Abstract
Dittrichia viscosa (L.) Greuter, a perennial weed of the Mediterranean area, was reported to be source of active substances. Here, by means of both ingestion and contact assays, the biological activity of three different extracts (n-hexane, methanol, and distilled water) of D. viscosa aerial part has been evaluated against Sitophilus granarius (L.) adults, an important pest of stored grains. Ingestion assays showed negligible mortality and food deterrence for all the extracts, whereas only a slight reduction of some nutritional parameters (relative growth rate, relative consumption rate, food efficiency conversion) was recorded for water extract. High contact toxicity was found only for the n-hexane extract (24 h median lethal dose LD50 = 53.20 μg/adult). This extract was further subfractioned by silica gel column chromatography and then by thin layer chromatography. Further contact toxicity bioassays highlighted two active subfractions which were analyzed by GC-MS. This revealed the occurrence, in both subfractions, of two major peaks that were identified as α- and γ- costic acid isomers. Moreover, D. viscosa active subfractions, did not cause acetylcholinesterase (AChE) inhibition; therefore, in the light of progressive limitation of compounds acting by this mechanism of action, D. viscosa represents a promising eco-sustainable source of natural products for pest control.
Subject terms: Entomology, Agroecology, Environmental impact
Introduction
The indiscriminate use of synthetic insecticides for several decades has led to the accumulation of toxic residues in the environment and food as well as to the development of resistant pest populations1. A clear example of that is represented by fenitrothion, a broad-spectrum organophosphorus pesticide acting on acetylcholinesterase (AChE), which, due to its extensive use, in some regions is classified as a common river water pollutant2. Therefore, the search for novel eco-friendly compounds safer for customers and workers is strongly required, as also recommended by both national and international legislations. In this context, the need for effective and biodegradable pesticides has created a significant market opportunity for alternative products3. Botanical pesticides offer a good alternative to traditional chemicals in Integrated Pest Management (IPM)4–6. In fact, their use reduces the risk to non-target organisms, due to their rapid degradation in the environment, and provides novel and multiple modes of action that reduce the probability of developing pest resistance1,7.
In this regard, the insecticidal activity of plants belonging to the Asteraceae family has been investigated in many previous studies8–10. Dittrichia viscosa (L.) Greuter (sin. Inula viscosa (L.) Aiton) is a perennial Mediterranean weed species of this family reported to be a source of bioactive compounds11,12, as also suggested by its use in folk medicine as an antidiabetic13, antipyretic and anti-inflammatory treatment14,15. More recently, D. viscosa extracts were proved to have a plethora of effects going from anti HIV-activity in an in vitro test16, to an abortifacient effect17 or protection against Helicobacter pylori18. In particular, besides antibacterial and antifungal activities19, extracts from this plant showed also toxicity against the nematode Meloidogyne javanica (Treub) Chitiwood20, the mite Tetranichus cinnabarinus (Boisduval)21, larvae of Tuta absoluta (Meyrick)22 and antifeedant effects against the aphid Myzus persicae (Sulzer)23. Moreover, many compounds were identified in different D. viscosa extracts, but very few of them have been associated with specific biological activity. However, until now, to the best of our knowledge, no studies have been carried out on the effects of D. viscosa against stored-product pests.
The granary weevil, Sitophilus granarius (L.), is one of the most damaging pests of stored grains that causes both quantitative and qualitative losses due to insect feeding on grains, alteration of nutritional and aesthetic value and contamination of commodities with insect bodies, excrement and mycotoxins that result from insect-promoted fungal growth during storage24–29. Control of the granary weevil is difficult due to the endophytic development of immature stages that are well protected within grains from pesticides, the increasing legislation limits to the use of some fumigants and broad-spectrum contact insecticides, and the increasing consumer demand for safe food30.
In this study, firstly the bioactivity of D. viscosa to S. granarius adults was evaluated by ingestion and contact toxicity assays with different plant extracts; later, by partially purifying the most active extract (n-hexane), the compounds present in the fraction retaining toxicity were identified. In parallel, both D. viscosa extracts and active subfractions were also evaluated for their effect on AChE in order to have a preliminary indication about their mechanism of action.
Results
Bioactivity of plant crude extracts
To gain a first insight into biological activity of D. viscosa against S. granarius adults, in a first series of experiments the ingestion toxicity and nutritional effects of plant extracts were evaluated by a flour disk bioassay31. Thus, S. granarius adults were fed for 5 days with flour disks treated with n-hexane, methanol or water extracts of plant aerial part, or the solvent alone (control). At the end of this period, mortality rate (%), insect relative growth rate (RGR), relative consumption rate (RCR), efficiency conversion of ingested food (ECI) and feeding deterrent index (FDI) were calculated (Tables 1–3). In the dose range tested, almost no ingestion toxicity was observed for all the extracts. Moreover, no significant differences in nutritional parameters were found in the treatment with n-hexane and methanol extracts. Only the highest dose of the aqueous extract caused a significant reduction of RGR and RCR indexes compared with control values, however the resulting ECI was not found to be significantly different from control. FDI was only slightly affected by different doses of the aqueous extract.
Table 2.
Mortality (Mort.), feeding deterrent index (FDI), relative growth rate (RGR), relative consumption rate (RCR) and efficiency conversion of ingested food (ECI) of S. granarius adults fed for 5 days on flour disks treated with increasing concentrations of D. viscosa methanol extract. No significant differences at P = 0.05 (Tukey HSD test) were found.
| Concentration (µg/disk) | Mort. (%) | FDI (%) ± S.E. | RGR (mg/mg/day) ± S.E. | RCR (mg/mg/day) ± S.E. | ECI (%) ± S.E. |
|---|---|---|---|---|---|
| 750.00 | 0 | 0.248 ± 4.612 | 0.026 ± 0.003 | 0.217 ± 0.006 | 12.008 ± 1.762 |
| 375.00 | 0 | −7.653 ± 4.668 | 0.024 ± 0.001 | 0.223 ± 0.007 | 10.747 ± 0.509 |
| 187.5 | 0 | −10.770 ± 4.946 | 0.020 ± 0.001 | 0.243 ± 0.008 | 8.433 ± 0.377 |
| 93.75 | 0 | −11.280 ± 4.096 | 0.023 ± 0.001 | 0.235 ± 0.009 | 9.901 ± 0.728 |
| 46.87 | 0 | −10.720 ± 3.893 | 0.025 ± 0.003 | 0.247 ± 0.011 | 10.007 ± 0.961 |
| Control | 0 | — | 0.023 ± 0.015 | 0.216 ± 0.017 | 8.742 ± 6.270 |
| F | 0.180 | 0.085 | 1.650 | 0.234 | |
| d.f. | 4 | 5 | 5 | 5 | |
| P | 0.350 | 0.994 | 0.185 | 0.944 |
Table 1.
