenton SA, et al. Peritonitis in continuous ambulatory peritoneal dialysis (CAPD): a multi‐centre randomized clinical trial comparing the Y connector disinfectant system to standard systems. Peritoneal Dialysis International 1989;9(3):159‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Crabtree 2003 {published data only}
- Crabtree JH, Burchette RJ, Siddiqi RA, Huen IT, Hadnott LL, Fishman A. The efficacy of silver‐ion implanted catheters in reducing peritoneal dialysis‐related infections. Peritoneal Dialysis International 2003;23(4):368‐74. [MEDLINE: ] [PubMed] [Google Scholar]
de Fijter 1992a {published data only}
- Fijter CW, Biemond A, Oe LP, Moesker HL, Verhoef J, Donker AJ, et al. Pharmacokinetics of ciprofloxacin after intraperitoneal administration in uninfected patients undergoing CCPD. Advances in Peritoneal Dialysis 1992;8:18‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Gadallah 2000 {published data only}
- Gadallah MF, Tamayo A, Sandborn M, Ramdeen G, Moles K. Role of intraperitoneal urokinase in acute peritonitis and prevention of catheter loss in peritoneal dialysis patients. Advances in Peritoneal Dialysis 2000;16:233‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Maiorca 1983 {published data only}
- Maiorca R, Cantaluppi A, Cancarini GC, Scalamogna A, Broccoli R, Graziani G, et al. Prospective controlled trial of a Y‐connector and disinfectant to prevent peritonitis in continuous ambulatory peritoneal dialysis. Lancet 1983;2(8351):642‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maiorca R, Cantaluppi A, Cancarini GC, Scalamogna A, Strada A, Graziani G, et al. 'Y' connector system for prevention of peritonitis in CAPD: a controlled study. Proceedings of the European Dialysis & Transplant Association 1983;20:223‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Naylor 1997 {published data only}
- Naylor M, Roe B. A study of the efficacy of dressings in preventing infections of continuous ambulatory peritoneal dialysis catheter exit sites. Journal of Clinical Nursing 1997;6(1):17‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oh 2000 {published data only}
- Klaus G, Baum H, Wuhl E, Schaefer F, European Pediatric Peritoneal Dialysis Study Group (EPPS). Efficacy of Mupirocin prophylaxis in reducing the incidence of peritoneal dialysis (PD)‐related Staphylococcus aureus infections in children on chronic PD: results of a double blind, placebo‐controlled trial [abstract]. Peritoneal Dialysis International 2002;22(1):149. [CENTRAL: CN‐00401508] [Google Scholar]
- Oh J, Baum H, Klaus G, Schaefer F. Nasal carriage of staphylococcus aureus in families of children on peritoneal dialysis. European Pediatric Peritoneal Dialysis Study Group (EPPS). Advances in Peritoneal Dialysis 2000;16:324‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Plum 1997a {published data only}
- Artic S, Busch T, Sahin K, Grabensee B, Plum J. Oral versus intraperitoneal application of clindamycin in tunnel infections: a prospective, randomized study in CAPD patients [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):260A‐1A. [PubMed] [Google Scholar]
- Plum J, Artik S, Busch T, Sahin K, Grabensee B. Oral versus intraperitoneal application of clindamycin in tunnel infections: a prospective, randomized study in CAPD patients. Peritoneal Dialysis International 1997;17(5):486‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Rodriguez‐Perez 1989 {published data only}
- Rodriguez‐Perez JC, Vega N, Plaza C, Fernandez A, Hortal L, Palop L. Prospective randomized trial with the use of antibiotic bonding to peritoneal catheters. A method to reduce tunnel and exit site infection in CAPD patients [abstract]. Kidney International 1989;36(1):154. [Google Scholar]
Thomae 1982 {published data only}
- Thomae U, Boos W, Adam D. Transperitoneal resorption of ampicillin, cefuroxim and gentamicin in continuous ambulatory peritoneal dialysis [Transperitoneale resorption von ampicillin, cefuroxim and gentamicin unter kontinuierlicher ambulanter peritonealdialyse]. Medizinische Welt 1982;33(5):182‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Trooskin 1990 {published data only}
- Trooskin SZ, Harvey RA, Lennard TW, Greco RS. Failure of demonstrated clinical efficacy of antibiotic‐bonded continuous ambulatory peritoneal dialysis (CAPD) catheters. Peritoneal Dialysis International 1990;10(1):57‐9. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
NCT02547103 {published data only}
- Ruengorn C. Efficacy and safety of local application of chlorhexidine gluconate versus mupirocin ointment in the prevention of peritoneal dialysis‐related infection: a pilot study, double‐ blind, stratified randomized controlled trial. www.clinicaltrials.gov/ct2/show/NCT02547103 (accessed 28 December 2016).
