Abstract
Background
High blood pressure is an important risk factor for cardiovascular disease, contributing to about 50% of cardiovascular events worldwide and 37% of cardiovascular‐related deaths in Western populations. Epidemiological studies suggest that cocoa‐rich products reduce the risk of cardiovascular disease. Flavanols found in cocoa have been shown to increase the formation of endothelial nitric oxide which promotes vasodilation and therefore blood pressure reduction. Here we update previous meta‐analyses on the effect of cocoa on blood pressure.
Objectives
To assess the effects on blood pressure of chocolate or cocoa products versus low‐flavanol products or placebo in adults with or without hypertension when consumed for two weeks or longer.
Search methods
This is an updated version of the review initially published in 2012. In this updated version, we searched the following electronic databases from inception to November 2016: Cochrane Hypertension Group Specialised Register, CENTRAL, MEDLINE and Embase. We also searched international trial registries, and the reference lists of review articles and included trials.
Selection criteria
Randomised controlled trials (RCTs) investigating the effects of chocolate or cocoa products on systolic and diastolic blood pressure in adults for a minimum of two weeks duration.
Data collection and analysis
Two review authors independently extracted data and assessed the risks of bias in each trial. We conducted random‐effects meta‐analyses on the included studies using Review Manager 5. We explored heterogeneity with subgroup analyses by baseline blood pressure, flavanol content of control group, blinding, age and duration. Sensitivity analyses explored the influence of unusual study design.
Main results
Thirty‐five trials (including 40 treatment comparisons) met the inclusion criteria. Of these, we added 17 trials (20 treatment comparisons) to the 18 trials (20 treatment comparisons) in the previous version of this updated review.
Trials provided participants with 30 to 1218 mg of flavanols (mean = 670 mg) in 1.4 to 105 grams of cocoa products per day in the active intervention group. The control group received either a flavanol‐free product (n = 26 treatment comparisons) or a low‐flavanol‐containing cocoa powder (range 6.4 to 88 mg flavanols (mean = 55 mg, 13 treatment comparisons; 259 mg, 1 trial).
Meta‐analyses of the 40 treatment comparisons involving 1804 mainly healthy participants revealed a small but statistically significant blood pressure‐reducing effect of flavanol‐rich cocoa products compared with control in trials of two to 18 weeks duration (mean nine weeks): Mean difference systolic blood pressure (SBP) (95% confidence interval (CI): ‐1.76 (‐3.09 to ‐0.43) mmHg, P = 0.009, n = 40 treatment comparisons, 1804 participants; Mean difference diastolic blood pressure (DBP) (95% CI): ‐1.76 (‐2.57 to ‐0.94) mmHg, P < 0.001, n = 39 treatment comparisons, 1772 participants.
Baseline blood pressure may play a role in the effect of cocoa on blood pressure. While systolic blood pressure was reduced significantly by 4 mmHg in hypertensive people (n = 9 treatment comparisons, 401 participants), and tended to be lowered in prehypertensive people (n= 8 treatment comparisons, 340 participants), there was no significant difference in normotensive people (n = 23 treatment comparisons, 1063 participants); however, the test for subgroup differences was of borderline significance (P = 0.08; I2 = 60%), requiring further research to confirm the findings.
Subgroup meta‐analysis by blinding suggested a trend towards greater blood pressure reduction in unblinded trials compared to double‐blinded trials, albeit statistically not significant. Further research is needed to confirm whether participant expectation may influence blood pressure results. Subgroup analysis by type of control (flavanol‐free versus low‐flavanol control) did not reveal a significant difference.
Whether the age of participants plays a role in the effect of cocoa on blood pressure, with younger participants responding with greater blood pressure reduction, needs to be further investigated.
Sensitivity analysis excluding trials with authors employed by trials sponsoring industry (33 trials, 1482 participants) revealed a small reduction in effect size, indicating some reporting bias.
Due to the remaining heterogeneity, which we could not explain in terms of blinding, flavanol content of the control groups, age of participants, or study duration, we downgraded the quality of the evidence from high to moderate.
Results of subgroup analyses should be interpreted with caution and need to be confirmed or refuted in trials using direct randomised comparisons.
Generally, cocoa products were highly tolerable, with adverse effects including gastrointestinal complaints and nausea being reported by 1% of participants in the active cocoa intervention group and 0.4% of participants in the control groups (moderate‐quality evidence).
Authors' conclusions
This review provides moderate‐quality evidence that flavanol‐rich chocolate and cocoa products cause a small (2 mmHg) blood pressure‐lowering effect in mainly healthy adults in the short term.
These findings are limited by the heterogeneity between trials, which could not be explained by prespecified subgroup analyses, including blinding, flavanol content of the control groups, age of participants, or study duration. However, baseline blood pressure may play a role in the effect of cocoa on blood pressure; subgroup analysis of trials with (pre)hypertensive participants revealed a greater blood pressure‐reducing effect of cocoa compared to normotensive participants with borderline significance.
Long‐term trials investigating the effect of cocoa on clinical outcomes are also needed to assess whether cocoa has an effect on cardiovascular events and to assess potential adverse effects associated with chronic ingestion of cocoa products.
Plain language summary
Effect of cocoa on blood pressure
Review question
We assessed the effect of cocoa products on blood pressure in adults when consumed daily for at least two weeks. We found 35 studies, covering 40 treatment comparisons.
Background
Dark chocolate and cocoa products are rich in chemical compounds called flavanols. Flavanols have attracted interest as they might help to reduce blood pressure, a known risk factor for cardiovascular disease (disorders of the heart and blood vessels). The blood pressure‐lowering properties of flavanols are thought to be related to widening of the blood vessels, caused by nitric oxide.
Study characteristics
Studies were short, mostly between two and12 weeks, with only one of 18 weeks. The studies involved 1804 mainly healthy adults. They provided participants with 30 to 1218 mg of flavanols (average of 670 mg) in 1.4 to 105 grams of cocoa products per day in the active intervention group. Seven of the studies were funded by companies with a commercial interest in their results, and the reported effect was slightly larger in these studies, indicating possible bias. The evidence is current to November 2016.
Key results
Meta‐analysis of 40 treatment comparisons revealed a small but statistically significant lowering of blood pressure (systolic and diastolic) of 1.8 mmHg. This small reduction in blood pressure might complement other treatment options and might contribute to reducing the risk of cardiovascular disease.
We investigated whether participants' blood pressure at the start of the study, their age, an awareness of group allocation (active or control), the flavanol content used in the control group, or how long the study lasted may explain variations between trials. While blood pressure status (high blood pressure or normal blood pressure) is a likely factor in the effect size of cocoa on blood pressure, the impact of other factors needs to be confirmed or rejected in further trials.
Side effects including digestive complaints and dislike of the trial product were reported by only 1% of people in the active cocoa intervention group and 0.4% of people in the control groups.
Longer‐term trials are needed to establish whether regularly eating flavanol‐rich cocoa products has a beneficial effect on blood pressure and cardiovascular health over time, and whether there are any side effects of long‐term use of cocoa products on a daily basis.
Quality of evidence
The evidence is of moderate quality. We were unable to identify any randomised controlled trials that tested the effect of long‐term daily use of cocoa products on blood pressure, and there were no trials that measured the health consequences of high blood pressure, such as heart attacks or strokes.
Summary of findings
Summary of findings for the main comparison. Flavanol‐rich cocoa products for blood pressure.
| Flavanol‐rich cocoa products for blood pressure | ||||||
| Patient or population: adults with or without hypertension Settings: Primary healthcare practice, community Intervention: flavanol‐rich cocoa products versus control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Flavanol‐rich cocoa products | |||||
| Systolic blood pressure clinical digital sphygmomanometer Follow‐up: mean 9 weeks | The mean systolic blood pressure ranged across control groups from 107 to 154 mm Hg | The mean systolic blood pressure in the intervention groups was 1.76 mmHg lower (3.09 to 0.43 lower) | 1804 (35 trials with 40 treatment comparisons) | ⊕⊕⊕⊕ moderate1,2,3,4 | ||
| Diastolic blood pressure clinical digital sphygmomanometer Follow‐up: mean 9 weeks | The mean diastolic blood pressure ranged across control groups from 66 to 92 mm Hg | The mean diastolic blood pressure in the intervention groups was 1.76 mmHg lower (2.57 to 0.94 lower) | 1772 (34 trials with 39 treatment comparisons) | ⊕⊕⊕⊕ moderate1,2,3,4 | ||
| Withdrawals due to adverse effects | 8 trials reported no withdrawals and no adverse effects. 9 trials reported adverse effects, including gastrointestinal complaints (cocoa groups: n = 8/760 (1%), control groups: n = 3/754 (0.4%)); dislike of the trial product (cocoa: n = 4/760; control: n = 1/754), headache (cocoa: n = 2/760; control: n = 1/754), and jitteriness (cocoa: n = 1/760, control: n = 0/754). | 1514 (31 trials) reported on withdrawals and adverse effects | ⊕⊕⊕⊕ moderate1,2,3,4 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1.Downgraded to moderate quality due to high heterogeneity which cannot be explained by subgroup analyses. SBP/DBP: I2 = 87%/78%.
2.Good quality across 40 treatment comparisons. Only 5 trials (12.5%) had 2 items at high risk of bias, 19 trials (47.5%) had 1 item at high risk of bias, and 16 trials (40%) had no items at high risk of bias. 17 trials were unblinded or single‐blinded. 7 industry‐sponsored trials had authors employed by industry. Only 4 trials (10%) had more than 20% attrition. We explored influence of trials with items at high risk of bias by subgroup and sensitivity analysis. 3.Statistically significant SBP: P = 0.009; DBP: P < 0.001. 4.Sensitivity analysis excluding treatment comparisons (n = 7) with authors employed by trials sponsoring industry revealed reduced effect size and statistical significance.
Background
Dark chocolate and flavanol‐rich cocoa products have attracted interest as an alternative treatment option for hypertension, a known risk factor for cardiovascular disease. Even small reductions in blood pressure may substantially reduce cardiovascular risk. Current guidelines strongly recommend integration of lifestyle modification and complementary treatment with the use of conventional blood pressure medications.
The interest in the effect of cocoa on blood pressure (BP) started with the discovery that an island population in Central America, the Kuna Indians, had a distinctively low rate of hypertension coupled with a consistent healthy low blood pressure unaffected by age (Hollenberg 2006; Kean 1944). The majority of the Kuna Indians live on the San Blas Island off Panama (population approximately 35,000); those Kuna Indians who migrated to the mainland had a higher prevalence of hypertension as well as an age‐dependent rise in blood pressure, implying that lifestyle factors such as diet rather than genetics play a protective role (McCullough 2006). Island‐dwelling Kuna Indians consume about three to four cups of cocoa drinks on average per day, while the mainland‐dwelling Kuna Indians consume up to 10 times less cocoa (McCullough 2006; Schroeter 2006). Average high salt intake was not associated with the differences in blood pressure (McCullough 2006). Mean blood pressure of the island‐dwelling adult Kuna Indians hovers around 110 mmHg systolic and 70 mmHg diastolic, while on the mainland the observed age‐related rise in blood pressure and prevalence of hypertension is comparable with that of Western populations (Hollenberg 2006).
Description of the condition
High blood pressure is a critically important risk factor for cardiovascular disease, attributable for 47% of ischaemic heart disease and 54% of stroke events worldwide (Lawes 2008). More than a third (37%) of cardiovascular deaths are attributed to hypertension in Western populations (Martiniuk 2007), and 13.5% globally (Lawes 2008). The association between cardiovascular risk and blood pressure levels is continuous (McInnes 2005) with the risk of ischaemic heart disease and stroke halved for every 20 mmHg reduction in systolic blood pressure (SBP) and 10 mmHg diastolic blood pressure (DBP) (Lewington 2002). Even small reductions in blood pressure may therefore reduce cardiovascular events at a population level.
However, a steady increase in SBP with age is expected, whereas DBP tends to fall after middle age, with studies in elderly and middle‐aged populations suggesting a nonlinear J‐ or U‐shaped relationship between blood pressure and mortality (Bangalore 2010; Denker 2013). Appropriate assessment of an individual’s BP status is important to guide whether antihypertension therapy is indicated or to avoid potential overtreatment.
Blood pressure levels are defined as:
Primary hypertension: SBP ≥ 140 mmHg or DBP ≥ 90 mm‐Hg
Prehypertension: SBP 120 ‐ 139 mmHg or DBP 80 ‐ 89 mmHg
Normotension: SBP < 120 mmHg or DBP < 80 mmHg, secondary hypertension
Description of the intervention
Cocoa is extracted from cacao beans, the fatty seeds of the Theobroma cacao tree. Cocoa is rich in flavanols, particularly epicatechin, catechin and procyanidins, proposed to be responsible for the blood pressure‐lowering effect (Corti 2009; Heiss 2010a). Flavanols are also found in other plant‐derived produce, including beans, apricots, blackberries, apples and tea leaves, albeit in a lower concentration than in cocoa products (460 ‐ 610 mg/kg of flavanol monomers; 4 ‐ 5 g/kg of flavanol polymers) (Fernandez‐Murga 2011; Hammerstone 2000). Flavanol intake is, however, also dependent on serving size, and flavanol content depends on the processing of the cacao beans and raw cocoa.
Traditionally cocoa was consumed as a cold unsweetened drink of raw dried cacao powder, often mixed with starch and spices by the native Latin‐American Indians, but this was considered bitter and unpalatable by the early European explorers, including Christopher Columbus in 1502 and Hernando Cortes in 1519. The Spanish brought cocoa to Europe, added sugar to it and heated the drink (Dillinger 2000; Lippi 2009). Subsequent roasting (up to 120 °C), mixing (conching), alkalising (dutching), adding sugar, milk, vanilla and lecithin emulsifiers make chocolate as we know it today (Beckett 2008). Various chocolate manufacturers have fine‐tuned the processing, leading to different flavours and smoothness of chocolates, but also to altered cocoa and flavanol content in various cocoa products.
Dark chocolate contains larger amounts of cocoa (50% ‐ 85%) than milk chocolate (20% ‐ 30%). Different processes influence the flavanol content of the cocoa in the chocolate; a 70% cocoa‐containing chocolate bar from one company therefore might not contain the same amount of flavanols and flavanol composition as a 70% chocolate bar from another company. Content and composition of flavanols depend on the variety and ripeness of cocoa beans used, as well as the manufacturing steps.
Fresh and fermented cocoa beans contain about 10% of flavanols (100 mg/g). The cocoa powder consumed by the Kuna Indians contains about 3.6% of flavanols, and cocoa‐rich dark chocolate on the market about 0.5% of flavanols (Chaitman 2006; Chevaux 2001). Moreover, heavy dutching (the alkalising of chocolate to pH 7 ‐ 8) can reduce the flavanol content to less than 10 mg per 100 grams (0.001%).
Research suggests that the monomeric portion of cocoa flavanols, epicatechin and catechin and to a lesser extent the polymeric flavanols, the procyanidins, are linked to blood pressure and vasoactive effects (Schroeter 2006). Modern processing of cacao reduces the monomeric flavanol content and influences the epicatechin/catechin ratio (Payne 2010). Fresh and fermented cocoa beans contain between 2.5 and 16.5 mg of epicatechin per gram, depending on the variety, the growing region and harvesting practices (Kim 1984; Wollgast 2000), whereas processed cocoa retains only 2% ‐ 18% of the original epicatechin, due to roasting and dutching (Payne 2010). Because of the large variation in flavanol content in chocolate and cocoa products, it is critical to compare the dosages of flavanols rather than simply the amounts of chocolate or administered cocoa products in clinical trials investigating the effect of cocoa on blood pressure.
How the intervention might work
The blood pressure‐lowering properties of cocoa have been linked to the formation of endothelial nitric oxide (NO) which promotes vasodilation and consequently lowers blood pressure. Increased NO production might be triggered by upregulation of NO‐synthase through the insulin‐mediated signalling pathway (Addison 2008). Insulin sensitivity has been shown to be improved after cocoa intake in a number of trials (Davison 2008a; Faridi 2008; Grassi 2005a; Grassi 2008), although Muniyappa 2008 did not confirm this. Secondly, cocoa flavanols have been shown to inhibit angiotensin converting enzyme (ACE) activity, and hence reduce blood pressure (Actis‐Goretta 2006; Persson 2011). Thirdly, there is evidence to suggest that cocoa flavanols have an indirect antioxidant effect within the cardiovascular system, upregulating NO‐synthase activity and hence reducing blood pressure (Fraga 2011; Keen 2005).
Why it is important to do this review
In the last decade, several clinical trials have investigated the effect of chocolate and cocoa products on blood pressure. This systematic review updates previous meta‐analyses by Taubert 2007a (including five trials), Desch 2010a (10 trials), Ried 2010 (15 trials), and updates a previous version of this Cochrane Review (20 treatment comparisons) (Ried 2012). In addition, we explore the influence of baseline blood pressure, type of control (flavanol dosage), age, duration, and trial quality, in particular blinding, on blood pressure outcomes.
Objectives
To assess the effects on blood pressure of chocolate or cocoa products versus low‐flavanol products or placebo in adults with or without hypertension when consumed for two weeks or longer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled parallel or cross‐over, single‐blind, double‐blind or open‐label trials of 14 days or longer duration that reported the clinical mean or median with or without standard deviation (SD) or standard error (SE) SBP or DBP at baseline, before and after intervention.
Types of participants
Adults, with no further restrictions.
Types of interventions
We included trials if the control group received an intervention, e.g. a placebo or a minimal dose of flavanol‐containing cocoa product.
We excluded:
Trials in which the control dose exceeds 25% cocoa polyphenols of the active dose
Trials testing isolated flavanols on blood pressure
Trials with a very high attrition rate (loss to follow‐up greater than 50%)
Types of outcome measures
Primary outcomes
Difference between cocoa and control group in systolic and diastolic blood pressure at final follow‐up, and adjusted for baseline differences.
Secondary outcomes
Number of participants who withdrew due to adverse effects or intolerance, and total adverse events.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on OVID for primary studies:
Cochrane Hypertension Group Specialised Register (1948 ‐ Nov 2016), Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 2), MEDLINE (1948 ‐ Nov 2016), Embase (1980 ‐ Nov 2016), and Food Science and Technology Abstracts (1969 ‐ Nov 2016).
International trial registries (clinicaltrials.gov; www.trialregister.nl; www.anzctr.org.au; www.controlled‐trials.com; www.apps.who.int/trialsearch/WHO clinical trials) for unpublished but completed studies investigating chocolate/cocoa for blood pressure.
We searched the electronic databases using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) with selected MeSH terms and free‐text terms, including cocoa, chocolate, blood pressure, and hypertension, with no language restrictions. The MEDLINE search strategy (Appendix 1) was translated into the Hypertension Group Specialised Register (Appendix 2), CENTRAL (Appendix 3), Embase (Appendix 4), and Food Science and Technology Abstracts (Appendix 5), using the appropriate controlled vocabulary as applicable, and the Database of Abstracts of Reviews of Effectiveness (DARE) and the Cochrane Database of Systematic Reviews for related reviews.
Searching other resources
We identified reference lists of all papers and relevant reviews.
We contacted authors of relevant papers regarding any further published or unpublished work.
We searched ISI Web of Science for papers which cite studies included in the review.
Data collection and analysis
Selection of studies
Two review authors independently assessed titles and abstracts of search results for relevant articles, and critically appraised the full text of relevant articles according to the inclusion criteria listed above. We resolved any discrepancies by discussion.
Data extraction and management
Two review authors independently extracted data using a standardised data extraction form and then cross‐checked them.
Assessment of risk of bias in included studies
Two review authors assessed the risks of bias for each trial by using the Cochrane tool for assessing risk of bias. This covers random sequence generation (selection bias), allocation concealment (selection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), and source of funding (other bias).
Measures of treatment effect
Mean difference in SBP/DBP in mmHg at final follow‐up, adjusted for baseline differences. We estimated the precision of mean differences as the standard deviation (SD) at final follow‐up.
When blood pressure measurements were reported in more than one position, the order of preference was: 1) sitting; 2) standing; and 3) supine.
When both clinical and ambulatory blood pressure measurements were available, the order of preference was: 1) clinical; 2) ambulatory.
Unit of analysis issues
If results are reported for several periods of follow‐up, we preferred the longest follow‐up from each study for comparison with baseline.
We conducted meta‐analysis of cross‐over trials by the generic invariance method, using mean differences and standard errors between outcome measurements (blood pressure) of experimental (cocoa) versus control groups. We extracted the mean (SE) blood pressure before and after intervention from tables, graphs, and text from individual studies included in the meta‐analysis.
In multiple‐arm studies, we included only the intervention arms and their comparable control arms in the meta‐analysis. Comparable intervention/control groups in multiple‐arm studies may have been stratified by age, body mass index (BMI), or blood markers. We avoided double‐counting of individual participants in the meta‐analysis.
Dealing with missing data
We contacted the authors of studies with missing information on mean SBP/DBP or SD or both in intervention and control groups and asked them to provide the missing data.
If standard errors were given instead of standard deviations, we calculated standard deviations at one time point with the formula SD = SE x square root of n. We assumed a correlation of 0.68 between the final follow‐up SBP/DBP results for the two treatment arms in a cross‐over trial, similar to previous meta‐analyses by Taubert 2007a and Desch 2010a.
If both standard deviations and standard errors were missing, we imputed standard deviations based on the information in the same trial or from other trials using the same intervention. We used the following hierarchy to impute standard deviation values:
standard deviation of blood pressure at end of treatment taken in a different position from that of the blood pressure data used
standard deviation of blood pressure at baseline
mean standard deviation of blood pressure at end of treatment from other trials using the same intervention
Assessment of heterogeneity
We assessed heterogeneity by the I2 statistic (Higgins 2003). We tested the following variables by subgroup analyses: baseline SBP or DBP, dosage of flavanols in the control group, age, study duration, and blinding.
Assessment of reporting biases
We assessed small‐study effects by funnel plots.
Data synthesis
For each study, we recorded the number of participants, mean difference, and the SE of intervention and control groups in Cochrane Review Manager 5 software. We used the generic inverse variance method to combine both parallel‐group and cross‐over trials, and the random‐effects model to incorporate heterogeneity.
Subgroup analysis and investigation of heterogeneity
We required at least four studies to conduct subgroup analysis.
We performed the following subgroup analyses:
Baseline SBP ≥ 140 mmHg versus SBP 130 ‐ 140 versus SBP < 130 mmHg
Baseline DBP ≥ 80 mmHg versus DBP < 80 mmHg
Flavanol‐free control versus low flavanol control
Double‐blind versus single‐blind/unblinded trials
Mean age < 50 years versus ≥ 50 years
Trial duration two to four weeks versus more than four weeks
We considered evidence of the differences found between subgroups to be stronger when the variation of the mean effects in the different subgroups was higher, as measured by the I2statistic for subgroup differences (e.g. I2 = 90% was considered more significant than I2 = 70%).
Sensitivity analysis
We tested the robustness of the results using the following sensitivity analyses:
Exclusion of trials using a unique study design compared to other trials (e.g. high flavanol content in the control group (20% ‐ 25%) compared to active group, close to threshold level for excluded trials (> 25% flavanol content in control group).
