Abstract
Background
Rapid implementation of robotic transabdominal surgery has resulted in the need for re‐evaluation of the most suitable form of anaesthesia. The overall objective of anaesthesia is to minimize perioperative risk and discomfort for patients both during and after surgery. Anaesthesia for patients undergoing robotic assisted surgery is different from anaesthesia for patients undergoing open or laparoscopic surgery; new anaesthetic concerns accompany robotic assisted surgery.
Objectives
To assess outcomes related to the choice of total intravenous anaesthesia (TIVA) or inhalational anaesthesia for adults undergoing transabdominal robotic assisted laparoscopic gynaecological, urological or gastroenterological surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016 Issue 5), Ovid MEDLINE (1946 to May 2016), Embase via OvidSP (1982 to May 2016), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1982 to May 2016) and the Institute for Scientific Information (ISI) Web of Science (1956 to May 2016). We also searched the International Standard Randomized Controlled Trial Number (ISRCTN) Registry and Clinical trials gov for ongoing trials (May 2016).
Selection criteria
We searched for randomized controlled trials (RCTs) including adults, aged 18 years and older, of both genders, treated with transabdominal robotic assisted laparoscopic gynaecological, urological or gastroenterological surgery and focusing on outcomes of TIVA or inhalational anaesthesia.
Data collection and analysis
We used standard methodological procedures of Cochrane. Study findings were not suitable for meta‐analysis.
Main results
We included three single‐centre, two‐arm RCTs involving 170 participants. We found one ongoing trial. All included participants were male and were undergoing radical robotic assisted laparoscopic radical prostatectomy (RALRP). The men were between 50 and 75 years of age and met criteria for American Society of Anesthesiologists physical classification scores (ASA) I, ll and III.
We found evidence showing no clinically meaningful differences in postoperative pain between the two types of anaesthetics (mean difference (MD) in visual analogue scale (VAS) scores at one to six hours was ‐2.20 (95% confidence interval (CI) ‐10.62 to 6.22; P = 0.61) in a sample of 62 participants from one study. Low‐quality evidence suggests that propofol reduces postoperative nausea and vomiting (PONV) over the short term (one to six hours after surgery) after RALRP compared with inhalational anaesthesia (sevoflurane, desflurane) (MD ‐1.70, 95% CI ‐2.59 to ‐0.81; P = 0.0002).
We found low‐quality evidence suggesting that propofol may prevent an increase in intraocular pressure (IOP) after pneumoperitoneum and steep Trendelenburg positioning compared with sevoflurane (MD ‐3.90, 95% CI ‐6.34 to ‐1.46; P = 0.002) with increased IOP from baseline to 30 minutes in steep Trendelenburg. However, it is unclear whether this surrogate outcome translates directly to clinical avoidance of ocular complications during surgery. No studies addressed the secondary outcomes of adverse effects, all‐cause mortality, respiratory or circulatory complications, cognitive dysfunction, length of stay or costs. Overall the quality of evidence was low to very low, as all studies were small, single‐centre trials providing unclear descriptions of methods.
Authors' conclusions
It is unclear which anaesthetic technique is superior ‐ TIVA or inhalational ‐ for transabdominal robotic assisted surgery in urology, gynaecology and gastroenterology, as existing evidence is scarce, is of low quality and has been generated from exclusively male patients undergoing robotic radical prostatectomy.
An ongoing trial, which includes participants of both genders with a focus on quality of recovery, might have an impact on future evidence related to this topic.
Plain language summary
Intravenous or inhalational anaesthesia for abdominal surgery assisted by a computerized surgical robot
This review assessed anaesthesia provided for computerized robotic assisted surgery in the abdomen via the veins or by breathing into the lungs (inhalation).
Review question
What are the advantages and disadvantages of using anaesthesia delivered via the veins compared with anaesthesia delivered by the lungs for robotic assisted abdominal surgery with a focus on managing pain, nausea and vomiting, adverse effects and associated costs?
Background
Computerized robotic assisted surgery in the abdomen is associated with reduced bleeding and less surgical trauma because incisions are small. With rapidly increasing use of computerized robotic assisted surgery has come the need to re‐evaluate the two main types of anaesthesia administration. Robotic surgery demands different positioning of the patient during the procedure and introduction of carbon dioxide gas into the abdominal cavity to create a better overview. These factors may affect the functioning heart and lungs and may alter pressure in the brain and in the eyes, with accompanying risk of adverse effects.
Study characteristics
The evidence is current to May 2016 and was provided by three small randomized controlled trials involving 170 males who had their prostate removed. These studies took place in hospitals in Korea and Turkey. We looked for differences in pain and postoperative nausea and vomiting (PONV) and changes in pressure inside the eyes between patients receiving anaesthetic via the veins or by inhalation.
Study funding sources
Studies were funded by the institution, or the funding sources were not stated.
Key results
Postoperative pain was no different between the two types of anaesthesia. Anaesthesia delivered through the veins reduced PONV at one to six hours after prostate surgery compared with anaesthesia provided via inhalation. One study showed that anaesthesia delivered through the veins prevented an increase in pressure inside the eyes during surgery compared with inhalational anaesthesia. We cannot conclude whether this means that anaesthesia provided via the veins reduces ocular complications, as no patients in this small study developed these complications.
Quality of the evidence
The quality of the evidence was considered low, as included studies were small and provided an unclear description of methods. All participants in these studies were men who were undergoing robotic assisted laparoscopic removal of the prostate. An ongoing trial includes men and women undergoing abdominal robotic surgery, also with a focus on the quality of recovery. Additional studies are needed.
Summary of findings
Summary of findings for the main comparison. Inhalational anaesthesia compared with intravenous anaesthesia for adults undergoing transabdominal robotic assisted laparoscopic surgery.
| Inhalational anaesthesia compared with intravenous anaesthesia for adults undergoing transabdominal robotic assisted laparoscopic surgery | ||||||
| Patient or population: adults undergoing transabdominal robotic assisted laparoscopic surgery Setting: Training and Research Hospital in Turkey and Univerity Hospital in South Korea Intervention: inhalational anaesthesia Comparison: intravenous anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with intravenous anaesthesia | Risk with inhalational anaesthesia | |||||
| Pain in the PACU (VAS) | Mean pain in the PACU (VAS) was 42.9 | MD 1 lower (9.82 lower to 7.82 higher) | ‐ | 62 (1 RCT) | ⊕⊕⊝⊝ LOWa | VAS 0 to 100, with higher score indicating greater pain |
| Pain at 1 to 6 hours (VAS) | Mean pain from 1 to 6 hours (VAS) was 42.5 | MD 2.2 lower (10.62 lower to 6.22 higher) | ‐ | 62 (1 RCT) | ⊕⊕⊝⊝ LOWa | VAS 0 to 100, with higher score indicating greater pain |
| Pain at 6 to 24 hours (VAS) | Mean pain from 6 to 24 hours (VAS) was 34.5 | MD 1 lower (7.51 lower to 5.51 higher) | ‐ | 62 (1 RCT) | ⊕⊕⊝⊝ LOWa | VAS 0 to 100, with higher score indicating greater pain |
| Presence of nausea | Study population | RR 0.39 (0.19 to 0.80) | 62 (1 RCT) | ⊕⊕⊝⊝ LOWa | ||
| 581 per 1000 | 226 per 1000 (110 to 465) | |||||
| Nausea intensity (VNRS) in PACU | Mean nausea intensity (VNRS) in PACU was 0.2 | MD 0.7 lower (1.35 lower to 0.05 lower) | ‐ | 62 (1 RCT) | ⊕⊕⊝⊝ LOWa | VNRS 0 to 10: 1 to 3 = mild, 4 to 6 = moderate, 7 to 10 = severe, or with retching and vomition |
| Nausea intensity (VNRS) at 1 to 6 hours | Mean nausea intensity (VNRS) at 1 to 6 hours was 0.4 | MD 1.7 lower (2.59 lower to 0.81 lower) | ‐ | 62 (1 RCT) | ⊕⊕⊝⊝ LOWa | VNRS 0 to 10: 1 to 3 = mild, 4 to 6 = moderate, 7 to 10 = severe, or with retching and vomition |
| Nausea‐vomiting rate at 1 hour | Study population | RR 23.00 (1.44 to 366.71) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Nausea‐vomiting rate at 3 hours | Study population | RR 2.00 (0.20 to 20.41) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 48 per 1000 | 95 per 1000 (10 to 972) | |||||
| Adverse effects: increase in IOP during maintenance of anaesthesia | Mean Increase in IOP during maintenance of anaesthesia was 2.1 | MD 3.9 lower (6.34 lower to 1.46 lower) | ‐ | 66 (1 RCT) | ⊕⊕⊝⊝ LOWa,d | No participants experienced ocular complications No included study reported other adverse events of interest |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; OR: odds ratio; RR: risk ratio; VAS: visual analogue scale; VNRS: verbal numerical rating scale. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aFew participants.
bUnclear allocation concealment and blinding.
cPower calculation for the study performed on mean difference in pH between groups and minimal relevant difference not stated; primary outcomes: heart rate, mean atrial pressure and values for oxygen and carbon dioxide.
dAddresses a restricted version of the review question.
Background
Description of the condition
The introduction of robotic assisted transabdominal surgery has resulted in the need for re‐evaluation of the most suitable form of anaesthesia. The objective of this approach is to minimize perioperative risk and discomfort for patients both during and after surgery. Anaesthesia for patients undergoing robotic assisted surgery is different from anaesthesia for patients undergoing open or laparoscopic surgery, and new anaesthetic concerns accompany robotic surgery. These concerns include physiological effects of fixed extreme positioning of the patient over a long time, pneumoperitoneum, restricted access to the patient during surgery and the need for carefully monitored relaxation of the patient. Regardless of the method of surgery performed, it is universal that the three anaesthetic qualities ‐ sleep, freedom from pain and muscle relaxation ‐ generally can be attained by two methods.
Total intravenous anaesthesia (TIVA), in which the patient receives all anaesthetic drugs through an intravenous line.
Inhalational anaesthesia, by which one of the drugs is delivered through the lungs.
This review addresses the advantages and disadvantages of TIVA compared with inhalational anaesthesia in transabdominal robotic assisted surgery.
Anaesthetists advocating for TIVA might highlight the lower risks of postoperative nausea and vomiting (PONV) and of pollution of the operating room and environment compared with anaesthesia gas (Vari 2010). However, those advocating for inhalational anaesthesia might focus on easier monitoring and regulation of delivered anaesthesia, faster emergence from anaesthesia and, although controversial in non‐cardiac surgery, the proposed cardioprotective effects of inhalational anaesthesia (Landoni 2009). How these circumstances influence patients in the context of transabdominal robotic assisted surgery must be considered.
Two issues in transabdominal robotic assisted surgery that might influence outcomes of anaesthesia are positioning and pneumoperitoneum. Extreme positioning, for example, steep Trendelenburg (in lower abdominal surgery) or steep anti‐Trendelenburg (in upper abdominal surgery), and insufflation of carbon dioxide in the abdominal cavity affect the patient's cardiovascular and respiratory systems, as well as intracerebral pressure. At the same time, the patient’s normal compensatory mechanisms are blunted by the anaesthesia. Absorption of carbon dioxide over abdominal tissue membranes, as well as upward pressure on the diaphragm and compromised venous return, is a constant challenge for the patient's respiratory and circulatory systems. These physiological disturbances are tolerated differently by patients according to their age, body mass index (BMI), medications and comorbidity. Furthermore, pneumoperitoneum is shown to be associated with multiple cardiovascular changes during laparoscopy, which may be exacerbated during steep Trendelenburg positioning, as reported in a study from 2007 (Danic 2007). The same study showed 68% decreased pulmonary compliance due to chest binding, steep 45‐degree Trendelenburg and high insufflation (Danic 2007).
Intravenous (IV) fluid administration during surgery may also influence the level of PONV (Voldby 2016) and may play a specific role in development of ischaemic optic neuropathy (ION) (Kan 2015).
Restricted access to the patient during often lengthy robotic assisted surgery demands careful preparation of IV lines and monitoring equipment, as well as focus on avoiding nerve damage and ocular injury (Awad 2012; Danic 2007; Kakar 2011; Sullivan 2008).