Additional references
Annigeri 2001
- Annigeri R, Conly J, Vas S, Dedier H, Prakashan K P, Bargman JM, et al. Emergence of mupirocin‐resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit‐site infection. Peritoneal Dialysis International 2001;21(6):554‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Campbell 2015
- Campbell DJ, Johnson DW, Mudge DW, Gallagher MP, Craig JC. Prevention of peritoneal dialysis‐related infections. Nephrology Dialysis Transplantation 2015;30(9):1461‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Canadian CAPD Clinical Trials Group 1989
- Anonymous. Peritonitis in continuous ambulatory peritoneal dialysis (CAPD): a multi‐centre randomized clinical trial comparing the Y connector disinfectant system to standard systems. Canadian CAPD Clinical Trials Group. Peritoneal Dialysis International 1989;9(3):159‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Cho 2014
- Cho Y, Johnson DW. Peritoneal dialysis‐related peritonitis: towards improving evidence, practices, and outcomes. American Journal of Kidney Diseases 2014;64(2):278‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 2004
- Choi P, Nemati E, Banerjee A, Preston E, Levy J, Brown E. Peritoneal dialysis catheter removal for acute peritonitis: a retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. American Journal of Kidney Diseases 2004;43(1):103‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chow 2005
- Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis‐related peritonitis. Peritoneal Dialysis International 2005;25(4):374‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Churchill 1997
- Churchill DN, Thorpe KE, Vonesh EF, Keshaviah PR. Lower probability of patient survival with continuous peritoneal dialysis in the United States compared with Canada. Canada‐USA (CANUSA) Peritoneal Dialysis Study Group. Journal of the American Society of Nephrology 1997;8(6):965‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collier 2008
- Collier M, Evans D, Farrington M, Gibbs E, Gould K, Jenkinson H, et al. Surgical site infection: prevention and treatment. Clinical guideline [CG74], 2008. www.nice.org.uk/guidance/cg74 (accessed 28 December 2016):1‐31.