'Summary of findings' table
The Table 1 summarises the magnitude of the effect of cocoa on systolic and diastolic blood pressure of the 35 RCTs including 40 treatment comparisons and 1804 adults, and rates the quality of the evidence using the GRADE system, by assessing potential within‐study biases and between‐study heterogeneity (Guyatt 2008).
Results
Description of studies
Results of the search
The updated Cochrane search strategy (inception to October 2015) using Scopus, PubMed and Embase, identified 254 potentially relevant publications which we assessed at the title/abstract level,in addition to the 136 articles in the previous review. Of 26 new potentially relevant trials (in 27 articles) assessed at the full‐text level, 17 new trials (20 new treatment comparisons, active vs control) met the inclusion criteria for meta‐analysis. Adding these to the 20 treatment comparisons in 18 trials from the previous version of this review (Ried 2012) gives a total of 40 treatment comparisons (from 35 trials) in the updated meta‐analysis. (Figure 1).
1.
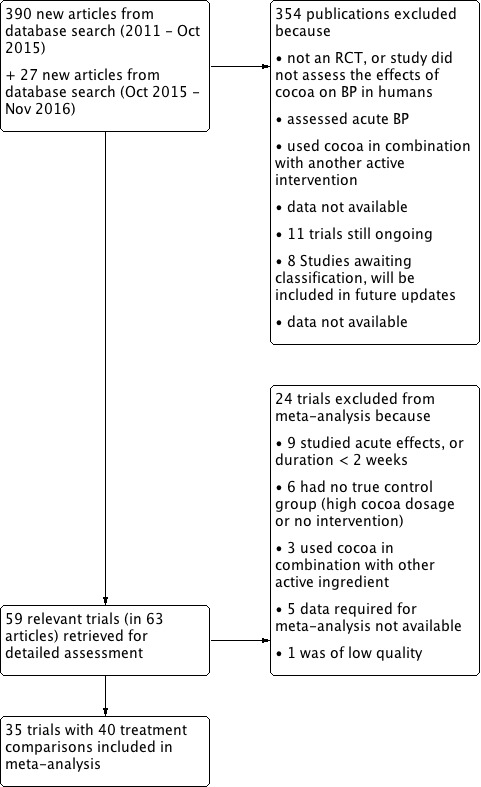
PRISMA Flow diagram
Included studies
We include 35 trials involving 1804 participants in this updated review.
Of the 35 trials, five contained two treatment arms with comparable non‐overlapping control groups, resulting in 40 bringing the number of treatment comparisons in the updated review. Trials with multiple treatment arms provided results stratified on the basis of blood pressure (normotensive/hypertensive) (Grassi 2005a), exercise (treatment only or in addition to exercise) (Davison 2008a), BMI (< 25, > 25 kg/m2) (Almoosawi 2012a), cholesterol (high, normal) (Sarria 2014), or age (young, elderly) (Heiss 2015a).
Eleven trials used commercially available chocolate and 24 trials used flavanol‐rich cocoa powder (tablet, bar, or powder mixed with water or milk) and compared the effect to a control group, which either took flavanol‐free placebo (white chocolate, milk or placebo pill) or low‐flavanol powder. The active intervention group received either dark chocolate of 3.6 to 105 grams (6 grams are equal to one piece of a 100‐gram dark chocolate bar) containing 50% to 90% cocoa, milk chocolate‐based confectionary (105 grams of < 10% cocoa) or flavanol‐enriched cocoa powder, containing a dosage of 30 to 1218 mg (mean = 670 mg) of flavanols per day. Trials ran between two weeks and 12 weeks, with a single trial ran 18 weeks.
Excluded studies
We excluded 24 trials from our meta‐analysis, because:
Trials investigated the acute effects within two hours after cocoa ingestion (n = 2)
The intervention period was less than two weeks (n = 7)
Trials did not have a true control group (n = 6)
The intervention was cocoa plus another active ingredient (n = 3)
Data required for meta‐analysis were not available (n = 5)
The trial was of low quality (n = 1)
See Figure 1; Characteristics of excluded studies table.
Ongoing studies
Eleven unpublished trials were identified in trial registries, they were either not completed at time of meta‐analysis or data were not yet available (Characteristics of ongoing studies).
Studies awaiting classification
Eight recent additional studies were found just before finalizing the updated review for publication (Characteristics of studies awaiting classification). These could potentially meet the inclusion criteria but in order to establish that it would require careful assessment. We chose not to include these studies in this update to avoid further delays in publication, but this will be done in a future update.
Risk of bias in included studies
'Risk of bias' assessments are summarised in Figure 2.
2.
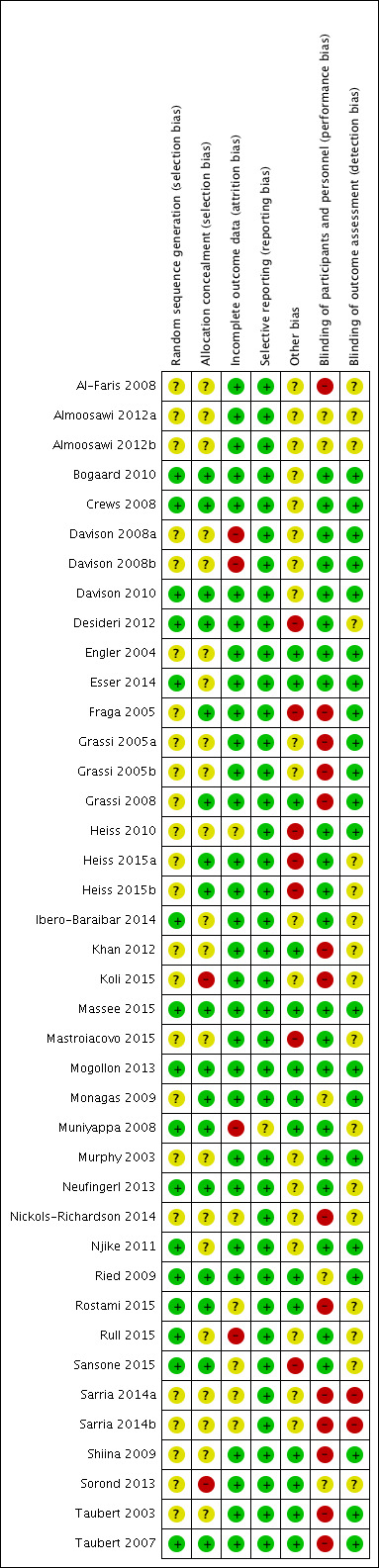
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Sixteen trials adequately described random sequence generation (Bogaard 2010; Crews 2008; Davison 2010; Desideri 2012; Esser 2014; Ibero‐Baraibar 2014; Massee 2015; Mogollon 2013; Muniyappa 2008; Neufingerl 2013; Njike 2011; Ried 2009; Rostami 2015; Rull 2015; Sansone 2015; Taubert 2007).
Random sequence generation was unclear in 19 trials (Al‐Faris 2008; Almoosawi 2012a (two treatment comparisons); Davison 2008a (two treatment comparisons); Engler 2004; Fraga 2005; Grassi 2005a (two treatment comparisons); Grassi 2008; Heiss 2010; Heiss 2015a (two treatment comparisons); Khan 2012; Koli 2015; Mastroiacovo 2015; Monagas 2009; Murphy 2003; Nickols‐Richardson 2014; Sarria 2014 (two treatment comparisons); Shiina 2009; Sorond 2013; Taubert 2003).
Allocation concealment
Eighteen trials described adequate allocation concealment (Bogaard 2010; Crews 2008; Davison 2010; Desideri 2012; Esser 2014; Fraga 2005; Grassi 2008; Heiss 2015a (two treatment comparisons); Massee 2015; Mogollon 2013; Monagas 2009; Muniyappa 2008; Neufingerl 2013; Ried 2009; Rostami 2015; Sansone 2015; Taubert 2007).
Seventeen trials provided insufficient information regarding allocation concealment (Al‐Faris 2008; Almoosawi 2012a; Davison 2008a (two treatment comparisons); Engler 2004; Grassi 2005a (two treatment comparisons); Heiss 2010; Ibero‐Baraibar 2014; Khan 2012; Mastroiacovo 2015; Murphy 2003; Nickols‐Richardson 2014; Njike 2011; Rull 2015; Sarria 2014 (two treatment comparisons); Shiina 2009; Sorond 2013; Taubert 2003).
Allocation was unconcealed in one trial (Koli 2015).
Blinding
Performance bias
Unblinded/ single‐blinded trials
Thirteen trials compared the cocoa group with unblinded controls using commercially available white chocolate, or only milk or water (Al‐Faris 2008; Fraga 2005; Grassi 2005a (two treatment comparisons); Grassi 2008; Khan 2012; Koli 2015; Monagas 2009; Nickols‐Richardson 2014; Rostami 2015; Sarria 2014 (two treatment comparisons); Shiina 2009; Taubert 2003; Taubert 2007).
One trial (Almoosawi 2012a; two treatment comparisons) reported a single‐blind design, with participants but not investigators probably blinded, as the placebo dark chocolate was matched in taste, texture, colour and macronutrient composition.
Double‐blinded trials
Thirteen trials used a low‐flavanol cocoa product as the control aiming to facilitate ‘blinding’ or ‘masking’ of participants to minimise any expectation bias or placebo effect (Crews 2008; Davison 2008a (two treatment comparisons); Davison 2010; Desideri 2012; Esser 2014; Heiss 2010; Mastroiacovo 2015; Mogollon 2013; Muniyappa 2008; Murphy 2003; Njike 2011; Rull 2015; Sorond 2013).
Eight trials used a blinded design with flavanol‐free control groups (Bogaard 2010; Engler 2004; Heiss 2015a (two treatment comparisons); Ibero‐Baraibar 2014; Massee 2015; Neufingerl 2013; Ried 2009; Sansone 2015).
Blinding was achieved in seven of the eight trials by matching taste, colour, texture, energy and nutrient components of the cocoa and placebo products. In addition, one trial (Ried 2009) compared the effect on blood pressure of dark chocolate or tomato extract capsules with placebo capsules. In this trial, blinding of the control group but not the dark chocolate group was assured, as participants in the control group did not know if they were allocated into an active or placebo capsule group.
Detection bias
One trial (Almoosawi 2012a; two treatment comparisons) reported adequate outcome assessment (n = 21), or did not report details but used standard blood pressure monitoring procedures (n = 16).
Incomplete outcome data
All but three trials (Davison 2008a (two treatment comparisons); Muniyappa 2008; Rull 2015) had less than 20% attrition.
Selective reporting
None of the trials was biased due to selective reporting. However, industry‐funding may have introduced a bias.
Other potential sources of bias
We found a small risk of publication bias, with slightly asymmetrical funnel plots, probably due to high heterogeneity of the 35 trials included in the meta‐analysis.
Involvement of industry‐sponsored studies may have influenced results. We therefore conducted a sensitivity analysis excluding trials (n = 6 trials) in which authors were employed by industry (Desideri 2012; Fraga 2005; Heiss 2010; Heiss 2015a (two comparisons); Mastroiacovo 2015; Sansone 2015) (see Analysis 7.1 and Analysis 7.2).
7.1. Analysis.
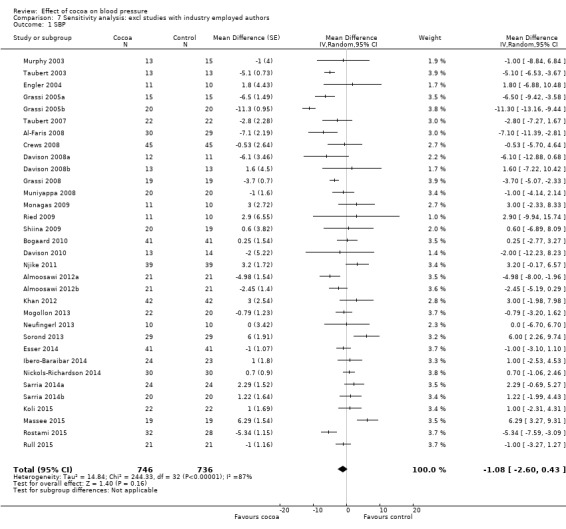
Comparison 7 Sensitivity analysis: excl studies with industry employed authors, Outcome 1 SBP.
7.2. Analysis.
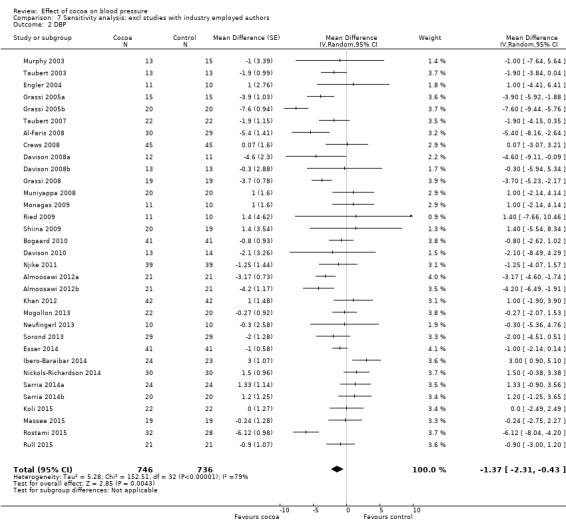
Comparison 7 Sensitivity analysis: excl studies with industry employed authors, Outcome 2 DBP.
Effects of interventions
See: Table 1
Meta‐analysis of all 40 treatment comparisons revealed a significant blood pressure‐reducing effect of flavanol‐rich cocoa products compared with control.
Mean difference systolic blood pressure (SBP) (95% confidence interval (CI)): ‐1.76 (‐3.09 to ‐0.43) mmHg, P = 0.009, 40 comparisons, 1804 participants; Mean difference diastolic blood pressure (DBP) (95% CI): ‐ 1.76 (‐2.57 to ‐0.94) mmHg, P < 0.001, 39 comparisons, 1772 participants.
Analysis 1.1, (Figure 3); Analysis 1.2, (Figure 4)
1.1. Analysis.
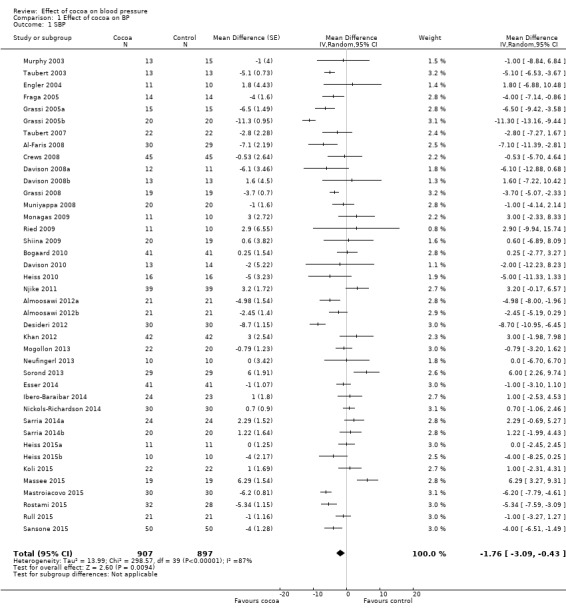
Comparison 1 Effect of cocoa on BP, Outcome 1 SBP.
3.
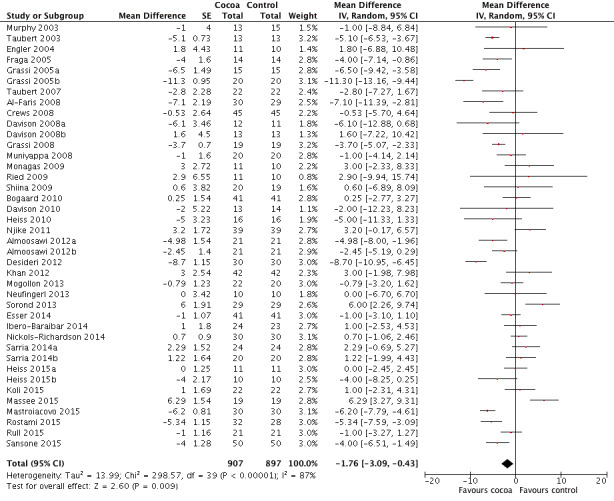
Forest plot of comparison: 1 Effect of cocoa on BP, outcome: 1.1 SBP.
1.2. Analysis.
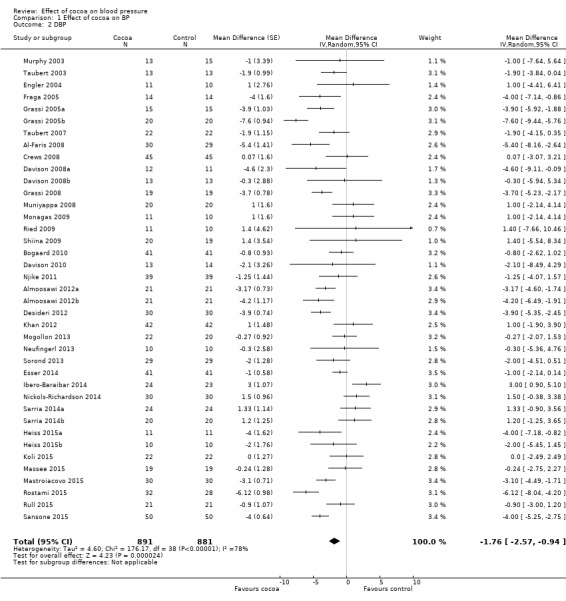
Comparison 1 Effect of cocoa on BP, Outcome 2 DBP.
4.
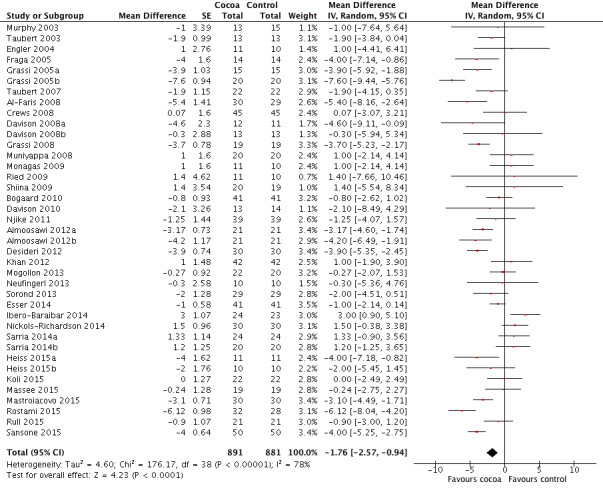
Forest plot of comparison: 1 Effect of cocoa on BP, outcome: 1.2 DBP.
Baseline blood pressure ‐ hypertensive, prehypertensive, normotensive
The previous versions of our review had revealed a difference in effect of cocoa products on blood pressure, depending on hypertension status at baseline. While blood pressure was significantly lowered in people with systolic hypertension (≥ 140 mmHg) or diastolic prehypertension (≥ 80 mmHg), there was no significant effect of cocoa on people with normal blood pressure (120/80 mmHg) (Ried 2010; Ried 2012).
Systolic blood pressure
The updated meta‐analysis (Analysis 2.1; Figure 5) shows a significant systolic blood pressure‐reducing effect in the hypertensive subgroup, a trend towards blood pressure reduction in the prehypertensive subgroup, and a small non‐significant effect in the normotensive subgroup:
2.1. Analysis.
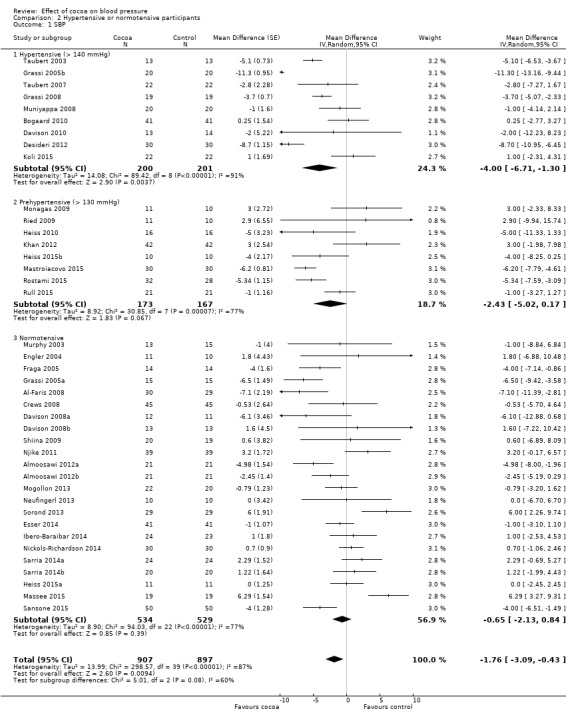
Comparison 2 Hypertensive or normotensive participants, Outcome 1 SBP.
5.
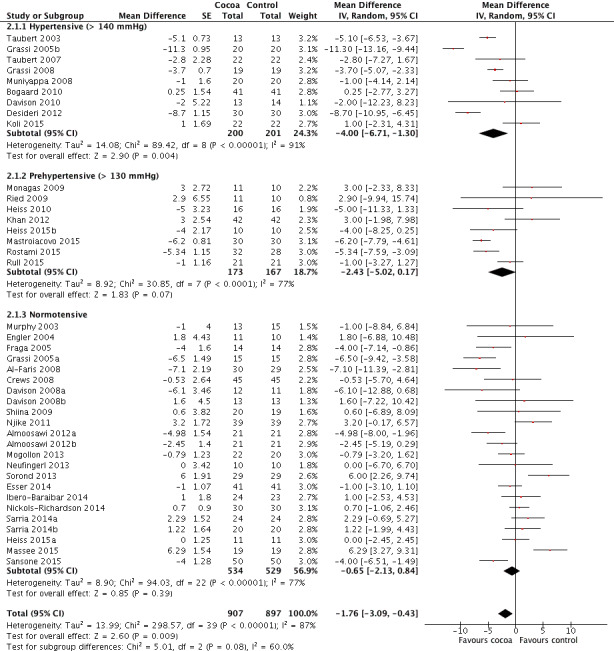
Forest plot of comparison: 2 Hypertensive or normotensive subjects, outcome: 2.1 SBP.
Hypertensive subgroup (baseline SBP > 140 mmHg): mean SBP difference (95% CI): ‐4.00 (‐6.71 to ‐1.30) mmHg, P = 0.004, 9 comparisons, 401 participants; Prehypertensive subgroup (baseline SBP > 130 mmHg): mean SBP difference (95% CI): ‐2.43 (‐5.02 to 0.17) mmHg, P = 0.07, 8 comparisons, 340 participants; Normotensive subgroup (baseline SBP < 130 mm Hg): mean SBP difference (95% CI): ‐0.65 (‐2.13 to 0.84) mmHg, P = 0.39, 23 comparisons, 1063 participants.
The 'Test for subgroup differences' (hypertensive/prehypertensive/normotensive) provided a trend between the subgroups with borderline significance: SBP: I2 = 60%, P = 0.08.
Notably, effect sizes in the hypertensive and prehypertensive subgroups were larger than the effect size of the main meta‐analysis including 40 trial comparisons (mean SBP differences (SE): ‐1.76 (1.3) mmHg).
Diastolic blood pressure
None of the trials in this meta‐analysis involved participants with hypertensive diastolic blood pressure (DBP > 90 mm Hg), so we undertook subgroup analysis by prehypertensive (mean DBP > 80 mm Hg) versus normotensive participants (mean DBP < 80 mmHg) (Analysis 2.2; Figure 6).