Refinement of surgical conditions for transabdominal robotic assisted surgery with reduced bleeding and potential advantages of lower rates of surgical trauma due to minor incisions make it possible to consider robotic assisted surgery for a broader patient population. Some of the patients subjected to robotic assisted surgery may be considered inoperable by traditional operating techniques, especially the growing patient population with a very high BMI (Stone 2010). This additional aspect of change in patient selection has to be considered when the type of anaesthesia is selected.
Robotic assisted surgery is used increasingly for treatment of patients with cancer in gynaecology (Mettler 2008), in urology (Sohn 2013) and in gastrointestinal oncology (Mak 2014; Papanikolaou 2014). Anaesthesia for robotic assisted surgery in the treatment of cancer involves another aspect of the choice of anaesthetic, namely, potentially different effects of inhalational agents and TIVA on the immune system, thereby influencing cancer recurrence (Fodale 2014). It is suspected that (particular) inhaled anaesthetics might be potentially genotoxic (Fodale 2014).
Use of robotic assisted surgery is expanding rapidly in the developed world. At present, two manufacturers provide the robotic surgical systems now available on the market (Paranjape 2014). One such system is the Zeus Surgical System (Computer Motion), and the other is the da Vinci Surgical System (Intuitive Surgical). The latter manufacturer claims that since 2000, the da Vinci Surgical System has been used in more than three million minimally invasive procedures performed worldwide (da Vinci 2016). The high cost and increasing volume of robotic assisted surgeries have implications for public health (Barbash 2010), as health services have limited budgets, and limited evidence has been provided by large multi‐centre randomized controlled trials (RCTs) to show which patients may benefit from robotic assisted surgery as opposed to conventional surgery (Gurusamy 2012; Liu 2014). Outcomes such as shorter length of stay, less postoperative pain, fewer episodes of PONV, faster recovery and return to habitual functioning after anaesthesia and surgery are of great importance for public health.
This review sought to compare two different anaesthesia techniques for transabdominal robotic assisted surgery with focus on postoperative patient comfort (pain, PONV, cardiovascular and respiratory complications), as well as patient safety.
Description of the intervention
For maintenance of anaesthesia, TIVA or a halogenated inhalational agent may be used in combination with intravenous drugs for a painless operation. Drugs used to keep the patient pain free are mainly opioid‐based but might also include a secondary analgesic or a regional anaesthetic administered as a central nervous blockade (Gerges 2006).
This review focused on the applicability of general anaesthesia provided for transabdominal robotic assisted surgery through intravenous infusion of a hypnotic and an analgesic agent compared with a halogenated inhalational agent together with an analgesic. For this review, we did not consider level of relaxation, combination with regional anaesthesia nor local infiltration. Instead, we focused on different aspects of delivery of these two types of anaesthesia regimens and included measures of pain, PONV, cardiovascular and pulmonary impact, effects on cerebral autoregulation, cognitive function, practical aspects, controllability and environmental effects.
How the intervention might work
Differences between the two types of anaesthesia that we have studied can be seen in their physiological properties and in practical delivery of these drugs. Although delivery of anaesthesia gas is easily monitored via the ventilator, pumps and intravenous lines associated with TIVA may be associated with error through accidental disconnection; furthermore, no direct feedback mechanism has been developed to monitor delivery. It is important to consider cardiovascular stability when drugs used for anaesthesia are selected for transabdominal robotic assisted surgery, during which patients are in non‐physiological positions such as the steep head‐down (Trendelenburg) or the steep head‐up (anti‐Trendelenburg) position for a long time. Traditionally, anaesthesia gas is considered to have a better safety profile than TIVA‐based anaesthesia in patients with cardiovascular morbidity (Landoni 2009) and may have cardioprotective properties during cardiac surgery (Hert 2003), although this might be controversial in cases of non‐cardiac surgery (Landoni 2009). For obese patients undergoing surgery such as knee arthroscopy, transurethral resection of the prostate or minor breast or hand surgery, inhalational anaesthetics (desflurane) may have a safer profile than propofol with regards to postoperative pulmonary function. This has been measured by surrogate outcomes such as oxyhaemoglobin saturation and lung function (Zoremba 2011). The combination of extreme positioning and pneumoperitoneum during most transabdominal robotic assisted procedures is known to affect intracerebral pressure (Kalmar 2010). For this reason, it is important that anaesthesia provided for this type of surgery preserves cerebral autoregulatory mechanisms to prevent cerebral events and postoperative cognitive dysfunction. Comparative studies of the use of sevoflurane versus propofol‐remifentanil for spinal or maxillofacial surgery have reported that propofol preserves cerebral autoregulation, but this is not the case when sevoflurane is used in higher concentrations (Conti 2006). Cerebral vascular responsiveness to carbon dioxide (CO2) is impaired during sevoflurane anaesthesia but not during anaesthesia with propofol‐remifentanil (Conti 2006).
Why it is important to do this review
Robotic assisted techniques are used increasingly for a wide variety of surgical specialities in the developed world (Barbash 2010). It is imperative that outcomes of anaesthesia for transabdominal robotic assisted surgery are known. Advantages and disadvantages must be clear to both clinicians and patients, so they can choose the most appropriate anaesthesia technique. At present, we know of isolated RCTs conducted to address this clinical question in specialized areas of the field (gynaecology, urology or gastroenterology) (Atallah 2009; Yoo 2012a; Yoo 2014a). We expect that this body of evidence will expand over the next few years, as several ongoing trials have been registered at http://clinicaltrials.gov/. (See Ongoing studies).
Objectives
To assess outcomes related to the choice of total intravenous anaesthesia (TIVA) or Inhalational anaesthesia for adults undergoing transabdominal robotic assisted laparoscopic gynaecological, urological or gastroenterological surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) conducted in any clinical or research setting in which management of anaesthesia for transabdominal robotic assisted laparoscopic surgery was the intervention.
Types of participants
We included RCTs with adult participants aged 18 years and older, of both genders, undergoing transabdominal robotic assisted gynaecological, urological or gastroenterological surgery.
Types of interventions
We included RCTs that provided the following interventions during transabdominal robotic assisted surgery.
TIVA (propofol and opioid‐based) versus inhalation‐based anaesthesia (isoflurane, desflurane or sevoflurane in combination with an opioid).
Types of outcome measures
Primary outcomes
Postoperative pain within 24 hours (as measured by the authors of included studies)
PONV within 24 hours (as measured by the authors of included studies)
Secondary outcomes
Adverse effects (as measured by the authors of included studies), for example, cerebral oedema, stroke and ocular complications (including changes in intraocular pressure as proxy for ocular complications)
All‐cause mortality within 90 days
Respiratory complications requiring treatment within 48 hours (as measured by the authors of included studies)
Circulatory complications requiring treatment within 48 hours (as measured by the authors of included studies)
Cognitive dysfunction (as measured by the authors of included studies)
Length of stay in the postoperative ward (as measured by the authors of included studies)
Costs (as measured by the authors of included studies)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016 Issue 5), Ovid MEDLINE (1946 to 17 May 2016), Embase via OvidSP (1982 to 17 May 2016), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1982 to 17 May 2016) and the Institute for Scientific Information (ISI) Web of Science (1956 to 17 May 2016).
We adapted our MEDLINE search strategy (Appendix 2) for searches of other databases. Search terms consisted of a combination of thesaurus‐based and free text terms for the interventions.
We imposed no language restrictions or limitations related to publication status.
Searching other resources
We searched the following resources in May 2016.
International Standard Randomized Controlled Trial Number (ISRCTN) Registry (http://www.controlled‐trials.com/).
ClinicalTrials.gov (http://www.clinicaltrials.gov/).
Data collection and analysis
Selection of studies
We have summarized the initial search for literature in the PRISMA study flow diagram (Figure 1).
1.
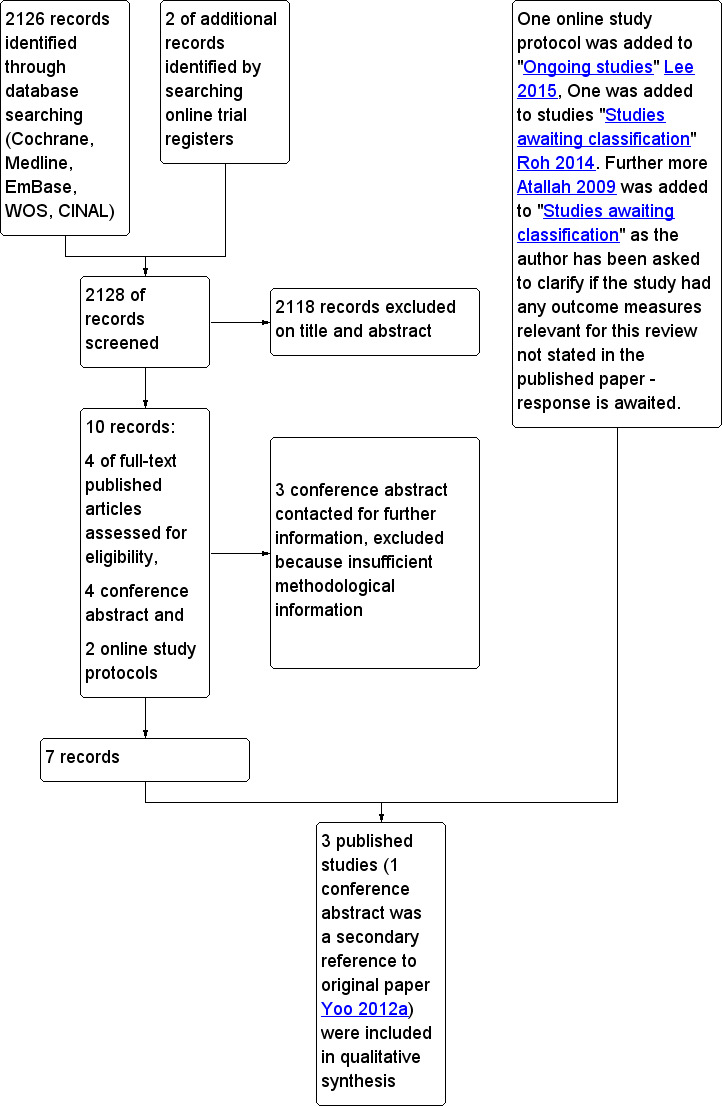
Study flow diagram.
We (SH and BD) reviewed titles and abstracts to begin to determine whether studies fulfilled the inclusion criteria. We (SH and BD) assessed the full‐text articles of eligible studies to decide if they were relevant for inclusion. We (SH and BD) resolved disagreements by discussion with a third review author (AM). If we found missing information or inconsistencies in the studies, we (SH) contacted the authors of those studies.
Data extraction and management
We (SH and BD) extracted data from published papers and from reports of original researchers. We (SH and BD) used a modified version of the Cochrane Anaesthesia, Emergency and Critical Care Group (ACE) data extraction form (see Appendix 6). This form includes data on participants, risk of bias, methods of randomization, blinding, reporting of outcomes, results and applicability. We recorded this information in the Characteristics of included studies, Characteristics of excluded studies and 'Risk of bias' sections of the review. We (SH and BD) resolved disagreements by discussion with a third review author (AM). We (SH) entered extracted data into Review Manager 5 (RevMan 5.3) for analysis and (BD) checked the accuracy of these data.
Assessment of risk of bias in included studies
We (SH and BD) independently assessed risk of bias in studies to be included using the criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We reported on the following domains.
Adequate sequence generation.
Allocation concealment.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other bias (e.g. baseline imbalances).
We (SH and BD) graded studies as having 'low risk', 'high risk' or 'unclear risk' of bias and resolved disagreements by discussion with a third review author (AM). We (SH and BD) created plots of risk of bias in Review Manager (RevMan 5.3).
We presented results in the 'Risk of bias' summary of review authors' judgements about each risk of bias item presented as percentages across all included studies (Figure 2) and in the 'Risk of bias' summary of review authors' judgements about each risk of bias item for each included study (Figure 3).
2.