Daly 2001
- Daly CD, Campbell MK, MacLeod AM, Cody DJ, Vale LD, Grant AM, et al. Do the Y‐set and double‐bag systems reduce the incidence of CAPD peritonitis? A systematic review of randomized controlled trials. Nephrology Dialysis Transplantation 2001;16(2):341‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dasgupta 1994
- Dasgupta MK. Silver peritoneal catheters reduce bacterial colonization. Advances in Peritoneal Dialysis 1994;10:195‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Davies 1989
- Davies SJ, Ogg CS, Cameron JS, Poston S, Noble WC. Staphylococcus aureus nasal carriage, exit‐site infection and catheter loss in patients treated with continuous ambulatory peritoneal dialysis (CAPD). Peritoneal Dialysis International 1989;9(1):61‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Digenis 1990
- Digenis GE, Abraham G, Savin E, Blake P, Dombros N, Sombolos K, et al. Peritonitis‐related deaths in continuous ambulatory peritoneal dialysis (CAPD) patients. Peritoneal Dialysis International 1990;10(1):45‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Farias 1994
- Farias MG, Soucie JM, McClellan W, Mitch WE. Race and the risk of peritonitis: an analysis of factors associated with the initial episode. Kidney International 1994;46(5):1392‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Figueiredo 2010
Fine 1994
- Fine A, Cox D, Bouw M. Higher incidence of peritonitis in native Canadians on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 1994;14(3):227‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Fried 1996
- Fried LF, Bernardini J, Johnston JR, Piraino B. Peritonitis influences mortality in peritoneal dialysis patients. Journal of the American Society of Nephrology 1996;7(10):2176‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fung 1996
- Fung LC, Khoury AE, Vas SI, Smith C, Oreopoulos DG, Mittelman MW. Biocompatibility of silver‐coated peritoneal dialysis catheter in a porcine model. Peritoneal Dialysis International 1996;16(4):398‐405. [MEDLINE: ] [PubMed] [Google Scholar]
Ghali 2011
- Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Peritoneal Dialysis International 2011;31(6):651‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Golper 1996
- Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, et al. Risk factors for peritonitis in long‐term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 Peritonitis and Catheter Survival Studies. American Journal of Kidney Diseases 1996;28(3):428‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heaf 2004
- Heaf J. Underutilization of peritoneal dialysis. JAMA 2004;291(6):740‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HICPAC 1995
- Hospital Infection Control Practices Advisory Committee (HICPAC). Recommendations for preventing the spread of vancomycin resistance.[Erratum appears in Infect Control Hosp Epidemiol 1995 Sep;16(9):498]. Infection Control & Hospital Epidemiology 1995;16(2):105‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holley 1993
- Holley JL, Bernardini J, Piraino B. A comparison of peritoneal dialysis‐related infections in black and white patients. Peritoneal Dialysis International 1993;13(1):45‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Jaar 2009
- Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrology 2009;10:3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2012
- Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. Journal of the American Society of Nephrology 2012;23(3):533‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2014
- Johnson DW, Badve SV, Pascoe EM, Beller E, Cass A, Clark C, et al. Antibacterial honey for the prevention of peritoneal‐dialysis‐related infections (HONEYPOT): a randomised trial. Lancet Infectious Diseases 2014;14(1):23‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keane 2000
- Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, et al. Adult peritoneal dialysis‐related peritonitis treatment recommendations: 2000 update.[Erratum appears in Perit Dial Int 2000 Nov‐Dec;20(6):828‐9]. Peritoneal Dialysis International 2000;20(4):396‐411. [MEDLINE: ] [PubMed] [Google Scholar]
Lim 2005
- Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non‐Aboriginal patients with end‐stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology 2005;10(2):192‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lloyd 2013
- Lloyd A, Tangri N, Shafer LA, Rigatto C, Perl J, Komenda P, et al. The risk of peritonitis after an exit site infection: a time‐matched, case‐control study. Nephrology Dialysis Transplantation 2013;28(7):1915‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Peritoneal Dialysis International 2004;24(4):340‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Moher 1998
Morton 2011
- Morton RL, Tong A, Webster AC, Snelling P, Howard K. Characteristics of dialysis important to patients and family caregivers: a mixed methods approach. Nephrology Dialysis Transplantation 2011;26(12):4038‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nessim 2009
- Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(7):1195‐200. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolph 1987