2.2. Analysis.
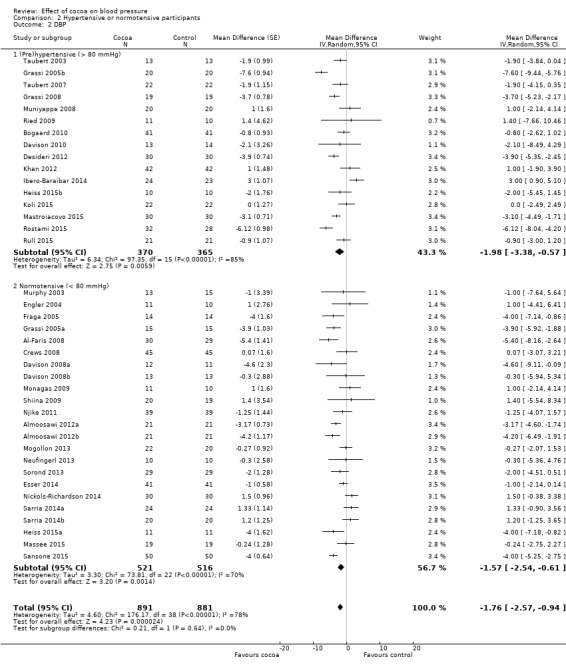
Comparison 2 Hypertensive or normotensive participants, Outcome 2 DBP.
6.
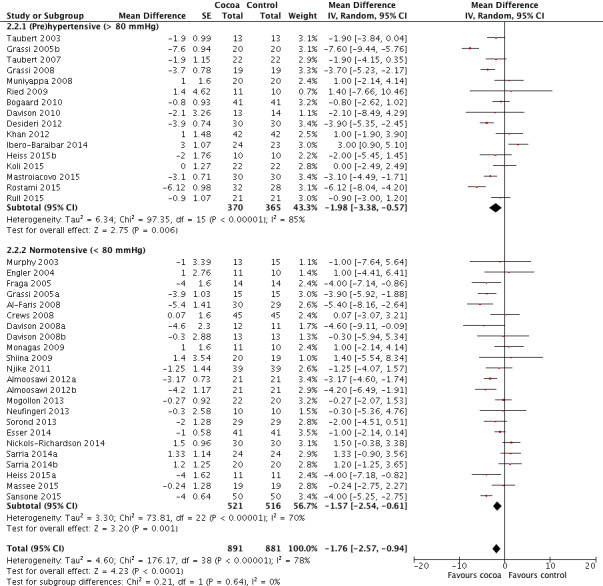
Forest plot of comparison: 2 Hypertensive or normotensive subjects, outcome: 2.2 DBP.
While a significant effect of cocoa on DBP was evident in both subgroups, there was no difference between the subgroups (I2 = 0%, P = 0.64). Prehypertensive subgroup (baseline DBP > 80 mmHg): mean DBP difference (95% CI): ‐1.98 (‐3.38 to ‐0.57) mmHg, P = 0.006, 16 comparisons, 735 participants; Normotensive subgroup (baseline DBP < 80 mmHg): mean DBP difference (95% CI): ‐1.57 (‐2.54 to ‐0.61) mmHg, P = 0.001, 23 comparisons, 1037 participants.
Dosage of flavanols and type of control group
Dosage of flavanol content was determined by two common standardised methods (Adamson 1999; Singleton 1965). We are reasonably confident that flavanol dosages are comparable.
Trials provided participants in the active group with 30 to 1218 mg of flavanols (mean = 670 mg) in 3.6 to 105 grams of cocoa products per day. The control group received either a flavanol‐free product (n = 26 treatment comparisons) or a low‐flavanol cocoa powder (n = 14 treatment comparisons). Flavanol dosage of low‐flavanol products in the control group ranged between 6.4 and 88 mg (mean = 45 mg), with one trial (Esser 2014) providing 259 mg flavanols in the control group per day.
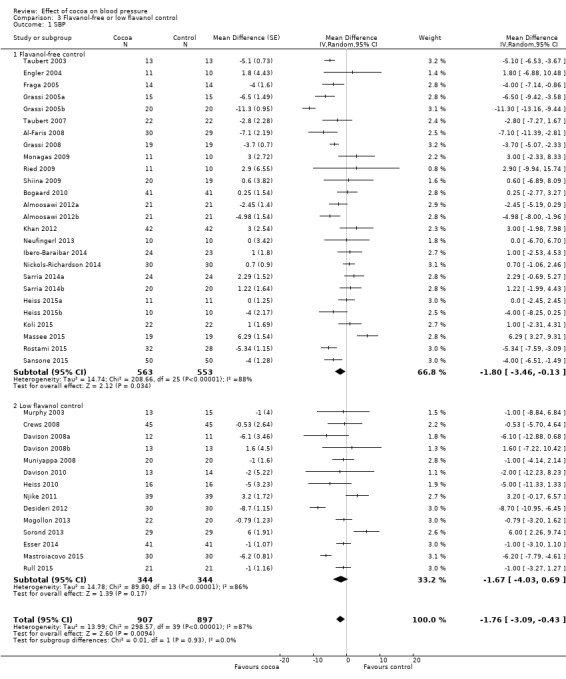
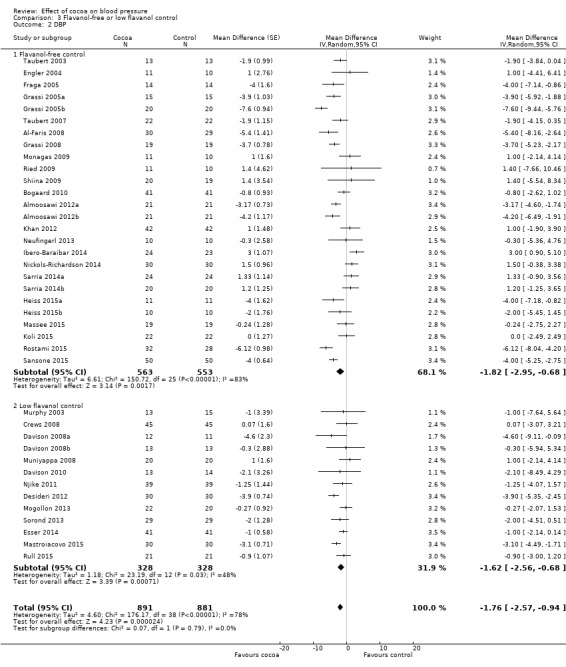
Meta‐analysis 3.1.1 and 3.2.1 of trials with true (flavanol‐free) control groups revealed a significant blood pressure‐reducing effect:
Mean difference SBP (95% CI): ‐1.80 (‐3.46 to ‐0.13) mmHg, P = 0.03, 26 comparisons, 1116 participants; Mean difference DBP (95% CI): ‐1.82 (‐2.95 to ‐0.68) mmHg, P = 0.002, 26 comparisons, 1116 participants.
Subgroup 3.1.2 and 3.2.2 analysis of trials with low‐flavanol control groups provided similar effect sizes: Mean difference SBP (95% CI): ‐1.67 (‐4.03 to 0.69) mmHg, P = 0.17, 14 comparisons, 688 participants; Mean difference DBP (95% CI): ‐1.62 (‐2.56 to ‐0.68) mmHg, P < 0.001, 13 comparisons, 656 participants. Similarity of subgroup findings was confirmed with the 'Test for subgroup differences' (flavanol‐free trials compared with low flavanol trials): I2 = 0%, P = 0.9 (no heterogeneity, no difference).
Sensitivity analysis of subgroup 2 (low‐flavanol control group) excluding the trial with very high flavanol content in the control group (Esser 2014), 1078 mg (active) versus 259 mg (24% of flavanol in the active group), did not change results appreciably.
Mean difference SBP (95% CI): ‐1.73 (‐4.35 to 0.90) mmHg, P = 0.20, 13 comparisons, 606 participants; Mean difference DBP (95% CI): ‐1.71 (‐2.77 to ‐0.65) mmHg, P = 0.002, 12 comparisons, 1690 participants.
Participants in nine of the 14 trials using low‐flavanol control groups received higher or similar dosages of flavanols (33 ‐ 259 mg flavanols) (Crews 2008; Davison 2008a; Davison 2010; Desideri 2012; Esser 2014; Mastroiacovo 2015; Mogollon 2013; Rull 2015) than the active intervention group in the trial by Taubert 2007 (30 mg flavanols; 0 mg flavanol control).
Blinding
We investigated whether blinding of participants and investigators may have played a role in the overall effect.
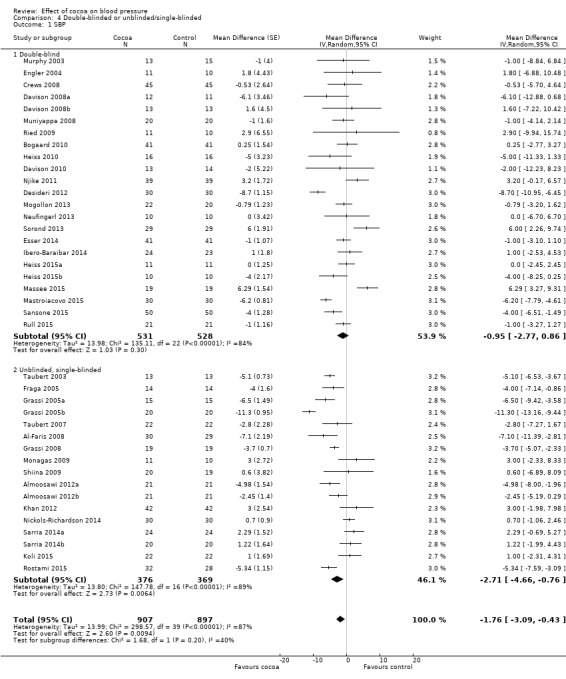
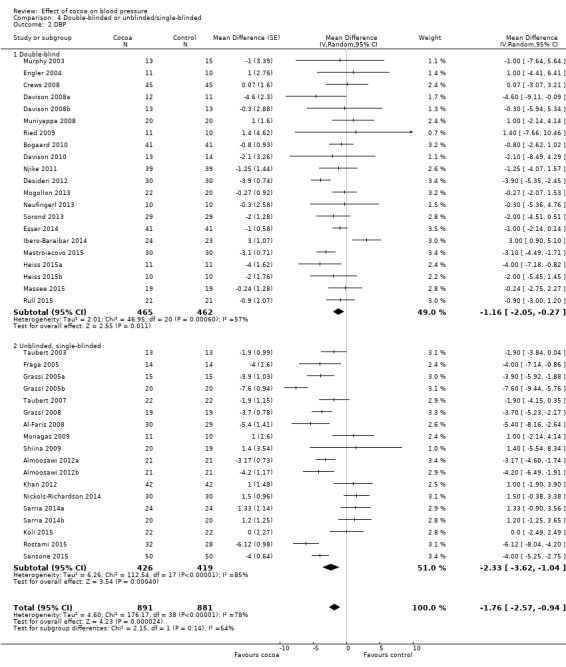
Subgroup analysis 4.1.1 and 4.2.1 of double‐blind trials provided a small effect size:
Mean difference SBP (95% CI): ‐0.95 (‐2.77 to 0.86) mm Hg, P = 0.30, 23 comparisons, 1059 participants; Mean difference DBP (95% CI): ‐1.16 (‐2.05 to ‐0.27) mm Hg, P = 0.01, 21 comparisons, 927 participants.
In contrast, subgroup analysis 4.1.2 and 4.2.2 of unblinded and single‐blinded trials revealed a greater effect size: Mean difference SBP (95% CI): ‐2.71 (‐4.66 to ‐0.76) mmHg, P < 0.001, 17 comparisons, 745 participants; Mean difference DBP (95% CI): ‐2.33 (‐3.62 to ‐1.04) mmHg, P < 0.001, 18 comparisons, 845 participants.
Nine out of the 23 comparisons (39%) in the double‐blind subgroup had flavanol‐free (0 mg) control groups, so differences between the blinding subgroups cannot be explained only by the type of control group. Instead, small changes in blood pressure can easily be influenced by participant expectation, as well as outcome measurement by unblinded investigators.
However, the 'Test for subgroup differences' (double‐blinded versus unblinded/single‐blinded) did not provide sufficient evidence for a genuine difference between the subgroups of SBP: I2 = 40.4%, P = 0.20.
Age
Subgroup differences by age were not statistically significant (I2 = 0%, P = 0.6).
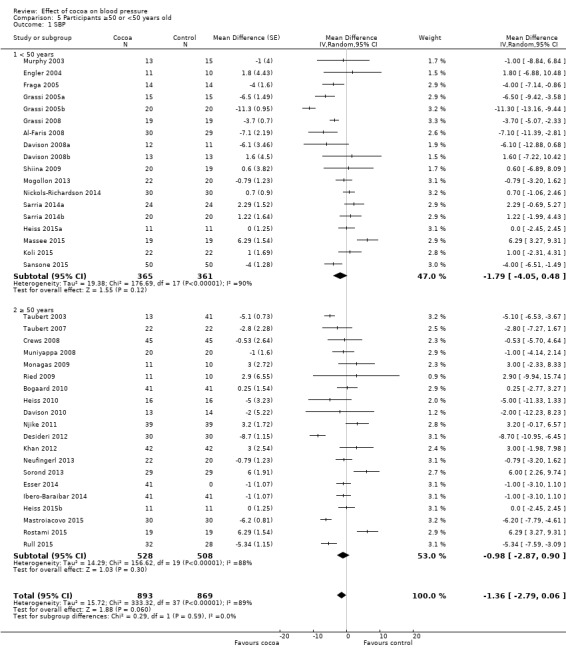
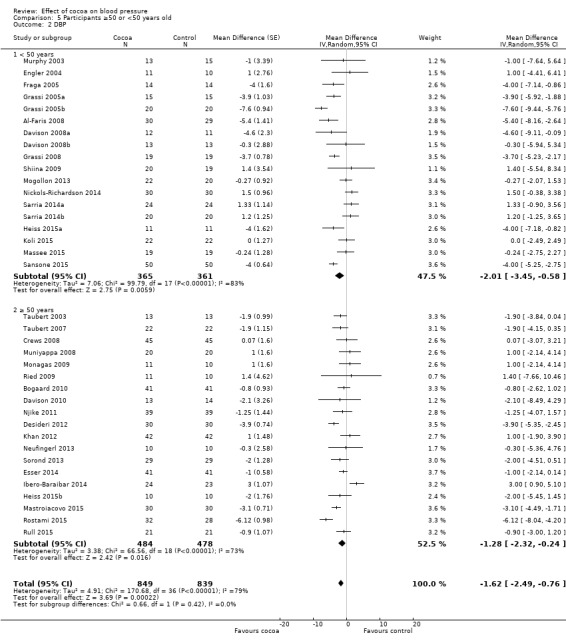
Subgroup analysis 5.1.1 and 5.2.1 of trials with younger participants (< 50 years):
Mean difference SBP (95% CI): ‐1.79 (‐4.05 to 0.48) mmHg, P = 0.12, 18 comparisons, 726 participants; Mean difference DBP (95% CI): ‐2.01 (‐3.45 to ‐0.58) mmHg, P 0.006, 18 comparisons, 726 participants.
Subgroup analysis 5.2.1 and 5.2.2 of trials with older participants (≥ 50 years):
Mean difference SBP (95% CI): ‐0.98 (‐2.87 to 0.90) mmHg, P = 0.30, 20 comparisons, 1036 participants; Mean difference DBP (95% CI): ‐1.28 (‐2.32 to ‐0.24) mmHg, P = 0.02, 19 comparisons, 962 participants.
One trial (Almoosawi 2012a; 2 treatment comparisons) did not provide participants' age details and was therefore excluded from this subgroup analysis.
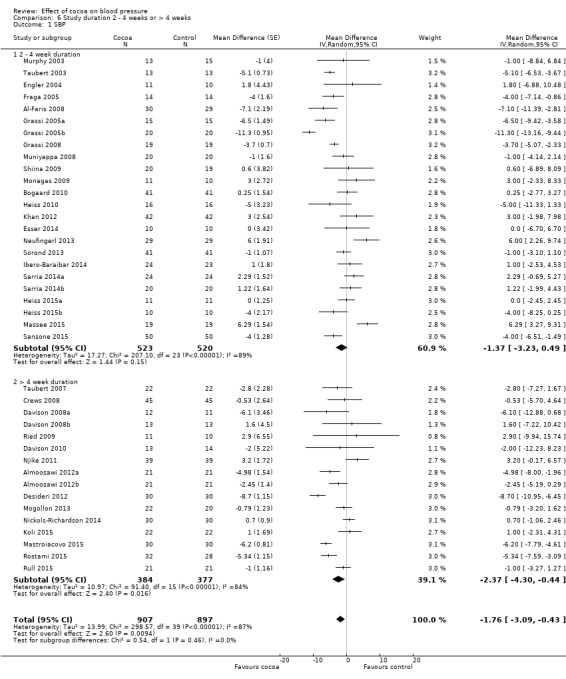
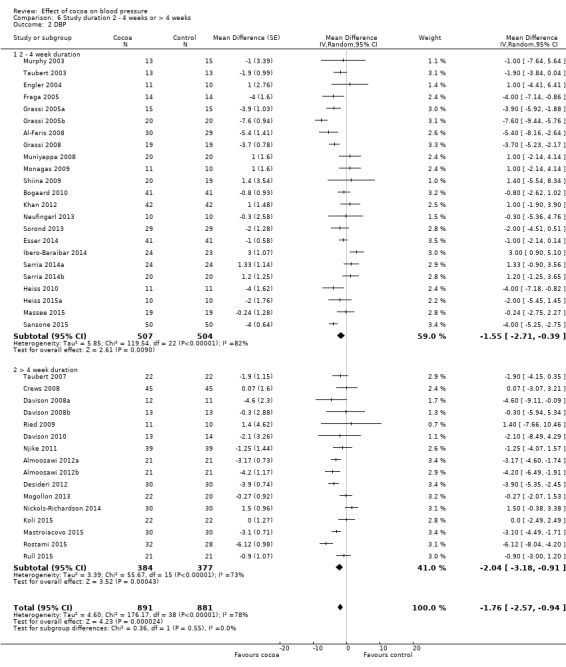
Duration
24 treatment comparisons were of two to four weeks duration, while 16 treatment comparisons were of six to 18 weeks duration (mean = 9 weeks).
We found no statistically significant difference between the subgroups by duration (I2 = 0%, P = 0.5).
Subgroup analysis 6.1.1 and 6.2.1 of trials of two to four weeks duration: Mean SBP difference (95% CI): ‐1.37 (‐3.23 to 0.49) mmHg, P = 0.15, 24 comparisons, 1043 participants; Mean DBP difference (95% CI): ‐1.55 (‐2.71 to ‐0.39) mmHg, P = 0.009, 23 comparisons, 1011 participants.
Subgroup analysis 6.1.2 and 6.2.2 of trials of 6 to 18 weeks duration: Mean SBP difference (95% CI): ‐2.37 (‐4.30 to ‐0.44) mmHg, P = 0.02, 16 comparisons, 761 participants; Mean DBP difference (95% CI): ‐2.04 (‐3.18 to ‐0.91) mmHg, P < 0.001, 16 comparisons, 761 participants.
Analysis 6.1; Analysis 6.2
Sensitivity analyses of all trials excluding those in which authors were employed by industry (n = 6) revealed a marked difference in results, reducing effect sizes and statistical significance, in particular for systolic blood pressure.
Mean difference SBP (95% CI): ‐1.08 (‐2.60 to 0.43) mmHg, P = 0.16, 33 comparisons, 1482 participants; Mean difference DBP (95% CI): ‐1.37 (‐2.31 to ‐0.43) mmHg, P = 0.004, 33 comparisons, 1482 participants.
Summary of secondary outcomes
We did not meta‐analyse withdrawals and adverse effects across trials, but we summarise them in Table 2.
1. Adverse events & withdrawals.
| Study |
Study design |
Participants Cocoa/ Control |
Withdrawn Cocoa/Control |
Reasons for withdrawal including adverse effects Cocoa/Control |
| Taubert 2003 | C | 13/13 | 0/0 | ‐ |
| Murphy 2003 | P | 13/15 | 3 in total | Family illness (2) Non‐compliance in final week (1) |
| Engler 2004 | P | 11/10 | 0/0 | ‐ |
| Fraga 2005 | C | 14/14 | 1/0 | No reason given |
| Grassi 2005a | C | 15/15 | 0/0 | ‐ |
| Grassi 2005b | C | 20/20 | 0/0 | ‐ |
| Taubert 2007 | P | 22/22 | 0/0 | ‐ |
| Crews 2008 | P | 45/45 | 6/5 | Gastrointestinal upset/headache/cold sweat (2/1) Bronchitis (1/0) Jitteriness/increased energy (1/0) Atrial arrhythmia/medication change (1/0) Dislike of study product (1/1) Family illness (0/1) Unspecified reason (0/1) No adherence to trial regimen (0/1) |
| Grassi 2008 | C | 19/19 | 0/0 | ‐ |
| Muniyappa 2008 | C | 20/20 | 5/4 | Lost to follow‐up (0/1) Discontinued intervention (4/2) due to Intolerance to treatment, family emergencies, personal problems excluded from analysis (1/1) |
| Davison 2008a | P | 12/11 | 7 in total | Time restrictions, personal circumstances (14) Non‐compliance (exercise or diet) (2) |
| Davison 2008b | P | 13/13 | 5 in total | |
| Al‐Faris 2008 | P | 30/29 | 0/0 | ‐ |
| Shiina 2009 | P | 20/19 | 0/0 | ‐ |
| Ried 2009 | P | 11/10 | 2/2 | Study product unpalatable (2/0) Gastrointestinal upset (0/1) Illness unrelated to study (0/1) |
| Monagas 2009 | C | 42/42 | 0/0 | Constipation (resolved with fibre intake) |
| Bogaard 2010 | C | 41/41 | 3 in total | Nausea (1) Headache (1) Arrythmia unrelated (1) Laxative effect (12/2) – did not withdraw |
| Heiss 2010 | C | 16/16 | 3 in total | Did not come to first visit |
| Davison 2010 | P | 13/14 | 7 in total | Mild gastric symptoms (1) Non‐compliance with study protocol (1) Withdrew due to personal circumstances (5) |
| Njike 2011 | C | 38/38 | 7 in total | Non‐compliance with study protocol (1) Withdrew for personal reasons (6) |
| Almoosawi 2012a | C | 21/21 | 1/1 | Personal reasons unrelated to study |
| Desideri 2012 | P | 30/30 | 0/1 | Gastric discomfort (1) |
| Khan 2012 | C | 42/42 | 1/0 | Constipation |
| Mogollon 2013 | P | 22/20 | 1/1 | Unrelated to study (1)/headache (1) |
| Neufingerl 2013 | P | 10/10 | 1/1 | Nausea (1)/unrelated (1) |
| Sorond 2013 | P | 29/29 | 1/1 | No details provided |
| Esser 2014 | C | 41/41 | 3 in total | Medical reasons (1), disliked chocolate (1), poor compliance (1) |
| Ibero‐Baraibar 2014 | P | 24/23 | 2/1 | Personal reason (2), poor compliance (1) |
| Nickols‐Richardson 2014 | P | 30/30 | 0/0 | None |
| Sarria 2014 (a) | C | 24/24 20/20 |
? | No information given |
| Heiss 2015 (a) | P | 11/11 10/10 |
0/0 | None |
| Massee 2015 | P | 19/19 | 1/1 | Personal reasons (1) |
| Rostami 2015 | P | 32/28 | 2/6 | No information given |
| Koli 2015 | C | 22/22 | 0/0 | No side effects reported |
| Mastroiacovo 2015 | P | 30/30 | 1/0 | Personal reasons (1) No side effects reported (1 gastric discomfort in IF (intermediate flavanol) group not included in this meta‐analysis) |
| Rull 2015 | C | 21/21 | 11 | No details provided |
| Sansone 2015 | P | 50/50 | ? | No information given |
C:Cross‐over P: Parallel
Four trials did not provide any information on reasons for withdrawals or adverse effects (Rostami 2015; Rull 2015; Sansone 2015; Sarria 2014). Out of 31 comparisons (1514 participants, cocoa groups: n = 760; control groups: n = 754) which provided information on withdrawals and adverse effects, eight trials reported no withdrawals and no adverse effects (Engler 2004; Grassi 2005a; Grassi 2008; Heiss 2015a; Koli 2015; Nickols‐Richardson 2014; Taubert 2003; Taubert 2007).