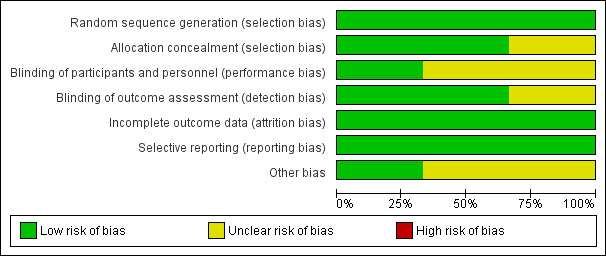
Risk of bias summary: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
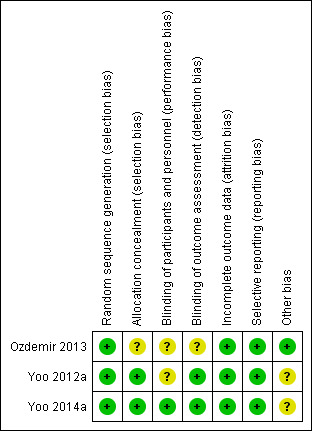
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We reported results in absolute numbers and percentages and as mean values with standard deviations (SDs) (see Differences between protocol and review section).
Unit of analysis issues
The unit of analysis consisted of participants included and randomly assigned. We included no cross‐over trials and no cluster‐randomized trials (see Differences between protocol and review section).
Dealing with missing data
We noted level of attrition in included studies and encountered no reports of missing data (see Differences between protocol and review section).
Assessment of heterogeneity
We did not assess heterogeneity statistically (see Differences between protocol and review section).
Assessment of reporting biases
When possible, we assessed within study reporting bias by seeking online protocols and comparing them with published studies. As we retrieved fewer than 10 studies, funnel plots were not relevant.
Data synthesis
We planned to use a fixed‐effect model if we identified moderate heterogeneity (I2 value 30% to 60%). If we identified substantial heterogeneity, (I2 value 50% to 90%), we would use a random‐effects model. We would analyse all statistics using Review Manager (RevMan 5.3). Further, we planned that if we identified considerable heterogeneity (I2 value 75% to 100%), we would not pool the data. We would instead summarize studies, present information in tables and describe data qualitatively. We would consider evidence of heterogeneity significant if we found P < 0.05 for the Chi2 test and 95% confidence intervals (CIs) for the I2 statistic. We did not test for heterogeneity in this review.
Included studies showed clinical diversity (clinical heterogeneity), as they focused on different outcomes and revealed variability in design and risk of bias (methodological diversity). Statistical heterogeneity covers variability in intervention effects (Higgins 2011), which we were not able to measure from data presented in the studies (see Table 1).
Differences in how outcomes are defined and measured are expected to lead to differences in observed effects of interventions, and as we have included only three studies, our investigation of heterogeneity is of questionable value (Higgins 2011). (See Differences between protocol and review.)
Subgroup analysis and investigation of heterogeneity
Originally, we planned to perform subgroup analysis, as the following subgroups might have outcomes different from those of the remaining population. Groups included gynaecology patients; urology patients; gastroenterology patients; and patients positioned with head up. However, this was not relevant, as included studies were homogenic in terms of participants ‐ all were men included in the urology group (see Differences between protocol and review).
Sensitivity analysis
We had planned that if we identified sufficient studies, we would perform a sensitivity analysis by comparing results with both inclusion and exclusion of RCTs classified as having 'low risk of bias', so we could decide whether our conclusions were robust (Higgins 2011). However, we found too few studies to do this (see Differences between protocol and review).
'Summary of findings' table and GRADE
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with the following specific outcomes.
Postoperative pain within 24 hours (as measured by the authors of included studies).
PONV within 24 hours (as measured by the authors of included studies).
Adverse effects (as measured by the authors of included studies) (e.g. cerebral oedema, stroke, ocular complications, including changes in intraocular pressure as proxy for ocular complications).
All‐cause mortality within 90 days.
Respiratory complications requiring treatment within 48 hours (as measured by the authors of included studies).
Circulatory complications requiring treatment within 48 hours (as measured by the authors of included studies).
Costs (as measured by the authors of included studies).
For our review, we constructed a 'Summary of findings' table using GRADE software. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessment of the quality of a body of evidence takes into consideration within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Guyatt 2008).
Results
Description of studies
Results of the search
We identified 2126 records through the database search, and we found two relevant ongoing studies in online trial databases. We excluded a total of 2118 records on review of titles and abstracts. During the database search, we found four relevant public conference abstracts (Calza 2011a; Calza 2011b; Choi 2013; Yoo 2012b). One study had been presented at two conferences (Calza 2011a; Calza 2011b); one (Yoo 2012b) was related to an included paper (Yoo 2012a); and one was provided only as a conference abstract (Choi 2013).
We contacted all authors of relevant abstracts (not duplicated in any paper) to request further information on their studies (Calza 2011a; Calza 2011b; Choi 2013) and to assess progress and publication status (Figure 1). We excluded all three of these, as study authors provided insufficient information for assessment of the quality of evidence. We excluded a fourth (Yoo 2012b) because it was already duplicated in the included published paper (Yoo 2012a).
Online, we discovered two ongoing topic‐relevant studies and included them in the review (Figure 1) as ongoing studies. We contacted the authors of the two ongoing studies to obtain up‐to‐date information on progress and publication status (Lee 2015; Roh 2014). We described Roh 2014 in the Studies awaiting classification section, and Lee 2015 in the Ongoing studies section.
Through the search, we identified four eligible studies, and we retrieved these four studies as full text (Figure 1). Atallah 2009 reported outcome measures that were not relevant for inclusion in the present review, as they described haemo‐respiratory dynamics, oxygenation and biochemical variables. We contacted study authors to ask it they had measured other outcomes relevant to this review. As we await their response, we have characterized this study as 'Awaiting classification'.
Included studies
We included three studies in this review (Ozdemir 2013; Yoo 2012a; Yoo 2014a) (see Characteristics of included studies). Yoo 2012a was reported in an original paper and as a conference proceeding (Yoo 2012b).
Design
The three included studies were single‐centre, two‐arm RCTs that were conducted in Korea (Yoo 2012a; Yoo 2014a) and in Turkey (Ozdemir 2013), respectively. They were conducted between 2010 (Yoo 2012a; Yoo 2014a) and 2012 (Ozdemir 2013). All three studies evaluated TIVA versus inhalational anaesthesia in men undergoing robotic assisted laparoscopic radical prostatectomy (RALRP) (Ozdemir 2013; Yoo 2012a; Yoo 2014a).
We found no RCTs in gynaecology or gastroenterology relevant for inclusion in this review.
Funding
Funding sources were the department (Yoo 2014a) or the hospital (Yoo 2012a) or were not stated (Ozdemir 2013).
Participants
The three studies included a total of 170 participants ‐ all men: 42 (Ozdemir 2013), 62 (Yoo 2012a) and 66 (Yoo 2014a), respectively. American Society of Anesthesiologists physical classification scores (ASA) were I to II (Yoo 2012a; Yoo 2014a) and l to lll (Ozdemir 2013). Participants' ages ranged from 50 to 70 years (Yoo 2012a; Yoo 2014a), and from 50 to 75 years (Ozdemir 2013). The indication for RALRP was not directly stated, but we assume it was early‐stage prostate cancer, as this is the conventional surgery for clinically localized cancer (Sampat 2015).
Exclusion criteria in the included studies varied according to the outcomes of interest. Ozdemir 2013 excluded men with neurological or psychological disease and severe cardiovascular disease, Yoo 2012a excluded participants with a history of motion sickness/PONV, use of antiemetics 24 hours before surgery, regular use of corticosteroids, chemotherapy within the previous four weeks, radiotherapy within the previous eight weeks, hepatic dysfunction, confirmed renal impairment and obesity with BMI > 35 kg/m2.
One study excluded men with baseline intraocular pressure (IOP) > 30 mmHg, diabetic retinopathy, cataract, uncontrolled hypertension, unstable angina or congestive heart failure; as well as participants having surgery or medication for glaucoma before surgery (Yoo 2014a).
Two studies excluded men with allergy towards the anaesthetic drugs (Ozdemir 2013; Yoo 2014a).
None of the three included studies reported significant differences in baseline measurements (Ozdemir 2013; Yoo 2012a; Yoo 2014a).
However, Ozdemir 2013 compared only age and duration of surgery.
Settings
All three studies were conducted at a university hospital (Ozdemir 2013; Yoo 2012a; Yoo 2014a).
Interventions
Investigators compared TIVA with propofol versus inhalational anaesthesia with sevoflurane in one study (Yoo 2014a), or versus desflurane in two studies (Ozdemir 2013; Yoo 2012a); in all three studies, the anaesthesia was combined with remifentanil in both groups.
Yoo 2012a gave antiemetic prophylaxis to participants in both groups.
One study defined delivery of the interventions according to weight (Ozdemir 2013); two studies defined delivery of the interventions by a target‐controlled infusion system and end‐tidal concentration of the inhalational anaesthetic (Yoo 2012a; Yoo 2014a).
Outcomes
The most common outcomes in the three included studies consisted of PONV (Ozdemir 2013; Yoo 2012a), circulatory condition (mean arterial pressure (MAP)) (Ozdemir 2013; Yoo 2014a) and respiratory condition (end‐tidal CO2 (ETCO2)) (Ozdemir 2013; Yoo 2014a) measured perioperatively. Other stated outcomes were intensity of pain and use of analgesics (Yoo 2012a), use of antiemetics (Yoo 2012a), change in intraocular pressure (Yoo 2014a), intraoperative blood gas values (Yoo 2014a), heart rate (HR) (Ozdemir 2013), central venous pressure (CVP) (Yoo 2014a), oxygen saturation (SpO2) (Ozdemir 2013), respiratory rate (Yoo 2014a), hydrogen ion concentration (pH) (Ozdemir 2013), Bispectral Index Score (Yoo 2014a), Aldrete Recovery Score (Ozdemir 2013) and patient satisfaction (Ozdemir 2013). Included studies reported only outcomes 1, 2 and 3 of interest in this review (as discussed in the Effects of interventions section). None of the included studies addressed outcomes 4 through 7 (see Table 1).
See Characteristics of included studies for details.
Excluded studies
We excluded three studies described only as conference abstracts (Calza 2011a; Calza 2011b; Choi 2013).
See Characteristics of excluded studies for details.
Ongoing studies
At http://clinicaltrials.gov/., we discovered one ongoing study (Lee 2015) conducted in Korea to test desflurane versus propofol for laparoscopic and robotic assisted laparoscopic gastrectomy; however, this study is not yet recruiting. The primary outcome is quality of recovery (Lee 2015). We contacted the principal investigator of this study but have not received a response.
See Characteristics of ongoing studies for details.
Awaiting classification
Two studies are awaiting classification (Atallah 2009; Roh 2014).
Roh 2014 is a completed study conducted in Korea to compare effects of propofol and desflurane on RALRP. We found this study on http://clinicaltrials.gov/, and the primary outcome was reported as change in malondialdehyde, interleukin‐1β, interleukin‐6 tumour necrotic factor and nitric oxide during operation. The study was completed in February 2015. We contacted the principal investigator to request further information without success.
Atallah 2009 is a completed small single‐centre, two‐arm RCT conducted in Egypt. This study compared anaesthesia with isoflurane (n = 8) versus ketamine‐midazolam‐fentanyl ‐ TIVA (n = 7) for patients (both genders) undergoing robotic assisted laparoscopic radical cystectomy and open surgery. Outcome measures included system organ function (primary), operative conditions (secondary) and recovery profiles (secondary). None of the reported outcomes comply with those required in the present review. We contacted study authors to request further information on additional outcomes and await their response.
See Characteristics of studies awaiting classification for details.
Risk of bias in included studies
Overall we assessed the three included studies as having low risk of bias, but two studies had several ambiguities (Ozdemir 2013; Yoo 2012a). Yoo 2012a included a third arm in the online study protocol, and Ozdemir 2013 based power calculations on a secondary outcome; bias assessment for these studies was difficult owing to limited reporting of study methods. Figure 2 and Figure 3 summarize risk of bias.
Allocation
All three included studies were RCTs that adequately described the method of randomization. Two studies used sealed envelopes (Ozdemir 2013; Yoo 2014a), and one used computer‐generated random numbers (Yoo 2012a). We considered all three included studies to have low risk of selection bias. One study did not state allocation concealment (Ozdemir 2013).
