Phenotypic antibiotic susceptibility testing in Mycobacterium tuberculosis is slow and cumbersome. Rapid molecular diagnostics promise to help guide therapy, but such assays rely on complete knowledge of the molecular determinants of altered antibiotic susceptibility. Recent genomic studies of antibiotic-resistant M. tuberculosis have identified several candidate loci beyond those already known to contribute to antibiotic resistance; however, efforts to provide experimental validation have lagged. Our study identifies a gene (Rv0565c) that is associated with resistance to the second-line antibiotic ethionamide at a population level. We then use bacterial genetics to show that the variants found in clinical strains of M. tuberculosis improve bacterial survival after ethionamide exposure.
KEYWORDS: genome-wide association study, Mycobacterium tuberculosis, antibiotic resistance, ethionamide, genome analysis, prothionamide
ABSTRACT
In Mycobacterium tuberculosis, recent genome-wide association studies have identified a novel constellation of mutations that are correlated with high-level drug resistances. Interpreting the functional importance of the new resistance-associated mutations has been complicated, however, by a lack of experimental validation and a poor understanding of the epistatic factors influencing these correlations, including strain background and programmatic variation in treatment regimens. Here we perform a genome-wide association analysis in a panel of Mycobacterium tuberculosis strains from China to identify variants correlated with resistance to the second-line prodrug ethionamide (ETH). Mutations in a bacterial monooxygenase, Rv0565c, are significantly associated with ETH resistance. We demonstrate that Rv0565c is a novel activator of ETH, independent of the two known activators, EthA and MymA. Clinically prevalent mutations abrogate Rv0565c function, and deletion of Rv0565c confers a consistent fitness benefit on M. tuberculosis in the presence of partially inhibitory doses of ETH. Interestingly, Rv0565c activity affects susceptibility to prothionamide (PTH), the ETH analog used in China, to a greater degree. Further, clinical isolates vary in their susceptibility to both ETH and PTH, to an extent that correlates with the total expression of ETH/PTH activators (EthA, MymA, and Rv0565c). These results suggest that clinical strains considered susceptible to ETH/PTH are not equally fit during treatment due to both Rv0565c mutations and more global variation in the expression of the prodrug activators.
INTRODUCTION
Even as the global incidence and mortality of tuberculosis disease (TB) have steadily decreased over the last several years, the incidence of multidrug-resistant TB (MDR-TB) is projected to rise over the next decade (1). The discrepancy in these trends is in part due to large differences in the efficacy of antibiotic treatment for drug-susceptible tuberculosis (DS-TB) versus MDR-TB; DS-TB is treated for 6 months with a combination of four potent first-line antibiotics capable of achieving a durable cure in ∼95% of patients (2, 3), while MDR-TB is treated using more complex and longer regimens that have significantly lower success rates (4).
Many factors are likely to contribute to the outcome of MDR-TB treatment, including the ability to choose antimicrobials to which the bacterium is fully susceptible. Regimen decisions are complicated by limited capacity to phenotypically measure second-line drug resistances in many parts of the world and an incomplete understanding of the genetic basis of altered treatment responses that would allow genotypic susceptibility testing (5). Defining a robust genotype-to-phenotype map for drug susceptibility in Mycobacterium tuberculosis has become feasible as whole-genome sequencing is applied to strains selected for antibiotic resistance in vitro as well as large numbers of clinical isolates for which drug susceptibility patterns are known (6, 7). Recently, genome-wide association (GWA) approaches have been applied to identify genetic determinants of drug resistance in several bacterial pathogens (8). These studies benefit from directly examining mutations that have evolved in the host environment with physiological levels and timing of antibiotic exposure. However, because most novel associations identified in these studies to date have not been experimentally validated, there is limited understanding of what phenotypes these mutations confer and how to incorporate these associations into treatment decisions. Determining the relationship between genotype and phenotype has been complicated by the inconsistent findings between GWA studies, perhaps due to differences in the methods used or biological differences between strain sets. Furthermore, recent work has suggested that many different forms of altered susceptibility are possible which are not captured by standard phenotypic drug susceptibility testing (9, 10).
Using various genome-wide association approaches, three recent studies have specifically looked for genetic loci with mutations correlating with resistance to the antimycobacterial agent ethionamide (ETH) (11–13). ETH, or its analog prothionamide (PTH), is an orally available second-line agent often incorporated into regimens for multidrug-resistant TB (14). ETH is a prodrug activated by the bacterial monooxygenase EthA (Rv3854c) (15) which then forms an ETH-NAD adduct capable of inhibiting the production of essential mycolic acids by targeting the enoyl acyl carrier protein reductase InhA (16). A wide range of mutations in ethA, in the promoter of the fabG1-inhA operon causing overexpression of inhA, or within inhA itself alter susceptibility to ETH (17, 18); however, these mutations do not explain resistance in all clinical isolates (5), suggesting that additional genes contribute to ETH susceptibility. Moreover, not all strains with EthA loss-of-function mutations are phenotypically ETH resistant (19), further suggesting that other ETH activators may exist. Recurrent loss-of-function mutants in genes encoding two additional bacterial monooxygenases, mymA (Rv3083) and Rv0565c, were identified in a large clinical data set from Russia (19), and a recent study implicated mymA in ETH susceptibility during in vitro growth, where mutation of either ethA or mymA conferred intermediate resistance to ETH, whereas the double mutant conferred a much higher level of resistance (20). In this study, Rv0565c deletion did not similarly alter ETH susceptibility (20). However, another study suggested that Rv0565c mutations were specifically correlated with ethA mutations in only one bacterial lineage (13) and proposed that Rv0565c mutations may be compensatory mutations reflecting the complexity of assessing genetic contributions to drug susceptibility.
Here we sought to identify genes in which mutations are significantly correlated with ETH resistance and determine their impact on ETH susceptibility. We used an algorithm that we recently developed, phyOverlap (9), to assess a set of 145 strains from China that had been phenotyped for ETH resistance. The most significant novel association with ETH resistance was Rv0565c. Given the strength of the association and the mixed data in the literature, we here experimentally define the role of Rv0565c in ETH/PTH susceptibility in M. tuberculosis. We demonstrate that Rv0565c mutations provide a subtle but consistent survival benefit in the face of ETH treatment in both Euro-American and East Asian lineage strains and have an unexpectedly stronger effect in PTH, which is used in place of ETH in China. We further demonstrate that clinical strains that are considered ETH susceptible vary in their total expression of the three ETH activators, EthA, MymA, and Rv0565c, and that total activator expression correlates well with bacterial growth restriction in sub-MICs of drug.
RESULTS
Mutations in Rv0565c are associated with ethionamide resistance in clinical isolates.
To identify genes and intergenic regions in which mutations are associated with ETH resistance in clinical isolates, we used phyOverlap (9) to perform genome-wide association testing on a set of sequenced M. tuberculosis strains from China for which drug susceptibility phenotypes were available (11). Eighteen genes and intergenic regions were associated with ETH resistance at the genome-wide level (q < 0.01). Among these were several genes in which mutations are known to confer resistance to ETH, including ethA and the inhA promoter (Fig. 1a). Genes conferring resistance to other clinically utilized antibiotics, including rifampin and isoniazid, were also identified, which is expected given that M. tuberculosis genomes are in complete linkage and ETH is used only in the setting of preexisting drug resistances.
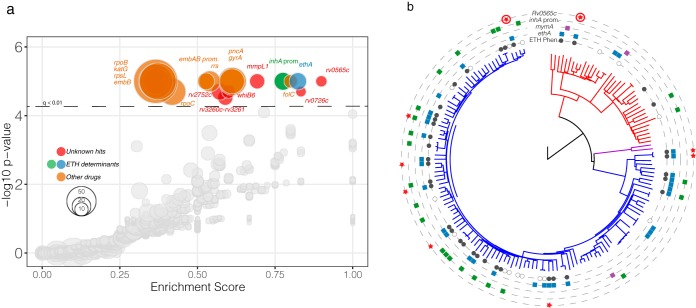
FIG 1.