In the remaining 23 comparisons, reasons for withdrawal included personal and trial‐unrelated reasons or adverse effects.
Withdrawals due to adverse effects were reported in nine trials (Bogaard 2010; Crews 2008; Davison 2010; Desideri 2012; Esser 2014; Khan 2012; Mogollon 2013; Neufingerl 2013; Ried 2009), including gastrointestinal complaints (cocoa groups: n = 8/760 (1%), control groups: n = 3/754 (0.4%)); dislike of the trial product (cocoa: n = 4/760; control: n = 1/754), headache (cocoa: n = 2/760; control: n = 1/754), and jitteriness (cocoa: n = 1/760, control: n = 0/754).
The product with a high theobromine content in one trial (Bogaard 2010) had a laxative effect (cocoa: n = 12/41, control: n = 2/41), but the affected participants completed the trial. Interestingly, two additional study groups in Neufingerl 2013, not included in this review, tested high theobromine content (850 mg or 1000 mg) and reported a high incidence of nausea, vomiting, headache, and diarrhoea (n = 7/20 participants).
While the potential effect on blood pressure is rather small, cocoa may have other cardiovascular benefits, including improved endothelial function and reduced vascular stiffness (Davison 2008a; Engler 2004; Grassi 2005a; Grassi 2008; Heiss 2010; Heiss 2015a; Mogollon 2013; Sansone 2015; Shiina 2009), as well as improved glucose metabolism and reduced insulin resistance, in particular in overweight or obese individuals (Almoosawi 2012a; Desideri 2012; Grassi 2005a; Grassi 2008; Mastroiacovo 2015; Muniyappa 2008; Nickols‐Richardson 2014). It may reduce triglyceride levels and oxidised LDL‐cholesterol (Almoosawi 2012a; Ibero‐Baraibar 2014; Khan 2012; Rostami 2015; Sarria 2014), decrease platelet aggregation (Murphy 2003; Rull 2015), reduce inflammation (Esser 2014; Monagas 2009), and improve cognitive function (Desideri 2012; Massee 2015; Mastroiacovo 2015; Sorond 2013).
Discussion
Summary of main results
Our updated meta‐analysis of 35 short‐term trials with 40 treatment comparisons involving 1804 mainly healthy individuals suggests flavanol‐rich cocoa products (mean 670 mg flavanols) to have a small but statistically significant effect in reducing blood pressure compared with control by 1.8 mmHg.
Heterogeneity was generally high. We explored reasons for heterogeneity in subgroup and sensitivity analyses.
Whilst subgroup meta‐analyses by baseline blood pressure indicated a larger average effect of cocoa in systolic hypertension compared with systolic prehypertension or normotension, the test for interaction was of borderline significance (Test for subgroups differences: I2 = 60%, P = 0.08). Further studies with hypertensive people are needed to confirm any significant interaction between baseline blood pressure and effect size. A significant blood pressure‐lowering effect of cocoa was evident in diastolic blood pressure, independent of status at baseline.
We investigated whether blinding may play a role. While meta‐analysis of trials with unblinded/single‐blinded trials revealed a greater systolic blood pressure‐reducing effect, compared to double‐blinded trials, the test for subgroup differences was statistically not significant. In addition, any differences cannot be explained by the type of control alone (flavanol‐free versus low flavanol control ), and may suggest an influence of participant expectations when unblinded to the intervention.
We found the effect of cocoa to be slightly attenuated by age, so that blood pressure reduction tended to be greater in younger individuals (mean age range 18 to 49 yrs; 18 trials) compared with older individuals (mean age range 50 to 73 yrs; 20 trials). While there was no statistically significance difference between subgroups, an age‐related difference in the effect of cocoa on blood pressure is biologically plausible. The age‐related effect might be associated with structural and biochemical changes in the arterial wall associated with aging (O'Rourke 1990) and subsequent vascular reactivity to stimuli. Age‐related changes include arterial stiffening together with decrease of elastin, and increase of collagen and glycosaminoglycans (O'Rourke 1990). In addition, endothelin‐1, a potent vasoconstrictor protein, is elevated in older adults (Donato 2009) and endothelial oxidative stress compromising nitric oxide availability is more pronounced in the elderly (Taddei 2001). Cocoa flavanols have been shown to reduce vascular resistance and arterial stiffness, and are potent scavengers of free radicals (Loke 2008; Schroeter 2006), which may lead to improved vascular function. In the short‐term studies included in our review the effect of cocoa on blood pressure might be more pronounced in younger individuals, due to the age‐related decrease in vascular reactivity to physiological stimuli such as cocoa flavanols.
Trial duration slightly influenced results, with greater effect sizes observed in the longer trials of six to 18 weeks compared to the shorter trials of two to four weeks, albeit not a statistically significant difference.
In this review, we assessed the flavanol content of cocoa products. Cocoa also contains the stimulant theobromine, which has been suggested to affect vasoactivity and thus blood pressure reduction in cocoa products (Kelly 2005). Theobromine is the bitter alkaloid of the cacao plant, and is also found in other plants, such as tea and the cola nut. Other similar compounds, the methylxanthines, include caffeine in coffee. However, analysis of the effect of cocoa on blood pressure by theobromine content was hindered by the lack of reporting of the theobromine content in a number of trials. Instead, ingestion of higher concentrations of theobromine have been associated with a higher rate of adverse effects, in particular nausea, vomiting, dizziness, and diarrhoea, as reported in a number of trials. It is also questionable whether chocolate and cocoa products are palatable if large amounts of theobromine are included. While some animals, such as dogs, might succumb to theobromine poisoning from as little as 50 grams of chocolate for a smaller dog and 400 grams for an average‐sized dog due to slow metabolism of theobromine (Strachan 1994), it is estimated that a 60 kg human would need to consume about 4.5 kg of dark chocolate containing natural theobromine to be poisoned (Rusconi 2010).
Sensitivity analysis of 33 treatment comparisons, excluding those with at least one of the authors employed by the trial sponsoring industry and with a commercial interest in the test cocoa product, revealed a reduced effect size and reduced statistical significance, alerting to a potential bias in reporting of results, and may explain some of the heterogeneity.
Overall completeness and applicability of evidence
Data were available for the 35 identified trials with 40 treatment comparisons fitting the inclusion criteria. We excluded two trials due to lack of data (Balzer 2008; Farouque 2006). Most trials studied healthy people with or without elevated blood pressure, including one trial of healthy pregnant women (Mogollon 2013). One trial (Heiss 2010) included people with coronary artery disease, three trials assessed individuals with impaired glucose tolerance or diabetes (Grassi 2008; Khan 2012; Rostami 2015), and one trial studied elderly people with mild cognitive impairment (Desideri 2012). Our findings are therefore applicable largely to healthy adults with or without hypertension. Our review included all types of cocoa products.
Our meta‐analysis contributes to the evidence for flavanol‐rich cocoa products being beneficial to cardiovascular health, albeit a modest effect. No long‐term trials investigating the effect of cocoa products on clinical outcomes are available to shed light on the effects of cocoa on cardiovascular events or long‐term adverse effects.
Quality of the evidence
We found a sufficient number of trials (35, with 40 treatment comparisons) and a reasonably large sample size (1804 participants) to generate meaningful meta‐analysis and to allow several subgroup analyses, exploring heterogeneity. Because of the large number of trials, many of high quality, and despite unexplained high heterogeneity, we consider the quality of the evidence to be moderate (Table 1). We explored heterogeneity in several subgroup analyses with a reasonable number of trials.
Potential biases in the review process
A strength of this updated review is the comprehensive literature search including several databases, trial registries and reference lists of included trials.
While we investigated heterogeneity in several subgroup analyses, we could not fully explain the variations in effect of cocoa on blood pressure. Continuing high levels of heterogeneity within subgroup analyses suggest that there may be a combination of factors, or additional ones beyond those we considered. It is possible that subgroups by age and hypertension status at baseline might be subject to ecological bias. The effect we found between studies might not hold within studies. However, analysis of individual patient data was not an approach that we adopted for this review.
Agreements and disagreements with other studies or reviews
While the effect on cocoa on systolic blood pressure is significant, noticeably, the effect sizes became smaller with the increasing number of studies compared to previous meta‐analyses. It is likely that a larger sample size provided a more unbiased result by reducing the influence of individual studies.
Ried 2012 (20 treatment comparisons): mean difference SBP (95% CI): ‐2.77 (‐4.72 to ‐0.82) mmHg, P = 0.005, 856 participants
Ried 2010 (15 treatment comparisons): mean difference SBP (95% CI): ‐3.16 (‐5.08 to ‐1.23) mmHg, P = 0.001, 578 participants
Desch 2010a (10 treatment comparisons): mean difference SBP (95% CI): ‐4.52 (‐5.87 to ‐3.16) mmHg, P < 001, 297 participants
Taubert 2007a (5 treatment comparisons): mean difference SBP (95% CI): ‐4.7 (‐7.6 to ‐1.8) mm‐Hg, P = 0.002, 97 participants
Overall reduction in diastolic blood pressure in our updated meta‐analysis is also smaller than reported in earlier versions of this review and previous meta‐analyses:
Ried 2012 (19 treatment comparisons): mean difference DBP (95% CI): ‐2.20 (‐3.46 to ‐0.93) mmHg, P = 0.006, 824 participants
Ried 2010 (15 treatment comparisons): mean difference DBP (95% CI): ‐2.02 (‐3.35 to 0.69) mmHg, P = 0.003, 578 participants
Desch 2010a (10 treatment comparisons): mean difference DBP (95% CI): ‐2.5 (‐3.90 to 1.20) mmHg, P < 0.001, 297 participants
Taubert 2007a (5 treatment comparisons): mean difference DBP (95% CI): ‐2.8 (‐4.80 to ‐0.80) mmHg, P = 0.006, 97 participants
Authors' conclusions
Implications for practice.
Our updated review provides moderate‐quality evidence that flavanol‐rich chocolate and cocoa products lower both systolic and diastolic blood pressure in mainly healthy adults by an average of 1.8 mmHg in the short term.
Our findings are limited by the heterogeneity between trials, which could not be explained by prespecified subgroup analyses, including blinding, flavanol content of the control groups, age of participants, or study duration. However, baseline blood pressure may play a role in the effect of cocoa on blood pressure, with subgroup analysis of trials with (pre)hypertensive participants revealing a greater blood pressure‐reducing effect of cocoa compared to normotensive participants.
Implications for research.
More trials are needed, designed to directly compare the effect of cocoa on specific population groups (e.g. hypertensive versus normotensive) to test the findings of our subgroup analyses.
Long‐term trials are needed investigating the effect of cocoa on clinical outcomes, to assess whether cocoa has an effect on cardiovascular events.
What's new
| Date | Event | Description |
|---|---|---|
| 2 May 2017 | Amended | fixed minor display error in forest plot for Analysis 1.1 |
History
Protocol first published: Issue 12, 2010 Review first published: Issue 8, 2012
| Date | Event | Description |
|---|---|---|
| 20 April 2017 | New search has been performed | 20 new treatment comparisons included, total of 40 treatment comparisons. |
| 20 April 2017 | New citation required but conclusions have not changed | Updated search |
Acknowledgements
We are thankful for the assistance by N Funabashi (Shiina 2009), D Grassi (Grassi 2008), and B van den Bogaard (Bogaard 2010), who provided unpublished data for inclusion in our meta‐analysis.
We would like to acknowledge the assistance and advice received from the Cochrane Hypertension Group.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 7 November 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (cacao$ or cocao$ or cocoa$ or chocolat$).mp. (5917) 2 exp cardiovascular diseases/ (2119273) 3 exp cardiovascular system/ (1138797) 4 cardiovascular.mp. (428184) 5 exp hypertension/ (239452) 6 (antihypertens$ or hypertens$).tw. (357352) 7 exp blood pressure/ (274194) 8 ((arterial or blood or diastolic or systolic) adj2 pressur?).tw. (297630) 9 (bloodpressur? or bp or dbp or sbp).tw. (139226) 10 or/2‐9 (3094934) 11 randomized controlled trial.pt. (434369) 12 controlled clinical trial.pt. (91859) 13 randomi?ed.ab. (398909) 14 placebo.ab. (166289) 15 clinical trials as topic/ (180579) 16 randomly.ab. (231524) 17 trial.ti. (144974) 18 or/11‐17 (1014610) 19 animals/ not (humans/ and animals/) (4303730) 20 18 not 19 (929627) 21 1 and 10 and 20 (161) 22 remove duplicates from 21 (151)
Appendix 2. Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register Search Date: 8 November 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1(cacao* or cocao* cocoa* or chocolat*) 179 #2RCT:DE 24183 #3 (Review OR Meta‐Analysis):MISC2 1164 #4 #1 AND (#2 OR #3) 129 ***************************
Appendix 3. CENTRAL search strategy
Database: Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 11 via the Cochrane Register of Studies Online Search Date: 7 November 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1(cacao* or cocao* or cocoa* or chocolat*)623 #2MESH DESCRIPTOR Cardiovascular Diseases EXPLODE ALL TREES73677 #3MESH DESCRIPTOR Cardiovascular System EXPLODE ALL TREES17870 #4cardiovascular*47208 #5MESH DESCRIPTOR Hypertension EXPLODE ALL TREES14248 #6(antihypertens* or hypertens*)42379 #7MESH DESCRIPTOR blood pressure EXPLODE ALL TREES24557 #8(arterial or blood or diastolic or systolic) NEAR2 pressur*59742 #9(bloodpressur* or bp or dbp or sbp)13514 #10#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9155268 #11#1 AND #10174
Appendix 4. Embase search strategy
Database: Embase <1974 to 2016 November 07> Search Date: 7 November 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (cacao$ or cocao$ or cocoa$ or chocolat$).mp. (9312) 2 exp cardiovascular disease/ (3576873) 3 exp cardiovascular system/ (1690837) 4 cardiovascular.mp. (814809) 5 exp hypertension/ (618867) 6 (antihypertens$ or hypertens$).tw. (536416) 7 exp blood pressure/ (504873) 8 ((arterial or blood or diastolic or systolic) adj2 pressur?).tw. (418083) 9 (bloodpressur? or bp or dbp or sbp).tw. (195852) 10 or/2‐9 (4650010) 11 randomized controlled trial/ (460216) 12 crossover procedure/ (53690) 13 double‐blind procedure/ (137595) 14 (randomi?ed or randomly).tw. (925570) 15 (crossover$ or cross‐over$).tw. (85589) 16 placebo.ab. (239247) 17 ((singl$ or doubl$) adj blind$).tw. (191478) 18 assign$.ab. (295579) 19 allocat$.ab. (107734) 20 or/11‐19 (1383382) 21 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5827297) 22 20 not 21 (1214819) 23 1 and 10 and 22 (326) 24 remove duplicates from 23 (303)
Appendix 5. Clinical Trials Registries
Database: ClinicalTrials.gov Search Date: 7 November 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: randomized Study type: Interventional Studies Intervention: cocoa OR chocolate Outcome Measures: blood pressure (40) *************************** Database: WHO International Clinical Trials Registry Platform Search Date: 8 November 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 random* AND blood pressure AND cocoa 5 #2 random* AND blood pressure AND chocolate 5 #3 random* AND hypertens* AND cocoa 7 #4 random* AND hypertens* AND chocolate 6 #5 random* AND cardiovasc* AND cocoa 8 #6 random* AND cardiovasc* AND chocolate 4 #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 35 #8 remove duplicates from #7 19 ***************************
Data and analyses
Comparison 1. Effect of cocoa on BP.
Comparison 2. Hypertensive or normotensive participants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 Hypertensive (> 140 mmHg) | 9 | 401 | Mean Difference (Random, 95% CI) | ‐4.00 [‐6.71, ‐1.30] |
| 1.2 Prehypertensive (> 130 mmHg) | 8 | 340 | Mean Difference (Random, 95% CI) | ‐2.43 [‐5.02, 0.17] |
| 1.3 Normotensive | 23 | 1063 | Mean Difference (Random, 95% CI) | ‐0.65 [‐2.13, 0.84] |
| 2 DBP | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 (Pre)hypertensive (> 80 mmHg) | 16 | 735 | Mean Difference (Random, 95% CI) | ‐1.98 [‐3.38, ‐0.57] |
| 2.2 Normotensive (< 80 mmHg) | 23 | 1037 | Mean Difference (Random, 95% CI) | ‐1.57 [‐2.54, ‐0.61] |
Comparison 3. Flavanol‐free or low flavanol control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 Flavanol‐free control | 26 | 1116 | Mean Difference (Random, 95% CI) | ‐1.80 [‐3.46, ‐0.13] |
| 1.2 Low flavanol control | 14 | 688 | Mean Difference (Random, 95% CI) | ‐1.67 [‐4.03, 0.69] |
| 2 DBP | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 Flavanol‐free control | 26 | 1116 | Mean Difference (Random, 95% CI) | ‐1.82 [‐2.95, ‐0.68] |
| 2.2 Low flavanol control | 13 | 656 | Mean Difference (Random, 95% CI) | ‐1.62 [‐2.56, ‐0.68] |
3.1. Analysis.
Comparison 3 Flavanol‐free or low flavanol control, Outcome 1 SBP.
3.2. Analysis.
Comparison 3 Flavanol‐free or low flavanol control, Outcome 2 DBP.
Comparison 4. Double‐blinded or unblinded/single‐blinded.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 Double‐blind | 23 | 1059 | Mean Difference (Random, 95% CI) | ‐0.95 [‐2.77, 0.86] |
| 1.2 Unblinded, single‐blinded | 17 | 745 | Mean Difference (Random, 95% CI) | ‐2.71 [‐4.66, ‐0.76] |
| 2 DBP | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 Double‐blind | 21 | 927 | Mean Difference (Random, 95% CI) | ‐1.16 [‐2.05, ‐0.27] |
| 2.2 Unblinded, single‐blinded | 18 | 845 | Mean Difference (Random, 95% CI) | ‐2.33 [‐3.62, ‐1.04] |
4.1. Analysis.
Comparison 4 Double‐blinded or unblinded/single‐blinded, Outcome 1 SBP.
4.2. Analysis.
Comparison 4 Double‐blinded or unblinded/single‐blinded, Outcome 2 DBP.
Comparison 5. Participants ≥50 or <50 years old.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 38 | 1762 | Mean Difference (Random, 95% CI) | ‐1.36 [‐2.79, 0.06] |
| 1.1 < 50 years | 18 | 726 | Mean Difference (Random, 95% CI) | ‐1.79 [‐4.05, 0.48] |
| 1.2 ≥ 50 years | 20 | 1036 | Mean Difference (Random, 95% CI) | ‐0.98 [‐2.87, 0.90] |
| 2 DBP | 37 | 1688 | Mean Difference (Random, 95% CI) | ‐1.62 [‐2.49, ‐0.76] |
| 2.1 < 50 years | 18 | 726 | Mean Difference (Random, 95% CI) | ‐2.01 [‐3.45, ‐0.58] |
| 2.2 ≥ 50 years | 19 | 962 | Mean Difference (Random, 95% CI) | ‐1.28 [‐2.32, ‐0.24] |
5.1. Analysis.
Comparison 5 Participants ≥50 or <50 years old, Outcome 1 SBP.
5.2. Analysis.
Comparison 5 Participants ≥50 or <50 years old, Outcome 2 DBP.
Comparison 6. Study duration 2 ‐ 4 weeks or > 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 2 ‐ 4 week duration | 24 | 1043 | Mean Difference (Random, 95% CI) | ‐1.37 [‐3.23, 0.49] |
| 1.2 > 4 week duration | 16 | 761 | Mean Difference (Random, 95% CI) | ‐2.37 [‐4.30, ‐0.44] |
| 2 DBP | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 2 ‐ 4 week duration | 23 | 1011 | Mean Difference (Random, 95% CI) | ‐1.55 [‐2.71, ‐0.39] |
| 2.2 > 4 week duration | 16 | 761 | Mean Difference (Random, 95% CI) | ‐2.04 [‐3.18, ‐0.91] |
6.1. Analysis.
Comparison 6 Study duration 2 ‐ 4 weeks or > 4 weeks, Outcome 1 SBP.
6.2. Analysis.
Comparison 6 Study duration 2 ‐ 4 weeks or > 4 weeks, Outcome 2 DBP.
Comparison 7. Sensitivity analysis: excl studies with industry employed authors.