Blinding
None of the three studies described blinding of participants (Ozdemir 2013; Yoo 2012a; Yoo 2014a) nor blinding of the anaesthetist. Two studies reported blinding of outcome assessors (Yoo 2012a; Yoo 2014a). Ozdemir 2013 did not describe blinding of outcome assessors.
Incomplete outcome data
None of the three included studies reported missing data among the relatively small samples of participants (Ozdemir 2013; Yoo 2012a; Yoo 2014a).
Selective reporting
All three included studies reported outcomes as stated in the Methods section (Ozdemir 2013; Yoo 2012a; Yoo 2014a), and two of the three studies were in agreement with available online study protocols (Yoo 2012a; Yoo 2014a).
Other potential sources of bias
Included studies had several other potential sources of bias. One study included a third arm in the online protocol that was not included in the published study (Bai 2011). The third comparison involved TIVA without antiemetic prophylaxis.
The power calculation in Ozdemir 2013 was based on a secondary outcome ‐ mean difference in pH between groups ‐ but investigators did not state whether the minimal clinically relevant difference was known. The primary outcome in this study was perioperative vital signs (HR, MAP, SpO2 and ETCO2). Furthermore, investigators reported only two baseline characteristics for the two groups: comorbidity and length of time in Trendenburg (Ozdemir 2013).
One study asked participants to rate their worst episode of nausea within a maximum of 24 hours after surgery (Yoo 2012a). This outcome may be at risk of recall bias, as noted by the study authors (Yoo 2012a).
Effects of interventions
See: Table 1
Primary outcomes
1. Postoperative pain within 24 hours (as measured by the authors of included studies)
Only one study assessed postoperative pain within 24 hours (n = 62) as a secondary outcome (Yoo 2012a). Researchers measured pain on a visual analogue scale (VAS) (0 = no pain and 100 = intolerable pain) during stay in the post‐anaesthesia care unit (PACU) at one to six hours, six to 24 hours and 24 to 48 hours postoperatively (Yoo 2012a). The mean difference (MD) in VAS at one to six hours was ‐2.20 (95% CI ‐10.62 to 6.22; P = 0.61) (see Table 2). Study authors concluded that they observed no difference in pain intensity or number of participants requiring rescue analgesics between the two groups at any time point (Yoo 2012a). Owing to the small series, we concluded that the quality of evidence must be considered low.
1. Effect estimates for single studies.
| Outcome | Study/Participant | Statistical method | Effect estimate [95% CI] | P value |
| Pain in the PACU (VAS) | 1/n = 62 | MD | ‐1.00 [‐9.82 to 7.82] | 0.82 |
| Pain at 1 to 6 hours (VAS) | 1/n = 62 | MD | ‐2.20 [‐10.62 to 6.22] | 0.61 |
| Pain at 6 to 24 hours (VAS) | 1/n = 62 | MD | ‐1.00 [‐7.51 to 5.51] | 0.76 |
| Presence of nausea | 1/n = 62 | RR | 0.39 [0.19 to 0.80] | 0.01 |
| Nausea intensity in PACU (VNRS) | 1/n = 62 | MD | ‐0.70 [‐1.35 to ‐0.05] | 0.03 |
| Nausea intensity at 1 to 6 hours (VNRS) | 1/n = 62 | MD | ‐1.70 [‐2.59 to ‐0.81] | 0.0002 |
| Nausea‐vomiting rate at 1 hour | 1/n = 42 | RR | 23.00 [1.44 to 366.71] | 0.03 |
| Nausea‐vomiting rate at 3 hours | 1/n = 42 | RR | 2.00 [0.20 to 20.41] | 0.56 |
| Adverse events: increase in IOP during maintenance of anaesthesia | 1/n = 66 | MD | ‐3.90 [‐6.34 to ‐1.46] | 0.002 |
CI: confidence interval; IOP: intraocular pressure; MD: mean difference; PACU: post‐anaesthesia care unit; RR: risk ratio; VAS: visual analogue scale; VNRS: verbal numerical rating scale.
2. PONV within 24 hours (as measured by the authors of included studies)
Two included studies (n = 104) used PONV as an outcome measure ‐ primary outcome measure in one (Yoo 2012a) and secondary outcome measure in the other (Ozdemir 2013) ‐ but measured PONV in different ways (number of participants with PONV or intensity and severity of PONV or rated by the Apfel score (Apfel 1999)). One study (n = 42) measured the number of participants experiencing nausea or vomiting at one, two and three hours after surgery (Ozdemir 2013). This study showed that the risk ratio (RR) at one hour was 23.00 (95% CI 1.44 to 366.71; P = 0.03) (highest rates in the sevoflurane group) for nausea or vomiting, or both, and after three hours was 2.00 (95% CI 0.20 to 20.41; P = 0.56) (Ozdemir 2013). This study had several unclear risks of bias such as selection bias, performance bias, detection bias and attrition bias and pooled frequencies for both nausea and vomiting (Figure 3). Owing to the small series and unclear allocation concealment and blinding, we concluded that the quality of evidence should be considered very low.
Yoo 2012a (n = 62) measured intensity and severity of PONV at one to six hours and six to 48 hours after surgery. Investigators scored participants before surgery by using the Apfel score, which is known to predict PONV (Apfel 1999). Baseline risk for PONV was assumed to be the same for both groups (mean 1.7, standard deviation (SD) 0.4 to 0.5) (Yoo 2012a). Researchers measured the intensity of nausea using an 11‐point verbal numerical rating scale (VNRS: 0 = no nausea and 10 = worst intolerable nausea) (Yoo 2012a). They defined nausea as VNRS ≥ 1, and PONV as the presence of nausea, retching or vomiting. They defined severity of PONV as mild: 1 to 3; moderate: 4 to 6; and severe: 7 to 10, or with retching or vomiting (Yoo 2012a). The RR for the presence of nausea was 0.39 (95% CI 0.19 to 0.80; P = 0.01) (highest in the desflurane group). The MD for intensity of nausea in the PACU was ‐ 0.70 (95% CI ‐1.35 to ‐0.05; P = 0.03) (highest in the desflurane group). At between one and six hours, the MD for intensity of nausea was ‐1.70 (95% CI ‐2.59 to ‐0.81; P = 0.0002) (highest in the desflurane group). Study authors concluded that participants receiving TIVA with propofol had a lower incidence and severity of PONV up to six hours after surgery compared with participants receiving desflurane as an anaesthetic (Yoo 2012a). After six hours, results showed no significant difference (Yoo 2012a). This study was at unclear risk of performance bias specifically (Figure 3). Again, owing to the small series, we considered the quality of evidence to be low. Both studies found, through a limited time effect (one hour and up to six hours), that TIVA ‐ propofol based ‐ was more effective than inhalation‐based anaesthesia in reducing PONV (Ozdemir 2013; Yoo 2012a).
Secondary outcomes
3. Adverse effects (as measured by the authors of included studies) (e.g. cerebral oedema, stroke, ocular complications)
Intraocular pressure
None of the three included studies addressed "adverse effects". However, we chose to include a study that examined changes in IOP as the primary outcome because changes in IOP could lead to ocular complications. Cases of permanent ocular damage occurring in previously healthy patients after RALRP with prolonged operative time have been reported. Extreme patient positioning may be a risk factor for ischaemic optic neuropathy (ION) after RALRP (Weber 2007). An increase in IOP may jeopardize retinal perfusion and may cause retinal ischaemia, especially among older patients with arteriosclerotic involvement in retinal perfusion (Yoo 2014a). Increased IOP is known to be a time‐dependent phenomenon that is worsened when combined with a steep Trendelenburg position (Awad 2009). Yoo 2014a measured changes in IOP during anaesthesia for robotic surgery as a primary outcome (n = 66). A blinded ophthalmologist measured IOP using a handheld tonometer (Yoo 2014a). This study showed similar IOP at baseline in participants receiving sevoflurane and those receiving propofol as well as a decline in IOP after induction of anaesthesia (Yoo 2014a) and increased IOP with pneumoperitoneum and Trendelenburg positioning (Yoo 2014a). During maintenance of anaesthesia, the level of IOP was higher in the sevoflurane group than in the propofol group (MD ‐3.90, 95% CI ‐6.34 to ‐1.46; P = 0.002) (Yoo 2014a). None of the participants in either group experienced ocular complications (Yoo 2014a). We judged this study to be at low risk of bias (Figure 3). However, owing to the small sample size and the restricted version of the review question, we concluded that the quality of evidence must be considered low. In conclusion, investigators found that propofol was better in attenuating the rise in IOP during RALRP with the steep Trendelenburg position when compared with sevoflurane (Yoo 2014a).
4. All‐cause mortality within 90 days
No study reported this outcome.
5. Respiratory complications requiring treatment within 48 hours (as measured by authors of included studies)
No study reported this outcome.
6. Circulatory complications requiring treatment within 48 hours (as measured by authors of included studies)
No study reported this outcome.
7.Costs (as measured by authors of included studies)
No study reported this outcome.
Discussion
Summary of main results
We found three studies including men undergoing robotic assisted laparoscopic radical prostatectomy (RALRP). These studies showed that propofol may reduce postoperative nausea and vomiting (PONV) over the short term (one to six hours after surgery) after RALRP compared with inhalational anaesthesia (sevoflurane, desflurane). Propofol may prevent an increase in intraocular pressure (IOP) after pneumoperitoneum and steep Trendelburg positioning compared with sevoflurane. However, it is unclear whether this surrogate outcome translates directly to clinical avoidance of ocular complications during surgery. We found evidence of no clinically meaningful effect on postoperative pain.
Few studies (Ozdemir 2013; Yoo 2012a; Yoo 2014a) have addressed the question of which type of anaesthesia ‐ inhalational or intravenous ‐ is preferable for different kinds of robotic assisted laparoscopic surgery. This is surprising given that the robotic technique is applied so widely in the developed world today. Several aspects of the anaesthesia technique are relevant for patient outcome and satisfaction, including cardiorespiratory and cerebral complications, as well as postoperative pain, nausea and vomiting.
None of the included studies reported adverse effects.
Owing to the number and quality of included studies, we did not pool data across studies and we did not test for publication bias.
Overall completeness and applicability of evidence
Evidence related to the superiority of inhalational or total intravenous anaesthesia (TIVA) for patients undergoing robotic assisted transabdominal laparoscopic surgery is incomplete, and, to our knowledge, only a few studies have been carried out; findings are applicable to a limited population: male patients undergoing radical robotic prostatectomy. It is unclear if findings of this review apply to women and to robotic surgery in gynaecology and gastroenterology. We have included limited data from three studies (Ozdemir 2013; Yoo 2012a; Yoo 2014a).
Quality of the evidence
The total body of evidence does not permit a robust conclusion on whether TIVA or inhalational anaesthesia provides better outcomes for adults undergoing transabdominal robotic assisted laparoscopic gynaecological, urological or gastroenterological surgery. The quality of evidence is generally considered low to very low owing to risk of bias and very small samples. The three studies included a total of 170 patients‐ all were men undergoing RALRP ‐ and all were small single‐centre randomized controlled trials (RCTs). The methodological quality of the included studies was difficult to assess, as details are reported poorly, so the predominant classification of bias was unclear. For Ozdemir 2013, it is unknown how allocations were concealed; blinding of staff, participants and outcome assessors was not described; and we could not consult an online protocol to assess for reporting bias. Thus it was not possible to assess risk of selection bias, performance bias, detection and attrition bias, and for baseline comparison, only data on age and operation time were presented (Ozdemir 2013). Data on comorbidity (diseases or symptoms related to nausea/vomiting) and time in Trendelenburg would have been relevant to report. The power calculation was performed on differences in pH between groups (Ozdemir 2013). The minimal clinically important difference was not stated, and it is surprising that the primary outcome was vital signs (heart rate (HR), mean arterial pressure (MAP), oxygen saturation (SpO2) and end‐tidal carbon dioxide (ETCO2)). We considered the quality of evidence from this study as very low, as it was a small study and allocation concealment and blinding were unclear. Yoo 2012a compared desflurane/remifentanil with propofol/remifentanil; all participants had ramosetron (antiemetic) administered at completion of surgery (Yoo 2012a), and the primary outcome was the incidence of nausea. It was unclear whether participants were blinded; therefore, we considered the study to be at unclear risk of performance bias. This study had an unknown source or potential source of bias, as the online protocol (Bai 2011) revealed a third arm in the original trial (including n = 93). The published paper did not mention the third arm described in the original study protocol (Yoo 2012a) (n = 62). Participants in this third arm received TIVA but without antiemetic prophylaxis (Bai 2011). The significance of this discrepancy between protocol and published paper is unknown. We considered the quality of evidence from this study as low owing to a small sample size (n = 62).