Mortality (Mort.), feeding deterrent index (FDI), relative growth rate (RGR), relative consumption rate (RCR) and efficiency conversion of ingested food (ECI) of S. granarius adults fed for 5 days on flour disks treated with increasing concentrations of D. viscosa n-hexane extract. No significant differences at P = 0.05 (Tukey HSD test) were found.
| Concentration (µg/disk) | Mort. (%) | FDI (%) ± S.E. | RGR (mg/mg/day) ± S.E. | RCR (mg/mg/day) ± S.E. | ECI (%) ± S.E. |
|---|---|---|---|---|---|
| 750.00 | 8 | −12.939 ± 6.924 | 0.020 ± 0.002 | 0.311 ± 0.007 | 6.543 ± 0.631 |
| 375.00 | 6 | −11.066 ± 7.622 | 0.025 ± 0.003 | 0.310 ± 0.012 | 8.042 ± 0.670 |
| 187.5 | 2 | −13.591 ± 6.605 | 0.020 ± 0.003 | 0.308 ± 0.011 | 6.302 ± 0.726 |
| 93.75 | 0 | −1.318 ± 10.068 | 0.021 ± 0.002 | 0.285 ± 0.032 | 7.578 ± 0.950 |
| 46.87 | 2 | −11.295 ± 5.552 | 0.042 ± 0.002 | 0.352 ± 0.044 | 10.295 ± 3.122 |
| Control | 0 | — | 0.015 ± 0.002 | 0.294 ± 0.012 | 4.913 ± 0.838 |
| F | 0.727 | 1.476 | 0.676 | 1.222 | |
| d.f. | 4 | 5 | 5 | 5 | |
| P | 0.584 | 0.234 | 0.646 | 0.32 |
Table 3.
Mortality (Mort.), feeding deterrent index (FDI), relative growth rate (RGR), relative consumption rate (RCR) and efficiency conversion of ingested food (ECI) of S. granarius adults fed for 5 days on flour disks treated with increasing concentrations of D. viscosa water extract. Values in the same column followed by the same letters are not significantly different at P = 0.05 (Tukey HSD test).
| Concentration (µg/disk) | Mort. (%) | FDI (%) ± S.E. | RGR (mg/mg/day) ± S.E. | RCR (mg/mg/day) ± S.E. | ECI (%) ± S.E. |
|---|---|---|---|---|---|
| 750.00 | 0 | 11.553 ± 2.871 b | 0.009 ± 0.002 a | 0.248 ± 0.008 a | 3.741 ± 0.722 a |
| 375.00 | 0 | 6.832 ± 3.962 ab | 0.011 ± 0.004 ab | 0.255 ± 0.011 ab | 4.195 ± 1.732 ab |
| 187.5 | 0 | −9.497 ± 6.794 a | 0.021 ± 0.001 ab | 0.296 ± 0.006 bc | 7.000 ± 0.236 ab |
| 93.75 | 0 | 1.635 ± 3.109 ab | 0.023 ± 0.002 b | 0.279 ± 0.008 abc | 8.165 ± 0.237 b |
| 46.87 | 0 | 7.019 ± 4.898 ab | 0.021 ± 0.003 ab | 0.279 ± 0.007 abc | 7.473 ± 0.881 ab |
| Control | 0 | — | 0.024 ± 0.004 b | 0.297 ± 0.015 c | 7.882 ± 1.098 ab |
| F | 3.142 | 4.372 | 4.591 | 3.586 | |
| d.f. | 4 | 5 | 5 | 5 | |
| P | 0.037 | 0.006 | 0.004 | 0.010 |
Having found poor or no effect of D. viscosa extracts by ingestion, in another series of experiments the contact toxicity of same extracts was evaluated by topic application32,33. Differently from water extract, which caused no mortality 24 h after treatment, a significant contact toxicity was recorded for both n-hexane and methanol extracts. However, a difference between the two active extracts was found, being the former much more active (80% mortality at the highest dose) than the latter (only 25% at the same dose). The LD50 value was 53.20 μg/adult for the n-hexane extract (Table 4).
Table 4.
Insect mortality, regression equation and LD50 value of D. viscosa aerial part extracts 24 h after topical application.
| D. viscosa extract (μg/adult) | Mortality rate (%)1 (mean ± SE) | Regression equation | X² | LD50 (μg/adult) |
|---|---|---|---|---|
| n-hexane | ||||
| 75.0 | 80.0 ± 11.6 a | y = 4.62 × −7.97 | 2.27 | 53.20 |
| 37.5 | 15.0 ± 9.6 b | |||
| 18.8 | 5.0 ± 5.0 b | |||
| 9.4 | 0.0 ± 0.0 b | |||
| 4.7 | 0.0 ± 0.0 b | |||
| 0.0 | 0.0 ± 0.0 b | |||
| F | 23.920 | |||
| d.f. | 5 | |||
| P | <0.01 | |||
| methanol | ||||
| 75.0 | 25.0 ± 12.6 a | NA | NA | NA |
| 37.5 | 10.0 ± 5.8 ab | |||
| 18.8 | 5 ± 5.0 b | |||
| 9.4 | 0.0 ± 0.0 b | |||
| 4.7 | 0.0 ± 0.0 b | |||
| 0.0 | 0.0 ± 0.0 b | |||
| F | 2.677 | |||
| d.f. | 5 | |||
| P | 0.06 | |||
| water | ||||
| 0.0–75.0 | 0 ± 0.0 | NA | NA | NA |
1Data were submitted to ANOVA followed by Tukey HSD test. For each extract, values followed by the same letters are not significantly different at P = 0.05. The mean adult weight was 1.983 ± 0.018 mg. NA, not applicable.
Partial purification of n-hexane extract and bioactivity of its subfractions
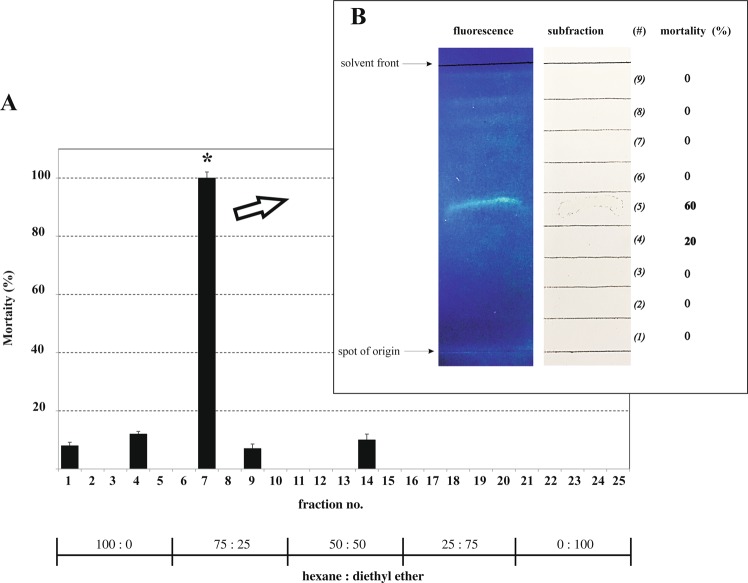
In the light of its highest contact toxicity, n-hexane extract was deeper analyzed in order to identify the active compounds contained in this mixture. Thus, an aliquot of this extract was purified by silica gel column chromatography and 25 fractions recovered and checked for contact toxicity against granary weevils. Toxicity was almost completely restricted to fraction 7 which returned a 100% mortality value (Fig. 1A). Therefore, this fraction 7 was further purified by means of thin layer chromatography and the recovered TLC subfractions tested on the insect as above (Fig. 1B). The highest insect mortality (60%) was obtained with the subfraction #5 which showed a clear fluorescent spot (Rf = 0.55). A lower toxicity was also found for subfraction #4, while no toxicity was observed for the other subfractions.