Genome-wide association with ETH resistance identifies Rv0565c. (a) Genome-wide associations for all genes and intergenic regions containing variants among the 145 strains with ETH susceptibility testing. Each gene or intergenic region is plotted by the enrichment score (see Materials and Methods) and phyOverlap P value. (b) The genome-wide SNP phylogeny of all 161 strains from Zhang et al. (11) rooted with M. canettii. Phenotypic ETH resistance is indicated in the innermost circle where open circles are strains for which phenotyping was not performed. Variants in the monooxygenase genes ethA, mymA, and Rv0565c as well as the promoter of inhA, excluding common-lineage-defining SNPs, are indicated in each ring. Circled stars in the Rv0565c ring represent isolates with indel mutations not included in the SNP analysis.
Among the significant genetic associations, mutations in Rv0565c were most strongly enriched in ETH-resistant isolates, almost perfectly overlapping with phenotypic ETH resistance. Mutations in this gene were identified as enriched in strains with ETH, ofloxacin, kanamycin, and capreomycin resistances in the original analysis of these strains (11). Among the 36 ETH-resistant strains in this data set, there were 6 independent SNPs in Rv0565c. No Rv0565c mutations were found in susceptible strains except the R110H variant that is shared by all isolates from the phylogenetically defined lineage 2, also known as the East Asian lineage (21). One additional significant gene, Rv0726c, mostly coincided with ETH resistance, while the remaining genes, including mmpL1, whiB6, and Rv2752c, and the Rv3260c-Rv3261 intergenic region were grouped closer to known determinants of resistance to other antibiotics (Fig. 1a), suggesting they may play a role in resistance to another agent.
To further characterize the genomic context in which Rv0565c mutations are found, we manually examined the sequence of Rv0565c, ethA, mymA, and the inhA promoter in all 145 strains for which ETH susceptibility was tested as well as the additional 16 strains in which no ETH phenotype was available (Fig. 1b; see also Table S1a to c in the supplemental material). We identified an additional two ETH-resistant isolates with frameshift mutations in Rv0565c, which were not included in the genome-wide SNP analysis (Fig. 1b, circled stars). Although Rv0565c mutations (except R110H) occurred specifically within ETH-resistant isolates, in every case these mutations cooccurred with ethA mutations (Fig. S1). Overall, mymA mutants were rare in this data set, occurring in only 3 isolates, two of which were scored as ETH susceptible while the other one was not tested. To examine whether Rv0565c mutations always occur in the background of ethA mutations, we expanded the analysis of Rv0565c and ethA to an additional 549 strains from China for which ETH susceptibility was not determined (9). Among these strains, we identified 20 strains with Rv0565c mutations, excluding the R110H site, of which only 10 strains also contained mutations within ethA, suggesting that Rv0565c mutations do not always occur subsequent to ethA mutation (Fig. S1; Table S2).
The overlap of Rv0565c and ethA mutations among strains from Zhang et al. (11) and Hicks et al. (9). The common, lineage-associated mutations Rv0565c-R110H, ethA-S266R, and ethA-P334A were excluded from this analysis. Download FIG S1, PDF file, 0.8 MB (825.8KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complete list of Rv0565c (a), mymA (b), and ethA (c) mutations found in the 161 strains from Zhang et al. (11) included in Fig. 1b. Download Table S1, XLSX file, 0.01 MB (14.2KB, xlsx) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genotypes of Rv0565c and ethA variants in the 549 strains analyzed from Hicks et al. (9) (Fig. S1, right panel). Download Table S2, XLSX file, 0.01 MB (12.5KB, xlsx) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Rv0565c expression confers hypersensitivity to ETH in Mycobacterium smegmatis by targeting inhA.
Based on sequence homology, Rv0565c is a Baeyer-Villiger monooxygenase in the same family as the known activators ethA and mymA (20). We hypothesized that Rv0565c may likewise be capable of activating ETH. To test this hypothesis, we first attempted to purify recombinant Rv0565c protein to assess biochemical function, but we were unable to obtain soluble protein. Therefore, we performed a genetic analysis of Rv0565c-related ETH susceptibility in the environmental mycobacterium M. smegmatis.
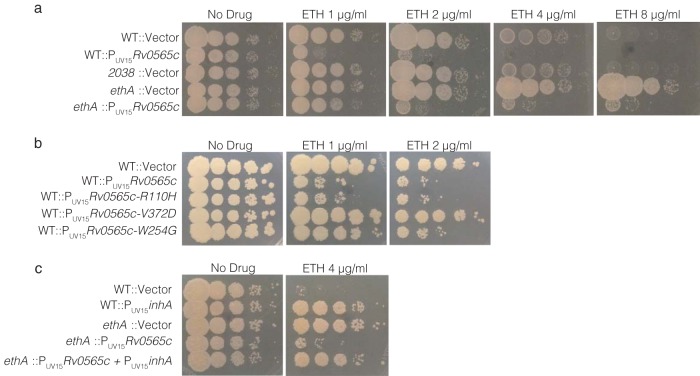
M. smegmatis contains genes homologous to ethA (Msmeg_6440) and Rv0565c (Msmeg_2038) but no mymA homolog. As expected, deletion of ethAMsmeg dramatically increased bacterial growth on increasing concentrations of ETH. Deletion of Msmeg_2038 did not alter ETH susceptibility (Fig. 2a). However, constitutive expression of Rv0565c under the strong UV15 promoter hypersensitized M. smegmatis to ETH, even in the absence of ethAMsmeg, suggesting that Rv0565c can act as an independent activator of ETH (Fig. 2a). Consistent with the model that Rv0565c directly acts on ETH rather than altering mycolic acid metabolism, constitutive expression of Rv0565c failed to sensitize M. smegmatis to isoniazid (INH), which shares the same target as ETH but is activated by the bacterial catalase-peroxidase katG (Fig. S2).
FIG 2.
Rv0565c activates ETH to target inhA in M. smegmatis. (a to c) Growth of indicated M. smegmatis strains was monitored by spot plating of serial 10-fold dilutions of the indicated strains on solid medium containing various concentrations of ETH. Images were taken after 3 days of growth.
Growth of indicated M. smegmatis strains was monitored by spot plating of serial 10-fold dilutions of the indicated strains on solid medium containing various concentrations of INH and ETH. Images were taken after 2.5 days of growth. Download FIG S2, PDF file, 8.4 MB (8.6MB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Although we identified two clinical isolates in which Rv0565c was the target of frameshifting mutations, several isolates contain SNPs for which the functional consequence is not obvious. Further, lineage 2 (East Asian) isolates all share an R110H variant in Rv0565c which could alter Rv0565c activity within this entire lineage. Using the hypersensitivity phenotype in M. smegmatis, we tested the effect of the common R110H variant as well as a panel of Rv0565c amino acid variants found in ETH-resistant M. tuberculosis strains. Overexpression of Rv0565c containing the R110H variant conferred hypersensitization to ETH that was indistinguishable from the WT allele, suggesting that R110H does not alter Rv0565c function (Fig. 2b). In contrast, strains overexpressing allelic variant R87G, A272S, R296L, or V372D behaved like a strain carrying an empty vector, suggesting these mutations confer near-complete loss of function. Strains expressing allelic variant H95D, W172S, W254G, or N291H grew more robustly on low doses of ETH but not higher doses, suggesting these alleles confer partial loss of function (Fig. 2b; Fig. S3).
Growth of indicated M. smegmatis strains was monitored by spot plating of serial 10-fold dilutions of the indicated strains on solid medium containing various concentrations of ETH. Images were taken after 2.5 days of growth. Download FIG S3, PDF file, 10.5 MB (10.8MB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ETH activated by ethA is known to target inhA (16); however, it is possible that Rv0565c activation of ETH targets another essential process. To test whether Rv0565c-activated ETH similarly targets InhA, we constructed two additional strains overexpressing inhA either in a wild-type background or in an ΔethA strain with strong constitutive expression of Rv0565c, in which all ETH activation occurs through the activity of Rv0565c. As expected, inhA overexpression or ethA deletion alone increased growth on a restrictive concentration of ETH. In the sensitized ΔethA::PUV15Rv0565c strain, where all ETH activation is provided by Rv0565c, inhA expression also increased growth on ETH, consistent with InhA being the target of ETH activated by Rv0565c (Fig. 2c).