Finally, the later Yoo study (Yoo 2014a) applied improved methods and was at low risk of bias in all categories, but study authors found no association between changes in IOP and ocular complications, as no participants in the study developed such complications. IOP is considered a questionable surrogate outcome. We considered the quality of evidence derived from this study as low, as the sample was small (n = 66) and evidence showed indirectness because the paper addressed a restricted version of the review question.
Potential biases in the review process
We conducted a thorough process to identify relevant studies, and we believe this review is comprehensive in identifying all eligible studies. We repeated the search several times during preparation of the review.
Publication bias is a possible cause of a small study effect ‐ a tendency for estimates of the intervention effect to be more beneficial in smaller studies (Higgins 2011) ‐ and this review suffers from including only small studies.
After identifying the studies, we decided against testing for heterogeneity, as none of these studies included similar outcomes measured in the same way.
We consider our inclusion criteria very broad so as not to exclude any studies that might carry some evidence related to the review question. We included a study (Yoo 2014a) with the surrogate outcome for ocular complications as changes in IOP.
We found that the literature supported the relevance of ocular complications, as a cohort study evaluating 1500 RALRPs reported that the most common anaesthesia‐related complication was corneal abrasion (Danic 2007): Corneal abrasion was seen in 3% of cases despite the use of eye tape. However, none of the participants developed long‐term sequelae (Danic 2007). A large register study including nearly one million participants undergoing hysterectomy and prostatectomy found a 6.5‐fold increased risk of corneal abrasion in patients undergoing robotic assisted surgery compared with those undergoing open surgery (Sampat 2015). However, none of the included studies in this review reported corneal abrasion.
Another potential source of bias is lack of response from study investigators or sponsors whom we have tried to reach for clarification of methods and additional study results.
This review may have a location bias (Higgins 2011), as all included studies were conducted in Korea (Yoo 2012a; Yoo 2014a) or in Turkey (Ozdemir 2013).
Agreements and disagreements with other studies or reviews
In light of the limited number of included studies, small sample sizes and inclusion of exclusively male participants undergoing robotic assisted laparoscopic surgery within urology, the superiority of TIVA or inhalational anaesthesia for transabdominal robotic surgery remains unclear to the review authors, but differences between inhalation anaesthetics and TIVA have previously been reported for other types of non‐robotic assisted surgery.
PONV
Gupta 2004 conducted a systematic review that focused on ambulatory anaesthesia in relation to mixed, gynaecological, orthopaedic, dental and eye surgery. Gupta 2004 included 16 papers and found that propofol induced less nausea and vomiting and reduced the need for antiemetics, and that early recovery after surgery was seen in patients receiving inhalation anaesthesia (Gupta 2004). Kumar 2014 conducted a systematic review and meta‐analysis of 18 RCTs testing propofol versus desflurane or sevoflurane in ambulatory surgery. The evidence was judged to be of low quality overall, but after analysing data from 1621 randomly assigned participants, review authors concluded that the occurrence of PONV was less in the propofol group (Kumar 2014). Data revealed no differences in post discharge nausea, vomiting and postoperative pain (Kumar 2014).
Pain (among other outcomes)
An RCT of participants undergoing elective laparoscopic cholecystectomy for benign gallbladder disease (n = 60) found that TIVA with propofol and alfentanil was associated with a significantly reduced rate of PONV and analgesic consumption, shortened recovery time and duration of hospitalization, accelerated onset of bowel movements and increased patient satisfaction compared with desflurane and alfentanil (Akkurt 2009). Pokkinen and colleagues conducted an RCT including 148 women who were randomized to propofol or sevoflurane anaesthesia during laparoscopic hysterectomy, and found no effect on the requirement of oxycodone nor on intensity of pain after surgery (Pokkinen 2014). Similarly, an RCT of participants (n = 80) undergoing laparoscopic cholecystectomy identified no differences in pain four hours after surgery among patients anaesthetized by propofol compared with those anaesthetized by isoflurane, desflurane or sevoflurane (Ortiz 2014). However, another RCT (n = 90) reported that propofol anaesthesia was associated with significantly less pain one‐half hour and one hour after surgery among participants undergoing gynaecological laparoscopy with planned opioid‐free postoperative analgesia (Li 2012).
Cognitive dysfunction
An RCT (n = 50) with participants undergoing cholecystolithotomy, colectomy or sigmoidectomy showed that sevoflurane‐based anaesthesia reduced the incidence of postoperative delirium compared with propofol‐based anaesthesia for laparoscopic surgery of long duration performed in elderly patients (Nishikawa 2004).
Other factors that might be influential
None of the three included studies (Ozdemir 2013; Yoo 2012a; Yoo 2014a) reported use of "air seal" for gas insufflation for pneumoperitoneum, but had it been utilized, the cardiorespiratory condition of patients during surgery might have been influenced. Previous findings in urology suggest that patients undergoing laparoscopic renal surgery using these valveless trocars (Airseal) had significantly less carbon dioxide (CO2) elimination and thus significantly lower CO2 absorption intraoperatively when compared with patients undergoing surgery with a standard trocar (Herati 2011). An increase in CO2 absorption can have several important clinical implications, including higher risk of respiratory acidosis and increased risk of formation of a gas embolism (Herati 2011).
Theoretically, IOP may change during robotic assisted laparoscopic surgery (Awad 2009; Hoshikawa 2014). IOP is known to increase with longer duration of steep head‐down positioning (Awad 2009; Hoshikawa 2014), and physicians are recommended to vigilantly observe ocular complications in patients who are positioned steep down for a long period. In a non‐systematic review of the pathophysiology and prevention of ocular complications in relation to RALRP, review authors recommended prevention of corneal abrasion by using occlusive dressings and prevention of the rarer ION by limiting time spent in the Trendelenburg position and by providing more judicious administration of IV fluids (Kan 2015). If patients have a history of severe ocular disease, an ophthalmologist should be consulted before robotic surgery is performed (Kan 2015). A study of laparoscopic surgery concluded that the impact of anaesthetics on IOP may change, depending on the surgical position. For laparoscopic surgery performed in the head‐down position, propofol may be helpful in preventing ocular hypertension (Hwang 2013).
Authors' conclusions
Implications for practice.
It is unclear whether TIVA is a superior anaesthetic compared with inhalational anaesthesia for transabdominal robotic assisted laparoscopic surgery in urology, gynaecology and gastroenterology, as existing evidence is scarce and of low quality and has been generated exclusively for male patients undergoing robotic radical prostatectomy. As robotic surgery becomes more and more routine, the duration of surgery is expected to diminish (Watt 2015), thereby reducing strain on patients caused by non‐physiological positioning. However, restricted access to patients remains an issue that compromises the anaesthesiologist's access for observing and treating patients during robotic assisted surgery. This may challenge the safety dimension of the choice of anaesthetics. As evidence and experience with transabdominal robotic assisted surgery for older and obese patients continue to be acquired, we expect that future trends will include a broader case mix of patients eligible for robotic assisted surgery. This scenario raises considerations for the anaesthesia provided during surgery, and patient outcomes must be followed closely in the future.
Implications for research.
Evidence is in the pipeline, and one online protocol (Lee 2015) describes an RCT planned to test desflurane and propofol in robotic assisted or laparoscopic gastrectomy for 84 patients of both sexes using the QoR‐40 score (obtained from a questionnaire) to measure the quality of recovery. Although it involves only a single centre and is a small series, results of this study will be of interest at the time of its planned completion in July 2016. Another online protocol (Roh 2014) documents an RCT including 50 participants (completed but not published) conducted to assess males undergoing RALRP with desflurane versus propofol and to test oxidative stress and nitric oxide.
We found no protocols for multi‐centre large RCTs in different specialities utilizing robotic assisted surgery. Additional RCTs are needed to determine the effects of TIVA versus inhalation anaesthesia on postoperative pain, PONV, adverse events, all‐cause mortality, respiratory and circulatory complications, cognitive dysfunction, length of post‐anaesthesia care unit (PACU) stay and costs.
In research within anesthesiology, it is tempting to choose surrogate outcomes, for example, "intraocular pressure" instead of "ocular complications", or "change in MAP" instead of "circulatory complications", as these measurements are easier to obtain. However, the correlation between surrogate and clinical outcomes might not be strong. Changes in intraocular pressure or MAP might have no clinical consequences for the patient. Surrogate endpoints can be easier to complete while not answering the essential question and do not closely reflect the target of treatment (Møller 2006). If a surrogate outcome should be used, the correlation between surrogate and actual outcomes must be strong, and the correlation must go both ways (Møller 2006). When surrogate measures are used, trials should be considered preliminary or hypothesis‐generating, and conclusions must be drawn with extreme care (Møller 2006). It would be best if future researchers would choose outcome measures that are clinically relevant and that measure how a patient feels, functions or survives (Møller 2006).
As adverse events are rare (or underreported), multi‐centre studies with greater power should prove significant.
Acknowledgements
We would like to thank Rodrigo Cavallazzi (Content Editor); Nathan Pace (Statistical Editor); and Daniel M Gainsburg, Hamdy Awad and André Börgers (Peer Reviewers) for their help and editorial advice during preparation of the protocol for this systematic review (Herling 2014), and Karen Hovhannisyan, Torben Jørgensen and Janne Vendt for assisting in the literature search for the review.