Characteristics of studies
Characteristics of included studies [author‐defined order]
Murphy 2003.
| Methods | P DB |
|
| Participants | Community setting, Melbourne, Australia Eligibility: healthy N = 28 Age: 43.5 Male: 53% Normotensive (mean baseline BP = 117/77 mmHg) |
|
| Interventions | 1. Cocoa tablets (234 mg flavanols/procyanidins)
2. Placebo tablets (< 6 mg cocoa flavanols/procyanidins); daily Duration: 28 days |
|
| Outcomes | SBP and DBP measured after 28 days. (No description of position of participant or which arm) Secondary outcome measure |
|
| Notes | Supported in part by Mars Inc, USA who supplied active tablets (CocoaPro; Mars Inc, Hackettstown, NJ) and placebo tablets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were separated into 2 groups that were sex‐matched and randomly assigned to consume either treatment. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.5% (4/32) loss to follow‐up: 1 did not to meet inclusion criteria, 2 withdrew because of family illnesses, and 1 failed to consume the specified number of tablets during the final week of the intervention. No other missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | industry‐supported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded (active and placebo tablets) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Taubert 2003.
| Methods | C SB |
|
| Participants | Community setting, Cologne, Germany Eligibility: healthy N = 13 Age: 55 ‐ 64 Male: 54% Hypertensive (Mean baseline BP = 153/84 mgHg) |
|
| Interventions | 1. 100 g dark chocolate (500 mg flavanols)
2. 90 g white chocolate (0 mg flavanols); daily Duration: 2 weeks |
|
| Outcomes | Seated SBP and DBP (left upper arm) measured daily Primary outcome measure |
|
| Notes | Sponsor not involved in data collection or analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to receive 14 consecutive daily doses of either treatment. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. No missing outcome data reported |
| Selective reporting (reporting bias) | Low risk | BP data were provided for all time points |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BP was recorded "in a blinded fashion" |
Engler 2004.
| Methods | P DB |
|
| Participants | Community setting, San Francisco, USA Eligibility: healthy N = 21 Age: 38 (21 ‐ 55) Male: 52% Normotensive (Mean baseline BP = 116/67 mmHg) |
|
| Interventions | 1. 46 g dark high flavanoid (213 mg procyanidin/46 mg epicatechin) chocolate
2. 46 g dark low flavanoid (trace procyanidin/epicatechin) chocolate; daily Duration: 2 weeks |
|
| Outcomes | Resting supine SBP and DBP after 2 weeks Secondary outcome measure |
|
| Notes | Funded by the University of California, San Francisco. Chocolate sourced from American Cocoa Research Institute, Vienna, VA. Sponsor not involved in data collection or analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Excellent compliance in all participants was documented by the return of all empty sample wrappers and by plasma epicatechin concentrations at 2 weeks |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each chocolate sample was provided in coded foil wrapped containers. Both high‐ and low‐flavonol chocolate bars were similar in physical appearance and taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Fraga 2005.
| Methods | C SB |
|
| Participants | Study dates: 10/00‐11/00 Community setting, Buenos Aires, Argentina Eligibility: young male active soccer players N = 28 Age: 18 (18 ‐ 21) Male: 100% Normotensive (mean baseline BP = 123/72 mmHg) |
|
| Interventions | 1. 105 g (168 mg flavanols) containing milk chocolate (M&M's)
2. 105 g cocoa butter chocolate (0 mg flavanols); daily Duration: 2 weeks |
|
| Outcomes | SBP and DBP measured daily. No description of position of participant or which arm Primary outcome measure |
|
| Notes | 3 authors from Mars. Funding supplied by the University of Buenos Aires and Argentinian government (ANPCYT). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | 2 treatments were provided in 105 g‐coded bags (1 daily dose) for 7‐day periods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.6% (1/28) loss to follow‐up; reason not reported |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry‐funded and authored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinding of participants (dark/white chocolate) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Grassi 2005a.
| Methods | C SB |
|
| Participants | Community setting, L'Aquila, Italy Eligibility: hypertensive N = 15 Age: 34 (SD = 7.6) Male: 47% Normotensive (mean baseline BP = 113/74 mgHg) |
|
| Interventions | 1. 100 g dark chocolate (500 mg flavanols)
2. 90 g white chocolate (0 mg flavanols); daily Duration: 15 days |
|
| Outcomes | Seated resting SBP and DBP after 15 days Primary outcome measure |
|
| Notes | Normotensive group; Influence of funding body unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP at start and end of study reported |
| Other bias | Unclear risk | Influence of funding body unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BP was measured always by the same physician who was unaware of the study design, results, and purpose |
Grassi 2005b.
| Methods | C SB |
|
| Participants | Community setting, L'Aquila, Italy Eligibility: hypertensive N = 15 Age: 34 (SD = 7.6) Male: 47% Normotensive (mean baseline BP = 113/74 mgHg) |
|
| Interventions | 1. 100 g dark chocolate (500 mg flavanols)
2. 90 g white chocolate (0 mg flavanols); daily Duration: 15 days |
|
| Outcomes | Seated resting SBP and DBP after 15 days Primary outcome measure |
|
| Notes | Hypertensive subgroup; Influence of funding body unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP at start and end of study reported |
| Other bias | Unclear risk | Influence of funding body unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BP was measured always by the same physician who was unaware of the study design, results, and purpose |
Taubert 2007.
| Methods | C SB |
|
| Participants | Study dates: 1/05‐12/16 Community setting, Cologne, Germany Eligibility: (pre‐)hypertensive N = 44 Age: 55 ‐ 75 Male: 45% Hypertensive (mean baseline BP = 148/86 mmHg) |
|
| Interventions | 1. 6.3 g dark chocolate (30 mg flavanols)
2. 5.6 g white chocolate (0 mg flavanols); daily Duration: 18 weeks |
|
| Outcomes | Seated resting SBP and DBP (left upper arm) after 6, 12, and 18 weeks Primary outcome |
|
| Notes | Funded by the University Hospital of Cologne, Germany. Funding body not involved in study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted randomisation in sex‐stratified blocks of 4 persons each, sequentially allocated to dark chocolate and white chocolate using a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | To conceal allocation from investigators, instructed trained staff at a separate site not involved with the trial generated and maintained the randomization list and prepared the chocolate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP data at start, during and end of study. |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants (dark/white chocolate) All clinical investigations, dietary assessments, laboratory tests, data collection, and data analysis were performed by physicians and trained staff who were blinded to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Participants received no information about their examination data and the exact objective of the study until trial completion. Participants were instructed that disclosing their group assignment to investigators would result in exclusion from the study. To further minimize the confounding influence of alerting reactions on BP, measurements were performed at a separate location outside the physician’s office and not associated with usual patient care." |
Al‐Faris 2008.
| Methods | P SB |
|
| Participants | Community setting, Riyadh University for girls, Saudi Arabia Eligibility: healthy Intervention: N = 30; age: 21 (SD = 2.0); male: 0% Control: N = 30; age: 22 (SD = 1.8); male: 0% Normotensive (mean baseline BP = 115.5/73 mmHg) |
|
| Interventions | 1. 100 g dark chocolate (50%; 500 mg flavanols)
2. 90 g white chocolate (0 mg flavanols); daily Duration: 15 days |
|
| Outcomes | Resting SBP and DBP (position not stated) after 15 days; Primary outcome measure | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Influence of funding body unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
Crews 2008.
| Methods | P DB |
|
| Participants | Community setting, Virginia, USA Eligibility: healthy N = 90 Age: 69 (SD = 8.3) Male: 42% Normotensive (mean baseline BP = 127.5/74.5 mmHg) |
|
| Interventions | 1. High‐flavanol dark chocolate bars (37.0 g; containing 60% cacao; 755 mg flavanols) and cocoa beverage (12 g cocoa)
2. Low‐flavanol ( 41 mg flavanols) placebos matched for appearance, smell, taste, and caloric content; daily. Duration: 6 weeks |
|
| Outcomes | Seated resting SBP and DBP (left upper arm) after 3 and 6 weeks | |
| Notes | Industry research grant. The authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation of the products was conducted by an independent researcher |
| Allocation concealment (selection bias) | Low risk | "The boxes and containers containing the products (and their randomization numbers, 1–101) were subsequently issued to participants in an ascending and sequential order as they entered the study (at the time of their pretreatment baseline assessments)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% (11 of 101) lost to follow‐up. 10 withdrew, 1 was excluded from analysis due to non‐compliance |
| Selective reporting (reporting bias) | Low risk | BP reported at start, middle, and end of study |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebos were matched for appearance (e.g. colour and quantity), smell, taste, and caloric content |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Davison 2008a.
| Methods | P DB |
|
| Participants | Study dates: 9/05‐12/16 Community setting, Adelaide, Australia Eligibility: sedentary, overweight Intervention: N = 12; age: 45 (SD = 4.4); male: 33% Control: N = 11; Age: 44 (SD = 4.4); male: 27% Normotensive (mean baseline BP = 124/76.5 mmHg) |
|
| Interventions | 1. HiFl drink (902 mg flavanols)
2. LoFl drink (36 mg flavanols); daily Duration: 12 weeks |
|
| Outcomes | Resting supine SBP and DBP at 6 and 12 weeks Primary outcome measure |
|
| Notes | no exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Volunteers were block‐matched into 2 groups according to BMI, gender, age and BP. The groups were then randomised to the daily consumption. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21% (14/65) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Change of BP from baseline reported |
| Other bias | Unclear risk | Manufacturer (Mars Inc.) provided the cocoa drinks and financial support |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Cocoa beverages were matched for taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Davison 2008b.
| Methods | P DB |
|
| Participants | Study dates: 9/05‐12/16 Community setting, Adelaide, Australia Eligibility: sedentary, overweight Intervention: N = 13; age: 45 (SD = 3.0); male: 31% Control: N = 13; age: 46 (SD = 4.0); male: 46% Normotensive (mean baseline BP = 124/76 mmHg) |
|
| Interventions | 1. HiFl drinks (902 mg flavanols); in addition to physical exercise
2. LoFl drinks (36 mg flavanols); daily; in addition to physical exercise Duration: 12 weeks |
|
| Outcomes | Resting supine SBP and DBP at 6 and 12 weeks Primary outcome measure |
|
| Notes | Intervention in addition to physical exercise; Manufacturer (Mars Inc.) provided the cocoa drinks and financial support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Volunteers were block‐matched into 2 groups according to BMI, gender, age and BP. The groups were then randomised to the daily consumption Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21% (14/65) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Change of BP from baseline reported |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Cocoa beverages were matched for taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Grassi 2008.
| Methods | C SB |
|
| Participants | Hospital outpatients setting, L'Aquila, Italy Eligibility: hypertensive, impaired glucose tolerance N = 19 Age: 45 (SD = 8) Male: 58% Hypertensive (Mean baseline BP = 141/91 mmHg) |
|
| Interventions | 1. 100 g flavanol‐rich dark chocolate bars (1080 mg flavanols)
2. 100 g flavanol‐free (0 mg) white chocolate bars; daily. Duration: 15 days |
|
| Outcomes | 24‐hour automated ambulatory SBP and DBP, in addition to seated SBP and DBP; after 15 days. Primary outcome measure |
|
| Notes | Supported by the Italian government (Ministero della Universita´e della Ricerca Scientifica) and the US government (USDA Agricultural Research Service). The dark chocolate bars were donated by the manufacturer. The authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "Chocolate doses for each subject were rolled in aluminium foil and administered in dated, sequentially numbered, nontransparent boxes not labelled with regard to content. Involved physicians and staff were unaware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants, only of personnel. Participants did not receive information regarding the chocolate and were instructed not to disclose their assigned group to investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Muniyappa 2008.
| Methods | C DB |
|
| Participants | Community setting, Bethesda, USA Eligibility: hypertensive N = 20 Age: 51 (SEM = 1.5) Male: 40% Hypertensive (mean baseline BP = 141/91 mmHg) |
|
| Interventions | 1. 31 g cocoa drink powder mixed in 150 mL warm water (902 mg flavanols)
2. 31 g matching placebo drink powder mixed in 150 mL warm water (28 mg total flavanols); daily Duration: 2 weeks |
|
| Outcomes | Resting (seated) SBP and DBP (on nondominant arm) measured 3 times a week Primary outcome measure |
|
| Notes | Supported by the US government (Intramural Research Program, NCCAM, NIH, and Office of Dietary Supplements, NIH). Cocoa and placebo preparations provided by manufacturer (Mars Inc.), not involved in research.The authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by NIH Clinical Center Pharmacy |
| Allocation concealment (selection bias) | Low risk | Assignment codes were not available to investigators until 20 participants completed the entire study and the database had been completed and secured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31% (9/29) participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | BP measured 3 times a week, but only outcomes at baseline and after 2 weeks treatment reported |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The cocoa and placebo drinks were similar in colour, taste, and packaging and participants were blinded to treatment assignment. Participant blinding was assessed by a questionnaire administered at the end of 6 wks that asked participants to indicate which treatment they believed they received during each of the 2 phases (cocoa, placebo, or uncertain)." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | In addition to monitoring BP in the outpatient clinic, participants were required to self‐monitor their blood pressure at home using a portable BP device |
Monagas 2009.
| Methods | C SB |
|
| Participants | Hospital outpatients setting, Barcelona, Spain Eligibility: diabetes, or >=3 CVD risk factors N = 25 Age: 70 Male: 45% Prehypertensive (mean baseline BP = 138/84 mmHg) |
|
| Interventions | 1. 40 g cocoa powder (495 mg flavanols) in milk
2. Only milk (0 mg flavanols); daily Duration: 4 weeks |
|
| Outcomes | Resting SBP and DBP (position not stated) after 4 weeks, Secondary outcome measure | |
| Notes | Supported by grants from the Spanish Ministries of Education and Science and Innovation. Funding body not involved in the study. No conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment achieved by using closed envelopes with correlative numbers by prespecified subgroups of sex and age |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention. |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants, but blinding of personnel: The clinical investigators and laboratory technicians were blinded to the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Ried 2009.
| Methods | P SB |
|
| Participants | Study dates: 6/07‐12/07 Community setting, Adelaide, Australia Eligibility: (pre‐)hypertensive Intervention: N = 11; age: 49 (SD = 12.2); male: 64% Control: N = 10; age: 58 (SD = 13.4); male: 50% Prehypertensive (mean baseline BP = 135.5/81 mmHg) |
|
| Interventions | 1. 50 g dark chocolate (70%) (750 mg flavanols)
2. Placebo pill (0 mg flavanols); daily Duration: 8 weeks |
|
| Outcomes | Resting supine SBP and DBP at 4 and 8 weeks Primary outcome measure |
|
| Notes | Chocolate provided by manufacturer (Haigh's Chocolates, Adelaide). Manufacturer did not provide funding and were not involved in study design, data collection, analysis or preparation of the manuscript. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated by permuted block randomisation using the SAS 9.1 software package. |
| Allocation concealment (selection bias) | Low risk | To conceal allocation from investigators, trained staff not involved in trial design and analysis handed out intervention packs to participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% (4/39) lost to follow‐up/ withdrawal |
| Selective reporting (reporting bias) | Low risk | BP data reported comprehensively |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants to chocolate was impractical, however blinding of participants in the capsule groups was achieved by identical packaging of active tomato extract and placebo capsules. Control group and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Shiina 2009.
| Methods | P SB |
|
| Participants | Community setting, Chiba, Japan Eligibility: males Intervention: N = 20; age: 29 (SD = 3.4); male: 100% Control: N = 19; age: 30 (SD = 4.5); male: 100% Normotensive (Mean baseline BP = 119/68.5 mm Hg) |
|
| Interventions | 1. 45 g dark chocolate (80%) (550 mg flavanols)
2. 35 g white chocolate (0 mg flavanols); daily Duration: 2 weeks |
|
| Outcomes | Resting SBP and DBP (position not stated) after 2 weeks; Secondary outcome measure | |
| Notes | Sponsor not involved in data collection and analysis. No conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded (dark/white chocolate) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Bogaard 2010.
| Methods | C DB |
|
| Participants | Study dates: 11/08‐10/09 Community setting, Amsterdam, Netherlands Eligibility: (pre‐)hypertensive n=41 Age: 62 (SD = 4.5) Male: 76% Hypertensive (mean baseline BP = 142/84 mmHg) |
|
| Interventions | 1. High flavanol drink (529 mg flavanols)
2. Low flavanol drink (0 mg flavanols); daily Duration: 3 weeks |
|
| Outcomes | Resting (seated) SBP and DBP (on nondominant arm) after 3 weeks; 24‐hour automated ambulatory SBP and DBP (on nondominant arm) after 3 weeks; Primary outcome measure | |
| Notes | Mean of theobromine‐enriched chocolate group (TEC) + natural dose theobromine chocolate group (NTC); Sponsored by manufacturer (Unilever); one co‐author (but none of the investigators) employed by Unilever; The contractual agreement between the Academic Medical Center and Unilever allowed the sponsor to review and comment on the article, but the investigators remained responsible for its contents and decision to submit the results for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Test product allocation and order of treatment were determined by a computer‐ generated randomised schedule |
| Allocation concealment (selection bias) | Low risk | Test products were provided in sequentially‐numbered sealed bottles |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% (2/42) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry‐funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The different test products all had similar taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All of the haemodynamic measurements were performed by a single investigator, blinded for treatment allocation |
Davison 2010.
| Methods | P DB |
|
| Participants | Community setting, San Franscisco, USA Eligibility: coronary artery disease Group 1 (33 mg flavanol): N = 14; age: 53 (SD = 6.7); male: 71% Group 2 (372 mg flavanol): N = 12; age: 56 (SD = 14.2); male: 58% Group 3 (712 mg flavanol) N = 13; age: 60 (SD = 13.7); male: 62% Group 4 (1052 mg flavanol): N = 13; sage: 57 (SD = 9.7); male: 54% Hypertensive (mean baseline BP = 144/85.5 mmHg) |
|
| Interventions | Cocoa drink containing 33 mg/372 mg flavanol/712 mg flavanol/1052 mg flavanol; daily Duration: 6 weeks |
|
| Outcomes | Seated clinic DBP and SBP (non‐dominant arm) after 3 and 6 weeks; 24‐hour automated ambulatory SBP and DBP (non‐dominant arm) after 3 and 6 weeks Primary outcome measure |
|
| Notes | Trial received funding from industry. The authors declared no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of groups was undertaken independently of group minimisation procedure by separate staff members of the research centre not otherwise involved with the trial |
| Allocation concealment (selection bias) | Low risk | Trial investigators remained blinded to treatment allocation until after the completion of data analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% (7/59) lost to follow‐up: 5 withdrawals, 1 exclusion due to non‐compliance (deliberate weight loss), 1 exclusion due to gastric complaints |
| Selective reporting (reporting bias) | Low risk | BP reported for each assessment point (baseline, week 3, week 6) |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The reconstituted cocoa beverages were matched for appearance and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Heiss 2010.
| Methods | C DB | |
| Participants | Community setting, San Franscisco, USA Eligibility: coronary artery disease N = 16 Age: 64 (SD = 3) Male: 19% Prehypertensive (mean baseline SBP = 131.5 mmHg; no DBP given) |
|
| Interventions | 1. High flavanol drink (750 mg flavanols)
2. Low flavanol (18 mg flavanols) drink; daily Duration: 4 weeks |
|
| Outcomes | Resting supine SBP and DBP after 30 days Tertiary outcome measure |
|
| Notes | This study was supported by a grant from the American Heart Association, and an unrestricted research grant from Mars, Inc. Two authors received funding from industry, and one author is employed by Mars. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation and dispensing of cocoa drinks were performed by the Department of Pharmacology. Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6% (1/17) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry‐funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All drinks were similar in taste. Participants and investigators were masked throughout the study with regard to flavanol content of the test drinks |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Njike 2011.
| Methods | C DB |
|
| Participants | Study dates: 08/05‐06/06 Community setting, Derby, USA Eligibility: overweight N = 38 Age = 52.5 (SD = 10.4) Male: 15% Normotensive (mean baseline BP = 123/68 mmHg) |
|
| Interventions | 1. High flavanol drink (805 mg flavanols)
2. Low flavanol (9 mg flavanols) drink; daily Duration: 6 weeks |
|
| Outcomes | Resting supine SBP and DBP after 6 weeks; Secondary outcome measure | |
| Notes | Grant funding from manufacturer Hershey. One author received speaker's fee. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 44 participants were randomly assigned using a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% (7/44) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
Almoosawi 2012a.
| Methods | C SB |
|
| Participants | Community setting, Cambridge, UK N=21 Age: not provided Male: 0% Normotensive (Mean baseline BP: 107/70 mm Hg) |
|
| Interventions | Polyphenol‐rich dark chocolate (500 mg polyphenol)
Polyphenol‐free /placebo dark chocolate The placebo was a dark chocolate matched for taste, texture, colour and macronutrient composition to the polyphenol‐rich DC, but which contained no polyphenols. Duration: 8 weeks |
|
| Outcomes | A validated automated A&D Medical UA‐767 BP monitor (A&D medical, San Jose, CA, USA) was used to measure BP after a rest of 10 min. Three values were taken at 2 min intervals Secondary |
|
| Notes | BMI < 25 (Subgroup 1); The authors declare no conflicts of interest. Funding source not given, except for a manufacturer supplying the chocolate products. The authors declare no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Following a 1‐week run‐in phase, eligible people were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/22 (5%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Funding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
Almoosawi 2012b.
| Methods | C SB |
|
| Participants | Community setting, Cambridge, UK N = 21 Age: not provided Male: 0% Normotensive (mean baseline BP = 119/76 mmHg) |
|
| Interventions | 1. Polyphenol‐rich dark chocolate (500 mg polyphenol)
2. Polyphenol‐free /placebo dark chocolate, matched for taste, texture, colour and macronutrient composition to the polyphenol‐rich DC, but which contained no polyphenols The placebo was a dark chocolate Duration: 8 weeks |
|
| Outcomes | As in Almoosawi 2012a | |
| Notes | BMI > 25 (Subgroup 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Following a 1‐week run‐in phase, eligible people were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/22 (5%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Funding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
Desideri 2012.
| Methods | P DB |
|
| Participants | Hospital setting: Alzheimer unit, L'Aquila, Italy Eligibility criteria: Mild cognitive impairment, Petersen criteria Intervention: N = 30; age: 71.2 (SD = 4.9); male: 47% Control: N = 30; age: 71.0 (SD = 4.5); male: 53% Hypertensive (mean baseline BP = 141/85 mmHg) |
|
| Interventions | 1. High flavanol drink (990 mg flavanols)
2. Very low flavanol drink (48 mg flavanols) Duration: 8 weeks |
|
| Outcomes | Seated rested SBP and DBP after 8 weeks; Secondary outcome measure |
|
| Notes | Study was supported by industry grant (Mars Inc), who supplied high/low flavanol powder. One of the authors is employed by Mars Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation of the products was conducted by an independent researcher |
| Allocation concealment (selection bias) | Low risk | Personnel not involved in the trial labelled identical boxes containing individual anonymised sachets. The boxes were subsequently issued to participants in an ascending and sequential order as they entered the study (at the time of their pre‐treatment baseline assessments). Neither the treating physicians, nor the participants were aware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant (1.1%) discontinued due to side effects |
| Selective reporting (reporting bias) | Low risk | BP reported at baseline and end of study |
| Other bias | High risk | Industry‐funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research staff, treating physicians, and the participants were blinded to treatment allocation. Drink powder was indistinguishable in taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
Khan 2012.
| Methods | C Open‐label, unblinded |
|
| Participants | Hospital setting, Barcelona, Spain Eligibility criteria: >= 3 risk factors CVD N = 42 Age: 69.7 (SD = 11.5) Male: 45% 78% hypertensive; mean baseline BP = 138/84 mmHg (pre‐hypertensive) |
|
| Interventions | 1. 40 cocoa powder (495 mg polyphenol incl. 56.5 mg epicatechin) in 500 ml skimmed milk
2. 500 ml skimmed milk (0 mg flavanols) Duration: 4 weeks |
|
| Outcomes | BP after 4 weeks Secondary outcome measure |
|
| Notes | Study was supported by grants from the Spanish Ministries of Education and Science and Innovation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information given |
| Allocation concealment (selection bias) | Unclear risk | No further information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to ‐follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at baseline and end of study periods |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants unblinded. No information of blinding of research staff given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
Mogollon 2013.