Rv0565c contributes to ETH susceptibility in M. tuberculosis.
Given the association between Rv0565c mutations and ETH resistance in clinical isolates as well as the ability of Rv0565c overexpression to confer susceptibility to ETH in M. smegmatis, we next assessed the effect of Rv0565c activity in M. tuberculosis. Similarly to M. smegmatis, overexpression of Rv0565c rendered the laboratory M. tuberculosis strain H37Rv hypersensitive to ETH, reducing the IC50 of ETH 36.6-fold as determined by alamarBlue reduction assay (Fig. 3a). Expression of the R110H allele conferred hypersensitivity similarly to expression of the wild-type allele, while the susceptibility of the strain expressing the V372D allele was indistinguishable from a strain carrying an empty expression vector.
FIG 3.
Rv0565c expression alters ETH susceptibility in H37Rv. (a) Growth of strains overexpressing Rv0565c wild-type or SNP-containing alleles in various concentrations of ETH as measured by alamarBlue reduction. Each point represents the mean and standard deviation from three technical replicates, and the line represents the nonlinear regression of growth dependent on concentration of antibiotic. (b) Comparison of the growth of H37Rv (+) and the H37Rv deletion of Rv0565c (Δ) at various concentrations of ETH. Each point represents the mean growth from three cultures within an experiment with each of four experimental replicates connected by a line. The number below each set of points is the average ratio of deletion over wild type across the 4 experiments. *, P < 0.05; **, P < 0.01, two-tailed paired t test comparing growth at each concentration.
Because the clinically prevalent mutations in Rv0565c appear to abrogate function, we next constructed an Rv0565c deletion strain in M. tuberculosis in the H37Rv background and compared its growth with wild-type H37Rv in ETH across a range of concentrations in which growth of the wild type is completely or partially inhibited. Concentrations which inhibited the growth of wild-type H37Rv were able to inhibit the growth of the ΔRv0565c strain. However, we observed that for a number of drug concentrations in which bacterial growth was partially inhibited (Fig. 3b), growth of the ΔRv0565c strain was modestly but significantly increased compared with the wild type across four biologically independent experiments performed on different days. As measured by area under the growth curve across concentrations (AUC), growth of the ΔRv0565c strain was on average 8.6% greater than the wild type (Fig. S4a and b) (range, 7.9% to 9.7%; two-tailed paired t test, P = 0.004).
(a and c) Representative graph of four biological replicates showing the growth of H37Rv and H37Rv Rv0565c deletion mutant (a) or 631 and 631 Rv0565c deletion mutant (c) in various concentrations of ETH. Points represent the mean from three technical replicates. (b and d) Paired area-under-the-curve measurements across 4 biological replicates for H37Rv (b) and strain 631 (d). The numbers to the right of each point represent the ratio of AUC of the deletion to wild type. **, P < 0.01, two-tailed paired t test of AUC values. Download FIG S4, PDF file, 0.8 MB (819.7KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Monooxygenase expression contributes to baseline ETH susceptibility in clinical isolates.
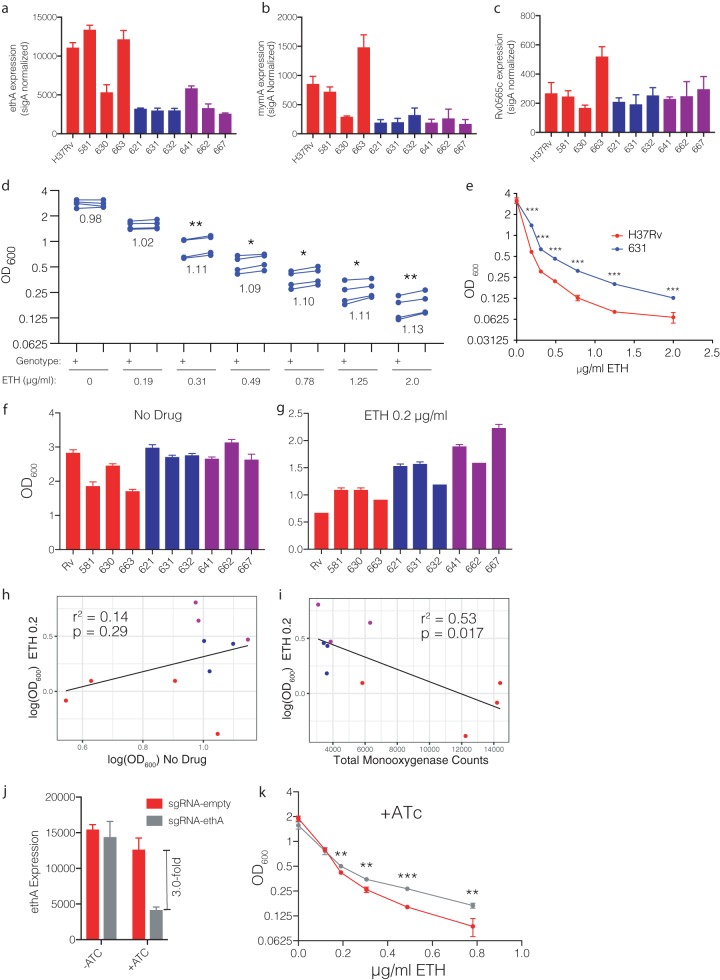
Given the modest contribution of Rv0565c deletion to ETH susceptibility in H37Rv (lineage 4), we next considered whether the magnitude of the effect of Rv0565c deletion might depend on strain background. One GWA study of ETH resistance failed to correlate Rv0565c mutations with ETH resistance overall; however, the authors found that Rv0565c mutations were correlated with ethA mutations specifically in lineage 2/East Asian isolates (13). In our data set, the majority of mutations likewise occurred in lineage 2/East Asian strains; however, given the fact that this set of strains from China is dominated by lineage 2/East Asian strains, it was not possible to comment on whether this skewing was biologically meaningful. One possible explanation for differences in selective pressure among different strains would be if monooxygenases were expressed at different levels, leading to different relative contributions to ETH susceptibility compared with H37Rv. Using a panel of previously characterized clinical isolates (22) from lineages 1, 2, and 4, we examined expression of ethA, mymA, and Rv0565c (Fig. 4a to c). We observed that the expression levels of ethA and mymA were highly variable among clinical isolates, with all three lineage 2 isolates expressing <30% of the amount of each gene compared to H37Rv. Expression of Rv0565c was much less variable. However, given the lower expression of both ethA and mymA in lineage 2/East Asian isolates, we hypothesized that as a greater fraction of the monooxygenase pool, Rv0565c might make a greater contribution to ETH susceptibility in lineage 2/East Asian strains. To test this hypothesis, we constructed an Rv0565c deletion mutant in the lineage 2 clinical isolate 631. Deletion of Rv0565c had a remarkably similar effect on ETH susceptibility in 631 as in H37Rv where growth was increased by ∼10% across a range of partially inhibitory concentrations, albeit across a wider range of concentrations (Fig. 4d). The AUC in partially inhibitory concentrations of ETH was increased on average by 11.5% (Fig. S4c and d) (range, 7.9% to 16.3%; two-tailed paired t test, P < 0.01). Thus, Rv0565c deletion conferred increased growth in partially inhibitory concentrations of ETH in both H37Rv and East Asian strain 631, but these data did not suggest a larger role for Rv0565c in lineage 2 isolates.
FIG 4.