Further, we would like to thank Rodrigo Cavallazzi (Content Editor); Nathan Pace (Statistical Editor); Hamdy Awad and Daniel M Gainsburg (Peer Reviewers) and Janet Wale (Consumer Editor) for their help and editorial advice during preparation of this systematic review.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Anesthesia] explode all trees #2 MeSH descriptor: [Anesthesia, General] explode all trees #3 MeSH descriptor: [Neuromuscular Blockade] explode all trees #4 MeSH descriptor: [Anesthesia, Local] explode all trees #5 MeSH descriptor: [Anesthetics, Local] explode all trees #6 MeSH descriptor: [Anesthesia, Epidural] explode all trees #7 MeSH descriptor: [Anesthesia, Inhalation] explode all trees #8 MeSH descriptor: [Anesthesia, Intravenous] explode all trees #9 MeSH descriptor: [Anesthetics, Intravenous] explode all trees #10 MeSH descriptor: [Nerve Block] explode all trees #11 MeSH descriptor: [Analgesia, Epidural] explode all trees #12 MeSH descriptor: [Analgesia, Patient‐Controlled] explode all trees #13 MeSH descriptor: [Anesthesia and Analgesia] explode all trees #14 MeSH descriptor: [Pain, Postoperative] explode all trees #15 MeSH descriptor: [Balanced Anesthesia] explode all trees #16 (an?esth* or analg*) or ((abdominis plane or neuromuscular) near/3 block*) #17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or (#13 and #14) or #15 or #16 #18 MeSH descriptor: [Robotics] explode all trees #19 MeSH descriptor: [Laparoscopy] explode all trees #20 MeSH descriptor: [Prostatectomy] explode all trees #21 MeSH descriptor: [General Surgery] explode all trees #22 MeSH descriptor: [Hysterectomy] explode all trees #23 MeSH descriptor: [Nephrectomy] explode all trees #24 MeSH descriptor: [Cholecystectomy] explode all trees #25 MeSH descriptor: [Cholecystectomy, Laparoscopic] explode all trees #26 MeSH descriptor: [Cystectomy] explode all trees #27 robotic* or (robot* near (surg* or operat* or prostatectomy or assist* or hysterectomy or nephrectomy or laparoscop* or cholecystectomy or cystectomy)) #28 (#18 and (#19 or #20 or #21 or #22 or #23 or #24 or #25 or #26)) or #27 #29 #17 and #28
Appendix 2. MEDLINE (OvidSP) search strategy
1. (exp Robotics/ and (exp Laparoscopy/ or exp Prostatectomy/ or exp General Surgery/ or exp Hysterectomy/ or exp Nephrectomy/ or exp Cholecystectomy/ or exp Cholecystectomy, Laparoscopic/ or exp Cystectomy/)) or robotic*.af. or (robot* adj5 (surg* or operat* or prostatectomy or assist* or hysterectomy or nephrectomy or laparoscop* or cholecystectomy or cystectomy or colectomy or colorectal or bariatric or gastro?intestinal or gastric* or cardia* )).mp. 2. exp Anesthesia/ or exp Anesthesia, General/ or exp Neuromuscular Blockade/ or exp Anesthesia, Local/ or Anesthetics, Local/ or Anesthesia, Epidural/ or exp Anesthesia, Inhalation/ or exp Anesthesia, Intravenous/ or exp Anesthetics, Intravenous/ or Nerve Block/ or exp Analgesia, Epidural/ or Analgesia, Patient‐Controlled/ or (exp Analgesia/ and exp Pain, Postoperative/) or exp Balanced Anesthesia/ or (an?esth* or analg*).mp. or ((abdominis plane or neuromuscular) adj3 block*).mp. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. Embase (OvidSP) search strategy
1. exp anesthesia/ or exp general anesthesia/ or exp neuromuscular blocking/ or exp local anesthesia/ or local anesthetic agent/ or epidural anesthesia/ or exp inhalation anesthesia/ or exp intravenous anesthesia/ or exp intravenous anesthetic agent/ or nerve block/ or exp patient controlled analgesia/ or (exp analgesia/ and exp postoperative pain/) or exp balanced anesthesia/ or (an?esth* or analg*).mp. or ((abdominis plane or neuromuscular) adj3 block*).mp. 2. (exp robotics/ and (exp Laparoscopy/ or exp Prostatectomy/ or exp General Surgery/ or exp Hysterectomy/ or exp nephrectomy/ or exp cholecystectomy/ or exp cystectomy/)) or robotic*.af. or (robot* adj3 (surg* or operat* or prostatectomy or assist* or hysterectomy or nephrectomy or laparoscop* or cholecystectomy or cystectomy)).mp. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. ISI Web of Science search strategy
#1 TS=(( analges* or an?esthe*) SAME (general or local or epidural or inhalation or intravenous or patient?controlled or balanced)) or TS=((nerve* or neuro*) SAME block*) or TS=(analgesia SAME pain SAME postoperative) or TI=(an?esth* or analg*) or TS=((abdominis plane or neuromuscular) SAME block*) #2 TS=(robot* SAME (surg* or operat* or prostatectomy or assist* or hysterectomy or nephrectomy or laparoscop* or cholecystectomy or cystectomy)) #3 TS=(random* or ((clinical or controlled) SAME trial*) or placebo* or multicenter* or prospective) or TS=((blind* or mask*) and (single or double or triple or treble)) #4 #1 and #2 and #3
Appendix 5. CINAHL (EBSCOhost) search strategy
S1 ( (MH "Anesthesia+") OR (MM "Anesthesia Induction") OR (MH "Anesthesia, General+") OR (MM "Anesthesia, Inhalation") OR (MM "Anesthesia, Intravenous") OR (MM "Anesthesia, Local") OR (MM "Analgesia, Epidural") OR (MH "Analgesics+") OR (MH "Anesthetics, General+") OR (MH "Anesthetics, Inhalation+") OR (MH "Anesthetics, Intravenous+") OR (MH "Anesthetics, Local+") ) OR TI ( an?esth* or analg* ) OR TX ( ((abdominis plane or neuromuscular) N3 block*) ) S2 ( ((MM "Robotics") and ((MM "Laparoscopy") OR (MH "Prostatectomy+") OR (MH "Hysterectomy+") OR (MM "Nephrectomy") OR (MH "Cholecystectomy+") OR (MM "Cystectomy") )) ) OR AB robotic* OR TX ( (robot* N3 (surg* or operat* or prostatectomy or assist* or hysterectomy or nephrectomy or laparoscop* or cholecystectomy or cystectomy)) ) S3 TX random* or ((clinical or controlled) N3 trial*) or placebo* or multicenter* or prospective or ((blind* or mask*) and (single or double or triple or treble)) S4 S1 and S2 and S3
Appendix 6. Data extraction form
Data collection form
| Review title or ID |
| Study ID(surname of first study author and year first full report of study was published, e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
|
Report ID (ID for this paper/abstract/report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized controlled trial | |||||
|
Participants: adults +18 years for transabdominal robotic assisted surgery in gynaecology, urology or gastroenterology |
||||||
| Types of interventions: TIVA vs inhalational anaesthesia | ||||||
|
Types of outcome measures: Primary outcomes:
Secondary outcomes:
|
||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description (Include comparative information for each group (i.e. intervention and controls) if available) |
Location in text (pg & ¶/fig/table) |
|
|
Population description (from which study participants were drawn) |
||
|
Setting (including location and social context) |
||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Method/s of recruitment of participants | ||
| Informed consent obtained | Yes/No/Unclear | |
| Notes: Clinical.gov. checked | ||
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
|
Unit of allocation (by individuals, clusters/groups ) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/obtained for study | Yes/No/Unclear | ||
| Notes: | |||
5. Risk of bias assessment
See Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (page 198)
| Domain | Risk of bias | Support for judgement |
Location in text (pg & ¶/fig/table) |
|||
| Low risk | High risk | Unclear risk | Non‐applicable | |||
|
Random sequence generation (selection bias) |
||||||
|
Allocation concealment (selection bias) |
||||||
|
Blinding of participants and personnel (performance bias) |
||||||
| (if required) | ||||||
|
Blinding of outcome assessment (detection bias) |
||||||
| (if required) | ||||||
|
Incomplete outcome data (attrition bias) |
||||||
|
Selective outcome reporting (reporting bias) |
OBS: check if there is a protocol in Clinical.trial.gov | |||||
| Other bias | ||||||
| Notes: | ||||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomized (or total pop. at start of study for NRCTs) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Severity of illness | ||
| Comorbidities | ||
| Other treatment received(additional to study intervention) | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention group 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomized to group (specify whether no. people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity, etc., if relevant) |
||
| Co‐interventions | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
| Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||
| Reanalysis possible? | Yes/No/Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||
| Reanalysis possible? | Yes/No/Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in intervention effects) | Yes/No/Unclear | |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes/No/Unclear | |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes/No/Unclear | |
| Notes: | ||
11. Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ozdemir 2013.
| Methods | Single‐centre, 2‐arm RCT Setting: training and research hospital in Istanbul Country: Turkey Groups: propofol and remifentanil vs sevoflurane and remifentanil Period: before 2012 ‐ not stated in paper |
|
| Participants |
Sample size: 42 Surgery: robotic assisted laparoscopic radical prostatectomy ‐ urology Included: males scheduled for RALRP with ASA I to III status, 50 to 75 years. Excluded: neurological or psychological disease, allergy to propofol, hypersensitivity or intolerance to opioids or to sevoflurane, severe pulmonary or cardiovascular system disease |
|
| Interventions | Propofol and remifentanil (n = 21) vs sevoflurane and remifentanil (n = 21) Procedure: robotic assisted radical prostatectomy |
|
| Outcomes |
HR: reported MAP: reported SpO2: reported ETCO2: reported pH: reported Aldrete Recovery Score (circulation, conscience, O2‐sat, respiration, activity): reported Nausea‐vomiting score: reported Patient satisfaction: reported |
|
| Notes | No imbalances stated at baseline; only age and operation time reported. No data on comorbidity (known disease or symptoms related to nausea or vomiting) nor on Trendelenburg time Time from anaesthesia to measurement of Aldrete score unclear Power calculation performed on mean difference in pH between groups; however, minimal relevant difference not stated; primary outcome measures: HR, MAP, SpO2 and ETCO2 Conclusion: TIVA provides early (1 and 2 hours) and better quality recovery and fewer side effects (nausea and vomiting) in robotic prostatectomy cases. Prolonged steep Trendelenburg position and CO2 pneumoperitoneum were well tolerated by both groups Funding: not stated Conflict of Interest: unknown |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers in sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Unknown how allocations were concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data stated |
| Selective reporting (reporting bias) | Low risk | All planned (as stated in paper) outcomes reported. No online protocol can be found for comparison |
| Other bias | Low risk | Same surgeon and anaesthetist for all procedures ‐ significance unknown |
Yoo 2012a.
| Methods | Single‐centre, 2‐arm RCT Setting: University College of Medicine (Hospital) Country: Korea Groups: TIVA with propofol vs desflurane Period: November 2010‐ May 2011 |
|
| Participants |
Number: 62 Surgery: robotic‐assisted laparoscopic radical prostatectomy ‐ urology Included: male between 50 and 70 years of age with ASA I or II Excluded: history of motion sickness/PONV, use of antiemetics 24 hours before surgery, regular use of corticosteroids, chemotherapy within 4 weeks, radiotherapy within 8 weeks, hepatic dysfunction, confirmed renal impairment or obesity (BMI > 35 kg/m2) |
|
| Interventions | TIVA with propofol and remifentanil and antiemetic prophylaxis (n = 31) vs desflurane and remifentanil and antiemetic prophylaxis (n = 31) Procedure: robotic assisted laparoscopic radical prostatectomy |
|
| Outcomes |
Incidence of nausea: reported Incidence of retching: reported Incidence of vomiting: reported Use of antiemetics: reported Severity of PONV: reported Intensity of pain ‐ VAS: reported Use of rescue analgesics: reported |
|
| Notes | A third arm with patients treated with TIVA without antiemetic prophylaxis not reported in the paper but reported in the online protocol (Bai 2011) Conclusion: TIVA with propofol reduced incidence and severity of PONV compared with desflurane in patients with low risk of PONV after RALRP until 6 hours after surgery Letter asking for further information on this study sent 2015 September 16. No reply Funding: faculty research grant of Yonsei University College of Medicine for 2007 (6‐2007‐0190) Conflict of Interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | A physician outside the trial performed randomization and assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants presumed blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout of participants |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Other bias | Unclear risk | A third arm with patients treated with TIVA without antiemetic prophylaxis not reported in the paper but reported in the online protocol |
Yoo 2014a.
| Methods | Single‐centre, 2‐arm RCT Setting: University Collage of Medicine (Hospital) Country: Korea Groups: TIVA with propofol vs inhalation anaesthesia with sevoflurane Period: May 2011‐March 2012 |
|
| Participants |
Sample size: 66 Surgery: robotic assisted laparoscopic radical prostatectomy Included: men > 50 years, ASA I and II Excluded: baseline IOP > 30 mmHg, diabetic retinopathy, cataract, known allergies to anaesthetic drugs, uncontrolled hypertension, unstable angina or congestive heart failure. Surgery or medication for glaucoma before RALRP |
|
| Interventions | TIVA with propofol (n = 33) vs inhalation anaesthesia with sevoflurane (n = 33) Procedure: robotic assisted radical prostatectomy |
|
| Outcomes |
Changes in IOP from baseline to 30 minutes after positioning in Trendelenburg with CO2 pneumoperitoneum: reported Number of men with IOP ≥ 24 at any time point: reported Ocular perfusion pressure: reported Arterial blood gas: reported MAP at all time points: reported CVP at all time points: reported Respiratory rate at all time points: reported ETCO2 at all time points: reported Bispectral Index Score at all time points: reported |
|
| Notes | Online protocol (Yoo 2014b) had additional exclusion criteria: illiteracy and retinal detachment Study finds a significant change in IOP in the sevoflurane group indicating that propofol is a more effective anaesthetic in attenuating the rise in IOP, thereby decreasing the risk for ocular hypoperfusion during steep Trendelenburg with pneumoperitoneum, which is used in transabdominal robotic assisted surgery. However, the study cannot show an association with ocular complications, as no participants in the study developed such a complication Administration of habitual medication and fasting before surgery not described Conclusion: Propofol is found to be better in attenuating the rise in IOP during RALRP with steep Trendelenburg position Funding: departmental sources Conflict of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Table of random numbers in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators but the anaesthesiologist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ophthalmologist and other outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Other bias | Unclear risk | Not known |
ASA: American Society of Anesthesiologists physical classification score; BMI: body mass index; CO2: carbon dioxide; CVP: central venous pressure; ETCO2: end‐tidal CO2; HR: heart rate; IOP: intraocular pressure; MAP: mean arterial pressure; mmHg: millimetres of mercury; pH: hydrogen ion concentration; PONV: postoperative nausea and vomiting; RALRP: robotic assisted laparoscopic radical prostatectomy; RCT: randomized controlled trial; SpO2: oxygen saturation; TIVA: total intravenous anaesthesia; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Calza 2011a | Insufficient information to assess study |
| Calza 2011b | Insufficient information to assess study |
| Choi 2013 | Insufficient information to assess study |
Characteristics of studies awaiting assessment [ordered by study ID]
Atallah 2009.