Figure 1.
(A) Mortality (%) of S. granarius adults 24 h after topic application of different fractions of D. viscosa n-hexane extract (0.5 μL/adult) obtained by silica gel column chromatography (*P < 0.05, n = 3). (B) Thin layer chromatography of fraction 7 and insect mortality (%) caused by the different TLC subfractions: aliquot (100 μL) of the active fraction (no. 7) showed in A was fractioned on a silica gel plate (n-hexane:diethyl ether, 80:20, as eluent) and observed by UV lamp (254–365 nm); recovered subfractions (2 mL) were concentrated (100 μL) and used for contact toxicity bioassay as in A.
Chemical analyses of active fractions
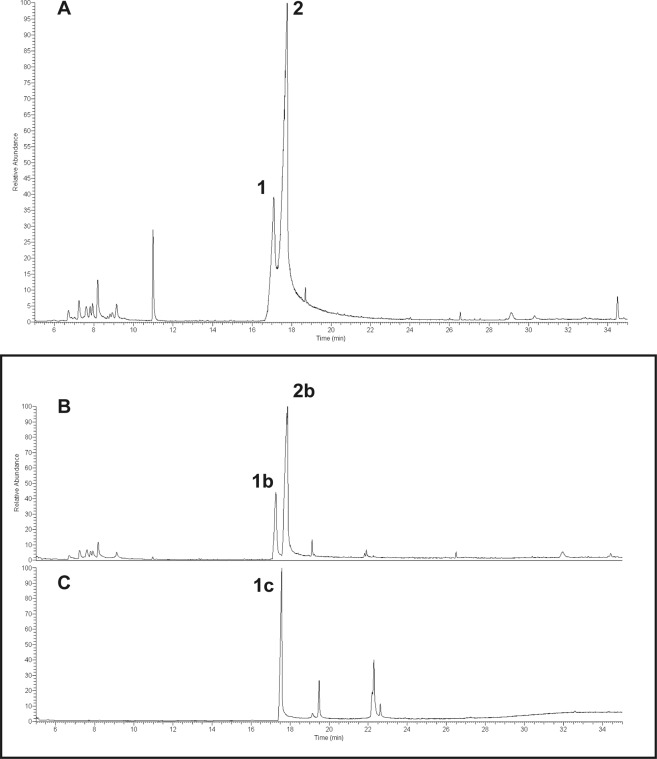
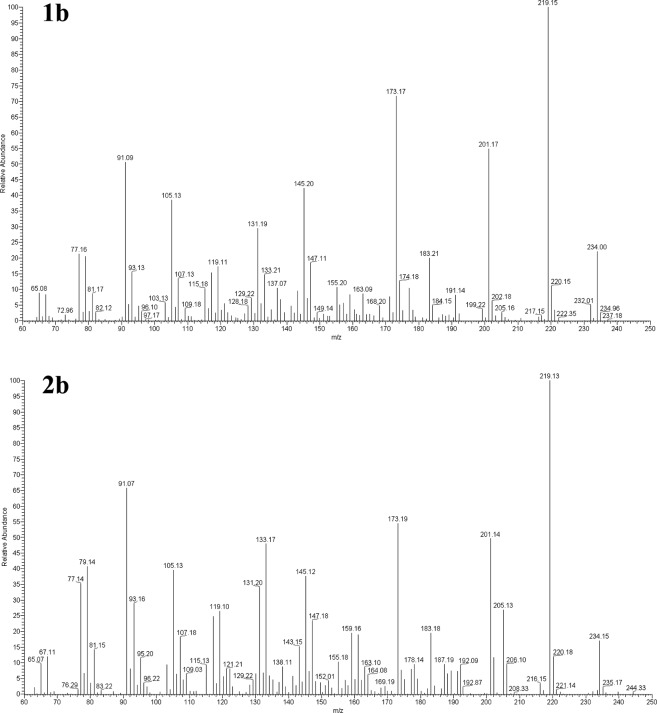
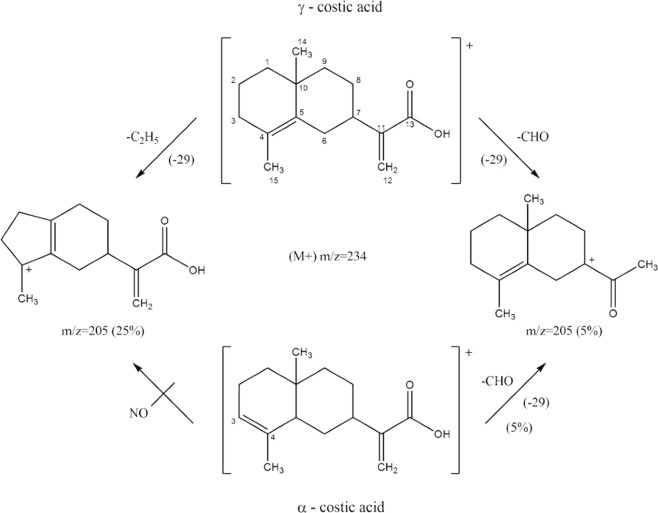
Having the biological assays identified the fraction and subfractions retaining n-hexane extract toxicity, a chemical characterization of these ones was carried out. The GC-MS analysis of fraction 7 revealed the presence of two main peaks (24.7 and 67.1% relative abundance, respectively) which were putatively identified as costic acid (Fig. 2A). Moreover, these peaks were also found in both bioactive subfractions (# 4 and 5), with more abundance in the most active one (Fig. 2B). To further ascertain the exact molecular identification of compounds revealed in Fig. 2A, a comparison with a standard of β-costic acid was carried out (Fig. 2C). Observing the mass spectra relative to peaks 1b and 2b (Fig. 3), there is a noticeable difference in mass fragment 205 (m/z). In fact, in spectrum 2b, this fragment (30%) is greater than that in spectrum 1b (5%). These differences are related to the structures of the two costic acid isomers. The mass fragment 205 observed in spectrum 2b comes from the molecular peak M+ (234) after the loss of a CHO (29) fragment or that of a C2H5 (29) fragment; the mass fragment 205 in spectrum 1b comes from the molecular peak M+ (234) after the loss of only the CHO molecule (29), whereas the other loss is prevented by the presence of double bond, in position (3–4) which prevents the formation of a pentane ring. Thus, the α-costic acid is assigned to peak 1b and the γ-costic acid isomer to peak 2b, respectively. The fragmentation scheme is shown in Fig. 4. Further, under these experimental conditions, and with this stationary phase, the order of elution of the costic acid isomers from the capillary column is α- (RT = 17.28 min), β- (RT = 17.57 min) and γ-costic acid (RT = 17.86 min), thus confirming what reported by other authors (in particular Fig. 8 of the cited paper)34.