Monooxygenase expression affects baseline ETH susceptibility. (a to c) Expression of the monooxygenase genes ethA, mymA, and Rv0565c as measured by NanoString and normalized to expression of sigA. Each bar represents the mean normalized expression from three separate cultures, and error bars represent the standard deviation. Colors correspond to lineage designation, with red for lineage 4, blue for lineage 2, and purple for lineage 1. (d) Comparison of the growth of strain 631 (+) and the 631 deletion of Rv0565c (Δ) at various concentrations of ETH. Each point represents the mean growth from three cultures within an experiment with each of four experimental replicates connected by a line. The number below each set of points is the average ratio of deletion over wild type across the 4 experiments. *, P < 0.05; **, P < 0.01, two-tailed paired t test comparing growth at each concentration. (e) Growth of H37Rv and strain 631 across a range of ETH concentrations. Each point represents the mean OD600 from three cultures and is representative of 4 experiments. ***, q < 0.001, two-tailed t tests with false-discovery rate correction for multiple tests. (f and g) Growth of clinical isolates as measured by OD600 after 7 days of growth in either drug-free medium (f) or medium containing 0.2 μg/ml ETH (g). (h and i) Correlation of growth in 0.2 μg/ml ETH with growth in drug-free medium (h) or with the sum of monooxygenase expression across strains (i). The r2 and P values represent the fit of the linear regression plotted on each graph. (j) Knockdown efficiency of ethA in H37Rv as measured by NanoString. Each bar represents the mean ethA expression from three independent cultures normalized to sigA expression with error bars representing the standard deviation. (k) Growth of the H37Rv ethA knockdown strain compared with an empty guide control in the presence of ATc across a range of ETH concentrations. Each point represents the mean from three cultures. **, q < 0.01; ***, q < 0.001, two-tailed t tests with false-discovery rate correction for multiple tests.
In these analyses, we noted that there was a substantial difference in the growth of wild-type H37Rv and M. tuberculosis strain 631 in the same ETH concentrations (Fig. 4e). Given the previously proposed model that EthA and MymA make independent contributions to ETH activation, we hypothesized that growth in partially inhibitory concentrations of ETH may be explained by the total expression of EthA, MymA, and Rv0565c among clinical isolates. Growth among strains was variable in the absence of antibiotics, as well as in the presence of 0.2 μg/ml ETH (Fig. 4f and g). However, growth in the presence of ETH was not statistically correlated with growth in no-drug medium (Fig. 4h) (r2 = 0.14, P = 0.29). In contrast, total monooxygenase expression was negatively correlated with growth in the presence of ETH (Fig. 4i) (r2 = 0.53, P = 0.017). Monooxygenase expression remained negatively correlated with growth in the presence of ETH even when controlling for growth in no-drug medium using multiple regression (no-drug growth, P = 0.24; monooxygenase expression, P = 0.020). This correlation was also observed at higher doses of ETH in which growth was further suppressed (Fig. S5). To further test the model that differences in the total level of monooxygenase expression affect M. tuberculosis growth in partially inhibitory concentrations of ETH, we conditionally knocked down ethA expression in H37Rv using CRISPR-mediated interference (23). Consistent with the model where baseline monooxygenase expression affects growth at low doses of ETH, modest (∼3-fold) ethA depletion significantly increased growth in partially inhibitory concentrations of ETH (Fig. 4j and k; Fig. S6). Importantly, our strains have no variants within ethA, its promoter, the ethA regulator ethR, or the putative promoter regions upstream of mymA and Rv0565c (22), which could explain the differences in monooxygenase expression levels (24). This finding suggests that there are as-yet-unrecognized genetic variants that underlie the differences in total monooxygenase expression and modulate ETH susceptibility.
(a) Growth of clinical isolates as measured by OD600 after 7 days of growth in medium containing 1.8 μg/ml ETH. (b) Correlation of growth in 1.8 μg/ml ETH with the sum of monooxygenase expression across strains. The r2 and P values represent the fit of the linear regression. Download FIG S5, PDF file, 0.4 MB (435.5KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth of the H37Rv ethA knockdown strain compared with an empty guide control in the absence of ATc induction across a range of ETH concentrations. Two-tailed t tests with false-discovery rate correction for multiple tests are annotated above each concentration. Download FIG S6, PDF file, 0.4 MB (395.8KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Rv0565c expression makes a significant contribution to prothionamide susceptibility.
We next considered other explanations for the reported association of Rv0565c mutations specifically with lineage 2/East Asian M. tuberculosis strains in a global data set. In China, the ethionamide analog prothionamide (PTH) is used in place of ETH for second-line treatment because PTH is manufactured in China (Y. Zhao, personal communication). These antibiotics are considered interchangeable in MDR regimen design (14); however, studies directly comparing isolate susceptibility to ETH with that to PTH consistently show a lower MIC for PTH (25, 26), suggesting that they are not completely biologically equivalent. To test whether Rv0565c may contribute more to PTH susceptibility than to ETH susceptibility, we repeated the characterization of M. tuberculosis Rv0565c mutants during exposure to PTH.
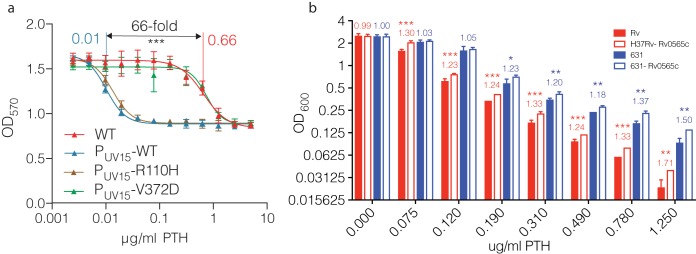
Consistent with the observed difference in ETH and PTH activity in previous studies of clinical isolates, the IC50 of PTH was 0.66 μg/ml compared with 2.23 μg/ml for ETH in H37Rv. Unexpectedly, overexpression of Rv0565c in H37Rv reduced the IC50 of PTH to 0.01 μg/ml, a change of 66-fold, which was 1.8-fold greater than the 36.6-fold change observed for ETH comparing the same strains (Fig. 5a). Deletion of Rv0565c in H37Rv likewise conferred a larger growth advantage in PTH than in ETH (Fig. 5b), where the deletion grew 23 to 71% more across partially inhibitory concentrations of PTH (Fig. 5b) and the AUC for the Rv0565c deletion mutant was on average 26.9% greater in partially inhibitory concentrations of PTH, which was significantly greater than the 8.6% increase in ETH (Tukey’s multiple-comparison test, adjusted P = 0.0003). This finding was replicated in East Asian strain 631, where deletion of Rv0565c increased growth by 18 to 50% in partially inhibitory concentrations (Fig. 5b) and increased the AUC in PTH by 20.7% compared with 11.5% in ETH (Tukey’s multiple-comparison test, adjusted P = 0.02). Taken together, these data suggest that Rv0565c activity has a greater effect on PTH susceptibility than on ETH susceptibility, perhaps due to enzyme affinity, and programmatic differences in the use of ETH versus PTH may explain geographic differences in the emergence of resistance mutations.
FIG 5.
Rv0565c expression more substantially alters PTH susceptibility. (a) Growth of strains overexpressing Rv0565c wild-type or SNP-containing alleles in various concentrations of PTH as measured by alamarBlue reduction. Each point represents the mean and standard deviation from three technical replicates, and the line represents the nonlinear regression of growth dependent on concentration of antibiotic. (b) Growth of H37Rv and strain 631 with their corresponding Rv0565c deletion mutants in various concentrations of PTH. Bar height represents the mean from three independent cultures with error bars showing standard deviations. The number above the bars is the ratios of the average of mutant over wild-type OD600. Data are representative of two experiments. *, q < 0.05; **, q < 0.01; ***, q < 0.001; two-tailed t tests with false-discovery rate correction for multiple tests.