| Methods | Single‐centre, 2‐arm RCT with computer‐generated randomization |
| Participants | Patients (mixed gender) undergoing robotic assisted laparoscopic radical cystectomy and open surgery n = 15 |
| Interventions | Anaesthesia with isoflurane (n = 8) for one group and with ketamine‐midazolam‐fentanyl ‐ TIVA (n = 7) for the other |
| Outcomes | System organ function (primary), operative conditions (secondary) (blood loss) and recovery profiles (secondary) Study reported heart rate, mean blood pressure, mean air way pressure, lung compliance, pH, arterial carbon dioxide tension, serum bicarbonate, growth hormone, cortisol, albumin, prothrombin and fibrinogen, bilirubin, aspartate aminotransferase and alanine aminotransferase levels |
| Notes |
Conclusion: TIVA was considered an advantage in shortening the duration of pneumoperitoneum without an increase in prothrombin and fibrinogen concentrations Funding: not stated Conflict of interest: not stated None of the reported outcomes comply with those required in the present review. Study authors were contacted in June 2016 as they may have measured other outcomes ‐ response awaited |
Roh 2014.
| Methods | Single‐centre, 2‐arm RCT, blinding (participant, caregiver, investigator, outcome assessor) |
| Participants | Males between 20 and 70 years undergoing robotic assisted laparoscopic radical prostatectomy under general anaesthesia Excluded: chronic renal failure; allergy to propofol, nuts or diuretics; vitamin C or E within 5 days before surgery; BMI > 30 kg/m2; inability to read consent form Estimated enrolment: 50 participants |
| Interventions | Anaesthesia with desflurane as primary anaesthetics guided by Bispectral Index Score (BIS) vs propofol infusion with target‐controlled infusion (TCI) device guided by BIS |
| Outcomes | Changes in serum malondialdehyde, interleukin‐1β, interleukin‐6, tumour necrotic factor, nitric oxide during operation (100 minutes after pneumoperitoneum, 10 minutes after decompression) Kidney function after anaesthesia (1 day after surgery) ‐ creatine measure |
| Notes |
Funding: not stated Conflict of interest: not stated |
BIS: Bispectral Index Score; BMI: body mass index; RCT: randomized controlled trial; TCI: target‐controlled infusion; TIVA: total intravenous anaesthesia.
Characteristics of ongoing studies [ordered by study ID]
Lee 2015.
| Trial name or title | Comparison of desflurane and propofol on quality of recovery in patients undergoing robotic assisted or laparoscopic gastrectomy |
| Methods | RCT, single‐centre, 2‐arm, double‐blind (participant and outcome assessor) |
| Participants | Both genders, > 20 years old, scheduled for laparoscopic or robotic gastrectomy, ASA I or II Estimated enrolment: 84 participants |
| Interventions | Anaesthesia with desflurane vs propofol during surgery |
| Outcomes | Quality of recovery within 24 hours of surgery measured with QoR‐40 score |
| Starting date | August 2015 |
| Contact information | Yonsei University, Korea. KI‐Young Lee, jjollong@yuhs.as |
| Notes |
Expected completion: July 2016 Letter asking for further information sent 16 September 2015. No reply |
ASA: American Society of Anesthesiologists physical classification score; QoR: quality of recovery; RCT: randomized controlled trial.
Differences between protocol and review
We made the following changes to the published protocol (Herling 2014).
Gitte W Lam is an additional author for this review.
"For adults" was added to the title to clarify study participants.
We planned to report event rates with 95% confidence intervals (CIs) and risk ratios (RRs) for adverse events, mortality and complications; however, the included studies did not produce data other than absolute numbers with standard deviations and percentages.
The unit of analysis consisted of participants included and randomly assigned; this was not stated in the protocol.
We planned to conduct intention‐to‐treat (ITT) analysis for all outcomes, as far as possible. For studies with more dropouts than estimated, we planned to contact trialists to request additional data on participants lost to follow‐up. We planned to perform a sensitivity analysis of best case versus worst case scenarios. However, we did not encounter reports of missing data and so noted the level of attrition in included studies.
We planned to assess heterogeneity between studies by visually inspecting forest plots. We planned to assess statistical heterogeneity of effect sizes by using the I2 statistic and the Chi2 test. The I2 statistic describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error (Higgins 2011). We planned to conduct a meta‐analysis if we visually (forest plot), statistically (I2 statistic) and clinically found heterogeneity acceptable (low enough). We planned to proceed with a meta‐analysis if populations, interventions, comparisons, measurements, time frames and settings were reasonably similar; if we identified three or more studies; and if the direction of effect of an intervention was consistent. All review authors will discuss and agree about when to conduct a meta‐analysis (Higgins 2011). We were not able to calculate effect size from the data needed to perform tests for statistical heterogeneity (seeTable 1). As we have included only three studies, the investigation of heterogeneity is of questionable value (Higgins 2011).
Subgroup analysis were not relevant, as the included studies were rather heterogenic in terms of participants ‐ all were male and were from urology.
"Change in intraocular pressure" has been added to the outcome post hoc: Adverse effects such as changes in intraocular pressure could lead to ocular complications.
As we found an insufficient number of studies, we were unable to perform a sensitivity analysis by comparing results with inclusion and with exclusion of RCTs classified to have 'low risk of bias' to decide whether our conclusions are robust (Higgins 2011).
As other bias, we added "baseline imbalances", as we noted these in one study.
Contributions of authors
Suzanne F Herling (SH), Bjørn Dreijer (BD), Gitte Wrist Lam (GL) Thordis Thomsen (TT), Ann Merete Møller (AM).
Conceiving of the review: SH, BD, TT and AM.
Co‐ordinating the review: SH.
Undertaking manual searches: SH and BD/Karen Hovhannisyan/Torben Jørgensen.
Screening search results: SH and BD.
Organizing retrieval of papers: SH.
Screening retrieved papers against inclusion criteria: SH and BD.
Appraising the quality of papers: SH, BD and AM.
Abstracting data from papers: SH and BD.
Writing to authors of papers for additional information: SH and BD.
Providing additional data about papers: SH and BD.
Obtaining and screening data on unpublished studies: SH and BD.
Managing data for the review: SH.
Entering data into Review Manager (RevMan 5.3): SH.
Analysing RevMan statistical data: SH.
Performing other statistical analysis not using RevMan: N/A.
Interpreting data: SH, BD, TT, GL and AM.
Making statistical inferences: N/A.
Writing the review: SH, BD, TT, GL and AM.
Securing funding for the review: AM.
Performing previous work that was the foundation of the present study: none of the authors of this review.
Serving as guarantor for the review (one author): AM.
Taking responsibility for reading and checking the review before submission: SH, BD, TT, GL and AM.
Sources of support
Internal sources
Department of Anaesthesiology, Copenhagen University Hospital, Herlev, Denmark.
External sources
None, Other.
Declarations of interest
Suzanne F Herling: none known.
Bjørn Dreijer: none known.
Thordis Thomsen: none known.
Gitte W Lam: none known.
Ann Merete Møller: none known.
New
References
References to studies included in this review
Ozdemir 2013 {published data only}
- Ozdemir M, Bakan N, Sahin K, Erbesler ZA, Tunca ST. The comparison of sevoflurane‐remifentanil and propofol‐remifentanil in robotic prostatectomies. Journal of Clinical and Analytical Medicine 2013;4:313‐7. [DOI: 10.4328/CAM.1018] [DOI] [Google Scholar]
Yoo 2012a {published data only}
- Yoo YC, Bai SJ, Lee KY, Shin S, Choi EK, Lee JW. Total intravenous anaesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot‐assisted laparoscopic radical prostatectomy: a prospective randomized trial. Yonsei Medical Journal 2012;53(6):1197‐202. [PUBMED: 23074122] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YC, Lee K‐Y, Choi EK, Bai S‐J. Total intravenous anaesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot‐assisted laparoscopic radical prostatectomy: prospective randomised trial. Conference. Paris, France: Lippincott Williams and Wilkins, From 6 to 9 June 2012. [DOI] [PMC free article] [PubMed]
Yoo 2014a {published data only}
- Yoo YC, Shin S, Choi EK, Kim CY, Choi YD, Bai SJ. Increase in intraocular pressure is less with propofol than with sevoflurane during laparoscopic surgery in the steep Trendelenburg position. Canadian Journal of Anaesthesia 2014;61(4):322‐9. [PUBMED: 24500661] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Calza 2011a {published data only}
- Calza E, Porpiglia F, Fiori C, Giannone A, Pusineri A, Meli A, et al. Intraocular pressure increase during robot assisted laparoscopic prostatectomy: balanced anesthesia versus total intravenous anesthesia. 26th Annual Congress of the European Association of Urology. Vienna Austria: Elsevier, Conference 3 to 22 March 2011.
Calza 2011b {published data only}
- Calza E, Porpiglia F, Fiori C, Giannone A, Pusineri A, Meli A, et al. Intraocular pressure increase during robot assisted laparoscopic prostatectomy: balanced anesthesia versus total intravenous anesthesia. Annual Meeting of the American Urological Association, AUA. Washington, DC, United States: Elsevier, Conference 14 to 19 May 2011.
Choi 2013 {published data only}
- Choi JH, Jung JW, Bang SR, Lee AR, Lee JJ. Changes in intraocular pressure during steep Trendelenburg position under propofol versus sevoflurane anesthesia. European Anaesthesiology Congress, EUROANAESTHESIA. Barcelona, Spain: Lippincott Williams and Wilkins, Conference Start: 20130601. Conference End: 20130604 (var. pagings).
References to studies awaiting assessment
Atallah 2009 {published data only}
- Atallah MM, Othman MM. Robotic laparoscopic radical cystectomy inhalational versus total intravenous anaesthesia: a pilot study. Middle East Journal of Anesthesiology 2009;20(2):257‐63. [PUBMED: 19583075] [PubMed] [Google Scholar]
Roh 2014 {published data only (unpublished sought but not used)}
- Roh G. The effect of propofol based total intravenous anaesthesia on oxidative stress and nitric oxide. https://clinicaltrials.gov/ct2/show/NCT02149628 Accessed 20 August 2015.
References to ongoing studies
Lee 2015 {published data only}
- Lee KY. Comparison of desflurane and propofol on quality of recovery in patients undergoing robotic or laparoscopic gastrectomy. ClinicalTrials.gov NCT02515968.