| Methods | P DB |
|
| Participants | Study dates: 7/08‐4/09 Hospital setting, Quebec, Canada Eligibility: pregnancy Intervention: N = 22; age: 28.7 (SD = 3.2); male: 0%, all pregnant women Control: N = 20 ; age: 29.8 (SD = 3.6); male: 0%, all pregnant women Normotensive (mean baseline BP = 109/69 mmHg) |
|
| Interventions | 1. High‐flavanol chocolate (400 mg flavanols)
2. Low‐flavanol chocolate (60 mg flavanols) Duration: 12 weeks |
|
| Outcomes | BP was measured by a trained, certified nurse blinded to treatment allocation, with an electronic monitor (Microlife 3 BTO‐A) after 15 mins of rest, back supported, arm supported at the heart level, and cuff placed on the left upper arm Primary outcome measure |
|
| Notes | All other authors declare that they have no conflicts of interest. Hospital employees | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation was generated using computer‐aided block randomisation (block size was kept secret), under the responsibility of an independent statistician |
| Allocation concealment (selection bias) | Low risk | Statistician undertook treatment allocation independently of the trial team. All clinical investigations, laboratory analyses, data collection and assessment were blinded to the randomisation allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women dropped out of the study for reasons not related to the intervention |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Chocolate placebo was identical to the experimental chocolate in its content for all other nutrients except for flavanols (including theobromine and caffeine contents), similar in taste and supplied in individual, opaque packaging |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinical investigations, laboratory analyses, data collection and assessment were blinded to the randomisation allocation |
Neufingerl 2013.
| Methods | P DB |
|
| Participants | Study dates: 12/10‐2/11 Community setting, Grenoble and Lyon, France Eligibility: <10% CVD risk on European risk chart Intervention: N = 10; age: 55.2 (SD = 8.2); male: 50% Control: N = 10; age: 55.4 (SD = 8.7); male: 50% Normotensive (mean baseline BP: 118/75 mmHg) |
|
| Interventions | 1. 6 g cocoa as chocolate‐flavoured (325 mg flavanoids) pasteurised acidified milk drink
2. Milk drink (0 mg flavanols) Duration: 4 weeks |
|
| Outcomes | 24‐hour ambulatory Mean BP | |
| Notes | 4‐group study, only cocoa and placebo group considered here, additional groups: theobromine only (850 mg), n = 10 and cocoa + theobromine (C+T) group, n = 10 (total theobromine 1000 mg); adverse events in n = 6 (C+T), N = 1 (T): nausea, vomiting, headache, diarrhoea, potentially related to high dose of theobromine. All authors were employed by Unilever R&D Vlaardingen at the time the research was conducted. Unilever has no products enriched with theobromine under development or on the market; however, it markets food products enriched with plant sterols to lower LDL cholesterol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐established blockwise randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Sequentially allocated by clinical investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at baseline and end of study |
| Other bias | Unclear risk | Industry‐supported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drinks supplied in identical tinted bottles that were packed individually for each participant in a neutral box and labelled with the participant code; participants were instructed not to pour the drink into a glass but to consume it directly out of the tinted bottle. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
Sorond 2013.
| Methods | P DB |
|
| Participants | Hospital setting, Neurology Research Unit, Boston, USA Eligibility: Hypertension N = 60 Age: 72.9 (SD = 5.4) yrs Male: 48% Normotensive (mean baseline BP = 125.5/69 mmHg) |
|
| Interventions | 1. Flavanol‐rich cocoa 1218 mg
2. Flavanol‐poor cocoa 26 mg; daily Duration: 4 weeks |
|
| Outcomes | BP mean of 3 measurements with automated cuff | |
| Notes | Controlled hypertensives (on BP medication); Supported by government grants from the National Institite on Aging and National Heart Lung and Blood Institute. Cocoa was provided by Mars Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided, unclear whether randomised |
| Allocation concealment (selection bias) | High risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: n = 2 (3%) |
| Selective reporting (reporting bias) | Low risk | BP at baseline, day 1 and end of the study |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
Esser 2014.
| Methods | C DB |
|
| Participants | Community setting, Wageningen, Netherlands Eligibility: overweight N = 41 Age: 63 (SD = 5) Male: 100% Normotensive (mean baseline BP = 128/79 mmHg) |
|
| Interventions | 1. High flavanol chocolate (1078mg flavanols)
2. Normal flavanol chocolate (259 mg flavanols), with a 4‐week washout between consumption periods Duration: 4 weeks |
|
| Outcomes | Brachial SBP, DBP, and heart rate (HR) were assessed automatically (Dinamap Pro 100; GE Healthcare, Little Chalfont, UK) for 10 mins with a 3‐min interval; Secondary outcome measure |
|
| Notes | Study was funded by Top Institute Food and Nutrition (Wageningen, The Netherlands). The chocolate used in this study was donated by Barry Callebaut (Lebbeke, Belgium). The authors declare no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by an independent research assistant using a computer‐generated table. We constructed 25 blocks with a size of 2." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/44 (7%) participants dropped out or were excluded, 1 due to medical reasons not related to the study, 1 due to dislike of the chocolate and 1 due to failure to adhere to the treatment |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Researchers as well as participants were blinded to randomisation until after data analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers as well as participants were blinded to randomisation until after data analysis. |
Ibero‐Baraibar 2014.
| Methods | P DB |
|
| Participants | Study dates: 3/12‐6/12 Community setting, Navarra, Spain Eligibility: overweight N = 47 Age: 57.3 (SD = 5.2) Male: 46% Normotensive (mean baseline BP: 120/80 mmHg) |
|
| Interventions | 1. Meals supplemented with 1.4 g/day cocoa extract (645 mg total polyphenols/414mg total flavanols)
2. Control meals (0 mg polyphenols) Duration: 4 weeks |
|
| Outcomes | BP was taken 3 times with automatic monitor (Intelli Sense. M6, OMRON Healthcare, Hoofddorp, Netherlands), to use the average value obtained from the last 2 measurements Secondary outcome measure |
|
| Notes | Co‐funded by food industry and government. Conducted at seemingly independent research institutions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed using the “random between 1 and 2” function in the Microsoft Office Excel (Microsoft Iberica, Spain) |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/50 (6%) participants dropped out or were excluded, 1 due to personal reasons and 2 due to failure to adhere to the treatment |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry co‐funded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boxes in which the meals were provided had the same appearance and differed only on the code label, ensuring the double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Nickols‐Richardson 2014.
| Methods | P Unblinded? |
|
| Participants | Study dates: 7/09‐7/10 Community setting, Pennsylvannia, USA Eligibility: overweight N = 60 Age: 35.9 (SEM = 0.8) Male: 0% Normotensive (mean baseline BP = 118/73 mmHg) |
|
| Interventions | 1. 236 mL natural cocoa beverage and 2.9 oz dark chocolate (270 mg flavanols)
2. 236 mL cocoa‐free vanilla beverage and non‐chocolate sweet snacks (0 mg flavanols) Duration: 18 weeks |
|
| Outcomes | Seated systolic and diastolic BP; Primary outcome measure |
|
| Notes | Co‐funded by food industry and public sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information given |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 85% of the women completed the intervention with no difference between DC and NC groups in discontinuation rate |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry co‐funded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded; no information on blinding of personnel given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Sarria 2014a.
| Methods | C unblinded |
|
| Participants | Community setting, Madrid, Spain N = 24 Age: 27 (SD = 8.4) Male: 46% Normotensive (Mean baseline BP: 116/72 mmHg) |
|
| Interventions | 1. Milk with cocoa (416 mg flavanols)
2. Milk only (0 mg flavanols) Duration: 4 weeks |
|
| Outcomes | Seated systolic and diastolic BP Secondary outcome measure |
|
| Notes | Subgroup: Normal cholesterol; Funded by food industry. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No further information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/50 withdrew due to personal, health or professional reasons (numbers not provided by intervention groups) |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Lack of blinding of participants and investigators |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding of participants and investigators |
Sarria 2014b.
| Methods | C Unblinded |
|
| Participants | Community setting, Madrid, Spain N = 20 Age: 30 (SD = 9) Male: 45% Normotensive (mean baseline BP = 121/76 mmHg) |
|
| Interventions | As in Sarria 2014a | |
| Outcomes | As in Sarria 2014a | |
| Notes | Subgroup: High cholesterol; Funded by food industry. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No further information on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/50 withdrew due to personal, health or professional reasons (numbers not provided by intervention groups) |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Lack of blinding of participants and investigators |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding of participants and investigators |
Heiss 2015a.
| Methods | P DB |
|
| Participants | Community setting, Duesseldorf, Germany Eligibility: healthy male N = 22 Age: 26 (SEM = 1) Male: 100% Normotensive (mean baseline BP: 120/77 mmHg) |
|
| Interventions | 1. Cocoa extract powder (900 mg flavanols) dissolved in water
2. Placebo powder (0 mg flavanols) dissolved in water Duration: 2 weeks |
|
| Outcomes | Office blood pressure was measured 3 times after 10 mins of rest using an automated clinical digital sphygmomanometer (Dynamap, Tampa, FL, USA) with appropriately sized cuff placed around the upper arm at heart level Primary outcome measure |
|
| Notes | Young subgroup; Co‐funded by food industry and public sources. One author employed by the company that manufactures and markets the specific cocoa powder used in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, no further information |
| Allocation concealment (selection bias) | Low risk | Anonymised sachets in alphanumeric order |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and all data were included in the analysis |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The beverage mixes were provided in sachets labelled with an alphanumeric identifier to enable a double‐masked study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Heiss 2015b.
| Methods | P DB |
|
| Participants | Community setting, Duesseldorf, Germany Eligibility: healthy male N = 20 Age: 60 (SEM = 2) Male: 100% Prehypertensive (mean baseline BP = 131/82 mmHg) |
|
| Interventions | 1. Cocoa extract powder (900 mg flavanols) dissolved in water
2. Placebo powder (0 mg flavanols) dissolved in water Duration: 2 weeks |
|
| Outcomes | as in Heiss 2015a | |
| Notes | Elderly subgroup; Co‐funded by food industry and public sources. One author employed by the company that manufactures and markets the specific cocoa powder used in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, no further information |
| Allocation concealment (selection bias) | Low risk | Anonymised sachets in alphanumeric order |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and all data were included in the analysis |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The beverage mixes were provided in sachets labelled with an alphanumeric identifier to enable a double‐masked study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Koli 2015.
| Methods | C Unblinded (no placebo, but reduced snack intake during study period) |
|
| Participants | Community setting, Helsinki, Finnland Eligibility: hypertensive N = 22 Age: 45.8 (SD = 8.3) Male: 64% Hypertensive (mean baseline BP = 142/89 mmHg) |
|
| Interventions | 1. 49 g dark chocolate (70% cacao, 603 mg flavanols)
2. Reduced intake of habitual snacks only (no placebo) (0 mg flavanols) Duration: 8 weeks |
|
| Outcomes | Clinical blood pressure and 24‐hr ambulatory BP monitor measured, no details given on assessment of clinical BP; Ambulatory 24‐hour blood pressure was monitored on a day of standard physical activity, with an adequate cuff for the size of the participant’s arm. Welch Allyn ABPM 6100 (Welch Allyn Inc, USA) validated according to the protocol of the Finnish Hypertension Society Primary outcome measure |
|
| Notes | Funded by Finnish chocolate manufacturer Oy Karl Fazer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants were randomly assigned to 1 of the 2 groups (denoting order of interventions) after stratification by sex and BMI. No details on random sequence generation provided |
| Allocation concealment (selection bias) | High risk | Participants knew which group they were in before/after cross‐over, not stated if researchers knew as well |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and all data were included in the analysis |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded, no placebo; unclear if investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Massee 2015.
| Methods | P DB |
|
| Participants | Study dates: 8/13‐9/14 Community setting, Melbourne, Australia Eligibility: healthy N = 38 Age: 24 (SD = 4.5) Male: 33% Normotensive (mean baseline BP = 119/71 mmHg) |
|
| Interventions | 1. Active cocoa tablet (3058 mg cacao seed extract, 250 mg catechin polyphenols)
2. Placebo tablet, identical in appearance, size, texture and colour to cocoa tablet, containing inert cellulose powder (0 mg polyphenols) Duration: 4 weeks |
|
| Outcomes | BP was assessed in a quiet, dedicated university laboratory following a 5‐min rest period completed by participants in the supine position on an examination bed; Secondary outcome measure |
|
| Notes | Funded from public or charitable sources. Cocoa and placebo tablets provided by supplement company, not involved in study design, data collection, analysis and publication. Authors declare no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to receive either active or placebo tablets using a computer‐generated permuted block randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Identical bottles in alphanumerical order, packaged offsite by staff not involved in participant recruitment and testing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% (2/40) lost to follow‐up, 1 each from intervention and control groups |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablet (Identical in appearance, size, texture and colour to cocoa tablet, containing inert cellulose powder). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blinding code was only revealed after analysis of the main study |
Mastroiacovo 2015.
| Methods | P DB |
|
| Participants | Study dates: 12/06‐7/08 Community setting, L'Aquila, Italy Eligibility: cognitively intact, Mini‐Mental‐State‐Examination Score < 27 N = 30 (high flavanol group), N = 30 (low flavanol group = control); (N = 30 intermediate flavanol group not included in this meta‐analysis) Age: 70 (SE = 0.8) Male: 43% Prehypertensive (mean baseline BP = 135/80 mmHg), incl. about 50% hypertensive |
|
| Interventions | 1. Dry dairy‐based beverage mixes with flavanol‐rich cocoa powder (993 mg flavanols, Cocoapro processed cocoa powder; Mars Inc)
2. Highly processed, alkalised cocoa powder (48 mg flavanols) Duration: 8 weeks |
|
| Outcomes | "Seated systolic and diastolic BP recorded in the morning with a validated oscillometric device with appropriately sized cuffs (Omron 705 CP; Omron Matsusaka) on the nondominant upper arm. These evaluations were performed by staff blinded to the study protocol. At each visit, participants rested 15 mins in a seated position, the first blood pressure measurement was taken but discarded, and the subsequent 3 consecutive blood pressure readings, taken at 3‐min intervals, were recorded. The average of these latter measures was considered for statistical analysis." Secondary outcome measure |
|
| Notes | One of the authors is employed by Mars Inc., a company with long‐term research and commercial interests in cocoa flavanols. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on random sequence generation given |
| Allocation concealment (selection bias) | Unclear risk | Neither the treating physicians nor the participants were aware of treatment allocation. No further details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 discontinued trial, 0 lost to follow‐up per group |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Food products were indistinguishable in appearance and had a flavanol content that was not obvious on the basis of flavour. Staff were blinded to the study protocol |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Rostami 2015.
| Methods | P SB |
|
| Participants | Study dates: 3/11‐2/12 Hospital setting, Tehran, Iran Eligibility: type‐2‐diabetes, hypertension Intervention: N = 32; age: 59 (SD = 9); male: 37.5% Control: N = 28; age: 57 (SD = 8); male: 42.9% Prehypertensive (Mean baseline BP = 137/86 mmHg) |
|
| Interventions | 1. 25 g chocolate containing 83% cocoa solids
2. Iso‐caloric white chocolate no flavanol content given Duration: 8 weeks |
|
| Outcomes | Systolic and diastolic blood pressure was reported on average of 2 properly measured in the right or left arm supported at the heart level of seated position after 10 mins of rest by a trained nurse using a mercury sphygmomanometer; Primary outcome measure |
|
| Notes | Funded by University. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation method |
| Allocation concealment (selection bias) | Low risk | The participants were given chocolate bars containing either dark chocolate or white chocolate in the same package by blinded person to the same colour and shape |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13% (8/60) lost to follow‐up: intervention group: n = 2; control group: n = 6 |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | SB only personnel‐blinded. The participants were given chocolate bars containing either dark chocolate or white chocolate in the same package by blinded person to the same colour and shape. Participants were aware unblinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Rull 2015.
| Methods | C DB |
|
| Participants | Community setting, London, UK Eligibility: hypertension N = 21 Age: 55 (SEM = 1.5) Male: 100% Prehypertensive (mean baseline BP = 135/85 mmHg) |
|
| Interventions | 1. 50 g high flavanol (1064 mg) dark chocolate bars
2. 50 g low flavanol (88 mg) dark chocolate bars Duration: 12 weeks |
|
| Outcomes | Ambulatory blood pressure measurements (24‐hour) were made during participant screening and at 6 and 12 weeks using a Spacelabs ABP monitor 90207 (Dolby UK, Stirling) | |
| Notes | This study was supported by a grant from Barry Callebaut Belgium NV to one of the authors (R. Corder). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was sent as a password‐protected file to Barry Callebaut, who prepared separate participant coded boxes for each phase of the study |
| Allocation concealment (selection bias) | Unclear risk | All interventions were provided in anonymised sachets |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up; 11/32 participants (34%) due to failure to attend the clinic on the required day, or BP monitor recording failure at either 6 or 12 weeks |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐control chocolate specifically manufactured, suggested to be similar in appearance to intervention, both plain foil wrapped. The investigators were blinded to the randomisation schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
Sansone 2015.
| Methods | P DB | |
| Participants | Study dates: 2/13‐8/14 Community / Hospital setting, Duesseldorf, Germany Eligibility: healthy N = 100 Age: 44.5 (SD = 8.5) M: 52.4% Normotensive (mean baseline BP = 123/77 mmHg) |
|
| Interventions | 1. High flavanol (450 mg) drink
2. Low flavanol (0 mg) drink; daily Duration: 4 weeks |
|
| Outcomes | Office BP was measured using an automated clinical digital sphygmomanometer (Dynamap) at the upper left arm in supine position, after 10 mins of rest in a quiet room with the arm at the heart level. 3 measurements were taken, the first discarded and the second and third averaged for further analysis. Secondary outcome measure |
|
| Notes | One of the authors is employed by Mars Inc., a company engaged in flavanol research and flavanol‐related commercial activities. None of the other authors has a conflict of interest to declare other than stated above. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 2 parallel groups by block randomisation |
| Allocation concealment (selection bias) | Low risk | All interventions were provided as drink powder in sachets to be freshly prepared by mixing with approximately 500 ml of water. The beverage mixes were provided in sachets (7 g = 1 serving) labelled with an alphanumeric identifier to enable a double‐masked study design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on compliance or dropouts reported |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were masked throughout the study for flavanol content of the test drinks |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment given |
BMI: body mass index BP: blood pressure C: cross‐over CVD: cardiovascular disease DB: double‐blind DBP: diastolic blood pressure P: parallel SB: single‐blind SBP: systolic blood pressure SD: standard deviation SEM: standard error of the mean
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
|---|---|
| Farouque 2006 | Data for meta‐analysis not available (mean SBP/DBP, SD) |
| Wang‐Polagruto 2006 | Low quality (50% lost to follow‐up, small sample size) |
| Flammer 2007 | Duration < 2 weeks, acute effects of cocoa, (heart transplant patients) |
| Balzer 2008 | Data for meta‐analysis not available (mean SBP/DBP, SD) |
| Erdman 2008 | High cocoa dosage in control group, cocoa+plant sterols vs cocoa; same study as Allen 2008 |
| Faridi 2008 | Duration < 2 weeks, acute effects of cocoa |
| Almoosawi 2010 | High cocoa dosage in control group |
| Berry 2010 | Duration < 2 weeks, acute effects of cocoa |
| Desch 2010 | Control group 25% flavanol content (6 g dark chocolate) vs intervention group (25 g dark chocolate) |
| Sudarma 2011 | No true control group: dark chocolate bar versus dark chocolate bar plus lycopene or dark chocolate bar plus lycosome |
| Curtis 2013 | Combination treatment of chocolate (850 mg flavanols) plus 100 mg isoflavones daily for 1 year in active group |
| D'Anna 2014 | Combination treatment of cocoa (30 mg) + isoflavanols (80 mg) + myo‐inositol (2g) in active group |
| Pereira 2014 | No intervention in control group |
| Petyaev 2014 | No true control group: flavanol/polyphenol content in active group intervention not provided; dietary polyphenol intake similar in active and control groups |
| West 2014 | Acute BP after 2 hours |
| Wirtz 2014 | Acute BP |
| Grassi 2015 | 5‐week cross‐over trial of different cocoa dosages and placebo, each taken 1 week |
| Lee 2016 | conference abstract only, insufficient information |
| Leyva‐Soto 2016 | conference abstract only, insufficient information |
| Suh 2014 | cohort study, not randomized, only conference abstract, insufficient information |
| Grassi 2016 | Duration < 2 weeks |
| Kuebler 2016 | Duration < 2 weeks |
| Sanguigni 2016 | Duration < 2 weeks |
| Sanchez‐Aguadero 2016 | Duration < 2 weeks, no separate chocolate intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
Campbell 2016.
| Methods | 6‐week clinical trial |
| Participants | nine panelists (age: 22.6 ± 1.7; BMI: 22.3 ± 2.1) |
| Interventions | chocolate‐protein beverages once per week, including placebo, whey protein isolate (WPI), low polyphenolic cocoa (LP), high polyphenolic cocoa (HP), LP‐WPI, and HP‐WPI |
| Outcomes | blood glucose and adiponectin levels, and hunger ratings at baseline and 0.5–4.0 h following beverage consumption |
| Notes |
De Palma 2016.
| Methods | single‐centre randomized double‐blind placebo‐controlled investigation with a crossover design |
| Participants | Thirty‐two patients with chronic HF, stable on guideline‐directed medical therapy, were randomized. Twenty‐four patients completed the study |
| Interventions | 50 g/day of high‐flavanol dark chocolate (HFDC; 1064 mg of flavanols/day) or low‐flavanol dark chocolate (LFDC; 88 mg of flavanols/day) for 4 weeks and then crossed over to consume the alternative dark chocolate for a further 4 weeks |
| Outcomes | reductions in N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) as an index of improved cardiac function. Changes in blood pressure. Effect on platelet function. |
| Notes | supported by a grant from Barry Callebaut Belgium NV |
Flammer 2012.
| Methods | 4 week double‐blind, randomized placebo‐controlled trial |
| Participants | Twenty‐two patients with stable CHF (NYHA ≥ II) and ejection fraction <50% have been randomized. Two patients dropped out during follow‐up. Twenty patients were included into the final analysis. |
| Interventions | two chocolate bars/day commercially available flavanol‐rich chocolate compared with cocoa‐liquor‐free control chocolate |
| Outcomes | endothelial function; platelet function; blood pressure; heart rate |
| Notes |
Noad 2016.
| Methods | 12‐week randomised controlled, single‐blinded dietary intervention design |
| Participants | 92 participants aged 40–65 years, with documented grade I (140–159/90–99 mm Hg) or grade II (160–179/100–109 mm Hg) hypertension |
| Interventions | The study commenced with a four‐week ‘run‐in phase’ for all participants, during which they were asked to consume two portions or less of F&V, and to exclude berries and dark chocolate (low‐polyphenol diet). At the end of this period, subjects were randomised to continue with the above low‐polyphenol diet for a further 8‐week ‘intervention period’ or to consume a high‐polyphenol diet of six portions F&V (including one portion of berries per day) and 50 g of dark chocolate per day |
| Outcomes | The primary endpoint was between‐group change in maximum FBF response to the endothelium‐dependent vasodilator, ACh. Secondary endpoints included between‐group change in self‐reported polyphenol‐rich food intake, between‐group change in biochemical markers of nutritional status and between‐group change in SBP and lipid profile |
| Notes | NCT01319786 |
Ottaviani 2015.