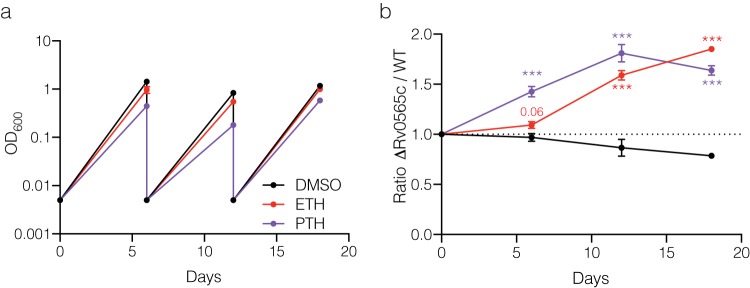
To further corroborate our finding that deletion of Rv0565c confers a meaningful growth advantage in the presence of antibiotic pressure, we performed long-term competition assays using strain 631 and the 631 Rv0565c deletion strain. Strains were cocultured in the absence of antibiotic selection or in the presence of ETH or PTH at 0.25 μg/ml, which partially inhibited growth (Fig. 6a). Using qPCR of genomic DNA targeting regions specific to each strain to quantify the relative abundance, we observed that by 6 days of exposure, the deletion mutant became 10% more abundant in ETH while in PTH it became 40% more abundant (Fig. 6b). This measurement was remarkably consistent with that taken by OD600 readings for individual strains (Fig. 4d and Fig. 5b). Upon redilution and continued growth, the differences in abundance expanded to 59% and 81% in ETH and PTH, respectively, by 12 days, and by 18 days the deletion mutant was 85% and 64% more abundant, respectively (Fig. 6b). These findings demonstrate that the relatively small advantage conferred by Rv0565c deletion can be expanded over longer antibiotic exposures.
FIG 6.
Rv0565c deletion in strain 631 confers a long-term growth benefit in ETH and PTH. (a) Growth of a pooled mixture of strain 631 and 631 Rv0565c deletion mutant in the indicated medium as measured by OD600. Strains were rediluted to 0.005 every 6 days. (b) The ratio of Rv0565c mutant over wild-type strain 631 over the course of treatment normalized to the starting library ratio on day 0. Each point represents the mean value from three qPCR technical replicates of three independent cultures with error bars showing the standard deviations among the three cultures. Each time point was compared with the input ratio by multiple t tests after two-way ANOVA and corrected for multiple hypothesis testing. ***, q < 0.001.
DISCUSSION
The intense selective pressure of antibiotic therapy against TB has led to the evolution of strains with a diverse set of mutations undermining treatment. Although the gold standard for measuring antibiotic susceptibility is growth-based phenotypic testing, genotype-based diagnostics are increasingly offering a more rapid and sensitive approach in cases where the full spectra of mutations underlying resistance are understood. For the widely used second-line antibiotic ethionamide (ETH), however, mutations in ethA and inhA and its promoter are able to predict phenotypic resistance with only ∼65% sensitivity (5). The recent discovery of ethA promoter mutants (27) and the implication of mymA in ETH susceptibility (20) promise to increase this sensitivity; however, it remains unclear whether this list is exhaustive. Here we examine the genomes of clinical isolates phenotyped for ETH resistance and identify several loci associated with resistance, including the monooxygenase Rv0565c. By overexpression of Rv0565c, we clearly demonstrate that it is an activator of ETH and PTH in M. tuberculosis, and mutations observed in clinical isolates confer partial or complete loss of function. Nevertheless, deletion of Rv0565c confers only a small growth advantage during treatment with ETH or PTH in vitro. This leads us to consider three alternate hypotheses regarding the impact of these mutations on antibiotic efficacy in vivo.
First, it is possible that our in vitro conditions poorly capture the true effect of Rv0565c activity in vivo and thus the effect of Rv0565c mutations. Expression of Rv0565c is low compared with ethA and mymA during growth in standard 7H9 medium, based on NanoString (Fig. 4) and qPCR analysis (data not shown). Thus, deletion of Rv0565c likewise can confer only a modest benefit in vitro. However, under any condition in which expression of Rv0565c is upregulated, wild-type strains may more closely resemble the Rv0565c overexpression strains, where introduction of a loss-of-function polymorphism may then dramatically increase resistance to ETH/PTH. Indeed, Rv0565c appears to be upregulated in macrophages while mymA is downregulated (28), suggesting that characterization of various monooxygenase mutants in infection models may help elucidate their relative roles in different contexts.
Second, although deletion of Rv0565c in the lineage 2 clinical isolate 631 failed to confer a more substantial shift in ETH IC50 compared with the laboratory strain H37Rv, it is possible that the balance of monooxygenase expression in other clinical isolates, even as measured in vitro, favors mutations in Rv0565c or mymA rather than the canonical activator ethA. Supporting this hypothesis, one study of serial isolates derived from individual patients during treatment identified acquisition of an Rv0565c mutation coinciding with the conversion from ETH susceptible to ETH resistant by standard phenotypic susceptibility testing (29).
Finally, it is possible that small changes in the ability of strains to grow in the presence of ETH or PTH may confer enough of an advantage to be selected for over 18 to 24 months of second-line antibiotic therapy. This selective advantage may not be trivial from the perspective of patient and caregiver, as Colangeli et al. recently demonstrated that small differences in isoniazid and rifampin MICs among supposedly susceptible strains could partially explain treatment outcome in a small cohort of patients (10). In this case, the differences in growth in partially inhibitory concentrations of ETH and PTH among clinical isolates based upon the expression of their monooxygenases may also prove an important determinant of treatment outcome. Examination of the ethA promoter and the known regulator ethR revealed no variation among the panel of clinical isolates which we studied; however, the underlying genetic determinants of ethA expression variation could now be identified using a genome-wide association approach directly correlating genetic variants with ethA expression.
In any of these three models, the integration of GWA and experimental data suggest the importance of incorporating Rv0565c mutations into decisions about the use of ethionamide and prothionamide in MDR treatment regimens. Although it is clear that high-level resistance as measured in vitro is a partial predictor of treatment response, small changes in MIC conferred by Rv0565c mutation and activator expression, as well as condition-dependent phenotypes experimentally defined in the context of genome-wide associations in clinical isolates, may allow us to refine treatment choices.
MATERIALS AND METHODS
Genomic data analysis and genetic association.
Genomic data were downloaded from the NCBI Sequence Read Archive accession numbers SRA065095 and PRJNA268900. To identify genetic variants, paired-end data were aligned against the H37Rv reference genome NC_00962.3 using the bwa mem algorithm implemented in bwa 0.9.7a (30), and variants were identified using the Genome Analysis Toolkit (31) version 3.5 haplotype caller set for haploid genomes and emitting reference confidence calls at base-pair resolution. Base-pair-resolution genomic variant call formats (gVCFs) were merged to make a master VCF from which variants were identified using the GenotypeGVCFs tool. For phylogenetic and genetic association analysis, the variants were filtered to retain only SNPs, which were annotated using snpEff (32). For phylogenetic analysis, the Mycobacterium canettii strain CIPT 140010059 genome (NC_15848.1) was used as an outgroup. To identify variants separating M. canettii from H37Rv, the wgsim function of SAMtools (33) was used to simulate 3 million 72-bp-paired end reads which were then analyzed like any other sample.
To construct the phylogenetic tree, SNPs in repetitive regions of the genome or regions known to confer antibiotic resistance were removed as previously described (9). To remove low-confidence calls, a manual threshold was set to call either a variant or reference alleles, where any site in any sample not achieving 10× read coverage with at least 80% of bases in agreement was masked as missing data. In addition, any site in which fewer than 90% of isolates achieved a high-confidence call was removed from the analysis. Phylogenetic tree reconstruction was performed using RAxML (34) v8.2.11 with the GTRCAT model of nucleotide evolution and rooted using M. canettii as the outgroup. Trees were visualized using the online interactive Tree of Life server (35). Ancestral sequence reconstruction was performed with RAxML v8.2.11 as well to determine the most probable ancestral allele at each SNP site. To determine the number of times each mutation evolved in the data set, the alleles were mapped back onto the whole-genome phylogenetic tree and the parsimony score was calculated with the Fitch algorithm as implemented in the R package Phangorn (36).
Genome-wide associations were calculated as previously described using the phyOverlap algorithm (9). Sites which differed from H37Rv only in M. canettii were excluded from analysis, as were synonymous mutations. Enrichment scores for each gene are calculated as the fraction of isolates containing a variant that are also resistant to ETH averaged across all sites within a gene weighted by the number of times a mutation evolves across the phylogenetic tree. This value is a standard output of phyOverlap. False-discovery rate correction was performed using the Benjamini-Hochberg procedure. Strains without a phenotypic drug susceptibility test (DST) for ethionamide (n = 15) were removed from this analysis.