Figure 2.
Total ion current chromatograms from GC-MS analysis of real samples: (A) fraction 7 of D. viscosa n-hexane extract obtained by silica gel column chromatography; (B) subfraction #5 obtained by TLC of fraction 7 showed in A; (C) a standard of β-costic (2 ng µL−1). Peak 1 and 1b = α-costic acid, Peak 2 and 2b = γ-costic acid, Peak 1c = β-costic acid.
Figure 3.
Mass spectra relative to peak 1b (α-costic acid), peak 2b (γ-costic acid) of Fig. 2. Scan acquisition in positive chemical ionization was from m/z 60 up to 400 a.m.u. at 1.68 scan s−1 and 70 eV.
Figure 4.
Characteristic fragmentation of α- and γ-costic acid isomers.
Finally, by comparison with β-costic acid standard, the amount of α- and γ-costic acid was found to be respectively 6.802 and 19.144 mg in the fraction 7, 0.471 and 1.283 mg in TLC subfraction #5, 0.188 and 0.513 mg in TLC subfraction #4. Accordingly, in contact toxicity assay (Fig. 1) the calculated dose of α- and γ-costic was respectively 3.40 and 9.57 μg/adult for fraction 7 (mortality 100%), 2.36 and 6.42 μg/adult for TLC subfraction #5 (mortality 60%), 0.94 and 2.57 μg/adult for TLC subfraction #4 (mortality 20%).
AChE assay
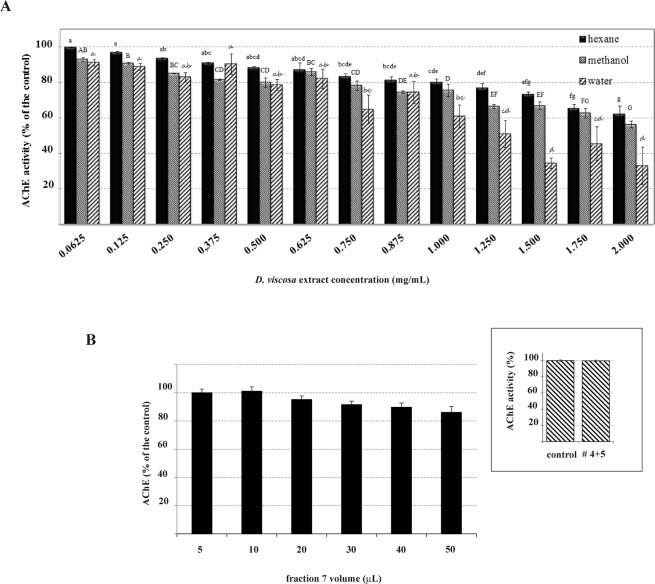
Being the impairment of nervous system function the main mechanism by which plant metabolites toxicity occurs35,36, the effect of plant extracts, fractions, and subfractions on the AChE, the most conserved mechanism in nervous transmission, was investigated. A progressive inhibition of pure commercial AChE was found at increasing concentration of each extract (Fig. 5). At maximum concentration checked (2 mg/mL) the water extract showed the highest capability (about 70% inhibition), whereas n-hexane extract was the less effective one (about 40% inhibition) (Fig. 5A). Differently, from plant n-hexane extract, no inhibition of this enzyme was registered for any silica gel column fractions (Supplementary Fig. S2). Moreover, to confirm the low anticholinesterase activity of n-hexane fraction, the effect of both fraction 7 and its active subfractions was further checked on AChE activity obtained from adult granary weevils (Fig. 5B). A very poor inhibition was found for increasing volumes of fraction 7, corresponding to 0.129–1.293 mg/ml range of costic acid (both isomers), whereas insect enzyme was found to be insensitive to the purified TLC active subfractions (Fig. 5B inset).
Figure 5.
(A) Mean values (±SE) of AChE activity obtained in the presence of different concentrations of n-hexane, methanol and water extracts of D. viscosa. Values (3 for each concentration) were calculated as % of the control (enzyme activity measured in the absence of plant extract). (B) Mean values (±SE) of S. granarius AChE obtained in the presence of either different volume of fraction 7 (whose content in costic acid was 25.9 µg/µL) or subfractions #4 + 5 (50 μL, about 0.6 mg costic acid) (inset). Different letters within the same font indicate a significant difference (P < 0.05).
Discussion
Results of this study confirm D. viscosa as an important source of biologically active compounds11,12 and, more importantly, suggest the possible application of this weed plant against stored-product pests. This consideration mostly relies on the high contact toxicity exerted by the n-hexane extract of D. viscosa aerial part against S. granarius adults. In fact, contact toxicity was low or absent respectively for methanol and water extracts and no ingestion toxicity was found for any of the three extracts. Marked differences in the toxicity of plant extracts obtained using organic solvents of different polarity was also observed in many previous studies9,32 including plant extracts of species belonging to the Asteraceae family10.
Nutritional parameters of insects fed with untreated flour disks (control) were in fairly good agreement with those reported in other studies37. Plant extracts exerted negligible effects on nutritional parameters of adult weevils except for the highest dose of water extract that caused a significant reduction of food intake (RCR) and relative growth rate (RGR) when compared to control. Since no ingestion toxicity was observed this alteration in nutritional parameters indicates the presence of antifeedant constituents in the water extract (rather than post-ingestion toxicants) which more likely act on the gustatory organs of the mouthparts38. However, chemical analysis of water extract is needed to support or refute this hypothesis, as well as for better understanding the variability registered with this extract for the other nutritional indexes. Moreover, the knowledge of both methanol and water extracts composition would shed some light on the different effects exerted by the three plant extracts.
On the other hand, the main outcome of this study remains the high contact toxicity of n-hexane extract. This toxic activity was comparable with those observed for n-hexane extract of Scrophularia canina L. (LD50 75.22 μg/adult)39 against the same insect species, and similar to those reported for extracts of other plants proposed to have potential toxic activity against the congener S. zeamais Motschulsky40,41.