- Nolph KD, Cutler SJ, Steinberg SM, Novak JW, Hirschman GH. Factors associated with morbidity and mortality among patients on CAPD. ASAIO Transactions 1987;33(2):57‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oxton 1994
- Oxton LL, Zimmerman SW, Roecker EB, Wakeen M. Risk factors for peritoneal dialysis‐related infections. Peritoneal Dialysis International 1994;14(2):137‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Perez‐Fontan 1993
- Perez‐Fontan M, Garcia‐Falcon T, Rosales M, Rodriguez‐Carmona A, Adeva M, Rodriguez‐Lozano I, et al. Treatment of Staphylococcus aureus nasal carriers in continuous ambulatory peritoneal dialysis with mupirocin: long‐term results. American Journal of Kidney Diseases 1993;22(5):708‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piraino 1989
- Piraino B, Bernardini J, Sorkin M. Catheter infections as a factor in the transfer of continuous ambulatory peritoneal dialysis patients to hemodialysis. American Journal of Kidney Diseases 1989;13(5):365‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piraino 1990
- Piraino B. A review of Staphylococcus aureus exit‐site and tunnel infections in peritoneal dialysis patients. American Journal of Kidney Diseases 1990;16(2):89‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piraino 1998
- Piraino B. Peritonitis as a complication of peritoneal dialysis. Journal of the American Society of Nephrology 1998;9(10):1956‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piraino 2000
- Piraino B. Staphylococcus aureus infections in dialysis patients: focus on prevention. ASAIO Journal 2000;46(6):S13‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piraino 2002
- Piraino B. ADEMEX: how should it change our practice? Adequacy of peritoneal dialysis in Mexico. Peritoneal Dialysis International 2002;22(5):552‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Piraino 2011
- Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD position statement on reducing the risks of peritoneal dialysis‐related infections. Peritoneal Dialysis International 2011;31(6):614‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salusky 1997
- Salusky IB, Holloway M. Selection of peritoneal dialysis for pediatric patients. Peritoneal Dialysis International 1997;17 Suppl 3:S35‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Schaefer 2003
- Schaefer F. Management of peritonitis in children receiving chronic peritoneal dialysis. Paediatric Drugs 2003;5(5):315‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sutherland 1985
- Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrobial Agents & Chemotherapy 1985;27(4):495‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thodis 2000
- Thodis E, Passadakis P, Panagoutsos S, Bacharaki D, Euthimiadou A, Vargemezis V. The effectiveness of mupirocin preventing Staphylococcus aureus in catheter‐related infections in peritoneal dialysis. Advances in Peritoneal Dialysis 2000;16:257‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Troidle 1998
- Troidle L, Gorban‐Brennan N, Kliger A, Finkelstein F. Differing outcomes of gram‐positive and gram‐negative peritonitis. American Journal of Kidney Diseases 1998;32(4):623‐8. [9774124] [DOI] [PubMed] [Google Scholar]
Troidle 2003
- Troidle L, Watnick S, Wuerth DB, Gorban‐Brennan N, Kliger AS, Finkelstein FO. Depression and its association with peritonitis in long‐term peritoneal dialysis patients. American Journal of Kidney Diseases 2003;42(2):350‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Biesen 2014
- Biesen W, Jorres A. Medihoney: let nature do the work?. Lancet Infectious Diseases 2014;14(1):2‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Diepen 2012
- Diepen AT, Tomlinson GA, Jassal SV. The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(8):1266‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2014
Ward 1986
- Ward RA, Chang BS. Systems and devices used for peritoneal dialysis. Medical Instrumentation 1986;20(2):85‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Woodrow 1997
- Woodrow G, Turney JH, Brownjohn AM. Technique failure in peritoneal dialysis and its impact on patient survival. Peritoneal Dialysis International 1997;17(4):360‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Woodrow 2010
- Woodrow G, Davies S. Peritoneal dialysis in CKD. 2010. www.renal.org/guidelines/modules/peritoneal‐dialysis‐in‐ckd#sthash.gkEx1Y5v.dpbs (accessed 28 December 2016).
References to other published versions of this review
Strippoli 2003
- Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Anti‐infective (antiseptics and antibiotics) agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004679] [DOI] [PubMed] [Google Scholar]
Strippoli 2004a
- Strippoli GF, Tong A, Johnson DW, Schena FP, Craig JC. Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004679.pub2] [DOI] [PubMed] [Google Scholar]
Strippoli 2004b
- Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Antimicrobial agents to prevent peritonitis in peritoneal dialysis: A systematic review of randomized controlled trials. American Journal of Kidney Diseases 2004;44(4):591‐603. [MEDLINE: ] [PubMed] [Google Scholar]