Further analysis of SNPs and indels affecting Rv0565c, mymA, and ethA shown in Fig. 1 and in Table S1a to c in the supplemental material was performed by manual inspection of the variant matrix produced by Genome Analysis Toolkit (GATK) which includes both types of variants.
To examine ethA, ethR, and their promoters in the panel of clinical isolates, the genomes assembled in the work of Carey et al. (22) were downloaded from the SRA database. Alignment was performed for each gene, including 100 bp upstream of the open reading frame, against all genomes using the online BLAST tool megablast with default settings (37).
Bacterial growth.
Mycobacterium smegmatis strains were grown and maintained in 7H9 Middlebrook medium supplemented with 0.2% glycerol, 5 g/liter BSA fraction V, 2 g/liter dextrose, 0.85 g/liter sodium chloride, and 0.05% Tween 80. Plating assays were performed on 7H10 Middlebrook medium supplemented with 0.5% glycerol, 5 g/liter BSA fraction V, 2 g/liter dextrose, 0.85 g/liter sodium chloride, and 0.05% Tween 80 and antibiotics at the indicated concentrations.
Mycobacterium tuberculosis was grown and maintained in 7H9 Middlebrook medium supplemented with 0.2% glycerol, 10% Middlebrook oleic acid-albumin-dextrose-catalase (OADC), and 0.05% Tween 20.
Strain and mutant construction.
M. smegmatis strains were constructed in the mc2155 strain background. Deletion of Msmeg_2038 and ethAMsmeg was performed using recombineering as described by Murphy et al. (38). Briefly, a hygromycin resistance cassette flanked with lox sites and then 500 bp of sequence flanking the start and stop codon of each gene was electroporated into mc2155 expressing the RecET phage proteins. Transformants were selected on hygromycin-containing medium and PCR validated for replacement of the gene of interest with the resistance cassette. For the Msmeg_2038 deletion, the hygromycin cassette was subsequently removed by expression of Cre recombinase. Overexpression strains of rv0565c and inhA were constructed by integration of a kanamycin-resistant plasmid at the L5 phage integration site containing the gene of interest under the control of the constitutive UV15 promoter.
H37Rv and strain 631 mutants with deletions of rv0565c were constructed using the ORBIT method (39). Strains carrying the recT expression vector pKM461 were cotransformed with the ORBIT oligonucleotide GATCTGGAGGGGCTGGCTGGATGTCCTGGCTACCCTGGTCGCTGATCCAGGATTTCAAGCGAGGTTCACG[GGTTTGTCTGGTCAACCACCGCGGTCTCAGTGGTGTACGGTACAAACC]TTGCTCCTGCGGTTCGTCGACGGATATCAAATGTCCGGTTGCCGCAATACGCTGAACGCCATGGCAGCCA (with the attB site bracketed) and pKM464, which results in a total deletion of rv0565c and replacement with the pKM464 hygromycin-resistant plasmid. Overexpression M. tuberculosis strains of Rv0565c and SNP variants were constructed by integration of a kanamycin-resistant plasmid at the L5 phage integration site containing the gene of interest under the control of the constitutive UV15 promoter.
CRISPR-mediated knockdown was performed as described by Rock et al. (23) using the pJR965 vector. The ethA knockdown strain was transformed with the guide targeting GGCGGCCGTGTACAGCGCCTG while the control strain was transformed with the unmodified pJR965.
Antibiotic susceptibility testing.
Antibiotic susceptibility testing was performed for M. smegmatis by growing the indicated strains to mid-logarithmic phase, diluting each to an OD600 of 0.2, and then spotting 2 μl (Fig. 2), 5 μl (Fig. S2), or 8 μl (Fig. S3) of serial 10-fold dilutions from undiluted to 10−4 on 7H10 agar as described above with antibiotics at the indicated concentrations. Images were taken after 2 to 3 days of growth as indicated in the figure legends.
Antibiotic susceptibility testing of M. tuberculosis strains overexpressing rv0565c was performed using the alamarBlue reduction to measure growth. Indicated strains were grown to mid-logarithmic phase and then seeded at an OD600 of 0.0005 in 200 μl of 7H9 OADC medium with antibiotics at the indicated concentration. After 4 days of growth with shaking at 37°C, 20 μl of alamarBlue (Bio-Rad) was added and the plates were incubated for an additional day. Reduction of alamarBlue was measured by absorbance at OD570.
Growth of Rv0565c deletion strains in antibiotics was performed in a similar manner. Indicated strains were grown to mid-logarithmic phase and then diluted to 0.01 in 600 μl of medium containing ETH or PTH at serial 1.6-fold dilutions. The concentrations of ETH (Fig. 3b) ranged from 2 to 0.12 μg/ml. For PTH (Fig. 5b), a lower range was used due to the increased potency of PTH, with the concentrations ranging from 1.25 to 0.075 μg/ml. Optical density was measured after 6 days of growth with shaking at 37°C. For experiments with CRISPR-mediated knockdown, anhydrous tetracycline (ATc) was added at a final concentration of 100 ng/ml to strains 2 days prior to dilution into antibiotic medium and was maintained throughout the assay. For clinical isolates, the protocol was slightly modified by reducing the input dilution to 0.005 and strains were allowed to grow for 7 days in drug-containing medium before final optical density was read.
Gene expression analysis.
RNA was extracted as previously described (22). Briefly, strains were grown to mid-logarithmic phase, spun down at 4,000 rpm for 10 min, and resuspended in TRIzol reagent. Cells were disrupted by bead beating, and 1/5 volume of chloroform was added. RNA was extracted from the TRIzol lysate using the Direct-zol miniprep kit (Zymo Research), with an additional in-tube DNA digestion with Turbo DNase (ThermoFisher Scientific) to remove residual DNA. Expression was assessed using the nCounter chemistry on the NanoString Sprint platform with 25 ng of total RNA as input. Expression of monooxygenases was normalized to expression of sigA within each sample using the nSolver software (NanoString Technologies). Hybridization probes used for transcript counting are included in Table S3.
NanoString probes for the detection of sigA, Rv0565c, mymA, and ethA (Fig. 4a to c). Download Table S3, XLSX file, 0.01 MB (9.7KB, xlsx) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Statistical analysis.
alamarBlue reduction curves (Fig. 3a and Fig. 5a) were analyzed in Prism 7 (GraphPad Software). Antibiotic concentrations were log transformed, and then nonlinear regression was performed using a 4-parameter curve to fit the OD570 values and estimate the IC50 for each strain with three technical replicates for each strain and drug concentration. Growth of strains at partially inhibitory concentrations of ETH and PTH in Fig. 3b and Fig. 4d was performed using three independent cultures across 4 separate experiments. Testing for differences in growth was assessed by two-tailed paired t tests with data from each of the 4 experiments paired to account for day-to-day variation in overall growth. Differences in growth shown in Fig. 4e and Fig. 5b were tested by multiple t tests comparing 3 cultures within a single experiment using the Benjamini, Krieger, and Yekutieli method as implemented by Prism 7. These measurements are representative of 4 and 2 experiments, respectively. Area-under-the curve (AUC) measurements shown in Fig. S4 were assessed for each biological replicate consisting of three technical replicates with separate wells for each strain. No-drug measurements were excluded from this analysis to measure the effect of genotype on growth in the presence of partially inhibitory concentrations of antibiotic. Comparison of growth among genotypes across biological replicates was performed with a two-sided paired t test of the AUC as implemented in Prism 7. Comparison of ETH and PTH AUC ratios was performed by one-way ANOVA followed by Tukey’s multiple-comparison test. For both H37Rv and 631, the 4 biological replicates comparing each with its corresponding deletion in ETH were contrasted with the 2 biological replicates in PTH.
Competition growth assays.