GC-MS analysis of active fractions strongly suggested costic acid, and in particular α- and γ- isomers, as the compound responsible for the contact toxicity of n-hexane extract against the granary weevil adults. Accordingly, an increasing insect mortality was observed in response to fractions containing increasing amounts of α- and γ- costic acid isomers. In this regard, the amount of costic acid (sum of both isomers) in fraction 7 was calculated to be 25.9 mg. Since this fraction comes from 187.5 mg of n-hexane extract loaded on silica gel column chromatography, it can be assumed that the amount of costic acid in this extract was at least 13.8%. Such a value is in fairly good agreement with the 10.6% found in a similar extract of the same plant by other authors42.
Identification of costic acid isomers in D. viscosa appears to be a controversial matter. In this study, the n-hexane extract was proved to contain α- and γ-costic acid, but not the β- isomer. On the contrary, this latter compound was identified and showed to be active against the mite Varroa destructor (Anderson and Trueman)43. However, in other studies some authors identified the α-, but not β-isomer44,45, whereas others identified both α- and β- isomers20,42. Whether these differences rely on different geographical region of plant growth, more unlikely on the different extraction conditions, is a matter of speculation.
In this study, topical application of a standard of β-costic acid in the dose range of 0.5–50 μg/adult did not cause insect mortality (data not reported), thus suggesting a strict dependence of the observed toxicity on the costic acid isomerism. Notice that this high specifity would not be unique since the same applies for alantolactone isomers of Inula helenium (L.) which showed a sharp decrease in toxic activity on both larvae and adults of Aedes aegypti due to the shift of double bond outside the carbon ring, as occurs for β-costic acid46. Future studies with α- and γ-costic acid standards, not commercially available at present, should clarify the relative contribution of each isomer to the contact toxicity of D. viscosa n-hexane extract.
Plant extracts investigated here showed a moderate dose dependent AChE inhibitory capability, in fair agreement with previous studies in which anticholinesterase activity for D. viscosa extracts and essential oil was reported47,48. However, the active fraction, and subfractions, of n-hexane extract induced a very poor inhibition of both insect and pure AChE, thus ruling out this mechanism of action. This lack of anticholinesterase activity appears of particular interest in the light of the continuous search for new compounds acting on target/s different from AChE49. It is known, in fact, that nervous transmission impairment, especially through AChE inhibition, have represented historically the main target of insecticides35. On the other hand, the occurrence of cholinergic system in almost all animals50, as well as the development of pesticide resistance and tolerance in insects mostly due to AChE mutation and/or duplication51, recently led to a sharp restriction of the use of AChE inhibitors52. Hence, results reported here suggest D. viscosa n-hexane extract as a valid natural and ecofriendly tool for the control of stored-product pests.
Materials and Methods
Insect
S. granarius was reared for several generations on wheat grains stored in glass cylindrical containers (Ø 15 × 15 cm) closed by metallic screen (1 mm mesh) and maintained in the dark in a climatic chamber set at 22±2 °C and 60±5% R.H. Adults two-four weeks old of mixed sexes were used for the experiments. For antifeedant and nutritional bioassays, insects were starved for 24 h before use.
Plant Material
About 300 plants of D. viscosa (100 plants/sampling) were collected during the flowering stage (October/November) from rural areas near Campobasso (Molise region, Italy) at 650 m a.s.l. (Supplementary Fig. S1). A voucher specimen was deposited in the herbarium of Department of Agricultural, Environmental and Food Sciences, University of Molise. For each plant, the aerial part containing at least 15 flowers and 10 leaves were dried and ground to a fine powder. Aliquots (50 g) of the dry powder were extracted with different solvents (600 mL) of increasing polarity, n-hexane, methanol, water (99% purity, Sigma-Aldrich Milan, Italy), for 24 h at room temperature. Each crude extract was centrifuged (30,000 × g, 15 min), and the supernatant paper filtered (Whatman No. 113). N-hexane and methanol extracts were dried under vacuum in a rotary evaporator (Laborota 4000, Heidolph, Germany) whereas water extract was lyophilized (Hetosicc FD 2.5, De Mori, Milan, Italy) until used. The residues obtained were 39.0, 129.7, and 192.3 g/kg dry weight for n-hexane, methanol and water extracts, respectively. Residues were stored at –20 °C until used.
Antifeedant and Nutritional Activity
Effects of D. viscosa extracts on the feeding activity and nutrition of granary weevil adults were evaluated by the flour disk bioassay31. Wheat flour (10 g) was uniformly suspended in distilled water (50 mL) by stirring. To obtain flour disks, aliquots (200 μL) of suspension were dropped onto a plastic Petri dish and left overnight at 26 ± 2 °C and 60 ± 5% R.H. to dry. In parallel, plant extracts samples were prepared by dissolving the residues of n-hexane, methanol and water extracts in n-hexane, acetone:methanol (1:1) and acetone:water (1:1), respectively. For each sample two-fold serial dilutions were prepared. Thus, disks were treated with sample solutions (5 μL) corresponding to different concentrations (750.00, 375.00, 187.50, 93.75, 46.87 μg/disk) or the solvent alone as control. Disks were held at room temperature for 2 h for solvent evaporation. In a pre-weighed glass vial (Ø 2.5 × 4.0 cm) 2 flour disks were introduced and the weight measured; later 10 group-weighed weevil adults were added and each vial was then re-weighed and maintained in the dark at 26 ± 2 °C, 60 ± 5% R.H. for 5 days. At the end of the test, for each glass vial, insects were removed, the number of dead insects recorded, and the weight of both the 2 flour disks residues and live insects were separately measured. As a control, glass vials containing treated flour disks but without insects were prepared to determine any decrease in weights due to evaporation of solvent and sample. For each sample concentration, as well as for control, 5 replicates were set up. The following nutritional indices40,53 for each replicate were calculated:
Relative Growth Rate (RGR) = (A − B)/B × day−1
Relative Consumption Rate (RCR) = D/B × day−1
Efficiency Conversion of Ingested food (ECI) = (RGR/RCR) × 100
Feeding Deterrence Index (FDI) (%) = [(C − T)/C] × 100
where A = mean weight (mg) of live insects on fifth day;
B = original mean weight (mg) of insects;
C = consumption of control disks;
D = biomass ingested (mg)/no. of living insects on the fifth day;
T = consumption of treated disks.
Data were submitted to ANOVA followed by Tukey’s HSD test for mean comparisons. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) v.23 for Windows (SPSS Inc., Chicago, IL).