Additional references
Akkurt 2009
- Akkurt BCO, Temiz M, Inanoglu K, Aslan A, Turhanoglu S, Asfuroglu Z, et al. Comparison of recovery characteristics, postoperative nausea and vomiting, and gastrointestinal motility with total intravenous anesthesia with propofol versus inhalation anesthesia with desflurane for laparoscopic cholecystectomy: a randomized controlled study. Current Therapeutic Research ‐ Clinical and Experimental 2009;70(2):94‐103. [PUBMED: 24683221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Apfel 1999
- Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross‐validations between two centers. Anesthesiology 1999;91(3):693‐700. [PUBMED: 10485781] [DOI] [PubMed] [Google Scholar]
Awad 2009
- Awad H, Santilli S, Ohr M, Roth A, Yan W, Fernandez S, et al. The effects of steep Trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesthesia and Analgesia 2009;109(2):473‐8. [PUBMED: 19608821] [DOI] [PubMed] [Google Scholar]
Awad 2012
- Awad H, Walker CM, Shaikh M, Dimitrova GT, Abaza R, O'Hara J. Anesthetic considerations for robotic prostatectomy: a review of the literature. Journal of Clinical Anesthesia 2012;24(6):494‐504. [PUBMED: 22986320 ] [DOI] [PubMed] [Google Scholar]
Bai 2011
- Bai SJ. The Effect of Total Intravenous Anesthesia With Propofol on Postoperative Nausea and Vomiting in Patients Undergoing Robot‐assisted Laparoscopic Radical Prostatectomy. clinicalTrials.gov/show/NCT01402622 (accessed 20 Juni 2014). www.clinicaltrials.gov. [ClinicalTrials.gov Identifier:NCT01402622]
Barbash 2010
- Barbash GI, Glied SA. The technology and health care costs ‐ the case of robot‐assisted surgery. New England Journal of Medicine 2010;363(8):701‐4. [PUBMED: 20818872] [DOI] [PubMed] [Google Scholar]
Conti 2006
- Conti A, Iacopino DG, Fodale V, Micalizzi S, Penna O, Santamaria LB. Cerebral haemodynamic changes during propofol‐remifentanil or sevoflurane anaesthesia: transcranial Doppler study under bispectral index monitoring. British Journal of Anaesthesia 2006;97(3):333‐9. [PUBMED: 16829673] [DOI] [PubMed] [Google Scholar]
da Vinci 2016
- Intuitive Surgical Inc. FAQ. http://www.intuitivesurgical.com/company/faqs.html# (accessed 3 March 2016).
Danic 2007
- Danic MJ, Chow M, Alexander G, Bhandari A, Menon M, Brown M. Anesthesia considerations for robotic‐assisted laparoscopic prostatectomy: a review of 1,500 cases. Journal of Robotic Surgery 2007;1(2):119‐23. [PUBMED: 25484947 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fodale 2014
- Fodale V, D'Arrigo MG, Triolo S, Mondello S, Torre D. Anesthetic techniques and cancer recurrence after surgery. The Scientific World Journal 2014;2014:ID 328513. [PUBMED: 24683330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerges 2006
- Gerges FJ, Kanazi GE, Jabbour‐Khoury SI. Anesthesia for laparoscopy: a review. Journal of Clinical Anesthesia 2006;18(1):67‐78. [PUBMED: 16517337] [DOI] [PubMed] [Google Scholar]
Gupta 2004
- Gupta A, Stierer T, Zuckerman R, Sakima N, Parker SD, Fleisher LA. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: a systematic review. Anesthesia and Analgesia 2004;98:632‐41. [PUBMED: 14980911] [DOI] [PubMed] [Google Scholar]
Gurusamy 2012
- Gurusamy KS, Samraj K, Fusai G, Davidson BR. Robot assistant versus human or another robot assistant in patients undergoing laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006578.pub3; PUBMED: 22972093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐CP, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herati 2011
- Herati AS, Andonian S, Rais‐Bahrami S, Atalla MA, Srinivasan AK, Richstone L, et al. Use of the valveless trocar system reduces carbon dioxide absorption during laparoscopy when compared with standard trocars. Urology 2011;77(5):1126‐32. [PUBMED: 20888033] [DOI] [PubMed] [Google Scholar]
Hert 2003
- Hert SG, Cromheecke S, Broecke PW, Mertens E, Blier IG, Stockman BA, et al. Recovery of myocardial function after coronary surgery in elderly high‐risk patients. Anesthesiology 2003;99(2):314‐23. [PUBMED: 12883404] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hoshikawa 2014
- Hoshikawa Y, Tsutsumi N, Ohkoshi K, Serizawa S, Hamada M, Inagaki K, et al. The effect of steep Trendelenburg positioning on intraocular pressure and visual function during robotic‐assisted radical prostatectomy. The British Journal of Ophthalmology 2014;98(3):305‐8. [PUBMED: 24064941 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2013
- Hwang JW, Oh AY, Hwang DW, Jeon YT, Kim YB, Park SH. Does intraocular pressure increase during laparoscopic surgeries? It depends on anesthetic drugs and the surgical position. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2013;23(2):229‐32. [PUBMED: 23579523] [DOI] [PubMed] [Google Scholar]
Kakar 2011
- Kakar PN, Jyotirmoy D, Mittal RP, Vijaya P. Robotic invasion of operation theatre and associated anaesthetic issues: a review. Indian Journal of Anaesthesia 2011;55(1):18‐25. [PUBMED: 21431048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalmar 2010
- Kalmar AF, Foubert L, Hendrickx JFA, Mottrie A, Absalom A, Mortier EP, et al. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. British Journal of Anaesthesia 2010;104(4):433‐9. [PUBMED: 20167583] [DOI] [PubMed] [Google Scholar]
Kan 2015
- Kan KM, Brown SE, Gainsburg DM. Ocular complications in robotic‐assisted prostatectomy: a review of pathophysiology and prevention. Minerva Anesthesiologica 2015;81(5):557‐66. [PubMed] [Google Scholar]
Kumar 2014
- Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta‐analysis. Anaesthesia 2014;69(10):1138‐50. [PUBMED: 24847783] [DOI] [PubMed] [Google Scholar]
Landoni 2009
- Landoni G, Fochi O, Tritapepe L, Guarracino F, Belloni I, Bignami E, et al. Cardiac protection by volatile aesthetics. A review. Minerva Anestesiologica 2009;75(5):269‐73. [PUBMED: 18987572] [PubMed] [Google Scholar]
Li 2012
- Li M, Mei W, Wang P, Yu Y, Qian W, Zhang ZG, et al. Propofol reduces early post‐operative pain after gynecological laparoscopy. Acta Anaesthesiologica Scandinavica 2012;56(3):368‐75. [PUBMED: 22192060] [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu H, Ta L, Lu D, Song H, Wang L, Shi G. Robot‐assisted surgery in gynaecology. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011422] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mak 2014
- Mak TW, Lee JF, Futaba K, Hon SS, Ngo DK, Ng SS. Robotic surgery for rectal cancer: a systematic review of current practice. World Journal of Gastrointestinal Oncology 2014;6(6):184‐93. [PUBMED: 24936229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mettler 2008
- Mettler L, Schollmeyer T, Boggess J, Magrina JF, Oleszczuk A. Robotic assistance in gynaecological oncology. Current Opinion in Oncology 2008;20(5):581–9. [DOI: 10.1097/CCO.0b013e328307c7ec; PUBMED: 19106665] [DOI] [PubMed] [Google Scholar]
Møller 2006
- Møller A, Pedersen T. Evidence‐based anaesthesia and intensive care. Cambridge, UK: Cambridge University Press, 2006. [ISBN: 13 978‐0‐521‐69052‐6] [Google Scholar]
Nishikawa 2004
- Nishikawa K, Nakayama M, Omote K, Namiki A. Recovery characteristics and post‐operative delirium after long‐duration laparoscope‐assisted surgery in elderly patients: propofol‐based vs. sevoflurane‐based anesthesia. Acta Anaesthesiologica Scandinavica 2004;48(2):162‐8. [PUBMED: 14995937 ] [DOI] [PubMed] [Google Scholar]
Ortiz 2014
- Ortiz J, Chang LC, Tolpin DA, Minard CG, Scott BG, Rivers JM. Randomized, controlled trial comparing the effects of anesthesia with propofol, isoflurane, desflurane and sevoflurane on pain after laparoscopic cholecystectomy. Brazilian Journal of Anesthesiology (Elsevier) 2014;64(3):145‐51. [PUBMED: 24907871 ] [DOI] [PubMed] [Google Scholar]
Papanikolaou 2014
- Papanikolaou IG. Robotic surgery for colorectal cancer: systematic review of the literature. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2014;24:478‐83. [PUBMED: 25054567] [DOI] [PubMed] [Google Scholar]
Paranjape 2014
- Paranjape S, Chhabra A. Anaesthesia for robotic surgery. Trends in Anaesthesia and Critical Care 2014;4(1):25‐31. [DOI: 10.1016/j.tacc.2013.10.003] [DOI] [Google Scholar]
Pokkinen 2014
- Pokkinen SM, Yli‐Hankala A, Kalliomäki M‐L. The effects of propofol vs. sevoflurane on post‐operative pain and need of opioid. Acta Anaesthesiologica Scandinavica 2014;58:1‐6. [PUBMED: 25039403] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sampat 2015
- Sampat A, Parakati I, Kunnavakkam R, Glick DB, Lee NK, Tenney M, et al. Corneal abrasion in hysterectomy and prostatectomy ‐ role of laparoscopic and robotic assistance. Anesthesiology 2015;122(5):994‐1001. [PUBMED: 25734923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohn 2013
- Sohn W, Lee HJ, Ahlering TE. Robotic surgery: review of prostate and bladder cancer. Cancer Journal (Sudbury, Mass.) 2013;19(2):133‐9. [PUBMED: 23528721 ] [DOI] [PubMed] [Google Scholar]
Stone 2010
- Stone P, Burnett A, Burton B, Roman J. Overcoming extreme obesity with robotic surgery. International Journal of Medical Robotics and Computer Assisted Surgery 2010;6:382‐5. [DOI: 10.1002/rcs.341; PUBMED: 20812220] [DOI] [PubMed] [Google Scholar]
Sullivan 2008
- Sullivan MJ, Frost EA, Lew MW. Anesthetic care of the patient for robotic surgery. Middle East Journal of Anesthesiology 2008;19(5):967‐82. [PUBMED: 18637599] [PubMed] [Google Scholar]
Vari 2010
- Vari A, Gazzanelli S, Cavallaro G, Toma G, Tarquini S, Guerra C, et al. Post‐operative nausea and vomiting (PONV) after thyroid surgery: a prospective, randomized study comparing totally intravenous versus inhalational anesthetics. The American Surgeon 2010;76(3):325‐8. [PUBMED: 20349666] [PubMed] [Google Scholar]
Voldby 2016
- Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting ‐ a clinical review. Journal of Intensive Care 2016;4:27. [PUBMED: 27087980 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 2015
- Watt SK, Jakobsen HL, Vogelsang R, Kromann‐Andersen B, Palle C, et al. Implementation of a multidisciplinary robotic centre in a high‐volume university hospital. DMJ 2015;62:1‐5. [PUBMED: 26183049] [PubMed] [Google Scholar]
Weber 2007
- Weber ED, Colyer MH, Lesser RL, Subramanian PS. Posterior ischemic optic neuropathy after minimally invasive prostatectomy. Journal of Neuro‐Ophthalmology 2007;27(4):285‐7. [PUBMED: 18090562] [DOI] [PubMed] [Google Scholar]
Yoo 2012b
- Yoo YC, Lee K‐Y, Choi EK, Bai S‐J. Total intravenous anaesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot‐assisted laparoscopic radical prostatectomy: prospective randomised trial. Paris, France: Lippincott Williams and Wilkins, Conference from 6 to 9 June 2012. [DOI] [PMC free article] [PubMed]
Yoo 2014b
- Yoo YC, Shin S, Choi EK, Kim CY, Choi YD, Bai SJ. A comparison of totally intravenous and inhalation anesthesia for intraocular pressure during robot‐assisted laparoscopic radical prostatectomy. www.clinicaltrials.gov (NCT01744262: accessed 20 June 2015). [clinicaltrials.gov identifier NCT01744262]
Zoremba 2011
- Zoremba M, Dette F, Hunecke T, Eberhart L, Braunecker S, Wulf H. A comparison of desflurane versus propofol: the effects on early postoperative lung function in overweight patients. Anesthesia and Analgesia 2011;113(1):63‐9. [PUBMED: 20966444] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Herling 2014
- Herling SF, Dreijer B, Thomsen T, Møller AM. Total intravenous anaesthesia versus inhalational anaesthesia for transabdominal robotic assisted laparoscopic surgery (Protocol). Cochrane Database of Systematic Reviews 2014;Issue 12(12):CD011387. [DOI: 10.1002/14651858.CD011387.] [DOI] [PMC free article] [PubMed] [Google Scholar]