Strain 631 and the 631 Rv0565c deletion were mixed 1:1 after expansion in antibiotic-free medium. The pooled mixture was then subcultured to an OD600 of 0.005 in 25 ml of medium containing ETH at 0.25 μg/ml, PTH at 0.25 μg/ml, or DMSO at an equal volume. Every 6 days for 18 days, the OD was measured and strains were rediluted to 0.005 in 25 ml of the corresponding medium. Simultaneously, genomic DNA (gDNA) was extracted from 10 ml of culture using a phenol-chloroform extraction protocol. Briefly, cultures were spun down at 4,000 rpm at room temperature. The supernatant was discarded, and pellets were resuspended in 700 μl TE-NaCl (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 50 mM NaCl) and transferred into bead-beating tubes. Three hundred microliters of 50:50 phenol-chloroform, pH 8, was then added, and cells were disrupted by two rounds of beating on a MagNA Lyser at setting 6.5 for 30 s each. Phase separation was performed by spinning in a microcentrifuge for 10 min at 4°C. The upper aqueous layer was transferred to a new tube to which RNase A was added at a final concentration of 80 μg/ml and allowed to incubate at 37°C for 30 min. Three hundred microliters of phenol-chloroform was then added again, and samples were removed from the BSL-3 laboratory. After another phase separation, the aqueous supernatant was mixed with 1/10 volume of 3 M sodium acetate, pH 5.2, and 1 volume of isopropanol. Samples were incubated at −20°C for >3 h and then precipitated by centrifugation at 14,000 rpm at 4°C for 15 min. DNA pellets were washed with 1 ml of 70% ethanol and resuspended in 10 mM Tris HCl, pH 8. DNA was quantified by Qubit assay, and then the relative abundance of each strain was measured by two qPCRs targeting the gene Rv0565c and the hygromycin resistance cassette, which are specific to the wild-type and Rv0565c deletion strains, respectively.
The abundance of each gene was measured for three independent cultures at each time point in technical qPCR triplicates using 0.45 ng of DNA as input. The relative abundance of each strain was calculated using the ΔΔCT method. First, the average CT of hygromycin was subtracted by the average Rv0565c CT for each culture, and then the average difference in the input library was subtracted from this value to account for differences at time zero. The fold abundance of mutant over wild type was then calculated as 2ΔΔCT in each culture. Each time point was compared with the input ratio by multiple two-tailed t tests after two-way ANOVA and corrected for multiple hypothesis testing using Prism 8. qPCR was performed on the Applied Biosystems Viia 7 platform using the iTaq Sybr Green supermix (Bio-Rad). The reaction mixtures were initially denatured for 3 min at 95°C, and then 40 cycles of PCR were performed with 30 s at 95°C and 45 s at 62°C followed by a melt curve. All samples exhibited unimodal melting profiles consistent with specific amplification. The Rv0565c detection primers were 5′-CAACTATGACGAGGGCTACAC-3′ and 5′-CCGATCACCACGATCTTCTT-3′. The hygromycin cassette detection primers were 5′-GTGACACAAGAATCCCTGTTACT-3′ and 5′-CCAGAATTCCTGGTCGTTCC-3′.
ACKNOWLEDGMENTS
We thank Michelle Gardner, who helped construct the Rv0565c deletion mutants in M. tuberculosis. We also thank Inna Krieger and James Sacchettini for their help in attempting to purify recombinant Rv0565c.
Support was provided by the National Institutes of Health grants 5U19AI109755-05 and 5U19AI107774-05 awarded to S.M.F.
A.F.C. was supported by NIH T32 AI007061, T32 CA009216, and K08 AI139339.
There are no competing financial interests to report.
Footnotes
Citation Hicks ND, Carey AF, Yang J, Zhao Y, Fortune SM. 2019. Bacterial genome-wide association identifies novel factors that contribute to ethionamide and prothionamide susceptibility in Mycobacterium tuberculosis. mBio 10:e00616-19. https://doi.org/10.1128/mBio.00616-19.
REFERENCES
- 1.Kendall EA, Azman AS, Cobelens FG, Dowdy DW. 2017. MDR-TB treatment as prevention: the projected population-level impact of expanded treatment for multidrug-resistant tuberculosis. PLoS One 12:e0172748. doi: 10.1371/journal.pone.0172748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, Odhiambo J, Amukoye E, Bah B, Kassa F, N’Diaye A, Rustomjee R, de Jong BC, Horton J, Perronne C, Sismanidis C, Lapujade O, Olliaro PL, Lienhardt C, OFLOTUB/Gatifloxacin for Tuberculosis Project. 2014. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 371:1588–1598. doi: 10.1056/NEJMoa1315817. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, Pappas F, Phillips PPJ, Nunn AJ, REMoxTB Consortium. 2014. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2018. WHO global tuberculosis report 2018. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/gtbr2018_main_text_28Feb2019.pdf. [Google Scholar]
- 5.Farhat MR, Sultana R, Iartchouk O, Bozeman S, Galagan J, Sisk P, Stolte C, Nebenzahl-Guimaraes H, Jacobson K, Sloutsky A, Kaur D, Posey J, Kreiswirth BN, Kurepina N, Rigouts L, Streicher EM, Victor TC, Warren RM, van Soolingen D, Murray M. 2016. Genetic Determinants of drug resistance in Mycobacterium tuberculosis and their diagnostic value. Am J Respir Crit Care Med 194:621–630. doi: 10.1164/rccm.201510-2091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safi H, Lingaraju S, Amin A, Kim S, Jones M, Holmes M, McNeil M, Peterson SN, Chatterjee D, Fleischmann R, Alland D. 2013. Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-β-D-arabinose biosynthetic and utilization pathway genes. Nat Genet 45:1190–1197. doi: 10.1038/ng.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker TM, Kohl TA, Omar SV, Hedge J, Del Ojo Elias C, Bradley P, Iqbal Z, Feuerriegel S, Niehaus KE, Wilson DJ, Clifton DA, Kapatai G, Ip CLC, Bowden R, Drobniewski FA, Allix-Béguec C, Gaudin C, Parkhill J, Diel R, Supply P, Crook DW, Smith EG, Walker AS, Ismail N, Niemann S, Peto TEA, Modernizing Medical Microbiology (MMM) Informatics Group. 2015. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect Dis 15:1193–1202. doi: 10.1016/S1473-3099(15)00062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falush D. 2016. Bacterial genomics: microbial GWAS coming of age. Nat Microbiol 1:16059. doi: 10.1038/nmicrobiol.2016.59. [DOI] [PubMed] [Google Scholar]
- 9.Hicks ND, Yang J, Zhang X, Zhao B, Grad YH, Liu L, Ou X, Chang Z, Xia H, Zhou Y, Wang S, Dong J, Sun L, Zhu Y, Zhao Y, Jin Q, Fortune SM. 2018. Clinically prevalent mutations in Mycobacterium tuberculosis alter propionate metabolism and mediate multidrug tolerance. Nat Microbiol 3:1032–1042. doi: 10.1038/s41564-018-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colangeli R, Jedrey H, Kim S, Connell R, Ma S, Chippada Venkata UD, Chakravorty S, Gupta A, Sizemore EE, Diem L, Sherman DR, Okwera A, Dietze R, Boom WH, Johnson JL, Mac Kenzie WR, Alland D, DMID 01-009/Tuberculosis Trials Consortium Study 22 Teams. 2018. Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med 379:823–833. doi: 10.1056/NEJMoa1715849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, Liu Z, Li C, Galwey N, Deng J, Zhou Y, Zhu Y, Gao Y, Wang T, Wang S, Huang Y, Wang M, Zhong Q, Zhou L, Chen T, Zhou J, Yang R, Zhu G, Hang H, Zhang J, Li F, Wan K, Wang J, Zhang X-E, Bi L. 2013. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet 45:1255–1260. doi: 10.1038/ng.2735. [DOI] [PubMed] [Google Scholar]
- 12.Desjardins CA, Cohen KA, Munsamy V, Abeel T, Maharaj K, Walker BJ, Shea TP, Almeida DV, Manson AL, Salazar A, Padayatchi N, O’Donnell MR, Mlisana KP, Wortman J, Birren BW, Grosset J, Earl AM, Pym AS. 2016. Genomic and functional analyses of Mycobacterium tuberculosis strains implicate ald in D-cycloserine resistance. Nat Genet 48:544–551. doi: 10.1038/ng.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coll F, Phelan J, Hill-Cawthorne GA, Nair MB, Mallard K, Ali S, Abdallah AM, Alghamdi S, Alsomali M, Ahmed AO, Portelli S, Oppong Y, Alves A, Bessa TB, Campino S, Caws M, Chatterjee A, Crampin AC, Dheda K, Furnham N, Glynn JR, Grandjean L, Minh Ha D, Hasan R, Hasan Z, Hibberd ML, Joloba M, Jones-López EC, Matsumoto T, Miranda A, Moore DJ, Mocillo N, Panaiotov S, Parkhill J, Penha C, Perdigão J, Portugal I, Rchiad Z, Robledo J, Sheen P, Shesha NT, Sirgel FA, Sola C, Oliveira Sousa E, Streicher EM, Helden PV, Viveiros M, Warren RM, McNerney R, Pain A, Clark TG. 2018. Genome-wide analysis of multi- and extensively drug-resistant Mycobacterium tuberculosis. Nat Genet 50:307–316. doi: 10.1038/s41588-017-0029-0. [DOI] [PubMed] [Google Scholar]