Contact toxicity
The contact toxicity of D. viscosa extracts to granary weevil adults was determined by topical application as reported in32,33,39 with minor differences. Residues of the n-hexane extract, its (sub)fractions, and β-costic acid (standard, Chem Faces, China) were dissolved in n-hexane, whereas methanol and water extracts were dissolved in acetone:methanol (1:1) and acetone:water (1:1), respectively. For each sample, two-fold serial dilutions for plant extracts (150.00–9.36 μg/μL) and β-costic acid standard (100.00–1.00 μg/μL) were prepared. A 0.5 μL droplet of each solution was applied onto the pronotum of an adult weevil in thanatosis using a Hamilton’s syringe (700 series, MicroliterTM Hamilton Company, USA). For each sample, 60 insects divided in 12 replicates were used. Concentrations were expressed as μg of sample per adult (average adult weight 1.98 ± 0.02 mg). Insects treated with solvent alone (n-hexane, acetone/methanol and acetone/water) were used as control. After topical application, the insects were confined in a Petri dish within a metal ring (Ø 4.0 × 2.5 cm) covered with metallic net (1 mm mesh) to prevent insects escape, provided with 5 wheat kernels and maintained in the dark at 26 ± 2 °C and 60 ± 5% R.H. The number of dead insects was recorded after 24 h. The percentage mortalities were transformed to arcsine square-root values for one-way analysis of variance (ANOVA). Treatment means were compared and separated by Tukey HSD test. The median lethal dose (LD50) values, the confidence upper and lower limits, regression equations and chi-square (χ2) values were calculated using probit analysis54.
Silica Gel Column Chromatography
Aliquots (250 mg) of the oily residue of the n-hexane extract were dissolved in n-hexane (1 mL) and 750 μL of this mixture (about 187.5 mg of n-hexane extract residue) were loaded on a glass chromatographic column (Ø 1.5 cm, 20 cm height) packed with n-hexane slurry of silica gel S previously activated (120 °C for 1 h). The column was eluted with increasing concentrations of diethyl ether in n-hexane and 25 fractions (20 mL each) were collected. Each fraction was first concentrated to 1 mL under a gentle flow of nitrogen, then, to remove diethyl ether, fractions were added with n-hexane (1.5 mL) and again concentrated (as above) to a final concentration of 1 mL. These solutions were tested for their contact toxicity against S. granarius adults and anti-AChE activity (see below). An aliquot (10 μL) of the active fraction was diluted 1:500 and analyzed in GC-MS.
Thin Layer Chromatography (TLC)
An aliquot (100 μL) of the most active fraction against insects was further fractioned on a silica gel plate (5 × 20 cm, thickness 0.25 cm, Merck) by means of an ascending chromatography using a mixture of diethyl ether (20%) in n-hexane as eluent. The plate was observed by UV lamp (UVSL-15, Multi-Band, California, USA) at long and short wave-UV (254, 365 nm, respectively) and divided into 9 subfractions which were recovered with 10 mL of n-hexane:diethyl ether (80:20). Each subfraction was concentrated to 2 mL and an aliquot (50 μL) diluted 1:50 was used for GC-MS analysis. To assess insecticidal activity, subfractions (1.95 mL) were further concentrated under a gentle flow of nitrogen to 100 μL.
GC/IT-MS Analysis and Quantification
A gas chromatograph Finnigan Trace GC Ultra equipped with an ion-trap (IT) mass spectrometry (MS) detector Polaris Q (Thermo Fisher Scientific, Waltham, MA), a Programmed Temperature Vaporizer (PTV) injector and a PC with a chromatography station Xcalibur (Thermo Fisher Scientific), was used. A fused-silica capillary column with chemically bonded phase (SE-54, 5% phenyl–95% dimethylpolysiloxane) was prepared in our laboratory55–57 with the following characteristics: 30 m × 0.25 mm i.d., N (theoretical plate number) 115,000 for n-dodecane at 90 °C; K’ (capacity factor) 6.7; df (film thickness) 0.23 μm; uopt (optimum linear velocity of carrier gas, hydrogen) 38.5 cm s−1, and UTE% (utilization of theoretical efficiency) 89%.
Helium was used both as carrier gas at a flow rate of 1 mL min−1 and dumping gas in the ion-trap at 0.3 mL min−1. The column oven temperature was programmed from 100 °C to 280 °C (5 min) at 6 °C min−1. Injected sample volume was of 1 μL. The PTV injector was performed in splitless mode: it was programmed from 110 °C to 290 °C at 800 °C min−1 and cooled after 4 min. The splitter valve was closed for 180 s. The transfer line and ion source were held at 270 °C and 250 °C, respectively. Scan acquisition in positive chemical ionization was from m/z 60 up to 400 a.m.u. at 1.68 scan s−1 and 70 eV. The costic acid isomer concentrations were obtained by calibration graphs of area (β-costic acid) plotted versus β-costic acid standard concentration (0.05, 0.1, 1.0, and 5.0 µg mL−1). The sample was quantified in triplicate.
AChE Assay
AChE activity was detected photometrically (λ = 412 nm, 25 °C) by means of a Jasco V-570 spectrophotometer (Tokyo, Japan) according to58 by using 5,5’dithio bis(2-nitrobenzoic) acid (DTNB). Briefly, about 0.01 EU from either commercial (from Electrophorus electricus, SIGMA) or insect enzyme, obtained by a crude extract of 100 adults59,60, were incubated in phosphate buffer (0.1 M, pH 8.00) plus DTNB (0.2 mM) either in the absence or in the presence of different aliquots of D. viscosa extracts and subfractions. For this assay, n-hexane extract and its active fraction were re-suspended in phosphate buffer by means of tween 20 (0.5%); control was made that tween 20 (up to 1%) did not affect AChE activity. Reaction was started by the addition of saturating concentration (2.5 mM) of acetylthiocholine iodide and the rate of absorbance change was obtained as tangent to the initial part of the progress curve. Results were expressed as % of the control (reaction rate measured in the absence of plant extract). Data were submitted to ANOVA followed by Tukey’s HSD test for mean comparisons.
Supplementary information
Acknowledgements
This work was financially supported by Ministero Italiano dell’Economia e delle Finanze, Ministero Italiano dell’Istruzione, dell’Università e della Ricerca Scientifica e Tecnologica and Assessorato Bilancio e Programmazione Regione Puglia (Program Art. 13 - DD prot. 713/Ric. 29 October 2010. Title: Innovative packaging solutions to extend shelf life of food products) to GSG, and by an internal fund of Department of Medicine and Health Sciences “V. Tiberio”, University of Molise to GP.
Author Contributions
G.R. and G.P. conceived and designed the experiments; G.R. and A.B. performed the experiments reported in Tables 1–4 and Figure 1; I.N. and M.V.R. carried out the experiments reported in Figures 2, 3; G.P. and A.B. performed experiments reported in Figure 5; G.R. and G.P. analyzed the data with the help of G.S.G. and A.D.C.; G.R., G.P. and G.S.G. wrote the paper.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giuseppe Rotundo, Email: rotundo@unimol.it.
Gianluca Paventi, Email: paventi@unimol.it.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42886-4.