| Methods | Part 1 was an open‐label, intake‐amount escalation study. Part 2 was a controlled, randomized, double‐masked, 2‐parallel‐arm dietary intervention study |
| Participants | 34 healthy adults aged 35‐55 years |
| Interventions | Part 1: consume escalating amounts of cocoa flavanol, ranging from 1000 to 2000 mg/d over 6 wk Part 2: consume for 12 consecutive weeks up to 2000 mg cocoa flavanol per day (n = 46) or a CF‐free control (n = 28) |
| Outcomes | Primary outcomes were blood pressure and platelet function, select metabolic variables, and the occurrence and severity of AEs. Secondary outcomes included plasma concentrations of CF‐derived metabolites and methylxanthines |
| Notes |
Pearson 2016.
| Methods | 12‐week randomised, controlled, parallel study |
| Participants | 102 non‐obese participants |
| Interventions | 4 arms: ˜1100 kJ/day for each of hazelnuts (42 g), chocolate (50 g), potato crisps (50 g), or no added snack food |
| Outcomes | Diet records, body composition, and physical activity were measured at baseline and week 12 |
| Notes |
Petrilli 2016.
| Methods | cross‐over, placebo‐controlled, double‐blind, randomized clinical trial |
| Participants | 92 individuals on antiretroviral therapy for at least six months and at viral suppression |
| Interventions | 65 g of chocolate or chocolate‐placebo or 3 g of yerba mate or mate‐placebo for 15 days each, alternating by a washout period of 15 days |
| Outcomes | data regarding anthropometry, inflammatory, oxidative and immunological parameters were collected at baseline, and at the end of each intervention regimen. High‐sensitivity C‐reactive protein, fibrinogen, lipid profile, white blood cell profile and thiobarbituric acid reactive substances were assessed |
| Notes |
Rassaf 2016.
| Methods | randomized, double‐blind, placebo‐controlled trial |
| Participants | Fifty‐seven participants with ESRD |
| Interventions | ingested CF‐rich beverages (900 mg CF per study day), compared with those ingesting CF‐free placebo |
| Outcomes | changes in flow–mediated dilation and hemodynamics |
| Notes | independent investigator–initiated trial without any commercial interest |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12607000239460.
| Trial name or title | The effect of long term intervention with cocoa flavanols on metabolic control and cardiovascular parameters in subjects with and without type 2 diabetes |
| Methods | Randomised controlled trial |
| Participants | Randomisation among groups with and without diabetes |
| Interventions | High flavanol supplement:low flavanol supplement |
| Outcomes | Systolic and diastolic blood pressure |
| Starting date | 2007 |
| Contact information | Dr Anne Reutens, Baker IDI Heart and Diabetes Institute, 250 Kooyong Road Caulfield VIC 3162, anne.reutens@bakeridi.edu.au |
| Notes | Sponsor: Mars Symbioscience, a division of Mars Incorporated |
Farhat 2012.
| Trial name or title | Effect of Polyphenol‐rich Dark Chocolate on Insulin Sensitivity in Normal Weight and Overweight Adults |
| Methods | Duration: 4 weeks Allocation: Randomized Intervention Model: Parallel Assignment Masking: Single Blind (Participant) |
| Participants | 61 Adults with no history of hypertension, diabetes and cardiovascular diseases
|
| Interventions | Experimental: Polyphenol‐rich Dark chocolate: Participants will be asked to consume 20g of dark chocolate containing 500mg of polyphenols daily for a period of 4 weeks Placebo Comparator: Placebo Dark chocolate: Participants will be asked to consume 20g of dark chocolate containing little or no polyphenols for a period of 4 weeks |
| Outcomes | Primary Outcome Measures: Determine if the consumption of DC rich in polyphenols can induce a change in insulin sensitivity [ Time Frame: Baseline and week 4 ]Insulin sensitivity will be determined by determined by HOMA‐IR (Homeostasis Model of Assessment ‐ Insulin Resistance) Secondary Outcome Measures: Determine if the consumption of DC rich in polyphenols can induce a change in glucose levels [ Time Frame: Baseline and week 4 ] Determine if the consumption of DC rich in polyphenols can induce a change in Lipid profile (TC, HDL, LDL & TG) [ Time Frame: Baseline and week 4 ] Determine if the consumption of DC rich in polyphenols can induce a change in oxidized LDL levels [ Time Frame: Baseline and week 4 ] Determine if the consumption of DC rich in polyphenols can induce a change in BMI and Waist circumference [ Time Frame: Baseline and week 4 ] Determine if the consumption of DC rich in polyphenols can induce a change in blood pressure [ Time Frame: Baseline and week 4 ] Determine if the consumption of DC rich in polyphenols can induce a change in salivary cortisol‐to‐cortisone ratio [ Time Frame: Baseline and week 4 ] Determine if the consumption of DC rich in polyphenols can induce a change in high sensitivity CRP [ Time Frame: Baseline and Week 4 ] |
| Starting date | March 2012 |
| Contact information | Grace Farhat, PhD research student, Queen Margaret University, Musselburgh, East Lothian, United Kingdom, EH21 6UU |
| Notes |
ISRCTN12092733.
| Trial name or title | Impact of High Energy Nutritional Supplement Drink (HENSD) consumed for five consecutive days on appetite, energy intake and cardiometabolic risk factors in underweight females |
| Methods | Single‐blinded randomised controlled crossover study |
| Participants | 22 Healthy women with body mass index of 17‐ 20 kg/m2 |
| Interventions | 1. HENSD (Scandishake, Chocolate, Nutricia) made up with 240 g of full fat milk, according to the manufacturer instructions (Nutricia, 2009) 2. Placebo (a low calorie drink prepared with 240 g of skimmed milk, 4 g of cocoa and two sweeteners) |
| Outcomes | Primary: 1. Fasting lipids, postprandial lipaemia, insulin resistance 2. Energy intake and body mass Secondary: 1. Appetite measures 2. Metabolic rate |
| Starting date | 12/02/2014 |
| Contact information | Dr Sadia Fatima Human Nutrition Section School of Medicine College of Medical Veterinary and Life Sciences (MVLS) New Lister Building Glasgow Royal Infirmary10‐16 Alexandra Parade. Glasgow G31 2ER United Kingdom ‐ s.fatima.1@research.gla.ac.uk |
| Notes |
ISRCTN32888088.
| Trial name or title | An investigation into the effects of chronic consumption of cocoa flavonoids on vascular function: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | 16 Non‐smoking postmenopausal women aged between 48 and 65 years |
| Interventions | cocoa powder |
| Outcomes | Primary: Blood pressure taken at the beginning and end of each intervention period. Secondary: Arterial stiffness, flow mediated dilatation, plasma ICAM‐1, VCAM‐1, C‐reactive protein, P‐selectin, 8‐isoprostane F2 α, lipids and urinary 8‐isoprostane F2 |
| Starting date | 24/08/2006 |
| Contact information | Dr Ummezeinab Mulla zeinab.mulla@imperial.ac.uk Professor Thomas Sanders tom.sanders@kcl.ac.uk |
| Notes |
NCT00125866.
| Trial name or title | The effect of cocoa flavanoids on blood pressure |
| Methods | RCT double‐blind parallel |
| Participants | Children, adults, elderly people with hypertension, n = 50 |
| Interventions | Flavonoid‐rich cocoa drink vs low‐flavanoid drink daily for 12 weeks |
| Outcomes | Primary: mean diff 24‐hour AMBP; Secondary: cholesterol, glucose, insulin, echocardiogram, PWV |
| Starting date | Sep 2005 |
| Contact information | Neil R Poulter, Imperial College London, Paddington, IK W21PG |
| Notes | Sponsor: MasterFoods |
NCT01276951.
| Trial name or title | Controlled clinical trial to determine the effective dose of cocoa in lowering blood pressure |
| Methods | RCT, double‐blind, parallel |
| Participants | Adults 18 ‐ 65 yrs, I‐II hypertension |
| Interventions | 6.5 g, 12 g, 25 g, or 50 g (change of groups every 2 weeks) of chocolate for 18 weeks |
| Outcomes | Primary: blood pressure inpatient |
| Starting date | 12/2008 |
| Contact information | Monica Lucia Giraldo Restrepo, Universidad de Antioquia, Colombia |
| Notes | Sponsor: Universidad de Antioquia |
NCT01754662.
| Trial name or title | A Pilot Study Investigating the Effects of the Combined Effects of Cocoa and Soy Polyphenols in a Soy Protein Matrix on Insulin Resistance and Cardiovascular Disease Risk in Type 2 Diabetes |
| Methods | 8‐week Randomised Placebo‐Controlled Double‐Blind Parallel Study |
| Participants | 84 Patients with type 2 diabetes controlled by diet or metformin only, Stable medication history for 3 months prior to screening visit, Age 45‐80 |
| Interventions | Soy protein with isoflavones and cocoa Soy protein alone with cocoa Soy protein with soy isoflavones Soy protein alone Placebo bar without soy protein, isoflavones or cocoa polyphenols |
| Outcomes | Primary: Insulin resistance, lipid profile Secondary: Cardiovascular risk, Isoflavones, Endothelial function |
| Starting date | October 2011 |
| Contact information | Stephen L Atkin, University of Hull |
| Notes |
NCT01882881.
| Trial name or title | Effects of Polyphenolic‐rich Dark Chocolate/Cocoa and Almonds on Cardiovascular Disease Risk Factors |
| Methods | Allocation: Randomized Intervention Model: Crossover Assignment Masking: Investigator Primary Purpose: Prevention |
| Participants | 48 Overweight and obese adults (BMI ≥25, ≤40 kg/m2) with moderately elevated LDL‐C between the 25‐95th percentile from NHANES: 105‐194 mg/dL for males; 98‐190 mg/dL for females |
| Interventions | Experimental: Dark Chocolate/Cocoa + Almond Diet Experimental: Almond Diet Experimental: Dark Chocolate/Cocoa Diet Active Comparator: Healthy American Control Diet |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
Other Outcome Measures:
|
| Starting date | March 2012 |
| Contact information | Penny Kris‐Etherton, Penn State University |
| Notes |
NCT02789761.
| Trial name or title | The Vascular and Cognitive Effects of Chronic High‐flavanol Intake in Healthy Males |
| Methods | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double Blind (Participant, Investigator) Primary Purpose: Prevention |
| Participants | 34 male adults (18 to 40 years) Body Mass Index 18.5‐27.5 kg/m2 Normal Blood pressure (< 150/90) Non‐smoker Regular exercise routine |
| Interventions | Active Comparator: High‐flavanol milk chocolate Placebo Comparator: Low‐flavanol milk chocolate |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | January 2016 |
| Contact information | Jeremy Paul Edward Spencer, University of Reading |
| Notes |
NCT02802904.
| Trial name or title | Multicountry Studies on the Effect of Positional Distribution of Fatty Acids at Triglyceride Backbone on Serum Lipids, Lipoprotein(a) and LDL‐subclasses in Healthy Malaysian Volunteers |
| Methods | 4 weeks Allocation: Randomized Intervention Model: Crossover Assignment Masking: Single Blind (Participant) |
| Participants | 42 Healthy adult male or female, aged 20‐50 years, BMI 18.5‐ 24.9 kg/m2 as per WHO Classification (1998) |
| Interventions | Experimental: Palm olein IV 64 Experimental: Cocoa butter Experimental: Virgin olive oil |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | January 2016 |
| Contact information | Malaysia Palm Oil Board |
| Notes |
NCT02845622.
| Trial name or title | Effects of Hazelnuts and Cocoa on Metabolic Parameters and Vascular Reactivity |
| Methods | 2 weeks Allocation: Randomized Intervention Model: Parallel Assignment Masking: Open Label Primary Purpose: Health Services Research |
| Participants | 61 adults (18 to 40 years) with BMI 18.5‐24.9 kg/m2 |
| Interventions | 1. Experimental: 30g peeled hazelnuts cream 2. Experimental: 30g unpeeled hazelnuts cream 3. Experimental: snack w/ 30g peeled hazelnuts 4. Experimental: snack w/ 2.5g cocoa powder 5. Experimental: snack w/ 30g peeled hazelnuts+2.5g cocoa 6. Placebo Comparator: empty snack |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | June 2014 |
| Contact information | Anna Ferrulli, Ospedale San Donato, Italy |
| Notes |
AMBP: ambulatory measurement of blood pressure PWV: pulse wave velocity
Differences between protocol and review
We added to the exclusion criteria: Trials of very low quality, specifically high losses to follow up of more than 50%, were excluded from meta‐analysis.
For clarity, we provided more detail of the approach for data analysis. We modified:
Primary outcome measure: 'Difference in systolic and diastolic blood pressure at final follow‐up between cocoa and control group, adjusted for baseline.' Previously, the protocol had read: 'Changes in systolic and diastolic blood pressure from baseline compared with control.'
Measurement of treatment effect: 'Mean difference in SBP/DBP in mmHg from baseline to final follow‐up, adjusted for baseline differences.' Previously, the protocol had read: 'Change of mean difference in SBP/DBP from baseline to follow‐up in mmHg.'
Dealing with missing data: '....We assumed a correlation of 0.68 between the final follow‐up SBP/DBP results for the two treatment arms in a cross‐over trial.' Previously, the protocol had read: 'We will assume a correlation of 0.68 for the standard deviation of the differences from baseline to follow‐up.'
-
We modified the imputation of standard deviations as follows:
standard deviation of blood pressure at end of treatment taken in a different position from that of the blood pressure data used
standard deviation of blood pressure at baseline
mean standard deviation of blood pressure at end of treatment from other trials using the same intervention.
Differences in versions of this review
The Ried 2012 version of this review incorporated a meta‐regression analysis which we have not conducted for this update, for practical reasons.
Contributions of authors
Search strategy, obtain copies of studies, study selection, extract data: KR, PF
Data entry into RevMan: KR
Analysis and interpretation: KR, PF
Draft of the review: KR with contributions from PF and NS
Sources of support
Internal sources
The University of Adelaide, Australia.
-
National Institute of Integrative Medicine, Australia.
First author is employed as Director of Research at NIIM
External sources
No sources of support supplied
Declarations of interest
KR has been an investigator on two randomised controlled trials included in this review (Ried 2009, Massee 2015). KR has no other conflict of interest to declare.
NS has been an investigator on one randomised controlled trial included in this review (Ried 2009). NS has no other conflict of interest to declare.
PF has no conflict of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Al‐Faris 2008 {published data only}
- Al‐Faris NA. Short‐term consumption of a dark chocolate containing flavanols is followed by a significant decrease in normotensive population. Pakistan Journal of Nutrition 2008;7(6):773‐81. [Google Scholar]
Almoosawi 2012a {published data only}
- Almoosawi S, Tsang C, Ostertag LM, Fyfe L, Al‐Dujaili EA. Differential effect of polyphenol‐rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: a randomized clinical trial. Food & Function 2012;3(10):1035‐43. [PUBMED: 22796902] [DOI] [PubMed] [Google Scholar]
Almoosawi 2012b {published data only}
- Almoosawi S, Tsang C, Ostertag LM, Fyfe L, Al‐Dujaili EA. Differential effect of polyphenol‐rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: a randomized clinical trial. Food & function 2012;3(10):1035‐43. [PUBMED: 22796902] [DOI] [PubMed] [Google Scholar]
Bogaard 2010 {published data only}
Crews 2008 {published data only}
- Crews WD Jr, Harrison DW, Wright JW. A double‐blind, placebo‐controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. American Journal of Clinical Nutrition 2008;87(4):872‐80. [DOI] [PubMed] [Google Scholar]
Davison 2008a {published data only}
- Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. International Journal of Obesity (Lond) 2008;32(8):1289‐96. [DOI] [PubMed] [Google Scholar]
Davison 2008b {published data only}
- Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. International Journal of Obesity (Lond) 2008;32(8):1289‐96. [DOI] [PubMed] [Google Scholar]
Davison 2010 {published data only}
- Davison K, Berry NM, Misan G, Coates AM, Buckley JD, Howe PR. Dose‐related effects of flavanol‐rich cocoa on blood pressure. Journal of Human Hypertension 2010;24(9):568‐76. [DOI] [PubMed] [Google Scholar]
Desideri 2012 {published data only}
- Desideri G, Kwik‐Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012;60(3):794‐801. [PUBMED: 22892813] [DOI] [PubMed] [Google Scholar]
Engler 2004 {published data only}
- Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, et al. Flavonoid‐rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. Journal of the American College of Nutrition 2004;23(3):197‐204. [DOI] [PubMed] [Google Scholar]
Esser 2014 {published data only}
- Esser D, Mars M, Oosterink E, Stalmach A, Muller M, Afman LA. Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2014;28(3):1464‐73. [PUBMED: 24302679] [DOI] [PubMed] [Google Scholar]
Fraga 2005 {published data only}
- Fraga CG, Actis‐Goretta L, Ottaviani JI, Carrasquedo F, Lotito SB, Lazarus S, et al. Regular consumption of a flavanol‐rich chocolate can improve oxidant stress in young soccer players. Clinical & Developmental Immunology 2005;12(1):11‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grassi 2005a {published data only}
- Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short‐term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. American Journal of Clinical Nutrition 2005;81(3):611‐4. [DOI] [PubMed] [Google Scholar]
- Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium‐dependent vasodilation in hypertensives. Hypertension 2005;46(2):398‐405. [DOI] [PubMed] [Google Scholar]
Grassi 2005b {published data only}
- Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short‐term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. American Journal of Clinical Nutrition 2005;81(3):611‐4. [DOI] [PubMed] [Google Scholar]
Grassi 2008 {published data only}
- Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, et al. Blood pressure is reduced and insulin sensitivity increased in glucose‐intolerant, hypertensive subjects after 15 days of consuming high‐polyphenol dark chocolate. Journal of Nutrition 2008;138(9):1671‐6. [DOI] [PubMed] [Google Scholar]
Heiss 2010 {published data only}
- Heiss C, Jahn S, Taylor M, Real WM, Angeli FS, Wong ML, et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. Journal of the American College of Cardiology 2010;56(3):218‐24. [DOI] [PubMed] [Google Scholar]
Heiss 2015a {published data only}
- Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez‐Mateos A, et al. Impact of cocoa flavanol intake on age‐dependent vascular stiffness in healthy men: a randomized, controlled, double‐masked trial. Age (Dordrecht, Netherlands) 2015;37(3):9794. [PUBMED: 26013912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heiss 2015b {published data only}
- Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez‐Mateos A, et al. Impact of cocoa flavanol intake on age‐dependent vascular stiffness in healthy men: a randomized, controlled, double‐masked trial. Age (Dordrecht, Netherlands) 2015;37(3):9794. [PUBMED: 26013912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibero‐Baraibar 2014 {published data only}
- Ibero‐Baraibar I, Abete I, Navas‐Carretero S, Massis‐Zaid A, Martinez JA, Zulet MA. Oxidised LDL levels decreases after the consumption of ready‐to‐eat meals supplemented with cocoa extract within a hypocaloric diet. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2014;24(4):416‐22. [PUBMED: 24462367] [DOI] [PubMed] [Google Scholar]
Khan 2012 {published data only}
- Khan N, Monagas M, Andres‐Lacueva C, Casas R, Urpi‐Sarda M, Lamuela‐Raventos RM, et al. Regular consumption of cocoa powder with milk increases HDL cholesterol and reduces oxidized LDL levels in subjects at high‐risk of cardiovascular disease. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2012;22(12):1046‐53. [PUBMED: 21550218] [DOI] [PubMed] [Google Scholar]
Koli 2015 {published data only}
- Koli R, Kohler K, Tonteri E, Peltonen J, Tikkanen H, Fogelholm M. Dark chocolate and reduced snack consumption in mildly hypertensive adults: an intervention study. Nutrition Journal 2015;14:84. [PUBMED: 26296850] [DOI] [PMC free article] [PubMed] [Google Scholar]
Massee 2015 {published data only}
- Massee LA, Ried K, Pase M, Travica N, Yoganathan J, Scholey A, et al. The acute and sub‐chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: a randomized, controlled trial. Frontiers in Pharmacology 2015;6:93. [PUBMED: 26042037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastroiacovo 2015 {published data only}
- Mastroiacovo D, Kwik‐Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study‐a randomized controlled trial. American Journal of Clinical Nutrition 2015;101(3):538‐48. [PUBMED: 25733639] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mogollon 2013 {published data only}
- Mogollon JA, Bujold E, Lemieux S, Bourdages M, Blanchet C, Bazinet L, et al. Blood pressure and endothelial function in healthy, pregnant women after acute and daily consumption of flavanol‐rich chocolate: a pilot, randomized controlled trial. Nutrition Journal 2013;12:41. [PUBMED: 23565841] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monagas 2009 {published data only}
- Monagas M, Khan N, Andres‐Lacueva C, Casas R, Urpi‐Sarda M, Llorach R, et al. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. American Journal of Clinical Nutrition 2009;90(5):1144‐50. [DOI] [PubMed] [Google Scholar]
Muniyappa 2008 {published data only}
- Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin‐mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. American Journal of Clinical Nutrition 2008;88(6):1685‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2003 {published data only}
- Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, et al. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. American Journal of Clinical Nutrition 2003;77(6):1466‐73. [DOI] [PubMed] [Google Scholar]
Neufingerl 2013 {published data only}
- Neufingerl N, Zebregs YE, Schuring EA, Trautwein EA. Effect of cocoa and theobromine consumption on serum HDL‐cholesterol concentrations: a randomized controlled trial. American Journal of Clinical Nutrition 2013;97(6):1201‐9. [PUBMED: 23595874] [DOI] [PubMed] [Google Scholar]
Nickols‐Richardson 2014 {published data only}
- Nickols‐Richardson SM, Piehowski KE, Metzgar CJ, Miller DL, Preston AG. Changes in body weight, blood pressure and selected metabolic biomarkers with an energy‐restricted diet including twice daily sweet snacks and once daily sugar‐free beverage. Nutrition Research and Practice 2014;8(6):695‐704. [PUBMED: 25489410] [DOI] [PMC free article] [PubMed] [Google Scholar]
Njike 2011 {published data only}
- Njike VY, Faridi Z, Shuval K, Dutta S, Kay CD, West SG, et al. Effects of sugar‐sweetened and sugar‐free cocoa on endothelial function in overweight adults. International Journal of Cardiology 2011;149(1):83‐8. [DOI] [PubMed] [Google Scholar]
Ried 2009 {published data only}
- Ried K, Frank OR, Stocks NP. Dark chocolate or tomato extract for prehypertension: a randomised controlled trial. BMC Complementary and Alternative Medicine 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rostami 2015 {published data only}