- 14.WHO. 2018. WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis. 2018 update. Pre-final text. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/2018/WHO.2018.MDR-TB.Rx.Guidelines.prefinal.text.pdf. [Google Scholar]
- 15.DeBarber AE, Mdluli K, Bosman M, Bekker LG, Barry CE. 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 97:9677–9682. doi: 10.1073/pnas.97.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Langley R, Gulten G, Dover LG, Besra GS, Jacobs WR, Sacchettini JC. 2007. Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med 204:73–78. doi: 10.1084/jem.20062100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 47:3799–3805. doi: 10.1128/AAC.47.12.3799-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilchèze C, Jacobs WR. 2014. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol Spectr 2:MGM2-0014-2013. doi: 10.1128/microbiolspec.MGM2-0014-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casali N, Nikolayevskyy V, Balabanova Y, Harris SR, Ignatyeva O, Kontsevaya I, Corander J, Bryant J, Parkhill J, Nejentsev S, Horstmann RD, Brown T, Drobniewski F. 2014. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat Genet 46:279–286. doi: 10.1038/ng.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant SS, Wellington S, Kawate T, Desjardins CA, Silvis MR, Wivagg C, Thompson M, Gordon K, Kazyanskaya E, Nietupski R, Haseley N, Iwase N, Earl AM, Fitzgerald M, Hung DT. 2016. Baeyer-Villiger monooxygenases EthA and MymA are required for activation of replicating and non-replicating Mycobacterium tuberculosis inhibitors. Cell Chem Biol 23:666–677. doi: 10.1016/j.chembiol.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, Parkhill J, Malla B, Berg S, Thwaites G, Yeboah-Manu D, Bothamley G, Mei J, Wei L, Bentley S, Harris SR, Niemann S, Diel R, Aseffa A, Gao Q, Young D, Gagneux S. 2013. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 45:1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey AF, Rock JM, Krieger IV, Chase MR, Fernandez-Suarez M, Gagneux S, Sacchettini JC, Ioerger TR, Fortune SM. 2018. TnSeq of Mycobacterium tuberculosis clinical isolates reveals strain-specific antibiotic liabilities. PLoS Pathog 14:e1006939. doi: 10.1371/journal.ppat.1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baulard AR, Betts JC, Engohang-Ndong J, Quan S, McAdam RA, Brennan PJ, Locht C, Besra GS. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J Biol Chem 275:28326–28331. [DOI] [PubMed] [Google Scholar]
- 25.Rüsch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. 2006. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol 44:688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schön T, Juréen P, Chryssanthou E, Giske CG, Sturegård E, Kahlmeter G, Hoffner S, Angeby KA. 2011. Wild-type distributions of seven oral second-line drugs against Mycobacterium tuberculosis. Int J Tuberc Lung Dis 15:502–509. doi: 10.5588/ijtld.10.0238. [DOI] [PubMed] [Google Scholar]
- 27.de Welzen L, Eldholm V, Maharaj K, Manson AL, Earl AM, Pym AS. 2017. Whole-transcriptome and -genome analysis of extensively drug-resistant Mycobacterium tuberculosis clinical isolates identifies downregulation of ethA as a mechanism of ethionamide resistance. Antimicrob Agents Chemother 61:e01461-17. doi: 10.1128/AAC.01461-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homolka S, Niemann S, Russell DG, Rohde KH. 2010. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog 6:e1000988. doi: 10.1371/journal.ppat.1000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merker M, Kohl TA, Roetzer A, Truebe L, Richter E, Rüsch-Gerdes S, Fattorini L, Oggioni MR, Cox H, Varaine F, Niemann S. 2013. Whole genome sequencing reveals complex evolution patterns of multidrug-resistant Mycobacterium tuberculosis Beijing strains in patients. PLoS One 8:e82551. doi: 10.1371/journal.pone.0082551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schliep KP. 2011. phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res 36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy KC, Papavinasasundaram K, Sassetti CM. 2015. Mycobacterial recombineering. Methods Mol Biol 1285:177–199. doi: 10.1007/978-1-4939-2450-9_10. [DOI] [PubMed] [Google Scholar]
- 39.Murphy KC, Nelson SJ, Nambi S, Papavinasasundaram K, Baer CE, Sassetti CM. 2018. ORBIT: a new paradigm for genetic engineering of mycobacterial chromosomes. mBio 9:e01467-18. doi: 10.1128/mBio.01467-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The overlap of Rv0565c and ethA mutations among strains from Zhang et al. (11) and Hicks et al. (9). The common, lineage-associated mutations Rv0565c-R110H, ethA-S266R, and ethA-P334A were excluded from this analysis. Download FIG S1, PDF file, 0.8 MB (825.8KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complete list of Rv0565c (a), mymA (b), and ethA (c) mutations found in the 161 strains from Zhang et al. (11) included in Fig. 1b. Download Table S1, XLSX file, 0.01 MB (14.2KB, xlsx) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genotypes of Rv0565c and ethA variants in the 549 strains analyzed from Hicks et al. (9) (Fig. S1, right panel). Download Table S2, XLSX file, 0.01 MB (12.5KB, xlsx) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth of indicated M. smegmatis strains was monitored by spot plating of serial 10-fold dilutions of the indicated strains on solid medium containing various concentrations of INH and ETH. Images were taken after 2.5 days of growth. Download FIG S2, PDF file, 8.4 MB (8.6MB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth of indicated M. smegmatis strains was monitored by spot plating of serial 10-fold dilutions of the indicated strains on solid medium containing various concentrations of ETH. Images were taken after 2.5 days of growth. Download FIG S3, PDF file, 10.5 MB (10.8MB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(a and c) Representative graph of four biological replicates showing the growth of H37Rv and H37Rv Rv0565c deletion mutant (a) or 631 and 631 Rv0565c deletion mutant (c) in various concentrations of ETH. Points represent the mean from three technical replicates. (b and d) Paired area-under-the-curve measurements across 4 biological replicates for H37Rv (b) and strain 631 (d). The numbers to the right of each point represent the ratio of AUC of the deletion to wild type. **, P < 0.01, two-tailed paired t test of AUC values. Download FIG S4, PDF file, 0.8 MB (819.7KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(a) Growth of clinical isolates as measured by OD600 after 7 days of growth in medium containing 1.8 μg/ml ETH. (b) Correlation of growth in 1.8 μg/ml ETH with the sum of monooxygenase expression across strains. The r2 and P values represent the fit of the linear regression. Download FIG S5, PDF file, 0.4 MB (435.5KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth of the H37Rv ethA knockdown strain compared with an empty guide control in the absence of ATc induction across a range of ETH concentrations. Two-tailed t tests with false-discovery rate correction for multiple tests are annotated above each concentration. Download FIG S6, PDF file, 0.4 MB (395.8KB, pdf) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
NanoString probes for the detection of sigA, Rv0565c, mymA, and ethA (Fig. 4a to c). Download Table S3, XLSX file, 0.01 MB (9.7KB, xlsx) .
Copyright © 2019 Hicks et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.