References
- 1.Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 2.Galal AAA, Ramadan RA, Metwally MMM, El-Sheikh SMA. Protective effect of N-acetylcysteine on fenitrothion-induced toxicity: The antioxidant status and metabolizing enzymes expression in rats. Ecotoxicol. Environ. Saf. 2019;171:502–510. doi: 10.1016/j.ecoenv.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Isman MB, Miresmailli S, Machial C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011;10:197–204. doi: 10.1007/s11101-010-9170-4. [DOI] [Google Scholar]
- 4.Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Pavela R, Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Zoubiri S, Baaliouamer A. Potentiality of plants as source of insecticide principles. J. Saudi Chem. Soc. 2014;18:925–938. doi: 10.1016/j.jscs.2011.11.015. [DOI] [Google Scholar]
- 7.Rajendran S, Sriranjini V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 2008;44:126–135. doi: 10.1016/j.jspr.2007.08.003. [DOI] [Google Scholar]
- 8.Secoy DM, Smith AE. Use of plants in control of agricultural and domestic pests. Econ. Bot. 1983;37:28–57. doi: 10.1007/BF02859304. [DOI] [Google Scholar]
- 9.Pascual-Villalobos MJ, Robledo A. Screening for anti-insect activity in Mediterranean plants. Ind. Crops Prod. 1998;8:183–194. doi: 10.1016/S0926-6690(98)00002-8. [DOI] [Google Scholar]
- 10.Boussaada O, et al. Insecticidal activity of some Asteraceae plant extracts against Tribolium confusum. Bull. Insectology. 2008;61:283–289. [Google Scholar]
- 11.Barrero AFAF, Herrador MMM, Arteaga P, Catalán JVJV. Dittrichia viscosa L. Greuter: Phytochemistry and biological activity. Nat. Prod. Commun. 2008;3:1799–1804. [Google Scholar]
- 12.Seca AML, Grigore A, Pinto DCGA, Silva AMS. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014;154:286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Yaniv Z, Dafni A, Friedman J, Palevitch D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987;19:145–151. doi: 10.1016/0378-8741(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 14.Barbetti P, Chiappini I, Fardella G, Menghini A. A new eudesmane acid from Dittrichia (Inula) viscosa. Planta Med. 1985;51:471. doi: 10.1055/s-2007-969564. [DOI] [PubMed] [Google Scholar]
- 15.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000;72:191–205. doi: 10.1016/S0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 16.Bedoya LM, Sanchez Palomino S, Abad MJ, Bermejo P, Alcami J. Screening of selected plant extracts for in vitro inhibitory activity on human immunodeficiency virus. Phyther. Res. 2002;16:550–554. doi: 10.1002/ptr.992. [DOI] [PubMed] [Google Scholar]
- 17.Al-Dissi NM, Salhab AS, Al-Hajj HA. Effects of Inula viscosa leaf extracts on abortion and implantation in rats. J. Ethnopharmacol. 2001;77:117–121. doi: 10.1016/S0378-8741(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 18.Stamatis G, et al. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J. Ethnopharmacol. 2003;88:175–179. doi: 10.1016/S0378-8741(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 19.Rhimi W, et al. Chemical composition, antibacterial and antifungal activities of crude Dittrichia viscosa (L.) greuter leaf extracts. Molecules. 2017;22:1–13. doi: 10.3390/molecules22070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oka Y, Ben-Daniel BH, Cohen Y. Nematicidal activity of powder and extracts of Inula viscosa. Nematology. 2001;3:735–742. doi: 10.1163/156854101750236286. [DOI] [Google Scholar]
- 21.Mansour F, et al. The potential of middle eastern flora as a source of new safe bio-acaricides to control Tetranychus cinnabarinus, the carmine spider mite. Phytoparasitica. 2004;32:66–72. doi: 10.1007/BF02980862. [DOI] [Google Scholar]
- 22.Allal-Benfekih, L., Bellatreche, M., Bounaceur, F., Tail, G. & Mostefaoui, H. First approach of using aqueous extracts of Inula viscosa, Salvia officinalis and Urtica urens for the control of Tuta absoluta (Lepidoptera, Gelechiidae) an invasive pest of tomato in Algeria. Les Cochenilles Ravag. Princ. ou Second. 9ème Conférence Int. sur les Ravag. en Agric. SupAgro, Montpellier, Fr. 25–27 octobre 2011 681–689 (2011).
- 23.Mamoci E, Cavoski I, Andres MF, Díaz CE, Gonzalez-Coloma A. Chemical characterization of the aphid antifeedant extracts from Dittrichia viscosa and Ferula communis. Biochem. Syst. Ecol. 2012;43:101–107. doi: 10.1016/j.bse.2012.02.012. [DOI] [Google Scholar]
- 24.Rajendran, S. In Encyclopedia of Pest Management (ed. Pimental, D.) 654–656 (Marcel Dekker, Inc., 2002).
- 25.Sauer DB, Storey CL, Walker DE. Fungal populations in US farming stored grain and their relationship tomoisture, storage time, regions and insect infestation. Phytopathology. 1984;74:1050–1053. doi: 10.1094/Phyto-74-1050. [DOI] [Google Scholar]
- 26.Magan N, Hope R, Cairns V, Aldred D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003;109:723–730. doi: 10.1023/A:1026082425177. [DOI] [Google Scholar]
- 27.Plarre R. An attempt to reconstruct the natural and cultural history of the granary weevil, Sitophilus granarius (Coleoptera: Curculionidae) Eur. J. Entomol. 2010;107:1–11. doi: 10.14411/eje.2010.001. [DOI] [Google Scholar]
- 28.Germinara GS, De Cristofaro A, Rotundo G. Repellents effectively disrupt the olfactory orientation of Sitophilus granarius to wheat kernels. J. Pest Sci. (2004). 2015;88:675–684. doi: 10.1007/s10340-015-0674-y. [DOI] [Google Scholar]
- 29.Dobie P, Kilminster AM. The susceptibility of triticale to post-harvest infestation by Sitophilus zeamais Motschulsky, Sitophilus oryzae (L.) and Sitophilus granarius (L.) J. Stored Prod. Res. 1978;14:87–93. doi: 10.1016/0022-474X(78)90003-6. [DOI] [Google Scholar]
- 30.Phillips TW, Throne JE. Biorational Approaches to managing stored-product insects. Annu. Rev. Entomol. 2010;55:375–397. doi: 10.1146/annurev.ento.54.110807.090451. [DOI] [PubMed] [Google Scholar]
- 31.Xie YS, Bodnaryk RP, Fields PG. A rapid and simple flour-disk bioassay for testing substances active against stored-product insects. Can. Entomol. 1996;128:865–875. doi: 10.4039/Ent128865-5. [DOI] [Google Scholar]
- 32.Germinara GS, Frontera AM, de Cristofaro A, Rotundo G. Insecticidal activity of different extracts from Scrophularia canina L. against Culex pipiens molestus Forskal (diptera, culicidae) J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes. 2011;46:473–479. doi: 10.1080/03601234.2011.583858. [DOI] [PubMed] [Google Scholar]
- 33.Germinara GS, et al. Bioactivities of Lavandula angustifolia essential oil against the stored grain pest Sitophilus granarius. Bull. Insectology. 2017;70:129–138. [Google Scholar]
- 34.Ramirez AM, et al. Biosynthesis of sesquiterpene lactones in pyrethrum (Tanacetum cinerariifolium) PLoS One. 2013;8:e65030. doi: 10.1371/journal.pone.0065030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casida John E. Hayes' Handbook of Pesticide Toxicology. 2010. Pest Toxicology; pp. 103–117. [Google Scholar]
- 36.Rattan RS. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010;29:913–920. doi: 10.1016/j.cropro.2010.05.008. [DOI] [Google Scholar]
- 37.Guedes NMP, Guedes RNC, Silva LB, Cordeiro EMG. Deltamethrin-induced feeding plasticity in pyrethroid-susceptible and -resistant strains of the maize weevil, Sitophilus zeamais. J. Appl. Entomol. 2009;133:524–532. doi: 10.1111/j.1439-0418.2009.01391.x. [DOI] [Google Scholar]
- 38.Sosa A, et al. Insecticidal effects of Vernonanthura nebularum against two economically important pest insects. Saudi J. Biol. Sci. 2018 doi: 10.1016/j.sjbs.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rotundo, G., Paventi, G. & Germinara, G. S. Attività insetticida di estratti di Scrophularia canina L. verso adulti di Sitophilus granarius (L.) (Coleoptera, Curculionidae) Tecnica Molitoria45, 90–96 (Chiarotti Editore, 2014).