- Rostami A, Khalili M, Haghighat N, Eghtesadi S, Shidfar F, Heidari I, et al. High‐cocoa polyphenol‐rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atherosclerosis 2015;11(1):21‐9. [PUBMED: 26089927] [PMC free article] [PubMed] [Google Scholar]
Rull 2015 {published data only}
- Rull G, Mohd‐Zain ZN, Shiel J, Lundberg MH, Collier DJ, Johnston A, et al. Effects of high flavanol dark chocolate on cardiovascular function and platelet aggregation. Vascular Pharmacology 2015;71:70‐8. [PUBMED: 25869509] [DOI] [PubMed] [Google Scholar]
Sansone 2015 {published data only}
- Sansone R, Rodriguez‐Mateos A, Heuel J, Falk D, Schuler D, Wagstaff R, et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double‐masked trial: the Flaviola Health Study. British Journal of Nutrition 2015;114(8):1246‐55. [PUBMED: 26348767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarria 2014a {published data only}
- Martinez‐Lopez S, Sarria B, Sierra‐Cinos JL, Goya L, Mateos R, Bravo L. Realistic intake of a flavanol‐rich soluble cocoa product increases HDL‐cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food & Function 2014;5(2):364‐74. [DOI] [PubMed] [Google Scholar]
- Sarria B, Martinez‐Lopez S, Sierra‐Cinos JL, Garcia‐Diz L, Mateos R, Bravo L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. British Journal of Nutrition 2014;111(1):122‐34. [PUBMED: 23823716] [DOI] [PubMed] [Google Scholar]
Sarria 2014b {published data only}
- Martinez‐Lopez S, Sarria B, Sierra‐Cinos JL, Goya L, Mateos R, Bravo L. Realistic intake of a flavanol‐rich soluble cocoa product increases HDL‐cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food & Function 2014;5(2):364‐74. [DOI] [PubMed] [Google Scholar]
- Sarria B, Martinez‐Lopez S, Sierra‐Cinos JL, Garcia‐Diz L, Mateos R, Bravo L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. British Journal of Nutrition 2014;111(1):122‐34. [PUBMED: 23823716] [DOI] [PubMed] [Google Scholar]
Shiina 2009 {published data only}
- Shiina Y, Funabashi N, Lee K, Murayama T, Nakamura K, Wakatsuki Y, et al. Acute effect of oral flavonoid‐rich dark chocolate intake on coronary circulation, as compared with non‐flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. International Journal of Cardiology 2009;131(3):424‐9. [DOI] [PubMed] [Google Scholar]
Sorond 2013 {published data only}
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology 2013;81(10):904‐9. [PUBMED: 23925758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taubert 2003 {published data only}
- Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA 2003;290(8):1029‐30. [DOI] [PubMed] [Google Scholar]
Taubert 2007 {published data only}
- Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA 2007;298(1):49‐60. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almoosawi 2010 {published data only}
- Almoosawi S, Fyfe L, Ho C, Al‐Dujaili E. The effect of polyphenol‐rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. British Journal of Nutrition 2010;103(6):842‐50. [DOI] [PubMed] [Google Scholar]
Balzer 2008 {published data only}
- Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, et al. Sustained benefits in vascular function through flavanol‐containing cocoa in medicated diabetic patients a double‐masked, randomized, controlled trial. Journal fo the American College of Cardiology 2008;51(22):2141‐9. [DOI] [PubMed] [Google Scholar]
Berry 2010 {published data only}
- Berry NM, Davison K, Coates AM, Buckley JD, Howe PR. Impact of cocoa flavanol consumption on blood pressure responsiveness to exercise. British Journal of Nutrition 2010;103(10):1480‐4. [DOI] [PubMed] [Google Scholar]
Curtis 2013 {published data only}
- Curtis PJ, Potter J, Kroon PA, Wilson P, Dhatariya K, Sampson M, et al. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin‐treated postmenopausal women with type 2 diabetes: a double‐blind randomized controlled trial. American Journal of Clinical Nutrition 2013;97(5):936‐42. [PUBMED: 23553151] [DOI] [PubMed] [Google Scholar]
D'Anna 2014 {published data only}
- D'Anna R, Santamaria A, Cannata ML, Interdonato ML, Giorgianni GM, Granese R, et al. Effects of a new flavonoid and Myo‐inositol supplement on some biomarkers of cardiovascular risk in postmenopausal women: a randomized trial. International Journal of Endocrinology 2014;2014:653561. [PUBMED: 25254044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Desch 2010 {published data only}
- Desch S, Kobler D, Schmidt J, Sonnabend, .Adams V, Sareban M, et al. Low vs. higher‐dose dark chocolate and blood pressure in cardiovascular high‐risk patients. American Journal of Hypertension 2010;23(6):694‐700. [DOI] [PubMed] [Google Scholar]
Erdman 2008 {published data only}
- Allen RR, Carson L, Kwik‐Uribe C, Evans EM, Erdman JW Jr. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. Journal of Nutrition 2008;138(4):725‐31. [DOI] [PubMed] [Google Scholar]
- Erdman JW Jr, Carson L, Kwik‐Uribe C, Evans EM, Allen RR. Effects of cocoa flavanols on risk factors for cardiovascular disease. Asia Pacific Journal of Clinical Nutrition 2008;17 Suppl 1:284‐7. [PubMed] [Google Scholar]
Faridi 2008 {published data only}
- Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. American Journal of Clinical Nutrition 2008;88(1):58‐63. [DOI] [PubMed] [Google Scholar]
Farouque 2006 {published data only}
- Farouque HM, Leung M, Hope SA, Baldi M, Schechter C, Cameron JD, et al. Acute and chronic effects of flavanol‐rich cocoa on vascular function in subjects with coronary artery disease: a randomized double‐blind placebo‐controlled study. Clinical Science (London) 2006;111(1):71‐80. [DOI] [PubMed] [Google Scholar]
Flammer 2007 {published data only}
- Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007;116(21):2376‐82. [DOI] [PubMed] [Google Scholar]
Grassi 2015 {published data only}
- Grassi D, Desideri G, Necozione S, Giosia P, Barnabei R, Allegaert L, et al. Cocoa consumption dose‐dependently improves flow‐mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. Journal of Hypertension 2015;33(2):294‐303. [PUBMED: 25380152] [DOI] [PubMed] [Google Scholar]
Grassi 2016 {published data only}
- Grassi D, Socci V, Tempesta D, Ferri C, Gennaro L, Desideri G, et al. Flavanol‐rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. Journal of Hypertension 2016;34(7):1298‐308. [DOI: 10.1097/HJH.0000000000000926] [DOI] [PubMed] [Google Scholar]
Kuebler 2016 {published data only}
- Kuebler U, Arpagaus A, Meister RE, Kanel R, Huber S, Ehlert U, et al. Dark chocolate attenuates intracellular pro‐inflammatory reactivity to acute psychosocial stress in men: a randomized controlled trial. Brain, Behavior, and Immunity 2016;57:200‐8. [DOI: 10.1016/j.bbi.2016.04.006] [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee Y, Berryman C, West S, Chen CYO, Blumberg J, Preston A, et al. Effects of polyphenolic‐rich dark chocolate and almonds on cardiovascular risk factors in overweight and obese adults. FASEB Journal. Conference: Experimental Biology 2016;30(no. 1 Supplement):293.1. [Google Scholar]
Leyva‐Soto 2016 {published data only}
- Leyva‐Soto A, Chavez‐Santoscoy RA, Lara‐Jacobo LR, Re‐Araujo D, Leal‐Orozco AE. Daily consumption of a dark chocolate containing flavanols prevents genotoxicity in buccal epithelial cells and improves biochemical parameters related to cardiovascular risk factors in young adults. FASEB Journal. Conference: Experimental Biology 2016;30(no. 1 Supplement):1176.19. [Google Scholar]
Pereira 2014 {published data only}
- Pereira T, Maldonado J, Laranjeiro M, Coutinho R, Cardoso E, Andrade I, et al. Central arterial hemodynamic effects of dark chocolate ingestion in young healthy people: a randomized and controlled trial. Cardiology Research and Practice 2014;2014:945951. [PUBMED: 24982813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petyaev 2014 {published data only}
- Petyaev IM, Dovgalevsky PY, Chalyk NE, Klochkov V, Kyle NH. Reduction in blood pressure and serum lipids by lycosome formulation of dark chocolate and lycopene in prehypertension. Food Science & Nutrition 2014;2(6):744‐50. [PUBMED: 25493193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez‐Aguadero 2016 {published data only}
- Sanchez‐Aguadero N, Garcia‐Ortiz L, Patino‐Alonso MC, Mora‐Simon S, Gomez‐Marcos MA, Alonso‐Dominguez R, et al. Postprandial effect of breakfast glycaemic index on vascular function, glycaemic control and cognitive performance (BGI study): study protocol for a randomised crossover trial. Trials 2016;17:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanguigni 2016 {published data only}
- Sanguigni V, Manco M, Sorge R, Gnessi L, Francomano D. Natural antioxidant ice cream acutely reduces oxidative stress and improves vascular function and physical performance in healthy individuals. Nutrition 2017;33:225‐33. [DOI: 10.1016/j.nut.2016.07.008] [DOI] [PubMed] [Google Scholar]
Sudarma 2011 {published data only}
- Sudarma V, Sukmaniah S, Siregar P. Effect of dark chocolate on nitric oxide serum levels and blood pressure in prehypertension subjects. Acta Medica Indonesiana 2011;43(4):224‐8. [PUBMED: 22156352] [PubMed] [Google Scholar]
Suh 2014 {published data only}
- Suh JH, Narayanan N, Laine‐Graves K, McCann JC, Shenvi SV, Shigenaga MK, et al. A high fiber nutrient dense supplement moves the metabolome in obese parent‐teen dyads. Circulation 2014;129(Suppl 1):AP279. [Google Scholar]
Wang‐Polagruto 2006 {published data only}
- Wang‐Polagruto JF, Villablanca AC, Polagruto JA, Lee L, Holt RR, Schrader HR, et al. Chronic consumption of flavanol‐rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. Journal of Cardiovascular Pharmacology 2006;47 Suppl 2:S177‐86; discussion S206‐9. [DOI] [PubMed] [Google Scholar]
West 2014 {published data only}
- West SG, McIntyre MD, Piotrowski MJ, Poupin N, Miller DL, Preston AG, et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. British Journal of Nutrition 2014;111(4):653‐61. [PUBMED: 24274771] [DOI] [PubMed] [Google Scholar]
Wirtz 2014 {published data only}
References to studies awaiting assessment
Campbell 2016 {published data only}
- Campbell CL, Foegeding EA, Harris GK. Cocoa and whey protein differentially affect markers of lipid and glucose metabolism and satiety. Journal of Medicinal Food 2016;19(3):219‐27. [10.1089/jmf.2015.0044] [DOI] [PubMed] [Google Scholar]
De Palma 2016 {published data only}
- Palma R, Sotto I, Wood EG, Khan NQ, Butler J, Johnston A, et al. Cocoa flavanols reduce N‐terminal pro‐B‐type natriuretic peptide in patients with chronic heart failure. ESC Heart Failure 2016;3(2):97‐106. [DOI: 10.1002/ehf2.12077] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flammer 2012 {published data only}
- Flammer AJ, Sudano I, Wolfrum M, Thomas R, Enseleit F, Périat D, et al. Cardiovascular effects of flavanol‐rich chocolate in patients with heart failure. European Heart Journal 2012;33(17):2172‐80. [DOI: 10.1093/eurheartj/ehr448] [DOI] [PubMed] [Google Scholar]
Noad 2016 {published data only}
- Noad RL, Rooney C, McCall D, Young IS, McCance D, McKinley MC, et al. Beneficial effect of a polyphenol‐rich diet on cardiovascular risk: a randomised control trial. Heart 2016;102(17):1371‐9. [DOI: 10.1136/heartjnl-2015-309218] [DOI] [PubMed] [Google Scholar]
Ottaviani 2015 {published data only}
- Ottaviani JI, Balz M, Kimball J, Ensunsa JL, Fong R, Momma TY, et al. Safety and efficacy of cocoa flavanol intake in healthy adults: a randomized, controlled, double‐masked trial. American Journal of Clinical Nutrition 2015;102(6):1425‐35. [DOI: 10.3945/ajcn.115.116178] [DOI] [PubMed] [Google Scholar]
Pearson 2016 {published data only}
- Pearson KR, Tey SL, Gray AR, Chisholm A, Brown RC. Energy compensation and nutrient displacement following regular consumption of hazelnuts and other energy‐dense snack foods in non‐obese individuals. European Journal of Nutrition 2017;56:1255. [DOI: 10.1007/s00394-016-1176-2] [DOI] [PubMed] [Google Scholar]
Petrilli 2016 {published data only}
- Petrilli AA, Souza SJ, Teixeira AM, Pontilho PM, Souza JM, Luzia LA, et al. Effect of chocolate and yerba mate phenolic compounds on inflammatory and oxidative biomarkers in HIV/AIDS individuals. Nutrients 2016;8(5):132. [DOI: 10.3390/nu8050132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rassaf 2016 {published data only}
- Rassaf T, Rammos C, Hendgen‐Cotta UB, Heiss C, Kleophas W, Dellanna F, et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double‐blind, randomized, placebo‐controlled trial. Clinical Journal of The American Society of Nephrology 2016;11(1):108‐18. [DOI: 10.2215/CJN.05560515] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12607000239460 {published data only}
- ACTRN12607000239460. The effect of long term intervention with cocoa flavanols on metabolic control and cardiovascular parameters in subjects with and without type 2 diabetes. www.anzctr.org.au/ACTRN12607000239460.aspx 2007.
Farhat 2012 {unpublished data only}
- NCT01749020, Farhat G. Effect of polyphenol‐rich dark chocolate on insulin sensitivity in normal weight and overweight adults. ClinicalTrials.gov: Identifier NCT01749020 Dec 10, 2012.
ISRCTN12092733 {published data only}
- ISRCTN12092733. Impact of High Energy Nutritional Supplement Drink (HENSD) consumed for five consecutive days on appetite, energy intake, and risk factors of cardiovascular diseases and type 2 diabetes. isrctn.com/ISRCTN12092733 2014. [DOI: 10.1186/ISRCTN12092733] [DOI]
ISRCTN32888088 {published data only}
- ISRCTN32888088. Effects of chronic consumption of cocoa flavonoids on vascular function. isrctn.com/ISRCTN32888088 2013. [DOI: 10.1186/ISRCTN32888088] [DOI]
NCT00125866 {unpublished data only}
- NCT00125866, Poulter N. The effect of cocoa flavonoids on blood pressure. ClinicalTrials.gov 16 April 2007; Vol. www.clinicaltrials.gov/ct2/show/NCT00125866.
NCT01276951 {unpublished data only}
- NCT01276951, Giraldo Restrepo. Controlled clinical trial to determine the effective dose of cocoa in lowering blood pressure. clinicaltrials.gov/ct2/show/NCT01276951 24 May 2010.
NCT01754662 {published data only}
- NCT01754662. Effects of combining cocoa and soy in type 2 diabetes [A pilot study investigating the effects of the combined effects of cocoa and soy polyphenolsin a soy protein matrix on insulin resistance and cardiovascular disease risk in type 2 diabetes ‐ a randomised placebo‐controlled double‐blind parallel study]. clinicaltrials.gov/show/NCT01754662 2012.
NCT01882881 {unpublished data only}
- NCT01882881, Kris‐Etherton P. Effects of polyphenolic‐rich dark chocolate/cocoa and almonds on cardiovascular disease risk factors. ClinicalTrials.gov : NCT01882881 Apr 15, 2013.
NCT02789761 {published data only}
- NCT02789761. The vascular and cognitive effects of chronic high‐flavanol intake in healthy males. ClinicalTrials.gov/show/NCT02789761 2016.
NCT02802904 {published data only}
- NCT02802904. Multicountry studies on the effect of positional distribution of fatty acids at triglyceride backbone on serum lipids, lipoprotein(a) and LDL‐subclasses in healthy Malaysian volunteers. ClinicalTrials.gov/show/NCT02802904 2016.
NCT02845622 {published data only}
- NCT02845622. Effects of hazelnuts and cocoa on metabolic parameters and vascular reactivity. clinicaltrials.gov/show/NCT02845622 2016.
Additional references
Actis‐Goretta 2006
- Actis‐Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol‐rich foods. Journal of Agricultural and Food Chemistry 2006;54:229‐34. [DOI] [PubMed] [Google Scholar]
Adamson 1999
- Adamson GE, Lazarus SA, Mitchell AE, Prior RL, Cao G, Jacobs, PH, et al. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. Journal of Agricultural and Food Chemistry 1999;47:4184‐8. [DOI] [PubMed] [Google Scholar]
Addison 2008
- Addison S, Stas S, Hayden MR, Sowers JR. Insulin resistance and blood pressure. Current Hypertension Reports 2008;10:319‐25. [DOI] [PubMed] [Google Scholar]
Bangalore 2010
- Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J‐curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. European Heart Journal 2010;31(23):2897‐908. [PUBMED: 20846991] [DOI] [PubMed] [Google Scholar]
Beckett 2008
- Beckett ST. The Science of Chocolate. Cambridge, UK: RSC Publishing, 2008. [Google Scholar]
Chaitman 2006
- Chaitman BR, Schmitz HH, Keen CL. Cocoa Flavanols and Cardiovascular Health. US Cardiology: Business Briefing 2006.
Chevaux 2001
- Chevaux KA, Jackson L, Elena Villar M, Hollenberg NK. Proximate, mineral and procyanidin content of certain foods and beverages consumed by the Kuna Amerinds of Panama. Journal of Food Composition and Analysis 2001;14:553‐63. [Google Scholar]
Corti 2009
- Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation 2009;119(10):1433‐41. [DOI] [PubMed] [Google Scholar]
Denker 2013
- Denker MG, Cohen DL. What is an appropriate blood pressure goal for the elderly: review of recent studies and practical recommendations. Clinical interventions in aging 2013;8:1505‐17. [PUBMED: 24255596] [DOI] [PMC free article] [PubMed] [Google Scholar]
Desch 2010a
- Desch S, Schmidt J, Kobler D, Sonnabend M, Eitel I, Sareban M, et al. Effect of cocoa products on blood pressure: systematic review and meta‐analysis. American Journal of Hypertension 2010;23(1):97‐103. [DOI] [PubMed] [Google Scholar]
Dillinger 2000
- Dillinger TL, Barriga P, Escarcega S, Jimenez M, Salazar Lowe D, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. Journal of Nutrition 2000;130(8S Suppl):2057S‐72S. [DOI] [PubMed] [Google Scholar]
Donato 2009
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: endothelin‐1 and endothelial nitric oxide synthase. American Journal of Physiology. Heart and Circulatory Physiology 2009;297:H425‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez‐Murga 2011
- Fernandez‐Murga L, Tarin JJ, Garcia‐Perez MA, Cano A. The impact of chocolate on cardiovascular health. Maturitas 2011;69:312‐21. [DOI] [PubMed] [Google Scholar]
Fraga 2011
- Fraga, CG, Oteiza, PI. Dietary flavonoids: role of (‐)‐epicatechin and related procyanidins in cell signaling. Free Radical Biology & Medicine 2011;51(4):813‐23. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck‐Ytter, Y, Alonso‐Coello, P, Schuenemann. HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations.. BMJ: British Medical Journal 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammerstone 2000
- Hammerstone JF, Lazarus SA, Schmitz HH. Procyanidin content and variation in some commonly consumed foods. Journal of Nutrition 2000;130:2086S‐92S. [DOI] [PubMed] [Google Scholar]
Heiss 2010a
- Heiss C, Kelm M. Chocolate consumption, blood pressure, and cardiovascular risk. European Heart Journal 2010;31:1554‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollenberg 2006
- Hollenberg NK. Vascular action of cocoa flavanols in humans: the roots of the story. Journal of Cardiovascular Pharmacology 2006;47 Suppl 2:S99‐102; discussion S119‐21. [DOI] [PubMed] [Google Scholar]
Kean 1944
- Kean BH. The BP of the Kuna Indians. American Journal of Tropical Medicine and Hygiene 1944;24:341. [Google Scholar]
Keen 2005
- Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH. Cocoa antioxidants and cardiovascular health. American Journal of Clinical Nutrition 2005;81(1):298S‐303. [DOI] [PubMed] [Google Scholar]
Kelly 2005
- Kelly CJ. Effects of theobromine should be considered in future studies. American Journal of Clinical Nutrition 2005;82(2):486‐7: Author reply American Journal of Clinical Nutrition 2005; 82(2)487‐8. [DOI] [PubMed] [Google Scholar]
Kim 1984
- Kim HC, Keeney PG. (‐)Epicatechin content in fermented and unfermented cocoa beans. Journal of Food Science 1984;49:1090‐2. [Google Scholar]
Lawes 2008
Lewington 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903‐13. [DOI] [PubMed] [Google Scholar]
Lippi 2009
- Lippi D. Chocolate and medicine: dangerous liaisons?. Nutrition 2009;25:1100‐3. [DOI] [PubMed] [Google Scholar]
Loke 2008
- Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (‐)‐epicatechin augment nitric oxide products and reduce endothelin‐1 acutely in healthy men. American Journal of Clinical Nutrition 2008;88:1018‐25. [DOI] [PubMed] [Google Scholar]
Martiniuk 2007
- Martiniuk AL, Lee CM, Lawes CM, Ueshima H, Suh I, Lam TH, et al. Hypertension: its prevalence and population‐attributable fraction for mortality from cardiovascular disease in the Asia‐Pacific region. Journal of Hypertension 2007;25(1):73‐9. [DOI] [PubMed] [Google Scholar]
McCullough 2006
- McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, et al. Hypertension, the Kuna, and the epidemiology of flavanols. Journal of Cardiovascular Pharmacology 2006;47 Suppl 2:S103‐9: Discussion: Journal of Cardiovascular Pharmacology 2006;48 Suppl 2:119‐21. [DOI] [PubMed] [Google Scholar]
McInnes 2005
- McInnes G T. Lowering blood pressure for cardiovascular risk reduction. Journal of Hypertension. Supplement 2005;23(1):S3‐8. [DOI] [PubMed] [Google Scholar]
O'Rourke 1990
- O'Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990;15:339‐47. [DOI] [PubMed] [Google Scholar]
Payne 2010
- Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. Journal of Agricultural and Food Chemistry 2010;58:10518‐27. [DOI] [PubMed] [Google Scholar]
Persson 2011
- Persson IA, Persson K, Hagg S, Andersson RG. Effects of cocoa extract and dark chocolate on angiotensin‐converting enzyme and nitric oxide in human endothelial cells and healthy volunteers‐‐a nutrigenomics perspective. Journal of Cardiovascular Pharmacology 2011;57:44‐50. [DOI] [PubMed] [Google Scholar]
Rusconi 2010
- Rusconi M, Conti A. Theobroma cacao L., the Food of the Gods: a scientific approach beyond myths and claims. Pharmacological Research 2010;61(1):5‐13. [DOI] [PubMed] [Google Scholar]
Schroeter 2006
- Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (‐)‐Epicatechin mediates beneficial effects of flavanol‐rich cocoa on vascular function in humans. PNAS 2006;103(4):1024‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singleton 1965
- Singleton VL, Rossi JA. Colorimetric of total phenolics with phosphomolybdic‐phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965;16:144‐58. [Google Scholar]
Strachan 1994
- Strachan ER, Bennett A. Theobromine poisoning in dogs. Veterinary Record 1994;134:284. [DOI] [PubMed] [Google Scholar]
Taddei 2001
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, et al. Age‐related reduction of NO availability and oxidative stress in humans. Hypertension 2001;38:274‐9. [DOI] [PubMed] [Google Scholar]
Taubert 2007a
- Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta‐analysis. Archives of Internal Medicine 2007;167(7):626‐34. [DOI] [PubMed] [Google Scholar]
Wollgast 2000
- Wollgast J, Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during manufacture of chocolate and methodolgy for identification and quantification. Food Research International (Ottawa, Ont.) 2000;33:423‐47. [Google Scholar]
References to other published versions of this review
Ried 2010
- Ried K, Sullivan T, Fakler P, Frank O R, Stocks NP. Does chocolate reduce blood pressure? A meta‐analysis. BMC Medicine 2010;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ried 2012
- Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD008893.pub2] [DOI] [PubMed] [Google Scholar]