- 40.Huang Y, Lam SL, Ho SH. Bioactivities of essential oil from Elletaria cardamomum (L.) Maton. to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst) J. Stored Prod. Res. 2000;36:107–117. doi: 10.1016/S0022-474X(99)00040-5. [DOI] [Google Scholar]
- 41.Chu SS, Liu QR, Liu ZL. Insecticidal activity and chemical composition of the essential oil of Artemisia vestita from China against Sitophilus zeamais. Biochem. Syst. Ecol. 2010;38:489–492. doi: 10.1016/j.bse.2010.04.011. [DOI] [Google Scholar]
- 42.Cohen Y, Wang W, Ben-Daniel B-H, Ben-Daniel Y. Extracts of Inula viscosa control downy mildew of grapes caused by Plasmopara viticola. Phytopathology. 2006 doi: 10.1094/PHYTO-96-0417. [DOI] [PubMed] [Google Scholar]
- 43.Sofou K, et al. Use of costic acid, a natural extract from Dittrichia viscosa, for the control of Varroa destructor, a parasite of the European honey bee. Beilstein J. Org. Chem. 2017;13:952–959. doi: 10.3762/bjoc.13.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontana G, La Rocca S, Passannanti S, Paternostro MP. Sesquiterpene compounds from Inula viscosa. Nat. Prod. Res. 2007;21:824–827. doi: 10.1080/14786410701415681. [DOI] [PubMed] [Google Scholar]
- 45.Andolfi A, et al. Inuloxins A-D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry. 2013;86:112–120. doi: 10.1016/j.phytochem.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Cantrell CL, Pridgeon JW, Fronczek FR, Becnel JJ. Structure-activity relationship studies on derivatives of eudesmanolides from Inula helenium as toxicants against Aedes aegypti larvae and adults. Chem. Biodivers. 2010;7:1681–1697. doi: 10.1002/cbdv.201000031. [DOI] [PubMed] [Google Scholar]
- 47.Albano SM, et al. Antioxidant, anti-5-lipoxygenase and antiacetylcholinesterase activities of essential oils and decoction waters of some aromatic plants. Rec. Nat. Prod. 2012;6:35–48. [Google Scholar]
- 48.Trimech I, et al. Evaluation of anti-oxidant and acetylcholinesterase activity and identification of polyphenolics of the invasive weed Dittrichia viscosa. Phytochem. Anal. 2014;25:421–428. doi: 10.1002/pca.2510. [DOI] [PubMed] [Google Scholar]
- 49.Casida JE. Pesticide Interactions: Mechanisms, Benefits, and Risks. J. Agric. Food Chem. 2017;65:4553–4561. doi: 10.1021/acs.jafc.7b01813. [DOI] [PubMed] [Google Scholar]
- 50.Pezzementi L, Chatonnet A. Evolution of cholinesterases in the animal kingdom. Chem. Biol. Interact. 2010;187:27–33. doi: 10.1016/j.cbi.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 51.Lee SH, Kim YH, Kwon DH, Cha DJ, Kim JH. Mutation and duplication of arthropod acetylcholinesterase: Implications for pesticide resistance and tolerance. Pestic. Biochem. Physiol. 2015;120:118–124. doi: 10.1016/j.pestbp.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Casida JE, Durkin KA. Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 2013;203:221–225. doi: 10.1016/j.cbi.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrar RR, Barbour JD, Kennedy GG. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 1989;82:593–598. doi: 10.1093/aesa/82.5.593. [DOI] [Google Scholar]
- 54.Finney, D. J. Probit Analysis, 3rd ed. (Cambridge University Press, 1971).
- 55.Russo MV, Goretti GC, Liberti A. A fast procedure to immobilize polyethylene glycols in glass capillary columns. J. High Resolut. Chromatogr. 1985;8:535–538. doi: 10.1002/jhrc.1240080911. [DOI] [Google Scholar]
- 56.Cartoni GP, Goretti G, Monticelli B, Russo MV. Evaluation of capillary gas chromatographic columns in series. Analytical application to lemon oil. J. Chromatogr. A. 1986;370:93–101. doi: 10.1016/S0021-9673(00)94677-6. [DOI] [Google Scholar]
- 57.Russo MV, Goretti G, Soriero A. Preparation and application of fused-silica capillary microcolumns (25–50 µm I.D.) in gas chromatography. Ann. Chim. 1996;86:115–124. [Google Scholar]
- 58.Ellman, G. L., Courtney, K. D., Andres, V. Jr. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7 (1961). [DOI] [PubMed]
- 59.Li F, Han Z. Purification and characterization of acetylcholinesterase from cotton aphid (Aphis gossypii glover) Arch. Insect Biochem. Physiol. 2002;51:37–45. doi: 10.1002/arch.10048. [DOI] [PubMed] [Google Scholar]
- 60.Paventi, G., Germinara GS, Passarella, S. & Rotundo, G. Inibizione dell’acetilcolinesterasi di Sitophilus granarius (L.) da estratti di Scrophularia canina L. in atti IX simposio la difesa antiparassitaria nelle industrie alimentari e la protezione degli alimenti (ed. Editori, C.) 97–103 (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.